- 1Department of Pharmacology, Institute of Pharmacy, I.M. Sechenov First Moscow State Medical University, Moscow, Russia
- 2Scientific Center of Genetics and Life Sciences, Sirius University of Science and Technology, Sirius, Russia
- 3Department of Medical Elementology, Peoples’ Friendship University of Russia (RUDN University), Moscow, Russia
- 4Department of Biosciences, School of Medicine, Nazarbayev University, Astana, Kazakhstan
Low-grade inflammation, both hypothalamic and systemic, sensitizes the neuroendocrine response to osmotic stimuli whose proximate cause is chronic underhydration common in older adults due to diminished thirst perception. These events drive persistent vasopressin (VP) release. VP exerts antidiuretic effects via renal V2 receptors and functions as a stress hormone through widely expressed V1a and V1b receptors. These latter actions are central to inappropriate activation of the hypothalamic-pituitary-adrenal axis observed in aging, as VP stimulates secretion of the adrenocorticotropic hormone. The resulting sustained elevations in circulating VP and cortisol contribute to metabolic, renal, and cardiovascular disorders that compromise health and lifespan in older individuals. This review reconciles the concept of microinflammation with recent molecular insights into hypothalamic osmosensitivity, proposing a model for the maladaptive hypersecretion of vasopressin in advanced age. This framework may inform the development of targeted interventions to normalize VP secretion, thereby mitigating the metabolic, cardiovascular, and renal diseases that disproportionately affect older adults.
Impact of vasopressin signaling on aging
Aging is associated with neuroendocrine dysregulation manifesting by increased levels of circulating vasopressin (VP) in a significant proportion of older adults (1–8). Elevated plasma VP levels measured by its stable surrogate marker copeptin have been associated with enhanced risk of cardiovascular, metabolic, and renal diseases disproportionally affecting older people (9–12). Chronic underhydration of older adults due to blunted thirst perception or impaired renal water conservation constitutes an obvious trigger for sustained VP secretion since antidiuresis is the principal function of VP, also referred to as the antidiuretic hormone (8, 13). Exaggerated VP secretion in older adults may be also related to the low-grade systemic or hypothalamic inflammation typically developing during aging because the major pro-inflammatory cytokines including the interleukin 1β (IL-1β), IL-2, and IL-6 function as potent VP secretagogues (14–24). Apart from the antidiuresis, VP acts as a stress hormone contributing to activation of the hypothalamic-pituitary-adrenal (HPA) axis and exerting peripheral vascular and metabolic effects on the blood pressure and systemic glucose availability (25). While the antidiuretic action of VP is mainly mediated via the renal vasopressin V2 receptor (V2R), the stress-related hormone effects depend on the V1b (V1b) and to a lesser extent on the V1a receptor types (25, 26). Sustained HPA hyperactivity has been closely associated with aging pathophysiology in human and animals (27–31). VP-deficient Brattleboro rats and V1bR-knockout mice exhibit attenuated baseline HPA activity and blunted HPA response to various stressors suggesting a significant role of VP in the HPA activation with potential implications for aging (32–35). Peripheral effects of VP may provoke insulin resistance, hyperglycemia, vasoconstriction, hypertension, and renal damage at long term (9, 10, 25, 36, 37). Therefore, sustained VP hypersecretion may compromise health at multiple levels.
In further sections we will outline potential triggers for exaggerated VP secretion with particular focus on the hypothalamic microinflammation and the low-grade systemic inflammation accompanying aging (14, 15) (Figure 1). We will consider a crosstalk between the osmotic and pro-inflammatory stimuli for VP release and integrate the recently identified molecular players in hypothalamic osmosensitivity to offer an updated model for maladaptive VP hypersecretion in advanced aging.
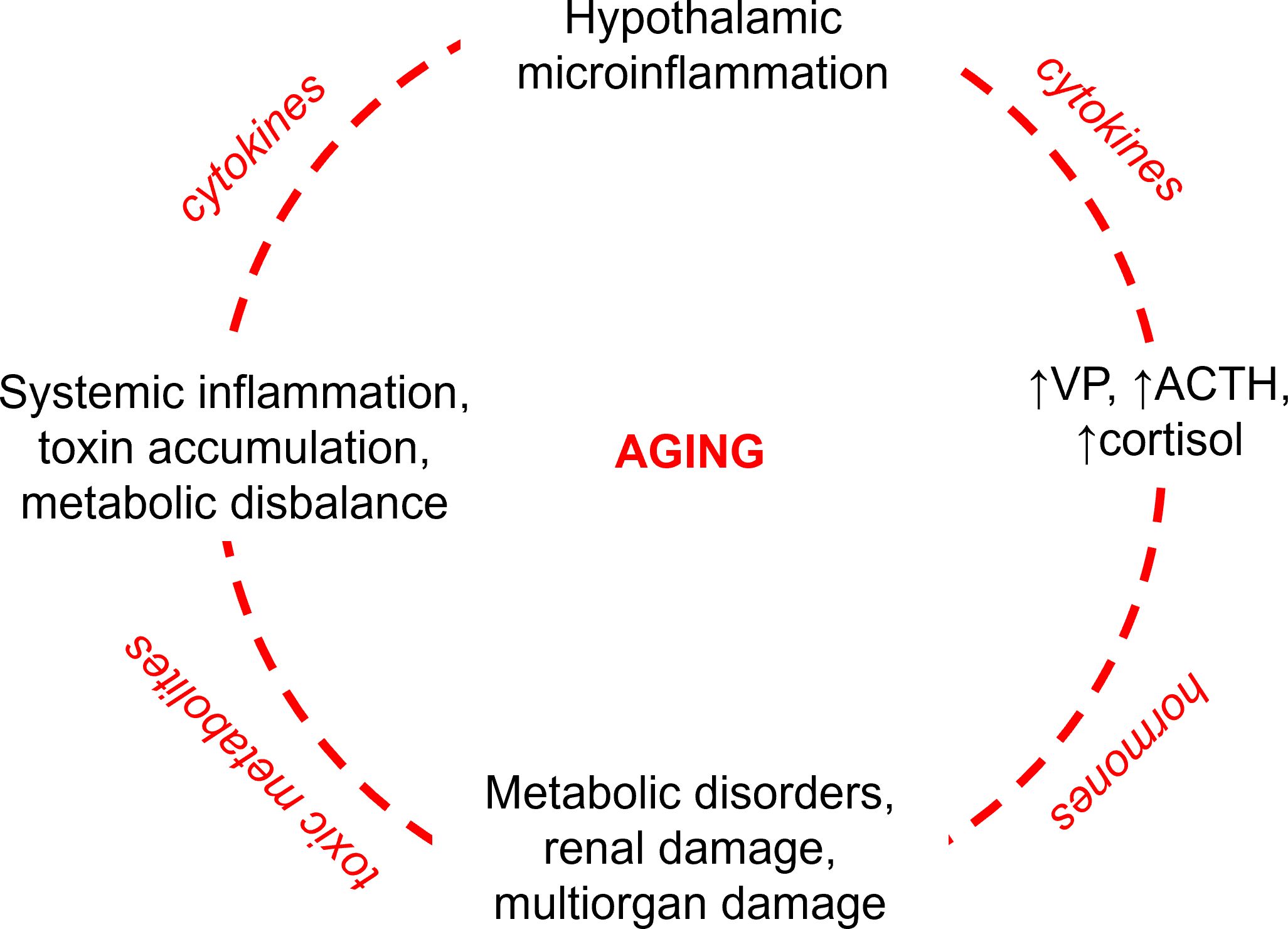
Figure 1. Vicious circle of pathophysiological events aggravating aging with focus on the role of vasopressin. Hypothalamic microinflammation induces sustained hyperactivity of the hypothalamic-pituitary-adrenal axis (HPA) enhancing central and peripheral secretion of vasopressin (VP) with ensuing sustained stimulation of the anterior pituitary producing the adrenocorticotropic hormone (ACTH) and adrenal glands producing cortisol. The resulting elevated levels of VP and cortisol provoke and aggravate systemic metabolic disorders such as diabetes mellitus, atherosclerosis, and hypertension with the ensuing renal and multiorgan damage. Impaired renal and cardiovascular performance lead to accumulation of toxic metabolites in the body and systemic inflammation. The latter aggravates the hypothalamic microinflammation by proinflammatory cytokines and toxic metabolites disrupting the brain-blood barrier.
Vasopressin biosynthesis and release in aging
VP is a hypothalamic-neurohypophyseal peptide hormone consisting of nine amino acids. Biosynthesis of the VP precursor takes place in magnocellular neurosecretory cells (MNCs) located within the supraoptic (SON), paraventricular (PVN), and accessory nuclei (AC) of hypothalamus and projecting to the posterior pituitary for hormone release into the peripheral blood (38). VP is also produced in a subset of hypothalamic parvocellular neurons which release the hormone into the hypophyseal portal circulation to potentiate the secretion of adrenocorticotropic hormone (ACTH) by the anterior pituitary (25, 39). The VP precursor (pre-provasopressin) contains VP at its N-terminus, the carrier protein neurophysin-2 in the middle, and a glycopeptide copeptin at the C-terminus (40). Proteolytic cleavage of the precursor results in secretion of VP and copeptin in equimolar amounts so that copeptin can serve as a surrogate marker of plasma VP levels (9). VP promotes antidiuresis thus preventing dehydration and playing the key role in water homeostasis (26). Accordingly, VP is secreted in response to increased plasma osmolality or reduced blood volume that occur during dehydration (26). Furthermore, secretion of VP increases in response to hyperthermia and certain pro-inflammatory cytokines. The thermal stimuli may enhance the osmosensitivity of MNCs thereby eliciting a greater VP release in response to smaller increments in plasma osmolality (41). Heat stress may further lead to activation of the hypothalamic-pituitary-adrenal (HPA) axis involving VP as a modulating hormone (25, 42, 43). Induction of VP secretion by thermal or inflammatory stress is at least in part mediated by pro-inflammatory cytokines such as IL-1β or IL-6 (24, 44). Inflammatory stress has been generally associated with stimulation of VP release (24). Thus, several non-osmotic triggers such as low blood pressure, hyperthermia, and inflammation contribute to the regulation of VP secretion in addition to its osmotic stimulation.
The aging process has been associated with sustained increase of baseline plasma VP levels in a significant proportion of older adults (1–8). Hypothalamic nuclei retain largely intact morphology during aging but the neuroendocrine functionality of VP-producing MNCs is altered (45, 46). Post-mortem evaluation of hypothalamic regions in human brains from younger vs. older individuals revealed similar VP mRNA levels but increased VP-positive cell numbers and size in aged brains (47–49). Enlarged size of the Golgi apparatus in PVN and SON of older (over 70 years of age) compared to younger individuals suggested enhanced activity of MNCs in the aged human brain as well (50). Notably, nearly intact morphology along with structural correlates of high MNCs activity were observed even in brain samples derived from individuals with Alzheimer’s disease history (49, 50). Therefore, unlike the most other brain regions, hypothalamic nuclei are resistant to the aging-dependent neurodegenerative alterations but exhibit signs of increased activity instead. Chronic activation of MNCs in advanced aging may be triggered by the pro-inflammatory signaling arising from hypothalamic microinflammation (14). Experimental studies in transgenic animals link the hypothalamic microinflammation to neuroendocrine disbalance affecting health and life span (14, 51). Pro-inflammatory cytokines including IL-1β, IL-2, and IL-6 were established as potent HPA activators and triggers of the VP secretion (16–22, 52, 53). Stimulation of MNCs by these cytokines promotes sustained peripheral VP secretion via the posterior pituitary leading to elevated plasma VP levels. Parallel activation of vasopressinergic parvocellular neurons in PVN enhances VP delivery to the anterior pituitary, where it potentiates the effect of the corticotropin-releasing hormone (CRH) in V1bR-expressing corticotrophs thereby co-stimulating the adrenocorticotropic hormone (ACTH) secretion (35, 53, 54). Effects of ACTH are further supported by the V1aR-mediated co-stimulation of the adrenal cortex leading to enhanced cortisol secretion in response to ACTH, as reported by studies ex vivo and in vivo (42, 55–58). Thus, the exaggerated VP secretion driven by hypothalamic microinflammation appears to play a role in HPA hyperactivity during aging (Figure 2).
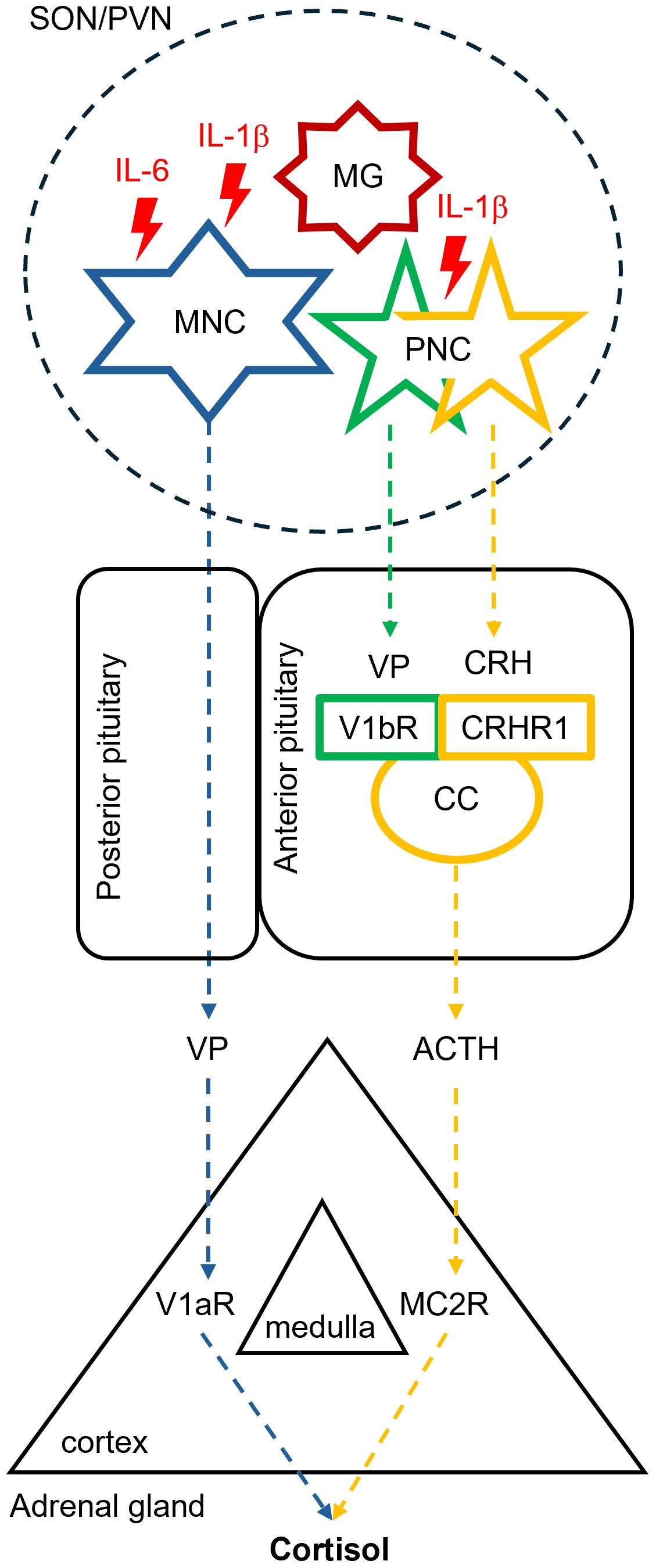
Figure 2. Putative effects of hypothalamic microinflammation on the neuroendocrine hypothalamic-pituitary-adrenal axis in advanced aging. Advanced aging is associated with enhanced hypothalamic release of pro-inflammatory cytokines including the interleukin 1β (IL-1β) and IL-6 by microglial cells. These interleukins enhance the excitability of neighboring vasopressin (VP)-producing magnocellular (MNC) and parvocellular neurosecretory cells (PNC) in the supraoptic (SON) and paraventricular hypothalamic nuclei (PVN). MNCs deliver an enhanced VP amount to the systemic circulation via the posterior pituitary, whereas PNCs release more VP along with the corticotropin releasing hormone (CRH) into the anterior pituitary. Activation of the corticotropin-producing cells (CC) residing in the anterior pituitary mediated by the vasopressin V1b receptor (V1bR) and CRH receptor type 1 (CRHR1) leads to enhanced secretion of the adrenocorticotropic hormone (ACTH). ACTH and VP synergistically stimulate cortisol secretion in the adrenal cortex via the melanocortin receptor type 2 (MC2R) and the vasopressin V1a receptor (V1aR), respectively.
HPA activation alongside the VP hypersecretion may be also provoked by pro-inflammatory cytokines derived from the systemic circulation as reported for IL-1β or IL-6 in human and animals (21, 59). IL-6 plasma levels tend to increase during aging, and this cytokine plays the key role in development of the low-grade systemic inflammation in advanced aging also known as “inflammaging” (15, 60, 61). The reasons for elevated IL-6 plasma levels are likely multifactorial and may combine cellular senescence, dysregulation of immune system, and chronic diseases or metabolic conditions prevalent in older adults (15, 62). Although IL-6 or IL-1β cannot easily cross the intact blood-brain barrier (BBB) under normal conditions, the circulating cytokines may signal via circumventricular organs (CVO) placed outside the BBB thus affecting the neuroendocrine and behavioral functions such as thirst and VP secretion (63–67). In addition, gradual BBB disruption associated with aging may increase the exposure of VP-producing hypothalamic nuclei to the pro-inflammatory cytokines from systemic circulation (8, 15, 62, 68).
Thirst vs. vasopressin secretion in aging
Adequate hydration of the body is critical to health. Physiological losses of water with urine, breath, sweat, and stool are balanced via behavioral adaptations driven by thirst sensation and ensuing water intake (69). The urine production by the kidneys constitutes the major route of daily water loss, disregarding atypical conditions such as fever or very high environmental temperatures resulting in extreme sweating (70). Intensive filtration of blood plasma in the kidneys is mandatory for rapid excretion of excessive or toxic water-soluble substances with the urine, whereas the ensuing tubular reabsorption of water prevents dehydration (71). Renal handling of water and electrolytes is under tight neuroendocrine control executed by VP (26). In advanced aging, water homeostasis is compromised both at the central and renal levels (72, 73). Blunted thirst sensation and impaired renal water conservation commonly occur in older adults leading to chronic underhydration and provoking sustained VP secretion (72).
Plasma hyperosmolality is the dominant stimulus for induction of both thirst and VP release, as the resultant intracellular dehydration is life-threatening and requires prompt normalization of the water homeostasis (26, 69). The osmosensory neurons triggering thirst reside in the forebrain regions known as the subfornical organ (SFO) and organum vasculosum of the lamina terminalis (OVLT), both regions belong to CVO placed outside of the blood-brain barrier and therefore directly exposed to changes in plasma osmolality (74). The osmotic stimulus for VP secretion originates from osmosensory SFO/OVLT neurons and converges with the intrinsic osmosensitivity of MNCs (75–77). The osmotic thresholds for thirst induction and VP release operate within a narrow range around 284–285 mOsmol/kg H2O in young healthy adults, as defined by several studies (8, 78, 79). Increments in plasma osmolality above that threshold range progressively trigger release of VP (~1 pg/ml per 1% osmolality) along with growing thirst perception (69, 80, 81). The close temporal association between VP release and thirst induction in young healthy adults promotes rapid rehydration with ensuing normalization of plasma osmolality and suppression of VP secretion.
Aging has been associated with blunted thirst perception but increased osmotic sensitivity towards VP release (8, 45, 82–84). The reasons for impaired thirst perception in older individuals are multifactorial including the low-grade systemic inflammation, since SFO and OVLT lack the blood-brain barrier and are directly exposed to the circulating pro-inflammatory cytokines and chemokines (8, 15, 62, 72, 85). In this context, administration of human IL-1β has been shown to suppress osmotic thirst in rats (66). Likewise, administration of the tumor necrosis factor (TNF) has been shown to reduce fluid intake in mice (86). However, these results were obtained with relatively high cytokine doses and thus model the acute inflammatory response rather than the low-grade inflammation in advanced aging. The available data on effects of pro-inflammatory cytokines on thirst perception in aging is still scarce. A recent study in aged individuals reported an inverse correlation between the skin hydration status and plasma levels of several pro-inflammatory cytokines including TNF, IL-1α, IL-1β, IL-6, and interferon γ (87). Although the topical water content in the skin partially reflects the global water homeostasis, serum osmolality is a more precise indicator of the body hydration status. Evaluation of serum cytokine profiles in healthy young vs. older adults showed no correlations between serum osmolality and circulating levels of IL-1β, IL-6, or TNF in one study (88). In view of the scarcity of data, further studies are awaited to clarify links between inflammaging and underhydration. Independently on the underlying mechanisms, impaired thirst perception and resultant chronic underhydration with moderately enhanced plasma osmolality persist in a significant proportion of older adults constituting an osmotic trigger for VP hypersecretion (2, 3, 5–8, 89–91).
Pro-inflammatory cytokines and vasopressin secretion in aging
The physiological task of hypothalamic cytokine signaling is to prime the response of MNCs to stress during temporary perturbations of homeostasis (17, 92). In contrast, sustained exposure of hypothalamic tissue to proinflammatory cytokines such as IL-1β, IL-6, or TNF may provoke maladaptive morphological and functional synaptic remodeling of MNCs resulting in their enhanced sensitivity and exaggerated response to osmotic stress in advanced aging (14, 45, 93). Such synaptic reorganization may be aggravated by chronic underhydration frequently occurring in aged individuals (94). IL-1β is the key cytokine adjusting the MNCs excitability as its local release into SON in response to osmotic stimuli accompanies VP secretion (92, 95). Both hypothalamic neuronal and microglia cells serve as IL-1β sources, whereas the functional IL-1 receptor type 1 (IL-1R1) is present in neuronal cells such as MNCs or vasopressinergic parvocellular neurons but absent in microglia cells (52, 53, 92, 96). The major signaling pathways downstream of IL-1R1 include the Nuclear Factor kappa B transcription factor (NF-kB) and Mitogen Activated Protein Kinase (MAPK). Binding of IL-1β to IL-1R1 triggers the canonical NF-kB signaling via inactivating phosphorylation of the NF-kB inhibitor (IkB) provided by the IkB kinase (also known as IKK) (97). The ensuing nuclear translocation of NF-kB drives expression of target genes, including interleukins and enzymes involved in prostaglandin biosynthesis, which amplify the initial IL-1β effect on MNCs and parvocellular VP neurons via autocrine and paracrine mechanisms (52). Prostaglandins, in particular the prostaglandin E2 (PGE2), affect several ion channel types via modulation of cytosolic cAMP or Ca2+ levels with the net effect of increased MNCs excitability (98). IL-1β-induced activation of MAPK signaling may amplify intracellular Ca²+ signals critical for vesicular VP release (95, 99). Stimulation of the p38-MAPK kinases may also promote the VP mRNA expression via phosphorylation of the cAMP response elements (CREB) and activator protein 1 (AP-1) (100). Hypothalamic effects of IL-1β may be potentiated by induction of the IL-6 production and release (101). IL-6 expression in PVN and SON is induced by dehydration and the cytokine is secreted by the posterior pituitary parallel to VP to support metabolic adaptations to the dehydration stress (102). Administration of recombinant IL-6 to healthy volunteers or cancer patients has been shown to stimulate VP, ACTH, and cortisol secretion suggesting that peripheral IL-6 affects the HPA axis and VP secretion in human (21, 103). Stimulation of the IL-6 receptor (IL-6R) in MNCs promotes VP secretion via the Mitogen-Activated Protein Kinase/Extracellular Signal Regulated Kinase (MAPK/ERK) kinase cascade (104). Activation of the cyclooxygenase 2 (COX-2) and induction of PGE2 synthesis is integrated in both IL-1β and IL-6 signaling pathways and stimulates VP secretion via prostaglandin E2 receptors expressed in the hypothalamus (105, 106). TNF may modulate VP secretion directly or via complex interactions with the endocannabinoid system (ECS) (107–109).
Chronic overexposure of MNCs to the aforementioned pro-inflammatory cytokines likely occurs in advanced aging due to hypothalamic microinflammation and systemic inflammaging (14, 15). These cytokines have been reported to reduce the threshold for MNCs depolarization via effects on several ion channel types. IL-1β has been shown to stimulate osmosensitive non-selective cation channels thus leading to influx of Ca2+, Na+, and K+ and increased excitability of MNCs (52, 92, 110). Although the identity of these channels remains to be clarified, members of the transient receptor potential vanilloid family (TRPV) emerge as appropriate candidates (77, 111–113). A functional N-terminal TRPV1 splice variant (ΔN-TRPV1) expressed in MNCs has been identified as a stretch-inactivated channel relevant for the intrinsic osmosensitivity of MNCs (114). Mechanical forces occurring during hyperosmotic stress and cell shrinkage are likely transduced on ΔN-TRPV1 via its C-terminal interaction with tubulin (115) (Figure 3A). Activation of ΔN-TRPV1 and the resulting Ca2+ influx induce the membrane depolarization with ensuing exocytotic VP release (114). The microtubular remodeling upon hyperosmotic stress further promotes the exocytotic membrane insertion of ΔN-TRPV1-containing vesicles via activation of the phospholipase C delta 1 (PLCδ1), as shown in mouse and rat MNCs (116). Thus, the initial ΔN-TRPV1-mediated calcium influx appears to trigger a positive feedback loop via PLCδ1 to amplify the ΔN-TRPV1 activity and MNC excitability upon sustained osmotic stress (117). According to this mechanism, IL-1β may sensitize the VP-producing MNCs thus causing stronger VP secretion in response to smaller increments in plasma osmolality, as has been reported in older adults (6, 8). The excitability of MNCs may be further potentiated by IL-6 since its increased hypothalamic expression in aged rat brains was linked to exaggerated VP release in response to immune challenge (118). The underlying molecular pathways and ion channels remain to be specified but may involve TRPV members or paracrine modulation of gap junction proteins in the surrounding astroglia (118–120). IL-6 receptor (IL-6R) is expressed in astrocytes also expressing the connexin 43 (Cx43), a gap junctional protein affecting the VP release via astrocyte-dependent uptake of neurotransmitters and neuropeptides and release of gliotransmitters (120–122).

Figure 3. Putative molecular mechanisms mediating osmosensitivity in vasopressin-producing magnocellular neurosecretory cells (A) and osmosensory circumventricular neurons (B). (A): The response of vasopressin-producing magnocellular neurosecretory cells (MNCs) to hyperosmotic stress is mediated by the N-terminal transient receptor potential vanilloid 1 splice variant (ΔN-TRPV1), which activated by microtubular (#) condensation during cell shrinkage transmitting mechanical forces for the channel activation. (B): In osmosensory neurons located in the subfornical organ (SFO) and organum vasculosum of the lamina terminalis (OVLT), hyperosmolality activates WNK1 in part by abrogating the inhibitory action of intracellular chloride ([Cl-]i). WNK1 signals via intermediate kinases, Ste20/SPS1-related proline/alanine-rich protein kinase (SPAK) and serum/glucocorticoid regulated kinase 1 (SGK1). The ensuing SPAK-mediated phosphorylation of the Na+-K+-2Cl- cotransporter type 1 (NKCC1) and K+-Cl- cotransporter type 2 (KCC2) produces reciprocal effects on their activity resulting in increased [Cl-]i and neuronal excitability in response to GABA. SGK1-mediated activation of the epithelial sodium channel (ENaC) promotes a depolarizing passive Na+ leak, whereas stimulation of voltage-gated potassium channels (Kv3) enables fast and precise neuron firing. Arrows point to activation, whereas T-shaped bars indicate inhibition. Created with BioRender.com.
In addition to the effects on MNCs excitability, the pro-inflammatory cytokines may affect the upstream osmosensory SFO/OVLT neurons projecting to vasopressin-secreting MNCs, as excitatory effects of IL-1β and TNF were documented in rodent CVO neurons (65, 123). Like in MNCs, studies in rats showed that IL-1β promotes depolarization of SFO neurons by activation of non-selective cation channels (64). The sensitivity of SFO/OVLT neurons to hyperosmotic stress is at least partially mediated by the with-no-lysine kinase 1 (WNK1), which detects molecular crowding during cell shrinkage thus acting as an intracellular osmolality sensor (124). Recent studies in mice link WNK1 activation to the VP secretion in response to plasma hyperosmolality (76, 125). WNK1 may affect neuronal excitability by modulation of several ion channels and transporters via intermediate kinases such as the STE20/SPS1-related proline/alanine-rich kinase (SPAK), the oxidative stress responsive kinase 1 (OSR1), or the serum- and glucocorticoid-inducible kinase 1 (SGK1) (126, 127). SPAK and OSR1 are homologous kinases targeting members of the electroneutral cation-coupled chloride cotransporters (CCC) family (128). Electrophysiological studies in rodent neurons showed that the Na+-K+-2Cl- cotransporter type 1 (NKCC1) acts as a chloride importer, whereas the K+-Cl- cotransporter type 2 (KCC2) is the major chloride exporter in mature neurons (129). Their functional interplay determines the intracellular Cl- concentration [Cl-]I, neuronal excitability, and the type of response to the γ-aminobutyric acid (GABA)-induced signaling, i.e. inhibitory or excitatory (130). Both NKCC1 and KCC2 are substrates for phosphorylation by SPAK/OSR1 with opposing functional effects: activating for NKCC1 but inhibitory for KCC2 (131, 132). Thus, the WNK1-SPAK/OSR1 signaling may lead to intracellular chloride accumulation (Figure 3B). Since GABAA receptor is a ligand-gated chloride channel, high [Cl-]I suppress an inhibitory while provoking an excitatory effect of the GABAergic signaling, depending on the neuron type. A tonic inhibitory effect of GABA-signaling on the VP secretion has been observed in vivo and in cell culture (133, 134). In contrast, other animal studies report an excitatory effect of GABA on the VP release especially under certain pathophysiological conditions such as sustained hyperosmotic stress, diabetes, or hypertension (135–140). This switch may be related to enhanced NKCC1 activity and suppressed KCC2 function in MNCs and/or osmosensory SFO/OVLT neurons (137–140). Since diabetes, hypertension, and enhanced plasma osmolality due to underhydration are prevalent in older adults, these factors are likely to contribute to VP hypersecretion in advanced aging. Notably, these pathophysiological conditions are generally accompanied by sustained increases in circulating IL-1β, IL-6, TNF levels (141–144).
Apart from SPAK/OSR1, WNK1 may affect VP secretion via SGK1 (126). WNK1 activates SGK1 thus preventing ubiquitination and degradation of the epithelial sodium channel (ENaC), whereas ENaC activity has been shown to reduce the threshold for MCNs depolarization in rodents (115, 126, 145). ENaC is further expressed in the osmosensitive SFO/OVLT neurons and may be involved in their activation upon hypernatremia (146). Studies in rodents and observations in human suggest that ENaC activity may contribute to enhanced VP secretion in response to high dietary salt intake (115, 147–151). High-threshold voltage-gated potassium channels (Kv3) belong to the downstream targets of the WNK1-SGK1 signaling as well (76, 125, 152, 153). Their WNK1-induced activation has been shown to support the repetitive firing in mouse osmosensory circumventricular neurons thus stimulating VP release via respective projections to MNCs (Figure 3B). IL-1β and IL-6 have been reported to stimulate ENaC in epithelial cells but the respective effects in neurons have received only minor attention so far (154, 155). Likewise, effects on these cytokines on Kv3 channels have not been extensively studied in the neuronal context.
Circadian VP secretion in aging
Healthy young adults exhibit a diurnal VP secretion pattern resulting in higher circulating VP levels at night and lower hormone levels during the day (156). The normal circadian rhythm of VP secretion is blunted in older people (157). Thus, insufficient rise of plasma VP levels at night may be involved in nocturia frequently reported by older people (157). Impaired renal response to the hormone may contribute to nocturia as well (73). Furthermore, VP is an established inducer of the ACTH secretion, which in turn stimulates production and secretion of the cortisol and aldosterone (158). The blunted circadian pattern of VP secretion may secondarily affect the diurnal rhythm of aldosterone or cortisol secretion in older individuals (159). Aldosterone secretion is also controlled by the renin-angiotensin system (RAS) activity but this regulatory pathway exhibits a gradual dissociation in advanced aging (160). Intact circadian pattern of the HPA neuroendocrine axis is crucial to global body metabolism, performance, and ability to elicit adequate stress responses (29, 161). VP is critically involved in maintaining the circadian rhythmic via its central and peripheral effects (162). The age-related circadian rhythm flattening results in decreased diurnal peaks but enhanced basal secretion of VP and cortisol which have been increasingly recognized as pathophysiological factors underlying diverse metabolic, renal, and cardiovascular disorders such as diabetes mellitus, atherosclerosis, or chronic kidney disease (163, 164). Notably, IL-1β has been shown to disrupt the pancreatic circadian rhythm with implications in the pathophysiology of diabetes mellitus (165). IL-1β-induced deterioration of the circadian rhythm has been also reported in articular cartilage during osteoarthritis (166). Characterization of IL-6-deficient mice revealed a role of IL-6 in the regulation of clock genes and behavioral rhythms of rest and activity (167). IL-6 secretion follows a biphasic circadian pattern in healthy young adults and is involved in the sleep/awake rhythm in human (168). Chronically elevated plasma IL-6 levels in cancer patients were associated with blunted diurnal variations in HPA activity (169). Taken together, sustained overexposure of the vasopressinergic system to pro-inflammatory cytokines because of hypothalamic microinflammation of inflammaging may contribute to flattening of the circadian VP secretion pattern but this assumption needs further experimental verification.
Therapeutic prospects for targeting VP signaling in aging
Elevated levels of circulating VP frequently occur in older adults because of chronic underhydration and maladaptive alterations in sensitivity and strength of the neuroendocrine response to hyperosmotic stress, the latter may be related to hypothalamic microinflammation and systemic inflammaging (8, 14, 15, 62). Excessive and prolonged VP signaling has been implicated in cardiovascular, metabolic, and renal diseases (9, 37, 170, 171). Lifestyle modifications such as regular physical activity, adequate hydration, and metabolic dietary therapies can prolong health and life span in older adults. Pharmacological approaches retarding aging processes have been actively investigated in the recent decades.
Water supplementation
Water supplementation to provide an adequate water intake is the obvious step towards prevention of dehydration and its negative consequences such as chronic stimulation of the VP system in older people. Indeed, several studies documented reduction of circulating VP levels in adults of different ages and health status receiving daily water supplementation, as judged by evaluation of the surrogate VP marker copeptin (172–175). Moreover, an adequate hydration and reduction of plasma copeptin levels were associated with improved glucose metabolism (172, 174, 175). However, excessive water intake bears a risk of euvolemic hyponatremia in older individuals since the aged kidney is limited in its capacity to excrete water (176). Therefore, a balanced water and electrolyte intake and regular monitoring of blood electrolytes is recommended in older individuals.
Physical exercise
Sedentary lifestyle during aging has been associated with increased incidence of cardiovascular and metabolic diseases such as hypertension, obesity, or diabetes, whereas regular physical activity mitigates these risks in part by suppressing the inflammaging (177, 178). Systematic aerobic training reduces the baseline levels of circulating IL-1β, IL-6 and TNF, i.e. the pro-inflammatory cytokines potentially contributing to sustained VP hypersecretion in advanced aging (178, 179). With respect to VP, physical exercise causes acute transient increases in plasma VP and copeptin levels both in younger and older individuals (180–182). These increases reflect the body response to acute physical stress and serve to maintain the water homeostasis. Although elevated VP/copeptin levels are typically accompanied by enhanced IL-1β and IL-6 levels during physical exercise, the exercise-induced VP release appears to be largely independent on these cytokines (181, 183). In contrast, resting VP levels remain unchanged during periods of systematic physical activity, as has been demonstrated in older men and women assigned to endurance exercise (184). Since regular physical activity exerts beneficial effects on the circadian rhythm in older individuals it is tempting to speculate that physical training may stabilize the circadian VP secretion pattern as well (185, 186). Taken together, regular, age-matched physical activity of moderate intensity represents a valuable non-pharmacological intervention promoting healthier aging (187). Habitual aerobic exercise has been shown to ameliorate the HPA hyperactivity in older individuals although the impact of VP herein remains to be clarified (188, 189).
Targeting inflammation
Derangement of VP signaling in advanced age is closely related with microinflammation of the hypothalamic tissue and increased production of pro-inflammatory cytokines such as IL-1β and IL-6 (14). Both cytokines are non-osmotic VP secretagogues (17, 18, 21, 190). Peripheral IL-1β or IL-6 induction in response to inflammation affects VP release as well, since these cytokines are able to disrupt the blood-brain barrier permeability and penetrate into the hypothalamic region (17, 67). IL-6 plasma levels tend to increase with aging independent on confounding factors such as major inflammatory diseases, whereas the circadian pattern of IL-6 secretion is flattened in older adults (61, 168, 191). The low-grade systemic inflammation promotes cellular senescence and metabolic disorders in aging (61). A growing repertoire and increasing availability of clinically approved IL-6 signaling inhibitors hold promise for retardation of aging-associated systemic and hypothalamic inflammatory processes triggering the inappropriate VP secretion (192). The extending clinical data pool from older individuals receiving IL-6 inhibiting drugs for treatment of autoimmune and inflammatory disorders may shed light on the utility of this strategy to manage age-related dysregulation in the VP system. Apart from pharmacological interventions, regular physical activity, especially aerobic exercise, has been established as a potent anti-inflammatory strategy to retard inflammaging (187).
Selective V1a or V1b receptor antagonists
While the urinary concentration depends on the renal V2 receptors, the unfavorable metabolic and vascular effects of VP in older adults are largely mediated by activation of the V1a and V1b receptors (25, 26, 36). Thus, antagonizing either V1a or V1b receptor could improve the body metabolism while preserving the antidiuretic VP action critical to adequate hydration. The therapeutic potential of V1a and V1b antagonists is burgeoning, although none of them has been tested in the clinical settings of aging and metabolic diseases (193). Conivaptan, a dual antagonist to the V1a and V2 receptor types, has been approved for correction of euvolemic and hypervolemic hyponatremia similar to the selective V2 receptor antagonist tolvaptan (194, 195). A selective V1a receptor antagonist balovaptan has been tested in patients with autism spectrum disorders (196). Selective suppression of the V1b receptor showed promising preclinical results in the field of neurologic stress-related disorders (197). Nevertheless, the available experimental data strongly suggests that targeting V1a or V1b signaling bears therapeutic potential for improved management of the aging-related pathophysiology (25, 36).
WNK-SPAK inhibitors
In view of the newly established role of the WNK1-SPAK/OSR1 signaling in hypothalamic osmosensitivity, selective pharmacological interventions in this pathway bear potential to blunt the excessive VP secretion in advanced aging (125). Inhibitors of SPAK and WNK kinases have been developed and showed therapeutic potential in animal models of hypertension and cystic fibrosis, although their utility in human requires further validation (198, 199). WNK1 fulfils multiple functions in immune cell biology ranging from the cell volume and motility to the regulation of cytokine production and pyroptosis (200). WNK inhibitors have been shown to exert toxic effects on the natural killer (NK) cells which may limit their therapeutic potential due to increased risk of malignancy (201). The cytotoxic effects of WNK inhibitors on the NK cells are likely mediated by disruption of the WNK1-OSR1 signaling, whereas selective inhibitors of SPAK downstream of WNK1 may have milder toxicity (201). Interestingly, WNK-dependent immunologic effects involve the mechanistic target of rapamycin (mTOR), whereas mTOR inhibitors have been shown to promote health and longevity in various animal models of aging (202). The mTOR signaling has been further shown to mediate some cell biological effects of VP such as autophagy inhibition (203). Therefore, beneficial effects of mTOR inhibitors may be partially related to improved cell metabolism due to disruption of the VP signaling.
Conclusions and perspectives
Increasing recognition of the hypothalamic microinflammation and systemic inflammaging as significant factors driving maladaptive neuroendocrine processes such as VP hypersecretion in aging opens new perspectives for targeted lifestyle and pharmacological interventions. Recent progress in identification of molecular networks governing the physiological VP secretion adds to the choice of potential candidates for pharmacological targeting of the VP system. The emerging solutions include anti-cytokine therapies, selective inhibitors of V1a or V1b receptors, and suppression of the WNK1-SPAK signaling.
Author contributions
KM: Conceptualization, Writing – original draft, Writing – review & editing. SL: Writing – original draft, Writing – review & editing. PS: Funding acquisition, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The study was supported by program-targeted funding from the Committee of Ministry of Science and Higher Education of the Republic of Kazakhstan “Prolonging healthy longevity: using new technologies and machine learning to control reversal of aging in old cells” (Grant No. BR24992900).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fendo.2025.1724342.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Li CH, Hsieh SM, and Nagai I. The response of plasma arginine vasopressin to 14h water deprivation in the elderly. Acta Endocrinologica. (1984) 105:314–7. doi: 10.1530/acta.0.1050314
2. Kirkland J, Lye M, Goddard C, Vargas E, and Davies I. Plasma arginine vasopressin in dehydrated elderly patients. Clin Endocrinol. (1984) 20:451–6. doi: 10.1111/j.1365-2265.1984.tb03441.x
3. Os I, Kjeldsen SE, Aakesson I, Skjøtø J, Eide I, Hjermann I, et al. Evidence of age-related variation in plasma vasopressin of normotensive men. Scandinavian J Clin Lab Invest. (1985) 45:263–8. doi: 10.3109/00365518509161004
4. Faull CM, Holmes C, and Baylis PH. Water balance in elderly people: is there a deficiency of vasopressin? Age Ageing. (1993) 22:114–20. doi: 10.1093/ageing/22.2.114
5. Johnson AG, Crawford GA, Kelly D, Nguyen TV, and Gyory AZ. Arginine vasopressin and osmolality in the elderly. J Am Geriatrics Soc. (1994) 42:399–404. doi: 10.1111/j.1532-5415.1994.tb07488.x
6. Davies I, O’Neill PA, Mclean KA, Catania J, and Bennett D. Age-associated alterations in thirst and arginine vasopressin in response to a water or sodium load. Age Ageing. (1995) 24:151–9. doi: 10.1093/ageing/24.2.151
7. Plasencia G, Luedicke JM, Nazarloo HP, Carter CS, and Ebner NC. Plasma oxytocin and vasopressin levels in young and older men and women: Functional relationships with attachment and cognition. Psychoneuroendocrinology. (2019) 110:104419. doi: 10.1016/j.psyneuen.2019.104419
8. Cowen LE, Hodak SP, and Verbalis JG. Age-associated abnormalities of water homeostasis. Endocrinol Metab Clin North Am. (2013) 42:349–70. doi: 10.1016/j.ecl.2013.02.005
9. Christ-Crain M. Vasopressin and Copeptin in health and disease. Rev Endocr Metab Disord. (2019) 20:283–94. doi: 10.1007/s11154-019-09509-9
10. Enhörning S, Bankir L, Bouby N, Struck J, Hedblad B, Persson M, et al. Copeptin, a marker of vasopressin, in abdominal obesity, diabetes and microalbuminuria: the prospective Malmö Diet and Cancer Study cardiovascular cohort. Int J Obes. (2013) 37:598–603. doi: 10.1038/ijo.2012.88
11. Wannamethee SG, Welsh P, Papacosta O, Lennon L, Whincup PH, and Sattar N. Copeptin, insulin resistance, and risk of incident diabetes in older men. J Clin Endocrinol Metab. (2015) 100:3332–9. doi: 10.1210/JC.2015-2362
12. Liber M, Sonnenblick M, and Munter G. Hypernatremia and copeptin levels in the elderly hospitalized patient. Endocrine Pract. (2016) 22:1429–35. doi: 10.4158/EP161240.OR
13. Tamma G, Goswami N, Reichmuth J, De Santo NG, and Valenti G. Aquaporins, vasopressin, and aging: current perspectives. Endocrinology. (2015) 156:777–88. doi: 10.1210/en.2014-1812
14. Cai D and Khor S. Hypothalamic microinflammation” Paradigm in aging and metabolic diseases. Cell Metab. (2019) 30:19–35. doi: 10.1016/j.cmet.2019.05.021
15. Franceschi C, Garagnani P, Parini P, Giuliani C, and Santoro A. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. (2018) 14:576–90. doi: 10.1038/s41574-018-0059-4
16. Burbach JPH, Luckman SM, Murphy D, and Gainer H. Gene regulation in the magnocellular hypothalamo-neurohypophysial system. Physiol Rev. (2001) 81:1197–267. doi: 10.1152/physrev.2001.81.3.1197
17. Palin K, Moreau ML, Sauvant J, Orcel H, Nadjar A, Duvoid-Guillou A, et al. Interleukin-6 activates arginine vasopressin neurons in the supraoptic nucleus during immune challenge in rats. Am J Physiology-Endocrinology Metab. (2009) 296:E1289–99. doi: 10.1152/ajpendo.90489.2008
18. Christensen JD, Hansen EW, and Fjalland B. Interleukin-1β stimulates the release of vasopressin from rat neurohypophysis. Eur J Pharmacol. (1989) 171:233–5. doi: 10.1016/0014-2999(89)90112-X
19. Diana A, Van Dam A-M, Winblad B, and Schultzberg M. Co-localization of interleukin-1 receptor type I and interleukin-1 receptor antagonist with vasopressin in magnocellular neurons of the paraventricular and supraoptic nuclei of the rat hypothalamus. Neuroscience. (1999) 89:137–47. doi: 10.1016/S0306-4522(98)00274-7
20. Summy-Long JY, Hu S, Pruss A, Chen X, and Phillips TM. Response of interleukin-1β in the magnocellular system to salt-loading. J Neuroendocrinol. (2006) 18:926–37. doi: 10.1111/j.1365-2826.2006.01490.x
21. Mastorakos G, Weber JS, Magiakou MA, Gunn H, and Chrousos GP. Hypothalamic-pituitary-adrenal axis activation and stimulation of systemic vasopressin secretion by recombinant interleukin-6 in humans: potential implications for the syndrome of inappropriate vasopressin secretion. J Clin Endocrinol Metab. (1994) 79:934–9. doi: 10.1210/jcem.79.4.7962300
22. Raber J and Bloom F. IL-2 induces vasopressin release from the hypothalamus and the amygdala: role of nitric oxide-mediated signaling. J Neurosci. (1994) 14:6187–95. doi: 10.1523/JNEUROSCI.14-10-06187.1994
23. Yasin SA, Costa A, Forsling ML, and Grossman A. Interleukin-1β and lnterleukin-6 stimulate neurohypophysial hormone release. vitro. J Neuroendocrinol. (1994) 6:179–84. doi: 10.1111/j.1365-2826.1994.tb00570.x
24. Chikanza IC, Petrou P, and Chrousos G. Perturbations of arginine vasopressin secretion during inflammatory stress: pathophysiologic implications. Ann New York Acad Sci. (2000) 917:825–34. doi: 10.1111/j.1749-6632.2000.tb05448.x
25. Koshimizu T, Nakamura K, Egashira N, Hiroyama M, Nonoguchi H, and Tanoue A. Vasopressin V1a and V1b receptors: from molecules to physiological systems. Physiol Rev. (2012) 92:1813–64. doi: 10.1152/physrev.00035.2011
26. Bankir L. Antidiuretic action of vasopressin: quantitative aspects and interaction between V1a and V2 receptor-mediated effects. Cardiovasc Res. (2001) 51:372–90. doi: 10.1016/S0008-6363(01)00328-5
27. Giordano R, Bo M, Pellegrino M, Vezzari M, Baldi M, Picu A, et al. Hypothalamus-pituitary-adrenal hyperactivity in human aging is partially refractory to stimulation by mineralocorticoid receptor blockade. J Clin Endocrinol Metab. (2005) 90:5656–62. doi: 10.1210/jc.2005-0105
28. Gupta D and Morley JE. Hypothalamic-pituitary-adrenal (HPA) axis and aging. In: Terjung R, editor. Comprehensive Physiology. Wiley (2014). p. 1495–510. doi: 10.1002/cphy.c130049
29. Gaffey AE, Bergeman CS, Clark LA, and Wirth MM. Aging and the HPA axis: Stress and resilience in older adults. Neurosci Biobehav Rev. (2016) 68:928–45. doi: 10.1016/j.neubiorev.2016.05.036
30. Atwood CS, Meethal SV, Liu T, Wilson AC, Gallego M, Smith MA, et al. Dysregulation of the hypothalamic-pituitary-gonadal axis with menopause and andropause promotes neurodegenerative senescence. J Neuropathol Exp Neurol. (2005) 64:93–103. doi: 10.1093/jnen/64.2.93
31. Veldhuis JD, Keenan DM, Liu PY, Iranmanesh A, Takahashi PY, and Nehra AX. The aging male hypothalamic–pituitary–gonadal axis: Pulsatility and feedback. Mol Cell Endocrinol. (2009) 299:14–22. doi: 10.1016/j.mce.2008.09.005
32. Zelena D, Földes A, Mergl Z, Barna I, Kovács KJ, and Makara GB. Effects of repeated restraint stress on hypothalamo-pituitary-adrenocortical function in vasopressin deficient Brattleboro rats. Brain Res Bull. (2004) 63:521–30. doi: 10.1016/j.brainresbull.2004.04.007
33. Makara GB, Varga J, Barna I, Pintér O, Klausz B, and Zelena D. The vasopressin-deficient brattleboro rat: lessons for the hypothalamo–pituitary–adrenal axis regulation. Cell Mol Neurobiol. (2012) 32:759–66. doi: 10.1007/s10571-012-9842-2
34. Zelena D, Domokos Á, Jain SK, Jankord R, and Filaretova L. The stimuli-specific role of vasopressin in the hypothalamus–pituitary–adrenal axis response to stress. J Endocrinol. (2009) 202:263–78. doi: 10.1677/JOE-09-0096
35. Tanoue A, Ito S, Honda K, Oshikawa S, Kitagawa Y, Koshimizu T, et al. The vasopressin V1b receptor critically regulates hypothalamic-pituitary-adrenal axis activity under both stress and resting conditions. J Clin Invest. (2004) 113:302–9. doi: 10.1172/JCI200419656
36. Lebedeva S, Margaryan A, Smolyarchuk E, Nedorubov A, Materenchuk M, Tonevitsky A, et al. Metabolic effects of vasopressin in pathophysiology of diabetic kidney disease. Front Endocrinol. (2023) 14:1176199. doi: 10.3389/fendo.2023.1176199
37. Bankir L, Bouby N, and Ritz E. Vasopressin: a novel target for the prevention and retardation of kidney disease? Nat Rev Nephrol. (2013) 9:223–39. doi: 10.1038/nrneph.2013.22
38. Zimmerman EA and Robinson AG. Hypothalamic neurons secreting vasopressin and neurophysin. Kidney Int. (1976) 10:12–24. doi: 10.1038/ki.1976.75
39. Stojiljković AS, Čupić Ž, Maćešić S, Ivanović-Šašić A, and Kolar-Anić L. Influence of arginine vasopressin on the ultradian dynamics of Hypothalamic-Pituitary-Adrenal axis. Front Endocrinol. (2022) 13:976323. doi: 10.3389/fendo.2022.976323
40. Gainer H. Precursors of vasopressin and oxytocin. In: Progress in Brain Research. Elsevier (1983). p. 205–15. doi: 10.1016/S0079-6123(08)64388-5
41. Sharif-Naeini R, Ciura S, and Bourque CW. TRPV1 gene required for thermosensory transduction and anticipatory secretion from vasopressin neurons during hyperthermia. Neuron. (2008) 58:179–85. doi: 10.1016/j.neuron.2008.02.013
42. Perraudin V, Delarue C, Lefebvre H, Contesse V, Kuhn JM, and Vaudry H. Vasopressin stimulates cortisol secretion from human adrenocortical tissue through activation of V1 receptors. J Clin Endocrinol Metab. (1993) 76:1522–8. doi: 10.1210/jcem.76.6.7684742
43. Stacey MJ, Delves SK, Britland SE, Allsopp AJ, Brett SJ, Fallowfield JL, et al. Copeptin reflects physiological strain during thermal stress. Eur J Appl Physiol. (2018) 118:75–84. doi: 10.1007/s00421-017-3740-8
44. Chang DM. The role of cytokines in heat stroke. Immunol Investigations. (1993) 22:553–61. doi: 10.3109/08820139309084183
45. Elsamad G, Mecawi AS, Pauža AG, Gillard B, Paterson A, Duque VJ, et al. Ageing restructures the transcriptome of the hypothalamic supraoptic nucleus and alters the response to dehydration. NPJ Aging. (2023) 9:12. doi: 10.1038/s41514-023-00108-2
46. Hofman MA. Lifespan changes in the human hypothalamus. Exp Gerontology. (1997) 32:559–75. doi: 10.1016/S0531-5565(96)00162-3
47. Lucassen PJ, Van Heerikhuize JJ, Guldenaar SEF, Pool CW, Hofman MA, and Swaab DF. Unchanged Amounts of Vasopressin mRNA in the Supraoptic and Paraventricular Nucleus during Aging and in Alzheimer’s Disease. J Neuroendocrinol. (1997) 9:297–305. doi: 10.1046/j.1365-2826.1997.t01-1-00583.x
48. Van Der Woude PF, Goudsmit E, Wierda M, Purba JS, Hofman MA, Bogte H, et al. No vasopressin cell loss in the human hypothalamus in aging and Alzheimer’s disease. Neurobiol Aging. (1995) 16:11–8. doi: 10.1016/0197-4580(95)80003-A
49. Lucassen PJ, Salehi A, Pool CW, Gonatas NK, and Swaab DF. Activation of vasopressin neurons in aging and alzheimer’s disease. J Neuroendocrinol. (1994) 6:673–9. doi: 10.1111/j.1365-2826.1994.tb00634.x
50. Lucassen PJ, Ravid R, Gonatas NK, and Swaab DF. Activation of the human supraptic and paraventricular nucleus neurons with aging and in Alzheimer’s disease as judged from increasing size of the Golgi apparatus. Brain Res. (1993) 632:105–13. doi: 10.1016/0006-8993(93)91144-H
51. Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, et al. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature. (2013) 497:211–6. doi: 10.1038/nature12143
52. Ferri CC, Yuill EA, and Ferguson AV. Interleukin-1β depolarizes magnocellular neurons in the paraventricular nucleus of the hypothalamus through prostaglandin-mediated activation of a non selective cationic conductance. Regul Peptides. (2005) 129:63–71. doi: 10.1016/j.regpep.2005.01.004
53. Ferri CC and Ferguson AV. Interleukin-1 β Depolarizes paraventricular nucleus parvocellular neurones. J Neuroendocrinol. (2003) 15:126–33. doi: 10.1046/j.1365-2826.2003.00870.x
54. Schmidt E, Janszen A, Wouterlood F, and Tilders F. Interleukin-1-induced long-lasting changes in hypothalamic corticotropin-releasing hormone (CRH)–neurons and hyperresponsiveness of the hypothalamus-pituitary-adrenal axis. J Neurosci. (1995) 15:7417–26. doi: 10.1523/JNEUROSCI.15-11-07417.1995
55. Grazzini E, Boccara G, Joubert D, Trueba M, Durroux T, Guillon G, et al. Vasopressin regulates adrenal functions by acting through different vasopressin receptor subtypes. In: Zingg HH, Bourque CW, and Bichet DG, editors. Vasopressin and Oxytocin. Advances in Experimental Medicine and Biology. Springer US, Boston, MA (1998). p. 325–34. doi: 10.1007/978-1-4615-4871-3_41
56. Bird IM, Nicol M, Williams BC, and Walker SW. Vasopressin stimulates cortisol secretion and phosphoinositide catabolism in cultured bovine adrenal fasciculata/reticularis cells. J Mol Endocrinol. (1990) 5:109–16. doi: 10.1677/jme.0.0050109
57. Brooks VL and Blakemore LJ. Vasopressin: a regulator of adrenal glucocorticoid production? Am J Physiology-Endocrinology Metab. (1989) 256:E566–72. doi: 10.1152/ajpendo.1989.256.4.E566
58. Schneider EG. Effect of vasopressin on adrenal steroidogenesis. Am J Physiology-Regulatory Integr Comp Physiol. (1988) 255:R806–11. doi: 10.1152/ajpregu.1988.255.5.R806
59. Matsuwaki T, Eskilsson A, Kugelberg U, Jönsson J-I, and Blomqvist A. Interleukin-1β induced activation of the hypothalamus–pituitary–adrenal axis is dependent on interleukin-1 receptors on non-hematopoietic cells. Brain Behavior Immun. (2014) 40:166–73. doi: 10.1016/j.bbi.2014.03.015
60. Vgontzas AN, Zoumakis M, Bixler EO, Lin H-M, Prolo P, Vela-Bueno A, et al. Impaired nighttime sleep in healthy old versus young adults is associated with elevated plasma interleukin-6 and cortisol levels: physiologic and therapeutic implications. J Clin Endocrinol Metab. (2003) 88:2087–95. doi: 10.1210/jc.2002-021176
61. Maggio M, Guralnik JM, Longo DL, and Ferrucci L. Interleukin-6 in aging and chronic disease: A magnificent pathway. Journals Gerontology. (2006) 61:575–84. doi: 10.1093/gerona/61.6.575
62. Li X, Li C, Zhang W, Wang Y, Qian P, and Huang H. Inflammation and aging: signaling pathways and intervention therapies. Sig Transduct Target Ther. (2023) 8:239. doi: 10.1038/s41392-023-01502-8
63. Vallières L, Lacroix S, and Rivest S. Influence of interleukin-6 on neural activity and transcription of the gene encoding corticotrophin-releasing factor in the rat brain: an effect depending upon the route of administration. Eur J Neurosci. (1997) 9:1461–72. doi: 10.1111/j.1460-9568.1997.tb01500.x
64. Desson SE and Ferguson AV. Interleukin 1beta modulates rat subfornical organ neurons as a result of activation of a non-selective cationic conductance. J Physiol. (2003) 550:113–22. doi: 10.1113/jphysiol.2003.041210
65. Wei S-G, Yu Y, and Felder RB. Blood-borne interleukin-1β acts on the subfornical organ to upregulate the sympathoexcitatory milieu of the hypothalamic paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol. (2018) 314:R447–58. doi: 10.1152/ajpregu.00211.2017
66. Osaka T, Kannan H, Kawano S, Ueta Y, and Yamashita H. Intraperitoneal administration of recombinant human interleukin-1β inhibits osmotic thirst in the rat. Physiol Behav. (1992) 51:1267–70. doi: 10.1016/0031-9384(92)90319-W
67. Swart RM, Hoorn EJ, Betjes MG, and Zietse R. Hyponatremia and inflammation: the emerging role of interleukin-6 in osmoregulation. Nephron Physiol. (2010) 118:p45–51. doi: 10.1159/000322238
68. Senatorov VV, Friedman AR, Milikovsky DZ, Ofer J, Saar-Ashkenazy R, Charbash A, et al. Blood-brain barrier dysfunction in aging induces hyperactivation of TGFβ signaling and chronic yet reversible neural dysfunction. Sci Transl Med. (2019) 11:eaaw8283. doi: 10.1126/scitranslmed.aaw8283
69. Leib DE, Zimmerman CA, and Knight ZA. Thirst. Curr Biol. (2016) 26:R1260–5. doi: 10.1016/j.cub.2016.11.019
70. McNeil-Masuka J and Boyer TJ. Insensible fluid loss. In: StatPearls. StatPearls Publishing, Treasure Island (FL (2025). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK544219/.
71. D’Acierno M, Fenton RA, and Hoorn EJ. The biology of water homeostasis. Nephrol Dial Transplant. (2025) 40(4):632–40. doi: 10.1093/ndt/gfae235
72. Begg DP. Disturbances of thirst and fluid balance associated with aging. Physiol Behav. (2017) 178:28–34. doi: 10.1016/j.physbeh.2017.03.003
73. Sands JM. Urine concentrating and diluting ability during aging. J Gerontol A Biol Sci Med Sci. (2012) 67:1352–7. doi: 10.1093/gerona/gls128
74. McKinley MJ, Denton DA, Ryan PJ, Yao ST, Stefanidis A, and Oldfield BJ. From sensory circumventricular organs to cerebral cortex: Neural pathways controlling thirst and hunger. J Neuroendocrinol. (2019) 31:e12689. doi: 10.1111/jne.12689
75. Brown CH, Bains JS, Ludwig M, and Stern JE. Physiological regulation of magnocellular neurosecretory cell activity: integration of intrinsic, local and afferent mechanisms. J Neuroendocrinol. (2013) 25:678–710. doi: 10.1111/jne.12051
76. Jin X, Xie J, Yeh C-W, Chen J-C, Cheng C-J, Lien C-C, et al. WNK1 promotes water homeostasis by acting as a central osmolality sensor for arginine vasopressin release. J Clin Invest. (2023) 133:e164222. doi: 10.1172/JCI164222
77. Sudbury JR, Ciura S, Sharif-Naeini R, and Bourque CW. Osmotic and thermal control of magnocellular neurosecretory neurons – role of an N-terminal variant of. trpv1. Eur J Neurosci. (2010) 32:2022–30. doi: 10.1111/j.1460-9568.2010.07512.x
78. Thompson CJ, Bland J, Burd J, and Baylis PH. The osmotic thresholds for thirst and vasopressin release are similar in healthy man. Clin Sci. (1986) 71:651–6. doi: 10.1042/cs0710651
79. Hughes F, Mythen M, and Montgomery H. The sensitivity of the human thirst response to changes in plasma osmolality: a systematic review. Perioper Med (Lond). (2018) 7:1. doi: 10.1186/s13741-017-0081-4
80. Fried LF and Palevsky PM. Hyponatremia and hypernatremia. Med Clinics North America. (1997) 81:585–609. doi: 10.1016/S0025-7125(05)70535-6
81. Zerbe RL, Miller JZ, and Robertson GL. The reproducibility and heritability of individual differences in osmoregulatory function in normal human subjects. J Lab Clin Med. (1991) 117:51–9.
82. Baylis PH. Osmoregulation and control of vasopressin secretion in healthy humans. Am J Physiology-Regulatory Integr Comp Physiol. (1987) 253:R671–8. doi: 10.1152/ajpregu.1987.253.5.R671
83. Keck ME, Hatzinger M, Wotjak CT, Landgraf R, Holsboer F, and Neumann ID. Ageing alters intrahypothalamic release patterns of vasopressin and oxytocin in rats. Eur J Neurosci. (2000) 12:1487–94. doi: 10.1046/j.1460-9568.2000.00030.x
84. Robertson GL and Rowe J. The effect of aging on neurohypophyseal function. Peptides. (1980) 1:159–62. doi: 10.1016/0196-9781(80)90113-8
85. Yao ST, McKinley MJ, and May CN. Circumventing a broken heart: cytokines and the subfornical organ. Am J Physiology-Heart Circulatory Physiol. (2017) 313:H729–31. doi: 10.1152/ajpheart.00480.2017
86. Biesmans S, Bouwknecht JA, Ver Donck L, Langlois X, Acton PD, De Haes P, et al. Peripheral administration of tumor necrosis factor-alpha induces neuroinflammation and sickness but not depressive-like behavior in mice. BioMed Res Int. (2015) 2015:716920. doi: 10.1155/2015/716920
87. Yang B, Lv C, Ye L, Wang Z, Kim Y, Luo W, et al. Stratum corneum hydration inversely correlates with certain serum cytokine levels in the elderly, possibly contributing to inflammaging. Immun Ageing. (2023) 20:7. doi: 10.1186/s12979-023-00331-1
88. Kim HO, Kim H-S, Youn J-C, Shin E-C, and Park S. Serum cytokine profiles in healthy young and elderly population assessed using multiplexed bead-based immunoassays. J Transl Med. (2011) 9:113. doi: 10.1186/1479-5876-9-113
89. McLEAN KA, O’Neill PA, Davies I, and Morris J. Influence of age on plasma osmolality: A community study. Age Ageing. (1992) 21:56–60. doi: 10.1093/ageing/21.1.56
90. Hooper L, Abdelhamid A, Ali A, Bunn DK, Jennings A, John WG, et al. Diagnostic accuracy of calculated serum osmolarity to predict dehydration in older people: adding value to pathology laboratory reports. BMJ Open. (2015) 5:e008846. doi: 10.1136/bmjopen-2015-008846
91. Kenney WL and Chiu P. Influence of age on thirst and fluid intake. Med Sci Sports Exercise. (2001) 33:1524–32. doi: 10.1097/00005768-200109000-00016
92. Summy-Long JY, Hu S, Long A, and Phillips TM. Interleukin-1beta release in the supraoptic nucleus area during osmotic stimulation requires neural function. J Neuroendocrinol. (2008) 20:1224–32. doi: 10.1111/j.1365-2826.2008.01783.x
93. Tasker JG, Di S, and Boudaba C. Functional synaptic plasticity in hypothalamic magnocellular neurons. Prog Brain Res. (2002) 139:113–9. doi: 10.1016/s0079-6123(02)39011-3
94. Di S and Tasker JG. Dehydration-induced synaptic plasticity in magnocellular neurons of the hypothalamic supraoptic nucleus. Endocrinology. (2004) 145:5141–9. doi: 10.1210/en.2004-0702
95. Landgraf R, Neumann I, Holsboer F, and Pittman QJ. Interleukin-1β Stimulates both central and peripheral release of vasopressin and oxytocin in the rat. Eur J Neurosci. (1995) 7:592–8. doi: 10.1111/j.1460-9568.1995.tb00663.x
96. Liu X, Nemeth DP, McKim DB, Zhu L, DiSabato DJ, Berdysz O, et al. Cell-type-specific interleukin 1 receptor 1 signaling in the brain regulates distinct neuroimmune activities. Immunity. (2019) 50:317–333.e6. doi: 10.1016/j.immuni.2018.12.012
97. Napetschnig J and Wu H. Molecular basis of NF-κB signaling. Annu Rev Biophys. (2013) 42:443–68. doi: 10.1146/annurev-biophys-083012-130338
98. Meves H. The action of prostaglandins on ion channels. Curr Neuropharmacol. (2006) 4:41–57. doi: 10.2174/157015906775203048
99. Dine J, Ducourneau VRR, Fénelon VS, Fossat P, Amadio A, Eder M, et al. Extracellular signal-regulated kinase phosphorylation in forebrain neurones contributes to osmoregulatory mechanisms. J Physiol. (2014) 592:1637–54. doi: 10.1113/jphysiol.2013.261008
100. Srinivasan D, Yen J-H, Joseph DJ, and Friedman W. Cell type-specific interleukin-1β Signaling in the CNS. J Neurosci. (2004) 24:6482–8. doi: 10.1523/JNEUROSCI.5712-03.2004
101. Van Dam A-M, Malinowsky D, Lenczowski MJP, Bartfai T, and Tilders FJH. Interleukin 1 (IL-1) type i receptors mediate activation of rat hypothalamus–pituitary–adrenal axis and interleukin 6 production as shown by receptor type selective deletion mutants of IL-1β. Cytokine. (1998) 10:413–7. doi: 10.1006/cyto.1997.0315
102. Ghorbel MT, Sharman G, Leroux M, Barrett T, Donovan DM, Becker KG, et al. Microarray analysis reveals interleukin-6 as a novel secretory product of the hypothalamo-neurohypophyseal system. J Biol Chem. (2003) 278:19280–5. doi: 10.1074/jbc.M209902200
103. Tsigos C, Papanicolaou DA, Defensor R, Mitsiadis CS, Kyrou I, and Chrousos GP. Dose effects of recombinant human lnterleukin-6 on pituitary hormone secretion and energy expenditure. Neuroendocrinology. (1997) 66:54–62. doi: 10.1159/000127219
104. Jankord R, Zhang R, Flak JN, Solomon MB, Albertz J, and Herman JP. Stress activation of IL-6 neurons in the hypothalamus. Am J Physiol Regul Integr Comp Physiol. (2010) 299:R343–351. doi: 10.1152/ajpregu.00131.2010
105. Turnbull AV, Prehar S, Kennedy AR, Little RA, and Hopkins SJ. Interleukin-6 is an afferent signal to the hypothalamo-pituitary-adrenal axis during local inflammation in mice. Endocrinology. (2003) 144:1894–906. doi: 10.1210/en.2002-220964
106. Ibrahim N, Shibuya I, Kabashima N, Sutarmo SV, Ueta Y, and Yamashita H. Prostaglandin E2 inhibits spontaneous inhibitory postsynaptic currents in rat supraoptic neurones via presynaptic EP receptors. J Neuroendocrinol. (1999) 11:879–86. doi: 10.1046/j.1365-2826.1999.00404.x
107. De Laurentiis A, Fernandez-Solari J, Mohn C, Burdet B, Zorrilla Zubilete MA, and Rettori V. The hypothalamic endocannabinoid system participates in the secretion of oxytocin and tumor necrosis factor-alpha induced by lipopolysaccharide. J Neuroimmunology. (2010) 221:32–41. doi: 10.1016/j.jneuroim.2010.02.006
108. Spicarova D and Palecek J. Tumor necrosis factor alpha sensitizes spinal cord TRPV1 receptors to the endogenous agonist N-oleoyldopamine. J Neuroinflamm. (2010) 7:49. doi: 10.1186/1742-2094-7-49
109. Benítez-Angeles M, Morales-Lázaro SL, Juárez-González E, and Rosenbaum T. TRPV1: structure, endogenous agonists, and mechanisms. Int J Mol Sci. (2020) 21:3421. doi: 10.3390/ijms21103421
110. Chakfe Y, Zhang Z, and Bourque CW. IL-1β directly excites isolated rat supraoptic neurons via upregulation of the osmosensory cation current. Am J Physiology-Regulatory Integr Comp Physiol. (2006) 290:R1183–90. doi: 10.1152/ajpregu.00716.2005
111. Sudbury JR and Bourque CW. Dynamic and permissive roles of TRPV1 and TRPV4 channels for thermosensation in mouse supraoptic magnocellular neurosecretory neurons. J Neurosci. (2013) 33:17160–5. doi: 10.1523/JNEUROSCI.1048-13.2013
112. Shenton FC and Pyner S. Transient receptor potential vanilloid type 4 is expressed in vasopressinergic neurons within the magnocellular subdivision of the rat paraventricular nucleus of the hypothalamus. J Comp Neurol. (2018) 526:3035–44. doi: 10.1002/cne.24514
113. Brown E, Brown CH, and Fronius M. Mechanosensitivity of TRPV channels: implications for vasopressin neuron activity. FASEB J. (2020) 34:1–1. doi: 10.1096/fasebj.2020.34.s1.03561
114. Zaelzer C, Hua P, Prager-Khoutorsky M, Ciura S, Voisin DL, Liedtke W, et al. ΔN-TRPV1: A molecular co-detector of body temperature and osmotic stress. Cell Rep. (2015) 13:23–30. doi: 10.1016/j.celrep.2015.08.061
115. Tasker JG, Prager-Khoutorsky M, Teruyama R, Lemos JR, and Amstrong WE. Advances in the neurophysiology of magnocellular neuroendocrine cells. J Neuroendocrinol. (2020) 32:e12826. doi: 10.1111/jne.12826
116. Haan KD, Park SJ, Nakamura Y, Fukami K, and Fisher TE. Osmotically evoked PLCδ1-dependent translocation of ΔN-TRPV1 channels in rat supraoptic neurons. iScience. (2023) 26:106258. doi: 10.1016/j.isci.2023.106258
117. Park SJ, Haan KD, Nakamura Y, Fukami K, and Fisher TE. PLCδ1 plays central roles in the osmotic activation of ΔN-TRPV1 channels in mouse supraoptic neurons and in murine osmoregulation. J Neurosci. (2021) 41:3579–87. doi: 10.1523/JNEUROSCI.2892-20.2021
118. Palin K, Moreau ML, Orcel H, Duvoid-Guillou A, Rabié A, Kelley KW, et al. Age-impaired fluid homeostasis depends on the balance of IL-6/IGF-I in the rat supraoptic nuclei. Neurobiol Aging. (2009) 30:1677–92. doi: 10.1016/j.neurobiolaging.2007.12.006
119. Lazzerini PE, Laghi-Pasini F, Acampa M, Srivastava U, Bertolozzi I, Giabbani B, et al. Systemic inflammation rapidly induces reversible atrial electrical remodeling: the role of interleukin-6–mediated changes in connexin expression. JAHA. (2019) 8:e011006. doi: 10.1161/JAHA.118.011006
120. Jiang S, Wang Y-Q, Xu C-F, Li Y-N, Guo R, and Li L. Involvement of connexin43 in the acute hyperosmotic stimulus-induced synthesis and release of vasopressin in the supraoptic nucleus of rats. Mol Med Rep. (2014) 10:2165–71. doi: 10.3892/mmr.2014.2400
121. Bobbo VC, Engel DF, Jara CP, Mendes NF, Haddad-Tovolli R, Prado TP, et al. Interleukin-6 actions in the hypothalamus protects against obesity and is involved in the regulation of neurogenesis. J Neuroinflamm. (2021) 18:192. doi: 10.1186/s12974-021-02242-8
122. Ohbuchi T, Haam J, and Tasker JG. Regulation of neuronal activity in hypothalamic vasopressin neurons. Interdiscip Inf Sci. (2015) 21:225–34. doi: 10.4036/iis.2015.B.07
123. Wei S-G, Zhang Z-H, Beltz TG, Yu Y, Johnson AK, and Felder RB. Subfornical organ mediates sympathetic and hemodynamic responses to blood-borne proinflammatory cytokines. Hypertension. (2013) 62:118–25. doi: 10.1161/HYPERTENSIONAHA.113.01404
124. Boyd-Shiwarski CR, Shiwarski DJ, Griffiths SE, Beacham RT, Norrell L, Morrison DE, et al. WNK kinases sense molecular crowding and rescue cell volume via phase separation. Cell. (2022) 185:4488–4506.e20. doi: 10.1016/j.cell.2022.09.042
125. Jin X, Xie J, and Huang C-L. WNK1 is a central osmolality sensor for arginine vasopressin release and acts through the OSR1/SPAK kinase cascade. Physiology. (2024) 39:443. doi: 10.1152/physiol.2024.39.S1.443
126. Xu B-E, Stippec S, Chu P-Y, Lazrak A, Li X-J, Lee B-H, et al. WNK1 activates SGK1 to regulate the epithelial sodium channel. Proc Natl Acad Sci. (2005) 102:10315–20. doi: 10.1073/pnas.0504422102
127. McCormick JA and Ellison DH. The WNKs: atypical protein kinases with pleiotropic actions. Physiol Rev. (2011) 91:177–219. doi: 10.1152/physrev.00017.2010
128. Alessi DR, Zhang J, Khanna A, Hochdörfer T, Shang Y, and Kahle KT. The WNK-SPAK/OSR1 pathway: master regulator of cation-chloride cotransporters. Sci Signal. (2014) 7:re3. doi: 10.1126/scisignal.2005365
129. Zhang S, Zhou J, Zhang Y, Liu T, Friedel P, Zhuo W, et al. The structural basis of function and regulation of neuronal cotransporters NKCC1 and KCC2. Commun Biol. (2021) 4:226. doi: 10.1038/s42003-021-01750-w
130. Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, et al. The K+/Cl– co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. (1999) 397:251–5. doi: 10.1038/16697
131. Friedel P, Kahle KT, Zhang J, Hertz N, Pisella LI, Buhler E, et al. WNK1-regulated inhibitory phosphorylation of the KCC2 cotransporter maintains the depolarizing action of GABA in immature neurons. Sci Signal. (2015) 8:ra65. doi: 10.1126/scisignal.aaa0354
132. Lee KL, Abiraman K, Lucaj C, Ollerhead TA, Brandon NJ, Deeb TZ, et al. Inhibiting with-no-lysine kinases enhances K+/Cl– cotransporter 2 activity and limits status epilepticus. Brain. (2022) 145:950–63. doi: 10.1093/brain/awab343
133. Sladek CD and Armstrong WE. γ-aminobutyric acid antagonists stimulate vasopressin release from organ-cultured hypothalamo-neurohypophyseal explants*. Endocrinology. (1987) 120:1576–80. doi: 10.1210/endo-120-4-1576
134. Brennan TJ, Morris M, and Haywood JR. GABA agonists inhibit the vasopressin-dependent pressor effects of central angiotensin II. Neuroendocrinology. (1984) 39:429–36. doi: 10.1159/000124016
135. Kim JS, Kim WB, Kim Y-B, Lee Y, Kim YS, Shen F-Y, et al. Chronic hyperosmotic stress converts GABAergic inhibition into excitation in vasopressin and oxytocin neurons in the rat. J Neurosci. (2011) 31:13312–22. doi: 10.1523/JNEUROSCI.1440-11.2011
136. Haam J, Popescu IR, Morton LA, Halmos KC, Teruyama R, Ueta Y, et al. GABA is excitatory in adult vasopressinergic neuroendocrine cells. J Neurosci. (2012) 32:572–82. doi: 10.1523/JNEUROSCI.3826-11.2012
137. Kim Y-B, Kim WB, Jung WW, Jin X, Kim YS, Kim B, et al. Excitatory GABAergic action and increased vasopressin synthesis in hypothalamic magnocellular neurosecretory cells underlie the high plasma level of vasopressin in diabetic rats. Diabetes. (2018) 67:486–95. doi: 10.2337/db17-1042
138. Kim Y-B, Kim YS, Kim WB, Shen F-Y, Lee SW, Chung HJ, et al. GABAergic excitation of vasopressin neurons: possible mechanism underlying sodium-dependent hypertension. Circ Res. (2013) 113:1296–307. doi: 10.1161/CIRCRESAHA.113.301814
139. Choe KY, Han SY, Gaub P, Shell B, Voisin DL, Knapp BA, et al. High salt intake increases blood pressure via BDNF-mediated downregulation of KCC2 and impaired baroreflex inhibition of vasopressin neurons. Neuron. (2015) 85:549–60. doi: 10.1016/j.neuron.2014.12.048
140. Thorsdottir D, Einwag Z, and Erdos B. BDNF shifts excitatory-inhibitory balance in the paraventricular nucleus of the hypothalamus to elevate blood pressure. J Neurophysiol. (2021) 126:1209–20. doi: 10.1152/jn.00247.2021
141. Chamarthi B, Williams GH, Ricchiuti V, Srikumar N, Hopkins PN, Luther JM, et al. Inflammation and hypertension: the interplay of interleukin-6, dietary sodium, and the renin-angiotensin system in humans. Am J Hypertension. (2011) 24:1143–8. doi: 10.1038/ajh.2011.113
142. Zhang W, Wang W, Yu H, Zhang Y, Dai Y, Ning C, et al. Interleukin 6 underlies angiotensin II–induced hypertension and chronic renal damage. Hypertension. (2012) 59:136–44. doi: 10.1161/HYPERTENSIONAHA.111.173328
143. Ramseyer VD and Garvin JL. Tumor necrosis factor-α: regulation of renal function and blood pressure. Am J Physiology-Renal Physiol. (2013) 304:F1231–42. doi: 10.1152/ajprenal.00557.2012
144. Plante TB, Juraschek SP, Howard G, Howard VJ, Tracy RP, Olson NC, et al. Cytokines, C-reactive protein, and risk of incident hypertension in the REGARDS study. Hypertension. (2024) 81:1244–53. doi: 10.1161/HYPERTENSIONAHA.123.22714
145. Teruyama R, Sakuraba M, Wilson LL, Wandrey NEJ, and Armstrong WE. Epithelial Na+ sodium channels in magnocellular cells of the rat supraoptic and paraventricular nuclei. Am J Physiol Endocrinol Metab. (2012) 302:E273–285. doi: 10.1152/ajpendo.00407.2011
146. Miller RL, Wang MH, Gray PA, Salkoff LB, and Loewy AD. ENaC-expressing neurons in the sensory circumventricular organs become c-Fos activated following systemic sodium changes. Am J Physiol Regul Integr Comp Physiol. (2013) 305:R1141–1152. doi: 10.1152/ajpregu.00242.2013
147. Sharma K, Haque M, Guidry R, Ueta Y, and Teruyama R. Effect of dietary salt intake on epithelial Na+ channels (ENaC) in vasopressin magnocellular neurosecretory neurons in the rat supraoptic nucleus. J Physiol. (2017) 595:5857–74. doi: 10.1113/JP274856
148. Levi DI, Wyrosdic JC, Hicks A-I, Andrade MA, Toney GM, Prager-Khoutorsky M, et al. High dietary salt amplifies osmoresponsiveness in vasopressin-releasing neurons. Cell Rep. (2021) 34:108866. doi: 10.1016/j.celrep.2021.108866
149. Costello HM, Krilis G, Grenier C, Severs D, Czopek A, Ivy JR, et al. High salt intake activates the hypothalamic–pituitary–adrenal axis, amplifies the stress response, and alters tissue glucocorticoid exposure in mice. Cardiovasc Res. (2023) 119:1740–50. doi: 10.1093/cvr/cvac160
150. Matsuguchi H, Schmid PG, Van Orden D, and Mark AL. Does vasopressin contribute to salt-induced hypertension in the Dahl strain? Hypertension. (1981) 3:174–81. doi: 10.1161/01.HYP.3.2.174
151. Tasevska I, Enhörning S, Burri P, and Melander O. High salt intake increases copeptin but salt sensitivity is associated with fluid induced reduction of copeptin in women. Int J Hypertens. (2014) 2014:641587. doi: 10.1155/2014/641587
152. Gamper N, Fillon S, Huber S, Feng Y, Kobayashi T, Cohen P, et al. IGF-1 up-regulates K+ channels via PI3-kinase, PDK1 and SGK1. Pflügers Arch - Eur J Physiol. (2002) 443:625–34. doi: 10.1007/s00424-001-0741-5
153. Zaika O, Palygin O, Tomilin V, Mamenko M, Staruschenko A, and Pochynyuk O. Insulin and IGF-1 activate K ir 4.1/5.1 channels in cortical collecting duct principal cells to control basolateral membrane voltage. Am J Physiology-Renal Physiol. (2016) 310:F311–21. doi: 10.1152/ajprenal.00436.2015
154. Mustafa SB, Hernandez TF, Johnson-Pais TL, Kumar PA, Petershack JA, Henson BM, et al. IL-1 promotes α-epithelial Sodium Channel (α-ENaC) expression in murine lung epithelial cells: involvement of NF-κB. J Cell Commun Signal. (2020) 14:303–14. doi: 10.1007/s12079-019-00533-7
155. Li K, Guo D, Zhu H, Hering-Smith KS, Hamm LL, Ouyang J, et al. Interleukin-6 stimulates epithelial sodium channels in mouse cortical collecting duct cells. Am J Physiol Regul Integr Comp Physiol. (2010) 299:R590–595. doi: 10.1152/ajpregu.00207.2009
156. George CPL, Messerli FH, Genest J, Nowaczynski W, Boucher R, Kuchel O, et al. Diurnal variation of plasma vasopressin in man*. J Clin Endocrinol Metab. (1975) 41:332–8. doi: 10.1210/jcem-41-2-332
157. Asplund R and Åberg H. Diurnal variation in the levels of antidiuretic hormone in the elderly. J Internal Med. (1991) 229:131–4. doi: 10.1111/j.1365-2796.1991.tb00320.x
158. Salata RA, Jarrett DB, Verbalis JG, and Robinson AG. Vasopressin stimulation of adrenocorticotropin hormone (ACTH) in humans. In vivo bioassay of corticotropin-releasing factor (CRF) which provides evidence for CRF mediation of the diurnal rhythm of ACTH. J Clin Invest. (1988) 81:766–74. doi: 10.1172/JCI113382
159. Tyagi S, Resnick NM, Clarkson BD, Zhang G, Krafty RT, Perera S, et al. Impact of sleep on chronobiology of micturition among healthy older adults. Am J Physiology-Renal Physiol. (2023) 325:F407–17. doi: 10.1152/ajprenal.00025.2023
160. Nanba K, Vaidya A, Williams GH, Zheng I, Else T, and Rainey WE. Age-related autonomous aldosteronism. Circulation. (2017) 136:347–55. doi: 10.1161/CIRCULATIONAHA.117.028201
161. Gardner MP, Lightman S, Sayer AA, Cooper C, Cooper R, Deeg D, et al. Dysregulation of the hypothalamic pituitary adrenal (HPA) axis and physical performance at older ages: An individual participant meta-analysis. Psychoneuroendocrinology. (2013) 38:40–9. doi: 10.1016/j.psyneuen.2012.04.016
162. Begemann K, Rawashdeh O, Olejniczak I, Pilorz V, De Assis LVM, Osorio-Mendoza J, et al. Endocrine regulation of circadian rhythms. NPJ Biol Timing Sleep. (2025) 2:10. doi: 10.1038/s44323-025-00024-6
163. Hauger RL, Thrivikraman KV, and Plotsky PM. Age-related alterations of hypothalamic-pituitary-adrenal axis function in male Fischer 344 rats. Endocrinology. (1994) 134:1528–36. doi: 10.1210/endo.134.3.8119195
164. Nakamura K, Velho G, and Bouby N. Vasopressin and metabolic disorders: translation from experimental models to clinical use. J Intern Med. (2017) 282:298–309. doi: 10.1111/joim.12649
165. Javeed N, Brown MR, Rakshit K, Her T, Sen SK, and Matveyenko AV. Proinflammatory cytokine interleukin 1β Disrupts β-cell circadian clock function and regulation of insulin secretion. Endocrinology. (2021) 162:bqaa084. doi: 10.1210/endocr/bqaa084
166. Pferdehirt L, Damato AR, Dudek M, Meng Q-J, Herzog ED, and Guilak F. Synthetic gene circuits for preventing disruption of the circadian clock due to interleukin-1–induced inflammation. Sci Adv. (2022) 8:eabj8892. doi: 10.1126/sciadv.abj8892
167. Monje FJ, Cicvaric A, Acevedo Aguilar JP, Elbau I, Horvath O, Diao W, et al. Disrupted ultradian activity rhythms and differential expression of several clock genes in interleukin-6-deficient mice. Front Neurol. (2017) 8:99. doi: 10.3389/fneur.2017.00099
168. Vgontzas AN, Bixler EO, Lin H-M, Prolo P, Trakada G, and Chrousos GP. IL-6 and its circadian secretion in humans. Neuroimmunomodulation. (2005) 12:131–40. doi: 10.1159/000084844
169. Jehn CF, Kühnhardt D, Bartholomae A, Pfeiffer S, Schmid P, Possinger K, et al. Association of IL-6, hypothalamus-pituitary-adrenal axis function, and depression in patients with cancer. Integr Cancer Ther. (2010) 9:270–5. doi: 10.1177/1534735410370036
170. Melander O. Vasopressin, from regulator to disease predictor for diabetes and cardiometabolic risk. Ann Nutr Metab. (2016) 68:24–8. doi: 10.1159/000446201
171. El Boustany R, Tasevska I, Meijer E, Kieneker LM, Enhörning S, Lefèvre G, et al. Plasma copeptin and chronic kidney disease risk in 3 European cohorts from the general population. JCI Insight. (2018) 3:121479. doi: 10.1172/jci.insight.121479
172. Clark AG, Parker EAD, Savla JS, Davy KP, and Davy BM. Is increased water consumption among older adults associated with improvements in glucose homeostasis? OJPM. (2013) 03:363–7. doi: 10.4236/ojpm.2013.35049
173. Sontrop JM, Huang S-H, Garg AX, Moist L, House AA, Gallo K, et al. Effect of increased water intake on plasma copeptin in patients with chronic kidney disease: results from a pilot randomised controlled trial. BMJ Open. (2015) 5:e008634. doi: 10.1136/bmjopen-2015-008634
174. Enhörning S, Brunkwall L, Tasevska I, Ericson U, Persson Tholin J, Persson M, et al. Water supplementation reduces copeptin and plasma glucose in adults with high copeptin: the H2O metabolism pilot study. J Clin Endocrinol Metab. (2019) 104:1917–25. doi: 10.1210/jc.2018-02195
175. Enhörning S, Tasevska I, Roussel R, Bouby N, Persson M, Burri P, et al. Effects of hydration on plasma copeptin, glycemia and gluco-regulatory hormones: a water intervention in humans. Eur J Nutr. (2019) 58:315–24. doi: 10.1007/s00394-017-1595-8
176. Filippatos TD, Makri A, Elisaf MS, and Liamis G. Hyponatremia in the elderly: challenges and solutions. CIA. (2017) 12:1957–65. doi: 10.2147/CIA.S138535
177. Booth FW, Laye MJ, and Roberts MD. Lifetime sedentary living accelerates some aspects of secondary aging. J Appl Physiol. (2011) 111:1497–504. doi: 10.1152/japplphysiol.00420.2011
178. Tayebi SM, Poorhabibi H, Heidary D, Amini MA, and Sadeghi A. Impact of aerobic exercise on chronic inflammation in older adults: a systematic review and meta-analysis. BMC Sports Sci Med Rehabil. (2025) 17:229. doi: 10.1186/s13102-025-01279-z
179. Ding Y and Xu X. Effects of regular exercise on inflammasome activation-related inflammatory cytokine levels in older adults: a systematic review and meta-analysis. J Sports Sci. (2021) 39:2338–52. doi: 10.1080/02640414.2021.1932279
180. Freund BJ, Shizuru EM, Hashiro GM, and Claybaugh JR. Hormonal, electrolyte, and renal responses to exercise are intensity dependent. J Appl Physiol. (1991) 70:900–6. doi: 10.1152/jappl.1991.70.2.900
181. Popovic M, Timper K, Seelig E, Nordmann T, Erlanger TE, Donath MY, et al. Exercise upregulates copeptin levels which is not regulated by interleukin-1. PLoS One. (2019) 14:e0217800. doi: 10.1371/journal.pone.0217800
182. Aakre KM, Kleiven Ø, Skadberg Ø, Bjørkavoll-Bergseth MF, Melberg T, Strand H, et al. The copeptin response after physical activity is not associated with cardiac biomarkers or asymptomatic coronary artery disease: The North Sea Race Endurance Exercise Study (NEEDED) 2013. Clin Biochem. (2018) 52:8–12. doi: 10.1016/j.clinbiochem.2017.10.007
183. Hew-Butler T, Jordaan E, Stuempfle KJ, Speedy DB, Siegel AJ, Noakes TD, et al. Osmotic and nonosmotic regulation of arginine vasopressin during prolonged endurance exercise. J Clin Endocrinol Metab. (2008) 93:2072–8. doi: 10.1210/jc.2007-2336
184. Carroll JF, Convertino VA, Wood CE, Graves JE, Lowenthal DT, and Pollock ML. Effect of training on blood volume and plasma hormone concentrations in the elderly. Med Sci Sports Exerc. (1995) 27:79–84. doi: 10.1249/00005768-199501000-00015
185. Panagiotou M, Michel S, Meijer JH, and Deboer T. The aging brain: sleep, the circadian clock and exercise. Biochem Pharmacol. (2021) 191:114563. doi: 10.1016/j.bcp.2021.114563
186. Shen B, Ma C, Wu G, Liu H, Chen L, and Yang G. Effects of exercise on circadian rhythms in humans. Front Pharmacol. (2023) 14:1282357. doi: 10.3389/fphar.2023.1282357
187. Woods JA, Wilund KR, Martin SA, and Kistler BM. Exercise, inflammation and aging. Aging Dis. (2012) 3:130–40.
188. Lucertini F, Ponzio E, Di Palma M, Galati C, Federici A, Barbadoro P, et al. High cardiorespiratory fitness is negatively associated with daily cortisol output in healthy aging men. PLoS One. (2015) 10:e0141970. doi: 10.1371/journal.pone.0141970
189. De Nys L, Anderson K, Ofosu EF, Ryde GC, Connelly J, and Whittaker AC. The effects of physical activity on cortisol and sleep: A systematic review and meta-analysis. Psychoneuroendocrinology. (2022) 143:105843. doi: 10.1016/j.psyneuen.2022.105843
190. Benrick A, Schéle E, Pinnock SB, Wernstedt-Asterholm I, Dickson SL, Karlsson-Lindahl L, et al. Interleukin-6 gene knockout influences energy balance regulating peptides in the hypothalamic paraventricular and supraoptic nuclei. J Neuroendocrinol. (2009) 21:620–8. doi: 10.1111/j.1365-2826.2009.01879.x
191. Ershler WB and Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. (2000) 51:245–70. doi: 10.1146/annurev.med.51.1.245
192. Choy EH, De Benedetti F, Takeuchi T, Hashizume M, John MR, and Kishimoto T. Translating IL-6 biology into effective treatments. Nat Rev Rheumatol. (2020) 16:335–45. doi: 10.1038/s41584-020-0419-z
193. Berl T and Schrier RW. Vasopressin antagonists in physiology and disease. In: Textbook of Nephro-Endocrinology. Elsevier (2018). p. 117–31. doi: 10.1016/B978-0-12-803247-3.00007-6
194. Verbalis. Conivaptan: Evidence supporting its therapeutic use in hyponatremia. CE. (2009) 83. doi: 10.2147/CE.S5997
195. Tzoulis P, Kaltsas G, Baldeweg SE, Bouloux P-M, and Grossman AB. Tolvaptan for the treatment of the syndrome of inappropriate antidiuresis (SIAD). Ther Adv Endocrinol Metab. (2023) 14:20420188231173327. doi: 10.1177/20420188231173327
196. Bolognani F, Del Valle Rubido M, Squassante L, Wandel C, Derks M, Murtagh L, et al. A phase 2 clinical trial of a vasopressin V1a receptor antagonist shows improved adaptive behaviors in men with autism spectrum disorder. Sci Transl Med. (2019) 11:eaat7838. doi: 10.1126/scitranslmed.aat7838
197. Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B, et al. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc Natl Acad Sci USA. (2002) 99:6370–5. doi: 10.1073/pnas.092012099
198. Kikuchi E, Mori T, Zeniya M, Isobe K, Ishigami-Yuasa M, Fujii S, et al. Discovery of novel SPAK inhibitors that block WNK kinase signaling to cation chloride transporters. J Am Soc Nephrol. (2015) 26:1525–36. doi: 10.1681/ASN.2014060560
199. Rehman T, Karp PH, Thurman AL, Mather SE, Jain A, Cooney AL, et al. WNK inhibition increases surface liquid pH and host defense in cystic fibrosis airway epithelia. Am J Respir Cell Mol Biol. (2022) 67:491–502. doi: 10.1165/rcmb.2022-0172OC
200. Mayes-Hopfinger L, Enache A, Xie J, Huang C-L, Köchl R, Tybulewicz VLJ, et al. Chloride sensing by WNK1 regulates NLRP3 inflammasome activation and pyroptosis. Nat Commun. (2021) 12:4546. doi: 10.1038/s41467-021-24784-4
201. Kim JS and Kehrl JH. Inhibition of WNK kinases in NK cells disrupts cellular osmoregulation and control of tumor metastasis. J Innate Immun. (2024) 16:451–69. doi: 10.1159/000540744
202. Mannick JB and Lamming DW. Targeting the biology of aging with mTOR inhibitors. Nat Aging. (2023) 3:642–60. doi: 10.1038/s43587-023-00416-y
Keywords: vasopressin, antidiuretic hormone, cytokine, interleukin - 1 β, interleukin-6, inflammaging, microinflammation
Citation: Mutig K, Lebedeva S and Singh PB (2025) Inflammation and vasopressin hypersecretion in aging. Front. Endocrinol. 16:1689787. doi: 10.3389/fendo.2025.1689787
Received: 20 August 2025; Accepted: 22 September 2025;
Published: 13 October 2025; Corrected: 23 October 2025.
Edited by:
Beatriz Merino, CEU San Pablo University, SpainReviewed by:
Charlotte Steenblock, Technical University Dresden, GermanyIsabelle Runkle De La Vega, San Carlos University Clinical Hospital, Spain
Copyright © 2025 Mutig, Lebedeva and Singh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kerim Mutig, bXV0aWcua0B0YWxhbnRpdXNwZWgucnU=; Prim B. Singh, cHJpbS5zaW5naEBudS5lZHUua3o=
 Kerim Mutig
Kerim Mutig Svetlana Lebedeva
Svetlana Lebedeva Prim B. Singh
Prim B. Singh