- 1Orthopedics and Traumatology Department of Integrated Traditional Chinese and Western Medicine, Tianjin Hospital, Tianjin, China
- 2Cardiothoracic Surgery Department Tianjin Hospital, Tianjin, China
Osteoporosis (OP) is a systemic bone disease characterized by reduced bone mass and deterioration of bone microarchitecture. Its critical complication, osteoporotic fractures (OPF), imposes a significant global disease burden. Macrophages, serving as central regulators within the osteoimmune microenvironment, dynamically modulate bone homeostasis and fracture healing through polarization (into pro-inflammatory M1 and reparative M2 phenotypes) and metabolic reprogramming. In OPF, OP-inducing factors (such as estrogen deficiency and aging) induce metabolic dysregulation in macrophages by disrupting the balance between glycolysis and oxidative phosphorylation (OXPHOS), causing aberrant succinate accumulation, and depleting NAD+ levels. This dysregulation disrupts the orderly transition from pro-inflammatory M1 to reparative M2 polarization, ultimately leading to insufficient inflammatory initiation in the early fracture phase and impaired osteogenic differentiation during later stages. Targeting this mechanism, innovative therapeutic strategies centered on macrophage metabolic reprogramming and polarization modulation are rapidly developing. These include nanocarriers for mitochondrial function restoration, bioactive coatings enabling time-programmed osseointegration, immunomodulatory smart hydrogels, and functionalized composite biomaterials. These strategies effectively promote osteoporotic bone regeneration by synergistically optimizing osteoimmune homeostasis and the osteoblast-osteoclast balance. This review systematically summarizes the immunometabolic mechanisms of macrophages in OPF and explores targeted intervention strategies, providing novel perspectives for the precision treatment of OPF.
1 Introduction
Osteoporosis (OP) is a systemic skeletal disorder marked by low bone mass, deteriorated bone microarchitecture, and consequently, increased bone fragility and fracture susceptibility (1). Its global prevalence is estimated at 19.7% (95% CI: 18.0%–21.4%) (2), rising to 21.7% among the elderly (95% CI: 18.8%–25.0%) (3). In 2019, OP incidence reached 41.5 million cases, reflecting a steady upward trend (4). Osteoporotic fractures (OPF) are a major complication of OP (5). Approximately 50% of women will experience at least one OPF during their lifetime (6). The associated annual global treatment costs are substantial, potentially reaching $25 billion USD (7).
Macrophages are highly heterogeneous immune cells that are key regulators of bone homeostasis within the osteoimmune microenvironment (8, 9). In response to local cues, they polarize into functionally distinct phenotypes (M1/M2) and release signaling molecules, including cytokines and exosomes. These signals modulate the activities of osteoblasts (OBs), osteoclasts (OCs), and bone marrow stromal cells (BMSCs) to maintain skeletal equilibrium (10–13). Furthermore, macrophages are indispensable for orchestrating bone repair following injury (14–16).
OPF is frequently complicated by delayed healing or non-union (17). While traditional theories of fracture repair have focused on biomechanics and OBs/OCs balance, emerging evidence underscores the central role of the osteoimmune microenvironment, where macrophages act as key orchestrators (18). Under the pathological state of osteoporosis, the impaired function of macrophages is an important mechanism of delayed healing of OPF (19). Therefore, deciphering the immunometabolic networks controlling macrophages in OPF and developing macrophage-targeted therapies to restore osteoimmune homeostasis may provide a promising avenue for addressing current treatment limitations and enabling precision intervention.
This review aims to systematically summarize the role of macrophages in the pathogenesis and progression of OPF. We will focus on the mechanisms underlying their immune polarization, metabolic reprogramming, and interactions with bone cells (OBs, OCs, BMSCs). We will elaborate on how OP-related pathological factors impair fracture healing by disrupting macrophage function. Furthermore, we will evaluate the potential and challenges of innovative therapies targeting macrophage immunometabolism for enhancing OPF repair. Ultimately, this review seeks to provide perspectives and a theoretical foundation for the future precision treatment of OPF.
2 Role of macrophages in bone homeostasis
Bone homeostasis depends on the dynamic balance between bone formation by OBs and bone resorption by OCs (20). Macrophages, as important immune effector cells, help maintain this equilibrium by directly influencing the activities of OBs, OCs, and BMSCs (21). Their functional impact on bone metabolism is largely determined by their polarization state, which can shift toward either the pro-inflammatory M1 or the anti-inflammatory M2 phenotype.
2.1 Regulation of OBs by macrophages
OBs are essential for bone formation (22). Macrophages influence OB differentiation and activity in a polarization-dependent manner through the secretion of various factors. M2 macrophages generally exert pro-osteogenic effects by releasing molecules such as BMP-2 and TGF-β1, which promote OBs differentiation and bone matrix mineralization via activation of the canonical Smad/Runx2 signaling pathway (23–26). In addition, M2-derived oncostatin M binds to the gp130 receptor and activates the JAK/STAT signaling pathway, thereby synergistically enhancing osteogenic differentiation (27). Specific M2 subsets (e.g., CD301b+ macrophages) also secrete IGF-1, promoting OBs differentiation via activation of the Akt/mTOR signaling pathway (28). Furthermore, M2 macrophages enhance OBs activity through chemokines (e.g., C-X-C motif chemokine ligand (CXCL) 3, CXCL6, CXCL14) and exosomes that modulate cytoskeletal and inflammatory pathways, and deliver osteogenic miRNAs such as miR-26a-5p and miR-21a-5p (29–33).
In contrast, M1 macrophages primarily inhibit OBs activity through the release of pro-inflammatory cytokines. Key effector molecules such as TNF-α and IL-1β suppress the expression of essential osteogenic transcription factors and inhibit the WNT/β-catenin signaling pathway, thereby impairing OBs differentiation and function (34–36). Although its role is complex, IL-6 in M1-dominant environments often indirectly suppresses osteogenesis, for example by upregulating TNF-α in OBs (37–40). Exosomes derived from M1 macrophages have also been implicated in the regulation of OB activity, though their precise mechanisms and functional distinctions from M2-derived exosomes remain to be fully elucidated (33, 41). It is also noteworthy that TNF-α exhibits a dual role: brief, low-level exposure can promote osteogenesis, while sustained, high-concentration exposure predominantly inhibits it (42).
2.2 Regulation of OCs by macrophages
OCs and macrophages share a common myeloid progenitor, which can lead to a competitive relationship during their differentiation. Macrophage polarization significantly influences osteoclastogenesis. While M1 macrophages are generally considered to promote osteoclastogenesis, their effects are highly context-dependent. Their key pro-inflammatory cytokine, TNF-α, strongly enhances RANKL-induced osteoclastogenesis and acts as an autocrine/paracrine factor in OCs formation (21, 43–46). IL-1β has also been shown to directly promote OCs formation and bone resorption in the presence of RANKL and M-CSF (47, 48). However, IFN-γ secreted by M1 macrophages inhibits osteoclastogenesis in in vitro RANKL-induced models (49), underscoring the context-dependent nature of M1-mediated regulation.
Conversely, M2 macrophages generally suppress osteoclastogenesis. They inhibit RANKL-induced OCs formation by interfering with TNF-α signaling and downregulating CSF2 expression (50). Characteristic anti-inflammatory cytokines, such as IL-10 and IL-4, directly impede osteoclast differentiation (10, 21). Furthermore, M2-derived exosomes serve as effective inhibitors by delivering miRNAs (e.g., miR-1227-5p) or modulating pathways such as STAT3 via CYLD (51, 52). Recent evidence indicates that these exosomes can metabolically reprogram osteoclast precursors—for example, by enhancing glutamine metabolism—and epigenetically downregulate osteoclastogenic genes, and may even promote their conversion into an M2-like phenotype. This establishes an important negative feedback loop that restrains bone resorption (53).
2.3 Crosstalk between macrophages and BMSCs
2.3.1 Influence of macrophages on BMSCs
Macrophage polarization significantly influences the osteogenic differentiation of BMSCs. M2 macrophages promote osteogenic differentiation and mineralization of BMSCs through the secretion of factors such as BMP-2 and TGF-β1. These ligands activate the Smad/Runx2 pathway in BMSCs, leading to upregulated expression of key osteogenic markers including ALP, OCN, and COL1A1 (54–58). Additionally, specific miRNAs (e.g., miR-26a-5p, miR-486, miR-381) packaged within M2-derived exosomes enhance osteogenic gene expression and contribute to bone repair (32, 59, 60).
The influence of M1 macrophages on BMSCs is primarily dependent on the inflammatory state of the microenvironment (61). Under low inflammatory conditions, M1 macrophages have been observed to promote osteogenic differentiation and enhance bone mineralization in co-culture systems (62, 63). This promotive effect may be mediated through the induction of high autophagy levels in BMSCs, which facilitates their migration and osteogenic commitment (64, 65).
Conversely, under high-inflammatory conditions, classical M1 macrophages strongly inhibit osteogenesis. They secrete elevated levels of TNF-α, IL-1β, and IL-6, which induce sustained activation of the NF-κB pathway in BMSCs (66–68). Furthermore, M1 macrophages can transfer oxidatively damaged mitochondria to BMSCs, disrupting redox homeostasis in the stem cells and thereby impairing osteogenic differentiation (69). Additionally, exosomes derived from hypoxia-induced M1 macrophages (e.g., those containing miR-222) have been shown to significantly reduce BMSCs viability and migratory capacity while promoting apoptosis (70). This contrast illustrates the context-dependent nature of M1 macrophage influence on bone formation.
2.3.2 Influence of BMSCs on macrophages
BMSCs are effective regulators of macrophage polarization. Under inflammatory conditions, BMSCs secrete factors such as prostaglandin E2 to promote macrophage transition from the pro-inflammatory M1 to the anti-inflammatory M2 phenotype, thereby enhancing the production of anti-inflammatory cytokines like IL-10 (71, 72). Furthermore, activated BMSCs can upregulate BMAL1 in macrophages via the KDM6B-BMAL1 axis, which suppresses the TLR2/NF-κB pathway, reduces pyroptosis, and ultimately lowers the M1/M2 ratio (73). BMSCs-derived exosomes—carrying molecules such as miR-27a-3p, miR-146a, and lncRNA-CAHM—promote M2 polarization while suppressing M1 polarization through inhibition of NF-κB signaling or direct targeting of downstream genes (74–77).
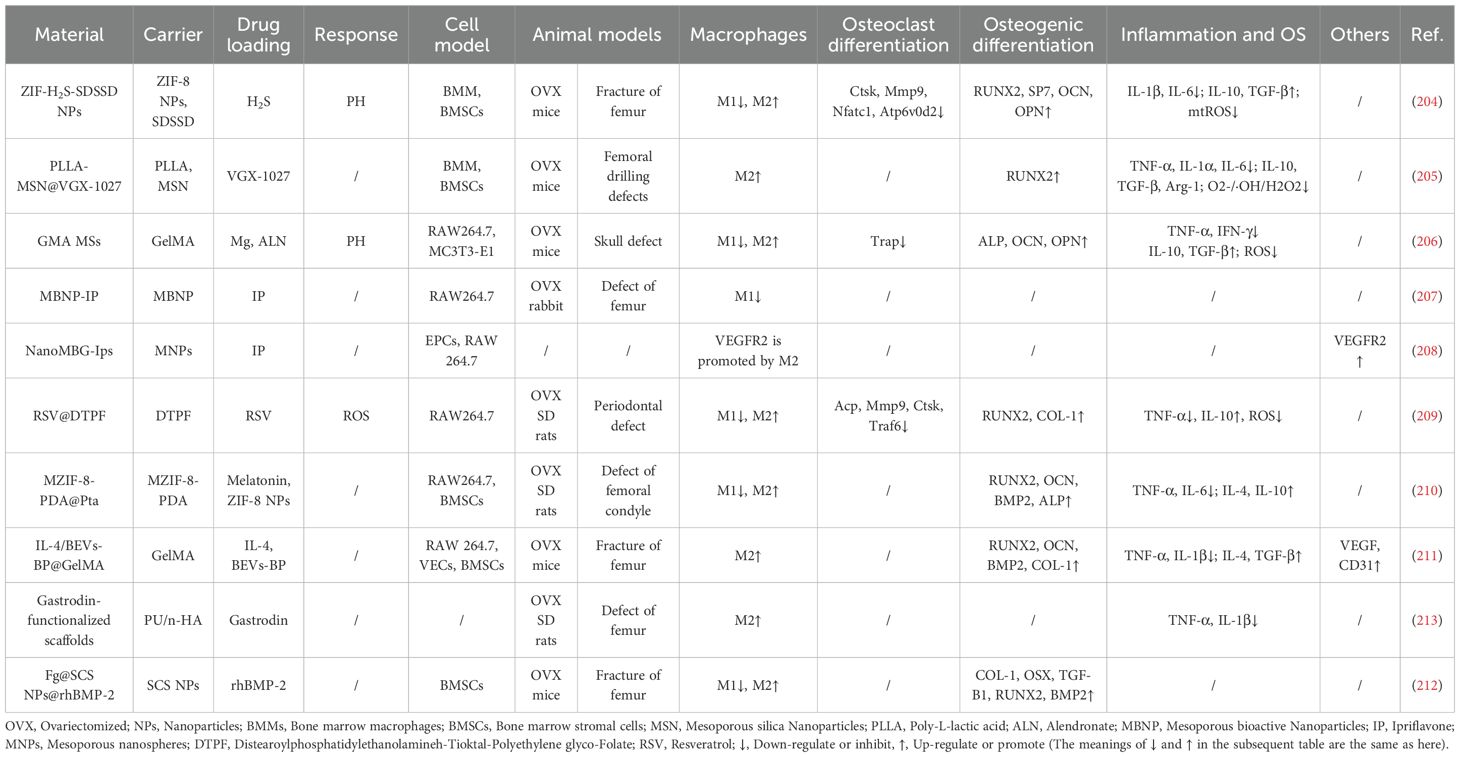
In summary, macrophages orchestrate bone remodeling through a complex network of cytokines, chemokines, and exosomes, dictated by their polarization state. Key pathways include BMP/Smad/Runx2 (osteogenesis), RANKL/RANK/OPG (osteoclastogenesis), and NF-κB (inflammation-mediated bone suppression). Typically, M2 macrophages promote bone formation by synergizing with OBs and BMSCs, while simultaneously inhibiting OCs activity. In contrast, M1 macrophages within inflammatory environments inhibit osteogenesis and potentiate osteoclastogenesis. This crosstalk is bidirectional; BMSCs provide critical feedback by secreting factors that promote macrophages toward the pro-regenerative M2 phenotype. Together, this dynamic feedback loop between macrophages, OBs, OCs, and BMSCs is fundamental to maintaining bone homeostasis (Figure 1), and its dysregulation is a pivotal immunometabolic mechanism driving OPF pathogenesis and progression.
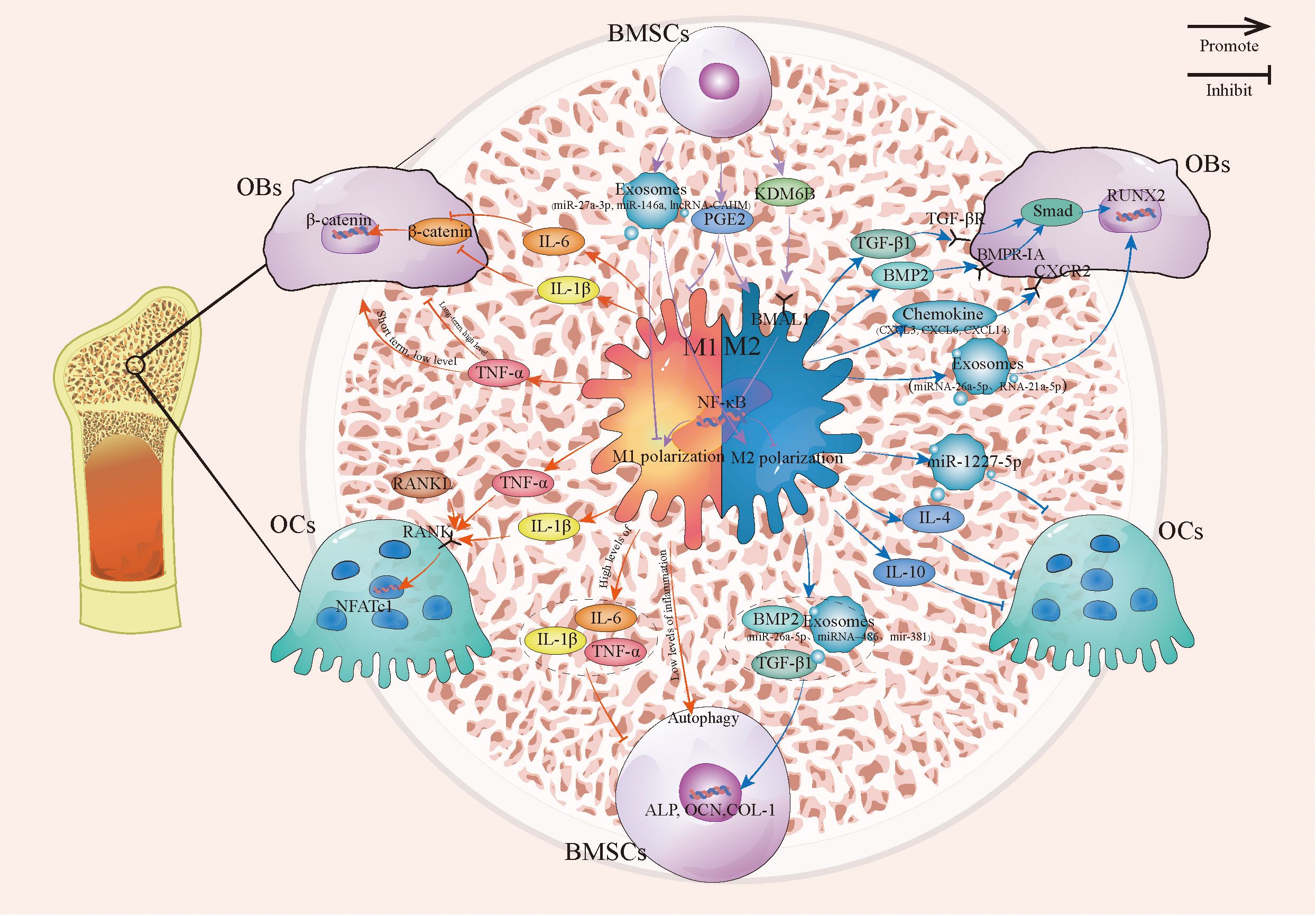
Figure 1. The connection between macrophages and OBs, OCs, and BMSCs. This image describes the relationship between macrophages and OBs, OCs, and BMSCs under different polarization states, including how M1 and M2 macrophages regulate OBs, OCs, and the mutual crosstalk between macrophages and BMSCs. BMSCs, Bone marrow stromal cells; OBs, Osteoblast; OCs, Osteoclast.
3 Macrophages in OP
OP is characterized by an imbalance in bone remodeling, resulting from enhanced bone resorption and reduced bone formation. Macrophages serve as central regulators of this process by modulating inflammatory status and bone homeostasis through immunometabolic reprogramming, which involves switching metabolic pathways and accumulating specific metabolites (78–81). Given the diverse etiologies of secondary osteoporosis, the following sections will adhere to the framework of primary OP to elucidate the role of the macrophage metabolism-polarization axis in OP pathogenesis (Figure 2).
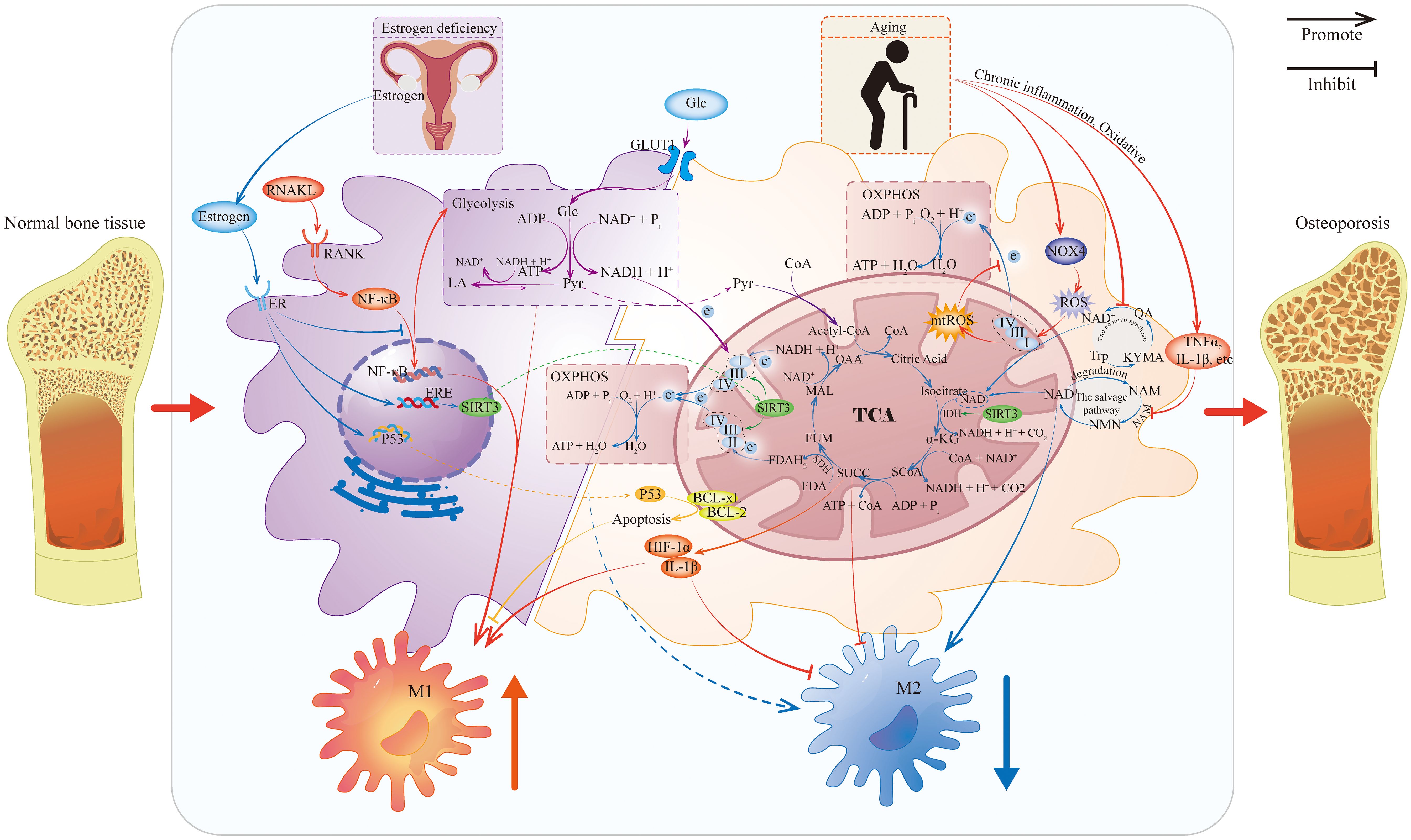
Figure 2. Metabolic reprogramming of macrophages caused by estrogen deficiency and aging. This figure illustrates how estrogen deficiency and aging affect macrophage metabolism and mediate macrophage polarization under OP. Because evidence of the role of gut microbiota and genetic mutations in the regulation of macrophages in OP is limited, it is not shown. IDH, Isocitrate Dehydrogenase; SDH, Succinate Dehydrogenase; CoA, Coenzyme A; OAA, Oxaloacetic Acid; MAL, Malic Acid; FUM, Fumaric Acid; SUCC, Succinic Acid; SCoA: Succinyl-CoA; α-KG, Alpha-Ketoglutaric Acid; TCA, Tricarboxylic Acid Cycle; Glc, Glucose; GLUT1, Glucose Transporter 1; Pyr, Pyruvic Acid; LA, Lactic Acid; ER, Estrogen Receptor; ERE, Estrogen Response Element; NAM, Nicotinamide; NMN, Nicotinamide Mononucleotide; NAMPT, Nicotinamide Phosphoribosyltransferase; Trp, Tryptophan; KYMA, Kynurenic Acid; QA, Quinolinic Acid.
3.1 Estrogen deficiency
The ovariectomized (OVX) mice model, which mimics postmenopausal osteoporosis, is widely used in related research. Studies using this model have shown an increased M1/M2 macrophage ratio in the bone marrow of osteoporotic mice. Under RANKL stimulation, aberrant differentiation of M2 macrophages into osteoclasts was observed, which contributed to enhanced bone resorption. Estrogen supplementation was found to reduce the M1 population and inhibit this macrophage-to-osteoclast transition (82, 83), indicating that estrogen has an important role in regulating macrophage polarization.
3.1.1 Estrogen deficiency and RANKL-induced metabolic dysregulation
Mechanistically, estrogen deficiency impairs the ability of ERα to inhibit NF-κB p65 nuclear translocation, thereby enhancing cellular responsiveness to RANKL (83). Under physiological conditions, RANKL stimulation rapidly upregulates glucose transporters and enhances glycolytic enzyme activity in macrophages to meet their energy demands (84). Concurrently, RANKL induces high expression of aconitate decarboxylase 1 (ACOD1), which promotes the conversion of isocitrate to itaconate. Itaconate acts as an inhibitor of succinate dehydrogenase (SDH), leading to succinate accumulation and a disruption of the tricarboxylic acid (TCA) cycle by blocking the conversion of succinate to fumarate (85).
Succinate contributes to enhanced bone resorption through several mechanisms. First, it inhibits prolyl hydroxylases (PHDs), thereby preventing the degradation of hypoxia-inducible factor 1-alpha (HIF-1α). HIF-1α stabilization upregulates the secretion of pro-inflammatory cytokines such as IL-1β, fostering a microenvironment that supports M1 polarization (86, 87). Second, succinate activates macrophages via the SUCNR1 receptor, promoting their polarization toward the pro-inflammatory M1 phenotype and further facilitating their differentiation into OCs (88). Additionally, ACOD1-mediated itaconate accumulation and SDH inhibition impair electron transport chain (ETC) function by reducing electron flux from succinate oxidation, which worsens metabolic dysregulation (85).
3.1.2 Estrogen deficiency and mitochondrial metabolic dysregulation
Upon binding to the ERα receptor on macrophages, estrogen upregulates the mitochondrial deacetylase SIRT3. SIRT3 enhances mitochondrial oxidative phosphorylation (OXPHOS) efficiency by deacetylating and activating mitochondrial ETC complexes (89, 90). At the same time, SIRT3 activates key TCA cycle enzymes, including isocitrate dehydrogenase and the pyruvate dehydrogenase complex. This activation facilitates the conversion of pyruvate and fatty acids to acetyl-CoA, promotes TCA cycle flux, and thereby enhances mitochondrial OXPHOS capacity (91, 92).
Furthermore, estrogen promotes the phosphorylation of p53 at Ser392 and facilitates its translocation to mitochondria. Within mitochondria, p53 binds directly to the anti-apoptotic proteins BCL-xL and BCL-2 through its DNA-binding domain. This interaction activates the mitochondrial apoptosis pathway, thereby reducing osteoclast formation (93, 94). In summary, by enhancing mitochondrial OXPHOS and suppressing glycolysis, estrogen attenuates M1 polarization of macrophages and limits osteoclastogenesis.
3.2 Aging
Aging is a major risk factor for chronic diseases, including metabolic disorders, cancer, and neurodegenerative diseases (95). In bone metabolism, aging is a primary driver of osteoporosis. As the global population ages, the associated disease burden is escalating (96, 97). Aging not only directly disrupts bone remodeling balance (98) but also accelerates bone loss through chronic inflammation and metabolic dysregulation (99, 100).
Aging remodels the immunometabolic program of macrophages, promoting a shift toward a pro-inflammatory phenotype. Experimental evidence shows a significant upregulation of M1 marker genes and an attenuated increase in M2 marker expression in bone marrow-derived macrophages from aged mice (101). Transcriptomic analysis of fracture callus further confirms that senescent macrophages exhibit dominant pro-inflammatory (M1) gene expression and dysregulation of immune-related networks (102). Critically, metabolic reprogramming is the central driver of this aging-associated polarization shift.
3.2.1 Mitochondrial dysfunction drives pro-inflammatory polarization
Mitochondria are particularly vulnerable to aging-related changes. In macrophages, aging impairs ETC function and OXPHOS capacity, and is accompanied by a reduction in mitochondrial spare respiratory capacity (103, 104). The aging process involves upregulation of NADPH oxidase 4, resulting in reactive oxygen species (ROS) accumulation that further disrupts mitochondrial energy metabolism (105, 106). This metabolic disturbance triggers a compensatory shift toward glycolysis. However, aging is associated with an overall decline in glycolytic flux and reduced succinate levels, which exacerbates macrophage dysfunction (107). Impaired mitochondrial OXPHOS hinders effective reprogramming toward the anti-inflammatory (M2) phenotype (108). At the same time, accumulated ROS activates the NF-κB pathway, enhancing inflammatory responses and promoting polarization toward the pro-inflammatory (M1) phenotype (109, 110).
3.2.2 NAD+ deficiency exacerbates energy metabolism imbalance
Aging is accompanied by a systemic decline in intracellular levels of the essential cofactor nicotinamide adenine dinucleotide (NAD+) (111–113). In macrophages under aging conditions, NAD+ synthesis becomes suppressed (113).
First, the de novo synthesis pathway is impaired. Macrophage de novo NAD+ synthesis originates from tryptophan metabolism via the kynurenine pathway (KP) (114). Upon immune challenge, indoleamine 2,3-dioxygenase 1 (IDO1) converts tryptophan into kynurenine, which is subsequently metabolized to quinolinic acid (115, 116). Quinolinic acid is then converted to nicotinic acid mononucleotide (NaMN) by quinolinic acid phosphoribosyltransferase, and NaMN is ultimately transformed into NAD+ via the Preiss-Handler pathway (117). Critically, in aging, innate immune challenges activate the upstream KP but restrict the downstream conversion of quinolinic acid to NAD+, resulting in dysfunction of the de novo synthesis pathway (114).
Second, the salvage pathway is impaired. This pathway serves as the primary source of NAD+ production during inflammatory stress. In it, NAD+ degradation products—primarily nicotinamide (NAM)—are converted to nicotinamide mononucleotide (NMN) by nicotinamide phosphoribosyltransferase (NAMPT). NMN is then transformed back into NAD+ by NMN adenylyltransferases (118–120). The expression of the rate-limiting enzyme NAMPT is induced by TNFα, IL-1β, LPS, IFNγ, and hypoxia—all factors that increase with age (121). Consequently, aging-associated inflammation can reduce NAD+ regeneration efficiency by dysregulating NAMPT.
NAD+ is an essential cofactor for the deacetylase activity of Sirtuin enzymes. The decline in NAD+ levels directly compromise SIRT activity (122, 123). Reduced SIRT3 activity impairs mitochondrial respiratory chain function and diminishes ATP synthesis, hindering the transition to the anti-inflammatory (M2) phenotype (124). Diminished SIRT3 activity also leads to impaired deacetylation of antioxidant enzymes like superoxide dismutase (SOD) 2, reducing cellular ROS-scavenging capacity and exacerbating oxidative stress (124, 125). Concurrently, decreased SIRT1 activity results in hyperactivation of the NF-κB pathway, promoting M1 polarization (126). In redox metabolism, NAD+ acts as a crucial electron carrier, accepting electrons from glycolysis and the TCA cycle to form NADH, which then transfers these electrons to the mitochondrial ETC for ATP production. Therefore, NAD+ deficiency exacerbates mitochondrial energy generation failure, ultimately disrupting macrophage phenotypic plasticity (127, 128).
3.2.3 Autophagy defects amplify mitochondrial dysfunction and inflammation
Autophagy plays a critical role in regulating macrophage metabolism and polarization (129, 130). Aging is associated with a significant decline in macrophage autophagy, potentially linked to suppressed expression of the autophagy-related gene ATG5 (131, 132). Impaired mitophagy leads to the accumulation of dysfunctional mitochondria, exacerbating ROS production and OXPHOS deficiency (133, 134). Under specific conditions like hyperglycemia, mitochondrial-derived ROS can induce lysosomal dysfunction, further obstructing autophagic flux and promoting M1 polarization (135).
3.2.4 Cellular senescence regulates polarization via metabolic reprogramming
Cellular senescence is frequently associated with downregulation of SIRT4 expression (136). In macrophages, SIRT4 modulates immune function by regulating branched-chain amino acid (BCAA) metabolism (137). BCAA catabolism depends on the branched-chain α-keto acid dehydrogenase (BCKDH) complex, whose activity requires functional dihydrolipoamide branched-chain transacylase E2 (DBT). SIRT4 ablation or downregulation leads to excessive itaconylation of DBT, reducing its enzymatic activity. This diminishes BCKDH activity, impairing BCAA breakdown and causing BCAA accumulation (137). The resulting BCAA buildup promotes pro-inflammatory polarization and impedes the transition to the anti-inflammatory phenotype (138, 139).
Although current studies have not directly established SIRT4 downregulation in senescent macrophages, the age-related decline in NAD+ may affect SIRT4 activity, potentially initiating this pathological cascade. Additionally, cellular senescence is marked by telomere damage; such telomere dysfunction disrupts mitochondrial metabolism in macrophages and activates the NLRP3 inflammasome through the PGC-1α/TNFAIP3 axis, thus promoting M1 polarization (140).
3.3 Gut microbiota
Gut dysbiosis represents a significant risk factor for OP (141, 142). Studies have revealed distinct compositional differences in gut microbiota between OP patients and healthy individuals (143), with proposed mechanisms involving microbial metabolites, immunoinflammatory modulation, and altered nutrient absorption (144–146). Although the precise mechanisms remain to be fully elucidated, the contribution of gut microbiota to OP pathogenesis has become increasingly evident, with macrophage polarization and metabolic reprogramming potentially serving as an intermediate link. Multiple microbial metabolites—including short-chain fatty acids (SCFA), bile acids, choline metabolites, indole derivatives, and vitamins—directly regulate macrophage polarization and metabolism (147).
SCFA including acetate, propionate, and butyrate, modulates bone metabolism through distinct mechanisms. For example, acetate reduces osteoclast numbers via T and B cells, while propionate and butyrate prevent OVX-induced bone loss by decreasing osteoclast formation (148). Although the regulatory effects of SCFA on macrophages have been documented in some studies—for instance, acetate enhances macrophage bactericidal activity (149). Propionate can promote the polarization of macrophages to anti-inflammatory M2 type by regulating the expression of transferrin receptor 1 and ferritin heavy chain 1 mediated by hypoxia-inducible factor (150). Butyrate can inhibit the M1 polarization of macrophages (151), the activation of inflammasomes, and reduce the production of osteoclasts, thereby improving osteolysis (152). However, the literature on the role of SCFA in regulating macrophage polarization and metabolic reprogramming in the context of OP is scarce and needs to be further explored.
In addition, bile acids derived from gut microbiota modulate macrophage polarization. For instance, under high-fat conditions, bile acids promote M1 polarization and pro-inflammatory cytokine production (153). They can also induce lipid peroxidation and suppress M2 polarization via the ROS/p38 MAPK/DGAT1 pathway, influencing disease processes such as acute myeloid leukemia (154). Trimethylamine oxide (TMAO), an oxidized metabolite of choline, enhances intracellular ROS levels and promotes osteoclast differentiation from macrophages (155). In contrast, indole-3-propionic acid inhibits osteoclast differentiation. Melatonin, a tryptophan-derived microbial metabolite, regulates TMAO metabolism and macrophage polarization, reduces inflammatory levels, and thereby ameliorates osteoporosis (156). These findings collectively indicate that gut microbiota metabolites can regulate both macrophage behavior and osteoporosis. However, direct evidence linking gut microbiota, their metabolites, macrophage polarization, and osteoporosis remains limited, suggesting that macrophages may act as an intermediate in the gut microbiota–OP axis, a hypothesis that requires further validation.
3.4 Genetic factors
Genetic predisposition is a major cause of primary OP. Mutations in genes such as WNT1, PLS3, and XYLT2 directly impair osteocyte function, thereby contributing to OP pathogenesis (157). However, the mechanistic links between genetic determinants and OP development remain unexplored from the perspective of macrophage biology, representing a promising avenue for future investigation.
4 Macrophage polarization and metabolic reprogramming during fracture healing
Fracture healing is a complex process involving three overlapping phases: inflammatory, repair, and remodeling (158). Throughout these stages, macrophages play a crucial role by coordinating inflammatory responses, clearing debris, promoting angiogenesis, stimulating bone formation, and guiding tissue remodeling (159–161) (Figure 3).
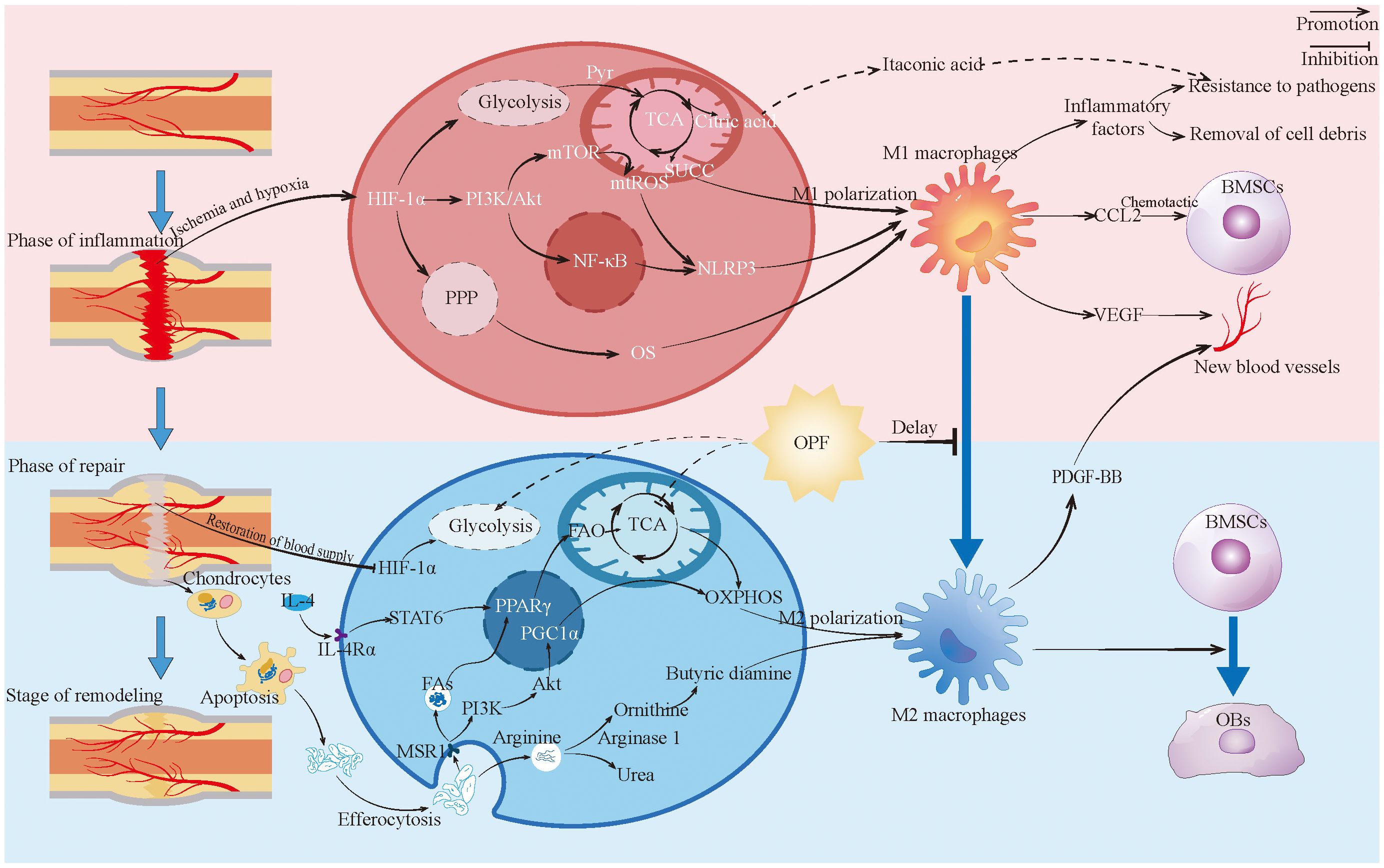
Figure 3. Macrophages in fracture healing. This figure depicts the changes and roles of macrophages during fracture healing, highlighting the metabolic reprogramming changes and contrasting with the characteristics of macrophages in the healing process of OPF. Pyr, Pyruvic acid; TCA, Tricarboxylic acid cycle; SUCC, Succinic acid; BMSCs, Bone marrow stromal cells; OPF, Osteoporotic fractures; PPP, Pentose phosphate pathway; MAR1, Macrophage scavenger receptor 1; Fas, Fatty acid; FAO, Fatty acid oxidation; OXPHOS, Oxidative phosphorylation; OBs, Bsteoblast.
4.1 Inflammatory phase
During the early inflammatory phase of fracture healing, disruption of the local blood supply creates a hypoxic microenvironment, leading to stabilization and elevated expression of HIF-1α (162). As a transcription factor, activated HIF-1α binds to promoters of multiple metabolic enzymes, upregulating glycolytic genes while suppressing OXPHOS (163, 164). This HIF-1α-driven glycolytic shift is a key mechanism promoting macrophage transition toward a pro-inflammatory phenotype (165). Although glycolysis generates pyruvate in macrophages, impaired TCA cycle flux leads to accumulation of citrate and succinate (166–168). Citrate is further metabolized to itaconate, which exerts antimicrobial effects, while succinate amplifies pro-inflammatory cytokine production in M1 macrophages (88, 169, 170). Under hypoxic conditions, HIF-1α overexpression also enhances the pentose phosphate pathway (PPP) in M1-like macrophages. The PPP generates nicotinamide adenine dinucleotide phosphate (NADPH), which helps modulate oxidative stress and sustain pro-inflammatory functions (165, 171–173). Furthermore, HIF-1α activates the NLRP3 inflammasome through the PI3K/AKT/mTOR signaling axis, thereby reinforcing the phenotypic effects of metabolic reprogramming (174).
M1 macrophages contribute to essential inflammatory responses by releasing cytokines such as TNF-α, IL-1β, IL-6, and CCL2, which aid in the clearance of pathogens and cellular debris (175). Through CCL2 secretion, they recruit BMSCs and promote prostaglandin E2 (PGE2) production via the COX-2–PGE2 axis. PGE2 then activates downstream signaling pathways in mesenchymal stem cells, facilitating osteogenic differentiation (62, 176). Concurrently, M1-derived vascular endothelial growth factor (VEGF) stimulates neovascularization, helping to alleviate hypoxia in the local microenvironment (177). Furthermore, within the early inflammatory milieu of fracture sites, IL-1β-stimulated M1 macrophages secrete inflammatory cytokines that enhance the expression and activity of nerve growth factor (NGF). By binding to tropomyosin receptor kinase A (TrkA) receptors, NGF promotes reinnervation, thereby supporting neural regeneration and functional recovery during bone repair (178–180).
4.2 Repair and remodeling phase
During the repair and remodeling phase, macrophages predominantly polarize toward the M2 phenotype (177). This phenotypic shift begins as early as the late inflammatory stage of fracture healing. The transition from M1 to M2 polarization appears to be facilitated by neovascularization that alleviates hypoxic conditions, leading to downregulation of glycolytic enzymes and restoration of OXPHOS in macrophages (177, 181, 182). Pro-inflammatory cytokines released by M1 macrophages during inflammation recruit immune cells; subsequently, microenvironmental cells (e.g., Th2 lymphocytes or BMSCs) secrete IL-4 and IL-10, modifying the immunoinflammatory milieu to further promote M2 polarization (183, 184). IL-4 activates the STAT6 pathway via IL-4Rα, inducing mitochondrial fatty acid oxidation (FAO) in M2 macrophages and upregulating the expression of CD36, CPT1, and PPARγ. This enhances mitochondrial respiratory chain activity and supports OXPHOS functionality (185, 186).
During endochondral ossification, macrophages phagocytose apoptotic chondrocytes, resulting in metabolic and polarization changes—a process referred to as efferocytosis (187–189). Studies have shown that apoptotic bodies derived from M2 macrophages contribute to regulating the M1/M2 balance (190). During efferocytosis, arginine from apoptotic cells is hydrolyzed by arginase 1 to produce ornithine and urea; ornithine is then catalyzed by ornithine decarboxylase (ODC) to generate putrescine, which enhances efferocytic efficiency and induces anti-inflammatory gene expression in macrophages (191, 192). Concurrently, the expression of macrophage scavenger receptor (MSR1) is upregulated. MSR1 activates the PI3K/AKT pathway, upregulates PGC1α expression, enhances mitochondrial OXPHOS, and promotes metabolic reprogramming along with M2 polarization (193). Moreover, fatty acids released from apoptotic chondrocytes can be taken up by macrophages via MSR1, subsequently activating PPAR-γ. This promotes fatty acid oxidation (FAO) in macrophages and stimulates BMP7 production, which facilitates osteogenic differentiation of BMSCs (194). These mechanisms act together to regulate macrophage metabolic reprogramming and polarization, thus supporting fracture healing.
In addition, M2 macrophages upregulate arginase-1 activity, shifting arginine metabolism away from the iNOS-dependent pathway—characteristic of M1 macrophages—and toward the synthesis of polyamines and proline. This metabolic shift promotes collagen deposition, cell proliferation, and angiogenesis (195, 196). Finally, PDGF-BB derived from M2 macrophages helps maintain neovascular stability, thereby supporting osteogenesis and nutrient delivery during bone remodeling (197).
Collectively, macrophages coordinate fracture healing through temporally regulated metabolic reprogramming and phenotypic switching (177). Initially, macrophages polarize toward the M1 phenotype via glycolytic metabolism, which enables clearance of necrotic debris, release of pro-inflammatory and chemotactic factors for BMSC recruitment, initiation of bone repair, and VEGF-mediated angiogenesis (198). As inflammation resolves, macrophage metabolism shifts toward OXPHOS, accompanied by a phenotypic transition from M1 to M2 (199). This transition further promotes osteogenic differentiation and mineral deposition by BMSCs, along with PDGF-BB release that stabilizes vasculature and consolidates newly formed bone (18). The metabolic plasticity of macrophages thus constitutes a regulatory hub for adapting to microenvironmental changes during bone regeneration (18).
4.3 Macrophages in OPF healing
In OPF healing, macrophage metabolism and polarization are disrupted by multiple pathological factors, leading to delayed union or nonunion (200). As described in earlier sections, estrogen deficiency impairs ERα-mediated inhibition of NF-κB p65 nuclear translocation, enhancing macrophage responsiveness to RANKL (83). RANKL stimulation induces high expression of ACOD1, promotes itaconate accumulation, and inhibits SDH, thereby disrupting the TCA cycle and leading to succinate accumulation (85). The accumulated succinate activates macrophages via the SUCNR1 receptor, driving polarization toward the pro-inflammatory M1 phenotype while suppressing OXPHOS, which is required for M2 polarization (88).
Experimental evidence supports this mechanism. Compared with the control group, the population of M2 macrophages in the callus of OVX mice was substantially reduced at 14 days post-fracture (DPF), concurrently showing decreased IL-4 secretion and a marked increase in IL-6 expression (201).
Aging exacerbates macrophage dysfunction through NAD+ deficiency, impaired autophagy, and cellular senescence, collectively leading to mitochondrial respiratory chain dysfunction, elevated oxidative stress, and enhanced inflammation—all of which favor M1 polarization. In aged rats with senile osteoporosis (SOP), transcriptomic analyses revealed significant upregulation of inflammation-related genes in macrophages. Serum levels of pro-inflammatory factors (IL-6 and TNF-α) were more than threefold higher than in young rats, whereas anti-inflammatory mediators such as IL-10 were markedly reduced (202, 203). These findings further support the concept that aging disrupts metabolic reprogramming and polarization in macrophages, thereby impairing osteoporotic fracture healing.
However, targeted studies examining the specific metabolic alterations in macrophages within the OPF microenvironment remain relatively limited. Key unresolved questions include whether macrophages in OPF exhibit metabolic dysregulation similar to that observed in osteoporosis alone, or whether these alterations are further exacerbated in the fracture context. Elucidating these mechanisms represents an important direction for future research on macrophage-centered interventions for OPF.
5 Therapeutic strategies targeting macrophages
As core regulators of the OPF immune microenvironment, macrophages directly orchestrate bone repair progression through their metabolic reprogramming and phenotypic polarization. Recent therapeutic advances in targeted macrophage therapies—leveraging innovative biomaterial design and delivery technologies—are demonstrating significant efficacy in modulating inflammatory responses and restoring osteoblast-osteoclast equilibrium.
To identify relevant therapeutic strategies, we conducted a literature search of the PubMed and Web of Science (WOS) databases using the following keywords: “Macrophages,” “Osteoporosis,” “Fracture,” “Bone defect,” “Bone healing,” “Fracture healing,” “Bone repair,” “Bone regeneration,” and “Osteogenesis.” The search was limited to publications from the last decade, up to July 4, 2024. After excluding review articles and irrelevant studies, 49 publications were included in the analysis (Figure 4).
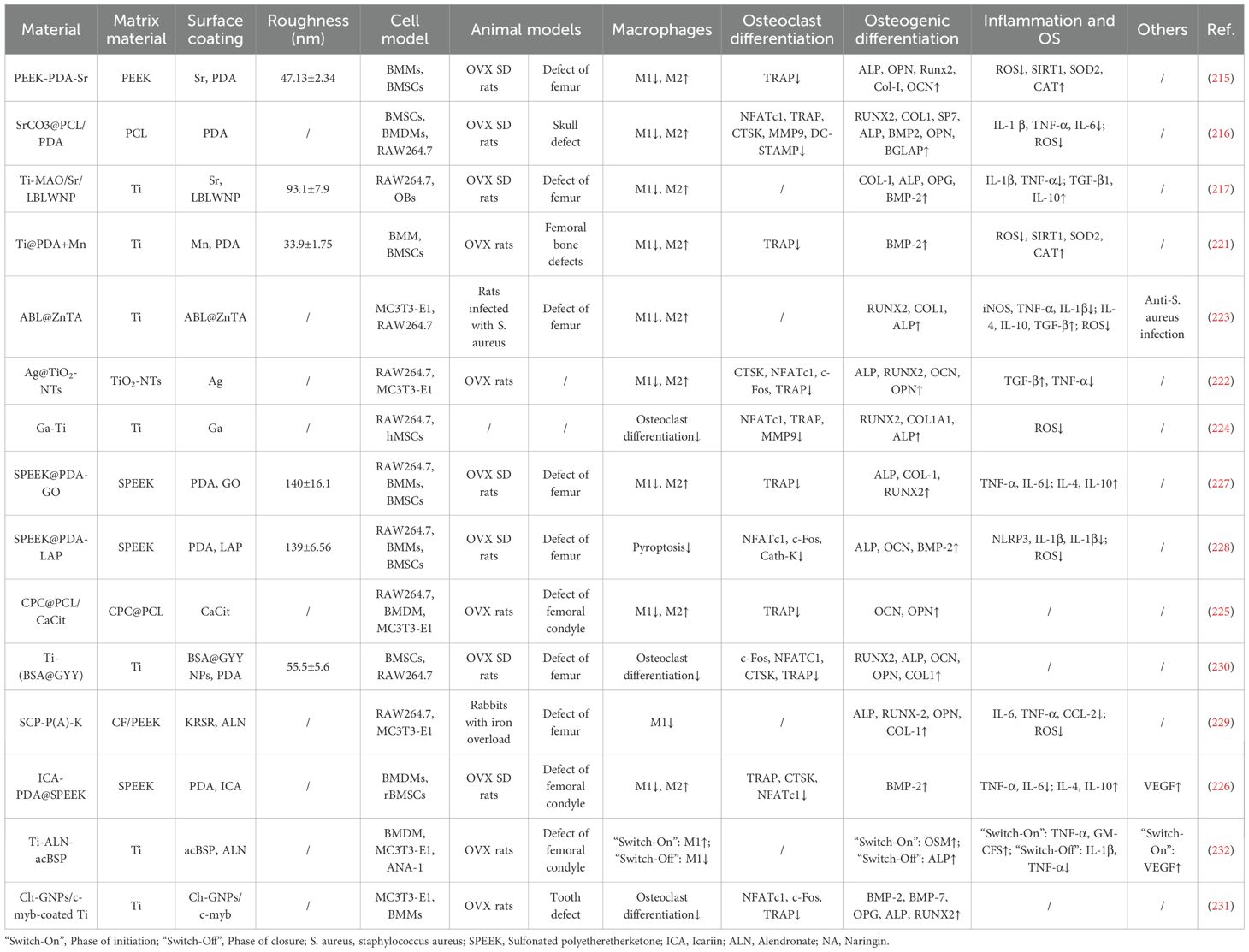
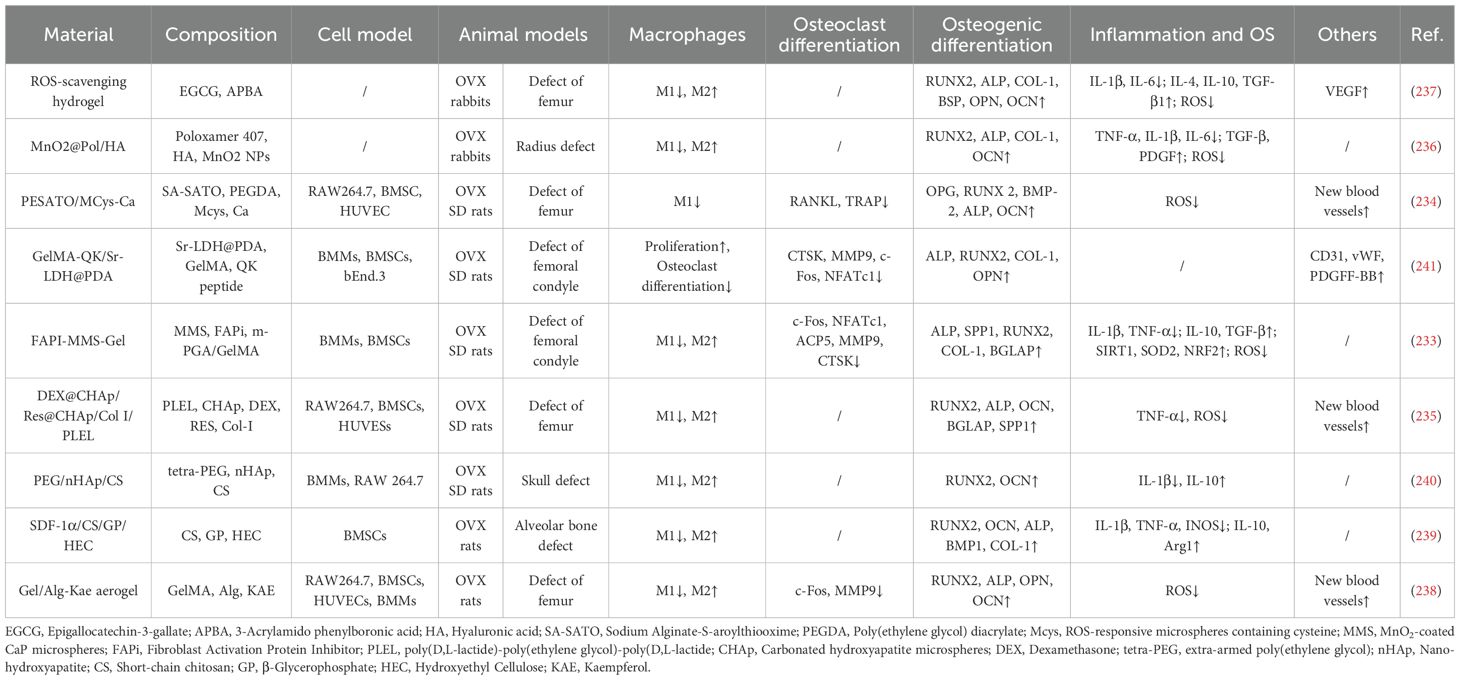
Based on the retrieved literature, this section systematically describes recent advances in drug delivery systems, surface modification technologies, hydrogels, and related biomaterials (Figure 5), with a focus on their mechanisms of action, therapeutic benefits, and translational challenges. The primary effects of different types of biomaterials on macrophage behavior are summarized in Table 1.
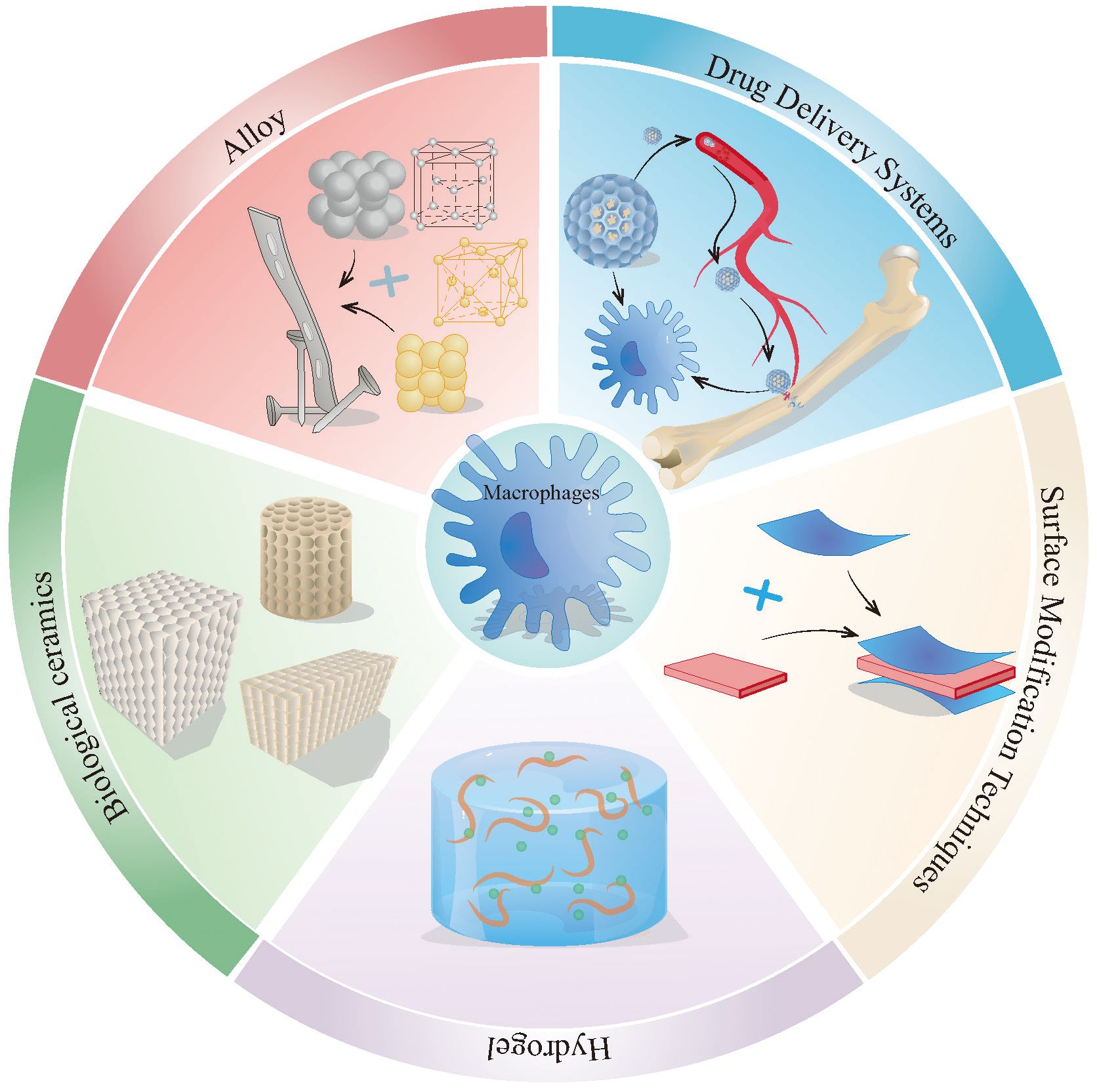
Figure 5. The method of treating osteoporotic fractures and bone defects by targeting macrophages. This figure describes the main approaches to target macrophages for the treatment of osteoporotic fractures and bone defects, including drug delivery systems, surface improvement technologies, hydrogels, alloys, bioceramics, etc. The alloy material in the picture uses the lattice of Zn and Cu. The actual alloy material may be made of other metals or more metals, and the lattice may not be the same as in the picture.
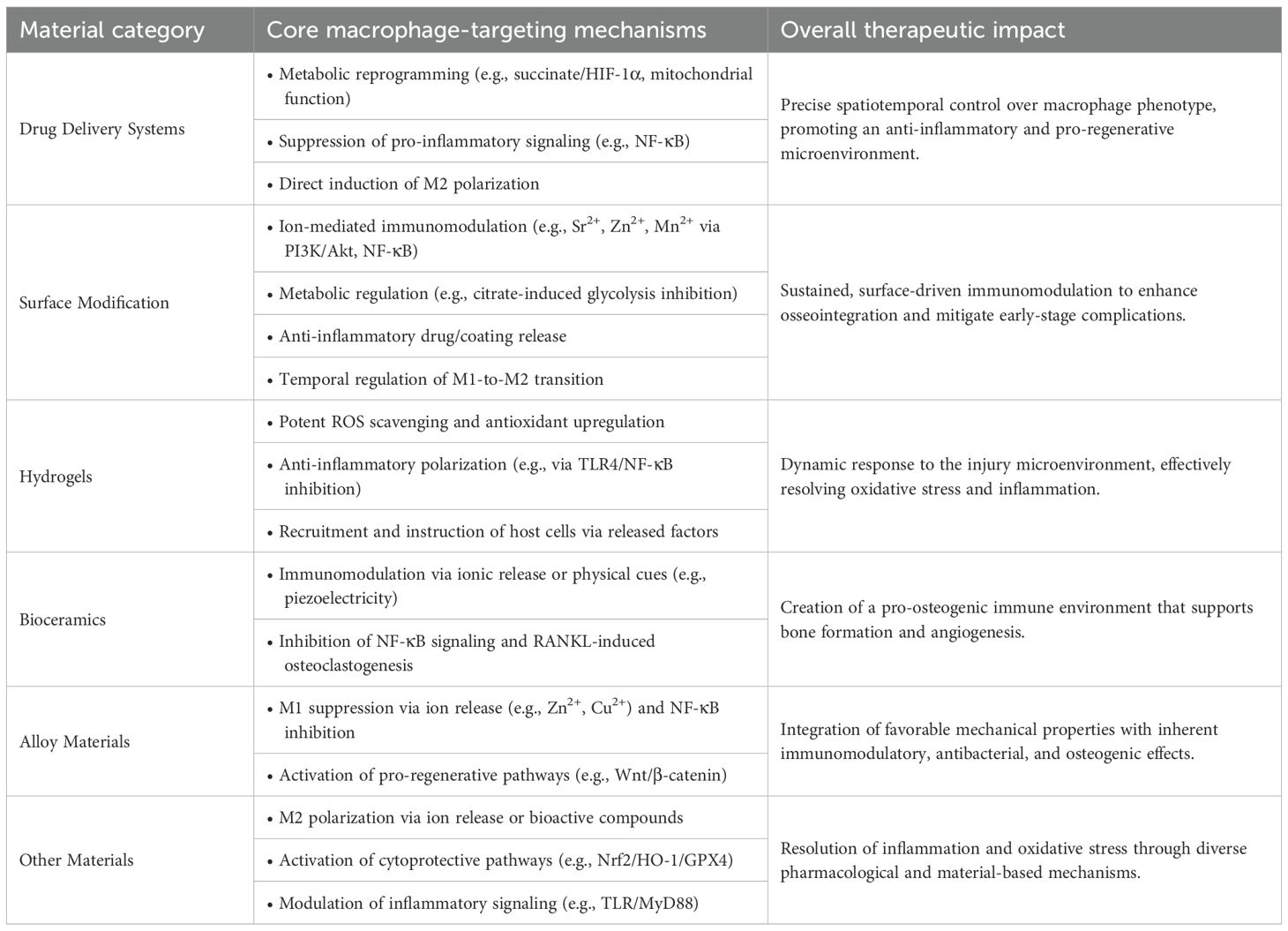
Table 1. Table of macrophage regulation strategies in osteoporotic bone repair based on biomaterials.
5.1 Drug delivery systems
Drug delivery systems use carriers to achieve active or passive targeting of macrophages, enabling precise modulation of their metabolism and polarization while improving drug bioavailability (Table 2).
5.1.1 Regulating metabolic reprogramming
Metabolic reprogramming acts as an intrinsic driver of macrophage phenotypic polarization. Targeting this process to modulate macrophage function offers a promising strategy for improving fracture healing under osteoporotic conditions.
For example, Qin et al. developed bone-targeted nanoparticles (ZIF−H2S−SDSSD) functionalized with SDSSD peptide for osseous delivery. These particles remain stable under physiological pH (7.4) with minimal Zn2+ release, but degrade under acidic conditions to release H2S and Zn2+ ions, achieving responsive drug release. The released H2S and Zn2+ alleviate inflammation by reducing succinate accumulation in the TCA cycle and suppressing HIF-1α expression. H2S also protects mitochondrial function by decreasing mitochondrial reactive oxygen species (mtROS) production. In vitro, ZIF-H2S-SDSSD promotes macrophage repolarization from the M1 to the M2 phenotype, downregulating pro-inflammatory mediators while upregulating anti-inflammatory factors. In vivo, the treatment alters the macrophage composition at fracture sites—decreasing CD86+ M1 macrophages and increasing CD206+ M2 macrophages—and reprograms cellular metabolism by attenuating glycolysis, enhancing TCA cycle flux, and reducing succinate accumulation. Additionally, the nanoparticles suppress osteoclast-related genes (e.g., Ctsk, Mmp9) and activate osteogenic markers (e.g., Runx2, OCN), significantly improving osseointegration (204).
Wang et al. developed poly (L-lactic acid) mesoporous silica nanoparticle (PLLA-MSN@VGX-1027) microspheres targeting mitochondrial function for immunomodulation. In vitro, the released VGX-1027 upregulates key proteins regulating mitochondrial dynamics (MFN2, OPA1, DRP1), enhances metabolic activity and ATP production, and thereby promotes M2 polarization. It also scavenges reactive oxygen species through intrinsic SOD/catalase activity, thereby reducing oxidative damage. In osteoporotic mice, the microspheres enhanced M2 macrophage infiltration at bone defect sites and accelerated regeneration via the macrophage–miR-5106–SIK2/3–Runx2 signaling axis. With sustained VGX-1027 release over 28 days, covering the critical healing phase, this approach demonstrates substantial therapeutic potential (205).
5.1.2 Regulating pro-inflammatory gene expression
Modulating macrophage phenotypic switching through inhibition of pro-inflammatory gene expression represents a viable therapeutic strategy. For instance, Cao et al. developed gelatin microspheres co-loaded with Mg2+ and alendronate (GMA MSs). The released Mg2+ suppresses M1 polarization by inhibiting the NF-κB signaling pathway, thereby improving the local inflammatory microenvironment through upregulation of IL-10 and TGF-β and downregulation of TNF-α and IFN-γ. In parallel, hydrogen (H2) generated during material degradation scavenges reactive oxygen species and alleviates oxidative stress, resulting in dual modulation of inflammatory and redox homeostasis. Furthermore, Mg2+ activates osteogenic gene expression, promoting osteogenic differentiation of MC3T3-E1 cells. In vivo studies demonstrated reduced osteoclast numbers at defect sites and markedly increased new bone formation (206).
In a similar approach, Arcos et al. developed mesoporous bioactive nanoparticles loaded with ipriflavone (MBNP-IP). This system was shown to suppress NF-κB expression in macrophages, reducing the secretion of pro-inflammatory cytokines such as IL-6 and TNF-α and thereby alleviating local inflammation. Its ability to promote osseointegration was further validated in OVX rabbit models (207). In parallel, Casarrubios et al. confirmed that ipriflavone-loaded nanospheres (NanoMBG-Ips) enhance VEGFR2 secretion by M2 macrophages, consequently accelerating angiogenesis in osteoporotic fracture healing (208).
5.1.3 Regulate macrophage polarization
Other biomaterial-based approaches modulate macrophage polarization to improve inflammatory and immune microenvironments and enhance osteogenesis. For example, Peng et al. developed folic acid-targeted liposomes (RSV@DTPF) that employ a ROS-responsive release mechanism to scavenge reactive oxygen species while promoting M2 polarization and rebalancing cytokine profiles—specifically reducing TNF-α and increasing IL-10. These liposomes also stimulate osteogenic differentiation of bone marrow stromal cells (BMSCs) while suppressing osteoclast-related genes (e.g., Acp, Mmp9, Ctsk, Traf6), thereby significantly inhibiting osteoclastogenesis and osteoclast maturation (209).
Liu et al. designed a tantalum-based scaffold (MZIF-8-PDA@PTa) that remodels the inflammatory microenvironment by inhibiting M1 polarization and promoting M2 transition in vitro, while also activating the p38-MAPK pathway to upregulate osteogenic markers such as Runx2 and Ocn. In OVX rat models, the scaffold markedly enhanced osseointegration (210). In a complementary approach, Zhou et al. developed an interleukin-4-loaded extracellular vesicle–gel system (IL-4/BEVs BP@GelMA) that effectively induces M2 polarization. In vivo results demonstrated synergistic upregulation of osteogenic factors (e.g., Runx2, Ocn) and angiogenic markers (e.g., VEGF, CD31), collectively accelerating fracture repair (211).
Furthermore, sulfated chitosan nanoparticles (Fg@SCS NPs@rhBMP-2) and gastrodin-functionalized scaffolds (PU/n-HA) have been shown to promote M2 polarization and resolve inflammation (212, 213). Additionally, PU/n-HA scaffolds effectively recruit BMSCs and enhance their activation through improved mitochondrial biogenesis and restored mitochondrial network homeostasis.
Drug delivery systems show considerable therapeutic potential; however, several limitations require attention. These include challenges in controlling release kinetics, minimizing off-target effects, and improving the physicochemical properties of drug carriers and synthetic bone matrices. Such issues currently hinder effective clinical translation. Future research should focus on developing materials with tunable release profiles—enabling both rapid and sustained drug release—and optimizing carrier systems to better navigate the complex bone microenvironment.
5.2 Surface modification techniques
Surface modification techniques alter implant surface properties through physical, chemical, or electrochemical methods to improve osseointegration, stimulate osteogenesis, reduce infection risks, and decrease complications (214). By functionalizing materials to modulate macrophage responses, these techniques enable improved macrophage regulation while maintaining the inherent physical properties of the base material (Table 3).
5.2.1 Metal ion-releasing coatings
Surface modification techniques enable the functionalization of materials to release specific ions—such as Sr2+, Mn2+, Zn2+, and Ag+—thereby modulating macrophage responses.
Sr2+ ions effectively modulate macrophage polarization. For instance, Zhang et al. developed Sr2+-anchored polyetheretherketone implants (PEEK-PDA-Sr), Du et al. designed strontium carbonate scaffolds (SrCO3@PCL/PDA), and Wang et al. fabricated nano-wogonin-composited strontium-doped titanium (Ti-MAO/Sr/LBL WNP). In vitro and in vivo studies demonstrated that these materials sustain Sr2+ release, effectively suppressing pro-inflammatory M1 polarization while promoting anti-inflammatory M2 polarization in macrophages. Additionally, they enhance osteogenic differentiation and inhibit osteoclast activity (215–217). Mechanistically, Sr2+ activates the PI3K/Akt signaling pathway to improve mitochondrial function while inhibiting NF-κB, thereby suppressing M1 polarization (216, 218).
Mn2+ ions exhibit context-dependent effects on macrophage polarization. While some studies report promotion of M1 polarization (219), others describe inhibition of M1 polarization (220). These varied outcomes appear to depend on chemical speciation, delivery system characteristics, and microenvironmental signals. Wang et al. developed Mn2+-modified titanium implants (Ti@PDA+Mn) that suppress M1 polarization and promote M2 polarization through scavenging reactive oxygen species and upregulating antioxidant genes (including SIRT1, SOD2, and CAT). This approach also effectively enhances osteogenic differentiation while inhibiting osteoclastogenesis (221).
Furthermore, silver nanotube coatings (Ag@TiO2-NTs) developed by Wang et al. enable sustained Ag+ release, which enhances macrophage autophagy and suppresses the NF-κB pathway (222). Separately, zinc-peptide metal-phenolic nanocoatings (ABL@ZnTA) designed by Xu et al. release Zn2+ under infectious conditions, inhibiting iNOS and TNF-α expression while promoting IL-10 and TGF-β secretion in macrophages, thereby achieving both antibacterial and osteogenic outcomes (223).
Leveraging the similar ionic behavior between Ga3+ and Fe3+, Piñera-A et al. modified titanium surfaces with gallium-doped perovskite layers. The released Ga3+ binds to transferrin and enters cells via transferrin receptor 1. This uptake competitively inhibits cellular iron absorption, disrupts iron metabolism, and accelerates the Fenton reaction, promoting ROS accumulation and ultimately leading to ferroptosis. Consequently, this process inhibits macrophage differentiation into osteoclasts (224). These functionalized coatings typically exhibit biphasic ion release profiles, characterized by an initial burst followed by a sustained release phase. Such release kinetics help suppress bacterial infection in early stages while providing continuous stimulation for bone regeneration in later phases.
5.2.2 Regulate metabolic reprogramming
Certain biomaterials modulate immune responses by regulating macrophage metabolism. For example, Wu et al. developed citrate-functionalized scaffolds (CPC@PCL/CaCit). Citrate released from these scaffolds binds to specific sites on key glycolytic enzymes, thereby inhibiting their activity and redirecting metabolic flux toward the TCA cycle. This metabolic shift enhances oxidative phosphorylation, supporting an M2-like polarization state (225).
5.2.3 Anti-inflammatory coatings
Certain materials function through anti-inflammatory mechanisms. For instance, Chai et al. developed an icariin-modified sulfonated polyetheretherketone system (ICA-PDA@SPEEK), where the loaded icariin exhibits anti-inflammatory properties by promoting M2 macrophage polarization, reducing pro-inflammatory cytokine secretion, and increasing anti-inflammatory factor production (226). Likewise, sulfonated polyetheretherketone modified with graphene oxide (SPEEK@PDA-GO) or laponite (SPEEK@PDA-LAP) inhibits macrophage pyroptosis and osteoclast activation through suppression of the STAT3-mediated NLRP3/caspase-1/IL-1β signaling axis (227, 228).
5.2.4 Coatings that inhibit osteoclast differentiation
Another category of functional coatings functions by suppressing osteoclast differentiation from macrophages. Zhou et al. designed an intelligent supramolecular coating (SCP-P(A)-K) that releases the anti-resorptive drug alendronate (ALN) to inhibit osteoclastogenesis (229). Similarly, Xia et al. developed a titanium-based system (Ti-(BSA@GYY)) that suppresses osteoclast differentiation through modulation of the OPG/RANKL pathway (230). Additionally, Takanche et al. reported that Ch-GNPs/c-myb nanoparticles inhibit RANKL signaling and the JNK pathway while blocking NF-κB nuclear translocation, thereby effectively preventing osteoclast differentiation of macrophages (231).
5.2.5 Temporally regulated macrophage modulation coatings
An emerging strategy involves the temporal regulation of macrophage function through smart coating design. Wang et al. developed a Ti-ALN-acBSP coating capable of sequentially regulating macrophage responses. During the early phase, the coating releases acidic bone sialoprotein (acBSP) to polarize macrophages toward the M1 phenotype. This establishes an appropriate inflammatory microenvironment while promoting the secretion of osteogenic factors such as oncostatin M and pro-angiogenic factors including VEGF, thereby initiating repair processes. In later stages, alkaline phosphatase secreted by osteoblasts triggers coating degradation, which induces apoptosis of pro-inflammatory macrophages, resolving local inflammation (232). This sequential immunomodulatory strategy better replicates the physiological shift in macrophage phenotypes during normal bone healing, providing a promising framework for designing biomaterials with temporally controlled macrophage-regulating properties.
5.3 Hydrogels
As localized delivery carriers, smart hydrogels enable dynamic release of active components in response to microenvironmental changes, while providing tunable mechanical properties and high biocompatibility (Table 4).
5.3.1 Antioxidant hydrogels
Numerous hydrogel systems demonstrate notable antioxidant properties. For instance, Chen et al. developed a methacrylated gelatin hydrogel incorporating a fibroblast activation protein inhibitor (FAPI) and MnO2 nanoparticles (FAPI-MMS-Gel). Both in vitro and in vivo studies confirmed that FAPI-MMS-Gel reduces reactive oxygen species (ROS) levels in bone marrow stromal cells and bone marrow macrophages (BMMs) by upregulating antioxidant genes such as SIRT1 and SOD2, while also suppressing the NF-κB pathway to promote M2 macrophage polarization (233). JJiang et al. designed an H2S-sustained-release hydrogel (PESATO/MCys-Ca) and demonstrated that its ROS-responsive H2S release significantly decreases intracellular ROS in macrophages, inhibits M1 polarization and osteoclast-related genes (e.g., Acp5, Mmp9), and upregulates osteogenic markers (e.g., Runx2, Ocn) (234). Li et al. developed a thermosensitive resveratrol/dexamethasone gel that effectively scavenges DPPH radicals and promotes macrophage transition from M1 to M2 phenotype (235). Other systems, including MnO2@Pol/HA (Ye et al.), EGCG/APBA composite hydrogel (Ding et al.), and kaempferol aerogel (Jin et al.), also efficiently eliminate ROS, promote the shift from M1 to M2 polarization, and reduce pro-inflammatory cytokine levels (e.g., TNF-α, IL-1β, IL-6) (236–238). Collectively, these antioxidant hydrogels alleviate inflammatory and oxidative stress, reduce M1 polarization while enhancing M2 polarization, thereby establishing a more favorable microenvironment for osseointegration in osteoporotic fractures and bone defects.
5.3.2 ROS-independent hydrogels
Some hydrogel materials function independently of ROS modulation. For example, Liu et al. developed an SDF-1α-functionalized chitosan hydrogel that promotes BMSCs migration and osteogenic differentiation in vitro, while suppressing M1 polarization and enhancing M2 macrophage polarization in vivo (239). Sun et al. designed a PEG/nHAp/CS hydrogel that inhibits M1 polarization and promotes M2 polarization by antagonizing the TLR4/NF-κB pathway, thereby shifting the local microenvironment toward an anti-inflammatory state. This hydrogel also enhances osteogenesis via the cAMP/PKA/CREB pathway. In OVX Sprague-Dawley rats with calvarial defects, implantation with PEG/nHAp/CS hydrogel increased the proportion of CD206+ M2 macrophages, decreased CD86+ M1 macrophages, and markedly reduced osteoclast numbers (240). He et al. developed an Sr-LDH/GelMA-QK hydrogel that promotes macrophage proliferation, suppresses osteoclast differentiation, enhances endothelial cell migration, and upregulates vasculogenesis-related genes (e.g., CD31, vWF). Animal studies demonstrated that this material inhibits osteoclast maturation, increases PDGF-BB release, and promotes H-type vessel formation to support osseointegration in osteoporotic bone (241). Additionally, Li et al. designed a Gel-Ale-Mg@PDA nanocomposite scaffold that induces M2 macrophage polarization through controlled Mg2+ release and promotes osteogenic differentiation of BMSCs (242).
While hydrogel-based strategies demonstrate therapeutic potential, further optimization is needed for hydrogel compositions and the controlled-release kinetics of bioactive molecules. Future studies should also clarify the complex interactions among hydrogels, macrophages, and other cell types during bone regeneration, particularly to address challenging pathological conditions (18, 243).
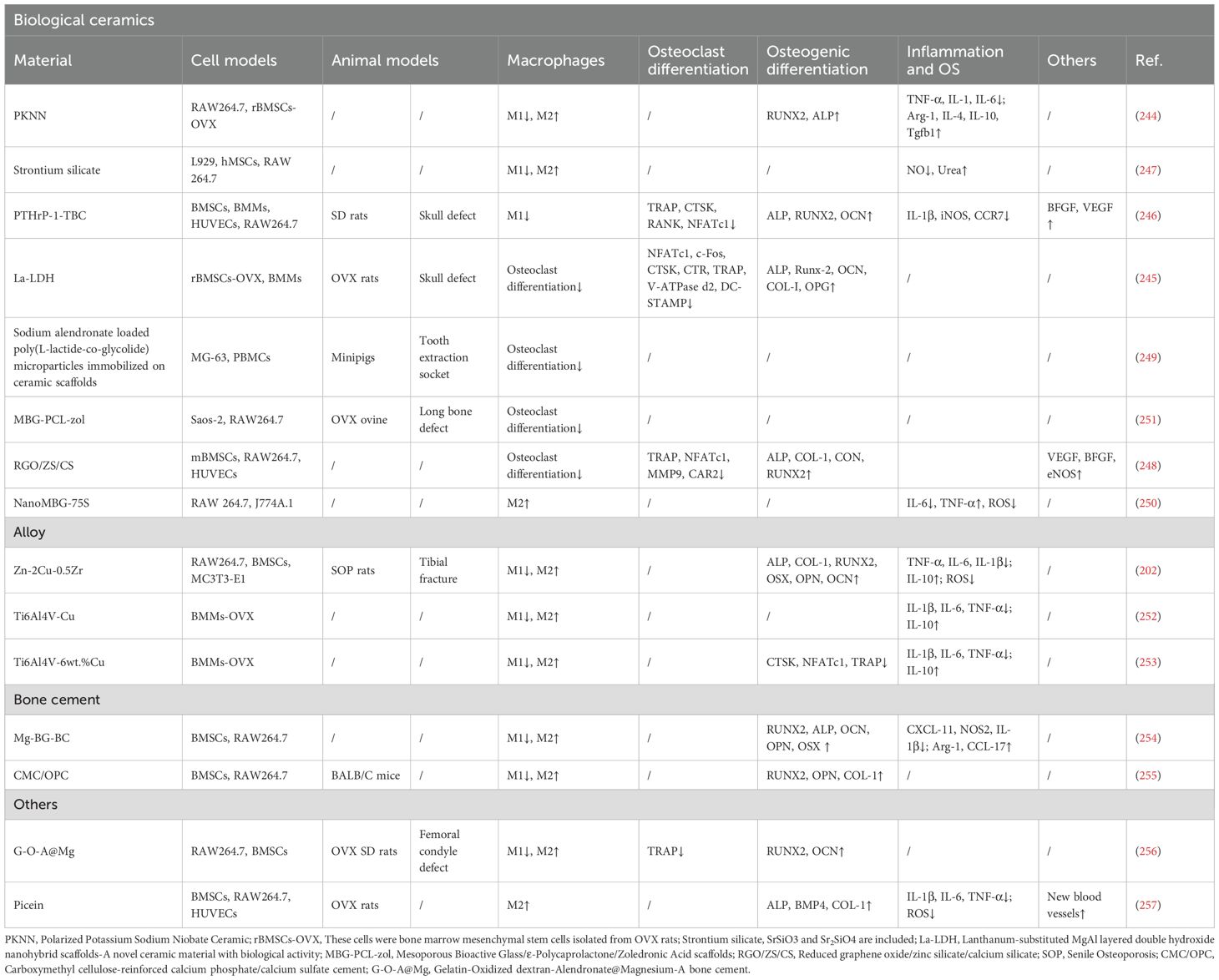
5.4 Biological ceramics
Although bioceramics were previously the main focus of research, current studies on bioceramic-assisted osseointegration have progressively shifted toward composite materials incorporating bioceramics (Table 5).
For example, Wang et al. developed a piezoelectric PKNN ceramic that maintains stable piezoelectric performance under physiological conditions, effectively reduces M1 macrophage polarization markers, promotes M2 phenotypic transition, and synergistically enhances osteogenic differentiation of BMSCs via the Runx2 pathway (244). Chu et al. designed a La-LDH nanohybrid scaffold that inhibits osteoclast differentiation of BMMs through suppression of NF-κB signaling, while upregulating the OPG/RANKL ratio to stimulate osteogenesis (245). The PTHrP-1-TBC scaffold enables sustained release of PTHrP-1, which inhibits M1 polarization and activates the pro-angiogenic factor VEGF, thus establishing a synergistic pro-angiogenic and osteoinductive microenvironment (246). Furthermore, strontium silicate particles (SrSiO3/Sr2SiO4) reduce M1 polarization and enhance M2 polarization, thereby promoting BMSCs migration (247). Xiong et al. developed a conductive RGO/ZS/CS scaffold that promotes BMSCs osteogenesis via silicon/zinc ion release and inhibits macrophage-derived osteoclastogenesis; its extracts significantly upregulate angiogenic genes (VEGF, bFGF) in HUVECs, thereby enhancing neovascularization (248). Rumian et al. immobilized AlN-loaded PLGA microparticles on a ceramic scaffold to form a composite material that inhibits osteoclast differentiation of macrophages (249). Feito et al. developed NanoMBG-75S, which reduces oxidative stress and promotes M2 polarization of macrophages (250). Meanwhile, Gómez-Cerezo et al. designed an MBG-PCL-zol scaffold that effectively inhibits osteoclastogenesis; however, in vivo studies indicate that high local concentrations of released zoledronic acid may trigger inflammatory responses, requiring further optimization (251).
Most bioceramic implants demonstrate favorable biocompatibility and bioactivity. Combined with modern additive manufacturing techniques, they allow precise design of pore structure and morphology, enhancing their functional versatility.
5.5 Alloy materials
Alloy materials represent a well-established class of implants used to enhance osseointegration (Table 5). For instance, Ji et al. developed a zinc-based alloy (Zn-2Cu-0.5Zr) in which released Zn2+ significantly reduces ROS levels in macrophages and suppresses pro-inflammatory cytokine secretion, while also activating the Wnt/β-catenin signaling pathway. Both in vitro and in vivo studies confirmed that this material promotes M2 macrophage polarization and accelerates bone regeneration (202). In another approach, copper-modified titanium alloy (Ti6Al4V-Cu) upregulates COMMD1 to inhibit NF-κB phosphorylation, thereby reshaping the macrophage polarization balance within inflammatory microenvironments. Concurrently, it suppresses osteoclast differentiation and promotes osteoblast-derived extracellular matrix (OBECM) formation (252, 253).
Alloy implants are widely employed in fracture and bone defect repair owing to their excellent mechanical properties and the capacity of released metal ions to exert antibacterial and osteogenic effects. Current developments in implant alloys emphasize biodegradable designs to avoid secondary surgical procedures. However, several challenges remain for biodegradable alloy materials. For instance, a standardized system for evaluating the cytotoxicity of degradable metals in vitro has not yet been established. The properties and biological effects of alloys vary with the types, proportions, and microstructure of constituent metals, making extensive experimental validation necessary to identify optimal compositions. In addition, the mechanical properties of such materials gradually decline during degradation, which may restrict their use in load-bearing sites and in the context of delayed fracture healing.
5.6 Other materials
Bone cement and certain specialized compounds have been shown to facilitate the repair of osteoporotic fractures or bone defects by regulating macrophage polarization (Table 5). For example, Dai et al. developed a magnesium-doped bone cement (Mg-BG-BC) that promotes the transition of macrophages from the M1 to the M2 phenotype through Mg2+ release and modulation of the TLR/MyD88 signaling pathway, while also enhancing the osteogenic differentiation of BMSCs (254). Separately, Li et al. introduced a novel bone cement formulation (CMC/OPC) that effectively induces M2 polarization of macrophages (255). In another approach, Zhao et al. constructed a G-O-A@Mg porous adhesive loaded with Mg2+ and alendronate, which modulates macrophage polarization while inhibiting osteoclast differentiation (256).
Furthermore, Huang et al. demonstrated that the natural compound picein (C14H18O7) exhibits notable anti-inflammatory and antioxidant properties. It promotes M2 macrophage polarization, suppresses ferroptosis in BMSCs, and enhances osteogenic differentiation through activation of the Nrf2/HO-1/GPX4 signaling pathway (257).
Several natural compounds have demonstrated potential in ameliorating osteoporosis and enhancing fracture healing, with certain compounds exhibiting modulatory effects on macrophage function. For instance, maltol—a natural compound derived from red ginseng—alleviates postmenopausal osteoporosis by promoting RNF213-mediated ubiquitination of CDK14 in macrophages, thereby suppressing M1 polarization and reducing TNFSF12-induced osteoblast apoptosis (258). Similarly, naringenin promotes M2 polarization of macrophages and reduces the secretion of pro-inflammatory factors, promoting bone formation while inhibiting bone resorption, thereby ameliorating pathological bone loss (259). Although these studies did not jointly analyze osteoporosis, fracture healing, and macrophage regulation, they suggest that such natural compounds may facilitate the healing of osteoporotic fractures and bone defects through macrophage-mediated mechanisms.
In addition, combining such natural compounds with other biomaterials represents a viable approach to broaden their therapeutic applicability and enhance treatment efficacy. For example, Zhou et al. developed a dual-targeted nanoplatform that delivers baicalein to fracture sites, where it reduces inflammation, promotes osteogenic differentiation of BMSCs, and accelerates fracture healing by inducing macrophage M2 polarization (260). Separately, Pan et al. constructed a PU/n-HA scaffold for sustained gastrodin release, thereby modulating macrophage responses and facilitating bone repair (213). These collective findings support the potential of natural compounds—either alone or integrated with biomaterials—as a macrophage-targeting strategy worthy of further investigation for managing osteoporotic fractures.
6 Challenges and future directions
Although macrophage-targeted therapies hold promise for treating OPF, their clinical translation faces several challenges. While current evidence indicates that macrophage metabolic reprogramming and polarization influence OP progression, the precise impact of key OP-inducing factors—such as aging and estrogen deficiency—on these cellular processes remains incompletely characterized in existing experimental models. Moreover, the spatiotemporal heterogeneity, polarization transitions, and metabolic adaptations of macrophage subpopulations during OPF healing have yet to be fully elucidated, requiring more systematic and high-resolution analysis in physiologically relevant settings.
Emerging research on the gut-bone and neuro-osteal axes has advanced our understanding of osteoporosis pathogenesis. However, their crosstalk with macrophage-mediated bone metabolism—particularly in the context of OPF—is not yet fully understood. Furthermore, the expression dynamics of macrophage subtypes and inflammatory mediators during fracture healing under osteoporotic conditions, such as in postmenopausal or aging models, have not yet been fully characterized. Deeper mechanistic studies are essential to clarify these regulatory networks and establish a solid foundation for future therapeutic development.
During normal fracture healing, macrophages require a coordinated transition from the pro-inflammatory M1 phenotype to the reparative M2 phenotype. While current therapeutic strategies primarily focus on promoting M2 polarization and suppressing M1 polarization, achieving optimal repair outcomes likely requires precise temporal regulation of macrophage phenotypic switching. Experimental evidence indicates that although existing approaches enhance osseointegration, greater therapeutic efficacy could be attained through dynamic control of polarization timing. For example, the Ti-ALN-acBSP coating enables temporal regulation of M1 macrophage responses through cellular crosstalk involving macrophages, OBs, and OCs, providing a promising paradigm for future temporally controlled strategies (232). However, most currently available biomaterials lack the capacity for precise spatiotemporal control over macrophage polarization, which may result in suboptimal bone regeneration and limited clinical efficacy in osteoporotic bone defects (205).
Concerning experimental models, most current studies on osteoporotic fractures and bone defects utilize animal models—particularly ovariectomized rats—to simulate human OPF conditions. However, these models do not fully recapitulate the human condition due to interspecies differences in both local microenvironment and mechanical properties, which may limit their translational relevance. Moreover, while ovariectomy-based models are widely used to simulate postmenopausal osteoporosis, research focusing on macrophage regulation in aged models remains limited, despite the higher clinical incidence of OPF in elderly populations. The pathogenic mechanisms underlying estrogen deficiency-induced osteoporosis differ substantially from those of age-related bone loss, which may influence the therapeutic efficacy of biomaterial-based interventions (203). Additionally, surgically created fractures and bone defects may not accurately mimic the pathophysiological processes of spontaneous osteoporotic fractures. These collective limitations reduce the clinical predictive value of current animal models and affect the generalizability of resulting findings (261).
Future studies should integrate spatial metabolomics with single-cell epigenetic sequencing to delineate the metabolism–epigenetics interaction network of macrophage subpopulations in OPF. Such multi-omics integration would improve understanding of macrophage dynamics and functional regulation during OPF healing, thereby informing targeted therapeutic strategies. For instance, Xue et al. demonstrated that combining single-cell RNA sequencing with spatial metabolomics can effectively elucidate how drug treatments alleviate tissue damage by reshaping macrophage metabolism and promoting M2 polarization. Their study provides a methodological paradigm for linking transcriptional programs with spatial metabolic microenvironments to unravel disease mechanisms (262). Adopting such an integrated approach in OPF research will help uncover the metabolic–epigenetic regulatory network of macrophages and offer reliable insights for precise immunomodulatory therapy.
Furthermore, more clinically relevant animal models of osteoporotic fracture should be established. These could include aging-based OPF models or patient-derived xenograft systems involving transplantation of bone marrow from elderly osteoporosis patients into immunodeficient mice, better replicating the human “inflammaging” microenvironment. Alternatively, advanced organoid models incorporating osteoporotic bone matrix, vascular networks, and patient-derived macrophages could be developed to enable longitudinal monitoring of microenvironmental dynamics during OPF healing.
7 Conclusions
In summary, this review systematically outlines the immunometabolic regulatory network of macrophages in OPF and its therapeutic relevance. We have highlighted how OP-related pathological factors—such as estrogen deficiency and aging—impair macrophage metabolic reprogramming and polarization dynamics through mechanisms including glycolysis/OXPHOS imbalance, succinate accumulation, and NAD+ deficiency, ultimately contributing to delayed fracture healing. A range of emerging macrophage-targeted strategies are thoroughly discussed, spanning metabolic regulatory nanocrystals (e.g., ZIF-H2S-SDSSD), temporally responsive implant coatings (e.g., Ti-ALN-acBSP), immunomodulatory hydrogels, and functionally enhanced biomaterials. These interventions facilitate bone regeneration by remodeling the osteoimmune microenvironment and rebalancing osteogenic and osteoclastic activities.
However, several research gaps remain: the spatiotemporal distribution of macrophage subsets during OPF healing is not yet fully mapped; existing aging-related OPF models exhibit translational limitations; insights into cross-system interactions such as the gut-bone axis and their link to macrophage metabolism are still insufficient; and most therapeutic systems lack precise temporal control over the M1-to-M2 transition.
Future research should prioritize the integration of spatial metabolomics with single-cell multi-omics profiling to establish more accurate inflammaging models, investigate novel neuro–immune and gut–bone regulatory targets, and develop smart biomaterials capable of dynamically guiding macrophage metabolism and polarization. Such advances will be crucial for translating immunometabolic precision therapies into clinical OPF management.
Author contributions
JX: Investigation, Writing – original draft, Visualization, Writing – review & editing. HL: Investigation, Writing – review & editing. HX: Visualization, Writing – review & editing. LX: Writing – review & editing. HZ: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Tianjin Youth Medical Rising Star Fund Project (No. TJSQNYXXR-D2-142).
Conflict of interest
The authors declare the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
acBSP: Acidic bone sialoprotein
ACOD1: Aconitate decarboxylase 1
ALN: Alendronate
BCAA: Branched-chain amino acid
BCKDH: Branched-chain α-keto acid dehydrogenase
BMMs: Bone marrow macrophages
BMSCs: Bone marrow stromal cells
CXCL: C-X-C motif chemokine ligand
DBT: Dihydrolipoamide branched-chain transacylase E2
DPF: Days post-fracture
ETC: Electron transport chain
FAO: Fatty acid oxidation
FAPI: Fibroblast activation protein inhibitor
Fas: Fatty acids
GO: Graphene oxide
LAP: Laponite
mtROS: Mitochondrial ROS
NADPH: Nicotinamide adenine dinucleotide phosphate
NAM: Nicotinamide
NaMN: Nicotinic acid mononucleotide
NAMPT: Nicotinamide phosphoribosyltransferase
NGF: Nerve growth factor
NMN: NAM mononucleotide
OBs: Osteoblasts
OCs: Osteoclasts
OP: Osteoporosis
OPF: Osteoporotic fracture
OVX: Ovariectomized
PGE2: Prostaglandin E2
PPP: Pentose phosphate pathway
RANKL: Receptor Activator of Nuclear Factor Kappa-B Ligand
ROS: Reactive oxygen species
SCFA: Short-chain fatty acids
SDH: Succinate dehydrogenase
SOD: Superoxide dismutase
SOP: Senile osteoporosis
TCA: Tricarboxylic acid
TrkA: Tropomyosin receptor kinase A
TMAO: Trimethylamine oxide
VEGF: Vascular endothelial growth factor
References
1. Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. (1993) 94:646–50. doi: 10.1016/0002-9343(93)90218-e
2. Xiao PL, Cui AY, Hsu CJ, Peng R, Jiang N, Xu XH, et al. Global, regional prevalence, and risk factors of osteoporosis according to the World Health Organization diagnostic criteria: a systematic review and meta-analysis. Osteoporosis Int. (2022) 33:2137–53. doi: 10.1007/s00198-022-06454-3
3. Salari N, Darvishi N, Bartina Y, Larti M, Kiaei A, Hemmati M, et al. Global prevalence of osteoporosis among the world older adults: a comprehensive systematic review and meta-analysis. J Orthopaedic Surg Res. (2021) 16:669. doi: 10.1186/s13018-021-02821-8
4. Zhu Z, Yu P, Wu Y, Wu Y, Tan Z, Ling J, et al. Sex specific global burden of osteoporosis in 204 countries and territories, from 1990 to 2030: an age-period-cohort modeling study. J Nutr Health Aging. (2023) 27:767–74. doi: 10.1007/s12603-023-1971-4
5. Shen Y, Huang X, Wu J, Lin X, Zhou X, Zhu Z, et al. The global burden of osteoporosis, low bone mass, and its related fracture in 204 countries and territories, 1990-2019. Front In Endocrinol. (2022) 13:882241. doi: 10.3389/fendo.2022.882241
6. Lems WF and Raterman HG. Critical issues and current challenges in osteoporosis and fracture prevention. An overview of unmet needs. Ther Adv Musculoskelet Dis. (2017) 9:299–316. doi: 10.1177/1759720X17732562
7. Gao Y, Chen N, Fu Z, and Zhang Q. Progress of Wnt signaling pathway in osteoporosis. Biomolecules. (2023) 13. doi: 10.3390/biom13030483
8. Kaur S, Raggatt LJ, Batoon L, Hume DA, Levesque JP, and Pettit AR. Role of bone marrow macrophages in controlling homeostasis and repair in bone and bone marrow niches. Semin Cell Dev Biol. (2017) 61:12–21. doi: 10.1016/j.semcdb.2016.08.009
9. Weivoda MM and Bradley EW. Macrophages and bone remodeling. J Bone Miner Res. (2023) 38:359–69. doi: 10.1002/jbmr.4773
10. Sun Y, Li J, Xie X, Gu F, Sui Z, Zhang K, et al. Macrophage-osteoclast associations: origin, polarization, and subgroups. Front Immunol. (2021) 12:778078. doi: 10.3389/fimmu.2021.778078
11. Horwood NJ. Macrophage polarization and bone formation: A review. Clin Rev Allergy Immunol. (2016) 51:79–86. doi: 10.1007/s12016-015-8519-2
12. Gu Q, Yang H, and Shi Q. Macrophages and bone inflammation. J Orthop Translat. (2017) 10:86–93. doi: 10.1016/j.jot.2017.05.002
13. Michalski MN and McCauley LK. Macrophages and skeletal health. Pharmacol Ther. (2017) 174:43–54. doi: 10.1016/j.pharmthera.2017.02.017
14. Mo Y, Zhao F, Lin Z, Cao X, Chen D, and Chen X. Local delivery of naringin in beta-cyclodextrin modified mesoporous bioactive glass promotes bone regeneration: from anti-inflammatory to synergistic osteogenesis and osteoclastogenesis. Biomater Sci. (2022) 10:1697–712. doi: 10.1039/d1bm01842f
15. Luo M, Zhao F, Liu L, Yang Z, Tian T, Chen X, et al. IFN-gamma/SrBG composite scaffolds promote osteogenesis by sequential regulation of macrophages from M1 to M2. J Mater Chem B. (2021) 9:1867–76. doi: 10.1039/d0tb02333g
16. Park JH, Seo YJ, Oh HS, and Byun JH. Effects of myeloid immune cells on the metabolic process of biomimetic bone regeneration. Life Sci. (2023) 334:122251. doi: 10.1016/j.lfs.2023.122251
17. Sanghani-Kerai A, McCreary D, Lancashire H, Osagie L, Coathup M, and Blunn G. Stem cell interventions for bone healing: fractures and osteoporosis. Curr Stem Cell Res Ther. (2018) 13:369–77. doi: 10.2174/1574888X13666180410160511
18. Wang C, Wu Q, Zhuang L, Chen Y, Zhang Q, Wu Y, et al. Immunometabolism of macrophages in the bone microenvironment: a new perspective for bone healing therapy. J Advanced Res. (2025) In Press. doi: 10.1016/j.j.Motorcycle2025.07.046
19. Saul D and Khosla S. Fracture healing in the setting of endocrine diseases, aging, and cellular senescence. Endocrine Rev. (2022) 43:984–1002. doi: 10.1210/endrev/bnac008
20. Kim J-M, Lin C, Stavre Z, Greenblatt MB, and Shim J-H. Osteoblast-osteoclast communication and bone homeostasis. Cells. (2020) 9. doi: 10.3390/cells9092073
21. Hu K, Shang Z, Yang X, Zhang Y, and Cao L. Macrophage polarization and the regulation of bone immunity in bone homeostasis. J Inflammation Res. (2023) 16:3563–80. doi: 10.2147/JIR.S423819
22. Ponzetti M and Rucci N. Osteoblast differentiation and signaling: established concepts and emerging topics. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22136651
23. Yu D, Guo S, Yu M, Liu W, Li X, Chen D, et al. Immunomodulation and osseointegration activities of Na(2)TiO(3) nanorods-arrayed coatings doped with different Sr content. Bioact Mater. (2022) 10:323–34. doi: 10.1016/j.bioactmat.2021.08.033
24. He J, Zhao D, Peng B, Wang X, Wang S, Zhao X, et al. A novel mechanism of Vildagliptin in regulating bone metabolism and mitigating osteoporosis. Int Immunopharmacol. (2024) 130:111671. doi: 10.1016/j.intimp.2024.111671
25. Nie Z, Hu Z, Guo X, Xiao Y, Liu X, de Bruijn JD, et al. Genesis of osteoclasts on calcium phosphate ceramics and their role in material-induced bone formation. Acta Biomater. (2023) 157:625–38. doi: 10.1016/j.actbio.2022.11.005
26. Chen T, Wu X, Zhang P, Wu W, Dai H, and Chen S. Strontium-doped hydroxyapatite coating improves osteo/angiogenesis for ameliorative graft-bone integration via the macrophage-derived cytokines-mediated integrin signal pathway. ACS Appl Mater Interfaces. (2024) 16:15687–700. doi: 10.1021/acsami.3c14904
27. Wang Q, Xu L, Willumeit-Romer R, and Luthringer-Feyerabend BJC. Macrophage-derived oncostatin M/bone morphogenetic protein 6 in response to Mg-based materials influences pro-osteogenic activity of human umbilical cord perivascular cells. Acta Biomater. (2021) 133:268–79. doi: 10.1016/j.actbio.2020.12.016
28. Wang C, Zhao Q, Chen C, Li J, Zhang J, Qu S, et al. CD301b(+) macrophage: the new booster for activating bone regeneration in periodontitis treatment. Int J Oral Sci. (2023) 15:19. doi: 10.1038/s41368-023-00225-4
29. Zhang Y, Wei J, Yu X, Chen L, Ren R, Dong Y, et al. CXCL chemokines-mediated communication between macrophages and BMSCs on titanium surface promotes osteogenesis via the actin cytoskeleton pathway. Mater Today Bio. (2023) 23:100816. doi: 10.1016/j.mtbio.2023.100816
30. Chen X, Wan Z, Yang L, Song S, Fu Z, Tang K, et al. Exosomes derived from reparative M2-like macrophages prevent bone loss in murine periodontitis models via IL-10 mRNA. J Nanobiotechnol. (2022) 20:110. doi: 10.1186/s12951-022-01314-y
31. Cai Z, Bai L, Li Q, Li Y, Cai X, and Lin Y. Gene-activating framework nucleic acid-targeted upregulating sirtuin-1 to modulate osteoimmune microenvironment for diabetic osteoporosis therapeutics. ACS Nano. (2024) 18:35214–29. doi: 10.1021/acsnano.4c08727
32. Bin-Bin Z, Da-Wa ZX, Chao L, Lan-Tao Z, Tao W, Chuan L, et al. M2 macrophagy-derived exosomal miRNA-26a-5p induces osteogenic differentiation of bone mesenchymal stem cells. J Orthop Surg Res. (2022) 17:137. doi: 10.1186/s13018-022-03029-0
33. Liu K, Luo X, Lv ZY, Zhang YJ, Meng Z, Li J, et al. Macrophage-derived exosomes promote bone mesenchymal stem cells towards osteoblastic fate through microRNA-21a-5p. Front Bioeng Biotechnol. (2021) 9:801432. doi: 10.3389/fbioe.2021.801432
34. Giraldo-Osorno PM, Wirsig K, Asa'ad F, Omar O, Trobos M, Bernhardt A, et al. Macrophage-to-osteocyte communication: Impact in a 3D in vitro implant-associated infection model. Acta Biomater. (2024) 186:141–55. doi: 10.1016/j.actbio.2024.08.005
35. Eger M, Hiram-Bab S, Liron T, Sterer N, Carmi Y, Kohavi D, et al. Mechanism and prevention of titanium particle-induced inflammation and osteolysis. Front Immunol. (2018) 9:2963. doi: 10.3389/fimmu.2018.02963
36. Osta B, Benedetti G, and Miossec P. Classical and paradoxical effects of TNF-α on bone homeostasis. Front In Immunol. (2014) 5:48. doi: 10.3389/fimmu.2014.00048
37. Li X, Zhou Z-Y, Zhang Y-Y, and Yang H-L. IL-6 contributes to the defective osteogenesis of bone marrow stromal cells from the vertebral body of the glucocorticoid-induced osteoporotic mouse. PloS One. (2016) 11:e0154677. doi: 10.1371/journal.pone.0154677
38. Malysheva K, de Rooij K, Lowik CW, Baeten DL, Rose-John S, Stoika R, et al. Interleukin 6/Wnt interactions in rheumatoid arthritis: interleukin 6 inhibits Wnt signaling in synovial fibroblasts and osteoblasts. Croat Med J. (2016) 57:89–98. doi: 10.3325/cmj.2016.57.89
39. Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PVN, Komm BS, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. (2005) 280:33132–40. doi: 10.1074/jbc.M500608200
40. Bodine PVN and Komm BS. Wnt signaling and osteoblastogenesis. Rev In Endocrine Metab Disord. (2006) 7:33–9. doi: 10.1007/s11154-006-9002-4
41. Wang D, Liu Y, Diao S, Shan L, and Zhou J. Long non-coding RNAs within macrophage-derived exosomes promote BMSC osteogenesis in a bone fracture rat model. Int J Nanomed. (2023) 18:1063–83. doi: 10.2147/IJN.S398446
42. Huang H, Zhao N, Xu X, Xu Y, Li S, Zhang J, et al. Dose-specific effects of tumor necrosis factor alpha on osteogenic differentiation of mesenchymal stem cells. Cell Proliferation. (2011) 44:420–7. doi: 10.1111/j.1365-2184.2011.00769.x
43. Xia Y, Inoue K, Du Y, Baker SJ, Reddy EP, Greenblatt MB, et al. TGFβ reprograms TNF stimulation of macrophages towards a non-canonical pathway driving inflammatory osteoclastogenesis. Nat Commun. (2022) 13:3920. doi: 10.1038/s41467-022-31475-1
44. Zha L, He L, Liang Y, Qin H, Yu B, Chang L, et al. TNF-α contributes to postmenopausal osteoporosis by synergistically promoting RANKL-induced osteoclast formation. Biomed Pharmacother = Biomed Pharmacotherapie. (2018) 102:369–74. doi: 10.1016/j.biopha.2018.03.080
45. Luo G, Li F, Li X, Wang Z-G, and Zhang B. TNF−α and RANKL promote osteoclastogenesis by upregulating RANK via the NF−κB pathway. Mol Med Rep. (2018) 17:6605–11. doi: 10.3892/mmr.2018.8698
46. AlQranei MS, Senbanjo LT, Aljohani H, Hamza T, and Chellaiah MA. Lipopolysaccharide- TLR-4 Axis regulates Osteoclastogenesis independent of RANKL/RANK signaling. BMC Immunol. (2021) 22:23. doi: 10.1186/s12865-021-00409-9
47. Otsuka Y, Kondo T, Aoki H, Goto Y, Kawaguchi Y, Waguri-Nagaya Y, et al. IL-1β promotes osteoclastogenesis by increasing the expression of IGF2 and chemokines in non-osteoclastic cells. J Pharmacol Sci. (2023) 151:1–8. doi: 10.1016/j.jphs.2022.10.007
48. Levescot A, Chang MH, Schnell J, Nelson-Maney N, Yan J, Martínez-Bonet M, et al. IL-1β-driven osteoclastogenic Tregs accelerate bone erosion in arthritis. J Clin Invest. (2021) 131. doi: 10.1172/JCI141008
49. Yamaguchi T, Movila A, Kataoka S, Wisitrasameewong W, Ruiz Torruella M, Murakoshi M, et al. Proinflammatory M1 macrophages inhibit RANKL-induced osteoclastogenesis. Infect Immun. (2016) 84:2802–12. doi: 10.1128/iai.00461-16
50. Zhou Y and Hu G. M2 macrophages-derived exosomes regulate osteoclast differentiation by the CSF2/TNF-alpha axis. BMC Oral Health. (2024) 24:107. doi: 10.1186/s12903-023-03842-x
51. Chen S, Liu J, and Zhu L. M2-like macrophage-derived exosomes inhibit osteoclastogenesis via releasing miR-1227-5p. Immunobiology. (2025) 230:152861. doi: 10.1016/j.imbio.2024.152861
52. Guo ZY, Yin NN, Li XF, Wang MM, Sui XN, Jiang CD, et al. Exosomes secreted from M2-polarized macrophages inhibit osteoclast differentiation via CYLD. Tissue Cell. (2025) 93:102645. doi: 10.1016/j.tice.2024.102645
53. Huang X, Lan Y, Shen J, Zhao X, Zhou Y, Wu W, et al. M2 macrophages secrete glutamate-containing extracellular vesicles to alleviate osteoporosis by reshaping osteoclast precursor fate. Mol Ther. (2024) 32:1158–77. doi: 10.1016/j.ymthe.2024.02.005
54. Lei SS, Huang XW, Li LZ, Wang XP, Zhang Y, Li B, et al. Explorating the mechanism of Epimedii folium-Rhizoma drynariae herbal pair promoted bone defects healing through network pharmacology and experimental studies. J Ethnopharmacol. (2024) 319:117329. doi: 10.1016/j.jep.2023.117329
55. Zhu XX, Meng XY, Chen G, Su JB, Fu X, Xu AJ, et al. Nesfatin-1 enhances vascular smooth muscle calcification through facilitating BMP-2 osteogenic signaling. Cell Commun Signal. (2024) 22:488. doi: 10.1186/s12964-024-01873-7
56. Cai G, Lu Y, Zhong W, Wang T, Li Y, Ruan X, et al. Piezo1-mediated M2 macrophage mechanotransduction enhances bone formation through secretion and activation of transforming growth factor-β1. Cell Proliferation. (2023) 56:e13440. doi: 10.1111/cpr.13440
57. Hwang YJ, Hwang HJ, Go H, Park N, and Hwang KA. Sword bean (Canavalia gladiata) pods induce differentiation in MC3T3-E1 osteoblast cells by activating the BMP2/SMAD/RUNX2 pathway. Nutrients. (2023) 15. doi: 10.3390/nu15204372
58. Balogh E, Toth A, Csiki DM, and Jeney V. Zinc ameliorates high pi and ca-mediated osteogenic differentiation of mesenchymal stem cells. Nutrients. (2024) 16. doi: 10.3390/nu16234012
59. Liu J, Sun Z, You Y, Zhang L, Hou D, Gu G, et al. M2 macrophage-derived exosomal miR-486-5p influences the differentiation potential of bone marrow mesenchymal stem cells and osteoporosis. Aging (Albany NY). (2023) 15:9499–520. doi: 10.18632/aging.205031
60. Zhu Y, Zhao S, Cheng L, Lin Z, Zeng M, Ruan Z, et al. Mg(2+) -mediated autophagy-dependent polarization of macrophages mediates the osteogenesis of bone marrow stromal stem cells by interfering with macrophage-derived exosomes containing miR-381. J Orthop Res. (2022) 40:1563–76. doi: 10.1002/jor.25189
61. Valles G, Bensiamar F, Maestro-Paramio L, Garcia-Rey E, Vilaboa N, and Saldana L. Influence of inflammatory conditions provided by macrophages on osteogenic ability of mesenchymal stem cells. Stem Cell Res Ther. (2020) 11:57. doi: 10.1186/s13287-020-1578-1
62. Lu LY, Loi F, Nathan K, Lin TH, Pajarinen J, Gibon E, et al. Pro-inflammatory M1 macrophages promote Osteogenesis by mesenchymal stem cells via the COX-2-prostaglandin E2 pathway. J Orthop Res. (2017) 35:2378–85. doi: 10.1002/jor.23553
63. Romero-López M, Li Z, Rhee C, Maruyama M, Pajarinen J, O'Donnell B, et al. Macrophage effects on mesenchymal stem cell osteogenesis in a three-dimensional in vitro bone model. Tissue Engineering Part A. (2020) 26:1099–111. doi: 10.1089/ten.TEA.2020.0041
64. Yang L, Xiao L, Gao W, Huang X, Wei F, Zhang Q, et al. Macrophages at Low-Inflammatory Status Improved Osteogenesis via Autophagy Regulation. Tissue Engineering Part A. (2024) 30:e766–79. doi: 10.1089/ten.TEA.2021.0015
65. Xia P, Wang X, Wang Q, Wang X, Lin Q, Cheng K, et al. Low-intensity pulsed ultrasound promotes autophagy-mediated migration of mesenchymal stem cells and cartilage repair. Cell Transplantation. (2021) 30. doi: 10.1177/0963689720986142
66. Deng L, Hu G, Jin L, Wang C, and Niu H. Involvement of microRNA-23b in TNF-alpha-reduced BMSC osteogenic differentiation via targeting runx2. J Bone Miner Metab. (2018) 36:648–60. doi: 10.1007/s00774-017-0886-8
67. Xie F, Teng L, Xu J, Lu J, Zhang C, Yang L, et al. Interleukin-10 modified bone marrow mesenchymal stem cells prevent hypertrophic scar formation by inhibiting inflammation. Pharmazie. (2020) 75:571–5. doi: 10.1591/ph.2020.0572
68. Wang F, Kong L, Wang W, Shi L, Wang M, Chai Y, et al. Adrenomedullin 2 improves bone regeneration in type 1 diabetic rats by restoring imbalanced macrophage polarization and impaired osteogenesis. Stem Cell Res Ther. (2021) 12:288. doi: 10.1186/s13287-021-02368-9
69. Cai W, Zhang J, Yu Y, Ni Y, Wei Y, Cheng Y, et al. Mitochondrial transfer regulates cell fate through metabolic remodeling in osteoporosis. Advanced Sci (Weinheim Baden-Wurttemberg Germany). (2023) 10:e2204871. doi: 10.1002/advs.202204871
70. Qi Y, Zhu T, Zhang T, Wang X, Li W, Chen D, et al. M1 macrophage-derived exosomes transfer miR-222 to induce bone marrow mesenchymal stem cell apoptosis. Lab Invest. (2021) 101:1318–26. doi: 10.1038/s41374-021-00622-5
71. Katagiri W, Takeuchi R, Saito N, Suda D, and Kobayashi T. Migration and phenotype switching of macrophages at early-phase of bone-formation by secretomes from bone marrow derived mesenchymal stem cells using rat calvaria bone defect model. J Dent Sci. (2022) 17:421–9. doi: 10.1016/j.jds.2021.08.012
72. Jiang Q, Bai G, Liu X, Chen Y, Xu G, Yang C, et al. 3D gelMA ICC scaffolds combined with SW033291 for bone regeneration by modulating macrophage polarization. Pharmaceutics. (2021) 13. doi: 10.3390/pharmaceutics13111934
73. Chen L, Yu C, Xu W, Xiong Y, Cheng P, Lin Z, et al. Dual-targeted nanodiscs revealing the cross-talk between osteogenic differentiation of mesenchymal stem cells and macrophages. ACS Nano. (2023) 17:3153–67. doi: 10.1021/acsnano.2c12440
74. Wang J, Huang R, Xu Q, Zheng G, Qiu G, Ge M, et al. Mesenchymal stem cell-derived extracellular vesicles alleviate acute lung injury via transfer of miR-27a-3p. Crit Care Med. (2020) 48:e599–610. doi: 10.1097/ccm.0000000000004315
75. Tavasolian F, Hosseini AZ, Soudi S, and Naderi M. miRNA-146a improves immunomodulatory effects of MSC-derived exosomes in rheumatoid arthritis. Curr Gene Ther. (2020) 20:297–312. doi: 10.2174/1566523220666200916120708
76. Ko JH and Oh JY. The effect of miR-146a on the gene expression of immunoregulatory cytokines in human mesenchymal stromal cells. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21186809
77. Li W, Xu Y, and Chen W. Bone mesenchymal stem cells deliver exogenous lncRNA CAHM via exosomes to regulate macrophage polarization and ameliorate intervertebral disc degeneration. Exp Cell Res. (2022) 421:113408. doi: 10.1016/j.yexcr.2022.113408
78. Chen Y, Bi K, Zhang C, Gu J, Yu Z, Lu J, et al. Identification of endoplasmic reticulum stress and mitochondrial dysfunction related biomarkers in osteoporosis. Hereditas. (2025) 162:21. doi: 10.1186/s41065-025-00387-7
79. Liu C, Cao Z, Zhang W, Tickner J, Qiu H, Wang C, et al. Lumichrome inhibits osteoclastogenesis and bone resorption through suppressing RANKL-induced NFAT activation and calcium signaling. J Cell Physiol. (2018) 233:8971–83. doi: 10.1002/jcp.26841
80. Mazzantini M and De Mattia G. The great challenge: bone fragility and environment. Clin Exp Rheumatol. (2024) 42:1714–9. doi: 10.55563/clinexprheumatol/7azn44
81. Karasik D, Rivadeneira F, and Johnson ML. The genetics of bone mass and susceptibility to bone diseases. Nat Rev Rheumatol. (2016) 12:323–34. doi: 10.1038/nrrheum.2016.48
82. Belboul A, Ashworth J, Fadel A, McLoughlin J, Mahmoud A, and El Mohtadi M. Estrogen induces the alternative activation of macrophages through binding to estrogen receptor-alpha. Exp Mol Pathol. (2025) 143:104971. doi: 10.1016/j.yexmp.2025.104971
83. Dou C, Ding N, Zhao C, Hou T, Kang F, Cao Z, et al. Estrogen deficiency-mediated M2 macrophage osteoclastogenesis contributes to M1/M2 ratio alteration in ovariectomized osteoporotic mice. J Bone Mineral Res. (2018) 33:899–908. doi: 10.1002/jbmr.3364
84. Ahn H, Lee K, Kim JM, Kwon SH, Lee SH, Lee SY, et al. Accelerated lactate dehydrogenase activity potentiates osteoclastogenesis via NFATc1 signaling. PLoS One. (2016) 11:e0153886. doi: 10.1371/journal.pone.0153886
85. Yu D, Gao Y, Luzarowski M, Seebach E, Heitkamp T, Börsch M, et al. Protein succinylome analysis identifies citrate synthase as a central regulator of osteoclast metabolic activity. FEBS J. (2025) 292:3736–54. doi: 10.1111/febs.70090
86. Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. (2013) 496:238–42. doi: 10.1038/nature11986
87. Jimi E, Nakamura I, Duong LT, Ikebe T, Takahashi N, Rodan GA, et al. Interleukin 1 induces multinucleation and bone-resorbing activity of osteoclasts in the absence of osteoblasts/stromal cells. Exp Cell Res. (1999) 247:84–93. doi: 10.1006/excr.1998.4320
88. Yang A, Wang J, Yang Z, Wang L, Li H, and Lei L. Hypoxic dental pulp stem cells-released succinate promotes osteoclastogenesis and root resorption. Int J Med Sci. (2024) 21:1155–64. doi: 10.7150/ijms.94972
89. Cantrell AC, Besanson J, Williams Q, Hoang N, Edwards K, Bishop GR, et al. Ferrostatin-1 specifically targets mitochondrial iron-sulfur clusters and aconitase to improve cardiac function in Sirtuin 3 cardiomyocyte knockout mice. J Mol Cell Cardiol. (2024) 192:36–47. doi: 10.1016/j.yjmcc.2024.05.003
90. Trinh D, Al Halabi L, Brar H, Kametani M, and Nash JE. The role of SIRT3 in homeostasis and cellular health. Front Cell Neurosci. (2024) 18:1434459. doi: 10.3389/fncel.2024.1434459
91. Zou X, Zhu Y, Park S-H, Liu G, O'Brien J, Jiang H, et al. SIRT3-mediated dimerization of IDH2 directs cancer cell metabolism and tumor growth. Cancer Res. (2017) 77:3990–9. doi: 10.1158/0008-5472.CAN-16-2393
92. Sun Y, Tian Z, Liu N, Zhang L, Gao Z, Sun X, et al. Exogenous H2S switches cardiac energy substrate metabolism by regulating SIRT3 expression in db/db mice. J Mol Med (Berlin Germany). (2018) 96:281–99. doi: 10.1007/s00109-017-1616-3
93. Marques-Carvalho A, Silva B, Pereira FB, Kim HN, Almeida M, and Sardão VA. Oestradiol and osteoclast differentiation: Effects on p53 and mitochondrial metabolism. Eur J Clin Invest. (2024) 54:e14195. doi: 10.1111/eci.14195
94. Wei H, Qu L, Dai S, Li Y, Wang H, Feng Y, et al. Structural insight into the molecular mechanism of p53-mediated mitochondrial apoptosis. Nat Commun. (2021) 12:2280. doi: 10.1038/s41467-021-22655-6
95. Vetrano DL, Calderón-Larrañaga A, Marengoni A, Onder G, Bauer JM, Cesari M, et al. An international perspective on chronic multimorbidity: approaching the elephant in the room. Journals Gerontol Ser A Biol Sci Med Sci. (2018) 73:1350–6. doi: 10.1093/gerona/glx178
96. Qadir A, Liang S, Wu Z, Chen Z, Hu L, and Qian A. Senile osteoporosis: the involvement of differentiation and senescence of bone marrow stromal cells. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21010349
97. He X-Y, Yu H-M, Lin S, and Li Y-Z. Advances in the application of mesenchymal stem cells, exosomes, biomimetic materials, and 3D printing in osteoporosis treatment. Cell Mol Biol Letters. (2021) 26:47. doi: 10.1186/s11658-021-00291-8
98. Zhong Y, Zhou X, Pan Z, Zhang J, and Pan J. Role of epigenetic regulatory mechanisms in age-related bone homeostasis imbalance. FASEB J. (2024) 38:e23642. doi: 10.1096/fj.202302665R
99. Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, et al. Geroscience: linking aging to chronic disease. Cell. (2014) 159:709–13. doi: 10.1016/j.cell.2014.10.039
100. Franceschi C, Garagnani P, Parini P, Giuliani C, and Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. (2018) 14:576–90. doi: 10.1038/s41574-018-0059-4
101. Gibon E, Loi F, Córdova LA, Pajarinen J, Lin T, Lu L, et al. Aging affects bone marrow macrophage polarization: relevance to bone healing. Regenerative Eng Trans Med. (2016) 2:98–104. doi: 10.1007/s40883-016-0016-5
102. Clark D, Brazina S, Yang F, Hu D, Hsieh CL, Niemi EC, et al. Age-related changes to macrophages are detrimental to fracture healing in mice. Aging Cell. (2020) 19:e13112. doi: 10.1111/acel.13112
103. Passos JF, Saretzki G, Ahmed S, Nelson G, Richter T, Peters H, et al. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PloS Biol. (2007) 5:e110. doi: 10.1371/journal.pbio.0050110
104. Pence BD and Yarbro JR. Aging impairs mitochondrial respiratory capacity in classical monocytes. Exp Gerontol. (2018) 108:112–7. doi: 10.1016/j.exger.2018.04.008
105. Vendrov AE, Lozhkin A, Hayami T, Levin J, Silveira Fernandes Chamon J, Abdel-Latif A, et al. Mitochondrial dysfunction and metabolic reprogramming induce macrophage pro-inflammatory phenotype switch and atherosclerosis progression in aging. Front In Immunol. (2024) 15:1410832. doi: 10.3389/fimmu.2024.1410832
106. Yegorov YE, Poznyak AV, Nikiforov NG, SoBenin IA, and Orekhov AN. The link between chronic stress and accelerated aging. Biomedicines. (2020) 8. doi: 10.3390/biomedicines8070198
107. Fix DK, Ekiz HA, Petrocelli JJ, McKenzie AM, Mahmassani ZS, O'Connell RM, et al. Disrupted macrophage metabolic reprogramming in aged soleus muscle during early recovery following disuse atrophy. Aging Cell. (2021) 20:e13448. doi: 10.1111/acel.13448
108. Van den Bossche J, Baardman J, Otto NA, van der Velden S, Neele AE, van den Berg SM, et al. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep. (2016) 17:684–96. doi: 10.1016/j.celrep.2016.09.008
109. Pryce BR, Oles A, Talbert EE, Romeo MJ, Vaena S, Sharma S, et al. Muscle inflammation is regulated by NF-κB from multiple cells to control distinct states of wasting in cancer cachexia. Cell Rep. (2024) 43:114925. doi: 10.1016/j.celrep.2024.114925
110. Liu L, Guo H, Song A, Huang J, Zhang Y, Jin S, et al. Progranulin inhibits LPS-induced macrophage M1 polarization via NF-кB and MAPK pathways. BMC Immunol. (2020) 21:32. doi: 10.1186/s12865-020-00355-y
111. Verdin E. NAD+ in aging, metabolism, and neurodegeneration. Sci (New York N.Y.). (2015) 350:1208–13. doi: 10.1126/science.aac4854
112. Poljšak B, Kovač V, Špalj S, and Milisav I. The central role of the NAD+ Molecule in the development of aging and the prevention of chronic age-related diseases: strategies for NAD+ Modulation. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24032959
113. Kim H-N, Ponte F, Warren A, Ring R, Iyer S, Han L, et al. A decrease in NAD+ contributes to the loss of osteoprogenitors and bone mass with aging. NPJ Aging Mech Dis. (2021) 7:8. doi: 10.1038/s41514-021-00058-7
114. Minhas PS, Liu L, Moon PK, Joshi AU, Dove C, Mhatre S, et al. Macrophage de novo NAD(+) synthesis specifies immune function in aging and inflammation. Nat Immunol. (2019) 20:50–63. doi: 10.1038/s41590-018-0255-3
115. Suchard MS and Savulescu DM. Nicotinamide pathways as the root cause of sepsis - an evolutionary perspective on macrophage energetic shifts. FEBS J. (2021) 289:955–64. doi: 10.1111/febs.15807
116. Moffett JR, Arun P, Puthillathu N, Vengilote R, Ives JA, Badawy AAB, et al. Quinolinate as a marker for kynurenine metabolite formation and the unresolved question of NAD+ Synthesis during inflammation and infection. Front In Immunol. (2020) 11:31. doi: 10.3389/fimmu.2020.00031
117. Zwickenpflug W, Hornung F, Hollaus A, Oswald MS, Chioato Z, Gudermann T, et al. Biosynthesis of vitamin B3 and NAD+: incubating HepG2 cells with the alkaloid myosmine. J Sci Food Agric. (2024) 104:6844–54. doi: 10.1002/jsfa.13513
118. Almeida KH, Avalos-Irving L, Berardinelli S, Chauvin K, and Yanez S. Novel carbon skeletons activate human NicotinAMide Phosphoribosyl Transferase (NAMPT) enzyme in biochemical assay. PloS One. (2023) 18:e0283428. doi: 10.1371/journal.pone.0283428
119. Li X, Li Y, Li F, Chen Q, Zhao Z, Liu X, et al. NAD+ Anabolism disturbance causes glomerular mesangial cell injury in diabetic nephropathy. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23073458
120. Galli U, Colombo G, Travelli C, Tron GC, Genazzani AA, and Grolla AA. Recent advances in NAMPT inhibitors: A novel immunotherapic strategy. Front In Pharmacol. (2020) 11:656. doi: 10.3389/fphar.2020.00656
121. Yarbro JR, Emmons RS, and Pence BD. Macrophage immunometabolism and inflammaging: roles of mitochondrial dysfunction, cellular senescence, CD38, and NAD. Immunometabolism. (2020) 2:e200026. doi: 10.20900/immunometab20200026
122. Grootaert MOJ and Bennett MR. Sirtuins in atherosclerosis: guardians of healthspan and therapeutic targets. Nat Rev Cardiol. (2022) 19:668–83. doi: 10.1038/s41569-022-00685-x
123. Tang J, Wang X, Chen S, Chang T, Gu Y, Zhang F, et al. Disruption of glucose homeostasis by bacterial infection orchestrates host innate immunity through NAD+/NADH balance. Cell Rep. (2024) 43:114648. doi: 10.1016/j.celrep.2024.114648
124. Smulan LJ, Martinez N, Kiritsy MC, Kativhu C, Cavallo K, Sassetti CM, et al. Sirtuin 3 downregulation in mycobacterium tuberculosis-infected macrophages reprograms mitochondrial metabolism and promotes cell death. MBio. (2021) 12. doi: 10.1128/mBio.03140-20
125. Mishra S, Welch N, Karthikeyan M, Bellar A, Musich R, Singh SS, et al. Dysregulated cellular redox status during hyperammonemia causes mitochondrial dysfunction and senescence by inhibiting sirtuin-mediated deacetylation. Aging Cell. (2023) 22:e13852. doi: 10.1111/acel.13852
126. Santos L, Benitez-Rosendo A, Bresque M, Camacho-Pereira J, Calliari A, and Escande C. Sirtuins: the NAD+-dependent multifaceted modulators of inflammation. Antioxidants Redox Signaling. (2023) 39:1185–208. doi: 10.1089/ars.2023.0295
127. Yang Y and Sauve AA. NAD(+) metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim Biophys Acta. (2016) 1864:1787–800. doi: 10.1016/j.bbapap.2016.06.014
128. Navas LE and Carnero A. Nicotinamide adenine dinucleotide (NAD) metabolism as a relevant target in cancer. Cells. (2022) 11. doi: 10.3390/cells11172627
129. Zhang Y, Morgan MJ, Chen K, Choksi S, and Liu Z-G. Induction of autophagy is essential for monocyte-macrophage differentiation. Blood. (2012) 119:2895–905. doi: 10.1182/blood-2011-08-372383
130. Vitaliti A, Reggio A, and Palma A. Macrophages and autophagy: partners in crime. FEBS J. (2025) 292:2957–72. doi: 10.1111/febs.17305
131. Liu R, Cui J, Sun Y, Xu W, Wang Z, Wu M, et al. Autophagy deficiency promotes M1 macrophage polarization to exacerbate acute liver injury via ATG5 repression during aging. Cell Death Discov. (2021) 7:397. doi: 10.1038/s41420-021-00797-2
132. Stranks AJ, Hansen AL, Panse I, Mortensen M, Ferguson DJ, Puleston DJ, et al. Autophagy controls acquisition of aging features in macrophages. J Innate Immun. (2015) 7:375–91. doi: 10.1159/000370112
133. Omarjee O, Mathieu A-L, Quiniou G, Moreews M, Ainouze M, Frachette C, et al. LACC1 deficiency links juvenile arthritis with autophagy and metabolism in macrophages. J Exp Med. (2021) 218. doi: 10.1084/jem.20201006
134. Hu L, Liu J, Peng J, Li X, Huang Z, Zhang C, et al. TREM2 alleviates neuroinflammation by maintaining cellular metabolic homeostasis and mitophagy activity during early inflammation. Dis (Basel Switzerland). (2025) 13. doi: 10.3390/diseases13020060
135. Yuan Y, Chen Y, Peng T, Li L, Zhu W, Liu F, et al. Mitochondrial ROS-induced lysosomal dysfunction impairs autophagic flux and contributes to M1 macrophage polarization in a diabetic condition. Clin Sci (Lond). (2019) 133:1759–77. doi: 10.1042/CS20190672
136. Lin S, Wu B, Hu X, and Lu H. Sirtuin 4 (Sirt4) downregulation contributes to chondrocyte senescence and osteoarthritis via mediating mitochondrial dysfunction. Int J Biol Sci. (2024) 20:1256–78. doi: 10.7150/ijbs.85585
137. Anderson KA, deSouza B, Castellano-Escuder P, Lin Z, Ilkayeva OR, Muehlbauer MJ, et al. SIRT4 controls macrophage function and wound healing through control of protein itaconylation in mice. bioRxiv. (2025). Available online at: https://www.biorxiv.org/content/10.1101/2025.05.12.653532v1 (Accessed October 13, 2025).
138. Huang H, Chen H, Yao Y, and Lou X. Branched-chain amino acids supplementation induces insulin resistance and pro-inflammatory macrophage polarization via INFGR1/JAK1/STAT1 signal pathway. Mol Med (Cambridge Mass.). (2024) 30:149. doi: 10.1186/s10020-024-00894-9
139. Lu M, Luo D, Zhang Z, Ouyang F, Shi Y, Hu C, et al. Branched-chain amino acid catabolism promotes M2 macrophage polarization. Front In Immunol. (2024) 15:1469163. doi: 10.3389/fimmu.2024.1469163
140. Kang Y, Zhang H, Zhao Y, Wang Y, Wang W, He Y, et al. Telomere dysfunction disturbs macrophage mitochondrial metabolism and the NLRP3 inflammasome through the PGC-1α/TNFAIP3 axis. Cell Rep. (2018) 22:3493–506. doi: 10.1016/j.celrep.2018.02.071
141. Seely KD, Kotelko CA, Douglas H, Bealer B, and Brooks AE. The human gut microbiota: A key mediator of osteoporosis and osteogenesis. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22179452
142. Hao L, Yan Y, Huang G, and Li H. From gut to bone: deciphering the impact of gut microbiota on osteoporosis pathogenesis and management. Front Cell Infect Microbiol. (2024) 14:1416739. doi: 10.3389/fcimb.2024.1416739
143. Wei M, Li C, Dai Y, Zhou H, Cui Y, Zeng Y, et al. High-throughput absolute quantification sequencing revealed osteoporosis-related gut microbiota alterations in han chinese elderly. Front Cell Infect Microbiol. (2021) 11:630372. doi: 10.3389/fcimb.2021.630372
144. Zhang YW, Wu Y, Liu XF, Chen X, and Su JC. Targeting the gut microbiota-related metabolites for osteoporosis: The inextricable connection of gut-bone axis. Ageing Res Rev. (2024) 94:102196. doi: 10.1016/j.arr.2024.102196
145. Jha SS, Jeyaraman N, Jeyaraman M, Ramasubramanian S, Muthu S, Santos GS, et al. Cross-talks between osteoporosis and gut microbiome. World J Orthop. (2025) 16:102274. doi: 10.5312/wjo.v16.i3.102274
146. Ding K, Hua F, and Ding W. Gut microbiome and osteoporosis. Aging Dis. (2020) 11:438–47. doi: 10.14336/ad.2019.0523
147. Lun H, Li P, Li J, and Liu F. The effect of intestinal flora metabolites on macrophage polarization. Heliyon. (2024) 10:e35755. doi: 10.1016/j.heliyon.2024.e35755
148. Lyu Z, Hu Y, Guo Y, and Liu D. Modulation of bone remodeling by the gut microbiota: a new therapy for osteoporosis. Bone Res. (2023) 11:31. doi: 10.1038/s41413-023-00264-x
149. MaChado MG, Patente TA, Rouillé Y, Heumel S, Melo EM, Deruyter L, et al. Acetate Improves the Killing of Streptococcus pneumoniae by Alveolar Macrophages via NLRP3 Inflammasome and Glycolysis-HIF-1α Axis. Front In Immunol. (2022) 13:773261. doi: 10.3389/fimmu.2022.773261
150. Yao T, Dong X, Lv J, Fu L, and Li L. Propionate alleviated colitis by modulating iron homeostasis to inhibit ferroptosis and macrophage polarization. Int Immunopharmacol. (2025) 162:115151. doi: 10.1016/j.intimp.2025.115151
151. Wu L, Seon GM, Kim Y, Choi SH, Vo QC, and Yang H-C. Enhancing effect of sodium butyrate on phosphatidylserine-liposome-induced macrophage polarization. Inflammation Res. (2022) 71:641–52. doi: 10.1007/s00011-022-01563-5
152. Wu Y-L, Zhang C-H, Teng Y, Pan Y, Liu N-C, Liu P-X, et al. Propionate and butyrate attenuate macrophage pyroptosis and osteoclastogenesis induced by CoCrMo alloy particles. Mil Med Res. (2022) 9:46. doi: 10.1186/s40779-022-00404-0
153. Wang L, Gong Z, Zhang X, Zhu F, Liu Y, Jin C, et al. Gut microbial bile acid metabolite skews macrophage polarization and contributes to high-fat diet-induced colonic inflammation. Gut Microbes. (2020) 12:1–20. doi: 10.1080/19490976.2020.1819155
154. Liu J, Wei Y, Jia W, Can C, Wang R, Yang X, et al. Chenodeoxycholic acid suppresses AML progression through promoting lipid peroxidation via ROS/p38 MAPK/DGAT1 pathway and inhibiting M2 macrophage polarization. Redox Biol. (2022) 56:102452. doi: 10.1016/j.redox.2022.102452
155. Wang N, Hao Y, and Fu L. Trimethylamine-N-oxide promotes osteoclast differentiation and bone loss via activating ROS-dependent NF-κB signaling pathway. Nutrients. (2022) 14. doi: 10.3390/nu14193955
156. Chen Y, Yang C, Deng Z, Xiang T, Ni Q, Xu J, et al. Gut microbially produced tryptophan metabolite melatonin ameliorates osteoporosis via modulating SCFA and TMAO metabolism. J Pineal Res. (2024) 76:e12954. doi: 10.1111/jpi.12954
157. Mäkitie RE, Kämpe AJ, Taylan F, and Mäkitie O. Recent discoveries in monogenic disorders of childhood bone fragility. Curr Osteoporosis Rep. (2017) 15:303–10. doi: 10.1007/s11914-017-0388-6
158. Claes L, Recknagel S, and Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. (2012) 8:133–43. doi: 10.1038/nrrheum.2012.1
159. Stefanowski J, Lang A, Rauch A, Aulich L, Köhler M, Fiedler AF, et al. Spatial distribution of macrophages during callus formation and maturation reveals close crosstalk between macrophages and newly forming vessels. Front In Immunol. (2019) 10:2588. doi: 10.3389/fimmu.2019.02588
160. Pajarinen J, Lin T, Gibon E, Kohno Y, Maruyama M, Nathan K, et al. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials. (2019) 196:80–9. doi: 10.1016/j.biomaterials.2017.12.025
161. Schlundt C, Fischer H, Bucher CH, Rendenbach C, Duda GN, and Schmidt-Bleek K. The multifaceted roles of macrophages in bone regeneration: A story of polarization, activation and time. Acta Biomater. (2021) 133:46–57. doi: 10.1016/j.actbio.2021.04.052
162. Gaber T, Dziurla R, Tripmacher R, Burmester GR, and Buttgereit F. Hypoxia inducible factor (HIF) in rheumatology: low O2! See what HIF can do! Ann Rheumatic Diseases. (2005) 64:971–80. doi: 10.1136/ard.2004.031641
163. Qiu B, Yuan P, Du X, Jin H, Du J, and Huang Y. Hypoxia inducible factor-1α is an important regulator of macrophage biology. Heliyon. (2023) 9:e17167. doi: 10.1016/j.heliyon.2023.e17167
164. Li S, Yuan H, Yang X-Z, Xu X, Yu W, Wu Y, et al. Synergistic antitumor immunotherapy via mitochondria regulation in macrophages and tumor cells by an iridium photosensitizer. ACS Cent Sci. (2025) 11:441–51. doi: 10.1021/acscentsci.4c02156
165. Wang T, Liu H, Lian G, Zhang SY, Wang X, and Jiang C. HIF1alpha-induced glycolysis metabolism is essential to the activation of inflammatory macrophages. Mediators Inflamm. (2017) 2017:9029327. doi: 10.1155/2017/9029327
166. Ryan DG and O'Neill LAJ. Krebs cycle reborn in macrophage immunometabolism. Annu Rev Immunol. (2020) 38:289–313. doi: 10.1146/annurev-immunol-081619-104850
167. He W, Heinz A, Jahn D, and Hiller K. Complexity of macrophage metabolism in infection. Curr Opin Biotechnol. (2021) 68:231–9. doi: 10.1016/j.copbio.2021.01.020
168. De Santa F, Vitiello L, Torcinaro A, and Ferraro E. The role of metabolic remodeling in macrophage polarization and its effect on skeletal muscle regeneration. Antioxid Redox Signal. (2019) 30:1553–98. doi: 10.1089/ars.2017.7420
169. Williams NC and O'Neill LAJ. A role for the Krebs cycle intermediate citrate in metabolic reprogramming in innate immunity and inflammation. Front Immunol. (2018) 9:141. doi: 10.3389/fimmu.2018.00141
170. Keiran N, Ceperuelo-Mallafre V, Calvo E, Hernandez-Alvarez MI, Ejarque M, Nunez-Roa C, et al. SUCNR1 controls an anti-inflammatory program in macrophages to regulate the metabolic response to obesity. Nat Immunol. (2019) 20:581–92. doi: 10.1038/s41590-019-0372-7
171. Sun B, Long Y, Xu G, Chen J, Wu G, Liu B, et al. Acute hypoxia modulate macrophage phenotype accompanied with transcriptome re-programming and metabolic re-modeling. Front Immunol. (2025) 16:1534009. doi: 10.3389/fimmu.2025.1534009
172. Wang YT, Trzeciak AJ, Rojas WS, Saavedra P, Chen YT, Chirayil R, et al. Metabolic adaptation supports enhanced macrophage efferocytosis in limited-oxygen environments. Cell Metab. (2023) 35:316–331.e6. doi: 10.1016/j.cmet.2022.12.005
173. Dionisio F, Tomas L, and Schulz C. Glycolytic side pathways regulating macrophage inflammatory phenotypes and functions. Am J Physiol Cell Physiol. (2023) 324:C558–c564. doi: 10.1152/ajpcell.00276.2022
174. Zhong W-J, Liu T, Yang H-H, Duan J-X, Yang J-T, Guan X-X, et al. TREM-1 governs NLRP3 inflammasome activation of macrophages by firing up glycolysis in acute lung injury. Int J Biol Sci. (2023) 19:242–57. doi: 10.7150/ijbs.77304
175. Yang Y, Wu A, Deng AN, Liu H, Lan Q, Mazhar M, et al. Macrophages after myocardial infarction: Mechanisms for repairing and potential as therapeutic approaches. Int Immunopharmacol. (2024) 143:113562. doi: 10.1016/j.intimp.2024.113562
176. Shinohara I, Tsubosaka M, Toya M, Lee ML, Kushioka J, Murayama M, et al. C-C motif chemokine ligand 2 enhances macrophage chemotaxis, osteogenesis, and angiogenesis during the inflammatory phase of bone regeneration. Biomolecules. (2023) 13. doi: 10.3390/biom13111665
177. Schlundt C, Sass RA, Bucher CH, Bartosch S, Hauser AE, Volk HD, et al. Complex spatio-temporal interplay of distinct immune and bone cell subsets during bone fracture healing. Cells. (2023) 13. doi: 10.3390/cells13010040
178. Meyers CA, Lee S, Sono T, Xu J, Negri S, Tian Y, et al. A neurotrophic mechanism directs sensory nerve transit in cranial bone. Cell Rep. (2020) 31:107696. doi: 10.1016/j.celrep.2020.107696
179. Li Z, Meyers CA, Chang L, Lee S, Li Z, Tomlinson R, et al. Fracture repair requires TrkA signaling by skeletal sensory nerves. J Clin Invest. (2019) 129:5137–50. doi: 10.1172/jci128428
180. Xu J, Li Z, Tower RJ, Negri S, Wang Y, Meyers CA, et al. NGF-p75 signaling coordinates skeletal cell migration during bone repair. Sci Adv. (2022) 8:eabl5716. doi: 10.1126/sciadv.abl5716
181. Mouton AJ, Aitken NM, Moak SP, do Carmo JM, da Silva AA, Omoto ACM, et al. Temporal changes in glucose metabolism reflect polarization in resident and monocyte-derived macrophages after myocardial infarction. Front Cardiovasc Med. (2023) 10:1136252. doi: 10.3389/fcvm.2023.1136252
182. Willenborg S, Sanin DE, Jais A, Ding X, Ulas T, Nüchel J, et al. Mitochondrial metabolism coordinates stage-specific repair processes in macrophages during wound healing. Cell Metab. (2021) 33:2398–414. doi: 10.1016/j.cmet.2021.10.004
183. Chow SK, Wong CH, Cui C, Li MM, Wong RMY, and Cheung WH. Modulating macrophage polarization for the enhancement of fracture healing, a systematic review. J Orthop Translat. (2022) 36:83–90. doi: 10.1016/j.jot.2022.05.004
184. He P, Dai M, Li Z, Wang X, Liu H, He Y, et al. Effect of connexin 43 in LPS/IL-4-induced macrophage M1/M2 polarization: An observational study. Med (Baltimore). (2024) 103:e37811. doi: 10.1097/md.0000000000037811
185. Huang SC-C, Everts B, Ivanova Y, O'Sullivan D, Nascimento M, Smith AM, et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. (2014) 15:846–55. doi: 10.1038/ni.2956
186. Gonzalez-Hurtado E, Lee J, Choi J, Selen Alpergin ES, Collins SL, Horton MR, et al. Loss of macrophage fatty acid oxidation does not potentiate systemic metabolic dysfunction. Am J Physiol Endocrinol Metab. (2017) 312:E381–93. doi: 10.1152/ajpendo.00408.2016
187. Ghahremani Piraghaj M, Soudi S, Ghanbarian H, Bolandi Z, Namaki S, and Hashemi SM. Effect of efferocytosis of apoptotic mesenchymal stem cells (MSCs) on C57BL/6 peritoneal macrophages function. Life Sci. (2018) 212:203–12. doi: 10.1016/j.lfs.2018.09.052
188. Murphy PS, Wang J, Bhagwat SP, Munger JC, Janssen WJ, Wright TW, et al. CD73 regulates anti-inflammatory signaling between apoptotic cells and endotoxin-conditioned tissue macrophages. Cell Death Differ. (2017) 24:559–70. doi: 10.1038/cdd.2016.159
189. Szondy Z, Sarang Z, Kiss B, Garabuczi E, and Koroskenyi K. Anti-inflammatory mechanisms triggered by apoptotic cells during their clearance. Front Immunol. (2017) 8:909. doi: 10.3389/fimmu.2017.00909
190. Qin L, Yang J, Su X, Xilan L, Lei Y, Dong L, et al. The miR-21-5p enriched in the apoptotic bodies of M2 macrophage-derived extracellular vesicles alleviates osteoarthritis by changing macrophage phenotype. Genes Dis. (2023) 10:1114–29. doi: 10.1016/j.gendis.2022.09.010
191. Yurdagul A, Kong N, Gerlach BD, Wang X, Ampomah P, Kuriakose G, et al. ODC (Ornithine decarboxylase)-dependent putrescine synthesis maintains MerTK (MER tyrosine-protein kinase) expression to drive resolution. Arteriosclerosis Thrombosis Vasc Biol. (2021) 41:e144–59. doi: 10.1161/ATVBAHA.120.315622
192. McCubbrey AL, McManus SA, McClendon JD, Thomas SM, Chatwin HB, Reisz JA, et al. Polyamine import and accumulation causes immunomodulation in macrophages engulfing apoptotic cells. Cell Rep. (2022) 38:110222. doi: 10.1016/j.celrep.2021.110222
193. Zhao S-J, Kong F-Q, Jie J, Li Q, Liu H, Xu A-D, et al. Macrophage MSR1 promotes BMSC osteogenic differentiation and M2-like polarization by activating PI3K/AKT/GSK3β/β-catenin pathway. Theranostics. (2020) 10:17–35. doi: 10.7150/thno.36930
194. Zheng Z-Y, Jiang T, Huang Z-F, Chu B, Gu J, Zhao X, et al. Fatty acids derived from apoptotic chondrocytes fuel macrophages FAO through MSR1 for facilitating BMSCs osteogenic differentiation. Redox Biol. (2022) 53:102326. doi: 10.1016/j.redox.2022.102326
195. Ji L, Zhao X, Zhang B, Kang L, Song W, Zhao B, et al. Slc6a8-mediated creatine uptake and accumulation reprogram macrophage polarization via regulating cytokine responses. Immunity. (2019) 51:272–284.e7. doi: 10.1016/j.immuni.2019.06.007
196. Xiao B, Liu Y, Chandrasiri I, Adjei-Sowah E, Mereness J, Yan M, et al. Bone-targeted nanoparticle drug delivery system-mediated macrophage modulation for enhanced fracture healing. Small. (2024) 20:e2305336. doi: 10.1002/smll.202305336
197. Xu Z, Wu L, Tang Y, Xi K, Tang J, Xu Y, et al. Spatiotemporal regulation of the bone immune microenvironment via dam-like biphasic bionic periosteum for bone regeneration. Advanced Healthcare Materials. (2023) 12:e2201661. doi: 10.1002/adhm.202201661
198. Wu AC, Raggatt LJ, Alexander KA, and Pettit AR. Unraveling macrophage contributions to bone repair. Bonekey Rep. (2013) 2:373. doi: 10.1038/bonekey.2013.107
199. Lu X, Gao J, Bao W, Xu J, Sun X, Wang Y, et al. Interaction of macrophages with bone healing microenvironment: mechanism and biomaterials. Tissue Engineering Part B Rev. (2023) 30:285–98. doi: 10.1089/ten.TEB.2023.0157
200. Li C, Xu W, Li L, Zhou Y, Yao G, Chen G, et al. Concrete-inspired bionic bone glue repairs osteoporotic bone defects by gluing and remodeling aging macrophages. Adv Sci (Weinh). (2024) 11:e2408044. doi: 10.1002/advs.202408044
201. Chen L, Cheng S, Sun K, Wang J, Liu X, Zhao Y, et al. Changes in macrophage and inflammatory cytokine expressions during fracture healing in an ovariectomized mice model. BMC Musculoskelet Disord. (2021) 22:494. doi: 10.1186/s12891-021-04360-z
202. Ji H, Shen G, Liu H, Liu Y, Qian J, Wan G, et al. Biodegradable Zn-2Cu-0.5Zr alloy promotes the bone repair of senile osteoporotic fractures via the immune-modulation of macrophages. Bioact Mater. (2024) 38:422–37. doi: 10.1016/j.bioactmat.2024.05.003
203. Clark D, Brazina S, Miclau T, Park S, Hsieh CL, Nakamura M, et al. Age-related changes to macrophage subpopulations and TREM2 dysregulation characterize attenuated fracture healing in old mice. Aging Cell. (2024) 23:e14212. doi: 10.1111/acel.14212
204. Qin Y, Zhang Z, Guo X, Li W, Xia W, Ge G, et al. A bone-targeting hydrogen sulfide delivery system for treatment of osteoporotic fracture via macrophage reprogramming and osteoblast-osteoclast coupling. Advanced Funct Materials. (2025) 35. doi: 10.1002/adfm.202418822
205. Wang X, Liu C, Wang M, Yin B, Ge Y, Shu L, et al. Multi-modal microcarriers reprogram mitochondrial metabolism and activate efferocytosis in macrophages for osteoporotic bone repair. Biomaterials. (2025) 322. doi: 10.1016/j.biomaterials.2025.123384
206. Cao J, Han Z, Sun S, Li C, Pan X, Li J, et al. Multi-core functional microspheres for osteoporotic bone defects therapy: from local treatment to systematic relief. Advanced Funct Materials. (2025) 35. doi: 10.1002/adfm.202500115
207. Arcos D, Gómez-Cerezo N, Saiz-Pardo M, de Pablo D, Ortega L, Enciso S, et al. Injectable mesoporous bioactive nanoparticles regenerate bone tissue under osteoporosis conditions. Acta Biomater. (2022) 151:501–11. doi: 10.1016/j.actbio.2022.07.067
208. Casarrubios L, Polo-Montalvo A, Serrano MC, Feito MJ, Vallet-Regí M, Arcos D, et al. Effects of ipriflavone-loaded mesoporous nanospheres on the differentiation of endothelial progenitor cells and their modulation by macrophages. Nanomaterials (Basel). (2021) 11. doi: 10.3390/nano11051102
209. Peng H, Qiu X, Cheng M, Zhao Y, Song L, Zhu B, et al. Resveratrol-loaded nanoplatform RSV@DTPF promote alveolar bone regeneration in OVX rat through remodeling bone-immune microenvironment. Chem Eng J. (2023) 476. doi: 10.1016/j.cej.2023.146615
210. Liu A, Liao H, Zhang X, Deng S, Wang C, Zhao Z, et al. A novel tantalum scaffold promoting osteoporotic osseointegration by controlled immune regulation. Int J Of Bioprinting. (2025) 11:474–93. doi: 10.36922/ijb.8595
211. Zhou G, Zhou Q, Li R, Sheng S, Gao Q, Zhou D, et al. Synthetically engineered bacterial extracellular vesicles and IL-4-encapsulated hydrogels sequentially promote osteoporotic fracture repair. ACS Nano. (2025) 19:16064–83. doi: 10.1021/acsnano.5c03106
212. Chen X, Wang S, Zhang X, Yu Y, Wang J, and Liu C. Dual-function injectable fibrin gel incorporated with sulfated chitosan nanoparticles for rhBMP-2-induced bone regeneration. Appl Materials Today. (2022) 26. doi: 10.1016/j.apmt.2021.101347
213. Pan S, Li Y, Wang L, Guan Y, Xv K, Li Q, et al. Microenvironment-optimized gastrodin-functionalized scaffolds orchestrate asymmetric division of recruited stem cells in endogenous bone regeneration. J Of Nanobiotechnol. (2024) 22. doi: 10.1186/s12951-024-02886-7
214. Cui C, Zhao Y, Bai Z, Yan J, Qin D, Peng H, et al. The effect of antibacterial-osteogenic surface modification on the osseointegration of titanium implants: A static and dynamic strategy. ACS Biomaterials Sci Engineering. (2024) 10:4093–113. doi: 10.1021/acsbiomaterials.3c01756
215. Zhang Y, Wang L, Long X, Yan C, Wang Q, Huang D, et al. Multi-functional PEEK implants enhance osseointegration in OVX rat by remodeling the bone immune microenvironment. Colloids And Surfaces B-Biointerfaces. (2025) 245. doi: 10.1016/j.colsurfb.2024.114219
216. Du J, Bao X, Wen J, Tang C, Wang C, Shi C, et al. SrCO3@PCL/PDA composite scaffold promote osteoporotic bone regeneration through immune regulation. Composites Part B-Engineering. (2025) 298. doi: 10.1016/j.compositesb.2025.112406
217. Wang D, Chen M-W, Wei Y-J, Geng W-B, Hu Y, Luo Z, et al. Construction of wogonin nanoparticle-containing strontium-doped nanoporous structure on titanium surface to promote osteoporosis fracture repair. Advanced Healthcare Materials. (2022) 11:e2201405. doi: 10.1002/adhm.202201405
218. Qiu H, Xiong H, Zheng J, Peng Y, Wang C, Hu Q, et al. Sr-incorporated bioactive glass remodels the immunological microenvironment by enhancing the mitochondrial function of macrophage via the PI3K/AKT/mTOR signaling pathway. ACS Biomaterials Sci Engineering. (2024) 10:3923–34. doi: 10.1021/acsbiomaterials.4c00228
219. Du Y, Mai Y, Liu Z, Lin G, Luo S, Guo C, et al. Synergistic provoking of pyroptosis and STING pathway by multifunctional manganese-polydopamine nano-immunomodulator for enhanced renal cell carcinoma immunotherapy. Advanced Healthcare Materials. (2025) 14:e2500141. doi: 10.1002/adhm.202500141
220. Yang W, Li K, Pan Q, Huang W, Xiao Y, Lin H, et al. An engineered bionic nanoparticle sponge as a cytokine trap and reactive oxygen species scavenger to relieve disc degeneration and discogenic pain. ACS Nano. (2024) 18:3053–72. doi: 10.1021/acsnano.3c08097
221. Wang L, Yan C, Wang F, Wang Q, Huang D, Yang X, et al. Bioactive manganese-decorated titanium implants for osteoporotic bone regeneration. Materials Today Advances. (2024) 24. doi: 10.1016/j.mtadv.2024.100542
222. Wang Z, Xiang P, Xu Z, Gu M, Zhang R, Li Y, et al. Modulating osteoclast activity and immune responses with ultra-low-dose silver nanoparticle-loaded TiO(2) nanotubes for osteoporotic bone regeneration. J Funct Biomater. (2025) 16. doi: 10.3390/jfb16050162
223. Xu L, Fang J, Pan J, Qi H, Yin Y, He Y, et al. Zinc finger-inspired peptide-metal-phenolic nanointerface enhances bone-implant integration under bacterial infection microenvironment through immune modulation and osteogenesis promotion. Bioactive Materials. (2024) 41:564–76. doi: 10.1016/j.bioactmat.2024.08.009
224. Pinera-Avellaneda D, Buxadera-Palomero J, Ginebra M-P, Ruperez E, and Manero JM. Gallium-doped thermochemically treated titanium reduces osteoclastogenesis and improves osteodifferentiation. Front In Bioengineering And Biotechnol. (2023) 11:1303313. doi: 10.3389/fbioe.2023.1303313
225. Wu X, Xia Y, Dai H, Hong C, Zhao Y, Wei W, et al. Metabolic control during macrophage polarization by a citrate-functionalized scaffold for maintaining bone homeostasis. Adv Healthc Mater. (2024) 13:e2400770. doi: 10.1002/adhm.202400770
226. Chai H, Sang S, Luo Y, He R, Yuan X, and Zhang X. Icariin-loaded sulfonated polyetheretherketone with osteogenesis promotion and osteoclastogenesis inhibition properties via immunomodulation for advanced osseointegration. J Of Materials Chem B. (2022) 10:3531–40. doi: 10.1039/d1tb02802b
227. Yang C, Zhu K, Cheng M, Yuan X, Wang S, Zhang L, et al. Graphene oxide-decorated microporous sulfonated polyetheretherketone for guiding osteoporotic bone regeneration. J Of Controlled Release. (2024) 374:15–27. doi: 10.1016/j.jconrel.2024.07.054
228. Yang C, Zhu K, Liu G, Yuan X, Wang Q, and Zhang X. Functionalized microporous sulfonated polyetheretherketone: Osteogenesis and anti-osteoclastogenesis effects via ROS and NLRP3-mediated pyroptosis in macrophages. Materials Design. (2024) 241. doi: 10.1016/j.matdes.2024.112901
229. Zhou X, Jiang J, Dang J, Wang Y, Hu R, Shen C, et al. Intelligent supramolecular modification for implants: endogenous regulation of bone defect repair in osteoporosis. Advanced Materials. (2024) 36. doi: 10.1002/adma.202406227
230. Xia Z, Sun X, Mu C, Wang K, Ma W, Yang W, et al. An enhanced osseointegration of titanium implants by H2S sustained-release coating via promoting osteogenesis and inhibiting osteoclastogenesis. Advanced Healthcare Materials. (2025) 14. doi: 10.1002/adhm.202404940
231. Takanche JS, Kim JE, Kim JS, Lee MH, Jeon JG, Park IS, et al. Chitosan-gold nanoparticles mediated gene delivery of c-myb facilitates osseointegration of dental implants in ovariectomized rat. Artif Cells Nanomed Biotechnol. (2018) 46:S807–s817. doi: 10.1080/21691401.2018.1513940
232. Wang Z, Niu Y, Tian X, Yu N, Yin X, Xing Z, et al. Switching on and off macrophages by a "Bridge-burning" Coating improves bone-implant integration under osteoporosis. Advanced Funct Materials. (2021) 31. doi: 10.1002/adfm.202007408
233. Chen Q, Li J, Han F, Meng Q, Wang H, Qiang W, et al. A multifunctional composite hydrogel that rescues the ROS microenvironment and guides the immune response for repair of osteoporotic bone defects. Advanced Funct Materials. (2022) 32. doi: 10.1002/adfm.202201067
234. Jiang L, Wu Y, Xu Z, Hou M, Chen S, Cheng C, et al. Harnessing hydrogen sulfide in injectable hydrogels that guide the immune response and osteoclastogenesis balance for osteoporosis treatment. Materials Today Bio. (2024) 29. doi: 10.1016/j.mtbio.2024.101338
235. Li J, Li L, Wu T, Shi K, Bei Z, Wang M, et al. An injectable thermosensitive hydrogel containing resveratrol and dexamethasone-loaded carbonated hydroxyapatite microspheres for the regeneration of osteoporotic bone defects. Small Methods. (2024) 8. doi: 10.1002/smtd.202300843
236. Ye Y, Zhong H, Huang S, Lai W, Huang Y, Sun C, et al. Reactive oxygen species scavenging hydrogel regulates stem cell behavior and promotes bone healing in osteoporosis. Tissue Eng And Regenerative Med. (2023) 20:981–92. doi: 10.1007/s13770-023-00561-w
237. Ding W, Zhou Q, Lu Y, Wei Q, Tang H, Zhang D, et al. ROS-scavenging hydrogel as protective carrier to regulate stem cells activity and promote osteointegration of 3D printed porous titanium prosthesis in osteoporosis. Front In Bioengineering And Biotechnol. (2023) 11:1103611. doi: 10.3389/fbioe.2023.1103611
238. Jin X, Huang P, Cao X, Wang X, Mao Z, and Li Z. Kaempferol-loaded mechanical-enhanced aerogel for augmenting osteoporotic bone regeneration. Materials Design. (2025) 253. doi: 10.1016/j.matdes.2025.113996
239. Liu Q, Wen Y, Qiu J, Zhang Z, Jin Z, Cao M, et al. Local SDF-1α application enhances the therapeutic efficacy of BMSCs transplantation in osteoporotic bone healing. Heliyon. (2020) 6. doi: 10.1016/j.heliyon.2020.e04347
240. Sun B, Wang H, Xiao B, Yan H, Wu H, Zhang R, et al. Bioactive composite hydrogel with effects of robust promoting osteogenesis and immunomodulation for osteoporotic bone regeneration. Chem Eng J. (2023) 476. doi: 10.1016/j.cej.2023.146743
241. He Y, Zeng F, Quan H, Liu L, Dai J, Jiang H, et al. Strontium-loaded multifunctional gelatin methacryloyl hydrogels for type-H vascularized bone regeneration under osteoporotic conditions. Materials Today Bio. (2025) 32. doi: 10.1016/j.mtbio.2025.101909
242. Li J, Wu J, Liu F, Li X, Yu P, Pan H, et al. Magnesium-organic framework-loaded bisphosphonate-functionalized gel scaffolds for enhanced bone regeneration. ACS Biomaterials Sci Engineering. (2023) 9:6849–59. doi: 10.1021/acsbiomaterials.3c01080
243. Mi B, Xiong Y, Lu L, Liao J, Liu G, and Zhao Y. Macrophage-mediated fracture healing: Unraveling molecular mechanisms and therapeutic implications using hydrogel-based interventions. Biomaterials. (2024) 305:122461. doi: 10.1016/j.biomaterials.2023.122461
244. Wang L, Zhou J, Jiang S, Deng X, Zhang W, and Lin K. Piezoelectricity regulating immune osteogenesis in osteoporosis. BME Front. (2025) 6:146. doi: 10.34133/bmef.0146
245. Chu M, Sun Z, Fan Z, Yu D, Mao Y, and Guo Y. Bi-directional regulation functions of lanthanum-substituted layered double hydroxide nanohybrid scaffolds via activating osteogenesis and inhibiting osteoclastogenesis for osteoporotic bone regeneration. Theranostics. (2021) 11:6717–34. doi: 10.7150/thno.56607
246. Wang Y, Hao Z, Zhang Y, Hu Y, Chen T, Yan F, et al. Recombinant PTH modification: A new strategy for a multifunctional CaP material to enhance bone regeneration. Composites Part B-Engineering. (2022) 247. doi: 10.1016/j.compositesb.2022.110289
247. Huang YR and Ding SJ. Exploring processing-structure-property relationships of chemically precipitated strontium silicate particles for medical applications. J Mater Chem B. (2025) 13:3990–4005. doi: 10.1039/d4tb02656j
248. Xiong K, Wu T, Fan Q, Chen L, and Yan M. Novel reduced graphene oxide/zinc silicate/calcium silicate electroconductive biocomposite for stimulating osteoporotic bone regeneration. ACS Appl Mater Interfaces. (2017) 9:44356–68. doi: 10.1021/acsami.7b16206
249. Rumian L, Wolf-Brandstetter C, Rossler S, Reczynska K, Tiainen H, Haugen HJ, et al. Sodium alendronate loaded poly(L-lactide-co-glycolide) microparticles immobilized on ceramic scaffolds for local treatment of bone defects. Regenerative Biomaterials. (2021) 8:293–302. doi: 10.1093/rb/rbaa012
250. Feito MJ, Casarrubios L, Oñaderra M, Gómez-Duro M, Arribas P, Polo-Montalvo A, et al. Response of RAW 264.7 and J774A.1 macrophages to particles and nanoparticles of a mesoporous bioactive glass: A comparative study. Colloids Surf B Biointerfaces. (2021) 208:112110. doi: 10.1016/j.colsurfb.2021.112110
251. Gómez-Cerezo N, Casarrubios L, Saiz-Pardo M, Ortega L, de Pablo D, Díaz-Güemes I, et al. Mesoporous bioactive glass/ε-polycaprolactone scaffolds promote bone regeneration in osteoporotic sheep. Acta Biomater. (2019) 90:393–402. doi: 10.1016/j.actbio.2019.04.019
252. Xu X, Lu Y, Zhou L, He M, Zhuo J, Zhong Q, et al. Tuning osteoporotic macrophage responses to favour regeneration by Cu-bearing titanium alloy in Porphyromonas gingivalis lipopolysaccharide-induced microenvironments. Regenerative Biomaterials. (2021) 8. doi: 10.1093/rb/rbaa045
253. Xu X, Lu Y, Yang X, Du Z, Zhou L, Li S, et al. Copper-modified ti6Al4 V suppresses inflammatory response and osteoclastogenesis while enhancing extracellular matrix formation for osteoporotic bone regeneration. ACS Biomater Sci Eng. (2018) 4:3364–73. doi: 10.1021/acsbiomaterials.8b00736
254. Dai Q, Wang Z, Liu C, Chen X, and Cao X. High performance injectable Mg doped bioactive glass bone cement for the regulation of osteogenic immune microenvironment. Biomaterials Advances. (2024) 160. doi: 10.1016/j.bioadv.2024.213864
255. Li X-D, Yan D-W, Ren H-H, Zhang Q-Y, and Yan Y-G. Fabricating biodegradable calcium phosphate/calcium sulfate cement reinforced with cellulose: in vitro and in vivo studies. J Of Materials Chem B. (2023) 11:303–15. doi: 10.1039/d2tb02191a
256. Zhao H, Wang L, Xi K, Wang W, Jiang X, Wang J, et al. In-situ pore forming bone adhesives targeting disordered osteoimmune microenvironment and bone metabolic balance to promote osteoporotic fracture healing. Chem Eng J. (2025) 510. doi: 10.1016/j.cej.2025.161315
257. Huang L, Wang J, Yu J, Bian M, Xiang X, Han G, et al. Picein alleviates oxidative stress and promotes bone regeneration in osteoporotic bone defect by inhibiting ferroptosis via Nrf2/HO-1/GPX4 pathway. Environ Toxicol. (2024) 39:4066–85. doi: 10.1002/tox.24239
258. Jin Z, Wei Y, Zhou Z, Fan Z, Huang Y, and Liu D. Mechanistic Insights into Maltol-Mediated Reversal of Postmenopausal Osteoporosis via Regulation of CDK14 Ubiquitination in Macrophages. J Agric Food Chem. (2025) 73:11730–55. doi: 10.1021/acs.jafc.5c00545
259. Zhou X, Zhang Z, Jiang W, Hu M, Meng Y, Li W, et al. Naringenin is a potential anabolic treatment for bone loss by modulating osteogenesis, osteoclastogenesis, and macrophage polarization. Front In Pharmacol. (2022) 13:872188. doi: 10.3389/fphar.2022.872188
260. Zhou W, Lin Z, Xiong Y, Xue H, Song W, Yu T, et al. Dual-targeted nanoplatform regulating the bone immune microenvironment enhances fracture healing. ACS Appl Materials Interfaces. (2021) 13:56944–60. doi: 10.1021/acsami.1c17420
261. Qi Z, Ye G, Liu Z, Zhang J, Xie W, Li Y, et al. A review of osteoporotic vertebral fracture animal models. BioMed Eng Online. (2025) 24:40. doi: 10.1186/s12938-025-01372-x
Keywords: osteoporotic fractures, macrophages, immunometabolism, metabolic reprogramming, polarization, targeted therapy
Citation: Xing J, Li H, Xia H, Xia L and Zhao H (2025) Macrophages in osteoporotic fractures: from immunometabolic mechanisms to precision therapeutic approaches. Front. Endocrinol. 16:1698647. doi: 10.3389/fendo.2025.1698647
Received: 03 September 2025; Accepted: 05 November 2025; Revised: 28 October 2025;
Published: 21 November 2025.
Edited by:
Jannis Bodden, TUM Klinikum Rechts der Isar, GermanyReviewed by:
Qingyu Zhang, Shandong Provincial Hospital, ChinaZhiguo Bi, First Affiliated Hospital of Jilin University, China
Danielle Cabral Bonfim, Federal University of Rio de Janeiro, Brazil
Copyright © 2025 Xing, Li, Xia, Xia and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongzhou Zhao, MTM5MjAyMzMyNTZAMTM5LmNvbQ==
 Jiahui Xing
Jiahui Xing Haibo Li
Haibo Li Honggang Xia
Honggang Xia Lilei Xia
Lilei Xia Hongzhou Zhao
Hongzhou Zhao