- 1Reproductive Medicine Center, Henan Provincial People’s Hospital, Zhengzhou, China
- 2Reproductive Medicine Center, People’s Hospital of Zhengzhou University, Zhengzhou, China
- 3Department of Obstetrics and Gynecology, The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Purpose: This study aimed to investigate the effect of serum vitamin D levels on in vitro fertilization and embryo transfer (IVF-ET) in patients with polycystic ovary syndrome (PCOS).
Methods: This retrospective cohort study included patients with PCOS who underwent IVF therapy and received fresh embryo transfer for the first time. The enrolled cohort was divided into two groups based on serum 25-hydroxyvitamin D (25(OH)D) levels: Vitamin D deficiency (25(OH)D < 20 ng/mL) and vitamin D replete-insufficiency (25(OH)D ≥ 20 ng/mL). The primary outcome was clinical pregnancy. The secondary outcomes were the number of oocytes retrieved, MII oocytes, fertilized embryos, available embryos, high-quality embryos, blastocysts formed, and live birth rates.
Results: This study included 613 patients who underwent their first IVF-ET cycle. Clinical pregnancy rates were significantly lower in patients with PCOS who had vitamin D deficiency than in those who had vitamin D replete-insufficiency [58.3% (211/367) versus 67.1% (163/246); P = 0.029]. Logistic regression, adjusted for endometrial thickness, progesterone, and vitamin D levels, demonstrated that serum vitamin D ≥ 20 ng/mL was independently associated with higher clinical pregnancy rates than the vitamin D deficiency group (odds ratio 1.48; 95% confidence interval, 1.02–2.32; P = 0.032). However, vitamin D deficiency did not significantly affect live birth rates (P = 0.57). We found no significant differences in the number of oocytes, MII oocytes, fertilized embryos, and the percentage of top-quality embryos between the two groups.
Conclusion: This study suggests that vitamin D deficiency leads to lower clinical pregnancy rates in patients with PCOS undergoing IVF-ET. Furthermore, the serum vitamin D level is independently associated with clinical pregnancy rates in patients with PCOS undergoing IVF-ET.
Introduction
Polycystic ovary syndrome (PCOS), with a prevalence of 5%–20% in women of reproductive age, is a common reproductive endocrine disorder and a leading cause of female infertility (1, 2). PCOS commonly presents with a combination of oligo-anovulation, hyperandrogenism, and polycystic ovarian morphology. A subset of affected individuals also exhibits concomitant metabolic disturbances, including insulin resistance, dyslipidemia, and broader metabolic dysfunction. Anovulation is a major cause of infertility in women with PCOS. Even when ovulatory cycles occur, these patients are at an elevated risk of infertility and miscarriage, potentially due to compromised oocyte quality and/or endometrial receptivity (3).
The precursor of vitamin D is synthesized in the skin upon exposure to sunlight. It is then sequentially hydroxylated in the liver and kidneys to form its active form, calcitriol (1,25-dihydroxyvitamin D), which mediates its genomic effects by binding to the vitamin D receptor (VDR). Gene for VDR is located on chromosome 12. Active VDR can regulate the target gene expression in many cell types, including those of the immune system. VDRs are present in calcium-regulating tissues and throughout the reproductive system, including the ovary, endometrium, placenta, and decidua in females as well as in the testes of males (4–6). Evidence indicates that vitamin D regulates key reproductive processes, including decidualization, implantation, and human chorionic gonadotropin (hCG) secretion (7). VDR expression in reproductive organs suggests a possible regulatory role for vitamin D in female reproductive physiology. Animal studies have demonstrated that compromised VDR expression correlates with impaired folliculogenesis, uterine hypoplasia, and reproductive impairments, including infertility and pregnancy complications (8–10). Moreover, clinical studies indicated that low vitamin D level is correlated with significantly higher risks of gestational diabetes, preeclampsia, recurrent pregnancy loss, and delivering small-for-gestational-age infants (11, 12).
Vitamin D deficiency is significantly more prevalent in patients with PCOS than in healthy controls and has been correlated with insulin resistance (IR), obesity, and metabolic syndrome (13, 14). Women with PCOS and vitamin D deficiency have lower ovulation and higher pregnancy loss (15, 16). Vitamin D supplementation improves reproductive outcomes, including the menstrual cycle, follicular development, and pregnancy rates in this population (14). Supporting evidence has also indicated that vitamin D levels affect IVF outcomes in patients with PCOS (17). Conversely, other studies have demonstrated no statistically significant correlation between pregnancy rates and serum or follicular 25(OH)D levels in women who underwent IVF (18).
However, emerging evidence has suggested a potential link between vitamin D status and reproductive outcomes (11, 19–22). Vitamin D deficiency is implicated in PCOS pathogenesis, though its causal role remains unclear (23). While it may increase live birth rates in PCOS patients (1, 24) and improve IVF outcomes in those with insulin resistance (15, 25), it is unknown if this is due to vitamin D’s metabolic improvements or direct effects on reproductive system. Conversely, high-dose supplementation of vitamin D may impair follicular development (14). The heterogeneity of PCOS itself, coupled with variations in clinical interventions and geographic differences, may account for the divergent conclusions reported across studies. The objective of this study was to investigate whether vitamin D levels impact pregnancy outcomes in infertile PCOS patients undergoing IVF.
Materials and methods
Study cohort
This retrospective cohort study included infertile women with PCOS. The patients underwent their first IVF cycle at the Henan Provincial People’s Hospital between June 2018 and December 2022. This study was included among 2,281 with PCOS who underwent their first IVF cycle at our center. A total of 613 patients were included in the final analysis. The inclusion criteria were as follows: (1) Patients diagnosed with PCOS following the 2023 updated Rotterdam diagnostic criteria, and (2) undergoing IVF stimulation and receiving fresh embryo transfer for the first time. The exclusion criteria were as follows: (1) Congenital or acquired uterine anomalies, (2) untreated hydrosalpinx, and (3) progesterone > 2.0 ng/mL on hCG trigger day. From the patients identified in the hospital database and screened for eligibility, 613 patients were included in the study. The study protocol was approved by the Reproductive Ethics Committee of Henan Provincial People’s Hospital (SYSZLL-2019110401).
Patient data collection
In this study, the following patient information was collected: age, duration of infertility, type of infertility, body mass index (BMI), fasting insulin (FINS), fasting glucose (FGP), and homeostasis model assessment of insulin resistance (HOMA-IR), was collected. Baseline data, including measurements of basic follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), progesterone (P), prolactin (PRL), testosterone (T), and anti-Müllerian hormone (AMH) levels, antral follicle count (AFC), and basal endometrial thickness, were collected. Ovarian stimulation characteristics and embryological outcomes were collected, including the controlled ovarian stimulation regimen, gonadotropin (Gn) dosage, number of retrieved oocytes, MII oocytes, available embryos, top-quality embryos, fertilization embryos. Progesterone levels and endometrial thickness were assessed on both the hCG trigger and embryo transfer days.
According to the clinically accepted ranges for vitamin D deficiency (26, 27) (< 20 ng/mL), participants who completed the study were divided into the following two groups: (1) vitamin D deficiency (25(OH)D < 20 ng/mL) and (2) vitamin D replete-insufficiency group (25(OH)D ≥ 20 ng/mL).
Each embryo was graded based on developmental speed, degree of fragmentation, and the evenness of the cleavage sphere. Embryos with 7–9 blastomeres, uniform cytoplasm, regular morphology, and < 10% fragmentation were considered high-quality embryos.
The primary outcome was the CPR, defined as the presence of an intrauterine sac with a gestational sac and fetal pole, and cardiac activity, at 28 and 35 days after embryo transfer. The secondary outcomes were the number of oocytes retrieved, MII oocytes, fertilized embryos, available embryos, high-quality embryos, blastocyst formation, and live birth rate. Serum vitamin D levels were measured before the patients entered IVF cycles using electrochemiluminescence. All patients underwent IVF-ET cycles using standardized regimens for pituitary downregulation and controlled ovarian hyperstimulation. The initial Gn dose (Gonal-f; EMD Serono, Geneva, Switzerland; 100–300 IU/day) was determined based on age, baseline FSH levels, and AFC, and subsequently adjusted according to ovarian response. Ovarian monitoring involved serial transvaginal ultrasonography and serum measurements of FSH, LH, E2, and P. When two to three follicles reached or exceeded 17/18 mm, hCG was administered. The hCG dose ranged from 4,000 to 10,000 IU based on the clinician’s concerns regarding ovarian hyperstimulation syndrome (OHSS). Transvaginal ultrasound-guided oocyte retrieval was performed 36–38 h following hCG injection. Fertilization was achieved via conventional insemination or intracytoplasmic sperm injection (ICSI). Fresh embryos were transferred under ultrasound guidance on day 3 or 5 after oocyte retrieval, if the endometrial thickness was ≥ 8 mm and the endometrium had homogeneous echogenicity. The number of embryos transferred during each procedure was determined based on the developmental stage of the embryos and the total number of viable embryos available for transfer. In fresh embryo transfer cycles, luteal phase support was initiated on the day of oocyte retrieval, consisting of oral dydrogesterone tablets twice a day (Duphaston, 10 mg per tablet, produced by Solvay Pharmaceuticals, Netherlands) in combination with 8% progesterone vaginal sustained-release gel once daily at a dose of 90 mg (Crinone, 90 mg per tube, manufactured by Merck Serono). Serum β-hCG levels > 50 IU/L were diagnosed as biochemical pregnancy 14 days after embryo transfer. Ultrasound examinations were performed 28 and 35 days after embryo transfer to define clinical pregnancy and rule out ectopic pregnancy.
Data analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) software (version 20.0; IBM Corp., Armonk, NY, USA). Continuous variables are presented as means, and categorical variables are presented as frequencies. The independent-samples T test and Mann–Whitney U test were used to assess normal and skewed distributional variables, respectively. Univariate and multivariate logistic regression analyses were performed to explore the effects of vitamin D on CPRs. Statistical significance was defined as P < 0.05.
Results
Patients’ baseline and ovarian stimulation characteristics
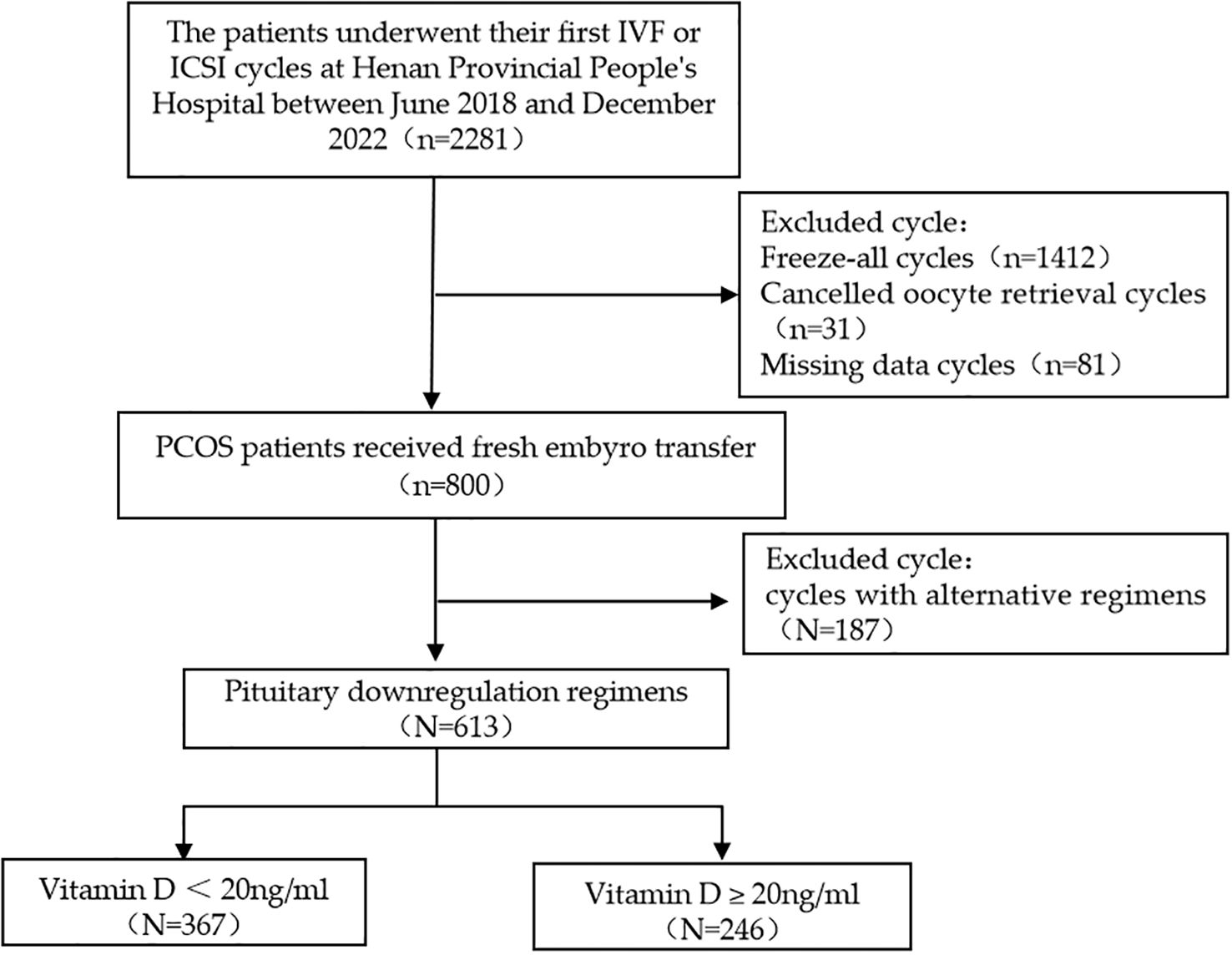
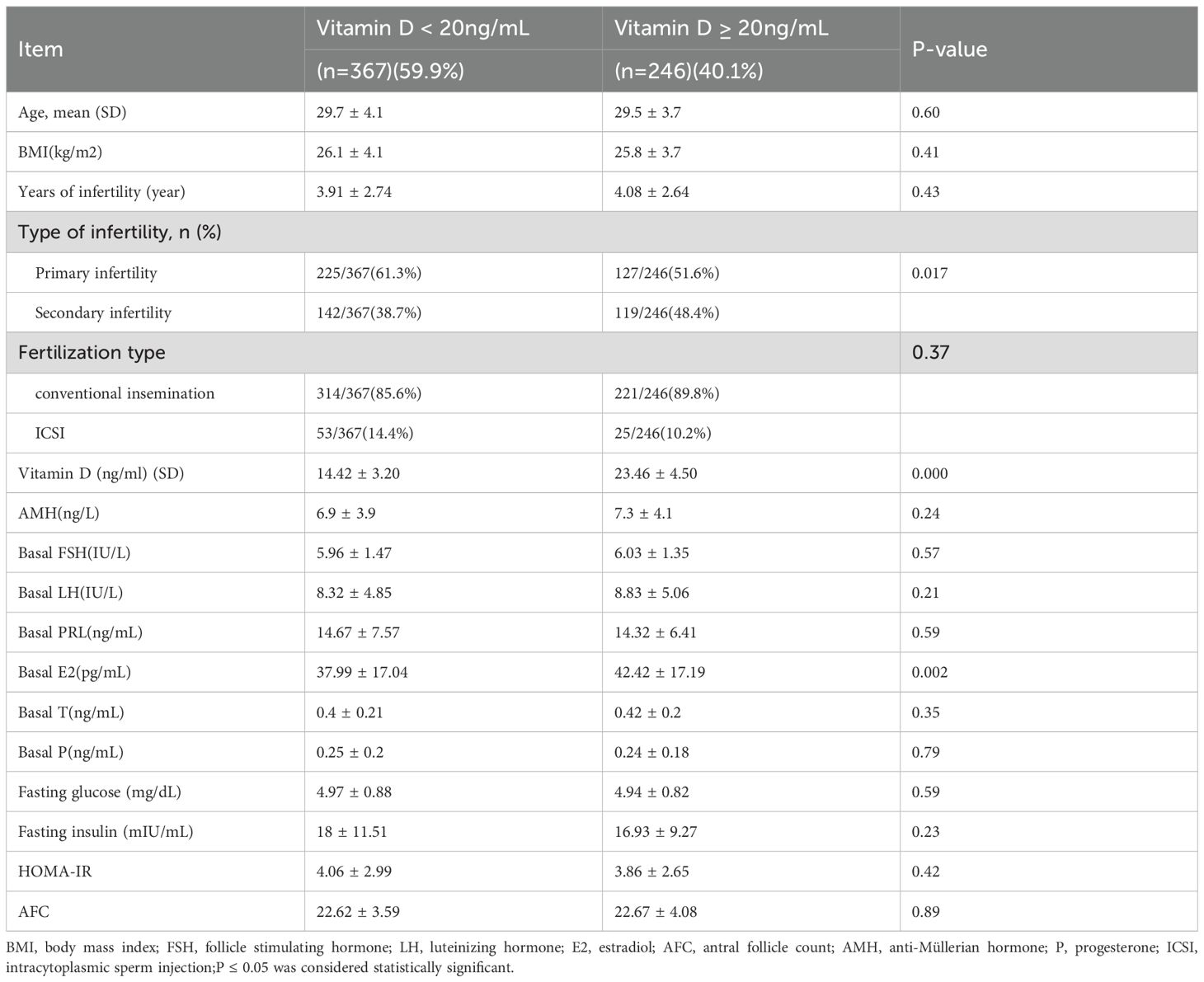
According to the inclusion and exclusion criteria, 613 patients were enrolled in this study (Figure 1). The baseline characteristics of the study population are presented in Table 1. Serum 25(OH)D levels were < 20 ng/mL in 367 women (59.9%) and ≥ 20 ng/mL in 246 women (40.1%). Baseline clinical characteristics, including age, BMI, duration of infertility, AMH, type of infertility, FGP, FINS, and HOMA-IR, were compared between women with vitamin D deficiency and those with 25(OH)D levels ≥ 20 ng/mL (Table 1). We found no statistically significant differences in Gn dose, duration of Gn treatment, and endometrial thickness on the day of hCG trigger between the two groups.
Embryological and clinical outcomes
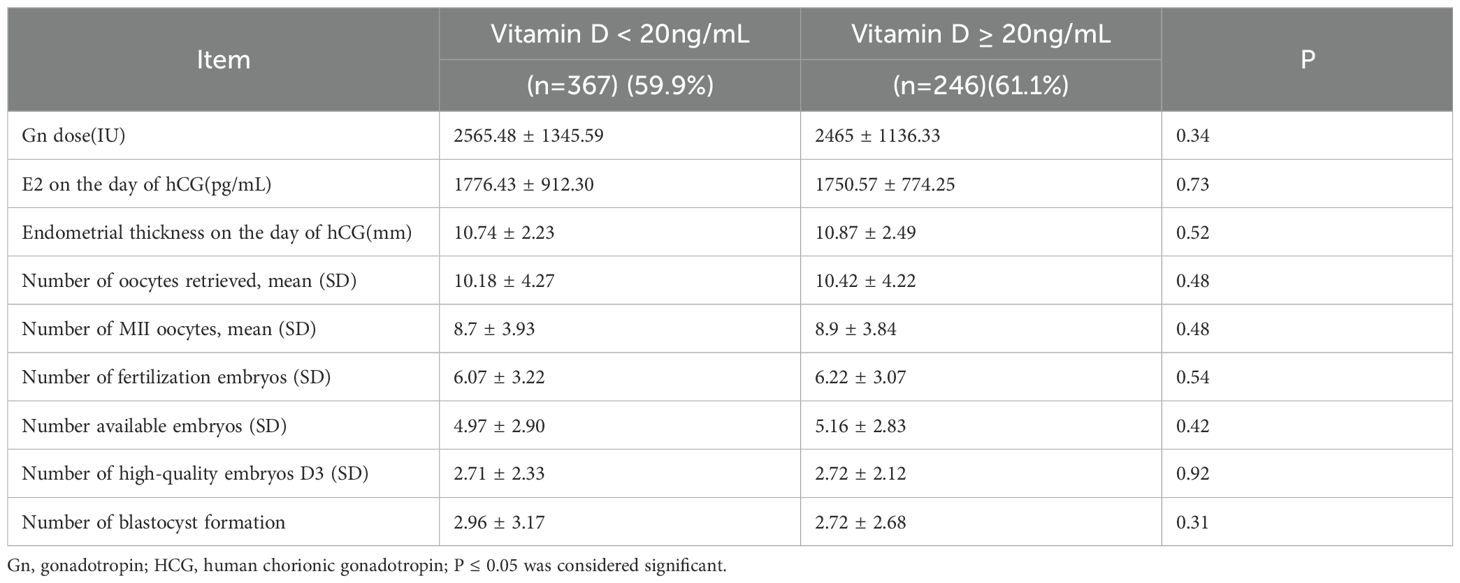
We observed no significant differences between vitamin D deficiency (25(OH)D < 20 ng/mL) and vitamin D replete-insufficiency (serum 25(OH)D ≥ 20 ng/mL) groups regarding the number of oocytes, MII oocytes, fertilized embryos, available embryos, number of high-quality embryos, and blastocyst formation (Table 2).
Pregnancy outcomes
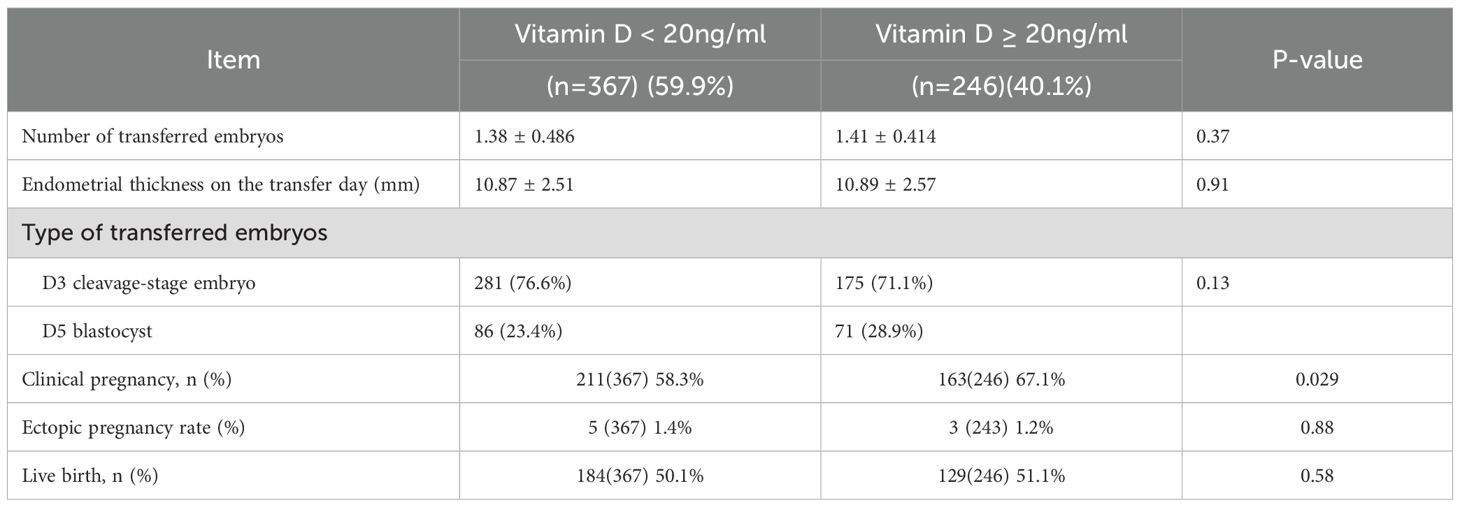
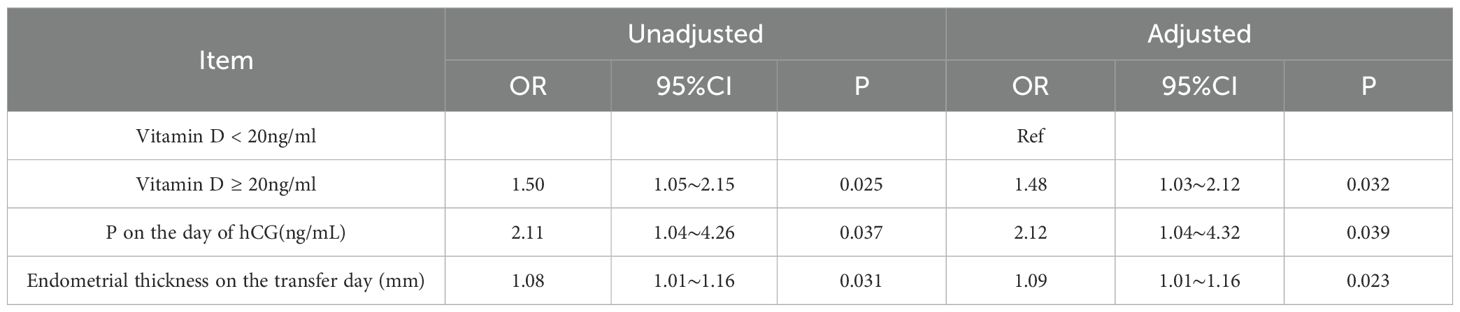
CPRs were significantly lower in patients with PCOS who had vitamin D deficiency than in those who had vitamin D replete-insufficiency [58.3% (211/367) versus 67.1% (163/246); P = 0.029; Table 3]. However, vitamin D deficiency did not significantly affect live birth rates (P = 0.58). The incidence of ectopic pregnancy did not differ between the two groups (P = 0.88). Additionally, there were no significant differences in the number or type of embryo transfers between the two groups. Logistic regression analysis was performed, incorporating serum vitamin D level, endometrial thickness on the day of embryo transfer, and progesterone level on the day of hCG administration. After adjusting for these covariates, a serum 25(OH)D level ≥ 20 ng/mL remained independently associated with increased CPR (Table 4). Specifically, the odds of achieving clinical pregnancy were 1.48 times higher in the vitamin D replete-insufficiency group than in the deficiency group (odds ratio [OR], 1.48; 95% confidence interval [CI], 1.02–2.32; P = 0.032).
Furthermore, logistic regression analysis revealed that endometrial thickness on the day of transfer and progesterone levels on the day of hCG administration were independently associated with CPRs (Table 4).
Discussion
In this cohort of women with PCOS who underwent IVF-ET treatment, vitamin D deficiency (serum 25(OH)D level < 20 ng/mL) was observed in 59.9% of the participants. This analysis revealed significantly reduced pregnancy rates among women with vitamin D deficiency compared to those with replete-insufficiency. Vitamin D levels did not affect IVF laboratory characteristics.
Vitamin D deficiency is a globally recognized condition globally. Research has indicated a correlation between vitamin D deficiency and an elevated risk of hypertension, infectious diseases, autoimmune diseases, and reproductive system disorders, including preeclampsia, miscarriage, and infertility (28). Halloran et al. reported that lower vitamin D levels impaired the fertility rate and affected neonatal growth in rats (8). Studies have revealed that vitamin D deficiency is more prevalent in PCOS, and lower vitamin D levels are a risk factor for PCOS development (10, 29–31). The association between vitamin D levels and IVF-ET outcomes, particularly CPRs, has been examined in several studies, with inconsistent evidence (10, 21, 32). Despite the ongoing controversy regarding the impact of vitamin D on assisted reproductive technology outcomes, this study investigated its role in infertile patients with PCOS by comparing fresh-cycle pregnancy rates across varying vitamin D levels.
Compromised vitamin D levels in PCOS have been associated with elevated AMH levels and metabolic abnormalities, such as elevated HOMA-IR, T level, and obesity (5, 33, 34). Additionally, vitamin D supplementation has been demonstrated to reduce AMH levels, thereby potentially enhancing folliculogenesis in PCOS (3, 33, 35). In this study, we found no association between vitamin D level and AMH, HOMA-IR, or BMI. Consistent with these research outcomes, a large retrospective investigation has indicated no correlation between AMH levels and vitamin D levels in patients with PCOS and the control group (23). Similarly, low serum vitamin D concentrations may be a consequence rather than the cause of obesity. It has been suggested that low levels of vitamin D and genes associated with reduced vitamin D concentrations exert a relatively minor influence on obesity (36). Vitamin D supplementation in women with PCOS could decrease the fasting glucose, HOMA-IR, and T level (37, 38). In this study, the two groups did not exhibit significant differences in HOMA-IR and T level. These findings are consistent with those of Bostanci et al. and Arslan et al. (23, 39). These non-significant differences may be attributed to the low vitamin D levels in both groups. Further studies are needed to determine whether additional vitamin D supplementation affects these indicators.
Studies have indicated that women with vitamin D deficiency and PCOS exhibit reduced ovulation rates during ovarian stimulation cycles (17, 19). This effect can be reversed with supplementation and potentially mediated by inducing vitamin D upregulation of the soluble receptor for advanced glycation end products, which binds circulating AGEs and inhibits their inflammatory deleterious effects in PCOS (13, 15, 40–42). Adequate vitamin D status is also associated with improved fertilization rates during IVF in women with PCOS (17). To date, the direct effect of vitamin D on the quality of human oocytes and embryos has not been investigated. In this study, the two groups shared a similar number of mature oocytes, 2PN embryos, available cleavage embryos, and blastocysts. These data indicate that vitamin D has no effect of vitamin D on egg maturation, fertilization rate, and embryo development. In slim PCOS patients, 25(OH)D3 correlated with embryo fertilization rates. However, 25(OH)D2 was associated with improved cleavage rate, top-quality day-3 embryos, and blastocyst formation in both PCOS and non-PCOS groups (17). This result suggests that the biological effects of different metabolites of vitamin D may vary across various systems, and their differential effects in the ovary require further clarification (17). The vitamin D levels detected in our study contained both 25(OH)D3 and 25(OH)D2. The varying conclusions across studies can be attributed to the specific vitamin D metabolite examined.
This study found no significant difference between vitamin D deficiency (< 20 ng/mL) and replete-insufficiency (≥ 20 ng/mL) groups in the number of embryos transferred or the type of embryos transferred. Likewise, endometrial thickness on the day of embryo transfer and progesterone levels on the day of hCG trigger were comparable between the two groups. However, CPR was significantly higher in the vitamin D replete-insufficiency group, suggesting that despite similar embryo quality and endometrial thickness, these patients may exhibit more favorable endometrial receptivity for embryo implantation. This aligns with studies that have reported a similar association between high vitamin D levels and improved reproductive outcomes. Sufficient serum levels of vitamin D (25(OH)D ≥ 30 ng/mL or 20 ng/mL) have been associated with higher CPRs in recipients of oocyte donation than in those with lower vitamin D levels (25(OH)D < 30 ng/mL or 20 ng/mL) (13, 43). Some studies have suggested that vitamin D may influence IVF outcomes by critically regulating endometrial homeobox 10 (HOXA10) expression (44). The HOXA10 gene is a key regulator of endometrial decidualization and immunomodulation essential for embryo implantation (45). A study reported that endometrial HOXA10 mRNA expression was significantly higher in women with PCOS who had sufficient serum vitamin D levels than in those with low serum vitamin D levels (44, 45). Besides its effect on HOXA10, vitamin D also plays an immunomodulatory role. In in vitro trophoblast experiments, vitamin D deficiency decreased the expression of hCG and increased the expression of tumor necrosis factor-alpha, interleukin-6, and interferon-gamma, thereby increasing the T-helper/T-cytotoxic cell ratio (46). Vitamin D deficiency is also associated with an increased prevalence of autoantibodies, including antiphospholipid antibodies, anti-thyroperoxidase antibodies, antibodies to nuclear antigens, and anti-ssDNA antibodies (47). Therefore, the higher pregnancy rate with vitamin D levels > 20 ng/mL may be partly due to the effect of vitamin D on endometrial receptivity.
In this study, we did not observe significant differences between the two groups in terms of basic metabolic parameters, such as BMI or fasting insulin levels, nor in embryonic data. Given the disparity in CPR between the two groups, we speculate that the underlying cause may be endometrial receptivity. The effect of vitamin D on endometrial receptivity can be determined only through an extensive combination of basic and clinical studies.
This study has several limitations. Maternal serum vitamin D levels were not monitored longitudinally until delivery, and potential vitamin D supplementation during this period was not assessed. Consequently, we did not draw definitive conclusions regarding the live birth rates. As a result, the findings regarding live birth rates should be interpreted cautiously. As this was a single-center retrospective analysis we cannot rule out the possibility of residual or unmeasured confounding factors, such as dietary habits, specific level of sun exposure, or using of supplements which could influence both vitamin D status and reproductive outcomes Moreover, studies have implied that vitamin D status is not a uniform condition, but is likely driven by distinct vitamin D metabolites, each exerting unique biological effects. This variability may explain the inconsistent findings and debates regarding the consequences of deficiency and the response to supplementation. Vitamin D measurement methods vary across studies, and vitamin D-binding protein polymorphisms have not been considered. Therefore, the relationship between vitamin D levels and pregnancy rate should be interpreted more carefully. These data indicate that vitamin D deficiency may not be a uniform condition, potentially influenced by various vitamin D metabolites and their resultant effects. This variability could explain the heterogeneity and ongoing debate regarding the impact of vitamin D deficiency and the efficacy of supplementation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Reproductive Ethics Committee of Henan Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
LY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. BH: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing, Conceptualization, Visualization. JK: Data curation, Formal analysis, Methodology, Writing – original draft. XX: Data curation, Formal analysis, Methodology, Writing – original draft. CZ: Conceptualization, Data curation, Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
We are grateful to the patients for their consent to use their data for research and analysis. We thank all the staff at our reproductive centers for their contributions to this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pal L, Zhang H, Williams J, Santoro NF, Diamond MP, Schlaff WD, et al. Vitamin D status relates to reproductive outcome in women with polycystic ovary syndrome: secondary analysis of a multicenter randomized controlled trial. J Clin Endocrinol Metab. (2016) 101:3027–35. doi: 10.1210/jc.2015-4352
2. Azziz R. Polycystic ovary syndrome. Obstet Gynecol. (2018) 132:321–36. doi: 10.1097/AOG.0000000000002698
3. Palomba S, Daolio J, and La Sala GB. Oocyte competence in women with polycystic ovary syndrome. Trends Endocrinol Metab. (2017) 28:186–98. doi: 10.1016/j.tem.2016.11.008
4. Muscogiuri G, Altieri B, De Angelis C, Palomba S, Pivonello R, Colao A, et al. Shedding new light on female fertility: The role of vitamin D. Rev Endocr Metab Disord. (2017) 18:273–83. doi: 10.1007/s11154-017-9407-2
5. Tian M, Zeng S, Cai S, Reichetzeder C, Zhang X, Yin C, et al. 25(OH)VitD and human endocrine and functional fertility parameters in women undergoing IVF/ICSI. Front Endocrinol. (2022) 13:986848. doi: 10.3389/fendo.2022.986848
6. Harkness LS and Bonny AE. Calcium and vitamin D status in the adolescent: key roles for bone, body weight, glucose tolerance, and estrogen biosynthesis. J Pediatr Adolesc Gynecol. (2005) 18:305–11. doi: 10.1016/j.jpag.2005.06.002
7. Knabl J, Vattai A, Ye Y, Jueckstock J, Hutter S, Kainer F, et al. Role of placental VDR expression and function in common late pregnancy disorders. Int J Mol Sci. (2017) 18:2340. doi: 10.3390/ijms18112340
8. Halloran BP and Deluca HF. Effect of vitamin D deficiency on fertility and reproductive capacity in the female rat. J Nutr. (1980) 110:1573–80. doi: 10.1093/jn/110.8.1573
9. Kinuta K, Tanaka H, Moriwake T, Aya K, Kato S, and Seino Y. Vitamin D is an important factor in estrogen biosynthesis of both female and Male gonads*. Endocrinology. (2000) 141:1317–24. doi: 10.1210/endo.141.4.7403
10. Cozzolino M, Busnelli A, Pellegrini L, Riviello E, and Vitagliano A. How vitamin D level influences in vitro fertilization outcomes: results of a systematic review and meta-analysis. Fertil Steril. (2020) 114:1014–25. doi: 10.1016/j.fertnstert.2020.05.040
11. Shahrokhi SZ, Ghaffari F, and Kazerouni F. Role of vitamin D in female reproduction. Clin Chim Acta. (2016) 455:33–8. doi: 10.1016/j.cca.2015.12.040
12. Rostami M, Tehrani FR, Simbar M, Bidhendi Yarandi R, Minooee S, Hollis BW, et al. Effectiveness of prenatal vitamin D deficiency screening and treatment program: a stratified randomized field trial. J Clin Endocrinol Metab. (2018) 103:2936–48. doi: 10.1210/jc.2018-00109
13. Banker M, Sorathiya D, and Shah S. Vitamin D deficiency does not influence reproductive outcomes of IVF-ICSI: a study of oocyte donors and recipients. J Hum Reprod Sci. (2017) 10:79. doi: 10.4103/jhrs.JHRS_117_16
14. Menichini D, Forte G, Orrù B, Gullo G, Unfer V, and Facchinetti F. The role of vitamin D in metabolic and reproductive disturbances of polycystic ovary syndrome: a narrative mini-review. Int J Vitam Nutr Res. (2022) 92:126–33. doi: 10.1024/0300-9831/a000691
15. Zhao J, Liu S, Wang Y, Wang P, Qu D, Liu M, et al. Vitamin D improves in-vitro fertilization outcomes in infertile women with polycystic ovary syndrome and insulin resistance. Minerva Med. (2019) 110(2019):199–208. doi: 10.23736/S0026-4806.18.05946-3
16. Aghadavod E, Mollaei H, Nouri M, and Hamishehkar H. Evaluation of relationship between body mass index with vitamin D receptor gene expression and vitamin D levels of follicular fluid in overweight patients with polycystic ovary syndrome. Int J Fertil Steril. (2017) 11(2017):105–111. doi: 10.22074/ijfs.2017.4704
17. Cunningham TK, Allgar V, Dargham SR, Kilpatrick E, Sathyapalan T, Maguiness S, et al. Association of vitamin D metabolites with embryo development and fertilization in women with and without PCOS undergoing subfertility treatment. Front Endocrinol. (2019) 10:13. doi: 10.3389/fendo.2019.00013
18. Firouzabadi RD, Rahmani E, Rahsepar M, and Firouzabadi MM. Value of follicular fluid vitamin D in predicting the pregnancy rate in an IVF program. Arch Gynecol Obstet. (2014) 289:201–6. doi: 10.1007/s00404-013-2959-9
19. Hasan HA, Barber TM, Cheaib S, and Coussa A. Preconception vitamin D level and In vitro fertilization: pregnancy outcome. Endocr Pract. (2023) 29:235–9. doi: 10.1016/j.eprac.2023.01.005
20. Lv SS, Wang JY, Wang XQ, Wang Y, and Xu Y. Serum vitamin D status and in vitro fertilization outcomes: a systematic review and meta-analysis. Arch Gynecol Obstet. (2016) 293:1339–45. doi: 10.1007/s00404-016-4058-1
21. Zhao J, Huang X, Xu B, Yan Y, Zhang Q, and Li Y. Whether vitamin D was associated with clinical outcome after IVF/ICSI: a systematic review and meta-analysis. Reprod Biol Endocrinol: RB&E. (2018) 16:13. doi: 10.1186/s12958-018-0324-3
22. Polyzos NP, Anckaert E, Guzman L, Schiettecatte J, Van Landuyt L, Camus M, et al. Vitamin D deficiency and pregnancy rates in women undergoing single embryo, blastocyst stage, transfer (SET) for IVF/ICSI. Hum Reprod. (2014) 29:2032–40. doi: 10.1093/humrep/deu156
23. Arslan E, Gorkem U, and Togrul C. Is there a relationship between vitamin D deficiency status and PCOS in infertile women? Geburtshilfe Frauenheilkd. (2019) 79:723–30. doi: 10.1055/a-0871-6831
24. Butts SF, Seifer DB, Koelper N, Senapati S, Sammel MD, Hoofnagle AN, et al. Vitamin D deficiency is associated with poor ovarian stimulation outcome in PCOS but not unexplained infertility. J Clin Endocrinol Metab. (2019) 104:369–78. doi: 10.1210/jc.2018-00750
25. Várbíró S, Takács I, Tűű L, Nas K, Sziva RE, Hetthéssy JR, et al. Effects of vitamin D on fertility, pregnancy and polycystic ovary syndrome—a review. Nutrients. (2022) 14:1649. doi: 10.3390/nu14081649
26. Amrein K, Scherkl M, Hoffmann M, Neuwersch-Sommeregger S, Köstenberger M, Tmava Berisha A, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. (2020) 74:1498–513. doi: 10.1038/s41430-020-0558-y
27. Demay MB, Pittas AG, Bikle DD, Diab DL, Kiely ME, Lazaretti-Castro M, et al. Vitamin D for the prevention of disease: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2024) 109:1907–47. doi: 10.1210/clinem/dgae290
28. Grant W, Wimalawansa S, Pludowski P, and Cheng R. Vitamin D: evidence-based health benefits and recommendations for population guidelines. Nutrients. (2025) 17:277. doi: 10.3390/nu17020277
29. Bindayel IA. Low vitamin D level in saudi women with polycystic ovary syndrome. Front Nutr. (2021) 8:611351. doi: 10.3389/fnut.2021.611351
30. Rudick B, Ingles S, Chung K, Stanczyk F, Paulson R, and Bendikson K. Characterizing the influence of vitamin D levels on IVF outcomes. Hum Reprod. (2012) 27:3321–7. doi: 10.1093/humrep/des280
31. Hu R, Li L, Liang L, Qi Y, Ma X, and Yang Y. 25(OH)D3 improves granulosa cell proliferation and IVF pregnancy outcomes in patients with endometriosis by increasing G2M+S phase cells. Reprod Biol Endocrinol. (2023) 21:115. doi: 10.1186/s12958-023-01165-8
32. Chu J, Gallos I, Tobias A, Tan B, Eapen A, and Coomarasamy A. Vitamin D and assisted reproductive treatment outcome: a systematic review and meta-analysis. Hum Reprod (Oxf Engl). (2018) 33:65–80. doi: 10.1093/humrep/dex326
33. Dastorani M, Aghadavod E, Mirhosseini N, Foroozanfard F, Zadeh Modarres S, Amiri Siavashani M, et al. The effects of vitamin D supplementation on metabolic profiles and gene expression of insulin and lipid metabolism in infertile polycystic ovary syndrome candidates for in vitro fertilization. Reprod Biol Endocrinol. (2018) 16:94. doi: 10.1186/s12958-018-0413-3
34. Ozkan S, Jindal S, Greenseid K, Shu J, Zeitlian G, Hickmon C, et al. Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertil Steril. (2010) 94:1314–9. doi: 10.1016/j.fertnstert.2009.05.019
35. Moridi I, Chen A, Tal O, and Tal R. The association between vitamin D and anti-müllerian hormone: a systematic review and meta-analysis. Nutrients. (2020) 12:1567. doi: 10.3390/nu12061567
36. Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki LT, et al. Causal relationship between obesity and vitamin D status: Bi-directional mendelian randomization analysis of multiple cohorts. PloS Med. (2013) 10:e1001383. doi: 10.1371/journal.pmed.1001383
37. March WA, Moore VM, Willson KJ, Phillips DIW, Norman RJ, and Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. (2010) 25:544–51. doi: 10.1093/humrep/dep399
38. Goodarzi MO, Dumesic DA, Chazenbalk G, and Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. (2011) 7:219–31. doi: 10.1038/nrendo.2010.217
39. Bostanci EI, Ozler S, Yilmaz NK, and Yesilyurt H. Serum 25-hydroxy vitamin D levels in turkish adolescent girls with polycystic ovary syndrome and the correlation with clinical/biochemical parameters. J Pediatr Adolesc Gynecol. (2018) 31:270–3. doi: 10.1016/j.jpag.2017.07.008
40. Garg D, Grazi R, Lambert-Messerlian GM, and Merhi Z. Correlation between follicular fluid levels of sRAGE and vitamin D in women with PCOS. J Assist Reprod Genet. (2017) 34:1507–13. doi: 10.1007/s10815-017-1011-6
41. Li J, Li M, Li Y, Zhao X, Guan Y, Zhang Y, et al. Do serum vitamin D levels affect assisted reproductive outcomes and perinatal outcomes in young non-PCOS patients? A retrospective study. Arch Gynecol Obstet. (2024) 309:2099–106. doi: 10.1007/s00404-024-07410-8
42. Merhi Z. Advanced glycation end products and their relevance in female reproduction. Hum Reprod. (2014) 29:135–45. doi: 10.1093/humrep/det383
43. Rudick BJ, Ingles SA, Chung K, Stanczyk FZ, Paulson RJ, and Bendikson KA. Influence of vitamin D levels on in vitro fertilization outcomes in donor-recipient cycles. Fertil Steril. (2014) 101:447–52. doi: 10.1016/j.fertnstert.2013.10.008
44. Shilpasree A, Kulkarni VB, Shetty P, Bargale A, Goni M, Oli A, et al. Induction of endometrial HOXA 10 gene expression by vitamin D and its possible influence on reproductive outcome of PCOS patients undergoing ovulation induction procedure. Indian J Endocrinol Metab. (2022) 26:252–8. doi: 10.4103/ijem.ijem_90_22
45. Ersahin SS and Ersahin A. Serum 25-hydroxyvitamin D correlates with endometrial HOXA10 mRNA expression. Eur Rev Med Pharmacol Sci. (2022) 26:3483–6. doi: 10.26355/eurrev_202205_28842
46. Díaz L, Noyola-Martínez N, Barrera D, Hernández G, Avila E, Halhali A, et al. Calcitriol inhibits TNF-α-induced inflammatory cytokines in human trophoblasts. J Reprod Immunol. (2009) 81:17–24. doi: 10.1016/j.jri.2009.02.005
Keywords: vitamin D, PCOS (polycystic ovary syndrome), infertility, IVF (in vitro fertilization), fresh embryo transfer (ET)
Citation: Yu L, Hu B, Kong J, Xu X and Zhang C (2025) Effect of vitamin D deficiency on clinical pregnancy outcomes in women with polycystic ovary syndrome undergoing in vitro fertilization. Front. Endocrinol. 16:1710004. doi: 10.3389/fendo.2025.1710004
Received: 21 September 2025; Accepted: 30 October 2025;
Published: 24 November 2025.
Edited by:
Hana Fakhoury, Alfaisal University, Saudi ArabiaReviewed by:
Tania M. Ortiga, Federal University of Rio de Janeiro, BrazilWilliam B. Grant, Sunlight Nutrition and Health Research Center, United States
Ali H. Hajeer, King Abdulaziz Medical City, Saudi Arabia
Copyright © 2025 Yu, Hu, Kong, Xu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cuilian Zhang, bHVja3l6Y2xAcXEuY29t
 Lan Yu
Lan Yu Bin Hu
Bin Hu Jing Kong
Jing Kong Xiaohang Xu
Xiaohang Xu Cuilian Zhang
Cuilian Zhang