Abstract
Introduction:
The prevalence of type 2 diabetes mellitus (T2DM) has more than doubled in the past 20 years. Most T2DM cases are preventable if risk factors are eliminated early. Hypertriglyceridemia (HTG) is also a potential but modifiable risk factor, and has a high prevalence as well. We aimed to investigate the dose-dependent effect of HTG on the development of T2DM.
Methods:
We carried out a systematic search in three databases (MEDLINE, Embase, and CENTRAL) on 9 November 2023. We investigated an adult population with different triglyceride levels (exposure). The outcome of interest was the development of T2DM. Pooled hazard ratio (HR), odds ratio (OR), and mean differences (MDs) with 95% confidence intervals (CIs) were calculated using a random-effects model. Risk of bias was assessed with the Quality In Prognosis Studies (QUIPS) tool. The protocol was registered in PROSPERO (CRD42023471288).
Results:
We identified 31,098 articles and included 101 in our meta-analysis. We found that people with HTG had more than a 1.5-fold higher risk (HR: 1.73 [1.31; 2.29]) of developing T2DM. Those who had their TG levels between 1.7 and 2.3 mmol/L had a 42% higher risk (HR: 1.42 [1.13; 1.79]), while those with TG levels above 2.3 mmol/L had an even higher risk for T2DM (HR: 1.82 [1.18; 2.87]) compared with patients with TG levels below 1.7 mmol/L. When investigating the hypertriglyceridemic waist phenotype, we found that only those with increased waist circumference had a higher risk in both sex groups among the different phenotype groups (female: HR: 2.86 [1.59; 5.14], male: HR: 3.31 [1.57; 7.27]).
Conclusion:
HTG is a dose-dependent risk factor for T2DM. Elevated waist circumference may have an even more important role in the development of T2DM than HTG.
Systematic review registration:
https://www.crd.york.ac.uk/prospero/, identifier CRD42023471288.
Highlights
Hypertriglyceridemia is a dose-dependent risk factor for T2DM.
Elevated waist circumference is a risk factor for T2DM and may have an even more important role in the development T2DM than hypertriglyceridemia.
Higher triglyceride-glucose index and triglyceride-to-high-density lipoprotein cholesterol ratio are associated with a higher risk of T2DM.
Introduction
According to the International Diabetes Foundation, in 2021, diabetes mellitus (DM) impacted over 530 million adults worldwide, and is projected to reach 783 million by 2045 (1). Type 2 diabetes mellitus (T2DM) accounts for more than 90% of all DM cases (1) and poses substantial health and economic challenges, including rising healthcare costs, an increased burden on healthcare systems, and a higher risk of complications, such as cardiovascular disease (CVD), neuropathy, retinopathy, and nephropathy, reducing quality of life and increasing mortality (1–3). The condition is caused by genetic, environmental, and lifestyle factors, particularly poor diet, physical inactivity, and obesity (1, 4). Notably, approximately 80% of T2DM cases could be prevented by addressing modifiable risk factors, including high body mass index (BMI) and inadequate fiber intake (4).
These same modifiable risk factors can also contribute to the development of metabolic syndrome, a constellation of metabolic abnormalities including central obesity, hypertension, dyslipidemia, and impaired glucose regulation (5). This syndrome is a well-established predictor of cardiovascular morbidity and mortality, and among its components, hypertriglyceridemia (HTG) plays a key role in promoting atherogenic dyslipidemia and systemic inflammation (5–8). Furthermore, severe HTG has been linked to acute pancreatitis, underscoring the clinical importance of managing elevated triglyceride levels (9).
In addition to its established associations with CVD and pancreatitis, HTG has been identified as a potential modifiable risk factor for T2DM (10–12). Elevated triglyceride levels have been associated with increased insulin resistance (IR) and beta-cell dysfunction, both of which are pivotal in the pathogenesis of T2DM (13).
In addition to triglyceride levels, several triglyceride-related indices are increasingly used in clinical practice and research as predictors of CVD and IR (14–16).
The triglyceride-glucose index (TyG), a surrogate marker of IR, has emerged as a potential predictor of T2DM. Although it is associated with the development of T2DM, its relationship appears to be non-linear, suggesting possible thresholds influencing its predictive value (14, 17, 18).
The triglyceride-to-high-density lipoprotein cholesterol ratio (TG/HDL-C), another triglyceride-related index, has demonstrated a linear association with the incidence of T2DM, making it a straightforward risk indicator of risk escalation (19, 20, 115).
In addition, the hypertriglyceridemic waist (HTGW) phenotype, characterized by elevated triglyceride levels and abdominal obesity, has been linked to IR and is investigated as a potential indicator of T2DM. This phenotype underscores the interplay between dyslipidemia and central obesity in metabolic dysfunction (16, 21, 22).
Triglyceride itself, a simple and accessible biomarker, offers significant utility in daily clinical practice without requiring complex calculations. Similarly, triglyceride-related indices (e.g., TyG and TG/HDL-C ratio) are easily computed and available for routine screening.
However, despite extensive research into the relationship between HTG and T2DM, the dose-dependent role of triglycerides in T2DM development has not been comprehensively evaluated. The existing literature predominantly focuses on dyslipidemia as a consequence of IR or diabetes, leaving a gap in understanding the causal and dose–response relationships in the prediabetic state (23, 24).
By identifying thresholds and dose-dependent relations, clinicians could better estimate the risk of T2DM and implement targeted interventions, such as scheduling screening visits with optimal frequency and tailoring preventive measures. We hypothesize that HTG may be a dose-dependent risk factor for T2DM, and its associated indices may be predictors of T2DM. Therefore, we aimed to investigate the dose-dependent effect of HTG on the development of T2DM.
Methods
Our systematic review and meta-analysis followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines (25) (Supplementary Table S1) and the Cochrane Handbook (26). The study protocol was registered with PROSPERO (27) under accession number CRD42023471288. The protocol deviation related to the PROSPERO registration is also reported in detail in the Supplementary Materials (Methods S1). The investigation was conducted under the Systems Education education-research model run by the Centre for Translational Medicine at Semmelweis University, Budapest (28).
Eligibility criteria and definitions
Our research aimed to investigate the effect of different triglyceride levels on the development of T2DM. We used the PEO framework to define eligibility criteria (29).
Population (P): adult patients without DM at baseline. Exposure (E): different levels of TG and triglyceride-related indices, such as TyG index, TG/HDL-C ratio, and HTGW phenotype. We used the definition of HTG, as reported in the guidelines and the articles included: >1.7 mmol/L (>150 mg/dL) (3, 4). TyG was calculated as the natural logarithm of [fasting glucose (mg/dL) × fasting triglycerides (mg/dL)/2]. Different cutoff values were used in the cohorts to categorize HTGW phenotypes; therefore, we used the category established based on the research for our analysis. In the case of HTGW, the population is divided into four groups: (1) normal TG level and normal waist circumference (control); (2) high TG level and normal waist circumference (HTNW); (3) normal TG and elevated waist circumference (NTGW); and (4) high TG level and elevated waist circumference.
Outcome (O): development of T2DM or prediabetes. Most studies used the following criteria to diagnose T2DM: fasting plasma glucose ≥7.0 mmol/L (126 mg/dL) and/or HbA1c ≥6.5% and/or the use of insulin or oral hypoglycemic agents. In some cases, the definition of newly diagnosed T2DM was different or missing; this uncertainty was taken into account during the risk of bias assessment.
Studies conducted in all publication years and all languages were included. Conference abstracts, reviews, case reports, and case series were excluded during the selection.
Information sources
Our systematic search was conducted on 9 November 2023 in three primary databases: MEDLINE (via Pubmed), Embase, and Cochrane (CENTRAL) without filters or restrictions. We used three domains: one for T2DM, one for TG, and a third for the required study types. For each database, we used a specific search key, which can be found in Supplementary Table S2.
Selection process
To remove duplicate records, we used the duplication removal tools of Endnote, followed by manual duplicate removal. We dealt with duplicate-free articles using the Rayyan tool. During the selection process, each article was reviewed by two independent authors (LH and DD/MB/AM/LV). Selection disagreements were resolved by consensus after discussion. First, title and abstract selection was performed, and then the studies included were screened by full text, where the reason for exclusion was recorded. Cohen’s kappa was calculated for each step to demonstrate the inter-rater agreement. Finally, we used the citation chaser tool for backward citation chase (Reference Chase) and dealt with the newly found articles similarly.
Data collection process
Data were extracted independently from the eligible articles by two authors (LH and DD/AM/LV) and entered into standardized Excel spreadsheets. In case of disagreement, the conflict was resolved by discussion. If an article lacked relevant data, a letter of inquiry was sent to the corresponding author for further information.
The following data were extracted: information on the publication [e.g., year and digital object identifier (DOI) number], country, study period, number of centers, study design, and follow-up time; information on the population (e.g., sex distribution, age, and BMI), the factor measured (e.g., TyG and HTGW), the outcome investigated (e.g., prediabetes and T2DM), and their definitions and assessment methods; the value of the factor as exposure and comparator factor; the statistical results reported [e.g., multivariate hazard ratios (HRs) and univariate odds ratios (ORs)]; and methods.
Study risk of bias assessment
Two co-authors (LH and DD/MB/AM/LV) independently assessed the risk of bias using the Quality In Prognosis Studies (QUIPS) tool (30). Disagreements were resolved by discussion, conducted separately for each outcome and factor. Further details on the Risk of Bias assessment methodology can be found in the Supplementary Materials (Methods S4).
Data synthesis
As we assumed considerable heterogeneity among the underlying population of the reported study results by the nature of the question under investigation, random-effects models were used in a frequentist framework.
Crude and adjusted OR were used as effect size measures for categorical outcomes. To calculate the crude ORs, we extracted the total number of patients and those with the event of interest in each group separately from the studies (called “raw data”). For adjusted OR and when the studies reported the crude OR directly, we used the published value with its confidence interval (CI) in the analysis. The difference between the means (MD) was used as an effect size measure for continuous outcomes. To calculate the study MDs and pooled MD, we extracted or estimated the sample size, the mean, and the corresponding standard deviation (SD) from each study (separately for each group). For time-to-event data, we used adjusted HR with 95% CI as the main measure of effect between the two groups of interest. We summarized the findings in forest plots.
Between-study heterogeneity was also described by the between-study variance (τ2) and Higgins and Thompson’s I (2–31) statistics. We directly reported the prediction interval only if the study number was over eight to ensure a meaningful estimation.
Small study publication bias was assessed by visual inspection of funnel plots and calculating Egger (for continuous, time-to-event data) or Harbord (for dichotomous) test p-value (32). However, we kept in mind that the diagnostic assessment of the test was limited, with less than 10 studies.
Potential outlier publications were explored using different influence measures and plots as recommended by Harrer et al. (33).
All statistical analyses were calculated by R software (34) using the meta (35) (v7.0.0) package for basic meta-analysis calculations and plots, and the dmetar (36) (v0.1.0) package for additional influental analysis calculations and plots. The package metafor (37) (v4.6.0) was used for the multilevel model.
For more details on calculations, data synthesis, publication bias assessment, and influential analyses, please read the detailed description in Supplementary Methods S3.
Results
Search and selection
Altogether, 31,098 articles were identified by searching three databases. After the selection process and reference checking, we included 145 studies, but only 101 articles were eligible for quantitative analysis. Because of overlapping populations, insufficient data for statistical analysis, and lack of poolable categories, the other 44 articles were included in the systematic review section and/or were used in forest plots only for visualization purposes. In the case of overlapping populations, the larger population and more recent publications were preferred, as described in more detail in the Supplementary Materials (Methods S2).
The PRISMA flow diagram with the calculated Cohen’s kappa values (Figure 1) provides detailed information on the selection (25).
Figure 1

PRISMA 2020 flow diagram for new systematic reviews that included searches of databases, registers, and other sources (25).
Basic characteristics of studies included
The basic characteristics of the studies included are provided in the Supplementary Materials (Supplementary Tables S3A, B), separately for articles included in the quantitative synthesis and separately for articles included only in the qualitative synthesis.
Triglyceride level
First, we analyzed patient groups with and without HTG (>1.7 mmol/L or = 150 mg/dL cutoff value). We found that according to our eight included (38–45) cohort studies with a total population of 19,525,661 participants, those with HTG at baseline had a more than 1.5-fold higher risk (HR: 1.73 [1.31; 2.29]) of the development of T2DM during the follow-up period (Figure 2). As we aimed to investigate the dose-dependent effect of TG level, we further analyzed two different TG level groups. According to the three included studies with a sample size of 29,485, participants with TG levels of 1.7–2.3 mmol/L had an almost 1.5-fold higher risk of incident diabetes (HR: 1.42 [1.13; 1.79]) and those with even higher TG levels (>2.3 mmol/L) had an even higher risk of developing diabetes (HR: 1.84 [1.18; 2.87]) compared with patients with TG levels below 1.7 mmol/L (Figure 2) (12, 46). This trend was also observed in the articles reporting OR (1.7–2.3 mmol/L: OR: 1.5 [1.01; 2.22], >2.3 mmol/L: OR: 2.44 [1.56; 3.80]) (Supplementary Figure S2) (47–57).
Figure 2
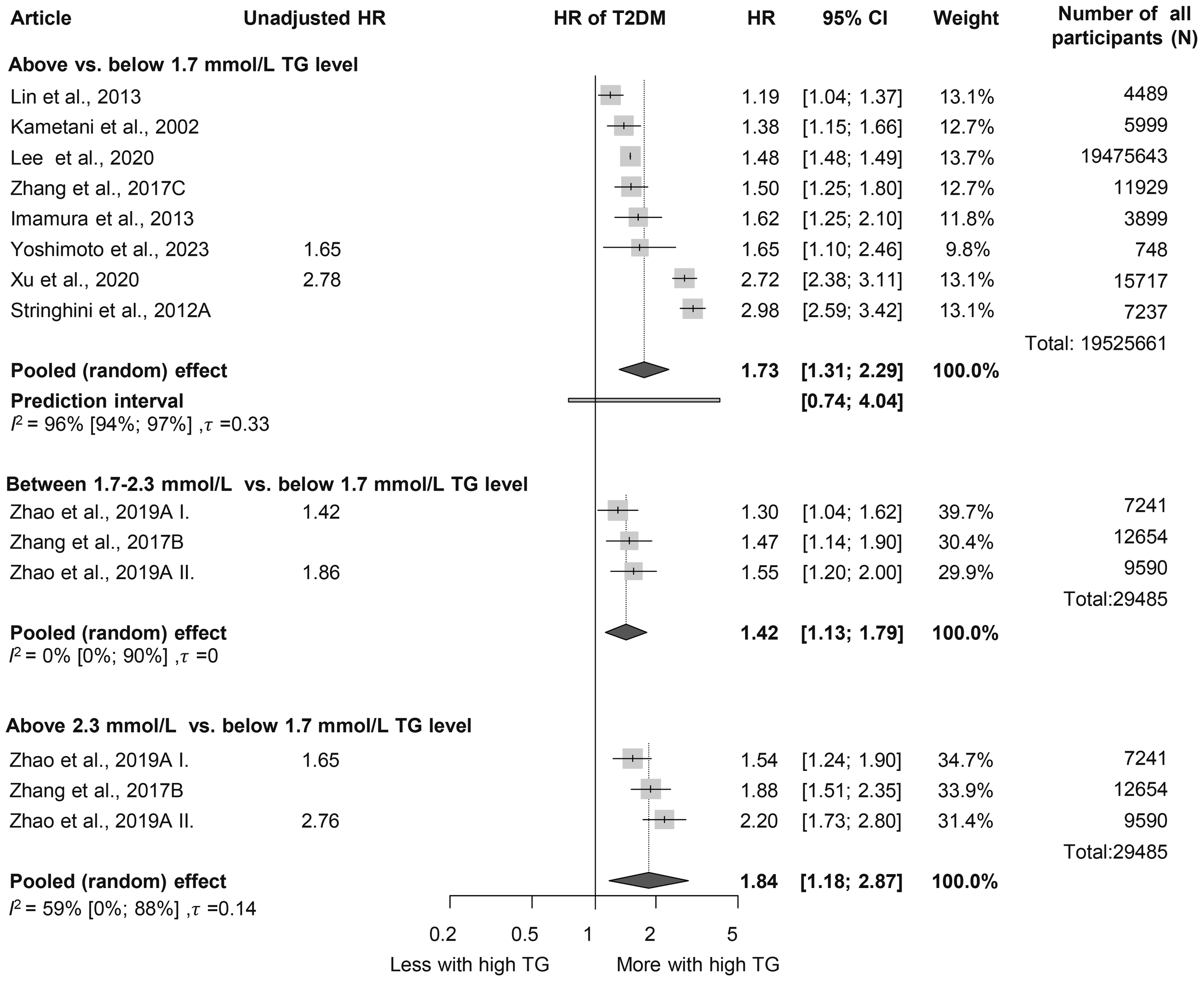
Hazard ratios for incident diabetes comparing groups with different baseline TG levels; results of three separate analyses (HR, hazard ratios; TG, triglyceride; T2DM, type 2 diabetes mellitus).
Analysis of baseline TG levels revealed that participants who developed diabetes had significantly higher TG levels, by nearly 0.5 mmol/L on average, compared to those who did not develop diabetes (MD: 0.46 mmol/L [0.41; 0.52 mmol/L]) (Figure 3, Supplementary Figure S3) (45, 47–50, 52, 54, 58–108). Those who developed prediabetes during follow-up had 0.31 mmol/L [0.06; 0.56 mmol/L] higher TG levels at baseline, which is a smaller difference than in those who developed T2DM (Supplementary Figure S4) (50, 58, 61, 89, 100, 109, 110). We also performed a subgroup analysis on the MD of TG levels in men and women; the MD was 0.50 mmol/L [0.37; 0.64 mmol/L] in women and 0.45 mmol/L [0.32; 0.58 mmol/L] in men (Supplementary Figure S5) (54, 63, 64, 68, 75, 80–82, 87, 90, 94, 104).
Figure 3

Mean differences (MD) in baseline TG levels, between patient groups with and without T2DM developed in the follow-up periods (MD, mean difference; TG, triglyceride; T2DM, type 2 diabetes mellitus; β, mean and SD are estimated mean and SD based on the quartiles in that study; c, male and female data from the same study were combined; $, mean and SD are estimated mean and SD based on the mean and SD given on the log scale variable in that study).
For every 50 mg/dL (0.56 mmol/L) increase in TG levels, the HR for incident diabetes was 1.08 [1.04; 1.13] based on six studies (Supplementary Figure S6) (39, 92, 97, 102, 111, 112) and we also visualized HRs corresponding to the SD increase in TG level in Supplementary Figure S6 (67, 79, 81, 87, 94, 95, 113, 114). The results of articles reporting OR also suggest that a 50-mg/dL increase in TG is a risk factor for T2DM (Supplementary Figure S7) (47, 66, 70, 71, 84, 90, 93, 99, 106, 115–119).
Hypertriglyceridemic waist phenotype
When assessing the HTGW phenotype, we found that participants with increased waist circumference but normal TG levels had a significantly elevated HR for diabetes in both sexes (women: HR: 2.86 [1.59; 5.14]; men: HR: 3.31 [1.51; 7.27]). Among those with HTG but normal waist circumference, HRs were lower (women: HR: 2.30 [0.66; 7.95]; men: HR: 1.82 [0.95; 3.48]). Participants with both elevated TG levels and waist circumference exhibited the highest risk, with more than a 3.5-fold increased risk in both sexes; however, these results were not significant (women: HR: 3.54 [0.52; 23.94]; men: HR: 3.51 [0.59; 20.84]) (Figure 4) (16, 21, 43).
Figure 4

Hazard ratios for incident diabetes in groups with elevated triglyceride or/and waist circumference compared to neither elevated group [within hypertriglyceridemic waist (HTGW) phenotype groups], divided by sex (female/male); results of six separate analyses (HR, hazard ratios; TG, triglyceride; T2DM, type 2 diabetes mellitus; NTNW, normal triglyceride level and waist circumference; HTNW, elevated triglyceride level and normal waist circumference; NTGW, normal triglyceride level and elevated waist circumference; HTGW, elevated triglyceride level and waist circumference; N, number of men or women in that phenotype group).
Triglyceride/high-density lipoprotein ratio
We evaluated the TG/HDL-C ratio as a risk factor for diabetes. Participants in the highest TG/HDL-C quartile (Q4) had a significantly higher HR for diabetes compared to those in the lowest quartile (Q1) (HR: 2.17 [1.53; 3.09]). This finding was consistent across sex groups (women: HR: 1.91 [1.08; 3.38]; men: HR: 1.65 [1.16; 2.35]) (Figure 5) (3, 15, 19, 64, 96, 120–126). Baseline TG/HDL-C ratios were also significantly higher among those with diabetes (MD: 0.65 [0.20; 1.10]) (Supplementary Figure S8) (64, 66, 102, 120, 127). Our results show that for a one-unit increase in TG/HDL-C ratio, the risk of developing diabetes is 1.14 [1.00; 1.31] (Supplementary Figure S9) (15, 102, 114, 125, 128).
Figure 5
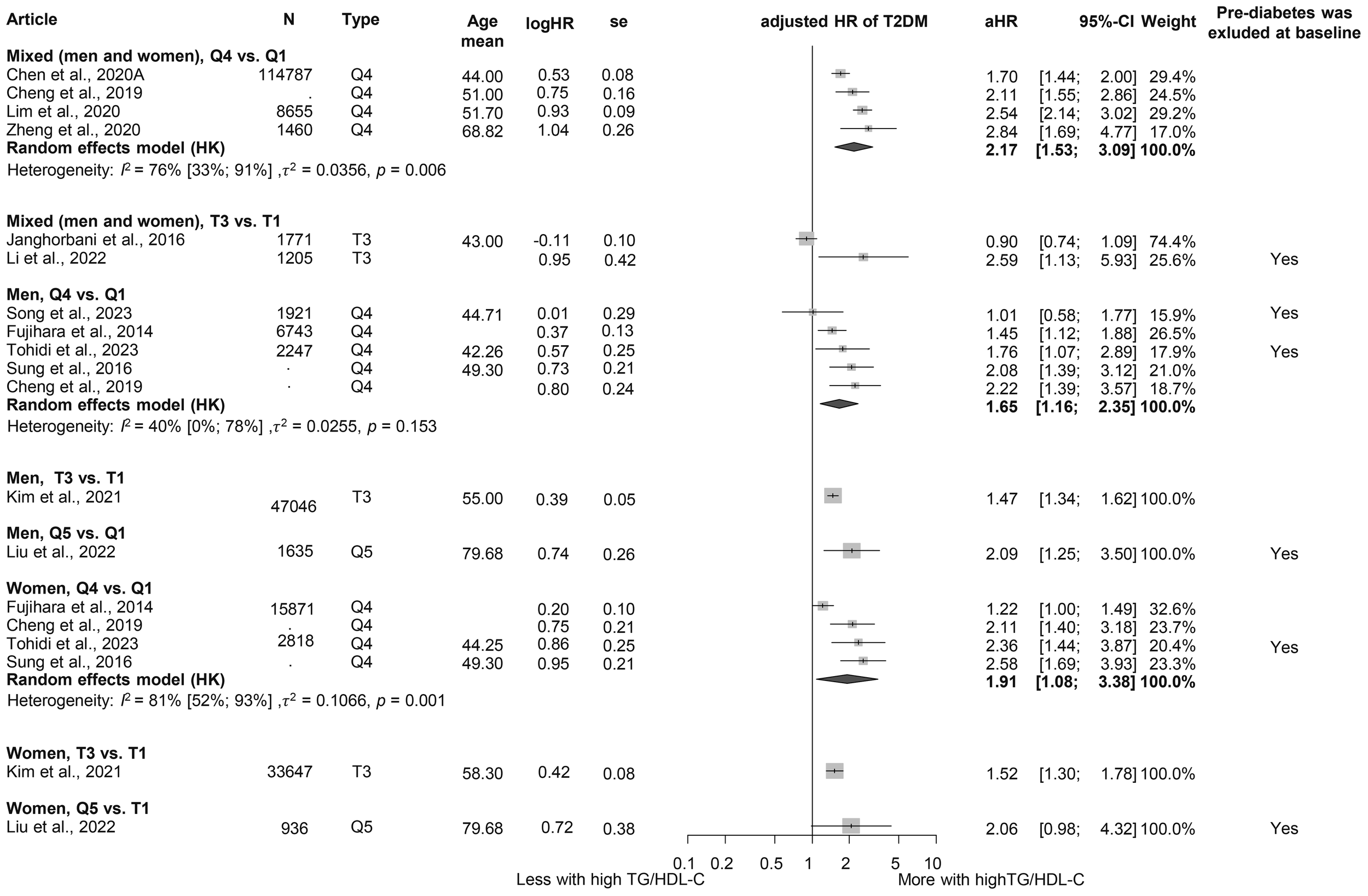
Hazard ratios for incident T2DM in groups with the highest triglyceride/high-density lipoprotein cholesterol (TG/HDL-C) ratio compared with the lowest TG/HDL-C ratio (HR, hazard ratio; T2DM, type 2 diabetes mellitus; TG/HDL-C, triglyceride/high-density lipoprotein cholesterol ratio; T1, 1st tertile; Q1, 1st quartile/1st quintile; T3, 3rd tertile; Q4, 4th quartile; Q5, 5th quintile; N, number of all participants or all male or all female participants in case of different sex groups).
Triglyceride-glucose index
When examining TyG, we obtained similar results as for the TG/HDL-C ratio. Those in the highest TyG group by population (quartile 4) had a higher HR for diabetes than those in the lowest TyG group (Q1), with more than a threefold increased risk (HR: 3.25 [1.91; 5.52]) (Figure 6) (3, 17, 18, 81, 121, 127, 129–133). This risk was higher than that observed for the TG/HDL-C ratio. The MD in baseline TyG between those who did and those who did not develop T2DM was 0.30 [0.21; 0.40] (Supplementary Figure S10) (3, 17, 18, 60, 81, 127, 134–136). An SD increase in the Tyg index was identified as a risk factor for developing T2DM (HR: 1.36 [1.15; 1.60]), whereas for a one-unit increase in this index, the HR was 1.91 [0.61; 5.98] (Supplementary Figure S11 (3, 18, 134, 137) and Supplementary Figure S12 (129, 132, 133, 138)).
Figure 6
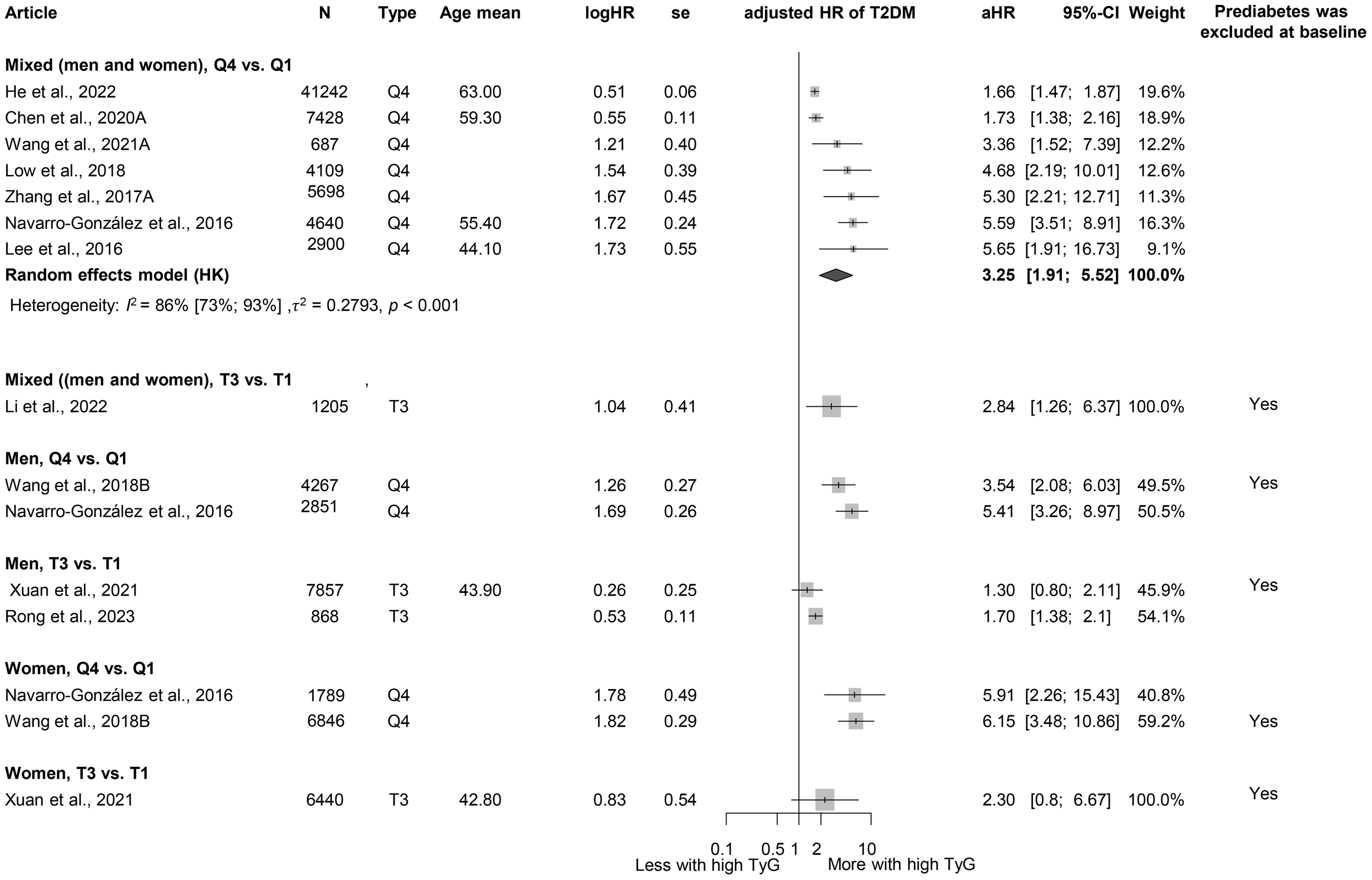
Hazard ratios for incident T2DM in groups with the highest triglyceride-glucose index (TyG) compared with groups with the lowest TyG index (HR, hazard ratio; T2DM, type 2 diabetes mellitus; TyG, triglyceride-glucose index; T1, 1st tertile; Q1, 1st quartile/1st quintile; T3, 3rd tertile; Q4, 4th quartile; N, number of all participants or all male or all female participants in case of different sex groups).
Heterogeneity, risk of bias assessment, and sensitivity analysis
Heterogeneity was generally high due to the large but diverse population and different follow-up durations of the relatively large number of cohorts included. The risk of study participation, outcome definition, and statistical analysis reporting was generally low, and potential bias was observed in terms of study attrition, prognostic factor measurement, and study confounding. The detailed results for risk of bias assessment separately for articles included in the quantitative analysis and qualitative analysis are available in the Supplementary Materials (Supplementary Figures S1A, B). There was no evidence of publication bias, and the leave-one-out analyses (where it was a meaningful enough study) showed either no statistically “influential” article or the effect of the statistically “influential” article was not clinically relevant.
Discussion
This is the first meta-analysis to examine not only individual TG levels and values but also several TG-related indices to explore the risk of diabetes in more depth. Our results suggest that triglyceride levels have a significant impact on the development of T2DM and highlight the role of different TG-related indices in this process. Our analysis suggests that the relation between TG levels and T2DM risk is dose-dependent. Although the link between HTG and T2DM has been extensively studied, most research has focused on dyslipidemia as a complication of IR or T2DM (23, 24). However, recently, with the emergence of more prediction models and research exploring T2DM risk factors, TG levels have been investigated from another perspective (139–144). In line with our own research findings, several previous and recent studies have found that higher triglyceride levels are associated with the development of DM and that HTG may act as a risk factor for T2DM (110, 145–158).
The underlying mechanisms potentially disrupt the metabolic pathways of free fatty acids (FFAs). Excess FFAs, along with resistin, tumor necrosis factor-α (TNF-α), interleukin-6, and other compounds released by enlarged adipose tissue, contribute to the development of IR, but elevated FFA levels can be triggered by increased TG production as well, further exacerbating IR (159, 160). Lipotoxicity, endoplasmic reticulum stress, and inflammation induced by dyslipidemia represent additional mechanisms that link TG abnormalities to IR (161).
Despite significant research efforts, the complex interactions between these conditions and their bidirectional influence remain to be further investigated.
While analyzing the HTGW phenotype, we found, based on the results of three cohorts, that only elevated waist circumference without HTG was a significant risk factor for T2DM in both sexes, and the highest HR was detected when both factors were elevated, consistent with previous publications (162–165). Prior studies also highlight the crucial importance of obesity and elevated adipose tissue in the development of IR and T2DM, which is partly due to the production of FFA and TNF-α and interleukins by the adipose tissue (159, 166, 167).
The TG/HDL-C ratio—an established marker of cardiovascular risk—has been shown to be associated with the incidence of IR, prediabetes, and even T2DM in previous studies (168–170). Consistent with our findings, a 2018 meta-analysis identified the TG/HDL-C ratio as an independent predictor of T2DM. The meta-analysis highlighted a dose–response relationship and reported a stronger association in female than in male patients, underscoring the importance of considering sex-specific differences in prevention strategies (15). Similarly, our analysis observed sex differences, with female patients demonstrating a higher risk of T2DM, aligning with these previous findings. In addition to the pathways already listed that lead to T2DM (such as lipotoxicity and inflammation), low HDL may directly mediate glucose, resulting in IR and T2DM (171, 172).
The TyG index has also emerged as a significant risk factor for T2DM, with an HR exceeding the HR of elevated TG/HDL-C ratio. This difference may be attributed to the inclusion of glucose—a direct marker of glucose metabolism—in the TyG index, making it a more specific indicator of glucose metabolism. The primary utility of the TyG index has previously been the risk of IR, but more recently, several studies have investigated and identified it as a risk factor for T2DM (173–176). Similar to our findings, in a previous meta-analysis, Pranata et al. also proved its impact on the development of T2DM; in their case, they found a non-linear association (14).
HTG management necessitates a multifaceted approach encompassing both lifestyle and pharmacological interventions. Lifestyle modifications, including a modest weight reduction of 5%–10%, dietary adjustments to reduce saturated fat intake, and increased dietary fiber consumption, have been shown to favorably influence triglyceride levels (177). In more severe cases, pharmacotherapy becomes essential. Statins, by inhibiting HMG-CoA reductase, limit hepatic cholesterol biosynthesis, thereby reducing triglyceride-rich lipoproteins. Fibrates activate peroxisome proliferator-activated receptors (PPARs), enhancing lipid metabolism and decreasing triglyceride levels. In addition, several novel therapeutic targets—such as apolipoprotein C3 and C2 inhibition, angiopoietin-like protein inhibition, and fibroblast growth factor analogs—emerge as promising future strategies for lowering triglycerides (8, 177).
In summary, our findings suggest that TG and TG-related indices have a strong impact on the development of T2DM. These metrics offer the advantage of being inexpensive and readily accessible; TG levels can be measured with routine blood tests, and other indices can be easily calculated using laboratory parameters. Although further research is warranted to explore their role in predicting T2DM, these markers hold promise for both healthcare systems and patients. Interventions targeting these pathways and the reduction of TG levels—such as dietary modifications, lifestyle interventions, or pharmacological agents such as fibrates—may mitigate the risk of elevated TG levels and, thus, also play a role in reducing T2DM risk.
Strengths and limitations
The strengths of our research include the transparent methodology, the large number of patients, and the fact that we were also able to analyze different TG levels and TG-related indices as well. One of our limitations related to generalization is the high heterogeneity. Our research aimed to examine the impact of TG on the development of T2DM, with an emphasis on the exclusion of both diabetic and prediabetic populations. However, most included studies lacked this distinction, which prevents separate analyses. Owing to the limited number of studies, publication bias and influence assessment of individual studies are of limited diagnostic value.
Implications for practice and research
Our research provides valuable insights with the potential to influence everyday clinical practices. As a result, it aligns with the principles of translational medicine, bridging the gap between scientific discovery and practical implementation (178, 179). Our results emphasize the importance of regular monitoring of accessible laboratory markers. On the basis of our findings, we recommend follow-ups to patients with elevated triglyceride levels and waist circumference to prevent diabetes. Further research should focus on effective lifestyle strategies to reduce TG levels and combat central obesity. Preventive programs are crucial to reducing the prevalence of chronic disease, and future studies should validate these markers in different populations and integrate them into risk guidelines for T2DM.
Conclusion
HTG is a dose-dependent risk factor for T2DM. Elevated waist circumference may play an even more important role in the development of T2DM than HTG. Both high TG-HDL-C ratio and high TyG index may be predictors of T2DM.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
LH: Conceptualization, Methodology, Project administration, Writing – original draft. ES: Methodology, Writing – review & editing. MO: Methodology, Writing – review & editing. DD: Data curation, Writing – review & editing. DV: Data curation, Formal Analysis, Writing – review & editing. TK: Data curation, Formal Analysis, Writing – review & editing. ES: Writing – review & editing. SV: Writing – review & editing. PH: Writing – review & editing. MB: Data curation, Writing – review & editing. AM: Data curation, Writing – review & editing. KV: Data curation, Writing – review & editing. PH: Conceptualization, Supervision, Writing – review & editing. AS: Conceptualization, Supervision, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The project was supported by the Centre for Translational Medicine, Semmelweis University, Hungarian Ministry of Innovation and Technology, National Research, Development and Innovation Fund (TKP2021-EGA-23 to Péter Hegyi), NKFIH project grants K131996 and K147265 (to Péter Hegyi), the University of Pécs Medical School Research Fund (300909 to Andrea Szentesi), and EKÖP-2024–11 New National Excellence Program of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund (to Szilárd Váncsa). The sponsors had no role in the design, data collection, analysis, interpretation, and manuscript preparation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1710007/full#supplementary-material
References
1
Federation ID. IDF Diabetes Atlas (2021). Brussels, Belgium. Available online at: https://www.diabetesatlas.org (Accessed December 15, 2024).
2
OngKLStaffordLKMcLaughlinSABoykoEJVollsetSESmithAEet al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. (2023) 402:203–34. doi: 10.1016/S0140-6736(23)01301-6
3
ChenCLLiuLLoKHuangJYYuYLHuangYQet al. Association between triglyceride glucose index and risk of new-onset diabetes among Chinese adults: findings from the China health and retirement longitudinal study. Front Cardiovasc Med. (2020) 7. doi: 10.3389/fcvm.2020.610322
4
SteinbrecherAMorimotoYHeakSOllberdingNJGellerKSGrandinettiAet al. The preventable proportion of type 2 diabetes by ethnicity: the multiethnic cohort. Ann Epidemiol. (2011) 21:526–35. doi: 10.1016/j.annepidem.2011.03.009
5
SaklayenMG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. (2018) 20:12. doi: 10.1007/s11906-018-0812-z
6
GalassiAReynoldsKHeJ. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. (2006) 119:812–9. doi: 10.1016/j.amjmed.2006.02.031
7
GrundySM. Hypertriglyceridemia, atherogenic dyslipidemia, and the metabolic syndrome. Am J Cardiol. (1998) 81:18b–25b. doi: 10.1016/S0002-9149(98)00033-2
8
ScicchitanoPAmatiFCicconeMMD’AscenziFImbalzanoELigaRet al. Hypertriglyceridemia: molecular and genetic landscapes. Int J Mol Sci. (2024) 25:6364. doi: 10.3390/ijms25126364
9
de PretisNAmodioAFrulloniL. Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management. U Eur Gastroenterol J. (2018) 6:649–55. doi: 10.1177/2050640618755002
10
BurchfielCMCurbJDRodriguezBLYanoKHwangLJFongKOet al. Incidence and predictors of diabetes in Japanese-American men the Honolulu heart program. Ann Epidemiol. (1995) 5:33–43. doi: 10.1016/1047-2797(94)00038-U
11
HaratiHHadaeghFSaadatNAziziF. Population-based incidence of type 2 diabetes and its associated risk factors: results from a six-year cohort study in Iran. BMC Public Health. (2009) 9:186. doi: 10.1186/1471-2458-9-186
12
ZhaoJZhangYWeiFSongJCaoZChenCet al. Triglyceride is an independent predictor of type 2 diabetes among middle-aged and older adults: a prospective study with 8-year follow-ups in two cohorts. J Trans Med. (2019) 17:403. doi: 10.1186/s12967-019-02156-3
13
MaMLiuHYuJHeSLiPMaCet al. Triglyceride is independently correlated with insulin resistance and islet beta cell function: a study in population with different glucose and lipid metabolism states. Lipids Health Disease. (2020) 19:121. doi: 10.1186/s12944-020-01303-w
14
PranataRHuangIIrvanLimMAVaniaR. The association between triglyceride-glucose index and the incidence of type 2 diabetes mellitus-a systematic review and dose-response meta-analysis of cohort studies. Endocrine. (2021) 74:254–62. doi: 10.1007/s12020-021-02780-4
15
ChengCLiuYSunXYinZLiHZhangMet al. Dose–response association between the triglycerides: High-density lipoprotein cholesterol ratio and type 2 diabetes mellitus risk: The rural Chinese cohort study and meta-analysis. J Diabetes. (2019) 11:183–92. doi: 10.1111/1753-0407.12836
16
HanKJLeeSYKimNHChaeHBLeeTHJangCMet al. Increased risk of diabetes development in subjects with the hypertriglyceridemic waist phenotype: a 4-year longitudinal study. Endocrinol Metab (Seoul). (2014) 29:514–21. doi: 10.3803/EnM.2014.29.4.514
17
LowSKhooKCJIrwanBSumCFSubramaniamTLimSCet al. The role of triglyceride glucose index in development of Type 2 diabetes mellitus. Diabetes Res Clin Pract. (2018) 143:43–9. doi: 10.1016/j.diabres.2018.06.006
18
WangZZhaoLHeS. Triglyceride–glucose index as predictor for future type 2 diabetes mellitus in a Chinese population in southwest China: a 15-year prospective study. Endocrine. (2021) 72:124–31. doi: 10.1007/s12020-020-02589-7
19
SongBWangKLuWZhaoXYaoTLiuTet al. A U-shaped association between the triglyceride to high-density lipoprotein cholesterol ratio and the risk of incident type 2 diabetes mellitus in Japanese men with normal glycemic levels: a population-based longitudinal cohort study. Front Endocrinol (Lausanne). (2023) 14:1180910. doi: 10.3389/fendo.2023.1180910
20
KosmasCERodriguez PolancoSBousvarouMDPapakonstantinouEJPeña GenaoEGuzmanEet al. The triglyceride/high-density lipoprotein cholesterol (TG/HDL-C) ratio as a risk marker for metabolic syndrome and cardiovascular disease. Diagnostics (Basel). (2023) 13:929. doi: 10.3390/diagnostics13050929
21
ZhangMGaoYChangHWangXLiuDZhuZet al. Hypertriglyceridemic-waist phenotype predicts diabetes: a cohort study in Chinese urban adults. BMC Public Health. (2012) 12:1081. doi: 10.1186/1471-2458-12-1081
22
RibeiroFBde Cássia da SilvaCVasquesACJZambonMPDe Bernardi RodriguesAMCamiloDFet al. Hypertriglyceridemic waist phenotype indicates insulin resistance in adolescents: validation study front hyperglycemic clamp-Brazilian Metabolic Syndrome Study (BRAMS). Diabetol Metab Syndrome. (2015) 7:A145. doi: 10.1186/1758-5996-7-S1-A145
23
SeghieriMTricòDNataliA. The impact of triglycerides on glucose tolerance: Lipotoxicity revisited. Diabetes Metab. (2017) 43:314–22. doi: 10.1016/j.diabet.2017.04.010
24
ZhuXWDengFYLeiSF. Meta-analysis of Atherogenic Index of Plasma and other lipid parameters in relation to risk of type 2 diabetes mellitus. Prim Care Diabetes. (2015) 9:60–7. doi: 10.1016/j.pcd.2014.03.007
25
PageMJMcKenzieJEBossuytPMBoutronIHoffmannTCMulrowCDet al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
26
ChandlerJHopewellS. Cochrane methods–twenty years experience in developing systematic review methods. Syst Rev. (2013) 2:76. doi: 10.1186/2046-4053-2-76
27
BoothAClarkeMDooleyGGhersiDMoherDPetticrewMet al. PROSPERO at one year: an evaluation of its utility. Syst Rev. (2013) 2:4. doi: 10.1186/2046-4053-2-4
28
HegyiPVarróA. Systems education can train the next generation of scientists and clinicians. Nat Med. (2024) 30:3399–400. doi: 10.1038/s41591-024-03315-w
29
MunnZSternCAromatarisELockwoodCJordanZ. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med Res Methodol. (2018) 18:5. doi: 10.1186/s12874-017-0468-4
30
HaydenJAvan der WindtDACartwrightJLCôtéPBombardierC. Assessing bias in studies of prognostic factors. Ann Intern Med. (2013) 158:280–6. doi: 10.7326/0003-4819-158-4-201302190-00009
31
HigginsJPTThompsonSG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
32
HarbordRMHarrisRJSterneJAC. Updated tests for small-study effects in meta-analyses. Stata J. (2009) 9:197–210. doi: 10.1177/1536867X0900900202
33
Harrer MCPFurukawaTEbertD. Doing Meta-Analysis with R: A Hands-On Guide. New York: Chapman and Hall/CRC (2021).
34
Team RC. A language and environment for statistical computing (2024). Vienna, Austria: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/ (Accessed January 10, 2025).
35
Meta SG. General package for meta-analysis(2024). Available online at: https://github.com/guido-s/meta/ (Accessed January 10, 2025).
36
CuijpersPFurukawaTEbertDD. Dmetar: Companion R Package For The Guide ‘Doing Meta-Analysis in R’ (2024). Available online at: http://dmetar.protectlab.org/ (Accessed January 10, 2025).
37
MetaforVW. Meta-analysis package for r(2024). Available online at: https://www.metafor-project.org (Accessed January 10, 2025).
38
ImamuraFMukamalKJMeigsJBLuchsingerJAIxJHSiscovickDSet al. Risk factors for type 2 diabetes mellitus preceded by β-cell dysfunction, insulin resistance, or both: The cardiovascular health study. Am J Epidemiol. (2013) 177(12):1418–29. doi: 10.1093/aje/kws440
39
KametaniTKoshidaHNagaokaTMiyakoshiH. Hypertriglyceridemia is an independent risk factor for development of impaired fasting glucose and diabetes mellitus: A 9-year longitudinal study in Japanese. Internal Med. (2002) 41:516–21. doi: 10.2169/internalmedicine.41.516
40
LeeMKHanKKimMKKohESKimESNamGEet al. Combinations of metabolic syndrome components and the risk of type 2 diabetes mellitus: A nationwide cohort study. Diabetes Res Clin Pract. (2020) 165:108237. doi: 10.1016/j.diabres.2020.108237
41
LinSXBerlinIYoungeRJinZSibleyCTSchreinerPet al. Does elevated plasma triglyceride level independently predict impaired fasting glucose? the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care. (2013) 36:342–7. doi: 10.2337/dc12-0355
42
StringhiniSTabakAGAkbaralyTNSabiaSShipleyMJMarmotMGet al. Contribution of modifiable risk factors to social inequalities in type 2 diabetes: Prospective Whitehall II cohort study. BMJ (Online). (2012) 345:e5452. doi: 10.1136/bmj.e5452
43
XuMHuangMQiangDGuJLiYPanYet al. Hypertriglyceridemic waist phenotype and lipid accumulation product: Two comprehensive obese indicators of waist circumference and triglyceride to predict type 2 diabetes mellitus in Chinese population. J Diabetes Res. (2020) 2020:9157430. doi: 10.1155/2020/9157430
44
YoshimotoMSakumaYOginoJIwaiRWatanabeSInoueTet al. Sex differences in predictive factors for onset of type 2 diabetes in Japanese individuals: A 15-year follow-up study. J Diabetes Invest. (2023) 14:37–47. doi: 10.1111/jdi.13918
45
ZhangMZhouJLiuYSunXLuoXHanCet al. Risk of type 2 diabetes mellitus associated with plasma lipid levels: The rural Chinese cohort study. Diabetes Res Clin Practice. (2018) 135:150–7. doi: 10.1016/j.diabres.2017.11.011
46
ZhangHWangCRenYWangBYangXZhaoYet al. A risk-score model for predicting risk of type 2 diabetes mellitus in a rural Chinese adult population: A cohort study with a 6-year follow-up. Diabetes/Metabolism Res Rev. (2017) 33:e2911. doi: 10.1002/dmrr.2911
47
BesharaACohenEGoldbergELilosPGartyMKrauseI. Triglyceride levels and risk of type 2 diabetes mellitus: A longitudinal large study. J Invest Med. (2016) 64:383–7. doi: 10.1136/jim-2015-000025
48
CharoensriSTurnsaketSPongchaiyakulC. Hypertriglyceridemia as an independent predictor for ten-year incidence of diabetes in thais. Vasc Health Risk Management. (2021) 17:519–25. doi: 10.2147/VHRM.S326500
49
GarbuzovaEVShcherbakovaLVRymarODKhudiakovaADShramkoVSRaginoYI. Triglycerides, obesity and education status are associated with the risk of developing type 2 diabetes in young adults, cohort study. J Personalized Med. (2023) 13:1403. doi: 10.3390/jpm13091403
50
LiuLGuanXYuanZZhaoMLiQZhangXet al. Different contributions of dyslipidemia and obesity to the natural history of type 2 diabetes: 3-year cohort study in China. J Diabetes Res. (2019) 2019:4328975. doi: 10.1155/2019/4328975
51
LiuMPanCJinM. A Chinese diabetes risk score for screening of undiagnosed diabetes and abnormal glucose tolerance. Diabetes Technol Ther. (2011) 13:501–7. doi: 10.1089/dia.2010.0106
52
MarottSCWNordestgaardBGTybjaerg-HansenABennM. Components of the metabolic syndrome and risk of type 2 diabetes. J Clin Endocrinol Metab. (2016) 101:3212–21. doi: 10.1210/jc.2015-3777
53
NishikawaTOkamuraTShimaAKawatsuYSugiyamaDKadotaAet al. Casual serum triglyceride as a predictor of premature type 2 diabetes mellitus: an 8-year cohort study of middle-aged Japanese workers. Diabetol Int. (2016) 7:252–8. doi: 10.1007/s13340-015-0241-z
54
NorbergMErikssonJWLindahlBAnderssonCRolandssonOStenlundHet al. A combination of HbA1c, fasting glucose and BMI is effective in screening for individuals at risk of future type 2 diabetes: OGTT is not needed. J Internal Med. (2006) 260:263–71. doi: 10.1111/j.1365-2796.2006.01689.x
55
SadeghiMTalaeiMParvaresh RiziEDianatkhahMOveisgharanSSarrafzadeganN. Determinants of incident prediabetes and type 2 diabetes in a 7-year cohort in a developing country: the Isfahan Cohort Study. J diabetes. (2015) 7:633–41. doi: 10.1111/1753-0407.12236
56
WangWLiuJSunJYWangMLiuJQiYet al. The association between the changes in triglyceride levels and the risk of incident type 2 diabetes mellitus: a 15 years followed-up results from the Chinese Multi-provincial Cohort Study. Zhonghua nei ke za zhi [Chinese J Internal medicine]. (2012) 51:516–9.
57
WannametheeSGPapacostaOWhincupPHThomasMCCarsonCLawlorDAet al. The potential for a two-stage diabetes risk algorithm combining non-laboratory-based scores with subsequent routine non-fasting blood tests: results from prospective studies in older men and women. Diabetes Med. (2011) 28:23–30. doi: 10.1111/j.1464-5491.2010.03171.x
58
AnjanaRMShanthi RaniCSDeepaMPradeepaRSudhaVDivya NairHet al. Incidence of diabetes and prediabetes and predictors of progression among Asian Indians: 10-year follow-up of the chennai urban rural epidemiology study (CURES). Diabetes Care. (2015) 38:1441–8. doi: 10.2337/dc14-2814
59
BoykoEJDe CourtenMZimmetPZChitsonPTuomilehtoJAlbertiKGMM. Features of the metabolic syndrome predict higher risk of diabetes and impaired glucose tolerance: A prospective study in Mauritius. Diabetes Care. (2000) 23:1242–8. doi: 10.2337/diacare.23.9.1242
60
ChamroonkiadtikunPAnanchaisarpTWanichanonW. The triglyceride-glucose index, a predictor of type 2 diabetes development: A retrospective cohort study. Primary Care Diabetes. (2020) 14:161–7. doi: 10.1016/j.pcd.2019.08.004
61
ChenNHuLKSunYDongJChuXLuYKet al. Associations of waist-to-height ratio with the incidence of type 2 diabetes and mediation analysis: Two independent cohort studies. Obes Res Clin Practice. (2023) 17:9–15. doi: 10.1016/j.orcp.2022.12.005
62
ChenNMuhammadIFLiZNilssonPMBornéY. Sex-specific associations of circulating uric acid with risk of diabetes incidence: A population-based cohort study from Sweden. Diabetes Metab Syndrome Obes. (2020) 13:4323–31. doi: 10.2147/DMSO.S273387
63
DotevallAJohanssonSWilhelmsenLRosengrenA. Increased levels of triglycerides, BMI and blood pressure and low physical activity increase the risk of diabetes in Swedish women. A prospective 18-year follow-up of the BEDA study. Diabetic Med. (2004) 21:615–22. doi: 10.1111/j.1464-5491.2004.01189.x
64
FujiharaKSugawaraAHeianzaYSairenchiTIrieFIsoHet al. Utility of the triglyceride level for predicting incident diabetes mellitus according to the fasting status and body mass index category: the Ibaraki Prefectural Health Study. J Atheroscler Thromb. (2014) 21:1152–69. doi: 10.5551/jat.22913
65
González-VillalpandoCDávila-CervantesCAZamora-MacorraMTrejo-ValdiviaBGonzález-VillalpandoME. Risk factors associated to diabetes in Mexican population and phenotype of the individuals who will convert to diabetes. Salud Publica Mex. (2014) 56:317–22. doi: 10.21149/spm.v56i4.7351
66
HeSWangSChenXJiangLPengYLiLet al. Higher ratio of triglyceride to high-density lipoprotein cholesterol may predispose to diabetes mellitus: 15-year prospective study in a general population. Metabolism: Clin Experimental. (2012) 61:30–6. doi: 10.1016/j.metabol.2011.05.007
67
HuHWangJHanXLiYMiaoXYuanJet al. Prediction of 5-year risk of diabetes mellitus in relatively low risk middle-aged and elderly adults. Acta Diabetologica. (2020) 57:63–70. doi: 10.1007/s00592-019-01375-w
68
HulseggeGSpijkermanAMWvan der SchouwYTBakkerSJLGansevoortRTSmitHAet al. Trajectories of metabolic risk factors and biochemical markers prior to the onset of type 2 diabetes: The population-based longitudinal Doetinchem study. Nutr Diabetes. (2017) 7:e270. doi: 10.1038/nutd.2017.23
69
HwangYCAhnHYParkSWParkCY. Apolipoprotein B and non-HDL cholesterol are more powerful predictors for incident type 2 diabetes than fasting glucose or glycated hemoglobin in subjects with normal glucose tolerance: a 3.3-year retrospective longitudinal study. Acta Diabetologica. (2014) 51:941–6. doi: 10.1007/s00592-014-0587-x
70
JeongJYKimJGKimBWMoonSSKimHSParkKGet al. Trend analysis of diabetic prevalence and incidence in a rural area of South Korea between 2003-2008. J Diabetes Invest. (2010) 1:184–90. doi: 10.1111/j.2040-1124.2010.00045.x
71
LeySHHarrisSBConnellyPWMamakeesickMGittelsohnJWoleverTMet al. Utility of non-high-density lipoprotein cholesterol in assessing incident type 2 diabetes risk. Diabetes Obes Metab. (2012) 14:821–5. doi: 10.1111/j.1463-1326.2012.01607.x
72
LuJManLamSWanQShiLHuoYChenLet al. High-coverage targeted lipidomics reveals novel serum lipid predictors and lipid pathway dysregulation antecedent to type 2 diabetes onset in normoglycemic Chinese adults. Diabetes Care. (2019) 42:2117–26. doi: 10.2337/dc19-0100
73
MackeyRHMoraSBertoniAGWasselCLCarnethonMRSibleyCTet al. Lipoprotein particles and incident type 2 diabetes in the multi-ethnic study of atherosclerosis. Diabetes Care. (2015) 38:628–36. doi: 10.2337/dc14-0645
74
MalmströmHWalldiusGCarlssonSGrillVJungnerIGudbjörnsdottirSet al. Elevations of metabolic risk factors 20 years or more before diagnosis of type 2 diabetes: Experience from the AMORIS study. Diabetes Obes Metab. (2018) 20:1419–26. doi: 10.1111/dom.13241
75
MustafinaSVRymarODShcherbakovaLVVerevkinEGPikhartHSazonovaOVet al. The risk of type 2 diabetes mellitus in a Russian population cohort according to data from the hapiee project. J Personalized Med. (2021) 11:1–15. doi: 10.3390/jpm11020119
76
OkadaKFurusyoNSawayamaYKanamotoYMurataMHayashiJ. Prevalence and risk factors for diabetes: a ten year follow-up study of the Yaeyama district of Okinawa. Fukuoka igaku zasshi = Hukuoka Acta medica. (2010) 101:215–24. doi: 10.1016/S1567-5688(10)70786-8
77
OnatAHergençGUyarelHCanGÖzhanH. Prevalence, incidence, predictors and outcome of type 2 diabetes in Turkey. Anadolu Kardiyoloji Dergisi. (2006) 6:314–21.
78
PangXYangSGuoXLiHZhangYWeiCet al. The association and mediating biomarkers of serum retinol in influencing the development of type 2 diabetes: A prospective cohort study in middle-aged and elderly population. Front Nutr. (2022) 9:831950. doi: 10.3389/fnut.2022.831950
79
PencinaKMPencinaMJDufresneLHolmesMThanassoulisGSnidermanAD. An adverse lipoprotein phenotype—hypertriglyceridaemic hyperapolipoprotein B—and the long-term risk of type 2 diabetes: a prospective, longitudinal, observational cohort study. Lancet Healthy Longevity. (2022) 3:e339–e46. doi: 10.1016/S2666-7568(22)00079-4
80
RathmannWStrassburgerKHeierMHolleRThorandBGianiGet al. Incidence of type 2 diabetes in the elderly German population and the effect of clinical and lifestyle risk factors: KORA S4/F4 cohort study. Diabetic Med. (2009) 26:1212–9. doi: 10.1111/j.1464-5491.2009.02863.x
81
RongLHouNHuJGongYYanSLiCet al. The role of TyG index in predicting the incidence of diabetes in Chinese elderly men: a 20-year retrospective study. Front Endocrinol. (2023) 14. doi: 10.3389/fendo.2023.1191090
82
SansSPadróTTuomilehtoJBadimonL. Incidence of diabetes and serum adipokines in Catalonian men: the ADIPOCAT study. Ann Med. (2013) 45:97–102. doi: 10.3109/07853890.2012.679958
83
WangTTLinBCuiWXZhangMZZhangYHZhangSY. Clustering of cardiovascular risk factors and diabetes: A prospective cohort study on the inner Mongolian population in China. Biomed Environ sciences: BES. (2018) 31:749–56. doi: 10.3967/bes2018.100
84
WuXYuZSuWIsquithDANeradilekMBLuNet al. Low levels of ApoA1 improve risk prediction of type 2 diabetes mellitus. J Clin Lipidol. (2017) 11:362–8. doi: 10.1016/j.jacl.2017.01.009
85
AbbasiACorpeleijnEGansevoortRTGansROBHillegeHLStolkRPet al. Role of HDL cholesterol and estimates of HDL particle composition in future development of type 2 diabetes in the general population: The PREVEND study. J Clin Endocrinol Metab. (2013) 98:E1352–E9. doi: 10.1210/jc.2013-1680
86
DuanMJDekkerLHCarreroJJNavisG. Using structural equation modeling to untangle pathways of risk factors associated with incident type 2 diabetes: the lifelines cohort study. Prev Sci. (2022) 23:1090–100. doi: 10.1007/s11121-022-01357-5
87
FreemanDJNorrieJCaslakeMJGawAFordILoweGDOet al. C-reactive protein is an independent predictor of risk for the development of diabetes in the west of Scotland coronary prevention study. Diabetes. (2002) 51:1596–600. doi: 10.2337/diabetes.51.5.1596
88
GagnonCLuZXMaglianoDJDunstanDWShawJEZimmetPZet al. Serum 25-hydroxyvitamin D, calcium intake, and risk of type 2 diabetes after 5 years: Results from a national, population-based prospective study (the Australian diabetes, obesity and lifestyle study). Diabetes Care. (2011) 34:1133–8. doi: 10.2337/dc10-2167
89
GongRLiuYLuoGLiuWJinZXuZet al. Associations of TG/HDL ratio with the risk of prediabetes and diabetes in Chinese adults: A Chinese population cohort study based on open data. Int J Endocrinol. (2021) 2021:9949579. doi: 10.1155/2021/9949579
90
HadaeghFHatamiMTohidiMSarbakhshPSaadatNAziziF. Lipid ratios and appropriate cut off values for prediction of diabetes: A cohort of Iranian men and women. Lipids Health Dis. (2010) 9:85. doi: 10.1186/1476-511X-9-85
91
HodgeAMJenkinsAJEnglishDRO’DeaKGilesGG. NMR-determined lipoprotein subclass profile predicts type 2 diabetes. Diabetes Res Clin Practice. (2009) 83:132–9. doi: 10.1016/j.diabres.2008.11.007
92
IchikawaTOkadaHHamaguchiMKurogiKMurataHItoMet al. Estimated small dense low-density lipoprotein-cholesterol and incident type 2 diabetes in Japanese people: Population-based Panasonic cohort study 13. Diabetes Res Clin Pract. (2023) 199:110665. doi: 10.1016/j.diabres.2023.110665
93
JacobsenBKBonaaKHNjolstadI. Cardiovascular risk factors, change in risk factors over 7 years, and the risk of clinical diabetes mellitus type 2: The Tromsø study. J Clin Epidemiol. (2002) 55:647–53. doi: 10.1016/S0895-4356(02)00404-3
94
JosephJSvartbergJNjølstadISchirmerH. Incidence of and risk factors for type-2 diabetes in a general population: the Tromsø Study. Scandinavian J Public Health. (2010) 38:768–75. doi: 10.1177/1403494810380299
95
KhalooPHasheminiaMTohidiMAbdiHMansourniaMAAziziFet al. Impact of 3-year changes in lipid parameters and their ratios on incident type 2 diabetes: Tehran lipid and glucose study. Nutr Metab. (2018) 15:50. doi: 10.1186/s12986-018-0287-6
96
KimJShinSJKimYSKangHT. Positive association between the ratio of triglycerides to high-density lipoprotein cholesterol and diabetes incidence in Korean adults. Cardiovasc Diabetol. (2021) 20:183. doi: 10.1186/s12933-021-01377-5
97
LiLWangZZhangMRuanHZhouLWeiXet al. New risk score model for identifying individuals at risk for diabetes in southwest China. Prev Med Rep. (2021) 24101618. doi: 10.1016/j.pmedr.2021.101618
98
LimNKParkSHChoiSJLeeKSParkHY. A risk score for predicting the incidence of type 2 diabetes in a middle-aged Korean cohort: the Korean genome and epidemiology study. Circ J. (2012) 76:1904–10. doi: 10.1253/circj.CJ-11-1236
99
Martínez-HervásSMorales-Suarez-VarelaMMAndrés-BlascoILara-HernándezFPeraita-CostaIRealJTet al. Developing a simple and practical decision model to predict the risk of incident type 2 diabetes among the general population: The Di@bet.es Study. Eur J Internal Med. (2022) 102:80–7. doi: 10.1016/j.ejim.2022.05.005
100
OhlsonLOLarssonBBjorntorpPErikssonHSvardsuddKWelinLet al. Risk factors for Type 2 (non-insulin-dependent) diabetes mellitus. Thirteen and one-half years of follow-up of the participants in a study of Swedish men born in 1913. Diabetologia. (1988) 31:798–805. doi: 10.1007/BF00277480
101
RiedigerNDClarkKLukianchukVRouletteJBruceS. Fasting triglycerides as a predictor of incident diabetes, insulin resistance and β-cell function in a Canadian First Nation. Int J circumpolar Health. (2017) 76:1310444. doi: 10.1080/22423982.2017.1310444
102
ShengGKuangMYangRZhongYZhangSZouY. Evaluation of the value of conventional and unconventional lipid parameters for predicting the risk of diabetes in a non-diabetic population. J Trans Med. (2022) 20:266. doi: 10.1186/s12967-022-03470-z
103
TanakaSIFujiokaYTsujinoTIshidaTHirataKI. Impact of serum cholesterol esterification rates on the development of diabetes mellitus in a general population. Lipids Health Dis. (2018) 17:180. doi: 10.1186/s12944-018-0822-5
104
VolSBalkauBLangeCCailleauMCogneauJLantieriOet al. Who should be screened for type 2 diabetes in France? A predictive score from the D.E.S.I.R. prospective study. Medecine Des Maladies Metaboliques. (2009) 3:198–202. doi: 10.1016/S1957-2557(09)71645-1
105
WangYKohWPSimXYuanJMPanA. Multiple biomarkers improved prediction for the risk of type 2 diabetes mellitus in Singapore Chinese men and women. Diabetes Metab J. (2019) 43:295–306. doi: 10.4093/dmj.2019.0020
106
WangYLKohWPTalaeiMYuanJMPanA. Association between the ratio of triglyceride to high-density lipoprotein cholesterol and incident type 2 diabetes in Singapore Chinese men and women. J Diabetes. (2017) 9:689–98. doi: 10.1111/1753-0407.12477
107
WangZHeSChenX. Capacity of different anthropometric measures to predict diabetes in a Chinese population in southwest China: a 15-year prospective study. Diabetic Med. (2019) 36:1261–7. doi: 10.1111/dme.14055
108
YangXYZhangMLuoXPWangJJYinLPangCet al. Body mass index, waist circumference and waist-to-height ratio associated with the incidence of type 2 diabetes mellitus: a cohort study. Zhonghua yu fang yi xue za zhi [Chinese J Prev medicine]. (2016) 50:328–33. doi: 10.3760/cma.j.issn.0253-9624.2016.04.009
109
OrisakaMNakaiKTominagaMSuwabeA. Risk factors for development of pre-diabetic state from normal glucose regulation. Tohoku J Exp Med. (2006) 210:279–83. doi: 10.1620/tjem.210.279
110
WangYWangLSuYZhongLPengB. Prediction model for the onset risk of impaired fasting glucose: a 10-year longitudinal retrospective cohort health check-up study. BMC Endocrine Disord. (2021) 21:211. doi: 10.1186/s12902-021-00878-4
111
NicholsGAHillierTABrownJB. Normal fasting plasma glucose and risk of type 2 diabetes diagnosis. Am J Med. (2008) 121:519–24. doi: 10.1016/j.amjmed.2008.02.026
112
QinHChenZZhangYWangLOuyangPChengLet al. Triglyceride to high-density lipoprotein cholesterol ratio is associated with incident diabetes in men: A retrospective study of Chinese individuals. J Diabetes Invest. (2020) 11:192–8. doi: 10.1111/jdi.13087
113
SkrettebergPTGryttenANGjertsenKGrundvoldIKjeldsenSEErikssenJet al. Triglycerides-diabetes association in healthy middle-aged men: modified by physical fitness? A long term follow-up of 1962 Norwegian men in the Oslo Ischemia Study. Diabetes Res Clin Pract. (2013) 101:201–9. doi: 10.1016/j.diabres.2013.06.001
114
YugeHOkadaHHamaguchiMKurogiKMurataHItoMet al. Triglycerides/HDL cholesterol ratio and type 2 diabetes incidence: Panasonic Cohort Study 10. Cardiovasc Diabetol. (2023) 22:308. doi: 10.1186/s12933-023-02046-5
115
CarvalhoLSFBenseñorIMNogueiraACCDuncanBBSchmidtMIBlahaMJet al. Increased particle size of triacylglycerol-enriched remnant lipoproteins, but not their plasma concentration or lipid content, augments risk prediction of incident type 2 diabetes. Diabetologia. (2021) 64:385–96. doi: 10.1007/s00125-020-05322-1
116
LeySHHarrisSBConnellyPWMamakeesickMGittelsohnJWoleverTMet al. Association of apolipoprotein B with incident type 2 diabetes in an aboriginal Canadian population. Clin Chem. (2010) 56:666–70. doi: 10.1373/clinchem.2009.136994
117
SeoMHBaeJCParkSERheeEJParkCYOhKWet al. Association of lipid and lipoprotein profiles with future development of type 2 diabetes in nondiabetic Korean subjects: A 4-year retrospective, longitudinal study. J Clin Endocrinol Metab. (2011) 96:E2050–E4. doi: 10.1210/jc.2011-1857
118
SungJLeeKSongYMLeeMKimJ. Genetic and baseline metabolic factors for incident diabetes and HbA1c at follow-up: The healthy twin study. Diabetes/Metabolism Res Rev. (2015) 31:376–84. doi: 10.1002/dmrr.2619
119
WangKGongMXieSZhangMZhengHZhaoXFet al. Nomogram prediction for the 3-year risk of type 2 diabetes in healthy mainland China residents. EPMA J. (2019) 10:227–37. doi: 10.1007/s13167-019-00181-2
120
JanghorbaniMAminiM. Utility of serum lipid ratios for predicting incident type 2 diabetes: the Isfahan Diabetes Prevention Study. Diabetes Metab Res Rev. (2016) 32:572–80. doi: 10.1002/dmrr.2770
121
LiXXueYDangYLiuWWangQZhaoYet al. Association of non-insulin-based insulin resistance indices with risk of incident prediabetes and diabetes in a Chinese rural population: A 12-year prospective study. Diabetes Metab Syndrome Obes. (2022) 15:3809–19. doi: 10.2147/DMSO.S385906
122
LimTKLeeHSLeeYJ. Triglyceride to HDL-cholesterol ratio and the incidence risk of type 2 diabetes in community dwelling adults: A longitudinal 12-year analysis of the Korean Genome and Epidemiology Study. Diabetes Res Clin Practice. (2020) 163:108150. doi: 10.1016/j.diabres.2020.108150
123
LiuHLiuJLiuJXinSLyuZFuX. Triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio, a simple but effective indicator in predicting type 2 diabetes mellitus in older adults. Front Endocrinol. (2022) 13. doi: 10.3389/fendo.2022.828581
124
SungKCParkHYKimMJReavenG. Metabolic markers associated with insulin resistance predict type 2 diabetes in Koreans with normal blood pressure or prehypertension. Cardiovasc Diabetol. (2016) 15:47. doi: 10.1186/s12933-016-0368-7
125
TohidiMAsgariSCharyASafieeSAziziFHadaeghF. Association of triglycerides to high-density lipoprotein cholesterol ratio to identify future prediabetes and type 2 diabetes mellitus: over one-decade follow-up in the Iranian population. Diabetol Metab Syndrome. (2023) 15:13. doi: 10.1186/s13098-023-00988-0
126
ZhengDLiHAiFSunFSinghMCaoXet al. Association between the triglyceride to high-density lipoprotein cholesterol ratio and the risk of type 2 diabetes mellitus among Chinese elderly: The Beijing Longitudinal Study of Aging. BMJ Open Diabetes Res Care. (2020) 8. doi: 10.1136/bmjdrc-2019-000811
127
WangBZhangMLiuYSunXZhangLWangCet al. Utility of three novel insulin resistance-related lipid indices for predicting type 2 diabetes mellitus among people with normal fasting glucose in rural China. J Diabetes. (2018) 10:641–52. doi: 10.1111/1753-0407.12642
128
ChenZHuHChenMLuoXYaoWLiangQet al. Association of Triglyceride to high-density lipoprotein cholesterol ratio and incident of diabetes mellitus: A secondary retrospective analysis based on a Chinese cohort study. Lipids Health Dis. (2020) 19:33. doi: 10.1186/s12944-020-01213-x
129
HeKZhangWHuXZhaoHSongRBaiKet al. Stronger associations of body mass index and waist circumference with diabetes than waist-height ratio and triglyceride glucose index in the middle-aged and elderly population: A retrospective cohort study. J Diabetes Res. (2022) 2022:9982390. doi: 10.1155/2022/9982390
130
LeeDYLeeESKimJHParkSEParkCYOhKWet al. Predictive value of triglyceride glucose index for the risk of incident diabetes: A 4-year retrospective longitudinal study. PloS One. (2016) 11:e0163465. doi: 10.1371/journal.pone.0163465
131
Navarro-GonzálezDSánchez-ÍñigoLPastrana-DelgadoJFernández-MonteroAMartinezJA. Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: The Vascular-Metabolic CUN cohort. Prev Med. (2016) 86:99–105. doi: 10.1016/j.ypmed.2016.01.022
132
XuanXHamaguchiMCaoQOkamuraTHashimotoYOboraAet al. U-shaped association between the triglyceride-glucose index and the risk of incident diabetes in people with normal glycemic level: A population-base longitudinal cohort study. Clin Nutr. (2021) 40:1555–61. doi: 10.1016/j.clnu.2021.02.037
133
ZhangMWangBLiuYSunXLuoXWangCet al. Cumulative increased risk of incident type 2 diabetes mellitus with increasing triglyceride glucose index in normal-weight people: The Rural Chinese Cohort Study. Cardiovasc Diabetol. (2017) 16:30. doi: 10.1186/s12933-017-0514-x
134
KuangMYangRHuangXWangCShengGXieGet al. Assessing temporal differences in the predictive power of baseline TyG-related parameters for future diabetes: an analysis using time-dependent receiver operating characteristics. J Trans Med. (2023) 21:299. doi: 10.1186/s12967-023-04159-7
135
LeeSHKwonHSParkYMHaHSJeongSHYangHKet al. Predicting the development of diabetes using the product of triglycerides and glucose: The chungju metabolic disease cohort (CMC) study. PloS One. (2014) 9:e90430. doi: 10.1371/journal.pone.0090430
136
ParkHMLeeHSLeeYJLeeJH. The triglyceride-glucose index is a more powerful surrogate marker for predicting the prevalence and incidence of type 2 diabetes mellitus than the homeostatic model assessment of insulin resistance. Diabetes Res Clin Pract. (2021) 180:109042. doi: 10.1016/j.diabres.2021.109042
137
TohidiMBaghbani-OskoueiAAhanchiNSAziziFHadaeghF. Fasting plasma glucose is a stronger predictor of diabetes than triglyceride–glucose index, triglycerides/high-density lipoprotein cholesterol, and homeostasis model assessment of insulin resistance: Tehran Lipid and Glucose Study. Acta Diabetologica. (2018) 55:1067–74. doi: 10.1007/s00592-018-1195-y
138
FuXLiuHLiuJLiNLiLKeDet al. Association between triglyceride-glucose index and the risk of type 2 diabetes mellitus in an older Chinese population aged over 75 years. Front Public Health. (2021) 9:796663.
139
AsgariSKhaliliDAziziFHadaeghF. External validation of the American prediction model for incident type 2 diabetes in the Iranian population. BMC Med Res methodol. (2023) 23:77. doi: 10.1186/s12874-023-01891-y
140
BalkauBLangeClFezeuLTichetJde Lauzon-GuillainBCzernichowSet al. Predicting diabetes: clinical, biological, and genetic approaches: data from the epidemiological study on the insulin resistance syndrome (DESIR). Diabetes Care. (2008) 31:2056–61. doi: 10.2337/dc08-0368
141
MannDMBertoniAGShimboDCarnethonMRChenHJennyNSet al. Comparative validity of 3 diabetes mellitus risk prediction scoring models in a multiethnic US cohort. Am J Epidemiol. (2010) 171:980–8. doi: 10.1093/aje/kwq030
142
YatsuyaHLiYHirakawaYOtaAMatsunagaMHaregotHEet al. A point system for predicting 10-year risk of developing type 2 diabetes mellitus in Japanese men: aichi workers’ Cohort study. J Epidemiol. (2018) 28:347–52. doi: 10.2188/jea.JE20170048
143
ZhangMZhangHWangCRenYWangBZhangLet al. Development and validation of a risk-score model for type 2 diabetes: A cohort study of a rural adult Chinese population. PloS One. (2016) 11:e0152054. doi: 10.1371/journal.pone.0152054
144
ZhouFZhaoZTianLZhengTGaoYChenTet al. Association of serum ferritin level with risk of incident abnormal glucose metabolism in southwestern China: a prospective cohort study. Biol Trace Element Res. (2016) 169:27–33. doi: 10.1007/s12011-015-0393-5
145
JosephJSvartbergJNjølstadISchirmerH. Risk factors for type 2 diabetes in groups stratified according to metabolic syndrome: a 10-year follow-up of the Tromsø Study. Eur J Epidemiol. (2011) 26:117–24. doi: 10.1007/s10654-010-9540-7
146
LeySHHarrisSBConnellyPWMamakeesickMGittelsohnJHegeleRAet al. Adipokines and incident type 2 diabetes in an Aboriginal Canadian [corrected] population: the Sandy Lake Health and Diabetes Project. Diabetes Care. (2008) 31:1410–5. doi: 10.2337/dc08-0036
147
MaglianoDJBarrELMZimmetPZCameronAJDunstanDWColagiuriSet al. Glucose indices, health behaviors, and incidence of diabetes in Australia: The Australian diabetes, obesity and lifestyle study. Diabetes Care. (2008) 31:267–72. doi: 10.2337/dc07-0912
148
OweiIUmekweNWanJDagogo-JackS. Plasma lipid levels predict dysglycemia in a biracial cohort of nondiabetic subjects: Potential mechanisms. Exp Biol Med. (2016) 241:1961–7. doi: 10.1177/1535370216659946
149
PengJZhaoFYangXPanXXinJWuMet al. Association between dyslipidemia and risk of type 2 diabetes mellitus in middle-aged and older Chinese adults: A secondary analysis of a nationwide cohort. BMJ Open. (2021) 11:e042821. doi: 10.1136/bmjopen-2020-042821
150
PerryIJWannametheeSGWalkerMKThomsonAGWhincupPHShaperAG. Prospective study of risk factors for development of non-insulin dependent diabetes in middle aged British men. Br Med J. (1995) 310:560–4. doi: 10.1136/bmj.310.6979.560
151
SaijoYOkadaHHamaguchiMHabuMKurogiKMurataHet al. The risk factors for development of type 2 diabetes: panasonic cohort study 4. Int J Environ Res Public Health. (2022) 19:571. doi: 10.3390/ijerph19010571
152
TiroshAShaiIBitzurRKochbaITekes-ManovaDIsraeliEet al. Changes in triglyceride levels over time and risk of type 2 diabetes in young men. Diabetes Care. (2008) 31:2032–7. doi: 10.2337/dc08-0825
153
ValdésSBotasPDelgadoEÁlvarezFCadórnigaFD. Does the new American Diabetes Association definition for impaired fasting glucose improve its ability to predict type 2 diabetes mellitus in Spanish persons? The Asturias study. Metabolism: Clin experimental. (2008) 57:399–403. doi: 10.1016/j.metabol.2007.10.017
154
YangWXingXLinH. Baseline hypertriglyceridemia, a risk factor for non-insulin dependent diabetes mellitus: a 6-year follow-up study of 432 nondiabetics. Zhonghua Nei Ke Za Zhi. (1995) 34:583–6.
155
MiljkovicICvejkusRAnPThyagarajanBChristensenKWojczynskiMet al. Low risk for developing diabetes among the offspring of individuals with exceptional longevity and their spouses. Front Clin Diabetes Healthc. (2022) 3:753986. doi: 10.3389/fcdhc.2022.753986
156
OlafsdottirEAspelundTSigurdssonGThorssonBBenediktssonRHarrisTBet al. Unfavourable risk factors for type 2 diabetes mellitus are already apparent more than a decade before onset in a population-based study of older persons: From the Age, Gene/Environment Susceptibility-Reykjavik Study (AGES-Reykjavik). Eur J Epidemiol. (2009) 24:307–14. doi: 10.1007/s10654-009-9343-x
157
ValdesSBotasPDelgadoEAlvarezFCadornigaFD. Population-based incidence of type 2 diabetes in northern Spain: the Asturias study. Diabetes Care. (2007) 30:2258–63. doi: 10.2337/dc06-2461
158
Szili-TorokTBakkerSJLTietgeUJF. Normal fasting triglyceride levels and incident type 2 diabetes in the general population. Cardiovasc Diabetol. (2022) 21:111. doi: 10.1186/s12933-022-01530-8
159
BodenG. Obesity and free fatty acids. Endocrinol Metab Clin North Am. (2008) 37:635–46, viii-ix. doi: 10.1016/j.ecl.2008.06.007
160
TenenbaumAKlempfnerRFismanEZ. Hypertriglyceridemia: a too long unfairly neglected major cardiovascular risk factor. Cardiovasc Diabetol. (2014) 13:159. doi: 10.1186/s12933-014-0159-y
161
LiNFuJKoonenDPKuivenhovenJASniederHHofkerMH. Are hypertriglyceridemia and low HDL causal factors in the development of insulin resistance? Atherosclerosis. (2014) 233:130–8. doi: 10.1016/j.atherosclerosis.2013.12.013
162
CarlssonACRisérusUÄrnlövJ. Hypertriglyceridemic waist phenotype is associated with decreased insulin sensitivity and incident diabetes in elderly men. Obesity. (2014) 22:526–9. doi: 10.1002/oby.20434
163
ChenDLiangZSunHLuCChenWWangHHXet al. Association between hypertriglyceridemic–waist phenotype and risk of type 2 diabetes mellitus in middle-aged and older chinese population: A longitudinal cohort study. Int J Environ Res Public Health. (2021) 18:9618. doi: 10.3390/ijerph18189618
164
HeSZhengYShuYHeJWangYChenX. Hypertriglyceridemic waist might be an alternative to metabolic syndrome for predicting future diabetes mellitus. PloS One. (2013) 8:e73292. doi: 10.1371/journal.pone.0073292
165
RenYLiuYSunXDengKWangCLiLet al. Hypertriglyceridemia-waist and risk of developing type 2 diabetes: The Rural Chinese Cohort Study. Sci Rep. (2017) 7:9072. doi: 10.1038/s41598-017-09136-x
166
DesprésJP. Intra-abdominal obesity: an untreated risk factor for Type 2 diabetes and cardiovascular disease. J Endocrinol Invest. (2006) 29:77–82.
167
FreemantleNHolmesJHockeyAKumarS. How strong is the association between abdominal obesity and the incidence of type 2 diabetes? Int J Clin Pract. (2008) 62:1391–6. doi: 10.1111/j.1742-1241.2008.01805.x
168
LiuHYanSChenGLiBZhaoLWangYet al. Association of the ratio of triglycerides to high-density lipoprotein cholesterol levels with the risk of type 2 diabetes: A retrospective cohort study in Beijing. J Diabetes Res. (2021) 2021:5524728. doi: 10.1155/2021/5524728
169
ShimodairaMMinemuraYNakayamaT. Elevated triglyceride/high-density lipoprotein-cholesterol ratio as a risk factor for progression to prediabetes: a 5-year retrospective cohort study in Japan. J Diabetes Metab Disord. (2023) 23:655–64. doi: 10.1007/s40200-023-01329-8
170
YoungKAMaturuALorenzoCLangefeldCDWagenknechtLEChenYIet al. The triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio as a predictor of insulin resistance, β-cell function, and diabetes in Hispanics and African Americans. J Diabetes Complications. (2019) 33:118–22. doi: 10.1016/j.jdiacomp.2018.10.018
171
HwangYCHayashiTFujimotoWYKahnSELeonettiDLMcNeelyMJet al. Differential association between HDL subclasses and the development of type 2 diabetes in a prospective study of Japanese Americans. Diabetes Care. (2015) 38:2100–5. doi: 10.2337/dc15-0625
172
DrewBGRyeKADuffySJBarterPKingwellBA. The emerging role of HDL in glucose metabolism. Nat Rev Endocrinol. (2012) 8:237–45. doi: 10.1038/nrendo.2011.235
173
JanghorbaniMAlmasiSZAminiM. The product of triglycerides and glucose in comparison with fasting plasma glucose did not improve diabetes prediction. Acta Diabetol. (2015) 52:781–8. doi: 10.1007/s00592-014-0709-5
174
LeeJWLimNKParkHY. The product of fasting plasma glucose and triglycerides improves risk prediction of type 2 diabetes in middle-aged Koreans. BMC Endocr Disord. (2018) 18:33. doi: 10.1186/s12902-018-0259-x
175
LiXLiGChengTLiuJSongGMaH. Association between triglyceride-glucose index and risk of incident diabetes: a secondary analysis based on a Chinese cohort study: TyG index and incident diabetes. Lipids Health Dis. (2020) 19:236. doi: 10.1186/s12944-020-01403-7
176
ParkBLeeHSLeeYJ. Triglyceride glucose (TyG) index as a predictor of incident type 2 diabetes among nonobese adults: a 12-year longitudinal study of the Korean Genome and Epidemiology Study cohort. Trans Res. (2021) 228:42–51. doi: 10.1016/j.trsl.2020.08.003
177
FiltzAPariharSGreenbergGSParkCMScottiALorenzattiDet al. New approaches to triglyceride reduction: Is there any hope left? Am J Prev Cardiol. (2024) 18:100648. doi: 10.1016/j.ajpc.2024.100648
178
HegyiPErőssBIzbékiFPárniczkyASzentesiA. Accelerating the translational medicine cycle: the Academia Europaea pilot. Nat Med. (2021) 27:1317–9. doi: 10.1038/s41591-021-01458-8
179
HegyiPPetersenOHHolgateSErőssBGaramiASzakácsZet al. Academia Europaea position paper on translational medicine: the cycle model for translating scientific results into community benefits. J Clin Med. (2020) 9:1532. doi: 10.3390/jcm9051532
Summary
Keywords
hypertriglyceridemia, HTG, type 2 diabetes mellitus, T2DM, risk factor
Citation
Havelda L, Szalai EÁ, Obeidat M, Dobszai D, Veres DS, Kói T, Sipter E, Váncsa S, Hegyi PJ, Bucur M, Molnár A, Vámossy KL, Hegyi P and Szentesi A (2025) Hypertriglyceridemia is a dose-dependent risk factor for type 2 diabetes mellitus: a systematic review and meta-analysis. Front. Endocrinol. 16:1710007. doi: 10.3389/fendo.2025.1710007
Received
21 September 2025
Accepted
20 October 2025
Published
05 November 2025
Volume
16 - 2025
Edited by
Hidetaka Hamasaki, Japanese Academy of Health and Practice, Japan
Reviewed by
Pietro Scicchitano, ASLBari - Azienda Sanitaria Localedella provincia di Bari (ASL BA), Italy
Ronald Pratama Adiwinoto, Hang Tuah University, Indonesia
Updates
Copyright
© 2025 Havelda, Szalai, Obeidat, Dobszai, Veres, Kói, Sipter, Váncsa, Hegyi, Bucur, Molnár, Vámossy, Hegyi and Szentesi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Szentesi, szentesiai@gmail.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.