- Department of Dermatology, The Second Xiangya Hospital of Central South University; Hunan Key Laboratory of Medical Epigenomics, The Second Xiangya Hospital of Central South University, Changsha, China
Systemic lupus erythematosus (SLE) is an autoimmune disease that involves multiple immune cells. Due to its complex pathogenesis, the effectiveness of traditional treatment methods is limited. Many patients have developed resistance to conventional treatment or are not sensitive to steroid and immunosuppressant therapy, and so emerging therapeutic antibodies have become an alternative and have been shown to work well in many patients with moderate and severe SLE. This review summarizes the biological agents that are in the preclinical and clinical trial study of SLE. In addition to the various monoclonal antibodies that have been studied for a long time, such as belimumab and rituximab, we focused on another treatment for SLE, bispecific antibodies (BsAbs) such as tibulizumab, which simultaneously targets multiple pathogenic cytokines or pathways. Although the application of BsAbs in cancer has been intensively studied, their application in autoimmune diseases is still in the infant stage. This unique combined mechanism of action may provide a novel therapeutic strategy for SLE.
Introduction
Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disease, and the pathogenesis involves genetic factors, epigenetics, environmental factors, which resulting in immune abnormalities. Immune abnormalities are mainly the loss of tolerance and sustained autoantibody production (1). The main immunological manifestations are the abnormal activation of T cells and B cells with abundant autoantibodies that form antigen-antibody complexes in tissues and organs, which results in damage and inflammation (2).
With a deepening understanding of the pathogenesis, targeted therapy has become a more promising treatment, especially for the patients who not respond to conventional treatments. Conventional treatments, mainly including glucocorticoids and immunosuppressants, have poor specificity and are prone to tolerance. SLE patients have an increase in multiple cytokines and auto-antibodies, and there may be significant differences in cytokine levels in different patients, such as I interferon (IFN) levels (3). This provides strong support for blocking specific cytokines or pathways with specific antibodies. In this review, we will summarize the existing biological agents, expound on their effects at different sites (Figure 1), and hope to shed light on future research to develop more targeted therapy.
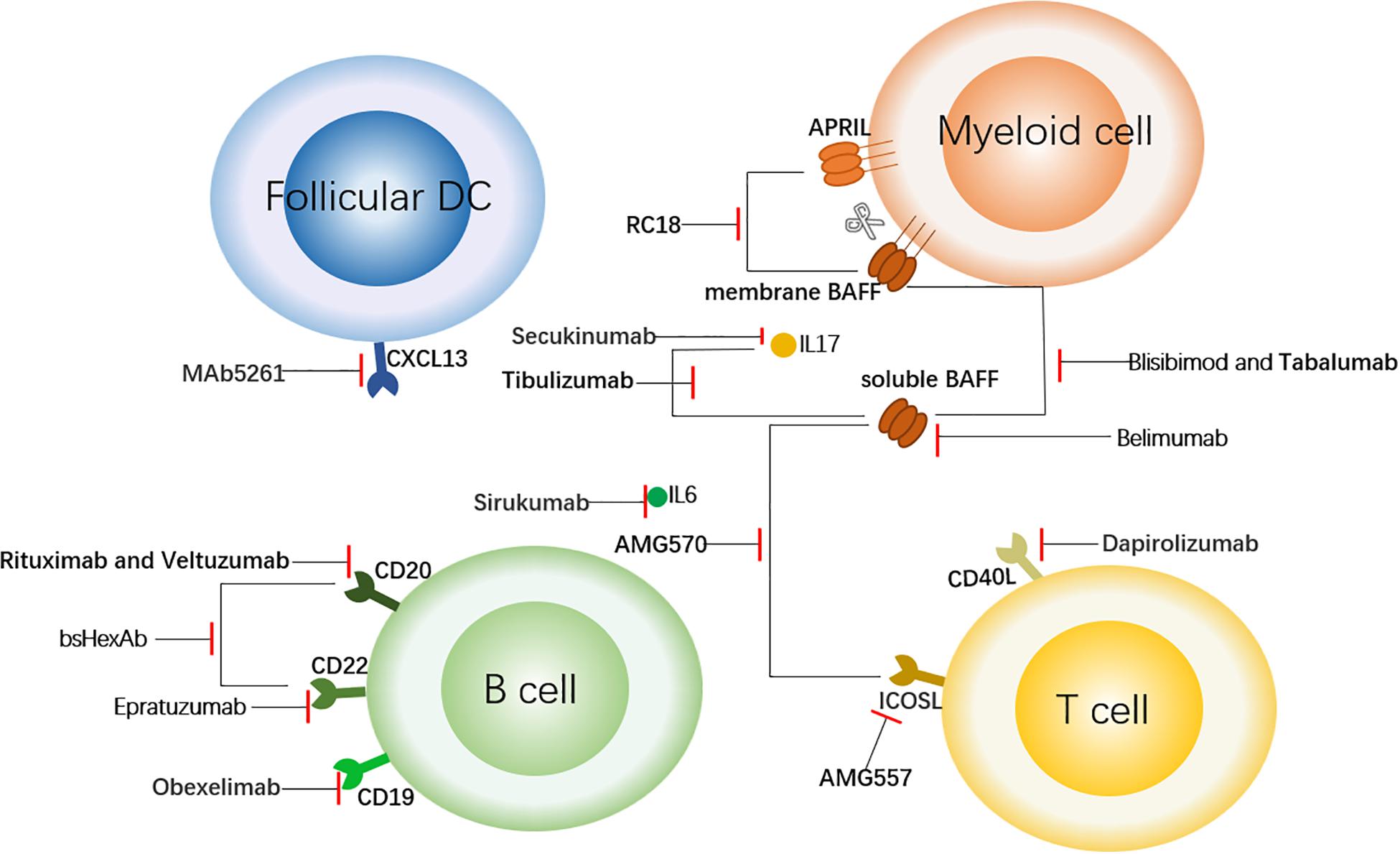
Figure 1. Targeted Therapy of SLE Centered on B Cells. This figure shows the sites of action of some therapeutic antibodies with a focus on B cells. The antibodies shown here bind to the surface molecules of B cells and down-regulate the immune response. In addition, to block the upstream factors regulating B cells (such as BAFF and APRIL) or downstream inflammatory factors such as IL6, so as to achieve the role of regulating immune response. The short red line indicates that the antibody has a blocking effect on the corresponding cell receptor or cytokine. follicular DC, follicular dendritic cell; CXCL13, chemokine ligand 13; APRIL, a proliferation-inducing ligand; BAFF, B cell activation factor; CD40L, CD40 ligand; and ICOSL, inducible T cell co-stimulator ligand.
Targeting B Cells
B cells are central to the pathogenesis of SLE. Dysregulation of transcription factors and cytokines in B cells and interaction between B-T cells can lead to abnormal maturation of B cells and the production of autoantibodies (4, 5). Targeted blocking of B-cell-related cytokines has an obvious effect on down-regulating the overly strong immune response.
BAFF/APRIL Inhibition
B cell activation factor (BAFF, or BLyS), which regulates the survival and maturation of B lymphocytes, is a member of the TNF family and has both a membrane form and soluble form (6). BAFF has been found to play an important role in the survival and differentiation of B cells in recent years. By binding to three different receptors, BAFF-R, TACI and BCMA, BAFF promotes B cell differentiation, maturation and class conversion, promoting the humoral immune response and participating in T cell activation (7, 8). APRIL (a proliferation-inducing ligand) is also a member of the TNF family, has high homology with BAFF, and binds to the receptors TACI and BCMA. Excessive expression of BAFF promotes the malignant proliferation of B cells and leads to autoimmune diseases (9).
Belimumab is a fully humanized IgG1 monoclonal antibody (mAb) that only binds to soluble BAFF and blocks its binding to the three receptors (10), directly reducing naive and transient B cells and indirectly inhibiting the function of IgD-CD27++ memory B cells and plasma cells (11). This is the first biological agent to be approved by the FDA for SLE. Early multicenter phase III clinical trials have shown that longterm use of high doses continuously improved serological indicators, reduced hormone dosage and reduced the risk of severe recurrence in SLE (12, 13). Real world study make us more comprehensive understanding of this drug. A retrospective study of 466 patients with active SLE found that the lower the baseline damage, the greater the probability of achieving remission, indicating the benefits of early medication for SLE (14). Currently Belimumab in childhood – onset systemic lupus erythematosus (cSLE) II period in the clinical trials have been successfully developed, and the efficacy is consistent with adults (15) (Table 1).
Tabalumab is a humanized IgG4 single-chain antibody that can bind to both membrane and soluble BAFF (16). In randomized phase II trials of rheumatoid arthritis (RA), treatment resulted in transient increases in the total number of B cells, naive B cells, and memory B cells (17). Two phase III studies evaluated the role of tabalumab in patients with moderate to severe SLE. One showed that although tabalumab treatment resulted in significant changes in the biological activity of anti-dsDNA, complement, B cells and immunoglobulin, the primary endpoint was not achieved (18). Another study showed that key secondary endpoints were not met and that side effects were depression and suicidality (19). In response to these results, tabalumab development has been discontinued.
Blisibimod is an antagonistic peptide-FC fusion protein that can specifically bind to both soluble and membrane BAFF (20). Antagonist peptide has the advantages of simple synthesis and little toxic. Compared to Bellimumab, blisibimod has a higher affinity for BAFF (21). A phase I clinical trial confirmed the safety in SLE patients with moderate disease activities and explained that its pharmacological effect is by reducing naive B cells (22). In a phase III trial involving 442 patients with systemic lupus erythematosus disease activity index (SLEDAI) scores greater than 10 (23), there was no significant difference in remission between the blisibimod group and the placebo group. However, blisibimod significantly reduced the urinary protein/creatinine ratio and improved the serological index.
Atacicept is a humanized recombinant soluble fusion protein that contains the extracellular ligand binding domain of TACI, which is fused into the Fc portion of human IgG1, blocking both APRIL, and BLys (24, 25). In a phase Ib clinical trial, the safety, tolerability, and biological activity of atacicept were demonstrated in patients with mild to moderate SLE (26). A phase IIb study involving 306 SLE patients showed evidence of efficacy, particularly in patients with high levels of disease activity (27).
RC18, also called telitacicept, is a novel recombinant TACI-Fc fusion protein that can binding to BAFF and APRIL. As a dual-targeting drug, it can inhibit the two cytokines of BAFF and APRIL at the same time, more effectively reduce the immune response, and achieve the purpose of treating autoimmune diseases. According to the published data by RemeGen, 249 SLE patients were enrolled to evaluate the efficacy and safety of telitacicept in the treatment of moderate to severe SLE subjects. The results showed that there was a statistically significant difference in the clinical response rate (SLE responder index, SRI-4) between the telitacicept group (79.2%) and the placebo group (32%), which reached the primary endpoint of the clinical trial (NCT02885610). It is expected to be on the market in China in 2020 and has been approved for a phase II clinical trial by the FDA, with a phase III trial still in recruiting (NCT04082416).
CD22/CD20 Inhibition
CD22 is a receptor on the surface of the B cell membrane and is initially expressed in naive B cells and also during the development of B cells; mature B cells have the highest CD22 expression, while plasma cells lack this surface molecule (28). CD22 can promote the proliferation and differentiation of B cells by regulating the signal transduction of the B cell receptor (BCR) (29). CD20 is a transmembrane calcium channel that is involved in the activation, proliferation and differentiation of B cells (30). CD20 exists in the late pre-B cells and goes through the maturation stage of B cells (31). Specifically, blocking these two B cell membrane surface receptors inhibits B cell proliferation and reduces the inflammatory response.
Epratuzumab is a humanized IgG1 mAb that targets CD22, which regulates B cell signals without a substantial reduction in the number of B cells (32). To date, seven clinical trials have examined the safety and efficacy of epratuzumab. Overall, these trials have demonstrated that epratuzumab is a well-tolerated drug with similar rates of adverse events, mainly infection and headache, in the placebo and epratuzumab groups (33). All tests showed an effect on B cells, and the number of B cells in peripheral blood decreased by 30–50%. Complement levels and autoantibody levels remained unchanged. Immunoglobulin levels stabilized, but data showed a 20% decrease in plasma IGM levels, which were not associated with infection (34–36).
Rituximab is a chimeric mAb with a human IgG1 domain and a mouse CD20 variable region (37). Rituximab is a classical B cell depletion therapy that has been approved for the treatment of RA. Although it failed trails in lupus nephritis (38), in a prospective observational study, 45/50 patients achieved complete remission (CR), or partial remission (PR) by a median time of 37 weeks (39). These results indicate that rituximab is still a promising therapy for the treatment of LN. A recent phase 2a, single-arm study involved 16 SLE patients with severe, refractory disease and they were treated with rituximab and belimumab. The responses are significant: 10/16 patients achieved low lupus disease activity, 11/16 reached renal responses. The combination therapy through complementary mechanisms, provides new insights in reducing the excessive autoreactive B lymphocytes (40). Another RCT of the combination of rituximab and belimumab is also under way (NCT03312907).
The complementary determinant region of veltuzumab is similar to that of rituximab. The binding activity and the effect on CDC were stronger than those of rituximab (41). Veltuzumab was effective in a patient with severe, drug-resistant SLE who did not respond to conventional treatment and was initially responsive to rituximab but subsequently deteriorated with high levels of anti-rituximab antibodies. After receiving veltuzumab treatment, the patient responded well, with decreased B cells and significantly improved clinical symptoms. Whether the application can be expanded is debatable (42).
CXCR5/CXCL13 Inhibition
CXCR5, which is expressed in Tfh cells, mature B cells, and Treg cells, is involved in B cell migration and the formation of germinal cells (GCs) and guides disease-causing double negative (DN) T cells into lymphoid organs and kidneys (43). In CXCR5-deficient lupus murine model, the migration of DN T cell to lymph nodes was reduced and the kidney was not infiltrated (44). CXCL13, a ligand of CXCR5, is expressed in follicular dendritic cells and macrophages in secondary lymphoid organs (45). Both molecules play an important role in the maturation and migration of B cells.
Numerous studies demonstrate that circulating CXCL13 level in patients with SLE increases and may act as a novel target in the treatment of SLE (46). MAb5261 is a humanized IgG mAb against CXCL13 in preclinical stage (47). After the treatment of MAb5261, the number of germinal centers decreased and it interfered with the transport of B cells to the spleen in mice models of RA and multiple sclerosis. Its role in SLE needs to be studied.
Targeting Costimulators
Immune activation of B cells requires the interaction of costimulatory signals with T cells, especially CD40/40L, CD28, Inducible T cell co-stimulator ligand (ICOSL), and CD80/CD86. Blocking this pathway indirectly inhibits the proliferation and activation of B cells and down-regulates autoantibody production, thus achieving a therapeutic effect (48, 49).
CD40 and CD40 ligand (CD40L) are a pair of costimulatory molecules. CD40L is mainly expressed in activated CD4+ cells and in monocytes, mast cells and basophils. After binding to CD40, which is expressed on the surface of B cells, CD40L regulates the interaction between CD4+ T cells and B cells, which is crucial for the activation, differentiation and memory generation of B cells (50–52). Dapirolizumab is an Fc peg-glycolated anti-CD40L antibody fragment (53). A phase I clinical trial that included 24 patients with SLE showed that the SRI-4 in the dapirolizumab group was obviously improved compared with that of the placebo group (5/12 vs 1/7) and the mechanism of gene expression changes was observed in blood RNA samples (54). A 24-week phase II trial is being recruited for (NCT02804763) to further study its efficiency in SLE.
Cytotoxic T lymphocyte associated protein 4 (CTLA4) is a receptor that is constitutively expressed in regulatory T cells and down-regulates the immune response when it binds to CD80 or CD86, which is expressed on the surface of antigen presenting cells (55). Abatacept is a recombinant protein composed of CTLA4 and immunoglobulin that binds to CD80/CD86 and inhibits the response pathway (56). Abatacept has been approved for arthritis and is currently being studied for SLE and lupus (57). A multicenter exploratory phase II clinical trial involving 175 SLE patients demonstrated its efficacy in SLE. The primary endpoint was the proportion of patients who deteriorated after steroid reduction began. After 12 months of follow-up, the rate of flares in the treatment group was 79.7%, and in the control group, it was 82.5%, which failed to reach the primary endpoint (58). However, given the pathogenesis of SLE, new clinical trials on abatacept should be designed to further confirm its potential use in SLE (57).
Inducible T cell co-stimulator ligand is highly expressed in CD4 and CD8 T cells in patients with SLE, leading to abnormal proliferation and activation of T cells and the generation of pathogenic autoantibodies (59). AMG557 is a mAb that binds to ICOSL. A phase Ib clinical trial showed its safety and potential curative effect (60). The phase II clinical trial of 112 patients showed that the KLH IgG reaction decreased significantly, but the KLH IgM reaction or IgG level had no obvious change. There were no significant changes in clinical features or other biological indicators (61).
CD80/CD86, a ligand of CD28 and CTLA4, plays a key role in autoimmune diseases and organ transplantation (62). There is no anti-CD80/CD86 antibody applied to clinical cases of SLE patients so far, but its application in follicular lymphoma has entered phase II clinical trials (63). Anti-CD86 (1D1) (64), a mAb that recognizes both human and mouse CD86, was used in the CGVHD-induced experimental lupus nephritis model. The data showed that blocking CD86 with 1D1 significantly alleviated proteinuria, autoantibody production, immune complex deposition, and renal parenchymal injury in mice.
Targeting Cytokines
In the pathogenesis of SLE, many cytokines not only mediate the immune response but also serve as markers of disease progression, inhibiting the corresponding immune stimulation, and reducing the immune response (65, 66).
IL-6 Inhibition
IL-6 is an important inflammatory factor that not only increases rapidly in the acute inflammatory response but also significantly up-regulates the immune response in immune diseases (67, 68). The increase in serum IL-6 levels is positively correlated with the disease activity of SLE (69). There is also a positive correlation with IL-17, which is why there have been studies on the bispecific antibody (BsAbs) of both factors.
Tocilizumab is a humanized anti-IL-6 receptor (IL-6R) mAb that blocks receptor binding to IL-6 (68). A phase I clinical trial involving 16 mild-to-moderate SLE patients studied the safety and efficacy of tocilizumab. The results showed that the level of resistant double-stranded DNA decreased by 47% and the disease activity significantly improved. However, tocilizumab resulted in a decrease in the absolute number of neutrophils, and the decrease was related to the dose of the drug, with 11 out of 16 patients becoming infected (70). The FDA has approved tocilizumab for the treatment of RA, but its use in SLE has not been well developed, given that it inhibits inflammatory responses and increases the risk of infection at the same time.
Sirukumab is a humanized mAb against IL-6 that neutralizes IL-6 in the blood and reduces inflammation (71). In a phase II clinical trial of lupus nephritis (72), the experimental group did not reach the expected endpoint, but urine protein decreased by 50% in 5/21 patients. More research need to be studied in lupus nephritis.
IL-17 Inhibition
IL-17A, a member of the IL-17 family, is secreted mainly by Th17 cells. In SLE, IL-17A collectively recruits and activates neutrophils with other cytokines to amplify the inflammatory response, exacerbate inflammation and injury in targeted organs, and enhance the immune response (73).
Secukinumab, an anti-IL-17A mAb, has shown some promise in Phase II trials for multiple autoimmune diseases, particularly psoriasis (74). In a case report, a woman with psoriasis vulgaris that was complicated with refractory lupus nephritis was treated with secukinumab for elevated Th17 cells in her peripheral blood and substantial IL-17 infiltration in her renal interstitium, despite resistance to conventional treatment (75). After starting secukinumab treatment, the condition of this patient was improved. Further research needs to be performed in SLE.
IFNα Inhibition
I interferon is a potent immune-stimulating factor produced by plasmacytoid DCs whose signaling pathway is mediated by type I interferon receptor (IFN R). In the pathogenesis of SLE, the activation of IFN system can be seen in most patients, manifesting an overexpression of type I IFN-regulated genes or an IFN signature (76). Blocking IFN suppresses the immune response and corrects the immune imbalance in SLE.
Sifalimumab is a humanized IgG1k mAb against IFN that is neutralized by binding to most subtypes of IFN (77). A phase IIb clinical trial that included 431 participants showed that only a group of patients with high levels of IFN SRI-4 significantly improved. Skin lupus erythematosus lesion area and severity index ——cutaneous lupus erythematosus disease area and severity index (CLASI) and joint count were significantly improved. No efficacy was found in reducing anti-dsDNA antibodies or improving C3/C4 levels, and subsequent exploratory analysis showed improvement in patients with low IFN expression (78). As with a recent multicenter phase II open-label study in Japan, the main adverse event was herpes zoster (78, 79). Although sifalimumab performed well in Phase II trial, its development was discontinued in favor of anifrolumab which had better results in phase II studies (80).
Rontalizumab is also a humanized IgG1 mAb against IFN, and clinical studies of rontalizumab have progressed to phase II. After observing 238 SLE patients for 24 weeks, it was found that although the primary and secondary endpoints were not reached, rontalizumab performed well in patients with low IFN signal measurements (ISMs), which was unexpected in terms of improving disease activity, reducing flares, and steroid reduction (81, 82). This is probably because of the difference in the mean trough concentrations of rontalizumab between the ISM-Low patients [56.5 (mu)g/mL] and ISM-High patients [39.4 (mu)g/mL], which may have contributed to the differential outcomes.
Anifrolumab is a humanized anti-IFNαR mAb that is effective in targeting IFNα (83). The first phase III trial of anifrolumab, TULIP-1, did not show significant influence at the primary endpoint according to SRI (84). But in TULIP -2, anifrolumab showed significant influence at the primary endpoint according to the BILAG-based combined lupus assessment (BICLA). The BICLA response rate of anifrolumab (48 weeks, 300 mg per 4 weeks) was 16.3 percentage points higher than placebo (47.8% and 31.5%, respectively) (85). This inconsistency in drug efficacy under different evaluation systems presents a challenge for the development of new drugs.
TNFα Inhibition
TNF is an important inflammatory factor that mediates the autoimmune response. The level of TNF reflects the disease activity (DA) level of SLE and is positively correlated with the activity of lupus nephritis (86).
Infliximab is a humanized mAb against TNF that neutralizes TNF in peripheral blood. Patients with refractory lupus nephritis (87) have improved DA and proteinuria in response to infliximab. However, its safety and efficacy in treating SLE need further study.
IL21/IL23 Inhibition
IL-21, a cytokine that is secreted by Th17 and Tfh cells, is highly expressed in the peripheral blood of SLE patients, induces the generation and differentiation of B cells and enhances the production of immunoglobulin (88). Up-regulation of IL-23 and its receptor has also been observed in lupus patients (89).
Ustekinumab is a mAb that acts on the IL12/IL23 subunit p40 is currently approved for use in psoriasis (90), with clinical trials in SLE underway. A phase II clinical trial involving 102 autoantibody-positive SLE patients who were receiving standard treatment showed (91) SRI-4 responses at 24 weeks in 37 (62%) of 60 ustekinumab patients and 14 (33%) of 42 placebo patients. The incidence of adverse events was higher in the ustekinumab group (78%) than in the placebo group (67%), with infection being the most common event.
Targeting Complement
Complement mediates the deposition of immune complexes, which further lead to the involvement and damage of the deposition site, and blocking the complement-mediated pathway and reducing the immune response is a way to alleviate the involvement of SLE organs (92).
Eculizumab is a humanized anti-C5 mAb (93). It specifically binds to human terminal complement protein C5 and blocks the release of inflammatory factor C5a and the formation of C5b-9 by inhibiting the cleavage of human complement C5 to C5a and C5b. In several case reports (94–96), all lupus nephritis patients with eculizumab showed improved renal function and normal complement.
Bispecific Antibodies in SLE
At present, there are around 100 BsAb drug candidates in clinical development (97), whereas only a dozen are associated with autoimmune disease (98). In SLE, in addition to two fusion proteins, atacicept and RC18, which are dual-target drugs, 5 BsAbs are in study (Table 2).
B cell activation factor is a critical target for these pending BsAbs, with more than half of the drugs designed to be targeted at it. This is due not only to its important role in the pathogenesis of SLE, but also to the confidence generated by Belimumab’s successful development (99). In addition, how to design cytokines into the network of dual targets is also a problem with research value.
Tibulizumab is a novel BsAb that is composed of two divalent antibodies that act independently and targets both BAFF and IL-17A (100) (Figure 2J). BAFF is not only involved in the activation of B cells but also promotes the proliferation of Th17 cells, thereby mediating the downstream immune response. IL-17, which is secreted by Th17 cells, in turn promotes inflammation (101). Blocking both IL-17 and BAFF has advantages that anti-17 mAbs and anti-BAFF mAbs alone cannot achieve (102). Tibulizumab effectively antagonizes BAFF and IL-17 in both cellular and live mouse models. In the Cynomolgus monkey model, the development and survival of B cells were inhibited, the circulatory function was complete, and the half-life was prolonged (100). A phase I clinical trial is currently ongoing to study the safety, tolerability, pharmacokinetics and pharmacodynamics of tibulizumab in Sjogren’s syndrome.
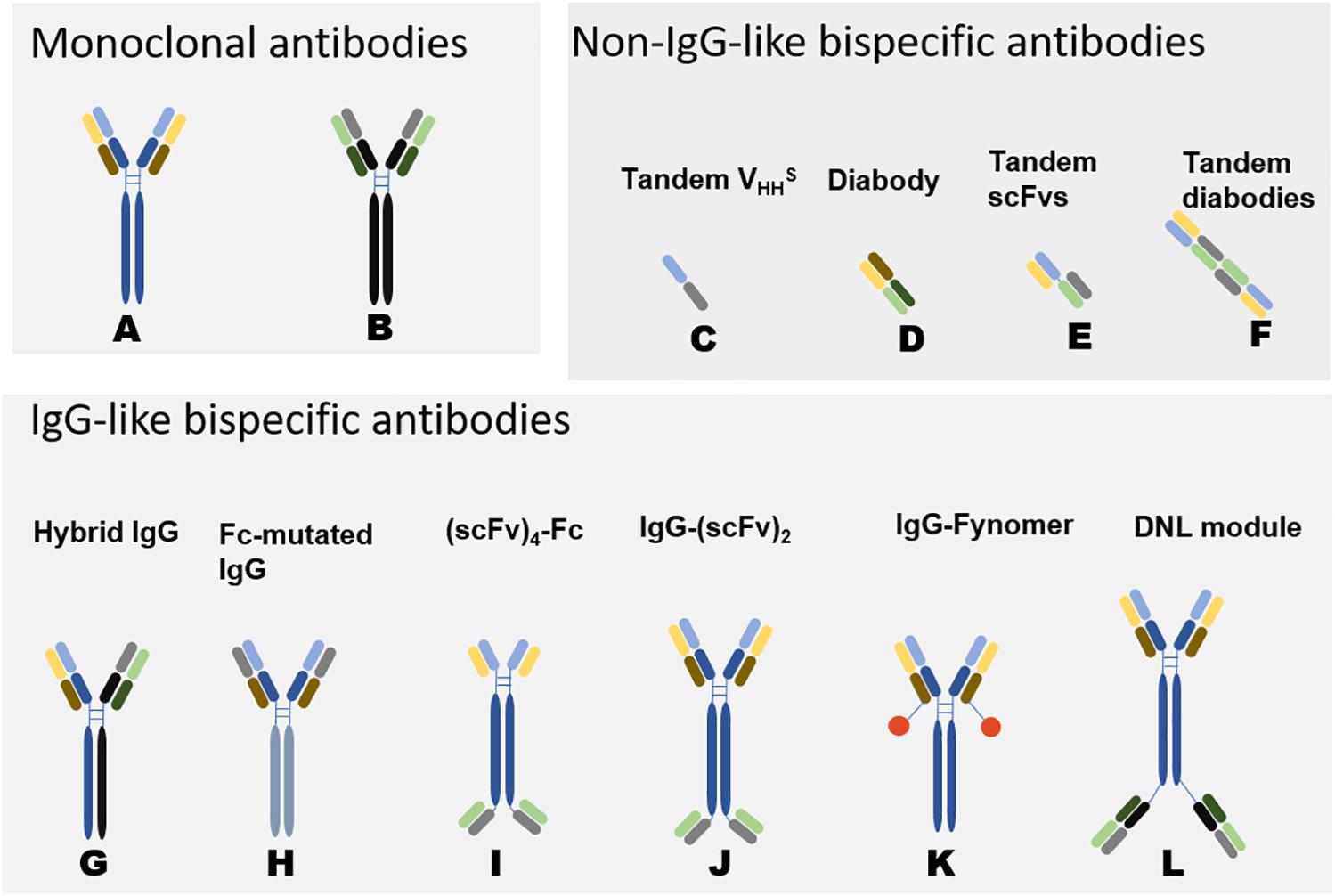
Figure 2. Schematic Diagram of Bispecific Antibodies (118). Natural antibodies are tetramers of two light chains (L) and two heavy chains (H) and contain two identical Fab domains with binding antigen sites and one Fc domain. Bispecific antibodies can be divided into two categories according to the presence or absence of Fc segments: non-IgG-like BsAbs and IgG-like BsAbs. Non-IgG-like BsAbs have low molecular weights and robust tissue penetration and exert effects through specific structural domains bound to antigens. However, due to their small molecular weights and lack of receptor-binding Fc structure, the antibodies cannot mediate antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) (118, 119). Because of random assembly of the different chains, the design of IgG-like BsAbs mainly focuses on how to solve the mismatch between two different heavy chains and the mismatch between heavy chains and light chains. IgG-like BsAbs have improved stability but strong immunogenicity. The development of genetic engineering technology has promoted the preparation of IgG-like BsAbs (120). (A, B) represent two different monoclonal antibodies. (C–L) are variants of (A, B), representing some common structures of bispecific antibodies. The origin of the light and heavy chains can be determined by their colors. The corresponding format name is marked above the antibody, different formats have their own characteristics in manufacturing and effect functions (30). DNL: In Dock-and-lock (DNL) method, antibody fragments are fused to heterodimerizing proteins.
AMG570 is a BsAb that targets ICOSL and BAFF for the treatment of autoimmune diseases such as SLE (103) (Figure 2J). The current research on AMG570 is still in the preclinical stage. Treatment with ICOSL/BAFF BsAb or combination therapy was more efficacious than that of a single ICOSL or BAFF inhibitor in a mouse lupus model. Dual ICOSL and BAFF inhibition was also effective in the mouse collagen-induced arthritis (CIA) model. In cynomolgus monkeys, B cells were reduced significantly after treatment with AMG570.
22∗-(20)-(20) is a bispecific hexadecavalent antibody (bsHexAb) that targets CD20 and CD22 (104). It is composed of the Fc of epratuzumab and four Fabs of veltuzumab, and a CD20-targeting immunocytokine, using the Dock-and-Lock (DNL) method (Figure 2L). This method combines recombinant engineering with site-specific conjugation, allowing the construction of various complex, yet defined, biostructures with multivalency and multispecificity (105). In vitro experiment, the 22∗-(20)-(20) mediates a broad and potent trogocytosis of multiple B-cell surface proteins with only moderate B-cell depletion compared to veltuzumab (104).
Obexelimab (XmAb5871) is a humanized Fc-engineered antibody that binds to CD19 on the B cell surface and has a better affinity for Fcγ receptor IIb (FcγRIIb) to inhibit the function and activation of B cells (106–108) (Figure 2H). CD19 is expressed in almost all stages of B cells, because of its wide expression, the use of therapeutic antibodies against CD19 in SLE is limited (109). In a phase II clinical trial involving 104 SLE patients, obexelimab showed some inhibition of disease activity. SLEDAI scores increased by no more than 4 points in 42% of patients in the treatment group compared with 23% in the placebo group.
MT-6194 is a bispecific antibody that targets both IL-17A and IL-6R using a gene fusion technique that combines the anti-IL-17A Fynomer 11L9C09 with anti-IL-6R tocilizumab light chain C-terminus (Figure 2K). Fynomer is a small protein, but it does not act as a drug on its own. Instead, it forms a fusion protein with an intact antibody molecule, allowing the complex to bind to two different targets simultaneously (110). Currently in preclinical studies, MT-6194 inhibits inflammation better than each cytokine alone in a mouse model of delayed hypersensitivity inflammation (111).
Bispecific Antibodies and Their Application in Other Diseases
Bispecific antibodies have been in development for some time. In 2014, blinatumomab (CD19 and CD3) became the first FDA-approved BsAb for the treatment of lymphoblastic leukemia (112). Emicizumab (Factor IX and Factor X), for the treatment of hemophilia, was marketed in 2017, becoming the first BsAb for a noncancer disease (113). Although BsAbs have been studied in various fields, such as infectious diseases, diabetes, and autoimmune diseases, their development is still in the early stage.
For autoimmune diseases, the pathogenesis involves complex immune abnormalities, involving multiple cytokines. Theoretically, this should be the ideal application of BsAbs, but in practice, it presents great challenges, mainly due to the following limitations. First, autoimmune diseases have very strong heterogeneity. The specific cytokines and cell levels in different patients vary greatly, which limits the clinical application of the corresponding antibody. Although these problems also exist with mAbs (Figures 2A,B), because monoclonal antibodies involve only one site and BsAbs involve two, BsAbs are more restricted in their application to the immune network. Second, immunogenicity limits the use of BsAbs. BsAbs are mostly fragment-based and nonnative formats, which may have stronger immunogenicity than simple IgG (114). Patients with autoimmune diseases have an overly strong immune response, which may produce anti-antibodies and crossreact with the use of biological products. At the same time, the immune complex formed by the double-targeting effect of BsAbs may be too large, and additional damage may be caused if deposition occurs. Third, there are many kinds of structures of BsAbs (Figures 2C–L), how to choose the most suitable form according to the needs of the target is a problem that needs to be studied.
Although there are some myths about the use and development of BsAbs, they also have obvious advantages. The first is the increase in the number of mechanisms of action (115), which can simultaneously target multiple activation pathways and more robustly inhibit immune responses. The second is that BsAbs are a special antibody mixture, and the ratio of the two antibodies is been determined at the very beginning. Therefore, it is possible to determine the safe dose, maximum dose and other issues during preliminary clinical trials. It is not necessary to consider the dose and effect of the two when using monoclonal antibodies in combination. The future of BsAbs is precision medicine. Once the production cost is greatly reduced and the research and development technology is fully mature, BsAbs and multiple antibodies can be customized according to the specific situation of each subtype or even each patient to achieve the relief of patients’ symptoms.
Conclusion
Biological therapies for SLE are diverse, covering all B cell-associated processes, from proliferation and differentiation to activation. There are some agents that work well, such as belimumab, rituximab and atacicept, on the market. RC18 is expected to be the world’s first dual-target biological drug for SLE. However, most of the biological agents are still in the phase II and III clinical stages or even in the preclinical stage, and have poor efficacy, side effects and other issues. In addition, many agents that have been widely used in other diseases are gradually broadening their indications and are being tested in SLE, but their efficacy needs further verification.
At present, biological agents are mainly used for patients with moderate and severe SLE. In the case that immunosuppressive agents and hormone therapy are ineffective, biological agents are used to control the disease (116). Therefore, there is still a question of whether the combination of drugs is reasonable. Rotalizumab is a good example for us to pay attention to the subgroup patients and give personalized treatment, according to the corresponding biological response. BsAbs are also currently being studied in SLE. The advantage of BsAbs is that blocking multiple activation pathways not only reduces the immune response but also changes the existing market (117, 118). Certainly, BsAbs against SLE are still in a relatively preliminary stage, and the specific dose problems need further clinical trials to be determined. Both mAbs and BsAbs have the problem of producing anti-antibodies, which leads to tolerance. Therefore, the use of therapeutic antibodies for in vitro immunosorbent therapy is also a promising application. Ustekinumab is also an insightful idea. Combined with the subunit of IL21/IL23, ustekinumab can affect the two up-regulated pathways. Finding more specific key targets is critical in the development of antibodies.
Author Contributions
BY wrote the manuscript. MZ did the editing. HW and QL revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 81972943 and 81830097) and Hunan Talent Young Investigator (No. 2019RS2012).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Tsokos G, Lo M, Costa Reis P, Sullivan K. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol. (2016) 12:716–30. doi: 10.1038/nrrheum.2016.186
2. Mohan C, Putterman C. Genetics and pathogenesis of systemic lupus erythematosus and lupus nephritis. Nat Rev Nephrol. (2015) 11:329–41. doi: 10.1038/nrneph.2015.33
3. Goulielmos G, Zervoua M, Vazgiourakis V, Ghodke-Puranik Y, Garyfallos A, Niewold T. The genetics and molecular pathogenesis of systemic lupus erythematosus (SLE) in populations of different ancestry. Gene. (2018) 668:59–72. doi: 10.1016/j.gene.2018.05.041
4. Yap D, Chan TB. Cell abnormalities in systemic Lupus Erythematosus and Lupus Nephritis-role in pathogenesis and effect of immunosuppressive treatments. Int J Mol Sci. (2019) 20:6231. doi: 10.3390/ijms20246231
5. Tanaka Y, Kubo S, Iwata S, Yoshikawa M, Nakayamada S. B cell phenotypes, signaling and their roles in secretion of antibodies in systemic lupus erythematosus. Clin Immunol. (2018) 186:21–5. doi: 10.1016/j.clim.2017.07.010
6. Karpusas M, Cachero T, Qian F, Boriack-Sjodin A, Mullen C, Strauch K, et al. Crystal structure of extracellular human BAFF, a TNF family member that stimulates B lymphocytes. J Mol Biol. (2002) 315:1145–54. doi: 10.1006/jmbi.2001.5296
7. Smulski C, Eibel H. BAFF and BAFF-Receptor in B Cell Selection and Survival. Front Immunol. (2018) 9:2285. doi: 10.3389/fimmu.2018.02285
8. Ferraccioli G, Gremese E. B cell activating factor (BAFF) and BAFF receptors: fakes and facts. Clin Exp Immunol. (2017) 190:291–2. doi: 10.1111/cei.13039
9. Vincent F, Morand E, Schneider P, Mackay F. The BAFF/APRIL system in SLE pathogenesis. Nat Rev Rheumatol. (2014) 10:365–73. doi: 10.1038/nrrheum.2014.33
10. Baker K, Edwards B, Main S, Choi G, Wager R, Halpern W, et al. Generation and characterization of LymphoStat-B,a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum. (2003) 48:3253–65. doi: 10.1002/art.11299
11. Nakayamada S, Tanaka Y. BAFF- and APRIL-targeted therapy in systemic autoimmune diseases. Inflamm Regen. (2016) 36:6. doi: 10.1186/s41232-016-0015-4
12. Furier P, Petrl M, Zamani O, Cervera R, Wallace D, Tegzová D, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. (2011) 63:3918–30. doi: 10.1002/art.30613
13. Zhang F, Bae S, Bass D, Chu M, Egginton S, Gordon D, et al. A pivotal phase III, randomised, placebo-controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan and South Korea. Ann Rheum Dis. (2018) 77:355–63. doi: 10.1136/annrheumdis-2017-211631
14. Gatto M, Saccon F, Zen M, Regola F, Fredi M, Andreoli L, et al. Early disease and low baseline damage as predictors of response to belimumab in patients with systemic Lupus Erythematosus in a real-life setting. Arthritis Rheumatol. (2020) 72:1314–24. doi: 10.1002/art.41253
15. Brunner H, Abud-Mendoza C, Viola D, Penades I, Levy D, Anton J, et al. Safety and efficacy of intravenous belimumab in children with systemic lupus erythematosus: results from a randomised, placebo-controlled trial. Ann Rheum Dis. (2020). doi: 10.1136/annrheumdis-2020-217101 [Epub ahead of print].
16. Manetta J, Bina H, Ryan P, Fox N, Witcher D, Kikly K. Generation and characterization of tabalumab, a human monoclonal antibody that neutralizes both soluble and membrane-bound B-cell activating factor. J Inflamm Res. (2014) 7:121–31. doi: 10.2147/JIR.S67751
17. Genovese M, Bojin S, Biagini I, Mociran E, Cristei D, Mirea G, et al. Tabalumab in rheumatoid arthritis patients with an inadequate response to methotrexate and naive to biologic therapy: a phase II, randomized, placebo-controlled trial. Arthritis Rheum. (2013) 65:880–9. doi: 10.1002/art.37820
18. Isenberg D, Petri M, Kalunian K, Tanaka Y, Urowitz M, Hoffman R, et al. Efficacy and safety of subcutaneous tabalumab in patients with systemic lupus erythematosus: results from ILLUMINATE-1, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled study. Ann Rheum Dis. (2016) 75:323–31. doi: 10.1136/annrheumdis-2015-207653
19. Merrill J, Vollenhoven R, Buyon J, Furie R, Stohl W, Morgan-Cox M, et al. Efficacy and safety of subcutaneous tabalumab, a monoclonal antibody to B-cell activating factor, in patients with systemic lupus erythematosus: results from ILLUMINATE-2, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled study. Ann Rheum Dis. (2016) 75:332–40. doi: 10.1136/annrheumdis-2015-207654
20. Hsu H, Khare S, Lee F, Miner K, Hu Y, Stolina M, et al. A novel modality of BAFF-specific inhibitor AMG623 peptibody reduces B-cell number and improves outcomes in murine models of autoimmune disease. Clin Exp Rheumatol. (2012) 30:197–201.
21. Lenert A, Niewold T, Lenert P. Spotlight on blisibimod and its potential in the treatment of systemic lupus erythematosus: evidence to date. Drug Des Devel Ther. (2017) 11:747–57. doi: 10.2147/DDDT.S114552
22. Stohl W, Merrill J, Looney R, Buyon J, Wallace D, Weisman M, et al. Treatment of systemic lupus erythematosus patients with the BAFF antagonist “peptibody” blisibimod (AMG 623/A-623): results from randomized, double-blind phase 1a and phase 1b trials. Arthritis Res Ther. (2015) 17:215. doi: 10.1186/s13075-015-0741-z
23. Merrill J, Shanahan W, Scheinberg M, Kalunian K, Wofsy D, Martin R, et al. Phase III trial results with blisibimod, a selective inhibitor of B-cell activating factor, in subjects with systemic lupus erythematosus (SLE): results from a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. (2018) 77:883–9. doi: 10.1136/annrheumdis-2018-213032
24. Dall’Era M, Chakravarty E, Wallace D, Genovese M, Weisman M, Kavanaugh A, et al. Reduced B lymphocyte and immunoglobulin levels after atacicept treatment in patients with systemic lupus erythematosus: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating trial. Arthritis Rheum. (2007) 56:4142–50. doi: 10.1002/art.23047
25. Cogollo E, Silva M, Isenberg D. Profile of atacicept and its potential in the treatment of systemic lupus erythematosus. Drug Des Devel Ther. (2015) 9:1331–9. doi: 10.2147/DDDT.S71276
26. Pena-Rossi C, Nasonov E, Stanislav M, Yakusevich V, Ershova O, Lomareva N, et al. An exploratory dose-escalating study investigating the safety, tolerability, pharmacokinetics and pharmacodynamics of intravenous atacicept in patients with systemic lupus erythematosus. Lupus. (2018) 18:547–55. doi: 10.1177/0961203309102803
27. Merrill J, Wallace D, Wax S, Kao A, Fraser P, Chang P, et al. Efficacy and safety of atacicept in patients with systemic Lupus Erythematosus: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled, parallel-arm, phase IIb study. Arthritis Rheumatol. (2018) 70:266–76. doi: 10.1002/art.40360
28. Daridon C, Blassfeld D, Reiter K, Mei H, Giesecke C, Goldenberg D, et al. Epratuzumab targeting of CD22 affects adhesion molecule expression and migration of B-cells in systemic lupus erythematosus. Arthritis Res Ther. (2010) 12:R204. doi: 10.1186/ar3179
29. Macauley M, Pfrengle F, Rademacher C, Nycholat C, Gale A, Drygalski A, et al. Antigenic liposomes displaying CD22 ligands induce antigen-specific B cell apoptosis. J Clin Invest. (2013) 123:3074–83. doi: 10.1172/JCI69187
30. Dorner T, Lipsky P. Beyond pan-B-cell-directed therapy – new avenues and insights into the pathogenesis of SLE. Nat Rev Rheumatol. (2016) 12:645–57. doi: 10.1038/nrrheum.2016.158
31. Du F, Mills E, Mao-Draayer Y. Next-generation anti-CD20 monoclonal antibodies in autoimmune disease treatment. Auto Immun Highlights. (2017) 8:12. doi: 10.1007/s13317-017-0100-y
32. Dorner T, Shock A, Goldenberg D, Lipsky P. The mechanistic impact of CD22 engagement with epratuzumab on B cell function: implications for the treatment of systemic lupus erythematosus. Autoimmun Rev. (2015) 14:1079–86. doi: 10.1016/j.autrev.2015.07.013
33. Geh D, Gordon C. Epratuzumab for the treatment of systemic lupus erythematosus. Expert Rev Clin Immunol. (2018) 14:245–58. doi: 10.1080/1744666X.2018.1450141
34. Strand V, Petri M, Kalunian K, Gordon C, Wallace D, Hobbs K, et al. Epratuzumab for patients with moderate to severe flaring SLE: health-related quality of life outcomes and corticosteroid use in the randomized controlled ALLEVIATE trials and extension study SL0006. Rheumatology. (2014) 53:502–11. doi: 10.1093/rheumatology/ket378
35. Wallace D, Kalunian K, Petri M, Strand V, Houssiau F, Pike M, et al. Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: results from EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled, multicentre study. Ann Rheum Dis. (2014) 73:183–90. doi: 10.1136/annrheumdis-2012-202760
36. Clowse M, Wallace D, Furie R, Petri M, Pike M, Leszczyński P, et al. Efficacy and safety of epratuzumab in moderately to severely active systemic lupus erythematosus: results from two phase III randomized, double-blind, placebo-controlled trials. Arthritis Rheumatol. (2017) 69:362–75. doi: 10.1002/art.39856
37. Kosits C, Callaghan M. Rituximab: a new monoclonal antibody therapy for non-Hodgkin’s lymphoma. Oncol Nurs Forum. (2000) 27:51–9.
38. Rovin B, Furie R, Latinis K, Looney R, Fervenza F, Sanchez-Guerrero J, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis assessment with rituximab study. Arthritis Rheum. (2012) 64:1215–26. doi: 10.1002/art.34359
39. Condon M, Ashby D, Pepper R, Cook H, Levy J, Griffith M, et al. Prospective observational single-centre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but no oral steroids. Ann Rheum Dis. (2013) 72:1280–6. doi: 10.1136/annrheumdis-2012-202844
40. Kraaij T, Kamerling S, de Rooij E, van Daele P, Bredewold O, Bakker J, et al. The NET-effect of combining rituximab with belimumab in severe systemic lupus erythematosus. J Autoimmun. (2018) 91:45–54. doi: 10.1016/j.jaut.2018.03.003
41. Robak T, Robak E. New anti-CD20 monoclonal antibodies for the treatment of B-cell lymphoid malignancies. BioDrugs. (2011) 25:13–25. doi: 10.2165/11539590-000000000-00000
42. Tahir H, Rohrer J, Bhatia A, Wegener W, Isenberg D. Humanized anti-CD20 monoclonal antibody in the treatment of severe resistant systemic lupus erythematosus in a patient with antibodies against rituximab. Rheumatology. (2005) 44:561–2. doi: 10.1093/rheumatology/keh533
43. Valentine K, Hoyer K. CXCR5+ CD8 T cells: protective or pathogenic? Front Immunol. (2019) 10:1322. doi: 10.3389/fimmu.2019.01322
44. Wiener A, Schippers A, Wagner N, Tacke F, Ostendorf T, Honke N, et al. CXCR5 is critically involved in progression of lupus through regulation of B cell and double-negative T cell trafficking. Clin Exp Immunol. (2016) 184:22–32. doi: 10.1111/cei.12791
45. Da Z, Li L, Zhu J, Gu Z, You B, Shan Y, et al. CXCL13 promotes proliferation of mesangial cells by combination with CXCR5 in SLE. J Immunol Res. (2016) 2016:2063985. doi: 10.1155/2016/2063985
46. Bao Y, Wang J, Dai Z, Mao Y, Wu J, Guo H, et al. Increased circulating CXCL13 levels in systemic lupus erythematosus and rheumatoid arthritis: a meta-analysis. Clin Rheumatol. (2020) 39:281–90. doi: 10.1007/s10067-019-04775-z
47. Klimatcheva E, Pandina T, Reilly C, Torno S, Bussler H, Scrivens M, et al. CXCL13 antibody for the treatment of autoimmune disorders. BMC Immunol. (2015) 16:6. doi: 10.1186/s12865-015-0068-1
48. Carreira P, Isenberg D. Recent developments in biologic therapies for the treatment of patients with systemic lupus erythematosus. Rheumatology. (2019) 58:382–7. doi: 10.1093/rheumatology/key064
49. Sharabi A, Tsokos G. T cell metabolism: new insights in systemic lupus erythematosus pathogenesis and therapy. Nat Rev Rheumatol. (2020) 16:100–12. doi: 10.1038/s41584-019-0356-x
50. Jiang H, Chess L. An integrated view of suppressor T cell subsets in immunoregulation. J Clin Invest. (2004) 114:1198–208. doi: 10.1172/JCI23411
51. Chen L, Flies D. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. (2013) 13:227–42. doi: 10.1038/nri3405
52. Kawabe T, Matsushima M, Hashimoto N, Imaizumi K, Hasegawa Y. CD40/CD40 ligand interactions in immune responses and pulmonary immunity. Nagoya J Med Sci. (2011) 73:69–78.
53. Tocoian A, Buchan P, Kirby H, Soranson J, Zamacona M, Walley R, et al. First-in-human trial of the safety, pharmacokinetics and immunogenicity of a PEGylated anti-CD40L antibody fragment (CDP7657) in healthy individuals and patients with systemic lupus erythematosus. Lupus. (2015) 24:1045–56. doi: 10.1177/0961203315574558
54. Chamberlain C, Colman P, Ranger A, Burkly L, Johnston G, Otoul C, et al. Repeated administration of dapirolizumab pegol in a randomised phase I study is well tolerated and accompanied by improvements in several composite measures of systemic lupus erythematosus disease activity and changes in whole blood transcriptomic profiles. Ann Rheum Dis. (2017) 76:1837–44. doi: 10.1136/annrheumdis-2017-211388
55. Tivol E, Borriello F, Schweitzer A, Lynch W, Bluestone J, Sharpe A. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. (1995) 3:541–7. doi: 10.1016/1074-7613(95)90125-6
56. National Institute of Diabetes and Digestive and Kidney Diseases. Abatacept. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases (2012).
57. Pimentel-Quiroz V, Ugarte-Gil M, Alarcon G. Abatacept for the treatment of systemic lupus erythematosus. Expert Opin Investig Drugs. (2016) 25:493–9. doi: 10.1517/13543784.2016.1154943
58. Merrill J, Burgos-Vargas R, Westhovens R, Chalmers A, D’Cruz D, Wallace D, et al. The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. (2010) 62:3077–87. doi: 10.1002/art.27601
59. Yang J, Zhang J, Cai Q, Zhao D, Wang J, Guo P, et al. Expression and function of inducible costimulator on peripheral blood T cells in patients with systemic lupus erythematosus. Rheumatology. (2005) 44:1245–54. doi: 10.1093/rheumatology/keh724
60. Cheng L, Amoura Z, Cheah B, Hiepe F, Sullivan B, Zhou L, et al. Brief report: a randomized, double-blind, parallel-group, placebo-controlled, multiple-dose study to evaluate AMG 557 in patients with systemic lupus erythematosus and active lupus arthritis. Arthritis Rheumatol. (2018) 70:1071–6. doi: 10.1002/art.40479
61. Sullivan B, Tsuji W, Kivitz A, Peng J, Arnold G, Boedigheimer M, et al. Inducible T-cell costimulator ligand (ICOSL) blockade leads to selective inhibition of anti-KLH IgG responses in subjects with systemic lupus erythematosus. Lupus Sci Med. (2016) 3:e000146. doi: 10.1136/lupus-2016-000146
62. Rau F, Dieter J, Luo Z, Priest S, Baumgarth N. B7-1/2 (CD80/CD86) direct signaling to B cells enhances IgG secretion. J Immunol. (2009) 183:7661–71. doi: 10.4049/jimmunol.0803783
63. Smith S, Schöder H, Johnson J, Jung S, Bartlett N, Cheson B, et al. The anti-CD80 primatized monoclonal antibody, galiximab, is well-tolerated but has limited activity in relapsed Hodgkin lymphoma: cancer and leukemia group B 50602 (Alliance). Leuk Lymphoma. (2013) 54:1405–10. doi: 10.3109/10428194.2012.744453
64. Han L, Shen L, Zhu Y, Qiu Y. A monoclonal antibody against CD86 and its protection in a murine lupus nephritis model of chronic graft-versus-host disease. Immunopharmacol Immunotoxicol. (2017) 39:285–91. doi: 10.1080/08923973.2017.1354878
65. Reynolds J, McCarthy E, Haque S, Ngamjanyaporn P, Sergeant J, Lee E, et al. Cytokine profiling in active and quiescent SLE reveals distinct patient subpopulations. Arthritis Res Ther. (2018) 20:173. doi: 10.1186/s13075-018-1666-0
66. Pranzatelli M. Advances in biomarker-guided therapy for pediatric- and adult-onset neuroinflammatory disorders: targeting chemokines/cytokines. Front Immunol. (2018) 9:557. doi: 10.3389/fimmu.2018.00557
67. Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. (2011) 1813:878–88. doi: 10.1016/j.bbamcr.2011.01.034
68. Yip R, Yim C. Role of interleukin 6 inhibitors in the management of rheumatoid arthritis. J Clin Rheumatol. (2019). doi: 10.1097/rhu.0000000000001293 [Epub ahead of print].
69. Tang Y, Tao H, Gong Y, Chen F, Li C, Yang X. Changes of serum IL-6, IL-17, and complements in systemic lupus erythematosus patients. J Interferon Cytokine Res. (2019) 39:410–5. doi: 10.1089/jir.2018.0169
70. Illei G, Shirota Y, Yarboro C, Daruwalla J, Tackey E, Takada K, et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum. (2010) 62:542–52. doi: 10.1002/art.27221
71. Thanarajasingam U, Niewold T. Sirukumab: a novel therapy for lupus nephritis? Expert Opin Invest Drugs. (2014) 23:1449–55. doi: 10.1517/13543784.2014.950837
72. Rovin BH, van Vollenhoven R, Aranow C, Wagner C, Gordon R, Zhuang Y, et al. A multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of treatment with sirukumab (CNTO 136) in patients with active lupus nephritis. Arthritis Rheum. (2016) 68:2174–83. doi: 10.1002/art.39722
73. Li D, Guo B, Wu H, Tan L, Chang C, Lu Q. Interleukin-17 in systemic lupus erythematosus: a comprehensive review. Autoimmunity. (2015) 48:353–61. doi: 10.3109/08916934.2015.1037441
74. Frieder J, Kivelevitch D, Menter A. Secukinumab: a review of the anti-IL-17A biologic for the treatment of psoriasis. Ther Adv Chronic Dis. (2017) 9:5–21. doi: 10.1177/2040622317738910
75. Satoh Y, Nakano K, Yoshinari H, Nakayamada S, Iwata S, Kubo S, et al. A case of refractory lupus nephritis complicated by psoriasis vulgaris that was controlled with secukinumab. Lupus. (2018) 27:1202–6. doi: 10.1177/0961203318762598
76. Chasset F, Arnaud L. Targeting interferons and their pathways in systemic lupus erythematosus. Autoimmun Rev. (2018) 17:44–52. doi: 10.1016/j.autrev.2017.11.009
77. Oganesyan V, Peng L, Woods R, Wu H, Dall’Acqua W. Structural insights into the neutralization properties of the fully human, anti-interferon monoclonal antibody sifalimumab. J Biol Chem. (2015) 290:14979–85. doi: 10.1074/jbc.M115.652156
78. Khamashta M, Merrill J, Werth V, Furie R, Kalunian K, Illei G, et al. Sifalimumab, an anti-interferon-alpha monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study. Ann Rheum Dis. (2016) 75:1909–16. doi: 10.1136/annrheumdis-2015-208562
79. Takeuchi T, Tanaka Y, Matsumura R, Saito K, Yoshimura M, Amano K, et al. Safety and tolerability of sifalimumab, an anti-interferon-alpha monoclonal antibody, in Japanese patients with systemic lupus erythematosus: a multicenter, phase 2, open-label study. Mod Rheumatol. (2020) 30:93–100. doi: 10.1080/14397595.2019.1583832
80. Anderson E, Furie R. Anifrolumab in systemic lupus erythematosus: current knowledge and future considerations. Immunotherapy. (2020) 12:275–86. doi: 10.2217/imt-2020-0017
81. Stohl W. Future prospects in biologic therapy for systemic lupus erythematosus. Nat Rev Rheumatol. (2013) 9:705–20. doi: 10.1038/nrrheum.2013.136
82. Kalunian K, Merrill J, Maciuca R, McBride J, Townsend M, Wei X, et al. A Phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-alpha) in patients with systemic lupus erythematosus (ROSE). Ann Rheum Dis. (2016) 75:196–202. doi: 10.1136/annrheumdis-2014-206090
83. Furie R, Khamashta M, Merrill J, Werth V, Kalunian K, Brohawn P, et al. Anifrolumab, an anti-interferon-alpha receptor monoclonal antibody, in moderate-to-severe systemic lupus erythematosus. Arthritis Rheumatol. (2017) 69:376–86. doi: 10.1002/art.39962
84. Morand E, Furie R, Tanaka Y, Bruce I, Askanase A, Richez C, et al. Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med. (2020) 382:211–21. doi: 10.1056/NEJMoa1912196
85. Furie R, Morand E, Bruce I, Manzi S, Kalunian K, Vital E, et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): a randomised, controlled, phase 3 trial. Lancet Rheumatol. (2019) 1:e208–19. doi: 10.1016/S2665-9913(19)30076-1
86. Idborg H, Eketjäll S, Pettersson S, Gustafsson J, Zickert A, Kvarnström M, et al. TNF-alpha and plasma albumin as biomarkers of disease activity in systemic lupus erythematosus. Lupus Sci Med. (2018) 5:e000260. doi: 10.1136/lupus-2018-000260
87. Matsumura R, Umemiya K, Sugiyama T, Sueishi M, Umibe T, Ichikawa K, et al. Anti-tumor necrosis factor therapy in patients with difficult-to-treat lupus nephritis: a prospective series of nine patients. Clin Exp Rheumatol. (2009) 27:416–21.
88. Long D, Chen Y, Wu H, Zhao M, Lu Q. Clinical significance and immunobiology of IL-21 in autoimmunity. J Autoimmun. (2019) 99:1–14. doi: 10.1016/j.jaut.2019.01.013
89. Dai H, He F, Tsokos G, Kyttaris V. IL-23 limits the production of IL-2 and promotes autoimmunity in lupus. J Immunol. (2017) 199:903–10. doi: 10.4049/jimmunol.1700418
90. Benson J, Sachs C, Treacy G, Zhou H, Pendley C, Brodmerkel C, et al. Therapeutic targeting of the IL-12/23 pathways: generation and characterization of ustekinumab. Nat Biotechnol. (2011) 29:615–24. doi: 10.1038/nbt.1903
91. van Vollenhoven R, Hahn B, Tsokos G, Wagner C, Lipsky P, Touma Z, et al. Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet. (2018) 392:1330–9. doi: 10.1016/S0140-6736(18)32167-6
92. Trouw L, Pickering M, Blom A. The complement system as a potential therapeutic target in rheumatic disease. Nat Rev Rheumatol. (2017) 13:538–47. doi: 10.1038/nrrheum.2017.125
93. National Institute of Diabetes and Digestive and Kidney Diseases. Eculizumab. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases (2012).
94. Lonze B, Singer A, Montgomery R. Eculizumab and renal transplantation in a patient with CAPS. N Engl J Med. (2010) 362:1744–5. doi: 10.1056/NEJMc0910965
95. Sun Z, Du X, Su L, Zhang X, Wang Y, Ren L, et al. Successful renal transplantation in a patient with atypical hemolytic uremic syndrome treated with eculizumab in China. Chin Med J (Engl). (2016) 129:1379–81. doi: 10.4103/0366-6999.182843
96. Lonze B, Zachary A, Magro C, Desai N, Orandi B, Dagher N, et al. Eculizumab prevents recurrent antiphospholipid antibody syndrome and enables successful renal transplantation. Am J Transplant. (2014) 14:459–65. doi: 10.1111/ajt.12540
97. Zhu Y, Shen R, Hao R, Wang S, Ho M. Highlights of antibody engineering and therapeutics 2019 in San Diego, USA: bispecific antibody design and clinical applications. Antib Ther. (2020) 3:146–54. doi: 10.1093/abt/tbaa012
98. Zhao Q. Bispecific antibodies for autoimmune and inflammatory diseases: clinical progress to date. BioDrugs. (2020) 34:111–9. doi: 10.1007/s40259-019-00400-2
99. Sanz I, Yasothan U, Kirkpatrick P. Belimumab. Nat Rev Drug Discov. (2011) 10:335–6. doi: 10.1038/nrd3436
100. Benschop R, Chow C, Tian Y, Nelson J, Barmettler B, Atwell S, et al. Development of tibulizumab, a tetravalent bispecific antibody targeting BAFF and IL-17A for the treatment of autoimmune disease. mAbs. (2019) 11:1175–90. doi: 10.1080/19420862.2019.1624463
101. Deng F, Chen J, Zheng J, Chen Y, Huang R, Yin J, et al. Association of BAFF and IL-17A with subphenotypes of primary Sjogren’s syndrome. Int J Rheum Dis. (2016) 19:715–20. doi: 10.1111/1756-185X.12569
102. Lam KLQ, Ko OKH, Zheng B, Lu L. Local BAFF gene silencing suppresses Th17-cell generation and ameliorates autoimmune arthritis. Proc Natl Acad Sci USA. (2008) 105:14993–8. doi: 10.1073/pnas.0806044105
103. Zhang M, Lee F, Kniza A, Jacobsen F, Yu S, Ishida K, et al. Development of an ICOSL and BAFF bispecific inhibitor AMG 570 for systemic lupus erythematosus treatment. Clin Exp Rheumatol. (2019) 37: 906–14.
104. Rossi E, Chang C, Goldenberg D. Anti-CD22/CD20 bispecific antibody with enhanced trogocytosis for treatment of Lupus. PLoS One. (2014) 9:e98315. doi: 10.1371/journal.pone.0098315
105. Rossi E, Chang C, Cardillo T, Goldenberg D. Optimization of multivalent bispecific antibodies and immunocytokines with improved in vivo properties. Bioconjug Chem. (2013) 24:63–71. doi: 10.1021/bc300488f
106. Murphy G, Isenberg D. New therapies for systemic lupus erythematosus – past imperfect, future tense. Nat Rev Rheumatol. (2019) 15:403–12. doi: 10.1038/s41584-019-0235-5
107. Chu S, Yeter K, Kotha R, Pong E, Miranda Y, Phung S, et al. Suppression of rheumatoid arthritis B cells by XmAb5871, an anti-CD19 antibody that coengages B cell antigen receptor complex and Fcgamma receptor IIb inhibitory receptor. Arthritis Rheumatol. (2014) 66:1153–64. doi: 10.1002/art.38334
108. Szili D, Cserhalmi M, Bankó Z, Nagy G, Szymkowski D, Sármay G. Suppression of innate and adaptive B cell activation pathways by antibody coengagement of FcgammaRIIb and CD19. mAbs. (2014) 6:991–9. doi: 10.4161/mabs.28841
109. Horton H, Chu S, Ortiz E, Pong E, Cemerski S, Leung I, et al. Antibody-mediated coengagement of FcgammaRIIb and B cell receptor complex suppresses humoral immunity in systemic lupus erythematosus. J Immunol. (2011) 186:4223–33. doi: 10.4049/jimmunol.1003412
110. Schlatter D, Brack S, Banner D, Batey S, Benz J, Bertschinger J, et al. Generation, characterization and structural data of chymase binding proteins based on the human Fyn kinase SH3 domain. mAbs. (2012) 4:497–508. doi: 10.4161/mabs.20452
111. Lyman M, Lieuw V, Richardson R, Timmer A, Stewart C, Granger S, et al. A bispecific antibody that targets IL-6 receptor and IL-17A for the potential therapy of patients with autoimmune and inflammatory diseases. J Biol Chem. (2018) 293:9326–34. doi: 10.1074/jbc.M117.818559
112. Przepiorka D, Ko C, Deisseroth A, Yancey C, Candau-Chacon R, Chiu H, et al. FDA approval: blinatumomab. Clin Cancer Res. (2015) 21:4035–9. doi: 10.1158/1078-0432.CCR-15-0612
114. Peyrin-Biroulet L, Demarest S, Nirula A. Bispecific antibodies: the next generation of targeted inflammatory bowel disease therapies. Autoimmun Rev. (2019) 18:123–8. doi: 10.1016/j.autrev.2018.07.014
115. Husain B, Ellerman D. Expanding the boundaries of biotherapeutics with bispecific antibodies. BioDrugs. (2018) 32:441–64. doi: 10.1007/s40259-018-0299-9
116. Chan A, Carter P. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol. (2010) 10:301–16. doi: 10.1038/nri2761
117. Fan G, Wang Z, Hao M, Li J. Bispecific antibodies and their applications. J Hematol Oncol. (2015) 8:130. doi: 10.1186/s13045-015-0227-0
118. Labrijn A, Janmaat M, Reichert J, Parren P. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. (2019) 18:585–608. doi: 10.1038/s41573-019-0028-1
119. Brinkmann U, Kontermann R. The making of bispecific antibodies. mAbs. (2017) 9:182–212. doi: 10.1080/19420862.2016.1268307
Keywords: SLE, belimumab, bispecific antibodies, tibulizumab, biological therapy
Citation: Yang B, Zhao M, Wu H and Lu Q (2020) A Comprehensive Review of Biological Agents for Lupus: Beyond Single Target. Front. Immunol. 11:539797. doi: 10.3389/fimmu.2020.539797
Received: 02 March 2020; Accepted: 01 September 2020;
Published: 02 October 2020.
Edited by:
Lazaros Ignatios Sakkas, University of Thessaly, GreeceReviewed by:
Trine N. Jorgensen, Case Western Reserve University, United StatesIoannis Parodis, Karolinska Institutet (KI), Sweden
Copyright © 2020 Yang, Zhao, Wu and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haijing Wu, Q2hyaXN3dTEwMTBAY3N1LmVkdS5jbg==; Q2hyaXN3dTEwMTBAMTI2LmNvbQ==; Qianjin Lu, cWlhbmx1NTg2MEBjc3UuZWR1LmNu; cWlhbmx1NTg2MEBnbWFpbC5jb20=
†These authors have contributed equally to this work
 Bingyi Yang
Bingyi Yang Ming Zhao†
Ming Zhao† Qianjin Lu
Qianjin Lu