- 1Division of Immunology, Department of Infectious Diseases, Faculty of Medicine, University of Miyazaki, Miyazaki, Japan
- 2Department of Dermatology, Faculty of Medicine, University of Miyazaki, Miyazaki, Japan
- 3Japan Agency for Medical Research and Development (AMED), Tokyo, Japan
- 4Department of Oral and Maxillofacial Surgery, Faculty of Medicine, University of Miyazaki, Miyazaki, Japan
- 5Department of Otolaryngology, Head and Neck Surgery, Faculty of Medicine, University of Miyazaki, Miyazaki, Japan
- 6Department of Biomedical Sciences, School of Medicine, Nazarbayev University, Nur-Sultan, Kazakhstan
- 7Division of Histochemistry and Cell Biology, Department of Anatomy, Faculty of Medicine, University of Miyazaki, Miyazaki, Japan
Atopic dermatitis (AD) is a common pruritic inflammatory skin disease characterized by impaired epidermal barrier function and dysregulation of Thelper-2 (TH2)-biased immune responses. While the lineage of conventional dendritic cells (cDCs) are implicated to play decisive roles in T-cell immune responses, their requirement for the development of AD remains elusive. Here, we describe the impact of the constitutive loss of cDCs on the progression of AD-like inflammation by using binary transgenic (Tg) mice that constitutively lacked CD11chi cDCs. Unexpectedly, the congenital deficiency of cDCs not only exacerbates the pathogenesis of AD-like inflammation but also elicits immune abnormalities with the increased composition and function of granulocytes and group 2 innate lymphoid cells (ILC2) as well as B cells possibly mediated through the breakdown of the Fms-related tyrosine kinase 3 ligand (Flt3L)-mediated homeostatic feedback loop. Furthermore, the constitutive loss of cDCs accelerates skin colonization of Staphylococcus aureus (S. aureus), that associated with disease flare. Thus, cDCs maintains immune homeostasis to prevent the occurrence of immune abnormalities to maintain the functional skin barrier for mitigating AD flare.
Introduction
Dendritic cells (DCs) are important professional antigen (Ag)-presenting cells (APCs) that play pleiotropic roles in integration and fine-tuning of the immune system, bridging between innate and adaptive immunity (1–3). DCs encompass the functionally distinguishable two principal lineages, classical or conventional DCs (cDCs) and plasmacytoid DCs (pDCs) (1–3). cDCs constitute unique APCs endowed with the unrivaled immunogenicity with remarkable expressions of major histocompatibility complex (MHC) and costimulatory molecules that license them for priming naïve T cells to differentiate into various types of effector T (Teff) cells (1–3). On the other hand, pDCs are characterized to secrete a large amount of type-I interferon (IFN) following recognition of viral nucleic acids through endosomal toll-like receptor (TLR)7/9 for the initiation of antiviral responses (3, 4). Conversely, DCs are also implicated to be crucial for generating immune tolerance, and that mechanism includes immune suppression by CD4+Foxp3+ regulatory T (Treg) cells under the homeostatic conditions and certain environmental conditions (3, 5–9).
Atopic dermatitis (AD) is a chronic relapsing inflammatory skin disorder associated with itchy eczematous skin lesion (10–12). The pathophysiology of AD is complex and results from impaired epidermal barrier function and cutaneous inflammation as well as a type 2 helper T (TH2)-skewed immune dysregulation with elevated serum immunoglobulin (Ig)E level and peripheral blood eosinophilia, caused by the interaction between genetic and environmental predispositions (10–15). Moreover, the pruritic inflammatory lesion of AD increases the susceptibility to microbial colonization such as infections to Staphylococcus aureus (S. aureus) that associated with disease flare (11, 12, 16–18). While the lineage of DCs have been considered to play decisive roles in the pathogenesis of cutaneous allergic diseases (19–29), the intrinsic role of cDCs in the maintenance of skin immune homeostasis in the steady-state conditions that impacts the onset of eczematous inflammation remains unclear.
In this study, we address how the congenital deficiency of CD11chi cDCs affects the development of experimental pruritic dermatitis by using binary transgenic (Tg) mice that constitutively lacked CD11chi cDCs (30). Unexpectedly, the constitutive loss of cDCs caused the immune dysregulation leading to the exacerbation of AD-like inflammation, which demonstrates the first time that cDCs mediate the maintenance of skin immune homeostasis mitigating the allergic skin disorders.
Materials and Methods
Mice
The following 6- to 12-week-old mice were used in this study. C57BL/6 mice (Japan Clea), B6.Cg-Tg(Itgax-cre)1-1Reiz/J mice (31) (CD11c-Cre mice; The Jackson Laboratory), and R26:lacZbpAfloxDTA mice (R-DTA mice) (32). R-DTA mice and CD11c-Cre mice, which had been backcrossed for ten generations on to C57BL/6 mice, were cross-mated for generating CD11c-Cre:R-DTA mice used as ΔCD11chi cDC mice, and their WT littermates were used as CD11chi cDC-sufficient control mice. B6.CD45.1+OT-I TCR Tg mice harboring OVA-specific CD8+ T cells (B6.CD45.1+OT-I mice) and B6.CD45.1+OT-II TCR Tg mice harboring OVA-specific CD4+ T cells (B6.CD45.1+OT-II mice) were generated as described previously (4, 33–36). All mice were bred and maintained in specific pathogen-free conditions in the animal facility at the University of Miyazaki. All experiments were performed in accordance with institutional guidelines and approved by the Animal Experiment Committee and Gene Recombination Experiment Committee at the University of Miyazaki.
Additional Methods
Tissue and cell isolation (4, 5, 9, 33–37), flow cytometry (4, 5, 9, 33–37), quantitative reverse transcription polymerase chain reaction (RT-PCR) (9, 36), measurement of serum Ig, Fms-related tyrosine kinase 3 ligand (Flt3L), and cytokines, AD-like inflammation (9, 13, 15, 37–39. 15), adoptive transfer (4, 33–36), histopathologic assessment (9, 37), immunohistochemical analysis, and bacterial culture (16) are described in Supplementary Material.
Statistical Analysis
Data are expressed as the mean ± s.d from three to ten individual samples in a single experiment, and we performed at least three independent experiments. The statistical significance of the differences between the values obtained was evaluated by two-sided paired student t-test or two-way ANOVA. A P value of <.05 was considered significant.
Results
Constitutive Loss of cDCs Alters the Immune Cell Composition Under Steady-State Conditions
To address the role of cDCs in the progression of AD-like inflammation, we generated a mouse model that constitutively lacks CD11chi cDCs (30) by crossing CD11c-Cre bacterial artificial chromosome (BAC) Tg mice (CD11c-Cre mice) (31) to mice that harbor the diphtheria toxin (DT) α chain (DTA) under control of a loxP-flanked stop cassette in the ubiquitously expressed ROSA26 locus (R-DTA mice) (32) to produce CD11c-Cre:R-DTA double-Tg mice, referred to as CD11c-Cre:R-DTA mice. Although similar absolute cell numbers of leukocytes were observed in spleen (Spl) and ear-draining lymph nodes (EDLNs) between wild-type (WT) littermates and CD11c-Cre:R-DTA mice (Figures S1A, B in Supplementary Materials), CD11c-Cre:R-DTA mice revealed almost complete elimination of CD11chi cDCs in Spl and EDLNs in the homeostatic conditions when compared with WT mice (Figures S1C, D, S2A in Supplementary Materials). Histological analysis confirmed the lack of CD11chi cDCs in Spl of CD11c-Cre:R-DTA mice (Figure S2B in Supplementary Materials). Furthermore, normal composition of pDCs was observed in Spl of CD11c-Cre:R-DTA mice, while their proportion were slightly reduced in EDLNs (Figures S1C, D in Supplementary Materials). On the other hand, epidermal LCs highly expressing CD11c were barely detected in ear epidermis of CD11c-Cre:R-DTA mice (Figures S2C–E in Supplementary Materials), that were differently observed in the previous report (30).
Collectively, CD11c-Cre:R-DTA mice shows the selective constitutive ablation of CD11chi cDC subsets (called “ΔCD11chi cDC mice” hereafter).
We also examined the influence of the constitutive elimination of CD11chi cDCs on the composition of leukocytes in lymphoid and peripheral tissues under steady-state condition. ΔCD11chi cDC mice displayed the reduced proportions of CD4+ T cells, CD8+ T cells, γδT cells, CD4+ST2+ cells known as pathogenic TH2 cells (36), CD4+Foxp3+ Treg cells, and natural killer T (NKT) cells, whereas they exhibited the enhanced proportions of neutrophils, eosinophils, and basophils/mast cells (MCs) in Spl as compared with WT mice (Figure S1C in Supplementary Materials). Similar results were observed in the proportions of CD4+ T cells, CD8+ T cells, CD4+Foxp3+ Treg cells, neutrophils, and eosinophils in EDLNs between WT mice and ΔCD11chi cDC mice (Figure S1D in Supplementary Materials).
Taken together, these results indicate that the constitutive absence of CD11chi cDCs leads to abnormal composition of leukocytes in lymphoid tissues.
Constitutive Loss of cDCs Causes the Spontaneous Inflammatory Responses
We compared the inflammatory status between WT mice and ΔCD11chi cDC mice in the homeostatic conditions. ΔCD11chi cDC mice exhibited the higher serum productions of tumor necrosis factor (TNF)-α, IFN-γ, interleukin (IL)-4, IL-5, IL-13, IL-17A, and Flt3L than WT mice (Figure S3A in Supplementary Materials). When compared with WT mice, ΔCD11chi cDC mice exhibited the enhanced expressions of Il4, Il13, Il33, Tarc, and S100a8 known as alarmin, whereas they showed the reduced expressions of Saa2 and Loricrin known as the barrier-related molecules in ear cutaneous tissues (Figure S3B in Supplementary Materials).
Taken together, these results indicate that the constitutive depletion of CD11chi cDCs triggers spontaneous inflammation in periphery and cutaneous tissues.
Constitutive Loss of cDCs Causes the Adaptive Immune Abnormality Under Steady-State Conditions
To determine the role of CD11chi cDCs in the initiation of Ag-specific T-cell responses, we adaptively transferred eFluor670-labelled OT-II+CD4+ T cells or OT-I+CD8+ T cells expressing the ovalbumin (OVA)-specific T-cell receptor (TCR) (4, 33–36) into WT mice or ΔCD11chi cDC mice, administrated OVA protein, and monitored their Ag-specific division in Spl and EDLNs. In contrast to the marked division of OT-II+CD4+ T cells or OT-I+CD8+ T cells in Spl and EDLNs in WT mice following systemic administration of OVA protein, their responses severely diminished in ΔCD11chi cDC mice (Figure S4 in Supplementary Materials).
Collectively, these results indicate that the constitutive deficiency of CD11chi cDCs abolishes the Ag-specific priming of T cells in vivo.
We also addressed the influence of the constitutive deficiency of CD11chi cDCs on the emergences of CD4+ Teff cells and innate lymphoid cells (ILCs) under steady state conditions. In Spl, ΔCD11chi cDC mice showed lower proportions of CD4+IFN-γ+ TH1 cells, CD4+IL-5+ TH2 cells, and CD4+IL-13+ TH2 cells than WT mice (Figure S5A in Supplementary Materials). Conversely, ΔCD11chi cDC mice displayed higher frequencies of Lin-Thy1.2+GATA3+ ILC2, Lin-Thy1.2+GATA3+IL-5+ ILC2, Lin-Thy1.2+GATA3+IL-13+ ILC2, Lin-RORγt+ ILC3, and Lin-RORγt+IL-17A+ ILC3 in Spl than WT mice (Figure S5B in Supplementary Materials). On the other hand, ΔCD11chi cDC mice exhibited higher or lower proportions of Lin-Thy1.2+GATA3+ ILC2 as well as Lin-Thy1.2+GATA3+IL-5+ ILC2 and Lin-Thy1.2+GATA3+IL-13+ ILC2 or CD4+IL-13+ TH2 cells in EDLNs (Figures S5C, D in Supplementary Materials).
Collectively, these results indicate that the constitutive loss of CD11chi cDCs enhances the generation of ILC2, while it impairs the initiation of Ag-specific T-cell responses and the generation of CD4+ Teff cells.
We further explored the influence of the constitutive ablation of CD11chi cDCs in B-cell responses in the homeostatic conditions. ΔCD11chi cDC mice showed more potent serum productions of IgG and IgE than WT mice (Figure S6A in Supplementary Materials). Furthermore, ΔCD11chi cDC mice displayed higher proportions of IgM+ B cells, B cells and IgE+ B cells in Spl and EDLNs (Figures S6B, C in Supplementary Materials). Similar results were observed in the frequencies of IgM+ B cells, B cells and IgE+ B cells in germinal center (GC) of Spl and EDLNs (Figures S6D, E in Supplementary Materials). On the other hand, ΔCD11chi cDC mice showed lower or higher proportion of IgM+ plasma cells or IgE+ plasma cells in bone marrow (BM) than WT mice (Figure S6F in Supplementary Materials).
Taken together, these results indicate that the constitutive deficiency of CD11chi cDCs promotes B-cell responses for the enhanced production of antibody (Ab) in the homeostatic conditions.
Constitutive Loss of cDCs Exacerbates the Development of AD-Like Inflammation
Lineage of DCs are believed to be required for the initiation and progression of the pathogenesis of cutaneous allergic diseases (19–29). We therefore sought to determine the effect of the constitutive elimination of CD11chi cDCs on the development of AD-like pathogenesis by using a low calcemic analogue of vitamin D3 known as MC903 (calcipotriol) (13, 15, 15, 38, 39). Unexpectedly, ΔCD11chi cDC mice exhibited a more prominent AD-like inflammation with significant scaling and thickening than WT mice upon topical application of MC903 on the ear skin (Figures 1A, B). Furthermore, histological analyses revealed that ΔCD11chi cDC mice displayed a more significant epidermal hyperplasia in ear skin as well as cutaneous infiltration of mononuclear cells, including MCs than WT mice following topical application of MC903 (Figures 1C–F).
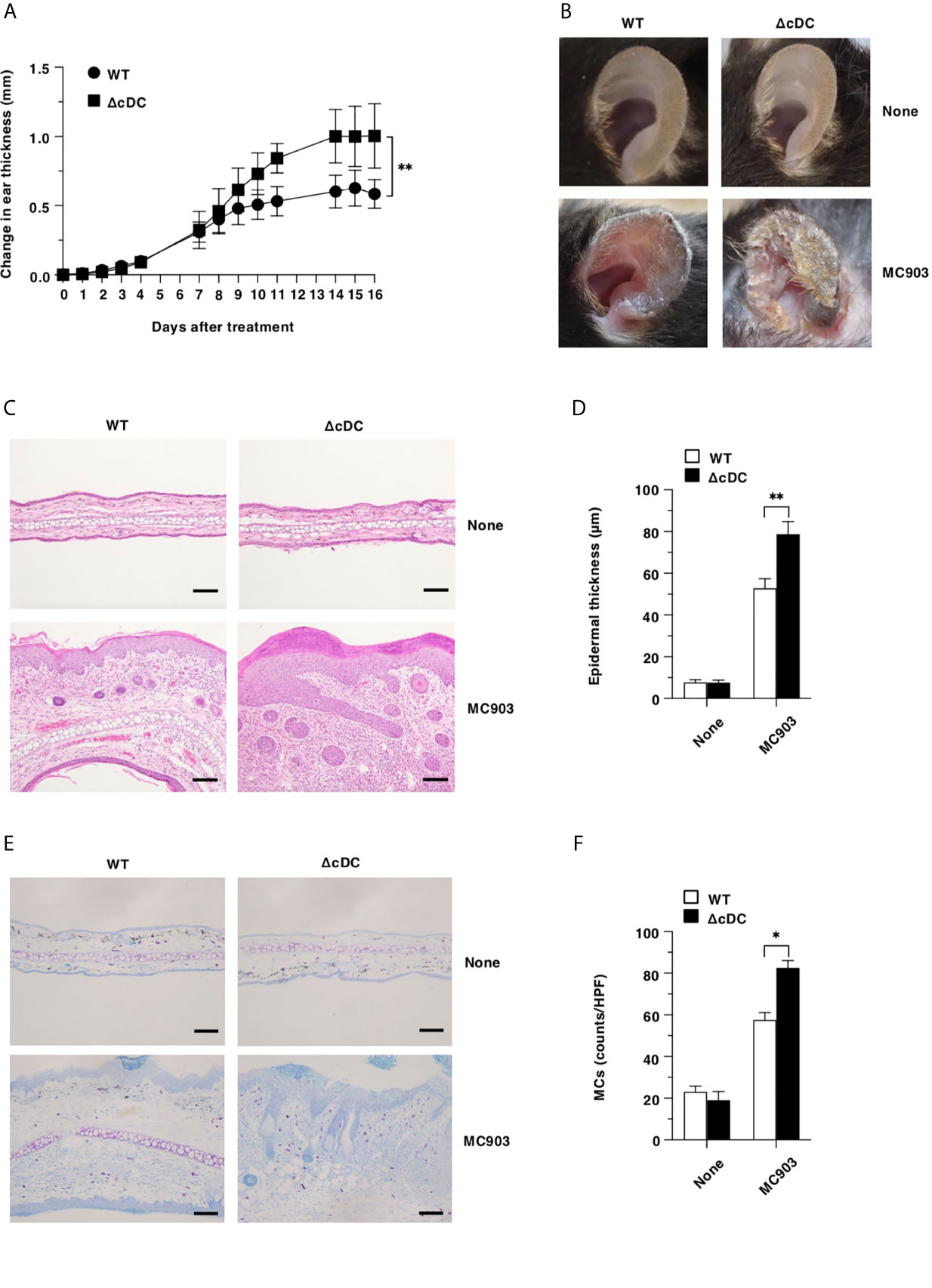
Figure 1 Constitutive loss of cDCs aggravates the development of AD-like inflammation. (A, B) WT mice and ΔCD11chi cDC mice (n = 14 per group) were treated topically with MC903 on the left ear skin. (A) Ear thickness was evaluated for 16 days. Data were obtained from fourteen individual samples in a single experiment. (B) Representative pictures of ear skin lesions at days 0 (None) and 16 (MC903). (C, D) Representative hematoxylin and eosin (H&E) sections (magnification; 200x) of ear skin (C), and epidermal thickness (D) was evaluated at days 0 (None) and 16 (MC903). Bars indicate 100 μm. Data were obtained from five individual samples in a single experiment. (E, F) Representative toluidine blue sections (magnification; 200x) for detecting MCs of ear skin (E), and the quantification of MCs of the skin (F) at days 0 (None) and 16 (MC903). Bars indicate 100 μm. HPF, high performance field. Data were obtained from three individual samples in a single experiment. *P < .05, **P < .01 compared with WT mice by two-sided paired student t-test. All data are representative of at least 3 independent experiments.
Collectively, these results indicate that the constitutive loss of CD11chi cDCs aggravates the development of AD-like inflammation.
Constitutive Loss of cDCs Promotes Type 2 Immune Responses Under Eczematous Inflammatory Conditions
We compared the constitutions of leukocytes in lymphoid and peripheral tissues between WT mice and ΔCD11chi cDC mice after topical application of MC903. ΔCD11chi cDC mice exhibited the reduced frequencies of CD4+ T cells, CD8+ T cells, γδT cells, CD4+ST2+ cells, CD4+Foxp3+ Treg cells, and NKT cells, while they exhibited the enhanced proportions of NK cells, pDCs, neutrophils, and eosinophils in Spl as compared with WT mice (Figure 2A). In EDLNs, ΔCD11chi cDC mice exhibited the reduced frequencies of T cell subsets, NKT cells, and pDCs, whereas they exhibited the enhanced proportions of B cells, NK cells, neutrophils, and basophils/MCs as compared with WT mice (Figure 2B). On the other hand, ΔCD11chi cDC mice exhibited lower or higher frequencies of γδT cells, eosinophils, and basophils/MCs or neutrophils in eczematous ear skin than WT mice (Figure 2C).
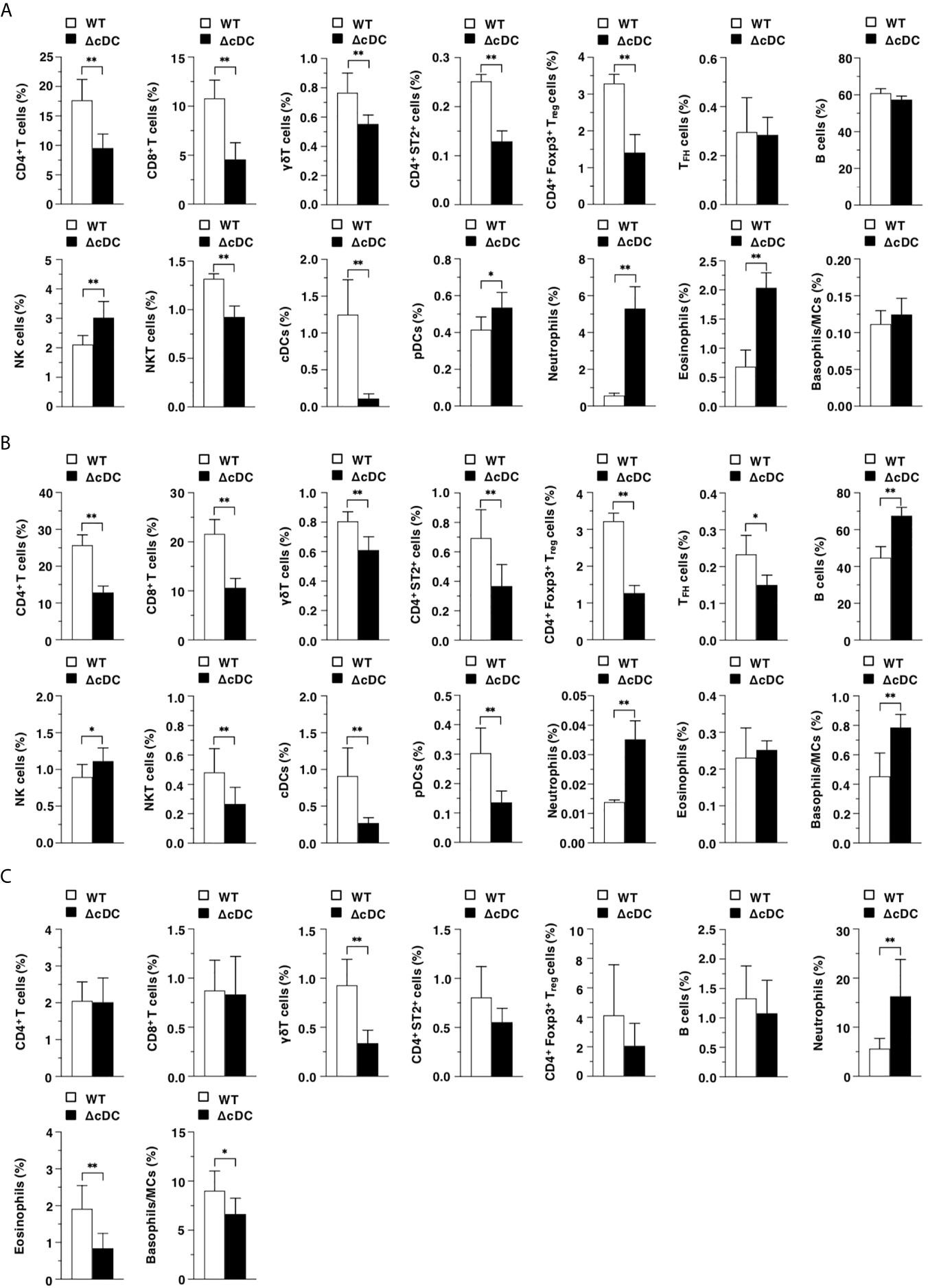
Figure 2 Constitutive loss of cDCs accelerates the AD-associated abnormal immune cell composition. The frequency of leukocytes in Spl (A), EDLNs (B), ear skin (C) obtained from WT mice and ΔCD11chi cDC mice (n = 10 per group) at 16 days after topical application with MC903. Data are obtained from ten individual samples in a single experiment. *P < .05, **P < .01 compared with WT mice by two-sided paired student t-test. All data are representative of at least 3 independent experiments.
Taken together, these results indicate that the constitutive absence of CD11chi cDCs accelerates the AD-associated abnormal composition of leukocytes in lymphoid tissues and eczematous skin lesion.
We next examined the differences in the inflammatory status between WT mice and ΔCD11chi cDC mice after topical application of MC903. ΔCD11chi cDC mice showed the higher serum productions of IFN-γ, IL-6, IL-22, and Flt3L than WT mice (Figure 3A). In ear eczematous tissues, ΔCD11chi cDC mice exhibited the higher or lower expressions of Il4, Il17a, Il33, Saa1, S100a8, and S100a9, or Tslp, Filaggrin, and Loricrin than WT mice (Figure 3B).
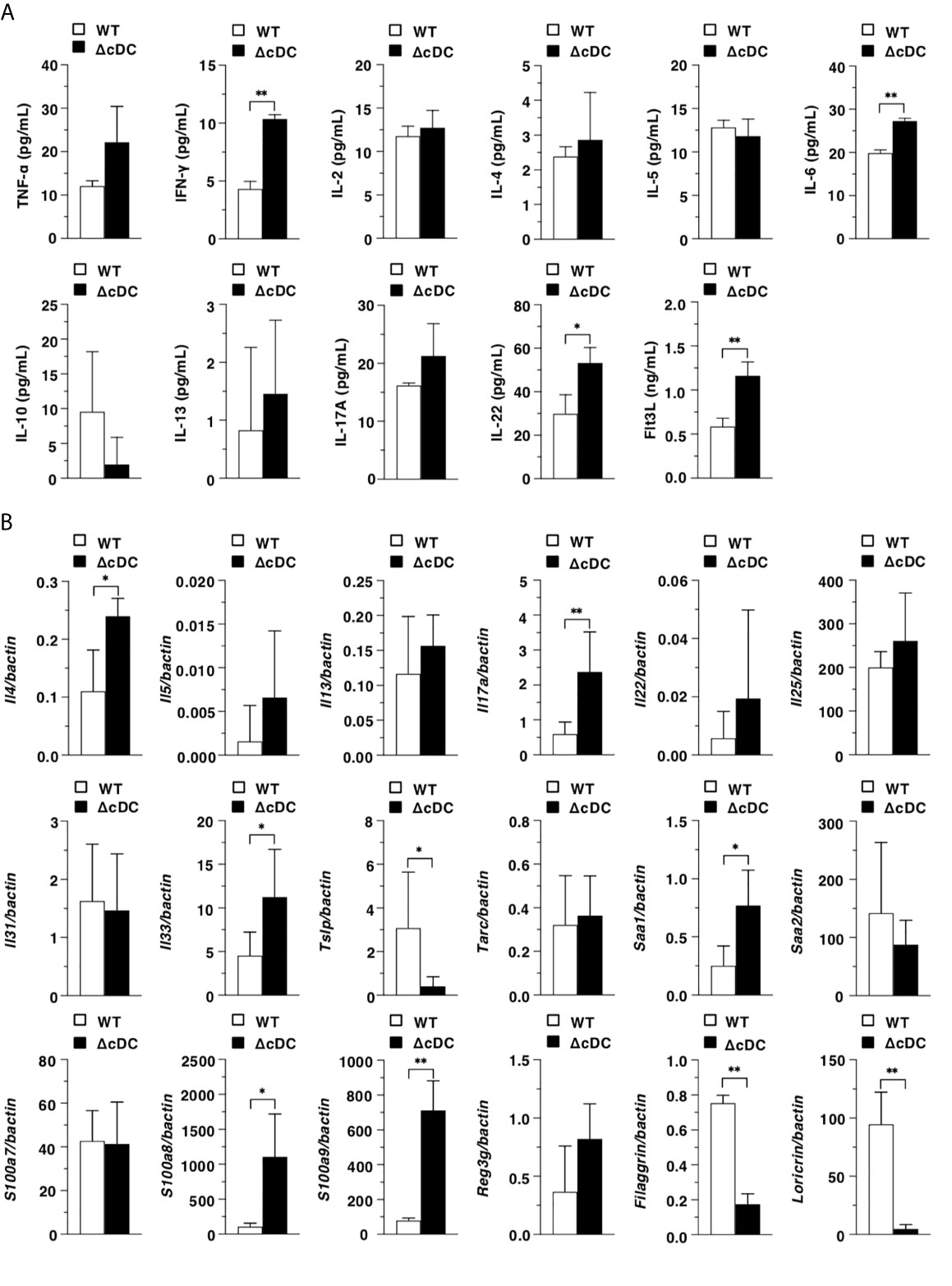
Figure 3 Constitutive loss of cDCs enhances the AD-associated inflammation. (A) Serum production of cytokines in WT mice and ΔCD11chi cDC mice (n = 4 per group) at 16 days after topical application with MC903. Data are obtained from four individual samples in a single experiment. (B) Transcriptional expression of inflammation- and epithelium-related molecules in ear skin obtained from WT mice and ΔCD11chi cDC mice (n = 9 per group) at 16 days after topical application with MC903. Data are obtained from nine individual samples in a single experiment. *P < .05, **P < .01 compared with WT mice by two-sided paired student t-test. All data are representative of at least 3 independent experiments.
Taken together, these results indicate that the constitutive depletion of CD11chi cDCs enhances the AD-associated inflammation in periphery and eczematous skin.
We also evaluated the differences in the generation of CD4+ Teff cells and ILCs in EDLNs between WT mice and ΔCD11chi cDC mice after topical treatment of MC903. The proportions of CD4+IFN-γ+ TH1 cells, CD4+IL-5+ TH2 cells, CD4+IL-13+ TH2 cells, Lin-T-bet+IFN-γ+ ILC1, Lin-RORγt+ ILC3, and Lin-RORγt+IL-17A+ ILC3 were decreased in ΔCD11chi cDC mice, whereas those of Lin-Thy1.2+GATA3+ ILC2, Lin-Thy1.2+GATA3+IL-5+ ILC2 and Lin-Thy1.2+GATA3+IL-13+ ILC2 were enhanced when compared with WT mice (Figure 4).
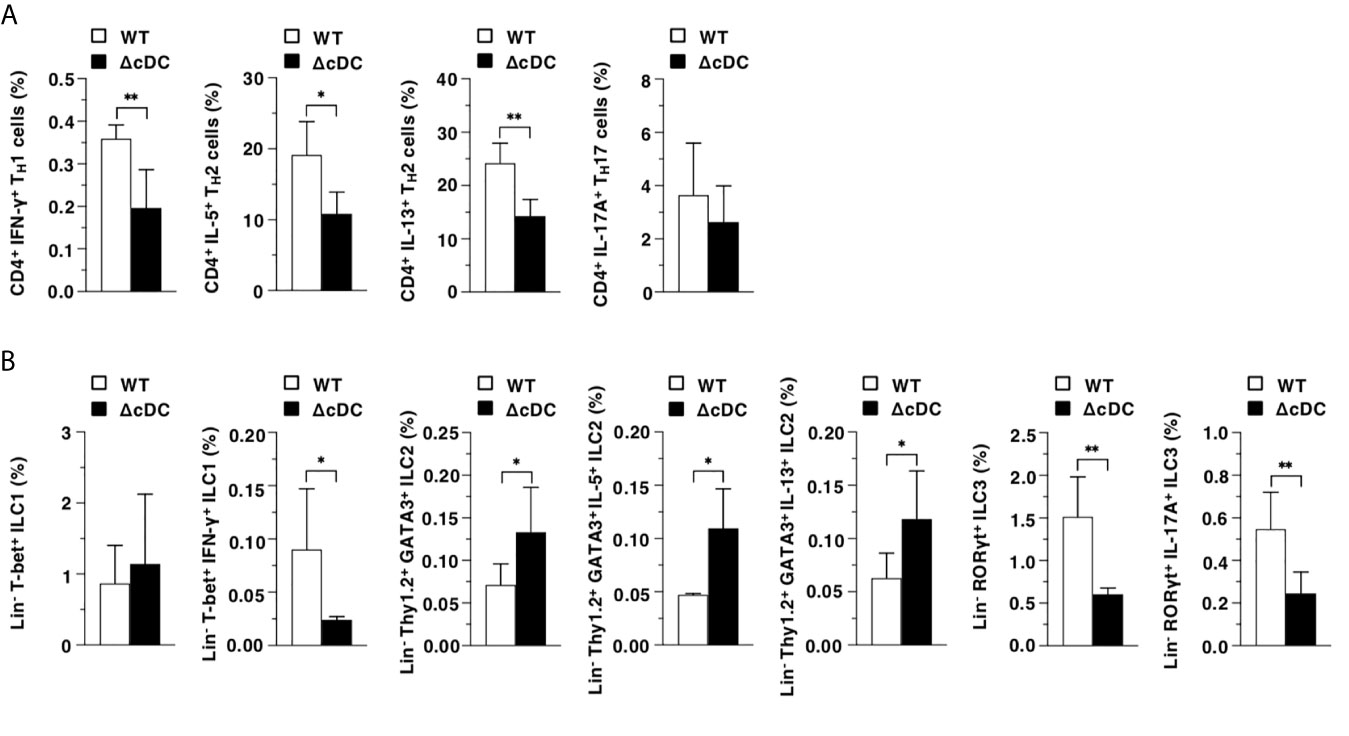
Figure 4 Constitutive loss of cDCs reciprocally controls the composition of CD4+ Teff cells and ILCs during onset of AD-like inflammation. The frequency of the subsets of T cells (A) and ILCs (B) in EDLNs obtained from WT mice and ΔCD11chi cDC mice (n = 6 per group) at 16 days after topical application with MC903. Data are obtained from six individual samples in a single experiment. *P < .05, **P < .01 compared with WT mice by two-sided paired student t-test. All data are representative of at least 3 independent experiments.
Collectively, these results indicate that the constitutive loss of CD11chi cDCs enhances the AD-associated generation of ILC2, while it inhibits the emergence of Teff cells and ILC3.
We further determined the influence of the constitutive ablation of CD11chi cDCs in B-cell responses during onset of AD-like inflammation. ΔCD11chi cDC mice retained higher serum productions of IgG and IgE than WT mice (Figure 5A). Furthermore, ΔCD11chi cDC mice showed higher proportions of IgM+ B cells, B cells and IgE+ B cells in Spl and EDLNs (Figures 5B, C). In addition, the frequencies of B cells and IgE+ B cells in GC of Spl, but not that in EDLNs, were enhanced in ΔCD11chi cDC mice when compared with WT mice (Figures 5D, E). On the other hand, ΔCD11chi cDC mice exhibited lower proportion of IgM+ plasma cells and IgE+ plasma cells in BM than WT mice (Figure 5F).
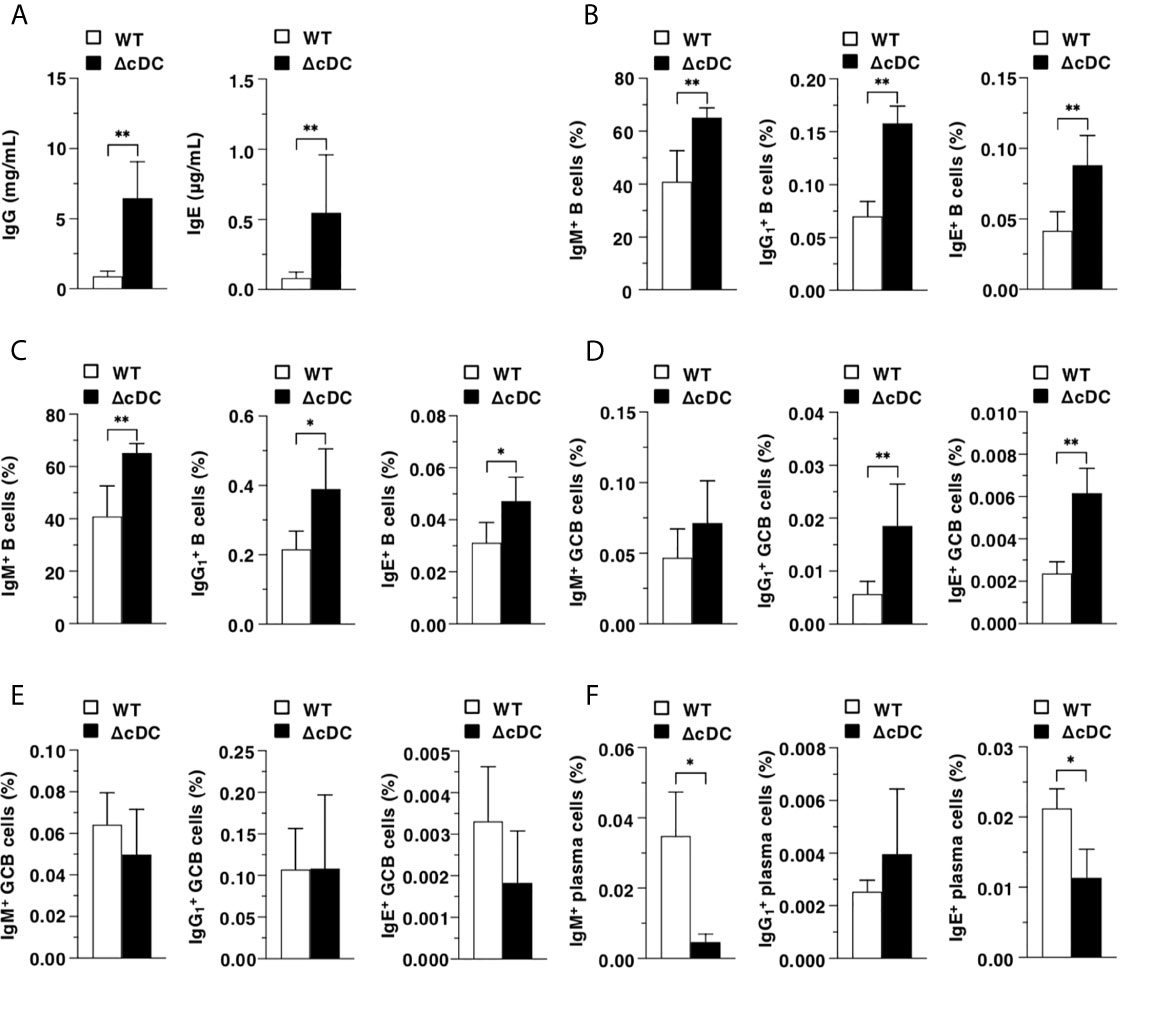
Figure 5 Constitutive loss of cDCs affects the AD-associated B-cell responses in during onset of AD-like inflammation. (A) Serum production of IgG and IgE in WT mice and ΔCD11chi cDC mice (n = 8 per group) at 16 days after topical application with MC903. (B–E) The frequency of the subsets of IgM+ B cells, B cells, and IgE+ B cells in whole (B, C) and GC (D, E) of Spl (B, D) and EDLNs (C, E) obtained from WT mice and ΔCD11chi cDC mice (n = 8 per group) at 16 days after topical application with MC903. (F) The frequency of the subsets of IgM+ plasma cells, plasma cells, and IgE+ plasma cells of BM obtained from WT mice and ΔCD11chi cDC mice (n = 8 per group) at 16 days after topical application with MC903. Data are obtained from eight individual samples in a single experiment. *P < .05, **P < .01 compared with WT mice by two-sided paired student t-test. All data are representative of at least 3 independent experiments.
Taken together, these results indicate that the constitutive deficiency of CD11chi cDCs strengthens B-cell responses for the reinforced secretion of Ab during progression of AD-like inflammation.
Constitutive Loss of cDCs Promotes the Colonization of S. aureus in Eczematous Skin
To address the differences in the colonization of S. aureus in eczematous lesion between WT mice and ΔCD11chi cDC mice after topical treatment of MC903, we cultured the homogenates of their ear skin on Mannitol salt agar (16) after topical application of MC903, and quantified colony-forming units (CFU) of S. aureus. While of S. aureus was barely detected in the ear skin surface of WT mice and ΔCD11chi cDC mice in the homeostatic conditions, ΔCD11chi cDC mice showed a significant outgrowth of S. aureus in the eczematous lesions as compared with WT mice upon topical treatment of MC903 (Figures 6A, B).
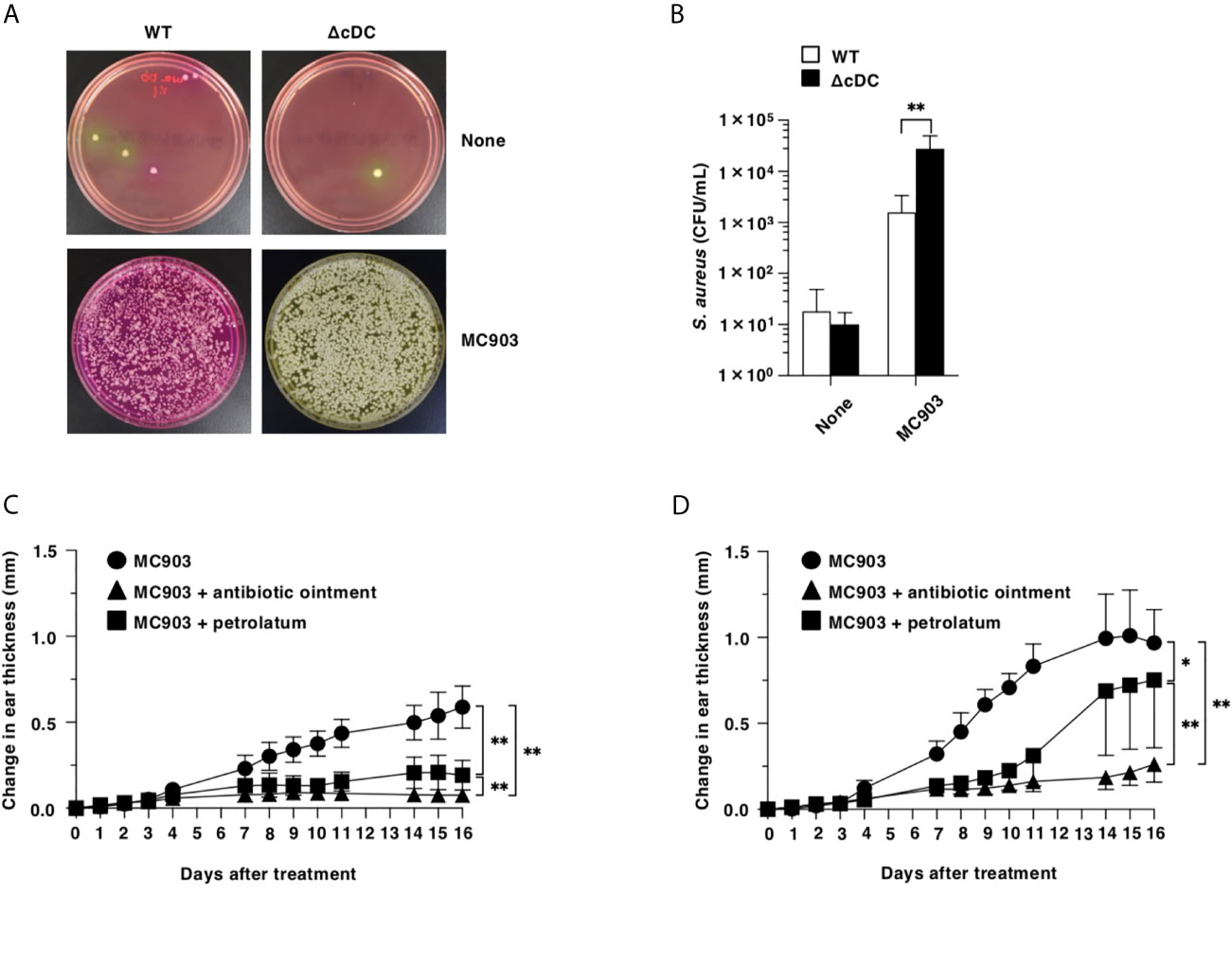
Figure 6 Constitutive loss of cDCs accelerates the colonization of S. aureus and the disruption of barrier function in eczematous skin. (A, B) Quantification of S. aureus cultured from the homogenates of ear skin of WT mice and ΔCD11chi cDC mice (n = 7 per group) at days 0 and 16 after topical application with MC903. (A) Representative pictures of Mannitol salt agar with egg yolk plates at days 0 (None) and 16 (MC903). (B) CFU of S. aureus at days 0 (None) and 16 (MC903) after topical application with MC903. **P <.01 compared with WT mice by two-sided paired student t-test. (C, D) WT mice (C) and ΔCD11chi cDC mice (D) (n = 6 per group) were treated topically with MC903 in combination with or without antibiotic ointment or petrolatum on the left ear skin. Ear thickness was evaluated for 16 days. Data are obtained from seven individual samples in a single experiment. *P < .05, **P < .01 compared with MC903-treated group or among groups. All data are representative of at least 3 independent experiments by two-way ANOVA.
We also examined the association of a dramatic increased colonization of S. aureus with the exacerbated AD-like inflammation under the constitutive lack of CD11chi cDCs (Figures 6C, D). Similarly observed in WT mice, the application of antibiotic ointment to ear skin after topical application of MC903 markedly attenuated the development of AD-like inflammation in ΔCD11chi cDC mice.
To investigate the role of the compromised skin barrier function for the enhanced eczematous inflammation under the constitutive deficiency of CD11chi cDCs, we coated the ear skin of ΔCD11chi cDC mice with petrolatum following topical application of MC903 (Figures 6C, D). Treatment of ΔCD11chi cDC mice with petrolatum prevented from forming eczematous lesions, while this treatment had a more prominent protecting effect in WT mice.
Taken together, these results indicate that the constitutive deficiency of CD11chi cDCs accelerates the colonization of S. aureus and the disruption of barrier function in eczematous skin for developing AD-like inflammation.
Discussion
While the lineage of DCs are believed to be crucial for the initiation and development of AD in humans and rodent models of AD-like inflammation, their intrinsic role for the onset of the eczematous inflammation remains to be determined. In this study, our findings reveal that CD11chi cDCs are dispensable for the development of AD-like inflammation, while the homeostatic feedback loop between CD11chi cDCs and other leukocytes prevents the progression of AD-like inflammation. Thus, CD11chi cDCs could maintain skin immune homeostasis in the steady-state conditions to protect from the exacerbating the onset of eczematous inflammation.
Analysis of ΔCD11chi cDC mice showed that the constitutive absence of CD11chi cDCs led to the abnormal composition of leukocytes in lymphoid tissues, that associated with the reduced or enhanced proportions of T cell-subsets or granulocytes under steady-state conditions. On the other hand, ΔCD11chi cDC mice exhibited an increased serum amounts of Flt3L in the homeostatic conditions. Since Flt3 is expressed on shared progenitors of lymphocytes and all myeloid cells during the early stages of hematopoiesis, Flt3L is critical for the expansion of certain hematopoietic progenitors and the generation of several mature leukocytes (40–42). Furthermore, it has been shown that Flt3+ common myeloid progenitors (CMPs) and their downstream Flt3+ progenitors gave rise to cDCs, which were only mature peripheral leukocytes expressing Flt3 (40, 43). Giving the importance of Flt3L for the development and homeostatic maintenance of all stages of cDC lineage, they might be responsible for major consumption of Flt3L in vivo. These phenomena led us to hypothesize that the absence of CD11chi cDCs causes the release of the excess amounts of Flt3L in periphery. Although this possibility remains elusive, the elevated level of Flt3L in the constitutive absence of CD11chi cDCs would mainly promotes the differentiation of granulocytes as well as other cell types, resulting in the abnormal composition of leukocytes in the steady-states.
We showed that the constitutive absence of CD11chi cDCs elevated the productions of cytokines related to type 2/type 17 immune responses in periphery and cutaneous tissues, linking to the spontaneous systemic and cutaneous inflammatory responses regardless of the homeostatic conditions. Furthermore, the constitutive loss of CD11chi cDCs enhanced or inhibited the expression levels of alarmin or the barrier-related molecules, that associated with cutaneous inflammation and barrier dysfunction (44, 45). Thus, these phenomena imply that the abnormal immune constitution might reduce their threshold of responsiveness to sense endogenous damage-associated molecular patterns (DAMPs) to trigger the inflammation in periphery and cutaneous tissues, and that affects epidermal functionality.
Given the impairment of Ag-specific priming of T cells and reduction in the generation of TH1 cells and TH2 cells in lymphoid tissues in the constitutive absence of CD11chi cDCs under steady-state conditions, CD11chi cDCs could be prerequisite for induction of Ag-specific Teff-responses. On the other hand, the constitutive deletion of CD11chi cDCs enhanced the proportions of ILC2 and ILC3 in lymphoid tissues in the homeostatic conditions. Having demonstrated the critical role of Flt3L for the homeostatic maintenance of ILC2 and ILC3 by acting on early ILC progenitors (42, 46), the increased serum amounts of Flt3L in the absence of CD11chi cDCs could accelerate the development of ILC2 and ILC3, leading to the promotion of type 2/type 17 immune responses in periphery and cutaneous tissues.
We showed that the constitutive ablation of CD11chi cDCs not only elevated serum productions of IgG and IgE but also enhanced the generation of IgM+ B cells, B cells, and IgE+ B cells in lymphoid tissues. It has been reported that Flt3 is expressed on pre-pro B cells and pre B cells, and Flt3L promotes the survival of Flt3+CD19- progenitors (41, 42), supporting a critical role for Flt3L in early B-cell development. On the other hand, it has been shown that ILC2 and ILC3 enhances B-cell proliferation and Ab production in T-cell independent manner (47–50). Therefore, the massive production of Flt3L under the deficiency of CD11chi cDCs could promote the generation of ILC2/ILC3 that stimulates B-cell responses. Taken together, our findings suggest that Flt3L mediates the homeostatic feedback loop in the context of CD11chi cDCs/ILC2/ILC3/B cells under the steady-state conditions.
Different from the implications based on the previous findings with the critical role of CD11chi cDCs for the induction of TH2-responses during the development of allergic cutaneous inflammation (19–29), we revealed the aggressive development of the MC903-induced AD-like inflammation under the constitutive absence of CD11chi cDCs, accompanied by the abnormality in the composition of leukocytes and the enforced peripheral and cutaneous inflammatory status as well as the barrier dysfunction in eczematous lesions. While the constitutive loss of CD11chi cDCs reduced the generation of TH1 cells and TH2 cells as well as ILC1 and ILC3 in lymphoid tissues during the development of MC903-induced AD-like inflammation, it not only promoted the generation of ILC2 in lymphoid tissues but also enhanced serum productions of IgG and IgE, linking with increased emergence of IgM+ B cells, B cells, and IgE+ B cells in lymphoid tissues and decreased generation of IgM+ plasma cells and IgE+ plasma cells. Given the sustained massive serum production of Flt3L under the constitutive deficiency of CD11chi cDCs, the breakdown of the Flt3L-mediated homeostatic feedback loop among CD11chi cDCs/granulocytes/ILC2/B cells could be responsible for the exacerbation of the eczematous inflammation.
The constitutive loss of CD11chi cDCs promoted the colonization of S. aureus in the eczematous ear skin, whereas the application of antibiotic ointment suppressed the progression of the MC903-induced AD-like inflammation. On the other hand, the treatment of the compromised skin barrier function with petrolatum mitigated the severity of the MC903-induced AD-like inflammation under the constitutive absence of CD11chi cDCs. Given the critical role of filaggrin in the formation of the functional skin barrier, the reduced expression of filaggrin caused the elevated skin pH during the development of AD, and that facilitates the colonization of S. aureus in the eczematous lesions, leading to the promotion of the eczematous inflammation (51–55). Therefore, the immune abnormality under the constitutive loss of CD11chi cDCs could enforce the eczematous inflammation in the skin accompanied by the reduced expression of filaggrin, and the disturbed skin barrier function could drive to the abundant colonization of S. aureus for exacerbation of the MC903-induced AD-like inflammation. Taken together, our findings suggest that the existence of CD11chi cDCs maintain the immune homeostasis, linking to the epidermal barrier function to prevent the massive colonization of S. aureus for protecting from AD flare.
In conclusion, our results unravel the unexpected role of CD11chi cDCs in the initiation and progression of AD-like inflammation. It has been highly appreciated that CD11chi cDCs are required for Ag-specific priming of CD4+ T cells to differentiate pathogenic TH2 cells for the development of AD (19–29). However, CD11chi cDCs could provide the Flt3L-mediated homeostatic feedback loop in the context of type 2 immune responses composed of granulocytes/ILC2/B cells, and that is critical link to skin barrier functions to limit the abundant colonization of S. aureus for protecting AD flare. Taken together, our findings propose that CD11chi cDCs maintain immune homeostasis to prevent the occurrence of the immune abnormalities skewing to type 2 immune responses for inhibiting the onset of eczematous inflammation. Thus, CD11chi cDCs and their feedback control may constitute the attractive targets for the intervention and treatment of allergic cutaneous disorders.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by The Animal Experiment Committee and Gene Recombination Experiment Committee at the University of Miyazaki.
Author Contributions
KS designed all experiments, analyzed data and wrote the manuscript. YN, ToF, TaF, TU, HT, JN, NM, and NC did experiments. DR, YH, and MA provided reagents and information. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a Grant-in-Aid for Scientific Research (B) (KS; 18H02670), for challenging Exploratory Research (KS; 16K15291), and for Young Scientists (B) (TU; 17K15027, ToF; 17K15732, and HT; 18K15194) from the Ministry of Education, Science and Culture of Japan, the Project for Cancer Research And Therapeutic Evolution (P-CREATE) from Japan Agency for Medical Research and development (AMED) (KS; 16cm0106307h0001, 17cm0106307h0002, 18cm0106307h0003, and 19cm0106307h0004), the Uehara Memorial Foundation (HT), Takeda Science Foundation (TU and ToF), the Naito Foundation (KS), Bristol-Myers Squibb Foundation Grants (KS), GSK Japan Research Grant 2016 (ToF), GSK Japan Research Grant 2017 (HT), GSK Japan Research Grant 2018 (TU), Daiichi Sankyo Foundation of Life Science (KS), Nipponham Foundation for the Future of Food (HT), and the Shin-Nihon Foundation of Advanced Medical Research (TU), and Nazarbayev University Faculty Development Grant (DR; 090118FD5310). GSK Japan was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all members of the animal facility at University of Miyazaki; Yumiko Sato and Rumi Sunachi for secretarial assistance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.712676/full#supplementary-material
Abbreviations
Ab, Antibody; AD, Atopic dermatitis; Ag, Antigen; APCs, Ag-presenting cells; BM, Bone marrow; cDCs, Conventional dendritic cells; CFU, Colony-forming units; CMPs, Common myeloid progenitors; DAMPs, Damage-associated molecular patterns; DT, Diphtheria toxin; DTA, DT α chain; EDLNs, Ear-draining lymph nodes; ELISA, Enzyme-linked immunosorbent assay; Flt3L, Fms-related tyrosine kinase 3 ligand; GC, Germinal center; HPF, High performance field, H&E, Hematoxylin and eosin, IFN, Interferon; Ig, Immunoglobulin; IL, Interleukin; ILCs, Innate lymphoid cells; i.p., Intraperitoneal; i.v., Intravenously; mAb, Monoclonal Ab; MCs, Mast cells; MHC, Major histocompatibility complex; NKT, Natural killer T; OVA, Ovalbumin; pDCs, Plasmacytoid DCs; PFA, Paraformaldehyde; RT-PCR, Reverse transcription polymerase chain reaction; Spl, Spleen; S. aureus, Staphylococcus aureus; TCR, T-cell receptor; Teff, Effector T; Tg, Transgenic; TH2 cells, Type 2 helper T; TLR, Toll-like receptor; TNF, Tumor necrosis factor; Treg, Regulatory T; WT, Wild-type.
References
1. Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, et al. Dendritic Cells, Monocytes and Macrophages: A Unified Nomenclature Based on Ontogeny. Nat Rev Immunol (2014) 14(8):571–78. doi: 10.1038/nri3712
2. Murphy TL, Grajales-Reyes GE, Wu X, Tussiwand R, Briseño CG, Iwata A, et al. Transcriptional Control of Dendritic Cell Development. Annu Rev Immunol (2016) 34:93–119. doi: 10.1146/annurev-immunol-032713-120204
3. Sato K, Uto T, Fukaya T, Takagi H. Regulatory Dendritic Cells. Curr Top Microbiol Immunol (2017) 410:47–71. doi: 10.1007/82_2017_60
4. Takagi H, Fukaya T, Eizumi K, Sato Y, Sato K, Shibazaki A, et al. Plasmacytoid Dendritic Cells are Crucial for the Initiation of Inflammation and T Cell Immunity In Vivo. Immunity (2011) 35(6):958–71. doi: 10.1016/j.immuni.2011.10.014
5. Fukaya T, Takagi H, Sato Y, Sato K, Eizumi K, Taya H, et al. Crucial Roles of B7-H1 and B7-DC Expressed on Mesenteric Lymph Node Dendritic Cells in the Generation of Antigen-Specific CD4+Foxp3+ Regulatory T Cells in the Establishment of Oral Tolerance. Blood (2010) 116(13):2266–76. doi: 10.1182/blood-2009-10-250472
6. Rescigno M. Dendritic Cells in Oral Tolerance in the Gut. Cell Microbiol (2011) 13(9):1312–8. doi: 10.1111/j.1462-5822.2011.01626.x
7. Bekiaris V, Persson EK, Agace WW. Intestinal Dendritic Cells in the Regulation of Mucosal Immunity. Immunol Rev (2014) 260(1):86–101. doi: 10.1111/imr.12194
8. Esterházy D, Loschko J, London M, Jove V, Oliveira TY, Mucida D. Classical Dendritic Cells Are Required for Dietary Antigen-Mediated Induction of Peripheral Treg Cells and Tolerance. Nat Immunol (2016) 17(5):545–55. doi: 10.1038/ni.3408
9. Uto T, Takagi H, Fukaya T, Nasu J, Fukui T, Miyanaga N, et al. Critical Role of Plasmacytoid Dendritic Cells in Induction of Oral Tolerance. J Allergy Clin Immunol (2018) 141(6):2156–67. doi: 10.1016/j.jaci.2017.11.048
10. Leung DYM. Atopic Dermatitis: The Skin as a Window Into the Pathogenesis of Chronic Allergic Diseases. J Allergy Clin Immunol (1995) 96(3):302–18. doi: 10.1016/s0091-6749(95)70049-8
11. Leung DYM, Bieber T. Atopic Dermatitis. Lancet (2003) 361(9352):151–60. doi: 10.1016/S0140-6736(03)12193-9
12. Leung DYM, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New Insights Into Atopic Dermatitis. J Clin Invest (2004) 113(5):651–57. doi: 10.1172/JCI21060
13. Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. TSLP Elicits IL-33-Independent Innate Lymphoid Cell Responses to Promote Skin Inflammation. Sci Transl Med (2013) 5(170):170ra16. doi: 10.1126/scitranslmed.3005374
14. Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, et al. A Role for IL-25 and IL-33-Driven Type-2 Innate Lymphoid Cells in Atopic Dermatitis. J Exp Med (2013) 13):2939–50. doi: 10.1084/jem.20130351
15. Moosbrugger-Martinz V, Schmuth M, Dubrac S. A Mouse Model for Atopic Dermatitis Using Topical Application of Vitamin D3 or of Its Analog MC903. Methods Mol Biol (2017) 1559:91–106. doi: 10.1007/978-1-4939-6786-5_8
16. Kobayashi T, Glatz M, Horiuchi K, Kawasaki H, Akiyama H, Kaplan DH, et al. Dysbiosis and Staphylococcus Aureus Colonization Drives Inflammation in Atopic Dermatitis. Immunity (2015) 42(4):756–66. doi: 10.1016/j.immuni.2015.03.014
17. Geoghegan JA, Irvine AD, Foster TJ. Staphylococcus Aureus and Atopic Dermatitis: A Complexand Evolving Relationship. Trends Microbiol (2018) 26(6):484–97. doi: 10.1016/j.tim.2017.11.008
18. Fyhrquist N, Muirhead G, Prast-Nielsen S, Jeanmougin M, Olah P, Skoog T, et al. Microbe-Host Interplay in Atopic Dermatitis and Psoriasis. Nat Commun (2019) 10(1):4703. doi: 10.1038/s41467-019-12253-y
19. Elentner A, Finke D, Schmuth M, Chappaz S, Ebner S, Malissen B, et al. Langerhans Cells Are Critical in the Development of Atopic Dermatitis-Like Inflammation and Symptoms in Mice. J Cell Mol Med (2009) 13(8B):2658–72. doi: 10.1111/j.1582-4934.2009.00797.x
20. Novak N, Koch S, Allam JP, Bieber T. Dendritic Cells: Bridging Innate and Adaptive Immunity in Atopic Dermatitis. J Allergy Clin Immunol (2010) 125(1):50–9. doi: 10.1016/j.jaci.2009.11.019
21. Hvid M, Vestergaard C, Kemp K, Christensen GB, Deleuran B, Deleuran M. IL-25 in Atopic Dermatitis: A Possible Link Between Inflammation and Skin Barrier Dysfunction? J Invest Dermatol (2011) 131(1):150–57. doi: 10.1038/jid.2010.277
22. Bieber T, Novak N, Herrmann N, Koch S. Role of Dendritic Cells in Atopic Dermatitis: An Update. Clin Rev Allergy Immunol (2011) 41(3):254–8. doi: 10.1007/s12016-010-8224-0
23. Ito T, Liu YJ, Arima K. Cellular and Molecular Mechanisms of TSLP Function in Human Allergic Disorders - TSLP Programs the “Th2 Code” in Dendritic Cells. Allergol Int (2012) 61(1):35–43. doi: 10.2332/allergolint.11-RAI-0376
24. Novak N. An Update on the Role of Human Dendritic Cells in Patients With Atopic Dermatitis. J Allergy Clin Immunol (2012) 29(4):879–86. doi: 10.1016/j.jaci.2012.01.062
25. Gros E, Novak N. Cutaneous Dendritic Cells in Allergic Inflammation. Clin Exp Allergy (2012) 42(8):1161–75. doi: 10.1111/j.1365-2222.2012.03964.x
26. Leyva-Castillo JM, Hener P, Michea P, Karasuyama H, Chan S, Soumelis V, et al. Skin Thymic Stromal Lymphopoietin Initiates Th2 Responses Through an Orchestrated Immune Cascade. Nat Commun (2013) 4:2847. doi: 10.1038/ncomms3847
27. Hammad H, Lambrecht BN. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity (2015) 43(1):29–40. doi: 10.1016/j.immuni.2015.07.007
28. Li C, Maillet I, Mackowiak C, Viala C, Di Padova F, Li M, et al. Experimental Atopic Dermatitis Depends on IL-33R Signaling via My88 in Dendritic Cells. Cell Death Dis (2017) 8(4):e2735. doi: 10.1038/cddis.2017.90
29. Hill DA, Spergel JM. The Atopic March: Critical Evidence and Clinical Relevance. Ann Allergy Asthma Immunol (2018) 120(2):131–37. doi: 10.1016/j.anai.2017.10.037
30. Birnberg T, Bar-On L, Sapoznikov A, Caton ML, Cervantes-Barragán L, Makia D, et al. Lack of Conventional Dendritic Cells Is Compatible With Normal Development and T Cell Homeostasis, But Causes Myeloid Proliferative Syndrome. Immunity (2008) 29(6):986–97. doi: 10.1016/j.immuni.2008.10.012
31. Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J Signaling Controls the Homeostasis Ofcd8- Dendritic Cells in the Spleen. J Exp Med (2007) 204(7):1653–64. doi: 10.1084/jem.2006264
32. Brockschnieder D, Pechmann Y, Sonnenberg-Riethmacher E, Riethmacher D. An Improvedmouse Line for Cre-Induced Cell Ablation Due to Diphtheria Toxin A, Expressed From the Rosa26 Locus. Genesis (2006) 44(7):322–7. doi: 10.1002/dvg.20218
33. Fukaya T, Murakami R, Takagi H, Sato K, Sato Y, Otsuka H, et al. Conditional Ablation of CD205+ Conventional Dendritic Cells Impacts the Regulation of T-Cell Immunity and Homeostasis In Vivo. Proc Natl Acad Sci USA (2012) 109(28):11288–93. doi: 10.1073/pnas.1202208109
34. Takagi H, Arimura K, Uto T, Fukaya T, Nakamura T, Choijookhuu N, et al. Plasmacytoid Dendritic Cells Orchestrate TLR7-Mediated Innate and Adaptive Immunity for the Initiation of Autoimmune Inflammation. Sci Rep (2016) 6:24477. doi: 10.1038/srep24477
35. Uto T, Fukaya T, Takagi H, Arimura K, Nakamura T, Kojima N, et al. Clec4A4 Is a Regulatory Receptor for Dendritic Cells That Impairs Inflammation and T-Cell Immunity. Nat Commun (2016) 7:11273. doi: 10.1038/ncomms11273
36. Miyanaga N, Takagi H, Uto T, Fukaya T, Nasu J, Fukui T, et al. Essential Role Ofsubmandibular Lymph Node Dendritic Cells in Protective Sublingual Immunotherapy Against Murine Allergy. Commun Biol (2020) 3(1):742. doi: 10.1038/s42003-020-01466-3
37. Fukaya T, Fukui T, Uto T, Takagi H, Nasu J, Miyanaga N, et al. Pivotal Role of IL-22 Binding Protein in the Epithelial Autoregulation of Interleukin-22 Signaling in the Control of Skin Inflammation. Front Immunol (2018) 9:1418. doi: 10.3389/fimmu.2018.01418
38. Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical Vitamin D3 and Low-Calcemic Analogs Induce Thymic Stromal Lymphopoietin in Mouse Keratinocytes and Trigger an Atopic Dermatitis. Proc Natl Acad Sci USA (2006) 103(31):11736–41. doi: 10.1073/pnas.060457510
39. Zhang Z, Hener P, Frossard N, Kato S, Metzger D, Li M, et al. Thymic Stromal Lymphopoietin Overproduced by Keratinocytes in Mouse Skin Aggravates Experimental Asthma. Proc Natl Acad Sci USA (2009) 106(5):1536–41. doi: 10.1073/pnas.0812668106
40. Karsunky H, Merad M, Cozzio A, Weissman IL, Manz MG. Flt3 Ligand Regulates Dendritic Cell Development From Flt3+ Lymphoid and Myeloid-Committed Progenitors to Flt3+ Dendritic Cells. vivo. J Exp Med (2003) 198(2):305–13. doi: 10.1084/jem.20030323
41. Antonysamy MA, Thomson AW. Flt3 Ligand (FL) and Its Influence on Immune Reactivity. Cytokine (2000) 12(2):87–100. doi: 10.1006/cyto.1999.0540
42. Tsapogas P, Mooney CJ, Brown G, Rolink A. The Cytokine Flt3-Ligand in Normal and Malignant Hematopoiesis. Int J Mol Sci (2017) 18(6):1115. doi: 10.3390/ijms18061115
43. Liu K, Nussenzweig MC. Origin and Development of Dendritic Cells. Immunol Rev (2010) 234(1):45–54. doi: 10.1111/j.0105-2896.2009.00879.x
44. Czarnowicki T, Krueger JG, Guttman-Yassky E. Skin Barrier and Immune Dysregulation in Atopic Dermatitis: An Evolving Story With Important Clinical Implications. J Allergy Clin Immunol Pract (2014) 2(4):371–9. doi: 10.1016/j.jaip.2014.03.006
45. Furue M. Regulation of Filaggrin, Loricrin, and Involucrin by IL-4, IL-13, IL-17a, IL-22, AHR, and NRF2: Pathogenic Implications in Atopic Dermatitis. Int J Mol Sci (2020) 21(15):5382. doi: 10.3390/ijms21155382
46. Baerenwaldt A, von Burg N, Kreuzaler M, Sitte S, Horvath E, Peter A, et al. Flt3 Ligand Regulates the Development of Innate Lymphoid Cells in Fetal and Adult Mice. J Immunol (2016) 196(6):2561–71. doi: 10.4049/jimmunol.1501380
47. Drake LY, Iijima K, Bartemes K, Kita H. Group 2 Innate Lymphoid Cells Promote an Early Antibody Response to a Respiratory Antigen in Mice. J Immunol (2016) 197(4):1335–42. doi: 10.4049/jimmunol.1502669
48. Wallrapp A, Riesenfeld SJ, Burkett PR, Kuchroo VK. Type 2 Innate Lymphoid Cells in the Induction and Resolution of Tissue Inflammation. Immunol Rev (2018) 286(1):53–73. doi: 10.1111/imr.12702
49. Akdis CA, Arkwright PD, Brüggen MC, Busse W, Gadina M, Guttman-Yassky E, et al. Type 2 Immunity in the Skin and Lungs. Allergy (2020) 75(7):1582–605. doi: 10.1111/all.14318
50. Magri G, Miyajima M, Bascones S, Mortha A, Puga I, Cassis L, et al. Innate Lymphoid Cells Integrate Stromal and Immunological Signals to Enhance Antibody Production by Splenic Marginal Zone B Cells. Nat Immunol (2014) 15(4):354–64. doi: 10.1038/ni.2830
51. Rippke F, Schreiner V, Doering T, Maibach HI. Stratum Corneum pH in Atopic Dermatitis: Impact on Skin Barrier Function and Colonization With Staphylococcus Aureus. Am J Clin Dermatol (2004) 5(4):217–23. doi: 10.2165/00128071-200405040-00002
52. Miajlovic H, Fallon PG, Irvine AD, Foster TJ. Effect of Filaggrin Breakdown Products on Growth of and Protein Expression by Staphylococcus Aureus. J Allergy Clin Immunol (2010) 126(6):1184–90.e3. doi: 10.1016/j.jaci.2010.09.015
53. van Drongelen V, Haisma EM, Out-Luiting JJ, Nibbering PH, El Ghalbzouri A. Reduced Filaggrin Expression Is Accompanied by Increased Staphylococcus Aureus Colonization of Epidermal Skin Models. Clin Exp Allergy (2014) 44(12):1515–24. doi: 10.1111/cea.12443
54. Nakatsuji T, Chen TH, Two AM, Chun KA, Narala S, Geha RS, et al. Staphylococcus Aureus Exploits Epidermal Barrier Defects in Atopic Dermatitis to Trigger Cytokine Expression. J Invest Dermatol (2016) 136(11):2192–200. doi: 10.1016/j.jid.2016.05.127
Keywords: atopic dermatitis, dendritic cells, type 2 immune responses, immune homeostasis, homeostatic feedback loop
Citation: Nishikawa Y, Fukaya T, Fukui T, Uto T, Takagi H, Nasu J, Miyanaga N, Riethmacher D, Choijookhuu N, Hishikawa Y, Amano M and Sato K (2021) Congenital Deficiency of Conventional Dendritic Cells Promotes the Development of Atopic Dermatitis-Like Inflammation. Front. Immunol. 12:712676. doi: 10.3389/fimmu.2021.712676
Received: 24 May 2021; Accepted: 01 July 2021;
Published: 27 July 2021.
Edited by:
Fabienne Anjuère, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceReviewed by:
Luc Van Kaer, Vanderbilt University, United StatesYan Zhou, The First Affiliated Hospital of Xi’an Jiaotong University, China
Priti Kumar Roy, Jadavpur University, India
Copyright © 2021 Nishikawa, Fukaya, Fukui, Uto, Takagi, Nasu, Miyanaga, Riethmacher, Choijookhuu, Hishikawa, Amano and Sato. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katsuaki Sato, a2F0c3Vha2lfc2F0b0BtZWQubWl5YXpha2ktdS5hYy5qcA==
†These authors have contributed equally to this work
 Yotaro Nishikawa
Yotaro Nishikawa Tomohiro Fukaya
Tomohiro Fukaya Takehito Fukui
Takehito Fukui Tomofumi Uto
Tomofumi Uto Hideaki Takagi
Hideaki Takagi Junta Nasu
Junta Nasu Noriaki Miyanaga
Noriaki Miyanaga Dieter Riethmacher
Dieter Riethmacher Narantsog Choijookhuu
Narantsog Choijookhuu Yoshitaka Hishikawa
Yoshitaka Hishikawa Masahiro Amano
Masahiro Amano Katsuaki Sato
Katsuaki Sato