- 1Molecular Immunology Unit, Swiss Tropical and Public Health Institute, Basel, Switzerland
- 2University of Basel, Basel, Switzerland
- 3Translational Medicine-Preclinical Safety, Novartis Institutes for Biomedical Research, Basel, Switzerland
- 4Novartis Pharma AG, Global Drug Development, London, United Kingdom
- 5Novartis Pharma AG, Global Drug Development, Basel, Switzerland
To assess the relative contribution of opsonisation by antibodies, classical and alternative complement pathways to pneumococcal phagocytosis, we analyzed killing of pneumococci by human blood leukocytes collected from vaccine-naïve and PCV13-vaccinated subjects. With serotype 4 pneumococci as model, two different physiologic opsonophagocytosis assays based on either hirudin-anticoagulated whole blood or on washed cells from EDTA-anticoagulated blood reconstituted with active serum, were compared. Pneumococcal killing was measured in the presence of inhibitors targeting the complement components C3, C5, MASP-2, factor B or factor D. The two assay formats yielded highly consistent and comparable results. They highlighted the importance of alternative complement pathway activation for efficient opsonophagocytic killing in blood of vaccine-naïve subjects. In contrast, alternative complement pathway inhibition did not affect pneumococcal killing in PCV13-vaccinated individuals. Independent of amplification by the alternative pathway, even low capsule-specific antibody concentrations were sufficient to efficiently trigger classical pathway mediated opsonophagocytosis. In heat-inactivated or C3-inhibited serum, high concentrations of capsule-specific antibodies were required to trigger complement-independent opsonophagocytosis. Our findings suggest that treatment with alternative complement pathway inhibitors will increase susceptibility for invasive pneumococcal infection in non-immune subjects, but it will not impede pneumococcal clearance in vaccinated individuals.
1 Introduction
The complement system is a complex protein network with essential functions during immunological and inflammatory processes (1). Complement activation induces direct killing of certain pathogens via insertion of the pore-forming membrane attack complex (MAC, C5b-9), and opsonizes invading bacteria for subsequent engulfment by phagocytes (1, 2). While MAC insertion into the outer membrane of Gram-negative bacteria like meningococci (Neisseria meningitidis) can kill them efficiently within minutes (1–3), Gram-positive bacteria are generally resistant to MAC-induced bacteriolysis, as the terminal complement complex cannot penetrate through the thick peptidoglycan layer of the Gram-positive cell wall (4, 5). The peptidoglycan layer itself, however, might not represent the only resistance mechanism of Gram-positive bacteria to MAC insertion, as recent publications additionally reported the release of specific proteins from Gram-positive bacteria that interfere with MAC assembly (5–7).
Streptococcus pneumoniae is a Gram-positive, extracellular, opportunistic pathogen, which may colonize the mucosa of the human upper respiratory tract (8). Although pneumococcal colonization typically manifests as a commensal relationship with its host (8, 9), pneumococci may also cause a range of invasive diseases including otitis media, sepsis, pneumonia and meningitis (8). Pneumococcal clearance from the bloodstream depends mostly on antibody-mediated opsonophagocytosis enhanced by classical complement pathway (CP)-mediated deposition of C3b on the bacterial cell surface with subsequent recognition by phagocyte C3 receptors (10, 11). Data from humans with homozygous C3 deficiencies further highlighted the importance of C3 during pneumococcal infections, as human C3 deficiency is associated with recurrent and life-threatening bacterial infections by encapsulated bacteria such as S. pneumoniae, N. meningitidis and Haemophilus influenza (12, 13). Deficiencies in alternative complement pathway (AP) components also increase susceptibility to bacterial infections. Factor D (fD) and properdin deficiencies are associated with meningococcal and pneumococcal diseases (14–18). Factor B (fB) deficiency has only been reported in two patients so far, which presented with recurring invasive pneumococcal and meningococcal disease (19, 20). The role of the lectin pathway (LP) during pneumococcal infection remains inconclusive (21), with large studies demonstrating no association between human LP deficiencies and the risk of pneumococcal infection (22, 23), despite affecting disease severity (22).
The pneumococcal polysaccharide capsule represents the major virulence determinant and the immunodominant surface structure of S. pneumoniae (8). After discovering the immunogenicity of purified pneumococcal polysaccharides, polyvalent pneumococcal polysaccharide vaccines (PPVs) were introduced – initially targeting 2 serotypes and progressing to the development of a 23-valent formulation in the early 1980s (24, 25). This eventually resulted in a coverage of up to 95% of circulating invasive pneumococcal strains depending on geographical location (26). PPVs have been demonstrated to be efficacious in preventing invasive pneumococcal disease in adults (27). However, in the elderly population, vaccine efficacy seems to be reduced, and protection against non-bacteremic pneumonia remains controversial (28–31). Together with the lack of immunogenicity of pure polysaccharide vaccines in children below the age of 2 years, PPVs provide suboptimal clinical efficacy for the two largest risk groups of pneumococcal disease (32).
Because of the poor immunogenicity of the T cell-independent pure polysaccharide vaccines in risk groups together with limited duration of protective antibody titers, pneumococcal conjugate vaccines (PCV) were developed (27, 32). By covalently conjugating capsular polysaccharides to immunogenic carrier proteins, the pneumococcal polysaccharides elicit T cell-dependent immune responses with improved immunological memory, which also reduce nasopharyngeal carriage (8, 32–35). In contrast to the PPV response, which relies on specific splenic B cell subsets that are not fully developed before the age of 2 (36, 37), PCVs elicit protective antibody responses in infants and children below the age of 2 (38). PCV13 was shown to provide significant protection against all vaccine serotypes – but serotype 3 – in young children (39) and also reduced vaccine-type pneumonia and invasive disease in the vaccinated elderly population over a 5-year period (40).
Dysregulation of complement activation causes a number of diseases, including paroxysmal nocturnal hemoglobinuria (PNH), C3 glomerulopathy (C3G) and atypical hemolytic uremic syndrome (aHUS) (41). Current treatment of such diseases includes prevention of the complement membrane attack complex (MAC) with monoclonal antibodies (mAbs) that bind to C5 (41). While MAC formation is involved in uncontrolled lysis of erythrocytes in some of these patients, it is also required for serum bactericidal activity (SBA) and therefore, terminal complement blockage increases the risk of invasive disease by encapsulated bacteria (42–45). This has led to the concept that compared to C5 inhibition, specific inhibition of the AP may reduce the infection risk.
To study complement-mediated opsonisation, phagocytosis and killing of encapsulated bacteria, an assay that incorporates active complement and living phagocytes is needed. Widely-used opsonophagocytosis assays are typically based on isolated phagocytes or cell lines, such as HL-60 cells and exogenous standardized complement sources, such as baby rabbit complement (46, 47). Focusing on isolated cell types, however, reduces the complexity found within whole blood and affects the biological properties of the investigated cell types (48). Recently, more physiological methods to study complement-mediated opsonophagocytosis of bacteria have been reported that circumvent these limitations (49, 50). Hirudin-anticoagulated whole blood – by irreversible direct thrombin inhibition (51) – can be directly deployed for opsonophagocytosis assays after venous puncture. It, therefore, represents a particularly physiological assay with minimal effects on complement activity (Figure 1A) (49). Hirudin is used to replace lepirudin (52, 53), which has been withdrawn from the market. Another physiological assay makes use of cells from ethylenediamine tetraacetic acid (EDTA)-anticoagulated whole blood, which are washed and reconstituted with active serum and Ca2+ and Mg2+ to override the effect of chelating bivalent cations by EDTA (50), which interferes with complement activation (Figure 1B) (49). Here we compared results obtained with these two physiological opsonophagocytosis assays and investigated the relative contribution of opsonisation by antibodies, classical, lectin, alternative and terminal complement pathways to pneumococcal opsonophagocytosis. Pneumococcal killing was measured in the presence of inhibitors against complement fB, fD, C3, C5 or the mannose-binding lectin–associated protease 2 (MASP-2).
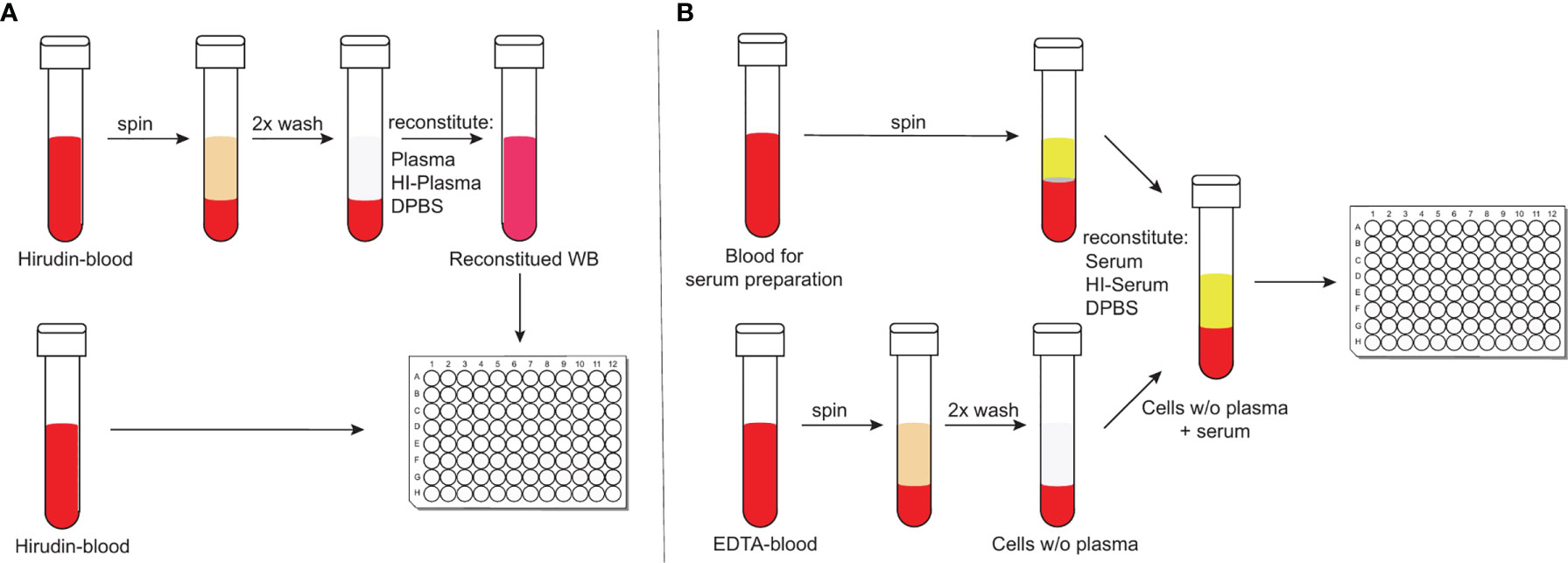
Figure 1 Schematic representation of the two physiological opsonophagocytosis assays. Hirudin-anticoagulated whole blood allows the assessment of bacterial killing in unprocessed whole blood or in reconstituted whole blood with either active or HI-plasma for internal controls (A). DPBS-washed cells from EDTA-anticoagulated blood can be reconstituted with active serum and represents another physiological opsonophagocytosis assay that can be used to analyze previously stored serum samples (B).
2 Methods
2.1 Ethical Approval
All experimental steps involving human specimens were approved by the Ethical Committee of Northwest and Central Switzerland (Ethikkommission Nordwest- und Zentralschweiz (EKNZ), Studie 2018-02341).
2.2 Measurement of Anti-Pneumococcal Capsule Antibody in Serum
Pneumococcal serotype-specific IgG concentrations were analyzed by the Vaccine Evaluation Unit (VEU) of Public Health England (PHE) in Manchester, UK, using the 007sp 12-plex pneumococcal IgG Bioplex assay. The lower limit of quantification (LLOQ) for all serotypes in the Pneumococcal IgG Bioplex assay was 0.10 µg/mL.
2.3 Bacterial Growth
A clinical isolate of Streptococcus pneumoniae (serotype 4, P1541) was used to assess complement-mediated opsonisation and phagocytic killing with the two blood assays. The pneumococcal strain was grown in 10 mL brain heart infusion broth (BHI, BD, Switzerland) for 7 h at 37°C. Subsequently, the culture was diluted 10-fold in fresh pre-warmed BHI broth and grown for 2 h at 37°C with 5% CO2 to logarithmic growth phase (OD600 ≈ 0.65). For opsonophagocytosis experiments, pneumococci were diluted in Dulbecco’s Phosphate-Buffered Saline containing MgCl2 and CaCl2 (DPBS, Sigma-Aldrich, Switzerland) to reach 8.4 ± 2.7 x 103 CFU/mL in hirudin assays or 2.8 ± 1.0 x 103 CFU/mL in ethylenediamine tetraacetic acid (EDTA) assays. CFUs were determined by serial dilution and plating on Columbia agar with 5% sheep blood (CSBA, BioMérieux, Switzerland). Neisseria meningitidis serogroup W case isolate 1682 (54) was grown in Frantz medium, as previously described (55). For opsonophagocytosis experiments, meningococci were diluted in DPBS to reach 3.5 ± 0.8 x 103 CFU/mL in DPBS before adding them to blood.
2.4 Opsonophagocytic Killing Assays
2.4.1 Blood Sampling
Study participants (age range 25-67, Table 1) had either been vaccinated previously with a 13-valent pneumococcal conjugate vaccine (PCV13, Prevenar 13, Pfizer; n=5) or were pneumococcal vaccine-naïve (n=4). Within this study, two physiological opsonophagocytic killing assays were compared using venous blood specimens from these nine healthy volunteers. The assay using hirudin-anticoagulated whole blood collected in S-monovette® 1.6 mL tubes (Sarstedt, Germany) was compared to the assay using EDTA-anticoagulated whole blood collected in BD Vacutainer® 4 mL tubes (BD, Switzerland) washed with DPBS and reconstituted with serum collected in Vacuette® CAT serum tubes (Greiner Bio-One, Switzerland). Blood from an additional volunteer, who had recently received a MenACWY vaccine booster immunization was used for control experiments analyzing meningococcal clearance.
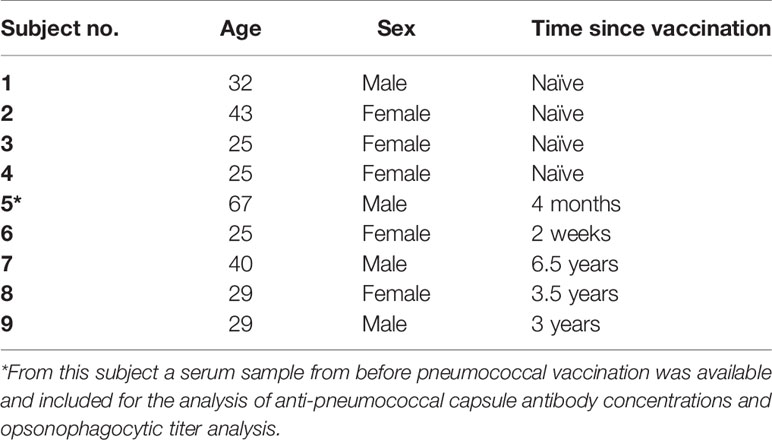
Table 1 Description of study participants with time since receiving the 13-valent pneumococcal conjugate vaccine (PCV13).
2.4.2 Hirudin Method
For the hirudin assay, 80 µL hirudin-anticoagulated whole blood was added per well in a 96-well flat bottom culture microplate (Falcon™, ThermoFisher Scientific, Switzerland) and 10 µL of complement inhibitors or DPBS were added prior to inoculation with 10 µL bacteria. For intrinsic controls, hirudin-anticoagulated whole blood was centrifuged at 1’000 x g for 10 minutes at 4°C, plasma was removed and a part of it was heat-inactivated (HI) at 56°C for 30 minutes. The cell fraction was washed with ice-cold DPBS and reconstituted with the previously determined amount of either active plasma or HI-plasma. From all intrinsic controls, 90 µL were added per well to the 96-well plate (Figure 1A).
2.4.3 EDTA Method
For the EDTA assay, serum collection tubes were kept at room temperature for 30 minutes to allow clotting. Tubes were spun at 1’700 x g at 4°C for 10 minutes and serum was transferred to 1.5 mL screw-cap microcentrifuge tubes. Tubes were either kept on ice, or stored at -80°C, while a fraction was heat-inactivated at 56°C for 30 minutes. EDTA-anticoagulated whole blood was centrifuged at 1’000 x g for 10 minutes at 4°C, washed twice with DPBS and reconstituted with the previously determined amount of either serum or HI-serum. As for the hirudin-assay, 80 µL of reconstituted blood and 10 µL of complement inhibitors or DPBS were added to a 96-well plate before inoculating with 10 µL of desired bacterial dilution. From all intrinsic controls, 90 µL were added per well to the 96-well plate. To analyze the effect of blood cells on bacterial killing without serum or plasma in both assays, cells were reconstituted with DPBS (Figure 1B).
2.4.4 Inhibitors
To investigate the contribution of different complement components to the killing of S. pneumoniae and N. meningitidis in the two blood assays, selected complement inhibitors of the alternative, lectin and terminal pathway were used. Inhibitors of factor B [Iptacopan, LNP023, Novartis (56)], factor D [CMS487, Novartis (56)], C3 (CP-40, Bachem) as well as an anti-C5 antibody [LFG316, tesidolumab, Novartis (57, 58)] and an anti-MASP-2 antibody [narsoplimab (59, 60)] were diluted in DPBS, added to the blood samples and incubated for 5 minutes at room temperature before adding the bacteria. Inhibitor concentrations tested for LNP023 and CMS487 were 1 and 10 µM, for C3, C5 and MASP-2 inhibitors 1 and 50 µg/mL.
2.4.5 Bacterial Clearance
After bacteria were added, the 96-well plates were incubated at 37°C with 5% CO2 and bacterial survival was analyzed by repeated sampling of 10 µL after 60, 180 and 300 minutes and plating on CSBA plates. Agar plates were subsequently incubated overnight at 37°C with 5% CO2, colonies were counted manually, and data converted into CFU per mL.
2.4.6 Opsonophagocytic Titer Determination
Opsonophagocytic titer were determined using the EDTA-based whole blood assay described above, but with modifications. In short, after 4-fold serial dilution of heat-inactivated sera in a 96-well flat bottom culture microplate leaving 25 µL diluted serum per well, 45 µL washed blood cells from a vaccine-naïve donor were added before adding 10 µL of diluted bacteria (as described above) and 20 µL of baby rabbit complement (Cedarlane). The 96-wells plates were incubated at 37°C with 5% CO2 on a rotator. Bacterial killing was analyzed at 60 minutes post inoculation. Opsonophagocytic titers represent the reciprocal of the serum dilution with >50% killing compared with bacterial growth in the complement control wells. Serum samples with undetectable titers, representing a titer lower than our lowest dilution (<4), obtained the value 2.
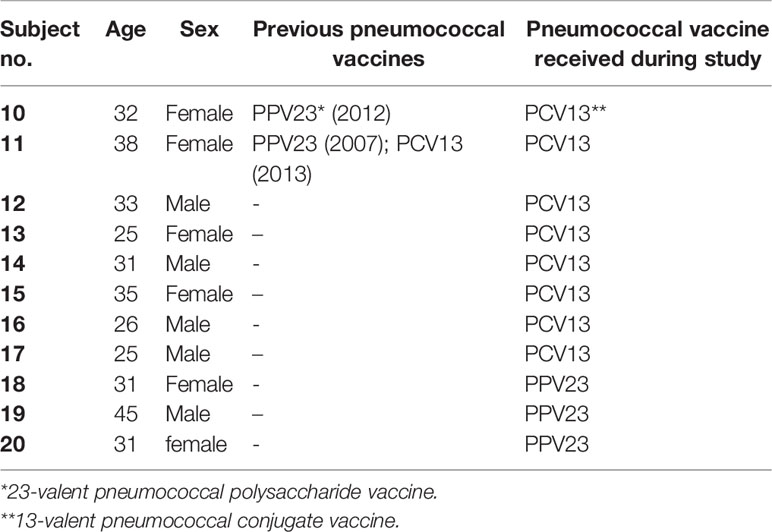
2.5 Longitudinal Study With Paired Pre and Post Vaccination Samples
Eleven healthy volunteers were recruited upon informed and written consent. Two subjects had previously been vaccinated with pneumococcal vaccines (Table 2). Eight subjects received a single dose of a 13-valent conjugate pneumococcal vaccine (Prevenar 13, PCV13, Pfizer) and three subjects received a single dose of a 23-valent unconjugated pneumococcal polysaccharide vaccine (Pneumovax 23, PPV23, MSD). Serum and blood from venous blood were taken before, 2 weeks and 2 months after vaccination. Pneumococcal serotype-specific IgG concentrations were analyzed as described above. Opsonophagocytic killing was assessed using the hirudin assays described above inoculated with a bacterial dilution containing 7.3 ± 3.1 x 103 CFU/mL.
2.6 Statistical Analysis
Differences of anti-pneumococcal immunoglobulin IgG between vaccine-naïve and previously vaccinated study participants were assessed using a two-tailed unpaired t test for parametric data on GraphPad Prism (Prism 8; GraphPad Software Inc., San Diego, USA). Difference in opsonophagocytic titers were assessed using a non-parametric Mann-Whitney test. Spearman’s non-parametric correlations was used to correlate the IgG levels with the opsonophagocytic titers. Pearson’s correlation was used to correlate serum anti-pneumococcal immunoglobulin IgG levels with the log-transformed numbers of surviving bacteria in presence of complement inhibitors. P values of ≤0.05 were considered to be statistically significant.
3 Results
3.1 Induction of Capsule Polysaccharide-Specific Antibodies by PCV-13 Vaccination
Serum of vaccine-naïve and PCV13-vaccinated subjects were analyzed for serotype 4 capsule polysaccharide-specific IgG. While sera of all five vaccine-naïve subjects showed IgG concentration below or at the lower limit of quantification (0.10 µg/mL), sera of five PCV13-vaccinated study participants contained between 0.78 and 8.41 µg/mL capsule polysaccharide-specific antibodies (Figure 2A). Detectable opsonophagocytic titers were only found in vaccinated subjects and ranged from 4 to 4096 with a median titer of 64 (Figure 2B). Opsonophagocytic titers and polysaccharide-specific IgG concentrations demonstrated a significant positive correlation (Spearman’s r = 0.927, p < 0.001, Figure 2C).
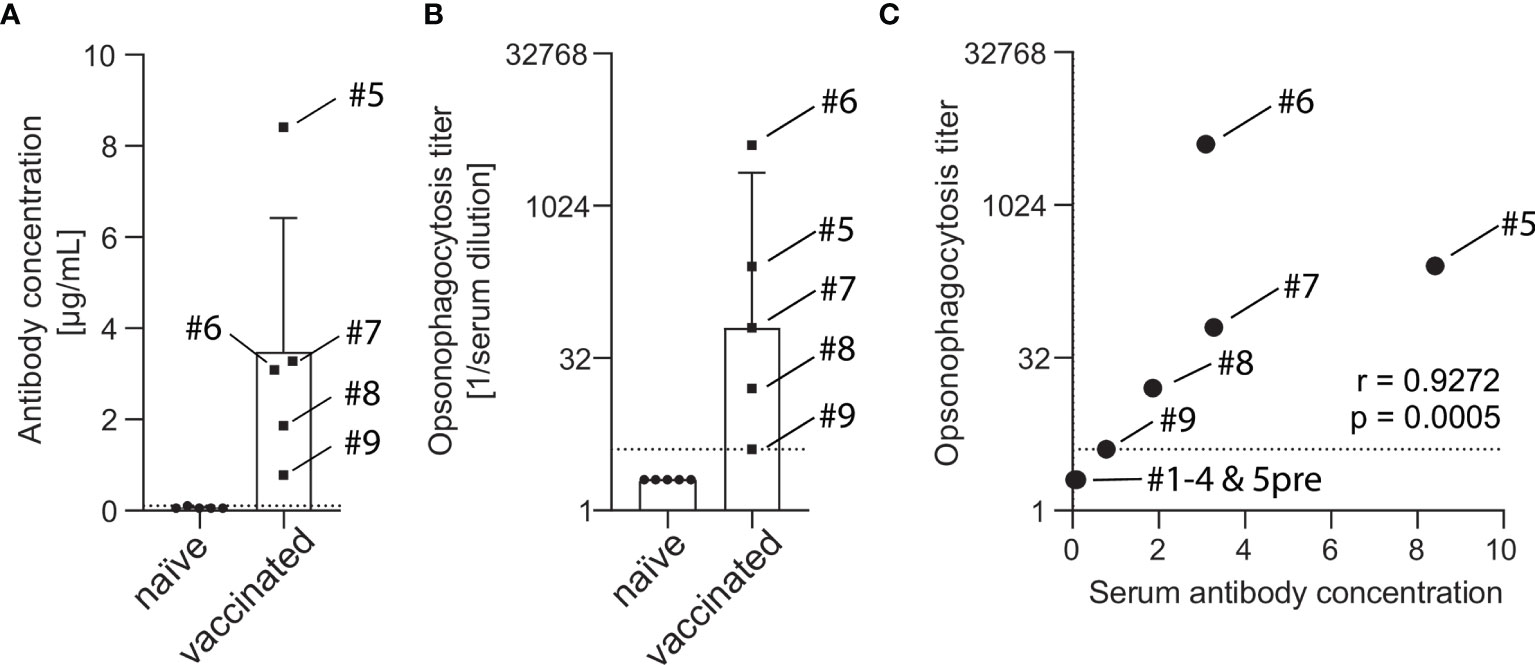
Figure 2 Concentration of capsule polysaccharide-specific IgG and opsonophagocytic titers in sera of vaccine-naïve and PCV13-vaccinated subjects. A pneumococcal IgG Bioplex assay was used to measure antibody concentrations in the serum of five vaccine-naïve and five PCV13-vaccinated subjects. Vaccinated study participants had statistically significant higher antibody concentrations than the vaccine-naive group (3.48 ± 2.93 µg/mL vs. 0.06 ± 0.02 µg/mL, p = 0.031). The box indicates the sample mean and the standard deviation and the dotted line represent the lower limit of quantification (A). Pneumococcal opsonophagocytic titer were determined using PBS-washed EDTA-anticoagulated blood cells reconstituted with diluted heat-inactivated serum and 20% active baby rabbit complement. Vaccinated study participants had statistically significant higher titers than the vaccine-naïve group (median titer 64 vs. 2, p = 0.0079). Boxes indicate sample median and interquartile range and the dotted line represents the lowest quantifiable opsonophagocytic titer (B). IgG levels and opsonophagocytic titers revealed a strong positive correlation (C). Serum from subject #5 before vaccination was available for these analyses.
3.2 Role of Antibodies and Complement Activation During Pneumococcal Opsonophagocytosis
To compare two physiological opsonophagocytosis assays based on either DPBS-washed cells from EDTA-anticoagulated blood reconstituted with active serum or hirudin-anticoagulated whole blood, we assessed bacterial killing in blood samples of nine healthy volunteers. Of these, four subjects were pneumococcal vaccine-naïve and five had received the 13-valent pneumococcal conjugate vaccine (PCV13). Both assays produced highly comparable results and demonstrated that active complement and/or high titers of capsule specific antibodies are required for phagocytic killing of pneumococci (Figure 3). Serum/plasma alone, blood cells alone and cells reconstituted with heat-inactivated serum/plasma did not cause bacterial killing, but rather allowed pneumococcal proliferation over the assay duration of 5 hours. Unprocessed whole blood from both PCV13-vaccinated and vaccine-naïve subjects or blood cells reconstituted with the corresponding active serum killed the pneumococci within 5 hours after inoculation in both assays (Figures 3A–D and 4). Pneumococcal killing might occur faster in samples from PCV13-vaccinated subjects, where a decreased colony count was detectable as early as one hour after inoculation. Notably, however, two vaccine-naïve subjects also showed complete killing within one hour after inoculation (Figures 4C, D). Pneumococcal proliferation in blood cell samples reconstituted with heat-inactivated serum/plasma from vaccinated, but not from vaccine-naïve subjects, was slightly reduced (Figures 3A–D).
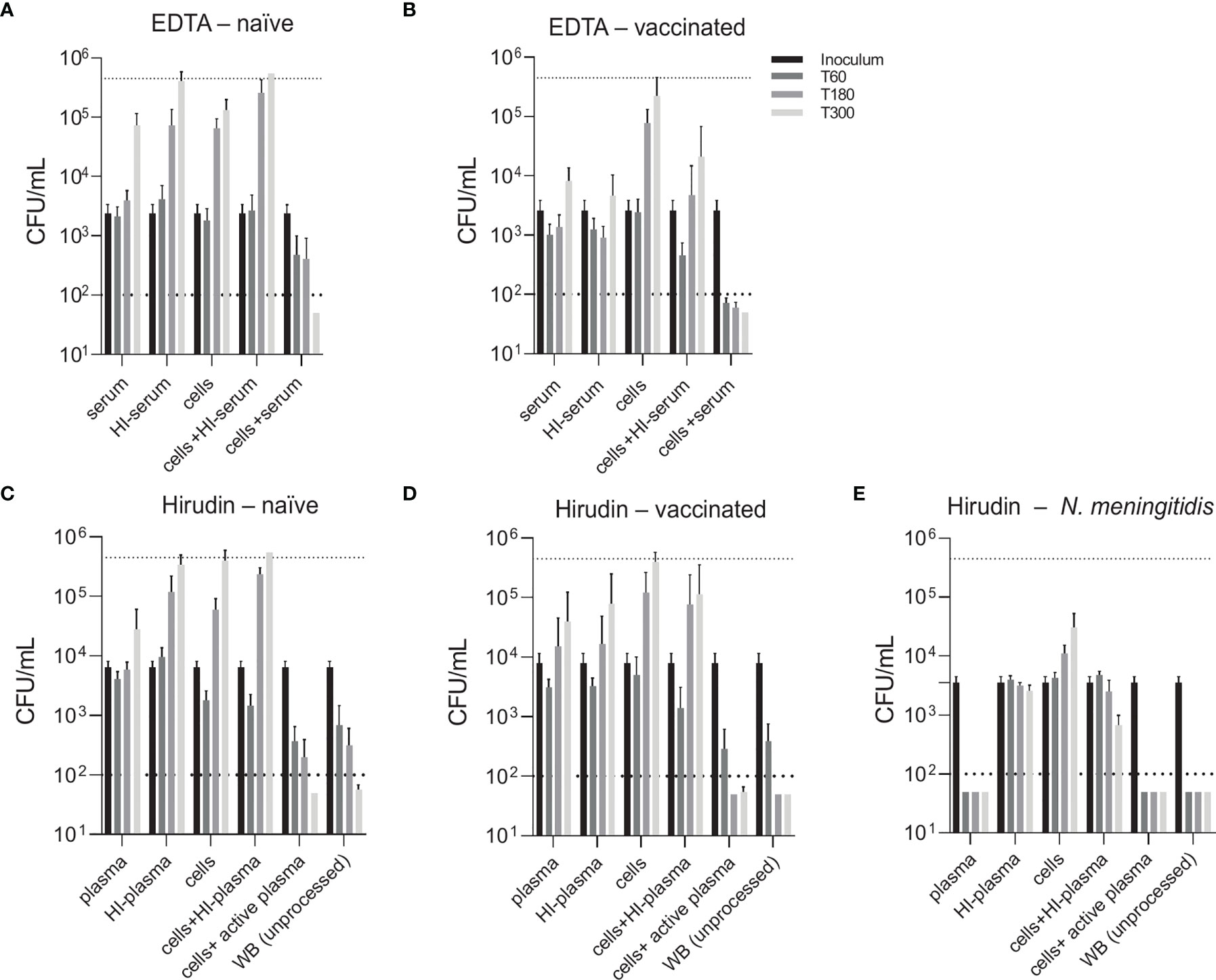
Figure 3 Contribution of active complement and antibodies to opsonophagocytic killing of pneumococci. Pneumococcal killing was assessed in either an assay based on DPBS-washed cells from EDTA-anticoagulated blood reconstituted with active or HI serum [EDTA assay; (A, B)] or in a hirudin-anticoagulated whole blood (WB) assay [Hirudin assay; (C, D)] comparing vaccine-naïve (A, C) and PCV13-vaccinated individuals (B, D). Cumulated results for all individuals per group are shown. Meningococcal killing in hirudin-anticoagulated whole blood of a MenACWY-vaccinated volunteer (E) served as a control for direct killing by the MAC. Bars indicate CFU/mL at start of inoculation and 60, 180 and 300 minutes after inoculation. Dashed lines demonstrate the lower detection limit ( < 100 CFU/mL) and the upper limit ( > 500’000 CFU/mL) for exact quantification. WB, whole blood; HI, heat-inactivated.
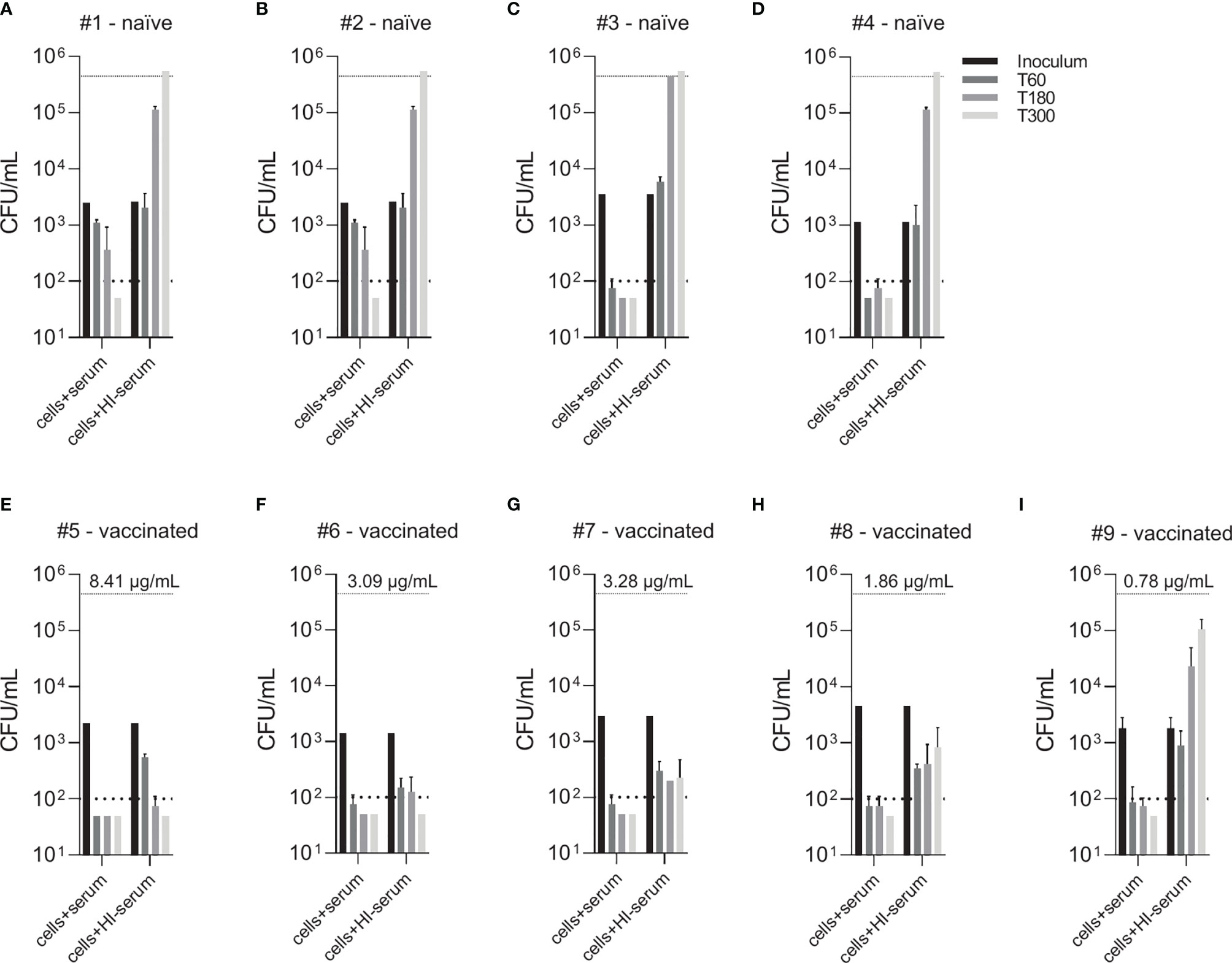
Figure 4 Effect of vaccine-induced anti-pneumococcal capsule antibodies in the absence of active complement. Pneumococcal killing was assessed in an opsonophagocytosis assay based on DPBS-washed cells from EDTA-anticoagulated blood reconstituted with active or HI serum, comparing individual vaccine-naïve (A–D) and PCV13-vaccinated individuals (E–I). Concentration of serotype 4 capsule polysaccharide-specific IgG in sera of vaccinated subjects is displayed. Bars indicate CFU/mL at time of bacterial inoculation and after 60, 180 and 300 minutes. Dashed lines demonstrate the lower detection limit ( < 100 CFU/mL) and the upper limit ( > 500’000 CFU/mL) for exact quantification.
Generally, the two assay formats yielded very similar results and highlighted the importance of active complement and/or antibodies for efficient opsonophagocytic killing of pneumococci. In contrast to these findings, a control experiment confirmed that, as expected, anti-capsular antibodies and active complement are sufficient for effective killing of the Gram-negative bacterium N. meningitidis in the absence of phagocytes (Figure 3E).
Comparison of individual subject data revealed that bacterial killing was also observed with blood cell samples reconstituted with heat-inactivated serum of vaccinated study participants, if the concentration of vaccine-induced anti-pneumococcal capsule antibodies was high (Figure 4). In particular, sera from subjects #5, 6 and 7 (Figures 4E–G), which contained capsule specific antibody concentrations >2 µg/mL, representing opsonophagocytic titers ≥64 (Figure 2), showed pneumococcal killing even in the absence of active complement, indicative for antibody-dependent, but complement-independent pneumococcal killing.
3.3 In Vaccine-Naïve Subjects, Pneumococcal Opsonophagocytosis Is Dependent on Alternative Pathway Activation
While blood from both PCV13-vaccinated and vaccine-naïve subjects induced pneumococcal killing after five hours of incubation (Figures 3, 4), striking differences were observed in the presence of AP inhibitors. In vaccine-naïve subjects, pneumococcal killing was completely abrogated when fB (with 10 µM LNP023) or fD (with either 1 or 10 µM CMS487) was inhibited (Figures 5A, C). In contrast, AP inhibition caused only a slight delay in killing at 3 hours and had no effect at 5 hours when blood samples from subjects who had previously been vaccinated with PCV13 were analyzed (Figures 5B, D). Correlation of anti-capsule antibody concentrations and pneumococcal clearance in presence of AP inhibition showed that all vaccinated subjects, independent of the anti-capsule antibody concentration, killed the pneumococci completely within 5 hours after bacterial inoculation (Figures 6A, B). Notably, complete pneumococcal clearance in presence of fD inhibitor was also observed in the non-immune subject #2 (Figure 6C), which was the only vaccine-naïve subject with detectable anti-capsule antibody levels (0.1 µg/mL).
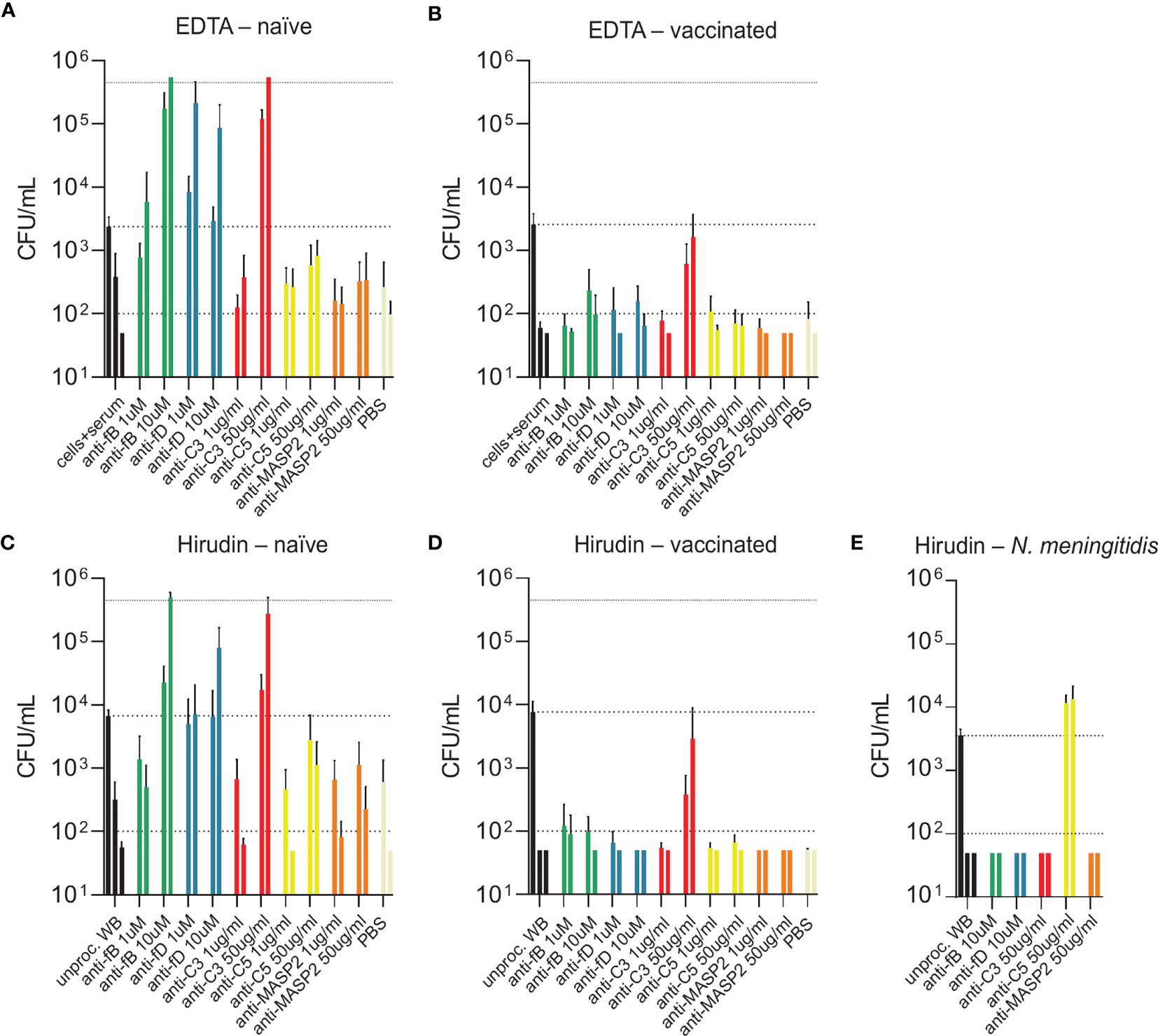
Figure 5 Bacterial opsonophagocytosis in the presence of complement inhibitors. Effects of different complement inhibitors on pneumococcal killing were assessed in parallel with either DPBS-washed cells from EDTA-anticoagulated blood reconstituted with active serum (A, B) or with hirudin-anticoagulated whole blood (C, D). Cumulated results for three individuals per group are shown. Meningococcal killing in the presence of complement inhibitors in hirudin-anticoagulated whole blood of a MenACWY-vaccinated volunteer (E) served as a control. Bars indicate bacterial CFU/mL at 3 and 5 h after inoculation (respectively, left and right bar for each condition). In the control conditions (black), the inoculum titer is depicted as the first bar and further indicated as dashed line. Other dashed lines demonstrate the lower detection limit and the upper limit for bacterial quantification. WB, whole blood.
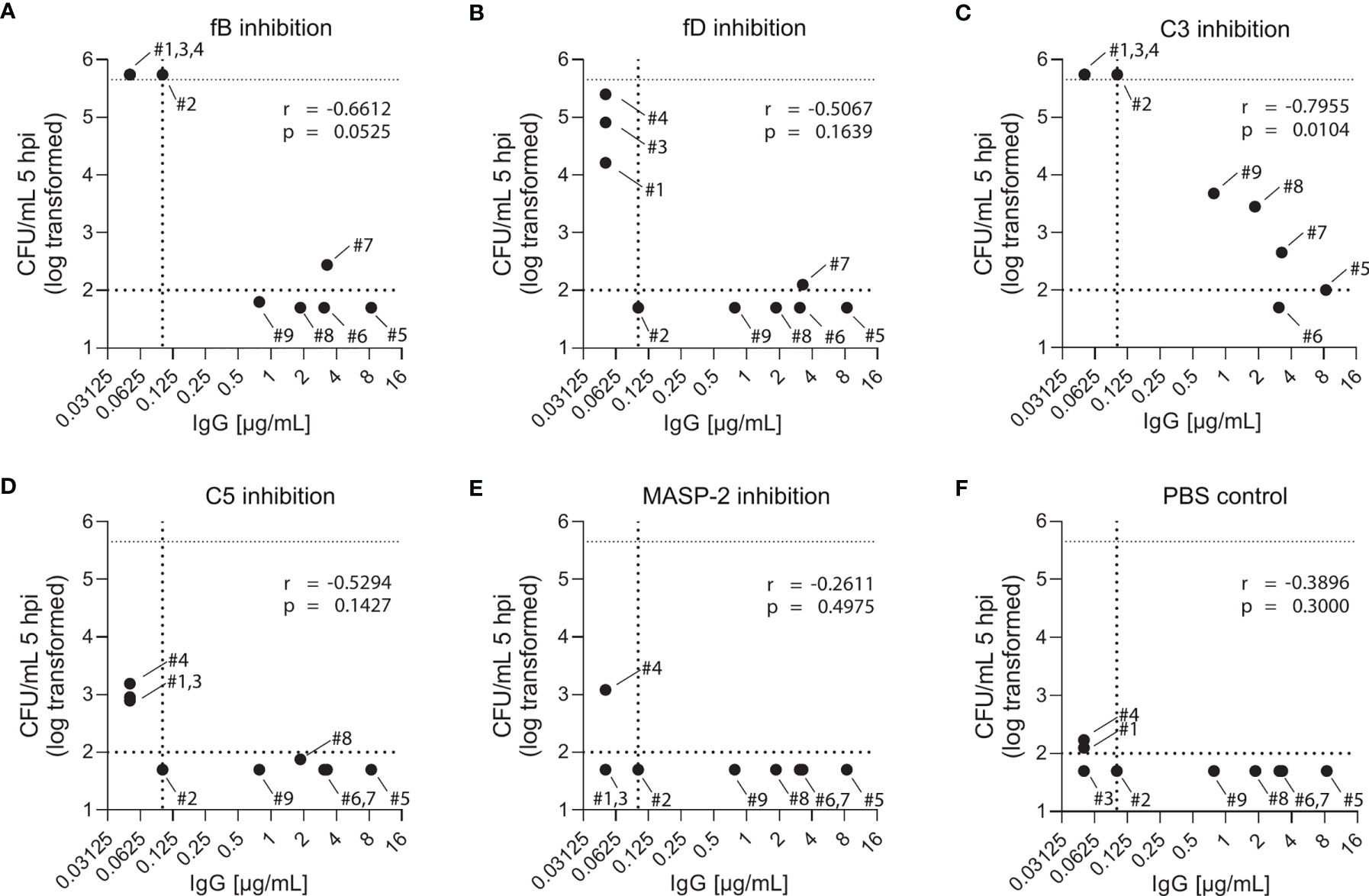
Figure 6 Effect of complement inhibition and anti-pneumococcal capsule antibody concentrations on pneumococcal killing in individual study participants. Pneumococcal killing was assessed in an opsonophagocytosis assay based on DPBS-washed cells from EDTA-anticoagulated blood reconstituted with active serum comparing individual vaccine-naïve and PCV13-vaccinated subjects in the presence of 10µM fB (A) or fD (B) inhibitor, 50 µg/mL C3 (C), C5 (D) or MASP-2 (E) inhibitor and PBS control (F). Log-transformed bacterial number in CFU/mL five hours after bacterial inoculation were correlated with capsule-specific IgG concentrations in serum of each individual study participant. Dashed horizontal lines demonstrate the lower detection limit and the upper limit for bacterial quantification, while the dashed vertical line represents lower limit for IgG concentration quantification. hpi: hours post inoculation.
Inhibition of C3 with CP-40 at a concentration of 50 µg/mL completely prevented pneumococcal killing in samples of vaccine-naïve participants (Figures 5A, C). In contrast, in samples of vaccinated individuals the effect depended on the concentration of the capsule-specific antibodies. While the cumulated results showed an overall impact of C3 inhibition (Figures 5B, D), detailed analyses of individual subjects provided evidence for complement-independent opsonophagocytic killing in participants with high concentrations of capsule-specific antibodies (Figure 6C). In reconstituted blood samples from subjects #5, 6 and 7, which contained antibody concentrations >2 µg/mL (Figure 2), efficient killing was observed when C3 was blocked (Figure 6C). In samples of the vaccinated subjects #8 and 9 with low antibody concentrations, bacterial proliferation was still contained, whereas inhibition of C3 provoked a massive proliferation of pneumococci in vaccine-naïve subjects (#1,2,3 and 4). A significant inverse correlation between serum IgG concentration and the number of surviving pneumococci 5 hours after inoculation was observed (Figure 6C), indicating that high levels of capsule-specific IgG are triggering pneumococcal opsonophagocytosis even in the absence of C3. Results of C3 inhibition were similar to obtained results with heat-inactivated sera (Figure 4).
Irrespective of the vaccination status, inhibition of MASP-2 or C5 did not substantially impair bacterial killing, except for a marginal effect of C5 in vaccine-naïve subjects (Figures 5A–D and Figures 6D, E). In control experiments, inhibition of C5 completely blocked killing of N. meningitidis with serum from a MenACWY-vaccinated individual (Figure 5E).
3.4 The Effect of Pneumococcal Vaccinations on Complement-Dependent Opsonophagocytosis
Eleven additional study participants were recruited and vaccinated with either PCV13 or PPV23 (Figures 7A, B). Pneumococcal opsonophagocytosis in the presence or absence of complement inhibitors was assessed before as well as two weeks and two months post vaccination. There was an increase in capsule polysaccharide-specific IgG concentrations upon vaccination in all eleven study participants (Figure 7C). Inhibition of C3 or the alternative pathway abrogated pneumococcal opsonophagocytosis in whole blood. The effect of fD inhibition was slightly less prominent in the PCV13 group, potentially due to pre-existing immunity in some subjects. Two weeks after vaccination, pneumococcal opsonophagocytic killing in whole blood was not prevented by inhibition of the alternative complement pathway. While inhibition of fD did not prevent complete pneumococcal killing, inhibition of fB slowed down pneumococcal killing slightly. Also C3 inhibition resulted in incomplete pneumococcal killing. Inhibition of C5 or MASP-2 did not affected pneumococcal killing. Opsonophagocytosis patterns observed with whole blood collected two weeks and two months after vaccinations were similar. Vaccination with PCV13 and PPV23 had similar effects.
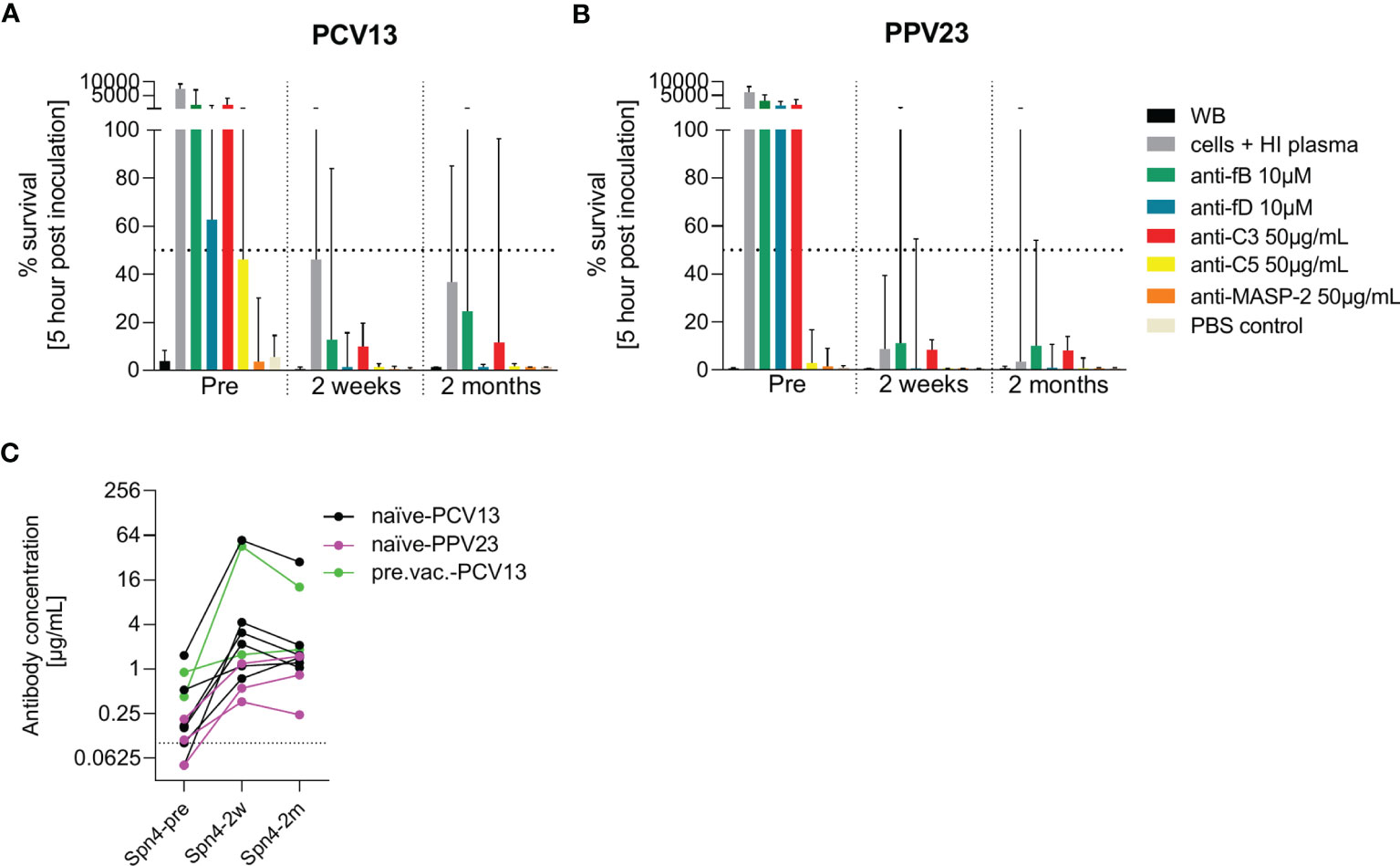
Figure 7 Effect of PCV13 and PPV23 vaccination on bacterial opsonophagocytosis in the presence of complement inhibitors. The effect of pneumococcal vaccination on complement-dependent opsonophagocytosis was assessed in hirudin-anticoagulated whole blood. Cumulated results for eight PCV-13 vaccinated subjects (A) and three PPV23 vaccinated participants (B) are shown. Bars indicate the median percentage of surviving bacteria five hours after inoculation. The dashed line represents 50% killing compared to the inoculum titer. Antibody concentrations in the serum of all study participants were measured before, two weeks and two months after vaccination by a pneumococcal IgG bioplex assays (C). The dashed line in (C) represents the lower limit of quantification. PCV13: 13-valent pneumococcal conjugate vaccine; PPV23: 23-valent pneumococcal polysaccharide vaccine; WB: whole blood, HI: heat-inactivated.
4. Discussion
Although there is general agreement that both complement and anti-pneumococcal antibodies contribute to opsonophagocytosis-mediated immune defense against S. pneumoniae (11, 61–63), only limited data are available on the relative contribution of complement- and direct antibody-mediated opsonisation. To address this, we compared two physiologic opsonophagocytosis assays adapted from recently published methods. A hirudin-anticoagulated whole blood assay (49) was directly compared to an assay based on cells from EDTA-anticoagulated blood, where plasma was removed, cells were washed and supplemented with active serum (50). The two assays yielded highly comparable results, with both assays demonstrating the relative importance of antibodies and of AP and CP activation for opsonophagocytic killing of pneumococci. A major advantage of the hirudin assay is that minimal processing results in highly physiologic conditions, furthermore representing an easy-to-use protocol. On the other hand, the serum-reconstituted EDTA assay allows analyzing the effect of different serum samples from different time points repeatedly and in direct comparison with the same cell preparation. However, this assay involves processing steps, which might affect the biology of investigated cell types and also affect complement activation through coagulation (48, 64). Depending on the scientific question addressed, one or the other assay might be more favorable to use.
Both assays showed that AP activation plays a key role in opsonophagocytosis in vaccine-naïve subjects, whereas inhibition of fB and fD did not affect pneumococcal killing in PCV13-vaccinated individuals, independent of anti-pneumococcal capsule antibody concentration and opsonophagocytic titer (Figures 6A, B). Even low anti-pneumococcal capsule antibody concentrations were sufficient to clear pneumococci from blood via antibody-mediated CP and/or Fc receptor-mediated phagocytosis. Opsonophagocytosis data with paired blood samples from study participants before and after vaccination confirmed these conclusions (Figures 7A, B).
Experiments with both heat-inactivated and C3-inhibited sera showed that in the absence of active complement, but in presence of high anti-capsule IgG serum concentrations, representing high opsonophagocytic titers, opsonophagocytosis is still efficient, which is indicative for complement-independent Fc receptor-mediated phagocytosis. Therapeutic inhibition of C3, however, might leave some vaccinated subjects with increased risk of acquiring pneumococcal infections, when vaccine-induced antibody levels are low (Figure 6C).
C5 cleavage, which induces MAC formation and release of the pro-inflammatory C5a anaphylatoxin is crucial for meningococcal clearance from whole blood (52). Control experiments with N. meningitidis confirmed this importance and showed that inhibition of C5 blocked meningococcal clearance in whole blood (Figure 5E), as previously reported (1–5, 52). Nevertheless, inhibiting C5 during experimental pneumococcal infection did not affect bacterial killing in vaccinated individuals and only marginally affected it in vaccine-naïve subjects. Furthermore, our data indicate that the lectin pathway may not be essential for immune defense against pneumococci even in vaccine-naïve subjects, all of whom presented some degree of natural immunity. This is in line with observational studies showing no association between LP deficiencies and the risk for acquiring pneumococcal infections (22, 23).
Limitations of this study include a relatively small initial sample size. However, main conclusions were fully verified by testing paired blood samples collected from subjects both before and after vaccination. Moreover, we focused on one pneumococcal serotype as blood volumes approved by the ethics committee did not allow to perform whole-blood assays with our panel of complement inhibitors for multiple serotypes.
Taken together, our results show that fB and fD inhibitors, which still allow complement activation through antibody-mediated CP, do not abrogate pneumococcal opsonophagocytosis in PCV13- and PPV23-vaccinated individuals. Hence, AP inhibitors can safely be developed in the clinic for potential treatments in patients with diseases involving the complement system. Treatment with AP inhibitors should be accompanied by vaccination with a pneumococcal vaccine to elicit anti-capsule antibodies and B cell memory responses that enable rapid humoral immune responses against pneumococcal infections.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethical Committee of Northwest and Central Switzerland (Ethikkommission Nordwest- und Zentralschweiz (EKNZ), Studie 2018-02341). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LM and EI contributed equally to this study during study conceptualization, data acquisition, analysis and interpretation as well as drafting the manuscript. GP contributed to study conceptualization and design, data analysis and interpretation and revised the manuscript. AS, CT, NZ, TH, and MK contributed to study conceptualization and design and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
GP received funding to conduct the study under a research agreement contract between Novartis Pharma AG and the Swiss Tropical and Public Health Institute.
Conflict of Interest
AS, CT, NZ, TH and MK are full-time employees of Novartis.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study received funding from Novartis Pharma AG. The funders initiated study design and decision to implement opsonophagocytosis assays to compare the activity of the tested drugs. The funders had no role in data collection and analysis.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are very grateful to all the study participants for donating blood and to the Swiss TPH medical and diagnostic personnel for performing the blood draws.
References
1. Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: A Key System for Immune Surveillance and Homeostasis. Nat Immunol (2010) 11:785–97. doi: 10.1038/ni.1923
2. Walport MJ. Complement: First of Two Parts. N Engl J Med (2001) 344:1058–66. doi: 10.1056/NEJM200104053441406
3. Heesterbeek DA, Bardoel BW, Parsons ES, Bennett I, Ruyken M, Doorduijn DJ, et al. Bacterial Killing by Complement Requires Membrane Attack Complex Formation via Surface-Bound C5 Convertases. EMBO J (2019) 38(4):e99852. doi: 10.15252/embj.201899852
4. Joiner KA, Brown EJ, Frank MM. Complement and Bacteria: Chemistry and Biology in Host Defense. Annu Rev Immunol (1984) 2:461–92. doi: 10.1146/annurev.iy.02.040184.002333
5. Berends ETM, Dekkers JF, Nijland R, Kuipers A, Soppe JA, van Strijp JAG, et al. Distinct Localization of the Complement C5b-9 Complex on Gram-Positive Bacteria. Cell Microbiol (2013) 15:1955–68. doi: 10.1111/cmi.12170
6. Akesson P, Sjöholm AG, Björck L. Protein SIC, a Novel Extracellular Protein of Streptococcus Pyogenes Interfering With Complement Function. J Biol Chem (1996) 271:1081–8. doi: 10.1074/jbc.271.2.1081
7. Laursen NS, Gordon N, Hermans S, Lorenz N, Jackson N, Wines B, et al. Structural Basis for Inhibition of Complement C5 by the SSL7 Protein From Staphylococcus Aureus. Proc Natl Acad Sci USA (2010) 107:3681–6. doi: 10.1073/pnas.0910565107
8. Weiser JN, Ferreira DM, Paton JC. Streptococcus Pneumoniae: Transmission, Colonization and Invasion. Nat Rev Microbiol (2018) 16:355–67. doi: 10.1038/s41579-018-0001-8
9. Bogaert D, De Groot R, Hermans PWM. Streptococcus Pneumoniae Colonisation: The Key to Pneumococcal Disease. Lancet Infect Dis (2004) 4:144–54. doi: 10.1016/S1473-3099(04)00938-7
10. Brown EJ, Hosea SW, Frank MM. The Role of Antibody and Complement in the Reticuloendothelial Clearance of Pneumococci From the Bloodstream. Rev Infect Dis (1983) 5:S797–805. doi: 10.1093/CLINIDS/5.SUPPLEMENT_4.S797
11. Andre GO, Converso TR, Politano WR, Ferraz LFC, Ribeiro ML, Leite LCC, et al. Role of Streptococcus Pneumoniae Proteins in Evasion of Complement-Mediated Immunity. Front Microbiol (2017) 8:224. doi: 10.3389/fmicb.2017.00224
12. Botto M, Kirschfink M, Macor P, Pickering MC, Würzner R, Tedesco F. Complement in Human Diseases: Lessons From Complement Deficiencies. Mol Immunol (2009) 46:2774–83. doi: 10.1016/j.molimm.2009.04.029
13. Pickering MC, Walport MJ. Links Between Complement Abnormalities and Systemic Lupus Erythematosus. Rheumatology (2000) 39:133–41. doi: 10.1093/rheumatology/39.2.133
14. El Sissy C, Rosain J, Vieira-Martins P, Bordereau P, Gruber A, Devriese M, et al. Clinical and Genetic Spectrum of a Large Cohort With Total and Sub-Total Complement Deficiencies. Front Immunol (2019) 10:1936. doi: 10.3389/fimmu.2019.01936
15. Biesma DH, Hannema AJ, van Velzen-Blad H, Mulder L, van Zwieten R, Kluijt I, et al. A Family With Complement Factor D Deficiency. J Clin Invest (2001) 108:233–40. doi: 10.1172/JCI12023
16. Hiemstra PS, Langeler E, Compier B, Keepers Y, Leijh PC, van den Barselaar MT, et al. Complete and Partial Deficiencies of Complement Factor D in a Dutch Family. J Clin Invest (1989) 84:1957–61. doi: 10.1172/JCI114384
17. Sprong T, Roos D, Weemaes C, Neeleman C, Geesing CLM, Mollnes TE, et al. Deficient Alternative Complement Pathway Activation Due to Factor D Deficiency by 2 Novel Mutations in the Complement Factor D Gene in a Family With Meningococcal Infections. Blood (2006) 107:4865–70. doi: 10.1182/blood-2005-07-2820
18. Ram S, Lewis LA, Rice PA. Infections of People With Complement Deficiencies and Patients Who Have Undergone Splenectomy. Clin Microbiol Rev (2010) 23:740–80. doi: 10.1128/CMR.00048-09
19. Slade C, Bosco J, Unglik G, Bleasel K, Nagel M, Winship I. Deficiency in Complement Factor B. N Engl J Med (2013) 369:1667–9. doi: 10.1056/NEJMc1306326
20. Gauthier A, Wagner E, Thibeault R, Lavoie A. A Novel Case of Complement Factor B Deficiency. J Clin Immunol (2021) 41(1):277–9. doi: 10.1007/s10875-020-00906-3
21. Ali YM, Lynch NJ, Haleem KS, Fujita T, Endo Y, Hansen S, et al. The Lectin Pathway of Complement Activation Is a Critical Component of the Innate Immune Response to Pneumococcal Infection. PloS Pathog (2012) 8:e1002793. doi: 10.1371/journal.ppat.1002793
22. Garcia-Laorden MI, Sole-Violan J, Rodriguez de Castro F, Aspa J, Briones ML, Garcia-Saavedra A, et al. Mannose-Binding Lectin and Mannose-Binding Lectin-Associated Serine Protease 2 in Susceptibility, Severity, and Outcome of Pneumonia in Adults. J Allergy Clin Immunol (2008) 122:368–74, 374.e1–2. doi: 10.1016/j.jaci.2008.05.037
23. Chapman SJ, Vannberg FO, Khor CC, Segal S, Moore CE, Knox K, et al. Functional Polymorphisms in the FCN2 Gene are Not Associated With Invasive Pneumococcal Disease. Mol Immunol (2007) 44:3267–70. doi: 10.1016/J.MOLIMM.2006.04.013
24. Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of Pneumococcal Vaccination in Adults: A Meta-Analysis. Can Med Assoc J (2009) 180:48–58. doi: 10.1503/cmaj.080734
25. Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for Preventing Pneumococcal Infection in Adults. Cochrane Database Syst Rev (2013) (1):CD000422. doi: 10.1002/14651858.CD000422.pub3
26. Kyaw MH, Clarke S, Edwards GFS, Jones IG, Campbell H. Serotypes/groups Distribution and Antimicrobial Resistance of Invasive Pneumococcal Isolates: Implications for Vaccine Strategies. Epidemiol Infect (2000) 125:561–72. doi: 10.1017/S0950268800004787
27. Shapiro ED, Berg AT, Schroeder D, Parcells V, Margolis A, Adair RK, et al. The Protective Efficacy of Polyvalent Pneumococcal Polysaccharide Vaccine. N Engl J Med (1991) 325:1453–60. doi: 10.1056/NEJM199111213252101
28. Jackson LA, Neuzil KM, Yu O, Benson P, Barlow WE, Adams AL, et al. Effectiveness of Pneumococcal Polysaccharide Vaccine in Older Adults. N Engl J Med (2003) 348:1747–55. doi: 10.1056/NEJMoa022678
29. Simberkoff MS, Cross AP, al-Ibrahim M, Baltch AL, Geiseler Pj, Nadler J, et al. Efficacy of Pneumococcal Vaccine in High-Risk Patients. N Engl J Med (1986) 315:1318–27. doi: 10.1056/NEJM198611203152104
30. Moore RA, Wiffen PJ, Lipsky BA. Are the Pneumococcal Polysaccharide Vaccines Effective? Meta-Analysis of the Prospective Trials. BMC Fam Pract (2000) 1:1. doi: 10.1186/1471-2296-1-1
31. Watson L, Wilson BJ, Waugh N. Pneumococcal Polysaccharide Vaccine: A Systematic Review of Clinical Effectiveness in Adults. Vaccine (2002) 20:2166–73. doi: 10.1016/S0264-410X(02)00112-3
32. Westerink MAJ, Schroeder HW, Nahm MH. Immune Responses to Pneumococcal Vaccines in Children and Adults: Rationale for Age-Specific Vaccination. Aging Dis (2012) 3:51–67.
33. Sings HL. Pneumococcal Conjugate Vaccine Use in Adults – Addressing an Unmet Medical Need for Non-Bacteremic Pneumococcal Pneumonia. Vaccine (2017) 35:5406–17. doi: 10.1016/j.vaccine.2017.05.075
34. Shiri T, Datta S, Madan J, Tsertsvadze A, Royle P, Keeling MJ, et al. Indirect Effects of Childhood Pneumococcal Conjugate Vaccination on Invasive Pneumococcal Disease: A Systematic Review and Meta-Analysis. Lancet Glob Heal (2017) 5:e51–9. doi: 10.1016/S2214-109X(16)30306-0
35. O’Brien KL, Millar EV, Zell ER, Bronsdon M, Weatherholtz R, Reid R, et al. Effect of Pneumococcal Conjugate Vaccine on Nasopharyngeal Colonization Among Immunized and Unimmunized Children in a Community-Randomized Trial. J Infect Dis (2007) 196:1211–20. doi: 10.1086/521833
36. Taillardet M, Haffar G, Mondière P, Asensio M, Pléau-Pison T, Burdin N, et al. Toll-Like Receptor Agonists Allow Generation of Long-Lasting Antipneumococcal Humoral Immunity in Response to a Plain Polysaccharidic Vaccine. J Infect Dis (2010) 202:470–9. doi: 10.1086/653739
37. Timens W, Boes A, Rozeboom-Uiterwijk T, Poppema S. Immaturity of the Human Splenic Marginal Zone in Infancy. Possible Contribution to the Deficient Infant Immune Response. J Immunol (1989) 143:3200–6.
38. Angoulvant F, Levy C, Grimprel E, Varon E, Lorrot M, Biscardi S, et al. Early Impact of 13-Valent Pneumococcal Conjugate Vaccine on Community-Acquired Pneumonia in Children. Clin Infect Dis (2014) 58:918–24. doi: 10.1093/cid/ciu006
39. Andrews NJ, Waight PA, Burbidge P, Pearce E, Roalfe L, Zancolli M, et al. Serotype-Specific Effectiveness and Correlates of Protection for the 13-Valent Pneumococcal Conjugate Vaccine: A Postlicensure Indirect Cohort Study. Lancet Infect Dis (2014) 14:839–46. doi: 10.1016/S1473-3099(14)70822-9
40. Patterson S, Webber C, Patton M, Drews W, Huijts SM, Bolkenbaas M, et al. Post Hoc Assessment of Duration of Protection in CAPiTA (Community Acquired Pneumonia Immunization Trial in Adults). Trials Vaccinol (2016) 5:92–6. doi: 10.1016/J.TRIVAC.2016.04.004
41. Mastellos DC, Ricklin D, Lambris JD. Clinical Promise of Next-Generation Complement Therapeutics. Nat Rev Drug Discov (2019) 18:707–29. doi: 10.1038/s41573-019-0031-6
42. Struijk GH, Bouts AHM, Rijkers GT, Kuin EAC, Ten Berge IJM, Bemelman FJ. Meningococcal Sepsis Complicating Eculizumab Treatment Despite Prior Vaccination. Am J Transplant (2013) 13:819–20. doi: 10.1111/ajt.12032
43. McNamara LA, Topaz N, Wang X, Hariri S, Fox L, Macneil JR. High Risk for Invasive Meningococcal Disease Among Patients Receiving Eculizumab (Soliris) Despite Receipt of Meningococcal Vaccine. Morb Mortal Wkly Rep (2017) 66:734–7. doi: 10.15585/mmwr.mm6627e1
44. Bouts A, Monnens L, Davin JC, Struijk G, Spanjaard L. Insufficient Protection by Neisseria Meningitidis Vaccination Alone During Eculizumab Therapy. Pediatr Nephrol (2011) 26:1919–20. doi: 10.1007/s00467-011-1929-3
45. Lewis LA, Panicker S, DeOliveira RB, Parry GC, Ram S. Effect of a C1s Inhibitor on the Efficacy of Anti-Capsular Antibodies Against Neisseria Meningitidis and Streptococcus Pneumoniae. ImmunoHorizons (2019) 3:519–30. doi: 10.4049/immunohorizons.1900031
46. Romero-Steiner S, Libutti D, Pais LB, Dykes J, Anderson P, Whitin JC, et al. Standardization of an Opsonophagocytic Assay for the Measurement of Functional Antibody Activity Against Streptococcus Pneumoniae Using Differentiated HL-60 Cells. Clin Diagn Lab Immunol (1997) 4:415–22. doi: 10.1128/cdli.4.4.415-422.1997
47. Fleck RA, Romero-Steiner S, Nahm MH. Use of HL-60 Cell Line to Measure Opsonic Capacity of Pneumococcal Antibodies. Clin Diagn Lab Immunol (2005) 12:19–27. doi: 10.1128/CDLI.12.1.19-27.2005
48. Watson F, Robinson JJ, Edwards SW. Neutrophil Function in Whole Blood and After Purification: Changes in Receptor Expression, Oxidase Activity and Responsiveness to Cytokines. Biosci Rep (1992) 12:123–33. doi: 10.1007/bf02351217
49. van der Maten E, de Jonge MI, de Groot R, van der Flier M, Langereis JD. A Versatile Assay to Determine Bacterial and Host Factors Contributing to Opsonophagocytotic Killing in Hirudin-Anticoagulated Whole Blood. Sci Rep (2017) 7:42137. doi: 10.1038/srep42137
50. van den Broek B, van Els CACM, Kuipers B, van Aerde K, Henriet SS, de Groot R, et al. Multi-Component Meningococcal Serogroup B (MenB)-4C Vaccine Induces Effective Opsonophagocytic Killing in Children With a Complement Deficiency. Clin Exp Immunol (2019) 198(3):381–9. doi: 10.1111/cei.13368
51. Xu Y, Wu W, Wang L, Chintala M, Plump AS, Ogletree ML, et al. Differential Profiles of Thrombin Inhibitors (Heparin, Hirudin, Bivalirudin, and Dabigatran) in the Thrombin Generation Assay and Thromboelastography. vitro Blood Coagul Fibrinol (2013) 24:332–8. doi: 10.1097/MBC.0b013e32835e4219
52. Konar M, Granoff DM. Eculizumab Treatment and Impaired Opsonophagocytic Killing of Meningococci by Whole Blood From Immunized Adults. Blood (2017) 130:891–9. doi: 10.1182/blood-2017-05-781450
53. Mollnes TE, Brekke O-L, Fung M, Fure H, Christiansen D, Bergseth G, et al. Essential Role of the C5a Receptor in E Coli-Induced Oxidative Burst and Phagocytosis Revealed by a Novel Lepirudin-Based Human Whole Blood Model of Inflammation. Blood (2002) 100:1869–77. doi: 10.1182/blood.V100.5.1869.h81702001869_1869_1877
54. Lamelas A, Hauser J, Dangy J-P, Hamid A-WM, Röltgen K, Abdul Sater MR, et al. Emergence and Genomic Diversification of a Virulent Serogroup W:ST-2881(CC175) Neisseria Meningitidis Clone in the African Meningitis Belt. Microb Genomics (2017) 3:e000120. doi: 10.1099/mgen.0.000120
55. Beernink PT, Ispasanie E, Lewis LA, Ram S, Moe GR. Granoff DM. A Meningococcal Native Outer Membrane Vesicle Vaccine With Attenuated Endotoxin and Overexpressed Factor H Binding Protein Elicits Gonococcal Bactericidal Antibodies. J Infect Dis (2019) 219:1130–7. doi: 10.1093/infdis/jiy609
56. Schubart A, Anderson K, Mainolfi N, Sellner H, Ehara T, Adams CM, et al. Small-Molecule Factor B Inhibitor for the Treatment of Complement-Mediated Diseases. Proc Natl Acad Sci USA (2019) 116:7926–31. doi: 10.1073/pnas.1820892116
57. Roguska M, Splawski I, Diefenbach-Streiber B, Dolan E, Etemad-Gilbertson B, Rondeau J-M, et al. Generation and Characterization of LFG316, A Fully-Human Anti-C5 Antibody for the Treatment of Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci (2014) 55:3433–3.
58. Zelek WM, Cole J, Ponsford MJ, Harrison RA, Schroeder BE, Webb N, et al. Complement Inhibition With the C5 Blocker LFG316 in Severe COVID-19. Am J Respir Crit Care Med (2020) 202:1304–8. doi: 10.1164/rccm.202007-2778LE
59. Lafayette RA, Rovin BH, Reich HN, Tumlin JA, Floege J, Barratt J. Safety, Tolerability and Efficacy of Narsoplimab, a Novel MASP-2 Inhibitor for the Treatment of IgA Nephropathy. Kidney Int Rep (2020) 5:2032–41. doi: 10.1016/j.ekir.2020.08.003
60. Elhadad S, Chapin J, Copertino D, Van Besien K, Ahamed J, Laurence J. MASP2 Levels are Elevated in Thrombotic Microangiopathies: Association With Microvascular Endothelial Cell Injury and Suppression by Anti-MASP2 Antibody Narsoplimab. Clin Exp Immunol (2021) 203:96–104. doi: 10.1111/cei.13497
61. Sorensen RU, Edgar JDM. Overview of Antibody-Mediated Immunity to S. Pneumoniae: Pneumococcal Infections, Pneumococcal Immunity Assessment, and Recommendations for IG Product Evaluation. Transfusion (2018) 58:3106–13. doi: 10.1111/trf.15044
62. Paterson GK, Orihuela CJ. Pneumococci: Immunology of the Innate Host Response. Respirology (2010) 15:1057–63. doi: 10.1111/j.1440-1843.2010.01814.x
63. Ren B, Li J, Genschmer K, Hollingshead SK, Briles DE. The Absence of PspA or Presence of Antibody to PspA Facilitates the Complement-Dependent Phagocytosis of Pneumococci. vitro Clin Vaccine Immunol (2012) 19:1574–82. doi: 10.1128/CVI.00393-12
Keywords: complement system, Streptococcus pneumoniae, opsonophagocytosis, alternative pathway inhibitor, microbial immunology, innate immunity
Citation: Muri L, Ispasanie E, Schubart A, Thorburn C, Zamurovic N, Holbro T, Kammüller M and Pluschke G (2021) Alternative Complement Pathway Inhibition Abrogates Pneumococcal Opsonophagocytosis in Vaccine-Naïve, but Not in Vaccinated Individuals. Front. Immunol. 12:732146. doi: 10.3389/fimmu.2021.732146
Received: 28 June 2021; Accepted: 23 September 2021;
Published: 11 October 2021.
Edited by:
Gee W. Lau, University of Illinois at Urbana-Champaign, United StatesReviewed by:
Luchang Zhu, Houston Methodist Research Institute, United StatesJingjun Lin, University of California, Davis, United States
Copyright © 2021 Muri, Ispasanie, Schubart, Thorburn, Zamurovic, Holbro, Kammüller and Pluschke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gerd Pluschke, Z2VyZC5wbHVzY2hrZUBzd2lzc3RwaC5jaA==
†These authors have contributed equally to this work and share first authorship
 Lukas Muri
Lukas Muri Emma Ispasanie
Emma Ispasanie Anna Schubart3
Anna Schubart3 Natasa Zamurovic
Natasa Zamurovic Michael Kammüller
Michael Kammüller Gerd Pluschke
Gerd Pluschke