Abstract
The tumour microenvironment (TME) presents a major block to anti-tumour immune responses and to effective cancer immunotherapy. The inflammatory mediators such as cytokines, chemokines, growth factors and prostaglandins generated in the TME alter the phenotype and function of dendritic cells (DCs) that are critical for a successful adaptive immune response against the growing tumour. In this mini review we discuss how tumour cells and the surrounding stroma modulate DC maturation and trafficking to impact T cell function. Fibroblastic stroma and the associated extracellular matrix around tumours can also provide physical restrictions to infiltrating DCs and other leukocytes. We discuss interactions between the inflammatory TME and infiltrating immune cell function, exploring how the inflammatory TME affects generation of T cell-driven anti-tumour immunity. We discuss the open question of the relative importance of antigen-presentation site; locally within the TME versus tumour-draining lymph nodes. Addressing these questions will potentially increase immune surveillance and enhance anti-tumour immunity.
Introduction
Anti-tumour immunity is the ability of the body’s immune system to recognise and eliminate tumour cells. This phenomenon has the potential to cure cancer even if cells are widely disseminated through multiple metastatic sites and has been harnessed to develop different immunotherapy drugs. With increased understanding of immune surveillance process by innate immune cells and discovery of T cell immune checkpoints, such as PD-1, PD-L1, and CTLA-4; cancer immunotherapy has significantly improved patient survival and quality of life (1–5). Treatments aim to promote successful infiltration and activation of antigen presenting cells and boost T-cells cytotoxic activity to promote anti-tumour immunity. However, despite promising results, not all tumour types or patients respond equally to immunotherapy (6–8). The major reasons for failure of immunotherapy are (1) reduced antigenicity (9–11) and (2) immunosuppressive tumour microenvironment (TME) (12–15). The TME is highly heterogeneous; consisting of tumour cells, stromal cells, extracellular matrix (ECM) and immune cell types including macrophages, dendritic cells, T and B lymphocytes, Natural killer (NK) cells, mast cells, myeloid derived suppressor cells (MDSCs) and neutrophils (16–20). The anti-tumour immune response relies on the antigen presenting cells (APCs) to prime naïve T cells. Tissue resident macrophages can activate T cells locally in the tumour; whereas dendritic cells (DCs), the professional APCs, are thought to migrate into the tumour draining lymph nodes (TDLNs) to prime T cells (21). However, immune surveillance by APCs and T-cell infiltration can be impaired by dynamic changes within the tumour microenvironment such as induction of chemokines, cytokines, growth factors, inflammation, ECM modulators and immune checkpoint proteins (22–27). This review focuses on the immunosuppressive properties of the TME and how these mechanisms alter activation, maturation and trafficking of dendritic cells to enable immune escape and tumour progression.
DC Maturation and DC Gene Signatures in Tumours
DCs are the professional APCs responsible for activation and maintenance of tumour-specific cytotoxicity by T cells (28, 29). Tumour infiltrating conventional DCs (cDC1 and cDC2) scan and phagocytose tumour antigens (30–32); and subsequently migrate to secondary lymphoid tissues to prime naïve CD8+ and CD4+ T cells (33–39). The phenotype and function of highly motile DCs is influenced by co-stimulatory molecules (CD80, CD86), chemokine receptors such as CCR7 and cell adhesion molecules (integrins, ICAM-1 and VCAM-1) (40–43). It has been well established that the interaction between CC chemokine receptor 7 (CCR7) upregulated on activated DCs and its ligand CC chemokine ligand 21 (CCL21) expressed by lymphatic endothelial cells (LECs) is essential for directional DC migration towards the lymph nodes (44–46). Upon entry to the LN, DCs use the C-type lectin CLEC-2 to migrate through the fibroblastic reticular network to reach the paracortex to stimulate the T cells (47–50). Secondary lymphoid tissues are structurally specialised to facilitate effective adaptive immune responses; however, the microenvironment of the tumour-draining lymph nodes (TDLNs) can be immune-suppressed in cancer patients and can display low DC count, defects in DC development, low levels of costimulatory molecules or accumulation of immature T cells (51, 52). DCs evaluated from TDLNs of an immunized B16F10 melanoma-bearing mice showed decreased functionality and expressed higher CD86 and lower CD206 levels (53). Similarly, in a study by Caronni et al., LNs draining lung tumours exhibited DCs with reduced antigen presentation due to downregulation of the SNARE VAMP3 and failed cytokine (IL12 and IFN-I) secretion. They reported lactic acid formation in the TME to be the main cause of DC function impairment (54). In addition, damage-associated molecular patterns (DAMPs) released from dying cells in the TME can also influence dendritic cells and other immune cells by interacting with toll-like receptors (TLRs) contributing to immunosuppressive phenotype (55). Lack of mature, migratory DCs in tumours correlates with poor prognosis in cancer patients and failure of immunotherapies (56–58). Recent development of single cell transcriptome profiling of tumour infiltrating DCs has proven to be a very powerful tool to map tumour-driven immune changes and to design future immune therapies leveraging DC biology. scRNA-seq studies on various human tumours, including non–small cell lung cancer (NSCLC) (59–62), head and neck squamous cell carcinoma (63), hepatocellular carcinoma (64), melanoma (65, 66), cutaneous squamous cell carcinoma (67), colorectal cancer (61, 68), ovarian cancer (61), and breast cancer (61) have identified tissue-specific DC subsets as well as those conserved across cancer types. By comparing tumour infiltrating DC states across various tumour studies, five major DC subsets have been defined that are conserved in most tumour types (69, 70) (Table 1). Four major ones are cDC1, cDC2, migratory DC3 (mDC3) and plasmacytoid DC (pDC); and the DC subset (DC5) that were less conserved, mostly contained cDC2 state (CD1C+) but additionally either expressed Langerhans cell-specific markers (CD201, CD1A) or monocyte markers (CD14, CD11b) such as in case of NSCLC (61, 62, 69, 70). DC5 were also referred as inflammatory DCs as these have phenotypic similarities to monocytes but are functionally different due to their cDC2-specific antigen presentation properties (71). On the other hand, classical monocytes (CD14+ CD16-) play a key role in tissue homeostasis and inflammation (72). Like monocytes, inflammatory DCs are also capable of releasing TNF-α and inducible nitric oxide synthase (iNOS) upon pathogen recognition. In addition, there is a subset of cDCs that induce antigen-specific tolerance in dLNs; known as regulatory DCs (DCregs) (73, 74). These are characterized by low MHC expression and therefore weaker antigen presentation capability to effector T cells. Instead, they can induce proliferation of regulatory T cells (Tregs) resulting in immune tolerance. These properties have led the use of DCregs in organ transplantations (75).
Table 1
| DC subsets | Markers |
|---|---|
| cDC1 | XCR1, CLEC9A, CADM1, CD141, CD103 |
| cDC2 | CD11b, SIRPa, CLEC10A, FCER1A, CD1c |
| mDC3 | MARCKS, CCL19, LAMP3, BATF3, CCR7, CD40 |
| pDC | TCf4, CXCR3, LILRA4, CLCEC4C, IRF7 |
| DC5 or inflammatory DCs | CD1c, CD201, CD1A, CD14 |
Overall cDC2 phenotype is the most abundant, while the other DC subtypes vary in each cancer type (61, 76). Single cell sequencing and clustering analysis have identified transcription factors underlying each DC phenotype, including BATF3 for cDC1s, CEBPB for cDC2s, NFKB2 for migratory cDCs and TCF4 for pDCs (61, 77). Another study reports differential expression of costimulatory molecules and immune checkpoints on different DC subsets present in the TME (78). Although these phenomena are tightly regulated, heterogeneity of TME can influence the transcriptional factor activity, expression of costimulatory molecules and hence DC maturation and/or migration (78–82). This new in-depth knowledge of DC gene signatures can facilitate the design of a favourable antitumour response or identification of response biomarkers for targeted therapies (83).
TME Factors Affecting DC Development in Tumours
Pro- and Anti-Inflammatory Factors
The immunosuppression of tumour-infiltrating DCs can be facilitated by various soluble factors secreted in the TME such as IL-6, IL-10, IDO, M-CSF, transforming growth factor-β1 (TGF-β1), PGE2, VEGF (Figure 1) (84–91); although promisingly some of these defects in DC development or function have been proven to be reversible in pre-clinical models and clinical trials (27, 91–94). Mature DC numbers or functions were improved leading to better immune control of the tumour in several mouse models: IL-6 KO mice (95); tumours treated with anti-VEGF antibody (96, 97); and treatment with anti-IL-8 monoclonal antibody (98, 99). On the other hand, pro-inflammatory cytokines such as IFN-α, IL-2, IL-15, IL-21 and GM-CSF are also present in the TME (Figure 1) that contribute to enhanced antigen priming, improved DC maturation and increased immune infiltration in tumours (100–103). Therefore, the complex balance of inflammatory signals in the TME is an area of intense research interest but is not trivial to target currently. One of the recent studies on human melanoma reported the correlation of pro-inflammatory cytokine FLT3L production (by NK cells) with abundant intratumoral stimulatory DCs, improved patient responsiveness to anti-PD-1 therapies and better overall survival (104).
Figure 1
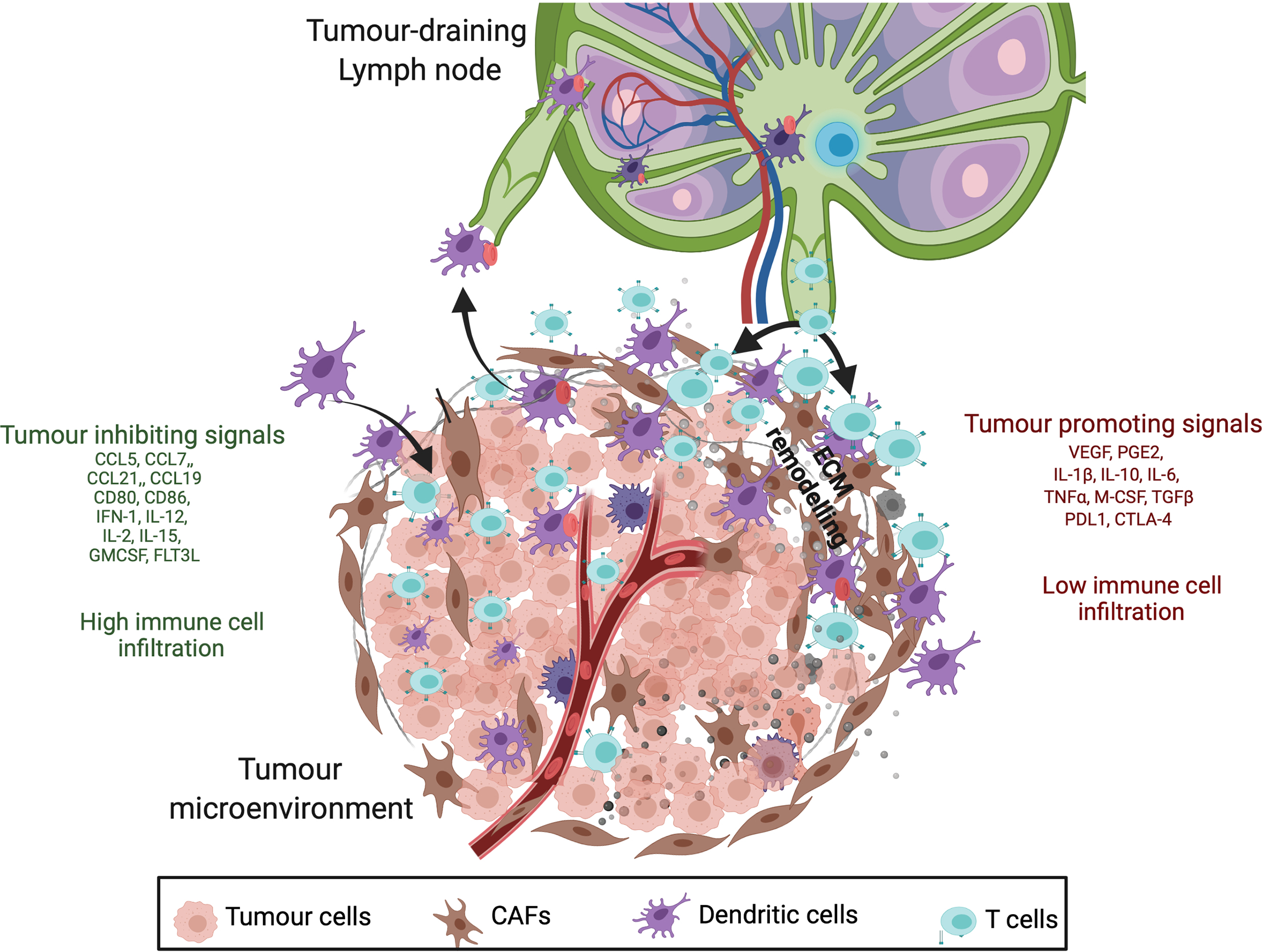
Cancer inhibitory and cancer-promoting signals within the tumour microenvironment (TME). Anti-tumour response is initiated by antigen recognition and trafficking by mature DCs to the tumour draining lymph node (TDLN) which involves upregulation of chemokine receptors (CCR7), MHC class II, co-stimulatory molecules (CD80 and CD86), inflammatory molecules (IL-12, INF-1) and adhesion molecules (ICAM-1) (listed in green). Having said that, immunosuppressive nature of TME secretes tumour promoting inflammatory mediators (listed in red) such as prostaglandin E2, cytokines (IL-10, IL-6, TGFß), chemokines (CXCL1) and growth factors (VEGF) that impede anti-tumour response by altering DC phenotype, T-cell infiltration and ECM remodelling. These differences result in poor surveillance by DCs and lower infiltration of T cells in tumours with immunosuppressive molecules (red).
The inflammatory factors described above can be derived from tumour cells, immune cells or stromal cells such as fibroblasts surrounding tumour (61, 88, 105, 106). Various subtypes of fibroblasts based on different tissue specific identity, localization, function, transcription factor expression, collagen factors, cancer hallmark genes etc. make up the total tumour mass. CAFs or cancer associated fibroblasts represent a major population in the TME of many solid tumours, however their origin and role in tumour progression is complex and they can generate pro-tumourigenic and anti-tumourigenic secretory factors. Phenotypically and functionally different CAF subtypes based on cell-surface markers such as podoplanin (PDPN), α-smooth muscle actin (αSMA), fibroblast-activated protein (FAP), fibroblast-specific protein-1 (FSP-1/S100A4), THY1 (also known as CD90), and platelet-derived growth factor receptor-α, and β (PDGFRα and PDGFRβ) have been associated with different tumour types, stages and patient survival (107–111). Recently, the ability of CAFs to modulate the immune responses has been discovered and is being explored to improve cancer therapies. CAFs also share some properties with fibroblasts in lymph nodes that already have a well-established role in DC migration (47, 112, 113); and therefore, parallels can be drawn between the two to better understand the DC trafficking in the TME. For example, PDPN present in fibroblasts interacts with CCL21 and promotes CCL21/CCR7 axis mediated DC migration in lymph node. This knowledge was exploited to study the role of PDPN+ CAFs under the influence of hypoxia in tumour progression (114). The study reported PDPN overexpression due to hypoxia in fact favoured invasion of CCR7+ tumour cell into CCL21+ peripheral lymph nodes leading to metastasis (114, 115). Tumours associated with hypoxia are immunosuppressive and lack high expression of CCL21 and therefore therapeutic use of recombinant chemokines (such as CCL21) to stimulate immune cell recognition in tumours is being considered as a novel treatment approach (116, 117). Also, more research is required to understand the transition of a ‘normal’ fibroblast into an immunosuppressive phenotype such as S100A4+ PDPN+ CAFs as reported in breast cancer patients (109) or into an inflammatory CAF (iCAF) phenotype producing IL-6, IL-10, and IDO (118, 119) linked to poor patient survival. Authors of Fang et al. (118) have shown the role of the urokinase-type plasminogen activator, PLAU in conversion of fibroblasts to iCAFs in esophgeal cancer (118), but much is still unknown about fibroblast differentiation in TME.
Tertiary Lymphoid Structures (TLS)
TLS are established at sites of chronic inflammation and can structurally and functionally resemble secondary lymphoid organs (120–122). Recent studies on murine models of TLS have shown the role of PDPN+ FAP+ immunofibroblasts in driving the development and expansion of TLSs (123, 124). These form part of the TME and can benefit from quick surveillance and locally primed immune response against tumour antigens (Figure 2). Occurrence of TLS correlated with high number of mature DCs, strong T-cell infiltration and long-term survival in human primary lung, breast, colorectal, melanoma and other tumours (120, 125–128). However, factors such as TLS location, tumour stage, tumour mutations, treatment history can affect immune cell infiltration and anti-tumour response (128, 129). The cells residing in TLS in tumours are known to express Th1, CD4, CD8, CD31, CD23, FOXP3, chemokines (CCL19, CCL21) and clusters of DC-Lamp+ mature dendritic cells (120, 130, 131) providing an immune-supportive niche (132–134). Typically, TLS at the periphery of the tumour have more organised and distinct DC/T-cell and B-cell zones than intratumoral TLS which contain mostly B cells (133). Future research understanding the immunological features of extratumoral versus intratumoral TLS will be useful to predict responsiveness to immunotherapy and overall survival.
Figure 2
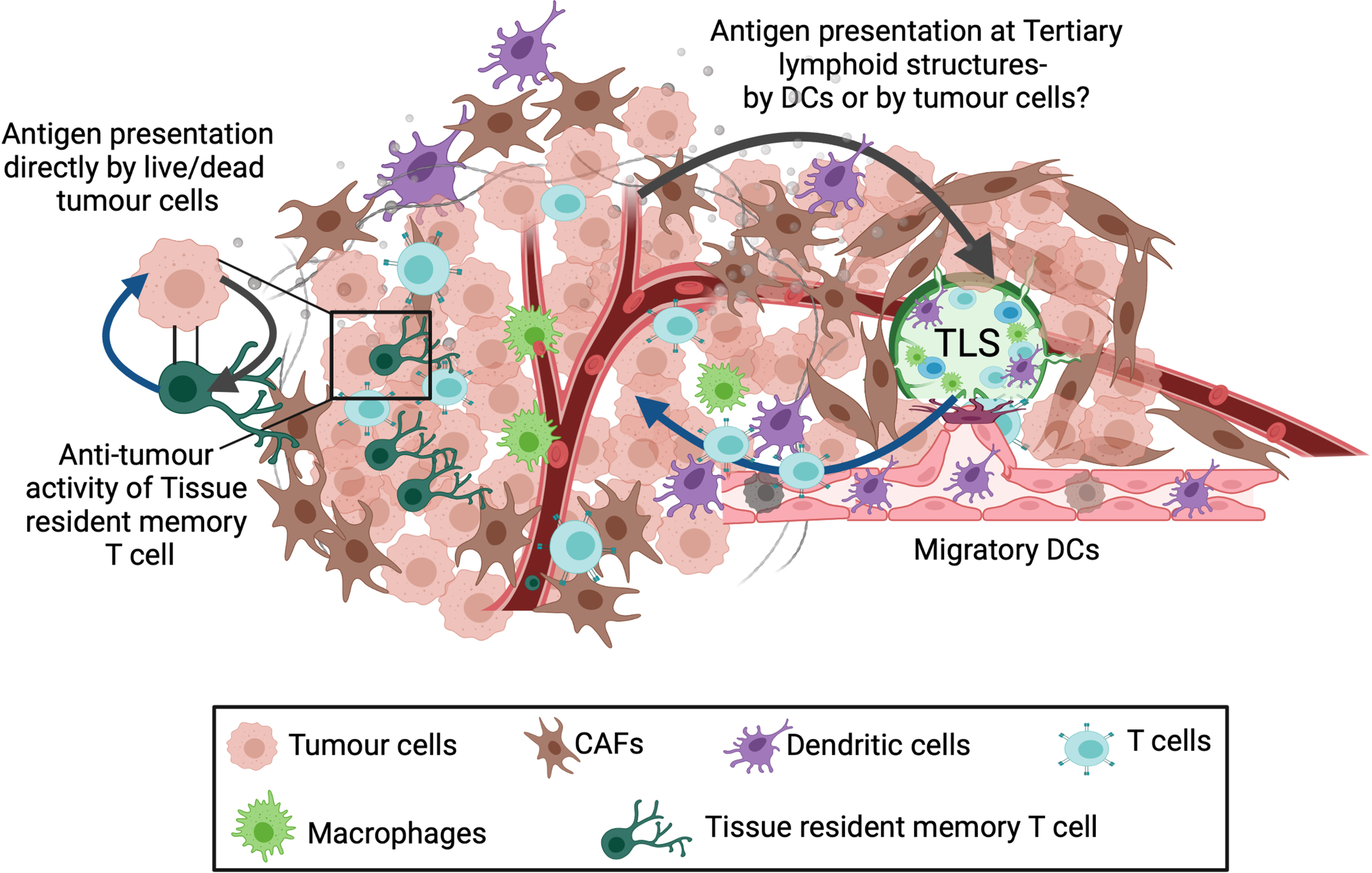
Alternate sites of antigen presentation and T-cell priming. Three different sites for presentation of tumour associated antigens have been described: Tumour draining lymph node (TDLN), Tertiary lymphoid structures (TLS) and Tissue resident memory T cells. A population of memory precursor cells are believed to differentiate into CD103+ tissue resident memory T cells. These cells reside in the tumour and can recognize tumour antigens followed by killing the target tumour cell. In addition, tertiary lymphoid structures (TLS) also present a potential site for T cell priming. TLSs are organised cell aggregates formed within or at tumour margins in response to local inflammation and numerous cell-cell interactions occurring within the TME. Since these contain various immune cell types, TLSs can activate local immune response against the tumour, however the mechanism for T-cell priming within the TLSs is unknown.
Immune Checkpoint Genes
The other group of molecules responsible for causing dysfunction in tumour-infiltrating DCs are immune checkpoint proteins PD-L1, PD-1, ILT2, CTLA4, TIM3 expressed by tumour cells or other immune cells (135–141). As mentioned before, expression of these inhibitory molecules is variable among DC subsets. For example, PD-1 and TIM-3 are mostly expressed on cDC1s; PD-1 expression specifically has been shown to inhibit NF-kB activation which is critical for DC functions including costimulatory molecule expression, antigen presentation and cytokine release leading to T cell inactivation (78, 135, 137, 139, 140). On contrary, ILT2 is expressed on pDCs and cDC2s, but not on cDC1s (78). The central goal of immunotherapies is inhibition of immune checkpoint genes and the expansion of mature cDCs and cytotoxic CD8+ T cells within tumours. It is associated with positive patient outcomes in multiple cancer types when combined with chemotherapy or radiotherapy treatments (28, 135, 142, 143). Despite this, many patients still fail to respond to immune checkpoint blockade. A better understanding of the role of inflammatory mediators in determining tumour progression will also provide therapeutic avenues to improve immunotherapy outcomes (144–147).
Different labs have reported direct inhibition of pro-tumourigenic inflammation in combination with immune checkpoint blockade as a powerful strategy to improve the patient survival rates (27, 148–150). One such example is the use of aspirin that blocks the COX-2/PGE2 pathway and has shown promising results in preclinical melanoma models (27, 149). Prostaglandin E2 (PGE2), catalysed by the enzyme COX-2 is elevated in many tumours (151) and plays a role in tumour evasion by directly inhibiting cytotoxic immune responses and subsequently mediates expression of other inflammatory molecules such as CXCL9, CXCL10, CXCR4, CXCL12, IDO1 and interferon (IFN)-γ (27, 144, 148, 150, 152–154). Induction of CXCL12, CXCR4 and IDO1 in tumours have been associated with accumulation of myeloid derived suppressor cells (90, 155). Moreover, direct interaction of EP2/EP4 receptors (present on DCs) with the available PGE2 can modulate DC maturation, metalloprotease-driven DC motility, and immune response in tumours (27, 149, 152, 156–158). Thus, targeting the inflammatory environment of the tumour is important to restore DC function to harvest the full potential of immunotherapy.
Leveraging DC Biology in Cancer Therapies
Anti-tumour immunity relies on cross-presentation of tumour antigens by DCs to elicit a CD8+ T cell response. Among various DC subsets, cDC1s (XCR1+, CD103+) play a critical role in anti-tumour immunity. CLEC9A, (also known as DNGR1) is highly expressed on cDC1s and binds necrotic cell debris and promotes antigen processing in tumours (159–161). One of the reasons for checkpoint blockade failure is poor antigen presentation due to absence of co-stimulatory molecules and therefore modulation of DC function could increase responses to these therapies. One method to address this issue is the development of DC vaccines for cancer treatment, bypassing the need to activate and mature DCs within the tumour. DC-based cancer vaccines work by recruiting ex-vivo generated dendritic cells (or monocyte derived patient DCs) that are genetically engineered, matured, and loaded with tumour-specific antigens (162–164) or by reprogramming endogenous DCs by injecting biomaterial-based scaffolds providing favourable microenvironment for the recruitment of activated DCs (165, 166). An ideal DC vaccine must be able to increase cross-presentation by DCs, express high levels of co-stimulatory molecules, induce tumour-specific T cells with high migratory and cytolytic capabilities. Furthermore, the use of dendritic growth factor Flt3L in combination with checkpoint inhibitors or DC vaccines has improved number of activated intratumoural cDC1s and enhanced anti-tumour immunity to BRAF and checkpoint blockade in preclinical models (167–170).
Presence of co-inhibitory signals (e.g., IL-10, IL-6, PGE2, TGF-β) or absence of co-stimulatory molecules (e.g. CD80 and CD86) can result in inefficient antigen presentation by DCs and poor induction of antigen-specific CD8+T cells. Therefore, inflammatory cytokines secreted by tumour cells and tumour-associated stroma have been identified as promising candidates to potentiate current immunotherapies including immune checkpoint blockade and CAR-T therapy (149, 171–173). Stroma present around most tumours can also magnify inflammation and impede DC phenotype (174–177) and hence manipulating stroma/DC crosstalk in the TME could help improve DC function.
Discussion
It is now established that tumours can exploit their surroundings to create an immunosuppressive microenvironment to control DC function within both the TME and TDLNs (178, 179). These signals including cytokines, chemokines, prostaglandins, growth factors, immune checkpoint genes, etc., may target different DC subsets infiltrating tumours and influence DC maturation, antigen uptake and DC migration (53, 180). Although the success of immunotherapy relies on enhanced T cell activity, activation of tumour-specific T cells cannot be achieved without prior antigen presentation by professional DCs. To overcome immunosuppressive signals, personalized vaccines loaded with patient-derived engineered DCs or delivery of innate stimulus such as TLR3 ligand or a STING agonist to DCs at the tumour site are being developed and have shown promising results (181, 182). Repurposing of existing anti-inflammatory drugs such as aspirin along with DC vaccines or immunotherapies has also been successfully tested in pre-clinical models (149).
This review also addresses the importance of local versus TDLN priming of anti-tumoural T cell responses. Tissue resident memory CD103+ CD8+ T cells residing in the non-lymphoid tissues have shown to provide local immunosurveillance and enhanced immune responses in melanoma, lung and breast tumours (183–187). Moreover, melanoma patients with higher resident T cell population responded better to anti-PD-1 immunotherapy with improved survival (188, 189). However, what is still unclear is how are tissue resident memory CD8+ T cells primed (Figure 2) and whether there is a distinct population of DCs required to activate them. Although the exact regulatory mechanisms remain to be explored further, it is hypothesized that crosstalk between tissue resident memory T cells, tumour cells, stromal cells and DCs within the TME potentiate secondary T-cell responses against tumours (Figure 2). This also opens discussion on the role of tumour associated tertiary lymphoid structures (TLSs) in intra-tumoural DC maturation; and sourcing T cells and B cells to the tumour (190). Although TLS has been positively correlated with anti-tumour responses, there are still many questions remain to be answered such as TLS composition and TLS induction at tumour site before TLS can be adopted as a predictive tool or as a therapeutic option. Our discussion demonstrates the importance of site of antigen presentation in DC maturation and trafficking which must be exploited therapeutically to enhance immune response against cancer.
Funding
This work is funded by Cancer Research UK (Career development fellowship CRUK-A19763 to SA) and Medical Research Council (MC-U12266B).
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Author contributions
YG and SA planned the concept and design of the review. YG and AK collected previous literature on the topic and drafted the article. YG made the figures. SA performed critical revision of the article. YG and SA edited the final version of the article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
APC, Antigen Presenting cells; LNs, Lymph nodes; TDLN, Tumour draining lymph node; TME, Tumour microenvironment; DCs, Dendritic cells; PGE2, Prostaglandin E2; ECM, Extracellular matrix; CAF, Cancer associated fibroblasts; TLS, Tertiary lymphoid structures.
References
1
Esfahani K Roudaia L Buhlaiga N del Rincon SV Papneja N Miller WH . A Review of Cancer Immunotherapy: From the Past, to the Present, to the Future. Curr Oncol (2020) 27:87–97. doi: 10.3747/co.27.5223
2
Koury J Lucero M Cato C Chang L Geiger J Henry D et al . Immunotherapies: Exploiting the Immune System for Cancer Treatment. J Immunol Res (2018) 2018:1–16. doi: 10.1155/2018/9585614
3
Larkin J Chiarion-Sileni V Gonzalez R Grob J-J Rutkowski P Lao CD et al . Five-Year Survival With Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med (2019) 381:1535–46. doi: 10.1056/NEJMoa1910836
4
Nogrady B . Game-Changing Class of Immunotherapy Drugs Lengthens Melanoma Survival Rates. Nature (2020) 580:S14–6. doi: 10.1038/d41586-020-01038-9
5
Zhao B Zhao H Zhao J . Efficacy of PD-1/PD-L1 Blockade Monotherapy in Clinical Trials. Ther Adv Med Oncol (2020) 12:1–22. doi: 10.1177/1758835920937612
6
Brahmer JR Tykodi SS Chow LQM Hwu W-J Topalian SL Hwu P et al . Safety and Activity of Anti–PD-L1 Antibody in Patients With Advanced Cancer. N Engl J Med (2012) 366:2455–65. doi: 10.1056/NEJMoa1200694
7
Haslam A Prasad V . Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Network Open (2019) 2:1–9. doi: 10.1001/jamanetworkopen.2019.2535
8
Royal RE Levy C Turner K Mathur A Hughes M Kammula US et al . Phase 2 Trial of Single Agent Ipilimumab (Anti-CTLA-4) for Locally Advanced or Metastatic Pancreatic Adenocarcinoma. J Immunother (2010) 33:828–33. doi: 10.1097/CJI.0b013e3181eec14c
9
Almand B Resser JR Lindman B Nadaf S Clark JI Kwon ED et al . Clinical Significance of Defective Dendritic Cell Differentiation in Cancer. Clin Cancer Res: An Off J Am Assoc Cancer Res (2000) 6:1755–66.
10
Balachandran VP Łuksza M Zhao JN Makarov V Moral JA Remark R et al . Identification of Unique Neoantigen Qualities in Long-Term Survivors of Pancreatic Cancer. Nature (2017) 551:512–6. doi: 10.1038/nature24462
11
Delp K Momburg F Hilmes C Huber C Seliger B . Functional Deficiencies of Components of the MHC Class I Antigen Pathway in Human Tumors of Epithelial Origin. Bone Marrow Transplant (2000) 25:S88–95. doi: 10.1038/sj.bmt.1702363
12
Bergmann C Strauss L Zeidler R Lang S Whiteside TL . Expansion of Human T Regulatory Type 1 Cells in the Microenvironment of Cyclooxygenase 2 Overexpressing Head and Neck Squamous Cell Carcinoma. Cancer Res (2007) 67:8865–73. doi: 10.1158/0008-5472.CAN-07-0767
13
Ene–Obong A Clear AJ Watt J Wang J Fatah R Riches JC et al . Activated Pancreatic Stellate Cells Sequester CD8+ T Cells to Reduce Their Infiltration of the Juxtatumoral Compartment of Pancreatic Ductal Adenocarcinoma. Gastroenterology (2013) 145:1121–32. doi: 10.1053/j.gastro.2013.07.025
14
Feig C Jones JO Kraman M Wells RJB Deonarine A Chan DS et al . Targeting CXCL12 From FAP-Expressing Carcinoma-Associated Fibroblasts Synergizes With Anti-PD-L1 Immunotherapy in Pancreatic Cancer. Proc Natl Acad Sci (2013) 110:20212–7. doi: 10.1073/pnas.1320318110
15
Kabacaoglu D Ciecielski KJ Ruess DA Algül H . Immune Checkpoint Inhibition for Pancreatic Ductal Adenocarcinoma: Current Limitations and Future Options. Front Immunol (2018) 9:1878. doi: 10.3389/fimmu.2018.01878
16
Chung W Eum HH Lee H-O Lee K-M Lee H-B Kim K-T et al . Single-Cell RNA-Seq Enables Comprehensive Tumour and Immune Cell Profiling in Primary Breast Cancer. Nat Commun (2017) 8:1–12. doi: 10.1038/ncomms15081
17
Kojima Y Acar A Eaton EN Mellody KT Scheel C Ben-Porath I et al . Autocrine TGF- and Stromal Cell-Derived Factor-1 (SDF-1) Signaling Drives the Evolution of Tumor-Promoting Mammary Stromal Myofibroblasts. Proc Natl Acad Sci (2010) 107:20009–14. doi: 10.1073/pnas.1013805107
18
Medrek C Pontén F Jirström K Leandersson K . The Presence of Tumor Associated Macrophages in Tumor Stroma as a Prognostic Marker for Breast Cancer Patients. BMC Cancer (2012) 12:1–9. doi: 10.1186/1471-2407-12-306
19
Smith HA Kang Y . The Metastasis-Promoting Roles of Tumor-Associated Immune Cells. J Mol Med (2013) 91:411–29. doi: 10.1007/s00109-013-1021-5
20
Spaeth EL Dembinski JL Sasser AK Watson K Klopp A Hall B et al . Mesenchymal Stem Cell Transition to Tumor-Associated Fibroblasts Contributes to Fibrovascular Network Expansion and Tumor Progression. PloS One (2009) 4:1–11. doi: 10.1371/journal.pone.0004992
21
Ruhland MK Roberts EW Cai E Mujal AM Marchuk K Beppler C et al . Visualizing Synaptic Transfer of Tumor Antigens Among Dendritic Cells. Cancer Cell (2020) 37:789–99. doi: 10.1016/j.ccell.2020.05.002
22
Aiello NM Bajor DL Norgard RJ Sahmoud A Bhagwat N Pham MN et al . Metastatic Progression Is Associated With Dynamic Changes in the Local Microenvironment. Nat Commun (2016) 7:1–9. doi: 10.1038/ncomms12819
23
Di Blasio S van Wigcheren GF Becker A van Duffelen A Gorris M Verrijp K et al . The Tumour Microenvironment Shapes Dendritic Cell Plasticity in a Human Organotypic Melanoma Culture. Nat Commun (2020) 11:1–17. doi: 10.1038/s41467-020-16583-0
24
Oba T Long MD Keler T Marsh HC Minderman H Abrams SI et al . Overcoming Primary and Acquired Resistance to Anti-PD-L1 Therapy by Induction and Activation of Tumor-Residing Cdc1s. Nat Commun (2020) 11:1–20. doi: 10.1038/s41467-020-19192-z
25
Poloso NJ Urquhart P Nicolaou A Wang J Woodward DF . PGE2 Differentially Regulates Monocyte-Derived Dendritic Cell Cytokine Responses Depending on Receptor Usage (EP2/EP4). Mol Immunol (2013) 54:284–95. doi: 10.1016/j.molimm.2012.12.010
26
Valenti R Huber V Iero M Filipazzi P Parmiani G Rivoltini L . Tumor-Released Microvesicles as Vehicles of Immunosuppression: Figure 1. Cancer Res (2007) 67:2912–5. doi: 10.1158/0008-5472.CAN-07-0520
27
Zelenay S van der Veen AG Böttcher JP Snelgrove KJ Rogers N Acton SE et al . Cyclooxygenase-Dependent Tumor Growth Through Evasion of Immunity. Cell (2015) 162:1257–70. doi: 10.1016/j.cell.2015.08.015
28
Broz ML Binnewies M Boldajipour B Nelson AE Pollack JL Erle DJ et al . Dissecting the Tumor Myeloid Compartment Reveals Rare Activating Antigen-Presenting Cells Critical for T Cell Immunity. Cancer Cell (2014) 26:638–52. doi: 10.1016/j.ccell.2014.09.007
29
Engelhardt JJ Boldajipour B Beemiller P Pandurangi P Sorensen C Werb Z et al . Marginating Dendritic Cells of the Tumor Microenvironment Cross-Present Tumor Antigens and Stably Engage Tumor-Specific T Cells. Cancer Cell (2012) 21:402–17. doi: 10.1016/j.ccr.2012.01.008
30
Hildner K Edelson BT Purtha WE Diamond M Matsushita H Kohyama M et al . Batf3 Deficiency Reveals a Critical Role for CD8 + Dendritic Cells in Cytotoxic T Cell Immunity. Science (2008) 322:1097–100. doi: 10.1126/science.1164206
31
Nakahara T Oba J Shimomura C Kido-Nakahara M Furue M . Early Tumor-Infiltrating Dendritic Cells Change Their Characteristics Drastically in Association With Murine Melanoma Progression. J Invest Dermatol (2016) 136:146–53. doi: 10.1038/JID.2015.359
32
Theisen DJ Davidson JT Briseño CG Gargaro M Lauron EJ Wang Q et al . WDFY4 Is Required for Cross-Presentation in Response to Viral and Tumor Antigens. Science (2018) 362:694–9. doi: 10.1126/science.aat5030
33
Allan RS Waithman J Bedoui S Jones CM Villadangos JA Zhan Y et al . Migratory Dendritic Cells Transfer Antigen to a Lymph Node-Resident Dendritic Cell Population for Efficient CTL Priming. Immunity (2006) 25:153–62. doi: 10.1016/j.immuni.2006.04.017
34
Binnewies M Mujal AM Pollack JL Combes AJ Hardison EA Barry KC et al . Unleashing Type-2 Dendritic Cells to Drive Protective Antitumor CD4+ T Cell Immunity. Cell (2019) 177:556–71. doi: 10.1016/j.cell.2019.02.005
35
Hirano N Butler MO Xia Z Ansén S von Bergwelt-Baildon MS Neuberg D et al . Engagement of CD83 Ligand Induces Prolonged Expansion of CD8+ T Cells and Preferential Enrichment for Antigen Specificity. Blood (2006) 107:1528–36. doi: 10.1182/blood-2005-05-2073
36
Plantinga M Guilliams M Vanheerswynghels M Deswarte K Branco-Madeira F Toussaint W et al . Conventional and Monocyte-Derived CD11b+ Dendritic Cells Initiate and Maintain T Helper 2 Cell-Mediated Immunity to House Dust Mite Allergen. Immunity (2013) 38:322–35. doi: 10.1016/j.immuni.2012.10.016
37
Pooley JL Heath WR Shortman K . Cutting Edge: Intravenous Soluble Antigen Is Presented to CD4 T Cells by CD8 – Dendritic Cells, But Cross-Presented to CD8 T Cells by CD8 + Dendritic Cells. J Immunol (2001) 166:5327–30. doi: 10.4049/jimmunol.166.9.5327
38
Schlitzer A McGovern N Teo P Zelante T Atarashi K Low D et al . IRF4 Transcription Factor-Dependent CD11b+ Dendritic Cells in Human and Mouse Control Mucosal IL-17 Cytokine Responses. Immunity (2013) 38:970–83. doi: 10.1016/j.immuni.2013.04.011
39
Steinman RM . Decisions About Dendritic Cells: Past, Present, and Future. Annu Rev Immunol (2012) 30:1–22. doi: 10.1146/annurev-immunol-100311-102839
40
Harjunpää H Llort Asens M Guenther C Fagerholm SC . Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front Immunol (2019) 10. doi: 10.3389/fimmu.2019.01078
41
Kobayashi D Endo M Ochi H Hojo H Miyasaka M Hayasaka H . Regulation of CCR7-Dependent Cell Migration Through CCR7 Homodimer Formation. Sci Rep (2017) 7:1–14. doi: 10.1038/s41598-017-09113-4
42
Morrison VL James MJ Grzes K Cook P Glass DG Savinko T . Loss of Beta2-Integrin-Mediated Cytoskeletal Linkage Reprogrammes Dendritic Cells to a Mature Migratory Phenotype. Nat Commun (2014) 5:1–26. doi: 10.1038/ncomms6359
43
Roberts EW Broz ML Binnewies M Headley MB Nelson AE Wolf DM et al . Critical Role for CD103 +/CD141 + Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell (2016) 30:324–36. doi: 10.1016/j.ccell.2016.06.003
44
Jang MH Sougawa N Tanaka T Hirata T Hiroi T Tohya K et al . CCR7 Is Critically Important for Migration of Dendritic Cells in Intestinal Lamina Propria to Mesenteric Lymph Nodes. J Immunol (2006) 176:803–10. doi: 10.4049/jimmunol.176.2.803
45
Russo E Teijeira A Vaahtomeri K Willrodt A-H Bloch JS Nitschké M et al . Intralymphatic CCL21 Promotes Tissue Egress of Dendritic Cells Through Afferent Lymphatic Vessels. Cell Rep (2016) 14:1723–34. doi: 10.1016/j.celrep.2016.01.048
46
Vaahtomeri K Brown M Hauschild R de Vries I Leithner AF Mehling M et al . Locally Triggered Release of the Chemokine CCL21 Promotes Dendritic Cell Transmigration Across Lymphatic Endothelia. Cell Rep (2017) 19:902–9. doi: 10.1016/j.celrep.2017.04.027
47
Acton SE Astarita JL Malhotra D Lukacs-Kornek V Franz B Hess PR et al . Podoplanin-Rich Stromal Networks Induce Dendritic Cell Motility via Activation of the C-Type Lectin Receptor CLEC-2. Immunity (2012) 37:276–89. doi: 10.1016/j.immuni.2012.05.022
48
Braun A Worbs T Moschovakis GL Halle S Hoffmann K Bölter J et al . Afferent Lymph–Derived T Cells and DCs Use Different Chemokine Receptor CCR7–dependent Routes for Entry Into the Lymph Node and Intranodal Migration. Nat Immunol (2011) 12:879–87. doi: 10.1038/ni.2085
49
Link A Vogt TK Favre S Britschgi MR Acha-Orbea H Hinz B et al . Fibroblastic Reticular Cells in Lymph Nodes Regulate the Homeostasis of Naive T Cells. Nat Immunol (2007) 8:1255–65. doi: 10.1038/ni1513
50
Peduto L Dulauroy S Lochner M Späth GF Morales MA Cumano A et al . Inflammation Recapitulates the Ontogeny of Lymphoid Stromal Cells. J Immunol (2009) 182:5789–99. doi: 10.4049/jimmunol.0803974
51
Núñez NG Tosello Boari J Ramos RN Richer W Cagnard N Anderfuhren CD et al . Tumor Invasion in Draining Lymph Nodes Is Associated With Treg Accumulation in Breast Cancer Patients. Nat Commun (2020) 11:1–15. doi: 10.1038/s41467-020-17046-2
52
Shinde P Fernandes S Melinkeri S Kale V Limaye L . Compromised Functionality of Monocyte-Derived Dendritic Cells in Multiple Myeloma Patients may Limit Their Use in Cancer Immunotherapy. Sci Rep (2018) 8:1–11. doi: 10.1038/s41598-018-23943-w
53
O’Melia MJ Rohner NA Manspeaker MP Francis DM Kissick HT Thomas SN . Quality of CD8+ T Cell Immunity Evoked in Lymph Nodes Is Compartmentalized by Route of Antigen Transport and Functional in Tumor Context. Sci Adv (2020) 6:1–16. doi: 10.1126/sciadv.abd7134
54
Caronni N Simoncello F Stafetta F Guarnaccia C Ruiz-Moreno JS Opitz B et al . Downregulation of Membrane Trafficking Proteins and Lactate Conditioning Determine Loss of Dendritic Cell Function in Lung Cancer. Cancer Res (2018) 78:1685–99. doi: 10.1158/0008-5472.CAN-17-1307
55
Wang X Ji J Zhang H Fan Z Zhang L Shi L et al . Stimulation of Dendritic Cells by DAMPs in ALA-PDT Treated SCC Tumor Cells. Oncotarget (2015) 6:44688–702. doi: 10.18632/oncotarget.5975
56
Bol KF Schreibelt G Rabold K Wculek SK Schwarze JK Dzionek A et al . The Clinical Application of Cancer Immunotherapy Based on Naturally Circulating Dendritic Cells. J ImmunoTher Cancer (2019) 7:1–13. doi: 10.1186/s40425-019-0580-6
57
Spranger S Luke JJ Bao R Zha Y Hernandez KM Li Y et al . Density of Immunogenic Antigens Does Not Explain the Presence or Absence of the T-Cell-Inflamed Tumor Microenvironment in Melanoma. Proc Natl Acad Sci USA (2016) 113:7759–68. doi: 10.1073/pnas.1609376113
58
Spranger S Bao R Gajewski TF . Melanoma-Intrinsic β-Catenin Signalling Prevents Anti-Tumour Immunity. Nature (2015) 523:231–5. doi: 10.1038/nature14404
59
Kim N Kim HK Lee K Hong Y Cho JH Choi JW et al . Single-Cell RNA Sequencing Demonstrates the Molecular and Cellular Reprogramming of Metastatic Lung Adenocarcinoma. Nat Commun (2020) 11:1–15. doi: 10.1038/s41467-020-16164-1
60
Maier B Leader AM Chen ST Tung N Chang C LeBerichel J et al . A Conserved Dendritic-Cell Regulatory Program Limits Antitumour Immunity. Nature (2020) 580:257–62. doi: 10.1038/s41586-020-2134-y
61
Qian J Olbrecht S Boeckx B Vos H Laoui D Etlioglu E et al . A Pan-Cancer Blueprint of the Heterogeneous Tumor Microenvironment Revealed by Single-Cell Profiling. Cell Res (2020) 30:745–62. doi: 10.1038/s41422-020-0355-0
62
Zilionis R Engblom C Pfirschke C Savova V Zemmour D Saatcioglu HD et al . Single-Cell Transcriptomics of Human and Mouse Lung Cancers Reveals Conserved Myeloid Populations Across Individuals and Species. Immunity (2019) 50:1317–34. doi: 10.1016/j.immuni.2019.03.009
63
Cillo AR Kürten CHL Tabib T Qi Z Onkar S Wang T et al . Immune Landscape of Viral- and Carcinogen-Driven Head and Neck Cancer. Immunity (2020) 52:183–99. doi: 10.1016/j.immuni.2019.11.014
64
Zhang Q He Y Luo N Patel SJ Han Y Gao R et al . Landscape and Dynamics of Single Immune Cells in Hepatocellular Carcinoma. Cell (2019) 179:829–45. doi: 10.1016/j.cell.2019.10.003
65
Brown CC Gudjonson H Pritykin Y Deep D Lavallée V-P Mendoza A et al . Transcriptional Basis of Mouse and Human Dendritic Cell Heterogeneity. Cell (2019) 179:846–63. doi: 10.1016/j.cell.2019.09.035
66
Nirschl CJ Suárez-Fariñas M Izar B Prakadan S Dannenfelser R Tirosh I et al . Ifnγ-Dependent Tissue-Immune Homeostasis Is Co-Opted in the Tumor Microenvironment. Cell (2017) 170:127–41. doi: 10.1016/j.cell.2017.06.016
67
Ji AL Rubin AJ Thrane K Jiang S Reynolds DL Meyers RM et al . Multimodal Analysis of Composition and Spatial Architecture in Human Squamous Cell Carcinoma. Cell (2020) 182:497–514. doi: 10.1016/j.cell.2020.05.039
68
Zhang L Li Z Skrzypczynska KM Fang Q Zhang W O’Brien SA et al . Single-Cell Analyses Inform Mechanisms of Myeloid-Targeted Therapies in Colon Cancer. Cell (2020) 181. doi: 10.1016/j.cell.2020.03.048
69
Cheng S Li Z Gao R Xing B Gao Y Yang Y et al . A Pan-Cancer Single-Cell Transcriptional Atlas of Tumor Infiltrating Myeloid Cells. Cell (2021) 184:792–809. doi: 10.1016/j.cell.2021.01.010
70
Gerhard GM Bill R Messemaker M Klein AM Pittet MJ . Tumor-Infiltrating Dendritic Cell States Are Conserved Across Solid Human Cancers. J Exp Med (2021) 218:1–13. doi: 10.1084/jem.20200264
71
Dutertre C-A Becht E Irac SE Khalilnezhad A Narang V Khalilnezhad S et al . Single-Cell Analysis of Human Mononuclear Phagocytes Reveals Subset-Defining Markers and Identifies Circulating Inflammatory Dendritic Cells. Immunity (2019) 51:573–89.e8. doi: 10.1016/j.immuni.2019.08.008
72
Auffray C Fogg D Garfa M Elain G Join-Lambert O Kayal S et al . Monitoring of Blood Vessels and Tissues by a Population of Monocytes With Patrolling Behavior. Science (2007) 317:666–70. doi: 10.1126/science.1142883
73
Boldison J da Rosa LC Davies J Wen L Wong FS . Dendritic Cells License Regulatory B Cells to Produce IL-10 and Mediate Suppression of Antigen-Specific CD8 T Cells. Cell Mol Immunol (2020) 17:843–55. doi: 10.1038/s41423-019-0324-z
74
Zhang Q Fujino M Iwasaki S Hirano H Cai S Kitajima Y et al . Generation and Characterization of Regulatory Dendritic Cells Derived From Murine Induced Pluripotent Stem Cells. Sci Rep (2015) 4:3979. doi: 10.1038/srep03979
75
Audiger C Rahman MJ Yun TJ Tarbell K Lesage S . The Importance of Dendritic Cells in Maintaining Immune Tolerance. J Immunol (2017) 198:2223–31. doi: 10.4049/jimmunol.1601629
76
del Prete A Sozio F Barbazza I Salvi V Tiberio L Laffranchi M et al . Functional Role of Dendritic Cell Subsets in Cancer Progression and Clinical Implications. Int J Mol Sci (2020) 21:1–24. doi: 10.3390/ijms21113930
77
Aibar S González-Blas CB Moerman T Huynh-Thu VA Imrichova H Hulselmans G et al . SCENIC: Single-Cell Regulatory Network Inference and Clustering. Nat Methods (2017) 14:7083–6. doi: 10.1038/nmeth.4463
78
Carenza C Calcaterra F Oriolo F di Vito C Ubezio M della Porta MG et al . Costimulatory Molecules and Immune Checkpoints Are Differentially Expressed on Different Subsets of Dendritic Cells. Front Immunol (2019) 10. doi: 10.3389/fimmu.2019.01325
79
Hernandez A Burger M Blomberg BB Ross WA Gaynor JJ Lindner I et al . Inhibition of NF-κb During Human Dendritic Cell Differentiation Generates Anergy and Regulatory T-Cell Activity for One But Not Two Human Leukocyte Antigen DR Mismatches. Hum Immunol (2007) 68:715–29. doi: 10.1016/j.humimm.2007.05.010
80
Medina BD Liu M Vitiello GA Seifert AM Zeng S Bowler T et al . Oncogenic Kinase Inhibition Limits Batf3-Dependent Dendritic Cell Development and Antitumor Immunity. J Exp Med (2019) 216:1359–76. doi: 10.1084/jem.20180660
81
Scholz F Grau M Menzel L Graband A Zapukhlyak M Leutz A et al . The Transcription Factor C/Ebpβ Orchestrates Dendritic Cell Maturation and Functionality Under Homeostatic and Malignant Conditions. Proc Natl Acad Sci (2020) 117:26328–39. doi: 10.1073/pnas.2008883117
82
Xiao X Yang G Bai P Gui S Nyuyen TMB Mercado-Uribe I et al . Inhibition of Nuclear Factor-Kappa B Enhances the Tumor Growth of Ovarian Cancer Cell Line Derived From a Low-Grade Papillary Serous Carcinoma in P53-Independent Pathway. BMC Cancer (2016) 16:1–13. doi: 10.1186/s12885-016-2617-2
83
Melaiu O Chierici M Lucarini V Jurman G Conti LA de Vito R et al . Cellular and Gene Signatures of Tumor-Infiltrating Dendritic Cells and Natural-Killer Cells Predict Prognosis of Neuroblastoma. Nat Commun (2020) 11:1–15. doi: 10.1038/s41467-020-19781-y
84
Brencicova E Jagger AL Evans HG Georgouli M Laios A Attard Montalto S et al . Interleukin-10 and Prostaglandin E2 Have Complementary But Distinct Suppressive Effects on Toll-Like Receptor-Mediated Dendritic Cell Activation in Ovarian Carcinoma. PloS One (2017) 12:1–24. doi: 10.1371/journal.pone.0175712
85
Imai K Minamiya Y Koyota S Ito M Saito H Sato Y et al . Inhibition of Dendritic Cell Migration by Transforming Growth Factor-β1 Increases Tumor-Draining Lymph Node Metastasis. J Exp Clin Cancer Res (2012) 31:1–9. doi: 10.1186/1756-9966-31-3
86
Llopiz D Ruiz M Infante S Villanueva L Silva L Hervas-Stubbs S et al . IL-10 Expression Defines an Immunosuppressive Dendritic Cell Population Induced by Antitumor Therapeutic Vaccination. Oncotarget (2017) 8:2659–71. doi: 10.18632/oncotarget.13736
87
Long J Hu Z Xue H Wang Y Chen J Tang F et al . Vascular Endothelial Growth Factor (VEGF) Impairs the Motility and Immune Function of Human Mature Dendritic Cells Through the VEGF Receptor 2-RhoA-Cofilin1 Pathway. Cancer Sci (2019) 110:2357–67. doi: 10.1111/cas.14091
88
Ohno Y Kitamura H Takahashi N Ohtake J Kaneumi S Sumida K et al . IL-6 Down-Regulates HLA Class II Expression and IL-12 Production of Human Dendritic Cells to Impair Activation of Antigen-Specific CD4+ T Cells. Cancer Immunol Immunother (2016) 65:193–204. doi: 10.1007/s00262-015-1791-4
89
Tauriello DVF Palomo-Ponce S Stork D Berenguer-Llergo A Badia-Ramentol J Iglesias M et al . Tgfβ Drives Immune Evasion in Genetically Reconstituted Colon Cancer Metastasis. Nature (2018) 554:538–43. doi: 10.1038/nature25492
90
Trabanelli S Lecciso M Salvestrini V Cavo M Očadlíková D Lemoli RM et al . PGE 2 -Induced IDO1 Inhibits the Capacity of Fully Mature DCs to Elicit an In Vitro Antileukemic Immune Response. J Immunol Res (2015) 2015:1–10. doi: 10.1155/2015/253191
91
Xu X Liu X Long J Hu Z Zheng Q Zhang C et al . Interleukin-10 Reorganizes the Cytoskeleton of Mature Dendritic Cells Leading to Their Impaired Biophysical Properties and Motilities. PloS One (2017) 12:1–15. doi: 10.1371/journal.pone.0172523
92
Ferrara N Carver-Moore K Chen H Dowd M Lu L O’Shea KS et al . Heterozygous Embryonic Lethality Induced by Targeted Inactivation of the VEGF Gene. Nature (1996) 380:439–42. doi: 10.1038/380439a0
93
Wakabayashi H Hamaguchi T Nagao N Kato S Iino T Nakamura T et al . Interleukin-6 Receptor Inhibitor Suppresses Bone Metastases in a Breast Cancer Cell Line. Breast Cancer (2018) 25:566–74. doi: 10.1007/s12282-018-0853-9
94
Yang J Yan J Liu B . Targeting VEGF/VEGFR to Modulate Antitumor Immunity. Front Immunol (2018) 9:978. doi: 10.3389/fimmu.2018.00978
95
Park S-J Nakagawa T Kitamura H Atsumi T Kamon H Sawa S et al . IL-6 Regulates In Vivo Dendritic Cell Differentiation Through STAT3 Activation. J Immunol (2004) 173:3844–54. doi: 10.4049/jimmunol.173.6.3844
96
Gabrilovich DI Ishida T Nadaf S Ohm JE Carbone DP . Antibodies to Vascular Endothelial Growth Factor Enhance the Efficacy of Cancer Immunotherapy by Improving Endogenous Dendritic Cell Function. Clin Cancer research : An Off J Am Assoc Cancer Res (1999) 5:2963–70.
97
Mashima T Wakatsuki T Kawata N Jang M-K Nagamori A Yoshida H et al . Neutralization of the Induced VEGF-A Potentiates the Therapeutic Effect of an Anti-VEGFR2 Antibody on Gastric Cancer In Vivo. Sci Rep (2021) 11:1–12. doi: 10.1038/s41598-021-94584-9
98
Bilusic M Heery CR Collins JM Donahue RN Palena C Madan RA et al . Phase I Trial of HuMax-IL8 (BMS-986253), an Anti-IL-8 Monoclonal Antibody, in Patients With Metastatic or Unresectable Solid Tumors. J ImmunoTher Cancer (2019) 7:1–8. doi: 10.1186/s40425-019-0706-x
99
Feijoó E Alfaro C Mazzolini G Serra P Peñuelas I Arina A et al . Dendritic Cells Delivered Inside Human Carcinomas Are Sequestered by Interleukin-8. Int J Cancer (2005) 116:275–81. doi: 10.1002/ijc.21046
100
Cauwels A van Lint S Paul F Garcin G de Koker S van Parys A et al . Delivering Type I Interferon to Dendritic Cells Empowers Tumor Eradication and Immune Combination Treatments. Cancer Res (2018) 78:463–74. doi: 10.1158/0008-5472.CAN-17-1980
101
Le DT Lutz E Uram JN Sugar EA Onners B Solt S et al . Evaluation of Ipilimumab in Combination With Allogeneic Pancreatic Tumor Cells Transfected With a GM-CSF Gene in Previously Treated Pancreatic Cancer. J Immunother (2013) 36:382–9. doi: 10.1097/CJI.0b013e31829fb7a2
102
Li Y Bleakley M Yee C . IL-21 Influences the Frequency, Phenotype, and Affinity of the Antigen-Specific CD8 T Cell Response. J Immunol (2005) 175:2261–9. doi: 10.4049/jimmunol.175.4.2261
103
Rhode PR Egan JO Xu W Hong H Webb GM Chen X et al . Comparison of the Superagonist Complex, ALT-803, to IL15 as Cancer Immunotherapeutics in Animal Models. Cancer Immunol Res (2016) 4:49–60. doi: 10.1158/2326-6066.CIR-15-0093-T
104
Barry KC Hsu J Broz ML Cueto FJ Binnewies M Combes AJ et al . A Natural Killer–Dendritic Cell Axis Defines Checkpoint Therapy–Responsive Tumor Microenvironments. Nat Med (2018) 24:1178–91. doi: 10.1038/s41591-018-0085-8
105
Bai W Zhang W Hu B . Vascular Endothelial Growth Factor Suppresses Dendritic Cells Function of Human Prostate Cancer. OncoTargets Ther (2018) 11:1267–74. doi: 10.2147/OTT.S161302
106
Cheng J Deng Y Yi H Wang G Fu B Chen W et al . Hepatic Carcinoma-Associated Fibroblasts Induce IDO-Producing Regulatory Dendritic Cells Through IL-6-Mediated STAT3 Activation. Oncogenesis (2016) 5:1–8. doi: 10.1038/oncsis.2016.7
107
Costa A Kieffer Y Scholer-Dahirel A Pelon F Bourachot B Cardon M et al . Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell (2018) 33:463–79. doi: 10.1016/j.ccell.2018.01.011
108
Elyada E Bolisetty M Laise P Flynn WF Courtois ET Burkhart RA et al . Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov (2019) 9:1102–23. doi: 10.1158/2159-8290.CD-19-0094
109
Friedman G Levi-Galibov O David E Bornstein C Giladi A Dadiani M et al . Cancer-Associated Fibroblast Compositions Change With Breast Cancer Progression Linking the Ratio of S100A4+ and PDPN+ CAFs to Clinical Outcome. Nat Cancer (2020) 1:692–708. doi: 10.1101/2020.01.12.903039
110
Rodda LB Lu E Bennett ML Sokol CL Wang X Luther SA et al . Single-Cell RNA Sequencing of Lymph Node Stromal Cells Reveals Niche-Associated Heterogeneity. Immunity (2018) 48:1014–28. doi: 10.1016/j.immuni.2018.04.006
111
Song Y-J Xu Y Deng C Zhu X Fu J Chen H et al . Gene Expression Classifier Reveals Prognostic Osteosarcoma Microenvironment Molecular Subtypes. Front Immunol (2021) 12:62376. doi: 10.3389/fimmu.2021.623762
112
Acton SE Farrugia AJ Astarita JL Mourão-Sá D Jenkins RP Nye E et al . Dendritic Cells Control Fibroblastic Reticular Network Tension and Lymph Node Expansion. Nature (2014) 514:498–502. doi: 10.1038/nature13814
113
Prados A Onder L Cheng H-W Mörbe U Lütge M Gil-Cruz C et al . Fibroblastic Reticular Cell Lineage Convergence in Peyer’s Patches Governs Intestinal Immunity. Nat Immunol (2021) 22:510–9. doi: 10.1038/s41590-021-00894-5
114
Tejchman A Lamerant-Fayel N Jacquinet J-C Bielawska-Pohl A Mleczko-Sanecka K Grillon C et al . Tumor Hypoxia Modulates Podoplanin/CCL21 Interactions in CCR7+ NK Cell Recruitment and CCR7+ Tumor Cell Mobilization. Oncotarget (2017) 8:31876–87. doi: 10.18632/oncotarget.16311
115
Hwang T-L Lee L-Y Wang C-C Liang Y Huang S-F Wu C-M . CCL7 and CCL21 Overexpression in Gastric Cancer Is Associated With Lymph Node Metastasis and Poor Prognosis. World J Gastroenterol (2012) 18:1249–56. doi: 10.3748/wjg.v18.i11.1249
116
Crola Da Silva C Lamerant-Fayel N Paprocka M Mitterrand M Gosset D Dus D et al . Selective Human Endothelial Cell Activation by Chemokines as a Guide to Cell Homing. Immunology (2009) 126:394–404. doi: 10.1111/j.1365-2567.2008.02906.x
117
Lin Y Sharma S John M . CCL21 Cancer Immunotherapy. Cancers (2014) 6:1098–110. doi: 10.3390/cancers6021098
118
Fang L Che Y Zhang C Huang J Lei Y Lu Z et al . PLAU Directs Conversion of Fibroblasts to Inflammatory Cancer-Associated Fibroblasts, Promoting Esophageal Squamous Cell Carcinoma Progression via uPAR/Akt/NF-κb/IL8 Pathway. Cell Death Discov (2021) 7:1–14. doi: 10.1038/s41420-021-00410-6
119
Öhlund D Handly-Santana A Biffi G Elyada E Almeida AS Ponz-Sarvise M et al . Distinct Populations of Inflammatory Fibroblasts and Myofibroblasts in Pancreatic Cancer. J Exp Med (2017) 214:579–96. doi: 10.1084/jem.20162024
120
Goc J Germain C Vo-Bourgais TKD Lupo A Klein C Knockaert S et al . Dendritic Cells in Tumor-Associated Tertiary Lymphoid Structures Signal a Th1 Cytotoxic Immune Contexture and License the Positive Prognostic Value of Infiltrating CD8+ T Cells. Cancer Res (2014) 74:705–15. doi: 10.1158/0008-5472.CAN-13-1342
121
Li Q Liu X Wang D Wang Y Lu H Wen S et al . Prognostic Value of Tertiary Lymphoid Structure and Tumour Infiltrating Lymphocytes in Oral Squamous Cell Carcinoma. Int J Oral Sci (2020) 12:1–8. doi: 10.1038/s41368-020-00092-3
122
Salem D Chelvanambi M Storkus WJ Fecek RJ . Cutaneous Melanoma: Mutational Status and Potential Links to Tertiary Lymphoid Structure Formation. Front Immunol (2021) 12:629519. doi: 10.3389/fimmu.2021.629519
123
Nayar S Campos J Smith CG Iannizzotto V Gardner DH Mourcin F et al . Immunofibroblasts Are Pivotal Drivers of Tertiary Lymphoid Structure Formation and Local Pathology. Proc Natl Acad Sci (2019) 116:13490–7. doi: 10.1073/pnas.1905301116
124
Rodriguez AB Peske JD Woods AN Leick KM Mauldin IS Meneveau MO et al . Immune Mechanisms Orchestrate Tertiary Lymphoid Structures in Tumors via Cancer-Associated Fibroblasts. Cell Rep (2021) 36:109422. doi: 10.1016/j.celrep.2021.109422
125
Dieu-Nosjean M-C Antoine M Danel C Heudes D Wislez M Poulot V et al . Long-Term Survival for Patients With Non–Small-Cell Lung Cancer With Intratumoral Lymphoid Structures. J Clin Oncol (2008) 26:4410–7. doi: 10.1200/JCO.2007.15.0284
126
Figenschau SL Fismen S Fenton KA Fenton C Mortensen ES . Tertiary Lymphoid Structures Are Associated With Higher Tumor Grade in Primary Operable Breast Cancer Patients. BMC Cancer (2015) 15:1–11. doi: 10.1186/s12885-015-1116-1
127
He W Zhang D Liu H Chen T Xie J Peng L et al . The High Level of Tertiary Lymphoid Structure Is Correlated With Superior Survival in Patients With Advanced Gastric Cancer. Front Oncol (2020) 10. doi: 10.3389/fonc.2020.00980
128
Jacquelot N Tellier J , Nutt SL Belz GT . Tertiary Lymphoid Structures and B Lymphocytes in Cancer Prognosis and Response to Immunotherapies. OncoImmunology (2021) 10(1):1900508. doi: 10.1080/2162402X.2021.1900508
129
Lin L Hu X Zhang H Hu H . Tertiary Lymphoid Organs in Cancer Immunology: Mechanisms and the New Strategy for Immunotherapy. Front Immunol (2019) 10:1. doi: 10.3389/fimmu.2019.00001
130
Barone F Nayar S Campos J Cloake T Withers DR Toellner K-M et al . IL-22 Regulates Lymphoid Chemokine Production and Assembly of Tertiary Lymphoid Organs. Proc Natl Acad Sci (2015) 112:11024–9. doi: 10.1073/pnas.1503315112
131
Fleige H Ravens S Moschovakis GL Bölter J Willenzon S Sutter G et al . IL-17–Induced CXCL12 Recruits B Cells and Induces Follicle Formation in BALT in the Absence of Differentiated FDCs. J Exp Med (2014) 211:643–51. doi: 10.1084/jem.20131737
132
Dieu-Nosjean M-C Goc J Giraldo NA Sautès-Fridman C Fridman WH . Tertiary Lymphoid Structures in Cancer and Beyond. Trends Immunol (2014) 35:571–780. doi: 10.1016/j.it.2014.09.006
133
Engelhard VH Rodriguez AB Mauldin IS Woods AN Peske JD Slingluff CL . Immune Cell Infiltration and Tertiary Lymphoid Structures as Determinants of Antitumor Immunity. J Immunol (2018) 200:432–42. doi: 10.4049/jimmunol.1701269
134
Sofopoulos M Fortis SP Vaxevanis CK Sotiriadou NN Arnogiannaki N Ardavanis A et al . The Prognostic Significance of Peritumoral Tertiary Lymphoid Structures in Breast Cancer. Cancer Immunol Immunother (2019) 68:1733–45. doi: 10.1007/s00262-019-02407-8
135
de Mingo Pulido Á. Gardner A Hiebler S Soliman H Rugo HS Krummel MF et al . TIM-3 Regulates CD103+ Dendritic Cell Function and Response to Chemotherapy in Breast Cancer. Cancer Cell (2018) 33:60–74. doi: 10.1016/j.ccell.2017.11.019
136
Dixon KO Tabaka M Schramm MA Xiao S Tang R Dionne D et al . TIM-3 Restrains Anti-Tumour Immunity by Regulating Inflammasome Activation. Nature (2021) 595:101–6. doi: 10.1038/s41586-021-03626-9
137
Karyampudi L Lamichhane P Krempski J Kalli KR Behrens MD Vargas DM et al . PD-1 Blunts the Function of Ovarian Tumor–Infiltrating Dendritic Cells by Inactivating NF-κb. Cancer Res (2016) 76:239–50. doi: 10.1158/0008-5472.CAN-15-0748
138
Oh SA Wu D-C Cheung J Navarro A Xiong H Cubas R et al . PD-L1 Expression by Dendritic Cells Is a Key Regulator of T-Cell Immunity in Cancer. Nat Cancer (2020) 1:681–91. doi: 10.1038/s43018-020-0075-x
139
Ouaaz F Arron J Zheng Y Choi Y Beg AA . Dendritic Cell Development and Survival Require Distinct NF-κb Subunits. Immunity (2002) 16:257–70. doi: 10.1016/s1074-7613(02)00272-8
140
Peng Q Qiu X Zhang Z Zhang S Zhang Y Liang Y et al . PD-L1 on Dendritic Cells Attenuates T Cell Activation and Regulates Response to Immune Checkpoint Blockade. Nat Commun (2020) 11:1–8. doi: 10.1038/s41467-020-18570-x
141
Wang XB Fan ZZ Anton D Vollenhoven A Ni ZH Chen XF et al . CTLA4 Is Expressed on Mature Dendritic Cells Derived From Human Monocytes and Influences Their Maturation and Antigen Presentation. BMC Immunol (2011) 12:1–8. doi: 10.1186/1471-2172-12-1
142
Ruffell B Chang-Strachan D Chan V Rosenbusch A Ho CMT Pryer N et al . Macrophage IL-10 Blocks CD8+ T Cell-Dependent Responses to Chemotherapy by Suppressing IL-12 Expression in Intratumoral Dendritic Cells. Cancer Cell (2014) 26:623–37. doi: 10.1016/j.ccell.2014.09.006
143
Sánchez-Paulete AR Cueto FJ Martínez-López M Labiano S Morales-Kastresana A Rodríguez-Ruiz ME et al . Cancer Immunotherapy With Immunomodulatory Anti-CD137 and Anti–PD-1 Monoclonal Antibodies Requires BATF3-Dependent Dendritic Cells. Cancer Discov (2016) 6:71–9. doi: 10.1158/1538-7445.AM2016-4908
144
Bonavita E Bromley CP Jonsson G Pelly VS Sahoo S Walwyn-Brown K et al . Antagonistic Inflammatory Phenotypes Dictate Tumor Fate and Response to Immune Checkpoint Blockade. Immunity (2020) 53:1215–29. doi: 10.1016/j.immuni.2020.10.020
145
Grivennikov SI Wang K Mucida D Stewart CA Schnabl B Jauch D et al . Adenoma-Linked Barrier Defects and Microbial Products Drive IL-23/IL-17-Mediated Tumour Growth. Nature (2012) 491:254–8. doi: 10.1038/nature11465
146
Hu Z Yang Y Zhao Y Huang Y . The Prognostic Value of Cyclooxygenase-2 Expression in Patients With Esophageal Cancer: Evidence From a Meta-Analysis. OncoTargets Ther (2017) 10:2893–901. doi: 10.2147/OTT.S134599
147
Shalapour S Karin M . Pas De Deux: Control of Anti-Tumor Immunity by Cancer-Associated Inflammation. Immunity (2019) 51:15–26. doi: 10.1016/j.immuni.2019.06.021
148
Grasso CS Tsoi J Onyshchenko M Abril-Rodriguez G Ross-Macdonald P Wind-Rotolo M et al . Conserved Interferon-γ Signaling Drives Clinical Response to Immune Checkpoint Blockade Therapy in Melanoma. Cancer Cell (2020) 38:500–15. doi: 10.1016/j.ccell.2020.08.005
149
Pelly VS Moeini A Roelofsen LM Bonavita E Bell CR Hutton C et al . Anti-Inflammatory Drugs Remodel the Tumor Immune Environment to Enhance Immune Checkpoint Blockade Efficacy. Cancer Discov (2021) 11(10):2602–19. doi: 10.1158/2159-8290.CD-20-1815
150
Zelenay S Reis e Sousa C . Reducing Prostaglandin E 2 Production to Raise Cancer Immunogenicity. OncoImmunology (2016) 5:1–3. doi: 10.1080/2162402X.2015.1123370
151
Zhang Y Tighe S Zhu Y-T . COX-2 Signaling in the Tumor Microenvironment. Adv Exp Med Biol (2020) 1277:87–104. doi: 10.1007/978-3-030-50224-9_6
152
Böttcher JP Bonavita E Chakravarty P Blees H Cabeza-Cabrerizo M Sammicheli S et al . NK Cells Stimulate Recruitment of Cdc1 Into the Tumor Microenvironment Promoting Cancer Immune Control. Cell (2018) 172:1022–37. doi: 10.1016/j.cell.2018.01.004
153
Wang D DuBois RN . The Role of COX-2 in Intestinal Inflammation and Colorectal Cancer. Oncogene (2010) 29:781–8. doi: 10.1038/onc.2009.421
154
Zhu Y Shi C Zeng L Liu G Jiang W Zhang X et al . High COX-2 Expression in Cancer-Associated Fibiroblasts Contributes to Poor Survival and Promotes Migration and Invasiveness in Nasopharyngeal Carcinoma. Mol Carcinogenesis (2020) 59:265–80. doi: 10.1002/mc.23150
155
Obermajer N Muthuswamy R Odunsi K Edwards RP Kalinski P . PGE 2 -Induced CXCL12 Production and CXCR4 Expression Controls the Accumulation of Human MDSCs in Ovarian Cancer Environment. Cancer Res (2011) 71:7463–70. doi: 10.1158/0008-5472.CAN-11-2449
156
Baratelli FE Heuzé-Vourc’h N Krysan K Dohadwala M Riedl K Sharma S et al . Prostaglandin E 2 -Dependent Enhancement of Tissue Inhibitors of Metalloproteinases-1 Production Limits Dendritic Cell Migration Through Extracellular Matrix. J Immunol (2004) 173:5458–66. doi: 10.4049/jimmunol.173.9.5458
157
Legler DF Krause P Scandella E Singer E Groettrup M . Prostaglandin E2 Is Generally Required for Human Dendritic Cell Migration and Exerts Its Effect via EP2 and EP4 Receptors. J Immunol (2006) 176:966–73. doi: 10.4049/jimmunol.176.2.966
158
Yen J-H Khayrullina T Ganea D . PGE2-Induced Metalloproteinase-9 is Essential for Dendritic Cell Migration. Blood (2008) 111:260–70. doi: 10.1182/blood-2007-05-090613
159
Canton J Blees H Henry CM Buck MD Schulz O Rogers NC et al . The Receptor DNGR-1 Signals for Phagosomal Rupture to Promote Cross-Presentation of Dead-Cell-Associated Antigens. Nat Immunol (2021) 22:140–53. doi: 10.1038/s41590-020-00824-x
160
Giampazolias E Schulz O Lim KHJ Rogers NC Chakravarty P Srinivasan N et al . Secreted Gelsolin Inhibits DNGR-1-Dependent Cross-Presentation and Cancer Immunity. Cell (2021) 184:4016–31. doi: 10.1016/j.cell.2021.05.021
161
Sancho D Joffre OP Keller AM Rogers NC Martínez D Hernanz-Falcón P et al . Identification of a Dendritic Cell Receptor That Couples Sensing of Necrosis to Immunity. Nature (2009) 458:899–903. doi: 10.1038/nature07750
162
Hsu FJ Benike C Fagnoni F Liles TM Czerwinski D Taidi B et al . Vaccination of Patients With B–cell Lymphoma Using Autologous Antigen–Pulsed Dendritic Cells. Nat Med (1996) 2:52–8. doi: 10.1038/nm0196-52
163
León B López-Bravo M Ardavín C . Monocyte-Derived Dendritic Cells Formed at the Infection Site Control the Induction of Protective T Helper 1 Responses Against Leishmania. Immunity (2007) 26:519–31. 10.1016/j.immuni.2007.01.017
164
Palucka AK Ueno H Connolly J Kerneis-Norvell F Blanck J-P Johnston DA et al . Dendritic Cells Loaded With Killed Allogeneic Melanoma Cells can Induce Objective Clinical Responses and MART-1 Specific CD8+ T-Cell Immunity. J Immunother (2006) 29:545–57. doi: 10.1097/01.cji.0000211309.90621.8b
165
Dranoff G Jaffee E Lazenby A Golumbek P Levitsky H Brose K et al . Vaccination With Irradiated Tumor Cells Engineered to Secrete Murine Granulocyte-Macrophage Colony-Stimulating Factor Stimulates Potent, Specific, and Long-Lasting Anti-Tumor Immunity. Proc Natl Acad Sci (1993) 90:3539–43. doi: 10.1073/pnas.90.8.3539
166
Mach N Dranoff G . Cytokine-Secreting Tumor Cell Vaccines. Curr Opin Immunol (2000) 12:571–5. doi: 10.1016/S0952-7915(00)00144-8
167
Bhardwaj N Friedlander PA Pavlick AC Ernstoff MS Gastman BR Hanks BA et al . Flt3 Ligand Augments Immune Responses to Anti-DEC-205-NY-ESO-1 Vaccine Through Expansion of Dendritic Cell Subsets. Nat Cancer (2020) 1:1204–17. doi: 10.1038/s43018-020-00143-y
168
Hammerich L Marron TU Upadhyay R Svensson-Arvelund J Dhainaut M Hussein S et al . Systemic Clinical Tumor Regressions and Potentiation of PD1 Blockade With in Situ Vaccination. Nat Med (2019) 25:814–24. doi: 10.1038/s41591-019-0410-x
169
Lai J Mardiana S House IG Sek K Henderson MA Giuffrida L et al . Adoptive Cellular Therapy With T Cells Expressing the Dendritic Cell Growth Factor Flt3L Drives Epitope Spreading and Antitumor Immunity. Nat Immunol (2020) 21:914–26. doi: 10.1038/s41590-020-0676-7
170
Salmon H Idoyaga J Rahman A Leboeuf M Remark R Jordan S et al . Expansion and Activation of CD103 + Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity (2016) 44:924–38. doi: 10.1016/j.immuni.2016.03.012
171
Hou J Karin M Sun B . Targeting Cancer-Promoting Inflammation — Have Anti-Inflammatory Therapies Come of Age? Nat Rev Clin Oncol (2021) 18:261–79. doi: 10.1038/s41571-020-00459-9
172
Ritter B Greten FR . Modulating Inflammation for Cancer Therapy. J Exp Med (2019) 216. doi: 10.1084/jem.20181739
173
Zappavigna S Cossu AM Grimaldi A Bocchetti M Ferraro GA Nicoletti GF et al . Anti-Inflammatory Drugs as Anticancer Agents. Int J Mol Sci (2020) 21:1–29. doi: 10.3390/ijms21072605
174
Chen X Song E . Turning Foes to Friends: Targeting Cancer-Associated Fibroblasts. Nat Rev Drug Discov (2019) 18:99–115. doi: 10.1038/s41573-018-0004-1
175
Kobayashi H Enomoto A Woods SL Burt AD Takahashi M Worthley DL . Cancer-Associated Fibroblasts in Gastrointestinal Cancer. Nat Rev Gastroenterol Hepatol (2019) 16:282–95. doi: 10.1038/s41575-019-0115-0
176
Liu T Han C Wang S Fang P Ma Z Xu L et al . Cancer-Associated Fibroblasts: An Emerging Target of Anti-Cancer Immunotherapy. J Hematol Oncol (2019). doi: 10.1186/s13045-019-0770-1
177
Ziani L Chouaib S Thiery J . Alteration of the Antitumor Immune Response by Cancer-Associated Fibroblasts. Front Immunol (2018) 9:414. doi: 10.3389/fimmu.2018.00414
178
Wang J-B Huang X Li F-R . Impaired Dendritic Cell Functions in Lung Cancer: A Review of Recent Advances and Future Perspectives. Cancer Commun (2019) 39(1):43. doi: 10.1186/s40880-019-0387-3
179
Wculek SK Cueto FJ Mujal AM Melero I Krummel MF Sancho D . Dendritic Cells in Cancer Immunology and Immunotherapy. Nat Rev Immunol (2020) 20(1):7–24. doi: 10.1038/s41577-019-0210-z
180
Martín-Fontecha A Sebastiani S Höpken UE Uguccioni M Lipp M Lanzavecchia A et al . Regulation of Dendritic Cell Migration to the Draining Lymph Node. J Exp Med (2003) 198:615–21. doi: 10.1084/jem.20030448
181
Perez CR de Palma M . Engineering Dendritic Cell Vaccines to Improve Cancer Immunotherapy. Nat Commun (2019) 10:1–10. doi: 10.1038/s41467-019-13368-y
182
Ramanjulu JM Pesiridis GS Yang J Concha N Singhaus R Zhang S-Y et al . Design of Amidobenzimidazole STING Receptor Agonists With Systemic Activity. Nature (2018) 564:439–43. doi: 10.1038/s41586-018-0705-y
183
Ganesan A-P Clarke J Wood O Garrido-Martin EM Chee SJ Mellows T et al . Tissue-Resident Memory Features Are Linked to the Magnitude of Cytotoxic T Cell Responses in Human Lung Cancer. Nat Immunol (2017) 18:940–50. doi: 10.1038/ni.3775
184
Malik BT Byrne KT Vella JL Zhang P Shabaneh TB Steinberg SM et al . Resident Memory T Cells in the Skin Mediate Durable Immunity to Melanoma. Sci Immunol (2017) 2.:1–24 doi: 10.1126/sciimmunol.aam6346
185
Park SL Buzzai A Rautela J Hor JL Hochheiser K Effern M et al . Tissue-Resident Memory CD8+ T Cells Promote Melanoma–Immune Equilibrium in Skin. Nature (2019) 565:366–71. doi: 10.1038/s41586-018-0812-9
186
Savas P Virassamy B Ye C Salim A Mintoff CP Caramia F et al . Single-Cell Profiling of Breast Cancer T Cells Reveals a Tissue-Resident Memory Subset Associated With Improved Prognosis. Nat Med (2018) 24:986–93. doi: 10.1038/s41591-018-0078-7
187
Schenkel JM Fraser KA Beura LK Pauken KE Vezys V Masopust D . Resident Memory CD8 T Cells Trigger Protective Innate and Adaptive Immune Responses. Science (2014) 346:98–101. doi: 10.1126/science.1254536
188
Edwards J Wilmott JS Madore J Gide TN Quek C Tasker A et al . CD103 + Tumor-Resident CD8 + T Cells Are Associated With Improved Survival in Immunotherapy-Naïve Melanoma Patients and Expand Significantly During Anti–PD-1 Treatment. Clin Cancer Res (2018) 24:3036–45. doi: 10.1158/1078-0432.CCR-17-2257
189
León-Letelier RA Castro-Medina DI Badillo-Godinez O Tepale-Segura A Huanosta-Murillo E Aguilar-Flores C et al . Induction of Progenitor Exhausted Tissue-Resident Memory CD8+ T Cells Upon Salmonella Typhi Porins Adjuvant Immunization Correlates With Melanoma Control and Anti-PD-1 Immunotherapy Cooperation. Front Immunol (2020) 11:583382. doi: 10.3389/fimmu.2020.583382
190
Sautès-Fridman C Petitprez F Calderaro J Fridman WH . Tertiary Lymphoid Structures in the Era of Cancer Immunotherapy. Nat Rev Cancer (2019) 19:1–21. doi: 10.1038/s41568-019-0144-6
Summary
Keywords
tumour microenvironment (TME), inflammatory cytokines, dendritic cells, anti-tumour immunity, draining lymph nodes, Tertiary Lymphoid Structures (TLS), immune infiltration
Citation
Gupta YH, Khanom A and Acton SE (2022) Control of Dendritic Cell Function Within the Tumour Microenvironment. Front. Immunol. 13:733800. doi: 10.3389/fimmu.2022.733800
Received
30 June 2021
Accepted
09 February 2022
Published
10 March 2022
Volume
13 - 2022
Edited by
Jörg Renkawitz, Ludwig Maximilian University of Munich, Germany
Reviewed by
Christine Moussion, Genentech, Inc., United States; Catherine Hedrick, La Jolla Institute for Immunology (LJI), United States; Toby Lawrence, King’s College London, United Kingdom
Updates
Copyright
© 2022 Gupta, Khanom and Acton.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yukti Hari Gupta, y.gupta@ucl.ac.uk; Sophie E. Acton, s.acton@ucl.ac.uk
This article was submitted to Molecular Innate Immunity, a section of the journal Frontiers in Immunology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.