Abstract
Objective:
To explore the efficacy and safety of Iguratimod intervention in Primary Sjogren’s syndrome (pSS).
Methods:
Many databases were searched to collect the RCTs. Three independent reviewers extracted data and assessed the quality of the studies based on the Cochrane Handbook. The statistical analysis was done by RevMan 5.3 and STATA. The quality of evidence was evaluated by GRADE tool.
Results:
Twenty-nine RCTs with 2258 participants were included in this review. The meta-analysis shows that: iguratimod experiment group can reduce the ESSPRI score (WMD -1.93 [-2.33, -1.52], P<0.00001), ESSDAI score (WMD -1.39 [-1.81, -0.98], P<0.00001), Schirmer’s test (WMD 1.77 [0.85, 2.70], P=0.0002), RF (WMD -5.78 [-7.59, -3.97], P<0.00001), and decrease the ESR level (WMD -7.05 [-9.84, -4.26], P<0.00001). Meanwhile, the summary result showed the addiction of Iguratimod may not increase the adverse events. The adverse events were mainly gastrointestinal discomfort, abnormal liver function, and rash and itching. The quality of evidence of adverse events was moderate. Referring to minimal clinically important difference (MCID), the improvement of ESSPRI is clinically significant, and the improvement of ESSDAI for patients older than 60 years old may be clinically significant.
Conclusion:
Based on current evidence, iguratimod can effectively reduce ESSPRI score, ESSDAI score, Schirmer’s test score and decrease systemic inflammatory response (such as ESR level and RF level) without increasing the probability of adverse events. The recommended course of treatment is at least 12 weeks.
Systematic Review Registration:
https://www.crd.york.ac.uk/prospero/display_record.php?, identifier CRD42020220770.
1 Introduction
Primary Sjogren’s syndrome (pSS) is a chronic inflammatory autoimmune disease that mainly involves exocrine glands (especially salivary glands and lacrimal glands), and is accompanied by chronic autoimmune diseases with visceral involvement [(1); Thorne and Sutcliffe 2005 (2)]. Its clinical manifestations are mainly dry eyes (keratoconjunctivitis sicca) and dry mouth (xerostomia) (2). The pathogenesis of Sjogren’s syndrome is still unclear. Pathology and serology, as well as the (albeit weak) association with HLA alleles, have shown that viral infection of the salivary glands leads to local cell death and the release of tissue autoantigens leading to the activation of autoreactive T cells and B cells. This ultimately leads to lymphocytic infiltration of the glands (lacrimal and salivary glands) (3–5). According to the patients with different levels of symptoms, the current treatment and management of primary Sjogren’s syndrome mainly include non-steroidal anti-inflammatory drugs, glucocorticoids, immunosuppressants, and biological inhibitors. When multiple organs are involved, a combination of the above drugs is often required (6–8), but the current treatment effect is still not good. Although the current comprehensive management strategies for pSS are entering the stage of biological targeted therapy, their efficacy in pSS is still imprecise. To date, two large randomized controlled trials (RCTs) have been conducted to evaluate the efficacy of rituximab in pSS (9, 10). The Devauchelle-Pensec et al. study included 120 patients to evaluate the efficacy of a single course of rituximab. However, the study did not meet the primary endpoint: at 24 weeks, the visual analogue scale (VAS, 0-100mm) did not decrease by 30mm in any two of the four measures (dryness, pain, fatigue and global assessment of disease activity). In addition, open-label studies of abatacept in pSS did not meet the primary efficacy outcome of improvement in ESSDAI score from baseline to week 24 (11, 12). At present, there is still no definite therapeutic drug for the clinical treatment of PSS. Iguratimod has shown an effect in the treatment of pSS, but different results have been reported in RCTs. Iguratimod, as a new anti-rheumatic drug (DMARDs) to improve the condition, is mainly used in rheumatoid arthritis in China and Japan (13–15). Iguratimod has a comprehensive immunomodulatory effect whether in cellular immunity (T cell) (16) or humoral immunity (B cell) (17). In addition, Iguratimod also has a powerful anti-inflammatory effect, such as TNF-α and interleukin factors (IL-6, IL-8, IL-17) and other inflammatory factors (18, 19).
Given that T cells and B cells play an important role in the pathogenesis of pSS (8), Iguratimod is a promising treatment for pSS. The current study also shows that Iguratimod can improve the EULAR Sjogren’s Syndrome Activity Index (ESSDAI) score and EULAR Sjogren’s Syndrome Reporting Patient Index (ESSPRI) score, and reduce the patient’s immune globulin, ESR, rheumatoid factor (RF) and B cell percentage (20). At present, some RCTs of Iguratimod in the treatment of pSS have been carried out clinically. A previous systematic review and meta-analysis evaluated the efficacy and safety of Iguratimod in the treatment of PSS, but the included RCTs were not enough and the quality was not high, and they did not include RCTs related to complications of PSS. Therefore, the conclusion may have limitations (21). Since Iguratimod was approved as a clinical recommended drug in China, a large number of RCTs have emerged, which may correct the results. Therefore, this study would conduct a systematic review and meta-analysis of Iguratimod combined with other therapies in the treatment of pSS to assess the efficacy and safety of Iguratimod in patients with pSS compared with other DMARDs.
2 Materials and Methods
2.1 Protocol
This systematic review and meta-analysis were conducted strictly in accordance with the protocol registered in PROSPERO (CRD42020220770) and PRISMA-guidelines (see Supplementary Materials).
2.2 Literature Search Strategy
The Chinese Science and Technology Periodical Database (VIP), Web of Science, EMBASE, Medline Complete, Pubmed, ClinicalTrials, Chinese Biomedical Database (CBM), Wan Fang Database, the China National Knowledge Infrastructure Databases (CNKI), Cochrane Library were searched to collect clinical studies on the treatment of Sjogren’s syndrome by Iguratimod. The retrieval time is up to 1st April 2022. The search formula of Pubmed and EMBASE is shown in Table S1 as an example. When searching the database, the constructed search formula is input to obtain search results.
2.3 Selection Criteria
2.3.1 Participants
Patients who have been clinically diagnosed as primary Sjogren’s syndrome, without limitation to gender, age, course of disease, etc.
2.3.2 Intervention and Control
The trial group received the treatment of iguratimod or combined with iguratimod on the basis of the control group. The control group received placebo treatment or conventional therapy.
2.3.3 Outcomes
(1) Primary outcome is Disease activity score (ESSPRI and ESSDAI). (2) Secondary outcomes are Schirmer’s test and Inflammation related indicators [ESR, CRP, rheumatoid factor (RF)]. (3) Adverse events. The minimal clinically important difference (MCID) of ESSDAI is decrease≥3 points, the MCID of ESSPRI is decrease≥1 point, and the MCID of Schirmer’s test is increase≥ 5mm (22).
2.3.4 Study Design
Randomized controlled trial, with no limitations to publication time, language, quality and publication status.
2.3.5 Exclusion Criteria
(1) Adolescents (under 18 years of age); (2) the participant is not human; (3) Non-original research literature. (4) Studies with only one author will be excluded, as RCTs often require multi-investigator collaboration.
2.4 Literature Screening, Data Extraction and Quality Assessment
Two researchers independently screened the literature, extracted data and cross-checked. If there is a disagreement, the decision will be made through discussion and negotiation by all three reviewers. When selecting documents, the title and abstract are read first. After excluding irrelevant documents, the remaining documents will be further read through the full text to determine whether to include them. The data extraction content mainly includes: (1) Basic information of the included research: research title, first author, year of publication, etc.; (2) Baseline characteristics of the research object; (3) Important information such as outcome indicators. Two researchers independently used the risk of bias assessment tool based on the Cochrane Handbook to assess the methodological quality of the study (23), and when they disagree, the results were determined through discussion and negotiation by all three reviewers. The risk of bias assessment tool includes: Selection bias (random sequence generation and allocation concealment), Performance bias, detection bias, reporting bias, other bias.
2.5 Statistical Analysis
Review Manager 5.3 software was used for statistical analysis. If the statistical variable is a continuous variable, mean difference (MD) and 95% confidence interval (CI) are used as effect indicators. If it is a dichotomous variable, relative risk (RR) and 95% CI are used as effect indicators. Heterogeneity among studies was assessed using Cochrane’s Q and I2 statistic (24). The fixed effect model would be used when P>0.1, I2<50%. We would explore the reasons for heterogeneity, perform the subgroup analysis or use the random effect model when P<0.1, I2>50%. The publication bias was detected by STATA 15 with Egger method (continuous variable) and Harbord methods (dichotomous variable) for primary outcomes. P>0.1 is considered to have no publication bias.
2.6 Sensitivity Analysis
Sensitivity analyses were conducted by STATA 15 for all outcomes if they meet the following criteria:
-
1. the random effects model was utilized;
-
2. the results of the fixed effect model are inconsistent with that of the random effect model.
3 Results
3.1 Results of the Search and Description of Included Trials
The total records identified through database searching and other sources were 210; 44 in CNKI, 51 in Wanfang, 42 in VIP, 36 in CBM, 6 in Pubmed, 4 in the Cochrane library, 3 in ClinicalTrials.gov, 4 in Embase, 8 in Medline, 12 in web of science. One hundred and seventy-nine (179) were excluded based on the title and abstract and 31 for more detailed evaluation. Two (2) of 31 records were excluded because they were not RCTs (20, 25), and 10 records were excluded because they only include one author (26–35) (Figure 1).
Figure 1
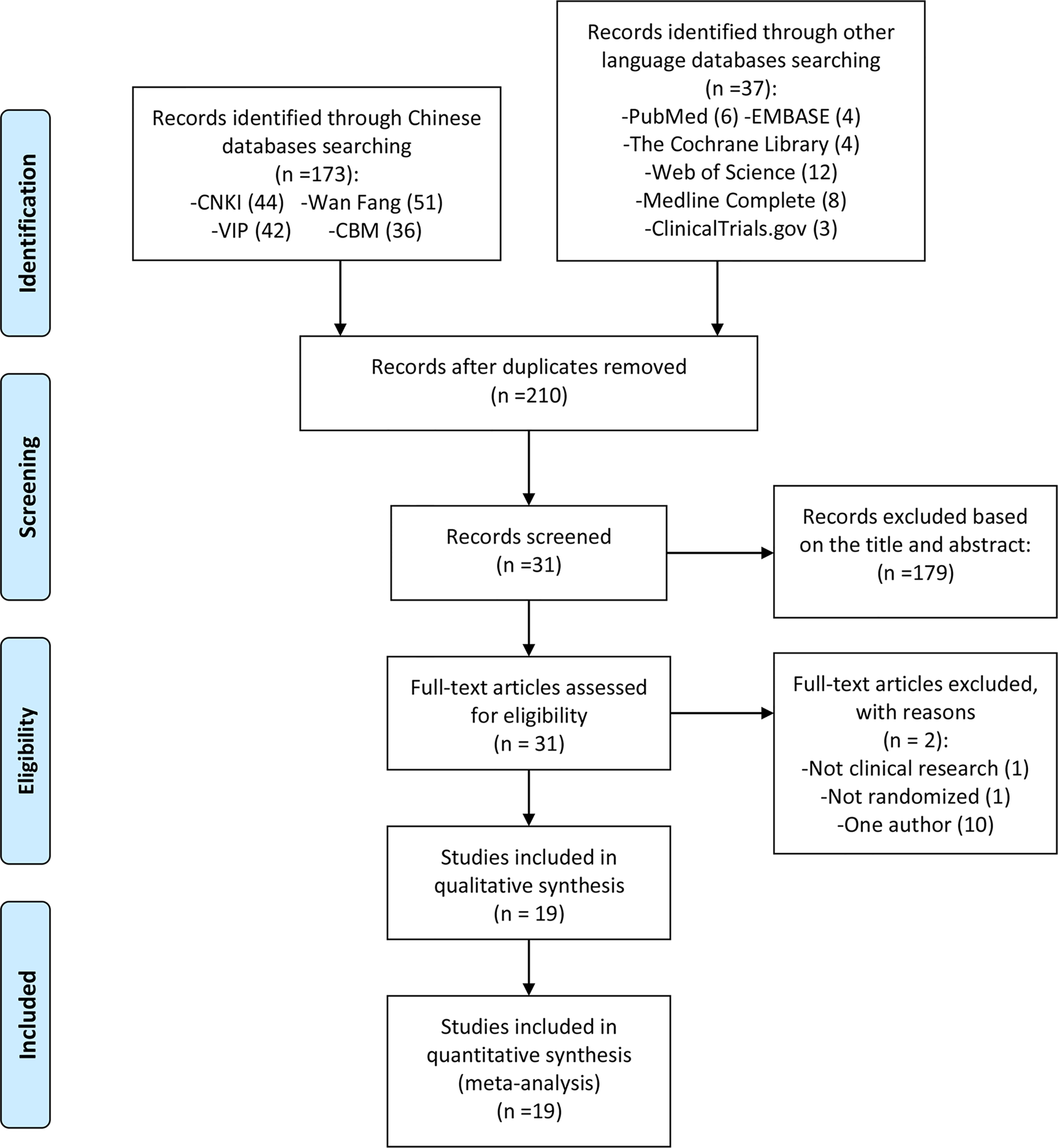
Flow diagram.
All RCTs comes from China. The scale of RCT is mostly between 40-80 participants. Only the control group of Shao et al. 2020 (36) used Placebo, Wang et al., 2019 (37), Xie et al. 2020 (38), Chen et al. 2022 (39) used Total Glucosides of Paeony + HCQ therapy, and the control group of the remaining studies used Conventional treatment. The ages involved in all RCTs are mostly between 25-70 years old, and the course of the disease is mostly within 30 years. The details of study characteristics are presented in Table 1.
Table 1
| Study | Country | Sample size | Intervention | Relevant outcomes | Mean age (years) | Course of disease (years) | ESSPRI | Duration | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial group | Control group | Trial group | Control group | Trial group | Control group | Trial group | Control group | Trial group | Control group | ||||
| Jiang et al. 2020 (40) | China | 25 | 25 | Conventional treatment + Iguratimod 50 mg Q.d. | Conventional treatment [10 mg prednisone, 400 mg hydroxychloroquine (HCQ), new hydrochloride bromine ethyl Q.d.] | EULAR Sjögren’s syndrome patient-reported index (ESSPRI), ESSDAI, Schirmer’s test, Adverse events | 29.3 ± 9.7 | 32.5 ± 11.5 | 7.5 ± 4.8 | 7.1 ± 6.6 | 7.36 ± 0.16 | 7.08 ± 0.15 | 12 weeks |
| Shao et al. 2020 (36) | China | 44 | 22 | Iguratimod 25mg B.i.d | Placebo | ESSPRI, ESR, ESSDAI, Adverse events | 49.5 ± 12.3 | 48.2 ± 11.5 | 12.8 ± 14.1 | 10.3 ± 15.2 | 4.5 ± 2.7 | 4.2 ± 2.4 | 24 weeks |
| Jiang et al. 2016 (41) | China | 30 | 30 | Conventional treatment + Iguratimod 50 mg Q.d. | Conventional treatment [8 mg prednisone Q.d. +200 mg HCQ B.i.d] | Rheumatoid factor (RF), ESR, Adverse events | 45.13 ± 12.11 | 46.33 ± 13.74 | 6.1 ± 2.3 | 4.9 ± 2.7 | – | – | 12 weeks |
| Wang et al. 2019 (37) | China | 32 | 32 | Iguratimod 25 mg B.i.d + Total Glucosides of Paeony 0.6g B.i.d. + HCQ 0.1g B.i.d | Total Glucosides of Paeony 0.6g B.i.d. + HCQ 0.1g B.i.d | ESSPRI, ESSDAI, Schirmer’s test, ESR, RF, Adverse events | 66.8 ± 7.7 | 65.3 ± 8.2 | 0.5-11 | 0.7-10 | 6.6 ± 1.5 | 7.2 ± 1.4 | 12 weeks |
| Jiang et al. 2014 (42) | China | 25 | 25 | Conventional treatment + Iguratimod 25 mg B.i.d. | Conventional treatment [5-10 mg prednisone Q.d. +200 mg HCQ B.i.d+Bromoethylsine 16mg T.i.d] | ESSPRI, ESSDAI, Schirmer’s test, Adverse events | 29.3 ± 9.7 | 32.5 ± 11.5 | 8-32 | 10-36 | 6.3 ± 1.5 | 7.1 ± 1.5 | 12 weeks |
| Bai et al. 2019(43) | China | 30 | 30 | Conventional treatment + Iguratimod 25 mg B.i.d. | Conventional treatment [8 mg methylprednisolone Q.d. +200 mg HCQ B.i.d]+ Leflunomide 50mg Q.d. | ESSPRI, ESSDAI, RF, ESR, Adverse events | 43 ± 21 | 43 ± 10 | 6.1 ± 2.3 | 5.75 ± 2.92 | 6.4 ± 1.4 | 7.2 ± 1.4 | 12 weeks |
| Li et al. 2018(44) | China | 34 | 34 | Conventional treatment + Iguratimod 25 mg B.i.d. | Conventional treatment [8 mg prednisone Q.d. +200 mg HCQ B.i.d] | ESSPRI, RF, ESR, Adverse events | 40.05 ± 3.16 | 40.02 ± 3.15 | 3.42 ± 0.26 | 3.15 ± 0.26 | 7.33 ± 1.01 | 7.24 ± 1.02 | 12 weeks |
| Li et al. 2020(45) | China | 23 | 23 | Conventional treatment + Iguratimod 25 mg B.i.d. | Conventional treatment [8 mg prednisone Q.d. +200 mg HCQ B.i.d] | ESSPRI, ESR, Adverse events | 46.29 ± 1.24 | 46.38 ± 1.37 | – | – | 7.23 ± 1.01 | 7.34 ± 1.02 | 12 weeks |
| Xie et al. 2020(38) | China | 38 | 38 | Iguratimod 25 mg B.i.d+Total Glucosides of Paeony 0.6g T.i.d. + HCQ 0.2g B.i.d | Total Glucosides of Paeony 0.6g T.i.d. + HCQ 0.2g B.i.d | ESR, CRP, Schirmer’s test, Adverse events | 57.3 ± 7.92 | 56.8 ± 8.44 | 8.8 ± 5.82 | 9.5 ± 4.86 | – | – | 24 weeks |
| Xia et al. 2017(46) | China | 50 | 50 | Iguratimod 25 mg B.i.d. + Methylprednisolone | HCQ 200mg B.i.d. + Methylprednisolone | ESR, RF | 42. 13 ± 9.97 | 42.08 ± 9.65 | – | – | – | – | 12 weeks |
| Xu et al. 2017(47) | China | 47 | 47 | Iguratimod 25 mg B.i.d. + Conventional treatment | Conventional treatment [8 mg methylprednisolone Q.d. +200 mg HCQ B.i.d] | ESSPRI, ESSDAI, ESR, RF, Schirmer’s test | 44.5 ± 13.2 | 45.3 ± 13.1 | 6.12 ± 1.82 | 5.96 ± 1.73 | 6.8 ± 1.7 | 7.3 ± 1.2 | 12 weeks |
| Luo et al. 2018(48) | China | 40 | 40 | Iguratimod 25 mg B.i.d. + Conventional treatment | Conventional treatment [8 mg methylprednisolone Q.d. +200 mg HCQ B.i.d] | ESR, RF, adverse events | 43.6 ± 10.5 | 45.2 ± 12.9 | 0.5-8 | – | – | 12 weeks | |
| Zhang and Shen(49) | China | 43 | 43 | Iguratimod 25 mg B.i.d. + Methylprednisolone 8 mg | 8 mg methylprednisolone Q.d. +200 mg HCQ B.i.d | ESSPRI, ESSDAI, ESR, RF, Schirmer’s test, adverse events | 40.35 ± 9.41 | 41.03 ± 10.01 | 2.31 ± 0.61 | 2.20 ± 0.52 | 7.28 ± 1.36 | 6.98 ± 1.27 | 12 weeks |
| Zhang et al. 2019(50) | China | 100 | 100 | Iguratimod 25 mg B.i.d. + Conventional treatment | Conventional treatment [Prednisone, HCQ, olfaction] | ESR, adverse events | 30.68 ± 3.51 | 31.00 ± 3.60 | 1.03 ± 0.22 | 1.08 ± 0.22 | – | – | 20 weeks |
| Liang et al., 2021(51) | China | 30 | 30 | Iguratimod 25 mg B.i.d. + Methylprednisolone 8 mg | 8 mg methylprednisolone Q.d. +200 mg HCQ B.i.d | ESSDAI, ESSPRI, ESR, CRP, adverse events | 45.16 ± 6.37 | 40.15 ± 6.65 | 3.59 ± 0.51 | 3.52 ± 0.50 | 6.96 ± 0.79 | 7.12 ± 0.95 | 16 weeks |
| Rao et al. 2022(52) | China | 43 | 43 | Iguratimod 25 mg B.i.d. + Conventional treatment | Conventional treatment [4 mg methylprednisolone Q.d. +200 mg HCQ B.i.d] | Schirmer’s test, ESR, RF | 51.8 ± 10.3 | 50.1 ± 9.9 | 2.0 ± 0.5 | 2.2 ± 0.6 | – | – | 12 weeks |
| Chen et al. 2022(39) | China | 62 | 62 | Iguratimod 25 mg B.i.d+Total Glucosides of Paeony 0.6g T.i.d. + HCQ 0.2g B.i.d | Total Glucosides of Paeony 0.6g T.i.d. + HCQ 0.2g B.i.d | ESSPRI, ESSDAI, ESR, RF | 68. 02 ± 3. 02 | 68. 50 ± 3. 05 | – | – | 7. 03 ± 1. 15 | 7. 04 ± 1. 20 | 12 weeks |
| Lu and Zhang(53) | China | 48 | 48 | Iguratimod 25 mg B.i.d+ HCQ 0.2g B.i.d | HCQ 0.2g B.i.d | ESR, RF, adverse events | 45. 52 ± 7. 48 | 44. 24 ± 8. 32 | 3.49 ± 0.26 | 3.42 ± 0.25 | – | – | 12 weeks |
| Jiang et al. 2021(54) | China | 24 | 22 | Iguratimod 25 mg B.i.d+ Chere Cunjing Granules | Chere Cunjing Granules (Traditional Chinese Medicine) | ESSPRI, ESSDAI, ESR, CRP, adverse events | 45.95 ± 11.52 | 48.92 ± 11.53 | 6.36 ± 6.33 | 4.30 ± 3.44 | 4.11 ± 1.10 | 3.93 ± 0.74 | 12 weeks |
The characteristics of the included studies.
3.2 Risk of Bias of Included Studies
The summary and graph of risk of bias ware shown in Figures 2, 3.
Figure 2
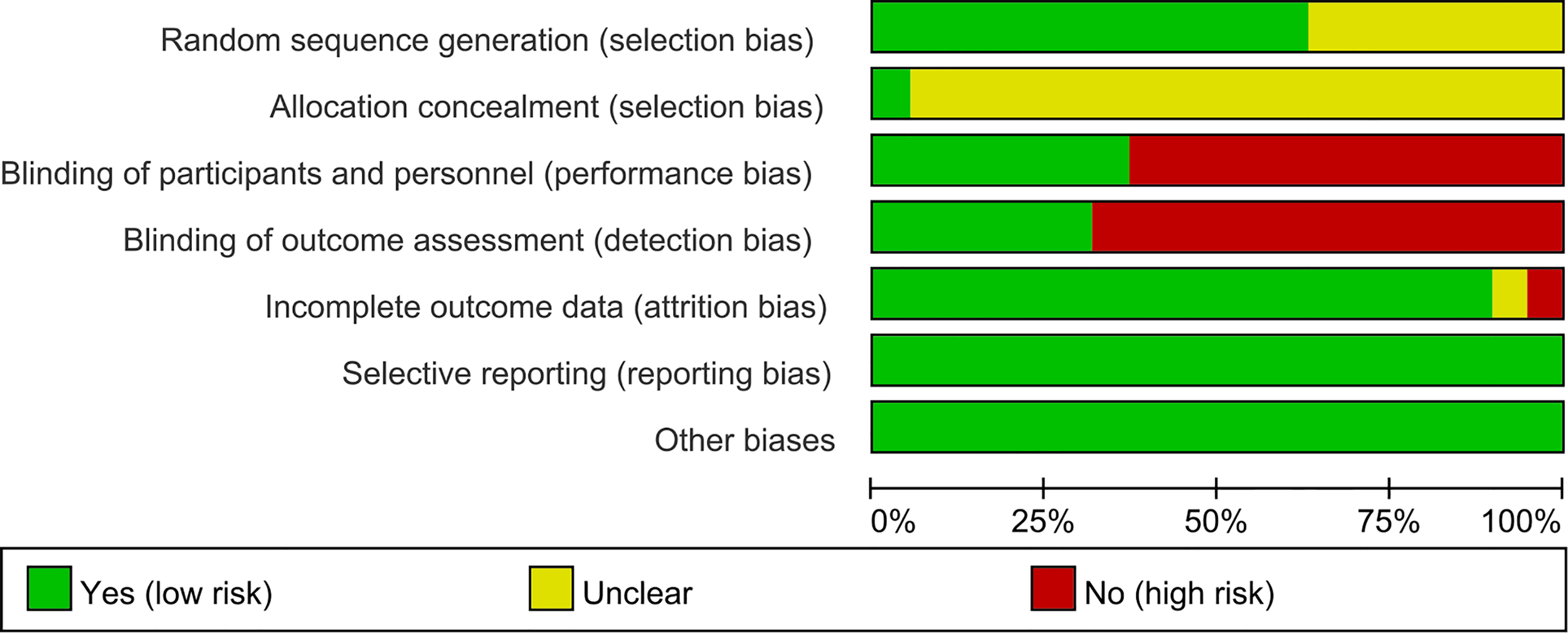
Risk of bias graph.
Figure 3
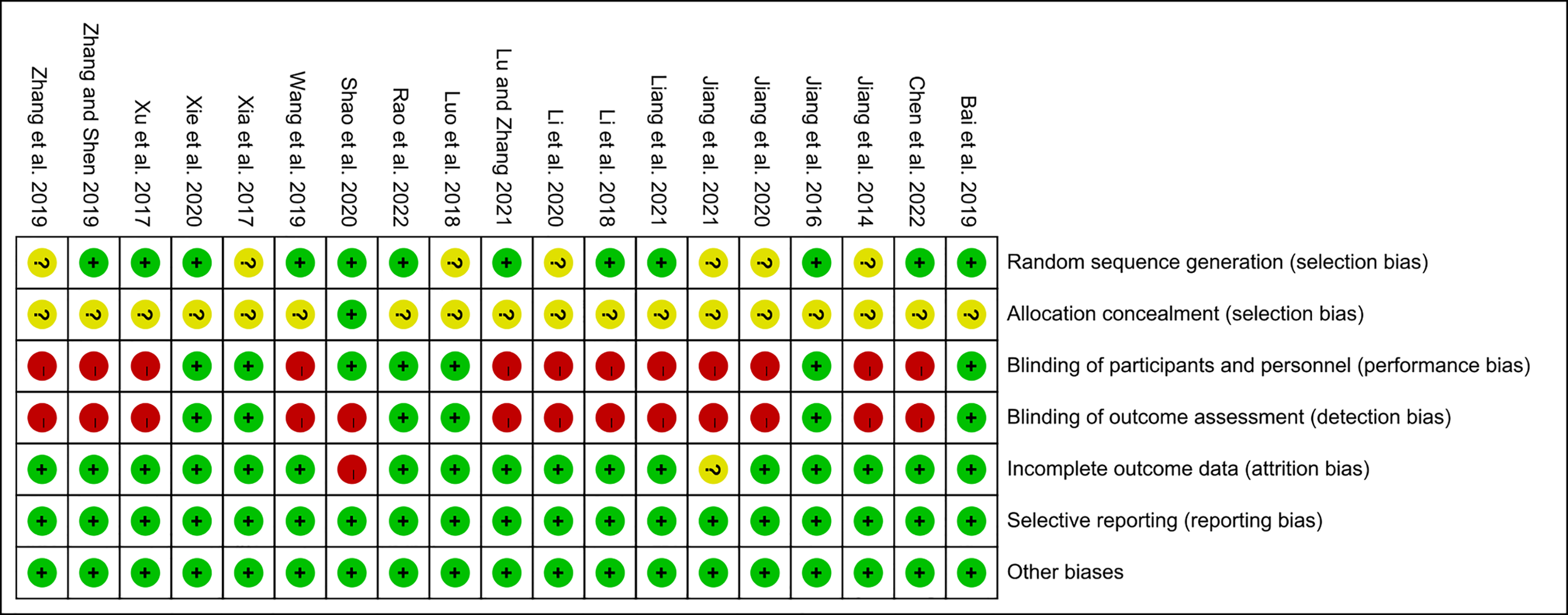
Risk of bias summary.
3.3 Primary Outcomes
Primary outcome is Disease activity score (ESSPRI and ESSDAI)
3.3.1 ESSPRI
Twelve RCTs (401 participants in experimental group and 392 participants in control group) reported ESSPRI. The heterogeneity test P<0.00001, I2 = 94%, indicating that the included studies are highly heterogeneous, and the random effects model was used for analysis. The summary result showed the ESSPRI in experimental group was lower (WMD -1.93 [-2.33, -1.52], P<0.00001; random effect model) (Figure 4). The publication bias detection suggests that the possibility of publication bias was small (P=0.514). Referring to MCID, the improvement in ESSPRI was greater than 1, suggesting that the difference between Iguratimod group and the control group is clinically significant.
Figure 4
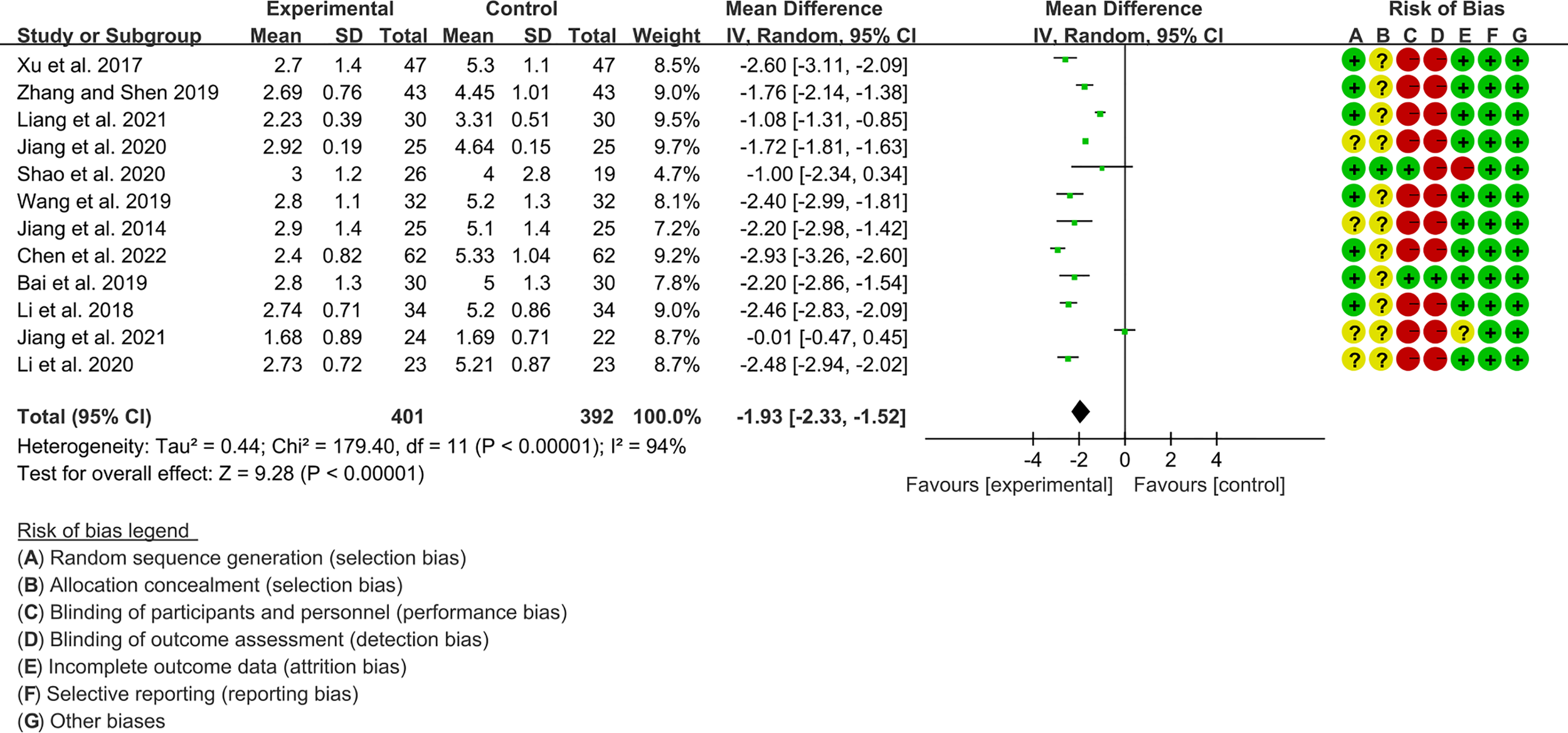
The results of ESSPRI.
3.3.2 ESSDAI
Ten RCTs (344 participants in experimental group and 335 participants in control group) reported ESSDAI. The heterogeneity test P<0.00001, I2 = 82%, indicating that the included studies are homogeneous, and the random effects model is used for analysis. The summary result showed the ESSDAI in experimental group was lower (WMD -1.39 [-1.81, -0.98], P<0.00001; random effect model) (Figure 5). The publication bias detection suggests that the possibility of publication bias was small (P=0.814). Referring to MCID, the improvement of ESSDAI was less than 3, suggesting that the difference between Iguratimod group and control group is of no clinically significant.
Figure 5
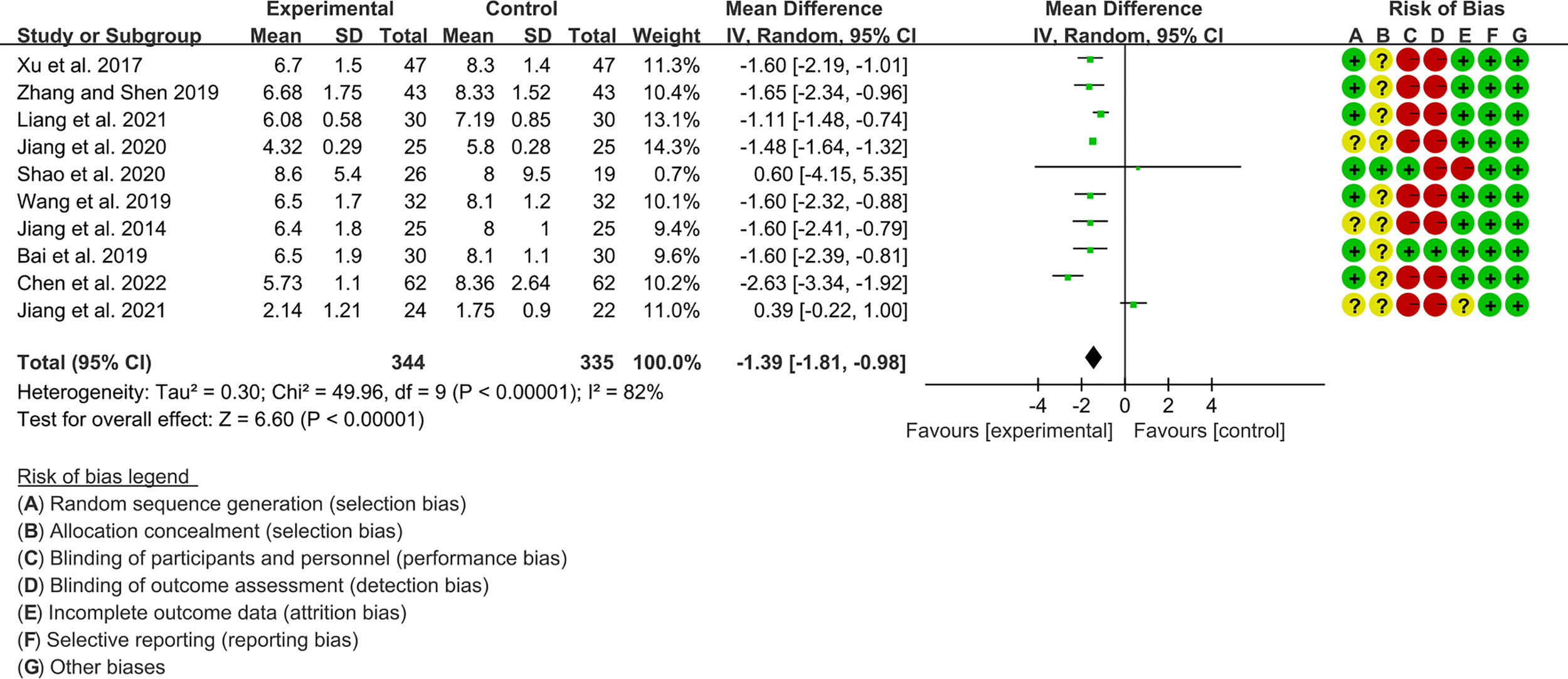
The results of ESSDAI.
3.4 Secondary Outcomes
3.4.1 Schirmer’s Test
Eight RCTs, involving 279 participants in experimental group and 272 participants in control group, reported Schirmer’s test. The heterogeneity test P<0.00001, I2 = 99%, indicating that the included studies are highly heterogeneous, and the random effects model was used for analysis. The summary result showed the Schirmer’s test in experimental group was higher (WMD 1.77 [0.85, 2.70], P=0.0002; random effect model) (Figure 6). The publication bias detection suggests that the possibility of publication bias was small (P=0.722). Referring to MCID, the improvement of Schirmer’s test was lower than 5 mm, suggesting that the difference between Iguratimod group and control group is of no clinically significant.
Figure 6
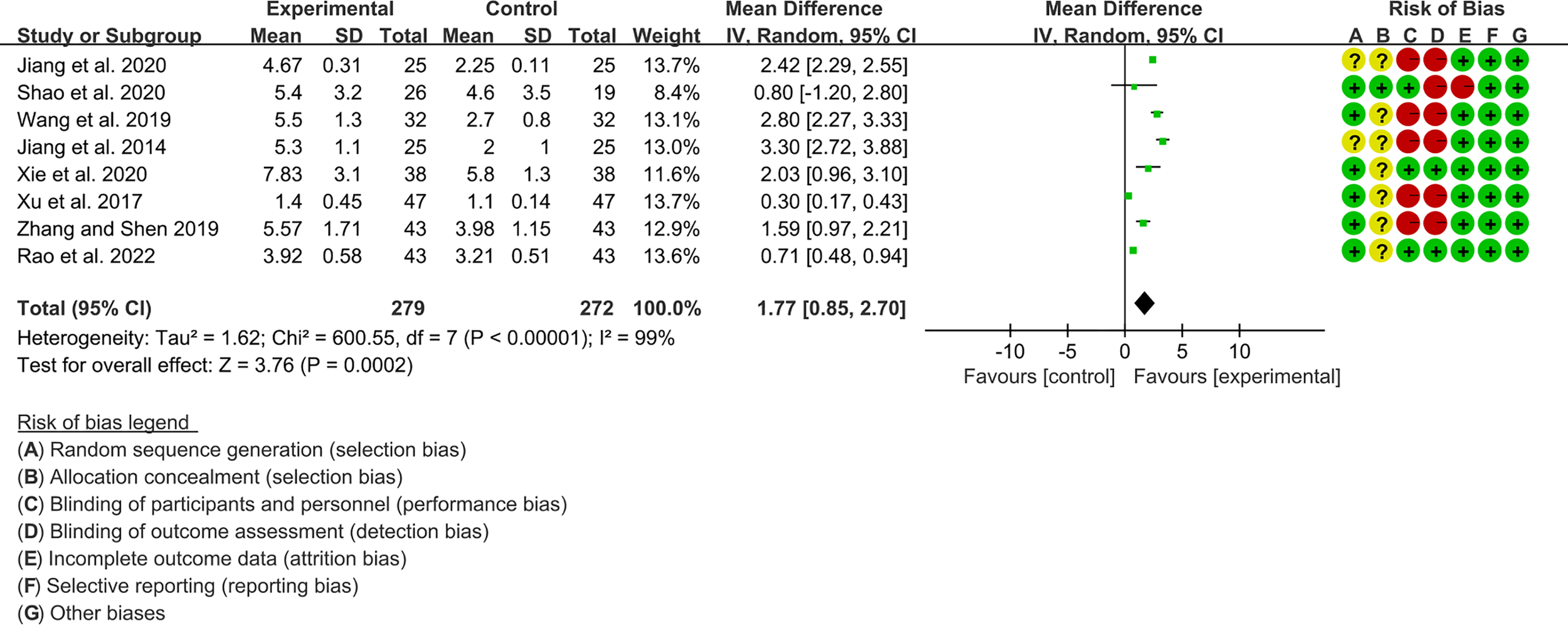
Schirmer’s test.
3.4.2 The Results of ESR
Fourteen RCTs (577 participants in experimental group and 568 participants in control group) reported ESR. The heterogeneity test P<0.00001, I2 = 96%, indicating that the included studies are highly heterogeneous, and the random effects model is used for analysis. The summary result showed the ESR in experimental group was lower (WMD -7.05 [-9.84, -4.26], P<0.00001; random effect model) (Figure 7).
Figure 7
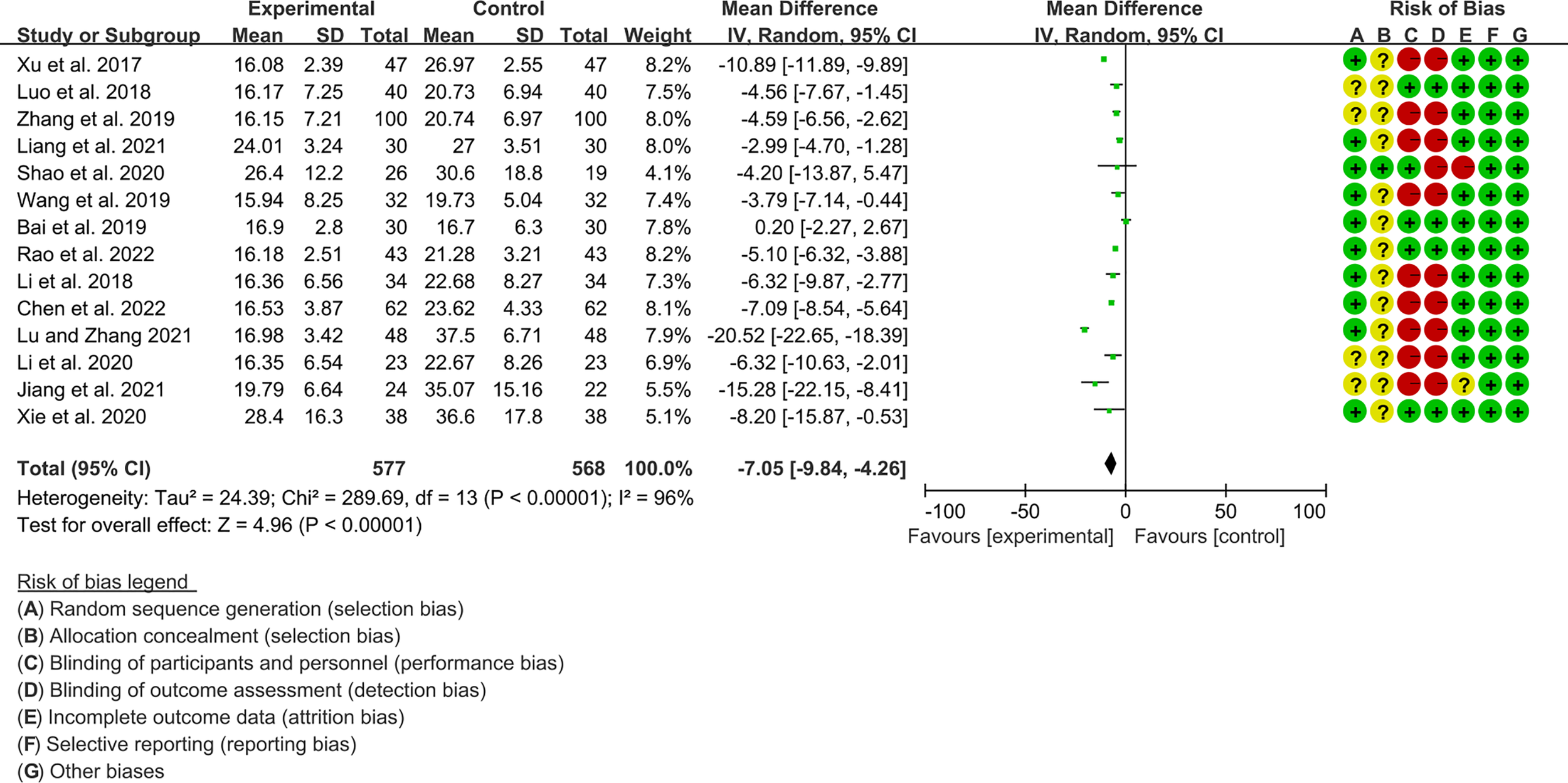
The results of ESR.
3.4.3 The Results of CRP
Four RCTs (122 participants in experimental group and 120 participants in control group) reported CRP. The heterogeneity test P<0.00001, I2 = 95%, indicating that the included studies are highly heterogeneous, and the random effects model is used for analysis. The summary result showed the CRP between the two groups has no statistical significance (WMD -0.95 [-2.22, 0.31], P=0.14; random effect model) (Figure 8).
Figure 8
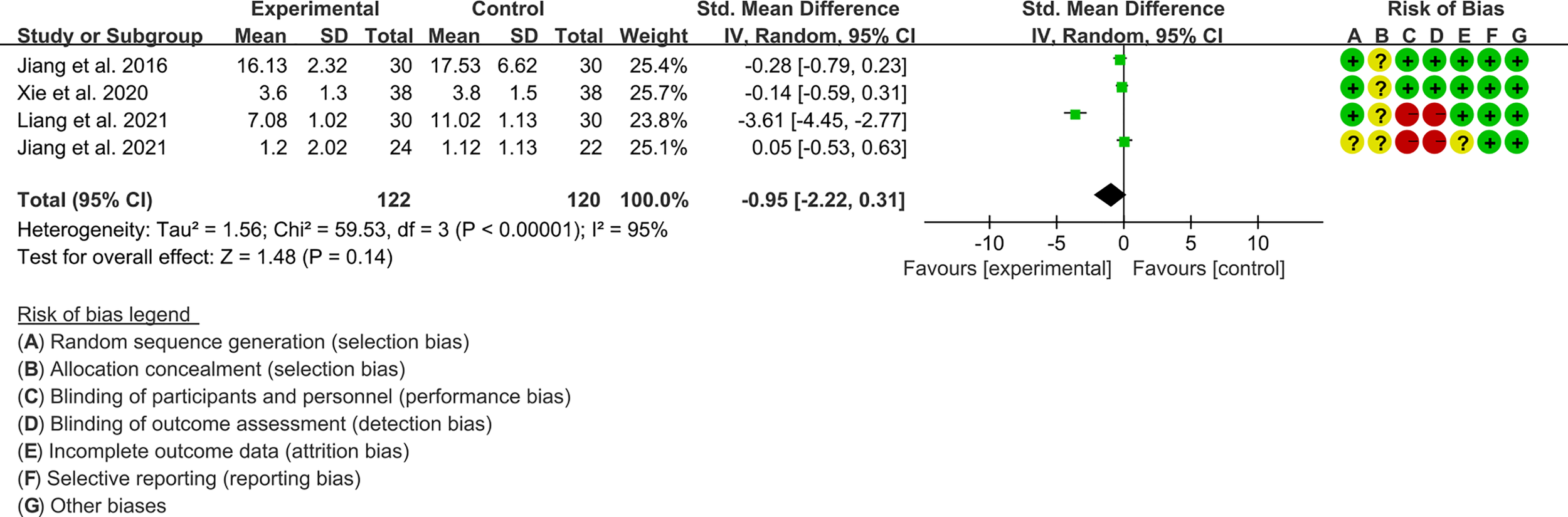
The results of CRP.
3.4.4 The Results of RF
Eleven RCTs (448 participants in experimental group and 448 participants in control group) reported RF. The heterogeneity test P<0.00001, I2 = 88%, indicating that the included studies are highly heterogeneous, and the random effects model is used for analysis. The summary result showed the RF in experimental group was lower (WMD -5.78 [-7.59, -3.97], P<0.00001; random effect model) (Figure 9).
Figure 9
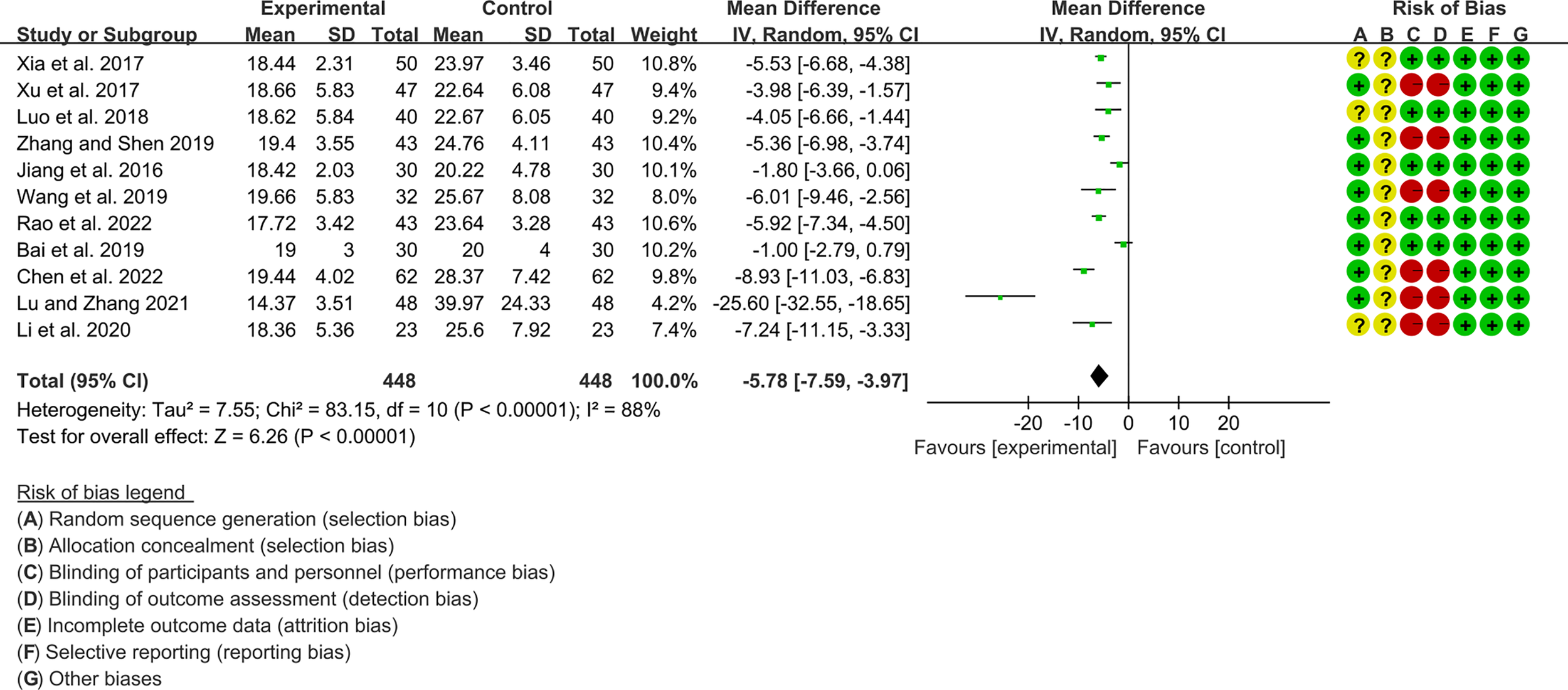
The results of RF.
3.5 Adverse Events
Fifteen RCTs (566 participants in experimental group and 542 participants in control group) reported adverse events. The heterogeneity test P=0.62, I2 = 0%, indicating that the included studies are heterogeneous, and the fix effects model is used for analysis. The summary result showed the adverse events had a downward trend in the experimental group, but the difference was critical (RR 0.88 [0.67, 1.16], P=0.37; fix effect model) (Figure 10). The publication bias detection suggests that the possibility of publication bias was small (P=0.632).
Figure 10
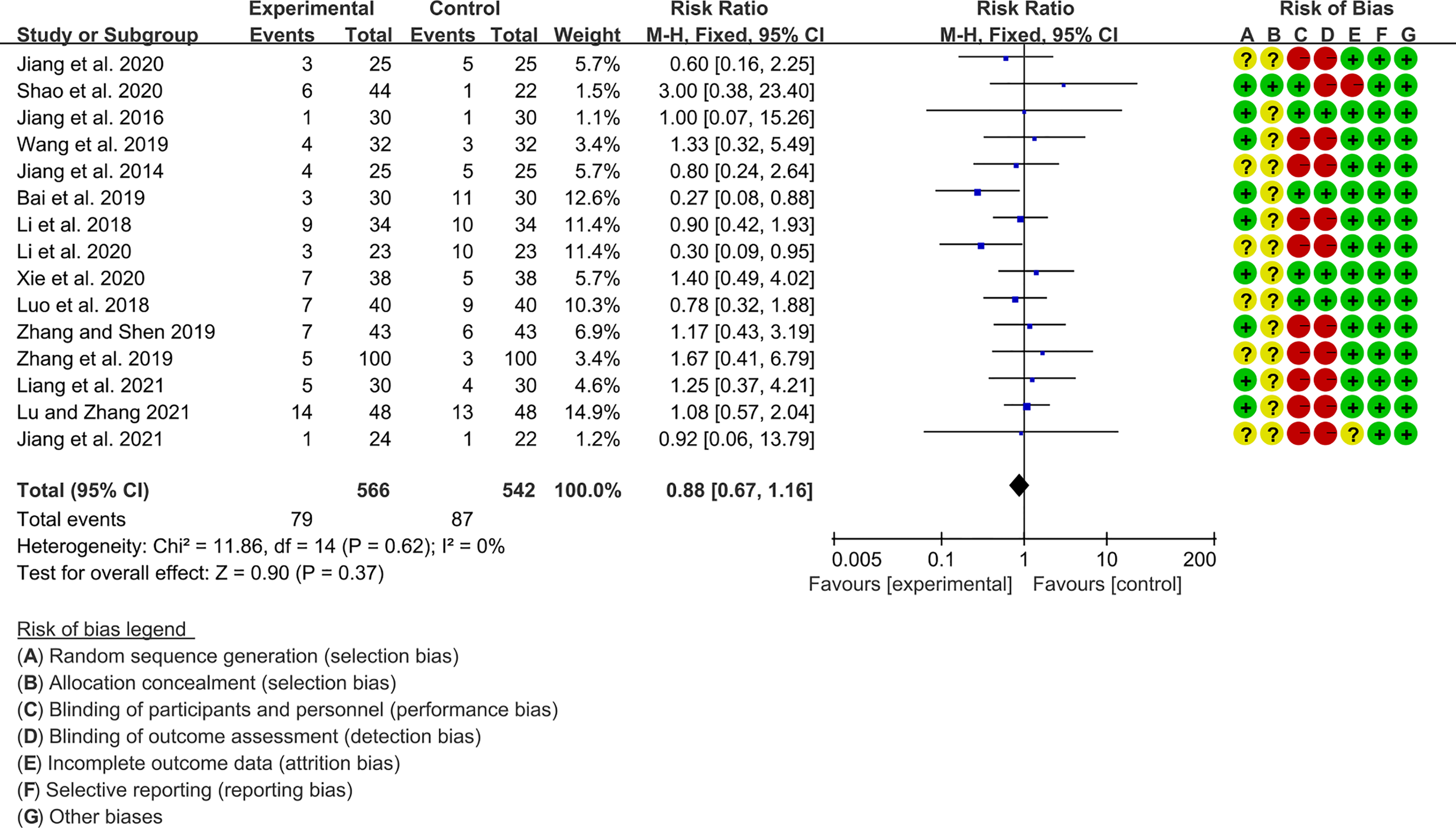
Adverse events.
Jiang et al., 2020 (40) showed that 1 case in the iguratimod group and 2 cases in the control group had mildly elevated liver enzyme levels; 2 cases in the iguratimod group and 3 cases in the control group developed diarrhea. Shao et al. 2020 (36) showed 2 cases of elevated liver enzymes in the iguratimod group, 2 cases of gastrointestinal discomfort (1 case in the placebo control group), 1 case of Diarrhoea, and 1 case of Skin rash. Jiang et al. 2016 (41) showed that there was 1 case of liver enzyme elevation and 1 case of leukopenia in the iguratimod group; 1 case of mild abdominal pain and diarrhea, and 1 case of skin pruritus in the control group. (37) showed that there was 1 case of mild liver function abnormality, 1 case of rash with itching, 1 case of diarrhea, and 1 case of leukopenia in the iguratimod group, and 2 cases of diarrhea and 1 case of rash with itching in the control group. Wang et al. 2019 (37) showed that 3 cases of gastrointestinal adverse reactions and 1 case of mild abnormal liver function in the iguratimod group; 2 cases of gastrointestinal discomfort, 2 cases of mild abnormal liver function, and 1 case of suspicious systemic rash in the control group. Bai et al., 2019 (43) showed that 1 case of iguratimod group had abnormal liver function (3 cases in the control group), 1 case with abnormal renal function (2 cases in the control group), and 1 case with abnormal gastrointestinal tract (3 cases in the control group); in addition, there were 3 cases of abnormal blood routine in the control group. Li et al. 2018 (44) showed 2 cases of gastrointestinal discomfort (4 cases in the control group), 1 case of abnormal liver function (1 case in the control group), 1 case of skin rash (2 cases in the control group), 1 case of skin rash (2 cases in the control group), skin itching occurred in 5 cases (3 cases in the control group) in the iguratimod group. Li et al. 2020 (45) showed that 1 case of iguratimod group had gastrointestinal discomfort (4 cases in the control group), 1 case of abnormal liver function (3 cases in the control group), and 1 case of skin rash (3 cases in the control group). Xie et al. 2020 (38) showed that 2 cases of abdominal distension, 2 cases of nausea and vomiting, and 3 cases of diarrhea occurred in the Ailamod group, while 1 case of abdominal distention, 2 cases of nausea and vomiting, 1 case of diarrhea, and 1 case of blurred vision occurred in the control group. Luo et al. 2018 (48) showed that 3 cases of gastrointestinal reactions (2 cases in the control group), 2 cases of rash (2 cases in the control group), 1 case of pruritus (2 cases in the control group), and 1 case of abnormal liver function in the iguratimod group (2 cases in the control group); there was also 1 case of leukopenia in the control group. Zhang and Shen 2019 (49) showed that there were 4 cases of gastrointestinal discomfort, 1 case of rash, 1 case of pruritus, and 1 case of leukopenia in the iguratimod group; in the control group, there were 3 cases of gastrointestinal discomfort, 2 cases of abnormal liver function, and 1 case of skin rash. Zhang et al. 2019 (50) showed that there were 2 cases of gastrointestinal discomfort (2 cases in the control group), 2 cases of abnormal liver function (1 case in the control group) and 1 case of skin rash in the iguratimod group. Liang et al., 2021 (51) showed that 2 cases of gastrointestinal symptoms (1 case in the control group), 2 cases of abnormal liver function (2 cases in the control group) and 1 case of skin itching (1 case in the control group) occurred in the iguratimod group. Lu and Zhang 2021 (53) showed 2 cases of gastrointestinal symptoms (3 cases in the control group), 2 cases of abnormal liver function (2 cases in the control group), 4 cases of skin rash (3 cases in the control group), and 5 cases of skin itching (4 cases in the control group), 1 case of dizziness (1 case in the control group) in the iguratimod group. Jiang et al. 2021 (54) showed that there was 1 case of diarrhea in each of the iguratimod group and the control group. Overall, adverse events were mainly gastrointestinal discomfort, abnormal liver function, and rash and itching.
3.6 Subgroup Analysis
Subgroup analyses were performed according to usage of iguratimod, age of patients, duration of intervention, and number of authors of RCTs (Table 2).
Table 2
| Subgroup | Outcomes | Overall effect | Heterogeneity test | Statistical method | Studies (N) | Sample size (N) | Figure | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MD | 95%CI | P | Tau2 | I2 (%) | P(Q) | ||||||
| ESSPRI-usage | Once a day | WMD -1.72 | [-1.81, -1.63] | P<0.000001 | – | – | – | Random | 1 | 50 | Fig S1a |
| Twice a day | WMD -1.94 | [-2.51, -1.38] | P<0.000001 | 0.82 | 94.37 | P<0.000001 | Random | 11 | 743 | ||
| ESSDAI-usage | Once a day | WMD -1.48 | [-1.64, -1.32] | P<0.000001 | – | – | – | Random | 1 | 50 | Fig S1b |
| Twice a day | WMD -1.38 | [-1.95, -0.81] | P<0.00001 | 0.57 | 83.32 | P<0.000001 | Random | 9 | 629 | ||
| Schirmer’s test-usage | Once a day | WMD 2.42 | [2.29, 2.55] | P<0.000001 | – | – | – | Random | 1 | 50 | Fig S1c |
| Twice a day | WMD 1.67 | [0.85, 2.50] | P=0.00007 | 1.07 | 96.75 | P<0.000001 | Random | 7 | 501 | ||
| CRP-usage | Once a day | SMD -0.28 | [-0.79, 0.23] | P=0.28 | – | – | – | Random | 1 | 60 | Fig S1d |
| Twice a day | SMD -1.20 | [-3.08, 0.67] | P=0.21 | 2.64 | 96.57 | P<0.000001 | Random | 3 | 182 | ||
| RF-usage | Once a day | WMD -1.80 | [-3.66, 0.06] | P=0.058 | – | – | – | Random | 1 | 60 | Fig S1e |
| Twice a day | WMD -6.21 | [-8.08, -4.34] | P<0.000001 | 7.20 | 87.21 | P<0.000001 | Random | 10 | 836 | ||
| Adverse events-usage | Once a day | RR 0.90 | [0.67, 1.19] | P=0.45 | – | 0 | P=0.49 | Fixed | 13 | 998 | Fig S1f |
| Twice a day | RR 0.67 | [0.20, 2.17] | P=0.50 | – | 0 | P=0.74 | Fixed | 2 | 110 | ||
| ESSPRI-age | > 60 years old | WMD -2.72 | [-3.23, -2.22] | P<0.000001 | 0.08 | 57.67 | P=0.12 | Random | 2 | 188 | Fig S2a |
| <60 years old | WMD -1.77 | [-2.18, -1.36] | P<0.000001 | 0.36 | 92.67 | P<0.000001 | Random | 10 | 605 | ||
| ESSDAI-age | > 60 years old | WMD -2.12 | [-3.13, -1.11] | P<0.0001 | 0.40 | 74.81 | P=0.046 | Random | 2 | 188 | Fig S2b |
| <60 years old | WMD -1.21 | [-1.65, -0.77] | P<0.000001 | 0.27 | 81.56 | P<0.00001 | Random | 8 | 491 | ||
| Schirmer’s test-age | > 60 years old | WMD 2.80 | [2.27, 3.33] | P<0.000001 | – | – | – | Random | 1 | 64 | Fig S2c |
| < 60 years old | WMD 1.62 | [0.63, 2.61] | P=0.001 | 1.62 | 98.95 | P<0.000001 | Random | 7 | 487 | ||
| ESR-age | > 60 years old | WMD -5.80 | [-8.96, -2.64] | P=0.0003 | 3.71 | 68.18 | P=0.08 | Random | 2 | 188 | Fig S2d |
| < 60 years old | WMD -7.34 | [-10.67, -4.01] | P=0.00002 | 30.14 | 96.13 | P<0.000001 | Random | 12 | 957 | ||
| RF-age | > 60 years old | WMD -7.80 | [-10.59, -5.02] | P<0.000001 | 2.14 | 50.14 | P=0.16 | Random | 2 | 188 | Fig S2e |
| < 60 years old | WMD -5.36 | [-7.31, -3.40] | P<0.000001 | 7.17 | 88.26 | P<0.000001 | Random | 9 | 708 | ||
| Adverse events-age | < 60 years old | RR 0.86 | [0.65, 1.15] | P=0.31 | – | 0 | P=0.57 | Fixed | 14 | 1044 | Fig S2f |
| > 60 years old | RR 1.33 | [0.32, 5.49] | P=0.69 | – | – | – | Fixed | 1 | 64 | ||
| ESSPRI-duration | 12 weeks | WMD -2.07 | [-2.52, -1.62] | P<0.000001 | 0.47 | 93.57 | P<0.000001 | Random | 10 | 688 | Fig S3a |
| 16 weeks | WMD -1.08 | [-1.31, -0.85] | P<0.000001 | – | – | – | Random | 1 | 60 | ||
| 24 weeks | WMD -1.00 | [-2.34, 0.34] | P=0.14 | – | – | – | Random | 1 | 45 | ||
| ESSDAI-duration | 12 weeks | WMD -1.46 | [-1.96, -0.96] | P<0.000001 | 0.41 | 84.88 | P<0.000001 | Random | 8 | 574 | Fig S3b |
| 16 weeks | WMD -1.11 | [-1.48, -0.74] | P<0.000001 | – | – | – | Random | 1 | 60 | ||
| 24 weeks | WMD 0.60 | [-4.15, 5.35] | P=0.8 | – | – | – | Random | 1 | 45 | ||
| Schirmer’s test-duration | 12 weeks | WMD 1.84 | [0.80, 2.88] | P=0.0005 | 1.64 | 99.17 | P<0.000001 | Random | 6 | 430 | Fig S3c |
| 24 weeks | WMD 1.72 | [0.67, 2.77] | P=0.0014 | 0.09 | 11.70 | P=0.29 | Random | 2 | 121 | ||
| ESR-duration | 12 weeks | WMD -7.84 | [-11.22, -4.45] | P<0.000001 | 27.16 | 96.33 | P<0.000001 | Random | 10 | 764 | Fig S3d |
| 12-24 weeks | WMD -3.71 | [-5.27, -2.15] | P<0.000001 | 0.40 | 31.01 | P=0.23 | Random | 2 | 260 | ||
| 24 weeks | WMD -6.65 | [-12.66, -0.64] | P=0.03 | 0.00 | 0.00 | P=0.53 | Random | 2 | 121 | ||
| CRP-duration | 12 weeks | SMD -0.14 | [-0.52, 0.25] | P=0.48 | 0.00 | 0.00 | P=0.41 | Random | 2 | 106 | Fig S3e |
| 16 weeks | SMD -3.61 | [-4.45, -2.77] | P<0.000001 | – | – | – | Random | 1 | 60 | ||
| 24 weeks | SMD -0.14 | [-0.59, 0.31] | P=0.54 | – | – | – | Random | 1 | 76 | ||
| Adverse events-duration | 12 weeks | RR 0.76 | [0.55, 1.03] | P=0.08 | – | 0 | P=0.60 | Fixed | 11 | 706 | Fig S3f |
| 12-24 weeks | RR 1.43 | [0.57, 3.58] | P=0.45 | – | 0 | P=0.76 | Fixed | 2 | 260 | ||
| 24 weeks | RR 1.74 | [0.68, 4.45] | P=0.25 | – | 0 | P=0.51 | Fixed | 2 | 142 | ||
| ESSPRI-number of authors | > 3 authors | WMD -1.70 | [-2.20, -1.21] | P<0.000001 | 0.42 | 93.87 | P<0.000001 | Random | 8 | 477 | Fig S4a |
| ≤ 3 authors | WMD -2.35 | [-2.93, -1.77] | P<0.000001 | 0.29 | 85.99 | P<0.0001 | Random | 4 | 316 | ||
| ESSDAI-number of authors | > 3 authors | WMD -1.15 | [-1.64, -0.65] | P<0.00001 | 0.30 | 83.94 | P<0.00001 | Random | 7 | 409 | Fig S4b |
| ≤ 3 authors | WMD -1.97 | [-2.63, -1.30] | P<0.000001 | 0.21 | 59.78 | P=0.08 | Random | 3 | 270 | ||
| Schirmer’s test-number of authors | > 3 authors | WMD 1.80 | [0.79, 2.81] | P=0.0005 | 1.68 | 99.00 | P<0.000001 | Random | 7 | 465 | Fig S4c |
| ≤ 3 authors | WMD 1.59 | [0.97, 2.21] | P<0.000001 | – | – | – | Random | 1 | 86 | ||
| ESR-number of authors | > 3 authors | WMD -6.28 | [-8.85, -3.72] | P<0.00001 | 12.97 | 91.55 | P<0.000001 | Random | 10 | 819 | Fig S4d |
| ≤ 3 authors | WMD -8.46 | [-17.15, 0.22] | P=0.056 | 76.47 | 98.28 | P<0.000001 | Random | 4 | 326 | ||
| RF-number of authors | > 3 authors | WMD -4.56 | [-5.93, -3.18] | P<0.000001 | 1.82 | 67.05 | P=0.01 | Random | 6 | 484 | Fig S4e |
| ≤ 3 authors | WMD -8.53 | [-13.04, -4.02] | P=0.0002 | 23.28 | 94.06 | P<0.000001 | Random | 5 | 412 | ||
| Adverse events-number of authors | > 3 authors | RR 1.05 | [0.73, 1.52] | P=0.78 | – | 0 | P=0.97 | Fixed | 11 | 820 | Fig S4f |
| ≤ 3 authors | RR 0.68 | [0.44, 1.04] | P=0.08 | – | 59.35 | P=0.06 | Fixed | 4 | 288 | ||
Subgroup analysis results.
3.6.1 Usage of Iguratimod
The included RCTs showed the use of iguratimod once or twice a day. Subgroup analysis showed that iguratimod 50 mg once a day and iguratimod 25 mg twice a day were both positive in Adverse events, CRP, ESSDAI, ESSPRI, and Schirmer’s test. However, the results of iguratimod once a day were negative, which was inconsistent with the results of iguratimod twice a day. Since there are too few RCTs in the “once a day” subgroup, more RCTs are needed to further test the results. Referring to MCID, the improvement of ESSPRI is clinically significant, while the improvement of ESSDAI and Schirmer’s test is of no clinically significant.
3.6.2 Age of Patients
RCTs were divided into “>60 years subgroup” and “<60 years old subgroup” according to the age of enrolled patients. Subgroup analyses showed that iguratimod was both effective and safe for people younger or older than 60. Referring to MCID, the improvement of ESSPRI is clinically significant, while the improvement of Schirmer’s test is of no clinically significant. Meanwhile, the improvement of ESSDAI in “> 60 years old” subgroup may be clinically significant.
3.6.3 Duration of Intervention
RCTs were divided into 12 weeks, 12-24weeks and 24weeks according to the duration of intervention. Strangely, the results for the “24 weeks” subgroup of ESSDAI and ESSPRI were negative. Since only 1 RCT is involved, its reliability is not high. More long-term RCTs are needed for further exploration. Referring to MCID, the improvement of ESSPRI in “12 weeks” subgroup is clinically significant, while the improvement of ESSDAI and Schirmer’s test is of no clinically significant.
3.6.4 Number of Authors of RCTs
Subgroup analysis was performed according to the authors greater than 3 or less than or equal to 3 to test the consistency of the results of the RCTs. The results of subgroup analysis showed that the results of the “>3 authors” subgroup and the “≤3 authors” subgroup were basically the same. Only ESR showed inconsistent results, which may be due to individual differences. Referring to MCID, the improvement of ESSPRI is clinically significant, while the improvement of ESSDAI and Schirmer’s test is of no clinically significant.
3.7 Sensitivity Analysis
We undertook sensitivity analysis for CRP (Figure 11). After we omitted the Liang et al., 2021, we found that the results changed significantly. This suggests that this outcome is not stable.
Figure 11
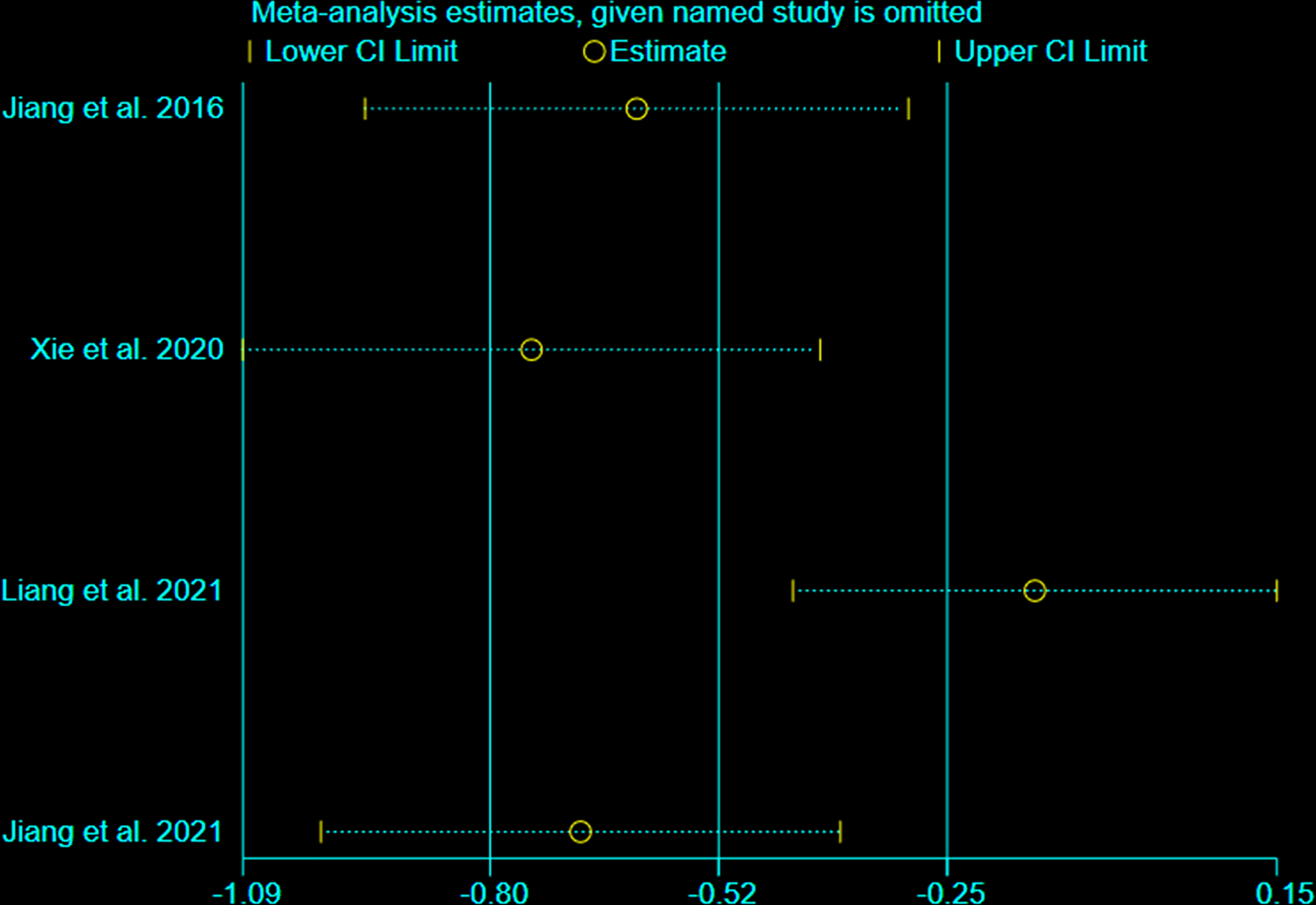
sensitivity analysis of CRP.
4 Discussion
4.1 Main Findings
This systematic review and meta-analysis included 19 RCTs with 1512 participants. The statistical analysis results showed that Iguratimod can reduce the ESSPRI score and ESSDAI score, and increase Schirmer’s test score; and the improvement of ESSPRI score is clinically significant. Iguratimod can also reduce systemic inflammation (decrease ESR level) and decrease RF. For CRP, this research showed that there was no statistical significance between experimental group and control group. Meanwhile, the addition of Iguratimod may not increase the occurrence of adverse events. The heterogeneity test found that the results of ESSPRI, ESSDAI, Schirmer’s test, ESR, CRP, RF are highly heterogeneous. After careful reading of the included RCTs, it is found that the interventions of these RCTs are not exactly the same, which may cause one of the sources of heterogeneity. For the results of CRP, the results of the fixed effect model are inconsistent with that of the random effect model; hence, the sensitivity analysis was performed on them. after we omitted the Liang et al., 2021, we found that the results changed significantly. This suggests that this outcome is not stable. We also used the Egger method and Harbord method to detect the publication bias of the main results, and showed that both P>0.1, indicating that there is no publication bias in the study. In addition, we performed subgroup analysis according to usage of iguratimod, age of patients, duration of intervention, and number of authors of RCTs. We found that the results of the subgroup analysis were basically consistent with the results of the pooled analysis. Although there are inconsistent results (for example, the results for the “24 weeks” subgroup of ESSDAI and ESSPRI were negative), it only contains 1 RCT, the reliability of the results is not high enough, and more RCTs are needed to confirm or correct the results. Interestingly, although the improvement in summary ESSDAI was not clinically significant, subgroup analysis showed that the results in the “>60 years” subgroup may be clinically significant. This suggests that iguratimod may have potential benefits for people over the age of 60.
4.2 Interpretation of the Outcomes
As a systemic autoimmune disease, pSS currently has a heavy burden of disease research, and there is currently no specific treatment for pSS. The current comprehensive clinical management mainly includes local treatment and systemic treatment according to the patient’s symptoms. For xerostomia, muscarinic agonists and artificial salivary replacement therapy are preferred without contraindications. For severe exocrine gland inflammation or involvement of extraglandular systems (such as skin, joints, muscles), systemic therapy (glucocorticoids, immunosuppressants, non-steroidal anti-inflammatory drugs, biological agents, etc.) is used. Although the above traditional anti-rheumatic drugs to improve the condition of the disease are supported by expert opinions, non-controlled studies and daily clinical practice, the effectiveness and safety of these anti-rheumatic drugs in the treatment of pSS still have not been verified by large-scale RCTs. According to the recommendations provided by the pSS diagnosis and treatment guidelines, hydroxychloroquine is the first-line drug for the treatment of inflammatory manifestations of the musculoskeletal system. A small dose/short course of glucocorticoids can be used for parotid gland enlargement, arthritis and skin vasculitis. Cyclophosphamide and azathioprine are effective drugs for the treatment of severe vasculitis or central nervous system involvement. Rituximab is used to treat pSS-related lymphoma. However, due to the many adverse reactions of non-steroidal anti-inflammatory drugs, glucocorticoids and the high price of biological preparations, there are high hopes for exploring new anti-rheumatic drugs to improve the disease. Current research shows that Iguratimod, as a new type of small molecule anti-rheumatic drug, has shown clinical effectiveness in the treatment of autoimmune diseases (such as rheumatoid arthritis and pSS). Its pharmacological mechanism is mainly related to inhibit the production of immunoglobulin and autoantibodies by B cells, down-regulate T cell-mediated cellular immunity, and inhibit various inflammatory factors (IL-1, IL-6, IL-8 and TNF) and the expression of inflammatory signal pathway (NF-KB signal pathway) [Jiang et al., 2020 (55)]. This research showed that Iguratimod combined with DMARDs can reduce the ESSPRI score, ESSDAI score, RF, and systemic inflammation (decrease the IgG and IgA level and ESR), and increase Schirmer’s test score, Saliva flow rate and PLT. Our previous open-label pilot study also showed that Iguratimod can reduce ESSDAI, RF and IgG level (56).
The results of this meta-analysis showed that the incidence of adverse reactions in the iguratimod test group was lower than that in the control group (P<0.05), indicating that iguratimod did not increase the adverse reaction rate and had better safety. Several meta-analysis of systematic reviews of iguratimod intervention in rheumatoid arthritis showed that Iguratimod is safe (57, 58). In addition, a number of clinical studies over 52 weeks have proved that Iguratimod can maintain good efficacy and safety regardless of long-term use as a single drug or a combination (59–64).
4.3 Quality of the Evidence
The GRADE tool was utilized to rate the quality of the evidence (65). According to the GRADE handbook (66), the evidence was judged to be high to low. The risk of bias associated with the unclear random sequence generation, allocation concealment, blinding and incomplete outcomes. Meanwhile, some of the evidence was downgraded because of their inconsistency and/or imprecision. The details were described in Table 3.
Table 3
| Iguratimod intervention in pSS | ||||||
|
Patient or population: patients with pSS Intervention: Iguratimod or combined with iguratimod on the basis of the control group Comparison: placebo treatment or conventional therapy |
||||||
| Outcomes | Illustrative comparative risks* (95% CI) |
Relative effect
(95% CI) |
No of Participants
(studies) |
Quality of the evidence
(GRADE) |
Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Primary outcomes | |||||
| Adverse events | Study population |
RR 0.88
(0.67 to 1.16) |
1108 (15 studies) |
⊕⊕⊕⊝ moderate1 |
||
| 161 per 1000 |
141 per 1000
(108 to 186) |
|||||
| Moderate | ||||||
| 140 per 1000 |
123 per 1000
(94 to 162) |
|||||
| ESSPRI | The mean esspri in the intervention groups was 1.93 lower (2.33 to 1.52 lower) |
793 (12 studies) |
⊕⊕⊝⊝ low1,2 |
|||
| ESSDAI | The mean essdai in the intervention groups was 1.39 lower (1.81 to 0.98 lower) |
679 (10 studies) |
⊕⊕⊝⊝ low1,2 |
|||
| Schirmer’s test | The mean schirmer’s test in the intervention groups was 1.77 higher (0.85 to 2.7 higher) |
551 (8 studies) |
⊕⊕⊝⊝ low1,2 |
|||
Summary of findings for the main comparison.
*The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI, Confidence interval; RR, Risk ratio;
GRADE Working Group grades of evidence.
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
1Downgraded one level due to serious risk of bias (random sequence generation, allocation concealment, blinding, incomplete outcomes) and most of the data comes from the RCTs with moderate risk of bias.
2Downgraded one level due to the probably substantial heterogeneity.
4.4 The Strengths of This Review
This is a comprehensive and newest systematic review and meta-analysis of RCTs on iguratimod intervention in pSS. This research not only found that the addition of iguratimod can improve the symptoms of pSS patients, but also showed that it does not increase adverse events. This research also conducted a quality assessment of primary outcomes to provide references for clinical applications and scientific research. Compared with previous systematic review and meta-analysis (21), the strengths of this review are: (1) Stricter inclusion and exclusion criteria were applied, excluding articles with only 1 author (even if they claimed to be an RCT). And the number of authors was used as a subgroup analysis to determine the consistency of the results of RCTs, and found that the results were consistent. (2) We introduced MCID as an assessment criterion for clinical efficacy and found that the improvement of ESSPRI is clinically significant, while the improvement of ESSDAI and Schirmer’s test is of no clinically significant. (3) In addition, subgroup analysis was performed according to usage of iguratimod, age of patients, duration of intervention. And it was found that iguratimod is effective whether 50mg Q.d or 25mg B.i.d; iguratimod is effective in people of all ages, but the improvement in ESSDAI may be more clinically significant in people older than 60 years than in people younger than 60 years. And based on the current evidence, it is speculated that the iguratimod intervention needs to continue for at least 12 weeks.
4.5 The Limitations of This Review
(1) The research area of all RCTs is in China and the participants are also Chinese. There is a lack of data from other countries and races. (2) Although there were 19 RCTs, the number of participants was small (The total number of participants does not exceed 4,000, and the number of participants in a single RCT does not exceed 150); some subgroups even had only 1 RCT. (3) Most RCTs failed to perform allocation concealment and blinding, which leads to a high risk of bias. (4) The quality of primary outcomes was also low; those outcomes was downgraded because of the serious risk of bias, the total sample size fails to meet the optimal information size. (5) For the subgroup analysis of age, due to the different age ranges involved in different RCTs, the subgroups can only be roughly divided into <60 years old and >60 years old, but cannot be divided into 30-40, 40-50,… and other levels. (6) Most of the RCTs were treated for 12 weeks, and there is a lack of observations on the efficacy of shorter (such as 4 weeks, 8 weeks) or longer treatment periods (48 weeks and more). This makes it difficult to evaluate the course of treatment finely.
4.6 Implications for Future Clinical Practice and Future Study
This systematic review and meta-analysis provides evidence of the use of the iguratimod in clinical practice. The results of this study support the application of iguratimod to pSS patients, because iguratimod can improve patients’ clinical symptoms (such as ESSPRI score, ESSDAI score, Schirmer’s test score) and systemic inflammatory response (decrease ESR level) and has good safety. From this point of view, iguratimod can be combined with a variety of DMARDs in the treatment of pSS, such as HCQ, and the combination therapy can have both efficacy and safety. However, further RCTs coming from different countries and races are needed to explore the efficacy and safety of iguratimod for other countries and races. More high-quality RCTs using blinding and allocation concealment are also demanded to reduce the risk of bias and generate more reliable and higher-quality evidence. In view of the small number of participants in the RCTs in this meta-analysis, more large-sample, multi-center RCT also expected so as to the findings of this research. In the future, more studies with courses of 4, 8, and 48 weeks, as well as more refined age-stratified studies, are needed to evaluate the efficacy and safety of IGU for different intervention times and for different age groups. More basic research about the iguratimod intervention in pSS are needed to explore the mechanisms of this medicine.
5 Conclusion
Based on current evidence, iguratimod can effectively reduce ESSPRI score, ESSDAI score, Schirmer’s test score and decrease systemic inflammatory response (such as ESR level and RF level) without increasing the probability of adverse events. And its improvement of ESSPRI has clinical significance, and the improvement of ESSDAI in people > 60 years old may have clinical significance. The recommended course of treatment is at least 12 weeks.
Funding
This work is supported by Natural Science Foundation of Changsha City (No. kq2202500).
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
LZ, GY, HC are responsible for the study concept and design. LZ, KY, WH, GY, QH and HC are responsible for the data collection, data analysis and interpretation; LZ and KY drafted the paper;HC and GY supervised the study; all authors participated in the analysis and interpretation of data and approved the final paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.924730/full#supplementary-material
References
1
Vivino FB . Sjogren's Syndrome: Clinical Aspects. Clin Immunol (2017) 182:48–54. doi: 10.1016/j.clim.2017.04.005
2
Thorne I Sutcliffe N . Sjögren's Syndrome. Br J Hosp Med (Lond). (2017) 78(8):438–42. doi: 10.12968/hmed.2017.78.8.438
3
Fox RI . Sjögren's Syndrome...Lancet (2005) 366(9482):321–31. doi: 10.1016/S0140-6736(05)66990-5
4
Cafaro G Croia C Argyropoulou OD Leone MC Orlandi M Finamore F et al . One Year in Review 2019: Sjögren's Syndrome. Clin Exp Rheumatol (2019) 37S1,118(3):3–15.
5
Jonsson R Brokstad KA Jonsson MV Delaleu N Skarstein K . Current Concepts on Sjögren's Syndrome - Classification Criteria and Biomarkers. Eur J Oral Sci (2018) 126:S1:37–48. doi: 10.1111/eos.12536
6
Marshall LL Stevens GA . Management of Primary Sjögren's Syndrome. Consult Pharm (2018) 33(12):691–701. doi: 10.4140/TCP.n.2018.691.
7
Chen X Wu H Wei W . Advances in the Diagnosis and Treatment of Sjogren's Syndrome. Clin Rheumatol (2018) 37(7):1743–9. doi: 10.1007/s10067-018-4153-8
8
Odani T Chiorini JA . Targeting Primary Sjögren's Syndrome. Mod Rheumatol (2019) 9(1):70–86. doi: 10.1080/14397595.2018.1546268
9
Devauchelle-Pensec V Mariette X Jousse-Joulin S Berthelot JM Perdriger A Puéchal X et al . Treatment of Primary Sjögren Syndrome With Rituximab: A Randomized Trial. Ann Intern Med (2014) 160(4):233–42. doi: 10.7326/m13-1085
10
Fisher BA Everett CC Rout J O'Dwyer JL Emery P Pitzalis C et al . Effect of Rituximab on a Salivary Gland Ultrasound Score in Primary Sjögren's Syndrome: Results of the TRACTISS Randomised Double-Blind Multicentre Substudy. Ann Rheum Dis (2018) 77(3):412–6. doi: 10.1136/annrheumdis-2017-212268
11
Baer AN Gottenberg JE St Clair EW Sumida T Takeuchi T Seror R et al . Efficacy and Safety of Abatacept in Active Primary Sjögren's Syndrome: Results of a Phase III, Randomised, Placebo-Controlled Trial. Ann Rheum Dis (2020) 80(3):339–48. doi: 10.1136/annrheumdis-2020-218599
12
Retamozo S Sisó-Almirall A Flores-Chávez A Ramos-Casals M Brito-Zerón P . An Update of Targeted Therapeutic Options for Primary Sjögren Syndrome: Current Status and Future Development. Expert Opin Pharmacother. (2021) 17):2359–71. doi: 10.1080/14656566.2021.1951224
13
Ishiguro N Yamamoto K Katayama K Kondo M Sumida T Mimori T Soen S et al . Concomitant Iguratimod Therapy in Patients With Active Rheumatoid Arthritis Despite Stable Doses of Methotrexate: A Randomized, Double-Blind, Placebo-Controlled Trial. Mod Rheumatol (2013) 23:430–9. doi: 10.3109/s10165-012-0724-8
14
Lü LJ Teng JL Bao CD Han XH Sun LY Xu JH et al . Safety and Efficacy of T-614 in the Treatment of Patients With Active Rheumatoid Arthritis: A Double Blind, Randomized, Placebo-Controlled and Multicenter Trial. Chin Med J (Engl) (2008) 121:615–9. doi: 10.1097/00029330-200804010-00008
15
Lu LJ Bao CD Dai M Teng JL Fan W Du F et al . Multicenter, Randomized, Double-Blind, Controlled Trial of Treatment of Active Rheumatoid Arthritis With T-614 Compared With Methotrexate. Arthritis Rheum (2009) 61:979–87. doi: 10.1002/art.24643
16
Xu Y Zhu Q Song J Liu H Miao Y Yang F et al . Regulatory Effect of Iguratimod on the Balance of Th Subsets and Inhibition of Inflammatory Cytokines in Patients With Rheumatoid Arthritis. Mediators Inflammation (2015) 2015:356040. doi: 10.1155/2015/356040
17
Tanaka K Yamamoto T Aikawa Y Kizawa K Muramoto K Matsuno H et al . Inhibitory Effects of an Anti-Rheumatic Agent T-614 on Immunoglobulin Production by Cultured B Cells and Rheumatoid Synovial Tissues Engrafted Into SCID Mice. Rheumatol Oxford (Oxford) (2003) 42(11):365–1371. doi: 10.1093/rheumatology/keg381
18
Aikawa Y Yamamoto M Yamamoto T Morimoto K Tanaka K . An Anti-Rheumatic Agent T-614 Inhibits NF-kappaB Activation in LPS- and TNF-Alpha-Stimulated THP-1 Cells Without Interfering With IkappaBalphadegradation. Inflamm Res (2002) 51(4):188–94. doi: 10.1007/PL00000291
19
Wang M Ni DH Wang XY Cao TP Liu D . Study on the Regulatory Effects of IGU on CD3+T Cells and Cytokines Il-6 in Patients With Rheumatoid Arthritis. Chin J Ctrl Endem Dis (2015) 30(4):310+2. doi: CNKI:SUN:DYBF.0.2016-12-015
20
Wang X Yuan X Wang Q Zhou Y Li X Wang G et al . Clinical Study of Effectiveness and Safety of Iguratimod in Treating Primary Sjogren’s Syndrome. Chin J Dis Control Prev (2018) 22(1):75–8. doi: 10.16462/j.cnki.zhjbkz.2018.01.017
21
Pu J Wang X Riaz F Zhang T Gao R Pan S et al . Effectiveness and Safety of Iguratimod in Treating Primary Sjögren's Syndrome: A Systematic Review and Meta-Analysis. Front Pharmacol (2021) 12:621208. doi: 10.3389/fphar.2021.621208
22
Seror R Baron G Camus M Cornec D Perrodeau E Bowman SJ et al . NECESSITY WP5 - STAR Development Working Group; NECESSITY WP5- STAR Development Working Group. Development and Preliminary Validation of the Sjögren's Tool for Assessing Response (STAR): A Consensual Composite Score for Assessing Treatment Effect in Primary Sjögren's Syndrome. Ann Rheum Dis (2022) 81: 979–989. doi: 10.1136/annrheumdis-2021-222054
23
Deeks JJ Higgins JP Altman DG . Chapter 8: Assessing Risk of Bias in Included Studies. In: HigginsJPGreenS, editors. Cochrane Handbook or Systematic Reviews of Interventions Version 5.1.0. UK: The Cochrane Collaboration (2020).
24
Deeks JJ Higgins JP Altman DG . Chapter 9: Analyzing Data and Undertaking Meta-Analyses. In: HigginsJPGreenS, editors. Cochrane Handbook for Systematic Reviews of Interventions. UK: The Cochrane Collaboration (2020).
25
Luo C Shi Y Chen X Wu L . Clinical Analysis of 46 Cases of Primary Sjogren’s Syndrome Treated by Ilamud. Chin J Pract Diagnosis Ther (2019) 33(12):1232–5. doi: 10.13507/j.issn.1674-3474.2019.12.024
26
Miao Y . Observation of the Changes of ESR, CRP, IgG, IgA, IgM Levels in Patients With Primary Sjogren’s Syndrome by Iramod. Electronic J Clin Med Literature (2018) 5(95):147+149.
27
Yu W . Clinical Analysis of Iramod in the Treatment of Primary Sjogren’s Syndrome. Guide to Chin Med (2020) 18(06):150. doi: CNKI:SUN:YYXK.0.2020-06-122
28
Gu J . Clinical Effect of Methylprednisolone Combined With Ilamod in the Treatment of Elderly Patients With Primary Sjogren’s Syndrome. Chin Natl Health Med (2020) 32(18):1–2+5. doi: 10.3969/j.issn.1672-0369.2020.18.001
29
Zhao L . Observation on the Effect of Methylprednisolone Combined With Iramud in the Treatment of Primary Sjogren’s Syndrome. Chin Natl Health Med (2019) 31(17):35–6. doi: CNKI:SUN:ZMYX.0.2019-17-015
30
Zhang X . Comparative Observation of Iguratimod and Hydroxychloroquine in the Treatment of Patients With Sjögren's Syndrome. Chin Med Guide (2019) 17(32):103. doi: 10.15912/j.cnki.gocm.2019.32.081
31
Zhao J . Comparison of the Efficacy and Safety of Iguratimod and Hydroxychloroquine in the Treatment of Sjögren's Syndrome. Chin Med Clin Med (2020) 20(21):3627–9. doi: 10.11655/zgywylc2020.21.040
32
Jia XQ . Analysis of the Effect of Iguratimod Combined With Hydroxychloroquine in the Treatment of Sjögren's Syndrome. Gansu Sci Technol (2020) 36(18):106–8.
33
Zhuang Z . To Explore the Effect of Iguratimod in the Treatment of Primary Sjögren's Syndrome. Special Health (2020) 31:100–1.
34
Wei D . Efficacy and Safety of Iguratimod and Hydroxychloroquine in the Treatment of Sjögren's Syndrome. Drugs and Clinical Practice (2019) 11:146–7. DOCTOR.
35
Gu J . Clinical Effect of Methylprednisolone Combined With Iguratimod in the Treatment of Primary Sjögren's Syndrome and its Influence on the Level of Immunoglobulin. Contemp Med (2022) 28(08):158–60. doi: 10.3969/j.issn.1009-4393.2022.08.057
36
Shao Q Wang S Jiang H Liu L . Efficacy and Safety of Iguratimod on Patients With Primary Sjögren's Syndrome: A Randomized, Placebo-Controlled Clinical Trial. Scand J Rheumatol (2020) 29:1–10. doi: 10.1080/03009742.2020.1809701
37
Wang Y Zhao F Ai X Liu Y Zhu Z . Observation on the Efficacy and Safety of Iramod in the Treatment of Primary Sjogren’s Syndrome in the Elderly. Geriatrics Health Care (2019) 25(02):209–13. doi: 10.3969/j.issn.1008-8296.2019.02.020
38
Xie H Liu Y Wang J Zeng C Zhou Y . Hydroxychloroquine Sulfate Combined With Total Glucosides of Paeony and Ilamod in the Treatment of Primary Sjogren’s Syndrome. Western Med (2020) 32(09):1358– 1362. doi: 10.3969/j.issn.1672-3511.2020.09.022
39
Chen Y Shen P Sun D . Analysis of Curative Effect of Iguratimod in the Treatment of Elderly Patients With pSS and its Effect on B Cell Activity and Secretion Level of Immunoglobulin G. Med Theory Pract (2022) 35(02):244–6. doi: 10.19381/j.issn.1001-7585.2022.02.025
40
Jiang W Zhang L Zhao Y He X Hu C Liu Y . The Efficacy and Mechanism for Action of Iguratimod in Primary Sjögren's Syndrome Patients. Int Ophthalmol (2020) 40(11):3059–65. doi: 10.1007/s10792-020-01490-6
41
Jiang D Bai Y Zhao L Zhang Y Chen Z . Observation on the Clinical Effect of Iramod Combined Treatment of Primary Sjogren’s Syndrome. Clin Misdiagnosis Mistreatment (2016) 29(08):90–3. doi: 10.3969/j.issn.1002-3429.2016.08.029
42
Jiang W Zhao Y Hui L Yi L Chen J . Observation on the Curative Effect of Iramod in the Treatment of Primary Sjogren’s Syndrome. Western Med (2014) 26(06):719–721 +724. doi: 10.3969/j.issn.1672-3511.2014.06.015
43
Bai J Jiao YW . Observation on the Clinical Effect of Iramod in the Treatment of Primary Sjogren’s Syndrome. Shanxi Med J (2019) 48(14):1724–6. doi: 10.3969/j.issn.1002-3429.2016.08.029
44
Li C Li R Liu H Cheng C Zhao T . Efficacy of Methylprednisolone Combined With Ilamod in the Treatment of Primary Sjogren’s Syndrome and its Effect on Immunoglobulin Levels. China Pharm (2018) 27( 14):35–7. doi: 10.3969/j.issn.1002-3429.2016.08.029
45
Li R Long H . Efficacy of Methylprednisolone Combined With Ilamod in the Treatment of Primary Sjogren’s Syndrome and its Effect on Immunoglobulin Levels. World Complex Med (2020) 6(05):189–91. doi: 10.11966/j.issn.2095-994X.2020.06.05.63
46
Xia Z Liu Y Meng F Fang Y Wu X Zhang C et al . Analysis of Clinical Effect of Iguratimod Combined Treatment on Primary Sjögren's Syndrome. China Health Nutr (2017) 27(034):263. doi: 10.3969/j.issn.1004-7484.2017.34.395
47
Xu D Lv X Cui P Ma S . Comparison of the Efficacy and Safety of Iguratimod and Hydroxychloroquine in the Treatment of Patients With Sjögren's Syndrome. J Difficult Difficult Dis (2017) 16(09):915–8. doi: 10.3969/j.issn.1671-6450.2017.09.013
48
Luo Q Guo D Yu Y Lin J . Efficacy and Safety of Iguratimod and Hydroxychloroquine in the Treatment of Patients With Sjögren's Syndrome. Clin Res Traditional Chin Med (2018) 10(24):94–5. doi: 10.3969/j.issn.1674-7860.2018.24.041
49
Zhang J Shen S . Efficacy and Mechanism of Iguratimod in the Treatment of Sjögren's Syndrome. Shaanxi Med J (2019) 048(004):452–5. doi: CNKI:SUN:SXYZ.0.2019-04-012
50
Zhang L Zhong M Li Q Guo X . Efficacy Evaluation of Iguratimod in the Treatment of Sjögren's Syndrome Complicated With Interstitial Pulmonary Disease. China Modern Med Appl (2019) 13(20):1–3. doi: 10.14164/j.cnki.cn11-5581/r.2019.20.001
51
Liang Z Wei F Ouyang C Feng M . Effects of Iguratimod on the Levels of ESR, CRP and Immunoglobulin in Patients With Primary Sjögren's Syndrome. Shanghai Med (2021) 42(01):32–5.
52
Rao Y Zhang W Lu T Xu J . Efficacy of Iguratimod in the Treatment of Sjögren's Syndrome Patients and its Influence on Immune Function. J Ningxia Med Univ (2022) 44(02):152–6. doi: 10.16050/j.cnki.issn1674-6309.2022.02.008
53
Lu T Zhang W . Clinical Efficacy of Iguratimod Combined With Hydroxychloroquine Sulfate in the Treatment of Primary Sjögren's Syndrome. J Clin Rational Med (2021) 14(31):55–7. doi: 10.15887/j.cnki.13-1389/r.2021.31.018
54
Jiang H . Clinical Observation of Shure Cunjin Granules Combined With Iguratimod in the Treatment of Primary Sjögren's Syndrome With High IgG[D]. Hunan University of Traditional Chin Med (2021). doi: 10.27138/d.cnki.ghuzc.2021.000018.(in chinese)
55
Jiang H Gao H Wang Q Wang M Wu B . Molecular Mechanisms and Clinical Application of Iguratimod: A Review. BioMed Pharmacother. (2020) 122:109704. doi: 10.1016/j.biopha.2019.109704
56
Chen H Qi X Li Y Wu Q Shi Q Wang L et al . Iguratimod Treatment Reduces Disease Activity in Early Primary Sjögren's Syndrome: An Open-Label Pilot Study. Mod Rheumatol (2021) 31(2):394–398. doi: 10.1080/14397595.2020.1789335
57
Shrestha S Zhao J Yang C et al . Relative Efficacy and Safety of Iguratimod Monotherapy for the Treatment of Patients With Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Clin Rheumatol (2020) 39(7):2139–50. doi: 10.1007/s10067-020-04986-9
58
Li J Mao H Liang Y Lu Y Chen S Yang N et al . Efficacy and Safety of Iguratimod for the Treatment of Rheumatoid Arthritis. Clin Dev Immunol (2013) 2013:310628. doi: 10.1155/2013/310628
59
Suto T Yonemoto Y Okamura K Sakane H Takeuchi K Tamura Y et al . The Three-Year Efficacy of Iguratimod in Clinical Daily Practice in Patients With Rheumatoid Arthritis. Mod Rheumatol (2019) 29(5):775–81. doi: 10.1080/14397595.2018.1510879
60
Wang X Ma C Li P Zhao F Bi L . Effects of Iguratimod on the Levels of Circulating Regulators of Bone Remodeling and Bone Remodeling Markers in Patients With Rheumatoid Arthritis. Clin Rheumatol (2017) 36(6):1369–77. doi: 10.1007/s10067-017-3668-8
61
Hara M Abe T Sugawara S Mizushima Y Hoshi K Irimajiri S et al . Long-Term Safety Study of Iguratimod in Patients With Rheumatoid Arthritis. Mod Rheumatol (2007) 17(1):10–6. doi: 10.3109/s10165-006-0543-x
62
Mimori T Harigai M Atsumi T Fujii T Kuwana M Matsuno H et al . Safety and Effectiveness of Iguratimod in Patients With Rheumatoid Arthritis: Final Report of a 52-Week, Multicenter Postmarketing Surveillance Study. Mod Rheumatol (2019) 29(2):314–23. doi: 10.1080/14397595.2018.1460230
63
Ishikawa K Ishikawa J . Iguratimod, a Synthetic Disease Modifying Anti-Rheumatic Drug Inhibiting the Activation of NF-κb and Production of RANKL: Its Efficacy, Radiographic Changes, Safety and Predictors Over Two Years' Treatment for Japanese Rheumatoid Arthritis Patients. Mod Rheumatol (2019) 29(3):418–29. doi: 10.1080/14397595.2018.1481565
64
Okamura K Yonemoto Y Suto T Okura C Takagishi K . Efficacy at 52 Weeks of Daily Clinical Use of Iguratimodin Patients With Rheumatoid Arthritis. Mod Rheumatol (2015) 25(4):534–9. doi: 10.3109/14397595.2014.998361
65
GRADEpro . GRADEpro Guideline Development Tool [Software]. McMaster University: Evidence Prime, Inc (2015).
66
Schünemann H Brożek J Guyatt G Oxman A eds. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. USA: The GRADE Working Group (2013).
Summary
Keywords
iguratimod, Primary Sjogren’s syndrome, systematic review, meta-analysis, randomized controlled trials
Citation
Zeng L, He Q, Yang K, Hao W, Yu G and Chen H (2022) A Systematic Review and Meta-Analysis of 19 Randomized Controlled Trials of Iguratimod Combined With Other Therapies for Sjogren’s Syndrome. Front. Immunol. 13:924730. doi: 10.3389/fimmu.2022.924730
Received
20 April 2022
Accepted
31 May 2022
Published
28 July 2022
Volume
13 - 2022
Edited by
Qiulun Lu, Nanjing Medical University, China
Reviewed by
Pankti Mehta, King George’s Medical University, India; Qi Zhou, Lanzhou University, China
Updates
Copyright
© 2022 Zeng, He, Yang, Hao, Yu and Chen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Chen, chhuaa332211@163.com; Liuting Zeng, zltab2016@hotmail.com; Qi He, heqi.ningxiang@outlook.com; Kailin Yang, yklab2014@hotmail.com
†These authors share first authorship
This article was submitted to Nutritional Immunology, a section of the journal Frontiers in Immunology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.