- 1Infectious Disease Epidemiology Group, Weill Cornell Medicine-Qatar, Cornell University, Doha, Qatar
- 2World Health Organization Collaborating Centre for Disease Epidemiology Analytics on HIV/AIDS, Sexually Transmitted Infections, and Viral Hepatitis, Weill Cornell Medicine–Qatar, Cornell University, Qatar Foundation – Education City, Doha, Qatar
- 3Department of Population Health Sciences, Weill Cornell Medicine, Cornell University, New York, NY, United States
- 4Department of Pathology, Sidra Medicine, Doha, Qatar
- 5Mathematics Program, Department of Mathematics, Statistics, and Physics, College of Arts and Sciences, Qatar University, Doha, Qatar
- 6Biomedical Research Center, QU Health, Qatar University, Doha, Qatar
- 7Department of Biomedical Science, College of Health Sciences, QU Health, Qatar University, Doha, Qatar
- 8Department of Public Health, College of Health Sciences, QU Health, Qatar University, Doha, Qatar
- 9Strategy Planning and Health Intelligence Primary Health Care Corporation, Doha, Qatar
- 10Hamad Medical Corporation, Doha, Qatar
- 11Wellcome-Wolfson Institute for Experimental Medicine, Queens University, Belfast, United Kingdom
- 12Department of Medicine, Weill Cornell Medicine, Cornell University, New York, NY, United States
- 13Ministry of Public Health, Doha, Qatar
In 2021, Qatar experienced considerable incidence of SARS-CoV-2 infection that was dominated sequentially by the Alpha, Beta, and Delta variants. Using the cycle threshold (Ct) value of an RT-qPCR-positive test to proxy the inverse of infectiousness, we investigated infectiousness of SARS-CoV-2 infections by variant, age, sex, vaccination status, prior infection status, and reason for testing in a random sample of 18,355 RT-qPCR-genotyped infections. Regression analyses were conducted to estimate associations with the Ct value of RT-qPCR-positive tests. Compared to Beta infections, Alpha and Delta infections demonstrated 2.56 higher Ct cycles (95% CI: 2.35-2.78), and 4.92 fewer cycles (95% CI: 4.67- 5.16), respectively. The Ct value declined gradually with age and was especially high for children <10 years of age, signifying lower infectiousness in small children. Children <10 years of age had 2.18 higher Ct cycles (95% CI: 1.88-2.48) than those 10-19 years of age. Compared to unvaccinated individuals, the Ct value was higher among individuals who had received one or two vaccine doses, but the Ct value decreased gradually with time since the second-dose vaccination. Ct value was 2.07 cycles higher (95% CI: 1.42-2.72) for those with a prior infection than those without prior infection. The Ct value was lowest among individuals tested because of symptoms and was highest among individuals tested as a travel requirement. Delta was substantially more infectious than Beta. Prior immunity, whether due to vaccination or prior infection, is associated with lower infectiousness of breakthrough infections, but infectiousness increases gradually with time since the second-dose vaccination.
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic continues with progressive viral evolution more than two years after it first emerged (1). Between January 18, 2021 and May 31, 2021, Qatar experienced a SARS-CoV-2 Alpha (2) (B.1.1.7) variant wave (3) that was immediately followed by a Beta (2) (B.1.351) variant wave (4). Starting in June 2021, the Delta (2) (B.1.617.2) variant dominated a prolonged low-incidence phase that persisted until November of 2021 (5–7). We investigated the effects of SARS-CoV-2 variant, age, vaccination, and prior infection on infectiousness of SARS-CoV-2 infections utilizing the threshold cycle (Ct) values from SARS-CoV-2 reverse transcription quantitative polymerase chain reaction (RT-qPCR) as the proxy for infectiousness.
In real-time PCR, the RT-qPCR Ct value refers to the number of amplification cycles at which fluorescent signals generated in a reaction crosses a pre-set, detection threshold. The higher the concentration of PCR targets in the reaction, the earlier the cycle it is detected and the lower the Ct value it generates (8). In RT-qPCR assays for detection of viruses such as SARS-CoV-2, Ct values are inversely proportional to the viral load, and are strongly correlated to the cultivability of the virus (9, 10). Therefore, RT-qPCR Ct values have been widely used to proxy SARS-CoV-2 infectiousness with lower Ct values indicating higher infectiousness and higher Ct values indicating lower infectiousness (10–15). Earlier studies found that lower Ct values (or higher viral loads) in samples of SARS-CoV-2 cases were associated with higher transmission rate and transmission risk, such as in studies of household transmission and among contacts of cases (11, 13). We assessed differences in Ct values in a random sample of 18,355 RT-qPCR-genotyped SARS-CoV-2 infections in relation to variant status, age, sex, vaccination status, prior infection status, and reason for testing.
Methods
Study population, data sources, and study design
This cross sectional study was conducted in the resident population of Qatar, applying a methodology used recently to investigate effects of the BA.1/BA.2 subvariant, vaccination, and prior infection on infectiousness of SARS-CoV-2 Omicron (2) (B.1.1.529) infections (16). Similarly, several effects on the infectiousness of SARS-CoV-2 infections were investigated including pre-Omicron variants (Alpha, Beta, and Delta), mRNA COVID-19 vaccination status [BNT162b2 (Pfizer-BioNTech) (17) and mRNA-1273 (Moderna) (18)], prior infection status, reason for RT-qPCR testing, study-period month of the RT-qPCR test (to account for the evolving phase of SARS-CoV-2 incidence), and demographic factors, including sex, age, and nationality.
The present study was conducted on a sample of 18,355 SARS-CoV-2 RT-qPCR-positive swabs that were collected randomly on a weekly basis from among all RT-qPCR-confirmed infections in Qatar between March 23, 2021 and November 6, 2021. These documented infections were RT-qPCR genotyped as part of a national project for surveillance of SARS-CoV-2 variants in Qatar (7, 19–21). Details of laboratory methods for RT-qPCR testing and variant ascertainment are provided in Section 1 of the Supplementary Material. Coronavirus disease 2019 (COVID-19) laboratory testing, vaccination, clinical infection, and demographic data for this population were extracted from the national, federated SARS-CoV-2 databases, which include all RT-qPCR testing, reason for RT-qPCR testing, COVID-19 vaccinations, and related demographic details since the start of the pandemic. Further description of Qatar’s national COVID-19 databases has been reported previously (4, 6, 7, 15, 22).
Every SARS-CoV-2 RT-qPCR test conducted in Qatar is classified based on the reason for testing (clinical symptoms, contact tracing, surveys or random testing campaigns, individual requests, routine healthcare testing, pre-travel, at port of entry, or other). RT-qPCR testing is performed at a mass scale and most infections are diagnosed not for appearance of symptoms, but because of routine testing (6). Qatar has unusually young, diverse demographics, in that only 9% of its residents are ≥50 years of age, and 89% are expatriates from over 150 countries (22, 23). Nearly all individuals were vaccinated in Qatar; however, vaccinations performed elsewhere were recorded in the health system at the port of entry upon arrival to Qatar, per national requirements.
For standardization of RT-qPCR Ct values, we analyzed only RT-qPCR-confirmed infections diagnosed with the TaqPath COVID-19 Combo Kit [Thermo Fisher Scientific, USA (24)]. For each individual, all RT-qPCR-positive swabs during the study period were included, provided that at least 90 days had elapsed between two consecutive positive swabs to avoid inclusion of positive results from the same infectious episode (25, 26). A summary measure was derived for the primary outcome, the RT-qPCR Ct value (9), by averaging Ct values of the N, ORF1ab, and S gene targets. This average Ct value was used as the dependent variable in all analyses.
Both vaccination status and prior infection status were ascertained at the time of the RT-qPCR test. Vaccination status was defined by the number of administered vaccine doses and months elapsed since the last vaccine dose, with one month defined as 30 days. Only individuals vaccinated with BNT162b2 (17) or mRNA-1273 (18) vaccines were included in the analyses, as these have been the vaccines of choice in the COVID-19 immunization program in Qatar (27–29). Rare occurrences of mixed vaccination regimens were excluded. Nearly all vaccinated persons received their second vaccine dose per protocol (17, 18) within 30 days of first dose. History of prior infection was defined as an RT-qPCR-positive test that occurred ≥90 days before the study RT-qPCR-positive test (4, 5, 30, 31). An RT-qPCR-positive test that occurred <90 days prior to the study RT-qPCR-positive test was not considered a prior infection, but was considered a category of its own. This is because the prior RT-qPCR-positive test and the study RT-qPCR-positive test may both reflect the same infection (25, 26). A small number of RT-qPCR tests that had no recorded Ct value were excluded from the analysis, but these constituted only 0.5% of all RT-qPCR-genotyped infections. Otherwise, data for the remaining study variables were complete.
Oversight
Hamad Medical Corporation and Weill Cornell Medicine-Qatar Institutional Review Boards approved this retrospective study with a waiver of informed consent. The study was reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. The STROBE checklist is found in Supplementary Table 1.
Statistical analysis
Frequency distributions and measures of central tendency were used to describe the study population with respect to a priori determined factors. These included SARS-CoV-2 variant, vaccination status (factoring dose number and months since vaccination), prior infection status, reason for RT-qPCR testing, study-period month of the RT-qPCR test, and demographic factors, age, sex, and nationality.
Association of each of these factors with Ct value was assessed using univariable linear regression analyses. Unadjusted β coefficients, 95% confidence intervals (CIs), and the F-test of overall covariate significance were reported. Adjusted β coefficients and associated 95% CIs and p-values were estimated using multivariable linear regression analyses that included all covariates in the model.
Two-sided p-value <0.05 indicated statistical significance. Interactions were not considered. Statistical analyses were conducted in STATA/SE version 16 (32).
Results
Figure 1 shows the process of selecting the study population and Table 1 describes study population characteristics. This was a national study involving a random sample of 18,355 RT-qPCR-confirmed SARS-CoV-2 infections. Therefore, the study population is broadly representative of the population of Qatar. The sample included 3,347 (18.2%) Alpha infections, 5,576 (30.4%) Beta infections, and 9,432 (51.4%) Delta infections (Table 1).
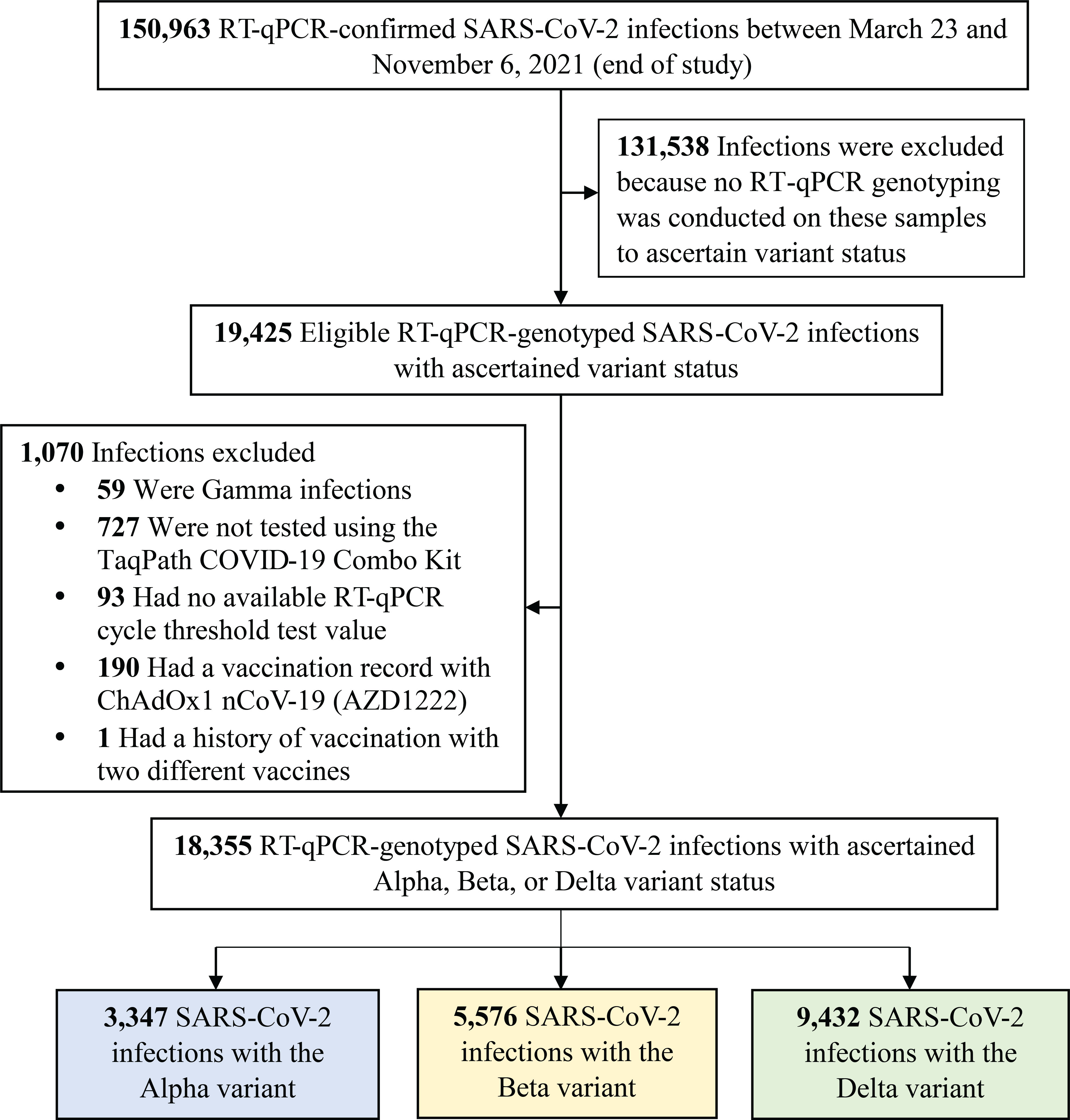
Figure 1 Flowchart describing the population selection process for investigating infectiousness of SARS-CoV-2 Alpha, Beta and Delta infections. Abbreviations: COVID-19, coronavirus disease 2019; RT-qPCR, real-time reverse-transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
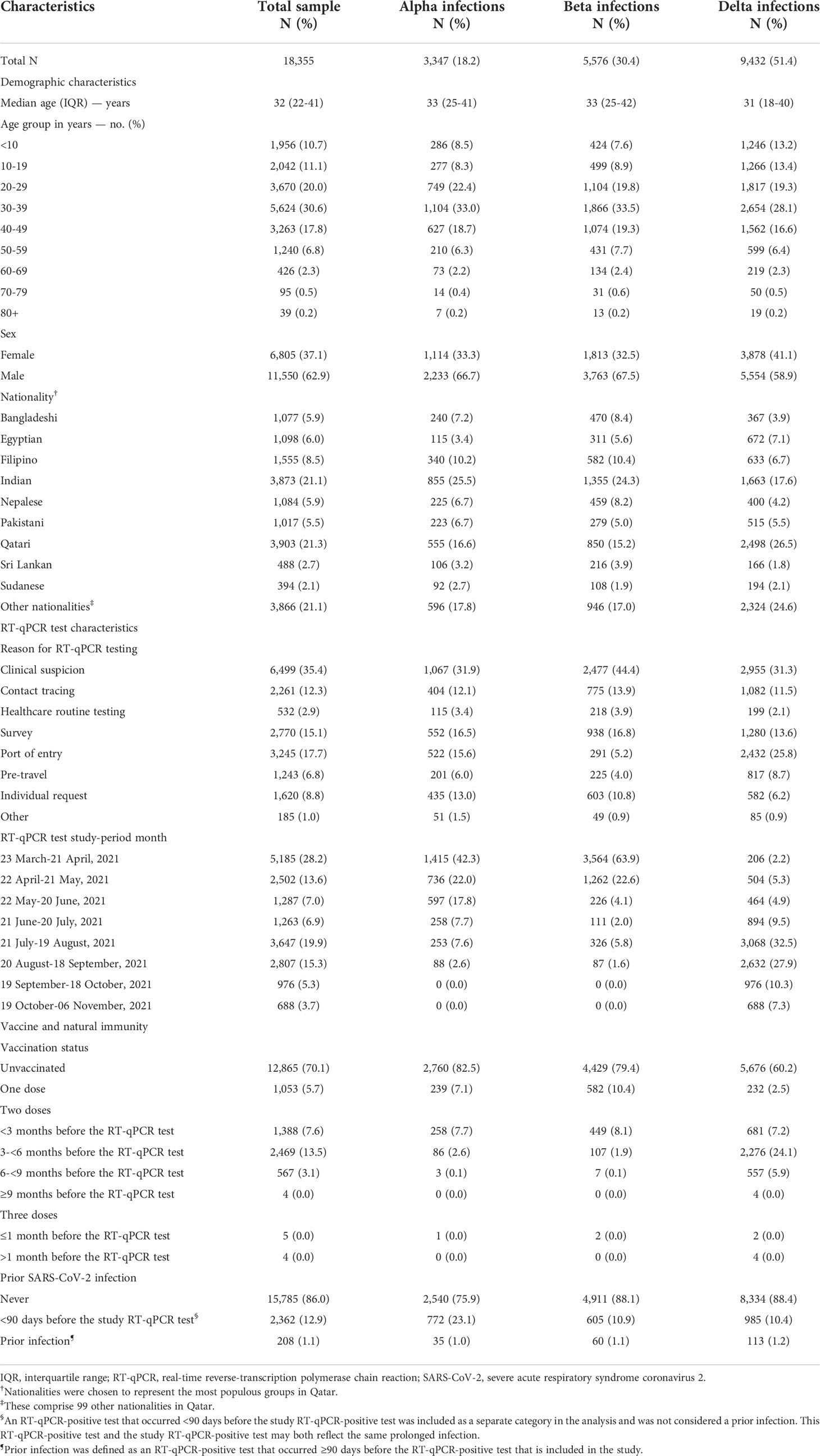
Table 1 Characteristics of the 18,355 RT-qPCR-genotyped SARS-CoV-2 infections between March 23 and November 6, 2021.
Compared to Beta infections, Alpha and Delta infections were associated with 2.56 higher Ct cycles (95% CI: 2.35-2.78), and 4.92 fewer cycles (95% CI: 4.67- 5.16) (Table 2), respectively, indicating the highest infectiousness for the Delta variant.
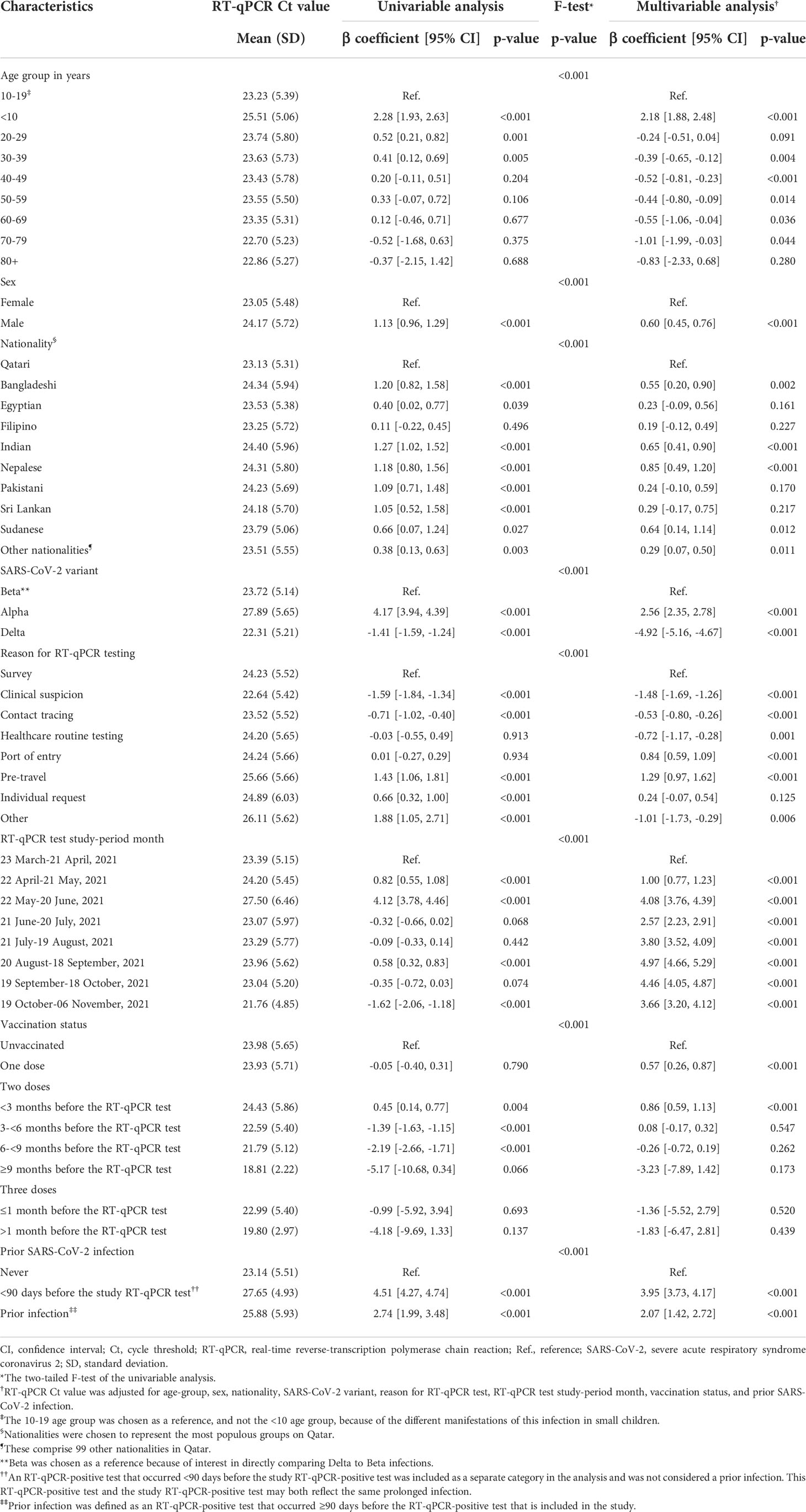
Table 2 Associations with RT-qPCR Ct value among the 18,355 RT-qPCR-genotyped SARS-CoV-2 infections between March 23 and November 6, 2021.
Ct value declined gradually with age and was especially high for children <10 years of age, signifying lower infectiousness of small children. Children <10 years of age had 2.18 higher Ct cycles (95% CI: 1.88-2.48) than those 10-19 years of age (Table 2). The 10-19 age group was chosen as a reference, and not the <10 age group, because of the different manifestations of this infection in small children. Males had higher Ct values than females and there were some differences in Ct value by nationality.
Compared to unvaccinated individuals, Ct value was higher among individuals who received one or two vaccine doses (Table 2). However, Ct value decreased gradually with time since second-dose vaccination. Very few individuals received a booster dose during the study period (Table 1) to allow for estimation of effect of booster vaccination on Ct value. Ct value was 2.07 cycles higher (95% CI: 1.42-2.72) for those with a prior infection compared to those without prior infection.
The Ct value was lowest when testing was performed due to suspicion of infection exposure (Table 2), such as appearance of symptoms or recent exposure to an infected person (contact tracing). The Ct value was highest for infections diagnosed because of routine testing for reasons unrelated to infection exposure, such as in a random survey or because of travel requirements. Stratified analyses for Alpha (Table 3), Beta (Table 4), and Delta (Table 5) infections suggested similar findings.
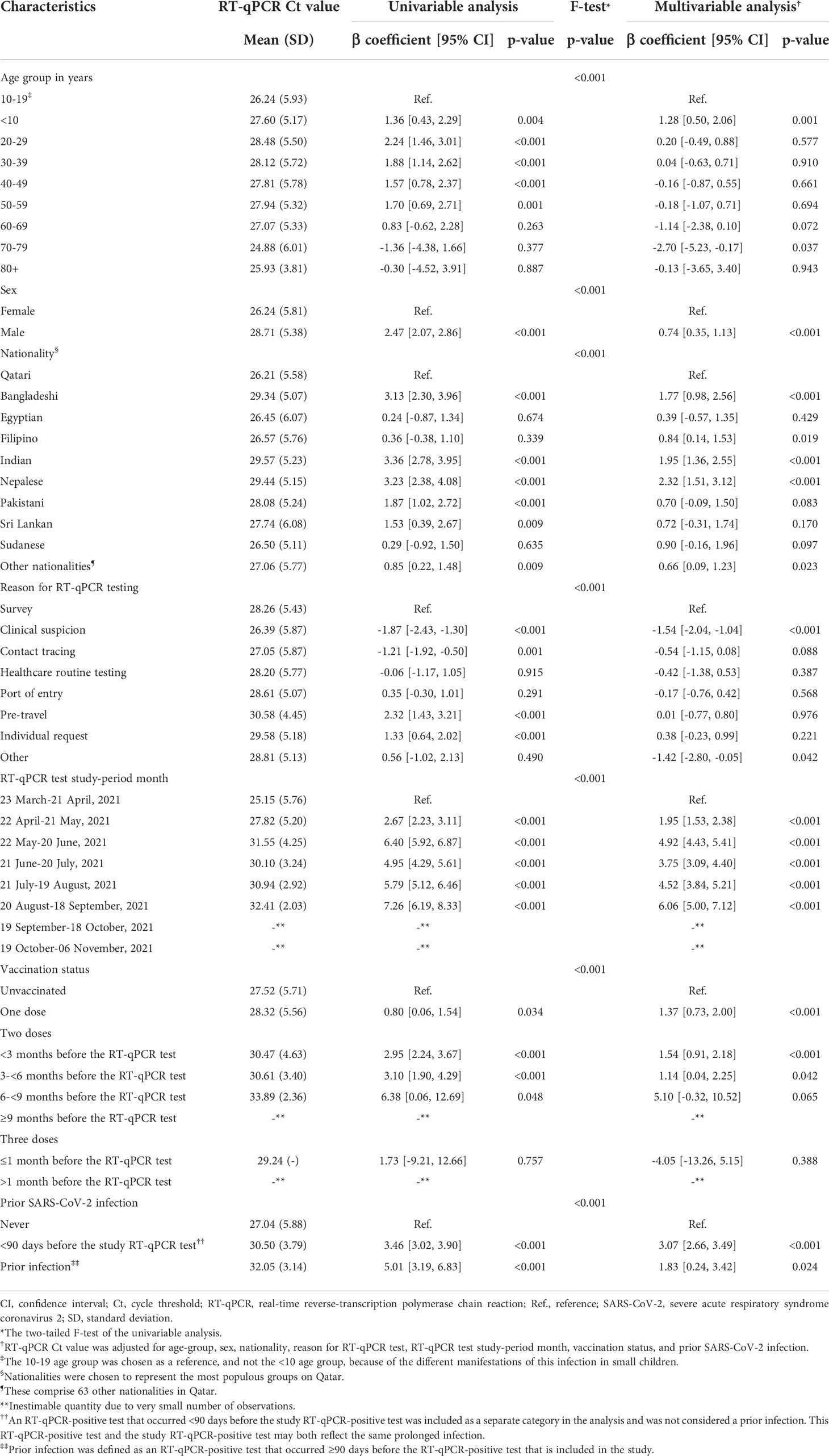
Table 3 Associations with RT-qPCR Ct value among 3,347 Alpha RT-qPCR-genotyped SARS-CoV-2 infections between March 23 and November 6, 2021.
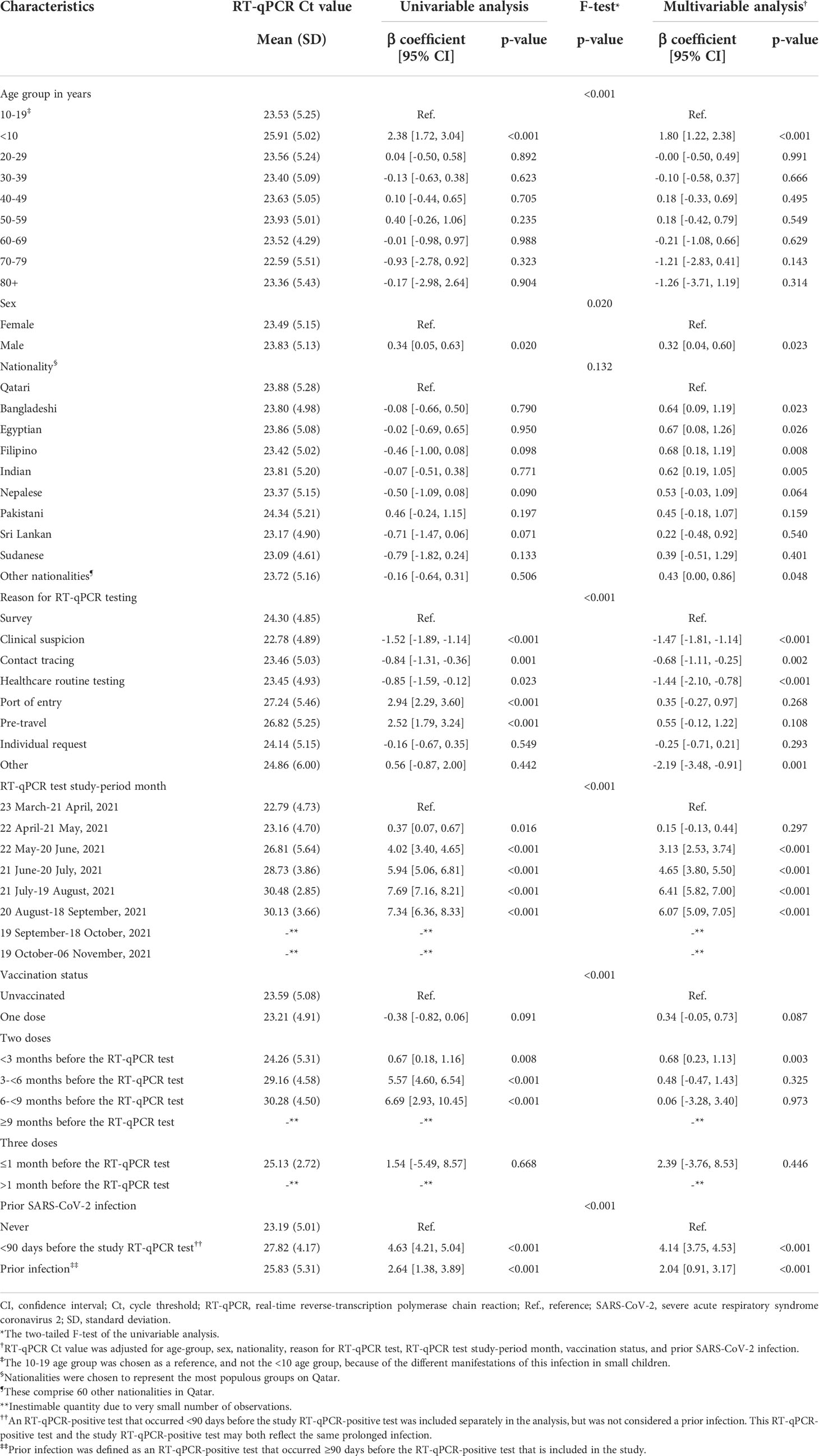
Table 4 Associations with RT-qPCR Ct value among 5,576 Beta RT-qPCR-genotyped SARS-CoV-2 infections between March 23 and November 6, 2021.
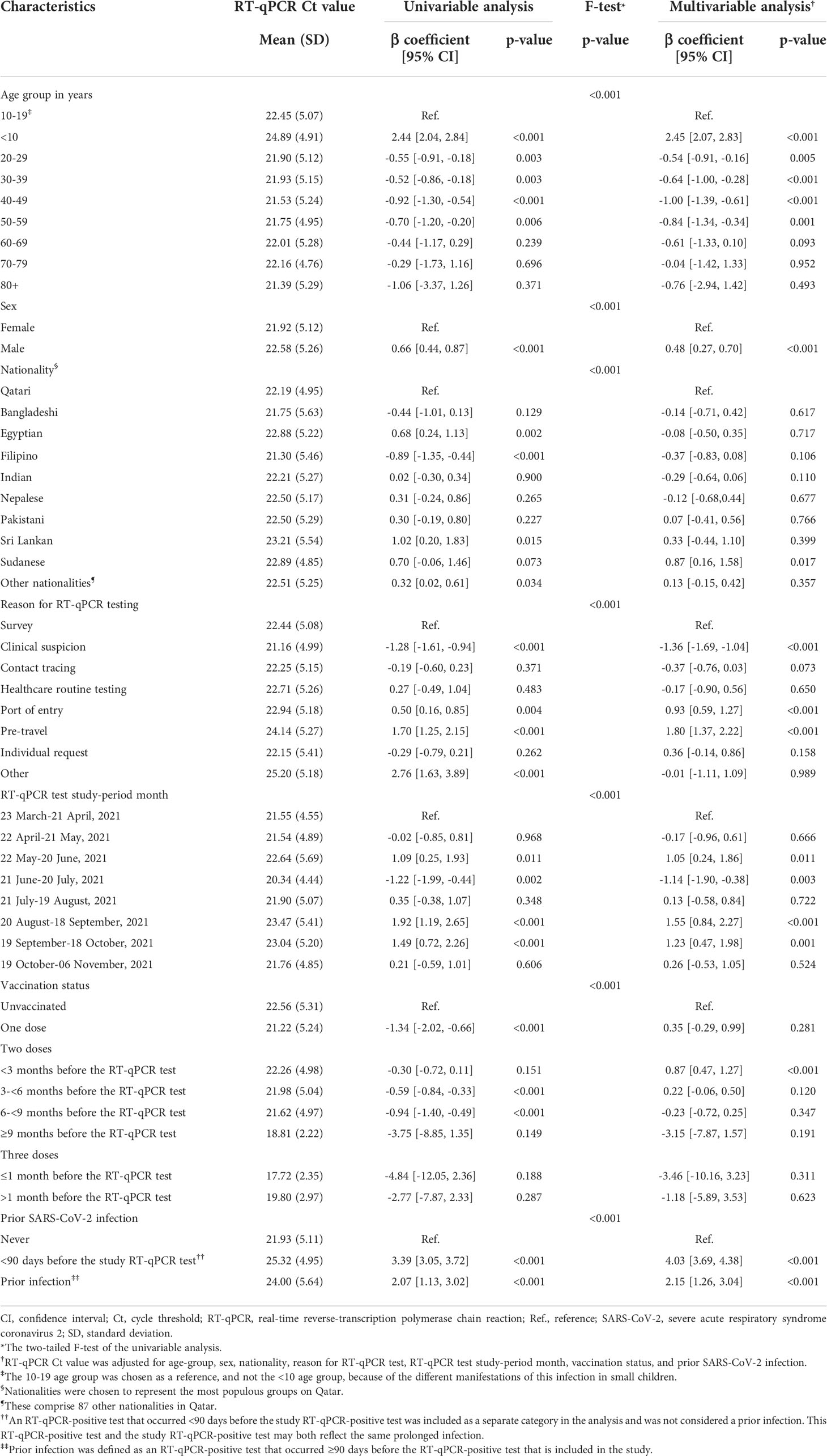
Table 5 Associations with RT-qPCR Ct value among 9,432 Delta RT-qPCR-genotyped SARS-CoV-2 infections between March 23 and November 6, 2021.
Discussion
Delta infections were associated with considerably lower Ct values than Beta infections, indicating higher infectiousness of this variant, perhaps because of higher viral load and/or longer duration of infection. This appears to be the first direct comparison of the infectiousness of Delta versus Beta infections, and supports the high infectiousness of the Delta variant compared to pre-Omicron variants such as Alpha, as reported previously (33, 34). This difference in viral load between Delta and Beta appears also to extend to severity of infection, as Delta infections were found associated with higher severity than Beta infections (35). Of note, Beta infections were also found earlier to be associated with higher severity than Alpha infections (36). Worth mentioning is that factors other than viral load, such as the variations in the spike proteins or any other differences in the biological properties of the viruses, may also affect the infectiousness of different SARS-CoV-2 variants (37).
Prior immunity against SARS-CoV-2 infection, whether due to vaccination or prior infection, was associated with higher Ct value at infection, and thus lower infectiousness of breakthrough infections. This confirms earlier findings (15, 16), and suggests that strength of immunity is manifest not only in protection against infection, but also against the infectiousness, if a breakthrough infection occurs (15). However, this effect appeared to depend on the time since the prior immunological event (Table 2). Ct values decreased gradually with time since second-dose vaccination, paralleling the established pattern of waning of vaccine effectiveness after the second dose (6, 38, 39).
Ct values decreased with age, perhaps reflecting slower virus clearance with aging (40) and confirming our earlier findings (16). Ct values were particularly high for infections among small children <10 years of age. This finding supports a lesser role for small children than adults in the transmission of infection, as suggested in studies of secondary transmission within households (41–43). There were differences in Ct value by sex and nationality, but these may be a consequence of different test-seeking behaviors for different socio-economic groups in Qatar’s diverse population. Ct values also varied by reason for testing, with lower Ct values of infections diagnosed because of suspicion of infection, and higher Ct values of infections diagnosed through routine testing unrelated to infection exposure. This finding also confirms our earlier finding for Omicron infections (16).
The study has limitations. RT-qPCR genotyping in Qatar started only after the Alpha wave peaked in the first week of March 2021 and thus many Alpha infections may have been older infections explaining the relatively higher Ct values of these infections compared to Delta or Beta infections. The study included only documented RT-qPCR-confirmed infections. It is possible that infections in those with a prior infection or those vaccinated are less likely to be diagnosed, perhaps because of minimal or no symptoms (15). Nevertheless, RT-qPCR testing in Qatar is done at a mass scale, where a significant proportion of the population is being tested weekly (6). The majority of infections are identified via routine testing and not because of appearance of symptoms (Table 1) (6).
Other limitations include the small number of individuals who received the booster dose by the end of the study precluding the estimation of effect of booster vaccination on Ct value and the inability to factor in the duration between symptom onset and RT-qPCR test (for symptomatic cases) as the date of symptom onset was not available. The study population consisted mostly of working-age adults; thus, the results may not be generalizable to the elderly.
In conclusion, the Delta variant appears substantially more infectious than the Beta variant, explaining its global reach in the pre-Omicron era. Infectiousness of SARS-CoV-2 infections increases with age, apparently reflecting slower virus clearance with aging. Prior immunity against SARS-CoV-2 infection, whether due to vaccination or prior infection, is associated with lower infectiousness of breakthrough infections. However, infectiousness of breakthrough infections increases gradually with time since second-dose vaccination, paralleling the waning of vaccine effectiveness after the second dose. These scientific insights enhance our understanding of the evolving epidemiology of the virus with implications for appropriate public health response against its different variants.
Data availability statement
The datasets presented in this article are not readily available because these are properties of the Qatar Ministry of Public Health that were provided to the researchers through a restricted-access agreement that prevents sharing the datasets with a third party or publicly. The data are available under restricted access for confidentiality. The raw data are protected and are not available due to data privacy laws. Aggregate data are available within the manuscript. Requests to access the datasets should be directed to Her Excellency the Minister of Public Health, https://www.moph.gov.qa/english/OurServices/eservices/Pages/Governmental-Health-Communication-Center.aspx.
Ethics statement
The studies involving human participants were reviewed and approved by Hamad Medical Corporation and Weill Cornell Medicine-Qatar Institutional Review Boards. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
SQ co-designed the study, performed the statistical analyses, and co-wrote the first draft of the article. PT and MH conducted the multiplex, RT-qPCR variant screening and viral genome sequencing. HC co-designed the study and supported the statistical analyses. LA-R conceived and co-designed the study, led the statistical analyses, and co-wrote the first draft of the article. All authors contributed to data collection and acquisition, database development, discussion and interpretation of the results, and to the writing of the manuscript. All authors have read and approved the final manuscript.
Acknowledgments
We acknowledge the many dedicated individuals at Hamad Medical Corporation, the Ministry of Public Health, the Primary Health Care Corporation, Qatar Biobank, Sidra Medicine, and Weill Cornell Medicine-Qatar for their diligent efforts and contributions to make this study possible. The authors are grateful for institutional salary support from the Biomedical Research Program and the Biostatistics, Epidemiology, and Biomathematics Research Core, both at Weill Cornell Medicine-Qatar, as well as for institutional salary support provided by the Ministry of Public Health, Hamad Medical Corporation, and Sidra Medicine. The authors are also grateful for the Qatar Genome Programme and Qatar University Biomedical Research Center for institutional support for the reagents needed for the viral genome sequencing. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the article. Statements made herein are solely the responsibility of the authors.
Conflict of interest
AB has received institutional grant funding from Gilead Sciences unrelated to the work presented in this paper.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.984784/full#supplementary-material
References
1. Subissi L, von Gottberg A, Thukral L, Worp N, Oude Munnink BB, Rathore S, et al. An early warning system for emerging sars-Cov-2 variants. Nat Med (2022):28:1110–5. doi: 10.1038/s41591-022-01836-w
2. World Health Organization. Tracking sars-Cov-2 variants. (2021). Available From: https://www.Who.Int/En/Activities/Tracking-Sars-Cov-2-Variants/.
3. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, Coyle P, Malek JA, Ahmed AA, et al. Introduction and expansion of the sars-Cov-2 B.1.1.7 variant and reinfections in Qatar: A nationally representative cohort study. PloS Med (2021) 18(12):e1003879. doi: 10.1371/journal.pmed.1003879
4. Chemaitelly H, Bertollini R, Abu-Raddad LJ. National study group for covid epidemiology. efficacy of natural immunity against sars-Cov-2 reinfection with the beta variant. N Engl J Med (2021) 385(27):2585–6. doi: 10.1056/NEJMc2110300
5. Altarawneh HN, Chemaitelly H, Hasan MR, Ayoub HH, Qassim S, AlMukdad S, et al. Protection against the omicron variant from previous sars-Cov-2 infection. N Engl J Med (2022) 386:1288–90. doi: 10.1056/NEJMc2200133
6. Chemaitelly H, Tang P, Hasan MR, AlMukdad S, Yassine HM, Benslimane FM, et al. Waning of Bnt162b2 vaccine protection against sars-Cov-2 infection in Qatar. N Engl J Med (2021) 385(24):e83. doi: 10.1056/NEJMoa2114114
7. Tang P, Hasan MR, Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, et al. Bnt162b2 and mrna-1273 covid-19 vaccine effectiveness against the sars-Cov-2 delta variant in Qatar. Nat Med (2021) 27(12):2136–43. doi: 10.1038/s41591-021-01583-4
8. Arya M, Shergill IS, Williamson M, Gommersall L, Arya N, Patel HR. Basic principles of real-time quantitative pcr. Expert Rev Mol Diagn (2005) 5(2):209–19. doi: 10.1586/14737159.5.2.209
9. Coyle PV, Al Molawi NH, Kacem MABH, El Kahlout RA, Al Kuwari E, Al Khal A, et al. Reporting of rt-pcr cycle threshold (Ct) values during the first wave of covid-19 in Qatar improved result interpretation in clinical and public health settings. J Med Microbiol (2022) 71(5). doi: 10.1099/jmm.0.001499
10. Singanayagam A, Patel M, Charlett A, Lopez Bernal J, Saliba V, Ellis J, et al. Duration of infectiousness and correlation with rt-pcr cycle threshold values in cases of covid-19, England, January to may 2020. Euro Surveill (2020) 25(32):2001483. doi: 10.2807/1560-7917.ES.2020.25.32.2001483
11. Lyngse FP, Mølbak K, Træholt Franck K, Nielsen C, Skov RL, Voldstedlund M, et al. Association between sars-Cov-2 transmissibility, viral load, and age in households. medRxiv(2021) 2021.02.28.21252608. doi: 10.1101/2021.02.28.21252608
12. Lee LYW, Rozmanowski S, Pang M, Charlett A, Anderson C, Hughes GJ, et al. Sars-Cov-2 infectivity by viral load, s gene variants and demographic factors and the utility of lateral flow devices to prevent transmission. Clin Infect Dis (2021) 74:407–15. doi: 10.1093/cid/ciab421
13. Marks M, Millat-Martinez P, Ouchi D, Roberts CH, Alemany A, Corbacho-Monné M, et al. Transmission of covid-19 in 282 clusters in Catalonia, Spain: A cohort study. Lancet Infect Dis (2021) 21(5):629–36. doi: 10.1016/s1473-3099(20)30985-3
14. Bullard J, Dust K, Funk D, Strong JE, Alexander D, Garnett L, et al. Predicting infectious severe acute respiratory syndrome coronavirus 2 from diagnostic samples. Clin Infect Dis (2020) 71(10):2663–6. doi: 10.1093/cid/ciaa638
15. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, Tang P, Coyle P, Hasan MR, et al. Relative infectiousness of sars-Cov-2 vaccine breakthrough infections, reinfections, and primary infections. Nat Commun (2022) 13(1):532. doi: 10.1038/s41467-022-28199-7
16. Qassim SH, Chemaitelly H, Ayoub HH, AlMukdad S, Tang P, Hasan MR, et al. Effects of Ba.1/Ba.2 subvariant, vaccination, and prior infection on infectiousness of sars-Cov-2 omicron infections. J Travel Med (2022). doi: 10.1093/jtm/taac068
17. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the Bnt162b2 mrna covid-19 vaccine. N Engl J Med (2020) 383:2603–15. doi: 10.1056/NEJMoa2034577
18. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mrna-1273 sars-Cov-2 vaccine. N Engl J Med (2021) 384(5):403–16. doi: 10.1056/NEJMoa2035389
19. . National project of surveillance for variants of concern and viral genome sequencing. Qatar viral genome sequencing data. data on randomly collected samples (2021). Available from: https://www.gisaid.org/phylodynamics/global/nextstrain/.
20. Benslimane FM, Al Khatib HA, Al-Jamal O, Albatesh D, Boughattas S, Ahmed AA, et al. One year of sars-Cov-2: Genomic characterization of covid-19 outbreak in Qatar. Front Cell Infect Microbiol (2021) 11:768883. doi: 10.3389/fcimb.2021.768883
21. Hasan MR, Kalikiri MKR, Mirza F, Sundararaju S, Sharma A, Xaba T, et al. Real-time sars-Cov-2 genotyping by high-throughput multiplex pcr reveals the epidemiology of the variants of concern in Qatar. Int J Infect Dis (2021) 112:52–4. doi: 10.1016/j.ijid.2021.09.006
22. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, Al Kanaani Z, Al Khal A, Al Kuwari E, et al. Characterizing the Qatar advanced-phase sars-Cov-2 epidemic. Sci Rep (2021) 11(1):6233. doi: 10.1038/s41598-021-85428-7
23. Planning and statistics authority-state of qatar. Qatar monthly statistics (2020). Available at: https://www.psa.gov.qa/en/pages/default.aspx (Accessed May 26, 2020).
24. Thermo Fisher Scientific. Taqpath™ Covid−19 Ce−Ivd Rt−Pcr kit instructions for use (2020). Available at: https://assets.thermofisher.com/tfs-assets/lsg/manuals/man0019215_taqpathcovid-19_ce-ivd_rt-pcr%20kit_ifu.pdf (Accessed December 02, 2020).
25. Choi B, Choudhary MC, Regan J, Sparks JA, Padera RF, Qiu X, et al. Persistence and evolution of sars-Cov-2 in an immunocompromised host. N Engl J Med (2020) 383(23):2291–3. doi: 10.1056/NEJMc2031364
26. Abu-Raddad LJ, Chemaitelly H, Malek JA, Ahmed AA, Mohamoud YA, Younuskunju S, et al. Two prolonged viremic sars-Cov-2 infections with conserved viral genome for two months. Infect Genet Evol (2021) 88:104684. doi: 10.1016/j.meegid.2020.104684
27. Abu-Raddad LJ, Chemaitelly H, Butt AA, National Study Group for Covid Vaccination. Effectiveness of the Bnt162b2 covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med (2021) 385(2):187–9. doi: 10.1056/NEJMc2104974
28. Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, Tang P, Hasan MR, et al. Mrna-1273 covid-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe covid-19 disease in Qatar. Nat Med (2021) 27(9):1614–21. doi: 10.1038/s41591-021-01446-y
29. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, Yassine HM, Benslimane FM, Al Khatib HA, et al. Association of prior sars-Cov-2 infection with risk of breakthrough infection following mrna vaccination in Qatar. JAMA (2021) 326(19):1930–9. doi: 10.1001/jama.2021.19623
30. Kojima N, Shrestha NK, Klausner JD. A systematic review of the protective effect of prior sars-Cov-2 infection on repeat infection. Eval Health Prof (2021) 44(4):327–32. doi: 10.1177/01632787211047932
31. Pilz S, Theiler-Schwetz V, Trummer C, Krause R, Ioannidis JPA. Sars-Cov-2 reinfections: Overview of efficacy and duration of natural and hybrid immunity. Environ Res (2022) 209:112911. doi: 10.1016/j.envres.2022.112911
33. Luo CH, Morris CP, Sachithanandham J, Amadi A, Gaston DC, Li M, et al. Infection with the sars-Cov-2 delta variant is associated with higher recovery of infectious virus compared to the alpha variant in both unvaccinated and vaccinated individuals(2021) (Accessed ciab986).
34. Wang Y, Chen R, Hu F, Lan Y, Yang Z, Zhan C, et al. Transmission, viral kinetics and clinical characteristics of the emergent sars-Cov-2 delta voc in guangzhou, China. EClinicalMedicine (2021) 40:101129. doi: 10.1016/j.eclinm.2021.101129
35. Butt AA, Dargham SR, Chemaitelly H, Al Khal A, Tang P, Hasan MR, et al. Severity of illness in persons infected with the sars-Cov-2 delta variant vs beta variant in Qatar. JAMA Intern Med (2022) 182(2):197–205. doi: 10.1001/jamainternmed.2021.7949
36. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, Yassine HM, Benslimane FM, Al Khatib HA, et al. Severity, criticality, and fatality of the sars-Cov-2 beta variant. Clin Infect Dis (2021) 75:e1188–91. doi: 10.1093/cid/ciab909
37. Motozono C, Toyoda M, Zahradnik J, Saito A, Nasser H, Tan TS, et al. Sars-Cov-2 spike L452r variant evades cellular immunity and increases infectivity. Cell Host Microbe (2021) 29(7):1124–36.e11. doi: 10.1016/j.chom.2021.06.006
38. Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, et al. Duration of effectiveness of vaccines against sars-Cov-2 infection and covid-19 disease: Results of a systematic review and meta-regression. Lancet (2022) 399(10328):924–44. doi: 10.1016/S0140-6736(22)00152-0
39. Abu-Raddad LJ, Chemaitelly H, Bertollini R, National Study Group for Covid Vaccination. Waning mrna-1273 vaccine effectiveness against sars-Cov-2 infection in Qatar. N Engl J Med (2022) 386:1091–93. doi: 10.1056/NEJMc2119432
40. Hansen CH, Michlmayr D, Gubbels SM, Molbak K, Ethelberg S. Assessment of protection against reinfection with sars-Cov-2 among 4 million pcr-tested individuals in Denmark in 2020: A population-level observational study. Lancet (2021) 397(10280):1204–12. doi: 10.1016/S0140-6736(21)00575-4
41. Zhu Y, Bloxham CJ, Hulme KD, Sinclair JE, Tong ZWM, Steele LE, et al. A meta-analysis on the role of children in severe acute respiratory syndrome coronavirus 2 in household transmission clusters. Clin Infect Dis (2021) 72(12):e1146–e53. doi: 10.1093/cid/ciaa1825
42. Davies NG, Klepac P, Liu Y, Prem K, Jit M, Group CC-W, et al. Age-dependent effects in the transmission and control of covid-19 epidemics. Nat Med (2020) 26:1205–11. doi: 10.1038/s41591-020-0962-9
Keywords: COVID-19, SARS-CoV-2 variant, vaccine, reinfection, breakthrough infection, immunity, epidemiology, PCR
Citation: Qassim SH, Hasan MR, Tang P, Chemaitelly H, Ayoub HH, Yassine HM, Al-Khatib HA, Smatti MK, Abdul-Rahim HF, Nasrallah GK, Al-Kuwari MG, Al-Khal A, Coyle P, Gillani I, Kaleeckal AH, Shaik RM, Latif AN, Al-Kuwari E, Jeremijenko A, Butt AA, Bertollini R, Al-Romaihi HE, Al-Thani MH and Abu-Raddad LJ (2022) Effects of SARS-CoV-2 Alpha, Beta, and Delta variants, age, vaccination, and prior infection on infectiousness of SARS-CoV-2 infections. Front. Immunol. 13:984784. doi: 10.3389/fimmu.2022.984784
Received: 02 July 2022; Accepted: 22 August 2022;
Published: 13 September 2022.
Edited by:
Herb Schellhorn, McMaster University, CanadaReviewed by:
Aminu S. Jahun, University of Cambridge, United KingdomNico Nagelkerke, United Arab Emirates University, United Arab Emirates
Copyright © 2022 Qassim, Hasan, Tang, Chemaitelly, Ayoub, Yassine, Al-Khatib, Smatti, Abdul-Rahim, Nasrallah, Al-Kuwari, Al-Khal, Coyle, Gillani, Kaleeckal, Shaik, Latif, Al-Kuwari, Jeremijenko, Butt, Bertollini, Al-Romaihi, Al-Thani and Abu-Raddad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laith J. Abu-Raddad, bGphMjAwMkBxYXRhci1tZWQuY29ybmVsbC5lZHU=
†These authors have contributed equally to this work
 Suelen H. Qassim1,2,3†
Suelen H. Qassim1,2,3† Patrick Tang
Patrick Tang Hadi M. Yassine
Hadi M. Yassine Hebah A. Al-Khatib
Hebah A. Al-Khatib Maria K. Smatti
Maria K. Smatti Hanan F. Abdul-Rahim
Hanan F. Abdul-Rahim Gheyath K. Nasrallah
Gheyath K. Nasrallah Mohamed Ghaith Al-Kuwari
Mohamed Ghaith Al-Kuwari Andrew Jeremijenko
Andrew Jeremijenko Adeel A. Butt
Adeel A. Butt Laith J. Abu-Raddad
Laith J. Abu-Raddad