Abstract
The gut microbiome is a heterogeneous population of microbes comprising viruses, bacteria, fungi, and protozoa. Such a microbiome is essential for sustaining host equilibrium, and its impact on human health can be altered by a variety of factors such as external variables, social behavior, age, nutrition, and genetics. Gut microbes’ imbalances are related to a variety of chronic diseases including cancer, obesity, and digestive disorders. Globally, recent findings show that intestinal microbes have a significant role in the formation of cardiovascular disease (CVD), which is still the primary cause of fatalities. Atherosclerosis, hypertension, diabetes, inflammation, and some inherited variables are all cardiovascular risk variables. However, studies found correlations between metabolism, intestinal flora, and dietary intake. Variations in the diversity of gut microbes and changes in their activity are thought to influence CVD etiology. Furthermore, the gut microbiota acts as an endocrine organ, producing bioactive metabolites such as TMA (trimethylamine)/TMAO (trimethylamine N-oxide), SCFA (short-chain fatty acids), and bile acids, which have a substantial impact on host wellness and disease by multiple mechanisms. The purpose of this overview is to compile current evidence highlighting the intricate links between gut microbiota, metabolites, and the development of CVD. It focuses on how intestinal dysbiosis promotes CVD risk factors such as heart failure, hypertension, and atherosclerosis. This review explores the normal physiology of intestinal microbes and potential techniques for targeting gut bacteria for CVD treatment using various microbial metabolites. It also examines the significance of gut bacteria in disease treatment, including supplements, prebiotics, probiotics, antibiotic therapies, and fecal transplantation, which is an innovative approach to the management of CVD. As a result, gut bacteria and metabolic pathways become increasingly attractive as potential targets for CVD intervention.
The significance of microbial-mediated metabolites in the emergence of CVD. CVD, cardiovascular disease; SCFAs, short-chain fatty acids; TMA, trimethylamine; TMAO, trimethylamine N-oxide; LPS, lipopolysaccharides.
Introduction
Understanding the evolution of the gut microbiota and its internal and external impacts on the intestine, as well as the risk factors for cardiovascular diseases (CVDs), such as metabolic syndrome, has attracted a great deal of attention (1, 2). Globally, CVDs are the primary causes of death, encompassing conditions including coronary artery disease (CAD), atherosclerosis, thrombosis, aneurysms, arterial hypertension, and cardiomyopathies that reinforce heart failure and cerebrovascular diseases (3–5). The current studies predict 17.5 million deaths per year by CVD, accounting for around 31% of all overall mortality (6). Among them, heart attack and stroke are directly linked to 85% of the cases (7, 8). Inflammation, dyslipidemia (i.e., elevated serum cholesterol, triglycerides, and low-density lipoproteins), and diabetes mellitus are prevalent pathological mechanisms and risk factors that can impact the progression and emergence of CVD (9, 10). In addition to hereditary factors, environmental factors such as nutrition and gut microbiota composition are going to play a significant influence in the development of CVDs. Furthermore, the rise of obesity and diabetes has been related to intestinal dysbiosis (11, 12), insulin resistance, and sedentary behaviors such as smoking, insufficient exercise, and poor nutrition are all identified risk variables for CVD (13, 14). The research into how the human gut microbiome affects CVD and metabolic diseases has expanded dramatically (15). Gut dysbiosis is a condition defined by changes in intestinal bacteria in adults, can be induced by a range of events such as dietary choices, environmental effects, intestinal infections, or external variables, and it can result in inflammation and metabolic disorders (16). The human gut microbiome comprises an array of over 10 trillion diverse microbes, encompassing bacteria, viruses, protozoa, methanogen archaea, and fungi. This collective term, microbiota, is synonymous with the entirety of these microbial inhabitants residing within the human body (17), while Actinobacteria, Firmicutes, Proteobacteria, and Bacteroides are the four major bacterial genera that comprise a healthy microbiota (18, 19). Before birth, an infant’s gut has very few germs (20), but after birth, the body begins to receive a steady stream of stimulation from the environment. It promotes a gradual rise in the number of bacteria in the colon, eventually leading to the formation of a dynamic and balanced balance in the gut microbiota (21).
The intestinal mucosal surface serves as the interface between the gut microbiota and performs a number of tasks that keep the intestinal epithelial barrier functioning (22). Endotoxins, microbes, and their byproducts can move more easily through the gut wall and into the bloodstream, where they can cause autoimmune disorders. Immune dysregulation and inflammation are at the basis of many CVDs, including atherosclerosis, myocardial infarction (MI), rhythm disorders, pericardial disease, cardiomyopathies, and heart failure (23). Moreover, it is important to highlight that the intestinal tract can be seen as an extensive and diverse ecosystem that produces a significant number of microbial metabolites (24). The host food is broken down by the gut flora into a variety of metabolically active products, including trimethylamine N-Oxide (TMAO), short-chain fatty acids (SCFAs), primary and secondary bile acids, tryptophan and indole derivatives, phenylacetylglutamine (PAGln), and branched-chain amino acids (BCAA), these may contribute to the CVD progression (25). Moreover, N-oxide TMA/TMAO and bile acids are fascinating biomarkers for CVD progression (26), however other gut microbiota components or similar chemicals should be investigated for use as early CVD markers (11). The loss of SCFA-producing intestinal microbes would disrupt the equilibrium of glucose metabolism, raising the risk of CVD (27). Trimethylamine stimulates macrophage stimulation, producing vascular damage; excessive TMAO levels owing to intestinal dysbiosis promote atherosclerosis, raise the risk of CAD, and hasten arterial plaque development that leads to cardiovascular disease (6). As shown in Figure 1, reducing dietary TMAO precursor intake is a promising strategy for lowering the risk of CVD due to the high amounts of trimethylamine (TMA) and TMAO generation by choline-induced gut flora (28, 29). Microbial sequencing analysis has emerged as a valuable tool for uncovering distinct gut microbiota patterns linked to cardiovascular disease CVD (30–32).
Figure 1

A diagram depicting the effect of gut bacteria and metabolites on CVD risk factors. SCFAs, short-chain fatty acids; TMAO, trimethylamine N-oxide.
The gut microbiota plays a crucial role in influencing overall health, either through direct mechanisms or indirect pathways. The intricate interactions involving variations in microbiome composition, metabolites, and CVD susceptibility underscore the significance of intestinal microbes as a novel modulator of CVD. The identified association between gut microbes and CVD suggests that modifying the intestinal microbiota could be beneficial in preventing and managing the development of CVD. Nutritional therapy, the use of pre/probiotics and antibiotics, fecal microbiota transplantation, TMAO reduction, and regular exercise are all current ways to manage gut bacteria to improve cardiovascular function (11). The latest study highlights the possible importance of microbial imbalance in CVD disorders. The advent of genomic and metabolomic technologies has allowed for more thorough characterization and molecular research of these microbiota and their metabolites. However, most evidence continues to indicate associations and the particular chemical processes driving a majority of visible events remain unidentified (33). Future studies focusing on microbe-microbe and microbe-host interactions could reveal how specific metabolites influence the disease process. It is also critical to have a better understanding of the bacterial mechanisms involved in the production of CVD-related metabolites, and also their functional roles. These results could provide a solid theoretical basis for the invention of therapeutic methods for CVD individuals. The present paper covers the usual composition and functional significance of intestinal bacteria and also provides new insights into the gut microbiota and its linked metabolites, which are implicated in CVDs. Scientific studies, putative biological explanations, and therapeutic outcomes are of significant interest to researchers. In addition, we discuss studies relating the gut microbiota to inflammatory processes, lipid metabolic disorders, and diabetes, all of which are linked to an elevated risk of cardiovascular disease. As a result, this overview focuses primarily on studying the role of gut microbiota-related metabolites and their therapeutic potential in CVDs, which may eventually provide more insight into the development of CVD prevention.
Gut microbiota and TMAO metabolite
The intestinal Bacteroidetes are one of the most significant bacterial colonies in the gastrointestinal microbiota. Despite their wide species composition, these cultures display stability in many gut regions, and some exhibit location-specific differentiation, mainly in the ascending colon (34). A total of around 1,000 different species of intestinal microbes, comprising about 1014, and bacterial-to-human cell ratio varied between 10:1 and 1:1 (35). In cardiovascular patients, more than 90% of these bacteria had an impact on the growth of Bacteroidetes and Firmicutes, keeping a stable Firmicutes/Bacteroidetes (F/B) ratio (36). The emergence of CVD is dependent on a compromised mucosal barrier and decreased intestinal mucosal barrier function, and is mostly caused by gram-negative microbes, such as lipopolysaccharide (LPS), which plays a significant role in the emergence of cardiometabolic diseases (37, 38). A high-fat diet has been shown to reduce gram-positive Bifidobacteria levels in the digestive system while increasing the amount of intestinal microbes that hold LPS, both of which contribute to obesity, the primary risk factor for CVD (39). The F/B ratio become a crucial role in the context of obesity, particularly in children (40). This ratio is linked to low-grade inflammation, which increases the probability of diabetes, a known risk factor for CVDs (41).
Because the gut acts as a bridge between them, the interaction between the host and the gut microbiota is crucial for preserving intestinal integrity. Several microbial metabolites have been linked to CVD (25) including bile acids, SCFAs, branched-chain amino acids, TMAO, tryptophan, and indole derivatives (42). The TMAO is formed when foods rich in choline, lecithin, and L-carnitine, primarily found in animal products, with limited plant-based sources, are ingested. In the gut, lecithin (comprising phosphatidylcholine, a choline source) and dietary choline are metabolized into TMA by the gut microbiota having specialized enzymes TMA lyses transcribed by cutC/cutD genes found in various bacterial strains. Recent data suggests that elevated circulating levels of TMAO are associated with an increased risk of CVD and mortality (43–46). Increased TMAO levels in the bloodstream encourage lipid accumulation in the arteries, which contributes to atherosclerosis. Figure 2 depicts how the inflammatory response influences the development of glucose intolerance, diabetes, and CVD (47–50). A dysbiotic microbe was found to decrease the amount of cholesterol eliminated by feces while increasing absorption and plasma levels of low-density lipoproteins, signaling that dysbiosis may increase the risk of atherosclerosis and CVD (39).
Figure 2

The gut microbiota of the target body’s functioning mechanisms. A low-fiber diet corresponds with decreased short-chain fatty acid butyrate formation, exacerbating dysbiosis and sustaining local and systemic inflammation via bacterial toxin leaks, most notably LPS. A modern Western diet strong in red meat promotes the synthesis of TMA by bacteria, which is then oxidized in the liver to the pro-atherosclerotic metabolite. CVD, cardiovascular disease; TMA, trimethylamine; TMAO, trimethylamine N-oxide.
Gut microbiota composition, diversity, and risk factors
The bacterial composition, diversity, and abundance are highly influenced by genetic changes in the host’s genome, and by external variables such as the host’s lifestyle, diet, sanitation, health, and the use of antibiotics and probiotics (51). The gut microbiota has small genetic differences in various parts of the intestine. Eckburg et al., (24) used metagenomic analysis to discover the gut bacterial community is made up of six phyla: Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Fusobacteria, and Verrucomicrobia, with the majority of the organisms in a healthy bacterial community being anaerobic population, as shown in Figure 3 (52). They inhabit unique biological niches on mucosal surfaces and in the gut lumen, where they form sophisticated biochemical interaction networks with both their hosts and with others (53). The synergistic interaction between the host species and the gut microbiome fosters the proliferation of beneficial microbes while inhibiting the growth of harmful bacteria (54). The gut flora regulates many bodily functions, such as providing metabolic fuel to the host, supporting growth and immune system regulation, removing harmful microbes, keeping intestinal wall integrity, and maintaining overall homeostasis (55). Microbial life has a significant impact on immune function and metabolism, with well-balanced gut microbiota playing an important role in the health of the host. (56). Inadequate dietary intake, excessive stress, significant life events, and antibiotic administration can all impact the diversity of the gut microbiota, leading to a disorder called dysbiosis (20). Elie Metchnikoff, a Russian immunologist and microbiologist renowned for his contributions to the understanding of the immune system, particularly the concept of phagocytosis, is not credited with coining the term “dysbiosis” (57, 58). An imbalance in the typical microbial composition (microbiota) of the colon or other bodily regions is described by this term, which is used in the current discipline of microbiology (59). In particular, Metchnikoff’s study has little work with the current concept of dysbiosis, which emerged as knowledge of the function of the human microbiome in health has increased (60). As seen in Figure 3, several risk factors were put out as potential causes of intestinal dysbiosis. There is a lot of literature known about the use of antibiotics, which has been seen to alter the composition of the gut’s microbiome and have both short-term and long-term effects (61–64). Obesity and high-fat and sugar meals are all related to persistent variations in the gut microbiota (65–68).
Figure 3

Gut microbiota composition (A) and diversity and Dysbiosis risk factors (B).
It is believed that external factors at various stages of life influence the formation of gut dysbiosis. The style of delivery, type of feeding, and hospital milieu are all related to the diversity of the bacteria during childhood (69, 70). Further, social stresses and exposure to xenobiotics including pesticides and heavy metals have been related to gut dysbiosis (71, 72). The emergence of a gut microbiome has a genetic basis as opposed to social factors, based on twin studies. Given that identical twins have nearly identical DNA, any changes in their gut microbes must be the result of non-genetic variables like food, medical history, or use of antibiotics (73, 74). The study of bacterial genomes has transformed the field of microbial research. Metagenomic sequencing and 16S rDNA sequencing are two types of sequencing that are frequently utilized to assess the abundance of microbial components. By focusing on the conserved sections that surround the hypervariable regions, the 16S sequencing approach can detect variations in bacterial genomes (75, 76).
Impact of gut microbiota on cardiovascular disease
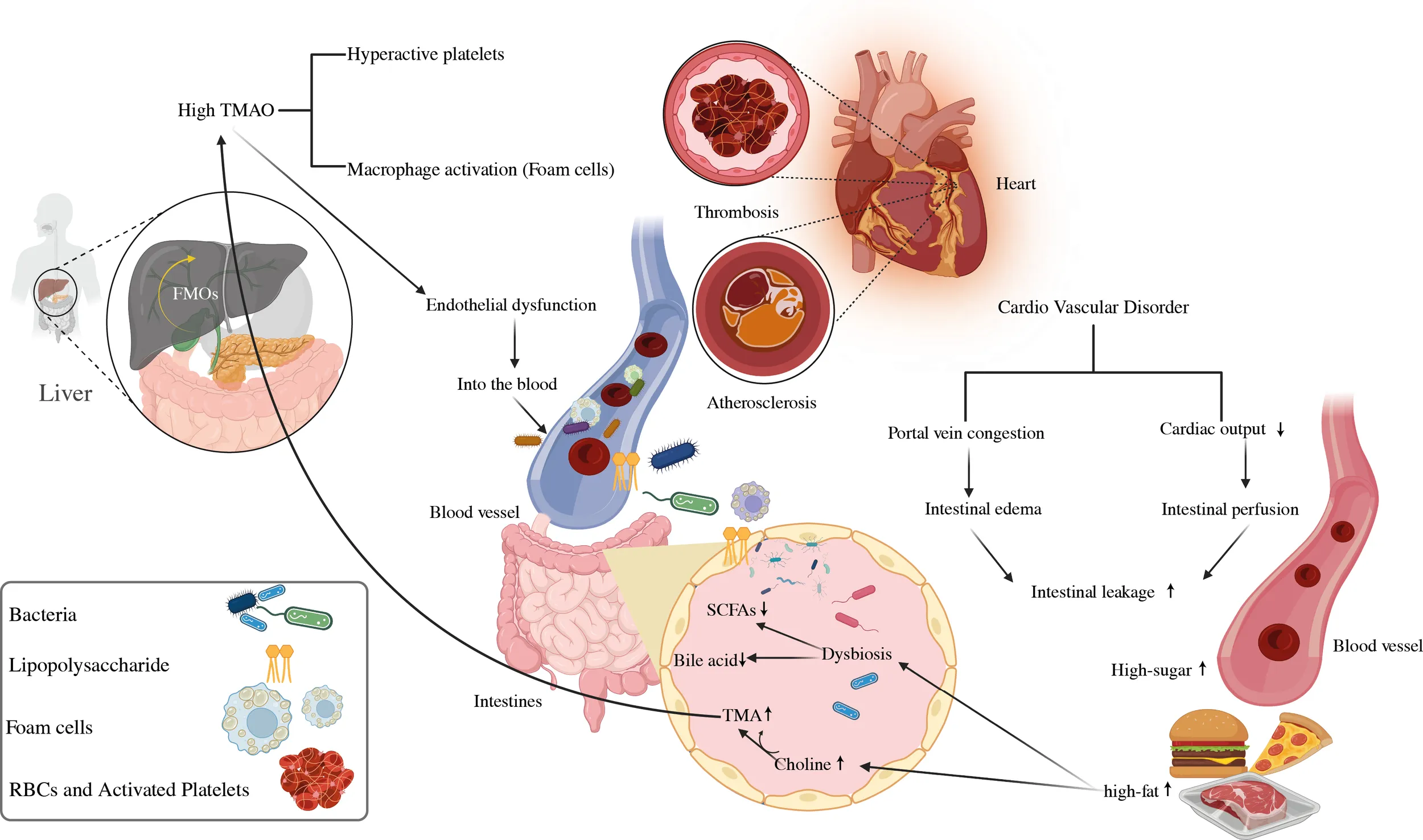
A broad spectrum of diseases is considered in CVD, including atherosclerosis, aortic valve disease, peripheral artery disease, hypertension, and stroke. Heart failure, hypertension, and atherosclerosis are associated with gut dysbiosis as shown in Table 1. In recent years, significant progress has been made in understanding how the gut microbiome impacts cardiovascular function and the development of these diseases. In this specific section, we intend to highlight several well-supported studies that deliver compelling evidence regarding the role of gut microbiota in the development of cardiovascular disease as depicted in Figure 4 (92).
Table 1
| Species | Technique | Modifications in gut microbial diversity attributed to diseases | References | |
|---|---|---|---|---|
| Decrease | Increase | |||
| Atherosclerosis and coronary artery disease | ||||
| Human | Metagenomics sequencing | Bactericides and Prevotella | Streptococcus and Escherichia | (77, 78) |
| Human | Terminal restriction fragment length polymorphism | Bactericides and Prevotella | Order Lactobacillales | (79) |
| Human | Metagenomics sequencing | Roseburia and Eubacterium | Collinsella | (31, 33) |
| Human | 16S sequencing | Clostridium, Faecalibacterium | Prevotella | (80) |
| Human | 16S sequencing | Burkholderia, Corynebacterium and Sediminibacterium, Comamonadaceae, Oxalobacteraceae, Rhodospirillaceae, Bradyrhizobiaceae and Burkholderiaceae | Curvibacter, Burkholderiales, Propionibacterium, Ralstonia | (33, 81) |
| Hypertension | ||||
| Human | Metagenomic sequencing | Prevotella and Klebsiella | (82) | |
| Human | Metagenomic sequencing | Roseburia spp., Faecalibacterium prausnitzii, | Klebsiella spp., Streptococcus spp., and Parabacteroides merdae | (83) |
| Human | 16S sequencing | Butyrate-producing bacteria Odoribacter | (84, 85) | |
| Heart failure | ||||
| Human | 16S sequencing | Blautia, Collinsella, uncl. Erysipelotrichaceae and uncl. Ruminococcaceae | (86, 87) | |
| Human | Incubation with a selective agar | Campylobacter, Shigella, Salmonella, Yersinia Enterocolitica, | (88) | |
| Human | 16S sequencing | Faecalibacterium | Lactobacillus | (89) |
| Human | Metagenomic sequencing | Faecalibacterium prausnitzii | Ruminococcusgnavus | (90) |
| Atrial fibrillation | ||||
| Human | Metagenomic sequencing | Faecalibacterium, Alistipes, Oscillibacter, and Bilophila | Ruminococcus, Streptococcus, and Enterococcus, | (91) |
Modifications in the diversity of the intestinal microbes attributed to CVD. CVD-related changes in the diversity of the gut’s microbes.
Figure 4

The contribution of the gastrointestinal microbiome to CVD. Choline, phosphatidylcholine, and carnitine are all available in high-cholesterol, high-fat diets. Intestinal microbes convert phosphatidylcholine in the diet to choline, which is then turned into trimethylamine. Hepatic flavin monooxygenases convert TMA to TMAO in the liver. By increasing atherosclerosis and generating agonist-induced platelet activation, TMAO promotes thrombosis. High levels of TMAO in the blood are linked to an increased risk of CVD. Furthermore, a high-fat diet raises the levels of microbe-associated molecular patterns like LPS. Increased intestinal uptake of microbe-associated molecular patterns results in metabolic endotoxemia and low-grade inflammation, both of which worsen atherosclerosis. TLR2 induces arterial thrombosis by increasing the interaction between von Willebrand factor and platelet integrin. Furthermore, intestinal bacteria convert carbs to SCFA and produced by gut microbial fermentation regulate blood pressure, a risk factor for CVD progression. FMO: flavin monooxygenases, SCFAs, short-chain fatty acids; TMAO, trimethylamine N-oxide; TLR2, toll-like receptor-2; LPS, lipopolysaccharide.
Gut bacteria; heart failure (HF) with microbial metabolites and current treatments
The disease termed heart failure is defined by the heart’s decreased ability to efficiently pump enough blood and oxygen to satisfy the demands of the body (93, 94). It serves as the final stage of multiple CVDs, which are highly prevalent, have significant mortality rates, and pose a significant threat to human well-being (95). While chronic exposure is defined by an altered inflammatory state related to pro-inflammatory aspects that are critical to the beginning of HF, immediate exposure is associated with a variety of inflammation-related symptoms (96). Our understanding of the pathophysiological processes behind HF has greatly increased. The recognition of the vital role of managing neurohumoral processes rather than focusing solely on changes in blood flow is a key shift in this understanding (97, 98). More evidence indicates the stomach is implicated in decreased heart rate and higher systemic congestion, both of which can contribute to intestinal mucosal ischemia and edema. As a result, bacterial translocation may be enhanced, allowing endotoxins into the bloodstream and contributing to the inflammation seen in HF patients (99). Niebauer et al. (100) conducted a study that revealed a significant association between peripheral edema in HF patients and higher plasma levels of endotoxins and inflammatory cytokines. Specifically, patients experiencing peripheral edema demonstrated elevated concentrations of these markers compared to those without edema. However, the study found a decrease in serum amounts of endotoxins but not cytokines following short-term diuretic treatment. This finding raises the prospect that edema and gut-associated inflammation in cardiac failure may be linked while diuretic medication might be effective (100). Pasini et al. (88) showed a recent comparison of the bacterial and fungal profiles in the feces of heart failure patients and the findings revealed people with chronic heart failure (CHF) had a greater risk of perilous bacterial growth compared with control group.
The presence of Candida, Campylobacter, and Shigella species was linked to 78.3% of CHF disease severity that had significantly greater intestinal permeability. Between TMAO and the risk of atherosclerosis, there was a strong positive correlation between gut permeability and right atrial pressure. An increased TMAO levels have been linked to poor outcomes in people with heart failure (101, 102). Scientists evaluated the levels of TMAO in 2,490 patients with chronic heart disease in the study with a 9.7-year follow-up. The data showed increasing TMAO levels coincided with increased rates of morbidity and mortality, particularly in HFrEF patients. This study suggests that TMAO could be utilized as a biomarker to predict poor outcomes in HfrEF patients (103). A recent meta-analysis offered that some reliable insights into the prognostic importance of TMAO in HF (102). The higher TMAO precursor trimethyl lysine (TML)-derived N, N, N-trimethyl-5-aminovaleric acid (TMAVA) synthesis by the gut microbiota was linked to a progressive reduction in fatty acid oxidation (104). Several studies have consistently reported that patients with HF exhibited a decrease in butyrate-producing bacteria, particularly within the Lachnospiraceaand Ruminococcaceae families (105). However, the absence of butyrate-producing microbes such Eubacterium Halli and Lachnospiracea is associated with higher mortality, increased inflammation, and severity of disease. This association implies that the abundance of these beneficial gut bacteria may have a major impact on the progress and results of cardiac failure (106). Dysbiosis has been consistently linked to reduced butyrate production in various heart failure cohorts (105). Additionally, bile acids, particularly secondary bile acids produced through the transformation by gut microbiota, play a crucial role in heart failure. Research has indicated a rise in secondary bile acids among individuals with CHF(97). Indoxyl sulfate produced by gut microbial metabolism, has also been linked to cardiac fibrosis and ventricular remodeling. These findings underscore the importance of the gut microbiota and its metabolites in heart health and offer possible therapeutic targets for heart failure management (107).
Atherosclerosis and therapeutic options
Atherosclerotic cardiovascular disease is a persistent inflammatory state primarily impacting sizeable and intermediate arteries. Numerous firmly established factors are correlated with atherosclerosis, including hypertension, dyslipidemia, advanced age, and smoking (108). This is characterized by the accumulation of low-density lipoprotein within the artery walls, leading to the formation of atheroma and distinct plaques consisting of proliferative fibrous tissue and calcifications (109, 110). Recent decades, there has been a growing interest in finding out how the gut microbiota plays a significant role in the emergence of atherosclerotic lesions (111). Koren et al. did an analysis using shotgun DNA sequencing focused on the gut metagenome that indicated significant changes in the diversity of gut microbial populations between patients with symptomatic atherosclerosis and those assumed to be healthy controls. These findings strongly suggest that the gut microbiota may play a significant role in the atherosclerosis (30, 31). Further, extensive metagenome-wide association research done on a cohort of 218 atherosclerosis patients and 187 healthy controls confirmed a link between the diseases with changed gut microbiome composition. In particular, the study found that people with atherosclerosis had significantly higher concentrations of Enterobacteriaceae, Ruminococcusgnavus, and Eggerthellalenta (112, 113). Introducing prebiotics and probiotic strains which enhance the production of SCFA and boost the diversity of beneficial microbes might be a valuable strategy in atherosclerosis prevention strategies (114). Various animal studies, including the work by Chan et al., (115) have explored the impact of probiotics and telmisartan on mitigating atherosclerosis induced by a high-fat diet, resulted in an increase in the Firmicutes to Bacteroidetes ratio. A diet rich in fats was found to decrease the prevalence of Eubacterium, Anaeroplasma, Oscillospira, Roseburia, and Dehalobacterium, while simultaneously elevating the quantities of Allobaculum, Clostridium, Lactobacillus, and Bifidobacteria (116). The findings show B. fragilis can produce extracellular vesicles (EVs), which are lipid bilayer particles. Human research has revealed a relationship between infectious and non-infectious disorders, as well as changes in the systemic levels of EVs derived from gut bacteria (117). It was identified as an essential cell-cell communicator with the potential to increase the knowledge of atherosclerotic disease, ranging from biomarkers to disease pathogenesis (118). Proteomic study has revealed unique protein compositions for EV subtypes, with some indicators assisting in the differentiation of EVs via biogenesis processes. Endosomal sorting complexes required for transport (ESCRT) proteins, Alix, and tetraspanins, for example, are exosome markers, whereas ectosome markers include Annexin A2/A5, ARF6, and Enolase 1 (119). EVs are involved in the immunological response, vascular remodeling, endothelial dysfunction, and apoptosis, which all contribute to atherosclerosis, and EVs in plasma may be useful as atherosclerosis indicators (120).
Scientists used the terminal restriction fragment length polymorphism technique for insight into the gut bacteria contents with coronary artery patients. Their research showed that the microbial diversity in the individuals studied experienced unique alterations. Notably, the levels of Bacteroides were decreased while the abundance of Lactobacillales and Clostridium subcluster XIVa increased in the fecal samples of people. These findings suggest the gut microbiota may contribute to the progression of coronary artery disease. Novel strategies for treating or preventing cardiovascular diseases may be developed as a result of a better understanding of such microbial modification (79). A significant negative association found between the decrease in (1) Eubacterium and the rise of inflammatory cytokines like matrix metalloproteinase-9 (MMP-9) and E-selectin; (2) Dehalobacterium and adipocyte fatty acid binding protein (A-FABP); and (3) Roseburia and MMP-9. This confirms a connection between imbalanced gut microbiota and the development of atherosclerosis (115). Similarly, Stepankova showed experiments signifying the beneficial impact of gut microbiota in inhibiting the atherosclerotic lesions progression (121).The aortas of the germ-free mice fed a low-cholesterol diet showed atherosclerotic plaques. The results of the study offer strong evidence that microorganisms suppress the progress of atherosclerosis (122).
On the contrary, systems depending on metabolism can be affected by gut dysbiosis, which alters a wide variety of metabolites and may have an effect on the onset and development of atherosclerosis (6). One of the several metabolites produced by the gut bacteria, TMAO, plays a crucial role in the formation of atherosclerosis (123). The accumulation of TMAO in the body has been related to an increase in the risk of atherosclerosis and cardiovascular diseases (124). The blood plasma levels of TMAO in mice with normal gut microbiota grew as they were fed a diet high in choline. In contrast, animals given the same choline-rich diet and antibiotic treatment, which changed their gut microbiota, had minimal TMAO amount (43). Compared to the control TMAO levels, the mice with greater TMAO levels displayed a higher amount of foam cell formation and the development of atherosclerotic plaques. The risk of sudden cardiovascular events is increased by TMAO linked to plaque vulnerability to its involvement in developing atherosclerosis (28, 47). High levels of TMAO in the bloodstream have been associated with conditions such as obesity, Type 2 diabetes mellitus, chronic kidney disease (CKD), and CVD (125, 126). Moreover, numerous other studies have shown that SCFAs may have a positive impact on atherosclerosis by suppressing inflammation (127, 128). As a result, SCFAs may reduce cholesterol levels and stop the host from developing lipid deposits. Dyslipidemia can result from decreased SCFA production, whereas probiotics (Lactobicili) are effective in reducing cholesterol. According to Dieck et al., (129) probiotic anti-cholesterolemic effect can be induced via bile salt hydrolysis (BSH), interference with hepatic de novo lipid synthesis by regulation of SCFA, or satiety hormones. It indicates that SCFAs may have a preventive effect by reducing the risk factors for cardiac disease (130).
Association of gut microbiome with hypertension (HTN)
Hypertension is a significant worldwide public health concern and stands as the foremost risk factor for cardiovascular diseases, leading to a substantial economic burden on society. Its epidemiology is defined by a high prevalence, notable levels of disability and mortality, and often insufficient awareness (131, 132). As of 2021, it was estimated that around 330 million people in China were affected by cardiovascular diseases, with approximately 245 million individuals having been diagnosed with hypertension (133). The causes of hypertension involve a combination of factors, including genetic predisposition, lifestyle choices, environmental influences, hormonal imbalances, inflammatory processes, and changes in hemodynamic mechanisms (134). The American College of Cardiology, the American Heart Association, and the European Society of Hypertension have collaboratively formulated behavioral guidelines aimed at maintaining optimal blood pressure levels, with a particular emphasis on non-pharmacological strategies (135). These include using the dietary methods to stop hypertension (DASH) diet, which highlights a high intake of fruits and vegetables while minimizing fat consumption, increasing physical activity through specific aerobic exercises, slicing off salt and alcohol consumption, losing weight, and increasing salt and alcohol consumption (136–138).
A small number of studies mostly in animal models have shown an explicit link between gut microbiota and the control of blood pressure (139–142). For instance, Yang et al. (140) conducted a study where they investigated changes in the fecal microbiota of animal models with hypertension, specifically comparing alterations in the spontaneously hypertensive rat and chronic angiotensin II infusion rat models. They observed a notable gut dysbiosis in hypertensive animals characterized by a decrease in microbial richness, diversity, and consistency (140). Kim (143) also found that among hypertension patients, the presence of butyrate-producing bacteria, such as Butyricimonas and anaerobic Corynebacterium, had significantly decreased. Studies have revealed a positive correlation between blood pressure and the levels of Ruminococcaceae, Streptococcus, and Turicibacter (140, 144, 145). The metabolite production derived from microbes may also be impacted by changes in gut microbiota. Since they are created by bacterial digestion of dietary fiber and are closely related to good health, SCFAs are of particular significance among these metabolites that are formed from microbes (146), play a vital role in the HTN development. A larger amount of research indicates the potential of SCFAs may effectively decrease the host’s blood pressure by interacting with G protein-coupled receptor 41 (147–149). Although TMAO is required for disease start, an animal model was first used to demonstrate the relationship between TMAO and CVD in 2011 (28). Recently, Wang et al. (150) have provided compelling evidence of a causal link between TMAO and its precursors with blood pressure by employing a Mendelian Randomization approach. Moreover, multiple studies have validated the strong association between elevated TMAO levels and an increased prevalence of hypertension (151–153). Ge et al. (153) proved that a rise of 5 and 10 mol/L in TMAO levels corresponded to a 9% and 20% escalation in the risk of hypertension, respectively. Apart from TMAO, other gut microbiota-derived metabolites, including corticosterone, H2S, choline, BAs, indole sulfate, and LPS, are also produced. The SCFAs, TMAO, BAs, H2S, and LPS metabolites have been closely linked to the development of hypertension. So, the intestinal microbiota may have an interconnected role in regulating blood pressure, and any disruptions in their function could be linked to hypertension. Studies have proposed that Lactobacillus probiotics might play a beneficial role in blood pressure regulation (154) Additionally, a meta-analysis has shown that probiotics treatment can lead to a significant reduction in blood pressure in patients (155).
Role of microbial derived metabolites and CVD
We will briefly discuss the association between trimethylamine N-Oxide and CVD, and focus on other microbial metabolites in this review as illustrated in Figure 5 (156).
Figure 5
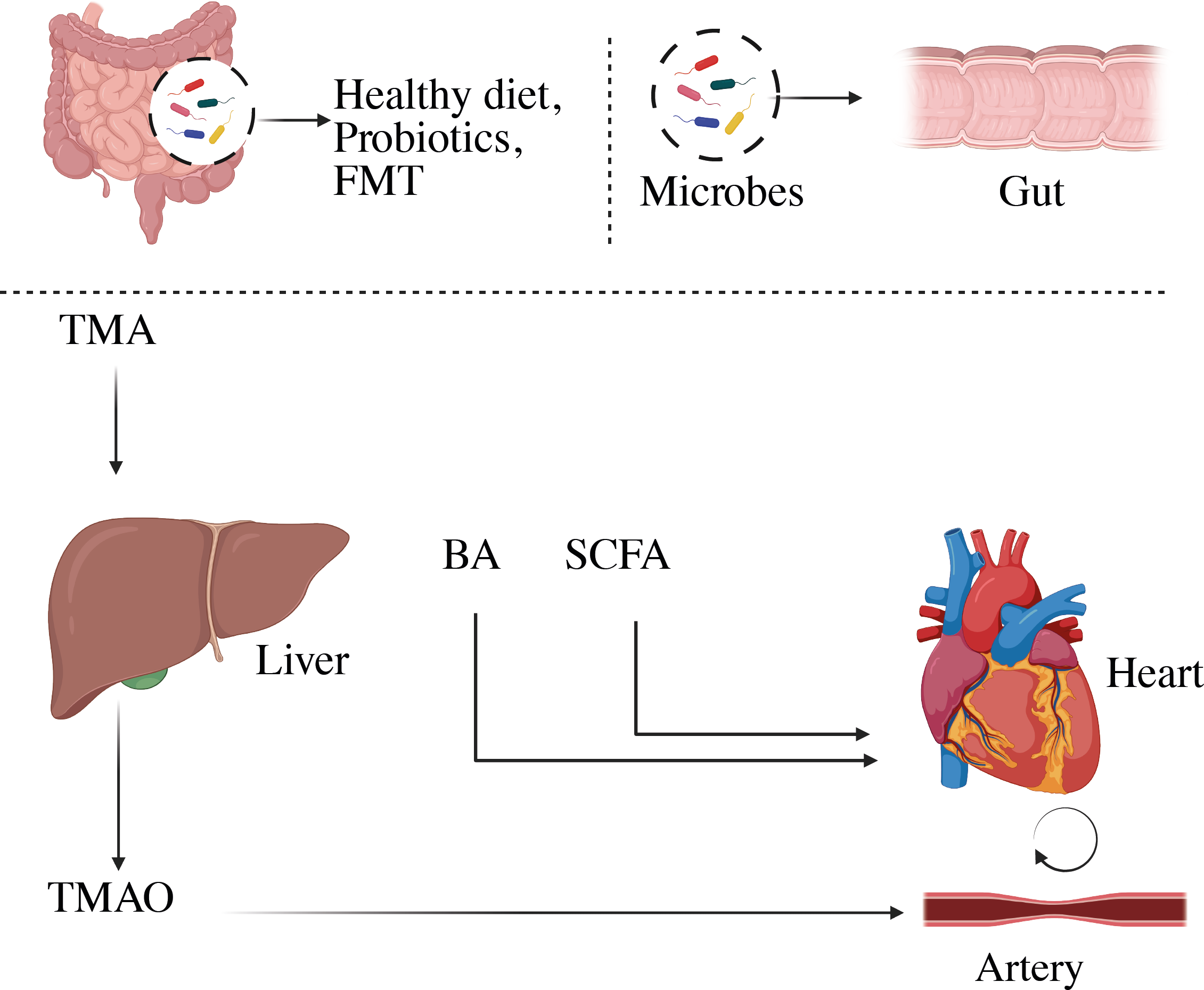
Representation of microbial-derived metabolites to CVD. Variations in the composition of the gut microbiome can change the metabolism, allowing bacteria or its fragments and metabolites to enter the circulation more easily. This can aggravate the pro-inflammatory milieu and produce metabolic disturbances, which can lead to CVD. BA, Bile acid; SCFA, short-chain fatty acids; TMA, trimethylamine; TMAO, trimethylamine-N-oxide.
Trimethylamine-N-oxide (TMAO) associated with CVD
The gut microbial digestion of phosphatidylcholine, the main dietary source of choline, was found to produce a proatherogenic metabolite called trimethylamine-N-oxide (157). Among the numerous physiologically active metabolites of microbial metabolism, TMAO is a biologically active molecule that has been linked to an increased risk of adverse cardiovascular events, including acute coronary syndrome (ACS), stroke, and mortality (47, 158, 159). TMAO production occurs secondary to the ingestion of nutrients containing the trimethylamine moiety, such as choline, phosphatidylcholine, and L-carnitine, all of which are found in high concentrations in animal products, including red meat, fish, milk, and eggs. The metabolism of these nutrients by microbial TMA lyases produces TMA, which enters the portal circulation, is oxidized to TMAO by hepatic flavin monooxygenases, primarily FMO3 (160), and subsequently enters the general circulation (28, 161). It is believed that TMAO may contribute to the development of atherosclerosis, following a proatherogenic pathway. Elevated levels of TMAO in the bloodstream have been positively associated with early atherosclerosis in humans. Moreover, monitoring TMAO levels can be useful in predicting the risk of mortality in patients with stable coronary artery disease and acute coronary syndrome (151, 162). Research has indicated that higher TMAO levels in the bloodstream are linked to the severity of peripheral artery disease and a greater risk of cardiovascular mortality among individuals affected by this condition (163).
In-depth analyses, including meta-analysis and dose-response studies, have further revealed that elevated plasma TMAO levels are associated with a higher occurrence of major adverse cardiovascular events in patients with coronary heart disease (164). Additionally, proinflammatory monocytes and elevated TMAO levels were substantially associated in stroke patients. According to Haghikia et al. (165) higher cardiovascular events such as myocardial infarction, recurrent stroke, and cardiovascular death were also linked to a raised TMAO plasma level. Numerous human investigations have also supported the involvement of TMAO in CVD. Compared to controls, patients with chronic heart failure had higher plasma levels of TMAO, choline, and betaine in a prospective observational analysis of stable CAD and healthy people (166). Similarly, in patients who experienced a myocardial infarction, TMAO was identified as an independent predictor of mortality at the two-year follow-up. The ratio stood at 1.21 (with a 95% confidence interval of 1.03-1.43, P = 0.023), as observed in a study involving 292 events (167). Another study conducted by Tang et al. (48), observed a correlation between elevated TMAO levels and a higher risk of major adverse cardiac events. However, the precise mechanisms by which TMAO affects cardiovascular disease have not been fully investigated.
Short-chain fatty acids and CVD and prevention strategies
The human digestive system cannot break down complex carbohydrates, such as dietary fiber, to support cell activity. Nevertheless, the gut microbiota can utilize fibers by fermenting them, resulting in the production of SCFAs (168). SCFAs are saturated fatty acids composed of carbon chains ranging from one to six carbons. Acetate, propionate, and butyrate are the main types of SCFAs found in the human body (169). The primary bacteria responsible for producing SCFAs are found in the clostridial clusters IV and XIVa within the Firmicutes phylum and include various species of bacteria such as Eubacterium, Roseburia, Faecalibacterium, and Coprococcus (170). It plays crucial roles in regulating anti-inflammatory responses, lipid metabolism, and gluconeogenesis. Notably, butyrate, one of the SCFAs, is considered a significant energy source for intestinal epithelial cells (171). A significant amount of research shows that SCFAs protect against heart failure and are essential for preserving the integrity of the intestinal barrier by encouraging mucus formation and reducing inflammation (172). The presence of high SCFA levels in fecal samples is linked to markers of hypertension, central obesity, and subclinical indicators of cardiometabolic disorders (173) and to the development of atherosclerosis (174).
Butyric acid in the diet effectively slowed the progression of atherosclerotic plaques in the arterial walls of mice missing apolipoprotein E (Apo-E) in a trial utilizing rodent as a model. The favorable benefits were obtained by slowing macrophage migration, boosting collagen deposition, and improving plaque stability (175). Multiple studies show that the SCFAs contribute to manage blood pressure. For example, when fecal material from hypertension human donors was introduced into germ-free mice vs normotensive donors, researchers saw an increase in blood pressure (176). SCFAs are linked to blood pressure regulation through G-protein coupled receptor (GPCR) pathways, specifically in renin secretion and blood control. SCFA activation of the olfactory receptor (Olfr) 78 and the free fatty acid receptor GPR41 causes an increase in blood pressure and a decrease in blood pressure, respectively. The acetate and propionate have antihypertensive properties due to their ability to reduce systemic inflammation and atherosclerotic lesions, both of which are independent predictors of hypertension (177). However, SCFAs have been implicated in causing damage to the organs affected by hypertension in mice infused with angiotensin II, indicating their role in hypertensive organ damage (178). As a result, a lot of evidence points to the gut microbial community’s influence on blood pressure regulation in the host, with SCFAs functioning as one of the microbial components that contribute to vasomotor tone and blood pressure regulation. Recent research has revealed more evidence that SCFAs play a role in a variety of CVD processes, including ischemia-reperfusion injury, heart repair after myocardial infarction, and arterial compliance impairment (179, 180).
Bile acid (BA) association with CVD and therapeutics
Bile acids are produced in the liver through the breakdown of cholesterol, are crucial in controlling the absorption of lipids. Primary and secondary BAs can be identified based on their structural features. BAs can also be classed as bound or free based on whether they are conjugated with glycine or taurine (181). In a healthy adult, the liver produces primary bile acids at a daily rate of 500 mg that constitute approximately 72.5% of the total bile acid pool; chenodeoxycholic acid comprises 35%, while cholic acid constitutes 37.5% (182, 183). The synthesis of bile acids occurs through two distinct pathways: the classic (or neutral) pathway and the alternative (or acidic) pathway, each regulated by a specific enzyme. Cholesterol 7-hydroxylase (CYP7A1) enzyme responsible for the classic pathway, whereas oxysterol 7-hydroxylase (CYP7B1) is involved in the alternative pathway (184). Bile acids are stored in the gallbladder and released during digestion into the small intestine. The primary role of bile acids is to emulsify dietary fats and fat-soluble vitamins, facilitating their absorption and transport in the digestive system. Primary bile acids released into the duodenum have a critical function in emulsifying food components and vitamins that are lipid-soluble, enabling their digestion and absorption (185). Secondary bile acids are created when bacterial enzymes change the primary bile acids, which make up roughly 27.5% of bile acid (186).
However, Deoxycholic acid accounts for 25% of the overall bile acid pool, while lithocholic acid and ursodeoxycholic acid collectively make up 2.5% of these bile acids. In a healthy individual, almost 95% of BAs are efficiently reabsorbed in the distal ileum, primarily due to the process of enterohepatic circulation (187). The bile acids that are reabsorbed in the distal ileum are then transported back to the liver to build an effective recycling process. Surprisingly, bile acids, which make up to 2-4 grams of the body’s total weight and play a crucial function, are controlled by a relatively tiny pool (188). This process happens multiple times a day, typically ranging from 5 to 10 cycles daily (189, 190). In order to prevent bile acids from building up to hepatotoxic levels and to limit their impact on cholesterol metabolism, the size of the bile pool is carefully managed through feedback regulation of bile acid synthesis (191). Bile acids also possess strong microbial activity and serve as signaling molecules, acting as ligands for nuclear receptors, thereby impacting various metabolic processes (192). For instance, Farnesoid X-receptor (FXR) activation leads to the suppression of the cholesterol 7a-hydroxylase enzyme. By regulating this enzyme, FXR helps maintain the balance of bile acid synthesis and contributes to the overall control of cholesterol metabolism (193). The gut microbiota plays a significant role in modifying primary bile acids through bacterial salt hydrolase activity. This enzymatic process involves removing the 2OH groups from primary bile acids, transforming them into secondary bile acids (194). Bacteria can lessen BA toxicity by increasing their solubility, giving the gut microbiota a way to defend itself. Additionally, the gut microbiota might change bile acids further before they return to the liver for reconjugation and rejoin the circulation (195). Bile acids serve as a crucial pathway for cholesterol elimination through excretion in feces helping to decrease circulating cholesterol levels and reduce the risk of plaque accumulation (193). However, alterations in the gut microbiome can influence the bile acid synthesis rate, potentially leading to increased plasma levels of LDL cholesterol and an elevated risk of atherosclerosis (196). Thus, maintaining a healthy gut microbiome is essential for regulating bile acid metabolism and its impact on cholesterol levels and cardiovascular health. Additionally, microbial metabolites such as tryptophan and indole have been identified to have significant roles in the development of cardiovascular diseases.
Therapeutic approaches to gut microbiome
The novel research implies that the gut microbiota plays a critical role in the progression of cardiovascular illnesses. Therapeutic techniques for influencing the composition and metabolic activity of the gut microbiota have been developed. As shown in Figure 6, these options include dietary changes, the use of probiotics and prebiotics, antibiotic treatments, and even fecal transplantation. Notably, these therapies have shown the potential to improve blood pressure control, restore lipid profiles to normal levels, and reduce body weight in people with cardiovascular disease (33). In a vicious cycle, the intricate interaction between dietary components and other variables affects the gut microbiota and pathogenesis of many cardiovascular diseases as shown in Figure 7 (168).
Figure 6
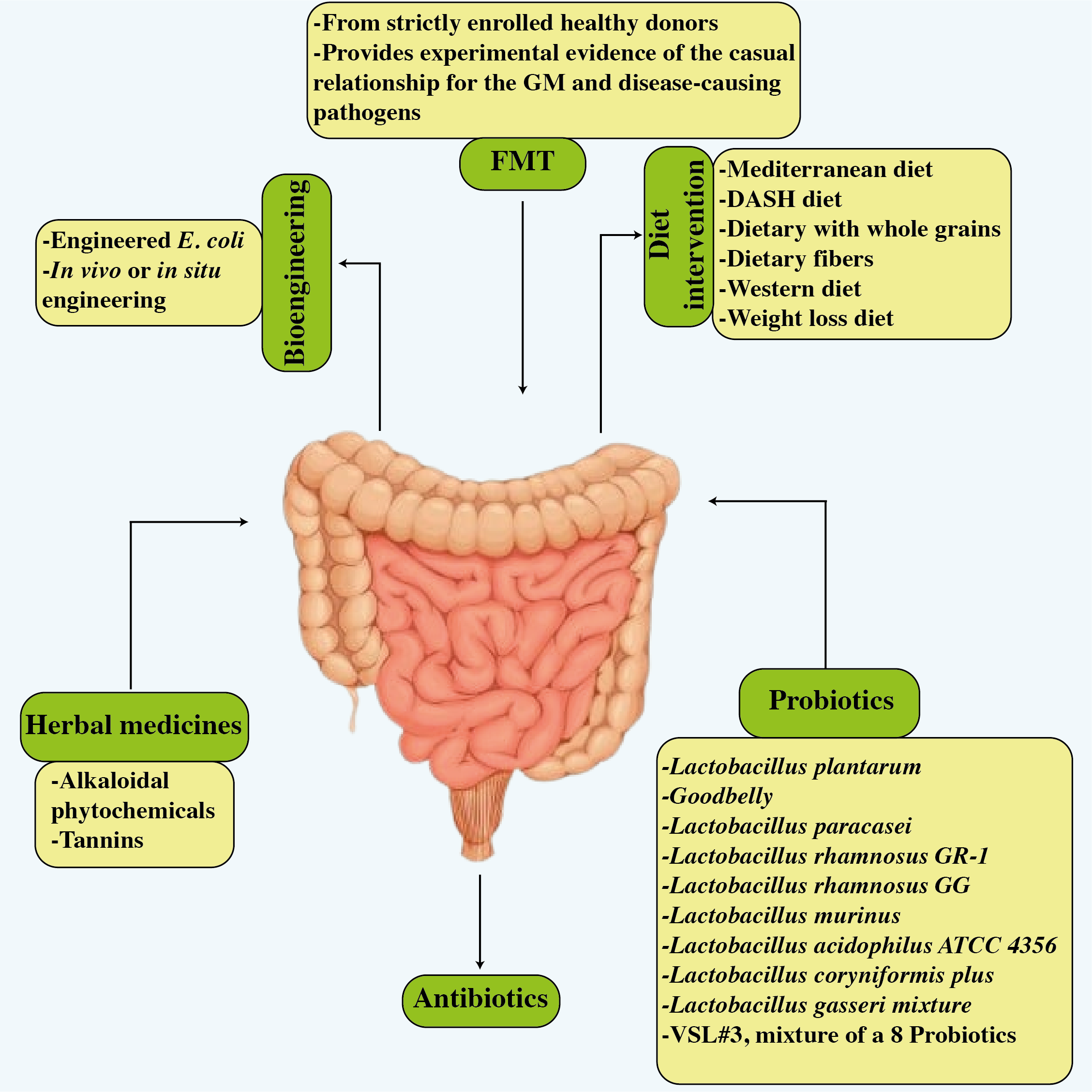
Potential treatments related to improved cardiovascular disease results and improved gut microbiota. The figure illustrates six strategies, i.e., dietary modifications, probiotics, antibiotics, FMT, bioengineering, and herbal treatment.
Figure 7
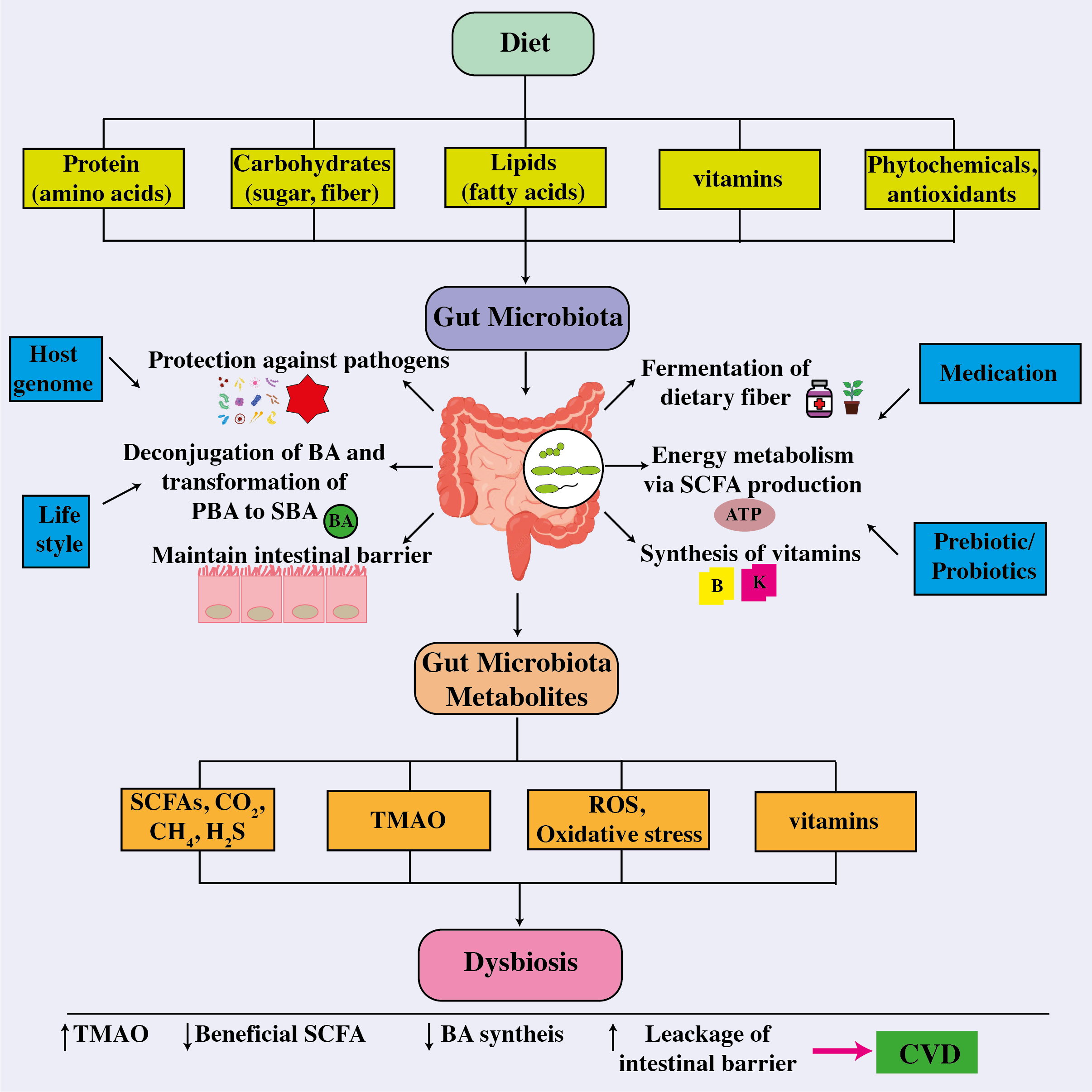
The correlation between the intestinal microbiome, metabolites, and cardiovascular disease. The intricate connection between dietary components absorbed and other factors influencing the gut microbiota, whose composition then influences their functionality and metabolite production and release, a disruption which leads to dysbiosis, thereby affecting host health and the onset and cause of different cardiovascular disorders. ATP, adenosine triphosphate; BA, bile acid; CO2, carbon dioxide; CH4, methane; CVD, cardiovascular disease; H2S, hydrogen sulfide; PBA, primary bile acid; ROS, reactive oxygen species; SBA, secondary bile acid; SCFA, short-chain fatty acids; TMAO, trimethylamine-N-oxide.
Dietary inventions
Numerous scientific studies have provided persuasive evidence supporting the idea that dietary interventions can significantly decrease the risk of cardiovascular problems (197, 198). Diets that frequently occur in Western industrialized societies that feature high consumption of red meat or animal proteins, saturated fats, and simple carbohydrates have been associated with an increased risk of CVD (199, 200). An increasing mass of evidence refers to the intestinal microbiota as a possible avenue for CVD treatment. Current clinical trials on microbe targeting for CVD therapy are summarized in Table 2 (39). Conversely, the composition of our diet can influence the structure and functioning of the gut microbiome (201). The gut microbiota is greatly influenced by essential food elements such as macronutrients, fiber, polyphenols, prebiotics, and probiotics, which also have an impact on the production and release of major gut microbiome metabolites including SCFAs (202). In a prior study, it was found that diets rich in fiber promote the growth of beneficial symbiotic microbes while preventing the spread of known infectious diseases (203). Moreover, the consumption of a high-fiber diet increased acetate-producing microbiota, which was associated with lower blood pressure and a reduction in cardiac hypertrophy and fibrosis (204). The mediterranean diet, which consists of a high intake of vegetables, fruits, grains, and legumes combined with a low intake of red meat and processed carbohydrates, has been shown to be effective in the prevention of CVD (205). This is mostly due to the high levels of antioxidants, nitrates, and fiber in this diet, and to the low levels of saturated/Trans fatty acids, salt, and phosphate. These elements are expected to reduce inflammation and oxidative stress, promote antioxidant activity, increase nitric oxide bioavailability, and microbiota modulation to improve vascular and cardiac function (206). The Western diet, compared to the Mediterranean diet, is known to raise CVD risk by lowering gut microbiota diversity and beneficial bacteria such as Bifidobacterium (207). A study involving mice fed a Western diet, the findings revealed higher plasma concentrations of TMAO and the development of cardiac dysfunction and heart fibrosis (208). The expression of pro-inflammatory cytokines (IL-10) and tumor necrosis factor (TNF-α) as well as interleukin-1 (IL-1), both of which are indicative of increased inflammation, was shown to be altered (208). In a study involving 153 volunteers from four cities in Italy, researchers found that the consumption of fruits, vegetables, and legumes is consistent with the Mediterranean diet led to an increase in fecal SCFA levels (209). This effect is due to fermentation by a greater abundance of bacteria from the Firmicutes and Bacteroidetes groups (209).
Table 2
| Models | Intervention | Result | Clinical ID |
|---|---|---|---|
| Diet | |||
| Overweight/obese individuals | Mediterranean diet | Positive | NCT03071718 |
| Patients with CAD | Moderate Alcohol Consumption | Positive | No report |
| Patients with CAD | Calorie restriction | Positive | IRCT20121028011288N15 |
| Patients with CAD | Lacto-Ovo-Vegetarian Diet | Positive | NCT02942628 |
| Overweight/obese individuals | Dietary fibers | Positive | NCT01719900 |
| Patients with CAD | Vegan Diet or the American Heart Association-Recommended Diet | Positive | NCT 02,135,939 |
| Patients with T2D | Dietary fibers | Positive | No report |
| Patients with heart failure | DASH diet | Positive | No report |
| Obese hypertensive patients | hypocaloric diet supplemented with probiotic cheese | Positive | ISRCTN76271778 |
| Probiotics | |||
| Subjects with metabolic syndrome | A. soehngenii | Positive | NTR-NL6630 |
| Patients with heart failure | Saccharomyces boulardii | Negative | NCT02637167 |
| Patients with CAD | Lactobacillus rhamnosus GG (LGG) | Positive | IRCT20121028011288N15 |
| Patients with MI | Lactobacillus Rhamnosus G | Positive | IRCT20121028011288N15 |
| Inulin | |||
| Patients with MI | Lactobacillus rhamnosus capsules | Positive | IRCT20121028011288N15 |
| Overweight/obese insulin-resistant volunteers | A. muciniphila | Positive | NCT02637115 |
| Patients with stable CAD | Lactobacillus plantarum 299v | Positive | NCT01952834 |
| Patients with heart failure | Saccharomyces boulardii | Positive | NCT01500343 |
| Subjects with high-normal blood pressure and mild hypertension | Lactobacillus helveticus | Positive | No report |
| Probiotics and Prebiotics | |||
| Patients with CAD | Lactobacillus Rhamnosus G and Inulin | Positive | IRCT20180712040438N4 |
| Healthy overweight or obese individuals | Polydextrose and Bifidobacterium animalis subsp | Positive | NCT01978691 |
| Prebiotics | |||
| Overweight to obese men | Inulin | Positive | NCT02009670 |
| Children with overweight or obesity | Oligofructose-enriched inulin | Positive | NCT02125955 |
| Mildly hypercholesterolemic individuals | β-glucan | Positive | NCT01408719 |
| Obese women | Inulin-type fructans© | Positive | NCT00616057 |
| Exercise | |||
| Patients with CAD | Bicycle ergometer | Negative | NCT01495091 |
| Patients with CAD | Exercise stress testing | Negative | NCT01495091 |
| Overweight participants | High-intensity interval training | Positive | ACTRN12617000472370 |
| Drug | |||
| Patients with T2D | Berberine and probiotics | Positive | NCT02861261 |
| Patients undergoing elective coronary angiography | broad-spectrum antibiotics | Positive | No report |
| Patients admitted with acute MI or unstable angina | Amoxicillin, metronidazole | Positive | No report |
| FMT | |||
| Hypertensive patients | Washed microbiota transplantation | Positive | No report |
| Obese patients | FMT capsules | Positive | NCT02741518 |
| Patients with metabolic syndrome | Vegan FMT | Positive | NTR 4338 |
| Patients with metabolic syndrome | FMT | Positive | NTR1776 |
Clinical trials targeting the gut bacteria in the treatment of cardiovascular diseases (39).
ATP, adenosine triphosphate; BA, bile acid; CO2, carbon dioxide; CH4, methane; CVD, cardiovascular disease; H2S, hydrogen sulfide; PBA, primary bile acid; ROS, reactive oxygen species; SBA, secondary bile acid; SCFA, short-chain fatty acids; TMAO, trimethylamine-N-oxide.
Following a mediterranean diet leads to reduced TMAO levels, thereby helping to prevent cardiovascular issues and heart failure (210, 211). A particular study revealed that incorporating ginger supplements into the diet influenced the gut microbiota composition, resulting in a notable rise in fatty acid metabolism (212). Moreover, the presence of miRNAs within ginger-derived exosome-like nanoparticles has the potential to modify bacterial gene expression, influencing the host genome (213). Currently, there is an obvious association between nutrition and intestinal microbes, resulting in varied gut bacterial communities across various diets and geographic locations. However, there is still a huge gap in our understanding of how our food influences the gut microbiome and the impact of the gut microbiota on general host health. More study is needed since nutrition is a low-cost, easy to manage strategy for the possible prevention, control, and management of cardiovascular disease.
Prebiotics and probiotics
The human colon is filled with probiotics, which are primarily made up of bifidobacteria and lactobacilli, and are essential for maintaining healthy immune systems, colon microflora, and the production of healthy compounds and also have ability to stop the spread of cancer, lower cholesterol, increase the synthesis of vital cytokines and vitamins, and prevent infections (214, 215). According to Gibson and Roberfroid, prebiotics are defined as non-digestible poly or oligosaccharides that have a positive impact on the host by selectively promoting the growth or activity of specific beneficial bacteria in the colon (216). The Lactobacillus Plantarum led to an improvement in the diversity of gut microbial flora, this consumption was linked to a reduction in the incidence of CVD incidents (217). Another study showed by Naruszewicz et al. (218), involving 36 healthy volunteers who were active smokers, revealed that there was a negative correlation between the intake of Lactobacillus Plantarum and various health markers, including blood pressure levels, fibrinogen levels, monocyte adhesion, and proinflammatory cytokine levels. These findings suggest that Lactobacillus Plantarum may have potential in the primary prevention of atherosclerosis. The normal or moderately elevated cholesterol levels in females experienced low LDL after consuming fermented milk containing Lactobacillus acidophilus and Bifidobacterium longum (219).
A recent study conducted by Catry et al.(220) revealed a 15-day supplementation of inulin-type fructans (ITFs) had a positive impact on endothelial function in the arteries of n-3 PUFA-depleted ApoE-/- mice. The improvement in endothelial function might be attributed to an increase in bacteria capable of producing nitric oxide, as it helps dilate blood vessels and improve blood flow, ultimately benefiting cardiovascular health. The findings highlight the ITFs potential in promoting cardiovascular well-being, particularly in the context of n-3 PUFA-depleted conditions (221). Similarly, a comprehensive analysis revealed that intake of isolated triterpene fraction yields favorable results on LDL cholesterol levels in the human (222). In addition to ITFs, beta-glucan supplements have also shown the capacity to lower total and LDL cholesterol levels while boosting endothelial vascular reactivity in people in the great health (223). It is crucial to recognize that prebiotics are formed up of a diverse range of chemicals that are controlled by different gut flora (224–227). A fiber-rich diet has been demonstrated to alter the gut microbiota by increasing acetate-producing bacteria, resulting in reduced gut dysbiosis and cardiovascular protection, most notably the transcription factor Egr1, are related to acetate regulation and govern CVD through inflammation, heart fibrosis, and hypertrophy (204).
Another prebiotic, beta-glucan was demonstrated to influence cholesterol levels and glucose homeostasis. A 2-month study that included a beta-glucan dietary plan indicated a significant reduction in LDL and total cholesterol levels. The endothelial function improved in healthy people, showing cardioprotective effects. These effects are mostly due to the production of beneficial SCFA by the gut flora (228). In animal tests, arabinoxylans showed potential as a possible prebiotic. It was discovered that their role in encouraging the growth of bifidobacteria and the production of propionate reduces cholesterol and fat deposition (206). Dietary arabinoxylan oligosaccharides raised bacterial populations and butyrate levels in stools in individuals (229). Probiotics help to improve human metabolism by boosting digestive enzyme output, suppressing bacterial enzyme activity, and lowering ammonia generation. Lactobacillus and Bifidobacterium have beneficial effects on intestinal barrier function and play a protective role in inflammatory diseases by modulating inflammatory and proinflammatory cytokines, which may potentially delay or improve CVD (230, 231). Akkermansia muciniphila is also renowned for its probiotic features, and it is related to glucose, insulin, and leptin, all of which have roles in the metabolism of lipids and glucose (232). However, Lactobacillus plantarum efficiently lowered LDL-C and total cholesterol levels while also inhibiting the formation of atherosclerotic plaques in hypercholesterolaemic individuals (233). The preceding research concentrated on the effects of prebiotics and probiotics on cardiovascular risk factors such as inflammation and hypertension, as well as impacts on glucose and lipid metabolism, rather than the direct benefit of atherosclerosis. However, considering their beneficial effect on several CVD risk variables, more research into how these medications affect the onset and progression of CVD is essential.
Antibiotics
In the regulation of host health, the gut microbiome has a huge impact on the host as a result of antibiotic usage. The use of antibiotics may damage the host’s health in a variety of methods, both directly and indirectly. This impact can alter a variety of bodily processes, including immunological control, metabolism, and ultimately general health (48, 234–237). A variety of antibiotics have shown evidence of affecting blood pressure and intestinal flora. A prime example is the drug minocycline, which has been studied for its capacity to alter the nature of the gut microbiota and control blood pressure (BP) in hypertensive rats (140). Using erythromycin, tetracycline, or doxycycline within the previous five years did not reduce the chance of developing a first MI, according to another population-based trial, and its authors disputed their efficacy in avoiding primary coronary heart disease (CHD) (238). Macrolides antibiotics, such as azithromycin, erythromycin, and clarithromycin, comprise a significant class of orally active antibiotics that function as bacteriostatic agents. In 2013, 51.5 million drugs for azithromycin were prescribed in the United States (239). The use of macrolide antibiotics is believed to increase the risk of cardiovascular diseases such as myocardial infarction (MI), ventricular tachyarrhythmias, and sudden cardiac death (SCD) (240, 241). Further, quadruple antibiotic therapy was shown to significantly lower high systolic and diastolic blood pressures in salt-induced hypertensive rats (242). It helps to realize the research on the effect of antibiotics on blood pressure regulation produced varying outcomes (243).
For instance, minocycline and vancomycin medication in rats led to lower Firmicutes levels in the gut, resulting in lower blood pressure in hypertensive rats. It’s noteworthy to note that identical antibiotic therapy actually raised blood pressure in salt-sensitive rats. This difference underlines the intricacy of the gut microbiota-blood pressure interaction and the significance of taking into account a variety of variables that may affect the results of such mediations (244). In a study by Rune et al., they found that ampicillin had the ability to lower mice’s levels of LDL and VLDL cholesterol. Atherosclerosis risk is associated with these forms of cholesterol. As a result, the mice’s aortic atherosclerotic lesions were reduced in size (245). Although a few studies provide promising results, indicating possible benefits in this regard, the efficiency of antibiotics for offering preventive benefits against CVD in trials involving patients is yet unknown (246). However, certain analyses have failed to demonstrate a distinct and obvious link between the use of antibiotics and protection against CVD, yielding unclear outcomes. As a result, more research and analysis are needed to determine whether antibiotics can significantly reduce the risk of cardiovascular disease (240). Furthermore, universal antibiotics can have a variety of impacts on the body, making methods of treating CVD with antibiotics contentious. While certain studies have suggested that taking antibiotics to treat CVD may have some benefits, their broad action can have a number of adverse reactions. Thus, any possible benefits of using antibiotics in treating CVD must be carefully balanced against any dangers and adverse effects that could result from their use. Before making antibiotics a common therapy choice, more study is required to better understand their precise mechanisms of action and potential advantages in the management of CVD.
Fecal microbiota transplantation as a prevention strategy
Fecal microbiota transplantation (FMT) is a therapeutic method intended to restore a healthy balance of gut microbiota in a recipient (247), by transferring fecal matter from a donor who is in a healthy condition (248). It gained a lot of attention for its safety and efficacy in therapeutic applications after being extensively studied in an array of mammalian species (249).The more complicated nature of propagating gut bacteria compared to those that inhabit the mouth cavity is one of the difficulties that FMT still challenges (250). The FMT involves transferring fecal matter from an adult donor to a recipient with an unbalanced intestinal microbiota and the fecal matter is rich in various microbial populations such as Clostridioides difficile (251, 252).This transplantation has shown promising results in treating several intestinal and other chronic diseases and has been researched as a viable therapeutic alternative in clinical applications (253). Notably, FMT has confirmed effectiveness in treating various conditions, including recurrent Clostridium difficile infection (254), inflammatory bowel disease (255), and irritable bowel syndrome (256). New research has explored the potential of FMT as a promising approach for addressing cardiometabolic disorders (257).
In 2013, the US Food and Drug Administration (FDA) granted its initial approval for FMT, specifically for managing recurrent Clostridium difficile infection. Since then, FMT has gained recognition as a therapy for a wide range of gastrointestinal as well as non-gastrointestinal conditions. However, there remains limited understanding of its mechanism of action and potential long-term side effects (258). The probable therapeutic effects of FMT have also been demonstrated in a number of animal models involving people with severe multiple sclerosis, autism, multidrug-resistant (MDR) infections, and multiple organ failure in seriously confined people (259–261). Recent findings have indicated a lower abundance of Clostridia strains that produce butyrate in the intestines with type 2 diabetes mellitus. Conversely, studies have shown a higher prevalence of non-butyrate-producing Clostridiales in these patients by demonstrated that both insulin sensitivity and levels of butyrate-producing intestinal microbiota significantly improved following microbiota transplantation (262). Experiments on mice raised the possibility of a brain-gut-microbiota axis that goes in each direction. Various neurological conditions like anxiety, depression, dementia such as Alzheimer’s, and Parkinson’s disorder are caused by an imbalance in this axis (263, 264).
Recently, Park et al. (265) from Inho University Hospital in Incheon, South Korea, used FMT to treat a 90-year-old woman who had severe CDI and Alzheimer’s dementia. Her fecal microbiota diversity drastically altered after the transplant, and her cognitive abilities significantly improved, according to a comparison of the results from before and after the procedure. The study also demonstrated a strong correlation between gut flora and cognitive function. Segal et al. (266) conducted distinct clinical research at Soroka University Medical Centre in Israel with six individuals suffering from both Parkinson’s disease and constipation. These patients were given treatment that included Fecal Microbiota Transplantation (FMT). Moreover, Doll et al.(267) used transplantation of fecal microbiota as add on therapy in two patients with major depression. After 4 weeks, both patient signs of depression improved, and study suggested that FMT be tested extensively for MDD treatment. It was found that transferring feces microbiota from healthy rats with normal heart rates to rats with naturally elevated levels produced positive results. The results included lower systolic blood pressure, enhanced blood vessel functionality, lower levels of oxidative stress and inflammation within blood vessels, and a more favorable balance between two unique types of immune cells, Th17 and Tregs (126, 268). However, the curative benefits of FMT can be linked to a broader variety of bacteria, viruses, fungi, and archaea that can engraft into the recipient host and increase the functional variety of a microbiota FMT is also being examined in almost 300 clinical trials for a variety of disease indications, including autoimmune diseases, neurological difficulties, cancer, host disease, and metabolic and gastrointestinal disorders. There is currently insufficient data to support the relevance of fecal microbiome transplantation about gut microbiota in human patients with CVD, necessitating more research in this field. Different approaches and processing variations, such as donor selection and testing, fecal microbiome transplantation via the upper gastrointestinal tract, enema, or colonoscopy, as well as short- and long-term patient monitoring for adverse effects and treatment efficacy, introduce new challenges to be investigated.
Exercise
Physical inactivity holds substantial significance as a risk factor for a range of metabolic disorders, and roughly 1/3 of world’s population contributes in inadequate levels of physical activity, which has implications for health (269). Statistics indicate that roughly 3.2million deaths annually can be attributed to inadequate levels of physical activity (270), with healthcare expenses amounting to $117 billion yearly, attributed to conditions resulting from a lack of exercise (271). People who adopt a sedentary lifestyle and fail to engage in regular physical activity are more prone to the development of cardiovascular disease (272), and who are less active face a 30-50% higher risk of developing high blood pressure (273). The researchers predicted that lack of exercise was responsible for 12% of myocardial infarctions (MI), a risk proportion that fell within high blood pressure (18%), CVD cases (6%) and diabetic mellitus (10%) recognized risk factors for heart disease whose incidence is also inversely related to physical activity levels (274, 275). The study confirmed that exercise can enrich the microflora diversity; improve the F/M ratio, which may contribute to weight loss, obesity-related pathologies, and gastrointestinal disorders; and stimulate the proliferation of bacteria, which can modulate mucosal immunity and maintain homeostasis (276–278). Research has demonstrated that exercise has the capacity to increase the levels of the bacterial metabolite known as butyrate (279).While human research in this area is limited, data from several laboratories, including our own, indicate that exercise training can exert a noteworthy influence on the gut microbiota in animal models (280–282). Moreover, the modifications in the gut microbiota brought about by exercise are linked to changes in the host’s physiology, such changes include metabolic rate modifications (283), immunity (280), and even behavior (281). Certainly, exercise training has been demonstrated to increase the concentrations of short-chain fatty acids derived from the gut microbiota in mouse models (284), comprising of two to six carbon atoms, play a crucial role as an energy source for various tissues and are associated with beneficial effects such as reducing inflammation (285), improving insulin sensitivity (286), and inducing the morphology of the central nervous system (287). Notably, levels of LPS are elevated in cardiovascular disease and specific cardio metabolic disorders (288). However, high-endurance training has been shown to have the potential to decrease plasma LPS levels. This suggests that exercise may have a positive impact on reducing inflammation associated with CVD and related metabolic conditions (289). A crucial observation to highlight is that the advantages provided by the gut microbiota due to exercise training were not enduring. This emphasizes the necessity for consistent and regular exercise to sustain a constructive impact on the gut microbiota and the associated health benefits (279). The findings highlighted the broad-ranging benefits of influencing the gut microbiota via physical exercise, stretching beyond the realm of cardiovascular health. Nonetheless, it’s important to recognize that substantial and enduring advantages necessitate prolonged periods and higher-intensity aerobic training. Participating in more extended and intense exercise sessions seems to be pivotal in achieving lasting enhancements in gut microbiota composition and the correlated health advantages for the individual. Consistently adhering to such exercise routines is pivotal for maximizing the influence on the gut microbiota and overall well-being (290). To protect the heart and arteries, physical activity can increase insulin sensitivity, reduce plasma dyslipidemia, proper raise blood pressure, decrease blood viscosity, promote endothelial nitric oxide generation, and improve leptin sensitivity. Furthermore, the preventive impact of exercise on the body involves not only laboratory animal models but also clinical studies, as proven by WHO recommendations (291). Numerous studies have illustrated a clear dose-response correlation between physical activity levels and a decreased incidence of CVD and characterized by reductions in factors such as blood pressure, body weight, oxidized low-density lipoprotein (ox-LDL), and improved glucose tolerance as physical activity increases (292, 293). Although it’s known that exercise protects against CVD by reducing sympathetic impulses, arterial pressure, and heart rate, increasing blood flow and endothelial NO production, causing vessel dilation, and decreasing inflammatory cytokines and oxygen radical formation, the precise processes that lead to transcriptional factor modifications are unknown. Future research could focus on the mechanisms of exercise’s protective effects on the heart and arteries.
Conclusions
The human intestine is the habitat of the most enormous and varied population of microbes. The main purpose of the gut microbes is to prevent the expansion of potentially lethal germs. However, there is a rising acknowledgment of the intestinal microbiota as a risk variable for developing cardiovascular disease (CVD). Metabolites derived from the gut microbiota, such as short-chain fatty acids, trimethylamine-N-oxide, bile acids, and polyphenols, are critical in maintaining normal cardiovascular function. When these metabolites are out of balance, it has the potential to contribute to an outbreak of CVD. Variations in the composition and diversity of the gut microbiota, known as dysbiosis, have been associated with disorders such as heart failure, atherosclerosis, hypertension, myocardial fibrosis, myocardial infarction, and coronary artery disease. However, the specific mechanisms behind these relationships are still unknown. As a result, the microbiota and its metabolites have emerged as a novel therapeutic target for both CVD prevention and treatment. Ongoing attempts are being made to widen the application of microbiota therapies not only for CVD but also for a variety of other human disorders. Innovations in genomic and metabolomic technology have enabled improved characterization and molecular research of bacteria and their metabolites. Individual microbiome may be profiled in the future utilizing metabolomic/biomarker analysis to measure individual health, potentially delivering specific guidance on food and lifestyle changes. Dietary treatments, the use of pre/probiotics and antibiotics, FMT, TMAO reduction, and regular exercise are current strategies for regulating gut bacteria to improve cardiac function. Further research on microbe-microbe and microbe-host associations may explain how specific metabolites affect the disease process. Improving our understanding of the complex interplay between gut microbiota, host characteristics, and therapeutic response is critical for developing breakthrough precision therapies for cardiovascular disease.
Statements
Author contributions
AL: Writing – original draft. AH: Writing – review & editing. MU: Writing – review & editing. SN: Writing – review & editing. MehrajU: Writing – review & editing. LZ: Writing – review & editing. AU: Writing – review & editing. KU: Writing – review & editing. WA: Writing – review & editing. GW: Writing – review & editing, Funding acquisition, Supervision.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (12032007, 31971242); the Science and Technology Innovation Project of JinFeng Laboratory, Chongqing, China (jfkyjf202203001); Chongqing University of Science and Technology, natural science fund surface project, (CSTB2023NSCQ-MSX1060)
Acknowledgments
We are thankful for the First Batch of Key Disciplines on Public Health in Chongqing and the Public Experiment Centre of State Bioindustrial Base (Chongqing), China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Hou K Wu Z-X Chen X-Y Wang J-Q Zhang D Xiao C et al . Microbiota in health and diseases. Signal transduction targeted Ther (2022) 7:135. doi: 10.1038/s41392-022-00974-4
2
Rahman MM Islam F Or-Rashid MH Mamun AA Rahaman MS Islam MM et al . The gut microbiota (microbiome) in cardiovascular disease and its therapeutic regulation. Front Cell Infection Microbiol (2022) 12:903570. doi: 10.3389/fcimb.2022.903570
3
Ding Y-N Tang X Chen H-Z Liu D-P . Epigenetic regulation of vascular aging and age-related vascular diseases. Aging Aging-Related Diseases: Mech Interventions (2018), 55–75. doi: 10.1007/978-981-13-1117-8_4
4
Velasquez MT Centron P Barrows I Dwivedi R Raj DS . Gut microbiota and cardiovascular uremic toxicities. Toxins (2018) 10:287. doi: 10.3390/toxins10070287
5
Ren S-C Chen X Gong H Wang H Wu C Li P-H et al . SIRT6 in vascular diseases, from bench to bedside. Aging Dis (2022) 13:1015. doi: 10.14336/AD.2021.1204
6
Hemmati M Kashanipoor S Mazaheri P Alibabaei F Babaeizad A Asli S et al . Importance of gut microbiota metabolites in the development of cardiovascular diseases (CVD). Life Sci (2023) 121947. doi: 10.1016/j.lfs.2023.121947
7
Marzullo P Di Renzo L Pugliese G De Siena M Barrea L Muscogiuri G et al . From obesity through gut microbiota to cardiovascular diseases: a dangerous journey. Int J Obes Suppl (2020) 10:35–49. doi: 10.1038/s41367-020-0017-1
8
Rehman S Rehman E Ikram M Jianglin Z . Cardiovascular disease (CVD): assessment, prediction and policy implications. BMC Public Health (2021) 21:1–14. doi: 10.1186/s12889-021-11334-2
9
Zoungas S Curtis AJ Mcneil JJ Tonkin AM . Treatment of dyslipidemia and cardiovascular outcomes: the journey so far—Is this the end for statins? Clin Pharmacol Ther (2014) 96:192–205. doi: 10.1038/clpt.2014.86
10
Haybar H Shokuhian M Bagheri M Davari N Saki N . Involvement of circulating inflammatory factors in prognosis and risk of cardiovascular disease. J Mol Cell Cardiol (2019) 132:110–9. doi: 10.1016/j.yjmcc.2019.05.010
11
Xu H Wang X Feng W Liu Q Zhou S Liu Q et al . The gut microbiota and its interactions with cardiovascular disease. Microbial Biotechnol (2020) 13:637–56. doi: 10.1111/1751-7915.13524
12
Almeida C Barata P Fernandes R . The influence of gut microbiota in cardiovascular diseases—a brief review. Porto Biomed J (2021) 6. doi: 10.1097/j.pbj.0000000000000106
13
Naghavi M Wang H Lozano R Davis A Liang X Zhou M . GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (2015) 385:117–71. doi: 10.1016/S0140-6736(14)61682-2
14
Mozaffarian D Benjamin EJ Go AS Arnett DK Blaha MJ Cushman M et al . Heart disease and stroke statistics—2016 update: a report from the American Heart Association. circulation (2016) 133:e38–e360.
15
Novakovic M Rout A Kingsley T Kirchoff R Singh A Verma V et al . Role of gut microbiota in cardiovascular diseases. World J Cardiol (2020) 12:110. doi: 10.4330/wjc.v12.i4.110
16
Degruttola AK Low D Mizoguchi A Mizoguchi E . Current understanding of dysbiosis in disease in human and animal models. Inflammation Bowel Dis (2016) 22:1137–50. doi: 10.1097/MIB.0000000000000750
17
Rinninella E Raoul P Cintoni M Franceschi F Miggiano G Gasbarrini A et al . What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms (2019) 7:14. doi: 10.3390/microorganisms7010014
18
Canfora EE Blaak EE . Acetate: a diet-derived key metabolite in energy metabolism: good or bad in context of obesity and glucose homeostasis? Curr Opin Clin Nutr Metab Care (2017) 20:477–83. doi: 10.1097/MCO.0000000000000408
19
Dekaboruah E Suryavanshi MV Chettri D Verma AK . Human microbiome: an academic update on human body site specific surveillance and its possible role. Arch Microbiol (2020) 202:2147–67. doi: 10.1007/s00203-020-01931-x
20
Jandhyala SM Talukdar R Subramanyam C Vuyyuru H Sasikala M Reddy DN . Role of the normal gut microbiota. World J gastroenterology: WJG (2015) 21:8787. doi: 10.3748/wjg.v21.i29.8787
21
Yatsunenko T Rey FE Manary MJ Trehan I Dominguez-Bello MG Contreras M et al . Human gut microbiome viewed across age and geography. nature (2012) 486:222–7. doi: 10.1038/nature11053
22
Ramezani A Raj DS . The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrology: JASN (2014) 25:657. doi: 10.1681/ASN.2013080905
23
Kumari S Taliyan R Dubey SK . Comprehensive review on potential signaling pathways involving the transfer of α-synuclein from the gut to the brain that leads to Parkinson’s disease. ACS Chem Neurosci (2023) 14:590–602. doi: 10.1021/acschemneuro.2c00730
24
Eckburg PB Bik EM Bernstein CN Purdom E Dethlefsen L Sargent M et al . Diversity of the human intestinal microbial flora. science (2005) 308:1635–8. doi: 10.1126/science.1110591
25
Wen Y Sun Z Xie S Hu Z Lan Q Sun Y et al . Intestinal flora derived metabolites affect the occurrence and development of cardiovascular disease. J Multidiscip Healthcare (2022), 2591–603. doi: 10.2147/JMDH.S367591
26
Li X Fan Z Cui J Li D Lu J Cui X et al . Trimethylamine N-oxide in heart failure: a meta-analysis of prognostic value. Front Cardiovasc Med (2022) 9:817396. doi: 10.3389/fcvm.2022.817396
27
He J Zhang P Shen L Niu L Tan Y Chen L et al . Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int J Mol Sci (2020) 21:6356. doi: 10.3390/ijms21176356
28
Wang Z Klipfell E Bennett BJ Koeth R Levison BS Dugar B et al . Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature (2011) 472:57–63. doi: 10.1038/nature09922
29
Ahmadmehrabi S Tang WW . Gut microbiome and its role in cardiovascular diseases. Curr Opin Cardiol (2017) 32:761. doi: 10.1097/HCO.0000000000000445
30
Koren O Spor A Felin J Fåk F Stombaugh J Tremaroli V et al . Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci (2011) 108:4592–8. doi: 10.1073/pnas.1011383107
31
Karlsson FH Fåk F Nookaew I Tremaroli V Fagerberg B Petranovic D et al . Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun (2012) 3:1245. doi: 10.1038/ncomms2266
32
Yamashiro K Tanaka R Urabe T Ueno Y Yamashiro Y Nomoto K et al . Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke. PloS One (2017) 12:e0171521.
33
Jin L Shi X Yang J Zhao Y Xue L Xu L et al . Gut microbes in cardiovascular diseases and their potential therapeutic applications. Protein Cell (2021) 12:346–59. doi: 10.1007/s13238-020-00785-9
34
Hemarajata P Versalovic J . Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Ther Adv Gastroenterol (2013) 6:39–51. doi: 10.1177/1756283X12459294
35
Sender R Fuchs S Milo R . Revised estimates for the number of human and bacteria cells in the body. PloS Biol (2016) 14:e1002533. doi: 10.1371/journal.pbio.1002533
36
Vaiserman A Romanenko M Piven L Moseiko V Lushchak O Kryzhanovska N et al . Differences in the gut Firmicutes to Bacteroidetes ratio across age groups in healthy Ukrainian population. BMC Microbiol (2020) 20:1–8. doi: 10.1186/s12866-020-01903-7
37
Zhi C Huang J Wang J Cao H Bai Y Guo J et al . Connection between gut microbiome and the development of obesity. Eur J Clin Microbiol Infect Dis (2019) 38:1987–98. doi: 10.1007/s10096-019-03623-x
38
Anhê FF Barra NG Cavallari JF Henriksbo BD Schertzer JD . Metabolic endotoxemia is dictated by the type of lipopolysaccharide. Cell Rep (2021) 36.
39
Wang L Wang S Zhang Q He C Fu C Wei Q . The role of the gut microbiota in health and cardiovascular diseases. Mol biomedicine (2022) 3:30. doi: 10.1186/s43556-022-00091-2
40
Indiani C.M.D.S.P. Rizzardi KF Castelo PM Ferraz LFC Darrieux M Parisotto TM . Childhood obesity and Firmicutes/Bacteroidetes ratio in the gut microbiota: a systematic review. Childhood Obes (2018) 14:501–9. doi: 10.1089/chi.2018.0040
41
Pascale A Marchesi N Govoni S Coppola A Gazzaruso C . The role of gut microbiota in obesity, diabetes mellitus, and effect of metformin: new insights into old diseases. Curr Opin Pharmacol (2019) 49:1–5. doi: 10.1016/j.coph.2019.03.011
42
Sanchez-Gimenez R Ahmed-Khodja W Molina Y Peiró OM Bonet G Carrasquer A et al . Gut microbiota-derived metabolites and cardiovascular disease risk: a systematic review of prospective cohort studies. Nutrients (2022) 14:2654. doi: 10.3390/nu14132654
43
Hoyles L Jiménez-Pranteda ML Chilloux J Brial F Myridakis A Aranias T et al . Metabolic retroconversion of trimethylamine N-oxide and the gut microbiota. Microbiome (2018) 6:1–14. doi: 10.1186/s40168-018-0461-0
44
Mutengo KH Masenga SK Mweemba A Mutale W Kirabo A . Gut microbiota dependant trimethylamine N-oxide and hypertension. Front Physiol (2023) 14:1075641. doi: 10.3389/fphys.2023.1075641
45
Shanmugham M Bellanger S Leo CH . Gut-derived metabolite, trimethylamine-N-oxide (TMAO) in cardio-metabolic diseases: detection, mechanism, and potential therapeutics. Pharmaceuticals (2023) 16:504. doi: 10.3390/ph16040504
46
Tacconi E Palma G De Biase D Luciano A Barbieri M De Nigris F et al . Microbiota effect on trimethylamine N-oxide production: from cancer to fitness—A practical preventing recommendation and therapies. Nutrients (2023) 15:563. doi: 10.3390/nu15030563
47
Koeth RA Wang Z Levison BS Buffa JA Org E Sheehy BT et al . Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med (2013) 19:576–85. doi: 10.1038/nm.3145
48
Tang WW Wang Z Levison BS Koeth RA Britt EB Fu X et al . Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. New Engl J Med (2013) 368:1575–84. doi: 10.1056/NEJMoa1109400
49
Lemos BS Medina-Vera I Malysheva OV Caudill MA Fernandez ML . Effects of egg consumption and choline supplementation on plasma choline and trimethylamine-N-oxide in a young population. J Am Coll Nutr (2018) 37:716–23. doi: 10.1080/07315724.2018.1466213
50
Jing L Zhang H Xiang Q Shen L Guo X Zhai C et al . Targeting trimethylamine N-oxide: A new therapeutic strategy for alleviating atherosclerosis. Front Cardiovasc Med (2022) 9:864600. doi: 10.3389/fcvm.2022.864600
51
Bu J Wang Z . Cross-talk between gut microbiota and heart via the routes of metabolite and immunity. Gastroenterol Res Pract (2018) 2018. doi: 10.1155/2018/6458094
52
Jiang C Hou X Gao X Liu P Guo X Hu G et al . The 16S rDNA high-throughput sequencing correlation analysis of milk and gut microbial communities in mastitis Holstein cows. BMC Microbiol (2023) 23:1–12. doi: 10.1186/s12866-023-02925-7
53
Shkoporov AN Hill C . Bacteriophages of the human gut: the “known unknown” of the microbiome. Cell Host Microbe (2019) 25:195–209. doi: 10.1016/j.chom.2019.01.017
54
Mukherjee S Joardar N Sengupta S Babu SPS . Gut microbes as future therapeutics in treating inflammatory and infectious diseases: lessons from recent findings. J Nutr Biochem (2018) 61:111–28. doi: 10.1016/j.jnutbio.2018.07.010
55
Jones RM . Focus: Microbiome: The influence of the gut microbiota on host physiology: In pursuit of mechanisms. Yale J Biol Med (2016) 89:285.
56
Neish A . Reviews in basic and clinical gastroenterology. Gastroenterology (2009) 136:65–80. doi: 10.1053/j.gastro.2008.10.080
57
Gordon S . Phagocytosis: the legacy of metchnikoff. Cell (2016) 166:1065–8. doi: 10.1016/j.cell.2016.08.017
58
Di Stefano M Santonocito S Polizzi A Mauceri R Troiano G Lo Giudice A et al . A reciprocal link between oral, gut microbiota during periodontitis: the potential role of probiotics in reducing dysbiosis-induced inflammation. Int J Mol Sci (2023) 24:1084. doi: 10.3390/ijms24021084
59
Mendoza-León MJ Mangalam AK Regaldiz A González-Madrid E Rangel-Ramírez MA Álvarez-Mardonez O et al . Gut microbiota short-chain fatty acids and their impact on the host thyroid function and diseases. Front Endocrinol (2023) 14.
60
Doskaliuk B . ÉLIE METCHNIKOFF’S LEGACY IN THE FIELD OF ORTHOBIOSIS. Anti-Aging Eastern Europe (2023) 2:54–8. doi: 10.56543/aaeeu.2023.2.1.10
61
Adamsson I Nord CE Lundquist P Sjöstedt S Edlund C . Comparative effects of omeprazole, amoxycillin plus metronidazole versus omeprazole, clarithromycin plus metronidazole on the oral, gastric and intestinal microflora in Helicobacter pylori-infected patients. J Antimicrobial Chemotherapy (1999) 44:629–40. doi: 10.1093/jac/44.5.629
62
Jernberg C Löfmark S Edlund C Jansson JK . Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J (2007) 1:56–66. doi: 10.1038/ismej.2007.3
63
Dethlefsen L Huse S Sogin ML Relman DA . The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PloS Biol (2008) 6:e280. doi: 10.1371/journal.pbio.0060280
64
Jakobsson HE Jernberg C Andersson AF Sjölund-Karlsson M Jansson JK Engstrand L . Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PloS One (2010) 5:e9836. doi: 10.1371/journal.pone.0009836
65
Ley RE Bäckhed F Turnbaugh P Lozupone CA Knight RD Gordon JI . Obesity alters gut microbial ecology. Proc Natl Acad Sci (2005) 102:11070–5. doi: 10.1073/pnas.0504978102
66
Turnbaugh PJ Ridaura VK Faith JJ Rey FE Knight R Gordon JI . The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Trans Med (2009) 1:6ra14–16ra14. doi: 10.1126/scitranslmed.3000322
67
Bisanz JE Upadhyay V Turnbaugh JA Ly K Turnbaugh PJ . Meta-analysis reveals reproducible gut microbiome alterations in response to a high-fat diet. Cell Host Microbe (2019) 26:265–272. e264. doi: 10.1016/j.chom.2019.06.013
68
Ju M Liu Y Li M Cheng M Zhang Y Deng G et al . Baicalin improves intestinal microecology and abnormal metabolism induced by high-fat diet. Eur J Pharmacol (2019) 857:172457. doi: 10.1016/j.ejphar.2019.172457
69
Rodríguez JM Murphy K Stanton C Ross RP Kober OI Juge N et al . The composition of the gut microbiota throughout life, with an emphasis on early life. Microbial Ecol Health Dis (2015) 26:26050. doi: 10.3402/mehd.v26.26050
70
Cong X Xu W Romisher R Poveda S Forte S Starkweather A et al . Focus: Microbiome: Gut microbiome and infant health: Brain-gut-microbiota axis and host genetic factors. Yale J Biol Med (2016) 89:299.
71
Tsiaoussis J Antoniou MN Koliarakis I Mesnage R Vardavas CI Izotov BN et al . Effects of single and combined toxic exposures on the gut microbiome: Current knowledge and future directions. Toxicol Lett (2019) 312:72–97. doi: 10.1016/j.toxlet.2019.04.014
72
Werbner M Barsheshet Y Werbner N Zigdon M Averbuch I Ziv O et al . Social-stress-responsive microbiota induces stimulation of self-reactive effector T helper cells. MSystems (2019) 4:e00292–00218. doi: 10.1128/mSystems.00292-18
73
Chang C-S Kao C-Y . Current understanding of the gut microbiota shaping mechanisms. J Biomed Sci (2019) 26:1–11. doi: 10.1186/s12929-019-0554-5
74
Cahana I Iraqi FA . Impact of host genetics on gut microbiome: Take-home lessons from human and mouse studies. Anim Models Exp Med (2020) 3:229–36. doi: 10.1002/ame2.12134
75
Carding S Verbeke K Vipond DT Corfe BM Owen LJ . Dysbiosis of the gut microbiota in disease. Microbial Ecol Health Dis (2015) 26:26191. doi: 10.3402/mehd.v26.26191
76
Peterson D Bonham KS Rowland S Pattanayak CW Consortium R Klepac-Ceraj V . Comparative analysis of 16S rRNA gene and metagenome sequencing in pediatric gut microbiomes. Front Microbiol (2021) 12:670336. doi: 10.3389/fmicb.2021.670336
77
Jie Z Xia H Zhong S-L Feng Q Li S Liang S et al . The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun (2017) 8:845. doi: 10.1038/s41467-017-00900-1
78
The HC Le S-NH . Dynamic of the human gut microbiome under infectious diarrhea. Curr Opin Microbiol (2022) 66:79–85. doi: 10.1016/j.mib.2022.01.006
79
Emoto T Yamashita T Kobayashi T Sasaki N Hirota Y Hayashi T et al . Characterization of gut microbiota profiles in coronary artery disease patients using data mining analysis of terminal restriction fragment length polymorphism: gut microbiota could be a diagnostic marker of coronary artery disease. Heart vessels (2017) 32:39–46. doi: 10.1007/s00380-016-0841-y
80
Aly AM Adel A El-Gendy AO Essam TM Aziz RK . Gut microbiome alterations in patients with stage 4 hepatitis C. Gut Pathog (2016) 8:1–12. doi: 10.1186/s13099-016-0124-2
81
Ziganshina EE Sharifullina DM Lozhkin AP Khayrullin RN Ignatyev IM Ziganshin AM . Bacterial communities associated with atherosclerotic plaques from Russian individuals with atherosclerosis. PloS One (2016) 11:e0164836. doi: 10.1371/journal.pone.0164836
82
Li Z Liu K Zhao J Yang L Chen G Liu A et al . Antibiotics in elderly Chinese population and their relations with hypertension and pulse pressure. Environ Sci pollut Res (2022) 29:67026–45. doi: 10.1007/s11356-022-20613-3
83
Yan Q Gu Y Li X Yang W Jia L Chen C et al . Alterations of the gut microbiome in hypertension. Front Cell infection Microbiol (2017) 7:381. doi: 10.3389/fcimb.2017.00381
84
Altemani F Barrett HL Gomez-Arango L Josh P Mcintyre HD Callaway LK et al . Pregnant women who develop preeclampsia have lower abundance of the butyrate-producer Coprococcus in their gut microbiota. Pregnancy hypertension (2021) 23:211–9. doi: 10.1016/j.preghy.2021.01.002
85
Cui X Wang X Chang X Bao L Wu J Tan Z et al . A new capacity of gut microbiota: Fermentation of engineered inorganic carbon nanomaterials into endogenous organic metabolites. Proc Natl Acad Sci (2023) 120:e2218739120. doi: 10.1073/pnas.2218739120
86
Luedde M Winkler T Heinsen FA Rühlemann MC Spehlmann ME Bajrovic A et al . Heart failure is associated with depletion of core intestinal microbiota. ESC Heart failure (2017) 4:282–90. doi: 10.1002/ehf2.12155
87
Lupu VV Adam Raileanu A Mihai CM Morariu ID Lupu A Starcea IM et al . The implication of the gut microbiome in heart failure. Cells (2023) 12:1158. doi: 10.3390/cells12081158
88
Pasini E Aquilani R Testa C Baiardi P Angioletti S Boschi F et al . Pathogenic gut flora in patients with chronic heart failure. JACC: Heart Failure (2016) 4:220–7. doi: 10.1016/j.jchf.2015.10.009
89
Kamo T Akazawa H Suda W Saga-Kamo A Shimizu Y Yagi H et al . Dysbiosis and compositional alterations with aging in the gut microbiota of patients with heart failure. PloS One (2017) 12:e0174099. doi: 10.1371/journal.pone.0174099
90
Cui X Ye L Li J Jin L Wang W Li S et al . Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci Rep (2018) 8:635. doi: 10.1038/s41598-017-18756-2
91
Zuo K Li J Li K Hu C Gao Y Chen M et al . Disordered gut microbiota and alterations in metabolic patterns are associated with atrial fibrillation. Gigascience (2019) 8:giz058. doi: 10.1093/gigascience/giz058
92
Ascher S Reinhardt C . The gut microbiota: an emerging risk factor for cardiovascular and cerebrovascular disease. Eur J Immunol (2018) 48:564–75. doi: 10.1002/eji.201646879
93
Ponikowski P Anker SD Alhabib KF Cowie MR Force TL Hu S et al . Heart failure: preventing disease and death worldwide. ESC Heart failure (2014) 1:4–25. doi: 10.1002/ehf2.12005
94
Rogers C Bush N . Heart failure: pathophysiology, diagnosis, medical treatment guidelines, and nursing management. Nurs Clinics (2015) 50:787–99.
95
Dickstein K Jaarsma T . Heart failure management programmes: delivering the message. (2005).
96
Shirazi LF Bissett J Romeo F Mehta JL . Role of inflammation in heart failure. Curr Atheroscl Rep (2017) 19:1–9. doi: 10.1007/s11883-017-0660-3
97
Dantzer R Cohen S Russo SJ Dinan TG . Resilience and immunity. Brain behavior Immun (2018) 74:28–42. doi: 10.1016/j.bbi.2018.08.010
98
Moshkelgosha S Masetti G Berchner-Pfannschmidt U Verhasselt HL Horstmann M Diaz-Cano S et al . Gut microbiome in BALB/c and C57BL/6J mice undergoing experimental thyroid autoimmunity associate with differences in immunological responses and thyroid function. Hormone Metab Res (2018) 50:932–41. doi: 10.1055/a-0653-3766
99
Sandek A Bauditz J Swidsinski A Buhner S Weber-Eibel J Von Haehling S et al . Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol (2007) 50:1561–9. doi: 10.1016/j.jacc.2007.07.016
100
Niebauer J Yolk ID Kemp M Dominguez M Schumann RR Rauchhaus M et al . Endotoxin and inunune activation in chronic heart failure: a prospective cohort study. Lancet (1999) 353:1838–42. doi: 10.1016/S0140-6736(98)09286-1
101
Tang WW Kitai T Hazen SL . Gut microbiota in cardiovascular health and disease. Circ Res (2017) 120:1183–96. doi: 10.1161/CIRCRESAHA.117.309715
102
Li H Liu W Lei Y Zhou H Wang P Li J . Professor jun li treating vascular dementia from mutual conclusion of phlegm and blood stasis. J Clin Nurs Res (2022) 6:67–75. doi: 10.26689/jcnr.v6i1.2904
103
Schuett K Kleber ME Scharnagl H Lorkowski S März W Niessner A et al . Trimethylamine-N-oxide and heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol (2017) 70:3202–4. doi: 10.1016/j.jacc.2017.10.064
104
Zhao M Wei H Li C Zhan R Liu C Gao J et al . Gut microbiota production of trimethyl-5-aminovaleric acid reduces fatty acid oxidation and accelerates cardiac hypertrophy. Nat Commun (2022) 13:1757. doi: 10.1038/s41467-022-29060-7
105
Trøseid M Andersen G.Ø Broch K Hov JR . The gut microbiome in coronary artery disease and heart failure: Current knowledge and future directions. EBioMedicine (2020) 52.
106
Kummen M Mayerhofer CC Vestad B Broch K Awoyemi A Storm-Larsen C et al . Gut microbiota signature in heart failure defined from profiling of 2 independent cohorts. J Am Coll Cardiol (2018) 71:1184–6. doi: 10.1016/j.jacc.2017.12.057
107
Wu C-C Hsieh M-Y Hung S-C Kuo K-L Tsai T-H Lai C-L et al . Serum indoxyl sulfate associates with postangioplasty thrombosis of dialysis grafts. J Am Soc Nephrology: JASN (2016) 27:1254. doi: 10.1681/ASN.2015010068
108
Mendelsohn AR Larrick JW . Dietary modification of the microbiome affects risk for cardiovascular disease. Rejuvenation Res (2013) 16:241–4. doi: 10.1089/rej.2013.1447
109
Bentzon JF Otsuka F Virmani R Falk E . Mechanisms of plaque formation and rupture. Circ Res (2014) 114:1852–66. doi: 10.1161/CIRCRESAHA.114.302721
110
Soehnlein O Libby P . Targeting inflammation in atherosclerosis—from experimental insights to the clinic. Nat Rev Drug Discovery (2021) 20:589–610. doi: 10.1038/s41573-021-00198-1
111
Forkosh E Ilan Y . The heart-gut axis: new target for atherosclerosis and congestive heart failure therapy. Open Heart (2019) 6:e000993. doi: 10.1136/openhrt-2018-000993
112
Guiducci L Nicolini G Forini F . Dietary patterns, gut microbiota remodeling, and cardiometabolic disease. Metabolites (2023) 13:760. doi: 10.3390/metabo13060760
113
Jie Z Zhu Q Zou Y Wu Q Qin M He D et al . A consortium of three-bacteria isolated from human feces inhibits formation of atherosclerotic deposits and lowers lipid levels in a mouse model. iScience (2023) 26. doi: 10.1016/j.isci.2023.106960
114
Markowiak-Kopeć P Śliżewska K . The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients (2020) 12:1107. doi: 10.3390/nu12041107
115
Chan YK Brar MS Kirjavainen PV Chen Y Peng J Li D et al . High fat diet induced atherosclerosis is accompanied with low colonic bacterial diversity and altered abundances that correlates with plaque size, plasma A-FABP and cholesterol: a pilot study of high fat diet and its intervention with Lactobacillus rhamnosus GG (LGG) or telmisartan in ApoE–/– mice. BMC Microbiol (2016) 16:1–13. doi: 10.1186/s12866-016-0883-4
116
Zhang H Jiang F Zhang J Wang W Li L Yan J . Modulatory effects of polysaccharides from plants, marine algae and edible mushrooms on gut microbiota and related health benefits: A review. Int J Biol Macromolecules (2022) 204:169–92. doi: 10.1016/j.ijbiomac.2022.01.166
117
Tulkens J Vergauwen G Van Deun J Geeurickx E Dhondt B Lippens L et al . Increased levels of systemic LPS-positive bacterial extracellular vesicles in patients with intestinal barrier dysfunction. Gut (2020) 69:191–3. doi: 10.1136/gutjnl-2018-317726
118
Dickhout A Koenen RR . Extracellular vesicles as biomarkers in cardiovascular disease; chances and risks. Front Cardiovasc Med (2018) 5:113. doi: 10.3389/fcvm.2018.00113
119
Brás IC Khani MH Riedel D Parfentev I Gerhardt E Van Riesen C et al . Ectosomes and exosomes are distinct proteomic entities that modulate spontaneous activity in neuronal cells. bioRxiv (2021). 2021.2006. 2024.449731.
120
Patel S Guo MK Abdul Samad M Howe KL . Extracellular vesicles as biomarkers and modulators of atherosclerosis pathogenesis. Front Cardiovasc Med (2023) 10:1202187. doi: 10.3389/fcvm.2023.1202187
121
Stepankova R Tonar Z Bartova J Nedorost L Rossman P Poledne R et al . Absence of microbiota (germ-free conditions) accelerates the atherosclerosis in ApoE-deficient mice fed standard low cholesterol diet. J Atheroscl Thromb (2010) 17:796–804. doi: 10.5551/jat.3285
122
Kiouptsi K Pontarollo G Todorov H Braun J Jäckel S Koeck T et al . Germ-free housing conditions do not affect aortic root and aortic arch lesion size of late atherosclerotic low-density lipoprotein receptor-deficient mice. Gut Microbes (2020) 11:1809–23. doi: 10.1080/19490976.2020.1767463
123
Gatarek P Kaluzna-Czaplinska J . Trimethylamine N-oxide (TMAO) in human health. EXCLI J (2021) 20:301.
124
Janeiro MH Ramírez MJ Milagro FI Martínez JA Solas M . Implication of trimethylamine N-oxide (TMAO) in disease: potential biomarker or new therapeutic target. Nutrients (2018) 10:1398. doi: 10.3390/nu10101398
125
Holmes E Li JV Marchesi JR Nicholson JK . Gut microbiota composition and activity in relation to host metabolic phenotype and disease risk. Cell Metab (2012) 16:559–64. doi: 10.1016/j.cmet.2012.10.007
126
Tang WW Wang Z Kennedy DJ Wu Y Buffa JA Agatisa-Boyle B et al . Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res (2015) 116:448–55. doi: 10.1161/CIRCRESAHA.116.305360
127
Li M Van Esch BC Henricks PA Folkerts G Garssen J . The anti-inflammatory effects of short chain fatty acids on lipopolysaccharide-or tumor necrosis factor α-stimulated endothelial cells via activation of GPR41/43 and inhibition of HDACs. Front Pharmacol (2018) 9:533. doi: 10.3389/fphar.2018.00533
128
Tayyeb JZ Popeijus HE Mensink RP Konings MC Mokhtar FB Plat J . Short-chain fatty acids (except hexanoic acid) lower NF-kB transactivation, which rescues inflammation-induced decreased apolipoprotein AI transcription in HepG2 cells. Int J Mol Sci (2020) 21:5088. doi: 10.3390/ijms21145088
129
Tom Dieck H Schön C Wagner T Pankoke HC Fluegel M Speckmann B . A synbiotic formulation comprising Bacillus subtilis DSM 32315 and L-alanyl-L-glutamine improves intestinal butyrate levels and lipid metabolism in healthy humans. Nutrients (2021) 14:143. doi: 10.3390/nu14010143
130
Larkin TA Astheimer LB Price WE . Dietary combination of soy with a probiotic or prebiotic food significantly reduces total and LDL cholesterol in mildly hypercholesterolaemic subjects. Eur J Clin Nutr (2009) 63:238–45. doi: 10.1038/sj.ejcn.1602910
131
Wu Y Jin A Xie G Wang L Liu K Jia G et al . The 20 most important and most preventable health problems of China: a Delphi consultation of Chinese experts. Am J Public Health (2018) 108:1592–8. doi: 10.2105/AJPH.2018.304684
132
Burström B Tao W . Social determinants of health and inequalities in COVID-19. European Public Health Association, Oxford, United Kingdom: Oxford University Press (2020).
133
Annual report on cardiovascular health and diseases in China. (2022) 37:553–78.
134
Brantsæter AL Myhre R Haugen M Myking S Sengpiel V Magnus P et al . Intake of probiotic food and risk of preeclampsia in primiparous women: the Norwegian Mother and Child Cohort Study. Am J Epidemiol (2011) 174:807–15. doi: 10.1093/aje/kwr168
135
Thomson A . Health professionals' knowledge and attitudes toward vitamin D in the general population, pregnancy, and infancy: a thesis presented in partial fulfilment of the requirements for the degree of Master of Science in Nutrition and Dietetics. Massey University, Albany, New Zealand: Massey University (2020).
136
Carey RM . 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA. (2017).
137
Williams B Mancia G Spiering W Agabiti Rosei E Azizi M Burnier M et al . 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. Blood Pressure (2018) 27:314–40. doi: 10.1080/08037051.2018.1527177
138
Cao L Li X Yan P Wang X Li M Li R et al . The effectiveness of aerobic exercise for hypertensive population: a systematic review and meta-analysis. J Clin Hypertension (2019) 21:868–76. doi: 10.1111/jch.13583
139
Mell B Jala VR Mathew AV Byun J Waghulde H Zhang Y et al . Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics (2015) 47:187–97. doi: 10.1152/physiolgenomics.00136.2014
140
Yang T Santisteban MM Rodriguez V Li E Ahmari N Carvajal JM et al . Gut dysbiosis is linked to hypertension. hypertension (2015) 65:1331–40. doi: 10.1161/HYPERTENSIONAHA.115.05315
141
Adnan S Nelson JW Ajami NJ Venna VR Petrosino JF Bryan RM Jr. et al . Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics (2017) 49:96–104. doi: 10.1152/physiolgenomics.00081.2016
142
Vickers NJ . Animal communication: when i’m calling you, will you answer too? Curr Biol (2017) 27:R713–5. doi: 10.1016/j.cub.2017.05.064
143
Kim S Goel R Kumar A Qi Y Lobaton G Hosaka K et al . Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci (2018) 132:701–18. doi: 10.1042/CS20180087
144
Zhang Z Zhao J Tian C Chen X Li H Wei X et al . Targeting the gut microbiota to investigate the mechanism of lactulose in negating the effects of a high-salt diet on hypertension. Mol Nutr Food Res (2019) 63:1800941. doi: 10.1002/mnfr.201800941
145
Guo H Hao Y Fan X Richel A Everaert N Yang X et al . Administration with quinoa protein reduces the blood pressure in spontaneously hypertensive rats and modifies the fecal microbiota. Nutrients (2021) 13:2446. doi: 10.3390/nu13072446
146
Huart J Leenders J Taminiau B Descy J Saint-Remy A Daube G et al . Gut microbiota and fecal levels of short-chain fatty acids differ upon 24-hour blood pressure levels in men. Hypertension (2019) 74:1005–13. doi: 10.1161/HYPERTENSIONAHA.118.12588
147
Le Poul E Loison C Struyf S Springael J-Y Lannoy V Decobecq M-E et al . Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem (2003) 278:25481–9. doi: 10.1074/jbc.M301403200
148
Pluznick JL Protzko RJ Gevorgyan H Peterlin Z Sipos A Han J et al . Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci (2013) 110:4410–5. doi: 10.1073/pnas.1215927110
149
Natarajan N Pluznick JL . From microbe to man: the role of microbial short chain fatty acid metabolites in host cell biology. Am J Physiology-Cell Physiol (2014) 307:C979–85. doi: 10.1152/ajpcell.00228.2014
150
Wang H Luo Q Ding X Chen L Zhang Z . Trimethylamine N-oxide and its precursors in relation to blood pressure: A mendelian randomization study. Front Cardiovasc Med (2022) 9:922441. doi: 10.3389/fcvm.2022.922441
151
Senthong V Wang Z Li XS Fan Y Wu Y Wilson Tang W et al . Intestinal microbiota-generated metabolite trimethylamine-N-oxide and 5-year mortality risk in stable coronary artery disease: the contributory role of intestinal microbiota in a COURAGE-like patient cohort. J Am Heart Assoc (2016) 5:e002816. doi: 10.1161/JAHA.115.002816
152
Suzuki T Heaney LM Bhandari SS Jones DJ Ng LL . Trimethylamine N-oxide and prognosis in acute heart failure. Heart (2016) 102:841–8. doi: 10.1136/heartjnl-2015-308826
153
Ge X Zheng L Zhuang R Yu P Xu Z Liu G et al . The gut microbial metabolite trimethylamine N-oxide and hypertension risk: a systematic review and dose–response meta-analysis. Adv Nutr (2020) 11:66–76. doi: 10.1093/advances/nmz064
154
Lewis-Mikhael A-M Davoodvandi A Jafarnejad S . Effect of Lactobacillusplantarum containing probiotics on blood pressure: A systematic review and meta-analysis. Pharmacol Res (2020) 153:104663. doi: 10.1016/j.phrs.2020.104663
155
Khalesi S Sun J Buys N Jayasinghe R . Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension (2014) 64:897–903. doi: 10.1161/HYPERTENSIONAHA.114.03469
156
Murphy K O’donovan AN Caplice NM Ross RP Stanton C . Exploring the gut microbiota and cardiovascular disease. Metabolites (2021) 11:493. doi: 10.3390/metabo11080493
157
Centner AM Khalili L Ukhanov V Kadyan S Nagpal R Salazar G . The role of phytochemicals and gut microbiome in atherosclerosis in preclinical mouse models. Nutrients (2023) 15:1212. doi: 10.3390/nu15051212
158
Zeisel SH Warrier M . Trimethylamine N-oxide, the microbiome, and heart and kidney disease. Annu Rev Nutr (2017) 37:157–81. doi: 10.1146/annurev-nutr-071816-064732
159
Li XS Obeid S Klingenberg R Gencer B Mach F Räber L et al . Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J (2017) 38:814–24. doi: 10.1093/eurheartj/ehw582
160
Bennett BJ De Aguiar Vallim TQ Wang Z Shih DM Meng Y Gregory J et al . Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab (2013) 17:49–60. doi: 10.1016/j.cmet.2012.12.011
161
Crisci G Israr MZ Cittadini A Bossone E Suzuki T Salzano A . Heart failure and trimethylamine N-oxide: time to transform a ‘gut feeling’in a fact? ESC Heart Failure (2023) 10:1. doi: 10.1002/ehf2.14205
162
Randrianarisoa E Lehn-Stefan A Wang X Hoene M Peter A Heinzmann SS et al . Relationship of serum trimethylamine N-oxide (TMAO) levels with early atherosclerosis in humans. Sci Rep (2016) 6:26745. doi: 10.1038/srep26745
163
Roncal C Martínez-Aguilar E Orbe J Ravassa S Fernandez-Montero A Saenz-Pipaon G et al . Trimethylamine-N-oxide (TMAO) predicts cardiovascular mortality in peripheral artery disease. Sci Rep (2019) 9:15580. doi: 10.1038/s41598-019-52082-z
164
Hoseini-Tavassol Z Ejtahed H-S Larijani B Hasani-Ranjbar S . Trimethylamine N-Oxide as a potential risk factor for non-communicable diseases: A systematic review. Endocrine Metab Immune Disorders-Drug Targets (Formerly Curr Drug Targets-Immune Endocrine Metab Disorders) (2023) 23:617–32. doi: 10.2174/1871530323666221103120410
165
Querio G Antoniotti S Geddo F Levi R Gallo MP . Modulation of endothelial function by TMAO, a gut microbiota-derived metabolite. Int J Mol Sci (2023) 24:5806. doi: 10.3390/ijms24065806
166
Trøseid M Ueland T Hov J Svardal A Gregersen I Dahl C et al . Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Internal Med (2015) 277:717–26. doi: 10.1111/joim.12328
167
Suzuki T Heaney LM Jones DJ Ng LL . Trimethylamine N-oxide and risk stratification after acute myocardial infarction. Clin Chem (2017) 63:420–8. doi: 10.1373/clinchem.2016.264853
168
Ahmad AF Dwivedi G O’gara F Caparros-Martin J Ward NC . The gut microbiome and cardiovascular disease: current knowledge and clinical potential. Am J Physiology-Heart Circulatory Physiol (2019) 317:H923–38. doi: 10.1152/ajpheart.00376.2019
169
Blacher E Levy M Tatirovsky E Elinav E . Microbiome-modulated metabolites at the interface of host immunity. J Immunol (2017) 198:572–80. doi: 10.4049/jimmunol.1601247
170
Fiorucci S Distrutti E . Bile acid-activated receptors, intestinal microbiota, and the treatment of metabolic disorders. Trends Mol Med (2015) 21:702–14. doi: 10.1016/j.molmed.2015.09.001
171
Donohoe DR Garge N Zhang X Sun W O'connell TM Bunger MK et al . The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab (2011) 13:517–26. doi: 10.1016/j.cmet.2011.02.018
172
Mayerhofer CC Kummen M Holm K Broch K Awoyemi A Vestad B et al . Low fibre intake is associated with gut microbiota alterations in chronic heart failure. ESC Heart Failure (2020) 7:456–66. doi: 10.1002/ehf2.12596
173
Chakaroun RM Olsson LM Bäckhed F . The potential of tailoring the gut microbiome to prevent and treat cardiometabolic disease. Nat Rev Cardiol (2023) 20:217–35. doi: 10.1038/s41569-022-00771-0
174
Aguilar EC Dos Santos LC Leonel AJ De Oliveira JS Santos EA Navia-Pelaez JM et al . Oral butyrate reduces oxidative stress in atherosclerotic lesion sites by a mechanism involving NADPH oxidase down-regulation in endothelial cells. J Nutr Biochem (2016) 34:99–105. doi: 10.1016/j.jnutbio.2016.05.002
175
Aguilar E Leonel A Teixeira L Silva A Silva J Pelaez J et al . Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFκB activation. Nutrition Metab Cardiovasc Dis (2014) 24:606–13. doi: 10.1016/j.numecd.2014.01.002
176
Li J Zhao F Wang Y Chen J Tao J Tian G et al . Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome (2017) 5:1–19. doi: 10.1186/s40168-016-0222-x
177
Masenga SK Hamooya B Hangoma J Hayumbu V Ertuglu LA Ishimwe J et al . Recent advances in modulation of cardiovascular diseases by the gut microbiota. J Hum Hypertension (2022) 36:952–9. doi: 10.1038/s41371-022-00698-6
178
Bartolomaeus H Balogh A Yakoub M Homann S Markó L Höges S et al . Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation (2019) 139:1407–21. doi: 10.1161/CIRCULATIONAHA.118.036652
179
Battson ML Lee DM Li Puma LC Ecton KE Thomas KN Febvre HP et al . Gut microbiota regulates cardiac ischemic tolerance and aortic stiffness in obesity. Am J Physiology-Heart Circulatory Physiol (2019) 317:H1210–20. doi: 10.1152/ajpheart.00346.2019
180
Tang TW Chen H-C Chen C-Y Yen CY Lin C-J Prajnamitra RP et al . Loss of gut microbiota alters immune system composition and cripples postinfarction cardiac repair. Circulation (2019) 139:647–59. doi: 10.1161/CIRCULATIONAHA.118.035235
181
Hanafi NI Mohamed AS Sheikh Abdul Kadir SH Othman MHD . Overview of bile acids signaling and perspective on the signal of ursodeoxycholic acid, the most hydrophilic bile acid, in the heart. Biomolecules (2018) 8:159. doi: 10.3390/biom8040159
182
Insull W Jr . Clinical utility of bile acid sequestrants in the treatment of dyslipidemia: a scientific review. South Med J (2006) 99:257–74. doi: 10.1097/01.smj.0000208120.73327.db
183
Yamaoka-Tojo M Tojo T Izumi T . Beyond cholesterol lowering: pleiotropic effects of bile acid binding resins against cardiovascular disease risk factors in patients with metabolic syndrome. Curr Vasc Pharmacol (2008) 6:271–81. doi: 10.2174/157016108785909698
184
Sun J Fan J Li T Yan X Jiang Y . Nuciferine protects against high-fat diet-induced hepatic steatosis via modulation of gut microbiota and bile acid metabolism in rats. J Agric Food Chem (2022) 70:12014–28. doi: 10.1021/acs.jafc.2c04817
185
Ahmed M . Functional, diagnostic and therapeutic aspects of bile. Clin Exp Gastroenterol (2022), 105–20. doi: 10.2147/CEG.S360563
186
Charach G Argov O Geiger K Charach L Rogowski O Grosskopf I . Diminished bile acids excretion is a risk factor for coronary artery disease: 20-year follow up and long-term outcome. Ther Adv Gastroenterol (2018) 11:1756283X17743420. doi: 10.1177/1756283X17743420
187
Choudhuri S Klaassen CD . Molecular regulation of bile acid homeostasis. Drug Metab Disposition (2022) 50:425–55. doi: 10.1124/dmd.121.000643
188
Raghunatha Reddy R . Biochemical studies on the antilithogenic effect of dietary fenugreek seeds (Trigonella foenum-graecum). India: University of Mysore (2010).
189
Packard C Shepherd J . The hepatobiliary axis and lipoprotein metabolism: effects of bile acid sequestrants and ileal bypass surgery. J Lipid Res (1982) 23:1081–98. doi: 10.1016/S0022-2275(20)38045-7
190
Einarsson K Ericsson S Ewerth S Reihner E Rudling M Ståhlberg D et al . Bile acid sequestrants: mechanisms of action on bile acid and cholesterol metabolism. Eur J Clin Pharmacol (1991) 40:S53–8. doi: 10.1007/BF03216291
191
Di Ciaula A Bonfrate L Baj J Khalil M Garruti G Stellaard F et al . Recent advances in the digestive, metabolic and therapeutic effects of farnesoid X receptor and fibroblast growth factor 19: from cholesterol to bile acid signaling. Nutrients (2022) 14:4950. doi: 10.3390/nu14234950
192
Yokota A Fukiya S Islam KS Ooka T Ogura Y Hayashi T et al . Is bile acid a determinant of the gut microbiota on a high-fat diet? Gut Microbes (2012) 3:455–9. doi: 610.4161/gmic.21216
193
Lau K Srivatsav V Rizwan A Nashed A Liu R Shen R et al . Bridging the gap between gut microbial dysbiosis and cardiovascular diseases. Nutrients (2017) 9:859. doi: 10.3390/nu9080859
194
Wang Z Zhao Y . Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell (2018) 9:416–31. doi: 10.1007/s13238-018-0549-0
195
Rowland I Gibson G Heinken A Scott K Swann J Thiele I et al . Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr (2018) 57:1–24. doi: 10.1007/s00394-017-1445-8
196
Jia B Zou Y Han X Bae J-W Jeon CO . Gut microbiome-mediated mechanisms for reducing cholesterol levels: Implications for ameliorating cardiovascular disease. Trends Microbiol (2023). doi: 10.1016/j.tim.2022.08.003
197
Cavalu S Banica F Gruian C Vanea E Goller G Simon V . Microscopic and spectroscopic investigation of bioactive glasses for antibiotic controlled release. J Mol Structure (2013) 1040:47–52. doi: 10.1016/j.molstruc.2013.02.016
198
Sindhu RK Goyal A Algın Yapar E Cavalu S . Bioactive compounds and nanodelivery perspectives for treatment of cardiovascular diseases. Appl Sci (2021) 11:11031. doi: 10.3390/app112211031
199
Myles IA . Fast food fever: reviewing the impacts of the Western diet on immunity. Nutr J (2014) 13:1–17. doi: 10.1186/1475-2891-13-61
200
Perler BK Friedman ES Wu GD . The role of the gut microbiota in the relationship between diet and human health. Annu Rev Physiol (2023) 85:449–68. doi: 10.1146/annurev-physiol-031522-092054
201
Rothschild D Weissbrod O Barkan E Kurilshikov A Korem T Zeevi D et al . Environment dominates over host genetics in shaping human gut microbiota. Nature (2018) 555:210–5. doi: 10.1038/nature25973
202
Danneskiold-Samsøe NB Barros HDDFQ Santos R Bicas JL Cazarin CBB Madsen L et al . Interplay between food and gut microbiota in health and disease. Food Res Int (2019) 115:23–31. doi: 10.1016/j.foodres.2018.07.043
203
Foye OT Huang I-F Chiou CC Walker WA Shi HN . Early administration of probiotic Lactobacillus acidophilus and/or prebiotic inulin attenuates pathogen-mediated intestinal inflammation and Smad 7 cell signaling. FEMS Immunol Med Microbiol (2012) 65:467–80. doi: 10.1111/j.1574-695X.2012.00978.x
204
Marques FZ Nelson E Chu P-Y Horlock D Fiedler A Ziemann M et al . High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation (2017) 135:964–77. doi: 10.1161/CIRCULATIONAHA.116.024545
205
Eckel R Jakicic J Ard J . 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013 Nov 12 [E-pub ahead of print. J Am Coll Cardiol (2014) 63:3027–8. doi: 10.1016/j.jacc.2013.11.003
206
Kerley CP . Dietary patterns and components to prevent and treat heart failure: a comprehensive review of human studies. Nutr Res Rev (2019) 32:1–27. doi: 10.1017/S0954422418000148
207
Battson ML Lee DM Jarrell DK Hou S Ecton KE Weir TL et al . Suppression of gut dysbiosis reverses Western diet-induced vascular dysfunction. Am J Physiology-Endocrinology Metab (2018) 314:E468–77. doi: 10.1152/ajpendo.00187.2017
208
Chen K Zheng X Feng M Li D Zhang H . Gut microbiota-dependent metabolite trimethylamine N-oxide contributes to cardiac dysfunction in western diet-induced obese mice. Front Physiol (2017) 8:139. doi: 10.3389/fphys.2017.00139
209
De Filippis F Pellegrini N Vannini L Jeffery IB La Storia A Laghi L et al . High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut (2016) 65:1812–21. doi: 10.1136/gutjnl-2015-309957
210
Papadaki A Martínez-González MÁ. Alonso-Gómez A Rekondo J Salas-Salvadó J Corella D et al . Mediterranean diet and risk of heart failure: results from the PREDIMED randomized controlled trial. Eur J Heart Failure (2017) 19:1179–85. doi: 10.1002/ejhf.750
211
Estruch R Ros E Salas-Salvadó J Covas M-I Corella D Arós F et al . Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. New Engl J Med (2018) 378:e34. doi: 10.1056/NEJMoa1800389
212
Wang J Wang P Li D Hu X Chen F . Beneficial effects of ginger on prevention of obesity through modulation of gut microbiota in mice. Eur J Nutr (2020) 59:699–718. doi: 10.1007/s00394-019-01938-1
213
Teng Y Ren Y Sayed M Hu X Lei C Kumar A et al . Plant-derived exosomal microRNAs shape the gut microbiota. Cell Host Microbe (2018) 24:637–652. e638. doi: 10.1016/j.chom.2018.10.001
214
Yeşilyurt N Yılmaz B Ağagündüz D Capasso R . Involvement of probiotics and postbiotics in the immune system modulation. Biologics (2021) 1:89–110. doi: 10.3390/biologics1020006
215
Singh D Singh A Kumar S . Probiotics: friend or foe to the human immune system. Bull Natl Res Centre (2023) 47:1–9. doi: 10.1186/s42269-023-01098-7
216
Gibson GR Roberfroid MB . Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr (1995) 125:1401–12. doi: 10.1093/jn/125.6.1401
217
Karlsson C Ahrné S Molin G Berggren A Palmquist I Fredrikson GN et al . Probiotic therapy to men with incipient arteriosclerosis initiates increased bacterial diversity in colon: a randomized controlled trial. Atherosclerosis (2010) 208:228–33. doi: 10.1016/j.atherosclerosis.2009.06.019
218
Naruszewicz M Johansson M-L Zapolska-Downar D Bukowska H . Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am J Clin Nutr (2002) 76:1249–55. doi: 10.1093/ajcn/76.6.1249
219
Andrade S Borges N . Effect of fermented milk containing Lactobacillus acidophilus and Bifidobacterium longum on plasma lipids of women with normal or moderately elevated cholesterol. J dairy Res (2009) 76:469–74. doi: 10.1017/S0022029909990173
220
Catry E Bindels LB Tailleux A Lestavel S Neyrinck AM Goossens J-F et al . Targeting the gut microbiota with inulin-type fructans: preclinical demonstration of a novel approach in the management of endothelial dysfunction. Gut (2018) 67:271–83. doi: 10.1136/gutjnl-2016-313316
221
Neyrinck AM Goossens J-F Lobysheva I Plovier H Essaghir A Demoulin J-B et al . Targeting the gut microbiota with inulin-type fructans: preclinical demonstration of a novel approach in the management of endothelial dysfunction. (2017).
222
Liu F Prabhakar M Ju J Long H Zhou H . Effect of inulin-type fructans on blood lipid profile and glucose level: a systematic review and meta-analysis of randomized controlled trials. Eur J Clin Nutr (2017) 71:9–20. doi: 10.1038/ejcn.2016.156
223
Cosola C De Angelis M Rocchetti MT Montemurno E Maranzano V Dalfino G et al . Beta-glucans supplementation associates with reduction in p-cresyl sulfate levels and improved endothelial vascular reactivity in healthy individuals. PloS One (2017) 12:e0169635. doi: 10.1371/journal.pone.0169635
224
Sonnenburg ED Smits SA Tikhonov M Higginbottom SK Wingreen NS Sonnenburg JL . Diet-induced extinctions in the gut microbiota compound over generations. Nature (2016) 529:212–5. doi: 10.1038/nature16504
225
Griffin NW Ahern PP Cheng J Heath AC Ilkayeva O Newgard CB et al . Prior dietary practices and connections to a human gut microbial metacommunity alter responses to diet interventions. Cell Host Microbe (2017) 21:84–96. doi: 10.1016/j.chom.2016.12.006
226
Holscher HD . Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes (2017) 8:172–84. doi: 10.1080/19490976.2017.1290756
227
Kootte RS Levin E Salojärvi J Smits LP Hartstra AV Udayappan SD et al . Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab (2017) 26:611–619. e616. doi: 10.1016/j.cmet.2017.09.008
228
Jayachandran M Chen J Chung SSM Xu B . A critical review on the impacts of β-glucans on gut microbiota and human health. J Nutr Biochem (2018) 61:101–10. doi: 10.1016/j.jnutbio.2018.06.010
229
Walton GE Lu C Trogh I Arnaut F Gibson GR . A randomised, double-blind, placebo controlled cross-over study to determine the gastrointestinal effects of consumption of arabinoxylan-oligosaccharides enriched bread in healthy volunteers. Nutr J (2012) 11:1–11. doi: 10.1186/1475-2891-11-36
230
Wang H Zhang W Zuo L Zhu W Wang B Li Q et al . Bifidobacteria may be beneficial to intestinal microbiota and reduction of bacterial translocation in mice following ischaemia and reperfusion injury. Br J Nutr (2013) 109:1990–8. doi: 10.1017/S0007114512004308
231
Jacouton E Chain F Sokol H Langella P Bermudez-Humaran LG . Probiotic strain Lactobacillus casei BL23 prevents colitis-associated colorectal cancer. Front Immunol (2017) 8:1553. doi: 10.3389/fimmu.2017.01553
232
Schneeberger M Everard A Gómez-Valadés AG Matamoros S Ramírez S Delzenne NM et al . Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep (2015) 5:16643. doi: 10.1038/srep16643
233
Fuentes MC Lajo T Carrión JM Cuné J . Cholesterol-lowering efficacy of Lactobacillus plantarum CECT 7527, 7528 and 7529 in hypercholesterolaemic adults. Br J Nutr (2013) 109:1866–72. doi: 10.1017/S000711451200373X
234
Cani PD Bibiloni R Knauf C Waget A Neyrinck AM Delzenne NM et al . Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet–induced obesity and diabetes in mice. Diabetes (2008) 57:1470–81. doi: 10.2337/db07-1403
235
Membrez M Blancher F Jaquet M Bibiloni R Cani PD Burcelin RG et al . Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. FASEB J (2008) 22:2416–26. doi: 10.1096/fj.07-102723
236
Kuczynski J Lauber CL Walters WA Parfrey LW Clemente JC Gevers D et al . Experimental and analytical tools for studying the human microbiome. Nat Rev Genet (2012) 13:47–58. doi: 10.1038/nrg3129
237
Fujisaka S Ussar S Clish C Devkota S Dreyfuss JM Sakaguchi M et al . Antibiotic effects on gut microbiota and metabolism are host dependent. J Clin Invest (2016) 126:4430–43. doi: 10.1172/JCI86674
238
Anderson JL Muhlestein JB . Antibiotic trials for coronary heart disease. Texas Heart Institute J (2004) 31:33.
239
Albert RK Schuller JL Network CCR . Macrolide antibiotics and the risk of cardiac arrhythmias. Am J Respir Crit Care Med (2014) 189:1173–80. doi: 10.1164/rccm.201402-0385CI
240
Cheng Y-J Nie X-Y Chen X-M Lin X-X Tang K Zeng W-T et al . The role of macrolide antibiotics in increasing cardiovascular risk. J Am Coll Cardiol (2015) 66:2173–84. doi: 10.1016/j.jacc.2015.09.029
241
Wong AY Root A Douglas IJ Chui CS Chan EW Ghebremichael-Weldeselassie Y et al . Cardiovascular outcomes associated with use of clarithromycin: population based study. bmj (2016) 352. doi: 10.1136/bmj.h6926
242
Yan X Jin J Su X Yin X Gao J Wang X et al . Intestinal flora modulates blood pressure by regulating the synthesis of intestinal-derived corticosterone in high salt-induced hypertension. Circ Res (2020) 126:839–53. doi: 10.1161/CIRCRESAHA.119.316394
243
Jose PA Raj D . Gut microbiota in hypertension. Curr Opin Nephrol hypertension (2015) 24:403. doi: 10.1097/MNH.0000000000000149
244
Galla S Chakraborty S Cheng X Yeo J Mell B Zhang H et al . Disparate effects of antibiotics on hypertension. Physiol Genomics (2018) 50:837–45. doi: 10.1152/physiolgenomics.00073.2018
245
Rune I Rolin B Larsen C Nielsen DS Kanter JE Bornfeldt KE et al . Modulating the gut microbiota improves glucose tolerance, lipoprotein profile and atherosclerotic plaque development in ApoE-deficient mice. PloS One (2016) 11:e0146439. doi: 10.1371/journal.pone.0146439
246
Li Z Wu Z Yan J Liu H Liu Q Deng Y et al . Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab Invest (2019) 99:346–57. doi: 10.1038/s41374-018-0091-y
247
Yang R Chen Z Cai J . Fecal microbiota transplantation: Emerging applications in autoimmune diseases. J Autoimmun (2023) 103038. doi: 10.1016/j.jaut.2023.103038
248
Tuniyazi M Hu X Fu Y Zhang N . Canine fecal microbiota transplantation: Current application and possible mechanisms. Veterinary Sci (2022) 9:396. doi: 10.3390/vetsci9080396
249
Valles-Colomer M Blanco-Míguez A Manghi P Asnicar F Dubois L Golzato D et al . The person-to-person transmission landscape of the gut and oral microbiomes. Nature (2023) 614:125–35. doi: 10.1038/s41586-022-05620-1
250
Yu D Meng X De Vos WM Wu H Fang X Maiti AK . Implications of gut microbiota in complex human diseases. Int J Mol Sci (2021) 22:12661. doi: 10.3390/ijms222312661
251
Settanni CR Ianiro G Bibbò S Cammarota G Gasbarrini A . Gut microbiota alteration and modulation in psychiatric disorders: Current evidence on fecal microbiota transplantation. Prog Neuropsychopharmacol Biol Psychiatry (2021) 109:110258. doi: 10.1016/j.pnpbp.2021.110258
252
Beyi AF Wannemuehler M Plummer PJ . Impacts of gut microbiota on the immune system and fecal microbiota transplantation as a re-emerging therapy for autoimmune diseases. Antibiotics (2022) 11:1093. doi: 10.3390/antibiotics11081093
253
Ooijevaar RE Terveer EM Verspaget HW Kuijper EJ Keller JJ . Clinical application and potential of fecal microbiota transplantation. Annu Rev Med (2019) 70:335–51. doi: 10.1146/annurev-med-111717-122956
254
Khan MY Dirweesh A Khurshid T Siddiqui WJ . Comparing fecal microbiota transplantation to standard-of-care treatment for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol (2018) 30:1309–17. doi: 10.1097/MEG.0000000000001243
255
D'odorico I Di Bella S Monticelli J Giacobbe DR Boldock E Luzzati R . Role of fecal microbiota transplantation in inflammatory bowel disease. J digestive Dis (2018) 19:322–34. doi: 10.1111/1751-2980.12603
256
El-Salhy M Mazzawi T . Fecal microbiota transplantation for managing irritable bowel syndrome. Expert Rev Gastroenterol Hepatol (2018) 12:439–45. doi: 10.1080/17474124.2018.1447380
257
Vrieze A Van Nood E Holleman F Salojärvi J Kootte RS Bartelsman JF et al . Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology (2012) 143:913–916. e917. doi: 10.1053/j.gastro.2012.06.031
258
Mahmoudi H Hossainpour H . Application and development of fecal microbiota transplantation in the treatment of gastrointestinal and metabolic diseases: A review. Saudi J gastroenterology: Off J Saudi Gastroenterol Assoc (2023) 29:3. doi: 10.4103/sjg.sjg_131_22
259
Innes AJ Mullish BH Ghani R Szydlo RM Apperley JF Olavarria E et al . Fecal microbiota transplant mitigates adverse outcomes seen in patients colonized with multidrug-resistant organisms undergoing allogeneic hematopoietic cell transplantation. Front Cell Infection Microbiol (2021) 11:684659. doi: 10.3389/fcimb.2021.684659
260
Li N Chen H Cheng Y Xu F Ruan G Ying S et al . Fecal microbiota transplantation relieves gastrointestinal and autism symptoms by improving the gut microbiota in an open-label study. Front Cell infection Microbiol (2021) 11:948.
261
Boussamet L Rajoka MSR Berthelot L . Microbiota, IgA and multiple sclerosis. Microorganisms (2022) 10:617. doi: 10.3390/microorganisms10030617
262
Arora T Tremaroli V . Therapeutic potential of butyrate for treatment of type 2 diabetes. Front Endocrinol (2021) 12:761834. doi: 10.3389/fendo.2021.761834
263
Knox EG Aburto MR Tessier C Nagpal J Clarke G O’driscoll CM et al . Microbial-derived metabolites induce actin cytoskeletal rearrangement and protect blood-brain barrier function. iscience (2022) 25. doi: 10.1016/j.isci.2022.105648
264
Su L Hong Z Zhou T Jian Y Xu M Zhang X et al . Health improvements of type 2 diabetic patients through diet and diet plus fecal microbiota transplantation. Sci Rep (2022) 12:1152. doi: 10.1038/s41598-022-05127-9
265
Park S-H Lee JH Shin J Kim J-S Cha B Lee S et al . Cognitive function improvement after fecal microbiota transplantation in Alzheimer’s dementia patient: A case report. Curr Med Res Opin (2021) 37:1739–44. doi: 10.1080/03007995.2021.1957807
266
Segal A Zlotnik Y Moyal-Atias K Abuhasira R Ifergane G . Fecal microbiota transplant as a potential treatment for Parkinson's disease–A case series. Clin Neurol Neurosurg (2021) 207:106791. doi: 10.1016/j.clineuro.2021.106791
267
Doll JP Vázquez-Castellanos JF Schaub A-C Schweinfurth N Kettelhack C Schneider E et al . Fecal microbiota transplantation (FMT) as an adjunctive therapy for depression—case report. Front Psychiatry (2022) 13:815422. doi: 10.3389/fpsyt.2022.815422
268
Luo X Han Z Kong Q Wang Y Mou H Duan X . Clostridium butyricum prevents dysbiosis and the rise in blood pressure in spontaneously hypertensive rats. Int J Mol Sci (2023) 24:4955. doi: 10.3390/ijms24054955
269
Park JH Moon JH Kim HJ Kong MH Oh YH . Sedentary lifestyle: overview of updated evidence of potential health risks. Korean J Family Med (2020) 41:365. doi: 10.4082/kjfm.20.0165
270
Wang L Lei J Wang R Li K . Non-traditional risk factors as contributors to cardiovascular disease. Rev Cardiovasc Med (2023) 24:134. doi: 10.31083/j.rcm2405134
271
Piercy KL Troiano RP Ballard RM Carlson SA Fulton JE Galuska DA et al . The physical activity guidelines for Americans. Jama (2018) 320:2020–8. doi: 10.1001/jama.2018.14854
272
Al-Saber A May A-N . Effect of mindful meditation, physical activity, and diet to reduce the risk to develop or reduce severity of cardiovascular diseases in Saudi Arabia: a systematic review. World J Cardiovasc Dis (2023) 13:46–72. doi: 10.4236/wjcd.2023.131005
273
Lippi G Henry BM Sanchis-Gomar F . Physical inactivity and cardiovascular disease at the time of coronavirus disease 2019 (COVID-19). Eur J Prev Cardiol (2020) 27:906–8. doi: 10.1177/2047487320916823
274
Lee I-M Shiroma EJ Lobelo F Puska P Blair SN Katzmarzyk PT . Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet (2012) 380:219–29. doi: 10.1016/S0140-6736(12)61031-9
275
Albalak G Stijntjes M Van Bodegom D Jukema JW Atsma DE Van Heemst D et al . Setting your clock: associations between timing of objective physical activity and cardiovascular disease risk in the general population. Eur J Prev Cardiol (2023) 30:232–40. doi: 10.1093/eurjpc/zwac239
276
Petriz BA Castro AP Almeida JA Gomes CP Fernandes GR Kruger RH et al . Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats. BMC Genomics (2014) 15:1–13. doi: 10.1186/1471-2164-15-511
277
Lambert JE Myslicki JP Bomhof MR Belke DD Shearer J Reimer RA . Exercise training modifies gut microbiota in normal and diabetic mice. Appl Physiology Nutrition Metab (2015) 40:749–52. doi: 10.1139/apnm-2014-0452
278
Jurdana M Maganja DB . Regular physical activity influences gut microbiota with positive health effects. (2023). doi: 10.5772/intechopen.110725
279
Allen JM Mailing LJ Niemiro GM Moore R Cook MD White BA et al . Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc (2018) 50:747–57. doi: 10.1249/MSS.0000000000001495
280
Choi JJ Eum SY Rampersaud E Daunert S Abreu MT Toborek M . Exercise attenuates PCB-induced changes in the mouse gut microbiome. Environ Health Perspect (2013) 121:725–30. doi: 10.1289/ehp.1306534
281
Kang SS Jeraldo PR Kurti A Miller MEB Cook MD Whitlock K et al . Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Mol neurodegeneration (2014) 9:1–12. doi: 10.1186/1750-1326-9-36
282
Allen JM Miller MEB Pence BD Whitlock K Nehra V Gaskins HR et al . Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6J mice. J Appl Physiol (2015). doi: 10.1152/japplphysiol.01077.2014
283
Evans CC Lepard KJ Kwak JW Stancukas MC Laskowski S Dougherty J et al . Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PloS One (2014) 9:e92193. doi: 10.1371/journal.pone.0092193
284
Matsumoto M Inoue R Tsukahara T Ushida K Chiji H Matsubara N et al . Voluntary running exercise alters microbiota composition and increases n-butyrate concentration in the rat cecum. Bioscience biotechnology Biochem (2008) 72:572–6. doi: 10.1271/bbb.70474
285
Kaczmarczyk MM Miller MJ Freund GG . The health benefits of dietary fiber: beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism (2012) 61:1058–66. doi: 10.1016/j.metabol.2012.01.017
286
Watterson KR Hudson BD Ulven T Milligan G . Treatment of type 2 diabetes by free fatty acid receptor agonists. Front Endocrinol (2014) 5:137. doi: 10.3389/fendo.2014.00137
287
De Vadder F Kovatcheva-Datchary P Goncalves D Vinera J Zitoun C Duchampt A et al . Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell (2014) 156:84–96. doi: 10.1016/j.cell.2013.12.016
288
Kallio KE Hätönen KA Lehto M Salomaa V Männistö S Pussinen PJ . Endotoxemia, nutrition, and cardiometabolic disorders. Acta diabetologica (2015) 52:395–404. doi: 10.1007/s00592-014-0662-3
289
Lira FS Rosa JC Pimentel GD Souza HA Caperuto EC Carnevali LC et al . Endotoxin levels correlate positively with a sedentary lifestyle and negatively with highly trained subjects. Lipids Health Dis (2010) 9:1–5. doi: 10.1186/1476-511X-9-82
290
Simmons A . Treated like animals: improving the lives of the creatures we own, eat and use. Wenlock Road, London, UK: Pelagic Publishing Ltd (2023).
291
Tian D Meng J . Exercise for prevention and relief of cardiovascular disease: prognoses, mechanisms, and approaches. Oxid Med Cell Longevity (2019) 2019. doi: 10.1155/2019/3756750
292
Li J Siegrist J . Physical activity and risk of cardiovascular disease—a meta-analysis of prospective cohort studies. Int J Environ Res Public Health (2012) 9:391–407. doi: 10.3390/ijerph9020391
293
Marchio P Guerra-Ojeda S Vila JM Aldasoro M Victor VM Mauricio MD . Targeting early atherosclerosis: a focus on oxidative stress and inflammation. Oxid Med Cell Longevity (2019) 2019. doi: 10.1155/2019/8563845
Summary
Keywords
CVD, HF, HTN, TMAO, SCFAs, FMT
Citation
Luqman A, Hassan A, Ullah M, Naseem S, Ullah M, Zhang L, Din AU, Ullah K, Ahmad W and Wang G (2024) Role of the intestinal microbiome and its therapeutic intervention in cardiovascular disorder. Front. Immunol. 15:1321395. doi: 10.3389/fimmu.2024.1321395
Received
14 October 2023
Accepted
08 January 2024
Published
26 January 2024
Volume
15 - 2024
Edited by
Nathella Pavan Kumar, National Institute of Research in Tuberculosis (ICMR), India
Reviewed by
Abdullah Saeed, City of Hope National Medical Center, United States
Farman Ullah Dawar, Kohat University of Science and Technology, Pakistan
Muhammad Shahid Riaz Rajoka, University of Alabama at Birmingham, United States
Muhammad Mohsin, Fujian Agriculture and Forestry University, China
Updates
Copyright
© 2024 Luqman, Hassan, Ullah, Naseem, Ullah, Zhang, Din, Ullah, Ahmad and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guixue Wang, wanggx@cqu.edu.cn
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.