- 1Department of Gynecologic Oncology, Roswell Park Comprehensive Cancer Center, Buffalo, NY, United States
- 2Department of Obstetrics and Gynecology, University of Rochester Medical Center, Rochester, NY, United States
- 3Department of Pharmaceutical Sciences, Northeastern University, Boston, MA, United States
- 4Department of Integrative Immunobiology, Duke University School of Medicine, Durham, NC, United States
- 5Department of Immunology, Roswell Park Comprehensive Cancer Center, Buffalo, NY, United States
Tertiary lymphoid structures (TLS) are organized ectopic lymphoid clusters of immune cells that develop in non-lymphoid tissue to promote antigen presentation, drive cytotoxic immune responses, and enhance humoral immunity via B cell clonal expansion. Their presence within the tumor microenvironment (TME) correlates with increased patient survival and an improved response to immune checkpoint inhibitors (ICIs), positioning TLS as potential predictive and prognostic biomarkers. Despite the widespread use of ICIs across various cancers, their effectiveness remains limited in gynecological malignancies, including ovarian cancer (OC), a notably challenging disease characterized by poor responses to both single and combination ICI therapies. Interestingly, the infiltration of T cells into the OC TME is linked to enhanced progression-free survival (PFS) and overall survival (OS), yet an immunosuppressive TME frequently impedes therapeutic efficacy, suggesting cell activity within localized immune niches can impact antitumor immunity. This review explores the roles of TLS, their maturity, functionality, identification, and related gene signatures; specific immune cells and cytokines that play a role in TLS formation and antitumor response; and other modifiable elements, including gut microbiota, that may drive improving OC survival by leveraging a TLS-driven antitumor response to bolster immunotherapy outcomes.
Introduction
Ovarian cancer (OC) represents the most lethal gynecologic malignancy in the United States, underscoring the need for innovative therapeutic strategies (1). OC is predominantly diagnosed at an advanced stage, where cytoreductive surgery and chemotherapy rarely produce curative benefit. The clinical trajectory for most patients is characterized by cycles of remission and relapse, with each remission period becoming progressively shorter until the disease develops resistance to chemotherapy or until significant toxicity arises (2). Given the limited clinical benefits of second line and subsequent therapies, a critical and ongoing need exists to develop novel therapeutic approaches.
Extensive evidence suggests OC is an immunogenic tumor that the host immune system can recognize. A higher infiltration of cytotoxic T cells within OC tumor islets is associated with significantly improved survival rates (3, 4). Tumor-specific T cell responses against multiple antigens overexpressed by OC, including folate receptor alpha (FRα), New York Esophageal Squamous Cell Carcinoma 1 (NY-ESO-1), p53, human epidermal growth factor receptor 2/neu (HER-2/neu), survivin, sperm surface protein 17 (Sp17), Wilms’ tumor 1 (WT1), transmembrane glycoprotein mucin 1 (MUC1), and melanoma-associated antigen-3 (MAGE-3), are quantifiable and highlight the potential for immunotherapy in treating OC (5–12). However, effective immunotherapy depends on the successful homing of functional tumor-specific cytotoxic T lymphocytes (CTLs) into tumors and chemotactic gradients within the tumor microenvironment (TME) (13–15) that support persistent immune cell infiltration or inflammatory function (8).
Even with the immunogenic nature of OC, immune checkpoint inhibitors (ICIs) show limited effectiveness in the relapsed/refractory setting, achieving response rates of only 8-15% (16–18). Currently, ICIs are approved solely for mismatch repair deficient (MMRd) OC or have high microsatellite instability (MSI) due to their inadequate effectiveness as single agents (19). While trials testing single agent antibodies targeting programmed cell death protein 1/program death-ligand 1(PD-1/PD-L1) or other ICIs in OC cancer patients have been disappointing (20), in a recent triple combination therapy clinical trial in advanced OC patients testing pembrolizumab, bevacizumab, and oral cyclophosphamide, one third of patients had a durable clinical benefit (DCB) (21–23). Comprehensive molecular, immunological, microbiome, and metabolic profiling analyses were performed on these patients’ biospecimens to assess response to this regimen. Increased T and B cell clusters and distinct microbial patterns with lipid and amino acid metabolism were linked to these patients with exceptional responses (23), compared to those with limited clinical benefit (LCB). Identifying reliable predictive biomarkers and who may benefit from immunotherapy would greatly contribute to patient selection for future immunotherapy clinical trials (21, 24–26).
Immunosuppression in ovarian cancer
Despite successful CTL infiltration, tumor heterogeneity and an immunosuppressive microenvironment often undermine the antitumor immune response, with increased recruitment of immunosuppressive cells, including regulatory T cells (Tregs), tumor associated macrophages (TAMs) and myeloid derived stem cells (MDSCs), indicative of poor survival outcomes in OC (27–29). Low tumor mutational burden (TMB) and neoantigen (NA) load (30), downregulation of major histocompatibility complex (MHC)-1 in tumor cells (31), lysophosphatidic acid inhibition of type 1 interferon (32), an immunosuppressive environment in the ascites (33–36), immune evasion promoted by cancer driver mutations, including TP53 and phosphatase and tensin homolog deleted on chromosome ten (PTEN) (37), and aberrant oncogenic signaling pathways, all contribute to ICI resistance across various cancers (38). High metabolic demands of rapidly proliferating cancer cells also exhaust key nutrients needed for immune cell function. Elevated glycolysis rates in tumor cells lead to metabolic exhaustion in effector T cells, while lactate accumulation inhibits natural killer (NK) cell activation. Furthermore, increased expression of indoleamine-2,3-dioxygenase in tumor cells depletes tryptophan levels, impairing cytotoxic T cell proliferation, and inducing T cell exhaustion through kynurenine production (39–42).
Tumor-extrinsic factors also play a significant role in dampening antitumor immunity. Inadequate infiltration of lymphocytes into tumor islets is often due to abnormal vasculature and chemokine gradients (43), as well as compensatory mechanisms like upregulating inhibitory immune checkpoint receptor signaling, including cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and lymphocyte activation gene 3 (LAG-3) (44, 45). Most recently, tertiary lymphoid structures (TLS) or organized ectopic lymphoid structures, that serve as hubs at sites of inflammation and support interactions between B and T lymphocytes and antigen-presenting cells (APCs) (46), has proved to be a major area of investigation (47). While the presence of TLS appears to be essential for coordinating robust antitumor responses, their absence impedes the integration of the humoral and cellular components of adaptive immunity, both of which have been deemed to be important for response to therapy. This limits immune activation and T-cell priming, thus underscoring the important role of TLS in effective tumor control. Given the critical role of TLS in enhancing antitumor immunity, this review will focus on presence and maturity of TLS, their significance on ICI efficacy and clinical trial outcomes, and the impact the gut microbiome and other factors have on antitumor immunity and TLS formation, especially in OC.
Tertiary lymphoid structures
TLS support interactions where specialized formations of B cells, CD4+ and CD8+ T cells, and antigen presenting dendritic cells (DC) come together, along with high endothelial venule cells (HEV), in a coordinated manner, supported by a stromal infrastructure (48). TLS were initially described in autoimmune diseases, chronic infections, solid tumors, age-related diseases, and graft rejection (49–52), and have roles in local autoantibody formation, antibody-mediated immune responses in infected organs, and allograft rejection. TLS do not exist under physiological conditions which contrasts with primary (bone marrow and thymus) or secondary lymphoid organs (SLO). SLO are embryonic in nature and involve encapsulated lymph nodes (LN), the spleen, Peyer’s Patches, as well as tonsils, the human appendix, and mucosal-associated lymphoid tissues. SLOs are dependent on specialized Lymphoid Tissue inducer (LTi) cells that help to drive embryonic mesenchymal tissue organizing cells into follicular dendritic cells (FDC) and fibroblast reticular cells (FRC), driven by CXCL13 and CCL19/CCL21, respectively. TLS are generated after birth by specific cells involved in the immune response at sites of inflammation and are not associated with a specific organ. Both SLO and TLS are involved in generation of antigen-specific immune responses, antigen recognition, and activation of B and T cells (46), as well as involvement with HEV, specialized structures which help facilitate movement of lymphocytes from the blood into tissue. Due to TLS being sites or hubs of inflamed tissue without encapsulation, they are exposed to tumor antigens (TA), cytokines, and other inflammatory signals, which prompt a humoral and cellular immune response.
The presence of TLS correlate with favorable disease outcomes in a variety of cancers, while in autoimmune and chronic age-related disease, TLS are associated with worse, more severe outcomes (52). TLS formation has been observed in almost all organ specific human autoimmune diseases including Sjogren syndrome (SjS) (53), lupus nephritis (54), type I Diabetes (55), Crohn’s disease (56) and rheumatoid arthritis (57), however the prevalence of TLS is variable (52). Understanding the involvement of TLS in chronic diseases, including cancer, is therefore important regarding how they are formed and mature to determine potential strategies to aid in disease management.
The autoimmune SjS has been used as a model to derive a spatial and cellular map of key components involved in the formation and function of TLS. Single cell RNA, tissue transcriptomics, and spatial proteomics have been used on salivary glands from SjS patients (58). It has been shown that TLS formation and maturation including a Germinal Center (GC) correlates with autoimmunity, a pathological humoral response, B cell hyperactivity, and development of B cell lymphoma. Results from these studies suggest a complex cellular landscape of TLS, an immunomodulatory pericyte population in SjS, and significant fibroblast diversity, including presence of tissue-resident fibroblasts (immunofibroblasts) that have features like FRC in SLOs. There are different signals involved in the production of CCL21 and CCL19 cytokines in fibroblasts vs. pericytes, as well as distinct properties related to GC in SLO vs TLS. Transcriptomic and proteomic analysis between the SLO and TLS revealed differences in lymphoid structures and enrichment of certain cell types in mature TLS (mTLS), including genes involved in inflammatory cell recruitment, inflammatory pathways, and co-stimulation (58).
These specialized stroma derived fibroblasts have been determined to be crucial for the structure of TLS (59). Fibroblasts have been shown to exhibit plasticity and specialization under inflammatory conditions (48). While TLS are primarily composed of lymphocytes and DC, TLS are also supported by a complex network of additional stromal cells, including endothelial cells, lymphatic vessels, nerves, and immunofibroblasts. Immunofibroblast progenitors have been shown to be present at sites where TLS are established. A mouse model of TLS has shown that under chronic inflammatory conditions, tissue resident fibroblasts can acquire an immunofibroblast phenotype, including expression of lymphoid chemokines, adhesion molecules, and lymphocyte factors which sustain B and T cell survival in tissue. This process includes 1. priming by tumor necrosis factor (TNF) and interferon (INF) family members, interleukin (IL)-13, IL-1 family cytokines, IL-17, and IL-22, resulting in upregulation of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule (VCAM1), and podoplanin-posititve (PDPN); 2. expansion (fibroblast proliferation); and 3. maturation (stable expression of lymphoid cytokines CXCL13, CCL19, CCL21, and lymphocyte survival factors, IL-7 or B cell activating factor) of the immunofibroblast network (48). Upregulation of cell adhesion molecules-ICAM1 and VCAM1 also appear to facilitate network interactions.
A variety of cell types are associated with TLS (Figure 1). These organized structures are composed of B cell-containing GCs and DC-lysosome-associated membrane glycoprotein-positive dendritic cells (DC-LAMP)+ for antigen presentation (60). TLS are also marked by peripheral T cell zones with CD4+/CD8+/Tfc cells and peripheral node addressin (PNAd+) HEVs (60–62). B lymphocytes which play an active role, express activation-induced deaminase, an enzyme vital to class switch recombination and somatic hypermutation, which leads to the production of antigen-specific antibodies (63). Inflammatory factors are directly implicated in TLS formation and released from activated T cells, macrophages (MP), and DCs in TLS (64, 65).
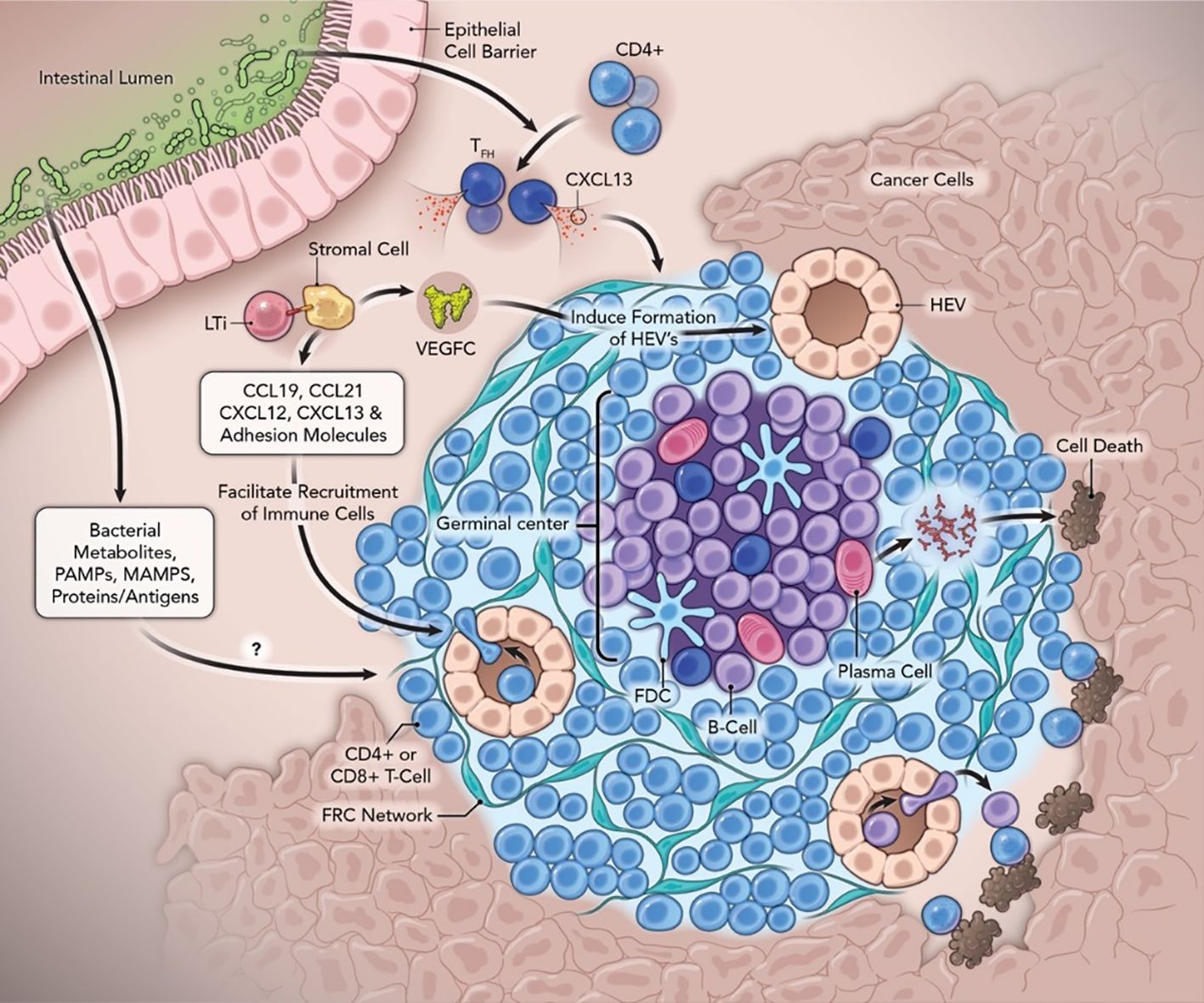
Figure 1. Neogenesis and function of tertiary lymphoid structures with the contribution of the gut microbiome in anti-cancer immunity. Abbreviations: FDC, follicular dendritic cells; Tfh, follicular helper T cells; FRC, fibroblastic reticular cells; GC, Germinal Centers; HEV, high endothelial venules; LTi, lymphoid tissue inducer cells; LTβR, lymphotoxin-β receptor; MAMPs, microbe-associated molecular patterns; PAMPs, pathogen-associated molecular patterns; PC, Plasma Cells; SHM, somatic hypermutation; TLS, tertiary lymphoid structure; VEGFC, vascular endothelial growth factor C.
TLS maturity
TLS maturation stage is a key determinant of TLS function and mTLS have been linked to improved survival and sensitivity to ICI in several cancers. Discussions are ongoing regarding how TLS are defined, their maturity, their increased levels of organization and complexity, and the involvement of immune cells to drive antitumor immunity more effectively, compared to loose immune aggregates of T and B cells observed in immature or “early” TLS. (66–68). As such, the careful classification of TLS and how their presence/location can impact patient outcome or treatment response represents active areas of ongoing investigation. For example, it has been proposed that B cells that accumulate in immature TLS can develop into regulatory B cells with immunosuppressive features that support tumor progression. In contrast, B cells found in mTLS associated with GC are activated and proliferate, undergo affinity maturation, as well as isotype switching, resulting in plasma cells (PC), which produce tumor-specific antibodies, resulting in improved survival and immunotherapy response (66).
At least three levels of organization for TLS have been described: Lymphoid aggregates with minimal organization and occasional DCs; Immature DCs with an organized T and B cell presence, a network of FDCs, no GC, and possibly HEVs; and mTLS with active GC and HEV, and full B and T cell zones for activation of cell proliferation and recruitment of immune cells (69). Vanhersecke has proposed a standardized method to screen mTLS in cancer samples using hematoxylin-eosin-saffron (HES) staining and immunohistochemistry (IHC) that can be applied to all specimens (70). Vanhersecke has previously defined only two TLS categories, including mTLS (secondary follicle-like TLS) with TLSs containing either a visible GC on HES staining or CD23+ FDC or immature TLS (including early aggregation and primary follicle-like TLS) (68, 70). Of note, the clinical specimen used for TLS detection (surgical vs. biopsy vs. metastatic) had an impact, with TLS identification more prevalent in larger tissue samples.
In gynecological cancers, including OC, Zhang et al. (71) classified these heterogenous TLS into 3 different categories: a very diffuse group of lymphoid aggregate cells containing stomal, dendritic, memory B, follicular B and T cells, and CD4+ and CD8+ T cells; an immature TLS, with more organization including follicular B cells and a CD4+ and CD8+ T cell zone surrounding them with HEV at the periphery, and finally, mTLS consisting of mainly B cell follicles with GC in a B cell zone with networks of CD21+FDCs near an adjacent T cell zone of CD4+ and CD8+ cells, surrounded by stromal and fibroblast cells, PC, and HEV (71).
The organization and maturity of TLS also appears to play a significant role in response to ICI in OC. High-grade serous ovarian cancer (HGSOC) have a low density of follicular helper T (Tfh) cells resulting in a limited number of mTLS, with accumulation of TIM3+PD-1+, rather than TCF1+PD1+ CD8+ T cells, which may at least in part promote ICI resistance in HGSOC patients (72). The quality of TLS, i.e., how well they are formed, have also recently been implicated in OC relapse (73). In addition to changes in T cell localization and increased glycoprotein PDPN+ cancer associated fibroblasts (CAF), which help regulate tumor development and activity of immune cells, malformed TLS-like aggregates and some lymphoid aggregates were associated with spatial patterns of early OC relapse, with PC allocated into compartments associated with TLS-like aggregates and CAFs, potentially accounting for context-dependent roles for PC in HGSOC. In addition, PDPN+ CAFs were frequently associated with partially organized immune cells.
TLS formation
It was thought TLS formation was similar to SLO induction involving LTi cells, hematopoietic cells with a critical immune function during embryonic development (74), which express lymphotoxin-α1β2 ligand and interact with the lymphotoxin-β receptor (LTβR) on lymphoid tissue organizer (LTo) cells to form aggregates. LTi cells interact with LTβR-expressing stromal cells, producing homeostatic chemokines, which are essential for an organization phase. TLS-associated cells are regulated during each phase of TLS formation by several categories of cytokines/chemokines that participate in stromal cell activation, LiTi cell aggregation, the loop between LTi and LTo, expansion of HEV and recruitment of lymphocytes, formation of T and B cell compartments, and GC formation with differentiation of B cells (63). These chemokines recruit B cells and T cells (via CXCL13, CCL19, CCL21) to form distinct immunological zones (75, 76). LTβR signaling also induces the production of VCAM-1, mucosal vascular addressin cell adhesion molecule-1, and ICAM-1, which further promote lymphangiogenesis (77).
It is now proposed T and B cells are surrogate LTi cells in TLS attracted to the inflammatory site by CXCL13 and IL-7 which activate LTo cells including stromal or immune cells via the TNF family receptor (78). LTo produce chemokines (CCL19 and CCL21 and CXCL10 and CXCL13) to attract immune cells near the site of immune activation and vascularization by establishing gradients which help guide the cells to the lymphoid structures. Adhesion molecules VCAM-1, ICAM-1, MAdCAM-1, and PNAd help circulating immune cells get from HEV to tissue and survival factors BAFF and IL-7 aid in B and T cell maturation and survival. It is thought that in OC, CD4+ T cells and DCs secrete cytokines as potential LTo cells (79).
In contrast to the pathway mentioned above, an alternative mechanistic pathway of lymphangiogenesis that generates TLS involves TNF superfamily member 14 (TNFSF14)/LIGHT, a cytokine produced by activated T cells (80, 81). LIGHT fused to a vascular targeting peptide has been found to normalize tumor blood vessels, activate CD4+/CD8+ T cells, and triggers TLS formation in immunotherapy-resistant pancreatic adenocarcinoma (64). Here, TLS were also found intratumorally instead of at the conventional tumor periphery, which could be due to vessel stabilization in deep tumor parenchyma and relocation of macrophages and effector T cells into the TME (64). In contrast, Tfh tumor-infiltrating lymphocytes (TILs), which secrete LIGHT, IL-21, and CXCL13, are sufficient to initiate TLS assembly, but at peritoneal tumor beds in OC murine models (82, 83)
Analysis of chemokine profiles in HGSOC supports the role of B cells in recruitment of DC-LAMP+, the latter which function in TA uptake and presentation for effective T cell priming (84). B cell clonal expansion correlates with an immunologic response in OC where CD20+ B cells that infiltrate tumors possess qualities of APCs, including surface expression of MHC class I/II, CD80, and CD86 (85, 86). Nielsen et al. suggested CD20+ TILs could serve as APCs to cytolytic CD8+ TILs, and the simultaneous presence of both B- and T-TIL subsets contribute to improved survival in OC patients (86). Montfort and colleagues demonstrated TLS present in OC omental specimens had an upregulated class-switched, memory B cell phenotype after neoadjuvant chemotherapy, suggesting that chemotherapy can regulate B cell functional status within TLS (84).
Detection of TLS
Several methods, including pathological diagnosis, to detect, characterize, and quantify TLS as a predictive and prognostic biomarker have been used including H&E, multiplex IHC and immunofluorescence, and transcriptomic means, however, a consensus method has not yet been determined. While H&E staining is easy, affordable, and widely accepted, there are some concerns including potential for bias, reproducibility among pathologists, and underrepresentation of TLS structures (71). Several markers have been used to identify and quantify key immunological players in TLS through IHC with mTLS identified by markers of T cells (CD3, CD8), B cells (CD20), CD21(B cell), PNAd (HEV), CD208 (DC), CD79A (B cells), PC (CD138), proliferation (Ki67), and cytokines (CXCL13/BCL6). Early and immature phenotypes include CD20, CD3, CD79A, CD8, and PNAd markers, while aggregates include CD20 and CD3 and CD68 MP (87). Additional methods, including IHC and immunofluorescence, are used to identify TLS through staining methods and spatial detection and visualization, respectively, of target proteins in the TLS.
Newer approaches have been used to automate and detect TLS. Multi-resolution deep learning based on HookNet-TLS, has been shown to automate TLS quantification, and identify GC in H&E- stained digital pathology slides. This approach was recently shown to characterize TLS and their prognostic relevance in lung squamous cell carcinoma, muscle invasive bladder cancer, and clear cell renal cell carcinoma (ccRCC). While TCGA slides for OC were not initially used to develop this tool, its availability may provide opportunities to validate it in the OC patient population (88). Multi-plexed whole specimen tissue imaging, 3D reconstruction, spatial statistics, and machine learning was used in a colorectal cancer (CRC) model to determine relevant morphological features associated with diagnostic and prognostic significance, including TLS (89). TLS were commonly interconnected, formed larger 3D structures or TLS networks, had graded molecular properties, and were found in patients’ samples at various locations.
Identifying new ways to effectively image TLS in the TME will be invaluable in advancing this field of study and may involve the use of multiple spatial -omic technologies including spatial transcriptomics, proteomics, and metabolomics (90) to identify TLS. Imaging-based approaches will also be important for identifying spatial patterns of TLS that may be associated with early relapse, presence of CAFs (73), risk stratification (91) or assessing recurrence of cancers through flow cytometry and co-detection by indexing (CODEX) (92). Li et al. have suggested several biomaterials that may be suitable for monitoring TLS (93), while three-dimensional imaging may also be an option to characterize TLS (94). Positron emission tomography (PET) using18-F-fluoro-2-deoxy-D-glucose (18F-FDG)(PET/FDG) tail vein injections, combined with computed tomography (CT) for anatomical localization and single photon emission computed tomography (SPECT) by intraperitoneal injection of 99mTC labeled Albumin Nanocoll (99mTC-Nanocoll), has been explored in murine models of systemic lupus erythematosus (SLE) to detect early kidney changes and TLS presence. While TLS were detected in pancreas and not kidney, future studies using new PET/SPECT tracer administration sites, together with more specific tracers in combination with magnetic resonance imaging (MRI), may make it possible to detect formation of TLS and LN for pre-clinical studies. This may be relevant in OC due to different sites of origin of OC. Additional opportunities may include CT-based radiomic models to non-invasively predict intratumoral TLS (iTLS) as demonstrated for hepatocellular carcinoma (HCC) (95) and invasive pulmonary adenocarcinoma (96), as well as the transfer learning radiomic model, an MRI-based model to detect iTLS in HCC which was found to correlate with favorable prognosis and responsiveness to combination therapy in the context of higher model scores (97).
Genomic signatures of TLS
Gene expression signatures have been used to identify TLS in a variety of cancers and determine their association to survival. A three-gene expression signature including IL-7, LTB, and CXCL13 was associated with LN neogenesis in human oral cancer, where higher grades of TLS were associated with improved disease-free survival (DFS) and overall survival (OS) (98). A unique 12-chemokine (CK) gene expression signature enriched for immune- and inflammation-related genes in primary CRC (99), identified TLS, and found an association with better patient survival independent of tumor staging. This gene signature has been used to confirm the presence of TLS in a variety of cancers including melanoma (100), breast (101), and bladder (102). A 12-gene signature associated with TLS derived from melanoma patient samples treated with ICI predicted clinical outcomes associated with B cells, immune cells, and CD8+ T cell-specific genes, including CXCL13 (103).
Recent OC studies have determined more involvement of TLS in immunotherapy responses. The presence of TLSs in OC and their potential clinical significance was also determined using the 12-CK signature (CCL2, CCL3, CCL4, CCL5, CCL8, CCL18, CCL19, CCL21, CXCL9, CXCL10, CXCL11, and CXCL13) previously identified (91). A 9-gene signature for TLS using a TCGA dataset was constructed and validated in the GSE140082 dataset (104). High expression of this gene signature (CETP, CCR7, SELL, LAMP3, CCL19, CXCL9, CXCL10, CXCL11, CXCL13) positively correlated with developed immune infiltration, and reduced immune escape with TLS associated with favorable responses to ICI in HGSOC patients. High-TLS clusters were characterized by better clinical prognosis, higher immune infiltration, more biological pathways, a higher TMB score, and higher expression of immune checkpoint (71). TLS strongly correlated with the immune-responsive microenvironment and were a favorable prognostic factor independent of other clinical characteristics. While the 12-gene CK signature associated with OC compares to TLS gene signatures from other cancers, additional genes appeared to be unique in the 9-gene signature, including CETP, CCR7, SELL, and LAMP 3. Using this signature, Lu et al. (105), showed TLS-high HGSOC tumors associated with better PFS, B cell maturation, and cytotoxic tumor-specific T cell activation and proliferation.
Recently, Chen et al. explored relevance of the 12-CK gene signature in gynecologic cancers and found it resonated most with cervical cancer, then endometrial cancer (EC), and finally OC (67). In addressing TLS in EC in biobanked samples from the PORTEC study, TLS associated with prognosis in only 2 of 4 EC subtypes: an excellent prognosis was associated with the ultra-mutated EC with DNA-polymerase epsilon exonuclease domain mutations (POLE mut), and an intermediate prognosis with hypermutated EC with MMRd (103, 106). TLSs were also found to assess recurrence with lower 5-year recurrence risk by 4-fold in EC patients (32.6% without TLS vs. 7.2% with TLS) (106). Mature TLS, presence of naïve-B, cycling/GC B cells, and antibody-secreting cells were also associated with L1CAM expression, a cell adhesion molecule independent of tumor expression, and what appears to be a specific biomarker for GC cells in mTLS. This might be a potential biomarker in OC, as well as EC. Note, in OC, TLS loss/dysfunction was linked to chromosome 4q deletion/DCAF15 amplification, whose copy number loss of IL15 and CXCL10 may limit TLS formation (105).
Overall, TLS related gene signatures may be relevant for OC patient stratification to different immunotherapies or responses. Specific gene signatures may not only predict the presence of TLS, involvement of immune markers, and clinical outcomes in different cancers, but may also select patients for specific immunotherapies based on molecular profiling of intratumoral lymphoid aggregates (65). Additional TLS-cancer-specific gene signatures may be forthcoming with artificial intelligence (AI)-driven assessment of large cancer-based datasets and further validation.
Clinical value of TLS
TLS have been identified in a variety of tumor models with characterizations of pro- and anti-tumorigenicity (46), however, the potential for TLS to serve as predictive ICI-based biomarkers is strengthened through clinical trials of solid tumors assessing TLS in immunological, pathological, and clinically relevant outcomes (Table 1), and serve as a potential model for OC. While there is a limited amount of OC clinical trials performed where TLS has been accessed as a biomarker, several studies have shown promising results (Table 2), including those that also indicate the importance B cells in survival and the anti-tumor response (61, 63, 107, 108). Immune and stromal transcriptomic profiles for melanoma patients who respond to neoadjuvant ICI therapy reveal TLS clusters with the highest B cell signature have significantly longer survival (109, 110). Additionally, co-occurrence of tumor-associated CD8+ T cells and CD20+ B cells improves survival in metastatic melanoma patient samples, where TLS in CD8+CD20+ tumors stained for CXCL13, CXCR5, and CD20, and B cell-enriched tumors had increased TCF-7 naive and/or memory T cell levels (111).
Pembrolizumab monotherapy was assessed in soft tissue sarcoma where one out of 5 sarcoma immune classes were characterized by high immune, TLS, and B cell lineage expression which significantly improved OS and overall response rate (ORR) (112). Sarcoma patients treated with pembrolizumab with low-dose cyclophosphamide had three times greater PFS in the TLS-enriched populations vs. all-comers (113), while NSCLC tumors from patients treated with either the PD-L1 inhibitor atezolizumab or chemotherapy showed B cells associated with extended OS following PD-L1 blockade. B cells and PC were also associated with TLS and organized lymphoid aggregates, with increased PC signatures predictive of OS in patients treated with atezolizumab only (114).
Combination immunotherapy trials have shown improved outcomes in some solid tumor cancers. Neoadjuvant anti-PD-L1 (durvalumab) plus anti-CTLA-4 (tremelimumab) treatment of cisplatin-ineligible patients with urothelial cancer (UC) had higher density TLS in pretreatment tumor specimens of responders (R) associated with longer recurrence-free survival (RFS) and OS (115). In previous neoadjuvant ipilimumab (anti-CTLA-4) and nivolumab (anti-PD-1) studies prior to surgical resection, while no correlation was observed between TLS quantity, TLS induction was seen in R, with immature TLSs higher in patient tumor specimens that did not have a complete response (CR) (116). Enriched FoxP3+ T cell-low TLS clusters were observed after anti-PD-1/CTLA-4 immunotherapy in unresponsive tumors in another study, while submucosal TLS had more pronounced T-helper cells, enrichment of early TLS, and displayed a lower fraction of secondary follicle-like TLS than TLS located in deeper tissue (117).
Other combination therapy, including immunotherapy, had varied results in smaller trials. Cryoablation plus tremelimumab modulated the immune microenvironment with increased immune cell infiltration in patients with clear cell (CC) metastatic renal cell carcinoma (mRCC); however, TLS were only observed in CC mRCC patients and not non-CC (118). An enhanced immune response was observed in R in a second trial where CC mRCC patients treated with nivolumab were enriched in circulating Tfh cells, TLS, and baseline NSwM B cells, and were also associated with improved OS and PFS (119). In a small single arm phase 1b study in 15 HCC patients testing neoadjuvant cabozantinib, a tyrosine kinases inhibitor, and nivolumab, TLS were found in R, with an enrichment in T effector cells, TLS, and CD138+ PC. Here, a distinctive spatial arrangement of B cells contributed to antitumor immunity in R vs. Non-responders (NR) (120).
Tertiary lymphoid structures in ovarian cancer
Several studies, including data collected through The Cancer Genome Atlas (TCGA), confirm a connection between TLS, B and T cells, and OC (Table 2). Increased patient survival was observed between tumor-infiltrating B cells and CD8+ T cells, offering possible mechanisms for B cell involvement in cellular immunity, including secreting polarized cytokines, serving as APCs, or organizing centers for TLS (121). B cells colocalized to large, well-organized TLS, while dense infiltrates of PC surrounded TLS, which are associated with the highest levels of CD8+, CD4+, and CD20+ TIL, as well as cytotoxicity-related gene products. TLS also correlated with CXCL13 gene expression, infiltration of T cells and B cells, and improved prognosis for HGSOC (79). The coexistence of CD8+ T cells and B cell lineages in the TME significantly improved the prognosis of HGSOC and correlated with the presence of TLS. CXCL13 expression predominantly coincided with CD4+ and CD8+ T cells in TILs, shifting from CD4+ T cells to CD21+ FDCs as TLS matured.
A phase II clinical trial with the hypomethylating agent guadecitabine and the anti-PD1 inhibitor pembrolizumab resulted in a clinical benefit rate (CBR) of 31.4% (8.6% partial responders and 22.9% stable disease) (122). Naive and/or central memory CD4+ T cells and classical monocytes were observed at a higher frequency in patients with a durable CBR, while TLS present in tumors were associated with a durable CBR and higher baseline density of CD8+ T cells and CD20+ B cells. Updated efficacy and OS survival data for an open-label single-arm nonrandomized phase 2 trial (NCT02853318) by Zsiros et al. (21) was presented in 2022 (22) for a triple combination therapy (pembrolizumab, bevacizumab, and oral metronomic cyclophosphamide) conducted in a heavily pre-treated OC patient population. Originally, an ORR of 47.5% was obtained, along with a median PFS of 10 months, which was over 4 times better than ORR (8%) or PFS (2.1 mo.) in single-agent ICI, and better than bevacizumab and cyclophosphamide combination therapy (25). Thirty percent of patients had disease control, with 95% having a clinical benefit, with limited toxicity and good quality of life (QoL) reported. Some exceptional R survived more than three years, and one up to five, with multi-omics performed on collected biospecimens from this study (23) suggesting involvement of TLS, humoral and cellular anti-tumor responses, and metabolomic pathways in DCB vs. LCB patients. These results contributed to changes in NCCN guidelines (123).
Additionally, in studies to predict immunotherapy response to PD-1 blockade, HGSOC samples from two patient cohorts and an HGSOC cohort from TCGA were analyzed to assess the relationship between TME and follicular cytotoxic CXCR5-CD8+ T cells. Again, high expression of CXCL13 was associated with prolonged survival, and, combined with CXCR5 and CD8+ cells, was an independent predictor for survival. CXCL13 also carried prognostic benefit, but only with TLS, and was also associated with CD20+ B cell clusters, predicting better patient survival when both were present (124). Along with the 9-gene and 12-gene CK signatures, the combinations of immune cell markers, as well as CXCL13, are the strongest biomarker front runners for prolonged survival in combination with mTLS.
Recently, MacFawn et al. (125), using spatial transcriptomics and multiplex immunofluorescence, demonstrated TLS in HGSOC differ significantly by anatomical site resulting in altered TLS activity and patient prognosis. Composition, function, activity, and number of TLS are distinct between OC originating in the ovary (OV) compared to the fallopian tube (FT) and omentum (OM) and this directly impacts TLS activity, immune function, and survival. Ovarian tumors originating from the FT and OM had more TLS that are more functionally active compared to OV, which are less mature and fewer in number, as well as have a reduced B to T cell ratio, fewer HEVs, and less GC, thus reduced antitumor immunity. Not only were B cells markedly reduced in OV compared to OM or FT, but there are decreased markers (MZB1+) of antibody production in OV. The TLS 12 CK-gene signature associated with improved survival was observed in FT and OM but indicated poor survival in OV. Another major finding was the impact of stromal cells in the OV TME (126). Cancer educated mesenchymal stem cells (CA-MSC) have been identified in the OV, where the CA-MSC signature appears to override the benefit of the TLS signature (125). These cells previously have been associated with reduced survival and metastasis (127). mTLS differences were also observed in adnexal tumors (PTs) and synchronous omental/peritoneal metastases (pMets) in HGSOC. pMets had more mTLS and immune components (CD8+ cytotoxic T cells, FOXP3+ Tregs and PD-1+ lymphocytes) than PT, but an independent prognostic impact of mTLS could not be obtained. A more immune cell rich outer zone of mTLS was observed in PTs, but not pMets. This suggests the anatomical site, as well as deciphering what is involved in immune response and evasion, are relevant when considering what signals to modulate to improve the antitumor response. TLS have also been assessed in HGSOC progression & early relapses using spatial heterogeneity. Spatial patterns associated with early relapses included changes in T cell location, TLS-like aggregates which appear malformed, and an increased presence of PDPN-CAFs. These poorly formed TLS aggregates are potential indicators of early relapse in HGSOC (73). Figure 2 summarizes factors that impact TLS in OC and identifies potential pathways or strategies that may be manipulated or explored that may increase TLS formation and improve therapeutic outcomes.
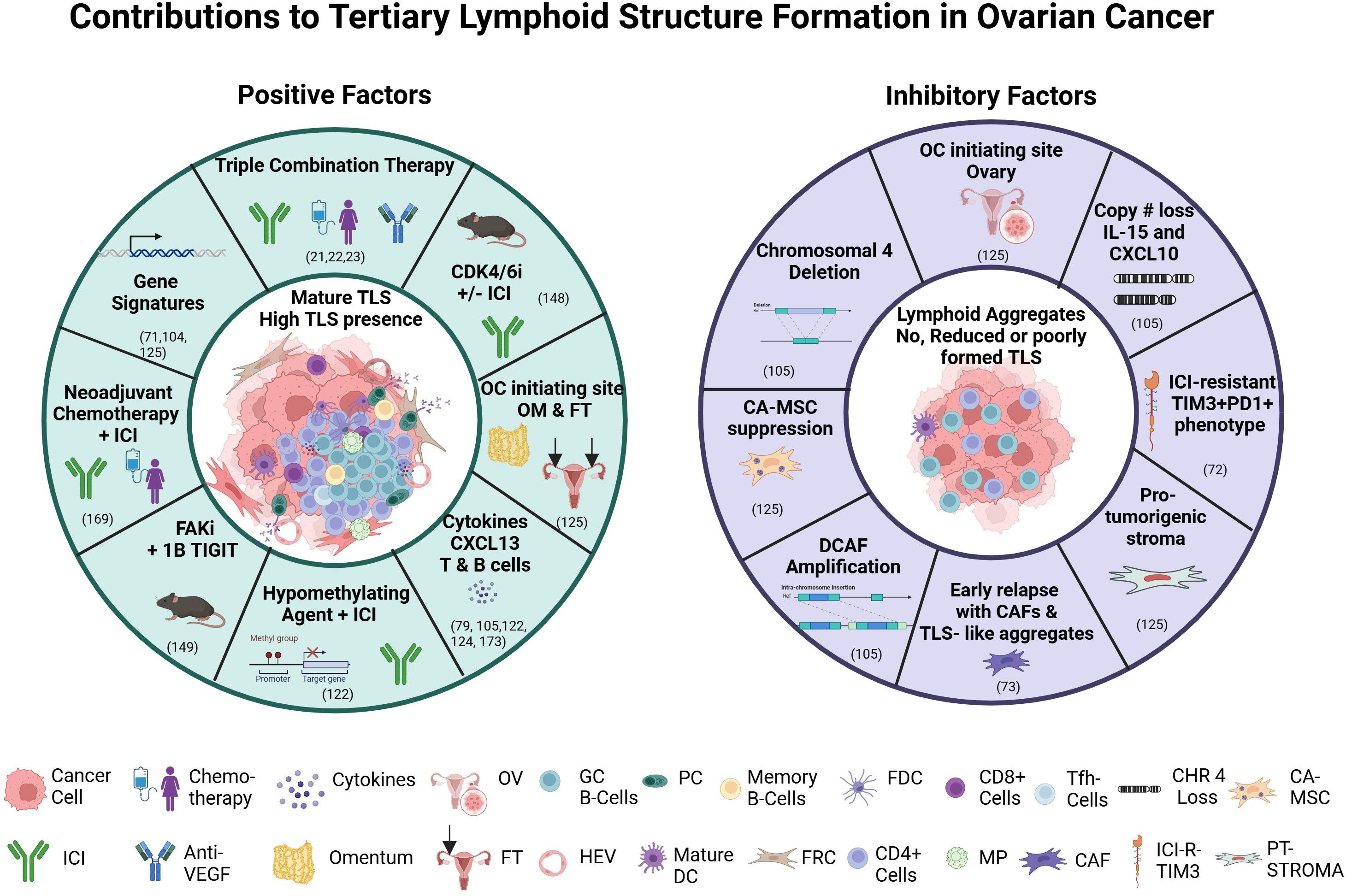
Figure 2. Factors contributing to or inhibiting tertiary lymphoid structure formation. Abbreviations: anti-PD-L1, Programmed death ligand 1; CDK 4/6i, cyclin dependent kinase 4/6 inhibitor; CAF, cancer associated fibroblasts; CA-MSC, cancer-educated mesenchymal stem cell; CHR 4, chromosome 4 loss; DC, dendritic cell; FAKi, focal adhesion kinase inhibitor; FDC, follicular dendritic cell; FRC, fibroblastic reticular cell; FT, fallopian tube; GC B-cells, germinal center B cells; HEV, high endothelial venule; ICI, immune checkpoint inhibitor; ICI-R-TIM3, ICI-Resistant TIM3; MP, macrophage; OM, omentum; OC, ovarian cancer; OV, ovary; PC, plasma cell; PT-STOMA, pro-tumorigenic stroma; TIGITi, T cell immunoreceptor with Ig and ITIM domain inhibitor; TIM3, T cell immunoglobulin and mucin domain 3;TLS, tertiary lymphoid structures; VEGF, vascular endothelial growth factor. Figure generated with BioRender.com.
While this review has predominantly focused on the role of TLS in the context of ICI, additional immunotherapeutic approaches and treatment strategies are in development for OC where TLS are likely to also play a functional role. Given that OC is readily infiltrated by T and NK cells, leveraging these cell subsets may represent particularly attractive therapeutic opportunities. This includes adoptive T cell therapies (ACT) or T cell activating modalities, such as the delivery of expanded TILs, or vaccine-primed T cells, infusion of engineered TCR-T or CAR-T cell therapies, as well as treatment with BiTEs to redirect T cells for tumor targeting (128, 129). A major focus of ACT-based modalities hinges on successful target identification, which includes in OC MUC 16, FRα, B7H3, mesothelin, and TAG72 (130), to name a few, as well as strategies to overcome inherent limitations of antitumor T cell responses, including lack of T cell persistence, tumor heterogeneity, loss of functionality, and impaired trafficking towards the TME, all of which may benefit from the presence of TLS. A recent publication from our group addressed some of these barriers to magnify therapeutic responses by using BiTE-secreting T cells targeting FRα present on most OC cells. Engineered T cells secreting FR-alpha engagers (FR-B T cells) not only effectively killed targeted OC cells but also redirected endogenous host T-cells to kill tumor cells (131). Follow up studies are currently underway to understand how TLS may influence the local organization of both transferred FR-B T cells and endogenous immune cells in the TME in orchestrating the antitumor response.
Other ACT strategies include using NK cells, which can kill cancer cells without prior sensitization and are not antigen dependent. Several studies have been conducted using murine models (132–134) to explore improving cytotoxicity and longevity of NK cells. One example, addition of cytokines IL-2, IL-15, and IL-18, promotes activation and proliferation of NK cells, as well as secretion of INF gamma and inhibition of OC cells (135). This strategy generated cytokine-induced memory-like NK cells. Additional IL-15 or IL-15 super agonist complexes (N-803 or ALT-803) also promoted proliferation of NK cells with additional secreted factors including TNFα, CXCL10, and CD107a, which may enhance the function of NK cells (132, 136, 137). Only a limited number of OC patients have been treated clinically with NK ACT (138, 139), with some patients achieving stable disease with mild side effects (139). Several pre-clinical NK studies propose strategies to maximize effects of NK ACT including genetic modifications, combination therapy with ICI, targeted therapies, cytokines, and CAR-NK constructs, which currently are in early phase clinical trials (140)
Therapeutic strategies to induce TLS
Because TLS appears to play a major role in the anti-tumor response, identifying ways to induce TLS appears to be an important strategy. Vaccination contributes to induction of TLS and T cell infiltration as evidenced in pancreatic and cervical cancer. For example, in pancreatic adenocarcinoma patients with GM-CSF secreting allogeneic vaccine (GVAX) +/- a single IV dose of daily oral cyclophosphamide, eighty-five percent of post-vaccination tumor specimens contained histologic evidence of TLS formation not observed pre-vaccination. T cell infiltration and TLS development occurred in TME, with five signaling pathways involved in regulating immune-cell activation and trafficking associated with improved postvaccination responses differentially expressed, including genes encoding integrins, chemokines/chemokine receptors, and members of the ubiquitin-proteasome system and NK-kappa B pathway (141). In cervical cancer patients, intramuscular injection of a vaccine against HPV16E6/E7 induced TLS (142). This suggests that TLS formation may be needed for optimal vaccine response, since cancer vaccines often have limited efficacy, but also vaccination may be a strategy to induce TLS formation.
Oncolytic virotherapy has been proposed to induce TLS especially with immune checkpoint blockade (ICB) to amplify antitumor immune responses to patients with cancer (143). Because many tumors do not form functional TLS structures, “designer LN” have been proposed via injection of genetically modified immune cells in biomaterial scaffolds (65). The success of bioengineered lymph structures may depend on cellular properties for effective migration, hydrostatic fluid pressures for regulation of lymph flow and extravasation, as well as safety for cancer patients (144).
TLS in murine models
Several murine models suggest key players and potential strategies for the induction of TLS or new therapeutic strategies +/- immunotherapy. He et al., 2022 (145) have shown an oncolytic adenovirus carrying mIL-15 effectively facilitated activation and infiltration of DC, T, and NK cells into the TME, and induced TLS and vascular normalization. This mechanism appears to be through induced activation of the STING-TBK1-IRF3 pathway in DCs, and not traditional pathways of TLS induction. IKZF1, a gene related to the development of OC, melanoma, breast, and liver cancer, has been proposed to be a key driver of the formation of immature TLS (146). Using CRISPR/Cas9 gene editing to generate novel ID8 derivatives that harbor single and double suppressor gene mutations (Trp53-/- or Trp53-/-; Brca2-/-) in HGSOC, slower orthotopic tumor growth was observed in the double mutant compared to Trp53-/-, with rich intra-tumoral TLS in CD3+ T cells (146). TGF-Beta mediated silencing of genomic organizer SATB1 promoted Tfh cell differentiation and formation of intra-tumoral TLS, providing additional insight into how TLS are generated within tumors (82). Accumulated tumor antigen-specific LIGHT+CXCL13+IL-21+ Tfh cells and TLS decreased tumor growth in a CD4+ T cell and CXCL13-dependent manner (82).
Finally, two murine studies that are TLS-driven may offer new therapeutic strategies for OC patients. Cdk4/6 inhibitors (CDK4/6i) promoted TLS formation and enhanced the immunotherapeutic effect of anti-PD-1 when used in combination. TLS were associated with a favorable prognosis following CDK4/6i treatment, which may provide a therapeutic option for OC patients when used either alone, or in combination with anti-PD-1 therapy (147). A second study used an oral, small molecule focal adhesion kinase (FAK) inhibitor, FAKi (VS-4718), alone, and in combination with a blocking antibody to the checkpoint protein T cell immunoglobulin and ITIM domain (TIGIT), an inhibitory receptor expressed on lymphocytes that interacts with CD155 expressed on APC or tumor cells to down-regulate T cell and NK cell functions. Using a Kras, Myc, FAK (KMF) syngeneic ovarian tumor mouse model, decreased tumor burden, suppressed ascites KMF-associated CD155/Polio Virus Receptor (PVR) levels, and increased peritoneal TILs were observed with blocked FAK activity. FAKi combined with the 1B4 TIGIT blocking antibody maintained elevated TIL levels, reduced TIGIT+ T reg cell levels, prolonged host survival, increased CXCL13 levels, and led to omental TLS formation. These results provide a rationale, especially considering different anatomical sites of OC origin playing a role in humoral and cellular immunity, conducting a FAKi/TIGIT blocking antibody pilot in HGSOC patients (148).
Influence of the gut microbiome on TLS formation and generation of antitumor immunity
Another inducer of TLS is the gut microbiome, which appears to play a role in modulating the host response to ICI. The microbiome, comprised of trillions of microorganisms lives in a symbiotic relationship with human hosts (149), and actively regulates aspects of host physiology, including immunity (150). The human gut microbiome appears to be associated with TLS, B cells, and the antitumor response (151–153), although the exact mechanism still needs to be elucidated. Advances in identifying potential important bacteria in the microbiome are due to culture-independent genomic sequencing (e.g., 16S rRNA sequencing) at the species and strain level (154). Recently, Helicobacter hepaticus, a single immunogenic commensal bacterium, controlled growth in a carcinogen-induced orthotopic CRC mouse model, challenging the premise multiple immune-potentiating microbes are required to shift the host immune system towards potent antitumor immune responses. Generated bacteria-specific CD4+ Tfh cells stimulated TLS formation within the tumor and surrounding areas, leading to increased tumor immune infiltration and reduced tumor burden (155). This manipulation at a single taxa level was sufficient to generate robust anti-tumor immunity and TLS formation. TLS induction has also been found in a retrospective study of 60 HCC cases which showed that in an intratumoral TLS group, Lachnoclostridium, Hungatella, Blautia, Gusobacterium, and Clostridium were increased (156).
The gut microbiome is also associated with clinical responses to anti-PD-1 immunotherapy in a variety of cancers (155, 157–163), however, no common overall microbial signal or metabolomic profile has been identified, even within cancers, indicating additional factors must be at play. Higher alpha diversity associated with longer PFS (151) (157) (162), and abundance of specific species has been observed. In melanoma, higher microbial community richness is associated with longer PFS, and abundance of specific Bacteroides species (Ruminococcus gnavus), while the Blautia producta strain is related to shorter PFS (162). PFS also correlated to metatranscriptomic expression of risk-associated pathways of L-rhamnose degradation, guanosine nucleotide biosynthesis, and B vitamin biosynthesis. Higher relative abundance of Ruminococcaceae family of bacteria were also found in melanoma R, as well as anabolic pathway enrichment and enhanced systemic and antitumor immunity, mediated by increased antigen presentation and improved effector T-cell function in the periphery and TME (151).
In two NSCLC studies, patients with high microbiome diversity at baseline (and throughout), had significantly prolonged PFS compared to those with low diversity, and also had a greater frequency of unique memory CD8+ T cell and NK cell subsets in the periphery, suggesting a strong association between gut microbiome diversity and specific immune-related systemic populations (157). In clinical response to anti-PD-1/PD-L1 in advanced-stage gastrointestinal (GI) cancer, an elevated Prevotella/Bacteroides ratio was observed in R. A differential abundance of pathways related to nucleoside and nucleotide biosynthesis, lipid biosynthesis, sugar metabolism, and fermentation to short-chain fatty acids (SCFA) was identified in patients’ samples exhibiting differential responses. Gut bacteria associated with SCFA production, including Eubacterium, Lactobacillus, and Streptococcus, were positively associated with anti-PD-1/PD-L1 response across different GI cancer types (161).
The gut microbiome of durable R was enriched for Akkermansia muciniphila and Ruminococcaceae species in HCC patients treated with anti-PD-1 therapy. In hepatobiliary cancers, seventy-four specific taxa, including Lachnospiraceae bacterium-GAM79 and Alistipes sp Marseille-P5997, were significantly enriched in the CBR group, which achieved longer PFS and OS than patients with lower bacterial abundance (158) and the CBR group was associated with energy metabolism. These bacterial, metabolomic, and immune signatures are potential biomarkers to explore in OC patients treated with immunotherapy. Recently, specific bacteria were identified, including Intestinimonas butyriciproducens and Anaerotignum propionicum (Clostridium propionicum), that were highly abundant both before and after treatment for OC patients that achieved DCB following a triple combination therapy (23). These butyrate-producing bacteria have previously been associated with an enhanced response to immunotherapy (164), and increases of these bacteria in the triple combination therapy were also linked to increased immune and TLS changes in the TME. These results may generate signatures for R and NR of specific combination therapies.
While such findings are encouraging, additional studies, including an improved understanding of the microbiome and metabolic pathways, are needed to delineate nuances of a patient’s likelihood to respond to ICI therapy (163), as well as to drive TLS formation. Opportunities exist for immunomodulation through dietary and gut microbial interventions (165). Manipulating the presence of different bacterial species or metabolic pathways/metabolites, with interventions such as fecal transplants, probiotic use, microbial antigens or proteins, and selective antibiotics, may generate TLS and more favorable patient outcomes using immunotherapeutic approaches.
Conclusion and future directions
Understanding the complex roles of TLS within the TME is vital for improving therapeutic outcome for cancer patients, especially for those with limited treatment options at late-stage disease, as is the case with OC patients. Unlike SLO which develop during embryogenesis, TLS develop in response to chronic inflammation and cancer, playing a crucial role in antitumor immunity by enhancing both humoral and cellular responses. Their presence, especially in intratumoral areas, has been linked to better outcomes following immunotherapy, indicating their potential as a predictive biomarker for treatment success. Determining how these biomarkers may be mechanistically linked to therapeutic response likely depends on several factors including the type of therapeutic intervention (chemotherapy, ICI, radiation, vaccine-based, ACT-based, chemokine strategies, etc.), single agent or combination therapies, sequence of combination drug administration, timepoints for biospecimen collection (Baseline, on treatment, end of treatment), TLS location and density, anatomical site, and specific cancer type. Further, these factors may differ between preclinical models and real-world settings, further complicating data interpretation and integration. Therefore, additional studies to validate TLS as a reliable biomarker for therapeutic response in OC will provide additional insights and guide future applications.
In the case of OC, several factors recently have been implicated that contribute to the complexity of the role of TLS. The anatomical site of origin of OC may contribute to not only the role that TLS play, but their potential as a biomarker for response to therapy. Cancers that develop in the OV appear to have less TLS, less B cells, and a reduced antitumor response, while TLS that originate in the FT or OM have increased TLS and more B cells, suggestive that the TME is more supportive of TLS formation and indicative of an antitumor response (125). These results not only suggest the importance of B cells in the antitumor response, but also suggest the OV has a more suppressive TME. In fact, CA-MSC present in the OV negatively impacts adaptive immunity and survival. This poses an important area for future studies to identify ways to mitigate their impact and improve TLS formation, function, and antitumor response.
Research into TLS has highlighted their influence on the balance between promoting and inhibiting tumor growth, driven by factors such as patient demographics, tumor characteristics, environmental exposures, and previous treatments. The dynamic interplay between these factors and therapeutic interventions, as well as the gut microbiome’s impact, points to the complexity of harnessing TLS for cancer therapy. While several means to induce TLS have been proposed, modulation of the microbiome through diet or probiotic efforts may be a low-cost way to affect immunity and response to ICI. Continued studies that assess fecal microbiome transplants may also be a viable option to enhance efficacy of ICI as more potential beneficial bacteria or pathways that contribute to OS in OC are identified (166).
Analysis of patients’ samples who achieved a DCB in a triple combination therapy, which included pembrolizumab, uncovered a tumor-immune-gut axis influencing immunotherapy outcomes in OC (23). Assessment of fecal biospecimens revealed several metabolites were altered in samples from DCB and LCB patients. Metabolic alterations were observed in pathways that impact immune cell function. For example, fatty acids, amino acids, indole, and purine biosynthetic pathways were altered (23). While this study had only 40 patients (21), it suggests the immune milieu and host-microbiome can be leveraged to improve antitumor response in future immunotherapy trials. Omics results identified a target, CD40 (23), that is currently in clinical trial (NCT05231122) using a CD40 agonist in combination with pembrolizumab and bevacizumab. It also suggests that strategically combining different therapies (immunotherapy, chemotherapy, PARP inhibitors, adoptive cell therapies, targeted therapies) may lead to generation of TLS and improved OS. Radiation therapy and chemotherapy have been shown to generate TLS as well. Radiation impacts infiltration of T cells and maturation of DC cells which can help drive TLS formation and reshape the TME (167). A study has shown encouraging results where neoadjuvant chemotherapy (paclitaxel and carboplatin) induced TLS formation in HGSOC suggesting that it may be used in combination with ICI to drive an anti-tumor response with increased TLS formation (168).
Several next step options to drive TLS may be through promising OC murine pre-clinical models such as the combination of cdki with ICI or use of FAK inhibitors +/- ICI. Additionally, combination therapies (ICI, chemotherapy, targeted therapy) that may modulate different components of the immune system may stimulate both the cellular and humoral response and provide a more robust DCB, as well as identify new therapeutic targets or pathways, potentially through multi-omics means. Vaccination strategies that may prime the immune response may also bolster an enhanced response through TLS generation as has been observed for PANC GM-CSF (GVAX) vaccination and HPV vaccination. CXCL13 approaches to introduce this powerful cytokine to drive TLS formation may also be a viable option in combination with ACT approaches. Finally, transcriptional assessment of different genes that may drive TLS formation may also prove to be beneficial in predicting a patient’s response by biospecimen analysis at baseline or over the course of treatment.
Advancing our knowledge of TLS, particularly in OC, and the factors affecting therapeutic response is critical. Future efforts focusing on exploiting TLS to improve immune infiltration, boost anti-tumor responses, and enhance patient responses to treatment, promise significant impact on cancer therapy optimization.
Author contributions
AV: Writing – review & editing, Writing – original draft. SH: Writing – review & editing. SC: Writing – original draft, Writing – review & editing. JC-G: Conceptualization, Writing – review & editing. AM: Writing – review & editing. EZ: Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Figure 1 printed with permission from the Roswell Park Comprehensive Cancer Center, ATLAS Program. Figure 2 was created using BioRender.com and has been included under publication license granted to Suzanne M. Hess, PhD.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lehrer EJ, Stoltzfus KC, Jones BM, Gusani NJ, Walter V, Wang M, et al. Trends in diagnosis and treatment of metastatic cancer in the United States. Am J Clin Oncol. (2021) 44:572–9. doi: 10.1097/COC.0000000000000866
2. Armstrong DK, Alvarez RD, Backes FJ, Bakkum-Gamez JN, Barroilhet L, Behbakht K, et al. NCCN guidelines(R) insights: ovarian cancer, version 3. 2022. J Natl Compr Canc Netw. (2022) 20:972–80. doi: 10.6004/jnccn.2022.0047
3. Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. (2003) 348:203–13. doi: 10.1056/NEJMoa020177
4. Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. (2005) 102:18538–43. doi: 10.1073/pnas.0509182102
5. Kalli KR, Krco CJ, Hartmann LC, Goodman K, Maurer MJ, Yu C, et al. An HLA-DR-degenerate epitope pool detects insulin-like growth factor binding protein 2-specific immunity in patients with cancer. Cancer Res. (2008) 68:4893–901. doi: 10.1158/0008-5472.CAN-07-6726
6. Knutson KL, Krco CJ, Erskine CL, Goodman K, Kelemen LE, Wettstein PJ, et al. T-cell immunity to the folate receptor alpha is prevalent in women with breast or ovarian cancer. J Clin Oncol. (2006) 24:4254–61. doi: 10.1200/JCO.2006.05.9311
7. Reuschenbach M, von Knebel Doeberitz M, and Wentzensen N. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother. (2009) 58:1535–44. doi: 10.1007/s00262-009-0733-4
8. Milne K, Barnes RO, Girardin A, Mawer MA, Nesslinger NJ, Ng A, et al. Tumor-infiltrating T cells correlate with NY-ESO-1-specific autoantibodies in ovarian cancer. PLoS One. (2008) 3:e3409. doi: 10.1371/journal.pone.0003409
9. Zhao J, Xu Z, Liu Y, Wang X, Liu X, Gao Y, et al. The expression of cancer-testis antigen in ovarian cancer and the development of immunotherapy. Am J Cancer Res. (2022) 12:681–94.
10. Takai N, Miyazaki T, Nishida M, Nasu K, and Miyakawa I. Expression of survivin is associated with Malignant potential in epithelial ovarian carcinoma. Int J Mol Med. (2002) 10:211–6. doi: 10.3892/ijmm.10.2.211
11. Wang L, Ma J, Liu F, Yu Q, Chu G, Perkins AC, et al. Expression of MUC1 in primary and metastatic human epithelial ovarian cancer and its therapeutic significance. Gynecol Oncol. (2007) 105:695–702. doi: 10.1016/j.ygyno.2007.02.004
12. Vermeij R, de Bock GH, Leffers N, Ten Hoor KA, Schulze U, Hollema H, et al. Tumor-infiltrating cytotoxic T lymphocytes as independent prognostic factor in epithelial ovarian cancer with wilms tumor protein 1 overexpression. J Immunother. (2011) 34:516–23. doi: 10.1097/CJI.0b013e31821e012f
13. Motz GT, Santoro SP, Wang LP, Garrabrant T, Lastra RR, Hagemann IS, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat medicine. (2014) 20:607–15. doi: 10.1038/nm.3541
14. Mukai S, Kjaergaard J, Shu S, and Plautz GE. Infiltration of tumors by systemically transferred tumor-reactive T lymphocytes is required for antitumor efficacy. Cancer Res. (1999) 59:5245–9.
15. Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. (1994) 76:301–14. doi: 10.1016/0092-8674(94)90337-9
16. Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. (2012) 366:2455–65. doi: 10.1056/NEJMoa1200694
17. Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. (2015) 33:4015–22. doi: 10.1200/JCO.2015.62.3397
18. Matulonis UA, Shapira-Frommer R, Santin AD, Lisyanskaya AS, Pignata S, Vergote I, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol. (2019) 30:1080–7. doi: 10.1093/annonc/mdz135
19. Gonzalez-Martin A and Sanchez-Lorenzo L. Immunotherapy with checkpoint inhibitors in patients with ovarian cancer: Still promising? Cancer. (2019) 125 Suppl 24:4616–22. doi: 10.1002/cncr.v125.s24
20. Ghisoni E, Morotti M, Sarivalasis A, Grimm AJ, Kandalaft L, Laniti DD, et al. Immunotherapy for ovarian cancer: towards a tailored immunophenotype-based approach. Nat Rev Clin Oncol. (2024) 21(11):801–7. doi: 10.1038/s41571-024-00937-4
21. Zsiros E, Lynam S, Attwood KM, Wang C, Chilakapati S, Gomez EC, et al. Efficacy and safety of pembrolizumab in combination with bevacizumab and oral metronomic cyclophosphamide in the treatment of recurrent ovarian cancer: A phase 2 nonrandomized clinical trial. JAMA Oncol. (2021) 7:78–85. doi: 10.1001/jamaoncol.2020.5945
22. Gaulin N, Chilakapati SR, and Zsiros E. Turning cold into hot: combination of pembrolizumab with bevacizumab and oral metronomic cyclophosphamide increases immune cell migration into the tumor microenvironment in responding patients with recurrent ovarian cancer (090). Gynecologic Oncology. (2022) 166:S61. doi: 10.1016/S0090-8258(22)01316-6
23. Rosario SR, Long MD, Chilakapati S, Gomez EC, Battaglia S, Singh PK, et al. Integrative multi-omics analysis uncovers tumor-immune-gut axis influencing immunotherapy outcomes in ovarian cancer. Nat Commun. (2024) 15:10609. doi: 10.1038/s41467-024-54565-8
24. Liu JF, Herold C, Gray KP, Penson RT, Horowitz N, Konstantinopoulos PA, et al. Assessment of combined nivolumab and bevacizumab in relapsed ovarian cancer: A phase 2 clinical trial. JAMA Oncol. (2019) 5:1731–8. doi: 10.1001/jamaoncol.2019.3343
25. Garcia AA, Hirte H, Fleming G, Yang D, Tsao-Wei DD, Roman L, et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol. (2008) 26:76–82. doi: 10.1200/JCO.2007.12.1939
26. Walsh CS, Kamrava M, Rogatko A, Kim S, Li A, Cass I, et al. Phase II trial of cisplatin, gemcitabine and pembrolizumab for platinum-resistant ovarian cancer. PLoS One. (2021) 16:e0252665. doi: 10.1371/journal.pone.0252665
27. Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat medicine. (2004) 10:942–9. doi: 10.1038/nm1093
28. Kryczek I, Wei S, Zhu G, Myers L, Mottram P, Cheng P, et al. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. (2007) 67:8900–5. doi: 10.1158/0008-5472.CAN-07-1866
29. Garlisi B, Lauks S, Aitken C, Ogilvie LM, Lockington C, Petrik D, et al. The complex tumor microenvironment in ovarian cancer: therapeutic challenges and opportunities. Curr Oncol. (2024) 31:3826–44. doi: 10.3390/curroncol31070283
30. Yarchoan M, Hopkins A, and Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. (2017) 377:2500–1. doi: 10.1056/NEJMc1713444
31. Cornel AM, Mimpen IL, and Nierkens S. MHC class I downregulation in cancer: underlying mechanisms and potential targets for cancer immunotherapy. Cancers (Basel). (2020) 12(7):1760–91. doi: 10.3390/cancers12071760
32. Chae CS, Sandoval TA, Hwang SM, Park ES, Giovanelli P, Awasthi D, et al. Tumor-derived lysophosphatidic acid blunts protective type I interferon responses in ovarian cancer. Cancer Discov. (2022) 12:1904–21. doi: 10.1158/2159-8290.CD-21-1181
33. Singel KL, Emmons TR, Khan ANH, Mayor PC, Shen S, Wong JT, et al. Mature neutrophils suppress T cell immunity in ovarian cancer microenvironment. JCI Insight. (2019) 4(5):e122311. doi: 10.1172/jci.insight.122311
34. Emmons TR, Giridharan T, Singel KL, Khan ANH, Ricciuti J, Howard K, et al. Mechanisms driving neutrophil-induced T-cell immunoparalysis in ovarian cancer. Cancer Immunol Res. (2021) 9:790–810. doi: 10.1158/2326-6066.CIR-20-0922
35. Song M, Sandoval TA, Chae CS, Chopra S, Tan C, Rutkowski MR, et al. IRE1alpha-XBP1 controls T cell function in ovarian cancer by regulating mitochondrial activity. Nature. (2018) 562:423–8. doi: 10.1038/s41586-018-0597-x
36. Kelleher RJ Jr., Balu-Iyer S, Loyall J, Sacca AJ, Shenoy GN, Peng P, et al. Extracellular vesicles present in human ovarian tumor microenvironments induce a phosphatidylserine-dependent arrest in the T-cell signaling cascade. Cancer Immunol Res. (2015) 3:1269–78. doi: 10.1158/2326-6066.CIR-15-0086
37. Walton J, Blagih J, Ennis D, Leung E, Dowson S, Farquharson M, et al. CRISPR/cas9-mediated trp53 and brca2 knockout to generate improved murine models of ovarian high-grade serous carcinoma. Cancer Res. (2016) 76:6118–29. doi: 10.1158/0008-5472.CAN-16-1272
38. Kobayashi Y, Lim SO, and Yamaguchi H. Oncogenic signaling pathways associated with immune evasion and resistance to immune checkpoint inhibitors in cancer. Semin Cancer Biol. (2020) 65:51–64. doi: 10.1016/j.semcancer.2019.11.011
39. Johnson RL, Cummings M, Thangavelu A, Theophilou G, de Jong D, and Orsi NM. Barriers to immunotherapy in ovarian cancer: metabolic, genomic, and immune perturbations in the tumour microenvironment. Cancers (Basel). (2021) 13(24):6231–79. doi: 10.3390/cancers13246231
40. Husain Z, Huang Y, Seth P, and Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol. (2013) 191:1486–95. doi: 10.4049/jimmunol.1202702
41. Amobi-McCloud A, Muthuswamy R, Battaglia S, Yu H, Liu T, Wang J, et al. IDO1 expression in ovarian cancer induces PD-1 in T cells via aryl hydrocarbon receptor activation. Front Immunol. (2021) 12:678999. doi: 10.3389/fimmu.2021.678999
42. Mellor AL and Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today. (1999) 20:469–73. doi: 10.1016/s0167-5699(99)01520-0
43. Schaaf MB, Garg AD, and Agostinis P. Defining the role of the tumor vasculature in antitumor immunity and immunotherapy. Cell Death Dis. (2018) 9:115. doi: 10.1038/s41419-017-0061-0
44. Kandalaft LE, Odunsi K, and Coukos G. Immunotherapy in ovarian cancer: are we there yet? J Clin Oncol. (2019) 37:2460–71. doi: 10.1200/JCO.19.00508
45. Huang RY, Francois A, McGray AR, Miliotto A, and Odunsi K. Compensatory upregulation of PD-1, LAG-3, and CTLA-4 limits the efficacy of single-agent checkpoint blockade in metastatic ovarian cancer. Oncoimmunology. (2017) 6:e1249561. doi: 10.1080/2162402X.2016.1249561
46. Colbeck EJ, Ager A, Gallimore A, and Jones GW. Tertiary lymphoid structures in cancer: drivers of antitumor immunity, immunosuppression, or bystander sentinels in disease? Front Immunol. (2017) 8:1830. doi: 10.3389/fimmu.2017.01830
47. Sun G and Liu Y. Tertiary lymphoid structures in ovarian cancer. Front Immunol. (2024) 15:1465516. doi: 10.3389/fimmu.2024.1465516
48. Asam S, Nayar S, Gardner D, and Barone F. Stromal cells in tertiary lymphoid structures: Architects of autoimmunity. Immunol Rev. (2021) 302:184–95. doi: 10.1111/imr.v302.1
49. Buckley CD, Barone F, Nayar S, Bénézech C, and Caamaño J. Stromal cells in chronic inflammation and tertiary lymphoid organ formation. Annu Rev Immunol. (2015) 33:715–45. doi: 10.1146/annurev-immunol-032713-120252
50. Mauri C and Menon M. The expanding family of regulatory B cells. Int Immunol. (2015) 27:479–86. doi: 10.1093/intimm/dxv038
51. Pitzalis C, Jones GW, Bombardieri M, and Jones SA. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol. (2014) 14:447–62. doi: 10.1038/nri3700
52. Sato Y, Silina K, van den Broek M, Hirahara K, and Yanagita M. The roles of tertiary lymphoid structures in chronic diseases. Nat Rev Nephrol. (2023) 19:525–37. doi: 10.1038/s41581-023-00706-z
53. Pers JO, Le Pottier L, Devauchelle V, Saraux A, and Youinou P. B lymphocytes in Sjogren’s syndrome. Rev Med Interne. (2008) 29:1000–6. doi: 10.1016/j.revmed.2008.01.018
54. Chang A, Henderson SG, Brandt D, Liu N, Guttikonda R, Hsieh C, et al. In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol. (2011) 186:1849–60. doi: 10.4049/jimmunol.1001983
55. Korpos E, Kadri N, Loismann S, Findeisen CR, Arfuso F, Burke GW 3rd, et al. Identification and characterisation of tertiary lymphoid organs in human type 1 diabetes. Diabetologia. (2021) 64:1626–41. doi: 10.1007/s00125-021-05453-z
56. Gomez-Nguyen A, Gupta N, Sanaka H, Gruszka D, Pizarro A, DiMartino L, et al. Chronic stress induces colonic tertiary lymphoid organ formation and protection against secondary injury through IL-23/IL-22 signaling. Proc Natl Acad Sci U S A. (2022) 119:e2208160119. doi: 10.1073/pnas.2208160119
57. Takemura S, Braun A, Crowson C, Kurtin PJ, Cofield RH, O’Fallon WM, et al. Lymphoid neogenesis in rheumatoid synovitis. J Immunol. (2001) 167:1072–80. doi: 10.4049/jimmunol.167.2.1072
58. Nayar S, Turner JD, Asam S, Fennell E, Pugh M, ColaFrancesco S, et al. Molecular and spatial analysis of tertiary lymphoid structures in Sjogren’s syndrome. Nat Commun. (2025) 16:5. doi: 10.1038/s41467-024-54686-0
59. Nayar S, Campos J, Smith CG, Iannizzotto V, Gardner DH, Mourcin F, et al. Immunofibroblasts are pivotal drivers of tertiary lymphoid structure formation and local pathology. Proc Natl Acad Sci U S A. (2019) 116:13490–7. doi: 10.1073/pnas.1905301116
60. Ruddle NH. High endothelial venules and lymphatic vessels in tertiary lymphoid organs: characteristics, functions, and regulation. Front Immunol. (2016) 7:491. doi: 10.3389/fimmu.2016.00491
61. Sautes-Fridman C, Verneau J, Sun CM, Moreira M, Chen TW, Meylan M, et al. Tertiary Lymphoid Structures and B cells: Clinical impact and therapeutic modulation in cancer. Semin Immunol. (2020) 48:101406. doi: 10.1016/j.smim.2020.101406
62. Zhao L, Jin S, Wang S, Zhang Z, Wang X, Chen Z, et al. Tertiary lymphoid structures in diseases: immune mechanisms and therapeutic advances. Signal Transduct Target Ther. (2024) 9:225. doi: 10.1038/s41392-024-01947-5
63. Sautès-Fridman C, Petitprez F, Calderaro J, and Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. (2019) 19:307–25. doi: 10.1038/s41568-019-0144-6
64. Johansson-Percival A, He B, Li ZJ, Kjellén A, Russell K, Li J, et al. De novo induction of intratumoral lymphoid structures and vessel normalization enhances immunotherapy in resistant tumors. Nat Immunol. (2017) 18:1207–17. doi: 10.1038/ni.3836
65. Zhu G, Falahat R, Wang K, Mailloux A, Artzi N, and Mulé JJ. Tumor-associated tertiary lymphoid structures: gene-expression profiling and their bioengineering. Front Immunol. (2017) 8:767. doi: 10.3389/fimmu.2017.00767
66. Fridman WH, Siberil S, Pupier G, Soussan S, and Sautes-Fridman C. Activation of B cells in Tertiary Lymphoid Structures in cancer: Anti-tumor or anti-self? Semin Immunol. (2023) 65:101703. doi: 10.1016/j.smim.2022.101703
67. Chen Y, Wu Y, Yan G, and Zhang G. Tertiary lymphoid structures in cancer: maturation and induction. Front Immunol. (2024) 15:1369626. doi: 10.3389/fimmu.2024.1369626
68. Vanhersecke L, Brunet M, Guegan JP, Rey C, Bougouin A, Cousin S, et al. Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression. Nat Cancer. (2021) 2:794–802. doi: 10.1038/s43018-021-00232-6
69. Teillaud JL, Houel A, Panouillot M, Riffard C, and Dieu-Nosjean MC. Tertiary lymphoid structures in anticancer immunity. Nat Rev Cancer. (2024) 24:629–46. doi: 10.1038/s41568-024-00728-0
70. Vanhersecke L, Bougouin A, Crombe A, Brunet M, Sofeu C, Parrens M, et al. Standardized pathology screening of mature tertiary lymphoid structures in cancers. Lab Invest. (2023) 103:100063. doi: 10.1016/j.labinv.2023.100063
71. Zhang K, Xie X, Zheng SL, Deng YR, Liao D, Yan HC, et al. Tertiary lymphoid structures in gynecological cancers: prognostic role, methods for evaluating, antitumor immunity, and induction for therapy. Front Oncol. (2023) 13:1276907. doi: 10.3389/fonc.2023.1276907
72. Kasikova L, Rakova J, Hensler M, Lanickova T, Tomankova J, Pasulka J, et al. Tertiary lymphoid structures and B cells determine clinically relevant T cell phenotypes in ovarian cancer. Nat Commun. (2024) 15:2528. doi: 10.1038/s41467-024-46873-w
73. Xu AM, Haro M, Walts AE, Hu Y, John J, Karlan BY, et al. Spatiotemporal architecture of immune cells and cancer-associated fibroblasts in high-grade serous ovarian carcinoma. Sci Adv. (2024) 10:eadk8805. doi: 10.1126/sciadv.adk8805
74. van de Pavert SA. Lymphoid Tissue inducer (LTi) cell ontogeny and functioning in embryo and adult. BioMed J. (2021) 44:123–32. doi: 10.1016/j.bj.2020.12.003
75. Randall TD, Carragher DM, and Rangel-Moreno J. Development of secondary lymphoid organs. Annu Rev Immunol. (2008) 26:627–50. doi: 10.1146/annurev.immunol.26.021607.090257
76. van de Pavert SA and Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol. (2010) 10:664–74. doi: 10.1038/nri2832
77. Honda K, Nakano H, Yoshida H, Nishikawa S, Rennert P, Ikuta K, et al. Molecular basis for hematopoietic/mesenchymal interaction during initiation of Peyer’s patch organogenesis. J Exp Med. (2001) 193:621–30. doi: 10.1084/jem.193.5.621
78. Schumacher TN and Thommen DS. Tertiary lymphoid structures in cancer. Science. (2022) 375:eabf9419. doi: 10.1126/science.abf9419
79. Ukita M, Hamanishi J, Yoshitomi H, Yamanoi K, Takamatsu S, Ueda A, et al. CXCL13-producing CD4+ T cells accumulate in the early phase of tertiary lymphoid structures in ovarian cancer. JCI Insight. (2022) 7(12):e157215. doi: 10.1172/jci.insight.157215
80. Lee Y, Chin RK, Christiansen P, Sun Y, Tumanov AV, Wang J, et al. Recruitment and activation of naive T cells in the islets by lymphotoxin beta receptor-dependent tertiary lymphoid structure. Immunity. (2006) 25:499–509. doi: 10.1016/j.immuni.2006.06.016
81. Lu TT and Browning JL. Role of the lymphotoxin/LIGHT system in the development and maintenance of reticular networks and vasculature in lymphoid tissues. Front Immunol. (2014) 5:47. doi: 10.3389/fimmu.2014.00047
82. Chaurio RA, Anadon CM, Lee Costich T, Payne KK, Biswas S, Harro CM, et al. TGF-beta-mediated silencing of genomic organizer SATB1 promotes Tfh cell differentiation and formation of intra-tumoral tertiary lymphoid structures. Immunity. (2022) 55:115–28.e9. doi: 10.1016/j.immuni.2021.12.007
83. Conejo-Garcia JR, Biswas S, Chaurio R, and Rodriguez PC. Neglected no more: B cell-mediated anti-tumor immunity. Semin Immunol. (2023) 65:101707. doi: 10.1016/j.smim.2022.101707
84. Montfort A, Pearce O, Maniati E, Vincent BG, Bixby L, Bohm S, et al. A strong B-cell response is part of the immune landscape in human high-grade serous ovarian metastases. Clin Cancer Res. (2017) 23:250–62. doi: 10.1158/1078-0432.CCR-16-0081
85. Biswas S, Mandal G, Payne KK, Anadon CM, Gatenbee CD, Chaurio RA, et al. IgA transcytosis and antigen recognition govern ovarian cancer immunity. Nature. (2021) 591:464–70. doi: 10.1038/s41586-020-03144-0
86. Nielsen JS, Sahota RA, Milne K, Kost SE, Nesslinger NJ, Watson PH, et al. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res. (2012) 18:3281–92. doi: 10.1158/1078-0432.CCR-12-0234
87. Khanal S, Wieland A, and Gunderson AJ. Mechanisms of tertiary lymphoid structure formation: cooperation between inflammation and antigenicity. Front Immunol. (2023) 14:1267654. doi: 10.3389/fimmu.2023.1267654
88. van Rijthoven M, Obahor S, Pagliarulo F, van den Broek M, Schraml P, Moch H, et al. Multi-resolution deep learning characterizes tertiary lymphoid structures and their prognostic relevance in solid tumors. Commun Med (Lond). (2024) 4:5. doi: 10.1038/s43856-023-00421-7
89. Lin JR, Wang S, Coy S, Chen YA, Yapp C, Tyler M, et al. Multiplexed 3D atlas of state transitions and immune interaction in colorectal cancer. Cell. (2023) 186:363–81.e19. doi: 10.1016/j.cell.2022.12.028
90. Walsh LA and Quail DF. Decoding the tumor microenvironment with spatial technologies. Nat Immunol. (2023) 24:1982–93. doi: 10.1038/s41590-023-01678-9
91. Gan X, Dong W, You W, Ding D, Yang Y, Sun D, et al. Spatial multimodal analysis revealed tertiary lymphoid structures as a risk stratification indicator in combined hepatocellular-cholangiocarcinoma. Cancer Lett. (2024) 581:216513. doi: 10.1016/j.canlet.2023.216513
92. Weed DT, Zilio S, McGee C, Marnissi B, Sargi Z, Franzmann E, et al. The tumor immune microenvironment architecture correlates with risk of recurrence in head and neck squamous cell carcinoma. Cancer Res. (2023) 83:3886–900. doi: 10.1158/0008-5472.CAN-23-0379
93. Li H, Lin WP, Zhang ZN, and Sun ZJ. Tailoring biomaterials for monitoring and evoking tertiary lymphoid structures. Acta Biomater. (2023) 172:1–15. doi: 10.1016/j.actbio.2023.09.028
94. Yang M, Che Y, Li K, Fang Z, Li S, Wang M, et al. Detection and quantitative analysis of tumor-associated tertiary lymphoid structures. J Zhejiang Univ Sci B. (2023) 24:779–95. doi: 10.1631/jzus.B2200605
95. Xu Y, Li Z, Yang Y, Li L, Zhou Y, Ouyang J, et al. A CT-based radiomics approach to predict intra-tumoral tertiary lymphoid structures and recurrence of intrahepatic cholangiocarcinoma. Insights Imaging. (2023) 14:173. doi: 10.1186/s13244-023-01527-1
96. Yu Y, Yang T, Ma P, Zeng Y, Dai Y, Fu Y, et al. Determining the status of tertiary lymphoid structures in invasive pulmonary adenocarcinoma based on chest CT radiomic features. Insights Imaging. (2025) 16:28. doi: 10.1186/s13244-025-01906-w
97. Long S, Li M, Chen J, Zhong L, Dai G, Pan D, et al. Transfer learning radiomic model predicts intratumoral tertiary lymphoid structures in hepatocellular carcinoma: a multicenter study. J Immunother Cancer. (2025) 13(3):e011126. doi: 10.1136/jitc-2024-011126
98. Li K, Guo Q, Zhang X, Dong X, Liu W, Zhang A, et al. Oral cancer-associated tertiary lymphoid structures: gene expression profile and prognostic value. Clin Exp Immunol. (2020) 199:172–81. doi: 10.1111/cei.13389
99. Coppola D, Nebozhyn M, Khalil F, Dai H, Yeatman T, Loboda A, et al. Unique ectopic lymph node-like structures present in human primary colorectal carcinoma are identified by immune gene array profiling. Am J Pathol. (2011) 179:37–45. doi: 10.1016/j.ajpath.2011.03.007
100. Messina JL, Fenstermacher DA, Eschrich S, Qu X, Berglund AE, Lloyd MC, et al. 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Sci Rep. (2012) 2:765. doi: 10.1038/srep00765
101. Prabhakaran S, Rizk VT, Ma Z, Cheng CH, Berglund AE, Coppola D, et al. Evaluation of invasive breast cancer samples using a 12-chemokine gene expression score: correlation with clinical outcomes. Breast Cancer Res. (2017) 19:71. doi: 10.1186/s13058-017-0864-z
102. Zhang C, Kang Y, Miao P, and Chang D. A novel genes-based signature with prognostic value and predictive ability to select patients responsive to Atezolizumab treatment in bladder cancer: an analysis on data from real-world studies. Transl Cancer Res. (2023) 12:2063–70. doi: 10.21037/tcr-23-220
103. Li R, Berglund A, Zemp L, Dhillon J, Putney R, Kim Y, et al. The 12-CK score: global measurement of tertiary lymphoid structures. Front Immunol. (2021) 12:694079. doi: 10.3389/fimmu.2021.694079
104. Hou Y, Qiao S, Li M, Han X, Wei X, Pang Y, et al. The gene signature of tertiary lymphoid structures within ovarian cancer predicts the prognosis and immunotherapy benefit. Front Genet. (2022) 13:1090640. doi: 10.3389/fgene.2022.1090640
105. Lu H, Lou H, Wengert G, Paudel R, Patel N, Desai S, et al. Tumor and local lymphoid tissue interaction determines prognosis in high-grade serous ovarian cancer. Cell Rep Med. (2023) 4:101092. doi: 10.1016/j.xcrm.2023.101092
106. Horeweg N, Workel HH, Loiero D, Church DN, Vermij L, Leon-Castillo A, et al. Tertiary lymphoid structures critical for prognosis in endometrial cancer patients. Nat Commun. (2022) 13:1373. doi: 10.1038/s41467-022-29040-x
107. Fridman WH, Meylan M, Petitprez F, Sun CM, Italiano A, and Sautes-Fridman C. B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome. Nat Rev Clin Oncol. (2022) 19:441–57. doi: 10.1038/s41571-022-00619-z
108. Peyraud F, Guegan JP, Vanhersecke L, Brunet M, Teyssonneau D, Palmieri LJ, et al. Tertiary lymphoid structures and cancer immunotherapy: From bench to bedside. Med. (2025) 6:100546. doi: 10.1016/j.medj.2024.10.023
109. Amaria RN, Reddy SM, Tawbi HA, Davies MA, Ross MI, Glitza IC, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. (2018) 24:1649–54. doi: 10.1038/s41591-018-0197-1
110. Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. (2020) 577:549–55. doi: 10.1038/s41586-019-1922-8
111. Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. (2020) 577:561–5. doi: 10.1038/s41586-019-1914-8
112. Petitprez F, de Reynies A, Keung EZ, Chen TW, Sun CM, Calderaro J, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. (2020) 577:556–60. doi: 10.1038/s41586-019-1906-8
113. Italiano A, Bessede A, Pulido M, Bompas E, Piperno-Neumann S, Chevreau C, et al. Pembrolizumab in soft-tissue sarcomas with tertiary lymphoid structures: a phase 2 PEMBROSARC trial cohort. Nat Med. (2022) 28:1199–206. doi: 10.1038/s41591-022-01821-3
114. Patil NS, Nabet BY, Muller S, Koeppen H, Zou W, Giltnane J, et al. Intratumoral plasma cells predict outcomes to PD-L1 blockade in non-small cell lung cancer. Cancer Cell. (2022) 40:289–300.e4. doi: 10.1016/j.ccell.2022.02.002
115. Gao J, Navai N, Alhalabi O, Siefker-Radtke A, Campbell MT, Tidwell RS, et al. Neoadjuvant PD-L1 plus CTLA-4 blockade in patients with cisplatin-ineligible operable high-risk urothelial carcinoma. Nat Med. (2020) 26:1845–51. doi: 10.1038/s41591-020-1086-y
116. van Dijk N, Gil-Jimenez A, Silina K, Hendricksen K, Smit LA, de Feijter JM, et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat Med. (2020) 26:1839–44. doi: 10.1038/s41591-020-1085-z
117. van Dijk N, Gil-Jimenez A, Silina K, van Montfoort ML, Einerhand S, Jonkman L, et al. The tumor immune landscape and architecture of tertiary lymphoid structures in urothelial cancer. Front Immunol. (2021) 12:793964. doi: 10.3389/fimmu.2021.793964
118. Campbell MT, Matin SF, Tam AL, Sheth RA, Ahrar K, Tidwell RS, et al. Pilot study of Tremelimumab with and without cryoablation in patients with metastatic renal cell carcinoma. Nat Commun. (2021) 12:6375. doi: 10.1038/s41467-021-26415-4
119. Carril-Ajuria L, Desnoyer A, Meylan M, Dalban C, Naigeon M, Cassard L, et al. Baseline circulating unswitched memory B cells and B-cell related soluble factors are associated with overall survival in patients with clear cell renal cell carcinoma treated with nivolumab within the NIVOREN GETUG-AFU 26 study. J Immunother Cancer. (2022) 10(5):e004885. doi: 10.1136/jitc-2022-004885
120. Ho WJ, Zhu Q, Durham J, Popovic A, Xavier S, Leatherman J, et al. Neoadjuvant cabozantinib and nivolumab converts locally advanced HCC into resectable disease with enhanced antitumor immunity. Nat Cancer. (2021) 2:891–903. doi: 10.1038/s43018-021-00234-4
121. Nielsen JS and Nelson BH. Tumor-infiltrating B cells and T cells: Working together to promote patient survival. Oncoimmunology. (2012) 1:1623–5. doi: 10.4161/onci.21650
122. Chen S, Xie P, Cowan M, Huang H, Cardenas H, Keathley R, et al. Epigenetic priming enhances antitumor immunity in platinum-resistant ovarian cancer. J Clin Invest. (2022) 132(14):e158800. doi: 10.1172/JCI158800
123. Liu J, Berchuck A, Backes FJ, Cohen J, Grisham R, Leath CA, et al. NCCN guidelines(R) insights: ovarian cancer/fallopian tube cancer/primary peritoneal cancer, version 3.2024. J Natl Compr Canc Netw. (2024) 22:512–9. doi: 10.6004/jnccn.2024.0052
124. Yang M, Lu J, Zhang G, Wang Y, He M, Xu Q, et al. CXCL13 shapes immunoactive tumor microenvironment and enhances the efficacy of PD-1 checkpoint blockade in high-grade serous ovarian cancer. J Immunother Cancer. (2021) 9(1):e001136. doi: 10.1136/jitc-2020-001136
125. MacFawn IP, Magnon G, Gorecki G, Kunning S, Rashid R, Kaiza ME, et al. The activity of tertiary lymphoid structures in high grade serous ovarian cancer is governed by site, stroma, and cellular interactions. Cancer Cell. (2024) 42(11):1864–81.e5. doi: 10.1016/j.ccell.2024.09.007
126. Bod L. Finding the right friends: Stromal composition influences TLS formation in ovarian cancer. Cancer Cell. (2024) 42:1811–2. doi: 10.1016/j.ccell.2024.10.004
127. Fan H, Atiya HI, Wang Y, Pisanic TR, Wang TH, Shih IM, et al. Epigenomic reprogramming toward mesenchymal-epithelial transition in ovarian-cancer-associated mesenchymal stem cells drives metastasis. Cell Rep. (2020) 33:108473. doi: 10.1016/j.celrep.2020.108473
128. Gitto SB, Ihewulezi CJN, and Powell DJ Jr. Adoptive T cell therapy for ovarian cancer. Gynecol Oncol. (2024) 186:77–84. doi: 10.1016/j.ygyno.2024.04.001
129. Mittica G, Capellero S, Genta S, Cagnazzo C, Aglietta M, Sangiolo D, et al. Adoptive immunotherapy against ovarian cancer. J Ovarian Res. (2016) 9:30. doi: 10.1186/s13048-016-0236-9
130. Wala JA and Hanna GJ. Chimeric antigen receptor T-cell therapy for solid tumors. Hematol Oncol Clin North Am. (2023) 37:1149–68. doi: 10.1016/j.hoc.2023.05.009
131. McGray AJR, Chiello JL, Tsuji T, Long M, Maraszek K, Gaulin N, et al. BiTE secretion by adoptively transferred stem-like T cells improves FRalpha+ ovarian cancer control. J Immunother Cancer. (2023) 11(6):e006863. doi: 10.1136/jitc-2023-006863
132. Felices M, Chu S, Kodal B, Bendzick L, Ryan C, Lenvik AJ, et al. IL-15 super-agonist (ALT-803) enhances natural killer (NK) cell function against ovarian cancer. Gynecol Oncol. (2017) 145:453–61. doi: 10.1016/j.ygyno.2017.02.028
133. Geller MA, Knorr DA, Hermanson DA, Pribyl L, Bendzick L, McCullar V, et al. Intraperitoneal delivery of human natural killer cells for treatment of ovarian cancer in a mouse xenograft model. Cytotherapy. (2013) 15:1297–306. doi: 10.1016/j.jcyt.2013.05.022
134. Uppendahl LD, Dahl CM, Miller JS, Felices M, and Geller MA. Natural killer cell-based immunotherapy in gynecologic Malignancy: A review. Front Immunol. (2017) 8:1825. doi: 10.3389/fimmu.2017.01825
135. Uppendahl LD, Felices M, Bendzick L, Ryan C, Kodal B, Hinderlie P, et al. Cytokine-induced memory-like natural killer cells have enhanced function, proliferation, and in vivo expansion against ovarian cancer cells. Gynecol Oncol. (2019) 153:149–57. doi: 10.1016/j.ygyno.2019.01.006
136. Hoogstad-van Evert JS, Maas RJ, van der Meer J, Cany J, van der Steen S, Jansen JH, et al. Peritoneal NK cells are responsive to IL-15 and percentages are correlated with outcome in advanced ovarian cancer patients. Oncotarget. (2018) 9:34810–20. doi: 10.18632/oncotarget.26199
137. Van der Meer JMR, Maas RJA, Guldevall K, Klarenaar K, de Jonge P, Evert JSH, et al. IL-15 superagonist N-803 improves IFNgamma production and killing of leukemia and ovarian cancer cells by CD34(+) progenitor-derived NK cells. Cancer Immunol Immunother. (2021) 70:1305–21. doi: 10.1007/s00262-020-02749-8
138. Hou Y, Zhao X, and Nie X. Enhancing the therapeutic efficacy of NK cells in the treatment of ovarian cancer (Review). Oncol Rep. (2024) 51(3):50. doi: 10.3892/or.2024.8709
139. Hoogstad-van Evert JS, Bekkers R, Ottevanger N, Jansen JH, Massuger L, and Dolstra H. Harnessing natural killer cells for the treatment of ovarian cancer. Gynecol Oncol. (2020) 157:810–6. doi: 10.1016/j.ygyno.2020.03.020
140. Rezvani K. Adoptive cell therapy using engineered natural killer cells. Bone Marrow Transplant. (2019) 54:785–8. doi: 10.1038/s41409-019-0601-6
141. Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res. (2014) 2:616–31. doi: 10.1158/2326-6066.CIR-14-0027
142. Maldonado L, Teague JE, Morrow MP, Jotova I, Wu TC, Wang C, et al. Intramuscular therapeutic vaccination targeting HPV16 induces T cell responses that localize in mucosal lesions. Sci Transl Med. (2014) 6:221ra13. doi: 10.1126/scitranslmed.3007323
143. Houel A, Foloppe J, and Dieu-Nosjean MC. Harnessing the power of oncolytic virotherapy and tertiary lymphoid structures to amplify antitumor immune responses in cancer patients. Semin Immunol. (2023) 69:101796. doi: 10.1016/j.smim.2023.101796
144. Aoyama S, Nakagawa R, Mule JJ, and Mailloux AW. Inducible tertiary lymphoid structures: promise and challenges for translating a new class of immunotherapy. Front Immunol. (2021) 12:675538. doi: 10.3389/fimmu.2021.675538
145. He T, Hao Z, Lin M, Xin Z, Chen Y, Ouyang W, et al. Oncolytic adenovirus promotes vascular normalization and nonclassical tertiary lymphoid structure formation through STING-mediated DC activation. Oncoimmunology. (2022) 11:2093054. doi: 10.1080/2162402X.2022.2093054
146. Yin S, Zhang C, and Gao F. Immature tertiary lymphoid structure formation was increased in the melanoma tumor microenvironment of IKZF1 transgenic mice. Transl Cancer Res. (2022) 11:2388–97. doi: 10.21037/tcr-22-1759
147. Feng W, Jiang D, Xu Y, Li Y, Chen L, Zhao M, et al. CDK4/6i enhances the antitumor effect of PD1 antibody by promoting TLS formation in ovarian cancer. Heliyon. (2023) 9:e19760. doi: 10.1016/j.heliyon.2023.e19760
148. Ozmadenci D, Shankara Narayanan JS, Andrew J, Ojalill M, Barrie AM, Jiang S, et al. Tumor FAK orchestrates immunosuppression in ovarian cancer via the CD155/TIGIT axis. Proc Natl Acad Sci U S A. (2022) 119:e2117065119. doi: 10.1073/pnas.2117065119
149. Lynch SV and Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. (2016) 375:2369–79. doi: 10.1056/NEJMra1600266
150. Zheng D, Liwinski T, and Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. (2020) 30:492–506. doi: 10.1038/s41422-020-0332-7
151. Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. (2018) 359:97–103. doi: 10.1126/science.aan4236
152. Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. (2018) 359:91–7. doi: 10.1126/science.aan3706
153. Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. (2015) 350:1084–9. doi: 10.1126/science.aac4255
154. Mas-Lloret J, Obon-Santacana M, Ibanez-Sanz G, Guino E, Pato ML, Rodriguez-Moranta F, et al. Gut microbiome diversity detected by high-coverage 16S and shotgun sequencing of paired stool and colon sample. Sci Data. (2020) 7:92. doi: 10.1038/s41597-020-0427-5
155. Overacre-Delgoffe AE, Bumgarner HJ, Cillo AR, Burr AHP, Tometich JT, Bhattacharjee A, et al. Microbiota-specific T follicular helper cells drive tertiary lymphoid structures and anti-tumor immunity against colorectal cancer. Immunity. (2021) 54:2812–24.e4. doi: 10.1016/j.immuni.2021.11.003
156. Zhao R, Li J, Chen B, Zhao J, Hu L, Huang K, et al. The enrichment of the gut microbiota Lachnoclostridium is associated with the presence of intratumoral tertiary lymphoid structures in hepatocellular carcinoma. Front Immunol. (2023) 14:1289753. doi: 10.3389/fimmu.2023.1289753
157. Jin Y, Dong H, Xia L, Yang Y, Zhu Y, Shen Y, et al. The diversity of gut microbiome is associated with favorable responses to anti-programmed death 1 immunotherapy in chinese patients with NSCLC. J Thorac Oncol. (2019) 14:1378–89. doi: 10.1016/j.jtho.2019.04.007
158. Mao J, Wang D, Long J, Yang X, Lin J, Song Y, et al. Gut microbiome is associated with the clinical response to anti-PD-1 based immunotherapy in hepatobiliary cancers. J Immunother Cancer. (2021) 9(12):e003334. doi: 10.1136/jitc-2021-003334
159. Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. (2018) 359:104–8. doi: 10.1126/science.aao3290
160. Newsome RC, Gharaibeh RZ, Pierce CM, da Silva WV, Paul S, Hogue SR, et al. Interaction of bacterial genera associated with therapeutic response to immune checkpoint PD-1 blockade in a United States cohort. Genome Med. (2022) 14:35. doi: 10.1186/s13073-022-01037-7
161. Peng Z, Cheng S, Kou Y, Wang Z, Jin R, Hu H, et al. The gut microbiome is associated with clinical response to anti-PD-1/PD-L1 immunotherapy in gastrointestinal cancer. Cancer Immunol Res. (2020) 8:1251–61. doi: 10.1158/2326-6066.CIR-19-1014
162. Peters BA, Wilson M, Moran U, Pavlick A, Izsak A, Wechter T, et al. Relating the gut metagenome and metatranscriptome to immunotherapy responses in melanoma patients. Genome Med. (2019) 11:61. doi: 10.1186/s13073-019-0672-4
163. Zheng Y, Wang T, Tu X, Huang Y, Zhang H, Tan D, et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer. (2019) 7:193. doi: 10.1186/s40425-019-0650-9
164. Dizman N, Meza L, Bergerot P, Alcantara M, Dorff T, Lyou Y, et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: a randomized phase 1 trial. Nat Med. (2022) 28:704–12. doi: 10.1038/s41591-022-01694-6
165. McKenzie ND, Ahmad S, Awada A, Kuhn TM, Recio FO, and Holloway RW. Prognostic features of the tumor microenvironment in high-grade serous ovarian cancer and dietary immunomodulation. Life Sci. (2023) 333:122178. doi: 10.1016/j.lfs.2023.122178
166. Kang YB and Cai Y. Faecal microbiota transplantation enhances efficacy of immune checkpoint inhibitors therapy against cancer. World J Gastroenterol. (2021) 27:5362–75. doi: 10.3748/wjg.v27.i32.5362
167. Li S, Chen K, Sun Z, Chen M, Pi W, Zhou S, et al. Radiation drives tertiary lymphoid structures to reshape TME for synergized antitumour immunity. Expert Rev Mol Med. (2024) 26:e30. doi: 10.1017/erm.2024.27
168. Lanickova T, Hensler M, Kasikova L, Vosahlikova S, Angelidou A, Pasulka J, et al. Chemotherapy drives tertiary lymphoid structures that correlate with ICI-responsive TCF1+CD8+ T cells in metastatic ovarian cancer. Clin Cancer Res. (2024) 31(1):164–80. doi: 10.1158/1078-0432.CCR-24-1594
169. Garaud S, Zayakin P, Buisseret L, Rulle U, Silina K, de Wind A, et al. Antigen specificity and clinical significance of igG and igA autoantibodies produced in situ by tumor-infiltrating B cells in breast cancer. Front Immunol. (2018) 9:2660. doi: 10.3389/fimmu.2018.02660
170. Ryan ST, Zhang J, Burner DN, Liss M, Pittman E, Muldong M, et al. Neoadjuvant rituximab modulates the tumor immune environment in patients with high risk prostate cancer. J Transl Med. (2020) 18:214. doi: 10.1186/s12967-020-02370-4
171. Cottrell TR and Taube JM. PD-L1 and emerging biomarkers in immune checkpoint blockade therapy. Cancer J. (2018) 24:41–6. doi: 10.1097/PPO.0000000000000301
172. Kroeger DR, Milne K, and Nelson BH. Tumor-infiltrating plasma cells are associated with tertiary lymphoid structures, cytolytic T-cell responses, and superior prognosis in ovarian cancer. Clin Cancer Res. (2016) 22:3005–15. doi: 10.1158/1078-0432.CCR-15-2762
173. Zhang L, Strange M, Elishaev E, Zaidi S, Modugno F, Radolec M, et al. Characterization of latently infected EBV+ antibody-secreting B cells isolated from ovarian tumors and Malignant ascites. Front Immunol. (2024) 15:1379175. doi: 10.3389/fimmu.2024.1379175
Keywords: tertiary lymphoid structures, ovarian cancer, immunotherapy, tumor microenvironment, gut microbiome, biomarkers
Citation: Varghese A, Hess SM, Chilakapati S, Conejo-Garcia JR, McGray AJR and Zsiros E (2025) Tertiary lymphoid structures: exploring opportunities to improve immunotherapy in ovarian cancer. Front. Immunol. 16:1473969. doi: 10.3389/fimmu.2025.1473969
Received: 31 July 2024; Accepted: 24 April 2025;
Published: 22 May 2025.
Edited by:
Kelsey P. Kubelick, University of Virginia, United StatesReviewed by:
Jichang Han, Washington University in St. Louis, United StatesJinhwan Kim, University of California, Davis, United States
Copyright © 2025 Varghese, Hess, Chilakapati, Conejo-Garcia, McGray and Zsiros. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emese Zsiros, ZW1lc2UuenNpcm9zQHJvc3dlbGxwYXJrLm9yZw==
†These authors share first authorship
 Aaron Varghese1,2†
Aaron Varghese1,2† Suzanne M. Hess
Suzanne M. Hess Shanmuga Chilakapati
Shanmuga Chilakapati A.J. Robert McGray
A.J. Robert McGray Emese Zsiros
Emese Zsiros