- 1Mediclinic Airport Road Hospital, Abu Dhabi, United Arab Emirates
- 2Molecular biology and Hematology, Abu Dhabi University, Abu Dhabi, United Arab Emirates
- 3College of Technological Innovation at Zayed University, Abu Dhabi, United Arab Emirates
- 4NMC Royal Hospital, Abu Dhabi, United Arab Emirates
- 5Department of Internal Medicine, Medical Research and Clinical Studies Institute, The National Research Center, Cairo, Egypt
- 6School of Health, Universidad Espíritu Santo-Ecuador, Samborondón, Guayas, Ecuador
- 7Respiralab Research Group, Guayaquil, Guayas, Ecuador
- 8Department of Clinical Biochemistry, Sher-i-Kashmir Institute of Medical Sciences (SKIMS), Srinagar, India
- 9Sheikh Khalifa Hospital, Al Fujairah, United Arab Emirates
- 10Molecular Cell Biology Unit, Division of Biochemistry, Department of Chemistry, Faculty of Science, Tanta University, Tanta, Egypt
Chemotherapeutic resistance is a major obstacle to chemotherapeutic failure. Cancer cell resistance involves several mechanisms, including epithelial-to-mesenchymal transition (EMT), signaling pathway bypass, drug efflux activation, and impairment of drug entry. P-glycoproteins (P-gp) are an efflux transporter that pumps chemotherapeutic drugs out of cancer cells, resulting in chemotherapeutic resistance. Several types of long noncoding RNA (lncRNAs) have been identified in resistant cancer cells, including ODRUL, MALAT1, and ANRIL. The high expression level of ODRUL is related to the induction of ATP-binding cassette (ABC) gene expression, resulting in the emergence of doxorubicin resistance in osteosarcoma. lncRNAs are observed to be regulators of drug transporters in cancer cells such as MALAT1 and ANRIL. Targeting P-gp expression using natural products is a new strategy to overcome cancer cell resistance and improve the sensitivity of resistant cells toward chemotherapies. This review validates the inhibitory effects of natural products on P-gp expression and activity using in silico molecular docking. In silico analysis showed that Delphinidin and Asparagoside-f are the most significant natural product inhibitors of p-glycoprotein-1. These inhibitors can reverse multi-drug resistance and induce the sensitivity of resistant cancer cells toward chemotherapy based on in silico molecular docking. It is important to validate that pre-elementary docking can be confirmed using in vitro and in vivo experimental data.
1 Introduction
Although there are many significant cancer treatments, many issues reduce the sensitivity and responsiveness toward chemotherapeutic drugs. There are many mechanisms for controlling cancer cell resistance and emergence of multidrug resistance, including overexpression of ABC transporters and p-glycoproteins (1). High expression levels of ABC transporters require ATP to efflux chemotherapeutic drugs from cells (2). P-glycoprotein (P-gp) is an efflux protein that is the main cause of Multidrug resistance (MDR) (3, 4). Juliano et coworkers observed P-gp expression on the surface of ovarian cells (5). ABCB1 encodes P‐gp, which consists of four domains: two nucleotide-binding domains and two transmembrane domains (6). P-gp is expressed at high levels on the surfaces of several cancers, including lung cancer, breast cancer, colon cancer, osteosarcoma, and hepatocellular carcinoma (7–9;MA et al., 2019;10, 11). P-gp transporters efflux chemotherapeutic agents from the cell, resulting in cancer cell resistance (12, 13). The chemotherapeutic substrates for P-gp transporters include paclitaxel, 5−fluorouracil, doxorubicin, and 5−fluorouracil (14, 15). Several studies have shown that lncRNAs are strongly associated with the emergence of multidrug resistance in several cancer cell types (16). Although lncRNA transcripts are > 200 nucleotides long, no protein-coding potential has been identified (17). Disturbances in lncRNA levels lead to chemoresistance in cancer cells (18, 19). lncRNAs regulate drug transporters in cancer cells, such as MALAT1 and ANRIL which control the expression of Multidrug resistance protein 1 (MRP1) and Multidrug resistance gene 1 (MDR1) (20). Both in vivo and in vitro studies have shown that MALAT1 is associated with the development of cisplatin-resistant A549 lung cancer cells. High expression levels of ANRIL induce cisplatin-resistant and 5-fluorouracil-resistant gastric cancer cells. In this context, inhibition of P-gp transporter expression decreases multidrug resistance and increases the responsiveness of resistant cancer cells toward chemotherapeutic drugs. Natural products can modulate cancer cell resistance by inhibiting P-gp transporter expression. P-glycoprotein is one of the chemoresistance mechanisms in cancer cells that causes long-term chemotherapeutic failure. lncRNAs, such as MALAT1, ANRIL, and ODRUL, are considered inducers of p-glycoprotein expression. Targeting P-gp expression is a significant strategy to overcome chemotherapeutic resistance and increase cancer cell sensitivity towards drugs. Because natural products are extracted from natural sources, they are considered favorable P-glycoprotein inhibitors without side effects. In this review, we describe several types of natural products that can increase the sensitivity of resistant cancer cells, which was confirmed by in silico molecular docking. In addition, this review describes the mechanisms of cancer drug resistance, different types of lncRNAs, and their relationship to chemotherapeutic resistance. This review presents an important step in the strategy to increase the responsiveness of resistant cancer cells; however, future in vitro and in vivo experiments are needed to confirm our preliminary docking results.
2 Mechanisms of cancer drug resistance
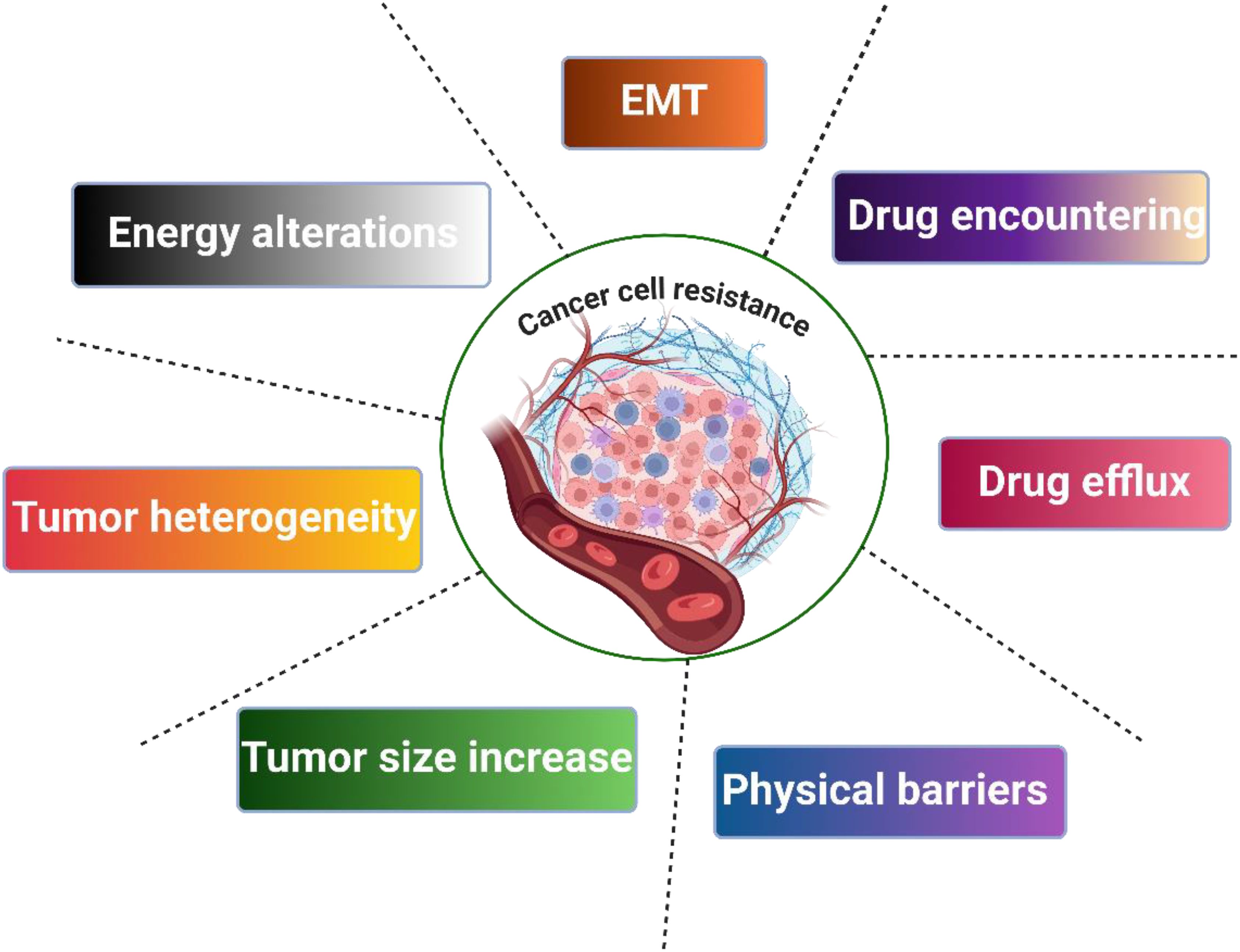
Drug resistance can be classified into two classes: primary and secondary. Primary resistance appears before the exposure of cancer cells to chemotherapy. Secondary resistance arises from adaptation of cancer cells to chemotherapy. Drug resistance commonly results from genomic alteration. There are different resistance mechanisms, including p-efflux transporters, inhibition of drug entry, and EMT, as shown in Figure 1 (21–24).
2.1 Tumor heterogeneity
Tumor heterogeneity is a significant factor underlying resistance to cancer drugs and is considered a fundamental feature of tumor progression and adaptation to different conditions. The evolutionary power of tumors is mainly linked to heterogeneity; colonies with more robust heterogeneity are favored during cancer progression and become dominant (25). Tumor heterogeneity can be attributed to both intrinsic and extrinsic factors. Generally, intrinsic factors are cellular in origin and can accumulate due to alterations in the levels of DNA, RNA, protein, epigenetics, and signal transduction. Genetic alterations include mutations, gene amplification, chromosomal aberrations, and miRNA gene changes. Alterations in transcriptomics, proteomics, and epigenetics could also originate from DNA alterations, which could modulate the cell cycle and its overall regulation (26). Additionally, modification of cancer stem cells (CSCs) supports heterogeneity, tumor plasticity, and resistance (27). Downregulation of pro-apoptotic molecules and upregulation of anti-apoptotic players participate in heterogeneity-associated resistance (28). Moreover, differences in signaling contribute to heterogeneity in cancer drug resistance heterogeneity (29). Tumors could possess a high level of stochasticity due to de novo differences in enzymatic signal transduction cascades that promote biological noise and subsequently develop feedback inhibition motifs to decrease biological noise (30).
Conversely, extrinsic or microenvironmental factors can promote heterogeneity and resistance through spatial differences in cells, blood supply, pH, hypoxia, and paracrine signaling (31, 32). Additionally, the vascular network and contact with cancer cells are arbitrary, resulting in fluctuations in the nutrient and metabolic status of different cancer cells (33).
2.2 Tumor burden and physical barriers
There is a significant association between tumor size and tumor resistance, and tumor size could be a determinant of tumor capacity to develop drug resistance mutations. Tumor growth and response to therapy have an inverse relationship with the growth rate (34). However, cancers can reveal spatial gradients that limit the blood supply and oxygen enrichment, creating an isolated hypoxic pro-tumorigenic environment with low contact with chemotherapy (34).
2.3 Tumor microenvironment
Tumor microenvironment (TME) interactions with tumor cells can aid in resistance. Tumors are not fully homogeneous but include different classes of cells and extracellular matrix (ECM), such as immune and inflammatory cells, fibroblast blood vessels, multiple nutrients, and signaling molecules (35). TME could be attributed to the pharmacological outcomes that force the cells to adapt to chemotherapy. One TME factor is pH; tumor cells typically exhibit a reversed pH gradient, with intracellular pH higher than extracellular pH, which promotes resistance to chemotherapy (36). Furthermore, alkaline pH modulates ion trapping, reduces drug efficacy, and helps cancer cells avoid apoptosis, promoting cell proliferation, tumor aggressiveness, invasion, and resistance to the immune response (37). Cycles of hypoxia, reoxygenation, and lack of O2 produce reactive oxygen species (ROS) that are associated with heterogeneity and resistance (38). In addition, EMT and CSCs have carcinogenic effects by helping cancer cells avoid apoptosis (39). Additionally, resistance could be attributed to TME attenuation of the immune clearance of cancer cells, and the TME could enhance resistance by inducing paracrine growth factors to mediate cancer cell growth (40). Chemotherapy pressure forces cells to possess a robust phenotype against stress (41). In addition, external pressure can modulate the expression of anti-apoptotic markers and epithelial-to-mesenchymal transition (EMT).
2.4 Cancer stem cells
The presence of stem cells in cancer tissues is linked to the resistance of many cancers, such as a long lifetime, high expression of drug exporters, elevated DNA repair mechanisms, and attenuated apoptosis (42). Stem-cell-dependent resistance is generally dependent on EMT machinery (43).
2.5 EMT
The EMT process is distinguished by the loss of both cell-cell contacts and apico–basal polarity associated with epithelial cells, ultimately acquiring mesenchymal features (44). The EMT program is mainly initiated by TME paracrine signaling by fibroblasts, macrophages, or immunocytes (44). EMT permits cancer cells to possess the ability to resist anticancer drugs and avoid apoptosis. EMT is characterized by elevation of transforming growth factor-beta (TGF-β), which significantly aids in resistance (45). EMT-linked transcription factors, including Twist1, Snail, Slug, ZEB, and FOXC2, are associated with drug resistance in cancer (46). These transcription factors support resistance by promoting drug efflux, such as ABC transporters, in addition to avoiding apoptosis via an immune response that shares similarities with the resistance profile of stem cancer cells (47–49). The EMT also has the capacity to self-renew and escape apoptosis. EMT is the initial step in escape from neighboring tissues and subsequent metastasis (50).
2.6 Drug manipulation
Drug efflux machinery, such as ABC transporter and efflux pump P-glycoprotein (P-gp), are enhanced through EMT, stem cells, miRNAs, and as a response to pharmaceutical pressure (51). Generally, drug uptake into cells occurs through diffusion through the plasma membrane (PM), transporter activity, and endocytosis. During cancer development, alterations are accompanied by changes in the lipid composition of the PM, such as phosphatidylserine (PS). In cancer cells, PS is exposed to an extracellular environment opposite to normal PM, which gives the cell more negative charge-altering drug entry (52, 53). Additionally, the attenuated pH of the extracellular media of cancer cells affects the ionization status of drugs and their entry (54). Drug entry is also dependent on several transporters called carriers (SLC), such as OATP1B3 and OCT6, which were found to be attenuated during treatment with doxorubicin and cisplatin (55). Moreover, elevated rigidity of endosomes affects the endocytosis process, thereby affecting drug entry (56, 57). In addition, alterations in drug targets, such as protein mutations or expression aberrations, suppress the robustness of targeted therapy. For instance, a missense variant in the epidermal growth factor receptor (EGFR) subsequently impairs the binding of gefitinib/erlotinib to the kinase (58).
2.7 Epigenetic alterations
Epigenetic modifications include methylation, histone modifications, and non-coding RNAs disturbances (59). Oncogene promoters can be demethylated and subsequently gene expression is elevated, as observed in several genes, including ID4, ERp29/MGMT, ETS-1,
and miR-663, which are involved in breast cancer resistance against several chemotherapeutics (60, 61). Similarly, the MDR1 and PD-L1/DNMT1 axes are hypomethylated in HCC cells treated with Doxorubicin and sorafenib, respectively (62). However, some gene promoters are hypermethylated, causing attenuation of gene expression and subsequent resistance, such as TGBI and ER-α, in breast cancer when Trastuzumab and Antiestrogen are administered, respectively (63, 64). Moreover, target genes and export pump functions can be enhanced by histone demethylation and acetylation (65). Furthermore, miRNA alterations that affect gene expression are involved in drug cancer resistance. For instance, miR-15b promotes resistance to cisplatin by targeting PEBP4- and RKIP-mediated EMT, similar to miR-27a (66). In addition, lncRNAs are overexpressed and promote proteins related to cancer drug resistance (67).
2.8 DNA damage repair
The DNA damage repair (DDR) machinery is controlled by several genes that are enhanced during cancer therapy, leading to resistance. Thus, impairment of the DDR can increase pharmaceutical sensitivity (68). O-6-Methylguanine-DNA Methyltransferase (MGMT) is responsible for the clearance of alkyl adducts from the O6 position of guanine; inactivation of this machinery was found to be a therapeutic target for sensitizing cells to O6-alkylating agents (69). DNA-dependent protein kinase (DNA-PK) is part of the double-strand break repair machinery (DSBs), and inhibition of this mechanism could promote radio/chemosensitivity of cancer cells (70).
2.9 Cell cycle
Irreversible cell arrest “senescence” could be provoked by several factors, including oncogenic genetic alterations, telomere erosion, and DNA damage linked to pharmaceutical therapy. However, the surviving cell populations may be more vigorous and highly proliferative (71). However, some cancer cells evade irreversible cell arrest and “senescence” by modifying apoptotic pathways to promote chemoresistance (72).
2.10 Energy alterations
Cancer cells develop characteristic metabolic phenotypes, especially glycolysis, known as the Warburg effect, which allows cancer cells to possess significantly higher intracellular ATP levels than normal cells of the same origin (73). Interestingly, cancer cell chemoresistance is correlated with ATP levels, and attenuation can sensitize cells (74). Increased cytosolic ATP levels are accompanied by elevated mitochondrial ATP levels in cancer- resistant cells. This phenomenon promotes drug efflux through ABC transporters, which in turn increases drug resistance (75). In contrast, the presence of extracellular ATP (eATP) in tumor cells is remarkably higher than that in normal cells, which is attributed to the elevated ATP produced from apoptosis and autophagy during therapy (76). Firstly, eATP can be transported to cells to promote resistance, as discussed earlier. Additionally, eATP signaling can promote EMT, cell growth, survival, and proliferation (77).
3 Drug efflux variations
49 of ATP-binding cassette transporters efflux chemical drugs from cancer cells, resulting in multidrug resistance (MDR). P-glycoprotein (P-gp), multi-drug-resistant associate protein (MRP), and adenosine triphosphate-binding cassette superfamily G member 2 (ABCG2) are the most common efflux transporters in ovarian and breast cancers (78). It has been observed that high expression levels of P-gp transporters in colorectal cancer and neuroblastoma lead to poor prognosis (79). P-gp transporters are encoded by the gene (MDR1) during the transformation of normal tissues into neoplastic tissues (80). Downstream receptors and proteins GTPase H-Ras, Mitogen-activated protein kinase 1/2 (MEK1/2), and Raf- 1 are involved in the mitogen-activated protein kinase (MAPK) pathway associated with high P-gp expression levels. On the other hand, Katayama, Imai, and their co-workers observed that inhibition of the extracellular signal-regulated kinase (ERK) pathway downregulates the expression level of P-gp (81, 82).
4 Effect of P-glycoprotein in tumor immunity
The high expression level of P-gp in immune cells induces their activation, modulation of their activity, and the release of cytokines. In the peripheral circulating system, the number of monocytes is very low, on the other hand, it increases in tissue tumor-infiltrating macrophages (83). The expression of P-gp in dendritic cells depends on its activation with a professional antigen (84, 85). Lloberas and his co-workers observed that using Valspodar to block P-gp prevented the maturation of dendritic cells and their activation markers CD80 and CD40 (85). Natural killer (NK (cells have a high P-gp expression level. There is a strong relationship between P-gp expression and the cytotoxic effects of NK. The high expression level of P-gp downregulates the cytotoxicity of NK cells by increasing the binding of Fas-mediated (Fas/FasL) P-gp+ NK cells to target cells. This triggers apoptosis of target cells by inducing the release of secretory granules with an inflammatory cytotoxic effect (86). In adaptive immunity, individual cell types determine the role of p-g expression. For example, in lymph nodes, the migration and transitional phenotype of B cells depends on the expression level of P-gp (87, 88). In CD4+T cells, Th1 and Th17 are effectors of T cells, and their inflammatory effect is associated with the expression level of P-gp. In contrast, the anti-inflammatory effect of T regulatory cells (Treg) limits the expression level of P-gp (89, 90). Kooij and his co-workers declared that memory (IL18Rα+CD161+CD62Llo) phenotype in CD8+T cells determines the expression level of P-gp (91). Bidirectional responses of P-gp expression were observed in CD8+ memory T cells in mucosal cells. In mucosal cells, P-gp normally effluxes xenobiotic toxins out of the cell; however, if a normal microbiome is distributed, this leads to enhanced effector responses and causes the emergence of autoimmune diseases such as Crohn’s disease. In acute myeloid leukemia, immune cells, including follicular lymphoma and B-cell lymphoma, boost P-gp expression levels, leading to chemotherapeutic resistance (92, 93). High MAP kinase/ERK signaling is associated with the induction of P-gp expression, which has a significant role in resistant myeloid leukemia (94). Ling et co-workers observed high P-gp expression levels in CD8+T cells derived from human colorectal cancer (95). In addition, chemoresistance in AML patients results from long-term chemotherapy and is correlated with the expression of CD4+CD161+P-gp+ T cells (96). On the other hand, Th17 and Th1 CD4+T-helper cells have been observed to trigger cytokine secretion, such as TNFα, and IL-17 which have anti-cancer activity (97). In this context, breast cancer cells have CD4+T-cells (CD4+CD73+T cells) that express P-gp and enhance the secretion of inflammatory and anticancer cytokines (98, 99). There is a conflicting role for P-gp in cancer cells, which is expressed in the pro-tumor effect of MΦ2-macrophage and the anti-cancer effect of NK-cell and Th17/CD4+T cells. Therefore, it is important to study the role of P-gp in immune cells.
5 lncRNAs related to p-glycoprotein
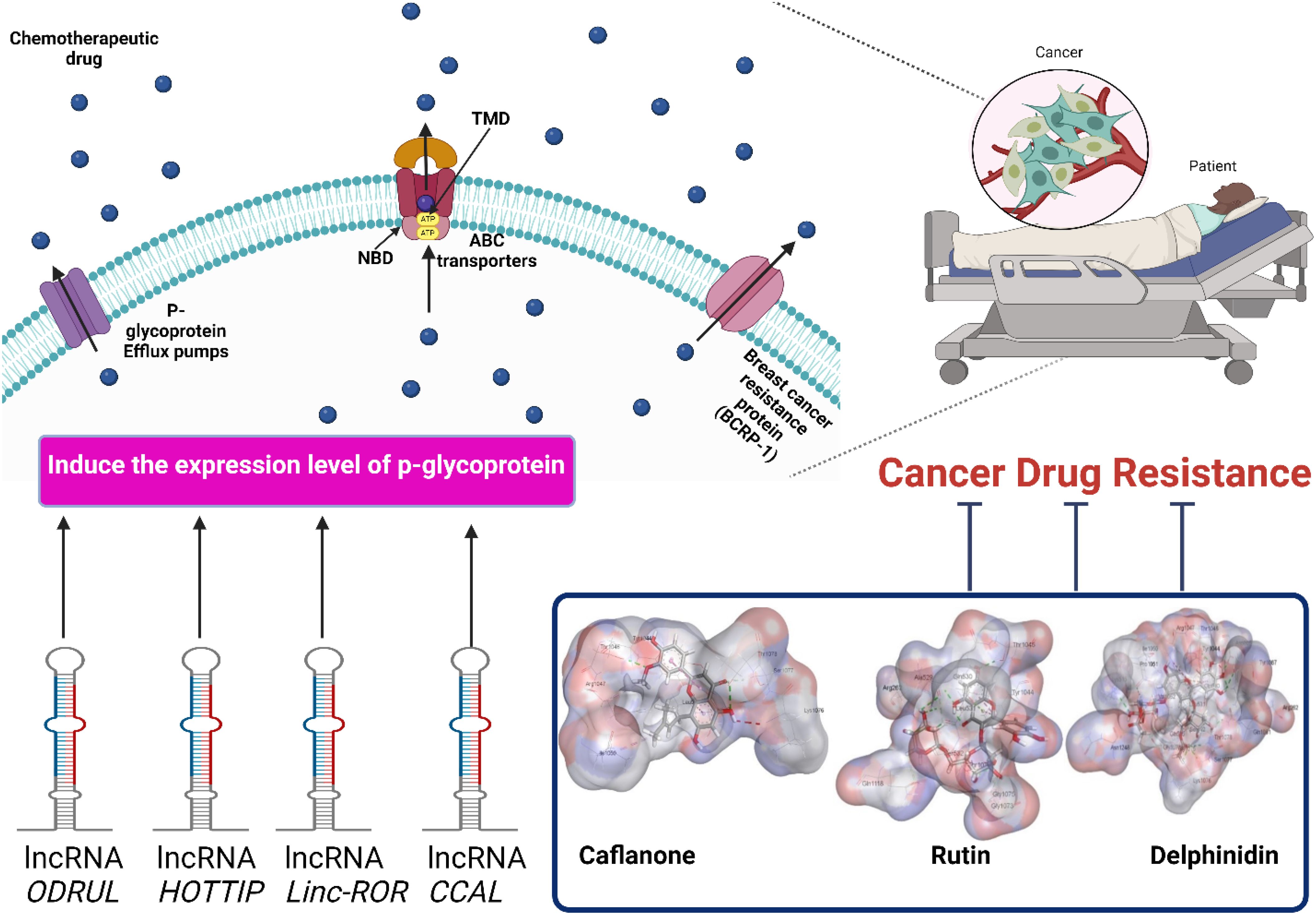
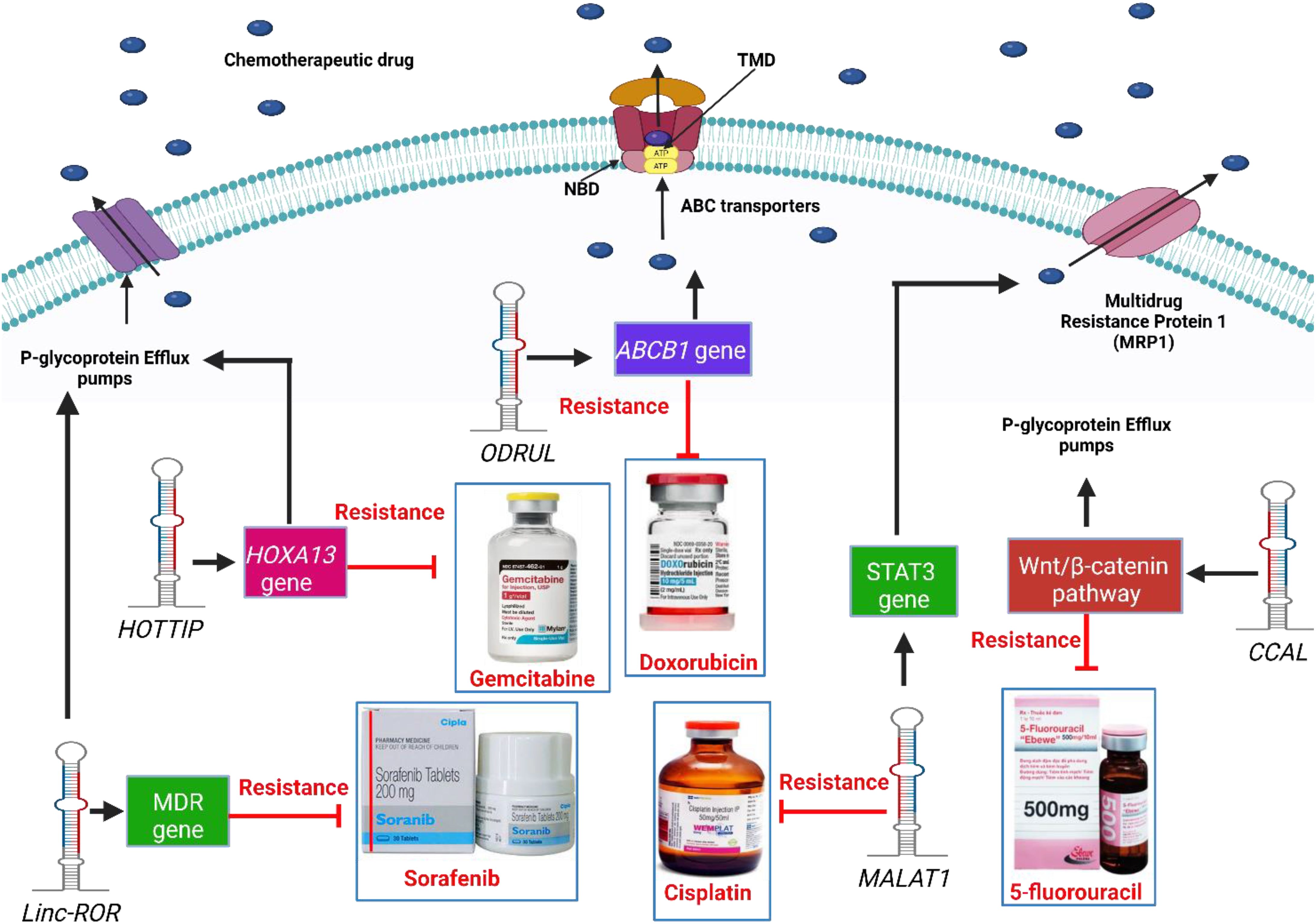
Several studies have shown that lncRNAs play a significant role in increasing the expression levels of P-gp transporters and inducing multidrug, as shown in Figure 2. ODRUL was observed to be highly expressed in osteosarcoma cell lines. The high expression level of ODRUL is related to the induction of ABCB1 gene and results in the emergence of doxorubicin resistance in osteosarcoma (100). Knockdown of the expression level of ODRUL leads to a decrease in the expression level of ABCB1 gene and improves the responsiveness of osteosarcoma toward doxorubicin (100). In addition, high expression levels of lncRNA HOTTIP have been observed in resistant pancreatic ductal adenocarcinoma (PDAC) (101). HOTTIP was associated with gemcitabine-resistant PDAC cells. In vitro and in vivo studies showed that HOTTIP induces proliferation, invasion, and gemcitabine resistance in cancer cells by modulating HOXA13 gene (101). HOTTIP knockdown improves the sensitivity of cancer cells toward gemcitabine (101). It was also observed that H19 mRNA in resistant HepG2 cells induces the expression of p-glycoprotein transporters. High levels of H19 mRNA induce doxorubicin resistance in HepG2 cells (102). Knockdown of H19 mRNA induces the responsiveness and sensitivity of resistant cancer cells toward doxorubicin by increasing its accumulation and toxicity in both resistant and normal hepatoma cancer cells (102). Furthermore, knockdown of H19 mRNA induces methylation of MDR1 and then decreases P-glycoprotein expression (102). Linc-ROR lncRNA is upregulated in sorafenib-resistant HCC cells towards sorafenib. Knockdown of linc-ROR induces sorafenib toxicity and cancer cell death (103). High expression levels of CCAL lncRNAs induce multidrug resistance in colorectal cancer cells (104). CCAL lncRNA triggers a decrease in the signaling AP-2α protein-activated Wnt/β-catenin pathway, leading to upregulation of p-glycoprotein expression (104). On the other hand, low expression levels of snaR induce chemotherapeutic resistance in colon cancer toward 5-fluorouracil (105). High snaR expression induces apoptosis in colon cancer cells (105). High expression levels of HOTAIR induce platinum resistance in ovarian cancer cells. A high level of HOTAIR repairs the DNA damaged by platinum therapy, which activates NF-κB signaling, resulting in chemoresistance (106). Fang et co-workers observed that a high expression level of MALAT1 is observed in cisplatin-resistant lung cancer. MALAT1 induces cisplatin efflux from cells through efflux transporters (MDR1 and MRP1) after STAT3 activation (20). Furthermore, ANRIL was observed to induce the expression of efflux transporter proteins in resistant gastric cancer cells. Efflux transporter proteins pump cisplatin and 5-fluorouracil (5-FU) from cells, resulting in chemoresistance in gastric cancer cells (107). ANRIL knockdown increased the responsiveness of resistant cancer cells toward chemotherapeutic drugs by decreasing the levels of transporter proteins (107). MRUL increases the expression of ABCB1, which induces multidrug resistance. ABCB1 gene is associated with efflux transporter proteins that pump doxorubicin out of cancer cells (108). ABCB1 knockdown reduces drug efflux, leading to drug accumulation, toxicity, and apoptosis (108). The high expression levels of linc-VLDLR is implicated in the expression of the ABCG2. Chemotherapeutic drugs, including doxorubicin, sorafenib, and camptothecin, induce the expression of linc-VLDLR in both inside cells and in extracellular vesicles (EVs). Knockdown of linc-VLDLR reduces the level of ABCG2 drug efflux transporters and decreases cancer cell proliferation (109). lncRNA XIST controls the expression of Serum- and Glucocorticoid-Regulated Kinase 1 (SGK1), which sponges miR-124, resulting in doxorubicin resistance in colorectal cancer cells (110). Knockdown of XIST decreases the expression of p-glycoproteins and improved the responsiveness of resistant cancer cells toward DOX (110). Hu et co-workers observed that high expression levels of KCNQ1OT1 are associated with oxaliplatin resistance in hepatoma cancer cells via the upregulation of efflux transporter genes, including MRP5, MDR1, and LRP1 (111). KCNQ1OT1 knockdown decreases gene-related resistance and cancer cell growth, invasion, and migration. A KCNQ1OT1 sponge with the 3′-UTR of miR-7-5p regulates the expression level of ABCC1 mRNA in hepatoma cancer cells (111). LINC00518 induces chemotherapeutic resistance in breast cancer cells by sponging miR- 199a (112). High levels of miR- 199a induce the expression of chemoresistant MRP1, resulting in paclitaxel, vincristine, and doxorubicin resistance in breast cancer. Knockdown of LINC00518 expression level induces breast cancer cell sensitivity toward chemotherapy (112). Bladder cancer-associated transcript-1 (BLACAT1) is observed in resistant gastric cancer cells (113). A high expression level of BLACAT1 is associated with oxaliplatin resistance in gastric cancer cells. In vitro and in vivo studies observed that knockdown of BLACAT1 downregulates the expression of ABCB1 protein and inhibits the proliferation of gastric cancer cells. miR-361 interacts with the 3′-UTR of BLACAT1 and ABCB1mRNA resulting in chemoresistance (113). In conclusion, targeting p-glycoprotein and chemoresistance associated with lncRNAs is a new strategy for improving the responsiveness of resistant cancer cells toward chemotherapy.
6 Phytochemicals target both lncRNAs and p-glycoprotein to overcome chemoresistance
Several studies have reported that different types of natural products act as P-gp inhibitors (114). Natural products decrease chemoresistance by targeting p-glycoprotein expression (Table 1) (114). Natural products are characterized by having groups of methoxy, allyloxy, or acetylamino substituents, chiral configuration at C-3, and chromanol scaffolds, which can modulate the activity of p-glycoproteins (159). Baicalin and baicalein are natural products derived from the root of Scutellaria baicalensis Georgi that can downregulate P-gp expression in Caco-2 cells. Baicalin and baicalein showed significant anti-cancer activity, with IC50 = 479-332 μg/mL against Caco-2 cells. Miao et coworkers reported that in vitro studies of baicalein showed that baicalein has a greater inhibitory effect against P-gp than baicalin due to the presence of a glucosyl group (116). Chalcone is a natural phenolic compound that is extracted from apples, tomatoes, and licorice. In vitro studies have shown that chalcone inhibits p-glycoprotein activity, resulting in improved sensitivity of cancer cells toward chemotherapy (160). Cyanidin is a flavonoid extracted from leaves, vegetables, and fruits such as grapes, cherries, apples, beans, and cabbage. Cyanidin is cytotoxic to cancer cells by inducing apoptosis (161). Kitagawa observed that cyanidin decreased the expression levels of P-gp proteins and decreased chemoresistance (162). Quercetin is a flavonoid extracted from onion skin that has antioxidant activity. Quercetin inhibits chemotherapy transport by suppressing ATPase activity of ABCB1 (163). Rutin is a flavonoid extracted from the papaya plant. In vitro studies have shown that rutin inhibits P-gp activity and improves the responsiveness of cancer cells toward paclitaxel (123). Curcumin is a natural polyphenolic compound extracted from Curcuma longa. Curcumin inhibits the activity of P-glycoprotein regardless of the substrate formulation in LS180 Cells (164). Cinnamyl acetate is a natural phenylpropanoid compound extracted from the cinnamon bark. Cinnamyl acetate inhibits the expression of P-gp transporters and decreases chemoresistance in cancer cells (165). Hesperidin is a natural flavonoid extracted from citrus fruit. Kong et co-workers observed that hesperidin suppressed P-gp expression and induced the accumulation of chemotherapy in A549 cancer cells (166). Ursolic acid (UA) is a natural triterpene extracted from Annurca apples. Ursolic acid inhibits cancer cell proliferation by inducing apoptosis and upregulating caspase levels in resistant hepatoma cancer cells (167). Kaempferol is a flavonoid extracted from tea, curly kale, and blueberries. Kaempferol inhibits P-gp activity and decreases multi-drug resistance in KB-V1 cells (130). Luteolin is a natural product produced by Helicteres hirsute. Luteolin triggers cell death in cancer cells expressing efflux transporter proteins (ABCG2 and P-gp) by inducing ROS generation and DNA damage via inhibition of the NF-kB signaling pathway and downregulation of anti-apoptotic markers (134a). Sarsasapogenin is a steroid compound extracted from Anemarrhena asphodeloides Bunge. Sarsasapogenin suppresses the inflammatory activity that results from lipopolysaccharide (168). Fisetin is a flavone that is extracted from strawberries and apples. Kaempferol significantly inhibits P-gp expression more than fisetin, resulting in the efficient accumulation of daunorubicin (169). Glycyrrhizin is a natural product that is extracted from liquid plants. Glycyrrhizin inhibits P-gp activity and interacts with its substrate to inhibit P-gp transport (170). Noscapine is an alkaloid extracted from Papaver somniferum. Noscapine has been observed to have an inhibitory effect on P-gp, resulting in the suppression of multi-drug resistance in cancer cells (143). Allicin is a natural product that is extracted from garlic. Allicin can overcome p-glycoprotein and BCRP activity and induce the accumulation of sulfadiazine and florfenicol (171). Gingerol has also been extracted from Zingiber officinale. Gingerol inhibits P-gp activity and induces the accumulation of 3-H digoxin in Caco-2 cells (172). Gallocatechin gallate was extracted from green tea leaves. Gallocatechin gallate interacts with P-gp and decreases multidrug resistance in cancer cells (173). In conclusion, natural products can act as significant inhibitors to overcome multi-drug resistance and improve the sensitivity of cancer cells toward chemotherapy. Mondal et et al. observed that mahanimbine induced P-gp ATPase activity and decreased cancer cell resistance (174). Diindolylmethane is a dietary bioactive compound that modulates the efflux of ABC transporters and improves the efficacy of Centchroman in breast cancer cells (126). In addition, in vitro and in vivo studies have shown that betulinic acid downregulates the expression level of MALAT1 which is associated with hepatoma-resistant cells. In addition, bharangin is a natural product with a quinone- methide structure derived from Pygmacopremna herbacea (175). Studies have shown that bharangin downregulates the expression of H19 lncRNA in resistant breast cancer cells. Curcumin also reduces H19 lncRNA expression, which is associated with resistance in MCF-7 breast cancer cells (175). Curcumin affects EMT biomarkers, including N-cadherin and E-cadherin levels. It reduced the levels of N-cadherin and increased the levels of E-cadherin. In vitro studies have shown that curcumin decreases renal cancer cell migration and invasion by downregulating the expression of HOTAIR (124). Resveratrol is extracted from pistachios, plums, grapes, and berries (176). The expression level of MALAT1 is increased in resistant colon cancer cells. Resveratrol decreases colon cancer resistance by downregulating MALAT1 and mediating the Wnt/β-catenin signaling pathway. Silibinin decreases the expression of HOTAIR by modulating the PI3K pathway (177). Therefore, the inhibitory effect of the natural products was validated by in silico molecular docking analysis; however, future in vitro and in vivo experiments are needed to confirm our preliminary docking results.
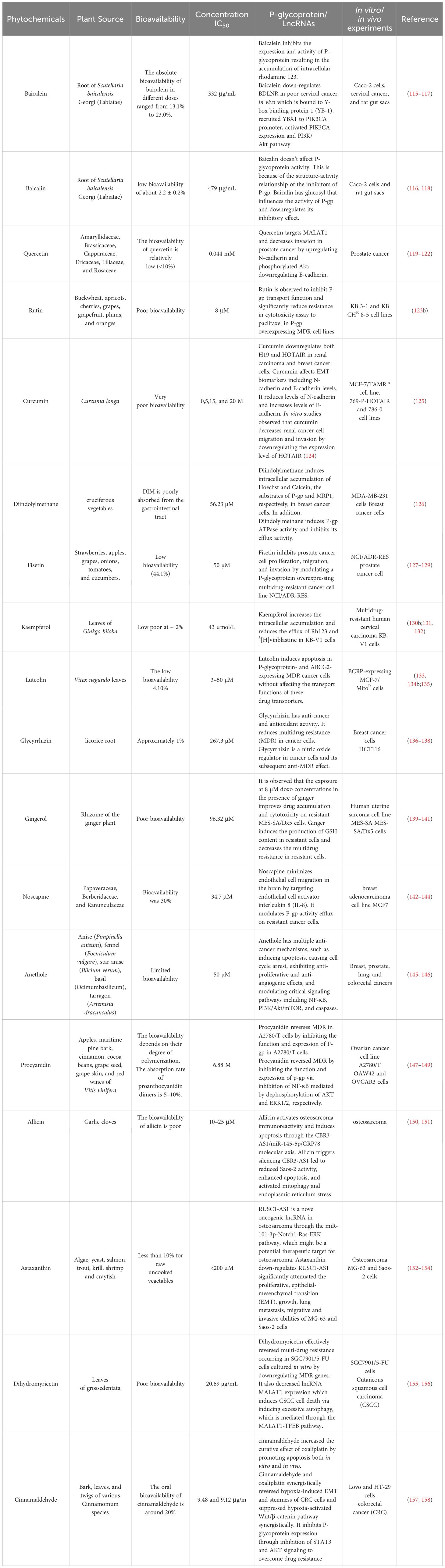
Table 1. Phytochemicals list that target P-glycoprotein and or LncRNAs to overcome chemoresistance in different cancer cells.
7 How specific lncRNAs regulate drug transporters like P-gp
The regulation of P-gp by lncRNAs typically occurs through various signaling pathways and mechanisms. Here’s a more specific look at how lncRNAs may regulate P-gp expression and function:
7.1 Transcriptional regulation
Some lncRNAs can regulate the transcription of the ABCB1 gene, which encodes P-gp, through various transcription factors. LncRNAs may act as scaffolds or guides for transcription factors or chromatin remodeling complexes to either promote or repress the expression of ABCB1 [1]. The lncRNA MALAT1 has been reported to influence the transcriptional regulation of drug resistance genes, including P-gp, in cancer cells. MALAT1 can interact with specific transcription factors and chromatin modifiers to enhance the expression of ABCB1, increasing the efflux of chemotherapeutic agents and promoting drug resistance [2].
7.2 Epigenetic regulation
lncRNAs can interact with chromatin-modifying complexes and enzymes to modify the chromatin structure and regulate the expression of P-gp through epigenetic mechanisms [3, 4]. This regulation often involves histone modifications or DNA methylation patterns at the ABCB1 gene locus. The lncRNA HOTAIR is known to regulate the expression of P-gp through epigenetic modifications. HOTAIR interacts with polycomb repressive complexes to silence genes that may inhibit the expression of P-gp, potentially increasing its activity in the context of drug resistance [4].
7.3 miRNA sponging
Many lncRNAs can act as miRNA sponges, sequestering specific microRNAs (miRNAs) that normally target the ABCB1 gene or its associated regulatory pathways. By binding to these miRNAs, lncRNAs prevent them from inhibiting the expression of P-gp [1]. The lncRNA H19 has been shown to sponge miR-675, which could normally suppress ABCB1 expression. By binding to miR-675, H19 indirectly promotes P-gp expression and drug resistance in certain cancers [5].
7.4 Interaction with signaling pathways
LncRNAs can modulate multiple signaling pathways, such as the PI3K/Akt, NF-kB, and MAPK pathways, which are known to regulate the expression of drug transporters like P-gp [6].
● PI3K/Akt Pathway: LncRNAs such as LncRNA-ATB have been shown to regulate the PI3K/Akt signaling pathway, which can enhance the expression of P-gp. This pathway is involved in cellular responses to stress and can influence drug transporter activity in cancer cells [7].
● NF-kB Pathway: Some lncRNAs, including LncRNA-MALAT1, may regulate the NF-kB signaling pathway, which is involved in inflammation and immune responses. NF-kB activation can also upregulate P-gp expression, particularly in the context of inflammation or drug resistance [8].
7.5 Post-transcriptional regulation
In addition to transcriptional regulation, lncRNAs can also affect P-gp at the post-transcriptional level. For instance, lncRNAs may modulate the stability of ABCB1 mRNA or influence its translation [1]. LncRNAs like TUG1 can regulate the stability of specific mRNAs through interactions with RNA-binding proteins, influencing the translation of P-gp and its levels in cells [1, 9].
7.6 Involvement in drug resistance mechanisms
lncRNAs are implicated in the development of drug resistance through their ability to modulate drug transporters like P-gp. For example, overexpression of certain lncRNAs can lead to increased P-gp activity, reducing the intracellular concentration of chemotherapeutic agents and thus contributing to resistance[10]. LncRNA-CCAT2 has been reported to be involved in chemoresistance by regulating the expression of P-gp. This lncRNA modulates the cellular response to chemotherapy drugs and enhances P-gp-mediated drug efflux [11].
8 Computational and preclinical studies of p-glycoprotein -1 in chemoresistance cancer cells
In this review, we validate the potential effects of natural products against p-glycoprotein-1 to understand their association with cancer cell resistance. To perform molecular docking and illustrate inhibitor reactions, we used both the Molecular Operating Environment software (MOE, 2015.10) and BIOVIA Discovery Studio Visualizer (178). We followed the steps of a previously reported procedure to illustrate the reactions of inhibitors with significant amino acids or protein hotspots (178–180). The 3D structures of the targeted proteins were obtained from the Protein Data Bank (PDB). As shown in Figure 3, docking of the human P-glycoprotein in the ATP-bound, outward-facing conformation was performed using PDB 6C0V for p-glycoprotein -1 inhibitors. The exact binding site of bioactive compounds is the active site at which the co-crystallized ligand binds. All structure minimizations were conducted until an RMSD gradient of 0.05 kcal··mol−1Å−1 with MMFF94x force field, and partial charges were automatically calculated. Furthermore, all water molecules were removed from the compounds and p-glycoprotein-1 was prepared for docking using the Protonate 3D protocol in MOE with default parameters. To calculate both docking and scoring, we employed the triangle Matcher placement method and London dG scoring function. First, self-docking of the cocrystallized ligand near the protein- binding site was performed to ensure the docking protocol steps. Subsequently, ligand-receptor interactions at the target protein-binding site for the reported natural products in the active site were studied using a validated docking protocol (RMSD < 2) to predict their binding approach and binding affinity. The inhibitory activity of the tested substances was compared with that of the most potent p-glycoprotein inhibitor (mifepristone) through computational analysis. The plausible modes of binding between these substances and their target binding sites were determined. Delphinidin 3,5-di(6-acetylglucoside) (docking score: S = -10.0549 kcal/mol) was found to have the most significant inhibitory activity in the p-glycoprotein-1 inhibitor group (Table 2), with a higher potency than that of the control (mifepristone) (S = -5.1600 kcal/mol). Delphinidin 3,5-di (6-acetyl glucoside) interacts with the p-glycoprotein-1 active site via hydrogen bonds with the LEU 531 H-donor, GLN535 H-donor, ASP 805 H-donor, ASP 805 H-donor, SER 1077 H-acceptor, and TYR 1044 pi-pi. In addition, asparagoside-f (docking score: S = -9.0916 kcal/mol) was found to have the 2nd highest inhibitory activity within the group, as shown in Table 2. Furthermore, Quercetin, caflanone, rutin, curcumin, kaempferol, and kazinol-f had more potent inhibitory effects than the control (mifepristone) (S = -5.1600 kcal/mol). Based on docking simulations, it can be concluded that these inhibitors can effectively inhibit p-glycoprotein-1 and are therefore considered potent drugs to treat chemoresistance or increase the responsiveness of cancer toward chemotherapy. Our molecular docking results represent the first step toward overcoming chemoresistance. In this context, future in vitro and in vivo, future experiments are required to confirm our results.
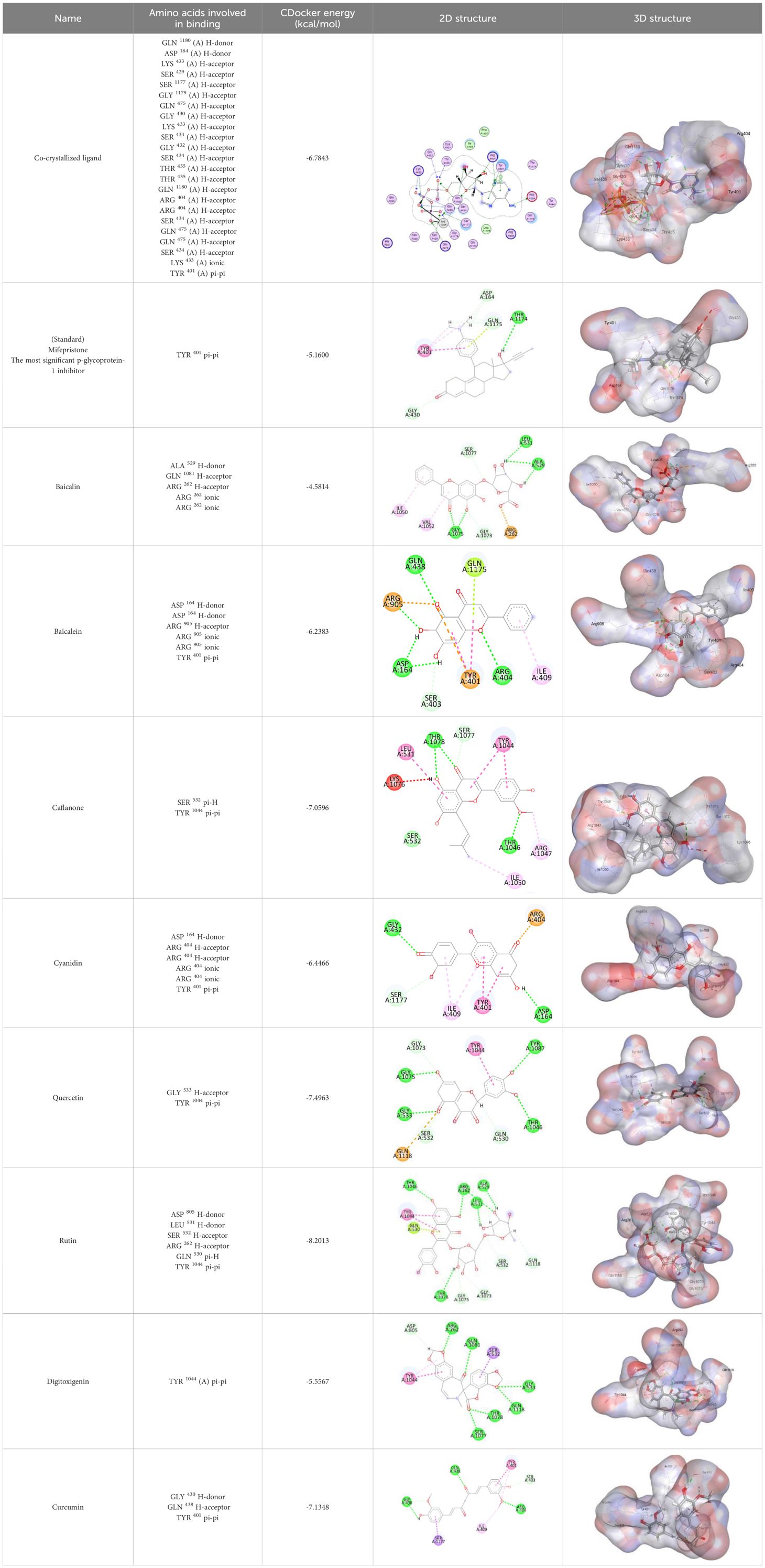
Table 2. Docking energy scores and amino acids involved in binding for Mifepristone, and the reported natural product inhibitors docked with the Molecular structure of human P-glycoprotein in the ATP-bound, outward-facing conformation (PDB: 6C0V).
9 Conclusion
Improving cancer cell responsiveness is a significant step toward enhancing the efficiency of chemotherapeutic drugs. Resistance to chemotherapy is one of the main causes of chemotherapeutic failure. P-gp is a membrane transporter that causes efflux of drugs from cancer cells and results in drug resistance. Several types of lncRNAs have been identified in resistant cancer cells, including ODRUL, MALAT1, and ANRIL. This review discusses the use of natural products as natural inhibitors of P-gp expression. In silico analysis showed that Delphinidin and Asparagoside-f are the most significant natural product inhibitors of p-glycoprotein-1 to overcome resistance. Our findings could open new hope in minimizing the immorality of chemoresistance and improving the outcome of several types of cancers.
Author contributions
MD: Conceptualization, Investigation, Resources, Supervision, Writing – original draft, Data curation. AH: Conceptualization, Supervision, Writing – original draft, Formal analysis, Funding acquisition, Writing – review & editing. FA-O: Writing – original draft, Writing – review & editing, Investigation, Software, Data curation, Methodology, Validation. WH: Investigation, Software, Writing – original draft, Writing – review & editing, Conceptualization, Formal analysis, Project administration, Resources, Supervision. IC-O: Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing. MG: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. GR: Investigation, Supervision, Validation, Writing – original draft, Writing – review & editing. SFE: Conceptualization, Formal analysis, Resources, Validation, Writing – review & editing. MAI: Conceptualization, Formal analysis, Resources, Validation, Writing – review & editing. TFI: Conceptualization, Formal analysis, Resources, Validation, Writing – review & editing. SSA: Conceptualization, Formal analysis, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
EMT: Epithelial-to-mesenchymal transition
lncRNA: Long non-coding RNA
P-gp: P-glycoproteins
ABC: ATP-binding cassette
MRP1: Multidrug resistance protein 1
MDR1: Multidrug resistance gene 1
CSCs: Cancer stem cells
ECM: Extracellular matrix
ROS: Reactive Oxygen Species
TGF-β: Transforming growth factor-beta
PM: Plasma membrane
PS: Phosphatidylserine
SLC: Several transporters named carriers
EGFR: Epidermal growth factor receptor
OATP1B3: Solute carrier organic anion transporter family member 1B3
OCT6: Organic cation transporter-6
ERP29: Endoplasmic reticulum protein 29
ID4: Inhibitor Of DNA Binding 4
MGMT: Methylated-DNA-protein-cysteine methyltransferase
ETS: ETS proto-oncogene 1
PD-L1: Programmed Cell Death Ligand 1
DNMT1: DNA methyltransferase 1
HCC: Hepatocellular carcinoma
TGBI: Transforming Growth Factor Beta Induced
ER-α: Estrogen Receptors Alpha
PEBP4: Phosphatidylethanolamine binding protein 4
RKIP: RAF-kinase inhibitor protein
DDR: DNA damage repair
MGMT: O-6-Methylguanine-DNA Methyltransferase
DNA-PK: DNA-dependent protein kinase
DSBs: Double-strand break repair machinery
eATP: Extracellular ATP
ABCG2: Adenosine triphosphate-binding cassette superfamily G member 2
MEK1/2: Mitogen-activated protein kinase 1/2
Raf- 1: RAF proto-oncogene
ERK: Extracellular signal-regulated kinase '
PDAC: ancreatic ductal adenocarcinoma
ODRUL: OS doxorubicin resistance-related upregulated lncRNA
HOTTIP: HOXA transcript at the distal tip
HOT AIR: HOX antisense intergenic RNA
LINC-ROR: Long Intergenic Non-Protein Coding RNA, Regulator of Reprogramming
CCAL: Colorectal cancer-associated lncRNA
MALAT1: metastasis-associated lung adenocarcinoma transcript 1
ANRIL: Antisense Noncoding RNA in the INK4 Locus
MRUL: MDR-related and upregulated lncRNA
EVs: Extracellular vesicles
Linc-VLDLR: Long intergenic non-coding RNA VLDLR
SGK1: Serum- and Glucocorticoid-Regulated Kinase 1
miRNA: microRNAs
XIST: X-inactive specific transcript
LINC00518: Long intergenic nonprotein coding RNA 518
BLACAT1: Bladder cancer-associated transcript-1
STAT1: Signal transducers and activators of transcription
References
1. Amawi H, Sim H-M, Tiwari AK, Ambudkar SV, Shukla S. ABC transporter-mediated multidrug-resistant cancer. Drug transporters Drug disposition effects toxicity. (2019), 549–80. doi: 10.1007/978-981-13-7647-4_12
2. Zhang H, Xu H, Ashby CR Jr., Assaraf YG, Chen ZS, Liu HM. Chemical molecular-based approach to overcome multidrug resistance in cancer by targeting P-glycoprotein (P-gp). Medicinal Res Rev. (2021) 41:525–55. doi: 10.1002/med.21739
3. Briz O, Perez-Silva L, Al-Abdulla R, Abete L, Reviejo M, Romero MR, et al. What “The Cancer Genome Atlas” database tells us about the role of ATP-binding cassette (ABC) proteins in chemoresistance to anticancer drugs. Expert Opin Drug Metab Toxicol. (2019) 15:577–93. doi: 10.1080/17425255.2019.1631285
4. He C, Sun Z, Hoffman RM, Yang Z, Jiang Y, Wang L, et al. P-glycoprotein overexpression is associated with cisplatin resistance in human osteosarcoma. Anticancer Res. (2019) 39:1711–8. doi: 10.21873/anticanres.13277
5. Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta (BBA)-Biomembranes. (1976) 455:152–62. doi: 10.1016/0005-2736(76)90160-7
6. Ibrahim MA, Abdeljawaad KA, Jaragh-Alhadad LA, Oraby HF, Atia MA, Alzahrani OR, et al. Potential drug candidates as P-glycoprotein inhibitors to reverse multidrug resistance in cancer: an in silico drug discovery study. J Biomolecular Structure Dynamics. (2023) 41:13977–92. doi: 10.1080/07391102.2023.2176360
7. Jeddi F, Soozangar N, Sadeghi MR, Somi MH, Shirmohamadi M, Eftekhar-Sadat A-T, et al. Nrf2 overexpression is associated with P-glycoprotein upregulation in gastric cancer. Biomedicine pharmacotherapy. (2018) 97:286–92. doi: 10.1016/j.biopha.2017.10.129
8. Chen Q, Liu X, Luo Z, Wang S, Lin J, Xie Z, et al. Chloride channel-3 mediates multidrug resistance of cancer by upregulating P-glycoprotein expression. J Cell Physiol. (2019) 234:6611–23. doi: 10.1002/jcp.v234.5
9. Liu T, Wei R, Zhang Y, Chen W, Liu H. Association between NF-κB expression and drug resistance of liver cancer. Oncol Lett. (2019) 17:1030–4. doi: 10.3892/ol.2018.9640
10. Ren H, Wang Z, Chen Y, Liu Y, Zhang S, Zhang T, et al. SMYD2-OE promotes oxaliplatin resistance in colon cancer through MDR1/P-glycoprotein via MEK/ERK/AP1 pathway. OncoTargets Ther. (2019) 12:2585–94. doi: 10.2147/OTT.S186806
11. Liu Y, Cao F, Xia F, Li J, Dong X, Guo Y, et al. Shc3 facilitates breast cancer drug resistance by interacting with ErbB2 to initiate ErbB2/COX2/MDR1 axis. Cancer Med. (2023) 12:10768–80. doi: 10.1002/cam4.v12.9
12. Chang Y-T, Lin Y-C, Sun L, Liao W-C, Wang CC, Chou C-Y, et al. Wilforine resensitizes multidrug resistant cancer cells via competitive inhibition of P-glycoprotein. Phytomedicine. (2020) 71:153239. doi: 10.1016/j.phymed.2020.153239
13. Liu M, Xu C, Qin X, Liu W, Li D, Jia H, et al. DHW-221, a dual PI3K/mTOR inhibitor, overcomes multidrug resistance by targeting P-glycoprotein (P-gp/ABCB1) and akt-mediated FOXO3a nuclear translocation in non-small cell lung cancer. Front Oncol. (2022) 12:873649. doi: 10.3389/fonc.2022.873649
14. Ma L, Luo M, Wu J, Yang J, Hong L. The combination therapy of HIF1α inhibitor LW6 and cisplatin plays an effective role on anti-tumor function in A549 cells. Neoplasma. (2019) 66:776. doi: 10.4149/neo_2018_180921N708
15. Yamamoto M, Suzuki S, Togashi K, Sanomachi T, Seino S, Kitanaka C, et al. AS602801 sensitizes ovarian cancer stem cells to paclitaxel by down-regulating MDR1. Anticancer Res. (2019) 39:609–17. doi: 10.21873/anticanres.13154
16. Kim J, Piao H-L, Kim B-J, Yao F, Han Z, Wang Y, et al. Long noncoding RNA MALAT1 suppresses breast cancer metastasis. Nat Genet. (2018) 50:1705–15. doi: 10.1038/s41588-018-0252-3
17. Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. (2009) 458:223–7. doi: 10.1038/nature07672
18. Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J neuro-oncology. (2013) 113:1–11. doi: 10.1007/s11060-013-1084-8
19. Silva A, Bullock M, Calin G. The clinical relevance of long non-coding RNAs in cancer. Cancers. (2015) 7:2169–82. doi: 10.3390/cancers7040884
20. Fang Z, Chen W, Yuan Z, Liu X, Jiang H. LncRNA-MALAT1 contributes to the cisplatin-resistance of lung cancer by upregulating MRP1 and MDR1 via STAT3 activation. Biomedicine Pharmacotherapy. (2018) 101:536–42. doi: 10.1016/j.biopha.2018.02.130
21. Alkafaas SS, Diab T, Shalaby T, Hessien M. Dexamethasone improves the responsiveness of hepatoma cells for both free and solvent containing paclitaxel in vitro. Egyptian J Biochem Mol Biol. (2019) 37:95–110.
22. Diab T, Alkafaas SS, Shalaby TI, Hessien M. Paclitaxel nanoparticles induce apoptosis and regulate txr1, cyp3a4 and cyp2c8 in breast cancer and hepatoma cells. Anti-Cancer Agents Medicinal Chem (Formerly Curr Medicinal Chemistry-Anti-Cancer Agents). (2020) 20:1582–91. doi: 10.2174/1871520620666200504071530
23. Alkafaas SS, Loutfy SA, Diab T, Hessien M. Vasopressin induces apoptosis but does not enhance the antiproliferative effect of dynamin 2 or PI3K/Akt inhibition in luminal A breast cancer cells. Med Oncol. (2022) 40:35. doi: 10.1007/s12032-022-01889-4
24. Chen S, Zhao Y, Liu S, Zhang J, Assaraf YG, Cui W, et al. Epigenetic enzyme mutations as mediators of anti-cancer drug resistance. Drug Resistance Updates. (2022) 61:100821. doi: 10.1016/j.drup.2022.100821
25. Chen J, Sprouffske K, Huang Q, Maley CC. Solving the puzzle of metastasis: the evolution of cell migration in neoplasms. PloS One. (2011) 6:e17933. doi: 10.1371/journal.pone.0017933
26. Hayford CE, Tyson DR, Robbins CJ 3rd, Frick PL, Quaranta V, Harris LA. An in vitro model of tumor heterogeneity resolves genetic, epigenetic, and stochastic sources of cell state variability. PloS Biol. (2021) 19:e3000797. doi: 10.1371/journal.pbio.3000797
27. Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B. The different mechanisms of cancer drug resistance: a brief review. Advanced Pharm Bull. (2017) 7:339. doi: 10.15171/apb.2017.041
28. Mirzayans R, Murray D. Intratumor heterogeneity and therapy resistance: contributions of dormancy, apoptosis reversal (Anastasis) and cell fusion to disease recurrence. Int J Mol Sci. (2020) 21:1–20. doi: 10.3390/ijms21041308
29. Sun XX, Yu Q. Intra-tumor heterogeneity of cancer cells and its implications for cancer treatment. Acta Pharmacol Sin. (2015) 36:1219–27. doi: 10.1038/aps.2015.92
30. Balázsi G, Van Oudenaarden A, Collins JJ. Cellular decision making and biological noise: from microbes to mammals. Cell. (2011) 144:910–25. doi: 10.1016/j.cell.2011.01.030
31. Fukumura D, Duda DG, Munn LL, Jain RK. Tumor microvasculature and microenvironment: novel insights through intravital imaging in pre-clinical models. Microcirculation. (2010) 17:206–25. doi: 10.1111/j.1549-8719.2010.00029.x
32. Asić K. Dominant mechanisms of primary resistance differ from dominant mechanisms of secondary resistance to targeted therapies. Crit Rev Oncol Hematol. (2016) 97:178–96. doi: 10.1016/j.critrevonc.2015.08.004
33. Fleischer JR, Jodszuweit CA, Ghadimi M, De Oliveira T, Conradi L-C. Vascular heterogeneity with a special focus on the hepatic microenvironment. Front Physiol. (2020) 11. doi: 10.3389/fphys.2020.591901
34. Vasan N, Baselga J, Hyman DM. A view on drug resistance in cancer. Nature. (2019) 575:299–309. doi: 10.1038/s41586-019-1730-1
35. Anderson NM, Simon MC. The tumor microenvironment. Curr Biol. (2020) 30:R921–r925. doi: 10.1016/j.cub.2020.06.081
36. Taylor S, Spugnini EP, Assaraf YG, Azzarito T, Rauch C, Fais S. Microenvironment acidity as a major determinant of tumor chemoresistance: Proton pump inhibitors (PPIs) as a novel therapeutic approach. Drug Resist Update. (2015) 23:69–78. doi: 10.1016/j.drup.2015.08.004
37. Icard P, Shulman S, Farhat D, Steyaert J-M, Alifano M, Lincet H. How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resistance Updates. (2018) 38:1–11. doi: 10.1016/j.drup.2018.03.001
38. Qian J, Rankin EB. Hypoxia-induced phenotypes that mediate tumor heterogeneity. Adv Exp Med Biol. (2019) 1136:43–55. doi: 10.1007/978-3-030-12734-3_3
39. Bao MH, Wong CC. Hypoxia, metabolic reprogramming, and drug resistance in liver cancer. Cells. (2021) 10:1–18. doi: 10.3390/cells10071715
40. Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. (2017) 168:707–23. doi: 10.1016/j.cell.2017.01.017
41. Crucitta S, Cucchiara F, Mathijssen R, Mateo J, Jager A, Joosse A, et al. Treatment-driven tumor heterogeneity and drug resistance: Lessons from solid tumors. Cancer Treat Rev. (2022) 104:102340. doi: 10.1016/j.ctrv.2022.102340
42. Pal B, Bayat Mokhtari R, Li H, Bhuyan R, Talukdar J, Sandhya S, et al. Abstract 251: Stem cell altruism may serve as a novel drug resistance mechanism in oral cancer. Cancer Res. (2016) 76:251–1. doi: 10.1158/1538-7445.AM2016-251
43. Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. (2017) 14:611–29. doi: 10.1038/nrclinonc.2017.44
44. Yang J, Antin P, Berx G, Blanpain C, Brabletz T, Bronner M, et al. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. (2020) 21:341–52. doi: 10.1038/s41580-020-0237-9
45. Oshimori N, Oristian D, Fuchs E. TGF-β promotes heterogeneity and drug resistance in squamous cell carcinoma. Cell. (2015) 160:963–76. doi: 10.1016/j.cell.2015.01.043
46. Lazarova D, Bordonaro M. ZEB1 mediates drug resistance and EMT in p300-deficient CRC. J Cancer. (2017) 8:1453–9. doi: 10.7150/jca.18762
47. Sun L, Ke J, He Z, Chen Z, Huang Q, Ai W, et al. HES1 promotes colorectal cancer cell resistance to 5-fu by inducing of EMT and ABC transporter proteins. J Cancer. (2017) 8:2802–8. doi: 10.7150/jca.19142
48. Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. (2019) 20:69–84. doi: 10.1038/s41580-018-0080-4
49. Williams ED, Gao D, Redfern A, Thompson EW. Controversies around epithelial-mesenchymal plasticity in cancer metastasis. Nat Rev Cancer. (2019) 19:716–32. doi: 10.1038/s41568-019-0213-x
50. De Las Rivas J, Brozovic A, Izraely S, Casas-Pais A, Witz IP, Figueroa A. Cancer drug resistance induced by EMT: novel therapeutic strategies. Arch Toxicol. (2021) 95:2279–97. doi: 10.1007/s00204-021-03063-7
51. Wang Y, Wang Y, Qin Z, Cai S, Yu L, Hu H, et al. The role of non-coding RNAs in ABC transporters regulation and their clinical implications of multidrug resistance in cancer. Expert Opin Drug Metab Toxicol. (2021) 17:291–306. doi: 10.1080/17425255.2021.1887139
52. Peetla C, Vijayaraghavalu S, Labhasetwar V. Biophysics of cell membrane lipids in cancer drug resistance: Implications for drug transport and drug delivery with nanoparticles. Adv Drug Delivery Rev. (2013) 65:1686–98. doi: 10.1016/j.addr.2013.09.004
53. Muley H, Fadó R, Rodríguez-Rodríguez R, Casals N. Drug uptake-based chemoresistance in breast cancer treatment. Biochem Pharmacol. (2020) 177:113959. doi: 10.1016/j.bcp.2020.113959
54. Alves AC, Ribeiro D, Nunes C, Reis S. Biophysics in cancer: The relevance of drug-membrane interaction studies. Biochim Biophys Acta (BBA) - Biomembranes. (2016) 1858:2231–44. doi: 10.1016/j.bbamem.2016.06.025
55. De Morrée ES, Böttcher R, Van Soest RJ, Aghai A, De Ridder CM, Gibson AA, et al. Loss of SLCO1B3 drives taxane resistance in prostate cancer. Br J Cancer. (2016) 115:674–81. doi: 10.1038/bjc.2016.251
56. Peetla C, Bhave R, Vijayaraghavalu S, Stine A, Kooijman E, Labhasetwar V. Drug resistance in breast cancer cells: biophysical characterization of and doxorubicin interactions with membrane lipids. Mol Pharm. (2010) 7:2334–48. doi: 10.1021/mp100308n
57. Nowak M, Klink M. The role of tumor-associated macrophages in the progression and chemoresistance of ovarian cancer. Cells. (2020) 9:1–12. doi: 10.3390/cells9051299
58. Chan BA, Hughes BG. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res. (2015) 4:36–54. doi: 10.3978/j.issn.2218-6751.2014.05.01
59. Bure I, Nemtsova M, Kuznetsova E. Histone modifications and non-coding RNAs: mutual epigenetic regulation and role in pathogenesis. Int J Mol Sci. (2022) 23:1–35. doi: 10.3390/ijms23105801
60. Ohata Y, Shimada S, Akiyama Y, Mogushi K, Nakao K, Matsumura S, et al. Acquired resistance with epigenetic alterations under long-term antiangiogenic therapy for hepatocellular carcinoma. Mol Cancer Ther. (2017) 16:1155–65. doi: 10.1158/1535-7163.MCT-16-0728
61. Romero-Garcia S, Prado-Garcia H, Carlos-Reyes A. Role of DNA methylation in the resistance to therapy in solid tumors. Front Oncol. (2020) 10.1152 doi: 10.3389/fonc.2020.01152
62. Fujiyoshi S, Honda S, Minato M, Ara M, Suzuki H, Hiyama E, et al. Hypermethylation of CSF3R is a novel cisplatin resistance marker and predictor of response to postoperative chemotherapy in hepatoblastoma. Hepatol Res. (2020) 50:598–606. doi: 10.1111/hepr.13479
63. Zhang J, Zhou C, Jiang H, Liang L, Shi W, Zhang Q, et al. ZEB1 induces ER-α promoter hypermethylation and confers antiestrogen resistance in breast cancer. Cell Death Dis. (2017) 8:e2732. doi: 10.1038/cddis.2017.154
64. Palomeras S, Diaz-Lagares Á., Viñas G, Setien F, Ferreira HJ, Oliveras G, et al. Epigenetic silencing of TGFBI confers resistance to trastuzumab in human breast cancer. Breast Cancer Res. (2019) 21:79. doi: 10.1186/s13058-019-1160-x
65. Asano T. Drug resistance in cancer therapy and the role of epigenetics. J Nippon Med School. (2020) 87:244–51. doi: 10.1272/jnms.JNMS.2020_87-508
66. Yin J, Zhao J, Hu W, Yang G, Yu H, Wang R, et al. Disturbance of the let-7/LIN28 double-negative feedback loop is associated with radio- and chemo-resistance in non-small cell lung cancer. PloS One. (2017) 12:e0172787. doi: 10.1371/journal.pone.0172787
67. Wei L, Wang X, Lv L, Liu J, Xing H, Song Y, et al. The emerging role of microRNAs and long noncoding RNAs in drug resistance of hepatocellular carcinoma. Mol Cancer. (2019) 18:147. doi: 10.1186/s12943-019-1086-z
68. Li L-Y, Guan Y-D, Chen X-S, Yang J-M, Cheng Y. DNA repair pathways in cancer therapy and resistance. Front Pharmacol. (2021) 11. doi: 10.3389/fphar.2020.629266
69. Maki Y, Murakami J, Asaumi J, Tsujigiwa H, Nagatsuka H, Kokeguchi S, et al. Role of O6-methylguanine-DNA methyltransferase and effect of O6-benzylguanine on the anti-tumor activity of cis-diaminedichloroplatinum(II) in oral cancer cell lines. Oral Oncol. (2005) 41:984–93. doi: 10.1016/j.oraloncology.2005.05.011
70. Ciszewski WM, Tavecchio M, Dastych J, Curtin NJ. DNA-PK inhibition by NU7441 sensitizes breast cancer cells to ionizing radiation and doxorubicin. Breast Cancer Res Treat. (2014) 143:47–55. doi: 10.1007/s10549-013-2785-6
71. Guillon J, Petit C, Toutain B, Guette C, Lelièvre E, Coqueret O. Chemotherapy-induced senescence, an adaptive mechanism driving resistance and tumor heterogeneity. Cell Cycle. (2019) 18:2385–97. doi: 10.1080/15384101.2019.1652047
72. Saleh T, Tyutyunyk-Massey L, Gewirtz DA. Tumor cell escape from therapy-induced senescence as a model of disease recurrence after dormancy. Cancer Res. (2019) 79:1044–6. doi: 10.1158/0008-5472.CAN-18-3437
73. Abbaszadeh Z, Çeşmeli S, Biray Avcı Ç. Crucial players in glycolysis: Cancer progress. Gene. (2020) 726:144158. doi: 10.1016/j.gene.2019.144158
74. Zhou Y, Tozzi F, Chen J, Fan F, Xia L, Wang J, et al. Intracellular ATP levels are a pivotal determinant of chemoresistance in colon cancer cells. Cancer Res. (2012) 72:304–14. doi: 10.1158/0008-5472.CAN-11-1674
75. Liu R, Chen Y, Liu G, Li C, Song Y, Cao Z, et al. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. (2020) 11:797. doi: 10.1038/s41419-020-02998-6
76. Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. (2017) 17:97–111. doi: 10.1038/nri.2016.107
77. Zanoni M, Pegoraro A, Adinolfi E, De Marchi E. Emerging roles of purinergic signaling in anti-cancer therapy resistance. Front Cell Dev Biol. (2022) 10:1006384. doi: 10.3389/fcell.2022.1006384
78. Cui H, Zhang J. ABC transporter inhibitors in reversing multidrug resistance to chemotherapy. Curr Drug Targets. (2015) 16:1356–71. doi: 10.2174/1389450116666150330113506
79. Iangcharoen P, Punfa W, Yodkeeree S, Kasinrerk W, Ampasavate C, Anuchapreeda S, et al. Anti-P-glycoprotein conjugated nanoparticles for targeting drug delivery in cancer treatment. Arch pharmacal Res. (2011) 34:1679–89. doi: 10.1007/s12272-011-1012-4
80. Abolhoda A, Wilson AE, Ross H, Danenberg PV, Burt M, Scotto KW. Rapid activation of MDR1 gene expression in human metastatic sarcoma after in vivo exposure to doxorubicin. Clin Cancer Res. (1999) 5:3352–6.
81. Imai Y, Ishikawa E, Asada S, Sugimoto Y. Estrogen-mediated post transcriptional down-regulation of breast cancer resistance protein/ABCG2. Cancer Res. (2005) 65:596–604. doi: 10.1158/0008-5472.596.65.2
82. Katayama K, Yoshioka S, Tsukahara S, Mitsuhashi J, Sugimoto Y. Inhibition of the mitogen-activated protein kinase pathway results in the down-regulation of P-glycoprotein. Mol Cancer Ther. (2007) 6:2092–102. doi: 10.1158/1535-7163.MCT-07-0148
83. Robinson K, Tiriveedhi V. Perplexing role of P-glycoprotein in tumor microenvironment. Front Oncol. (2020) 10:265. doi: 10.3389/fonc.2020.00265
84. Van De Ven R, De Jong MC, Reurs AW, Schoonderwoerd AJ, Jansen G, Hooijberg JH, et al. Dendritic cells require multidrug resistance protein 1 (ABCC1) transporter activity for differentiation. J Immunol. (2006) 176:5191–8. doi: 10.4049/jimmunol.176.9.5191
85. Lloberas N, Rama I, Llaudó I, Torras J, Cerezo G, Cassis L, et al. Dendritic cells phenotype fitting under hypoxia or lipopolysaccharide; adenosine 5′-triphosphate-binding cassette transporters far beyond an efflux pump. Clin Exp Immunol. (2013) 172:444–54. doi: 10.1111/cei.12067
86. Klimecki WT, Taylor CW, Dalton WS. Inhibition of cell-mediated cytolysis and P-glycoprotein function in natural killer cells by verapamil isomers and cyclosporine A analogs. J Clin Immunol. (1995) 15:152–8. doi: 10.1007/BF01543107
87. Wirths S, Lanzavecchia A. ABCB1 transporter discriminates human resting naive B cells from cycling transitional and memory B cells. Eur J Immunol. (2005) 35:3433–41. doi: 10.1002/eji.200535364
88. Tsujimura S, Adachi T, Saito K, Kawabe A, Tanaka Y. Relevance of P-glycoprotein on CXCR4+ B cells to organ manifestation in highly active rheumatoid arthritis. Modern Rheumatol. (2018) 28:276–86. doi: 10.1080/14397595.2017.1341458
89. Pendse SS, Briscoe DM, Frank MH. P-glycoprotein and alloimmune T-cell activation. Clin Appl Immunol Rev. (2003) 4:3–14. doi: 10.1016/S1529-1049(03)00007-2
90. Zhao J, Cao Y, Lei Z, Yang Z, Zhang B, Huang B. Selective depletion of CD4+ CD25+ Foxp3+ regulatory T cells by low-dose cyclophosphamide is explained by reduced intracellular ATP levels. Cancer Res. (2010) 70:4850–8. doi: 10.1158/0008-5472.CAN-10-0283
91. Kooij G, Kroon J, Paul D, Reijerkerk A, Geerts D, van der Pol SM, et al. P-glycoprotein regulates trafficking of CD8+ T cells to the brain parenchyma. Acta neuropathologica. (2014) 127:699–711. doi: 10.1007/s00401-014-1244-8
92. Gerrard G, Payne E, Baker RJ, Jones DT, Potter M, Prentice HG, et al. Clinical effects and P-glycoprotein inhibition in patients with acute myeloid leukemia treated with zosuquidar trihydrochloride, daunorubicin and cytarabine. Haematologica. (2004) 89:782–90.
93. Yoshimori M, Takada H, Imadome KI, Kurata M, Yamamoto K, Koyama T, et al. P-glycoprotein is expressed and causes resistance to chemotherapy in EBV-positive T-cell lymphoproliferative diseases. Cancer Med. (2015) 4:1494–504. doi: 10.1002/cam4.494
94. Shen H, Xu W, Luo W, Zhou L, Yong W, Chen F, et al. Upregulation of mdr1 gene is related to activation of the MAPK/ERK signal transduction pathway and YB-1 nuclear translocation in B-cell lymphoma. Exp Hematol. (2011) 39:558–69. doi: 10.1016/j.exphem.2011.01.013
95. Ling L, Lin Y, Zheng W, Hong S, Tang X, Zhao P, et al. Circulating and tumor-infiltrating mucosal associated invariant T (MAIT) cells in colorectal cancer patients. Sci Rep. (2016) 6:20358. doi: 10.1038/srep20358
96. Alsuliman A, Muftuoglu M, Khoder A, Ahn Y-O, Basar R, Verneris MR, et al. A subset of virus-specific CD161+ T cells selectively express the multidrug transporter MDR1 and are resistant to chemotherapy in AML. Blood J Am Soc Hematol. (2017) 129:740–58. doi: 10.1182/blood-2016-05-713347
97. Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. (2009) 31:787–98. doi: 10.1016/j.immuni.2009.09.014
98. Frank MH, Denton MD, Alexander SI, Khoury SJ, Sayegh MH, Briscoe DM. Specific MDR1 P-glycoprotein blockade inhibits human alloimmune T cell activation in vitro. J Immunol. (2001) 166:2451–9. doi: 10.4049/jimmunol.166.4.2451
99. Antonioli L, Yegutkin GG, Pacher P, Blandizzi C, Haskó G. Anti-CD73 in cancer immunotherapy: awakening new opportunities. Trends Cancer. (2016) 2:95–109. doi: 10.1016/j.trecan.2016.01.003
100. Zhang C-L, Zhu K-P, Shen G-Q, Zhu Z-S. A long non-coding RNA contributes to doxorubicin resistance of osteosarcoma. Tumor Biol. (2016) 37:2737–48. doi: 10.1007/s13277-015-4130-7
101. Li Z, Zhao X, Zhou Y, Liu Y, Zhou Q, Ye H, et al. The long non-coding RNA HOTTIP promotes progression and gemcitabine resistance by regulating HOXA13 in pancreatic cancer. J Trans Med. (2015) 13:1–16. doi: 10.1186/s12967-015-0442-z
102. Tsang W, Kwok T. Riboregulator H19 induction of MDR1-associated drug resistance in human hepatocellular carcinoma cells. Oncogene. (2007) 26:4877–81. doi: 10.1038/sj.onc.1210266
103. Takahashi K, Yan IK, Kogure T, Haga H, Patel T. Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer. FEBS Open Bio. (2014) 4:458–67. doi: 10.1016/j.fob.2014.04.007
104. Ma Y, Yang Y, Wang F, Moyer M-P, Wei Q, Zhang P, et al. Long non-coding RNA CCAL regulates colorectal cancer progression by activating Wnt/β-catenin signaling pathway via suppression of activator protein 2α. Gut. (2016) 65:1494–504. doi: 10.1136/gutjnl-2014-308392
105. Lee H, Kim C, Ku J-L, Kim W, Yoon SK, Kuh H-J, et al. A long non-coding RNA snaR contributes to 5-fluorouracil resistance in human colon cancer cells. Molecules Cells. (2014) 37:540–6. doi: 10.14348/molcells.2014.0151
106. Özeş AR, Miller DF, Özeş ON, Fang F, Liu Y, Matei D, et al. NF-κB-HOTAIR axis links DNA damage response, chemoresistance and cellular senescence in ovarian cancer. Oncogene. (2016) 35:5350–61. doi: 10.1038/onc.2016.75
107. Tirosh I, Izar B, Prakadan SM, Wadsworth MH, Treacy D, Trombetta JJ, et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. (2016) 352:189–96. doi: 10.1126/science.aad0501
108. Wang Y, Zhang D, Wu K, Zhao Q, Nie Y, Fan D. Long noncoding RNA MRUL promotes ABCB1 expression in multidrug-resistant gastric cancer cell sublines. Mol Cell Biol. (2014) 34:3182–93. doi: 10.1128/MCB.01580-13
109. Takahashi K, Yan IK, Wood J, Haga H, Patel T. Involvement of extracellular vesicle long noncoding RNA (linc-VLDLR) in tumor cell responses to chemotherapy. Mol Cancer Res. (2014) 12:1377–87. doi: 10.1158/1541-7786.MCR-13-0636
110. Zhu J, Zhang R, Yang D, Li J, Yan X, Jin K, et al. Knockdown of long non-coding RNA XIST inhibited doxorubicin resistance in colorectal cancer by upregulation of miR-124 and downregulation of SGK1. Cell Physiol Biochem. (2018) 51:113–28. doi: 10.1159/000495168
111. Hu H, Yang L, Li L, Zeng C. Long non-coding RNA KCNQ1OT1 modulates oxaliplatin resistance in hepatocellular carcinoma through miR-7-5p/ABCC1 axis. Biochem Biophys Res Commun. (2018) 503:2400–6. doi: 10.1016/j.bbrc.2018.06.168
112. Chang L, Hu Z, Zhou Z, Zhang H. Linc00518 contributes to multidrug resistance through regulating the MiR-199a/MRP1 axis in breast cancer. Cell Physiol Biochem. (2018) 48:16–28. doi: 10.1159/000491659
113. Wu X, Zheng Y, Han B, Dong X. Long noncoding RNA BLACAT1 modulates ABCB1 to promote oxaliplatin resistance of gastric cancer via sponging miR-361. Biomedicine Pharmacotherapy. (2018) 99:832–8. doi: 10.1016/j.biopha.2018.01.130
114. Wang Y-J, Patel BA, Anreddy N, Zhang Y-K, Zhang G-N, Alqahtani S, et al. Thiazole-valine peptidomimetic (TTT-28) antagonizes multidrug resistance in vitro and in vivo by selectively inhibiting the efflux activity of ABCB1. Sci Rep. (2017) 7:42106. doi: 10.1038/srep42106
115. Tian S, He G, Song J, Wang S, Xin W, Zhang D, et al. Pharmacokinetic study of baicalein after oral administration in monkeys. Fitoterapia. (2012) 83:532–40. doi: 10.1016/j.fitote.2011.12.019
116. Miao Q, Wang Z, Zhang Y, Miao P, Zhao Y, Zhang Y, et al. In vitro potential modulation of baicalin and baicalein on P-glycoprotein activity and expression in Caco-2 cells and rat gut sacs. Pharm Biol. (2016) 54:1548–56. doi: 10.3109/13880209.2015.1107744
117. Yu X, Yang Y, Li Y, Cao Y, Tang L, Chen F, et al. Baicalein inhibits cervical cancer progression via downregulating long noncoding RNA BDLNR and its downstream PI3 K/Akt pathway. Int J Biochem Cell Biol. (2018) 94:107–18. doi: 10.1016/j.biocel.2017.11.009
118. Ibrahim A, Nasr M, El-Sherbiny IM. Baicalin as an emerging magical nutraceutical molecule: Emphasis on pharmacological properties and advances in pharmaceutical delivery. J Drug Delivery Sci Technol. (2022) 70:103269. doi: 10.1016/j.jddst.2022.103269
119. Vellosa JCR, Regasini LO, Khalil NM, Bolzani VDS, Khalil OA, Manente FA, et al. Antioxidant and cytotoxic studies for kaempferol, quercetin and isoquercitrin. Eclética quimica. (2011) 36:07–20. doi: 10.1590/S0100-46702011000200001
120. Lu X, Chen D, Yang F, Xing N. Quercetin inhibits epithelial-to-mesenchymal transition (EMT) process and promotes apoptosis in prostate cancer via downregulating lncRNA MALAT1. Cancer Manage Res. (2020) 12:1741–50. doi: 10.2147/CMAR.S241093
121. Kandemir K, Tomas M, Mcclements DJ, Capanoglu E. Recent advances on the improvement of quercetin bioavailability. Trends Food Sci Technol. (2022) 119:192–200. doi: 10.1016/j.tifs.2021.11.032
122. Wang M, Chen X, Yu F, Zhang L, Zhang Y, Chang W. The targeting of noncoding RNAs by quercetin in cancer prevention and therapy. Oxid Med Cell Longevity. (2022) 2022:4330681. doi: 10.1155/2022/4330681
123. Mohana S, Ganesan M, Agilan B, Karthikeyan R, Srithar G, Beaulah Mary R, et al. Screening dietary flavonoids for the reversal of P-glycoprotein-mediated multidrug resistance in cancer. Mol Biosyst. (2016) 12:2458–70. doi: 10.1039/C6MB00187D
124. Pei C-S, Wu H-Y, Fan F-T, Wu Y, Shen C-S, Pan L-Q. Influence of curcumin on HOTAIR-mediated migration of human renal cell carcinoma cells. Asian Pacific J Cancer Prev. (2014) 15:4239–43. doi: 10.7314/APJCP.2014.15.10.4239
125. Zamani M, Sadeghizadeh M, Behmanesh M, Najafi F. Dendrosomal curcumin increases expression of the long non-coding RNA gene MEG3 via up-regulation of epi-miRs in hepatocellular cancer. Phytomedicine. (2015) 22:961–7. doi: 10.1016/j.phymed.2015.05.071
126. Penta D, Mondal P, Natesh J, Meeran SM. Dietary bioactive diindolylmethane enhances the therapeutic efficacy of centchroman in breast cancer cells by regulating ABCB1/P-gp efflux transporter. J Nutr Biochem. (2021) 94:108749. doi: 10.1016/j.jnutbio.2021.108749
127. Mukhtar E, Adhami VM, Sechi M, Mukhtar H. Dietary flavonoid fisetin binds to β-tubulin and disrupts microtubule dynamics in prostate cancer cells. Cancer Lett. (2015) 367:173–83. doi: 10.1016/j.canlet.2015.07.030
128. Afroze N, Pramodh S, Shafarin J, Bajbouj K, Hamad M, Sundaram MK, et al. Fisetin deters cell proliferation, induces apoptosis, alleviates oxidative stress and inflammation in human cancer cells, HeLa. Int J Mol Sci. (2022) 23:1–20. doi: 10.3390/ijms23031707
129. Szymczak J, Cielecka-Piontek J. Fisetin—In search of better bioavailability—From macro to nano modifications: A review. Int J Mol Sci. (2023) 24:14158. doi: 10.3390/ijms241814158
130. Limtrakul P, Khantamat O, Pintha K. Inhibition of P-glycoprotein function and expression by kaempferol and quercetin. J Chemother. (2005) 17:86–95. doi: 10.1179/joc.2005.17.1.86
131. Barve A, Chen C, Hebbar V, Desiderio J, Saw CLL, Kong AN. Metabolism, oral bioavailability and pharmacokinetics of chemopreventive kaempferol in rats. Biopharmaceutics Drug disposition. (2009) 30:356–65. doi: 10.1002/bdd.677
132. Zhu L, Xue L. Kaempferol suppresses proliferation and induces cell cycle arrest, apoptosis, and DNA damage in breast cancer cells. Oncol Res. (2019) 27:629. doi: 10.3727/096504018X15228018559434
133. Sarawek S, Derendorf H, Butterweck V. Pharmacokinetics of luteolin and metabolites in rats. Natural product Commun. (2008) 3:1934578X0800301218. doi: 10.1177/1934578X0800301218
134. Rao PS, Satelli A, Moridani M, Jenkins M, Rao US. Luteolin induces apoptosis in multidrug resistant cancer cells without affecting the drug transporter function: involvement of cell line-specific apoptotic mechanisms. Int J Cancer. (2012) 130:2703–14. doi: 10.1002/ijc.26308
135. Çetinkaya M, Baran Y. Therapeutic potential of luteolin on cancer. Vaccines. (2023) 11:554. doi: 10.3390/vaccines11030554
136. Yamamura Y, Santa T, Kotaki H, Uchino K, Sawada Y, Iga T. Administration-route dependency of absorption of glycyrrhizin in rats: intraperitoneal administration dramatically enhanced bioavailability. Biol Pharm Bull. (1995) 18:337–41. doi: 10.1248/bpb.18.337
137. Kim M, Park SC, Lee DY. Glycyrrhizin as a nitric oxide regulator in cancer chemotherapy. Cancers. (2021) 13:1–22. doi: 10.3390/cancers13225762
138. Han Y, Sheng W, Liu X, Liu H, Jia X, Li H, et al. Glycyrrhizin ameliorates colorectal cancer progression by regulating NHEJ pathway through inhibiting HMGB1-induced DNA damage response. Sci Rep. (2024) 14:24948. doi: 10.1038/s41598-024-76155-w
139. Angelini A, Conti P, Ciofani G, Cuccurullo F, Di Ilio C. Modulation of multidrug resistance P-glycoprotein activity by antiemetic compounds in human doxorubicin-resistant sarcoma cells (MES-SA/Dx-5): implications on cancer therapy. J Biol regulators homeostatic Agents. (2013) 27:1029–37.
140. Salari Z, Khosravi A, Pourkhandani E, Molaakbari E, Salarkia E, Keyhani A, et al. The inhibitory effect of 6-gingerol and cisplatin on ovarian cancer and antitumor activity: In silico, in vitro, and in vivo. Front Oncol. (2023) 13:1098429. doi: 10.3389/fonc.2023.1098429
141. Samota MK, Rawat M, Kaur M, Garg D. Gingerol: extraction methods, health implications, bioavailability and signaling pathways. Sustain Food Technology. (2024) 2:1652–69. doi: 10.1039/D4FB00135D
142. Jackson T, Chougule MB, Ichite N, Patlolla RR, Singh M. Antitumor activity of noscapine in human non-small cell lung cancer xenograft model. Cancer chemotherapy Pharmacol. (2008) 63:117–26. doi: 10.1007/s00280-008-0720-z
143. Muthiah D, Henshaw GK, Debono AJ, Capuano B, Scammells PJ, Callaghan R. Overcoming P-glycoprotein–mediated drug resistance with noscapine derivatives. Drug Metab Disposition. (2019) 47:164–72. doi: 10.1124/dmd.118.083188
144. Askari VR, Rahmanian-Devin P, Rahimi VB, Jaafari MR, Golmohammadzadeh S, Sanei-Far Z. Noscapine, an emerging medication for different diseases: a mechanistic review. London: Hindawi (2021).
145. Semlali A, Ajala I, Beji S, Al-Zharani MM, Rouabhia M. Synergistic effect of anethole and platinum drug cisplatin against oral cancer cell growth and migration by inhibiting MAPKase, beta-catenin, and NF-κB pathways. Pharmaceuticals. (2023) 16:700. doi: 10.3390/ph16050700
146. Raposo A, Raheem D, Zandonadi RP, Suri N, Olukosi A, De Lima BR, et al. Anethole in cancer therapy: Mechanisms, synergistic pHyungseo Bobbyotential, and clinical challenges. Biomedicine Pharmacotherapy. (2024) 180:117449. doi: 10.1016/j.biopha.2024.117449
147. Ou K, Gu L. Absorption and metabolism of proanthocyanidins. J Funct Foods. (2014) 7:43–53. doi: 10.1016/j.jff.2013.08.004
148. Lee Y. Cancer chemopreventive potential of procyanidin. Toxicological Res. (2017) 33:273–82. doi: 10.5487/TR.2017.33.4.273
149. Xue B, Qualls C, Lanthiez A, Lu Q-Y, Yang J, Lee R-P, et al. The effects of a grape seed procyanidin extract on cytochrome P450 3A4 activity and inflammatory mediators in the lungs of heavy active and former smokers. Int J Mol Sci. (2024) 25:13105. doi: 10.3390/ijms252313105
150. Talib WH, Baban MM, Azzam AO, Issa JJ, Ali AY, Alsuwais AK, et al. Allicin and cancer hallmarks. Molecules. (2024) 29:1–20. doi: 10.3390/molecules29061320
151. Xie W, Ma F, Dou L, Chang W, Yuan D, Zhang Z, et al. Allicin affects immunoreactivity of osteosarcoma cells through lncRNA CBR3-AS1. Heliyon. (2024) 10:1–12. doi: 10.1016/j.heliyon.2024.e31971
152. Jiang R, Zhang Z, Zhong Z, Zhang C. Long-non-coding RNA RUSC1-AS1 accelerates osteosarcoma development by miR-101-3p-mediated Notch1 signaling pathway. J Bone Oncol. (2021) 30:100382. doi: 10.1016/j.jbo.2021.100382
153. Liao Y, Wang H, Li S, Xue Y, Chen Y, Adu-Frimpong M, et al. Preparation of astaxanthin-loaded composite micelles with coaxial electrospray technology for enhanced oral bioavailability and improved antioxidation capability. J Sci Food Agric. (2024) 104:1408–19. doi: 10.1002/jsfa.v104.3
154. Xu Y, Jiang C. Astaxanthin suppresses the Malignant behaviors of nasopharyngeal carcinoma cells by blocking PI3K/AKT and NF-κB pathways via miR-29a-3p. Genes Environ. (2024) 46:10. doi: 10.1186/s41021-024-00304-w
155. Tan M, Jiang B, Wang H, Ouyang W, Chen X, Wang T, et al. Dihydromyricetin induced lncRNA MALAT1-TFEB-dependent autophagic cell death in cutaneous squamous cell carcinoma. J Cancer. (2019) 10:4245–55. doi: 10.7150/jca.32807
156. Wu M, Jiang M, Dong T, Xu L, Lv J, Xue M, et al. Reversal effect of dihydromyricetin on multiple drug resistance in SGC7901/5-FU cells. Asian Pac J Cancer Prev. (2020) 21:1269–74. doi: 10.31557/APJCP.2020.21.5.1269
157. Wu C, Zhuang Y, Zhou J-Y, Liu S-L, Wang R-P, Shu P. Cinnamaldehyde enhances apoptotic effect of oxaliplatin and reverses epithelial-mesenchymal transition and stemnness in hypoxic colorectal cancer cells. Exp Cell Res. (2019) 111500:1–11. doi: 10.1016/j.yexcr.2019.111500
158. Banerjee S, Banerjee S. Anticancer potential and molecular mechanisms of cinnamaldehyde and its congeners present in the cinnamon plant. Physiologia. (2023) 3:173–207. doi: 10.3390/physiologia3020013
159. Zhao X, Di J, Luo D, Vaishnav Y, Kamal Nuralieva N, Verma D, et al. Recent developments of P-glycoprotein inhibitors and its structure–activity relationship (SAR) studies. Bioorganic Chem. (2024) 143:106997. doi: 10.1016/j.bioorg.2023.106997
160. Le MT, Trinh DT, Ngo TD, Tran-Nguyen VK, Nguyen DN, Hoang T, et al. Chalcone derivatives as potential inhibitors of P-glycoprotein and norA: an in silico and in vitro study. BioMed Res Int. (2022) 2022:9982453. doi: 10.1155/2022/9982453
161. Olivas-Aguirre FJ, Rodrigo-García J, Martínez-Ruiz NDR, Cárdenas-Robles AI, Mendoza-Díaz SO, Álvarez-Parrilla E, et al. Cyanidin-3-O-glucoside: Physical-chemistry, foodomics and health effects. Molecules. (2016) 21:1–30. doi: 10.3390/molecules21091264
162. Kitagawa S. Inhibitory effects of polyphenols on p-glycoprotein-mediated transport. Biol Pharm Bull. (2006) 29:1–6. doi: 10.1248/bpb.29.1
163. Singh K, Patil RB, Patel V, Remenyik J, Hegedűs T, Goda K. Synergistic inhibitory effect of quercetin and cyanidin-3O-sophoroside on ABCB1. Int J Mol Sci. (2023) 24:1–24. doi: 10.3390/ijms241411341
164. Flory S, Männle R, Frank J. The inhibitory activity of curcumin on P-glycoprotein and its uptake by and efflux from LS180 cells is not affected by its galenic formulation. Antioxidants (Basel). (2021) 10:1–23. doi: 10.3390/antiox10111826
165. Rajaei N, Rahgouy G, Panahi N, Razzaghi-Asl N. Bioinformatic analysis of highly consumed phytochemicals as P-gp binders to overcome drug-resistance. Res Pharm Sci. (2023) 18:505–16. doi: 10.4103/1735-5362.383706
166. Kong W, Ling X, Chen Y, Wu X, Zhao Z, Wang W, et al. Hesperetin reverses P−glycoprotein−mediated cisplatin resistance in DDP−resistant human lung cancer cells via modulation of the nuclear factor−κB signaling pathway. Int J Mol Med. (2020) 45:1213–24. doi: 10.3892/ijmm.2020.4485
167. Zhang D-M, Tang PM-K, Chan JY-W, Lam H-M, Au SW-N, Kong S-K, et al. Anti-proliferative effect of ursolic acid on multidrug resistant hepatoma cells R-HepG2 by apoptosis induction. Cancer Biol Ther. (2007) 6:1377–85. doi: 10.4161/cbt.6.9.4528
168. Mustafa NH, Sekar M, Fuloria S, Begum MY, Gan SH, Rani N, et al. Chemistry, biosynthesis and pharmacology of sarsasapogenin: A potential natural steroid molecule for new drug design, development and therapy. Molecules. (2022) 27:1–26. doi: 10.3390/molecules27062032
169. Kitagawa S, Nabekura T, Takahashi T, Nakamura Y, Sakamoto H, Tano H, et al. Structure-activity relationships of the inhibitory effects of flavonoids on P-glycoprotein-mediated transport in KB-C2 cells. Biol Pharm Bull. (2005) 28:2274–8. doi: 10.1248/bpb.28.2274
170. Yoshida N, Koizumi M, Adachi I, Kawakami J. Inhibition of P-glycoprotein-mediated transport by terpenoids contained in herbal medicines and natural products. Food Chem Toxicol. (2006) 44:2033–9. doi: 10.1016/j.fct.2006.07.003
171. Wang X, Wang Y, Fang C, Gong Q, Huang J, Zhang Y, et al. Allicin affects the pharmacokinetics of sulfadiazine and florfenicol by downregulating the expression of jejunum P-gp and BCRP in broilers. Poultry Sci. (2022) 101:101947. doi: 10.1016/j.psj.2022.101947
172. Zhang W, Lim LY. Effects of spice constituents on P-glycoprotein-mediated transport and CYP3A4-mediated metabolism in vitro. Drug Metab Dispos. (2008) 36:1283–90. doi: 10.1124/dmd.107.019737
173. Sugihara N, Tsutsui Y, Tagashira T, Choshi T, Hibino S, Kamishikiryou J, et al. The ability of gallate and pyrogallol moieties of catechins to inhibit P-glycoprotein function. J Funct Foods. (2011) 3:298–304. doi: 10.1016/j.jff.2011.05.005
174. Mondal P, Natesh J, Salam A, Meeran SM. Mahanimbine isolated from Murraya koenigii inhibits P-glycoprotein involved in lung cancer chemoresistance. Bioorganic Chem. (2022) 129:106170. doi: 10.1016/j.bioorg.2022.106170
175. Rajabi S, Rajani HF, Mohammadkhani N, Ramírez-Coronel AA, Maleki M, Maresca M, et al. Long non-coding RNAs as novel targets for phytochemicals to cease cancer metastasis. Molecules. (2023) 28:987. doi: 10.3390/molecules28030987
176. Gupta SC, Kannappan R, Reuter S, Kim JH, Aggarwal BB. Chemosensitization of tumors by resveratrol. Ann New York Acad Sci. (2011) 1215:150–60. doi: 10.1111/j.1749-6632.2010.05852.x
177. Chen L-L, Carmichael GG. Decoding the function of nuclear long non-coding RNAs. Curr Opin Cell Biol. (2010) 22:357–64. doi: 10.1016/j.ceb.2010.03.003
178. Environment MO. (2015). 1010 Sherbrooe St. West, Suite910, Montreal, QC, Canada: Chemical Computing Group Inc., 10.
179. Alkafaas SS, Abdallah AM, Ghosh S, Loutfy SA, Elkafas SS, Abdel Fattah NF, et al. Insight into the role of clathrin-mediated endocytosis inhibitors in SARS-CoV-2 infection. Rev Med Virol. (2023) 33:e2403. doi: 10.1002/rmv.v33.1
Keywords: chemoresistance, p-glycoprotein-1, lncRNA, efflux transporters, in silico analysis
Citation: Diab M, Hamdi A, Al-Obeidat F, Hafez W, Cherrez-Ojeda I, Gador M, Rashid G, Elkhazin SF, Ibrahim MA, Ismail TF and Alkafaas SS (2025) Discovery of drug transporter inhibitors tied to long noncoding RNA in resistant cancer cells; a computational model -in silico- study. Front. Immunol. 16:1511029. doi: 10.3389/fimmu.2025.1511029
Received: 14 October 2024; Accepted: 26 March 2025;
Published: 25 April 2025.
Edited by:
Noha Mousaad Elemam, University of Sharjah, United Arab EmiratesReviewed by:
Hauke Busch, University of Lübeck, GermanyPriya Mondal, Central Food Technological Research Institute (CSIR), India
Copyright © 2025 Diab, Hamdi, Al-Obeidat, Hafez, Cherrez-Ojeda, Gador, Rashid, Elkhazin, Ibrahim, Ismail and Alkafaas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amel Hamdi, YW1lbC5oYW1kaUBhZHUuYWMuYWU=; Wael Hafez, V2FlbC5oYWZlekBubWMuYWU=; Feras Al-Obeidat, ZmVyYXMuYWwtb2JlaWRhdEB6dS5hYy5hZQ==
†These authors have contributed equally to this work and share first authorship
 Mohanad Diab
Mohanad Diab Amel Hamdi
Amel Hamdi Feras Al-Obeidat
Feras Al-Obeidat Wael Hafez
Wael Hafez Ivan Cherrez-Ojeda
Ivan Cherrez-Ojeda Muneir Gador
Muneir Gador Gowhar Rashid
Gowhar Rashid Sana F. Elkhazin
Sana F. Elkhazin Mahmad Anwar Ibrahim
Mahmad Anwar Ibrahim Tarek Farag Ismail
Tarek Farag Ismail Samar Sami Alkafaas
Samar Sami Alkafaas