- 1Department of General Surgery, Peking Union Medical College Hospital, Peking Union Medical College, Beijing, China
- 2Department of Gastrocolorectal Surgery, General Surgery Center, The First Hospital of Jilin University, Changchun, Jilin, China
- 3Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 4Guangdong Institute of Gastroenterology, Department of General Surgery, Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Diseases, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China
- 5Department of Colorectal Surgery, Changhai Hospital, Naval Medical University, Shanghai, China
- 6Department of General Surgery, China-Japan Friendship Hospital, No.2 East Yinghua Road, Chaoyang District, Beijing, China
- 7Department of Colorectal Surgery, Zhejiang Cancer Hospital, 1 Banshan East Road, Hangzhou, Zhejiang, China
- 8Department of General Surgery, Beijing Friendship Hospital, Capital Medical University & National Clinical Research Center for Digestive Diseases, Beijing, China
Background: The growing use of immune checkpoint inhibitors (ICIs) in the neoadjuvant treatment of colorectal cancer (CRC) has highlighted immune-related adverse events (irAEs) as a major concern. This study aimed to investigate the characteristics of irAEs.
Methods: This study was a retrospective, multicenter, registry-based investigation conducted in China, including 148 patients who developed irAEs after neoadjuvant immunotherapy between September 2020 and March 2024. The study analyzed the types, severity, risk factors and management strategies of irAEs. Data were collected on patient demographics, tumor assessments, neoadjuvant therapy regimens, and irAEs. Statistical analyses were conducted to identify the characteristics of irAEs and to assess their impact on surgical outcomes.
Results: Among the 148 patients, a total of 203 irAEs were documented, primarily affecting the skin, endocrine system, and liver. Most irAEs (95.6%) were mild-to-moderate in severity and were effectively managed with symptomatic treatment. Hepatotoxicity was the most frequent irAE, notably associated with the combination of radiotherapy and the CAPOX chemotherapy regimen. The severity of irAEs did not affect surgical complexity or postoperative complications.
Conclusion: Neoadjuvant immunotherapy combined with chemoradiotherapy demonstrates a favorable safety profile, with most irAEs being manageable. The findings support the clinical feasibility of combined regimens in CRC treatment, emphasizing the need for individualized management and extended follow-up for late-onset or chronic irAEs.
Highlights
● Most immune side effects are mild, manageable, and don’t complicate surgery.
● Study supports tailored management of irAEs for safer CRC immunotherapy integration.
● Immune-related side effects common with neoadjuvant immunotherapy for CRC.
● Hepatotoxicity is the most frequent irAE, linked to chemoradiotherapy.
Introduction
Colorectal cancer (CRC) is a common digestive system malignancy, ranking second in the overall incidence of cancer and fifth in overall mortality rate in China (1). Immune checkpoint inhibitors (ICIs), particularly inhibitors targeting programmed cell death protein-1 (PD-1) and cytotoxic T lymphocyte-associated protein-4 (CTLA-4), have shown promising therapeutic potential for patients with locally advanced rectal cancer (LARC) and metastatic CRC (2, 3). However, most CRC cases are microsatellite stable (MSS)/mismatch repair proficient (pMMR), limiting the effectiveness of ICIs as monotherapy. Patients undergoing neoadjuvant therapy tend to have better immune function, stronger antigen exposure, and a longer immune memory (4). Combining immunotherapy with chemotherapy or radiotherapy has emerged as a promising new strategy for CRC treatment (5).
With the application of ICIs in CRC treatment, immune-related adverse events (irAEs) have become a significant concern (6). These toxic reactions are caused by nonspecific immune system activation and can affect various organs or systems, including the skin, digestive, endocrine, and musculoskeletal systems. Common manifestations include rashes, thyroid dysfunction, and elevated liver enzymes (7). Radiotherapy and chemotherapy can enhance the effectiveness of immunotherapy by releasing tumor-associated antigens through tumor cell destruction (8). The risk factors for different types of irAEs remain unclear, particularly the role of combining immunotherapy with chemoradiotherapy. The impact of irAEs on surgical safety and postoperative complications is not yet well understood.
Existing research on irAE occurrence has largely been limited by small sample sizes, with a predominant focus on single types of adverse events rather than a comprehensive evaluation. Current insights are primarily derived from clinical trials, where irAEs are often treated as secondary outcomes and studied without a comprehensive investigation into their diversity and complexity (3, 9–15). As a result, a comprehensive understanding of the irAE profile in CRC neoadjuvant immunotherapy is limited, emphasizing the need for larger and more systematic evaluations.
This study therefore aims to characterize irAEs in CRC patients receiving neoadjuvant immunotherapy and to assess the safety of combining ICIs with chemoradiotherapy. By analyzing data from a large, multicenter registry cohort, this investigation will provide evidence on the safety of these combination therapies, thereby supporting future clinical decision-making and improving irAE management.
Methods
Study design and participants
This study was a retrospective, multicenter, registry-based investigation of CRC patients who developed irAEs during neoadjuvant immunotherapy. This cohort study involved eight tertiary referral centers across China from September 2020 to March 2024. The enrollment flowchart is shown in Figure 1. Eligible participants included CRC patients aged 18 years or older. All patients received ICIs as part of their treatment regimen, including PD-1 inhibitors (tislelizumab, sintilimab, toripalimab, and pembrolizumab) and the PD-1/CTLA-4 bispecific antibody cadonilimab. For analysis, patients were grouped by mechanism of ICIs (PD-1 inhibition vs. PD-1/CTLA-4 dual blockade). The diagnosis of irAEs was determined by the adverse event management teams at each participating center, while the treatment plan was decided by the physicians at each center. A database named IRAE-NCRC (irae-ncrc.com/crc/user/login) was established to consolidate data from these centers. After patient information was collected, professional physicians at the main center performed a secondary screening of the data, referencing the guidelines for managing immune checkpoint inhibitor-related toxicities, with the goal of retaining only those adverse events related to ICIs. The ethics committees of Peking Union Medical College Hospital (PUMCH) (ID: I-24PJ0024) and each participating center approved the study. Patients were monitored from the initiation of immunotherapy to at least six months post-treatment completion.

Figure 1. Flowchart of Patient Selection and Classification of irAEs. irAEs, immune-related adverse events; CRC, colorectal cancer.
Data collection
Data were collected and entered into the database by principal investigators at each participating center, with periodic quality checks conducted by PUMCH. The following data were collected: demographics [age at CRC diagnosis, gender, comorbidities, and body mass index (BMI)], oncological assessments (biopsy results, rectal MRI, TNM stage, expression of MMR protein, and postoperative pathology). Additionally, baseline blood test results, detailed neoadjuvant therapy regimens, and comprehensive surgical details were documented.
In this study, early-onset irAEs were defined as those occurring within three months of starting ICIs, while late-onset irAEs were defined as those occurring after three months from treatment initiation or after treatment cessation (16). Each irAE was characterized by its type, time of onset, severity, management strategy, and outcome. Adverse events were categorized and graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. Grades were defined as follows: Grade I (asymptomatic or mild symptoms), Grade II (moderate symptoms requiring minimal intervention), Grade III (severe symptoms), Grade IV (life-threatening symptoms), and Grade V (fatal events).
Statistical
Descriptive statistics were employed to summarize patient demographics, baseline characteristics, and management strategies for adverse events. Categorical variables were presented as frequencies (%) and compared using the Chi-square or Fisher’s exact test. Continuous variables were analyzed based on their distribution: normally distributed data were expressed as mean ± standard deviation (SD) and analyzed via Student’s t-test, while non-normally distributed data were presented as median (Q1, Q3) and analyzed with the Mann-Whitney U test. A p-value of less than 0.05 was considered statistically significant. Indicators with a p-value less than 0.1 were included in a multivariate logistic regression analysis. Given the multiple comparisons involved in this study, a Bonferroni correction was applied to control for type I error. All statistical analyses were conducted using SPSS version 26.0 (IBM Corporation, Chicago, IL), employing modules for categorical and continuous data analysis.
Results
Characteristics of population and treatment regimens
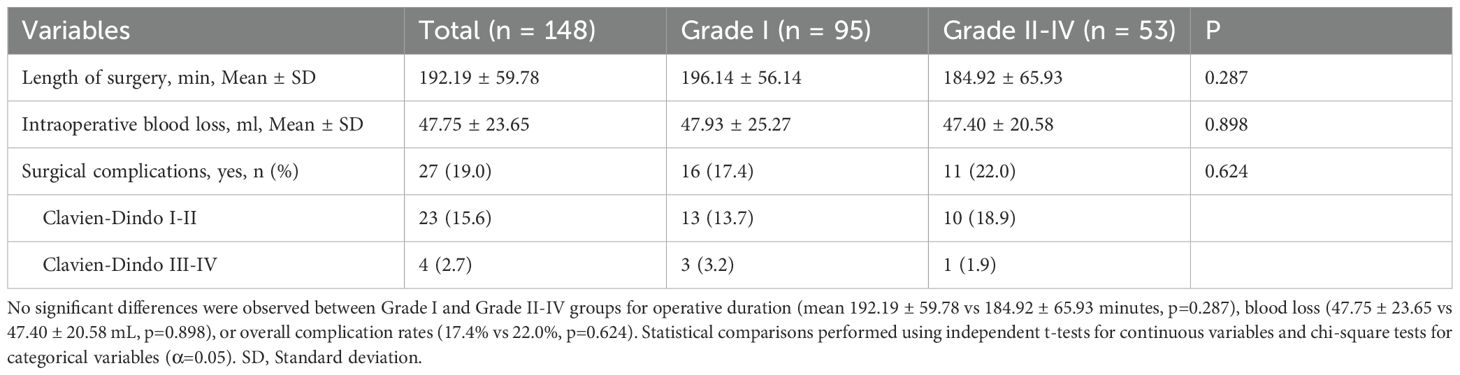
This study included a cohort of 148 CRC patients who developed irAEs following treatment with ICIs. Among these patients, 94 (63.5%) were male, with a median age of 60 years (52, 67). Most patients were diagnosed with rectal cancer (93.2%) and had MSS/pMMR status (78.4%) (Table 1). The neoadjuvant therapy regimens administered were summarized in Table 2. Most patients (125, 84.5%) received a combination of ICIs and chemoradiotherapy. The primary regimen among these patients was capecitabine plus oxaliplatin (CAPOX) in combination with long-course radiotherapy (LCRT). A smaller proportion of patients (6.8%) received immunotherapy as a monotherapy. PD-1 inhibitors were the predominant ICIs used in the study cohort (93.2%). Among the different ICIs regimens, we observed distinct patterns of irAEs associated with specific agents. Tislelizumab-treated patients predominantly exhibited endocrine and cutaneous toxicities, whereas hepatic adverse events were more frequent with Sintilimab and Cadonilimab therapy, a bispecific PD-1/CTLA-4 antibody administered to 10 patients (6.8%). Pembrolizumab was primarily associated with cutaneous manifestations (Figure 2).

Table 1. Baseline characteristics and clinical outcomes stratified by irAE severity in CRC patients receiving ICIs.
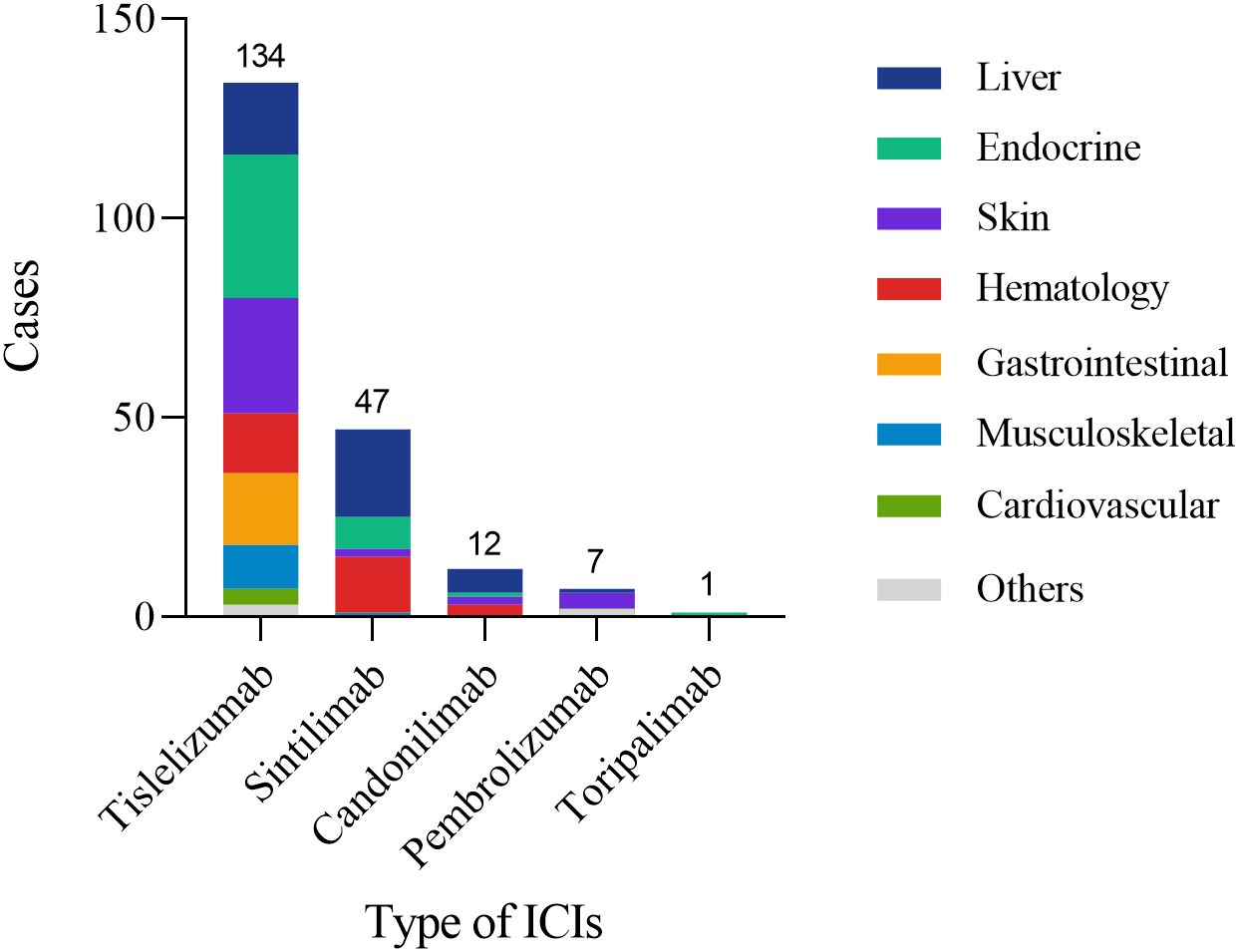
Figure 2. Organ distribution of irAEs among patients receiving different ICIs. The figure displays the number of patients experiencing irAEs for each ICI (Tislelizumab: 134; Sintilimab: 47; Camrelizumab: 12; Pembrolizumab: 7; Toripalimab: 1) and their distribution across affected organ systems (Liver, Endocrine, Skin, Hematology, Gastrointestinal, Musculoskeletal, Cardiovascular, Others).
Surgical approaches and complications
Of the 148 patients, 141 (95.3%) underwent surgical procedures, with laparoscopic surgery as the most commonly employed technique (130 cases, 87.8%). Laparoscopic procedures included the Dixon procedure (64.9%), transanal total mesorectal excision (taTME) (14.9%), and the Miles procedure (2.0%). Seven patients (4.7%) opted for a non-surgical, watch-and-wait approach, mainly driven by a preference for sphincter preservation.
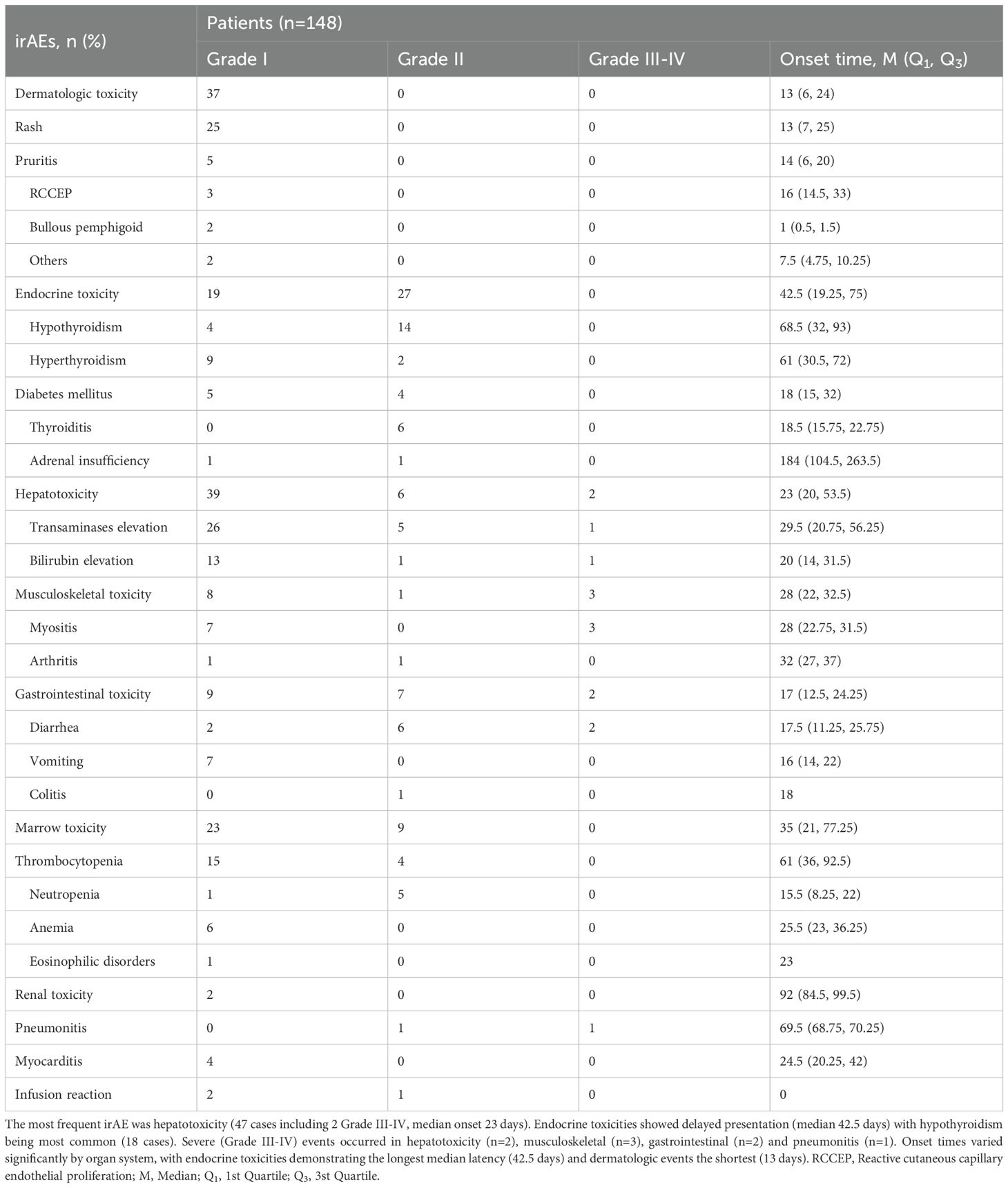
Postoperative complications were observed in 27 patients (19.0%) and were classified according to the Clavien–Dindo system. Among these, 23 cases were Grade I–II complications, while only four patients experienced Grade III–IV complications (Table 3). In terms of intraoperative metrics, Average surgery duration was 192.2 ± 59.8 minutes, and mean blood loss was 47.75 ± 23.65 ml. To assess the influence of irAEs on surgical complexity and outcomes, patients were categorized into two groups based on irAE severity: mild (Grade I) and moderate-to-severe (Grades II–IV). No statistically significant differences between these two groups in terms of surgery duration, intraoperative blood loss, or the incidence of postoperative complications were observed (Table 3). Pathological complete response (pCR) was achieved in 52 patients (36.6%) (Table 3). Additionally, 62 patients (44.0%) received adjuvant therapy, with 5 patients continuing ICIs as part of their adjuvant treatment (Supplementary eTable 1).
Characteristic of irAEs
A total of 203 clinically significant irAEs were documented in this study among 148 patients receiving ICIs, affecting multiple organ systems (Figure 3). Among these patients, 67.6% experienced only one type of irAE, 28.4% (42 patients) had two, and 4.1% (6 patients) experienced three or more irAEs. No specific risk factors were identified for the occurrence of multiple irAEs (Supplementary eTable 5). Most irAEs were mild (Grade I) or moderate (Grade II), affecting various organs such as the skin, endocrine system, liver, and musculoskeletal system, and resolved with standard symptomatic treatments (Table 4, Figure 3).
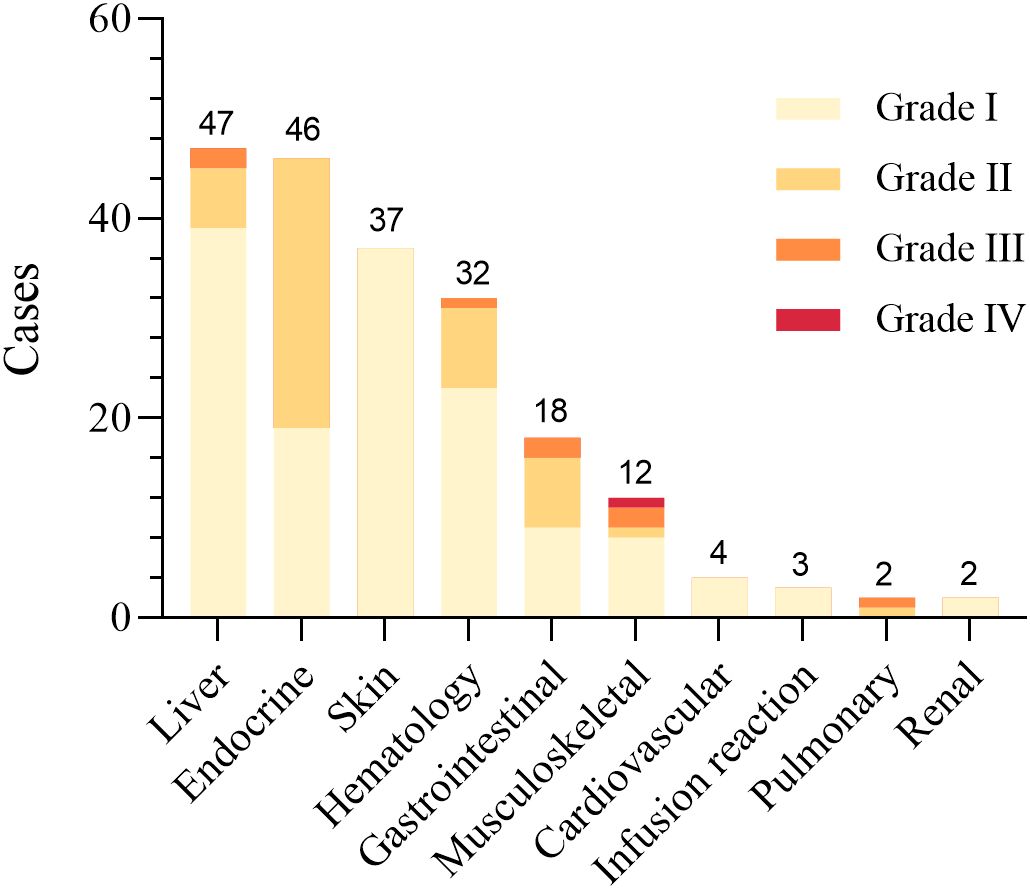
Figure 3. Distribution and severity grading of irAEs by organ systems in CRC patients receiving ICIs. The figure categorizes irAEs into organ systems and presents severity grading (Grade I-IV). The data demonstrate hepatic toxicity as the predominant irAE (n=47), with a subset manifesting severe clinical presentations. Endocrine toxicities (n=46) exhibited exclusively mild-to-moderate severity (Grade I-II), while dermatologic manifestations (n=37) were uniformly classified as Grade I events. Detailed results are shown in Table 4.
Hepatotoxicity, primarily Grade I-II reactions, was the most common irAE, affecting 47 patients (23.2%) with a median onset of 23 days (20, 53.5). 25 patients experienced spontaneous resolution without treatment, and 21 improved with hepatoprotective drugs. One patient developed Grade III transaminase elevation, resolving after multidisciplinary treatment with corticosteroids and immunosuppressants. A higher incidence of hepatotoxicity was observed in patients receiving short-course radiotherapy (SCRT) or the CAPOX chemotherapy regimen, whereas a history of hepatitis or number of immunotherapy cycles was not a risk factor (Supplementary eTable 2). Multivariate analysis showed that combined SCRT was an independent risk factor for hepatic toxicity [p=0.015, OR (95% CI) = 7.92 (1.50 ~ 41.84), Ref: LCRT].
Endocrine irAEs were the second most common, affecting 46 patients (22.7%) with a median onset of 42.5 days (19.25–75), which was significantly later than other irAEs. The most prevalent endocrine irAEs were hypothyroidism (18 cases), hyperthyroidism (11 cases), and thyroiditis (6 cases), often detected as asymptomatic thyroid function abnormalities in routine lab tests. Patients with thyroiditis often progressed from hyperthyroidism to hypothyroidism, requiring hormone replacement. All thyroid dysfunction cases were Grade I-II and did not require treatment modification. In addition, 9 patients developed elevated blood glucose levels, with 5 resolving through lifestyle modifications and 4 requiring oral hypoglycemic medications, though no serious complications like diabetic ketoacidosis occurred. Patients with a history of autoimmune diseases, particularly Hashimoto’s thyroiditis, were at increased risk for endocrine irAEs (Supplementary eTable 3).
Dermatologic toxicities were observed in 35 patients (17.2%), with a median onset of 13 days (6, 24), earlier than most other adverse reactions. Common presentations included rash (25 cases) and pruritus (5 cases), primarily categorized as Grade I, requiring only topical creams or antihistamines for relief. More severe cases, such as bullous pemphigoid (2 cases), led to treatment delays until symptoms were managed. Reactive cutaneous capillary endothelial proliferation (RCCEP), observed exclusively in pembrolizumab-treated patients, resolved spontaneously without intervention. No risk factors were found for skin toxicity (Supplementary eTable 4).
Musculoskeletal irAEs, such as asymptomatic creatine kinase elevations or mild myositis, were observed in 12 patients (5.9%) with a median onset time of 28 (22, 32.5) days. While most cases were mild, three severe instances required high-dose corticosteroids and immunosuppressive therapy due to symptoms like muscle weakness and dysphagia, leading to permanently discontinuation of ICIs. 1 patient with pre-existing osteoarthritis experienced worsening joint pain, managed with corticosteroids.
Additionally, there were some rare but severe adverse events, including cardiac and pulmonary toxicity. In this study, four patients developed mild cardiac toxicity, manifested by elevated myocardial enzymes and slight changes in electrocardiogram, but without obvious clinical symptoms. After discontinuing ICIs, these values gradually returned to normal, and no further treatment was affected. Two other patients experienced pulmonary toxicity, with mild dyspnea and cough, which improved following treatment with corticosteroids and immunoglobulin.
Late-onset irAEs
</u>In total, 46 patients experienced late-onset irAEs, with a median onset of 78 days (range: 63.5–94.0), primarily affecting the endocrine and hepatic systems. The latest recorded case of a late-onset irAE was adrenal insufficiency, observed 343 days after the initiation of immunotherapy. These late-onset irAEs were significantly associated with SCRT and CAPOX therapy combinations (Supplementary eTable 6). However, further multivariate analysis did not identify those as independent risk factors for late-onset irAEs.
Discussion
This study collected clinical information on CRC patients who experienced irAEs during neoadjuvant immunotherapy combined with chemoradiotherapy. Our aim was to analyze the characteristics of these irAEs. To date, this is the largest retrospective clinical study on irAEs associated with neoadjuvant immunotherapy for CRC. Our findings reveal that while irAEs are common in combined treatments, they are manageable and do not increase postoperative complications significantly.
In our study, hepatotoxicity was the most frequent irAE, contrasting with previous studies where dermatologic and endocrine toxicities are predominant in immunotherapy (17). The hepatotoxicity observed in our study, frequently manifesting as asymptomatic transaminase elevations, may be attributed either to direct hepatic injury caused by chemoradiotherapy or to synergistic immune activation resulting from its combination with immunotherapy (18, 19). Compared with LCRT, SCRT regimens may elevate hepatotoxicity risk, possibly through enhanced anti-tumor immune activation (20). For high-risk patients, adjusting the treatment regimen, such as using LCRT, may be advisable. Our study found that patients with autoimmune diseases did not have a higher risk of high-grade irAEs, suggesting that such conditions may not contraindicate immunotherapy. However, both our findings and those of Quan et al. indicate that pre-existing autoimmune diseases may be reactivated or exacerbated by ICIs (21). Thus, enhanced monitoring and management are essential for patients with a history of autoimmune diseases. Our study also found that dermatologic irAEs occurred earlier than other irAEs, while endocrine irAEs had a later onset. This suggests that the timing of irAEs varies greatly across different target organs, which may help us further understand the mechanisms underlying their occurrence.
Despite being PD-1 inhibitors, different ICIs exhibited distinct irAE patterns. Consistent with findings from the TORCH study (22), our data demonstrated a significantly higher incidence of hepatotoxicity with sintilimab treatment, potentially attributable to its frequent combination with SCRT regimens. In contrast, pembrolizumab was predominantly linked to dermatologic toxicities, likely attributable to its characteristic induction of RCCEP. Combining two ICIs targeting different pathways may create a synergistic effect, increasing the severity and range of adverse reactions (23, 24). In our study, a portion of patients were treated with Cadonilimab; however, there was no evidence to suggest that the severity of irAEs was increased in this group. Studies suggest that administering ICIs after radiotherapy may enhance immune response by promoting antigen release from tumor cell destruction (25). However, our study found no significant difference in irAE severity between concurrent and sequential administration of ICIs with chemoradiotherapy, suggesting that both approaches are feasible.
Although some researchers have suggested that ICIs may activate the immune system, potentially leading to enhanced inflammatory responses in surrounding tissues, which could obscure tissue planes and increase surgical difficulty, as well as impair wound healing (26), our study did not find that severe irAEs significantly impact operative time or the incidence of postoperative complications. On the contrary, in our study, the overall incidence of surgery-related complications across all grades in patients who experienced irAEs was lower than the rates reported in other studies for patients receiving neoadjuvant therapy (13, 27). This could be attributed to the relatively low frequency of ICI treatment during neoadjuvant therapy, leading to a milder inflammatory response. Additionally, neoadjuvant therapy significantly reduces tumor size, which may contribute to lowering surgical difficulty. Another concern is that the occurrence of irAEs may potentially delay or even prevent surgery in these patients (28). However, in our cohort, even patients with grade II or higher irAEs did not experience delays or discontinuation of surgery.
The onset of irAEs usually occurs later than chemotherapy and radiotherapy adverse reactions and can arise at any stage of treatment, with the potential for recurrence (29, 30). In our study, 46 patients experienced late-onset irAEs, primarily endocrine toxicities, which is consistent with findings from other studies (31). Some studies have found that ICI combination therapy increases the incidence of late-onset irAEs (32); however, our study did not identify any predictive factors.
Our analysis showed that for most Grade I-II irAEs, symptomatic treatment alone was effective, avoiding delays or cessation of immunotherapy. For Grade III or higher reactions, immunotherapy was paused and managed with corticosteroids or immunosuppressants, yielding favorable outcomes with this severity-based approach. All patients with Grade IV adverse reactions permanently discontinued ICIs. For other patients, ICIs were restarted once adverse reactions subsided to Grade I, with a reassessment of treatment benefits.
In summary, this study characterized the types, severity, and management of irAEs, confirming the safety of ICIs in CRC neoadjuvant treatment. These results will help optimize personalized treatment for CRC by identifying high-risk populations more likely to experience specific irAEs. Patients receiving concurrent chemoradiotherapy may be at higher risk for hepatotoxicity, while those with autoimmune diseases may require closer monitoring. Future research should focus on elucidating the mechanisms of irAEs and identifying predictive biomarkers. Long-term, prospective, and multicenter studies with diverse patient populations, especially older patients and those with comorbidities, are needed to assess the impact of irAE management on survival, quality of life, and the long-term incidence of irAEs.
Limitations
This study has several limitations, primarily due to its retrospective, registry-based design, which may introduce selection and information biases. Additionally, the relatively young and healthy profile of our cohort may limit representativeness for older or comorbid populations, and the short follow-up period may have restricted the detection of late-onset and chronic irAEs.
Conclusion
This study demonstrates the safety and feasibility of combining ICIs with chemoradiotherapy as neoadjuvant treatment for CRC. Most irAEs are manageable, mild-to-moderate in severity, and do not increase postoperative complications with appropriate symptomatic management. Our findings support the development of tailored management plans for CRC patients receiving ICIs and chemoradiotherapy and underscore the importance of continuous monitoring and proactive management of irAEs, particularly for high-risk groups. However, large-scale prospective studies remain necessary to further clarify irAE risks across diverse populations and to optimize personalized treatment strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
The ethics committee of Peking Union Medical College Hospital (PUMCH). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the low-risk nature of the study, its retrospective design, and the use of deidentified data. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article because Given the low-risk nature of the study, its retrospective design, and the use of deidentified data, the requirement for patient consent was waived.
Author contributions
CW: Data curation, Formal Analysis, Methodology, Writing – original draft. QW: Data curation, Investigation, Resources, Writing – review & editing. JZ: Data curation, Methodology, Writing – review & editing. AZ: Data curation, Investigation, Writing – review & editing. XW: Data curation, Investigation, Writing – review & editing. GY: Conceptualization, Data curation, Investigation, Writing – review & editing. LZ: Data curation, Resources, Writing – review & editing. YZ: Data curation, Resources, Writing – review & editing. WC: Methodology, Writing – review & editing. XQ: Investigation, Validation, Writing – review & editing. LS: Software, Writing – review & editing. YG: Software, Writing – review & editing. XZ: Investigation, Validation, Writing – review & editing. GBL: Investigation, Writing – review & editing. YA: Formal Analysis, Investigation, Resources, Writing – review & editing. HC: Investigation, Writing – review & editing. XX: Investigation, Validation, Writing – review & editing. JT: Investigation, Validation, Writing – review & editing. GL: Conceptualization, Funding acquisition, Writing – review & editing. HY: Conceptualization, Data curation, Investigation, Resources, Writing – review & editing. WZ: Data curation, Investigation, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by National High Level Hospital Clinical Research Funding (No. 2022-PUMCH-C-005).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1529637/full#supplementary-material
References
1. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J. (2022) 135:584–90. doi: 10.1097/cm9.0000000000002108
2. Hu H, Kang L, Zhang J, Wu Z, Wang H, Huang M, et al. Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): a single-centre, parallel-group, non-comparative, randomised, phase 2 trial. Lancet Gastroenterol Hepatol. (2022) 7:38–48. doi: 10.1016/S2468-1253(21)00348-4
3. Chalabi M, Fanchi LF, Dijkstra KK, Van den Berg JG, Aalbers AG, Sikorska K, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. (2020) 26:566–76. doi: 10.1038/s41591-020-0805-8
4. Zhu J, Lian J, Xu B, Pang X, Ji S, Zhao Y, et al. Neoadjuvant immunotherapy for colorectal cancer: Right regimens, right patients, right directions? Front Immunol. (2023) 14:1120684. doi: 10.3389/fimmu.2023.1120684
5. Salvatore L, Bensi M, Corallo S, Bergamo F, Pellegrini I, Rasola C, et al. Phase II study of preoperative (PREOP) chemoradiotherapy (CTRT) plus avelumab (AVE) in patients (PTS) with locally advanced rectal cancer (LARC): The AVANA study. J Clin Oncol. (2021) 39:3511–1. doi: 10.1200/JCO.2021.39.15_suppl.3511
6. Wang SJ, Dougan SK, and Dougan M. Immune mechanisms of toxicity from checkpoint inhibitors. Trends Cancer. (2023) 9:543–53. doi: 10.1016/j.trecan.2023.04.002
7. Postow MA, Sidlow R, and Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. New Engl J Med. (2018) 378:158–68. doi: 10.1056/NEJMra1703481
8. Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, et al. Low-dose irradiation programs macrophage differentiation to an iNOS⁺/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. (2013) 24:589–602. doi: 10.1016/j.ccr.2013.09.014
9. Ren Z, Qin S, Meng Z, Chen Z, Chai X, Xiong J, et al. A phase 2 study of camrelizumab for advanced hepatocellular carcinoma: two-year outcomes and continued treatment beyond first RECIST-defined progression. Liver Cancer. (2021) 10:500–9. doi: 10.1159/000516470
10. Rahma OE, Yothers G, Hong TS, Russell MM, You YN, Parker W, et al. Use of total neoadjuvant therapy for locally advanced rectal cancer: initial results from the pembrolizumab arm of a phase 2 randomized clinical trial. JAMA Oncol. (2021) 7:1225–30. doi: 10.1001/jamaoncol.2021.1683
11. Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. (2017) 18:1182–91. doi: 10.1016/S1470-2045(17)30422-9
12. Lin Z, Cai M, Zhang P, Li G, Liu T, Li X, et al. Phase II, single-arm trial of preoperative short-course radiotherapy followed by chemotherapy and camrelizumab in locally advanced rectal cancer. J Immunother Cancer. (2021) 9(11):e003554. doi: 10.1136/jitc-2021-003554
13. Hu H, Zhang J, Li Y, Wang X, Wang Z, Wang H, et al. Neoadjuvant chemotherapy with oxaliplatin and fluoropyrimidine versus upfront surgery for locally advanced colon cancer: the randomized, phase III OPTICAL trial. J Clin oncology: Off J Am Soc Clin Oncol. (2024) 42(25):2978–88. doi: 10.1200/jco.23.01889
14. Morton D, Seymour M, Magill L, Handley K, Glasbey J, Glimelius B, et al. Preoperative chemotherapy for operable colon cancer: mature results of an international randomized controlled trial. J Clin oncology: Off J Am Soc Clin Oncol. (2023) 41:1541–52. doi: 10.1200/jco.22.00046
15. Bando H, Tsukada Y, Inamori K, Togashi Y, Koyama S, Kotani D, et al. Preoperative Chemoradiotherapy plus Nivolumab before Surgery in Patients with Microsatellite Stable and Microsatellite Instability-High Locally Advanced Rectal Cancer. Clin Cancer Res. (2022) 28:1136–46. doi: 10.1158/1078-0432.CCR-21-3213
16. Lerner A, Lee AJX, Yan H, Van Griethuysen J, Bartlett AD, Veli M, et al. A multicentric, retrospective, real-world study on immune-related adverse events in patients with advanced non-small cell lung cancers treated with pembrolizumab monotherapy. Clin Oncol (R Coll Radiol). (2024) 36:193–9. doi: 10.1016/j.clon.2024.01.009
17. Ma B and Anandasabapathy N. Immune checkpoint blockade and skin toxicity pathogenesis. J Invest Dermatol. (2022) 142:951–9. doi: 10.1016/j.jid.2021.06.040
18. Luo Y, Zeng Z, Liu Y, and Liu A. Reflecting on the cardiac toxicity in non-small cell lung cancer in the era of immune checkpoint inhibitors therapy combined with thoracic radiotherapy. Biochim Biophys Acta Rev Cancer. (2023) 1878:189008. doi: 10.1016/j.bbcan.2023.189008
19. Galluzzi L, Humeau J, Buqué A, Zitvogel L, and Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. (2020) 17:725–41. doi: 10.1038/s41571-020-0413-z
20. Morisada M, Clavijo PE, Moore E, Sun L, Chamberlin M, Van Waes C, et al. PD-1 blockade reverses adaptive immune resistance induced by high-dose hypofractionated but not low-dose daily fractionated radiation. Oncoimmunology. (2018) 7:e1395996. doi: 10.1080/2162402x.2017.1395996
21. Quan L, Liu J, Wang Y, Yang F, Yang Z, Ju J, et al. Exploring risk factors for endocrine-related immune-related adverse events: Insights from meta-analysis and Mendelian randomization. Hum Vaccines immunotherapeutics. (2024) 20:2410557. doi: 10.1080/21645515.2024.2410557
22. Xia F, Wang Y, Wang H, Shen L, Xiang Z, Zhao Y, et al. Randomized phase II trial of immunotherapy-based total neoadjuvant therapy for proficient mismatch repair or microsatellite stable locally advanced rectal cancer (TORCH). J Clin oncology: Off J Am Soc Clin Oncol. (2024) 42:3308–18. doi: 10.1200/jco.23.02261
23. Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. (2016) 13:473–86. doi: 10.1038/nrclinonc.2016.58
24. Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. (2009) 15:5379–88. doi: 10.1158/1078-0432.Ccr-09-0265
25. Yang L, Cui X, Wu F, Chi Z, Xiao L, Wang X, et al. The efficacy and safety of neoadjuvant chemoradiotherapy combined with immunotherapy for locally advanced rectal cancer patients: a systematic review. Front Immunol. (2024) 15:1392499. doi: 10.3389/fimmu.2024.1392499
26. Pignot G, Thiery-Vuillemin A, Walz J, Lang H, Bigot P, Werle P, et al. Nephrectomy after complete response to immune checkpoint inhibitors for metastatic renal cell carcinoma: A new surgical challenge? Eur Urol. (2020) 77:761–3. doi: 10.1016/j.eururo.2019.12.018
27. Lin ZY, Zhang P, Chi P, Xiao Y, Xu XM, Zhang AM, et al. Neoadjuvant short-course radiotherapy followed by camrelizumab and chemotherapy in locally advanced rectal cancer (UNION): early outcomes of a multicenter randomized phase III trial. Ann Oncol. (2024) 35:882–91. doi: 10.1016/j.annonc.2024.06.015
28. Ghoreifi A, Vaishampayan U, Yin M, Psutka SP, and Djaladat H. Immune checkpoint inhibitor therapy before nephrectomy for locally advanced and metastatic renal cell carcinoma: A review. JAMA Oncol. (2024) 10:240–8. doi: 10.1001/jamaoncol.2023.5269
29. Emiloju OE and Sinicrope FA. Neoadjuvant immune checkpoint inhibitor therapy for localized deficient mismatch repair colorectal cancer: A review. JAMA Oncol. (2023) 9:1708–15. doi: 10.1001/jamaoncol.2023.3323
30. Goodman RS, Lawless A, Woodford R, Fa'ak F, Tipirneni A, Patrinely JR, et al. Long-term outcomes of chronic immune-related adverse events from adjuvant anti-PD-1 therapy for high-risk resected melanoma. J Clin Oncol. (2023) 41:9591–1. doi: 10.1200/JCO.2023.41.16_suppl.9591
31. Noseda R, Bedussi F, Giunchi V, Fusaroli M, Raschi E, and Ceschi A. Reporting of late-onset immune-related adverse events with immune checkpoint inhibitors in VigiBase. J immunotherapy Cancer. (2024) 12(11):e009902. doi: 10.1136/jitc-2024-009902
Keywords: immune checkpoint inhibitors, colorectal neoplasms, neoadjuvant therapy, drug toxicities, immunotherapy
Citation: Wang C, Wang Q, Zhou J, Zhou A, Wu X, Yu G, Zhou L, Zhu Y, Chen W, Qiu X, Sun L, Gong Y, Zhang X, Li G, An Y, Chen H, Xie X, Tao J, Lin G, Yao H and Zhang W (2025) Insights into immune-related adverse events in colorectal cancer patients receiving neoadjuvant immunotherapy: findings from a multicenter registry study. Front. Immunol. 16:1529637. doi: 10.3389/fimmu.2025.1529637
Received: 17 November 2024; Accepted: 14 May 2025;
Published: 09 June 2025.
Edited by:
Yinghong Wang, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Shiva Nickaria, The University of Manchester, United KingdomJustin Balko, Vanderbilt University Medical Center, United States
Copyright © 2025 Wang, Wang, Zhou, Zhou, Wu, Yu, Zhou, Zhu, Chen, Qiu, Sun, Gong, Zhang, Li, An, Chen, Xie, Tao, Lin, Yao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guole Lin, bGluZ3VvbGVAMTI2LmNvbQ==; Hongwei Yao, eWFvaG9uZ3dlaUBjY211LmVkdS5jbg==; Wei Zhang, d2VpemhhbmcyMDAwY25AMTYzLmNvbQ==
†These authors have contributed equally to this work
 Chentong Wang1†
Chentong Wang1† Quan Wang
Quan Wang Aiping Zhou
Aiping Zhou Xiaojian Wu
Xiaojian Wu Guanyu Yu
Guanyu Yu Yuping Zhu
Yuping Zhu Weijie Chen
Weijie Chen Liting Sun
Liting Sun Xiao Zhang
Xiao Zhang Ganbin Li
Ganbin Li Guole Lin
Guole Lin Hongwei Yao
Hongwei Yao Wei Zhang
Wei Zhang