- Department of Dermatology, Xijing Hospital, Fourth Military Medical University, Xi’an, Shaanxi, China
The B cell-activating factor (BAFF) system, comprising two ligands and three receptors, plays a pivotal role in adaptive and innate immunity, driving autoimmunity through dysregulated B and T cell survival, differentiation, and cytokine production. This review synthesizes evidence linking BAFF system overexpression to multiple autoimmune diseases, including systemic lupus erythematosus (SLE), Sjögren’s syndrome (SS), bullous pemphigoid (BP), pemphigus vulgaris (PV), and alopecia areata (AA), where elevated BAFF system molecule levels correlate with autoantibody titers, disease activity, and post-B cell depletion relapse. BAFF-targeted therapies have demonstrated efficacy in reducing disease activity in SLE and SS. Key challenges include interspecies receptor expression discrepancies and context-dependent signalling cascades. Emerging strategies, such as sequential therapy with rituximab followed by belimumab, show promise in treating refractory autoimmune diseases such as BP and PV by counteracting the post-depletion BAFF surge. Despite progress, mechanistic gaps in BAFF-mediated crosstalk between innate and adaptive immunity, as well as interspecies-specific pathogenesis warrant further investigation using humanized disease models and single-cell transcriptomic profiling. This review underscores the therapeutic potential of BAFF system modulation while advocating for disease-specific clinical trials to optimize precision-therapeutic targeting in autoimmune diseases.
1 Introduction
The B cell-activating factor (BAFF) system can be synthesized in various cell types and consists of two ligands, BAFF and A proliferation-inducing ligand (APRIL), along with three receptors, BAFF receptor (BAFFR), B cell maturation antigen (BCMA), and transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) (1). As a key component of the BAFF system, BAFF was originally discovered as a fundamental survival cytokine for B cells. BAFF can maintain B cell survival, autoreactive B cell selection, class switch recombination, and the maintenance of long-lived plasma cells (PC) (2). Additionally, increasing evidence has shown that the BAFF system can promote T cell survival, differentiation, as well as regulate other immune cells (3–8).
Research in both humans and mouse models has identified that the BAFF system is a vital player in autoimmunity pathogenesis (9, 10). Overexpression of BAFF system molecules has been detected in patients with various types of autoimmune diseases, including systemic lupus erythematosus (SLE), Sjögren’s syndrome (SS), systemic sclerosis (SSc), bullous pemphigoid, pemphigus vulgaris, and alopecia areata, and may be involved in the pathogenesis of these diseases (11–20). Therefore, BAFF system molecules are considered potential therapeutic targets for autoimmune diseases. Monoclonal antibodies have been developed to target single or dual BAFF system molecules, and belimumab has been approved for use in SLE (21). Moreover, several clinical studies on these mAbs are underway, with positive results for SS and SSc (22–24).
In this review, we describe the key structural and biological features of the BAFF system as well as its functional implications in the pathogenesis of autoimmune diseases. We also highlight that therapies targeting the BAFF system are a promising strategy for treating different autoimmune diseases and warrant further investigation.
2 B cell-activating factor system
2.1 Ligands
B cell-activating factor (BAFF) and its structural homologue A proliferation-inducing ligand (APRIL) are type II transmembrane proteins belonging to the tumour necrosis factor (TNF) cytokine superfamily (25, 26). Although they share the same structure, their functions are quite different. BAFF can be synthesized as a membrane-bound form (mBAFF) and converted into a secreted form (sBAFF), which is the main form in circulation (27, 28). BAFF is mainly expressed by immune cells, such as monocytes, macrophages, dendritic cells, and neutrophils (29, 30). Moreover, BAFF can be produced by non-immune cells, including adipocytes, keratinocytes, and intestinal epithelial cells (31, 32). BAFF expression is upregulated by interleukin (IL)-10, interferons (IFNs), toll-like receptor (TLR) agonists, granulocyte colony-stimulating factor (G-CSF), and by the activation of interferon regulatory factors (IRFs), such as IRF1 and IRF2. Conversely, IRF4 and IRF8 negatively regulate BAFF expression (33, 34). APRIL can also be synthesized by various types of immune cells in both membrane-bound and secreted form. Similarly, APRIL expression is regulated by IL-10, IFNs, G-CSF, TLR, and IRFs (9, 35–37).
2.2 Receptors
BAFF receptor (BAFFR), B cell maturation antigen (BCMA), and transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) are type III membrane proteins with distinct but complementary effects (38, 39). These three receptors, like their ligands, can be converted from the membrane-bound form into the secreted form (40–42). BCMA and TACI share two common ligands, BAFF and APRIL, whereas BAFFR has only one ligand, BAFF (43, 44). Moreover, BCMA shows a lower affinity for BAFF compared with that for APRIL, whereas TACI has an equal affinity for both ligands (45). In contrast to the widespread expression of ligands, the expression of these three receptors is restricted to specific immune cells. Although B cells can express all three receptors, their expression varies during B cell maturation stage (37, 46, 47). In contrast to B cells, human T cells only express BAFFR, whereas mouse T cells express BAFFR and TACI (47–49).
2.3 B cell-activating factor function in autoimmunity
B cell-activating factor (BAFF) system molecules modulate a variety of biological processes, including cell survival, differentiation, and other effector functions, as demonstrated in some autoimmune diseases, such as systemic lupus erythematosus (SLE), Sjögren’s syndrome (SS), and systemic sclerosis (SSc) (50–52). BAFF receptor (BAFFR) interaction with BAFF activates both the classical and alternative transcription factor nuclear factor-kappa B (NF-κB) pathways, whereas B cell maturation antigen (BCMA) and transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) linked to BAFF only activates the classical NF-κB pathway (29, 53). Furthermore, BAFF activates the phosphoinositide-3-kinase (PI3K) dependent signalling cascade to support cell survival (54) (Figure 1). However, most immunobiological findings related to molecules of the BAFF system have been obtained using transgenic mouse models.
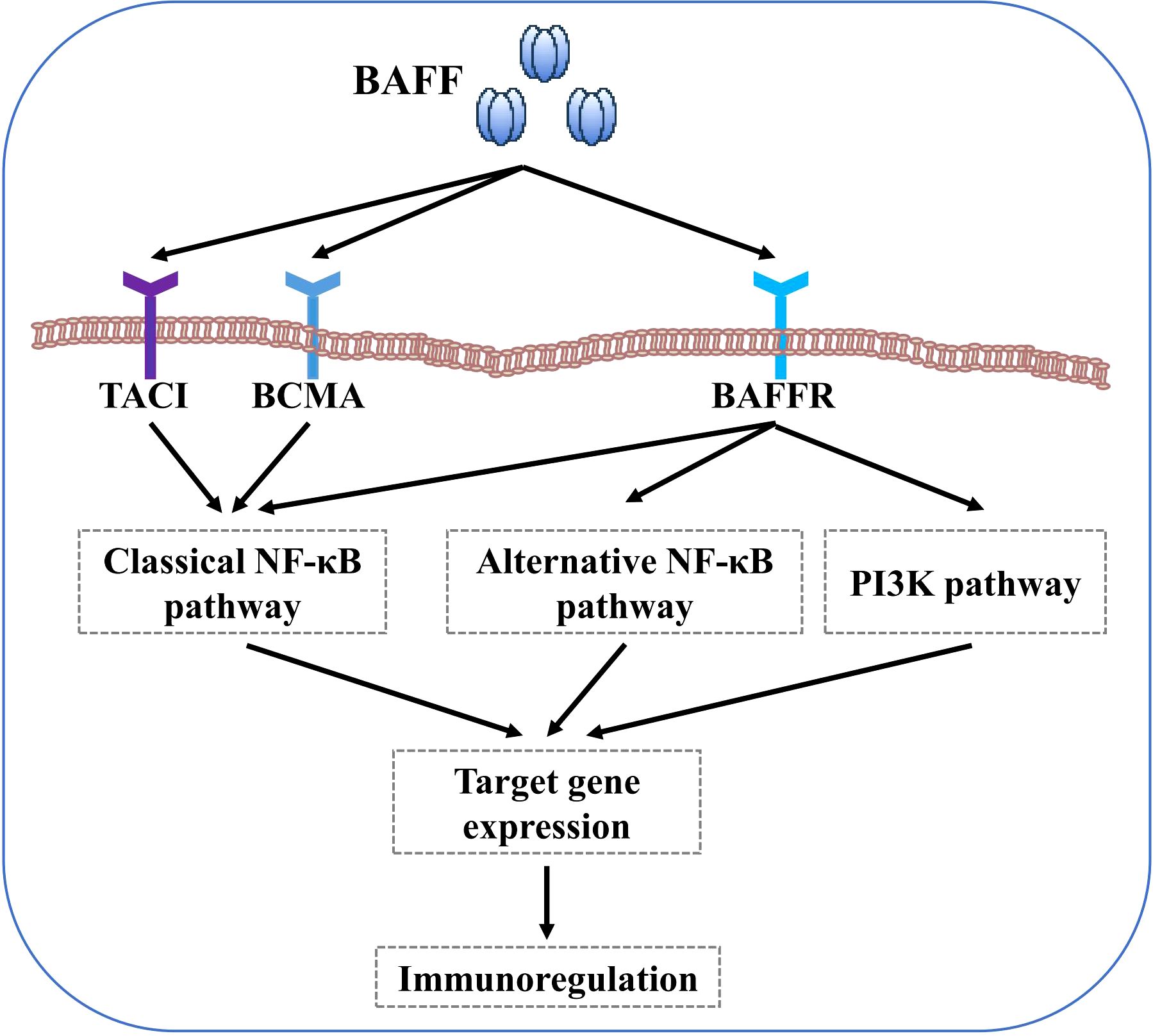
Figure 1. BAFF signaling pathways. BAFF binding to BAFFR activates both the classical and alternative NF-κB pathways. In contrast, BAFF interaction with BCMA and TACI only activates the classical NF-κB pathway. Furthermore, BAFF binding to BAFFR also activates the PI3K pathways.
2.3.1 Function of BAFF in adaptive immunity
BAFF system molecules play an important role in adaptive immunity (Figure 2). As discussed in Section 2.2, the differential expression of the three receptors during B cell maturation stage is related to their individual functions. BAFFR expression begins in transitional B cells, preventing premature apoptosis via BAFFR-dependent pro-survival signals (47, 55–58). BCMA expression is restricted to long-lived bone marrow plasma cell (PC) and plasmablasts, where it supports PC formation, maintenance, and differentiation while preserving antigen presentation (59–63). TACI is mostly expressed by marginal zone B cells, activated B cells, switched memory B cells, and PCs. TACI negatively regulates early B cell maturation and mediates PC generation, maintenance, and differentiation, as well as T cell-independent immunoglobulin (Ig) isotype conversion and release (64–68). Mouse models underscore BAFF’s essential role in B cell physiology, responsiveness, and autoimmunity. BAFF transgenic mice (BAFF Tg mice), which overexpress BAFF, exhibit SLE and SS-like manifestations, such as increased peripheral mature B cell numbers, immune globulin (Ig) deposits in the kidney, and enlarged lymphoid organs (69). Conversely, BAFFR-mutant or BAFF-deficient mice show significantly reduced peripheral mature B cells and impaired immune responses (38, 70–72). Compared to BAFF, APRIL overexpression or APRIL deficiency does not cause remarkable abnormalities during B cell maturation (73, 74). Additionally, APRIL-deficient mice show impaired class-switching to IgA and enhanced IgG responses to T-dependent antigens (33, 75). TACI-deficient mice exhibit pseudo-autoimmune traits, with increased B cell numbers, elevated autoantibody-producing cells, and diminished T cell-independent humoral responses, indicating that TACI may negatively regulate B cells (65, 76, 77). Finally, the vital role of BCMA in the survival of long-term bone marrow PCs has been confirmed in BCMA-deficient mice (59). Despite significant advances in understanding BAFF’s effects on B cells, many aspects require further investigation.
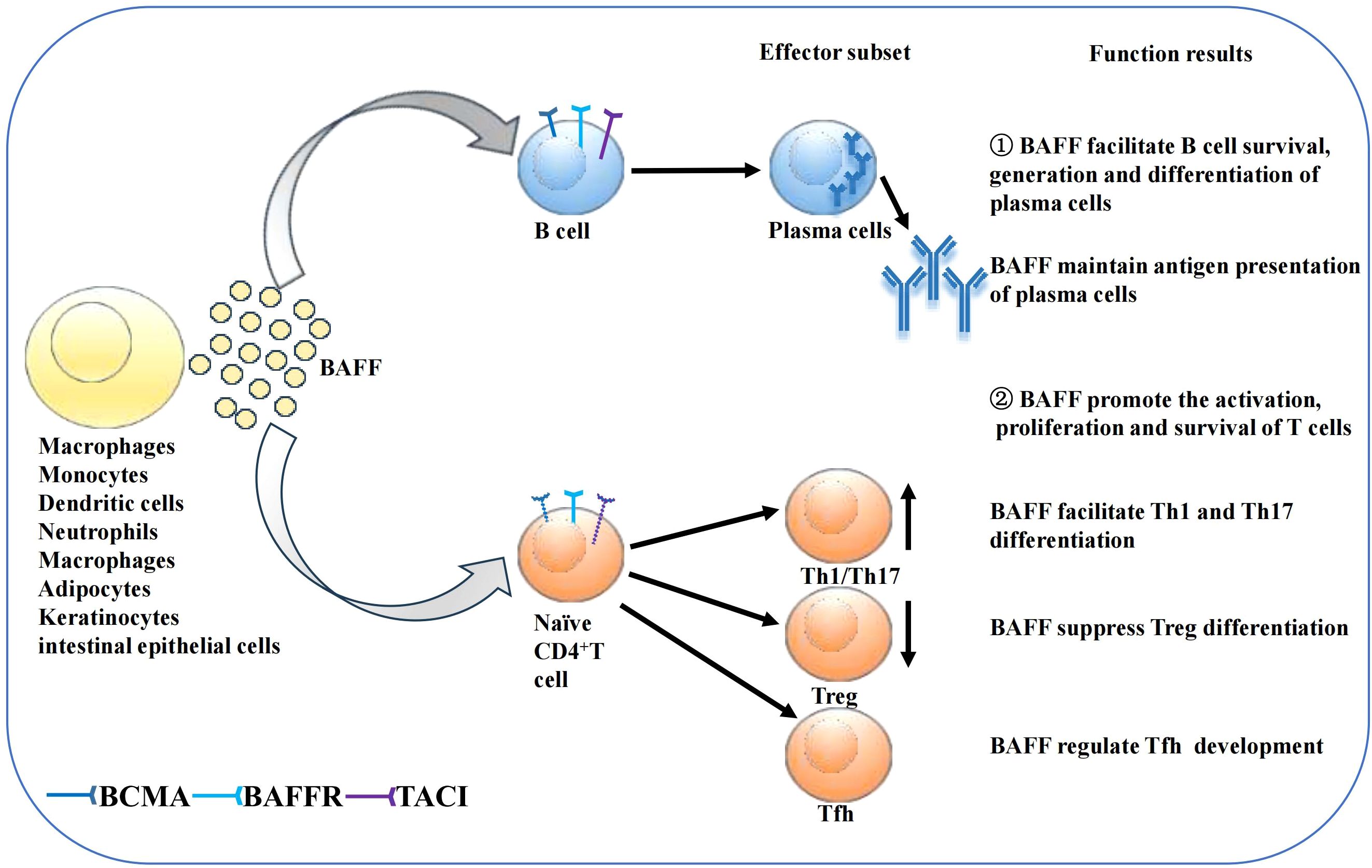
Figure 2. Function of BAFF on B cells and T cells. Secreted BAFF can be synthesized by several kinds of cells as shown in the figure. BAFF then promotes B cell survival, generation and differentiation of plasma cells, supports antigen presentation of PCs. BAFF also affects T cell survival and proliferation. BAFF may promote the differentiation of Th1 and Th17 cells while suppressing Treg differentiation. Moreover, BAFF regulate Tfh development.
The BAFF system also affects T cell activation, proliferation, and survival by acting as a costimulatory signal together with TCR in both effector and naïve T cells (4, 78, 79). BAFF-deficient mice develop reduced quantities of effector T cells, while APRIL-deficient mice show normal proliferation, differentiation, and T cell function (74, 80). BAFF augments T cell stimulation by increasing costimulatory molecules expression in antigen-presenting cells and by upregulating the expression of BAFFR and TACI in murine T cells (3, 81). BAFF, which interacts with BAFFR on T cells, can promote the activation and proliferation of CD4+ T cells through the PI3K/Akt pathway (3, 49). However, treatment with anti-BAFFR antibodies enhances the cytolytic function of human CD4+ and CD8+ T cells, a discrepancy likely attributable to varying BAFF concentrations (82). Notably, BAFF may facilitate T-helper (Th) 1 and Th 17 cell differentiation and suppress regulatory T cell differentiation (83–86). Moreover, the BAFF system regulates follicular helper T (Tfh) cells through the noncanonical NF-κB pathway by mediating the expression of inducible costimulatory ligand expression on B cells (87). TACI-deficient mice exhibit increased quantities of Tfh cells in their spleens after T cell-dependent antigen immunisation, largely owing to the upregulation of inducible costimulatory ligand on TACI-deficient B cells (88). However, the effect of the BAFF system on T cells remains unclear and requires further investigation.
2.3.2 Function of BAFF in innate immune cells
Although evidence is minimal, BAFF also affects other immune cells. Monocytes, which are a source of BAFF, can also be regulated by BAFF. Along with augmented release of proinflammatory cytokines, BAFF strongly promotes monocyte survival and differentiation into macrophages by activating the NF-κB pathway (6, 89). It induces human myeloid dendritic cell maturation, increasing costimulatory molecule expression and inflammatory cytokine secretion (7). Furthermore, regulation of the BAFF system in megakaryocytic cells and natural killer (NK) cells has also been reported. However, no BAFF receptors are expressed on NK cells, suggesting that NK cells are indirectly regulated by BAFF (8, 90, 91).
2.3.3 Future directions on BAFF
Although BAFF system molecules play a central role in regulating adaptive immunity, their context-specific mechanisms remain poorly understood. A major translational challenge arises from species-specific differences in receptor expression: BAFFR is found exclusively on human T cells, whereas murine models show co-expression of BAFFR and TACI in T cells. These discrepancies complicate the extrapolation of findings from animal studies to human biology. Additionally, paradoxical observations—such as anti-BAFFR antibodies enhancing human T cell cytotoxicity while BAFF simultaneously promotes Th17 polarization—highlight the complexity of BAFF-mediated signaling pathways. To bridge this translational gap, B-hBAFF/hBAFFR transgenic mouse models could provide a critical platform. These models would allow researchers to simulate human-specific receptor signaling by selectively blocking interference from TACI in murine T cells. Such systems would be particularly valuable for studying BAFF-driven pathologies like SLE -associated kidney damage or SS salivary gland dysfunction, enabling direct validation of therapeutic targets in a humanized context. Cutting-edge technologies like spatial transcriptomics combined with single-cell ATAC-seq could further clarify the spatiotemporal dynamics of BAFFR, TACI, and BCMA expression within disease microenvironments. For example, mapping these receptors in SLE renal follicular regions might reveal how they coordinate with Tfh cell expansion and plasma cell differentiation, offering mechanistic insights into disease progression. Beyond adaptive immunity, BAFF also influences innate immunity by modulating myeloid cells and indirectly regulating NK cells. However, its dual roles in driving inflammation versus supporting tissue repair remain unclear. Future studies should prioritize myeloid-specific BAFFR knockout models to dissect BAFF’s effects on macrophage and dendritic cell function. Equally important is investigating receptor-independent BAFF-NK interactions, such as potential signaling through heparan sulfate proteoglycans or extracellular vesicles, which could uncover novel immunoregulatory pathways. By addressing these gaps, researchers can unravel BAFF’s multifaceted roles in immunity and inflammation, paving the way for targeted therapies in autoimmune and inflammatory diseases.
3 Pathogenic role of the B cell-activating factor system in autoimmune diseases
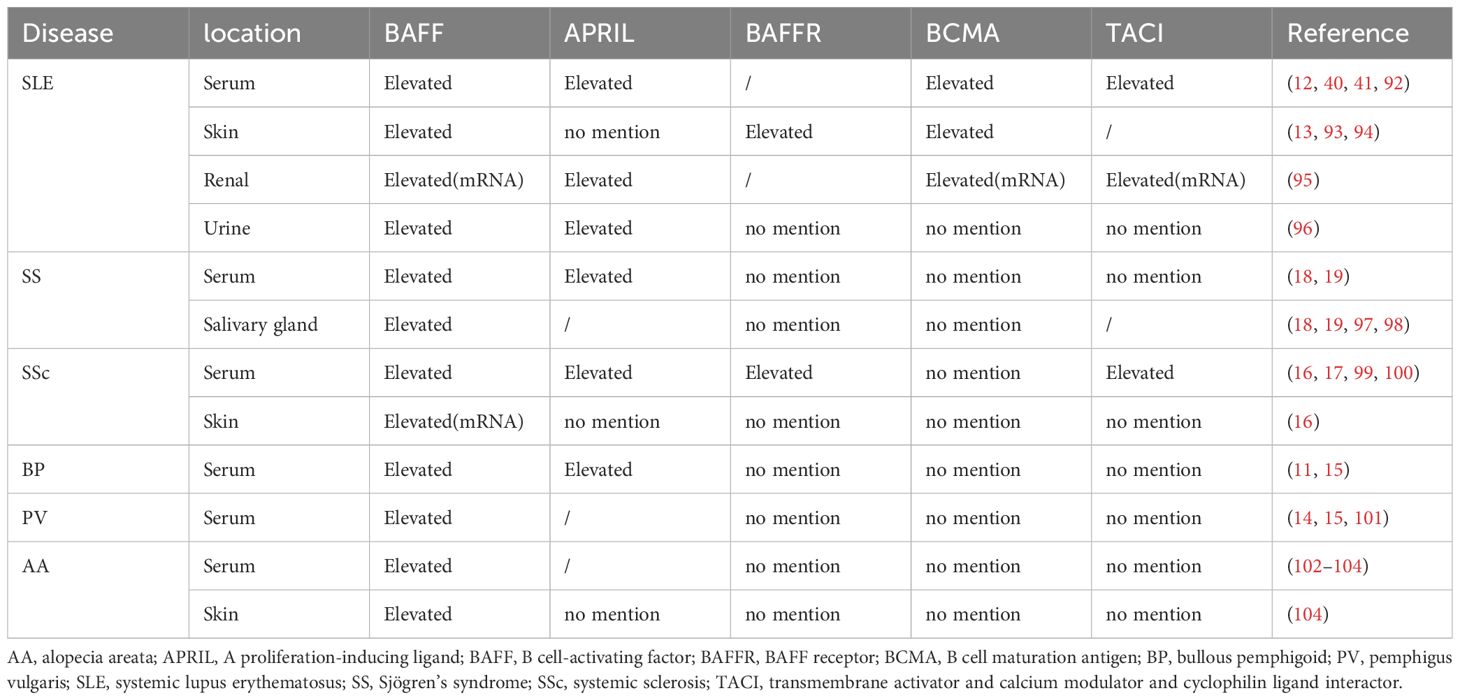
Overexpression of B cell-activating factor (BAFF) system molecules has been detected in patients with various types of autoimmune diseases, such as systemic lupus erythematosus (SLE), Sjögren’s syndrome (SS), systemic sclerosis (SSc), bullous pemphigoid (BP), pemphigus vulgaris (PV), and alopecia areata (AA) (Figure 3). Additionally, elevated circulating BAFF levels correlate with autoantibody titers in patients with SLE and SSc. Furthermore, studies on animal models have highlighted that BAFF system molecules participate in regulating immune cells, promoting systemic autoimmunity, and mediating the occurrence and development of autoimmune diseases. Here, we discuss the expression and the roles of BAFF system molecules in autoimmune diseases pathogenesis (Table 1).
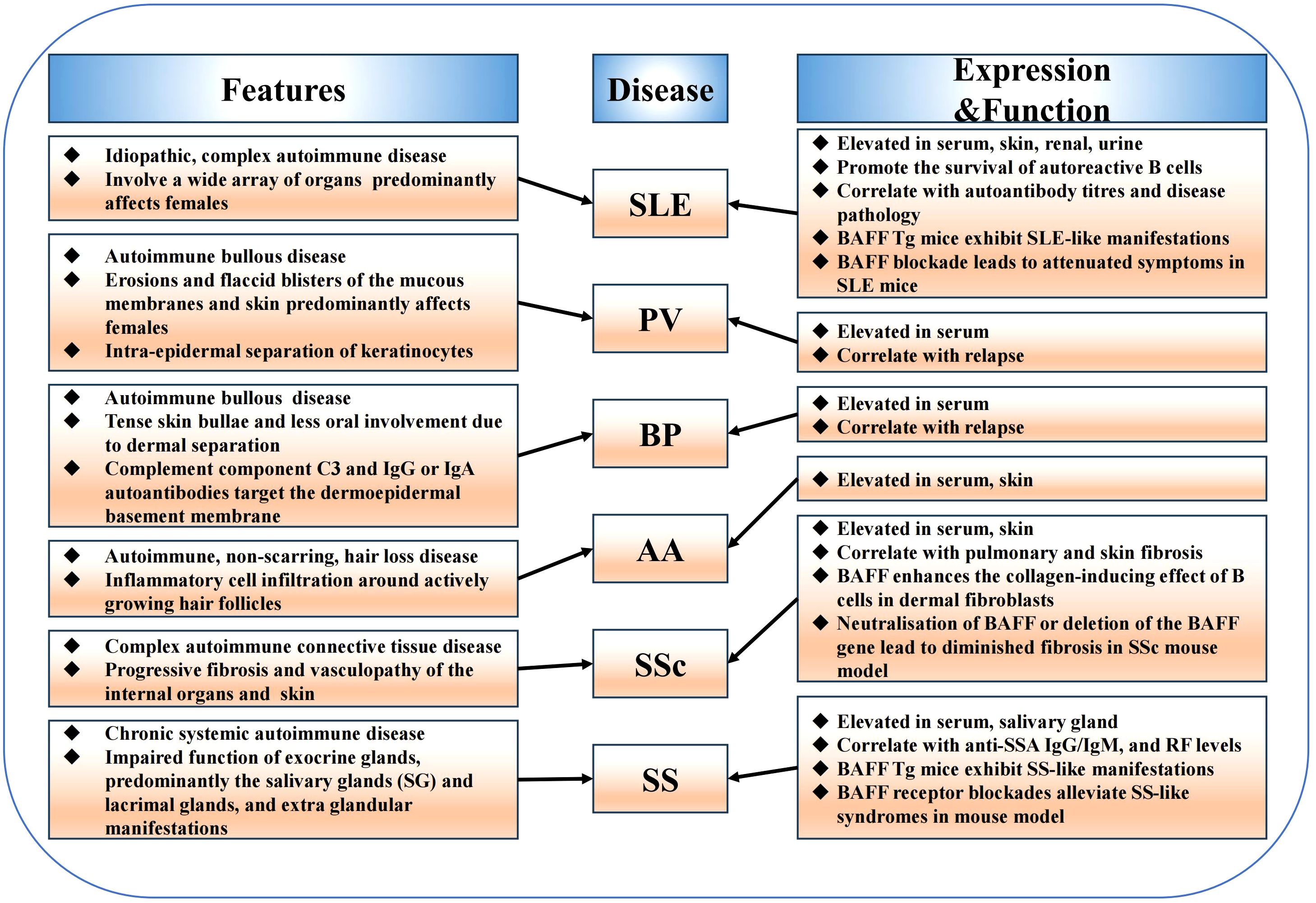
Figure 3. The function of BAFF system in autoimmune diseases. BAFF system molecules are elevated in various autoimmune diseases including systemic lupus erythematosus, Sjögren’s syndrome, systemic sclerosis, bullous pemphigoid, pemphigus vulgaris, alopecia areata, and are involved in the pathogenesis of these diseases. Furthermore, mouse models have highlighted the role of the BAFF system in regulating immune cells, promoting systemic autoimmunity, and mediating the occurrence and development of autoimmune diseases.
3.1 Systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is an idiopathic, complex autoimmune disease that involves a wide array of organs and predominantly affects females (105). Approximately 70% of the patients experience some degree of skin involvement (106). Elevated BAFF levels have been detected in the skin, serum, urine, and kidneys of patients with SLE, with correlations to disease pathology and autoantibody titres in both human and murine models (12, 107–110). However, the correlation between circulating BAFF levels and anti-double-stranded DNA (anti-dsDNA) titers remains controversial. This contradiction may be attributed to the different detection methods (111, 112). BAFF Tg mice with BAFF overexpression exhibit SLE-like manifestations, such as hypergammaglobulinemia, increased serum immune complexes, levels of rheumatoid factor (RF) and anti-dsDNA, as well as renal Ig deposits (69). Moreover, BAFF overexpression has been observed in other spontaneous SLE-prone mice (39). Consistently, BAFF blockade attenuates symptoms and disease activity in SLE mice, thereby improving survival (113, 114). Excess BAFF may participate in the development of SLE through supporting autoreactive B cells survival (115). Lupus nephritis (LN) is a severe complication of SLE, characterized by kidney inflammation due to autoimmune-mediated damage, primarily affecting the glomeruli. Glomerular APRIL and BAFF levels are significantly elevated in patients with LN (95). Furthermore, BAFF levels in the kidneys of LN mice are correlated with disease activity and the histopathological activity index (116). BAFF promotes LN by inducing a tertiary lymphoid structure in the kidney and modulating the position of glomerular T cells (117). In addition to LN, cutaneous symptoms are also observed in many patients with SLE. The levels of BAFF and its three receptors—BAFFR, BCMA, and TACI—are increased in patients with cutaneous lupus erythematosus (CLE). In these patients, BAFF is mainly expressed in keratinocytes, whereas the three receptors are mainly expressed in the lymphoid cells. Moreover, BAFF expression is significantly upregulated after stimulation with immunostimulatory DNA motifs in cultured keratinocytes (13, 94, 118).
3.2 Sjögren’s syndrome
Sjögren’s syndrome (SS) is a chronic systemic autoimmune disease characterized by impaired function of exocrine glands and extra glandular manifestations (119). Notably, patients with SS exhibit elevated serum and SG levels of BAFF. In addition, increased BAFF levels are strongly correlated with anti-SSA IgG/IgM and RF levels (18, 98, 120). As mentioned in Section 3.1, BAFF Tg mice overexpressing BAFF exhibit SLE-like manifestations. However, as they age, these mice acquire features of SS, such as glandular inflammation and structural destruction (18). Early BAFFR blockade alleviates SS-like syndromes in mice (121). Moreover, salivary epithelial cells in patients can secrete different forms of BAFF to participate in the pathogenesis of SS, and BAFF can also promote epithelial cell survival through autocrine signalling (122). Additionally, studies in patients with SS and mouse models have shown that BAFF is involved in the formation of germinal centre-like structures, which are important in SS pathogenesis (123–126).
3.3 Systemic sclerosis
Systemic sclerosis (SSc) is a complex autoimmune connective tissue disease characterized by progressive fibrosis and vasculopathy of the internal organs and skin (127). The serum levels of APRIL and BAFF are increased in patients with SSc and positively correlated with skin and pulmonary fibrosis, respectively (16, 17). Furthermore, adding anti-IgM and BAFF to a co-culture of dermal fibroblasts and peripheral B cells isolated from patients with SSc showed that BAFF enhanced the collagen-inducing effect of B cells in dermal fibroblasts (128). Tight skin (TSK/+) mice, which are genetic a murine model of SSc, develop cutaneous fibrosis and autoimmunity. Intriguingly, circulating BAFF levels and skin fibrotic cytokines are elevated in the TSK/+ mouse model. Moreover, BAFF antagonists enhance the expression of anti-fibrotic cytokines, therefore inhibiting autoantibody production, skin fibrosis, and fibrotic cytokine expression in TSK/+ mice (129). Notably, neutralisation of BAFF or deletion of the BAFF gene led to diminished fibrosis in a bleomycin-induced model of pulmonary fibrosis (130). Furthermore, a small pilot study using BLM to suppress BAFF showed an improvement in skin hardening in patients with SSc (51). The results of these studies show that the pathogenesis of SSc is complicated and involves various environmental and genetic factors, warranting further investigation in future studies.
3.4 Bullous pemphigoid
Bullous pemphigoid (BP) is an autoimmune bullous skin disease characterized by tense skin bullae and less oral involvement. These manifestations may be due to the presence of complement component C3 and IgG autoantibodies, which target the dermo–epidermal basement membrane, resulting in dermal separation (131). One study revealed increased levels of circulating BAFF in patients with BP (15). Furthermore, flow cytometric analysis confirmed elevated BAFF expression in memory and naïve B cells in patients with BP (132). Conversely, another study found that the levels of circulating BAFF molecules in healthy controls were comparable with those in patients with BP (133). This discrepancy is likely attributable to differences in disease duration. Serum APRIL levels are also increased in patients with BP (11). RTX is a third-line treatment for BP and has clinical benefits for severe BP. One study found that serum BAFF level increased after RTX treatment in patients with BP. Additionally, serum BAFF levels increased before the peripheral B cell number returned to normal, implying a relapse (134). Dipeptidyl peptidase 4 inhibitors (DPP4is), commonly used for the treatment of type 2 diabetes, increase the risk of BP (DPP4is-associated BP). BAFF expression is higher in regular BP skin (not associated with DPP4is) compared to that in DPP4i-associated BP skin (135). However, few studies have explored the role of BAFF in BP pathogenesis, with studies largely restricted to phenomenological observations. Thus, further investigation is required to determine whether and how BAFF is involved in BP pathogenesis.
3.5 Pemphigus vulgaris
Pemphigus vulgaris (PV) is an autoimmune bullous disease characterized by erosions and flaccid blisters of the skin and the mucous membranes owing to the intra-epidermal separation of keratinocytes (136). While some studies report elevated serum APRIL levels in patients with PV, others show no significant differences, casting doubt on its role (11, 14). A similar inconsistency is observed with serum BAFF levels in PV (15, 101, 137), likely due to variations in sample sizes across studies. Rituximab (RTX), a targeting B-lymphocyte CD20 mAb, is the most common B cell-depleting therapy for bullous dermatoses. One study found that circulating BAFF levels were significantly increased after RTX treatment, which normalised upon the recovery of peripheral CD19+ B cells (138). Another reported higher baseline BAFF levels in patients with PV than in healthy controls, with levels rising further after 3 months of RTX treatment, suggesting a link between BAFF and PV immunopathogenesis (137). Although the B cell-depleting agent RTX is effective for patients with PV, relapses are frequent. Hebert et al. observed that most relapses occurred precisely when autoreactive B cells reappeared and BAFF serum levels increased, suggesting that relapse after RTX therapy might be attribute to a raise in circulating BAFF levels and the reappearance of autoreactive B cells (101). Taken together, the role of the BAFF system in PV remains unclear, and further studies are required to elucidate its role in PV.
3.6 Alopecia areata
Alopecia areata (AA) is a non-scarring autoimmune hair loss disorder characterized by inflammatory cell infiltration around actively growing hair follicles (20). Circulating BAFF levels are elevated in patients with AA with more than three lesions (102). This phenomenon was confirmed by a later study, which also revealed increased tissue BAFF levels in patients with AA. Furthermore, BAFF and TH17 synergistically participate in the pathogenesis of AA (104). While research on BAFF’s role in AA remains limited, its significance cannot be overlooked.
4 Targeting B cell-activating factor system molecules for autoimmune diseases therapy
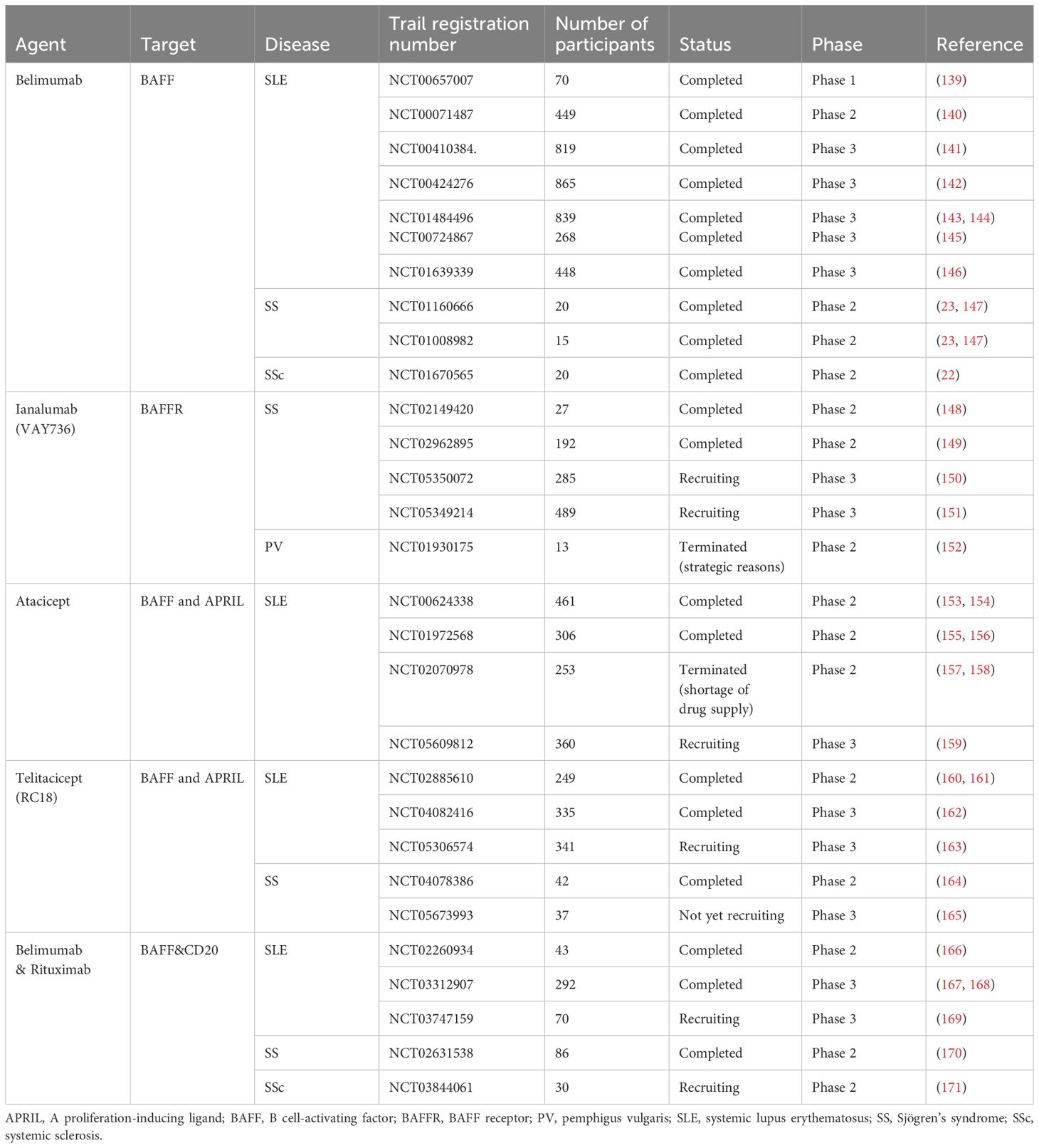
Since their discovery in 1999, studies targeting single or dual B cell-activating factor (BAFF) system molecules have been conducted (Figure 4). Belimumab (BLM) is a fully humanised recombinant IgG1λ mAb that antagonises the biological activity of sBAFF by preventing its interaction with receptors. BLM was approved for systemic lupus erythematosus (SLE) therapy in 2011, suggesting that BAFF system molecules are potential targets for the treatment of several autoimmune diseases. Several clinical trials are ongoing, with encouraging results for sjögren’s syndrome (SS) and systemic sclerosis (SSc), reinforcing BAFF-targeting therapies as a potential strategy for autoimmune diseases treatment. Below, we summarize several BAFF system-targeting therapies for autoimmune diseases (Table 2).
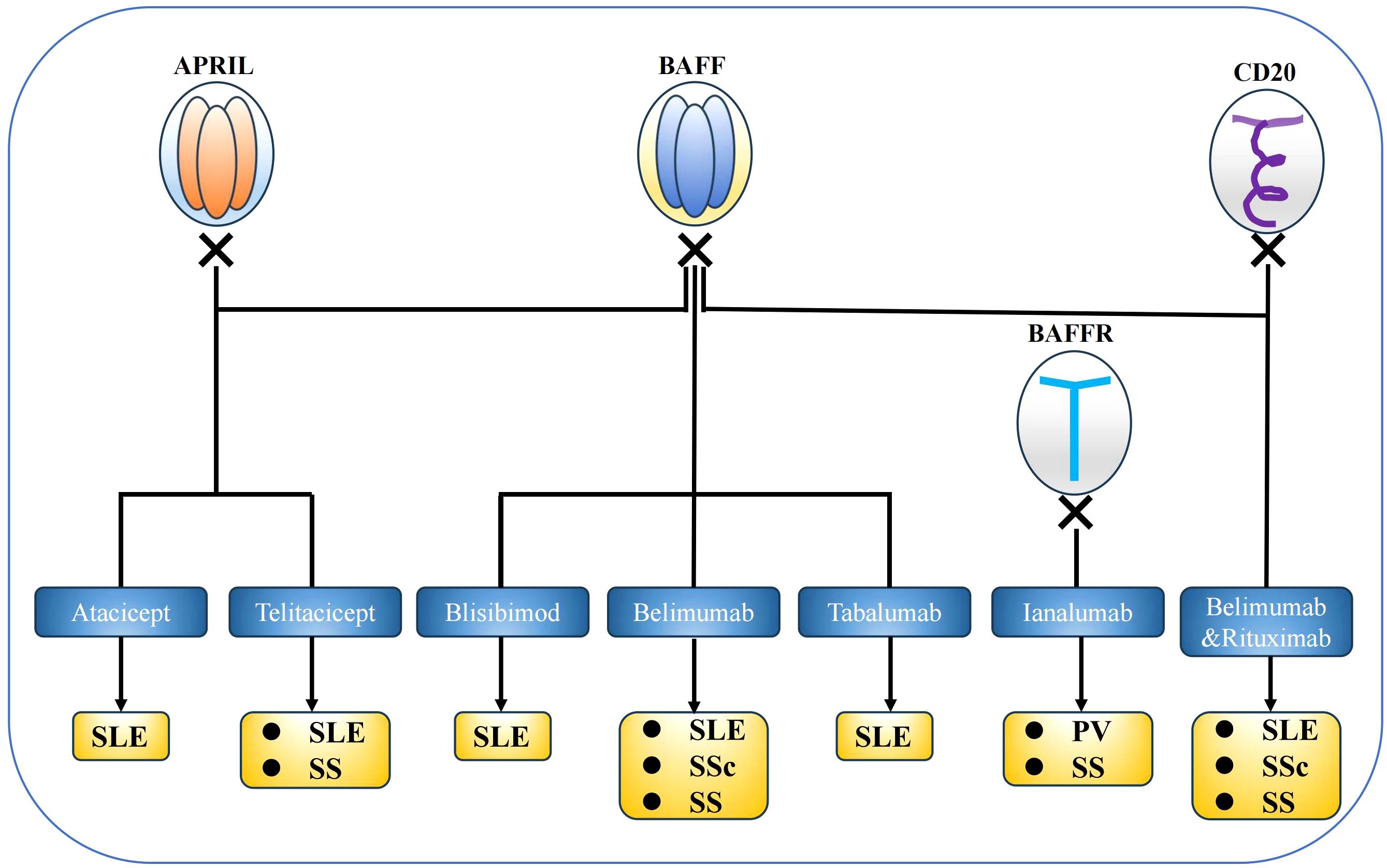
Figure 4. BAFF system targeted therapies. Several experiments that target BAFF system molecules have been conducted in mouse models and clinical settings, and belimumab was approved for systemic lupus erythematosus therapy. BAFF system molecules are considered potential targets for the treatment of several autoimmune diseases.
4.1 B cell-activating factor-targeted therapies
The efficacy and safety of belimumab (BLM) in systemic lupus erythematosus (SLE) were validated across multiple pivotal clinical trials (139–146). In phase III trials for active SLE, intravenous BLM combined with standard therapy significantly outperformed placebo in SLE Responder Index (SRI) rates at week 52 in the BLISS-76 and BLISS-SC trials (141, 142). Subcutaneous administration further improved outcomes in moderate-to-severe SLE, with an SRI-4 response rate of 61.4% vs 48.4% and a 49% reduction in severe flare risk (143). Notably, hypocomplementemic/anti-dsDNA+ patients exhibited enhanced benefits alongside corticosteroid-sparing effects (144). Long-term extension data confirmed sustained efficacy over 7 years: SRI response increased from 41.9% (year 1) to 75.6% (year 7), with 31.4% mean prednisone reduction and 83.2% CD20+ B cell depletion, while maintaining stable safety profiles (145). In LN management, BLM combined with standard therapy significantly improved primary and complete renal responses, reducing renal-related event/death risk by 49% (146). These findings led to FDA approval for SLE in 2011 and an expanded indication for active LN in 2020, cementing BLM as a key LN therapy (146, 172).
The use of BLM in patients with sjögren’s syndrome (SS) has also been assessed. In a clinical study, there was a significant improvement in clinical manifestations and biomarkers of B cell activation in patients with SS who were treated long-term with BLM (23, 147). Furthermore, a clinical study assessed the safety and efficacy of BLM in patients with SSc who received background mycophenolate mofetil. This study found a significantly improvement of clinical symptoms in the BLM group compared with the placebo group (22). Overall, the results are encouraging and justify further randomised controlled studies with larger populations.
The use of BLM after rituximab (RTX) for the treatment of SLE with bullous pemphigoid (BP) has also been reported. For example, one patient with SLE overlapping with BP achieved significant clinical remission and steroid sparing after RTX-BLM sequential treatment, suggesting that a combination therapy of anti-CD20 and anti-BAFF mAbs might maintain longer B cell depletion and clinical remission (172). In addition, a case of pemphigus vulgaris (PV) was successfully treated with BLM after a failed steroid therapy course. After four cycles of BLM treatment, clinical symptoms and autoantibody levels were significantly decreased in the patient, highlighting the effectiveness of BLM in treating PV (173).
4.2 B cell-activating factor receptor-targeted therapies
Ianalumab (VAY736), a fully human anti-B cell-activating factor receptor (BAFFR) mAb, has two action mechanisms: direct depletion of BAFFR+ B cells and competitive BAFFR blockade, leading to B cell apoptosis (149). In a clinical study, ianalumab was used as a single-dose treatment for patients with sjögren’s syndrome. The results showed that ianalumab reduced clinical manifestations, B cell activation biomarker expression, and serum Ig light chain levels and augmented the salivary flow rate in sjögren’s syndrome (148). Moreover, a dose-finding study confirmed a dose-related decrease in disease activity (149). However, Novartis terminated a clinical trial of VAY736 in patients with pemphigus vulgaris prior to its completion for strategic reasons (152, 174).
4.3 B cell-activating factor- and a proliferation-inducing ligand-targeted therapies
4.3.1 Atacicept
Atacicept is a recombinant soluble fusion protein that targets both B cell-activating factor (BAFF) and a proliferation-inducing ligand (APRIL) (175). A phase II study showed that patients with systemic lupus erythematosus (SLE) administered with 150 mg atacicept experienced a lower flare rate than those administered with a placebo (153). Moreover, a post-hoc analysis of this study demonstrated a dose–response relationship between atacicept concentrations and reduced flare rates, which further confirmed the efficiency of atacicept (154). Additionally, a phase IIb ADDRESS II trial demonstrated the dose-dependent efficacy of atacicept (75/150 mg) in treating active SLE, with 75 mg achieving a significant SRI-4 response and reduced flare risk in high-activity subgroups, while maintaining placebo-comparable safety (155, 156). Subsequently, a long-term extension of this study was conducted. Although it was terminated early owing to drug supply shortage, atacicept exhibited an acceptable safety and efficacy during the study period (157, 158). Although these clinical trials yielded positive results, further research is needed to evaluate the safety and effectiveness of atacicept for treating SLE.
4.3.2 Telitacicept
Telitacicept (RC18) is a novel, recombinant transmembrane activator and calcium modulator and cyclophilin ligand interactor-Fc fusion protein. Similar to atacicept, telitacicept can bind to BAFF and APRIL simultaneously. The safety and efficacy of telitacicept have been assessed in patients with SLE in a phase IIb trial, and it succeeded in meeting the primary endpoint, validating its safety (160, 161). Telitacicept was approved for treating active SLE in China in 2021 (176). A subsequent phase III randomised, double-blind, placebo-controlled trial involving 335 patients demonstrated robust efficacy and safety of weekly subcutaneous telitacicept combined with standard therapy compared to placebo (162). These promising results have driven global multicentre clinical trials, some of which are currently recruiting. Moreover, the use of telitacicept in patients with SS was assessed in a phase II study and yielded positive results, with significant improvements in clinical manifestations (164). Ongoing phase III trials aim to further evaluate its efficacy (177).
4.4 Belimumab and rituximab combination therapy
Despite the demonstrated efficacy of rituximab (RTX) in autoimmune diseases therapy, frequent relapses remain a clinical challenge. Several studies have investigated this issue, revealing that serum B cell-activating factor (BAFF) levels rise significantly during B cell depletion post-RTX and return to baseline upon B cell recovery. Given that excessive BAFF has been shown to rescue self-reactive B cells from apoptosis, these findings suggest that the recovery of self-reactive B cells may be attributed to elevated BAFF levels following B cell depletion (178, 179). These findings led to investigations of sequential therapy with RTX followed by belimumab (BLM). For instance, a multicentre randomised trial in patients with refractory lupus nephritis demonstrated that adding BLM to RTX/cyclophosphamide therapy was safe and modulated B cell reconstitution more effectively than B cell depletion alone (166). However, the phase III BLISS-BELIEVE trial found that sequential subcutaneous BLM with a single RTX cycle did not achieve superior disease control at week 52 or clinical remission at week 64 compared to BLM plus placebo. Nonetheless, this combination significantly reduced anti-dsDNA antibodies, modulated B cell subsets, and prolonged disease control duration, warranting further investigation (167, 168). In sjögren’s syndrome (SS), sequential RTX-BLM therapy improved clinical outcomes compared to monotherapies without compromising safety (170, 180). Collectively, although current studies on sequential therapy are limited, these findings highlight its potential as a promising therapeutic strategy that warrants further research to explore its long-term effects and mechanisms.
Emerging BAFF-targeted therapies for systemic lupus erythematosus (SLE), SS, and systemic sclerosis show translational promise in understudied autoimmune diseases. In bullous pemphigoid (BP), elevated BAFF molecule levels in memory B cells and post-RTX surges correlate with relapse, while DPP4 inhibitor-associated cases exhibit reduced BAFF molecule levels, implicating pathogenic heterogeneity (134, 135). Dual BAFF/APRIL inhibitors and SLE-validated B cell maturation antigen chimeric antigen receptor T cell immunotherapy therapies may counteract BP autoantibody pathology. For pemphigus vulgaris, post-RTX BAFF resurgence aligns with B cell recovery (101, 137, 138); sequential use of RTX-BLM or transmembrane activator and calcium modulator and cyclophilin ligand interactor agonists could limit autoreactivity. In alopecia areata, BAFF elevation is linked to Th17 inflammation (104), supporting the potential for telitacicept or JAK/BAFF dual inhibition. Disease-specific trials are critical to refine BAFF-axis modulation strategies across autoimmune diseases.
5 Conclusions
The B cell-activating factor (BAFF) system plays an indispensable role in autoimmunity. Increasing numbers of clinical trials targeting BAFF antagonism have yielded promising results, leading to the approval of BLM for active systemic lupus erythematosus. However, despite these advances, the understanding of BAFF’s role in autoimmune diseases pathogenesis remains in its early stages, leaving many aspects yet to be explored. In terms of targeted BAFF therapy for autoimmune diseases, there is a shortage of available drugs; therefore, additional clinical trials with larger sample sizes are required to identify new targeted drugs.
Author contributions
LL: Writing – original draft. SXS: Writing – original draft. SS: Writing – original draft. ED: Writing – original draft. GW: Writing – review & editing. HF: Writing – review & editing. HQ: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82173410).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
AA: alopecia areata
anti-dsDNA: anti–double-stranded DNA
APRIL: A proliferation-inducing ligand
BAFF: B cell-activating factor
BAFFR: BAFF receptor
BAFF Tg mice: BAFF transgenic mice
BCMA: B cell maturation antigen
BLM: belimumab
BP: bullous pemphigoid
CLE: cutaneous lupus erythematosus
DPP4is: dipeptidyl peptidase 4 inhibitors
ESSDAI: European League Against Rheumatism Sjögren’s Syndrome Disease Activity Index
ESSPRI: European League Against Rheumatism Sjögren’s Syndrome Patient Reported Index
G-CSF: granulocyte colony-stimulating factor
Ig: immunoglobulin
IFNs: interferons
IL: interleukin
LN: lupus nephritis
IRFs: interferon regulatory factors
mAbs: monoclonal antibodies
mBAFF: membrane-bound form BAFF
NF-κB: nuclear factor-kappa B
NK: natural killer
PC: plasma cell
PI3K: phosphoinositide-3-kinase
PV: pemphigus vulgaris
RF: rheumatoid factor
RTX: rituximab
sBAFF: soluble form BAFF
SG: salivary gland
SLE: systemic lupus erythematosus
SS: Sjögren’s syndrome
SSc: systemic sclerosis
TACI: transmembrane activator and calcium modulator and cyclophilin ligand interactor
Tfh: follicular helper T
Th: T-helper
TLR: toll-like receptor
TNF: tumour necrosis factor
TSK/+: tight skin
References
1. Mackay F and Schneider P. Cracking the BAFF code. Nat Rev Immunol. (2009) 9:491–502. doi: 10.1038/nri2572
2. Mackay F and Browning JL. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol. (2002) 2:465–75. doi: 10.1038/nri844
3. Hu S, Wang R, Zhang M, Liu K, Tao J, Tai Y, et al. BAFF promotes T cell activation through the BAFF-BAFF-R-PI3K-Akt signaling pathway. BioMed Pharmacother. (2019) 114:108796. doi: 10.1016/j.biopha.2019.108796
4. Chen M, Lin X, Liu Y, Li Q, Deng Y, Liu Z, et al. The function of BAFF on T helper cells in autoimmunity. Cytokine Growth Factor Rev. (2014) 25:301–5. doi: 10.1016/j.cytogfr.2013.12.011
5. Mackay F and Leung H. The role of the BAFF/APRIL system on T cell function. Semin Immunol. (2006) 18:284–9. doi: 10.1016/j.smim.2006.04.005
6. Chang SK, Arendt BK, Darce JR, Wu X, and Jelinek DF. A role for BLyS in the activation of innate immune cells. Blood. (2006) 108:2687–94. doi: 10.1182/blood-2005-12-017319
7. Chang SK, Mihalcik SA, and Jelinek DF. B lymphocyte stimulator regulates adaptive immune responses by directly promoting dendritic cell maturation. J Immunol. (2008) 180:7394–403. doi: 10.4049/jimmunol.180.11.7394
8. Quah PS, Sutton V, Whitlock E, Figgett WA, Andrews DM, Fairfax KA, et al. The effects of B-cell-activating factor on the population size, maturation and function of murine natural killer cells. Immunol Cell Biol. (2022) 100:761–76. doi: 10.1111/imcb.v100.10
9. Vincent FB, Saulep-Easton D, Figgett WA, Fairfax KA, and Mackay F. The BAFF/APRIL system: emerging functions beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev. (2013) 24:203–15. doi: 10.1016/j.cytogfr.2013.04.003
10. Zhang Y, Tian J, Xiao F, Zheng L, Zhu X, Wu L, et al. B cell-activating factor and its targeted therapy in autoimmune diseases. Cytokine Growth Factor Rev. (2022) 64:57–70. doi: 10.1016/j.cytogfr.2021.11.004
11. Watanabe R, Fujimoto M, Yazawa N, Nakashima H, Asashima N, Kuwano Y, et al. Increased serum levels of a proliferation-inducing ligand in patients with bullous pemphigoid. J Dermatol Sci. (2007) 46:53–60. doi: 10.1016/j.jdermsci.2006.12.008
12. Koyama T, Tsukamoto H, Miyagi Y, Himeji D, Otsuka J, Miyagawa H, et al. Raised serum APRIL levels in patients with systemic lupus erythematosus. Ann Rheum Dis. (2005) 64:1065–7. doi: 10.1136/ard.2004.022491
13. Chen Y, Yang M, Long D, Li Q, Zhao M, Wu H, et al. Abnormal expression of BAFF and its receptors in peripheral blood and skin lesions from systemic lupus erythematosus patients. Autoimmunity. (2020) 53:192–200. doi: 10.1080/08916934.2020.1736049
14. Torkamani EKSN and Naseri M. Investigation of serum APRIL and BAFF levels in pemphigus vulgaris patients in Southern Iran. Iran J Dermatol. (2011) 14:16–9.
15. Asashima N, Fujimoto M, Watanabe R, Nakashima H, Yazawa N, Okochi H, et al. Serum levels of BAFF are increased in bullous pemphigoid but not in pemphigus vulgaris. Br J Dermatol. (2006) 155:330–6. doi: 10.1111/j.1365-2133.2006.07305.x
16. Matsushita T, Hasegawa M, Yanaba K, Kodera M, Takehara K, and Sato S. Elevated serum BAFF levels in patients with systemic sclerosis: enhanced BAFF signaling in systemic sclerosis B lymphocytes. Arthritis Rheum. (2006) 54:192–201. doi: 10.1002/art.21526
17. Matsushita T, Fujimoto M, Hasegawa M, Tanaka C, Kumada S, Ogawa F, et al. Elevated serum APRIL levels in patients with systemic sclerosis: distinct profiles of systemic sclerosis categorized by APRIL and BAFF. J Rheumatol. (2007) 34:2056–62.
18. Groom J, Kalled SL, Cutler AH, Olson C, Woodcock SA, Schneider P, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjögren’s syndrome. J Clin Invest. (2002) 109:59–68. doi: 10.1172/JCI0214121
19. Vosters JL, Roescher N, Polling EJ, Illei GG, and Tak PP. The expression of APRIL in Sjogren’s syndrome: aberrant expression of APRIL in the salivary gland. Rheumatol (Oxford). (2012) 51:1557–62. doi: 10.1093/rheumatology/kes080
20. Zaaroura H, Gilding AJ, and Sibbald C. Biomarkers in alopecia Areata: A systematic review and meta-analysis. Autoimmun Rev. (2023) 22:103339. doi: 10.1016/j.autrev.2023.103339
21. Ahmed S. Belimumab in kidney transplantation. Lancet. (2019) 393:874. doi: 10.1016/S0140-6736(18)33074-5
22. Gordon JK, Martyanov V, Franks JM, Bernstein EJ, Szymonifka J, Magro C, et al. Belimumab for the treatment of early diffuse systemic sclerosis: results of a randomized, double-blind, placebo-controlled, pilot trial. Arthritis Rheumatol. (2018) 70:308–16. doi: 10.1002/art.40358
23. Mariette X, Seror R, Quartuccio L, Baron G, Salvin S, Fabris M, et al. Efficacy and safety of belimumab in primary Sjögren’s syndrome: results of the BELISS open-label phase II study. Ann Rheum Dis. (2015) 74:526–31. doi: 10.1136/annrheumdis-2013-203991
24. Lee DSW, Rojas OL, and Gommerman JL. B cell depletion therapies in autoimmune disease: advances and mechanistic insights. Nat Rev Drug Discovery. (2021) 20:179–99. doi: 10.1038/s41573-020-00092-2
25. Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. (1999) 285:260–3. doi: 10.1126/science.285.5425.260
26. Hahne M, Kataoka T, Schröter M, Hofmann K, Irmler M, Bodmer JL, et al. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J Exp Med. (1998) 188:1185–90. doi: 10.1084/jem.188.6.1185
27. Nardelli B, Belvedere O, Roschke V, Moore PA, Olsen HS, Migone TS, et al. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood. (2001) 97:198–204. doi: 10.1182/blood.V97.1.198
28. Bossen C, Tardivel A, Willen L, Fletcher CA, Perroud M, Beermann F, et al. Mutation of the BAFF furin cleavage site impairs B-cell homeostasis and antibody responses. Eur J Immunol. (2011) 41:787–97. doi: 10.1002/eji.201040591
29. Mackay F and Ambrose C. The TNF family members BAFF and APRIL: the growing complexity. Cytokine Growth Factor Rev. (2003) 14:311–24. doi: 10.1016/S1359-6101(03)00023-6
30. Huard B, Arlettaz L, Ambrose C, Kindler V, Mauri D, Roosnek E, et al. BAFF production by antigen-presenting cells provides T cell co-stimulation. Int Immunol. (2004) 16:467–75. doi: 10.1093/intimm/dxh043
31. Alexaki VI, Notas G, Pelekanou V, Kampa M, Valkanou M, Theodoropoulos P, et al. Adipocytes as immune cells: differential expression of TWEAK, BAFF, and APRIL and their receptors (Fn14, BAFF-R, TACI, and BCMA) at different stages of normal and pathological adipose tissue development. J Immunol. (2009) 183:5948–56. doi: 10.4049/jimmunol.0901186
32. Woo SJ, Im J, Jeon JH, Kang SS, Lee MH, Yun CH, et al. Induction of BAFF expression by IFN-γ via JAK/STAT signaling pathways in human intestinal epithelial cells. J Leukoc Biol. (2013) 93:363–8. doi: 10.1189/jlb.0412210
33. Sakai J and Akkoyunlu M. The role of BAFF system molecules in host response to pathogens. Clin Microbiol Rev. (2017) 30:991–1014. doi: 10.1128/CMR.00046-17
34. Leah E. Crosstalk in RA synovia-TLR3-BAFF axis sustains B-cell activation. Nat Rev Rheumatol. (2011) 7:559. doi: 10.1038/nrrheum.2011.122
35. Chu VT, Enghard P, Riemekasten G, and Berek C. In vitro and in vivo activation induces BAFF and APRIL expression in B cells. J Immunol. (2007) 179:5947–57. doi: 10.4049/jimmunol.179.9.5947
36. Huard B, McKee T, Bosshard C, Durual S, Matthes T, Myit S, et al. APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J Clin Invest. (2008) 118:2887–95. doi: 10.1172/JCI33760
37. Ullah MA and Mackay F. The BAFF-APRIL system in cancer. Cancers (Basel). (2023) 15. doi: 10.3390/cancers15061791
38. Thompson JS, Bixler SA, Qian F, Vora K, Scott ML, Cachero TG, et al. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. (2001) 293:2108–11. doi: 10.1126/science.1061965
39. Gross JA, Johnston J, Mudri S, Enselman R, Dillon SR, Madden K, et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. (2000) 404:995–9. doi: 10.1038/35010115
40. Laurent SA, Hoffmann FS, Kuhn PH, Cheng Q, Chu Y, Schmidt-Supprian M, et al. γ-Secretase directly sheds the survival receptor BCMA from plasma cells. Nat Commun. (2015) 6:7333. doi: 10.1038/ncomms8333
41. Hoffmann FS, Kuhn PH, Laurent SA, Hauck SM, Berer K, Wendlinger SA, et al. The immunoregulator soluble TACI is released by ADAM10 and reflects B cell activation in autoimmunity. J Immunol. (2015) 194:542–52. doi: 10.4049/jimmunol.1402070
42. Meinl E, Thaler FS, and Lichtenthaler SF. Shedding of BAFF/APRIL receptors controls B cells. Trends Immunol. (2018) 39:673–6. doi: 10.1016/j.it.2018.07.002
43. Day ES, Cachero TG, Qian F, Sun Y, Wen D, Pelletier M, et al. Selectivity of BAFF/BLyS and APRIL for binding to the TNF family receptors BAFFR/BR3 and BCMA. Biochemistry. (2005) 44:1919–31. doi: 10.1021/bi048227k
44. Wu Y, Bressette D, Carrell JA, Kaufman T, Feng P, Taylor K, et al. Tumor necrosis factor (TNF) receptor superfamily member TACI is a high affinity receptor for TNF family members APRIL and BLyS. J Biol Chem. (2000) 275:35478–85. doi: 10.1074/jbc.M005224200
45. Bossen C and Schneider P. BAFF, APRIL and their receptors: structure, function and signaling. Semin Immunol. (2006) 18:263–75. doi: 10.1016/j.smim.2006.04.006
46. Magliozzi R, Marastoni D, and Calabrese M. The BAFF/APRIL system as therapeutic target in multiple sclerosis. Expert Opin Ther Targets. (2020) 24:1135–45. doi: 10.1080/14728222.2020.1821647
47. Ng LG, Sutherland AP, Newton R, Qian F, Cachero TG, Scott ML, et al. B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. J Immunol. (2004) 173:807–17. doi: 10.4049/jimmunol.173.2.807
48. Scapini P, Hu Y, Chu CL, Migone TS, Defranco AL, Cassatella MA, et al. Myeloid cells, BAFF, and IFN-gamma establish an inflammatory loop that exacerbates autoimmunity in Lyn-deficient mice. J Exp Med. (2010) 207:1757–73. doi: 10.1084/jem.20100086
49. Ye Q, Wang L, Wells AD, Tao R, Han R, Davidson A, et al. BAFF binding to T cell-expressed BAFF-R costimulates T cell proliferation and alloresponses. Eur J Immunol. (2004) 34:2750–9. doi: 10.1002/eji.200425198
50. Möckel T, Basta F, Weinmann-Menke J, and Schwarting A. B cell activating factor (BAFF): Structure, functions, autoimmunity and clinical implications in Systemic Lupus Erythematosus (SLE). Autoimmun Rev. (2021) 20:102736. doi: 10.1016/j.autrev.2020.102736
51. Maciejewska M, Sikora M, Alda-Malicka R, and Czuwara J. Future treatment options in systemic sclerosis-potential targets and ongoing clinical trials. J Clin Med. (2022) 11. doi: 10.3390/jcm11051310
52. Zhan Q, Zhang J, Lin Y, Chen W, Fan X, Zhang D, et al. Pathogenesis and treatment of Sjogren’s syndrome: Review and update. Front Immunol. (2023) 14:1127417. doi: 10.3389/fimmu.2023.1127417
53. Mackay F, Figgett WA, Saulep D, Lepage M, and Hibbs ML. B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunol Rev. (2010) 237:205–25. doi: 10.1111/j.1600-065X.2010.00944.x
54. Smulski CR and Eibel H. BAFF and BAFF-receptor in B cell selection and survival. Front Immunol. (2018) 9:2285. doi: 10.3389/fimmu.2018.02285
55. Smith SH and Cancro MP. Cutting edge: B cell receptor signals regulate BLyS receptor levels in mature B cells and their immediate progenitors. J Immunol. (2003) 170:5820–3. doi: 10.4049/jimmunol.170.12.5820
56. Rodig SJ, Shahsafaei A, Li B, Mackay CR, and Dorfman DM. BAFF-R, the major B cell-activating factor receptor, is expressed on most mature B cells and B-cell lymphoproliferative disorders. Hum Pathol. (2005) 36:1113–9. doi: 10.1016/j.humpath.2005.08.005
57. Hsu BL, Harless SM, Lindsley RC, Hilbert DM, and Cancro MP. Cutting edge: BLyS enables survival of transitional and mature B cells through distinct mediators. J Immunol. (2002) 168:5993–6. doi: 10.4049/jimmunol.168.12.5993
58. Mihalcik SA, Huddleston PM 3rd, Wu X, and Jelinek DF. The structure of the TNFRSF13C promoter enables differential expression of BAFF-R during B cell ontogeny and terminal differentiation. J Immunol. (2010) 185:1045–54. doi: 10.4049/jimmunol.1001120
59. O'Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. (2004) 199:91–8. doi: 10.1084/jem.20031330
60. Avery DT, Kalled SL, Ellyard JI, Ambrose C, Bixler SA, Thien M, et al. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest. (2003) 112:286–97. doi: 10.1172/JCI18025
61. Underhill GH, George D, Bremer EG, and Kansas GS. Gene expression profiling reveals a highly specialized genetic program of plasma cells. Blood. (2003) 101:4013–21. doi: 10.1182/blood-2002-08-2673
62. Tarte K, Zhan F, De Vos J, Klein B, and Shaughnessy J Jr. Gene expression profiling of plasma cells and plasmablasts: toward a better understanding of the late stages of B-cell differentiation. Blood. (2003) 102:592–600. doi: 10.1182/blood-2002-10-3161
63. Peperzak V, Vikström I, Walker J, Glaser SP, LePage M, Coquery CM, et al. Mcl-1 is essential for the survival of plasma cells. Nat Immunol. (2013) 14:290–7. doi: 10.1038/ni.2527
64. Schuepbach-Mallepell S, Das D, Willen L, Vigolo M, Tardivel A, Lebon L, et al. Stoichiometry of heteromeric BAFF and APRIL cytokines dictates their receptor binding and signaling properties. J Biol Chem. (2015) 290:16330–42. doi: 10.1074/jbc.M115.661405
65. Mantchev GT, Cortesão CS, Rebrovich M, Cascalho M, and Bram RJ. TACI is required for efficient plasma cell differentiation in response to T-independent type 2 antigens. J Immunol. (2007) 179:2282–8. doi: 10.4049/jimmunol.179.4.2282
66. Ozcan E, Garibyan L, Lee JJ, Bram RJ, Lam KP, Geha RS, et al. Transmembrane activator, calcium modulator, and cyclophilin ligand interactor drives plasma cell differentiation in LPS-activated B cells. J Allergy Clin Immunol. (2009) 123:1277–1286.e1275. doi: 10.1016/j.jaci.2009.03.019
67. Tsuji S, Cortesão C, Bram RJ, Platt JL, and Cascalho M. TACI deficiency impairs sustained Blimp-1 expression in B cells decreasing long-lived plasma cells in the bone marrow. Blood. (2011) 118:5832–9. doi: 10.1182/blood-2011-05-353961
68. Karpusas M, Cachero TG, Qian F, Boriack-Sjodin A, Mullen C, Strauch K, et al. Crystal structure of extracellular human BAFF, a TNF family member that stimulates B lymphocytes. J Mol Biol. (2002) 315:1145–54. doi: 10.1006/jmbi.2001.5296
69. Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. (1999) 190:1697–710. doi: 10.1084/jem.190.11.1697
70. Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. (1999) 189:1747–56. doi: 10.1084/jem.189.11.1747
71. Gorelik L, Gilbride K, Dobles M, Kalled SL, Zandman D, Scott ML, et al. Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells. J Exp Med. (2003) 198:937–45. doi: 10.1084/jem.20030789
72. Rahman ZS, Rao SP, Kalled SL, and Manser T. Normal induction but attenuated progression of germinal center responses in BAFF and BAFF-R signaling-deficient mice. J Exp Med. (2003) 198:1157–69. doi: 10.1084/jem.20030495
73. Stein JV, López-Fraga M, Elustondo FA, Carvalho-Pinto CE, Rodríguez D, Gómez-Caro R, et al. APRIL modulates B and T cell immunity. J Clin Invest. (2002) 109:1587–98. doi: 10.1172/JCI0215034
74. Varfolomeev E, Kischkel F, Martin F, Seshasayee D, Wang H, Lawrence D, et al. APRIL-deficient mice have normal immune system development. Mol Cell Biol. (2004) 24:997–1006. doi: 10.1128/MCB.24.3.997-1006.2004
75. Castigli E, Scott S, Dedeoglu F, Bryce P, Jabara H, Bhan AK, et al. Impaired IgA class switching in APRIL-deficient mice. Proc Natl Acad Sci U.S.A. (2004) 101:3903–8. doi: 10.1073/pnas.0307348101
76. Mackay F and Schneider P. TACI, an enigmatic BAFF/APRIL receptor, with new unappreciated biochemical and biological properties. Cytokine Growth Factor Rev. (2008) 19:263–76. doi: 10.1016/j.cytogfr.2008.04.006
77. Yan M, Wang H, Chan B, Roose-Girma M, Erickson S, Baker T, et al. Activation and accumulation of B cells in TACI-deficient mice. Nat Immunol. (2001) 2:638–43. doi: 10.1038/89790
78. Mackay F and Mackay CR. The role of BAFF in B-cell maturation, T-cell activation and autoimmunity. Trends Immunol. (2002) 23:113–5. doi: 10.1016/S1471-4906(01)02159-7
79. Huard B, Schneider P, Mauri D, Tschopp J, and French LE. T cell costimulation by the TNF ligand BAFF. J Immunol. (2001) 167:6225–31. doi: 10.4049/jimmunol.167.11.6225
80. Gross JA, Dillon SR, Mudri S, Johnston J, Littau A, Roque R, et al. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. impaired B cell maturation in mice lacking BLyS. Immunity. (2001) 15:289–302. doi: 10.1016/S1074-7613(01)00183-2
81. Salazar-Camarena DC, Ortíz-Lazareno P, Marín-Rosales M, Cruz A, Muñoz-Valle F, Tapia-Llanos R, et al. BAFF-R and TACI expression on CD3+ T cells: Interplay among BAFF, APRIL and T helper cytokines profile in systemic lupus erythematosus. Cytokine. (2019) 114:115–27. doi: 10.1016/j.cyto.2018.11.008
82. Bloom DD, Reshetylo S, Nytes C, Goodsett CT, and Hematti P. Blockade of BAFF receptor BR3 on T cells enhances their activation and cytotoxicity. J Immunother. (2018) 41:213–23. doi: 10.1097/CJI.0000000000000209
83. Lai Kwan Lam Q, King Hung Ko O, Zheng BJ, and Lu L. Local BAFF gene silencing suppresses Th17-cell generation and ameliorates autoimmune arthritis. Proc Natl Acad Sci U S A. (2008) 105:14993–8. doi: 10.1073/pnas.0806044105
84. Granato A, Hayashi EA, Baptista BJ, Bellio M, and Nobrega A. IL-4 regulates Bim expression and promotes B cell maturation in synergy with BAFF conferring resistance to cell death at negative selection checkpoints. J Immunol. (2014) 192:5761–75. doi: 10.4049/jimmunol.1300749
85. Zheng Y, Gallucci S, Gaughan JP, Gross JA, and Monestier M. A role for B cell-activating factor of the TNF family in chemically induced autoimmunity. J Immunol. (2005) 175:6163–8. doi: 10.4049/jimmunol.175.9.6163
86. Jeon Y, Lim JY, Im KI, Kim N, and Cho SG. BAFF blockade attenuates acute graft-versus-host disease directly via the dual regulation of T- and B-cell homeostasis. Front Immunol. (2022) 13:995149. doi: 10.3389/fimmu.2022.995149
87. Hu H, Wu X, Jin W, Chang M, Cheng X, and Sun SC. Noncanonical NF-kappaB regulates inducible costimulator (ICOS) ligand expression and T follicular helper cell development. Proc Natl Acad Sci U S A. (2011) 108:12827–32. doi: 10.1073/pnas.1105774108
88. Ou X, Xu S, and Lam KP. Deficiency in TNFRSF13B (TACI) expands T-follicular helper and germinal center B cells via increased ICOS-ligand expression but impairs plasma cell survival. Proc Natl Acad Sci U S A. (2012) 109:15401–6. doi: 10.1073/pnas.1200386109
89. Allman WR, Dey R, Liu L, Siddiqui S, Coleman AS, Bhattacharya P, et al. TACI deficiency leads to alternatively activated macrophage phenotype and susceptibility to Leishmania infection. Proc Natl Acad Sci U S A. (2015) 112:E4094–4103. doi: 10.1073/pnas.1421580112
90. Zhang W, Wen L, Huang X, Liang J, Gao W, Zhang S, et al. hsBAFF enhances activity of NK cells by regulation of CD4(+) T lymphocyte function. Immunol Lett. (2008) 120:96–102. doi: 10.1016/j.imlet.2008.07.005
91. Levy RA, Gonzalez-Rivera T, Khamashta M, Fox NL, Jones-Leone A, Rubin B, et al. 10 Years of belimumab experience: What have we learnt? Lupus. (2021) 30:1705–21. doi: 10.1177/09612033211028653
92. Zhang J, Roschke V, Baker KP, Wang Z, Alarcón GS, Fessler BJ, et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol. (2001) 166:6–10. doi: 10.4049/jimmunol.166.1.6
93. Erazo-Martínez V, Tobón GJ, and Cañas CA. Circulating and skin biopsy-present cytokines related to the pathogenesis of cutaneous lupus erythematosus. Autoimmun Rev. (2023) 22:103262. doi: 10.1016/j.autrev.2022.103262
94. Wenzel J, Landmann A, Vorwerk G, and Kuhn A. High expression of B lymphocyte stimulator in lesional keratinocytes of patients with cutaneous lupus erythematosus. Exp Dermatol. (2018) 27:95–7. doi: 10.1111/exd.2018.27.issue-1
95. Neusser MA, Lindenmeyer MT, Edenhofer I, Gaiser S, Kretzler M, Regele H, et al. Intrarenal production of B-cell survival factors in human lupus nephritis. Mod Pathol. (2011) 24:98–107. doi: 10.1038/modpathol.2010.184
96. Phatak S, Chaurasia S, Mishra SK, Gupta R, Agrawal V, Aggarwal A, et al. Urinary B cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL): potential biomarkers of active lupus nephritis. Clin Exp Immunol. (2017) 187:376–82. doi: 10.1111/cei.12894
97. Lombardi T, Moll S, Youinou P, Pers JO, Tzankov A, Gabay C, et al. Absence of up-regulation for a proliferation-inducing ligand in Sjögren’s sialadenitis lesions. Rheumatol (Oxford). (2011) 50:1211–5. doi: 10.1093/rheumatology/ker016
98. Daridon C, Devauchelle V, Hutin P, Le Berre R, Martins-Carvalho C, Bendaoud B, et al. Aberrant expression of BAFF by B lymphocytes infiltrating the salivary glands of patients with primary Sjögren’s syndrome. Arthritis Rheum. (2007) 56:1134–44. doi: 10.1002/art.22458
99. Abdo MS, Mohammed RH, Raslan HM, and Gaber SM. Serum B-cell activating factor assessment in a population of Egyptian patients with systemic sclerosis. Int J Rheum Dis. (2013) 16:148–56. doi: 10.1111/apl.2013.16.issue-2
100. Erdő-Bonyár S, Rapp J, Szinger D, Minier T, Kumánovics G, Czirják L, et al. Ligation of TLR homologue CD180 of B cells activates the PI3K/Akt/mTOR pathway in systemic sclerosis and induces a pathological shift in the expression of BAFF receptors. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23126777
101. Hebert V, Maho-Vaillant M, Golinski ML, Petit M, Riou G, Boyer O, et al. Modifications of the BAFF/BAFF-receptor axis in patients with pemphigus treated with rituximab versus standard corticosteroid regimen. Front Immunol. (2021) 12:666022. doi: 10.3389/fimmu.2021.666022
102. Kuwano Y, Fujimoto M, Watanabe R, Ishiura N, Nakashima H, Ohno Y, et al. Serum BAFF and APRIL levels in patients with alopecia areata. J Dermatol Sci. (2008) 50:236–9. doi: 10.1016/j.jdermsci.2008.01.004
103. Gregoriou S, Papafragkaki D, Kontochristopoulos G, Rallis E, Kalogeromitros D, and Rigopoulos D. Cytokines and other mediators in alopecia areata. Mediators Inflammation. (2010) 2010:928030. doi: 10.1155/2010/928030
104. Elela MA, Gawdat HI, Hegazy RA, Fawzy MM, Abdel Hay RM, Saadi D, et al. B cell activating factor and T-helper 17 cells: possible synergistic culprits in the pathogenesis of Alopecia Areata. Arch Dermatol Res. (2016) 308:115–21. doi: 10.1007/s00403-016-1617-z
105. Caielli S, Wan Z, and Pascual V. Systemic lupus erythematosus pathogenesis: interferon and beyond. Annu Rev Immunol. (2023) 41:533–60. doi: 10.1146/annurev-immunol-101921-042422
106. Ribero S, Sciascia S, Borradori L, and Lipsker D. The cutaneous spectrum of lupus erythematosus. Clin Rev Allergy Immunol. (2017) 53:291–305. doi: 10.1007/s12016-017-8627-2
107. Morel J, Roubille C, Planelles L, Rocha C, Fernandez L, Lukas C, et al. Serum levels of tumour necrosis factor family members a proliferation-inducing ligand (APRIL) and B lymphocyte stimulator (BLyS) are inversely correlated in systemic lupus erythematosus. Ann Rheum Dis. (2009) 68:997–1002. doi: 10.1136/ard.2008.090928
108. Hegazy M, Darwish H, Darweesh H, El-Shehaby A, and Emad Y. Raised serum level of APRIL in patients with systemic lupus erythematosus: correlations with disease activity indices. Clin Immunol. (2010) 135:118–24. doi: 10.1016/j.clim.2009.12.012
109. Petri M, Stohl W, Chatham W, McCune WJ, Chevrier M, Ryel J, et al. Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis Rheum. (2008) 58:2453–9. doi: 10.1002/art.23678
110. McCarthy EM, Lee RZ, J NG, Smith S, Cunnane G, Doran MF, et al. Elevated B lymphocyte stimulator levels are associated with increased damage in an Irish systemic lupus erythematosus cohort. Rheumatol (Oxford). (2013) 52:1279–84. doi: 10.1093/rheumatology/ket120
111. Elbirt D, Asher I, Mahlab-Guri K, Bezalel-Rosenberg S, Edelstein V, and Sthoeger Z. BLyS levels in sera of patients with systemic lupus erythematosus: clinical and serological correlation. Isr Med Assoc J. (2014) 16:491–6.
112. Howe HS, Thong BYH, Kong KO, Chng HH, Lian TY, Chia FL, et al. Associations of B cell-activating factor (BAFF) and anti-BAFF autoantibodies with disease activity in multi-ethnic Asian systemic lupus erythematosus patients in Singapore. Clin Exp Immunol. (2017) 189:298–303. doi: 10.1111/cei.12975
113. Ding H, Wang L, Wu X, Yan J, He Y, Ni B, et al. Blockade of B-cell-activating factor suppresses lupus-like syndrome in autoimmune BXSB mice. J Cell Mol Med. (2010) 14:1717–25. doi: 10.1111/j.1582-4934.2009.00817.x
114. Thorn M, Lewis RH, Mumbey-Wafula A, Kantrowitz S, and Spatz LA. BAFF overexpression promotes anti-dsDNA B-cell maturation and antibody secretion. Cell Immunol. (2010) 261:9–22. doi: 10.1016/j.cellimm.2009.10.004
115. Vincent FB, Morand EF, Schneider P, and Mackay F. The BAFF/APRIL system in SLE pathogenesis. Nat Rev Rheumatol. (2014) 10:365–73. doi: 10.1038/nrrheum.2014.33
116. Schwarting A, Relle M, Meineck M, Föhr B, Triantafyllias K, Weinmann A, et al. Renal tubular epithelial cell-derived BAFF expression mediates kidney damage and correlates with activity of proliferative lupus nephritis in mouse and men. Lupus. (2018) 27:243–56. doi: 10.1177/0961203317717083
117. Kang S, Fedoriw Y, Brenneman EK, Truong YK, Kikly K, and Vilen BJ. BAFF Induces Tertiary Lymphoid Structures and Positions T Cells within the Glomeruli during Lupus Nephritis. J Immunol. (2017) 198:2602–11. doi: 10.4049/jimmunol.1600281
118. Chong BF, Tseng LC, Kim A, Miller RT, Yancey KB, and Hosler GA. Differential expression of BAFF and its receptors in discoid lupus erythematosus patients. J Dermatol Sci. (2014) 73(3):216–24. doi: 10.1016/j.jdermsci.2013.11.007
119. Brito-Zeron P, Baldini C, Bootsma H, Bowman SJ, Jonsson R, Mariette X, et al. Sjogren syndrome. Nat Rev Dis Primers. (2016) 2:16047. doi: 10.1038/nrdp.2016.47
120. Mariette X, Roux S, Zhang J, Bengoufa D, Lavie F, Zhou T, et al. The level of BLyS (BAFF) correlates with the titre of autoantibodies in human Sjögren’s syndrome. Ann Rheum Dis. (2003) 62:168–71. doi: 10.1136/ard.62.2.168
121. Sharma A, Kiripolsky J, Klimatcheva E, Howell A, Fereidouni F, Levenson R, et al. Early BAFF receptor blockade mitigates murine Sjogren’s syndrome: Concomitant targeting of CXCL13 and the BAFF receptor prevents salivary hypofunction. Clin Immunol. (2016) 164:85–94. doi: 10.1016/j.clim.2016.01.015
122. Lahiri A, Varin MM, Le Pottier L, Pochard P, Bendaoud B, Youinou P, et al. Specific forms of BAFF favor BAFF receptor-mediated epithelial cell survival. J Autoimmun. (2014) 51:30–7. doi: 10.1016/j.jaut.2014.02.004
123. Le Pottier L, Devauchelle V, Fautrel A, Daridon C, Saraux A, Youinou P, et al. Ectopic germinal centers are rare in Sjogren’s syndrome salivary glands and do not exclude autoreactive B cells. J Immunol. (2009) 182:3540–7. doi: 10.4049/jimmunol.0803588
124. Varin MM, Le Pottier L, Youinou P, Saulep D, Mackay F, and Pers JO. B-cell tolerance breakdown in Sjögren’s syndrome: focus on BAFF. Autoimmun Rev. (2010) 9:604–8. doi: 10.1016/j.autrev.2010.05.006
125. Carrillo-Ballesteros FJ, Palafox-Sánchez CA, Franco-Topete RA, Muñoz-Valle JF, Orozco-Barocio G, Martínez-Bonilla GE, et al. Expression of BAFF and BAFF receptors in primary Sjögren’s syndrome patients with ectopic germinal center-like structures. Clin Exp Med. (2020) 20:615–26. doi: 10.1007/s10238-020-00637-0
126. Ding J, Zhang W, Haskett S, Pellerin A, Xu S, Petersen B, et al. BAFF overexpression increases lymphocytic infiltration in Sjögren’s target tissue, but only inefficiently promotes ectopic B-cell differentiation. Clin Immunol. (2016) 169:69–79. doi: 10.1016/j.clim.2016.06.007
127. Volkmann ER, Andreasson K, and Smith V. Systemic sclerosis. Lancet. (2023) 401:304–18. doi: 10.1016/S0140-6736(22)01692-0
128. François A, Chatelus E, Wachsmann D, Sibilia J, Bahram S, Alsaleh G, et al. B lymphocytes and B-cell activating factor promote collagen and profibrotic markers expression by dermal fibroblasts in systemic sclerosis. Arthritis Res Ther. (2013) 15:R168. doi: 10.1186/ar4352
129. Matsushita T, Fujimoto M, Hasegawa M, Matsushita Y, Komura K, Ogawa F, et al. BAFF antagonist attenuates the development of skin fibrosis in tight-skin mice. J Invest Dermatol. (2007) 127:2772–80. doi: 10.1038/sj.jid.5700919
130. Francois A, Gombault A, Villeret B, Alsaleh G, Fanny M, Gasse P, et al. B cell activating factor is central to bleomycin- and IL-17-mediated experimental pulmonary fibrosis. J Autoimmun. (2015) 56:1–11. doi: 10.1016/j.jaut.2014.08.003
131. Schmidt E and Zillikens D. Pemphigoid diseases. Lancet. (2013) 381:320–32. doi: 10.1016/S0140-6736(12)61140-4
132. Qian H, Kusuhara M, Li X, Tsuruta D, Tsuchisaka A, Ishii N, et al. B-cell activating factor detected on both naive and memory B cells in bullous pemphigoid. Exp Dermatol. (2014) 23:596–605. doi: 10.1111/exd.2014.23.issue-8
133. Matsushita T, Hasegawa M, Matsushita Y, Echigo T, Wayaku T, Horikawa M, et al. Elevated serum BAFF levels in patients with localized scleroderma in contrast to other organ-specific autoimmune diseases. Exp Dermatol. (2007) 16:87–93. doi: 10.1111/j.1600-0625.2006.00485.x
134. Hall RP, Streilein RD 3rd, Hannah DL, McNair PD, Fairley JA, Ronaghy A, et al. Association of serum B-cell activating factor level and proportion of memory and transitional B cells with clinical response after rituximab treatment of bullous pemphigoid patients. J Invest Dermatol. (2013) 133:2786–8. doi: 10.1038/jid.2013.236
135. Tuusa J, Kokkonen N, Mattila A, Huilaja L, Varpuluoma O, Rannikko S, et al. Dipeptidyl peptidase 4 inhibitor–Associated bullous pemphigoid is characterized by an altered expression of cytokines in the skin. J Invest Dermatol. (2023) 143:78–86.e12. doi: 10.1016/j.jid.2022.07.006
136. Schmidt E, Kasperkiewicz M, and Joly P. Pemphigus. Lancet. (2019) 394:882–94. doi: 10.1016/S0140-6736(19)31778-7
137. Daneshvar E, Tavakolpour S, Mahmoudi H, Daneshpazhooh M, Teimourpour A, Aslani S, et al. Elevated serum level of B-cell activating factor (BAFF) after rituximab therapy in pemphigus vulgaris patients suggests a possible therapeutic efficacy of B-cell depletion therapies combined with anti-BAFF agents. Int J Dermatol. (2023) 62:567–74. doi: 10.1111/ijd.16363
138. Nagel A, Podstawa E, Eickmann M, Müller HH, Hertl M, and Eming R. Rituximab mediates a strong elevation of B-cell-activating factor associated with increased pathogen-specific IgG but not autoantibodies in pemphigus vulgaris. J Invest Dermatol. (2009) 129:2202–10. doi: 10.1038/jid.2009.27
139. Furie R, Stohl W, Ginzler EM, Becker M, Mishra N, Chatham W, et al. Biologic activity and safety of belimumab, a neutralizing anti-B-lymphocyte stimulator (BLyS) monoclonal antibody: a phase I trial in patients with systemic lupus erythematosus. Arthritis Res Ther. (2008) 10:R109. doi: 10.1186/ar2506
140. Wallace DJ, Stohl W, Furie RA, Lisse JR, McKay JD, Merrill JT, et al. A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arthritis Rheum. (2009) 61:1168–78. doi: 10.1002/art.24699
141. Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzová D, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. (2011) 63:3918–30. doi: 10.1002/art.30613
142. Navarra SV, Guzmán RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. (2011) 377:721–31. doi: 10.1016/S0140-6736(10)61354-2
143. Doria A, Stohl W, Schwarting A, Okada M, Scheinberg M, van Vollenhoven R, et al. Efficacy and safety of subcutaneous belimumab in anti-double-stranded DNA-positive, hypocomplementemic patients with systemic lupus erythematosus. Arthritis Rheumatol. (2018) 70:1256–64. doi: 10.1002/art.2018.70.issue-8
144. Stohl W, Schwarting A, Okada M, Scheinberg M, Doria A, Hammer AE, et al. Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus: A fifty-two-week randomized, double-blind, placebo-controlled study. Arthritis Rheumatol. (2017) 69:1016–27. doi: 10.1002/art.40049
145. Furie RA, Wallace DJ, Aranow C, Fettiplace J, Wilson B, Mistry P, et al. Long-term safety and efficacy of belimumab in patients with systemic lupus erythematosus: A continuation of a seventy-six-week phase III parent study in the United States. Arthritis Rheumatol. (2018) 70:868–77. doi: 10.1002/art.40439
146. Furie R, Rovin BH, Houssiau F, Malvar A, Teng YKO, Contreras G, et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med. (2020) 383:1117–28. doi: 10.1056/NEJMoa2001180
147. De Vita S, Quartuccio L, Seror R, Salvin S, Ravaud P, Fabris M, et al. Efficacy and safety of belimumab given for 12 months in primary Sjögren’s syndrome: the BELISS open-label phase II study. Rheumatol (Oxford). (2015) 54:2249–56. doi: 10.1093/rheumatology/kev257
148. Dörner T, Posch MG, Li Y, Petricoul O, Cabanski M, Milojevic JM, et al. Treatment of primary Sjögren’s syndrome with ianalumab (VAY736) targeting B cells by BAFF receptor blockade coupled with enhanced, antibody-dependent cellular cytotoxicity. Ann Rheum Dis. (2019) 78:641–7. doi: 10.1136/annrheumdis-2018-214720
149. Bowman SJ, Fox R, Dörner T, Mariette X, Papas A, Grader-Beck T, et al. Safety and efficacy of subcutaneous ianalumab (VAY736) in patients with primary Sjögren’s syndrome: a randomised, double-blind, placebo-controlled, phase 2b dose-finding trial. Lancet. (2022) 399:161–71. doi: 10.1016/S0140-6736(21)02251-0
150. Two-arm study to assess efficacy and safety of Ianalumab (VAY736) in patients with active Sjogren’s syndrome (NEPTUNUS-1). Available online at: https://clinicaltrials.gov/ct2/results?pg=1&load=cart&id=NCT05350072.
151. Three-arm study to assess efficacy and safety of Ianalumab (VAY736) in patients with active Sjogren’s syndrome (NEPTUNUS-2). Available online at: https://clinicaltrials.gov/ct2/results?pg=1&load=cart&id=NCT05349214.
152. Study of efficacy and safety of VAY736 in patients with pemphigus vulgaris. Available online at: https://clinicaltrials.gov/ct2/show/NCT01930175?term=VAY736&draw=6&rank=10.
153. Isenberg D, et al. Efficacy and safety of atacicept for prevention of flares in patients with moderate-to-severe systemic lupus erythematosus (SLE): 52-week data (APRIL-SLE randomised trial). Ann Rheum Dis. (2015) 74:2006–15. doi: 10.1136/annrheumdis-2013-205067
154. Gordon C, Wofsy D, Wax S, Li Y, Pena Rossi C, Isenberg D, et al. Post hoc analysis of the phase II/III APRIL-SLE study: association between response to atacicept and serum biomarkers including BLyS and APRIL. Arthritis Rheumatol. (2017) 69:122–30. doi: 10.1002/art.39809
155. Efficacy and safety of atacicept in systemic lupus erythematosus (ADDRESS II) . Available online at: https://clinicaltrials.gov/ct2/results?pg=1&load=cart&id=NCT01972568.
156. Merrill JT, Wallace DJ, Wax S, Kao A, Fraser PA, Chang P, et al. Efficacy and safety of atacicept in patients with systemic lupus erythematosus: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled, parallel-arm, phase IIb study. Arthritis Rheumatol. (2018) 70:266–76. doi: 10.1002/art.40360
157. Long-term safety and tolerability of atacicept (Long-term Follow-Up of Participant Who Participated in ADDRESS II). Available online at: https://clinicaltrials.gov/ct2/show/NCT02070978?id=NCT02070978&draw=2&rank=1&load=cart.
158. Wallace DJ, Isenberg DA, Morand EF, Vazquez-Mateo C, Kao AH, Aydemir A, et al. Safety and clinical activity of atacicept in the long-term extension of the phase 2b ADDRESS II study in systemic lupus erythematosus. Rheumatol (Oxford). (2021) 60:5379–89. doi: 10.1093/rheumatology/keab115
159. Atacicept in subjects with active lupus nephritis (COMPASS) (COMPASS) . Available online at: https://clinicaltrials.gov/ct2/results?pg=1&load=cart&id=NCT05609812.
160. Nie Y, Zhao L, and Zhang X. B cell aberrance in lupus: the ringleader and the solution. Clin Rev Allergy Immunol. (2022) 62:301–23. doi: 10.1007/s12016-020-08820-7
161. Study of RC18 administered subcutaneously to subjects with Systemic Lupus Erythematosus(SLE). Available online at: https://clinicaltrials.gov/ct2/show/NCT02885610?id=NCT02885610&draw=2&rank=1&load=cart.
162. Study of recombinant human B lymphocyte(RC18) administered subcutaneously to subjects with Systemic Lupus Erythematosus(SLE). Available online at: https://clinicaltrials.gov/ct2/results?pg=1&load=cart&id=NCT04082416.
163. A study of telitacicept for the treatment of moderately to severely active systemic lupus erythematosus . Available online at: https://clinicaltrials.gov/ct2/results?pg=1&load=cart&id=NCT05306574.
164. A study of TACI-antibody fusion protein injection (RC18) in subjects with primary Sjögren’s syndrome . Available online at: https://clinicaltrials.gov/ct2/results?pg=1&load=cart&id=NCT04078386.
165. A study to evaluate the efficacy and safety of telitacicept in subjects with active primary Sjogren’s syndrome. Available online at: https://clinicaltrials.gov/ct2/show/NCT05673993?id=NCT05673993&draw=2&rank=1&load=cart.
166. Atisha-Fregoso Y, Malkiel S, Harris KM, Byron M, Ding L, Kanaparthi S, et al. Phase II randomized trial of rituximab plus cyclophosphamide followed by belimumab for the treatment of lupus nephritis. Arthritis Rheumatol. (2021) 73:121–31. doi: 10.1002/art.41466
167. Teng YKO, Bruce IN, Diamond B, Furie RA, van Vollenhoven RF, Gordon D, et al. Phase III, multicentre, randomised, double-blind, placebo-controlled, 104-week study of subcutaneous belimumab administered in combination with rituximab in adults with systemic lupus erythematosus (SLE): BLISS-BELIEVE study protocol. BMJ Open. (2019) 9:e025687. doi: 10.1136/bmjopen-2018-025687
168. Aranow C, Allaart CF, Amoura Z, Bruce IN, Cagnoli PC, Chatham WW, et al. Efficacy and safety of sequential therapy with subcutaneous belimumab and one cycle of rituximab in patients with systemic lupus erythematosus: the phase 3, randomised, placebo-controlled BLISS-BELIEVE study. Ann Rheum Dis. (2024) 83:1502–12. doi: 10.1136/ard-2024-225686
169. Synergetic B-cell immunomodulation in SLE - 2nd study. (SynBioSe-2) . Available online at: https://clinicaltrials.gov/ct2/results?pg=1&load=cart&id=NCT03747159.
170. Mariette X, Barone F, Baldini C, Bootsma H, Clark KL, De Vita S, et al. A randomized, phase II study of sequential belimumab and rituximab in primary Sjögren’s syndrome. JCI Insight. (2022) 7. doi: 10.1172/jci.insight.163030
171. Belimumab and Rituximab combination therapy for the treatment of diffuse cutaneous systemic sclerosis . Available online at: https://clinicaltrials.gov/ct2/results?pg=1&load=cart&id=NCT03844061.
172. Petricca L, Gigante MR, Paglionico A, Costanzi S, Vischini G, Di Mario C, et al. Rituximab followed by belimumab controls severe lupus nephritis and bullous pemphigoid in systemic lupus erythematosus refractory to several combination therapies. Front Med (Lausanne). (2020) 7:553075. doi: 10.3389/fmed.2020.553075
173. Ruan J, Zhang X, and Zheng M. Belimumab for the treatment of pemphigus. Dermatol Ther. (2022) 35:e15621. doi: 10.1111/dth.15621
174. Altman EM. Novel therapies for pemphigus vulgaris. Am J Clin Dermatol. (2020) 21:765–82. doi: 10.1007/s40257-020-00544-w
175. Kappos L, Hartung HP, Freedman MS, Boyko A, Radü EW, Mikol DD, et al. Atacicept in multiple sclerosis (ATAMS): a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Neurol. (2014) 13:353–63. doi: 10.1016/S1474-4422(14)70028-6
176. Dhillon S. Telitacicept: first approval. Drugs. (2021) 81:1671–5. doi: 10.1007/s40265-021-01591-1
177. A study to evaluate the efficacy and safety of telitacicept in subjects with active primary Sjogren’s syndrome . Available online at: https://clinicaltrials.gov/ct2/results?pg=1&load=cart&id=NCT05673993.
178. Lavie F, Miceli-Richard C, Ittah M, Sellam J, Gottenberg JE, and Mariette X. Increase of B cell-activating factor of the TNF family (BAFF) after rituximab treatment: insights into a new regulating system of BAFF production. Ann Rheum Dis. (2007) 66:700–3. doi: 10.1136/ard.2006.060772
179. Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. (2004) 20:785–98. doi: 10.1016/j.immuni.2004.05.010
Keywords: B cell-activating factor, autoimmune diseases, targeted therapies, autoimmunity, b cells, T cells
Citation: Li L, Shen S, Shao S, Dang E, Wang G, Fang H and Qiao H (2025) The role of B cell-activating factor system in autoimmune diseases: mechanisms, disease implications, and therapeutic advances. Front. Immunol. 16:1538555. doi: 10.3389/fimmu.2025.1538555
Received: 03 December 2024; Accepted: 21 May 2025;
Published: 06 June 2025.
Edited by:
Soheil Tavakolpour, Dana–Farber Cancer Institute, United StatesReviewed by:
Guilherme Ramires De Jesús, Rio de Janeiro State University, BrazilSébastien Calbo, Neuromusculaires et THErapies Régénératrices (PANTHER), France
Copyright © 2025 Li, Shen, Shao, Dang, Wang, Fang and Qiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongjiang Qiao, eGpob25namlhbmdxQGZtbXUuZWR1LmNu; Hui Fang, ZmFuZ2h1aTg4MjIxQDE2My5jb20=; Gang Wang, eGp3Z2FuZ0BmbW11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Liang Li
Liang Li Shengxian Shen
Shengxian Shen Shuai Shao
Shuai Shao Erle Dang
Erle Dang Gang Wang
Gang Wang Hui Fang
Hui Fang Hongjiang Qiao
Hongjiang Qiao