- 1Department of Pharmacy, Cancer Hospital of China Medical University, Liaoning Cancer Hospital and Institute, Shenyang, Liaoning, China
- 2Department of General Surgery, Cancer Hospital of China Medical University, Liaoning Cancer Hospital and Institute, Shenyang, Liaoning, China
- 3Department of Colorectal Surgery, Cancer Hospital of China Medical University, Liaoning Cancer Hospital and Institute, Shenyang, Liaoning, China
Background: Immunotherapy has become the first-line treatment for metastatic mismatch repair deficient (dMMR) colorectal cancer. The efficacy and safety of neoadjuvant immunotherapy for the treatment of non-metastatic dMMR colorectal cancer remain unclear. In this article, we explore the clinical effect and safety of neoadjuvant immunotherapy for non-metastatic dMMR colorectal cancer.
Methods: We collected clinical data from the databases (PubMed, Wanfang Embase, Cochrane Library, and China National Knowledge Infrastructure databases) up to November 2024. The primary outcomes of major pathological response (MPR), pathological complete response (pCR), and other outcomes were analyzed for the final results. The secondary outcomes (pCR rates for the subgroups) were also analyzed.
Results: We included 21 articles with 628 non-metastatic dMMR colorectal cancers. A pCR was found in 320/480 (66.6%) patients [effect size (ES): 0.70, 95% CI: 0.66 to 0.74] with the fixed-effects model and little heterogeneity. A MPR was found in 388/452 (85.8%) patients (ES: 0.86, 95% CI: 0.81 to 0.91) with the fixed-effects model and little heterogeneity. In the subgroup analysis, pCR rates were similar in the T1-T3 group and T4a-T4b group in the fixed-effects model with minimal heterogeneity (OR: 0.76, 95% CI: 0.48 to 1.22). The pCR rates were similar in the colon cancer group and rectal cancer group in the fixed-effects model with minimal heterogeneity (OR: 1.41, 95% CI: 0.39 to 5.12). Similar pCR rates were found in the immune monotherapy group and immune therapy plus chemotherapy group (OR: 0.74, 95% CI: 0.26 to 2.10) with the fixed-effects model and little heterogeneity.
Conclusion: Neoadjuvant immunotherapy achieves high rates of pCR and MPR for non-metastatic dMMR colorectal cancer. For locally advanced T4 stage dMMR colorectal cancer, neoadjuvant immunotherapy can still achieve good pCR rates. Neoadjuvant immune monotherapy can achieve good pCRs rates, avoiding the toxic side effects caused by combined dual immunotherapy and chemo(radio)therapy. Neoadjuvant immunotherapy could be another treatment option for non-metastatic dMMR colorectal cancer.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42024594173.
Background
The high incidence rate of colorectal cancer (CRC) has seriously threatened human health (1). CRC can be classified into mismatch repair-deficient (dMMR) CRC and mismatch repair-proficient (pMMR) CRC based on the absence of MMR protein expression (2). Microsatellite instability (MSI) status, detected by polymerase chain reaction (PCR), is divided into microsatellite stable (MSS) and microsatellite instability-high (MSI-H). dMMR/MSI-H CRC accounted for approximately 15% of non-metastatic CRC (3, 4). Neoadjuvant therapy is an important treatment mode for CRC, especially for rectal cancer (5, 6). The FOxTROT study reported that most dMMR/MSI-H colon cancer patients had little response to neoadjuvant chemotherapy (FOLFOX or CAPOX) (7). Some relevant retrospective studies showed that dMMR/MSI-H rectal cancer had disease progression with neoadjuvant chemotherapy (fluorouracil plus oxaliplatin), but there was no disease progression in the pMMR/MSS group with neoadjuvant chemotherapy (8).
The KEYNOTE-016 study (NCT01876511) indicated that immunotherapy benefits patients with metastatic dMMR/MSI-H CRC (9). The original NICHE study cohort reported a major pathological response (MPR) rate of 95% and a pathological complete response (pCR) rate of 60% for non-metastatic dMMR/MSI-H colon cancer patients after neoadjuvant immunotherapy (10). The European Society of Oncology (ESMO) guidelines recommend pembrolizumab or nivolumab ± ipilimumab as the first-line treatment for dMMR/MSI-H metastatic CRC. Immunotherapy is superior to chemotherapy in terms of survival benefits, quality of life, and tolerability (11). Although some studies have confirmed the efficacy of neoadjuvant immunotherapy for locally advanced dMMR/MSI-H CRC, there is no relevant guideline recommending whether neoadjuvant immunotherapy can be used in non-metastatic dMMR/MSI-H CRC (12). Current evidence primarily comes from small phase II trials, which showed high pathological response rates but lacked long-term survival outcomes. Existing guidelines for non-metastatic dMMR/MSI-H CRC have lagged behind emerging evidence, reflecting gaps in evidence quality, protocol standardization, and practical implementation (2). Guidelines have hesitated to endorse neoadjuvant immunotherapy without Phase III validation.
This study focused on dMMR/MSI-H specifically due to its unique biological characteristics, including high tumor mutational burden (TMB) and increased neoantigen production, which are associated with enhanced immunogenicity and responsiveness to immune checkpoint inhibitors (13). These features make dMMR/MSI-H tumors particularly susceptible to immunotherapy, as demonstrated by higher objective response rates and durable clinical benefits in clinical trials.
Non-dMMR/MSI-H patients have a low response rate to immunotherapy (<5%). MMR/MSI status is recommended to be routinely tested (via immunohistochemistry or molecular testing) before initiating immunotherapy to identify potential beneficiaries. This testing helps avoid unnecessary toxicity and economic burden (14). Therefore, we collected relevant articles on neoadjuvant immunotherapy for non-metastatic dMMR/MSI-H CRC and tried to explain the safety and efficacy of neoadjuvant immunotherapy for non-metastatic dMMR/MSI-H CRC.
Methods
Literature search
This systematic review was registered on the PROSPERO website (CRD42024594173). Supplementary Material 1 provides the details. We performed the systematic review according to the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) (Supplementary Material 2).
We obtained relevant articles from various databases (Embase, PubMed, Cochrane Library, China National Knowledge Infrastructure (CNKI), and Wanfang databases). We searched the literature up to November 2024. The search terms were “colorectal cancer” and “mismatch repair-deficient” and “neoadjuvant immunotherapy” or “PD-1” or “CTLA-4”.
The PICO (population, intervention, comparator, and outcomes) model was followed to guide our literature research for the subgroup analysis. We used one of the subgroup analyses as an example. The population included non-metastatic dMMR/MSI-H CRC patients. The intervention was the colon cancer group. The comparator was the rectal cancer group. The outcome was a pCR.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) non-metastatic dMMR/MSI-H CRC; (2) prospective study, retrospective study, single-arm study, cohort study, and RCTs; (3) the included patients received neoadjuvant immunotherapy.
The exclusion criteria were as follows: (1) pMMR/MSS or metastatic CRC; (2) case reports, meeting, letter, and other unsuitable article types; (3) no neoadjuvant immunotherapy.
In meta-analyses, conference abstracts and case reports are excluded due to considerations of methodological rigor and result reliability. Conference abstracts typically provide only preliminary results and lack detailed study designs, methodological descriptions, and complete results. Case reports have low evidence levels, lack scientific inferential value, and carry a high risk of bias. The PRISMA guidelines explicitly recommend prioritizing the inclusion of peer-reviewed full-text publications and excluding sources with incomplete or unverified information (conference abstracts and case reports) (15).
Data extraction and quality assessment
Two reviewers (HXC and FJW) searched the relevant studies in the literature and sorted the useful clinical data independently with the help of the revised version of MINORS (methodological index for non-randomized studies). The revised version of MINORS was used for the quality assessment of observational or non-randomized studies (16). The third reviewer (GYZ) resolved the inconsistencies between the above two authors.
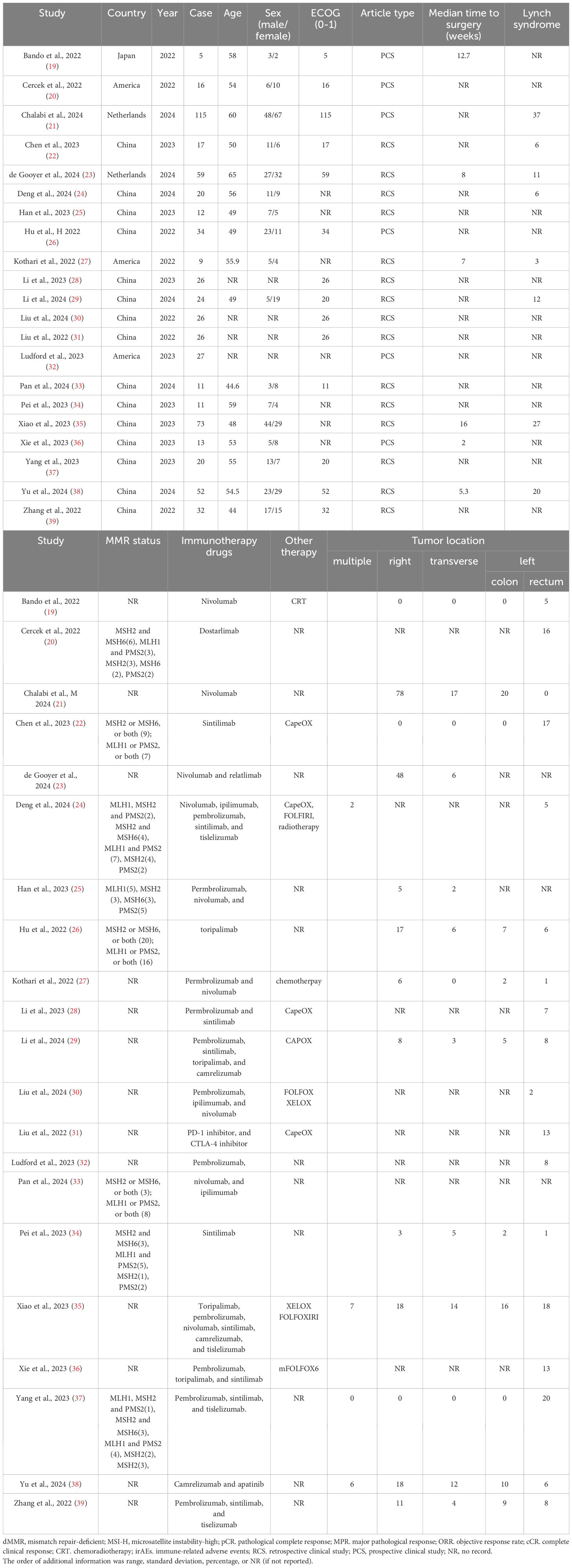
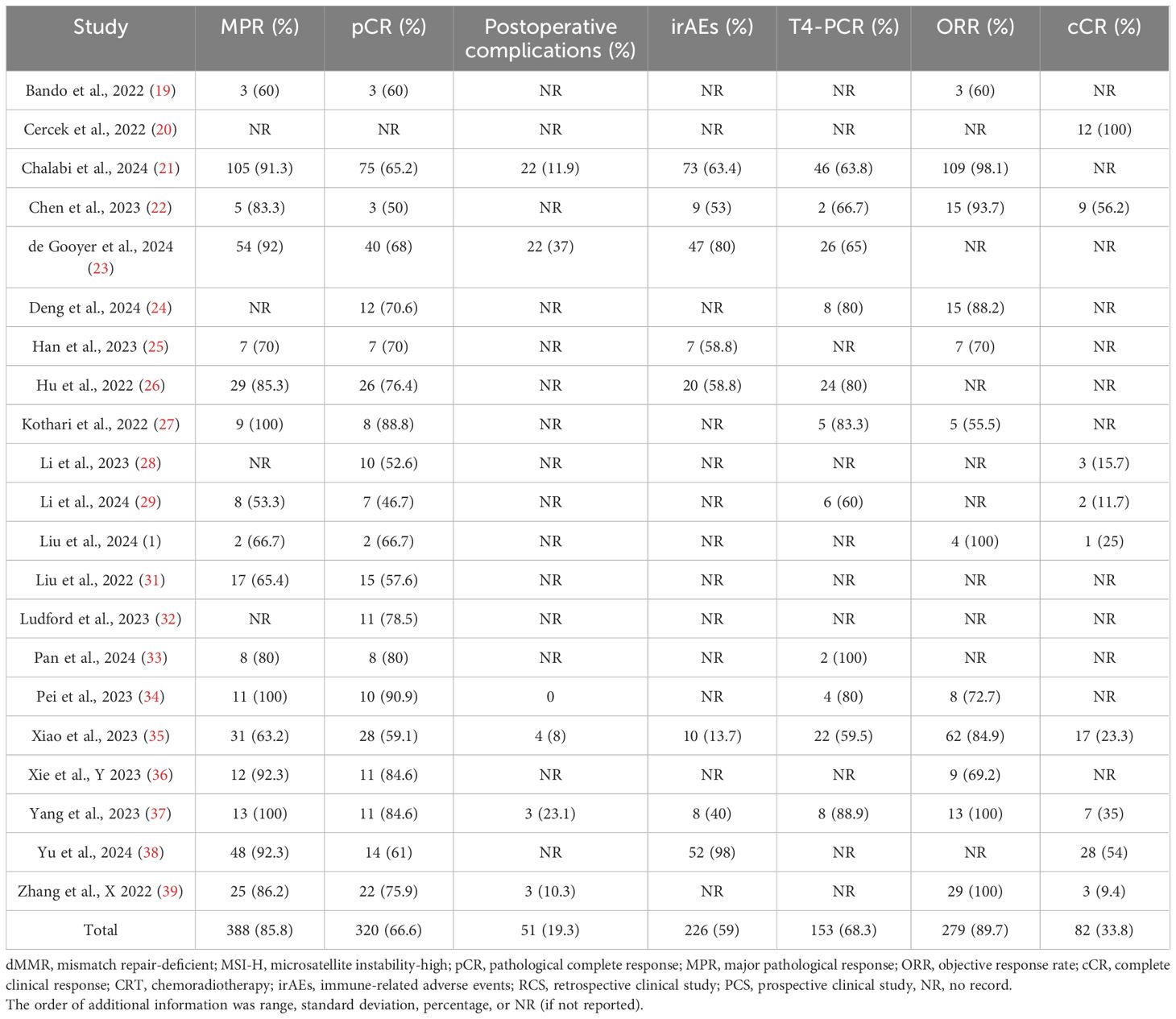
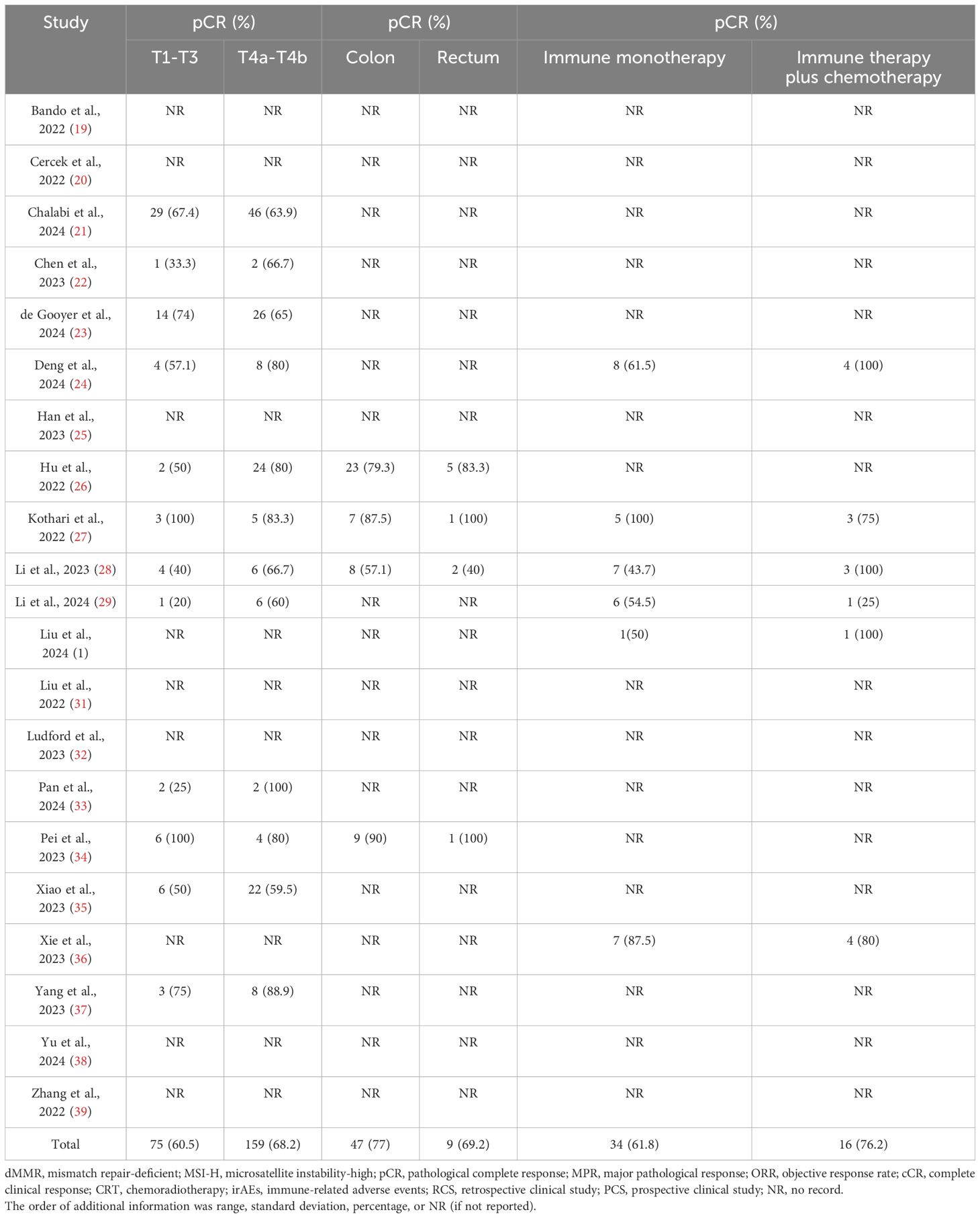
Tables 1–3 showed the baseline data (such as sex, country, age, and MMR status), the primary outcomes (MPR, pCR, and so on), and secondary outcomes (pCR rates for the subgroups). The details of the clinical stage and neoadjuvant immunotherapy plan are shown in Supplementary Materials 3, 4, respectively. No further information was obtained from the relevant authors of the included studies.
Statistical analysis
We used STATA software to perform statistical analysis on single-group binary variables. The effect size (ES) in the results typically reflected the overall effect derived from specific statistical approaches, such as Logit transformation or Freeman–Tukey double arcsine transformation (17). For the statistical analysis of two-group binary variables (colon vs. rectal cancer group), we utilized the risk ratio {RR=[a/(a+b)]/[c/(c+d)]} or odds ratio (OR=a*d/b*c) to compare the relative risks of event occurrence between the two groups using RevMan software. A random effects model and fixed effects model were used to analyze the data with high heterogeneity (I2≧50%) and low heterogeneity (I2<50%), respectively. The fixed-effects model had high statistical power, with narrow confidence intervals, making the results more “significant” and suitable for idealized scenarios. However, in studies with high heterogeneity, it could lead to false-positive results. In contrast, the random-effects model produced more conservative statistical results, which were more robust and better suited for heterogeneous scenarios. Nevertheless, it had lower statistical power and was sensitive to small sample sizes, making it more aligned with real-world situations (18). Publication bias was assessed using funnel plots.
Results
Study selection
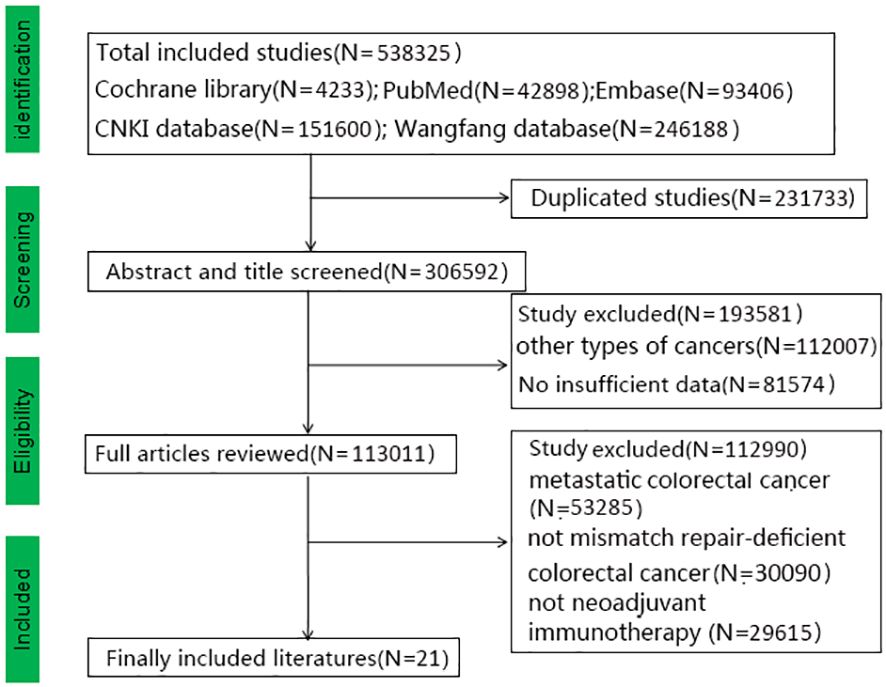
We obtained 538,325 relevant studies from the medical databases. According to the inclusion and exclusion criteria, we excluded studies on metastatic colorectal cancer (N=53,285) and those that did not include mismatch repair-deficient colorectal cancer (N=30,090) and neoadjuvant immunotherapy (N=29,615) (Figure 1). In total, 21 articles with 628 patients with non-metastatic dMMR colorectal cancer were collected, including 474 (75.5%) with colon cancer and 154 (24.5%) with rectal cancers (19–39). Table 1 shows the baseline data of the included studies. Eight studies reported MMR status. Alterations in MSH2 were most common while pathogenic alterations in MSH6, MLH1, and PMS2 were also present. There were 15 retrospective studies (13 Eastern studies and 2 Western studies) and 6 prospective studies (3 Eastern studies and 3 Western studies). All the patients had an Eastern Cooperative Oncology Group Performance Status (ECOG) score of 0-1. All the included studies achieved 12–14 points with high-moderate quality according to MINORS standard and the specific information is provided in Supplementary Material 5. Chalabi et al. (2024) and Chalabi et al. (2022) collected the same patients (the first patient was enrolled in March 2017), therefore this study did not include Chalabi et al. (2022) (10, 21). Li et al. (2023) included colorectal cancer, duodenal cancer, gastric cancer, and colorectal liver metastases (28). We selected the eligible patients according to the criteria, which could result in some missing data (such as postoperative complications, MRP, and others). Pembrolizumab, nivolumab, and sintilimab were the most used immunotherapy drugs. Other immunotherapy drugs, such as dostarlimab, toripalimab, camrelizumab, tiselizumab, and others, were also used for colorectal cancer. Bando (2022) used neoadjuvant immunotherapy combined with chemoradiotherapy (capecitabine and radiation to a dose of 50.4 Gy), while neoadjuvant immunotherapy combined with chemotherapy (CapeOX or FOLFOXIRI) was used in some studies (Kothari, 2022; Xiao, 2023; Liu, 2022; Li, 2023) (19, 27, 28, 31, 35).
According to the RECIST criteria, the objective response rate (ORR) was 89.7% after neoadjuvant immunotherapy. A pCR and cCR were observed in 320 (66.6%) and 82 (33.8%) patients after neoadjuvant immunotherapy, respectively. Table 2 shows the information on the primary outcomes [pCR, MPR, postoperative complications, immune-related adverse events (irAEs), T4-pCR, and ORR]. Table 3 shows the information on the secondary outcomes, i.e., pCR rates between different subgroups.
Primary outcomes: pCR, MPR, postoperative complications, immune-related adverse events, T4-pCR, and ORR
The pCR rate was reported by 20 studies, and 320/480 (66.6%) patients had a pCR (ES: 0.70, 95% CI: 0.66 to 0.74, P<0.01, chi2 = 29.69, P>0.05, I2 = 36%, Figure 2) and there was low heterogeneity, thus the fixed-effects model was used. MPR was reported by 17 studies. An MPR was found in 388/452 (85.8%) patients (ES: 0.86, 95% CI: 0.81 to 0.91, P<0.01, chi2 = 31.31, P<0.05, I2 = 48.9%, Figure 2), and there was low heterogeneity, thus, the fixed-effects model was used.

Figure 2. Primary outcomes of neoadjuvant immunotherapy for non-metastatic MMR colorectal cancer. (A) pathological complete response (PCRS) (B) major pathological response(MPR): (C) objective response rate (ORR): (D) PCRe for 14 stage : (E) postoperative (F) immune-related adverse events (irAEs).
In total, 51 cases of postoperative complications occurred. The postoperative complications rate was estimated at 19.3% (ES: 0.18, 95% CI: 0.07 to 0.29, P<0.01, chi2 = 17.50, P<0.01, I2 = 82.9%, Figure 2) with the random-effects model and there was high heterogeneity. The irAE rate was estimated at 59% (226/383) (ES: 0.58, 95% CI: 0.31 to 0.85, P<0.01, chi2 = 404.9, P<0.01, I2 = 98.3%, Figure 2) with the random-effects model and there was high heterogeneity.
The T4-pCR rate was reported in 11 studies. A T4-pCR was found in 153/224 (68.3%) patients (ES: 0.70, 95% CI: 0.64 to 0.76, P<0.01, chi2 = 11.1, P=0.35, I2 = 9.9%, Figure 2) with the fixed-effects model and there was low heterogeneity. ORR was reported by 12 studies and it was found in 279/311 (89.7%) patients (ES: 0.88, 95% CI: 0.81 to 0.94, P<0.01, chi2 = 31.6, P<0.01, I2 = 65.2%, Figure 2) with the random-effects model. There was high heterogeneity.
Secondary outcomes (subgroup analysis): pCR for T1-T3 vs. T4a-T4b, pCR for colon cancer vs. rectal cancer, and pCR for immune monotherapy vs. immunetherapy plus chemotherapy
In total, 12 studies reported the clinical data of pCR for a T1-T3 group and a T4a-T4b group. The pCR rate was similar in the two groups in the fixed-effects model with minimal heterogeneity (OR: 0.76, 95% CI: 0.48 to 1.22, P=0.26, chi2 = 9.75, P=0.55, I2 = 0%, Figure 3). Four studies reported the clinical data of pCR for the colon cancer group and rectal cancer group. The pCR rate was similar in the two groups in the fixed-effects model with minimal heterogeneity (OR: 1.41, 95% CI: 0.39 to 5.12, P=0.6, chi2 = 0.43, P=0.93, I2 = 0%, Figure 3). Six studies reported the clinical data of pCR for an immune monotherapy group and an immune therapy plus chemotherapy group. The pCR rate was similar in the two groups in the fixed-effects model with little heterogeneity (OR: 0.74, 95% CI: 0.26 to 2.10, P=0.57, chi2 = 5.29, P=0.38, I2 = 5%, Figure 3).
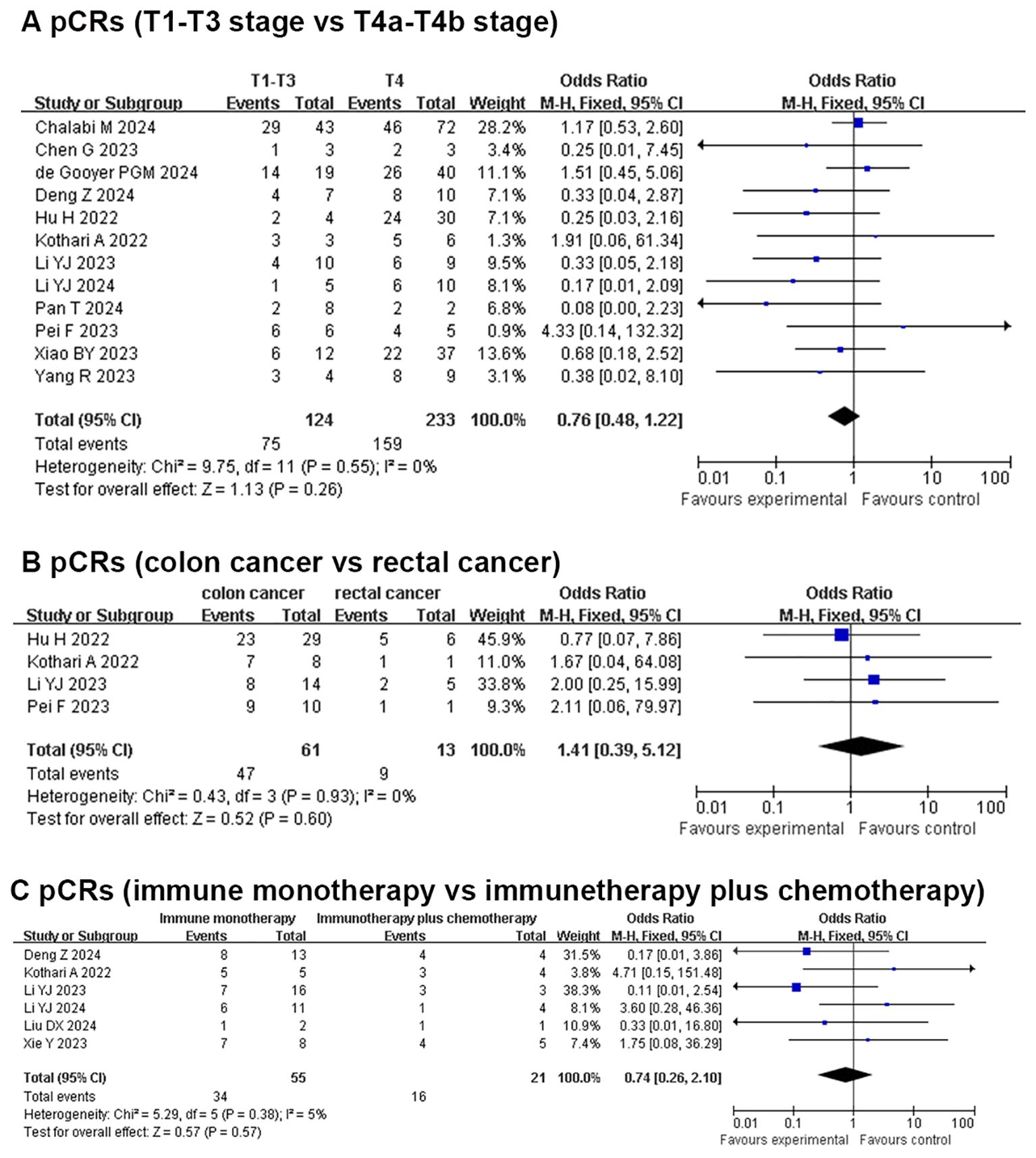
Figure 3. Secondary outcomes of neoadjuvant immunotherapy for non-metastatic dMMR colorectal cancer. (A) PCRs (T1-T3 stage vs T4a-T4b stage); (B) PCRs (colon cancer vs rectal cancer); (C) PCRs (immune monotherapy vs immunetherapy plus chemotherapy).
Publication bias
The publication bias was visualized using RevMan 5.0 software with the clinical data of pCR. We found that the points were evenly distributed in the forest plot.
Discussion
The DNA mismatch repair system is a testing system composed of multiple interacting proteins. The system recognizes and repairs base mismatches that occur during DNA replication after activation. The loss of function of any protein in MMR can cause defects in the MMR system, leading to the accumulation of microsatellite errors and making it susceptible to being attacked by the immune system (40, 41). Therefore, immunotherapy can mobilize immune cells to effectively kill tumor cells in dMMR/MSI-H tumors. The KEYNOTE-177 study demonstrated that immunotherapy improves survival time and ORR for metastatic dMMR/MSI-H CRC. The National Comprehensive Cancer Network (NCCN) guidelines recommend pembrolizumab as the first-line treatment of metastatic/unresectable dMMR/MSI-H CRC (Category 1 recommendation) (42).
At present, for some malignant tumors (such as melanoma, urothelial carcinoma, and other tumors), immunotherapy is not only applied in the first-line treatment for metastatic tumors but has also achieved significant clinical results in neoadjuvant immunotherapy (43, 44). A clinical case report reported two patients with locally advanced rectal cancer with dMMR/MSI-H status who received neoadjuvant immunotherapy (nivolumab) and achieved a complete response (CR) (45). The NICHE study showed that all the patients with dMMR/MSI-H colon cancer responded to neoadjuvant nivolumab plus ipilimumab with a high pCR rate (46). As the efficacy and safety of neoadjuvant immunotherapy for non-metastatic dMMR/MSI-H CRC has not been fully elucidated, we tried to use the available clinical data to explain the safety and clinical effects of neoadjuvant immunotherapy for non-metastatic dMMR/MSI-H CRC.
Outcome results
pCR, MPR, postoperative complications, immune-related adverse events, T4-pCR, and ORR
The pCR rate for neoadjuvant chemo(radio)therapy for CRC was approximately 5%–15%. The pCR rate for neoadjuvant immunotherapy was 36% for non-metastatic CRC, while the pCR rate for neoadjuvant immunotherapy was 59.6% for non-metastatic dMMR/MSI-H CRC (47, 48). In our study, the pCR rate for neoadjuvant immunotherapy was 66.9% for non-metastatic dMMR/MSI-H CRC. Especially with the addition of the NICHE-2 study, the increased clinical sample sizes made the results more convincing. In Rahma et al.’s study, it was further confirmed that neoadjuvant immunotherapy had higher pCR and R0 resection rates than neoadjuvant chemoradiotherapy (49).
Loria et al. found that 50% to 91% of non-metastatic dMMR CRC with neoadjuvant immunotherapy could achieve ypT0N0 pathology (50). In our previous research, we further confirmed the excellent clinical efficacy of neoadjuvant immunotherapy (51). The pCR rate for neoadjuvant immunotherapy for locally advanced T4 stage dMMR/MSI-H CRC was 68.2%, which was exciting news for partially locally advanced T4b tumors (52). For locally advanced T4b tumors with dMMR/MSI-H status, the NCCN recommends neoadjuvant immunotherapy, while the short courses of neoadjuvant immune checkpoint inhibition will continue to expand with more ongoing clinical trials. The MPR and ORR rates were 85.8% and 89.7%, respectively, in our study. Compared with the clinical statistics of neoadjuvant chemo(radio)therapy, MPR and ORR were significantly improved. These data reflected the effectiveness of neoadjuvant immunotherapy at the pathological and imaging levels. For locally advanced T4b tumors with dMMR/MSI-H status, neoadjuvant immunotherapy can cause tumor downgrading and transform unresectable T4b tumors into resectable T1-T4a tumors, which is beneficial for complete resection and achieving curative effects.
Adverse events and postoperative complications
The most common irAEs were endocrine disorders, skin disease, gastrointestinal reactions, and others (53). The incidence of irAEs was 59% in our study, similar to the incidence of irAEs reported in other studies (54). Most irAEs were grade I-II with mild clinical reactions or laboratory abnormalities and could be treated symptomatically. The incidence of severe irAEs was very low, including hepatic damage, neurological damage, cardiac toxicity, and others. Severe irAEs required immediate discontinuation and timely treatment. Therefore, choosing a suitable immunotherapy drug, dosage, and administration time could effectively avoid the occurrence of irAEs.
The incidence of postoperative complications was 19.3%, and most postoperative complications were abdominal infection and anastomotic leakage (grade I-II). Chalabi et al.’s (2024) study reported grade III postoperative complications with an incidence rate of 10%. Most patients with postoperative complications were discharged smoothly with conservative symptomatic treatment (55).
One multicenter retrospective study reported that 76 patients with locally advanced rectal cancer were given 45 Gy for pelvis tumors, and 52.5 to 57.5 Gy for local tumors. The pCR rate was 27.8%, the incidence of surgical complications was 18.1%, and the incidence of grade 3-4 adverse reactions was 10.5% (56). Immunotherapy was different from chemo(radio)therapy and did not increase tissue edema and fibrosis, which was beneficial for surgery. Based on the above results, we speculate that neoadjuvant immunotherapy did not significantly increase postoperative complications, but more studies are still needed to confirm this.
Subgroup analysis: pCR for T1-T3 vs. T4a-T4b, pCR for colon cancer vs. rectal cancer, and pCR for immune monotherapy vs. immunetherapy plus chemotherapy
The pCR rate was similar in the T1-T3 group (60.5%) and the T4a-T4b group (68.2%) after neoadjuvant immunotherapy without statistical difference. For locally advanced T4b patients with dMMR/MSI-H status, neoadjuvant immunotherapy was beneficial for tumor progression and achieving curative resection (57). The MRP and pCR rates were 85.4% and 59.5% in T4b tumor with neoadjuvant immunotherapy, as reported by Xiao et al. (2023), with an acceptable safety profile and a low recurrence rate (35). The incidence of irAEs and surgical complications was similar to other studies. Therefore, we speculate that neoadjuvant immunotherapy could also preserve organs, reduce surgical trauma, and avoid combined organ resection, especially for non-metastatic unresectable T4b CRC with dMMR/MSI-H status.
The rectum is located in the pelvic cavity, with an embryonic origin in the hindgut, and its anatomical structure is complex (proximity to the anal sphincter and rich neurovascular supply), making surgery and radiotherapy challenging. Some patients require a permanent colostomy due to low tumor location necessitating anal resection. In contrast, a right hemicolectomy (originating from the embryonic midgut) and left hemicolectomy (originating from the hindgut) were associated with relatively lower surgical difficulty, did not involve permanent colostomy, and caused less trauma to patients (58). The proportion of MSI-H was higher in right-sided colon cancer (approximately 15%–20%) compared to rectal cancer (approximately 5%–10%). In our study, the pCR rate was similar in colon cancer (77%) and rectal cancer (69.2%) after neoadjuvant immunotherapy, and there was no statistical difference between the two groups. The pCR rate for neoadjuvant immunotherapy was much higher than the pCR rate for neoadjuvant chemo(radio)therapy. High pCRs rate of neoadjuvant immunotherapy could improve the rectal sphincter preservation rate without surgery, especially for low advanced rectal cancer (59). It could eliminate the trauma caused by surgery, improve the quality of life and avoid adverse events such as anterior resection syndrome and abdominal infections. In addition, watch and wait (W&W) strategy may be safe for rectal cancer patients with cCR status after neoadjuvant immunotherapy, anal examination, colonoscopy and MRI were need to completed regularly (60).
In our study, the pCR rate was similar in the immune monotherapy group (61.8%) and immune therapy plus chemotherapy group (76.2%). Yang et al. (2023) reported the efficacy of PD-1 monotherapy for locally advanced rectal cancer with dMMR/MSI-H status and the pCR and MRP rates were 84.6% and 100%, respectively (37). Li et al. (2024) pointed out that neoadjuvant immune monotherapy could achieve a high tumor complete response in initially resected difficult dMMR/MSI-H CRC. Neoadjuvant immune monotherapy could achieve cCR status, even for stage IV colorectal cancer (29). The KEYNOTE-177 trial demonstrated that pembrolizumab monotherapy as first-line treatment for dMMR/MSI-H metastatic CRC achieved a median progression-free survival (PFS) of 16.5 months (vs. 8.2 months with chemotherapy) and a 3-year overall survival rate of 55%vs. 48.6%. The incidence of grade 3-4 immune-related adverse events (irAEs) was approximately 14-20%,primarily including colitis (8%) and hepatitis (5%) (61). The treatment was well-tolerated and particularly suitable for elderly patients (>70 years) or those with complications. Immune monotherapy is considered a highly effective and low-toxicity first-line option.
In contrast, combination immunotherapy aimed for higher response rates at the cost of increased toxicity. The CheckMate-142 trial showed that nivolumab plus ipilimumab in metastatic dMMR/MSI-H CRC resulted in an ORR of 69%, a CR rate of 13%, and durable responses. However, the incidence of grade 3-4 irAEs was 32% (12% colitis, 10% hepatitis), with a significantly increased need for hospitalization or immunosuppressive interventions (62). The KEYNOTE-651 trial indicated that pembrolizumab combined with FOLFOX chemotherapy as a first-line treatment for MSS CRC achieved an ORR of 45% but with a notable increase in grade 3-4 neutropenia (50%) and diarrhea (15%). Local radiotherapy combined with PD-1 inhibitors can activate the “abscopal effect,” but the risk of radiation enteritis (10%–20%) combined with immune-related colitis was a concern (63). Based on the above results, we speculated that patients with non-metastatic dMMR/MSI-H CRC could also benefit greatly from immune monotherapy. Immune monotherapy was the preferred first-line option due to its high efficacy and low toxicity, while combination therapy was reserved for high-risk patients requiring rapid symptom relief.
Novelty of the study and outlook for RCTs
We attempted to use the latest research to explore the clinical efficacy of neoadjuvant immunotherapy for non-metastatic dMMR/MSI-H CRC. Especially with the addition of the NICHE-2 study, the clinical data have become larger than ever before. We explored many observation indicators (pCR, MPR, postoperative complications, irAEs, and others) to clarify the advantages and disadvantages of neoadjuvant immunotherapy for non-metastatic dMMR/MSI-H CRC. We conducted a subgroup analysis and found that patients with T4 stage non-metastatic dMMR/MSI-H CRC can achieve a high pCR rate, and immune monotherapy could achieve good clinical efficacy.
Existing clinical trials focused primarily on monotherapy or combination therapy (PD-1 and CTLA-4). We expect more multi-arm randomized controlled trials (two-arm or three-arm designs) will clarify the advantages and disadvantages of different combination regimens (dual immunotherapy and immunotherapy combined with chemotherapy or targeted therapy) and define the efficacy-toxicity balance of these regimens. Long-term follow-up could reveal the curative potential and resistance mechanisms of immunotherapy (64). Since some dMMR/MSI-H patients did not respond well to immunotherapy, more refined stratification biomarkers are needed to enable personalized treatment based on multi-omics biomarkers.
Neoadjuvant immunotherapy plan, prognosis, and resistance mechanisms
PD-L1, PD-1, or CTLA-4 monoclonal antibodies can block tumor immune escape and restore the anticancer function of the autoimmune system (65). PD-1 blockade (pembrolizumab, nivolumab, sintilimab, toripalimab, camrelizumab, and others) and CTLA-4 blockade (ipilimumab and others) have been applied in immunotherapy for colorectal cancer, while the application of PD1 blockade is more widespread than CTLA-4 blockade. Many studies recommend using PD1 for 4-6 cycles, such as pembrolizumab (200mg every 3 weeks), nivolumab (240 mg every 2 weeks), dostarlimab (500 mg every 3 weeks), and others. The next treatment plan will then be determined based on relevant examinations. Chalabi et al. (2024) reported that PD-1 (two nivolumab 3 mg/kg) combined with CTLA-4 (single ipilimumab 1 mg/kg) blockade achieved a pCR rate of 68% and an MRP rate of 95% (21). At the same time, the incidence of irAEs was 63%, which was higher than that of the monotherapy group. Neoadjuvant immune monotherapy can reduce the irAEs of dual immunotherapy and chemo(radio)therapy. The results of ongoing clinical research could ascertain the direction of the immunotherapy treatment mode and a suitable population. There was no consensus on the clinical treatment of neoadjuvant immunotherapy combined with neoadjuvant radiotherapy (SCRT or IMRT, 25-50.4 Gy) and chemotherapy (FOLFOX or CAPOX) for non-metastatic dMMR/MSI-H CRC. Unlike pMMR/MSS CRC, neoadjuvant immunotherapy can achieve an effective clinical treatment effect for non-metastatic dMMR/MSI-H CRC. The selection of immunotherapy drugs, treatment cycles, and treatment plans was still inconclusive, and further research is needed to explore this.
Traditional chemotherapy agents (such as oxaliplatin and fluorouracil) were relatively low in cost and the drugs were covered under national medical insurance reimbursement programs with single-cycle costs typically ranging from ¥ 3,000- ¥ 5,000. However, chemotherapy required multiple treatment cycles (usually 4–6 cycles), and the total cost increased significantly when combined with expenses for imaging monitoring and side-effect management (bone marrow suppression and gastrointestinal reactions). In contrast, PD-1/PD-L1 inhibitors (such as pembrolizumab and camrelizumab) had higher per-cycle costs (approximately ¥15,000–¥30,000) and most were not fully covered by insurance, resulting in a higher out-of-pocket burden for patients. Nevertheless, immunotherapy required shorter treatment durations (typically 2–4 cycles) and it demonstrated rapid tumor regression for dMMR/MSI-H CRC, which reduced the complexity of subsequent surgery. Hence, neoadjuvant immunotherapy was potentially more cost-effective for dMMR/MSI-H CRC. Despite higher upfront drug costs, the pCRs and MRP rate was significantly superior to chemotherapy. It could reduce surgical difficulty and the risk of postoperative complications, thereby reducing long-term medical expenses.
Due to neoadjuvant immunotherapy being in the exploratory stage, there was no specific consensus on drug regimens, cycles, and dosages. At this time, PD-1, PD-L1, and CTLA-4 inhibitors are commonly used in clinical treatment, but the endpoints of the studies were similar, including MRP, PCR, ORR, OS, and other indicators. Therefore, we extracted common efficacy endpoints (ORR, MRP, PCR, OS, etc.) for analysis and identified the advantages and disadvantages of neoadjuvant immunotherapy for non-metastatic dMMR CRC (66). We aimed to standardize the dosage and treatment course and adopted subgroup analysis and sensitivity analysis to evaluate inconsistencies. When significant heterogeneity was observed, we used a random-effects model to combine studies with different designs or schemes, striving to minimize heterogeneity.
The cCR rate was 31.4% in our study. This was significantly higher than that of patients receiving neoadjuvant chemotherapy or pMMR patients with neoadjuvant immunotherapy. Cercek et al. (2022) reported that 12 patients had achieved cCR and no case had progression or recurrence during follow-up (6 to 25 months) (20). Pei et al. (2023) reported that all the patients in their study had no recurrence or metastasis for 335 days in non-metastatic dMMR/MSI-H CRC after neoadjuvant immunotherapy, while Xiao et al. (2023) found that there was no recurrence or metastasis for 2 years (34, 35). The NICHE-1 trial was the first phase II clinical study to investigate neoadjuvant immunotherapy in early-stage non-metastatic colon cancer. For dMMR/MSI-H patients, the trial demonstrated a major MPR rate of 95% (19/20) and pCRs rate of 60% (12/20). With a median follow-up of 25 months, none of the dMMR/MSI-H patients experienced recurrence, resulting in a 100% recurrence-free survival (RFS) rate (10). Neoadjuvant immunotherapy in dMMR/MSI-H CRC demonstrated remarkable pCRs rate and short-term survival advantages, but its conversion to long-term survival benefits still required higher-level evidence. Future efforts needed to focus on refinement of biomarkers, optimization of combination regimens, and long-term follow-up to validate the causal relationship between pathological responses and survival outcomes.
In dMMR/MSI-H tumors, although immunotherapy significantly improved the prognosis of some patients, approximately 20%–40% of patients exhibited primary or secondary resistance. These resistance mechanisms were complex, involving tumor-intrinsic characteristics, microenvironment regulation, and host immune status (67). Loss or mutation of HLA class I molecules and insufficient antigen diversity leads to antigen presentation defects, resulting in immune escape. Activation of oncogenic signaling pathways (Wnt/β-catenin pathway activation and IFN-γ signaling defects) and DNA methylation or chromatin remodeling contribute to tumor resistance (68, 69). Therefore, future research is needed to reveal resistance dynamics through multi-omics analysis (genomics, transcriptomics, and immunomics) and develop precise combination strategies to facilitate early identification of resistance and optimize treatment decisions.
Limitations
There were several limitations in the meta-analysis. First, inconsistencies between the neoadjuvant immunotherapy drugs and regimens included in the article may affect the results. Second, limited clinical data for neoadjuvant immunotherapy for non-metastatic dMMR/MSI-H CRC could affect the results. Third, single-arm studies without a control group can often enroll highly selected patients (those with good performance status, normal organ function, and others), which limits the generalizability of the results to real-world populations and increases the risk of selection bias. Single-arm studies focus primarily on efficacy endpoints such as ORR, MPR, and pCR, and do not assess PFS and OS (overall survival), which had certain limitations and could affect the robustness of conclusions.
Conclusion
Based on the above results, neoadjuvant immunotherapy was found to increase MPR and pCR rates for patients with non-metastatic dMMR/MSI-H CRC. Neoadjuvant immunotherapy did not increase the incidence of postoperative complications and irAEs, while neoadjuvant immune monotherapy can further reduce irAEs compared with dual immunotherapy combined with chemo(radio)therapy. For patients with locally advanced T4 stage dMMR/MSI-H CRC, neoadjuvant immunotherapy can still achieve good pCR rates. We expect that precise neoadjuvant immunotherapy drugs and regimens will be developed, which will increase MPR and pCR rates and other outcomes. furthermore, we also look forward to the emergence of more RCTs that can confirm the clinical effects of neoadjuvant immunotherapy for non-metastatic dMMR/MSI-H CRC. Neoadjuvant immunotherapy could be another treatment option for non-metastatic dMMR/MSI-H CRC, especially for locally advanced T4 stage dMMR/MSI-H CRC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
H-XC: Data curation, Investigation, Writing – original draft. X-QY: Software, Writing – original draft. G-yZ: Data curation, Formal analysis, Writing – original draft. F-jW: Investigation, Writing – review & editing. XL: Software, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Cancer Hospital of China Medical University. The funding project is Natural Science Foundation of Liaoning Province Fund of China (grant nos.2020-MS-060) and Pilot Cancer Research Fund Project of CSCO (Chinese society of clinical oncology; grant nos. Y-2019AZQN-0035).
Acknowledgments
We thank the Cancer Hospital of China Medical University and Liaoning Cancer Hospital and Institute for their technical assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1540751/full#supplementary-material.
Abbreviations
pCR, pathological complete response; MPR, major pathological response; MINORS, methodological index for non-randomized studies; ORR, objective response rate; RCT, randomized controlled trial; PNCT, prospective comparative nonrandomized trial; RNCT, retrospective nonrandomized controlled trials; cCR, complete clinical response; CNKI, China National Knowledge Infrastructure; dMMR, mismatch repair deficient; MSI-H, microsatellite instability-high; pMMR, mismatch repair proficient; MSS, microsatellite stable; CI, confidence interval; OR, Odds ratio; ES, Effect size; T1, T1 stage; T2, T2 stage; T3, T3 stage; T4, T4 stage; RCTs, randomized controlled trials; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PFS, progression-free survival; OS, overall survival; SMD, Standardized mean difference.
References
1. Jacobsson M, Wagner V, Kanneganti S. Screening for colorectal cancer. Surg Clin North Am. (2024) 104:595–607. doi: 10.1016/j.suc.2023.11.009
2. Taieb J, Svrcek M, Cohen R, Basile D, Tougeron D, Phelip JM. Deficient mismatch repair/microsatellite unstable colorectal cancer: Diagnosis, prognosis and treatment. Eur J Cancer. (2022) 175:136–57. doi: 10.1016/j.ejca.2022.07.020
3. Grillo F, Angerilli V, Parente P, Vanoli A, Luchini C, Sciallero S, et al. Prevalence and type of MMR expression heterogeneity in colorectal adenocarcinoma: therapeutic implications and reporting. Virchows Arch. (2024) 485:131–5. doi: 10.1007/s00428-023-03726-z
4. Greco L, Rubbino F, Dal Buono A, Laghi L. Microsatellite instability and immune response: from microenvironment features to therapeutic actionability-lessons from colorectal cancer. Genes (Basel). (2023) 14:1169. doi: 10.3390/genes14061169
5. Smith HG, Nilsson PJ, Shogan BD, Harji D, Gambacorta MA, Romano A, et al. Neoadjuvant treatment of colorectal cancer: comprehensive review. BJS Open. (2024) 8:zrae038. doi: 10.1093/bjsopen/zrae038
6. Daprà V, Airoldi M, Bartolini M, Fazio R, Mondello G, Tronconi MC, et al. Total neoadjuvant treatment for locally advanced rectal cancer patients: where do we stand? Int J Mol Sci. (2023) 24:12159. doi: 10.3390/ijms241512159
7. FOxTROT Collaborating Group. Risk of bowel obstruction in patients undergoing neoadjuvant chemotherapy for high-risk colon cancer: A nested case-control-matched analysis of an international, multicenter, randomized controlled trial (FOxTROT). Ann Surg. (2024) 280:283–93. doi: 10.1097/SLA.0000000000006145
8. Cercek A, Dos Santos Fernandes G, Roxburgh CS, Ganesh K, Ng S, Sanchez-Vega F, et al. Mismatch repair-deficient rectal cancer and resistance to neoadjuvant chemotherapy. Clin Cancer Res. (2020) 26:3271–9. doi: 10.1158/1078-0432.CCR-19-3728
9. Franke AJ, Skelton WP, Starr JS, Parekh H, Lee JJ, Overman MJ, et al. Immunotherapy for colorectal cancer: A review of current and novel therapeutic approaches. J Natl Cancer Inst. (2019) 111:1131–41. doi: 10.1093/jnci/djz093
10. Chalabi M, Fanchi LF, Dijkstra KK, Van den Berg JG, Aalbers AG, Sikorska K, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. (2020) 26:566–76. doi: 10.1038/s41591-020-0805-8
11. Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. (2023) 34:10–32. doi: 10.1016/j.annonc.2022.10.003
12. Yang L, Cui X, Wu F, Chi Z, Xiao L, Wang X, et al. The efficacy and safety of neoadjuvant chemoradiotherapy combined with immunotherapy for locally advanced rectal cancer patients: a systematic review. Front Immunol. (2024) 15:1392499. doi: 10.3389/fimmu.2024.1392499
13. Ambrosini M, Rousseau B, Manca P, Artz O, Marabelle A, André T, et al. Immune checkpoint inhibitors for POLE or POLD1 proofreading-deficient metastatic colorectal cancer. Ann Oncol. (2024) 35:643–55. doi: 10.1016/j.annonc.2024.03.009
14. Altshuler E, Franke AJ, Skelton WP4, Feely M, Wang Y, Lee JH, et al. Impact of institutional universal microsatellite-instability (MSI) reflex testing on molecular profiling differences between younger and older patients with colorectal cancer. Clin Colorectal Cancer. (2023) 22:153–9. doi: 10.1016/j.clcc.2022.09.004
15. Beach SR, Gomez-Bernal F, Huffman JC, Fricchione GL. Alternative treatment strategies for catatonia: A systematic review. Gen Hosp Psychiatry. (2017) 48:1–19. doi: 10.1016/j.genhosppsych.2017.06.011
16. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
17. Lin L, Xu C. Arcsine-based transformations for meta-analysis of proportions: Pros, cons, and alternatives. Health Sci Rep. (2020) 3:e178. doi: 10.1002/hsr2.178
18. Min L, Ablitip A, Wang R, Luciana T, Wei M, Ma X. Effects of exercise on gut microbiota of adults: A systematic review and meta-analysis. Nutrients. (2024) 16:1070. doi: 10.3390/nu16071070
19. Bando H, Tsukada Y, Inamori K, Togashi Y, Koyama S, Kotani D, et al. Preoperative Chemoradiotherapy plus Nivolumab before Surgery in Patients with Microsatellite Stable and Microsatellite Instability-High Locally Advanced Rectal Cancer. Clin Cancer Res. (2022) 28:1136–46. doi: 10.1158/1078-0432
20. Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med. (2022) 386:2363–76. doi: 10.1056/NEJMoa2201445
21. Chalabi M, Verschoor YL, Tan PB, Balduzzi S, Van Lent AU, Grootscholten C, et al. Neoadjuvant immunotherapy in locally advanced mismatch repair-deficient colon cancer. N Engl J Med. (2024) 390:1949–58. doi: 10.1056/NEJMoa2400634
22. Chen G, Jin Y, Guan WL, Zhang RX, Xiao WW, Cai PQ, et al. Neoadjuvant PD-1 blockade with sintilimab in mismatch-repair deficient, locally advanced rectal cancer: an open-label, single-centre phase 2 study. Lancet Gastroenterol Hepatol. (2023) 8:422–31. doi: 10.1016/S2468-1253(22)00439-3
23. de Gooyer PGM, Verschoor YL, van den Dungen LDW, Balduzzi S, Marsman HA, Geukes Foppen MH, et al. Neoadjuvant nivolumab and relatlimab in locally advanced MMR-deficient colon cancer: a phase 2 trial. Nat Med. (2024) 30:3284–90. doi: 10.1038/s41591-024-03250-w
24. Deng Z, Luo Y, Chen X, Pan T, Rui Y, Hu H, et al. Pathological response following neoadjuvant immunotherapy and imaging characteristics in dMMR/MSI-H locally advanced colorectal cancer. Front Immunol. (2024) 15:1466497. doi: 10.3389/fimmu.2024.1466497
25. Han K, Tang JH, Liao LE, Jiang W, Sui QQ, Xiao BY, et al. Neoadjuvant immune checkpoint inhibition improves organ preservation in T4bM0 colorectal cancer with mismatch repair deficiency: A retrospective observational study. Dis Colon Rectum. (2023) 66:e996–e1005. doi: 10.1097/DCR.0000000000002466
26. Hu H, Kang L, Zhang J, Wu Z, Wang H, Huang M, et al. Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): a single-centre, parallel-group, non-comparative, randomised, phase 2 trial. Lancet Gastroenterol Hepatol. (2022) 7:38–48. doi: 10.1016/S2468-1253(21)00348-4
27. Kothari A, White MG, Peacock O, Kaur H, Palmquist SM, You N, et al. Pathological response following neoadjuvant immunotherapy in mismatch repair-deficient/microsatellite instability-high locally advanced, non-metastatic colorectal cancer. Br J Surg. (2022) 109:489–92. doi: 10.1093/bjs/znac050
28. Li YJ, Liu XZ, Yao YF, Chen N, Li ZW, Zhang XY, et al. Efficacy and safety of preoperative immunotherapy in patients with mismatch repair-deficient or microsatellite instability-high gastrointestinal Malignancies. World J Gastrointest Surg. (2023) 15:222–33. doi: 10.4240/wjgs.v15.i2.222
29. Li Y, Tan L, Chen N, Liu X, Liang F, Yao Y, et al. Neoadjuvant immunotherapy alone for patients with locally advanced and resectable metastatic colorectal cancer of dMMR/MSI-H status. Dis Colon Rectum. (2024) 67:1413–22. doi: 10.1097/DCR.0000000000003290
30. Liu DX, Li DD, He W, Ke CF, Jiang W, Tang JH, et al. PD-1 blockade in neoadjuvant setting of DNA mismatch repair-deficient/microsatellite instability-high colorectal cancer. Oncoimmunology. (2020) 9:1711650. doi: 10.1080/2162402X.2020.1711650
31. Liu XZ, Xiong Z, Xiao BY, Yu GY, Li YJ, Yao YF, et al. Multicenter real-world study on safety and efficacy of neoadjuvant therapy in combination with immunotherapy for colorectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi. (2022) 25:219–27. doi: 10.3760/cma.j.cn441530-20220228-00070
32. Ludford K, Ho WJ, Thomas JV, Raghav KPS, Murphy MB, Fleming ND, et al. Neoadjuvant pembrolizumab in localized microsatellite instability high/deficient mismatch repair solid tumors. J Clin Oncol. (2023) 41:2181–90. doi: 10.1200/JCO.22.01351
33. Pan T, Yang H, Wang WY, Rui YY, Deng ZJ, Chen YC, et al. Neoadjuvant immunotherapy with ipilimumab plus nivolumab in mismatch repair deficient/microsatellite instability-high colorectal cancer: A preliminary report of case series. Clin Colorectal Cancer. (2024) 23:104–10. doi: 10.1016/j.clcc.2024.01.002
34. Pei F, Wu J, Zhao Y, He W, Yao Q, Huang M, et al. Single-agent neoadjuvant immunotherapy with a PD-1 antibody in locally advanced mismatch repair-deficient or microsatellite instability-high colorectal cancer. Clin Colorectal Cancer. (2023) 22:85–91. doi: 10.1016/j.clcc.2022.11.004
35. Xiao BY, Zhang X, Cao TY, Li DD, Jiang W, Kong LH, et al. Neoadjuvant immunotherapy leads to major response and low recurrence in localized mismatch repair-deficient colorectal cancer. J Natl Compr Canc Netw. (2023) 21:60–66.e5. doi: 10.6004/jnccn.2022.7060
36. Xie Y, Lin J, Zhang N, Wang X, Wang P, Peng S, et al. Prevalent pseudoprogression and pseudoresidue in patients with rectal cancer treated with neoadjuvant immune checkpoint inhibitors. J Natl Compr Canc Netw. (2023) 21:133–142.e3. doi: 10.6004/jnccn.2022.7071
37. Yang R, Wu T, Yu J, Cai X, Li G, Li X, et al. Locally advanced rectal cancer with dMMR/MSI-H may be excused from surgery after neoadjuvant anti-PD-1 monotherapy: a multiple-center, cohort study. Front Immunol. (2023) 14:1182299. doi: 10.3389/fimmu.2023.1182299
38. Yu JH, Xiao BY, Li DD, Jiang W, Ding Y, Wu XJ, et al. Neoadjuvant camrelizumab plus apatinib for locally advanced microsatellite instability-high or mismatch repair-deficient colorectal cancer (NEOCAP): a single-arm, open-label, phase 2 study. Lancet Oncol. (2024) 25:843–52. doi: 10.1016/S1470-2045(24)00203-1
39. Zhang X, Yang R, Wu T, Cai X, Li G, Yu K, et al. Efficacy and safety of neoadjuvant monoimmunotherapy with PD-1 inhibitor for dMMR/MSI⁃H locally advanced colorectal cancer: A single-center real-world study. Front Immunol. (2022) 13:913483. doi: 10.3389/fimmu.2022.913483
40. Fan WX, Su F, Zhang Y, Zhang XL, Du YY, Gao YJ, et al. Oncological characteristics, treatments and prognostic outcomes in MMR-deficient colorectal cancer. Biomark Res. (2024) 12:89. doi: 10.1186/s40364-024-00640-7
41. Challoner BR, Woolston A, Lau D, Buzzetti M, Fong C, Barber LJ, et al. Genetic and immune landscape evolution in MMR-deficient colorectal cancer. J Pathol. (2024) 262:226–39. doi: 10.1002/path.6228
42. Agarwal P, Le DT, Boland PM. Immunotherapy in colorectal cancer. Adv Cancer Res. (2021) 151:137–96. doi: 10.1016/bs.acr.2021.03.002
43. Carlino MS, Larkin J, Long GV. Immune checkpoint inhibitors in melanoma. Lancet. (2021) 398:1002–14. doi: 10.1016/S0140-6736(21)01206-X
44. Hsu MM, Balar AV. PD-1/PD-L1 combinations in advanced urothelial cancer: rationale and current clinical trials. Clin Genitourin Cancer. (2019) 17:e618–26. doi: 10.1016/j.clgc.2019.03.009
45. Zhang J, Cai J, Deng Y, Wang H. Complete response in patients with locally advanced rectal cancer after neoadjuvant treatment with nivolumab. Oncoimmunology. (2019) 8:e1663108. doi: 10.1080/2162402X.2019.1663108
46. Zhang X, Wu T, Cai X, Dong J, Xia C, Zhou Y, et al. Neoadjuvant immunotherapy for MSI-H/dMMR locally advanced colorectal cancer: new strategies and unveiled opportunities. Front Immunol. (2022) 13:795972. doi: 10.3389/fimmu.2022.795972
47. Kanani A, Veen T, Søreide K. Neoadjuvant immunotherapy in primary and metastatic colorectal cancer. Br J Surg. (2021) 108:1417–25. doi: 10.1093/bjs/znab342
48. Tan S, Ou Y, Huang S, Gao Q. Surgical, oncological, and functional outcomes of local and radical resection after neoadjuvant chemotherapy or chemoradiotherapy for early- and mid-stage rectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis. (2023) 38:132. doi: 10.1007/s00384-023-04433-6
49. Rahma OE, Yothers G, Hong TS, Russell MM, You YN, Parker W, et al. Use of total neoadjuvant therapy for locally advanced rectal cancer: initial results from the pembrolizumab arm of a phase 2 randomized clinical trial. JAMA Oncol. (2021) 7:1225–30. doi: 10.1001/jamaoncol.2021.1683
50. Loria A, Ammann AM, Olowokure OO, Paquette IM, Justiniano CF. Systematic review of neoadjuvant immunotherapy for mismatch repair deficient locally advanced colon cancer: an emerging strategy. Dis Colon Rectum. (2024) 67:762–71. doi: 10.1097/DCR.0000000000003263
51. Zhou L, Yang XQ, Zhao GY, Wang FJ, Liu X. Meta-analysis of neoadjuvant immunotherapy for non-metastatic colorectal cancer. Front Immunol. (2023) 14:1044353. doi: 10.3389/fimmu.2023.1044353
52. Nakamura D, Yanagita T, Fujii Y, Watanabe K, Suzuki T, Ushigome H, et al. Unanticipated pathological clearance in two cases of clinical T4b dMMR/MSI-h advanced colorectal cancer: the potential of immune checkpoint inhibitors despite positive positron-emission tomography results. Surg Case Rep. (2024) 10:105. doi: 10.1186/s40792-024-01894-x
53. Men Q, Duan Y, Pei F, Yao Q, He W, Zhao Y, et al. PD-1 blockade combined with chemotherapy and bevacizumab in DNA mismatch repair-proficient/microsatellite stable colorectal liver metastases. J Gastrointest Oncol. (2024) 15:1534–44. doi: 10.21037/jgo-23-940
54. Su A, Pedraza R, Kennecke H. Developments in checkpoint inhibitor therapy for the management of deficient mismatch repair (dMMR) rectal cancer. Curr Oncol. (2023) 30:3672–83. doi: 10.3390/curroncol30040279
55. But-Hadzic J, Anderluh F, Brecelj E, Edhemovic I, Secerov-Ermenc A, Hudej R, et al. Acute toxicity and tumor response in locally advanced rectal cancer after preoperative chemoradiation therapy with shortening of the overall treatment time using intensity-modulated radiation therapy with simultaneous integrated boost: A phase 2 trial. Int J Radiat Oncol Biol Phys. (2016) 96:1003–10. doi: 10.1016/j.ijrobp.2016.08.031
56. Garcia-Aguilar J, Patil S, Gollub MJ, Kim JK, Yuval JB, Thompson HM, et al. Organ preservation in patients with rectal adenocarcinoma treated with total neoadjuvant therapy. J Clin Oncol. (2022) 40:2546–56. doi: 10.1200/JCO.22.00032
57. Trojan J, Stintzing S, Haase O, Koch C, Ziegler P, Demes M, et al. Complete pathological response after neoadjuvant short-course immunotherapy with ipilimumab and nivolumab in locally advanced MSI-H/dMMR rectal cancer. Oncologist. (2021) 26:e2110–4. doi: 10.1002/onco.13955
58. Duraes LC, Steele SR, Valente MA, Lavryk OA, Connelly TM, Kessler H. Right colon, left colon, and rectal cancer have different oncologic and quality of life outcomes. Int J Colorectal Dis. (2022) 37:939–48. doi: 10.1007/s00384-022-04121-x
59. Manuel-Vázquez A, Tristán MS, Miguel Martín Martínez J, Luis Ramos Rodríguez J. Neoadjuvant treatment in locally advanced rectal cancer with deficient mismatch repair (dMMR): a paradigm shift. Cir Esp (Engl Ed). (2025) 103:118–20. doi: 10.1016/j.cireng.2024.10.003
60. Feferman Y, Verheij FS, Williams H, Omer DM, Pappou EP, Wei IH, et al. Outcomes of distal rectal cancer patients who did not qualify for watch-and-wait: comparison of intersphincteric resection versus abdominoperineal resection. Ann Surg Oncol. (2025) 32:128–36. doi: 10.1245/s10434-024-16316-3
61. André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt CJA, et al. Pembrolizumab versus chemotherapy in microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer: 5-year follow-up from the randomized phase III KEYNOTE-177 study. Ann Oncol. (2025) 36:277–84. doi: 10.1016/j.annonc.2024.11.012
62. Overman MJ, Gelsomino F, Aglietta M, Wong M, Limon Miron ML, Leonard G, et al. Nivolumab plus relatlimab in patients with previously treated microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the phase II CheckMate 142 study. J Immunother Cancer. (2024) 12:e008689. doi: 10.1136/jitc-2023-008689
63. Kim R, Tehfe M, Kavan P, Chaves J, Kortmansky JS, Chen EX, et al. Pembrolizumab plus mFOLFOX7 or FOLFIRI for microsatellite stable/mismatch repair-proficient metastatic colorectal cancer: KEYNOTE-651 cohorts B and D. Clin Colorectal Cancer. (2024) 23:118–127.e6. doi: 10.1016/j.clcc.2024.03.001
64. Powell MA, Cibula D, O’Malley DM, Boere I, Shahin MS, Savarese A, et al. Efficacy and safety of dostarlimab in combination with chemotherapy in patients with dMMR/MSI-H primary advanced or recurrent endometrial cancer in a phase 3, randomized, placebo-controlled trial (ENGOT-EN6-NSGO/GOG-3031/RUBY). Gynecol Oncol. (2025) 192:40–9. doi: 10.1016/j.ygyno.2024.10.022
65. Stein A, Simnica D, Schultheiß C, Scholz R, Tintelnot J, Gökkurt E, et al. PD-L1 targeting and subclonal immune escape mediated by PD-L1 mutations in metastatic colorectal cancer. J Immunother Cancer. (2021) 9:e002844. doi: 10.1136/jitc-2021-002844
66. Fu X, Huang J, Zhu J, Fan X, Wang C, Deng W, et al. Prognosis and immunotherapy efficacy in dMMR&MSS colorectal cancer patients and an MSI status predicting model. Int J Cancer. (2024) 155:766–75. doi: 10.1002/ijc.34946
67. Flecchia C, Auclin E, Alouani E, Mercier M, Hollebecque A, Turpin A, et al. Primary resistance to immunotherapy in patients with a dMMR/MSI metastatic gastrointestinal cancer: who is at risk? An AGEO real-world study. Br J Cancer. (2024) 130:442–9. doi: 10.1038/s41416-023-02524-3
68. Rocha MR, Castillo-Medina YK, de Lima Coelho BM, Rios LLL, Morgado-Diaz JA. Wnt/beta-catenin pathway as a link between therapy resistance-driven epithelial-mesenchymal transition and stemness in colorectal cancer. Cell Biol Int. (2025) 49:154–60. doi: 10.1002/cbin.12270
Keywords: neoadjuvant immunotherapy, non-metastatic colorectal cancer, mismatch repair-deficient, pCR, meta-analysis.
Citation: Cui H-X, Yang X-Q, Zhao G-y, Wang F-j and Liu X (2025) The neoadjuvant immunotherapy for non-metastatic mismatch repair-deficient colorectal cancer: a systematic review. Front. Immunol. 16:1540751. doi: 10.3389/fimmu.2025.1540751
Received: 06 December 2024; Accepted: 07 April 2025;
Published: 01 May 2025.
Edited by:
Aristotle A. Chatziioannou, Biomedical Research Foundation of the Academy of Athens (BRFAA), GreeceReviewed by:
Stavros P. Papadakos, Laiko General Hospital of Athens, GreeceGuillaume Mestrallet, Icahn School of Medicine at Mount Sinai, United States
Copyright © 2025 Cui, Yang, Zhao, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Liu, bGl1eGluNTYyNjg1NUBzaW5hLmNvbQ==
 Hong-Xia Cui
Hong-Xia Cui Xiao-Quan Yang2
Xiao-Quan Yang2 Xin Liu
Xin Liu