- 1Department of General Surgery, Hepatic-biliary-pancreatic Institute, The Second Hospital & Clinical Medical School, Lanzhou University, Lanzhou, Gansu, China
- 2Department of Liver Disease, The Second Hospital & Clinical Medical School, Lanzhou University, Lanzhou, Gansu, China
Cancer is a significant public health problem worldwide, and its morbidity and mortality are challenging to improve, which is an important obstacle to prolonging life expectancy. Cytotoxic drugs have been used in anti-cancer therapy since the 1940s. They play an important role in tumor therapy. However, drug resistance and systemic toxicity often limit its application. Combination or synergistic chemotherapy can promote therapeutic effects and reduce toxicity. Quercetin (QUE) is a natural flavonoid widely found in fruits and vegetables. It has anti-cancer, anti-inflammatory, antioxidant, and neuroprotective properties. An increasing number of studies have found that the combination of QUE and chemotherapy drugs has a chemosensitization effect. To a certain extent, it can inhibit the side effects of chemotherapeutic drugs, such as nephrotoxicity, cardiotoxicity, reproductive toxicity, and neurotoxicity, which has attracted great attention. The immune system plays a significant role in tumor development. Notably, several studies have revealed that QUE plays an immunomodulatory role by promoting the differentiation of anti-cancer immune cells and inhibiting immune checkpoint expression. In conclusion, current studies have emphasized the potential of QUE in chemosensitization, reduction of toxic side effects, and enhancement of the anti-cancer immune response. However, more preclinical and clinical cohort studies are needed to determine QUE’s efficacy, mechanism, optimal formulation, and long-term effects in synergistic chemotherapy and immunomodulatory effects.
1 Introduction
Cancer is a complex genetic disease, and multiple genetic and environmental factors have influenced its occurrence and development. It arises from dysregulation of cell proliferation and concomitant genomic modifications (1). Unfortunately, cancer prevalence continues to increase to the extent that it has become the second leading cause of death worldwide after heart disease (2). About 20 million new cancer cases were reported in 2022, including nearly 9.7 million deaths (3). With the development of cancer research, our understanding of the biological characteristics and treatment options for cancer continues to evolve. Several therapies have been used to treat cancer, including surgery, immunotherapy, radiotherapy, chemotherapy, endocrine therapy, and various RNA molecules (4). Among these, chemotherapy is a relatively effective option, particularly for advanced cancers. Unfortunately, chemotherapy cannot wholly eradicate all cancer cells, and cancer cells may reappear within a short period (5). Chemotherapeutic drugs also have a significant impact on normal cells, causing inevitable damage to the body during chemotherapy, such as alopecia, gastrointestinal toxicity, nephrotoxicity, and neurotoxicity (6). Therefore, improving the sensitivity to chemotherapy drugs and reducing side effects is a continuing concern in cancer drug therapy.
Quercetin (QUE) is a flavonol compound that is insoluble in water but soluble in alcohol and lipids (7). It is rich in grapes, cherries, buckwheat, berries, Onions, citrus fruits, and so on (8). Many in vitro and in vivo studies have demonstrated the effects of QUE in cancer treatment. It has been reported (9) that QUE has many anti-cancer properties, including the ability to inhibit proliferation, anti-angiogenesis, induce apoptosis, and inhibit mitotic processes (10), as well as many benefits such as antioxidant (7, 11), anti-inflammatory (12), neuroprotection (13), antimicrobial (14), anti-allergy (15), and immune regulation functions. QUE shows strong potential for adjuvant chemotherapy because of its low toxicity and multi-target nature (16). Many studies have shown the potential of QUE to prevent drug resistance and sensitize cancer cells to chemotherapeutic agents by modulating key factors associated with tumor growth and progression, such as inhibition of anti-apoptotic proteins, activation of pro-apoptotic proteins, and inhibition of cell cycle proteins (16). In addition, a growing body of evidence highlights the potential of QUE as a natural compound to prevent chemotherapy-induced cardiotoxicity (17), nephrotoxicity (18), and neurotoxicity (19). Figure 1 illustrates the chemotherapeutic drugs that synergize with QUE and the side effects mitigated by QUE.
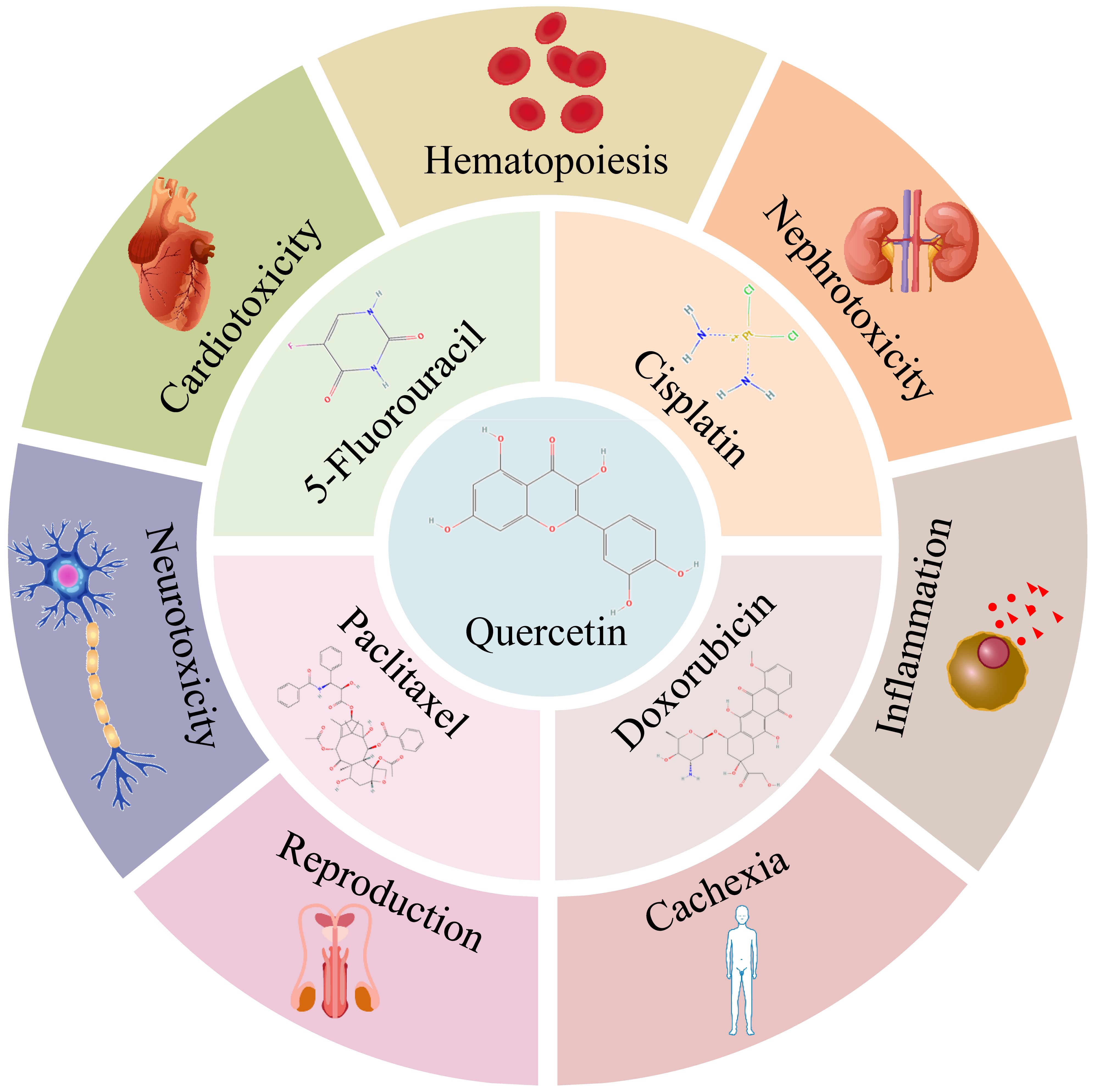
Figure 1. QUE synergizes with 5-Fluorouracil, cisplatin, doxorubicin, and paclitaxel and alleviates side effects of chemotherapy. [Chemical structures were obtained from Pubchem (20)].
Tumor development and progression depend on the interactions between cancer cells and multiple cell types surrounding the tumor, constituting the tumor microenvironment (TME) (21). The immune system is an important factor that controls the occurrence and development of tumors (22) and is an emerging field of tumor therapy. Cancer cells usually must evade immune surveillance to grow and metastasize indefinitely (22). Therefore, developing immunogenic therapies to reverse immune surveillance and activate anti-cancer responses is promising. Several compounds have been shown to interact with subsets of immune cells to stimulate anti-cancer immune responses (23). It has been reported (24) that QUE promotes activation of the host immune system and anti-cancer immune response, inhibiting tumor growth and possibly a new anti-cancer immunotherapy.
This review summarizes the data obtained from various studies to explore the benefits of QUE in combination with various chemotherapeutic agents, which manifest in two aspects: enhanced chemotherapy sensitivity and reduced toxic side effects of chemotherapy. The effect of QUE on tumor immunity is also discussed. This review seeks to contribute to the advancement of QUE and improved treatment outcomes for cancer patients.
2 The chemosensitization effect of QUE in cancer chemotherapeutic
The mechanisms of chemotherapeutic drugs are complex and include effects on DNA structure, inhibition of nucleic acid synthesis, DNA replication, and interference with mitotic tubulin (6). The mechanisms of acquired drug resistance in tumor cells usually manifest in several aspects (25): (1) The balance between drug absorption and excretion is disrupted, decreasing intracellular drug levels. (2) As a protective process, autophagy inhibits tumor cell apoptosis. (3) Induction of immunosuppression. Combining chemotherapy drugs with QUE has shown synergistic or sensitizing effects and higher cytotoxic effects on tumor cells.
2.1 5-Fluorouracil
5-FU is a metabolic inhibitor that interferes with nucleic acid metabolism by inhibiting cancer cells’ thymidylate synthase (TS) activity, blocking their DNA synthesis, and inhibiting their proliferation. Since 1957, it has been widely used for various types of cancer, such as colorectal cancer (CRC), breast cancer (BC), and gastric cancer (GC) (26). Despite its many advantages, its clinical application is limited because of the progressive development of drug resistance after chemotherapy (26). Therefore, new therapeutic strategies to suppress drug resistance and improve the response to 5-FU are urgently needed.
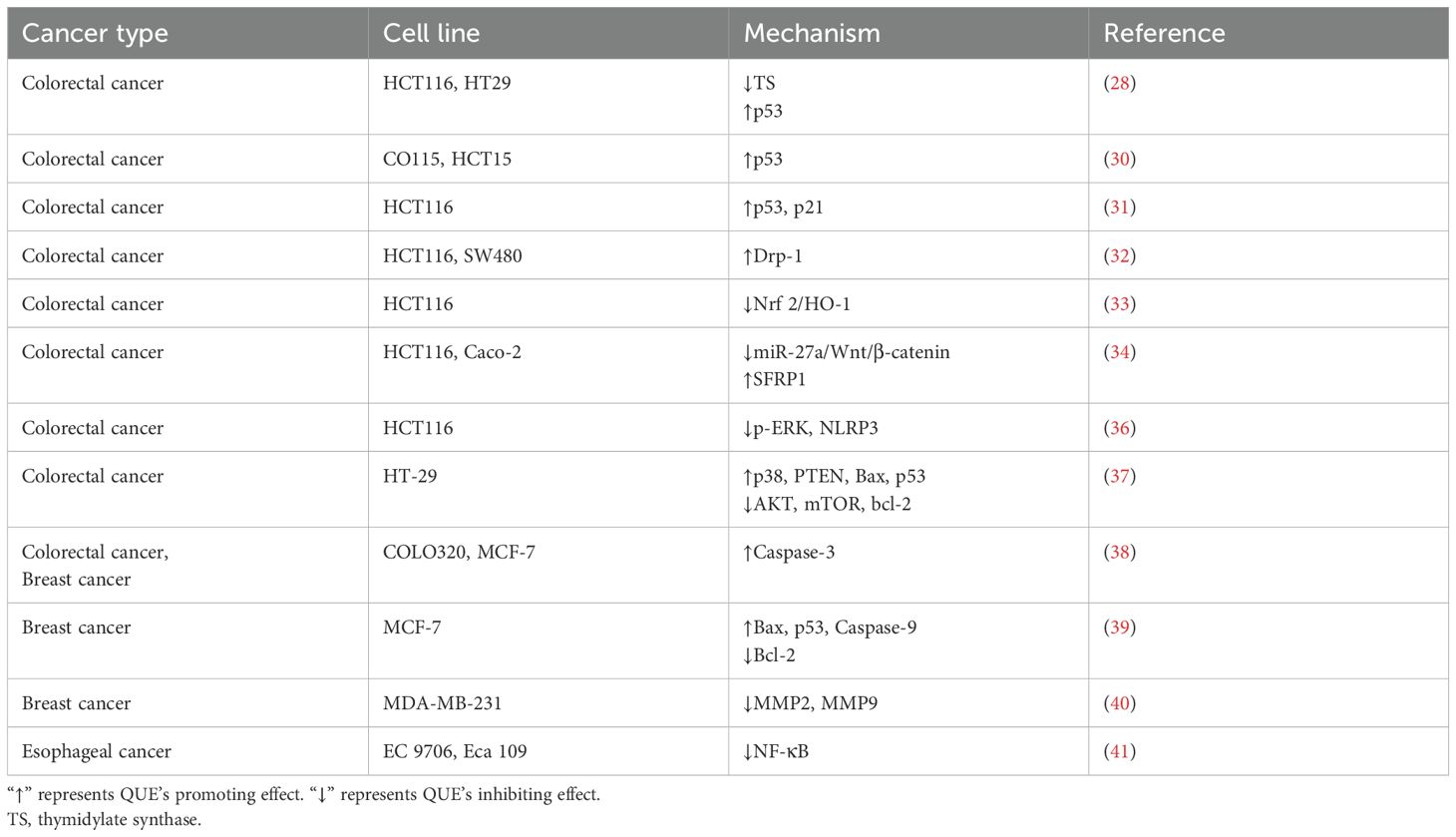
The 5-FU-resistant cells express high levels of TS. 5-FU administration for 24 h can increase the expression level of bound TS in HCT116 and HT29 CRC cells and inhibit the expression of p53, thereby tolerating 5-FU (27). The combination of QUE and 5-FU significantly attenuated 5-FU-induced upregulation of TS expression and enhanced the 5-FU effect by inducing mitochondrial dysfunction and increasing the expression levels of p53 (28). p53 is closely related to 5-FU resistance, and its mutation reduces apoptosis and cell cycle inhibition (29). Another study found that QUE could activate p53 expression in CO115 and HCT15 CRC cells, rendering these two cells sensitive to 5-FU (30). Das et al. used QUE and 5-FU to construct chitosan nanoparticles, which induced apoptosis and cell cycle arrest at the G0/G1 phase in HCT116 CRC cells by activating the p53/p21 axis (31). In addition, Li et al. found that QUE enhanced the sensitivity of HCT116 and SW480 cells to 5-FU by inducing autophagy and up-regulating the expression of Drp-1, resulting in mitochondrial fragmentation (32). Tang et al. constructed a 5-FU-resistant HCT116 cell line and found that QUE reversed 5-FU resistance by inhibiting the Nrf 2/HO-1 pathway (33). In addition, QUE combined with 5-FU enhanced the inhibitory effect of 5-FU on the proliferation of HCT116 and Caco-2 cells by down-regulating miR-27a/Wnt/β-catenin signaling and up-regulating SFRP1 expression (34). NLRP3 is an inflammasome, and its activation is closely related to tumorigenesis and tumor-related inflammation (35). Lee et al. found that after resistin-induced resistance to 5-FU in CRC cells, the phosphorylation level of p-ERK increased to promote NLRP3 expression, indicating that NLRP3 expression increased the resistance of HCT116 cells to 5-FU (36). Therefore, QUE could enhance the cytotoxic effect of 5-FU on HCT116 cells by inhibiting ERK/NLRP3 (36). QUE synergistically enhanced the sensitivity of HT-29 CRC cells to 5-FU by up-regulating the expression of p38, PTEN, Bax, and p53, but down-regulating the expression of AKT, mTOR and bcl-2 (37). As a prodrug form of 5-FU, capecitabine is used as monotherapy in a variety of advanced or metastatic cancers. When combined with capecitabine, QUE increased the killing effect of capecitabine on COLO320 CRC cells and MCF-7 BC cells, and arrested the cell cycle by inducing mitochondrial depolarization and promoting the expression of Caspase-3, while it had no cytotoxicity on normal mouse fibroblast 3T3-L1 cells (38).
In BC, 5-FU combined with QUE further promoted the apoptosis of MCF-7 cells by increasing the expression of Bax, Caspase-9, and p53 genes and decreasing the expression of bcl-2 genes, indicating that QUE could enhance the sensitivity of 5-FU to BC (39). In addition, the combination of QUE and 5-FU had a better inhibitory effect on the migration ability and the expression of MMP2 and MMP9 in highly invasive MDA-MB-231 cells than 5-FU alone (40). In addition, QUE enhanced the cytotoxic effect of 5-FU on EC9706 and Eca109 esophageal cancer cells by inhibiting NF-κB (41). Roman initially found (42) that QUE could enhance the cytotoxic effect of 5-FU on A375 melanoma cells but did not explore the mechanism. To explore the relationship between QUE and 5-FU pharmacokinetics, Tavakoli et al. intraperitoneally injected QUE and 5-FU into rats. Subsequently, blood samples were collected to evaluate the pharmacokinetic parameters using high-performance liquid chromatography, and it was found that QUE significantly increased the plasma concentration of 5-FU and improved the availability of 5-FU (43).
In conclusion, the present study suggests that QUE sensitizes CRC and BC cells to 5-FU by regulating the expression of apoptotic proteins (Table 1). It has been established that QUE positively affects the combination of 5-FU, but the specific mechanism deserves further exploration. We found that the sensitivity to 5-FU is related to the inflammasome NLRP3, which is an interesting topic. In addition, current studies have focused on the role of QUE combined with 5-FU in CRC and BC, but 5-FU is suitable for a variety of tumors such as GC (44) and hepatocellular carcinoma (HCC) (45). Subsequent studies are needed to explore the role of QUE combined with 5-FU in these tumors.
2.2 Doxorubicin
Doxorubicin (DOX) is one of the most effective chemotherapeutic drugs for BC treatment. It is a naturally occurring anthracycline antibiotic that prevents DNA replication by inhibiting DNA topoisomerase II (46). Drug resistance is also one of the main reasons for DOX treatment failure, leading to poor prognosis and survival (47). Solving the drug resistance of DOX is a new hope for cancer treatment.
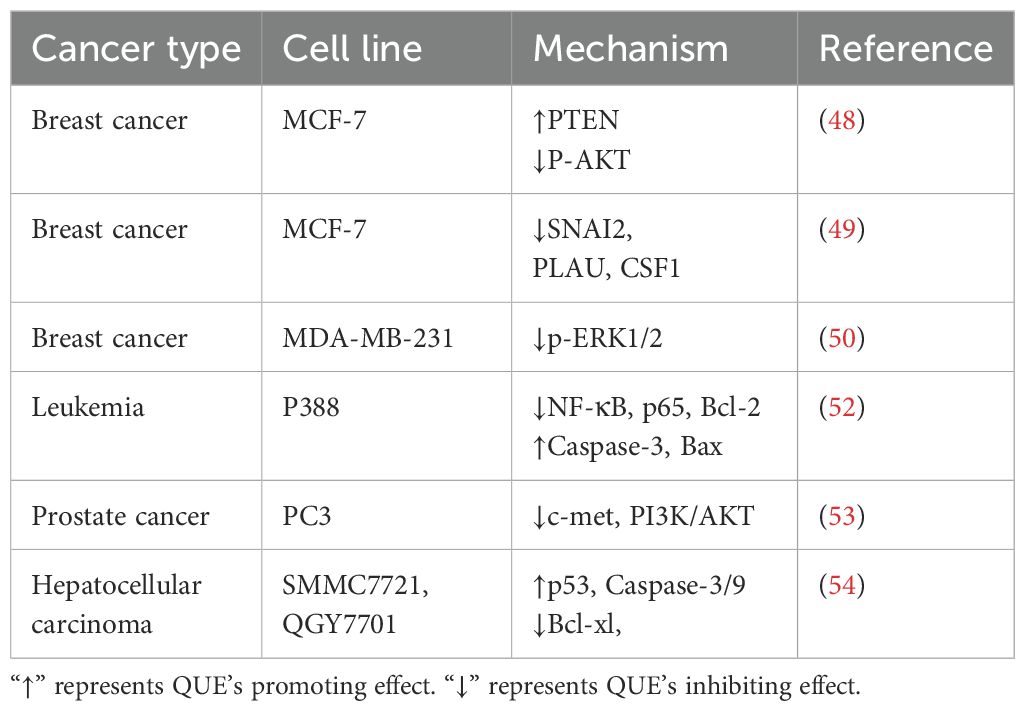
Li et al. found that non-toxic doses of QUE enhanced the toxic effect of DOX on MCF-7 BC cells by up-regulating PTEN and inhibiting p-AKT phosphorylation (48). QUE also reversed DOX resistance in the MCF-7/DOX-resistant BC cell line by down-regulating the SNAI2, PLAU, and CSF1 genes (49). Triple-negative breast cancer (TNBC) has a poor response to endocrine and anti-HER2 therapies, and chemotherapy is the primary treatment in addition to surgical resection. A study reported that QUE could improve DOX and cyclophosphamide (CTX) chemosensitivity by inhibiting ROS production and the p-ERK1/2 pathway in TNBC cells MDA-MB-231 (50). In HOS and MG-63 osteosarcoma cells, the combination of QUE and DOX was shown to reverse DOX resistance in osteosarcoma by increasing mitochondrial biogenesis (51). Similarly, QUE enhanced the killing effect of DOX on P388 leukemia cells by inhibiting the expression of NF-κB, p65, and Bcl-2 but by activating Bax and Caspase-3 (52). For the acquired DOX-resistant PC3 prostate cancer cell line, QUE was found to reverse its resistance by inhibiting c-Met and PI3K/AKT signaling pathways (53). In SMMC7721 and QGY7701 HCC cells, QUE promoted the expression of p53, inhibited Bcl-xl, and up-regulated the expression of Caspase-3/9, which enhanced the effect of DOX on promoting apoptosis (54). Li et al. constructed a hyaluronic acid (HA)-modified zeolitic imidazolate framework for loading DOX and QUE, prioritizing targeting HEPPG2/DOX resistant cells overexpressed CD44. Moreover, the released QUE reshaped the TME by reducing the content of collagen and α-SMA in tumor tissues and significantly increased the anti-cancer effect of DOX (55). In addition, QUE loading with lectin-stabilized polymeric micelles enhanced DOX growth inhibition in CT26 CRC cells and reduced cardiotoxicity (56).
Many studies have reported an augmentation effect of QUE on DOX chemotherapy regimens (Table 2). The known mechanism of QUE increases the apoptotic process of tumor cells, but this mechanism is complex, and further studies are needed to confirm this.
2.3 Cisplatin
In 1965, Cisplatin (CIS) was found to inhibit cell division. It blocks the synthesis of DNA, mRNA, and protein synthesis by interacting with purine bases on gDNA or mitochondrial DNA, inhibiting DNA replication and promoting cell apoptosis (57). CIS is widely used in many types of tumors, such as lung, ovarian, breast, bladder, and testicular cancers, but drug resistance and multi-organ toxicity limit its practical use (58).
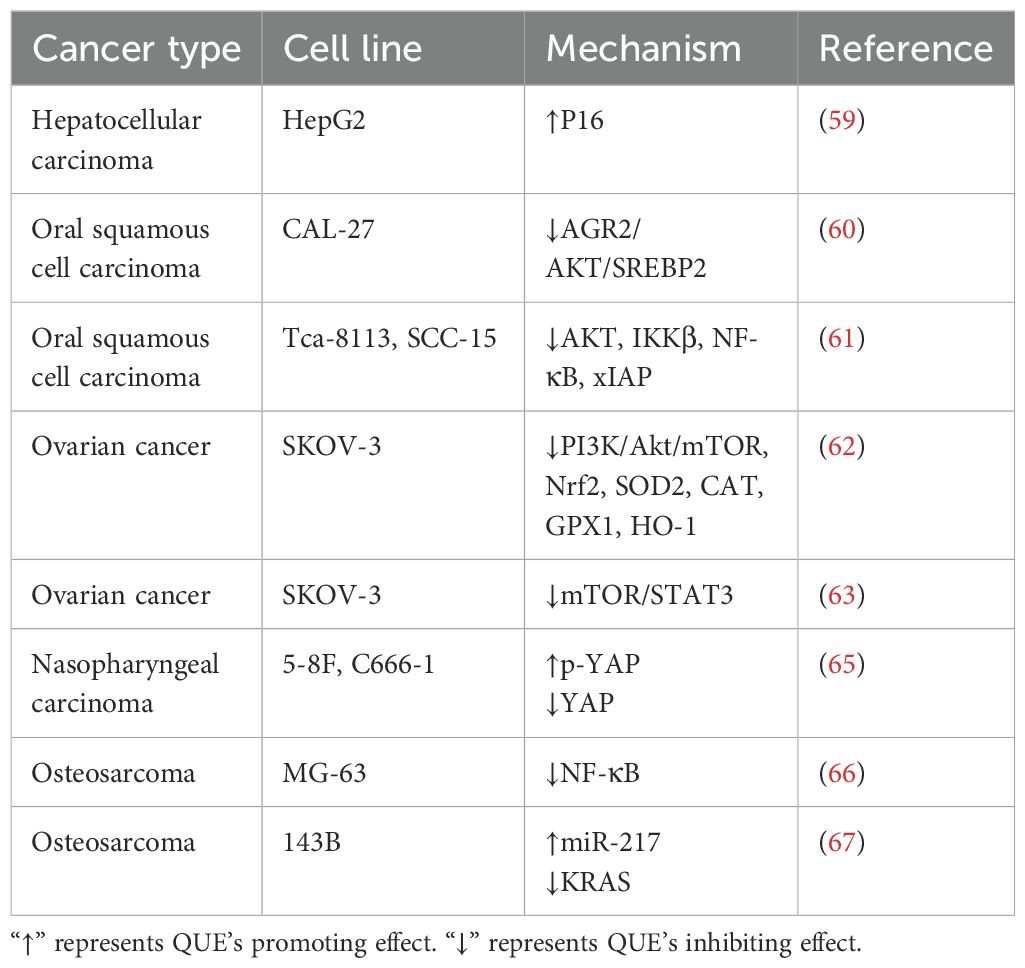
A previous study has found that QUE enhanced the growth inhibition of CIS in HepG2 HCC cells by up-regulating p16 expression (59). For oral squamous cell carcinoma (OSCC), QUE inhibited cholesterol metabolism by inhibiting the AGR2/AKT/SREBP2 signaling pathway, enhancing CIS sensitivity to CIS-resistant CAL-27 cells (60). QUE pre-treatment of Tca-8113 and SCC-15 OSCC lines enhanced CIS cytotoxicity by inhibiting AKT and IKKβ phosphorylation, the NF-κB pathway, and expression of the anti-apoptotic protein xIAP (61). In ovarian cancer cells, QUE enhanced the CIS sensitivity of the CIS-resistant cell line SKOV-3/CIS by inhibiting the expression of SOD2, catalase (CAT), GPX1, HO-1, Nrf 2, and PI3K/Akt/mTOR (62). Hasan et al. found that QUE can down-regulate the mTOR/STAT3 signal of the SKOV-3/CIS cell line, make it sensitive to CIS, and promote apoptosis (63). The results of a network pharmacological analysis by Ji et al. suggested that QUE can potentially enhance the sensitivity of CIS to cervical cancer (64). Similarly, QUE reversed CIS resistance in CIS-resistant nasopharyngeal carcinoma cell lines 5-8F and C666-1 by inhibiting YAP protein expression and activating the p-YAP phosphorylation (65). In addition, compared with CIS alone, QUE promoted apoptosis induced by CIS by down-regulating NF-κB levels in osteosarcoma cells MG-63 (66). For the 143B osteosarcoma cell line, QUE enhanced the sensitivity of CIS to 143B cells by down-regulating KRAS expression (67). The same effect was observed in the BC cell line (EMT6) (68).
As an effective chemotherapeutic agent, CIS is used to treat various human cancers. Drug resistance is an important challenge in tumor treatment. Owing to QUE’s multi-target and multi-activity characteristics of QUE, combination therapy with CIS has achieved some success (Table 3). Therefore, the co-delivery of QUE and CIS drug delivery systems has attracted increasing attention, which could pave the way for developing novel strategies to overcome CIS resistance.
2.4 Paclitaxel
Paclitaxel (PTX) is a natural anti-cancer compound that is a taxane chemotherapeutic drug. It prevents cell division by stabilizing β-tubulin heterodimers in the microtubules, preventing depolymerization, and inhibiting the G2/M phase of the cell cycle, leading to cell death. It is often combined with platinum-based chemotherapy regimens for the treatment of various malignancies (69).
A study found that low-dose QUE combined with PTX had more potent inhibitory effects on the proliferation, cell cycle, and migration of PC-3 prostate cancer cells than PTX alone, whereas this combination promoted apoptosis (70). This effect was also observed in SKOV3, A2780, EFO27, and OVCAR-3 ovarian cancer cells (69, 71). Further mechanistic studies revealed that the synergistic effect was mediated by the down-regulation of ERBB2 and BIRC5 and the up-regulation of Caspase-3 expression (69). Network pharmacology analysis and experimental verification by Yang et al. showed that QUE increased the sensitivity of the PTX-resistant BC cell line MCF-7/PTX to PTX by inhibiting the EGFR/ERK axis (72). Due to hydrophobicity, QUE and PTX’s therapeutic efficacy is limited (73). Wang et al. demonstrated growth inhibition in PTX-resistant A549 lung cancer cells by inhibiting AKT and ERK phosphorylation using a chitosan nanoparticle-coated QUE and PTX co-delivery system (73).
However, the oral administration of PTX remains problematic owing to its low oral bioavailability (<10%), which has been attributed to its poor solubility in digestive fluid, intestinal permeability, first-pass effect, and the abundant presence of drug efflux protein in the gastrointestinal tract (74). Therefore, developing a co-delivery system of QUE and PTX can potentially improve the availability of PTX and inhibit its efflux.
2.5 Multidrug resistance
The resistance of cancer cells to various anti-cancer drugs with different structures and mechanisms of action is called Multidrug resistance (MDR). MDR is a major cause of chemotherapy failure. Its mechanism of action mainly includes DNA repair, regulation of drug targets, metabolic modification, and reduction of drug concentration through the up-regulation of ATP-binding cassette (ABC) protein, such as P-gp (ABCB1), MRP1 (ABCC1), and MRP2 (ABCC2) (75). ABC protein, an ATP-driven drug efflux transporter, is distributed on the surface of tumor cells as an efflux pump to reduce the effect of chemotherapy drugs so that tumor cells form a unique defense against chemotherapy drugs (75). Therefore, inhibition of ABC efflux transporter activity or inhibition of its expression is very promising for improving the chemotherapeutic effect of tumors.
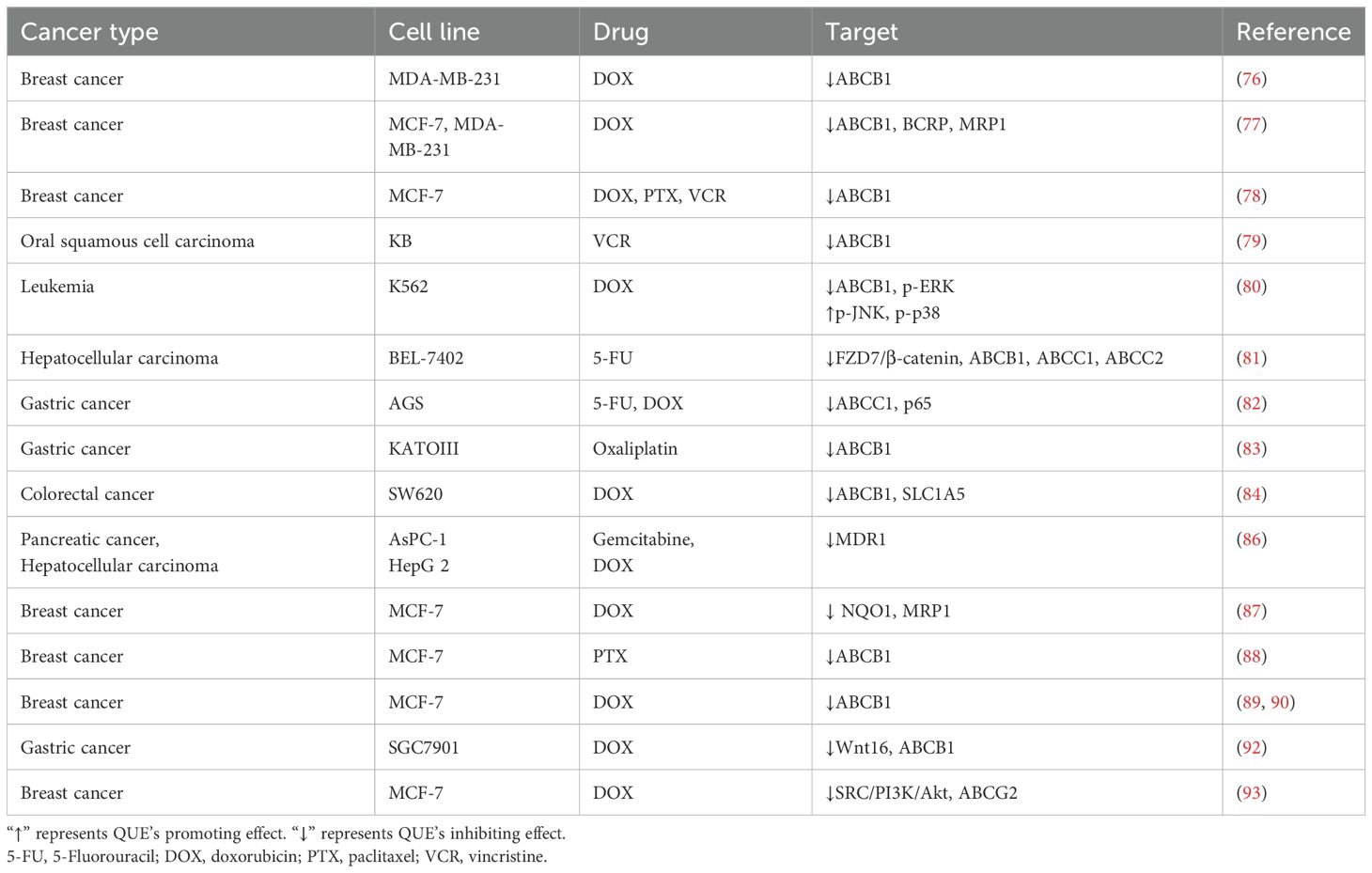
The mechanism of the chemosensitizing effect of QUE is complex, and several studies have found that it has a favorable inhibitory effect on MDR (Table 4). However, pre-treatment of MDA-MB-231/MDR1 cells with QUE reduced DOX efflux and increased DOX accumulation by down-regulating ABCB1, thereby accelerating cell apoptosis (76). When low concentrations of QUE were used in combination with DOX, QUE increased the accumulation of DOX in MCF-7 and MDA-MB-231 cells by down-regulating the expression of ABCB1, BCRP, and MRP1 and promoted the killing effect of DOX on BC cells, especially BC stem cells (77). Another study combined QUE with DOX, PTX, and vincristine (VCR) on MCF-7 and BC stem cells and confirmed that QUE could down-regulate ABCB1 and inhibit the nuclear translocation of YB-1 to kill BC stem cells when combined with these three drugs (78). QUE reversed the VCR resistance of OSCC KB/VCR cells by inhibiting ABCB1 expression in a concentration-dependent manner (79). In addition, QUE reversed MDR in the leukemia cell line K562/DOX by activating p-JNK and p-p38 and inhibiting p-ERK signaling to inhibit ABCB1 expression (80). Moreover, QUE was found to reverse the MDR of BEL-7402/5-FU cells by inhibiting the expression of ABCB1, ABCC1, and ABCC2 by inhibiting the FZD7/β-catenin pathway (81). In addition, overexpression of CYR61 in AGS cells could up-regulate ABCC1, and QUE treatment suppressed CYR61/ABCC1 expression to suppress MDR (82). Moreover, QUE significantly down-regulated ABCB1 expression in KATOIII/OxR, an oxaliplatin-resistant GC cell line, and increased the apoptosis rate of KATOIII/OxR cells (83). Zhou et al. used metabolomics to detect that QUE increased the sensitivity of DOX-selective ABCB1 overexpression SW620 CRC cells to DOX by inhibiting SLC1A5 expression from antagonizing glutamine metabolism and inhibiting ABCB1 transporter activity (84). MDR1 is also an energy-dependent efflux pump that reduces intracellular drug concentrations and triggers drug resistance (85). QUE combined with gemcitabine and DOX could alleviate chemoresistance of pancreatic cancer AsPC-1 and HCC cells HepG 2 by inhibiting the activity of MDR1 (86).
A phytosome (nano-QUE) prepared with a mixture of QUE and lecithin enhanced DOX-induced apoptosis in MCF-7 BC cells by reducing the expression of NQO1 and MRP1 (87). Liu et al. constructed a QUE co-loaded and chondroitin sulfate-coated mesoporous silica nanoparticles co-delivery system to be absorbed by the CD44 receptor in MCF-7 cells, thereby inhibiting the ABCB1 activity of MCF-7 cells and reducing the efflux of PTX to reverse PTX resistance (88). Another DOX-encapsulated ph-responsive nano-micelle, which is based on chitosan, QUE, and citraconic anhydride, could escape from lysosomes and rapidly release DOX and QUE in the cytoplasm, inhibiting ABCB1 and enhancing the inhibitory effect of DOX on MCF-7/DOX cells (89). Zhang et al. constructed DOX and QUR co-loaded gold nanocages, which have a strong cytotoxic effect on MCF-7/DOX cells by inducing a large amount of ROS production and inhibiting ABCB1 expression (90). ABCB1, which is highly expressed on the surface of intestinal cells, can promote the efflux of DOX, inhibiting DOX from being absorbed by intracellular pathways (91). Mu et al. synthesized QUE-chitosan conjugate, which has high water solubility and opens the tight junctions of Caco-2 colon cells, improving DOX bioavailability and promoting DOX absorption (91). A (HA)-modified silica nanoparticle used to co-deliver QUE and DOX was able to target CD44-overexpressing SGC7901 GC cells, inhibit the expression of Wnt16 and ABCB1, remodel the TME, and reverse MDR (92). A β-cyclodextrin that encapsulates DOX and QUE effectively overcomes the MDR of MCF-7/DOX cells by inhibiting SRC/PI3K/Akt signaling and ABCG2 (93).
Table 4 illustrates the mechanism by which QUE inhibits MDR to chemotherapeutic agents and demonstrates the potential of QUE in reversing MDR. In conclusion, various nano drug delivery systems enhanced the transcellular transport of DOX and showed inhibitory effects on the ABC protein. Although these drug carrier systems have shown sound inhibitory effects on MDR, they rarely report the toxicity of these drug carrier systems to normal cells or organisms. However, this may need to be further investigated.
2.6 Summary
Although various important mechanisms have been found to enhance the sensitivity of QUE to chemotherapeutic drugs, including the regulation of tumor-related signaling pathways and the inhibition of MDR proteins, the diverse causes of drug resistance still need to be further explored. Cancer stem cells (CSC) in the TME are also a key cause of tumor drug resistance (26). Conventional drug therapy mainly targets highly proliferating and mature cancer cells but does not satisfactorily affect quiescent and poorly differentiated CSC cell populations, resulting in tumor cell resuscitation (26). Currently, there is a lack of research on the effects of QUE on CSC, and this may be a new area. Therefore, the effect of QUE combined with chemotherapy drugs on the CSC population is worth exploring in the future, and perhaps a new understanding of the synergistic effect of chemotherapy and QUE. In addition, some studies (94, 95) have found that QUE combined with mycophenolic acid can enhance its anti-cancer effect by increasing the half-life of drugs. However, the effect of QUE on the bioavailability of other chemotherapeutic drugs warrants further investigation in animal models and clinical trials.
3 The effect of QUE on the side effects of chemotherapy
Chemotherapy is mainly used for palliative care and adjuvant treatment after surgery. However, chemotherapy is associated with several serious side effects, including early symptoms of toxicity and late symptoms of chronic toxicity (96). According to the WHO classification, its intensity can be mild (grade 1), moderate (grade 2), severe (grade 3), or life-threatening or disabling (grade 4). Immediate toxicity effects can be seen in the skin, hair, bone marrow, blood, gastrointestinal tract, and kidneys. However, all body organs can be affected, including vital organs such as the heart, lungs, and brain. Grade 3 and 4 neurotoxicity can induce somnolence, paresthesia, paralysis, ataxia, spasticity, and coma (96). Additionally, the chronic effects of chemotherapy include drug resistance, carcinogenicity, and reproductive toxicity. However, several preclinical studies have shown that QUE effectively reduces chemotherapy-induced toxicity due to its complex pharmacological activities.
3.1 Nephrotoxicity
The key roles of the kidney include the secretion, reabsorption, filtration, bioactivation, and excretion of most substances that enter biological systems, making it a good target for many drugs. Increased and sustained exposure to multiple drugs and drug metabolites can damage the functional units of the kidney, including the vasculature, tubules, and glomeruli. Drug-induced nephrotoxicity is a major cause of acute kidney disease. The toxicity of 5-FU is mainly manifested in liver and kidney toxicity, central nervous system toxicity, and heart toxicity. Ali et al. found that the combination of telmisartan and QUE can significantly reduce the levels of tissue KIM-1, NGAL, cys-C, new inflammatory markers of kidney injury [such as Neutrophil/Lymphocyte (NLP), Monocyte/Lymphocyte (MLR), and Platelets/Lymphocyte ratios (PLR)], and uric acid, which has renoprotective effects against 5-FU-induced nephrotoxicity (97). DOX also causes renal injury, which is mainly characterized by glomerular and tubulointerstitial inflammation and renal fibrosis. In a mice model, urinary creatinine (UCr) was decreased, and 24-hour volume output, creatinine (Cr), urea (UREA), and urine protein were increased after being treated by DOX (98). QUE can up-regulate AKT1, Raf, MEK, and p-ERK signals, restore mitochondrial structure, reduce apoptosis, and inhibit inflammatory factors and Ang II, alleviating DOX-induced kidney injury in mice (98). Under a light microscope, DOX caused renal tubular dilatation, tubular vacuolization, glomerular vacuolization, reduction of Bowman’s space, thickening of Bowman’s capsule, and interstitial infiltration, which were alleviated by QUE treatment (99). Khalil et al. showed that QUE can attenuate DOX-induced elevation of renal nitric oxide (NO), TNF-α, and myeloperoxidase (MPO) activities and ameliorate podocyte injury (100). In addition, QUE can inhibit the accumulation of malondialdehyde (MDA) and the increase in glutathione peroxidase (GPX) levels induced by DOX and CTX in rats and reduce the oxidative damage to the liver and kidney caused by chemotherapy drugs (101). Seker et al. also found that QUE alleviated kidney injury caused by CTX by up-regulating MAPK/ERK phosphorylation and inhibiting NF-κB (18).
The most common side effect of CIS is nephrotoxicity, with acute kidney injury (AKI) as a common manifestation. It has been established that CIS-associated AKI is closely related to the process of ferroptosis, a cell death induced by iron-mediated lipid peroxidation (102). Shi et al. found that QUE alleviated CIS-induced AKI by inhibiting ferroptosis and cupreptosis (102). Tan et al. found that QUE can significantly inhibit LPS-induced secretion of IL-1β, IL-6, and TNF-α from bone marrow-derived macrophages and reduce the Mincle/Syk/NF-κB signaling pathway and Cr, blood urea nitrogen (BUN), IL-1β, IL-6 and TNF-α levels in AKI model rats, alleviating CIS-induced AKI (103). QUE-loaded chitosan nanoparticles were used to reduce IL-18 and KIM-1 levels in renal tissues of CIS-intoxicated rats and improved histological changes such as renal interstitial nephritis, glomerular atrophy, tubular dilatation, cell degeneration, and necrosis caused by CIS (104).
3.2 Cardiotoxicity
Cardiotoxicity induced by chemotherapeutic drugs may be a persistent phenomenon. The first is damage to the cardiomyocytes, and this is followed by left ventricular ejection fraction (LVEF), which, if left untreated, can gradually lead to symptomatic heart failure (105). In in vivo studies, QUE combined with DOX resulted in less DOX accumulation in AC16 cardiomyocytes and less toxicity in AC16 cells (77). In another experiment, QUE pre-treatment protected primary cardiomyocytes exposed to DOX by inhibiting oxidative stress and improving mitochondrial function by up-regulating 14-3-3γ expression (106). Bmi-1 can directly regulate oxidative stress levels (107). Dong et al. revealed that QUE inhibited apoptosis, mitochondrial dysfunction, ROS production, and DNA double-strand breaks in DOX-induced H9c2 rat cardiomyocytes by inhibiting Bid, p53, and oxidase (p47 and Nox1) and up-regulating the expression of Bcl-2 and Bmi-1. Furthermore, in vivo experiments confirmed this effect (107). In addition, the proteomic analysis showed that QUE promoted damage repair of cardiomyocytes by regulating metabolic activation, protein folding, and cytoskeletal remodeling to inhibit the cardiotoxicity of DOX (108). DOX combined with CTX is the most commonly used chemotherapy regimen for TNBC; however, this regimen can cause severe damage to cardiomyocytes. Zhang et al. reported that QUE up-regulated the p-ERK and c-Myc and down-regulated Caspase-3 in H9c2 cells with DOX-CTX treated, which protected cardiomyocytes (50).
The cardiotoxicity of 5-FU can be shown to increase the expression of cardiac proinflammatory cytokines (TNF-α and IL-1β), NF-κB, and Caspase-3 to enhance the apoptosis of cardiac cells, whereas QUE can significantly improve this effect in rats with cardiotoxicity induced by 5-FU (109). Dorostkar et al. used DOX liposomes combined with free QUE to inhibit body weight loss and creatine phosphokinase-MB (CK-MB), lactate dehydrogenase (LDH), and MDA induced by DOX in rats while increasing the activities of GPX, CAT, and superoxide dismutase (SOD) in the left ventricle, which reduces heart tissue damage and cardiomyocyte apoptosis (110). Similarly, in a nude mouse transplanted tumor model of leukemia cells P388, QUE alleviated the myocardial damage caused by DOX by regulating the activities of GPX, SOD, and MDA to clear reactive oxygen species (52). In addition, Zakaria et al. revealed that QUE combined with DOX can reduce the toxicity of DOX in the heart of rats by increasing the expression of PCG-1α, PPARα, and AMPKα2 and down-regulating serum troponin, CK-MB, and creatine phosphokinase (CPK) (111). In rat models of chronic DOX treatment, DOX significantly reduced systolic ventricular septal thickness, LV posterior wall thickness, and LVEF (17). QUE administration in this rat significantly improved LVEF and NT-proBNP levels, alleviating the cardiotoxicity of DOX by alleviating oxidative stress (17). Nrf2 is a major regulator of oxidative stress signaling and is widely expressed in the cardiovascular system, regulating the expression of antioxidant genes and other cell-protective kinases (112). In DOX-induced rat cardiomyopathy models, QUE reduced elevated blood pressure and left ventricular dysfunction, inhibited the increase in CK-MB and LDH, maintained cell membrane integrity, and enhanced myocardial antioxidant capacity by up-regulating Nrf2 expression to restore biochemical and histological abnormalities of the myocardium (112). The cardiotoxicity of CIS can lead to congestive heart failure and sudden cardiac death in patients treated with CIS (113). However, QUE can reduce ROS-mediated mitochondrial damage and inflammation by regulating the Nrf2/HO-1 and p38 MAPK/NF-Bp 65/IL-8 signaling pathways, thereby antagonizing cardiotoxicity induced by CIS (113).
3.3 Neurotoxicity
The neurotoxicity of chemotherapeutic drugs manifests as both peripheral and central neurotoxicity. The main symptoms of central toxicity include encephalopathy, posterior reversible encephalopathy syndrome, and cognitive impairment (114). Peripheral neurotoxicity often presents as acrodynia, sensory neuropathy, and sometimes sensory ataxic movement (115). DOX, as a widely used anti-cancer drug, can cause damage to the cerebral cortex and hippocampus. QUE combined with DOX can significantly reduce oxidative stress, neuroinflammation, synaptic plasticity damage, and DNA damage in the cerebral cortex and hippocampus (19). It has been reported that DOX can mediate the production of free radicals in brain tissue, increase lipid peroxidation and protein oxidation, weaken the antioxidant defense system, and aggravate oxidative stress, leading to neuropsychological changes (116). In addition, in a rat model, DOX combined with QUE alleviated corticosterone excess, oxidative stress in brain tissue, and anxiety-depression-like behavior (117).
Peripheral nervous system damage caused by chemotherapy is a side effect of CIS (118). Unay et al. used QUE to intervene in PC12 nerve cells exposed to CIS and found that QUE reduced CIS-induced apoptosis of PC12 cells, changes in mitochondrial membrane potential, and LDH activity (118). Therefore, QUE can potentially reduce CIS-induced peripheral nervous system lesions (118). Reactive oxygen and nitrogen species produced after CIS treatment cause severe damage to the cell membrane of hair cells, leading to irreversible neurological hearing loss (119). In a zebrafish model, QUE mitigated CIS-induced neuronal hair cell apoptosis (120). In a rat model, QUE combined with CIS reduced hair cell loss, vascular stripe, and spiral ganglion degeneration in rats and had a protective effect against CIS (119). Severe painful sensory neuropathy often occurs during PTX treatment, which necessitates the termination of chemotherapy, leading to further deterioration of the quality of life and survival of cancer patients. A study confirmed in vivo and in vitro that QUE inhibited PTX-induced histamine release from RBL-2 H3 cells and inhibited plasma histamine levels in rats treated with PTX (121). Histamine released by mast cells is a neurotransmitter closely related to pain transduction (122). QUE can dose-dependently increase the threshold of thermal hyperalgesia and mechanical pain in animal models treated with PTX (121). In addition, QUE dose-dependently inhibited increased PKCϵ and TRPV1 expression levels in the spinal cord and dorsal root ganglion of PTX-treated animal models. QUE also inhibited the translocation of PKCϵ from the cytoplasm to the membrane in the spinal cord and dorsal root segments treated with PTX (121).
3.4 Hematopoietic and immune system toxicity
Previous studies have reported toxic effects of DOX on hematopoietic and immune systems (123, 124). DOX combined with QUE significantly improved serum lgG, lgM, lgE, and DNA damage in rat models and weakened spleen cell inflammation, apoptosis, and lymphocyte proliferation inhibition (125). In addition, the combination of QUE and DOX significantly enhanced white blood cells and lymphocytes in a rat model (117). One of the important factors that limit the clinical application of CIS is the side effect of myelosuppression, which manifests as a decrease in the number of bone marrow cells. In rats treated with CIS+QUE, QUE increased hematopoietic growth factors (GM-CSF, SCF, and IL-9) and simultaneously inhibited hematopoietic inhibitors (TNF-α and TGF-β1), thereby increasing the differentiation of white blood cells, platelets, neutrophils, erythrocytes, and lymphocytes (126).
3.5 Reproductive toxicity
DOX has been reported to have serious effects on the reproductive system, such as impaired sperm production, vacuolar degeneration, abnormal sperm morphology, reduced number of primordial follicles, and follicular morphological variation (127). In an animal experiment, the reproductive toxicity of DOX was shown to reduce the number of follicles, the volume of ovaries and their associated uterus, the number of uterine layers, and the volume of related structures in female rats. At the same time, QUE combined with vitamin E significantly alleviated these adverse effects (128). Similarly, DOX administration reduced spermatogenesis and significantly reduced spermatogenic cells in the rat model. In contrast, QUE administration resulted in significant regeneration of most spermatogenic tubule epithelial cells and a uniform arrangement of spermatogenic cells, indicating that QUE alleviated the testicular toxicity of DOX (129). CIS is also toxic to the testes. In animal models, CIS was observed to cause degeneration of the seminiferous tubule epithelium, cell detachment from the basal membrane, giant cell formation, cell loss, atrophy, vacuolation, and perivascular fibrosis (130). QUE combined with CIS can reduce atrophy, giant cell formation, and vacuolization, thereby improving testicular injury (130).
3.6 Cachexia
Cancer often causes cachexia in patients, with approximately 80% of cancer patients with cachexia not surviving, and 20% of cancer-related deaths are attributed to cachexia (131). 5-FU treatment of C26 tumor-bearing mice resulted in weight and muscle mass loss and mitochondrial function damage, while QUE administration alleviated muscle cross-sectional area loss and improved skeletal muscle mitochondrial size and number, which improved chemotherapy-induced skeletal muscle atrophy (132). Chemotherapy drugs can also cause abnormal lipid metabolism and lead to fat loss, which reduces the quality of life of patients with cancer and exacerbates the shortening of survival. CIS has been shown to promote fat degradation and oxidation in mice and impair fat production and lipid deposition (133). Lin et al. found that the intraperitoneal injection of QUE weakened CIS-induced fat loss in tumor-bearing mice (134). Moreover, in vitro experiments showed that CIS promoted fat metabolism in 3T3-Ll adipocytes, whereas QUE inhibited this effect (134).
3.7 Mucositis and hand-foot syndrome
5-FU inhibits cell division and proliferation; therefore, it non-selectively targets cells with rapidly dividing capacity, such as tumor and mucosal cells (135). Approximately 80% of patients receiving 5-FU develop mucositis, characterized by apoptosis and mucosal degeneration, which limits nutrient intake and drug application (136). In a CRC mouse model of 5-FU-induced colitis, treatment with QUE significantly reduced bloody stool and diarrhea symptoms and restored colon length (28). Further exploration confirmed that QUE reduced the expression of IL-6, TNF-α, and IL-10 in the serum and IL-1β, IL-6, IL-13, and TNF in colon tissue, thereby reducing the inflammatory response (28). A QUE nano-emulsion improved 5-FU-induced colitis by down-regulating NF-κB and HIF-α expression and inhibiting ROS production (137). Histological observations showed that QUE increased villus width, villus diameter, and intestinal muscle thickness, restoring the histopathological changes caused by 5-FU (137). Oral mucositis is a common side effect of 5-FU combination chemotherapy that often leads to oral swelling, erythema, and ulcers, seriously affecting the patient’s quality of life. QUE nanoparticles constructed by Lotfi et al. also alleviated hemorrhage and inflammatory cell infiltration in tongue tissue and inhibited the expression of NF-κB in the oral mucosa (138). Hand-foot syndrome, also known as palmoplantar erythrodysesthesia or acral erythema, is often induced by drugs such as DOX, 5-FU, or capecitabine (139). A collagen matrix incorporated with QUE inhibited the apoptosis of keratinocytes by down-regulating Caspase-3. It increased the antioxidant capacity by down-regulating the levels MDA in the skin tissue, thereby alleviating capecitabine-induced hand-foot syndrome in rats (140).
3.8 Summary
Although chemotherapy is associated with serious side effects, it remains the primary treatment option for cancer. As mentioned above, QUE reduces the toxicity of chemotherapeutic drugs (Table 5). In terms of the mechanism, QUE is mainly achieved by inhibiting ROS production, anti-oxidation, and normal cell apoptosis due to QUE’s potent antioxidant, anti-inflammatory, and neuroprotective abilities. However, the low water solubility of QUE lowers its oral availability, and more drug delivery systems need to be developed to maximize its bioavailability. In addition, the currently reported effects and mechanisms of QUE inhibition of chemotherapy side effects are limited to animals and cells, and future clinical studies are needed to evaluate its effectiveness and safety further.
4 Immunomodulation effect of QUE in TME
Innate and adaptive immunity in the TME plays a regulatory role in the recognition and killing of tumor cells. The immune escape of cancer cells and immunosuppressive microenvironment are key factors that support tumor progression. Therefore, changing the inhibitory TME and inducing an anti-cancer immune response is an attractive development direction for tumor immunotherapy.
Zhu et al. revealed that QUE up-regulated the expression of IL-2 and IFN-γ and down-regulated IL-10 in a nude mouse transplanted tumor model of TNBC 4T1 cells and increased the ratio of CD4+ and CD8+ cell differentiation in the transplanted tumor (141). Similarly, Manni found in this model that QUE can significantly increase the infiltration level of natural killer (NK) cells and decrease the infiltration level of Treg cells (142). Further mechanistic studies confirmed that QUE inhibited the content of Treg cells and the secretion of the immunosuppressive factor IL-10 in 4T1 transplanted tumor mice by inhibiting the IL-6/JAK2/STAT3 signaling pathway; however, it enhanced the cytotoxicity of CD8+T cells and NK cells, promoting anti-cancer immunity in the TME (143). Administration of QUE, resveratrol, and curcumin in 4T1 tumor-bearing mice increased the recruitment of T cells, reduced the accumulation of neutrophils and macrophages in the TME, and inhibited the development of tumor-infiltrating lymphocytes into immunosuppressive cell subgroups. Specifically, QUE’s drug combination inhibited CD4+T cells differentiate to Th2, tumor-associated neutrophils differentiate to N2 type, and tumor-associated macrophages differentiate to M2 type (144). Another study on CRC (145) revealed that QUE targeting CXCL8 inhibited macrophage polarization towards M2 and thus inhibited CRC progression. It helps reverse the dilemma of immunosuppression in TME and makes TME progress toward immune activation (144). When QUE acts on the TME of melanoma, it not only inhibits the proliferation and migration of B16 cells but also increases the number of M1 macrophages and CD8+ T cells by inhibiting PDK1/CD47 signals and promoting the secretion of IL-2 and IFN-γ by CD8+ T cells, thereby inhibiting the growth of melanoma (146). Myeloid-derived suppressor cells (MDSC) can promote tumor growth in the TME and are potential therapeutic targets (147). QUE inhibited the increase in MDSCs in CRC mouse models (28).
Immune checkpoint inhibitors have opened up new avenues for the treatment of tumors. Combining QUE and anti-PD-1 antibodies can significantly inhibit the expression of PD-L1, IL-4, and IL-6 and raise the expression of CD8a, CD4, CD11b, and IFN-γ in HCC (148). After the intervention of QUE in the H22 HCC cell BALB/c mouse transplanted tumor model, the expression levels of granulocyte colony-stimulating factor and PD-L1 were decreased, and the proportion of CD86+ cells was increased. In contrast, the proportion of CD206+ cells was decreased, indicating that QUE promoted the polarization of macrophages towards the M1 type (149). At the same time, QUE could inhibit the PD-1/PD-L1 interaction after the intervention of transplanted tumor mice, which increases the expression of CD8, GZMB, and IFN-γ and weakens the inhibitory effect of PD-L1 on T cells (150). In addition, Que inhibited the growth of BC cells by up-regulating the protein levels of IFN-γ-R, p-JAK2, and p-STAT1 and decreased the expression of PD-L1, promoting the differentiation of γδ T cells into Vδ2 T cell subgroups (151).
Jing et al. established the folic acid-modified liposome QUE, which can inhibit the expression of PD-L1 in osteosarcoma by inhibiting JAK2/STAT3 signaling, antagonizing the proliferation and immune escape of osteosarcoma cells (152). QUE-ferrum nanoparticles constructed by Li et al. inhibited the expression of the PD-L1 receptor by inhibiting the phosphorylation of JAK2 and STAT3. They remodeled the extracellular matrix by down-regulating α-SMA, which could capture tumor antigens and deliver them to tumor drainage lymph nodes, increasing the activation of antigen-presenting cells (153). Similarly, Hong et al. constructed a nanoprodrug self-assembled from polyethylene glycol-poly-4-borono-l-phenylalanine conjugating with QUE. The released QUE can reduce the high expression of PD-L1 on the surface of tumor cells, thus promoting the infiltration of cytotoxic T lymphocytes into the TME and enhancing the effect of tumor immunotherapy (154). Microsatellite-stable CRC is resistant to immunotherapies. A nanoformulated co-delivery of QUE and alantolactone can up-regulate dendritic cells’ co-stimulatory signals (MHCII and CD86) to initiate anti-cancer T lymphocyte response and cytokine secretion. In contrast, the number of Treg cells and MDSC was significantly reduced. This QUE drug delivery system changed the inhibitory TME and promoted the systemic memory anti-cancer response (155).
In conclusion, QUE has shown great potential for modulating tumor immune responses. It enhances the anti-cancer immune response by reducing Treg cell differentiation, promoting CD8+T cell differentiation, inducing macrophages to M1-type polarization, and inhibiting the PD-1/PD-L1 immune checkpoint (Figure 2). However, these roles and mechanisms need to be further explored and validated. Therefore, QUE may be a potential tumor immunotherapy option in the future.
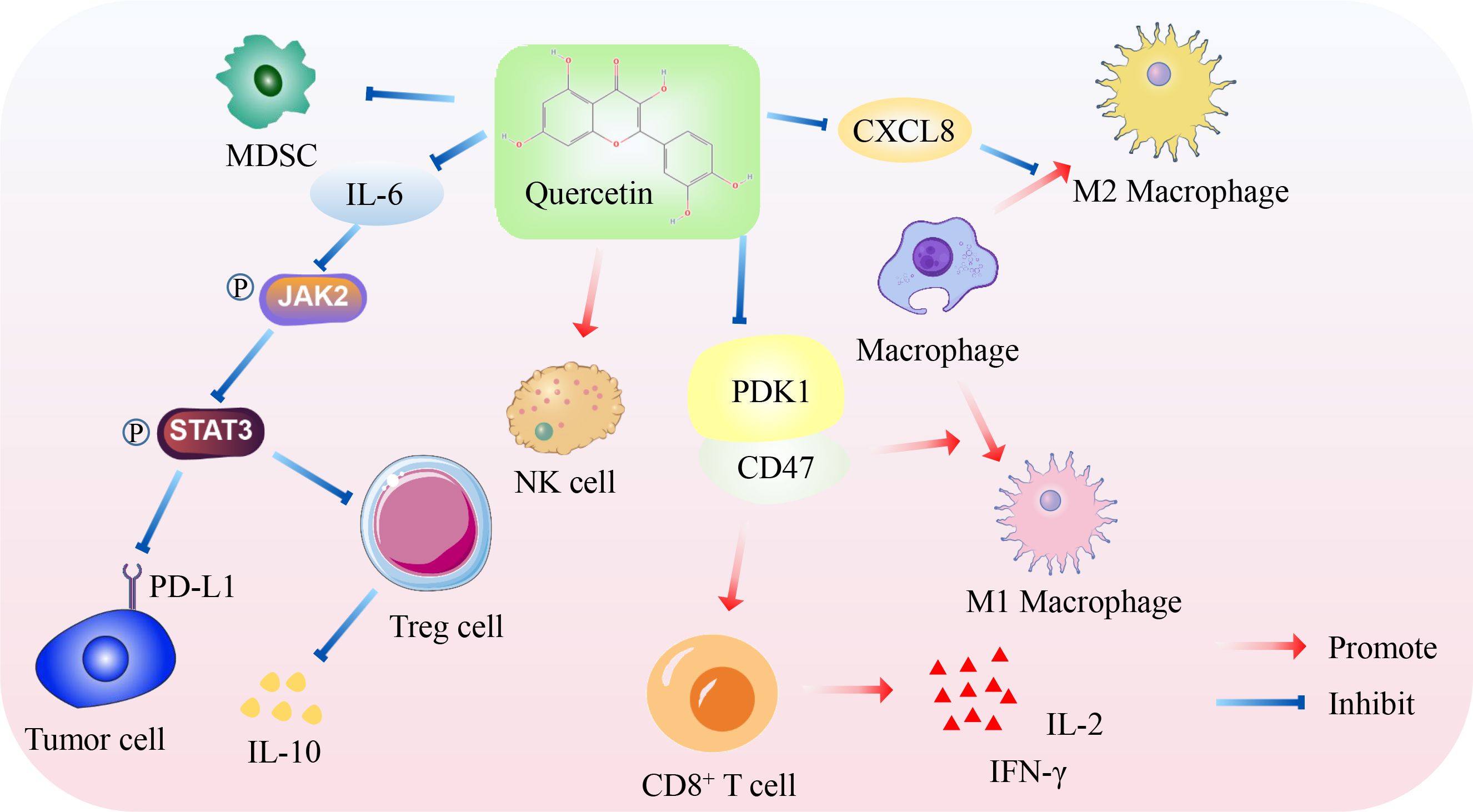
Figure 2. Mechanism of QUE promoting anti-cancer immunity. On the one hand, QUE inhibits PD-L1 expression of tumor cells, Treg cell differentiation, and immunosuppressive factor IL-10 secretion by inhibiting JAK2/STAT3 pathway. On the other hand, QUE inhibits the PDK1/CD47 axis to promote the differentiation of M1 macrophages and CD8+T cells and promote the secretion of IL-2 and IFN-γ by CD8+T cells. In addition, QUE inhibits immunosuppressive cells, such as M2 macrophages and MDSC, but increases the cytotoxicity of NK cells.
5 Clinical trials
Numerous clinical studies have shown that QUE is beneficial for various diseases such as myocardial infarction (156), upper respiratory tract infection (157), rheumatoid arthritis (158), nonalcoholic fatty liver disease (159), wound healing (160), polycystic ovary syndrome (161), and sarcoidosis (162). Regarding anti-cancer, a clinical study (163) showed that QUE combined with curcumin significantly reduced the size and number of adenomas in patients with familial adenomatous polyposis without showing toxicity to the patients. In addition, a cohort study (164) of two groups of healthy, non-smoking male subjects whose diet was supplemented with QUE or placebo found that plasma TIMP1 mRNA and protein levels were significantly decreased in subjects supplementation with QUE, suggesting that QUE may be a cancer chemopreventive agent. Another prospective randomized controlled study (165) investigated whether QUE could increase green tea polyphenols’ bioavailability in prostate cancer patients. However, QUE did not show any significant effect compared with the placebo. We searched the ClinicalTrials.gov database (https://www.clinicaltrials.gov/) with the keywords “ Quercetin” and “Cancer.” Surprisingly, we found that clinical trials have been completed to investigate the effects of QUE on radiation-induced cystitis, chemotherapy-induced oral mucositis, and cancer-associated Fanconi anemia. In addition, there are several ongoing studies investigating the efficacy and safety of QUE in combination with tislelizumab and dasatinib in patients with head and neck squamous cell carcinoma, the efficacy of QUE and green tea in combination with docetaxel in castration-resistant prostate cancer, and the efficacy of QUE in combination with dasatinib in reversing chemotherapy resistance in TNBC.
We look forward to publishing these clinical trial reports to reveal the potential applications of QUE in cancer. However, there is still a lack of clinical trials focusing on the effect of QUE on chemotherapeutic drugs and the immune status of cancer patients. In the future, more prospective randomized controlled trials with larger sample sizes are needed to explore QUE’s benefits on chemotherapy and cancer patients’ immune status.
6 Conclusions and future prospects
Despite significant advances in treatment methods in recent years, cancer remains one of the most serious diseases, causing a large number of deaths each year. Although chemotherapy is considered an effective cancer treatment, its side effects and the development of drug resistance in tumor cells pose significant obstacles to its application. QUE is a widely distributed flavonoid with chemosensitization properties, and it alleviates the toxic side effects combined with various common chemotherapeutic agents. However, the optimal ratio of QUE to chemotherapeutic drugs remains an interesting topic. How to use the proportion of QUE and chemotherapy drugs to achieve an optimal point between anti-cancer effects and reducing toxic side effects may be a problem that needs to be considered in the future. Dormant CSC in tumor tissue escapes the killing of chemotherapeutic drugs and immune cells, which is one of the key factors in chemotherapy resistance and relapse. However, there is still a lack of understanding of the effect of QUE combined with chemotherapeutic drugs on CSC, which is worth exploring. However, existing studies support the idea that QUE promotes an anti-cancer immune response. However, at present, the study of QUE on tumor immunity is in the initial stage, and further multi-omics methods are needed to reveal the influence of QUE on the TME landscape in animal models. The low solubility and bioavailability of QUE and some chemotherapeutic agents limit their application in cancer therapy. Nanoformulation strategies, such as the use of nanoparticles and liposomes, have been implemented, and these strategies have shown excellent results in enhancing chemotherapy sensitivity and mitigating side effects by improving solubility and cellular uptake of QUE and chemotherapy drugs. However, nanocarriers may also be toxic, and attention should be paid to the toxicological properties of nanomaterials. Biodegradable and biocompatible materials should be given a higher priority. Ultimately, more clinical trials are required to evaluate the benefits of QUE in chemotherapy and patient immune status. We believe that with the continuous disclosure of the synergistic effect of QUE with chemotherapy drugs, the mechanism of immune regulation, and the further development of nanomaterials, adjuvant therapy of QUE against tumors will show broad prospects.
Author contributions
HD: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. FW: Writing – review & editing. WH: Writing – review & editing. YL: Funding acquisition, Writing – review & editing. XX: Funding acquisition, Writing – review & editing. LZ: Funding acquisition, Writing – review & editing. YZ: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by the Gansu Province University Industrial Support Plan (2023CYZC-05), the Cuiying Technology Innovation Project of Lanzhou University Second Hospital (CY2022-MS-B04, CY2022-QN-A15), the Doctoral Students Training Research Fund of Lanzhou University Second Hospital (YJS-BD-32), and the Natural Science Foundation of Gansu Province (23JRRA0992).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Biswas P, Kaium MA, Islam Tareq MM, Tauhida SJ, Hossain MR, Siam LS, et al. The experimental significance of isorhamnetin as an effective therapeutic option for cancer: A comprehensive analysis. BioMed Pharmacother. (2024) 176:116860. doi: 10.1016/j.biopha.2024.116860
2. You W and Henneberg M. Cancer incidence increasing globally: The role of relaxed natural selection. Evol Appl. (2018) 11:140–52. doi: 10.1111/eva.12523
3. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
4. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, and Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
5. Behranvand N, Nasri F, Zolfaghari Emameh R, Khani P, Hosseini A, Garssen J, et al. Chemotherapy: a double-edged sword in cancer treatment. Cancer Immunol Immunother. (2022) 71:507–26. doi: 10.1007/s00262-021-03013-3
6. Li B, Shao H, Gao L, Li H, Sheng H, and Zhu L. Nano-drug co-delivery system of natural active ingredients and chemotherapy drugs for cancer treatment: a review. Drug Deliv. (2022) 29:2130–61. doi: 10.1080/10717544.2022.2094498
7. Anand David AV, Arulmoli R, and Parasuraman S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn Rev. (2016) 10:84–9. doi: 10.4103/0973-7847.194044
8. Sultana B and Anwar F. Flavonols (kaempeferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chem. (2008) 108:879–84. doi: 10.1016/j.foodchem.2007.11.053
9. Dharmendra K and Pramod Kumar S. Quercetin: A comprehensive review. Curr Nutr Food Sci. (2024) 20:143–66. doi: 10.2174/1573401319666230428152045
10. Reyes-Farias M and Carrasco-Pozo C. The anti-cancer effect of quercetin: molecular implications in cancer metabolism. Int J Mol Sci. (2019) 20:3177. doi: 10.3390/ijms20133177
11. Hollman PC and Katan MB. Absorption, metabolism and health effects of dietary flavonoids in man. BioMed Pharmacother. (1997) 51:305–10. doi: 10.1016/s0753-3322(97)88045-6
12. Morikawa K, Nonaka M, Narahara M, Torii I, Kawaguchi K, Yoshikawa T, et al. Inhibitory effect of quercetin on carrageenan-induced inflammation in rats. Life Sci. (2003) 74:709–21. doi: 10.1016/j.lfs.2003.06.036
13. Islam MR, Al-Imran MIK, Zehravi M, Sweilam SH, Mortuza MR, Gupta JK, et al. Targeting signaling pathways in neurodegenerative diseases: Quercetin’s cellular and molecular mechanisms for neuroprotection. Anim Model Exp Med. (2025) 1068. doi: 10.1002/ame2.12551
14. Yang D, Wang T, Long M, and Li P. Quercetin: its main pharmacological activity and potential application in clinical medicine. Oxid Med Cell Longev. (2020) 2020:8825387. doi: 10.1155/2020/8825387
15. Vollmannová A, Bojňanská T, Musilová J, Lidiková J, and Cifrová M. Quercetin as one of the most abundant represented biological valuable plant components with remarkable chemoprotective effects - A review. Heliyon. (2024) 10:e33342. doi: 10.1016/j.heliyon.2024.e33342
16. Khatoon E, Banik K, Harsha C, Sailo BL, Thakur KK, Khwairakpam AD, et al. Phytochemicals in cancer cell chemosensitization: Current knowledge and future perspectives. Semin Cancer Biol. (2022) 80:306–39. doi: 10.1016/j.semcancer.2020.06.014
17. Dulf PL, Coadă CA, Florea A, Moldovan R, Baldea I, Dulf DV, et al. Mitigating doxorubicin-induced cardiotoxicity through quercetin intervention: an experimental study in rats. Antioxidants (Basel). (2024) 13:1068. doi: 10.3390/antiox13091068
18. Seker U, Kavak DE, Dokumaci FZ, Kizildag S, and Irtegun-Kandemir S. The nephroprotective effect of Quercetin in Cyclophosphamide-induced renal toxicity might be associated with MAPK/ERK and NF-κB signal modulation activity. Drug Chem Toxicol. (2024) 47:1165–74. doi: 10.1080/01480545.2024.2347541
19. El-Shetry ES, Ibrahim IA, Kamel AM, and Abdelwahab OA. Quercetin mitigates doxorubicin-induced neurodegenerative changes in the cerebral cortex and hippocampus of rats; insights to DNA damage, inflammation, synaptic plasticity. Tissue Cell. (2024) 87:102313. doi: 10.1016/j.tice.2024.102313
20. Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, et al. PubChem 2023 update. Nucleic Acids Res. (2023) 51:D1373–d1380. doi: 10.1093/nar/gkac956
21. Hanahan D and Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
22. Petroni G, Buqué A, Zitvogel L, Kroemer G, and Galluzzi L. Immunomodulation by targeted anticancer agents. Cancer Cell. (2021) 39:310–45. doi: 10.1016/j.ccell.2020.11.009
23. Zhang Z, Yu X, Wang Z, Wu P, and Huang J. Anthracyclines potentiate anti-tumor immunity: A new opportunity for chemoimmunotherapy. Cancer Lett. (2015) 369:331–5. doi: 10.1016/j.canlet.2015.10.002
24. Bandyopadhyay S, Romero JR, and Chattopadhyay N. Kaempferol and quercetin stimulate granulocyte-macrophage colony-stimulating factor secretion in human prostate cancer cells. Mol Cell Endocrinol. (2008) 287:57–64. doi: 10.1016/j.mce.2008.01.015
25. Luo S, Yue M, Wang D, Lu Y, Wu Q, and Jiang J. Breaking the barrier: Epigenetic strategies to combat platinum resistance in colorectal cancer. Drug Resist Updat. (2024) 77:101152. doi: 10.1016/j.drup.2024.101152
26. Sethy C and Kundu CN. 5-Fluorouracil (5-FU) resistance and the new strategy to enhance the sensitivity against cancer: Implication of DNA repair inhibition. BioMed Pharmacother. (2021) 137:111285. doi: 10.1016/j.biopha.2021.111285
27. Yang C, Song J, Hwang S, Choi J, Song G, and Lim W. Apigenin enhances apoptosis induction by 5-fluorouracil through regulation of thymidylate synthase in colorectal cancer cells. Redox Biol. (2021) 47:102144. doi: 10.1016/j.redox.2021.102144
28. Yang C, Song J, Park S, Ham J, Park W, Park H, et al. Targeting thymidylate synthase and tRNA-derived non-coding RNAs improves therapeutic sensitivity in colorectal cancer. Antioxidants (Basel). (2022) 11:2158. doi: 10.3390/antiox11112158
29. Benhattar J, Cerottini JP, Saraga E, Metthez G, and Givel JC. p53 mutations as a possible predictor of response to chemotherapy in metastatic colorectal carcinomas. Int J Cancer. (1996) 69:190–2. doi: 10.1002/(sici)1097-0215(19960621)69:3<190::Aid-ijc7>3.0.Co;2-v
30. Xavier CP, Lima CF, Rohde M, and Pereira-Wilson C. Quercetin enhances 5-fluorouracil-induced apoptosis in MSI colorectal cancer cells through p53 modulation. Cancer Chemother Pharmacol. (2011) 68:1449–57. doi: 10.1007/s00280-011-1641-9
31. Das S, Saha M, Mahata LC, China A, Chatterjee N, and Das Saha K. Quercetin and 5-Fu Loaded Chitosan Nanoparticles Trigger Cell-Cycle Arrest and Induce Apoptosis in HCT116 Cells via Modulation of the p53/p21 Axis. ACS Omega. (2023) 8:36893–905. doi: 10.1021/acsomega.3c03933
32. Li M, Fan J, Hu M, Xu J, He Z, and Zeng J. Quercetin enhances 5-fluorouracil sensitivity by regulating the autophagic flux and inducing drp-1 mediated mitochondrial fragmentation in colorectal cancer cells. Curr Mol Pharmacol. (2024) 17:e18761429283717. doi: 10.2174/0118761429283717231222104730
33. Tang Z, Wang L, Chen Y, Zheng X, Wang R, Liu B, et al. Quercetin reverses 5-fluorouracil resistance in colon cancer cells by modulating the NRF2/HO-1 pathway. Eur J Histochem. (2023) 67:3719. doi: 10.4081/ejh.2023.3719
34. Terana GT, Abd-Alhaseeb MM, Omran GA, and Okda TM. Quercetin potentiates 5-fluorouracil effects in human colon cancer cells through targeting the Wnt/β-catenin signalling pathway: the role of miR-27a. Contemp Oncol (Pozn). (2022) 26:229–38. doi: 10.5114/wo.2022.120361
35. Lin TY, Tsai MC, Tu W, Yeh HC, Wang SC, Huang SP, et al. Role of the NLRP3 inflammasome: insights into cancer hallmarks. Front Immunol. (2020) 11:610492. doi: 10.3389/fimmu.2020.610492
36. Lee KC, Wu KL, Yen CK, Chang SF, Chen CN, and Lu YC. Inhibition of NLRP3 by fermented quercetin decreases resistin-induced chemoresistance to 5-fluorouracil in human colorectal cancer cells. Pharmaceuticals (Basel). (2022) 15:798. doi: 10.3390/ph15070798
37. Erdoğan MK, Ağca CA, and Aşkın H. Quercetin and luteolin improve the anticancer effects of 5-fluorouracil in human colorectal adenocarcinoma in vitro model: A mechanistic insight. Nutr Cancer. (2022) 74:660–76. doi: 10.1080/01635581.2021.1900301
38. Rani Inala MS and Pamidimukkala K. Amalgamation of quercetin with anastrozole and capecitabine: A novel combination to treat breast and colon cancers - An in vitro study. J Cancer Res Ther. (2023) 19:S93–s105. doi: 10.4103/jcrt.JCRT_599_20
39. Mawalizadeh F, Mohammadzadeh G, Khedri A, and Rashidi M. Quercetin potentiates the chemosensitivity of MCF-7 breast cancer cells to 5-fluorouracil. Mol Biol Rep. (2021) 48:7733–42. doi: 10.1007/s11033-021-06782-3
40. Roshanazadeh M, Babaahmadi Rezaei H, and Rashidi M. Quercetin synergistically potentiates the anti-metastatic effect of 5-fluorouracil on the MDA-MB-231 breast cancer cell line. Iran J Basic Med Sci. (2021) 24:928–34. doi: 10.22038/ijbms.2021.56559.12629
41. Chuang-Xin L, Wen-Yu W, Yao C, Xiao-Yan L, and Yun Z. Quercetin enhances the effects of 5-fluorouracil-mediated growth inhibition and apoptosis of esophageal cancer cells by inhibiting NF-κB. Oncol Lett. (2012) 4:775–8. doi: 10.3892/ol.2012.829
42. Roman A, Smeu A, Lascu A, Dehelean CA, Predescu IA, Motoc A, et al. Quercetin enhances 5-fluorouracil-driven cytotoxicity dose-dependently in A375 human melanoma cells. Life (Basel). (2024) 14:1685. doi: 10.3390/life14121685
43. Tavakoli Pirzaman A, Aghajanian S, Mansoori R, Al EAA, Ebrahimzadeh M, Moghadamnia AA, et al. Interaction of quercetin and 5-fluorouracil: cellular and pharmacokinetic study. Toxicol Mech Methods. (2023) 33:502–11. doi: 10.1080/15376516.2023.2188928
44. Liu J, Yuan Q, Guo H, Guan H, Hong Z, and Shang D. Deciphering drug resistance in gastric cancer: Potential mechanisms and future perspectives. BioMed Pharmacother. (2024) 173:116310. doi: 10.1016/j.biopha.2024.116310
45. Ding H, Wang Y, and Zhang H. CCND1 silencing suppresses liver cancer stem cell differentiation and overcomes 5-Fluorouracil resistance in hepatocellular carcinoma. J Pharmacol Sci. (2020) 143:219–25. doi: 10.1016/j.jphs.2020.04.006
46. Smith L, Watson MB, O’Kane SL, Drew PJ, Lind MJ, and Cawkwell L. The analysis of doxorubicin resistance in human breast cancer cells using antibody microarrays. Mol Cancer Ther. (2006) 5:2115–20. doi: 10.1158/1535-7163.Mct-06-0190
47. AbuHammad S and Zihlif M. Gene expression alterations in doxorubicin resistant MCF7 breast cancer cell line. Genomics. (2013) 101:213–20. doi: 10.1016/j.ygeno.2012.11.009
48. Li SZ, Qiao SF, Zhang JH, and Li K. Quercetin increase the chemosensitivity of breast cancer cells to doxorubicin via PTEN/akt pathway. Anticancer Agents Med Chem. (2015) 15:1185–9. doi: 10.2174/1871520615999150121121708
49. Almohammad Aljabr B, Zihlif M, Abu-Dahab R, and Zalloum H. Effect of quercetin on doxorubicin cytotoxicity in sensitive and resistant human MCF7 breast cancer cell lines. BioMed Rep. (2024) 20:58. doi: 10.3892/br.2024.1745
50. Zhang P, Zhang J, Zhao L, Li S, and Li K. Quercetin attenuates the cardiotoxicity of doxorubicin-cyclophosphamide regimen and potentiates its chemotherapeutic effect against triple-negative breast cancer. Phytother Res. (2022) 36:551–61. doi: 10.1002/ptr.7342
51. Giacomini I, Cortini M, Tinazzi M, Baldini N, Cocetta V, Ragazzi E, et al. Contribution of mitochondrial activity to doxorubicin-resistance in osteosarcoma cells. Cancers (Basel). (2023) 15:1370. doi: 10.3390/cancers15051370
52. Han Y, Yu H, Wang J, Ren Y, Su X, and Shi Y. Quercetin alleviates myocyte toxic and sensitizes anti-leukemic effect of adriamycin. Hematology. (2015) 20:276–83. doi: 10.1179/1607845414y.0000000198
53. Shu Y, Xie B, Liang Z, and Chen J. Quercetin reverses the doxorubicin resistance of prostate cancer cells by downregulating the expression of c-met. Oncol Lett. (2018) 15:2252–8. doi: 10.3892/ol.2017.7561
54. Wang G, Zhang J, Liu L, Sharma S, and Dong Q. Quercetin potentiates doxorubicin mediated antitumor effects against liver cancer through p53/Bcl-xl. PloS One. (2012) 7:e51764. doi: 10.1371/journal.pone.0051764
55. Li M, Yue X, Wang Y, Zhang J, Kan L, and Jing Z. Remodeling the tumor microenvironment to improve drug permeation and antitumor effects by co-delivering quercetin and doxorubicin. J Mater Chem B. (2019) 7:7619–26. doi: 10.1039/c9tb02131k
56. Chang CE, Hsieh CM, Huang SC, Su CY, Sheu MT, and Ho HO. Lecithin-stabilized polymeric micelles (L(sb)PMs) for delivering quercetin: pharmacokinetic studies and therapeutic effects of quercetin alone and in combination with doxorubicin. Sci Rep. (2018) 8:17640. doi: 10.1038/s41598-018-36162-0
57. Ghosh S. Cisplatin: The first metal based anticancer drug. Bioorg Chem. (2019) 88:102925. doi: 10.1016/j.bioorg.2019.102925
58. Dasari S, Njiki S, Mbemi A, Yedjou CG, and Tchounwou PB. Pharmacological effects of cisplatin combination with natural products in cancer chemotherapy. Int J Mol Sci. (2022) 23:1532. doi: 10.3390/ijms23031532
59. Zhao JL, Zhao J, and Jiao HJ. Synergistic growth-suppressive effects of quercetin and cisplatin on HepG2 human hepatocellular carcinoma cells. Appl Biochem Biotechnol. (2014) 172:784–91. doi: 10.1007/s12010-013-0561-z
60. Wang XJ, Zhang P, and Chen L. Quercetin represses cholesterol metabolism and mitigates resistance to cisplatin in oral squamous cell carcinoma by regulating AGR2/AKT/SREBP2 axis. Heliyon. (2024) 10:e37518. doi: 10.1016/j.heliyon.2024.e37518
61. Li X, Guo S, Xiong XK, Peng BY, Huang JM, Chen MF, et al. Combination of quercetin and cisplatin enhances apoptosis in OSCC cells by downregulating xIAP through the NF-κB pathway. J Cancer. (2019) 10:4509–21. doi: 10.7150/jca.31045
62. Hasan AAS, Kalinina EV, Tatarskiy VV, Volodina YL, Petrova АS, Novichkova MD, et al. Suppression of the antioxidant system and PI3K/akt/mTOR signaling pathway in cisplatin-resistant cancer cells by quercetin. Bull Exp Biol Med. (2022) 173:760–4. doi: 10.1007/s10517-022-05626-9
63. Hasan AA, Kalinina E, Nuzhina J, Volodina Y, Shtil A, and Tatarskiy V. Potentiation of cisplatin cytotoxicity in resistant ovarian cancer SKOV3/cisplatin cells by quercetin pre-treatment. Int J Mol Sci. (2023) 24:10960. doi: 10.3390/ijms241310960
64. Ji H, Li K, Xu W, Li R, Xie S, and Zhu X. Prediction of the mechanisms by which quercetin enhances cisplatin action in cervical cancer: A network pharmacology study and experimental validation. Front Oncol. (2021) 11:780387. doi: 10.3389/fonc.2021.780387
65. Li T and Li Y. Quercetin acts as a novel anti-cancer drug to suppress cancer aggressiveness and cisplatin-resistance in nasopharyngeal carcinoma (NPC) through regulating the yes-associated protein/Hippo signaling pathway. Immunobiology. (2023) 228:152324. doi: 10.1016/j.imbio.2022.152324
66. Ahmadi M, Valizadeh A, Bazavar M, and Yousefi B. Investigating the role of quercetin in increasing the rate of cisplatin-induced apoptosis via the NF-κB pathway in MG-63 cancer cells. Drug Res (Stuttg). (2022) 72:385–9. doi: 10.1055/a-1842-7424
67. Zhang X, Guo Q, Chen J, and Chen Z. Quercetin enhances cisplatin sensitivity of human osteosarcoma cells by modulating microRNA-217-KRAS axis. Mol Cells. (2015) 38:638–42. doi: 10.14348/molcells.2015.0037
68. Liu H, Lee JI, and Ahn TG. Effect of quercetin on the anti-tumor activity of cisplatin in EMT6 breast tumor-bearing mice. Obstet Gynecol Sci. (2019) 62:242–8. doi: 10.5468/ogs.2019.62.4.242
69. Ji H, Zhang Z, Chen C, Xu W, Liu T, Dong Y, et al. The impact of quercetin and paclitaxel combination on ovarian cancer cells. iScience. (2024) 27:110434. doi: 10.1016/j.isci.2024.110434
70. Zhang X, Huang J, Yu C, Xiang L, Li L, Shi D, et al. Quercetin enhanced paclitaxel therapeutic effects towards PC-3 prostate cancer through ER stress induction and ROS production. Onco Targets Ther. (2020) 13:513–23. doi: 10.2147/ott.S228453
71. Maciejczyk A and Surowiak P. Quercetin inhibits proliferation and increases sensitivity of ovarian cancer cells to cisplatin and paclitaxel. Ginekol Pol. (2013) 84:590–5. doi: 10.17772/gp/1609
72. Yang Y, Yan J, Huang J, Wu X, Yuan Y, Yuan Y, et al. Exploring the mechanism by which quercetin re-sensitizes breast cancer to paclitaxel: network pharmacology, molecular docking, and experimental verification. Naunyn Schmiedebergs Arch Pharmacol. (2023) 396:3045–59. doi: 10.1007/s00210-023-02510-9
73. Wang Y, Yu H, Wang S, Gai C, Cui X, Xu Z, et al. Targeted delivery of quercetin by nanoparticles based on chitosan sensitizing paclitaxel-resistant lung cancer cells to paclitaxel. Mater Sci Eng C Mater Biol Appl. (2021) 119:111442. doi: 10.1016/j.msec.2020.111442
74. Wang X, Chen Y, Dahmani FZ, Yin L, Zhou J, and Yao J. Amphiphilic carboxymethyl chitosan-quercetin conjugate with P-gp inhibitory properties for oral delivery of paclitaxel. Biomaterials. (2014) 35:7654–65. doi: 10.1016/j.biomaterials.2014.05.053
75. Chen Z, Shi T, Zhang L, Zhu P, Deng M, Huang C, et al. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. (2016) 370:153–64. doi: 10.1016/j.canlet.2015.10.010
76. Liu S, Li R, Qian J, Sun J, Li G, Shen J, et al. Combination therapy of doxorubicin and quercetin on multidrug-resistant breast cancer and their sequential delivery by reduction-sensitive hyaluronic acid-based conjugate/d-α-tocopheryl poly(ethylene glycol) 1000 succinate mixed micelles. Mol Pharm. (2020) 17:1415–27. doi: 10.1021/acs.molpharmaceut.0c00138
77. Li S, Yuan S, Zhao Q, Wang B, Wang X, and Li K. Quercetin enhances chemotherapeutic effect of doxorubicin against human breast cancer cells while reducing toxic side effects of it. BioMed Pharmacother. (2018) 100:441–7. doi: 10.1016/j.biopha.2018.02.055
78. Li S, Zhao Q, Wang B, Yuan S, Wang X, and Li K. Quercetin reversed MDR in breast cancer cells through down-regulating P-gp expression and eliminating cancer stem cells mediated by YB-1 nuclear translocation. Phytother Res. (2018) 32:1530–6. doi: 10.1002/ptr.6081
79. Yuan Z, Wang H, Hu Z, Huang Y, Yao F, Sun S, et al. Quercetin inhibits proliferation and drug resistance in KB/VCR oral cancer cells and enhances its sensitivity to vincristine. Nutr Cancer. (2015) 67:126–36. doi: 10.1080/01635581.2015.965334
80. Chen FY, Cao LF, Wan HX, Zhang MY, Cai JY, Shen LJ, et al. Quercetin enhances adriamycin cytotoxicity through induction of apoptosis and regulation of mitogen-activated protein kinase/extracellular signal-regulated kinase/c-Jun N-terminal kinase signaling in multidrug-resistant leukemia K562 cells. Mol Med Rep. (2015) 11:341–8. doi: 10.3892/mmr.2014.2734
81. Chen Z, Huang C, Ma T, Jiang L, Tang L, Shi T, et al. Reversal effect of quercetin on multidrug resistance via FZD7/β-catenin pathway in hepatocellular carcinoma cells. Phytomedicine. (2018) 43:37–45. doi: 10.1016/j.phymed.2018.03.040
82. Hyun HB, Moon JY, and Cho SK. Quercetin suppresses CYR61-mediated multidrug resistance in human gastric adenocarcinoma AGS cells. Molecules. (2018) 23:209. doi: 10.3390/molecules23020209
83. Zhao G and Xue S. Mechanism of quercetin as a multidrug-resistant reversing compound in oxaliplatin-resistant gastric-cancer cell lines. Altern Ther Health Med. (2023) 29:54–9.
84. Zhou Y, Zhang J, Wang K, Han W, Wang X, Gao M, et al. Quercetin overcomes colon cancer cells resistance to chemotherapy by inhibiting solute carrier family 1, member 5 transporter. Eur J Pharmacol. (2020) 881:173185. doi: 10.1016/j.ejphar.2020.173185
85. Xia CQ and Smith PG. Drug efflux transporters and multidrug resistance in acute leukemia: therapeutic impact and novel approaches to mediation. Mol Pharmacol. (2012) 82:1008–21. doi: 10.1124/mol.112.079129
86. Hassan S, Peluso J, Chalhoub S, Idoux Gillet Y, Benkirane-Jessel N, Rochel N, et al. Quercetin potentializes the respective cytotoxic activity of gemcitabine or doxorubicin on 3D culture of AsPC-1 or HepG2 cells, through the inhibition of HIF-1α and MDR1. PloS One. (2020) 15:e0240676. doi: 10.1371/journal.pone.0240676
87. Minaei A, Sabzichi M, Ramezani F, Hamishehkar H, and Samadi N. Co-delivery with nano-quercetin enhances doxorubicin-mediated cytotoxicity against MCF-7 cells. Mol Biol Rep. (2016) 43:99–105. doi: 10.1007/s11033-016-3942-x
88. Liu M, Fu M, Yang X, Jia G, Shi X, Ji J, et al. Paclitaxel and quercetin co-loaded functional mesoporous silica nanoparticles overcoming multidrug resistance in breast cancer. Colloids Surf B Biointerfaces. (2020) 196:111284. doi: 10.1016/j.colsurfb.2020.111284
89. Mu Y, Wu G, Su C, Dong Y, Zhang K, Li J, et al. pH-sensitive amphiphilic chitosan-quercetin conjugate for intracellular delivery of doxorubicin enhancement. Carbohydr Polym. (2019) 223:115072. doi: 10.1016/j.carbpol.2019.115072
90. Zhang Z, Xu S, Wang Y, Yu Y, Li F, Zhu H, et al. Near-infrared triggered co-delivery of doxorubicin and quercetin by using gold nanocages with tetradecanol to maximize anti-tumor effects on MCF-7/ADR cells. J Colloid Interface Sci. (2018) 509:47–57. doi: 10.1016/j.jcis.2017.08.097
91. Mu Y, Fu Y, Li J, Yu X, Li Y, Wang Y, et al. Multifunctional quercetin conjugated chitosan nano-micelles with P-gp inhibition and permeation enhancement of anticancer drug. Carbohydr Polym. (2019) 203:10–8. doi: 10.1016/j.carbpol.2018.09.020
92. Fang J, Zhang S, Xue X, Zhu X, Song S, Wang B, et al. Quercetin and doxorubicin co-delivery using mesoporous silica nanoparticles enhance the efficacy of gastric carcinoma chemotherapy. Int J Nanomed. (2018) 13:5113–26. doi: 10.2147/ijn.S170862
93. Pawar CS, Balamurugan K, Baskar S, Prasad NR, and Khan HA. Enhancing chemosensitivity in drug-resistant breast cancer cells using β-cyclodextrin-loaded quercetin and doxorubicin inclusion complex via modulating SRC/PI3K/akt pathway. Appl Biochem Biotechnol. (2025). doi: 10.1007/s12010-025-05219-y
94. Patel G, Thakur NS, Kushwah V, Patil MD, Nile SH, Jain S, et al. Mycophenolate co-administration with quercetin via lipid-polymer hybrid nanoparticles for enhanced breast cancer management. Nanomedicine. (2020) 24:102147. doi: 10.1016/j.nano.2019.102147
95. Patel G, Thakur NS, Kushwah V, Patil MD, Nile SH, Jain S, et al. Liposomal delivery of mycophenolic acid with quercetin for improved breast cancer therapy in SD rats. Front Bioeng Biotechnol. (2020) 8:631. doi: 10.3389/fbioe.2020.00631
96. Schirrmacher V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int J Oncol. (2019) 54:407–19. doi: 10.3892/ijo.2018.4661
97. Ali HH, Ahmed ZA, and Aziz TA. Effect of telmisartan and quercetin in 5 fluorouracil-induced renal toxicity in rats. J Inflammation Res. (2022) 15:6113–24. doi: 10.2147/jir.S389017
98. Yufang W, Mingfang L, Nan H, and Tingting W. Quercetin-targeted AKT1 regulates the Raf/MEK/ERK signaling pathway to protect against doxorubicin-induced nephropathy in mice. Tissue Cell. (2023) 85:102229. doi: 10.1016/j.tice.2023.102229
99. Yagmurca M, Yasar Z, and Bas O. Effects of quercetin on kidney injury induced by doxorubicin. Bratisl Lek Listy. (2015) 116:486–9. doi: 10.4149/bll_2015_092
100. Khalil SR, Mohammed AT, Abd El-Fattah AH, and Zaglool AW. Intermediate filament protein expression pattern and inflammatory response changes in kidneys of rats receiving doxorubicin chemotherapy and quercetin. Toxicol Lett. (2018) 288:89–98. doi: 10.1016/j.toxlet.2018.02.024
101. Kocahan S, Dogan Z, Erdemli E, and Taskin E. Protective effect of quercetin against oxidative stress-induced toxicity associated with doxorubicin and cyclophosphamide in rat kidney and liver tissue. Iran J Kidney Dis. (2017) 11:124–31.
102. Shi M, Mobet Y, and Shen H. Quercetin attenuates acute kidney injury caused by cisplatin by inhibiting ferroptosis and cuproptosis. Cell Biochem Biophys. (2024) 82:2687–99. doi: 10.1007/s12013-024-01379-6
103. Tan RZ, Wang C, Deng C, Zhong X, Yan Y, Luo Y, et al. Quercetin protects against cisplatin-induced acute kidney injury by inhibiting Mincle/Syk/NF-κB signaling maintained macrophage inflammation. Phytother Res. (2020) 34:139–52. doi: 10.1002/ptr.6507
104. Bakr AF, El-Shiekh RA, Mahmoud MY, Khalil HMA, Alyami MH, Alyami HS, et al. Efficacy of quercetin and quercetin loaded chitosan nanoparticles against cisplatin-induced renal and testicular toxicity via attenuation of oxidative stress, inflammation, and apoptosis. Pharmaceuticals (Basel). (2024) 17:1384. doi: 10.3390/ph17101384
105. Cardinale D, Iacopo F, and Cipolla CM. Cardiotoxicity of anthracyclines. Front Cardiovasc Med. (2020) 7:26. doi: 10.3389/fcvm.2020.00026
106. Chen X, Peng X, Luo Y, You J, Yin D, Xu Q, et al. Quercetin protects cardiomyocytes against doxorubicin-induced toxicity by suppressing oxidative stress and improving mitochondrial function via 14-3-3γ. Toxicol Mech Methods. (2019) 29:344–54. doi: 10.1080/15376516.2018.1564948
107. Dong Q, Chen L, Lu Q, Sharma S, Li L, Morimoto S, et al. Quercetin attenuates doxorubicin cardiotoxicity by modulating Bmi-1 expression. Br J Pharmacol. (2014) 171:4440–54. doi: 10.1111/bph.12795
108. Chen JY, Hu RY, and Chou HC. Quercetin-induced cardioprotection against doxorubicin cytotoxicity. J BioMed Sci. (2013) 20:95. doi: 10.1186/1423-0127-20-95
109. Lokman MS, Althagafi HA, Alharthi F, Habotta OA, Hassan AA, Elhefny MA, et al. Protective effect of quercetin against 5-fluorouracil-induced cardiac impairments through activating Nrf2 and inhibiting NF-κB and caspase-3 activities. Environ Sci Pollut Res Int. (2023) 30:17657–69. doi: 10.1007/s11356-022-23314-z
110. Dorostkar H, Haghiralsadat BF, Hemati M, Safari F, Hassanpour A, Naghib SM, et al. Reduction of doxorubicin-induced cardiotoxicity by co-administration of smart liposomal doxorubicin and free quercetin: in vitro and in vivo studies. Pharmaceutics. (2023) 15:1920. doi: 10.3390/pharmaceutics15071920
111. Zakaria N, Khalil SR, Awad A, and Khairy GM. Quercetin reverses altered energy metabolism in the heart of rats receiving adriamycin chemotherapy. Cardiovasc Toxicol. (2018) 18:109–19. doi: 10.1007/s12012-017-9420-4
112. Sharma A, Parikh M, Shah H, and Gandhi T. Modulation of Nrf2 by quercetin in doxorubicin-treated rats. Heliyon. (2020) 6:e03803. doi: 10.1016/j.heliyon.2020.e03803
113. Wang SH, Tsai KL, Chou WC, Cheng HC, Huang YT, Ou HC, et al. Quercetin mitigates cisplatin-induced oxidative damage and apoptosis in cardiomyocytes through nrf2/HO-1 signaling pathway. Am J Chin Med. (2022) 50:1281–98. doi: 10.1142/s0192415x22500537
114. Taillibert S, Le Rhun E, and Chamberlain MC. Chemotherapy-related neurotoxicity. Curr Neurol Neurosci Rep. (2016) 16:81. doi: 10.1007/s11910-016-0686-x
115. Staff NP, Grisold A, Grisold W, and Windebank AJ. Chemotherapy-induced peripheral neuropathy: A current review. Ann Neurol. (2017) 81:772–81. doi: 10.1002/ana.24951
116. Joshi G, Aluise CD, Cole MP, Sultana R, Pierce WM, Vore M, et al. Alterations in brain antioxidant enzymes and redox proteomic identification of oxidized brain proteins induced by the anti-cancer drug adriamycin: implications for oxidative stress-mediated chemobrain. Neuroscience. (2010) 166:796–807. doi: 10.1016/j.neuroscience.2010.01.021
117. Merzoug S, Toumi ML, and Tahraoui A. Quercetin mitigates Adriamycin-induced anxiety- and depression-like behaviors, immune dysfunction, and brain oxidative stress in rats. Naunyn Schmiedebergs Arch Pharmacol. (2014) 387:921–33. doi: 10.1007/s00210-014-1008-y
118. Unay S and Bilgin MD. Investigation of effects of quercetin and low-level laser therapy in cisplatin-induced in vitro peripheral neuropathy model. Lasers Med Sci. (2023) 38:49. doi: 10.1007/s10103-023-03718-0
119. Gündoğdu R, Erkan M, Aydın M, Sönmez MF, Vural A, Kökoğlu K, et al. Assessment of the effectiveness of quercetin on cisplatin-induced ototoxicity in rats. J Int Adv Otol. (2019) 15:229–36. doi: 10.5152/iao.2019.5902
120. Lee SK, Oh KH, Chung AY, Park HC, Lee SH, Kwon SY, et al. Protective role of quercetin against cisplatin-induced hair cell damage in zebrafish embryos. Hum Exp Toxicol. (2015) 34:1043–52. doi: 10.1177/0960327114567766
121. Gao W, Zan Y, Wang ZJ, Hu XY, and Huang F. Quercetin ameliorates paclitaxel-induced neuropathic pain by stabilizing mast cells, and subsequently blocking PKCϵ-dependent activation of TRPV1. Acta Pharmacol Sin. (2016) 37:1166–77. doi: 10.1038/aps.2016.58
122. Rosa AC and Fantozzi R. The role of histamine in neurogenic inflammation. Br J Pharmacol. (2013) 170:38–45. doi: 10.1111/bph.12266
123. Owumi SE, Nwozo SO, Arunsi UO, Oyelere AK, and Odunola OA. Co-administration of Luteolin mitigated toxicity in rats’ lungs associated with doxorubicin treatment. Toxicol Appl Pharmacol. (2021) 411:115380. doi: 10.1016/j.taap.2020.115380
124. Nugroho AE, Hermawan A, Nastiti K, Suven S, Elisa P, and Hadibarata T. Immunomodulatory effects of hexane insoluble fraction of Ficus septica Burm. F. @ in doxorubicin-treated rats. Asian Pac J Cancer Prev. (2012) 13:5785–90. doi: 10.7314/apjcp.2012.13.11.5785
125. Farag MR, Moselhy AAA, El-Mleeh A, Aljuaydi SH, Ismail TA, Di Cerbo A, et al. Quercetin alleviates the immunotoxic impact mediated by oxidative stress and inflammation induced by doxorubicin exposure in rats. Antioxidants (Basel). (2021) 10:1906. doi: 10.3390/antiox10121906
126. Chuang CH, Lin YC, Yang J, Chan ST, and Yeh SL. Quercetin supplementation attenuates cisplatin induced myelosuppression in mice through regulation of hematopoietic growth factors and hematopoietic inhibitory factors. J Nutr Biochem. (2022) 110:109149. doi: 10.1016/j.jnutbio.2022.109149
127. Mohan UP, BT P, Iqbal STA, and Arunachalam S. Mechanisms of doxorubicin-mediated reproductive toxicity - A review. Reprod Toxicol. (2021) 102:80–9. doi: 10.1016/j.reprotox.2021.04.003
128. Samare-Najaf M, Zal F, Safari S, Koohpeyma F, and Jamali N. Stereological and histopathological evaluation of doxorubicin-induced toxicity in female rats’ ovary and uterus and palliative effects of quercetin and vitamin E. Hum Exp Toxicol. (2020) 39:1710–24. doi: 10.1177/0960327120937329
129. Ahmed ZA, Abtar AN, Othman HH, and Aziz TA. Effects of quercetin, sitagliptin alone or in combination in testicular toxicity induced by doxorubicin in rats. Drug Des Devel Ther. (2019) 13:3321–9. doi: 10.2147/dddt.S222127
130. Bostancıeri N, Taşlidere A, Elbe H, and Taşlıdere E. Protective effects of quercetin against testis damage caused by cisplatin. Biotech Histochem. (2022) 97:180–4. doi: 10.1080/10520295.2021.1924405
131. Arthur ST, Van Doren BA, Roy D, Noone JM, Zacherle E, and Blanchette CM. Cachexia among US cancer patients. J Med Econ. (2016) 19:874–80. doi: 10.1080/13696998.2016.1181640
132. VanderVeen BN, Cardaci TD, Cunningham P, McDonald SJ, Bullard BM, Fan D, et al. Quercetin improved muscle mass and mitochondrial content in a murine model of cancer and chemotherapy-induced cachexia. Nutrients. (2022) 15:102. doi: 10.3390/nu15010102
133. Garcia JM, Scherer T, Chen JA, Guillory B, Nassif A, Papusha V, et al. Inhibition of cisplatin-induced lipid catabolism and weight loss by ghrelin in male mice. Endocrinology. (2013) 154:3118–29. doi: 10.1210/en.2013-1179
134. Lin YC, Chen LW, Chen YC, Chan ST, Liao JW, and Yeh SL. Quercetin attenuates cisplatin-induced fat loss. Eur J Nutr. (2021) 60:1781–93. doi: 10.1007/s00394-020-02371-5
135. Cool JC, Dyer JL, Xian CJ, Butler RN, Geier MS, and Howarth GS. Pre-treatment with insulin-like growth factor-I partially ameliorates 5-fluorouracil-induced intestinal mucositis in rats. Growth Horm IGF Res. (2005) 15:72–82. doi: 10.1016/j.ghir.2004.12.002
136. Symonds RP. Treatment-induced mucositis: an old problem with new remedies. Br J Cancer. (1998) 77:1689–95. doi: 10.1038/bjc.1998.279
137. Lotfi M, Kazemi S, Shirafkan F, Hosseinzadeh R, Ebrahimpour A, Barary M, et al. The protective effects of quercetin nano-emulsion on intestinal mucositis induced by 5-fluorouracil in mice. Biochem Biophys Res Commun. (2021) 585:75–81. doi: 10.1016/j.bbrc.2021.11.005
138. Lotfi M, Kazemi S, Ebrahimpour A, Shirafkan F, Pirzadeh M, Hosseini M, et al. Protective effect of quercetin nanoemulsion on 5-fluorouracil-induced oral mucositis in mice. J Oncol. (2021) 2021:5598230. doi: 10.1155/2021/5598230
139. Miller KK, Gorcey L, and McLellan BN. Chemotherapy-induced hand-foot syndrome and nail changes: a review of clinical presentation, etiology, pathogenesis, and management. J Am Acad Dermatol. (2014) 71:787–94. doi: 10.1016/j.jaad.2014.03.019
140. Mashaly ME, Kotb HM, Abdin AA, and Hedya SE. Thymidine phosphorylase as a molecular target for quercetin-incorporated collagen matrix in the prevention of hand-foot syndrome induced by capecitabine in rats. Can J Physiol Pharmacol. (2023) 101:340–8. doi: 10.1139/cjpp-2022-0439
141. Zhu Q, Yang L, Yang H, Han Y, Chen Y, and He Y. Quercetin alleviates the progression of breast cancer-related depression via inhibiting the pyroptosis and promoting the immune response. Mediators Inflamm. (2022) 2022:8011988. doi: 10.1155/2022/8011988
142. Manni A, Sun YW, Schell TD, Lutsiv T, Thompson H, Chen KM, et al. Complementarity between microbiome and immunity may account for the potentiating effect of quercetin on the antitumor action of cyclophosphamide in a triple-negative breast cancer model. Pharmaceuticals (Basel). (2023) 16:1422. doi: 10.3390/ph16101422
143. Liao Y, Xie X, Zhang C, Zhong H, Shan L, Yu P, et al. Quercetin exerts anti-tumor immune mechanism by regulating IL-6/JAK2/STAT3 signaling pathway to deplete Treg cells. Toxicon. (2024) 243:107747. doi: 10.1016/j.toxicon.2024.107747
144. Li C, Xu Y, Zhang J, Zhang Y, He W, Ju J, et al. The effect of resveratrol, curcumin and quercetin combination on immuno-suppression of tumor microenvironment for breast tumor-bearing mice. Sci Rep. (2023) 13:13278. doi: 10.1038/s41598-023-39279-z
145. Wang W, Lin F, Shi S, Yu Y, Lin M, Lian W, et al. Investigating the role of quercetin, an active ingredient in bazhen decoction, in targeting CXCL8 to inhibit macrophage M2 polarization and reshape the immunological microenvironment of colorectal cancer. Chem Biol Drug Des. (2025) 105:e70047. doi: 10.1111/cbdd.70047
146. Li X, He X, Lin B, Li L, Deng Q, Wang C, et al. Quercetin limits tumor immune escape through PDK1/CD47 axis in melanoma. Am J Chin Med. (2024) 52:541–63. doi: 10.1142/s0192415x2450023x
147. Law AMK, Valdes-Mora F, and Gallego-Ortega D. Myeloid-derived suppressor cells as a therapeutic target for cancer. Cells. (2020) 9:561. doi: 10.3390/cells9030561
148. Wu R, Xiong J, Zhou T, Zhang Z, Huang Z, Tian S, et al. Quercetin/anti-PD-1 antibody combination therapy regulates the gut microbiota, impacts macrophage immunity and reshapes the hepatocellular carcinoma tumor microenvironment. Front Biosci (Landmark Ed). (2023) 28:327. doi: 10.31083/j.fbl2812327
149. Wu R, Zhou T, Xiong J, Zhang Z, Tian S, Wang Y, et al. Quercetin, the ingredient of xihuang pills, inhibits hepatocellular carcinoma by regulating autophagy and macrophage polarization. Front Biosci (Landmark Ed). (2022) 27:323. doi: 10.31083/j.fbl2712323
150. Jing L, Lin J, Yang Y, Tao L, Li Y, Liu Z, et al. Quercetin inhibiting the PD-1/PD-L1 interaction for immune-enhancing cancer chemopreventive agent. Phytother Res. (2021) 35:6441–51. doi: 10.1002/ptr.7297
151. Qiu D, Yan X, Xiao X, Zhang G, Wang Y, Cao J, et al. To explore immune synergistic function of Quercetin in inhibiting breast cancer cells. Cancer Cell Int. (2021) 21:632. doi: 10.1186/s12935-021-02345-5
152. Jing D, Wu W, Chen X, Xiao H, Zhang Z, Chen F, et al. Quercetin encapsulated in folic acid-modified liposomes is therapeutic against osteosarcoma by non-covalent binding to the JH2 domain of JAK2 Via the JAK2-STAT3-PDL1. Pharmacol Res. (2022) 182:106287. doi: 10.1016/j.phrs.2022.106287
153. Li L, Zhang M, Liu T, Li J, Sun S, Chen J, et al. Quercetin-ferrum nanoparticles enhance photothermal therapy by modulating the tumor immunosuppressive microenvironment. Acta Biomater. (2022) 154:454–66. doi: 10.1016/j.actbio.2022.10.008
154. Hong K, Cao J, Jiang W, Deng W, Huang G, Huang T, et al. A nanodrug provokes antitumor immune responses via synchronous multicellular regulation for enhanced cancer immunotherapy. J Colloid Interface Sci. (2024) 678:750–62. doi: 10.1016/j.jcis.2024.09.016
155. Zhang J, Shen L, Li X, Song W, Liu Y, and Huang L. Nanoformulated codelivery of quercetin and alantolactone promotes an antitumor response through synergistic immunogenic cell death for microsatellite-stable colorectal cancer. ACS Nano. (2019) 13:12511–24. doi: 10.1021/acsnano.9b02875
156. Dehghani F, Sezavar Seyedi Jandaghi SH, Janani L, Sarebanhassanabadi M, Emamat H, and Vafa M. Effects of quercetin supplementation on inflammatory factors and quality of life in post-myocardial infarction patients: A double blind, placebo-controlled, randomized clinical trial. Phytother Res. (2021) 35:2085–98. doi: 10.1002/ptr.6955
157. Heinz SA, Henson DA, Austin MD, Jin F, and Nieman DC. Quercetin supplementation and upper respiratory tract infection: A randomized community clinical trial. Pharmacol Res. (2010) 62:237–42. doi: 10.1016/j.phrs.2010.05.001
158. Javadi F, Ahmadzadeh A, Eghtesadi S, Aryaeian N, Zabihiyeganeh M, Rahimi Foroushani A, et al. The effect of quercetin on inflammatory factors and clinical symptoms in women with rheumatoid arthritis: A double-blind, randomized controlled trial. J Am Coll Nutr. (2017) 36:9–15. doi: 10.1080/07315724.2016.1140093
159. Li N, Cui C, Xu J, Mi M, Wang J, and Qin Y. Quercetin intervention reduced hepatic fat deposition in patients with nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled crossover clinical trial. Am J Clin Nutr. (2024) 120:507–17. doi: 10.1016/j.ajcnut.2024.07.013
160. Gallelli G, Cione E, Serra R, Leo A, Citraro R, Matricardi P, et al. Nano-hydrogel embedded with quercetin and oleic acid as a new formulation in the treatment of diabetic foot ulcer: A pilot study. Int Wound J. (2020) 17:485–90. doi: 10.1111/iwj.13299
161. Vaez S, Parivr K, Amidi F, Rudbari NH, Moini A, and Amini N. Quercetin and polycystic ovary syndrome; inflammation, hormonal parameters and pregnancy outcome: A randomized clinical trial. Am J Reprod Immunol. (2023) 89:e13644. doi: 10.1111/aji.13644
162. Boots AW, Drent M, de Boer VC, Bast A, and Haenen GR. Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin Nutr. (2011) 30:506–12. doi: 10.1016/j.clnu.2011.01.010
163. Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, Wexner SD, et al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. (2006) 4:1035–8. doi: 10.1016/j.cgh.2006.03.020
164. Morrow DM, Fitzsimmons PE, Chopra M, and McGlynn H. Dietary supplementation with the anti-tumour promoter quercetin: its effects on matrix metalloproteinase gene regulation. Mutat Res. (2001) 480-481:269–76. doi: 10.1016/s0027-5107(01)00184-1
Keywords: quercetin, cancer, 5-fluorouracil, doxorubicin, nephrotoxicity, neurotoxicity, immune cells
Citation: Deng H, Wei F, Han W, Li Y, Xu X, Zhang L and Zhang Y (2025) Synergistic chemotherapy and immunomodulatory effects of Quercetin in cancer: a review. Front. Immunol. 16:1547992. doi: 10.3389/fimmu.2025.1547992
Received: 19 December 2024; Accepted: 02 May 2025;
Published: 26 May 2025.
Edited by:
Omowumi Adewale, Osun State University, NigeriaReviewed by:
Gopal Patel, Lakshmi Narain College of Pharmacy, IndiaMiguel Pereira-Silva, University of Texas MD Anderson Cancer Center, United States
Copyright © 2025 Deng, Wei, Han, Li, Xu, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Youcheng Zhang, emhhbmd5Y2htZEAxMjYuY29t
 Hongyang Deng
Hongyang Deng Fengxian Wei
Fengxian Wei Wei Han1
Wei Han1 Xiaodong Xu
Xiaodong Xu Lingyi Zhang
Lingyi Zhang Youcheng Zhang
Youcheng Zhang