Abstract
Background:
extensive-stage small cell lung cancer (ES-SCLC) were the majority of SCLC patients. Recently the combination of chemotherapy with immune checkpoint inhibitors (ICIs) have emerged as the new first-line treatment standard for ES-SCLC. However, the specific patient populations that are most likely to benefit from this treatment remains to be clearly identified making the establishment of baseline biomarkers critical.
Methods:
We recruited ES-SCLC patients who were treated at the First Affiliated Hospital of Zhengzhou University and conducted a propensity score-matched analysis (PSM). And used the Kaplan-Meier (K-M) method and Cox proportional hazards regression to compare the survival outcomes. In addition, univariate and multivariate COX regression analyses were conducted to identify predictors.
Results:
After-PSM, chemotherapy group had a longer median overall survival (mOS) of 15.23 months (95%CI: 14.00-17.87) and hazard ratio (HR) of 0.576 (95% confidence interval (CI): 0.404-0.821), P=0.002), and the median progression free survival (mPFS) in the chemotherapy group was shorter: 6.05months (95%CI: 4.33-7.87), HR=0.707 (95%CI: 0.526 -0.950, P=0.021) compared to before PSM. Multivariate analysis confirmed that Eastern Cooperative Oncology Group performance status (ECOG PS) =1 (HR: 2.36, 95% CI: 1.38-4.03, P=0.002) and brain metastases (HR: 2.08, 95% CI: 1.05-4.14, P=0.038) were independent prognostic factors for PFS, and only systemic inflammation response index (SIRI)> 2.63 (HR: 0.06, 95% CI: 0.01-0.29, P<0.001) was an independent prognostic factor for OS.
Conclusion:
our findings indicate that incorporating ICIs into first-line chemotherapy significantly improves PFS and OS in ES-SCLC patients, while maintaining safety. Moreover, poor ECOG PS, brain metastases, and high SIRI at baseline may serve as valuable prognostic indicators for disease progression and survival in ES-SCLC patients undergoing first-line chemotherapy plus ICIs. It is worth noting that these findings should be interpreted as hypothesis-generating, not definitive clinical conclusions.
1 Introduction
Lung cancer is the most prevalent and deadliest form of cancer both globally and in China (1). In approximately 15% of lung cancer cases, the diagnosis is small cell lung cancer (SCLC). This subtype of lung cancer is defined by a high proliferation rate, early development of metastasis, and an unfavorable prognosis (2). Consequently, the 5-year survival rate for patients diagnosed with SCLC is reported to be only 5% (3). Approximately 30% of SCLC cases are classified as limited-stage SCLC (LS-SCLC), while the majority are extensive-stage SCLC (ES-SCLC) (4). Notably, many SCLC patients are diagnosed with ES-SCLC at the initial presentation, in contrast to those with LS-SCLC, studies have demonstrated that ES-SCLC has a shorter median overall survival (mOS) and a less favorable prognosis, with a 2-year survival rate of merely 2% (5, 6).
Major breakthrough in the treatment of SCLC have been limited over an extended period. For ES-SCLC, the standard therapeutic approach remains systemic chemotherapy. This regimen typically comprises platinum-based compounds in conjunction with etoposide (7). ES-SCLC demonstrates a high degree of initial sensitivity to chemotherapy, resulting in notable response rates. However, this condition is also marked by a high recurrence rate and an unfavorable prognosis. The mOS with standard therapy is only 9–10 months, underscoring the need for novel therapeutic approaches (8). A recent Phase 3 clinical trial demonstrated that first-line durvalumab (a programmed death-ligand 1 (PDL-1) inhibitor) in combination with platinum-etoposid significantly improved OS was observed in patients with ES-SCLC treated with the combination regimen compared to platinum-etoposide alone, and the mOS was 12.9 months versus 10.5 months, respectively. These findings suggest that the combination of durvalumab and etoposide may represent a new standard of care for first-line treatment of ES-SCLC (9). Similarly, another Phase 3 clinical trial showed statistically significant clinical benefits and a manageable safety profile when combining a tirellizumab (programmed death 1 (PD-1) inhibitor) with chemotherapy. Therefore, the addition of immune checkpoint inhibitors (ICIs) (including PD-1 and PDL-1) to platinum-based chemotherapy has altered the treatment paradigm for ES-SCLC. Over five years of follow-up, chemotherapy combined ICIs with have shown sustained benefits, with a significant increase in five-year survival rates by 12% (10).
Currently, the Chinese Consensus on Immunotherapy for Small Cell Lung Cancer (2024 edition) has endorsed the combination of chemotherapy with ICIs as the new first-line treatment standard for ES-SCLC (11). However, the majority of patients experience disease progression within 6 months of initiating first-line therapy. To date, there is no definitive and reliable method to identify patients who are most likely to benefit from this treatment approach, making it crucial to establish baseline biomarkers. Recently, several novel immunoinflammatory and nutritional indicators have been utilized in combination therapy to predict prognosis in non-small cell lung cancer (NSCLC), such as the prognostic nutritional index (PNI), platelet-to-lymphocyte ratio (PLR), lung immune prognostic index (LIPI), neutrophil-to-lymphocyte ratio (NLR) and lymphocyte-to-monocyte ratio (LMR) (12–14). However, limited studies have explored the use of these indicators as biomarkers for predicting the prognosis of ES-SCLC patients treated with ICIs combined with chemotherapy.
In this study, we evaluated the efficacy of ICIs in combination with chemotherapy compared to chemotherapy alone in the clinical management of ES-SCLC, focusing on endpoints such as OS, progression free survival (PFS), disease control rate (DCR), objective response rate (ORR), and treatment-related adverse events (AEs), to provide a reference for clinicians. To ensure the comparability of baseline characteristics, propensity score matching (PSM) was employed. Additionally, to identify independent prognostic factors among patients treated with chemotherapy plus ICIs for ES-SCLC, univariate and multivariate COX regression analyses were performed. These factors may encompass demographic characteristics, clinical features at initial diagnosis, tumor markers, serum immunity, inflammation, and nutritional indicators.
2 Methods
2.1 ES-SCLC patients selection
This retrospective study was conducted in accordance with the revised Declaration of Helsinki and received approval from the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (Ethics Approval Number: 2024-KY-0189-002, Ethics Approval Date: 2024-03-11). Patients diagnosed with SCLC who received treatment at the First Affiliated Hospital of Zhengzhou University between January 2020 and April 2022 were included in this study. Diagnosis of SCLC was confirmed through definitive cytological and histological evidence, verified by the clinician responsible for data collection. Standard first-line chemotherapy comprised etoposide plus platinum, with or without ICIs. ES-SCLC was defined according to the criteria established by the Veterans Administration Lung Study Group (VALG) (15). Patients with ES-SCLC who received only 1–2 cycles of treatment, had a history of other malignancies, underwent surgical intervention, or patients with an Eastern Cooperative Oncology Group performance status (ECOG PS) greater than 1 were excluded from the study. The follow-up period extended until September 1, 2024. Due to the retrospective nature of this study, informed consent was waived. All patient personal information was maintained with strict confidentiality.
2.2 Data collection and evaluation
To evaluate the efficacy of first-line chemotherapy with or without ICIs in patients with ES-SCLC, we utilized the Kaplan-Meier (K-M) method to PFS and OS. Furthermore, imaging assessments were performed for each treatment regimen, and responses were evaluated according to the Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1. This allowed us to assess the therapeutic outcomes during first-line treatment and subsequently calculate the ORR and DCR. Specifically, ORR was calculated as (complete response (CR) + partial response (PR))/(CR + PR + stable disease (SD) + progressive disease (PD)), while DCR was determined as (CR + PR + SD)/(CR + PR + SD + PD). Adverse events (immune-related (ir) AEs and other adverse drug reactions) graded using the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 5.0, and irAEs mainly included suggestive of immune-mediated events (e.g., skin rash, pneumonitis, thyroid dysfunction, nephritis, hepatitis, and hypophysitis).
In this study, we collected comprehensive baseline information prior to first-line treatment, including demographic data (age, sex), lifestyle factors (smoking history), performance status (ECOG PS), and radiotherapy history. Additionally, we recorded the baseline TNM stage and laboratory test results, such as leukocytes, lymphocytes, monocytes, neutrophils, lactate dehydrogenase (LDH), platelets, serum albumin, and various tumor markers. Based on these data, we calculated several composite indicators:
The NLR is calculated as the neutrophil count divided by the lymphocyte count. The derived neutrophil-to-lymphocyte ratio (dNLR) is determined by dividing the neutrophil count by the difference between the white blood cell count and the neutrophil count. The LMR is obtained by dividing the lymphocyte count by the monocyte count. The PLR is computed as the platelet count divided by the lymphocyte count. The systemic immune-inflammation index (SII) is defined as the product of the platelet count and the neutrophil count, divided by the lymphocyte count. The systemic inflammation response index (SIRI) is calculated as the product of the neutrophil count and the monocyte count, divided by the lymphocyte count. The PNI is given by the sum of serum albumin concentration (in g/L) and five times the total number of peripheral blood lymphocytes. The LIPI is assessed using baseline LDH levels and dNLR to categorize patients into three prognostic groups: poor (dNLR > 3 and LDH > ULN), intermediate (dNLR > 3 or LDH > ULN) and good (dNLR ≤ 3 and LDH ≤ upper limit of normal [ULN]). In addition, ULN was used to convert LDH and tumor markers into categorical variables, and the best cutoff values for SII, LMR, PLR, SIRI, PNI, and PAR were determined by the “surv_cutpoint” function.
2.3 Statistical analysis
Descriptive analysis (frequency and percentage) was conducted to summarize the baseline characteristics of ES-SCLC patients across different treatment groups. Statistical comparisons were performed using the χ2 test and the Kruskal-Wallis test as appropriate. To analyze survival data, the K-M method was employed, and differences in survival curves were assessed using the log-rank test.
Subgroup analyses were based on several variables. To mitigate the risk of false positive findings due to multiple comparisons across these subgroups, we applied false discovery rate (FDR) correction using the Benjamini-Hochberg procedure to all subgroup interaction P-values and within-subgroup treatment effect P-values.
PSM was performed using a 2:1 optimal matching method with a tolerance level of 0.05. The covariates included in the matching process were age, sex, smoking status, ECOG PS, Tumor primary site, radiotherapy, tumor size, node metastasis, extrathoracic metastasis, adrenal gland metastasis, brain metastasis, bone metastasis, liver metastasis, pleural metastasis, other sites, total bilirubin and uric acid. A logistic regression model was used to estimate the propensity scores and the standardized differences (SMD) and love plot are used to assess the balance between the two groups.
To identify predictors of OS and PFS in ES-SCLC patients treated with chemotherapy combined with ICIs, univariate and multivariate COX regression analyses were conducted to evaluate the data. Variables that exhibited a univariate P-value < 0.05 were included in the multivariate COX regression analysis using bidirectional stepwise selection. All statistical analyses were performed using R software (version 4.4.2) and SPSS version 25.0, with a significance level set at P < 0.05.
3 Results
3.1 Basic characteristics of ES-SCLC patients
Initially, a total of 308 ES-SCLC patients were identified as shown in Figure 1. After applying the inclusion and exclusion criteria, 253 ES-SCLC patients were included for evaluation. Among these, 179 patients received chemotherapy alone while 74 patients received chemotherapy combined with ICIs. Table 1 summarizes the baseline characteristics of the 253 patients: 119 (47.04%) were aged ≥65 years, 77.47% were male, and 60.87% had a history of smoking. The majority of tumor size (69.87%) were larger than 5 cm, and most metastatic sites were extrathoracic (183 patients). The top three metastatic sites were bone (71 patients), liver (68 patients), and brain (34 patients). Prior to PSM, some baseline characteristics differed between the chemotherapy and chemotherapy combined with ICIs. For instance, the ECOG SMD was 0.1266, which was greater than 0.1, indicating an imbalance between the two groups. After PSM, the ECOG SMD decreased to 0.0878. Moreover, the SMDs of other covariates also showed a certain degree of decrease (Supplementary Table S1, Supplementary Figures S1, S2).
Figure 1

Diagram of the patient’s selection process. SCLC, Small cell lung cancer; ES, extensive-stage; ICIs, immune checkpoint inhibitors.
Table 1
| Variables | Subcategories | n (%) All patients (n = 253) | Before matching n (%) | After matching n (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Chemotherapy (n = 179) | Chemotherapy +ICIs (n = 74) | P | Chemotherapy (n =148) | Chemotherapy +ICIs (n = 74) | P | |||
| Age | <65 | 134 (52.96) | 92 (51.40) | 42 (56.76) | 0.437 | 76 (51.35) | 42 (56.76) | 0.447 |
| ≥65 | 119 (47.04) | 87 (48.60) | 32 (42.24) | 72 (48.65) | 32 (42.24) | |||
| Sex | Male | 196 (77.47) | 142 (79.33) | 54 (72.97) | 0.271 | 115 (77.70) | 54 (72.97) | 0.436 |
| Female | 57 (22.53) | 37 (20.67) | 20 (27.03) | 33 (22.30) | 20 (27.03) | |||
| Smoke | NO | 99 (39.13) | 69 (38.55) | 30 (40.54) | 0.768 | 54 (36.49) | 30 (40.54) | 0.557 |
| YES | 154 (60.87) | 110 (61.45) | 44 (59.46) | 94 (63.51) | 44 (59.46) | |||
| ECOG PS |
0 | 97 (38.34) | 62 (34.64) | 35 (47.30) | 0.060 | 57 (38.51) | 35 (47.30) | 0.210 |
| 1 | 156 (61.66) | 117 (65.36) | 39 (52.70) | 91 (61.49) | 39 (52.70) | |||
| Primary site |
Left | 113 (44.67) | 79 (44.13) | 34 (45.95) | 0.792 | 63 (42.57) | 34 (45.95) | 0.632 |
| Right | 135 (53.36) | 97 (54.19) | 40 (54.05) | 85 (57.43) | 40 (54.05) | |||
| Radiotherapy | NO | 170 (67.19) | 120 (67.04) | 50 (67.37) | 0.935 | 102 (68.92) | 50 (67.37) | 0.838 |
| YES | 83 (34.78) | 59 (32.96) | 24 (32.43) | 46 (31.08) | 24 (32.43) | |||
| Tumor size |
< 5cm | 88 (65.22) | 58 (32.40) | 30 (40.54) | 0.216 | 55 (37.16) | 30 (40.54) | 0.625 |
| ≥5cm | 165 (69.87) | 121 (67.60) | 44 (59.46) | 93 (62.84) | 44 (59.46) | |||
| Node metastases |
NO/ LOCAL |
149 (58.89) | 107 (59.78) | 42 (56.76) | 0.657 | 90 (60.81) | 42 (56.76) | 0.562 |
| Distant | 104 (41.11) | 72 (40.22) | 32 (43.24) | 58 (39.19) | 32 (43.24) | |||
| Extrathoracic metastases |
NO | 70 (27.67) | 53 (29.61) | 17 (22.97) | 0.283 | 39 (26.35) | 17 (22.97) | 0.585 |
| YES | 183 (72.33) | 126 (70.39) | 57 (77.03) | 109 (73.65) | 57 (77.03) | |||
| Adrenalgland metastases |
NO | 223 (88.14) | 157 (87.71) | 66 (89.19) | 0.741 | 129 (87.16) | 66 (89.19) | 0.663 |
| YES | 20 (11.86) | 22 (12.29) | 8 (10.81) | 19 (12.84) | 8 (10.81) | |||
| Brain metastases |
NO | 219 (86.56) | 155 (86.59) | 64 (86.49) | 0.982 | 129 (87.16) | 64 (86.49) | 0.888 |
| YES | 34 (13.44) | 24 (13.41) | 10 (13.51) | 19 (12.84) | 10 (13.51) | |||
| Bone metastases |
NO | 182 (71.94) | 127 (70.95) | 55 (74.32) | 0.587 | 106 (71.62) | 55 (74.32) | 0.671 |
| YES | 71 (28.06) | 52 (29.05) | 19 (25.68) | 42 (28.38) | 19 (25.68) | |||
| Liver metastases |
NO | 185 (73.12) | 132 (73.74) | 53 (71.62) | 0.729 | 106 (71.62) | 53 (71.62) | 1.000 |
| YES | 68 (26.88) | 47 (26.26) | 21 (28.38) | 42 (28.38) | 21 (28.38) | |||
| Pleural metastases |
NO | 237 (93.68) | 169 (94.41) | 68 (91.89) | 0.453 | 139 (93.92) | 68 (91.89) | 0.571 |
| YES | 16 (6.32) | 10 (5.59) | 6 (8.11) | 9 (6.08) | 6 (8.11) | |||
| Other sites |
NO | 233 (91.30) | 168 (93.85) | 63 (85.14) | 0.025 | 137 (92.57) | 63 (85.14) | 0.081 |
| YES | 22 (8.70) | 11 (6.15) | 11 (14.86) | 11 (7.43) | 11 (14.86) | |||
| Total bilirubin |
Normal | 220 (87.96) | 153 (85.47) | 67 (90.54) | 0.276 | 131 (88.51) | 67 (90.54) | 0.647 |
| Unnormal | 33 (13.04) | 26 (14.53) | 7 (9.46) | 17 (11.49) | 7 (9.46) | |||
| Uric acid | Normal | 186 (73.52) | 129 (72.07) | 57 (77.03) | 0.416 | 108 (72.97) | 57 (77.03) | 0.515 |
| Unnormal | 67 (26.48) | 50 (27.93) | 17 (22.97) | 40 (27.03) | 17 (22.97) | |||
Baseline characteristics before and after propensity score matching according to first-line therapy (chemotherapy/chemotherapy+ICIs).
SCLC, Small cell lung cancer; ICIs, immune checkpoint inhibitors; ECOG PS, Eastern Cooperative Oncology Group performance status.
3.2 Survival outcome before and after PSM
Before PSM, we used K-M curves to analyze the survival outcomes comparing the chemotherapy group and the chemotherapy plus ICIs group, as illustrated in Figure 2A. The mOS in the chemotherapy plus ICIs group was 19.54 months (95% CI: 16.80-26.63), compared to 15.03 months (95% CI: 13.80-16.37) in the chemotherapy group, with a hazard ratio (HR) of 0.563 (95% CI: 0.412-0.768, P < 0.001). Similarly, the median mPFS in the chemotherapy plus ICIs group was 9.44 months (95% CI: 7.30-11.93), compared to 6.03 months (95% CI: 4.70-7.87) in the chemotherapy group, with an HR of 0.521 (95% CI: 0.388-0.698, P < 0.001) (Figure 2B).
Figure 2

Survival outcomes of chemotherapy/chemotherapy +ICIs. (A) Kaplan-Meier curves of OS stratified by chemotherapy/chemotherapy +ICIs; (B) Kaplan-Meier curves of PFS stratified by chemotherapy/chemotherapy +ICIs; (C) Forest plot of subgroup analysis of OS; (D) Forest plot of subgroup analysis of PFS; OS, overall survival; PFS, progression free survival; SCLC, Small cell lung cancer; ICIs, immune checkpoint inhibitors; HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status.
We then conducted a subgroup analysis based on baseline information, as shown in Figures 2C, D, Supplementary Table S2. Even though chemotherapy combined with ICIs was beneficial in both OS and PFS, some subgroups of people did not show statistically significant benefit, regardless of OS or PFS. For example, Metastases = NO (OS: FDR-adjusted P=0.939, PFS: FDR-adjusted P=0.583); Brain metastases (OS: FDR-adjusted P=0.332, PFS: FDR-adjusted P=0.516). Pleural metastases (OS: FDR-adjusted P=0.855, PFS: FDR-adjusted P=0.054). Finally, we took patients receiving chemotherapy combined with ICIs as the baseline, and based on the baseline information, 2:1 matching was performed for ES-SCLC patients receiving chemotherapy alone.
K-M curves demonstrated results consistent with those before PSM, the mOS in the chemotherapy group was 15.23 months (95% CI: 13.90-17.23), compared to 19.54 months (95% CI: 16.80-26.63) in the chemotherapy plus ICIs group, with a hazard ratio (HR) of 0.575 (95% CI: 0.418-0.791, P < 0.001, Figure 3A). Similarly, the median mPFS in the chemotherapy was 6.71 months (95% CI: 5.97-7.73), compared to 9.44 months (95% CI: 7.30-11.93) in the chemotherapy plus ICIs group, with an HR of 0.524 (95% CI: 0.388-0.708, P < 0.001, Figure 3B).
Figure 3

Survival outcomes of chemotherapy/chemotherapy +ICIs after PSM. (A) Kaplan-Meier curves of OS stratified by chemotherapy/chemotherapy +ICIs after PSM; (B) Kaplan-Meier curves of PFS stratified by chemotherapy/chemotherapy +ICIs after PSM; OS, overall survival; PFS, progression free survival; PSM, propensity score matching; ICIs, immune checkpoint inhibitors; HR, hazard ratio; CI, confidence interval.
3.3 Response and treatment-related AEs
Treatment response was evaluated in 253 patients, as showed in Figure 4A. The DCR and ORR for all ES-SCLC patients were 85.38% and 45.85%, respectively. Specifically, the DCR and ORR for the chemotherapy group were 82.68% and 40.78%, while those for the chemotherapy plus ICIs group were 91.89% and 58.11%, respectively. The difference in ORR between the two groups was statistically significant (P=0.012), whereas the difference in DCR was not (P=0.059). Additionally, there were statistically significant differences between the two groups in PR, SD, and PD rates (P=0.034). Subsequently, we compared the ORR and DCR between PD-1 and PDL -1 inhibitors, finding no statistically significant differences (ORR: P=0.480; DCR: P=0.394; overall: P=0.633).
Figure 4

Tumor response and treatment-related AEs in all ES-SCLC patients. (A) Tumor response in all ES-SCLC patients. (B) Treatment-related AEs in all ES-SCLC patients. (C) irAEs in all ES-SCLC patients (D) irAEs in chemotherapy group and chemotherapy plus ICIs group ES, extensive-stage; AEs, adverse events; ICIs, immune checkpoint inhibitors; PR, partial response; SD, stable disease; ORR, objective response rate; DCR, disease control rate; PD-1, programmed cell death protein 1; PDL -1, programmed cell death ligand 1. irAEs, immune-related adverse events.
Regarding treatment-related AEs, 92.89% of the 253 patients experienced AEs, with the majority being Grade 1 (61.26%) and a smallest proportion being Grade 4 (2.77%). No Grade 5 events, which are considered life-threatening, were reported. In contrast to the treatment response, there was no significant difference in AEs incidence between the chemotherapy group and the chemotherapy plus ICIs group (P=0.491), nor between PD-1 and PDL-1 (P=0.184, Figure 4B). Then we performed a reanalysis of the irAEs in all SCLC patients. The results are presented in Figures 4C, D. Among all SCLC patients who experienced AEs, 41.48% had irAEs. Gastritis and colitis were the most prevalent, accounting for 52.53%, and the irAEs in the chemotherapy plus ICIs group was 40.54%, which was higher than that in the chemotherapy alone group (36.31%).
3.4 Prognostic factors of OS and PFS in ES-SCLC patients treated with chemotherapy plus ICIs
Univariate and multivariate COX regression analyses were conducted for the chemotherapy plus ICIs treatment group, with clinical outcomes defined as death and disease progression. The aim was to identify baseline characteristics, serum inflammatory markers, and tumor markers associated with survival. The results are summarized in Table 2. The univariate analysis revealed that ECOG PS=1 (HR: 2.37, 95% CI: 1.38-4.06, P=0.002) and brain metastases (HR: 2.10, 95% CI: 1.05-4.18, P=0.036) were associated with PFS in patients receiving first-line chemotherapy plus ICIs. Multivariate analysis confirmed that ECOG PS=1 (HR: 2.36, 95% CI: 1.38-4.03, P=0.002) and brain metastases (HR: 2.08, 95% CI: 1.05-4.14, P=0.038) were independent prognostic factors for PFS.
Table 2
| Variables | Subcategories | PFS-Univariate analysis | PFS-Multivariae analysis | OS- Univariate analysis | OS- Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|---|---|
| P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | ||
| Age | <65 | ||||||||
| ≥65 | 0.276 | 0.76 (0.47 ~ 1.24) |
0.280 | 0.74 (0.43 ~ 1.28) |
|||||
| Sex | Male | ||||||||
| Female | 0.920 | 0.97 (0.57 ~ 1.67) |
0.956 | 0.98 (0.52 ~ 1.85) |
|||||
| Smoke | NO | ||||||||
| YES | 0.179 | 1.40 (0.86 ~ 2.28) |
0.106 | 1.60 (0.91 ~ 2.82) |
|||||
| ECOG PS |
0 | ||||||||
| 1 | 0.002 | 2.37 (1.38 ~ 4.06) |
0.002 | 2.36 (1.38 ~ 4.03) |
0.428 | 1.25 (0.72 ~ 2.17) |
|||
| Primary site |
Left | ||||||||
| Right | 0.639 | 1.12 (0.69 ~ 1.82) |
0.983 | 1.01 (0.59 ~ 1.72) |
|||||
| Radiotherapy | NO | ||||||||
| YES | 0.706 | 1.10 (0.66 ~ 1.83) |
0.302 | 0.73 (0.40 ~ 1.33) |
|||||
| Tumor size | < 5cm | ||||||||
| ≥5cm | 0.452 | 1.21 (0.74 ~ 1.98) |
0.438 | 0.81 (0.47 ~ 1.39) |
|||||
| Node metastases |
NO/LOCAL | ||||||||
| Distant | 0.113 | 0.67 (0.41 ~ 1.10) |
0.064 | 0.59 (0.34 ~ 1.03) |
|||||
| Extrathoracic metastases |
NO | ||||||||
| YES | 0.201 | 0.69 (0.40 ~ 1.21) |
0.108 | 0.62 (0.34 ~ 1.11) |
|||||
| Adrenalgland metastases |
NO | ||||||||
| YES | 0.880 | 1.06 (0.48 ~ 2.33) |
0.962 | 1.02 (0.41 ~ 2.58) |
|||||
| Brain metastases |
NO | ||||||||
| YES | 0.036 | 2.10 (1.05 ~ 4.18) |
0.037 | 2.08 (1.05 ~ 4.14) |
0.953 | 0.98 (0.44 ~ 2.18) |
|||
| Bone metastases |
NO | ||||||||
| YES | 0.408 | 0.79 (0.45 ~ 1.39) |
0.631 | 1.16 (0.63 ~ 2.15) |
|||||
| Liver metastases | NO | ||||||||
| YES | 0.771 | 0.92 (0.54 ~ 1.57) |
0.108 | 1.61 (0.90 ~ 2.88) |
|||||
| Pleural metastases | NO | ||||||||
| YES | 0.751 | 0.87 (0.38 ~ 2.03) |
0.862 | 1.09 (0.39 ~ 3.05) |
|||||
| Other sites | NO | ||||||||
| YES | 0.897 | 0.95 (0.47 ~ 1.93) |
0.754 | 1.14 (0.51 ~ 2.52) |
|||||
| ICIs | PD-1 | ||||||||
| PD-L1 | 0.464 | 1.19 (0.74 ~ 1.92) |
0.943 | 0.98 (0.57-1.68) |
|||||
| LDH | ≤245 | ||||||||
| >245 | 0.831 | 1.07 (0.56 ~ 2.07) |
0.707 | 1.17 (0.52 ~ 2.60) |
|||||
| CEA | ≤5 | ||||||||
| >5 | 0.725 | 1.10 (0.65 ~ 1.85) |
0.667 | 1.14 (0.63 ~ 2.04) |
|||||
| Cyfran21 | ≤3.3 | ||||||||
| >3.3 | 0.193 | 1.52 (0.81 ~ 2.86) |
0.915 | 0.96 (0.48 ~ 1.95) |
|||||
| CA125 | ≤35 | ||||||||
| >35 | 0.805 | 0.93 (0.54 ~ 1.61) |
0.469 | 1.26 (0.67 ~ 2.35) |
|||||
| CA19-9 | ≤37 | ||||||||
| >37 | 0.174 | 1.58 (0.82 ~ 3.05) |
0.627 | 0.82 (0.37 ~ 1.81) |
|||||
| CA72-4 | ≤7 | ||||||||
| >7 | 0.652 | 0.82 (0.34 ~ 1.95) |
0.029 | 2.71 (1.11 ~ 6.62) |
|||||
| NSE | ≤16.3 | ||||||||
| >16.3 | 0.647 | 1.19 (0.56 ~ 2.54) |
0.388 | 1.51 (0.59 ~ 3.88) |
|||||
| SII | ≤146.11 | ||||||||
| >146.11 | 0.648 | 0.88 (0.50 ~ 1.54) |
0.028 | 0.44 (0.22 ~ 0.92) |
|||||
| NLR | ≤4 | ||||||||
| >4 | 0.917 | 0.97 (0.57 ~ 1.67) |
0.047 | 0.50 (0.25 ~ 0.99) |
0.080 | 2.61 (0.89 ~7.66) |
|||
| dNLR | ≤3 | ||||||||
| >3 | 0.617 | 0.85 (0.44 ~ 1.62) |
0.176 | 0.58 (0.26 ~ 1.28) |
|||||
| LIPI | Good | ||||||||
| Intermediate | 0.837 | 0.93 (0.45 ~ 1.90) |
0.887 | 1.06 (0.45 ~ 2.50) |
|||||
| Poor | 0.303 | 1.77 (0.60 ~ 5.25) |
0.940 | 1.05 (0.30 ~ 3.65) |
|||||
| LMR | ≤2.01 | ||||||||
| >2.01 | 0.974 | 0.99 (0.56 ~ 1.74) |
0.008 | 2.90 (1.33 ~ 6.34) |
|||||
| PLR | ≤288.42 | ||||||||
| >288.42 | 0.965 | 1.02 (0.48 ~ 2.13) |
0.057 | 0.36 (0.13 ~ 1.03) |
|||||
| SIRI | ≤2.63 | ||||||||
| >2.63 | 0.965 | 0.99 (0.56 ~ 1.75) |
<0.001 | 0.22 (0.09 ~ 0.53) |
<0.001 | 0.06 (0.01 ~ 0.29) |
|||
| PAR | ≤6.00 | ||||||||
| >6.00 | 0.668 | 1.15 (0.61 ~ 2.15) |
0.055 | 0.46 (0.20 ~ 1.02) |
|||||
| PNI | ≤40.45 | ||||||||
| >40.45 | 0.120 | 0.53 (0.24 ~ 1.18) |
0.151 | 2.00 (0.78 ~ 5.17) |
Univariate and multivariate analyses of progression-free survival and overall survival in ES-SCLC patients who received first-line chemotherapy +ICIs.
OS, overall survival; PFS, progression free survival; ICIs, immune checkpoint inhibitors. HR, hazard ratio; CI, confidence interval; ES, extensive-stage; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; NLR, neutrophil-to-lymphocyte ratio; dNLR, derived Neutrophil to Lymphocyte Ratio; LIPI, lung immune prognostic index; LMR, lymphocyte to monocyte ratio; PLR, platelet to lymphocyte ratio; PAR, platelet to albumin ratio; PNI, prognostic nutrition index; SII, systemic immune-inflammation index; SIRI, systemic inflammation response index; CEA, carcinoembryonic antigen; NSE, Neuron-specific enolase; CA125, Cancer antigen 125; CA153, Carbohydrate antinegen 15-3; Cyfran21, cytokeratin 19 fragments.
In contrast, the univariate analysis for OS showed that elevated CA72-4 (>7 U/mL, HR: 2.71, 95% CI: 1.11-6.62, P=0.029), SII > 146.11 (HR: 0.44, 95% CI: 0.22-0.92, P=0.028), NLR > 4 (HR: 0.50, 95% CI: 0.25-0.99, P=0.047), LMR> 2.01 (HR: 2.90, 95% CI: 1.33-6.34, P=0.008), and SIRI > 2.63 (HR: 0.29, 95% CI: 0.09-0.31, P<0.001) were associated with OS. However, multivariate analysis indicated that only SIRI > 2.63 (HR: 0.06, 95% CI: 0.01-0.29, P<0.001) was an independent prognostic factor for OS.
In addition, survival analysis was conducted to compare the outcomes of ES-SCLC patients treated with PD-1 inhibitors versus those treated with PDL-1 inhibitors. As shown in Figure 5A, the HR for OS between the PD-1 and PDL-1 groups was 0.980 (95% CI: 0.573-1.678, P=0.942), but the wide CI crossing the null value (HR=1) precludes definitive conclusions regarding equivalence or difference between groups.
Figure 5
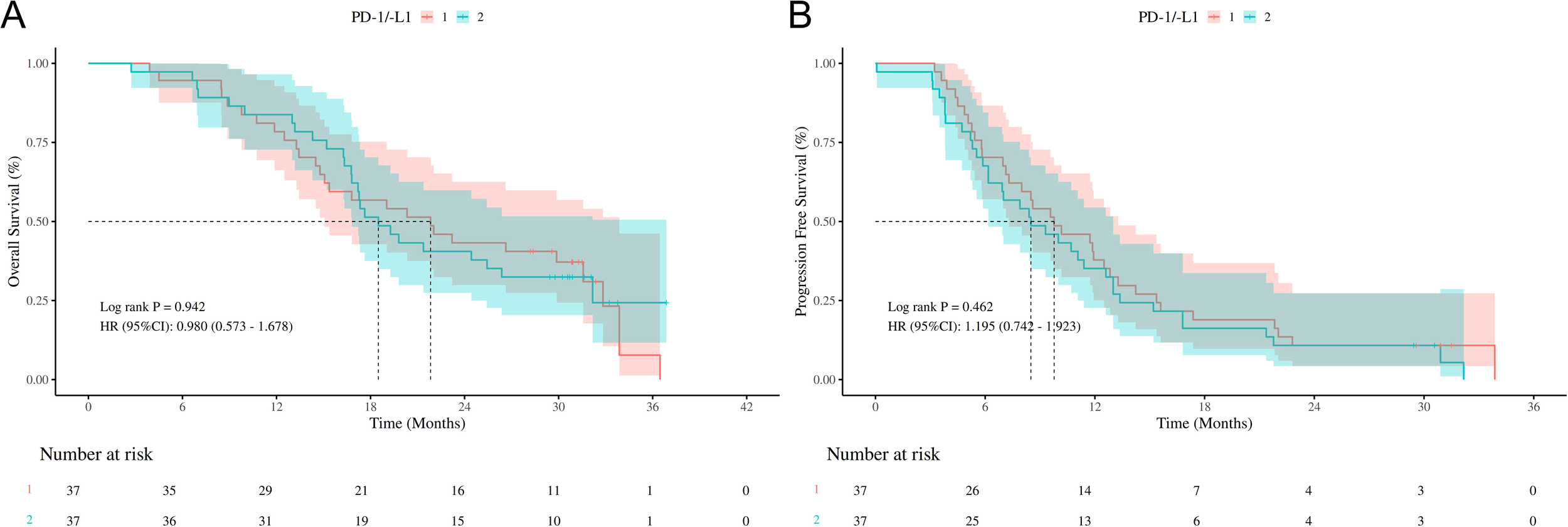
Survival outcomes of different ICIs (PD-1/PDL-1) (A) Kaplan-Meier curves of OS stratified by different ICIs (PD-1/PDL-1); (B) Kaplan-Meier curves of PFS stratified by different ICIs (PD-1/PDL-1); OS, overall survival; PFS, progression free survival; ICIs, immune checkpoint inhibitors. HR, hazard ratio; CI, confidence interval; PD-1, programmed cell death protein 1; PDL-1, programmed cell death ligand 1.
Similarly, as depicted in Figure 5B, the HR for PFS between the two groups was 1.195 (95% CI: 0.742-1.923, P=0.462), suggesting inconclusive evidence due to the imprecise estimate reflected by the wide CI.
4 Discussion
ES-SCLC accounts for the vast majority of all SCLC patients and is characterized by poor prognosis and limited treatment. In the past 30 years, the main treatment has been chemotherapy based on platinum-based drugs (etoposide + cisplatin/carboplatin), but the long-term survival is low, 5-year survival rate <7% (16). However, the application of some ICIs (such as durvalumab and atezolizumab) in first-line treatment has prolonged the OS of ES-SCLC patients, which brings a new dawn to the long-term unchanged treatment mode of ES-SCLC and is also a milestone in the change of treatment mode. These include CASPIAN (9), IMpower133 (17), ASTRUM005 (18), Checkmate032 (19), and Keynote028 (20).
Based on the more encouraging and effective results of multi clinical trials, many countries have adopted ICIs (PD-1/PDL-1 inhibitor) combined with chemotherapy as the first-line treatment for ES-SCLC and included it in the relevant treatment guidelines (11, 21). However, in the clinical management of ES-SCLC, the efficacy of first-line chemotherapy in combination with ICIs remains an area worthy of further investigation. And the role of baseline data (mainly tumor markers, serum immunoinflammatory and nutritional indicators, etc.) in evaluating the prognosis of patients with ES-SCLC treated by such methods is still unclear. To investigate the efficacy and safety of first-line chemotherapy combined with ICIs in patients with ES-SCLC, as well as to identify biomarkers associated with patient prognosis, we conducted a retrospective matched-pair analysis.
In this retrospective study, we analyzed data from super-large medical centers in central China. The results indicated that the K-M curves before and after PSM demonstrated that patients with ES-SCLC who received ICIs combined with chemotherapy had significantly higher OS and PFS, along with fewer AEs. Notably, although some baseline covariates were no longer significantly different before-PSM, they continued to influence the final outcomes. After-PSM, patients with ES-SCLC who received only chemotherapy experienced a longer mOS and mPFS, likely due to diminished differences in baseline covariates and confounders among treatment groups (22). This aligns with clinical trial findings: durvalumab, a PD-1 inhibitor, combined with chemotherapy significantly increased mOS and mPFS, while AEs did not differ significantly from chemotherapy alone, occurring at rates of 32% and 36%, respectively (9).Meanwhile, our data indicate that the ORR in the chemotherapy plus ICIs group was 58.11%, which was significantly higher than that in the chemotherapy group (40.7%). This finding is consistent with the results of the CASPIAN trial: the chemotherapy plus durvalumab group demonstrated a significant improvement in the ORR compared to the chemotherapy group (23). Regarding DCR, the 91.89% in the chemotherapy plus CIs group was higher than the 82.68% in the chemotherapy group, which is consistent with the findings of the IMpower133 trial (24).
Our subgroup analysis revealed that patients without extrathoracic metastases, brain metastases, or pleural metastases did not benefit from the combination therapy, whereas most other subgroups did. Meta-analyses based on multiple clinical trials also showed that combination therapy for ES-SCLC patients with brain metastases was not superior to chemotherapy alone (25, 26), consistent with our findings. Additionally, Jin et al.’s study indicated that pleural metastasis remains the predominant mode of failure following first-line chemotherapy plus ICIs for ES-SCLC, suggesting that thoracic radiotherapy might be an effective strategy (27).
Our study demonstrated no significant difference in outcomes between SCLC cases treated with PD-1 inhibitors and those treated with PDL-1 inhibitors when combined with chemotherapy and ICIs. Although the mOS and mPFS were slightly longer for PD-1 inhibitors, no direct clinical trials have compared these two types of drugs. A network meta-analysis that synthesized data from multiple randomized controlled trials found no significant differences in OS (HR 0.96, 95% CI: 0.72-1.30), PFS (HR 0.83, 95% CI: 0.72-1.30), or ORR (OR 1.39, 95% CI: 0.66-2.50) between PD-1 inhibitor + chemotherapy and PDL-1 inhibitor + chemotherapy. Bayesian ranking analysis suggested a trend toward longer OS with PDL-1 inhibitor + chemotherapy compared to PD-1 inhibitor + chemotherapy (28). Similarly, Pillai et al.’s study on NSCLC indicated that the efficacy and toxicity profiles of PD-1 and PDL-1 inhibitors appeared similar, although immune-related adverse events were slightly more frequent in patients receiving PD-1 inhibitors (29). The question of whether PD-1 and PDL-1 inhibitors result in different clinical outcomes in SCLC remains unresolved, and further studies are warranted.
Our multivariate analysis revealed that a poor ECOG PS (1 vs 0) and brain metastases were independent prognostic factors for PFS in ES-SCLC patients treated with chemotherapy plus ICIs. Previous studies have demonstrated that an ECOG PS of 3–4 versus 0–1 is independently associated with long-term prognosis in SCLC patients receiving first-line chemotherapy (30). However, despite Phase III clinical trials excluding patients with ECOG PS ≥2, one study indicated that chemotherapy plus ICIs could extend PFS in patients with ECOG PS 2 or 3 extensive-stage SCLC (31). Subgroup analysis within this study, however, showed no benefit for patients with ECOG PS 1 from chemotherapy plus ICIs, possibly due to inconsistencies in the control group. Moreover, when comparing the efficacy between ECOG PS 2–3 and ECOG PS 0–1 in SCLC patients undergoing chemotherapy plus ICIs, no significant differences were observed in PFS or OS (32). In patients with ES-SCLC and an ECOG PS less than 2, an ECOG PS of 1 compared to 0 was identified as an independent risk factor for PFS (33), which aligns with our findings, and a potential explanation is that the physical condition significantly influences the development of the tumor (34).
Brain metastases are among the most frequent sites of distant spread in ES-SCLC, and patients diagnosed with brain metastases typically face a less favorable prognosis. Whole-brain radiotherapy remains the standard treatment for ES-SCLC brain metastases. Consistent with several clinical trials, including the CASPIAN trial (HR=0.69, 95% CI: 0.35-1.31) (23) and the ETHER701 trial (HR=0.64, 95% CI: 0.29-1.41) (35), our study found that ES-SCLC patients with brain metastases did not benefit from immunotherapy. Furthermore, a real-world study revealed that patients treated with PD-1/PD-L1 inhibitors showed better control of intracranial disease and experienced a reduced rate of intracranial progression in comparison to ES-SCLC patients who received only chemotherapy (36). This may be attributed to the active immune microenvironment in SCLC brain metastases: a study of 32 SCLC brain metastasis samples revealed that 93.8% had lymphocyte infiltration, 75% expressed PDL-1, and 34.4% had PDL-1 expression exceeding 5% (37). Retrospective studies have also indicated that ES-SCLC patients with brain metastases who received immunotherapy in addition to chemotherapy and radiotherapy had significantly higher PFS compared to those treated with chemotherapy plus radiotherapy alone (38). In this study, baseline brain metastases in ES-SCLC patients receiving chemotherapy plus ICIs were identified as an independent prognostic factor for PFS, underscoring the importance of monitoring brain metastases in ES-SCLC patients prior to chemotherapy plus ICIs. Furthermore, Li et al.’s study showed that the combination therapy of albumin-bound paclitaxel effectively treated ES-SCLC patients with brain metastases, achieving an ORR of 36.6% (39). Additional research is warranted to explore more effective treatment options.
Inflammation greatly contributes to the development and progression of cancer, impacting every stage of tumorigenesis. Within this process, cancer cells interact complexly with neighboring stromal and inflammatory cells to form the inflammatory tumor microenvironment (TME) (40). Several novel inflammatory and nutritional biomarkers, including the NLR, LMR, SIRI, and SI, have emerged as significant prognostic indicators in various cancers such as cervical (41), esophageal (42), gastric (43), and breast cancer (44). These markers provide valuable insights into disease progression and patient outcomes. However, the prognostic significance of these indicators in patients with ES-SCLC undergoing immunotherapy remains unclear. Our univariate analysis revealed that baseline NLR > 4, SIRI > 2.63, and SII > 146.11 were significantly associated with LMR > 2.01 in patients with ES-SCLC treated with chemotherapy plus ICIs. Multivariate analysis further indicated that a high SIRI was a protective factor for OS.
This finding is inconsistent with the results of some studies. Yilmaz et al. demonstrated that in ES-SCLC, multivariate analysis identified high SIRI as a risk factor for both PFS and OS prognosis; however, this study did not differentiate between various treatment modalities (45). In LS-SCLC patients undergoing combined radiotherapy plus ICId, a high SIRI was identified as an independent risk factor for OS (46). Conversely, in ES-SCLC patients treated with chemotherapy plus ICIs, a high SIRI was not associated with OS prognosis (33). These inconsistencies suggest that SIRI’s role varies across different stages of SCLC and treatment methods, potentially due to the impact of immunotherapy on the TME. In solid tumors, monocytes predominantly develop into immunosuppressive tumor-associated macrophages (TAMs), which results in an anti-tumor immune reaction that strongly complements immune checkpoint inhibition (47). Therefore, in the context of immunotherapy, monocyte differentiation and immunotherapy may exhibit synergistic effects. However, further research is needed to confirm these findings.
There are several limitations in this study that warrant consideration. 1, one of the major limitations of this study is the relatively small sample size (n = 74). Despite making every possible effort to ensure the representativeness of the sample and the quality of the data during the design phase, the small sample size might impact the statistical power and external validity of the results. To evaluate whether our research design has sufficient statistical power, we performed a retrospective power calculation. Nevertheless, due to the absence of a formal power analysis during the design stage, we were unable to report specific power values. Under these circumstances, we suggest that future studies carry out detailed power calculations during the design phase to guarantee an adequate sample size for detecting the expected effect size.
2, the relatively short follow-up period may have influenced the results; therefore, longer-term follow-up studies are necessary to validate these findings.
Given that ICIs therapy has only recently been included in the diagnosis and treatment guidelines in China, future studies with larger sample sizes are required to draw more robust conclusions.
3, this study employed a retrospective design. Although PSM was used to balance baseline characteristics, some residual confounding bias may still exist.
5 Conclusion
In summary, our findings indicate that incorporating immunotherapy into first-line chemotherapy significantly improves PFS and OS in ES-SCLC patients, while maintaining safety. In addition, poor ECOG PS, brain metastases, and high SIRI at baseline may serve as valuable prognostic indicators for disease progression and survival in ES-SCLC patients. These factors provide a reliable reference for clinicians to assess ES-SCLC patients outcomes in the context of chemotherapy plus ICIs treatment regimens. It is worth noting that these findings should be interpreted as hypothesis-generating, not definitive clinical conclusions.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the First Affiliated Hospital of Zhengzhou University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Due to the retrospective nature of this study, informed consent from the patients who were enrolled in it was not required, and patients’ information was secure.
Author contributions
BJ: Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Visualization, Writing – original draft. CZ: Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft. FZ: Writing – review & editing. XS: Writing – review & editing. YD: Writing – review & editing. XW: Writing – review & editing. BW: Writing – review & editing. HW: Writing – review & editing. QH: Conceptualization, Data curation, Visualization, Writing – review & editing. SC: Conceptualization, Data curation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Wu Jieping Medical Foundation (NO. 320.6750.2024-16-4).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1562458/full#supplementary-material
Supplementary Figure 1Comparison of data before and after propensity score match (PSM).
Supplementary Figure 2Love plot before and after PSM.
References
1
Leiter A Veluswamy RR Wisnivesky JP . The global burden of lung cancer: current status and future trends. Nat Rev Clin Oncol. (2023) 20:624–39. doi: 10.1038/s41571-023-00798-3
2
Rudin CM Brambilla E Faivre-Finn C Sage J . Small-cell lung cancer. Nat Rev Dis Primers. (2021) 7:3. doi: 10.1038/s41572-020-00235-0
3
Semenova EA Nagel R Berns A . Origins, genetic landscape, and emerging therapies of small cell lung cancer. Genes Dev. (2015) 29:1447–62. doi: 10.1101/gad.263145.115
4
Faivre-Finn C Snee M Ashcroft L Appel W Barlesi F Bhatnagar A et al . Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. (2017) 18:1116–25. doi: 10.1016/S1470-2045(17)30318-2
5
Iams WT Porter J Horn L . Immunotherapeutic approaches for small-cell lung cancer. Nat Rev Clin Oncol. (2020) 17:300–12. doi: 10.1038/s41571-019-0316-z
6
Lally BE Urbanic JJ Blackstock AW Miller AA Perry MC . Small cell lung cancer: have we made any progress over the last 25 years? Oncologist. (2007) 12:1096–104. doi: 10.1634/theoncologist.12-9-1096
7
Farago AF Keane FK . Current standards for clinical management of small cell lung cancer. Transl Lung Cancer Res. (2018) 7:69–79. doi: 10.21037/tlcr.2018.01.16
8
Demedts IK Vermaelen KY van Meerbeeck JP . Treatment of extensive-stage small cell lung carcinoma: current status and future prospects. Eur Respir J. (2010) 35:202–15. doi: 10.1183/09031936.00105009
9
Goldman JW Dvorkin M Chen Y Reinmuth N Hotta K Trukhin D et al . Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. (2021) 22:51–65. doi: 10.1016/S1470-2045(20)30539-8
10
Cheng Y Fan Y Zhao Y Huang D Li X Zhang P et al . Tislelizumab plus platinum and etoposide versus placebo plus platinum and etoposide as first-line treatment for extensive-stage SCLC (RATIONALE-312): A multicenter, double-blind, placebo-controlled, randomized, phase 3 clinical trial. J Thorac Oncol. (2024) 19:1073–85. doi: 10.1016/j.jtho.2024.03.008
11
Experts Committee on Small-Cell Lung Cancer of Committee of the Chinese Society of Clinical Oncology (CSCO), Multidisciplinary Cancer Diagnosis and Treatment Committee of Chinese Medical Doctor Association . Consensus on immunotherapy for small cell lung cancer (2024 edition). Zhonghua Zhong Liu Za Zhi. (2024) 46:1241–51. doi: 10.3760/cma.j.cn112152-20240905-00383
12
Veccia A Dipasquale M Kinspergher S Caffo O . Prognostic role of inflammatory and nutritional biomarkers in non-small-cell lung cancer patients treated with immune checkpoint inhibitors alone or in combination with chemotherapy as first-line. Cancers (Basel). (2024) 16(22):3871. doi: 10.3390/cancers16223871
13
Guo L Li J Wang J Chen X Cai C Zhou F et al . Prognostic role of dynamic changes in inflammatory indicators in patients with non-small cell lung cancer treated with immune checkpoint inhibitors-a retrospective cohort study. Transl Lung Cancer Res. (2024) 13:1975–87. doi: 10.21037/tlcr-24-637
14
Shi Z Zheng D Tang X Du Y . Correlation of immune inflammatory indices and nutritional risk index with prognosis in patients with non-small cell lung cancer. Am J Transl Res. (2023) 15:4100–9.
15
Micke P Faldum A Metz T Beeh KM Bittinger F Hengstler JG et al . Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer–what limits limited disease? Lung Cancer. (2002) 37:271–6. doi: 10.1016/S0169-5002(02)00072-7
16
Tian Y Ma J Jing X Zhai X Li Y Guo Z et al . Radiation therapy for extensive-stage small-cell lung cancer in the era of immunotherapy. Cancer Lett. (2022) 541:215719. doi: 10.1016/j.canlet.2022.215719
17
Liu SV Reck M Mansfield AS Mok T Scherpereel A Reinmuth N et al . Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol. (2021) 39:619–30. doi: 10.1200/JCO.20.01055
18
Cheng Y Han L Wu L Chen J Sun H Wen G et al . Effect of first-line serplulimab vs placebo added to chemotherapy on survival in patients with extensive-stage small cell lung cancer: the ASTRUM-005 randomized clinical trial. Jama. (2022) 328:1223–32. doi: 10.1001/jama.2022.16464
19
Antonia SJ López-Martin JA Bendell J Ott PA Taylor M Eder JP et al . Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. (2016) 17:883–95. doi: 10.1016/S1470-2045(16)30098-5
20
Ott PA Elez E Hiret S Kim DW Morosky A Saraf S et al . Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase ib KEYNOTE-028 study. J Clin Oncol. (2017) 35:3823–9. doi: 10.1200/JCO.2017.72.5069
21
Melosky BL Leighl NB Dawe D Blais N Wheatley-Price PF Chu QS et al . Canadian consensus recommendations on the management of extensive-stage small-cell lung cancer. Curr Oncol. (2023) 30:6289–315. doi: 10.3390/curroncol30070465
22
Austin PC . An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. (2011) 46:399–424. doi: 10.1080/00273171.2011.568786
23
Paz-Ares L Dvorkin M Chen Y Reinmuth N Hotta K Trukhin D et al . Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. (2019) 394:1929–39. doi: 10.1016/S0140-6736(19)32222-6
24
Horn L Mansfield AS Szczęsna A Havel L Krzakowski M Hochmair MJ et al . First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. (2018) 379:2220–9. doi: 10.1056/NEJMoa1809064
25
Landre T Chouahnia K Des Guetz G Duchemann B Assié JB Chouaïd C . First-line immune-checkpoint inhibitor plus chemotherapy versus chemotherapy alone for extensive-stage small-cell lung cancer: a meta-analysis. Ther Adv Med Oncol. (2020) 12:1758835920977137. doi: 10.1177/1758835920977137
26
Kang W Cheng J Pan L Zhan P Liu H Lv T et al . Heterogeneity between subgroups of first-line chemoimmunotherapy for extensive-stage small cell lung cancer patients: a meta-analysis and systematic review. Front Oncol. (2024) 14:1334957. doi: 10.3389/fonc.2024.1334957
27
Kim D Kim HJ Wu HG Lee JH Kim S Kim TM et al . Intrathoracic progression is still the most dominant failure pattern after first-line chemo-immunotherapy in extensive-stage small-cell lung cancer: implications for thoracic radiotherapy. Cancer Res Treat. (2024) 56:430–41. doi: 10.4143/crt.2023.931
28
Liu W Yu L Feng Y Huang S Hua Y Peng M et al . Which is more suitable for first-line treatment of extensive-stage small cell lung cancer, PD-L1 inhibitors versus PD-1 inhibitors? A systematic review and network meta-analysis. Clin Respir J. (2024) 18:e13804. doi: 10.1111/crj.13804
29
Pillai RN Behera M Owonikoko TK Kamphorst AO Pakkala S Belani CP et al . Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: A systematic analysis of the literature. Cancer. (2018) 124:271–7. doi: 10.1002/cncr.v124.2
30
Lattuca-Truc M Timsit JF Levra MG Ruckly S Villa J Dumas I et al . Trends in response rate and survival in small-cell lung cancer patients between 1997 and 2017. Lung Cancer. (2019) 131:122–7. doi: 10.1016/j.lungcan.2019.03.028
31
Agarwal M Liu A Langlais BT Leventakos K Yu NY Almquist D et al . Chemoimmunotherapy as the first-line treatment for patients with extensive-stage small-cell lung cancer and an ECOG performance status 2 or 3. Clin Lung Cancer. (2023) 24:591–7. doi: 10.1016/j.cllc.2023.05.005
32
Agarwal M Liu A Almquist D Langlais BT Leventakos K Yu NY et al . Chemoimmunotherapy in patients with extensive-stage small cell lung cancer and a poor performance status. Cancer. (2023) 129:3546–53. doi: 10.1002/cncr.v129.22
33
Xie J Chen M Han H Xu K Qiu G Lin X et al . Clinical impact of first-line PD-1 or PD-L1 inhibitors combined with chemotherapy in extensive-stage small cell lung cancer patients: A real-world multicenter propensity score-matched study. Thorac Cancer. (2023) 14:1327–38. doi: 10.1111/1759-7714.14874
34
Jang RW Caraiscos VB Swami N Banerjee S Mak E Kaya E et al . Simple prognostic model for patients with advanced cancer based on performance status. J Oncol Pract. (2014) 10:e335–41. doi: 10.1200/JOP.2014.001457
35
Cheng Y Chen J Zhang W Xie C Hu Q Zhou N et al . Benmelstobart, anlotinib and chemotherapy in extensive-stage small-cell lung cancer: a randomized phase 3 trial. Nat Med. (2024) 30:2967–76. doi: 10.1038/s41591-024-03132-1
36
Namgung Y Lee K Choi J Lee SY Kang EJ . 520P Role of atezolizumab in controlling CNS progression in ES-SCLC. Ann Oncol. (2023) 34:S1673–S4. doi: 10.1016/j.annonc.2023.10.599
37
Huang L Chen S Liu H Meng L Liu C Wu X et al . PD-L1 inhibitors combined with whole brain radiotherapy in patients with small cell lung cancer brain metastases: Real-world evidence. Cancer Med. (2024) 13:e7125. doi: 10.1002/cam4.v13.7
38
Chang J Jing X Hua Y Geng K Li R Lu S et al . Programmed cell death 1 pathway inhibitors improve the overall survival of small cell lung cancer patients with brain metastases. J Cancer Res Clin Oncol. (2023) 149:1825–33. doi: 10.1007/s00432-022-04121-y
39
Li X Wu D Peng Y Tang J Wu Y . The efficacy and safety of albumin-bound paclitaxel combined with anlotinib and PD-1/L1 inhibitors for treating patients with extensive-stage small cell lung cancer and brain metastasis: A retrospective cohort study. Cancer Med. (2024) 13:e70449. doi: 10.1002/cam4.v13.23
40
Greten FR Grivennikov SI . Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. (2019) 51:27–41. doi: 10.1016/j.immuni.2019.06.025
41
Chen Q Zhai B Li J Wang H Liu Z Shi R et al . Systemic immune-inflammatory index predict short-term outcome in recurrent/metastatic and locally advanced cervical cancer patients treated with PD-1 inhibitor. Sci Rep. (2024) 14:31528. doi: 10.1038/s41598-024-82976-6
42
Su J Li Y Tan S Cheng T Luo Y Zhang L . Pretreatment neutrophil-to-lymphocyte ratio is associated with immunotherapy efficacy in patients with advanced cancer: a systematic review and meta-analysis. Sci Rep. (2025) 15:446. doi: 10.1038/s41598-024-84890-3
43
Wu Q Zhao H . Prognostic and clinicopathological role of pretreatment systemic inflammation response index (SIRI) in gastric cancer: a systematic review and meta-analysis. World J Surg Oncol. (2024) 22:333. doi: 10.1186/s12957-024-03602-3
44
Pang J Ding N Liu X He X Zhou W Xie H et al . Prognostic value of the baseline systemic immune-inflammation index in HER2-positive metastatic breast cancer: exploratory analysis of two prospective trials. Ann Surg Oncol. (2025) 32:750–9. doi: 10.1245/s10434-024-16454-8
45
Yilmaz H Yersal Ö . Prognostic significance of novel inflammatory markers in extensive-stage small-cell lung cancer. J Cancer Res Ther. (2022) 18:691–6. doi: 10.4103/jcrt.jcrt_1937_21
46
Kucuk A Ozkan EE Eskici Oztep S Mertsoylu H Pehlivan B Selek U et al . The influence of systemic inflammation response index on survival outcomes of limited-stage small-cell lung cancer patients treated with concurrent chemoradiotherapy. J Oncol. (2020) 2020:8832145. doi: 10.1155/2020/8832145
47
Devalaraja S To TKJ Folkert IW Natesan R Alam MZ Li M et al . Tumor-derived retinoic acid regulates intratumoral monocyte differentiation to promote immune suppression. Cell. (2020) 180:1098–114.e16. doi: 10.1016/j.cell.2020.02.042
Summary
Keywords
ES-SCLC, chemotherapy, ICIs, PSM, COX, prognostic
Citation
Jia B, Zhou C, Zhao F, Song X, Ding Y, Wang X, Wu B, Wang H, Hu Q and Chen S (2025) Efficacy and safety of first-line chemotherapy combined with immune checkpoint inhibitors for extensive-stage small cell lung cancer patients: a real-world propensity score matching study. Front. Immunol. 16:1562458. doi: 10.3389/fimmu.2025.1562458
Received
17 January 2025
Accepted
28 July 2025
Published
13 August 2025
Volume
16 - 2025
Edited by
Hongfei Jiang, Qingdao University, China
Reviewed by
Li-Yue Sun, Fudan University, China
Bishnu Joshi, Gel4Med, Inc. (A Harvard Innovation Labs Company), United States
Updates
Copyright
© 2025 Jia, Zhou, Zhao, Song, Ding, Wang, Wu, Wang, Hu and Chen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Quanman Hu, quanmanhu@163.com; Shuaiyin Chen, sychen@zzu.edu.cn
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.