- 1Department of Clinical Medicine, University of Bergen, Bergen, Norway
- 2Neuro-SysMed, Department of Neurology, Haukeland University Hospital, Bergen, Norway
- 3Department of General Biochemistry, Faculty of Biology and Environmental Protection, University of Lodz, Lodz, Poland
- 4Faculty of Medicine, Friedrich-Alexander-University Erlangen-Nuremberg (FAU), Erlangen, Germany
Multiple sclerosis (MS) is a chronic inflammatory and neurodegenerative disease of the nervous system and a main cause of neurological disability in young adults. Most disease-modifying therapies are administrated as long-term maintenance therapies and may, thereby, increase the risk of infections and other immune-mediated side effects. In the last years, several cerebrospinal fluid and soluble blood biomarkers have been suggested as potential key tools for diagnosis, prognosis, and treatment monitoring of MS. Recently, the specific ability of brain-derived blood extracellular vesicles (EVs) that cross the blood-brain barrier into the bloodstream, reflecting the current immune status of the central nervous system, has kindled interest as potential biomarkers. In this review, we discuss the current trends of clinical brain-derived blood biomarkers, with a special focus on the emerging role of brain-derived blood EVs in MS.
1 Introduction
Almost 3 million people worldwide are affected by multiple sclerosis (MS), an immune-mediated inflammatory and degenerative disease of the central nervous system (CNS) (1). From a clinical perspective, MS is highly heterogeneous with most patients (85%–90%) experiencing an initial relapsing-remitting course (RRMS) marked by episodic inflammation and, if not treated effectively, followed by a secondary progressive (SPMS) phase, associated with gradual increasing disability (2). Epidemiological data suggest that Epstein–Barr virus is a prerequisite for developing MS, but the underlying pathogenic mechanisms are still unclear (3, 4).
The MS diagnosis relies on the combination of clinical and paraclinical findings, with no single definitive diagnostic test available (5). Currently, it is essential to determine inflammatory immune-mediated damage affecting at least two distinct regions (dissemination in space) of the CNS at varied time points (dissemination in time) to establish an MS diagnosis. Since the incorporation in the diagnostic criteria (1983), magnetic resonance imaging (MRI) of the brain and spinal cord holds a pivotal role in the diagnostic process. In addition, cerebrospinal fluid (CSF) analysis detecting intrathecal immunoglobulin G (IgG) synthesis was highlighted in the update of the diagnostic criteria of MS in 2017 (5).
Recent advancements have shed light on detecting brain-derived proteins at remarkably low concentrations in blood, paving the way for the exploration of early blood-based biomarkers in MS (6). Specific markers of immunopathological processes including neuroaxonal damage [neurofilament light chain (NfL)] and astrocyte activation [glial fibrillary acidic protein (GFAP)] are already rapidly emerging (7, 8). Extracellular vesicles (EVs) are defined as membrane-bound particles, released from virtually all cell types, with a sophisticated sorting mechanism of their cargo inclusive of lipids, proteins, and nucleic acids, in addition to carrying specific membrane proteins, mainly reflecting their donor cell. This peculiarity, plus their ability to cross the blood-brain barrier (BBB) into the blood stream, increased stability, and involvement in the regulation of both the immune system and CNS homeostasis, features brain-derived blood EVs, as improved biomarkers in CNS diseases, including MS (9–12). This review aims to summarize the current CSF and blood biomarkers in MS, discussing the unmet needs and future perspectives.
2 MS pathogenesis and fluid biomarkers
In the early stages of MS, the recurrent invasion of T and B cells in the brain and spinal cord drives a cascade of pathophysiological processes within the CNS (13). Several fluid biomarkers have emerged as effective indicators of this complex interaction, which contributes to the diverse clinical manifestations observed in the disease (14). Early episodes of acute focal inflammation, demyelination, and axonal damage, driven by infiltrating immune cells (macrophages, CD8+ T cells, CD4+ T cells, B cells, and plasma cells), could be typically detected through conventional MRI, showing new lesions in T2-weighted and/or T1-weighted gadolinium enhancing lesions (15, 16). Infiltrating immune cells are attracted to the CNS by several chemotactic factors such as chemokine (C-X-C motif) ligand 13 (CXCL13) for B cells (Figure 1) (17).
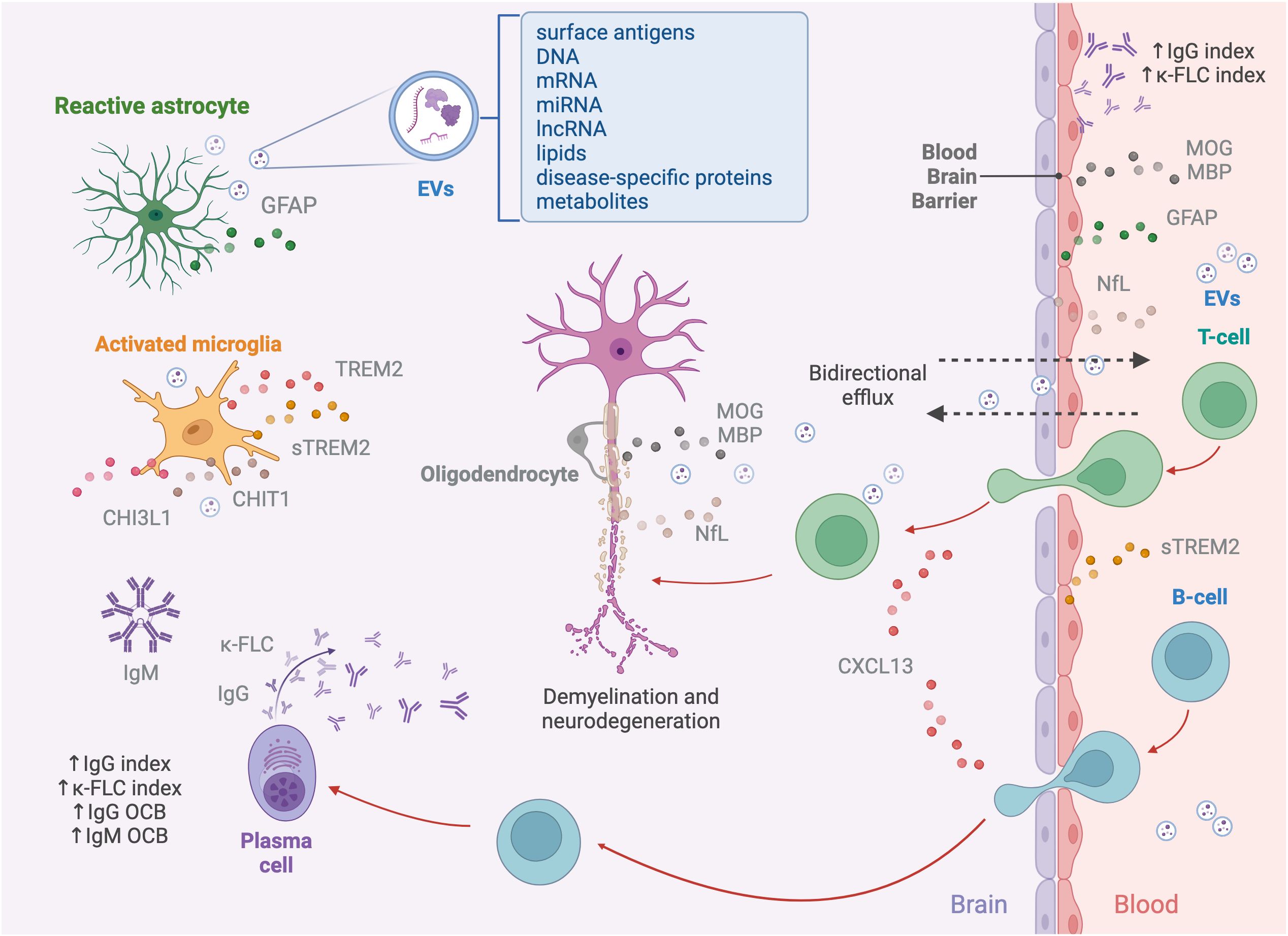
Figure 1. Pathophysiology of multiple sclerosis and associated biomarkers. The pathogenesis of MS begins with immune cells, including macrophages, autoreactive T cells targeting myelin, B cells, and plasma cells, infiltrating the CNS through a dysfunctional BBB. Lymphocyte recruitment is mediated by chemokines like CXCL13, which specifically attracts B cells. Within the CNS, T and B cells interact, to amplify the immune response, with T cells secreting cytokines and B cells acting as APCs. Activated B cells differentiate into plasma cells, producing immunoglobulins, including IgG and IgM and releasing κ-FLCs. The ongoing neuroinflammation leads to demyelination, axonal damage, and neurodegeneration, marked by NfL, which is released into the interstitial space, CSF, and bloodstream as a result of axonal injury, along with MOG and MBP, major proteins of the myelin sheet of oligodendrocytes. Resident immune cells within the CNS, such as microglia and astrocytes, contribute to the disruption of axonal integrity and synaptic function. While activated, microglia and astrocytes release various mediators into the CSF, including sTREM2, CHIT1, and CHI3L1. Additionally, astrocyte damage results in the release of structural proteins, such as GFAP, into the CSF and bloodstream. Another source of biomarkers reflecting pathological processes occurring in the CNS are EVs. These nanovesicles, secreted by various cell types, including neurons, astrocytes, microglia, and oligodendrocytes, carry a diverse molecular cargo, such as surface antigens, DNA, mRNA, miRNA, lncRNA, lipids, disease-specific proteins, and metabolites, acting as mediators of intercellular communication. The bidirectional efflux of EVs and soluble biomarkers across the compromised BBB enables their detection in peripheral fluids.
Invading T and B cells closely interact within the CNS (16, 17). In contrast to T cells, the immune pathways involving B cell activation have, so far, served as the most robust fluid biomarkers for MS. Mature plasma cells secrete IgG and IgM antibodies intrathecally, also leading to release of free light chains (due to a mismatch between immunoglobulin light- and heavy-chain synthesis) (18, 19). This inflammatory process results in axonal damage and release of neuronal markers like NfL (20). Over time, there is worsening of disability and accumulation of neurological deficits in the absence of concurrent relapses defined as “progression independent of relapse activity” (PIRA) (21). Underlying mechanism driving PIRA is increasingly understood as a pathophysiological continuum of the early “relapsing” phase driven by a chronic “smouldering” inflammatory process compartmentalized within the CNS, characterized by innate immune cells and astrocytes (22). Recent studies on positron emission tomography (PET) employing radioligands for innate immunity activation assessment have revealed an interestingly high prevalence of smouldering component in MS lesions (23). Chronically active MS lesions are slowly expanding over time or as paramagnetic rim lesions, expressing a dense network of activated iron-laden microglia/macrophages (24). Activated microglia and astrocytes release various mediators into the CSF, such as soluble triggering receptor expressed on myeloid cells 2 (sTREM2), chitinase 1 (CHIT1), chitinase-3–like protein 1 (CHI3L1), and GFAP, impacting axon, synaptic integrity, and function (25–30).
The critical role of the complement system in MS is underlined with the complement and Ig deposition across all areas of demyelination regardless of the plaque subtype, including complement-mediated myelin phagocytosis implying its importance once the disease is established. In progressive MS and long-standing disease patients, white matter plaques were consistently positive for complement proteins (C3, factor B, and C1q), regulators (factor H, C1inh, and clusterin) and activation products [C3b, iC3b, C4d, and terminal complement complex (TCC)] providing evidence that, once established, progression of inflammation in MS may not rely on infiltrating cells but rather on innate immune mechanisms including complement activation (31, 32).
EVs are pivotal in the intricate communication of neurons and glial cells of the CNS system holding neuroprotective and homeostatic effects but may have detrimental effects under pathological conditions (33, 34). EVs derived from T cells containing chemokine CCL5 and arachidonic acid can increase the expression of intercellular adhesion molecule 1 (ICAM-1) on endothelial cells and of Mac-1 on monocytes, contributing to the dysfunction of the BBB, leading to immune infiltration, a characteristic of MS pathogenesis (35–37). Dendritic cell (DCs) derived EVs carry cell surface molecules like major histocompatibility complex (MHC), ICAM-1, and other costimulatory molecules, which could aid in T-cell activation (38). EVs from activated microglia express pro-inflammatory mediators (Tumor Necrosis Factor-alpha (TNF-α) and Interleukin-1 (IL-1)) exhibiting a distinct proteomic profile enforcing inflammatory stimuli throughout the CNS (39). Recent studies show the role of astrocyte-derived EVs in the regulation of T-cell secretion and biomarker utility of myelin basic protein (MBP) and myelin oligodendrocyte glycoprotein (MOG) content in oligodendrocytes-derived EVs (40). Most immune cell–derived EVs seem to be significantly higher in treatment naïve relapsing MS patients with low disability, and their functions might depend on the physiological environment, despite limited changes in circulating immune cells (33).
3 MS fluid biomarkers—current trends and beyond
The diagnostic criterion for MS underscores the importance of both MRI and biofluid biomarkers emphasizing the pivotal role of accurate diagnosis, prognosis, and treatment response in the management of the disease (5). In addition to advancements in MRI techniques (7-T MRI, PET, magnetization transfer imaging, diffusion tensor imaging, and myelin water imaging), integrating biofluid biomarkers would be beneficial because of their ability to directly reflect the pathophysiological processes involved in the MS disease course (41). Cumulative evidence shows that the blood-based biomarker sNfL can predict relapses in relapsing MS patients, whereas CSF IgM oligoclonal bands, CHI3L1, and GFAP seem to be associated with a more progressive phenotype. Different aspects of microglial involvement (CHIT1 and sTREM2), astroglia pathology (CHI3L1 and GFAP), B-cell–related pathology (CXCL13), and neuroaxonal damage (sNfL) have been evaluated in several studies aiding in classifying MS disease activity (Table 1) (25–30). Brain-derived blood EVs (L1CAM, MOG, and GLAST) serve as potential windows into the CNS reflecting the underlying MS-related pathophysiology (Table 1) (33).
Certain limitations of the emerging fluid biomarkers intrude their clinical transition. For example, NfL is a promising biomarker but with limited diagnostic use due to its unspecific increase in the blood connected to several neurological conditions (42). EVs hold potential as biomarkers; however, existing knowledge gaps in terms of EVs biology, biodistribution, and assay standardization are yet to be fully elucidated (33). Although MS fluid biomarkers hold a promising frontier, addressing standardization, data validation, and accessibility are key in resolving ongoing challenges. Composite scoring with integrated clinical and MRI metrics [e.g., the MAGNIMS score or no evidence of disease activity 3 (NEDA-3) and NEDA-4] and multimodal biomarker profiling (CSF and blood-based biomarkers with neuroimaging) may be a way forward in MS management (41). Furthermore, artificial intelligence (automated lesion detection and improved diagnostic accuracy) holds transformative potential in enhancing clinical decision-making.
In conclusion, despite the limitations, the recent advances within the field hold a promising frontier, giving a paradigm shift from the conventional CSF (oligoclonal banding) analysis to a new era of brain-derived blood biomarkers (NfL, GFAP, and EVs), enabling improved longitudinal disease monitoring and personalized treatment.
Author contributions
SA: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. KM: Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. RB: Investigation, Writing – review & editing. SM: Investigation, Writing – review & editing. CK: Investigation, Writing – review & editing. OT: Investigation, Project administration, Writing – review & editing. KMM: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by and the Research Council of Norway through its Centers of Excellence funding scheme (Grant Number 288164).
Acknowledgments
The figure was created with BioRender (BioRender.com).
Conflict of interest
K-MM has received speaker honoraria from Biogen, Novartis, or Sanofi and has participated in clinical trials organized by Biogen, Merck, Novartis, Roche, and Sanofi. OT has participated in advisory boards and received speaker honoraria from Biogen, Merck, Novartis, Teva, Roche, Sanofi, and Bristol Myers Squibb and has participated in clinical trials organized by Merck, Novartis, Roche, and Sanofi.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
MS, multiple sclerosis; CNS, central nervous system; BBB, blood-brain barrier; CXCL13, chemokine (C-X-C motif) ligand 13; APCs, antigen-presenting cells; κ-FLCs, kappa-free light chains; NfL, neurofilament light chain; CSF, cerebrospinal fluid; sTREM2, soluble triggering receptor expressed on myeloid cells 2; CHIT1, chitotriosidase 1; CHI3L1, chitinase-3–like protein 1; GFAP, glial fibrillary acidic protein; EVs, extracellular vesicles; miRNA, microRNA; lncRNA, long non-coding RNA; MOG, myelin oligodendrocyte glycoprotein; MBP, myelin basic protein.
References
1. Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult Scler. (2020) 26:1816–21.
2. Thompson AJ, Baranzini SE, Geurts J, Hemmer B, and Ciccarelli O. Multiple sclerosis. Lancet. (2018) 391:1622–36.
3. Fernández-Fournier M, López-Molina M, Torres Iglesias G, Botella L, Chamorro B, Laso-García F, et al. Antibody content against epstein–barr virus in blood extracellular vesicles correlates with disease activity and brain volume in patients with relapsing–remitting multiple sclerosis. Int J Mol Sci. (2023) 24:14192.
4. Mrad MF, Saba ES, Nakib L, and Khoury SJ. Exosomes from subjects with multiple sclerosis express EBV-derived proteins and activate monocyte-derived macrophages. Neurol Neuroimmunology Neuroinflammation. (2021) 8:e1004.
5. Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurology. (2018) 17:162–73.
6. Teunissen CE, Kimble L, Bayoumy S, Bolsewig K, Burtscher F, Coppens S, et al. Methods to discover and validate biofluid-based biomarkers in neurodegenerative dementias. Mol Cell Proteomics. (2023) 22:100629.
7. Meier S, Willemse EAJ, Schaedelin S, Oechtering J, Lorscheider J, Melie-Garcia L, et al. Serum glial fibrillary acidic protein compared with neurofilament light chain as a biomarker for disease progression in multiple sclerosis. JAMA Neurology. (2023) 80:287–97.
8. Di Filippo M, Gaetani L, Centonze D, Hegen H, Kuhle J, Teunissen CE, et al. Fluid biomarkers in multiple sclerosis: from current to future applications. Lancet Regional Health – Europe. (2024) 44.
9. Gurunathan S, Kang MH, Jeyaraj M, Qasim M, and Kim JH. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. (2019) 8.
10. Huo L, Du X, Li X, Liu S, and Xu Y. The emerging role of neural cell-derived exosomes in intercellular communication in health and neurodegenerative diseases. Front Neurosci. (2021) 15.
11. Mycko MP and Baranzini SE. microRNA and exosome profiling in multiple sclerosis. Mult Scler. (2020) 26:599–604.
12. Hornung S, Dutta S, and Bitan G. CNS-derived blood exosomes as a promising source of biomarkers: opportunities and challenges. Front Mol Neurosci. (2020) 13.
13. Filippi M, Bar-Or A, Piehl F, Preziosa P, Solari A, Vukusic S, et al. Multiple sclerosis. Nat Rev Dis Primers. (2018) 4:43.
14. Engelhardt B, Comabella M, and Chan A. Multiple sclerosis: Immunopathological heterogeneity and its implications. Eur J Immunol. (2022) 52:869–81.
15. Rocca MA, Preziosa P, Barkhof F, Brownlee W, Calabrese M, De Stefano N, et al. Current and future role of MRI in the diagnosis and prognosis of multiple sclerosis. Lancet Regional Health – Europe. (2024) 44.
16. Dendrou CA, Fugger L, and Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. (2015) 15:545–58.
17. Novakova L, Axelsson M, Malmeström C, Zetterberg H, Blennow K, Svenningsson A, et al. NFL and CXCL13 may reveal disease activity in clinically and radiologically stable MS. Multiple Sclerosis Related Disord. (2020) 46:102463.
18. Hegen H, Walde J, Berek K, Arrambide G, Gnanapavan S, Kaplan B, et al. Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis. Multiple Sclerosis J. (2022) 29:169–81.
19. Hegen H, Arrambide G, Gnanapavan S, Kaplan B, Khalil M, Saadeh R, et al. Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A consensus statement. Multiple Sclerosis J. (2022) 29:182–95.
20. Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, and Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurology Neurosurg Psychiatry. (2019) 90:870–81.
21. Kappos L, Wolinsky JS, Giovannoni G, Arnold DL, Wang Q, Bernasconi C, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurology. (2020) 77:1132–40.
22. Tur C, Carbonell-Mirabent P, Cobo-Calvo Á, Otero-Romero S, Arrambide G, Midaglia L, et al. Association of early progression independent of relapse activity with long-term disability after a first demyelinating event in multiple sclerosis. JAMA Neurology. (2023) 80:151–60.
23. Hamzaoui M, Garcia J, Boffa G, Lazzarotto A, Absinta M, Ricigliano VAG, et al. Positron emission tomography with [F]-DPA-714 unveils a smoldering component in most multiple sclerosis lesions which drives disease progression. Ann Neurology. (2023) 94:366–83.
24. Jäckle K, Zeis T, Schaeren-Wiemers N, Junker A, van der Meer F, Kramann N, et al. Molecular signature of slowly expanding lesions in progressive multiple sclerosis. Brain. (2020) 143:2073–88.
25. Hinsinger G, Galéotti N, Nabholz N, Urbach S, Rigau V, Demattei C, et al. Chitinase 3-like proteins as diagnostic and prognostic biomarkers of multiple sclerosis. Multiple Sclerosis J. (2015) 21:1251–61.
26. Cantó E, Tintoré M, Villar LM, Costa C, Nurtdinov R, Álvarez-Cermeño JC, et al. Chitinase 3-like 1: prognostic biomarker in clinically isolated syndromes. Brain. (2015) 138:918–31.
27. Steinacker P, Verde F, Fang L, Feneberg E, Oeckl P, Roeber S, et al. Chitotriosidase (CHIT1) is increased in microglia and macrophages in spinal cord of amyotrophic lateral sclerosis and cerebrospinal fluid levels correlate with disease severity and progression. J Neurology Neurosurg Psychiatry. (2018) 89:239–47.
28. Filipello F, Goldsbury C, You SF, Locca A, Karch CM, and Piccio L. Soluble TREM2: Innocent bystander or active player in neurological diseases? Neurobiol Dis. (2022) 165:105630.
29. Högel H, Rissanen E, Barro C, Matilainen M, Nylund M, Kuhle J, et al. Serum glial fibrillary acidic protein correlates with multiple sclerosis disease severity. Multiple Sclerosis J. (2018) 26:210–9.
30. Abdelhak A, Foschi M, Abu-Rumeileh S, Yue JK, D’Anna L, Huss A, et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat Rev Neurology. (2022) 18:158–72.
31. Breij EC, Brink BP, Veerhuis R, Van den Berg C, Vloet R, Yan R, et al. Homogeneity of active demyelinating lesions in established multiple sclerosis. Ann Neurology: Off J Am Neurological Assoc Child Neurol Society. (2008) 63:16–25.
32. Ingram G, Loveless S, Howell OW, Hakobyan S, Dancey B, Harris CL, et al. Complement activation in multiple sclerosis plaques: an immunohistochemical analysis. Acta neuropathologica Commun. (2014) 2:1–15.
33. Pistono C, Osera C, Cuccia M, and Bergamaschi R. Roles of extracellular vesicles in multiple sclerosis: from pathogenesis to potential tools as biomarkers and therapeutics. Sclerosis. (2023) 1:91–112.
34. Schnatz A, Müller C, Brahmer A, and Krämer-Albers EM. Extracellular Vesicles in neural cell interaction and CNS homeostasis. FASEB Bioadv. (2021) 3:577–92.
35. Sáenz-Cuesta M, Osorio-Querejeta I, and Otaegui D. Extracellular vesicles in multiple sclerosis: what are they telling us? Front Cell Neurosci. (2014) 8:100.
36. Barry OP, Kazanietz MG, Pratico D, and FitzGerald GA. Arachidonic acid in platelet microparticles up-regulates cyclooxygenase-2-dependent prostaglandin formation via a protein kinase C/mitogen-activated protein kinase-dependent pathway. J Biol Chem. (1999) 274:7545–56.
37. Quandt J and Dorovini-Zis K. The beta chemokines CCL4 and CCL5 enhance adhesion of specific CD4+ T cell subsets to human brain endothelial cells. J Neuropathology Exp Neurology. (2004) 63:350–62.
38. Segura E, Nicco C, Lombard B, Véron P, Raposo G, Batteux F, et al. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. (2005) 106:216–23.
39. Aires ID, Ribeiro-Rodrigues T, Boia R, Ferreira-Rodrigues M, Girão H, Ambrósio AF, et al. Microglial extracellular vesicles as vehicles for neurodegeneration spreading. Biomolecules. (2021) 11:770.
40. Agliardi C, Guerini FR, Zanzottera M, Bolognesi E, Picciolini S, Caputo D, et al. Myelin basic protein in oligodendrocyte-derived extracellular vesicles as a diagnostic and prognostic biomarker in multiple sclerosis: a pilot study. Int J Mol Sci. (2023) 24:894.
41. Anderhalten L, Wohlrab F, and Paul F. Emerging MRI and biofluid biomarkers in the diagnosis and prognosis of multiple sclerosis. Lancet Regional Health – Europe. (2024) 44.
42. eBioMedicine. Blood biomarkers for multiple sclerosis: neurofilament light chain and beyond. eBioMedicine. (2024) 104.
43. Link H and Huang Y-M. Oligoclonal bands in multiple sclerosis cerebrospinal fluid: An update on methodology and clinical usefulness. J Neuroimmunology. (2006) 180:17–28.
44. McLean BN, Luxton RW, and Thompson EJ. A study of immunoglobulin G in the cerebrospinal fluid of 1007 patients with suspected neurological disease using isoelectric focusing and the Log IgG-Index. A comparison and diagnostic applications. Brain. (1990) 113:1269–89.
45. Lunding J, Midgard R, and Vedeler CA. Oligoclonal bands in cerebrospinal fluid:a comparative study of isoelectric focusing, agarose gel electrophoresis and IgG index. Acta Neurologica Scandinavica. (2000) 102:322–5.
46. Arrambide G, Espejo C, Carbonell-Mirabent P, Dieli-Crimi R, Rodríguez-Barranco M, Castillo M, et al. The kappa free light chain index and oligoclonal bands have a similar role in the McDonald criteria. Brain. (2022) 145:3931–42.
47. Rojas Juan I, Patrucco L, and Cristiano E. Oligoclonal bands and MRI in clinically isolated syndromes: predicting conversion time to multiple sclerosis. J Neurol. (2010) 257:1188–91.
48. Berek K, Bsteh G, Auer M, Di Pauli F, Grams A, Milosavljevic D, et al. Kappa-free light chains in CSF predict early multiple sclerosis disease activity. Neurol Neuroimmunology Neuroinflammation. (2021) 8:e1005.
49. Dekeyser C, De Kesel P, Cambron M, Vanopdenbosch L, Van Hijfte L, Vercammen M, et al. Inter-assay diagnostic accuracy of cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis. Front Immunol. (2024).
50. Duell F, Evertsson B, Al Nimer F, Sandin Å, Olsson D, Olsson T, et al. Diagnostic accuracy of intrathecal kappa free light chains compared with OCBs in MS. Neurol Neuroimmunology Neuroinflammation. (2020) 7:e775.
51. Ning L and Wang B. Neurofilament light chain in blood as a diagnostic and predictive biomarker for multiple sclerosis: A systematic review and meta-analysis. PloS One. (2022) 17:e0274565.
52. Freedman MS, Gnanapavan S, Booth RA, Calabresi PA, Khalil M, Kuhle J, et al. Guidance for use of neurofilament light chain as a cerebrospinal fluid and blood biomarker in multiple sclerosis management. EBioMedicine. (2024) 101:104970.
53. Bäckström D, Linder J, Jakobson Mo S, Riklund K, Zetterberg H, Blennow K, et al. NfL as a biomarker for neurodegeneration and survival in Parkinson disease. Neurology. (2020) 95:e827–e38.
54. Sahrai H, Norouzi A, Hamzehzadeh S, Majdi A, Kahfi-Ghaneh R, and Sadigh-Eteghad S. SIMOA-based analysis of plasma NFL levels in MCI and AD patients: a systematic review and meta-analysis. BMC Neurology. (2023) 23:331.
55. Urbano T, Maramotti R, Tondelli M, Gallingani C, Carbone C, Iacovino N, et al. Comparison of serum and cerebrospinal fluid neurofilament light chain concentrations measured by ella™ and lumipulse™ in patients with cognitive impairment. Diagnostics. (2024) 14:2408.
56. Vecchio D, Puricelli C, Malucchi S, Virgilio E, Martire S, Perga S, et al. Serum and cerebrospinal fluid neurofilament light chains measured by SIMOA™, Ella™, and Lumipulse™ in multiple sclerosis naïve patients. Mult Scler Relat Disord. (2024) 82:105412.
57. Magliozzi R, Mazziotti V, Montibeller L, Pisani AI, Marastoni D, Tamanti A, et al. Cerebrospinal fluid igM levels in association with inflammatory pathways in multiple sclerosis patients. Front Cell Neurosci. (2020) 14.
58. Mandrioli J, Sola P, Bedin R, Gambini M, and Merelli E. A multifactorial prognostic index in multiple sclerosis. J Neurology. (2008) 255:1023–31.
59. Villar LM, Casanova B, Ouamara N, Comabella M, Jalili F, Leppert D, et al. Immunoglobulin M oligoclonal bands: biomarker of targetable inflammation in primary progressive multiple sclerosis. Ann Neurol. (2014) 76:231–40.
60. Villar LM, Sádaba MC, Roldán E, Masjuan J, González-Porqué P, Villarrubia N, et al. Intrathecal synthesis of oligoclonal IgM against myelin lipids predicts an aggressive disease course in MS. J Clin Invest. (2005) 115:187–94.
61. Villar LM, González-Porqué P, Masjuán J, Alvarez-Cermeño JC, Bootello A, and Keir G. A sensitive and reproducible method for the detection of oligoclonal IgM bands. J Immunol Methods. (2001) 258:151–5.
62. Pike SC, Gilli F, and Pachner AR. The CXCL13 index as a predictive biomarker for activity in clinically isolated syndrome. Int J Mol Sci. (2023) 24.
63. Lucchini M, De Arcangelis V, Piro G, Nociti V, Bianco A, De Fino C, et al. CSF CXCL13 and chitinase 3-like-1 levels predict disease course in relapsing multiple sclerosis. Mol Neurobiology. (2023) 60:36–50.
64. Khademi M, Kockum I, Andersson ML, Iacobaeus E, Brundin L, Sellebjerg F, et al. Cerebrospinal fluid CXCL13 in multiple sclerosis: a suggestive prognostic marker for the disease course. Mult Scler. (2011) 17:335–43.
65. DiSano KD, Gilli F, and Pachner AR. Intrathecally produced CXCL13: A predictive biomarker in multiple sclerosis. Mult Scler J Exp Transl Clin. (2020) 6:2055217320981396.
66. Mohammed MS, Al-Rubae'i SHN, Rheima AM, and Al-Kazazz FF. A novel sandwich ELISA method for quantifying CHI3L1 in blood serum and cerebrospinal fluid multiple sclerosis patients using sustainable photo-irradiated zero-valence gold nanoparticles. Results Chem. (2024) 11:101856.
67. Pérez-Miralles F, Prefasi D, García-Merino A, Gascón-Giménez F, Medrano N, Castillo-Villalba J, et al. CSF chitinase 3-like-1 association with disability of primary progressive MS. Neurol Neuroimmunol Neuroinflamm. (2020) 7.
68. Floro S, Carandini T, Pietroboni AM, De Riz MA, Scarpini E, and Galimberti D. Role of chitinase 3–like 1 as a biomarker in multiple sclerosis. Neurol Neuroimmunology Neuroinflammation. (2022) 9:e1164.
69. Cantó E, Reverter F, Morcillo-Suárez C, Matesanz F, Fernández O, Izquierdo G, et al. Chitinase 3-like 1 plasma levels are increased in patients with progressive forms of multiple sclerosis. Mult Scler. (2012) 18:983–90.
70. Oldoni E, Smets I, Mallants K, Vandebergh M, Van Horebeek L, Poesen K, et al. CHIT1 at diagnosis reflects long-term multiple sclerosis disease activity. Ann Neurol. (2020) 87:633–45.
71. Beliën J, Swinnen S, D’hondt R, Verdú de Juan L, Dedoncker N, Matthys P, et al. CHIT1 at diagnosis predicts faster disability progression and reflects early microglial activation in multiple sclerosis. Nat Commun. (2024) 15:5013.
72. Rabin A, Bello E, Kumar S, Zeki DA, Afshari K, Deshpande M, et al. Targeted proteomics of cerebrospinal fluid in treatment naïve multiple sclerosis patients identifies immune biomarkers of clinical phenotypes. Sci Rep. (2024) 14:21793.
73. Comabella M, Fernández M, Martin R, Rivera-Vallvé S, Borrás E, Chiva C, et al. Cerebrospinal fluid chitinase 3-like 1 levels are associated with conversion to multiple sclerosis. Brain. (2010) 133:1082–93.
74. Ioannides ZA, Csurhes PA, Swayne A, Foubert P, Aftab BT, and Pender MP. Correlations between macrophage/microglial activation marker sTREM-2 and measures of T-cell activation, neuroaxonal damage and disease severity in multiple sclerosis. Mult Scler J Exp Transl Clin. (2021) 7:20552173211019772.
75. Piccio L, Buonsanti C, Cella M, Tassi I, Schmidt RE, Fenoglio C, et al. Identification of soluble TREM-2 in the cerebrospinal fluid and its association with multiple sclerosis and CNS inflammation. Brain. (2008) 131:3081–91.
76. Öhrfelt A, Axelsson M, Malmeström C, Novakova L, Heslegrave A, Blennow K, et al. Soluble TREM-2 in cerebrospinal fluid from patients with multiple sclerosis treated with natalizumab or mitoxantrone. Mult Scler. (2016) 22:1587–95.
77. Ashton NJ, Suárez-Calvet M, Heslegrave A, Hye A, Razquin C, Pastor P, et al. Plasma levels of soluble TREM2 and neurofilament light chain in TREM2 rare variant carriers. Alzheimer’s Res Ther. (2019) 11:94.
78. Cignarella F, Filipello F, Bollman B, Cantoni C, Locca A, Mikesell R, et al. TREM2 activation on microglia promotes myelin debris clearance and remyelination in a model of multiple sclerosis. Acta Neuropathol. (2020) 140:513–34.
79. Sun M, Liu N, Xie Q, Li X, Sun J, Wang H, et al. A candidate biomarker of glial fibrillary acidic protein in CSF and blood in differentiating multiple sclerosis and its subtypes: A systematic review and meta-analysis. Mult Scler Relat Disord. (2021) 51:102870.
80. Barro C, Healy BC, Liu Y, Saxena S, Paul A, Polgar-Turcsanyi M, et al. Serum GFAP and nfL levels differentiate subsequent progression and disease activity in patients with progressive multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. (2023) 10.
81. Rosenstein I, Nordin A, Sabir H, Malmeström C, Blennow K, Axelsson M, et al. Association of serum glial fibrillary acidic protein with progression independent of relapse activity in multiple sclerosis. J Neurol. (2024) 271:4412–22.
82. Schindler P, Aktas O, Ringelstein M, Wildemann B, Jarius S, Paul F, et al. Glial fibrillary acidic protein as a biomarker in neuromyelitis optica spectrum disorder: a current review. Expert Rev Clin Immunol. (2023) 19:71–91.
83. Simrén J, Weninger H, Brum WS, Khalil S, Benedet AL, Blennow K, et al. Differences between blood and cerebrospinal fluid glial fibrillary Acidic protein levels: The effect of sample stability. Alzheimers Dement. (2022) 18:1988–92.
84. Sheremata WA, Jy W, Delgado S, Minagar A, McLarty J, and Ahn Y. Interferon-beta1a reduces plasma CD31+ endothelial microparticles (CD31+EMP) in multiple sclerosis. J Neuroinflammation. (2006) 3:23.
85. Sáenz-Cuesta M, Haritz I, Tamara C-T, Maider M-C, Iñaki O-Q, Alvaro P, et al. Circulating microparticles reflect treatment effects and clinical status in multiple sclerosis. Biomarkers Med. (2014) 8:653–61.
86. Galazka G, Mycko MP, Selmaj I, Raine CS, and Selmaj KW. Multiple sclerosis: Serum-derived exosomes express myelin proteins. Mult Scler. (2018) 24:449–58.
87. Moseley CE, Virupakshaiah A, Forsthuber TG, Steinman L, Waubant E, and Zamvil SS. MOG CNS autoimmunity and MOGAD. Neurol Neuroimmunol Neuroinflamm. (2024) 11:e200275.
88. D’Anca M, Fenoglio C, Buccellato FR, Visconte C, Galimberti D, and Scarpini E. Extracellular vesicles in multiple sclerosis: role in the pathogenesis and potential usefulness as biomarkers and therapeutic tools. Cells. (2021) 10.
89. Bhargava P, Nogueras-Ortiz C, Chawla S, Bæk R, Jørgensen MM, and Kapogiannis D. Altered levels of toll-like receptors in circulating extracellular vesicles in multiple sclerosis. Cells. (2019) 8.
90. Bhargava P, Nogueras-Ortiz C, Kim S, Delgado-Peraza F, Calabresi PA, and Kapogiannis D. Synaptic and complement markers in extracellular vesicles in multiple sclerosis. Mult Scler. (2021) 27:509–18.
91. Nogueras-Ortiz CJ, Eren E, Yao P, Calzada E, Dunn C, Volpert O, et al. Single-extracellular vesicle (EV) analyses validate the use of L1 Cell Adhesion Molecule (L1CAM) as a reliable biomarker of neuron-derived EVs. J Extracell Vesicles. (2024) 13:e12459.
92. Li D, Zou S, Huang Z, Sun C, and Liu G. Isolation and quantification of L1CAM-positive extracellular vesicles on a chip as a potential biomarker for Parkinson’s Disease. J Extracell Vesicles. (2024) 13:e12467.
93. Mazzucco M, Mannheim W, Shetty SV, and Linden JR. CNS endothelial derived extracellular vesicles are biomarkers of active disease in multiple sclerosis. Fluids Barriers CNS. (2022) 19:13.
94. Martinsen V and Kursula P. Multiple sclerosis and myelin basic protein: insights into protein disorder and disease. Amino Acids. (2022) 54:99–109.
95. Sáenz-Cuesta M, Alberro A, Muñoz-Culla M, Osorio-Querejeta I, Fernandez-Mercado M, Lopetegui I, et al. The first dose of fingolimod affects circulating extracellular vesicles in multiple sclerosis patients. Int J Mol Sci. (2018) 19.
96. Verderio C, Muzio L, Turola E, Bergami A, Novellino L, Ruffini F, et al. Myeloid microvesicles are a marker and therapeutic target for neuroinflammation. Ann Neurol. (2012) 72:610–24.
97. Geraci F, Ragonese P, Barreca MM, Aliotta E, Mazzola MA, Realmuto S, et al. Differences in intercellular communication during clinical relapse and gadolinium-enhanced MRI in patients with relapsing remitting multiple sclerosis: A study of the composition of extracellular vesicles in cerebrospinal fluid. Front Cell Neurosci. (2018) 12:418.
Keywords: multiple sclerosis (MS), cerebrospinal fluid (CSF), brain-derived blood biomarkers, extracellular vesicles (EVs), magnetic resonance imaging (MRI)
Citation: Anandan S, Maciak K, Breinbauer R, Mostafavi S, Kvistad CE, Torkildsen O and Myhr KM (2025) Brain-derived blood biomarkers in multiple sclerosis—current trends and beyond. Front. Immunol. 16:1569503. doi: 10.3389/fimmu.2025.1569503
Received: 31 January 2025; Accepted: 26 May 2025;
Published: 16 June 2025.
Edited by:
Stella E. Tsirka, Stony Brook University, United StatesReviewed by:
Luisa María Villar, Ramón y Cajal University Hospital, SpainCopyright © 2025 Anandan, Maciak, Breinbauer, Mostafavi, Kvistad, Torkildsen and Myhr. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shamundeeswari Anandan, U2hhbXVuZGVlc3dhcmkuQW5hbmRhbkB1aWIubm8=; c2FtYW5hbmRoYW5AZ21haWwuY29t
 Shamundeeswari Anandan
Shamundeeswari Anandan Karina Maciak
Karina Maciak Regina Breinbauer
Regina Breinbauer Sepideh Mostafavi2
Sepideh Mostafavi2 Oivind Torkildsen
Oivind Torkildsen