- 1Department of General Surgery, The Sixth People’s Hospital of Huizhou, Huizhou, China
- 2Department of Internal Medicine, The Sixth People’s Hospital of Huizhou, Huizhou, China
Colorectal cancer (CRC) is among the most prevalent and lethal cancers globally, accounting for approximately 10% of all cancer cases and deaths. Regulatory T (Treg) cells, which accumulate in CRC tissue, suppress anti-tumor immune responses and facilitate tumor progression. This review discusses Treg cell origins and functions, along with the mechanisms by which Tregs influence CRC development. In addition, we highlight therapeutic strategies targeting Tregs-such as immune checkpoint inhibitors and combinatorial approaches-to enhance effector T cell responses. A deeper understanding of Treg-mediated immunosuppression in CRC may inform the design of more effective immunotherapies and precision medicine strategies.
1 Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide, contributing to 10% of cancer cases and deaths globally (1). It is a heterogeneous disease, with genetic and molecular variations affecting clinical outcomes and treatment responses (2, 3). Approximately 70% of CRC cases are sporadic, while 20%-30% are familial, with one-third linked to highly penetrant genetic mutations, referred to as hereditary CRC. The remaining familial cases involve low-expressivity susceptibility genes and polymorphisms influenced by environmental or genetic factors (4–6). Chronic inflammation, particularly in inflammatory bowel disease (IBD), also increases CRC risk (7, 8). Colorectal polyps or adenomas, arising from epithelial cells, are precursors to CRC, where genetic and epigenetic changes lead to malignant transformation, progressing through the adenoma-carcinoma sequence (2, 3).
Host immune status significantly influences CRC development. Immune cells play a critical role in tumor immunity by suppressing immune effector cells in the tumor microenvironment (TME) (9–12), facilitating immune evasion and tumor growth (13–17). Studies show increased Treg infiltration in CRC tissues compared to normal tissues, correlating with higher TNM stages and elevated mRNA expression of Foxp3, IL-10, and TGF-β1 in tumor-infiltrating CD4+ T cells (18). Peripheral blood Treg levels are also higher in CRC patients, with greater Treg infiltration in tumor-adjacent tissues and regional lymph nodes than in distant or non-regional nodes (19, 20). Depleting Tregs can enhance cancer immunotherapy efficacy (21), but risks autoimmune diseases by disrupting immune tolerance (22, 23). Effective immunotherapy should selectively target TME Tregs while preserving peripheral Tregs to avoid autoimmune responses (24–26).
While CRC treatment primarily relies on surgery, radiotherapy, and chemotherapy, targeted therapies and immunotherapies are increasingly utilized, advancing “immuno-precision medicine.” Tregs typically promote tumor progression by suppressing anti-tumor immunity but may also exert anti-inflammatory protective effects, particularly in early tumorigenesis, where excessive inflammation could drive malignancy (27). The dual roles of Tregs in CRC remain incompletely understood, necessitating further research to develop context-specific immunotherapeutic strategies. This review introduced the role of Treg cells in CRC and progress in immunotherapy targeting Treg cells.
2 Origins and classification of Treg cells
Treg cells are classified based on their developmental origins into natural regulatory T cells and peripherally induced regulatory T cells (28–31). Thymus-derived Treg cells, formally known as naturally occurring Treg (nTreg) cells, develop in the thymus from CD4+ single-positive thymocytes and are considered to exhibit a high affinity for self-peptide major histocompatibility complex (MHC) molecules through their T cell receptor (TCR) (32). In contrast, peripheral Treg (pTreg) cells are generated in the periphery following antigen encounter under the influence of various factors, such as IL-2 and TGF-β (33). The pathways for the generation of Treg cells in the TME differ from those in normal tissues. In the TME, multiple factors interact to influence immune cells generation (34, 35). Chemokines recruit Treg cells from the thymus, bone marrow, lymph nodes, and peripheral blood to the tumor site, where they proliferate. Dysfunctional antigen-presenting cells can also induce the differentiation and proliferation of Treg cells. Moreover, suppressive molecules in the TME can directly convert CD4+CD25+ T cells into CD4+CD25+ FoxP3+ Treg cells (36).
Treg cells are integral to immune tolerance and anti-inflammatory responses, comprising both CD4+ and CD8+ subtypes (37). Both subsets exhibit functional differences, with a prominent distinction in their recognition of antigens. CD8+ Treg cells recognize antigens presented by MHC-I molecules, which are expressed on nearly all nucleated cells, enabling them to be activated by virtually any cell and exert inhibitory functions. In contrast, CD4+ Treg cells are activated only by cells expressing MHC-II molecules (37). Foxp3, a member of the forkhead transcription factor family, is a key intracellular marker and the principal regulatory factor of Treg cells (38, 39). Human Foxp3+ CD4+ T cells can be classified into three subsets based on the expression of CD4, CD45RA, CD25, and Foxp3: naïve/resting Treg cells, effector/activated Treg (eTreg) cells, and non-Treg cells. Although Foxp3+ T cells can be induced from conventional T cells via TCR stimulation, these induced cells typically secrete inflammatory cytokines and lack immunosuppressive capabilities. In contrast, specific cytokines or microbiota can promote the differentiation of CD4+CD25- T cells into functional Treg cells with immunosuppressive properties. Conversely, certain cytokines or specific microbiota can induce Treg cells with immune-suppressive functions from CD4+CD25-T cells. In contrast, non-Treg cells do not possess immune-suppressive functions but instead produce inflammatory cytokines, such as interferon (IFN)-γ and IL-17 (36, 40).
3 Function of Treg cells
Treg cells possess two major functional characteristics: immune tolerance and immune suppression. Immune tolerance refers to the inability of Treg cells to respond to high concentrations of interleukin (IL)-2 stimulation alone, solid-phase coating, or soluble anti-CD3 monoclonal antibodies (mAb), as well as the lack of response to combined CD3 and CD8 monoclonal antibody stimulation. Additionally, Treg cells themselves do not secrete IL-2. Immune suppression refers to the ability of activated Treg cells to nonspecifically suppress the activation and proliferation of T cells through various mechanisms, including inhibition of both CD4+ and CD8+ T cells.
The immune suppressive function of Treg cells is mediated through multiple mechanisms, including downregulation of co-stimulatory signals (41), IL-2 consumption, secretion of immunosuppressive cytokines (e.g., IL-10 and IL-35) (42, 43), and production of immunosuppressive metabolites. Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) is crucial for Treg cell-mediated suppression, as it prevents aberrant autoimmune responses and excessive immune activation, thereby protecting the host from autoimmune attacks (44, 45). Treg cells also inhibit anti-tumor immune responses, especially tumor antigen-specific T cell responses (46). IL-2 is essential for effector T cell activation and survival. The high-affinity IL-2 receptor, comprising CD25 (α-chain), CD122 (β-chain), and CD132 (γ-chain), is expressed on most Treg cells, which consume IL-2 and limit its availability in the TME. This depletion impairs effector T cell activation (47). IL-10 and IL-35 further suppress T cell function by downregulating MHC and co-stimulatory molecules on antigen-presenting cells (APCs) (42).
Treg cells also exhibit granzyme-dependent immune suppression, releasing granzymes and perforins that promote cytolysis by NK cells and cytotoxic T lymphocytes (CTLs) (48). In the TME (49), Treg cells amplify in an antigen-specific manner, displaying distinct T cell receptor (TCR) repertoires compared to conventional CD4+ T cells (50). Recent studies have shown that Treg cells within the TME are highly activated and phenotypically differentiated (51). Treg cells strongly suppress anti-tumor immune responses through the function of hypoxia-inducible factor 1α (HIF-1α). In terms of metabolism, anti-tumor immune cells such as CD8+ T cells primarily utilize glycolysis for activation. Tumor cells consume glucose, reducing blood glucose levels in the TME (Warburg effect), thereby inhibiting the activation process of CD8+ T cells (52). Meanwhile, Treg cells proliferate and exert their immunosuppressive functions by metabolizing abundant lactate (53) and fatty acids (54) in the TME. In glucose-enriched TME, such as in liver metastatic lesions, Treg cells are activated by large amounts of lactate. Consequently, Treg cells acquire high programmed cell death protein-1 (PD-1) expression, and their activation is further enhanced by anti-PD-1 monoclonal antibody (mAb) treatment, leading to resistance to PD-1/PD-L1 blockade therapy (55). Besides, lymphocyte activation gene-3 (LAG-3) and T-cell immunoglobulin and mucin-domain containing-3 (TIM-3) are emerging immune checkpoints that can further contribute to T cell exhaustion and Treg-mediated immunosuppression in CRC (56).
4 Role of Treg cells in CRC
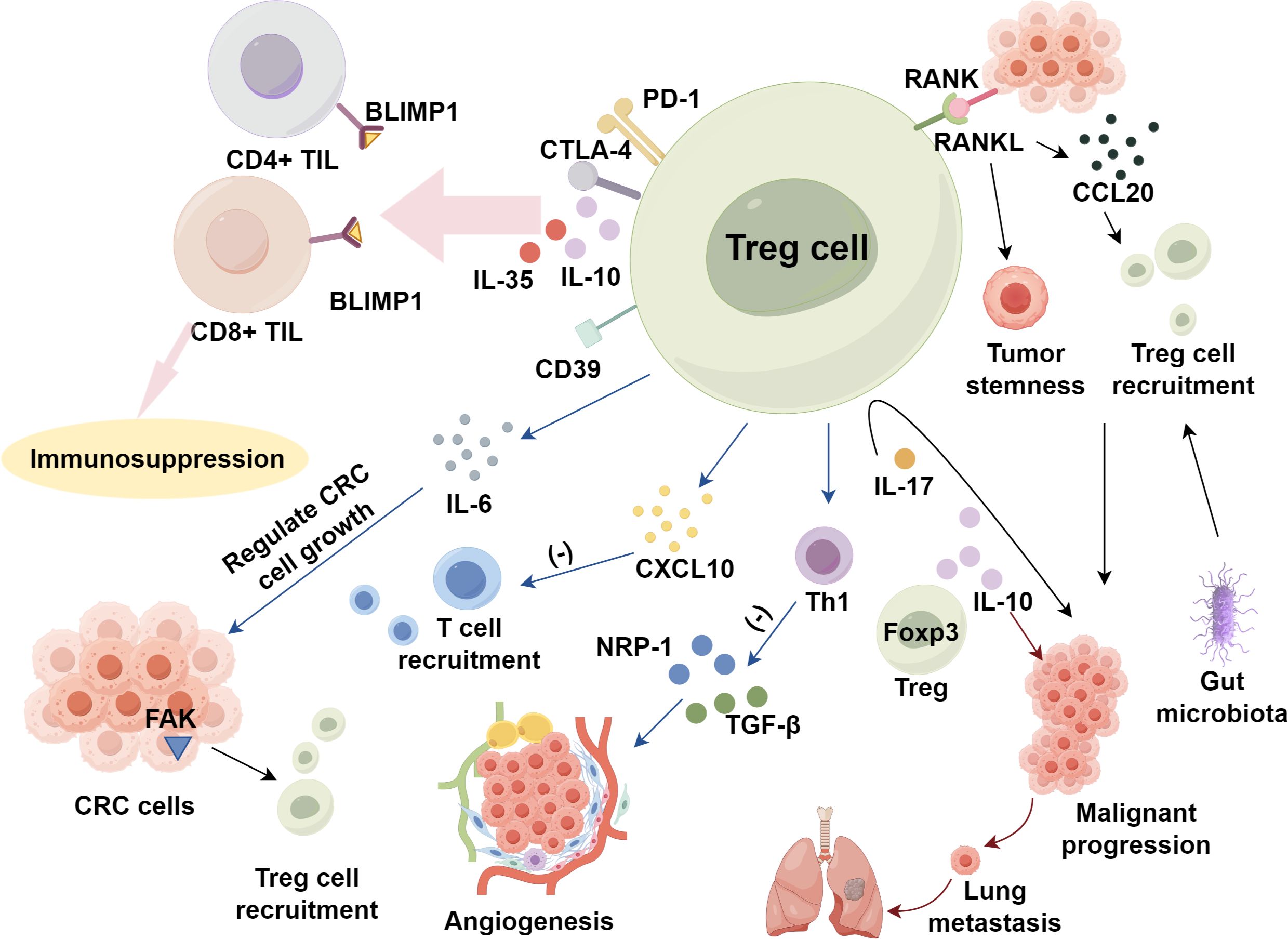
In CRC, Tregs heighten their suppressive function by elevating surface molecules such as CD39, CTLA-4, and PD-1 (57, 58), and by secreting IL-10 and IL-35, which jointly modulate the B-lymphocyte-induced maturation protein 1 (BLIMP1) inhibitory axis in CD4+ and CD8+ TILs (59). Tregs also dampen T cell recruitment to tumor sites by reducing CXC chemokine ligand 10 (CXCL10) (60) and can regulate CRC cell growth via IL-6 modulation (61). Additionally, by suppressing Th1 cell activity, Tregs indirectly or directly promote CRC angiogenesis through the inhibition of Th1-derived angiogenesis inhibitors, such as transforming growth factor-beta (TGF-β), and the production of angiogenic factors like neuropilin-1 (NRP-1) (62, 63). Foxp3+ Treg-derived IL-10 promotes lung metastasis in CRC by acting on both Foxp3+ Tregs and myeloid cells (64). However, the role of IL-23R signaling in Treg cells has been found to differ significantly between murine models of sporadic and inflammation-associated CRC. Inflammatory factors are important in diseases’ development and progression (65–68). In inflammation-related CRC, IL-23R signaling in Tregs suppresses carcinogenesis, whereas in sporadic CRC, it facilitates tumor development. These findings underscore the importance of the underlying etiological factors in CRC, which may have distinct impacts on the disease’s progression (69).
Despite the fact that the immunosuppressive role facilitates tumor cell evasion of anti-tumor immunity (70–73), some studies suggest that, at least in the early stages of inflammation-associated tumorigenesis, Treg cells may inhibit tumor progression by suppressing inflammatory responses (74). However, the impact of FoxP3+ Tregs on CRC prognosis remains controversial. Some studies indicate a correlation between Treg infiltration and favorable prognosis (75), while others suggest an association with poor prognosis (76–78). Saito et al. (76) reported that this discrepancy might be due to the existence of different FoxP3+ T cell subsets, namely eTregs and non-Tregs, which are difficult to distinguish through immunohistochemistry. Compared to CRC dominated by non-Treg infiltration, CRC patients with high eTreg infiltration exhibit worse prognosis.
CRC cells and the TME further regulate Treg accumulation by secreting chemokines and cytokines (79). Focal adhesion kinase (FAK) in CRC cells enhances Treg recruitment (80). While the signaling of TNF receptor superfamily member 11a (TNFRSF11a, RANK) and its ligand TNF receptor superfamily member 11 (TNFRSF11, RANKL) induces CRC cells to produce CC motif chemokine ligand 20 (CCL20), leading to Treg recruitment through CCL20-CCR6 pathway and promoting tumor stemness and malignant progression (81). IL-17 directly activates Tregs, enhancing their maturation and functionality. This signaling mechanism forms a negative feedback loop that regulates inflammation, which contributes to cancer progression in CRC (82). Furthermore, gut microbiota may participate in immune suppression by promoting Treg accumulation (83) (Figure 1).
5 Targeting Treg cells in CRC immunotherapy
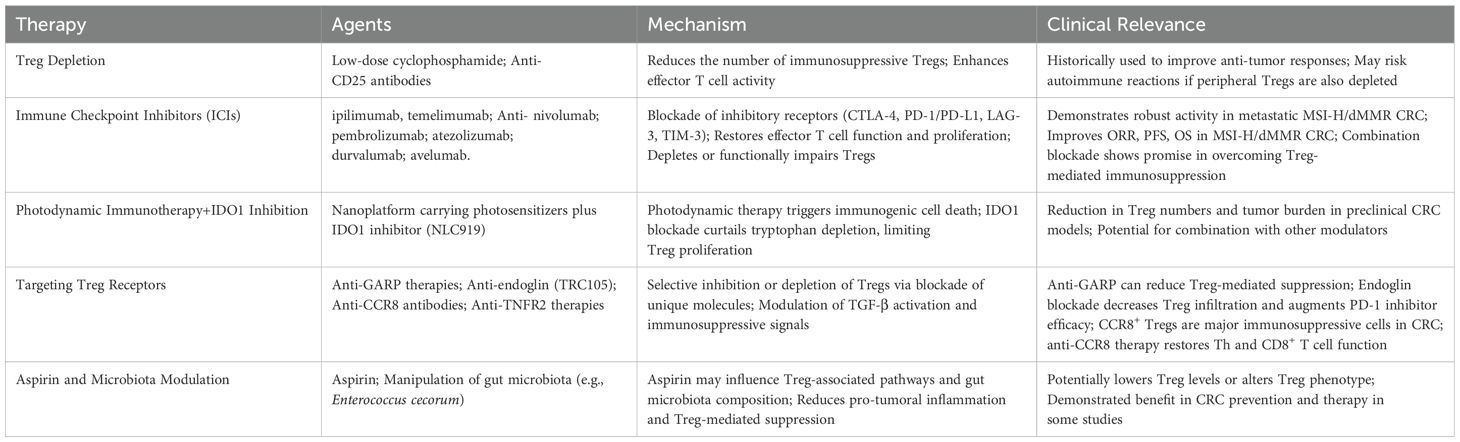
Peripheral immune tolerance mediated by Treg cells presents a significant challenge in immunotherapy. Therefore, to eliminate the immunosuppressive activity of Tregs during treatment, strategies such as depleting or inhibiting Tregs are often considered. These approaches aim to enhance the activity and accumulation of effector T cells, thereby achieving the therapeutic goal of immunotherapy. Studies have indicated that since the 20th century, researchers have attempted to treat cancer through Treg depletion, using low-dose cyclophosphamide or targeting specific surface markers such as CD25 (36). Immune checkpoint inhibitors have emerged as a prominent area of research in recent years, demonstrating substantial efficacy in various cancers, including metastatic MSI-H/dMMR CRC (84, 85). It has been confirmed that Tregs can express several surface markers, including CTLA-4, PD-1, and its ligand PD-L1, further highlighting the importance of targeting Tregs in immunotherapy (86). Moreover, combinatorial therapies targeting Treg surface receptors, such as glycoprotein A repetitions predominant (GARP), are under investigation (87, 88).
5.1 Immune checkpoint inhibitors
ICIs, such as CTLA-4 and PD-1/PD-L1 inhibitors, are widely used in cancer treatment, with MSI-H/dMMR identified as a biomarker for their efficacy. CTLA-4, expressed on activated T cells, inhibits T cell activation by competing with CD28 for CD80/CD86 binding, thereby promoting tumor cell survival. Anti-CTLA-4 therapy, like ipilimumab and temelimumab, induces Treg depletion through antibody-dependent cytotoxicity, enhancing T cell proliferation and antitumor immunity (58). These inhibitors are primarily used for metastatic melanoma, non-small cell lung cancer, renal, and bladder cancers, and in CRC, they are often combined with PD-1/PD-L1 inhibitors (86, 89).
PD-1, interacting with its ligands PD-L1 and PD-L2, suppresses T cell function, contributing to T cell exhaustion (36, 90, 91). PD-1/PD-L1 inhibitors, including pembrolizumab, nivolumab, durvalumab, atezolizumab, and avelumab, can restore effector T cell function and increase the CD8+ Treg ratio to negatively regulate Treg numbers (86). Recent clinical studies have shown that metastatic MSI-H/dMMR CRC is more likely to benefit from PD-1 inhibitors, whereas microsatellite stable (MSS)/mismatch repair proficient (pMMR) CRC shows minimal response (84). Andre et al. (92) evaluated pembrolizumab as a first-line treatment compared with standard chemotherapy in metastatic dMMR CRC, showing that pembrolizumab improved median progression-free survival (PFS), with fewer grade 3-5 drug-related adverse events than standard chemotherapy. In CRC, targeting LAG-3 or TIM-3 in combination with PD-1 or CTLA-4 blockade could potentially synergize to overcome Treg-mediated immunosuppression, thereby enhancing antitumor immunity (86, 93). These newer checkpoint molecules represent promising targets for combination immunotherapies aiming to modulate Tregs in the TME.
Several clinical studies have investigated low-dose ipilimumab combined with nivolumab in metastatic MSI-H/dMMR CRC, reporting objective response rates of 55-69%, with improved PFS and overall survival (OS) compared to PD-1 inhibitors alone (87, 94). The combination of low-dose ipilimumab and nivolumab has demonstrated robust and durable clinical benefits, potentially providing a new first-line treatment option for metastatic MSI-H/dMMR CRC patients. Additionally, combining PD-1 inhibitors with vascular endothelial growth factor receptor (VEGFR) inhibitors offers another therapeutic option for CRC. Activation of VEGF and its receptor VEGFR is associated with the immunosuppressive tumor microenvironment, contributing to tumor immune evasion by upregulating inhibitory immune checkpoint expression and recruiting immunosuppressive cells, including Tregs and myeloid-derived suppressor cells (95). A retrospective study on the use of VEGFR inhibitor regorafenib combined with PD-1 inhibitors in MSS/pMMR CRC patients revealed that while no objective responses were observed, the disease control rate was 78.3% (96). PLCG2 is an important oncogene and prognostic biomarker (97). Targeting PLCG2 can inhibit tumor progression, regulate the tumor immune microenvironment, and enhance immune checkpoint blockade therapy in CRC (98). Elevated TNF-α levels in the CRC tumor microenvironment promote the upregulation of CCR8 on Tregs via the TNFR2/NF-κB signaling pathway and FOXP3 transcription factor activity. Inhibition or depletion of TNFR2 significantly reduces the infiltration of CCR8+ Tregs, which in turn suppresses tumor progression and improves the efficacy of anti-PD1 therapy (99). In recent clinical trials (NCT04126733), regorafenib plus nivolumab have shown encouraging activity in CRC patients (100).
5.2 Photodynamic immunotherapy combined with IDO1 inhibition
To achieve efficient immunotherapy, Hu et al. designed a supramolecular prodrug nanocarrier for multiple immune modulators. By integrating photosensitizers, such as hyaluronic acid, magnesium phthalocyanine A, and indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor NLC919 into the nanocarrier, the combination of photodynamic immunotherapy and IDO1 blockade led to a reduction in Treg numbers and inhibited tumor growth in a CRC mouse model (CT26), extending survival (101, 102). Further studies are needed to explore whether this nanoplatform can improve the efficacy of other immune modulators.
5.3 Targeting Treg surface receptors and markers
Glycoprotein A repetitions predominant (GARP) is expressed on the surface of activated Tregs, where it binds and activates TGF-β, playing a role in disease progression and immune evasion. Salem et al. (88) observed that Tregs lacking GARP had a reduced ability to suppress inflammation, leading to improved antitumor immunity and slower tumor progression in a colitis-associated colorectal cancer model. However, a study by Vermeersch et al. (103) in a murine colorectal cancer model showed that knocking out GARP did not delay tumor growth, suggesting that the absence of GARP is insufficient to affect Treg-mediated immunosuppressive activity. The potential of GARP inhibitors as a therapeutic target in CRC requires further investigation. Endoglin, an auxiliary receptor for TGF-β, is highly expressed on endothelial cells and cancer-associated fibroblasts. Schoonderwoerd et al. (104) reported that endoglin is highly expressed on Tregs in both murine and human CRC tissues, but absent from conventional CD4+ T cells. Anti-endoglin antibody (TRC105) has shown inhibition of angiogenesis and tumor metastasis in animal models of breast cancer (105). In CRC animal models, TRC105 treatment reduced Treg numbers in tumors, and the combination of TRC105 with PD-1 inhibitors significantly enhanced the efficacy of PD-1 inhibitors in CRC models (subcutaneous, orthotopic, and chemically induced) (104). The chemokine receptor CCR8 is selectively expressed on tumor-infiltrating Tregs. CCR8+ Tregs play a crucial role in the immune-suppressive tumor microenvironment in CRC by inhibiting the function of CD4+ Th and CD8+ T cells. Anti-CCR8 antibody therapy, targeting tumor-infiltrating CCR8+ Tregs, has the potential to restore the function of CD4+ Th and CD8+ T cells in CRC, thereby inducing antitumor immunity (106). Aspirin as an antitumor agent, influence key processes such as apoptosis, proliferation, metastasis, and senescence in cancer cells (107). Aspirin may help prevent colorectal cancer by modulating the levels of Enterococcus cecorum and TIGIT+ Treg cells, highlighting its potential therapeutic value in CRC treatment (108) (Table 1).
6 Conclusion
Treg cells play a pivotal role in the immune landscape of CRC, facilitating tumor progression by suppressing anti-tumor immune responses and promoting tumor immune evasion. Their accumulation within the TME is driven by various factors, including chemokine signaling, cytokine modulation, and interactions with CRC cells. While Treg cells may offer protective effects in the early stages of inflammation-associated tumorigenesis, their overall contribution to CRC progression is largely detrimental. The dual nature of Treg cell functions, including both immune suppression and regulation of inflammatory responses, underscores the complexity of targeting Tregs in CRC therapy. Future research is urgently required to disentangle how Tregs switch from an anti-inflammatory role to a predominantly pro-tumor function over the course of CRC development. Elucidating the molecular and cellular underpinnings of this transition could pave the way for innovative immunotherapies that selectively counteract the immunosuppressive properties of Tregs while preserving their beneficial impacts on early-stage inflammation. Such targeted strategies hold the promise of improving clinical outcomes and advancing precision medicine in CRC. Besides, the development of therapies aimed at selectively depleting or inhibiting Treg cells within the TME, while preserving their peripheral functions, holds great promise for enhancing the efficacy of immunotherapy in CRC. Future research should focus on identifying specific Treg cell subsets, optimizing therapeutic strategies, and understanding the interplay between Treg cells and other immune components in the TME to achieve better clinical outcomes for CRC patients.
Author contributions
HY: Writing – original draft. RY: Writing – original draft. ML: Writing – original draft. DL: Writing – original draft, Writing – review & editing. YX: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Figure was drawn via FigDraw website.
Conflict of interest
The authors declare that no competing financial interests or commercial relationships have influenced the research presented herein.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Singh A. Global burden of five major types of gastrointestinal cancer. Prz Gastroenterol. (2024) 19:236–54. doi: 10.5114/pg.2024.141834
2. Yamauchi M, Lochhead P, Morikawa T, Huttenhower C, Chan AT, Giovannucci E, et al. Colorectal cancer: a tale of two sides or a continuum? Gut. (2012) 61:794–7. doi: 10.1136/gutjnl-2012-302014doi: 10.1136/gutjnl-2012-302014
3. Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. (2009) 361:2449–60. doi: 10.1056/NEJMra0804588
4. Pearlman R, Frankel WL, Swanson B, Zhao W, Yilmaz A, Miller K, et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA Oncol. (2017) 3:464–71. doi: 10.1001/jamaoncol.2016.5194
5. Stigliano V, Sanchez-Mete L, Martayan A, Anti M. Early-onset colorectal cancer: a sporadic or inherited disease? World J Gastroenterol. (2014) 20:12420–30. doi: 10.3748/wjg.v20.i35.12420
6. Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. (2010) 138:2044–58. doi: 10.1053/j.gastro.2010.01.054
7. Grivennikov SI. Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol. (2013) 35:229–44. doi: 10.1007/s00281-012-0352-6
8. Sturlan S, Oberhuber G, Beinhauer BG, Tichy B, Kappel S, Wang J, et al. Interleukin-10-deficient mice and inflammatory bowel disease associated cancer development. Carcinogenesis. (2001) 22:665–71. doi: 10.1093/carcin/22.4.665
9. Xie H, Xi X, Lei T, Liu H, Xia Z. CD8(+) T cell exhaustion in the tumor microenvironment of breast cancer. Front Immunol. (2024) 15:1507283. doi: 10.3389/fimmu.2024.1507283
10. Deng Y, Shi M, Yi L, Naveed Khan M, Xia Z, Li X. Eliminating a barrier: Aiming at VISTA, reversing MDSC-mediated T cell suppression in the tumor microenvironment. Heliyon. (2024) 10:e37060. doi: 10.1016/j.heliyon.2024.e37060
11. Zhang X, Zhang P, Cong A, Feng Y, Chi H, Xia Z, et al. Unraveling molecular networks in thymic epithelial tumors: deciphering the unique signatures. Front Immunol. (2023) 14:1264325. doi: 10.3389/fimmu.2023.1264325
12. Xia Z, Chen S, He M, Li B, Deng Y, Yi L, et al. Editorial: Targeting metabolism to activate T cells and enhance the efficacy of checkpoint blockade immunotherapy in solid tumors. Front Immunol. (2023) 14:1247178. doi: 10.3389/fimmu.2023.1247178
13. Zhang J, Peng G, Chi H, Yang J, Xie X, Song G, et al. CD8 + T-cell marker genes reveal different immune subtypes of oral lichen planus by integrating single-cell RNA-seq and bulk RNA-sequencing. BMC Oral Health. (2023) 23:464. doi: 10.1186/s12903-023-03138-0
14. Zhang X, Zhuge J, Liu J, Xia Z, Wang H, Gao Q, et al. Prognostic signatures of sphingolipids: Understanding the immune landscape and predictive role in immunotherapy response and outcomes of hepatocellular carcinoma. Front Immunol. (2023) 14:1153423. doi: 10.3389/fimmu.2023.1153423
15. Xiong J, Chi H, Yang G, Zhao S, Zhang J, Tran LJ, et al. Revolutionizing anti-tumor therapy: unleashing the potential of B cell-derived exosomes. Front Immunol. (2023) 14:1188760. doi: 10.3389/fimmu.2023.1188760
16. Gong X, Chi H, Strohmer DF, Teichmann AT, Xia Z, Wang Q. Exosomes: A potential tool for immunotherapy of ovarian cancer. Front Immunol. (2022) 13:1089410. doi: 10.3389/fimmu.2022.1089410
17. Zhao Y, Wei K, Chi H, Xia Z, Li X. IL-7: A promising adjuvant ensuring effective T cell responses and memory in combination with cancer vaccines? Front Immunol. (2022) 13:1022808. doi: 10.3389/fimmu.2022.1022808
18. Ma H, Gao W, Sun X, Wang W. STAT5 and TET2 cooperate to regulate FOXP3-TSDR demethylation in CD4(+) T cells of patients with colorectal cancer. J Immunol Res. (2018) 2018:6985031. doi: 10.1155/2018/6985031
19. Krijgsman D, de Vries NL, Skovbo A, Andersen MN, Swets M, Bastiaannet E, et al. Characterization of circulating T-, NK-, and NKT cell subsets in patients with colorectal cancer: the peripheral blood immune cell profile. Cancer Immunol Immunother. (2019) 68:1011–24. doi: 10.1007/s00262-019-02343-7
20. Kazama K, Otake J, Satoyoshi T, Shiozawa M, Sugano N, Sato S, et al. Distribution of regulatory T-cells and other phenotypes of T-cells in tumors and regional lymph nodes of colorectal cancer patients. In Vivo. (2020) 34:849–56. doi: 10.21873/invivo.11848
21. Nishikawa H, Koyama S. Mechanisms of regulatory T cell infiltration in tumors: implications for innovative immune precision therapies. J Immunother Cancer. (2021) 9:e002591corr1. doi: 10.1136/jitc-2021-002591
22. Liu Y, Zheng P. Preserving the CTLA-4 checkpoint for safer and more effective cancer immunotherapy. Trends Pharmacol Sci. (2020) 41:4–12. doi: 10.1016/j.tips.2019.11.003
23. Larocca C, Kupper TS, LeBoeuf NR. Mogamulizumab forecast: clearer patients, with a slight chance of immune mayhem. Clin Cancer Res. (2019) 25:7272–4. doi: 10.1158/1078-0432.CCR-19-2742
24. Jin W, Yang Q, Chi H, Wei K, Zhang P, Zhao G, et al. Ensemble deep learning enhanced with self-attention for predicting immunotherapeutic responses to cancers. Front Immunol. (2022) 13:1025330. doi: 10.3389/fimmu.2022.1025330
25. Chi H, Gao X, Xia Z, Yu W, Yin X, Pan Y, et al. FAM family gene prediction model reveals heterogeneity, stemness and immune microenvironment of UCEC. Front Mol Biosci. (2023) 10:1200335. doi: 10.3389/fmolb.2023.1200335
26. Ren Q, Zhang P, Lin H, Feng Y, Chi H, Zhang X, et al. A novel signature predicts prognosis and immunotherapy in lung adenocarcinoma based on cancer-associated fibroblasts. Front Immunol. (2023) 14:1201573. doi: 10.3389/fimmu.2023.1201573
27. Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. (2017) 27:109–18. doi: 10.1038/cr.2016.151
28. Lee W, Lee GR. Transcriptional regulation and development of regulatory T cells. Exp Mol Med. (2018) 50:e456. doi: 10.1038/emm.2017.313
29. Sharma A, Rudra D. Emerging functions of regulatory T cells in tissue homeostasis. Front Immunol. (2018) 9:883. doi: 10.3389/fimmu.2018.00883
30. Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression - implications for anticancer therapy. Nat Rev Clin Oncol. (2019) 16:356–71. doi: 10.1038/s41571-019-0175-7
31. Shen K, Wang H, Xue S, Wang L, Ren M, Gao Z, et al. Genome-wide screening and immune landscape suggest a potential-m6A-related lncRNA risk signature for predicting prognosis of melanoma. Ann Transl Med. (2022) 10:241. doi: 10.21037/atm-21-4402
32. Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. (2012) 30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623
33. Dominguez-Villar M, Hafler DA. Regulatory T cells in autoimmune disease. Nat Immunol. (2018) 19:665–73. doi: 10.1038/s41590-018-0120-4
34. Wang Y, Wang J, Liu J, Zhu H. Immune-related diagnostic markers for benign prostatic hyperplasia and their potential as drug targets. Front Immunol. (2024) 15:1516362. doi: 10.3389/fimmu.2024.1516362
35. Wang JF, Wang JS, Liu Y, Ji B, Ding BC, Wang YX, et al. Knockdown of integrin beta1 inhibits proliferation and promotes apoptosis in bladder cancer cells. Biofactors. (2025) 51:e2150. doi: 10.1002/biof.2150
36. Ohue Y, Nishikawa H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target?Cancer Sci. (2019) 110:2080–9. doi: 10.1111/cas.2019.110.issue-7
37. Flippe L, Bezie S, Anegon I, Guillonneau C. Future prospects for CD8(+) regulatory T cells in immune tolerance. Immunol Rev. (2019) 292:209–24. doi: 10.1111/imr.v292.1
38. Hori S, Nomura T, Sakaguchi SJS. Control of regulatory T cell development by the transcription factor Foxp3. Science. (2003) 299:1057–61. doi: 10.1126/science.1079490
39. Fontenot J, Gavin M, Rudensky AJJI. Pillars Article: Foxp3 programs the development and function of CD4, CD25, regulatory T cells.J Immunol. (2017) 198:986–92. doi: 10.1093/jimmunol/198.3.986
40. Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. (2009) 30:899–911. doi: 10.1016/j.immuni.2009.03.019
41. Maeda Y, Nishikawa H, Sugiyama D, Ha D, Hamaguchi M, Saito T, et al. Detection of self-reactive CD8(+) T cells with an anergic phenotype in healthy individuals. Science. (2014) 346:1536–40. doi: 10.1126/science.aaa1292
42. Sawant DV, Hamilton K, Vignali DA. Interleukin-35: expanding its job profile. J Interferon Cytokine Res. (2015) 35:499–512. doi: 10.1089/jir.2015.0015
43. Wang Y, Wang X. A pan-cancer analysis of heat-shock protein 90 beta1(HSP90B1) in human tumours. Biomolecules. (2022) 12:1377. doi: 10.3390/biom12101377
44. Boyman O, Cho JH, Sprent J. The role of interleukin-2 in memory CD8 cell differentiation. Adv Exp Med Biol. (2010) 684:28–41. doi: 10.1007/978-1-4419-6451-9_3
45. Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. (2008) 322:271–5. doi: 10.1126/science.1160062
46. Yano H, Andrews LP, Workman CJ, Vignali DAA. Intratumoral regulatory T cells: markers, subsets and their impact on anti-tumor immunity. Immunology. (2019) 157:232–47. doi: 10.1111/imm.2019.157.issue-3
47. Dikiy S, Li J, Bai L, Jiang M, Janke L, Zong X, et al. A distal Foxp3 enhancer enables interleukin-2 dependent thymic Treg cell lineage commitment for robust immune tolerance. Immunity. (2021) 54:931–946 e911. doi: 10.1016/j.immuni.2021.03.020
48. Hariyanto AD, Permata TBM, Gondhowiardjo SA. Role of CD4(+)CD25(+)FOXP3(+) T(Reg) cells on tumor immunity. Immunol Med. (2022) 45:94–107. doi: 10.1080/25785826.2021.1975228
49. Wang Y, Zhu H, Wang X. Prognosis and immune infiltration analysis of endoplasmic reticulum stress-related genes in bladder urothelial carcinoma. Front Genet. (2022) 13:965100. doi: 10.3389/fgene.2022.965100
50. Ahmadzadeh M, Pasetto A, Jia L, Deniger DC, Stevanovic S, Robbins PF, et al. Tumor-infiltrating human CD4(+) regulatory T cells display a distinct TCR repertoire and exhibit tumor and neoantigen reactivity. Sci Immunol. (2019) 4:eaao4310. doi: 10.1126/sciimmunol.aao4310
51. Itahashi K, Irie T, Yuda J, Kumagai S, Tanegashima T, Lin YT, et al. BATF epigenetically and transcriptionally controls the activation program of regulatory T cells in human tumors. Sci Immunol. (2022) 7:eabk0957. doi: 10.1126/sciimmunol.abk0957
52. Zhang L, Romero P. Metabolic control of CD8(+) T cell fate decisions and antitumor immunity. Trends Mol Med. (2018) 24:30–48. doi: 10.1016/j.molmed.2017.11.005
53. Watson MJ, Vignali PDA, Mullett SJ, Overacre-Delgoffe AE, Peralta RM, Grebinoski S, et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature. (2021) 591:645–51. doi: 10.1038/s41586-020-03045-2
54. Lim SA, Wei J, Nguyen TM, Shi H, Su W, Palacios G, et al. Lipid signalling enforces functional specialization of T(reg) cells in tumours. Nature. (2021) 591:306–11. doi: 10.1038/s41586-021-03235-6
55. Kumagai S, Koyama S, Itahashi K, Tanegashima T, Lin YT, Togashi Y, et al. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell. (2022) 40:201–218 e209. doi: 10.1016/j.ccell.2022.01.001
56. Olguín JE, Medina-Andrade I, Rodríguez T, Rodríguez-Sosa M, Terrazas LIJC. Relevance of regulatory T cells during colorectal cancer development. Cancers (Basel). (2020) 12:1888. doi: 10.3390/cancers12071888
57. Ahlmanner F, Sundstrom P, Akeus P, Eklof J, Borjesson L, Gustavsson B, et al. CD39(+) regulatory T cells accumulate in colon adenocarcinomas and display markers of increased suppressive function. Oncotarget. (2018) 9:36993–7007. doi: 10.18632/oncotarget.26435
58. Sasidharan Nair V, Elkord EJI. biology c: Immune checkpoint inhibitors in cancer therapy: a focus on T-regulatory cells. Immunol Cell Biol. (2018) 96:21–33. doi: 10.1111/imcb.2018.96.issue-1
59. Sawant DV, Yano H, Chikina M, Zhang Q, Liao M, Liu C, et al. Adaptive plasticity of IL-10(+) and IL-35(+) T(reg) cells cooperatively promotes tumor T cell exhaustion. Nat Immunol. (2019) 20:724–35. doi: 10.1038/s41590-019-0346-9
60. Akeus P, Szeponik L, Ahlmanner F, Sundstrom P, Alsen S, Gustavsson B, et al. Regulatory T cells control endothelial chemokine production and migration of T cells into intestinal tumors of APC(min/+) mice. Cancer Immunol Immunother. (2018) 67:1067–77. doi: 10.1007/s00262-018-2161-9
61. De Simone V, Franze E, Ronchetti G, Colantoni A, Fantini MC, Di Fusco D, et al. Th17-type cytokines, IL-6 and TNF-alpha synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene. (2015) 34:3493–503. doi: 10.1038/onc.2014.286
62. Shen E, Zhao K, Wu C, Yang B. The suppressive effect of CD25+Treg cells on Th1 differentiation requires cell-cell contact partially via TGF-beta production. Cell Biol Int. (2011) 35:705–12. doi: 10.1042/CBI20100528
63. Chaudhary B, Elkord E. Novel expression of Neuropilin 1 on human tumor-infiltrating lymphocytes in colorectal cancer liver metastases. Expert Opin Ther Targets. (2015) 19:147–61. doi: 10.1517/14728222.2014.977784
64. Shiri AM, Fard-Aghaie M, Bedke T, Papazoglou ED, Sabihi M, Zazara DE, et al. Foxp3 + Treg-derived IL-10 promotes colorectal cancer-derived lung metastasis. Sci Rep. (2024) 14:30483. doi: 10.1038/s41598-024-80437-8
65. Zhai X, Zhang H, Xia Z, Liu M, Du G, Jiang Z, et al. Oxytocin alleviates liver fibrosis via hepatic macrophages. JHEP Rep. (2024) 6:101032. doi: 10.1016/j.jhepr.2024.101032
66. Xiao J, Lin H, Liu B, Xia Z, Zhang J, Jin J. Decreased S1P and SPHK2 are involved in pancreatic acinar cell injury. biomark Med. (2019) 13:627–37. doi: 10.2217/bmm-2018-0404
67. Xiao J, Huang K, Lin H, Xia Z, Zhang J, Li D, et al. Mogroside II(E) inhibits digestive enzymes via suppression of interleukin 9/interleukin 9 receptor signalling in acute pancreatitis. Front Pharmacol. (2020) 11:859. doi: 10.3389/fphar.2020.00859
68. Zhang H, Xia T, Xia Z, Zhou H, Li Z, Wang W, et al. KIF18A inactivates hepatic stellate cells and alleviates liver fibrosis through the TTC3/Akt/mTOR pathway. Cell Mol Life Sci. (2024) 81:96. doi: 10.1007/s00018-024-05114-5
69. Jacobse J, Pilat JM, Li J, Brown RE, Kwag A, Buendia MA, et al. Distinct roles for interleukin-23 receptor signaling in regulatory T cells in sporadic and inflammation-associated carcinogenesis. Front Oncol. (2023) 13:1276743. doi: 10.3389/fonc.2023.1276743
70. Wang L, Shen K, Gao Z, Ren M, Wei C, Yang Y, et al. Melanoma Derived Exosomes Amplify Radiotherapy Induced Abscopal Effect via IRF7/I-IFN Axis in Macrophages. Adv Sci (Weinh). (2024) 11:e2304991. doi: 10.1002/advs.202304991
71. Wei C, Sun W, Shen K, Zhong J, Liu W, Gao Z, et al. Delineating the early dissemination mechanisms of acral melanoma by integrating single-cell and spatial transcriptomic analyses. Nat Commun. (2023) 14:8119. doi: 10.1038/s41467-023-43980-y
72. Liu T, Li C, Zhang J, Hu H, Li C. Unveiling efferocytosis-related signatures through the integration of single-cell analysis and machine learning: a predictive framework for prognosis and immunotherapy response in hepatocellular carcinoma. Front Immunol. (2023) 14:1237350. doi: 10.3389/fimmu.2023.1237350
73. Zhang C, Sun D, Li C, Liu Y, Zhou Y, Zhang J. Development of cancer-associated fibroblasts subtype and prognostic model in gastric cancer and the landscape of tumor microenvironment. Int J Biochem Cell Biol. (2022) 152:106309. doi: 10.1016/j.biocel.2022.106309
74. Erdman SE, Sohn JJ, Rao VP, Nambiar PR, Ge Z, Fox JG, et al. CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res. (2005) 65:3998–4004. doi: 10.1158/0008-5472.CAN-04-3104
75. Nakagawa K, Tanaka K, Homma Y, Nojiri K, Kumamoto T, Takeda K, et al. Low infiltration of peritumoral regulatory T cells predicts worse outcome following resection of colorectal liver metastases. Ann Surg Oncol. (2015) 22:180–6. doi: 10.1245/s10434-014-3974-1
76. Saito T, Nishikawa H, Wada H, Nagano Y, Sugiyama D, Atarashi K, et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. (2016) 22:679–84. doi: 10.1038/nm.4086
77. Sideras K, Galjart B, Vasaturo A, Pedroza-Gonzalez A, Biermann K, Mancham S, et al. Prognostic value of intra-tumoral CD8(+)/FoxP3(+) lymphocyte ratio in patients with resected colorectal cancer liver metastasis. J Surg Oncol. (2018) 118:68–76. doi: 10.1002/jso.v118.1
78. Liu Y, Cheng L, Song X, Li C, Zhang J, Wang L. A TP53-associated immune prognostic signature for the prediction of the overall survival and therapeutic responses in pancreatic cancer. Math Biosci Eng. (2022) 19:191–208. doi: 10.3934/mbe.2022010
79. Wang D, Yang L, Yu W, Wu Q, Lian J, Li F, et al. Colorectal cancer cell-derived CCL20 recruits regulatory T cells to promote chemoresistance via FOXO1/CEBPB/NF-kappaB signaling. J Immunother Cancer. (2019) 7:215. doi: 10.1186/s40425-019-0701-2
80. Bian ZQ, Luo Y, Guo F, Huang YZ, Zhong M, Cao H. Overexpressed ACP5 has prognostic value in colorectal cancer and promotes cell proliferation and tumorigenesis via FAK/PI3K/AKT signaling pathway. Am J Cancer Res. (2019) 9:22–35.
81. Ouyang J, Hu S, Zhu Q, Li C, Kang T, Xie W, et al. RANKL/RANK signaling recruits Tregs via the CCL20-CCR6 pathway and promotes stemness and metastasis in colorectal cancer. Cell Death Dis. (2024) 15:437. doi: 10.1038/s41419-024-06806-3
82. Theune WC, Chen J, Theune EV, Ye X, Menoret A, Vella AT, et al. Interleukin-17 directly stimulates tumor infiltrating Tregs to prevent cancer development. Front Immunol. (2024) 15:1408710. doi: 10.3389/fimmu.2024.1408710
83. Kikuchi T, Mimura K, Ashizawa M, Okayama H, Endo E, Saito K, et al. Characterization of tumor-infiltrating immune cells in relation to microbiota in colorectal cancers. Cancer Immunol Immunother. (2020) 69:23–32. doi: 10.1007/s00262-019-02433-6
84. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. (2017) 357:409–13. doi: 10.1126/science.aan6733
85. Ma B, Qin L, Sun Z, Wang J, Tran LJ, Zhang J, et al. The single-cell evolution trajectory presented different hypoxia heterogeneity to reveal the carcinogenesis of genes in clear cell renal cell carcinoma: Based on multiple omics and real experimental verification. Environ Toxicol. (2024) 39:869–81. doi: 10.1002/tox.24009
86. Toor SM, Murshed K, Al-Dhaheri M, Khawar M, Abu Nada M, Elkord E. Immune checkpoints in circulating and tumor-infiltrating CD4(+) T cell subsets in colorectal cancer patients. Front Immunol. (2019) 10:2936. doi: 10.3389/fimmu.2019.02936
87. Morse MA, Overman MJ, Hartman L, Khoukaz T, Brutcher E, Lenz HJ, et al. Safety of nivolumab plus low-dose ipilimumab in previously treated microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer. Oncologist. (2019) 24:1453–61. doi: 10.1634/theoncologist.2019-0129
88. Salem M, Wallace C, Velegraki M, Li A, Ansa-Addo E, Metelli A, et al. GARP dampens cancer immunity by sustaining function and accumulation of regulatory T cells in the colon. Cancer Res. (2019) 79:1178–90. doi: 10.1158/0008-5472.CAN-18-2623
89. Shen K, Song W, Wang H, Wang L, Yang Y, Hu Q, et al. Decoding the metastatic potential and optimal postoperative adjuvant therapy of melanoma based on metastasis score. Cell Death Discovery. (2023) 9:397. doi: 10.1038/s41420-023-01678-6
90. Yang H, Li Z, Zhu S, Wang W, Zhang J, Zhao D, et al. Molecular mechanisms of pancreatic cancer liver metastasis: the role of PAK2. Front Immunol. (2024) 15:1347683. doi: 10.3389/fimmu.2024.1347683
91. Li C, Wirth U, Schardey J, Ehrlich-Treuenstatt VV, Bazhin AV, Werner J, et al. An immune-related gene prognostic index for predicting prognosis in patients with colorectal cancer. Front Immunol. (2023) 14:1156488. doi: 10.3389/fimmu.2023.1156488
92. Andre T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. (2020) 383:2207–18. doi: 10.1056/NEJMoa2017699
93. Rastin F, Javid H, Oryani MA, Rezagholinejad N, Afshari AR, Karimi-Shahri M. Immunotherapy for colorectal cancer: Rational strategies and novel therapeutic progress. Int Immunopharmacol. (2024) 126:111055. doi: 10.1016/j.intimp.2023.111055
94. Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. (2018) 36:773–9. doi: 10.1200/JCO.2017.76.9901
95. Rahma OE, Hodi FS. The intersection between tumor angiogenesis and immune suppression. Clin Cancer Res. (2019) 25:5449–57. doi: 10.1158/1078-0432.CCR-18-1543
96. Li J, Cong L, Liu J, Peng L, Wang J, Feng A, et al. The efficacy and safety of regorafenib in combination with anti-PD-1 antibody in refractory microsatellite stable metastatic colorectal cancer: A retrospective study. Front Oncol. (2020) 10:594125. doi: 10.3389/fonc.2020.594125
97. Yang Y, Yang Y, Huang H, Song T, Mao S, Liu D, et al. PLCG2 can exist in eccDNA and contribute to the metastasis of non-small cell lung cancer by regulating mitochondrial respiration. Cell Death Dis. (2023) 14:257. doi: 10.1038/s41419-023-05755-7
98. Zhou X, Lin J, Shao Y, Zheng H, Yang Y, Li S, et al. Targeting PLCG2 suppresses tumor progression, orchestrates the tumor immune microenvironment and potentiates immune checkpoint blockade therapy for colorectal cancer. Int J Biol Sci. (2024) 20:5548–75. doi: 10.7150/ijbs.98200
99. Guo Y, Xie F, Liu X, Ke S, Chen J, Zhao Y, et al. Blockade of TNF-alpha/TNFR2 signalling suppresses colorectal cancer and enhances the efficacy of anti-PD1 immunotherapy by decreasing CCR8+T regulatory cells. J Mol Cell Biol. (2024) 16:mjad067. doi: 10.1093/jmcb/mjad067
100. Fakih M, Raghav KPS, Chang DZ, Larson T, Cohn AL, Huyck TK, et al. Regorafenib plus nivolumab in patients with mismatch repair-proficient/microsatellite stable metastatic colorectal cancer: a single-arm, open-label, multicentre phase 2 study. EClinicalMedicine. (2023) 58:101917. doi: 10.1016/j.eclinm.2023.101917
101. Hu X, Hou B, Xu Z, Saeed M, Sun F, Gao Z, et al. Supramolecular prodrug nanovectors for active tumor targeting and combination immunotherapy of colorectal cancer. Adv Sci (Weinh). (2020) 7:1903332. doi: 10.1002/advs.201903332
102. Wang J, Zuo Z, Yu Z, Chen Z, Tran LJ, Zhang J, et al. Collaborating single-cell and bulk RNA sequencing for comprehensive characterization of the intratumor heterogeneity and prognostic model development for bladder cancer. Aging (Albany NY). (2023) 15:12104–19. doi: 10.18632/aging.205166
103. Vermeersch E, Lienart S, Collignon A, Lucas S, Gallimore A, Gysemans C, et al. Deletion of GARP on mouse regulatory T cells is not sufficient to inhibit the growth of transplanted tumors. Cell Immunol. (2018) 332:129–33. doi: 10.1016/j.cellimm.2018.07.011
104. Schoonderwoerd MJA, Koops MFM, Angela RA, Koolmoes B, Toitou M, Paauwe M, et al. et al: targeting endoglin-expressing regulatory T cells in the tumor microenvironment enhances the effect of PD1 checkpoint inhibitor immunotherapy. Clin Cancer Res. (2020) 26:3831–42. doi: 10.1158/1078-0432.CCR-19-2889
105. Paauwe M, Heijkants RC, Oudt CH, van Pelt GW, Cui C, Theuer CP, et al. Endoglin targeting inhibits tumor angiogenesis and metastatic spread in breast cancer. Oncogene. (2016) 35:4069–79. doi: 10.1038/onc.2015.509
106. Chen Q, Shen M, Yan M, Han X, Mu S, Li Y, et al. Targeting tumor-infiltrating CCR8(+) regulatory T cells induces antitumor immunity through functional restoration of CD4(+) T(convs) and CD8(+) T cells in colorectal cancer. J Transl Med. (2024) 22:709. doi: 10.1186/s12967-024-05518-8
107. Miao R, Xu X, Wang Z, Liu S, Qu K, Chen W, et al. Synergistic effect of nutlin-3 combined with aspirin in hepatocellular carcinoma HepG2 cells through activation of Bcl-2/Bax signaling pathway. Mol Med Rep. (2018) 17:3735–43. doi: 10.3892/mmr.2017.8346
Keywords: colorectal cancer, regulatory T cells, chemokines, immune suppression, immunotherapy, immune checkpoint inhibitor
Citation: Yu H, Yang R, Li M, Li D and Xu Y (2025) The role of Treg cells in colorectal cancer and the immunotherapy targeting Treg cells. Front. Immunol. 16:1574327. doi: 10.3389/fimmu.2025.1574327
Received: 10 February 2025; Accepted: 28 March 2025;
Published: 16 April 2025.
Edited by:
Zhiheng Lin, Shanghai University of Traditional Chinese Medicine, ChinaReviewed by:
Kangjie Shen, Fudan University, ChinaCopyright © 2025 Yu, Yang, Li, Li and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dan Li, bGQxMzc2NjA1MzQ5MUAxNjMuY29t; Yuanqing Xu, aHp4dXl1YW5xaW5nQDE2My5jb20=
†These authors have contributed equally to this work
 Hanqing Yu1†
Hanqing Yu1† Yuanqing Xu
Yuanqing Xu