- 1Division of Infectious Diseases, Department of Medicine, National University Health System, Singapore, Singapore
- 2Infectious Diseases Translational Research Programme and Department of Microbiology and Immunology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
- 3Biosafety Level 3 Core Facility, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
- 4Genome Institute of Singapore, Agency for Science, Technology and Research (ASTAR), Singapore, Singapore
- 5Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, Singapore
- 6Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, United Kingdom
- 7Infectious Diseases Research Laboratory, National Centre for Infectious Diseases, Singapore, Singapore
- 8Cardiovascular Research Institute, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
- 9ASTAR Infectious Diseases Labs (ASTAR ID Labs), Agency for Science, Technology and Research (ASTAR), Singapore, Singapore
- 10Bioinformatic Institute, ASTAR (Agency for Science, Technology and Research), Singapore, Singapore
- 11Department of Anaesthesia, National University Health System, Singapore, Singapore
- 12Collaborative and Translation Unit for Hand, Foot and Mouth Disease (HFMD), Institute for Molecular and Cell Biology, Agency for Science, Technology and Research (ASTAR), Singapore, Singapore
- 13Department of Hematology-Oncology, National University Cancer Institute of Singapore (NCIS), National University Health System, Singapore and Cancer Science Institute of Singapore, National University of Singapore, Singapore, Singapore
- 14Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
- 15Department of Biochemistry, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
- 16Rural Clinical School, Toowoomba Regional Clinical Unit, The University of Queensland, Brisbane, QLD, Australia
Background: Genome-wide associative studies can potentially uncover novel pathways which modulate anti-viral immune responses against SARS-CoV-2 or identify drivers of severe disease. To date, these studies have yielded loci mostly in non-functional domains of unknown biological significance and invariably require large sample sizes, potentially missing lower frequency variants, especially in under-represented or minority populations.
Methods: To identify unique genetic traits predisposing to severe COVID-19 in Asians, we employed an alternative strategy using whole exome sequencing of representative cohort of severe versus mild COVID-19 patients. Candidate gene variants were identified by performing logistic regression against top genetic principal components, prioritised for missense variants with likely causal impact. Then, functional sequelae of variants were replicated in-vitro and re-validated in patients ex vivo to demonstrate causality between genotype and clinical phenotype.
Results: Of 136 COVID-19 patients in Singapore (of whom 25% had severe disease), a single nucleotide polymorphism rs2980619 (p.L252F substitution) belonging to NudC-Domain-Containing-1 (NUDCD1) was highly-placed. Homozygous bearers of variant p.L252F had higher (3.97x) odds of severe disease. Age >50 years and male sex were significant covariates which increased the odds of severe disease by 3.38x and 3.16x, respectively. We showed in-vitro that variant p.L252F reduced NUDCD1 activity, leading to reduced antiviral signalling through RNA helicase DHX15 and antiviral signalling adaptor MAVS, reduced activation of NFκB components RelB and p65, and resultant 1-log higher SARS-CoV-2 viral load compared to wild type (L252) cells. Patients bearing p.L252F had lower NUDCD1, MAVS, and RelB expressions, affirming the above findings.
Conclusion: A gene variant of NUDCD1 influences COVID-19 severity in Asians through interacting with DHX15 and MAVS, affecting effective response against SARS-CoV-2.
Introduction
COVID-19 disease manifestations are protean, from asymptomatic infection to severe disease with organ failure. Amongst the clinical factors identified, age confers the clearest risk for severe disease whereby patients >65 years old are at 5x higher risk of hospitalisation and 65x higher risk of death, alongside ethnicity, chronic kidney and lung diseases, and malignancy (1). However, through the 3 years of the pandemic, we had seen younger patients who were previously well, with COVID-19 who required supplemental oxygen or mechanical ventilation in the ICU; conversely, elderly SARS-CoV-2-infected patients with ailments had passed through relatively unscathed. These observations point to additional contributions by intrinsic host traits which dictate susceptibility to the SARS-CoV-2 virus.
To date, genome wide association studies (GWAS) involving tens of thousands of subjects have identified more than 20 loci associated with increased susceptibility or severity to SARS-CoV-2 infection (as described in Table 1). The majority of these loci lie in purported non-functional domains which, despite some showing robust association, have unascertained biological significance. GWAS utilises pre-established genotypic database (derived primarily from Caucasian cohorts) to measure association between disease phenotype and gene variant. However, the strength of the computed association can be compromised as allele frequencies decrease. Furthermore, GWAS can miss yet-to-be-identified novel variants (e.g. in Asians) not incorporated into existing genotyping arrays (18), as exemplified by the recent identification of DOCK2 (dedicator of cytokinesis 2) in the Japanese COVID-19 cohort (11). Whole exome or genome sequencing can enhance the detection of rare variants. Studying the effect of low frequency or rare variants invariably requires large sample sizes, often running into tens of thousands lest the study risks being under-powered. The requirement of such large numbers for GWAS necessitates incorporation of multi-national cohorts as extraordinarily exemplified by the COVID-19 Host Genetics Initiative, which recently identified the TLR7 gene amongst others as complicit in severe COVID-19 (19). In such a trans-national setting, however, minority ethnic-specific predisposing traits may be missed, as has been exemplified by the COVID-19 Host Genetics Initiative whereby East Asians constitute only 10% of the overall study population (20).
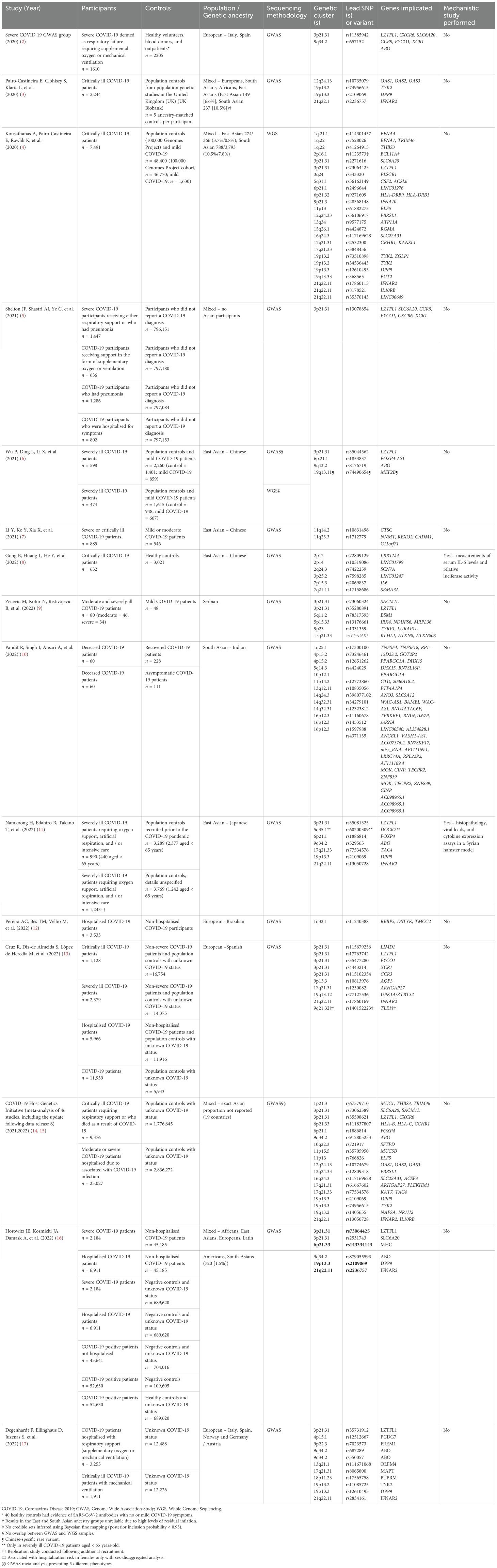
Table 1. Published genetic studies examining susceptibility to severe COVID-19 disease as a whole or part of the study.
Acknowledging the challenges faced studying genetic predisposition in minority cohorts and in a resource- or cost-limited setting, one practical strategy would be to sequence a representative study cohort and validate the findings through mechanistic immunological and virological studies. To build upon findings from molecular epidemiological associative studies, it is imperative to replicate the functional sequelae of genotypic variation in experimental studies. This helps strengthen and demonstrate causality between genotype and clinical phenotype (21). This will be critical to our understanding to discover new pathways underlying the pathological processes against SARS-CoV-2 or drivers of severe disease state. We set about using this strategy to identify novel immune traits predisposing to severe COVID-19 in patients in Singapore.
Methods
Patient recruitment
Patients were recruited from the National Centre for Infectious Diseases, Singapore. We obtained 136 COVID-19 patient DNA specimens, of whom 25% had severe disease as defined by requiring oxygen or ventilation in ICU. The study protocols 2012/00917 and 2020/00435 were approved by the Domain Specific Research Board of National Healthcare Group, Singapore.
Whole exome sequencing (WES)
DNA concentration and purity were quantified after extraction. 1µg of genomic DNA was fragmented to give a distribution of 200–500 base pairs. Library preparation was done using Kapa DNA HTP Library Prep Kit. Hybridisation of adapter ligated DNA was performed at 47°C, for 72h, to biotin-labelled probe included in the Nimblegen SeqCap EZ Human Exome Kit. Libraries were sequenced using the Illumina Hiseq4000 sequencing system and paired-end 151bp reads were generated for analysis.
Bioinformatics analysis for WES
For genomic base calling, we followed best practice guidelines implemented in the GATK workflow (22). Raw sequencing reads from FASTQ files were aligned to the GRCh37 reference genome using the Burrows-Wheeler Aligner (BWA) program (23). Following alignment, Picard tools was used to label read groups and mark PCR duplicates to generate BAM files (24). The resulting BAM files were sorted and indexed. Local realignment around indels was performed to correct misalignments, and base quality score recalibration was applied to adjust the quality scores, aligning them more closely with the true probability of base mismatches in relation to the reference genome. Variant joint-calling was performed with HaplotypeCaller from GATK (version 4.5.0.0, GCCcore-12.3.0, Java-v17) using dbSNP reference b37, dbSNP version 137, with -ERC flag. Genotypes of individual chromosomes were called in parallel separately using GenomicsDBImport and GenotypeGVCFs functions to jointly aggregate multiple samples from previously single-sample haplotype calling. Following which, genotypes of all samples across all chromosomes were merged using the MergeVcfs function (22). Sites/variants were filtered by depth, base quality score, strand-bias and Hardy-Weinberg Equilibrium. High-quality variants were annotated using the Variant Effect Predictor program (25) to understand population-scale, ethnic-specific allele frequencies, variant-to-gene mappings, variant effect types, protein consequences and in silico functional consequence predictions. Identification of candidate gene was adopted from Ambry’s clinical variant classification scheme (26). We performed a logistic regression with oxygen requirement as end point in PLINK v1.9, adjusting for age and gender (27). Our sample size was powered to identify a minimum odds ratio (OR) of 1.8 at 80% power and 5% significance level. In view of the modest sample size and the intention to follow up with comprehensive laboratory confirmation, we did not apply multiple-testing correction for the significance level, but rather relied on filters by effect sizes (OR>1.8), minimum allele frequency (MAF) filters (MAF>0.4 across multiple ancestry populations) to ensure applicability for general population, and a preference for variants that associates with gene expression across multiple genes (number of expression quantitative trait loci [eQTL] > 5), which is more likely to represent a potential critical key role.
Cloning and transfection
Candidate gene variants were amplified from human ORFeome v5.1 and cloned into pEGFP-C1 backbone using Gibson cloning with EGFP at the C-terminus. Mutant alleles were introduced by Gibson Assembly via overlapping primers targeting the mutant site (28). Mutant proteins were transfected into HEK293 cells to assay for expression and localisation, followed by SARS-CoV-2 infection in A549-ACE2 cells.
Cell culture transfection and infection
A549-ACE2 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco), supplemented with 10% foetal bovine serum (FBS) in 5% CO2 at 37°C. Cells were seeded into 24-well plates (ThermoFisher) at 105 cells/well. Transfection of empty vector (EV), wild type NUDCD1 and NUDCD1 p.L252F variant were performed using Lipofectamine™ 3000 (Thermo Fisher Scientific) according to manufacturer’s protocol. After 48 hours of transfection, the cells were infected with wild type SARS-CoV-2 at MOI of 1 at 37°C with 5% CO2 in a BSL-3 facility. After 1h, the wells were topped up with 500µl of DMEM supplemented with 2% FBS and incubated for 48h. The supernatants were collected for plaque assay and ELISA.
Plaque assay
Supernatants from infected cells were serially diluted 10-fold, added onto A549-ACE2 cells, and incubated for 1h at 37°C with 5% CO2. The inoculum was removed, overlaid with plaque medium containing 1.2% Avicel CL-611 (DuPont) with 2% FBS. After 3 days, the cells were fixed with 4% formaldehyde and stained with 0.5% crystal violet. Virus titre was calculated using the following formula:
Cytokine detection
ELISA for IL-6, TNFα and IFNα were performed according to manufacturer’s instructions (Invitrogen). Absorbance was measured with the Infinite M200PRO (Tecan) plate reader at 450 nm. The concentration of analytes was calculated from the respective standard curves and multiplied by the dilution factor.
Western blotting
Infected cells were washed with ice-cold PBS and lysates collected at the indicated times post-infection with lysis buffer (1% NP-40, 2mM EDTA, 10% glycerol, 150mM NaCl, 50mM Tris HCl) supplemented with protease inhibitors (SigmaFAST) and phosphatase inhibitors (Roche PhosStop easy pack). Cell lysates were heat-inactivated at 65°C for 10min and protein concentration was determined by Bradford assay (Bio-Rad). Each 20μg of lysates was heated at 95°C for 5min, separated by SDS-PAGE (10%) and transferred to polyvinylidene difluoride membranes. Blots were blocked with 5% non-fat milk probed with following antibodies: NUDCD1 (1:500; Novus biologicals), DHX15 (1:500; sc271686; Santa cruz Biotechnology), anti-MAVS (1:500; A5764; ABclonal), anti-p65 (1:500; sc372; ScB), anti-RelB (1:1000; 10544S; Cell Signalling Technology), anti-IRF-3 (1:1000; D83B9; CST) and anti β-actin (1:1000; sc47778; ScB). Primary antibodies were incubated overnight at 4°C. Primary antibodies binding was detected using horseradish peroxidase-conjugated secondary antibodies (anti-rabbit, anti-mouse IgG, CST) by incubating at room temperature for 1h. Blots were visualised using Clarity Western ECL blotting substrate (Bio-Rad). The blots were stripped using Thermo Scientific Restore western blot stripping buffer (cat#21059) for reuse.
Co-immunoprecipitation
Total proteins extracted after infection were used for co-immunoprecipitation and western blotting. DHX15 antibody was tagged with Dynabeads Protein G for magnetic selection and co-immunoprecipitated proteins were detected by western blotting using NUDCD1 and MAVS antibodies.
Total RNA isolation and quantitative real-time PCR
RNA extraction was performed using TRIzol reagent (Invitrogen) according to manufacturer’s instructions. For cDNA synthesis, reverse transcription was carried out using iScript™ cDNA Synthesis Kit (Bio-Rad) and qPCR was performed using GoTaq qPCR Mastermix (Promega Corporation) in CFX connect thermocycler (Bio-Rad). Primer sequences for human NUDCD1 were: 5’-CTACTCTGGCAACCACACTCCA-3’ (forward) and 5’-CTCACAAAGGGCTGCATACGAG-3’ (reverse). Beta-2-microglobulin (B2M) was used as internal control: 5’-ATGAGTATGCCTGCCGTGTG-3’ (forward) and 5’-CCAAATGCGGCATCTTCAAAC-3’ (reverse). qPCR signals were quantified comparing cycle threshold (Ct) values of target gene with Ct values of reference gene B2M. Based on comparative Ct method, mean relative mRNA expression was calculated. The values were expressed as ratio of fold increase to control cell mRNA levels.
RNA interference
The siRNA for NUDCD1 (hs.Ri.NUDCD1.13.1) was purchased from Integrated DNA Technologies. To inhibit NUDCD1 expression, cells were seeded (105 cells/well) in 24-well plates in antibiotic-free medium. After 24h, the siRNA was transfected into cells using Lipofectamine™ 3000 Reagent (Thermo Fisher) according to manufacturer’s protocol. A scrambled (sc) siRNA (5’-AUGUUUGGAAGUGGAACCC-3’ and 5´-UAGGGUGUACCCGUAAUAG-3´) was used as negative control.
Modeling of NUDCD1 docking
The most probable site of docking was determined using diffdock 4 https://github.com/gcorso/DiffDock). From the range of possible results, the result that had most highly probable structure across 10 runs was selected as input for PyMOL (https://pymol.org/2/) (29). PyMOL visualisation settings were set to showcase the possible hydrophobic and electrostatic interactions at the most possible docking site.
Statistical analysis
Statistical analyses were performed using GraphPad Prism (version 8.0.2), and RStudio (version 2023.03.1 + 446) and denoted in the figure legends. The χ² test was used to examine differences between patients with and without the NUDCD1 variant, as well as differences between patients with severe disease versus those who did not. To examine the relationship of the NUDCD1 genotype on disease severity, a logistic regression model was fitted using stepwise variable selection for the outcome of oxygen requirement. We included NUDCD1 genotype as well as clinical features of age, sex, ethnicity, and the presence of chronic pulmonary disease as model covariates. Statistical significance was defined as P<0.05.
Results
Whole exome sequencing identified NUDCD1 variant which increased likelihood of COVID-19 disease severity
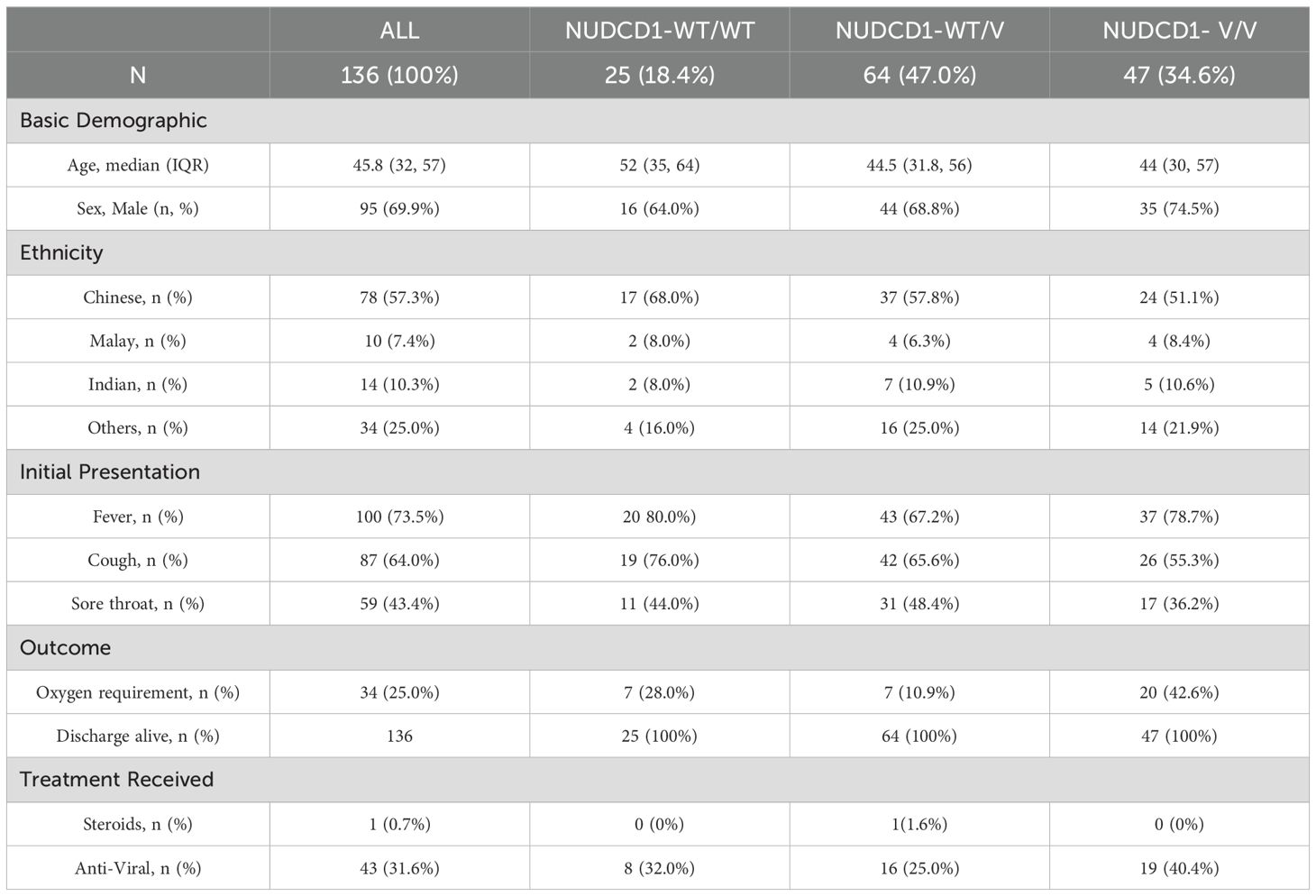
We performed WES on 136 COVID-19 patients in Singapore as described in Table 2, of whom 95 (69.9%) were males and mean age was 45.8 years (range 32-57). The patients recruited here are representative of the Asian ethnic distribution of Singapore. Thirty-four of the patients (25%) had severe COVID-19 disease based on requirement for oxygen supplementation or ventilation, of whom 14 (10.3%) required ICU admission. Presenting symptoms, treatment received, length of stay are as described in Table 2. There were no mortalities.
We performed logistic regression using endpoint of requiring oxygen supplementation in PLINK, adjusting for age and gender. We started with 12,041,822 variants across all patients, with 258,963 variants remaining with P<0.05 and after 100% call rate filter. Prioritisation of missense variants with odds ratio (OR) greater than 1.8 narrowed the field to 1,629 variants. Our aim had been to identify common variants with potential wider applicability to the study population and targeting gene expression in association with multiple gene sets implying potential key biological activity. With these intents, applying a MAF>0.4 in the dataset filtered down to 188 common variants and further selection of those with >5 eQTL hits had led to 58 remaining variants eventually. For practicality, a final filter pointing to potential applicability to worldwide population was applied: we further selected variants with MAF>0.4 across diverse ethnic 1KG cohorts (AFR, AMR, ASN, EUR). This narrowed the list to 18 variants across 14 loci. Figure 1A outlines the number of variants remaining at each step of filtering process. The SNP rs2980619 belonging to NudC Domain Containing 1 (NUDCD1) emerged as a shortlisted gene variant of interest. NUDCD1 is ubiquitously expressed in the body, including the lungs and immune cells (30, 31). It lies on exon 5 of chromosome 8, position 110302047, with thymine (T) as its reference base for the wild type (WT) allele. The alternative base known from Genome Aggregation Database (gnomAD) is guanine (G) for the variant (V) SNP; the primary reference T allele frequency is 41.8% and primary alternative G allele frequency is 58.2%. The switch of T-to-G results in an amino acid change from leucine (L) to phenylalanine (F) at position 252 (p.L252F) of the mature protein (Figures 1B, C).
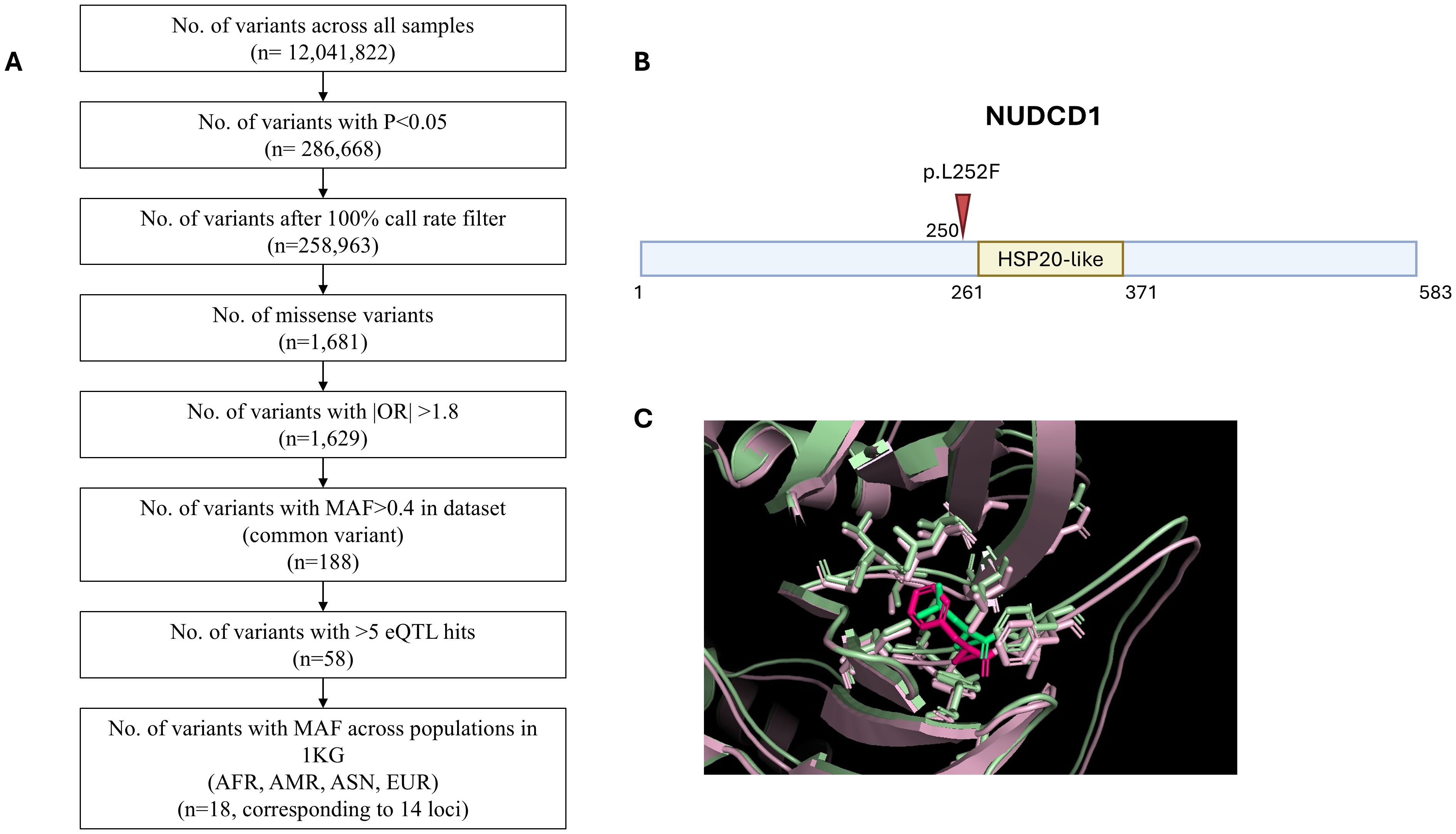
Figure 1. Whole exome sequencing identifies NUDCD1 polymorphism in influencing COVID-19 disease severity. (A) Filtering strategy. (B) Schematic representation of the p.L252F mutation (arrow) in the NUDCD1 amino acid sequence. Putative HSP20-like domain of NUDCD1 from InterPro (https://www.ebi.ac.uk/interpro/). (C) Superimposed protein structures of the wild type NUDCD1 and p.L252F variant.
In the study population, 18.4%, 47.0% and 34.6% of the patients were homozygous WT(L252)/WT(L252), heterozygous WT(L252)/V(p.L252F) and homozygous V(p.L252F)/V(p.L252F), respectively, for NUDCD1. Further stratification of the patient demographics and clinical status based on the respective NUDCD1 genotypes (WT/WT, WT/V, V/V) are detailed in Table 2. Univariate analysis revealed the NUDCD1 V/V genotype, age >50 years old, and male gender to be linked to severe disease (Table 3). Possession of the homozygous V/V genotype increased the odds of severe disease by 3.97x (95% confidence interval [CI] 1.76-8.94, P< 0.001). Age >50 years and male sex were significant covariates which increased the odds of severe disease by 3.38x (95% CI 1.48-7.68, P=0.003) and 3.16x (95% CI 1.13 – 8.88, P=0.036), respectively (Table 3). Ethnicity differences and the presence of chronic pulmonary comorbidities were not statistically significant in the model.
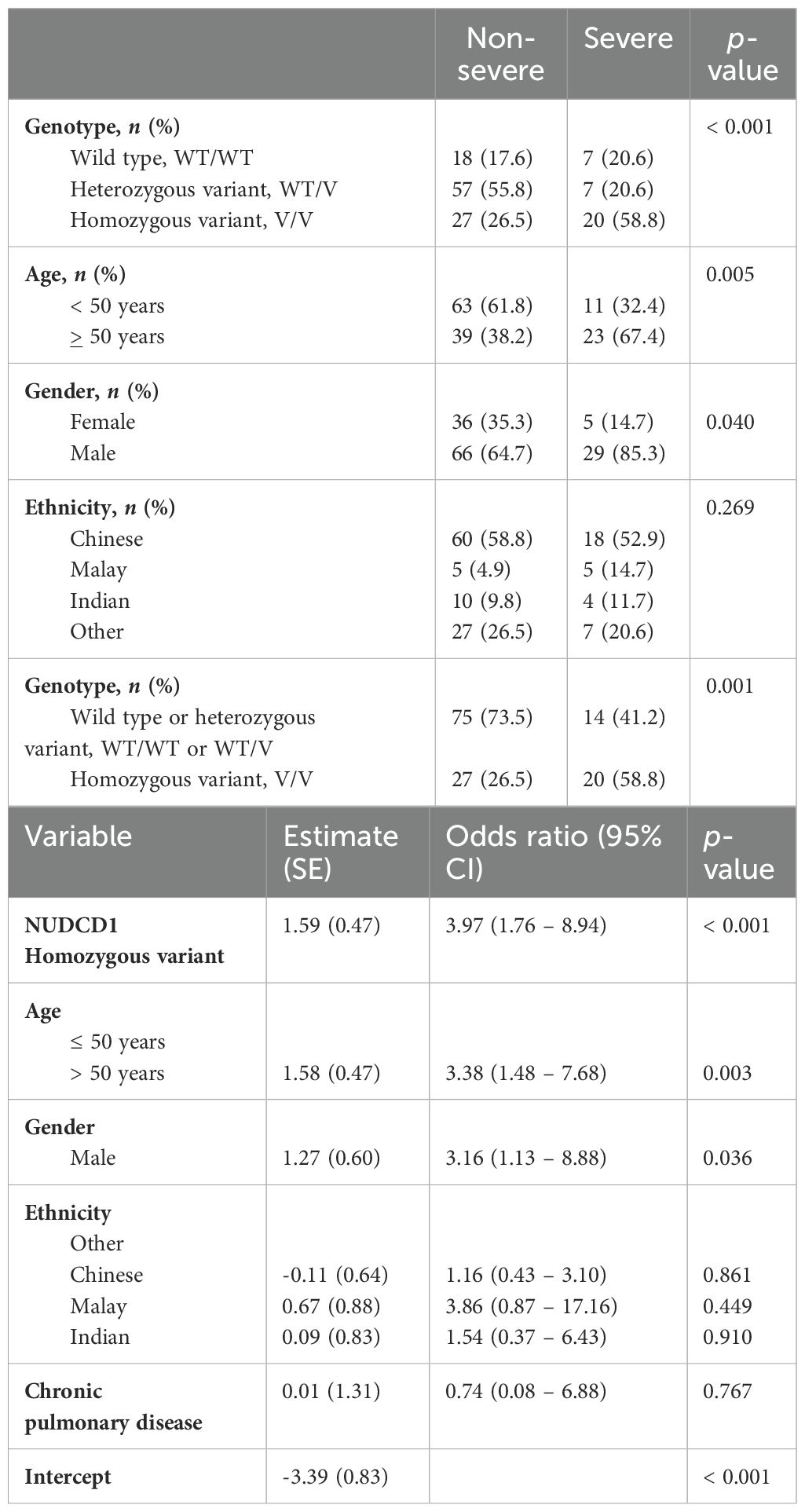
Table 3. Logistic regression with stepwise fitting of NUDCD1 genotype, age, gender and presence of chronic pulmonary diseases as covariates for risk estimates and odds ratio of severe COVID-19 disease.
NUDCD1 p.L252F variant resulted in reduced protein expression and attenuated activation of DHX15 and MAVS
NUDCD1 is located in the cytosol and is thought to be involved in modulation of the immune system process and linked to autoimmune atherosclerosis (32). This gene and its protein function are not understood in the context of COVID-19 or infections per se. To study its role in disease causation, WT NUDCD1 (L252) and its non-synonymous variant carrying the alternative allele G (NUDCD1 p.L252F) were cloned and transfected into A549-ACE2 cells. Cells transfected with the NUDCD1 p.L252F variant showed diminished NUDCD1 expression compared to WT NUDCD1 (L252) (Figure 2A).
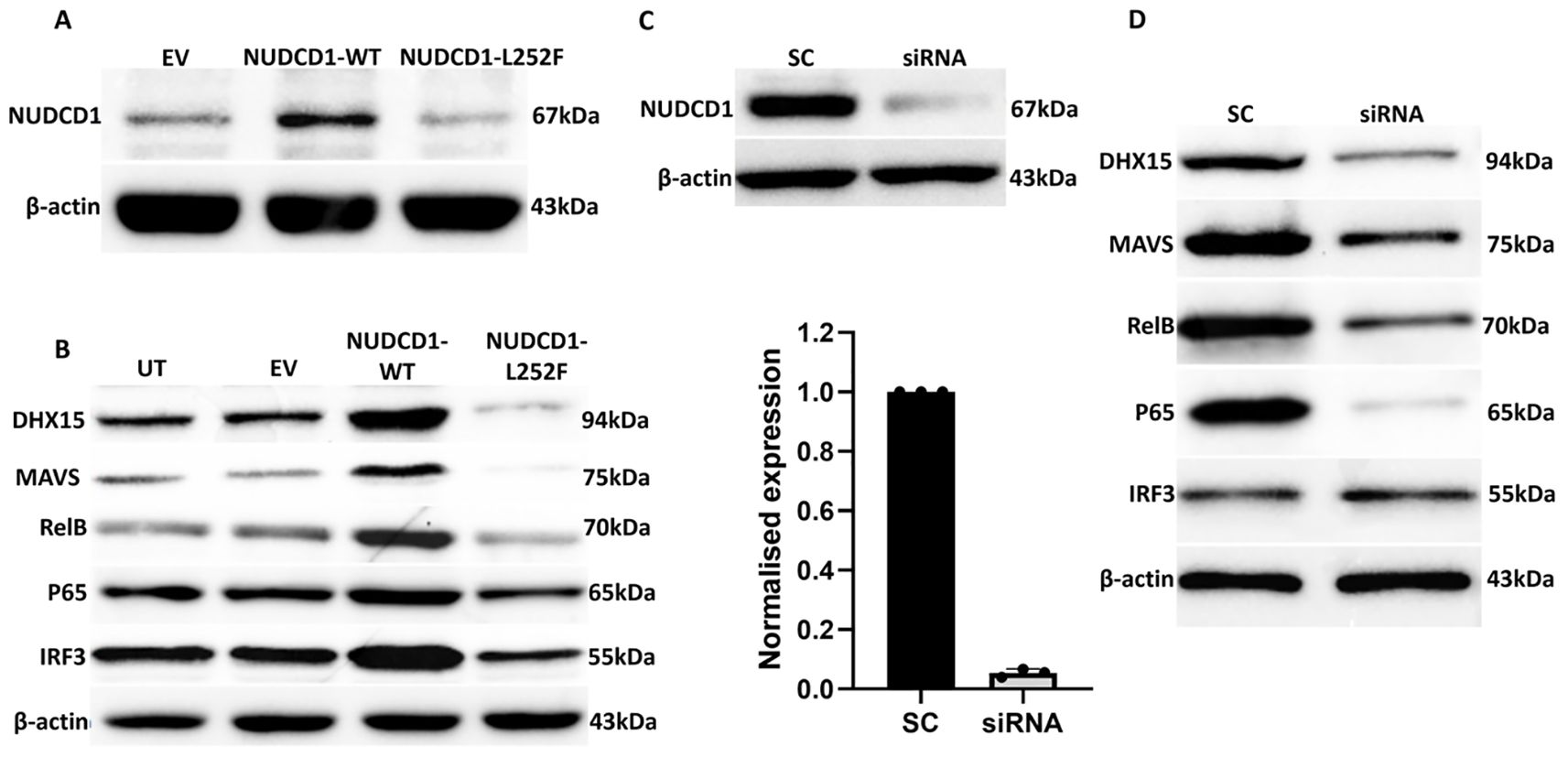
Figure 2. The NUDCD1 p.L252F variant results in reduced protein expression and attenuated activation of DHX15 and MAVS. (A) Diminished levels of NUDCD1 in NUDCD1 p.L252F-transfected A549-ACE2 cells compared to wild type NUDCD1-transfected cells. β-actin was used as a loading control. (B) Reduced protein levels of DHX15, MAVS, RelB and p65 in NUDCD1 p.L252F-transfected A549-ACE2 cells. IRF3 levels remained similar between the wild type and p.L252F NUDCD1 variant. β-actin was used as a loading control. UT: untransfected; EV: empty vector. (C) Efficiency of NUDCD1 siRNA knockdown determined by Western blot and qRT-PCR. (D) Reduced protein levels of DHX15, MAVS, RelB and p65 in NUDCD1 knockdown. β-actin was used as a loading control. SC: scrambled siRNA, negative control.
Recently, NUDCD1 was reported to have a putative relationship with the RNA helicase DEAH-Box Helicase 15 (DHX15) (33). The latter is involved in defence against RNA viruses which is mediated through MAVS (Mitochondrial Antiviral Signalling Protein) (34). MAVS is critical in triggering type I interferon response against viruses including Coronaviruses (35). The convergence of these findings, yet to be reported, may explain a role of NUDCD1 in COVID-19 disease.
We demonstrated that WT NUDCD1 (L252) had higher NUDCD1 protein expression than NUDCD1 p.L252F. In turn, WT NUDCD1 (L252)-transfected cells induced greater expression of DHX15 and MAVS than variant NUDCD1 p.L252F cells. Given that the induction of innate antiviral response by MAVS is through NFκB, we investigated whether components of the NFκB complex requisite for cytokine production were affected. Our results demonstrated that the alternative NUDCD1 p.L252F variant led to reduced activation of NFκB components RelB and p65 (Figure 2B). In contrast, IRF3 was less affected by altered NUDCD1 function.
To validate the consequence of reduced NUDCD1 activation such as in the setting of NUDCD1 p.L252F, we utilised siRNA knockdown against NUDCD1 in A549-ACE2 cells. Effective silencing of NUDCD1 (Figure 2C) resulted in diminished induction of DHX15 and MAVS, and similarly reduced RelB and p65 expression (Figure 2D), as observed with NUDCD1 p.L252F cells. Taken together, our findings suggest that the diminished NUDCD1 activity by the p.L252F variant resulted in decreased effector antiviral response against SARS-CoV-2.
NUDCD1 p.L252F cells show increased susceptibility to infection by SARS-CoV-2 and reduced cytokine production
To elicit the virologic sequelae of altered NUDCD1 function in its susceptibility to SARS-CoV-2 infection, we transfected A549-ACE2 cells with plasmid expressing either WT NUDCD1 (L252) or variant NUDCD1 p.L252F and infected the cells with the SARS-CoV-2 virus. We observed that cells transfected with WT NUDCD1 (L252) were more resistant to SARS-CoV-2 than the NUDCD1 p.L252F variant. The NUDCD1 p.L252F-transfected cells sustained significantly higher viral load by approximately 1-log10 compared to WT NUDCD1 (L252)-transfected cells (Figure 3A). In addition, we observed that NUDCD1 p.L252F cells produced significantly lower levels of IL-6 compared with WT NUDCD1 (L252) cells (Figure 3B), in line with findings of reduced MAVS and RelB/p65 activation in NUDCD1 p.L252F cells (Figure 2B). Using infected cell lysates, co-immunoprecipitation of DHX15 with NUDCD1 and MAVS demonstrated that in NUDCD1 p.L252F-tranfected cells, there were decreased NUDCD1 and MAVS interaction with DHX15 (Figure 3C). These results suggest that the NUDCD1 p.L252F variant leads to impairment of NUDCD1 function through its decreased interaction with DHX15 and MAVS, resulting in increased SARS-CoV-2 susceptibility.

Figure 3. NUDCD1 p.L252F cells show increased susceptibility to infection by SARS-CoV-2 and reduced interleukin 6 production. (A) SARS-CoV-2 viral load from A549-ACE2 cells transfected with plasmids expressing either the wild type or p.L252F NUDCD1 variant (n=3). Statistical significance was performed with unpaired t-test. **: P<0.01. (B) Interleukin 6 levels from SARS-CoV-2 infected cell supernatant (n=3). Statistical significance was performed with unpaired t-test. **: P<0.01. (C) Association of NUDCD1 and MAVS with DHX15 by co-immunoprecipitation from SARS-CoV-2 infected cell lysates.
Patients bearing NUDCD1 p.L252F have reduced NUDCD1 expression
To re-validate the above findings, PBMCs from SARS-CoV-2-infected patients who bore WT NUDCD1 (L252) or variant NUDCD1 p.L252F were tested. Notably, cells of patients with NUDCD1 p.L252F showed marked reduction of NUDCD1, MAVS and RelB expression (Figures 4A, B), in line with the in vitro findings.
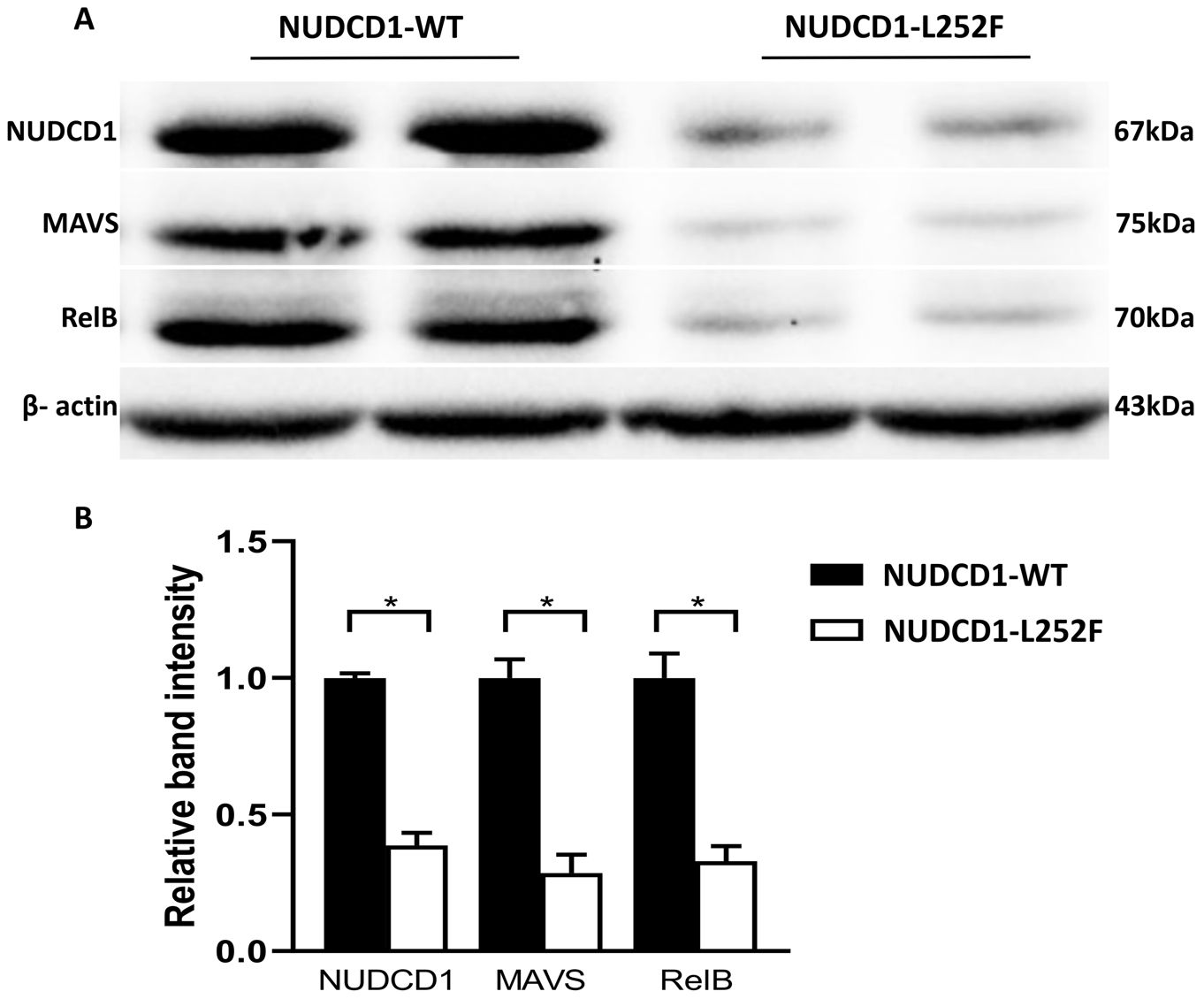
Figure 4. Patients bearing the NUDCD1 p.L252F variant have reduced NUDCD1 protein expression. (A) Diminished NUDCD1 expression from patient PBMCs with the p.L252F variant. Likewise, MAVS and RelB were attenuated. β-actin was used as a loading control. (B) Respective densitometric analysis of western blot result. Western blots were normalised to β-actin and densitometric analysis was performed using image processing software. Values are mean ± standard error of the mean of L252 (n=4) and p.L252F (n=5). Statistical significance was performed using Mann-Whitney U test. *: P<0.05.
Overall, the infection studies align with findings from genetic and mechanistic studies. WT NUDCD1 (L252) is protective against severe COVID-19. A non-synonymous switch from T to alternative G led to altered protein function in NUDCD1 p.L252F, which compromised upfront antiviral effector response through DHX15, MAVS and NFκB complex. This diminished innate response hampered effective clearance of virus and resulted in higher SARS-CoV-2 viral load and consequently more severe COVID-19 disease.
Discussion
The distinction of true disease-causing variants from the numerous potentially functional variants derived from genomic association is critical (36). With high throughput data arising from current genomic arrays and sequencing platforms, assignment of causality through demonstration of direct evidence of pathogenicity is all the more vital to weed out false-positive variants which can be retained despite the varied filtering strategies (37). Conversely, potential true disease-causing variants may be missed out in the absence of a fully or even intermediate penetrant phenotype, or if the study sample size, be it on resource-restricted or minority grounds, fails to attain the power of genetic homogeneity and statistical strength (21, 38). This calls for additional tiers of evidence to demonstrate the damaging (or protective) impact of genetic variants in disease pathophysiology. The guidelines by MacArthur proposed that genetic associative findings of gene variants significantly enriched in cases compared with controls be strengthened by informatic in silico data and very importantly, experimental evidence (36, 39). Experimental studies ought to demonstrate biologically-plausible causal role of the specified variant through techniques involving gene disruption and manoeuvres to recapitulate the observed phenotype in in vitro, ex vivo, animal experimental setting or as applicable, in the patient cohort of interest. We had sought to fulfil these requisite criteria to link molecular epidemiology to pathogenesis with emphasis on validation given that this had not been widely attempted in the context of severe COVID-19 disease.
Our WES output had yielded NUDCD1 rs2980619 as a top candidate amongst enriched variants which emerged from comparing patients with severe and mild COVID-19 disease. There was an odds ratio difference of 3.97x higher likelihood of severe disease in subjects homozygous for NUDCD1 p.L252F, of which the reference allele was protective. This lesser known gene was first described in the context as a tumour antigen (also known as Chronic Myelogenous Leukemia Tumour Antigen 66 [CML66]), although it has been perceived as being an immunogenic epitope capable of evoking host immune response (40, 41). Besides carcinogenesis, the parental NUDC (Nuclear Distribution Protein C) has been shown to be involved in haematopoiesis, cell cycle progression and inflammation (42). NUDCD1 is now known to be ubiquitously expressed in almost all major organs and particularly at higher levels in white blood cells and lung tissues as seen on both mRNA and proteomics arrays as well as in ciliated epithelium of bronchial mucosa (43–46). The viability of NUDCD1’s involvement in COVID-19 disease was supported by prior description of NUDCD1 as a high-placed candidate amongst a catalogue of genes listed in a COVID-19 severity association study though this track was not further pursued, as well as its described link with an RNA helicase DHX15 with ascribed antiviral role (33, 47).
The consequential non-synonymous allele switch from T to G results in the amino acid substitution p.L252F, leading to decreased expression of the protein. Furthermore, p.L252F lies (5 amino acids) proximal to the HSP20-like chaperon domain of NUDCD1 (48). HSP20 as a family of small heat shock proteins (sHSP) serve as molecular chaperons, which are activated to mitigate states of molecular stress through its multiple regulatory roles within the nucleus including inhibition of caspase-3 and apoptosis (49, 50). This molecular chaperon function of the NUDC family is further extended through the demonstration of its interaction with DEAD/DEAH box helicases, and more specifically recently, co-association studies showing unique NUDCD1 interaction with DHX15, a DEAD/DEAH box helicase (33, 51). In our study, we further demonstrate the interaction of NUDCD1 with DHX15 experimentally through co-immunoprecipitation. The attenuated NUDCD1 expression from p.L252F led to diminished DHX15 and MAVS activities. DHX15 is a well-recognised viral RNA sensor essential for type I interferon which can trigger MAVS, a critical adaptor for virus-induced signalling through NFκB (34, 52). This interaction with DHX15 and MAVS was replicated by our targeted silencing of NUDCD1.
We strengthened these mechanistic findings to the SARS-CoV-2-infected patients whereby bearers of NUDCD1 p.L252F had indeed lower NUDCD1, MAVS and RelB expression compared with those who were homozygous for the WT allele. The patients had been recruited prior to introduction of SARS-CoV-2 immunisation v.i.z. the immune trait elicited was independent of the vaccine effect. As much as may be speculated if effective vaccination might mitigate against severe COVID-19 disease, this unique unvaccinated study cohort provides us with the opportunity to study host susceptibility to SARS-CoV-2 infection and disease process unadulterated. Further to the widely held perception directly linking the said hyperinflammatory phase of SARS-CoV-2 infection to severe disease, our findings rather, suggest that the critical determinant of severe disease is the ability of the host immunity to mount an adequate immune response upon early encounter with the SARS-CoV-2 virus. As exemplified by the hypomorphic NUDCD1 p.L252F variant and the compromised antiviral effectors DHX15 and MAVS, the resultant diminished proinflammatory cytokines and type I interferon facilitated SARS-CoV-2 virus proliferation. Our findings here are as schematically summarised in Figure 5. In turn, it is the higher SARS-CoV-2 viral burden which triggers the host immunity to an exaggerated hyperinflammatory response.
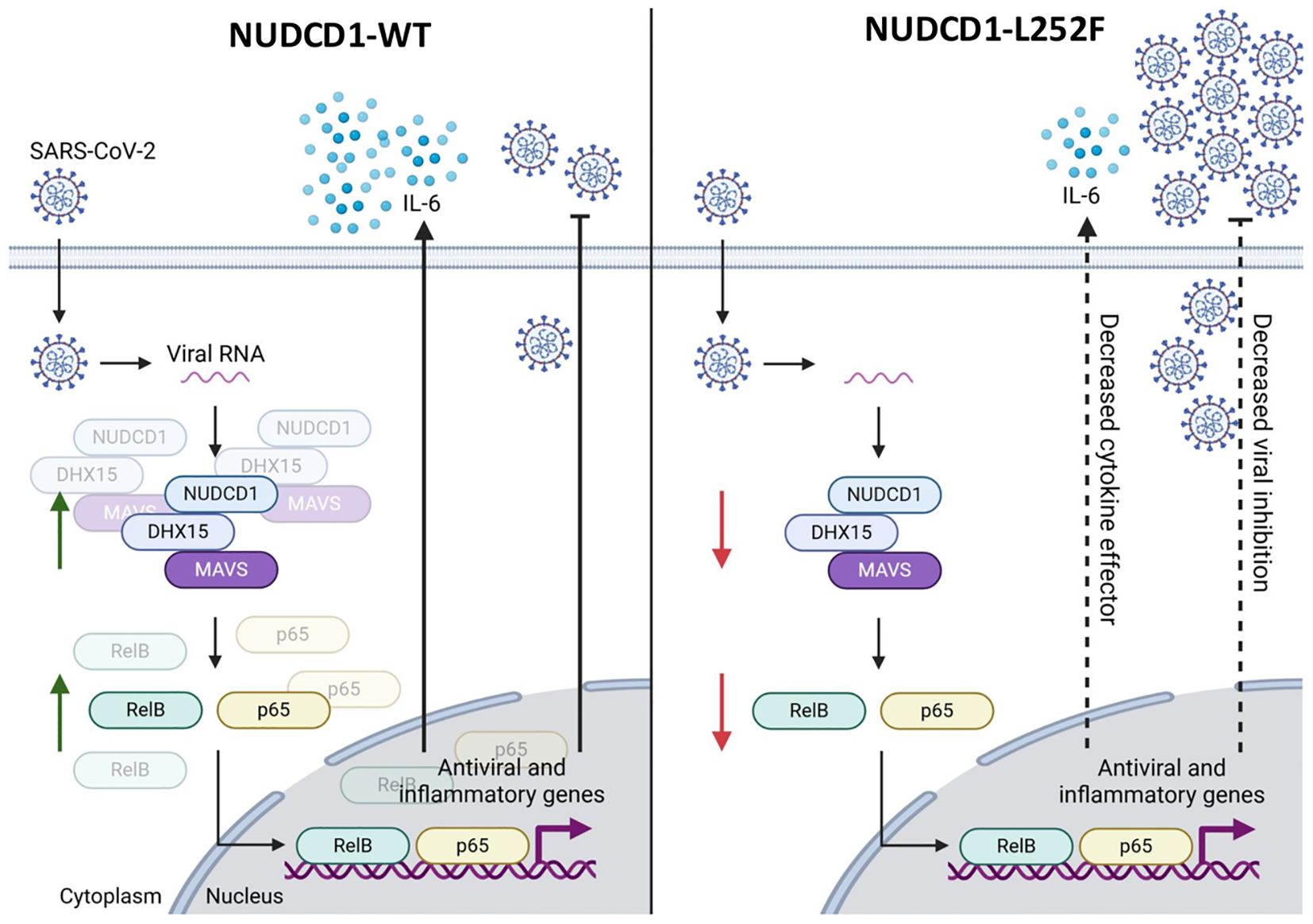
Figure 5. The NUDCD1 p.L252F variant impairs antiviral signalling and cytokine response during SARS-CoV-2 infection. This schematic illustrates the differential immune response to SARS-CoV-2 infection mediated by wild type NUDCD1 (left) and variant NUDCD1 p.L252F (right). (Left) Upon SARS-CoV-2 infection, viral RNA is detected in the cytoplasm by the protein complex involving NUDCD1, DHX15, and MAVS. This complex facilitates the activation of transcription factors RelB and p65, which translocate into the nucleus and drive the expression of antiviral and inflammatory genes, including IL-6. The result is effective cytokine signalling and viral inhibition. (Right) The variant impairs the assembly or function of the NUDCD1-DHX15-MAVS complex, reducing the activation of RelB and p65. This leads to lower cytokine effector function, as exemplified by decreased IL-6 and reduced viral inhibition, allowing for increased viral replication. Green arrows indicate upregulation or activation, while red arrows indicate downregulation or impaired signalling. Created with www.biorender.com.
Indeed, over the years of this pandemic, we have now a better understanding that different therapeutics are to be deployed for the different phases of SARS-CoV-2 infection: use of antivirals for early infection, and immune modulators like steroids in the later ensuing hyperinflammatory phase. Our study supports the stance of adopting an antiviral and immune-augmenting strategy at the earliest opportunity following infection by the SARS-CoV-2 virus. This has been as demonstrated by use of the antiviral remdesivir (53) or the immunostimulatory pegylated interferon lambda (54) in early COVID-19 infection which led to reduced hospitalisation risks. And yet in another major clinical study of patients at high risk of severe disease progression, early combination remdesivir and interferon beta-1b treatment shortened viral shedding, alleviated symptoms faster and reduced hospitalisation (55).
SARS-CoV-2 infections will persist in the years to come. In tandem with ongoing epidemiological interests in chasing the dynamic evolution of the circulating virus strains, continued studies to better understand the other critical aspect determining disease outcome v.i.z. the host and underlying traits predisposing to severe disease are important. This is achieved through understanding pathogenesis to guide development of new treatment or management strategies including genetic stratification tests, which may be less challenging and more fruitful than chasing a moving target with monoclonal antibody development against a continuously evolving virus. Of the discovery studies to date on the genetic factors affecting SARS-CoV-2 infection risk which have been mainly associative in method, we have elicited a novel gene variant of NUDCD1 in Asian patients which influences disease severity. This has been validated mechanistically to highlight its functional role and disease causation.
Data availability statement
The data presented in the study are deposited in the NCBI Sequence Read Archive (SRA) under BioProject ID PRJNA1266378, and are available at the following link: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1266378.
Ethics statement
The studies involving humans were approved by National Healthcare Group Domain Specific Research Board (No.2012/00917 and No.2020/00435). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AA: Methodology, Validation, Writing – review & editing. DT: Investigation, Methodology, Validation, Writing – review & editing. YS: Methodology, Resources, Writing – review & editing. ML: Formal analysis, Software, Visualization, Writing – review & editing. SR: Methodology, Project administration, Writing – review & editing. JY: Software, Visualization, Writing – review & editing. SL: Project administration, Validation, Writing – review & editing. S-WF: Conceptualization, Visualization, Writing – review & editing. CJML: Formal analysis, Methodology, Visualization, Writing – review & editing. JL: Methodology, Software, Validation, Writing – review & editing. LG: Software, Writing – review & editing. WK: Supervision, Validation, Writing – review & editing. YD: Supervision, Validation, Visualization, Writing – review & editing. QS: Data curation, Formal analysis, Writing – review & editing. ZT: Methodology, Writing – review & editing, Investigation, Visualization. RT: Methodology, Visualization, Writing – review & editing. CL: Validation, Writing – review & editing. JC: Investigation, Resources, Visualization, Writing – review & editing. AS: Conceptualization, Methodology, Writing – review & editing. SP: Formal analysis, Writing – review & editing. WC: Supervision, Visualization, Writing – review & editing. LR: Conceptualization, Methodology, Writing – review & editing. DL: Conceptualization, Project administration, Writing – review & editing. LN: Investigation, Resources, Writing – review & editing. KT: Resources, Supervision, Writing – original draft. RF: Methodology, Software, Writing – review & editing. CM: Methodology, Resources, Writing – review & editing. BY: Software, Supervision, Visualization, Writing – review & editing. LC: Conceptualization, Funding acquisition, Investigation, Resources, Writing – original draft, Formal analysis, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The BSL-3 work was supported by funds from the National Medical Research Council (NMRC), Singapore, NMRC/CG/M009/2017 NUH/NUHS (NUSMed BSL-3), NUS Reimagine Research Grant (NUSMed BSL-3) and NUSMed BSL3 S3RT Grant (NUSMed BSL-3). Subject recruitment and sample collection were funded by the NMRC, Singapore. L.Y.A.C. was supported by the Clinician Scientist Awards (CSA Senior Investigator and Investigator) from NMRC, Singapore, the National University Health System, Singapore and the National Research Foundation, Singapore.
Acknowledgments
We acknowledge assistance rendered by members of the COVID-19 Study Group, A*STAR Infectious Diseases Labs (A*STAR ID Labs), Agency for Science, Technology and Research (A*STAR), Singapore. We thank the NUS Medicine BSL-3 Core Facility team for their operational support in BSL-3 related experiments. We thank Prof Linfa Wang, Prof Eng Eong Ooi and Dr Naiyang Fu from Duke-NUS Medical School, Singapore, for their advice around this project. We are also grateful to Prof Linfa Wang for providing the A549-ACE2 cells, and NUS Infectious Diseases Translational Research Programme for providing the SARS-CoV-2 virus used in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. da Silva JF, Hernandez-Romieu AC, Browning SD, Bruce BB, Natarajan P, Morris SB, et al. COVID19 clinical phenotypes: presentation and temporal progression of disease in a cohort of hospitalized adults in Georgia, United States. Open Forum Infect Dis. (2021). doi: 10.1093/ofid/ofaa596
2. Severe Covid-19 GWAS Group, Ellinghaus D, Degenhardt F, Bujanda L, Buti M, Albillos A, et al. Genomewide association study of severe covid-19 with respiratory failure. N Engl J Med. (2020) 383:1522–34. doi: 10.1056/NEJMoa2020283
3. Pairo-Castineira E, Clohisey S, Klaric L, Bretherick AD, Rawlik K, Pasko D, et al. Genetic mechanisms of critical illness in COVID-19. Nature. (2021) 591:92–8. doi: 10.1038/s41586-020-03065-y
4. Kousathanas A, Pairo-Castineira E, Rawlik K, Stuckey A, Odhams CA, Walker S, et al. Whole-genome sequencing reveals host factors underlying critical COVID-19. Nature. (2022) 607:97–103. doi: 10.1038/s41586-022-04576-6
5. Shelton JF, Shastri AJ, Ye C, Weldon CH, Filshtein-Sonmez T, Coker D, et al. Trans-ancestry analysis reveals genetic and nongenetic associations with COVID-19 susceptibility and severity. Nat Genet. (2021) 53:801–8. doi: 10.1038/s41588-021-00854-7
6. Wu P, Ding L, Li X, Liu S, Cheng F, He Q, et al. Trans-ethnic genome-wide association study of severe COVID-19. Commun Biol. (2021) 4:1034. doi: 10.1038/s42003-021-02549-5
7. Li Y, Ke Y, Xia X, Wang Y, Cheng F, Liu X, et al. Genome-wide association study of COVID-19 severity among the Chinese population. Cell Discov. (2021) 7:76. doi: 10.1038/s41421-021-00318-6
8. Gong B, Huang L, He Y, Xie W, Yin Y, Shi Y, et al. A genetic variant in IL-6 lowering its expression is protective for critical patients with COVID-19. Signal Transduct Target Ther. (2022) 7:112. doi: 10.1038/s41392-022-00923-1
9. Zecevic M, Kotur N, Ristivojevic B, Gasic V, Skodric-Trifunovic V, Stjepanovic M, et al. Genome-wide association study of COVID-19 outcomes reveals novel host genetic risk loci in the Serbian population. Front Genet. (2022) 13:911010. doi: 10.3389/fgene.2022.911010
10. Pandit R, Singh I, Ansari A, Raval J, Patel Z, Dixit R, et al. First report on genome wide association study in western Indian population reveals host genetic factors for COVID-19 severity and outcome. Genomics. (2022) 114:110399. doi: 10.1016/j.ygeno.2022.110399
11. Namkoong H, Edahiro R, Takano T, Nishihara H, Shirai Y, Sonehara K, et al. DOCK2 is involved in the host genetics and biology of severe COVID-19. Nature. (2022) 609:754–60. doi: 10.1038/s41586-022-05163-5
12. Pereira AC, Bes TM, Velho M, Marques E, Jannes CE, Valino KR, et al. Genetic risk factors and COVID-19 severity in Brazil: results from BRACOVID study. Hum Mol Genet. (2022) 31:3021–31. doi: 10.1093/hmg/ddac045
13. Cruz R, Diz-de Almeida S, López de Heredia M, Quintela I, Ceballos FC, Pita G, et al. Novel genes and sex differences in COVID-19 severity. Hum Mol Genet. (2022) 31:3789–806. doi: 10.1093/hmg/ddac132
14. COVID-19 Host Genetics Initiative. Mapping the human genetic architecture of COVID-19. Nature. (2021) 600:472–7. doi: 10.1038/s41586-021-03767-x
15. COVID-19 Host Genetics Initiative. A first update on mapping the human genetic architecture of COVID-19. Nature. (2022) 608:E1–E10. doi: 10.1038/s41586-022-04826-7
16. Horowitz JE, Kosmicki JA, Damask A, Sharma D, Roberts GHL, Justice AE, et al. Genome-wide analysis provides genetic evidence that ACE2 influences COVID-19 risk and yields risk scores associated with severe disease. Nat Genet. (2022) 54:382–92. doi: 10.1038/s41588-021-01006-7
17. Degenhardt F, Ellinghaus D, Juzenas S, Lerga-Jaso J, Wendorff M, Maya-Miles D, et al. Detailed stratified GWAS analysis for severe COVID-19 in four European populations. Hum Mol Genet. (2022) 31:3945–66. doi: 10.1093/hmg/ddac158
18. Wu D, Dou J, Chai X, Bellis C, Wilm A, Shih CC, et al. Large-scale whole-genome sequencing of three diverse Asian populations in Singapore. Cell. (2019) 179:736–749 e15. doi: 10.1016/j.cell.2019.09.019
19. Butler-Laporte G, Povysil G, Kosmicki JA, Cirulli ET, Drivas T, Furini S, et al. Exome-wide association study to identify rare variants influencing COVID-19 outcomes: Results from the Host Genetics Initiative. PloS Genet. (2022) 18:e1010367. doi: 10.1371/journal.pgen.1010367
20. Initiative C-HG. The COVID-19 Host Genetics Initiative, a global initiative to elucidate the role of host genetic factors in susceptibility and severity of the SARS-CoV-2 virus pandemic. Eur J Hum Genet. (2020) 28:715–8. doi: 10.1038/s41431-020-0636-6
21. Casanova JL, Conley ME, Seligman SJ, Abel L, and Notarangelo LD. Guidelines for genetic studies in single patients: lessons from primary immunodeficiencies. J Exp Med. (2014) 211:2137–49. doi: 10.1084/jem.20140520
22. McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsk A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. (2010) 20:1297–303. doi: 10.1101/gr.107524.110
23. Li H and Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. (2009) 25:1754–60. doi: 10.1093/bioinformatics/btp324
24. Picard Toolkit. Broad institute, gitHub repository. Broad Institute (2019). Available at: https://broadinstitute.github.io/picard/ (Accessed June 14, 2021).
25. McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GR, Thormann A, et al. The ensembl variant effect predictor. Genome Biol. (2016) 17:122. doi: 10.1186/s13059-016-0974-4
26. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. (2015) 17:405–24. doi: 10.1038/gim.2015.30
27. Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. (2007) 81:559–75. doi: 10.1086/519795
28. Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA 3rd, and Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. (2009) 6:343–5. doi: 10.1038/nmeth.1318
29. Schrödinger L and DeLano W. PyMOL . Available online at: http://www.pymol.org/pymol (Accessed April 28, 2023).
30. GTEx portal NUDCD1 . Available online at: https://gtexportal.org/home/gene/NUDCD1 (Accessed October 18, 2023).
31. ProteomicsDB. NudC domain-containing protein 1 . Available online at: https://www.proteomicsdb.org/protein/78018/expression (Accessed October 18, 2023).
32. Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, Fishilevich S, et al. The geneCards suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinf. (2016) 54:1 30 1–1 30 33. doi: 10.1002/cpbi.5
33. Asselin-Mullen P, Chauvin A, Dubois ML, Drissi R, Lévesque D, Boisvert FM, et al. Protein interaction network of alternatively spliced NudCD1 isoforms. Sci Rep. (2017) 7:12987. doi: 10.1038/s41598-017-13441-w
34. Mosallanejad K, Sekine Y, Ishikura-Kinoshita S, Kumagai K, Nagano T, Matsuzawa A, et al. The DEAH-box RNA helicase DHX15 activates NF-kappaB and MAPK signaling downstream of MAVS during antiviral responses. Sci Signal. (2014) 7:ra40. doi: 10.1126/scisignal.2004841
35. Wu B and Hur S. How RIG-I like receptors activate MAVS. Curr Opin Virol. (2015) 12:91–8. doi: 10.1016/j.coviro.2015.04.004
36. MacArthur DG, Manolio TA, Dimmock DP, Rehm HL, Shendure J, Abecasis GR, et al. Guidelines for investigating causality of sequence variants in human disease. Nature. (2014) 508:469–76. doi: 10.1038/nature13127
37. Pedersen BS, Brown JM, Dashnow H, Wallace AD, Velinder M, Tristani-Firouzi M, et al. Effective variant filtering and expected candidate variant yield in studies of rare human disease. NPJ Genom Med. (2021) 6:60. doi: 10.1038/s41525-021-00227-3
38. Auer PL and Lettre G. Rare variant association studies: considerations, challenges and opportunities. Genome Med. (2015) 7:16. doi: 10.1186/s13073-015-0138-2
39. Garcia FAO, de Andrade ES, and Palmero EI. Insights on variant analysis in silico tools for pathogenicity prediction. Front Genet. (2022) 13:1010327. doi: 10.3389/fgene.2022.1010327
40. Yang XF, Wu CJ, McLaughlin S, Chillemi A, Wang KS, Canning C, et al. CML66, a broadly immunogenic tumor antigen, elicits a humoral immune response associated with remission of chronic myelogenous leukemia. Proc Natl Acad Sci U S A. (2001) 98:7492–7. doi: 10.1073/pnas.131590998
41. Yan Y, Phan L, Yang F, Talpaz M, Yang Y, Xiong Z, et al. A novel mechanism of alternative promoter and splicing regulates the epitope generation of tumor antigen CML66-L. J Immunol. (2004) 172:651–60. doi: 10.4049/jimmunol.172.1.651
42. Miller BA, Zhang MY, Gocke CD, De Souza C, Osmani AH, Lynch C, et al. A homolog of the fungal nuclear migration gene nudC is involved in normal and Malignant human hematopoiesis. Exp Hematol. (1999) 27:742–50. doi: 10.1016/S0301-472X(98)00074-5
43. Consortium GT. The genotype-tissue expression (GTEx) project. Nat Genet. (2013) 45:580–5. doi: 10.1038/ng.2653
44. Wu C, Orozco C, Boyer J, Leglise M, Goodale J, Batalov S, et al. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. (2009) 10:R130. doi: 10.1186/gb-2009-10-11-r130
45. Schmidt T, Samaras P, Frejno M, Gessulat S, Barnert M, Kienegger H, et al. ProteomicsDB. Nucleic Acids Res. (2018) 46:D1271–81. doi: 10.1093/nar/gkx1029
46. Gocke CD, Osmani SA, and Miller BA. The human homologue of the Aspergillus nuclear migration gene nudC is preferentially expressed in dividing cells and ciliated epithelia. Histochem Cell Biol. (2000) 114:293–301. doi: 10.1007/s004180000197
47. Shao Y, Saredy J, Xu K, Sun Y, Saaoud F, Drummer C, et al. Endothelial immunity trained by coronavirus infections, DAMP stimulations and regulated by anti-oxidant NRF2 may contribute to inflammations, myelopoiesis, COVID-19 cytokine storms and thromboembolism. Front Immunol. (2021) 12:653110. doi: 10.3389/fimmu.2021.653110
48. InterPro. HSP20-like chaperone . Available online at: http://www.ebi.ac.uk/interpro/entry/InterPro/IPR008978 (Accessed October 10, 2023).
49. Garcia-Ranea JA, Mirey G, Camonis J, and Valencia A. p23 and HSP20/alpha-crystallin proteins define a conserved sequence domain present in other eukaryotic protein families. FEBS Lett. (2002) 529:162–7. doi: 10.1016/S0014-5793(02)03321-5
50. Sin YY and Baillie GS. Heat shock protein 20 (HSP20) is a novel substrate for protein kinase D1 (PKD1). Cell Biochem Funct. (2015) 33:421–6. doi: 10.1002/cbf.v33.7
51. Taipale M, Tucker G, Peng J, Krykbaeva I, Lin ZY, Larsen B, et al. A quantitative chaperone interaction network reveals the architecture of cellular protein homeostasis pathways. Cell. (2014) 158:434–48. doi: 10.1016/j.cell.2014.05.039
52. Xing J, Zhou X, Fang M, Zhang E, Minze LJ, and Zhang Z. DHX15 is required to control RNA virus-induced intestinal inflammation. Cell Rep. (2021) 35:109205. doi: 10.1016/j.celrep.2021.109205
53. Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, et al. Early remdesivir to preventProgression to severe covid-19 in outpatients. N Engl J Med. (2022) 386:305–15. doi: 10.1056/NEJMoa2116846
54. Reis G, Moreira Silva EAS, Medeiros Silva DC, Thabane L, Campos VHS, Ferreira TS, et al. Early treatment with pegylated interferon lambda for covid-19. N Engl J Med. (2023) 388:518–28. doi: 10.1056/NEJMoa2209760
55. Tam AR, Zhang RR, Lung KC, Liu R, Leung KY, Liu D, et al. Early treatment of high-risk hospitalized coronavirus disease 2019 (COVID-19) patients with a combination of interferon beta-1b and remdesivir: A phase 2 open-label randomized controlled trial. Clin Infect Dis. (2023) 76:e216–26. doi: 10.1093/cid/ciac523
Keywords: SARS-CoV-2, innate immunity, DEAH-Box helicase, type I interferon, Southeast Asia
Citation: Anusha Amali A, Tay DJW, Seow Y, Loh M, Ravikumar S, Yu JJ, Loong SSE, Fong SW, Lee CJM, Lim JJC, Gan LH, Koh WLC, Ding Y, Sam QH, Tan Z, Tan RYM, Lua CB, Chu JJH, Singhal A, Prabhakar S, Chng WJ, Renia L, Lye DCB, Ng LFP, Tan KS, Foo R, Lee CCM, Young B and Chai LYA (2025) Novel NUDCD1 gene variant predisposes to severe COVID-19 disease in Asians through modulation of antiviral DHX15- and MAVS-mediated signalling. Front. Immunol. 16:1581293. doi: 10.3389/fimmu.2025.1581293
Received: 21 February 2025; Accepted: 15 April 2025;
Published: 04 June 2025.
Edited by:
Xiaoyu Zhao, Fudan University, ChinaReviewed by:
Valentina Di Salvatore, University of Catania, ItalyKarthikeyan Subbarayan, Martin Luther University of Halle-Wittenberg, Germany
Copyright © 2025 Anusha Amali, Tay, Seow, Loh, Ravikumar, Yu, Loong, Fong, Lee, Lim, Gan, Koh, Ding, Sam, Tan, Tan, Lua, Chu, Singhal, Prabhakar, Chng, Renia, Lye, Ng, Tan, Foo, Lee, Young and Chai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chang Chuan Melvin Lee, bWVsdmluLmxlZUB1cS5lZHUuYXU=; Louis Yi Ann Chai, Y2hhaWxvdWlzQGhvdG1haWwuY29t
†These authors have contributed equally to this work
‡These authors have contributed equally to this work
§These authors have contributed equally to this work
 Aseervatham Anusha Amali1†
Aseervatham Anusha Amali1† Douglas Jie Wen Tay
Douglas Jie Wen Tay Yiqi Seow
Yiqi Seow Marie Loh
Marie Loh Shaun Seh Ern Loong
Shaun Seh Ern Loong Siew Wai Fong
Siew Wai Fong Chang Jie Mick Lee
Chang Jie Mick Lee Qi Hui Sam
Qi Hui Sam Justin Jang Hann Chu
Justin Jang Hann Chu Laurent Renia
Laurent Renia Lisa F. P. Ng
Lisa F. P. Ng Kai Sen Tan
Kai Sen Tan Louis Yi Ann Chai
Louis Yi Ann Chai