- 1Department of Microbiology and Immunology, University of Nevada, Reno School of Medicine, Reno, NV, United States
- 2Department of Microbiology and Immunology, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand
Melioidosis, caused by the Gram-negative bacterium Burkholderia pseudomallei, is a severe infectious disease that is responsible for a significant amount of morbidity and mortality in endemic areas. While the majority of melioidosis cases occur in Southeast Asia, South Asia and Northern Australia, the disease is being increasingly recognized across tropical and subtropical regions worldwide. Due to diagnostic and treatment challenges as well as the potential misuse of B. pseudomallei as a biothreat agent, an effective vaccine is critically needed. Over the years, numerous different strategies have been explored to develop melioidosis vaccines. Based on the choice of protective antigens, many of the resulting candidates would also be predicted to provide some level of protection against Burkholderia mallei, the etiologic agent of glanders. In this review, we examine the different approaches that have recently been used to develop melioidosis vaccine candidates, highlighting both traditional and emerging vaccine platform technologies. Using these approaches, several promising melioidosis and glanders candidates have been identified with pre-clinical animal studies providing valuable insights into the immunogenic and protective capacities of these potential vaccines. Collectively, this review summarizes recent advancements in melioidosis vaccine research and highlights critical findings that will help guide a path toward the development of a safe, effective and affordable vaccine to combat disease caused by B. pseudomallei.
1 Introduction
Burkholderia pseudomallei is the causative agent of melioidosis, a severe infectious disease that is known to be endemic in 45 countries in Southeast Asia, South Asia, the Middle East, Africa, Central America, and South America (1, 2). Regions where the disease is being detected are increasing and models predict an additional 34 countries where it is likely present but yet to be reported (2). Notably, there have been recent reports of locally acquired cases of melioidosis in the U.S. making this disease more widespread than previously appreciated (3). In a study published in 2016, the estimated incidence of disease was ~165,000 cases per year worldwide with ~89,000 associated deaths (2). Melioidosis has a wide range of clinical manifestations that vary from chronic localized infections to acute pneumonias and fulminant sepsis. As a result of poor diagnostics, a lack of clinical and laboratory expertise in endemic regions, and misdiagnosis due to diverse clinical presentations, the disease is severely underreported (4–10).
B. pseudomallei is a motile, facultative-intracellular, Gram-negative pathogen that is found in moist soils, surface waters and untreated potable water systems in tropical and subtropical regions (2, 11). Typical routes of inoculation for humans include inhalation, ingestion, and percutaneous inoculation (11, 12). B. pseudomallei is inherently resistant to a wide range of antibiotics including β-lactams, aminoglycosides, macrolides, and polymyxins. Effective treatment typically involves intravenous ceftazidime or meropenem for 10 to 14 days followed by oral co-trimoxazole for 3 to 6 months (11, 13–16). Previous studies have shown that antibiotic-resistant strains of B. pseudomallei can develop during the course of treatment and can lead to poor outcomes (11, 15, 17, 18). Even with appropriate treatment, B. pseudomallei infections cause significant morbidity and mortality in endemic regions.
Burkholderia mallei is a closely related pathogen that is transmitted to humans from solipeds (i.e. horses, mules, donkeys) and causes glanders. B. mallei is a genetically similar species that evolved from B. pseudomallei via genome reduction (19, 20). These two facultative-intracellular pathogens express similar key virulence factors including lipopolysaccharide (LPS), capsular polysaccharide (CPS), the bsa type III secretion system (T3SS-3) and the cluster 1 type IV secretion system (T6SS-1) (21–25). Because of this, it is conceivable that a vaccine could be designed to provide protective immunity against both melioidosis and glanders (26–30). Currently, B. pseudomallei and B. mallei are considered potential biothreat agents that are categorized as Tier 1 select agents by the U.S. Centers for Disease Control and Prevention (CDC) (31, 32). Historically, B. mallei was used as a biological weapon in the American Civil War and World War II (27, 33).
An effective vaccine for immunization against melioidosis and glanders would be an important countermeasure for both public health and biodefense purposes. This review examines advances in melioidosis and glanders vaccine development over the past seven years, and focuses on work encompassing live-attenuated vaccines (LAVs), glycoconjugate-based and protein-based subunit vaccines, outer membrane vesicle (OMV) vaccines, nanoparticle-based vaccines, virus-like particle (VLP) vaccines, as well as DNA and viral vector-based vaccines (Figure 1). While there are currently no licensed vaccines available to protect against disease caused by these bacterial pathogens, several of the platforms discussed in this review have been shown to provide significant protection in animal models of melioidosis and/or glanders (Table 1; Figure 2).
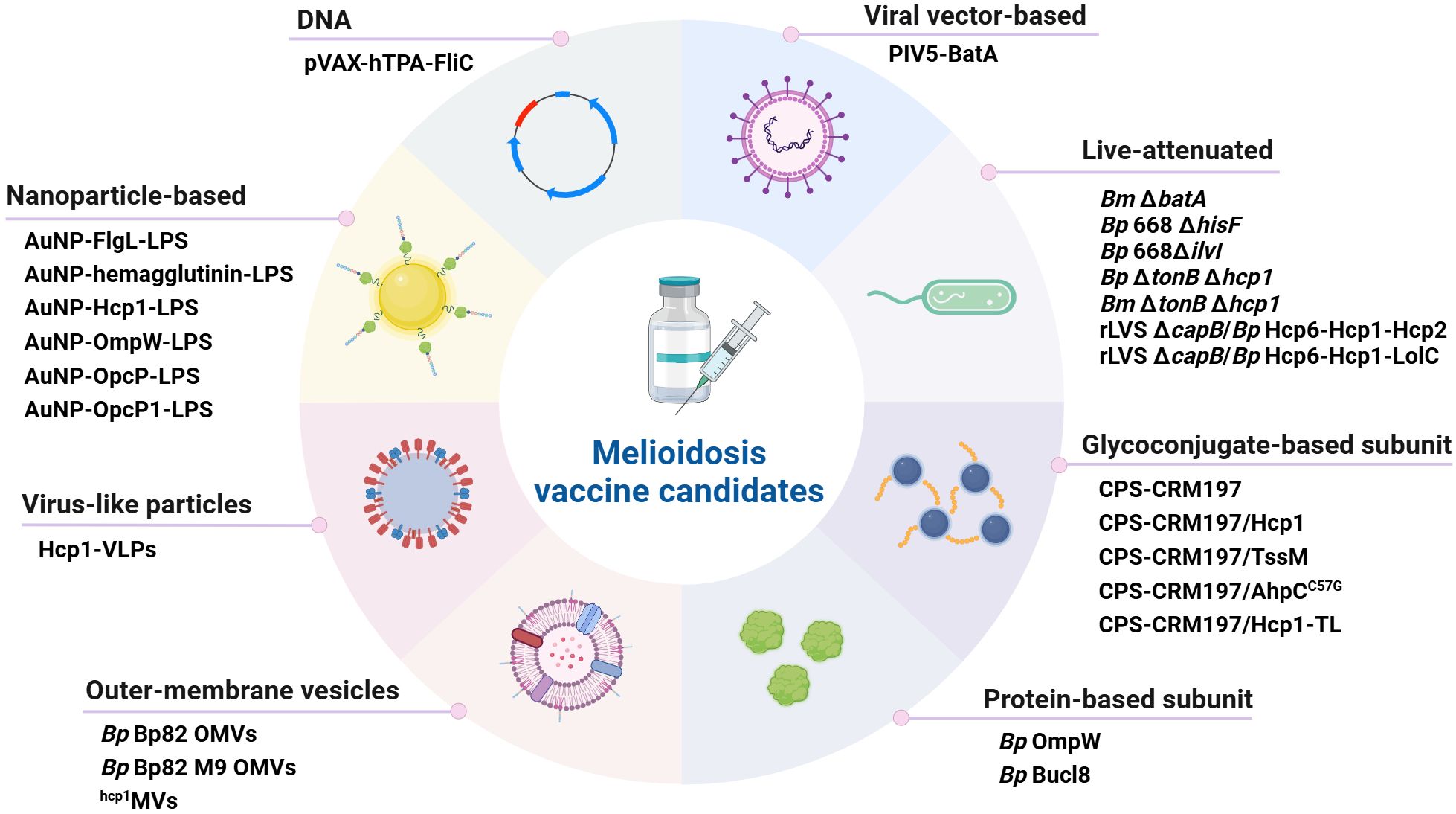
Figure 1. Classification of melioidosis vaccine candidates. Several different platforms have recently been used to develop a variety of promising vaccine candidates. The main types of vaccines that have been developed are i) live-attenuated, ii) glycoconjugate-based subunit, iii) protein-based subunit, iv) outer membrane vesicle, v) nanoparticle-based, vi) virus-like particles, vii) DNA and viii) viral vector-based. Bp, B. pseudomallei; Bm, B. mallei. Created in BioRender. Sengyee, S (2025). https://BioRender.com/1ypkjdc.
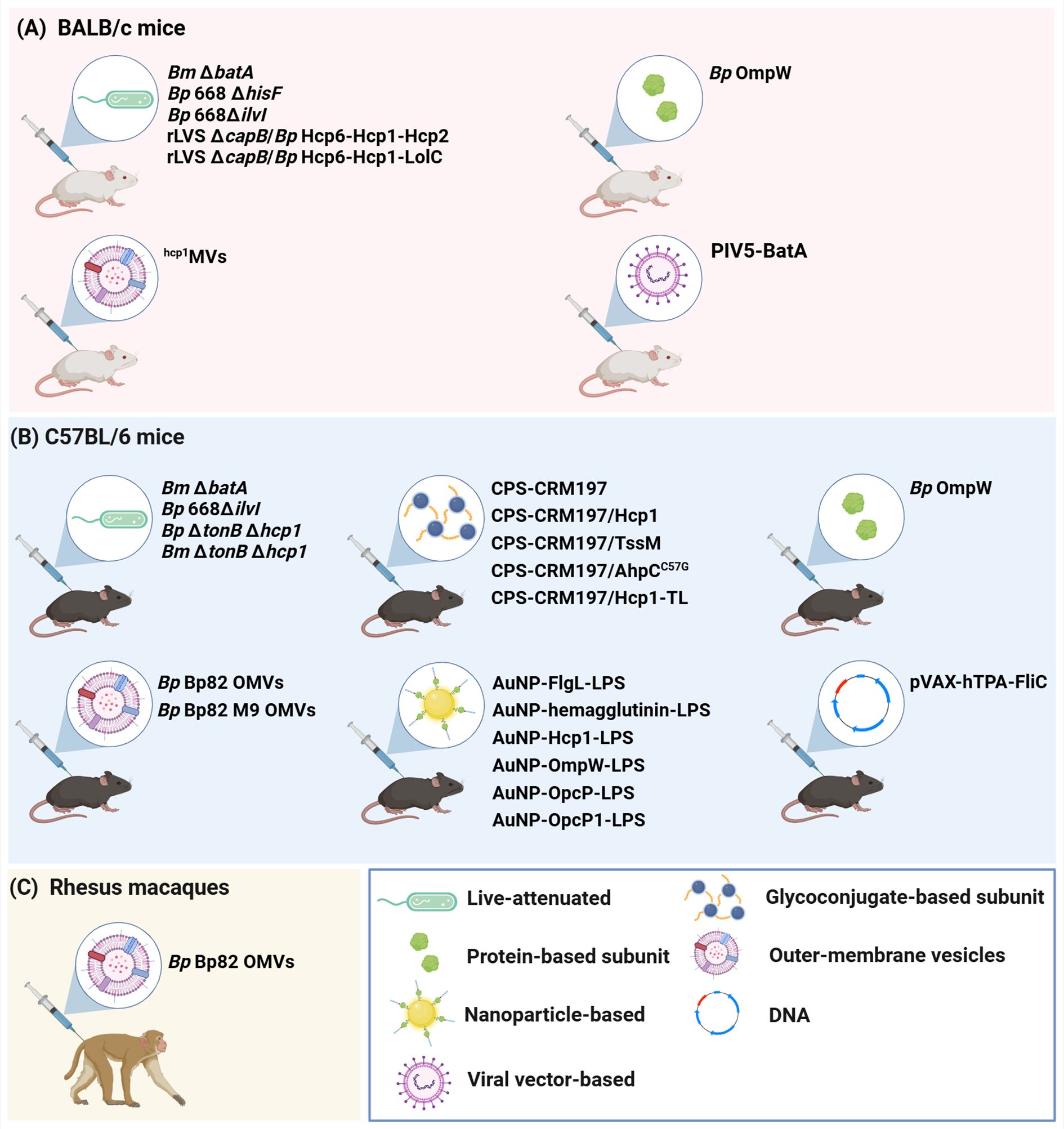
Figure 2. Animal models used for the development of melioidosis vaccine candidates. Three main animal models have been used to evaluate the immunogenicity and protective capacity of the melioidosis vaccine candidates discussed in this review. These are (A) BALB/c mice, (B) C57BL/6 mice and (C) Rhesus macaques. Bp, B. pseudomallei; Bm, B. mallei. Created in BioRender. Sengyee, S. (2025) https://BioRender.com/wdjhqlt.
2 Vaccine platforms
2.1 Live-attenuated vaccines
LAV strains designed to provide protection against melioidosis and/or glanders have been developed and evaluated in both BALB/c and C57BL/6 mouse models (Table 1). While some LAV strains have been shown to induce protective immunity against B. pseudomallei, these types of vaccines have associated risks including the possibilities of reversion to wild-type virulence and development of latent infections (34). Recently, B. mallei ΔbatA, which harbors a mutation in the autotransporter protein BatA, was used to immunize mice against lethal intratracheal challenges with B. mallei and B. pseudomallei (35). Immunization of BALB/c and C57BL/6 mice with 104 CFU of this LAV strain stimulated robust antibody responses and resulted in 56-100% and 67-100% survival against challenges with B. mallei and B. pseudomallei, respectively (Table 1) (35). Furthermore, analysis of humoral immune responses from BALB/c mice immunized with ΔbatA demonstrated that robust B. mallei-specific IgG titers were generated with a strong Th1-bias (as evidenced by high IgG2a/IgG1 ratios) and that the serum enhanced uptake of opsonized bacteria as well as promoted effective intracellular killing by macrophages (35). In addition, passive transfer of the immune serum to mice provided equivalent levels of protection to ΔbatA immunized mice when they were challenged intratracheally with B. mallei or B. pseudomallei (35).
Several different single gene auxotrophs constructed in B. pseudomallei MSHR668 have been evaluated as LAVs in BALB/c mice with the most effective strains being B. pseudomallei 668 ΔhisF and 668 ΔilvI (36). Subcutaneous immunization of BALB/c mice with two doses of B. pseudomallei 668 ΔhisF or 668 ΔilvI demonstrated similar levels of protection against intraperitoneal challenges with a 50-fold median lethal dose (MLD50) of B. pseudomallei K96243, with survival rates of 100% at day 25 or 21, respectively, and 50% at day 60 (Table 1) (36). The serum levels of B. pseudomallei-specific IgG were similar in mice immunized with B. pseudomallei 668 ΔhisF and 668 ΔilvI (36). Upon re-stimulation, splenocytes obtained from mice immunized with B. pseudomallei 668 ΔhisF or 668 ΔilvI displayed significantly increased IFN-γ cytokine responses compared to the phosphate buffered saline control group, suggesting that cellular immune responses contribute to protection against B. pseudomallei infection (36).
Additionally, the B. pseudomallei 668 ΔilvI LAV was evaluated in C57BL/6 mice, and results revealed that subcutaneous immunizations with this strain conferred survival rates of 40-60% and 85% at day 60 against lethal aerosol challenges of B. pseudomallei and B. mallei, respectively (Table 1) (37). IgG responses were measured against killed whole-cells and purified B. pseudomallei O-polysaccharide (OPS) and shown to correlate with protection in B. pseudomallei 668 ΔilvI-immunized C57BL/6 mice. Analysis of cytokine profiles of lung homogenates obtained post-challenge with B. pseudomallei K96243 revealed that the levels of IFN-γ and IL-22 had increased significantly suggesting that these cytokines correlated with protective immunity in the surviving mice (37). Recently, a combination of B. pseudomallei 668 ΔilvI vaccination and co-trimoxazole treatment delivered every 12 hours for either 7 or 21 days demonstrated improved protection in C57BL/6 mice against an inhalational challenge with B. pseudomallei K96243 compared to immunized mice with no post-exposure antibiotic co-treatment. This combined approach provided 80-100% survival for up to 86 days post challenge (38).
Safety concerns associated with LAV strains include tolerance induction, autoimmune exacerbation, and reversion to wild type virulence. Because of this, introducing attenuating mutations at multiple sites is preferred to reduce the chances of reversion to virulent phenotypes (39). To address this concern, strains harboring mutations in the tonB and hcp1 genes were constructed in both B. pseudomallei K96243 and B. mallei ATCC 23344. These ΔtonB Δhcp1 double mutants were deficient in iron acquisition, intracellular spread, and ability to stimulate multinucleated giant cell formation (40–43). Immunization of C57BL/6 mice with the B. pseudomallei ΔtonB Δhcp1 strain stimulated strong Th1-biased humoral immune responses (IgG2a > IgG1) when serum was titered against irradiated B. pseudomallei K96243. Additionally, robust IFN-γ, TNF-α and IL-17A cytokine production was observed in cell supernatants following the re-stimulation of splenocytes with heat-killed B. pseudomallei K96243 whole cell lysates (40). Upon a lethal inhalational challenge with B. pseudomallei, 100% of the mice immunized with B. pseudomallei ΔtonB Δhcp1 survived until day 27, exhibited low bacterial loads (less than 20 CFU/organ) and minimal pathological changes in lungs, livers, and spleens (Table 1) (40). When mice depleted of CD4+ or CD8+ T cells were immunized with B. pseudomallei ΔtonB Δhcp1 and then challenged with B. pseudomallei K96243 results demonstrated that the absence of these T cells did not significantly affect the levels of survival, suggesting that protective immunity against B. pseudomallei primarily correlated with humoral immune responses in this study (40).
Recently, the cross-protective properties of B. mallei ΔtonB Δhcp1 have been examined in mouse models of both glanders and melioidosis (42, 43). B. mallei ΔtonB Δhcp1 provided C57BL/6 mice with 100% protection at day 21 against both intranasal and inhalational challenges of B. mallei ATCC 23344, and 87.5% protection at day 27 following an inhalational challenge with B. pseudomallei K96243 (Table 1) (42). The surviving mice demonstrated significant reductions in bacterial burdens in the lungs, livers, and spleens with 87.5% and 50% sterilizing immunity in the intranasal and inhalational challenge experiments, respectively (42). Immunization with B. mallei ΔtonB Δhcp1 stimulated high levels of B. mallei-specific IgG, IgG1, and IgG2a in serum as well as robust IFN-γ and IL-17A cytokine responses in cell supernatants following re-stimulation of splenocytes with heat-killed B. mallei ATCC 23344 or B. pseudomallei K96243 whole cell lysates (42). Consistent with previous findings, depletion of CD4+ or CD8+ T cells showed no difference in levels of protection or bacterial burdens in immunized mice. These results supported that humoral immune responses play a major role in the protective capacity of the ΔtonB Δhcp1 LAV strains (40, 42).
Khakhum et al. also evaluated the immune correlates of protection following the immunization of C57BL/6 mice with B. pseudomallei ΔtonB Δhcp1 and B. mallei ΔtonB Δhcp1. Their results confirmed that both LAV strains elicited strong B. pseudomallei-specific serum IgM, IgG2b, and IgG2c responses that promoted bacterial uptake and enhanced bacterial killing by macrophages (43). However, passive transfer of serum from mice immunized with the ΔtonB Δhcp1 LAV strains to naïve mice did not provide protection against inhalational challenges with B. pseudomallei K96243 (43). Interestingly, the ΔtonB Δhcp1 LAV strains stimulated robust mucosal immune responses in the lungs, particularly IgA as well as Th1- and Th17-like CD4+ T cell responses. Histological analysis of lung tissues from immunized mice challenged with B. pseudomallei revealed only mild to moderate lung inflammation, suggesting that controlled immune activation stimulated protective immunity (43).
A more recent study by Tullius et al., used derivatives of the Francisella tularensis Live Vaccine Strain (LVS) ΔcapB that were engineered to express B. pseudomallei antigens as novel vaccine candidates (44). LVS, derived from F. tularensis subsp. holarctica, is a less virulent subspecies of F. tularensis that has been previously used to construct vaccine candidates for tularemia, anthrax, plague, and COVID-19 (44). LVS ΔcapB, a mutant lacking a putative capsule synthesis gene, expressing the B. pseudomallei T6SS proteins Hcp1, Hcp2, or Hcp6, or the membrane protein LolC were constructed in different combinations and evaluated for their immunogenicity and protective capacity in BALB/c mice. LVS ΔcapB alone or expressing two, three or four B. pseudomallei proteins (rLVS ΔcapB/Bp proteins) were used to immunize mice via either an intranasal or an intradermal route and then challenged intranasally with 5 LD50 of B. pseudomallei 1026b. The mice that were immunized intradermally with rLVS ΔcapB/Bp-Hcp6-Hcp1 or rLVS ΔcapB/Bp-Hcp6-Hcp2 exhibited 88% survival at day 42 post-challenge, while groups that were immunized with rLVS ΔcapB/Bp-LolC-Hcp1 or rLVS ΔcapB/Bp-LolC-Hcp2 exhibited only 38% survival over the same timeframe (Table 1). The use of the rLVS ΔcapB/Bp-Hcp6-Hcp1 and rLVS ΔcapB/Bp-Hcp6-Hcp2 strains resulted in high levels of sterilizing immunity with 75% and 50% of the mice surviving until day 42, respectively (44).
Since rLVS ΔcapB/Bp-Hcp6-Hcp1 showed promising results with high survival rates and sterilizing immunity, rLVS ΔcapB/Bp vaccine candidates expressing three antigens (Hcp6-Hcp1-Hcp2 or Hcp6-Hcp1-LolC) were constructed. Mice immunized intradermally with rLVS ΔcapB/Bp-Hcp6-Hcp1-Hcp2 or rLVS ΔcapB/Bp-Hcp6-Hcp1-LolC and then challenged with 4 LD50 of B. pseudomallei 1026b intranasally yielded survival rates of 88% and 75%, respectively (Table 1). Additionally, intranasal administration of rLVS ΔcapB/Bp-Hcp6-Hcp1-Hcp2 or rLVS ΔcapB/Bp-Hcp6-Hcp1-LolC provided 75-100% protection against intranasal challenges with 6 LD50 of B. pseudomallei 1026b (Table 1). Notably, intranasal delivery of the various rLVS ΔcapB/Bp vaccine candidates proved to be superior to intradermal delivery and provided robust protection against both low and high challenge doses (Table 1) (44). As observed in previous studies, humoral immunity appeared to dominate the B. pseudomallei antigen-specific immune responses, as all groups immunized with rLVS ΔcapB/Bp strains generated strong serum IgG titers against Hcp1, Hcp6, and LolC. However, no significant increases in antigen-specific T-cell responses were observed (44). While these findings highlight the promising nature of rLVS ΔcapB/Bp vaccine candidates, particularly in inducing robust humoral immunity, the lack of significant T-cell responses suggests that further refinement to enhance cellular immunity may be necessary for optimizing protection against melioidosis.
2.2 Subunit vaccines
Subunit vaccines are composed of one or more purified antigens that induce protective immune responses and are typically formulated with immune-stimulating adjuvants. Adjuvants are used to enhance both humoral and cellular immune responses against the antigens by activating innate immune receptors, promoting antigen uptake and processing/presentation, and stimulating Th1-, Th2- and/or Th17-like responses. For instance, Alhydrogel is known to promote potent Th2-like responses, while monophosphoryl lipid A and CpG oligodeoxynucleotides (CpG) are associated with development of strong Th1-like responses (45–47). Subunit vaccines have several advantages over the use of LAVs including that they are safe, antigenically defined, and pose minimal risk to immunocompromised individuals following immunization. Additionally, they allow for the selection of conserved protective antigens that are capable of eliciting robust immune responses against multiple species or strains within a species (48–51). Limitations of subunit vaccines compared to other platforms, however, are their need for adjuvants and the requirement for multiple doses to achieve optimal protection. Furthermore, production of antigens for these types of vaccines can be technically challenging and costly.
Some of the most recently developed melioidosis subunit vaccines consist of glycoconjugate-based and protein-based formulations (48–54). Glycoconjugate-based subunit vaccines are produced via the covalent linkage of bacterial polysaccharides to carrier proteins to facilitate linked recognition and promote the development of T cell-dependent type immune responses against the polysaccharide component of these hybrid immunogens (48–52). A non-toxic mutant of diphtheria toxin, cross-reacting material 197 (CRM197) is a commonly used protein carrier for glycoconjugate vaccines. CRM197 is used in Haemophilus influenza type b, Streptococcus pneumoniae and Neisseria meningitidis conjugate vaccines, as well as in the Burkholderia polysaccharide-based glycoconjugate vaccine candidates discussed here (55, 56).
Two important and highly conserved polysaccharide antigens expressed by both B. pseudomallei and B. mallei are the virulence associated 6-deoxyheptan CPS (21, 25, 50, 57–60) and the O-polysaccharide (OPS) moieties of LPS (25, 26, 58, 61, 62). Previous studies have shown that immunization with purified Burkholderia CPS and/or LPS provides high levels of protection in mouse models of melioidosis (26, 58, 62). Several studies have also found that CPS- and OPS-specific monoclonal antibodies (mAbs) provide protection against intraperitoneal B. pseudomallei challenges in rats and/or BALB/c mice (30, 63, 64). Additionally, it has been observed that anti-OPS antibody responses were significantly higher in melioidosis survivors in northeastern Thailand compared to individuals who succumbed to infection (65).
Since B. pseudomallei and B. mallei express structurally similar OPS moieties, these antigens are considered as potential candidates for use in the development of glycoconjugate vaccines that could provide protection against both melioidosis and glanders (66). Tamigney Kenfack et al. demonstrated that OPS-specific mAbs exhibited strong interactions with the 6-deoxytalose residue of the 3-O-methylated terminal disaccharides of B. mallei or B. pseudomallei OPS (67). In their study, they constructed synthetic oligosaccharide conjugates (SOC-6 and SOC-7) which represented the terminal disaccharides of B. mallei or B. pseudomallei OPS linked to CRM197, and then evaluated their immunogenicity in BALB/c mice. These synthetic OPS-based glycoconjugates stimulated high levels of antigen-specific IgG, with SOC-6 eliciting higher titers than SOC-7 (67). Enzyme-linked immunosorbent assay (ELISA) results showed that culture-confirmed Thai melioidosis patient samples were also reactive to these synthetic OPS-based glycoconjugates, suggesting that synthetic or native OPS may potentially be useful as a vaccine antigen (67).
CPS is also an attractive antigen for glycoconjugate vaccine development since it is highly conserved in virulent isolates of B. pseudomallei and B. mallei (21, 25, 50, 57–60). When conjugated to the carrier protein CRM197 to form the glycoconjugate CPS-CRM197, T cell dependent-like responses are raised against the polysaccharide component of the molecule, resulting in high-titer CPS-specific antibody responses in C57BL/6 mice (48, 49). In addition to polysaccharides, B. pseudomallei and B. mallei also express several conserved protein antigens including the T6SS-1 associated hemolysin coregulated protein 1 (Hcp1) and the deubitiquinase (TssM), which have been shown to be immunogenic in animal models and correlate with survival in melioidosis patients from Thailand (68, 69).
A recent study focused on the development of CPS-based glycoconjugate subunit vaccine candidates assessed the immunogenicity and protective capacity of CPS-CRM197 when combined with either Hcp1 or TssM. Immunization of C57BL/6 mice with CPS-CRM197, CPS-CRM197 plus Hcp1, or CPS-CRM197 plus TssM, all formulated with Alhydrogel and CpG (ODN 2006) resulted in 67%, 100%, and 80% protection, respectively, at 35 days following an acute inhalational challenge with B. pseudomallei K96243 (Table 1) (48). Notably, 70% of the mice immunized with CPS-CRM197 plus Hcp1 formulation that survived the duration of the experiment had no culturable bacteria in lungs, livers, or splenic tissues. All three test groups produced high titer IgM and IgG responses against CPS. Mice immunized with CPS-CRM197 plus Hcp1 or CPS-CRM197 plus TssM stimulated high-titer IgM and IgG against their respective recombinant Burkholderia proteins. Further analysis of immune serum showed that antibody responses to all antigens were Th1/Th2 balanced based on the IgG2b/IgG1 ratios. Robust IFN-γ-secreting T responses were also observed when splenocytes were re-stimulated with either Hcp1 or TssM (48).
Another highly immunogenic protein that has been identified as a potential vaccine candidate is alkyl hydroperoxide reductase subunit C (AhpC), which is involved in protecting cells from oxidative damage (70). Previous studies have shown that enhanced T-cell responses to AhpC correlate with survival in melioidosis patients, highlighting its potential as a protective antigen (71). In recent studies, CPS-CRM197 plus AhpC harboring an active site mutation (AhpCC57G) formulated with Alhydrogel and CpG (ODN 2006) was used to immunize C57BL/6 mice prior to an inhalational challenge with B. pseudomallei K96243 (49). This formulation elicited high levels of protection, with 70% of immunized mice surviving to day 35 (Table 1) (49). Survival rates were significantly higher than the adjuvant-only control mice but were lower than the levels of protection observed in prior studies using CPS-CRM197 plus Hcp1 or TssM (48). CPS-CRM197 plus AhpCC57G immunized mice produced high titer CPS- and AhpCC57G-specific serum IgG responses, and robust IFN-γ-, IL-5-, and IL-17-secreting T cell responses following the re-stimulation of splenocytes against AhpCC57G (49).
More recently, CPS-CRM197 was used to immunize C57BL/6 mice along with Hcp1-TL and AhpCC57G, or Hcp1-TL alone, both formulated with Alhydrogel and CpG (ODN 2006) (37). Mice in the CPS-CRM197 plus Hcp1-TL/AhpCC57G and CPS-CRM197 plus Hcp1-TL groups were challenged with B. pseudomallei K96243 via an inhalational route, and resulted in survival rates of 50% and 80% by day 60, respectively (Table 1) (37). In a second study, mice immunized with CPS-CRM197 plus Hcp1-TL were challenged via an inhalational route with B. mallei FMH and B. pseudomallei MSHR5855, and showed survival rates of 80% and 35% by day 60, respectively (Table 1) (37). In addition, the CPS-CRM197 plus Hcp1-TL formulation was shown to produce similar levels of protection, cellular and humoral immune responses, and sterilizing immunity when compared to the LAV strain 668 ΔilvI (37).
Extending upon these studies, novel intervention strategies that layer vaccination and post-exposure antibiotic treatment have been conducted with CPS-CRM197-based subunit vaccine candidates. When C57BL/6 mice were immunized with CPS-CRM197 plus Hcp1-TL/AhpCC57G or CPS-CRM197 plus Hcp1-TL, both formulated with Alhydrogel and CpG (ODN 2006), in combination with co-trimoxazole treatment and then challenged with B. pseudomallei K96243 via an inhalational route, 90-100% of mice survived to day 86 (37, 38). CPS-CRM197 plus Hcp1 was also evaluated in combination with the fluoroquinolone antibiotic, finafloxacin, against inhalational challenges of B. pseudomallei K96243 in BALB/c mice (72). In this study, mice were immunized subcutaneously with CPS-CRM197 plus Hcp1 formulated with Alhydrogel alone or CPS-CRM197 plus Hcp1 formulated with Alhydrogel and CpG (ODN 2006) and finafloxacin treatment was initiated at 36 or 48 h post-challenge. Notably, the formulation resulted in a synergistic effect only when CpG (ODN 2006) was included and when finafloxacin treatment was started at 48 h post-challenge. Mice that were immunized with CPS-CRM197 plus Hcp1 formulated with Alhydrogel and CpG (ODN 2006) and treated with finafloxacin exhibited 80% survival up to 35 days post challenge with B. pseudomallei K96243. In contrast, groups that were immunized with CPS-CRM197 plus Hcp1 formulated with Alhydrogel alone and then treated with finafloxacin showed only 40% survival (72).
Several B. pseudomallei protein antigens including LolC, PotF, OppA, and various outer membrane proteins (e.g., Omp3, Omp7, Omp85 and OmpW) have been identified and evaluated as potential candidates for use in protein-based subunit vaccines (53, 73–75). Casey et al. assessed the protective efficacy of OmpW formulated with the Sigma-adjuvant system (SAS), which is composed of monophosphoryl lipid A (TLR-4 ligand) and trehalose dicorynomycolate (a C-type lectin mincle receptor ligand), in both BALB/c and C57BL/6 mouse models (53). Intraperitoneal immunization of mice with SAS-adjuvanted OmpW followed by lethal intraperitoneal challenges of B. pseudomallei 576 resulted in 75% survival in both BALB/c mice (day 21) and C57BL/6 mice (day 80) (Table 1) (53). Immunization with SAS-adjuvanted OmpW elicited strong serum antibody responses, along with IFN-γ-secreting CD4+, CD8+, natural killer, and natural killer T cell responses against OmpW in non-insulin-resistant C57BL/6J and insulin-resistant C57BL/6J mouse models of Type 2 diabetes (53, 76). While SAS is a highly effective adjuvant, it has not been approved for human use.
Tomás-Cortázar et al. proposed CAF01 as a promising adjuvant, since it has a proven human safety profile and has demonstrated efficacy against various intracellular pathogens, such as tuberculosis and malaria (54). In their study, C57BL/6J mice were immunized subcutaneously with CAF01-adjuvanted OmpW or with CAF01 alone. Quantitation of serum antibody levels indicated balanced Th1/Th2 responses based on the IgG2a/IgG1 ratios. Upon re-stimulation with B. pseudomallei OmpW, splenocytes obtained from mice immunized with CAF01-adjuvanted OmpW demonstrated robust OmpW-specific Th1 (IFN-γ), Th2 (IL-4), and Th17 (IL-17) responses (54). CAF01-adjuvanted OmpW was found to stimulate equivalent or superior immune responses when compared to OmpW combined with the SAS adjuvant, making it a promising candidate for future studies (54, 76). These studies suggested that B. pseudomallei OmpW adjuvanted with CAF01 has the potential to be an effective vaccine candidate for melioidosis. However, the protective capacity of this vaccine formulation still needs to be evaluated in animal challenge experiments to determine its protective efficacy as a melioidosis vaccine candidate.
Another candidate antigen that has been investigated as a potential protein-based subunit vaccine candidate is the outer membrane protein Burkholderia collagen-like 8 (Bucl8). Bucl8 is composed of two main components (i) a periplasmic α- and outer membrane β-barrels (ii) an extended extracellular portion composed of a collagen (CL) domain and a non-collagenous carboxyl terminal (Ct) region (77). As part of a novel tetrapartite efflux pump, Bucl8 plays a crucial role in fusaric acid resistance, fibrinogen binding, and optimal growth, making it an attractive target for vaccine development (77). Additionally, homology modelling has identified extracellular loops 1 and 2 (L1 and L2) on the β-barrel, and the extended extracellular CL-Ct portion as promising vaccine antigens (77, 78). In studies with CD-1 mice, subcutaneous immunization with recombinant proteins Bucl8-CL/Ct or synthetic peptide L1- or L2-CRM197 conjugates promoted strong Th2 (IgG1) antibody responses against the corresponding proteins or peptides (78). Interestingly, peptide-conjugate L1 elicited significantly higher antibody titers compared to L2, suggesting differential immunogenicity between the two loops (78). However, this subcutaneous immunization failed to provide protection against an intranasal challenge with B. thailandensis strain E264, suggesting that the lack of mucosal immunity may have contributed to this failure (78, 79). To enhance mucosal immunity, intranasal immunization with L1-CRM197 formulated with fluorinated cyclic diguanosine monophosphate (FCDG) was tested (79). This approach also failed to protect CD-1 mice against an intranasal challenge of 8 × 105 CFU of B. thailandensis strain E264 (79). While Bucl8 showed promise as a subunit vaccine candidate further optimization in these studies additional testing using a B. pseudomallei challenge is necessary to further evaluate its potential as a subunit vaccine candidate for melioidosis.
2.3 Outer membrane vesicle vaccines
OMVs are non-infectious vesicles that are constitutively secreted by Gram-negative bacteria (80). They are composed of numerous virulence factors and Toll-like receptor agonists that aid in the activation of immune cells and are, thus, self-adjuvating (81, 82). The use of OMVs as a vaccine platform is desirable as it is inherently safer than LAVs due to the absence of self-replicative capacity. Previously, OMV vaccines have provided protection and elicited robust immune responses against Klebsiella pneumoniae, Neisseria meningitidis, and Bordetella pertussis (83–85). Recent work has shown that OMVs derived from B. pseudomallei provided protection against lethal inhalational challenges of B. pseudomallei and B. mallei in C57BL/6 mice and NHP models of infection (86–88). OMVs derived from B. pseudomallei 1026b provided BALB/c mice with 67% and 60% protection in lethal sepsis and pulmonary infection models, respectively, but did not result in sterilizing immunity (86, 87).
The same research group recently demonstrated that OMVs derived from the select agent-excluded strain B. pseudomallei Bp82, a ΔpurM mutant of strain 1026b, provided cross-protection against inhalational challenges of B. mallei in both C57BL/6 mice and NHPs (Table 1) (89, 90). Mice immunized with the OMV vaccine generated high titer OMV-specific and B. mallei-specific serum IgG responses as well as robust B. mallei-specific Th1/Th17 CD4+ and CD8+ T cell responses (89). C57BL/6 mice immunized with B. pseudomallei Bp82-derived OMVs displayed humoral and cellular immune responses that were comparable to mice immunized with B. pseudomallei Bp82 when used as a LAV strain. In challenge experiments, immunization with Bp82 derived OMVs resulted in 80% of mice surviving to day 30. Rhesus macaques immunized with OMVs and then challenged with B. mallei displayed sub-clinical infections with pulmonary lesions and mild bronchopneumonia, with 100% of the animals surviving up to the 30 day study endpoint (Table 1) (89). High levels of B. mallei-specific and OMV-specific serum IgG were also observed in immunized Rhesus macaques when compared to saline only controls. There were no detectable differences in cellular immune responses between OMV- and control animals (89). This OMV platform is comparable in immunogenicity and protective capacity to B. pseudomallei Bp82 when used as a LAV strain and displayed cross-protection to B. mallei, but similar to previous work failed to produce sterilizing immunity (86, 87, 89).
More recently, the OMV platform was improved upon by generating OMVs from B. pseudomallei Bp82 grown in M9 minimal media (M9 OMV) (91). This nutrient-limiting media mimics the intracellular environment of a macrophage, enriching OMVs with intracellular-stage proteins associated with virulence and key immune targets that are predicted to be important for providing sterilizing immunity. One immunogenic protein found to be enriched in M9 OMVs as compared to earlier OMVs is Hcp1, a component of T6SS-1 (68, 69, 91). Following immunization with B. pseudomallei Bp82 LAV or M9 OMVs derived from B. pseudomallei Bp82, C57BL/6 mice were challenged with B. pseudomallei K96243 via an inhalational route (91). The M9 OMV vaccine conferred 100% protection at day 30, and spleens collected at the study endpoint yielded no culturable bacteria (Table 1) (91). M9 OMV immunized mice produced significantly higher IgG titers to OMVs and whole inactivated bacteria than mice immunized with B. pseudomallei Bp82 LAV. Similar trends were observed for cellular immune responses in that IFN-γ- and IL-17-secreting CD4+ T cells and IFN-γ-secreting CD8+ T cells were higher in M9 OMV immunized mice than B. pseudomallei Bp82 LAV immunized mice (91). These results demonstrate that M9 OMVs not only offer improved immunogenicity and protection comparable to LAVs but also represent a promising vaccine candidate that may be capable of achieving sterilizing immunity.
Previous work has demonstrated that Hcp1 elicits strong IFN-γ-secreting T cell responses that correlate with survival in melioidosis patients (68). Building upon this, a recent study engineered a Staphylococcus aureus strain, RN4220-Δagr/pdhB-hcp1, to produce Hcp1-loaded OMVs (92). This involved the construction of an in-frame fusion of the hcp1 gene from B. pseudomallei BPC006 with the gene encoding a major vesicular component in S. aureus RN4220-Δagr. To generate B. pseudomallei Hcp1-loaded membrane vesicles (hcp1MVs), RN4220-Δagr/pdhB-hcp1 was cultured and subjected to a series of centrifugation and filtration steps. BALB/c mice were immunized with three doses of hcp1MVs alone or hcp1MVs formulated with Freund’s adjuvant and then challenged with B. pseudomallei BPC006 via the intraperitoneal route. hcp1MVs- and hcp1MVs/Freund’s adjuvant-immunized mice displayed 60% and 70% survival over 21 days, respectively (Table 1) (92). Following immunization, mice that received hcp1MVs/Freund’s adjuvant displayed the highest titer IgG responses to Hcp1 (92). This study suggests that OMVs loaded with B. pseudomallei antigens may be potential melioidosis vaccine candidates when combined with an appropriate adjuvant.
2.4 Nanoparticle-based vaccines
Gold nanoparticles (AuNPs) are promising candidates for various biological applications due to their unique physical properties, ease of synthesis, capacity for bioconjugation with protein or polysaccharide antigens as well as their utility as vaccine delivery systems (93, 94). AuNPs covalently coupled to one of three different proteins (Hcp1, Hc fragment of tetanus toxin, or flagellin) and LPS purified from the lowly-pathogenic species, B. thailandensis, have been evaluated in BALB/c mice for protection against glanders (95). These vaccine candidates induced LPS-specific IgG responses and provided 60-90% survival at day 35 following a lethal inhalational challenge with B. mallei strain ATCC 23344 (95). When evaluated in Rhesus macaques, an AuNP glycoconjugate vaccine composed of B. thailandensis LPS conjugated to flagellin formulated with Alhydrogel generated high titer LPS- and protein-specific IgG responses, however, 50% survival was observed against a B. mallei challenge (96).
Recently, a reverse vaccinology approach was employed to identify novel protein candidates that are conserved in both B. pseudomallei and B. mallei for potential use in AuNP glycoconjugate-based vaccines. Candidates were selected based on predicted antigenicity and validated by confirming their reactivity with melioidosis sera from humans and mice (97–102). Three promising candidates (FlgL, hemagglutinin, and Hcp1) were identified and individually conjugated to an AuNP-glycoconjugate platform along with B. thailandensis LPS (100). The AuNP-glycoconjugate formulations were used alone or in combination with the three proteins (AuNP-combo-LPS) to subcutaneously immunize C57BL/6 mice. When intranasally challenged with 3.4 LD50 of B. pseudomallei K96243, mice receiving AuNP-FlgL-LPS and AuNP-combo-LPS demonstrated the highest levels of protection with 90% and 100% survival at day 35, respectively (Table 1). Groups receiving AuNP-hemagglutinin-LPS and AuNP-Hcp1-LPS exhibited 20% and 10% survival, at day 35, respectively (Table 1) (100). Surviving mice in all groups demonstrated a significant reduction of bacterial loads in lungs compared to the adjuvant-only control group (100).
Additional AuNP-glycoconjugate vaccine candidates have been developed by incorporating predicted immunogenic proteins such as OmpW, OpcP, and OpcP1 along with previously identified antigens (101, 102). AuNP-protein-LPS candidates, comprised of different proteins coupled to AuNPs and LPS were tested in mouse models of glanders (101). C57BL/6 mice that received AuNP-OpcP-LPS, AuNP-OmpW-LPS, AuNP-hemagglutinin-LPS or AuNP-Hcp1-LPS demonstrated high level protection (90-100% survival) at day 35 following an intranasal challenge with 2 LD50 of B. mallei ATCC 23344 (Table 1). Since the protection afforded by AuNP-OpcP-LPS, AuNP-OmpW-LPS or AuNP-hemagglutinin-LPS was significantly higher than the adjuvant control group, these formulations were further evaluated using a higher challenge dose (50 LD50). An AuNP-glycoconjugate vaccine containing a combination of three proteins (OpcP, OmpW, and hemagglutinin) and LPS resulted in 100% survival at 35 days following an intranasal challenge with 50 LD50 of B. mallei ATCC 23344, while AuNP-OpcP-LPS, AuNP-OmpW-LPS, or AuNP-hemagglutinin-LPS resulted in 50-80% survival (Table 1) (101). Analysis of humoral immune responses showed that serum from mice immunized with OpcP- and OmpW-formulations exhibited high LPS- and protein-specific IgG2c levels, indicating a Th1-biased (IgG2c > IgG1) immune response. The immune serum was associated with enhanced macrophage-mediated phagocytosis of B. mallei ATCC 23344 and reduced bacterial adherence to murine lung epithelial cells (101).
The effectiveness of different AuNP-protein-LPS candidates against B. pseudomallei has also been evaluated in C57BL/6 mice (102). Specifically, mice immunized with AuNP-OpcP-LPS or AuNP-OpcP1-LPS demonstrated 90% and 30% protection at day 35, respectively, against a 6 LD50 intranasal challenge of B. pseudomallei K96243 (Table 1). Upon initial experimentation, the combination of AuNP-OpcP-LPS and AuNP-OpcP1-LPS were further evaluated and deemed to be the most effective, with 100% survival at day 35 post-challenge with 5 LD50 of B. pseudomallei K96243 (Table 1). Most of the surviving mice had low bacterial loads in lungs, livers, and spleens with only a few pathological lesions. AuNP-OpcP-LPS, AuNP-OpcP1-LPS, and AuNP-OpcP-LPS/AuNP-OpcP1-LPS elicited robust LPS- and protein-specific IgG responses which promoted macrophage uptake of B. pseudomallei K96243. Additionally, immunized mice demonstrated high levels of LPS- and protein-specific IgG and IgA in their lungs as well as mixed Th1 and Th17 biased protein-specific cytokine responses upon splenocyte re-stimulation (102).
2.5 Virus-like particle vaccines
Virus-like particles (VLPs) are protein-based nanoparticles that are frequently used as carriers in conjugate vaccine platforms and for delivering immunotherapies (103). These particles are non-infectious due to lack of genetic material necessary to replicate but may be engineered to express immunogenic antigens that elicit robust B cell responses (104). A recent study employed the external decoration approach by displaying Hcp1 protein on the surface of P22 VLPs (105). Mice immunized with conjugated Hcp1-VLPs demonstrated robust Hcp1-specific IgG, IgG1, IgG2c, and IgA titers, irrespective of low (5 μg) or high (10 μg) doses of Hcp1-VLPs, compared to mice that received PBS or unconjugated VLPs as controls. The serum obtained from Hcp1-VLPs immunized mice enhanced antibody responses and promoted phagocytosis of opsonized bacteria by macrophages (105). Future animal challenge studies are needed to evaluate the protective capacity of Hcp1-VLPs against B. pseudomallei.
2.6 DNA vaccines
Plasmid-based DNA vaccines are designed to deliver genes encoding specific antigens that can induce humoral and cellular immune responses against pathogens and are considered cost-effective and amenable to large-scale manufacture (106–109). Recently, a B. pseudomallei flagellin (FliC) plasmid DNA vaccine, pVAX-hTPA-FliC, was evaluated in C57BL/6 mice using either a rapid dermal tattoo or an intranasal delivery system. Following an intranasal challenge with B. pseudomallei 1026b, a single intranasal immunization with pVAX-hTPA-FliC was more successful than dermal tattoo delivery in reducing bacterial loads, pulmonary cytokine levels (TNF-α, IL-6, CXCL1), plasma cytokine levels (TNF-α, IL-6, IFN-γ), lung pathology scores, systemic inflammation, and organ damage. However, a single intranasal immunization failed to elicit detectable anti-FliC IgG responses. Results demonstrated that only 53% survival was observed in mice receiving intranasal immunization with pVAX-hTPA-FliC at 14 days post-challenge with B. pseudomallei 1026b (Table 1) (110). As DNA vaccines against B. pseudomallei have not been previously explored, future studies are needed to focus on the optimization of vaccine formulations and routes of administration to provide robust humoral and cellular immune responses needed for protection against melioidosis.
2.7 Viral vector-based vaccines
Viral vector-based vaccines are designed to deliver genes encoding specific antigens into host cells (111). These types of vaccines can elicit immune responses without the need for an adjuvant and are amenable to large-scale and cost-effective production (112, 113). Viral vector-based vaccine platforms have been developed for immunization against melioidosis and glanders using Parainfluenza virus 5 (PIV5) as the vector to deliver the conserved B. mallei autotransporter protein BatA (PIV5-BatA) to target cells. Following an inhalational challenge with 5 LD50 B. pseudomallei K96243, BALB/c mice immunized with a single intranasal dose of PIV5-BatA displayed survival rates of 80% on day 10 and 60% day 35 (Table 1) (114). Of the surviving mice, 78% and 44% had no culturable bacteria in their lungs and spleen, respectively. Additionally, PIV5-BatA provided 84% survival at day 10 and 74% survival at day 40 against an inhalational challenge with B. mallei ATCC 23344 (Table 1) (114). Analysis of immune responses revealed that BatA-specific IgG and IFN-γ-secreting T cell responses were critical for providing protection (114). While the PIV5-BatA vaccine candidate showed significant promise for providing immunity against B. pseudomallei and B. mallei, further studies will be needed to evaluate the longevity of protection and the need for booster doses, which could influence the overall efficacy of this platform in preventing melioidosis and glanders.
3 Conclusions and future directions
Since B. pseudomallei is a facultative-intracellular bacterium, it is anticipated that protective immunity against this pathogen will be complex. Several studies support that humoral immune responses are important for controlling early stages of an infection (extracellular phase) whereas cellular immune responses are important for controlling later stages of an infection (intracellular phase). It is expected, therefore, that a vaccine that elicits both types of responses will be required to provide full protection against disease. This review summarizes the various approaches that have been used to develop melioidosis vaccine candidates over the past seven years. While significant progress has been made in this area, the development of a broadly effective vaccine continues to be challenging (60, 115–118). Several factors are likely responsible for this including that B. pseudomallei 1) is a highly virulent pathogen that requires specialized permissions, facilities and containment practices to be studied, 2) expresses an impressive array of virulence factors that enables it to survive and replicate in a variety of different cell types and tissues and 3) exhibits a multifaceted lifestyle that enables it to avoid clearance by host immune defenses.
At present, mouse models remain the primary means for evaluating melioidosis vaccine efficacy, with BALB/c and C57BL/6 mice commonly being used for acute and chronic infection studies, respectively. Using these models, several live-attenuated, glycoconjugate-based and/or protein-based subunit, OMV, nanoparticle-based, and viral vector-based vaccine candidates have yielded promising results. Robust protection against lethal doses of B. pseudomallei have been observed with some of these vaccines, however, sterilizing immunity has proven difficult to achieve especially during protracted challenge studies. There is strong evidence to support that high titer, opsonizing IgG responses specific for B. pseudomallei CPS are critical for controlling early stages of infection (48). Furthermore, there appears to be a correlation with the most promising melioidosis vaccine candidates and their ability to stimulate robust Th1- and Th17-like humoral and cellular immune responses. Such observations are consistent with studies demonstrating that melioidosis patient survival correlates with strong IFN-γ secreting T cell responses against B. pseudomallei protein antigens (68). Further studies are required, however, to better establish correlates of antigen-induced immunity to guide the rational design of future melioidosis vaccines.
Newer technologies, including mRNA vaccines and the use of in silico methodologies to guide the design of multi-epitope-based peptide vaccines, may also represent novel approaches for immunization against melioidosis (119–121). The benefits of these platforms include low production costs, scalability, and the ability to induce robust humoral and cellular immune responses. A limitation of both approaches, however, is their inability to express non-protein antigens, specifically polysaccharides, which have been proven to be important components of several vaccine platforms described in this review. To address this issue, it will be important to identify and use proteins or B cell epitopes that can stimulate protective opsonizing and complement-activating immune responses, similar to those elicited by OPS and CPS antigens.
Moving forward, efforts should be placed on 1) defining specific correlates of immunity associated with efficacious vaccines, 2) investigating how different adjuvants and immune-modulators can be used to potentiate protective immune responses, 3) optimizing dosing and routes of immunization and 4) the use of immunocompromised mouse models (e.g. diabetic mice). Although mice have been invaluable for pre-clinical evaluation of melioidosis vaccines, studies using higher-order animal species (e.g. NHPs) will likely also be necessary to assess the safety and immunogenicity of lead vaccine candidates prior to their advancement into human clinical trials.
An effective vaccine aimed at reducing the incidence and severity of melioidosis in endemic regions would be predicted to improve morbidity and mortality rates as well as decrease healthcare costs. Individuals who are vaccinated may have a lower risk of developing severe symptoms or complications and have a reduced need for prolonged antibiotic therapy (122). Effective vaccine candidates should be considered for their ability to generate protective immunity in high risk populations such as individuals with diabetes, chronic lung or kidney disease, thalassemia, other immunocompromising conditions and the elderly (123). Furthermore, future studies should not only focus on safety, immunogenicity, durability and efficacy but also consider stability, cost-effectiveness and accessibility for use in endemic regions worldwide (60, 115, 116).
To date, good progress has been made by the few research groups that have taken on the challenge of developing a safe, affordable, and efficacious melioidosis vaccine. Based on recent successes, the melioidosis research community is optimistic that this can be achieved but also acknowledges that significant obstacles must be overcome for this to happen. Unless funding agencies, public health officials, and government policymakers recognize the true burden of melioidosis in countries where it is endemic, and implement strategies to combat this disease, a licensed vaccine will remain elusive.
Author contributions
SS: Conceptualization, Funding acquisition, Visualization, Writing – original draft, Writing – review & editing. SW: Conceptualization, Writing – original draft, Writing – review & editing. IR: Conceptualization, Writing – original draft, Writing – review & editing. MB: Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing. PB: Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded in part by the Defense Threat Reduction Agency under the Rapid Assessment of Platform Technologies to Expedite Response (RAPTER) program (award no. HDTRA1242031), Defense Threat Reduction Agency contract number HDTRA1-18-C-0062, Medical CBRN Defense Consortium contract number MCDC-18-04-11–004 and the Springboard Science and Research Fund, University of Nevada, Reno School of Medicine.
Acknowledgments
The authors thank Dr. Traci Pals and Dr. Bob Webb for their support of this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views expressed in this article are those of the authors and do not reflect the official policy or position of the US Department of Defense or the US Government.
References
1. Currie BJ, Dance DA, and Cheng AC. The global distribution of Burkholderia pseudomallei and melioidosis: an update. Trans R Soc Trop Med Hyg. (2008) 102 Suppl 1:S1–4. doi: 10.1016/S0035-9203(08)70002-6
2. Limmathurotsakul D, Golding N, Dance DA, Messina JP, Pigott DM, Moyes CL, et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol. (2016) 1:15008. doi: 10.1038/nmicrobiol.2015.8
3. Petras JK, Elrod MG, Ty MC, Dawson P, O’Laughlin K, Gee JE, et al. Locally acquired melioidosis linked to environment - mississippi, 2020-2023. N Engl J Med. (2023) 389:2355–62. doi: 10.1056/NEJMoa2306448
4. Meumann EM, Cheng AC, Ward L, and Currie BJ. Clinical features and epidemiology of melioidosis pneumonia: results from a 21-year study and review of the literature. Clin Infect Dis. (2012) 54:362–9. doi: 10.1093/cid/cir808
5. Chantratita N, Phunpang R, Yarasai A, Dulsuk A, Yimthin T, Onofrey LA, et al. Characteristics and one year outcomes of melioidosis patients in northeastern Thailand: A prospective, multicenter cohort study. Lancet Reg Health Southeast Asia. (2023) 9:100118. doi: 10.1016/j.lansea.2022.100118
6. Churuangsuk C, Chusri S, Hortiwakul T, Charernmak B, and Silpapojakul K. Characteristics, clinical outcomes and factors influencing mortality of patients with melioidosis in southern Thailand: A 10-year retrospective study. Asian Pac J Trop Med. (2016) 9:256–60. doi: 10.1016/j.apjtm.2016.01.034
7. Dubey DK, Bano N, Dubey M, Sangwan P, Mitra SK, Kulshrestha V, et al. A case series of melioidosis: an underdiagnosed infection. IDCases. (2023) 31:e01685. doi: 10.1016/j.idcr.2023.e01685
8. Hoffmaster AR, AuCoin D, Baccam P, Baggett HC, Baird R, Bhengsri S, et al. Melioidosis diagnostic workshop, 2013. Emerg Infect Dis. (2015) 21:e141045. doi: 10.3201/eid2102.141045
9. Hemarajata P, Baghdadi JD, Hoffman R, and Humphries RM. Burkholderia pseudomallei: challenges for the clinical microbiology laboratory. J Clin Microbiol. (2016) 54:2866–73. doi: 10.1128/JCM.01636-16
10. Peacock SJ, Schweizer HP, Dance DA, Smith TL, Gee JE, Wuthiekanun V, et al. Management of accidental laboratory exposure to Burkholderia pseudomallei and B. Mallei. Emerg Infect Dis. (2008) 14:e2. doi: 10.3201/eid1407.071501
11. Wiersinga WJ, Currie BJ, and Peacock SJ. Melioidosis. N Engl J Med. (2012) 367:1035–44. doi: 10.1056/NEJMra1204699
12. Limmathurotsakul D, Kanoksil M, Wuthiekanun V, Kitphati R, deStavola B, Day NP, et al. Activities of daily living associated with acquisition of melioidosis in northeast Thailand: A matched case-control study. PLoS Negl Trop Dis. (2013) 7:e2072. doi: 10.1371/journal.pntd.0002072
13. Barnes KB, Steward J, Thwaite JE, Lever MS, Davies CH, Armstrong SJ, et al. Trimethoprim/sulfamethoxazole (Co-trimoxazole) prophylaxis is effective against acute murine inhalational melioidosis and glanders. Int J Antimicrob Agents. (2013) 41:552–7. doi: 10.1016/j.ijantimicag.2013.02.007
14. Chetchotisakd P, Chierakul W, Chaowagul W, Anunnatsiri S, Phimda K, Mootsikapun P, et al. Trimethoprim-sulfamethoxazole versus trimethoprim-sulfamethoxazole plus doxycycline as oral eradicative treatment for melioidosis (MERTH): A multicentre, double-blind, non-inferiority, randomised controlled trial. Lancet. (2014) 383:807–14. doi: 10.1016/S0140-6736(13)61951-0
15. Dance DA, Wuthiekanun V, Chaowagul W, and White NJ. The antimicrobial susceptibility of pseudomonas pseudomallei. Emergence of resistance in vitro and during treatment. J Antimicrob Chemother. (1989) 24:295–309. doi: 10.1093/jac/24.3.295
16. Rhodes KA and Schweizer HP. Antibiotic resistance in Burkholderia species. Drug Resist Update. (2016) 28:82–90. doi: 10.1016/j.drup.2016.07.003
17. Fen SHY, Tandhavanant S, Phunpang R, Ekchariyawat P, Saiprom N, Chewapreecha C, et al. Antibiotic susceptibility of clinical Burkholderia pseudomallei isolates in northeast Thailand during 2015–2018 and the genomic characterization of beta-lactam-resistant isolates. Antimicrob Agents Chemother. (2023) 95:e02230-20. doi: 10.1128/AAC.02230-20
18. Sivabalan P, Satyaputra F, Gassiep I, Forde B, Frazer J, Glover M, et al. Meropenem-resistant Burkholderia pseudomallei: A concerning single case in Australia with no prior meropenem exposure. Access Microbiol. (2024) 6:000619.v4. doi: 10.1099/acmi.0.000619.v4
19. Nierman WC, DeShazer D, Kim HS, Tettelin H, Nelson KE, Feldblyum T, et al. Structural flexibility in the Burkholderia mallei genome. Proc Natl Acad Sci U S A. (2004) 101:14246–51. doi: 10.1073/pnas.0403306101
20. Losada L, Ronning CM, DeShazer D, Woods D, Fedorova N, Kim HS, et al. Continuing Evolution of Burkholderia mallei through Genome Reduction and Large-Scale Rearrangements. Genome Biol Evol. (2010) 2:102–16. doi: 10.1093/gbe/evq003
21. DeShazer D, Waag DM, Fritz DL, and Woods DE. Identification of a Burkholderia mallei polysaccharide gene cluster by subtractive hybridization and demonstration that the encoded capsule is an essential virulence determinant. Microb Pathog. (2001) 30:253–69. doi: 10.1006/mpat.2000.0430
22. Ulrich RL and DeShazer D. Type III secretion: A virulence factor delivery system essential for the pathogenicity of Burkholderia mallei. Infect Immun. (2004) 72:1150–4. doi: 10.1128/IAI.72.2.1150-1154.2004
23. Burtnick MN, DeShazer D, Nair V, Gherardini FC, and Brett PJ. Burkholderia mallei cluster 1 type VI secretion mutants exhibit growth and actin polymerization defects in raw 264.7 murine macrophages. Infect Immun. (2010) 78:88–99. doi: 10.1128/IAI.00985-09
24. Burtnick MN and Brett PJ. Burkholderia mallei and Burkholderia pseudomallei Cluster 1 Type VI Secretion System Gene Expression Is Negatively Regulated by Iron and Zinc. PLoS One. (2013) 8:e76767. doi: 10.1371/journal.pone.0076767
25. Galyov EE, Brett PJ, and DeShazer D. Molecular Insights into Burkholderia pseudomallei and Burkholderia mallei Pathogenesis. Annu Rev Microbiol. (2010) 64:495–517. doi: 10.1146/annurev.micro.112408.134030
26. Moustafa DA, Scarff JM, Garcia PP, Cassidy SK, DiGiandomenico A, Waag DM, et al. Recombinant salmonella expressing Burkholderia mallei LPS O antigen provides protection in a murine model of melioidosis and glanders. PLoS One. (2015) 10:e0132032. doi: 10.1371/journal.pone.0132032
27. Sarkar-Tyson M, Smither SJ, Harding SV, Atkins TP, and Titball RW. Protective Efficacy of Heat-Inactivated B. Thailandensis, B. mallei or B. pseudomallei against Experimental Melioidosis and Glanders. Vaccine. (2009) 27:4447–51. doi: 10.1016/j.vaccine.2009.05.040
28. Schell MA, Zhao P, and Wells L. Outer Membrane Proteome of Burkholderia pseudomallei and Burkholderia mallei from Diverse Growth Conditions. J Proteome Res. (2011) 10:2417–24. doi: 10.1021/pr1012398
29. Whitlock GC, Deeraksa A, Qazi O, Judy BM, Taylor K, Propst KL, et al. Protective Response to Subunit Vaccination against Intranasal Burkholderia mallei and B. pseudomallei Challenge. Proc Vaccinol. (2010) 2:73-7. doi: 10.1016/j.provac.2010.03.013
30. Zhang S, Feng SH, Li B, Kim HY, Rodriguez J, Tsai S, et al. In Vitro and in Vivo Studies of Monoclonal Antibodies with Prominent Bactericidal Activity against Burkholderia pseudomallei and Burkholderia mallei. Clin Vaccine Immunol. (2011) 18:825–34. doi: 10.1128/CVI.00533-10
31. Centers for Disease C, Prevention DoH, and Human S. Possession, use, and transfer of select agents and toxins; biennial review. Final Rule. Fed Regist. (2012) 77:61083–115.
32. Rotz LD, Khan AS, Lillibridge SR, Ostroff SM, and Hughes JM. Public health assessment of potential biological terrorism agents. Emerg Infect Dis. (2002) 8:225–30. doi: 10.3201/eid0802.010164
33. Cheng AC, Dance DA, and Currie BJ. Bioterrorism, glanders and melioidosis. Euro Surveill. (2005) 10:E1–2; author reply E1-2. doi: 10.2807/esm.10.03.00528-en
34. Pasetti MF, Cuberos L, Horn TL, Shearer JD, Matthews SJ, House RV, et al. An improved Francisella tularensis live vaccine strain (LVS) is well tolerated and highly immunogenic when administered to rabbits in escalating doses using various immunization routes. Vaccine. (2008) 26:1773–85. doi: 10.1016/j.vaccine.2008.01.005
35. Zimmerman SM, Dyke JS, Jelesijevic TP, Michel F, Lafontaine ER, and Hogan RJ. Antibodies against in Vivo-Expressed Antigens Are Sufficient to Protect against Lethal Aerosol Infection with Burkholderia mallei and Burkholderia pseudomallei. Infect Immun. (2017) 85:e00102-17. doi: 10.1128/IAI.00102-17
36. Amemiya K, Dankmeyer JL, Biryukov SS, Trevino SR, Klimko CP, Mou SM, et al. Deletion of two genes in Burkholderia pseudomallei MSHR668 that target essential amino acids protect acutely infected BALB/c mice and promote long term survival. Vaccines (Basel). (2019) 7:196. doi: 10.3390/vaccines7040196
37. Biryukov SS, Cote CK, Klimko CP, Dankmeyer JL, Rill NO, Shoe JL, et al. Evaluation of two different vaccine platforms for immunization against melioidosis and glanders. Front Microbiol. (2022) 13:965518. doi: 10.3389/fmicb.2022.965518
38. Klimko CP, Shoe JL, Rill NO, Hunter M, Dankmeyer JL, Talyansky Y, et al. Layered and Integrated Medical Countermeasures against Burkholderia pseudomallei Infections in C57bl/6 Mice. Front Microbiol. (2022) 13:965572. doi: 10.3389/fmicb.2022.965572
39. Detmer A and Glenting J. Live bacterial vaccines-a review and identification of potential hazards. Microb Cell Fact. (2006) 5:23. doi: 10.1186/1475-2859-5-23
40. Khakhum N, Bharaj P, Myers JN, Tapia D, Kilgore PB, Ross BN, et al. Burkholderia pseudomallei ΔtonB Δhcp1 Live Attenuated Vaccine Strain Elicits Full Protective Immunity against Aerosolized Melioidosis Infection. mSphere. (2019) 4:e00570-18. doi: 10.1128/mSphere.00570-18
41. Hatcher CL, Mott TM, Muruato LA, Sbrana E, and Torres AG. Burkholderia mallei CLH001 Attenuated Vaccine Strain Is Immunogenic and Protects against Acute Respiratory Glanders. Infect Immun. (2016) 84:2345–54. doi: 10.1128/IAI.00328-16
42. Khakhum N, Bharaj P, Myers JN, Tapia D, Walker DH, Endsley JJ, et al. Evaluation of Burkholderia mallei ΔtonB Δhcp1 (CLH001) as a live attenuated vaccine in murine models of glanders and melioidosis. PLoS Negl Trop Dis. (2019) 13:e0007578. doi: 10.1371/journal.pntd.0007578
43. Khakhum N, Bharaj P, Walker DH, Torres AG, and Endsley JJ. Antigen-specific antibody and polyfunctional T cells generated by respiratory immunization with protective Burkholderia ΔtonB Δhcp1 live attenuated vaccines. NPJ Vaccines. (2021) 6:72. doi: 10.1038/s41541-021-00333-4
44. Tullius MV, Bowen RA, Back PS, Maslesa-Galic S, Nava S, and Horwitz MA. LVS ΔcapB-Vectored Multiantigenic Melioidosis Vaccines Protect against Lethal Respiratory Burkholderia pseudomallei Challenge in Highly Sensitive BALB/c Mice. mBio. (2024) 15:e0018624. doi: 10.1128/mbio.00186-24
45. Li H, Nookala S, and Re F. Aluminum hydroxide adjuvants activate caspase-1 and induce IL-1beta and IL-18 release. J Immunol. (2007) 178:5271–6. doi: 10.4049/jimmunol.178.8.5271
46. Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, and Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. (2007) 316:1628–32. doi: 10.1126/science.1138963
47. Hou B, Reizis B, and DeFranco AL. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity. (2008) 29:272–82. doi: 10.1016/j.immuni.2008.05.016
48. Burtnick MN, Shaffer TL, Ross BN, Muruato LA, Sbrana E, DeShazer D, et al. Development of subunit vaccines that provide high-level protection and sterilizing immunity against acute inhalational melioidosis. Infect Immun. (2018) 86:e00724-17. doi: 10.1128/IAI.00724-17
49. Schmidt LK, Orne CE, Shaffer TL, Wilson SM, Khakhum N, Torres AG, et al. Development of melioidosis subunit vaccines using an enzymatically inactive Burkholderia pseudomallei AhpC. Infect Immun. (2022) 90:e0022222. doi: 10.1128/iai.00222-22
50. Scott AE, Burtnick MN, Stokes MG, Whelan AO, Williamson ED, Atkins TP, et al. Burkholderia pseudomallei Capsular Polysaccharide Conjugates Provide Protection against Acute Melioidosis. Infect Immun. (2014) 82:3206–13. doi: 10.1128/IAI.01847-14
51. Scott AE, Christ WJ, George AJ, Stokes MG, Lohman GJ, Guo Y, et al. Protection against experimental melioidosis with a synthetic manno-heptopyranose hexasaccharide glycoconjugate. Bioconjug Chem. (2016) 27:1435–46. doi: 10.1021/acs.bioconjchem.5b00525
52. Burtnick MN, Heiss C, Roberts RA, Schweizer HP, Azadi P, and Brett PJ. Development of capsular polysaccharide-based glycoconjugates for immunization against melioidosis and glanders. Front Cell Infect Microbiol. (2012) 2:108. doi: 10.3389/fcimb.2012.00108
53. Casey WT, Spink N, Cia F, Collins C, Romano M, Berisio R, et al. Identification of an OmpW Homologue in Burkholderia pseudomallei, a Protective Vaccine Antigen against Melioidosis. Vaccine. (2016) 34:2616–21. doi: 10.1016/j.vaccine.2016.03.088
54. Tomas-Cortazar J, Quinn C, Corcoran N, Blanco A, Christensen D, and McClean S. Bp ompW antigen administered with CAF01 adjuvant stimulates comparable T cell responses to sigma adjuvant system. Vaccine X. (2024) 17:100438. doi: 10.1016/j.jvacx.2024.100438
55. Broker M, Costantino P, DeTora L, McIntosh ED, and Rappuoli R. Biochemical and biological characteristics of cross-reacting material 197 (CRM197), a non-toxic mutant of diphtheria toxin: use as a conjugation protein in vaccines and other potential clinical applications. Biologicals. (2011) 39:195–204. doi: 10.1016/j.biologicals.2011.05.004
56. Rappuoli R, De Gregorio E, and Costantino P. On the mechanisms of conjugate vaccines. Proc Natl Acad Sci U S A. (2019) 116:14–6. doi: 10.1073/pnas.1819612116
57. Reckseidler SL, DeShazer D, Sokol PA, and Woods DE. Detection of bacterial virulence genes by subtractive hybridization: identification of capsular polysaccharide of Burkholderia pseudomallei as a major virulence determinant. Infect Immun. (2001) 69:34–44. doi: 10.1128/IAI.69.1.34-44.2001
58. Nelson M, Prior JL, Lever MS, Jones HE, Atkins TP, and Titball RW. Evaluation of lipopolysaccharide and capsular polysaccharide as subunit vaccines against experimental melioidosis. J Med Microbiol. (2004) 53:1177–82. doi: 10.1099/jmm.0.45766-0
59. Reckseidler-Zenteno SL, DeVinney R, and Woods DE. The capsular polysaccharide of Burkholderia pseudomallei contributes to survival in serum by reducing complement factor C3b deposition. Infect Immun. (2005) 73:1106–15. doi: 10.1128/IAI.73.2.1106-1115.2005
60. Titball RW, Burtnick MN, Bancroft GJ, and Brett P. Burkholderia pseudomallei and Burkholderia mallei Vaccines: Are We Close to Clinical Trials? Vaccine. (2017) 35:5981–9. doi: 10.1016/j.vaccine.2017.03.022
61. Wiersinga WJ, van der Poll T, White NJ, Day NP, and Peacock SJ. Melioidosis: Insights into the Pathogenicity of Burkholderia pseudomallei. Nat Rev Microbiol. (2006) 4:272–82. doi: 10.1038/nrmicro1385
62. Ngugi SA, Ventura VV, Qazi O, Harding SV, Kitto GB, Estes DM, et al. Lipopolysaccharide from Burkholderia Thailandensis E264 provides protection in a murine model of melioidosis. Vaccine. (2010) 28:7551–5. doi: 10.1016/j.vaccine.2010.08.058
63. Brett PJ and Woods DE. Structural and immunological characterization of Burkholderia pseudomallei O-polysaccharide-flagellin protein conjugates. Infect Immun. (1996) 64:2824–8. doi: 10.1128/iai.64.7.2824-2828.1996
64. AuCoin DP, Reed DE, Marlenee NL, Bowen RA, Thorkildson P, Judy BM, et al. Polysaccharide Specific Monoclonal Antibodies Provide Passive Protection against Intranasal Challenge with Burkholderia pseudomallei. PLoS One. (2012) 7:e35386. doi: 10.1371/journal.pone.0035386
65. Charuchaimontri C, Suputtamongkol Y, Nilakul C, Chaowagul W, Chetchotisakd P, Lertpatanasuwun N, et al. Antilipopolysaccharide II: an antibody protective against fatal melioidosis. Clin Infect Dis. (1999) 29:813–8. doi: 10.1086/520441
66. Heiss C, Burtnick MN, Roberts RA, Black I, Azadi P, and Brett PJ. Revised Structures for the Predominant O-Polysaccharides Expressed by Burkholderia pseudomallei and Burkholderia mallei. Carbohydr Res. (2013) 381:6–11. doi: 10.1016/j.carres.2013.08.013
67. Tamigney Kenfack M, Mazur M, Nualnoi T, Shaffer TL, Ngassimou A, Bleriot Y, et al. Deciphering Minimal Antigenic Epitopes Associated with Burkholderia pseudomallei and Burkholderia mallei Lipopolysaccharide O-Antigens. Nat Commun. (2017) 8:115. doi: 10.1038/s41467-017-00173-8
68. Sengyee S, Yarasai A, Janon R, Morakot C, Ottiwet O, Schmidt LK, et al. Melioidosis patient survival correlates with strong IFN-γ Secreting T cell responses against Hcp1 and TssM. Front Immunol. (2021) 12:698303. doi: 10.3389/fimmu.2021.698303
69. Pumpuang A, Phunpang R, Ekchariyawat P, Dulsuk A, Loupha S, Kwawong K, et al. Distinct classes and subclasses of antibodies to hemolysin co-regulated protein 1 and O-polysaccharide and correlation with clinical characteristics of melioidosis patients. Sci Rep. (2019) 9:13972. doi: 10.1038/s41598-019-48828-4
70. Loprasert S, Sallabhan R, Whangsuk W, and Mongkolsuk S. Compensatory Increase in AhpC Gene Expression and Its Role in Protecting Burkholderia pseudomallei against Reactive Nitrogen Intermediates. Arch Microbiol. (2003) 180:498–502. doi: 10.1007/s00203-003-0621-9
71. Dunachie SJ, Jenjaroen K, Reynolds CJ, Quigley KJ, Sergeant R, Sumonwiriya M, et al. Infection with Burkholderia pseudomallei - immune correlates of survival in acute melioidosis. Sci Rep. (2017) 7:12143. doi: 10.1038/s41598-017-12331-5
72. Barnes KB, Brett P, Burtnick M, Vente A, Bentley C, Richards MI, et al. Layering vaccination with antibiotic therapy results in protection and clearance of Burkholderia pseudomallei in BALB/c mice. Infect Immun. (2024) 92:e0045523. doi: 10.1128/iai.00455-23
73. Harland DN, Chu K, Haque A, Nelson M, Walker NJ, Sarkar-Tyson M, et al. Identification of a lolC homologue in Burkholderia pseudomallei, a novel protective antigen for melioidosis. Infect Immun. (2007) 75:4173–80. doi: 10.1128/IAI.00404-07
74. Hara Y, Mohamed R, and Nathan S. Immunogenic burkholderia pseudomallei outer membrane proteins as potential candidate vaccine targets. PLoS One. (2009) 4:e6496. doi: 10.1371/journal.pone.0006496
75. Su YC, Wan KL, Mohamed R, and Nathan S. Immunization with the recombinant Burkholderia pseudomallei outer membrane protein omp85 induces protective immunity in mice. Vaccine. (2010) 28:5005–11. doi: 10.1016/j.vaccine.2010.05.022
76. Tomas-Cortazar J, Bossi L, Quinn C, Reynolds CJ, Butler DK, Corcoran N, et al. Bp OmpW antigen stimulates the necessary protective T-cell responses against melioidosis. Front Immunol. (2021) 12:767359. doi: 10.3389/fimmu.2021.767359
77. Grund ME, Choi SJ, McNitt DH, Barbier M, Hu G, LaSala PR, et al. Burkholderia Collagen-Like Protein 8, Bucl8, Is a Unique Outer Membrane Component of a Putative Tetrapartite Efflux Pump in Burkholderia pseudomallei and Burkholderia mallei. PLoS One. (2020) 15:e0242593. doi: 10.1371/journal.pone.0242593
78. Grund ME, Kramarska E, Choi SJ, McNitt DH, Klimko CP, Rill NO, et al. Predictive and experimental immunogenicity of Burkholderia collagen-like protein 8-derived antigens. Vaccines (Basel). (2021) 9:49. doi: 10.3390/vaccines9111219
79. Grund M, Choi SJ, Powell L, and Lukomski S. Intranasal immunization with a bucl8-based vaccine ameliorates bacterial burden and pathological inflammation, and promotes an IgG2a/b dominant response in an outbred mouse model of Burkholderia infection. Front Immunol. (2023) 14:1177650. doi: 10.3389/fimmu.2023.1177650
80. Gerritzen MJH, Martens DE, Wijffels RH, van der Pol L, and Stork M. Bioengineering bacterial outer membrane vesicles as vaccine platform. Biotechnol Adv. (2017) 35:565–74. doi: 10.1016/j.bioteChadv.2017.05.003
81. Kulp A and Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. (2010) 64:163–84. doi: 10.1146/annurev.micro.091208.073413
82. Ellis TN and Kuehn MJ. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol Biol Rev. (2010) 74:81–94. doi: 10.1128/MMBR.00031-09
83. Li W, Hu Y, Zhang Q, Hua L, Yang Z, Ren Z, et al. Development of drug-resistant Klebsiella pneumoniae vaccine via novel vesicle production technology. ACS Appl Mater Interfaces. (2021) 13:32703–15. doi: 10.1021/acsami.1c06701
84. Holst J, Oster P, Arnold R, Tatley MV, Naess LM, Aaberge IS, et al. Vaccines against meningococcal serogroup B disease containing outer membrane vesicles (OMV): lessons from past programs and implications for the future. Hum Vaccin Immunother. (2013) 9:1241–53. doi: 10.4161/hv.24129
85. Balhuizen MD, Veldhuizen EJA, and Haagsman HP. Outer membrane vesicle induction and isolation for vaccine development. Front Microbiol. (2021) 12:629090. doi: 10.3389/fmicb.2021.629090
86. Nieves W, Asakrah S, Qazi O, Brown KA, Kurtz J, Aucoin DP, et al. A Naturally Derived Outer-Membrane Vesicle Vaccine Protects against Lethal Pulmonary Burkholderia pseudomallei Infection. Vaccine. (2011) 29:8381–9. doi: 10.1016/j.vaccine.2011.08.058
87. Nieves W, Petersen H, Judy BM, Blumentritt CA, Russell-Lodrigue K, Roy CJ, et al. A Burkholderia pseudomallei Outer Membrane Vesicle Vaccine Provides Protection against Lethal Sepsis. Clin Vaccine Immunol. (2014) 21:747–54. doi: 10.1128/CVI.00119-14
88. Petersen H, Nieves W, Russell-Lodrigue K, Roy CJ, and Morici LA. Evaluation of a Burkholderia pseudomallei outer membrane vesicle vaccine in nonhuman primates. Proc Vaccinol. (2014) 8:38–42. doi: 10.1016/j.provac.2014.07.007
89. Baker SM, Davitt CJH, Motyka N, Kikendall NL, Russell-Lodrigue K, Roy CJ, et al. A Burkholderia pseudomallei Outer Membrane Vesicle Vaccine Provides Cross Protection against Inhalational Glanders in Mice and Non-Human Primates. Vaccines (Basel). (2017) 5:49. doi: 10.3390/vaccines5040049
90. Propst KL, Mima T, Choi KH, Dow SW, and Schweizer HPA. Burkholderia pseudomallei ΔpurM mutant is avirulent in immunocompetent and immunodeficient animals: candidate strain for exclusion from select-agent lists. Infect Immun. (2010) 78:3136–43. doi: 10.1128/IAI.01313-09
91. Baker SM, Settles EW, Davitt C, Gellings P, Kikendall N, Hoffmann J, et al. Burkholderia pseudomallei OMVs derived from infection mimicking conditions elicit similar protection to a live-attenuated vaccine. NPJ Vaccines. (2021) 6:18. doi: 10.1038/s41541-021-00281-z
92. Zhu K, Li G, Li J, Zheng M, Peng X, Rao Y, et al. Hcp1-loaded staphylococcal membrane vesicle vaccine protects against acute melioidosis. Front Immunol. (2022) 13:1089225. doi: 10.3389/fimmu.2022.1089225
93. Shah M, Badwaik VD, and Dakshinamurthy R. Biological applications of gold nanoparticles. J Nanosci Nanotechnol. (2014) 14:344–62. doi: 10.1166/jnn.2014.8900
94. Aminabad NS, Farshbaf M, and Akbarzadeh A. Recent advances of gold nanoparticles in biomedical applications: state of the art. Cell Biochem Biophys. (2019) 77:123–37. doi: 10.1007/s12013-018-0863-4
95. Gregory AE, Judy BM, Qazi O, Blumentritt CA, Brown KA, Shaw AM, et al. A Gold Nanoparticle-Linked Glycoconjugate Vaccine against Burkholderia mallei. Nanomedicine. (2015) 11:447–56. doi: 10.1016/j.nano.2014.08.005
96. Torres AG, Gregory AE, Hatcher CL, Vinet-Oliphant H, Morici LA, Titball RW, et al. Protection of non-human primates against glanders with a gold nanoparticle glycoconjugate vaccine. Vaccine. (2015) 33:686–92. doi: 10.1016/j.vaccine.2014.11.057
97. Hizbullah, Nazir Z, Afridi SG, Shah M, Shams S, and Khan A. Reverse vaccinology and subtractive genomics-based putative vaccine targets identification for Burkholderia pseudomallei Bp1651. Microb Pathog. (2018) 125:219–29. doi: 10.1016/j.micpath.2018.09.033
98. He Y, Xiang Z, and Mobley HL. Vaxign: the first web-based vaccine design program for reverse vaccinology and applications for vaccine development. J BioMed Biotechnol. (2010) 2010:297505. doi: 10.1155/2010/297505
99. Capelli R, Peri C, Villa R, Nithichanon A, Conchillo-Sole O, Yero D, et al. Bpsl1626: Reverse and Structural Vaccinology Reveal a Novel Candidate for Vaccine Design against Burkholderia pseudomallei. Antibodies (Basel). (2018) 7:26. doi: 10.3390/antib7030026
100. Muruato LA, Tapia D, Hatcher CL, Kalita M, Brett PJ, Gregory AE, et al. Use of Reverse Vaccinology in the Design and Construction of Nanoglycoconjugate Vaccines against Burkholderia pseudomallei. Clin Vaccine Immunol. (2017) 24:e00206-17. doi: 10.1128/CVI.00206-17
101. Tapia D, Sanchez-Villamil JI, and Torres AG. Multicomponent Gold Nano-Glycoconjugate as a Highly Immunogenic and Protective Platform against Burkholderia mallei. NPJ Vaccines. (2020) 5:82. doi: 10.1038/s41541-020-00229-9
102. Tapia D, Sanchez-Villamil JI, Stevenson HL, and Torres AG. Multicomponent Gold-Linked Glycoconjugate Vaccine Elicits Antigen-Specific Humoral and Mixed TH1-TH17 Immunity, Correlated with Increased Protection against Burkholderia pseudomallei. mBio. (2021) 12:e0122721. doi: 10.1128/mBio.01227-21
103. Comas-Garcia M, Colunga-Saucedo M, and Rosales-Mendoza S. The role of virus-like particles in medical biotechnology. Mol Pharm. (2020) 17:4407–20. doi: 10.1021/acs.molpharmaceut.0c00828
104. Frietze KM, Peabody DS, and Chackerian B. Engineering virus-like particles as vaccine platforms. Curr Opin Virol. (2016) 18:44–9. doi: 10.1016/j.coviro.2016.03.001
105. Khakhum N, Baruch-Torres N, Stockton JL, Chapartegui-Gonzalez I, Badten AJ, Adam A, et al. Decoration of Burkholderia Hcp1 protein to virus-like particles as a vaccine delivery platform. Infect Immun. (2024) 92:e0001924. doi: 10.1128/iai.00019-24
106. Myhr AI. DNA vaccines: regulatory considerations and safety aspects. Curr Issues Mol Biol. (2017) 22:79–88. doi: 10.21775/cimb.022.079
107. Hobernik D and Bros M. DNA vaccines-how far from clinical use? Int J Mol Sci. (2018) 19:3605. doi: 10.3390/ijms19113605
108. Li L and Petrovsky N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert Rev Vaccines. (2016) 15:313–29. doi: 10.1586/14760584.2016.1124762
109. Eusebio D, Neves AR, Costa D, Biswas S, Alves G, Cui Z, et al. Methods to improve the immunogenicity of plasmid DNA vaccines. Drug Discov Today. (2021) 26:2575–92. doi: 10.1016/j.drudis.2021.06.008
110. Lankelma JM, Wagemakers A, Birnie E, Haak BW, Trentelman JJA, Weehuizen TAF, et al. Rapid DNA Vaccination against Burkholderia pseudomallei Flagellin by Tattoo or Intranasal Application. Virulence. (2017) 8:1683–94. doi: 10.1080/21505594.2017.1307485
111. Travieso T, Li J, Mahesh S, Mello J, and Blasi M. The use of viral vectors in vaccine development. NPJ Vaccines. (2022) 7:75. doi: 10.1038/s41541-022-00503-y
112. Chen Z. Parainfluenza virus 5-vectored vaccines against human and animal infectious diseases. Rev Med Virol. (2018) 28:e1965. doi: 10.1002/rmv.1965
113. Ura T, Okuda K, and Shimada M. Developments in viral vector-based vaccines. Vaccines (Basel). (2014) 2:624–41. doi: 10.3390/vaccines2030624
114. Lafontaine ER, Chen Z, Huertas-Diaz MC, Dyke JS, Jelesijevic TP, Michel F, et al. The Autotransporter Protein BatA Is a Protective Antigen against Lethal Aerosol Infection with Burkholderia mallei and Burkholderia pseudomallei. Vaccine X. (2019) 1:100002. doi: 10.1016/j.jvacx.2018.100002
115. Morici L, Torres AG, and Titball RW. Novel multi-component vaccine approaches for Burkholderia pseudomallei. Clin Exp Immunol. (2019) 196:178–88. doi: 10.1111/cei.13286
116. Peacock SJ, Limmathurotsakul D, Lubell Y, Koh GC, White LJ, Day NP, et al. Melioidosis vaccines: A systematic review and appraisal of the potential to exploit biodefense vaccines for public health purposes. PloS Negl Trop Dis. (2012) 6:e1488. doi: 10.1371/journal.pntd.0001488
117. Khakhum N, Chapartegui-Gonzalez I, and Torres AG. Combating the great mimicker: latest progress in the development of Burkholderia pseudomallei vaccines. Expert Rev Vaccines. (2020) 19:653–60. doi: 10.1080/14760584.2020.1791089
118. Chapartegui-Gonzalez I, Bowser S, Torres AG, and Khakhum N. Recent progress in Shigella and Burkholderia pseudomallei vaccines. Pathogens. (2021) 10:1353. doi: 10.3390/pathogens10111353
119. Bergstrom C, Fischer NO, Kubicek-Sutherland JZ, and Stromberg ZR. mRNA vaccine platforms to prevent bacterial infections. Trends Mol Med. (2024) 30:524–6. doi: 10.1016/j.molmed.2024.02.013
120. Ghaffar SA, Tahir H, Muhammad S, Shahid M, Naqqash T, Faisal M, et al. Designing of a Multi-Epitopes Based Vaccine against Haemophilius parainfluenzae and Its Validation through Integrated Computational Approaches. Front Immunol. (2024) 15:1380732. doi: 10.3389/fimmu.2024.1380732
121. Zhu Y, Shi J, Wang Q, Zhu Y, Li M, Tian T, et al. Novel Dual-Pathogen Multi-Epitope mRNA Vaccine Development for Brucella melitensis and Mycobacterium tuberculosis in Silico Approach. PLoS One. (2024) 19:e0309560. doi: 10.1371/journal.pone.0309560
122. Limmathurotsakul D, Funnell SG, Torres AG, Morici LA, Brett PJ, Dunachie S, et al. Consensus on the development of vaccines against naturally acquired melioidosis. Emerg Infect Dis. (2015) 21:e141480. doi: 10.3201/eid2106.141480
Keywords: Burkholderia pseudomallei, Burkholderia mallei, melioidosis, glanders, vaccine, review
Citation: Sengyee S, Weiby SB, Rok IT, Burtnick MN and Brett PJ (2025) Melioidosis vaccines: recent advances and future directions. Front. Immunol. 16:1582113. doi: 10.3389/fimmu.2025.1582113
Received: 23 February 2025; Accepted: 09 June 2025;
Published: 24 June 2025.
Edited by:
Evan Skowronski, TMG Biosciences, LLC, United StatesReviewed by:
V. L. S. Prasad Burra, K. L. University, IndiaMegan Grund, Cleveland Clinic, United States
Copyright © 2025 Sengyee, Weiby, Rok, Burtnick and Brett. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paul J. Brett, cGJyZXR0QG1lZC51bnIuZWR1; Mary N. Burtnick, bWJ1cnRuaWNrQG1lZC51bnIuZWR1
 Sineenart Sengyee
Sineenart Sengyee Sarah B. Weiby
Sarah B. Weiby Ivory T. Rok
Ivory T. Rok Mary N. Burtnick
Mary N. Burtnick Paul J. Brett
Paul J. Brett