- Department of Gynecology, Chengdu Pidu District Hospital of Traditional Chinese Medicine, Chengdu, China
Human papillomavirus (HPV), a double-stranded DNA virus linked to various malignancies, poses a significant global public health challenge. In cervical cancer, persistent infection with high-risk HPV genotypes, particularly HPV-16 and HPV-18, initiates immune evasion mechanisms within the tumor microenvironment. The polarization of tumor-associated macrophages (TAMs) from M1 to M2 phenotypes promotes cervical carcinogenesis, metastasis, and therapeutic resistance via establishing an immunosuppressive microenvironment. This review provides a comprehensive overview of HPV-induced immune evasion pathways, including MHC downregulation, T-cell impairment, regulatory T cell induction, and cGAS-STING pathway inhibition. Furthermore, describe the pivotal role of TAMs in cervical cancer progression, focusing on their phenotypic plasticity, pro-tumoral functions, and potential as therapeutic targets. By elucidating these cellular and molecular dynamics, this review aims to support advanced research. Targeting TAM polarization through immunotherapies and nanomedicine-based strategies represents a promising strategy for enhancing patient outcomes.
1 Introduction
Human papillomavirus (HPV) induces hyperproliferation of squamous epithelium in human skin and mucous membranes (1). High-risk types, notably HPV-16 and HPV-18, are strongly linked to malignant tumors such as cervical, anal, and oral cancers, collectively termed HPV-related tumors. While the immune system typically clears HPV in immunocompetent individuals, certain immune evasion strategies enable progression to malignancy. Recent studies have detailed HPV’s mechanisms to evade host adaptive immunity, closely associated with HPV-related tumor development.
Cervical cancer has seen significant advancements in prevention and diagnosis, including vaccines, HPV detection, cytological screening, and colposcopy. Persistent infection with high-risk HPV-16 and HPV-18 is the primary driver of cervical cancer. The tumor microenvironment (TME) (2–5), composed of tumor cells, bone marrow-derived cells, and stromal cells, fosters an immunosuppressive microenvironment that promotes tumor immune evasion, growth, and metastasis (6–8). Despite these insights, treatment outcomes for advanced and recurrent cervical cancer remain suboptimal. TAMs within the TME play a pivotal role in tumorigenesis and progression by promoting invasion, migration, angiogenesis, and suppressing anti-tumor immunity (9, 10). This review consolidates current knowledge on TAM polarization, their functional roles, and therapeutic targets in cervical cancer, aiming to advance research on its progression and treatment.
2 Mechanisms of HPV-induced evasion of host adaptive immune responses
2.1 MHC downregulation to prevent cytotoxic T lymphocyteactivation
CTLs are crucial in anti-tumor immunity, eliminating mutated or tumor cells through perforin-granzyme and Fas-FasL/TNF-TNFR pathways. Their ability to recognize exogenous peptides is governed by MHC class I and II molecules, with MHC contributing to variability in immune responses—a critical regulatory mechanism. Tumors frequently harbor mutations that disrupt MHC antigen presentation pathways (11). Genome-wide association studies have identified HA locus mutations potentially influencing susceptibility to HPV-related cancers. HPV employs gene expression modulation to inhibit antigen presentation, preventing viral antigens from being displayed on MHC class I molecules during infection. Specifically, the HPV E5 protein induces late endosome alkalization, disrupting the trafficking of peptide-MHC class I complexes to the cell surface and hindering the presentation of tumor-associated antigens, thereby suppressing CTL activation (12). In head and neck squamous cell carcinoma (HNSCC), HPV E5 overexpression confers CTL resistance by reducing antigen presentation, while treatment with the HPV E5 inhibitor gemcitabine can enhance MHC class I expression and mitigates this effect (13).
2.2 Regulation of CD4+ T cell activation
CD4+ T cells regulate CTL activation and secrete cytokines and chemokines that support anti-tumor immunity. In murine models, CD4+ T cell depletion impairs CTL responses to the HPV E7 protein (14). Clinically, HIV-infected patients with CD4+ deficiency exhibit a higher incidence of HPV-related cancers. In cervical cancer, CD4+ T cells exhibit impaired responses to HPV peptides, contributing to tumor immune tolerance (15). Anti-HPV activity is associated with CD4+ T cell phenotypes rather than quantity, with the CD4+CD161+ subset linked to improved survival (16, 17). In HPV-related oral cancers, CD4+ T cell abundance does not predict prognosis (18). Cervical cancer patients display a Th2-skewed CD4+ response and reduced IFN-γ levels (19). Conversely, in HPV-related oropharyngeal carcinoma, Th1 responses mediated by CD161+ and CD103+ T cells are prognostic (16). Furthermore, stromal fibroblasts secrete CCL20, driving pro-tumorigenic Th17 responses during invasive carcinoma progression, although their persistence across stages remains unclear (20).
2.3 Induction of regulatory T cell generation
Tregs suppress immune responses, disrupting normal host immunity. HPV-related tumors induce Tregs and other regulatory cells to inhibit anti-tumor immunity. Clinically, Treg levels are higher in HPV-infected individuals than in healthy controls, with chronic infections showing greater Treg elevations than those who clear the virus, suggesting impaired cellular immunity allows persistent infection (21, 22). Cervical HPV infections are typically localized to lesions with rare viremia, making local immunity crucial for infection outcomes. In cervical cancer, low-proliferative Foxp3+ Tregs are present at primary tumors, metastatic lymph nodes, and peripheral blood, indicating systemic immune tolerance and potential tumor metastasis (23). Studies on Treg levels in HPV-positive versus HPV-negative HNSCC are inconsistent (24, 25). However, the Treg/CD8+ T cell ratio remains a prognostic marker in HNSCC irrespective of HPV status (26).
2.4 Regulation of the cGAS-STING pathway
The cGAS-STING pathway senses exogenous DNA by synthesizing cGAMP from ATP and GTP, triggering STING, IRFs, and NF-κB to induce interferons and pro-inflammatory cytokines. While it mediates antiviral effects against DNA viruses, HPV evades this defense through mechanisms like HPV18 E7’s LCXCE domain blocking STING (27) and HPV16 E7 destabilizing STING via NLRX1 (28). Depletion of NLRX1 boosts type I interferon-dependent T cell infiltration and tumor suppression (29). In HPV16-positive HNSCC, the LCXCE domain of HPV16 E7 may disrupt cGAS-STING signaling (30). STING expression correlates positively with tumor-infiltrating lymphocytes and improved survival outcomes (31). HPV-positive HNSCC exhibits higher STING mRNA levels than HPV-negative cases, suggesting partial pathway activation despite HPV-mediated inhibition. STING activation may also enhance cetuximab-induced NK cell activity, driving tumor regression (32).
2.5 PD-1/PD-L1 immune checkpoint regulation
The PD-1/PD-L1 immune checkpoint is a conserved inhibitory mechanism regulating immune responses and plays a key role in inducing tumor immune tolerance during tumorigenesis (33–36). HPV-related tumors upregulate PD-1/PD-L1 to enhance immune tolerance. Preliminary analysis of 27 HNSCC tumors shows higher PD-L1 expression in HPV-positive versus HPV-negative tumors (30). In a study of 214 oropharyngeal cancer patients, 85.2% of HPV-positive cancers expressed PD-L1 compared to 57.1% of HPV-negative ones, and HPV-positive tumors also exhibited greater T cell infiltration (31). Additionally, IFN-γ secretion by T cells upregulates PD-L1 expression, potentially intensifying the inflammatory response in the tumor microenvironment. Analyses of TCGA and MSK-IMPACT cohorts indicate that HPV-positive status is a superior predictor of HNSCC outcomes compared to immune checkpoint inhibitor responses, independent of PD-L1 levels, and correlates with higher expression of inflammatory genes and CD8+ T cell infiltration in HPV-positive HNSCC tumors (37).
In summary, HPV-infected tumor cells evade host adaptive immunity through multiple mechanisms, including downregulating MHC molecules, suppressing CD4+ T cells, inducing regulatory T cells, and disrupting signaling pathways, thereby facilitating tumorigenesis. Establishing effective cellular immunity is essential for eliminating persistent HPV infections. Further investigation into HPV-related immune evasion will aid the development of vaccines, anti-tumor therapies, and prognostic tools.
3 The role of TAMs in cervical cancer
3.1 Phenotypic alterations of macrophages during cervical cancer progression
Macrophages exhibit a strong correlation with cervical intraepithelial neoplasia (CIN) progression, with their prevalence increasing linearly alongside tumor advancement (38). Human HPV manipulates innate and adaptive immune responses through various mechanisms, enabling immune evasion and persistent infection. In low-grade CIN (CIN I-II) associated with HPV, pro-inflammatory cytokines and infiltrating inflammatory cells are markedly reduced, fostering malignant transformation. While immune cell infiltration escalates from high-grade CIN (CIN III) to invasive cervical cancer, immune suppression endures, with the phenotype and function of infiltrating immune cells being tightly regulated.
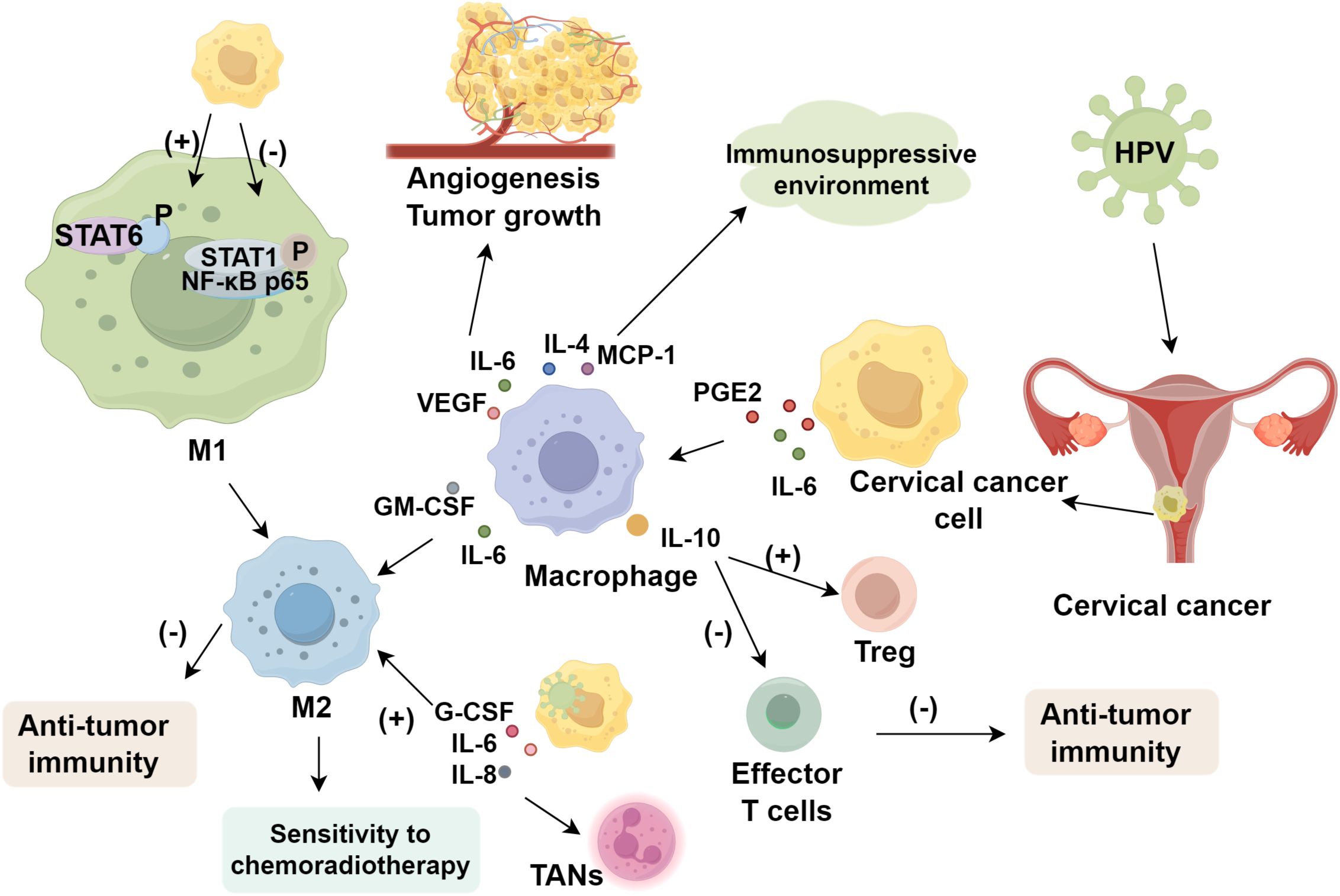
Macrophage phenotypes dynamically shift across cervical cancer stages (39), contributing to tumor proliferation, invasion, and metastasis through multiple pathways. Within the cervical cancer microenvironment, monocyte differentiation into dendritic cells is impaired, while prostaglandin E2 (PGE2) and interleukin-6 (IL-6) secreted by cancer cells induce differentiation into M2 macrophages (40). Cervical cancer cells convert M1 to M2 macrophages, consistent with the high prevalence of M2 types in the tumor microenvironment (41). Supernatants from cancer cell lines stimulate macrophages to secrete elevated IL-6, IL-10, MCP-1, IL-8, GM-CSF, PDGF-AA, PDGF-BB, and VEGF. IL-6 and VEGF promote angiogenesis and tumor growth, while IL-4, MCP-1, and other cytokines create an immunosuppressive environment. GM-CSF and IL-6 synergistically enhance M2 polarization (42). Furthermore, cervical cancer cells reduce STAT1 and NF-κB p65 phosphorylation in M1 macrophages while increasing STAT6 phosphorylation to activate M2 macrophages, thereby impairing macrophage-mediated anti-tumor immunity.
3.2 TAM polarization facilitates HPV-induced cervical cancer progression
HPV comprises three genomic regions: six early genes (E1, E2, E4, E5, E6, E7), two late genes (L1, L2), and a non-coding region. Persistent infection with high-risk HPV types is the primary driver of cervical cancer. Studies demonstrates a positive correlation between cervical cancer progression and the expression of CD68+ or CD163+ macrophages, with macrophage infiltration increasing linearly with high-risk HPV infections (43). In HPV-positive cervical cancer specimens, M2-type macrophages are significantly enriched and exhibit elevated expression of antigen presentation genes, including CD74 and HLA-A (44). P-selectin glycoprotein ligand 1 (PSGL-1) in macrophages reprograms their function to activate T cells, contributing to anti-tumor immunity. In HPV16/18-positive CIN tissues, PSGL-1 expression is closely linked to infection status, lesion severity, immune infiltration, and prognosis (45). The HPV E6/E7 genes suppress CCL20/MIP3α secretion, impairing Langerhans cell migration (46), while HPV-positive cells secrete elevated levels of G-CSF, IL-6, IL-8, promoting the recruitment of tumor-associated neutrophils and M2 macrophages (47).
3.3 Impact of tumor-associated macrophages on cervical cancer prognosis
Increased TAM infiltration is associated with poorer patient prognosis (48–50). High-risk HPV infection is the primary cause of cervical cancer; however, immune responses against HPV antigens eliminate most infections and precursor lesions, with only a minority of infected individuals developing persistent infections leading to malignancy. Interleukin-10 (IL-10) inhibits the production of other cytokines, such as IL-2, IFN-γ, IL-12, and TNF-α, and is associated with downregulation of MHC class I molecules, resulting in reduced Th1 responses. In cervical cancer, TAMs secrete IL-10, inducing the proliferation of HPV-specific regulatory T cells and suppressing the anti-tumor activity of effector T cells, thereby contributing to adverse prognosis. During tumorigenesis and progression, cancer cells proliferate by absorbing nutrients via blood vessels, a process closely linked to TAMs (38). TAMs promote tumor angiogenesis and metastasis, suggesting that TAMs may facilitate the extensive growth of new blood vessels through factor secretion, synergistically promoting the malignant progression of cervical cancer (51).
TAMs are closely associated with recurrence and metastasis in cervical cancer. CD163 and CD68 are common TAM markers. The increased CD163+ macrophage count was significantly associated with reduced recurrence-free survival, whereas CD68+ macrophages were not correlated with recurrence in Stage I squamous cell carcinoma of the cervix (52). Moreover, M2-polarized TAMs reduce sensitivity to chemoradiotherapy, and a higher M1/M2 ratio independently predicts unfavorable survival. Targeting macrophage polarization to prevent M2 differentiation effectively impedes tumor progression (53, 54), thereby improving cervical cancer prognosis (Figure 1).
4 Therapeutic targeting of TAMs
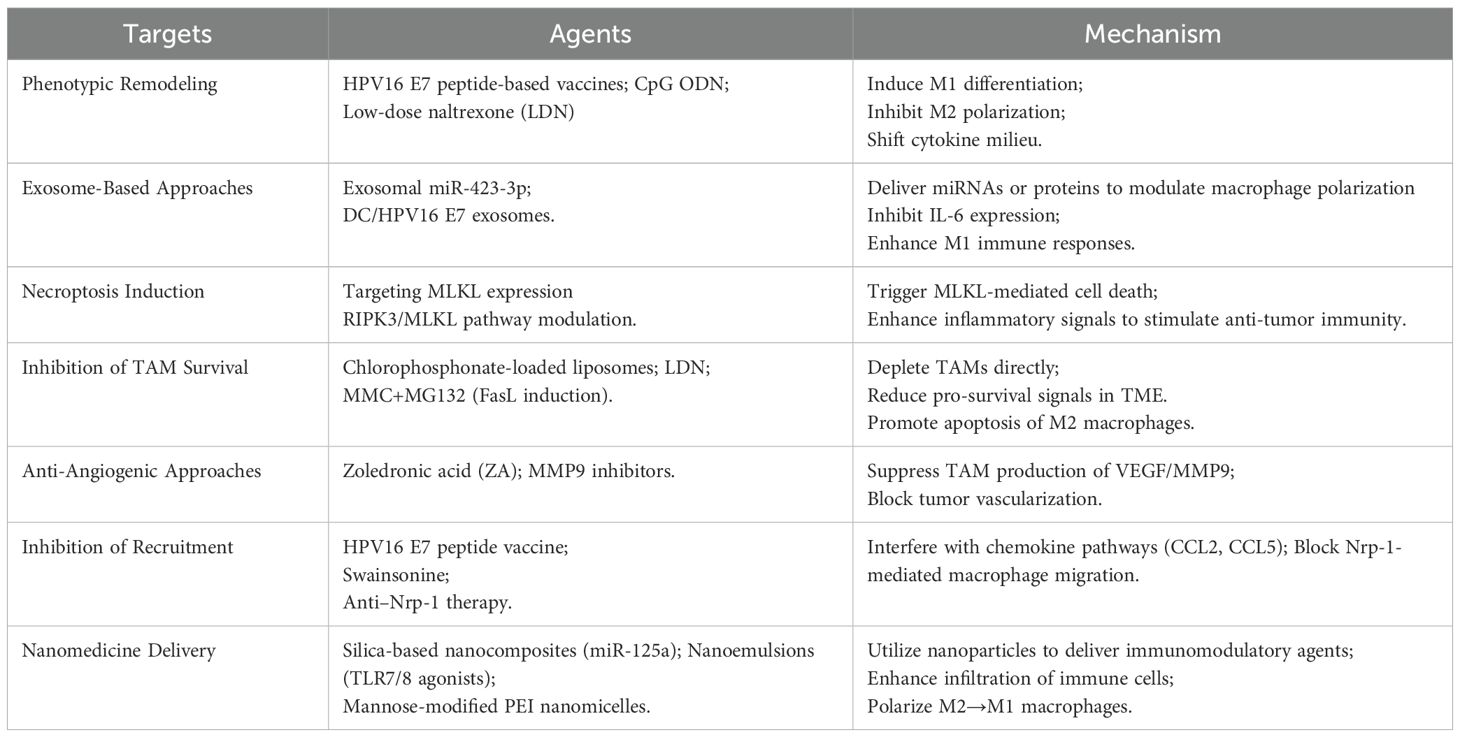
4.1 Phenotypic remodeling of TAMs
M1-type macrophages show anti-tumor, pro-inflammatory roles in cervical cancer, whereas M2-type macrophages correlate with poor prognosis and are more prevalent in cervical cancer tissues than non-tumorous samples (55, 56). 6Because macrophages are highly plastic, promoting M1 polarization and inhibiting M2 differentiation effectively treats cervical cancer via phenotypic remodeling. Such a strategy fosters monocyte differentiation into M1, curbs M2 polarization, and represents a viable therapeutic intervention for cervical cancer.
4.1.1 Vaccines
To date, three prophylactic HPV vaccines, like bivalent, quadrivalent, and nonavalent, have been approved for preventing common HPV infections (57). However, current prophylactic HPV vaccines cannot eliminate existing HPV infections or interfere with the progression of precancerous lesions to malignancy (58). CHE YX et al. (59) employed a therapeutic vaccine containing HPV16 E7 43-77 peptides and adjuvants to treat cervical cancer in murine models, observing a downregulation of IL-10 and TGF-β expression in M2-type macrophages and an upregulation of CXCL9 and CXCL10 chemokines in M1-type macrophages. This induced significant reduction in the percentage of M2-type TAMs, decreased tumor microvascular density, and reduced Ki-67 indices, suggesting a macrophage shift from M2 to M1. Similarly, Sonia et al. (60) demonstrated that HPV16 E7 peptides and CpG ODN reduced TAMs and genital tumor growth. Moreover, low-dose naltrexone decreases the number of M2-type macrophages and reduces serum IL-10 secretion, thereby inhibiting cervical cancer progression (61).
4.1.2 TAM-derived exosomes
Exosomes are extracellular vesicles containing nucleic acids, proteins, metabolites, and other bioactive molecules (62, 63), mediating “crosstalk” between tumor and immune cells, including TAMs. They serve as valuable mediators for TAM-targeted immunotherapy. In cervical cancer, tumor-derived exosomes promote TAM polarization toward M1, reducing PD-1 expression (64). Exosome-derived miR-423-3p targets CDK4/p-STAT3, silencing IL-6 and inhibiting M2 polarization (65). Inflammatory factors widely involve in diseases’ progression (66–69). In DC/HPV16 E7, silencing CAT2 in exosomes induces M1 differentiation, increasing IL-12 and TNF-α and curtailing tumor growth (70). SEPT9 methylation confers radio-resistance via miR-375 “crosstalk” to TAMs, favoring M2 polarization and tumor progression (71). TAM-derived exosomes also affect ferroptosis by transferring miR-660-5p through the IL-4/IL-13/p-STAT6 pathway, suppressing ALOX15 (72). Due to their wide distribution, abundance, stability, and plasticity, exosome-based technologies hold significant promise for cervical cancer therapy. Hence, understanding their mechanisms is essential for developing novel therapeutic strategies and improving patient prognosis in cervical cancer. By harnessing these vesicles, clinicians may optimize treatment outcomes.
4.1.3 Mixed lineage kinase domain-like protein
Necroptosis, a programmed cell death mechanism, triggers pro-inflammatory cytokines and anti-tumor immunity, thereby contributing to tumor necrosis. Hence, molecules in necroptosis pathways are promising therapeutic targets (73). MLKL, the key necroptotic effector, undergoes RIPK3-mediated phosphorylation, oligomerizes, relocates to the plasma membrane, and disrupts membrane integrity, a hallmark of necroptosis (74). Cervical cancer cells may limit M1 macrophage polarization by reducing macrophage necroptosis, particularly in HPV-positive contexts (75). Plasma MLKL and HPV DNA are readily measurable in clinical labs, suggesting their combined detection in cervical cancer diagnosis and disease monitoring. Additionally, blocking MLKL expression might regulate macrophage polarization, offering a novel therapeutic strategy for cervical cancer. Future studies are further needed to elucidate MLKL’s clinical role in both prevention and therapy.
4.2 Inhibition of TAM viability
TAMs drive cervical cancer proliferation, invasion, and metastasis, making their depletion a key therapeutic strategy. Metastasis is initiated by tumor cell dissemination and the acquisition of invasive capabilities, often mediated by epithelial-mesenchymal transition (EMT). The TME, comprising TAMs, extracellular matrix, hypoxia, and tumor-mesenchymal interactions, fosters EMT (76). Low-dose naltrexone (LDN) has been shown to reduce TAMs in vitro, suppress M2-type macrophage-mediated cervical cancer proliferation, invasion, and migration, promotes apoptosis, and inhibits EMT (61), positioning it as a potential adjunct therapy targeting TAM survival to curb tumor progression. Chlorophosphonates induce TAM apoptosis and inhibit tumor growth in HPV16 E6/E7-expressing TC-1 murine models without affecting TC-1 cell viability (77). Additionally, mitomycin C (MMC) combined with MG132 enhances FasL expression in TAMs both in vivo and in vitro, amplifying their bystander effect on cervical cancer cells and suppressing tumor growth.
Angiogenesis is a critical process in tumor progression. DiNardo et al. (78) reported that zoledronic acid (ZA) in K14-HPV16 transgenic female mice suppresses MMP9 in TAMs, reduces VEGF-receptor binding, and suppresses angiogenesis, thereby inhibiting cervical cancer cell proliferation and metastasis. Moreover, chlorophosphonate-loaded liposomes deplete TAMs, boost tumor-specific CD8+ T-cell responses, and inhibit TC-1 tumor growth in murine models (79).
4.4 Regulation of TAM recruitment to the microenvironment
4.4.1 Interference with chemokines to inhibit M2 macrophage recruitment
Cytokines and chemokines within the TME drive monocyte and macrophage recruitment to tumors and their subsequent differentiation into the TAM phenotype. As previously discussed, administering a cervical cancer vaccine containing HPV16 E7 43-77 peptides to TC-1 murine models alters the immune microenvironment, decreases the expression of chemokines such as CCL2 and CCL5, suppresses myeloid-derived suppressor cell (MDSC) recruitment to tumors, and significantly reduces M2-TAMs within tumors (59). The natural anticancer compound swainsonine competitively inhibits α-mannosidase, modulates TAM phenotypes, and suppresses the secretion of CCL2 and IL-10, thereby reducing macrophage recruitment within the microenvironment (80). Under hypoxic conditions in cervical cancer, high expression of neuropilin-1 (Nrp-1) is significantly associated with TAM recruitment and migratory function. Researchers have found that interfering with Nrp-1 expression markedly impairs TAM migration (81).
4.4.2 Nanomedicine delivery systems to enhance immune cell recruitment
Nanomedicine-based immunotherapy harnesses nanoparticles to deliver drugs or immune cells, enhancing efficacy and minimizing toxicity. Targeting TAMs in cervical cancer shows promise, exemplified by silica-based nanocomposites with miR-125a that shift macrophages to M1, reducing tumor growth (82). Nanoemulsions with TLR7/8 agonists increase M1/M2 ratios in bone marrow-derived macrophages, elevating MCP-1/CCL2 and MIP-1α/CCL3 to bolster immune cell recruitment (83). In HPV E6/E7-positive TC-1 tumor models, mannose-modified polyethyleneimine nanomicelles loaded with R-848 target dendritic cells and macrophages in draining lymph nodes, polarizing M2 to M1 macrophages, activating CD8+ T cells, and enhancing anti-tumor responses (84) (Table 1).
5 Conclusion
In conclusion, TAMs in cervical cancer predominantly exhibit an M2-like phenotype, contributing to tumor progression, immune suppression, and therapy resistance, while strategies targeting TAM reprogramming toward an M1 phenotype or reducing M2 polarization show promising therapeutic potential. Future research should focus on elucidating the molecular mechanisms of TAM plasticity, exploring combinational therapies such as vaccines and immune checkpoint inhibitors, and investigating HPV-mediated macrophage manipulation to develop novel immunomodulatory approaches. Clinically, TAM-targeted therapies could complement existing treatments, with biomarkers like CD163 and IL-10 guiding personalized interventions, ultimately improving tumor control and patient outcomes through optimized multimodal strategies.
Author contributions
ZC: Writing – original draft. BZ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gong X, Chi H, Xia Z, Yang G, Tian G. Advances in HPV-associated tumor management: Therapeutic strategies and emerging insights. J Med Virol. (2023) 95:e28950. doi: 10.1002/jmv.28950
2. Xia Z, Chen S, He M, Li B, Deng Y, Yi L, et al. Editorial: Targeting metabolism to activate T cells and enhance the efficacy of checkpoint blockade immunotherapy in solid tumors. Front Immunol. (2023) 14:1247178. doi: 10.3389/fimmu.2023.1247178
3. Zhang X, Zhang P, Cong A, Feng Y, Chi H, Xia Z, et al. Unraveling molecular networks in thymic epithelial tumors: deciphering the unique signatures. Front Immunol. (2023) 14:1264325. doi: 10.3389/fimmu.2023.1264325
4. Deng Y, Shi M, Yi L, Naveed Khan M, Xia Z, Li X. Eliminating a barrier: Aiming at VISTA, reversing MDSC-mediated T cell suppression in the tumor microenvironment. Heliyon. (2024) 10:e37060. doi: 10.1016/j.heliyon.2024.e37060
5. Xie H, Xi X, Lei T, Liu H, Xia Z. CD8(+) T cell exhaustion in the tumor microenvironment of breast cancer. Front Immunol. (2024) 15:1507283. doi: 10.3389/fimmu.2024.1507283
6. Mo Y, Ma J, Zhang H, Shen J, Chen J, Hong J, et al. Prophylactic and therapeutic HPV vaccines: current scenario and perspectives. Front Cell Infect Microbiol. (2022) 12:909223. doi: 10.3389/fcimb.2022.909223
7. Zhang J, Peng G, Chi H, Yang J, Xie X, Song G, et al. CD8 + T-cell marker genes reveal different immune subtypes of oral lichen planus by integrating single-cell RNA-seq and bulk RNA-sequencing. BMC Oral Health. (2023) 23:464. doi: 10.1186/s12903-023-03138-0
8. Wang Y, Li C, He J, Zhao Q, Zhou Y, Sun H, et al. Multi-omics analysis and experimental validation of the value of monocyte-associated features in prostate cancer prognosis and immunotherapy. Front Immunol. (2024) 15:1426474. doi: 10.3389/fimmu.2024.1426474
9. Anfray C, Ummarino A, Andon FT, Allavena P. Current strategies to target tumor-associated-macrophages to improve anti-tumor immune responses. Cells. (2019) 9:46. doi: 10.3390/cells9010046
10. Choi Y, Lee D, Kim NY, Seo I, Park NJ, Chong GO. Role of tumor-associated macrophages in cervical cancer: integrating classical perspectives with recent technological advances. Life (Basel). (2024) 14:443. doi: 10.3390/life14040443
11. Reeves E, James E. Antigen processing and immune regulation in the response to tumours. Immunology. (2017) 150:16–24. doi: 10.1111/imm.2017.150.issue-1
12. de Freitas AC, de Oliveira THA, Barros MR Jr., Venuti A. hrHPV E5 oncoprotein: immune evasion and related immunotherapies. J Exp Clin Cancer Res. (2017) 36:71. doi: 10.1186/s13046-017-0541-1
13. Miyauchi S, Sanders PD, Guram K, Kim SS, Paolini F, Venuti A, et al. HPV16 E5 mediates resistance to PD-L1 blockade and can be targeted with rimantadine in head and neck cancer. Cancer Res. (2020) 80:732–46. doi: 10.1158/0008-5472.CAN-19-1771
14. Saito Y, Komori S, Kotani T, Murata Y, Matozaki T. The role of type-2 conventional dendritic cells in the regulation of tumor immunity. Cancers (Basel). (2022) 14:1976. doi: 10.3390/cancers14081976
15. de Jong A, van Poelgeest MI, van der Hulst JM, Drijfhout JW, Fleuren GJ, Melief CJ, et al. Human papillomavirus type 16-positive cervical cancer is associated with impaired CD4+ T-cell immunity against early antigens E2 and E6. Cancer Res. (2004) 64:5449–55. doi: 10.1158/0008-5472.CAN-04-0831
16. Welters MJP, Ma W, Santegoets S, Goedemans R, Ehsan I, Jordanova ES, et al. Intratumoral HPV16-specific T cells constitute a type I-oriented tumor microenvironment to improve survival in HPV16-driven oropharyngeal cancer. Clin Cancer Res. (2018) 24:634–47. doi: 10.1158/1078-0432.CCR-17-2140
17. Santegoets SJ, van Ham VJ, Ehsan I, Charoentong P, Duurland CL, van Unen V, et al. The anatomical location shapes the immune infiltrate in tumors of same etiology and affects survival. Clin Cancer Res. (2019) 25:240–52. doi: 10.1158/1078-0432.CCR-18-1749
18. Oguejiofor K, Hall J, Slater C, Betts G, Hall G, Slevin N, et al. Stromal infiltration of CD8 T cells is associated with improved clinical outcome in HPV-positive oropharyngeal squamous carcinoma. Br J Cancer. (2015) 113:886–93. doi: 10.1038/bjc.2015.277
19. Bais AG, Beckmann I, Lindemans J, Ewing PC, Meijer CJ, Snijders PJ, et al. A shift to a peripheral Th2-type cytokine pattern during the carcinogenesis of cervical cancer becomes manifest in CIN III lesions. J Clin Pathol. (2005) 58:1096–100. doi: 10.1136/jcp.2004.025072
20. Walch-Ruckheim B, Mavrova R, Henning M, Vicinus B, Kim YJ, Bohle RM, et al. Stromal Fibroblasts Induce CCL20 through IL6/C/EBPbeta to Support the Recruitment of Th17 Cells during Cervical Cancer Progression. Cancer Res. (2015) 75:5248–59. doi: 10.1158/0008-5472.CAN-15-0732
21. Adurthi S, Krishna S, Mukherjee G, Bafna UD, Devi U, Jayshree RS. Regulatory T cells in a spectrum of HPV-induced cervical lesions: cervicitis, cervical intraepithelial neoplasia and squamous cell carcinoma. Am J Reprod Immunol. (2008) 60:55–65. doi: 10.1111/j.1600-0897.2008.00590.x
22. Heeren AM, Koster BD, Samuels S, Ferns DM, Chondronasiou D, Kenter GG, et al. High and interrelated rates of PD-L1+CD14+ antigen-presenting cells and regulatory T cells mark the microenvironment of metastatic lymph nodes from patients with cervical cancer. Cancer Immunol Res. (2015) 3:48–58. doi: 10.1158/2326-6066.CIR-14-0149
23. van der Burg SH, Piersma SJ, de Jong A, van der Hulst JM, Kwappenberg KM, van den Hende M, et al. Association of cervical cancer with the presence of CD4+ regulatory T cells specific for human papillomavirus antigens. Proc Natl Acad Sci U.S.A. (2007) 104:12087–92. doi: 10.1073/pnas.0704672104
24. Heusinkveld M, Goedemans R, Briet RJ, Gelderblom H, Nortier JW, Gorter A, et al. Systemic and local human papillomavirus 16-specific T-cell immunity in patients with head and neck cancer. Int J Cancer. (2012) 131:E74–85. doi: 10.1002/ijc.v131.2
25. Jie HB, Gildener-Leapman N, Li J, Srivastava RM, Gibson SP, Whiteside TL, et al. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br J Cancer. (2013) 109:2629–35. doi: 10.1038/bjc.2013.645
26. Nasman A, Romanitan M, Nordfors C, Grun N, Johansson H, Hammarstedt L, et al. Tumor infiltrating CD8+ and Foxp3+ lymphocytes correlate to clinical outcome and human papillomavirus (HPV) status in tonsillar cancer. PloS One. (2012) 7:e38711. doi: 10.1371/journal.pone.0038711
27. Lau L, Gray EE, Brunette RL, Stetson DB. DNA tumor virus oncogenes antagonize the cGAS-STING DNA-sensing pathway. Science. (2015) 350:568–71. doi: 10.1126/science.aab3291
28. Luo X, Donnelly CR, Gong W, Heath BR, Hao Y, Donnelly LA, et al. HPV16 drives cancer immune escape via NLRX1-mediated degradation of STING. J Clin Invest. (2020) 130:1635–52. doi: 10.1172/JCI129497
29. Shaikh MH, Bortnik V, McMillan NA, Idris A. cGAS-STING responses are dampened in high-risk HPV type 16 positive head and neck squamous cell carcinoma cells. Microb Pathog. (2019) 132:162–5. doi: 10.1016/j.micpath.2019.05.004
30. Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. (2013) 73:1733–41. doi: 10.1158/0008-5472.CAN-12-2384
31. Hong AM, Ferguson P, Dodds T, Jones D, Li M, Yang J, et al. Significant association of PD-L1 expression with human papillomavirus positivity and its prognostic impact in oropharyngeal cancer. Oral Oncol. (2019) 92:33–9. doi: 10.1016/j.oraloncology.2019.03.012
32. Lu S, Concha-Benavente F, Shayan G, Srivastava RM, Gibson SP, Wang L, et al. STING activation enhances cetuximab-mediated NK cell activation and DC maturation and correlates with HPV(+) status in head and neck cancer. Oral Oncol. (2018) 78:186–93. doi: 10.1016/j.oraloncology.2018.01.019
33. Jin W, Yang Q, Chi H, Wei K, Zhang P, Zhao G, et al. Ensemble deep learning enhanced with self-attention for predicting immunotherapeutic responses to cancers. Front Immunol. (2022) 13:1025330. doi: 10.3389/fimmu.2022.1025330
34. Wang Y, Wang J, Liu J, Zhu H. Immune-related diagnostic markers for benign prostatic hyperplasia and their potential as drug targets. Front Immunol. (2024) 15:1516362. doi: 10.3389/fimmu.2024.1516362
35. Wang Y, Zhu H, Wang X. Prognosis and immune infiltration analysis of endoplasmic reticulum stress-related genes in bladder urothelial carcinoma. Front Genet. (2022) 13:965100. doi: 10.3389/fgene.2022.965100
36. Wang Y, Wang X. A pan-cancer analysis of heat-shock protein 90 beta1(HSP90B1) in human tumours. Biomolecules. (2022) 12:1377. doi: 10.3390/biom12101377
37. Wang J, Sun H, Zeng Q, Guo XJ, Wang H, Liu HH, et al. HPV-positive status associated with inflamed immune microenvironment and improved response to anti-PD-1 therapy in head and neck squamous cell carcinoma. Sci Rep. (2019) 9:13404. doi: 10.1038/s41598-019-49771-0
38. Jiang S, Yang Y, Fang M, Li X, Yuan X, Yuan J. Co-evolution of tumor-associated macrophages and tumor neo-vessels during cervical cancer invasion. Oncol Lett. (2016) 12:2625–31. doi: 10.3892/ol.2016.5014
39. Ding H, Cai J, Mao M, Fang Y, Huang Z, Jia J, et al. Tumor-associated macrophages induce lymphangiogenesis in cervical cancer via interaction with tumor cells. APMIS. (2014) 122:1059–69. doi: 10.1111/apm.2014.122.issue-11
40. Heusinkveld M, de Vos van Steenwijk PJ, Goedemans R, Ramwadhdoebe TH, Gorter A, Welters MJ, et al. M2 macrophages induced by prostaglandin E2 and IL-6 from cervical carcinoma are switched to activated M1 macrophages by CD4+ Th1 cells. J Immunol. (2011) 187:1157–65. doi: 10.4049/jimmunol.1100889
41. Sanchez-Reyes K, Bravo-Cuellar A, Hernandez-Flores G, Lerma-Diaz JM, Jave-Suarez LF, Gomez-Lomeli P, et al. Cervical cancer cell supernatants induce a phenotypic switch from U937-derived macrophage-activated M1 state into M2-like suppressor phenotype with change in Toll-like receptor profile. BioMed Res Int. (2014) 2014:683068. doi: 10.1155/2014/683068
42. Sanchez-Reyes K, Pedraza-Brindis EJ, Hernandez-Flores G, Bravo-Cuellar A, Lopez-Lopez BA, Rosas-Gonzalez VC, et al. The supernatant of cervical carcinoma cells lines induces a decrease in phosphorylation of STAT-1 and NF-kappaB transcription factors associated with changes in profiles of cytokines and growth factors in macrophages derived from U937 cells. Innate Immun. (2019) 25:344–55. doi: 10.1177/1753425919848841
43. Chen XJ, Han LF, Wu XG, Wei WF, Wu LF, Yi HY, et al. Clinical significance of CD163+ and CD68+ Tumor-associated macrophages in high-risk HPV-related cervical cancer. J Cancer. (2017) 8:3868–75. doi: 10.7150/jca.21444
44. Li C, Liu D, Zhao Y, Ding Y, Hua K. Diverse intratumoral heterogeneity and immune microenvironment of two HPV-related cervical cancer types revealed by single-cell RNA sequencing. J Med Virol. (2023) 95:e28857. doi: 10.1002/jmv.28857
45. Lin Y, Huang S, Qi Y, Xie L, Jiang J, Li H, et al. PSGL-1 is a novel tumor microenvironment prognostic biomarker with cervical high-grade squamous lesions and more. Front Oncol. (2023) 13:1052201. doi: 10.3389/fonc.2023.1052201
46. Caberg JH, Hubert P, Herman L, Herfs M, Roncarati P, Boniver J, et al. Increased migration of Langerhans cells in response to HPV16 E6 and E7 oncogene silencing: role of CCL20. Cancer Immunol Immunother. (2009) 58:39–47. doi: 10.1007/s00262-008-0522-5
47. Alvarez KLF, Beldi M, Sarmanho F, Rossetti RAM, Silveira CRF, Mota GR, et al. Local and systemic immunomodulatory mechanisms triggered by Human Papillomavirus transformed cells: a potential role for G-CSF and neutrophils. Sci Rep. (2017) 7:9002. doi: 10.1038/s41598-017-09079-3
48. Heeren AM, Punt S, Bleeker MC, Gaarenstroom KN, van der Velden J, Kenter GG, et al. Prognostic effect of different PD-L1 expression patterns in squamous cell carcinoma and adenocarcinoma of the cervix. Mod Pathol. (2016) 29:753–63. doi: 10.1038/modpathol.2016.64
49. Raiha MR, Puolakkainen PA. Tumor-associated macrophages (TAMs) as biomarkers for gastric cancer: A review. Chronic Dis Transl Med. (2018) 4:156–63. doi: 10.1016/j.cdtm.2018.07.001
50. Zhai X, Zhang H, Xia Z, Liu M, Du G, Jiang Z, et al. Oxytocin alleviates liver fibrosis via hepatic macrophages. JHEP Rep. (2024) 6:101032. doi: 10.1016/j.jhepr.2024.101032
51. Petrillo M, Zannoni GF, Martinelli E, Pedone Anchora L, Ferrandina G, Tropeano G, et al. Polarisation of tumor-associated macrophages toward M2 phenotype correlates with poor response to chemoradiation and reduced survival in patients with locally advanced cervical cancer. PloS One. (2015) 10:e0136654. doi: 10.1371/journal.pone.0136654
52. Carus A, Ladekarl M, Hager H, Nedergaard BS, Donskov F. Tumour-associated CD66b+ neutrophil count is an independent prognostic factor for recurrence in localised cervical cancer. Br J Cancer. (2013) 108:2116–22. doi: 10.1038/bjc.2013.167
53. Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. (2014) 41:49–61. doi: 10.1016/j.immuni.2014.06.010
54. Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. (2015) 27:462–72. doi: 10.1016/j.ccell.2015.02.015
55. Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel). (2014) 6:1670–90. doi: 10.3390/cancers6031670
56. Zhang M, He Y, Sun X, Li Q, Wang W, Zhao A, et al. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res. (2014) 7:19. doi: 10.1186/1757-2215-7-19
57. Kim HJ, Kim HJ. Current status and future prospects for human papillomavirus vaccines. Arch Pharm Res. (2017) 40:1050–63. doi: 10.1007/s12272-017-0952-8
58. Hoppe-Seyler K, Bossler F, Braun JA, Herrmann AL, Hoppe-Seyler F. The HPV E6/E7 oncogenes: key factors for viral carcinogenesis and therapeutic targets. Trends Microbiol. (2018) 26:158–68. doi: 10.1016/j.tim.2017.07.007
59. Che Y, Yang Y, Suo J, An Y, Wang X. Induction of systemic immune responses and reversion of immunosuppression in the tumor microenvironment by a therapeutic vaccine for cervical cancer. Cancer Immunol Immunother. (2020) 69:2651–64. doi: 10.1007/s00262-020-02651-3
60. Domingos-Pereira S, Galliverti G, Hanahan D, Nardelli-Haefliger D. Carboplatin/paclitaxel, E7-vaccination and intravaginal CpG as tri-therapy towards efficient regression of genital HPV16 tumors. J Immunother Cancer. (2019) 7:122. doi: 10.1186/s40425-019-0593-1
61. Liu N, Ma M, Qu N, Wang R, Chen H, Hu F, et al. Low-dose naltrexone inhibits the epithelial-mesenchymal transition of cervical cancer cells in vitro and effects indirectly on tumor-associated macrophages in vivo. Int Immunopharmacol. (2020) 86:106718. doi: 10.1016/j.intimp.2020.106718
62. Gong X, Chi H, Strohmer DF, Teichmann AT, Xia Z, Wang Q. Exosomes: A potential tool for immunotherapy of ovarian cancer. Front Immunol. (2022) 13:1089410. doi: 10.3389/fimmu.2022.1089410
63. Xiong J, Chi H, Yang G, Zhao S, Zhang J, Tran LJ, et al. Revolutionizing anti-tumor therapy: unleashing the potential of B cell-derived exosomes. Front Immunol. (2023) 14:1188760. doi: 10.3389/fimmu.2023.1188760
64. Ren J, Li L, Yu B, Xu E, Sun N, Li X, et al. Extracellular vesicles mediated proinflammatory macrophage phenotype induced by radiotherapy in cervical cancer. BMC Cancer. (2022) 22:88. doi: 10.1186/s12885-022-09194-z
65. Yan X, Zhang S, Jia J, Yang J, Song Y, Duan H. Exosomal MiR-423-3p inhibits macrophage M2 polarization to suppress the Malignant progression of cervical cancer. Pathol Res Pract. (2022) 235:153882. doi: 10.1016/j.prp.2022.153882
66. Xiao J, Lin H, Liu B, Xia Z, Zhang J, Jin J. Decreased S1P and SPHK2 are involved in pancreatic acinar cell injury. biomark Med. (2019) 13:627–37. doi: 10.2217/bmm-2018-0404
67. Xiao J, Huang K, Lin H, Xia Z, Zhang J, Li D, et al. Mogroside II(E) inhibits digestive enzymes via suppression of interleukin 9/interleukin 9 receptor signalling in acute pancreatitis. Front Pharmacol. (2020) 11:859. doi: 10.3389/fphar.2020.00859
68. Zhang H, Xia T, Xia Z, Zhou H, Li Z, Wang W, et al. KIF18A inactivates hepatic stellate cells and alleviates liver fibrosis through the TTC3/Akt/mTOR pathway. Cell Mol Life Sci. (2024) 81:96. doi: 10.1007/s00018-024-05114-5
69. Wang JF, Wang JS, Liu Y, Ji B, Ding BC, Wang YX, et al. Knockdown of integrin beta1 inhibits proliferation and promotes apoptosis in bladder cancer cells. Biofactors. (2025) 51:e2150. doi: 10.1002/biof.2150
70. Zhang G, Liao Y, Pan X, Zhang X. Exosomes from HPV-16 E7-pulsed dendritic cells prevent the migration, M1 polarization, and inflammation of macrophages in cervical cancer by regulating catalase 2 (CAT2). Ann Transl Med. (2022) 10:217. doi: 10.21037/atm-21-6998
71. Jiao X, Zhang S, Jiao J, Zhang T, Qu W, Muloye GM, et al. Promoter methylation of SEPT9 as a potential biomarker for early detection of cervical cancer and its overexpression predicts radioresistance. Clin Epigenet. (2019) 11:120. doi: 10.1186/s13148-019-0719-9
72. Luo Y, Chen Y, Jin H, Hou B, Li H, Li X, et al. The suppression of cervical cancer ferroptosis by macrophages: The attenuation of ALOX15 in cancer cells by macrophages-derived exosomes. Acta Pharm Sin B. (2023) 13:2645–62. doi: 10.1016/j.apsb.2023.03.025
73. Radogna F, Dicato M, Diederich M. Cancer-type-specific crosstalk between autophagy, necroptosis and apoptosis as a pharmacological target. Biochem Pharmacol. (2015) 94:1–11. doi: 10.1016/j.bcp.2014.12.018
74. Meng MB, Wang HH, Cui YL, Wu ZQ, Shi YY, Zaorsky NG, et al. Necroptosis in tumorigenesis, activation of anti-tumor immunity, and cancer therapy. Oncotarget. (2016) 7:57391–413. doi: 10.18632/oncotarget.10548
75. Li L, Yu S, Zang C. Low necroptosis process predicts poor treatment outcome of human papillomavirus positive cervical cancers by decreasing tumor-associated macrophages M1 polarization. Gynecol Obstet Invest. (2018) 83:259–67. doi: 10.1159/000487434
76. Su Q, Fan M, Wang J, Ullah A, Ghauri MA, Dai B, et al. Sanguinarine inhibits epithelial-mesenchymal transition via targeting HIF-1alpha/TGF-beta feed-forward loop in hepatocellular carcinoma. Cell Death Dis. (2019) 10:939. doi: 10.1038/s41419-019-2173-1
77. Singh SV, Ajay AK, Mohammad N, Malvi P, Chaube B, Meena AS, et al. Proteasomal inhibition sensitizes cervical cancer cells to mitomycin C-induced bystander effect: the role of tumor microenvironment. Cell Death Dis. (2015) 6:e1934. doi: 10.1038/cddis.2015.292
78. DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, et al. Durable remissions with ivosidenib in IDH1-Mutated relapsed or refractory AML. N Engl J Med. (2018) 378:2386–98. doi: 10.1056/NEJMoa1716984
79. Lepique AP, Daghastanli KR, Cuccovia IM, Villa LL. HPV16 tumor associated macrophages suppress antitumor T cell responses. Clin Cancer Res. (2009) 15:4391–400. doi: 10.1158/1078-0432.CCR-09-0489
80. Silveira CRF, Cipelli M, Manzine C, Rabelo-Santos SH, Zeferino LC, Rodriguez Rodriguez G, et al. Swainsonine, an alpha-mannosidase inhibitor, may worsen cervical cancer progression through the increase in myeloid derived suppressor cells population. PloS One. (2019) 14:e0213184. doi: 10.1371/journal.pone.0213184
81. Chen XJ, Wu S, Yan RM, Fan LS, Yu L, Zhang YM, et al. The role of the hypoxia-Nrp-1 axis in the activation of M2-like tumor-associated macrophages in the tumor microenvironment of cervical cancer. Mol Carcinog. (2019) 58:388–97. doi: 10.1002/mc.22936
82. Yang L, Li F, Cao Y, Liu Q, Jing G, Niu J, et al. Multifunctional silica nanocomposites prime tumoricidal immunity for efficient cancer immunotherapy. J Nanobiotechnology. (2021) 19:328. doi: 10.1186/s12951-021-01073-2
83. Kim SY, Kim S, Kim JE, Lee SN, Shin IW, Shin HS, et al. Lyophilizable and multifaceted toll-like receptor 7/8 agonist-Loaded nanoemulsion for the reprogramming of tumor microenvironments and enhanced cancer immunotherapy. ACS Nano. (2019) 13:12671–86. doi: 10.1021/acsnano.9b04207
84. Mohapatra A, Rajendrakumar SK, Cherukula K, Park MS, Padmanaban S, Vasukuty A, et al. A sugar modified amphiphilic cationic nano-adjuvant ceased tumor immune suppression and rejuvenated peptide vaccine induced antitumor immunity in cervical cancer. Biomater Sci. (2023) 11:1853–66. doi: 10.1039/D2BM01715F
Keywords: macrophage, cervical cancer, HPV, immune response, TAM recruitment, vaccine, diagnosis
Citation: Chen Z and Zhao B (2025) The role of tumor-associated macrophages in HPV induced cervical cancer. Front. Immunol. 16:1586806. doi: 10.3389/fimmu.2025.1586806
Received: 03 March 2025; Accepted: 25 March 2025;
Published: 08 April 2025.
Edited by:
Haixia Zhu, Nantong Tumor Hospital, ChinaReviewed by:
Zhijia Xia, Chongqing Medical University, ChinaCopyright © 2025 Chen and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Binzhu Zhao, Wmhhb2JpbnpodUBjZHV0Y20uZWR1LmNu
†These authors have contributed equally to this work
 Zeping Chen
Zeping Chen Binzhu Zhao*†
Binzhu Zhao*†