- 1Department of Genetics and Life Sciences, Sirius University of Science and Technology, Sirius, Russia
- 2Institute of Neuroscience, National Research Lobachevsky State University of Nizhny Novgorod, Nizhny Novgorod, Russia
The secretome of immune cells is currently a major focus in both diagnostic and therapeutic contexts. Cell-free therapeutic agents attract even more attention in cancer immunotherapy research, as their properties are comparable to, and sometimes surpass, those of cell-based immunotherapy. This is particularly evident when dendritic cell-based vaccines are compared with dendritic cell-derived exosomes (dexosomes). However, there is still significant potential for further research and optimization. We propose incorporating immunogenic cell death stimuli into the production of dendritic cell-derived exosomes in order to improve their effectiveness as a cell-free anti-cancer treatment. In this review, we suggest a new strategy to enhance the immunogenic potential of dexosomes, as well as summarize and compare immunogenic proprieties of dendritic cells and dendritic cells-derived exosomes as anti-cancer agents.
1 Introduction
In recent decades, the concept of strengthening cancer patients’ immune systems has gained recognition. The idea of self-healing seems elegant and straightforward. The first instance of cancer immunotherapy was reported by William Coley in 1891, who noted that some cancer patients experienced spontaneous remission when they developed a Streptococcus skin infection, erysipelas. He then used an injectable mixture of live and inactivated bacteria, specifically Streptococcus pyogenes and Serratia marcescens, to develop a treatment (1). Later, in the 1950-1960s, the idea of anti-cancer immunoediting was introduced, setting the stage for further research on immunotherapy. It postulated that the immune system can both inhibit and stimulate tumor growth. In 1990, the Food and Drug Administration (FDA) approved a bacteria-based vaccine (alive/protein-containing) Bacillus Calmette-Guérin for bladder cancer, marking one of the first successful applications of an immunotherapy agent (2, 3). Sipuleucel-T—the first therapeutic cell-based anti-cancer vaccine—was approved in 2011 for metastatic castration-resistant prostate cancer. This milestone in cell immunotherapy paved the way for further advances in vaccine development. A pinnacle of achievement in cell immunotherapy has been the development of chimeric antigen receptor (CAR) T cells. CAR-T therapy has been used to treat various cancer types, including the first FDA-approved CAR-T drug tisagenlecleucel against acute lymphoblastic leukemia, axicabtagene ciloleucel (Yescarta) against non-Hodgkin lymphoma, and idecabtagene vicleucel (Abecma) against multiple myeloma (4).
Nevertheless, there are still several barriers to the widespread application of cell immunotherapy, such as its high cost and low accessibility, as well as immune system fatigue (5). Furthermore, cell immunotherapy is often regarded as an adjuvant, supportive treatment, or last resort when other clinical approaches prove insufficient or totally ineffective. A therapy based on membrane-bound vesicles could be the next step in the fight against cancer. As a result, researchers are becoming more interested in the idea of using immune cell derivatives as anti-cancer agents: these medications are potentially more effective, less ethically constrained, and more commercially viable.
One of the prospective therapeutic cell-free agents is dendritic cells-derived exosomes (DEX). In the first part of the article, we comprehensively describe dendritic cells as therapeutic anti-cancer agents. The second half focuses on a detailed description of DEX proprieties, including their advantages and limitations, and compares them with dendritic cells in the context of cancer treatment. Furthermore, a novel strategy to increase the immunogenic potential of DEXs is proposed.
2 Discovery of dendritic cells: a new step in understanding antigen presentation
Dendritic cells (DCs) show great promise as a therapeutic agent, progressing steadily through clinical trials and being increasingly incorporated into conventional therapies and combination strategies to treat different types of solid tumors (6, 7).
In 1973, Ralph Steinman and Zanvil Cohn were the first to identify DCs in the mouse spleen (8). The unique morphology of DCs and high expression of major histocompatibility complex (MHC) molecules led to their classification as a distinct type of antigen-presenting cells (APCs) (9, 10). In the late 1990s, DCs were established as essential for linking the innate and adaptive immune system via the presentation of processed antigens to T cells (11, 12). Since those findings had been reported, the idea of employing DCs to treat cancer emerged.
2.1 Advances and challenges in dendritic cell-based cancer immunotherapy: timeline of clinical research and application
The clinical exploration of DCs as therapeutic agents began with clinical trials focusing on their ability to present tumor antigens to T cells. Despite objective tumor response rates being typically moderate, below 15%, early research showed that DC-based vaccines could elicit immune responses in cancer patients. By the late 1990s, as antigen-presenting proprieties of DCs had been described, various clinical trials commenced to assess the efficacy of DCs in treating diverse malignancies, including melanoma and prostate cancer. For instance, a notable study published in the Journal of Experimental Medicine in 1999 illustrated that vaccination with peptide-pulsed mature DCs could expand specific cytotoxic T cells (CTLs) and induce regression of metastases in patients with advanced melanoma (13, 14).
The understanding of DCs’ roles has evolved over the years: researchers have shown that DC vaccines may increase overall survival rates in some patients, despite the initially limited responses to these vaccines. This has changed the way clinical effectiveness is evaluated. New strategies have emerged, including next-generation DC vaccines designed to enhance immunogenicity and combination therapies integrating DC vaccination with other cancer treatments (14–16).
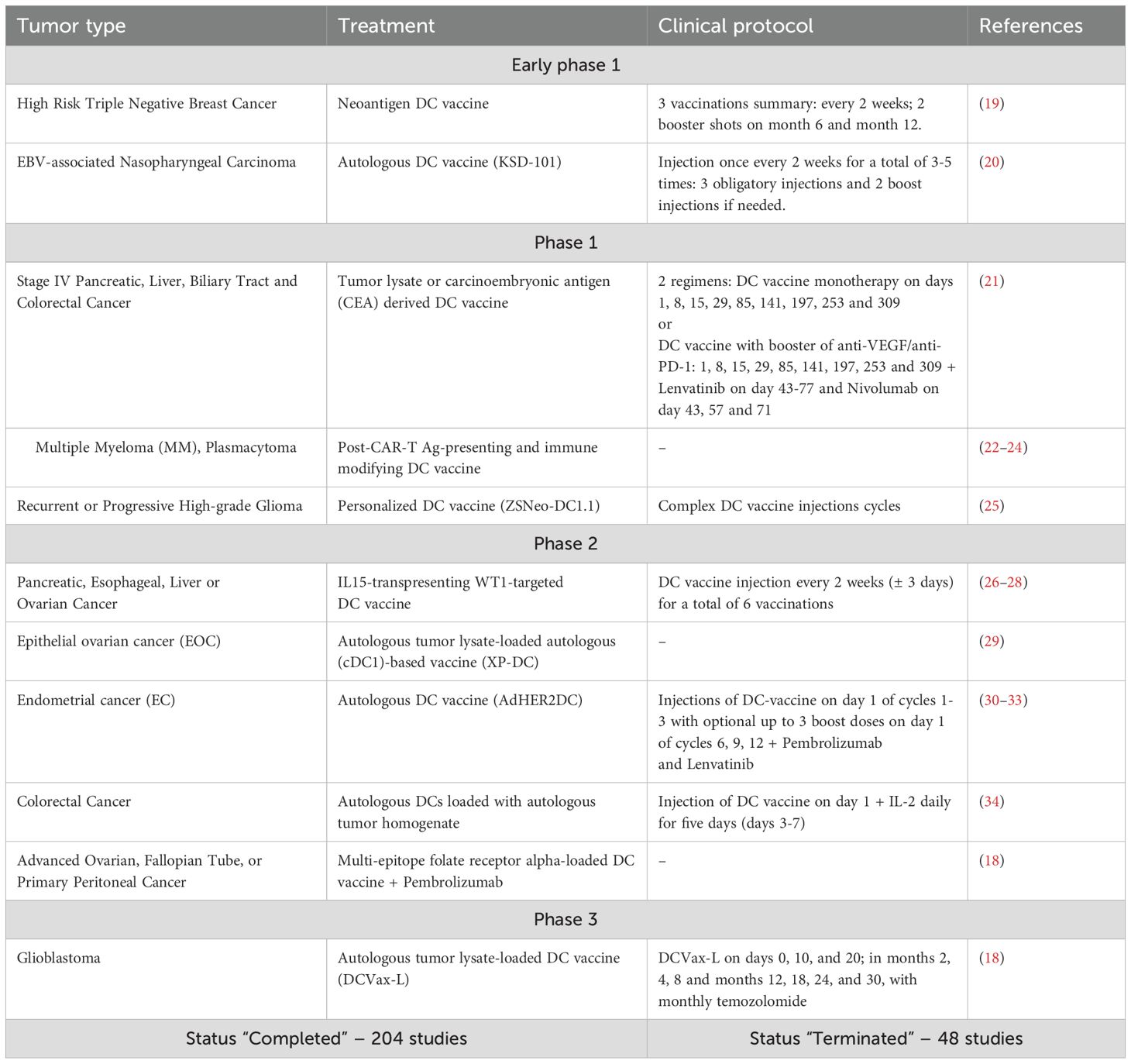
As of the end of 2024, the ClinicalTrials.gov database by the National Library of Medicine included 69 active or recruiting clinical trials on DC-based or DC-targeted mono- or combined immunotherapy: 5 studies were in the early phase I, 40 – phase I, 37 – phase II, and none were in phase III or IV. The ‘Completed’ and ‘Terminated’ statuses were assigned to 204 and 48 studies, respectively (17).
Not registered in this database, a phase III anti-glioma DC vaccine trial has shown that autologous tumor lysate-loaded DC vaccine (DCVax-L) increased the median overall survival up to 19,3 months, achieving clinical and statistical significance in comparison with matched, contemporaneous external controls (18) (Table 1).
2.2 Expanding role of dendritic cell vaccines in cancer therapy: mechanisms, combinatorial strategies, and future directions
Such a strong research interest in DCs is understandable and well-justified, as they have significant potential both as a standalone treatment and in combination with other immunotherapeutic cancer medications and chemotherapy (35, 36). It is clear today that DC vaccination is an important and expanding subject, with continuous research being done to increase its overall clinical efficacy and application versatility (37).
DCs are recognized as the most effective APCs, capable of inducing both innate and adaptive immune responses. They demonstrate exceptional proficiency in processing and presenting tumor antigens to T cells, which is crucial for initiating a robust immune response against cancer (38, 39). By presenting tumor-specific antigens, DCs can stimulate the production of cytotoxic T lymphocytes that specifically target and eliminate cancer cells (40). Clinical trials have repeatedly shown that DC-based treatments are safe, even for patients whose cancer has progressed. DCs are therefore a good choice for many patients who might not respond well to conventional therapies. DC-based therapy can promote immune responses that result in persistent remissions, offering hope for long-lasting results (41).
The effectiveness of treatment increases when DC-based immunotherapy is combined with other modalities, such as immune checkpoint inhibitors (ICIs). This combined approach has demonstrated encouraging results in various cancers, e.g., melanoma, non-Hodgkin lymphoma, and breast cancer, including enhanced immune responses and better clinical outcomes. However, the percentage of cancer patients who benefit from ICIs is still low (42). These advantages of DCs motivate researchers to create specific, targeted, and highly effective DC-based vaccines against different malignancies.
2.3 Dendritic cells-based vaccines: general practiсe and innovative strategies
To date, there is only one DC-based vaccine approved for clinical usage. Sipuleucel-T (Provenge) is an FDA-approved vaccine against metastatic castration-resistant prostate cancer. It is a DC vaccine based on autologous cancer cell stimulation, with prostatic acid phosphatase (PAP) as the main target. According to the clinical trial results, overall survival increased by nearly 6.5 times after vaccination compared to the placebo, and side effects, such as fever and chills, were rare (43). The clinical protocol involves ex vivo incubation of autologous DCs with a recombinant antigen protein combines PAP and GM-CSF. Yet, the vaccine application is limited by strict requirements for sterility, transportation, and manufacturing conditions. Only 50 centers around the world are capable of providing this kind of therapy (44).
The general principle of DC vaccine design is to induce the activation and maturation of the patient’s or donor’s DCs ex vivo with the primary tumor cell lysates, followed by their administration via subcutaneous injection. The first step is to obtain DC, for which the patient’s mobilized blood is the ideal choice. Mobilization is an inevitable step because the number of DC progenitors in the blood is low, about 0.2% of the total peripheral blood mononuclear cells. To increase the proportion of DCs and progenitor cells (e.g., CD34+ hematopoietic stem cells), differentiation stimuli like granulocyte colony-stimulating factor (G-CSF) or granulocyte-macrophage colony-stimulating factor (GM-CSF) must be used (45). Next, DCs should be purified using leukapheresis or other similar methods. The remaining cells are then cultured in a specific cytokine environment (e.g., GM-CSF, IL-4, and/or TNF-α) to promote DC differentiation and maturation (46, 47). Once DCs reach a mature state, they are pulsed with specific tumor lysates and re-injected to the patient as a subcutaneous vaccine, with the follow-up for non-specific inflammation-like reactions and side effects, which are usually limited to fever, redness, local pain, and mild swelling (43, 48). Interestingly, DCs that are currently used as therapeutic agents can be pulsed not only with autologous tumor cell lysates or homogenates, but also with messenger RNA (mRNA), tumor-associated antigens (TAA), proteins, or modified nanoparticles, while cytokine cocktails are often used as maturation stimuli (49–53).
Some extraordinary examples of DC vaccines have been observed in clinical applications. The N.N. Petrov National Medical Research Center of Oncology (Saint-Petersburg, Russia) provides a promising example of treatment with a DC vaccine. There, DC vaccines are used as a routine method for melanoma: bone marrow-derived progenitors and photosensitizers are injected intratumorally and then activated by light. According to in vitro and animal studies, it might be immunogenic cell death of primary melanoma cells in vivo that activates DCs and stimulates specific immune response, but the results have not been published yet (54). The same scientific group also described a clinical case of effective DC vaccination against pediatric H3 K27M-mutant diffuse midline glioma. They observed a dynamic increase in the proportion of T-lymphocytes (CD3+CD19-) and natural killer (NK) cells (CD3-CD16+56+). There were steady trends in the proliferation of activated HLA-DR+ T helper cells (CD3+CD4+) and CTLs (CD3+CD8+). At the same time, the proportion of regulatory subpopulations—both CD3+CD4+CD25highCD127low T regulatory (Treg) cells and CD3+CD16+56+ NKT cells—showed no significant growth. The profile of immune response is presented in this article but the fate of the patient is not clear (55). The use of the DC vaccine against soft tissue sarcoma, colon cancer, and kidney cancer is mentioned as well, but no data has been published in a scientific format (56).
As many strategies have emerged, some researchers are striving to improve well-described and, more importantly, clinically used approaches to lysate-pulsed DC vaccines. One fundamental principle that could be integrated into clinical practice is the targeted induction of immunogenic cell death (ICD) during lysate preparation. Immunogenic cell death is a type of regulated cell death characterized by cell stress, the release or surface exposure of damage-associated molecular patterns (DAMPs), activation of antigen-presenting cells, especially DCs, and, as a result, enhanced specific T-cell immune response. It has been shown that DCs pulsed with lysates of immunogenically killed cells are more effective than DCs pulsed with necrotic cell lysates both in vitro and in vivo (57–60). ICD was first described as immunogenic apoptosis in 2005 and was later expanded to include other types of cell death, such as ferroptosis, pyroptosis, necroptosis, and other modalities (61, 62).
2.4 Challenges in dendritic cell immunotherapy: overcoming tumor microenvironment barriers – focus on exosomes
The many advantages of DCs in cancer therapy include specific immune response activation via antigen presentation, long-lasting immunity, less harmful side effects compared to chemo- or radiotherapy, and high compatibility with other therapeutic approaches (37, 42, 63, 64). However, many limitations have yet to be overcome. Thus, the tumor immune microenvironment (TIME) has a strong immunosuppressive DC-targeted profile. The subset of DCs typically used in immunotherapy due to accessibility issues — monocyte-derived DCs from mobilized peripheral blood — have lower efficacy in anti-tumor response than conventional DCs, previously known as myeloid DCs derived from a common DC progenitor (65–67). As described earlier, mobilization of monocytes and hematopoietic stem cells is critical for DC vaccination because of the constraints of autologous transplantation (68, 69). Here, researchers and clinical practitioners face yet another problem: the immune system of cancer patients develops fatigue and exhaustion as a consequence of chemotherapy and radiotherapy, cytokine imbalances, long-term bone marrow injury, and comorbidities (70, 71). Furthermore, limited lymph node infiltration in tumor-bearing individuals contributes to immunosuppression. If professional APCs cannot effectively penetrate the lymph nodes, they may fail to activate the full potential of T cells, resulting in a weaker targeted anti-tumor response.
Altered chemokine expression, phenotypic changes, challenges in identifying target antigens for DC stimulation, the risk of both immunosuppression and overstimulation, and the weakened immune status of late-stage cancer patients all similarly affect treatment outcomes (72–77). To overcome these limitations, novel immunotherapeutic strategies must be developed. Among emerging options, DC-derived exosomes show particular promise as an innovative approach to immunotherapy.
3 Dendritic cell-derived exosomes – cell-free saviors?
3.1 Exosomes as nanoscale mediators: content and structure
Exosomes are nanosized extracellular vesicles, typically ranging from 30 to 150 nm in diameter. They can be secreted by various cell types both under normal conditions and in response to external stimuli or due to acquired impairments (78). Exosomes play a crucial role in intercellular communication by transporting proteins, lipids, and nucleic acids from donor to recipient cells. This unique capability makes exosomes promising candidates for targeted drug delivery systems in therapeutic applications. Exosomes are primarily composed of a lipid bilayer, proteins (including heat shock proteins (Hsp) and membrane transport proteins), and nucleic acids (mRNAs and microRNAs). The lipid bilayer protects the cargo from degradation and facilitates fusion with target cell membranes (79–81). Exosome uptake by recipient cells involves several mechanisms, including receptor-ligand interactions, membrane fusion, and endocytosis. The specific uptake pathway can vary depending on the type of recipient cell and the surface proteins present on the exosomes (79, 82, 83).
3.2 Dendritic cell-derived exosomes: mechanisms of antigen presentation
One of the most impressive properties of DEX surface membranes, contributing to their immunogenic potential, is the presence of markers involved in specific antigen processing and presentation. DC-derived exosomes were first described in the context of their immunological function in 1998. This discovery was made by Zitvogel et al., who demonstrated that MHC-II enriched exosomes could present peptide–MHC II complexes to T cells, thus activating specific immune responses (84, 85). Worth noting that MHC-expressing exosomes secreted from B lymphocytes were detected and described two years earlier, in 1996, by Raposo et al. who first reported that extracellular vesicles are able to present antigens to T cells (86).
Usually, DC-derived exosomes are 30–150 nm extracellular vesicles that transport and deliver molecular signals, carry a variety of receptors, and inhibit immune surveillance (87, 88). They can present TAA to T cells, promoting a robust immune response. Interestingly, DEXs have a stimulatory effect on APCs termed ‘cross-dressing’. Exosomes from DCs transfer MHC-peptide complexes directly to other APCs, enhancing their ability to stimulate T-cell responses against tumors (89–91). Studies indicate that exosomes derived from DCs can efficiently capture and present antigens, leading to improved activation of CD8+ T cells compared to direct antigen presentation by DCs.
The DEX specific content and surface profile can induce a T-cell immune response either directly or indirectly (87, 92). The indirect pathway involves antigen cross-presentation via the MHC-peptide complex on exosomes, followed by reprocessing of antigen in DCs. Then, DCs present newly recognized peptides to T cells. In contrast, the direct pathway implies immediate interaction between T cells and MHC complexes on the surface of the DEXs (88, 93, 94).
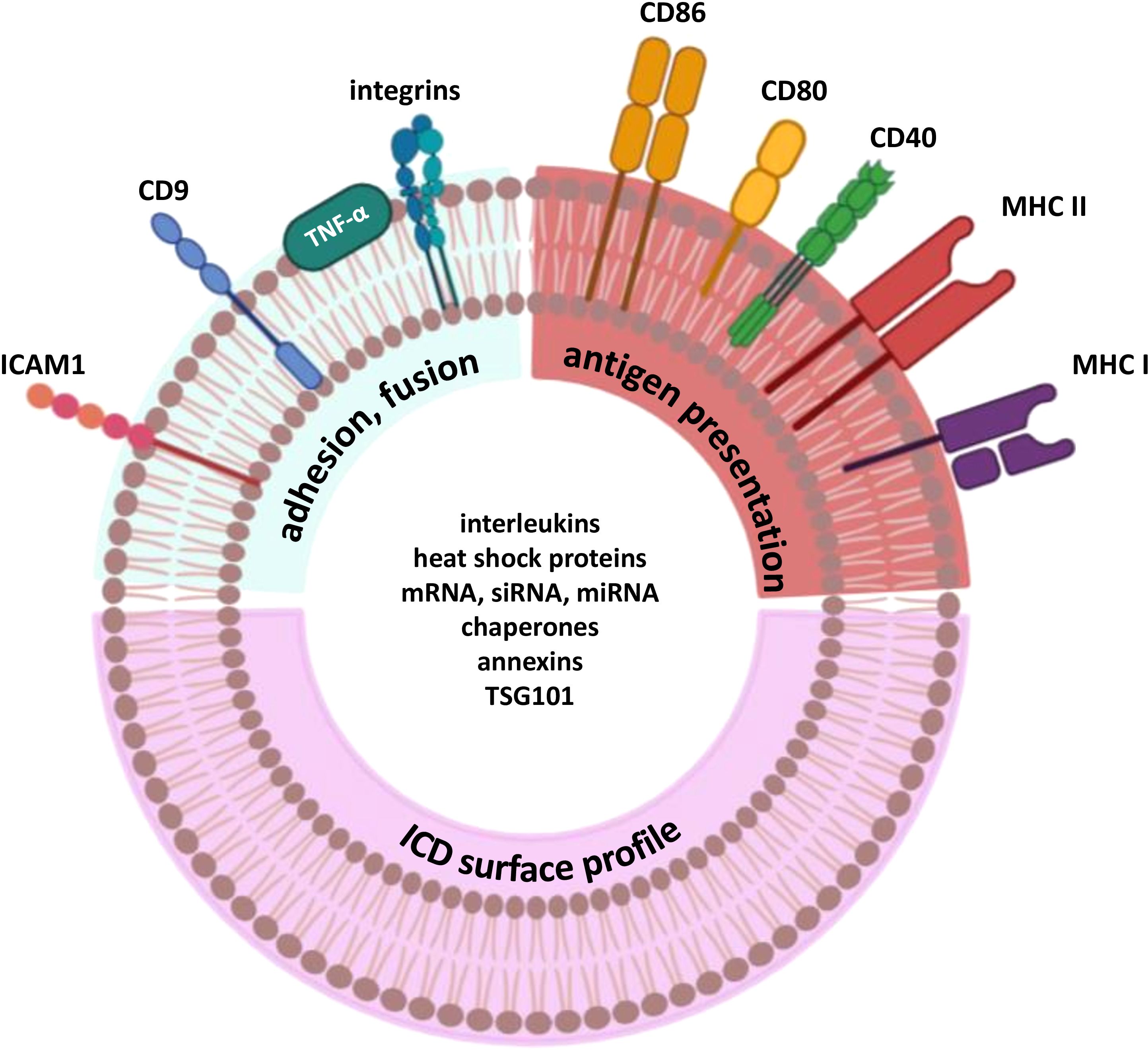
Interestingly, DEXs are able to make the tumor more attractive for immune cells. A variety of strategies was described for breast carcinoma, melanoma, and hepatocellular carcinoma (89, 95–97). DEXs contain a rich array of MHC molecules, both class I and II, and co-stimulatory signals crucial for effective T-cell activation. Furthermore, it has been found that DEX membranes are enriched with MHC molecules compared to DCs (98). Major surface molecules responsible for antigen presentation on DCs are also found on DEX: MHC-I, MHC-II, CD80, CD86, CD40, etc. (87). Notably, co-stimulatory molecules B7.1 and B7.2, originally very important in T-cell response activation via different interactions, have also been identified on DEX. Additionally, intercellular adhesion molecule 1 (ICAM-1) has been shown to be essential for T-cell priming, as its expression as a co-stimulatory molecule is critical for the immunogenic properties of DEX in the anti-tumor immune response (92).
3.3 Dexosomes: next step in overcoming the limitations of DC-vaccines
Derived from cells, exosomes naturally have high biocompatibility, which makes them suitable for clinical applications (82) and a possible answer to the limitations of DC vaccine therapy. DEXs have a more targeted action: their sizes range from 30 to 150 nm, allowing them to migrate and internalize within lymph nodes. DEXs are also less susceptible to the TIME due to their stable composition and phenotype, which remains unaffected by immunosuppressive immune and tumor cells (87, 99–101). There are reports that DCs activated in vitro can switch into an immunosuppressive phenotype associated with a weaker immunotherapy effect (102, 103). In the TIME, DEXs are able to change pro-tumor immune cells into anti-tumor cells. Exosomes from activated DCs can induce a pro-inflammatory environment, essential for effective anti-tumor immunity. They achieve this by enhancing the expression of pro-inflammatory cytokines and promoting the maturation of other DCs, which in turn can stimulate T-cell responses against tumors (104–107). Exosomes influence the tumor microenvironment (TME) by altering the behavior of stromal cells, such as fibroblasts and immune cells. For instance, they can induce the secretion of cytokines and chemokines that recruit more immune cells to the tumor site, thereby creating an environment conducive to immune attack (108). Additionally, exosomes can inhibit the immunosuppressive functions of Tregs and myeloid-derived suppressor cells, further enhancing anti-tumor immunity (Figure 1) (109, 110). T cells can independently attract DEX via the LFA-1 molecule, potentially enhancing the immune response (111). The interaction of DEXs with CD4+ results in MHC I expression in the CD4+ population and facilitates CTL transition.
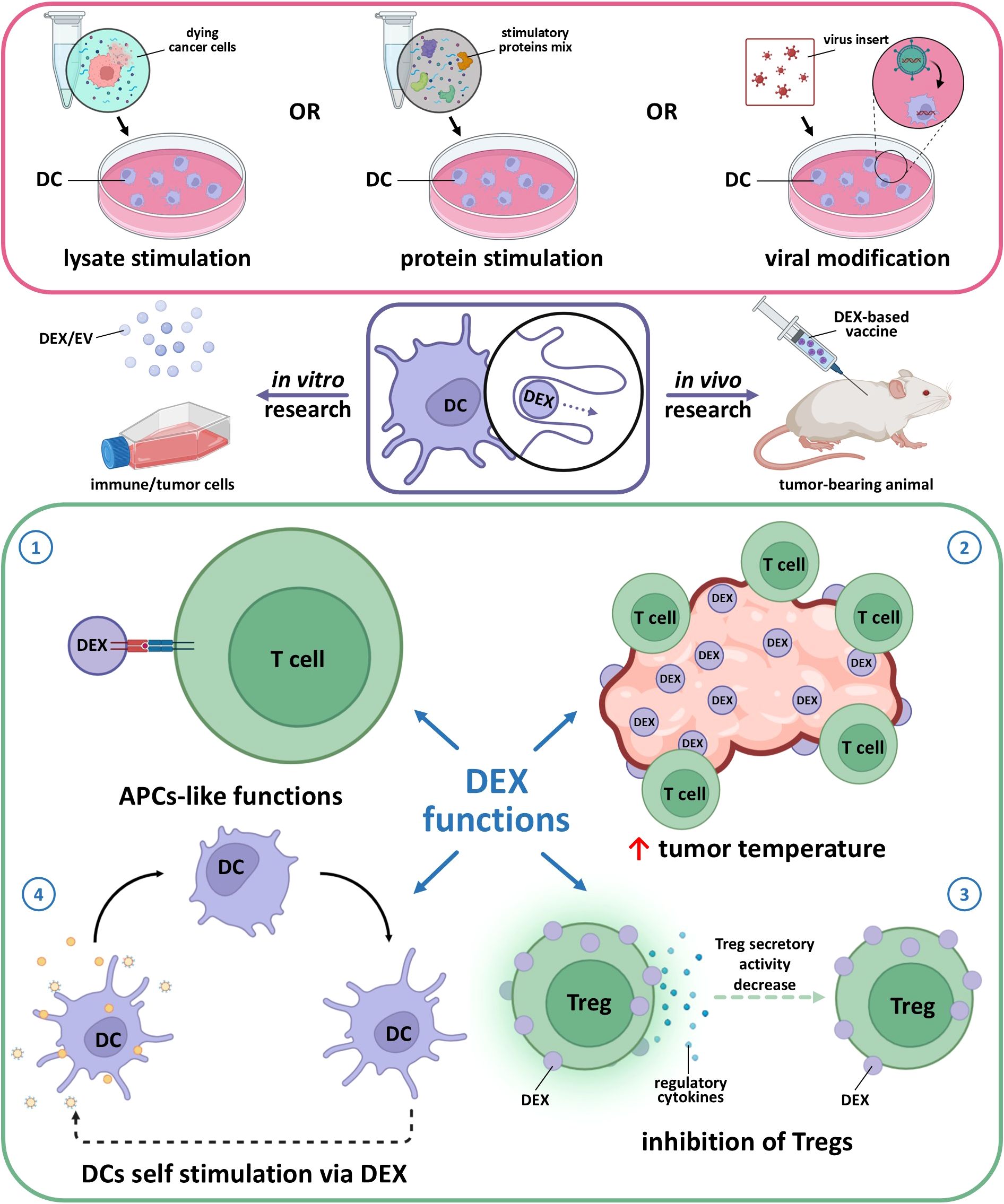
Figure 1. Main effects of dendritic cell-derived exosomes (DEX) obtained from mature stimulated dendritic cells. 1 – APC-like function assumes DEX interaction with T cells via MHC-TCR complex for antigen presentation as it happens in case of cellular (DC-T cell) contact. 2 – DEX are able to recruit immune cells to the tumor same as entice DC from TME to act as anti-tumor active cells. 3 – modified DEX (e.g. caring FasL, TNF-α) are able to suppress Treg pro-tumor activity via MHC-TCR interaction along with shifting the balance away from Treg dominance to effector T cells activation as DEX express co-stimulatory molecules. 4 – DEX express integrins, ICAM-1, and tetraspanins (CD9, CD63, CD81) which enable efficient binding to and uptake by other DCs, also DC-derived EVs are able to transfer peptide-MHC complex to recipient DCs, which then reprocess and present these antigens, amplifying the immune response.
Importantly, exosomes can easily penetrate biological barriers such as the blood-brain barrier or blood-tumor barrier (112, 113). This could be leveraged in brain tumor treatment (114), as not all immunotherapeutic and chemotherapeutic drugs can penetrate these barriers to accumulate in the target tumor (115). For example, temozolomide, a standard chemotherapy drug for glioblastoma multiforme, only reaches about 20% of its blood concentration within the brain (115).
Romagnoli et al. have hypothesized that tumor cells treated with DEX could induce anti-tumor effects de novo and stimulate migration of both primary and modified immune cells and interact with them. Through a series of complex incubation steps using DC+tumor-derived exosomes, activated DC+T-cells, and interferon-γ (IFN-γ)-producing T-cell detection, it was elegantly demonstrated that tumor cells treated with DC-derived exosomes become more attractive targets for existing immune effector cells (89).
Furthermore, we should mention the economic benefits of DC-derived exosomes. During the manufacturing process, DEX can be easily stored at -80˚ C for 3–6 months, their homogeneity is sufficient for massive production, and as subcellular components, exosomes have significantly fewer ethical restrictions in clinical usage (92, 116–118). The main features and effects of DEXs are illustrated in Figure 1.
3.4 Challenges and considerations in dendritic cell-derived exosome production: standardization and optimization
There are currently no standardized guidelines or criteria for DEX manufacturing (119). While it is evident that DCs are the source of dexosomes, several key factors are overlooked. For instance, the subtype of DCs producing DEXs is often disregarded. Furthermore, methods for identifying intravesicular content are complex and costly. Thus, more aspects and methods should be reviewed to improve standardization, optimization, and applicability.
The common DEX production process begins with ex vivo DC cultivation and stimulation of their mature phenotype as one of the most critical points that determine the effectiveness of immune system activation by DEXs. The subsequent manipulations include the incubation of DCs with tumor lysates or proteins and several genetic, e.g., viral, modifications of DCs (Figure 2A). After exposure to antigenic stimuli, DEXs are collected from the culture media and washed several times before analysis (87, 120) (Figure 2B). Typical DEX analysis includes several techniques (Figure 2C) (121–123). Scanning electron microscopy is used to assess the morphology of extracellular vesicles, which is crucial for understanding their biological functions and potential applications in diagnostics and therapeutics. Nanoparticle tracking analysis allows for rapid and precise measurement of vesicle size and concentration. Dynamic light scattering helps assess biophysical properties of extracellular vesicles, while immunoblotting is used to valuate purity and detect different proteins and markers they carry.
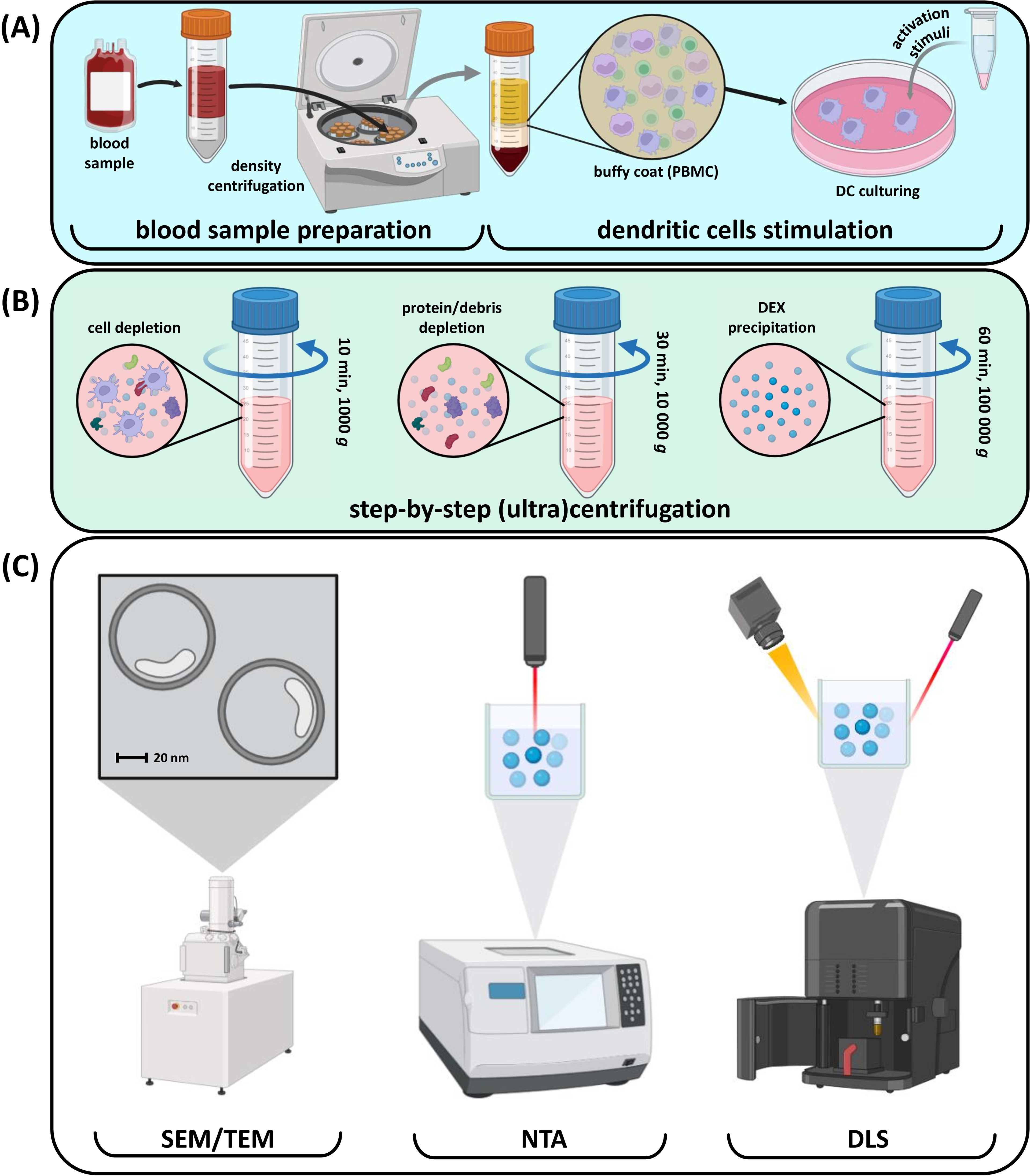
Figure 2. Common steps involved in DEX production and quality control. (A) Blood processing and DC stimulation; (B) Сonsecutive DEX purification with centrifugation; (C) Purified DEX size and quality control.
In vivo-focused research includes additional methods like flow cytometry for phenotype detection and mass spectrometry for identifying internal DEX content (124). After purification, DEXs can be used as active vesicles in various applications.
Purity of exosomes is a critical factor in the context of both in vitro and in vivo studies. Inappropriate characterization of purified EVs might cause the lack of efficacy, false assays interpretation and additional immunological load on organism. So, there are several strategies to prevent the production of unearmarked EVs. On of the strategies include the usage of culture medium depleted of bovine serum exosomes. This method implies ultracentrifugation for serum-derived exosomes precipitation and following culture medium preparation without pelleted exosomes (125). Another strategy is based on the known size of DEX. To improve purity and separate exosomes from other vesicles and protein aggregates, density gradient ultracentrifugation could be used. The goal product typically located in one of the fractions depending on target size of the EV (126). Immunoaffinity isolation is a precise and high-selective method of DEX isolation. Magnetic beads coated with antibodies against exosome-specific surface markers (e.g., MHC-II molecules) could be used while selective DC-derived exosomes capturing (127).
To ensure the safety and efficacy of dendritic cell-derived exosomes for different patients, strict quality control measures throughout production should be implemented. Firstly, criteria for dendritic cell vaccination could be applied in the context of the ability to get the cells from the blood through the general volume of DC and DC precursors measurement. As the source of the DEX are dendritic cells, so the first criteria should be the ability to culture and stimulate autologous dendritic cells (128). The second criteria could be presented as a list of requirements for detailed EVs characterization (e.g. identity, purity, structure and sterility), functional compliance and storage (98, 129). Adhering to guidelines and regulations set forth by organizations like the International Society for Extracellular Vesicles (ISEV) and the European Network on Micro-vesicles and Exosomes in Health and Disease (ME-HaD) is another step to the clinical safety of DEX-based treatment (130).
The versatility of DEX remains an important but largely unexplored area of research. Most studies report obtaining DEXs from allogeneic DCs, and graft-versus-host reactions have not been described. This raises the question of HLA typing for DEX, which is another challenge researchers have to face. Overall, the use of DEXs derived from non-donor DCs is still under discussion. Addressing these questions will require approaches rooted in autoimmune research and allogeneic transplantation algorithms.
3.5 Current status and preclinical advancements in DEX-based anti-cancer therapy
As of 2024, the clinical trials database (https://clinicaltrials.gov/) lists only two clinical trials related to anti-cancer treatment with DC-derived exosomes. The first is a completed phase II clinical trial for unresectable non-small cell lung cancer. However, the results have not yet been published and no data on survival or effectiveness are available. Still, the trial included a well-documented clinical protocol involving stimulatory proteins for DC activation, cyclophosphamide treatment, and intradermal injections of DEX—administered weekly for four weeks and followed by injections every two weeks for six weeks (131). The second study was completed at the end of 2023 and investigated DEX therapy for bladder cancer but details on the DEX design and clinical protocol are not available (131). Notably, most clinical studies focus on the role of exosomes of different origin as diagnostic molecules for various diseases.
In contrast to the limited number of clinical trials, likely due to the novelty of this approach, numerous fundamental and pre-clinical studies have been described. Thus, exosomes enriched with chaperones proved an effective way of glioma treatment in mice. The chaperone-rich lysate contained at least four chaperone proteins, including Hsp70 and Hsp90, calreticulin, and glucose-regulated protein 94 (GRP94), which made the treatment more effective (132). Lu et al. showed that DEX derived from α-fetoprotein-expressing DCs activated a specific anti-tumor response against hepatocellular carcinoma and increased the number of CD8+ T cells in the TME. Furthermore, CD8+ cells gradually increased the level of immunostimulatory cytokines, such as IL-2 and INF-γ, while also reducing CD25+ expression and CD25+FoxP3+ the proportion of Tregs, as well as lowering IL-10 and TGF-β in tumor sites (97). Another DEX-based approach has been used for cervical cancer. This study presented a protocol for DEXs engineered via DC stimulation with the HPV early antigen 7 protein, the main target antigen for this type of tumors (133). In human gastric adenocarcinoma, the CTL immune response was induced using either tumor lysate or RNA to treat DCs, with tumor RNA being more effective. Exosomes were then obtained and injected as a vaccine. In a phase I clinical trial, DEXs derived from autologous plasmacytoid DCs have shown mixed effectiveness against melanoma. Only one patient in this study had a pronounced T-cell response, but researchers observed a high proportion of infiltrating NK cells and proposed that DEXs are important for NK activation (134). This finding was later supported by multiple studies and another phase I clinical trial (88, 135, 136). Combination of DEXs with anti-CTLA-4 ICI treatment was able to strongly enhance the efficacy of immune response (137). Using chimeric DEX-like exosomes for glioblastoma treatment in the orthotopic model, researchers found an increase in tumor-infiltrating T-cells, a better Ki-67response, and the maintenance of memory T cells (138). For gastric cancer, hybrid exosomes were engineered. DEXs modified with a chemotherapeutic agent induced an effective anti-cancer immune response in a murine model (139).
3.6 Immunogenic cell death as a strategy for to enhance dendritic cell-derived exosome-based cancer therapy
As described above, immunogenic cell death (ICD) is a form of regulated cell death. It is characterized by exposure to DAMPs released from dying tumor cells, followed by the activation of specific immunity (61, 140). We propose DEX therapy could benefit from employing core determinants of ICD. First, the cell should undergo stress adaptation as it leads to intracellular changes and shapes the immunogenic profile of cell death mediated by therapeutic modifications. The second determinant is that dying cells should be taken up by antigen-presenting DCs rather than macrophages, to promote an effective immune response. The third important point is the sufficient antigenicity of the dying cells. The last determinant is the adjuvant effect, which involves the release of DAMPs as immune-stimulating molecules (141).
With various methods of its induction available, ICD is an elegant cell death pathway to be used in research and development. This cell death modality can be activated using classical inducers, such as chemotherapeutic drugs, e.g., anthracyclines, cyclophosphamide, bortezomib, radiotherapy (142), or photodynamic therapy when immunogenic photosensitizers (PS) are used, e.g. porphyrazines, G-chlorin, and nanocarriers with PS (143–145). Interestingly, there are some rarely used methods of ICD induction, like cold atmospheric plasma (146) or natural compounds capsaicin and curcumin (147). Since immunogenic apoptosis was first described by Guido Kroemer’s group in 2005, the number of studies focusing on ICD has increased rapidly. As of 17th December, 2024, PubMed database includes 5,262 articles mentioning immunogenic cell death (61, 148). Given that immunogenic cell death markers include cell surface proteins, they may also be exposed on extracellular vesicles in a similar manner. We further suggest that DEXs could be enriched with immunogenic surface proteins, as this has been observed for MHC molecules (98). While the DEX classical phenotype has been described, there is no data on their inner and surface composition in the context of ICD-mediated DEX (Figure 3). As MHC and co-stimulatory molecules are common for the majority of DEX, some of inner molecules like Hsp and annexins might be detected as DAMPs which play a key role in immune system response during ICD-based activation (61, 87). On the other hand, the specific surface profile after ICD stimuli has not been clearly described which opens a wide area for the research of dendritic cell-derived exosomes in the context of therapy search.
ICD proprieties suggest that immunogenically killed cells could be applied in the production of DC-derived exosomes, as this strategy could potentially enhance the therapeutic efficacy of extracellular vesicles (Figure 4). The content of DEXs is well characterized and was discussed earlier in this review. However, the impact of immunogenically killed cells on the resulting ICD-DEX remains largely unexplored, highlighting the need for further studies.
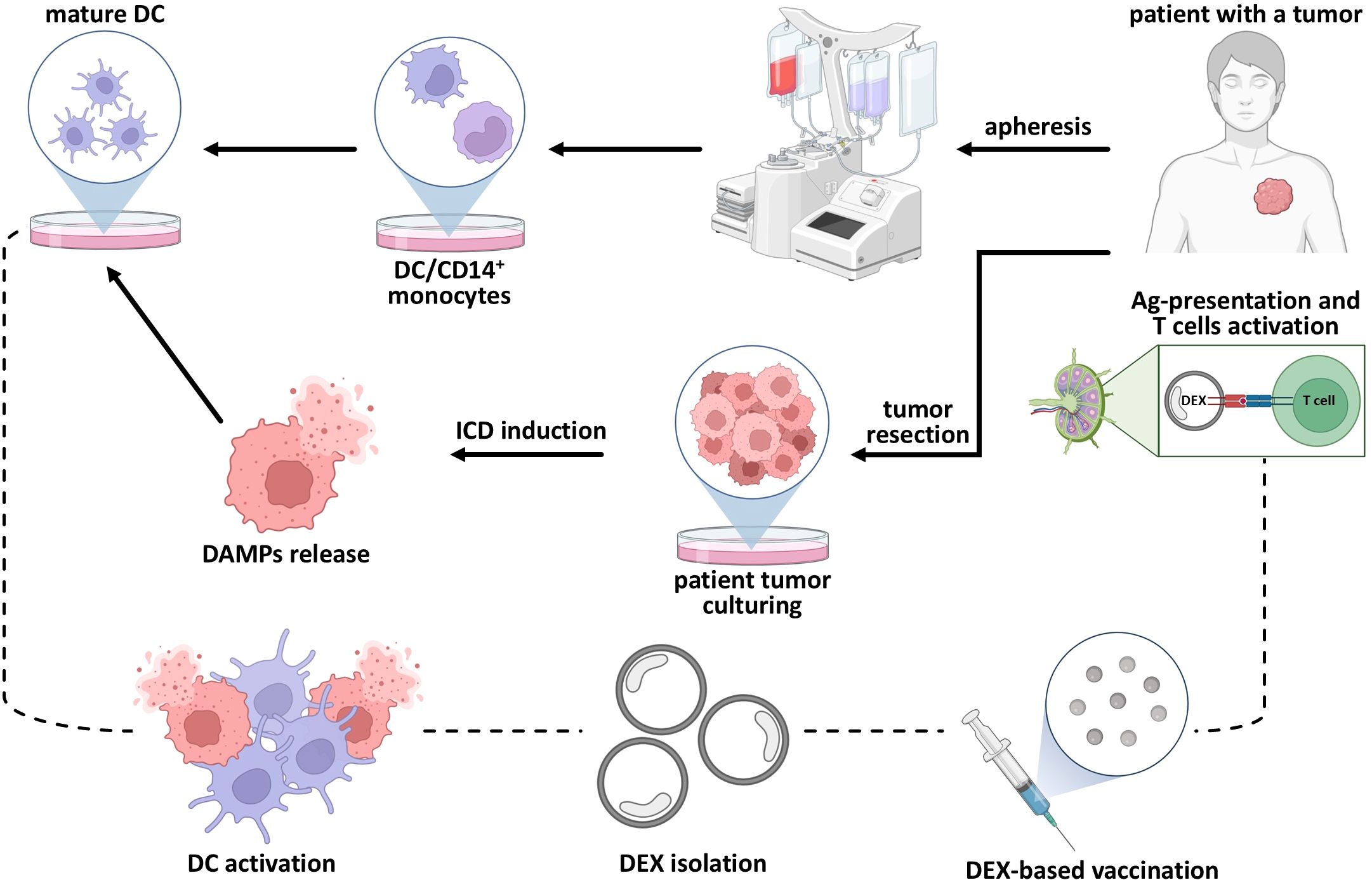
Figure 4. Combination of ICD and DEX-based vaccination as a strategy for anti-tumor treatment. Classical ICD maturation stimuli include immunogenically killed cells loaded on the surface of dendritic cell which activates cytotoxic T cells responsible for specific anti-tumor response. After immunogenically-killed cells DC stimulation the emission of DEX could be observed. So, the DEX-based immunization implies collecting this ICD-mediated dexosomes and using them as an anti-tumor vaccine.
3.7 Challenges and strategies to enhance the anti-tumor efficacy of dendritic cell-derived exosomes in cancer immunotherapy
Dexosomes promote a strong T cell response in both in vitro and in vivo models. DEXs are often described as vesicles with strong antigen-presenting properties, yet DEX immunotherapy faces challenges in clinical trials and treatment integration (92, 120). Most clinical trials listed in databases do not progress to the next phases or are even terminated without providing results (88), and clinical applications seem to have stuck in a holding pattern. On the other hand, fundamental research has explored DEX properties, content, and their so-called phenotype, or surface molecule profile, in depth. Therefore, this matter calls for explanation.
First, DEXs are not living cells, so they cannot actively migrate to the tumor or lymph nodes like DCs. Instead, exosomes are transported from peripheral tissues through lymphatic vessels and nodes in complex with lymphatic endothelial cells (149). Second, the complexity of DEX composition can vary significantly, making it difficult to standardize the treatment and manufacturing process (150). Interestingly, artificially generated DC subtypes, e.g. cDC obtained after PIB-induced transformation, might become a prospective source of DEX in context of both routine and novel manufacturing designs. Furthermore, dendritic cells subtypes obtained with INF-γ simulation show solid efficacy and immunogenicity. Exosomes derived from DC stimulated with IFN-γ show imposing therapeutic effect in combination with chemotherapeutic treatment of non-small cell lung cancer. Controlled stimulation of dendritic cells phenotypes might become one of the solutions on the way to standardization of DEX manufacturing and immunogenicity enhancement of therapy (151–153). Still discussing the possibility of using PIB-induced or other derived DC is important in context of the ability to use them as full-fledged alternative of DEX source as this transformation strategy might face the problems of incomplete functional maturation, variable reprogramming efficiency, reduced proliferative activity, and genetic and epigenetic barriers (154). DEX are easy to store and transport, but they still originate from DCs derived from live donors or cancer patients, which requires complex invasive procedures. The ‘quality’ of the original biospecimens and the source and subtypes of DCs also play an important role in the treatment success (155). The last limitation we would like to highlight is the lack of pre-selection criteria. There is currently no standardized set of criteria for selecting patients who would benefit most from DEX therapy, which complicates the identification of optimal candidates (156). This is particularly evident in clinical trials when the immune backgrounds of patients and treatment strategies differ a lot in each case.
All these limitations of DEXs necessitate modifications to enhance their anti-tumor properties. Therefore, researchers propose strategies to address these challenges and improve existing methods. DEXs are more compatible with different modifications like genetic engineering, surface treatment, and exogenous cargo loading. In particular, genetic engineering enables target ligand expression and can be employed as a therapeutic strategy. For instance, overexpression of several proteins in precursor DCs results in CD8+ reprogramming and an enhanced CTL response following DEX treatment. Another approach is to inhibit the PD-1/PD-L1 pathway, which tumors exploit to evade immune detection. PD-1 is a T-cell checkpoint protein engaged by its ligand PD-L1 that inhibits T-cell activation. Parental DCs transfected with a plasmid can successfully express anti-PD-1 antibodies. Alternatively, DEXs can be loaded with anti-PD-1 antibodies. Thus, anti-PD-1 antibodies in combination with DEXs can significantly enhance T cell activity against tumors (92, 157). Additionally, antigens or adjuvant molecules can be directly conjugated to the cell surface. As demonstrated in macrophages, such a modification could be utilized to modify DEX in future research (158). Preconditioning DCs with cytokines, such as IFN-γ, can enhance the immunogenicity of the resulting exosomes. IFN-γ preconditioning promotes DC maturation and increases the pro-inflammatory cytokine production, which can improve the efficacy of DEXs against tumors (150). DEXs can be loaded with specific molecules endogenously by using viral vectors or plasmids to modify parental cells (159).
4 Conclusion
Over 25 years, since the first description of DEX functions and pilot in vivo experiments, DC-derived exosomes are still under active research focus. DEXs have the potential to control, suppress, and target tumors in vitro and in vivo. Due to these properties, DEX are therapeutically promising cell-derived elements that can efficiently present antigens, overcome immunosuppression in the tumor microenvironment, and enhance immunogenicity (85, 89, 98). In addition, although DEX-based drugs have reached the stage of clinical trials, they were not successful for a number of reasons. DEXs are cell-derived products rather than viable cells. The main benefit of DEX-based therapy is that the exosomes cannot be recruited by immune cells in the tumor microenvironment. Furthermore, some studies show that they can reprogram host’s immune cells previously recruited by tumors. However, despite all DEX benefits, there is great room for further research and improvements. For example, it has never been described how the cells that died via the ICD pathway impact the efficacy of the resulting exosomes. We propose that DEX obtained following an ICD stimulus can promote a significantly more effective response against a tumor, as demonstrated by DC vaccines in a variety of murine models (57, 160–162). The ICD-DEX approach requires further study and validation, both in vitro and in vivo. If successful, it could become an appealing option for clinical practice, not only as a monotherapy or a part of multicomponent treatment but also as an agent that enhances the immune response through its impact on the tumor microenvironment.
Author contributions
TR: Conceptualization, Investigation, Visualization, Writing – original draft. VT: Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the state program of the ‘Sirius’ Federal Territory ‘Scientific and technological development of the “Sirius” Federal Territory’ (Agreement No. 20–03 dated 27.09.2024).
Acknowledgments
We would like to thank Maria Saviuk from Ghent University for her valuable contribution to this work, her kind help with figures and feedback on the manuscript draft.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhang Y and Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. (2020) 17:807–21. doi: 10.1038/s41423-020-0488-6
2. Mukherjee N, Wheeler KM, and Svatek RS. Bacillus Calmette–Guérin treatment of bladder cancer. Curr Opin Urol. (2019) 29:181–8. doi: 10.1097/MOU.0000000000000595
3. Eno J. Immunotherapy through the years. J Adv Pract Oncol. (2017) 8:747–53. doi: 10.6004/jadpro.2017.8.7.8
4. Mitra A, Barua A, Huang L, Ganguly S, Feng Q, and He B. From bench to bedside: the history and progress of CAR T cell therapy. Front Immunol. (2023) 14. doi: 10.3389/fimmu.2023.1188049
5. Duarte A da SS, Zangirolami AB, Santos I, Niemann FS, Honma HN, Amaro EC, et al. Production of dendritic cell vaccines using different methods with equivalent results: Implications for emerging centers. Hematol Transfus Cell Ther. (2024) 46:30–5. doi: 10.1016/j.htct.2022.11.006
6. Hotchkiss KM, Batich KA, Mohan A, Rahman R, Piantadosi S, and Khasraw M. Dendritic cell vaccine trials in gliomas: Untangling the lines. Neuro Oncol. (2023) 25:1752–62. doi: 10.1093/neuonc/noad088
7. Qian D, Li J, Huang M, Cui Q, Liu X, and Sun K. Dendritic cell vaccines in breast cancer: Immune modulation and immunotherapy. Biomed Pharmacother. (2023) 162:114685. doi: 10.1016/j.biopha.2023.114685
8. Steinman RM and Cohn ZA. Identification of A novel cell type in peripheral lymphoid organs of mice. J Exp Med. (1973) 137:1142–62. doi: 10.1084/jem.137.5.1142
9. Zanna MY, Yasmin AR, Omar AR, Arshad SS, Mariatulqabtiah AR, Nur-Fazila SH, et al. Review of dendritic cells, their role in clinical immunology, and distribution in various animal species. Int J Mol Sci. (2021) 22:8044. doi: 10.3390/ijms22158044
10. Soto JA, Gálvez NMS, Andrade CA, Pacheco GA, Bohmwald K, Berrios RV, et al. The role of dendritic cells during infections caused by highly prevalent viruses. Front Immunol. (2020) 11. doi: 10.3389/fimmu.2020.01513
11. Liu K. Dendritic cells. In: Encyclopedia of Cell Biology. Elsevier (2016). p. 741–9. doi: 10.1016/B978-0-12-394447-4.30111-0
12. Mellman I. Dendritic cells: master regulators of the immune response. Cancer Immunol Res. (2013) 1:145–9. doi: 10.1158/2326-6066.CIR-13-0102
13. Thurner B, Haendle I, Röder C, Dieckmann D, Keikavoussi P, Jonuleit H, et al. Vaccination with mage-3a1 peptide–pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. (1999) 190:1669–78. doi: 10.1084/jem.190.11.1669
14. Anguille S, Smits EL, Lion E, van Tendeloo VF, and Berneman ZN. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. (2014) 15:e257–67. doi: 10.1016/S1470-2045(13)70585-0
15. Sabado RL, Balan S, and Bhardwaj N. Dendritic cell-based immunotherapy. Cell Res. (2017) 27:74–95. doi: 10.1038/cr.2016.157
16. Gardner A, de Mingo Pulido Á, and Ruffell B. Dendritic cells and their role in immunotherapy. Front Immunol. (2020) 11. doi: 10.3389/fimmu.2020.00924
17. U.S. National Library of Medicine. Search of: Cancer | vaccine | Dendritic Cells - List Results - ClinicalTrials.gov. Available online at: https://clinicaltrials.gov/search?cond=Cancer&term=vaccine&intr=Dendritic%20Cells&aggFilters=status:act%20rec (Accessed December 27, 2024).
18. Liau LM, Ashkan K, Brem S, Campian JL, Trusheim JE, Iwamoto FM, et al. Association of autologous tumor lysate-loaded dendritic cell vaccination with extension of survival among patients with newly diagnosed and recurrent glioblastoma. JAMA Oncol. (2023) 9:112. doi: 10.1001/jamaoncol.2022.5370
19. U.S. National Library of Medicine. NCT06097793: Dendritic cell-based cancer vaccine study. Available online at: https://clinicaltrials.gov/study/NCT06097793?cond=Cancer&term=vaccine&intr=Dendritic%20Cells&aggFilters=phase:0,status:act%20rec&rank=5 (Accessed November 5, 2024).
20. U.S. National Library of Medicine. NCT05767684: Phase 1 study of dendritic cell-based cancer vaccine. Available online at: https://clinicaltrials.gov/study/NCT05767684?cond=Cancer&term=vaccine&intr=Dendritic%20Cells&aggFilters=phase:1,status:act%20rec&rank=5 (Accessed November 5, 2024).
21. U.S. National Library of Medicine. NCT06435910: Phase 1 study of dendritic cell-based cancer vaccine. Available online at: https://clinicaltrials.gov/study/NCT06435910?cond=Cancer&term=vaccine&intr=Dendritic%20Cells&aggFilters=phase:1,status:act%20rec&rank=7publications (Accessed November 5, 2024).
22. Han S, Wang B, Cotter MJ, Yang LJ, Zucali J, Moreb JS, et al. Overcoming immune tolerance against multiple myeloma with lentiviral calnexin-engineered dendritic cells. Mol Ther. (2008) 16:269–79. doi: 10.1038/sj.mt.6300369
23. Ayed AO, Chang LJ, and Moreb JS. Immunotherapy for multiple myeloma: Current status and future directions. Crit Rev Oncol Hematol. (2015) 96:399–412. doi: 10.1016/j.critrevonc.2015.06.006
24. U.S. National Library of Medicine. NCT06253234: Phase 1 study of dendritic cell-based cancer vaccine. Available online at: https://clinicaltrials.gov/study/NCT06253234?cond=Cancer&term=vaccine&intr=Dendritic%20Cells&aggFilters=phase:1,status:act%20rec&rank=10 (Accessed November 5, 2024).
25. U.S. National Library of Medicine. NCT05964361: Phase 1 study of dendritic cell-based cancer vaccine. Available online at: https://clinicaltrials.gov/study/NCT05964361?cond=Cancer&term=vaccine&intr=Dendritic%20Cells&aggFilters=phase:1,status:act%20rec&rank=6 (Accessed November 5, 2024).
26. Van den Bergh JM, Lion E, Van Tendeloo VF, and Smits EL. IL-15 receptor alpha as the magic wand to boost the success of IL-15 antitumor therapies: The upswing of IL-15 transpresentation. Pharmacol Ther. (2017) 170:73–9. doi: 10.1016/j.pharmthera.2016.10.012
27. Van den Bergh J, Willemen Y, Lion E, Van Acker H, De Reu H, Anguille S, et al. Transpresentation of interleukin-15 by IL-15/IL-15Rα mRNA-engineered human dendritic cells boosts antitumoral natural killer cell activity. Oncotarget. (2015) 6:44123–33. doi: 10.18632/oncotarget.v6i42
28. U.S. National Library of Medicine. NCT05773859: Phase 1 study of dendritic cell-based cancer vaccine. Available online at: https://clinicaltrials.gov/study/NCT05773859?cond=Cancer&term=vaccine&intr=Dendritic%20Cells&aggFilters=phase:1,status:act%20rec&rank=8 (Accessed November 5, 2024).
29. U.S. National Library of Medicine. NCT06253494: Phase 2 study of dendritic cell-based cancer vaccine. Available online at: https://clinicaltrials.gov/study/NCT06253494?cond=Cancer&term=vaccine&intr=Dendritic%20Cells&aggFilters=phase:2,status:act%20rec&rank=32study-overview (Accessed November 5, 2024).
30. Konecny GE, Santos L, Winterhoff B, Hatmal M, Keeney GL, Mariani A, et al. HER2 gene amplification and EGFR expression in a large cohort of surgically staged patients with nonendometrioid (type II) endometrial cancer. Br J Cancer. (2009) 100:89–95. doi: 10.1038/sj.bjc.6604814
31. Makker V, Colombo N, Casado Herráez A, Santin AD, Colomba E, Miller DS, et al. Lenvatinib plus pembrolizumab for advanced endometrial cancer. New Engl J Med. (2022) 386:437–48. doi: 10.1056/NEJMoa2108330
32. Constantine GD, Kessler G, Graham S, and Goldstein SR. Increased incidence of endometrial cancer following the women’s health initiative: an assessment of risk factors. J Womens Health. (2019) 28:237–43. doi: 10.1089/jwh.2018.6956
33. U.S. National Library of Medicine. NCT02919644: Phase 2 study of dendritic cell-based cancer vaccine. Available online at: https://clinicaltrials.gov/study/NCT02919644?cond=Cancer&term=vaccine&intr=Dendritic%20Cells&aggFilters=phase:2,status:act%20rec&rank=17 (Accessed November 7, 2024).
34. U.S. National Library of Medicine. NCT05920798: Phase 2 study of dendritic cell-based cancer vaccine. Available online at: https://clinicaltrials.gov/study/NCT05920798?cond=Cancer&term=vaccine&intr=Dendritic%20Cells&aggFilters=phase:2,status:act%20rec&rank=33study-overview\ (Accessed November 9, 2024).
35. Truxova I, Hensler M, Skapa P, Halaska MJ, Laco J, Ryska A, et al. Rationale for the Combination of Dendritic Cell-Based Vaccination Approaches With Chemotherapy Agents. (2017). Academic Press, pp. 115–56. pp. 115–56. doi: 10.1016/bs.ircmb.2016.09.003
36. Koukourakis IM and Koukourakis MI. Combining the past and present to advance immuno-radiotherapy of cancer. Int Rev Immunol. (2023) 42:26–42. doi: 10.1080/08830185.2021.1974020
37. Najafi S and Mortezaee K. Advances in dendritic cell vaccination therapy of cancer. Biomed Pharmacother. (2023) 164:114954. doi: 10.1016/j.biopha.2023.114954
38. Théry C and Amigorena S. The cell biology of antigen presentation in dendritic cells. Curr Opin Immunol. (2001) 13:45–51. doi: 10.1016/S0952-7915(00)00180-1
39. Murphy TL and Murphy KM. Dendritic cells in cancer immunology. Cell Mol Immunol. (2022) 19:3–13. doi: 10.1038/s41423-021-00741-5
40. Fu C and Jiang A. Dendritic cells and CD8 T cell immunity in tumor microenvironment. Front Immunol. (2018) 9. doi: 10.3389/fimmu.2018.03059
41. Constantino J, Gomes C, Falcão A, Cruz MT, and Neves BM. Antitumor dendritic cell–based vaccines: lessons from 20 years of clinical trials and future perspectives. Trans Res. (2016) 168:74–95. doi: 10.1016/j.trsl.2015.07.008
42. Zanotta S, Galati D, De Filippi R, and Pinto A. Enhancing dendritic cell cancer vaccination: the synergy of immune checkpoint inhibitors in combined therapies. Int J Mol Sci. (2024) 25:7509. doi: 10.3390/ijms25147509
43. Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. New Engl J Med. (2010) 363:411–22. doi: 10.1056/NEJMoa1001294
44. Anassi E and Ndefo UA. Sipuleucel-T (provenge) injection: the first immunotherapy agent (vaccine) for hormone-refractory prostate cancer. P T. (2011) 36:197–202.
45. Choi D, Perrin M, Hoffmann S, Chang AE, Ratanatharathorn V, Uberti J, et al. Dendritic cell-based vaccines in the setting of peripheral blood stem cell transplantation: CD34+ cell-depleted mobilized peripheral blood can serve as a source of potent dendritic cells. Clin Cancer Res. (1998) 4:2709–16.
46. Saxena M and Bhardwaj N. Re-emergence of dendritic cell vaccines for cancer treatment. Trends Cancer. (2018) 4:119–37. doi: 10.1016/j.trecan.2017.12.007
47. Nair S, Archer GE, and Tedder TF. Isolation and generation of human dendritic cells. Curr Protoc Immunol. (2012) 99:7.32.1–7.32.23. doi: 10.1002/0471142735.2012.99.issue-1
48. Ito Y and Noguchi K. Abstract A48: Subcutaneous injection of aGalCer loaded mature dendritic cells at near regional lymph node area leads tumor regression in the patients with Malignant tumor. Cancer Immunol Res. (2022) 10:A48–8. doi: 10.1158/2326-6074.TUMIMM22-A48
49. Islam MA, Rice J, Reesor E, Zope H, Tao W, Lim M, et al. Adjuvant-pulsed mRNA vaccine nanoparticle for immunoprophylactic and therapeutic tumor suppression in mice. Biomaterials. (2021) 266:120431. doi: 10.1016/j.biomaterials.2020.120431
50. Dörrie J, Schaft N, Schuler G, and Schuler-Thurner B. Therapeutic cancer vaccination with ex vivo RNA-transfected dendritic cells—An update. Pharmaceutics. (2020) 12:92. doi: 10.3390/pharmaceutics12020092
51. Wang QT, Nie Y, Sun SN, Lin T, Han RJ, Jiang J, et al. Tumor-associated antigen-based personalized dendritic cell vaccine in solid tumor patients. Cancer Immunol Immunother. (2020) 69:1375–87. doi: 10.1007/s00262-020-02496-w
52. Ding Z, Li Q, Zhang R, Xie L, Shu Y, Gao S, et al. Personalized neoantigen pulsed dendritic cell vaccine for advanced lung cancer. Signal Transduct Target Ther. (2021) 6:26. doi: 10.1038/s41392-020-00448-5
53. Watanabe A, Yamashita K, Fujita M, Arimoto A, Nishi M, Takamura S, et al. Vaccine based on dendritic cells electroporated with an exogenous ovalbumin protein and pulsed with invariant natural killer T cell ligands effectively induces antigen-specific antitumor immunity. Cancers (Basel). (2021) 14:171. doi: 10.3390/cancers14010171
54. Kit OIVDIMTIFEMMAP. Method for treatment of primary patients with locally advanced cervical cancer. Russia: Russian Federal Service for Intellectual Property (2017). p. RU2616531C1.
55. Kuleva S, Borokshinova K, Baldueva I, Nekhaeva T, Artemyeva A, Efremova N, et al. Experience with multitargeted antitumor vaccine in a child with diffuse midline glioma, H3 K27M-mutant. Vopr Onkol. (2023) 69:555–64. doi: 10.37469/0507-3758-2023-69-3-555-564
56. National Research Oncology Center. Anti-cancer therapy. Available online at: https://www.niioncologii.ru/en/patients/additional-information/anti-cancer/ (Accessed December 16, 2024).
57. Garg AD, Vandenberk L, Koks C, Verschuere T, Boon L, Van Gool SW, et al. Dendritic cell vaccines based on immunogenic cell death elicit danger signals and T cell–driven rejection of high-grade glioma. Sci Transl Med. (2016) 8:328ra27–328ra27. doi: 10.1126/scitranslmed.aae0105
58. Aaes TL, Kaczmarek A, Delvaeye T, De Craene B, De Koker S, Heyndrickx L, et al. Vaccination with necroptotic cancer cells induces efficient anti-tumor immunity. Cell Rep. (2016) 15:274–87. doi: 10.1016/j.celrep.2016.03.037
59. Efimova I, Catanzaro E, van der Meeren L, Turubanova VD, Hammad H, Mishchenko TA, et al. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J Immunother Cancer. (2020) 8:e001369. doi: 10.1136/jitc-2020-001369
60. Liu P, Zhao L, Zitvogel L, Kepp O, and Kroemer G. Immunogenic cell death (ICD) enhancers—Drugs that enhance the perception of ICD by dendritic cells. Immunol Rev. (2024) 321:7–19. doi: 10.1111/imr.v321.1
61. Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. (2005) 202:1691–701. doi: 10.1084/jem.20050915
62. Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. (2018) 25:486–541. doi: 10.1038/s41418-017-0012-4
63. Pittet MJ, Di Pilato M, Garris C, and Mempel TR. Dendritic cells as shepherds of T cell immunity in cancer. Immunity. (2023) 56:2218–30. doi: 10.1016/j.immuni.2023.08.014
64. Jahani V, Yazdani M, Badiee A, Jaafari MR, and Arabi L. Liposomal celecoxib combined with dendritic cell therapy enhances antitumor efficacy in melanoma. J Controlled Release. (2023) 354:453–64. doi: 10.1016/j.jconrel.2023.01.034
65. Chow KV, Lew AM, Sutherland RM, and Zhan Y. Monocyte-derived dendritic cells promote Th polarization, whereas conventional dendritic cells promote Th proliferation. J Immunol. (2016) 196:624–36. doi: 10.4049/jimmunol.1501202
66. Kolostova K, Pospisilova E, Matkowski R, Szelachowska J, and Bobek V. Immune activation of the monocyte-derived dendritic cells using patients own circulating tumor cells. Cancer Immunol Immunother. (2022) 71:2901–11. doi: 10.1007/s00262-022-03189-2
67. Kushwah R and Hu J. Complexity of dendritic cell subsets and their function in the host immune system. Immunology. (2011) 133:409–19. doi: 10.1111/j.1365-2567.2011.03457.x
68. Schroeder MA, Rettig MP, Lopez S, Christ S, Fiala M, Eades W, et al. Mobilization of allogeneic peripheral blood stem cell donors with intravenous plerixafor mobilizes a unique graft. Blood. (2017) 129:2680–92. doi: 10.1182/blood-2016-09-739722
69. Karpova D, Rettig MP, and DiPersio JF. Mobilized peripheral blood: an updated perspective. F1000Res. (2019) 8:2125. doi: 10.12688/f1000research
70. Plackoska V, Shaban D, and Nijnik A. Hematologic dysfunction in cancer: Mechanisms, effects on antitumor immunity, and roles in disease progression. Front Immunol. (2022) 13. doi: 10.3389/fimmu.2022.1041010
71. Hacker ED, Fink AM, Peters T, Park C, Fantuzzi G, and Rondelli D. Persistent fatigue in hematopoietic stem cell transplantation survivors. Cancer Nurs. (2017) 40:174–83. doi: 10.1097/NCC.0000000000000405
72. Xiao Z, Wang R, Wang X, Yang H, Dong J, He X, et al. Impaired function of dendritic cells within the tumor microenvironment. Front Immunol. (2023) 14. doi: 10.3389/fimmu.2023.1213629
73. Tesone AJ, Svoronos N, Allegrezza MJ, and Conejo-Garcia JR. Pathological mobilization and activities of dendritic cells in tumor-bearing hosts: challenges and opportunities for immunotherapy of cancer. Front Immunol. (2013) 4. doi: 10.3389/fimmu.2013.00435
74. Kim CW, Kim KD, and Lee HK. The role of dendritic cells in tumor microenvironments and their uses as therapeutic targets. BMB Rep. (2021) 54:31–43. doi: 10.5483/BMBRep.2021.54.1.224
75. Rao Q, Ma G, Li M, Wu H, Zhang Y, Zhang C, et al. Targeted delivery of triptolide by dendritic cell-derived exosomes for colitis and rheumatoid arthritis therapy in murine models. Br J Pharmacol. (2023) 180:330–46. doi: 10.1111/bph.v180.3
76. Veglia F and Gabrilovich DI. Dendritic cells in cancer: the role revisited. Curr Opin Immunol. (2017) 45:43–51. doi: 10.1016/j.coi.2017.01.002
77. Lozano M, Cid J, Benitez-Ribas D, and Otero MJ. Technical challenges in the manufacture of dendritic cell cancer therapies. Eur Oncol Haematol. (2019) 15:22. doi: 10.17925/EOH.2019.15.1.22
78. Kalluri R and LeBleu VS. The biology, function, and biomedical applications of exosomes. Sci (1979). (2020) 367:eaau6977. doi: 10.1126/science.aau6977
79. Huang J, Xu Y, Wang Y, Su Z, Li T, Wu S, et al. Advances in the study of exosomes as drug delivery systems for bone-related diseases. Pharmaceutics. (2023) 15:220. doi: 10.3390/pharmaceutics15010220
80. Chen H, Wang L, Zeng X, Schwarz H, Nanda HS, Peng X, et al. Exosomes, a new star for targeted delivery. Front Cell Dev Biol. (2021) 9. doi: 10.3389/fcell.2021.751079
81. Sun Y, Liu G, Zhang K, Cao Q, Liu T, and Li J. Mesenchymal stem cells-derived exosomes for drug delivery. Stem Cell Res Ther. (2021) 12:561. doi: 10.1186/s13287-021-02629-7
82. Bunggulawa EJ, Wang W, Yin T, Wang N, Durkan C, Wang Y, et al. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnol. (2018) 16:81. doi: 10.1186/s12951-018-0403-9
83. Familtseva A, Jeremic N, and Tyagi SC. Exosomes: cell-created drug delivery systems. Mol Cell Biochem. (2019) 459:1–6. doi: 10.1007/s11010-019-03545-4
84. Harding CV, Heuser JE, and Stahl PD. Exosomes: Looking back three decades and into the future. J Cell Biol. (2013) 200:367–71. doi: 10.1083/jcb.201212113
85. Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell derived exosomes. Nat Med. (1998) 4:594–600. doi: 10.1038/nm0598-594
86. Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. (1996) 183:1161–72. doi: 10.1084/jem.183.3.1161
87. Luo S, Chen J, Xu F, Chen H, Li Y, and Li W. Dendritic cell-derived exosomes in cancer immunotherapy. Pharmaceutics. (2023) 15:2070. doi: 10.3390/pharmaceutics15082070
88. Ghorbaninezhad F, Alemohammad H, Najafzadeh B, Masoumi J, Shadbad MA, Shahpouri M, et al. Dendritic cell-derived exosomes: A new horizon in personalized cancer immunotherapy? Cancer Lett. (2023) 562:216168. doi: 10.1016/j.canlet.2023.216168
89. Romagnoli GG, Zelante BB, Toniolo PA, Migliori IK, and Barbuto JAM. Dendritic Cell-Derived Exosomes may be a Tool for Cancer Immunotherapy by Converting Tumor Cells into Immunogenic Targets. Front Immunol. (2015) 5. doi: 10.3389/fimmu.2014.00692
90. Lindenbergh MFS, Wubbolts R, Borg EGF, van ‘T Veld EM, Boes M, and Stoorvogel W. Dendritic cells release exosomes together with phagocytosed pathogen; potential implications for the role of exosomes in antigen presentation. J Extracell Vesicles. (2020) 9. doi: 10.1080/20013078.2020.1798606
91. Xia J, Miao Y, Wang X, Huang X, and Dai J. Recent progress of dendritic cell-derived exosomes (Dex) as an anti-cancer nanovaccine. Biomed Pharmacother. (2022) 152:113250. doi: 10.1016/j.biopha.2022.113250
92. Tian H and Li W. Dendritic cell-derived exosomes for cancer immunotherapy: hope and challenges. Ann Transl Med. (2017) 5:221–1. doi: 10.21037/atm.2017.02.23
93. Chistiakov DA, Grechko AV, Orekhov AN, and Bobryshev YV. An immunoregulatory role of dendritic cell-derived exosomes versus HIV-1 infection: take it easy but be warned. Ann Transl Med. (2017) 5:362–2. doi: 10.21037/atm.2017.06.34
94. You L, Mao L, Wei J, Jin S, Yang C, Liu H, et al. The crosstalk between autophagic and endo-/exosomal pathways in antigen processing for MHC presentation in anticancer T cell immune responses. J Hematol Oncol. (2017) 10:165. doi: 10.1186/s13045-017-0534-8
95. Näslund TI, Gehrmann U, Qazi KR, Karlsson MCI, and Gabrielsson S. Dendritic cell–derived exosomes need to activate both T and B cells to induce antitumor immunity. J Immunol. (2013) 190:2712–9. doi: 10.4049/jimmunol.1203082
96. Damo M, Wilson DS, Simeoni E, and Hubbell JA. TLR-3 stimulation improves anti-tumor immunity elicited by dendritic cell exosome-based vaccines in a murine model of melanoma. Sci Rep. (2015) 5:17622. doi: 10.1038/srep17622
97. Lu Z, Zuo B, Jing R, Gao X, Rao Q, Liu Z, et al. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J Hepatol. (2017) 67:739–48. doi: 10.1016/j.jhep.2017.05.019
98. Pitt JM, André F, Amigorena S, Soria JC, Eggermont A, Kroemer G, et al. Dendritic cell–derived exosomes for cancer therapy. J Clin Invest. (2016) 126:1224–32. doi: 10.1172/JCI81137
99. Barnwal A, Gaur V, Sengupta A, Tyagi W, Das S, and Bhattacharyya J. Tumor antigen-primed dendritic cell-derived exosome synergizes with colony stimulating factor-1 receptor inhibitor by modulating the tumor microenvironment and systemic immunity. ACS Biomater Sci Eng. (2023) 9:6409–24. doi: 10.1021/acsbiomaterials.3c00469
100. Gupta D, Liang X, Pavlova S, Wiklander OPB, Corso G, Zhao Y, et al. Quantification of extracellular vesicles in vitro and in vivo using sensitive bioluminescence imaging. J Extracell Vesicles. (2020) 9. doi: 10.1080/20013078.2020.1800222
101. Czajka-Francuz P, Prendes MJ, Mankan A, Quintana Á, Pabla S, Ramkissoon S, et al. Mechanisms of immune modulation in the tumor microenvironment and implications for targeted therapy. Front Oncol. (2023) 13. doi: 10.3389/fonc.2023.1200646
102. Laoui D, Keirsse J, Morias Y, Van Overmeire E, Geeraerts X, Elkrim Y, et al. The tumour microenvironment harbours ontogenically distinct dendritic cell populations with opposing effects on tumour immunity. Nat Commun. (2016) 7:13720. doi: 10.1038/ncomms13720
103. Fernández-Delgado I, Calzada-Fraile D, and Sánchez-Madrid F. Immune regulation by dendritic cell extracellular vesicles in cancer immunotherapy and vaccines. Cancers (Basel). (2020) 12:3558. doi: 10.3390/cancers12123558
104. Huang Y, Liu K, Li Q, Yao Y, and Wang Y. Exosomes Function in Tumor Immune Microenvironment. (2018). pp. 109–22. pp. 109–22. Springer, Cham. doi: 10.1007/978-3-319-74470-4_7
105. Boussadia Z, Zanetti C, and Parolini I. Role of microenvironmental acidity and tumor exosomes in cancer immunomodulation. Transl Cancer Res. (2020) 9:5775–86. doi: 10.21037/tcr.2020.03.69
106. Wang S and Shi Y. Exosomes derived from immune cells: the new role of tumor immune microenvironment and tumor therapy. Int J Nanomed. (2022) 17:6527–50. doi: 10.2147/IJN.S388604
107. Zhang H, Wang S, Sun M, Cui Y, Xing J, Teng L, et al. Exosomes as smart drug delivery vehicles for cancer immunotherapy. Front Immunol. (2023) 13. doi: 10.3389/fimmu.2022.1093607
108. Li I and Nabet BY. Exosomes in the tumor microenvironment as mediators of cancer therapy resistance. Mol Cancer. (2019) 18:32. doi: 10.1186/s12943-019-0975-5
109. Jin Y, Xing J, Xu K, Liu D, and Zhuo Y. Exosomes in the tumor microenvironment: Promoting cancer progression. Front Immunol. (2022) 13. doi: 10.3389/fimmu.2022.1025218
110. Yang E, Wang X, Gong Z, Yu M, Wu H, and Zhang D. Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal Transduct Target Ther. (2020) 5:242. doi: 10.1038/s41392-020-00359-5
111. Nolte-’t Hoen ENM, Buschow SI, Anderton SM, Stoorvogel W, and Wauben MHM. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. (2009) 113:1977–81. doi: 10.1182/blood-2008-08-174094
112. Xu M, Feng T, Liu B, Qiu F, Xu Y, Zhao Y, et al. Engineered exosomes: desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics. (2021) 11:8926–44. doi: 10.7150/thno.62330
113. Osaid Z, Haider M, Hamoudi R, and Harati R. Exosomes interactions with the blood–brain barrier: implications for cerebral disorders and therapeutics. Int J Mol Sci. (2023) 24:15635. doi: 10.3390/ijms242115635
114. Khatami SH, Karami N, Taheri-Anganeh M, Taghvimi S, Tondro G, Khorsand M, et al. Exosomes: promising delivery tools for overcoming blood-brain barrier and glioblastoma therapy. Mol Neurobiol. (2023) 60:4659–78. doi: 10.1007/s12035-023-03365-0
115. Rui Y and Green JJ. Overcoming delivery barriers in immunotherapy for glioblastoma. Drug Delivery Transl Res. (2021) 11:2302–16. doi: 10.1007/s13346-021-01008-2
116. Mendt M, Kamerkar S, Sugimoto H, McAndrews KM, Wu CC, Gagea M, et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight. (2018) 3. doi: 10.1172/jci.insight.99263
117. Chen YS, Lin EY, Chiou TW, and Harn HJ. Exosomes in clinical trial and their production in compliance with good manufacturing practice. Tzu Chi Med J. (2020) 32:113. doi: 10.4236/cm.2020.113007
118. Tan F, Li X, Wang Z, Li J, Shahzad K, and Zheng J. Clinical applications of stem cell-derived exosomes. Signal Transduct Target Ther. (2024) 9:17. doi: 10.1038/s41392-023-01704-0
119. Shimon MB, Shapira S, Seni J, and Arber N. The big potential of small particles: lipid-based nanoparticles and exosomes in vaccination. Vaccines (Basel). (2022) 10:1119. doi: 10.3390/vaccines10071119
120. Wahlund CJE, Güclüler G, Hiltbrunner S, Veerman RE, Näslund TI, and Gabrielsson S. Exosomes from antigen-pulsed dendritic cells induce stronger antigen-specific immune responses than microvesicles in vivo. Sci Rep. (2017) 7:17095. doi: 10.1038/s41598-017-16609-6
121. Shamshiripour P, Rahnama M, Nikoobakht M, Rad VF, Moradi AR, and Ahmadvand D. Extracellular vesicles derived from dendritic cells loaded with VEGF-A siRNA and doxorubicin reduce glioma angiogenesis in vitro. J Controlled Release. (2024) 369:128–45. doi: 10.1016/j.jconrel.2024.03.042
122. Soo CY, Song Y, Zheng Y, Campbell EC, Riches AC, Gunn-Moore F, et al. Nanoparticle tracking analysis monitors microvesicle and exosome secretion from immune cells. Immunology. (2012) 136:192–7. doi: 10.1111/j.1365-2567.2012.03569.x
123. Khan MA, Anand S, Deshmukh SK, Singh S, and Singh AP. Determining the Size Distribution and Integrity of Extracellular Vesicles by Dynamic Light Scattering. (2022). pp. 165–75. pp. 165–75. Humana, New York, NY. doi: 10.1007/978-3-319-74470-4_7
124. Simhadri VR, Reiners KS, Hansen HP, Topolar D, Simhadri VL, Nohroudi K, et al. Dendritic cells release HLA-B-associated transcript-3 positive exosomes to regulate natural killer function. PloS One. (2008) 3:e3377. doi: 10.1371/journal.pone.0003377
125. Théry C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, et al. Molecular characterization of dendritic cell-derived exosomes. J Cell Biol. (1999) 147:599–610. doi: 10.1083/jcb.147.3.599
126. Popović M and de Marco A. Canonical and selective approaches in exosome purification and their implications for diagnostic accuracy. Transl Cancer Res. (2018) 7:S209–25. doi: 10.21037/tcr.2017.08.44
127. Théry C, Amigorena S, Raposo G, and Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. (2006) 30:3.22.1–3.22.29. doi: 10.1002/0471143030.2006.30.issue-1
128. Nguyen XD, Eichler H, Sucker A, Hofmann U, SChadendorf D, and Klüter H. Collection of autologous monocytes for dendritic cell vaccination therapy in metastatic melanoma patients. Transfusion (Paris). (2002) 42:428–32. doi: 10.1046/j.1525-1438.2002.00067.x
129. Ahn SH, Ryu SW, Choi H, You S, Park J, and Choi C. Manufacturing therapeutic exosomes: from bench to industry. Mol Cells. (2022) 45:284–90. doi: 10.14348/molcells.2022.2033
130. Thakur A and Rai D. Global requirements for manufacturing and validation of clinical grade extracellular vesicles. J liquid biopsy. (2024) 6:100278. doi: 10.1016/j.jlb.2024.100278
131. U.S. National Library of Medicine. NCT05559177: Study of dendritic cells derived exosomes (MT-EXO) in cancer patients. Available online at: https://clinicaltrials.gov/study/NCT05559177?cond=Cancer&term=dendritic%20cells%20exosomes&rank=2study-overview (Accessed November 14, 2024).
132. Bu N, Wu H, Zhang G, Zhan S, Zhang R, Sun H, et al. Exosomes from dendritic cells loaded with chaperone-rich cell lysates elicit a potent T cell immune response against intracranial glioma in mice. J Mol Neurosci. (2015) 56:631–43. doi: 10.1007/s12031-015-0506-9
133. Chen S, Lv M, Fang S, Ye W, Gao Y, and Xu Y. Poly(I:C) enhanced anti-cervical cancer immunities induced by dendritic cells-derived exosomes. Int J Biol Macromol. (2018) 113:1182–7. doi: 10.1016/j.ijbiomac.2018.02.034
134. Escudier B, Dorval T, Chaput N, André F, Caby MP, Novault S, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J Transl Med. (2005) 3:10. doi: 10.1186/1479-5876-3-10
135. Viaud S, Terme M, Flament C, Taieb J, André F, Novault S, et al. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: A role for NKG2D ligands and IL-15Rα. PLoS One. (2009) 4:e4942. doi: 10.1371/journal.pone.0004942
136. Hazrati A, Soudi S, Malekpour K, Mahmoudi M, Rahimi A, Hashemi SM, et al. Immune cells-derived exosomes function as a double-edged sword: role in disease progression and their therapeutic applications. biomark Res. (2022) 10:30. doi: 10.1186/s40364-022-00374-4
137. Phung CD, Pham TT, Nguyen HT, Nguyen TT, Ou W, Jeong JH, et al. Anti-CTLA-4 antibody-functionalized dendritic cell-derived exosomes targeting tumor-draining lymph nodes for effective induction of antitumor T-cell responses. Acta Biomater. (2020) 115:371–82. doi: 10.1016/j.actbio.2020.08.008
138. Bao P, Gu H, Ye J, He J, Zhong Z, Yu A, et al. Chimeric exosomes functionalized with STING activation for personalized glioblastoma immunotherapy. Advanced Sci. (2024) 11. doi: 10.1002/advs.202306336
139. Li Y, Tian L, Zhao T, and Zhang J. A nanotherapeutic system for gastric cancer suppression by synergistic chemotherapy and immunotherapy based on iPSCs and DCs exosomes. Cancer Immunol Immunother. (2023) 72:1673–83. doi: 10.1007/s00262-022-03355-6
140. Arimoto Ki, Miyauchi S, Liu M, and Zhang DE. Emerging role of immunogenic cell death in cancer immunotherapy. Front Immunol. (2024) 15. doi: 10.3389/fimmu.2024.1390263
141. Galluzzi L, Kepp O, Hett E, Kroemer G, and Marincola FM. Immunogenic cell death in cancer: concept and therapeutic implications. J Transl Med. (2023) 21:162. doi: 10.1186/s12967-023-04017-6
142. Sprooten J, Laureano RS, Vanmeerbeek I, Govaerts J, Naulaerts S, Borras DM, et al. Trial watch: chemotherapy-induced immunogenic cell death in oncology. Oncoimmunology. (2023) 12. doi: 10.1080/2162402X.2023.2219591
143. Turubanova VD, Mishchenko TA, Balalaeva IV, Efimova I, Peskova NN, Klapshina LG, et al. Novel porphyrazine-based photodynamic anti-cancer therapy induces immunogenic cell death. Sci Rep. (2021) 11:7205. doi: 10.1038/s41598-021-86354-4
144. Tanaka M, Kataoka H, Yano S, Sawada T, Akashi H, Inoue M, et al. Immunogenic cell death due to a new photodynamic therapy (PDT) with glycoconjugated chlorin (G-chlorin). Oncotarget. (2016) 7:47242–51. doi: 10.18632/oncotarget.v7i30
145. Alzeibak R, Mishchenko TA, Shilyagina NY, Balalaeva IV, Vedunova MV, and Krysko DV. Targeting immunogenic cancer cell death by photodynamic therapy: past, present and future. J Immunother Cancer. (2021) 9:e001926. doi: 10.1136/jitc-2020-001926
146. Troitskaya O, Novak D, Varlamov M, Biryukov M, Nushtaeva A, Kochneva G, et al. Immunological effects of cold atmospheric plasma-treated cells in comparison with those of cells treated with lactaptin-based anticancer drugs. Biophysica. (2022) 2:266–80. doi: 10.3390/biophysica2030025
147. Amiri M, Molavi O, Sabetkam S, Jafari S, and Montazersaheb S. Stimulators of immunogenic cell death for cancer therapy: focusing on natural compounds. Cancer Cell Int. (2023) 23:200. doi: 10.1186/s12935-023-03058-7
148. U.S. National Library of Medicine. Search results for “immunogenic cell death” (2005-2025), sorted by publication date. Available online at (Accessed January 11, 2024).
149. Srinivasan S, Vannberg FO, and Dixon JB. Lymphatic transport of exosomes as a rapid route of information dissemination to the lymph node. Sci Rep. (2016) 6:24436. doi: 10.1038/srep24436
150. Jung I, Shin S, Baek MC, and Yea K. Modification of immune cell-derived exosomes for enhanced cancer immunotherapy: current advances and therapeutic applications. Exp Mol Med. (2024) 56:19–31. doi: 10.1038/s12276-023-01132-8
151. Rosa FF, Pires CF, Kurochkin I, Halitzki E, Zahan T, Arh N, et al. Single-cell transcriptional profiling informs efficient reprogramming of human somatic cells to cross-presenting dendritic cells. Sci Immunol. (2022) 7. doi: 10.1126/sciimmunol.abg5539
152. Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology. (2016) 5. doi: 10.1080/2162402X.2015.1071008
153. Viaud S, Ploix S, Lapierre V, Théry C, Commere PH, Tramalloni D, et al. Updated technology to produce highly immunogenic dendritic cell-derived exosomes of clinical grade. J Immunother. (2011) 34:65–75. doi: 10.1097/CJI.0b013e3181fe535b
154. Zimmermannova O, Ferreira AG, Ascic E, Velasco Santiago M, Kurochkin I, Hansen M, et al. Restoring tumor immunogenicity with dendritic cell reprogramming. Sci Immunol. (2023) 8. doi: 10.1126/sciimmunol.add4817
155. Cintolo JA, Datta J, Mathew SJ, and Czerniecki BJ. Dendritic cell-based vaccines: barriers and opportunities. Future Oncol. (2012) 8:1273–99. doi: 10.2217/fon.12.125
156. Yao Y, Fu C, Zhou L, Mi QS, and Jiang A. DC-derived exosomes for cancer immunotherapy. Cancers (Basel). (2021) 13:3667. doi: 10.3390/cancers13153667
157. Liu C, Liu X, Xiang X, Pang X, Chen S, Zhang Y, et al. A nanovaccine for antigen self-presentation and immunosuppression reversal as a personalized cancer immunotherapy strategy. Nat Nanotechnol. (2022) 17:531–40. doi: 10.1038/s41565-022-01098-0
158. Nie W, Wu G, Zhang J, Huang L, Ding J, Jiang A, et al. Responsive exosome nano-bioconjugates for synergistic cancer therapy. Angewandte Chemie Int Edition. (2020) 59:2018–22. doi: 10.1002/anie.201912524
159. Lu Y, Mai Z, Cui L, and Zhao X. Engineering exosomes and biomaterial-assisted exosomes as therapeutic carriers for bone regeneration. Stem Cell Res Ther. (2023) 14:55. doi: 10.1186/s13287-023-03275-x
160. Vedunova M, Turubanova V, Vershinina O, Savyuk M, Efimova I, Mishchenko T, et al. DC vaccines loaded with glioma cells killed by photodynamic therapy induce Th17 anti-tumor immunity and provide a four-gene signature for glioma prognosis. Cell Death Dis. (2022) 13:1062. doi: 10.1038/s41419-022-05514-0
161. Redkin TS, Sleptsova EE, Turubanova VD, Saviuk MO, Lermontova SA, Klapshina LG, et al. Dendritic cells pulsed with tumor lysates induced by tetracyanotetra(aryl)porphyrazines-based photodynamic therapy effectively trigger anti-tumor immunity in an orthotopic mouse glioma model. Pharmaceutics. (2023) 15:2430. doi: 10.3390/pharmaceutics15102430
Keywords: extracellular vesicles, dendritic cell-derived exosomes, immunotherapy, cancer treatment, immunogenic cell death
Citation: Redkin T and Turubanova V (2025) Dendritic cell-derived exosomes as anti-cancer cell-free agents: new insights into enhancing immunogenic effects. Front. Immunol. 16:1586892. doi: 10.3389/fimmu.2025.1586892
Received: 03 March 2025; Accepted: 07 May 2025;
Published: 28 May 2025.
Edited by:
Ling Ni, Tsinghua University, ChinaCopyright © 2025 Redkin and Turubanova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tikhon Redkin, cmVka2luLnRzQHRhbGFudGl1c3BlaC5ydQ==
 Tikhon Redkin
Tikhon Redkin Victoria Turubanova
Victoria Turubanova