- 1Interventions and Clinical Trials Department, Ifakara Health Institute, Bagamoyo, Tanzania
- 2Nelson Mandela African Institution of Science and Technology, Arusha, Tanzania
- 3European Vaccine Initiative, Heidelberg, Germany
- 4Transmission-blocking Malaria Vaccine Group, Jenner Institute, University of Oxford, Oxford, United Kingdom
- 5Laboratory of Malaria and Vector Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, MD, United States
Transmission-blocking vaccines are among the novel tools under development for malaria control and elimination. Understanding the human immune response to the sexual stages of Plasmodium falciparum is essential for progressing transmission-blocking vaccine development. A serosurvey was conducted in Tanzania, from May to August 2022 among 290 participants, consisting of 114 children (5–12 years), 44 adolescents (13–17 years), and 132 adults (18–45 years). The participants were tested for malaria parasites using quantitative polymerase chain reaction, and standardized enzyme-linked immunosorbent assays were performed to detect the presence of IgG antibodies against transmission-blocking target antigens—Pfs230D1M, Pfs48/45, and Pfs25. A set of 10 plasma samples that were reactive to Pfs230DIM and/or Pfs48/45 were tested individually for transmission-reducing activity via standard membrane feeding assays. Of the participants tested, 56% (157/281) had detectable Pfs230D1M antibodies, and 49% (141/290) were positive for Pfs48/45 IgG. Approximately 30% were seropositive for both. However, Pfs25 IgG was not detected in any of the 117 participants tested. The seroprevalence for Pfs230D1M and Pfs48/45 IgG increased significantly with participants’ age, with adults more likely to have antibodies than children: Pfs230D1M (adjusted odds ratio: 3.16, 95% confidence interval: 1.81–5.53, p-value ≤ 0.0001) and Pfs45/48 (OR: 3.06, 95% CI: 1.79–5.25, p ≤ 0.0001). There was no significant difference in antibody titers for Pfs230D1M and Pfs48/45 antibodies across age groups. A significant transmission-reducing activity was observed in 2/10 participants, who were highly reactive to Pfs230D1M and Pfs48/45. Naturally acquired antibody responses to both full-length Pfs48/45 and Pfs230D1M proteins are prevalent and appeared to be stable, suggesting that semi-immune populations may be ideal to evaluate boosting transmission-blocking vaccine candidates.
1 Introduction
Malaria continues to represent a major public health burden globally, leading to 263 million cases and 597,000 deaths, with the heaviest burden carried by sub-Saharan Africa (1–5). Tanzania accounted for 4% of the global malaria deaths reported in 2023 (2), and the Tanzania National Malaria Strategic Plan aims to reduce the malaria burden in moderate- and high-risk areas from 15% to less than 7.5% prevalence in 2025 and to further reduce transmission in low prevalence areas to less than 0.5% Plasmodium falciparum parasite rate (pfpr) in 2025 (6). Although the current control methods have led to substantial reductions in malaria morbidity and mortality, progress has slowed, and thus, additional novel tools that may not be impacted by the limitations of the current vector control tools are required (1). Vaccination against malaria is a promising novel tool that is required for a sustainable impact on the disease burden and elimination (5). The licensed malaria vaccines, R21/Matrix-M and RTS,S/AS01, are designed to target the liver stages of the Plasmodium falciparum parasites in the human host (7–9). However, the Plasmodium parasite has a complex life cycle, involving stages within the human host and the female Anopheles mosquito. Interventions that can target other stages of the parasite life cycle (10) are therefore needed. Transmission-blocking vaccines (TBVs) offer a unique approach of interrupting the development of malaria parasites in mosquitoes (11) and could complement the available interventions in controlling and eliminating malaria.
Malaria transmission occurs when at least one male and one female gametocyte are ingested by female Anopheles mosquitoes during a blood meal (12). The gametocytes and gametes that egress from red blood cells within the mosquito midgut express specific proteins on their surfaces (13). These surface proteins play an important role in the fertilization, maturation, and colonization of malaria parasites in mosquitoes (14, 15). The parasite surface proteins are categorized into two groups: pre-fertilization and post-fertilization proteins (16, 17), and they form the basis of malaria TBV development. Pfs48/45 and Pfs230 are the most studied P. falciparum pre-fertilization antigens and are expressed on both gametocytes and gametes. Pfs230 protein is expressed in both male and female gametocytes and appears on the surface of gametes as a complex with Pfs48/45 (18). Pfs48/45 is highly essential for male and female gamete fusion to form zygotes (19). A proportion of gametocytes die in the human host without being passed on to the mosquitoes, thereby exposing the gametocyte surface antigens to the human immune system. Naturally acquired anti-gametocyte immunity develops after repeated exposure to gametocytes. Based on the production of antibodies against numerous fragments covering Pfs230, Pfs230 domain 1 (Pfs230D1M) has been confirmed as the most robust region for antibody targeting (20). The functional Pfs48/45 antibodies bind to all three domains of Pfs48/45 protein with the strongest functional activity targeting epitopes in domains 1 and 3 (21). The most advanced P. falciparum post-fertilisation antigen, Pfs25 (22, 23) is expressed on zygotes and ookinetes, solely in the mosquito vector, but transcripts may occur in circulating gametocytes (24). Though Pfs25 is not a target of naturally acquired immunity in humans, vaccine-induced antibodies have been shown to inhibit oocyst development, which provides a basis for the development of Pfs25 as a TBV (25–27). Transmission-blocking antibodies have been shown to interrupt the fertilization of gametes in the mosquito midgut and the development of transmissible forms of the parasite (28–30).
Naturally acquired antibodies to Pfs230 and Pfs48/45 proteins have been shown to significantly reduce mosquito infection rates by >90% in standard membrane feeding assays (30). However, there is limited knowledge on the prevalence and variation of these antibodies in different malaria endemic settings and how they relate to factors that influence transmission, including age, parasitemia, and transmission intensity, among other factors. Assessment of naturally induced anti-gametocyte responses to parasite target proteins is of paramount importance for progressing the development of transmission-blocking vaccines, especially in malaria-endemic settings. Here, we determined the seroprevalence, levels, and potential functionality of naturally acquired antibodies to P. falciparum transmission-blocking target antigens Pfs230D1M and full-length Pfs48/45, as a baseline for a phase 1 clinical evaluation of a transmission-blocking vaccine candidate, Pfs25-IMX313/Matrix-M, in Bagamoyo, Tanzania.
2 Methods
2.1 Study area
The study was conducted in Bagamoyo District, located in the coastal region of Tanzania. The district represents heterogeneous malaria transmission, with malaria prevalence in the general population estimated to be between 7% and 39% (31, 32). Five sites in Bagamoyo District with varying malaria transmission intensities categorized following the guidelines of the U.S. President’s Malaria Initiative (PMI) (32) were surveyed: Bagamoyo township, Fukayosi, and Yombo with low to moderate (5% to 30% malaria infection prevalence) and Wami-Mkoko and Miono with high malaria transmission intensity (>30% malaria infection prevalence) (37). The malaria transmission intensity is usually high during and after the long rainy season (33), which usually occurs from March to June. The majority of the reported cases are caused by P. falciparum, but other Plasmodium species have also been reported (31, 34–36). Reported vector control practices employed in the study area are bed nets with 69% coverage (households with one bed net for every two persons) and house modification with 65% of the households with screened windows and 13% with closed eaves (38). There was no mass drug administration done in the study area.
2.2 Study design and population
A serosurvey was conducted between May and August 2022. A total number of 290 participants aged 5–45 years, without malaria clinical symptoms, were enrolled using simple random sampling from primary schools, peripheral dispensaries, and community-based malaria testing camps. Participants were invited for antibody and malaria testing following mass sensitization by village health teams. Among the 290 recruited participants, Pfs48/45 IgG was tested in all, Pfs230D1M and Pfs48/45 IgG in 281, and all three antigens (Pfs48/45, Pfs230D1M, Pfs25) in 117. Antibody persistence was assessed in 57 participants, sampled five times from July 2022 to March 2023, following baseline sampling in June 2022. Antibody functionality was assessed in 10 of the 57 participants via standard membrane feeding assays.
2.3 Blood sample collection and preservation
Blood samples were collected into 2.0-mL tubes containing ethylenediaminetetraacetic acid (EDTA). A volume of 200 µL was immediately preserved in 600 µL of 1× DNA/RNA Shield™ (Zymo Research, Irvine, USA) for nucleic acid extraction to detect circulating malaria parasites in each participant, using quantitative polymerase chain reaction (qPCR). A volume of 300 µL of the collected plasma was used for detecting total IgG antibodies against Pfs230D1M, Pfs48/45, and Pfs25 antigens. The remainder of the 2-mL blood sample was reserved for determining antibody functionality via standard membrane feeding assays.
2.4 Recombinant proteins
Production of both full-length Pfs48/45 (39) and Pfs25 (40) has been described previously (39, 40). A truncated recombinant version of Pfs230, termed Pfs230D1M (41), was based on the full-length 3D7 sequence (GenBank™ accession number XP_001349600.1), comprising residues Ser542 to Gly736 with a single Asn to Gln mutation at position 585 to remove an N-glycosylation site. Pfs230D1M was expressed in the laboratory as a secreted protein using the pPIC9K vector (Invitrogen, California, USA) in Pichia pastoris yeast (Invitrogen). All proteins contained a C-terminal EPEA motif to allow for the use of C-tag-based affinity chromatography (Thermo Fisher Scientific, UK) for the initial capture, followed by a gel filtration polishing step on a HiLoad 16/600 Superdex 200 PG column (Cytiva, Uppsala, Sweden).
2.5 The standardized enzyme-linked immunosorbent assay
The enzyme-linked immunosorbent assay (ELISA) was performed on plasma samples to detect and measure levels of total IgG to recombinant Pfs48/45, Pfs230D1M, and Pfs25 antigens following the protocol described previously (42). Briefly, ELISA plate wells were coated overnight with 50 μL of either Pfs48/45, Pfs230D1M, or Pfs25 recombinant proteins at a concentration of 2 μg/mL. The plates were blocked with StartingBlock™ T20 (PBS) Blocking Buffer (Thermo Fisher Scientific, UK) for 1 h before 50 μL of the diluted test plasma samples were added into the ELISA microtiter plate in triplicate. The plates were then incubated for 2 h at room temperature before a detecting antibody [goat anti-human IgG (γ-chain) conjugated to alkaline phosphatase] was added. The plates were incubated at room temperature for 1 h, and 100 µL of the pNPP with diethanolamine substrate was added to each well to produce fluorescence. The substrate was left to develop for 25 min, and the absorbance at 405 nm was read using the BioTek microplate reader (ELx808) and Gen5 software (v3.04). Between each incubation step, plates were washed six times with 1× PBS + 0.05% Tween 20. The ELISAs were performed using a standard curve and internal positive controls from reference samples. Known anti-Pfs25 serum from De Graaf et al. (43) was used as a reference positive control for Pfs25 ELISAs. The Pfs230D1M and Pfs48/45 reference plasmas from 20 donors (10 donors for each) were prepared separately as described previously (42). Briefly, Pfs230D1M or Pfs48/45 reactive plasma was pooled and optimized at a 1:800 dilution in StartingBlock™ T20 (PBS) Blocking Buffer. For each plate, a total of six wells were incubated with a positive control and negative control sera from unexposed United Kingdom (UK) donors. Wells containing no test sera were used to deduct background reactivity from each sample. A seropositivity cutoff point was set at an OD above the mean + 2SDs of a panel of UK donors. A standard curve and Gen5 ELISA software v3.04 (BioTek, UK) were used to convert the OD405 of individual test samples into arbitrary units (AU).
2.5.1 Antibody avidity determination by ELISA
Sodium thiocyanate (NaSCN, a chaotropic agent)-based avidity assays were conducted to estimate the avidity of antibody responses before the assessment of antibody functionality according to a protocol previously described (44). Briefly, each plasma was diluted based on standardized ELISA readouts to reach an optical density (OD) of 1. The plasma was added into ELISA plate wells coated overnight with 50 µL/well of 2 µg/mL Pfs230D1M or Pfs48/45 protein. Concentrations of the NaSCN chaotropic agent, ranging from 0 to 7 M, were added in increasing concentration down the plate. The plates were incubated at RT for 15 min, and a detecting secondary antibody was added, followed by pNPP with diethanolamine substrate (100 µL/well). The plates were allowed to develop before being read at 405 nm OD on BioTek ELx808 microplate reader with Gen5 software. Avidity index (mol/L) was determined as the concentration of NaSCN which gave 50% of OD values compared to no NaSCN. The binding of antibodies with lesser avidity to the antigen is disrupted at concentrations of NaSCN lower than for antibodies with greater avidity.
2.6 Ex vivo functionality of Pfs230D1M and/or Pfs48/45-positive IgG
The NF54 laboratory strain of P. falciparum gametocytes was used to evaluate the functional activity of Pfs230D1M and Pfs48/45 antibodies. Gametocyte cultures were maintained according to a previously published protocol (45). Briefly, cultures were initiated at an asexual parasitemia of 0.15%–0.2% and hematocrit of 5% in 10 mL complete medium (RPMI-1640 with 6 g/L of HEPES, 50 mg/L of hypoxanthine, 2.5 g/L of sodium bicarbonate, and 10% human serum). Cultures were maintained for 16 days under controlled gas conditions (90% nitrogen, 5% carbon dioxide, 5% oxygen), with daily media changes. More than three identical replicate cultures were maintained. For each experiment, at least two replicates were pooled based on stage V gametocytemia and exflagellation levels. The culture medium was replaced with normal human serum, normal red blood cells (RBCs) at a stage V gametocytemia of approximately 0.15%, and 50% hematocrit (1 mL of serum:1 mL of RBCs). The male-to-female gametocyte ratio was stabilized at 1:2–3. The human serum and RBCs used were obtained from Interstate Blood Bank, Inc., USA.
The ability of total IgG positive for Pfs230D1M and/or Pfs48/45 antibodies to reduce P. falciparum infections in mosquitoes was assessed using standard membrane feeding assays (SMFAs) as previously described in Miura et al. (45).
Total IgG from individual samples was purified using Protein G affinity chromatography and reconstituted to final concentrations of 40 mg/mL in PBS. The purified human IgG (at 10 mg/mL) was then mixed with NF54 P. falciparum gametocytes at 30°C and immediately fed to 4–6-day-old laboratory-reared Anopheles stephensi mosquitoes via a Parafilm® membrane. Mouse monoclonal antibody 4B7 at a concentration of 93.8 μg/mL was used as a positive control. The negative control was either a purified IgG (10 mg/mL) from pooled malaria naive human sera (SMFA-1) or that (0.75 mg/mL) from a pooled normal mouse serum (SMFA-2). The mosquito midguts were dissected 8 days post-feeding and examined microscopically to assess oocyst intensities in the test and control assays. A total of 20 blood-fed mosquitoes per volunteer were dissected for every SMFA round. Transmission-reducing activity (TRA), a measure of percentage (%) reduction in oocyst intensity, was calculated relative to the respective control IgG tested in the same assay. TRA was calculated as follows: 100 × {1 − (mean number of oocysts in the test IgG)/(mean number of oocysts in the control IgG)} (46). The average oocysts and % infectivity for the negative control IgGs were 34 oocysts per mosquito and 100% infectivity in SMFA-1 and 19 oocysts and 98% in SMFA-2.
2.7 Total parasites and gametocyte detection by RT-qPCR
Total parasitemia was determined by qPCR performed on the blood samples preserved in DNA/RNA Shield™ (47, 48), using CFX96 real-time qPCR thermocycler as detailed in Mulamba et al. (37). Genomic DNA was extracted from Plasmodium-positive blood samples using a Quick-DNA Miniprep Plus Kit (Zymo Research, USA) and eluted in 50 µL of elution buffer. The qPCR targeting the Pan-Plasmodium 18S rRNA and P. falciparum-specific varATS sequences was performed. This approach enhances both the specificity and sensitivity for detecting P. falciparum and non-falciparum Plasmodium species.
To determine gametocytemia, a combination of two independent Plasmodium targets—the female gametocyte-specific marker (CCp4) and the male gametocyte-specific marker (PfMGET) from Meerstein−Kessel et al.—was used (49). Ribonucleic acid (RNA) was extracted from all qPCR-positive samples, using the Quick-RNA™ MiniPrep Plus kit (Zymo Research). The protocol was adapted according to the manufacturer’s instructions as previously described (31). Plasmodium falciparum gametocytes were detected using a multiplex qRT-PCR assay described previously by Meerstein−Kessel et al. (49) as detailed in Mulamba et al. (37).
2.8 Statistical analysis
Data were recorded in Excel (Microsoft, 2016) and analyzed using Stata version 16 (50) and GraphPad Prism version 10 (GraphPad Software Inc., California, USA). Descriptive analysis was conducted on the demographic characteristics of the participants and the status (negative or positive) in relation to gametocyte and antibody presence using proportions and their respective 95% confidence intervals (CIs). The antibody responses were descriptively analyzed using geometric mean (95% confidence interval) and presented in tables. Pearson correlation coefficient (PCC) was used to assess the correlation between the log of parasite density and the log of antibody titers and the correlation between the titers of Pfs230D1M and Pfs48/45 antibodies. Binary logistic regression was used to estimate the association between antibody titers and the following factors: age groups (5–12, 13–17, and 18–45 years), sex (male and female), and location (low and high transmission) of the participants. The likelihood ratio test was also performed to assess the influence of each of the variables in the multivariate model. For the SMFA results, a zero-inflated negative binomial model was used (45). Binary logistic regression was also employed to assess the association between parasite positivity in relation to antibody seropositivity, with demographic characteristics of age groups, sex, and location of the participants adjusted for fixed effects. Furthermore, the change in Pfs230D1M and Pfs48/45 antibodies over time was explored using mixed-effect gamma regression, given the non-normal distribution of the data, and unique identification adjusted for random effects for the unadjusted models. The adjusted models included age groups, sex, and location of the participants as fixed effects.
3 Results
3.1 Study participants
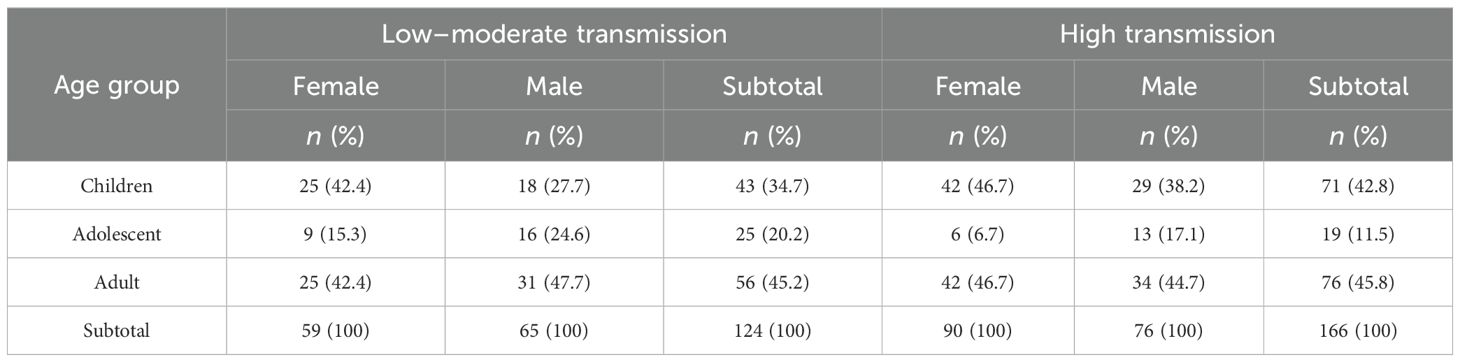
Of the 290 participants recruited, 114 (39%) were children (5–12 years), 44 (15%) adolescents (13–17 years), and 132 (46%) adults (18–45 years). The average age (years) in each age group was 9, 15, and 31 years, for children, adolescents, and adults, respectively. The proportion of female participants was 51%. Recruitment varied between low [124 (43%)] and high [166 (57%)] malaria transmission sites (Table 1).
3.2 Prevalence of anti-Pfs230D1M and anti-Pfs48/45 antibodies
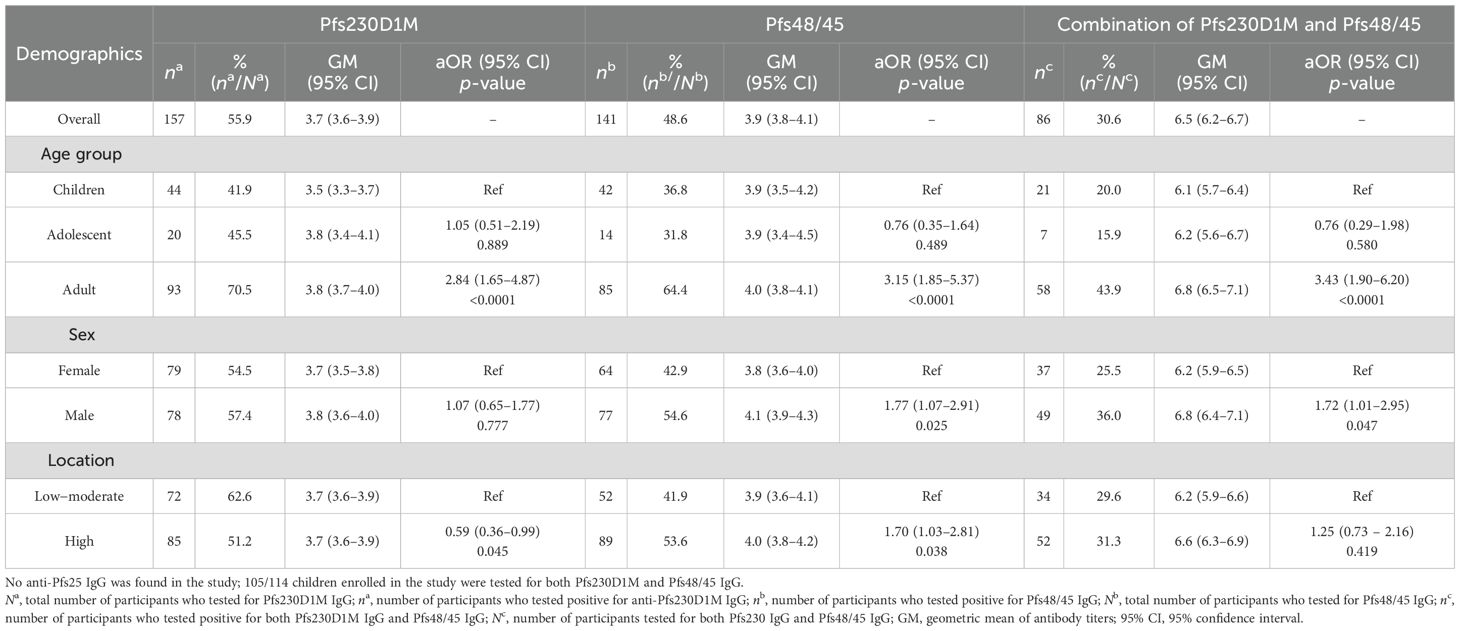
Overall, 56% (157/281) and 49% (141/290) of the participants were categorized as seropositive for anti-Pfs230D1M and anti-Pfs48/45 IgG, respectively. Approximately 30% (86/281) of the participants were seropositive for both Pfs230D1M and anti-Pfs48/45 IgG (Table 2). There was no anti-Pfs25 IgG detected among the 117 participants tested. The seroprevalence for anti-Pfs230D1M and anti-Pfs48/45 IgG increased significantly with participants’ age, with adults more likely to have antibodies than children—Pfs230D1M [adjusted OR 3.18 (95% CI: 1.85–5.57), p ≤ 0.0001] and Pfs45/48 [OR 3.18 (95% CI: 1.83–5.29), p ≤ 0.0001]—reflecting cumulative exposure to infection (Table 2). There was a significant positive correlation between titers for anti-Pfs48/45 and anti-Pfs230D1M IgG (R-value: 0.3021, p = 0.00001; Figure 1A).
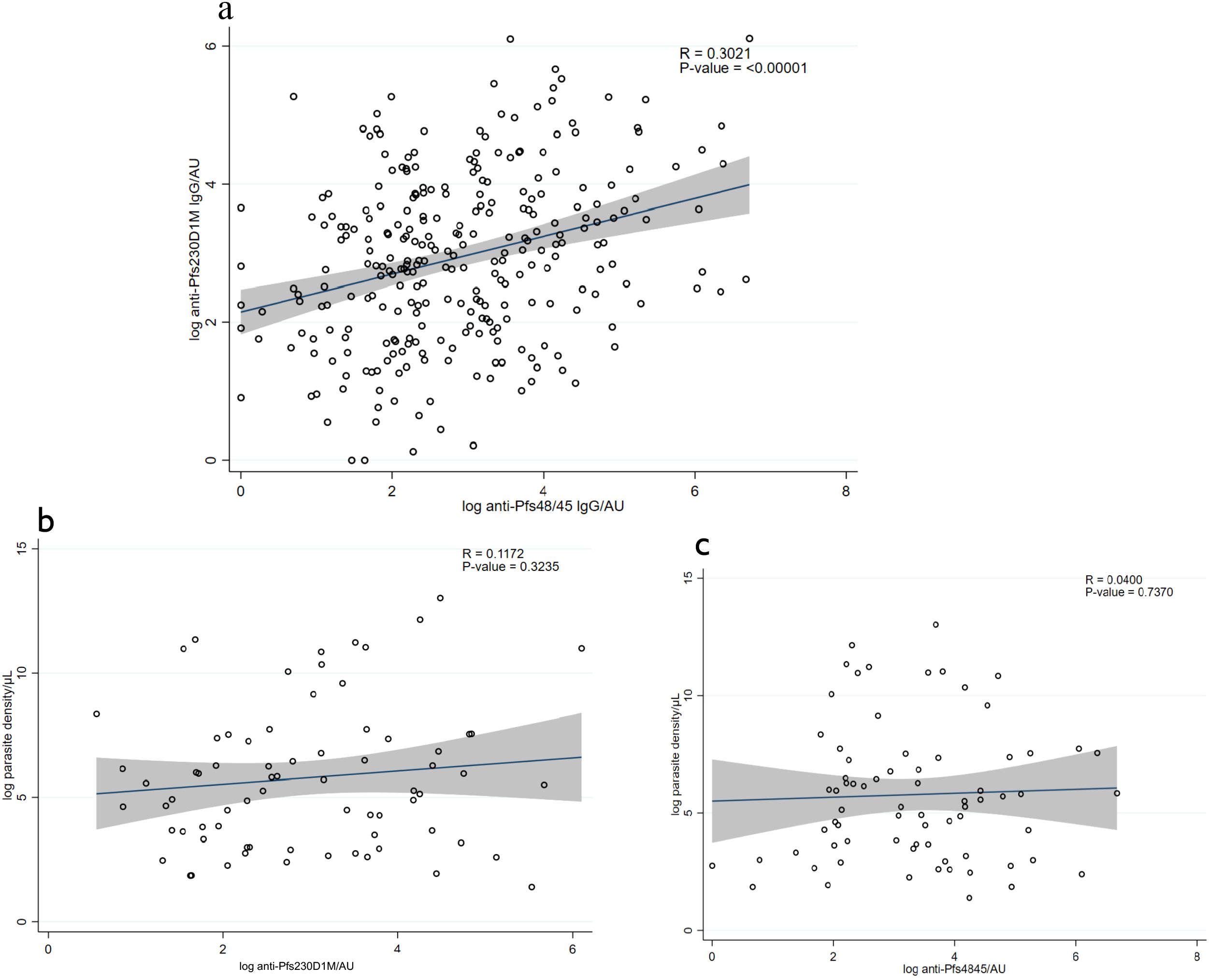
Figure 1. Correlation between antibody levels and parasite density. (A) Correlation between anti-Pfs230DIM antibody levels and anti-Pfs48/45 levels in the figure. (B) Correlation between anti-Pfs230D1M antibody levels and malaria parasite density. (C) Correlation between anti-Pfs48/45 levels and parasite density. The solid line shows the predicted mean from the linear regression, and the shaded area is the confidence interval.
3.3 Parasite positivity in relation to antibody seropositivity
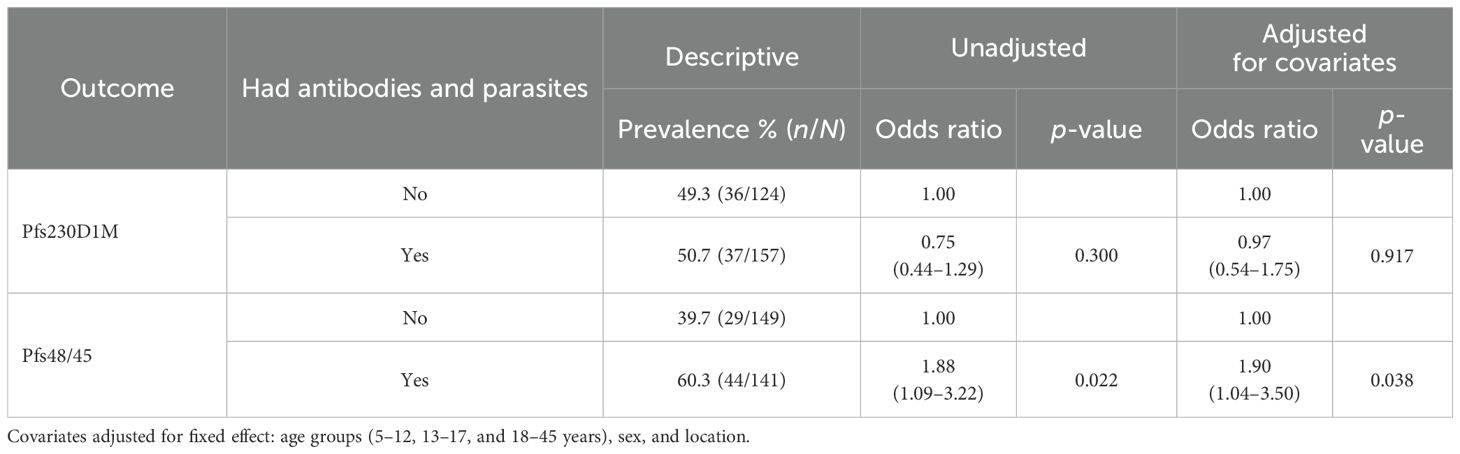
The total parasite and gametocyte positivity was 23.5% and 5.6%, respectively, and has already been published elsewhere (37). There was a significant association between Pfs48/45 and parasite positivity (Table 3).
3.3.1 Correlation between antibody levels and parasite density
There was no correlation between the antibody titers and total parasite density, as indicated in Figures 1B, C: Pfs230D1M antibody titers and parasite density (R-value: 0.1172, p-value = 0.3235) and anti-Pfs48/45 titers and parasite density (R-value: 0.0451; p-value = 0.7046).
3.4 Persistence of Pfs230D1M and Pfs48/45 antibodies over time
To assess the persistence of anti-Pfs230D1M and anti-Pfs48/45 over time, a group of 33 adults and 24 children, conveniently selected from high and low transmission areas, were sampled at five different time points spanning over a period of 10 months in addition to baseline (June 2022) sampling. Of the 57 participants included in the longitudinal analysis, 39 originated from high transmission areas and 18 from low transmission areas (Supplementary Table S1). We were unable to sample adolescents during the 10-month period. Seropositive participants at baseline remained seropositive at each of the later time points, and their geometric mean antibody responses are shown in Figure 2. There was no significant difference in Pfs230D1M antibody responses over time, while a significant decrease in titers for Pfs48/45 antibodies between August 2022 and March 2023 was shown. Participants who were seronegative at baseline remained seronegative at all time points and were not included in the analysis.

Figure 2. Geometric mean anti-Pfs230D1M and anti-Pfs48/45 IgG titer in seropositive adults and children over a 10-month-period: (A) anti-Pfs230D1M total IgG responses; n = 31 (8 children and 23 adults); (B) anti-Pfs48/45 total IgG responses; n = 26 (9 children and 17 adults), and antibody responses are shown on the left y-axes. The error bars indicate the lowest and highest antibody titer. Average precipitation at each time point is indicated by blue bars (right y-axes). The sampling time points were between June 2022 and March 2023.
3.5 Small proportion of participants positive for Pfs230D1M and Pfs48/45 antibodies showed significant TRA
Purified IgGs from baseline plasma samples from 10 participants reactive to Pfs230D1M and/or Pfs48/45 antigens by ELISA were individually tested for TRA via a single SMFA. The 10 participants selected were from high transmission areas and included two children and eight adults, for whom adequate plasma samples had been collected. Individual antibody responses and avidity indices of the 10 participants are shown in Figures 3A, B. Three participants (N006, N008, and N019) were reactive to both Pfs230D1M and Pfs48/45 antigens. Two participants (N001 and N021) were reactive to the Pfs48/45 antigen only. Five participants (N026, N055, N027, N028, and N036) were reactive to Pfs230D1M antigen only. Only two participants (N006, N008), who were adults, demonstrated significant TRA (Figure 3C; Supplementary Table S2). Independent SMFA performed for N006 and N008 confirmed their significant inhibitory activity (Figure 3D).
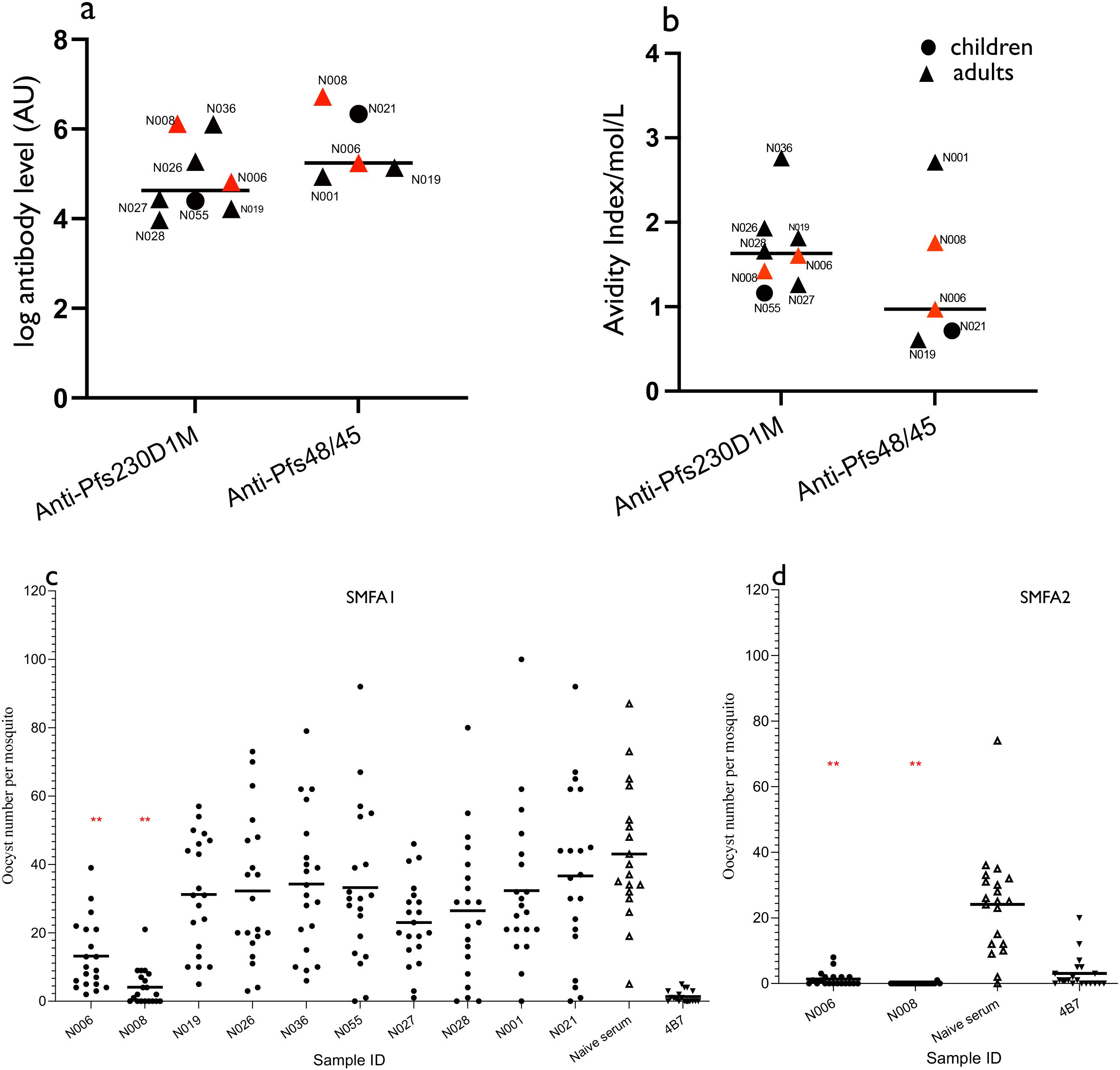
Figure 3. Antibody levels, antibody avidity, and oocyst intensity in participants assessed for antibody functionality. (A) Pfs230D1M and Pfs48/45 individual and median Pfs230D1M and Pfs48/45 antibody responses. (B) Individual and median avidity indices of total Pfs230D1M and Pfs48/45 IgG. Plasma samples were tested individually, and increasing concentrations of NaSCN (0, 1, 2, 3,4,5, 6, 7, and 8 M) were used prior to the development of the ELISA reaction. Results for both ID N006 and N008 are in red. IC50 = concentration of NaSCN needed to reduce antibodies by 50%. (C, D) Oocyst counts following SMFA with purified IgGs from Pfs230D1M and/or Pfs48/45-positive plasmas. Purified IgG (10 mg/mL) was mixed with P. falciparum NF54 cultured gametocytes and fed to mosquitoes (n = 20 per test assay), which were dissected 7 days post-feeding to determine oocyst intensity. Purified IgG from a pool of naive sera was used as negative control. Monoclonal Pfs25 antibodies (4B7) was used as the positive control. The asterisk means statistically significant difference (p-value < 0.05) between the negative control and test samples. All 10 samples were tested in SMFA1 (C), and only two samples were tested in SMFA2 (D).
4 Discussion
The present study is the first to determine the prevalence of naturally acquired antibodies to Pfs230D1M and Pfs48/45 transmission-blocking parasite proteins in Bagamoyo. These observations need to be taken into consideration in the planning of vaccine trials that have to account for pre-existing immunity and potential immune boosting following natural antigen exposure. We found strong evidence of naturally acquired antibody responses against P. falciparum gametocyte antigens—Pfs230D1M and Pfs48/45—as previously reported (28, 33, 51, 52). All the participants tested for anti-Pfs25-specific IgG were seronegative, most likely because Pfs25 transcripts are thought to be translated only in mosquito midgut post-blood ingestion.
The higher seroprevalence of Pfs230D1M and Pfs48/45 antibodies in adults compared to children is most likely due to repeated exposure to gametocytes over the years, as previously observed (28, 51). There was no significant difference in anti-Pfs48/45 and anti Pfs230D1M titers detected in children, adolescents, and adults, but a significant difference was observed in seroprevalence between areas of low and high transmission. This indicates a potential role for recent parasite exposure in the presence of Pfs48/45-specific immunity.
A large proportion of participants demonstrated positive anti-Pfs230D1M or anti-Pfs48/45 IgG responses in the absence of gametocytes or parasite infection at the time of sample collections. The detected anti-gametocyte responses were most likely resulting from immune responses from previous gametocyte exposure prior to sampling. This observation suggests that antibodies may serve as markers of gametocyte exposure, rather than biomarkers of active gametocytemia. However, 30%–40% of adults were ELISA-negative for Pfs230D1M or Pfs48/45 antigens, but it is highly likely that they have been infected multiple times. Concurrent gametocytes may reflect the development of anti-gametocyte responses, but a direct association may not always be observed (28, 51). Anti-gametocyte antibodies induced before sample collection may persist for several weeks after gametocyte clearance. On the other hand, antibody induction/boosting to circulating gametocytes at the time of sampling may take longer, e.g., after their death/destruction and subsequent clearance (33, 51).
The 10-month period over which anti-gametocyte responses were measured coincided with the end of a long rainy season and the start of the next rainy season. Data from this period indicated that both anti-Pfs230D1M and anti-Pfs48/45 IgG responses were stable, although this study did not answer whether the stable IgG responses were due to longevity of antibody response, repeat gametocyte exposures, or a mixture of both. More detailed longitudinal studies should be undertaken with a bigger sample size and larger age range to firmly establish the age dependency of anti-gametocyte malaria immunity as well as provide more evidence on the longevity of these responses.
The lack of significant inhibition of oocyst intensity by individuals who were reactive to either Pfs230 or Pfs48/45 antigens suggests that the antibodies could be binding to non-blocking epitopes or that the antibody levels were not sufficient to affect oocyst reduction in the mosquitoes. The observation of high transmission-reducing activity in the two samples reactive to both Pfs48/45 and Pfs230D1M antigens is similar to reports from previous field studies (33), and the observed TRA may be attributed to an additive or synergistic effect of antibodies to two different antigens. However, it is also possible that these two individuals were reactive to an unknown transmission-blocking antigen, and further investigations are required to fully understand the immune responses promoting the observed TRA in the two participants.
Several limitations were encountered in this study; the participants were recruited from only five locations, which were easily accessible, and this may have restricted the study area and sample size. In addition, we were unable to perform antibody depletion assays to ascertain if the TRA observed in the two participants was promoted by Pfs48/45 or Pfs230D1M IgG. Despite these limitations, the prevalence of naturally acquired antibodies to Pfs48/45 and Pfs230D1M in this study bodes well for the potential of vaccine-induced immunity against these antigens as suggested by other studies (53, 54). A recent phase 1 trial of Pfs48/45 and Pfs230 vaccine candidates in Burkina Faso found that vaccine-specific responses boosted pre-existing immunity against respective antigens (55), which contrasts prior findings that exposure to P. falciparum might diminish subsequent boosting by vaccination (56). These experiences indicate the need for transmission-blocking vaccine evaluation in malaria-endemic settings to take into account naturally acquired immune responses to the sexual-stage vaccine antigens. Naturally acquired antibody responses to both Pfs48/45 and Pfs230D1M proteins are highly prevalent and appear to be stable, suggesting that semi-immune populations may be ideal to evaluate boosting transmission-blocking immunity by transmission-blocking vaccine candidates.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Institutional Review Board (IRB) of Ifakara Health Institute (IHI/IRB/No: 29 -2021), and by the National Institute for Medical Research (NIMR), Tanzania (NIMR/HQ/R.8a/Vol. IX/3716). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for.
Author contributions
CM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing, Project administration, Resources, Supervision, Validation. WK: Data curation, Writing – review & editing. IM: Writing – review & editing. LL: Writing – review & editing. JK: Writing – review & editing. IN: Writing – review & editing. OO: Writing – review & editing. DM: Methodology, Resources, Validation, Writing – review & editing. AO: Project administration, Resources, Supervision, Writing – review & editing. CW: Conceptualization, Methodology, Resources, Supervision, Validation, Writing – review & editing. KK: Writing – review & editing, Supervision, Software. KM: Writing – review & editing, Data curation, Methodology, Conceptualization, Formal analysis, Validation, Resources. CL: Writing – review & editing, Methodology, Supervision, Funding acquisition, Resources.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. CM was awarded a PhD fellowship by the European Vaccine Initiative (EVI), an organization funded by the European & Developing Countries Clinical Trials Partnership (EDCTP) through the Multi-Stage Malaria Vaccine Consortium: field efficacy testing of a malaria vaccine targeting all four stages of the parasite’s life cycle (MMVC, Grant Agreement Number: RIA2016V-1649). The SMFA activity was supported by the Division of Intramural Research (DIR), National Institute of Allergy and Infectious Diseases (NIAID).
Acknowledgments
We are grateful to the volunteers for their participation in the study and to Dr. Mwajuma Chemba and teams at the IHI’s Clinical Trials Department, Immunology and Malaria Transmission Laboratories, for their assistance during fieldwork, data collection, and wet laboratory analysis. We also thank Prof. Sumi Biswas and Ms. Sarah Silk at the Jenner Institute, the University of Oxford, for their technical assistance with the standard curve setup during Pfs230D1M and Pfs48/45 ELISAs.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1589061/full#supplementary-material
Abbreviations
bp, base pairs; DNA, deoxyribonucleic acid; CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; NIMR, National Institute of Medical Research; nm, nanometer; OD, optical density; Pfs, Plasmodium falciparum surface antigen; qPCR, quantitative polymerase chain reaction; RBC, red blood cell; RNA, ribonucleic acid; qPCR, reverse transcription quantitative polymerase chain reaction; TBV, transmission-blocking vaccines; TRA, transmission-reducing activity; WHO, World Health Organization.
References
1. WHO. World malaria report 2020: 20 years of global progress and challenges. Geneva: World Health Organization (WHO). (2020).
6. PROGRAMME NMC. National malaria strategic plan 2014–2020: abridged version. Dar es salaam, Tanzania: Programme NMC. Dar es salaam The Government of Tanzania (2014).
7. Datoo MS, Dicko A, Tinto H, Ouédraogo J-B, Hamaluba M, Olotu A, et al. Safety and efficacy of malaria vaccine candidate R21/Matrix-M in African children: a multicentre, double-blind, randomised, phase 3 trial. Lancet. (2024) 403:533–44. doi: 10.1016/S0140-6736(23)02511-4
8. RTS, S Clinical Trials Partnership. Efficacy and safety of RTS, S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. (2015) 386:31–45. doi: 10.1016/S0140-6736(15)60721-8
9. White MT, Verity R, Griffin JT, Asante KP, Owusu-Agyei S, Greenwood B, et al. Immunogenicity of the RTS, S/AS01 malaria vaccine and implications for duration of vaccine efficacy: secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect Dis. (2015) 15:1450–8. doi: 10.1016/S1473-3099(15)00239-X
10. Brod F, Miura K, Taylor I, Li Y, Marini A, Salman AM, et al. Combination of RTS, S and Pfs25-IMX313 induces a functional antibody response against malaria infection and transmission in mice. Front Immunol. (2018) 9:2780. doi: 10.3389/fimmu.2018.02780
11. Smith RC, Vega-Rodríguez J, and Jacobs-Lorena M. The Plasmodium bottleneck: malaria parasite losses in the mosquito vector. Memórias do Instituto Oswaldo Cruz. (2014) 109:644–61. doi: 10.1590/0074-0276130597
12. Reuling IJ, Van De Schans LA, Coffeng LE, Lanke K, Meerstein-Kessel L, Graumans W, et al. A randomized feasibility trial comparing four antimalarial drug regimens to induce Plasmodium falciparum gametocytemia in the controlled human malaria infection model. Elife. (2018) 7:e31549. doi: 10.7554/eLife.31549
13. Acquah FK, Adjah J, Williamson KC, and Amoah LE. Transmission-blocking vaccines: old friends and new prospects. Infection Immun. (2019) 87:e00775–00718. doi: 10.1128/IAI.00775-18
14. Gerloff DL, Creasey A, Maslau S, and Carter R. Structural models for the protein family characterized by gamete surface protein Pfs230 of Plasmodium falciparum. Proc Natl Acad Sci. (2005) 102:13598–603. doi: 10.1073/pnas.0502378102
15. Tibúrcio M, Niang M, Deplaine G, Perrot S, Bischoff E, Ndour PA, et al. A switch in infected erythrocyte deformability at the maturation and blood circulation of Plasmodium falciparum transmission stages. Blood J Am Soc Hematol. (2012) 119:e172–80. doi: 10.1182/blood-2012-03-414557
16. Carter R, Miller L, Rener J, Kaushal D, Kumar N, Graves PM, et al. Target antigens in malaria transmission blockling immunity. Philos Trans R Soc London. B Biol Sci. (1984) 307:201–13. doi: 10.1098/rstb.1984.0120
17. Vermeulen AN, Ponnudurai T, Beckers P, Verhave J, Smits M, and Meuwissen J. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J Exp Med. (1985) 162:1460–76. doi: 10.1084/jem.162.5.1460
18. Kumar N. Target antigens of malaria transmission blocking immunity exist as a st able membrane bound complex. Parasite Immunol. (1987) 9:321–35. doi: 10.1111/j.1365-3024.1987.tb00511.x
19. van Dijk MR, Janse CJ, Thompson J, Waters AP, Braks JA, Dodemont HJ, et al. A central role for P48/45 in malaria parasite male gamete fertility. Cell. (2001) 104:153–64. doi: 10.1016/S0092-8674(01)00199-4
20. Tachibana M, Miura K, Takashima E, Morita M, Nagaoka H, Zhou L, et al. Identification of domains within Pfs230 that elicit transmission blocking antibody responses. Vaccine. (2019) 37:1799–806. doi: 10.1016/j.vaccine.2019.02.021
21. Fabra-García A, Hailemariam S, de Jong RM, Janssen K, Teelen K, van de Vegte-Bolmer M, et al. Highly potent, naturally acquired human monoclonal antibodies against Pfs48/45 block Plasmodium falciparum transmission to mosquitoes. Immunity. (2023) 56:406–419. e407. doi: 10.1016/j.immuni.2023.01.009
22. Nikolaeva D, Draper SJ, and Biswas S. Toward the development of effective transmission-blocking vaccines for malaria. Expert Rev Vaccines. (2015) 14:653–80. doi: 10.1586/14760584.2015.993383
23. Wu Y, Sinden RE, Churcher TS, Tsuboi T, and Yusibov V. Development of malaria transmission-blocking vaccines: from concept to product. Adv Parasitol. (2015) 89:109–52. doi: 10.1016/bs.apar.2015.04.001
24. Miao J, Fan Q, Parker D, Li X, Li J, and Cui L. Puf mediates translation repression of transmission-blocking vaccine candidates in malaria parasites. PloS Pathog. (2013) 9:e1003268. doi: 10.1371/journal.ppat.1003268
25. Duffy PE and Kaslow DC. A novel malaria protein, Pfs28, and Pfs25 are genetically linked and synergistic as falciparum malaria transmission-blocking vaccines. Infection Immun. (1997) 65:1109–13. doi: 10.1128/iai.65.3.1109-1113.1997
26. Stowers AW, Keister DB, Muratova O, and Kaslow DC. A region of Plasmodium falciparum antigen Pfs25 that is the target of highly potent transmission-blocking antibodies. Infect Immun. (2000) 68:5530–8. doi: 10.1128/IAI.68.10.5530-5538.2000
27. Gozar MMG, Price VL, and Kaslow DC. Saccharomyces cerevisiae-secreted fusion proteins Pfs25 and Pfs28 elicit potent Plasmodium falciparum transmission-blocking antibodies in mice. Infection Immun. (1998) 66:59–64. doi: 10.1128/IAI.66.1.59-64.1998
28. Drakeley C, Bousema J, Akim N, Teelen K, Roeffen W, Lensen A, et al. Transmission-reducing immunity is inversely related to age in Plasmodium falciparum gametocyte carriers. Parasite Immunol. (2006) 28:185–90. doi: 10.1111/j.1365-3024.2005.00818.x
29. Bousema JT, Roeffen W, Kolk M, de Vlas SJ, van de Vegte-Bolmer M, MJ B, et al. Rapid onset of transmission-reducing antibodies in javanese migrants exposed to malaria in papua, Indonesia. Am J Trop Med Hyg. (2006) 74(3):425–31. doi: 10.4269/ajtmh.2006.74.425
30. Stone WJ, Dantzler KW, Nilsson SK, Drakeley CJ, Marti M, Bousema T, et al. Naturally acquired immunity to sexual stage P. falciparum parasites. Parasitol. (2016) 143:187–98. doi: 10.1017/S0031182015001341
31. Hofer LM, Kweyamba PA, Sayi RM, Chabo MS, Maitra SL, Moore SJ, et al. Malaria rapid diagnostic tests reliably detect asymptomatic Plasmodium falciparum infections in school-aged children that are infectious to mosquitoes. Parasites Vectors. (2023) 16:217. doi: 10.1186/s13071-023-05761-w
33. Bousema T, Roeffen W, Meijerink H, Mwerinde H, Mwakalinga S, van Gemert G-J, et al. The dynamics of naturally acquired immune responses to Plasmodium falciparum sexual stage antigens Pfs230 & Pfs48/45 in a low endemic area in Tanzania. PloS One. (2010) 5:e14114. doi: 10.1371/journal.pone.0014114
34. Mwangonela Z. Field evaluation of a novel one step malaria pf and pf/pv rapid diagnostic tests in Pwani region. Tanzania: NM-AIST (2023).
35. Tarimo BB, Nyasembe VO, Ngasala B, Basham C, Rutagi IJ, Muller M, et al. Seasonality and transmissibility of Plasmodium ovale in Bagamoyo District, Tanzania. Parasites Vectors. (2022) 15:1–6. doi: 10.1186/s13071-022-05181-2
36. Popkin-Hall ZR, Seth MD, Madebe RA, Budodo R, Bakari C, Francis F, et al. Malaria species positivity rates among symptomatic individuals across regions of differing transmission intensities in Mainland Tanzania. J Infect Dis. (2024) 229(4):959–68. doi: 10.1093/infdis/jiad522
37. Mulamba C, Odufuwa OG, Kweyamba PA, Lazaro LO, Chabo MS, Kamage JJ, et al. Plasmodium falciparum gametocyte burden in a Tanzanian heterogeneous transmission setting. Malaria J. (2025) 24:54. doi: 10.1186/s12936-025-05270-4
38. Odufuwa OG, Ross A, Mlacha YP, Juma O, Mmbaga S, Msellemu D, et al. Household factors associated with access to insecticide-treated nets and house modification in Bagamoyo and Ulanga districts, Tanzania. Malaria J. (2020) 19:1–13. doi: 10.1186/s12936-020-03303-8
39. Ko K-T, Lennartz F, Mekhaiel D, Guloglu B, Marini A, Deuker DJ, et al. Structure of the malaria vaccine candidate Pfs48/45 and its recognition by transmission blocking antibodies. Nat Commun. (2022) 13:5603. doi: 10.1038/s41467-022-33379-6
40. Li Y, Leneghan DB, Miura K, Nikolaeva D, Brian IJ, Dicks MD, et al. Enhancing immunogenicity and transmission-blocking activity of malaria vaccines by fusing Pfs25 to IMX313 multimerization technology. Sci Rep. (2016) 6:18848. doi: 10.1038/srep18848
41. MacDonald NJ, Nguyen V, Shimp R, Reiter K, Herrera R, Burkhardt M, et al. Structural and immunological characterization of recombinant 6-cysteine domains of the Plasmodium falciparum sexual stage protein Pfs230. J Biol Chem. (2016) 291:19913–22. doi: 10.1074/jbc.M116.732305
42. Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A, and Long CA. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine. (2008) 26:193–200. doi: 10.1016/j.vaccine.2007.10.064
43. De Graaf H, Payne RO, Taylor I, Miura K, Long CA, Elias SC, et al. Safety and immunogenicity of ChAd63/MVA Pfs25-IMX313 in a phase I first-in-human trial. Front Immunol. (2021) 12:694759. doi: 10.3389/fimmu.2021.694759
44. Pullen G, Fitzgerald MG, and Hosking C. Antibody avidity determination by ELISA using thiocyanate elution. J Immunol Methods. (1986) 86:83–7. doi: 10.1016/0022-1759(86)90268-1
45. Miura K, Deng B, Tullo G, Diouf A, Moretz SE, Locke E, et al. Qualification of standard membrane-feeding assay with Plasmodium falciparum malaria and potential improvements for future assays. PloS One. (2013) , 8. doi: 10.1371/journal.pone.0057909
46. Miura K, Swihart BJ, Deng B, Zhou L, Pham TP, Diouf A, et al. Transmission-blocking activity is determined by transmission-reducing activity and number of control oocysts in Plasmodium falciparum standard membrane-feeding assay. Vaccine. (2016) 34:4145–51. doi: 10.1016/j.vaccine.2016.06.066
47. Kamau E, Alemayehu S, Feghali KC, Saunders D, and Ockenhouse CF. Multiplex qPCR for detection and absolute quantification of malaria. PloS One. (2013) 8:e71539. doi: 10.1371/journal.pone.0071539
48. Hofmann N, Mwingira F, Shekalaghe S, Robinson LJ, Mueller I, and Felger I. Ultra-sensitive detection of Plasmodium falciparum by amplification of multi-copy subtelomeric targets. PloS Med. (2015) 12:e1001788. doi: 10.1371/journal.pmed.1001788
49. Meerstein-Kessel L, Andolina C, Carrio E, Mahamar A, Sawa P, Diawara H, et al. A multiplex assay for the sensitive detection and quantification of male and female Plasmodium falciparum gametocytes. Malaria J. (2018) 17:1–11. doi: 10.1186/s12936-018-2584-y
51. Bousema J, Drakeley C, Kihonda J, Hendriks J, Akim N, Roeffen W, et al. A longitudinal study of immune responses to Plasmodium falciparum sexual stage antigens in Tanzanian adults. Parasite Immunol. (2007) 29:309–17. doi: 10.1111/j.1365-3024.2007.00948.x
52. Ouédraogo AL, Roeffen W, Luty AJ, de Vlas SJ, Nebie I, Ilboudo-Sanogo E, et al. Naturally acquired immune responses to Plasmodium falciparum sexual stage antigens Pfs48/45 and Pfs230 in an area of seasonal transmission. Infection Immun. (2011) 79:4957–64. doi: 10.1128/IAI.05288-11
53. Theisen M, Roeffen W, Singh SK, Andersen G, Amoah L, van de Vegte-Bolmer M, et al. A multi-stage malaria vaccine candidate targeting both transmission and asexual parasite life-cycle stages. Vaccine. (2014) 32:2623–30. doi: 10.1016/j.vaccine.2014.03.020
54. Farrance CE, Rhee A, Jones RM, Musiychuk K, Shamloul M, Sharma S, et al. A plant-produced Pfs230 vaccine candidate blocks transmission of Plasmodium falciparum. Clin Vaccine Immunol. (2011) 18:1351–7. doi: 10.1128/CVI.05105-11
55. Tiono AB, Plieskatt JL, Ouedraogo A, Soulama BI, Miura K, Bougouma EC, et al. A randomized first-in-human phase I trial of differentially adjuvanted Pfs48/45 malaria vaccines in Burkinabé adults. J Clin Invest. (2024) 134. doi: 10.1172/JCI175707
Keywords: malaria transmission, transmission-blocking vaccines, Plasmodium falciparum, gametocytes, Tanzania, Pfs25, Pfs230D1M, and Pfs48/45
Citation: Mulamba C, Kalinga WF, Mtaka I, Lazaro LO, Kamage J, Nkumama I, Odufuwa OG, Kreppel K, Mekhaiel D, Miura K, Long CA, Olotu AI and Williams C (2025) Seroprevalence of antibodies to Plasmodium falciparum transmission-blocking target proteins Pfs230D1M and Pfs48/45 in Tanzanian populations of diverse malaria transmission intensity. Front. Immunol. 16:1589061. doi: 10.3389/fimmu.2025.1589061
Received: 06 March 2025; Accepted: 01 August 2025;
Published: 11 September 2025.
Edited by:
Takafumi Tsuboi, Ehime University, JapanReviewed by:
Wang Nguitragool, Mahidol University, ThailandJordan Plieskatt, State Serum Institute (SSI), Denmark
Copyright © 2025 Mulamba, Kalinga, Mtaka, Lazaro, Kamage, Nkumama, Odufuwa, Kreppel, Mekhaiel, Miura, Long, Olotu and Williams. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chris Williams, Y2hyaXMud2lsbGlhbXNAbmRtLm94LmFjLnVr
 Charles Mulamba
Charles Mulamba Wilmina F. Kalinga
Wilmina F. Kalinga Ivanny Mtaka
Ivanny Mtaka Linda O. Lazaro2
Linda O. Lazaro2 Olukayode G. Odufuwa
Olukayode G. Odufuwa David Mekhaiel
David Mekhaiel Ally I. Olotu
Ally I. Olotu Chris Williams
Chris Williams