- 1Department of Gastrointestinal Surgery, The First Hospital of China Medical University, Shenyang, Liaoning, China
- 2Shenyang Medical Nutrition Clinical Medical Research Center, Shenyang, Liaoning, China
- 3Department of Pulmonary and Critical Care Medicine, Institute of Respiratory Disease, The First Hospital of China Medical University, Shenyang, Liaoning, China
Colorectal cancer (CRC) has the highest incidence in the Asia-Pacific region, accounting for 51.8% of global cases. Despite early screening methods like colonoscopy, CT, and MRI, 20-25% of patients are diagnosed at advanced stages, with some having liver metastasis. Personalized treatments, including targeted and immunotherapy, are crucial for metastatic or recurrent CRC. Circulating tumor cells (CTC), emerging as a non-invasive biomarker, play a key role in assessing metastasis and prognosis. CTC count is linked to CRC stage, microsatellite instability (MSI-H), and drug resistance, and is valuable in evaluating the response to immune checkpoint inhibitors (ICIs). Immune cells in the tumor microenvironment influence CTC behavior, impacting metastasis, immune evasion, and resistance. Advances in CTC detection and genetic markers offer new possibilities for early diagnosis and personalized treatment.
1 Introduction
Colorectal cancer (CRC) incidence is the highest in the Asia-Pacific region, accounting for 51.8% of the global burden. With a growing population, a high Human Development Index (HDI), and rapid economic growth, the region faces an increasing challenge (1). Early screening methods, including colonoscopy, abdominal computed tomography (CT), and magnetic resonance imaging (MRI), can reduce the risk of colorectal cancer (2–4). Approximately 20-25% of patients are diagnosed at stage IV during their initial examination, with 10-15% presenting with colorectal cancer liver metastasis (CRCLM) (5). Additionally, among patients undergoing curative surgery for CRC, about 40% experience recurrence, primarily in the form of either local or distant metastasis (6, 7). For patients with metastatic or recurrent CRC, personalized treatment options, such as targeted therapy or immunotherapy, are essential for preventive treatment (8, 9). Biomarkers to guide the selection of the most appropriate therapy include tumor histology, such as KRAS/BRAF mutations, HER2 amplification, and microsatellite instability-high (MSI-H). Previous assessments were based on postoperative pathology. In contrast, liquid biopsy (LB) and circulating tumor cell (CTC) assessment offer a non-invasive and easily accessible technique that can improve personalized treatment before surgery (10).
Circulating tumor cells (CTCs) are emerging tumor biomarkers, referring to somatic cells that detach from the primary tumor and migrate into the circulatory system, which can lead to liver metastasis via the hepatic portal vein (11). CTC count has been established as an independent prognostic factor for patients with metastatic CRC (12). CTCs were first discovered by Thomas Ashworth in 1869, but it was not until the 1970s, with the rapid development of molecular biology technologies, that the enrichment and characterization of CTCs became feasible (13). Furthermore, the NCCN guidelines recognize the importance of CTCs in preoperative screening for central nervous system cancers, as well as in guiding treatment decisions for advanced prostate and breast cancers (14–17).
Immunotherapy for colorectal cancer (CRC) works by blocking immune checkpoint (IC) pathways. Cancer cells can disguise themselves as normal cells through the IC pathways (Figure 1) (18). Tumor cells can express inhibitory ligands such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death ligand 1 (PD-L1), which send “stop” signals to active T cells, enabling the tumor to escape cell-mediated immunity. These studies have driven medical advancements, ushering in the era of precision medicine. The development of monoclonal antibodies targeting PD-1 (nivolumab and pembrolizumab) and PD-L1 (durvalumab and atezolizumab) has made enhanced antitumor immunity possible. Immunotherapy can improve clinical outcomes and extend overall survival (OS) (19, 20). However, only a small subset of CRC patients can benefit from immune checkpoint therapy (ICT) (21). Only those with MSI-H or defective mismatch repair (dMMR) in their CRC tumors demonstrate favorable treatment responses (22). This is due to insertions or deletions of nucleotides that can lead to DNA or microsatellite repeats. The accumulation of these mutations results in the generation of novel neoantigens, which can be recognized by the host immune system (20). Therefore, preoperative screening to identify patients who are suitable for immune checkpoint inhibitors (ICIs) treatment is crucial for the management of advanced colorectal cancer.
2 Clinical significance of circulating tumor cells in colorectal cancer
2.1 The relationship between MSI-H and CTCs
Colorectal cancers (CRCs) with microsatellite instability-high (MSI-H) are considered to have a better prognosis. The level of MSI-H in CRC is associated with the extent of tumor-infiltrating lymphocytes (TILs). The presence of TILs may partially restrict tumor cell metastasis, potentially by reducing the release of CTCs (23). Microsatellites refer to short tandem repeat sequences scattered throughout the genome (comprising 1–6 or more base pairs, typically repeated 5 to 50 times). When base-pair mismatches or replication errors occur frequently, they are termed microsatellite instability. The accumulation of genetic mutations produces additional tumor antigens, enabling the possibility of immunotherapy. The incidence of MSI-H in CRC is approximately 10–15% (24). However, in clinical practice, the detection rate of MSI-H may fall below 10%, attributed to the high costs and technical complexity of microsatellite testing. This results in many patients missing the opportunity for immunotherapy. Several recent studies have demonstrated the feasibility of identifying immunotherapy-eligible patients more affordably and efficiently by analyzing circulating tumor cells in the blood. Immunotherapy involves the use of immune checkpoint inhibitors (ICIs) to specifically block immune checkpoints such as PD-L1, CTLA-4, and CD47, thereby disrupting the immunosuppressive tumor microenvironment (25).
2.2 Perioperative CTC dynamics and MSI status differences
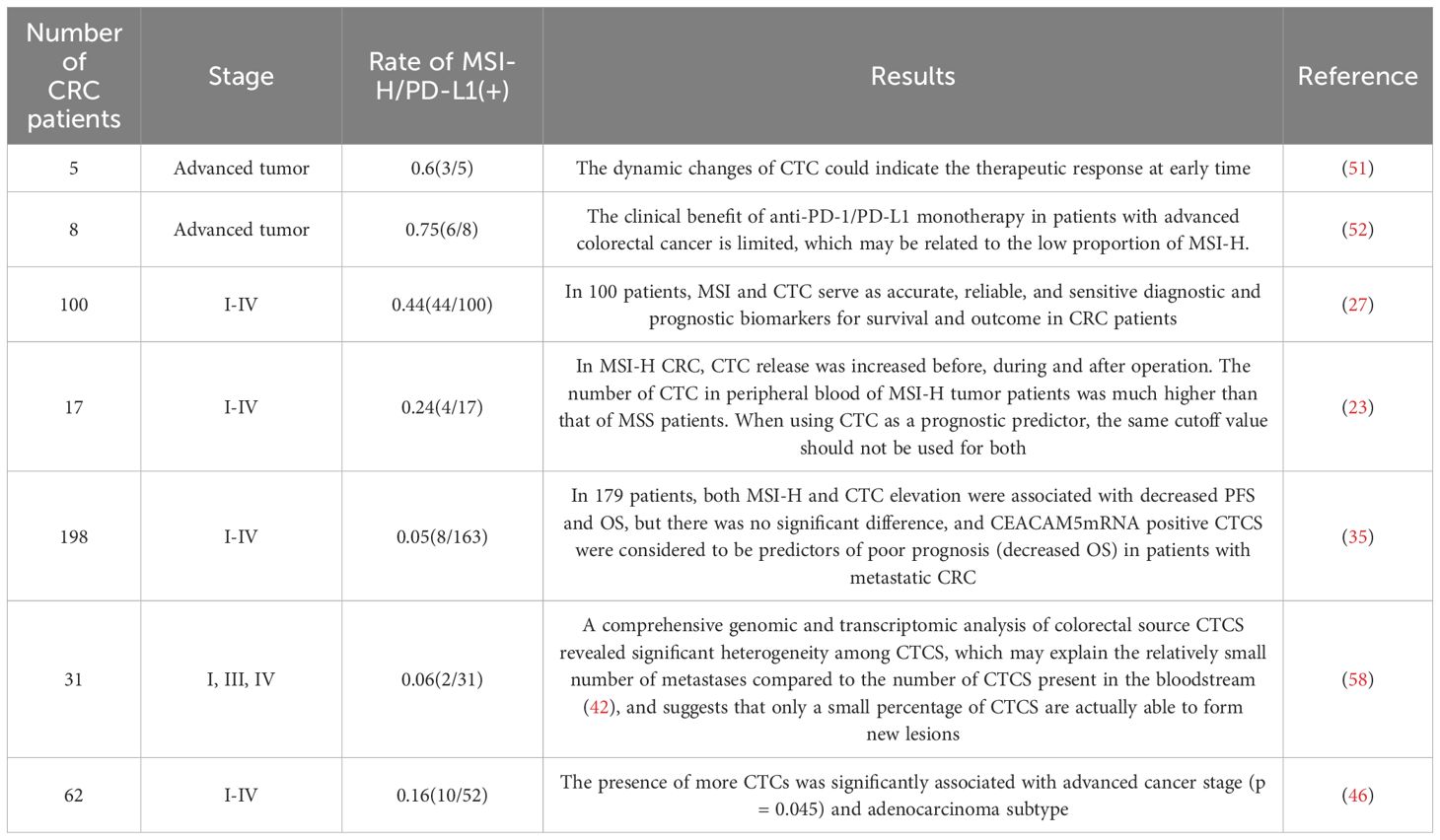
The count of circulating tumor cells (CTCs) correlates with tumor stage, showing statistically significant differences in peripheral blood measurements at various stages and time points (preoperative, intraoperative, postoperative) (26). Notably, the dynamic trends of perioperative CTC counts differed significantly between patients with MSI-H tumors and those with microsatellite−stable (MSS) tumors. The mechanical manipulation–induced tumor cell shedding effect during surgery was pronounced in the MSI-H subtype, with a median CTC count of 37.8, which was significantly higher than the 23.7 observed in the MSS group (P = 0.0328). Postoperative dynamic monitoring revealed a rapid decline in CTC counts from 24 hours to one month after surgery in MSI-H patients, whereas MSS patients exhibited persistently low-level fluctuations or no significant change (23, 27). Overall, perioperative CTC counts were higher in MSI-H patients compared to MSS patients (27).
This finding contradicts intuition, as MSI-H is associated with better survival outcomes, while a CTC count >3 is linked to poorer prognosis. Toh JWT et al. showed the median CTC count for 13 MSS colorectal cancer (CRC) patients at preoperative, intraoperative, and postoperative time points was 1. Conversely, MSI-H CRC patients had median CTC counts exceeding 10 at all measured time points. This paradoxical result was not fully explained in the study. The authors proposed a hypothesis: CTCs shed from MSI-H tumors retain microsatellite instability and carry more immunogenic antigens, potentially eliciting stronger immune responses in peripheral blood and enhancing lymphocyte immunogenicity (23). Studies have indicated that although peripheral CTCs in MSI−H CRC patients are relatively more abundant, their “quality” and functional status may differ from those in MSS patients: CTCs originating from MSI−H tumors harbor indel−induced frameshift mutations that profoundly alter protein amino acid sequences, endowing them with highly immunogenic neoantigens that are readily recognized and cleared by activated T cells, and their survival and metastatic potential may be lower than those of MSS−derived CTCs (28). High PD-L1 expression on the surface of CTCs can bind to PD-1 on T cells, terminating downstream T-cell receptor (TCR) signaling and CD28 co-stimulation, thereby transiently suppressing naïve effector T cells, though its suppressive effect on memory T cells is limited. Consequently, truly metastatic CTC clones may be effectively eliminated by memory T cells (29, 30). Moreover, tumor cell stemness characteristics and inflammatory cytokines (e.g., TNFα, IL-6) promote upregulation of adhesion molecules on tumor cells, facilitating the formation of CTC clusters in peripheral blood (31, 32). Within CTC clusters derived from MSI−H tumors, heterogeneous tumor mutation burdens (TMB) aggregate, leading to clusters containing increased apoptotic markers, which may limit their distant metastatic potential (33).
2.3 MSI-dependent prognostic utility of CTCs
This raises important questions: Do CTCs shed from MSI-H and MSS CRC patients have equivalent metastatic potential? Should MSI-H and MSS patients share the same CTC cutoff values? Nearly all clinical studies employ a uniform threshold, namely the FDA-approved CellSearch criterion of ≥3 CTCs per 7.5 mL of blood as an adverse prognostic indicator in metastatic CRC (34). Alsayed et al. proposed that postoperative CTC levels remaining ≥4 cells per 7 mL of blood constitute an independent prognostic factor for overall survival (OS) (27). Toh et al. found that a preoperative median CTC count >10 in MSI-H patients remained associated with favorable prognosis, whereas >3 CTCs in MSS patients indicated adverse outcomes (23). In practice, investigators may stratify CTC counts into categories of 0 vs. ≥1, 3, 4, or 5 (depending on study design), but no studies have specifically calibrated or stratified these cutoffs by MSI subtype. However, Messaritakis et al. developed a molecular assay for CTC detection based on carcinoembryonic antigen-like cellular adhesion molecule 5 (CEACAM5) (35). They found that CEACAM5 mRNA–positive circulating tumor cells (CTCs) were associated with reduced overall survival (11.2 months vs. 19.6 months) and poorer clinical outcomes in patients with MSI−H metastatic CRC (mCRC). Although current evidence for MSI-H CRC is limited, existing studies suggest that CEACAM5-positive CTCs in MSI-H patients may predict poorer clinical outcomes. And post-treatment reduction in CTC burden may be associated with improved prognosis. Therefore, stratifying patients solely on the basis of a CTC count >3 cells/mL—without accounting for MSI status—is inadvisable. In MSI-high colorectal cancer patients, the clinical value of CTC enumeration should be interpreted in conjunction with phenotypic and molecular characteristics of the circulating tumor cells.
Given the limited number and small sample sizes of current studies, the overall evidence remains incomplete. Large-scale, prospective studies are needed to validate whether dynamic changes in CTCs during treatment (including pre- and post-surgery, chemotherapy, or immunotherapy) can reliably predict recurrence risk, progression-free survival, or overall survival in MSI-H CRC patients. Additionally, development of more CTC-related biomarkers is necessary to assess the metastatic and invasive potential of individual shed tumor cells.
Current prognostic models based on CTC counts (e.g., ≥3 per 7.5 mL) do not distinguish MSI status, potentially leading to over-risk stratification of MSI-H patients. In clinical practice, management of MSI-H colorectal cancer patients should incorporate CTC functional characteristics (e.g., PD-L1 expression or CEACAM5 mRNA positivity) and dynamic monitoring of CTC count changes pre- and postoperatively as well as before and after adjuvant therapy to optimize personalized management strategies. Ultimately, integrating these functional CTC assessments with traditional clinicopathological factors will enable more accurate risk stratification, reducing unnecessary interventions in low-risk MSI H individuals and ensuring high-risk patients receive timely escalation of care.
2.4 Tumor site and CTC biological characteristics
Tumor location also influences CTC counts, a phenomenon observed in many clinical studies. Left- and right-sided colon cancers differ in tumor characteristics due to disparities in embryological origin, gene expression, and clinical behavior. From an embryological perspective, the demarcation between the left and right colon lies at the distal third of the transverse colon (36). During the fourth week of gestation, the endoderm of the fetus folds and rotates, forming the foregut (blind-ending cranially), hindgut (blind-ending caudally), and midgut, which remains open to the yolk sac via the vitelline duct. The midgut develops into the jejunum, ileum, cecum, ascending colon, and two-thirds of the transverse colon. The hindgut forms the remaining third of the transverse colon, descending colon, and sigmoid colon (37). Anatomically, the right colon is primarily supplied by the superior mesenteric artery, while the left colon is perfused by the inferior mesenteric artery. At the genetic level, right-sided colon cancers often exhibit distinct genetic mutations, higher PD-L1 expression, and elevated microsatellite instability, which may lead to increased infiltration of CD8+ tumor-infiltrating lymphocytes (TILs). Notably, stage II right-sided colon cancers have a higher likelihood of MSI-H (38).
3 The role of CTCs in immunotherapy
3.1 Expression of PD-L1 in CTCs and mechanisms of immune resistance
Programmed death-ligand 1 (PD-L1), an immunosuppressive protein, is regulated by colorectal tumors (39). PD-L1 expression is fundamentally regulated by the MAPK and PI3K/AKT signaling pathways (40). In addition to the intrinsic regulation by signaling pathways within tumor cells, studies on CTCs have further revealed how tumors promote immune evasion through the expression of immune checkpoints.
Research on CTCs has revealed that oncogenes and tumor suppressor genes facilitate immune evasion by promoting immune checkpoint expression. Previous studies have shown that PD−L1 expression is inversely correlated with KRAS mutations in colorectal cancer, particularly in MSI−H tumors (41). KRAS-mediated repression of interferon regulatory factor 2 (IRF2) results in high expression of CXCL3, which binds to CXCR2 on myeloid-derived suppressor cells (MDSCs) and regulates the immune responses in colorectal cancers (42). KRAS mutations in colorectal cancer are commonly associated with a MSS phenotype and poor response to single−agent immune checkpoint inhibitors; notably, in the KEYNOTE−177 trial, MSI−H CRC patients harboring KRAS or NRAS mutations did not benefit from ICI monotherapy (43). In CTCs with KRAS mutations, the CTLA-4 gene is also highly expressed, with a positive correlation between KRAS and CTLA-4. MDSCs can secrete immunosuppressive factors such as IL−10 and TGF−β to induce Treg expansion, and these CTLA−4–high Tregs further inhibit CD8+ T−cell responses (44, 45). Collectively, these findings indicate that KRAS mutations drive immune evasion in colorectal cancer through multifaceted mechanisms, including CXCL3-mediated MDSC recruitment via IRF2 suppression, PD-L1 downregulation in MSI-H tumors, and CTLA-4-dependent Treg expansion, ultimately dampening anti-tumor CD8+ T-cell responses and immune escape (46).
3.2 Predictive value of PD-L1 expression in CTCs for treatment response
CTCs exhibiting high PD-L1 expression serve as predictive biomarkers, suggesting potential sensitivity to anti-PD-1/PD-L1 monotherapy in these patients (47). Additionally, their presence indicates a persistent immunosuppressive state within the tumor microenvironment (48). Given this dual role, clinicians managing such patients should move beyond monotherapy paradigms. Rational combination strategies—integrating chemotherapy, targeted therapies, or dual immune checkpoint blockade—can synergistically disrupt immune evasion mechanisms, thereby augmenting treatment response and circumventing resistance pathways (49).
The PD-L1-specific inhibitor pembrolizumab has been employed in numerous clinical trials for over 30 cancers, including gastric cancer, colorectal cancer, head and neck cancer, and melanoma (50). In the study by Yue et al., colorectal cancer (CRC) patients undergoing PD-1 blockade therapy with IB1308 were stratified into four groups based on PD-L1 expression levels on circulating tumor cells(CTCs): PD-L1negative (MFI<50), PD-L1low (50≤MFI<100), PD-L1medium (100≤MFI<150), and PD-L1high (MFI≥150). This study was the first to propose a PD-L1 expression cutoff value of 20% for CTCs, revealing that patients with PD-L1highCTCs (≥20% abundance) achieved a significantly higher objective response rate (ORR: 64% vs. 14%, P<0.001) and prolonged median progression-free survival (4.27 vs. 2.07 months, HR=3.342, P=0.002) compared to those below the threshold. Longitudinal monitoring demonstrated that dynamic reductions in PD-L1high CTC counts correlated with therapeutic efficacy (63.6% of disease control patients showed declines, P=0.007), whereas stable or elevated PD-L1high CTCs predicted progression (84.2% of PD cases) (51). The study conducted by Tan et al. demonstrated that PD-L1 is not only broadly applicable in immunotherapy but also serves as a predictive biomarker. Patients with high baseline PD-L1 expression on circulating tumor cells (CTCs) who received anti-PD-1/PD-L1 monoclonal antibodies combined with conventional chemotherapy regimens showed significantly prolonged progression-free survival (median PFS: 4.9 months vs. 2.2 months, P < 0.0001) (52). The detection method involves isolating CTCs from blood samples using EpCAM antibodies or other surface markers, combined with CD45 for leukocyte exclusion, followed by immunofluorescence staining with PD-L1-specific antibodies (e.g., clones D84TX, 22C3, or KN802) to evaluate PD-L1 expression.
A study evaluated the efficacy of the oral multikinase inhibitor regorafenib based on PD-L1 expression in CTCs obtained from peripheral blood. This study leveraged the advantage of CTCs in reflecting tumor heterogeneity. CTCs were detected in nearly all metastatic colorectal cancer patients (38/40, 95%). Among 17 patients with tumor progression following regorafenib treatment, shorter progression-free survival (PFS) and overall survival (OS) were observed, with PD-L1(+) CTCs present in their blood. These findings suggest that PD-L1-positive tumors may develop resistance to regorafenib. Importantly, such resistance could be detected via CTCs as early as one month after initiating treatment, enabling timely adjustments to therapeutic strategies (53). Regorafenib inhibits the PI3K/AKT/mTOR and RAF/MEK/ERK signaling cascades, thereby inducing immunogenic cell death in tumor cells and promoting the release of ATP, high-mobility group box 1(HMGB1), and other damage‐associated molecular patterns. Concomitantly, these activate T cells to secrete IFN-γ, which, via the JAK–STAT pathway, upregulates PD-L1 expression and engenders an “immune editing” effect that maintains PD-L1 exposure on residual tumor cells, thus furnishing targets for subsequent immunotherapy (54, 55). Consequently, in patients harboring PD-L1–positive CTCs, regorafenib monotherapy demonstrates limited efficacy, whereas its combination with immune checkpoint inhibitors yields significantly enhanced therapeutic responses (56).
3.3 CTCs and microsatellite status in multimodal therapy
Circulating tumor cells (CTCs) play a crucial role in monitoring disease progression (PD) and serve as important biomarkers for prognostic assessment and intermediate response evaluation in immunotherapy. CTCs are integral to prognostic stratification in colorectal cancer (CRC) patients, with numerous studies validating their clinical utility. Previously, Bahnassy et al. conducted a prospective cohort study involving 44 CRC patients (Stages I–IV) to evaluate the prognostic significance of changes in CTC counts and microsatellite instability (MSI) profiles before and after curative surgery. The study revealed that a sustained postoperative decline in CTCs, combined with MSI-high (MSI-H) status, served as independent indicators of better progression-free survival (PFS) and overall survival (OS) in advanced-stage patients (P<0.001) (27). This could be attributed to the dynamic evolution of tumor lesions influenced by time, treatment, and surgical intervention, wherein immunotherapy stimulates the expansion of tumor subclones, resulting in changes in the number and molecular characteristics of CTCs (57). In this review, we summarize several studies related to circulating tumor cells (CTCs) in colorectal cancer (CRC). The findings not only underscore the potential value of CTC counts but also provide deeper insights into the feasibility of utilizing CTCs as a liquid biopsy tool (Table 1).
4 Mechanisms of immune evasion by circulating tumor cells in the tumor microenvironment
4.1 Macrophage-CTC interaction
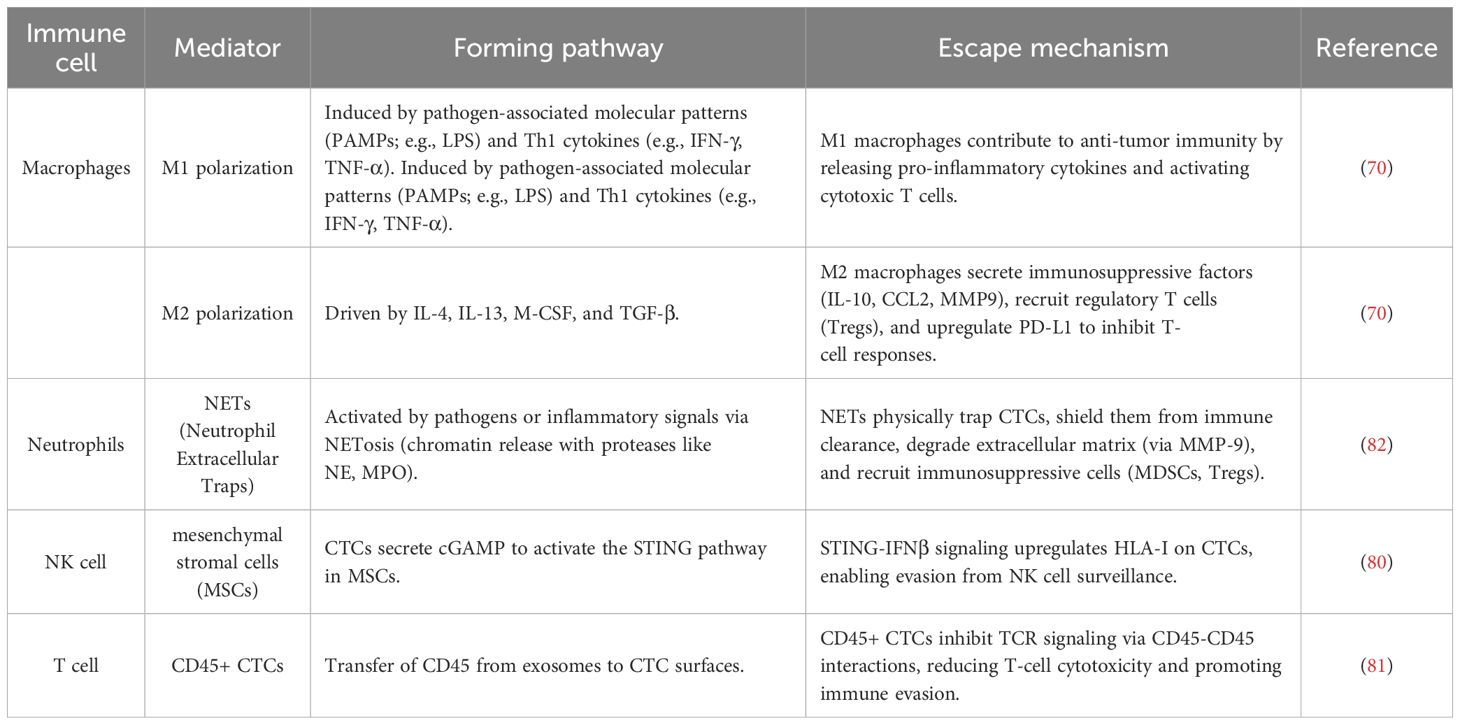
Circulating tumor cells (CTCs), as critical mediators of primary tumors and distant metastases, play a pivotal role in cancer initiation, progression, and metastasis through interactions with the tumor microenvironment (TME) (Figure 1) (59). The TME in colorectal cancer comprises stromal and immune cells that regulate immune suppression and inflammation (60). Tumor-associated macrophages (TAMs), the predominant immune cells in the TME, exhibit functional polarization toward either the M1 or M2 phenotype, modulated by tumor and stromal signals. Advances in gene expression profiling, morphology, and single-cell RNA sequencing have provided deeper insights into TAM heterogeneity (61). In the TME, M1 macrophage polarization is induced by recognizing pathogen-associated molecular patterns (e.g., lipopolysaccharide [LPS]) and type 1 helper T cell (Th1) cytokines, such as interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α). M1 macrophages primarily function in innate immunity to combat infections and tumors. In contrast, M2 polarization is driven by interleukin-4 (IL-4), interleukin-13 (IL-13), macrophage colony-stimulating factor (M-CSF), and transforming growth factor-β (TGF-β), contributing to pro-tumor characteristics (62, 63). Most TAMs exhibit the M2 phenotype, significantly promoting tumor cell survival, proliferation, and immune evasion by enhancing immunosuppression. This ultimately leads to cancer progression, chemoresistance, and metastasis. Within the TME, signals such as IL-10, CCL2, CSF-1, VEGF, and IL-6 secreted by cancer cells, adaptive immune cells, fibroblasts, and TAMs recruit and differentiate monocytes into M2-like TAMs (64, 65).

Figure 1. Shed tumor cells in peripheral blood can evade immune surveillance and immune killing through various mechanisms. ECM, extracellular matrix; NETs, neutrophil extracellular traps; Tregs: regulatory T cells; MDSCs, myeloid derived suppressor cells; IL-10, interleukin-10; TGF-β, Transforming Growth Factor-β; FoxQ1, forkhead box Q1; CCL2, C–C motif chemokine ligand 2; M2-polarized tumor-associated macrophages; PD-L1, programmed death-ligand 1; PD-1, programmed cell death protein 1.
In vitro and in vivo studies suggest that CTCs may originate from the fusion of tumor cells with hematopoietic or myeloid cells, particularly macrophages (66). This intercellular interaction relies on the formation of membrane protrusions, which facilitate signal exchange over short distances (tens of micrometers) and long distances (hundreds of micrometers) through direct cell contact. Among these structures, the most extensively studied are protrusions derived from filopodia, including cytochromes and tunneling nanotubes (TNTs). TNTs represent the extended filopodia, connecting previously non-adjacent cells through a process referred to as “protrusion elongation” (67). M2−polarized macrophages more readily form membrane protrusions and fuse with tumor cells via tunneling nanotubes than M1 macrophages. Wei et al. showed that M2−derived IL−6 activates the JAK2/STAT3 pathway in tumor cells, leading to STAT3 nuclear translocation and repression of miR−506−3p, which upregulates FoxQ1. FoxQ1 induces epithelial–mesenchymal transition (EMT), yielding CTCs with enhanced invasiveness, and drives C-C motif chemokine ligand 2 (CCL2) secretion to recruit more M2 macrophages, creating a pro−metastatic feedback loop (68).
Recent studies have revealed that TNT-mediated paracrine signaling and juxtacrine interactions between tumor cells and macrophages play crucial roles in shared molecular pathways that enhance cell migration and invasion. The interaction between macrophages and tumor cells in the TME predominantly depends on classical paracrine mechanisms (69). Specifically, macrophages secrete epidermal growth factor (EGF), which binds to epidermal growth factor receptors (EGFR) on tumor cells, activating downstream signaling pathways and inducing colony-stimulating factor 1 (CSF-1) secretion. CSF-1 binds to its receptor to recruit macrophages to tumor sites. This paracrine loop, mediated by tumor cell-secreted CSF-1 and macrophage-secreted EGF, is critical in facilitating the co-migration of tumor cells and macrophages toward blood vessels. Once tumor cells enter the bloodstream, they can be detected as circulating hybrid cells or CTCs (70).
4.2 Neutrophils-CTC interaction
Surgery-induced dissemination of circulating tumor cells (CTCs) and the accompanying inflammatory response promote the growth and metastasis of occult tumors by constructing a supportive tumor microenvironment (TME) (71). Neutrophil extracellular traps(NETs), composed of depolymerized DNA fibers modified by proteins secreted by activated neutrophils, capture CTCs in cases of severe postoperative sepsis. This fosters early adhesion of tumor cells to distant organs, creating favorable conditions for tumor metastasis (72). These CTC-neutrophil clusters form a “shield” around tumor cells, not only physically isolating CTCs but also suppressing NK cell-mediated cytotoxicity. Additionally, they promote tumor cell evasion by releasing cytokines such as IL-1B, MMP-9, and HMGB1 (62, 73).
The significance of NETs-related genes in immunotherapy and cancer treatment has garnered increasing attention. Studies have demonstrated that the expression levels of NETs-related genes (e.g., H3Cit, NE, MPO) are closely associated with an immunosuppressive microenvironment and the response to cancer immunotherapy (74). During immune checkpoint inhibitor therapy, changes in the expression of these genes can serve as indicators of immune response intensity. Transcriptome analyses have identified 19 genes associated with NETs formation, leading to the establishment of a NETs scoring system. This score has been found to negatively correlate with cancer patient prognosis (75, 76). Furthermore, the expression levels of NETs-related genes across different tumor types are intricately linked to mechanisms of tumor immune evasion, immunosuppression, and cancer progression. In CTC-related studies, the interaction between NETs and CTCs promote tumor invasion, metastasis, and immune evasion through multiple mechanisms. First, NETs physically entrap CTCs, enhancing their intravascular retention and adhesion while shielding them from immune clearance. Concurrently, NETs release proteolytic enzymes—such as matrix metalloproteinase-9 (MMP-9) and neutrophil elastase—that degrade the extracellular matrix, thereby creating a permissive niche for tumor cell invasion and migration. They also secrete cytokines like interleukin-8 (IL-8) and transforming growth factor-β (TGF-β), which induce EMT in CTCs and further augment their migratory and invasive capabilities. Moreover, NET-associated high-mobility group box 1 (HMGB1) activates the RAGE and TLR4 signaling pathways to drive the release of pro-inflammatory mediators (e.g., CXCL2, IL-8), recruiting additional neutrophils and establishing a pro-metastatic inflammatory microenvironment. In the realm of immune regulation, NETs discharge immunosuppressive factors—including programmed death ligand-1 (PD-L1), reactive oxygen species (ROS), and arginase-1 (ARG1)—to inhibit T cell and natural killer (NK) cell cytotoxicity, while fostering the accumulation of myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs), thereby remodeling the tumor microenvironment into an immunosuppressive state (77, 78).
4.3 Other immune cells
Circulating tumor cells (CTCs) evade immune surveillance through multiple mechanisms, facilitating tumor metastasis and modulating the function of immune cells. First, CTCs escape NK cell surveillance by upregulating HLA-I through the cGAS-STING-IFNβ-HLA signaling pathway (79). Additionally, CTCs enhance immune evasion by upregulating N-cadherin, a natural ligand of the NK cell receptor KLRG1. The interaction between N-cadherin and KLRG1 inhibits NK cell cytotoxicity, induces NK cell differentiation, and increases the proportion of KLRG1+ cells, leading to NK cell exhaustion and impaired antitumor efficacy (80). Furthermore, CTCs achieve immune evasion through interactions with T cells, particularly via exosome-derived CD45 transferred to the tumor cell surface, forming CD45+ CTCs. These CD45+ CTCs inhibit TCR signaling through CD45-CD45 interactions with T cells, reducing T-cell cytotoxic responses and accelerating tumor metastasis (81). These findings elucidate the complex interaction mechanisms between CTCs and immune cells, providing new insights into tumor immune evasion and highlighting potential therapeutic targets for CTCs-targeted immunotherapy. The mechanisms of CTC immune evasion involving various immune cells are summarized in Table 2.
5 Innovation and progress in circulating tumor cell screening technologies
5.1 Screening and application of novel genetic markers
Liquid biopsy has emerged as a transformative approach in oncology, encompassing diverse biomarkers such as circulating tumor DNA (ctDNA), microRNAs (miRNAs), extracellular vesicles (EVs), and CTCs to guide precision medicine (83). These components collectively provide a non-invasive window into tumor dynamics, enabling real-time monitoring of disease progression and therapeutic response. Among these, CTCs hold unique value as intact cellular entities that reflect both genetic and functional characteristics of tumors, offering insights into metastasis and immune evasion mechanisms.
In CTC screening, common genetic mutations associated with colorectal cancer include KRAS and BRAF mutations. KRAS mutations lead to the activation of KRAS protein, which promotes tumor cell proliferation. BRAF gene mutations, in the B-Raf proto-oncogene serine/threonine kinase, are closely related to metastasis and drug resistance. However, the mutation rates of KRAS and BRAF in colorectal cancer patients are only 40% and 10%, respectively (84–86). Advances in proteomic technologies, such as those highlighted in hepatocellular carcinoma (HCC) biomarker research, could enhance CTC characterization in CRC (87). For example, mass spectrometry and pathway analysis—tools pivotal for uncovering PI3K/AKT/mTOR dysregulation in HCC—could likewise characterize post-translational modifications and protein interactions in CTCs, thereby refining prognostic assessments and therapeutic strategies in CRC. Notably, CTC detection shows a 77% concordance with tumor tissue profiling (88). The application of DNA microarray technology not only allows for the detection of mutated genes that are masked by wild-type DNA in contaminating leukocytes, but it also offers lower costs and higher sensitivity. With the help of DNA microarrays, research can progress to the coding level. Commonly analyzed mutations include KRAS mutations in exon 2 (codons 12 and 13), 3 (codon 61), and 4 (codon 146), NRAS mutations in exon 2 (codons 12 and 13), and BRAF mutations in exon 15 (V600E) (89).
Cluster differentiation (CD) markers are emerging as potential targets. The expression of CD45 in CTCs is consistent with that in corresponding tumor tissues, while CD47 expression is significantly upregulated and closely associated with immune evasion by cancer cells (90, 91). Mass spectrometry has been used to localize protein glycosylation, revealing that cancer-associated glycans such as Sialyl-Tn (STn) are expressed in most advanced gastrointestinal cancers, including colorectal cancer, but are minimally expressed or absent in normal tissues. Glycans, by modulating the activity of cell-surface glycosyltransferases in tumor cells, induce aberrant glycosylation and the emergence of truncated glycan epitopes (e.g., Tn and Sialyl-Tn). These truncated glycan structures contribute to enhanced metastatic potential, invasive capacity, and immune evasion (92, 93). In CTCs, STn expression is considered one of the primary drivers of metastasis and a significant downstream biological target. Studies have shown that STn(+) CTCs can also be captured, with an incidence three times higher than that of EpCAM(+) CTCs (94).
5.2 Breakthroughs in emerging screening technologies: microfluidics and molecular aptamers
5.2.1 CellSearch system
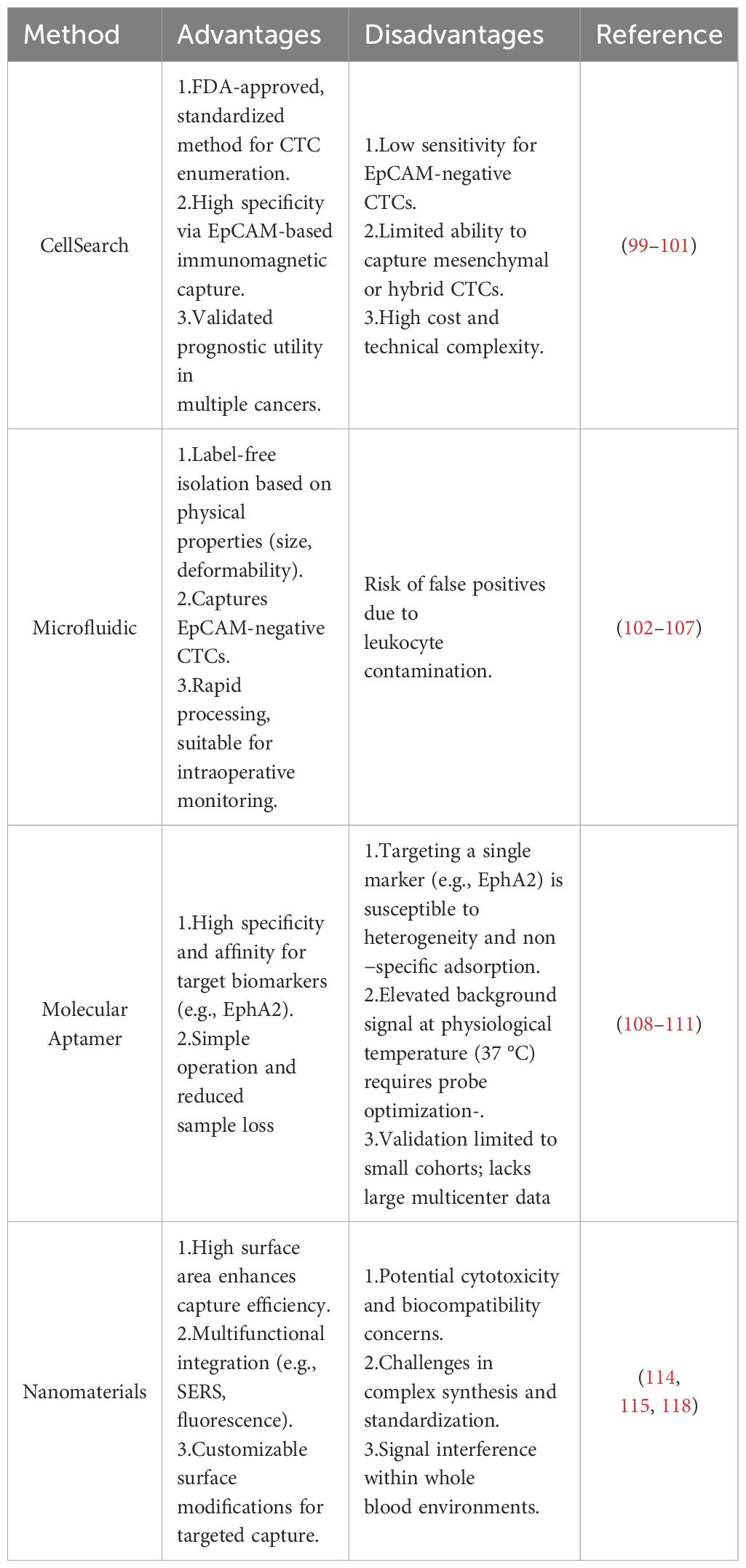
Counting circulating tumor cells (CTCs) is technically challenging, as CTCs constitute less than 0.004% of all mononuclear cells (95). The CellSearch System (Veridex) is the most widely utilized antibody-based isolation technology and the only method approved by the U.S. Food and Drug Administration (FDA) for detecting CTCs in the blood of patients with certain cancers (96). The CellSearch System enriches tumor cells using ferromagnetic beads coated with EpCAM antibodies. EpCAM stands for Epithelial Cell Adhesion Molecule. Colorectal cancer arises from the epithelial cells lining the colon or rectum, and these tumor cells are highly likely to express EpCAM on their surface (97). Consequently, EpCAM offers high specificity, as colorectal carcinoma cells generally retain epithelial characteristics even during metastasis. In contrast, CD45 serves as a pan−leukocyte marker for negative selection, effectively labeling and depleting the vast majority of immune cells to minimize contamination (98). These magnetic beads specifically bind to EpCAM-positive CTCs, capturing them and isolating them from the patient’s peripheral blood (99). Flow cytometry is subsequently used to further sort out and remove leukocytes, ultimately isolating individual CTCs (100). CellSearch is currently the most established CTC‐enrichment method, and the genomic mutational profile of isolated CTCs is largely concordant with that of the primary tumor. However, its low sensitivity has constrained the informational value of CTC enumeration in this disease (101).
5.2.2 Microfluidics-based technology
Microfluidics-based cell sorting methods leverage fluid dynamics and external forces (such as magnetic fields, electric fields, acoustic waves, and optical forces) to separate cells based on their physical and biological properties (102). Microfluidic technology can organize CTCs into monolayers in a few minutes (103). Yang et al. developed a label-free wedge-shaped microfluidic chip called CTC-Δchip. This enrichment technique relies on size-based filtration, using nano- to micron-scale pores to isolate CTCs, which are larger and stiffer in shape, from blood cells (104). The self-assembled cell array (SACA) has been demonstrated as a reliable platform for CTC enumeration. When combined with a 3D-microDialysis chip, SACA enables image analysis to be completed in under 4 hours and exhibits high sensitivity in detecting one CTC among 105^55 cells. Additionally, SACA combined with carcinoembryonic antigen (CEA) serves as a powerful risk stratification tool. Patients with preoperative CTC counts >4 and CEA levels >5 ng/mL had poorer progression-free survival (PFS) compared to others (105). Microfluidic technology, as a biophysical isolation method, exploits the intrinsic physical properties of CTCs and blood cells. It enables the capture of EpCAM‐negative CTCs, which often exhibit greater invasiveness and metastatic potential, while bypassing the labor‐intensive, multi‐step analyses required by biochemical isolation and thereby significantly shortening enrichment time—making it well suited for real‐time intraoperative monitoring (106). However, megakaryocytes or activated leukocytes of similar size may be misclassified as CTCs. Issues related to false positives (specificity) and false negatives (sensitivity) remain major challenges for immunomagnetic detection technologies in CTC isolation (107).
5.2.3 Application of molecular aptamers in CTC detection
W3 is an aptamer that has long been regarded as a predictive factor for colorectal cancer (CRC) metastasis. A molecular beacon based on W3 (MAB-W3-3G) can act as a molecular probe, specifically capturing CTCs in the bloodstream (108). The molecular aptamer beacon combines the advantages of both aptamers and molecular beacons, offering not only the specificity of aptamers in detecting targets but also the convenience of molecular beacons in operationally detecting those targets (109). Lu et al. (2023) used SELEX technology to obtain the aptamer W3 from CRC cells and employed W3 as a specific recognition probe for the molecular beacon (MAB). In the stem region of MAB, some base sequences were modified to maintain stability, and the shortest sequence out of four variants was selected, named W3-3. This was further used to construct a monoclonal antibody, MAB-W3-3G. In a real blood sample validation using 14 healthy blood donors and 28 CRC patients, no positive cells were detected in the blood samples from healthy donors. In 28 CRC patient samples, 75% of the patients tested positive for CTCs (21/28), indicating that MAB-W3-3G-based imaging can specifically detect cancer cells in whole blood. Notably, the number of CTCs in metastatic patients was significantly higher than in non-metastatic patients (6.4 ± 2.0, n = 8 vs 2.3 ± 0.5, n = 20) (110). The W3 aptamer can be conjugated to either quantum-dot probes or molecular beacons (MAB-W3-3G) to enable one-step capture and quantification of metastatic CTCs in patient blood, and it also selectively recognizes EphA2‐bearing exosomes, achieving a detection sensitivity of 8.4×106 particles/ml. Moreover, W3 exhibits excellent stability in both plasma and culture medium—retaining full activity over a 3-hour period—and is compatible with live‐cell imaging and microplate‐based fluorescence assays, offering operational simplicity and reduced sample loss (111). However, as it targets only a single EphA2 marker, it is prone to interference from tumor cell expression heterogeneity and nonspecific adsorption in whole blood (108). Additionally, MAB-W3-3G shows elevated background signals at 37 °C, indicating that further probe optimization is required. Its performance has thus far been validated only in a small cohort, underscoring the need for larger, multicenter clinical trials to assess diagnostic concordance and prognostic value (110).
5.2.4 Applications of nanomaterials in biomedicine
Nanomaterials are widely used in the biomedical field due to their unique physicochemical properties, such as high surface area and good biocompatibility (112). Common nanomaterials include gold, magnetic, and silica-based materials, which typically exist in the form of nanoparticles, nanostructures, or nanowires (113). Gold nanomaterials, owing to their excellent conductivity, stability, and increased surface area, are able to effectively interact with various biomolecules. They are commonly used in molecular detection and imaging applications, such as fluorescence imaging and Raman spectroscopy. Furthermore, the high biocompatibility of gold nanoparticles allows them to enter the body and be used for the separation and detection of CTCs (circulating tumor cells) and CCSCs (cancer stem cells) (114). In related studies, gold nanorods, when combined with targeted antibodies like EpCAM, CD44, etc., utilize surface-enhanced Raman scattering (SERS) technology to achieve highly sensitive and multiplexed detection of CTCs from blood samples (115). Silica-based nanomaterials are widely used in CTC detection platforms due to their excellent chemical stability and biocompatibility (116). The nanoparticles or nanostructures of silica materials enhance interactions with cells, improving the efficiency of CTC capture. They can also be integrated with optical detection technologies and microfluidic devices to provide higher sensitivity and specificity (117).
Despite the development of various commercial CTC detection systems, which have made progress in laboratory settings, these methods have not been widely adopted in clinical practice due to certain limitations. Future clinical studies should address how to improve CTC capture efficiency, simplify identification methods, reduce cell loss, and optimize the clinical applicability of nanotechnology to enhance the practical value of CTC detection in early cancer diagnosis, monitoring, and therapy(Table 3) (118).
6 Current status and challenges
Liquid biopsy, particularly the detection of circulating tumor cells (CTCs), has shown tremendous potential in the immunotherapy of colorectal cancer (CRC). However, challenges remain in improving the sensitivity and specificity of screening due to the short half-life of CTCs in circulation and the significantly higher concentrations of CTCs in the portal vein/mesenteric vein blood compared to central venous blood (119, 120). Furthermore, the tumor heterogeneity of CRC further complicates CTC detection (121). CRC exhibits significant intra-tumoral and inter-tumoral heterogeneity, with phenotypic and genotypic differences between metastatic and primary lesions. This makes CTC capture and analysis more complex. Such heterogeneity not only affects the efficiency of CTC detection but also limits their utility as prognostic and predictive biomarkers (122). For example, the metastatic routes and hemodynamic changes in tumors may lead to different biological characteristics of CTCs, increasing the technical difficulty of detecting them (123). Additionally, current methods for CTC isolation and characterization are still immature, and there is a lack of standardized operating procedures (SOPs). Differences in reagents, equipment, and operational procedures used in different laboratories make the reproducibility and comparability of research results difficult. Therefore, developing standardized operating procedures (SOPs) and validation methods is crucial for the widespread application of liquid biopsy technologies (124).
To overcome these challenges, scientists are developing liquid biopsy technologies with higher sensitivity and specificity. One of the current focuses of research is the improvement of CTC separation techniques and the use of multi-biomarker combined analysis. Traditional CTC capture methods typically rely on surface markers, such as epithelial cell adhesion molecule (EpCAM). However, these methods are prone to selective bias. As a result, researchers are exploring multi-molecular marker-based combined analyses to enhance the comprehensiveness and accuracy of CTC capture (125–127). Multi-marker strategies not only improve CTC capture efficiency but also address the limitations of relying on a single marker, which may miss specific types of CTCs (128). Yu et al. noted that the integration of genomics, transcriptomics, proteomics, and metabolomics enables the construction of comprehensive molecular profiles for early tumor detection and therapeutic intervention. These approaches—particularly when coupled with artificial intelligence (AI) and machine learning (ML)–driven data analysis—enhance biomarker discovery by detecting subtle patterns within heterogeneous datasets, thereby facilitating real-time monitoring of treatment response and disease progression (129). The integration of AI and ML technologies provides new insights into the analysis of liquid biopsy data. These technologies are capable of efficiently processing complex multidimensional data, enabling researchers to extract valuable clinical information from liquid biopsies (130, 131). For example, AI algorithms can be used to analyze the morphological features of CTCs, their gene expression profiles, and their relationship with tumor progression, thereby improving the accuracy of liquid biopsies in tumor diagnosis, prognosis evaluation, and monitoring treatment responses (132).
In addition to technical advancements, conducting multicenter, large-scale clinical studies and fostering multidisciplinary collaboration are essential for transitioning liquid biopsy technologies from research to clinical practice (133, 134). Currently, the clinical application of liquid biopsy in colorectal cancer is still in its early stages. Although some clinical studies have shown that liquid biopsy has potential for early screening, treatment response prediction, and prognosis evaluation of CRC, its diagnostic accuracy and sensitivity still face many challenges (135). For example, liquid biopsy may struggle with detecting mutations with low allele frequencies, such as microsatellite instability (MSI), due to insufficient sensitivity when mutation frequencies are too low, especially in early-stage tumors or localized cancers (low tumor mutational burden, TMB). Additionally, current liquid biopsy technologies, particularly CTC detection based on next-generation sequencing (NGS), are limited by low signal-to-noise ratios and sample contamination (e.g., non-tumor cells such as immune and hematopoietic cells in the blood) (136–138). Furthermore, combining liquid biopsy with other components, such as circulating tumor DNA (ctDNA), may significantly enhance the sensitivity and specificity of early colorectal cancer detection (139). This approach could further help identify advanced colorectal cancer patients who are suitable for immunotherapy or surgical resection (140).
7 Conclusion and future prospects
This article summarizes the clinical significance of circulating tumor cells (CTC) in the immune treatment of colorectal cancer (CRC), particularly in the roles of prognosis assessment, immune escape, drug resistance mechanisms, and tumor microenvironment (TME) interactions. Research shows that CTCs have significant potential in treatment monitoring and immunotherapy in CRC patients. MSI-H patients often exhibit higher CTC counts, which may suggest better prognoses in response to immunotherapy. Additionally, the role of PD-L1 positive CTCs in immune resistance further validates the potential of CTCs as predictors of drug response.
Looking forward, the use of CTCs as a liquid biopsy tool for CRC, particularly in personalized treatment and early screening, still holds vast developmental potential. With the discovery of new genetic markers and advancements in screening technologies, CTCs could provide more precise prognosis predictions and treatment response evaluations for CRC patients.
Author contributions
YF: Conceptualization, Data curation, Formal Analysis, Writing – original draft. GL: Methodology, Supervision, Writing – review & editing. QC: Methodology, Visualization, Writing – original draft. JZ: Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The “Xingliao Talent Program “Medical Expert Project (No. YXMJ-LJ-08);Bei Jing Huikang Charity Foundation Project (No. BHCF20240321).
Acknowledgments
The Figure 1 used in this manuscript was created using the FigDraw platform (www.figdraw.com) and has been authorized for use. It is hereby confirmed that the displayed figure has received explicit permission from FigDraw for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung JJY, Chiu HM, Lieberman D, Kuipers EJ, Rutter MD, Macrae F, et al. Third asia-pacific consensus recommendations on colorectal cancer screening and postpolypectomy surveillance. Gut. (2022) 71:2152–66. doi: 10.1136/gutjnl-2022-327377
2. Bretthauer M, Loberg M, Wieszczy P, Kalager M, Emilsson L, Garborg K, et al. Effect of colonoscopy screening on risks of colorectal cancer and related death. N Engl J Med. (2022) 387:1547–56. doi: 10.1056/NEJMoa2208375
3. Lin JS, Perdue LA, Henrikson NB, Bean SI, and Blasi PR. Screening for colorectal cancer: updated evidence report and systematic review for the us preventive services task force. JAMA. (2021) 325:1978–98. doi: 10.1001/jama.2021.4417
4. Carney BW, Gholami S, Fananapazir G, Sekhon S, Lamba R, Loehfelm TW, et al. Utility of combined gadoxetic acid and ferumoxytol-enhanced liver mri for preoperative detection of colorectal cancer liver metastases: A pilot study. Acta Radiol. (2023) 64:1357–62. doi: 10.1177/02841851221136499
5. Adam R, de Gramont A, Figueras J, Kokudo N, Kunstlinger F, Loyer E, et al. Managing synchronous liver metastases from colorectal cancer: A multidisciplinary international consensus. Cancer Treat Rev. (2015) 41:729–41. doi: 10.1016/j.ctrv.2015.06.006
6. Gvozdenovic A and Aceto N. Emp1-positive cells found guilty of metastatic relapse in colorectal cancer. Dev Cell. (2022) 57:2673–4. doi: 10.1016/j.devcel.2022.11.017
7. Tsai KY, Huang PS, Chu PY, Nguyen TNA, Hung HY, Hsieh CH, et al. Current applications and future directions of circulating tumor cells in colorectal cancer recurrence. Cancers (Basel). (2024) 16(13):2316. doi: 10.3390/cancers16132316
8. Thibaudin M, Fumet JD, Chibaudel B, Bennouna J, Borg C, Martin-Babau J, et al. First-line durvalumab and tremelimumab with chemotherapy in ras-mutated metastatic colorectal cancer: A phase 1b/2 trial. Nat Med. (2023) 29:2087–98. doi: 10.1038/s41591-023-02497-z
9. Patel SP, Alonso-Gordoa T, Banerjee S, Wang D, Naidoo J, Standifer NE, et al. Phase 1/2 study of monalizumab plus durvalumab in patients with advanced solid tumors. J Immunother Cancer. (2024) 12(2):e007340. doi: 10.1136/jitc-2023-007340
10. Kolencik D, Narayan S, Thiele JA, McKinley D, Gerdtsson AS, Welter L, et al. Circulating tumor cell kinetics and morphology from the liquid biopsy predict disease progression in patients with metastatic colorectal cancer following resection. Cancers (Basel). (2022) 14(3):642. doi: 10.3390/cancers14030642
11. Yang X, Zhang Z, and Bi X. A nomogram for predicting colorectal cancer liver metastasis using circulating tumor cells from the first drainage vein. Eur J Surg Oncol. (2024) 50:108579. doi: 10.1016/j.ejso.2024.108579
12. Zhang W, Xu F, Yao J, Mao C, Zhu M, Qian M, et al. Single-cell metabolic fingerprints discover a cluster of circulating tumor cells with distinct metastatic potential. Nat Commun. (2023) 14:2485. doi: 10.1038/s41467-023-38009-3
13. Galvis MM, Romero CS, Bueno TO, and Teng Y. Toward a new era for the management of circulating tumor cells. Adv Exp Med Biol. (2021) 1286:125–34. doi: 10.1007/978-3-030-55035-6_9
14. Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ, et al. Breast cancer-major changes in the american joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. (2017) 67:290–303. doi: 10.3322/caac.21393
15. Nabors LB, Portnow J, Ahluwalia M, Baehring J, Brem H, Brem S, et al. Central nervous system cancers, version 3.2020, nccn clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2020) 18:1537–70. doi: 10.6004/jnccn.2020.0052
16. Schaeffer EM, Srinivas S, Adra N, An Y, Barocas D, Bitting R, et al. Prostate cancer, version 4.2023, nccn clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2023) 21:1067–96. doi: 10.6004/jnccn.2023.0050
17. Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, et al. Breast cancer, version 3.2022, nccn clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20:691–722. doi: 10.6004/jnccn.2022.0030
18. Manso T, Kushwaha A, Abdollahi N, Duroux P, Giudicelli V, and Kossida S. Mechanisms of action of monoclonal antibodies in oncology integrated in imgt/mab-db. Front Immunol. (2023) 14:1129323. doi: 10.3389/fimmu.2023.1129323
19. Sharma P, Siddiqui BA, Anandhan S, Yadav SS, Subudhi SK, Gao J, et al. The next decade of immune checkpoint therapy. Cancer Discov. (2021) 11:838–57. doi: 10.1158/2159-8290.CD-20-1680
20. Thomas J, Leal A, and Overman MJ. Clinical development of immunotherapy for deficient mismatch repair colorectal cancer. Clin Colorectal Cancer. (2020) 19:73–81. doi: 10.1016/j.clcc.2020.02.002
21. Wang DK, Zuo Q, He QY, and Li B. Targeted immunotherapies in gastrointestinal cancer: from molecular mechanisms to implications. Front Immunol. (2021) 12:705999. doi: 10.3389/fimmu.2021.705999
22. Mei WJ, Mi M, Qian J, Xiao N, Yuan Y, and Ding PR. Clinicopathological characteristics of high microsatellite instability/mismatch repair-deficient colorectal cancer: A narrative review. Front Immunol. (2022) 13:1019582. doi: 10.3389/fimmu.2022.1019582
23. Toh JWT, Lim SH, MacKenzie S, de Souza P, Bokey L, Chapuis P, et al. Association between microsatellite instability status and peri-operative release of circulating tumour cells in colorectal cancer. Cells. (2020) 9(2):425. doi: 10.3390/cells9020425
24. Ierano C, Righelli D, D’Alterio C, Napolitano M, Portella L, Rea G, et al. In pd-1+ Human colon cancer cells nivolumab promotes survival and could protect tumor cells from conventional therapies. J Immunother Cancer. (2022) 10(3):e004032. doi: 10.1136/jitc-2021-004032
25. Dompe C, Chojnowska A, Ramlau R, Nowicki M, Alix-Panabieres C, and Budna-Tukan J. Unveiling the dynamics of circulating tumor cells in colorectal cancer: from biology to clinical applications. Front Cell Dev Biol. (2024) 12:1498032. doi: 10.3389/fcell.2024.1498032
26. Hardingham JE, Grover P, Winter M, Hewett PJ, Price TJ, and Thierry B. Detection and clinical significance of circulating tumor cells in colorectal cancer–20 years of progress. Mol Med. (2015) 21 Suppl 1:S25–31. doi: 10.2119/molmed.2015.00149
27. Alsayed A, Salem SE, El Serafi MM, Abdellateif MS, Zekri AN, Mohanad M, et al. Assessment of the circulating tumor cells and microsatellite instability in colorectal cancer patients: prognostic and diagnostic value. Onco Targets Ther. (2021) 14:1937–51. doi: 10.2147/OTT.S292551
28. Ambrosini M, Manca P, Nasca V, Sciortino C, Ghelardi F, Seligmann JF, et al. Epidemiology, pathogenesis, biology and evolving management of msi-H/dmmr cancers. Nat Rev Clin Oncol. (2025) 22(6):385-407. doi: 10.1038/s41571-025-01015-z
29. Lin X, Kang K, Chen P, Zeng Z, Li G, Xiong W, et al. Regulatory mechanisms of pd-1/pd-L1 in cancers. Mol Cancer. (2024) 23:108. doi: 10.1186/s12943-024-02023-w
30. Gu X, Wei S, and Lv X. Circulating tumor cells: from new biological insights to clinical practice. Signal Transduct Target Ther. (2024) 9:226. doi: 10.1038/s41392-024-01938-6
31. Fumagalli A, Oost KC, Kester L, Morgner J, Bornes L, Bruens L, et al. Plasticity of lgr5-negative cancer cells drives metastasis in colorectal cancer. Cell Stem Cell. (2020) 26:569–78 e7. doi: 10.1016/j.stem.2020.02.008
32. Schuster E, Taftaf R, Reduzzi C, Albert MK, Romero-Calvo I, and Liu H. Better together: circulating tumor cell clustering in metastatic cancer. Trends Cancer. (2021) 7:1020–32. doi: 10.1016/j.trecan.2021.07.001
33. Li Q, Geng S, Luo H, Wang W, Mo YQ, Luo Q, et al. Signaling pathways involved in colorectal cancer: pathogenesis and targeted therapy. Signal Transduct Target Ther. (2024) 9:266. doi: 10.1038/s41392-024-01953-7
34. Magri V, Marino L, Nicolazzo C, Gradilone A, De Renzi G, De Meo M, et al. Prognostic role of circulating tumor cell trajectories in metastatic colorectal cancer. Cells. (2023) 12(8):1172. doi: 10.3390/cells12081172
35. Messaritakis I, Sfakianaki M, Vogiatzoglou K, Koulouridi A, Koutoulaki C, Mavroudis D, et al. Evaluation of the role of circulating tumor cells and microsatellite instability status in predicting outcome of advanced crc patients. J Pers Med. (2020) 10(4):235. doi: 10.3390/jpm10040235
36. Bergen ES, Scherleitner P, Ferreira P, Kiesel B, Muller C, Widhalm G, et al. Primary tumor side is associated with prognosis of colorectal cancer patients with brain metastases. ESMO Open. (2021) 6:100168. doi: 10.1016/j.esmoop.2021.100168
37. Malone JC, Arbor TC, and Shah AB. Embryology, Midgut. In StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing. (2025).
38. De Renzi G, Gaballo G, Gazzaniga P, and Nicolazzo C. Molecular biomarkers according to primary tumor location in colorectal cancer: current standard and new insights. Oncology. (2021) 99:135–43. doi: 10.1159/000510944
39. Han Y, Liu D, and Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. (2020) 10:727–42.
40. Ju X, Zhang H, Zhou Z, and Wang Q. Regulation of pd-L1 expression in cancer and clinical implications in immunotherapy. Am J Cancer Res. (2020) 10:1–11.
41. Lou E, Xiu J, Baca Y, Nelson AC, Weinberg BA, Beg MS, et al. Expression of immuno-oncologic biomarkers is enriched in colorectal cancers and other solid tumors harboring the A59t variant of kras. Cells. (2021) 10(6):1275. doi: 10.3390/cells10061275
42. Liao W, Overman MJ, Boutin AT, Shang X, Zhao D, Dey P, et al. Kras-irf2 axis drives immune suppression and immune therapy resistance in colorectal cancer. Cancer Cell. (2019) 35:559–72 e7. doi: 10.1016/j.ccell.2019.02.008
43. Xu M, Zhao X, Wen T, and Qu X. Unveiling the role of kras in tumor immune microenvironment. BioMed Pharmacother. (2024) 171:116058. doi: 10.1016/j.biopha.2023.116058
44. Dong S, Guo X, Han F, He Z, and Wang Y. Emerging role of natural products in cancer immunotherapy. Acta Pharm Sin B. (2022) 12:1163–85. doi: 10.1016/j.apsb.2021.08.020
45. Wang L, Lynch C, Pitroda SP, Piffko A, Yang K, Huser AK, et al. Radiotherapy and immunology. J Exp Med. (2024) 221(7):e20232101. doi: 10.1084/jem.20232101
46. Aktar S, Hamid FB, Gamage SMK, Cheng T, Parkneshan N, Lu CT, et al. Gene expression analysis of immune regulatory genes in circulating tumour cells and peripheral blood mononuclear cells in patients with colorectal carcinoma. Int J Mol Sci. (2023) 24(5):5051. doi: 10.3390/ijms24055051
47. He Y, Zhu M, Lai X, Zhang H, and Jiang W. The roles of pd-L1 in the various stages of tumor metastasis. Cancer Metastasis Rev. (2024) 43:1475–88. doi: 10.1007/s10555-024-10189-4
48. Wang NH, Lei Z, Yang HN, Tang Z, Yang MQ, Wang Y, et al. Radiation-induced pd-L1 expression in tumor and its microenvironment facilitates cancer-immune escape: A narrative review. Ann Transl Med. (2022) 10:1406. doi: 10.21037/atm-22-6049
49. Malkawi W, Lutfi A, Afghan MK, Shah LM, Costandy L, Ramirez AB, et al. Circulating tumour cell enumeration, biomarker analyses, and kinetics in patients with colorectal cancer and other gi Malignancies. Front Oncol. (2023) 13:1305181. doi: 10.3389/fonc.2023.1305181
50. Li L, Yu D, Yang J, Zhang F, Zhang D, Lin Z, et al. Significant response to pembrolizumab plus lenvatinib in epstein-barr-virus-associated intrahepatic cholangiocarcinoma: A case report. Cancer Biol Ther. (2024) 25:2338644. doi: 10.1080/15384047.2024.2338644
51. Yue C, Jiang Y, Li P, Wang Y, Xue J, Li N, et al. Dynamic change of pd-L1 expression on circulating tumor cells in advanced solid tumor patients undergoing pd-1 blockade therapy. Oncoimmunology. (2018) 7:e1438111. doi: 10.1080/2162402X.2018.1438111
52. Tan Z, Yue C, Ji S, Zhao C, Jia R, Zhang Y, et al. Assessment of pd-L1 expression on circulating tumor cells for predicting clinical outcomes in patients with cancer receiving pd-1/pd-L1 blockade therapies. Oncologist. (2021) 26:e2227–e38. doi: 10.1002/onco.13981
53. Raimondi L, Raimondi FM, Di Benedetto L, Cimino G, and Spinelli GP. Pd-L1 expression on circulating tumour cells may be predictive of response to regorafenib in patients diagnosed with chemorefractory metastatic colorectal cancer. Int J Mol Sci. (2020) 21(18):6907. doi: 10.3390/ijms21186907
54. Sun B, Chen H, Wang X, and Chen T. Regorafenib induces bim-mediated intrinsic apoptosis by blocking akt-mediated foxo3a nuclear export. Cell Death Discov. (2023) 9:37. doi: 10.1038/s41420-023-01338-9
55. Dirven I, Pierre E, Vander Mijnsbrugge AS, Vounckx M, Kessels JI, and Neyns B. Regorafenib combined with braf/mek inhibitors for the treatment of refractory melanoma brain metastases. Cancers (Basel). (2024) 16(23):4083. doi: 10.3390/cancers16234083
56. Okpalanwaka IF, Daugherity EA, McCormick AL, Anderson TS, Smith SL, Lawrence C, et al. A pd-L1/cd3 bispecific antibody enhances the anti-tumor effects of regorafenib against colon cancer. Mol Cancer Ther. (2025). doi: 10.1158/1535-7163.MCT-24-1015
57. Rzhevskiy A, Kapitannikova A, Malinina P, Volovetsky A, Aboulkheyr Es H, Kulasinghe A, et al. Emerging role of circulating tumor cells in immunotherapy. Theranostics. (2021) 11:8057–75. doi: 10.7150/thno.59677
58. Steinert G, Scholch S, Niemietz T, Iwata N, Garcia SA, Behrens B, et al. Immune escape and survival mechanisms in circulating tumor cells of colorectal cancer. Cancer Res. (2014) 74:1694–704. doi: 10.1158/0008-5472.CAN-13-1885
59. Shasha T, Gruijs M, and van Egmond M. Mechanisms of colorectal liver metastasis development. Cell Mol Life Sci. (2022) 79:607. doi: 10.1007/s00018-022-04630-6
60. Lin A, Zhang J, and Luo P. Crosstalk between the msi status and tumor microenvironment in colorectal cancer. Front Immunol. (2020) 11:2039. doi: 10.3389/fimmu.2020.02039
61. Jain N, Srinivasarao DA, Famta P, Shah S, Vambhurkar G, Shahrukh S, et al. The portrayal of macrophages as tools and targets: A paradigm shift in cancer management. Life Sci. (2023) 316:121399. doi: 10.1016/j.lfs.2023.121399
62. El-Kenawi A, Hanggi K, and Ruffell B. The immune microenvironment and cancer metastasis. Cold Spring Harb Perspect Med. (2020) 10(4):a037424. doi: 10.1101/cshperspect.a037424
63. Inagaki K, Kunisho S, Takigawa H, Yuge R, Oka S, Tanaka S, et al. Role of tumor-associated macrophages at the invasive front in human colorectal cancer progression. Cancer Sci. (2021) 112:2692–704. doi: 10.1111/cas.14940
64. Yunna C, Mengru H, Lei W, and Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. (2020) 877:173090. doi: 10.1016/j.ejphar.2020.173090
65. Karlsson S and Nystrom H. The extracellular matrix in colorectal cancer and its metastatic settling - alterations and biological implications. Crit Rev Oncol Hematol. (2022) 175:103712. doi: 10.1016/j.critrevonc.2022.103712
66. Dietz MS, Sutton TL, Walker BS, Gast CE, Zarour L, Sengupta SK, et al. Relevance of circulating hybrid cells as a non-invasive biomarker for myriad solid tumors. Sci Rep. (2021) 11:13630. doi: 10.1038/s41598-021-93053-7
67. Manjunath Y, Porciani D, Mitchem JB, Suvilesh KN, Avella DM, Kimchi ET, et al. Tumor-cell-macrophage fusion cells as liquid biomarkers and tumor enhancers in cancer. Int J Mol Sci. (2020) 21(5):1872. doi: 10.3390/ijms21051872
68. Wei C, Yang C, Wang S, Shi D, Zhang C, Lin X, et al. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer. (2019) 18:64. doi: 10.1186/s12943-019-0976-4
69. Friedman-DeLuca M, Karagiannis GS, Condeelis JS, Oktay MH, and Entenberg D. Macrophages in tumor cell migration and metastasis. Front Immunol. (2024) 15:1494462. doi: 10.3389/fimmu.2024.1494462
70. Magri V, De Renzi G, Marino L, De Meo M, Siringo M, Gelibter A, et al. Circulating cancer-associated macrophage-like cells as a blood-based biomarker of response to immune checkpoint inhibitors. Int J Mol Sci. (2024) 25(7):3752. doi: 10.3390/ijms25073752
71. Ren J, He J, Zhang H, Xia Y, Hu Z, Loughran P, et al. Platelet tlr4-erk5 axis facilitates net-mediated capturing of circulating tumor cells and distant metastasis after surgical stress. Cancer Res. (2021) 81:2373–85. doi: 10.1158/0008-5472.CAN-20-3222
72. Pereira-Veiga T, Schneegans S, Pantel K, and Wikman H. Circulating tumor cell-blood cell crosstalk: biology and clinical relevance. Cell Rep. (2022) 40:111298. doi: 10.1016/j.celrep.2022.111298
73. Hu C, Long L, Lou J, Leng M, Yang Q, Xu X, et al. Ctc-neutrophil interaction: A key driver and therapeutic target of cancer metastasis. BioMed Pharmacother. (2024) 180:117474. doi: 10.1016/j.biopha.2024.117474
74. Mizuno R, Kawada K, Itatani Y, Ogawa R, Kiyasu Y, and Sakai Y. The role of tumor-associated neutrophils in colorectal cancer. Int J Mol Sci. (2019) 20(3):529. doi: 10.3390/ijms20030529
75. Saini M, Szczerba BM, and Aceto N. Circulating tumor cell-neutrophil tango along the metastatic process. Cancer Res. (2019) 79:6067–73. doi: 10.1158/0008-5472.CAN-19-1972
76. Garrido-Navas C, de Miguel-Perez D, Exposito-Hernandez J, Bayarri C, Amezcua V, Ortigosa A, et al. Cooperative and escaping mechanisms between circulating tumor cells and blood constituents. Cells. (2019) 8(11):1382. doi: 10.3390/cells8111382
77. Sionov RV. Leveling up the controversial role of neutrophils in cancer: when the complexity becomes entangled. Cells. (2021) 10(9):2486. doi: 10.3390/cells10092486
78. Fatima S, Ma Y, Safrachi A, Haider S, Spring KJ, Vafaee F, et al. Harnessing liquid biopsies to guide immune checkpoint inhibitor therapy. Cancers (Basel). (2022) 14(7):1669. doi: 10.3390/cancers14071669
79. Liu X, Song J, Zhang H, Liu X, Zuo F, Zhao Y, et al. Immune checkpoint hla-E:Cd94-nkg2a mediates evasion of circulating tumor cells from nk cell surveillance. Cancer Cell. (2023) 41:272–87 e9. doi: 10.1016/j.ccell.2023.01.001
80. Yi Y, Qin G, Yang H, Jia H, Zeng Q, Zheng D, et al. Mesenchymal stromal cells increase the natural killer resistance of circulating tumor cells via intercellular signaling of cgas-sting-ifnbeta-hla. Adv Sci (Weinh). (2024) 11:e2400888. doi: 10.1002/advs.202400888
81. Yang C, Wang X, To KKW, Cui C, Luo M, Wu S, et al. Circulating tumor cells shielded with extracellular vesicle-derived cd45 evade T cell attack to enable metastasis. Signal Transduct Target Ther. (2024) 9:84. doi: 10.1038/s41392-024-01789-1
82. Ma Y, Wei J, He W, and Ren J. Neutrophil extracellular traps in cancer. MedComm (2020). (2024) 5:e647. doi: 10.1002/mco2.647
83. Bao Y, Zhang D, Guo H, and Ma W. Beyond blood: advancing the frontiers of liquid biopsy in oncology and personalized medicine. Cancer Sci. (2024) 115:1060–72. doi: 10.1111/cas.16097
84. Zhu G, Pei L, Xia H, Tang Q, and Bi F. Role of oncogenic kras in the prognosis, diagnosis and treatment of colorectal cancer. Mol Cancer. (2021) 20:143. doi: 10.1186/s12943-021-01441-4
85. Grothey A, Fakih M, and Tabernero J. Management of braf-mutant metastatic colorectal cancer: A review of treatment options and evidence-based guidelines. Ann Oncol. (2021) 32:959–67. doi: 10.1016/j.annonc.2021.03.206
86. Ciombor KK, Strickler JH, Bekaii-Saab TS, and Yaeger R. Braf-mutated advanced colorectal cancer: A rapidly changing therapeutic landscape. J Clin Oncol. (2022) 40:2706–15. doi: 10.1200/JCO.21.02541
87. Yu B and Ma W. Biomarker discovery in hepatocellular carcinoma (Hcc) for personalized treatment and enhanced prognosis. Cytokine Growth Factor Rev. (2024) 79:29–38. doi: 10.1016/j.cytogfr.2024.08.006
88. Denis JA, Patroni A, Guillerm E, Pepin D, Benali-Furet N, Wechsler J, et al. Droplet digital pcr of circulating tumor cells from colorectal cancer patients can predict kras mutations before surgery. Mol Oncol. (2016) 10:1221–31. doi: 10.1016/j.molonc.2016.05.009
89. Damin F, Galbiati S, Soriani N, Burgio V, Ronzoni M, Ferrari M, et al. Analysis of kras, nras and braf mutational profile by combination of in-tube hybridization and universal tag-microarray in tumor tissue and plasma of colorectal cancer patients. PloS One. (2018) 13:e0207876. doi: 10.1371/journal.pone.0207876
90. Saadi S, Aarab M, Tabyaoui I, and Jouti NT. Circulating tumor cells in colorectal cancer - a review of detection methods and clinical relevance. Contemp Oncol (Pozn). (2023) 27:123–31. doi: 10.5114/wo.2023.133740
91. Musina AM, Zlei M, Mentel M, Scripcariu DV, Stefan M, Anitei MG, et al. Evaluation of circulating tumor cells in colorectal cancer using flow cytometry. J Int Med Res. (2021) 49:300060520980215. doi: 10.1177/0300060520980215
92. Ferreira JA, Magalhaes A, Gomes J, Peixoto A, Gaiteiro C, Fernandes E, et al. Protein glycosylation in gastric and colorectal cancers: toward cancer detection and targeted therapeutics. Cancer Lett. (2017) 387:32–45. doi: 10.1016/j.canlet.2016.01.044
93. Lima L, Neves M, Oliveira MI, Dieguez L, Freitas R, Azevedo R, et al. Sialyl-tn identifies muscle-invasive bladder cancer basal and luminal subtypes facing decreased survival, being expressed by circulating tumor cells and metastases. Urol Oncol. (2017) 35:675 e1– e8. doi: 10.1016/j.urolonc.2017.08.012
94. Neves M, Azevedo R, Lima L, Oliveira MI, Peixoto A, Ferreira D, et al. Exploring sialyl-tn expression in microfluidic-isolated circulating tumour cells: A novel biomarker and an analytical tool for precision oncology applications. N Biotechnol. (2019) 49:77–87. doi: 10.1016/j.nbt.2018.09.004
95. Yao S, Han Y, Yang M, Jin K, and Lan H. Integration of liquid biopsy and immunotherapy: opening a new era in colorectal cancer treatment. Front Immunol. (2023) 14:1292861. doi: 10.3389/fimmu.2023.1292861
96. Rushton AJ, Nteliopoulos G, Shaw JA, and Coombes RC. A review of circulating tumour cell enrichment technologies. Cancers (Basel). (2021) 13(5):970. doi: 10.3390/cancers13050970
97. Panda SS, Lee CC, Geevimaan K, Chen KC, Yang SH, Shen CN, et al. Intracellular domain of epithelial cell adhesion molecule induces wnt receptor transcription to promote colorectal cancer progression. J BioMed Sci. (2024) 31:72. doi: 10.1186/s12929-024-01057-y
98. Chiu SY, Hsieh CH, You JF, Chu PY, Hung HY, Chu PH, et al. Enhancing prediction performance by add-on combining circulating tumor cell count, cd45(Neg) epcam(Neg) cell count on colorectal cancer, advance, and metastasis. Cancers (Basel). (2021) 13(11):2521. doi: 10.3390/cancers13112521
99. Lu X, Tan S, Wu M, Ju H, Liang X, and Li P. Evaluation of a new magnetic bead as an integrated platform for systematic ctc recognition, capture and clinical analysis. Colloids Surf B Biointerfaces. (2021) 199:111542. doi: 10.1016/j.colsurfb.2020.111542
100. Ma S, Zhou M, Xu Y, Gu X, Zou M, Abudushalamu G, et al. Clinical application and detection techniques of liquid biopsy in gastric cancer. Mol Cancer. (2023) 22:7. doi: 10.1186/s12943-023-01715-z
101. Alves JM, Estevez-Gomez N, Valecha M, Prado-Lopez S, Tomas L, Alvarino P, et al. Comparative analysis of capture methods for genomic profiling of circulating tumor cells in colorectal cancer. Genomics. (2022) 114:110500. doi: 10.1016/j.ygeno.2022.110500
102. Lin S, Feng D, Han X, Li L, Lin Y, and Gao H. Microfluidic platform for omics analysis on single cells with diverse morphology and size: A review. Anal Chim Acta. (2024) 1294:342217. doi: 10.1016/j.aca.2024.342217
103. Bhat MP, Thendral V, Uthappa UT, Lee KH, Kigga M, Altalhi T, et al. Recent advances in microfluidic platform for physical and immunological detection and capture of circulating tumor cells. Biosensors (Basel). (2022) 12(4):220. doi: 10.3390/bios12040220
104. Yang C, Chen F, Wang S, and Xiong B. Circulating tumor cells in gastrointestinal cancers: current status and future perspectives. Front Oncol. (2019) 9:1427. doi: 10.3389/fonc.2019.01427
105. Chu HY, Lu LS, Cho W, Wu SY, Chang YC, Lin CP, et al. Enumerating circulating tumor cells with a self-assembled cell array (Saca) chip: A feasibility study in patients with colorectal cancer. Cancers (Basel). (2019) 11(1):56. doi: 10.3390/cancers11010056
106. Carneiro A, Piairo P, Teixeira A, Ferreira D, Cotton S, Rodrigues C, et al. Discriminating epithelial to mesenchymal transition phenotypes in circulating tumor cells isolated from advanced gastrointestinal cancer patients. Cells. (2022) 11(3):376. doi: 10.3390/cells11030376
107. Sun H, Hu N, and Wang J. Application of microfluidic technology in antibody screening. Biotechnol J. (2022) 17:e2100623. doi: 10.1002/biot.202100623
108. Li W, Wu CC, Wang S, Zhou L, Qiao L, Ba W, et al. Identification of the target protein of the metastatic colorectal cancer-specific aptamer W3 as a biomarker by aptamer-based target cells sorting and functional characterization. Biosens Bioelectron. (2022) 213:114451. doi: 10.1016/j.bios.2022.114451
109. Moutsiopoulou A, Broyles D, Dikici E, Daunert S, and Deo SK. Molecular aptamer beacons and their applications in sensing, imaging, and diagnostics. Small. (2019) 15:e1902248. doi: 10.1002/smll.201902248
110. Lu Y, Li X, Liu Y, Li J, Chen Z, Meng X, et al. Novel molecular aptamer beacon for the specific simultaneous analysis of circulating tumor cells and exosomes of colorectal cancer patients. Anal Chem. (2023) 95:1251–61. doi: 10.1021/acs.analchem.2c04017
111. Chen Y, Yang Y, Feng J, Carrier AJ, Tyagi D, Yu X, et al. A universal monoclonal antibody-aptamer conjugation strategy for selective non-invasive bioparticle isolation from blood on a regenerative microfluidic platform. Acta Biomater. (2022) 152:210–20. doi: 10.1016/j.actbio.2022.09.001
112. Jia F, Wang Y, Fang Z, Dong J, Shi F, Zhang W, et al. Novel peptide-based magnetic nanoparticle for mesenchymal circulating tumor cells detection. Anal Chem. (2021) 93:5670–5. doi: 10.1021/acs.analchem.1c00577
113. Chowdhury T, Cressiot B, Parisi C, Smolyakov G, Thiebot B, Trichet L, et al. Circulating tumor cells in cancer diagnostics and prognostics by single-molecule and single-cell characterization. ACS Sens. (2023) 8:406–26. doi: 10.1021/acssensors.2c02308
114. Farkas B and de Leeuw NH. A perspective on modelling metallic magnetic nanoparticles in biomedicine: from monometals to nanoalloys and ligand-protected particles. . Materials (Basel). (2021) 14(13):3611. doi: 10.3390/ma14133611
115. Yun Y, Kim S, Lee SN, Cho HY, and Choi JW. Nanomaterial-based detection of circulating tumor cells and circulating cancer stem cells for cancer immunotherapy. Nano Converg. (2024) 11:56. doi: 10.1186/s40580-024-00466-x
116. Nayl AA, Abd-Elhamid AI, Aly AA, and Brase S. Recent progress in the applications of silica-based nanoparticles. RSC Adv. (2022) 12:13706–26. doi: 10.1039/d2ra01587k
117. Kefayat A, Sartipzadeh O, Molaabasi F, Amiri M, Gholami R, Mirzadeh M, et al. Microfluidic system consisting of a magnetic 3d-printed microchannel filter for isolation and enrichment of circulating tumor cells targeted by anti-her2/mof @Ferrite core-shell nanostructures: A theranostic ctc dialysis system. Anal Chem. (2024) 96:4377–84. doi: 10.1021/acs.analchem.3c03567
118. Li Z, Qin C, Zhao B, Li T, Zhao Y, Zhang X, et al. Circulating tumor cells in pancreatic cancer: more than liquid biopsy. Ther Adv Med Oncol. (2024) 16:17588359241284935. doi: 10.1177/17588359241284935
119. Mjahed RB, Astaras C, Roth A, and Koessler T. Where are we now and where might we be headed in understanding and managing brain metastases in colorectal cancer patients? Curr Treat Options Oncol. (2022) 23:980–1000. doi: 10.1007/s11864-022-00982-0
120. Ko SW and Yoon SB. Clinical implications and perspectives of portal venous circulating tumor cells in pancreatic cancer. World J Gastrointest Oncol. (2023) 15:632–43. doi: 10.4251/wjgo.v15.i4.632
121. Kilgour E, Rothwell DG, Brady G, and Dive C. Liquid biopsy-based biomarkers of treatment response and resistance. Cancer Cell. (2020) 37:485–95. doi: 10.1016/j.ccell.2020.03.012
122. Gruijs M, Zeelen C, Hellingman T, Smit J, Borm FJ, Kazemier G, et al. Detection of circulating tumor cells using the attune nxt. Int J Mol Sci. (2022) 24(1):21. doi: 10.3390/ijms24010021
123. Xu J, Liao K, Yang X, Wu C, and Wu W. Using single-cell sequencing technology to detect circulating tumor cells in solid tumors. Mol Cancer. (2021) 20:104. doi: 10.1186/s12943-021-01392-w
124. Ho HY, Chung KK, Kan CM, and Wong SC. Liquid biopsy in the clinical management of cancers. Int J Mol Sci. (2024) 25(16):8594. doi: 10.3390/ijms25168594
125. Lopresti A, Malergue F, Bertucci F, Liberatoscioli ML, Garnier S, DaCosta Q, et al. Sensitive and easy screening for circulating tumor cells by flow cytometry. JCI Insight. (2019) 5(14):e128180. doi: 10.1172/jci.insight.128180
126. Pei H, Li L, Wang Y, Sheng R, Wang Y, Xie S, et al. Single-cell phenotypic profiling of ctcs in whole blood using an integrated microfluidic device. Anal Chem. (2019) 91:11078–84. doi: 10.1021/acs.analchem.9b01647
127. Ai J, Huang Y, Yin Z, Deng Y, Yan L, Liao J, et al. Sea anemone-inspired conducting polymer sensing platform for integrated detection of tumor protein marker and circulating tumor cell. Adv Healthc Mater. (2024) 13:e2401305. doi: 10.1002/adhm.202401305
128. Francescangeli F, Magri V, De Angelis ML, De Renzi G, Gandini O, Zeuner A, et al. Sequential isolation and characterization of single ctcs and large ctc clusters in metastatic colorectal cancer patients. Cancers (Basel). (2021) 13(24):6362. doi: 10.3390/cancers13246362
129. Yu B, Shao S, and Ma W. Frontiers in pancreatic cancer on biomarkers, microenvironment, and immunotherapy. Cancer Lett. (2025) 610:217350. doi: 10.1016/j.canlet.2024.217350
130. Liu Y, Zugazagoitia J, Ahmed FS, Henick BS, Gettinger SN, Herbst RS, et al. Immune cell pd-L1 colocalizes with macrophages and is associated with outcome in pd-1 pathway blockade therapy. Clin Cancer Res. (2020) 26:970–7. doi: 10.1158/1078-0432.CCR-19-1040
131. Zhang P, Draz MS, Xiong A, Yan W, Han H, and Chen W. Immunoengineered magnetic-quantum dot nanobead system for the isolation and detection of circulating tumor cells. J Nanobiotechnology. (2021) 19:116. doi: 10.1186/s12951-021-00860-1
132. Wu CY, Fu JY, Wu CF, Hsieh MJ, Liu YH, Liu HP, et al. Malignancy prediction capacity and possible prediction model of circulating tumor cells for suspicious pulmonary lesions. J Pers Med. (2021) 11(6):444. doi: 10.3390/jpm11060444
133. Aranda E, Vieitez JM, Gomez-Espana A, Gil Calle S, Salud-Salvia A, Grana B, et al. Folfoxiri plus bevacizumab versus folfox plus bevacizumab for patients with metastatic colorectal cancer and >/=3 circulating tumour cells: the randomised phase iii visnu-1 trial. ESMO Open. (2020) 5:e000944. doi: 10.1136/esmoopen-2020-000944
134. Hao YJ, Chang LW, Yang CY, Lo LC, Lin CP, Jian YW, et al. The rare circulating tumor microemboli as a biomarker contributes to predicting early colorectal cancer recurrences after medical treatment. Transl Res. (2024) 263:1–14. doi: 10.1016/j.trsl.2023.07.011
135. Crisafulli G. Liquid biopsy and challenge of assay heterogeneity for minimal residual disease assessment in colon cancer treatment. Genes (Basel). (2025) 16(1):71. doi: 10.3390/genes16010071
136. Sastre J, Orden V, Martinez A, Bando I, Balbin M, Bellosillo B, et al. Association between baseline circulating tumor cells, molecular tumor profiling, and clinical characteristics in a large cohort of chemo-naive metastatic colorectal cancer patients prospectively collected. Clin Colorectal Cancer. (2020) 19:e110–e6. doi: 10.1016/j.clcc.2020.02.014
137. Abdallah EA, Souza ESV, Braun AC, Gasparini VA, Kupper BEC, Tariki MS, et al. A higher platelet-to-lymphocyte ratio is prevalent in the presence of circulating tumor microemboli and is a potential prognostic factor for non-metastatic colon cancer. Transl Oncol. (2021) 14:100932. doi: 10.1016/j.tranon.2020.100932
138. Tieng FYF, Abu N, Lee LH, and Ab Mutalib NS. Microsatellite instability in colorectal cancer liquid biopsy-current updates on its potential in non-invasive detection, prognosis and as a predictive marker. Diagnostics (Basel). (2021) 11(3):544. doi: 10.3390/diagnostics11030544
139. van ‘t Erve I, Medina JE, Leal A, Papp E, Phallen J, Adleff V, et al. Metastatic colorectal cancer treatment response evaluation by ultra-deep sequencing of cell-free DNA and matched white blood cells. Clin Cancer Res. (2023) 29:899–909. doi: 10.1158/1078-0432.CCR-22-2538
140. Bidard FC, Kiavue N, Ychou M, Cabel L, Stern MH, Madic J, et al. Circulating tumor cells and circulating tumor DNA detection in potentially resectable metastatic colorectal cancer: A prospective ancillary study to the unicancer prodige-14 trial. Cells. (2019) 8(6):516. doi: 10.3390/cells8060516
Keywords: circulating tumor cell, colorectal cancer, microsatellite instability (MSI), immunotherapy, drug resistance, immune microenvironment
Citation: Feng Y, Liu G, Cai Q and Zhou J (2025) Unveiling the dual role of circulating tumor cells in colorectal cancer immunotherapy: a comprehensive review of biomarker utility and immune microenvironment crosstalk. Front. Immunol. 16:1591359. doi: 10.3389/fimmu.2025.1591359
Received: 12 March 2025; Accepted: 22 May 2025;
Published: 06 June 2025.
Edited by:
Wenxue Ma, University of California, San Diego, United StatesReviewed by:
Kimia Kardani, Massachusetts General Hospital and Harvard Medical School, United StatesMagalie Dosset, University of California, San Diego, United States
Copyright © 2025 Feng, Liu, Cai and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianping Zhou, empwaGFtYUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Yifan Feng
Yifan Feng Gang Liu
Gang Liu Qixue Cai
Qixue Cai Jianping Zhou
Jianping Zhou