- 1Department of Orthopaedics, Lanzhou University Second Hospital, Lanzhou, Gansu, China
- 2Orthopaedics Clinical Medicine Research Center of Gansu Province, Lanzhou, Gansu, China
- 3Intelligent Orthopedics Industry Technology Center of Gansu Province, Lanzhou, Gansu,, China
Osteoarthritis (OA) is a chronic joint disease characterized by cartilage degradation, inflammation, and bone structural changes, leading to significant disability. Current therapeutic strategies, including traditional treatments and stem cell-based therapies, face limitations such as inability to prevent disease progression, immunogenic rejection, and tumorigenic risks. Extracellular vesicle (EVs), nanoscale membrane-bound vesicles secreted by cells, has emerged as a promising cell-free therapeutic approach due to their low immunogenicity, stability, and ability to mediate intercellular communication. This review summarizes the roles of EVs derived from various cell types, including cartilage progenitor cells (CPCs), bone marrow mesenchymal stem cells (BMSCs), synovial mesenchymal stem cells (SMSCs), adipose-derived stem cells (ADSCs), and immune cells, in OA pathogenesis and treatment. EVs exhibit multifaceted therapeutic potential, including immunomodulation, chondrocyte regeneration, and anti-inflammatory effects. Additionally, EVs serve as diagnostic biomarkers, offering non-invasive early detection of OA. Despite their promise, challenges such as scalability, targeting efficiency, and safety concerns remain. This review highlights the potential of EVs as both therapeutic agents and diagnostic tools, paving the way for innovative OA management strategies.
1 Introduction
Osteoarthritis (OA), as a prevalent chronic whole-joint disease, is characterized by low-grade systemic inflammation, degeneration of joint-associated tissues (such as articular cartilage), and ultimately, bone structural alterations leading to disability (1, 2). The degradation of articular cartilage is recognized as a hallmark of OA. Clinical factors such as trauma, obesity, and congenital abnormalities contribute to pathological conditions that impair the load-bearing capacity of cartilage and lead to chronic diseases such as OA (3, 4). With the aging population and rising obesity rates, the incidence of OA is increasing, imposing a substantial burden on individuals and socioeconomic systems. However, current therapeutic strategies for OA remain limited, encompassing both conventional treatments and stem cell-based therapies (5, 6). Traditional OA management includes non-surgical interventions, such as surgical procedures, and nonsteroidal anti-inflammatory drugs, including advanced-stage joint replacement (7–9). Unfortunately, these methods fail to address early disease initiation, halt cartilage degradation, or promote tissue regeneration (10). Novel treatment approaches, particularly those involving stem cell applications, encounter substantial obstacles such as immune rejection risks and potential tumor formation (11). Consequently, comprehensive insights into the causative elements and biological processes driving OA pathogenesis are crucial for formulating enhanced prevention and treatment approaches.
Extracellular vesicle (EVs) are nanoscale membrane-bound vesicles actively secreted by cells (12), capable of delivering genetic information from donor cells and mediating intercellular communication (13). EVs are produced through various biological processes, with their formation primarily stemming from the plasma membrane, which contributes to their minimal immunogenic properties (14, 15). These vesicles not only inherit most of the functional attributes of their parental cells but also circumvent several associated challenges, such as immune-compatibility, stability, heterogeneity, and the maintenance of stemness (16). Given the substantial limitations and risks associated with both conventional and stem cell-based therapies, EVs have garnered increasing attention as a cell-free therapeutic strategy for OA (1). Accumulating evidence suggests that EVs play a crucial and multifaceted role in OA pathogenesis, diagnosis, and treatment. This review provides a comprehensive overview of the role of EVs from various sources in OA and their potential applications in OA therapy.
2 The roles of different EVs in OA
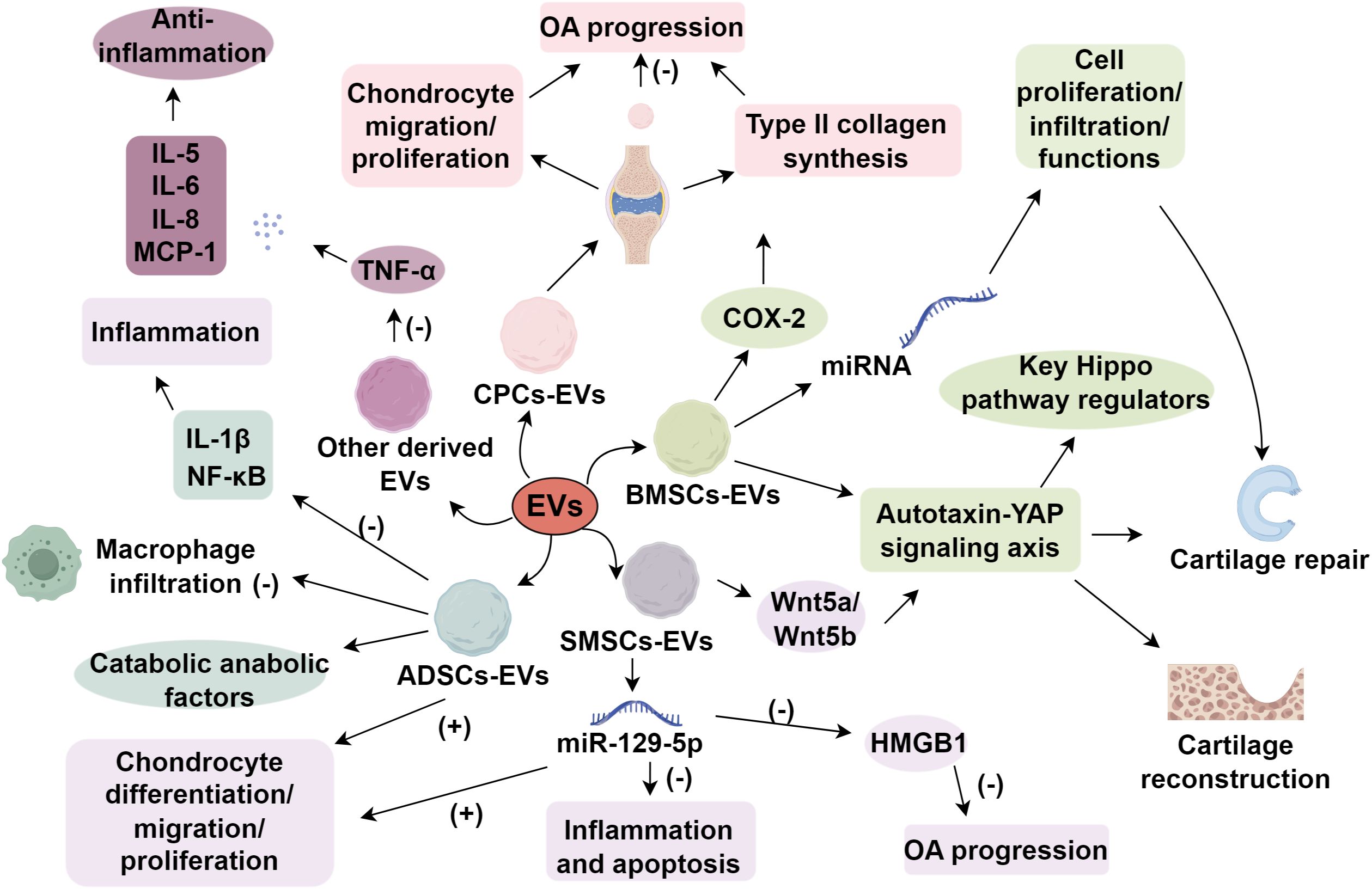
EVs derived from various cell types exert distinct roles in the treatment of OA. In normal physiological processes, OA repair and tissue regeneration encompass multiple mechanisms, including immune regulation, pain management, reduction of chondrocyte aging and metabolic imbalance, as well as stimulation of cartilage cell renewal (17, 18). In addition to their direct effects on chondrocytes, EVs interact extensively with synovial fibroblasts, the synovium, and subchondral bone. By modulating synovial fibroblast activity, EVs can reduce production of pro-inflammatory cytokines and inhibit the recruitment of immune cells (19). EVs also influence subchondral bone remodeling by regulating osteoclastogenesis and osteoblast activities via pathways such as RANKL-RANK-OPG (20). Furthermore, EV cargo containing proteolytic enzymes or their inhibitors can reshape the local extracellular matrix (ECM) environment, balancing matrix synthesis and degradation (21). Through this multidimensional interplay, EVs help restore joint homeostasis, thereby exerting regenerative and anti-inflammatory actions across multiple tissue interfaces in OA. EVs derived from various cellular origins demonstrate distinct biological characteristics. The following analysis focuses on the functional contributions of EVs produced by different cell types in OA.
2.1 Synovial mesenchymal stem cells derived EVs
Synovial mesenchymal stem cells have been demonstrated to attenuate OA progression. With superior proliferation and chondrogenic potential, SMSCs facilitate cartilage repair by accelerating chondrocyte proliferation (22). SMSCs promote cartilage repair by accelerating chondrocyte proliferation and differentiation, and their chondrogenic potential is attributed to several key factors (23). Furthermore, SMSCs exhibit tissue specificity for cartilage regeneration, underscoring their potential applications in cartilage repair (24). SMSCs express high levels of chondrogenic markers, including Sox9, collagen type II (COL2A1), and aggrecan, which are essential for cartilage formation (25). Mechanistically, SMSCs exhibit enhanced chondrogenic differentiation through the activation of the Wnt signaling pathway, which mediate the activation of the Yes-associated protein (YAP) pathway in chondrocytes (26–29). These signaling cascades stimulate chondrocyte proliferation and matrix synthesis, promoting cartilage regeneration.
A study revealed that extracellular vesicles secreted by lipopolysaccharide (LPS)-preconditioned SMSCs (LPS-pre-EVs) promote chondrocyte proliferation and migration while inhibiting apoptosis. This effect is primarily mediated by suppressing IL-1β-induced aggrecan and COL2A1 degradation and reducing ADAMTS5 expression (30). In a murine OA model, LPS-preconditioned EVs delayed early OA progression and prevented OA-induced knee cartilage damage in vivo. Given the reduced expression of miR-129-5p and the upregulation of HMGB1 in OA patients and IL-1β-induced chondrocytes, which stimulate inflammatory and apoptotic responses (31, 32). Exosomes are a subtype of EVs with a diameter typically ranging from 30 to 150 nm (33). Another study has demonstrated that SMSC-derived exosomes (SMSC-Exo) with high miR-129-5p expression significantly alleviated chondrocyte inflammation and apoptosis, whereas low miR-129-5p expression exacerbated IL-1β-mediated chondrocyte inflammation and apoptosis. This mechanism is primarily mediated through the suppression of HMGB1 release by miR-129-5p in SMSC-Exo, thereby inhibiting IL-1β-induced OA pathogenesis (34). Furthermore, SMSC-derived extracellular vesicles were found to contain abundant Wnt5a and Wnt5b, which activate YAP through non-canonical Wnt signaling pathways (35). Collectively, these findings establish SMSC-EVs as a promising acellular therapeutic strategy for OA, capable of simultaneously promoting chondrocyte differentiation, migration, and proliferation while inhibiting apoptotic processes.
2.2 EVs derived from bone marrow mesenchymal stem cells
BMSCs are a population of multipotent stem cells with adipogenic, osteogenic, and chondrogenic potential (36). They exhibit regenerative capacity and immunomodulatory functions and have been utilized in treating inflammatory and degenerative diseases such as OA, rheumatoid arthritis, and bone defects (36, 37). BMSC-EVs exert their anti-inflammatory and regenerative effects by modulating pathways such as NF-κB, which governs the release of pro-inflammatory cytokines (38). Besides, BMSC-EVs significantly mitigate IL-1β-induced suppression of chondrocyte proliferation and motility in vitro (39). Furthermore, EV-based treatment effectively counteracts the IL-1β-mediated upregulation of MMP13 and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS), while preventing the downregulation of collagen type II and aggrecan (35). Among EVs explored for OA therapy, BMSC-derived EVs (BMSC-EVs) are the most extensively studied. A recent study demonstrated that BMSC-EVs induce cartilage reconstruction in OA via the autotaxin-YAP signaling axis. Specifically, sEVs-autotaxin promotes cartilage repair and upregulates key Hippo pathway regulators (40). BMSC-EVs also facilitate cartilage defect repair by promoting cell proliferation and infiltration and modulating cellular functions through various miRNAs (41, 42). When co-cultured with OA chondrocytes, BMSC-EVs exhibit high expression of cyclooxygenase-2 (COX-2) and pro-inflammatory interleukins while inhibiting tumor necrosis factor-α (TNF-α)-induced collagen degradation and promoting ACAN and collagen II synthesis (43). Collectively, BMSC-EVs display substantial regenerative and immunoregulatory properties in OA cartilage, making them an ideal candidate for OA treatment.
2.3 EVs derived from cartilage progenitor cells
CPCs possess high self-renewal capacity and chondrogenic potential (44). Cellular populations with mesenchymal stem cell (MSC)-like properties, particularly CPCs and cartilage-derived stem cells, play pivotal roles in both cartilage formation and its regulatory processes due to their oligopotent differentiation capacity (45). Serving as cartilage progenitor cells, CPCs play a pivotal role in maintaining cartilage homeostasis. Moreover, CPCs may significantly impact OA progression by mitigating chondrocyte proliferation and cartilage formation (46). Changes in their spatial organization during OA development indicate their potential role in mediating cellular interactions among articular cartilage, subchondral bone, and adjacent joint components (44). An in vitro study demonstrated that CPCs effectively reduce inflammatory cytokines (IL-6, MCP-1, and IL-1β) and matrix metalloproteinases (MMPs) while significantly upregulating collagen II expression in IL-1β-induced human chondrocytes (47). Additionally, CPCs exhibit lower expression of the transcription factor RUNX2, which is essential for chondrocyte terminal differentiation and calcified bone formation (48). Consequently, CPCs resist hypertrophy and continuously produce hyaline-like cartilage.
A recent study applied CPC-derived EVs (CPC-EVs) in OA for the first time, comparing their therapeutic effects with MRL-EVs and normal murine EVs. This study also investigated the impact of CPC-EVs and MRL-EVs on chondrocyte proliferation and migration in vitro and in vivo (49). Unlike BMSC-EVs, which primarily act through the autotaxin-YAP signaling axis and Hippo pathway to induce cartilage reconstruction (40), CPC-EVs exhibit a more direct ECM-modulatory role, enhancing type II collagen synthesis in inner meniscal fibrochondrocytes and promoting cellular regeneration without significant involvement of hypertrophic pathways (50, 51). Recent studies have demonstrated that EVs derived from CPC-EVs exhibit a preferential localization to cartilage tissue following intra-articular injection (52). These EVs have been shown to promote matrix anabolism and inhibit inflammatory responses, at least partially by blocking STAT3 activation, thereby enhancing cartilage repair mechanisms. Intra-articular secreted EVs from CPCs may impede OA progression, paving the way for novel therapeutic strategies.
2.4 EVs derived from adipose mesenchymal stem cells
ADSCs possess regenerative capabilities akin to BMSCs. However, ADSCs have gained increasing attention due to the ease of adipose tissue harvesting and the relatively simple cell isolation process, yielding approximately 500 times more stem cells than bone marrow (53). Several studies have investigated the therapeutic potential of ADSCs in OA (54, 55). Early studies demonstrated that intra-articular injection of ADSCs confers anti-inflammatory, antioxidative, and chondroprotective effects (56, 57). Recent findings indicate that ADSC-derived EVs (ADSC-EVs) primarily function by preserving chondrocytes and suppressing inflammation. ADSC-EVs promote human OA chondrocyte proliferation and migration while modulating catabolic and anabolic factors and effectively preventing macrophage infiltration into synovial tissue (58). Inflammatory factors are pivotal in inflammatory diseases progression (59, 60). ADSC-derived EVs regulate gene expression and protein secretion in chondrocytes and synoviocytes, effectively counteracting IL-1β-induced inflammatory responses and mitigating NF-κB pathway-mediated inflammatory and catabolic environments, offering a promising strategy for OA treatment (61). Additionally, miRNAs present in ADSC-EVs have been implicated in OA pathogenesis (62). Hence, ADSC-EVs should be considered a potential therapeutic approach for OA.
2.5 EVs derived from other cell types
Comprehensive investigations into EVs from diverse cellular origins are essential for developing robust therapeutic strategies and advancing our understanding of OA pathogenesis. Immune cells, such as neutrophils and macrophages, influence the inflammatory milieu and chondrocyte senescence and metabolism in OA. Compared to stem cell-derived EVs, immune cell-derived EVs typically exhibit simpler functionality, potentially minimizing adverse effects (63). Neutrophil-derived EVs have been shown to be internalized by fibroblast-like synoviocytes in OA patients, thereby downregulating TNF-α-induced inflammatory cytokines such as IL-5, IL-6, IL-8, and MCP-1, exerting an anti-inflammatory effect (64). However, not all immune cell-derived EVs exhibit protective roles (65). Recent studies have revealed that EVs secreted by pro-inflammatory macrophages in the osteoarthritic synovium can carry potent inflammatory cargo, such as IL-1β, contributing to local joint inflammation and cartilage degradation (66). These IL-1β+ macrophage-derived EVs can enhance the activation of fibroblast-like synoviocytes and upregulate MMPs and other catabolic mediators, thereby exacerbating synovial inflammation and OA progression (67, 68). This highlights the dual, context-dependent nature of immune-derived EVs, which can either attenuate or aggravate OA pathology depending on their cellular origin and microenvironmental stimuli. Moreover, antler stem cell-derived exosomes (ASC-Exos) restore heterochromatin stability and rejuvenate senescent MSCs, offering a novel OA treatment strategy (69). While EVs from various sources present diverse therapeutic potentials for OA, rigorous preclinical studies, including long-term efficacy assessments and safety evaluations, are imperative before clinical translation (Figure 1).
3 Application of EVs in osteoarthritis treatment
3.1 Potential of EVs as therapeutic carriers
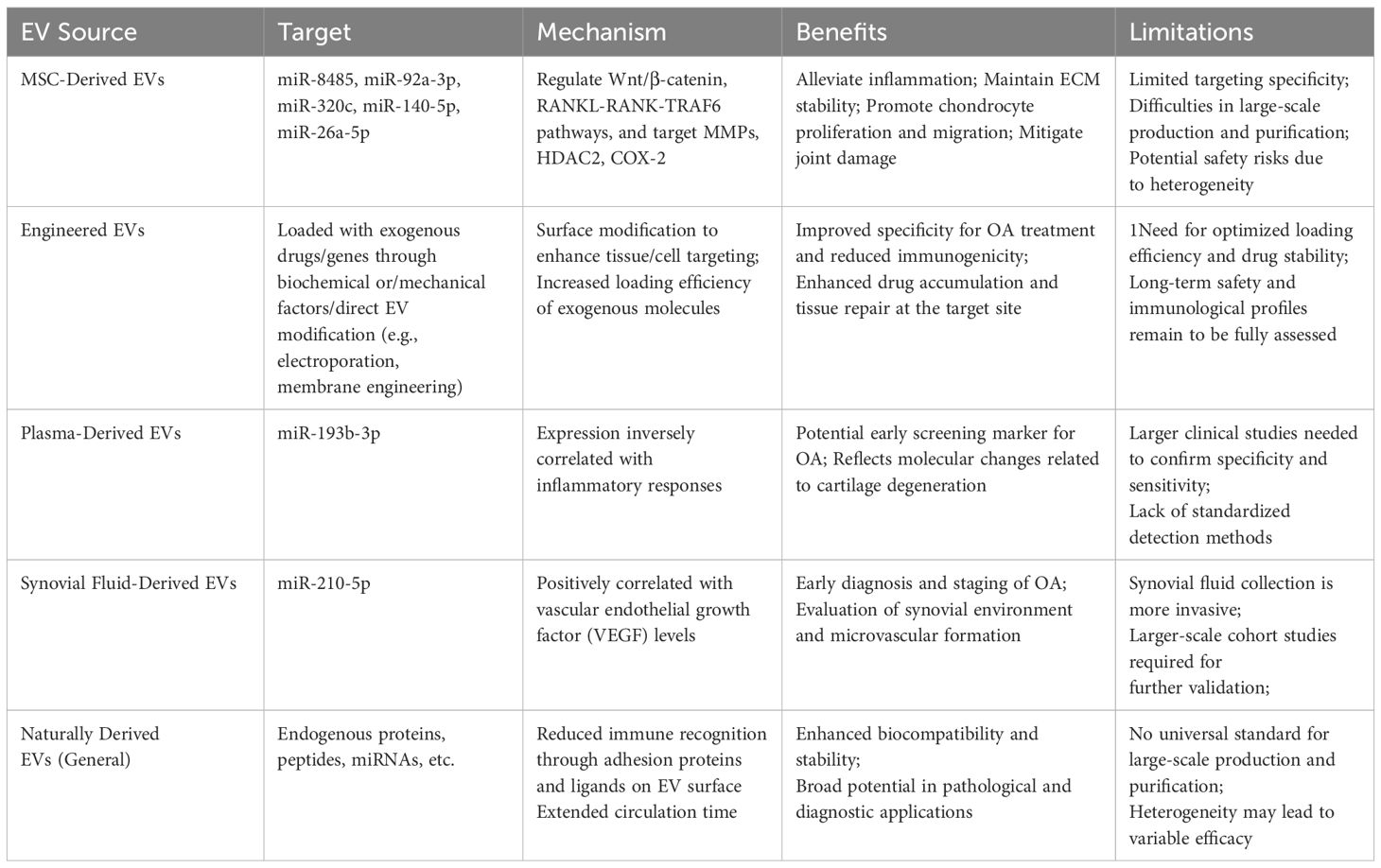
Natural EVs are widely investigated for osteoarthritis therapy due to their accessibility and effectiveness in promoting cartilage regeneration and joint repair, yet challenges such as poor targeting, scalability, and safety hinder clinical translation, driving research into engineered EVs as optimized drug delivery systems (70, 71). Currently, engineering strategies are being developed to improve EV-mediated drug delivery, targeting precision, and therapeutic efficacy (72). Methods for loading exogenous molecules into EVs can be broadly categorized into two approaches (73, 74). The first approach involves modifying donor cells using biochemical factors or mechanical factors. The second strategy entails direct EV modifications, including exogenous cargo loading and membrane engineering.
Moreover, numerous studies have demonstrated that MSC-derived exosomes contain bioactive molecules, such as miR-8485, miR-95a-5p, miR-320c, miR-150-5p, and miR-26a-5p, which regulate pathways such as Wnt/β-catenin and RANKL-RANK-TRAF6 by targeting proteins including MMPs, histone deacetylase 2 (HDAC2), and COX-2. These regulatory effects help mitigate inflammation, maintain ECM stability, reduce OA chondrocyte apoptosis, and promote chondrocyte proliferation and migration, thereby alleviating joint damage (75–81). Furthermore, immune responses within joint cartilage play a crucial role in OA progression. Evidence suggests that MSC-derived exosomes exert immunomodulatory effects by suppressing T lymphocytes while activating B lymphocytes, highlighting their potential in immune regulation and making this a promising avenue for future research (82). Notwithstanding their generally low immunogenicity, EVs may still pose immunological risks under certain conditions. If EVs carry unexpected alloantigens pathogen-derived components, or pro-inflammatory molecules (83, 84), they could inadvertently trigger immune activation, particularly in individuals with underlying autoimmune disorders or compromised immune tolerance. Hence, stringent purification, characterization, and screening protocols are vital to minimizing adverse immunologic reactions in EV-based therapies.
3.2 Application of EVs as diagnostic biomarkers
Currently, OA diagnosis relies on clinical symptoms and physical examinations, with imaging techniques such as radiography used as supplementary tools when necessary (85). However, early-stage OA is often asymptomatic, and treatment becomes increasingly challenging as the disease progresses. Therefore, there is an urgent need for biomarkers to facilitate early diagnosis (86). Blood sampling provides a minimally invasive approach, and given that EVs remain stable in body fluids, they hold promise as screening tools for early-stage OA. EV-based diagnostics could enable non-invasive assessment of joint burden and potential risk factors before imaging-confirmed OA manifestations. Additionally, early biomarker-based diagnosis could offer insights into cellular and molecular alterations, facilitating timely and targeted interventions. EVs are promising novel biomarkers due to their ability to encapsulate donor cell-derived molecular signatures while maintaining strong stability within the circulatory system (86). Compared with existing OA biomarkers such as cartilage oligomeric matrix protein (COMP) (87), C-reactive protein (CRP) (88), and certain pro-inflammatory cytokines (89), EV-derived markers may offer higher sensitivity and specificity by virtue of their cell-type-specific cargo (90). Their encapsulated structure not only prolongs half-life in the circulation but also preserves the integrity of proteins and nucleic acids that can be degraded in free form. Additionally, EV-based biomarkers may allow for earlier detection of OA by capturing dynamic changes in gene expression and protein composition within the joint environment, thus providing a more refined molecular fingerprint of disease progression (91). Studies have demonstrated that plasma-derived exosomal miR-193b-3p expression is significantly reduced in OA patients compared to healthy individuals, mirroring findings from degenerative cartilage samples (92). Furthermore, previous research has indicated an inverse correlation between serum miR-142-5p expression and inflammatory responses, suggesting that plasma exosomal miR-142-5p represents a potential biomarker for OA (93). However, given that these miRNAs also exhibit differential expression patterns in rheumatoid arthritis (94), the development of a multi-miRNA panel would significantly improve the accuracy of clinical differential diagnosis between these two arthritic conditions.
The advancement of EV-derived biomarker systems, incorporating diverse molecular components such as genetic material, protein complexes, lipid structures, and carbohydrate molecules, has demonstrated significant potential across multiple medical domains, especially in cancer research, metabolic syndrome investigations, and neurological disorder studies (95–97). A notable example is the ExoDx™ Lung test, which was the first EV-based biomarker to undergo clinical trials in 2016 for detecting EML4-ALK mutations in lung cancer diagnosis (98). This milestone underscored the superior diagnostic potential of EVs. In the context of OA, synovial EVs have been identified as markers for differentiating disease stages, further expanding their diagnostic value (99). Initial studies on EVs as OA biomarkers emerged from mechanistic investigations, revealing differential miRNA and other nucleic acid expressions between healthy individuals and OA patients (100). Subsequently, EVs were found to facilitate OA subtype differentiation, introducing a novel paradigm for clinical diagnosis (101). Furthermore, the isolation of exosomes derived from biological fluids such as blood and urine, followed by the characterization of their cargo, has emerged as a promising approach for identifying biochemical biomarkers to predict cartilage degeneration and assess the progression of joint-related diseases. Recent studies have extracted synovial fluid-derived exosomes from patients with OA and performed RNA sequencing analysis on their miRNA content, revealing a significant upregulation of miR-210-5p (102). Further investigations have demonstrated that miR-210 is markedly upregulated in synovial fluid samples from both early- and late-stage OA patients and is positively correlated with VEGF levels. These findings suggest that the upregulation of miR-210 in synovial fluid may occur at the early stages of OA, highlighting its potential as a non-invasive and early diagnostic biomarker for identifying individuals at risk of developing OA and enabling rapid disease detection (103). EVs have already been employed in clinical research for disease diagnosis, progression monitoring, and prognostic evaluation. These findings underscore the robust potential of EVs as biomarkers, and it is anticipated that EV-based OA biomarker applications will be realized in clinical practice in the near future (Table 1).
4 Conclusion
OA remains a significant global health challenge, with current therapeutic strategies offering limited efficacy in halting disease progression or promoting cartilage regeneration. EVs have emerged as a promising alternative, offering a cell-free approach to OA treatment with advantages such as low immunogenicity, stability, and the ability to mediate intercellular communication. EVs derived from various cell types, including CPCs, BMSCs, SMSCs, ADSCs, and immune cells, demonstrate diverse therapeutic potentials, including immunomodulation, chondrocyte regeneration, and anti-inflammatory effects.
Furthermore, EVs hold significant promise as diagnostic biomarkers, enabling early detection and monitoring of OA progression through non-invasive methods. However, challenges such as scalability, targeted delivery, and safety concerns must be addressed before clinical translation. Future research should focus on optimizing EV-based therapies, improving their targeting efficiency, and conducting rigorous preclinical and clinical trials to ensure their efficacy and safety. Overall, EVs represent a transformative approach to OA management, offering hope for more effective prevention, diagnosis, and treatment of OA.
Author contributions
CS: Writing – original draft. FT: Writing – original draft. YX: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by The National Natural Science Foundation of China (82060405 and 82360436); Lanzhou Science and Technology Plan Program (2021-RC-102); Natural Science Foundation of Gansu Province (22JR5RA943, 22JR5RA956 and 23JRRA1500); Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (CY2021-MS-A07, CY2022-MS-A19).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yin B, Ni J, Witherel CE, Yang M, Burdick JA, Wen C, et al. Harnessing tissue-derived extracellular vesicles for osteoarthritis theranostics. Theranostics. (2022) 12:207–31. doi: 10.7150/thno.62708
2. Wu P. Unveiling metabolic signatures in osteoarthritis progression through non-targeted metabolomics analysis: A paradigm shift in diagnosis and treatment prospects. Phenomics. (2024) 4:525–6. doi: 10.1007/s43657-024-00177-7
3. Wu X, Fan X, Crawford R, Xiao Y, and Prasadam I. The metabolic landscape in osteoarthritis. Aging Dis. (2022) 13:1166–82. doi: 10.14336/AD.2021.1228
4. Sun S, Chen M, Zhang T, Wang Y, Shen W, Zhang T, et al. Identification of key factors in cartilage tissue during the progression of osteoarthritis using a non-targeted metabolomics strategy. Phenomics. (2024) 4:227–33. doi: 10.1007/s43657-023-00123-z
5. Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC, and Zhang CQ. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. (2017) 7:180–95. doi: 10.7150/thno.17133
6. Zhou JF, Xiong Y, Kang X, Pan Z, Zhu Q, Goldbrunner R, et al. Application of stem cells and exosomes in the treatment of intracerebral hemorrhage: an update. Stem Cell Res Ther. (2022) 13:281. doi: 10.1186/s13287-022-02965-2
7. Vaishya R, Pariyo GB, Agarwal AK, and Vijay V. Non-operative management of osteoarthritis of the knee joint. J Clin Orthop Trauma. (2016) 7:170–6. doi: 10.1016/j.jcot.2016.05.005
8. Sharif MU, Aslam HM, Iftakhar T, and Abdullah M. Pathophysiology of cartilage damage in knee osteoarthritis and regenerative approaches toward recovery. J Bone Jt Dis. (2024) 39:32–44. doi: 10.4103/jbjd.jbjd_2_24
9. Cai W, Zhang Y, Jin W, Wei S, Chen J, Zhong C, et al. Procyanidin B2 ameliorates the progression of osteoarthritis: An in vitro and in vivo study. Int Immunopharmacol. (2022) 113:109336. doi: 10.1016/j.intimp.2022.109336
10. Ferreira RM, Duarte JA, and Goncalves RS. Non-pharmacological and non-surgical interventions to manage patients with knee osteoarthritis: An umbrella review. Acta Reumatol Port. (2018) 43:182–200.
11. Palermi S, Gnasso R, Belviso I, Iommazzo I, Vecchiato M, Marchini A, et al. Stem cell therapy in sports medicine: current applications, challenges and future perspectives. J Basic Clin Physiol Pharmacol. (2023) 34:699–706. doi: 10.1515/jbcpp-2023-0200
12. Xiong J, Chi H, Yang G, Zhao S, Zhang J, Tran LJ, et al. Revolutionizing anti-tumor therapy: unleashing the potential of B cell-derived exosomes. Front Immunol. (2023) 14:1188760. doi: 10.3389/fimmu.2023.1188760
13. Li Y, Guo X, Guo S, Wang Y, Chen L, Liu Y, et al. Next generation sequencing-based analysis of mitochondrial DNA characteristics in plasma extracellular vesicles of patients with hepatocellular carcinoma. Oncol Lett. (2020) 20:2820–8. doi: 10.3892/ol.2020.11831
14. Li R, Wang H, Wang X, Yang Y, Zhong K, Zhang X, et al. MSC-EVs and UCB-EVs promote skin wound healing and spatial transcriptome analysis. Sci Rep. (2025) 15:4006. doi: 10.1038/s41598-025-87592-6
15. Guo Y, Liu Q, Yang J, Gao Y, and Liu Y. The role of stem cell-derived exosomes in regulating pyroptosis for disease therapy. Stem Cell Res Ther. (2025) 16:386. doi: 10.1186/s13287-025-04519-8
16. Zhou T, Yuan Z, Weng J, Pei D, Du X, He C, et al. Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol. (2021) 14:24. doi: 10.1186/s13045-021-01037-x
17. Zheng L, Zhang Z, Sheng P, and Mobasheri A. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res Rev. (2021) 66:101249. doi: 10.1016/j.arr.2020.101249
18. Grassel S and Aszodi A. Osteoarthritis and cartilage regeneration: focus on pathophysiology and molecular mechanisms. Int J Mol Sci. (2019) 20:6156. doi: 10.3390/ijms20246156
19. Ni Z, Zhou S, Li S, Kuang L, Chen H, Luo X, et al. Exosomes: roles and therapeutic potential in osteoarthritis. Bone Res. (2020) 8:25. doi: 10.1038/s41413-020-0100-9
20. Chen W, Wang Q, Tao H, Lu L, Zhou J, Wang Q, et al. Subchondral osteoclasts and osteoarthritis: new insights and potential therapeutic avenues. Acta Biochim Biophys Sin (Shanghai). (2024) 56:499–512. doi: 10.3724/abbs.2024017
21. Nawaz M, Shah N, Zanetti BR, Maugeri M, Silvestre RN, Fatima F, et al. Extracellular vesicles and matrix remodeling enzymes: the emerging roles in extracellular matrix remodeling, progression of diseases and tissue repair. Cells. (2018) 7:167. doi: 10.3390/cells7100167
22. Tang G, Asou Y, Matsumura E, Nakagawa Y, Miyatake K, Katagiri H, et al. Short cytoplasmic isoform of IL1R1/CD121a mediates IL1beta induced proliferation of synovium-derived mesenchymal stem/stromal cells through ERK1/2 pathway. Heliyon. (2022) 8:e09476. doi: 10.1016/j.heliyon.2022.e09476
23. Harrell CR, Markovic BS, Fellabaum C, Arsenijevic A, and Volarevic V. Mesenchymal stem cell-based therapy of osteoarthritis: Current knowledge and future perspectives. BioMed Pharmacother. (2019) 109:2318–26. doi: 10.1016/j.biopha.2018.11.099
24. Jones BA and Pei M. Synovium-derived stem cells: a tissue-specific stem cell for cartilage engineering and regeneration. Tissue Eng Part B Rev. (2012) 18:301–11. doi: 10.1089/ten.teb.2012.0002
25. Kitahashi T, Kogawa R, Nakamura K, and Sekiya I. Integrin β1, PDGFRβ, and type II collagen are essential for meniscus regeneration by synovial mesenchymal stem cells in rats. Sci Rep. (2022) 12:14148. doi: 10.1038/s41598-022-18476-2
26. Li F, Xu Z, Xie Z, Sun X, Li C, Chen Y, et al. Adipose mesenchymal stem cells-derived exosomes alleviate osteoarthritis by transporting microRNA -376c-3p and targeting the WNT-beta-catenin signaling axis. Apoptosis. (2023) 28:362–78. doi: 10.1007/s10495-022-01787-0
27. Kong R, Zhang J, Ji L, Yu Y, Gao J, and Zhao D. Synovial mesenchymal stem cell-derived exosomal microRNA-320c facilitates cartilage damage repair by targeting ADAM19-dependent Wnt signalling in osteoarthritis rats. Inflammopharmacology. (2023) 31:915–26. doi: 10.1007/s10787-023-01142-y
28. Jiang L, Li J, Zhang C, Shang Y, and Lin J. YAP−mediated crosstalk between the Wnt and Hippo signaling pathways (Review). Mol Med Rep. (2020) 22:4101–6. doi: 10.3892/mmr.2020.11529
29. Nakako Y, Hasegawa K, Fujii S, Kami Y, Sakamoto T, Sakamoto M, et al. Wnt/β-catenin-YAP axis in the pathogenesis of primary intraosseous carcinoma NOS, deriving from odontogenic keratocyst. Pathol Res Pract. (2024) 260:155420. doi: 10.1016/j.prp.2024.155420
30. Duan A, Shen K, Li B, Li C, Zhou H, Kong R, et al. Extracellular vesicles derived from LPS-preconditioned human synovial mesenchymal stem cells inhibit extracellular matrix degradation and prevent osteoarthritis of the knee in a mouse model. Stem Cell Res Ther. (2021) 12:427. doi: 10.1186/s13287-021-02507-2
31. Ma R, Chen X, Ma Y, Bai G, and Li DS. MiR-129-5p alleviates myocardial injury by targeting suppressor of cytokine signaling 2 after ischemia/reperfusion. Kaohsiung J Med Sci. (2020) 36:599–606. doi: 10.1002/kjm2.12211
32. Wenzhao L, Jiangdong N, Deye S, Muliang D, Junjie W, Xianzhe H, et al. Dual regulatory roles of HMGB1 in inflammatory reaction of chondrocyte cells and mice. Cell Cycle. (2019) 18:2268–80. doi: 10.1080/15384101.2019.1642680
33. Gong X, Chi H, Strohmer DF, Teichmann AT, Xia Z, and Wang Q. Exosomes: A potential tool for immunotherapy of ovarian cancer. Front Immunol. (2022) 13:1089410. doi: 10.3389/fimmu.2022.1089410
34. Qiu M, Liu D, and Fu Q. MiR-129-5p shuttled by human synovial mesenchymal stem cell-derived exosomes relieves IL-1beta induced osteoarthritis via targeting HMGB1. Life Sci. (2021) 269:118987. doi: 10.1016/j.lfs.2020.118987
35. Pakdaman Kolour SS, Nematollahi S, Dehbozorgi M, Fattahi F, Movahed F, Esfandiari N, et al. Extracecellulr vesicles (EVs) microRNAs (miRNAs) derived from mesenchymal stem cells (MSCs) in osteoarthritis (OA); detailed role in pathogenesis and possible therapeutics. Heliyon. (2025) 11:e42258. doi: 10.1016/j.heliyon.2025.e42258
36. Wang M, Dai T, Meng Q, Wang W, and Li S. Regulatory effects of miR-28 on osteogenic differentiation of human bone marrow mesenchymal stem cells. Bioengineered. (2022) 13:684–96. doi: 10.1080/21655979.2021.2012618
37. Bulati M, Miceli V, Gallo A, Amico G, Carcione C, Pampalone M, et al. The immunomodulatory properties of the human amnion-derived mesenchymal stromal/stem cells are induced by INF-gamma produced by activated lymphomonocytes and are mediated by cell-to-cell contact and soluble factors. Front Immunol. (2020) 11:54. doi: 10.3389/fimmu.2020.00054
38. Nie H and Jiang Z. Bone mesenchymal stem cell-derived extracellular vesicles deliver microRNA-23b to alleviate spinal cord injury by targeting toll-like receptor TLR4 and inhibiting NF-kappaB pathway activation. Bioengineered. (2021) 12:8157–72. doi: 10.1080/21655979.2021.1977562
39. He L, He T, Xing J, Zhou Q, Fan L, Liu C, et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res Ther. (2020) 11:276. doi: 10.1186/s13287-020-01781-w
40. Wang Y, Zhao M, Li W, Yang Y, Zhang Z, Ma R, et al. BMSC-derived small extracellular vesicles induce cartilage reconstruction of temporomandibular joint osteoarthritis via autotaxin-YAP signaling axis. Front Cell Dev Biol. (2021) 9:656153. doi: 10.3389/fcell.2021.656153
41. Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, and Toh WS. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. (2018) 156:16–27. doi: 10.1016/j.biomaterials.2017.11.028
42. Cosenza S, Ruiz M, Toupet K, Jorgensen C, and Noel D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. (2017) 7:16214. doi: 10.1038/s41598-017-15376-8
43. Vonk LA, van Dooremalen SFJ, Liv N, Klumperman J, Coffer PJ, Saris DBF, et al. Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration. In Vitro Theranostics. (2018) 8:906–20. doi: 10.7150/thno.20746
44. Jiang Y and Tuan RS. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat Rev Rheumatol. (2015) 11:206–12. doi: 10.1038/nrrheum.2014.200
45. Mejia J, Salisbury E, Sonnet C, Gugala Z, Olmsted-Davis EA, and Davis AR. A replicating stem-like cell that contributes to bone morphogenetic protein 2-induced heterotopic bone formation. Stem Cells Transl Med. (2021) 10:623–35. doi: 10.1002/sctm.20-0378
46. Yamasaki K, Nakasa T, Miyaki S, Ishikawa M, Deie M, Adachi N, et al. Expression of MicroRNA-146a in osteoarthritis cartilage. Arthritis Rheum. (2009) 60:1035–41. doi: 10.1002/art.24404
47. Kim J, Tran AN, Lee JY, Park SH, Park SR, Min BH, et al. Human fetal cartilage-derived progenitor cells exhibit anti-inflammatory effect on IL-1beta-mediated osteoarthritis phenotypes. In Vitro Tissue Eng Regener Med. (2022) 19:1237–50. doi: 10.1007/s13770-022-00478-w
48. Vinod E, Parameswaran R, Ramasamy B, and Kachroo U. Pondering the potential of hyaline cartilage-derived chondroprogenitors for tissue regeneration: A systematic review. Cartilage. (2021) 13:34S–52S. doi: 10.1177/1947603520951631
49. Wang R, Jiang W, Zhang L, Xie S, Zhang S, Yuan S, et al. Intra-articular delivery of extracellular vesicles secreted by chondrogenic progenitor cells from MRL/MpJ superhealer mice enhances articular cartilage repair in a mouse injury model. Stem Cell Res Ther. (2020) 11:93. doi: 10.1186/s13287-020-01594-x
50. Betensky DJ, Chen MD, Trivedi J, Desai S, Twomey-Kozak J, Wen S, et al. Extracellular vesicles from cartilage progenitors stimulate type II collagen expression and wound healing in meniscal cells. J Orthop Res. (2025) 43:682–91. doi: 10.1002/jor.26013
51. Li W, Shi Z, Jing H, Dou Y, Liu X, Zhang M, et al. Streamlined metal-based hydrogel facilitates stem cell differentiation, extracellular matrix homeostasis and cartilage repair in male rats. Nat Commun. (2025) 16:4344. doi: 10.1038/s41467-025-59725-y
52. González-Rodríguez A, De Toro FJ, Jorge-Mora A, Fernandez-Pernas P, Rivadulla CP, Fraga M, et al. Targeting osteoarthritis with small extracellular vesicle therapy: potential and perspectives. Front Bioeng Biotechnol. (2025) 13:1570526. doi: 10.3389/fbioe.2025.1570526
53. Sanghani-Kerai A, Black C, Cheng SO, Collins L, Schneider N, Blunn G, et al. Clinical outcomes following intra-articular injection of autologous adipose-derived mesenchymal stem cells for the treatment of osteoarthritis in dogs characterized by weight-bearing asymmetry. Bone Joint Res. (2021) 10:650–8. doi: 10.1302/2046-3758.1010.BJR-2020-0540.R1
54. Damia E, Chicharro D, Lopez S, Cuervo B, Rubio M, Sopena JJ, et al. Adipose-derived mesenchymal stem cells: are they a good therapeutic strategy for osteoarthritis? Int J Mol Sci. (2018) 19:1926. doi: 10.3390/ijms19071926
55. Song Y, Du H, Dai C, Zhang L, Li S, Hunter DJ, et al. Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regener Med. (2018) 13:295–307. doi: 10.2217/rme-2017-0152
56. ter Huurne M, Schelbergen R, Blattes R, Blom A, de Munter W, Grevers LC, et al. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. (2012) 64:3604–13. doi: 10.1002/art.34626
57. Desando G, Cavallo C, Sartoni F, Martini L, Parrilli A, Veronesi F, et al. Intra-articular delivery of adipose derived stromal cells attenuates osteoarthritis progression in an experimental rabbit model. Arthritis Res Ther. (2013) 15:R22. doi: 10.1186/ar4156
58. Woo CH, Kim HK, Jung GY, Jung YJ, Lee KS, Yun YE, et al. Small extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. J Extracell Vesicles. (2020) 9:1735249. doi: 10.1080/20013078.2020.1735249
59. Zhai X, Zhang H, Xia Z, Liu M, Du G, Jiang Z, et al. Oxytocin alleviates liver fibrosis via hepatic macrophages. JHEP Rep. (2024) 6:101032. doi: 10.1016/j.jhepr.2024.101032
60. Xie H, Xi X, Lei T, Liu H, and Xia Z. CD8(+) T cell exhaustion in the tumor microenvironment of breast cancer. Front Immunol. (2024) 15:1507283. doi: 10.3389/fimmu.2024.1507283
61. Cavallo C, Merli G, Borzi RM, Zini N, D’Adamo S, Guescini M, et al. Small Extracellular Vesicles from adipose derived stromal cells significantly attenuate in vitro the NF-kappaB dependent inflammatory/catabolic environment of osteoarthritis. Sci Rep. (2021) 11:1053. doi: 10.1038/s41598-020-80032-7
62. Ragni E, Perucca Orfei C, De Luca P, Vigano M, Colombini A, Lugano G, et al. miR-22-5p and miR-29a-5p Are Reliable Reference Genes for Analyzing Extracellular Vesicle-Associated miRNAs in Adipose-Derived Mesenchymal Stem Cells and Are Stable under Inflammatory Priming Mimicking Osteoarthritis Condition. Stem Cell Rev Rep. (2019) 15:743–54. doi: 10.1007/s12015-019-09899-y
63. Liu Z, Zhuang Y, Fang L, Yuan C, Wang X, and Lin K. Breakthrough of extracellular vesicles in pathogenesis, diagnosis and treatment of osteoarthritis. Bioact Mater. (2023) 22:423–52. doi: 10.1016/j.bioactmat.2022.10.012
64. Zhan D, Cross A, Wright HL, Moots RJ, Edwards SW, and Honsawek S. Internalization of neutrophil-derived microvesicles modulates TNFalpha-stimulated proinflammatory cytokine production in human fibroblast-like synoviocytes. Int J Mol Sci. (2021) 22:7409. doi: 10.3390/ijms22147409
65. Ghosh P, Sasaki K, Pulido Ruiz IA, King KE, Weinman SA, and Wozniak AL. Inflammatory macrophage to hepatocyte signals can be prevented by extracellular vesicle reprogramming. J Cell Sci. (2023) 136:jcs260691. doi: 10.1242/jcs.260691
66. Kodidela S, Sinha N, Kumar A, Zhou L, Godse S, and Kumar S. Extracellular vesicles released from macrophages modulates interleukin-1β in astrocytic and neuronal cells. Sci Rep. (2023) 13:3005. doi: 10.1038/s41598-023-29746-y
67. Ni Z, Kuang L, Chen H, Xie Y, Zhang B, Ouyang J, et al. The exosome-like vesicles from osteoarthritic chondrocyte enhanced mature IL-1β production of macrophages and aggravated synovitis in osteoarthritis. Cell Death Dis. (2019) 10:522. doi: 10.1038/s41419-019-1739-2
68. Liu B, Xian Y, Chen X, Shi Y, Dong J, Yang L, et al. Inflammatory fibroblast-like synoviocyte-derived exosomes aggravate osteoarthritis via enhancing macrophage glycolysis. Adv Sci (Weinh). (2024) 11:e2307338. doi: 10.1002/advs.202307338
69. Lei J, Jiang X, Li W, Ren J, Wang D, Ji Z, et al. Exosomes from antler stem cells alleviate mesenchymal stem cell senescence and osteoarthritis. Protein Cell. (2022) 13:220–6. doi: 10.1007/s13238-021-00860-9
70. Liang F, Zheng Y, Zhao C, Li L, Hu Y, Wang C, et al. Microalgae-derived extracellular vesicles synergize with herbal hydrogel for energy homeostasis in osteoarthritis treatment. ACS Nano. (2025) 19:8040–57. doi: 10.1021/acsnano.4c16085
71. Zhan J, Zou J, Pang Q, Chen Z, Liu J, Liu S, et al. MSCs-EVs harboring OA immune memory reprogram macrophage phenotype via modulation of the mt-ND3/NADH-CoQ axis for OA treatment. J Nanobiotechnol. (2025) 23:140. doi: 10.1186/s12951-025-03216-1
72. Armstrong JP, Holme MN, and Stevens MM. Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano. (2017) 11:69–83. doi: 10.1021/acsnano.6b07607
73. Liao W, Du Y, Zhang C, Pan F, Yao Y, Zhang T, et al. Exosomes: The next generation of endogenous nanomaterials for advanced drug delivery and therapy. Acta Biomater. (2019) 86:1–14. doi: 10.1016/j.actbio.2018.12.045
74. Esmaeili A, Hosseini S, and Baghaban Eslaminejad M. Engineered-extracellular vesicles as an optimistic tool for microRNA delivery for osteoarthritis treatment. Cell Mol Life Sci. (2021) 78:79–91. doi: 10.1007/s00018-020-03585-w
75. Pourakbari R, Khodadadi M, Aghebati-Maleki A, Aghebati-Maleki L, and Yousefi M. The potential of exosomes in the therapy of the cartilage and bone complications; emphasis on osteoarthritis. Life Sci. (2019) 236:116861. doi: 10.1016/j.lfs.2019.116861
76. Wang Y, Yu D, Liu Z, Zhou F, Dai J, Wu B, et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther. (2017) 8:189. doi: 10.1186/s13287-017-0632-0
77. Sun H, Hu S, Zhang Z, Lun J, Liao W, and Zhang Z. Expression of exosomal microRNAs during chondrogenic differentiation of human bone mesenchymal stem cells. J Cell Biochem. (2019) 120:171–81. doi: 10.1002/jcb.27289
78. Mao G, Zhang Z, Hu S, Zhang Z, Chang Z, Huang Z, et al. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res Ther. (2018) 9:247. doi: 10.1186/s13287-018-1004-0
79. Chen Z, Wang H, Xia Y, Yan F, and Lu Y. Therapeutic potential of mesenchymal cell-derived miRNA-150-5p-expressing exosomes in rheumatoid arthritis mediated by the modulation of MMP14 and VEGF. J Immunol. (2018) 201:2472–82. doi: 10.4049/jimmunol.1800304
80. Li Z, Wang Y, Xiang S, Zheng Z, Bian Y, Feng B, et al. Chondrocytes-derived exosomal miR-8485 regulated the Wnt/beta-catenin pathways to promote chondrogenic differentiation of BMSCs. Biochem Biophys Res Commun. (2020) 523:506–13. doi: 10.1016/j.bbrc.2019.12.065
81. Mao G, Hu S, Zhang Z, Wu P, Zhao X, Lin R, et al. Exosomal miR-95-5p regulates chondrogenesis and cartilage degradation via histone deacetylase 2/8. J Cell Mol Med. (2018) 22:5354–66. doi: 10.1111/jcmm.13808
82. Zhang HG, Liu C, Su K, Yu S, Zhang L, Zhang S, et al. A membrane form of TNF-alpha presented by exosomes delays T cell activation-induced cell death. J Immunol. (2006) 176:7385–93. doi: 10.4049/jimmunol.176.12.7385
83. Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. (2014) 28:970–3. doi: 10.1038/leu.2014.41
84. Peng Q and Mu H. The potential of protein-nanomaterial interaction for advanced drug delivery. J Control Release. (2016) 225:121–32. doi: 10.1016/j.jconrel.2016.01.041
85. Hunter DJ and Bierma-Zeinstra S. Osteoarthritis. Lancet. (2019) 393:1745–59. doi: 10.1016/S0140-6736(19)30417-9
86. Maehara M, Toyoda E, Takahashi T, Watanabe M, and Sato M. Potential of exosomes for diagnosis and treatment of joint disease: towards a point-of-care therapy for osteoarthritis of the knee. Int J Mol Sci. (2021) 22:2666. doi: 10.3390/ijms22052666
87. Verma P and Dalal K. Serum cartilage oligomeric matrix protein (COMP) in knee osteoarthritis: a novel diagnostic and prognostic biomarker. J Orthop Res. (2013) 31:999–1006. doi: 10.1002/jor.22324
88. Sowers M, Jannausch M, Stein E, Jamadar D, Hochberg M, and Lachance L. C-reactive protein as a biomarker of emergent osteoarthritis. Osteoarthritis Cartilage. (2002) 10:595–601. doi: 10.1053/joca.2002.0800
89. Daghestani HN and Kraus VB. Inflammatory biomarkers in osteoarthritis. Osteoarthritis Cartilage. (2015) 23:1890–6. doi: 10.1016/j.joca.2015.02.009
90. Budd E, Nalesso G, and Mobasheri A. Extracellular genomic biomarkers of osteoarthritis. Expert Rev Mol Diagn. (2018) 18:55–74. doi: 10.1080/14737159.2018.1415757
91. Anderson JR, Johnson E, Jenkins R, Jacobsen S, Green D, Walters M, et al. Multi-omic temporal landscape of plasma and synovial fluid-derived extracellular vesicles using an experimental model of equine osteoarthritis. Int J Mol Sci. (2023) 24:14888. doi: 10.3390/ijms241914888
92. Meng F, Li Z, Zhang Z, Yang Z, Kang Y, Zhao X, et al. MicroRNA-193b-3p regulates chondrogenesis and chondrocyte metabolism by targeting HDAC3. Theranostics. (2018) 8:2862–83. doi: 10.7150/thno.23547
93. Zeng G, Deng G, Xiao S, and Li F. Fibroblast-like synoviocytes-derived exosomal PCGEM1 accelerates IL-1β-induced apoptosis and cartilage matrix degradation by miR-142-5p/RUNX2 in chondrocytes. Immunol Invest. (2022) 51:1284–301. doi: 10.1080/08820139.2021.1936010
94. Jiang X, Wei Z, Wang C, Wang Q, Zhang Y, and Wu C. A four-miRNA-based diagnostic signature for rheumatoid arthritis. Dis Markers. (2022) 2022:6693589. doi: 10.1155/2022/6693589
95. Chan L, Chung CC, Hsieh YC, Wu RM, and Hong CT. Plasma extracellular vesicle tau, beta-amyloid, and alpha-synuclein and the progression of Parkinson’s disease: a follow-up study. Ther Adv Neurol Disord. (2023) 16:17562864221150329. doi: 10.1177/17562864221150329
96. Krishnan SR and Bebawy M. Circulating biosignatures in multiple myeloma and their role in multidrug resistance. Mol Cancer. (2023) 22:79. doi: 10.1186/s12943-022-01683-w
97. Cheng L and Hill AF. Therapeutically harnessing extracellular vesicles. Nat Rev Drug Discov. (2022) 21:379–99. doi: 10.1038/s41573-022-00410-w
98. Sheridan C. Exosome cancer diagnostic reaches market. Nat Biotechnol. (2016) 34:359–60. doi: 10.1038/nbt0416-359
99. Zhao Y and Xu J. Synovial fluid-derived exosomal lncRNA PCGEM1 as biomarker for the different stages of osteoarthritis. Int Orthop. (2018) 42:2865–72. doi: 10.1007/s00264-018-4093-6
100. Li Q, Wu M, Fang G, Li K, Cui W, Li L, et al. MicroRNA−186−5p downregulation inhibits osteoarthritis development by targeting MAPK1. Mol Med Rep. (2021) 23:253. doi: 10.3892/mmr.2021.11892
101. Kolhe R, Hunter M, Liu S, Jadeja RN, Pundkar C, Mondal AK, et al. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci Rep. (2017) 7:2029. doi: 10.1038/s41598-017-01905-y
102. Wu X, Crawford R, Xiao Y, Mao X, and Prasadam I. Osteoarthritic subchondral bone release exosomes that promote cartilage degeneration. Cells. (2021) 10:251. doi: 10.3390/cells10020251
Keywords: osteoarthritis, extracellular vesicles, mesenchymal stem cells, stem cell-derived exosomes, therapeutic carriers, diagnostic biomarkers, anti-inflammation
Citation: Sun C, Teng F and Xia Y (2025) Extracellular vesicles in osteoarthritis: mechanisms, therapeutic potential, and diagnostic applications. Front. Immunol. 16:1595095. doi: 10.3389/fimmu.2025.1595095
Received: 17 March 2025; Accepted: 25 July 2025;
Published: 13 August 2025.
Edited by:
Antonia Ru-Jia Sun, Queensland University of Technology, AustraliaReviewed by:
Lisa Jia Tran, Ludwig Maximilian University of Munich, GermanyCopyright © 2025 Sun, Teng and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yayi Xia, eGlheWF5aTIwMjVAMTYzLmNvbQ==
 Chongxiao Sun1,2,3
Chongxiao Sun1,2,3 Yayi Xia
Yayi Xia