- 1Department of Ultrasound Imaging, Renmin Hospital of Wuhan University, Wuhan, Hubei, China
- 2Hubei Key Laboratory of Digestive System Disease, Renmin Hospital of Wuhan University, Wuhan, Hubei, China
- 3General Surgery Laboratory, Renmin Hospital of Wuhan University, Wuhan, Hubei, China
- 4Department of Anesthesiology, Renmin Hospital of Wuhan University, Wuhan, Hubei, China
- 5Department of General Surgery, Renmin Hospital of Wuhan University, Wuhan, Hubei, China
Objective: This investigation seeks to examine the association between spleen volume and prognosis in cancer patients undergoing immune checkpoint inhibitor (ICI) treatment.
Methods: We performed a retrospective analysis involving 61 patients diagnosed with hepatocellular carcinoma (HCC) who received ICIs at our institution. We evaluated the relationship between baseline splenic volume and its changes during ICI therapy concerning overall survival (OS) and progression-free survival (PFS) using a log-rank test. To identify relevant literature, we searched databases such as PubMed, EMBASE, the Cochrane Library, and Google Scholar up until February 20, 2024. The primary metrics assessed were hazard ratios (HR) for both OS and PFS, with pooled estimates and corresponding 95% confidence intervals (CIs) calculated.
Results: Within our study population, findings demonstrated a significantly decreased OS (HR: 2.02, 95% CI: 1.08–3.77, p = 0.027) and PFS (HR: 1.84, 95% CI: 1.05–3.21, p = 0.032) in HCC patients with a high baseline spleen volume, compared to individuals with lower spleen volumes. Additionally, HCC patients who experienced an increase in spleen volume during ICI therapy exhibited significantly poorer OS (HR: 2.27, 95% CI: 1.17–4.41, p = 0.016) and PFS (HR: 2.40, 95% CI: 1.30–4.41, p = 0.005) than those whose spleen volume decreased. The meta-analysis results revealed that subjects with higher spleen volumes had a significantly reduced OS (HR: 1.74, 95% CI: 1.12–2.72, p = 0.014) and PFS (HR: 1.35, 95% CI: 1.15–1.58, p < 0.001) compared to counterparts with lower volumes. Furthermore, the data clearly highlighted that patients with increases in splenic volume faced significantly poorer clinical outcomes, as indicated by reduced OS (HR: 1.83, 95% CI: 1.36–2.46, p < 0.001) and PFS (HR: 1.70, 95% CI: 1.28–2.25, p < 0.001) relative to those with decreases in splenic size.
Conclusion: A higher baseline spleen volume and an increase in spleen volume during ICI therapy were predictors of a poor prognosis in cancer patients treated with ICI.
1 Introduction
Immune checkpoints, which encompass both stimulatory and inhibitory signals, regulate the immune response and protect neoplastic cells from immune detection (1–3). In recent years, oncology has experienced notable progress, highlighted by the emergence of immune checkpoint inhibitors (ICIs) and various immunotherapeutic approaches (4–7). The use of ICIs has become vital in treating various cancers, offering a distinct survival advantage compared to traditional treatments, including chemotherapy and radiation (4–7). While conventional chemotherapy primarily targets cancerous cells to disrupt their cycle, ICIs consist of antibodies that target PD-1, PD-L1, or CTLA-4. This mechanism interrupts critical regulatory signals that inhibit immune activity in the tumor microenvironment (TME) (4–8). As a result, ICIs reduce immune suppression, allowing tumor-specific T cells to trigger an antitumor response by leveraging the patient’s immune capabilities against the cancer (4–7).
The efficacy of ICI treatment varies significantly among different cancer types, typically ranging from 10% to 40%. Most patients ultimately experience progression despite an initial favorable response (9, 10). Furthermore, the negative effects associated with immune responses triggered by ICI treatment can be severe or even life-threatening (11). Consequently, early identification of patients unlikely to benefit from ICI therapy has become a critical focus in oncology, aimed at preventing ineffective therapy and reducing the risk of adverse reactions (12, 13). At present, intra-tumoral PD-L1 assays are commonly employed as biomarkers to inform ICI treatment (14, 15). However, the clinical predictive value of PD-L1 in practice is limited due to its heterogeneous expression across tumor tissues (16). Additional immune-related biomarkers, such as tumor mutation burden, are also used for companion diagnostics (17–20). Nevertheless, the individual effectiveness of these markers in predicting treatment outcomes is restricted (17, 18). Moreover, establishing standardized criteria for the quantification of these biomarkers presents significant challenges. Thus, the discovery of new prognostic markers that can improve outcomes for cancer patients receiving ICIs is critically important.
The spleen is the largest lymphoid organ in humans, containing diverse populations of immune cells. Previous studies suggest that individuals with splenomegaly may experience splenic dysfunction along with alterations in the immune microenvironment. This abnormal enlargement could potentially affect the efficacy of ICIs, influenced by imbalances in the immune microenvironment. However, the predictive effect of baseline spleen volume or changes in spleen volume on the efficacy of ICI in cancer patients remains controversial. This study aims to offer valuable insights by systematically consolidating all existing evidence, thereby improving our comprehension of the clinical significance of spleen volume in forecasting the prognosis of cancer patients treated with ICIs. To the best of our knowledge, this represents the first meta-analysis examining the role of spleen volume in predicting prognosis in cancer patients receiving ICI.
2 Methods
2.1 Study cohort and data collection
The institutional review board approved this study. Due to its retrospective nature, informed consent was not required. We conducted a retrospective analysis of patients with hepatocellular carcinoma (HCC) who received immunotherapy and angiogenesis blockade therapy at our institution between December 2020 and June 2022. The immunotherapy treatments included anti-PD-1 and anti-PD-L1 agents. Eligible patients had diagnosed HCC, with a baseline computed tomography (CT) scan performed within four weeks before the start of treatment. Patients were included if they had at least one measurable lesion, as defined by RECIST version 1.1. Exclusion criteria included prior immunotherapy exposure and the absence of a pretreatment CT scan.
Comprehensive data were collected from patient medical records, encompassing demographic details (age, sex), Eastern Cooperative Oncology Group performance status (ECOG PS), hepatitis etiology, liver cirrhosis, Barcelona Clinic Liver Cancer (BCLC) classification, Child–Pugh classification, tumor count, macrovascular invasion, treatment line, modified albumin-bilirubin grade, and AFP levels. Tumor progression was assessed according to RECIST version 1.1. Follow-up CT imaging was performed every one to three months after the initiation of treatment. Progression-free survival (PFS) was defined as the duration from the start of ICI therapy to death or disease progression, while overall survival (OS) was measured from the start of ICI therapy until death.
2.2 Spleen volume estimation
Spleen volume was assessed using CT imaging, following the method outlined in a previous study (21). The spleen’s maximal width (W) was determined by measuring the largest diameter across any transverse section, while the thickness at the hilum (Th) was defined as the distance between the inner and outer borders of the spleen on a plane perpendicular to the width and intersecting the hilum. Additionally, the spleen length (L) was recorded from abdominal CT scans. Spleen volume was calculated using the following formula: Spleen volume = 30 + 0.58 (W × L × Th).
2.3 Search strategy and inclusion/exclusion criteria
An electronic search was initiated on February 1, 2025, across several bibliographic databases, including PubMed, EMBASE, and the Cochrane Library. The search incorporated a range of key terms such as “immune checkpoint inhibitors” [Mesh], “PD-1 inhibitors,” “PD-L1 inhibitors,” “CTLA-4 inhibitors,” “splenomegaly” [Mesh], “splenic volume,” “spleen volume,” and “enlarged spleen,” with a focus on all relevant fields. Only studies published in English involving human participants were included. Detailed search strategies are outlined in Supplementary Material 1. In addition, grey literature was sourced from Google Scholar, and reference lists of eligible studies were manually reviewed. All search results, both electronic and manual, were consolidated in Covidence software for streamlined data management, in accordance with Cochrane collaboration guidelines.
Inclusion criteria were as follows: (i) studies involving cancer patients, (ii) administration of ICIs as the therapeutic approach, (iii) evaluation of the association between baseline spleen volume (categorized into high and low groups) and changes in spleen volume and prognosis, and (iv) documentation of at least one outcome measure, including OS or PFS. Exclusion criteria included studies utilizing methodologies such as animal models, literature reviews, case reports, or conference abstracts and studies lacking hazard ratios (HRs) for evaluating outcomes based on published or text data. In cases of overlapping patient cohorts, preference was given to studies that provided comprehensive data and adhered to rigorous research methodologies.
2.4 Data extraction and quality assessment
During the data collection phase, we extracted key details such as author information, year of publication, study period, country, cancer type, treatment regimens, sample size, and gender, along with splenic volume-related parameters (including measurement tool and calculation method). HRs and their respective 95% confidence intervals (CIs) were primarily obtained from multivariate analyses. In cases where these were unavailable, data were derived from univariate analyses or extracted from survival curves using Engauge Digitizer software (22).
To evaluate the quality of the included observational studies, the Newcastle-Ottawa Scale (NOS) was used, with studies scoring six or more points classified as high quality (23). The quality assessment considered nine criteria across three domains: patient selection, study comparability, and outcome evaluation. All aspects of the process, including literature retrieval, screening, data extraction, and quality assessment, were independently performed by two researchers, with any disagreements resolved through discussions with the senior author.
Two independent reviewers conducted the screening and data extraction processes to reduce bias and ensure accuracy. Any discrepancies were resolved through discussion or consultation with a third reviewer.
2.5 Statistical methods
The Cox proportional-hazards model and the Kaplan-Meier method were used to assess survival curves across different groups. Meta-analysis was conducted using Stata version 18.0, with results visually represented through forest plots. Heterogeneity was assessed using Cochran’s Q test and the I² statistic, with significant variation defined as a p-value < 0.1 or an I² value > 50%. In cases of significant heterogeneity, a random-effects model based on the DerSimonian-Laird approach was applied; otherwise, a fixed-effects model using the Inverse Variance method was used. Publication bias was evaluated through Egger’s regression test (24) and Begg’s test (25). Sensitivity analyses were performed to test the robustness of the findings by sequentially excluding individual studies (26). Additionally, subgroup analyses were conducted based on different body composition assessment techniques. Statistical significance was set at a two-tailed p-value of < 0.05.
3 Results
3.1 Patient characteristics
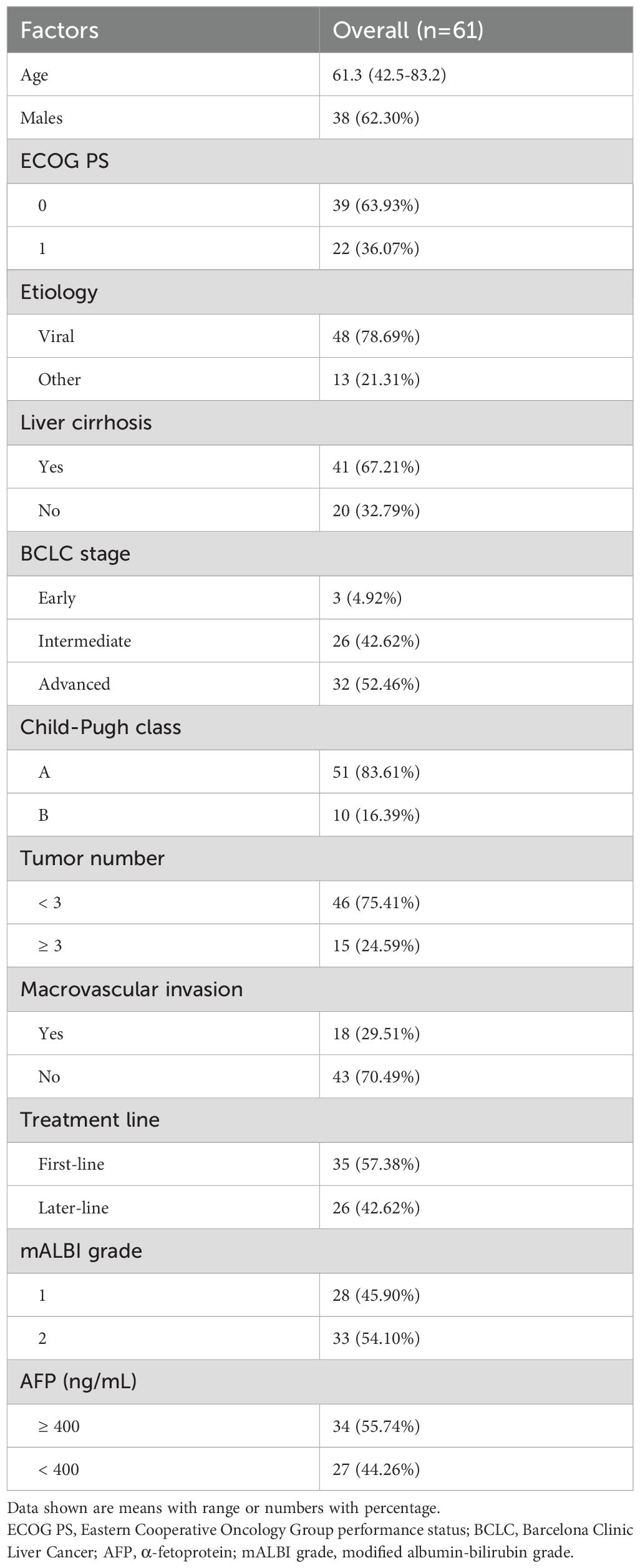
Table 1 outlines the characteristics of the patient cohort. The median age was 61.3 years, with a range from 42.5 to 83.2 years. Within the cohort, 38 patients (62.3%) were male. The ECOG PS was 0 in 39 patients (63.93%) and 1 in 22 patients (36.07%). Viral infections were present in 48 individuals (78.69%), and liver cirrhosis was identified in 41 patients (67.21%). The distribution of Barcelona Clinic Liver Cancer stages was as follows: early stage (n=3, 4.92%), intermediate stage (n=26, 42.62%), and advanced stage (n=32, 52.46%).
3.2 Relationship between baseline splenic volume, changes in splenic volume, and prognosis
The median basal splenic volume of all patients was 198 mL (range: 118–369). We divided the cohort into two groups based on the cutoff value for the median pretreatment splenic volume. Survival curves revealed significantly shorter OS (HR: 2.02, 95% CI: 1.08–3.77, p = 0.027, Figure 1A) and PFS (HR: 1.84, 95% CI: 1.05–3.21, p = 0.032; Figure 1B) in HCC patients with high baseline spleen volume compared to those with low spleen volume. Additionally, we also found that HCC patients with an increase in spleen volume during ICI treatment exhibited worse OS (HR: 2.27, 95% CI: 1.17–4.41, p = 0.016; Figure 1C) and PFS (HR: 2.40, 95% CI: 1.30–4.41, p = 0.005; Figure 1D) compared to those with a decrease in spleen volume. Therefore, data from our center indicate that HCC patients with high baseline spleen volume or an increase in spleen volume during ICI treatment have a worse prognosis.
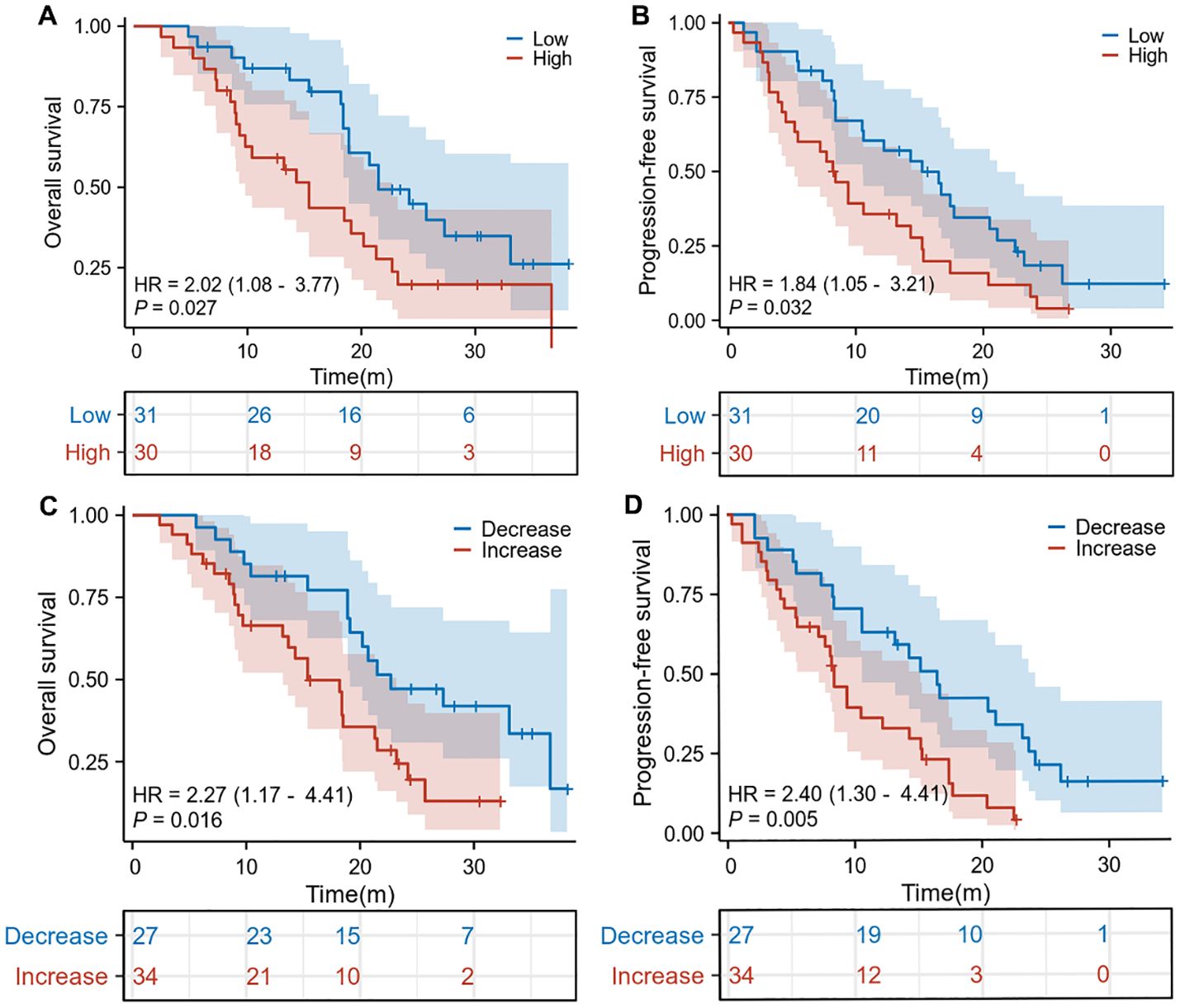
Figure 1. The Kaplan-Meier curve of overall survival (A) and progression-free survival (B) in the pretreatment high splenic volume and low splenic volume in our cohorts. The Kaplan-Meier curve of overall survival (C) and progression-free survival (D) according to the relative change compared to baseline splenic volume in our cohorts. HR, hazard ratio; CI, confidence interval.
3.3 Search results and included studies
The initial search strategy, combined with manual screening, identified 365 potentially relevant articles. After removing 40 duplicates, 287 articles were excluded based on title and abstract screening for non-compliance with the selection criteria. A full-text evaluation of the remaining 38 articles resulted in the exclusion of 29 that did not meet the required standards. Ultimately, 9 studies (27–35) were included in the final analysis (Figure 2).
3.4 Study characteristics
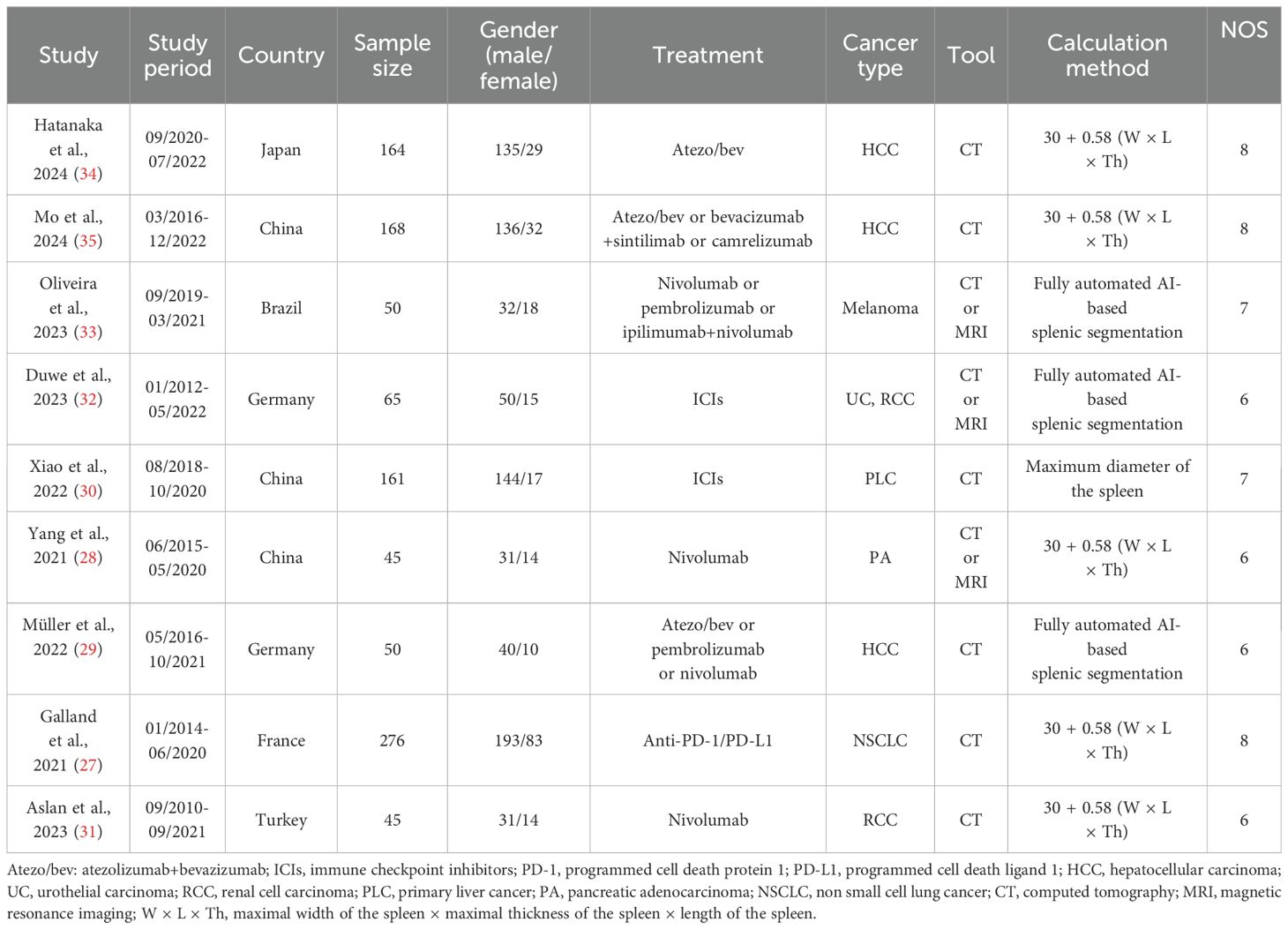
Table 2 summarizes the key features of the studies analyzed in this research. A total of 1,024 participants were included, of whom 77.34% were male, with individual study sample sizes ranging from 45 to 276. Geographically, three studies were conducted in China, two in Germany, and one each in Brazil, France, Japan, and Turkey. To evaluate spleen volume, all studies employed either computed tomography (CT) or magnetic resonance imaging (MRI). These studies were exclusively retrospective in design, with NOS scores ranging from 6 to 8, indicating a low likelihood of bias (Table 1).
3.5 Baseline spleen volume and overall survival and progression-free survival
In this investigation, we analyzed data from nine studies involving a total of 917 patients to assess the impact of pre-treatment spleen volumes on OS and PFS in cancer patients receiving ICIs. The results indicated that individuals with high spleen volume experienced significantly reduced OS (HR: 1.74, 95% CI: 1.12–2.72, p = 0.014, Figure 3A) and PFS (HR: 1.35, 95% CI: 1.15–1.58, p < 0.001, Figure 3B) compared to those with low spleen volume. The analysis of OS using the Cochran Q test and I² statistics (I² = 59.8%, p = 0.015) revealed considerable heterogeneity across the studies. Consequently, we employed a random-effects model for these analyses. In contrast, the evaluation of PFS studies did not show significant heterogeneity (I² = 35.7%, p = 0.144); thus, a fixed-effects model was considered appropriate.
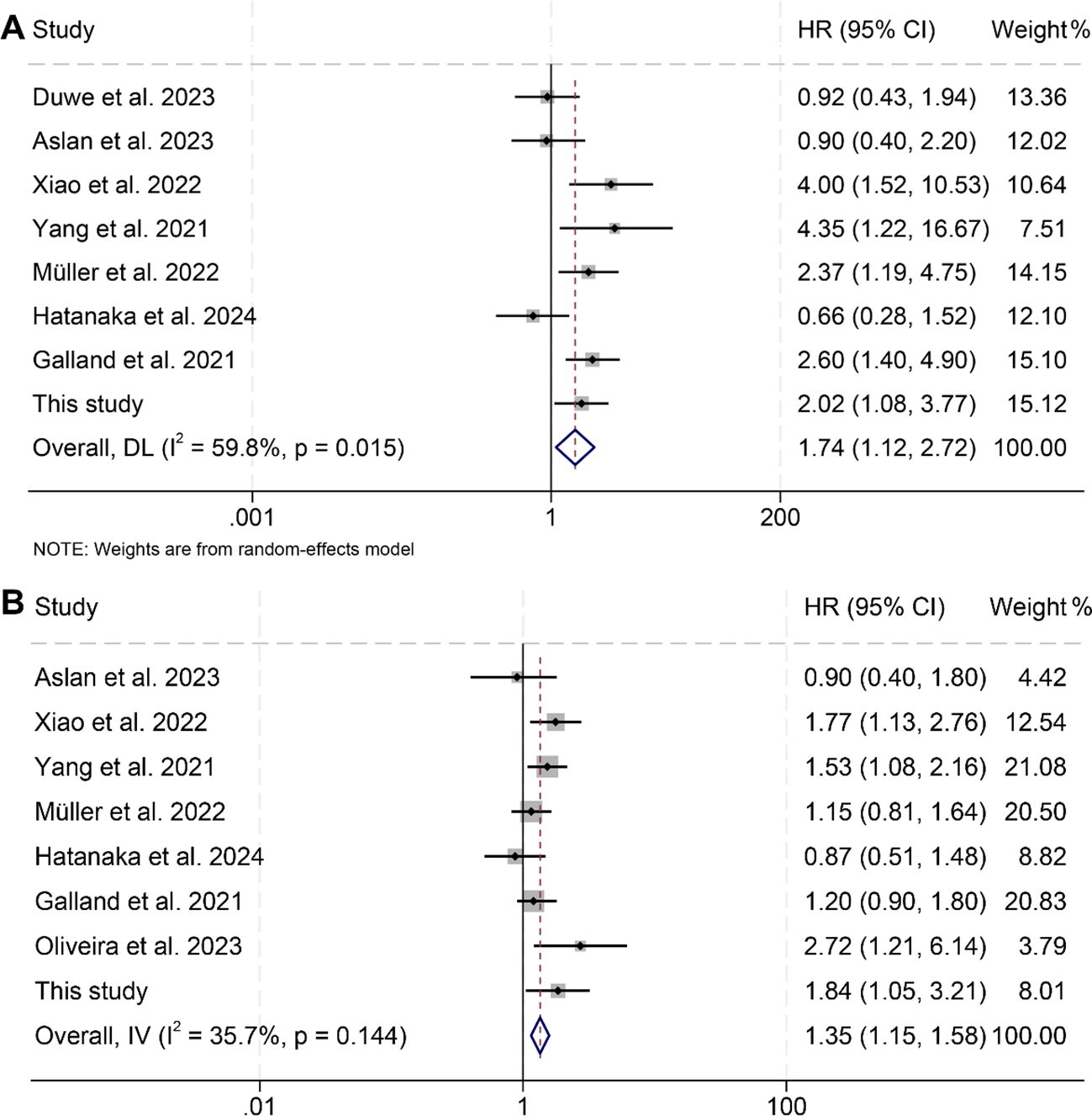
Figure 3. Forest plots illustrating the relationship between spleen volume and overall survival (A) and progression-free survival (B). HR, hazard ratio; CI, confidence interval; DL, DerSimonian and Laird; IV, inverse-variance model.
The assessment of potential publication bias was performed using funnel plots, along with Begg’s and Egger’s tests. The results showed no significant bias concerning OS (Egger’s test: p = 0.913; Begg’s test: p = 0.711; Supplementary Figure S1A) or PFS (Egger’s test: p = 0.651; Begg’s test: p = 0.902; Supplementary Figure S1B). Our sensitivity analysis, which systematically excluded each study one at a time, demonstrated the consistent stability and robustness of the pooled HRs for both OS and PFS (Figures 4A, B).
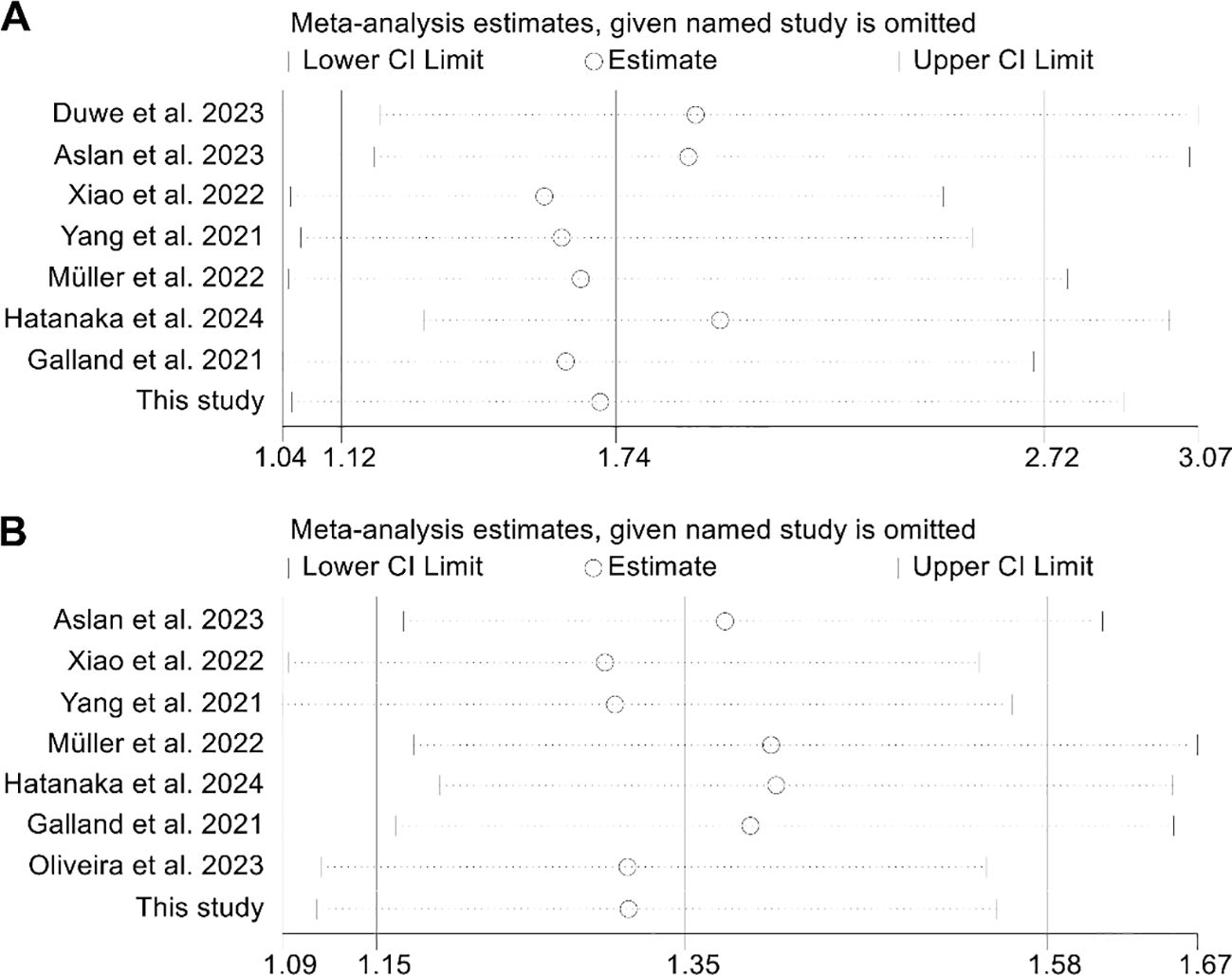
Figure 4. Sensitivity analysis of the relationship between spleen volume and overall survival (A) and progression-free survival (B). HR, hazard ratio; CI, confidence interval.
3.6 Changes in splenic volume and overall survival and progression-free survival
An analysis was further conducted to investigate the correlation between changes in splenic volume and OS as well as PFS among cancer patients, incorporating data from seven studies with a total of 829 subjects. Notably, these studies exhibited minimal heterogeneity for both OS (I² = 12.5%, p = 0.334) and PFS (I² = 0, p = 0.420), supporting the use of a fixed-effects model for the analysis. The results clearly indicated that patients with increases in splenic volume faced significantly worse outcomes, with reduced OS (HR: 1.83, 95% CI: 1.36–2.46, p < 0.001, Figure 5A) and PFS (HR: 1.70, 95% CI: 1.28–2.25, p < 0.001, Figure 5B) compared to those with decreases in splenic volume.
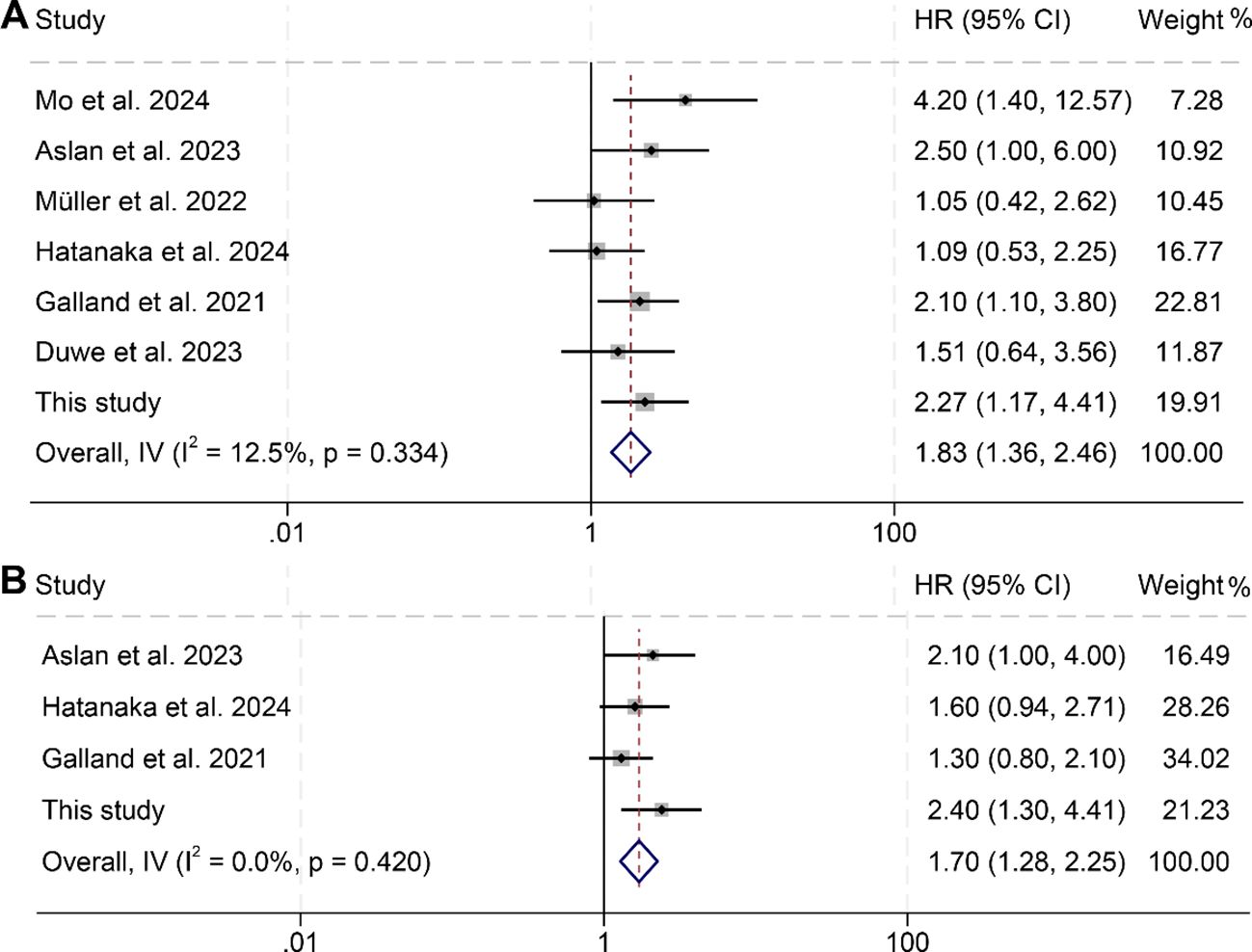
Figure 5. Forest plots illustrating the relationship between changes in splenic volume and overall survival (A) as well as progression-free survival (B). HR, hazard ratio; CI, confidence interval; IV, inverse-variance model.
Publication bias was assessed using funnel plots, along with Begg’s and Egger’s tests. The findings showed no significant bias for OS (Egger’s test: p = 0.753; Begg’s test: p = 0.548; Supplementary Figure S2A) or PFS (Egger’s test: p = 0.129; Begg’s test: p = 0.308; Supplementary Figure S2B). Additionally, sensitivity analysis, which sequentially excluded individual studies, reaffirmed the stability and robustness of the pooled HRs (Figures 6A, B).
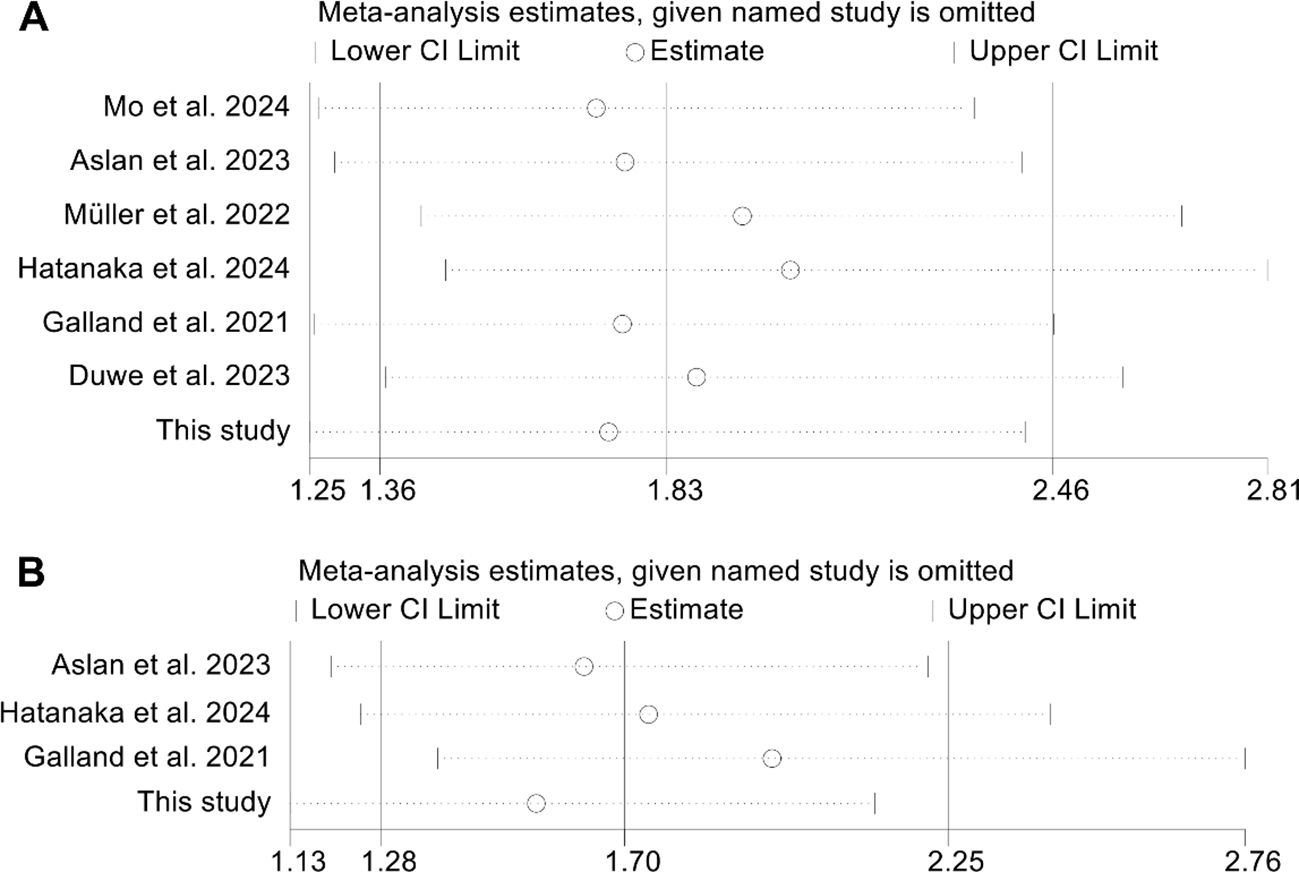
Figure 6. Sensitivity analysis of the relationship between changes in splenic volume and overall survival (A) and progression-free survival (B). HR, hazard ratio; CI, confidence interval.
4 Discussion
In our queue, we found that a higher baseline spleen volume and an increase in spleen volume during ICI therapy were predictors of a poor prognosis in HCC cancer patients treated with ICI. Considering the controversies between different studies, we further included nine studies for meta-analysis and the conclusions were consistent with the findings of our cohort.
The spleen plays a vital role in regulating hematopoiesis and immune responses, making it a key focus for assessing the effectiveness of immunotherapy across various cancer types (36). In animal models, a significant accumulation of myeloid-derived suppressor cells (MDSCs) has been noted in the spleen, resulting in splenomegaly (37, 38). Additionally, certain clinical studies have shown a correlation between MDSC levels and splenic volume (39). Measuring splenic volume is both quick and straightforward in clinical settings.
Recent studies have identified an increase in MDSCs as a significant factor contributing to resistance against immunotherapy (40). This heterogeneous group consists of immature, immunosuppressive myeloid progenitor cells. The prevalence of MDSCs is heightened in the spleen, bloodstream of cancer patients, and within the TME across various malignancies. Their elevation in these areas is influenced by chemokines, growth factors, and cytokines secreted by tumors (41, 42). MDSCs are known for their role in fostering immunotherapy resistance by suppressing the functions of natural killer cells and T cells, as well as by activating immunosuppressive regulatory T cells. These pathological cells create an immunosuppressive environment through the excessive production of interleukin-10, transforming growth factor-β, arginase, and nitric oxide within the TME. Additionally, they promote tumor progression by expressing surface receptors that inhibit T cell activity (41, 43–46). Research shows that targeting and inactivating Tregs or MDSCs can restore the anticancer efficacy of ICIs (47–49). Furthermore, a study involving RCC indicated that combining MDSC-targeted therapy with IL-2 treatment enhances the response to immunotherapy (50). So far, the mechanism by which splenomegaly affects the curative effect of ICI has not been reported. We suggest that the relationship between splenomegaly and MDSCs may partly explain our conclusions.
Splenomegaly serves as a valuable predictor due to the straightforward, accessible, non-invasive, and cost-effective imaging methods available for assessing spleen size. Previous research indicated that high-affinity neoantigens are associated with improved OS in individuals diagnosed with HCC (51). However, the analysis of neoantigens was performed through whole-exome sequencing, a method that is both expensive and not readily available in clinical settings. Additionally, the expression of PD-L1 has been linked to the effectiveness of ICIs (52). Nonetheless, PD-L1 levels were assessed using immunohistochemistry, a technique that is invasive and not easily accessible in clinical practice. Another investigation found that immune-related adverse events could predict the effectiveness of ICIs (53). While these adverse events do not occur in every patient, spleen size can be reliably measured through imaging techniques for all individuals. Therefore, we believe that the assessment of spleen volume is a very meaningful indicator to predict the efficacy of ICI treatment.
The single-center cohort predominantly consisted of HCC patients with underlying cirrhosis, mainly of viral etiology, which is commonly associated with portal hypertension and resultant splenomegaly. Baseline splenomegaly in these patients may partly reflect the presence and severity of portal hypertension, a factor that could have independently contributed to poorer survival outcomes. We intentionally chose not to exclude these patients or set a mean baseline splenic volume cutoff in order to preserve the real-world clinical scenario, despite the potential confounding effect. Importantly, our meta-analysis, which incorporated a larger and more diverse patient cohort, supports the overall conclusions drawn from our findings. Nonetheless, future studies with more detailed stratification are warranted to further delineate the specific impact of portal hypertension and related factors on patient outcomes.
This meta-analysis has certain limitations that must be acknowledged. First, it is crucial to recognize that all studies included were retrospective cohort designs, which may restrict their statistical validity. Second, the limited number of studies analyzed hindered our ability to perform subgroup analyses for specific cancer types and ICIs. Third, the cut-off values for the same diagnostic criteria varied among the studies. Finally, due to the limited sample size in our single-center data, multivariate analysis was not performed. Therefore, to draw more robust conclusions, there is an urgent need for a global, multicenter study to explore the impact of splenic volume on the outcomes of cancer patients receiving ICIs.
5 Conclusion
A higher baseline spleen volume and an increase in spleen volume during ICI therapy were predictors of a poor prognosis in cancer patients treated with ICI.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by Ethics Committee of Renmin Hospital of Wuhan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YZ: Methodology, Writing – original draft, Investigation, Conceptualization, Formal Analysis, Resources, Data curation, Visualization. XF: Writing – original draft, Conceptualization, Formal Analysis, Software, Data curation, Methodology, Investigation, Visualization. LZ: Methodology, Investigation, Resources, Writing – original draft, Visualization. QZ: Visualization, Validation, Project administration, Conceptualization, Supervision, Writing – review & editing. WW: Validation, Conceptualization, Writing – original draft, Supervision, Project administration, Visualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank all the medical staff who contributed to the maintenance of the medical record database.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1598484/full#supplementary-material
References
1. Hanahan D and Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
2. Yuan Q, Deng D, Pan C, Ren J, Wei T, Wu Z, et al. Integration of transcriptomics, proteomics, and metabolomics data to reveal HER2-associated metabolic heterogeneity in gastric cancer with response to immunotherapy and neoadjuvant chemotherapy. Front Immunol. (2022) 13:951137. doi: 10.3389/fimmu.2022.951137
3. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. (2012) 12:252–64. doi: 10.1038/nrc3239
4. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. (2019) 381:1535–46. doi: 10.1056/NEJMoa1910836
5. Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. (2015) 372:311–9. doi: 10.1056/NEJMoa1411087
6. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. (2015) 373:23–34. doi: 10.1056/NEJMoa1504030
7. Cheng W, Fu D, Xu F, and Zhang Z. Unwrapping the genomic characteristics of urothelial bladder cancer and successes with immune checkpoint blockade therapy. Oncogenesis. (2018) 7:2. doi: 10.1038/s41389-017-0013-7
8. Li Z, Liu Y, Huang X, Wang Q, Fu R, Wen X, et al. F. Nucleatum enhances oral squamous cell carcinoma proliferation via E-cadherin/β-Catenin pathway. BMC Health. (2024) 24:518. doi: 10.1186/s12903-024-04252-3
9. Ribas A and Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. (2018) 359:1350–5. doi: 10.1126/science.aar4060
10. O’Donnell JS, Teng MWL, and Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. (2019) 16:151–67. doi: 10.1038/s41571-018-0142-8
11. Dolladille C, Ederhy S, Sassier M, Cautela J, Thuny F, Cohen AA, et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol. (2020) 6:865–71. doi: 10.1001/jamaoncol.2020.0726
12. Zhang L, Feng J, Kuang T, Chai D, Qiu Z, Deng W, et al. Blood biomarkers predict outcomes in patients with hepatocellular carcinoma treated with immune checkpoint Inhibitors: A pooled analysis of 44 retrospective sudies. Int Immunopharmacol. (2023) 118:110019. doi: 10.1016/j.intimp.2023.110019
13. Zhao J, Li D, Xie S, Deng X, Wen X, Li J, et al. Nomogram for predicting prognosis of patients with metastatic melanoma after immunotherapy: A Chinese population-based analysis. Front Immunol. (2022) 13:1083840. doi: 10.3389/fimmu.2022.1083840
14. Ren J, Wang A, Liu J, and Yuan Q. Identification and validation of a novel redox-related lncRNA prognostic signature in lung adenocarcinoma. Bioengineered. (2021) 12:4331–48. doi: 10.1080/21655979.2021.1951522
15. Havel JJ, Chowell D, and Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. (2019) 19:133–50. doi: 10.1038/s41568-019-0116-x
16. Zhou KI, Peterson B, Serritella A, Thomas J, Reizine N, Moya S, et al. Spatial and Temporal Heterogeneity of PD-L1 Expression and Tumor Mutational Burden in Gastroesophageal Adenocarcinoma at Baseline Diagnosis and after Chemotherapy. Clin Cancer Res. (2020) 26:6453–63. doi: 10.1158/1078-0432.CCR-20-2085
17. Jørgensen JT. Companion and complementary diagnostics: clinical and regulatory perspectives. Trends Cancer. (2016) 2:706–12. doi: 10.1016/j.trecan.2016.10.013
18. Schalper KA, Carleton M, Zhou M, Chen T, Feng Y, Huang SP, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med. (2020) 26:688–92. doi: 10.1038/s41591-020-0856-x
19. Wang Y, Wang J, Liu J, and Zhu H. Immune-related diagnostic markers for benign prostatic hyperplasia and their potential as drug targets. Front Immunol. (2024) 15:1516362. doi: 10.3389/fimmu.2024.1516362
20. Pang ZQ, Wang JS, Wang JF, Wang YX, Ji B, Xu YD, et al. JAM3: A prognostic biomarker for bladder cancer via epithelial-mesenchymal transition regulation. Biomol Biomed. (2024) 24:897–911. doi: 10.17305/bb.2024.9979
21. Prassopoulos P, Daskalogiannaki M, Raissaki M, Hatjidakis A, and Gourtsoyiannis N. Determination of normal splenic volume on computed tomography in relation to age, gender and body habitus. Eur Radiol. (1997) 7:246–8. doi: 10.1007/s003300050145
22. Deng J, Huang Y, Yu K, Luo H, Zhou D, and Li D. Changes in the gut microbiome of patients with esophageal cancer: A systematic review and meta-analysis based on 16S gene sequencing technology. Microb Pathog. (2024) 193:106784. doi: 10.1016/j.micpath.2024.106784
23. Lilong Z, Kuang T, Li M, Li X, Hu P, Deng W, et al. Sarcopenia affects the clinical efficacy of immune checkpoint inhibitors in patients with gastrointestinal cancers. Clin Nutr. (2024) 43:31–41. doi: 10.1016/j.clnu.2023.11.009
24. Egger M, Davey Smith G, Schneider M, and Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
25. Begg CB and Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
26. Zhang L, Xia Z, Li Z, Zhang J, Wang K, and Wang W. Influence of body fat tissue on outcomes in patients undergoing hepatectomy or liver transplantation: a systematic review and meta-analysis. Int J Surg. (2025) 111:1167–81. doi: 10.1097/JS9.0000000000001864
27. Galland L, Lecuelle J, Favier L, Fraisse C, Lagrange A, Kaderbhai C, et al. Splenic volume as a surrogate marker of immune checkpoint inhibitor efficacy in metastatic non small cell lung cancer. Cancers (Basel). (2021) 13(12):3020. doi: 10.3390/cancers13123020
28. Yang SH, Lu LC, Kao HF, Chen BB, Kuo TC, Kuo SH, et al. Negative prognostic implications of splenomegaly in nivolumab-treated advanced or recurrent pancreatic adenocarcinoma. Oncoimmunology. (2021) 10:1973710. doi: 10.1080/2162402X.2021.1973710
29. Müller L, Gairing SJ, Kloeckner R, Foerster F, Weinmann A, Mittler J, et al. Baseline splenic volume outweighs immuno-modulated size changes with regard to survival outcome in patients with hepatocellular carcinoma under immunotherapy. Cancers. (2022) 14(15):3574. doi: 10.3390/cancers14153574
30. Xiao LS, Hu CY, Cui H, Li RN, Hong C, Li QM, et al. Splenomegaly in predicting the survival of patients with advanced primary liver cancer treated with immune checkpoint inhibitors. Cancer Med. (2022) 11:4880–8. doi: 10.1002/cam4.v11.24
31. Aslan V, Karabörk Kılıç AC, Özet A, Üner A, Günel N, Yazıcı O, et al. The role of spleen volume change in predicting immunotherapy response in metastatic renal cell carcinoma. BMC Cancer. (2023) 23:1045. doi: 10.1186/s12885-023-11558-y
32. Duwe G, Müller L, Ruckes C, Fischer ND, Frey LJ, Börner JH, et al. Change in splenic volume as a surrogate marker for immunotherapy response in patients with advanced urothelial and renal cell carcinoma-evaluation of a novel approach of fully automated artificial intelligence based splenic segmentation. Biomedicines. (2023) 11(9):2482. doi: 10.3390/biomedicines11092482
33. Oliveira Taveira M, de Barros ESMJ, and Vieira Barbosa Pinto PN. Imaging-calculated splenic volume is associated with response in melanoma patients treated with immunotherapy. Immunotherapy. (2023) 15:343–51. doi: 10.2217/imt-2022-0222
34. Hatanaka T, Saito N, Kakizaki S, Hiraoka A, Tada T, Kariyama K, et al. Factors affecting an increase in spleen volume and association of spleen volume variation with the clinical outcomes of atezolizumab and bevacizumab treatment for hepatocellular carcinoma: A retrospective analysis. Oncology. (2024) 103(2):94–106. doi: 10.1159/000541002
35. Mo Z, Lv L, Mai Q, Li Q, He J, Zhang T, et al. Prognostic model for unresectable hepatocellular carcinoma treated with dual PD-1 and angiogenesis blockade therapy. J Immunother Cancer. (2024) 12(1):e008191. doi: 10.1136/jitc-2023-008191
36. Wang Q, Huang X, Zhou S, Ding Y, Wang H, Jiang W, et al. IL1RN and PRRX1 as a prognostic biomarker correlated with immune infiltrates in colorectal cancer: evidence from bioinformatic analysis. Int J Genomics. (2022) 2022:2723264. doi: 10.1155/2022/2723264
37. Bronte V and Pittet MJ. The spleen in local and systemic regulation of immunity. Immunity. (2013) 39:806–18. doi: 10.1016/j.immuni.2013.10.010
38. Ramudo L, Yubero S, Manso MA, Vicente S, and De Dios I. Signal transduction of MCP-1 expression induced by pancreatitis-associated ascitic fluid in pancreatic acinar cells. J Cell Mol Med. (2009) 13:1314–20. doi: 10.1111/j.1582-4934.2008.00529.x
39. Limagne E, Euvrard R, Thibaudin M, Rébé C, Derangère V, Chevriaux A, et al. Accumulation of MDSC and th17 cells in patients with metastatic colorectal cancer predicts the efficacy of a FOLFOX-bevacizumab drug treatment regimen. Cancer Res. (2016) 76:5241–52. doi: 10.1158/0008-5472.CAN-15-3164
40. Wang Q, Wang H, Ding Y, Wan M, and Xu M. The role of adipokines in pancreatic cancer. Front Oncol. (2022) 12:926230. doi: 10.3389/fonc.2022.926230
41. Gabrilovich DI. Myeloid-derived suppressor cells. Cancer Immunol Res. (2017) 5:3–8. doi: 10.1158/2326-6066.CIR-16-0297
42. Liu C, Liu R, Wang B, Lian J, Yao Y, Sun H, et al. Blocking IL-17A enhances tumor response to anti-PD-1 immunotherapy in microsatellite stable colorectal cancer. J Immunother Cancer. (2021) 9(1):e001895. doi: 10.1136/jitc-2020-001895
43. Pastaki Khoshbin A, Eskian M, Keshavarz-Fathi M, and Rezaei N. Roles of myeloid-derived suppressor cells in cancer metastasis: immunosuppression and beyond. Arch Immunol Ther Exp (Warsz). (2019) 67:89–102. doi: 10.1007/s00005-018-0531-9
44. Veglia F, Sanseviero E, and Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol. (2021) 21:485–98. doi: 10.1038/s41577-020-00490-y
45. Ye B, Wang Q, Zhu X, Zeng L, Luo H, Xiong Y, et al. Single-cell RNA sequencing identifies a novel proliferation cell type affecting clinical outcome of pancreatic ductal adenocarcinoma. Front Oncol. (2023) 13:1236435. doi: 10.3389/fonc.2023.1236435
46. Li Z, Li J, Bai X, Huang X, and Wang Q. Tumor microenvironment as a complex milieu driving cancer progression: a mini review. Clin Transl Oncol. (2024) 27(5):1943–52. doi: 10.1007/s12094-024-03697-w
47. Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med. (2014) 6:237ra67. doi: 10.1126/scitranslmed.3007974
48. Taylor NA, Vick SC, Iglesia MD, Brickey WJ, Midkiff BR, McKinnon KP, et al. Treg depletion potentiates checkpoint inhibition in claudin-low breast cancer. J Clin Invest. (2017) 127:3472–83. doi: 10.1172/JCI90499
49. Gide TN, Wilmott JS, Scolyer RA, and Long GV. Primary and acquired resistance to immune checkpoint inhibitors in metastatic melanoma. Clin Cancer Res. (2018) 24:1260–70. doi: 10.1158/1078-0432.CCR-17-2267
50. Haka AS, Volynskaya Z, Gardecki JA, Nazemi J, Lyons J, Hicks D, et al. In vivo margin assessment during partial mastectomy breast surgery using raman spectroscopy. Cancer Res. (2006) 66:3317–22. doi: 10.1158/0008-5472.CAN-05-2815
51. Liu T, Tan J, Wu M, Fan W, Wei J, Zhu B, et al. High-affinity neoantigens correlate with better prognosis and trigger potent antihepatocellular carcinoma (HCC) activity by activating CD39(+)CD8(+) T cells. Gut. (2021) 70:1965–77. doi: 10.1136/gutjnl-2020-322196
52. Khemlina G, Ikeda S, and Kurzrock R. The biology of Hepatocellular carcinoma: implications for genomic and immune therapies. Mol Cancer. (2017) 16:149. doi: 10.1186/s12943-017-0712-x
Keywords: immune checkpoint inhibitors, cancer, splenic volume, prognosis, hepatocellular carcinoma
Citation: Zhang Y, Fu X, Zhang L, Zhou Q and Wang W (2025) Splenic volume as a predictor of survival in cancer patients treated with immune checkpoint inhibitors. Front. Immunol. 16:1598484. doi: 10.3389/fimmu.2025.1598484
Received: 23 March 2025; Accepted: 12 May 2025;
Published: 30 May 2025.
Edited by:
Qi Wang, Jiangsu University, ChinaReviewed by:
Xiaocui Liang, Wuhan Hospital of Traditional Chinese and Western Medicine, ChinaChong Jin, Taizhou Central Hospital, China
Volkan Aslan, Gulhane Training and Research Hospital, Türkiye
Copyright © 2025 Zhang, Fu, Zhang, Zhou and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing Zhou, cWluZ3pob3VAd2h1LmVkdS5jbg==; Weixing Wang, d2FuZ3d4QHdodS5lZHUuY24=
†These authors have contributed equally to this work
 Yunhua Zhang
Yunhua Zhang Xin Fu4†
Xin Fu4† Lilong Zhang
Lilong Zhang Qing Zhou
Qing Zhou Weixing Wang
Weixing Wang