- 1School of Medicine, Zhongnan Hospital of Wuhan University, Wuhan, China
- 2Department of Radiation and Medical Oncology, Zhongnan Hospital of Wuhan University, Wuhan, China
- 3Hubei Key Laboratory of Tumor Biological Behavior, Zhongnan Hospital of Wuhan University, Wuhan, China
- 4Hubei Provincial Clinical Research Center for Cancer, Zhongnan Hospital of Wuhan University, Wuhan, China
Cancers, with its rising incidence strongly linked to human papillomavirus (HPV) infection, particularly HPV16. HPV-induced OPSCC (HPV-OPSCC) exhibits distinct biological behaviors, including a high propensity for early lymphatic metastasis, occurring in most of cases, often presenting as cystic lymph node changes. The rising incidence of HPV-positive OPSCC is associated with specific mechanisms, particularly the characteristic biological behaviors driven by the E6/E7 oncoproteins: E7 disrupts cell cycle control by degrading pRb protein, while E6 inhibits apoptotic pathways through ubiquitination-mediated degradation of p53. Despite advances in treatment, HPV-OPSCC poses unique challenges due to its complex tumor microenvironment and immune interactions. Tertiary lymphoid structures (TLS) within the tumor microenvironment play a critical role in modulating anti-tumor immunity, correlating with improved clinical outcomes. Recent advances in immunotherapy, such as immune checkpoint inhibitors and HPV-specific vaccines, have shown promise in enhancing patient survival. This review explores the mechanisms of HPV-driven carcinogenesis, the clinical and molecular features of lymphatic metastasis, and the emerging role of TLS and immunotherapeutic strategies in HPV-OPSCC. By analyzing existing evidence, this review seeks to clarify the distinct biological features of HPV-associated oropharyngeal squamous cell carcinoma (HPV-OPSCC) and guide the development of novel treatment strategies aimed at enhancing clinical outcomes for patients. (OPSCC)
1 Introduction
With current estimates indicating approximately 25% of HNSCC cases originating in the oropharynx, OPSCC nowadays represents a substantial disease burden characterized via continuously escalating incidence rates. Escalating incidence rates of HPV (notably HPV16) infection, recognized as a key etiological factor in oropharyngeal malignancy formation, constitute the major determinant of this upward trend (1). Despite advances in treatment, including surgery, radiotherapy, and chemotherapy, HPV-OPSCC presents unique challenges, particularly its propensity for early lymphatic metastasis, which occurs in most of cases and significantly influences on prognosis (2, 3). Lymphatic metastasis is a hallmark of HPV-OPSCC progression, often manifesting as cystic changes in neck lymph nodes and exhibiting distinct patterns of spread compared to HPV-negative OPSCC (2). Studies have found that the spatial proximity between CD8+ T cells and PD-L1+ macrophages is enhanced in HPV-positive tumors, suggesting that immune escape may promote the lymphatic metastasis of cancer cells, which is associated with the high rate of lymphatic metastasis in HPV-OPSCC (4). In the management of early-stage human papillomavirus-associated oropharyngeal squamous cell carcinoma (HPV-OPSCC) with postoperative radiotherapy (PORT), lymphovascular invasion (LVI) has been identified as an independent adverse prognostic factor, potentially linked to its role in promoting lymphatic metastasis (5). HPV genome integration into host DNA can activate nearby oncogenes. Viral integration also induces genomic instability. These effects collectively promote lymphatic metastasis (6). The tumor microenvironment, including tertiary lymphoid structures (TLS), plays a critical role in modulating immune responses and shaping lymphatic metastatic behaviors. TLS are ectopic lymphoid formations and have been associated with improved clinical outcomes in HPV-OPSCC due to their role in enhancing local anti-tumor immunity (7). Recent advances in immunotherapy, particularly immune checkpoint inhibitors and HPV-specific vaccines, have shown promise in improving outcomes for HPV-OPSCC patients (8). However, the association between HPV status and immunotherapy response has not been fully elucidated, and validated biomarkers are currently lacking. This review aims summarizes the mechanisms of lymphatic metastasis in HPV-OPSCC, and the emerging role of TLS and immunotherapeutic strategies in HPV-OPSCC. By systematically consolidating contemporary evidence, this synthesis not only deciphers the distinct molecular landscape of HPV-OPSCC, but also establishes foundational knowledge for refining precision medicine strategies in clinical oncology.
2 Mechanisms of HPV-induced OPSCC
2.1 HPV-driven cell transformation and oncogene activation in OPSCC
While the precise oncogenic pathways of HPV in OPSCC remain incompletely characterized, accumulating evidence from translational studies strongly implicates HPV16 as the predominant etiological agent in oropharyngeal carcinogenesis. The high-risk HPV E6 and E7 genes are pivotal drivers of cell transformation, especially in basal squamous epithelial cells. For HPV types 16 and 18, both E6 and E7 proteins play essential roles in preventing senescence in human primary keratinocytes, potentially activating oncogenes and inducing carcinogenic cell proliferation (9, 10). E6 forms a complex as E6-AP, binding to p53 and promoting its degradation, thereby accelerating cell division and malignant transformation (11). Meanwhile, the E7 oncoprotein interacts with the retinoblastoma protein (pRb), disrupting its association with E2F transcription factors and facilitating unregulated progression into the S-phase of the cell cycle (12). The limited oncogenic potential demonstrated by HPV16/18 E2-E4-E5 genomic segments, which becomes further attenuated upon viral genome integration, may mechanistically explain their predominant association with non-malignant epithelial transformations rather than invasive carcinomas (13).
Some researchers propose that the cis-activating effect of HPV DNA on adjacent host genes has been postulated by investigators as a potential mechanism driving oncogenic transformation processes (11, 14). Hu et al. found that the E6/E7 proteins regulate the activity of key enzymes in the aerobic glycolysis pathway by influencing the binding of IGF2BP2 to the MYC m6A site (overexpression of IGF2BP2 in E6/E7-knockout CC cells). Ultimately, this reduces the glycolytic flux, leading to a decrease in cancer cell proliferation (15). Besides, hu et al. suggest that oncogenic human papillomaviruses (HPVs) could generate cir-cRNAs, some of which encompass the E7 oncogene (circE7), which is N6-methyladenosine (m6A) modified, preferentially localized to the cytoplasm, associated with polysomes. CircE7 can be translated and produce E7 oncoprotein (16). Other studies have noted that the integration of HPV DNA into chromosomes is a significant inducer of p53 gene mutations and may also lead to its overexpression (17). Palefsky et al. discovered that HPV16 infection is a cause of cellular transformation, likely due to alterations in the p53 and retinoblastoma proteins(pRb) triggered by HPV E6 and E7 (18). The cumulative evidence establishes HPV E6/E7’s pivotal function in p53 and pRb pathway disruption, which initiates cellular transformation and oncogenic signaling cascades, thereby mechanistically underpinning OPSCC pathogenesis.
2.2 Synergistic effects of HPV and other carcinogenic factors
Although HPV alone can induce malignant cell transformation, its carcinogenicity is often enhanced by synergistic interactions with other factors (14, 17). Oral tissues, exposed to various physical, chemical, and microbial agents, are particularly susceptible to the combined effects of HPV with smoking, alcohol, trauma, fungal infections, or other viruses, contributing to oral malignancies (17). Animal models confirm HPV’s role as a cofactor in carcinogenesis, with smoking facilitating HPV invasion and colonization in oral tissues (19). Additionally, HPV, smoking, and alcohol collectively induce oral cancer, suggesting a synergistic interaction. HPV’s carcinogenicity may also be influenced by hormone levels and immune status. For instance, The observed elevation in HPV prevalence among gravid populations, combined with augmented cervical carcinogenesis risk in prolonged contraceptive users, implies endocrine-mediated potentiation of viral genome duplication and mitotic activity (17, 19). Elevated hormone levels may enhance viral DNA expression, favoring carcinogenesis. Furthermore, higher HPV infection rates and tumor incidence in immunocompromised individuals, such as kidney transplant and acquired immunodeficiency syndromes (AIDS) patients, indicate latent HPV reactivation under such conditions (19).
3 Characteristics, detection and treatment of lymphatic metastasis in HPV-OPSCC
HPV-OPSCC often lacks early symptoms, and the existence of precancerous lesions remains controversial, with no such lesions found in 4,095 healthy individuals (20). Clinical data show that the lymphatic metastasis rate of HPV-OPSCC can exceed 90%, significantly higher than that of HPV-negative OPSCC (3). Additionally, Two-thirds of patients present with a neck mass, indicating lymphatic metastasis at diagnosis, creating a diagnostic and therapeutic blind spot (21).
3.1 Characteristics of lymphatic metastasis in HPV-OPSCC
Compared with HPV-negative OPSCC, HPV-OPSCC typically presents with smaller primary lesions but is more likely to develop early lymphatic metastasis, often manifesting as cystic changes in metastatic neck lymph nodes (cystic metastases). HPV-OPSCC also exhibits a distinctive pattern of lymph node invasion: the involved lymph nodes tend to be large and prone to extracapsular spread (2). Although HPV-OPSCC and HPV-negative OPSCC differ substantially in terms of their lymphatic metastasis rate and time to metastasis, they share similar locations and numbers of invaded lymph nodes, suggesting a fundamentally similar route of lymphatic invasion (22). Given the different pathogenic mechanisms and biological behaviors of HPV-OPSCC and HPV-negative OPSCC, the 8th edition of the American Joint Committee on Cancer (AJCC) staging manual has established an independent TNM staging system for HPV-OPSCC (21).
3.2 Detection of lymphatic metastasis in HPV-OPSCC
Positron emission tomography-computed tomography (PET-CT) is currently one of the most employed clinical methods for detecting lymphatic metastasis in head and neck tumors (23). However, a clinical study by Snyder et al. reported a misdiagnosis rate of 36–43% in detecting lymphatic metastasis in 49 HPV-OPSCC cases using PET-CT, with challenges in accurately determining the number and size of affected lymph nodes (24). Some researchers have established predictive models based on clinical presentation and imaging results to evaluate the risk of lymphatic metastasis in HPV-OPSCC patients; however, these models require validation through prospective studies (25). Fine-needle aspiration (FNA) biopsy is also insufficient for accurate detection, as aspirates from cystic metastatic lymph nodes in HPV-OPSCC resemble fluid from benign cystic lesions, potentially leading to diagnostic omissions (26). High-risk HPV testing on FNA samples is recommended for patients presenting with unexplained neck masses to determine whether these lesions might represent HPV-OPSCC metastases (26). HPV-RNA in situ hybridization has shown promise, with an 88.9% concordance rate with p16 immunohistochemical staining in detecting HPV in neck FNA samples (27).
3.3 Treatment and prognosis of lymphatic metastasis in HPV-OPSCC
Although HPV-associated oropharyngeal squamous cell carcinoma (HPV-OPSCC) is categorized as a distinct disease entity in the AJCC 8th edition staging system, current clinical management remains largely similar to that for HPV-negative OPSCC because of limited high-quality clinical evidence justifying disease-specific therapeutic strategies. (28). Patients with early HPV-OPSCC can undergo surgical monotherapy; however, most patients present with lymphatic metastasis and extracapsular spread at diagnosis, necessitating combined surgery, radiotherapy, and chemotherapy (28). It is important to emphasize that patients with lymphatic metastasis have a poorer prognosis compared to those without nodal involvement, underscoring the need to further elucidate the mechanisms of lymphatic metastasis and develop targeted interventions. Despite early and high rates of lymphatic metastasis, the overall prognosis of HPV-OPSCC is markedly better than that of HPV-negative OPSCC. A recent clinical study found that the 3-year overall survival rate was significantly higher (93%) among HPV-OPSCC patients than among HPV-negative OPSCC patients with a smoking history (46.2%) (28). The number of invaded lymph nodes exerts minimal effect on overall survival in HPV-OPSCC, contrasting sharply with other head and neck malignancies (29). The distinct pathobiological profile of HPV-OPSCC necessitates urgent elucidation of molecular mediators governing its preferential lymphatic dissemination, a prerequisite for optimizing nodal disease management protocols.
4 Exploration of lymphatic metastatic mechanisms in HPV-OPSCC
Metastatic colonization of lymphatic systems is mechanistically governed by synergistic contributions from genomic instability, epithelial-mesenchymal transition, and chemokine-mediated navigation. Seeing that the marked differences in lymphatic metastasis between HPV-OPSCC and HPV-negative OPSCC, several studies have sought to elucidate the specific mechanisms underlying lymphatic metastasis in HPV-OPSCC.
4.1 HPV+ OPSCC and epithelial–mesenchymal transition
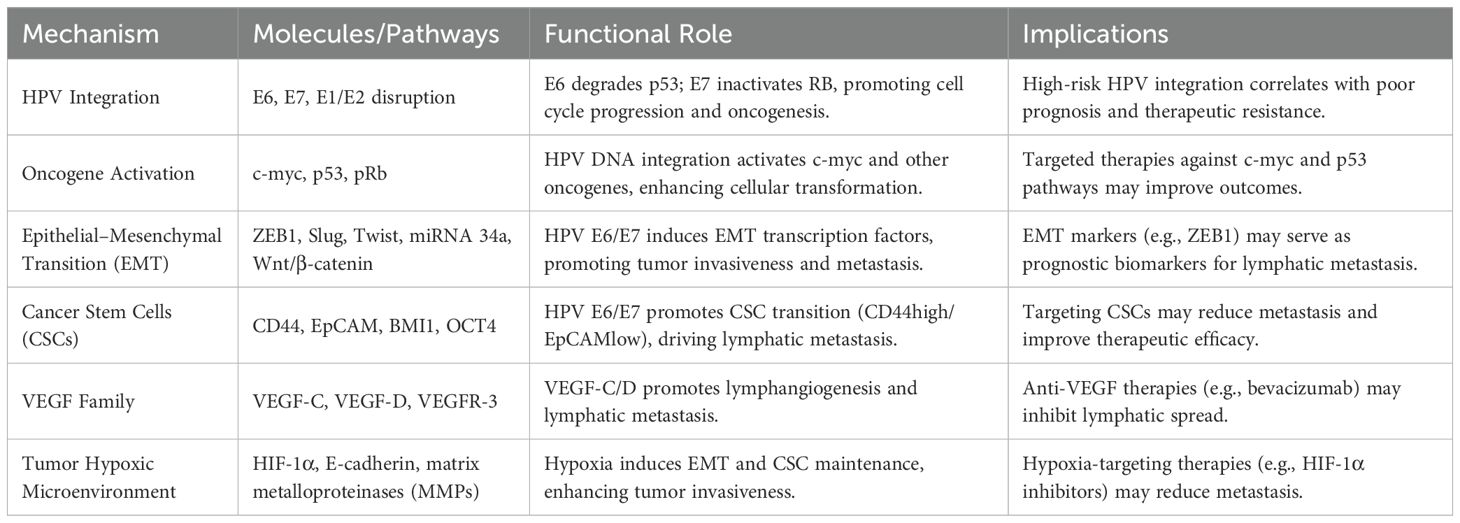
Despite HPV-OPSCC’s sensitivity to radiotherapy and chemotherapy, its high lymphatic metastasis incidence remains paradoxical. EMT is critical for lymphatic metastasis (30). HPV E6/E7 upregulates EMT transcription factors (Slug, Twist, ZEB1, ZEB2), enhancing tumor proliferation and invasiveness (31). Nevertheless, PRKCZ, an oncogenic factor, promotes HPV-OPSCC progression but is inhibited by HPV E6-induced hypermethylation, suppressing EMT (32). Recent studies indicate that miRNA 34a significantly inhibits tumor stem cell proliferation, invasiveness, and EMT in HNSCC, with reduced miRNA 34a levels correlating with increased tumor invasiveness (33). The oncogenic proteins E6 and E7 of HPV can also enhance the tendency of tumor cells to metastasize to lymph nodes by activating the pathways related to epithelial-mesenchymal transition (EMT), such as the Wnt/β-catenin pathway (33). Cancer stem cells (CSCs) are pivotal for EMT and tumor metastasis, with markers like ALDH1A1, CD44, CD98, BMI1, and OCT4. Gunduz et al. reported lower CD44 and CD98 expression in HPV+ OPSCC specimens (34), while another study found reduced BMI1 expression in HPV+ OPSCC (35). Zhang et al. found that HPV-OPSCC contains more CSCs than HPV-negative OPSCC, with HPV E6 degrading p53 or blocking its acetylation, increasing CSC numbers and tumor invasiveness (36). Hufbauer et al. showed that HPV E6/E7 mediates the transition from stationary to migratory CSCs by regulating CD44 and EpCAM, promoting lymphatic metastasis in HPV-OPSCC (37). It shows that the intricate interplay between cancer stem cell plasticity and microRNA-mediated regulatory networks in HPV-driven oncogenesis is compellingly demonstrated by these experimental observations.
4.2 HPV-OPSCC and the vascular endothelial growth factor family
The VEGF family, particularly VEGF-C, VEGF-D, and their receptor VEGFR-3, plays a critical role in lymphangiogenesis and lymphatic metastasis in various malignancies, including HNSCC (38). Elevated VEGF-C/D expression in HNSCC correlates with increased peritumoral lymphatic vessel density (LVD), lymphatic metastasis, and poor prognosis, with VEGF-C serving as a predictive marker for metastasis (39, 40). In HPV-related tumors, HPV16-E6 seems to induce VEGF expression independently of TP53 inactivation, using the SP1 transcription factor for E6-mediated induction of the VEGF promoter (41). However, Baruah et al. found that circulating VEGF levels in HPV-OPSCC were similar to healthy controls, with no significant difference in VEGF-D expression among HPV-OPSCC, HPV-negative OPSCC, and healthy groups (42). This low VEGF expression contrasts with the lymphatic invasiveness of HPV-OPSCC, raising questions about whether HPV-OPSCC forms specific lymphatic metastases via VEGF-C-mediated lymphangiogenesis (Table 1).
4.3 HPV-OPSCC and the tumor hypoxic microenvironment
A hypoxic tumor microenvironment leads to elevated levels of hypoxia-inducible factor (HIF), which promotes EMT and increased secretion of matrix metalloproteinases by inducing downstream target genes, thereby enhancing tumor invasiveness (43). HPV-associated oropharyngeal squamous cell carcinoma (HPV-OPSCC) tumor cells may demonstrate hypoxia-inducible factor (HIF)-mediated suppression of the activation of E-cadherin and concurrent EMT, promoting increased invasive potential. Under hypoxic conditions, CSCs can maintain stemness and self-renewal capacity under the stimulation of HIF-1, further enhancing tumor invasiveness (43). However, another study showed that the tumor microenvironment of HPV-OPSCC lacks significant hypoxia, with minimal HIF expression, but displays significantly increased neovascular density around the cancer cells (44). This finding suggests that HPV-OPSCC may possess a unique mechanism of hypoxia.
5 Relationship between HPV-induced OPSCC and the immune microenvironment
The relationship between HPV-driven oropharyngeal squamous cell carcinoma (OPSCC) and the tumor immune microenvironment has been extensively characterized in contemporary research. HPV-positive OPSCC exhibits significantly higher infiltration of CD8+ T cells within the tumor microenvironment compared to HPV-negative cases, correlating with improved patient prognosis (45). Furthermore, the activation of interferon signaling pathways, such as IFN-γ, in HPV-positive OPSCC enhances tumor immunogenicity, promoting antigen presentation and T cell activation (46). However, despite the heightened immune activity in HPV-positive OPSCC, tumor cells can evade immune surveillance through mechanisms such as upregulation of PD-L1 expression. A study revealed that PD-L1 expression is significantly higher in HPV-positive OPSCC, potentially mediated by the HPV oncoprotein, which contributes to immune evasion (47).
Additionally, the tumor microenvironment in HPV-positive OPSCC is characterized by increased infiltration of immunosuppressive cells, including tumor-associated macrophages (TAMs) and regulatory T cells (Tregs). These cells secrete cytokines such as IL-10 and TGF-β, which suppress effector T cell function and facilitate immune escape (32, 47). Although PD-1/PD-L1 inhibitors show efficacy in some patients, the presence of regulatory T cells (Tregs) can promote an immunosuppressive microenvironment that limits therapeutic effectiveness (48). Studies have shown that Tregs further exacerbate the formation of an immunosuppressive microenvironment by recruiting and activating other immunosuppressive cells, such as myeloid-derived suppressor cells (MDSCs) and M2 macrophages (49). Tregs suppress the activation and cytotoxic function of CD8+ T cells by secreting inhibitory cytokines and through direct cell-cell contact, thereby enabling tumor cells to evade immune-mediated elimination (50, 51). Due to the immunosuppressive function of Tregs, single-target immunotherapy is difficult to be effective. Therefore, some strategies that jointly target Tregs are needed. Studies have found that IDO1 inhibitors can regulate the development and activation of Treg cells and granulocyte-derived suppressor cells (MDSCs), and inhibit effector T cells and natural killer (NK) cells. IDO1 can also promote the neovascularization of tumors by regulating the production of interferon-gamma (IFN-γ) and interleukin-6 (IL-6) (52, 53). Therefore, the combined use of IDO1 inhibitor and the checkpoint inhibitor can enhance the efficacy of cancer immunotherapy (54). In summary, Tregs promote the formation of an immunosuppressive microenvironment in HPV-OPSCC through multiple mechanisms, thereby driving immune evasion and resistance to immunotherapy. Targeting Tregs represents a promising therapeutic strategy to overcome these challenges and achieve breakthroughs in HPV-OPSCC treatment.
HPV viral antigens induce CD161+ CTL subsets that co-express activation markers (e.g., IFN-γ) and exhaustion markers (e.g., PD-1, CTLA-4), forming an immunosuppressive microenvironment to facilitate lymphatic metastasis. Other research indicates that integrins on the surface of tumor-derived exosomes (TDEs), such as α6β4, specifically target lymphatic endothelial cells, thereby activating EMT and inhibit T - cell function and establishing a pre-metastatic microenvironment that directs tumor cells to colonize within the lymph nodes (55). Therefore, despite demonstrating a high degree of immunogenicity in HPV-positive oropharyngeal squamous cell carcinoma (OPSCC), the immunosuppressive processes inherent to the tumor microenvironment continue to present a major obstacle to successful immunotherapeutic interventions.
6 Debate of tertiary lymphoid structures in HPV-induced OPSCC
Tertiary lymphoid structures (TLS) are ectopic immune aggregates in HPV-induced oropharyngeal squamous cell carcinoma (HPV-OPSCC) that exhibit dual roles in tumor immunity. While TLS formation may correlate with improved prognosis by supporting cytotoxic lymphocyte activity, their functional dysfunction-driven by immunosuppressive mechanisms like regulatory T cell infiltration and cytokine dysregulation-can paradoxically promote immune evasion and immunotherapy resistance. We next examine these conflicting roles and explore therapeutic strategies targeting TLS reprogramming to enhance treatment efficacy in HPV-OPSCC.
6.1 Role of tertiary lymphoid structures in HPV-induced OPSCC
TLS form from lymphoid and stromal cell aggregation in non-secondary lymphoid organs under pathological conditions, including autoimmune diseases, infections, transplant rejection, and malignancies (56). Persistent inflammation upregulates chemokines (e.g., CXCL13, IL-7), attracting lymphoid tissue inducer (LTi) cells (e.g., Th17, B cells, M1 macrophages) to inflamed sites (57, 58). LTα1β2 on LTi cells interacts with LTβR and IL-17 receptors on stromal cells, inducing VEGF-C release, high endothelial venule (HEV) formation, and adhesion molecule expression (VCAM-1, ICAM-1) (59). Macrophages and endothelial cells secrete IL-36γ, further enhancing VCAM-1, ICAM-1, and chemokines (IL-8, CCL2, CCL20), promoting HEV recruitment of lymphocytes and TLS maturation (60).
The role of TLS in HPV-positive OPSCC has garnered increasing attention. A study found that the presence of TLS in HPV-positive OPSCC is associated with higher CD8+ T cell infiltration and improved clinical outcomes (61). Tertiary lymphoid structures (TLS), residing within the tumor microenvironment, represent ectopic lymphoid formations that actively promote localized anti-tumor immune activity. In HPV-positive OPSCC, B cells and T cells within TLS collaborate to generate HPV-specific antibodies and cytotoxic T cell (CTL) responses, thereby enhancing anti-tumor immunity. The formation and maintenance of TLS are closely linked to the expression of chemokines such as CXCL13 and CCL21. High CXCL13 expression is associated with TLS formation and function in HPV-positive OPSCC, providing a theoretical basis for targeting chemokine networks to enhance TLS activity (56). Additionally, follicular helper T cells (Tfh) within TLS play a critical role in B cell differentiation and antibody production, further amplifying anti-tumor immune responses (62). However, TLS functionality is not uniformly effective across all patients, as some exhibit dysfunctional TLS characterized by B cell exhaustion or T cell impairment. Therefore, it is meaningful to further explore and focus on elucidating the mechanisms underlying the dysfunction of tertiary lymphoid structures (TLS), and to develop targeted strategies to restore their anti-tumor activity.
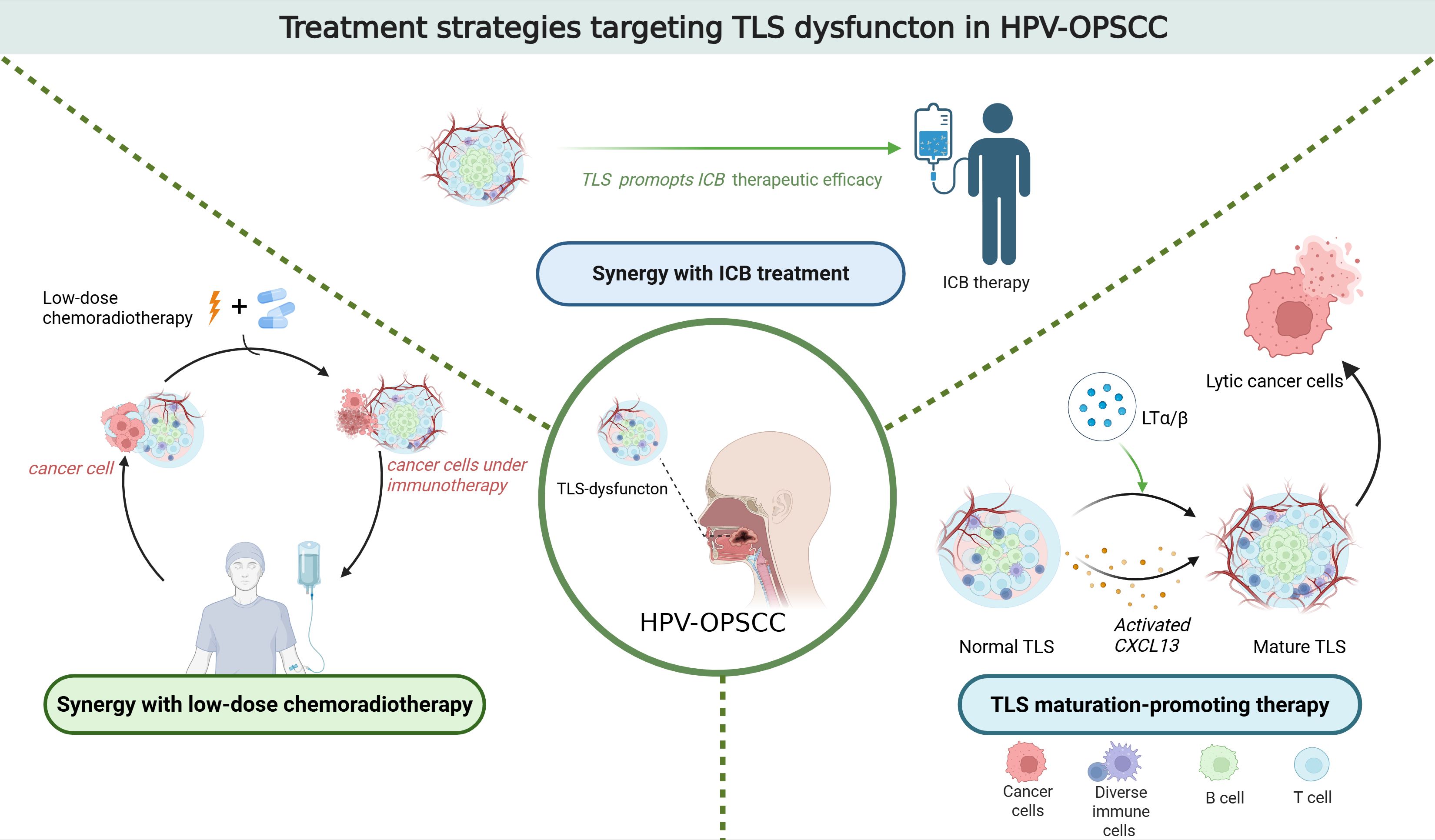
6.2 The mechanism of TLS dysfunction and treatment strategies in HPV-induced OPSCC
Immune checkpoint blockade (ICB) targeting PD-1/PD-L1 is a prominent immunotherapy. It has demonstrated significant efficacy in treating OPSCC. However, the overall response rate to PD-1/PD-L1 blockade in OPSCC remains below 20% (63). Studies have revealed that CD20+ B cells predominantly localize within TLS (64). Moreover, the presence of CD20+ B cells is associated with a more favorable prognosis for patients with OPSCC (65). The induction of TLS formation enhances the response to PD-1 blockade treatment in HPV-OPSCC mouse models. Therefore, promoting TLS formation may improve the response rates of HPV-OPSCC patients to ICB therapy (66). A study found that by regulating chemokines such as CXCL13 or lymphoid tissue inducers like LTα/β, the maturation and function of TLS can be promoted, and the anti-tumor immunity can be enhanced (67). Another study found that the PD-1 inhibitor pembrolizumab is effective in enhancing the response to immunotherapy. It demonstrated clinically meaningful anti-tumour activity in recurrent or metastatic squamous cell carcinoma of the head and neck (68).
In HPV-OPSCC, the maturity of TLS is closely linked to their functional capacity. Immature TLS may fail to effectively facilitate antigen presentation and lymphocyte activation, thereby compromising immunotherapy efficacy. In contrast, mature TLS are enriched with memory B cells, plasma cells, and CD4+ T cells, accompanied by upregulated expression of B-cell activation-related genes. Consequently, promoting TLS maturation could enhance the therapeutic response to immunotherapy in HPV-OPSCC (69).
Besides, in HPV-associated oropharyngeal squamous cell carcinoma (HPV-OPSCC), dysfunction of TLS may be linked to a significant reduction in CD8+ T cells and B lymphocytes within the tumor microenvironment. This association is particularly pronounced in cases of disease recurrence following chemotherapy or radiotherapy (70). Further research revealed T cell hat conventional chemoradiotherapy may disrupt TLS integrity, leading to deterioration of the local immune microenvironment. Consequently, this impairment reduces response rates to immunotherapy. Given favorable prognosis of HPV-OPSCC patients, decreasing therapeutic intensity-such as reducing radiation doses and avoiding chemotherapy, and minimizing overtreatment-could preserve TLS functionality and sustain antitumor immunity (71). A recent study found that neoadjuvant chemotherapy (NAC) can enhance the HPV-specific T cell response of the primary tumor and improve the survival rate of patients (72) (Figure 1).
7 Immunotherapeutic strategies for HPV-induced OPSCC
HPV-positive OPSCC shows stronger responses to immunotherapy, especially immune checkpoint inhibitors. The KEYNOTE-048 clinical trial reported that the PD-1 inhibitor pembrolizumab significantly improved overall survival in patients with HPV-positive OPSCC (61). Additionally, HPV vaccines have shown promise in treating HPV-associated OPSCC. A study in demonstrated that an HPV E6/E7 vaccine induced robust HPV-specific T cell responses and led to tumor regression in some patients (8). Despite these advancements, resistance to immunotherapy remains a significant challenge. Research identified that immunosuppressive cells, such as Tregs and myeloid-derived suppressor cells (MDSCs), within the tumor microenvironment can inhibit T cell function, contributing to immunotherapy resistance (73). To overcome these limitations, current research focuses on developing multimodal treatment approaches that combine immune checkpoint inhibitors with radiotherapy or chemotherapy to counteract therapeutic resistance and improve clinical outcomes. Furthermore, adoptive T cell therapies, such as chimeric antigen receptor (CAR) T cell therapy and tumor-infiltrating lymphocyte (TIL) therapy, have shown potential in clinical trials. For instance, a study demonstrated that CAR-T cells targeting HPV E6/E7 exhibited potent anti-tumor activity in HPV-positive OPSCC (74). These emerging immunotherapeutic strategies offer new hope for patients with HPV-positive OPSCC, underscoring the need for continued research to optimize treatment outcomes.
8 Conclusion
HPV-positive oropharyngeal squamous cell carcinoma (HPV-OPSCC) represents a distinct clinical and molecular entity within head and neck cancers, characterized by a high propensity for early lymphatic metastasis and a unique tumor-immune microenvironment. The coordinated engagement of HPV-derived oncogenic factors, immune regulation, and lymphotropic metastatic processes serves to elucidate the pathobiological complexity inherent in virally mediated tumor development. While tertiary lymphoid structures (TLS) have emerged as critical modulators of anti-tumor immunity, their functional heterogeneity emphasizes the need for further mechanistic studies to harness their full therapeutic potential.
Recent advancements in immunotherapy, particularly immune checkpoint blockade and HPV-targeted vaccines, have reshaped the treatment landscape, demonstrating promising efficacy in HPV-OPSCC. However, immune evasion mechanisms, including regulatory T cell infiltration and PD-L1 upregulation, continue to pose significant challenges, necessitating the development of combination strategies to strengthen therapeutic responsiveness. Future investigations should prioritize the refinement of prognostic biomarkers, enhance the development of immunotherapeutic approaches, and systematically characterize the molecular mechanisms governing lymph node metastasis. By integrating these insights, the field can move toward more precise and durable treatment strategies, ultimately improving patient outcomes in HPV-OPSCC.
Author contributions
XL: Investigation, Resources, Funding acquisition, Writing – original draft, Writing – review & editing, Software. HQ: Writing – review & editing. QW: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Health Commission of Hubei Province Scientific Research Project (No. WJ2023M068), Leading Discipline of Oncology Construction Project of Zhongnan Hospital of Wuhan University (No. XKJS202005), Youth Interdisciplinary Special Fund of Zhongnan Hospital of Wuhan University (No. ZNQNJC2022003), Knowledge Innovation Program of Wuhan-Shuguang Project (No. 2023020201020510), Translational Medicine and Interdisciplinary Research Joint Fund of Zhongnan Hospital of Wuhan University (ZNJC202322), Clinical Research Special Fund of Zhongnan Hospital of Wuhan University (2025LCYJZX-MS009, 2025LCYJZX-ZD005).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AJCC, the American Joint Committee on Cancer; CAR, chimeric antigen receptor; CTL, cytotoxic T cell; CSCs, Cancer stem cells; circE7, E7 oncogene; FNA, Fine-needle aspiration; HEV, high endothelial venule; HIF, hypoxia-inducible factor; HPV, human papillomavirus; HPV-OPSCC, HPV-induced OPSCC; ICB, Immune checkpoint blockade; ICIs, immune checkpoint inhibitors; IFN-γ, interferon-gamma; IL-6, interleukin-6; LTi, lymphoid tissue inducer; LVD, lymphatic vessel density; LVI, lymphovascular invasion; MDSCs, myeloid-derived suppressor cells; (m6A), N6-methyladenosine; NAC, neoadjuvant chemo-therapy; OPSCC, Oropharyngeal squamous cell carcinoma; PET-CT, Positron emission tomography-computed tomography; pRb, the retinoblastoma protein; TAMs, tumor-associated macrophages; TDEs, Tumor-derived exosomes; TLS, Tertiary lymphoid structures; Tregs, regulatory T cells; Tfh, follicular helper T cells; TIL, tumor-infiltrating lymphocyte.
References
1. Tanaka TI and Alawi F. Human papillomavirus and oropharyngeal cancer. Dent Clin North Am. (2018) 62:111–20. doi: 10.1016/j.cden.2017.08.008
2. Bauwens L, Baltres A, Fiani DJ, Zrounba P, Buiret G, Fleury B, et al. Prevalence and distribution of cervical lymph node metastases in HPV-positive and HPV-negative oropharyngeal squamous cell carcinoma. Radiother Oncol. (2021) 157:122–9. doi: 10.1016/j.radonc.2021.01.028
3. Lechner M, Liu J, Masterson L, and Fenton TR. HPV-associated oropharyngeal cancer: epidemiology, molecular biology and clinical management. Nat Rev Clin Oncol. (2022) 19:306–27. doi: 10.1038/s41571-022-00603-7
4. Wei Y, Xu T, Li C, Zhou X, Qian W, Shen C, et al. CD161 characterizes an inflamed subset of cytotoxic T lymphocytes associated with prolonged survival in human papillomavirus-driven oropharyngeal cancer. Cancer Immunol Res. (2023) 11:306–19. doi: 10.1158/2326-6066.CIR-22-0454
5. Hong SA, Armstrong AT, Snow K, Walker RJ, and Massa ST. Association of adjuvant radiation and survival in human papilloma virus-positive oropharynx squamous cell carcinoma with lymphovascular invasion as the sole adverse pathologic feature. Head Neck. (2024) 46:1043–50. doi: 10.1002/hed.27740
6. Zhou L, Qiu Q, Zhou Q, Li J, Yu M, Li K, et al. Long-read sequencing unveils high-resolution HPV integration and its oncogenic progression in cervical cancer. Nat Commun. (2022) 13:2563. doi: 10.1038/s41467-022-30190-1
7. Germain C, Gnjatic S, and Dieu-Nosjean MC. Tertiary lymphoid structure-associated B cells are key players in anti-tumor immunity. Front Immunol. (2015) 6:67. doi: 10.3389/fimmu.2015.00067
8. Morris VK, Jazaeri A, Westin SN, Pettaway C, George S, Huey RW, et al. Phase II trial of MEDI0457 and durvalumab for patients with recurrent/metastatic human papillomavirus-associated cancers. Oncologist. (2023) 28:618–23. doi: 10.1093/oncolo/oyad085
9. Chiba I, Shindoh M, Yasuda M, Yamazaki Y, Amemiya A, Sato Y, et al. Mutations in the p53 gene and human papillomavirus infection as significant prognostic factors in squamous cell carcinomas of the oral cavity. Oncogene. (1996) 12:1663–8.
10. Haraf DJ, Nodzenski E, Brachman D, Mick R, Montag A, Graves D, et al. Human papilloma virus and p53 in head and neck cancer: clinical correlates and survival. Clin Cancer Res. (1996) 2:755–62.
11. Chatterjee R, Mukhopadhyay D, Chakraborty RN, and Mitra RB. Evaluation of argyrophilic nucleolar organizer regions (AgNORs) in oral carcinomas in relation to human papillomavirus infection and cytokinetics. J Oral Pathol Med. (1997) 26:310–4. doi: 10.1111/j.1600-0714.1997.tb00221.x
12. Chung CH and Gillison ML. Human papillomavirus in head and neck cancer: its role in pathogenesis and clinical implications. Clin Cancer Res. (2009) 15:6758–62. doi: 10.1158/1078-0432.CCR-09-0784
13. Li X and Coffino P. High-risk human papillomavirus E6 protein has two distinct binding sites within p53, of which only one determines degradation. J Virol. (1996) 70:4509–16. doi: 10.1128/jvi.70.7.4509-4516.1996
14. van Oijen MG, Tilanus MG, Medema RH, and Slootweg PJ. Expression of p21 (Waf1/Cip1) in head and neck cancer in relation to proliferation, differentiation, p53 status and cyclin D1 expression. J Oral Pathol Med. (1998) 27:367–75. doi: 10.1111/j.1600-0714.1998.tb01969.x
15. Hu C, Liu T, Han C, Xuan Y, Jiang D, Sun Y, et al. HPV E6/E7 promotes aerobic glycolysis in cervical cancer by regulating IGF2BP2 to stabilize m(6)A-MYC expression. Int J Biol Sci. (2022) 18:507–21. doi: 10.7150/ijbs.67770
16. Zhao J, Lee EE, Kim J, Yang R, Chamseddin B, Ni C, et al. Transforming activity of an oncoprotein-encoding circular RNA from human papillomavirus. Nat Commun. (2019) 10:2300. doi: 10.1038/s41467-019-10246-5
17. Schifter M, Jones AM, and Walker DM. Epithelial p53 gene expression and mutational analysis, combined with growth fraction assessment, in oral lichen planus. J Oral Pathol Med. (1998) 27:318–24. doi: 10.1111/j.1600-0714.1998.tb01963.x
18. Werness BA, Levine AJ, and Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. (1990) 248:76–9. doi: 10.1126/science.2157286
19. Warnakulasuriya KA, Tavassoli M, and Johnson NW. Relationship of p53 overexpression to other cell cycle regulatory proteins in oral squamous cell carcinoma. J Oral Pathol Med. (1998) 27:376–81. doi: 10.1111/j.1600-0714.1998.tb01970.x
20. Palmer E, Newcombe RG, Green AC, Kelly C, Noel Gill O, Hall G, et al. Human papillomavirus infection is rare in nonmalignant tonsil tissue in the UK: implications for tonsil cancer precursor lesions. Int J Cancer. (2014) 135:2437–43.
21. Huang SH, O’Sullivan B, and Waldron J. The current state of biological and clinical implications of human papillomavirus-related oropharyngeal cancer. Semin Radiat Oncol. (2018) 28:17–26. doi: 10.1016/j.semradonc.2017.08.007
22. Amsbaugh MJ, Yusuf M, Cash E, Silverman C, Wilson E, Bumpous J, et al. Distribution of cervical lymph node metastases from squamous cell carcinoma of the oropharynx in the era of risk stratification using human papillomavirus and smoking status. Int J Radiat Oncol Biol Phys. (2016) 96:349–53. doi: 10.1016/j.ijrobp.2016.06.2450
23. Wong WL. PET-CT for staging and detection of recurrence of head and neck cancer. Semin Nucl Med. (2021) 51:13–25. doi: 10.1053/j.semnuclmed.2020.09.004
24. Snyder V, Goyal LK, Bowers EMR, Kubik M, Kim S, Ferris RL, et al. PET/CT poorly predicts AJCC 8th edition pathologic staging in HPV-related oropharyngeal cancer. Laryngoscope. (2021) 131:1535–41. doi: 10.1002/lary.v131.7
25. Joo L, Bae YJ, Choi YJ, Lee YS, Chung SR, Suh CH, et al. Prediction model for cervical lymph node metastasis in human papillomavirus-related oropharyngeal squamous cell carcinomas. Eur Radiol. (2021) 31:7429–39. doi: 10.1007/s00330-021-07766-4
26. Civantos FJ, Vermorken JB, Shah JP, Rinaldo A, Suárez C, Kowalski LP, et al. Metastatic squamous cell carcinoma to the cervical lymph nodes from an unknown primary cancer: management in the HPV era. Front Oncol. (2020) 10:593164. doi: 10.3389/fonc.2020.593164
27. Daneshpajouhnejad P, Miller JA, and Maleki Z. Diagnostic utility of high-risk human papillomavirus mRNA in situ hybridisation in squamous cell carcinoma of unknown primary in the head and neck and implementing American Society of Clinical Oncology guideline recommendations. Cytopathology. (2020) 31:547–54. doi: 10.1111/cyt.12896
28. Pfister DG, Spencer S, Adelstein D, Adkins D, Anzai Y, Brizel DM, et al. Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2020) 18:873–98. doi: 10.6004/jnccn.2020.0031
29. Moya-Plana A, Mangin D, Blanchard P, Obongo R, Casiraghi O, Bidault F, et al. Prognostic value and therapeutic implications of nodal involvement in head and neck mucosal melanoma. Head Neck. (2021) 43:2325–31. doi: 10.1002/hed.26694
30. Ren H, He G, Lu Z, He Q, Li S, Huang Z, et al. Arecoline induces epithelial-mesenchymal transformation and promotes metastasis of oral cancer by SAA1 expression. Cancer Sci. (2021) 112:2173–84. doi: 10.1111/cas.v112.6
31. Jung YS, Kato I, and Kim HR. A novel function of HPV16-E6/E7 in epithelial-mesenchymal transition. Biochem Biophys Res Commun. (2013) 435:339–44. doi: 10.1016/j.bbrc.2013.04.060
32. Wang HF, Wang SS, Tang YJ, Chen Y, Zheng M, Tang YL, et al. The double-edged sword-how human papillomaviruses interact with immunity in head and neck cancer. Front Immunol. (2019) 10:653. doi: 10.3389/fimmu.2019.00653
33. Hu J, Ji Y, Miao T, Zheng S, Cui X, Hu J, et al. HPV 16 E6 promotes growth and metastasis of esophageal squamous cell carcinoma cells in vitro. Mol Biol Rep. (2023) 50:1181–90. doi: 10.1007/s11033-022-07952-7
34. Gunduz M, Gunduz E, Tamagawa S, Enomoto K, and Hotomi M. Cancer stem cells in oropharyngeal cancer. Cancers (Basel). (2021) 2:13. doi: 10.3390/cancers13153878
35. Mohamed H, Hagström J, Jouhi L, Atula T, Almangush A, Mäkitie A, et al. The expression and prognostic value of stem cell markers Bmi-1, HESC5:3, and HES77 in human papillomavirus-positive and -negative oropharyngeal squamous cell carcinoma. Tumour Biol. (2019) 41:1010428319840473. doi: 10.1177/1010428319840473
36. Zhang M, Kumar B, Piao L, Xie X, Schmitt A, Arradaza N, et al. Elevated intrinsic cancer stem cell population in human papillomavirus-associated head and neck squamous cell carcinoma. Cancer. (2014) 120:992–1001. doi: 10.1002/cncr.v120.7
37. Hufbauer M, Maltseva M, Meinrath J, Lechner A, Beutner D, Huebbers CU, et al. HPV16 increases the number of migratory cancer stem cells and modulates their miRNA expression profile in oropharyngeal cancer. Int J Cancer. (2018) 143:1426–39. doi: 10.1002/ijc.v143.6
38. Yang Y and Cao Y. The impact of VEGF on cancer metastasis and systemic disease. Semin Cancer Biol. (2022) 86:251–61. doi: 10.1016/j.semcancer.2022.03.011
39. Li X, Song D, Liu H, Wang Z, Ma G, Yu M, et al. Expression levels of VEGF-C and VEGFR-3 in renal cell carcinoma and their association with lymph node metastasis. Exp Ther Med. (2021) 21:554. doi: 10.3892/etm.2021.9986
40. Ravaggi A, Gambino A, Ferrari F, Olivari A, Zanotti L, Romani C, et al. VEGF-D serum level as a potential predictor of lymph node metastasis and prognosis in vulvar squamous cell carcinoma patients. Front Oncol. (2022) 12:818613. doi: 10.3389/fonc.2022.818613
41. Uzun S, Korkmaz Y, Wuerdemann N, Arolt C, Puladi B, Siefer OG, et al. Comprehensive analysis of VEGFR2 expression in HPV-positive and -negative OPSCC reveals differing VEGFR2 expression patterns. Cancers (Basel) Oct. (2021) 18:13. doi: 10.3390/cancers13205221
42. Baruah P, Lee M, Wilson PO, Odutoye T, Williamson P, Hyde N, et al. Impact of p16 status on pro- and anti-angiogenesis factors in head and neck cancers. Br J Cancer. (2015) 113:653–9. doi: 10.1038/bjc.2015.251
43. Knuth J, Sharma SJ, Würdemann N, Holler C, Garvalov BK, Acker T, et al. Hypoxia-inducible factor-1α activation in HPV-positive head and neck squamous cell carcinoma cell lines. Oncotarget. (2017) 8:89681–91. doi: 10.18632/oncotarget.20813
44. Hanns E, Job S, Coliat P, Wasylyk C, Ramolu L, Pencreach E, et al. Human Papillomavirus-related tumours of the oropharynx display a lower tumour hypoxia signature. Oral Oncol. (2015) 51:848–56. doi: 10.1016/j.oraloncology.2015.06.003
45. Partlová S, Bouček J, Kloudová K, Lukešová E, Zábrodský M, Grega M, et al. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology. (2015) 4:e965570. doi: 10.4161/21624011.2014.965570
46. Yan S, Zhang X, Lin Q, Du M, Li Y, He S, et al. Deciphering the interplay of HPV infection, MHC-II expression, and CXCL13(+) CD4(+) T cell activation in oropharyngeal cancer: implications for immunotherapy. Cancer Immunol Immunother. (2024) 73:206. doi: 10.1007/s00262-024-03789-0
47. Wu J, Pang X, Yang X, Zhang M, Chen B, Fan H, et al. M1 macrophages induce PD-L1(hi) cell-led collective invasion in HPV-positive head and neck squamous cell carcinoma via TNF-α/CDK4/UPS14. J Immunother Cancer. (2023) 11. doi: 10.1136/jitc-2023-007670
48. Huppert LA, Green MD, Kim L, Chow C, Leyfman Y, Daud AI, et al. Tissue-specific Tregs in cancer metastasis: opportunities for precision immunotherapy. Cell Mol Immunol. (2022) 19:33–45. doi: 10.1038/s41423-021-00742-4
49. Shan Y, Xie T, Sun Y, Lu Z, Topatana W, Juengpanich S, et al. Lipid metabolism in tumor-infiltrating regulatory T cells: perspective to precision immunotherapy. biomark Res. (2024) 12:41. doi: 10.1186/s40364-024-00588-8
50. Gao H, Zhou Y, and Chen X. Tregs and platelets play synergistic roles in tumor immune escape and inflammatory diseases. Crit Rev Immunol. (2022) 42:59–69. doi: 10.1615/CritRevImmunol.2023047234
51. Saleh R and Elkord E. Treg-mediated acquired resistance to immune checkpoint inhibitors. Cancer Lett. (2019) 457:168–79. doi: 10.1016/j.canlet.2019.05.003
52. Liu M, Wang X, Wang L, Ma X, Gong Z, Zhang S, et al. Targeting the IDO1 pathway in cancer: from bench to bedside. J Hematol Oncol. (2018) 11:100. doi: 10.1186/s13045-018-0644-y
53. Tang K, Wu YH, Song Y, and Yu B. Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors in clinical trials for cancer immunotherapy. J Hematol Oncol. (2021) 14:68. doi: 10.1186/s13045-021-01080-8
54. Kannan B, Jayaseelan VP, and Arumugam P. Immunotherapy for oral cancer treatment through targeting of IDO1 and its pathway. J Stomatol Oral Maxillofac Surg. (2023) 124:101375. doi: 10.1016/j.jormas.2022.101375
55. Wee I, Syn N, Sethi G, Goh BC, and Wang L. Role of tumor-derived exosomes in cancer metastasis. Biochim Biophys Acta Rev Cancer. (2019) 1871:12–9. doi: 10.1016/j.bbcan.2018.10.004
56. Sautès-Fridman C, Lawand M, Giraldo NA, Kaplon H, Germain C, Fridman WH, et al. Tertiary lymphoid structures in cancers: prognostic value, regulation, and manipulation for therapeutic intervention. Front Immunol. (2016) 7:407. doi: 10.3389/fimmu.2016.00407
57. Denton AE, Innocentin S, Carr EJ, Bradford BM, Lafouresse F, Mabbott NA, et al. Type I interferon induces CXCL13 to support ectopic germinal center formation. J Exp Med. (2019) 216:621–37. doi: 10.1084/jem.20181216
58. van de Pavert SA and Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol. (2010) 10:664–74. doi: 10.1038/nri2832
59. Jacquelot N, Tellier J, Nutt Sl, and Belz Gt. Tertiary lymphoid structures and B lymphocytes in cancer prognosis and response to immunotherapies. Oncoimmunology. (2021) 10:1900508. doi: 10.1080/2162402X.2021.1900508
60. Weinstein AM, Giraldo NA, Petitprez F, Julie C, Lacroix L, Peschaud F, et al. Association of IL-36γ with tertiary lymphoid structures and inflammatory immune infiltrates in human colorectal cancer. Cancer Immunol Immunother. (2019) 68:109–20. doi: 10.1007/s00262-018-2259-0
61. Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. (2019) 394:1915–28. doi: 10.1016/S0140-6736(19)32591-7
62. Colbeck EJ, Ager A, Gallimore A, and Jones GW. Tertiary lymphoid structures in cancer: drivers of antitumor immunity, immunosuppression, or bystander sentinels in disease? Front Immunol. (2017) 8:1830. doi: 10.3389/fimmu.2017.01830
63. Morad G, Helmink BA, Sharma P, and Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. (2021) 184:5309–37. doi: 10.1016/j.cell.2021.09.020
64. Schumacher TN and Thommen DS. Tertiary lymphoid structures in cancer. Science. (2022) 375:eabf9419. doi: 10.1126/science.abf9419
65. Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med. (2014) 189:832–44. doi: 10.1164/rccm.201309-1611OC
66. Li H, Zhu SW, Zhou JJ, Chen DR, Liu J, Wu ZZ, et al. Tertiary lymphoid structure raises survival and immunotherapy in HPV(-) HNSCC. J Dent Res. (2023) 102:678–88. doi: 10.1177/00220345231151685
67. Wang M, Zhai R, Wang M, Zhu W, Zhang J, Yu M, et al. Tertiary lymphoid structures in head and neck squamous cell carcinoma improve prognosis by recruiting CD8(+) T cells. Mol Oncol. (2023) 17:1514–30. doi: 10.1002/1878-0261.13403
68. Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. (2016) 17:956–65. doi: 10.1016/S1470-2045(16)30066-3
69. Xu S, Han C, Zhou J, Yang D, Dong H, Zhang Y, et al. Distinct maturity and spatial distribution of tertiary lymphoid structures in head and neck squamous cell carcinoma: implications for tumor immunity and clinical outcomes. Cancer Immunol Immunother. (2025) 74:107. doi: 10.1007/s00262-025-03952-1
70. Krupar R. The tumor microenvironment-relay station for prognosis and therapy response. Pathol (Heidelb). (2022) 43:141–7. doi: 10.1007/s00292-022-01159-0
71. Chen AM. De-escalated radiation for human papillomavirus virus-related oropharyngeal cancer: Who, why, what, where, when, how, how much and what next? Radiother Oncol. (2024) 200:110373. doi: 10.1016/j.radonc.2024.110373
72. Samaniego C, Friedman J, Yang X, Badger C, Shaver T, Samankan S, et al. Neoadjuvant chemotherapy enhances tumor-specific T cell immunity in patients with HPV-associated oropharyngeal cancer. Head Neck. (2023) 45:2294–302. doi: 10.1002/hed.27463
73. Gabrilovich DI and Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. (2009) 9:162–74. doi: 10.1038/nri2506
Keywords: oropharyngeal carcinoma, human papillomavirus, lymphatic metastasis, immune microenvironment, immunotherapy
Citation: Li X, Qiu H and Wu Q (2025) Lymphatic dissemination of HPV-positive oropharyngeal squamous cell carcinoma: underlying mechanisms and treatment innovations. Front. Immunol. 16:1601572. doi: 10.3389/fimmu.2025.1601572
Received: 28 March 2025; Accepted: 19 May 2025;
Published: 02 June 2025.
Edited by:
Haixia Zhu, Nantong Tumor Hospital, ChinaReviewed by:
Xin Zhang, Affiliated Foshan Hospital of Southern Medical University, ChinaCopyright © 2025 Li, Qiu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Qiu, cWl1aHVpem55eUB3aHUuZWR1LmNu; Qiuji Wu, d3VxaXVqaUAxMjYuY29t
 Xinyu Li
Xinyu Li Hui Qiu
Hui Qiu Qiuji Wu
Qiuji Wu