- 1Center of Basic Medical Research, Institute of Medical Innovation and Research, Peking University Third Hospital, Beijing, China
- 2Cancer Center of Peking University Third Hospital, Peking University Third Hospital, Beijing, China
- 3Beijing Key Laboratory for Interdisciplinary Research in Gastrointestinal Oncology (BLGO), Peking University Third Hospital, Beijing, China
- 4Biobank, Institute of Medical Innovation and Research, Peking University Third Hospital, Beijing, China
Programmed death-ligand 1 (PD-L1) carried by tumor-derived exosomes has emerged as a critical mediator of immune evasion and resistance to immune checkpoint blockade therapy. Unlike membrane-bound PD-L1, exosomal PD-L1 is systemically distributed and capable of suppressing T cell activity at distant sites. This review summarizes the current understanding of exosomal PD-L1 biogenesis, its immunosuppressive mechanisms, and its clinical relevance across multiple cancer types. We highlight its potential as a non-invasive biomarker for predicting therapeutic response and monitoring disease progression. Compared with tissue-based PD-L1 assessment, exosomal PD-L1 offers advantages in accessibility and dynamic reflection of tumor immune status. However, challenges remain regarding standardization of detection methods and clinical interpretation. Future directions include the integration of exosomal PD-L1 profiling into immunotherapy decision-making and the development of therapeutic strategies targeting exosome secretion. These insights may contribute to overcoming resistance in immunologically inert tumors and advancing precision oncology.
1 Introduction
Immune checkpoint blockade (ICB) therapies, particularly those targeting the programmed cell death protein 1 (PD-1) and its ligand PD-L1, have revolutionized cancer treatment by restoring anti-tumor immunity in a subset of patients (1). Despite their success, less than 30% of patients can achieve durable clinical benefit, and both primary and acquired resistance remain major obstacles to broader efficacy (2–5). This underscores a critical need for reliable, minimally invasive biomarkers that can predict therapeutic response and guide personalized immunotherapy strategies.
To date, biomarkers such as PD-L1 expression in tumor tissues, tumor mutational burden (TMB), and microsatellite instability (MSI) have been investigated with varying predictive value. However, the clinical application of these markers is limited by tumor heterogeneity, dynamic expression, sampling bias, and the invasive nature of tissue biopsies (6–8). Furthermore, pan-cancer analyses have demonstrated wide variability in response rates to anti-PD-1/PD-L1 therapies—even within the same tumor type—suggesting that existing tissue-based indicators do not fully capture systemic immune escape mechanisms (9).
Exosomal PD-L1 (exo-PD-L1), a membrane-bound form of PD-L1 secreted via tumor-derived exosomes, has emerged as a promising biomarker with distinct advantages (9). Exosomes are small extracellular vesicles that facilitate intercellular communication by transporting bioactive molecules, including PD-L1, to immune cells (10, 11). Unlike static tumor PD-L1 measurements, exo-PD-L1 levels in peripheral blood provide a dynamic and systemic snapshot of tumor-mediated immune suppression (12). Elevated circulating exo-PD-L1 has been associated with poor prognosis, resistance to ICB, and increased tumor burden across various cancers (9, 13–15).
In this review, we explore the biological mechanisms underlying exo-PD-L1 secretion and its immunosuppressive role in the tumor microenvironment. We summarize current detection strategies and assess the clinical significance of exo-PD-L1 as a predictive biomarker across cancer types. Finally, we highlight future research directions and potential applications of exo-PD-L1 in enhancing ICB precision, overcoming resistance, and advancing personalized cancer immunotherapy.
2 Biogenesis and immune regulation of exosomal PD-L1
2.1 Biogenesis and secretion of exo-PD-L1
Exosomes are biologically active, lipid bilayer nano-vesicles. Current evidence suggests that exo-PD-L1 originates from the plasma membrane. Consequently, it is widely believed that early endosomes formed via cellular membrane endocytosis serve as the source of exo-PD-L1.
The process begins with the inward invagination of the parent cell’s plasma membrane, leading to the closure and formation of early endosomes. Within these early endosomes, intraluminal vesicles (ILVs) are generated through multiple inward budding events, ultimately resulting in the formation of mature multivesicular bodies (MVBs) containing ILVs. During MVB formation, certain endosomal proteins and other cellular components, not destined for lysosomal degradation, are selectively sorted into ILVs. Upon maturation, MVBs fuse with the cellular membrane, releasing the vesicles as exosomes, which typically range in size from 30 to 150 nm (10, 11). They are secreted by all active cells and are found in various bodily fluids (16). Exosomes’ cargo typically includes a diverse array of RNA, DNA, proteins, miRNA, metabolites, and other bioactive molecules (17).These cargoes mediate intercellular signaling, information exchange, and immune modulation by transporting their contents to recipient cells (10, 18–22).
Importantly, during the formation of intraluminal vesicles (ILVs), membrane proteins such as PD-L1 are incorporated with preserved topological orientation. This means that transmembrane proteins located on the plasma membrane of the parent cell are embedded into the ILV membrane such that their extracellular domains face the lumen of the ILV. Upon exosome release, the ILV becomes an exosome, and its membrane orientation flips relative to the cytoplasm, thereby exposing PD-L1 on the outer surface of the exosome. This membrane topology conservation ensures that exo-PD-L1 retains its ability to engage PD-1 receptors on recipient T cells, thereby mediating immunosuppressive interactions post-secretion (9, 23, 24).
2.2 Function of exo-PD-L1
Given the conserved membrane orientation of PD-L1 on exosomal surfaces, exo-PD-L1 is functionally positioned to interact with PD-1 on T cells and other immune populations. Interaction of exo-PD-L1 with PD-1 on immune cells triggers PD-1-mediated intracellular signaling, inhibiting PI3K-AKT and MAPK pathways, thereby restricting T cell proliferation, activation, and survival (6–8). Prolonged exposure to elevated levels of exo-PD-L1 leads to T cell exhaustion, impacting long-term immune responses and reducing responsiveness to tumors, thereby facilitating immune escape. The interaction between TCR and MHC molecules is essential for the first signal required to induce T cell activation. On the exosome surface, the presence of MHC-I can enhance PD-L1-induced T cell dysfunction. Therefore, exo-PD-L1 can more effectively induce T cell dysfunction (8, 14, 25). Removing exosomal PD-L1 can effectively enhance the sensitivity of mouse tumor models to anti-PD-L1 immune checkpoint therapy (4, 9, 13). This finding suggests potential therapeutic strategies, which will be discussed in the next section regarding the mechanisms of immune suppression induced by exosomal PD-L1.
Furthermore, extensive experiments demonstrate that interferon-gamma significantly increases exo-PD-L1 release. Exo-PD-L1 release is considered as an immune evasion mechanism in response to interferon-gamma secreted by CD8+ T cells, macrophages, and natural killer cells. In vitro studies show that exo-PD-L1 also reduces secretion of interferon-gamma, tumor necrosis factor-alpha, granzyme B, and perforin from T cells (4, 13–15). This suggests that tumor cells can counteract CD8+ T cell function during their effector phase by exo-PD-L1, inhibiting cytokine production and cytotoxic granule exocytosis without requiring direct cell-to-cell interactions (Figure 1). Recent studies have revealed that tumor-derived exosomes carrying PD-L1 can promote T cell senescence through lipid metabolism reprogramming (26). This process involves metabolic shifts in T cells that accelerate their aging process, impairing their ability to produce cytokines and perform cytotoxic functions. These changes contribute to long-term immune suppression, further enhancing tumor immune evasion (26–28).The lipid metabolism alterations induced by exo-PD-L1 result in T cell dysfunction and senescence, diminishing their effectiveness in combating tumors. By promoting T cell aging, exo-PD-L1 not only suppresses immediate immune responses but also impairs the ability of T cells to mount sustained anti-tumor immunity (26, 29). This new mechanism of T cell senescence adds to the understanding of how tumors exploit exosomal PD-L1 to evade immune surveillance, underscoring the potential of targeting this pathway for therapeutic intervention.
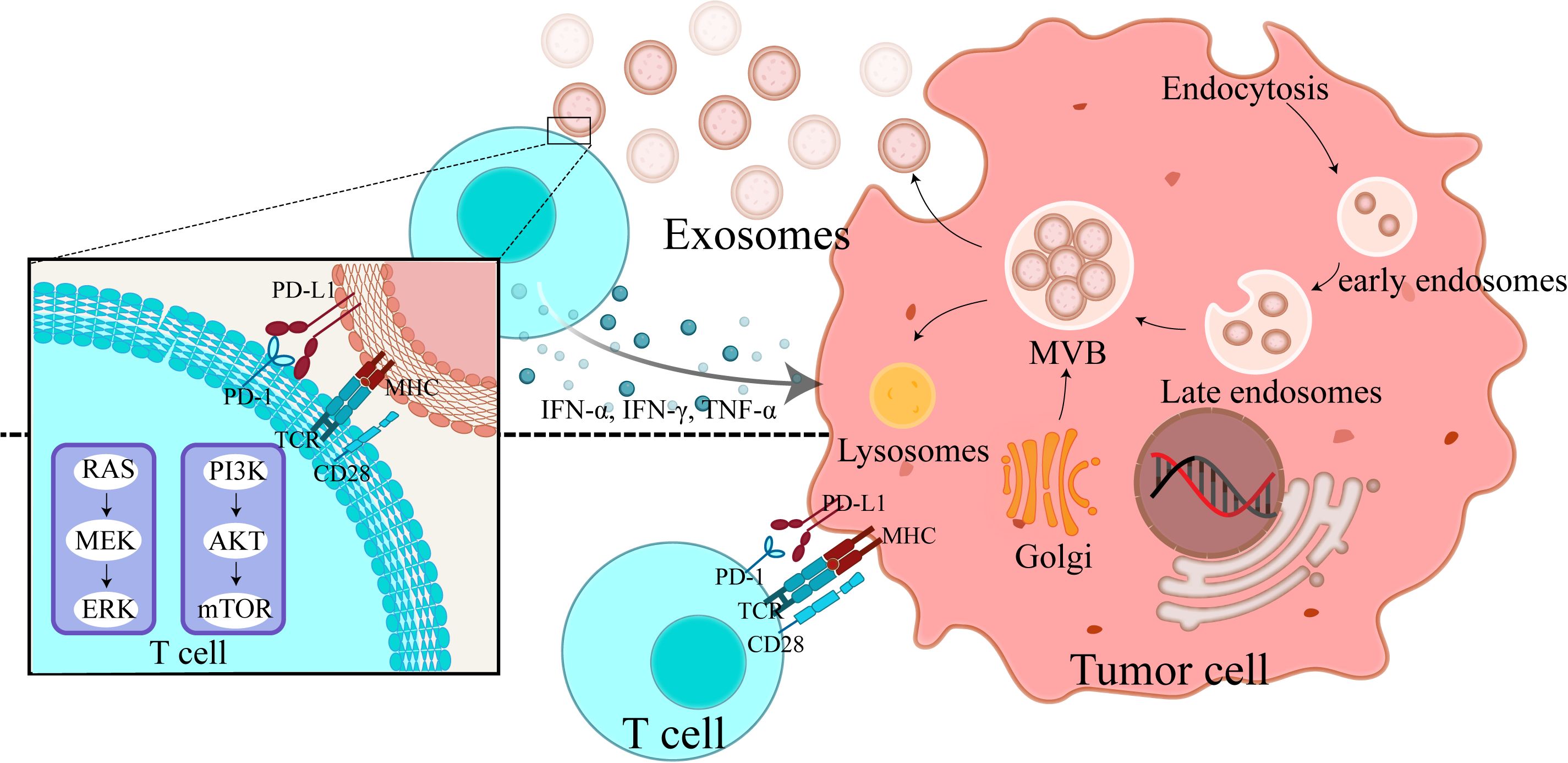
Figure 1. Exosomal PD-L1 release mechanism and its impact on T cell function: tumor cell-mediated immune evasion. This figure provides a detailed depiction of the exo-PD-L1 release mechanism from tumor cells and its impact on T cell function. It depicts exosome biogenesis, starting from plasma membrane invagination, progressing to the formation of multivesicular bodies, and culminating in the release of PD-L1-containing exosomes. Additionally, it shows how cytokines—IFN-α, IFN-γ, and TNF-α—stimulate exosome release and enhance PD-L1 expression on tumor cells. Two key interactions are highlighted (1): the direct binding of PD-L1 on tumor cells to T cells, resulting in T cell inhibition, and (2) the interaction of T cells with PD-L1 on exosomes, leading to immune suppression over longer distances. This figure demonstrates how exo-PD-L1 contributes to immune evasion and how cytokine stimulation affects its release and expression.
In summary, exo-PD-L1 mediates multifaceted immunosuppressive effects, including the inhibition of T cell activation, the promotion of exhaustion and senescence, and the suppression of cytokine secretion and cytotoxic granule release. Emerging evidence also suggests that exo-PD-L1 may influence the function of antigen-presenting cells and regulatory T cells, warranting further investigation.
2.3 Factor regulating exo-PD-L1
Tumor heterogeneity is a major factor influencing the effectiveness of exo-PD-L1 as a reliable biomarker for predicting immunotherapy outcomes. This heterogeneity can manifest at various levels, including tumor type, immune cell infiltration, genetic mutations, and even the tumor microenvironment (TME), all of which can affect exo-PD-L1 expression. The levels of exo-PD-L1 in circulation may vary significantly due to these factors, limiting its predictive value across different cancer types and patients (30).
For example, ovarian cancer, often referred to as a ‘cold tumor’, is characterized by low immune cell infiltration, particularly T cells. As a result, exo-PD-L1 levels in ovarian cancer patients may be lower than in cancers with higher immune infiltration, such as melanoma or non-small cell lung cancer (NSCLC). Similarly, in tumors with significant genetic mutations or TME dysregulation, the regulation of PD-L1 expression on both tumor cells and exosomes may vary, leading to inconsistent levels of exo-PD-L1 in the blood.
Additionally, the tumor’s immune microenvironment plays a crucial role in modulating exo-PD-L1 secretion (31). In cancers with an immunosuppressive TME, such as pancreatic cancer, high levels of cytokines and other immune modulators might promote the secretion of exo-PD-L1 by tumor cells and immune cells. Conversely, tumors with a more pro-inflammatory TME might show a different pattern of exo-PD-L1 expression.
Moreover, patient-specific factors, including genetic predispositions, prior treatments, and the overall immune status of the individual, can contribute to differences in exo-PD-L1 levels (9). For instance, patients with a history of autoimmune diseases or those who are immunosuppressed might exhibit altered exo-PD-L1 dynamics, further complicating the biomarker’s predictive value.
2.4 Exo-PD-L1 in tumor progression and immunotherapy monitoring
The cargo of extracellular vesicles (EVs) provides a holistic snapshot of the patient’s immune status (8, 13, 32). exo-PD-L1 correlates with tumor progression in various cancers such as melanoma, breast cancer, head and neck squamous cell carcinoma, and glioblastoma (9, 13, 24, 33). Notably, elevated exo-PD-L1 expression is significantly associated with advanced tumor stages, highlighting its pivotal role in fueling tumor growth and metastasis. Previous studies utilizing various murine models have consistently shown that the introduction of tumor-derived EVs amplifies the metastatic propensity of primary tumors and exacerbates overall tumor burden (34–36).
Therefore, targeting exogenous exosomes may provide a novel strategy to overcome tumor resistance to anti-PD-L1 therapies (37). Monitoring circulating exo-PD-L1 levels serves as a biomarker for tumor response to immunotherapy. The dynamic changes in exo-PD-L1 levels during treatment could be used to predict the likelihood of a sustained immune response and inform adjustments to therapy, ultimately optimizing individualized treatment regimens (38).
3 Exo-PD-L1 in the tumor immune suppression
The tumor microenvironment (TME) is a complex ecosystem composed of various immune cells, stromal components, and signaling molecules that collectively influence tumor progression and immune escape (31, 39). Exosomal PD-L1 (exo-PD-L1) has become a key player in mediating immune suppression within the TME (9, 40). Secreted by both tumor cells and infiltrating immune cells, exo-PD-L1 can modulate the immune response by transferring immune checkpoint molecules to surrounding immune cells, primarily T cells. This process contributes to immune evasion and aids tumor progression by inhibiting T cell activation and function (9, 14).
3.1 Exo-PD-L1 and immune suppression
Exo-PD-L1, primarily released by tumor cells and tumor-associated macrophages (TAMs), plays a pivotal role in shaping the immune landscape of the TME. Tumor-derived exosomes carry PD-L1 to the surface of immune cells, such as T cells and dendritic cells, and bind to the PD-1 receptor, effectively inhibiting their activity. By doing so, exo-PD-L1 fosters a suppressive immune microenvironment that prevents T cells from recognizing and attacking tumor cells. This immune evasion mechanism is crucial for tumor survival, particularly in the context of immunotherapy resistance (39).
In addition to its effects on T cells, exo-PD-L1 can also influence the activity of other immune modulators within the TME. For example, tumor-derived exosomal PD-L1 has been shown to promote the polarization of macrophages toward the M2 (immunosuppressive) phenotype, thereby contributing to a more suppressive tumor immune microenvironment and facilitating immune escape (41, 42). This reprogramming is linked to the secretion of cytokines such as TGF-β, which suppresses T cell activity and promotes tumor growth (43, 44). TAM-derived exosomes, containing miR-21 and other microRNAs, can further exacerbate immune suppression by inhibiting T cell proliferation and inducing the recruitment of regulatory T cells (Tregs) to the tumor site (45, 46).
3.2 Exo-PD-L1 and resistance to immunotherapy
The ability of tumors to evade immune surveillance by secreting exo-PD-L1 is a significant factor in the development of resistance to immune checkpoint inhibitors (ICIs), such as anti-PD-1/PD-L1 therapies. While ICIs are designed to block the PD-1/PD-L1 interaction and restore T cell function, the presence of exo-PD-L1 in the TME can counteract these therapies by maintaining an inhibitory signaling axis that diminishes T cell responses. Exo-PD-L1 thus represents a critical mechanism of “immune checkpoint resistance” in the context of ICI treatment. Additionally, exo-PD-L1 can act as a decoy by competitively binding anti-PD-L1 antibodies, thereby reducing their availability to block membrane-bound PD-L1 on tumor cells. This mechanism contributes to the limited efficacy of checkpoint inhibitors in some patients (14, 47).
Moreover, exosomes from tumor cells can also promote immune suppression by facilitating the transfer of other immune checkpoint molecules such as VISTA (V-domain Ig-containing suppressor of T cell activation), which can act synergistically with PD-L1 to inhibit T cell function (48, 49). This underscores the complexity of the immune evasion mechanisms in the TME, where multiple immune checkpoints are concurrently regulated, often rendering ICI therapy less effective.
3.3 Advantages of exo PD-L1 as a predictive biomarker for ICB response
While section 2.5 presented clinical evidence supporting exo-PD-L1 as a biomarker for tumor progression and immunotherapy response, this section highlights the comparative advantages of exo-PD-L1 over other circulating and tissue-based biomarkers, such as IHC-PD-L1, ctDNA, and soluble PD-L1.
The limited efficacy of current biomarkers in predicting immune checkpoint blockade (ICB) response reflects the complexity of tumor-immune interactions, encompassing genomic, spatial, and immunologic heterogeneity. Conventional markers—such as PD-L1 immunohistochemistry (IHC), tumor mutational burden (TMB), and microsatellite instability (MSI)—offer static, region-specific snapshots that often fail to represent dynamic immune status or therapeutic adaptation (33–36)
Liquid biopsy approaches have sought to overcome these limitations. While circulating tumor DNA (ctDNA) provides mutational insights, it does not reflect real-time immune suppression and is affected by tumor shedding kinetics (37). Soluble PD-L1 (sPD-L1), though easily detectable in plasma, lacks stability and functional specificity due to its unbound, cleaved nature (38, 39).
By contrast, exosomal PD-L1 (exo-PD-L1) represents a membrane-bound, functionally active form of PD-L1, selectively packaged via Rab27a and ESCRT-dependent pathways (9). Exosomes preserve PD-L1 integrity and extend its immunosuppressive reach beyond the tumor, notably trafficking to lymph nodes and modulating systemic immunity.
Clinically, high baseline exo-PD-L1 levels correlate with poor prognosis and early resistance to ICB in NSCLC and melanoma, while dynamic increases post-treatment predict durable responses—even before imaging confirmation (9). Unlike static biomarkers, exo-PD-L1 integrates upstream oncogenic signals (e.g., IFN-γ/JAK-STAT), microenvironmental factors (e.g., hypoxia), and downstream immune suppression, serving as both a functional and temporal indicator of tumor immune escape (40).
In sum, exo-PD-L1 is a mechanistically distinct biomarker with potential for real-time monitoring and stratification in precision immunotherapy (40).
4 Clinical implications of exosomal PD-L1 across cancer types
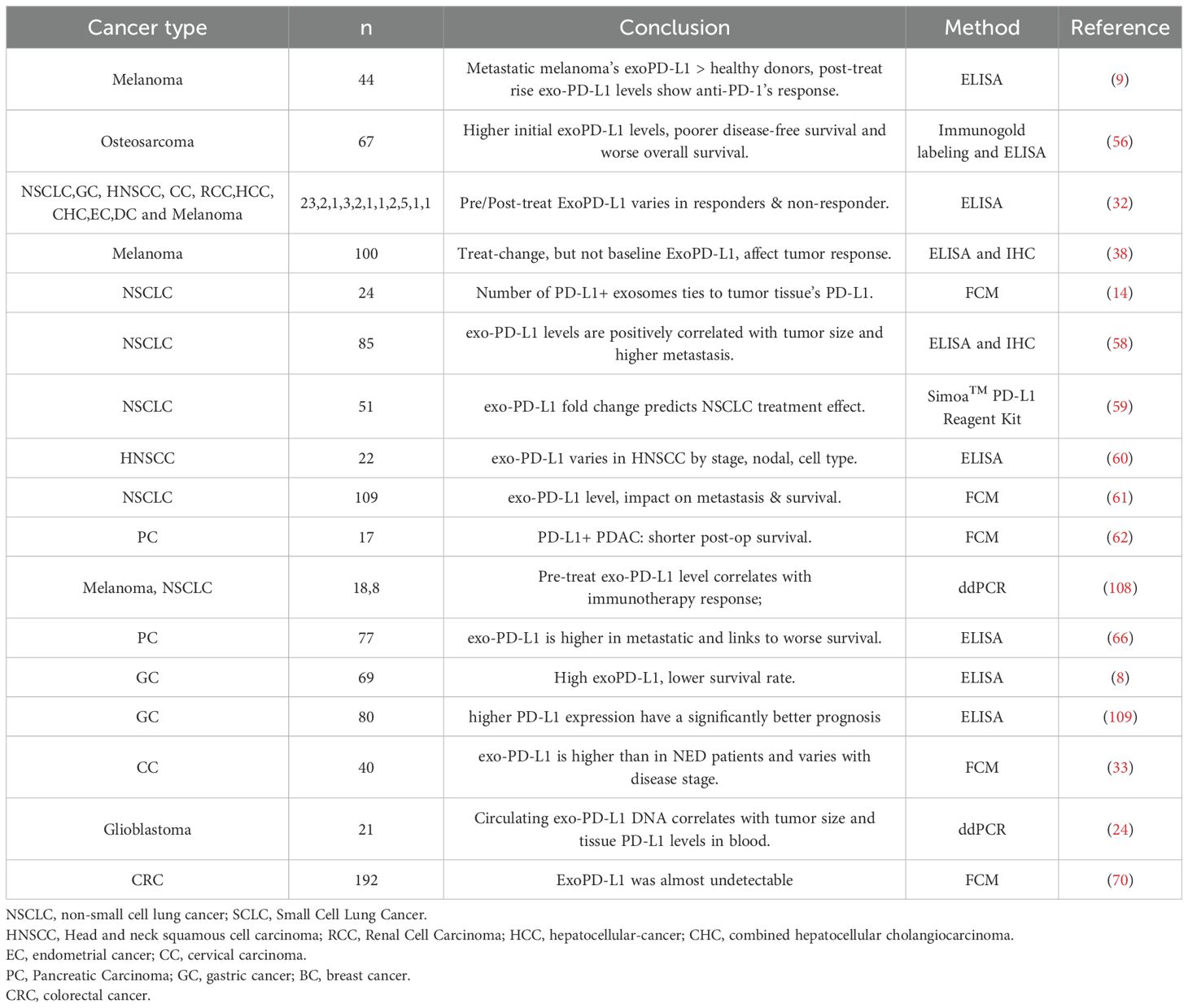
In recent years, immune checkpoint blockade (ICB) therapies targeting CTLA-4, PD-1, and PD-L1 have shown promising efficacy in various cancers, including melanoma, NSCLC, and renal cell carcinoma (50–53). However, sustained responses are observed in fewer than 30% of patients, with minimal benefit in malignancies like ovarian and prostate cancer (54, 55) (Table 1).
Circulating exo-PD-L1 reflects the immunosuppressive tumor microenvironment and may serve as an indicator of ICB responsiveness (8, 9, 32). Nonetheless, its predictive value varies across cancer types due to tumor heterogeneity and individual immune differences. These challenges are elaborated in subsequent sections.
Research on exo-PD-L1 is most advanced in melanoma, where early increases in circulating exo-PD-L1 during PD-1 blockade have been shown to distinguish responders from non-responders, reflecting T cell reactivation. In metastatic melanoma, a 2.43-fold increase in exo-PD-L1 correlates with favorable outcomes, despite the lack of consistent association with tumor PD-L1 expression (9). Additionally, metastatic melanoma patients exhibit higher exo-PD-L1 levels than healthy donors. Similar patterns have been observed in NSCLC, supporting its cross-tumor relevance as a treatment response biomarker.
Further studies show that melanoma patients with pre-treatment exo-PD-L1 levels below 25.96 pg/mL have better survival rates (56). Post-treatment, exo-PD-L1 levels rise more significantly in responders than in non-responders (32)., whereas total or non-exosomal PD-L1 levels show no such distinction (9, 57). An early increase in exo-PD-L1 during therapy may serve as a dynamic marker for patient stratification (57). Moreover, exo-PD-L1 levels correlate with tumor burden and IFN-γ levels, reinforcing their value in prognosis and treatment monitoring (9, 38).
4.1 Exo-PD-L1 in NSCLC
In NSCLC studies, exo-PD-L1 in patient plasma strongly correlates with positive tumor PD-L1 expression (14). Exosomal miR-5684 and miR-125b-5p are significantly lower in patients’ peripheral blood compared to healthy donors (20). Exo-PD-L1 levels were significantly higher in NSCLC patients compared to healthy donors, especially in those with advanced tumor features, while sPD-L1 levels showed no significant difference between the two groups (58). In another NSCLC report, a fold change in exo-PD-L1 of ≥ 1.86 was associated with better therapeutic outcomes and overall survival (OS) (59).
4.2 Exo-PD-L1 in cervical cancer
In cervical cancer patients, non-recurrent individuals had higher levels of tumor-enriched CD3- exoPD-L1 before treatment, which significantly decreased five weeks post-treatment (60, 61). Conversely, recurrent patients showed an increase in tumor-enriched exoPD-L1 and a decrease in CD3+ exoPD-L1 levels at week 5 of treatment (61).
4.3 Exo-PD-L1 in pancreatic ductal adenocarcinoma
In pancreatic ductal adenocarcinoma, exo-PD-L1 is associated with poor prognosis (62). Higher levels are linked to unresectable tumors and shorter survival. miRNA-196a, miRNA-1246, miRNA-191, miRNA-21, miR-451a, and miRNA-483-3p are elevated in exosomes or liquid biopsies (63–65). In NSCLC, higher extracellular vesicle PD-L1 mRNA levels before treatment are related to better responses. In pancreatic cancer, serum exo-PD-L1 is higher in metastatic patients, and elevated levels mean worse survival (66).
4.4 Exo-PD-L1 in gastric cancer
In gastric cancer, preoperative soluble PD-L1 levels aren’t linked to clinical outcomes (8). Higher circulating PD-L1 may indicate malignancy, but its correlation with staging and prognosis is inconsistent. In advanced cases, tumor tissue PD-L1 is higher than in healthy tissues and ties to differentiation and lymph node metastasis. In adenocarcinoma, high PD-L1 expression means better prognosis. Exo-PD-L1 signals mean poor post-treatment outcomes and is an early gastric adenocarcinoma prognostic factor related to tumor staging (66).
4.5 Exo-PD-L1 in head and neck squamous cell carcinoma
In head and neck squamous cell carcinoma studies, exo-PD-L1 serves as a marker of poor outcomes following surgery or chemoradiotherapy (58). The level of exo-PD-L1 correlates with disease activity (33). However, studies on glioblastoma indicate that exo-PD-L1 levels cannot distinguish glioblastoma patients from healthy donors (24, 34, 35).
4.6 Exo-PD-L1 in “cold tumor”
Although research on exo-PD-L1 has provided valuable insights in many cancer types, its role in cold tumors like ovarian cancer remains poorly understood. Ovarian cancer, often described as a ‘cold tumor’ due to its lack of immune cell infiltration, shows minimal response to immune checkpoint blockade (ICB) therapies. Preliminary studies on exo-PD-L1 in ovarian cancer suggest that low levels of exo-PD-L1 may contribute to this poor response, but further research is needed to better understand its potential as a biomarker in this setting. Ovarian cancer’s limited immune response is thought to be due to the absence of a robust T-cell infiltrate, which limits the effectiveness of PD-1/PD-L1 blockade therapies. Some preliminary data suggest that ovarian cancer patients exhibit relatively low exo-PD-L1 levels, which might contribute to the poor response observed in clinical settings (54, 55). However, further research is needed to understand the role of exo-PD-L1 in this and other less-responsive cancers.
Accurate diagnosis, staging, and prognosis assessment are crucial for enhancing immune therapy response. Immunohistochemistry (IHC) detection of tumor PD-L1 expression, tumor mutational burden (TMB) and microsatellite instability (MSI) are the most commonly used biomarkers for predicting immune therapy response (67–69). However, methodological differences among studies and the dynamic regulation of PD-L1 expression have led to conflicting evidence, limiting the use of tumor PD-L1 expression as an exclusionary biomarker (69). Thus, liquid biopsy may reemerge as a non-invasive tool for screening candidate factors influencing clinical outcomes of immune therapy. Exo-PD-L1 provides a platform to assess cancer patients’ immune status non-invasively. However, correlating exo-PD-L1 levels with tumor PD-L1 expression remains challenging.
In conclusion, exo-PD-L1 shows potential in tumor staging, detection, and aiding ICB therapy. However, a universal standard for evaluation does not exist across different cancers. Future research must focus on analyzing the relationship between PD-L1 and exo-PD-L1 in various cancer tissues, exploring the statistical relationships of exoPD-L1 in different tumors, standardizing detection techniques, and establishing critical ratio for exoPD-L1 and tumor PD-L1 values to differentiate patient conditions and prognosis (70). Additionally, translating these findings into clinical practice requires overcoming significant challenges in standardizing exo-PD-L1 measurement and interpretation across different tumor types and clinical settings.
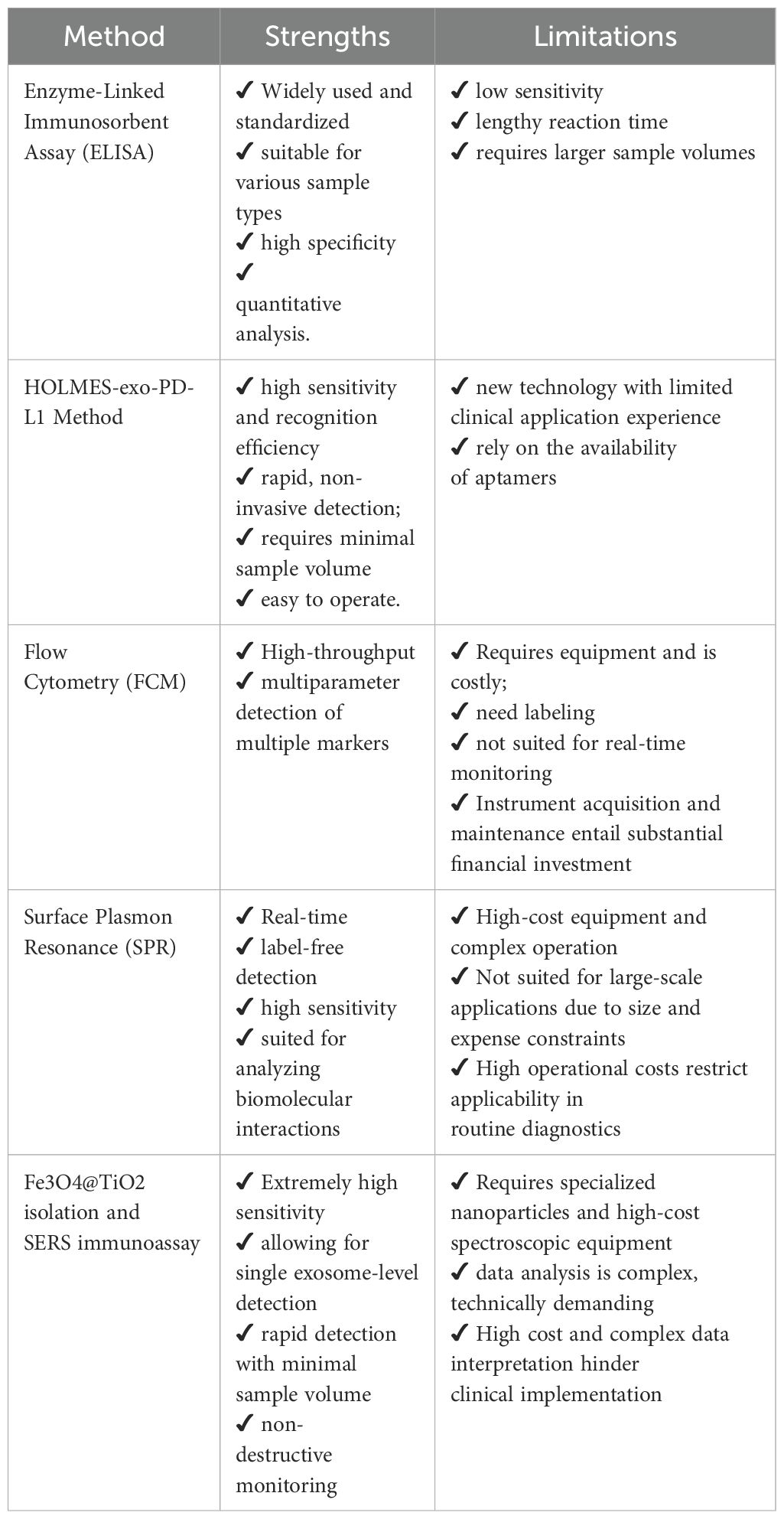
5 Exosomal PD-L1 detection technologies
Peripheral blood is crucial for liquid biopsy, aiding in disease subtyping pre - treatment and minimal residual disease monitoring post - therapy. Exosomes in peripheral blood have higher concentrations and better stability, biocompatibility, low immunogenicity, and minimal toxicity than ctDNA and CTCs. As lipid bilayer vesicles, they’re less affected by the environment and degradation, helping distinguish tumor-derived exosomes from normal ones. In cancer patients, exosomes from serum are analyzed for immune-suppressive molecules like PD-L1, FasL, TRAIL, IL-10, and TGF-β1. In ovarian cancer, ascites and plasma can be analyzed for soluble cytokines via ELISA and CBA, and exosomes from them examined for immune checkpoint molecules like exo-PD-L1 or exo-Gal-9 (12).
5.1 Exosome isolation methods
Exosomes are small extracellular vesicles that play a crucial role in intercellular communication, and their detection and analysis are essential for various clinical applications. Due to their small size and high heterogeneity, ultracentrifugation has long been the predominant method for exosome separation. This technique involves differential centrifugation to remove cells and debris, followed by high-speed centrifugation (up to 100,000 g) to isolate microvesicles, ultimately obtaining purified exosomes. However, the time-consuming nature of this method in clinical settings has led to the development of faster techniques, such as affinity-based purification kits. These kits employ antibodies coupled with magnetic beads targeting exosome surface markers (e.g., CD63, CD9, CD81) or proteins with T-cell immunoglobulin and mucin domains (e.g., TIM4) for efficient exosome isolation (71). Recent advancements in flow cytometry, such as Nano-FACS, have been adapted for exosome analysis, validating the potential clinical applications of these rapid separation methods for exosomes and their surface markers, including PD-L1 (71).
5.2 PD-L1 detection techniques
Current methods for detecting PD-L1 on tumor-derived exosomes primarily involve ultracentrifugation coupled with ELISA, though these suffer from low efficiency and sensitivity, limiting widespread clinical use (72, 73). Recent advancements include the development of HOLMES-exoPD-L1, a homogeneous, low-volume, high-efficiency, and high-sensitivity quantitative method for exosome PD-L1 detection (74). This technique combines PD-L1 aptamers with HOLMES technology, eliminating the need for separation and offering significantly higher sensitivity and faster detection compared to traditional ELISA methods (74, 75).
In addition, there exist several conventional techniques for the detection of exo-PD-L1 (exo-PD-L1). Electron microscopy and immunoelectron microscopy can serve as qualitative methods to confirm the presence of exoPD-L1. The percentage of exoPD-L1 can be measured using nanoscale flow cytometry or conventional flow cytometry with the aid of magnetic or latex beads. The relative quantification of PD-L1 levels is often assessed through relative fluorescence intensity. Western blotting is used to evaluate the total PD-L1 protein levels within exosomes. Both flow cytometry and immunoblotting provide semi-quantitative measurements. Absolute quantification can be achieved through enzyme-linked immunosorbent assay (ELISA). However, it is important to note that Western blotting and ELISA have detection limits and may not be suitable for detecting low abundance of exoPD-L1 in the early stages of cancer (76). In addition to Western blotting (WB) and ELISA, devices such as the Exo-Counter can directly quantify exosomes expressing specific membrane molecules using as little as 10–50 microliters of plasma or bodily fluid samples without the need for exosome isolation. These advancements hold promise for meeting the future demands of rapid clinical diagnostics (77, 78).
To address ELISA limitations, the nano plasmonic extravesicular (nPLEX) assay has been developed, utilizing modified surface plasmon resonance (SPR) biosensors and compact SPR biosensors (79, 80). This approach enables real-time detection of exoPD-L1 in 50 µL serum samples, demonstrating enhanced detection sensitivity (80).
Additionally, a rapid and precise method for detecting exo-PD-L1 directly from clinical samples has been established using Fe3O4@TiO2 separation and surface-enhanced Raman scattering (SERS) immunoassays. This approach significantly enhances both the separation efficiency and detection sensitivity of exosomes (54, 81).
Despite challenges posed by inconsistent exosome separation procedures, quality control, and storage methods, emerging microfluidic-based separation technologies (e.g., nanoparticle platforms) are being investigated as next-generation solutions for effective exosome isolation (82–85). As these technical challenges are addressed, the potential of exosomes in cancer therapy is expected to be further realized (85) (Figure 2; Table 2).
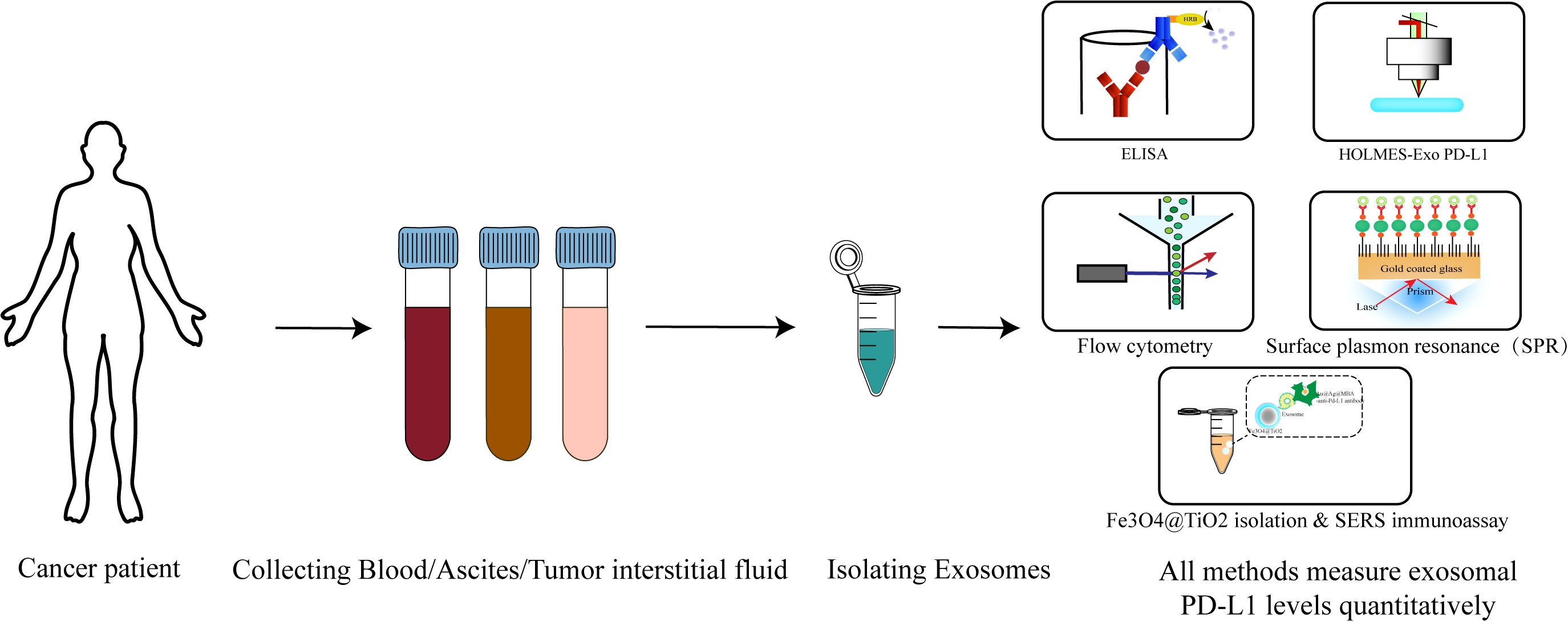
Figure 2. Detection process of exo-PD-L1: from sample collection to modern detection techniques. The diagram outlines the process for detecting exo-PD-L1, a biomarker for cancer diagnostics. The workflow begins with the collection of patient samples, which may include peripheral blood, tumor tissue fluid, or ascites (in cases such as ovarian cancer) (1). Following sample collection, exosomes are isolated using various methods such as ultracentrifugation, affinity-based purification, or advanced techniques like Nano-FACS (2). Once exosomes are purified, the presence of PD-L1 on their surface is detected using several techniques. Traditional methods include enzyme-linked immunosorbent assay (ELISA). Recent advancements feature high-sensitivity approaches such as HOLMES-exo-PD-L1, nano plasmonic extravesicular (nPLEX) assay, and surface-enhanced Raman scattering (SERS) immunoassays (4). These modern techniques offer improved detection sensitivity and efficiency. The figure highlights the steps from sample collection through exosome isolation to the detection of exo-PD-L1, reflecting the current state-of-the-art in exosome-based cancer diagnostics.
6 Therapeutic applications and targeting strategies
By alleviating the suppression of T cells, reducing exosome secretion can potentially restore anti-tumor T cell responses. However, therapies specifically targeting exosomal PD-L1 (exo-PD-L1) are still in their nascent stages, with no direct exo-PD-L1-targeting treatments currently available. Current approaches predominantly focus on inhibiting exosome production, thereby indirectly lowering exo-PD-L1 levels.
6.1 Inhibition of exosome biogenesis and secretion
Exosome release can be inhibited using antibodies, chemical inhibitors, and genetic manipulation, enhancing cancer treatment efficacy (86, 87). Exosome formation, cargo sorting, and secretion rely on the Endosomal Sorting Complex Required for Transport (ESCRT) mechanism. High-throughput screening of 4,580 compounds identified five effective inhibitors: tipifarnib, naftifine, clomipramine, ketoconazole, and miconazole (88). These suppress exosome production by downregulating ESCRT-dependent proteins (88–90).Dimethyl amiloride (DMA) inhibits exosome release by targeting H+/Na+ and Na+/Ca2+ channels, reducing exosome-induced immunosuppression and enhancing anti-tumor efficacy, making it a promising chemotherapy approach (91). GW4869 and spiroepoxide inhibit neutral sphingomyelinase (nSMase) via ESCRT-independent mechanisms (92), blocking exosome secretion. As ceramide biosynthesis inhibitors, GW4869 suppresses exosome secretion in 293T cells and inhibits ovarian cancer exosome release and cell invasion (93).
6.2 Combination with immune checkpoint inhibitors
Combining exosome inhibition with immune checkpoint blockade has emerged as a promising therapeutic strategy to enhance antitumor immunity. By reducing circulating exo-PD-L1 levels, such combinations may enhance the accessibility and efficacy of anti-PD-1/PD-L1 antibodies within the tumor microenvironment. In breast cancer mouse models, treatment with sulfamethoxazole, macitentan, and anti–PD-L1 antibodies significantly decreased plasma exo-PD-L1 levels, reactivated cytotoxic T cells, and led to reduced tumor growth and metastasis (94–96). These findings suggest that exosome-mediated immune suppression may be reversible and that targeting exo-PD-L1 could enhance responsiveness to ICIs.
6.3 Alternative physical approaches
In addition to pharmacologic inhibition, several physical approaches have been investigated for exosome elimination. Autophagy induction has been shown to suppress exosome biogenesis by promoting degradation of multivesicular bodies. On the other hand, extracorporeal removal techniques, such as blood purification systems and ultrafiltration, offer non-invasive methods to physically eliminate exosomes from circulation. These technologies are still in preclinical evaluation but have demonstrated potential advantages over drug-based therapies, including lower systemic toxicity and broader applicability across tumor types (97). Nevertheless, their specificity for exo-PD-L1 remains to be established (97).
Regulating exo-PD-L1 expression is complex. Pre-clinical studies on exo-PD-L1-targeting strategies are promising, but more research is required to solve challenges and explore their combined clinical effectiveness (4). Further investigations are necessary to identify precise, clinically feasible approaches that can modulate exo-PD-L1 without disrupting essential exosome functions.
As these strategies advance toward clinical translation, both efficacy and the spectrum of on- and off-target effects must be rigorously benchmarked. First-generation chemical blockers of exosome biogenesis—such as the nSMase2 inhibitor GW4869 and the RAB27A-JFC1 interaction disruptor Nexinhib-20—display only cell-line-restricted activity and fail to curb vesicle release in prostate (PC3) and other cancer models or to produce an in-vivo benefit in the MC38 syngeneic tumor (90, 98). Compounds that more globally perturb vesicle trafficking, for example dimethyl-amiloride, also interfere with lipid metabolism or ion transport and may introduce unintended immunotoxicity (99). Notably, small-molecule nSMase2 blockade with GW4869 has been linked to phosphatidylserine-dependent cytotoxicity in myeloma cells and memory impairment in mice, while Nexinhib-20 suppresses β2-integrin activation in neutrophils, raising infection or bleeding concerns. Dimethyl-amiloride (100), a classic Na+/H+-exchanger blocker, perturbs cardiomyocyte ion homeostasis at micromolar doses (101–103). These emerging data underscore the need for structure-guided optimization and rigorous toxicology before first-in-human trials. Likewise, bulk physical-removal approaches—such as extracorporeal plasma exchange—risk indiscriminately depleting exosomes with homeostatic roles in immune regulation or tissue repair (104). Therefore, future work should aim to improve the specificity, tolerability, and context-dependent application of these interventions to ensure clinical safety and efficacy.
7 Future perspectives, challenges, and conclusions
Exosomal PD-L1 (exo-PD-L1) plays a critical role in tumor initiation, progression, and immune suppression. Tumor-derived exo-PD-L1 binds to PD-1 receptors on T cells, thereby inhibiting anti-tumor immune responses both locally and systemically. Moreover, exo-PD-L1 may serve as a molecular sink for immune checkpoint inhibitors by sequestering anti-PD-L1 antibodies in the circulation, limiting their effective concentration within the tumor microenvironment. By integrating exosome analysis into personalized treatment strategies, clinicians can tailor ICI treatment plans based on exo-PD-L1 levels, improving treatment efficiency and accurately predicting patient responses.
7.1 Limitations and challenges
Despite its emerging clinical value, the use of exosomal PD-L1 (exo-PD-L1) as a predictive biomarker for immune checkpoint blockade (ICB) therapy remains constrained by several critical limitations. One major challenge lies in its cellular origin: exo-PD-L1 is not exclusively secreted by tumor cells but may also be derived from immune and stromal cells, thereby compromising its tumor specificity and interpretative precision (75). Furthermore, the relationship between exo-PD-L1 levels and therapeutic response has proven inconsistent across different cancer types, undermining its reliability as a universal biomarker (76). Technical and practical hurdles also present significant barriers to clinical translation. The absence of standardized isolation and quantification protocols contributes to inter-study variability, while the lack of established clinical thresholds impedes its integration into routine diagnostic workflows (56, 77, 78). In addition, many of the most sensitive and specific detection methods—such as surface plasmon resonance (SPR) biosensing, SERS-based assays, and nanoparticle-enhanced platforms—require sophisticated instrumentation, specialized reagents, and high operational costs, which substantially limit their accessibility in standard clinical settings (Table 2). Together, these issues highlight the need for methodological harmonization, cost reduction, and further clinical validation before exo-PD-L1 can be reliably adopted in precision oncology.
7.2 Exo-PD-L1/IHC-PD-L1 ratio as a predictive model for immunotherapy
An important avenue for future research is to explore the prognostic and predictive potential of the exosomal PD-L1 to tumor PD-L1 ratio (exo-PD-L1/IHC-PD-L1) in the context of immune checkpoint inhibitor (ICI) therapy. While exosomal PD-L1 and tumor PD-L1 have each been individually investigated as biomarkers, this ratio may offer enhanced discriminatory power by integrating both systemic and tumor-localized immunosuppressive cues. Incorporating this dynamic ratio into future stratification models could improve patient selection and inform more personalized immunotherapeutic strategies. (Figure 3) Additionally, a comprehensive evaluation of tissue PD-L1, exo-PD-L1, and soluble PD-L1 is essential for companion diagnostics. Emerging studies suggest that tumor metabolic regulation and nanotechnology-based strategies could influence PD-L1 expression and secretion, offering potential tools to indirectly modulate exo-PD-L1 levels (36, 105–107).
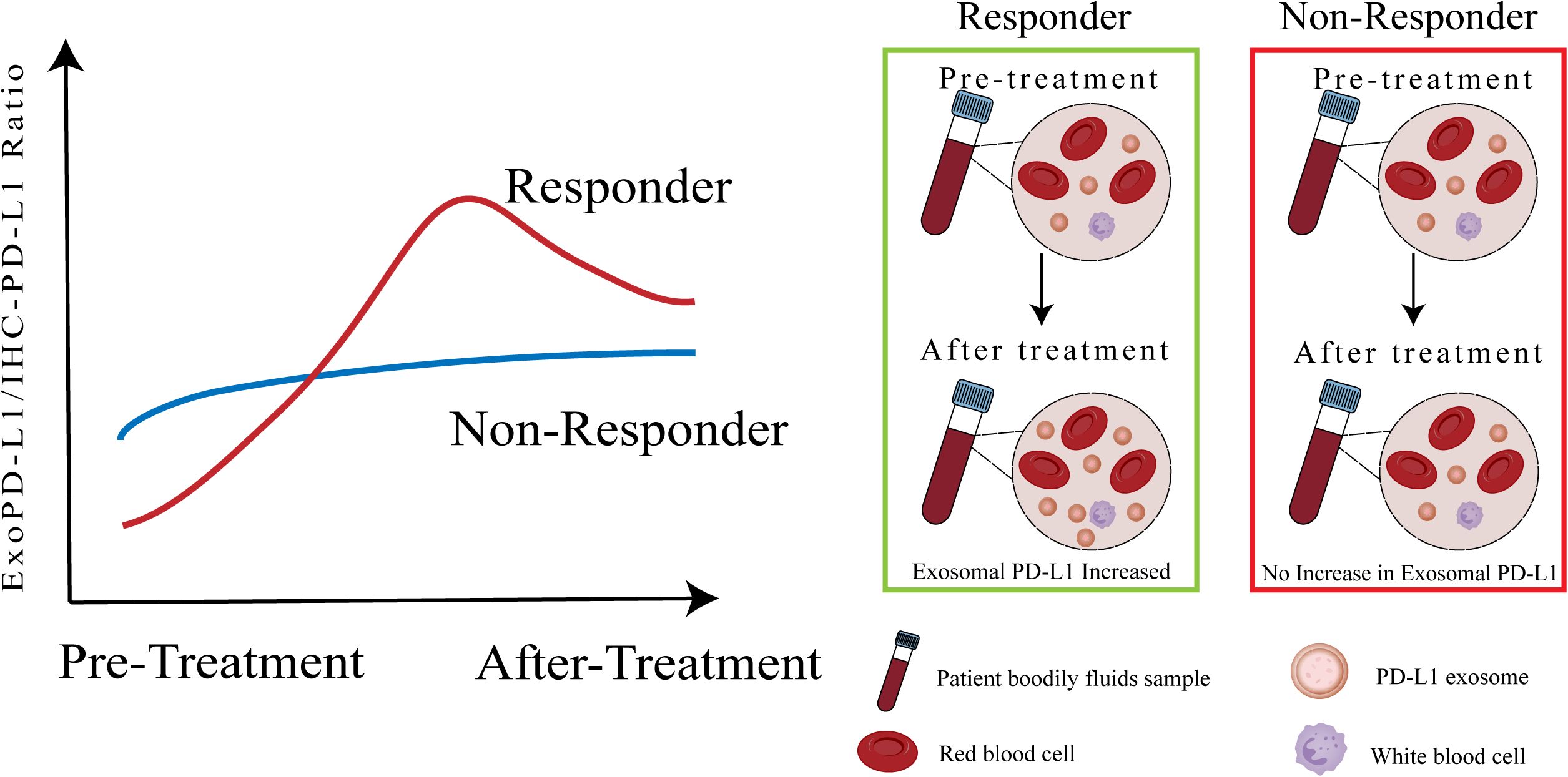
Figure 3. Exo-PD-L1/IHC-PD-L1 ratio as a potential biomarker for predicting immune checkpoint blockade therapy response. This figure illustrates the proposed utility of the ratio between circulating exosomal PD-L1 and tumor PD-L1 expression (exo-PD-L1/IHC-PD-L1) as a dynamic and potentially more informative biomarker for predicting clinical response to ICB therapy. Unlike static, single-site measurements, this ratio may better capture the interplay between localized immune escape and systemic immunomodulation. In responders, a low baseline ratio followed by a marked post-treatment increase in exosomal PD-L1 reflects enhanced immune activation and treatment efficacy. In contrast, non-responders tend to display a high baseline ratio with minimal change after therapy, indicating persistent immune suppression. This ratio-based model may enable early patient stratification and guide the design of personalized immunotherapeutic strategies, though further validation is needed.
7.3 Applications of exo-PD-L1 in precision oncology
A fascinating avenue is the potential of exo-PD-L1 as an early warning system for predicting organ-specific metastasis. By analyzing exo-PD-L1 levels from body fluids, researchers could potentially infer which organ may be the next site of metastatic spread. However, this approach still requires extensive validation, as identifying definitive biomarkers for organ-specific metastasis remains challenging. Additionally, monitoring exo-PD-L1 dynamics could support combination therapies that reprogram cold tumors into responsive ones, improving ICB therapy outcomes in resistant cancers (36).
7.4 Conclusion
In advancing anti-PD-1/PD-L1 immune checkpoint blockade therapies, it is essential to consider both PD-L1 on tumor cell surfaces and exo-PD-L1 levels for a comprehensive evaluation of patient conditions. In conclusion, exo-PD-L1 shows great promise as a multifaceted biomarker in immune checkpoint blockade therapy. By integrating exo-PD-L1 levels into clinical decision-making, we can potentially refine personalized treatment regimens, enhance the precision of immunotherapies, and ultimately improve patient outcomes.
Author contributions
TK: Writing – review & editing, Writing – original draft. XM: Writing – review & editing. HW: Writing – review & editing. XY: Writing – review & editing. YS: Writing – review & editing. ZG: Writing – review & editing. JS: Writing – review & editing. LX: Writing – review & editing. JY: Writing – review & editing, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by General Program of National Natural Science Foundation of China (No.82072870), General Program of Natural Science Foundation of Beijing (No.7202224), Innovation and Transformation Fund of Peking University Third Hospital (BYSYZHKC2022110), and Youth Program of National Natural Science Foundation of China (No. 82303801).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-pd-1 antibody in cancer. N Engl J Med. (2012) 366:2443–54. doi: 10.1056/NEJMoa1200690
2. Topalian SL, Drake CG, and Pardoll DM. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell. (2015) 27:450–61. doi: 10.1016/j.ccell.2015.03.001
3. Chen L and Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. (2013) 13:227–42. doi: 10.1038/nri3405
4. Poggio M, Hu T, Pai CC, Chu B, Belair CD, Chang A, et al. Suppression of exosomal pd-L1 induces systemic anti-tumor immunity and memory. Cell. (2019) 177:414–27.e13. doi: 10.1016/j.cell.2019.02.016
5. Sharma P, Hu-Lieskovan S, Wargo JA, and Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. (2017) 168:707–23. doi: 10.1016/j.cell.2017.01.017
6. Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, et al. T cell costimulatory receptor cd28 is a primary target for pd-1-mediated inhibition. Science. (2017) 355:1428–33. doi: 10.1126/science.aaf1292
7. Sun C, Mezzadra R, and Schumacher TN. Regulation and function of the pd-L1 checkpoint. Immunity. (2018) 48:434–52. doi: 10.1016/j.immuni.2018.03.014
8. Fan Y, Che X, Qu J, Hou K, Wen T, Li Z, et al. Exosomal pd-L1 retains immunosuppressive activity and is associated with gastric cancer prognosis. Ann Surg Oncol. (2019) 26:3745–55. doi: 10.1245/s10434-019-07431-7
9. Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal pd-L1 contributes to immunosuppression and is associated with anti-pd-1 response. Nature. (2018) 560:382–6. doi: 10.1038/s41586-018-0392-8
10. Pegtel DM and Gould SJ. Exosomes. Annu Rev Biochem. (2019) 88:487–514. doi: 10.1146/annurev-biochem-013118-111902
11. Czystowska-Kuzmicz M and Whiteside TL. The potential role of tumor-derived exosomes in diagnosis, prognosis, and response to therapy in cancer. Expert Opin Biol Ther. (2021) 21:241–58. doi: 10.1080/14712598.2020.1813276
12. Whiteside TL. Exosomes and tumor-mediated immune suppression. J Clin Invest. (2016) 126:1216–23. doi: 10.1172/jci81136
13. Yang Y, Li CW, Chan LC, Wei Y, Hsu JM, Xia W, et al. Exosomal pd-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth. Cell Res. (2018) 28:862–4. doi: 10.1038/s41422-018-0060-4
14. Kim DH, Kim H, Choi YJ, Kim SY, Lee JE, Sung KJ, et al. Exosomal pd-L1 promotes tumor growth through immune escape in non-small cell lung cancer. Exp Mol Med. (2019) 51:1–13. doi: 10.1038/s12276-019-0295-2
15. Li L, Cao B, Liang X, Lu S, Luo H, Wang Z, et al. Microenvironmental oxygen pressure orchestrates an anti- and pro-tumoral γδ T cell equilibrium via tumor-derived exosomes. Oncogene. (2019) 38:2830–43. doi: 10.1038/s41388-018-0627-z
16. Keller S, Ridinger J, Rupp AK, Janssen JW, and Altevogt P. Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med. (2011) 9:86. doi: 10.1186/1479-5876-9-86
17. Ruivo CF, Adem B, Silva M, and Melo SA. The biology of cancer exosomes: insights and new perspectives. Cancer Res. (2017) 77:6480–8. doi: 10.1158/0008-5472.Can-17-0994
18. Meldolesi J. Exosomes and ectosomes in intercellular communication. (2018) 28(8):pR435-R444. doi: 10.1016/j.cub.2018.01.059
19. Zhang L and Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim Biophys Acta Rev Cancer. (2019) 1871:455–68. doi: 10.1016/j.bbcan.2019.04.004
20. Li S, Yi M, Dong B, Jiao Y, Luo S, and Wu K. The roles of exosomes in cancer drug resistance and its therapeutic application. Clin Transl Med. (2020) 10:e257. doi: 10.1002/ctm2.257
21. Lyu T, Wang Y, Li D, Yang H, Qin B, Zhang W, et al. Exosomes from bm-mscs promote acute myeloid leukemia cell proliferation, invasion and chemoresistance via upregulation of S100a4. Exp Hematol Oncol. (2021) 10:24. doi: 10.1186/s40164-021-00220-7
22. Boyiadzis M and Whiteside TL. Information transfer by exosomes: A new frontier in hematologic Malignancies. Blood Rev. (2015) 29:281–90. doi: 10.1016/j.blre.2015.01.004
23. Théry C, Zitvogel L, and Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. (2002) 2:569–79. doi: 10.1038/nri855
24. Ricklefs FL, Alayo Q, Krenzlin H, Mahmoud AB, Speranza MC, Nakashima H, et al. Immune evasion mediated by pd-L1 on glioblastoma-derived extracellular vesicles. Sci Adv. (2018) 4:eaar2766. doi: 10.1126/sciadv.aar2766
25. Wortzel I, Dror S, Kenific CM, and Lyden D. Exosome-mediated metastasis: communication from a distance. Dev Cell. (2019) 49:347–60. doi: 10.1016/j.devcel.2019.04.011
26. Ma F, Liu X, Zhang Y, Tao Y, Zhao L, Abusalamah H, et al. Tumor extracellular vesicle-derived pd-L1 promotes T cell senescence through lipid metabolism reprogramming. Sci Transl Med. (2025) 17:eadm7269. doi: 10.1126/scitranslmed.adm7269
27. Liu X, Hartman CL, Li L, Albert CJ, Si F, Gao A, et al. Reprogramming lipid metabolism prevents effector T cell senescence and enhances tumor immunotherapy. Sci Transl Med. (2021) 13. doi: 10.1126/scitranslmed.aaz6314
28. Tang Y, Chen Z, Zuo Q, and Kang Y. Regulation of cd8+ T cells by lipid metabolism in cancer progression. Cell Mol Immunol. (2024) 21:1215–30. doi: 10.1038/s41423-024-01224-z
29. Liu L, Hao Z, Yang X, Li Y, Wang S, and Li L. Metabolic reprogramming in T cell senescence: A novel strategy for cancer immunotherapy. Cell Death Discov. (2025) 11:161. doi: 10.1038/s41420-025-02468-y
30. Shimada Y, Matsubayashi J, Kudo Y, Maehara S, Takeuchi S, Hagiwara M, et al. Serum-derived exosomal pd-L1 expression to predict anti-pd-1 response and in patients with non-small cell lung cancer. Sci Rep. (2021) 11:7830. doi: 10.1038/s41598-021-87575-3
31. Ma J, Cen Q, Wang Q, Liu L, and Zhou J. Exosomes released from pd-L1(+) tumor associated macrophages promote peritoneal metastasis of epithelial ovarian cancer by up-regulating T cell lipid metabolism. Biochem Biophys Rep. (2023) 36:101542. doi: 10.1016/j.bbrep.2023.101542
32. Zhang C, Fan Y, Che X, Zhang M, Li Z, Li C, et al. Anti-pd-1 therapy response predicted by the combination of exosomal pd-L1 and cd28. Front Oncol. (2020) 10:760. doi: 10.3389/fonc.2020.00760
33. Theodoraki MN, Yerneni SS, Hoffmann TK, Gooding WE, and Whiteside TL. Clinical significance of pd-L1(+) exosomes in plasma of head and neck cancer patients. Clin Cancer Res. (2018) 24:896–905. doi: 10.1158/1078-0432.Ccr-17-2664
34. Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma Exosomes Educate Bone Marrow Progenitor Cells toward a Pro-Metastatic Phenotype through Met. Nat Med. (2012) 18:883–91. doi: 10.1038/nm.2753
35. Wen SW, Sceneay J, Lima LG, Wong CS, Becker M, Krumeich S, et al. The biodistribution and immune suppressive effects of breast cancer-derived exosomes. Cancer Res. (2016) 76:6816–27. doi: 10.1158/0008-5472.Can-16-0868
36. Daassi D, Mahoney KM, and Freeman GJ. The importance of exosomal pdl1 in tumour immune evasion. Nat Rev Immunol. (2020) 20:209–15. doi: 10.1038/s41577-019-0264-y
37. Zhou K, Guo S, Li F, Sun Q, and Liang G. Exosomal pd-L1: new insights into tumor immune escape mechanisms and therapeutic strategies. Front Cell Dev Biol. (2020) 8:569219. doi: 10.3389/fcell.2020.569219
38. Cordonnier M, Nardin C, Chanteloup G, Derangere V, Algros MP, Arnould L, et al. Tracking the evolution of circulating exosomal-pd-L1 to monitor melanoma patients. J Extracell Vesicles. (2020) 9:1710899. doi: 10.1080/20013078.2019.1710899
39. Zhong W, Lu Y, Han X, Yang J, Qin Z, Zhang W, et al. Upregulation of exosome secretion from tumor-associated macrophages plays a key role in the suppression of anti-tumor immunity. Cell Rep. (2023) 42:113224. doi: 10.1016/j.celrep.2023.113224
40. Wang C, Zhou L, Kang X, Huang CH, Gao Z, Shen J, et al. A nanoplasmonic cell-on-a-chip for in situ monitoring of pd-L1(+) exosome-mediated immune modulation. Biosens Bioelectron. (2025) 277:117293. doi: 10.1016/j.bios.2025.117293
41. Yuan Y, Jiao P, Wang Z, Chen M, Du H, Xu L, et al. Endoplasmic reticulum stress promotes the release of exosomal pd-L1 from head and neck cancer cells and facilitates M2 macrophage polarization. Cell Commun Signal. (2022) 20:12. doi: 10.1186/s12964-021-00810-2
42. Lu X, Shen J, Huang S, Liu D, and Wang H. Tumor cells-derived exosomal pd-L1 promotes the growth and invasion of lung cancer cells in vitro via mediating macrophages M2 polarization. Eur J Histochem. (2023) 67(3):274–82. doi: 10.4081/ejh.2023.3784
43. Gunassekaran GR, Poongkavithai Vadevoo SM, Baek MC, and Lee B. M1 macrophage exosomes engineered to foster M1 polarization and target the il-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages. Biomaterials. (2021) 278:121137. doi: 10.1016/j.biomaterials.2021.121137
44. Wang P, Wang H, Huang Q, Peng C, Yao L, Chen H, et al. Exosomes from M1-polarized macrophages enhance paclitaxel antitumor activity by activating macrophages-mediated inflammation. Theranostics. (2019) 9:1714–27. doi: 10.7150/thno.30716
45. Yin C, Han Q, Xu D, Zheng B, Zhao X, and Zhang J. Sall4-mediated upregulation of exosomal mir-146a-5p drives T-cell exhaustion by M2 tumor-associated macrophages in hcc. Oncoimmunology. (2019) 8:1601479. doi: 10.1080/2162402x.2019.1601479
46. Chen X, Ying X, Wang X, Wu X, Zhu Q, and Wang X. Exosomes derived from hypoxic epithelial ovarian cancer deliver microrna-940 to induce macrophage M2 polarization. Oncol Rep. (2017) 38:522–8. doi: 10.3892/or.2017.5697
47. Chen J, Yang J, Wang W, Guo D, Zhang C, Wang S, et al. Tumor extracellular vesicles mediate anti-pd-L1 therapy resistance by decoying anti-pd-L1. Cell Mol Immunol. (2022) 19:1290–301. doi: 10.1038/s41423-022-00926-6
48. Fu J, Li S, Ma H, Yang J, Pagnotti GM, Brown LM, et al. The checkpoint inhibitor pd-1h/vista controls osteoclast-mediated multiple myeloma bone disease. Nat Commun. (2023) 14:4271. doi: 10.1038/s41467-023-39769-8
49. Vanmeerbeek I, Naulaerts S, Sprooten J, Laureano RS, Govaerts J, Trotta R, et al. Targeting conserved tim3(+)Vista(+) tumor-associated macrophages overcomes resistance to cancer immunotherapy. . Sci Adv. (2024) 10:eadm8660. doi: 10.1126/sciadv.adm8660
50. Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (Checkmate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. (2018) 19:1480–92. doi: 10.1016/s1470-2045(18)30700-9
51. Pacheco JM, Camidge DR, Doebele RC, and Schenk E. A changing of the guard: immune checkpoint inhibitors with and without chemotherapy as first line treatment for metastatic non-small cell lung cancer. Front Oncol. (2019) 9:195. doi: 10.3389/fonc.2019.00195
52. Wei Y, Du Q, Jiang X, Li L, Li T, Li M, et al. Efficacy and safety of combination immunotherapy for Malignant solid tumors: A systematic review and meta-analysis. Crit Rev Oncol Hematol. (2019) 138:178–89. doi: 10.1016/j.critrevonc.2019.04.008
53. Postow MA, Callahan MK, and Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. (2015) 33:1974–82. doi: 10.1200/jco.2014.59.4358
54. Pang Y, Shi J, Yang X, Wang C, Sun Z, and Xiao R. Personalized detection of circling exosomal pd-L1 based on fe(3)O(4)@Tio(2) isolation and sers immunoassay. Biosens Bioelectron. (2020) 148:111800. doi: 10.1016/j.bios.2019.111800
55. Menzies AM, Johnson DB, Ramanujam S, Atkinson VG, Wong ANM, Park JJ, et al. Anti-pd-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. (2017) 28:368–76. doi: 10.1093/annonc/mdw443
56. Wang J, Guo W, Wang X, Tang X, Sun X, and Ren T. Circulating exosomal pd-L1 at initial diagnosis predicts outcome and survival of patients with osteosarcoma. Clin Cancer Res. (2023) 29:659–66. doi: 10.1158/1078-0432.Ccr-22-2682
57. Farhood B, Najafi M, and Mortezaee K. Cd8(+) cytotoxic T lymphocytes in cancer immunotherapy: A review. J Cell Physiol. (2019) 234:8509–21. doi: 10.1002/jcp.27782
58. Li C, Li C, Zhi C, Liang W, Wang X, Chen X, et al. Clinical significance of pd-L1 expression in serum-derived exosomes in nsclc patients. J Transl Med. (2019) 17:355. doi: 10.1186/s12967-019-2101-2
59. Yang Q, Chen M, Gu J, Niu K, Zhao X, Zheng L, et al. Novel biomarkers of dynamic blood pd-L1 expression for immune checkpoint inhibitors in advanced non-small-cell lung cancer patients. Front Immunol. (2021) 12:665133. doi: 10.3389/fimmu.2021.665133
60. Theodoraki MN, Hoffmann TK, and Whiteside TL. Separation of plasma-derived exosomes into cd3((+)) and cd3((-)) fractions allows for association of immune cell and tumour cell markers with disease activity in hnscc patients. Clin Exp Immunol. (2018) 192:271–83. doi: 10.1111/cei.13113
61. Theodoraki MN, Yerneni S, Gooding WE, Ohr J, Clump DA, Bauman JE, et al. Circulating exosomes measure responses to therapy in head and neck cancer patients treated with cetuximab, ipilimumab, and imrt. Oncoimmunology. (2019) 8:1593805. doi: 10.1080/2162402x.2019.1593805
62. Lux A, Kahlert C, Grützmann R, and Pilarsky C. C-met and pd-L1 on circulating exosomes as diagnostic and prognostic markers for pancreatic cancer. Int J Mol Sci. (2019) 20. doi: 10.3390/ijms20133305
63. Xu YF, Hannafon BN, Zhao YD, Postier RG, and Ding WQ. Plasma exosome mir-196a and mir-1246 are potential indicators of localized pancreatic cancer. Oncotarget. (2017) 8:77028–40. doi: 10.18632/oncotarget.20332
64. Goto T, Fujiya M, Konishi H, Sasajima J, Fujibayashi S, Hayashi A, et al. An elevated expression of serum exosomal microrna-191, - 21, -451a of pancreatic neoplasm is considered to be efficient diagnostic marker. BMC Cancer. (2018) 18:116. doi: 10.1186/s12885-018-4006-5
65. Abue M, Yokoyama M, Shibuya R, Tamai K, Yamaguchi K, Sato I, et al. Circulating mir-483-3p and mir-21 is highly expressed in plasma of pancreatic cancer. Int J Oncol. (2015) 46:539–47. doi: 10.3892/ijo.2014.2743
66. Park SJ, Park JY, Shin K, Hong TH, Lee M, Kim Y, et al. Clinical significance of serum-derived exosomal pd-L1 expression in patients with advanced pancreatic cancer. BMC Cancer. (2023) 23:389. doi: 10.1186/s12885-023-10811-8
67. Lin H, Wei S, Hurt EM, Green MD, Zhao L, Vatan L, et al. Host expression of pd-L1 determines efficacy of pd-L1 pathway blockade-mediated tumor regression. J Clin Invest. (2018) 128:805–15. doi: 10.1172/jci96113
68. Jiang C, Cao S, Li N, Jiang L, and Sun T. Pd-1 and pd-L1 correlated gene expression profiles and their association with clinical outcomes of breast cancer. Cancer Cell Int. (2019) 19:233. doi: 10.1186/s12935-019-0955-2
69. Patel SP and Kurzrock R. Pd-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. (2015) 14:847–56. doi: 10.1158/1535-7163.Mct-14-0983
70. He Y, Zhang X, Zhu M, He W, Hua H, Ye F, et al. Soluble pd-L1: A potential dynamic predictive biomarker for immunotherapy in patients with proficient mismatch repair colorectal cancer. J Transl Med. (2023) 21:25. doi: 10.1186/s12967-023-03879-0
71. Morales-Kastresana A, Musich TA, Welsh JA, Telford W, Demberg T, Wood JCS, et al. High-fidelity detection and sorting of nanoscale vesicles in viral disease and cancer. J Extracell Vesicles. (2019) 8:1597603. doi: 10.1080/20013078.2019.1597603
72. Liu F, Vermesh O, Mani V, Ge TJ, Madsen SJ, Sabour A, et al. The exosome total isolation chip. ACS Nano. (2017) 11:10712–23. doi: 10.1021/acsnano.7b04878
73. Yang F, Liao X, Tian Y, and Li G. Exosome separation using microfluidic systems: size-based, immunoaffinity-based and dynamic methodologies. Biotechnol J. (2017) 12. doi: 10.1002/biot.201600699
74. Huang M, Yang J, Wang T, Song J, Xia J, Wu L, et al. Homogeneous, low-volume, efficient, and sensitive quantitation of circulating exosomal pd-L1 for cancer diagnosis and immunotherapy response prediction. Angew Chem Int Ed Engl. (2020) 59:4800–5. doi: 10.1002/anie.201916039
75. Lee HH, Wang YN, Xia W, Chen CH, Rau KM, Ye L, et al. Removal of N-linked glycosylation enhances pd-L1 detection and predicts anti-pd-1/pd-L1 therapeutic efficacy. Cancer Cell. (2019) 36:168–78.e4. doi: 10.1016/j.ccell.2019.06.008
76. Yu ZL, Liu JY, and Chen G. Small extracellular vesicle pd-L1 in cancer: the knowns and unknowns. NPJ Precis Oncol. (2022) 6:42. doi: 10.1038/s41698-022-00287-3
77. Kabe Y, Suematsu M, Sakamoto S, Hirai M, Koike I, Hishiki T, et al. Development of a highly sensitive device for counting the number of disease-specific exosomes in human sera. Clin Chem. (2018) 64:1463–73. doi: 10.1373/clinchem.2018.291963
78. Jiang S, Zhang W, Cao Q, Rahbar M, Cooke W, Ono M, et al. Exocounter assays identify women who may develop early-onset preeclampsia from 12.5 μl first-trimester serum by characterizing placental small extracellular vesicles. Hypertension. (2023) 80:1439–51. doi: 10.1161/hypertensionaha.123.20907
79. Enderle D, Spiel A, Coticchia CM, Berghoff E, Mueller R, Schlumpberger M, et al. Characterization of rna from exosomes and other extracellular vesicles isolated by a novel spin column-based method. PloS One. (2015) 10:e0136133. doi: 10.1371/journal.pone.0136133
80. Liu C, Zeng X, An Z, Yang Y, Eisenbaum M, Gu X, et al. Sensitive detection of exosomal proteins via a compact surface plasmon resonance biosensor for cancer diagnosis. ACS Sens. (2018) 3:1471–9. doi: 10.1021/acssensors.8b00230
81. Wang R, Yang Y, Huang J, and Yao Y. The detection of exosomal pd-L1 in peripheral blood. Methods Mol Biol. (2023) 2695:195–212. doi: 10.1007/978-1-0716-3346-5_13
82. Heath N, Grant L, De Oliveira TM, Rowlinson R, Osteikoetxea X, Dekker N, et al. Rapid isolation and enrichment of extracellular vesicle preparations using anion exchange chromatography. Sci Rep. (2018) 8:5730. doi: 10.1038/s41598-018-24163-y
83. Lin S, Yu Z, Chen D, Wang Z, Miao J, Li Q, et al. Progress in microfluidics-based exosome separation and detection technologies for diagnostic applications. Small. (2020) 16:e1903916. doi: 10.1002/smll.201903916
84. Ha D, Yang N, and Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. (2016) 6:287–96. doi: 10.1016/j.apsb.2016.02.001
85. Rider MA, Hurwitz SN, and Meckes DG Jr. Extrapeg: A polyethylene glycol-based method for enrichment of extracellular vesicles. Sci Rep. (2016) 6:23978. doi: 10.1038/srep23978
86. Napoletano C, Bellati F, Landi R, Pauselli S, Marchetti C, Visconti V, et al. Ovarian cancer cytoreduction induces changes in T cell population subsets reducing immunosuppression. J Cell Mol Med. (2010) 14:2748–59. doi: 10.1111/j.1582-4934.2009.00911.x
87. Battaglia A, Fossati M, Buzzonetti A, Scambia G, and Fattorossi A. A robust immune system conditions the response to abagovomab (Anti-idiotypic monoclonal antibody mimicking the ca125 protein) vaccination in ovarian cancer patients. Immunol Lett. (2017) 191:35–9. doi: 10.1016/j.imlet.2017.09.006
88. Datta A, Kim H, McGee L, Johnson AE, Talwar S, Marugan J, et al. High-throughput screening identified selective inhibitors of exosome biogenesis and secretion: A drug repurposing strategy for advanced cancer. Sci Rep. (2018) 8:8161. doi: 10.1038/s41598-018-26411-7
89. Yin Z, Yu M, Ma T, Zhang C, Huang S, Karimzadeh MR, et al. Mechanisms underlying low-clinical responses to pd-1/pd-L1 blocking antibodies in immunotherapy of cancer: A key role of exosomal pd-L1. J Immunother Cancer. (2021) 9. doi: 10.1136/jitc-2020-001698
90. Johnson JL, Ramadass M, He J, Brown SJ, Zhang J, Abgaryan L, et al. Identification of neutrophil exocytosis inhibitors (Nexinhibs), small molecule inhibitors of neutrophil exocytosis and inflammation: druggability of the small gtpase rab27a. J Biol Chem. (2016) 291:25965–82. doi: 10.1074/jbc.M116.741884
91. Savina A, Furlán M, Vidal M, and Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. (2003) 278:20083–90. doi: 10.1074/jbc.M301642200
92. Lallemand T, Rouahi M, Swiader A, Grazide MH, Geoffre N, Alayrac P, et al. Nsmase2 (Type 2-neutral sphingomyelinase) deficiency or inhibition by gw4869 reduces inflammation and atherosclerosis in apoe(-/-) mice. Arterioscler Thromb Vasc Biol. (2018) 38:1479–92. doi: 10.1161/atvbaha.118.311208
93. Wang G, Xie L, Li B, Sang W, Yan J, Li J, et al. A nanounit strategy reverses immune suppression of exosomal pd-L1 and is associated with enhanced ferroptosis. Nat Commun. (2021) 12:5733. doi: 10.1038/s41467-021-25990-w
94. Lee CH, Bae JH, Choe EJ, Park JM, Park SS, Cho HJ, et al. Macitentan improves antitumor immune responses by inhibiting the secretion of tumor-derived extracellular vesicle pd-L1. Theranostics. (2022) 12:1971–87. doi: 10.7150/thno.68864
95. Shin JM, Lee CH, Son S, Kim CH, Lee JA, Ko H, et al. Sulfisoxazole elicits robust antitumour immune response along with immune checkpoint therapy by inhibiting exosomal pd-L1. Adv Sci (Weinh). (2022) 9:e2103245. doi: 10.1002/advs.202103245
96. Im EJ, Lee CH, Moon PG, Rangaswamy GG, Lee B, Lee JM, et al. Sulfisoxazole inhibits the secretion of small extracellular vesicles by targeting the endothelin receptor A. Nat Commun. (2019) 10:1387. doi: 10.1038/s41467-019-09387-4
97. Marleau AM, Chen CS, Joyce JA, and Tullis RH. Exosome removal as a therapeutic adjuvant in cancer. J Transl Med. (2012) 10:134. doi: 10.1186/1479-5876-10-134
98. Phuyal S, Hessvik NP, Skotland T, Sandvig K, and Llorente A. Regulation of exosome release by glycosphingolipids and flotillins. FEBS J. (2014) 281:2214–27. doi: 10.1111/febs.12775
99. Luberto C, Hassler DF, Signorelli P, Okamoto Y, Sawai H, Boros E, et al. Inhibition of tumor necrosis factor-induced cell death in mcf7 by a novel inhibitor of neutral sphingomyelinase. J Biol Chem. (2002) 277:41128–39. doi: 10.1074/jbc.M206747200
100. Liu W, Cronin CG, Cao Z, Wang C, Ruan J, Pulikkot S, et al. Nexinhib20 inhibits neutrophil adhesion and β(2) integrin activation by antagonizing rac-1-guanosine 5’-triphosphate interaction. J Immunol. (2022) 209:1574–85. doi: 10.4049/jimmunol.2101112
101. Soliman M. Dimethyl amiloride, a na+-H+ Exchange inhibitor, and its cardioprotective effects in hemorrhagic shock in in vivo resuscitated rats. J Physiol Sci. (2009) 59:175–80. doi: 10.1007/s12576-009-0024-z
102. Vuckovic S, Vandyke K, Rickards DA, McCauley Winter P, Brown SHJ, Mitchell TW, et al. The cationic small molecule gw4869 is cytotoxic to high phosphatidylserine-expressing myeloma cells. Br J Haematol. (2017) 177:423–40. doi: 10.1111/bjh.14561
103. Risner ML, Ribeiro M, McGrady NR, Kagitapalli BS, Chamling X, Zack DJ, et al. Neutral sphingomyelinase inhibition promotes local and network degeneration in vitro and in vivo. Cell Commun Signal. (2023) 21:305. doi: 10.1186/s12964-023-01291-1
104. Oya K, Shen LT, Maruo K, and Matsusaka S. Plasma exchange may enhance antitumor effects by removal of soluble programmed death-ligand 1 and extracellular vesicles: preliminary study. . Biomedicines. (2022) 10. doi: 10.3390/biomedicines10102483
105. Zhou Z, Wang H, Li J, Jiang X, Li Z, and Shen J. Recent progress, perspectives, and issues of engineered pd-L1 regulation nano-system to better cure tumor: A review. Int J Biol Macromol. (2024) 254:127911. doi: 10.1016/j.ijbiomac.2023.127911
106. Xiong W, Qi L, Jiang N, Zhao Q, Chen L, Jiang X, et al. Metformin liposome-mediated pd-L1 downregulation for amplifying the photodynamic immunotherapy efficacy. ACS Appl Mater Interfaces. (2021) 13:8026–41. doi: 10.1021/acsami.0c21743
107. Zhou Z, Liu Y, Jiang X, Zheng C, Luo W, Xiang X, et al. Metformin modified chitosan as a multi-functional adjuvant to enhance cisplatin-based tumor chemotherapy efficacy. Int J Biol Macromol. (2023) 224:797–809. doi: 10.1016/j.ijbiomac.2022.10.167
108. Del Re M, Marconcini R, Pasquini G, Rofi E, Vivaldi C, Bloise F, et al. Pd-L1 mrna expression in plasma-derived exosomes is associated with response to anti-pd-1 antibodies in melanoma and nsclc. Br J Cancer. (2018) 118:820–4. doi: 10.1038/bjc.2018.9
Keywords: exosomal PD-L1, cancer biomarkers, early detection, liquid biopsy, immune checkpoint blockade
Citation: Kansha T, Ma X, Wang H, Yu X, Song Y, Guo Z, Song J, Xue L and Yang J (2025) Exosomal PD-L1 detection in cancer predictive biomarker for response to immune checkpoint blockade therapy. Front. Immunol. 16:1603855. doi: 10.3389/fimmu.2025.1603855
Received: 01 April 2025; Accepted: 02 June 2025;
Published: 03 July 2025.
Edited by:
Fernando Torres Andón, Institute of Biomedical Research of A Coruña (INIBIC), SpainReviewed by:
Vasyl Nagibin, National Academy of Sciences of Ukraine, UkraineZaigang Zhou, Wenzhou Medical University, China
Copyright © 2025 Kansha, Ma, Wang, Yu, Song, Guo, Song, Xue and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lixiang Xue, bGl4aWFuZ3h1ZUBoc2MucGt1LmVkdS5jbg==; Jianling Yang, amlhbmxpbmd5YW5nQHBrdS5lZHUuY24=
†These authors have contributed equally to this work
 Tetsuichi Kansha
Tetsuichi Kansha Xiaojuan Ma1,2,3†
Xiaojuan Ma1,2,3† Hao Wang
Hao Wang Zhengyang Guo
Zhengyang Guo Jiagui Song
Jiagui Song Lixiang Xue
Lixiang Xue Jianling Yang
Jianling Yang