- 1Department of Hematology and Oncology, Shanghai Children’s Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 2Department of Hematology and Oncology, Shanghai Children’s Medical Center, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 3Department of Pediatric Hematology and Oncology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
- 4Department of Hematology and Oncology, Anhui Children’s Hospital, Hefei, China
Background: Blinatumomab, a bispecific T-cell engager targeting CD3+ and CD19+, promotes T cell–mediated cytotoxicity against B-cell precursor acute lymphoblastic leukemia (B-ALL). While its efficacy is established in relapsed/refractory (R/R) disease, its role as preemptive therapy for minimal residual disease (MRD)–positive patients or those experiencing chemotherapy delays remains undefined. Predictors of treatment failure also require further investigation.
Methods: In this multicenter retrospective study, 105 patients who received blinatumomab were enrolled. Of these, 30 had R/R ALL, 21 were in complete remission (CR) with MRD positivity (CR-MRDpos), and 54 experienced chemotherapy delays. Eight patients received blinatumomab directly as reinduction therapy and 22 patients received burden-reduction chemotherapy prior to blinatumomab. In total, 11 children were in R/R status and 40 were in CR-MRDpos before treatment. Patients were subsequently bridged to stem cell transplantation, chimeric antigen receptor T-cell therapy (CAR-T), or protocol continuation. Treatment response was analyzed across CR-MRDpos, R/R, and CR with MRD negativity (CR-MRDneg). Immune reconstitution profiles (T-cell subsets, cytokine dynamics), cytogenetic markers, and clinical outcomes were assessed to identify predictors of treatment resistance.
Results: The CR rate was 81.8% in R/R and 82.5% in CR-MRDpos patients (P = 1.000). Of 74 courses with CR-MRDneg, 73 remained MRD-negative during treatment. Univariate analysis revealed poor cytogenetics (P = 0.0001), CD19+ B-cell loss (P = 0.046), and BCR-ABL1 positivity (P = 0.002) as predictors of poor response. Cox regression analysis identified high MRD (P = 0.014), BCR/ABL1 (P = 0.065), and poor cytogenetics (P = 0.025) as independent risk factors. Blinatumomab significantly increased CD3+ T cells [0.96 (0.03–3.79) to 1.13 (0.26–7.74) ×109/L, P = 0.016], along with CD4+ [0.35 (0.01–1.39) to 0.47 (0.07–2.94) ×109/L] and CD8+ T cells [0.41 (0.01–2.39) to 0.56 (0.07–6.07) ×109/L] (P = 0.005 and P = 0.006, respectively).The 1-year event-free survival for CR-MRDneg, CR-MRDpos, and R/R patients was 97.8% ± 2.2%, 86.7% ± 6.2%, and 73.3% ± 8.1%, respectively (P = 0.001), while overall survival was 97.8% ± 2.2%, 100%, and 93.3% ± 4.6% (P = 0.029).
Conclusions: Blinatumomab effectively clears MRD as preemptive therapy and serves as a bridging strategy during chemotherapy delays in pediatric B-ALL, while maintaining high response rates in R/R cases.
Introduction
Survival rates for B-cell acute lymphoblastic leukemia (B-ALL) have significantly improved in recent years. However, approximately 15% of children with B-ALL experience relapse after frontline chemotherapy (1). Based on the site and timing of relapses, these children are classified as having standard- or high-risk first-relapse B-ALL (2).
Blinatumomab is a bispecific T-cell–engaging antibody that binds CD3+ T cells and CD19+ leukemia cells, inducing cytotoxic immune responses that lyse CD19-expressing B cells via activated T cells (3). Meta-analyses have demonstrated the potent therapeutic efficacy and favorable safety profile of blinatumomab in children with relapsed/refractory (R/R) B-ALL (4). It has also induced high rates of complete minimal residual disease (MRD) response in both adults and children with molecularly resistant B-ALL.
Nevertheless, 10%–15% of patients exhibit primary resistance to blinatumomab. Emerging evidence has identified several mechanisms contributing to treatment failure. Elevated levels of regulatory T cells, characterized by CD4/CD25/FOXP3 expression and interleukin-10 (IL-10)–mediated suppression of T-cell proliferation, have been associated with reduced response (5). Increased expression of programmed death ligand 1 (PD-L1), the binding ligand of the inhibitory checkpoint molecule programmed death 1 (PD-1), has also been linked to impaired T-cell function and diminished efficacy (6). Additionally, KMT2A-rearranged ALL lineage switch may induce resistance (7, 8). Lower blast counts in bone marrow (BM) (≤50%) have been associated with better response than higher disease burden (9, 10).
Despite these insights, the mechanisms underlying blinatumomab resistance remain incompletely elucidated. With the expanding application of blinatumomab as frontline preemptive therapy, particularly for patients with persistent complete remission with MRD positivity (CR-MRDpos) or chemotherapy delays, its therapeutic scope has broadened. In this study, we comprehensively analyzed treatment response across three cohorts: CR-MRDpos, R/R, and CR with MRD negativity (CR-MRDneg). Through systematic evaluation of immune reconstitution profiles (T-cell subsets, cytokine dynamics), cytogenetic markers, and clinical outcomes, we aimed to identify key predictors of treatment resistance.
Methods
Patients
This multicenter retrospective study was approved by the institutional review boards (No. 2025R022-E01) following discussion in a multicenter advisory panel across four pediatric medical centers: Shanghai Children’s Hospital, Shanghai Children’s Medical Center, Anhui Children’s Hospital, and Shandong Provincial Hospital. Patients ≤18 years old with R/R B-ALL and MRD positivity at any time who received blinatumomab therapy were enrolled. Patients with chemotherapy intolerance or severe infection who received blinatumomab as bridging therapy were enrolled as the control group. The exclusion criteria were as follows: (i) patients with severe infection and cardiac, liver, or kidney insufficiency who had an expected survival time of less than 3 months; and (ii) those who received blinatumomab for fewer than 7 days. Patient enrollment lasted from September 2021 to June 2024, with follow-up through March 2025. A total of 105 patients were enrolled.
Treatment strategy
Blinatumomab was administered via a stepwise dose-escalation protocol during the initial cycle: 5 μg/m²/day as continuous intravenous infusion on days 2–7, followed by escalation to 15 μg/m²/day for a total cycle duration of 14–28 days. The infusion duration depended on family financial conditions and physician discretion. In some cases, BM aspiration was performed on day 15, and treatment was discontinued upon achieving BM remission. Each treatment cycle was separated by a 14-day treatment break.
Dexamethasone prophylaxis (5 mg/m²/day for 1 day) was routinely administered. Subsequent treatment cycles commenced directly at 15 μg/m²/day. Adverse events (AEs) were managed according to the manufacturer’s instructions. BM assessment was performed upon completion of the infusion cycle. Intrathecal injections of methotrexate, cytarabine, and dexamethasone were administered before, during, or after blinatumomab cycles. Upon achieving BM remission, patients proceeded to hematopoietic stem cell transplantation (HSCT), continued the original protocol, or received alternative treatment. Non-responders were transitioned to salvage protocols, chimeric antigen receptor T-cell (CAR-T) therapy, or palliative HSCT, as clinically indicated.
Some patients received reinduction therapy to reduce tumor burden. The reinduction therapy followed the initial induction regimen: dexamethasone 6 mg/m² on days 1–4; vincristine 1.5 mg/m² on days 5, 12, 19, and 26; prednisone 45 mg/m² on days 5–28; daunorubicin 25 mg/m² on days 5 and 12; and peg-asparaginase 2,000 U/m² on days 6 and 26.
For patients with Philadelphia chromosome–positive (Ph+) disease, a tyrosine kinase inhibitor (TKI) was added, with dasatinib 80 mg/m² preferred. Bridging chemotherapy prior to blinatumomab included induction chemotherapy or continued consolidation chemotherapy consisting of cyclophosphamide 1,000 mg/m² on day 1, cytarabine 50 mg/m² on days 1–7, and mercaptopurine 40 mg/m² on days 1–7.
Definition
Patients were divided into three groups: CR-MRDneg, CR-MRDpos, and R/R. Response was categorized as either cytological CR or MRD CR. Cytological CR was defined as <5% BM blasts in patients with R/R status. MRD CR, detected by flow cytometry (FCM), was defined as a reduction in MRD to <0.01% or maintenance of MRD negativity in patients with CR-MRDpos. No response was defined as partial remission (PR) or no remission (NR) in R/R patients, and persistent MRD ≥0.01% in patients with CR-MRDpos.
Poor cytogenetics were defined as KMT2Ar, BCR-ABL1, and TCF3-HLF, according to the Chinese Children’s Cancer Group ALL (CCCG-ALL) 2015 protocol. Event-free survival (EFS) was defined as the time from diagnosis to relapse, death, secondary cancer, or last contact for those who were event-free. Overall survival (OS) was defined as the time from diagnosis to death from any cause or last contact if alive.
Cytokine detection
Serum concentrations of target cytokines [IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-17, interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and IFN-α] were measured using a multiplex microsphere-based flow immunofluorescence assay (12-cytokine kits, Raisecare, China) according to the manufacturer’s instructions. Cytokines were assessed before blinatumomab infusion, at the onset of cytokine release syndrome (CRS) or immune effector cell–associated neurotoxicity syndrome (ICANS), and at the end of blinatumomab treatment.
T- cell and B-cell subsets
Basic lymphocyte subpopulations were analyzed using a FACSCalibur flow cytometer (BD Biosciences) and reported as both percentages and absolute counts. FCM with CellQuest software (BD Biosciences) was used for analysis of lymphocyte subsets (CD3/CD45/CD4/CD8/CD16CD56/CD19, BD Biosciences), including T cells (CD3+CD45+), cytotoxic T cells (CD3+CD8+CD45+), helper T cells (CD3+CD4+CD45+), NK cells (CD16+CD56+CD3−CD45+), and B cells (CD19+CD45+). A total of 15,000 lymphocytes were acquired for analysis. Data were collected at baseline and on days 14, 21, and 28 (end of treatment). T-cell activation magnitude was defined as the difference between post-blinatumomab and baseline (pre-treatment) measurements.
Statistical analysis
Quantitative data with a Gaussian distribution were presented as mean ± standard deviation (SD) and compared using the t-test. Non-normally distributed data were presented as medians with full ranges. Comparisons between two groups were performed using the Mann–Whitney U test or Wilcoxon matched-pairs test. Comparisons involving two or more factors were conducted using one-way or two-way analysis of variance (ANOVA). Categorical variables were presented as percentages and compared using Fisher’s exact test or the chi-square (χ²) test. Patients lost to follow-up were censored at the last date they were known to be alive. OS and EFS were estimated by the Kaplan–Meier method, and curves were compared using the log-rank test. The Cox proportional hazards model was used for univariate and multivariate analyses. All statistical analyses were performed using GraphPad Prism version 9 (GraphPad Software Inc., La Jolla, CA, USA) and SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). All tests were two-sided, and P < 0.05 was considered statistically significant.
Results
Patients’ characteristics
A total of 105 patients with B-ALL received blinatumomab across 125 cycles, following standardized clinical practice. The median age was 72 months (range, 5–210 months). Sixty-four patients (61%) were male and 41 (39%) were female.
Thirty cases were diagnosed with R/R ALL, including 23 with relapse and seven with induction failure. Eight patients received blinatumomab directly as reinduction therapy, and 22 patients received burden-reduction chemotherapy prior to blinatumomab. Among these, three patients failed to reduce tumor burden, with blast levels remaining above 5%.
Ten patients achieved CR-MRDpos, and nine patients achieved CR-MRDneg. An additional 21 patients had CR-MRDpos following induction or developed MRD positivity during treatment. Fifty-four patients received blinatumomab due to chemotherapy delay caused by chemotherapy intolerance or severe AEs, among whom nine patients had CR-MRDpos.
At the initial cycle of blinatumomab, 40 patients had CR-MRDpos, 11 patients had R/R status, and 54 patients had MRD negativity. Among the latter, four cases had BM blasts ranging from 5%–9.5%, while seven had blasts ≥20%. For these 51 patients, the median BM blast percentage was 2% and the median MRD percentage was 0.52% (Table 1). In patients achieving MRD negativity, a total of 74 cycles were administered. The demographic and clinical characteristics of the R/R, CR-MRDpos, and CR-MRDneg groups are summarized in Table 1.
Response rate
The CR rate of R/R patients was 81.8% (9/11), while the MRD-negative CR rate was 72.7% (8/11). MRD CR was achieved in 33 of 40 cases (82.5%) with CR-MRDpos. The overall CR response rate was 82.4%.
Among the R/R and CR-MRDpos patients, 31 received a 14-day infusion and 20 received a 3–4-week infusion. The response rate was 83.9% for the 2-week regimen and 80% for the 3–4-week regimen.
Of the 74 MRD-negative cycles, 73 patients remained MRD negative until the follow-up day. Only one patient experienced central nervous system (CNS) relapse 14 months after blinatumomab.
In nine cases with NR after the first blinatumomab cycle, three received a second cycle of blinatumomab, two patients underwent HSCT (achieving CR), three patients with persistent MRD continued chemotherapy (achieving CR), and one patient discontinued treatment and subsequently died. Among the three patients receiving a second cycle of blinatumomab, one achieved CR and bridged to HSCT. The remaining two patients failed to achieve remission and continued treatment with CAR-T. All three patients remained alive.
T-cell response after blinatumomab
CD3+ T cell activation
We compared data obtained before and after 2–4 weeks of blinatumomab infusion. The data exhibited a non-Gaussian distribution and were expressed as median (range). Comparisons were performed using the Wilcoxon matched-pairs test. Detailed data are shown in Supplementary Table S1.
The absolute count of CD3+ T cells significantly increased from 0.96 (0.03–3.79) × 109/L to 1.13 (0.26–7.74) ×109/L (P = 0.016; Figure 1A). The CD3+ percentage also rose significantly (P = 0.008; Supplementary Table S1, Figure 1D).
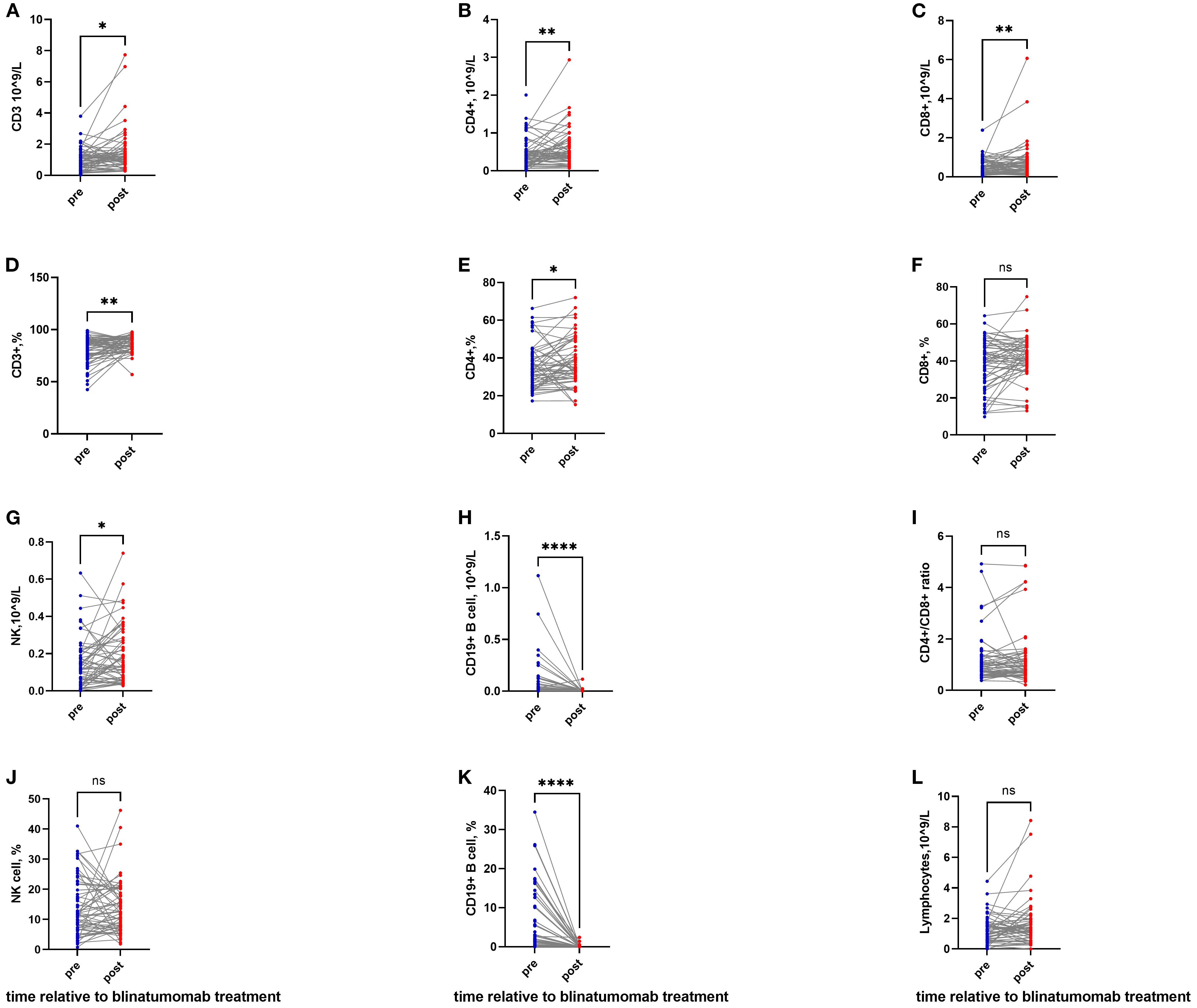
Figure 1. T-cell activation and B-cell depletion following blinatumomab therapy. (A, D) The absolute count and percentage of CD3+ T cells significantly increased after blinatumomab. (B, E) The absolute count and percentage of CD4+ T cells significantly increased after blinatumomab. (C, F) The absolute count of CD8+ T cells elevated significantly, while the percentage remained stable. (G, J) NK cell counts showed a significant increase, though the percentage remained unchanged. (H, K) B cells were depleted profoundly in both absolute and percentage. (I) The CD4+/CD8+ ratio exhibited a non-significant decrease. (L) Total lymphocyte count remained stable throughout treatment. *P< 0.05; **P< 0.01; ****P< 0.0001.
CD4+ and CD8+ T cells also increased, from 0.35 (0.01–1.39) to 0.47 (0.07–2.94) ×109/L, and from 0.41 (0.01–2.39) to 0.56 (0.07–6.07) ×109/L, respectively (P = 0.005 and P = 0.006; Figures 1B, C). The percentages of CD4+ and CD8+ T cells also increased (P = 0.025 and P = 0.054; Supplementary Table S1, Figures 1E, F).
The CD4/CD8 ratio exhibited a nonsignificant decrease from 0.85 (0.37–4.92) to 0.78 (0.21–4.84) (P = 0.532; Figure 1I). The CD16+CD56+/CD3− NK cell count increased from 0.12 (0.00–0.63) ×109/L to 0.15 (0.03–0.74) ×109/L (P = 0.024; Figure 1G), although the percentage remained unchanged (Figure 1J).
B cells were completely depleted, with absolute counts decreasing from 0.007×109/L (0.00–1.12) to undetectable levels 0.00×109/L (0.00–0.11) (P <0.0001; Figure 1H), and percentages falling from 1.39% (0.00–34.48) to 0% (0.00–2.43) (P < 0.0001; Figure 1K). Total lymphocyte counts remained stable throughout the observation period.
In patients receiving a 14-day infusion, CD3+, CD4+, and CD8+ T cells had already increased compared with baseline (Supplementary Table S1). A subset of NK cells also expanded significantly, from 0.12 (0.00–0.63) to 0.18 (0.03–0.74) ×109/L (P = 0.013).
In patients receiving a 21/28-day infusion, CD3+ T cells continued to increase, rising from 0.80 (0.03–1.86) to 1.01 (0.33–7.74) ×109/L (P = 0.033; Supplementary Table S1). CD4+ T cells showed a sustained elevation from 0.42 (0.01–0.72) to 0.47 (0.12–1.25) ×109/L (P = 0.092), while CD8+ and NK cells declined (P = 0.470 and P = 0.850; Supplementary Table S1).
Levels of Immunoglobulin (Ig)
Levels of G, IgA, and IgM were assessed before and after therapy. Following blinatumomab, IgG levels went down from 9.23 (2.28–18.50) to 7.05 (1.61–18.00) g/L (P = 0.0005). IgA levels and IgM levels also declined from 0.78 (0.12–2.39) to 0.27 (0.03–0.92) g/L (P < 0.0001) and from 0.41 (0.11-1.31) to 0.19 (0.01- 0.56) g/L, respectively (both P < 0.0001).
CD3+ T cell activation in MRDpos+R/R and MRDneg patients
When patients with R/R and CR-MRDpos status were compared with those with CR-MRDneg, notable differences in the immune cell repertoire were observed. The data exhibited a non-Gaussian distribution and were expressed as median (range), with comparisons performed using the Mann–Whitney U test.
The increase in CD3+ T cells was significantly greater in the R/R + CR-MRDpos group than in the CR-MRDneg group [0.57 (−1.08 to 6.07) vs. 0.12 (-1.83 to 3.34)×109/L; P = 0.047; Figure 2A]. CD4+ cells expanded more significantly in the R/R + CR-MRDpos group than in the CR-MRDneg group [0.24 (−0.55 to 1.81) vs. 0.03 (−0.73 to 1.19) × 10^9/L; P = 0.039; Figure 2B]. In contrast, no significant difference in CD8+ T-cell expansion was observed between the two cohorts [0.16 (−0.48 to 5.21) vs. 0.11 (−0.58 to 1.33) × 10^9/L; P = 0.174; Figure 2C].
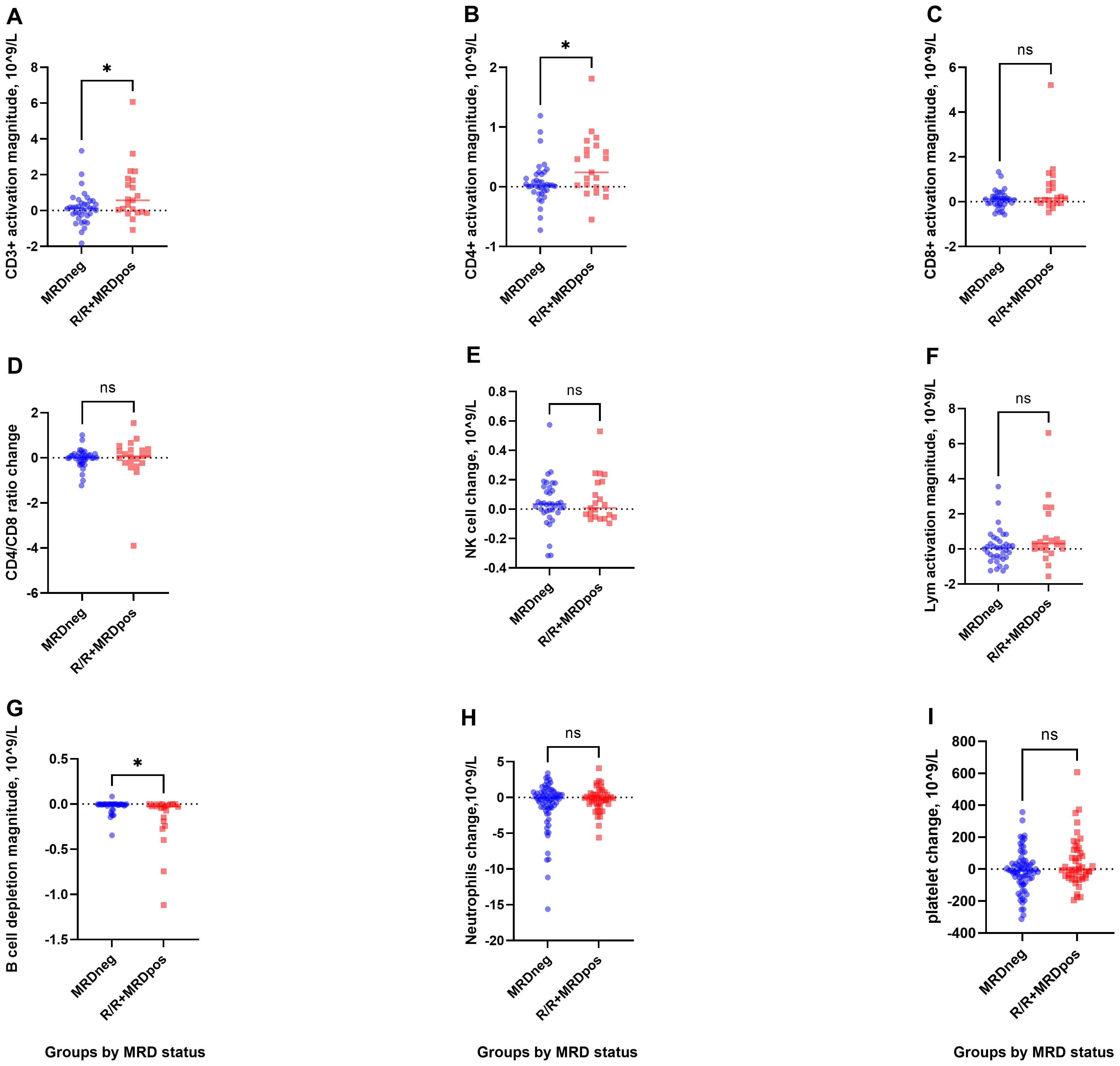
Figure 2. T-cell activation and B-cell depletion in R/R+MRDpos and CR-MRDneg groups. (A, B), Greater increases in CD3+ and CD4+ T-cell counts were observed in the R/R+MRDpos cohort. (C-F), CD8+, CD4+/CD8+ ratio, NK cells, and lymphocytes showed mild fluctuations. (G), Enhanced B-cell depletion was observed in R/R+MRDpos patients. (H, I), No significant intergroup differences were observed in neutrophils or platelets. *P< 0.05.
Enhanced B-cell eradication was observed in the R/R + CR-MRDpos group compared with the CR-MRDneg group [−0.03 (−1.12 to 0.00) vs. −0.004 (−0.35 to 0.08)×109/L, P = 0.017; Figure 2G]. No significant intergroup differences were detected in NK cells, total lymphocytes, CD4+/CD8+ ratio, neutrophils, or platelets (Figures 2D–F, H, I; Supplementary Table S2).
Cytokine level following CRS
At the onset of CRS, significant elevations in serum levels of IL-2, IL-5, IL-10, and IFN-γ were observed. The data were expressed as median (range), and comparisons were performed using the Wilcoxon matched-pairs test.
IL-10 increased from 2.4 (0.3–8.3) to 2.4 (0.3–34.3) pg/mL (P < 0.0001; Figure 3A). IL-5 rose from 2.7 (0.3–9.6) to 2.7 (0.5–205.6) pg/mL (P = 0.0006; Figure 3B). IFN-γ increased from 4.6 (1.0–84.5) to 12.3 (1.3–328.3) pg/mL (P = 0.003; Figure 3C). IL-2 rose from 2.4 (0.5–17.3) to 2.4 (0.7–22.8) pg/mL (P = 0.001; Figure 3D).

Figure 3. Cytokine dynamics during blinatumomab therapy. (A-D) IL-10, IL-5, IFN-γ, and IL-2 increased significantly at CRS-onset. (E-H) IL-6, IFN-α, IL-1β, and IL-8 levels remained stable during blinatumomab. (I-L) No significant alterations occurred in IL-17, IL-12p70, TNF-α, and IL-4. **P< 0.01; ***P< 0.001; ****P< 0.0001.
No significant changes were observed in IL-6, IL-8, IL-4, IL-1β, IL-12p70, IL-17, TNF-α, or IFN-α between pre-blinatumomab and CRS onset (Figures 3E–L).
Overall survival and event free survival
In total, nine patients had NR after the first cycle of blinatumomab. During follow-up, four relapses and one death were documented. Among the relapses, three occurred in the R/R group and one in a CR-MRDpos patient with subsequent MRD reversion to positivity. Of these, three were BM recurrences and one was a CNS relapse. Additionally, one CR-MRDneg patient died from CNS-invasive aspergillosis.
In patients with and without R/R status, the 1-year EFS rates were 73.3% ± 8.1% and 93.3% ± 2.9%, respectively (P = 0.0004; Figure 4A). The 1-year OS rates were 93.3% ± 4.6% and 98.6% ± 1.3%, respectively (P = 0.009; Figure 4B).

Figure 4. Survival outcomes of ALL patients receiving blinatumomab. (A) EFS in patients with and without R/R ALL. (B) OS in patients with and without R/R ALL. (C) EFS in patients with CR-MRDneg, CR-MRDpos, and R/R ALL. (D) OS in patients with CR-MRDneg, CR-MRDpos, and R/R ALL.
In patients with CR-MRDneg and CR-MRDpos status, the 1-year EFS rates were 97.8% ± 2.2% and 86.7% ± 6.2%, respectively (P = 0.001; Figure 4C). The 1-year OS rates were 97.8% ± 2.2% and 100%, respectively (P = 0.029; Figure 4D).
In patients with R/R and CR-MRDpos status at initial classification, the 1-year EFS was 94.0% ± 3.4% for those achieving MRD negativity versus 10.0% ± 9.5% for those not achieving MRD negativity after blinatumomab (P < 0.0001). The corresponding 1-year OS rates were 100% and 78.8% ± 13.4% (P = 0.001).
In the R/R ALL subgroup, the 1-year EFS was 87.5% ± 6.8% for patients who achieved MRD negativity after blinatumomab therapy, compared with 16.7% ± 15.2% for those who remained MRD positive (P < 0.0001). The 1-year OS rates were 100% and 66.7% ± 19.2%, respectively (P = 0.002).
In the CR-MRDpos cohort, the 1-year EFS was 100% for patients who achieved MRD negativity versus 0% for those who remained MRD positive (P < 0.0001). The 1-year OS was 100% in both groups (P = 1.000).
Risk factors of treatment failure
Among the 51 cases with CR-MRDpos or R/R status, nine cases failed to respond. Univariable analysis revealed poor cytogenetics, BCR-ABL1 fusion, and low absolute B-cell count as risk factors for treatment failure (Table 2). Multivariable analysis using a Cox regression model further demonstrated that high MRD level (P = 0.014), BCR-ABL1 fusion (P = 0.065), and poor cytogenetics (P = 0.025) were independent risk factors.
To evaluate the impact on MRD negativization, univariable analysis revealed poor cytogenetics (P = 0.0003), BCR-ABL1 fusion (P = 0.004), and low absolute B-cell count (P = 0.065) as risk factors (Supplementary Table S3). The Cox regression model showed that high MRD level (P = 0.014) and poor cytogenetics (P = 0.009) were independent risk factors.
In the initial cohort of 30 patients with R/R disease, 22 patients receiving bridging therapy demonstrated a CR rate of 86.4% (19/22), while the CR rate was 75.0% (6/8) in patients without bridging therapy (P = 0.589). Details of bridging therapy and response for R/R patients are presented in Supplementary Table S4. A subset of eight patients received bridging chemotherapy following induction therapy, with cyclophosphamide administered at doses of either 1,000 mg/m² or 300 mg/m². No statistically significant difference in CR rate was observed between patients receiving cyclophosphamide-containing bridging chemotherapy (87.5%, 7/8) and those who did not undergo lymphodepleting chemotherapy (81.8%, 18/22; P = 1.000; Supplementary Table S4).
Adverse events
Each patient received one to four courses of blinatumomab, with a total of 125 cycles across the entire cohort. The most common AEs were CRS and hematologic toxicity. The incidence of severe CRS and ICANS in the R/R and CR-MRDpos groups was comparable to that in the CR-MRDneg group (3.9% vs. 0% and 0% vs. 5.4%, respectively; P = 0.146 and P = 0.399; Table 3).
Among the six patients who developed ICANS, the median age was 160 months, significantly older than that of patients without ICANS [160.5 (69–210) vs. 73 (5–212) months; P = 0.014]. Four patients underwent serum cytokine profiling both before and at ICANS onset, while three patients additionally received cerebrospinal fluid (CSF) cytokine profiling. During ICANS onset, CSF showed a white blood cell count of 0–40 cells/μL, albumin levels between 300–783 mg/L, and cytokine profiling in three patients revealed elevations of IL-5, IL-6, and IL-8, while IL-2, IL-10, and IFN-γ remained within reference ranges. Meanwhile, most serum cytokines showed no abnormal elevations; however, elevated IL-8 was observed. Detailed data are shown in Table 4.
Five of six patients with ICANS underwent T-cell subset analysis. A more pronounced inversion of the CD4+/CD8+ ratio—particularly with higher CD8+ proportions, together with lower absolute B-cell counts and reduced B-cell percentages—was implicated in a higher risk of ICANS (Supplementary Table S5).
Severe neutropenia occurred more frequently in the CR-MRDneg group compared with the high-MRD group (32.4% vs. 17.4%; P = 0.003). Thrombocytopenia was more common in the R/R plus MRDpos group than in the CR-MRDneg group (16.4% vs. 1.4%; P = 0.003).
Discussion
Blinatumomab, the first bispecific T-cell engager approved for R/R B-ALL, has demonstrated remarkable clinical outcomes across multiple cohorts. In our study, we observed a high overall response rate of 82.4% in B-ALL, including both R/R and MRDpos cases. The 1-year EFS and OS for R/R patients were 73.3% and 93.3%, respectively. These favorable outcomes may be attributed to the robust response to blinatumomab, often followed by HSCT or CAR-T therapy. Compared with standard salvage chemotherapy, patients with R/R B-ALL treated with blinatumomab exhibited significantly improved OS (11, 12). In the Children’s Oncology Group AALL1331 study, patients with low-risk first relapse of B-ALL were randomized to receive either chemotherapy cycles or chemotherapy intercalated with three blocks of blinatumomab. The 4-year disease-free survival (DFS)/OS for 255 patients were 61.2% and 90.4% in the blinatumomab group, compared with 49.5% and 79.6% in the chemotherapy group (P = 0.089 and 0.11) (13). In another study evaluating blinatumomab as consolidation, children with high-risk first-relapse B-ALL were randomized to receive one cycle of blinatumomab or a third course of consolidation chemotherapy prior to HSCT. A higher MRD remission rate was observed in the blinatumomab group compared with chemotherapy (90% [44/49] vs. 54% [26/48]), along with improved EFS (14). In a randomized phase 3 clinical trial, patients received either two cycles of blinatumomab or two cycles of multiagent chemotherapy after reinduction chemotherapy, followed by transplantation. With a median follow-up of 2.9 years, 2-year DFS and OS were superior in the blinatumomab group compared with the chemotherapy group (54.4% vs. 39.0%, P = 0.03; 71.3% vs. 58.4%, P = 0.02) (15).
Persistence or recurrence of CR-MRDpos was mainly attributed to delayed MRD clearance and subsequent re-emergence, primarily due to adverse cytogenetic profiles and delays in chemotherapy administration. In our study, these patients received blinatumomab as a preemptive intervention. Although CR-MRDpos patients demonstrated inferior 1-year EFS compared with CR-MRDneg patients (86.7% vs. 97.8%), their outcomes were better than those of R/R patients. Notably, the 1-year OS for CR-MRDpos patients was 100%, higher than the 97.7% observed in CR-MRDneg patients. These findings indicate that blinatumomab was both safe and effective in patients with chemotherapy intolerance or resistance. In a matched cohort study evaluating blinatumomab as an alternative to intensive post-remission chemotherapy for chemotherapy-intolerant or resistant patients, comparable 2-year EFS and OS rates were seen between the blinatumomab-treated cohort (n = 80) and conventional chemotherapy controls (n = 192): 95% vs. 90% and 97% vs. 94%, respectively (16).
The mechanisms underlying blinatumomab resistance remain incompletely understood. Our study identified adverse cytogenetics, BCR-ABL1 fusion, and low absolute CD19+ B-cell counts as significant predictors of treatment failure. Furthermore, elevated MRD burden, BCR-ABL1 positivity, and high-risk cytogenetic profiles emerged as independent risk factors. Previous studies have shown that lower tumor burden is associated with higher CR rates (14, 17, 18), which in turn influence DFS and OS (18). Consistent with prior findings, our data confirm the association between elevated MRD levels and suboptimal treatment response (19). Our findings also suggest that low absolute CD19+ B-cell count contributes to blinatumomab resistance. In line with earlier investigations, pre-blinatumomab absolute lymphocyte count (ALC) and the MRD/ALC ratio were associated with MRD response. Analysis revealed prognostic associations for pre-blinatumomab MRD level, ALC, MRD/ALC ratio, and post-blinatumomab MRD remission with OS and EFS (19). Among the poor cytogenetics, BCR-ABL1 fusion was the main predictor of blinatumomab resistance in our cohort.
Outcomes in patients with Philadelphia chromosome (Ph)–positive ALL have improved with the use of TKIs. A chemotherapy-free induction and consolidation regimen combining dasatinib and blinatumomab reported a high induction CR rate of 98%. The molecular response at the end of dasatinib induction therapy (29%) increased to 60% after two cycles of blinatumomab (20). Nevertheless, its application remains rare in the pediatric setting.
The MRD monitoring in this research was performed using FCM and applied to all cases, with a sensitivity of 0.01%. The MRD cut-off was appropriate according to the recommendation of the 2024 European LeukemiaNet (ELN) (21). FCM can be applied to most ALL cases (>90%), and the results are promptly available. Molecular MRD monitoring of fusion genes (e.g., BCR-ABL1) has a sensitivity of around 0.01%. However, its accuracy is hampered by the variability in the number of RNA transcripts in leukemic cells. In extremely low-burden cases, novel techniques such as digital droplet PCR and next-generation sequencing (NGS) could be used. The use of qPCR measurement of clonal immunoglobulin/T-cell receptor (IG/TR) in Ph’- ALL could be more precise, as recommended in ELN 2024. Despite the promising efficacy of blinatumomab, its impact on host immune cell dynamics remains incompletely understood. T-cell activation may play a critical role in modulating blinatumomab responsiveness. To address this, we systematically characterized the immune cell repertoire at baseline, throughout treatment, and post-therapy. Our immunophenotyping data demonstrated significant temporal expansion of CD3+, CD8+, CD4+ T cells, and NK cells by day 14, with sustained elevation through days 21–28, consistent with prior observations (22). Recent studies have further elucidated blinatumomab-mediated modulation of peripheral blood T-cell subset distribution during therapy (20, 23–25). Circulating T cells were found to decrease within the first day of infusion and then recover to baseline after approximately one week, likely due to increased T-cell adhesion to blood vessel endothelium (25). During the T-cell activation phase, we observed near-complete depletion of circulating B lymphocytes across nearly all cases. In vitro coculture experiments have shown that blinatumomab can induce redirected lysis of CD19+ B lymphocytes and malignant B-cell lines by previously resting peripheral T cells (26).
Notably, blinatumomab exhibited differential immunomodulatory effects across distinct patient subgroups, with marked variations observed between R/R and CR-MRDpos patients compared with CR-MRDneg (chemotherapy-intolerant) patients. Analysis revealed significantly greater activation of CD3+ and CD4+ T cells in the R/R and CR-MRDpos cohorts relative to the CR-MRDneg group. Our cohort showed that the proportion of T cells was not related to response. An interesting case series reported by Duminuco indicated that a higher proportion of baseline T lymphocytes achieved MRD negativity more frequently, though without statistical significance (P = 0.06) (27). Concomitantly, more profound depletion of B cells was seen in the R/R and CR-MRDpos groups, likely attributable to enhanced CD19-directed cytolysis of malignant B-cell populations by activated T cells. Following blinatumomab, decreased IgG levels were noted among the tested patients. Taken together, these findings indicate that blinatumomab induces transient but significant immunophenotypic remodeling, characterized by preferential expansion of CD3+ and CD4+ T-cell compartments. However, no statistically significant differences were observed in NK cell counts, total lymphocytes, or CD8+ T-cell populations at the end of the cycle compared with pretreatment levels.
Relapses occurred in four patients during follow-up, with three in the R/R group and one in the CR-MRDpos group. Two cases developed CD19-negative relapses, a well-documented mechanism of blinatumomab resistance, with reported incidence rates ranging from 8% to 35% in clinical studies (28–30). The relapse pattern included isolated CNS involvement in one patient (25%). Two cases represented early treatment failure, with relapses occurring within one month of blinatumomab initiation. The remaining two patients experienced late relapse at nine and 14 months post-therapy, respectively.
The safety profile observed in this study was consistent with the AE spectrum reported in prior clinical trials. No treatment-related mortality was reported. CRS of grade ≥3 severity occurred in two patients (1.6%), exclusively within the R/R cohort. Neurological events of grade ≥3 were documented in four patients, all in the CR-MRDneg group, with older age identified as a significant risk factor for neurotoxicity. The incidence of grade ≥3 CRS and neurotoxicity was 1.6% and 3.2%, aligning with published safety data reporting severe CRS and neurotoxicity incidences of 1–3.1% and 1–7%, respectively (13, 15, 31). Grade 3–4 neutropenia was more common in the CR-MRDneg population than in R/R patients, while any-grade thrombocytopenia was more frequently observed in the combined R/R and CR-MRDpos groups compared with CR-MRDneg cases. Overall, the incidence of severe AEs remained low, and no blinatumomab-related death was observed.
Blinatumomab demonstrated encouraging results in children with R/R ALL and MRD-positive disease. Notably, it emerged as a particularly valuable therapeutic option for chemotherapy-intolerant patients or those with severe concurrent infections, serving as an effective bridging therapy to maintain durable MRD negativity. Treatment failure occurred in approximately 10–15% of cases, with growing evidence suggesting that intrinsic disease biology, including specific cytogenetic abnormalities and immunophenotypic profiles, significantly influences therapeutic response. Comprehensive pretreatment evaluation incorporating high-risk genetic markers and quantitative CD19+ B-cell assessment may facilitate more precise identification of therapy-sensitive and therapy-resistant subgroups, potentially informing risk-adapted treatment strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Shanghai Children’s Hospital ethics committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
NZ: Data curation, Writing – original draft, Conceptualization, Funding acquisition. WH: Writing – review & editing, Data curation. YD: Writing – review & editing, Data curation. JW: Data curation, Writing – review & editing. LQ: Writing – review & editing, Project administration. DW: Writing – review & editing, Project administration. BL: Project administration, Writing – review & editing. JS: Project administration, Writing – review & editing. SS: Supervision, Conceptualization, Writing – review & editing. HJ: Supervision, Writing – review & editing, Conceptualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by grants from Shanghai Children’s Hospital (No. 2020XKZX30 and 2020YLYM09).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1607138/full#supplementary-material
References
1. Hunger SP and Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. (2015) 373:1541–52. doi: 10.1056/NEJMra1400972
2. Locatelli F, Schrappe M, Bernardo ME, and Rutella S. How I treat relapsed childhood acute lymphoblastic leukemia. Blood. (2012) 120:2807–16. doi: 10.1182/blood-2012-02-265884
3. Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. (2008) 321:974–7. doi: 10.1126/science.1158545
4. Chen B, Zou Z, Zhang Q, Chen K, Zhang X, Xiao D, et al. Efficacy and safety of blinatumomab in children with relapsed/refractory B cell acute lymphoblastic leukemia: A systematic review and meta-analysis. Front Pharmacol. (2023) 13:1032664. doi: 10.3389/fphar.2022.1032664
5. Duell J, Dittrich M, Bedke T, Mueller T, Eisele F, Rosenwald A, et al. Frequency of regulatory T cells determines the outcome of the T-cell-engaging antibody blinatumomab in patients with B-precursor ALL. Leukemia. (2017) 31:2181–90. doi: 10.1038/leu.2017.41
6. Köhnke T, Krupka C, Tischer J, Knösel T, and Subklewe M. Increase of PD-L1 expressing B-precursor ALL cells in a patient resistant to the CD19/CD3-bispecific T cell engager antibody blinatumomab. J Hematol Oncol. (2015) 8:111. doi: 10.1186/s13045-015-0213-6
7. Haddox CL, Mangaonkar AA, Chen D, Shi M, He R, Oliveira JL, et al. Blinatumomab-induced lineage switch of B-ALL with t(4:11)(q21;q23) KMT2A/AFF1 into an aggressive AML: pre- and post-switch phenotypic, cytogenetic and molecular analysis. Blood Cancer J. (2017) 7:e607. doi: 10.1038/bcj.2017.89
8. Wölfl M, Rasche M, Eyrich M, Schmid R, Reinhardt D, and Schlegel PG. Spontaneous reversion of a lineage switch following an initial blinatumomab-induced ALL-to-AML switch in MLL-rearranged infant ALL. Blood Adv. (2018) 2:1382–5. doi: 10.1182/bloodadvances.2018018093
9. Zhao Y, Aldoss I, Qu C, Crawford JC, Gu Z, Allen EK, et al. Tumor-intrinsic and -extrinsic determinants of response to blinatumomab in adults with B-ALL. Blood. (2021) 137:471–84. doi: 10.1182/blood.2020006287
10. Queudeville M, Stein AS, Locatelli F, Ebinger M, Handgretinger R, Gökbuget N, et al. Low leukemia burden improves blinatumomab efficacy in patients with relapsed/refractory B-cell acute lymphoblastic leukemia. Cancer. (2023) 129:1384–93. doi: 10.1002/cncr.34667
11. Kantarjian H, Stein A, Gökbuget N, Fielding AK, Schuh AC, Ribera JM, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. (2017) 376:836–47. doi: 10.1056/NEJMoa1609783
12. Martinelli G, Boissel N, Chevallier P, Ottmann O, Gökbuget N, Rambaldi A, et al. Long-term follow-up of blinatumomab in patients with relapsed/refractory Philadelphia chromosome-positive B-cell precursor acute lymphoblastic leukaemia: final analysis of ALCANTARA study. Eur J Cancer. (2021) 146:107–14. doi: 10.1016/j.ejca.2020.12.022
13. Hogan LE, Brown PA, Ji L, Xu X, Devidas M, Bhatla T, et al. Children’s oncology group AALL1331: phase III trial of blinatumomab in children, adolescents, and young adults with low-risk B-cell ALL in first relapse. J Clin Oncol. (2023) 41:4118–29. doi: 10.1200/JCO.22.02200
14. Locatelli F, Zugmaier G, Rizzari C, Morris JD, Gruhn B, Klingebiel T, et al. Effect of blinatumomab vs chemotherapy on event-free survival among children with high-risk first-relapse B-cell acute lymphoblastic leukemia: A randomized clinical trial. JAMA. (2021) 325:843–54. doi: 10.1001/jama.2021.0987
15. Brown PA, Ji L, Xu X, Devidas M, Hogan LE, Borowitz MJ, et al. Effect of postreinduction therapy consolidation with blinatumomab vs chemotherapy on disease-free survival in children, adolescents, and young adults with first relapse of B-cell acute lymphoblastic leukemia: A randomized clinical trial. JAMA. (2021) 325:833–42. doi: 10.1001/jama.2021.0669
16. Hodder A, Mishra AK, Enshaei A, Baird S, Elbeshlawi I, Bonney D, et al. Blinatumomab for first-line treatment of children and young persons with B-ALL. J Clin Oncol. (2024) 42:907–14. doi: 10.1200/JCO.23.01392
17. Locatelli F, Zugmaier G, Mergen N, Bader P, Jeha S, Schlegel PG, et al. Blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia: results of the RIALTO trial, an expanded access study. Blood Cancer J. (2020) 10:77. doi: 10.1038/s41408-020-00342-x
18. Cabannes-Hamy A, Brissot E, Leguay T, Huguet F, Chevallier P, Hunault M, et al. High tumor burden before blinatumomab has a negative impact on the outcome of adult patients with B-cell precursor acute lymphoblastic leukemia. A real-world study by the GRAALL. Haematologica. (2022) 107:2072–80. doi: 10.3324/haematol.2021.280078
19. Essa MF, Abdellatif R, Elimam N, Ballourah W, Alsudairy R, Alkaiyat M, et al. Outcomes of blinatumomab based therapy in children with relapsed, persistent, or refractory acute lymphoblastic leukemia: a multicenter study focusing on predictors of response and post-treatment immunoglobulin production. Pediatr Hematol Oncol. (2022) 39:613–28. doi: 10.1080/08880018.2022.2049936
20. Foà R, Bassan R, Vitale A, Elia L, Piciocchi A, Puzzolo MC, et al. Dasatinib-blinatumomab for ph-positive acute lymphoblastic leukemia in adults. N Engl J Med. (2020) 383:1613–23. doi: 10.1056/NEJMoa2016272
21. Gökbuget N, Boissel N, Chiaretti S, Dombret H, Doubek M, Fielding A, et al. Management of ALL in adults: 2024 ELN recommendations from a European expert panel. Blood. (2024) 143:1903–30. doi: 10.1182/blood.2023023568
22. Klinger M, Brandl C, Zugmaier G, Hijazi Y, Bargou RC, Topp MS, et al. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood. (2012) 119:6226–33. doi: 10.1182/blood-2012-01-400515
23. Ocadlikova D, Lussana F, Fracchiolla N, Bonifacio M, Santoro L, Delia M, et al. Blinatumomab differentially modulates peripheral blood and bone marrow immune cell repertoire: A Campus ALL study. Br J Haematol. (2023) 203:637–50. doi: 10.1111/bjh.19104
24. Gaballa MR, Banerjee P, Milton DR, Jiang X, Ganesh C, Khazal S, et al. Blinatumomab maintenance after allogeneic hematopoietic cell transplantation for B-lineage acute lymphoblastic leukemia. Blood. (2022) 139:1908–19. doi: 10.1182/blood.2021013290
25. Nägele V, Kratzer A, Zugmaier G, Holland C, Hijazi Y, Topp MS, et al. Changes in clinical laboratory parameters and pharmacodynamic markers in response to blinatumomab treatment of patients with relapsed/refractory ALL. Exp Hematol Oncol. (2017) 6:14. doi: 10.1186/s40164-017-0074-5
26. Löffler A, Kufer P, Lutterbüse R, Zettl F, Daniel PT, Schwenkenbecher JM, et al. A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. (2000) 95:2098–103. doi: 10.1182/blood.V95.6.2098
27. Duminuco A, Markovic U, Parrinello NL, Lo Nigro L, Mauro E, Vetro C, et al. Potential clinical impact of T-cell lymphocyte kinetics monitoring in patients with B cell precursors acute lymphoblastic leukemia treated with blinatumomab: a single-center experience. Front Immunol. (2023) 14:1195734. doi: 10.3389/fimmu.2023.1195734
28. Aldoss I, Song J, Stiller T, Nguyen T, Palmer J, O’Donnell M, et al. Correlates of resistance and relapse during blinatumomab therapy for relapsed/refractory acute lymphoblastic leukemia. Am J Hematol. (2017) 92:858–65. doi: 10.1002/ajh.24783
29. Mejstríková E, Hrusak O, Borowitz MJ, Whitlock JA, Brethon B, Trippett TM, et al. CD19-negative relapse of pediatric B-cell precursor acute lymphoblastic leukemia following blinatumomab treatment. Blood Cancer J. (2017) 7:659. doi: 10.1038/s41408-017-0023-x
30. Jabbour E, Düll J, Yilmaz M, Khoury JD, Ravandi F, Jain N, et al. Outcome of patients with relapsed/refractory acute lymphoblastic leukemia after blinatumomab failure: no change in the level of CD19 expression. Am J Hematol. (2018) 93:371–4. doi: 10.1002/ajh.24987
Keywords: blinatumomab, B-cell acute lymphoblastic leukemia, children, minimal residual disease, relapsed/refractory, T cell activation
Citation: Zhang N, Hu W, Dai Y, Wang J, Qu L, Wang D, Liu B, Shao J, Shen S and Jiang H (2025) Blinatumomab demonstrates MRD eradication in MRD-positive/chemotherapy-delayed pediatric B-ALL and high response in relapsed/refractory cases: a multicenter cohort study. Front. Immunol. 16:1607138. doi: 10.3389/fimmu.2025.1607138
Received: 07 April 2025; Accepted: 28 August 2025;
Published: 18 September 2025.
Edited by:
Miroslawa Puskulluoglu, Maria Sklodowska-Curie National Research Institute of Oncology, PolandReviewed by:
Uros Markovic, University Hospital Polyclinic Vittorio Emanuele, ItalyMalgorzata Czogala, Jagiellonian University Medical College, Poland
Copyright © 2025 Zhang, Hu, Dai, Wang, Qu, Wang, Liu, Shao, Shen and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuhong Shen, c2hlbnNodWhvbmdAc2NtYy5jb20uY24=; Hui Jiang, amh1aTAxMTFAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Na Zhang
Na Zhang Wenting Hu
Wenting Hu Yunpeng Dai
Yunpeng Dai Jian Wang4†
Jian Wang4† Bingju Liu
Bingju Liu Shuhong Shen
Shuhong Shen


