- 1Department of Pharmacy, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Hubei Province Clinical Research Center for Precision Medicine for Critical Illness, Wuhan, China
- 3School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Cancer immunotherapy has emerged as a powerful strategy for clinical treatment of malignant cancers. Despite the advances, cancer immunotherapy has met several challenges such as the limited efficacy to small subsets of patients, and serious autoimmune side effects. Cancer vaccines that target neoantigens to direct and amplify immune responses against tumors, have shown their efficacy and safety in preclinical and clinical researches. The developed cancer vaccines mainly contained peptide vaccines, mRNA vaccine, cell vaccine and oncolytic virus vaccine. In the last decade, both peptide based vaccines and vesicle based vaccines have attracted enormous attention for personalized vaccine development due to their potent efficacy in different tumor models. Peptide based vesicles are one kind of vesicles that are modified with functional peptides to enhance the efficiency of immune response and anti-cancer effect. In this review, we will introduce the basic characteristics, classification and biological application of vesicles or peptide based cancer vaccines respectively. Then the design and construction of peptide based vesicles will be summarized. Finally, we concluded the biological applications of peptide based vesicles in various cancer types and analyzed the key obstacles to overcome for their clinical applications. We hope this review could provide a better understanding of the construction of peptide based vesicles and their prospects for clinical applications.
1 Introduction
Immunotherapy aims to enhance the natural immune system to eliminate malignant cells. The advent of cancer immunotherapy has a profound impact on the field of cancer treatment, significantly prolonging the survival of patients with malignant tumors and improving their quality of life (1). However, few patients can benefit from the currently available immunotherapies and many patients suffer from serious immune-related adverse events (2). In recent years, various forms of immunotherapy showed great potential for cancer immunotherapy, such as CAR-T cell therapy, peptide vaccines, mRNA vaccines, dendritic cell (DC) vaccines, oncolytic viruses (OVs) et al., shown in Figure 1 (3–5).
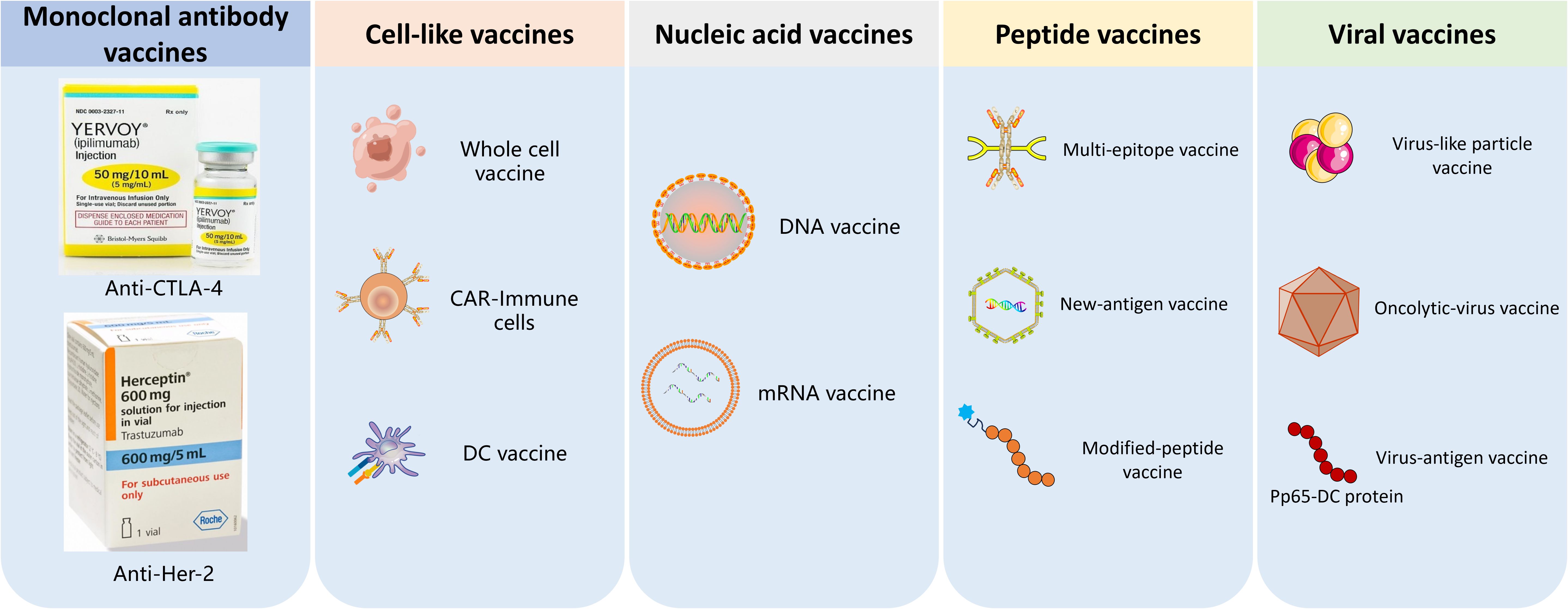
Figure 1. The classification of tumor vaccines. 1) Monoclonal antibodies, 2) Cell-like vaccines such as CRT-immune cells vaccines and DC vaccine; 3) Nucleic acid vaccines,; 4) Peptide vaccines; 5) oncolytic-virus vaccine and virus-antigen vaccine.
Extracellular vesicles have been proven to contribute to the remodeling of the immune-suppressive tumor microenvironment (TME), thereby influencing the efficacy of immunotherapy. In order to satisfy different demands for cancer therapy, extracellular vesicles are engineered by various methods, among which peptide based vesicles exhibit significant potential, characterized by key benefits from functional peptides such as high specificity for tumor targeting, excellent biocompatibility, and robust immune regulatory capabilities (6). Thus, peptide based vesicles are modified by various peptides which can modify the vesicle surface to accurately recognize receptors on tumor cell membranes, thereby enhancing drug accumulation in tumor tissues (6). Meanwhile, peptide based vesicles possess the capacity to modulate Therapeutic Drug Monitoring (TDM). It can counteract the immunosuppressive state in TDM by regulating the functions of tumor-associated immune cells. For example, IL4RPep-1 peptide (CRKRLDRNC) modified exosomes can specifically target M2-type tumor-associated macrophages (TAMs) and facilitate their conversion into anti-tumor M1-type macrophages (7). Furthermore, peptide vesicles exhibit excellent compatibility with the human physiological environment and are less prone to induce immunological rejection. They also possess significant biodegradability. After completing their drug delivery mission, peptide vesicles can spontaneously degrade into harmless small molecules within the body, which are then eliminated through standard metabolic pathways. This prevents long-term accumulation and mitigates the risk of long-term toxicity associated with residual materials (7). The properties of peptide based vesicles make them as potential drug candidate for cancer therapy. In this review, we will first introduce the function of peptide vaccines and vesicle vaccines respectively, and then introduce the design and construction of peptide based vesicles, next review their anti-cancer application, and last discuss their challenges for clinical translation and potential solutions to overcome them, shown as Figure 2.
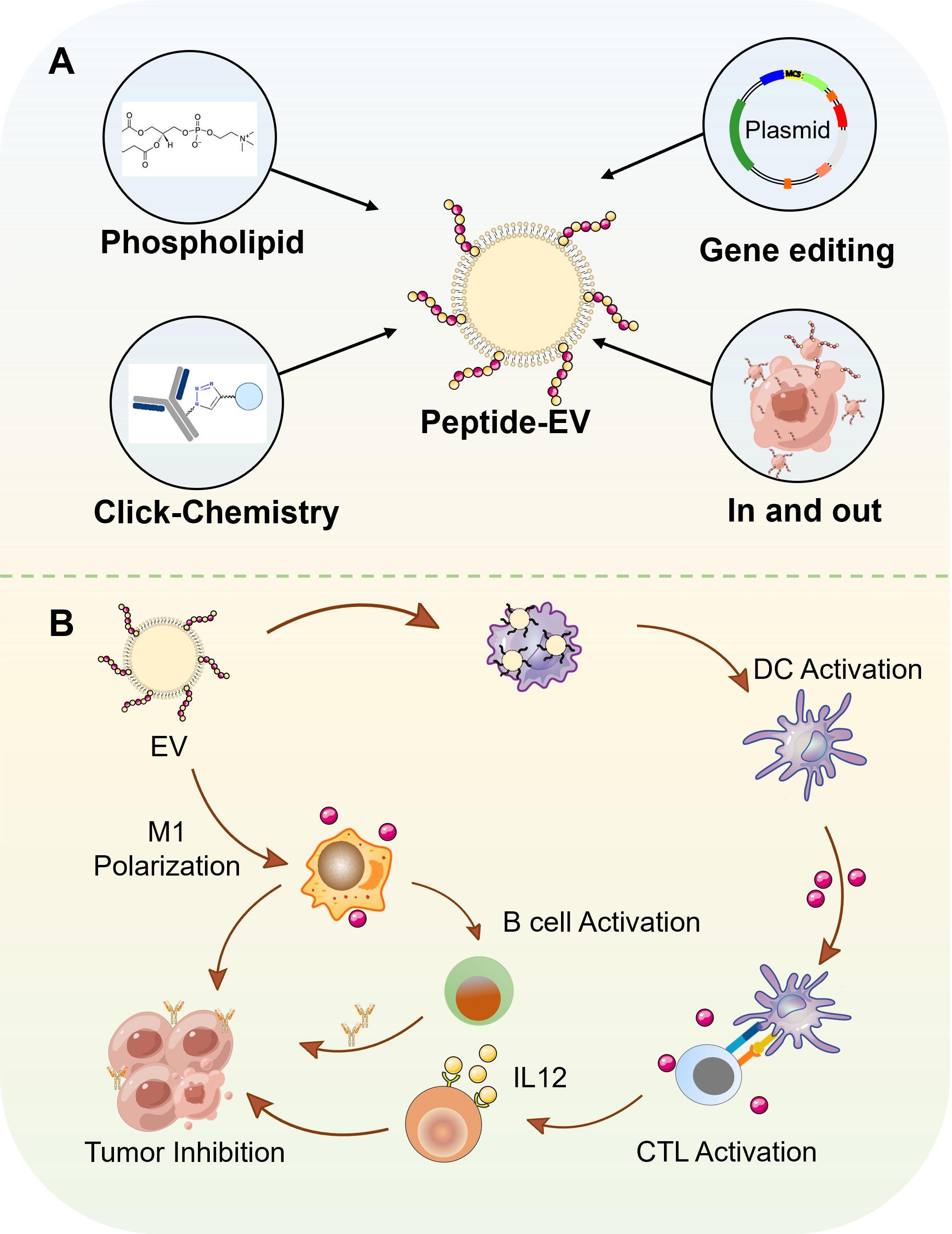
Figure 2. The various types of peptide-based vesicles and their application in cancer immunotherapy. (A) Preparation methods of peptide-based vesicles. 1) Phospholipid-modified peptide insertion. 2) Click chemistry-mediated conjugation. 3) Genetically engineered expression. 4) Endocytosis-exocytosis strategy. (B) Application of peptide-based vesicles in tumor immunotherapy. Peptide-based vesicles were internalized by DCs. Mature DCs present antigenic peptide-MHC complexes to naive T cells, activating cytotoxic T lymphocytes that migrate to tumor sites and induce apoptosis in malignant cells. In addition, M2-like tumor-associated macrophages uptake peptide-based vesicles, leading to their reprogramming into pro-inflammatory M1 phenotypes. M1 macrophages secrete cytokines to directly kill tumor cells. Furthermore, M1 macrophages further present tumor-associated antigens to B cells, promoting their differentiation into plasma cells.
2 Roles of vesicles in cancer immunotherapy
2.1 Basic characteristics of extracellular vesicles
EVs are one kind of nanometer-sized spherical hollow structures that can carry bioactive molecules and deliver them to recipient cells (8). Classic EVs can be broadly classified into exosomes, microvesicles, and apoptotic bodies. Exosomes, with a diameter of 50–100 nm, can be released by resting or stimulated cells and can transfer mRNA, miRNA, and oncogenic receptors, exhibiting antigen presentation, immune activation, and immune suppression activities (9) (10). Microvesicles, measuring 100–1000 nm in diameter, are produced by platelets (11), red blood cells (12), or epithelial cells (13) through outward budding of cell membranes and have procoagulant functions. Apoptotic bodies, with a diameter of 1–5 micrometers, are generated during cell apoptosis and can transfer DNA and oncogenes, presenting T-cell epitopes to immune cells when ingested by phagocytes, thereby exerting immune suppressive effects (8).
2.2 Immunological functions of cellular vesicles
Although most cells possess the ability to produce extracellular vesicles (EVs), not all EVs derived from cells can be used as carriers for drug delivery. The standards for drug delivery included the production yield, surface protein properties, size, and the composition in the vesicles. Currently, several cell types have been explored as potential donor sources for EVs used in drug delivery, such as dendritic cells (DC) (14), macrophage (15), tumor cells (9), red blood cells et al. (16) (12).
Tumor cell-derived EVs (TEVs), especially autologous TEVs, carry a similar repertoire of tumor antigens, co-stimulatory molecules, and DNA fragments as their parental cells (17, 18). This property can elicit robust T-cell-dependent anti-tumor immune responses and has demonstrated therapeutic effects in melanoma mouse models (19), hepatocellular carcinoma (20), and colon cancer (21). Compared to EVs produced by non-cancerous cells, TEVs can achieve tumor cell-specific targeting through intrinsic homotypic adhesion mediated by membrane surface antigens (22). In tumor therapy, TEVs play a significant role, such as enabling deep tumor penetration for drug delivery (23), exhibiting high specific homing capabilities, and activating the signal transducer and activator of transcription 3 pathway (24). Additionally, the in situ generation of micron-sized tumor cell-derived vesicles serves as an autologous tumor vaccine to enhance systemic immune responses (25), and functional DNA-modified cancer cell membrane vesicles are used as targeted vaccines for tumor immunotherapy (26). However, the role of TEVs in promoting cancer progression by enhancing cell proliferation and evading apoptosis, inducing angiogenesis, metabolic reprogramming, enhancing invasion and metastasis, and evading immune surveillance has been well-documented (27). Therefore, unlike exosomes from other sources, TEVs can be a double-edged sword when used as therapeutic agents in cancer treatment. A thorough elucidation of their formation, secretion, and network functions is urgently needed to realize this attractive and promising cancer treatment strategy, requiring more extensive in vivo studies with larger sample sizes to investigate the effectiveness and safety of TEVs as future drug delivery systems (DDS) (28).
Dendritic cells (DCs) are fundamental immune cells essential for antigen presentation and T cell activation. DC-derived vesicles (DEVs) maintain the basic immune stimulating ability of DCs (e.g., antigen presentation to T cells) (29). DEVs production processes are amenable to strict regulation and monitoring (e.g., easy determination of their composition and MHC-I and MHC-II contents) and pose lower risks associated with feasible cell or viral therapies (e.g., in vivo replication risks) (30, 31). In recent decades, DEV-based therapies have been widely applied in immunotherapy and drug delivery. For instance, in breast cancer treatment, DEVs can enhance cancer cell sensitivity to immune checkpoint inhibitors and prevent recurrence of resected tumors (15). DEVs can overcome biological barriers like the blood-brain barrier (BBB), making them attractive for future drug delivery (32).
Macrophage-derived EVs express functional immune regulatory proteins including MHC class I and II (33), preferentially inducing Th1-type (cell-mediated) immune responses that direct T cells to attack abnormal cells (e.g. cancer cells) or cells infected by intracellular parasites (34, 35). Macrophage-derived EVs also have extensive applications in tumor treatment. For example, macrophage-derived exosomes are thought to transfer miR-365, a key regulator of gemcitabine resistance in pancreatic cancer (36). M1-like macrophage-derived EVs (M1 EVs) are used to treat glioblastoma multiforme (37). In photodynamic therapy (PDT), fusion of M1 EVs with thylakoid membranes of natural plants imparts active tumor targeting ability to M1 EVs. Therefore, macrophage-derived EVs offer promising new strategies for tumor treatment.
Besides these cells, there are other candidates for drug delivery vesicles, such as those derived from red blood cells (RBCs) (38, 39), natural killer (NK) cells (40) and T cells (41). The CD47 on RBC-derived EVs interacts with its receptor, signal regulatory protein alpha (SIRPa) on macrophages, protecting the RBC-derived EVs from clearance by initiating a “don’t eat me” signal (42). NK cell-derived EVs contain tumor necrosis factor-α and granzyme B, exhibiting cytotoxic effects on glioma cells (40) and melanoma- cells (43) with no significant side effects in vitro and in vivo. Furthermore, studies have found that activated CD8+ T cells from healthy mice release cytotoxic EVs, leading to a significant reduction in tumor invasion and metastasis (44). EVs derived from CD4+ T cells enhance the anti-tumor response of CD8+ T cells by augmenting their proliferation and activity without affecting regulatory T cells.
Among various sources of EVs, RBCs exhibit distinct advantages in safety and scalable production due to their relatively low content of cellular components and ease of procurement (45). TEVs, while amenable to scalable production through in vitro expansion of tumor cells, possess surface markers enriched with tumor-specific antigens that may enhance tumor-targeting efficacy (23). However, their content (proteins, nucleic acids, etc.) may carry oncogenic risks, necessitating further improvements in biosafety (46). Immune cell-derived EVs (e.g., DCs, macrophages, or NK cells) retain functional biological properties inherent to their parent cells, enabling tailored therapeutic applications. Nevertheless, the scalability of immune cell-derived EVs remains constrained due to stringent ex vivo expansion requirements and high costs associated with isolating immune cells from biological systems (47). These factors collectively highlight the need to balance source-specific advantages with technical and safety considerations for clinical translation.
3 The role of peptides in tumor immunotherapy
3.1 Peptides as antigens
Peptide vaccines can be divided into two categories based on their activation functions, one group stimulates the innate immune system by interacting with tumor-associated macrophages (TAM), dendritic cells (DC), neutrophils, and natural killer (NK) cells, while the other group can activate the adaptive immune system by interacting with T cells and B cells (48–50).
For tumor-associated macrophages (TAM) in the innate immune system, TAMs exhibit two phenotypic activation states: the antitumor M1 and the protumor M2 (51–55). Currently, the main strategies to block M2-TAM activity involve inhibiting the recruitment of macrophages to tumors and converting M2-TAMs to M1-TAM. For example, researchers developed a biohybrid material with the ability to immunologically regulate TAM cell populations, using vascular endothelial growth factor (VEGF) mRNA interference-M2 targeting peptide. This material primarily blocks M2-TAM activity and cancer cell growth by inhibiting VEGF-related signaling pathways and triggering host immune responses that lead to sustained tumor regression, and it can also generate long-lasting antitumor immune memory (56).
As for DCs, Wang et al. selected the TRP2 peptide and the dodecamer CPP (AAVLLPVLLAAP) to prolong the presentation of MHC class I-restricted self-peptides on dendritic cells (DCs), thereby enhancing antitumor immune responses. CPP1 can effectively deliver the TRP2 peptide into mature DCs and retain the full capacity of DCs to present MHC-peptide complexes to antigen-specific T cells over an extended period. They demonstrated that immunizing mice with DCs loaded with TRP2-CPP1 conjugate led to complete protection against B16 tumor suppression, and lung metastasis inhibition (57).
The human body has three principal subtypes of mature T cells: cytotoxic T lymphocytes (CTLs), helper T cells, and regulatory T cells (Tregs).Immune checkpoint blockade is one of the major immunotherapies (58–64), which precisely targets tumor cells by blocking immune checkpoints such as CTLA-4 and PD-1. Researchers have discovered peptides that inhibit the PD-1/PD-L1 interaction and reactivate T cell function against tumor cells, including peptide-57, CLP001/CLP002, and PD-L1 Pep-1/PD-L1 Pep-2. These peptides not only reawaken T cells via their PD-L1 inhibiting activity but also utilize PD-L1 as a tumor target to deliver chemotherapeutic agents specifically to tumors exhibiting elevated PD-L1 expression.
3.2 Peptides as immune modulators
Anti-cancer peptide(ACP) are bioactive peptides that inhibit cancer cell growth via a spectrum of mechanisms. Lytic peptides are toxic molecules that kill cancer cells by disrupting cell membrane. Recent studies revealed that the cell fragments by lytic peptides can act as tumor antigens to trigger the immune response. For example, lytic peptide EP-100 offers a unique therapeutic option for patients demonstrating insufficient responses to immunotherapy for ovarian cancer (65). EP-100 is a synthetic fusion peptide composed of an LHRH ligand and a lytic peptide (CLIP-71) that specifically binds the LHRH receptor (LHRH-R) (66). As an immune enhancer, it induces PD-L1 synthesis in neoplastic cells, therefore altering the tumor microenvironment. This leads to an augmentation of immune cells that facilitate tumor lysis (CD8+ T cells, NK cells, dendritic cells, and macrophages) while diminishing immunosuppressive cells (Tregs, B cells, and mMDSCs). Targeted ACPs can inhibit immune-related signal pathways to modulate immune response. For example, A new peptide-based PROTAC has been developed to combat the prevalent resistance to PD-1/PD-L1 inhibitors in clinical contexts by degrading PD-1 or PD-L1, thus inducing cancer cell apoptosis (67).
3.3 Application of peptides as carriers in tumor immunotherapy
Peptide nanoparticles are widely acknowledged as an effective approach in cancer immunotherapy because of their exceptional stability and significant capability for delivering peptide antigens and immunological adjuvants (68, 69). Peptides and their derivatives can self-assemble into one-dimensional fibers or nanofibers, which can then interlace to form hydrogels or nanoparticles, enabling the targeted release of peptides and adjuvants (69). Collier et al. developed a vaccine utilizing the Q11 self-assembling domain (ac-qqkfqfqqfeqq-am) produced from chicken ovalbumin (OVA323-339) to incorporate MUC1-derived peptides (70). These immunizations stimulate the production of potent antibodies specifically targeting breast cancer cells. To improve the application of this technique in clinical therapy, Huang et al. created a novel synthetic self-adjuvant vaccine using a self-assembling Q11 domain (71). This vaccine can produce fibrous structures under mild conditions and display multivalent B-cell epitopes, thereby markedly enhancing their immunogenicity.
4 The role of peptide-based vesicle for cancer immunotherapy
Vesicles play dual roles in immune activation and anti-tumor treatment by serving as an autologous tumor vaccine to enhance systemic immune responses and a good vehicle for drug delivery. However, the limited tumor selectivity and immune stimulating ability have hindered their broad applications. As we mentioned above, functional peptides can act as warheads for tumor selective penetration, as peptide vaccines to enhance tumor immune response, as immune modulators to inhibit immune-related signal pathways. These properties can be used to overcome the limitations of vesicle based application. In this part, we will introduce the construction and applications of peptide based vesicles, particularly the roles of peptides in vesicles to enhance therapeutic effect.
4.1 Forms of peptide-based vesicle
The principal techniques for constructing peptide-based vesicles encompass direct loading via phosphatidylation or click chemistry; surface modification of gene-edited cellular vesicles; and the administration of peptides to immune cells, followed by the preparation of vesicles from these cells to commence the antigen presentation process (72).
Zhu et al. chemically crosslinked a dibenzobicyclooctyne (DBCO) moiety to the surface of dendritic cell-derived extracellular vesicles (EVs) and subsequently reacted it with azide-functionalized MUC1 glycopeptide by click chemistry, thereby covalently affixing MUC1 to the EV surface (73). As for lipid insertion, Ye et al. introduced a noteworthy methodology (74). The 4F-KLA-LDL peptide was synthesized by combining the pro-apoptotic peptide KLA with an LDL-targeting peptide, which selectively binds to the overexpressed LDL receptors on blood-brain barrier (BBB) and glioblastoma (GBM) cell lines. Based on the molecular recognition between phospholipids on EV and ApoA-I mimetic peptides, They developed methotrexate (MTX)-loaded EVs functionalized with 4F-KLA-LDL peptide, which can target low-density lipoprotein (LDL) on GBM cells and enhance the transport of the pro-apoptotic peptide KLA and methotrexate (MTX) to U87 glioma cells. As for genetic manipulation, EVs are typically equipped with these peptides in EV donor cells using transfection or retroviral/lentiviral infection (75). For instance, Ohno et al. reported a technique involving the expression of a fusion protein within HEK-293T cells using a retroviral plasmid (76). This fusion protein consists of the transmembrane domain of the platelet-derived growth factor receptor and a peptide that targets the epidermal growth factor receptor (EGFR), resulting in EGFR-targeted extracellular vesicles (EVs). These electric vehicles are engineered to transport the anti-cancer miRNA let-7 straight to breast tumor cells. As for the chemical engineering approach to covalently conjugate peptides to EVs, Nakase et al. chemically synthesize stearyl-modified octaarginine peptide solid-phase peptide synthesis. The stearyl group functioned as an anchoring unit for membrane insertion. This method facilitated straightforward alteration of the exosome membrane to promote macropinocytosis, markedly increasing cellular absorption of extracellular vesicles (EVs) and enabling efficient intracellular transport of the artificially encapsulated ribosome-inactivating protein saporin through EVs, therefore resulting in tumor cell apoptosis (77). Nevertheless, the severe chemical treatment of EV surfaces, which may result in detrimental functional degradation, has hindered the widespread adoption of these methods (78). As for affinity conjugation, there are various methods that use EV-binding peptides or antibodies to coat EVs. However, these conjugations are transient and unstable. He et al. designed chiral peptide Au (I) infinite covalent polymers (DPAICP) using D-peptides and Au³+26. They then ultracentrifuged milk-derived extracellular vesicles (ME) membranes with lactoprotein, embedded the chiral peptide supramolecular assemblies into the ME membrane, and obtained an artificial milk DPAICP@ME with pharmaceutical and absorbable properties. This approach restores the p53 signaling pathway for cancer therapy while further activating T cells and enhancing the efficacy of anti-PD-1 immunotherapy (79). As mentioned earlier, existing surface modification methods for EVs have various drawbacks in terms of safety, stability, and integrity (80). A stable and gentle method for EV coupling has been developed by Pham et al. who devised a novel technique utilizing protein ligases (including sortase A and OaAEP1 ligase) to covalently attach EVs to high-copy-number targeting moieties. The conjugation of EVs with EGFR-targeting peptides or anti-EGFR nanobodies facilitates their accumulation in EGFR-positive cancer cells both in vitro and in vivo. Furthermore, this methodology is applicable for conjugating EVs with peptides and nanobodies targeting other receptors, such as HER2 and SIRPα (81).
4.2 Anti-cancer applications of peptide-based vesicle
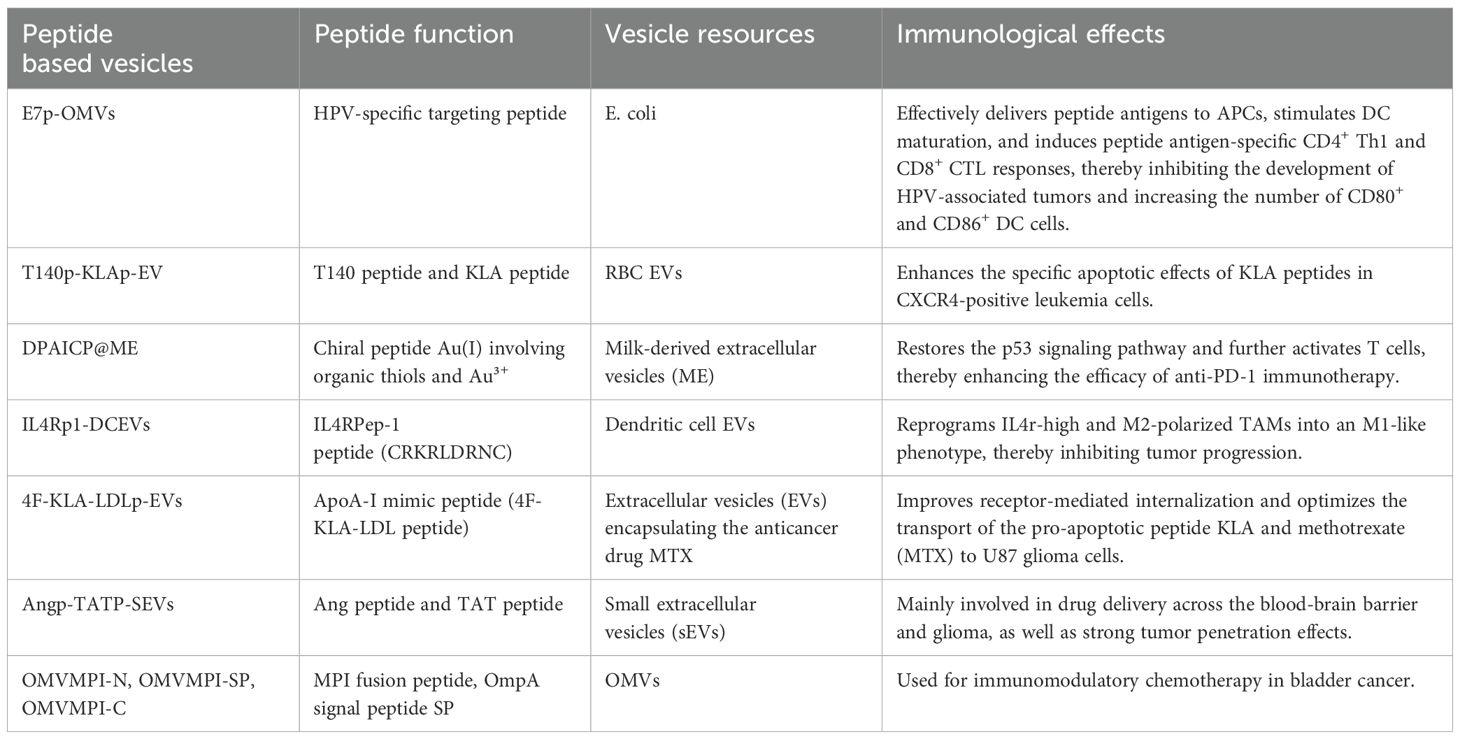
Different peptide based vesicles had quite different functions for cancer immunotherapy, which are concluded in Table 1. The synergistic effect of peptide based vesicles can be categorized into three parts: enhancing immune response by peptide antigen or targeted peptide, augmenting anti-cancer effect of toxic peptides, improving tumor targeted delivery by peptide ligands.
4.2.1 Enhancing immune response
The immune response of vesicles can be enhanced by peptide antigens or PD-L1 targeting peptides. For example, in cervical cancer, E7p-OMVs, entails the introduction of a plasmid encoding the peptide antigen E7p (amino acids 44-62) with CTL and Th cell epitopes into E. coli cells (6). This method facilitates the in vivo creation of E7p-encapsulated natural bacterial outer membrane vesicles (OMVs), which effectively transport peptide antigens to antigen-presenting cells (APCs), therefore impeding the progression of HPV-associated malignancies (6). In osteosarcoma, Wu et al. discovered that the interaction between NPM PD-L1 and IGFBP3 activates mTOR signaling and promotes osteosarcoma tumor growth through PGK1-mediated phosphorylation enhancement (82). They generated a PD-L1 phosphorylation-mimetic peptide incorporating the S279 location and encapsulated it within cRGD-modified RBCM vesicles to create peptide@cRGD-M. An effective peptide@cRGD-M nanoparticle method for osteosarcoma treatment was created by integrating erythrocyte membrane therapy with peptide therapy, thereby enhancing the anti-cancer effect.
4.2.2 Augmenting anti-cancer effect of toxic peptides
In bladder cancer, Ren et al. reported a bioengineered OMV-based platform using bacterial OMVs as nanocarriers to encapsulate toxic MPI fusion peptides generated by genetic engineering (83). MPI was conjugated to both the C- and N-termini of the fusion peptide to facilitate membrane integration. As MPI-N could not be encapsulated by OMVs, EVs were utilized to encapsulate MPI-N, which was introduced with the OmpA signal peptide SP. Three bioengineered outer membrane vesicles (OMVs) were ultimately produced: OMVMPI-N (with minimal MPI-N), OMVMPI-SP (with MPI-N obstructed by SP), and OMVMPI-C. These were utilized for immunomodulatory chemotherapy in bladder cancer, resulting in good therapeutic outcomes and biosafety. In leukemia, T140-KLA-EV was synthesized by covalently attaching T140 and KLA peptides to pre-existing RBCEV membrane proteins utilizing OaAEP1Cys247Ala. This construct diminishes the infiltration of leukemia cells in the spleen by augmenting the specific apoptotic effects of KLA peptides in CXCR4-positive leukemia cells, consequently decelerating disease progression and improving overall survival (84).
4.2.3 Improving tumor targeted delivery by peptide ligands
In glioblastoma, it has been documented that neuron-specific rabies virus glycoprotein (RVG) peptide-modified sEVs provide an efficient tissue-targeting delivery mechanism for the treatment of glioblastoma and Alzheimer’s disease (85, 86). Additionally, Zhu et al. developed functional Ang/TAT-sEVs-Dox by modifying sEVs with Ang peptide and TAT peptide (87). This system targets the blood-brain barrier and glioblastoma, penetrating both the barrier and the tumor. In lung cancer, Pham et al. conjugated RBCEVs with EGFR-targeting peptides using sortase A and OaAEP1 ligase (81). This method facilitates the targeted absorption of RBCEVs by EGFR-positive cells. Additionally, RBCEVs treated with paclitaxel (PTX) demonstrated substantial antitumor efficacy at low dosages (10–20 times lower than therapeutically equivalent doses) against EGFR-positive lung cancer. This technique is likewise pertinent to the conjugation of extracellular vesicles with peptides and nanobodies that target alternative receptors (e.g., HER2 and SIRPα) for precise medication delivery to pertinent malignancies. In prostate cancer, Diao et al. reported a novel strategy using cationic membrane-penetrating peptide TAT to encapsulate siRNA into EVs (87). Three TAT peptides were co-expressed with DRBD as a 3TD (TAT-TAT-TAT-DRBD) chimeric protein. The sequence-independent binding of DRBD enabled the multiplexing of siRNA for targeting several genes, yielding more potent therapeutic effects compared to single-gene targeting inhibitors. The concurrent siRNA-mediated silencing of the FLOH1, NKX3, and DHRS7 genes demonstrated considerable promise for enhancing CRPC treatment, offering a novel approach for CRPC therapy.
5 The challenges and future direction of peptide based vesicles
Peptide based vesicles, as an innovative approach for cancer immunotherapy, have shown considerable promise in drug delivery and immunotherapeutic applications. Nonetheless, their clinical application encounters several obstacles, including safety, immunogenicity, stability, targeting ability, drug releasing, size and product preparation and manufacturing. Safety stands as the paramount concern in advancing peptide based vesicles toward clinical applications. Cell-derived vesicles inherently carry biological information from their parent cells, which endows these vesicles with unique biological functions while simultaneously introducing potential safety hazards. For instance, vesicles originating from tumor cells carry genetic material from their parent tumor cells, posing a latent carcinogenic risk. Current research strategies predominantly focus on isolating exosomes or fabricating vesicles through cell membrane extraction. However, these methodologies inevitably amplify procedural complexity and compromise product uniformity, thereby representing significant challenges in therapeutic development. As for the intrinsic low immunogenicity of peptide vesicles, combination therapy could be a method to overcome it. Currently, researchers have identified many peptides with strong affinity for PD-L1 or CTLA-4 using phage display method. These peptides can be affixed to the surface of vesicles and transported to the tumor microenvironment. Peptide vesicles can transport immunomodulatory molecules, such as cytokines or short interfering RNAs, and deliver them to the tumor microenvironment via targeted administration, thereby altering the immune milieu. Concerning the scalability of peptide-vesicle conjugates, enzymatic techniques exhibit a certain degree of transformability for extracellular vesicles produced from alternative cellular sources (81). This presents novel concepts for the synthesis of various peptide-vesicle conjugates and offers direction for the formulation of peptide-vesicle combinations. In addition, size and product preparation and manufacturing is an essential factor to consider, the current vesicle separation method is mainly ultracentrifugation, however, this method is expensive and the sample processing capacity per batch is limited. The dimensions of peptide based vesicles substantially influence their in vivo dispersion and targeting efficacy. Larger vesicles may encounter difficulties in traversing the thick tumor extracellular matrix (ECM), whereas smaller vesicles may be swiftly eliminated. Furthermore, size heterogeneity may result in unpredictable medication release. To tackle these challenges, various strategies may be employed: optimizing synthesis processes, such as solvent-switching or self-assembly techniques, to accurately regulate vesicle size, creating intelligent responsive designs that leverage pH or temperature variations to modulate vesicle behavior, or utilizing nanoencapsulation to improve stability and control release.
6 Conclusion and discussion
Peptide-based vesicles have versatile roles in cancer immunotherapy due to the incorporation of peptides into vesicles, including the immune checkpoint blockade, modulating the tumor microenvironment, enhancing their delivery specificity, activating immune cells et al. As extracellular vesicles (EVs) lack target-specificity, peptide ligands targeting cancer cell surface can be used for efficient EV delivery. For example, Tin et al. conjugated EVs with an epidermal growth factor receptor (EGFR)-targeting peptide and found EGFR targeting EVs facilitates their accumulation in EGFR-positive cancer cells both in vitro and in vivo. This peptide based vesicles significantly increases drug efficacy in a xenografted mouse model of EGFR-positive lung cancer at a low dose (81). The anti-cancer peptides can also be loaded into vesicles to enhance their cancer immunotherapeutic effects. For example, Tang et al. developed cRGD-functionalized chimaeric vehicle for LTX-315 delivery, which in combination with CpG adjuvant and anti-PD-1 boost immunotherapy of malignant B16F10 melanoma in mice (88). This combination was proved to secret IL-6, IFN-γ and TNF-α, tumor infiltration of CD8+CTLs and Th, and induction of TEM and TCMin spleen. Peptide antigens are good tools to enhance the cancer immunity of vesicles. For example, peptide antigen E7p modified EVs could effectively transport peptide antigens to antigen-presenting cells (APCs), promote dendritic cell (DC) maturation, and elicit peptide antigen-specific CD4+ T helper 1 (Th1) and CD8+ cytotoxic T lymphocyte (CTL) responses, therefore impeding the progression of HPV-associated malignancies.
This article provides a detailed overview of the applications and underlying mechanisms of vesicles from different cell sources in cancer therapy, as well as the application of peptides with immune activation and modulation functions in cancer treatment. From preparation methods to application mechanisms, the research on peptide-vesicle composite carriers in cancer immunotherapy is further explored. However, despite extensive research by many scholars on the application of peptide-vesicle composite carriers in cancer treatment, their clinical application still faces many obstacles. Future research should focus on how to further improve targeting to tumor tissues, enhance biocompatibility, simplify the formulation process, streamline storage and transportation conditions, and improve biosafety. These challenges need to be overcome through further research and technological innovation to promote the successful clinical application of peptide-modified vesicles.
Author contributions
YY: Conceptualization, Formal analysis, Software, Validation, Writing – original draft. JL: Investigation, Software, Writing – original draft. YM: Investigation, Software, Writing – original draft. CS: Conceptualization, Supervision, Validation, Writing – review & editing. DW: Conceptualization, Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by National Natural Science Foundation of China (22007033).
Acknowledgments
We would like to thank everyone who supported in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Szeto GL and Finley S D. Integrative approaches to cancer immunotherapy. Trends Cancer. (2019) 5:400–10. doi: 10.1016/j.trecan.2019.05.010
2. Martin JD, Cabral H, Stylianopoulos T, and Jain R K. Improving cancer immunotherapy using nanomedicines: progress, opportunities and challenges. Nat Rev Clin Oncol. (2020) 17:251–66. doi: 10.1038/s41571-019-0308-z
3. Zhang Y and Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. (2020) 17:807–21. doi: 10.1038/s41423-020-0488-6
4. Liu C, Shi Q, Huang X, Koo S, Kong N, and Tao W. mRNA-based cancer therapeutics. Nat Rev Cancer. (2023) 23:526–43. doi: 10.1038/s41568-023-00586-2
5. Wang S, Liang B, Wang W, Li L, Feng N, Zhao Y, et al. Viral vectored vaccines: design, development, preventive and therapeutic applications in human diseases. Signal transduction targeted Ther. (2023) 8:149. doi: 10.1038/s41392-023-01408-5
6. Chen H, Zheng X, Li L, Huang L, Huang W, and Ma Y. Peptide-based therapeutic HPV cancer vaccine synthesized via bacterial outer membrane vesicles. Int J nanomedicine. (2023) 18:4541–54. doi: 10.2147/ijn.S416706
7. Gangadaran P, Gunassekaran GR, Rajendran RL, Oh JM, Vadevoo SMP, Lee HW, et al. Interleukin-4 receptor targeting peptide decorated extracellular vesicles as a platform for in vivo drug delivery to thyroid cancer. Biomedicines. (2022) 10:1987. doi: 10.3390/biomedicines10081978
8. van Niel G, D’Angelo G, and Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. (2018) 19:213–28. doi: 10.1038/nrm.2017.125
9. Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, and Lyden D. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell. (2016) 30:836–48. doi: 10.1016/j.ccell.2016.10.009
10. Marar C, Starich B, and Wirtz D. Extracellular vesicles in immunomodulation and tumor progression. Nat Immunol. (2021) 22:560–70. doi: 10.1038/s41590-021-00899-0
11. Lazar S and Goldfinger L E. Platelets and extracellular vesicles and their cross talk with cancer. Blood. (2021) 137:3192–200. doi: 10.1182/blood.2019004119
12. Blow F and Buck A H. Extracellular vesicles from malaria-infected red blood cells: not all are secreted equal. EMBO Rep. (2022) 23:e55499. doi: 10.15252/embr.202255499
13. Díaz-Garrido N, Badia J, and Baldomà L. Microbiota-derived extracellular vesicles in interkingdom communication in the gut. J extracellular vesicles. (2021) 10:e12161. doi: 10.1002/jev2.12161
14. Schioppa T, Gaudenzi C, Zucchi G, Piserà A, Vahidi Y, Tiberio L, et al. Extracellular vesicles at the crossroad between cancer progression and immunotherapy: focus on dendritic cells. J Trans Med. (2024) 22:691. doi: 10.1186/s12967-024-05457-4
15. Ding JY, Chen MJ, Wu LF, Shu GF, Fang SJ, Li ZY, et al. Mesenchymal stem cell-derived extracellular vesicles in skin wound healing: roles, opportunities and challenges. Military Med Res. (2023) 10:36. doi: 10.1186/s40779-023-00472-w
16. Meng W, He C, Hao Y, Wang L, Li L, and Zhu G. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug delivery. (2020) 27:585–98. doi: 10.1080/10717544.2020.1748758
17. Qiu X, Li Z, Han X, Zhen L, Luo C, Liu M, et al. Tumor-derived nanovesicles promote lung distribution of the therapeutic nanovector through repression of Kupffer cell-mediated phagocytosis. Theranostics. (2019) 9:2618–36. doi: 10.7150/thno.32363
18. Rahbarghazi R, Jabbari N, Sani NA, Asghari R, Salimi L, Kalashani SA, et al. Tumor-derived extracellular vesicles: reliable tools for Cancer diagnosis and clinical applications. Cell communication signaling: CCS. (2019) 17:73. doi: 10.1186/s12964-019-0390-y
19. Mannavola F, Tucci M, Felici C, Passarelli A, D’Oronzo S, and Silvestris F. Tumor-derived exosomes promote the in vitro osteotropism of melanoma cells by activating the SDF-1/CXCR4/CXCR7 axis. J Trans Med. (2019) 17:230. doi: 10.1186/s12967-019-1982-4
20. Moris D, Beal EW, Chakedis J, Burkhart RA, Schmidt C, Dillhoff M, et al. Role of exosomes in treatment of hepatocellular carcinoma. Surg Oncol. (2017) 26:219–28. doi: 10.1016/j.suronc.2017.04.005
21. Teng Y, Ren Y, Hu X, Mu J, Samykutty A, Zhuang X, et al. MVP-mediated exosomal sorting of miR-193a promotes colon cancer progression. Nat Commun. (2017) 8:14448. doi: 10.1038/ncomms14448
22. Aslan C, Maralbashi S, Salari F, Kahroba H, Sigaroodi F, Kazemi T, et al. Tumor-derived exosomes: Implication in angiogenesis and antiangiogenesis cancer therapy. J Cell Physiol. (2019) 234:16885–903. doi: 10.1002/jcp.28374
23. Gao M, Sun Q, Zhang R, Shan G, Zhang H, Peng R, et al. Extracellular vesicles-hitchhiking boosts the deep penetration of drugs to amplify anti-tumor efficacy. Biomaterials. (2025) 314:122829. doi: 10.1016/j.biomaterials.2024.122829
24. Peng L, Sferruzza G, Yang L, Zhou L, and Chen S. CAR-T and CAR-NK as cellular cancer immunotherapy for solid tumors. Cell Mol Immunol. (2024) 21:1089–108. doi: 10.1038/s41423-024-01207-0
25. Guo Y, Wang SZ, Zhang X, Jia HR, Zhu YX, Zhang X, et al. In situ generation of micrometer-sized tumor cell-derived vesicles as autologous cancer vaccines for boosting systemic immune responses. Nat Commun. (2022) 13:6534. doi: 10.1038/s41467-022-33831-7
26. Liu B, Yang Y, Chao Y, Xiao Z, Xu J, Wang C, et al. Equipping cancer cell membrane vesicles with functional DNA as a targeted vaccine for cancer immunotherapy. Nano Lett. (2021) 21:9410–8. doi: 10.1021/acs.nanolett.1c02582
27. Meng W, Hao Y, He C, Li L, and Zhu G. Exosome-orchestrated hypoxic tumor microenvironment. Mol Cancer. (2019) 18:57. doi: 10.1186/s12943-019-0982-6
28. Sun W, Luo JD, Jiang H, and Duan D D. Tumor exosomes: a double-edged sword in cancer therapy. Acta pharmacologica Sin. (2018) 39:534–41. doi: 10.1038/aps.2018.17
29. Andre F, Escudier B, Angevin E, Tursz T, and Zitvogel L. Exosomes for cancer immunotherapy. Ann oncology: Off J Eur Soc Med Oncol. (2004) 15 Suppl:4iv141–4. doi: 10.1093/annonc/mdh918
30. Zhang B, Yin Y, Lai RC, and Lim S K. Immunotherapeutic potential of extracellular vesicles. Front Immunol. (2014) 5:5518. doi: 10.3389/fimmu.2014.00518
31. Gabrilovich DI, Ciernik IF, and Carbone D P. Dendritic cells in antitumor immune responses. I. Defective antigen presentation in tumor-bearing hosts. Cell Immunol. (1996) 170:101–10. doi: 10.1006/cimm.1996.0139
32. Khan AR, Yang X, Fu M, and Zhai G. Recent progress of drug nanoformulations targeting to brain. J Controlled release: Off J Controlled Release Soc. (2018) 291:37–64. doi: 10.1016/j.jconrel.2018.10.004
33. Pitt JM, André F, Amigorena S, Soria JC, Eggermont A, Kroemer G, et al. Dendritic cell-derived exosomes for cancer therapy. J Clin Invest. (2016) 126:1224–32. doi: 10.1172/jci81137
34. Segura E, Amigorena S, and Théry C. Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses. Blood cells molecules Dis. (2005) 35:89–93. doi: 10.1016/j.bcmd.2005.05.003
35. Yao Y, Chen L, Wei W, Deng X, Ma L, and Hao S. Tumor cell-derived exosome-targeted dendritic cells stimulate stronger CD8+ CTL responses and antitumor immunities. Biochem Biophys Res Commun. (2013) 436:60–5. doi: 10.1016/j.bbrc.2013.05.058
36. Binenbaum Y, Fridman E, Yaari Z, Milman N, Schroeder A, Ben David G, et al. Transfer of miRNA in macrophage-derived exosomes induces drug resistance in pancreatic adenocarcinoma. Cancer Res. (2018) 78:5287–99. doi: 10.1158/0008-5472.Can-18-0124
37. Li Y, Basar R, Wang G, Liu E, Moyes JS, Li L, et al. KIR-based inhibitory CARs overcome CAR-NK cell trogocytosis-mediated fratricide and tumor escape. Nat Med. (2022) 28:2133–44. doi: 10.1038/s41591-022-02003-x
38. Kuo WP, Tigges JC, Toxavidis V, and Ghiran I. Red blood cells: A source of extracellular vesicles. Methods Mol Biol (Clifton N.J.). (2017) 1660:15–22. doi: 10.1007/978-1-4939-7253-1_2
39. Zhang KL, Wang YJ, Sun J, Zhou J, Xing C, Huang G, et al. Artificial chimeric exosomes for anti-phagocytosis and targeted cancer therapy. Chem Sci. (2019) 10:1555–61. doi: 10.1039/c8sc03224f
40. Zhu L, Oh JM, Gangadaran P, Kalimuthu S, Baek SH, Jeong SY, et al. Targeting and therapy of glioblastoma in a mouse model using exosomes derived from natural killer cells. Front Immunol. (2018) 9:824. doi: 10.3389/fimmu.2018.00824
41. Lu J, Wu J, Tian J, and Wang S. Role of T cell-derived exosomes in immunoregulation. Immunologic Res. (2018) 66:313–22. doi: 10.1007/s12026-018-9000-0
42. Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. (2014) 35:2383–90. doi: 10.1016/j.biomaterials.2013.11.083
43. Zhu L, Kalimuthu S, Gangadaran P, Oh JM, Lee HW, Baek SH, et al. Exosomes derived from natural killer cells exert therapeutic effect in melanoma. Theranostics. (2017) 7:2732–45. doi: 10.7150/thno.18752
44. Seo N, Shirakura Y, Tahara Y, Momose F, Harada N, Ikeda H, et al. Activated CD8(+) T cell extracellular vesicles prevent tumour progression by targeting of lesional mesenchymal cells. Nat Commun. (2018) 9:435. doi: 10.1038/s41467-018-02865-1
45. Bai S, Wang Z, Zhang Y, Yang Y, Wei Y, Luo Y, et al. iRGD-TRP-PK1-modified red blood cell membrane vesicles as a new chemotherapeutic drug delivery and targeting system in head and neck cancer. Theranostics. (2025) 15:86–102. doi: 10.7150/thno.99481
46. Zhang C, Qin C, Dewanjee S, Bhattacharya H, Chakraborty P, Jha NK, et al. Tumor-derived small extracellular vesicles in cancer invasion and metastasis: molecular mechanisms, and clinical significance. Mol Cancer. (2024) 23:18. doi: 10.1186/s12943-024-01932-0
47. Kalluri R. The biology and function of extracellular vesicles in immune response and immunity. Immunity. (2024) 57:1752–68. doi: 10.1016/j.immuni.2024.07.009
48. Chen J, Yao Y, Gong C, Yu F, Su S, Chen J, et al. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell. (2011) 19:541–55. doi: 10.1016/j.ccr.2011.02.006
49. Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A, and Moon J J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat materials. (2017) 16:489–96. doi: 10.1038/nmat4822
50. Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. (1998) 4:321–7. doi: 10.1038/nm0398-321
51. Gjertsen MK, Bakka A, Breivik J, Saeterdal I, Solheim BG, Søreide O, et al. Vaccination with mutant ras peptides and induction of T-cell responsiveness in pancreatic carcinoma patients carrying the corresponding RAS mutation. Lancet (London England). (1995) 346:1399–400. doi: 10.1016/s0140-6736(95)92408-6
52. Gordon S and Martinez F O. Alternative activation of macrophages: mechanism and functions. Immunity. (2010) 32:593–604. doi: 10.1016/j.immuni.2010.05.007
53. Martinez FO, Helming L, and Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. (2009) 27:451–83. doi: 10.1146/annurev.immunol.021908.132532
54. Varin A and Gordon S. Alternative activation of macrophages: immune function and cellular biology. Immunobiology. (2009) 214:630–41. doi: 10.1016/j.imbio.2008.11.009
55. Gao S, Yang D, Fang Y, Lin X, Jin X, Wang Q, et al. Engineering nanoparticles for targeted remodeling of the tumor microenvironment to improve cancer immunotherapy. Theranostics. (2019) 9:126–51. doi: 10.7150/thno.29431
56. Conde J, Bao C, Tan Y, Cui D, Edelman ER, Azevedo HS, et al. Dual targeted immunotherapy via in vivo delivery of biohybrid RNAi-peptide nanoparticles to tumour-associated macrophages and cancer cells. Advanced Funct materials. (2015) 25:4183–94. doi: 10.1002/adfm.201501283
57. Wang RF and Wang H Y. Enhancement of antitumor immunity by prolonging antigen presentation on dendritic cells. Nat Biotechnol. (2002) 20:149–54. doi: 10.1038/nbt0202-149
58. Desrichard A, Snyder A, and Chan T A. Cancer neoantigens and applications for immunotherapy. Clin Cancer Res. (2016) 22:807–12. doi: 10.1158/1078-0432.Ccr-14-3175
59. Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. New Engl J Med. (2015) 372:2006–17. doi: 10.1056/NEJMoa1414428
60. Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. New Engl J Med. (2011) 364:2119–27. doi: 10.1056/NEJMoa1012863
61. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. New Engl J Med. (2015) 372:2521–32. doi: 10.1056/NEJMoa1503093
62. Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. New Engl J Med. (2013) 369:134–44. doi: 10.1056/NEJMoa1305133
63. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. New Engl J Med. (2015) 373:123–35. doi: 10.1056/NEJMoa1504627
64. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. New Engl J Med. (2015) 372:2509–20. doi: 10.1056/NEJMoa1500596
65. Kim MS, Ma S, Chelariu-Raicu A, Leuschner C, Alila HW, Lee S, et al. Enhanced immunotherapy with LHRH-R targeted lytic peptide in ovarian cancer. Mol Cancer Ther. (2020) 19:2396–406. doi: 10.1158/1535-7163.Mct-20-0030
66. Curtis KK, Sarantopoulos J, Northfelt DW, Weiss GJ, Barnhart KM, Whisnant JK, et al. Novel LHRH-receptor-targeted cytolytic peptide, EP-100: first-in-human phase I study in patients with advanced LHRH-receptor-expressing solid tumors. Cancer chemotherapy Pharmacol. (2014) 73:931–41. doi: 10.1007/s00280-014-2424-x
67. Dai MY, Shi YY, Wang AJ, Liu XL, Liu M, and Cai H B. High-potency PD-1/PD-L1 degradation induced by Peptide-PROTAC in human cancer cells. Cell Death Dis. (2022) 13:924. doi: 10.1038/s41419-022-05375-7
68. Koutsopoulos S, Unsworth LD, Nagai Y, and Zhang S. Controlled release of functional proteins through designer self-assembling peptide nanofiber hydrogel scaffold. Proc Natl Acad Sci United States America. (2009) 106:4623–8. doi: 10.1073/pnas.0807506106
69. Lyu Y and Azevedo H S. Supramolecular hydrogels for protein delivery in tissue engineering. Molecules (Basel Switzerland). (2021) 26:873. doi: 10.3390/molecules26040873
70. Rudra JS, Tian YF, Jung JP, and Collier J H. A self-assembling peptide acting as an immune adjuvant. Proc Natl Acad Sci United States America. (2010) 107:622–7. doi: 10.1073/pnas.0912124107
71. Huang ZH, Shi L, Ma JW, Sun ZY, Cai H, Chen YX, et al. A totally synthetic, self-assembling, adjuvant-free MUC1 glycopeptide vaccine for cancer therapy. J Am Chem Soc. (2012) 134:8730–3. doi: 10.1021/ja211725s
72. Kostyushev D, Kostyusheva A, Brezgin S, Smirnov V, Volchkova E, Lukashev A, et al. Gene editing by extracellular vesicles. Int J Mol Sci. (2020) 21:7362. doi: 10.3390/ijms21197362
73. Zhu H, Wang K, Wang Z, Wang D, Yin X, Liu Y, et al. An efficient and safe MUC1-dendritic cell-derived exosome conjugate vaccine elicits potent cellular and humoral immunity and tumor inhibition in vivo. Acta biomaterialia. (2022) 138:491–504. doi: 10.1016/j.actbio.2021.10.041
74. Ye Z, Zhang T, He W, Jin H, Liu C, Yang Z, et al. Methotrexate-loaded extracellular vesicles functionalized with therapeutic and targeted peptides for the treatment of glioblastoma multiforme. ACS Appl materials interfaces. (2018) 10:12341–50. doi: 10.1021/acsami.7b18135
75. EL Andaloussi S, Mäger I, Breakefield XO, and Wood M J. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. (2013) 12:347–57. doi: 10.1038/nrd3978
76. Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol therapy: J Am Soc Gene Ther. (2013) 21:185–91. doi: 10.1038/mt.2012.180
77. Nakase I, Noguchi K, Fujii I, and Futaki S. Vectorization of biomacromolecules into cells using extracellular vesicles with enhanced internalization induced by macropinocytosis. Sci Rep. (2016) 6:34937. doi: 10.1038/srep34937
78. Armstrong JP, Holme MN, and Stevens M M. Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS nano. (2017) 11:69–83. doi: 10.1021/acsnano.6b07607
79. He W, Zhang Z, Yang W, Zheng X, You W, Yao Y, et al. Turing milk into pro-apoptotic oral nanotherapeutic: De novo bionic chiral-peptide supramolecule for cancer targeted and immunological therapy. Theranostics. (2022) 12:2322–34. doi: 10.7150/thno.70568
80. Jayasinghe MK, Pirisinu M, Yang Y, Peng B, Pham TT, Lee CY, et al. Surface-engineered extracellular vesicles for targeted delivery of therapeutic RNAs and peptides for cancer therapy. Theranostics. (2022) 12:3288–315. doi: 10.7150/thno.68667
81. Pham TC, Jayasinghe MK, Pham TT, Yang Y, Wei L, Usman WM, et al. Covalent conjugation of extracellular vesicles with peptides and nanobodies for targeted therapeutic delivery. J extracellular vesicles. (2021) 10:e12057. doi: 10.1002/jev2.12057
82. Wu W, Guo H, Jing D, Zhang Z, Zhang Z, Pu F, et al. Targeted delivery of PD-L1-derived phosphorylation-mimicking peptides by engineered biomimetic nanovesicles to enhance osteosarcoma treatment. Advanced healthcare materials. (2022) 11:e2200955. doi: 10.1002/adhm.202200955
83. Ren C, Li Y, Cong Z, Li Z, Xie L, and Wu S. Bioengineered bacterial outer membrane vesicles encapsulated Polybia-mastoparan I fusion peptide as a promising nanoplatform for bladder cancer immune-modulatory chemotherapy. Front Immunol. (2023) 14:1129771. doi: 10.3389/fimmu.2023.1129771
84. Yang R, Wong YH, Nguyen GKT, Tam JP, Lescar J, and Wu B. Engineering a catalytically efficient recombinant protein ligase. J Am Chem Soc. (2017) 139:5351–8. doi: 10.1021/jacs.6b12637
85. Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, and Wood M J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. (2011) 29:341–5. doi: 10.1038/nbt.1807
86. Zhu Q, Ling X, Yang Y, Zhang J, Li Q, Niu X, et al. Embryonic stem cells-derived exosomes endowed with targeting properties as chemotherapeutics delivery vehicles for glioblastoma therapy. Advanced Sci (Weinheim Baden-Wurttemberg Germany). (2019) 6:1801899. doi: 10.1002/advs.201801899
87. Zhu Z, Zhai Y, Hao Y, Wang Q, Han F, Zheng W, et al. Specific anti-glioma targeted-delivery strategy of engineered small extracellular vesicles dual-functionalised by Angiopep-2 and TAT peptides. J extracellular vesicles. (2022) 11:e12255. doi: 10.1002/jev2.12255
88. Xia Y, Wei J, Zhao S, Guo B, Meng F, Klumperman B, et al. Systemic administration of polymersomal oncolytic peptide LTX-315 combining with CpG adjuvant and anti-PD-1 antibody boosts immunotherapy of melanoma. J Controlled release: Off J Controlled Release Soc. (2021) 336:262–73. doi: 10.1016/j.jconrel.2021.06.032
Keywords: cancer immunotherapy, peptide vaccine, vesicle, peptide based vesicle, anti-cancer peptide
Citation: Yu Y, Lyu J, Muhadaisi Y, Shi C and Wang D (2025) Peptide based vesicles for cancer immunotherapy: design, construction and applications. Front. Immunol. 16:1609162. doi: 10.3389/fimmu.2025.1609162
Received: 10 April 2025; Accepted: 08 May 2025;
Published: 27 May 2025.
Edited by:
Yufen Xiao, University of Texas Southwestern Medical Center, United StatesReviewed by:
Zexiang Chen, University of Texas Southwestern Medical Center, United StatesCopyright © 2025 Yu, Lyu, Muhadaisi, Shi and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongyuan Wang, d2FuZ2R5MjAxOUBodXN0LmVkdS5jbg==; Chen Shi, d2h4aGNoZW5AMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yulin Yu1,2†
Yulin Yu1,2† Chen Shi
Chen Shi Dongyuan Wang
Dongyuan Wang