- 1Department of Infectious Diseases, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Institute of Infectious Diseases and Immunity, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Despite the successful implementation of prophylactic vaccines, hepatitis B virus (HBV) continues to affect over 350 million individuals globally. It remains a predominant etiology of end-stage liver pathologies, including liver cirrhosis and hepatocellular carcinoma (HCC). While nucleos(t)ide analog (NUC) therapies effectively suppress viral replication, functional cure is achieved in less than 1% of patients annually. Given that viral clearance fundamentally requires reconstitution of antiviral immunity, emerging therapeutic paradigms necessitate combinatorial strategies integrating direct-acting antiviral agents with immunomodulatory interventions. Substantial research efforts have been directed toward elucidating the immunological mechanisms underlying HBV persistence during chronic infection. This review systematically summarizes the functional impairment of innate immune populations and unconventional T cell subsets across distinct clinical phases of chronic HBV infection, and characterizes longitudinal immune reconstitution patterns following antiviral treatments. Our review identifies potential immunological biomarkers and provides a mechanistic framework for developing targeted immunotherapies to achieve durable HBV control.
1 Introduction
Hepatitis B virus (HBV) remains a major global health challenge, chronically infecting an estimated 296 million people worldwide (1). Persistent HBV infection poses a significant risk for progression to end-stage liver diseases including cirrhosis, liver failure, and hepatocellular carcinoma. Current first-line antiviral therapies comprise two distinct modalities: pegylated interferon-α (PEG-IFN-α) and nucleos(t)ide analogs (NUCs). While PEG-IFN-α demonstrates the potential to induce HBsAg seroclearance in 10-30% of patients within defined treatment durations, its clinical utility is constrained by frequent severe adverse effects and the necessity for subcutaneous administration (2). In contrast, NUCs have gained widespread acceptance due to their oral dosing regimen and favorable safety profile. Despite these advantages, NUCs exhibit limited efficacy in achieving functional cure (defined as HBsAg loss) and require careful clinical management. Premature treatment discontinuation may trigger virological relapse with subsequent hepatic flares, and prolonged therapy raises concerns about indefinite or even lifelong medication dependency.
Emerging evidence suggests that sustained virological responses via antiviral treatments are accompanied by dynamic modulations of immune cell phenotypes and functional states (3). Notably, the interplay between antiviral therapy and immune reconstitution remains incompletely characterized, particularly regarding innate immunity components and unconventional T cell populations. This review systematically summarized current knowledge on the immunomodulatory effects of NUCs and PEG-IFN-α on temporal changes in innate immune cells (including NK cells, macrophages, and dendritic cells) and unconventional T cell responses during treatment. By integrating these findings, we aim to identify possible immune intervention for HBV immune therapy.
2 Partial functional recovery of innate immune cells following antiviral therapy
The innate immune system serves as the critical first line of defense against pathogens and plays a pivotal role in initiating and shaping subsequent adaptive immune responses. Beyond direct antiviral effector functions, innate immune cells are essential for antigen presentation, cytokine production and modulating the activation and function of HBV-specific T and B lymphocytes (4). While extensive researches have focused on the dysfunction and restoration of adaptive HBV-specific immunity during antiviral therapy (5), the longitudinal dynamics and functional reconstitution of innate immune cells remain relatively less explored. A deeper understanding of how current antivirals impact these innate compartments is crucial for revealing potential mechanisms to break immune tolerance and achieving functional cure.
2.1 Dendritic cells
Dendritic cells (DCs), as professional antigen-presenting cells, play a pivotal role as critical mediators bridging innate and adaptive immunity. Human DCs are broadly categorized into three main types, including monocyte-derived DCs (moDCs), plasmacytoid DCs (pDCs), and conventional DCs (cDCs) (6). MoDCs, characterized by the surface markers CD14, FcγRI (CD64), and FcεRI, become activated primarily under inflammatory conditions (7). In contrast, pDCs are identified by their expression of CD123, CD303 (BDCA2), and CD304 (BDCA4). These cells specialize in robust type I interferon (IFN-I) production in response to single-stranded viral RNA and DNA, a function mediated through pattern recognition receptors (PRRs) such as Toll-like receptor (TLR)-9 (8). The cDC population, often referred to as myeloid DCs (mDCs) in literature, consists of two principal subsets: cDC1s and cDC2s. cDC1s express CD141 (BDCA3) and excel at cross-presenting exogenous antigens on MHC class I molecules to activate CD8+ T cells. Conversely, cDC2s, which express CD1c/BDCA1 and CD172a, primarily present antigens on MHC class II molecules to stimulate CD4+ T cells (7).
Emerging evidence reveals profound DC dysfunction during chronic HBV infection, with distinct pathophysiological manifestations across disease phases. Studies demonstrate reduced mDC frequencies alongside elevated B7-H1 (PD-L1) expression on mDCs in chronic hepatitis B (CHB) patients (9, 10). Similarly, decreased peripheral pDC percentages, reduced TLR9 expression, and impaired CpG-induced IFN-α responses are observed in CHB patients compared to healthy controls (HCs) (11–15). Notably, Ouaguia et al. have reported higher pDC frequencies in CHB livers than those in HCs, while liver cDCs remain comparable (16). This dysfunction extends to disrupted crosstalk between pDCs and natural killer (NK) cell, evidenced by impaired cytotoxic activation of NK cells in CHB patients (17). Beyond classical DC subsets, recent studies by Li et al. have identified expanded circulating follicular DCs (FDCs; CD14+ CD21high)in chronic HBV patients compared to HCs (18).
Both circulating and intrahepatic cDC2s from HBV-infected patients exhibit reduced CD40/CD80 expression, whereas peripheral and hepatic pDCs display elevated CD40 levels compared to HCs (16). Altered expression of co-stimulatory/co-inhibitory molecules on DCs is prominent in CHB that co-stimulatory molecules (OX40L and 4-1BBL) are downregulated on peripheral pDCs and cDC1s, while PD-L1 expression on cDC2s and pDCs inversely correlates with HBV DNA (16). CD86 expression on pDCs is elevated in both immune-tolerant (IT) and immune-active (IA) phases compared to controls, with IA patients showing higher CD86 levels and enhanced IFN-α2 production (19). In addition, TGF-β1 significantly elevate within intrahepatic cDC2s and pDCs of IT patients compared to other disease stages or HCs (20). Metabolic disturbances are also evident, as Dumolard et al. have recently demonstrated dysregulated glycolysis and oxidative phosphorylation (OXPHOS) in hepatic cDC1s and pDCs across HBV infection stages (20). Furthermore, peripheral DCs from IT patients show significantly reduced levels of free cholesterol, lipid rafts, and LDL receptor (LDLR) compared to HCs. This lipid raft impairment, potentially influenced by HBsAg, can be partially restored by lipophilic statins, which also enhances the antigen-presentation ability of DCs. (21). Improtantly, functional recovery of DCs emerges in disease resolution phases, with inactive carriers (IC) demonstrating superior DC functionality over IT patients through increased expression of CD80, CD86, HLA-DR and IL-12 (22).
Functional impairments are further highlighted by TLR stimulation assays. Chronic HBV patients show significantly reduced production of IL-12p40/70 and TNFα by cDC2s, IFNα/TNFα/IFNλ1 by pDCs, and IFNλ1/TNFα/IL-12p40/70 by cDC1s compared to HCs (16). In contrast, intrahepatic DCs from CHB patients retain full functionality upon TLR triggering, producing pro-inflammatory cytokines at levels comparable to HCs (16). Furthermore, study on purified peripheral moDCs from CHB patients reveals heightened activation. The expression of both MHCII and co-stimulated molecules (CD80, CD86) as well as the cytokines (TNF-α, IL-10, IL-12) secretion in the purified peripheral moDCs from CHB patients are significantly higher than those from HCs when co-cultured with supernatant of HepG2.2.15 cells (23). Interestingly, enhanced autophagy is also observed in mo-DCs from chronic HBV patients compared to healthy donors upon re-exposure to HBV (23).
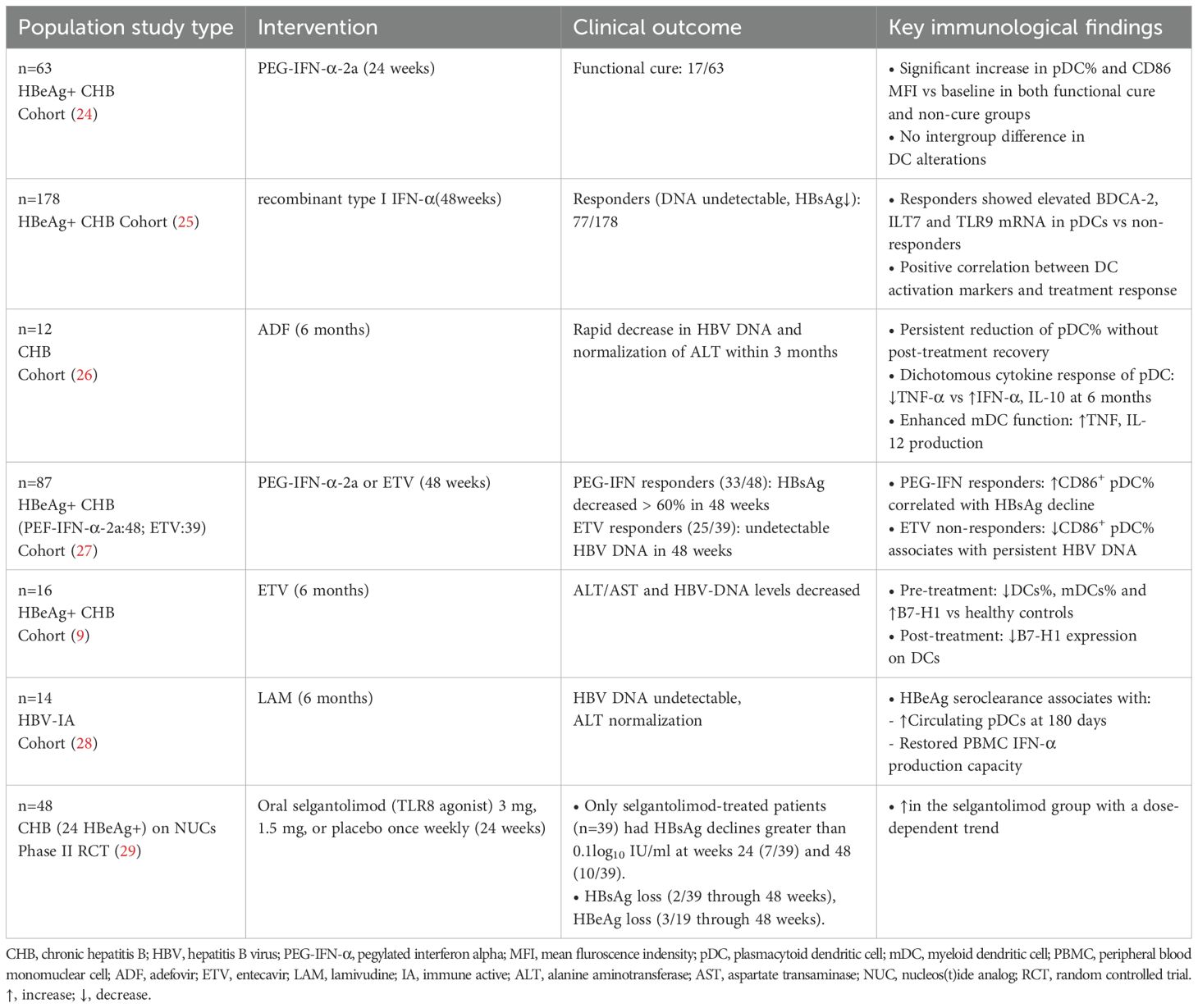
The immunomodulatory effects of antiviral therapies on DC populations exhibit substantial heterogeneity across clinical studies (Table 1). One study has illustrated that entecavir (ETV) therapy significantly reduces B7-H1 expression on peripheral DCs in CHB patients through suppression of HBcAg-mediated AKT/ERK/p38 signaling pathways (9). Furthermore, another study has shown that ETV induces early pDC proliferation (12-24 weeks), while CD86 is downregulated on pDCs in HBV DNA non-responders (27). Six-month therapy of adefovir dipivoxil (ADF) restores mDC frequency and enhances their capacity to produce TNF and IL-12, whereas the frequency and TNF-α and IL-10 secretion of pDCs remain refractory (26).
Interferon-based regimens reveal distinct immunostimulatory patterns. PEG-IFN-α-2a treatment induces sustained CD86 upregulation on pDCs in patients achieving functional cure (24). Moreover, the frequency of pDC increases at week 24 post-treatment in the functional cure group (24). Consistently, Cao et al. have found that HBsAg decline significantly associates with CD86 elevation on pDCs during IFN-α treatment (27). Mechanistically, IFN-α treatment enhances hepatic pDC expansion and upregulates TLR-9 mRNA in peripheral blood mononuclear cells (PBMCs) of virological responders (25). A recent clinical trial of selgantolimod (TLR8 agonist) has demonstrated significant increase of peripheral pDCs, with a dose-dependent trend (29). Taken together, critical analysis identifies three determinants of DC functional restoration, including baseline DC subset characteristic, different antiviral agents, and variant duration of treatment and observation.
2.2 Monocytes
Monocytes, originating from common myeloid progenitors (CMPs) in the bone marrow, constitute approximately 10% of human peripheral leukocytes and perform multifaceted functions in homeostasis and inflammation (30). In humans, two functionally distinct subsets are recognized. CD14++ CD16- “migratory” monocytes are capable of tissue infiltration, and CD14+ CD16+ “patrolling” monocytes maintain vascular surveillance (31).
Chronic HBV exposure induces immunoregulatory reprogramming of monocytes. Monocytes from chronically infected individuals demonstrate elevated expression of TNF-α, IL-10, TGF-β, PD-L1, Gal-9 and HLA-E compared to HCs (32, 33). Notably, PD-L1 upregulation on monocytes is particularly pronounced in HBeAg-positive patients (34, 35). Furthermore, the hepatic compartment of chronic HBV (CHB) patients shows an enrichment of monocytes expressing Gal-9 and PD-L1 compared to HCs (33). Functionally, monocytes from IT patients and HBeAg-positive or -negative CHB patients demonstrate suppressed signaling through TLR2, TLR4, and TLR9 compared to ICs and HCs. This functional impairment is accompanied by reduced production of IL-12, TNF-α, and IL-6, as well as diminished phagocytic capacity and oxidative response (36–38). Moreover, PD-L1- and Gal-9-expressing monocytes in CHB contribute to the dysregulation of both adaptive and innate immune responses (33). Another study has revealed significantly downregulated expression of membrane-bound CD163, a monocyte activation marker, on circulating monocytes from both treatment-naïve CHB patients and those achieving HBsAg loss compared to HCs (37). Conversely, circulating soluble CD163 (sCD163) levels are elevated in CHB patients with significant inflammation (A≥2) or fibrosis (F≥2) (37).
Emerging evidence suggests antiviral interventions may partially reverse HBV-induced monocyte dysfunction, though therapeutic outcomes remain heterogeneous. After one year of treatment, tenofovir disoproxil fumarate (TDF) fails to restore monocyte functionality, as evidenced by unchanged monocyte subset distribution and proportions expressing PD-LI, Gal-9, TLR-2, IL-12, IL-10, CD64, and iNOS before and after treatment (33, 38), whereas responders to Peg-IFN-α and ETV demonstrate partial TLR9 expression recovery on monocytes (36). Intrahepatic transcriptomics reveal elevation of hepatic monocytes after 24-week PEG-IFN-α treatment (39). Recent single-cell analyses reveal that PEG-IFN-α reduces proportions of pro-inflammotory CD14+ and CD16+ monocytes, accompanied by systemic immune reprogramming from TNF-α-dominant to IFN-α-driven transcriptional profiles (40). Consistently, NUC-treated patients exhibit upregulated expression of TLR-associated genes LY6E and STK4 on monocytes compared to ICs (41). A recent clinical trial of selgantolimod (TLR8 agonist) has demonstrated significant increase of peripheral CD14+ classical monocytes, with a dose-independent trend (29). Collectively, these findings position monocytes as pivotal mediators of HBV immunopathogenesis. While current antivirals show partial efficacy in reversing monocyte dysfunction, stratified interventions targeting subset-specific reprogramming are needed to achieve functional cure.
2.3 Myeloid-derived suppressor cells
Myeloid-derived suppressor cells (MDSCs), constituting less than 1% of myeloid cells in healthy individuals (42), are a heterogeneous population of immunosuppressive myeloid cells comprising two functionally distinct subsets, polymorphonuclear MDSCs (PMN-MDSCs) and monocytic MDSCs (M-MDSCs) (43, 44). In human PBMCs, these subsets are phenotypically characterized as CD11b+ CD14− CD15+/CD66b+ (PMN-MDSC) and CD11b+CD14+HLA-DR−/loCD33+CD15− (M-MDSC) (45). MDSCs undergo significant expansion under pathological conditions, suppressing T cell responses and promoting disease progression through multiple mechanisms (46).
Several clinical studies demonstrate remarkable expansion of circulating MDSCs in CHB patients compared to HCs (47–49) The frequency of MDSCs positively correlates with HBV DNA load, HBeAg levels and HBsAg levels (48, 50). Both M-MDSC and granulocytic-MDSC (gMDSCs) from different phases of CHB expressed high TGF-β and IL-10 (51). Notably, purified M-MDSCs from HBeAg-positive patients exhibit enhanced suppression of CD4+/CD8+ T cell proliferation and IFN-γ production compared to those from HBeAg-negative individuals (52). Moreover, gMDSCs expressing arginase expand during high viral replication phases, impairing T cell function via arginase-dependent pathways (53). Notably, an enrichment of PD-L1/Arg/iNOS expressing hepatic MDSCs is observed in CHB patients compared to HCs (51). A recent single-cell RNA sequencing of PBMC has shown that a CD14+ cluster with an MDSC-like phenotype predominantly accumulates in patients with CHB, with high expression of genes with immunoregulatory functions (54).
Apart from peripheral immune suppression, MDSCs also contribute to central tolerance via chemokine-mediated trafficking. HBsAg upregulates CCR9 expression on M-MDSCs through ERK1/2-IL-6 signaling, facilitating thymic homing via CCL25 chemotaxis (55). This process enables peripheral HBsAg transport to thymic medulla, ultimately inducing clonal deletion of HBsAg-specific CD8+ thymocytes, a mechanism predominant in pediatric CHB patients (55). Collectively, these findings unveal MDSCs as central orchestrators of HBV-induced immune tolerance through peripheral and thymic mechanisms, offering potential targets for therapeutic intervention.
Current evidence suggests suboptimal efficacy of NUCs in reconstituting MDSC homeostasis. One-year TDF monotherapy fails to restore MDSC frequency and the secretion of IL-10 and TGF-β or improve HBV-specific T-cell responses (51, 56). Strikingly, patients achieving functional cure through PEG-IFN-α-2a display substantial M-MDSC reduction (57). Consistently, targeting MDSCs with all-trans retinoic acid restores HBV-specific CD4+ and CD8+ T cell proliferation and IFN-γ production in CHB patients (50).
2.4 NK cells
As critical effectors of innate immunity, NK cells mediate rapid antiviral and antitumor responses. In humans, NK cell populations are traditionally classified into CD56dim (cytotoxic) and CD56bright (immunoregulatory) subsets based on CD56 and CD16 surface marker expression (58). NK cells exhibit dual roles in HBV immunity, balancing antiviral defense mechanisms and immunopathogenic potential through liver injury (59). During acute HBV infection (AHB), peripheral CD56bright NK cells undergo significant expansion (60) and display an activated phenotype characterized by upregulated activation receptors (NKp30, NKp44, NKp46 and NKG2C), activation markers (CD38 and HLA-DR), and cytotoxic mediators like TRAIL, alongside downregulation of inhibitory receptors (CD158a/b and NKG2A) (61). Elevated CD107a expression and robust IFN-γ production upon IL-12+ IL-18 or K562 stimulation have also been observed in peripheral CD56bright NK cells during acute HBV (61). Notably, CD56dim NK cell-mediated antibody-dependent cellular cytotoxicity (ADCC) associates with early HBsAg clearance in AHB (62). Temporal analyses, however, reveal transient suppression of IFN-γ and TNF-α production during peak viremia, with functional recovery upon viral resolution (63).
In chronic HBV infection, phenotypic and functional defects of NK cells are well-documented. Discrepancies in circulating NK cell frequencies across studies reflect population heterogeneity and clinical phase variations (64–66). Progressive NK cell dysfunction has been observed in chronic HBV infection, characterized by reduced expression of activating receptors (e.g. NKG2D), increased inhibitory checkpoint molecules (PD-1, Tim-3, CD94) (67), with the frequency of intrahepatic PD-1+ NK cells being the highest in HBeAg+ HBV patients (68). This dysfunction is further marked by attenuated antiviral cytokine (IFN-γ, TNF-α) secretion (69, 70), and elevated immunosuppressive IL-10/TGF-β1 production (71, 72). Conversely, NK cells may negatively regulate HBV-specific T cells through TRAIL-R2-mediated lysis (73). Furthermore, the activation of NK cells driven by proinflammatory cytokines (IFN-α, IL-12, IL-15, IL-8) also exacerbates liver inflammation via NKG2D/TRAIL/IFN-γ-mediated hepatocyte damage, particularly in IA phase (64, 74–76). This pathogenic role is supported by a positive correlation between intrahepatic NK cell accumulation and histological inflammation severity (77). Furthermore, TRAIL expression on CD56bright NK cells positively correlates with liver inflammation and ALT flare (65, 71, 75). Intrahepatic analyses of a recent single-cell RNA sequencing demonstrate that the CXCR6+ NCAM1+ CD160high liver-resident NK-cell cluster with a significant higher expression of IL-32 within the HBsAg-high group compared to HBsAg-low group (78).
Functional analyses reveal discrepancies in NK cell cytotoxic activity. While NK cells from IA patients exhibit enhanced TNF-α, IFN-γ, and CD107a production compared to HCs (75, 79), cytokine-mediated functional exhaustion has been reported following IL-2 and IL-12 or IL-21 stimulation (71, 80, 81). Conversely, other studies illustrate preserved cytotoxic function of NK cells, as evidenced by intact K562 lysis capacity (82, 83).
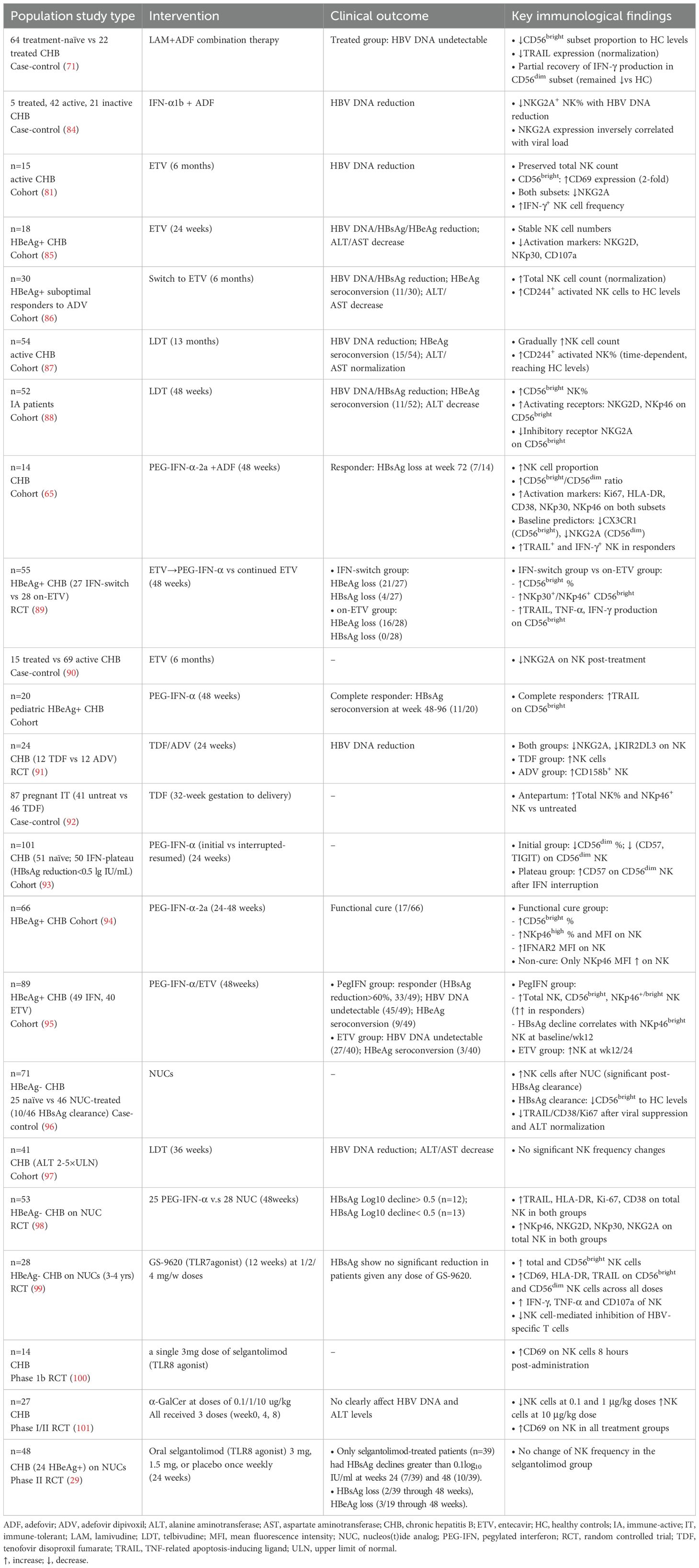
Emerging evidence reveals the multifaceted immunomodulatory effects of antiviral therapies on NK cells in CHB (Table 2). NUCs and PEG-IFN-α therapies have demonstrated marked heterogeneity across studies regarding capacity to reshape NK cell quantity, phenotype, and function, influenced by treatment duration, therapeutic agents, and patient-specific factors. A recent randomized controlled trial has observed significant upregulation of activation markers (TRAIL, HLA-DR, Ki-67, CD38) and receptors (NKp46, NKG2D, NKp30, NKG2A) on total NK cells—irrespective of HBsAg decline magnitude (98). However, some studies report transient expansion of immunoregulatory CD56bright subset during NUC therapy, with normalization post-HBsAg clearance (88, 96), while other investigations document static or even reduced NK cell counts in NUC-treated cohorts, including telbivudine (LDT) and ETV (81, 97). Intrahepatic transcriptomics reveal no alteration of hepatic NK cells after 24-week PEG-IFN-α treatment (39).
The phenotype of NK cells varies among different studies following antiviral therapy. Inhibitory receptors such as NKG2A and KIR2DL3 demonstrate progressive downregulation in tandem with viral suppression under NUC therapy (88, 90). Consistently, activation receptors(NKp30, NKp46, and NKG2D) exhibit temporal upregulation patterns that parallel HBsAg clearance trajectories (88, 92, 94, 95). ETV monotherapy transiently suppresses NKG2D and NKp30 expression on NK cells in HBeAg-positive patients (85), whereas therapeutic regimen switching (ADV to ETV) enhances CD244+ activated NK subsets (86). PEG-IFN-α induces TRAIL upregulation on CD56bright NK cells in complete responders (102), while LAM-ADV combination therapy restores TRAIL expression without rescuing IFN-γ production deficits in CD56dim subsets (71). Intriguingly, ETV treatment enhances CD69 expression and IFN-γ production specifically within CD56bright NK populations (81). Furthermore, PEG-IFN-α discontinuation in plateau-phase patients reduces exhaustion markers (CD57, TIGIT) on CD56dim NK (93). Several clinical trials of novel immunotherapies exhibit prominent alteration on NK cells. GS-9620 (TLR-7 agonist) rapidly upregulates NK activation markers (CD69, TRAIL, HLA-DR) and enhances effector functions (IFN-γ, TNF-α, degranulation) (99). Preliminary research of selgantolimod (TLR-8 agonist) activates NK cells as well, evidenced by CD69 expression (100), while another phase II trial demonstrates no alteration in circulating NK cell frequencies (29). α-GalCer modulates NK cell frequencies bidirectionally (decreasing at lower doses, increasing at 10 μg/kg), and effectively increases CD69 expression (101). Collectively, these findings highlight the critical role of NK cells in antiviral immunity, with treatment-induced phenotypic remodeling potentially serving as a biomarker for therapeutic efficacy.
3 Effects of antiviral therapies on unconventional T cells
Unconventional T cells (UTCs) represent a heterogeneous group of non-classical MHC-restricted lymphocytes that recognize non-peptide, non-polymorphic antigens. This family includes γδ T cells, invariant natural killer T (iNKT) cells, mucosal-associated invariant T (MAIT) cells, and CD4/CD8 double-negative T cells (103). UTCs orchestrate rapid antimicrobial responses through producing potent cytokines (e.g. IFN-γ, TNF-α, IL-17) and exerting cytotoxicity during early infection phases, prior to conventional αβ T cell activation (104). Beyond pathogen defense, UTCs contribute to chronic inflammation and tissue homeostasis (105). UTCs account for 10–30% of peripheral T cell populations in adult (106). These cells predominantly reside at mucosal sites and notably enriched in the human liver, positioning them as key sentinels and early responders in HBV infection (107, 108). Despite their potential significance in hepatic immunity, the impact of chronic HBV infection and subsequent antiviral therapy on the frequency, phenotype, and function of distinct UTC subsets is less comprehensively characterized compared to conventional HBV-specific CD4+ and CD8+ T cells. Investigating the dynamics and restoration of UTCs during treatment is vital, as these cells may contribute uniquely to viral control, immunopathology, and offer novel immunological insights or biomarkers for therapeutic efficacy and the development of combined immunotherapies aimed at functional cure.
3.1 MAIT cells
MAIT cells are characterized by their semi-invariant TCR α-chain (usually Vα7.2–Jα33/12/20 in humans) and restriction to the MHC-I-related protein MR1 (103, 109), which presents microbial riboflavin (vitamin B2) and folate (vitamin B9) derivatives (110). MAIT cells constitute approximately 5% of circulating T cells (111) but are enriched in mucosal tissues, representing up to 45% of hepatic T lymphocytes (109). Upon activation, they predominantly secrete IFN-γ and TNF, with a minor subset producing IL-17A (109).
The frequency, phenotype and cytokine production of MAIT cells exhibits conflicting patterns across studies in CHB patients. Several studies have reported reduced circulating MAIT cells in CHB compared to HCs (112, 113), whereas another study documents comparable levels (114). MAIT cell reduction is also observed in patients with HBV-related acute-on-chronic liver failure (115). Mechanistically, this reduction potentially attributes to conjugated bilirubin-mediated apoptosis of MAIT cells (113). Several studies have documented the upregulation of activation markers (CD69, HLA-DR, CD38), immunosenescence marker CD57, and inhibitory receptors (PD-1, CTLA-4) on peripheral MAIT cells in CHB compared to HCs (113, 116, 117). However, another study demonstrates reduced expression of CD69 on MAIT cells in CHB patients (118). Notably, CD69 expression on MAIT cells correlates positively with HBV viral load, while inhibitory markers (PD-1 and CTLA-4) on MAIT cells show negative correlation with HBV DNA levels (115, 116). Further functional assessments show enhanced IFN-γ and Granzyme B secretion from MAIT cells in CHB patients than those in HCs upon anti-CD28/E.coli co-stimulation (114, 118), whereas combined stimulation of IL-12 and IL-18 yields impaired IFN-γ responses in CHB patients (119). Single-cell transcriptomics identify two hepatic MAIT subsets in CHB, T7(CD3+SLC4A10+TNFAIP3+) cells displaying proinflammatory cytokine secretion and immune cell recruitment capacities, and T6(CD3+SLC4A10+TNFAIP3-) cells with impaired antiviral function (120). The progressive shift toward T6 predominance during advanced hepatic inflammation highlights MAIT cell dysfunction in chronic HBV pathogenesis (120). These findings collectively illustrate the complex duality of MAIT cell responses in CHB, balancing protective immunity with inflammation-driven exhaustion. Longitudinal analyses suggest preserved MAIT cell frequencies during NUC therapy (114, 121). Nevertheless, treatment-induced normalization of CD38 activation marker expression implies partial recovery of MAIT cell functionality, though complete phenotypic and functional restoration remains to be established (114). A phase 1b clinical trial of selgantolimod (TLR8 agonist) shows the elevation of CD69 on MAIT after a single dose (100).
3.2 γδ T cells
γδ T cells are defined by their unique TCR consisting of a γ-chain and a δ-chain, which enables antigen recognition independent of MHC class I/II molecules (103). Two major subsets exist in humans, Vδ1+ and Vδ2+ γδ T cells (122). Vδ1+ cells, characterized by pairing of the Vδ1 chain with diverse Vγ family members (Vγ2/3/4/5/8/9) (123) predominantly reside in mucosal and epithelial tissues such as intestinal epithelium (124), skin (125, 126), spleen and liver. In contrast, Vδ2+ cells typically express an invariant Vγ9 chain paired with Vδ2 (127), constituting 50–95% of circulating γδ T cells in human peripheral blood (128, 129). These cells are activated through phosphoantigen recognition via butyrophilin 3A1 (BTN3A1) (130, 131), triggering rapid secretion of cytotoxic molecules and Th1 cytokines (IFN-γ and TNF-α) to combat malignancies and microbial pathogens (132, 133). Additionally, Vδ3+ T cells have been found in the periphery which only consist about 0.2% of γδ T cells, while in the liver they are more abundant. Limited studies on this subset show their capacity to secret Th1, Th2 and Th17 cytokines (134).
Acute HBV infection significantly reduces peripheral γδ T cell proportions and absolute counts compared to CHB and HCs, negatively correlating with serum ALT (135). AHB patients exhibit heightened activation profiles in circulating γδ T cells compared to HCs, characterized by upregulated CD38, HLA-DR, granzyme B, CD107a, and distinct transcriptional polarization as Tbet+/hi Eomesdim Vδ1 subsets and Tbetdim Eomeshi Vδ2 subsets (135, 136). Concurrently, intrahepatic γδ T cells accumulate in inflamed liver lobules during AHB (135), a phenomenon recapitulated in acute HBV murine models where hepatic γδ T cell expansion coincides with early-stage IFN-β production (137).
In chronic HBV infection, peripheral γδ T cells are significantly reduced in CHB patients relative to HCs (138), particularly in severe liver inflammation (ALT>3×ULN) (139). However, one study reports comparable γδ T cell frequencies between symptomatic CHB and HCs (140), and some studies document elevated Vδ1 T cell percentages in CHB (138, 141). Hepatic γδ T cells, particularly the Vδ2 subset, decrease in CHB patients, especially within the IA group (138). Analysis of paired samples further reveals markedly lower hepatic Vδ2 T cell levels than their peripheral counterparts in IA patients (138).
The phenotype and function of γδ T cells varies among different studies. Elevated exhaustion markers (PD-1, Tim-3 and Lag-3) and activation markers (CD69, CD38 and HLA-DR) levels are frequently reported in CHB (140, 142). Paradoxically, Chang et al. have observed decreased PD-1, CD38, Ki-67, Tim-3, and CD158a expression on Vδ2 T cells from CHB patients compared to HCs (136). Intriguingly, PD-1 expression on circulating Vδ2+ cells inversely correlates with serum 25(OH)D3 levels in CHB (142). PMA/ionomycin stimulation enhances IFN-γ/granzyme B/TNF-α co-expression on γδ T cells from CHB patients (136, 141). However, another study describes suppressed IFN-γ secretion of γδ T cells, but can be reversible by Tim-3/Lag-3 blockade (136, 140). Functional cytotoxicity assays reveal impaired γδ T cell-mediated lysis of HBV-infected hepatocytes in symptomatic CHB compared to HCs, though asymptomatic carriers retain partial cytolytic activity than symptomatic patients (139).
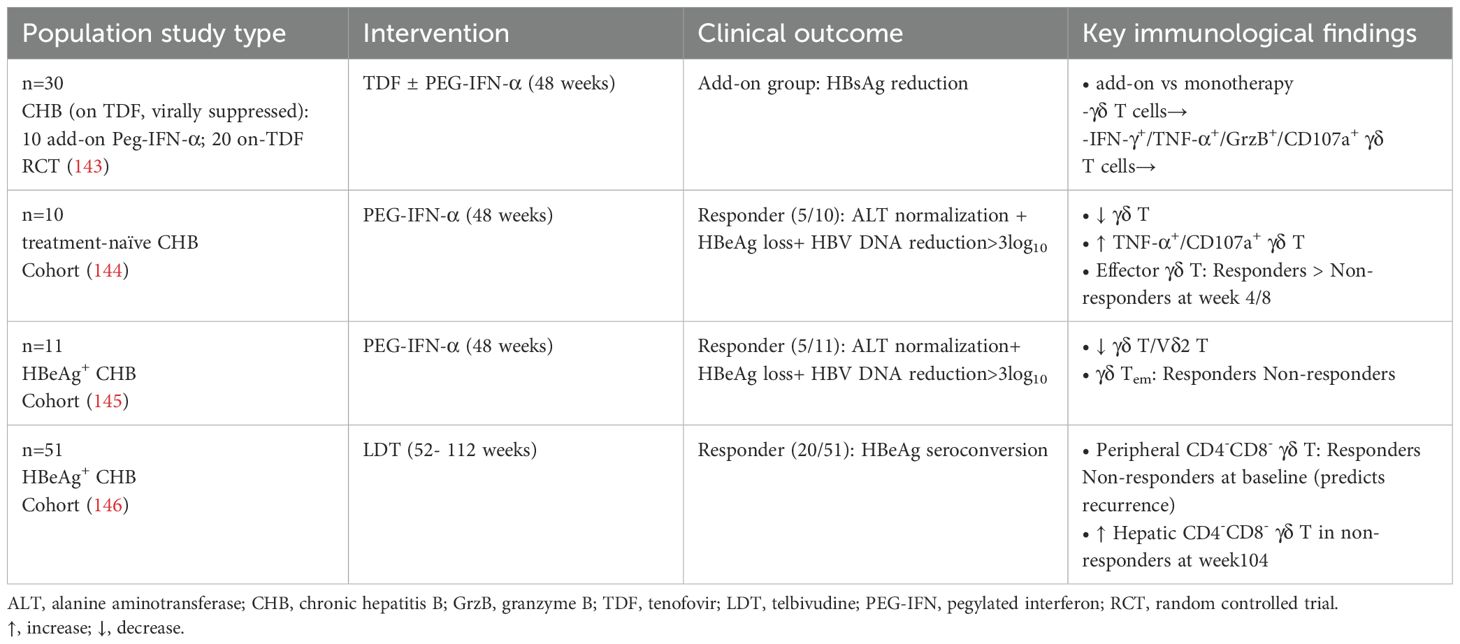
The impact of antiviral therapies on γδ T cell populations in CHB treatment presents complex immunological modifications (Table 3). A randomized controlled trial has revealed TDF/PEG-IFN-α combination therapy in HBV-suppression patients exhibits no significant alterations in γδ T cell frequencies or their functional capacity to produce IFN-γ/TNF-α/granzyme B/CD107a (143). Conversely, PEG-IFN-α monotherapy reduces γδ T cell numbers, accompanied by enhanced TNF-α/CD107a expression (144, 145). Furthermore, treatment responders exhibit distinct γδ T cell differentiation patterns characterized by transient early effector cell expansion and reduced Tem subsets (145). Longitudinal monitoring of LDT therapy suggests that elevated baseline CD4⁻CD8⁻ γδ T cells predict non-response and virologic relapse (146). Collectively, these findings underscore the heterogeneity of γδ T cell responses during CHB therapy, with dynamic changes in subsets and functional markers correlating with treatment efficacy and relapse risk.
3.3 NKT cells
Natural killer T (NKT) cells constitute a specialized lymphocyte population distinguished by their recognition of lipid antigens presented through the CD1d molecule (103). These CD1d-restricted cells are broadly classified into two subsets, invariant NKT (iNKT) cells and diverse (type II) NKT cells. iNKT cells are characterized by a semi-invariant TCR architecture, featuring a conserved α chain rearrangement Vα24-Jα18 paired with limited β chain diversity Vβ11 in humans (103). This unique TCR configuration enables iNKT cells to detect both endogenous and exogenous lipid antigens, including the prototypical α-galactosylceramide (α-GalCer), presented via the MHC-I-like CD1d molecule (103). Additionally, iNKT cells can be activated in a TCR-independent manner through innate cytokines like IL-12 and IL-18 (147). In contrast to their invariant counterparts, type II NKT cells possess highly diverse αβ TCR repertoires while maintaining CD1d-restricted lipid antigen specificity (148). Current understanding of type II NKT cell functionality remains limited, though emerging evidence suggests their involvement in both immunoregulatory and pathogenic responses through distinct lipid antigen recognition pathways (149).
Chronic HBV infection markedly alters homeostasis and function of iNKT cells. Both peripheral and hepatic iNKT cells are significantly reduced in CHB patients compared to HCs, with negative correlation between circulating iNKT cell counts and liver injury severity (143, 150, 151). Furthermore, CD4- iNKT cells are reduced in CHB, especially in those with detectable HBV DNA levels (151). Functional analyses reveal complex dysregulation of CD1d-iNKT axis in chronic HBV infection. Despite hepatic CD1d upregulation, the CD1d-iNKT system remains unactivated in CHB, showing impaired α-Galcer responses (150, 152). Surface marker profiling unveils a complex phenotype characterized by increased expression of NKG2A (153) and activation markers (CD69, CD38, HLA-DR) (150) alongside elevated exhaustion markers (Tim-3, PD-1) and reduced CD28 co-stimulation in both peripheral and hepatic iNKT cells from CHB patients compared to HCs (154). However, one study reports no significant upregulation in circulating or hepatic iNKT populations (150). Functional restoration is achieved in vitro through Tim-3/PD-1 blockade or CD28 activation (154). Other studies reveal that enhanced chemokine receptor expression (CCR5 and CCR6) and elevated Fas and FasL levels on peripheral iNKT cells from CHB (150). Moreover, IFN-γ+ NKT cells positively correlated with ALT levels and inversely correlated with HBV DNA (79, 155). Besides, other studies report diminished IL-4 and IFN-γ production in iNKT cells from CHB patients, partially reversible by exogenous IL-2 and IL-15 (150, 153, 154), while other studies find comparable cytokine production post-stimulation across disease phases upon stimulation of α-GalCer and PMA (156, 157).
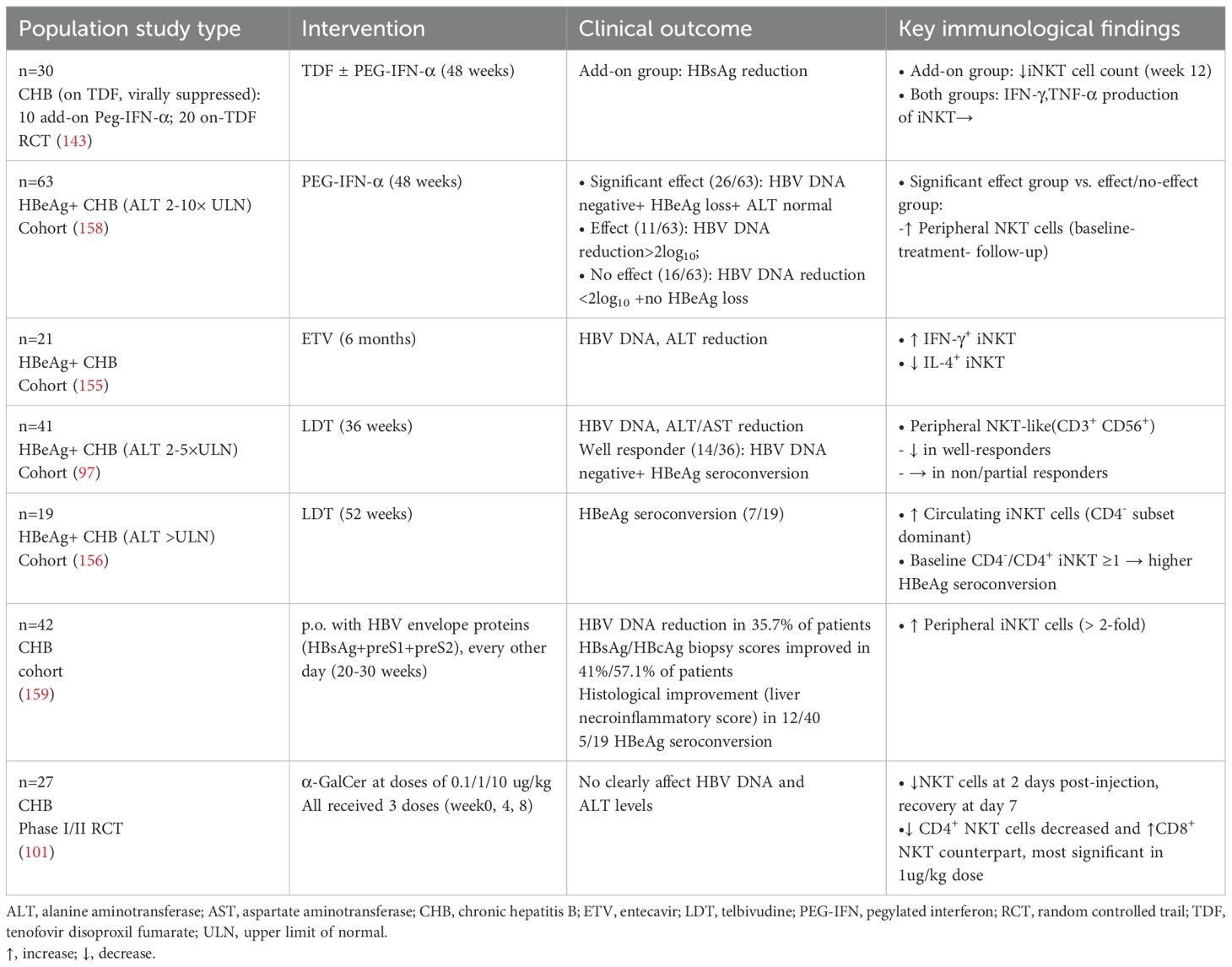
Antiviral therapies elicit heterogeneous modulation of NKT cells (Table 4). PEG-IFN-α add-on TDF therapy reduces peripheral iNKT frequencies without altering cytokine profiles (IFN-γ and TNF-α) (143). However, other studies observe that PEG-IFN-α or LDT monotherapy conversely increase iNKT frequencies (156, 158). Notably, baseline iNKT frequencies predict sustained response to PEG-IFN-α monotherapy in HBeAg-positive patients (158). ETV treatment differentially modulates iNKT subsets, enhancing IFN-γ+ while reducing IL-4+ iNKT cells during six-month treatment (155). LDT therapy selectively reduces peripheral CD3+CD56+ NKT-like cells in treatment responders instead of non-responders (97). Longitudinal analyses further reveal post-treatment expansion of circulating CD4− iNKT subsets, with baseline elevations in the CD4−/CD4+ iNKT cell ratio correlating with HBeAg seroconversion (156). Novel immunotherapies reveal distinct mechanisms. Oral HBV envelope proteins trigger a >2-fold increase in iNKT frequency alongside improved histology and seroconversion (159), while α-GalCer administration transiently suppresses total NKT cells at 2 days post-injection (recovering by day 7) and drives a shift toward CD8+ predominance, most prominently at the 1 μg/kg dose (101). Collectively, these findings highlight the heterogeneity of NKT cell responses across therapeutic regimens, emphasizing NKT cells as potential biomarkers for therapeutic stratification and outcome prediction in CHB management.
4 Conclusion
Chronic HBV infection induces broad immune dysfunction across innate (DCs, monocytes, MDSCs, NK cells) and unconventional T cell populations (MAIT, γδ T, NKT cells), characterized by inhibitory receptor upregulation, suppressed cytotoxicity, and immunosuppressive cytokine profiles (Figure 1). While NUCs demonstrate limited immunorestorative capacity, PEG-IFN-α exhibits superior efficacy in reversing DC/monocyte dysfunction, reducing MDSC accumulation, and partially restoring NK/unconventional T cell activity (Figure 1). Critically, the currently limited evidence base (summarized in Tables 1-4) reveals a paucity of prospective studies tracking innate immune dynamics during NUC therapy, hindering comprehensive understanding of functional restoration in these compartments. Future studies should prioritize intrahepatic immune profiling, given the profound functional and phenotypic disparities between circulating and liver-resident immune cells in chronic HBV infection.
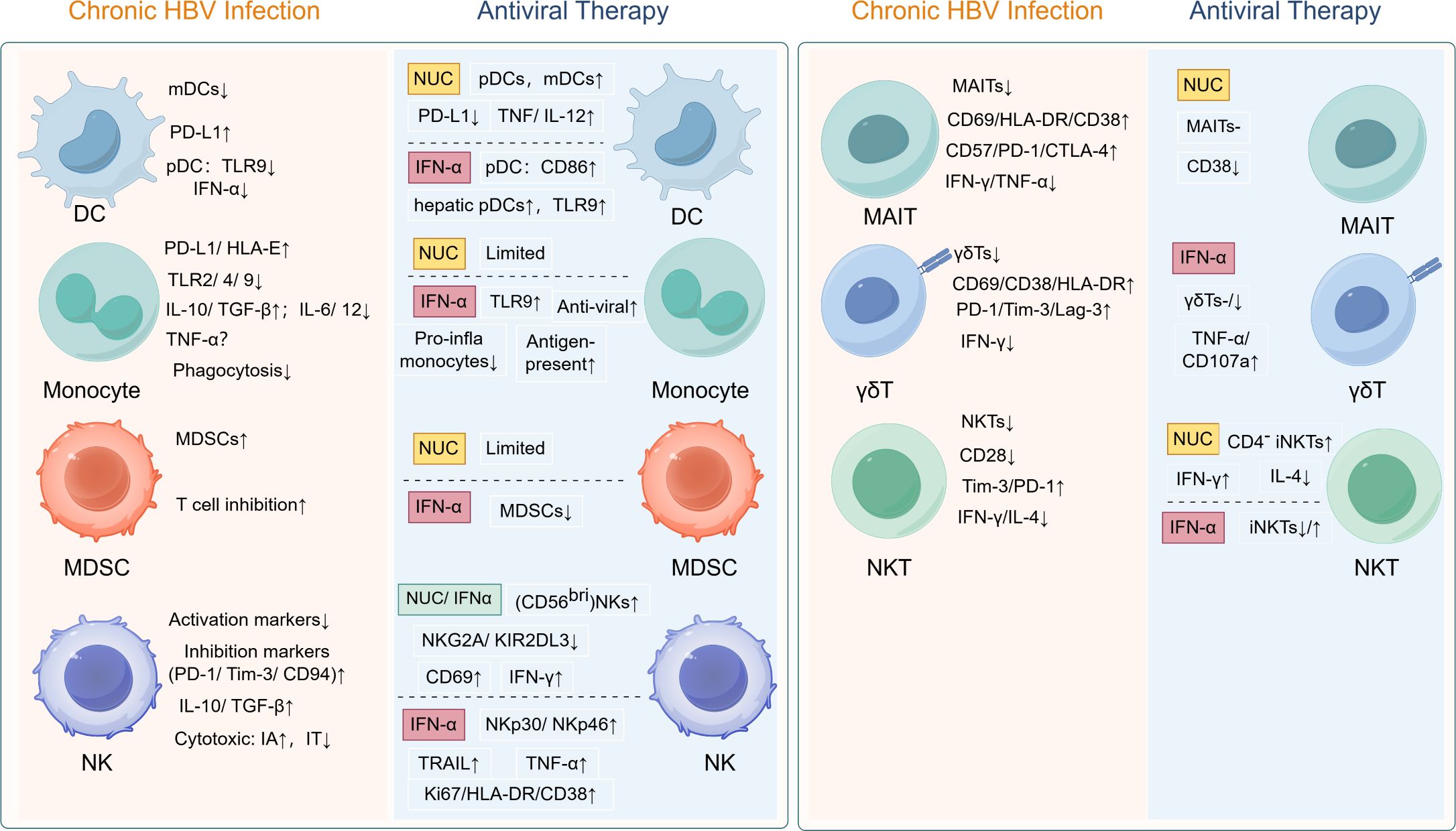
Figure 1. Treatment-induced immune reconstitution in chronic HBV: restoring functionality of dysregulated innate immune and unconventional T cells. HBV, hepatitis B virus; DC, dendritic cell; pDC, plasmacytoid dendritic cell; mDC, myeloid dendritic cell; PD-L1, programmed death ligand-1; TLR, Toll-like recptor; IFN, interferon; HLA, human leukocyte antigen; IL, interleukin; TGF-β, transforming growth factor-beta; TNF-α, tumor necrosis factor-alpha; MDSC, myeloid-derived suppressor cell; NK, natural killer cell; PD-1; programmed cell death protein-1; Tim-3, T-cell immunoglobulin and mucin-domain containing-3; IA, immune active phase; IT, immune tolerant phase; NKG2A, natural killer group 2 member A; KIR2DL3, killer cell immunoglobulin like receptor, two Ig domains and long cytoplasmic tail 3; TRAIL, TNF-related apoptosis-inducing ligand; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; Lag-3, lymphocyte-activation gene 3; MAIT, mucosal-associated invariant T cell; NKT, natural killer T cell.
Emerging immunomodulatory agents show promise in restoring antiviral immunity. For instance, TLR agonists like selgantolimod (TLR8 agonist) remodel the intrahepatic immune microenvironment by activating MAIT and NK cells (160). Combination therapies pairing immunomodulators (anti PD-1/PD-L1, TLR agonists, therapeutic vaccines and monoclonal antibodies) and viral-targeting agents (siRNA, core protein allosteric modulators (CpAMs) and virus entry inhibitors) represent a theoretically powerful strategy to overcome monotherapy limitations in achieving HBV functional cure (161). While several clinical studies confirm the efficacy of such combinations (162–164), their underlying immune mechanisms remain inadequately explored. The success of combination strategies will likely depend on identifying immunological biomarkers and implementing high-dimensional immune profiling to enable precise patient selection (165, 166). In summary, advancing immune-focused combinatorial regimens within precision medicine frameworks is essential to overcome HBV’s potent immunosuppressive mechanisms.
Author contributions
YS: Writing – original draft, Writing – review & editing. SL: Writing – review & editing. YD: Writing – original draft, Funding acquisition, Conceptualization, Writing – review & editing. XZ: Conceptualization, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the grant of National Key Research and Development Program of China (2022YFC2305100) and National Natural Science Foundation of China (82302508).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
ADCC: Antibody-Dependent Cellular Cytotoxicity
ADF: Adefovir Dipivoxil
AHB: Acute Hepatitis B
AKT: Protein Kinase B
ALT: Alanine Aminotransferase
Arg1: Arginase-1
AST: Aspartate Aminotransferase
AVT: Antiviral Therapy
BDCA-2: Blood Dendritic Cell Antigen-2
BTN3A1: Butyrophilin Subfamily 3 Member A1
CCL25: C-C Motif Chemokine Ligand 25
CCR5/CCR6: C-C Chemokine Receptor Type 5/6
CD: Cluster of Differentiation
cDC: Conventional Dendritic Cell
CHB: Chronic Hepatitis B
CTLA-4: Cytotoxic T-Lymphocyte-Associated Protein 4
CX3CR1: CX3C Chemokine Receptor 1
DAAs: Direct-Acting Antiviral Agents
DBIL: Direct Bilirubin
DC: Dendritic Cell
ERK: Extracellular Signal-Regulated Kinase
ETV: Entecavir
FDC: Follicular Dendritic Cell
gMDSC: Granulocytic Myeloid-Derived Suppressor Cell
GZ: Gray Zone
HBeAg: Hepatitis B e Antigen
HBsAg: Hepatitis B Surface Antigen
HBV: Hepatitis B Virus
HCC: Hepatocellular Carcinoma
HC: Healthy Controls
HLA: Human Leukocyte Antigen
IA: Immune-Active Phase
IC: Inactive Carrier Phase
IFN: Interferon
IFNAR2: Interferon Alpha/Beta Receptor Subunit 2
IL: Interleukin
IT: Immune-Tolerant Phase
KIR: Killer-Cell Immunoglobulin-Like Receptor
LAG-3: Lymphocyte-Activation Gene 3
LAM: Lamivudine
LDT: Telbivudine
LY6E: Lymphocyte Antigen 6E
MAIT: Mucosal-Associated Invariant T Cells
mDC: Myeloid Dendritic Cell
MDSC: Myeloid-Derived Suppressor Cell
MFI: Mean Fluorescence Intensity
M-MDSC: Monocytic Myeloid-Derived Suppressor Cell
moDC: Monocyte Dendritic Cell
MR1: MHC Class I-Related Gene Protein
MyD88: Myeloid Differentiation Primary Response 88
NF-κB: Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells
NK: Natural Killer Cells
NKG2A/D: Natural Killer Group 2 Member A/D
NKT: Natural Killer T Cells
NUC: Nucleos(t)ide Analog
OXPHOS: Oxidative Phosphorylation
PBMC: Peripheral Blood Mononuclear Cell
PD-1: Programmed Cell Death Protein 1
PD-L1: Programmed Death-Ligand 1
PEG-IFN-α: Pegylated Interferon-Alpha
pDC: Plasmacytoid Dendritic Cell
PMN-MDSC: Polymorphonuclear Myeloid-Derived Suppressor Cell
sCD163: Soluble CD163
STAT3: Signal Transducer and Activator of Transcription 3
STK4: Serine/Threonine Kinase 4
Tbet: T-box Transcription Factor TBX21
TCR: T-Cell Receptor
TDF: Tenofovir Disoproxil Fumarate
TGF-β: Transforming Growth Factor Beta
Th1: T Helper 1 Cells
Tim-3: T-cell Immunoglobulin and Mucin-Domain Containing-3
TLR: Toll-Like Receptor
TNF-α: Tumor Necrosis Factor-Alpha
TRAIL: TNF-Related Apoptosis-Inducing Ligand
ULN: Upper Limit of Normal
UTCs: Unconventional T Cells
Vδ1/Vδ2: T-Cell Receptor Delta Variable Segments.
References
1. Jeng W-J, Papatheodoridis GV, and Lok ASF. Hepatitis B. Lancet Lond Engl. (2023) 401:1039–52. doi: 10.1016/S0140-6736(22)01468-4
2. Dusheiko G, Agarwal K, and Maini MK. New approaches to chronic hepatitis B. N Engl J Med. (2023) 388:55–69. doi: 10.1056/NEJMra2211764
3. Zheng J-R, Wang Z-L, and Feng B. Hepatitis B functional cure and immune response. Front Immunol. (2022) 13:1075916. doi: 10.3389/fimmu.2022.1075916
4. Chiale C, Marchese AM, and Robek MD. Innate immunity and HBV persistence. Curr Opin Virol. (2021) 49:13–20. doi: 10.1016/j.coviro.2021.04.003
5. Iannacone M and Guidotti LG. Immunobiology and pathogenesis of hepatitis B virus infection. Nat Rev Immunol. (2022) 22:19–32. doi: 10.1038/s41577-021-00549-4
6. Collin M and Bigley V. Human dendritic cell subsets: An update. Immunology. (2018) 154:3–20. doi: 10.1111/imm.12888
7. Mildner A and Jung S. Development and function of dendritic cell subsets. Immunity. (2014) 40:642–56. doi: 10.1016/j.immuni.2014.04.016
8. Villar J and Segura E. Decoding the heterogeneity of human dendritic cell subsets. Trends Immunol. (2020) 41:1062–71. doi: 10.1016/j.it.2020.10.002
9. Li M, Zhou Z-H, Sun X-H, Zhang X, Zhu X-J, Jin S-G, et al. Hepatitis B core antigen upregulates B7-H1 on dendritic cells by activating the AKT/ERK/P38 pathway: A possible mechanism of hepatitis B virus persistence. Lab Investig J Tech Methods Pathol. (2016) 96:1156–64. doi: 10.1038/labinvest.2016.96
10. Ferrando-Martinez S, Huang K, Bennett AS, Sterba P, Yu L, Suzich JA, et al. HBeAg seroconversion is associated with a more effective PD-L1 blockade during chronic hepatitis B infection. JHEP Rep Innov Hepatol. (2019) 1:170–8. doi: 10.1016/j.jhepr.2019.06.001
11. Vincent IE, Zannetti C, Lucifora J, Norder H, Protzer U, Hainaut P, et al. Hepatitis B virus impairs TLR9 expression and function in plasmacytoid dendritic cells. PLoS One. (2011) 6:e26315. doi: 10.1371/journal.pone.0026315
12. Xu N, Yao H-P, Lv G-C, and Chen Z. Downregulation of TLR7/9 leads to deficient production of IFN-α from plasmacytoid dendritic cells in chronic hepatitis B. Inflammation Res Off J Eur Histamine Res Soc Al. (2012) 61:997–1004. doi: 10.1007/s00011-012-0493-z
13. Xu Y, Hu Y, Shi B, Zhang X, Wang J, Zhang Z, et al. HBsAg inhibits TLR9-mediated activation and IFN-alpha production in plasmacytoid dendritic cells. Mol Immunol. (2009) 46:2640–6. doi: 10.1016/j.molimm.2009.04.031
14. Woltman AM, Op den Brouw ML, Biesta PJ, Shi CC, and Janssen HLA. Hepatitis B virus lacks immune activating capacity, but actively inhibits plasmacytoid dendritic cell function. PLoS One. (2011) 6:e15324. doi: 10.1371/journal.pone.0015324
15. Sukriti S, Trehanpati N, Kumar M, Pande C, Hissar SS, and Sarin SK. Functionally aberrant dendritic cell subsets and expression of DC-SIGN differentiate acute from chronic HBV infection. Hepatol Int. (2016) 10:916–23. doi: 10.1007/s12072-016-9763-0
16. Ouaguia L, Leroy V, Dufeu-Duchesne T, Durantel D, Decaens T, Hubert M, et al. Circulating and hepatic BDCA1+, BDCA2+, and BDCA3+ dendritic cells are differentially subverted in patients with chronic HBV infection. Front Immunol. (2019) 10:112. doi: 10.3389/fimmu.2019.00112
17. Martinet J, Dufeu-Duchesne T, Bruder Costa J, Larrat S, Marlu A, Leroy V, et al. Altered functions of plasmacytoid dendritic cells and reduced cytolytic activity of natural killer cells in patients with chronic HBV infection. Gastroenterology. (2012) 143:1586–1596.e8. doi: 10.1053/j.gastro.2012.08.046
18. Li X, Zhang Q, Zhang W, Ye G, Ma Y, Wen C, et al. Expanded circulating follicular dendritic cells facilitate immune responses in chronic HBV infection. J Transl Med. (2020) 18:417. doi: 10.1186/s12967-020-02584-6
19. Li M-H, Zhang L, Zhang D, Cao W-H, Qi T-L, Hao H-X, et al. Plasmacytoid dendritic cell function and cytokine network profiles in patients with acute or chronic hepatitis B virus infection. Chin Med J (Engl). (2018) 131:43–9. doi: 10.4103/0366-6999.221275
20. Dumolard L, Gerster T, Chuffart F, Decaens T, Hilleret M-N, Larrat S, et al. HBV and HBsAg strongly reshape the phenotype, function, and metabolism of DCs according to patients’ clinical stage. Hepatol Commun. (2025) 9:e0625. doi: 10.1097/HC9.0000000000000625
21. Zhao H, Yu Y, Wang Y, Zhao L, Yang A, Hu Y, et al. Cholesterol accumulation on dendritic cells reverses chronic hepatitis B virus infection-induced dysfunction. Cell Mol Immunol. (2022) 19:1347–60. doi: 10.1038/s41423-022-00939-1
22. Lin C, Zou H, and Wang S. Hepatitis B e antigen seroconversion is related with the function of dendritic cells in chronic hepatitis B virus infection. Gastroenterol Res Pract. (2014) 2014:413952. doi: 10.1155/2014/413952
23. Xu H, Kang J, Zhong S, Chen M, Hu P, Ren H, et al. Function and autophagy of monocyte-derived dendritic cells is affected by hepatitis B virus infection. BMC Immunol. (2023) 24:31. doi: 10.1186/s12865-023-00571-2
24. Cao W, Xie S, Zhang L, Bi X, Lin Y, Yang L, et al. Expression of functional molecule on plasmacytoid dendritic cells is associated with HBsAg loss in HBeAg-positive patients during PEG-IFN α-2a treatment. Front Immunol. (2022) 13:891424. doi: 10.3389/fimmu.2022.891424
25. Chen Y, Yang J-E, Tang J-M, Mao Q-G, Zheng Q-Z, and Zheng Y. Predictive value of plasmacytoid dendritic cells and toll-like receptor-9 regarding the treatment efficacy of interferon-α in HBeAg-positive chronic hepatitis B patients. Exp Ther Med. (2019) 18:4541–6. doi: 10.3892/etm.2019.8161
26. van der Molen RG, Sprengers D, Biesta PJ, Kusters JG, and Janssen HLA. Favorable effect of adefovir on the number and functionality of myeloid dendritic cells of patients with chronic HBV. Hepatol Baltim Md. (2006) 44:907–14. doi: 10.1002/hep.21340
27. Cao W-H, Li M-H, Pan CQ, Lu Y, Zhang L, Ran C-P, et al. Quantitation of plasmacytoid dendritic cells in chronic hepatitis B patients with HBeAg positivity during PEG-IFN and entecavir therapy. J Interferon Cytokine Res Off J Int Soc Interferon Cytokine Res. (2018) 38:197–205. doi: 10.1089/jir.2018.0014
28. Duan X-Z, Wang M, Li H-W, Zhuang H, Xu D, and Wang F-S. Decreased frequency and function of circulating plasmocytoid dendritic cells (pDC) in hepatitis B virus infected humans. J Clin Immunol. (2004) 24:637–46. doi: 10.1007/s10875-004-6249-y
29. Gane EJ, Dunbar PR, Brooks AE, Zhang F, Chen D, Wallin JJ, et al. Safety and efficacy of the oral TLR8 agonist selgantolimod in individuals with chronic hepatitis B under viral suppression. J Hepatol. (2023) 78:513–23. doi: 10.1016/j.jhep.2022.09.027
30. Hettinger J, Richards DM, Hansson J, Barra MM, Joschko A-C, Krijgsveld J, et al. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol. (2013) 14:821–30. doi: 10.1038/ni.2638
31. Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, et al. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. (2013) 39:599–610. doi: 10.1016/j.immuni.2013.08.007
32. Li H, Zhai N, Wang Z, Song H, Yang Y, Cui A, et al. Regulatory NK cells mediated between immunosuppressive monocytes and dysfunctional T cells in chronic HBV infection. Gut. (2018) 67:2035–44. doi: 10.1136/gutjnl-2017-314098
33. Dey D, Biswas S, Pal S, Nandi S, Khatun N, Jha R, et al. Monocyte-derived galectin-9 and PD-L1 differentially impair adaptive and innate immune response in chronic HBV infection and their expression remain unaltered after antiviral therapy. Front Immunol. (2024) 15:1474853. doi: 10.3389/fimmu.2024.1474853
34. Han Y, Li J, Jiang L, Xu Q, Liu B, Jin K, et al. Regulation of B7-H1 expression on peripheral monocytes and IFN-γ secretion in T lymphocytes by HBeAg. Cell Immunol. (2013) 283:25–30. doi: 10.1016/j.cellimm.2013.05.009
35. Geng L, Jiang G, Fang Y, Dong S, Xie H, Chen Y, et al. B7-H1 expression is upregulated in peripheral blood CD14+ monocytes of patients with chronic hepatitis B virus infection, which correlates with higher serum IL-10 levels. J Viral Hepat. (2006) 13:725–33. doi: 10.1111/j.1365-2893.2006.00746.x
36. Huang Y-W, Hsu C-K, Lin S-C, Wei S-C, Hu J-T, Chang H-Y, et al. Reduced toll-like receptor 9 expression on peripheral CD14+ monocytes of chronic hepatitis B patients and its restoration by effective therapy. Antivir Ther. (2014) 19:637–43. doi: 10.3851/IMP2762
37. Xie P, Yao B, Huang D, Chen Y, Gong Q, and Zhang X. Soluble CD163 and CD163 expression on monocytes associated with chronic hepatitis B inflammation and HBsAg loss. J Clin Transl Hepatol. (2022) 10:1059–67. doi: 10.14218/JCTH.2021.00496
38. Dey D, Pal S, Chakraborty BC, Baidya A, Bhadra S, Ghosh R, et al. Multifaceted defects in monocytes in different phases of chronic hepatitis B virus infection: Lack of restoration after antiviral therapy. Microbiol Spectr. (2022) 10:e0193922. doi: 10.1128/spectrum.01939-22
39. Li N, Yu K, Dong M, Wang J, Yang F, Zhu H, et al. Intrahepatic transcriptomics reveals gene signatures in chronic hepatitis B patients responded to interferon therapy. Emerg Microbes Infect. (2022) 11:1876–89. doi: 10.1080/22221751.2022.2100831
40. Jiang P, Jia H, Qian X, Tang T, Han Y, Zhang Z, et al. Single-cell RNA sequencing reveals the immunoregulatory roles of PegIFN-α in patients with chronic hepatitis B. Hepatol Baltim Md. (2024) 79:167–82. doi: 10.1097/HEP.0000000000000524
41. Montanari NR, Conceição-Neto N, Van Den Wyngaert I, Van Oord GW, Groothuismink ZMA, Van Tilburg S, et al. Differential gene expression, irrespective of circulating hepatitis B surface antigen levels, between inactive carrier and nucleos(t)ide analogue-treated hepatitis B virus patients. J Infect Dis. (2022) 225:1471–6. doi: 10.1093/infdis/jiaa614
42. Tesi RJ. MDSC; the most important cell you have never heard of. Trends Pharmacol Sci. (2019) 40:4–7. doi: 10.1016/j.tips.2018.10.008
43. Gabrilovich DI, Bronte V, Chen S-H, Colombo MP, Ochoa A, Ostrand-Rosenberg S, et al. The terminology issue for myeloid-derived suppressor cells. Cancer Res. (2007) 67:425. doi: 10.1158/0008-5472.CAN-06-3037
44. Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. (2008) 111:4233–44. doi: 10.1182/blood-2007-07-099226
45. Dumitru CA, Moses K, Trellakis S, Lang S, and Brandau S. Neutrophils and granulocytic myeloid-derived suppressor cells: Immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol Immunother. (2012) 61:1155–67. doi: 10.1007/s00262-012-1294-5
46. Hegde S, Leader AM, and Merad M. MDSC: Markers, development, states, and unaddressed complexity. Immunity. (2021) 54:875–84. doi: 10.1016/j.immuni.2021.04.004
47. Lu L-R, Liu J, Xu Z, Zhang G-L, Li D-C, and Lin C-S. Expression and clinical significance of myeloid derived suppressor cells in chronic hepatitis B patients. Asian Pac J Cancer Prev APJCP. (2014) 15:4367–72. doi: 10.7314/apjcp.2014.15.10.4367
48. Lv Y, Cui M, Lv Z, Lu J, Zhang X, Zhao Z, et al. Expression and significance of peripheral myeloid-derived suppressor cells in chronic hepatitis B patients. Clin Res Hepatol Gastroenterol. (2018) 42:462–9. doi: 10.1016/j.clinre.2018.04.002
49. Zhang H, Guan S, Yang K, Ye J, Yan K, Pan Y, et al. the frequency of peripheral blood CD14(+)HLA-DR(-/low) MDSCs is negatively correlated with the inflammation in patients with chronic hepatitis B. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi Chin J Cell Mol Immunol. (2015) 31:1387–1390, 1395.
50. Fang Z, Li J, Yu X, Zhang D, Ren G, Shi B, et al. Polarization of monocytic myeloid-derived suppressor cells by hepatitis B surface antigen is mediated via ERK/IL-6/STAT3 signaling feedback and restrains the activation of T cells in chronic hepatitis B virus infection. J Immunol Baltim Md 1950. (2015) 195:4873–83. doi: 10.4049/jimmunol.1501362
51. Pal S, Dey D, Chakraborty BC, Nandi M, Khatun M, Banerjee S, et al. Diverse facets of MDSC in different phases of chronic HBV infection: Impact on HBV-specific T-cell response and homing. Hepatol Baltim Md. (2022) 76:759–74. doi: 10.1002/hep.32331
52. Yang F, Yu X, Zhou C, Mao R, Zhu M, Zhu H, et al. Hepatitis B e antigen induces the expansion of monocytic myeloid-derived suppressor cells to dampen T-cell function in chronic hepatitis B virus infection. PLoS Pathog. (2019) 15:e1007690. doi: 10.1371/journal.ppat.1007690
53. Pallett LJ, Gill US, Quaglia A, Sinclair LV, Jover-Cobos M, Schurich A, et al. Metabolic regulation of hepatitis B immunopathology by myeloid-derived suppressor cells. Nat Med. (2015) 21:591–600. doi: 10.1038/nm.3856
54. Huang L, Ye B, Cao F, Ruan B, and Li X. Single-cell atlas of the peripheral immune response in patients with chronic hepatitis B. J Med Virol. (2025) 97:e70360. doi: 10.1002/jmv.70360
55. Fang Z, Zhang Y, Zhu Z, Wang C, Hu Y, Peng X, et al. Monocytic MDSCs homing to thymus contribute to age-related CD8+ T cell tolerance of HBV. J Exp Med. (2022) 219:e20211838. doi: 10.1084/jem.20211838
56. Pal S, Nandi M, Dey D, Chakraborty BC, Shil A, Ghosh S, et al. Myeloid-derived suppressor cells induce regulatory T cells in chronically HBV infected patients with high levels of hepatitis B surface antigen and persist after antiviral therapy. Aliment Pharmacol Ther. (2019) 49:1346–59. doi: 10.1111/apt.15226
57. Zhang Y, Han J, Zhang X, Li F, Guo Y, He J, et al. Lower frequency of MDSCs was significantly related to functional cure in CHB patients treated with peginterferon. Liver Int Off J Int Assoc Study Liver. (2023) 43:329–39. doi: 10.1111/liv.15489
58. Mace EM. Human natural killer cells: Form, function, and development. J Allergy Clin Immunol. (2023) 151:371–85. doi: 10.1016/j.jaci.2022.09.022
59. Maini MK and Peppa D. NK cells: A double-edged sword in chronic hepatitis B virus infection. Front Immunol. (2013) 4:57. doi: 10.3389/fimmu.2013.00057
60. Lunemann S, Malone DFG, Hengst J, Port K, Grabowski J, Deterding K, et al. Compromised function of natural killer cells in acute and chronic viral hepatitis. J Infect Dis. (2014) 209:1362–73. doi: 10.1093/infdis/jit561
61. Zhao J, Li Y, Jin L, Zhang S, Fan R, Sun Y, et al. Natural killer cells are characterized by the concomitantly increased interferon-γ and cytotoxicity in acute resolved hepatitis B patients. PLoS One. (2012) 7:e49135. doi: 10.1371/journal.pone.0049135
62. Yu W-H, Cosgrove C, Berger CT, Cheney PC, Krykbaeva M, Kim AY, et al. ADCC-mediated CD56DIM NK cell responses are associated with early HBsAg clearance in acute HBV infection. Pathog Immun. (2018) 3:2–18. doi: 10.20411/pai.v3i1.228
63. Dunn C, Peppa D, Khanna P, Nebbia G, Jones M, Brendish N, et al. Temporal analysis of early immune responses in patients with acute hepatitis B virus infection. Gastroenterology. (2009) 137:1289–300. doi: 10.1053/j.gastro.2009.06.054
64. Wang Y, Wang W, Shen C, Wang Y, Jiao M, Yu W, et al. NKG2D modulates aggravation of liver inflammation by activating NK cells in HBV infection. Sci Rep. (2017) 7:88. doi: 10.1038/s41598-017-00221-9
65. Stelma F, de Niet A, Tempelmans Plat-Sinnige MJ, Jansen L, Takkenberg RB, Reesink HW, et al. Natural killer cell characteristics in patients with chronic hepatitis B virus (HBV) infection are associated with HBV surface antigen clearance after combination treatment with pegylated interferon alfa-2a and adefovir. J Infect Dis. (2015) 212:1042–51. doi: 10.1093/infdis/jiv180
66. Liu N, Liu B, Zhang L, Li H, Chen Z, Luo A, et al. Recovery of circulating CD56dim NK cells and the balance of Th17/treg after nucleoside analog therapy in patients with chronic hepatitis B and low levels of HBsAg. Int Immunopharmacol. (2018) 62:59–66. doi: 10.1016/j.intimp.2018.06.043
67. Ju Y, Hou N, Meng J, Wang X, Zhang X, Zhao D, et al. T cell immunoglobulin- and mucin-domain-containing molecule-3 (tim-3) mediates natural killer cell suppression in chronic hepatitis B. J Hepatol. (2010) 52:322–9. doi: 10.1016/j.jhep.2009.12.005
68. Dumolard L, Hilleret M-N, Costentin C, Mercey-Ressejac M, Sturm N, Gerster T, et al. Differences in the intrahepatic expression of immune checkpoint molecules on T cells and natural killer cells in chronic HBV patients. Front Immunol. (2024) 15:1489770. doi: 10.3389/fimmu.2024.1489770
69. Shi CC, Tjwa ETTL, Biesta PJ, Boonstra A, Xie Q, Janssen HLA, et al. Hepatitis B virus suppresses the functional interaction between natural killer cells and plasmacytoid dendritic cells. J Viral Hepat. (2012) 19:e26–33. doi: 10.1111/j.1365-2893.2011.01496.x
70. Ghosh S, Nandi M, Pal S, Mukhopadhyay D, Chakraborty BC, Khatun M, et al. Natural killer cells contribute to hepatic injury and help in viral persistence during progression of hepatitis B e-antigen-negative chronic hepatitis B virus infection. Clin Microbiol Infect. (2016) 22:733.e9–733.e19. doi: 10.1016/j.cmi.2016.05.009
71. Peppa D, Micco L, Javaid A, Kennedy PTF, Schurich A, Dunn C, et al. Blockade of immunosuppressive cytokines restores NK cell antiviral function in chronic hepatitis B virus infection. PLoS Pathog. (2010) 6:e1001227. doi: 10.1371/journal.ppat.1001227
72. Sun C, Fu B, Gao Y, Liao X, Sun R, Tian Z, et al. TGF-β1 down-regulation of NKG2D/DAP10 and 2B4/SAP expression on human NK cells contributes to HBV persistence. PLoS Pathog. (2012) 8:e1002594. doi: 10.1371/journal.ppat.1002594
73. Peppa D, Gill US, Reynolds G, Easom NJW, Pallett LJ, Schurich A, et al. Up-regulation of a death receptor renders antiviral T cells susceptible to NK cell-mediated deletion. J Exp Med. (2013) 210:99–114. doi: 10.1084/jem.20121172
74. Dunn C, Brunetto M, Reynolds G, Christophides T, Kennedy PT, Lampertico P, et al. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J Exp Med. (2007) 204:667–80. doi: 10.1084/jem.20061287
75. Zhang Z, Zhang S, Zou Z, Shi J, Zhao J, Fan R, et al. Hypercytolytic activity of hepatic natural killer cells correlates with liver injury in chronic hepatitis B patients. Hepatol Baltim Md. (2011) 53:73–85. doi: 10.1002/hep.23977
76. Chen Y, Sun R, Jiang W, Wei H, and Tian Z. Liver-specific HBsAg transgenic mice are over-sensitive to poly(I:C)-induced liver injury in NK cell- and IFN-gamma-dependent manner. J Hepatol. (2007) 47:183–90. doi: 10.1016/j.jhep.2007.02.020
77. Zheng Q, Zhu YY, Chen J, Ye YB, Li JY, Liu YR, et al. Activated natural killer cells accelerate liver damage in patients with chronic hepatitis B virus infection. Clin Exp Immunol. (2015) 180:499–508. doi: 10.1111/cei.12597
78. Beudeker BJB, Osmani Z, van Oord GW, Groothuismink ZMA, de Knegt RJ, Hoogenboezem RM, et al. Association of HBsAg levels with differential gene expression in NK, CD8 T, and memory B cells in treated patients with chronic HBV. JHEP Rep Innov Hepatol. (2024) 6:100980. doi: 10.1016/j.jhepr.2023.100980
79. Gu Y, Lian Y, Zheng Q, Huang Z, Gu L, Bi Y, et al. Association among cytokine profiles of innate and adaptive immune responses and clinical-virological features in untreated patients with chronic hepatitis B. BMC Infect Dis. (2020) 20:509. doi: 10.1186/s12879-020-05233-x
80. Oliviero B, Varchetta S, Paudice E, Michelone G, Zaramella M, Mavilio D, et al. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology. (2009) 137:1151–1160, 1160.e1–7. doi: 10.1053/j.gastro.2009.05.047
81. Tjwa ETTL, van Oord GW, Hegmans JP, Janssen HLA, and Woltman AM. Viral load reduction improves activation and function of natural killer cells in patients with chronic hepatitis B. J Hepatol. (2011) 54:209–18. doi: 10.1016/j.jhep.2010.07.009
82. Wang W-T, Zhao X-Q, Li G-P, Chen Y-Z, Wang L, Han M-F, et al. Immune response pattern varies with the natural history of chronic hepatitis B. World J Gastroenterol. (2019) 25:1950–63. doi: 10.3748/wjg.v25.i16.1950
83. de Groen RA, Hou J, van Oord GW, Groothuismink ZMA, van der Heide M, de Knegt RJ, et al. NK cell phenotypic and functional shifts coincide with specific clinical phases in the natural history of chronic HBV infection. Antiviral Res. (2017) 140:18–24. doi: 10.1016/j.antiviral.2017.01.007
84. Li F, Wei H, Wei H, Gao Y, Xu L, Yin W, et al. Blocking the natural killer cell inhibitory receptor NKG2A increases activity of human natural killer cells and clears hepatitis B virus infection in mice. Gastroenterology. (2013) 144:392–401. doi: 10.1053/j.gastro.2012.10.039
85. Zhao P-W, Jia F-Y, Shan Y-X, Ji H-F, Feng J-Y, Niu J-Q, et al. Downregulation and altered function of natural killer cells in hepatitis B virus patients treated with entecavir. Clin Exp Pharmacol Physiol. (2013) 40:190–6. doi: 10.1111/1440-1681.12048
86. Zhang L, Wang Q, Zhao P, Hu X, and Jiang Y. Effects of entecavir on peripheral blood lymphocyte profiles in chronic hepatitis B patients with suboptimal responses to adefovir. Clin Exp Pharmacol Physiol. (2014) 41:514–23. doi: 10.1111/1440-1681.12245
87. Ma L, Cai Y-J, Yu L, Feng J-Y, Wang J, Li C, et al. Treatment with telbivudine positively regulates antiviral immune profiles in Chinese patients with chronic hepatitis B. Antimicrob Agents Chemother. (2013) 57:1304–11. doi: 10.1128/AAC.02181-12
88. Chen T, Zhu L, Shi A, Ding L, Zhang X, Tan Z, et al. Functional restoration of CD56bright NK cells facilitates immune control via IL-15 and NKG2D in patients under antiviral treatment for chronic hepatitis B. Hepatol Int. (2017) 11:419–28. doi: 10.1007/s12072-017-9803-4
89. Shi A, Zhang X, Xiao F, Zhu L, Yan W, Han M, et al. CD56bright natural killer cells induce HBsAg reduction via cytolysis and cccDNA decay in long-term entecavir-treated patients switching to peginterferon alfa-2a. J Viral Hepat. (2018) 25:1352–62. doi: 10.1111/jvh.12946
90. Ma Q, Dong X, Liu S, Zhong T, Sun D, Zong L, et al. Hepatitis B e antigen induces NKG2A+ natural killer cell dysfunction via regulatory T cell-derived interleukin 10 in chronic hepatitis B virus infection. Front Cell Dev Biol. (2020) 8:421. doi: 10.3389/fcell.2020.00421
91. Lv J, Jin Q, Sun H, Chi X, Hu X, Yan H, et al. Antiviral treatment alters the frequency of activating and inhibitory receptor-expressing natural killer cells in chronic hepatitis B virus infected patients. Mediators Inflammation. (2012) 2012:804043. doi: 10.1155/2012/804043
92. Wang F, Xie S, Ran C, Hao H, Jiang T, Deng W, et al. Effect of antiviral therapy during pregnancy on natural killer cells in pregnant women with chronic HBV infection. Front Immunol. (2022) 13:893628. doi: 10.3389/fimmu.2022.893628
93. Bi X, Xie S, Wu S, Cao W, Lin Y, Yang L, et al. Changes of natural killer cells’ phenotype in patients with chronic hepatitis B in intermittent interferon therapy. Front Immunol. (2023) 14:1116689. doi: 10.3389/fimmu.2023.1116689
94. Cao W, Lu H, Zhang L, Wang S, Deng W, Jiang T, et al. Functional molecular expression of nature killer cells correlated to HBsAg clearance in HBeAg-positive chronic hepatitis B patients during PEG-IFN α-2a therapy. Front Immunol. (2022) 13:1067362. doi: 10.3389/fimmu.2022.1067362
95. Cao W, Li M, Zhang L, Lu Y, Wu S, Shen G, et al. The characteristics of natural killer cells in chronic hepatitis B patients who received PEGylated-interferon versus entecavir therapy. BioMed Res Int. (2021) 2021:2178143. doi: 10.1155/2021/2178143
96. Boni C, Lampertico P, Talamona L, Giuberti T, Invernizzi F, Barili V, et al. Natural killer cell phenotype modulation and natural killer/T-cell interplay in nucleos(t)ide analogue-treated hepatitis e antigen-negative patients with chronic hepatitis B. Hepatol Baltim Md. (2015) 62:1697–709. doi: 10.1002/hep.28155
97. Diao H, He J, Zheng Q, Chen J, Cui G, Wei Y, et al. A possible role for NKT-like cells in patients with chronic hepatitis B during telbivudine treatment. Immunol Lett. (2014) 160:65–71. doi: 10.1016/j.imlet.2014.03.013
98. Vecchi A, Rossi M, Tiezzi C, Fisicaro P, Doselli S, Gabor EA, et al. HBcrAg values may predict virological and immunological responses to pegIFN-α in NUC-suppressed HBeAg-negative chronic hepatitis B. Gut. (2024) 73:1737–48. doi: 10.1136/gutjnl-2024-332290
99. Boni C, Vecchi A, Rossi M, Laccabue D, Giuberti T, Alfieri A, et al. TLR7 agonist increases responses of hepatitis B virus-specific T cells and natural killer cells in patients with chronic hepatitis B treated with nucleos(T)ide analogues. Gastroenterology. (2018) 154:1764–1777.e7. doi: 10.1053/j.gastro.2018.01.030
100. Ayithan N, Ghosh A, Dwivedi A, Wallin JJ, Tan SK, Chen D, et al. Oral selective TLR8 agonist selgantolimod induces multiple immune cell responses in humans. Viruses. (2021) 13:2400. doi: 10.3390/v13122400
101. Woltman AM, Ter Borg MJ, Binda RS, Sprengers D, von Blomberg BME, Scheper RJ, et al. Alpha-galactosylceramide in chronic hepatitis B infection: Results from a randomized placebo-controlled phase I/II trial. Antivir Ther. (2009) 14:809–18. doi: 10.3851/IMP1295
102. Ning L, Huang X, Xu Y, Yang G, Yang J, Fu Q, et al. Boosting of hepatitis B virus-specific T cell responses after pegylated-interferon-α-2a therapy for hepatitis B e antigen-positive pediatric patients. J Interferon Cytokine Res Off J Int Soc Interferon Cytokine Res. (2019) 39:740–51. doi: 10.1089/jir.2019.0042
103. Pellicci DG, Koay H-F, and Berzins SP. Thymic development of unconventional T cells: How NKT cells, MAIT cells and γδ T cells emerge. Nat Rev Immunol. (2020) 20:756–70. doi: 10.1038/s41577-020-0345-y
104. Constantinides MG and Belkaid Y. Early-life imprinting of unconventional T cells and tissue homeostasis. Science. (2021) 374:eabf0095. doi: 10.1126/science.abf0095
105. Darrigues J, Almeida V, Conti E, and Ribot JC. The multisensory regulation of unconventional T cell homeostasis. Semin Immunol. (2022) 61–64:101657. doi: 10.1016/j.smim.2022.101657
106. Loh L, Gherardin NA, Sant S, Grzelak L, Crawford JC, Bird NL, et al. Human mucosal-associated invariant T cells in older individuals display expanded TCRαβ clonotypes with potent antimicrobial responses. J Immunol Baltim Md 1950. (2020) 204:1119–33. doi: 10.4049/jimmunol.1900774
107. Yang Zhou J. Innate immunity and early liver inflammation. Front Immunol. (2023) 14:1175147. doi: 10.3389/fimmu.2023.1175147
108. Maini MK and Gehring AJ. The role of innate immunity in the immunopathology and treatment of HBV infection. J Hepatol. (2016) 64:S60–70. doi: 10.1016/j.jhep.2016.01.028
109. Dusseaux M, Martin E, Serriari N, Péguillet I, Premel V, Louis D, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. (2011) 117:1250–9. doi: 10.1182/blood-2010-08-303339
110. Corbett AJ, Eckle SBG, Birkinshaw RW, Liu L, Patel O, Mahony J, et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. (2014) 509:361–5. doi: 10.1038/nature13160
111. Gherardin NA, Souter MN, Koay H-F, Mangas KM, Seemann T, Stinear TP, et al. Human blood MAIT cell subsets defined using MR1 tetramers. Immunol Cell Biol. (2018) 96:507–25. doi: 10.1111/imcb.12021
112. Dias J, Hengst J, Parrot T, Leeansyah E, Lunemann S, Malone DFG, et al. Chronic hepatitis delta virus infection leads to functional impairment and severe loss of MAIT cells. J Hepatol. (2019) 71:301–12. doi: 10.1016/j.jhep.2019.04.009
113. Liu Y, Zhu P, Wang W, Tan X, Liu C, Chen Y, et al. Mucosal-associated invariant T cell dysregulation correlates with conjugated bilirubin level in chronic HBV infection. Hepatol Baltim Md. (2021) 73:1671–87. doi: 10.1002/hep.31602
114. Boeijen LL, Montanari NR, de Groen RA, van Oord GW, van der Heide-Mulder M, de Knegt RJ, et al. Mucosal-associated invariant T cells are more activated in chronic hepatitis B, but not depleted in blood: Reversal by antiviral therapy. J Infect Dis. (2017) 216:969–76. doi: 10.1093/infdis/jix425
115. Xue H, Li H, Ju L-L, Han X-D, Cheng T-C, Luo X, et al. Mucosal-associated invariant T cells in hepatitis B virus-related liver failure. World J Gastroenterol. (2020) 26:4703–17. doi: 10.3748/wjg.v26.i31.4703
116. Vimali J, Yong YK, Murugesan A, Tan HY, Zhang Y, Ashwin R, et al. Chronic viral infection compromises the quality of circulating mucosal-associated invariant T cells and follicular T helper cells via expression of inhibitory receptors. Front Biosci Landmark Ed. (2024) 29:128. doi: 10.31083/j.fbl2903128
117. Yong YK, Saeidi A, Tan HY, Rosmawati M, Enström PF, Batran RA, et al. Hyper-expression of PD-1 is associated with the levels of exhausted and dysfunctional phenotypes of circulating CD161++TCR iVα7.2+ mucosal-associated invariant T cells in chronic hepatitis B virus infection. Front Immunol. (2018) 9:472. doi: 10.3389/fimmu.2018.00472
118. Yong YK, Tan HY, Saeidi A, Rosmawati M, Atiya N, Ansari AW, et al. Decrease of CD69 levels on TCR Vα7.2+CD4+ innate-like lymphocytes is associated with impaired cytotoxic functions in chronic hepatitis B virus-infected patients. Innate Immun. (2017) 23:459–67. doi: 10.1177/1753425917714854
119. Huang W, He W, Shi X, Ye Q, He X, Dou L, et al. Mucosal-associated invariant T-cells are severely reduced and exhausted in humans with chronic HBV infection. J Viral Hepat. (2020) 27:1096–107. doi: 10.1111/jvh.13341
120. Shao L, Zhao H, Guo R, Cheng J, Lu X, and Fan X. Biopsy-based single-cell transcriptomics reveals MAIT cells as potential targets for controlling fibrosis-related liver inflammation due to chronic hepatitis-B infection. Clin Transl Med. (2022) 12:e1073. doi: 10.1002/ctm2.1073
121. Ju L, Xue H, Luo X, Wang Y, Chen L, Shao J, et al. Loss of mucosal-associated invariant T cell in patients with chronic hepatitis B virus-infected cirrhosis. Acta Med Mediterr. (2019) 35:3349–53. doi: 10.19193/0393-6384_2019_6_527
122. Nguyen CT, Maverakis E, Eberl M, and Adamopoulos IE. γδ T cells in rheumatic diseases: From fundamental mechanisms to autoimmunity. Semin Immunopathol. (2019) 41:595–605. doi: 10.1007/s00281-019-00752-5
123. Hu Y, Hu Q, Li Y, Lu L, Xiang Z, Yin Z, et al. γδ T cells: Origin and fate, subsets, diseases and immunotherapy. Signal Transduct Target Ther. (2023) 8:434. doi: 10.1038/s41392-023-01653-8
124. Deusch K, Lüling F, Reich K, Classen M, Wagner H, and Pfeffer K. A major fraction of human intraepithelial lymphocytes simultaneously expresses the gamma/delta T cell receptor, the CD8 accessory molecule and preferentially uses the V delta 1 gene segment. Eur J Immunol. (1991) 21:1053–9. doi: 10.1002/eji.1830210429
125. Ebert LM, Meuter S, and Moser B. Homing and function of human skin gammadelta T cells and NK cells: Relevance for tumor surveillance. J Immunol Baltim Md 1950. (2006) 176:4331–6. doi: 10.4049/jimmunol.176.7.4331
126. Toulon A, Breton L, Taylor KR, Tenenhaus M, Bhavsar D, Lanigan C, et al. A role for human skin-resident T cells in wound healing. J Exp Med. (2009) 206:743–50. doi: 10.1084/jem.20081787
127. Davey MS, Willcox CR, Hunter S, Kasatskaya SA, Remmerswaal EBM, Salim M, et al. The human Vδ2+ T-cell compartment comprises distinct innate-like Vγ9+ and adaptive Vγ9- subsets. Nat Commun. (2018) 9:1760. doi: 10.1038/s41467-018-04076-0
128. Wragg KM, Tan H-X, Kristensen AB, Nguyen-Robertson CV, Kelleher AD, Parsons MS, et al. High CD26 and low CD94 expression identifies an IL-23 responsive Vδ2+ T cell subset with a MAIT cell-like transcriptional profile. Cell Rep. (2020) 31:107773. doi: 10.1016/j.celrep.2020.107773
129. Provine NM, Binder B, FitzPatrick MEB, Schuch A, Garner LC, Williamson KD, et al. Unique and common features of innate-like human Vδ2+ γδT cells and mucosal-associated invariant T cells. Front Immunol. (2018) 9:756. doi: 10.3389/fimmu.2018.00756
130. Vavassori S, Kumar A, Wan GS, Ramanjaneyulu GS, Cavallari M, El Daker S, et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human γδ T cells. Nat Immunol. (2013) 14:908–16. doi: 10.1038/ni.2665
131. Sandstrom A, Peigné C-M, Léger A, Crooks JE, Konczak F, Gesnel M-C, et al. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vγ9Vδ2 T cells. Immunity. (2014) 40:490–500. doi: 10.1016/j.immuni.2014.03.003
132. Chen CY, Yao S, Huang D, Wei H, Sicard H, Zeng G, et al. Phosphoantigen/IL2 expansion and differentiation of Vγ2Vδ2 T cells increase resistance to tuberculosis in nonhuman primates. PLoS Pathog. (2013) 9:e1003501. doi: 10.1371/journal.ppat.1003501
133. Qaqish A, Huang D, Chen CY, Zhang Z, Wang R, Li S, et al. Adoptive transfer of phosphoantigen-specific γδ T cell subset attenuates mycobacterium tuberculosis infection in nonhuman primates. J Immunol Baltim Md 1950. (2017) 198:4753–63. doi: 10.4049/jimmunol.1602019
134. Cieslak SG and Shahbazi R. Gamma delta T cells and their immunotherapeutic potential in cancer. biomark Res. (2025) 13:51. doi: 10.1186/s40364-025-00762-6
135. Jia Z-H, Li Y-Y, Wang J-Y, Zhang J-Y, Huang A, Guo X-D, et al. Activated γδ T cells exhibit cytotoxicity and the capacity for viral clearance in patients with acute hepatitis B. Clin Immunol Orlando Fla. (2019) 202:40–8. doi: 10.1016/j.clim.2019.03.005
136. Chang K-M, Traum D, Park J-J, Ho S, Ojiro K, Wong DK, et al. Distinct phenotype and function of circulating Vδ1+ and Vδ2+ γδT-cells in acute and chronic hepatitis B. PLoS Pathog. (2019) 15:e1007715. doi: 10.1371/journal.ppat.1007715
137. Chang L, Wang L, Ling N, Peng H, and Chen M. Increase in liver γδ T cells with concurrent augmentation of IFN-β production during the early stages of a mouse model of acute experimental hepatitis B virus infection. Exp Ther Med. (2020) 19:67–78. doi: 10.3892/etm.2019.8197
138. Wu X, Zhang J-Y, Huang A, Li Y-Y, Zhang S, Wei J, et al. Decreased Vδ2 γδ T cells associated with liver damage by regulation of Th17 response in patients with chronic hepatitis B. J Infect Dis. (2013) 208:1294–304. doi: 10.1093/infdis/jit312
139. Chen M, Zhang D, Zhen W, Shi Q, Liu Y, Ling N, et al. Characteristics of circulating T cell receptor gamma-delta T cells from individuals chronically infected with hepatitis B virus (HBV): An association between V(delta)2 subtype and chronic HBV infection. J Infect Dis. (2008) 198:1643–50. doi: 10.1086/593065
140. Gogoi D, Borkakoty B, Biswas D, Yadav K, and Patel V. Characteristics of circulatory γδ T cells in patients with symptomatic chronic hepatitis B infection. Viral Immunol. (2021) 34:483–90. doi: 10.1089/vim.2020.0314
141. Chen M, Hu P, Peng H, Zeng W, Shi X, Lei Y, et al. Enhanced peripheral γδT cells cytotoxicity potential in patients with HBV-associated acute-on-chronic liver failure might contribute to the disease progression. J Clin Immunol. (2012) 32:877–85. doi: 10.1007/s10875-012-9678-z
142. Li K, Lu E, Wang Q, Xu R, Yuan W, Wu R, et al. Serum vitamin D deficiency is associated with increased risk of γδ T cell exhaustion in HBV-infected patients. Immunology. (2024) 171:31–44. doi: 10.1111/imm.13696
143. Cannizzo ES, Tincati C, Binda F, Ronzi P, Cazzaniga FA, Antinori S, et al. Unconventional T cells in chronic hepatitis B patients on long-term suppressive therapy with tenofovir followed by a peg-IFN add-on strategy: A randomized study. J Viral Hepat. (2018) 25:381–90. doi: 10.1111/jvh.12820
144. Chen M, Hu P, Ling N, Peng H, Lei Y, Hu H, et al. Enhanced functions of peripheral γδ T cells in chronic hepatitis B infection during interferon α treatment in vivo and in vitro. PLoS One. (2015) 10:e0120086. doi: 10.1371/journal.pone.0120086
145. Liu K, Shen YX, Ling N, Lei Y, Hu P, Ren H, et al. changes and clinical significance of γδT cells in peripheral blood of patients with chronic hepatitis B during pegylated interferon α-2a treatment. Zhonghua Gan Zang Bing Za Zhi Zhonghua Ganzangbing Zazhi Chin J Hepatol. (2018) 26:365–70. doi: 10.3760/cma.j.issn.1007-3418.2018.05.010
146. Lai Q, Ma S, Ge J, Huang Z, Huang X, Jiang X, et al. TCRγδ(+)CD4(-)CD8(-) T cells suppress the CD8(+) T-cell response to hepatitis B virus peptides, and are associated with viral control in chronic hepatitis B. PLoS One. (2014) 9:e88475. doi: 10.1371/journal.pone.0088475
147. Brigl M, Tatituri RVV, Watts GFM, Bhowruth V, Leadbetter EA, Barton N, et al. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med. (2011) 208:1163–77. doi: 10.1084/jem.20102555
148. Rhost S, Sedimbi S, Kadri N, and Cardell SL. Immunomodulatory type II natural killer T lymphocytes in health and disease. Scand J Immunol. (2012) 76:246–55. doi: 10.1111/j.1365-3083.2012.02750.x
149. Kato S, Berzofsky JA, and Terabe M. Possible therapeutic application of targeting type II natural killer T cell-mediated suppression of tumor immunity. Front Immunol. (2018) 9:314. doi: 10.3389/fimmu.2018.00314
150. Tan X, Ding Y, Zhu P, Dou R, Liang Z, Yang D, et al. Elevated hepatic CD1d levels coincide with invariant NKT cell defects in chronic hepatitis B virus infection. J Immunol Baltim Md 1950. (2018) 200:3530–8. doi: 10.4049/jimmunol.1701801
151. Wu X, Zhao W, Miao Q, Shi S, Wei B, Luo L, et al. CCR2+TREM-1+ monocytes promote natural killer T cell dysfunction contributing towards HBV disease progression. Immunol Res. (2024) 72:938–47. doi: 10.1007/s12026-024-09495-4
152. Conroy MJ, Mac Nicholas R, Grealy R, Taylor M, Otegbayo JA, O’Dea S, et al. Circulating CD56dim natural killer cells and CD56+ T cells that produce interferon-γ or interleukin-10 are expanded in asymptomatic, E antigen-negative patients with persistent hepatitis B virus infection. J Viral Hepat. (2015) 22:335–45. doi: 10.1111/jvh.12299
153. Wu S, Li M, Sun X, Zhou Z, Zhu X, Zhang X, et al. phenotypic and functional features of NK and NKT cells in chronic hepatitis B. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi Chin J Cell Mol Immunol. (2015) 31:821–5.
154. Yang Z, Lei Y, Chen C, Ren H, and Shi T. Roles of the programmed cell death 1, T cell immunoglobulin mucin-3, and cluster of differentiation 288 pathways in the low reactivity of invariant natural killer T cells after chronic hepatitis B virus infection. Arch Virol. (2015) 160:2535–45. doi: 10.1007/s00705-015-2539-3
155. Li M, Zhou Z-H, Sun X-H, Zhang X, Zhu X-J, Jin S-G, et al. The dynamic changes of circulating invariant natural killer T cells during chronic hepatitis B virus infection. Hepatol Int. (2016) 10:594–601. doi: 10.1007/s12072-015-9650-0
156. Jiang X, Zhang M, Lai Q, Huang X, Li Y, Sun J, et al. Restored circulating invariant NKT cells are associated with viral control in patients with chronic hepatitis B. PLoS One. (2011) 6:e28871. doi: 10.1371/journal.pone.0028871
157. Zhu H, Zhang Y, Liu H, Zhang Y, Kang Y, Mao R, et al. Preserved function of circulating invariant natural killer T cells in patients with chronic hepatitis B virus infection. Med (Baltimore). (2015) 94:e961. doi: 10.1097/MD.0000000000000961
158. Huang F, Lu MH, Gong HY, and Xiong ZP. Changes in peripheral blood natural killer T cells in hepatitis B e antigen-positive chronic hepatitis B patients and efficacy prediction after pegylated interferon therapy. Genet Mol Res GMR. (2015) 14:4932–8. doi: 10.4238/2015.May.11.26
159. Safadi R, Israeli E, Papo O, Shibolet O, Melhem A, Bloch A, et al. Treatment of chronic hepatitis B virus infection via oral immune regulation toward hepatitis B virus proteins. Am J Gastroenterol. (2003) 98:2505–15. doi: 10.1111/j.1572-0241.2003.07700.x
160. Jo J, Tan AT, Ussher JE, Sandalova E, Tang X-Z, Tan-Garcia A, et al. Toll-like receptor 8 agonist and bacteria trigger potent activation of innate immune cells in human liver. PLoS Pathog. (2014) 10:e1004210. doi: 10.1371/journal.ppat.1004210
161. Wu D, Kao J-H, Piratvisuth T, Wang X, Kennedy PTF, Otsuka M, et al. Update on the treatment navigation for functional cure of chronic hepatitis B: Expert consensus 2.0. Clin Mol Hepatol. (2025) 31:S134–64. doi: 10.3350/cmh.2024.0780
162. Tak WY, Chuang W-L, Chen C-Y, Tseng K-C, Lim Y-S, Lo G-H, et al. Phase ib/IIa randomized study of heterologous ChAdOx1-HBV/MVA-HBV therapeutic vaccination (VTP-300) as monotherapy and combined with low-dose nivolumab in virally-suppressed patients with CHB. J Hepatol. (2024) 81:949–59. doi: 10.1016/j.jhep.2024.06.027
163. Buti M, Heo J, Tanaka Y, Andreone P, Atsukawa M, Cabezas J, et al. Sequential peg-IFN after bepirovirsen may reduce post-treatment relapse in chronic hepatitis B. J Hepatol. (2025) 82:222–34. doi: 10.1016/j.jhep.2024.08.010
164. Hou J, Zhang W, Xie Q, Hua R, Tang H, Morano Amado LE, et al. Xalnesiran with or without an immunomodulator in chronic hepatitis B. N Engl J Med. (2024) 391:2098–109. doi: 10.1056/NEJMoa2405485
165. Gehring AJ, Mendez P, Richter K, Ertl H, Donaldson EF, Mishra P, et al. Immunological biomarker discovery in cure regimens for chronic hepatitis B virus infection. J Hepatol. (2022) 77:525–38. doi: 10.1016/j.jhep.2022.02.020
Keywords: hepatitis B virus, antiviral treatment, dendritic cell (DC), monocyte, natural killer (Nk) cell, MAIT (mucosal-associated invariant T) cell, γδT cell, NKT (natural killer T) cell
Citation: Shu Y, Li S, Du Y and Zheng X (2025) Anti-HBV treatment partially restores the dysfunction of innate immune cells and unconventional T cells during chronic HBV infection. Front. Immunol. 16:1611976. doi: 10.3389/fimmu.2025.1611976
Received: 15 April 2025; Accepted: 23 June 2025;
Published: 04 July 2025.
Edited by:
Saba Valadkhan, Case Western Reserve University, United StatesReviewed by:
Zuzana Macek Jilkova, Centre Hospitalier Universitaire de Grenoble, FranceRiddhi Sharma, The Institute of Liver and Biliary Sciences (ILBS), India
Copyright © 2025 Shu, Li, Du and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Zheng, eGluekBodXN0LmVkdS5jbg==; Yanqin Du, eWFucWluZHVAMTYzLmNvbQ==
†These authors have contributed equally to this work and share senior authorship
 Yiwen Shu
Yiwen Shu Sumeng Li
Sumeng Li Yanqin Du
Yanqin Du Xin Zheng
Xin Zheng