- 1Department of Respiratory and Critical Care Medicine, The First People’s Hospital of Lianyungang, Affiliated Hospital of Xuzhou Medical College, Lianyungang, China
- 2Department of Laboratory Medicine, The First People’s Hospital of Lianyungang, Affiliated Hospital of Xuzhou Medical College, Lianyungang, China
With the widespread clinical application of immune checkpoint inhibitors (ICIs), the treatment of lung squamous cell carcinoma (LUSC) has entered a new era, characterized by equal emphasis on precision medicine and immunotherapy. Among these, programmed cell death protein-1 (PD-1) inhibitors have demonstrated significant efficacy in prolonging patient survival. However, while immunotherapy provides substantial clinical benefits, it may also induce immune-related adverse events (irAEs). We report a case of a 74-year-old male with LUSC who developed reversible pulmonary bullae following camrelizumab treatment. The patient presented with a one-year history of cough. Chest CT revealed a right hilar mass (11×10cm) with pleural effusion. Histopathological analysis of EBUS-TBNA specimens confirmed squamous cell carcinoma. Comprehensive systemic evaluation established the diagnosis of right lung squamous cell carcinoma (cT4N3M1a, stage IVA). The patient received albumin-bound paclitaxel and carboplatin in combination with camrelizumab. During treatment, the patient developed a known immune-related adverse event, interstitial pneumonitis, as well as a previously unreported complication, pulmonary bullae. After discontinuation of camrelizumab and initiation of glucocorticoid therapy (methylprednisolone), the pulmonary bullae showed significant resolution. We believe that the formation of these reversible pulmonary bulla may be associated with two mechanisms. First, immune-mediated airway inflammation and mucus-induced airway obstruction. Second, microvascular or small pulmonary vessel thrombosis leading to localized ischemic injury, which may allow thrombi to enter the airway lumen. Both mechanisms may contribute to a “One-way valve” effect, resulting in alveolar overdistension and bulla formation. This case suggests that pulmonary bullae may represent a rare pulmonary irAE associated with camrelizumab. It provides new clinical insights into immune-related pulmonary complications and offers a valuable reference for the management of similar cases.
Introduction
Camrelizumab (SHR-1210) is a humanized IgG4κ monoclonal antibody targeting programmed cell death protein-1 (PD-1). It binds to the PD-1 with high affinity, blocking its interaction with PD-L1 and PD-L2. This blockade relieves immunosuppression, restores T-cell immune activity, and enhances the body’s anti-tumour immune response (1–3). Clinical studies have demonstrated that camrelizumab, as an immune checkpoint inhibitor (ICI), shows significant therapeutic efficacy in patients with lung squamous cell carcinoma (LUSC) and has been widely adopted in both first-line and second-line treatment settings (4–7). However, the application of ICIs may trigger various immune-related adverse events (irAEs) due to excessive activation of the immune system (8). Previous studies have reported that common adverse effects of camrelizumab include reactive cutaneous capillary endothelial proliferation (RCCEP), anemia, pyrexia, fatigue, hypothyroidism, proteinuria, and checkpoint inhibitor-related pneumonitis (2, 9, 10). At present, there are no reports indicating that camrelizumab may cause pulmonary bullae. However, we identified a case of reversible pulmonary bullae that was highly related to camrelizumab.
Case reports
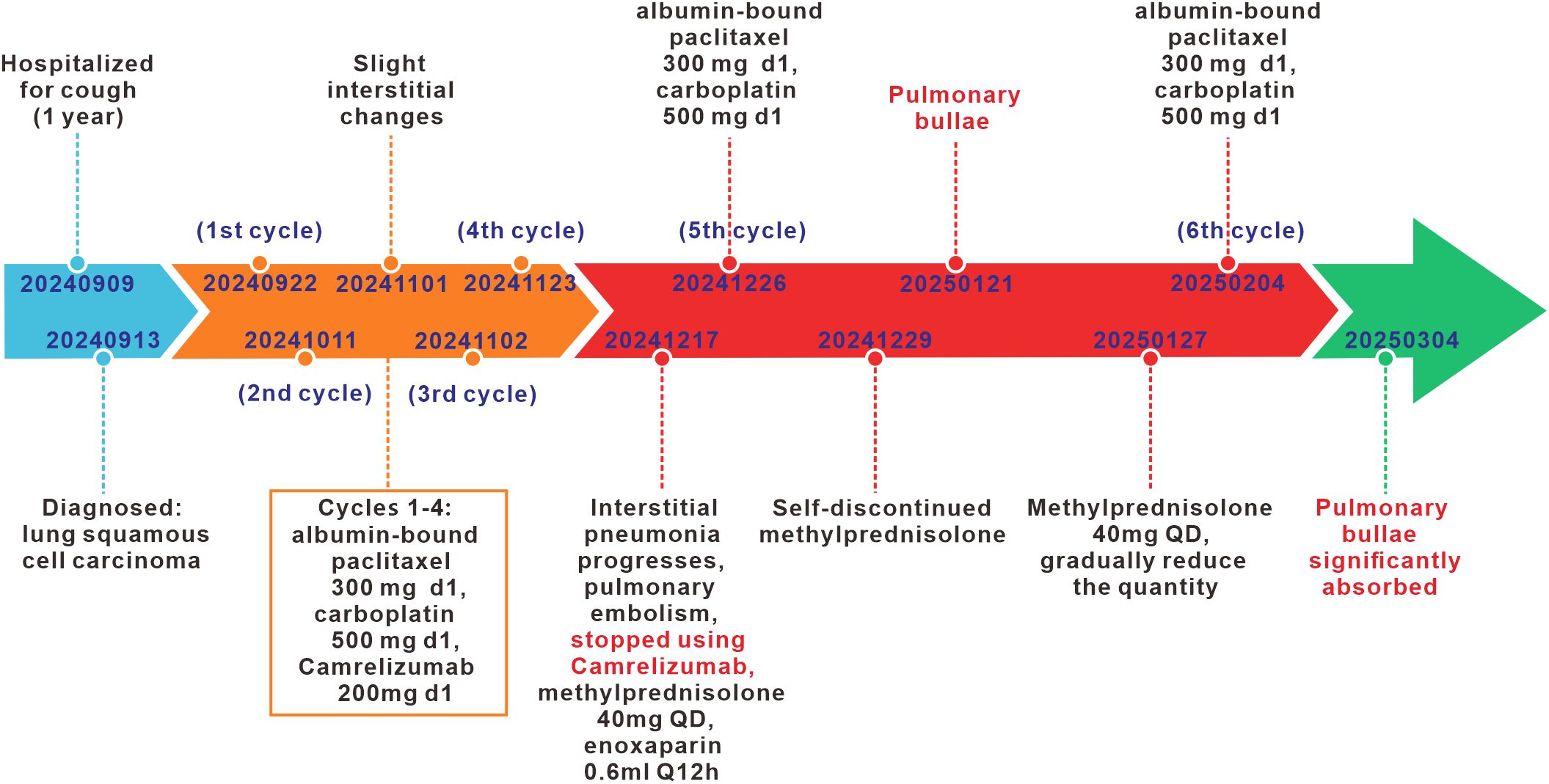
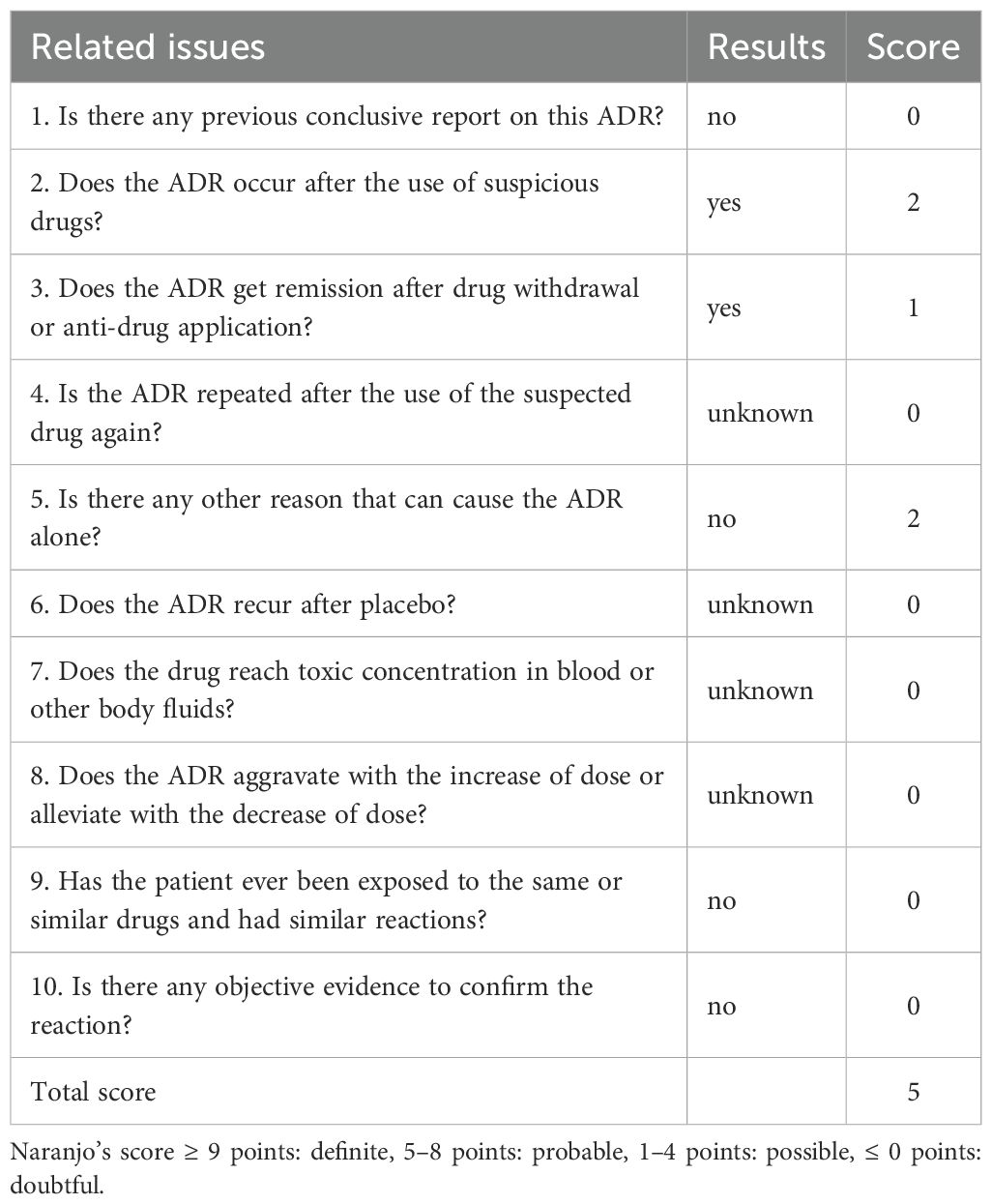
A 74-year-old male patient was admitted with a one-year history of persistent cough, without sputum production or shortness of breath. He had a history of smoking (30 pack-years of smoking) but had quit 20 years prior. He denied any family history of lung cancer or other hereditary diseases. Physical examination revealed a clear mental status, no palpable superficial lymphadenopathy, diminished breath sounds in the right lung with audible moist rales. Cardiac auscultation revealed regular rhythm without murmurs in any valvular area. The abdomen was soft and non-tender, with no palpable hepatosplenomegaly. No edema was noted in the lower limbs. Contrast-enhanced chest CT showed a mass (11×10cm) in the right hilar region; localized bronchial narrowing and occlusion; marked heterogeneous enhancement on contrast imaging; enlarged mediastinal lymph nodes; inflammatory changes in the right lung; and a right-sided pleural effusion (Figure 1A). Serum tumour markers revealed elevated cytokeratin 19 fragment (39.10 ng/ml) and CEA (12.60 ng/ml). Bronchoscopy revealed external compression and obstruction of the right lower lobe bronchus. EBUS revealed an abnormal echogenic lesion in the right lower lobe. The lesion had well-defined margins, a heterogeneous internal echotexture, and clearly visible vascular structures. Fine-needle aspiration was performed while carefully avoiding the vascular areas (Figure 1B). EBUS-TBNA biopsy showed squamous cell carcinoma on histopathological examination (Figure 1C). Immunohistochemistry results: TTF-1 (−), NapsinA (−), P40 (3+), CK5/6 (3+), Ki67 (10%+), CK7 (+), CD34 (−) (Figure 1D). To further determine the disease stage, right-sided thoracic drainage was performed. Cytology of pleural fluid was negative for malignant cells, but pleural fluid CEA was significantly elevated (128 ng/ml), raising strong suspicion of pleural metastasis. Abdominal contrast-enhanced CT revealed a left renal cyst, prostatic calcification, and a small amount of pelvic effusion. Brain MRI indicated mild lacunar infarction. Cervical and supraclavicular ultrasound showed no significant lymphadenopathy. Whole-body bone scintigraphy and rib MRI revealed no evidence of bone metastases. Based on the AJCC 9th edition TNM staging system, the patient was diagnosed with stage IVA right lung squamous cell carcinoma (cT4N3M1a) (Figure 2A). Following multidisciplinary team (MDT) consultation, the patient received two cycles of chemotherapy with albumin-bound paclitaxel (0.3 g on day 1) and carboplatin (500 mg on day 1), administered every 3 weeks, combined with camrelizumab (200 mg on day 1, Q3W). According to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, follow-up imaging indicated partial remission, and CT scans revealed mild interstitial changes in the right lung (Figure 2B). After two additional cycles of the same regimen, the patient developed exertional dyspnea. Laboratory tests revealed an elevated D-dimer level (3626 ng/ml). Computed tomography pulmonary angiography (CTPA) indicated right lower pulmonary artery embolism, as well as interstitial changes in both lungs, which had progressed compared to previous imaging (Figure 1E). Immune-related pneumonitis is highly suspected. According to the immune-related adverse event management guidelines, the patient discontinued camrelizumab and was administered methylprednisolone 40 mg QD for anti-inflammatory treatment, along with enoxaparin for anticoagulation and symptomatic treatment. The patient completed the fifth cycle of chemotherapy with albumin-bound paclitaxel (0.3 g on day 1) and carboplatin (500 mg on day 1) and, after discharge, self-discontinued methylprednisolone. Follow-up chest CT revealed multiple pulmonary bullae in the right lung (Figure 2C). The development of pulmonary bullae following interstitial pneumonitis suggests a strong likelihood of an immune-related cause. Methylprednisolone 40 mg QD treatment was reinitiated, with gradual tapering (total course approximately 6 weeks). During this period, the sixth cycle of chemotherapy was completed with albumin-bound paclitaxel (0.3 g on day 1) and carboplatin (500 mg on day 1). Follow-up CT showed significant improvement in both interstitial pneumonia and pulmonary bullae (Figure 2D) (Timeline summarizing the patient’s treatment progression see Figure 3). According to the Naranjo Adverse Drug Reaction Probability Scale (Table 1), the reversible pulmonary bullae were deemed “most likely” related to camrelizumab.
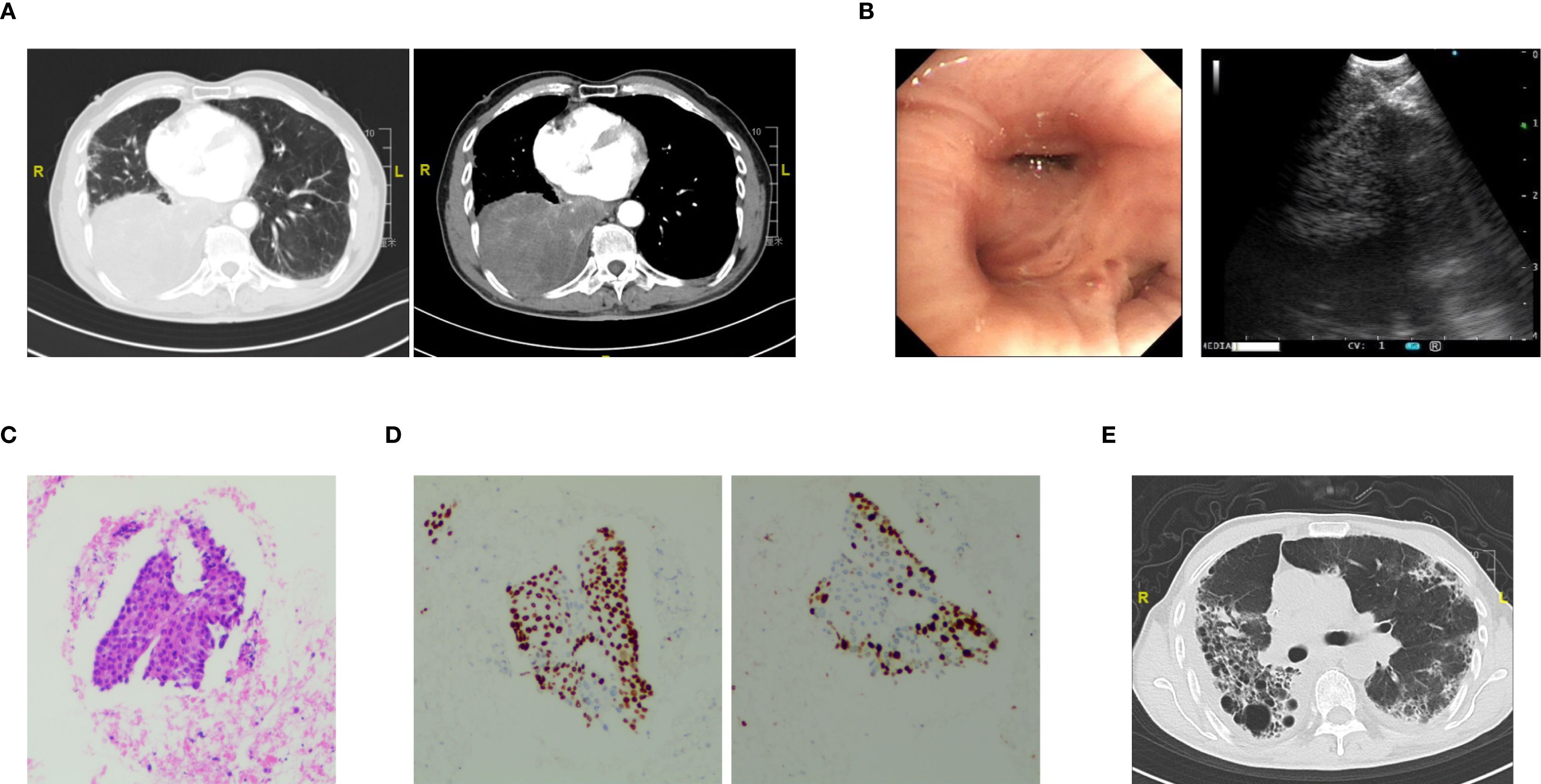
Figure 1. The diagnostic workup on initial visit. (A) Contrast-enhanced chest CT showing a right hilar mass with bronchial narrowing and right-sided pleural effusion. (B) Bronchoscopic view showing extrinsic compression and obstruction of the right lower lobe bronchus (Left). EBUS image showing a hypoechoic lesion in the right lower lobe with clear boundaries, heterogeneous internal echo, and visible thick blood vessels; fine-needle aspiration was performed while avoiding vascular structures (Right). (C) EBUS-TBNA revealing cytological features consistent with squamous cell carcinoma. (D) Immunohistochemical staining of biopsy specimens showing strong positivity for P40 (left) and Ki-67 (right). (E) Chest CT scan showing bilateral interstitial pneumonitis after four cycles of chemotherapy combined with camrelizumab.
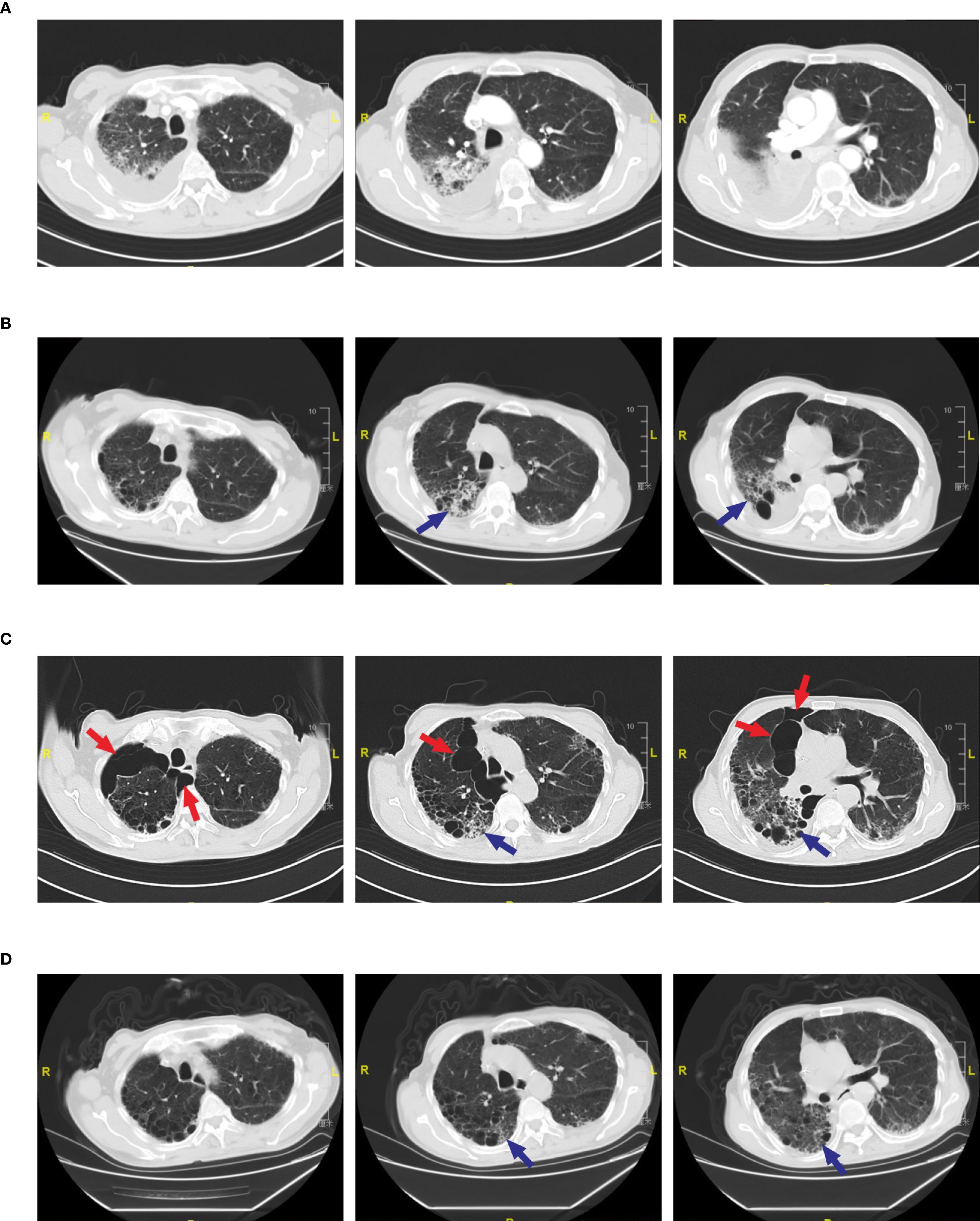
Figure 2. Radiological changes in pulmonary bullae during treatment. (A) Pre-treatment CT scan showing no evidence of pulmonary bullae. (B) CT scan after two cycles of camrelizumab showing no pulmonary bullae. Blue arrows indicate interstitial pneumonia in the right lung. (C) CT scan after four cycles of camrelizumab.(Left) Red arrows indicate multiple pulmonary bullae in the right upper lobe, with the largest measuring 8.75 cm in diameter; (Middle) Red arrows indicate multiple pulmonary bullae in the right upper lobe; blue arrows indicate interstitial pneumonia. (Right) Red arrows indicate multiple pulmonary bullae in the right middle lobe, with the largest measuring 4.85 cm in diameter; blue arrows indicate interstitial pneumonia. (D) CT scan following methylprednisolone therapy showing resolution of pulmonary bullae and improvement of interstitial pneumonia. Blue arrow indicates interstitial pneumonia.
Discussion
Although the incidence of pulmonary irAEs is relatively low, their clinical severity is not to be ignored. With the clinical application of immune checkpoint inhibitors (ICIs), an increasing number of rare pulmonary toxicities have been gradually recognized. These toxicities include interstitial pneumonia, ground glass opacities, eosinophilic pneumonia, pulmonary sarcoidosis, organizing pneumonia (OP), adult respiratory distress syndrome, and lung cavitation (11–14). In the present case, the patient developed interstitial pneumonia and previously unreported pulmonary bullae following camrelizumab treatment. The imaging features, clinical symptoms, and temporal progression suggest that pulmonary bullae may represent a rare form of pulmonary irAE. Notably, a previous report described a patient with metastatic squamous cell carcinoma (SCC) who developed bullae-like changes in the lungs on CT imaging after receiving 12 cycles of the PD-1 inhibitor nivolumab (15). This case provides important support for our findings and suggests that immune checkpoint inhibitors may induce similar pulmonary toxicities.
PD-1/PD-L1 inhibitors enhance anti-tumor immune activity by relieving T cell exhaustion, but this widespread immune activation is not entirely tumor-specific and can also induce inflammatory responses in normal tissues (16, 17). Camrelizumab enhances the immune response of Th1 and Th17 helper T cells by inhibiting the PD-1 pathway. At the same time, it suppresses the function of regulatory T cells (Tregs). This disruption of immune tolerance may lead to excessive local inflammation in the lungs (18). IL-17 and other pro-inflammatory cytokines secreted by Th17 cells can drive the recruitment of neutrophils to the lung tissue. Upon activation, these cells release elastase and other tissue-degrading enzymes, leading to the destruction of the alveolar wall structure (19). Additionally, activated CD8+ cytotoxic T lymphocytes may also directly mediate damage to alveolar epithelial cells by releasing perforin and granzymes (20). We believe that the mechanism behind this leads to alveolar wall rupture and the fusion of multiple alveoli, resulting in the formation of irreversible pulmonary bullae. However, in this case, the patient showed significant resolution of pulmonary bullae on imaging after discontinuing camrelizumab and initiating corticosteroid therapy, suggesting that the bullae may be reversible. We further speculate that the reversible pulmonary bullae in this case may not be caused by typical alveolar elastic fiber destruction. Instead, they may be caused by another mechanism: immune checkpoint inhibitors can trigger small airway inflammation, edema, or mucus plugging. These changes may lead to airway obstruction and create a “one-way valve” effect, causing distal alveolar air trapping and hyperinflation. This process can ultimately lead to the formation of pulmonary bullae (21, 22). After PD-1 inhibitors activate T cells, they may cause immune-mediated injury to the pulmonary capillary endothelium, leading to increased vascular permeability and pulmonary edema, which in turn affects pulmonary compliance and ventilation distribution, ultimately inducing regional alveolar overinflation (23). In addition, after the tumor was controlled, the patient developed pulmonary embolism. Anticoagulation therapy was administered, and the treatment regimen was adjusted to discontinue camrelizumab while continuing chemotherapy, resulting in clinical improvement. This suggests that the pulmonary embolism may be related to immune therapy rather than tumor progression. It is possible that immune-mediated small vessel vasculitis or a hypercoagulable state led to microvascular or capillary thrombosis in the lungs, resulting in ventilation/perfusion (V/Q) mismatch. Under conditions of relative hyperventilation, reduced perfusion, and localized ischemic injury, microvascular thrombi may enter the airway lumen and act as a “one-way valve.” This one-way valve mechanism causes distal alveolar overdistension, ultimately leading to the formation of pulmonary bullae (24–26). In summary, the mechanisms outlined above may synergistically contribute to immune-related lung injury, resulting in either irreversible or reversible pulmonary bullae.
We reviewed the patient’s treatment history. The patient has a history of smoking but quit 20 years ago. Prior to treatment, the patient had no long-term respiratory symptoms such as persistent sputum production or dyspnea. Imaging studies showed no evidence of emphysema or pulmonary bullae. Considering that the patient had no chronic obstructive pulmonary disease (COPD), it is therefore presumed that the formation of pulmonary bullae is a new onset. During treatment, the patient developed a fever with a CRP level of 110.27 mg/L. Six respiratory pathogen tests, PCT, sputum smear, sputum culture, and blood culture all showed no abnormalities. The patient received antimicrobial therapy with piperacillin-tazobactam (4.5 g Q12H). However, after 72 hours, the fever persisted and PCT level showed no increase, suggesting that the fever was unlikely to be infection-related. After switching to methylprednisolone treatment, the patient’s body temperature returned to normal. This suggests that the fever was likely due to an inflammatory response related to immunotherapy, and the formation of pulmonary bullae is not considered to be caused by infection. During the subsequent chemotherapy, the patient did not experience any further infectious symptoms, which further excluding infection-related fever and infection as causes of the pulmonary bullae. Although paclitaxel and carboplatin can cause pulmonary toxicity, their primary manifestations are diffuse pulmonary fibrosis or interstitial changes, with bulla formation being rare (27). After the sixth cycle of chemotherapy, a follow-up CT scan showed significant absorption and improvement of the pulmonary bullae. This finding suggests that the formation of pulmonary bullae is unrelated to the chemotherapy. It is noteworthy that although irAEs commonly occur during the early phase of treatment, they may also develop in the later stages or even after discontinuation of therapy (28). In this case, the patient developed interstitial pneumonia after receiving camrelizumab immunotherapy combined with chemotherapy. Camrelizumab was discontinued, and methylprednisolone treatment was initiated. However, the patient self-discontinued methylprednisolone after discharge, and follow-up CT scans revealed multiple pulmonary bullae in the right lung. This suggested progression of immune-related lung injury. After the patient received education, methylprednisolone was resumed according to the prescribed regimen. Follow-up CT scans showed significant absorption of the pulmonary bullae and marked improvement in interstitial pneumonia. These findings further strengthen the causal inference that the pulmonary bullae were immune-related. In conclusion, we propose that pulmonary bullae may represent a rare form of pulmonary irAE.
This case provides important insights into the identification of a new form of pulmonary adverse reaction related to immunotherapy, and also suggests the need for early recognition and prompt management of atypical irAEs in clinical practice. However, as this is a single case observation, a definitive causal relationship cannot be fully established. Further validation through large-scale, multicenter studies is required to elucidate the potential association and underlying mechanisms between immunotherapy and pulmonary bulla formation. Future efforts should focus on advancing mechanistic research and improving radiologic recognition to optimize risk management strategies for immunotherapy, thereby enhancing survival outcomes and quality of life in patients with lung cancer.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YW: Writing – original draft. SW: Writing – original draft. QL: Writing – review & editing. QC: Writing – review & editing. JS: Writing – review & editing. HZ: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This report was supported by Research Project of the First People’s Hospital of Lianyungang (L202205).
Acknowledgments
We thank the patient’s relatives for providing permission to share the patient’s information.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen Z, Lu X, and Koral K. The clinical application of camrelizumab on advanced hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. (2020) 14:1017–24. doi: 10.1080/17474124.2020.1807939
2. Markham A and Keam SJ. Camrelizumab: first global approval. Drugs. (2019) 79:1355–61. doi: 10.1007/s40265-019-01167-0
3. Mo H, Huang J, Xu J, Chen X, Wu D, Qu D, et al. Safety, anti-tumour activity, and pharmacokinetics of fixed-dose SHR-1210, an anti-PD-1 antibody in advanced solid tumours: a dose-escalation, phase 1 study. Br J Cancer. (2018) 119:538–45. doi: 10.1038/s41416-018-0100-3
4. Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med. (2021) 9:305–14. doi: 10.1016/S2213-2600(20)30365-9
5. Yang JJ, Huang C, Fan Y, Pan H, Feng J, Jiang L, et al. Camrelizumab in different PD-L1 expression cohorts of pre-treated advanced or metastatic non-small cell lung cancer: a phase II study. Cancer Immunol Immunother. (2022) 71:1393–402. doi: 10.1007/s00262-021-03091-3
6. Ren S, Chen J, Xu X, Jiang T, Cheng Y, Chen G, et al. Camrelizumab plus carboplatin and paclitaxel as first-line treatment for advanced squamous NSCLC (CameL-sq): A phase 3 trial. J Thorac Oncol. (2022) 17:544–57. doi: 10.1016/j.jtho.2021.11.018
7. Zhao D, Bi M, Cheng X, Wang S, Cheng H, Xia X, et al. Camrelizumab-based therapies for the treatment of advanced lung cancer: a prospective, open-label, multicenter, observational, real-world study. Front Immunol. (2025) 16:1494708. doi: 10.3389/fimmu.2025.1494708
8. O’Kane GM, Labbé C, Doherty MK, Young K, Albaba H, and Leighl NB. Monitoring and management of immune-related adverse events associated with programmed cell death protein-1 axis inhibitors in lung cancer. Oncologist. (2017) 22:70–80. doi: 10.1634/theoncologist.2016-0164
9. Wang F, Qin S, Sun X, Ren Z, Meng Z, Chen Z, et al. Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial. J Hematol Oncol. (2020) 13:47. doi: 10.1186/s13045-020-00886-2
10. D’Andréa G, Lassalle S, Guevara N, Mograbi B, and Hofman P. From biomarkers to therapeutic targets: the promise of PD-L1 in thyroid autoimmunity and cancer. Theranostics. (2021) 11:1310–25. doi: 10.7150/thno.50333
11. Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. (2017) 35:709–17. doi: 10.1200/JCO.2016.68.2005
12. Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. (2017) 5:95. doi: 10.1186/s40425-017-0300-z
13. Bukamur H, Katz H, Alsharedi M, Alkrekshi A, Shweihat YR, and Munn NJ. Immune checkpoint inhibitor-related pulmonary toxicity: focus on nivolumab. South Med J. (2020) 113:600–5. doi: 10.14423/SMJ.0000000000001166
14. Bukamur H, Alkrekshi A, Katz H, Alsharedi M, Shweihat YR, and Munn NJ. Immune checkpoint inhibitor-related pulmonary toxicity: A comprehensive review, part II. South Med J. (2021) 114:614–9. doi: 10.14423/SMJ.0000000000001295
15. Rampinelli C, Spitaleri G, Passaro A, Pochesci A, Ancona E, and De Marinis F. Lung tissue injury as an atypical response to nivolumab in non-small cell lung cancer. Am J Respir Crit Care Med. (2017) 196:1349–50. doi: 10.1164/rccm.201705-0875IM
16. Sullivan RJ and Weber JS. Immune-related toxicities of checkpoint inhibitors: mechanisms and mitigation strategies. Nat Rev Drug Discov. (2022) 21:495–508. doi: 10.1038/s41573-021-00259-5
17. Wang H, Guo X, Zhou J, Li Y, Duan L, Si X, et al. Clinical diagnosis and treatment of immune checkpoint inhibitor-associated pneumonitis. Thorac Cancer. (2020) 11:191–7. doi: 10.1111/1759-7714.13240
18. Yin J, Wu Y, Yang X, Gan L, and Xue J. Checkpoint inhibitor pneumonitis induced by anti-PD-1/PD-L1 therapy in non-small-cell lung cancer: occurrence and mechanism. Front Immunol. (2022) 13:830631. doi: 10.3389/fimmu.2022.830631
19. Rapoport BL, Shannon VR, Cooksley T, Johnson DB, Anderson L, Blidner AG, et al. Pulmonary toxicities associated with the use of immune checkpoint inhibitors: an update from the immuno-oncology subgroup of the neutropenia, infection & Myelosuppression study group of the multinational association for supportive care in cancer. Front Pharmacol. (2021) 12:743582. doi: 10.3389/fphar.2021.743582
20. Williams M, Todd I, and Fairclough LC. The role of CD8 + T lymphocytes in chronic obstructive pulmonary disease: a systematic review. Inflammation Res. (2021) 70:11–8. doi: 10.1007/s00011-020-01408-z
21. Mitropoulou G, Daccord C, Sauty A, Pasche A, Egger B, Aedo Lopez V, et al. Immunotherapy-induced airway disease: A new pattern of lung toxicity of immune checkpoint inhibitors. Respiration. (2020) 99:181–6. doi: 10.1159/000504968
22. Akbari O, Stock P, Singh AK, Lombardi V, Lee WL, Freeman GJ, et al. PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions. Mucosal Immunol. (2010) 3:81–91. doi: 10.1038/mi.2009.112
23. Herold S, Gabrielli NM, and Vadász I. Novel concepts of acute lung injury and alveolar-capillary barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. (2013) 305:L665–81. doi: 10.1152/ajplung.00232.2013
24. Chiang CH, Osataphan S, Chang YC, Chi KY, Chiang CH, Chang Y, et al. Anticoagulation for the treatment of immune checkpoint inhibitor-associated venous thromboembolism. Blood Adv. (2024) 8:4803–6. doi: 10.1182/bloodadvances.2024013869
25. Kaptein FHJ, Kroft LJM, Hammerschlag G, Ninaber MK, Bauer MP, Huisman MV, et al. Pulmonary infarction in acute pulmonary embolism. Thromb Res. (2021) 202:162–9. doi: 10.1016/j.thromres.2021.03.022
26. Dodaro S and Winston D. Tracheobronchial thrombus. Am J Forensic Med Pathol. (2025) 46:e3–4. doi: 10.1097/PAF.0000000000000969
27. Shen J, Wen Z, Lin J, and Su H. Case report: Idiopathic pulmonary fibrosis induced by nab-paclitaxel: A rare complication. Front Pharmacol. (2023) 14:1094844. doi: 10.3389/fphar.2023.1094844
Keywords: lung squamous cell carcinoma, camrelizumab, immune checkpoint inhibitors, immune-related adverse events, reversible pulmonary bullae
Citation: Wang Y, Wang S, Li Q, Cui Q, Song J and Zheng H (2025) Case Report: A case of reversible pulmonary bullae induced by camrelizumab: a new immune-related adverse event. Front. Immunol. 16:1619702. doi: 10.3389/fimmu.2025.1619702
Received: 28 April 2025; Accepted: 15 September 2025;
Published: 30 September 2025.
Edited by:
Rui Meng, Huazhong University of Science and Technology, ChinaReviewed by:
Hesong Wang, Fourth Hospital of Hebei Medical University, ChinaVivek Patel, Roquette Frères, France
Saran Feng, Shandong Provincial Qianfoshan Hospital, China
Copyright © 2025 Wang, Wang, Li, Cui, Song and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiafu Song, eWlmYW5zb25nMTIzQDE2My5jb20=; Hong Zheng, MzQ4MDAyMjY0QHFxLmNvbQ==
 Yun Wang
Yun Wang Shaoshan Wang2
Shaoshan Wang2