- Future Industries Institute, University of South Australia, Adelaide, SA, Australia
Granzyme K (GzmK) is an immune-secreted serine protease typically expressed at low levels but elevated in response to tissue injury and disease. Known as an orphan granzyme due to limited scientific investigation, this tryptase is being redefined as having important roles in inflammation and disease pathogenesis. Multiple GzmK expressing CD8+ T cell subsets are being identified with augmented expression and important roles in disease. Traditionally recognized as a mediator of cytotoxic lymphocyte-mediated cell death, GzmK’s role is being recharacterized through multiple recently released studies focused on newly identified extracellular mechanisms of action. These studies identify GzmK to be inflammatory, being able to trigger pro-inflammatory cytokine release, enhance immune cell recruitment, exacerbate the immune response to bacterial infections, and activate complement. In multiple disease states, dysregulated GzmK expression and potential accumulation in the extracellular space directly contributes to impaired health outcomes, thereby suggesting downregulation may prevent disease severity. GzmK is therefore emerging as a therapeutic target, potentially valuable in sepsis, pulmonary disease, inflammatory skin disease, rheumatoid arthritis and even aging.
1 Introduction
1.1 Granzymes
Granule-secreted enzymes (granzymes) are a family of serine proteases identified to mediate cell death by natural killer cells and cytotoxic T lymphocytes (1–5). There are five human granzymes, comprising tryptases granzyme A (GzmA) and GzmK, aspartase GzmB, chymase GzmH, and metase GzmM. Despite sharing structural sequence homology and a conserved secondary structure (6, 7), granzymes exhibit distinct substrates and varied roles in both healthy tissues and pathologic ones in multiple disease modalities. GzmA and GzmB are the most extensively studied granzymes, while the others are less well elucidated, thus referred to as ‘orphan’ granzymes. In recent years, several emerging studies have focused on GzmK, revealing significant implications in various diseases and offering new insights into mechanisms of action. As a result, investigating GzmK has become an exciting area of active research.
1.2 Granzyme K
GZMK, the human GzmK gene (EC: 3.4.21) is located on chromosome 5.q11.2 and encodes a 264 amino acid protein. Also known as granzyme-3, fragmentin-3, or NK-tryptase-2, GzmK is synthesized in the rough endoplasmic reticulum as a zymogen precursor and then stored in granules, where it is associated with the proteoglycan serglycin. To become proteolytically active, the proteinase cathepsin C (also known as dipeptidyl peptidase I) performs NH2-terminal processing (8). As a highly cationic tryptase-like protease, GzmK cleaves after basic amino acids, preferentially after positions 6 and 9 but also after positions 7 and 8 (9). Since both GzmA and GzmK are tryptases, have closely related three-dimensional structures (9) and share some common substrates, GzmK was long considered a redundant enzyme to GzmA. GZMK is located near GZMA on chromosome 5, likely due to gene duplication. However, the idea that GzmK is merely redundant to GzmA is now rejected, as GzmK has unique substrates and functions that distinguish it from GzmA. GzmK and GzmA also shows wide structural variation around the active subsites (10).
1.3 Granzyme K expression is elevated or augmented in multiple disease states
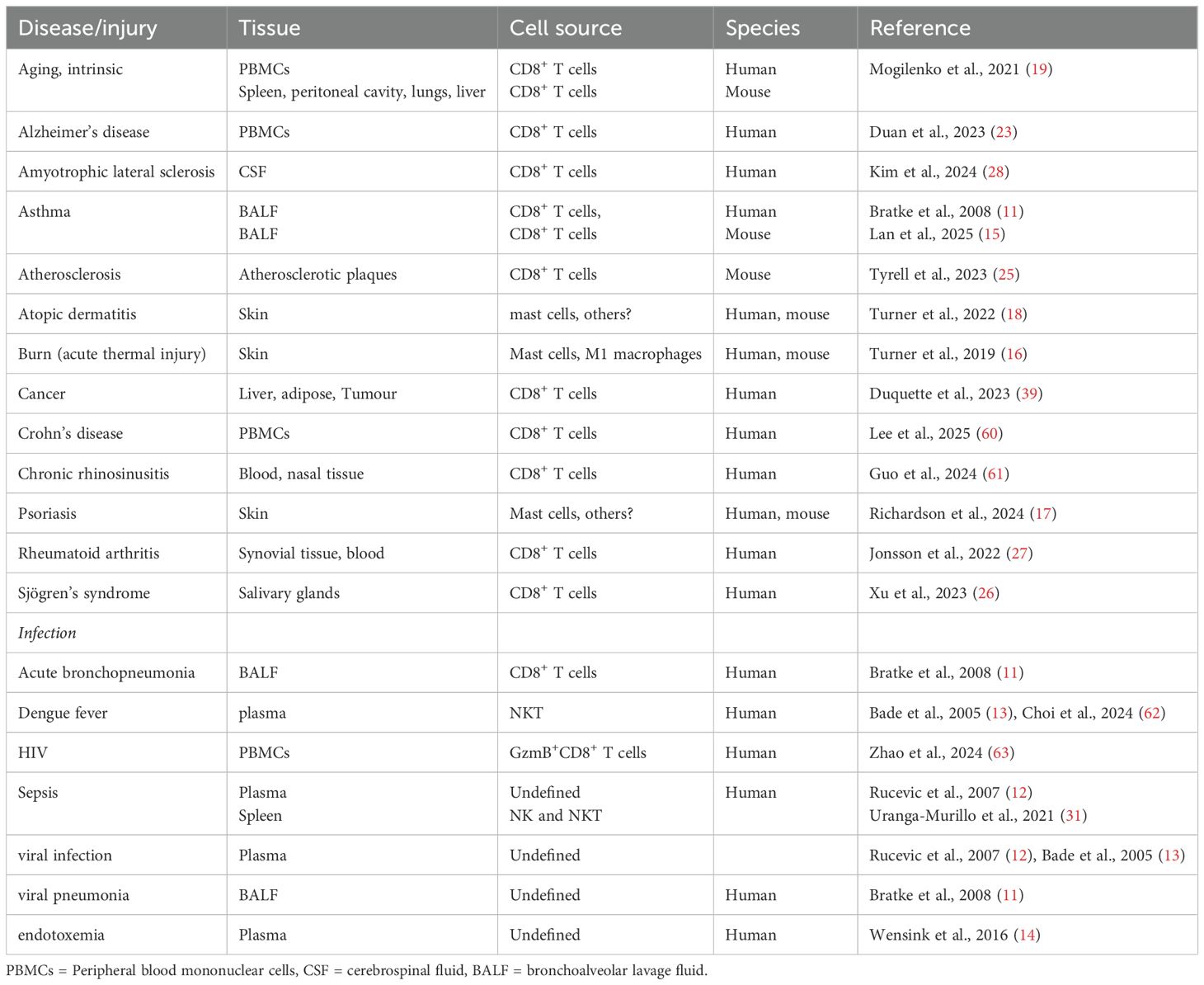
GzmK detection in plasma and tissues is low in healthy conditions but becomes elevated in response to disease/tissue injury, specifically accumulating in regions of inflammation (Table 1). GzmK is elevated in bronchoalveolar lavage fluid from acute bronchopneumonia patients and allergic asthma subject’s post-allergen challenge, but not mild chronic obstructive pulmonary disease (11). Plasma GzmK is elevated in patients experiencing sepsis (12) and Dengue fever (13). In renal transplant patients with immunosuppressive therapy and suffering from cytomegalovirus infection, plasma GzmK is elevated and associated directly with the infection (13). GzmK is also transiently elevated in circulation following lipopolysaccharide (LPS) administration (14). GzmK is only released upon stimulation with Pseudomonas aeruginosa, but not Escherichia coli BL21, and Neisseria meningitidis, suggesting upregulation is pathogen specific. Tissue GzmK levels predict chronic rhinosinusitis-associated nasal polyp recurrence and asthma comorbidity (15). GzmK is also elevated in skin damaged by acute burn injury (16), and lesions in the inflammatory skin diseases, psoriasis (17) and atopic dermatitis (18).
Specific immune cell populations, and in particular T cells, have augmented GzmK expression in response to certain disease states, including rheumatoid arthritis, amyotrophic lateral sclerosis, and aging. A subset of GzmK+ exhausted memory T cells (Taa) has been identified to accumulate with age in the lung, liver, peritoneal cavity, and spleen (19). Separately, GzmK+ CD8+ T cells were found to be higher in the plasma of older adults (20). Humans seropositive for cytomegalovirus exhibit higher GzmK+ CD8+ T cells. GzmK+ NK cell frequency is inversely correlated with antibody titers pre-and post-influenza vaccination. GzmK+ T cells are increased in both cancer and inflammaging, including squamous cell carcinoma (21), melanoma (22), Alzheimer’s disease (23), and atherosclerosis (24, 25). In Sjögren’s syndrome patients, there is an increased proportion of CXCR6+GzmK+CD8+ T cells in the peripheral blood, with these displaying an activated phenotype (26). In rheumatoid arthritis, GzmK+ CD8 T cells are enriched, with these greater than 10% of all live cells in inflamed RA synovium (27). GzmK+ cytotoxic T cells were also found to be a major CD8+ T cell population in gut samples from Crohn’s disease patients and bronchoalveolar lavage fluid samples from COVID-19 patients, with these enriched in diseased tissue but also found in circulation (27). Finally, there is a higher proportion of CD8+GzmKhi effector memory T cells in the cerebrospinal fluid of patients with amyotrophic lateral sclerosis (28).
1.4 Granzyme K contributes to disease
The development of a GzmK knockout (GzmK-/-) mouse (29) has allowed elucidation of the biological role of this protease in a variety of disease states. Comparing GzmK-/- and GzmA-/- mice has allowed confirmation that there is the lack of overlap between the functions of GzmK and GzmA. GzmK-/- mice exposed to Chikungunya virus infection displayed reduced foot swelling, although this is less than observed in GzmA-/- mice (30). Sepsis scores are also reduced in GzmK-/- mice compared to WT mice, however, only GzmA-/- mice have improved survival (31).
In acute burn injury, GzmK-/- mice resolved inflammation faster, and improved wound closure, quality of healing, and scar strength compared to wild-type mice (16). Separately, in oxazolone-dermatitis (18) and imiquimod-psoriasis (17) models of inflammatory skin disease, severity is reduced in GzmK-/- mice. In the dermatitis mice, GzmK-/- mice display reduced scaling, erosions and erythema, with an associated improvement in angiogenesis and decreased microvascular damage. In the psoriasis mice, GzmK-/- mice have reduced plaque formation, less erythema, and decreased epidermal thickening. Using a different GzmK-/- mouse, this time with skin exposed to imiquimod to induce skin inflammation, there is decreased erythema, scaling and skin thickness (15). In mouse asthma models, GzmK knockdown or pharmacological inhibition decreased tissue pathology and restored lung function (15). GzmK-/- mice display reduced arthritis severity and dermatitis with reduced complement activation.
2 Different schools of thought: intracellular versus extracellular roles for GzmK
2.1 Intracellular roles for granzyme K
Historically, all granzymes were believed to mediate cytotoxic lymphocyte-mediated cell death. Upon target cell engagement, granules release their granzyme payload into the immunological synapse. The pore-forming protein perforin is released in conjunction with these granzymes and facilitates granzymes entry into the target cells. Once internalized, granzymes induce cell death through caspase-independent or -dependent mechanisms (reviewed in (32)). The specific details related to these mechanisms remain unclear and have not been independently confirmed. As such, this remains an area of controversial area, especially the idea that GzmK contributes to cell killing, and has been questioned by several independent studies (33).
2.2 Extracellular roles for granzyme K
There are three main key indicators that GzmK is released from cells and into the extracellular space: 1/leakage from the immunological synapse, 2/secretion from non-cytotoxic and possibly non-immune cells, and 3/interaction with extracellular substrates. Notably, the GzmK+ CD8 T cells found to have a relatively increased expression in multiple disease states minimally express cytotoxic markers (27), suggesting extracellular roles may be especially important in disease.
2.2.1 Leakage from immunological synapse
GzmK is expressed in diverse populations of cytotoxic cells, including CD8+ T cells (γδ T cells, mucosal-associated invariant T (MAIT) cells, a subset of non-MAIT CD8 T cells, CD8+GzmKhi T cells, and CD45RO+CCR7+ and CD45RO+CCR7- CD8 T cells) natural killer cells (CD56bright and invariant NKT) and cytotoxic CD4+ T cells (27, 28, 34–36). Following target-cell engagement and granzyme release into the immunological synapse, only an estimated two thirds are internalized with the remainder dispersed into the extracellular milieu (37). Recently, CD8+ T cells were found to secrete GzmK in the absence of T cell receptor stimulation, supporting constitutive synthesis and secretion (38).
2.2.2 Non-cytotoxic cells express and secrete GzmK
Multiple GzmK+ cells are non-cytotoxic, with these cell types secreting no perforin and/or unable to form immunological synapses (39). These cell types include macrophages (16), non-cytotoxic CD56bright CD16− natural killer cells (35), and mast cells (17, 18). In cultured macrophages, GzmK is constitutively secreted from M1 but not MØ or M2a macrophages (16). In dual GzmK/TBO stained mast cells, extracellular GzmK+ vesicles were also observed following degranulation (18).
2.2.3 GzmK cleaves extracellular substrates
GzmK is potently inhibited in human plasma by the inter-alpha-inhibitor protein complex (IαIp), leading to speculation of the existence of extracellular GzmK substrates (11). Multiple extracellular substrates have now been identified within the extracellular matrix and on cell surface membranes. These include cleavage of Protease-Activated Receptors (PAR) (40), complement C2 and C4 (38), LPS (41), syndecan-1 (18) and decorin (18). The use of degradomics and other techniques will allow further identification of additional extracellular substrates.
In summation, the data now suggests extracellular GzmK as having an emerging role in disease pathogenesis, likely more so than GzmK-driven cell-mediated cytotoxicity. Fundamental to this idea is the need for a better understanding of the extent extracellular GzmK accumulates in diseased tissue, what kinds of tissues, and whether the amount of accumulation directly correlates to disease severity.
3 Current research gaps: does extracellular GzmK accumulate in diseased tissue?
In injured/inflamed tissues collected from a variety of diseases, GzmK positive cells are clearly elevated (16–18). Numerous GzmK expressing cell types have been identified in vitro, with multiple found to secrete GzmK under specific culture conditions (16, 38). Moreover, constitutive secretion of GzmK has been described in a population of CD8+ T cells (38). In response to tissue injury and inflammation, it is therefore extremely likely a pool of extracellular GzmK will accumulate. However, due to limitations in the sensitivity of immunohistochemistry, there is an inability to accurately detect extracellular granzymes within these tissues. This makes conclusions about the effect of GzmK accumulation difficult to separate between cellular GzmK or that present extracellularly. There have been recent advances and tools are emerging for the detection of other granzymes in multiple biological samples. Recently, fluorescence-energy resonance-transfer (FRET)-based peptide probes (FAM-peptide-DABCYL) were developed to detect GzmA activity in serum and tissue lysates (42). The development of similar tools for GzmK detection would be enormously useful to elucidate how GzmK accumulates in a range of tissue types. The ability to better understand how GzmK accumulates in disease would inform the development of therapeutic approaches, including inhibitor design.
4 Current research gaps: how important is GzmK’s pro-inflammatory role?
Emerging evidence over recent years has established GzmK as having pro-inflammatory properties (Figure 1). This is, in part, due to its ability to binds to LPS (41), induce pro-inflammatory cytokine expression (18, 40, 41, 43, 44), facilitate immune cell recruitment (44), and activate complement (35). GzmK has also been identified as being a key contributor to inflammaging (19). The most well described mechanisms will be discussed below.
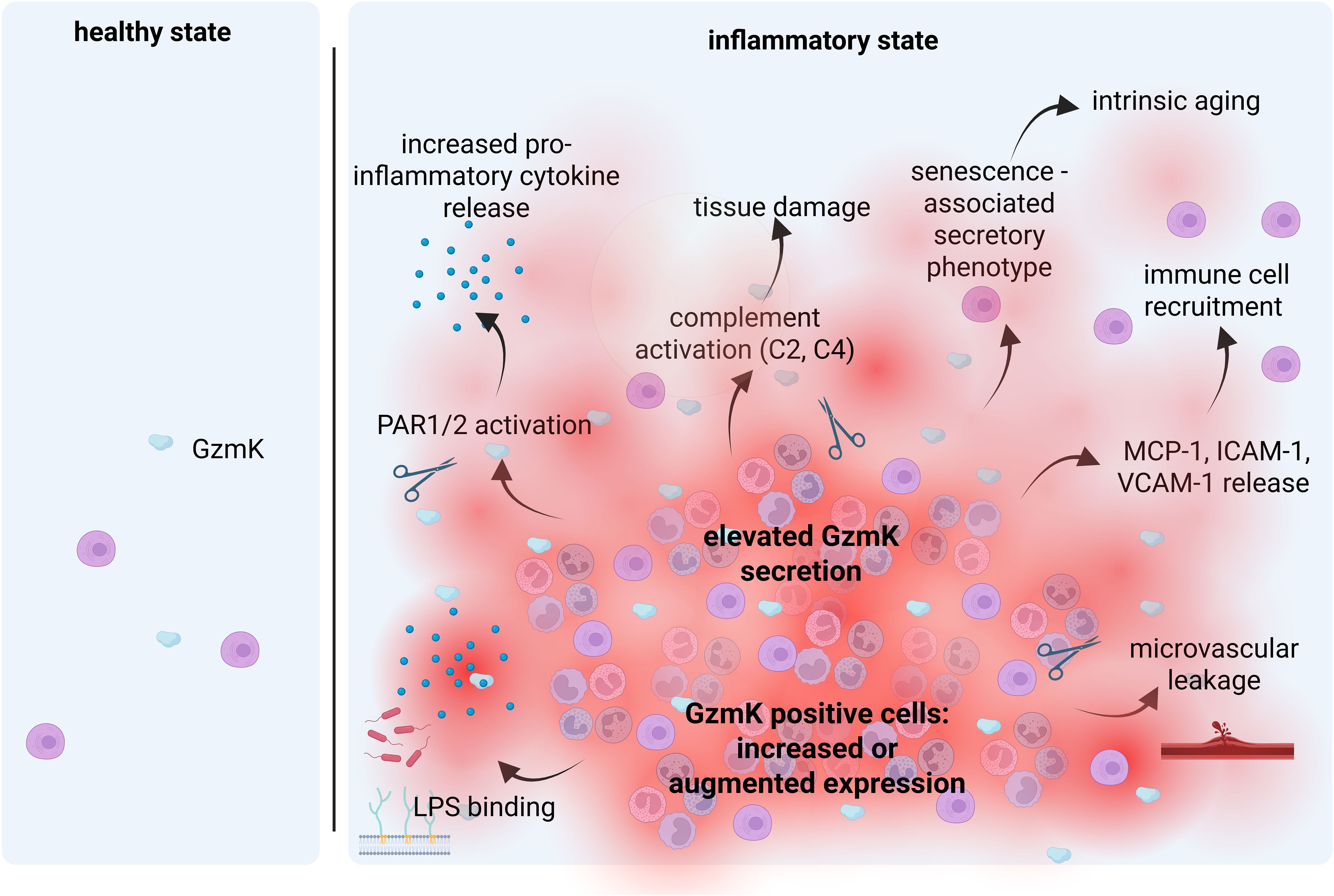
Figure 1. Main mechanisms involved in the GzmK-mediated inflammatory process. There is negligible GzmK in healthy tissues but is elevated in response to tissue injury and inflammation. Enhanced GzmK secretion leads to enhanced immune cell recruitment, elevated pro-inflammatory cytokine detection, complement activation and cell senescence. Reproduced with permission from BioRender.
4.1 LPS
Human GzmK has been demonstrated to bind to both purified LPS and LPS on Gram-negative bacterial cell walls (41). GzmK modulates toll-like receptor 4 (TLR4) signaling in immune cells, leading to increased pro-inflammatory cytokine expression, including TNF-α from monocytes and IL-1β from macrophages. Together, extracellular GzmK contributes directly to the immune response to bacterial infections. However, based on studies in GzmK-/- mice, the contribution of GzmK to overall disease severity in response to infection appears to be less than other immune-secreted proteases (i.e., GzmA) (29, 30).
4.2 Protease-activated receptor
PARs, a subfamily of G protein-coupled receptors, mediate the cellular effects of proteinases. Comprising PAR1, 2, 3 and 4, they have unique but sometimes overlapping roles in inflammation, hemostasis, and thrombosis (45). Multiple studies confirm GzmK to cleave and activate PAR1 (40, 44). This leads to increase pro-inflammatory cytokine secretion and has been observed in multiple cell types, including peritoneal macrophages and cultured M1 macrophages (IL-1β) (16, 46), lung fibroblasts (IL-6, IL-8) (40), keratinocytes and skin fibroblasts (IL-6) (16), and endothelial cells (IL-6) (44). These observations are supported in vivo, where GzmK-/- mice with acute burns display decreased IL-1β and IL-6 compared to WT mice (16). GzmK-mediated PAR1 activation in endothelial cells also increases the expression of intercellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1, and monocyte chemotactic protein (MCP)-1 (44). This led to increased monocyte attachment to endothelial cells, suggesting GzmK as an immune cell attractant. In support, thermal injured GzmK-/- mice wounds display reduced ICAM-1, VCAM-1, and MCP-1 expression in combination with lower macrophage detection (16).
More recently, GzmK is reported to cleave and activate PAR2 (18, 43). Similarly to PAR1, GzmK-mediated PAR2 activation increases pro-inflammatory cytokine expression (IL-6 and IL-8), which is observed in epithelial cells (43). GzmK activation of PAR2 is separately demonstrated through detection of cleavage on the surface of a reporter cell (nLuc–PAR–eYFP CHO) (18). Mechanistically, GzmK-mediated PAR2 cleavage led to recruitment of β-arrestin and phosphorylation of ERK (43). Notably, both GzmK and trypsin cleave PAR2 at the same location, however, GzmK is unable to induce a classical Ca2+ flux.
Multiple naturally expressed proteases have also been described to cleave PAR1 and/or PAR2, including trypsin, plasmin, kallikreins, neutrophil elastase, mast cell tryptase, tissue factor/factor VIIa/factor Xa, activated protein C, and matrix metalloproteinase-1 (47). Some of these proteases may be dysregulated in disease, whilst others are tightly regulated. As an example, although matrix metalloproteinase-1 is elevated in response to tissue injury, it is tightly regulated by tissue inhibitors of metalloproteinases (TIMPs), thereby limiting its ability to act uncontrolled. To better understand the role of GzmK in disease, future studies must therefore elucidate the relative contributions these proteases play in PAR activation and under what conditions does GzmK have the greatest impact. This includes identifying whether GzmK accumulates and increases its proteolytic activity in response to sustained inflammation.
4.3 Complement
Lymphocyte-derived GzmK is emerging as having a key role in complement activation (38). GzmK mediates activation through the cleavage of C2 and C4. Ultimately, this results in the generation of C3a, C3b, C4b, and C5a, which are key effectors of complement. This has been observed in vivo in rheumatoid arthritis patients, where regions of complement activation correspond to increased GzmK detection (38). Moreover, in arthritis and dermatitis mice, GzmK-mediated complement activation reportedly contributes to disease progression.
Together, it is now clear GzmK mediates a pro-inflammatory phenotype, occurring through multiple and distinct pathways. GzmK will therefore likely have important pathologic roles in multiple disease modalities characterized by inflammation.
5 Future developments in the field: will pharmacological inhibition of GzmK reduce disease?
Based on its pro-inflammatory and overall pathogenic effects in multiple disease states, GzmK is emerging as a therapeutic target. Although GzmK inhibitory agents have been described, none are highly specific and with each capable of inhibiting other proteases. IαIp is a naturally occurring physiological inhibitor of GzmK. Found in human and mouse plasma, Plasma IαIp levels are inversely correlated with extracellular GzmK and disease severity in sepsis patients (12). This suggests IαIp to provide a regulatory mechanism (at least in circulation) for limiting the detrimental effects of extracellular GzmK, likely in response to increased GzmK secretion during pro-inflammatory events. IαIp, which also inhibits trypsin, chymotrypsin, plasmin, neutrophil elastase, and cathepsin G (48), has been assessed therapeutically in conditions where there is increased inflammation. Circulating IαIp levels are higher in healthy volunteers than severe sepsis patients (49), thus IαIp delivery was assessed in mice as a potential sepsis treatment. Intravenous IαIp increased survival after an intravenous challenge of Escherichia coli (49). In a separate study, intraperitoneal IαIp delivery improved survival to nearly 90% in both LPS induced sepsis and with live bacterial infections (50). IαIp also improved survival after cecal ligation and puncture (51, 52). Intraperitoneal IαIp has additionally been evaluated for anthrax, lacking improved survival outcomes (53). However, combining IαIp and moxifloxacin did improved survival compared to controls including moxifloxacin alone.
The light chain of IαIp, also called bikunin, contains the GzmK inhibitory activity (54), suggesting it may alternatively be used therapeutically. Bikunin is cross-linked in the IαIp complex and requires partial proteolytic degradation to activate. Following cleavage, active bikunin is rapidly cleared from circulation by glomerular filtration and receptor-mediated uptake (55). In rats, intravenous bikunin injection has a half-life of only 10 min. This may account for free bikunin only representing about 2% of total plasma bikunin (reported in (8)). As such, the limited half-life of bikunin may be limiting for therapeutic use unless improved delivery strategies are implemented.
Other non-specific synthetic GzmK inhibitors have been identified, including Phe-Pro-Arg-chloromethyl ketone (PFR-CK), PefablocSC, phenylmethylsulfonyl fluoride, and benzamidine (56, 57). In mice with asthma, PFR-CK, which also inhibits plasma kallikrein, factor XIIa (58) and granzyme A (59), was recently assessed (intraperitoneally every second day), displaying decreased airway eosinophil infiltration, reduced goblet cell hyperplasia, and improved lung function (15). Together, although the number of studies is limited, pharmacological inhibition of GzmK has potential as a therapeutic and warrants further investigation.
6 Discussion
It is now clear GzmK has important roles in disease pathogenesis, but many questions remain. More work is required to better understand the relative contributions of different cell types, especially the various CD8+ T cell subsets, to the presence of GzmK in diseased tissue. We need better tools to assess extracellular GzmK accumulation in damaged tissues and if elevated, what tissues display the greatest increase. Although GzmK’s role in numerous mechanisms have been described, we need to better uncover GzmK substrates and how increased proteolytic cleavage of these substrates contributes to disease. A greater knowledge of novel substrates will likely lead to the identification of additional mechanisms of action. Remaining a controversial issue, we need to establish the relative contribution of GzmK’s catalytic activity to overall pro-inflammatory mediation. Finally, we need to evaluate the therapeutic potential of pharmacological GzmK inhibition. A better grasp of how GzmK contributes to disease will guide the design of these therapeutics and help select the specific diseases to focus on.
Author contributions
CT: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The author acknowledges funding support including grants-in-aid from DEBRA-Australia and Epidermolysis Bullosa Research Partnership.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Masson D, Nabholz M, Estrade C, and Tschopp J. Granules of cytolytic T-lymphocytes contain two serine esterases. EMBO J. (1986) 5:1595–600. doi: 10.1002/j.1460-2075.1986.tb04401.x
2. Masson D and Tschopp J. A family of serine esterases in lytic granules of cytolytic T lymphocytes. Cell. (1987) 49:679–85. doi: 10.1016/0092-8674(87)90544-7
3. Tschopp J, Masson D, and Schafer S. Inhibition of the lytic activity of perforin by lipoproteins. J Immunol (Baltimore Md: 1950). (1986) 137:1950–3. doi: 10.4049/jimmunol.137.6.1950
4. Lobe CG, Finlay BB, Paranchych W, Paetkau VH, and Bleackley RC. Novel serine proteases encoded by two cytotoxic T lymphocyte-specific genes. Sci (New York NY). (1986) 232:858–61. doi: 10.1126/science.3518058
5. Trapani JA. Granzymes: a family of lymphocyte granule serine proteases. Genome Biol. (2001) 2:Reviews3014. doi: 10.1186/gb-2001-2-12-reviews3014
6. Bovenschen N, Quadir R, van den Berg AL, Brenkman AB, Vandenberghe I, Devreese B, et al. Granzyme K displays highly restricted substrate specificity that only partially overlaps with granzyme A. J Biol Chem. (2009) 284:3504–12. doi: 10.1074/jbc.M806716200
7. Plasman K, Demol H, Bird PI, Gevaert K, and Van Damme P. Substrate specificities of the granzyme tryptases A and K. J Proteome Res. (2014) 13:6067–77. doi: 10.1021/pr500968d
8. Wilharm E, Parry MA, Friebel R, Tschesche H, Matschiner G, Sommerhoff CP, et al. Generation of catalytically active granzyme K from Escherichia coli inclusion bodies and identification of efficient granzyme K inhibitors in human plasma. J Biol Chem. (1999) 274:27331–7. doi: 10.1074/jbc.274.38.27331
9. Hink-Schauer C, Estébanez-Perpiñá E, Wilharm E, Fuentes-Prior P, Klinkert W, Bode W, et al. The 2.2-A crystal structure of human pro-granzyme K reveals a rigid zymogen with unusual features. J Biol Chem. (2002) 277:50923–33. doi: 10.1074/jbc.M207962200
10. Sattar R, Ali SA, and Abbasi A. Bioinformatics of granzymes: sequence comparison and structural studies on granzyme family by homology modeling. Biochem Biophys Res Commun. (2003) 308:726–35. doi: 10.1016/S0006-291X(03)01458-X
11. Bratke K, Klug A, Julius P, Kuepper M, Lommatzsch M, Sparmann G, et al. Granzyme K: a novel mediator in acute airway inflammation. Thorax. (2008) 63:1006–11. doi: 10.1136/thx.2007.091215
12. Rucevic M, Fast LD, Jay GD, Trespalcios FM, Sucov A, Siryaporn E, et al. Altered levels and molecular forms of granzyme k in plasma from septic patients. Shock (Augusta Ga). (2007) 27:488–93. doi: 10.1097/01.shk.0000246905.24895.e5
13. Bade B, Lohrmann J, ten Brinke A, Wolbink AM, Wolbink GJ, ten Berge IJ, et al. Detection of soluble human granzyme K in vitro and in vivo. Eur J Immunol. (2005) 35:2940–8. doi: 10.1002/eji.200526249
14. Wensink AC, Wiewel MA, Jongeneel LH, Boes M, van der Poll T, Hack CE, et al. Granzyme M and K release in human experimental endotoxemia. Immunobiology. (2016) 221:773–7. doi: 10.1016/j.imbio.2016.02.006
15. Lan F, Li J, Miao W, Sun F, Duan S, Song Y, et al. GZMK-expressing CD8+ T cells promote recurrent airway inflammatory diseases. Nature. (2025) 638:490–8. doi: 10.1038/s41586-024-08395-9
16. Turner CT, Zeglinski MR, Richardson KC, Zhao H, Shen Y, Papp A, et al. Granzyme K expressed by classically activated macrophages contributes to inflammation and impaired remodeling. J Invest Dermatol. (2019) 139:930–9. doi: 10.1016/j.jid.2018.09.031
17. Richardson KC, Aubert A, Turner CT, Nabai L, Hiroyasu S, Pawluk MA, et al. Granzyme K mediates IL-23-dependent inflammation and keratinocyte proliferation in psoriasis. Front Immunol. (2024) 15:1398120. doi: 10.3389/fimmu.2024.1398120
18. Turner CT, Zeglinski MR, Boivin W, Zhao H, Pawluk MA, Richardson KC, et al. Granzyme K contributes to endothelial microvascular damage and leakage during skin inflammation. Br J Dermatol. (2022) 189(3):279–91. doi: 10.1093/bjd/ljac017
19. Mogilenko DA, Shpynov O, Andhey PS, Arthur L, Swain A, Esaulova E, et al. Comprehensive profiling of an aging immune system reveals clonal GZMK(+) CD8(+) T cells as conserved hallmark of inflammaging. Immunity. (2021) 54:99–115.e12. doi: 10.1016/j.immuni.2020.11.005
20. Verschoor CP, Picard E, Andrew MK, Haynes L, Loeb M, Pawelec G, et al. NK- and T-cell granzyme B and K expression correlates with age, CMV infection and influenza vaccine-induced antibody titres in older adults. Front Aging. (2022) 3:1098200. doi: 10.3389/fragi.2022.1098200
21. Luoma AM, Suo S, Wang Y, Gunasti L, Porter CBM, Nabilsi N, et al. Tissue-resident memory and circulating T cells are early responders to pre-surgical cancer immunotherapy. Cell. (2022) 185:2918–35.e29. doi: 10.1016/j.cell.2022.06.018
22. Chang JW, Huang CJ, Huang WK, Wang YC, Hsieh JJ, Chang YY, et al. Genomic and tumour microenvironmental biomarkers of immune checkpoint inhibitor response in advanced Taiwanese melanoma. Clin Transl Immunol. (2023) 12:e1465. doi: 10.1002/cti2.v12.8
23. Duan T, Chu J, and Hu F. Identification of peripheral blood GZMK (+) CD8 (+) T cells as biomarkers of alzheimer’s disease based on single-cell transcriptome. Sichuan Da Xue Xue Bao Yi Xue Ban. (2023) 54:863–73. doi: 10.12182/20230960107
24. Depuydt MAC, Prange KHM, Slenders L, Örd T, Elbersen D, Boltjes A, et al. Microanatomy of the human atherosclerotic plaque by single-cell transcriptomics. Circ Res. (2020) 127:1437–55. doi: 10.1161/CIRCRESAHA.120.316770
25. Tyrrell DJ, Wragg KM, Chen J, Wang H, Song J, Blin MG, et al. Clonally expanded memory CD8(+) T cells accumulate in atherosclerotic plaques and are pro-atherogenic in aged mice. Nat Aging. (2023) 3:1576–90. doi: 10.1038/s43587-023-00515-w
26. Xu T, Zhu HX, You X, Ma JF, Li X, Luo PY, et al. Single-cell profiling reveals pathogenic role and differentiation trajectory of granzyme K+CD8+ T cells in primary Sjögren’s syndrome. JCI Insight. (2023) 8(8):e167490. doi: 10.1172/jci.insight.167490
27. Jonsson AH, Zhang F, Dunlap G, Gomez-Rivas E, Watts GFM, Faust HJ, et al. Granzyme K(+) CD8 T cells form a core population in inflamed human tissue. Sci Transl Med. (2022) 14:eabo0686. doi: 10.1126/scitranslmed.abo0686
28. Kim HJ, Ban JJ, Kang J, Im HR, Ko SH, Sung JJ, et al. Single-cell analysis reveals expanded CD8(+) GZMK (high) T cells in CSF and shared peripheral clones in sporadic amyotrophic lateral sclerosis. Brain Commun. (2024) 6:fcae428. doi: 10.1093/braincomms/fcae428
29. Joeckel LT, Allison CC, Pellegrini M, Bird CH, and Bird PI. Granzyme K-deficient mice show no evidence of impaired anti-viral immunity. Immunol Cell Biol. (2017) 95(8):676–83. doi: 10.1038/icb.2017.35
30. Wilson JA, Prow NA, Schroder WA, Ellis JJ, Cumming HE, Gearing LJ, et al. RNA-Seq analysis of chikungunya virus infection and identification of granzyme A as a major promoter of arthritic inflammation. PloS Pathog. (2017) 13:e1006155. doi: 10.1371/journal.ppat.1006155
31. Uranga-Murillo I, Tapia E, Garzón-Tituaña M, Ramirez-Labrada A, Santiago L, Pesini C, et al. Biological relevance of Granzymes A and K during E. coli sepsis. Theranostics. (2021) 11:9873–83. doi: 10.7150/thno.59418
32. Bouwman AC, van Daalen KR, Crnko S, Ten Broeke T, and Bovenschen N. Intracellular and extracellular roles of granzyme K. Front Immunol. (2021) 12:677707. doi: 10.3389/fimmu.2021.677707
33. Joeckel LT and Bird PI. Are all granzymes cytotoxic in vivo? Biol Chem. (2014) 395:181–202. doi: 10.1515/hsz-2013-0238
34. Koga R, Maehara T, Aoyagi R, Munemura R, Murakami Y, Doi A, et al. Granzyme K- and amphiregulin-expressing cytotoxic T cells and activated extrafollicular B cells are potential drivers of IgG4-related disease. J Allergy Clin Immunol. (2024) 153:1095–112. doi: 10.1016/j.jaci.2023.11.916
35. Donado CA, Jonsson AH, Theisen E, Zhang F, Nathan A, Rupani KV, et al. Granzyme K drives a newly-intentified pathway of complement activation. bioRxiv. (2024) 26:2024.05.22.595315. doi: 10.1101/2024.05.22.595315
36. Jonsson AH. Granzyme K(+) CD8 T cells in autoimmunity. Best Pract Res Clin Rheumatol. (2024) 38:101930. doi: 10.1016/j.berh.2024.101930
37. Isaaz S, Baetz K, Olsen K, Podack E, and Griffiths GM. Serial killing by cytotoxic T lymphocytes: T cell receptor triggers degranulation, re-filling of the lytic granules and secretion of lytic proteins via a non-granule pathway. Eur J Immunol. (1995) 25:1071–9. doi: 10.1002/eji.1830250432
38. Donado CA, Theisen E, Zhang F, Nathan A, Fairfield ML, Rupani KV, et al. Granzyme K activates the entire complement cascade. Nature. (2025) 641(8061):211–21. doi: 10.1038/s41586-025-08713-9
39. Duquette D, Harmon C, Zaborowski A, Michelet X, O’Farrelly C, Winter D, et al. Human granzyme K is a feature of innate T cells in blood, tissues, and tumors, responding to cytokines rather than TCR stimulation. J Immunol (Baltimore Md: 1950). (2023) 211:633–47. doi: 10.4049/jimmunol.2300083
40. Cooper DM, Pechkovsky DV, Hackett TL, Knight DA, and Granville DJ. Granzyme K activates protease-activated receptor-1. PloS One. (2011) 6:e21484. doi: 10.1371/journal.pone.0021484
41. Wensink AC, Kemp V, Fermie J, García Laorden MI, van der Poll T, Hack CE, et al. Granzyme K synergistically potentiates LPS-induced cytokine responses in human monocytes. Proc Natl Acad Sci United States America. (2014) 111:5974–9. doi: 10.1073/pnas.1317347111
42. Senan-Salinas A, Comas L, Esteban P, Garzón-Tituaña M, Cheng Z, Santiago L, et al. Selective detection of active extracellular granzyme A by using a novel fluorescent immunoprobe with application to inflammatory diseases. ACS Pharmacol Trans Sci. (2024) 7:1474–84. doi: 10.1021/acsptsci.4c00065
43. Kaiserman D, Zhao P, Rowe CL, Leong A, Barlow N, Joeckel LT, et al. Granzyme K initiates IL-6 and IL-8 release from epithelial cells by activating protease-activated receptor 2. PloS One. (2022) 17:e0270584. doi: 10.1371/journal.pone.0270584
44. Sharma M, Merkulova Y, Raithatha S, Parkinson LG, Shen Y, Cooper D, et al. Extracellular granzyme K mediates endothelial activation through the cleavage of protease-activated receptor-1. FEBS J. (2016) 283:1734–47. doi: 10.1111/febs.2016.283.issue-9
45. Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. (2005) 3:1800–14. doi: 10.1111/j.1538-7836.2005.01377.x
46. Joeckel LT, Wallich R, Martin P, Sanchez-Martinez D, Weber FC, Martin SF, et al. Mouse granzyme K has pro-inflammatory potential. Cell Death Differ. (2011) 18:1112–9. doi: 10.1038/cdd.2011.5
47. Heuberger DM and Schuepbach RA. Protease-activated receptors (PARs): mechanisms of action and potential therapeutic modulators in PAR-driven inflammatory diseases. Thromb J. (2019) 17:4. doi: 10.1186/s12959-019-0194-8
48. Potempa J, Kwon K, Chawla R, and Travis J. Inter-alpha-trypsin inhibitor. Inhibition spectrum of native and derived forms. J Biol Chem. (1989) 264:15109–14. doi: 10.1016/S0021-9258(18)63818-9
49. Lim YP, Bendelja K, Opal SM, Siryaporn E, Hixson DC, and Palardy JE. Correlation between mortality and the levels of inter-alpha inhibitors in the plasma of patients with severe sepsis. J Infect Dis. (2003) 188:919–26. doi: 10.1086/jid.2003.188.issue-6
50. Singh K, Zhang LX, Bendelja K, Heath R, Murphy S, Sharma S, et al. Inter-alpha inhibitor protein administration improves survival from neonatal sepsis in mice. Pediatr Res. (2010) 68:242–7. doi: 10.1203/PDR.0b013e3181e9fdf0
51. Wu R, Cui X, Lim YP, Bendelja K, Zhou M, Simms HH, et al. Delayed administration of human inter-alpha inhibitor proteins reduces mortality in sepsis. Crit Care Med. (2004) 32:1747–52. doi: 10.1097/01.CCM.0000132903.14121.0E
52. Yang S, Lim YP, Zhou M, Salvemini P, Schwinn H, Josic D, et al. Administration of human inter-alpha-inhibitors maintains hemodynamic stability and improves survival during sepsis. Crit Care Med. (2002) 30:617–22. doi: 10.1097/00003246-200203000-00021
53. Opal SM, Lim YP, Cristofaro P, Artenstein AW, Kessimian N, Delsesto D, et al. Inter-alpha inhibitor proteins: a novel therapeutic strategy for experimental anthrax infection. Shock (Augusta Ga). (2011) 35:42–4. doi: 10.1097/SHK.0b013e3181e83204
54. Wachter E and Hochstrasser K. Kunitz-type proteinase inhibitors derived by limited proteolysis of the inter-alpha-trypsin inhibitor, IV. The amino acid sequence of the human urinary trypsin inhibitor isolated by affinity chromatography. Hoppe Seylers Z Physiol Chem. (1981) 362:1351–5. doi: 10.1515/bchm2.1981.362.2.1351
55. Sjöberg EM, Blom A, Larsson BS, Alston-Smith J, Sjöquist M, and Fries E. Plasma clearance of rat bikunin: evidence for receptor-mediated uptake. Biochem J. (1995) 308:881–7. doi: 10.1042/bj3080881
56. Wilharm E, Tschopp J, and Jenne DE. Biological activities of granzyme K are conserved in the mouse and account for residual Z-Lys-SBzl activity in granzyme A-deficient mice. FEBS Lett. (1999) 459:139–42. doi: 10.1016/S0014-5793(99)01200-4
57. Jackson DS, Fraser SA, Ni LM, Kam CM, Winkler U, Johnson DA, et al. Synthesis and evaluation of diphenyl phosphonate esters as inhibitors of the trypsin-like granzymes A and K and mast cell tryptase. J Med Chem. (1998) 41:2289–301. doi: 10.1021/jm970543s
58. Adams GN, LaRusch GA, Stavrou E, Zhou Y, Nieman MT, Jacobs GH, et al. Murine prolylcarboxypeptidase depletion induces vascular dysfunction with hypertension and faster arterial thrombosis. Blood. (2011) 117:3929–37. doi: 10.1182/blood-2010-11-318527
59. Metkar SS, Menaa C, Pardo J, Wang B, Wallich R, Freudenberg M, et al. Human and mouse granzyme A induce a proinflammatory cytokine response. Immunity. (2008) 29:720–33. doi: 10.1016/j.immuni.2008.08.014
60. Lee Y, Baek J, Kim Y, Hwang SW, Lee JL, Park SH, et al. P1291 Transcriptional heterogeneity of granzyme K CD8 T Cells in Crohn’s Disease: Implications for tissue inflammation. J Crohns Colitis. (2025) 19:i2327–i9. doi: 10.1093/ecco-jcc/jjae190.1465
61. Guo C-L, Wang C-S, Wang Z-C, Liu F-F, Liu L, Yang Y, et al. Granzyme K+CD8+ T cells interact with fibroblasts to promote neutrophilic inflammation in nasal polyps. Nat Commun. (2024) 15:10413. doi: 10.1038/s41467-024-54685-1
62. Choi Y, Saron WA, O’Neill A, Senanayake M, Wilder-Smith A, Rathore AP, et al. NKT cells promote Th1 immune bias to dengue virus that governs long-term protective antibody dynamics. J Clin Invest. (2024) 134(18):e169251. doi: 10.1172/JCI169251
Keywords: granzyme, inflammation, serine protease, CD8 lymphocytes +, cytotoxicity and immune system
Citation: Turner CT (2025) Pro-inflammatory granzyme K contributes extracellularly to disease. Front. Immunol. 16:1620670. doi: 10.3389/fimmu.2025.1620670
Received: 30 April 2025; Accepted: 05 June 2025;
Published: 18 June 2025.
Edited by:
Eva M. Galvez, Spanish National Research Council (CSIC), SpainReviewed by:
Julian Pardo, Fundacion Agencia Aragonesa para la Investigacion y el Desarrollo, SpainCopyright © 2025 Turner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christopher T. Turner, Y2hyaXMudHVybmVyQHVuaXNhLmVkdS5hdQ==
 Christopher T. Turner
Christopher T. Turner