- 1Department of Biomedical and Molecular Sciences, Queen’s University, Kingston, ON, Canada
- 2Centre for Neuroscience Studies, Queen’s University, Kingston, ON, Canada
Microglia have emerged as central players in the pathophysiology of traumatic spinal cord injury (SCI). The purpose of this brief review is to highlight the evolution of knowledge on the role of microglia in SCI. We explore the initial discovery of macrophages and their role in SCI lesions, followed by how microglia were examined and distinguished from monocyte-derived macrophages. We then discuss findings from studies that mapped and manipulated microglia in experimental SCI, made possible through technological advances in genetic, pharmacological, and bioinformatic approaches. We also highlight the importance of considering how the timing and location of microglia activation shapes neuroinflammation, synaptic plasticity and intraspinal circuit remodelling. Finally, as microglia research continues to flourish, we consider how microglia could be harnessed therapeutically to promote repair and functional recovery of motor, sensory, and autonomic systems after SCI.
Early descriptions of macrophages in SCI lesions
Macrophages are the most abundant immune cell type found in clinical and experimental spinal cord injury (SCI) lesions (1–3). This rich population is derived from at least two phenotypically similar but ontogenetically distinct sources: circulating monocyte-derived macrophages that originate from the spleen and bone marrow, and tissue-resident microglia that originate from the embryonic yolk sac (4–8). Because the macrophage response to SCI is prolific and conserved across species, macrophage-targeting therapies hold great potential to repair the injured spinal cord if the role of both blood-borne and tissue-resident macrophage populations can be deciphered. Research over the last century has made great strides toward this goal (Figure 1).
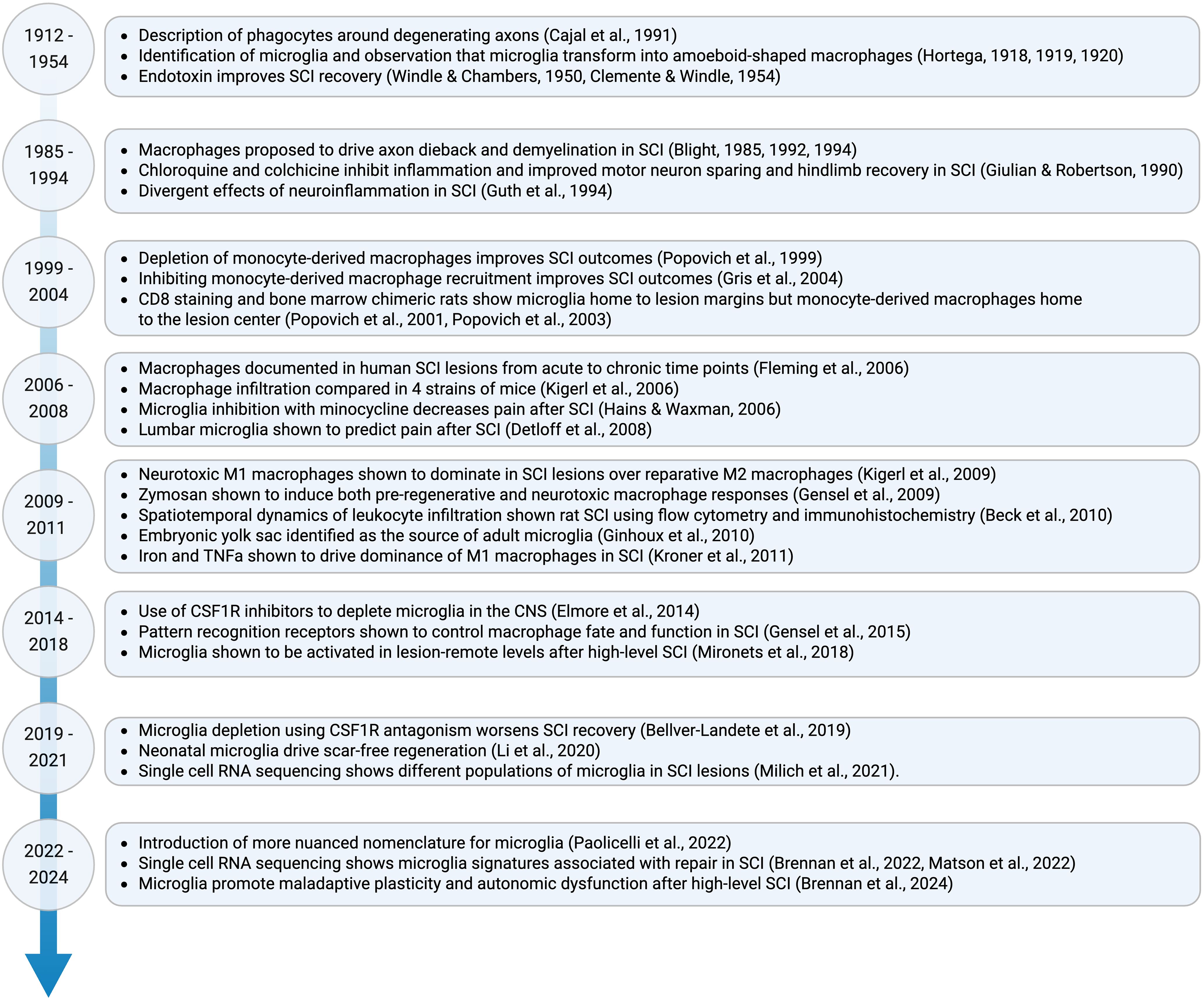
Figure 1. Timeline of major discoveries on the role of microglia in SCI. Due to space restrictions only select papers are shown.
Initial descriptions of macrophages in SCI were made in the early 1900’s by Spanish neuroanatomist Santiago Ramón y Cajal. In spinal tissue sections from cats, dogs, and rabbits with contusion, laceration, or transection SCI, Cajal observed rapid ‘traumatic degeneration’ – dystrophic axon bulbs that were thought to underlie central nervous system (CNS) regeneration failure (9). Cajal remarked that the centers and peripheral stumps of these degenerated and blebbing nerves were a ‘pasture-ground for phagocytes’ (9–12). Cajal’s silver staining techniques were not able to determine the origin, phenotype, or functional repertoire of lesion-associated phagocytes, though he accurately predicted that most of the cells directly around dystrophic axons originated from the blood (12). After over 100 iterations of protocol development, Cajal’s contemporary, Pío del Río Hortega, integrated lithium carbonate with silver nitrate staining and formalin-ammonium bromide fixation methods to precipitate silver carbonate (13). Using this method, the cytoplasmic expansions of cells with a tiny soma and branched processes could be distinguished from astrocytes and neurons in the intact CNS (14, 15). As these cells were smaller than other glia and exhibited shorter, finer processes, they were called microglia (14, 15). Hortega noted that microglia could migrate, phagocytose, and undergo morphological transformation, increasing their soma to become amoeboid-shaped macrophages (16, 17). However, at the time it was impossible to distinguish microglia from infiltrating, monocyte-derived macrophages in CNS lesions, or to determine their functional role in CNS injury. Despite the discovery that CNS lesions were rich in macrophages, neuroimmune research stagnated for the next three decades. This was because the study of glia and phagocytosis was limited to morphological characterizations with insufficient tools to assess function. Also, electrical properties could not be detected in glial or immune cells at the time, making them less attractive to study than neuronal action potentials. Third, glia were still largely considered as ‘connective tissue’ that simply held nervous elements together (18). Fortunately, this view would dramatically change in future years.
Functional roles for macrophages in SCI repair
In the 1950s, an unexpected discovery highlighted functional interactions between neuronal, immune, and glial cells that rejuvenated neuroimmune research. Injection of Priomen, a crude pyrogen used to study mechanisms of thermal regulation, improved functional recovery after SCI in dogs (19). Macrophage profiles were detected adjacent to newly sprouting nerve fibers, extending their processes around demyelinated axons, with their cell bodies laden with lipid debris months and years post-SCI (19, 20). Studies three decades later in rats with SCI found that injection of bacterial endotoxin also enhanced macrophage accumulation and functional recovery (21). The beneficial effects of macrophages were thought to be mediated by the removal of cellular debris required to stimulate tissue revascularization and reconstruction (21, 22). However, the beneficial effects of endotoxin were augmented by simultaneous injection with anti-inflammatory steroids (21, 22). This was among the first observations showing the divergent effects of neuroinflammatory cells in SCI. Data from subsequent studies in the early 1990’s in different species also showed that the inflammatory response, which was known to involve macrophages, could be harmful to SCI motor, sensory and autonomic recovery (23, 24). For example, chloroquine and colchicine decreased the number of macrophages and improved motor neuron sparing, hindlimb recovery, and bladder function when given to rabbits six hours after ischemic SCI (24), although effects on specific motor or autonomic neuron subtypes were not identified. Similarly, injection of silica dust to suppress macrophage function improved sparing of myelinated axons in the dorsal horn of guinea pigs with lateral compression SCI (23). In the 1980’s and early 1990’s, electron and light microscopy studies of axons in contusion lesions revealed that the number of intact axons decreases over 2–7 days (d) post-injury, coinciding with invasion of macrophages (25, 26). However, the specific macrophage subsets, neurons they interacted with, and intracellular signaling pathways affected by these broad-acting immune-modulatory strategies was not fully understood.
In the 2000’s it became appreciated that intraspinal macrophages have the potential to promote both tissue injury and repair in SCI, and that these seemingly divergent effects are not necessarily mutually exclusive (27–30). The injured spinal cord is rich in damage-associated molecular patterns (DAMPs), including heat shock proteins, necrotic cell debris, extracellular matrix products (fibronectin, hyaluronic acid), high-mobility group box 1, and mRNA, that can activate macrophage pattern recognition receptors (PRRs). Stochastic interactions between DAMPs and macrophage PRRs have the capacity to control the functional fate of monocyte-derived macrophages and microglia in SCI lesions (31, 32). Indeed, the phenotype of intraspinal macrophages changes as the lesion environment evolves (33).
Although more dimensional descriptions of macrophages are now used to better capture the phenotypic and functional heterogeneity of macrophages (34), a linear scale was initially used to describe intraspinal macrophages. Macrophages were often described as being activated on a continuum from ‘pro-inflammatory/M1’ to ‘anti-inflammatory/M2’ macrophages (33). M1 macrophages express more iNOS, CD86 and CD16/32, and are activated by endotoxin, interferon (IFN)-γ and tumor necrosis factor (TNF)-α. M2 macrophages express more CD206, Arginase-1 and CD16, and are activated by IL-4 and IL-13. In SCI, M1 macrophages drive neuron death and axon dieback, whereas M2 macrophages can promote neuron survival and axon outgrowth even across grown-inhibitory gradients containing chondroitin sulphate proteoglycans (33). In line with this, blocking M2 macrophage recruitment worsens motor recovery and increases lesion size (35). The typical ratio of M1:M2 macrophages in SCI is ~50:50 until 7 d post-injury, but unfortunately, M1 macrophages dominate after 14 d post-injury (33, 36), and transplanted M2-polarized macrophages differentiate into M1 macrophages (12, 33). The reason that harmful M1 macrophages ultimately dominate SCI lesions was a mystery until a seminal study showed that intraspinal iron and TNF are powerful signals that prevent phagocytosis-mediated conversion from M1 to M2 macrophages (37).
However, pro-inflammatory macrophage activation is not exclusively detrimental. This was demonstrated by combining intraspinally injected zymosan, a glucan polysaccharide found in yeast and potent macrophage activator, with transplantation of dorsal root ganglion (DRG) cells into the same spinal cord (38). Zymosan triggers a florid macrophage response and drives DRG axon outgrowth through the release of macrophage-derived neurotrophins and growth factors [e.g., brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), and glial cell line-derived neurotrophic factor (GDNF)] (29, 38, 39). However, enhanced axonal outgrowth induced by zymosan occurs concurrently with axon loss and neuron death near reactive macrophages (38). This is likely because zymosan can have paradoxical roles depending on which PRR(s) it activates. Specifically, zymosan can bind to both dectin-1, a C-type lectin receptor (CLR), and toll-like receptor 2 (TLR2). The activation of dectin-1 on intraspinal macrophages drives zymosan-induced axonal dieback and increases lesion size (40). Conversely, the activation of TLR2 using a TLR2 antagonist, which also triggers macrophage activation, increases axon density and reduces axon retraction from the lesion site (40, 41). These data are reminiscent of observations made decades earlier using crude pyrogens and endotoxin (19–22), which activate TLR2. The potential to manipulate macrophage functional plasticity to promote repair of the injured spinal cord is the subject of several excellent reviews (12, 42–49), although monocyte-derived macrophages and microglia are often considered together.
Mapping the location of monocyte-derived macrophages vs. microglia in SCI
As it became evident that macrophages had significant but complex roles in SCI pathophysiology, subsequent efforts sought to better understand macrophage heterogeneity, beginning with distinguishing microglia from monocyte-derived macropahges. Adoption of specific tools, including targeted antibody labeling, bone marrow chimeras, and transgenic reporter mice, enabled more precise mapping of the niches that monocyte-derived macrophages vs. microglia occupy within SCI lesions (Figure 2). Monoclonal antibody staining to CD8 showed that hematogenous macrophages home to central necrotic regions of lesion cavitation after rat spinal cord injury (50). Bone marrow chimeric rats demonstrated that microglia are activated rapidly after SCI and are present around the injury site, whereas monocyte-derived macrophages exclusively infiltrate the central gray matter lesion, and to a lesser extent the subpial white matter, peaking recruitment around 7 d post-SCI (51). Lys-EGFP-ki mice (which express enhanced green fluorescent protein (EGF) in mature myeloid lineage cells but not microglia) showed that at six weeks after compression SCI, monocyte-derived macrophages reside in the lesion epicentre, but microglia are at the lesion margins (52). Lys-EGFP-ki mice were also used to show that microglia are the first macrophage population to contact degenerating axons in vivo (within minutes). After ~ 3 d post-injury, monocyte-derived macrophages become the main cell type contacting dying axons, but they process phagocytic material less effectively than microglia (53). Studies using Cx3cr1gfp/+>WT bone marrow chimeric mice also confirmed that monocyte recruitment is delayed relative to microglia, peaking around 7d post-SCI, and that these cells home to the central gray matter (5, 54). More recent studies using tamoxifen-inducible conditional transgenic reporter mice (Cx3cr1creER::R26-TdT) to selectively label microglia showed that microglia rapidly die but then proliferate extensively during the first two weeks post-SCI (55). These proliferating microglia home to the interface between infiltrating leukocytes and astrocytes (55). The homing of monocyte-derived macrophages and microglia to distinct alcoves of SCI lesions suggests that the developmental origin of macrophages dictates which lesion-associated ligands they are exposed to, and their functional effects on surrounding tissue (51).
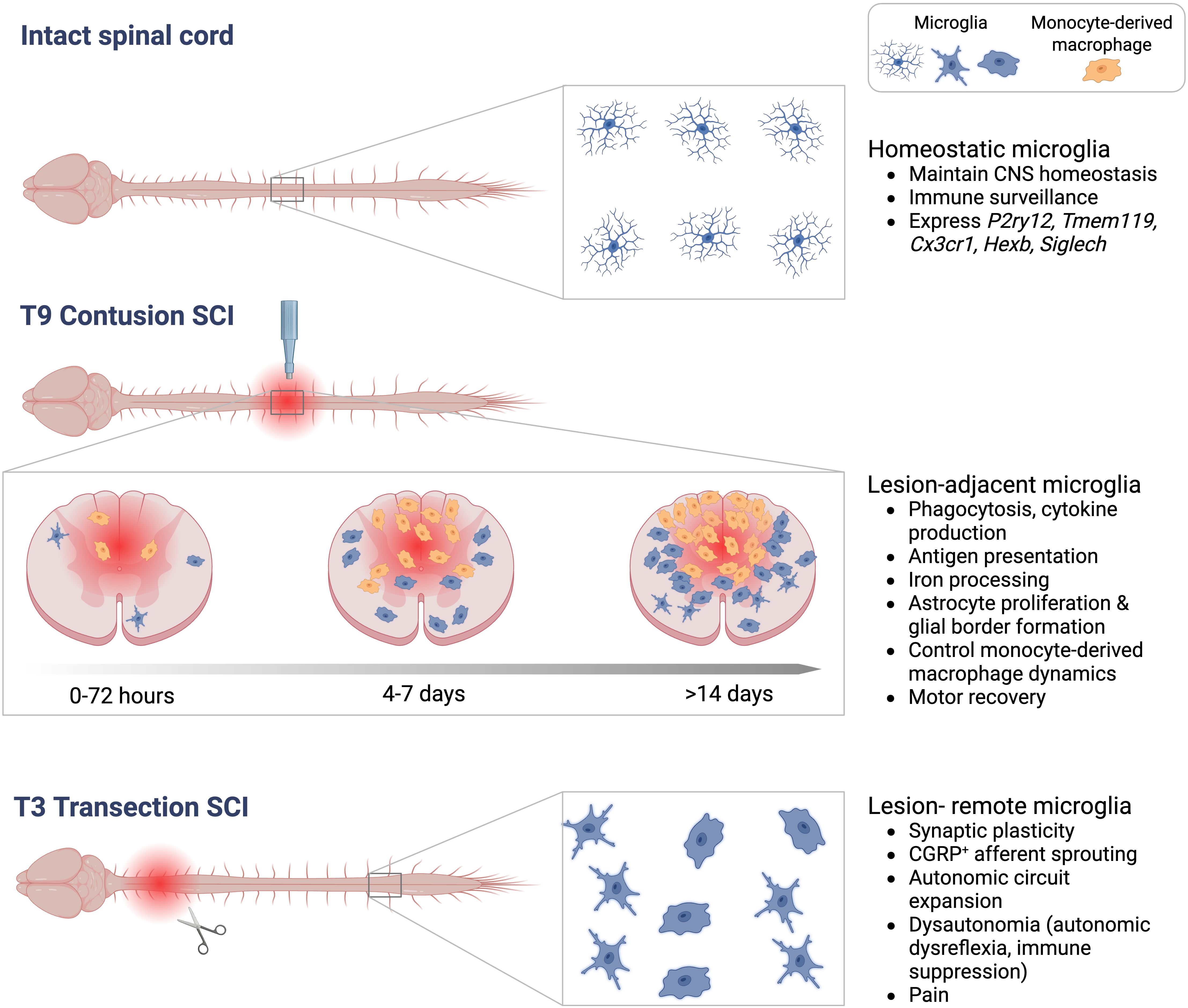
Figure 2. Schematic showing different activation states and functions of microglia. Top<: Microglia tile throughout the intact spinal cord and exhibit a ramified morphology. Middle: Microglia adjacent to a T9 contusion SCI become phagocytic and stimulate cytokine production, coordinate astrogliosis and the inflammatory response to drive motor recovery. Microglia and monocyte-derived macrophages home to specific regions of SCI lesions in a time-dependent manner. Bottom: After a T3 transection SCI, microglia in lesion-remote thoracic and lumbar segments drive maladaptive plasticity after high-level SCI.
Distinguishing the function of monocyte-derived macrophages and microglia using targeted cell depletion strategies
Although both monocyte-derived macrophages and microglia have the capacity to drive repair or secondary injury, the use of more precise strategies to deplete specific macrophage populations provided evidence that blood-borne macrophages are mostly harmful to the injured spinal cord, whereas tissue resident microglia are mostly beneficial. Intravenously injected liposome-encapsulated clodronate depletes monocyte-derived macrophages and improves hindlimb locomotion, preserves myelinated axons, decreases cavitation, and enhances axon sprouting in the lesion (56–58). The tissue damage and macrophage activation induced by zymosan can also be partially reversed by injecting clodronate-encapsulated liposomes (38). Radiation bone marrow chimeric rats also confirmed that hematogenous macrophages are the principal effectors of zymosan-induced axonal pathology (59). In vivo studies and time-lapse imaging in cultured dorsal root ganglion neurons showed that monocyte-derived macrophages physically interact with dystrophic axons and drive their retraction (60). Like hematogenous macrophage depletion, blocking recruitment of circulating myeloid cells into SCI lesions via intravenous injection of a neutralizing antibody to CD11d integrin or CD49d/CD29 integrin improves motor performance, myelin preservation, and axon sparing in rodent SCI (61–63).
In 2014, colony stimulating factor 1 receptor (CSF1R) inhibitors became available to deplete microglia without depleting monocyte-derived macrophages (64). These tools have now been used by several groups to interrogate the role of microglia in contusion SCI. The data show that pharmacological microglia depletion impairs motor recovery by disrupting several naturally occurring neuroprotective processes (55, 65, 66). Microglia-dependent protective functions include: JAK/STAT3-dependent astroglial proliferation and protective astroglial border formation, promoting neuronal survival, releasing neurotrophins, axon regeneration, and oligodendrocyte precursor cell survival (55, 65–69) (Figure 2). Microglia depletion also significantly delays the entry of monocyte-derived macrophages into spinal lesions. When monocyte-derived macrophages do arrive, they disperse throughout ventrolateral white matter regions that would normally be spared, and hinder motor recovery (66). This is in line with data showing that blocking the centripedal migration and sequestration of monocyte-derived macrophages to the central lesion core by worsens tissue sparing and functional recovery from SCI (70–72). Increasing microglial proliferation by local delivery of macrophage-colony stimulating factor (M-CSF) reduces lesion size and enhances functional recovery (55). Similarly, engineering microglia to overexpress BDNF, using Cx3cr1creER::BDNF or Tmem119::BDNF transgenic mice, reduces inflammation, neuronal death, and increases angiogenesis and motor recovery in mice with T10 crush SCI (67). A protective role of microglia on spinal vasculature was also demonstrated in an aortic cross-clamp model of ischemic SCI. Serial injections of lipopolysaccharide (LPS) prior to SCI ‘prime’ microglia and prevents ischemia-induced paralysis; LPS-induced neuroprotection is reversed by microglia depletion (73). IL-1-dependent microglia-endothelial cell interactions are critical in mediating this neuroprotective program (73). Collectively, studies using microglia-specific depletion strategies consistently show that, in contrast to monocyte-derived macrophages, microglia drive repair and regenerative processes after SCI.
Transcriptional responses of microglia to SCI
Since boosting the beneficial functions of long-lived microglia in vivo could be a novel therapeutic strategy for SCI, it is critical to understand the mechanisms through which microglia drive CNS repair. Research in recent years has taken advantage of RNA sequencing technologies to provide more granular insight as to how microglia coordinate inflammation, neuroprotection, and tissue repair in SCI. Bulk RNA sequencing of spinal cord homogenates showed that >50% of the top 1000 genes that are increased by SCI require microglia presence (66). Gene ontology analysis showed that these genes are responsible for microglia proliferation, phagocytosis, cytokine production, endocytosis, and/or protein secretion (e.g., Aif1, Ccl2, Ccl3, Ccl5, CD14, Cd36, Osm, Pycard, Syk, Tgfb1, Tlr2, Tlr4, Tnf, Trem2) (66, 74). The beneficial effects of microglia in SCI are partly mediated through phagocytosis and cytokine production, since the worsened phenotype of microglia-depleted mice can be rescued by reconstituting the lesion environment with recombinant CCL2 and a TLR2 agonist, effectively reprograming monocyte-derived macrophages to become less destructive (66). These data are in line with observations that efficient phagocytic clearance of myelin debris and apoptotic cell material is required for tissue repair, remyelination, and axon regeneration after SCI (44–47).
Single cell RNA sequencing datasets also show that microglia coordinate SCI repair by dynamically changing their transcriptional phenotype. In the intact spinal cord, microglia mainly express homeostatic genes, including P2ry12, Tmem119, Hexb, Siglech, and Cx3cr1 (66, 75, 76). However, microglia in the injured spinal cord adopt several injury-associated transcriptional phenotypes, including genes that control cell lipid phagocytosis (e.g., Cd68, Clec7a, Ctsd, Ctsz, Trem2, Apoe), iron processing (e.g., Fth1, Ftl1), interferon production (e.g., Ifit1, Ifit2, Ifit 3, Irf7), and antigen-binding and processing (e.g., H2-Ab1, H2-Eb1, CD74, Cd93, Cd38) (66, 75–78). These phenotypes shift in proportion over time, but can be found in acute (1–3 d), subacute (7 d) and chronic (one month) time points (66, 75). Evaluating the transcriptional profile of other cell types in the lesion shows that microglia are also required for astrocytes to increase genes that drive cytoplasmic translation, response to interleukin-4, and immune responses (e.g. Tmsb4x, Fth1, Apoe) (66). Transcriptional analysis of monocyte-derived macrophages shows that without microglia present, monocyte-derived macrophages express more genes that could promote inflammation and neurotoxicity (e.g., Cd86, Cd36, Clec12a) (33, 66, 79). The induction of these transcriptional programs by microglia explains why astroglial and monocyte-derived macrophage responses to SCI are disrupted without microglia.
CSF1R inhibition combined with single cell RNA sequencing also revealed how microglia control axon regeneration in the injured young vs. adult CNS. Mice at postnatal day two exhibit scar-free healing and axon regeneration across the lesion site (69). Microglia are critical for neonatal spinal cord regeneration, as microglia depletion prevents axon regeneration across the lesion site (69). Single cell RNA sequencing showed that neonatal microglia secrete extracellular matrix bridge proteins (e.g. Fn1, Thbs1) that ligate the crushed spinal cord ends, then produce peptidase and endopeptidase inhibitors (e.g. Cstb, Stfa1, Serpin6a, Anxa1) that drive resolution of inflammation (69). Transplantation of neonatal microglia or peptidase inhibitor-treated microglia into adult lesions improves axon growth and tissue repair (69). Regeneration-associated bridging microglia are much less abundant in the adult spinal cord and express higher levels of CD68 and lower levels of P2y12, which is thought to dampen their ability to promote regeneration in the adult spinal cord (69, 80).
Interestingly, a recent study showed that if microglia are depleted and then allowed to repopulate the inflammatory environment of chronic SCI lesions, they return with a more pro-inflammatory and pro-regenerative phenotype than the original microglia (81). In this study, CSF1R was inhibited from 7–9 weeks post-SCI and then the inhibitor was withdrawn from week 9–12 to allow microglia to repopulate (81). Microglia depletion reduced expression of inflammatory genes (e.g. C1qb, Ccl12) (81). In comparison, forcing microglia turnover increased extracellular matrix genes (e.g. Ncam1, Cadm3, L1cam) and neuronal transcripts (e.g. App, Nptn, Nf1, Nrxn1), which were associated with increased density of β3-tubulin+ axons in the lesions (81).
We anticipate that ongoing sequencing studies will continue to shed light on mechanisms of biological heterogeneity as a function of time post-injury, injury level, injury severity, proximity to the lesion, biological sex, age, and other environmental or therapeutic factors. These data could then be harnessed to provide new microglia-dependent targets that could be co-opted to develop tailored microglia-dependent therapeutics.
Lesion-remote microglia shape intraspinal plasticity after SCI
Although most research has focused on lesion-adjacent microglia and their role in neuroinflammation, microglia distant to the lesion can also become activated and shape spinal circuitry to affect functional outcomes from SCI (Figure 2). The role of microglia in synaptic plasticity and circuit remodeling was recently shown to be critical for the development of autonomic dysregulation after SCI (82). A high-level SCI above the major sympathetic outflow (spinal level T6) disinhibits sympathetic preganglionic neurons (SPNs) from descending brainstem control. Consequently, remarkable synaptic plasticity, axonal sprouting and autonomic circuit expansion occurs within circuits that control lymphoid and endocrine organs (83–84). This leads to a condition called dysautonomia, which manifests in the cardiovascular system as autonomic dysreflexia, in the immune system as immune-depression syndrome, and in the endocrine system as metabolic syndrome (83, 84). In T3 transection SCI, microglia increase in number and adopt hypertrophic, amoeboid-shaped morphologies in thoracic and lumbar spinal segments centimeters away from the lesion (3, 82, 85, 86). Microglia activation in lesion-remote regions is triggered by the activity of disinhibited glutamatergic interneurons; silencing excitatory neuron activity by blocking Vglut2 activity, or blocking calcium channel α2δ-1 signaling, prevents microglia hyperplasia and hypertrophy (82). These interventions also prevent maladaptive synaptic plasticity, circuit formation and dysautonomia (82, 87, 88).
To determine if microglia have a causal role in maladaptive plasticity and dysautonomia, microglia were depleted pharmacologically using CSF1R antagonism or genetically using Cx3cr1creERxR26iDTR mice. These experiments showed that microglia depletion blocks structural and functional plasticity of autonomic circuits after high-level SCI (82). Specifically, microglia depletion prevents SCI-induced excitatory synaptogenesis and loss of inhibitory synapses, decreases sprouting of lumbar CGRP+ afferents, and prevents the expansion of neuronal circuits that innervate lymphoid and endocrine tissues (82). Consequently, indices of dysautonomia (i.e., autonomic dysreflexia, splenic atrophy, antigen-specific antibody production), are also improved by microglia depletion in high-level SCI. Mechanistically, microglia strip inhibitory synapses from SPNs and the interneurons they connect to, lowering their threshold for activation and excitatory circuit formation. The Trem2 receptor is at least partially required for this response (82). Other studies have shown that inhibition of soluble TNFα, which is predominantly produced by microglia, prevents maladaptive structural plasticity and autonomic dysregulation after high-level SCI (85, 89).
Lesion-remote microglia are also thought to drive thermal and mechanical hypersensitivity post-SCI. Activation of lumbar microglia is associated with phosphorylation of p38 MAP kinase, elevated TNFα and IL-1β levels, and induction of allodynia after SCI (90). The inhibition of lesion-remote microglia using minocycline prevents hyperresponsiveness of lumbar dorsal horn neurons, p38 MAP kinase and blocks SCI-induced pain (91). Thus, in designing strategies to manipulate microglia therapeutically, it is important to not only consider the protective role of lesion-adjacent microglia in coordinating neuroinflammation, but also the pathological role of lesion-remote microglia in aberrant signaling that drives dysautonomia and pain.
The future: microglia-targeting strategies to repair the injured spinal cord
There are now several genetic and pharmacological approaches being actively explored to manipulate microglia to promote tissue repair and functional recovery from SCI. Microglia transplantation (69, 92, 93) in specific CNS regions is possible through local intraparenchymal injections, although whether their phenotype and function remains long-term is unknown. A more targeted approach is to use lipid-polymer-hybridized-nanoparticles (LPNPs) to deliver siRNA within defined CNS regions to modify microglial gene expression (94). This technique harnesses the fact that microglia are the primary phagocytes in the CNS, and selectively phagocytose biocompatible nanoparticles loaded with siRNA and either Rhodamine B or Alexa555-conjugated gold nanoparticles tracers, allowing microglial fate-mapping alongside gene manipulation (94). However, this technique requires fully functional phagocytosis pathways (i.e., ‘find me’, and ‘eat me’ signals), which may themselves be modified by pathology.
An alternative approach is to use adeno-associated viral (AAV) vectors containing, for example, Iba1 promoter regions to transduce microglia in vivo (95–97). AAV viral vectors have successfully modified microglial gene expression and disease outcomes in various neurodegenerative diseases and peripheral neuropathies (95–100). Since SCI has a less complex progression staging and timing of diagnosis than these conditions, it should be possible to time the delivery of AAV therapies to target specific microglia-dependent neuroinflammatory events. However, since SCI lesions have a larger contingent of peripheral immune cells than chronic neurodegenerative lesions, AAV technologies may not be as effective in distinguishing and targeting microglia vs. monocyte-derived macrophages in SCI. However, a recent study used a combinatorial genetic and surgical strategy to chronically target microglia with region specificity, without affecting peripheral macrophages (101). Specifically, a tamoxifen metabolite (endoxifen) was administered to Cx3cr1creERT2 or TMEM119creERT2 mice. Sustained microglia gene manipulation was achieved by delivering endoxifen through osmotic pumps attached to fine cannulas made of stainless steel or microfluidic polymer fibers (101).
Microglia are also central components of various other therapeutic strategies in development for SCI. For example, the gut microbiome influences microglial immunosurveillance, phenotype, and synaptic remodeling, suggesting that microglia could also be co-opted non-invasively through strategies targeting the gut-brain axis (102, 103). Epigenetic changes (e.g. DNA methylation, histone deactylation) impact microglia responses and represent a novel therapeutic avenue (104). Microglia-targeting therapies have also been shown to boost the efficacy of other interventions, such as rehabilitation training (105). We expect that in future years, these and many other strategies centered on microglia biology will emerge as flourishing fields to enhance recovery after SCI, and potentially other types of CNS trauma.
Author contributions
ES: Writing – original draft, Writing – review & editing. FB: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The laboratory of FHB was supported by the Natural Sciences and Engineering Council of Canada (2023-04519 and 2023-00174), the Craig H. Neilsen Foundation (994510), the Wings for Life Spinal Cord Research Foundation (WFL-CA-05/23), the Banting foundation, the J.P. Bickell foundation, and Queen’s University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, and Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain J Neurol. (2010) 133:433–47. doi: 10.1093/brain/awp322
2. Kigerl KA, McGaughy VM, and Popovich PG. Comparative analysis of lesion development and intraspinal inflammation in four strains of mice following spinal contusion injury. J Comp Neurol. (2006) 494:578–94. doi: 10.1002/cne.20827
3. Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, et al. The cellular inflammatory response in human spinal cords after injury. Brain J Neurol. (2006) 129:3249–69. doi: 10.1093/brain/awl296
4. Mazzitelli JA, Smyth LCD, Cross KA, Dykstra T, Sun J, Du S, et al. Cerebrospinal fluid regulates skull bone marrow niches via direct access through dural channels. Nat Neurosci. (2022) 25:555–60. doi: 10.1038/s41593-022-01029-1
5. Blomster LV, Brennan FH, Lao HW, Harle DW, Harvey AR, and Ruitenberg MJ. Mobilisation of the splenic monocyte reservoir and peripheral CX3CR1 deficiency adversely affects recovery from spinal cord injury. Exp Neurol. (2013) 247:226–40. doi: 10.1016/j.expneurol.2013.05.002
6. Kettenmann H, Hanisch U-K, Noda M, and Verkhratsky A. Physiology of microglia. Physiol Rev. (2011) 91:461–553. doi: 10.1152/physrev.00011.2010
7. Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. (2010) 330:841–5. doi: 10.1126/science.1194637
8. Patel AA, Zhang Y, Fullerton JN, Boelen L, Rongvaux A, Maini AA, et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J Exp Med. (2017) 214:1913–23. doi: 10.1084/jem.20170355
9. Cajal SR, DeFelipe J, and Jones EG. Cajal’s Degeneration and Regeneration of the Nervous System. London: Oxford University Press (1991).
10. Ramon y Cajal S. Sobre un nuevo proceder de impregnación de la neuroglía y sus resultados en los centros nerviosos del hombre y animales. Trab Lab Invest Biol. (1913) 11:103–12.
11. Popovich PG. Neuroimmunology of traumatic spinal cord injury: a brief history and overview. Exp Neurol. (2014) 258:1–4. doi: 10.1016/j.expneurol.2014.05.001
12. Silver J, Schwab ME, and Popovich PG. Central nervous system regenerative failure: role of oligodendrocytes, astrocytes, and microglia. Cold Spring Harb Perspect Biol. (2014) 7:a020602. doi: 10.1101/cshperspect.a020602
13. Del Rio Hortega P. Noticia de un nuevo y fácil método para la coloración de la neuroglia y el tejido conjuntivo. Trab Lab Invest Biol. (1918) 15:367–78.
14. Del Rio Hortega P. El “Tercer Elemento” de los Centros Nerviosos. I. La Microglía en Estado Normal. Bol Soc Esp Biol VIII. (1919), 67–82.
15. Pérez-Cerdá F, Sánchez-Gómez MV, and Matute C. Pío del Río Hortega and the discovery of the oligodendrocytes. Front Neuroanat. (2015) 9:92. doi: 10.3389/fnana.2015.00092
16. Del Rio Hortega P. Estudios sobre la neuroglía. La microglía y su transformación en células en bastoncito y cuerpos granuloadiposos. Trab Lab Invest Biol. (1920) 18:37–82.
17. Augusto-Oliveira M, Arrifano G de P, Leal-Nazaré CG, Chaves-Filho A, Santos-Sacramento L, Lopes-Araujo A, et al. Morphological diversity of microglia: Implications for learning, environmental adaptation, ageing, sex differences and neuropathology. Neurosci Biobehav Rev. (2025) 172:106091. doi: 10.1016/j.neubiorev.2025.106091
18. Fan X and Agid Y. At the origin of the history of glia. Neuroscience. (2018) 385:255–71. doi: 10.1016/j.neuroscience.2018.05.050
19. Windle WF and Chambers WW. Regeneration in the spinal cord of the cat and dog. J Comp Neurol. (1950) 93:241–57. doi: 10.1002/cne.900930206
20. Clemente CD and Windle WF. Regeneration of severed nerve fibers in the spinal cord of the adult cat. J Comp Neurol. (1954) 101:691–731. doi: 10.1002/cne.901010304
21. Guth L, Zhang Z, DiProspero NA, Joubin K, and Fitch MT. Spinal cord injury in the rat: treatment with bacterial lipopolysaccharide and indomethacin enhances cellular repair and locomotor function. Exp Neurol. (1994) 126:76–87. doi: 10.1006/exnr.1994.1043
22. Guth L, Zhang Z, and Roberts E. Key role for pregnenolone in combination therapy that promotes recovery after spinal cord injury. Proc Natl Acad Sci U.S.A. (1994) 91:12308–12. doi: 10.1073/pnas.91.25.12308
23. Blight AR. Effects of silica on the outcome from experimental spinal cord injury: implication of macrophages in secondary tissue damage. Neuroscience. (1994) 60:263–73. doi: 10.1016/0306-4522(94)90220-8
24. Giulian D and Robertson C. Inhibition of mononuclear phagocytes reduces ischemic injury in the spinal cord. Ann Neurol. (1990) 27:33–42. doi: 10.1002/ana.410270107
25. Blight AR. Macrophages and inflammatory damage in spinal cord injury. J Neurotrauma. (1992) 9 Suppl 1:S83–91.
26. Blight AR. Delayed demyelination and macrophage invasion: a candidate for secondary cell damage in spinal cord injury. Cent Nerv Syst Trauma J Am Paralys Assoc. (1985) 2:299–315. doi: 10.1089/cns.1985.2.299
27. Donnelly DJ and Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. (2008) 209:378–88. doi: 10.1016/j.expneurol.2007.06.009
28. Popovich PG and Longbrake EE. Can the immune system be harnessed to repair the CNS? Nat Rev Neurosci. (2008) 9:481–93. doi: 10.1038/nrn2398
29. Benowitz LI and Popovich PG. Inflammation and axon regeneration. Curr Opin Neurol. (2011) 24:577–83. doi: 10.1097/WCO.0b013e32834c208d
30. Stoll G, Jander S, and Schroeter M. Detrimental and beneficial effects of injury-induced inflammation and cytokine expression in the nervous system. Adv Exp Med Biol. (2002) 513:87–113. doi: 10.1007/978-1-4615-0123-7_3
31. Kigerl KA, Lai W, Wallace LM, Yang H, and Popovich PG. High mobility group box-1 (HMGB1) is increased in injured mouse spinal cord and can elicit neurotoxic inflammation. Brain Behav Immun. (2018) 72:22–33. doi: 10.1016/j.bbi.2017.11.018
32. Kigerl KA, de Rivero Vaccari JP, Dietrich WD, Popovich PG, and Keane RW. Pattern recognition receptors and central nervous system repair. Exp Neurol. (2014) 258:5–16. doi: 10.1016/j.expneurol.2014.01.001
33. Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, and Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci Off J Soc Neurosci. (2009) 29:13435–44. doi: 10.1523/JNEUROSCI.3257-09.2009
34. Paolicelli RC, Sierra A, Stevens B, Tremblay M-E, Aguzzi A, Ajami B, et al. Microglia states and nomenclature: A field at its crossroads. Neuron. (2022) 110:3458–83. doi: 10.1016/j.neuron.2022.10.020
35. Shechter R, Miller O, Yovel G, Rosenzweig N, London A, Ruckh J, et al. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity. (2013) 38:555–69. doi: 10.1016/j.immuni.2013.02.012
36. Gordon S and Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. (2005) 5:953–64. doi: 10.1038/nri1733
37. Kroner A, Greenhalgh AD, Zarruk JG, Passos Dos Santos R, Gaestel M, and David S. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron. (2014) 83:1098–116. doi: 10.1016/j.neuron.2014.07.027
38. Gensel JC, Nakamura S, Guan Z, van Rooijen N, Ankeny DP, and Popovich PG. Macrophages promote axon regeneration with concurrent neurotoxicity. J Neurosci Off J Soc Neurosci. (2009) 29:3956–68. doi: 10.1523/JNEUROSCI.3992-08.2009
39. Batchelor PE, Porritt MJ, Martinello P, Parish CL, Liberatore GT, Donnan GA, et al. Macrophages and Microglia Produce Local Trophic Gradients That Stimulate Axonal Sprouting Toward but Not beyond the Wound Edge. Mol Cell Neurosci. (2002) 21:436–53. doi: 10.1006/mcne.2002.1185
40. Gensel JC, Wang Y, Guan Z, Beckwith KA, Braun KJ, Wei P, et al. Toll-like receptors and dectin-1, a C-type lectin receptor, trigger divergent functions in CNS macrophages. J Neurosci Off J Soc Neurosci. (2015) 35:9966–76. doi: 10.1523/JNEUROSCI.0337-15.2015
41. Stivers NS, Pelisch N, Orem BC, Williams J, Nally JM, and Stirling DP. The toll-like receptor 2 agonist Pam3CSK4 is neuroprotective after spinal cord injury. Exp Neurol. (2017) 294:1–11. doi: 10.1016/j.expneurol.2017.04.012
42. Kroner A and Rosas Almanza J. Role of microglia in spinal cord injury. Neurosci Lett. (2019) 709:134370. doi: 10.1016/j.neulet.2019.134370
43. Zha X, Zheng G, Skutella T, Kiening K, Unterberg A, and Younsi A. Microglia: a promising therapeutic target in spinal cord injury. Neural Regener Res. (2025) 20:454–63. doi: 10.4103/NRR.NRR-D-23-02044
44. Milich LM, Ryan CB, and Lee JK. The origin, fate, and contribution of macrophages to spinal cord injury pathology. Acta Neuropathol (Berl). (2019) 137:785–97. doi: 10.1007/s00401-019-01992-3
45. David S, Greenhalgh AD, and Kroner A. Macrophage and microglial plasticity in the injured spinal cord. Neuroscience. (2015) 307:311–8. doi: 10.1016/j.neuroscience.2015.08.064
46. Gensel JC and Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. (2015) 1619:1–11. doi: 10.1016/j.brainres.2014.12.045
47. Brennan FH and Popovich PG. Emerging targets for reprograming the immune response to promote repair and recovery of function after spinal cord injury. Curr Opin Neurol. (2018) 31:334–44. doi: 10.1097/WCO.0000000000000550
48. David S and Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. (2011) 12:388–99. doi: 10.1038/nrn3053
49. Kong X and Gao J. Macrophage polarization: a key event in the secondary phase of acute spinal cord injury. J Cell Mol Med. (2017) 21:941–54. doi: 10.1111/jcmm.13034
50. Popovich PG, van Rooijen N, Hickey WF, Preidis G, and McGaughy V. Hematogenous macrophages express CD8 and distribute to regions of lesion cavitation after spinal cord injury. Exp Neurol. (2003) 182:275–87. doi: 10.1016/s0014-4886(03)00120-1
51. Popovich PG and Hickey WF. Bone marrow chimeric rats reveal the unique distribution of resident and recruited macrophages in the contused rat spinal cord. J Neuropathol Exp Neurol. (2001) 60:676–85. doi: 10.1093/jnen/60.7.676
52. Mawhinney LA, Thawer SG, Lu W-Y, Rooijen Nv, Weaver LC, Brown A, et al. Differential detection and distribution of microglial and hematogenous macrophage populations in the injured spinal cord of lys-EGFP-ki transgenic mice. J Neuropathol Exp Neurol. (2012) 71:180–97. doi: 10.1097/NEN.0b013e3182479b41
53. Greenhalgh AD and David S. Differences in the phagocytic response of microglia and peripheral macrophages after spinal cord injury and its effects on cell death. J Neurosci Off J Soc Neurosci. (2014) 34:6316–22. doi: 10.1523/JNEUROSCI.4912-13.2014
54. Donnelly DJ, Longbrake EE, Shawler TM, Kigerl KA, Lai W, Tovar CA, et al. Deficient CX3CR1 signaling promotes recovery after mouse spinal cord injury by limiting the recruitment and activation of Ly6Clo/iNOS+ macrophages. J Neurosci Off J Soc Neurosci. (2011) 31:9910–22. doi: 10.1523/JNEUROSCI.2114-11.2011
55. Bellver-Landete V, Bretheau F, Mailhot B, Vallières N, Lessard M, Janelle M-E, et al. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat Commun. (2019) 10:518. doi: 10.1038/s41467-019-08446-0
56. Lee SM, Rosen S, Weinstein P, van Rooijen N, and Noble-Haeusslein LJ. Prevention of both neutrophil and monocyte recruitment promotes recovery after spinal cord injury. J Neurotrauma. (2011) 28:1893–907. doi: 10.1089/neu.2011.1860
57. Iannotti CA, Clark M, Horn KP, van Rooijen N, Silver J, and Steinmetz MP. A combination immunomodulatory treatment promotes neuroprotection and locomotor recovery after contusion SCI. Exp Neurol. (2011) 230:3–15. doi: 10.1016/j.expneurol.2010.03.010
58. Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, and Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. (1999) 158:351–65. doi: 10.1006/exnr.1999.7118
59. Popovich PG, Guan Z, McGaughy V, Fisher L, Hickey WF, and Basso DM. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J Neuropathol Exp Neurol. (2002) 61:623–33. doi: 10.1093/jnen/61.7.623
60. Horn KP, Busch SA, Hawthorne AL, van Rooijen N, and Silver J. Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J Neurosci Off J Soc Neurosci. (2008) 28:9330–41. doi: 10.1523/JNEUROSCI.2488-08.2008
61. Geremia NM, Bao F, Rosenzweig TE, Hryciw T, Weaver L, Dekaban GA, et al. CD11d antibody treatment improves recovery in spinal cord-injured mice. J Neurotrauma. (2012) 29:539–50. doi: 10.1089/neu.2011.1976
62. Gris D, Marsh DR, Oatway MA, Chen Y, Hamilton EF, Dekaban GA, et al. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J Neurosci Off J Soc Neurosci. (2004) 24:4043–51. doi: 10.1523/JNEUROSCI.5343-03.2004
63. Fleming JC, Bao F, Chen Y, Hamilton EF, Relton JK, and Weaver LC. Alpha4beta1 integrin blockade after spinal cord injury decreases damage and improves neurological function. Exp Neurol. (2008) 214:147–59. doi: 10.1016/j.expneurol.2008.04.024
64. Elmore MRP, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. (2014) 82:380–97. doi: 10.1016/j.neuron.2014.02.040
65. Fu H, Zhao Y, Hu D, Wang S, Yu T, and Zhang L. Depletion of microglia exacerbates injury and impairs function recovery after spinal cord injury in mice. Cell Death Dis. (2020) 11:528. doi: 10.1038/s41419-020-2733-4
66. Brennan FH, Li Y, Wang C, Ma A, Guo Q, Li Y, et al. Microglia coordinate cellular interactions during spinal cord repair in mice. Nat Commun. (2022) 13:4096. doi: 10.1038/s41467-022-31797-0
67. Zeng F, Li Y, Li X, Gu X, Cao Y, Cheng S, et al. Microglia overexpressing brain-derived neurotrophic factor promote vascular repair and functional recovery in mice after spinal cord injury. Neural Regener Res. (2024) 21:365–76. doi: 10.4103/NRR.NRR-D-24-00381
68. Zhou Z-L, Xie H, Tian X-B, Xu H-L, Li W, Yao S, et al. Microglial depletion impairs glial scar formation and aggravates inflammation partly by inhibiting STAT3 phosphorylation in astrocytes after spinal cord injury. Neural Regener Res. (2023) 18:1325–31. doi: 10.4103/1673-5374.357912
69. Li Y, He X, Kawaguchi R, Zhang Y, Wang Q, Monavarfeshani A, et al. Microglia-organized scar-free spinal cord repair in neonatal mice. Nature. (2020) 587:613–8. doi: 10.1038/s41586-020-2795-6
70. Ye J, Shan F, Xu X, Liang C, Zhang N, Hu H, et al. Centripetal migration and prolonged retention of microglia promotes spinal cord injury repair. J Neuroinflamm. (2025) 22:77. doi: 10.1186/s12974-025-03411-9
71. Kobayakawa K, Ohkawa Y, Yoshizaki S, Tamaru T, Saito T, Kijima K, et al. Macrophage centripetal migration drives spontaneous healing process after spinal cord injury. Sci Adv. (2019) 5:eaav5086. doi: 10.1126/sciadv.aav5086
72. Zhou X, Wahane S, Friedl M-S, Kluge M, Friedel CC, Avrampou K, et al. Microglia and macrophages promote corralling, wound compaction and recovery after spinal cord injury via Plexin-B2. Nat Neurosci. (2020) 23:337–50. doi: 10.1038/s41593-020-0597-7
73. Freria CM, Brennan FH, Sweet DR, Guan Z, Hall JC, Kigerl KA, et al. Serial systemic injections of endotoxin (LPS) elicit neuroprotective spinal cord microglia through IL-1-dependent cross talk with endothelial cells. J Neurosci Off J Soc Neurosci. (2020) 40:9103–20. doi: 10.1523/JNEUROSCI.0131-20.2020
74. Noristani HN, Gerber YN, Sabourin J-C, Le Corre M, Lonjon N, Mestre-Frances N, et al. RNA-seq analysis of microglia reveals time-dependent activation of specific genetic programs following spinal cord injury. Front Mol Neurosci. (2017) 10:90. doi: 10.3389/fnmol.2017.00090
75. Milich LM, Choi JS, Ryan C, Cerqueira SR, Benavides S, Yahn SL, et al. Single-cell analysis of the cellular heterogeneity and interactions in the injured mouse spinal cord. J Exp Med. (2021) 218:e20210040. doi: 10.1084/jem.20210040
76. Matson KJE, Russ DE, Kathe C, Hua I, Maric D, Ding Y, et al. Single cell atlas of spinal cord injury in mice reveals a pro-regenerative signature in spinocerebellar neurons. Nat Commun. (2022) 13:5628. doi: 10.1038/s41467-022-33184-1
77. John RK, Vogel SP, Zia S, Lee KV, Nguyen AT, Torres-Espin A, et al. Reawakening inflammation in the chronically injured spinal cord using lipopolysaccharide induces diverse microglial states. J Neuroinflamm. (2025) 22:56. doi: 10.1186/s12974-025-03379-6
78. Hakim R, Zachariadis V, Sankavaram SR, Han J, Harris RA, Brundin L, et al. Spinal cord injury induces permanent reprogramming of microglia into a disease-associated state which contributes to functional recovery. J Neurosci Off J Soc Neurosci. (2021) 41:8441–59. doi: 10.1523/JNEUROSCI.0860-21.2021
79. Zhu Y, Lyapichev K, Lee DH, Motti D, Ferraro NM, Zhang Y, et al. Macrophage transcriptional profile identifies lipid catabolic pathways that can be therapeutically targeted after spinal cord injury. J Neurosci Off J Soc Neurosci. (2017) 37:2362–76. doi: 10.1523/JNEUROSCI.2751-16.2017
80. Li C, Wu Z, Zhou L, Shao J, Hu X, Xu W, et al. Temporal and spatial cellular and molecular pathological alterations with single-cell resolution in the adult spinal cord after injury. Signal Transduct Target Ther. (2022) 7:65. doi: 10.1038/s41392-022-00885-4
81. Stewart AN, Bosse-Joseph CC, Kumari R, Bailey WM, Park KA, Slone VK, et al. Nonresolving neuroinflammation regulates axon regeneration in chronic spinal cord injury. J Neurosci Off J Soc Neurosci. (2025) 45:e1017242024. doi: 10.1523/JNEUROSCI.1017-24.2024
82. Brennan FH, Swarts EA, Kigerl KA, Mifflin KA, Guan Z, Noble BT, et al. Microglia promote maladaptive plasticity in autonomic circuitry after spinal cord injury in mice. Sci Transl Med. (2024) 16:eadi3259. doi: 10.1126/scitranslmed.adi3259
83. DiSabato DJ, Marion CM, Mifflin KA, Alfredo AN, Rodgers KA, Kigerl KA, et al. System failure: Systemic inflammation following spinal cord injury. Eur J Immunol. (2024) 54:e2250274. doi: 10.1002/eji.202250274
84. Rodgers KA, Kigerl KA, Schwab JM, and Popovich PG. Immune dysfunction after spinal cord injury - A review of autonomic and neuroendocrine mechanisms. Curr Opin Pharmacol. (2022) 64:102230. doi: 10.1016/j.coph.2022.102230
85. Mironets E, Osei-Owusu P, Bracchi-Ricard V, Fischer R, Owens EA, Ricard J, et al. Soluble TNFα Signaling within the Spinal Cord Contributes to the Development of Autonomic Dysreflexia and Ensuing Vascular and Immune Dysfunction after Spinal Cord Injury. J Neurosci Off J Soc Neurosci. (2018) 38:4146–62. doi: 10.1523/JNEUROSCI.2376-17.2018
86. Freria CM, Hall JCE, Wei P, Guan Z, McTigue DM, and Popovich PG. Deletion of the fractalkine receptor, CX3CR1, improves endogenous repair, axon sprouting, and synaptogenesis after spinal cord injury in mice. J Neurosci Off J Soc Neurosci. (2017) 37:3568–87. doi: 10.1523/JNEUROSCI.2841-16.2017
87. Brennan FH, Noble BT, Wang Y, Guan Z, Davis H, Mo X, et al. Acute post-injury blockade of α2δ-1 calcium channel subunits prevents pathological autonomic plasticity after spinal cord injury. Cell Rep. (2021) 34:108667. doi: 10.1016/j.celrep.2020.108667
88. Noble BT, Brennan FH, Wang Y, Guan Z, Mo X, Schwab JM, et al. Thoracic VGluT2+ Spinal interneurons regulate structural and functional plasticity of sympathetic networks after high-level spinal cord injury. J Neurosci Off J Soc Neurosci. (2022) 42:3659–75. doi: 10.1523/JNEUROSCI.2134-21.2022
89. Mironets E, Fischer R, Bracchi-Ricard V, Saltos TM, Truglio TS, O’Reilly ML, et al. Attenuating neurogenic sympathetic hyperreflexia robustly improves antibacterial immunity after chronic spinal cord injury. J Neurosci Off J Soc Neurosci. (2020) 40:478–92. doi: 10.1523/JNEUROSCI.2417-19.2019
90. Detloff MR, Fisher LC, McGaughy V, Longbrake EE, Popovich PG, and Basso DM. Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp Neurol. (2008) 212:337–47. doi: 10.1016/j.expneurol.2008.04.009
91. Hains BC and Waxman SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci Off J Soc Neurosci. (2006) 26:4308–17. doi: 10.1523/JNEUROSCI.0003-06.2006
92. Li P, Zhao J, Ma Y, Wang L, Liang S, Fan F, et al. Transplantation of miR-145a-5p modified M2 type microglia promotes the tissue repair of spinal cord injury in mice. J Transl Med. (2024) 22:724. doi: 10.1186/s12967-024-05492-1
93. Akhmetzyanova ER, Mukhamedshina YO, Zhuravleva MN, Galieva LR, Kostennikov AA, Garanina EE, et al. Transplantation of microglia in the area of spinal cord injury in an acute period increases tissue sparing, but not functional recovery. Front Cell Neurosci. (2018) 12:507. doi: 10.3389/fncel.2018.00507
94. Guo S, Cázarez-Márquez F, Jiao H, Foppen E, Korpel NL, Grootemaat AE, et al. Specific silencing of microglial gene expression in the rat brain by nanoparticle-based small interfering RNA delivery. ACS Appl Mater Interfaces. (2022) 14:5066–79. doi: 10.1021/acsami.1c22434
95. Stamataki M, Rissiek B, Magnus T, and Körbelin J. Microglia targeting by adeno-associated viral vectors. Front Immunol. (2024) 15:1425892. doi: 10.3389/fimmu.2024.1425892
96. Serrano C, Cananzi S, Shen T, Wang L-L, and Zhang C-L. Simple and highly specific targeting of resident microglia with adeno-associated virus. iScience. (2023) 27:110706. doi: 10.1016/j.isci.2024.110706
97. Okada Y, Hosoi N, Matsuzaki Y, Fukai Y, Hiraga A, Nakai J, et al. Development of microglia-targeting adeno-associated viral vectors as tools to study microglial behavior in vivo. Commun Biol. (2022) 5:1224. doi: 10.1038/s42003-022-04200-3
98. Zhou L, Wang Y, Xu Y, Zhang Y, and Zhu C. A comprehensive review of AAV-mediated strategies targeting microglia for therapeutic intervention of neurodegenerative diseases. J Neuroinflamm. (2024) 21:232. doi: 10.1186/s12974-024-03232-2
99. Ayers JI, Fromholt S, Sinyavskaya O, Siemienski Z, Rosario AM, Li A, et al. Widespread and efficient transduction of spinal cord and brain following neonatal AAV injection and potential disease modifying effect in ALS mice. Mol Ther J Am Soc Gene Ther. (2015) 23:53–62. doi: 10.1038/mt.2014.180
100. Li Q, Yang Z, Wang K, Chen Z, and Shen H. Suppression of microglial Ccl2 reduces neuropathic pain associated with chronic spinal compression. Front Immunol. (2023) 14:1191188. doi: 10.3389/fimmu.2023.1191188
101. Stranahan AM, Tabet A, and Anikeeva P. Region-specific targeting of microglia in vivo using direct delivery of tamoxifen metabolites via microfluidic polymer fibers. Brain Behav Immun. (2024) 115:131–42. doi: 10.1016/j.bbi.2023.09.021
102. Huang Y, Wu J, Zhang H, Li Y, Wen L, Tan X, et al. The gut microbiome modulates the transformation of microglial subtypes. Mol Psychiatry. (2023) 28:1611–21. doi: 10.1038/s41380-023-02017-y
103. Abdel-Haq R, Schlachetzki JCM, Glass CK, and Mazmanian SK. Microbiome-microglia connections via the gut-brain axis. J Exp Med. (2019) 216:41–59. doi: 10.1084/jem.20180794
104. York EM, Petit A, and Roskams AJ. Epigenetics of neural repair following spinal cord injury. Neurother J Am Soc Exp Neurother. (2013) 10:757–70. doi: 10.1007/s13311-013-0228-z
Keywords: microglia, astrogliosis, neurotrauma, demyelination, axon regeneration
Citation: Swarts EA and Brennan FH (2025) Evolving insights on the role of microglia in neuroinflammation, plasticity, and regeneration of the injured spinal cord. Front. Immunol. 16:1621789. doi: 10.3389/fimmu.2025.1621789
Received: 01 May 2025; Accepted: 28 July 2025;
Published: 19 August 2025.
Edited by:
Fengying Xu, People’s Liberation Army Navy 971 Hospital, ChinaReviewed by:
Tana Sue Pottorf, Emory University, United StatesCopyright © 2025 Swarts and Brennan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Faith H. Brennan, ZmFpdGguYnJlbm5hbkBxdWVlbnN1LmNh
 Emily A. Swarts
Emily A. Swarts Faith H. Brennan
Faith H. Brennan