- 1Centre for Malaria Elimination, Institute of Tropical Medicine, Mount Kenya University, Thika, Kenya
- 2Division of Malaria Research, Proteo-Science Center, Ehime University, Matsuyama, Japan
- 3School of Pure and Applied Sciences, Mount Kenya University, Thika, Kenya
- 4Division of Cell-Free Sciences, Proteo-Science Center, Ehime University, Matsuyama, Japan
Plasmodium falciparum infection in pregnancy leads to substantial maternal and infant morbidity and mortality. Such an infection may result in placental malaria (PM) due to P. falciparum-infected red blood cells adhering to the placenta via parasite-derived ligands. Despite the risk of infection being the same for women of all gravidities, the risk of poor birth outcomes is highest in primigravida women as they lack protective antibodies against placental malaria-associated parasites. Thus, understanding how specific P. falciparum antigens interact with the host’s immune system during the first and subsequent pregnancies may provide insights into the immunopathology of malaria and guide vaccine target prioritization. In this study, we assessed human antibody responses to 698 P. falciparum recombinant antigens derived from different antigen families among Kenyan primigravida and multigravida women. Consistent with existing literature, we observed high immunoreactivity across the different antigen families, with the number of antigens identified by sera from pregnant women increasing with gravidity. Antibody response analysis selected 3 antigens: PF3D7_1301800 (SURFIN 13.1), PF3D7_0424400 (SURFIN 4.2), and PF3D7_1252100 (rhoptry neck antigen 3 domain) as statistically significant in multigravida. While all five VAR2CSA domains were immunoreactive with seroprevalence of 42 - 62% and correlated with the selected antigens, which suggests co-acquisition, none had statistical significance in association with gravidity. Thus, although further characterization of the selected antigens will be required, this study may provide insights into targets that could be prioritized for vaccine development to reduce risks associated with malaria in pregnancy.
Introduction
In regions of malaria endemicity, pregnant women are at high risk of Plasmodium falciparum infection, which could lead to infection of the placenta leading to placental malaria (PM) (1). PM accounts for about 10,000 maternal and 200,000 infant deaths worldwide, mostly due to inflammatory and adverse outcomes, namely severe maternal anemia and low birth weight (2). With over 25 million pregnancies at risk of infection in sub-Saharan Africa and loss of efficacy of sulfadoxine-pyrimethamine intermittent preventive treatment during pregnancy due to the emergence of P. falciparum resistance to the drug (3), it calls for the development of new interventions. Malaria vaccines offer the best approach for reduced transmission in addition to other tools currently available for malaria control, including vector control, chemoprophylaxis, prompt diagnosis, and use of effective anti-malarial drugs (4).
Association between levels of antibodies and the risk of clinical malaria has pointed to an important role of P. falciparum antigens in children and malaria in pregnancy (MiP) (5–7). However, the effector mechanisms of these antibodies are incompletely understood. In pregnancy, multigravida mothers are at lower risk of PM-associated complications mainly due to the acquisition of protective antibodies that are thought to prevent the accumulation of infected red blood cells (iRBCs) in the intervillous space of the placenta (8). The majority of studies, including vaccine trials, focus on understanding and evaluating the role played by VAR2CSA, a unique member of P. falciparum erythrocyte membrane protein 1 (PfEMP1) family that is upregulated in placental malaria-associated parasites, that has been associated with adverse pregnancy outcomes by its interaction with placental chondroitin sulphate A (CSA) (9). It was recently demonstrated that Cameroonian women who were negative for PM at delivery had significantly higher antibody levels to the Full-length VAR2CSA (FV2) region of VAR2CSA throughout the pregnancy and that women with a high proportion of high avidity antibody to the FV2 region during the second trimester had a reduced risk of having PM at delivery (10). Indeed, VAR2CSA is the only antigen under consideration as a malaria vaccine for malaria in pregnancy (11–14). However, recent studies have identified some invariant red blood cell plasmodium surface proteins co-expressed with VAR2CSA such as; PF3D7_0424000 and PF3D7_0936900, which are Poly-Helical Interspersed Sub-Telomeric (PHIST) exported proteins, PF3D7_0202400 a Plasmodium Translation Enhancing Factor (PTEF) and the P. falciparum chondroitin sulfate A ligand, (PfCSA-L; PF3D7_1001000) whose mechanism in PM pathogenesis could be explored (15–17). There is inconsistent data regarding the molecules to target for vaccine development, highlighting the urgent need for in-depth studies to identify the ideal proteins of P. falciparum that can generate functionally protective antibodies against MiP. This study aims to identify these target proteins of P. falciparum in a region of stable malaria transmission in Kenya, with the goal of exploring their potential in the development of placental malaria vaccines.
Materials and methods
Study population
The serum samples used in this study were obtained from a well-characterized biobank of a prospective cohort of pregnant Kenyan women (n=53) between 12–18 weeks of gestation visiting Webuye County Hospital, Bungoma County, Kenya. The county is located in a malaria hyperendemic region where residents have been exposed to malaria infection since childhood (18, 19). The study included women who were under routine intermittent preventive treatment in pregnancy (IPTp) with sulfadoxine-pyrimethamine (IPTp-SP), and who were in the first or early second trimester of pregnancy. Serum samples were collected 4 weeks after the IPTp, immediately frozen, and shipped on dry ice to the laboratories at Mount Kenya University, where they were carefully stored uninterrupted in a -80°C deep freezer. Each serum sample was linked to its corresponding anonymized demographic data and pregnancy outcome information gathered during both scheduled and unscheduled hospital visits. The study excluded women with conditions such as tuberculosis or other known comorbidities.
Production of a P. falciparum parasite antigen library
The assayed antigens, produced using the wheat germ cell-free system (WGCFS), consisted of broad range of asexual blood-stage proteins (BSP; n = 158) and variable surface antigens (VSAs);P. falciparum erythrocyte membrane protein 1 (PfEMP1): Duffy binding–like domains (DBL; n= 163) and cysteine-rich interdomain regions (CIDR; n = 108), repetitive interspersed family proteins (RIFINs; n =182), surface-associated interspersed gene family proteins (SURFINs; n = 33), and subtelomeric variable open reading frame proteins (STEVORs; n= 54) as previously reported (20–22). VSAs are predominantly expressed on the surface of infected erythrocytes during the trophozoite-schizont stages. These 698 Plasmodium falciparum antigens included in this study were prioritized based on their previously reported serological reactivity, functional relevance in parasite-host interactions (e.g., cytoadherence, immune evasion), and expression during the asexual blood stage—the primary stage associated with clinical disease.
Briefly, the transcription templates for the 698 proteins were prepared from genomic DNA or complementary DNA of P. falciparum 3D7 strain amplified by polymerase chain reaction using high fidelity PrimeSTAR DNA polymerase (Takara Bio, Kusatsu, JP), and cloned into pEU plasmid vector (CellFree Sciences, Matsuyama, Japan) with In-Fusion HD Cloning Kit. The N-terminus His-tagged mono-biotinylated recombinant proteins were expressed by the WGCFS (21). Protein expression was confirmed by Western blot analysis using HRP-labelled streptoavidin (21).
Antibody quantification by AlphaScreen assay
To assess the level of acquisition of anti-P. falciparum-specific antibodies in the malaria-exposed pregnant women cohort, we performed an AlphaScreen assay (PerkinElmer) with all the 698 recombinant proteins as described (23). The AlphaScreen assay readout was presented as AlphaScreen Counts (ASC). To standardize assay variability, serially diluted biotinylated rabbit IgG (PerkinElmer) was included in each plate. The assays were run in randomized order.
Statistical analysis
The data analysis was performed using the R software (Version 4.2.1, R Foundation for Statistical Computing). The antigen seropositivity cut-off value to human sera was set above the assay background readout, and an antigen was considered immunoreactive if more than 10% of the participants had ASC levels above the seropositivity cut-off. A linear regression model was used to evaluate significant associations of antibody breadth based on age and hemoglobin levels. Kruskal–Wallis test with pairwise adjustments was used to evaluate the association of antibody breadth between primigravida and multigravida women groups. Then, a volcano scatter plot was generated; which is a graphical representation of a differential antibody response analysis to evaluate and represent changes in antibody response to P. falciparum antigens between primigravida and multigravida. P < 0.05 was considered significant.
Results
Characteristics of the pregnant women in this study
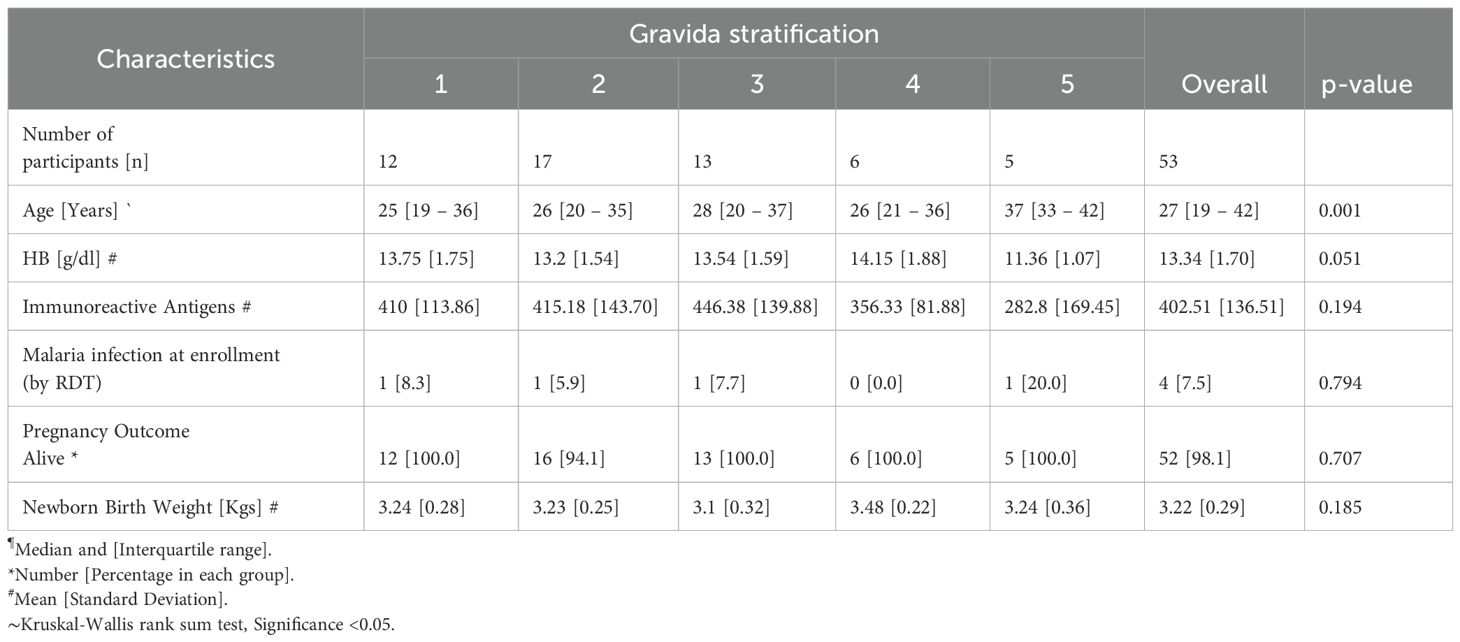
The serum samples used in this prospective study were obtained from a cohort of pregnant women visiting Webuye Level IV Hospital, Bungoma County, Kenya (n = 53). The participants were spread across gravida 1 to 5 and were aged between 19–42 years. Their ages were significantly different across gravida groups (Kruskal Wallis test, p < 0.05) (Table 1). The overall mean hemoglobin level was 13.3 g/dl. There were four P. falciparum-positive malarial infections at enrollment by rapid diagnostic test (RDT). The majority of the births had normal newborn birth weight, with a mean of 3.22kg. Only one miscarriage was observed.
Seroprevalence of serum antibodies to various P. falciparum antigens
To determine the seroprevalence among the pregnant women in the cohort, we evaluated the reactivities of the serum obtained from the pregnant women (n=53) against a library of recombinant antigens (n=698) (Supplementary Table S1). The seroprevalence variedsignificantly among the antigen groups: BSP (3.8-100%), CIDR domains (7.6-94.3%), DBL domains (1.9-100%), RIFIN (1.9-94.3%), STEVORs (1.9-77.4%) and SURFINs (22.6-96.2%) (Figure 1). The DBLs had the highest seroprevalence, with a median of 64.2%, while STEVORs had the lowest median of 40.6%. All the SURFINs were immunoreactive with a seroprevalence above the threshold cut-off point of 10%. Only a small number of BSP, CIDR, DBL, RIFIN, and STEVOR were not immunoreactive (Kruskal-Wallis rank; P< 0.05). The observed antibodies against the P. falciparum library indicated that this population was previously exposed to malaria infection. Therefore, the observed outcomes were influenced by the abundance or efficacy of this immunity.
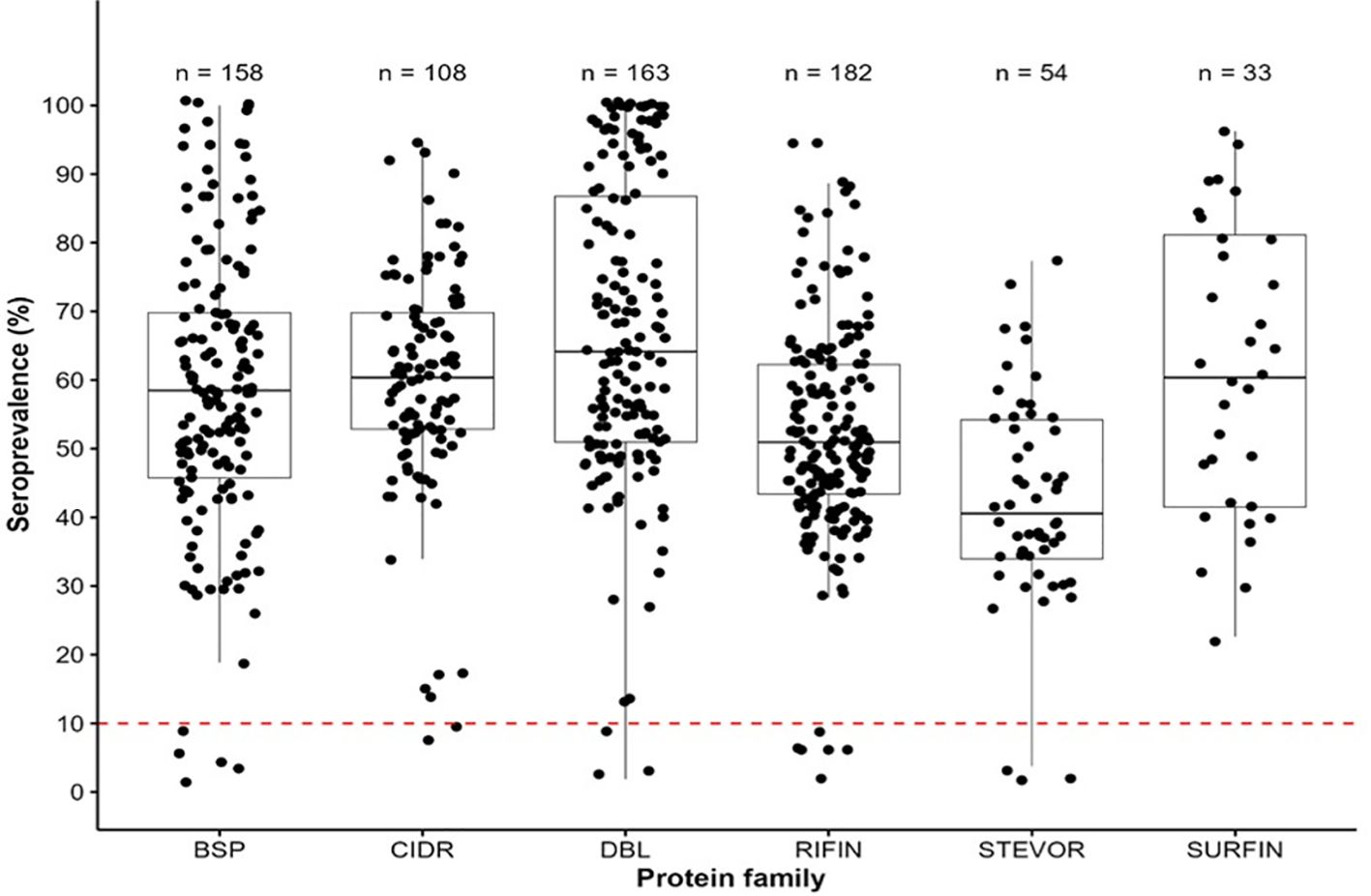
Figure 1. Immunoreactivity and seroprevalence of antibodies to Pf antigen families (BSPs, CIDRs, DBLs, RIFINs, STEVORs, and SURFINs). (Antibody immunoreactivity in the cohort of pregnant women to asexual blood stage antigens (BSP), CIDR (cysteine-rich interdomain regions of PfEMP1), DBL (Duffy binding–like domains of PfEMP1), RIFIN (repetitive interspersed family proteins), SURFIN (surface-associated interspersed gene family proteins) and STEVOR (subtelomeric variable open reading frame proteins). Box plots illustrate the overall medians of each protein group at the horizontal line per group. The dashed red horizontal line indicates a 10% seroprevalence that was set as the antigens immunoreactivity cut-off point.
Relationship between antibody breadth with age, hemoglobin levels, gravida, and malaria infection status
To determine the relationship between the antibody breadth (number of antigens recognized) by age, hemoglobin levels, gravida, and malaria infection status for each participant in the prospective study, we carried out association analysis. The overall median and range of antigens recognized by the participants were 419 and 87- 652, respectively. Further, we generated a linear regression model for the antibody breadth versus the age of participants (Figure 2A). No significant correlation was found between antibody breadth and participants’ age (Spearman’s correlation, R = -0.079, P=0.58).
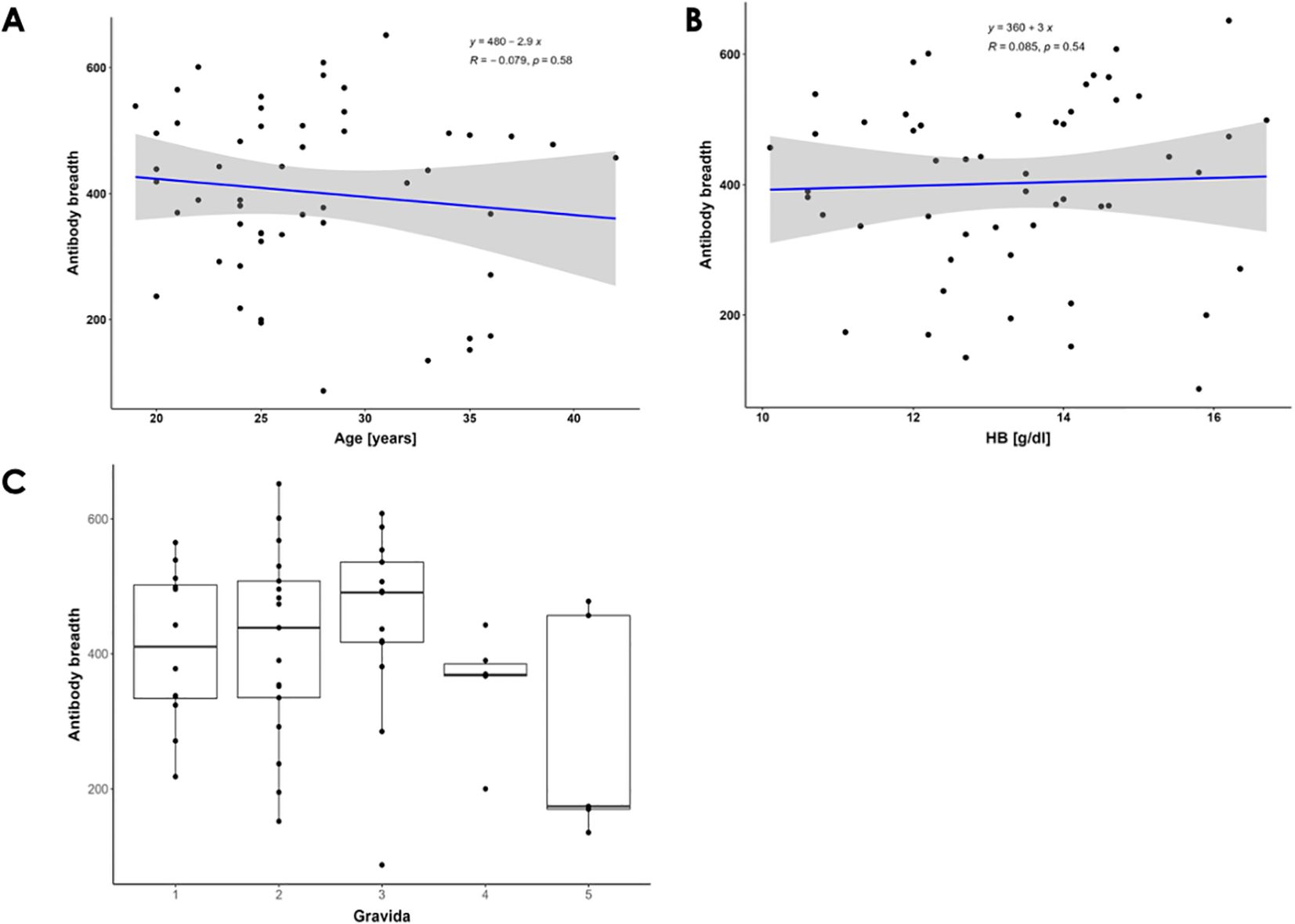
Figure 2. Breadth of Antibody Responses Against P. falciparum Antigens. (A) Correlation between the breadth of antibody responses and pregnant women's ages. Spearman's correlation coefficients (R) and significance P-values are shown. The blue line represents the linear regression line, while the gray area indicates the 95% confidence interval. There is a non-significant negative correlation between the age of participants and the number of P. falciparum antigens recognized. (B) Correlation between the breadth of antibody responses and hemoglobin levels. The blue line represents the linear regression line. There is a weak, non-significant positive correlation between participants' serum antibodies and hemoglobin levels. (C) Distribution of breadth of antibody responses across gravidae 1 to 5. The box plots illustrate the overall medians of antigens recognized (indicated by the horizontal line) per gravida. The medians are: gravida 1 = 410.5, gravida 2 = 439, gravida 3 = 491, gravida 4 = 369, and gravida 5 = 174.
Additionally, PM is characterized by reduced plasma hemoglobin levels due to hemolysis of infected and uninfected red blood cells (24). In this study, the pregnant women cohort showed normal hemoglobin levels above 10 g/dl. We generated a linear regression model for the antibody breadth versus the hemoglobin levels of the participants (Figure 2B). No significant correlation was found (Spearman R = 0.085, P = 0.54) between participants’ serum antibodies and hemoglobin levels.
Finally, since the risk of malaria-associated poor birth outcomes decreases with increasing gravidity, making gravida a clear indicator of PM immunity, we assessed the antibody responses in women of different gravida (25, 26). We found that the number of P. falciparum antigens recognized by participants’ antibodies increased with the participants’ gravidity up to gravida 3 (Figure 2C) (8). This suggests that specific multiple antigens may play a role in protective immunity against PM. We further evaluated the effect of malaria status in multigravida women. Only 3 women had positive malaria infection at enrollment. Positive malaria cases showed a trend of increased antibody response across all protein families except in the STEVOR family, where the median for both positive and negative cases was equal (Supplementary Figure S1) (26).
Differential antibody response analysis by gravida
In order to identify which parasite antigens may be responsible for the variation in antibody response between pregnant women, we created a volcano scatter plot to analyze the differential antibody response. This analysis revealed differences in the antibody response to P. falciparum antigens between primigravida women (gravida 1) and multigravida women (gravida 2 and 3). We did not include data from women (gravida 4 and 5) due to the limited number of samples available. The mean antibody responses to three of the 698 were significantly higher in multigravida women than in primigravida women (Figure 3; Supplementary Table S2). Of the three antigen domains, two were SURFIN domains; (PF3D7_1301800 - SURFIN 13.1 and PF3D7_0424400 - SURFIN 4.2, and one was a blood stage protein; (PF3D7_1252100 - rhoptry neck protein 3). However, primigravida women responded significantly to a greater number of P. falciparum proteins compared to the multigravida women. Although, two domains of VAR2CSA namely; PF3D7_1200600_CIDRpam and PF3D7_1200600_DBLe10 were recognized by in multigravida women, no significant antibody responses to this antigen was observed.
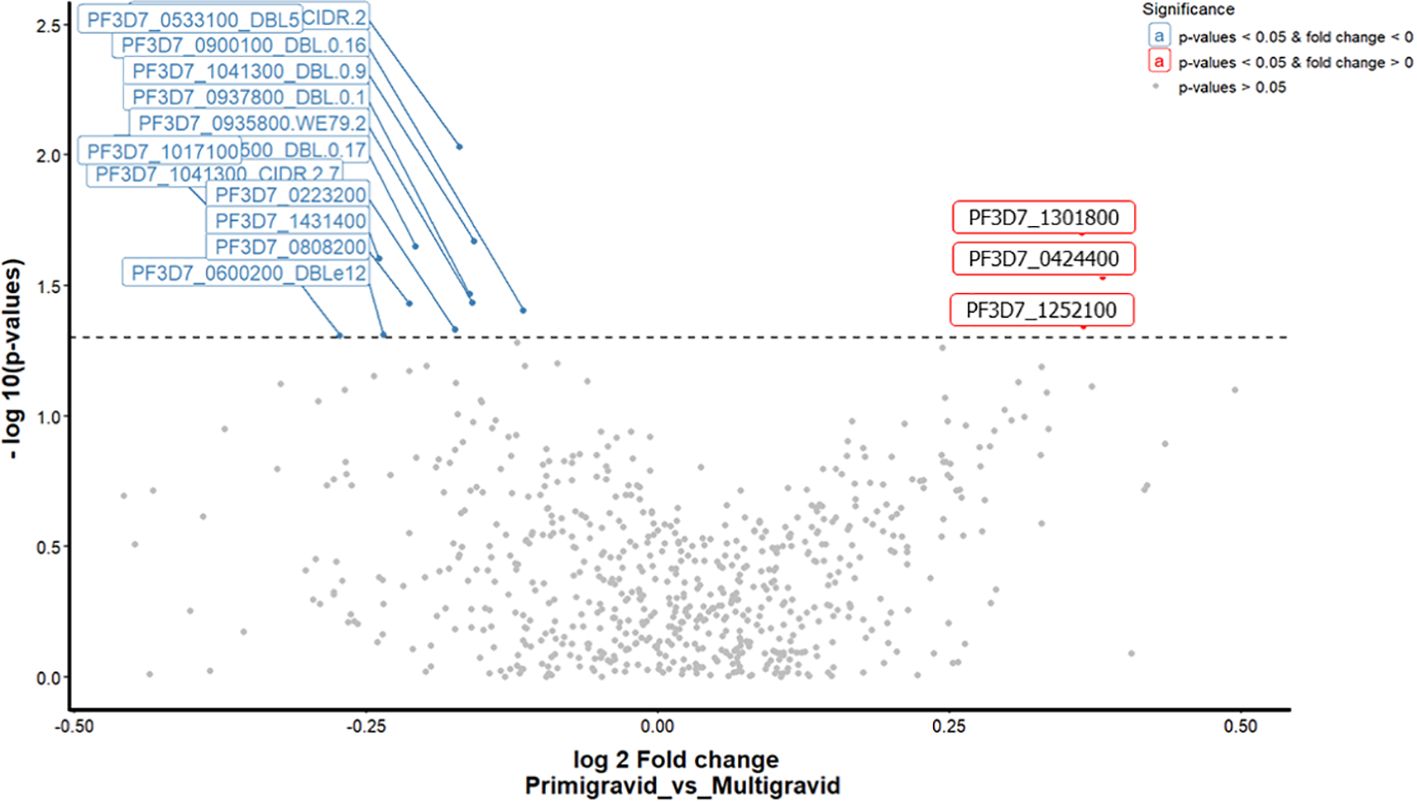
Figure 3. A volcano scatter plot showing differential antibody response analysis between primigravida vs multigravida. This graph indicates the fold change on the x-axis and p-values on the y-axis. The points represent individual mean ASC counts of antigen domains colored according to significance by gravida (Significant values p<0.05, fold change < 0 (blue colour), p<0.05, fold change >0 (red colour), and non - significant values > 0.05 grey colour). The total significant antigens by fdr unadjusted analysis are (13 primigravida and 3 multigravida).
Discussion
In malaria-endemic regions, there is a strong negative association between gravidity and poor birth outcomes from placental malaria (PM) (26) such that, poor birth outcomes are mainly experienced by primigravida women. Although there is evidence showing that the VAR2CA mediates the adhesion of P. falciparum parasite to CSA in placenta and that the acquisition of antibodies against this VAR2CSA are parity-dependent, direct epidemiological proof that VAR2CSA antibodies prevent PM and associated adverse pregnancy and birth outcomes has been inconsistent (27). Therefore, this study aimed to investigate antibody responses in a cohort of pregnant women and found a high seroprevalence against antigens expressed in various stages of the malaria parasite’s life cycle, specifically those expressed in the merozoite stage (BSP), and on the surface of iRBCs (PfEMP1, RIFIN, SURFIN, and STEVOR).
Consistent with previous studies, women with successive pregnancies had a wider breadth of antibody response to a broad range of parasite proteins with an increase in gravidity, for gravida 1 to 3 (26–29). The analysis of the breadth of antibody response to the antigen library was further extrapolated based on age, hemoglobin levels and the differential antibody response based on gravida (Figure 3). Through these analyses, we explored the key antigenic targets that are attributed to the observed differences in gravidity namely; PF3D7_1301800 (SURFIN 13.1) and PF3D7_0424400 (SURFIN 4.2), and PF3D7_1252100 (rhoptry neck protein 3, PfRON3). These antigens had seropositivity of 30%, 62%, and 69%, respectively, in the pregnant women cohort. SURFIN family antigens have been reported as targets of naturally acquired immunity against malaria infections (21). The PF3D7_1301800 (SURFIN 13.1) is understudied but has been predicted to be involved in cell surface adhesion, hence supporting the sequestration of iRBCs (30). On the other hand, PF3D7_0424400 (SURFIN 4.2) has been established to colocalize with PfEMP1 on the iRBCs membrane as well as the merozoite surface, hence it could also be important for parasite growth in the blood-stage development (29, 30). PfRON3 (blood stage antigen) has been shown to form a complex with P. falciparum rhoptry-associated membrane antigen (PfRAMA), resulting in a novel PfRON3/PfRAMA rhoptry antigen complex on the P. falciparum merozoite. The complex has a role in the invasion of red blood cells by merozoites (31, 32).
In most studies on placental malaria, VAR2CSA—a member of the PfEMP1 family—has been intensively investigated as a vaccine candidate using laboratory parasite strains (10, 33). In contrast, our study did not identify VAR2CSA domains among the key antigens associated with gravidity, although PF3D7_1200600_DBLe10 and PF3D7_1200600_DBLpam3 were immunoreactive in 60% and 45% of participants, respectively. Such discrepancy with prior findings may be attributed to differences in the study design employed, notably the timing of antibody measurement, since VAR2CSA‐specific IgG may peak later in pregnancy or postpartum and be missed in cross-sectional assays like the one used in this study (26, 34). Another factor could be population genetics, given that VAR2CSA exhibits extensive allelic diversity and gene duplication in African field isolates this may have lead to potential underestimation of antibody responses when using laboratory strain–derived antigens in prior studies (35, 36). Methodological differences, such as the use of truncated versus full-length VAR2CSA constructs, assay platforms, and antigen folding can further influence epitope presentation and detection sensitivity (37, 38).
Our findings therefore challenge the universality of the current VAR2CSA-focused vaccine strategies, that is, PRIMVAC and PAMVAC, which aim to elicit inhibitory IgG against specific CSA-binding domains (39, 40). To broaden protective coverage across diverse field isolates, inclusion of multiple VAR2CSA variants or epitopes and supplementary antigens such as SURFIN 13.1, SURFIN 4.2 and PfRON3 recognized in this study may be warranted. Ultimately, multicomponent vaccine formulations that combine VAR2CSA domains with other blood-stage or surface antigens hold promise for achieving parity-independent protection against placental malaria (39).
On the other hand, primigravida women participants in this study responded significantly to a greater number of P. falciparum proteins. Previous studies have shown that primigravida women are more susceptible to malaria infection during pregnancy (41) because of factors such as pregnancy-related immunosuppression (42) and more recently, the fact that the placenta provides a unique environment for malaria parasite sequestration inducing increased antibody response in women who get pregnant for the first time (43). This placental sequestration of P. falciparum is mediated primarily by variant surface antigens on iRBCs, most notably PfEMP1; a family encoded by the var gene repertoire of approximately 50–60 distinct genes per haploid genome (43, 44). Expression of var genes is strictly mutually exclusive, where, only a single PfEMP1 variant is displayed at any given time, through heterochromatin silencing and locus repositioning mechanisms (43, 45),. Periodic switching among var genes drives antigenic variation, allowing the parasite to evade host antibodies and maintain chronic malarial infection (43). In our cohort of primigravida women, seven of the thirteen differentially recognized antigens were PfEMP1 variants, underscoring the extensive heterogeneity of var expression among field isolates and its influence on parity‐dependent immune responses time (46). The most significant seropositive antigen among the PfEMP1 recognized in primigravida women was PF3D7_0533100 (var1csa), which was annotated as a pseudogene in the 3D7 strain. However, interestingly, Cabral et al. (44) reported that its expression was not involved in the allelic mutual exclusion of var gene transcription, which may explain its highly significant expression in the majority of women in this study.
In summary, this study evaluated a library of 698 P. falciparum antigens alongside an extensive analysis of clinical data encompassing various outcomes, such as gravidity, maternal anemia, and birth weight. However, due to the study’s limited sample size, identifying protective associations may have been challenging, particularly among women with a higher gravidity. Nevertheless, the study provides evidence that pregnant women in malaria-endemic areas have significant levels of antibodies against both merozoite and iRBC surface antigens. These antibodies could potentially reduce the risk of adverse outcomes associated with placental malaria, such as malaria-associated anemia and low birth weight, and contribute to overall normal pregnancy outcomes. Further research is needed to validate the identified antigenic targets using a larger sample size. It is also crucial to investigate their role in the opsonization of parasitized red blood cells for phagocytosis, opsonization of merozoites, and complement fixation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Scientific and Ethics Research Committee (ISERC) of Mount Kenya University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LM: Visualization, Writing – review & editing, Writing – original draft, Formal Analysis, Methodology, Investigation. SM: Formal Analysis, Writing – review & editing, Visualization. HW: Writing – review & editing, Methodology, Investigation. HN: Investigation, Methodology, Writing – review & editing. PL: Supervision, Writing – review & editing. TT: Funding acquisition, Writing – review & editing, Writing – original draft. ET: Supervision, Methodology, Writing – review & editing, Writing – original draft, Conceptualization, Funding acquisition. JG: Investigation, Conceptualization, Writing – review & editing, Funding acquisition, Supervision. BK: Writing – review & editing, Funding acquisition, Formal Analysis, Supervision, Methodology, Data curation, Investigation, Conceptualization, Writing – original draft, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. BK is an EDCTP Fellow under EDCTP2 programme supported by the European Union grant number TMA2020CDF-3203. JG was supported by Royal Society, Future Leaders African Independent Researchers (FLAIR) Scheme (FLR/R1/201314). This work was also supported in part by JSPS KAKENHI (Grant Nos. JP23KK0140, JP24K02273) and AMED under grant number (JP23wm0325038, JP25jm0210110). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
We appreciate the study volunteers from Webuye Level IV Hospital, Bungoma county; and thank the research teams at Centre for Malaria Elimination, Mount Kenya University, for their technical assistance in processing the field samples.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1622435/full#supplementary-material
Supplementary Figure 1 | A box plot showing the malaria infection status versus the median antibody responses across the protein families in multigravida women. Positive malaria cases showed increased antibody response across all protein families except in STEVOR family where the median for both positive and negative cases was equal.
References
1. Moya-Alvarez V, Abellana R, and Cot M. Pregnancy-associated malaria and malaria in infants: An old problem with present consequences. Malar J. (2014) 13(13):271. doi: 10.1186/1475-2875-13-271
2. Desai M, ter Kuile FO, Nosten F, McGready R, ASamoa K, Brabin B, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. (2007) 7:93–104. doi: 10.1016/S1473-3099(07)70021-X
3. van Eijk AM, Larsen DA, Kayentao K, Koshy G, Slaughter DEC, Roper C, et al. Effect of Plasmodium falciparum sulfadoxine-pyrimethamine resistance on the effectiveness of intermittent preventive therapy for malaria in pregnancy in Africa: a systematic review and meta-analysis. Lancet Infect Dis. (2019) 19:546–56. doi: 10.1016/S1473-3099(18)30732-1
4. Rogerson SJ and Aitken EH. Progress towards vaccines to protect pregnant women from malaria. EBioMedicine. (2019) 42:12–3. doi: 10.1016/j.ebiom.2019.03.042
5. Chandrasiri UP, Fowkes FJI, Beeson JG, Richards JS, Kamiza S, Maleta K, et al. Association between malaria immunity and pregnancy outcomes among Malawian pregnant women receiving nutrient supplementation. Malar J. (2016) 15:547. doi: 10.1186/s12936-016-1597-7
6. Mayor A, Dobaño C, Nhabomba A, Guinovart C, Jiménez A, Manaca MN, et al. IgM and IgG against Plasmodium falciparum lysate as surrogates of malaria exposure and protection during pregnancy. Malar J. (2018) 17:182. doi: 10.1186/s12936-018-2331-4
7. Ndam NT, Denoeud-Ndam L, Doritchamou J, Viwami F, Salanti A, Nielsen MA, et al. Protective Antibodies against Placental Malaria and Poor Outcomes during Pregnancy, Benin. Emerg Infect Dis. (2015) 21:813–23. doi: 10.3201/eid2105.141626
8. Ismail MR, Ordi J, Menendez C, Ventura PJ, Aponte JJ, Kahigwa E, et al. Placental pathology in malaria: A histological, immunohistochemical, and quantitative study. Hum Pathol. (2000) 31:85–93. doi: 10.1016/S0046-8177(00)80203-8
9. Zakama AK, Ozarslan N, and Gaw SL. Placental malaria. Curr Trop Med Rep. (2020) 7:162–71. doi: 10.1007/s40475-020-00213-2
10. Tutterrow YL, Salanti A, Smith AM, and Pagano JD. High avidity antibodies to full-length VAR2CSA correlate with absence of placental malaria. PLoS One. (2012) 7:40049. doi: 10.1371/journal.pone.0040049
11. Duffy PE and Patrick Gorres J. Malaria vaccines since 2000: progress, priorities, products. NPJ Vaccines. (2020) 5:48. doi: 10.1038/s41541-020-0196-3
12. Chêne A, Houard S, Nielsen MA, Hundt S, D'Alessio F, Sirima SB, et al. Clinical development of placental malaria vaccines and immunoassays harmonization: a workshop report. Malar J. (2016) 15:476. doi: 10.1186/s12936-016-1527-8
13. Gangnard S, Chêne A, Dechavanne S, Srivastava A, Avril M, Smith JD, et al. VAR2CSA binding phenotype has ancient origin and arose before Plasmodium falciparum crossed to humans: implications in placental malaria vaccine design. Sci Rep. (2019) 9:16978. doi: 10.1038/s41598-019-53334-8
14. Gamain B, Chêne A, Viebig NK, Tuikue Ndam N, and Nielsen MA. Progress and insights toward an effective placental malaria vaccine. Front Immunol. (2021) 12:634508. doi: 10.3389/fimmu.2021.634508
15. Rotich AK, Takashima E, Yanow SK, Gitaka J, and Kanoi BN. Towards identification and development of alternative vaccines against pregnancy-associated malaria based on naturally acquired immunity. Front Trop Diseases. (2022) 3:1–9. doi: 10.3389/fitd.2022.988284
16. Keitany GJ, Jenkins BJ, Obiakor HT, Daniel S, Muehlenbachs A, Semblat JP, et al. An invariant protein that colocalizes with VAR2CSA on plasmodium falciparum-infected red cells binds to chondroitin sulfate A. J Infect Dis. (2022) 225:2011–22. doi: 10.1093/infdis/jiab550
17. Francis SE, Malkov VA, Oleinikov AV, Rossnagle E, Wendler JP, Mutabingwa TK, et al. Six genes are preferentially transcribed by the circulating and sequestered forms of plasmodium falciparum parasites that infect pregnant women. Infect Immun. (2007) 75:4838. doi: 10.1128/IAI.00635-07
18. Kepha S, Nikolay B, Nuwaha F, Mwandawiro CS, Nankabirwa J, Ndibazza J, et al. Plasmodium falciparum parasitaemia and clinical malaria among school children living in a high transmission setting in western Kenya. Malar J. (2016) 15:157. doi: 10.1186/s12936-016-1176-y
19. Gitaka J, Natecho A, Mwambeo HM, Gatungu DM, Githanga D, and Abuya T. Evaluating quality neonatal care, call Centre service, tele-health and community engagement in reducing newborn morbidity and mortality in Bungoma county, Kenya. BMC Health Serv Res. (2018) 18:493. doi: 10.1186/s12913-018-3293-5
20. Kanoi BN, Nagaoka H, Morita M, White MT, Palacpac NMQ, Ntege EH, et al. Comprehensive analysis of antibody responses to Plasmodium falciparum erythrocyte membrane protein 1 domains. Vaccine. (2018) 36:6826–33. doi: 10.1016/j.vaccine.2018.08.058
21. Kanoi BN, Nagaoka H, White MT, Morita M, Palacpac NMQ, Ntege EH, et al. Global repertoire of human antibodies against plasmodium falciparum RIFINs, SURFINs, and STEVORs in a malaria exposed population. Front Immunol. (2020) 11:893. doi: 10.3389/fimmu.2020.00893
22. Takashima E, Kanoi BN, Nagaoka H, Morita M, Hassan I, Palacpac NMQ, et al. Meta-analysis of human antibodies against plasmodium falciparum variable surface and merozoite stage antigens. Front Immunol. (2022) 13:2793. doi: 10.3389/fimmu.2022.887219
23. Hassan I, Kanoi BN, Nagaoka H, Sattabongkot J, Udomsangpetch R, Tsuboi T, et al. High-throughput antibody profiling identifies targets of protective immunity against P. falciparum Malaria Thailand. Biomolecules. (2023) 13:1267. doi: 10.3390/biom13081267
25. Rogerson SJ, Hviid L, Duffy PE, Leke RF, and Taylor DW. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect Diseases. (2007) 7:105–17. doi: 10.1016/S1473-3099(07)70022-1
26. Cutts JC, Agius PA, Lin Z, Powell R, Moore K, Draper B, et al. Pregnancy-specific malarial immunity and risk of malaria in pregnancy and adverse birth outcomes: a systematic review. BMC Med. (2020) 18:14. doi: 10.1186/s12916-019-1467-6
27. Mutabingwa TK, Bolla MC, Li JL, Domingo GJ, Li X, Fried M, et al. Maternal malaria and gravidity interact to modify infant susceptibility to malaria. PLoS Med. (2005) 2:1260–8. doi: 10.1371/journal.pmed.0020407
28. Kinyanjui SM, Bejon P, Osier FH, Bull PC, and Marsh K. What you see is not what you get: Implications of the brevity of antibody responses to malaria antigens and transmission heterogeneity in longitudinal studies of malaria immunity. Malar J. (2009) 8:1–8. doi: 10.1186/1475-2875-8-242
29. Oakley MSM, Kumar S, Anantharaman V, Zheng H, Mahajan B, Haynes JD, et al. Molecular factors and biochemical pathways induced by febrile temperature in intraerythrocytic plasmodium falciparum parasites. Infection immunity. (2007) 75:2012–25. doi: 10.1128/IAI.01236-06
30. Winter G, Kawai S, Haeggström M, Kaneko O, von Euler A, Kawazu S, et al. SURFIN is a polymorphic antigen expressed on Plasmodium falciparum merozoites and infected erythrocytes. J Exp Med. (2005) 201:1853–63. doi: 10.1084/jem.20041392
31. Ito D, Chen JH, Takashima E, Hasegawa T, Otsuki H, Takeo S, et al. Identification of a novel RAMA/RON3 rhoptry protein complex in plasmodium falciparum merozoites. Front Cell Infect Microbiol. (2021) 10:836. doi: 10.3389/fcimb.2020.605367
32. Richards JS, Arumugam TU, Reiling L, Healer J, Hodder AN, Fowkes FJI, et al. Identification and prioritization of merozoite antigens as targets of protective human immunity to plasmodium falciparum malaria for vaccine and biomarker development. J Immunol. (2013) 191:795–809. doi: 10.4049/jimmunol.1300778
33. Salanti A, Staalsoe T, Lavstsen T, Jensen ATR, Sowa MPK, Arnot DE, et al. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol Microbiol. (2003) 49:179–91. doi: 10.1046/j.1365-2958.2003.03570.x
34. McLean ARD, Opi DH, Stanisic DI, Cutts JC, Feng G, Ura A, et al. High antibodies to VAR2CSA in response to malaria infection are associated with improved birthweight in a longitudinal study of pregnant women. Front Immunol. (2021) 12:644563. doi: 10.3389/fimmu.2021.644563
35. Renn JP, Doritchamou JYA, Tentokam BCN, Morrison RD, Cowles MV, Burkhardt M, et al. Allelic variants of full-length VAR2CSA, the placental malaria vaccine candidate, differ in antigenicity and receptor binding affinity. Commun Biol. (2021) 4:1309. doi: 10.1038/s42003-021-02787-7
36. Benavente ED, Oresegun DR, de Sessions PF, Walker EM, Roper C, Dombrowski JG, et al. Global genetic diversity of var2csa in Plasmodium falciparum with implications for malaria in pregnancy and vaccine development. Sci Rep. (2018) 8:1–8. doi: 10.1038/s41598-018-33767-3
37. Doritchamou JYA, Herrera R, Aebig JA, Morrison R, Nguyen V, Reiter K, et al. VAR2CSA domain-specific analysis of naturally acquired functional antibodies to plasmodium falciparum placental malaria. J Infect Dis. (2016) 214:577. doi: 10.1093/infdis/jiw197
38. Ma R, Salinas ND, Orr-Gonzalez S, Richardson B, Ouahes T, Torano H, et al. Structure-guided design of VAR2CSA-based immunogens and a cocktail strategy for a placental malaria vaccine. PLoS Pathog. (2024) 20:e1011879. doi: 10.1371/journal.ppat.1011879
39. Sirima S odiomon B, Richert L, Chêne A, Konate AT, Campion C, Dechavanne S, et al. PRIMVAC vaccine adjuvanted with Alhydrogel or GLA-SE to prevent placental malaria: a first-in-human, randomised, double-blind, placebo-controlled study. Lancet Infect Dis. (2020) 20:585–97. doi: 10.1016/S1473-3099(19)30739-X
40. Mordmüller B, Sulyok M, Egger-Adam D, Resende M, de Jongh WA, Jensen MH, et al. First-in-human, randomized, double-blind clinical trial of differentially adjuvanted PAMVAC, A vaccine candidate to prevent pregnancy-associated malaria. Clin Infect Diseases. (2019) 69:1509–16. doi: 10.1093/cid/ciy1140
41. Ataíde R, Mayor A, and Rogerson SJ. Malaria, primigravidae, and antibodies: Knowledge gained and future perspectives. Trends Parasitol. (2014) 30:85–94. doi: 10.1016/j.pt.2013.12.007
42. Menendez C. Malaria during pregnancy: A priority area of malaria research and control. Parasitol Today. (1995) 11:178–83. doi: 10.1016/0169-4758(95)80151-0
43. Rowe JA and Kyes SA. The role of Plasmodium falciparum var genes in malaria in pregnancy. Mol Microbiol. (2004) 53:1011. doi: 10.1111/j.1365-2958.2004.04256.x
44. Cabral FJ, Vianna LG, Medeiros MM, Carlos BC, Martha RD, Silva NM, et al. Immunoproteomics of Plasmodium falciparum-infected red blood cell membrane fractions. Mem Inst Oswaldo Cruz. (2017) 112:850–6. doi: 10.1590/0074-02760170041
45. Bull PC and Abdi AI. The role of PfEMP1 as targets of naturally acquired immunity to childhood malaria: prospects for a vaccine. Parasitology. (2016) 143:171. doi: 10.1017/S0031182015001274
Keywords: malaria in pregnancy, placental malaria. gravida, antibodies, immunity, bloodstage proteins
Citation: Mwai L, Musundi S, Waweru H, Nagaoka H, Limbua PG, Tsuboi T, Takashima E, Gitaka J and Kanoi BN (2025) Exploring the P. falciparum antigens associated with reduced risk of malaria in pregnancy. Front. Immunol. 16:1622435. doi: 10.3389/fimmu.2025.1622435
Received: 03 May 2025; Accepted: 24 June 2025;
Published: 14 July 2025.
Edited by:
Fabrizio Bruschi, University of Pisa, ItalyReviewed by:
Yaw Aniweh, University of Ghana, GhanaSonalika Kar, National Institute of Malaria Research (ICMR), India
Copyright © 2025 Mwai, Musundi, Waweru, Nagaoka, Limbua, Tsuboi, Takashima, Gitaka and Kanoi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bernard N. Kanoi, Ymthbm9pQG1rdS5hYy5rZQ==; Jesse Gitaka, amdpdGFrYUBta3UuYWMua2U=
 Lucy Mwai
Lucy Mwai Sebastian Musundi
Sebastian Musundi Harrison Waweru
Harrison Waweru Hikaru Nagaoka
Hikaru Nagaoka Purity Gacheri Limbua
Purity Gacheri Limbua Takafumi Tsuboi
Takafumi Tsuboi Eizo Takashima
Eizo Takashima Jesse Gitaka
Jesse Gitaka Bernard N. Kanoi
Bernard N. Kanoi