- 1The Feinstein Institutes for Medical Research, Northwell Health, Manhasset, NY, United States
- 2Departments of Emergency Medicine and Molecular Medicine, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, NY, United States
- 3Department of Surgery, University of Texas (UT) Southwestern Medical Center, Dallas, TX, United States
Sepsis, a life-threatening organ dysfunction caused by a dysregulated host response to infection, presents a major clinical challenge. While the complex interplay of inflammatory mediators and immune cells during sepsis is increasingly understood, the role of neurotransmitters, particularly dopamine, in modulating the innate immune response is emerging as a crucial area of investigation. Dopamine, traditionally recognized for its role in the central nervous system, acts as an endogenous regulator of innate immunity, significantly influencing the course and outcome of sepsis. In this mini-review, we highlight our recent finding of dopamine’s critical role in regulating aconitate decarboxylase 1 (ACOD1) in sepsis.
Introduction
Sepsis is a life-threatening condition that arises when the body’s response to infections triggers a dysregulated production of various inflammatory cytokines (1). It accounts for almost 20% of total deaths worldwide (2), and annually cost >$60 billion in the U.S. alone. Animal models remain indispensable in sepsis research, providing a controlled environment to unravel complex pathophysiological mechanisms, identify therapeutic targets, and evaluate novel interventions (3). The intricate interplay of various innate immune cells, inflammatory mediators, and signaling pathways contributes to the pathogenesis and progression of sepsis (4, 5). While the inflammatory cascade initiated by the innate immune system is crucial for pathogen clearance, its dysregulation can lead to detrimental systemic inflammation and subsequent immunosuppression (6, 7). Understanding the intricate mechanisms governing this immune response is critical for developing effective therapeutic strategies.
Dopamine (DA), traditionally recognized for its role in the central nervous system (8), acts as an endogenous regulator of innate immunity through a complex interplay with various immune cells expressing dopamine receptors, primarily D1-like (DRD1 and DRD5) and D2-like (DRD2, DRD3, and DRD4) receptors (9), thereby significantly influencing the course and outcome of sepsis. Depending on the receptor subtype and the specific context, dopamine can trigger intracellular signaling cascades that can either enhance or suppress immune cell activities.
Dopamine as a bridge between the nervous and innate immune systems
Dopamine (DA) is a central nervous system (CNS) neurotransmitter involved in the control of several key functions, such as cognition and movement. In the periphery, DA is produced by neuroendocrine cells, the adrenal glands, and neuronal fibers, and influence functions like blood pressure, sodium balance and adrenal and renal functions (10, 11), as well as glucose homeostasis and body weight (12). In addition to its primary role in the CNS, DA is now recognized as a key modulator of the innate immune response in sepsis. For instance, dopamine is produced and released by various types of immune cells including lymphocytes, macrophages, peripheral blood mononuclear cells (PBMCs), and dendritic cells (8, 13–17) during inflammation (18). This localized production suggests dopamine’s involvement in modulating immune responses through paracrine or autocrine signaling, regulating both neurological and immunological responses (16, 19, 20).
It is plausible that dopamine release is tightly regulated and triggered by specific immunological stimuli, such as pathogen-associated molecular pattern molecules (PAMPs) or damage-associated molecular pattern molecules (DAMPs). Understanding the temporal dynamics of dopamine production—when and for how long it is released in response to specific immune challenges—is crucial. Furthermore, different tissues might exhibit varying abundances of these cells, leading to tissue-specific effects of dopamine signaling. Therefore, a deeper understanding of this interplay between temporal and spatial dynamics of dopamine production is essential for developing targeted therapies that effectively harness the immunomodulatory potential of dopamine signaling in immune-related diseases. This includes further research into the precise regulation of dopamine release, variations in its production across different immune cell types and tissues, and the intricate relationship between neuronal and immune-derived dopamine.
DA interacts with D1-like (DRD1, DRD5) and D2-like (DRD2, DRD3, DRD4) receptors on immune cells, triggering specific intracellular signaling cascades and influencing immune cell activity depending on receptor subtype and the specific immune cell involved. For instance, DA modulates innate immunity, in part, by influencing neutrophil functions. As the first line of defense against invading pathogens, neutrophils are crucial for bacterial clearance. Expressing DRD3, DRD5, and to a lesser extent, DRD2 and DRD4 dopamine receptors (21), neutrophils are responsive to dopamine signaling. For instance, acting via D1-like receptors, DA inhibits neutrophil chemotaxis and phagocytosis (22), potentially mitigating excessive inflammation and tissue damage (23). While this inhibition may be beneficial in early sepsis by attenuating an overwhelming inflammatory response, prolonged suppression of neutrophil activity can compromise bacterial clearance and increase the risk of secondary infections. Furthermore, DA reduces neutrophil activity by decreasing endothelial adherence, reactive oxygen species and cytokine production (18), and impairing cell migration and phagocytosis (22, 24–27).
Human monocytes predominantly express DRD2 and DRD3, with lower expression of DRD4 and DRD5 (21). Consequently, DA suppresses LPS-mediated NF-κB activation and cytokine production in these cells (28). Additionally, DA modulates macrophage polarization towards the M2 phenotype, which contribute to tissue repair and resolution of inflammation (9), suggesting a potential role for DA in the later stages of sepsis by fostering an anti-inflammatory action. In vivo, DA and its agonists suppress inflammatory responses in mice, reducing LPS-induced production of IL-12p40 (29), TNF (30), IFN-γ, and nitric oxide in macrophages (31) primarily via D2-like receptors (DRD2/DRD3/DRD4) (32). Conversely, DA stimulates the production of anti-inflammatory cytokines, such as IL-10, in macrophages (32, 33), mounting an anti-inflammatory response.
Therapeutic potential of dopamine-based agents
DA exerts cardiovascular effects by acting on α- and β-adrenergic receptors, increasing cardiac output, systemic vascular resistance, and blood pressure (34), thereby counteracting the hypotension and hypoperfusion characteristic of organ dysfunction. Consequently, DA is often a first-line vasopressor in sepsis and septic shock during overwhelming immune responses to bacterial infections (35). While both DA and norepinephrine (NE) are commonly used as first-line vasopressors in the treatment of septic shock (36–39), NE may demonstrate superior efficacy in clinical settings (37, 40).
In vivo, pharmacological DA administration modulates the secretion of hormones such as prolactin (41, 42), restores hepatic blood flow (43), and improves hemodynamics by increasing blood pressure/flow and causing vasodilatation (34). DA suppresses systemic inflammation by blocking the TRAF6/NF-κB pathway via a DRD5 receptor-mediated signaling axis involving ARRB2 and PP2A (44). Consistently, a dopamine D1-like receptor-specific agonist improves survival of septic mice, partly by inhibiting TNF, IL-1β (45), IL-6, and IFN-γ (46). Similarly, electroacupuncture of the sciatic nerve increases adrenal DA production in mice, which subsequently acts on DRD1 receptors to reduce systemic inflammation and protect against lethal sepsis (47). Clinically, low-dose DA may benefit splanchnic blood flow and oxygen consumption in patients with septic shock (48), aligning with the potential therapeutic benefits of dopaminergic agonists in septic diabetic patients by controlling both hyperglycemia and systemic inflammation (49). However, high-dose DA, compared to norepinephrine, is associated with increased arrhythmic events and mortality (50–52). Independent of norepinephrine (NE) use, DA administration is associated with higher mortality (51, 53) and a greater incidence of arrhythmic events compared to NE administration (40).
While the role of dopamine in modulating immune responses is increasingly recognized, it is presently unclear whether systemic and locally produced dopamine exert distinct effects on immune function during sepsis. Addressing this important question is crucial for refining our understanding of sepsis pathophysiology and developing targeted therapeutic interventions. As aforementioned, systemic dopamine, primarily derived from the nervous system, circulates throughout the body and can interact with dopamine receptors expressed on various immune cells. These interactions can modulate immune cell activity, including cytokine production, phagocytosis, and lymphocyte proliferation. In the context of sepsis, extensive evidence indicates that DA might play a protective role by modulating inflammatory responses (43, 46, 54). In contrast to systemic dopamine, locally produced dopamine is synthesized and released by immune cells themselves, acting within the immediate microenvironment. This localized production allows for precise and targeted modulation of immune responses within specific tissues or at the site of infection, influencing the activity of neighboring immune cells.
The distinct effects of systemic versus local dopamine in sepsis may stem from several factors. First, the concentration of dopamine at the site of action may differ significantly. Locally produced dopamine can achieve high concentrations within the immune microenvironment, potentially exceeding those achieved by circulating dopamine. Second, the specific dopamine receptor subtypes expressed on different immune cell populations and within different tissues may vary, leading to diverse downstream effects. Finally, the interplay between dopamine and other signaling molecules present in the local microenvironment, such as cytokines and chemokines, could further influence the net effect of dopamine on immune function. Therefore, disentangling the roles of systemic and locally produced dopamine in sepsis requires sophisticated experimental approaches. For instance, studies using conditional knockout mice, where dopamine production is selectively ablated in specific cell types or tissues, could help elucidate the distinct contributions of systemic and local dopamine. Furthermore, in vitro studies using co-culture systems of immune cells and other relevant cell types, such as endothelial cells, can provide valuable insights into the interplay between dopamine and other signaling pathways within the immune microenvironment.
Therefore, understanding the differential effects of systemic versus locally produced dopamine in sepsis has significant implications for developing targeted therapeutic strategies. Manipulating dopamine signaling pathways could offer novel approaches to modulating immune function and improving outcomes in sepsis patients. For instance, selectively enhancing local dopamine production by immune cells at the site of infection could promote bacterial clearance and dampen excessive inflammation. Conversely, modulating systemic dopamine levels or targeting specific dopamine receptor subtypes might be beneficial in mitigating the systemic inflammatory response and preventing organ damage. Therefore, deciphering the distinct roles of systemic and locally produced dopamine in sepsis is a critical area of future research. This knowledge will not only enhance our understanding of the complex immunopathology of sepsis but also pave the way for developing innovative therapeutic strategies that harness the immunomodulatory potential of dopamine signaling.
Novel role of DA in the regulation of ACOD1 expression
Aconitate decarboxylase 1 (ACOD1, also known as immune-responsive gene 1, IRG1) is a critical regulator of immunometabolism and inflammation, particularly in the context of infection and injury. While initially recognized for its role in generating the anti-inflammatory metabolite itaconate, ACOD1’s functions have proven to be multifaceted, encompassing both itaconate-dependent and -independent mechanisms (Figure 1). Initially, the well-characterized function of ACOD1 relates to its catalysis of cis-aconitate to itaconate within the mitochondria. This activity is markedly upregulated in macrophages and other immune cells upon stimulation with inflammatory stimuli like lipopolysaccharide (LPS) (55). Itaconate, in turn, exerts a range of anti-inflammatory effects through multiple mechanisms such as: 1) competitive inhibition of succinate dehydrogenase (SDH), leading to succinate accumulation and stabilization of hypoxia-inducible factor-1α (HIF-1α) which promotes anti-inflammatory gene expression (56); 2) direct alkylation of proteins like Kelch-like ECH-associated protein 1 (KEAP1), resulting in activation of nuclear factor erythroid 2-related factor 2 (Nrf2) and subsequent antioxidant and anti-inflammatory responses (Figure 1) (57); and 3) inhibition of glycolysis, contributing to the metabolic reprogramming of activated immune cells (58). Collectively, through these mechanisms enable itaconate to dampen inflammation and promotes tissue repair.
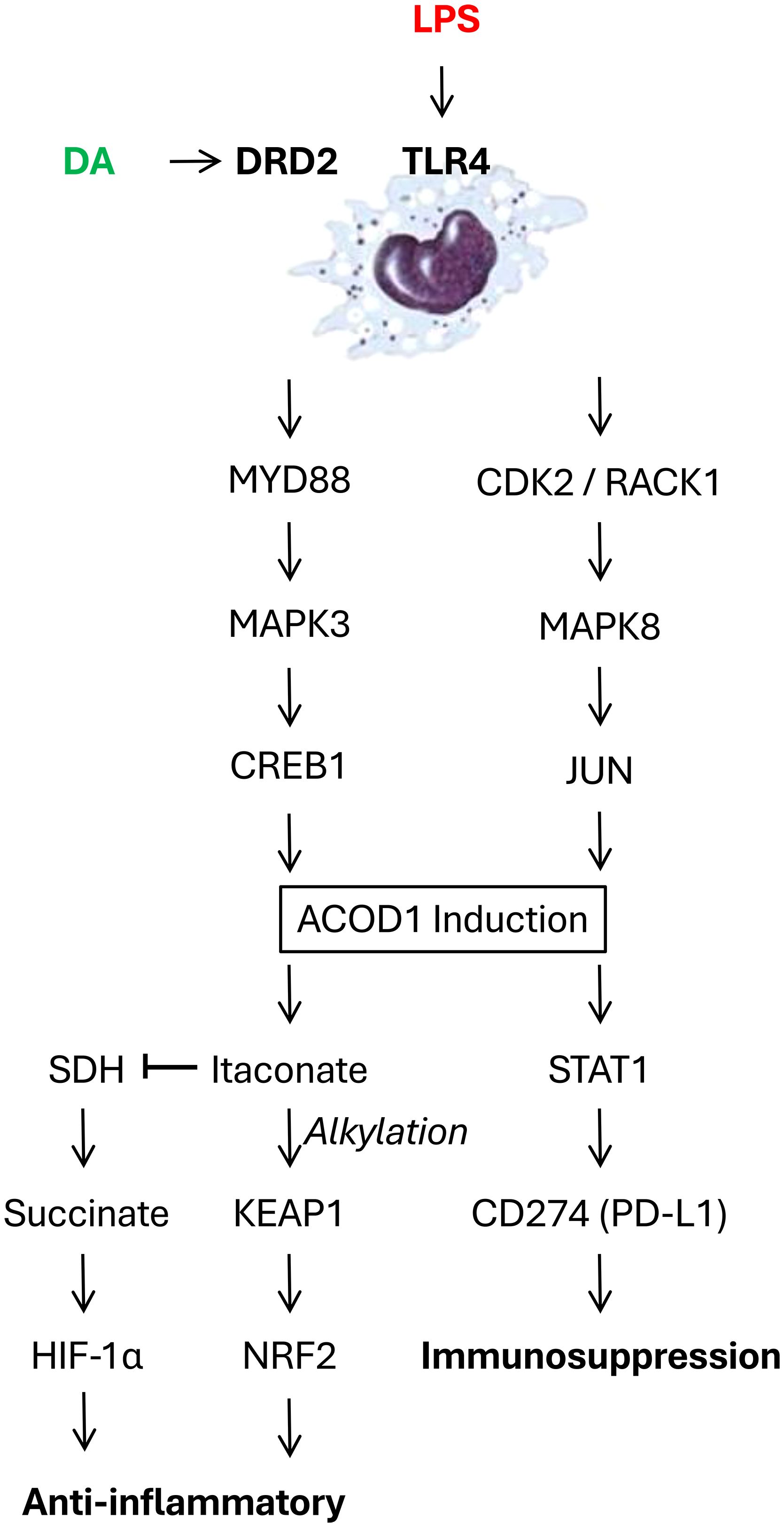
Figure 1. Dopamine (DA) counter-regulates bacterial endotoxins (LPS)-induced aconitate decarboxylase 1 (ACOD 1) expression in innate immune cells. LPS engages Toll-like receptor 4 (TLR4), activating either the MYD88-MAPK3-CREB1 or CDK2-RACK1-MAPK8-JUN signaling pathway, ultimately upregulating ACOD1 expression in innate immune cells. This ACOD1 upregulation increases itaconate production, which exerts anti-inflammatory effects by promoting direct alkylating Kelch-like ECH-associated protein 1 (KEAP1), activating nuclear factor erythroid 2-related factor 2 (Nrf2) and driving antioxidant and anti-inflammatory responses. Concurrently, ACOD1 upregulation also competitively inhibits succinate dehydrogenase (SDH), resulting in succinate accumulation which stabilizes hypoxia-inducible factor-1α (HIF-1α) and promotes anti-inflammatory gene expression. Furthermore, ACOD1 can promote the expression of the immune checkpoint inhibitor CD274 (PD-L1) via STAT1 activation. The engagement of DRD2 by dopamine or agonists can disrupt TLR4-MYD88 interaction, inhibit ACOD1 expression, thereby conferring protection against lethal sepsis.
Our recent research has uncovered itaconate-independent functions of ACOD1 (59), adding complexity to its immunoregulatory role in sepsis. Specifically, an LPS-stimulated, JUN-regulated, pro-inflammatory function of ACOD1 has been identified, involving an interplay between CDK2, RACK1, and MAPK8 (59). Mechanistically, LPS triggers the formation of a CDK2-RACK1-MAPK8 complex, leading to MAPK8 activation and JUN phosphorylation (Figure 1). Phosphorylated JUN then translocates to the nucleus and promotes ACOD1 expression (Figure 1) (59, 60). In a mouse model of sepsis induced by cecal ligation and puncture (CLP), global or myeloid-specific genetic knockout of either CDK2 or ACOD1 significantly improved survival (59), attenuating systemic inflammation, organ dysfunction, and coagulopathy. These protective effects have been observed even in the absence of itaconate production, supporting the existence of itaconate-independent mechanisms. Pharmacological inhibition of CDK2, a kinase upstream of ACOD1, with dinaciclib has replicated these benefits in CLP and other clinically relevant sepsis models (e.g., E. coli and S. pneumoniae) (59), suggesting that targeting the CDK2-ACOD1 axis may be a promising therapeutic strategy.
Recently, we have identified a key role for DA in regulating ACOD1 expression through a comprehensive screening of neurotransmitters for their ability to modulate LPS-induced expression of ACOD1 (54). Dopamine’s inhibitory effect was observed at both the mRNA and protein levels, suggesting a mechanism of transcriptional regulation. Furthermore, we pinpointed DRD2 as the specific receptor mediating dopamine’s inhibitory effect on ACOD1 expression (Figure 1) (54). Consistently, DRD2 knockdown and knockout reversed dopamine’s suppression on ACOD1 expression, while the DRD2 agonist ropinirole mimicked dopamine’s effect (54). These findings establish DRD2 as a crucial receptor in mediating DA’s immunomodulatory function in sepsis, opening avenues for targeted therapeutic interventions.
To elucidate the molecular mechanism underlying dopamine-mediated suppression of ACOD1 expression, we assessed the potential involvement of the TLR4-MYD88-MAPK3-CREB1 signaling pathway. A transcription factor, CREB1, was identified as a critical regulator of ACOD1 expression (54). Dopamine, acting through DRD2, inhibits CREB1 phosphorylation at Ser133, thereby suppressing ACOD1 transcription (Figure 1). Upstream of CREB1, the canonical TLR4-MYD88-MAPK3 pathway also plays a central role, with dopamine disrupting the interaction between TLR4-MYD88 interaction, leading to decreased MAPK3 activation and subsequent reduction in CREB1 phosphorylation (Figure 1) (54).
While ACOD1 is known for its role in producing the anti-inflammatory metabolite itaconate (61), our study also revealed an itaconate-independent function of ACOD1 in regulating the expression of CD274 (PD-L1), a crucial immune checkpoint inhibitor (Figure 1). ACOD1 promotes CD274 expression via STAT1 activation (54). Consequently, dopamine, by inhibiting ACOD1 upregulation, indirectly suppresses CD274 expression. This finding has significant implications for understanding the immunosuppressive phase of sepsis, as CD274 contributes to T-cell exhaustion and dysfunction. This itaconate-independent role of ACOD1 highlights its multifaceted involvement in immune regulation. At present, it is entirely unknow whether DA inhibits CD274 expression partly by inhibiting itaconate-independent activity of ACOD1.
We also explored the therapeutic potential of modulating DA signaling in sepsis. Pramipexole, a DRD2 agonist, conferred a significant protection in mouse models of endotoxemia and polymicrobial sepsis (54). Even when administered after the onset of sepsis, pramipexole significantly improved survival rates, reduced pro-inflammatory cytokine levels, attenuated organ damage, and downregulated ACOD1 and CD274 expression (54). These results suggest that enhancing dopamine signaling through DRD2 agonism, specifically using pramipexole, could represent a promising therapeutic strategy for sepsis.
These preclinical findings were corroborated by clinical data from sepsis patients. Non-survivors exhibited lower circulating dopamine levels and higher ACOD1 expression in peripheral blood mononuclear cells compared to survivors (54). This inverse correlation between dopamine and ACOD1 expression in human sepsis underscores the clinical relevance of our animal studies. The observed association of ACOD1 and CD274 with inflammatory markers in patients further reinforces the potential role of this axis in sepsis pathogenesis (54). However, larger and well-controlled clinical trials are needed to evaluate the efficacy and safety of DRD2 agonists, like pramipexole, in diverse sepsis patient populations, considering factors like sepsis stage, infection source, and comorbidities.
Conclusions
Our recent studies have confirmed a crucial immunoregulatory role for dopamine in sepsis via the DRD2-TLR4-ACOD1-CD274 axis. Dopamine, acting through DRD2, inhibits the TLR4-MYD88-MAPK3 pathway, suppressing CREB1 phosphorylation and downregulating ACOD1 (Figure 1). This, in turn, impacts both the inflammatory and immunosuppressive phases of sepsis, influencing cytokine production, and ultimately animal survival. While these new findings offer promising new avenues for sepsis treatment, further investigation is crucial to translate these findings into clinical practice. It will be important to fully elucidate the complex interplay of dopamine and innate immunity in sepsis, including the roles of specific dopamine receptor subtypes, dopamine production by immune cells, and its impact on distinct immune cell subsets. A more comprehensive understanding of dopamine’s multifaceted effects on the innate immune response during sepsis is essential for developing effective therapeutic strategies.
Author contributions
HW: Writing – original draft, Writing – review & editing. RK: Writing – review & editing. DT: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Research in Dr. Haichao Wang’s laboratory was partly supported by the National Institutes of Health (NIH) grants R01AT005076 and R35GM145331. Research in Dr. Rui Kang’s laboratory was partly supported by the National Institutes of Health (NIH) grant R01CA211070. Research in Dr. Daolin Tang’s laboratory was partly supported by the National Institutes of Health (NIH) grant R01GM127791.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
2. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the global burden of disease study. Lancet. (2020) 395:200–11. doi: 10.1016/S0140-6736(19)32989-7
3. Wang H, Ayala A, Aziz M, Billiar TR, Deutschman CS, Jeyaseelan S, et al. Value of animal sepsis research in navigating the translational labyrinth. Front Immunol. (2025) 16:1593342. doi: 10.3389/fimmu.2025.1593342
4. Tindal EW, Armstead BE, Monaghan SF, Heffernan DS, and Ayala A. Emerging therapeutic targets for sepsis. Expert Opin Ther Targets. (2021) 25:175–89. doi: 10.1080/14728222.2021.1897107
5. Li J, Zhu CS, He L, Qiang X, Chen W, and Wang H. A two-decade journey in identifying high mobility group box 1 (Hmgb1) and procathepsin L (Pcts-L) as potential therapeutic targets for sepsis. Expert Opin Ther Targets. (2023) 27:575–91. doi: 10.1080/14728222.2023.2239495
6. Rittirsch D, Flierl MA, and Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. (2008) 8:776–87. doi: 10.1038/nri2402
7. Hotchkiss RS, Monneret G, and Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. (2013) 13:862–74. doi: 10.1038/nri3552
8. Sarkar C, Basu B, Chakroborty D, Dasgupta PS, and Basu S. The immunoregulatory role of dopamine: an update. Brain Behav Immun. (2010) 24:525–8. doi: 10.1016/j.bbi.2009.10.015
9. Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, et al. Dopamine controls systemic inflammation through inhibition of nlrp3 inflammasome. Cell. (2015) 160:62–73. doi: 10.1016/j.cell.2014.11.047
10. Tayebati SK, Lokhandwala MF, and Amenta F. Dopamine and vascular dynamics control: present status and future perspectives. Curr Neurovasc Res. (2011) 8:246–57. doi: 10.2174/156720211796558032
11. Jose PA, Eisner GM, and Felder RA. Regulation of blood pressure by dopamine receptors. Nephron Physiol. (2003) 95:19–27. doi: 10.1159/000073676
12. Rubí B and Maechler P. Minireview: new roles for peripheral dopamine on metabolic control and tumor growth: let’s seek the balance. Endocrinology. (2010) 151:5570–81. doi: 10.1210/en.2010-0745
13. Basu S and Dasgupta PS. Dopamine, a neurotransmitter, influences the immune system. J Neuroimmunol. (2000) 102:113–24. doi: 10.1016/s0165-5728(99)00176-9
14. Levite M. Dopamine and T cells: dopamine receptors and potent effects on T cells, dopamine production in T cells, and abnormalities in the dopaminergic system in T cells in autoimmune, neurological and psychiatric diseases. Acta Physiol (Oxf). (2016) 216:42–89. doi: 10.1111/apha.12476
15. Thomas Broome S, Louangaphay K, Keay KA, Leggio GM, Musumeci G, and Castorina A. Dopamine: an immune transmitter. Neural Regener Res. (2020) 15:2173–85. doi: 10.4103/1673-5374.284976
16. Cosentino M, Fietta AM, Ferrari M, Rasini E, Bombelli R, Carcano E, et al. Human cd4+Cd25+ Regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood. (2007) 109:632–42. doi: 10.1182/blood-2006-01-028423
17. Cosentino M, Zaffaroni M, Marino F, Bombelli R, Ferrari M, Rasini E, et al. Catecholamine production and tyrosine hydroxylase expression in peripheral blood mononuclear cells from multiple sclerosis patients: effect of cell stimulation and possible relevance for activation-induced apoptosis. J Neuroimmunol. (2002) 133:233–40. doi: 10.1016/s0165-5728(02)00372-7
18. Beck G, Brinkkoetter P, Hanusch C, Schulte J, van Ackern K, van der Woude FJ, et al. Clinical review: immunomodulatory effects of dopamine in general inflammation. Crit Care. (2004) 8:485–91. doi: 10.1186/cc2879
19. Nakano K, Higashi T, Takagi R, Hashimoto K, Tanaka Y, and Matsushita S. Dopamine released by dendritic cells polarizes th2 differentiation. Int Immunol. (2009) 21:645–54. doi: 10.1093/intimm/dxp033
20. Cosentino M, Zaffaroni M, Ferrari M, Marino F, Bombelli R, Rasini E, et al. Interferon-gamma and interferon-beta affect endogenous catecholamines in human peripheral blood mononuclear cells: implications for multiple sclerosis. J Neuroimmunol. (2005) 162:112–21. doi: 10.1016/j.jneuroim.2005.01.019
21. McKenna F, McLaughlin PJ, Lewis BJ, Sibbring GC, Cummerson JA, Bowen-Jones D, et al. Dopamine receptor expression on human T- and B-lymphocytes, monocytes, neutrophils, eosinophils and nk cells: A flow cytometric study. J Neuroimmunol. (2002) 132:34–40. doi: 10.1016/s0165-5728(02)00280-1
22. Wenisch C, Parschalk B, Weiss A, Zedwitz-Liebenstein K, Hahsler B, Wenisch H, et al. High-dose catecholamine treatment decreases polymorphonuclear leukocyte phagocytic capacity and reactive oxygen production. Clin Diagn Lab Immunol. (1996) 3:423–8. doi: 10.1128/cdli.3.4.423-428.1996
23. Marino F, Pinoli M, Rasini E, Martini S, Luini A, Pulze L, et al. Dopaminergic inhibition of human neutrophils is exerted through D1-like receptors and affected by bacterial infection. Immunology. (2022) 167:508–27. doi: 10.1111/imm.13550
24. Pinoli M, Marino F, and Cosentino M. Dopaminergic regulation of innate immunity: A review. J Neuroimmune Pharmacol. (2017) 12:602–23. doi: 10.1007/s11481-017-9749-2
25. Sookhai S, Wang JH, Winter D, Power C, Kirwan W, and Redmond HP. Dopamine attenuates the chemoattractant effect of interleukin-8: A novel role in the systemic inflammatory response syndrome. Shock. (2000) 14:295–9. doi: 10.1097/00024382-200014030-00009
26. Matsuoka T. A sedative effect of dopamine on the respiratory burst in neonatal polymorphonuclear leukocytes. Pediatr Res. (1990) 28:24–7. doi: 10.1203/00006450-199007000-00006
27. Trabold B, Gruber M, and Fröhlich D. Functional and phenotypic changes in polymorphonuclear neutrophils induced by catecholamines. Scand Cardiovasc J. (2007) 41:59–64. doi: 10.1080/14017430601085948
28. Bergquist J, Ohlsson B, and Tarkowski A. Nuclear factor-kappa B is involved in the catecholaminergic suppression of immunocompetent cells. Ann N Y Acad Sci. (2000) 917:281–9. doi: 10.1111/j.1749-6632.2000.tb05394.x
29. Haskó G, Szabó C, Németh ZH, and Deitch EA. Dopamine suppresses il-12 P40 production by lipopolysaccharide-stimulated macrophages via a beta-adrenoceptor-mediated mechanism. J Neuroimmunol. (2002) 122:34–9. doi: 10.1016/s0165-5728(01)00459-3
30. Haskó G, Szabó C, Merkel K, Bencsics A, Zingarelli B, Kvetan V, et al. Modulation of lipopolysaccharide-induced tumor necrosis factor-alpha and nitric oxide production by dopamine receptor agonists and antagonists in mice. Immunol Lett. (1996) 49:143–7. doi: 10.1016/0165-2478(96)02494-7
31. Chi DS, Qui M, Krishnaswamy G, Li C, and Stone W. Regulation of nitric oxide production from macrophages by lipopolysaccharide and catecholamines. Nitric Oxide. (2003) 8:127–32. doi: 10.1016/s1089-8603(02)00148-9
32. Matalka KZ, Attallah LJ, Qinna NA, and Alhussainy T. Dopamine selectively modulates lipopolysaccharide-induced tnf-alpha, ifn-gamma and il-10 within mice tissues. Neuro Endocrinol Lett. (2011) 32:176–86.
33. Tarazona R, González-García A, Zamzami N, Marchetti P, Frechin N, Gonzalo JA, et al. Chlorpromazine amplifies macrophage-dependent il-10 production in vivo. J Immunol. (1995) 154:861–70. doi: 10.4049/jimmunol.154.2.861
34. McDonald RH Jr., Goldberg LI, McNay JL, and Tuttle EP Jr. Effect of dopamine in man: augmentation of sodium excretion, glomerular filtration rate, and renal plasma flow. J Clin Invest. (1964) 43:1116–24. doi: 10.1172/jci104996
35. Zhang Z and Chen K. Vasoactive agents for the treatment of sepsis. Ann Transl Med. (2016) 4:333. doi: 10.21037/atm.2016.08.58
36. Loeb HS, Winslow EB, Rahimtoola SH, Rosen KM, and Gunnar RM. Acute hemodynamic effects of dopamine in patients with shock. Circulation. (1971) 44:163–73. doi: 10.1161/01.cir.44.2.163
37. De Backer D, Creteur J, Silva E, and Vincent JL. Effects of dopamine, norepinephrine, and epinephrine on the splanchnic circulation in septic shock: which is best? Crit Care Med. (2003) 31:1659–67. doi: 10.1097/01.Ccm.0000063045.77339.B6
38. Vincent JL and de Backer D. The international sepsis forum’s controversies in sepsis: my initial vasopressor agent in septic shock is dopamine rather than norepinephrine. Crit Care. (2003) 7:6–8. doi: 10.1186/cc1851
39. Sharma VK and Dellinger RP. The international sepsis forum’s controversies in sepsis: my initial vasopressor agent in septic shock is norepinephrine rather than dopamine. Crit Care. (2003) 7:3–5. doi: 10.1186/cc1835
40. De Backer D, Aldecoa C, Njimi H, and Vincent JL. Dopamine versus norepinephrine in the treatment of septic shock: A meta-analysis*. Crit Care Med. (2012) 40:725–30. doi: 10.1097/CCM.0b013e31823778ee
41. Bernton EW, Meltzer MS, and Holaday JW. Suppression of macrophage activation and T-lymphocyte function in hypoprolactinemic mice. Science. (1988) 239:401–4. doi: 10.1126/science.3122324
42. Zhu XH, Zellweger R, Wichmann MW, Ayala A, and Chaudry IH. Effects of prolactin and metoclopramide on macrophage cytokine gene expression in late sepsis. Cytokine. (1997) 9:437–46. doi: 10.1006/cyto.1996.0186
43. Townsend MC, Schirmer WJ, Schirmer JM, and Fry DE. Low-dose dopamine improves effective hepatic blood flow in murine peritonitis. Circ Shock. (1987) 21:149–53. doi: 10.1097/00005373-199910000-00014
44. Wu Y, Hu Y, Wang B, Li S, Ma C, Liu X, et al. Dopamine uses the drd5-arrb2-pp2a signaling axis to block the traf6-mediated nf-κb pathway and suppress systemic inflammation. Mol Cell. (2020) 78:42–56.e6. doi: 10.1016/j.molcel.2020.01.022
45. Tanaka K, Choudhury ME, Kikuchi S, Takeda I, Umakoshi K, Miyaue N, et al. A dopamine D1-like receptor-specific agonist improves the survival of septic mice. iScience. (2024) 27:109587. doi: 10.1016/j.isci.2024.109587
46. Oberbeck R, Schmitz D, Wilsenack K, Schüler M, Husain B, Schedlowski M, et al. Dopamine affects cellular immune functions during polymicrobial sepsis. Intensive Care Med. (2006) 32:731–9. doi: 10.1007/s00134-006-0084-y
47. Torres-Rosas R, Yehia G, Peña G, Mishra P, del Rocio Thompson-Bonilla M, Moreno-Eutimio MA, et al. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med. (2014) 20:291–5. doi: 10.1038/nm.3479
48. Meier-Hellmann A, Bredle DL, Specht M, Spies C, Hannemann L, and Reinhart K. The effects of low-dose dopamine on splanchnic blood flow and oxygen uptake in patients with septic shock. Intensive Care Med. (1997) 23:31–7. doi: 10.1007/s001340050287
49. Feketeova E, Li Z, Joseph B, Shah R, Spolarics Z, and Ulloa L. Dopaminergic control of inflammation and glycemia in sepsis and diabetes. Front Immunol. (2018) 9:943. doi: 10.3389/fimmu.2018.00943
50. De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. (2010) 362:779–89. doi: 10.1056/NEJMoa0907118
51. Ventura AM, Shieh HH, Bousso A, Góes PF, de Cássia FOFI, de Souza DC, et al. Double-blind prospective randomized controlled trial of dopamine versus epinephrine as first-line vasoactive drugs in pediatric septic shock. Crit Care Med. (2015) 43:2292–302. doi: 10.1097/ccm.0000000000001260
52. Xu B and Peter O. Dopamine versus noradrenaline in septic shock. Australas Med J. (2011) 4:571–4. doi: 10.4066/amj.2011.761
53. Sakr Y, Reinhart K, Vincent JL, Sprung CL, Moreno R, Ranieri VM, et al. Does dopamine administration in shock influence outcome? Results of the sepsis occurrence in acutely ill patients (Soap) study. Crit Care Med. (2006) 34:589–97. doi: 10.1097/01.Ccm.0000201896.45809.E3
54. Wang N, Liu J, Wu R, Chen F, Zhang R, Yu C, et al. A neuroimmune pathway drives bacterial infection. Sci Adv. (2025) 11:eadr2226. doi: 10.1126/sciadv.adr2226
55. Michelucci A, Cordes T, Ghelfi J, Pailot A, Reiling N, Goldmann O, et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci U.S.A. (2013) 110:7820–5. doi: 10.1073/pnas.1218599110
56. Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces il-1β through hif-1α. Nature. (2013) 496:238–42. doi: 10.1038/nature11986
57. Mills EL, Ryan DG, Prag HA, Dikovskaya D, Menon D, Zaslona Z, et al. Itaconate is an anti-inflammatory metabolite that activates nrf2 via alkylation of keap1. Nature. (2018) 556:113–7. doi: 10.1038/nature25986
58. Peace CG and O’Neill LA. The role of itaconate in host defense and inflammation. J Clin Invest. (2022) 132. doi: 10.1172/jci148548
59. Wu R, Liu J, Wang N, Zeng L, Yu C, Chen F, et al. Aconitate decarboxylase 1 is a mediator of polymicrobial sepsis. Sci Transl Med. (2022) 14:eabo2028. doi: 10.1126/scitranslmed.abo2028
60. Chen F, Wu R, Liu J, Kang R, Li J, and Tang D. The sting1-myd88 complex drives acod1/irg1 expression and function in lethal innate immunity. iScience. (2022) 25:104561. doi: 10.1016/j.isci.2022.104561
Keywords: dopamine, sepsis, aconitate decarboxylase 1, CD274, innate immunity
Citation: Wang H, Kang R and Tang D (2025) Dopamine as an endogenous regulator of innate immunity in sepsis. Front. Immunol. 16:1625368. doi: 10.3389/fimmu.2025.1625368
Received: 08 May 2025; Accepted: 20 June 2025;
Published: 09 July 2025.
Edited by:
Guirong Wang, Upstate Medical University, United StatesReviewed by:
Qun Sophia Zang, Loyola University Chicago, United StatesLin Zou, University of Maryland, United States
Copyright © 2025 Wang, Kang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haichao Wang, aHdhbmdAbm9ydGh3ZWxsLmVkdQ==; Daolin Tang, RGFvbGluLlRhbmdAVVRTb3V0aHdlc3Rlcm4uZWR1
 Haichao Wang
Haichao Wang Rui Kang
Rui Kang Daolin Tang
Daolin Tang