- 1College of Dental Medicine, Qatar University (QU) Health, Qatar University, Doha, Qatar
- 2Department of Biomedical Sciences, College of Health Sciences, Qatar University (QU) Health, Qatar University, Doha, Qatar
- 3Biomedical Research Center, Qatar University (QU) Health, Qatar University, Doha, Qatar
- 4College of Medicine, Qatar University (QU) Health, Qatar University, Doha, Qatar
- 5Department of Pharmaceutical Sciences, College of Pharmacy, Qatar University (QU) Health, Qatar University, Doha, Qatar
Oral squamous cell carcinoma (OSCC) is a heterogeneous malignant neoplasm characterized by intricate molecular pathways and a varied genetic landscape, resulting in a diminished 5-year survival rate. Due to this complexity, many trials of emerging therapies are failing to improve the outcome and survival rate of OSCC, posing a great challenge in the management of this cancer. This review examines the key molecular pathways, genetic susceptibility, and the influence of the microbiome in the advancement of OSCC. Furthermore, it analyses contemporary therapeutic approaches, their limitations, and prospects, especially the incorporation of immunotherapy. The discussion will also encompass the difficulties in turning research findings into successful therapeutic applications and enhancing the patient’s quality of life.
1 Introduction
According to the GLOBOCAN estimates, in 2022, Lip and oral cavity cancers ranked as the 15th leading cause of death due to cancer and resulted in 188,230 deaths per year (1). The most common type of cancer in the oral cavity is the squamous cell carcinoma. This malignancy, which accounts for 90% of oral cancers, arises from the epithelial lining of the larynx, pharynx, and oral cavity (2). Oral squamous cell carcinoma (OSCC) is classified under the category of head and neck carcinoma (3).
OSCC causes functional impairments and disfigurations, which include taste, speech, and swallowing difficulties that substantially influence the patient’s quality of life (4, 5). In addition, notable rates of suicidal attempts and ideations have been observed among patients with oral cancers (6).
The development of OSCC is primarily associated with excessive alcohol and tobacco consumption (2), along with other risk factors such as genetic predisposition, epigenetic factors, viruses such as Human papillomavirus (HPV) and Epstein–Barr virus (EBV), gender, race, age, diet, nutrition and consumption of betel nuts, Fanconi anemia and dyskeratosis congenita, as well as immune disorders and certain immunosuppressive agents (7). Furthermore, chronic mucosal trauma due to ill-fitting dentures, sharp teeth, faulty restorations, or implants, and intraoral contact with metal dental restorations were reported to increase the risk of OSCC (8, 9).
OSCC is commonly treated with surgery, chemotherapy, and radiotherapy, depending on the stage. However, over the past few years, immunotherapy (e.g., programmed death-1 (PD-1) immune checkpoint inhibitors) has also emerged as a potential therapy in refractory and relapsed Head and neck squamous cell carcinoma (HNSCC) (10).
Despite the advancements in the treatment modalities of OSCC, there has been no noticeable 5-year survival improvement in recent years. In comparison to other cancers, OSCC was found to have a poor 5-year survival rate of around 35% in India and 50% in Europe. The 5-year-survival rates for this disease overall ranged between 80% for localized, early detected cancers to 20% in the case of regional lymph node involvement, driven by the stage at which the disease was diagnosed (10–12). A recent study reported an approximate 59.3% 5-year survival rate of OSCC, with around 45.7% of cases experiencing metastasis or postoperative recurrences (13). In addition, a recently published, long-term, retrospective observational study that evaluated OSCC risk factors and epidemiology in patients with invasive OSCC over 39 years indicated that 40% of the patients developed recurrence and that the survival rate did not improve over time (14). Furthermore, the Global Cancer Observatory (GCO) has projected that by the year 2040, the incidence of OSCC would increase by roughly 40%, along with a growth in mortality (15). This lack of progress in our management of the disease is attributed to a gap in understanding the complex challenges associated with the genetics, molecular pathways, and treatment modalities of OSCC and their limitations. Therefore, we aim in this review to explore the complex relationship between the genetic landscape and with underlying key molecular pathways of OSCC, their mechanisms, and crosstalk in OSCC. We also provide an overview of positive and detrimental risk factors of the disease, the currently available treatment modalities, and their limitations, and offer insights into the translation of research findings into applicable clinical practices, allowing for tailored personalized medicine.
2 Genomic alterations and pathway dysregulation in OSCC
Next-generation sequencing (NGS) offers comprehensive details about the mutations defined in OSCC. These studies investigate the different genetic changes that happen in OSCC and how they affect tumor suppressors, oncogenes, and epigenetic regulators (16, 17). Out of the many OSCC common mutations, the TP53 (tumor protein 53) gene is one of the most frequently altered. In about 60% to 80% of OSCC cases, alterations occur in TP53 that lead to oncogenesis (18), resulting in the uncontrolled division of malignant cells, as well as genomic instability, immune suppression, and treatment resistance. These genomic modifications are detected in the early stages of OSCC (19, 20). About 70% of the cases that were examined had genetic alterations in the receptor tyrosine kinases (RTKs), mitogen-activated protein kinase (MAPK), and Phosphoinositide 3-Kinase (PI3-K) signaling pathways (19). RTKs, particularly those from the ERBB family—Epidermal Growth Factor Receptor and ERBB2-4—often drive epithelial cancers. Ligand-binding RTK phosphorylation leads to the activation of various downstream signaling pathways, like the RAS-RAF-MEK-ERK MAPK pathway responsible for cell growth and survival (21). Amplification or somatic mutations were cited as common in RTKs (19) and were common in OSCC samples (21). However, although mutations in RTKs, MAPK, and PI3-K are linked to a poor prognosis, survival outcomes are influenced by multiple factors, including treatment history and immune microenvironment composition, requiring cautious interpretation (21). PI3-K signaling is controlled by oncogenic mutations in PIK3CA (Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha) that are often found in OSCC, though the rates of occurrence vary by race (22, 23). These mutations increase the kinase activity, which results in the constitutive phosphorylation of Ak strain transforming (Akt) or Protein Kinase B (PKB), leading to oncogenic transformation (24). Functional research corroborates mutation assertions in Akt activation, establishing it as a therapeutic target, irrespective of the variability in the reported incidence of PIK3CA mutations. The alteration in OSCC is also noted to be NOTCH signaling mediation (25, 26), which underlines the further complexity of its role in tumor development (27). Some populations show positive NOTCH1 variation mutations in a range of 18% to 45% (28, 29). A study of Japanese OSCC patients found that NOTCH1 mutations were the second most common change, occurring in 25.5% of the time. This is higher than the 17.8% frequency observed in the HNSCC dataset (19). Several studies suggest that alterations in the oncogene NOTCH1 can act either as tumor-promoting or tumor-suppressing, depending on the cell type. Loss-of-function mutations in NOTCH1, NOTCH2, NOTCH3, and SRY-Box Transcription Factor 2 (SOX2) (30) inhibit squamous differentiation, subsequently leading to cancerous growth. Moreover, alterations in FBXW7, which regulates NOTCH activity via degradation, further destabilize the Notch system (31). Whole-exome sequencing of OSCC samples has shown frequent alterations in FAT1 (FAT Atypical Cadherin 1) (32) and CASP8 (Caspase 8) (33). These mutations have been suggested to increase the likelihood of OSCC proliferation, migration, and resistance to apoptosis (31). Beyond classical oncogenic signaling, alterations in chromatin remodeling genes underscore the role of epigenetic dysregulation in OSCC heterogeneity. Chromosome accessibility and transcriptional regulation are affected by mutations in KMT2D (lysine-specific methyltransferase 2D), also known as Mixed-Lineage Leukemia 2 (MLL2), a histone methyltransferase that causes H3K4 (Histone 3 Lysine 4) methylation (34). Although their exact role in OSCC is unknown, further mutations in SYNE1 (Syne Homolog 1) and TAFIL [TATA-Box Binding Protein (TBP)-Associated Factor 1-Like] are linked to chromatin remodeling abnormalities (19). Notably, mutations in MLL, MLL3, and other histone modifiers are frequently detected in HPV-associated oropharyngeal cancers, suggesting a potential virus-driven epigenetic reprogramming mechanism (35, 36). The long-tailed distribution of low-frequency mutations in chromatin regulators reveals the genetic complexity of OSCC, requiring functional validation of epigenetic targets (19). Further mutations in SYNE1 and TAF1L are linked to chromatin remodeling problems (37), but no one knows exactly what role they play in OSCC. Notably, changes in MLL, MLL3, and other histone modifiers are often found in oropharyngeal cancers that are linked to HPV. This suggests that the virus may change epigenetics (35, 36). The long-tailed distribution of low-frequency mutations in chromatin regulators reveals the genetic complexity of OSCC, requiring functional validation of epigenetic targets (37).
3 Epigenetics of OSCC
Epigenetics plays a detrimental role in the development, progression, and treatment resistance of OSCC. It changes the way genes are expressed without interfering with the DNA sequence. These changes encompass DNA methylation, histone modifications, and miRNA regulation (38, 39).
3.1 DNA methylation
DNA methylation involves the addition of a methyl group to cytosine residues in CpG islands, habitually steering to gene silencing. In OSCC, aberrant DNA methylation patterns are witnessed in the hypermethylation of tumor suppressor genes (TSGs) and hypomethylation of oncogenes (40).
3.1.1 Hypermethylation of TSGs
Hypermethylation of CpG islands in tumor suppressor gene (TSG) promoters silences their expression, accelerating tumor growth and progression. In OSCC, several genes are frequently hypermethylated (41, 42). Cell cycle regulators, such as p16 (CDKN2A) and p15 (CDKN2B), are key regulators of the G1/S checkpoint, and their silencing leads to unchecked cell cycle progression. Apoptosis-related genes, including DAPK1 and p73 (Tumor protein 73), are inactivated through hypermethylation, disrupting apoptotic pathways and promoting tumor survival. DNA repair genes, such as MGMT and MLH1 (MutL homolog 1), play crucial roles in DNA repair, and their silencing contributes to genomic instability, driving carcinogenesis.
3.1.2 Hypomethylation of oncogenes
Conversely, hypomethylation activates oncogenes by increasing their transcription. EGFR (Epidermal Growth Factor Receptor) overexpression, driven by hypomethylation, activates oncogenic pathways such as RAS/RAF/MEK/ERK and PI3-K/Akt/mTOR, which promote tumor proliferation, survival, angiogenesis, and resistance to therapy (43). Survivin, an anti-apoptotic protein, is upregulated through hypomethylation, enhancing tumor aggressiveness by inhibiting programmed cell death (44).
3.2 Histone modifications
Histone modifications regulate chromatin structure and gene expression by altering the accessibility of DNA to transcriptional machinery. These modifications, including acetylation, methylation, and phosphorylation, are crucial in OSCC epigenetics (45).
3.2.1 Histone acetylation
Histone acetylation, regulated by histone acetyltransferases (HATs) and histone deacetylases (HDACs), plays an imperative role in gene expression by restraining chromatin structure. HATs add acetyl groups to lysine residues on histones, leading to chromatin relaxation and transcriptional activation, while HDACs remove these groups, triggering chromatin condensation and gene repression (45). In OSCC, the acetylation of histones H3 and H4 is particularly noteworthy, as dysregulated acetylation patterns are concomitant with tumor progression (46, 47). Moreover, HDAC inhibitors (HDACis), such as Trichostatin A (TSA) and Vorinostat (SAHA), have revealed potential in reestablishing normal acetylation levels and resuscitating TSGs, proffering a promising therapeutic approach (45).
3.2.2 Histone methylation
Histone methylation can either activate or repress gene transcription, reliant on the specific lysine residue modified. For example, H3K4me3 is correlated with active transcription, and its dysregulation may endorse oncogene expression, while H3K9me2 is connected to transcriptional repression, with increased levels associating with poor prognosis in OSCC. Additionally, abnormal histone methylation patterns, driven by dysregulated histone methyltransferases (HMTs) and demethylases (HDMs), contribute to OSCC aggressiveness. Furthermore, overexpression of enhancer of zeste homolog 2 (EZH2), a histone methyltransferase, is mostly associated with poor prognosis and increased invasiveness. Targeting these enzymes with inhibitors like DZNep has shown promise in preclinical studies, emphasizing histone methylation as a potential therapeutic target in OSCC (40, 48–50).
3.2.3 Histone phosphorylation
Phosphorylation of histones H3 and H4 plays an imperative role in OSCC progression by influencing chromatin dynamics, mitotic processes, and transcriptional regulation. Histone H3 phosphorylation at serine 10 (H3S10ph) is linked to both chromosome condensation during mitosis and transcriptional activation, with low levels associated with cervical lymph node metastasis. Similarly, histone H4 phosphorylation at serine 1 (H4S1) and high levels of H4K12 acetylation (H4K12ac) are linked with metastasis, gender, and alcohol consumption. These modifications influence chromatin remodeling, gene regulation, and cell cycle control, making them potential biomarkers for OSCC prognosis (46, 51).
3.3 MicroRNA dysregulation
miRNAs are small non-coding RNAs that regulate gene expression post-transcriptionally, and their dysregulation plays a significant role in OSCC progression. Oncogenic miRNAs (oncomiRs), such as miR-21 and miR-181b, are overexpressed in OSCC, encouraging malignant transformation by downregulating the mRNA expression of tumor suppressors like PTEN (Phosphatase and Tensin Homolog) and PDCD4 (Programmed Cell Death 4) and heightening inflammation-driven tumor progression. Conversely, tumor-suppressive miRNAs, including miR-133a/b and miR-34a, are often downregulated, escorting oncogene activation, such as PKM2 (Pyruvate Kinase M2)-driven glycolysis and Bcl-2 (B-cell leukemia/lymphoma 2 protein)-mediated apoptosis resistance. In Addition, miRNAs contribute to immune evasion, with miR-200c downregulation facilitating Epithelial-Mesenchymal Transition (EMT) and immune resistance through PD-L1 (Programmed death-ligand 1) modulation. Epigenetic silencing via hypermethylation further exacerbates miRNA dysregulation, highlighting their potential as both biomarkers and therapeutic targets in OSCC (52–55).
4 OSCC risk factors and preventive measures
Several risk factors were identified to contribute to the development of OSCC (56). Conversely, there are various protective factors and strategies that can serve as preventive measures against OSCC, and these will be explored in this section (57).
4.1 Risk factors
The most relevant among all is tobacco use and alcohol consumption, accounting for 70-80% of all HNSCCs (7). Their combined effect can synergistically increase the risk of oral cancer by around 35% (58). Tobacco smoking inhibits systemic immune functions and causes epigenetic alterations in the oral epithelial cells. In addition, its metabolites can initiate oxidative stress and induce oral squamous cell carcinoma in tissues (59). Tobacco has been shown to activate pathways implicated in OSCC. This includes for instance GLUT-1 (glucose transporter 1), which plays a role in enhancing cancer cell growth and survival, p16 (AKA cyclin-dependent kinase Inhibitor 2A (CDKN2A) (60), which is involved in cell cycle progression and proliferation, and p53 (tumor protein 53), which is important for DNA repair and cell cycle arrest. In addition, PI3-K (phosphatidylinositol 3-kinase) which is involved in cell survival, proliferation, and metabolism, DAPK (death-associated protein kinase), which has a role in tumor suppression and apoptosis, and MGMT (O6-methylguanine-DNA methyltransferase), which is involved in cell genomic stability and DNA repair are also implicated in OSCC (59).
Ethanol contained in alcoholic beverages is classified as carcinogenic (61). OSCC has been inextricably connected with the consumption of alcohol (62). The molecular mechanisms by which alcohol contributes to OSCC include oxidative stress-mediated DNA damage, inflammation, and immune dysregulation through the production of chemokines and cytokines (63), epigenetic modulations (64), and a modification of pivotal signaling pathways implicated in cell proliferation, angiogenesis, and apoptosis, such as the Nuclear Factor-kappa B (NF-κB) and Wnt signaling pathway/beta-catenin (Wnt/β-catenin) (62).
Genetic and epigenetic factors have an important association with OSCC. The primary genes identified earlier to be up-regulated significantly in OSCC early stages are CPA6 (Carboxypeptidase A6), TNC (Tenascin-C), FMO2 (Flavin-Containing Monooxygenase 2), and SIAT1 (Sialyltransferase 1). In addition, the expression of the LGI1 (Leucine-Rich, Glioma-Inactivated 1) gene was found to be enhanced in healthy surrounding mucosa, nevertheless, it did not show differential expression in cancerous tissue biopsies (65). More recent studies indicated other genetic contributors to the development of HNSCC, including several DNA repair enzymes, Fanconi anemia-associated genes, apoptotic pathway members, and cell-cycle control proteins, which were suggested to modulate HNSCC susceptibility (66).
It is crucial to emphasize the risks associated with using heavy metals such as titanium, chromium, gold (67), silver, cobalt, palladium, nickel, tin, and copper, as well as alloys containing silver-tin and mercury, which are commonly used in dental amalgams (68). For instance, chromium and cadmium are reported to induce oxidative deterioration in biological macromolecules, and cadmium can replace zinc in the DNA binding domains of zinc finger and hence trigger cancer (69, 70). Senevirathna et al. observed significantly higher cadmium, chromium, copper, cobalt, and zinc serum concentrations in patients with OSCC and oral potentially malignant disorders (OPMD) (69). A recent in vitro study demonstrated that iron and nickel induce the generation of reactive oxygen species (ROS), whereas manganese and chromium were associated with both ROS production and single-strand breaks in DNA. The cytotoxic effects identified in the analyzed eluates were attributed to iron, aluminum, manganese, and chromium (71). The accumulation of heavy metals in saliva can lead to DNA damage, cytokine production, oxidative stress, and inflammation. Silver, chromium, and nickel have been shown to suppress fibroblast proliferation. Elevated levels of metal ions such as nickel, cobalt, titanium, and chromium are associated with hypersensitivity reactions, chronic inflammation, cell death, and mutations. Notably, some studies have reported that Ni2+ induces the production of inflammatory genes, such as interleukin (IL)-8, in primary human monocytes (72). Other major risk factors of OSCC include genetic predisposition, viruses such as HPV and EBV, gender, race, age, diet, nutrition, and consumption of betel (Arecal) nut (7). According to the GCO, OSCC appears to affect males more than females, and the susceptibility increases in men ranging from middle-aged to elderly (3). However, recent reports indicate a rising trend in OSCC cases among patients under the age of 45 (73). Furthermore, the use of Areca nut was found to have a significant correlation with OSCC. This phenomenon was predominantly observed in the Pacific and East Asian populations (15). Furthermore, current data support the association of Fanconi anemia and dyskeratosis congenita, wherein OSCC is predominantly inherited. A correlation between immune disorders, such as human immunodeficiency virus (HIV) in smokers and immunosuppressive medications, and HNSCC has been demonstrated, as both are considered possible risk factors (7).
4.2 Protective factors and preventive measures
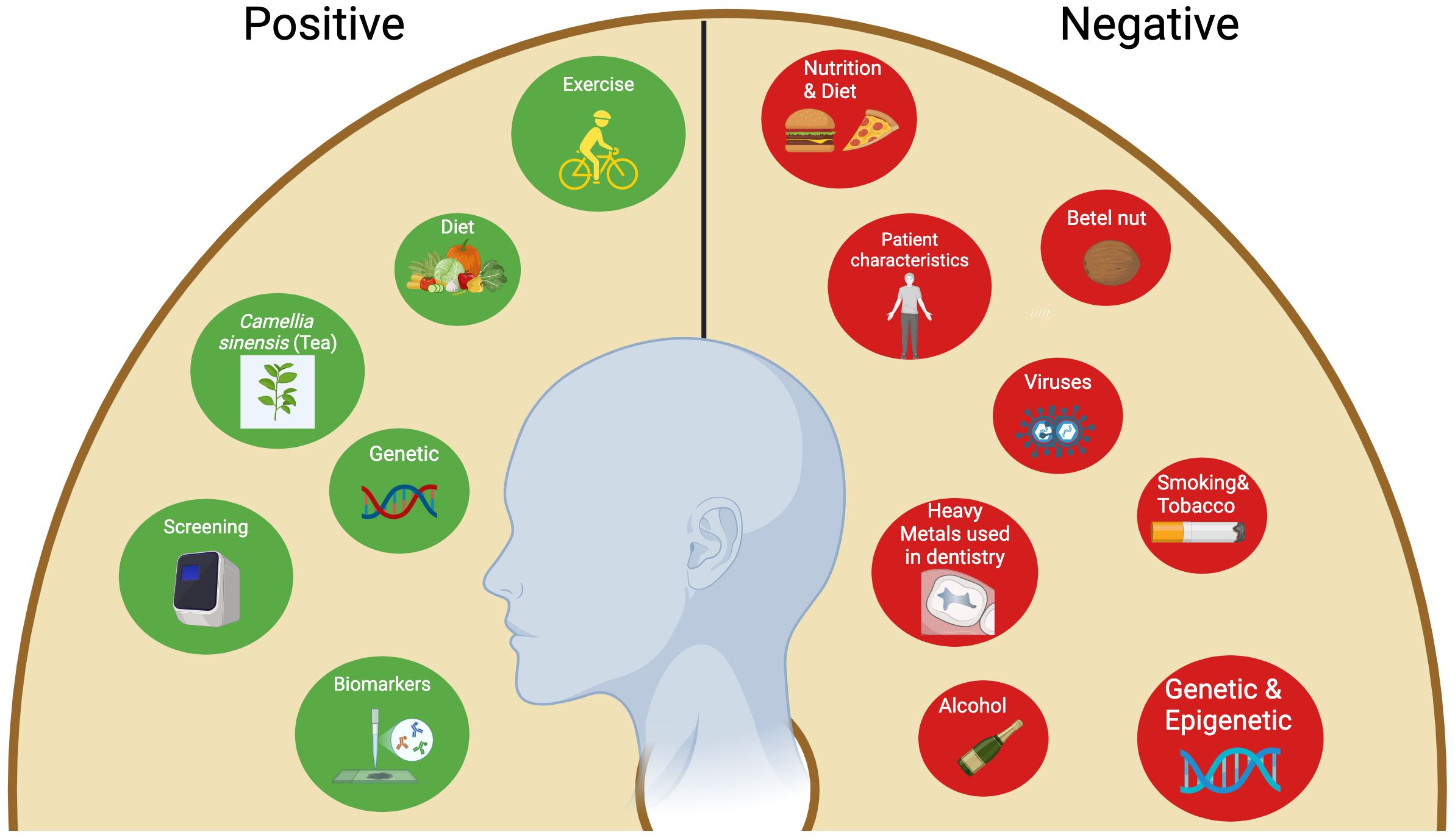
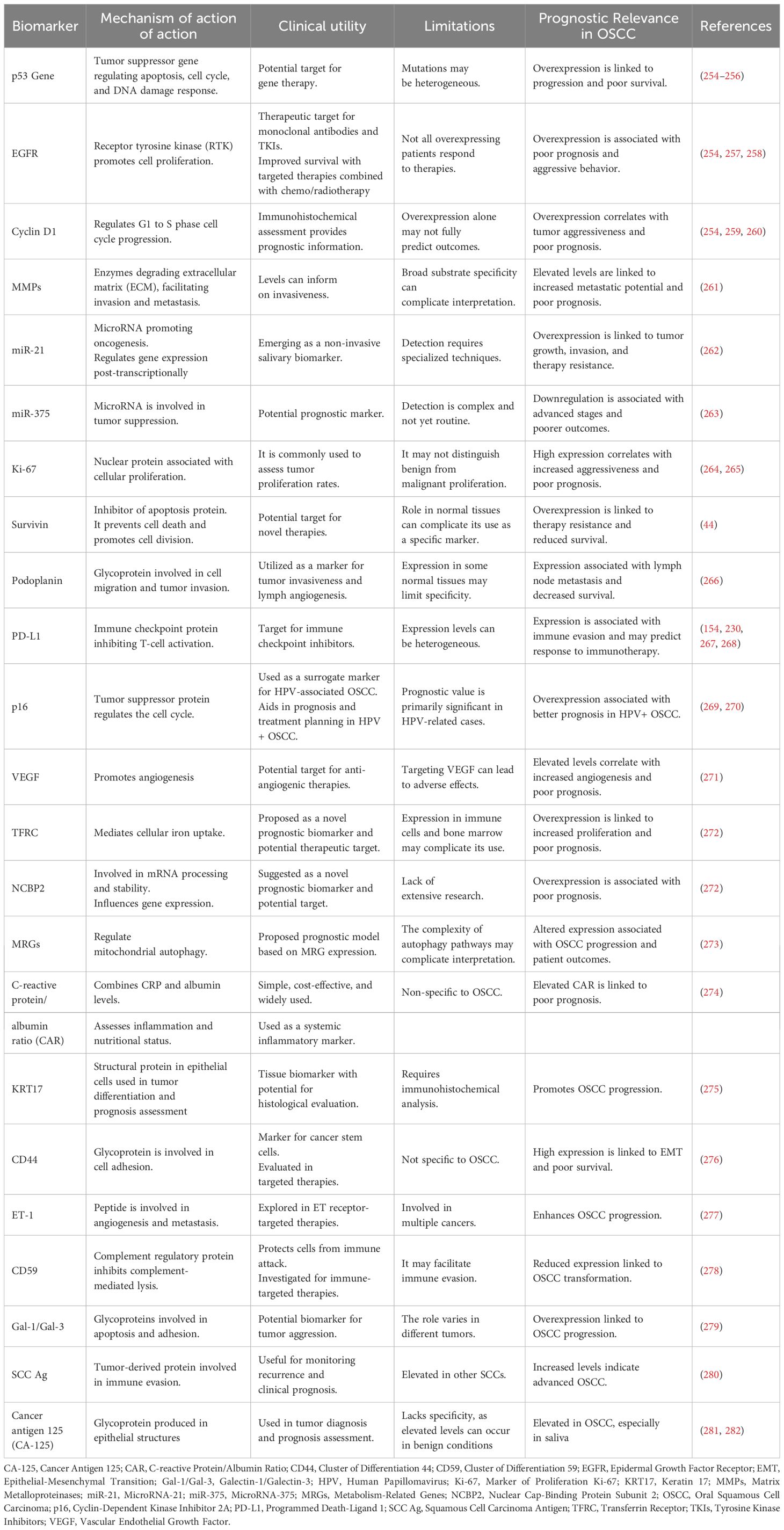
Conversely, several preventive strategies to address this hazardous situation have been documented. A portion of them are factors susceptible to change through lifestyle improvements. The others are impervious and genetic. The former mostly entails avoiding modifiable deleterious risk factors such as tobacco use, alcohol intake, and betel quid (57). Furthermore, maintaining a balanced, healthy diet that contains fresh vegetables and fruits, good mouth hygiene practices, avoiding direct exposure to the sun, and regular exercise are all advisable practices (74, 75). The mechanism by which dietary regimens mitigate the risk of oral cancers is believed to be by affecting metabolic genes and detoxifying enzymes (74). Some studies reported that tea (Camellia sinensis) comprises components able to inhibit oral cavity carcinogenesis via a mechanism involving the reduction of cell proliferation and induction of cell apoptosis (76, 77). Another pivotal preventive measure for oral cancers is performing screening tests such as VEL scope, Orascoptic DK, Micolux/DL, VizilitePlus, Vizilite, and OralCDx. These screening tools assist in the prediction and early detection of oral cancers (78). The utilization of efficient biomarkers is a crucial element in the early detection of oral malignancies. These encompass processes, substances, or structures quantified in products or the body that can forecast or affect the occurrence of an event. Biomarkers associated with oral cancer encompass salivary indicators for OSCC diagnosis, including sphinganine, S-carboxy-methyl-L-cysteine, phytosphingosine, and L-phenylalanine. Furthermore, at the genetic level, CD34 expression serves as a marker for recurrence detection, whereas the genomic markers ITGB4 (Integrin Subunit Beta 4) and ITGA3 (Integrin Subunit Alpha 3) are regarded as drivers of distant metastasis and prognosis (57). In Europe, a multicenter study conducted by Hashibe et al. revealed a positive relationship between the allele ADH1B*2 and alcohol drinkers, as it highlighted a protective effect of this allele in drinkers in comparison to the allele ADH1B*1/*1 (79). Figure 1 summarizes the risk factors associated with OSCC.
5 Molecular mechanisms underlying OSCC
OSCC can occur through many molecular mechanisms, such as genetic mutations in oncogenes and tumor suppressor genes, which will lead to uncontrolled cell proliferation and evasion of apoptosis, epigenetic modifications, which play an important role in the regulation of gene expression in OSCC, thus lead to the silencing of tumor suppressor genes and activation of oncogenes. Chronic Inflammation also contributes to the development and progression of OSCC. Inflammatory cytokines and growth factors promote tumor growth and angiogenesis. Epithelial-mesenchymal transition is aided by various signaling pathways and transcription factors, considered a key step in cancer metastasis. Angiogenesis, as OSCC cells secrete angiogenic factors that stimulate the growth of blood vessels, provides the tumor with nutrients and oxygen. Immune Evasion as OSCC cells can evade the immune system through various mechanisms, which allows the tumor to escape immune surveillance and continue to grow unnoticed. Metabolic Reprogramming, where Cancer cells often undergo metabolic changes to support their rapid proliferation, can be targeted for therapeutic interventions. Microenvironment interactions, as crosstalk between cancer cells and the microenvironment, can promote tumor growth, invasion, and metastasis. Understanding these complex molecular mechanisms is essential for developing targeted therapies and improving the prognosis for patients with OSCC (80–84). Figure 2 summarizes the main molecular pathways implicated in the initiation and progression of OSCC.
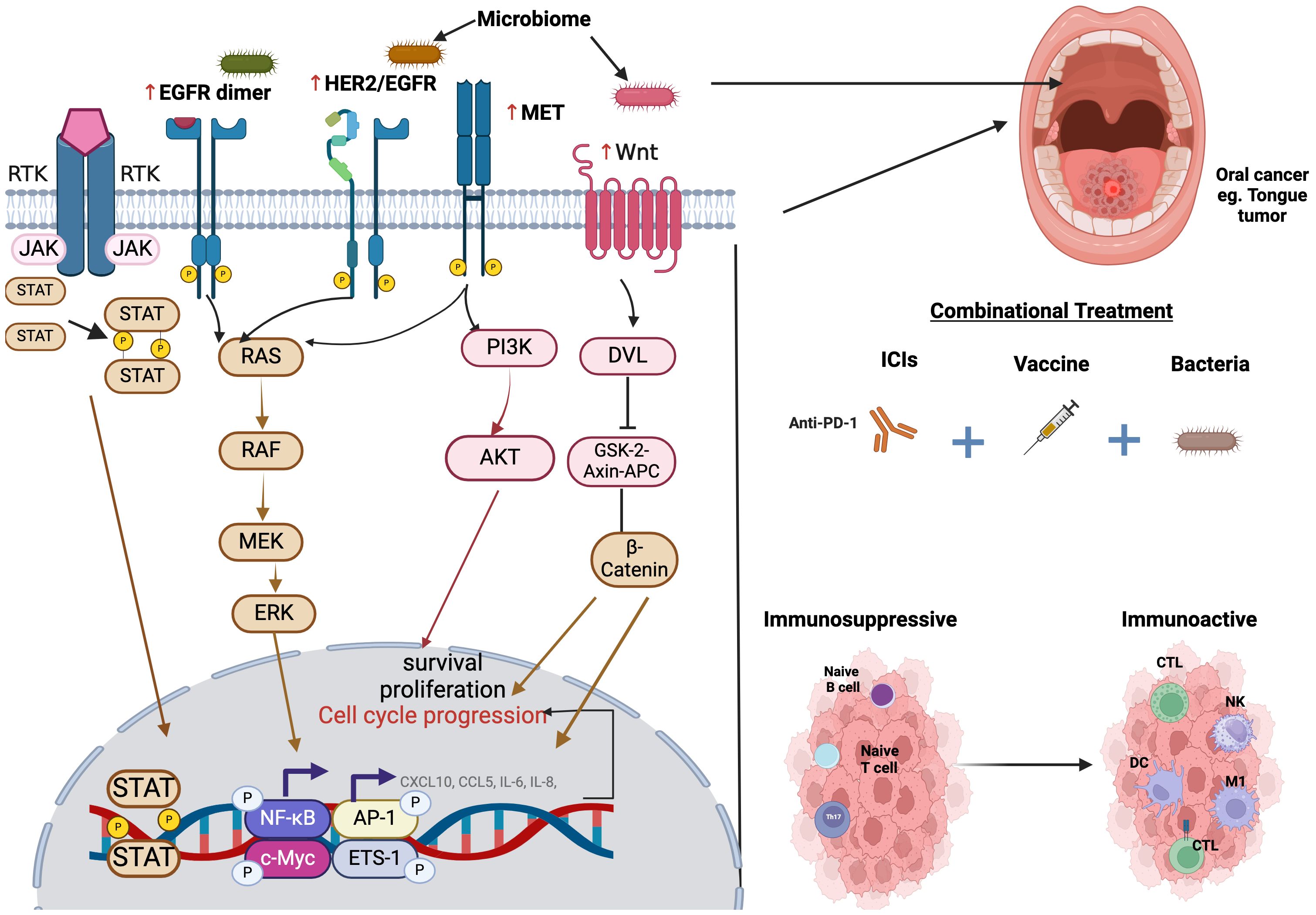
Figure 2. Illustration of the molecular pathways implicated in cancer initiation and progression. The microbiome and other signals influence receptor tyrosine kinase (RTK) signaling, leading to the activation of EGFR, HER2/EGFR, and MET, which in turn trigger downstream pathways such as JAK-STAT, RAS-RAF-MEK-ERK, PI3-K-Akt, and Wnt/β-catenin signaling. These pathways promote cell survival, proliferation, and cell cycle progression, contributing to tumorigenesis. Additionally, a combinational treatment approach incorporating beneficial bacteria, immune checkpoint inhibitors (such as anti-PD-1), and cancer vaccines may help transform an immunosuppressive or “cold” tumor into an immune-active or “hot” tumor. This transformation is characterized by enhanced infiltration of cytotoxic T lymphocytes (CTLs), natural killer (NK) cells, dendritic cells (DCs), and M1 macrophages, ultimately fostering a more effective anti-tumor immune response.
5.1 G-Protein coupled receptor-related pathways: Wnt/B-catenin-EGFR pathway
5.1.1 Wingless/integrated and beta-catenin signaling pathway
The Wnt/β-catenin pathway is a critical signaling pathway that plays a role in various physiological processes, including embryogenesis and tissue regeneration. This pathway is tightly regulated in the human body, as its dysregulation leads to cancer (85). The Wnt pathway diverges into canonical (β-catenin dependent) and non-canonical (β-catenin independent) (86). In the absence of the Wnt signal, the scaffolding protein disheveled (DVL) is not recruited to the Frizzled receptor. This allows the destruction complex [Axin (Axis inhibition protein), GSK-3 (Glycogen synthase kinase 3), and APC (Adenomatous Polyposis Coli)] to sequester β-catenin in the cytoplasm and target it for degradation (86). Conversely, in the presence of the Wnt signal, disheveled is activated by Frizzled receptor (FZD) and co-receptor LRP5/6. Consequently, the destruction complex is dissociated, and β-catenin will be translocated to the nucleus, interacting with TCF/LEF to open genes involved in cell cycle progression and proliferation (86). Many studies highlighted the role of the Wnt pathway in OSCC progression, invasion, and migration, which is mediated through aberrant regulation of β-catenin (87–89). Wnt1 signaling activates the canonical pathway, inhibits apoptosis, and promotes cell invasion in OSCC (90). Li et al. demonstrated that OSCC progression, invasion, and migration are potentially initiated by Wnt7B (non-canonical) through Wnt/β-catenin activation, and its abrogation reverses these effects (91). Another Wnt subtype, Wnt 7A, is stimulated by EGF through Akt, promoting the Wnt7A/β-catenin pathway to enhance Matrix metalloproteinase-9 (MMP9) expression. These events stimulate cell invasion in OSCC (92). In HNSCC, Huang et al. suggested that Wnt7A is associated with cancer progression and survival independently of β-catenin, instead activating FZD7/JAK1 (Janus Kinase 1)/STAT3 (Signal Transducer and Activator of Transcription 3) axis (93). Collectively, these studies provide evidence of potential prognostic markers and a therapeutic target for precision medicine; however, further studies are needed to elucidate the molecular pathways through which Wnt7A and Wnt7B exert their effects. A study investigating the role of CDK5RAP2 (Cyclin-Dependent Kinase 5 Regulatory Subunit Associated Protein 2) in OSCC demonstrated that Wnt/β-catenin stimulates OSCC progression and cell pluripotency through CDK5RAP2 (94). This study highlights a stemness-associated prognostic factor and provides insight into the regulation of pluripotent cell populations in OSCC. Independent of Wnt/β-catenin activation and E-cadherin downregulation, Wnt5A, a non-canonical activator, enhanced OSCC progression. In this study, Prgomet et al. identified Wnt5A as a potential biomarker for the progression of OSCC from dysplasia to oral cancer (95). A pioneering study examined the link between β-catenin expression and immune cell infiltration in OSCC and showed that accumulated nuclear β-catenin is associated with a reduction in CD8+ Tumor-infiltrating lymphocytes (TIL) in the tumor microenvironment (TME), particularly in the stroma and tumor (96). Furthermore, β-catenin expression correlated with low PD-L1 tumor expression, indicating immune evasion and fostering an immunosuppressive TME (96). The results of this study warrant further investigation, underscoring a pivotal pathway through which cancer can be targeted and eradicated. Mounting evidence emphasizes the interplay between Wnt/β-catenin, the microbiome, and cancer (97–99). In OSCC, an in vivo study demonstrated that Fusobacterium nucleatum (F. nucleatum), through the CDH1/β-catenin pathway, enhanced OSCC proliferation (100). Another study revealed a sophisticated interplay between host oral microbiota and imbalanced gene expression in OSCC development. This multi-omics study identified 20 perturbed CpG methylated promoter regions in host genes that were associated with an increased abundance of harmful bacteria in tumors. Furthermore, in vitro work in the same study uncovered the mechanism by which F. nucleatum induced tumor dissemination. This occurred through upregulating the SNAI2 (Snail Family Transcriptional Repressor 2) gene, which resulted in crosstalk between the E-cadherin/β-catenin pathway, tumor necrosis factor (TNF)-α/NF-кB signaling, and extracellular remodeling, leading to EMT (101). Zhou et al. demonstrated that intra-tumoral colonization with pathogenic bacteria, such as Streptococcus mutans, enhances the production of onco-metabolites like kynurenic acid and IL-1β that lead to T cell exhaustion in TME and undermine the effectiveness of immune checkpoint blockade (anti-PD-L1) and IL-1β inhibitors (102). Consequently, modulating the oral microbiota, targeting its metabolites, and unraveling the interplay between the oral microbiome, the TME, host gene expression regulation, and underlying molecular pathways could serve as a prophylactic and therapeutic intervention for OSCC.
5.1.2 The EGFR pathway
The transmembrane EGFR receptor belongs to the ErbB family of RTKs, which also includes ErbB2 (HER-2/Neu), ErbB3 (HER-3), and ErbB4 (HER-4). EGFR is overexpressed in up to 90% of OSCC cases and plays a crucial role in other malignancies. The activation of EGFR is frequently observed in oncogenic pathways linked to the etiology of oral cancers, worsening survival rates, and elevating the risk of lymph node metastases. Activated EGFR initiates several signaling pathways, including the ERK and STAT3 pathways, which promote cell proliferation, tumor progression, and survival. Dysregulation of the EGFR signaling cascade has been shown to critically affect the progression and development of HNSCCs, including OSCC. Many studies highlight EGFR’s role in OSCC progression and chemoresistance, further emphasizing its oncogenic potential. Any disruption in EGFR is associated with the inhibition of cell proliferation and suppression of oncogenic signaling pathways such as PI3-K/Akt and Myc (Myelocytomatosis Oncogene) in OSCC cells (103–105). Additionally, radiation therapy resistance in OSCC is closely related to EGFR-mediated signaling pathways. Radiation can induce EGFR translocation to the nucleus, where it functions as a transcription factor, leading to radio-resistance (106). Furthermore, genetic polymorphism in the EGFR gene, such as rs2227983, predicts increased susceptibility and progression of OSCC (107). Understanding the significance of the EGFR-mediated signaling pathways in OSCC will help in devising more effective treatment methods.
5.1.3 Mesenchymal-epithelial transition pathway
The stimulation of the MET signaling response facilitates tumor development, angiogenesis, and metastasis in numerous cancers. Recent studies indicated that either MET, its ligand HGF (hepatocyte growth factor), or both were expressed in 80% of HNSCC cases. Seiwert et al. identified MET overexpression in 84% of the analyzed cases of HNSCC in their study. In contrast, the suppression of MET signaling leads to a decrease in cellular proliferation, migration, motility, and angiogenesis, underscoring its vital role in tumor growth (108).
5.1.4 MET and EGFR crosstalk
The MET signaling pathway interacts with other essential signaling pathways. EGFR and MET function as upstream regulators of the MAPK (RAS/RAF/MEK/ERK) and PI3-K/Akt/mTOR signaling pathways. Multiple systems have documented functional interactions between MET and other receptors, including EGFR, ERBB2, and IGF1R (Insulin-Like Growth Factor 1 Receptor). This interaction is regarded as a pivotal mechanism that initiates cancer growth and confers resistance to therapy. A previous study by Stabile et al. that integrated the EGFR inhibitor erlotinib with the MET inhibitor PF04217903 in a c-SRC (Cellular Sarcoma Kinase) expressing cell line demonstrated a significant reduction in cell viability and tumor volume. The findings indicate that resistance to anti-EGFR therapy, including monoclonal antibodies, may arise from the functional interaction between EGFR and MET in the cells of HNSCC patients. Consequently, there is a growing interest in formulating novel techniques that facilitate the concurrent use of MET and EGFR inhibitors, particularly in instances when MET serves as a targetable co-driver, such as in EGFR-mutant cancers. The implementation of this combinatorial approach may improve therapeutic efficacy and diminish resistance mechanisms (109).
5.1.5 RAS/RAF/MAPK pathway
The RAS/RAF/MAPK pathway is pivotal in the pathophysiology of OSCC since it is active in over 50% of human oral cancer cases, indicating its substantial influence on the pathogenesis and progression of OSCC (110). The RAS/RAF/MAPK pathway is initiated by the binding of growth factors, such as EGF (epidermal growth factor), to RTKs, leading to the activation of RAS proteins. This activation subsequently recruits and phosphorylates RAF proteins, which activate MEK (Mitogen-Activated Protein Kinase Kinase) and ERK (Extracellular Signal-Regulated Kinase), ultimately regulating various cellular processes, including survival, proliferation, and migration (111). The RAS/RAF/MAPK pathway interacts intricately with other signaling cascades in OSCC. The PI3-K/Akt/mTORC1 (mechanistic Target of Rapamycin Complex 1) pathway and the RAS/RAF/MAPK pathway interact, resulting in the dysregulation seen in numerous human malignancies (112). This interaction is mediated by different feedback loops and shared components, such as the GAB docking proteins, which contribute to both RAS activation and PI3-K signaling (21). BRAF protein, a member of the RAF family, plays a significant role in OSCC progression. Unlike RAS mutations, BRAF mutations specifically initiate the MAPK signaling pathway, which in turn will promote tumor progression (113, 114). This specificity makes BRAF a strategic target for OSCC therapies. Recent studies have uncovered complex regulatory mechanisms within the RAS/RAF/MAPK pathway in OSCC. For example, during the process of activation, RAF proteins can form dimers or higher-order multimers, which are crucial for signal transduction and can be targeted for OSCC treatment. Furthermore, the RAS/RAF/MAPK pathway interacts with the PI3-K/Akt/mTOR (Mammalian Target of Rapamycin) pathway, facilitated by several feedback loops and common elements, such as the GAB docking proteins, which play a role in both RAS activation and PI3-K signaling. The complex interplay of positive feedforward and negative feedback loops profoundly influences signal patterns in OSCC cells (112). Understanding the intricate mechanics of the RAS/PAF/MAPK pathway in OSCC is crucial for creating successful targeted therapeutics. Presently, extensive research is concentrated on elucidating the relationships among diverse signaling cascades and integrating therapeutic methods that target multiple pathway components to enhance therapeutic efficacy and circumvent potential resistance mechanisms in OSCC patients.
5.2 Inflammatory pathways associated with OSCC
It is predicted that about 15% of all cancers are ascribed to various inflammatory processes, and mounting evidence supports the association between OSCC and chronic inflammation (115). This section will examine the most deleterious inflammatory pathways implicated in the stages of OSCC. This will significantly aid in customizing a targeted therapy that can combat the disease with minimal compromises.
5.2.1 Nuclear factor kappa-light-chain-enhancer of activated B-cells
NF-κB is one of the chief inflammatory transcription factors, and its aberrant expression has been connected to oncogenesis (116). It is extensively expressed in various cancers, and it regulates the expression of a variety of genes in the pathways related to cell survival, inflammation, tumorigenesis, proliferation, and therapeutic resistance (117–120). NF-κB initiates its function upon activation by lipopolysaccharide (LPS), TNF-α, or IL-1 (118). Following activation, the transcription factor facilitates the expression of many cytokines, including IL-8, IL-6, and IL-1, hence predisposing the individual to inflammation. In OSCC, these transcription factors are constitutively activated, resulting in the overexpression of inflammatory genes CXCL10, CCL5, IL-6, and IL-8, which are the primary factors responsible for the inflammatory infiltrate observed in the TME (120). NF-κB has a crucial role in defining the malignant phenotype of oral cancer by promoting angiogenesis (121), invasion (122), and metastasis (123).
5.2.2 Activator protein 1
AP-1 is another important transcription factor complex involved in the regulation of cell survival and death. It consists of Jun protein homodimers, or Jun and Fos proteins heterodimers (124), and is activated by the cytokine IL-1. AP-1 regulates the expression of several genes involved in activities such as proliferation, DNA synthesis, cellular invasion, and apoptosis (115, 125, 126). The high expression and activation of this pathway were associated with drug resistance, complicated by the drug therapy regimens (125), making it an interesting target for cancer therapy (126). Upon activation by IL-1, AP-1 triggers the release of IL-8, hence facilitating cell proliferation and survival in HSNCC. Another study indicated that AP-1 may facilitate the activation of Bcl-2 expression. This is implicated in the resistance to chemoradiation and the inhibition of apoptosis in recurring oral tumors exhibiting radio- and chemo-resistance (127). This indicates that targeting IL-1, which promotes the AP-1 pathway, may overcome the chemoradiation resistance observed in OSCC.
5.2.3 Cytokine receptors JAK/STAT
The Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway is a key signaling mechanism cytokine receptors use to transmit signals from the cell surface to the nucleus (128). This pathway is critical for cellular growth, differentiation, and apoptosis (129). Aberrant activation of this pathway, particularly STAT3, has been implicated in different oncogenic processes, including cell proliferation, survival, angiogenesis, invasion, metastasis, and immune evasion (130). Concerning cell proliferation and survival in OSCC, the binding of IL-6 to its receptor activates the JAK2/STAT3 signaling pathway, and elevated levels of IL-6 in OSCC tissues are positively associated with the expression of SRY-box transcription factor 4 (Sox4), both of which contribute to OSCC proliferation and survival (131). In OSCC, hyperactivation of STAT3 leads to increased expression of pro-survival and proliferative genes such as cyclin D1 and Bcl-xL. Cyclin D1 promotes cell cycle progression (132), while Bcl-xL is anti-apoptotic; its overexpression raises the threshold for apoptosis, allowing tumor cells to resist various physiological death signals (133). Moreover, Cyclin D1, in conjunction with cyclin-dependent kinases (CDKs) such as CDK4 and CDK6, endorses cell cycle progression by phosphorylating the retinoblastoma protein (pRb), so attenuating transcriptional repression of E2F target genes necessary for DNA synthesis (134, 135). Furthermore, STAT3 wields its pro-proliferative effects by suppressing the transcription of CDK inhibitors such as p21 and p27, which are essential negative regulators of cell cycle progression (136). The downregulation of these inhibitors confiscates critical cell cycle checkpoints, compounding cell proliferation and the likelihood of mutation accumulation (137). For angiogenesis, upon activation, STAT3 binds to the VEGF (Vascular endothelial growth factor) promoter, the most potent pro-angiogenic factor, which results in VEGF production, which results in increased microvessel density (MVD) within tumors by stimulating endothelial cell proliferation, migration, and new blood vessel formation (138). Elevated VEGF levels show a relationship with aggressive OSCC phenotypes and poor prognosis due to enhanced tumor vascularization and metastasis (139, 140). Additionally, the STAT3-VEGF axis was tested by pre-clinical studies, indicating that inhibition of STAT3 downregulates VEGF expression and suppresses angiogenesis in xenograft models (141–143).
The stimulation of the JAK/STAT3 pathway enhances the invasiveness and metastatic potential of tumor cells by: a) The activation of STAT3 in OSCC cells results in elevated expression of MMP-9 (matrix metalloproteinase-9), a crucial enzyme that destroys the extracellular matrix (ECM), hence promoting cancer cell invasion and metastasis. IL-22 and IL-6 facilitate OSCC cell migration and invasion through the activation of the JAK/STAT3/MMP-9 signaling pathway (137, 144); and b) EMT is a pivotal biological process facilitating OSCC metastasis. STAT3 facilitates EMT in OSCC cells by downregulating E-cadherin, wherein the active JAK2/STAT3 signaling pathway diminishes E-cadherin expression, hence decreasing cell-cell adhesion and fostering a more migratory phenotype. The activation of STAT3 boosts the expression of mesenchymal markers, including N-cadherin and E-box binding zinc finger protein 2 (ZEB2). This alteration in cadherin expression is a characteristic of EMT and increases cell motility (137, 145).
Upon activation, STAT3 signaling modulates immune responses by several mechanisms, which are: a) Modulation of myeloid-derived suppressor cells (MDSCs). MDSCs are immunosuppressive cells that hinder T-cell activation and foster tumor growth; the hyperactivation of STAT3 heightens the recruitment and expansion of MDSCs within the TME. What’s more, STAT3 upregulates chemokines like CXCL1 (C-X-C Motif Chemokine Ligand 1) and attracts MDSCs to tumor sites. Hence, inhibition of STAT3 has been shown to reduce MDSC populations, thereby enhancing antitumor immunity (137, 142, 146); b) Polarization of tumor-associated macrophages (TAMs). TAMs play a decisive role in cancer progression, they can be polarized into M1 (anti-tumor) or M2 (pro-tumor) phenotypes, with M2 TAMs dominating in the TME (147, 148). STAT3 activation enhances the expression of CD39 and CD73 on monocytes, driving the conversion of extracellular ATP into adenosine, and this inaugurates an immunosuppressive microenvironment that endorses the differentiation of monocytes into MDSCs through the CD39/CD73-adenosine signaling pathway (149). In OSCC tissues, phosphorylated STAT3 (p-STAT3) is positively concurrent with markers of MDSCs, such as CD33 and CD14; high p-STAT3 levels are associated with increased MDSC accumulation and enhanced immunosuppressive activity in the TME (146); c) Suppression of Dendritic Cell (DC) Function: STAT3 hyperactivation disrupts DC maturation, weakening their ability to present antigens and activate T cells, while immature DCs accumulate in the TME due to STAT3 signaling and immunosuppressive factors, including regulatory T-cells, tumor-associated macrophages, and hypoxic conditions. These factors cooperatively preserve DCs in an immature state, further aggravating immune suppression in TME (146, 150); and d) Downregulation of Cytotoxic T-cell Responses: STAT3 activation plays a fundamental role in suppressing cytotoxic CD8+ T-cell responses, empowering tumor immune evasion, this ensues through the induction of immunosuppressive cytokines such as IL-10 and TGF-β, which create an inhibitory TME that impairs T-cell activity (151). As mentioned earlier, STAT3 also promotes the expression of survival genes like Bcl-xL and cyclin D1, further protecting OSCC cells from immune-mediated destruction (132). Additionally, STAT3 signaling fosters the recruitment of immunosuppressive cells, such as regulatory T-cells and myeloid-derived suppressor cells, while reducing immune-activating cytokines like IL-2 (151, 152). e) Upregulation of Immune Checkpoints: Immune checkpoint molecule expression is significantly upregulated by hyperactivation of STAT3, particularly PD-L1, which suppresses T-cell activity and facilitates immune evasion. In OSCC, increased PD-L1 expression is closely linked to heightened STAT3 signaling, as STAT3 directly controls PD-L1 transcription. This interaction creates an immunosuppressive TME by suppressing cytotoxic T-cell activity and facilitating tumor progression (153, 154). Collectively, these mechanisms contribute to OSCC progression and resistance to immune surveillance, highlighting STAT3 as a key therapeutic target for restoring antitumor immunity in OSCC patients.
6 Implications of the microbiota in OSCC
OSCC has a distinct microbial signature, while the oral microbiome plays a significant role in its onset. The gut microbiome, although potentially important, is not well-characterized (155). Recent studies revealed reduced microbial diversity and changes in the relative abundance of certain bacterial taxa in OSCC tissues compared to healthy ones. This dysbiosis in the oral environment may contribute to the etiopathogenesis of OSCC (156). The involvement of oral microbiota in OSCC pathogenesis is driven by interconnected mechanisms, including chronic inflammation, direct cellular effects, metabolite production, and modulation of key signaling pathways.
6.1 Chronic inflammation
Chronic inflammation is a recognized pathway contributing to OSCC drive (157). It is initiated by the persistence of microbial dysbiosis, which activates the recruitment of pro-inflammatory cytokines and immune cells (158). The relationship between chronic inflammation and dysbiosis appears to be bidirectional: persistent infections that manifest as chronic inflammation can perturb the oral microbiota, leading to dysbiosis. Conversely, dysbiosis, characterized by a shift in the bacterial population toward more pro-inflammatory species, can itself trigger inflammation (159). For example, TNF-α, IL-1, IL-6, and IL-8 have shown potential as diagnostic and prognostic biomarkers for OSCC (115). Among the bacteria linked with inflammation are Porphyromonas gingivalis (P. gingivalis) and F. nucleatum, both of which are known to induce the production of pro-inflammatory cytokines such as TNF-α, IL-1, IL-6, IL-8, as well as activation of NF-κB and NLRP3 pathways (160–162). Therefore, mitigating and controlling inflammation, as well as stabilizing the microbiome landscape, is key to reducing the risk of OSCC initiation from these causes.
6.2 Direct cellular effects
A major direct cellular effect of the oral microbiome in OSCC is the stimulation of cell proliferation and invasion. Hebshi et al. identified the F. nucleatum subspecies polymorphum as the most significantly overrepresented species in OSCC tumors (163). This bacterium promotes cellular invasion and is particularly associated with OSCC cases in non-smokers and non-drinkers. Thus, it highlights its role in carcinogenesis independent of traditional risk factors (164). F. nucleatum can adhere to and invade epithelial and endothelial cells, disrupting β-catenin signaling, which leads to increased oncogene expression and enhanced cancer cell proliferation (165, 166). The oral microbiome also contributes to OSCC metastasis by modulating local immune responses and facilitating cancer cell metastasis to distant sites. For instance, P. gingivalis, a common oral bacterium, enhances the invasive potential of OSCC cells by activating MMPs and inducing EMT (162). In line with this, F. nucleatum induces oral cancer metastasis to the lungs through activation of intracellular autophagic mechanisms. Additionally, ECM remodeling was observed. F. nucleatum enhances metastasis via the production of outer membrane vesicles (OMVs), which are key drivers of N-cadherin upregulation and E-cadherin downregulation, thereby promoting metastatic progression (167). Oral bacteria are linked to resistance to chemotherapy and radiotherapy in OSCC treatment. F. nucleatum, for example, induces autophagy in OSCC cells, protecting them from the cytotoxic effects of chemotherapeutic drugs. This bacteria-driven chemoresistance can lead to treatment failure and poor outcomes for OSCC patients (166, 168). A study conducted by Liu et al. demonstrated that F. nucleatum altered autophagy in oral cancer by inducing autophagosome formation through the upregulation of LC3 and ATG7 gene expression. Notably, knockdown of ATG7 reversed the effect of F. nucleatum, rendering the cancer cells more sensitive to chemotherapy (169). In addition to the aforementioned bacteria, a plethora of other microbial species have been implicated in dysbiosis and the initiation and progression of oral cancer; however, a detailed discussion of these is beyond the scope of this review.
6.3 Metabolite production
The role of microbiome metabolite production in OSCC is an emerging area of research (170). Studies have shown that oral microbiota from OSCC patients, when exposed to ethanol, can produce carcinogenic levels of acetaldehyde, with higher levels observed in smokers (171). Through metabolomic analysis of saliva from Japanese OSCC patients, Oshima et al. identified potential biomarkers such as choline, branched-chain amino acids, urea, and 3-hydroxybutyric acid (172). This establishes an association between these metabolites and OSCC; however, direct evidence of their oncogenic potential remains limited, and further studies are required to validate their mechanistic role in tumor progression.
Similarly, research on South American OSCC patients revealed alterations in metabolic pathways such as the malate-aspartate shuttle, beta-alanine metabolism, and Warburg effect. Potential salivary biomarkers from this study include malic acid, maltose, protocatechuic acid, lactose, 2-ketoadipic acid, and catechol (173). The variations observed in metabolite profiles across these populations likely reflect underlying differences in genetic makeup, environmental exposures, diet, and microbiome composition, highlighting the importance of region-specific biomarker validation. Collectively, these findings expand our understanding of OSCC pathophysiology and underscore the promise of salivary metabolomics in early diagnosis, though further studies are needed to establish causal links and clinical utility.
6.4 Modulation of key signaling pathways
The oral microbiome influences key signaling pathways, contributing to the initiation and progression of OSCC. One such pathway is the E-cadherin/β-catenin signaling pathway, which is notably affected by F. Nucleatum. Activation of this pathway by F. Nucleatum triggers the EMT, enhancing oncogene expression and promoting cancer cell proliferation (101, 174). Moreover, microbial metabolites activate the PI3-K/Akt pathway, increasing cancer cell survival and resistance to apoptosis. The pro-inflammatory cytokine TNF-α further drives EMT by upregulating MMP-2 and MMP-9, processes regulated by NF-κB, Akt, and PI3-K signaling pathways. This results in enhanced invasion and metastasis of OSCC cells (115).
Another critical pathway disrupted in OSCC is the JAK/STAT pathway, with P. gingivalis playing a significant role by activating STAT3. This activation has several key implications (137, 175, 176). STAT3 activity inhibits immunological responses, allowing P. gingivalis and tumor cells to avoid detection and elimination. It also increases the transcription of genes involved in cell proliferation and survival, accelerating tumor growth. Furthermore, STAT3 activation promotes the development of new blood vessels, encouraging tumor growth and metastasis. Both F. nucleatum and P. gingivalis are highly enriched in OSCC and may synergize with genetic and environmental factors to drive tumorigenesis. Understanding these mechanisms provides valuable insights for early detection, prognostic evaluation, and the development of targeted therapies for OSCC.
7 Emerging therapeutic strategies in OSCC
OSCC is an aggressive neoplasm, and traditional therapeutic modalities, such as surgery, chemotherapy, and radiation therapy, whether used alone or in combination, provide considerable challenges and notable side effects in practical applications (177). Contemporary therapeutic approaches involve using anticancer agents such as 5-fluorouracil, paclitaxel, cisplatin, and docetaxel monotherapies or synergistic combinations. Nonetheless, their toxicity to normal cells is significant when administered intravenously, as they exhibit non-specific distribution throughout the body, leading to considerable harm to healthy tissues and resulting in severe adverse reactions. The limitations of oral chemotherapy are further underscored by the low solubility, permeability, and inadequate bioavailability of these anticancer agents in physiological fluids (177). Consequently, advancing novel therapeutic regimens or refining existing methodologies is paramount for enhancing human health and ensuring survival in the face of oral cancer and its associated tissues. The dysfunction of OSCC pathways is crucial because it enables excessive cell growth, longevity, and metastasis (178). The management of OSCC also incorporates EGFR monoclonal antibodies and tyrosine kinase inhibitors (TKIs) (179). For recurrent and metastatic OSCC, cetuximab, a monoclonal antibody against the extracellular domain of EGFR, is given with chemotherapy and radiation (180). By halting tumor cell growth and spread, cetuximab helps in the deactivation of EGFR while stimulating the internalization and degradation of the receptor. Research shows that cetuximab administration improves outcomes during chemoradiotherapy, proven to be beneficial for progression-free survival (PFS) and overall survival (OS) among patients suffering from OSCC (181, 182). Noting the challenges associated with cetuximab resistance, which is frequently associated with mutations such as EGFRvIII, there is a considerable need for better patient stratification along different lines of treatment (183). Compared to cetuximab, nimotuzumab is an EGFR-targeting monoclonal antibody that has lower immunogenicity and thus has fewer side effects, especially skin toxicity (184). Using chemotherapy and radiotherapy along with nimotuzumab is effective in advanced OSCC, thus supporting its use as an alternative treatment (185). TKIs mark a milestone in the development of targeted cancer therapies as they reduce the damage expected with non-targeted approaches, especially the collateral injury inflicted on healthy tissues (186). TKIs like gefitinib, erlotinib, and afatinib block the downstream signaling required for tumor proliferation by binding to the intracellular domain of EGFR. Gefitinib was the first active EGFR-targeting-TKI and has shown moderate response rates during OSCC monotherapy, ranging from 1.4% to 10.6% (187). Despite these figures, gefitinib appears to have better clinical outcomes when combined with chemotherapy, achieving a median PFS of 4.8 months and an OS of 9.5 months (188). The response rates and survival with erlotinib combined with platinum and radiation therapy exceeded those of each therapy given alone (189, 190). Afatinib is a second-generation irreversible EGFR inhibitor aimed at both the wild-type and mutant EGFR, including the EGFRvIII mutation. It has shown comparable anti-tumor activity as cetuximab in phase II trials (191). Considering these results, afatinib may be an effective treatment option for EGFR-driven OSCC, particularly for non-responders to prior EGFR-directed therapies (179). The rapid increase in tumor cell population is associated with the development of new blood vessels that are critical sources of nutrients, oxygen, and growth factors needed for tumor maintenance and proliferation of tumor cells (192, 193). Angiogenesis is regulated by the VEGF pathway, which is crucial in OSCC. Monoclonal antibodies, particularly bevacizumab (194), along with multi-kinase inhibitors like sorafenib and vandetanib (195), target and block VEGF receptors, thus reducing angiogenesis and tumor growth. Bevacizumab, a humanized monoclonal antibody targeting VEGF-A, has demonstrated efficacy in many malignancies, including colorectal and non-small cell lung cancer. In OSCC, the combination of cetuximab, cisplatin, and radiation with Bevacizumab has enhanced outcomes and elevated progression-free and OS rates (196, 197). Nonetheless, particularly in combinatorial therapies, the danger of hemorrhage persists as a significant issue (198, 199). Sorafenib, a multi-kinase inhibitor, targets many receptors, including VEGFR1-3, PDGFR-β, and c-Kit, which facilitate tumor cell proliferation and angiogenesis (200). OSCC tumor cells demonstrate increased susceptibility to chemotherapy and radiation when administered sorafenib, suggesting its potential use in combination therapy (200, 201). Nonetheless, its application requires validation through direct efficacy assessments on OSCC in further clinical investigations (201).
Vandetanib is an inhibitor of the recently discovered tyrosine kinases, VEGFR-2 and EGFR, that demonstrated efficacy in preclinical investigations of OSCC. Vandetanib, in conjunction with cisplatin and radiation, has markedly diminished tumor burden, metastases, and microvessel density, indicating its potential application as both a chemopreventive and therapeutic drug for OSCC (202, 203). The survival, proliferation, and chemotherapy resistance of OSCC tumors are exacerbated by the often-disturbed PI3-K/Akt/mTOR signaling pathway. Preclinical and clinical research have identified a viable candidate in mTOR suppressor drugs, such as temsirolimus, which have demonstrated efficacy in anti-tumor preclinical models. Everolimus, a rapamycin derivative, has demonstrated efficacy when utilized in conjunction with other targeted medicines by augmenting radiosensitivity and tumor suppression (204). The observed clinical benefits were not significant, with certain trials indicating only moderate enhancements in PFS and OS (205). The therapeutic combination of mTOR inhibitors and EGFR inhibitors appears to be superior; nonetheless, the emergence of significant toxicities complicates patient management, necessitating heightened attention to detail. Mutations in NOTCH1 that result in aberrant Notch signaling are crucial in the carcinogenesis and development of OSCC. Research is underway on gamma-secretase inhibitors to evaluate their potential in inhibiting the Notch signaling system. This strategy may prove advantageous in OSCC tumors marked by dysregulated Notch activity, hence providing viable alternatives for refractory OSCC instances that resist other treatment modalities (179).
The effective management and treatment of OSCC rely on several essential components, including CDKs and their function in regulating the cell cycle and cellular transcription (206). The overexpression of these kinases, particularly PCNA (proliferating cell nuclear antigen) and cyclin B1, correlates with increased OSCC metastasis and poor prognoses (207). Flavopiridol, ribociclib, and palbociclib are investigational drugs for cancer treatment due to their roles as CDK inhibitors. Although flavopiridol has demonstrated potential in preclinical investigations, its clinical use is significantly restricted due to side effects. Other CDK inhibitors, like palbociclib and ribociclib, have yielded inconsistent clinical outcomes in OSCC patients despite their established efficacy in advanced breast cancer (208). To date, few effective CDK inhibitors have been produced due to the highly homologous ATP-binding clefts on the CDK surface, complicating the targeting of a specific type. The creation of isoform-specific inhibitors is crucial (209).
Different approaches are also being explored. One therapeutic approach utilizes cyclooxygenase-2 (COX-2) inhibitors and endostatin (210). COX-2 exerts an oncogenic influence predominantly through the secretion of its pro-inflammatory mediators (211). COX-2 overexpression is frequently observed in several malignancies, especially in OSCC, and it significantly contributes to tumor proliferation, immune evasion, and angiogenesis. Celecoxib, a selective COX-2 inhibitor, has demonstrated efficacy in inhibiting the invasion, metastasis, and migration of OSCC cells. The concomitant administration of celecoxib and EGFR inhibitors, such as cetuximab, has demonstrated enhanced therapeutic efficacy due to the synergistic inhibition of COX-2 and EGFR (212–214), representing a significant treatment strategy for OSCC (179). Endostatin, an angiogenesis inhibitor, has demonstrated anti-tumor effects by causing apoptosis in endothelial cells and inhibiting blood vessel development. Endostatin has demonstrated the potential to diminish lymph node metastases and may serve as a viable adjunctive therapy for OSCC in conjunction with chemotherapy for refractory or treatment-resistant tumors (215, 216).
Systemic toxicity commonly accompanies traditional treatments like chemotherapy, and radiotherapy can cause significant side effects, including damage to healthy tissues. Limited therapeutic effect as some patients may not respond adequately to conventional treatments, leading to persistent or recurrent disease. Drug resistance is where cancer cells can develop resistance to chemotherapy drugs, reducing their effectiveness over time. Treatments can affect a patient’s quality of life and lead to complications such as dry mouth, inflammation of the mucous membranes, and restricted jaw movement. High-cost advanced treatments like targeted therapy and immunotherapy can be expensive for many patients. A delayed diagnosis may lead to poorer outcomes for patients diagnosed at later stages. Despite significant efforts in advancing immunotherapy for OSCC, the persistently low 5-year survival rate remains a major concern. This is largely due to the urgent need to better understand the genetic landscape (specifically identifying tumor-specific antigens) through the integration of sequencing technologies and bioinformatics, improve stratification scores, and gain a comprehensive insight into tumor-tumor, tumor-stromal, and microbiota interactions within the TME. Such knowledge is essential for developing personalized treatments that could improve overall survival and quality of life for patients. Furthermore, combination therapies are emerging as a promising approach to enhance survival and address the challenges in treating this stubborn disease. These limitations highlight the need for ongoing research and development of more effective, less toxic, and accessible treatment options for OSCC (10, 217, 218).
7.1 Potential therapeutic targets based on epigenetic modifications
Epigenetic modifications are critical in the development of OSCC and display potential targets for therapy and biomarkers. DNA methylation inhibitors like 5-Azacytidine and Decitabine can reverse the hypermethylation of TSG, while histone deacetylase (HDAC) inhibitors such as Trichostatin A and Vorinostat help restore normal histone acetylation (219). On the other hand, miRNA-based therapies, including synthetic miRNA mimics and antagomiRs, show promise in reactivating tumor-suppressing miRNAs or blocking oncogenic ones (220, 221). Recent studies indicate that combination therapies, including HDAC inhibitors and cisplatin, enhance chemosensitivity in OSCC cells. Novel targets, including DOT1L (Disruptor of Telomeric Silencing 1-Like), are linked to cancer stem cell invasion and cisplatin resistance, whereas selective mTOR inhibitors in conjunction with HDAC inhibitors are under investigation (222). Salivary miRNAs, particularly miR-423-5p, have emerged as potential prognostic biomarkers, as elevated levels in preoperative saliva are associated with reduced disease-free survival in OSCC patients (220, 223).
7.2 Immunotherapy in OSCC: current progress, challenges, and future directions
A significant advancement in cancer therapy has been made by utilizing the immune system to identify and eliminate tumors. Cancer immunotherapeutic strategies encompass vaccinations, adoptive T-cell treatment, and immune checkpoint blockade therapy (224). Given the essential role that the immune system plays in the progression and management of OSCC, early strategies aimed at exploiting immunity to mitigate cancer garnered widespread attention. A groundbreaking advancement in this field was the U.S. Food and Drug Administration (FDA) approval of T-VEC (Talimogene) in 2015, an HSV-1-based oncolytic virus armed with GM-CSF (Granulocyte-Macrophage Colony-Stimulating Factor), for the treatment of melanoma (225). Since then, T-VEC has been investigated for use in breast cancer (226), pancreatic cancer, and HNSCC (227). One year later, in 2016, the FDA approved another transformative innovation, immune checkpoint inhibitors (ICI), for the treatment of metastatic and inoperable HNSCC patients. This transformative modality demonstrated robust efficacy in HNSCC patients, paving the way for an effective treatment for this heterogeneous solid tumor (228, 229). The efficacy of ICIs is limited to patients possessing particular biomarkers and is contingent upon the expression levels of immune checkpoints like PD-L1 (230, 231). Mechanistically, anti-PD-1/PD-L1 antibodies (also known as PD-1/PD-L1 inhibitors) work by restoring T-cell activity within the TME. In the KEYNOTE-048, a phase III randomized clinical trial, pembrolizumab (an anti-PD-1 monoclonal antibody) was evaluated as monotherapy and in combination with chemotherapy, compared to cetuximab with chemotherapy (230). The results demonstrated that pembrolizumab combined with chemotherapy significantly improved overall survival, surpassing cetuximab plus chemotherapy in PD-L1-positive patients with HNSCC, including those with OSCC (230). This study established that the combination of pembrolizumab with platinum and 5-fluorouracil is both safe and effective and is now considered an appropriate first-line treatment for this patient group. However, treatment responses remain largely confined to biomarker-defined subgroups, highlighting the importance of predictive markers such as tumor mutational burden (TMB). Understanding the TME, the dynamics of immune-infiltrating cells, the genetic landscape, and immune profiling is crucial for tailoring treatments for cancer patients, including those with OSCC. Numerous clinical trials investigating a wide range of immunotherapeutic treatments have been completed, with many still ongoing. Table 1 provides a comparison of different immunotherapeutic modalities, highlighting their pros and cons. Harrington et al. conducted a multicenter phase Ib clinical trial involving 36 patients with recurrent/metastatic squamous cell carcinoma of the head and neck. The trial aimed to investigate the potential of enhancing treatment with the oncolytic vaccine, T-VEC, in combination with ICI, pembrolizumab, following a low response to monotherapy with ICI treatment (ClinicalTrials.gov Identifier: NCT02626000) (232). This combination was shown to be safe compared to treatment with pembrolizumab alone. However, the trial was not advanced to phase III as initially planned (232). Mell et al. conducted a phase I clinical trial to evaluate the safety profile of GL-ONC1, a recombinant vaccinia virus that encodes three transgenes: Ruc-GFP, β-glucuronidase, and β-galactosidase. This double-stranded DNA platform is regarded as highly promising for its capacity to selectively lyse tumor cells while preserving normal tissue (233). Its short, well-defined life cycle, efficient cell-to-cell spreading, and ample use as a smallpox vaccine contribute to its high safety profile (234, 235). This Phase I study was conducted to determine the maximum tolerated dose (MTD), identify dose-limiting toxicities, and recommend a dose for a Phase II study of GL-ONC1 when administered intravenously in combination with chemoradiotherapy for primary non-metastatic HNSCC patients. GL-ONC1 was found to be safe and well-tolerated when given intravenously and demonstrated improvements in OS and PFS (233). A phase II trial is, however, warranted for this oncolytic vaccine platform (233). Nevertheless, no FDA-approved oncolytic virus has been approved exclusively for OSCC due to the disease’s complexity and the limited data from preclinical research. However, oncolytic vaccines are gaining attention, with recent research and preclinical studies in this area. In a commentary, Raja emphasized the potential of oncolytic vaccines to revolutionize OSCC treatment and improve patients’ lives in the future (236). In line with this, preclinical trials investigating the efficacy of oncolytic viruses in OSCC have been conducted. Gohara et al. demonstrated that OBP-301, in combination with radiotherapy, is a novel therapy effective in treating radioresistant OSCC (237). This vaccine, which uses an adenoviral backbone encoding the human telomerase reverse transcriptase (hTERT) promoter (238)—exclusively expressed in cancer cells—shows promise in enhancing treatment efficacy. The observed effects are attributed to the STAT3-Bcl-Xl axis, which regulates autophagy and apoptosis, thereby improving radiosensitivity (237). OBP-301 warrants further investigation in refractory OSCC patients and those resistant to standard therapies. Despite advancements in understanding the OSCC tumor immune microenvironment and the promising outcomes of various immunotherapeutic strategies, significant challenges remain in translating these findings into practical clinical applications (239). The process of selecting and evaluating the effectiveness of immunotherapy, whether used alone or in combination with other treatments, requires improved methodologies to assess synergy and minimize side effects (240). Moreover, improving long-term survival with multi-agent immunotherapy strategies requires a careful balance between efficacy and toxicity to provide lasting benefits (239). Furthermore, regulatory obstacles pose significant challenges in approving and clinical application of innovative immunotherapies, consequently hindering their broader adoption (240).
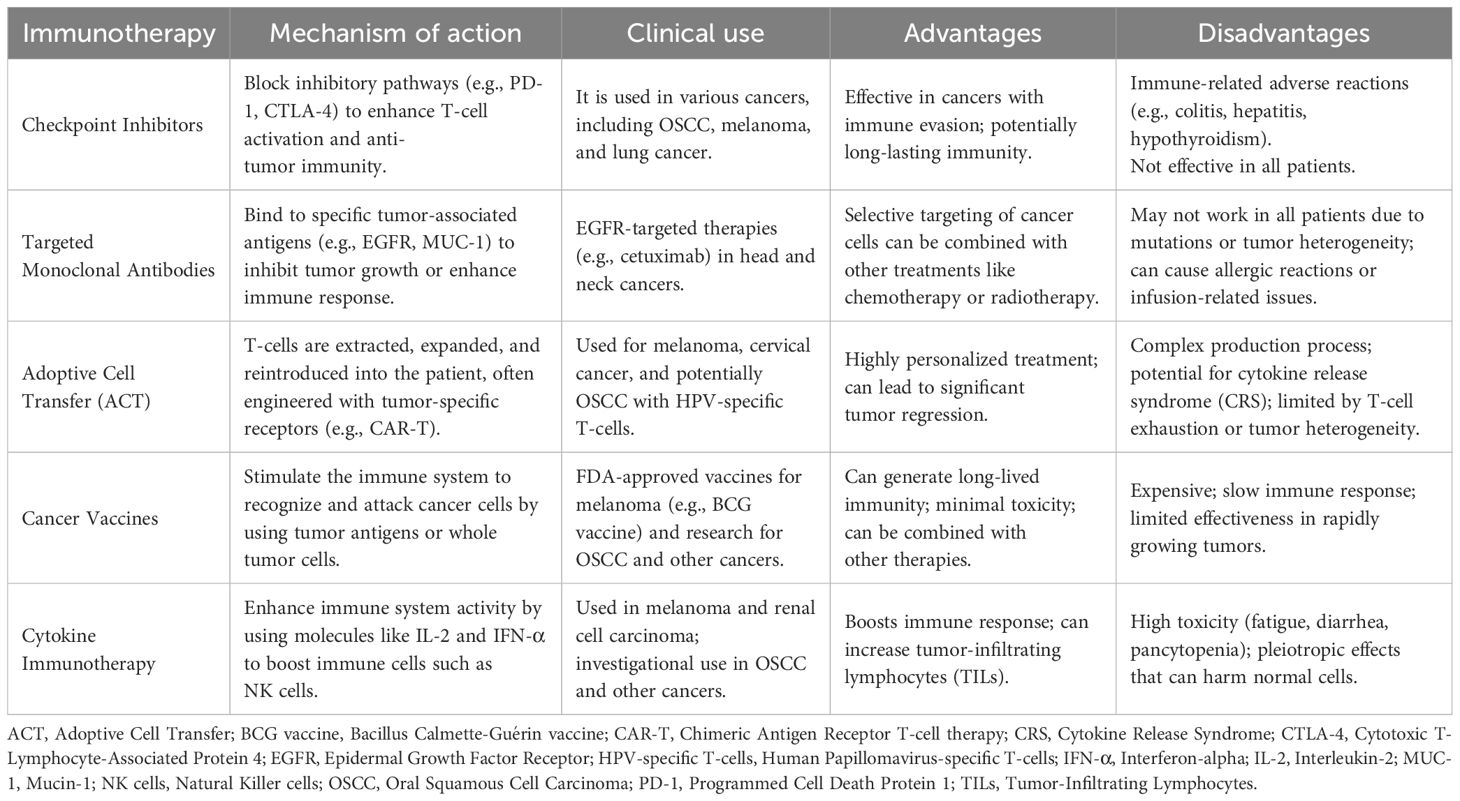
Table 1. Comparison of various immunotherapeutic modalities: mechanistic action, advantages, and disadvantages.
7.3 Potential therapeutic targets
The recent identification of several possible therapeutic targets for OSCC presents novel treatment avenues for this complex cancer. EGFR inhibition: Previous research indicated that OSCC cells frequently demonstrate an overexpression of EGFR. Consequently, inhibiting the receptors is a viable treatment alternative, as the antibodies, cetuximab, have previously received approval for use. Furthermore, it was shown that gefitinib, an EGFR TKI, markedly enhances the efficacy of cisplatin against OSCC cells. Research demonstrates that gefitinib reduces the activity of EGFR and the downstream signaling pathways, ERK and Akt, in a dose-dependent manner (241). Recent research indicated that EGFR amplification is prevalent among younger patients diagnosed with OSCC, suggesting that targeting these receptors may be advantageous for this group (242). Vascular Endothelial Growth Factor Receptor (VEGFR) Inhibition: The signaling mediated by the VEGFR is essential for the progression of OSCC. Research revealed further anti-proliferative effects in OSCC cells when the VEGFR inhibitor vatalanib was administered in conjunction with other EGFR inhibitors. The most compelling finding arose from the EGFR-knockout OSCC cells, which exhibited elevated expression of VEGFR2, suggesting a potential compensatory mechanism (105). Nanoparticle-based drug delivery: Nanoparticles have been utilized in the treatment of OSCC, demonstrating enhanced drug bioavailability and selective tumor targeting, which results in improved biocompatibility, efficacy, and reduced systemic toxicity and side effects associated with oral cancer therapy. A range of nanoparticles has been examined for their efficacy against OSCC, including:
● Polymeric nanoparticles: Polymers of both natural and synthetic sources, such as polylactic acid, polyglycolic acid, polysaccharides, polycaprolactone, and polyethylene glycol, are used to entrap or adhere to the therapeutic agent (243).
● Liposomes: Artificial vesicles made of a phospholipid bilayer capable of encapsulating therapeutic agents of both hydrophilic and hydrophobic nature to the targeted site of action. Since the frugs loaded on liposomes can be identified by reticuloendothelial systems, polyethylene glycol can be used to cover the liposomal surface (244).
● Gold nanoparticles (AuNPs): They are characterized by good biocompatibility and high permeability, thus, AuNPs have been used in many biomedical fields. AuNPs have many geometric shapes, such as nano shells, nanorods, nanosphere, and nanocages, making them effective carriers for the therapeutic agents to the target site (243).
● Hydrogel: A three-dimensional porous material that is injected directly into the site of injury, thus avoiding the need for intravenous administration of small NPs and providing the advantage of controlled release of the drug (243, 245).
Combination therapy has been shown to enhance outcomes for individuals with OSCC by integrating multiple treatment modalities. The “EXTREME” regimen, utilized as the primary treatment for recurrent HNSCC cases, integrates cetuximab with platinum and 5-FU, followed by maintenance cetuximab. Alternative combinations, including taxanes with cisplatin and cetuximab, have demonstrated considerable therapeutic efficacy (246). Recent endeavors have focused on evaluating the effectiveness of augmenting epigenetic modifiers, such as 5-aza-2’-deoxycytidine (5-AZA-2CdR), in conjunction with conventional chemotherapy drugs like cisplatin. This approach alters the methylation patterns of critical DNA repair and drug-resistance genes, potentially enhancing chemotherapy efficacy (247, 248). These targeted and combination therapeutic approaches exhibit promising advancements in OSCC treatment outcomes; nevertheless, extensive research is necessary to enhance their efficacy and reduce potentially harmful effects.
8 Personalized medicine in the treatment of OSCC
Recently emerging approaches in personalized medicine demonstrate encouraging advancements in biomarker identification and targeted therapeutics. Recent studies demonstrated the potential of minimally invasive biomarkers, including circulating tumor cells (CTCs) and molecular targets, in facilitating early diagnosis and monitoring therapy responses (10). Much research concentrated on targeting specific cellular receptors, such as EGFR. A previous study indicated that younger OSCC patients (below 50 years of age) had EGFR amplification and increased RNA levels. This discovery indicates that EGFR amplification will serve as a significant therapeutic target for this patient subgroup (249). The advancement and progression of OSCC are significantly affected by epigenetic regulation, serving as a crucial mechanism for the formulation of epigenetic-targeted therapies, including DNA methyltransferase (DNMT) inhibitors and HDAC inhibitors, which mitigate aberrant gene expression and address drug resistance (250). Personalized medicine may also integrate advanced imaging techniques, including confocal microscopy (CM) and optical coherence tomography (OCT), which assist in evaluating suspicious oral lesions and various histological stages of OSCC. These methods are non-invasive, facilitating early tumor detection and precise treatment planning (251, 252). Combined therapy targeting several pathways has also been investigated. They evaluated the possibility of EGFR and Cortactin co-expression as predictors for dual-target treatment in OSCC. The study determined that the co-expression of EGFR and Cortactin was associated with poor survival rates, suggesting the potential for dual protein targeting in advanced OSCC cases (253). Research continues to be conducted to identify reliable biomarkers and effective therapeutic combinations. We anticipate that the integration of multi-omics data with artificial intelligence will enhance the complexity of data processing, resulting in improved discovery of novel therapy targets and prediction of treatment results. Nevertheless, the consistent implementation of biomarker testing and personalized treatment remains a difficulty yet to be resolved for clinical applications.
8.1 Transformative role of personalized medicine in OSCC immunotherapy
Molecular profiling encompasses the analysis of tumors at the cellular level, employing genomic, proteomic, and metabolomic approaches. These technologies can help discover targetable immunotherapy genetic aberrations along with their associated prognostic markers. Biomarker characterization, such as the expression of PD-L1 and tumor mutational burden (TMB), may enrich the patient populations for whom immunotherapy is likely to be successful. Table 2 presents the types of biomarkers and their clinical relevance in OSCC. The administration of anti-PD-L1 antibody therapy has been crucial for the management of OSCC (283, 284). Recent studies have examined PD-L1 in specimens from patients who received immunotherapy to identify cases likely to respond to the treatment. For example, Emancipator et al. (285) determined that a “combined positive score”, defined as the ratio of PD-L1 expressing cells (both cancerous and immune) to the total number of viable cancer cells, multiplied by 100, is effective in assessing the response to pembrolizumab (285). In other investigations, PD-L1 expression was substantially linked with the response to the anti-PD-L1 antibody durvalumab (286, 287). These investigations established that a threshold of 25% of cancer cells exhibiting PD-L1 staining is considered appropriate for evaluating patients’ responses to durvalumab immunotherapy (286, 287). Some work has been conducted on the predictive significance of tumor mutational burden (TMB) in relation to immunotherapy, specifically with pembrolizumab. Cristescu et al. indicated that the conjunction of elevated TMB and T cell inflamed gene expression profiles correlates with improved survival outcomes in patients who received pembrolizumab (288). A tumor mutational burden of 10 mutations per megabase is frequently the criterion for initiating treatment with pembrolizumab, although its widespread application remains controversial. Strickler et al. consistently account for the potentially confounding influence of prior cytotoxic chemotherapy, which may elevate TMB, rather than formulating treatment recommendations solely based on this variable (289). Moreover, personalized medicine enables the development of interventions tailored to the unique characteristics of a patient’s tumor, potentially enhancing efficacy. For several cancers, including OSCC, a reliable approach for assessing tumor TILs in H&E-stained sections has been established (290). A study by Almangush et al. demonstrated the significance of assessing stromal TILs to forecast overall survival, disease-specific survival, and disease-free survival rates in patients with OSCC, particularly in early-stage oral tongue cancer cases. Such simple methods of TIL evaluation should be studied in validation studies with larger sample sizes and could improve the evaluation of the impact of immunotherapy on the patients (291). Such simple methods of TIL evaluation should be studied in validation studies with larger sample sizes and could improve evaluating the impact of immunotherapy on the patients. By understanding the molecular profile of a patient’s tumor, clinicians can design combination therapies that enhance the effectiveness of immunotherapy. For example, combining checkpoint inhibitors with other treatments like chemotherapy or radiotherapy. Personalized medicine has the potential to improve patient outcomes by providing more precise and effective treatments paving the way for more effective and individualized treatment strategies in OSCC, offering hope for better outcomes and reduced side effects (292–294).
9 Challenges in translating molecular research into clinical applications for the management of OSCC
Integrating therapeutic science into clinical care for OSCC has proven to be a complex endeavor even with the advancements made in molecular oncology. Implementing novel drugs and biomarker testing into standard clinical practice is hindered by several scientific, logistical, and patient-focused challenges that restrict the adoption of precision medicine in OSCC treatment. Significant obstacles stem from tumor heterogeneity, including genetic, epigenetic, and molecular differences within and between individuals (295, 296).
This variety impedes the establishment of common therapeutic markers and contributes to discrepancies in treatment response. For instance, some regions within a singular tumor may contain different abundances of cancer-associated fibroblasts (CAFs), immune cells, and ECM constituents, which lead to regional differences in treatment efficacy (297). Such diversity requires customized therapeutic approaches that consider specific features of individual tumors’ microenvironments rather than generic approaches (295). Moreover, the implication of the TME on OSCC growth and treatment resistance is another barrier. Composed of an intricate network of immune cells, fibroblasts, ECM building blocks, and hypoxic areas, it regulates tumor behavior through a complex interplay. The identification and validation of effective biomarkers for TME-directed therapies remain an important focus of the research. Other authors have shown markers like fibroblast activation protein (FAP) for the possible evidence of activity of CAF in OSCC that may help guide treatment aimed at altering the interactions between stromal tissue and the tumor (298). The possibility of identifying these biomarkers in patients, which is more challenging, may enhance the accuracy of treatment by enabling the monitoring and modification of treatment plans based on the changes within the TME in real-time (295). Another challenge that negatively impacts the efficacy of immune checkpoint inhibitors and EGFR inhibitors is resistance to targeted therapy. There may be intrinsic or acquired resistance because of tumor mutations, activation of compensatory survival pathways, or the existence of TME that promotes evasion of the immune response.
The latter introduces further resistance by recruiting immunosuppressive cell types, such as MDSCs, and changing cellular metabolism (295). There are efforts to propose a combination therapy directed at tumor-supporting pathways and the environment that supports these pathways. The attempt to improve therapy effectiveness already includes MDSC-targeting strategies and immune checkpoint blockade in combination with CAF-disrupting agents (299–302).
Targeted molecular therapies represent a step forward, but there are still numerous risks regarding their side effects (303). Using immune checkpoint drugs, EGFR inhibitors (304), and kinase-targeted medicines (305) can have grave consequences for side effects, which might diminish patients’ quality of life and compliance. For instance, cetuximab, an EGFR-targeted medication, has been associated with severe skin toxicity, while erlotinib, a TKI targeting EGFR, inhibits the effector’s action, which leads to the development of drug resistance, and while doing so, immune checkpoint inhibitors could bring about immunological side effects (304). Other contraindications are gastrointestinal disorders like diarrhea, the probable sequel of increased epithelial cell turnover and mucosal damage, fatigue, nausea, vomiting, and changes in electrolyte balance (306, 307). Managing these toxicities requires carefully balancing and enhancing therapeutic effectiveness with maintaining the patient’s well-being, which is especially challenging in OSCC patients who frequently suffer from significant functional and cosmetic deficits because of tumor burden and previous treatments. A lack of standardized treatment protocols that integrate the newest advances in molecular-targeted therapy is yet another issue. Cetuximab, an EGFR inhibitor, is utilized in conjunction with chemotherapy (308), radiation (309), and ICIs such as anti-PDL-1 (310). However, optimal administration methods, sequencing of treatments, and patient selection criteria remain under investigation (311). Clinical decision-making is further complex, and the integration of new treatment options into standard practice is constrained for these reasons. A holistic strategy, encompassing molecular analysis, biomarker-driven patient categorization, resistance-targeted combination therapy, and supportive care for treatment-related toxicity, is essential. The implementation of novel research into effective and customized OSCC treatment will be enhanced by establishing standardized treatment protocols grounded in developing clinical evidence.
10 Recent highlights from key studies
Despite the recent breakthroughs in immunotherapeutic treatments and their potential enhanced effects when combined with other treatment modalities or conventional therapies, traditional approaches such as surgery, radiotherapy, chemotherapy, or chemoradiotherapy remain the gold standard for patients with OSCC. Table 3 summarizes selected completed and ongoing clinical trials investigating treatment strategies in OSCC. A recent clinical trial involving patients with high-risk recurrent OSCC and extranodal extension greater than 2 mm showed improved prognosis in those who received postoperative treatment with either radiotherapy or chemoradiotherapy (320). Additionally, conventional therapies have been investigated in clinical trials in combination with immunotherapy to enhance the efficacy of immune-based treatments and improve the 5-year survival rate in early-stage, advanced, invasive, and non-metastatic oral squamous cell carcinoma (OSCC). In a two-arm, open-label phase II clinical trial (NCT05821452), Chen et al. hypothesized that combining chemotherapy with camrelizumab, an anti-PD-1 antibody, may enhance the immune response in locally advanced unresectable esophageal squamous carcinoma. Chemotherapy is proposed to prime the immune system by activating tumor antigens, thereby reducing tumor immune evasion (321). This could result in a stronger immune response, reduced side effects, and allow for lower doses and shorter treatment durations (321). Ultimately, this approach aims to shrink the tumor and enable surgical resection in cases initially deemed inoperable. The trial is expected to conclude by the end of May 2026. In line with this, a randomized two-arm phase II clinical trial by Liu et al. investigated the efficacy of combining neoadjuvant immunotherapy (camrelizumab, an anti-PD-1 inhibitor) with or without docetaxel-cisplatin-5-fluorouracil (TPF) chemotherapy in patients with resectable locally advanced OSCC (316). The study reported that the combination therapy achieved significantly improved outcomes, including a major pathological response rate of 76.4% and a 2-year event-free survival rate of 91.2%, both of which were markedly higher than those observed with camrelizumab monotherapy, which yielded survival rates of 14.7% and 52.9%, respectively (316). These results suggest that neoadjuvant immunotherapy combined with chemotherapy is both feasible and safe in patients with resectable OSCC (322). In the context of neoadjuvant therapy, a pilot clinical trial for early-stage OSCC investigated the use of imiquimod, a toll-like receptor 7 agonist. The treatment resulted in a 50 percent reduction in tumor cell count in 60 percent of patients post-treatment, along with an immune-related pathological response in the same proportion of patients (322). This tumor reduction was attributed to increased activity of functional helper T cells and cytotoxic T cells. These findings suggest that neoadjuvant immunotherapy may represent a promising strategy for managing OSCC at early stages (322). Collectively, these recent clinical studies highlight the growing potential of immunotherapy, particularly in the neoadjuvant setting and in combination with other modalities, as a promising strategy for managing OSCC. While conventional treatments remain the cornerstone of care, emerging evidence supports the integration of immunotherapeutic agents to enhance response rates, improve survival outcomes, and potentially enable surgical intervention in previously unresectable cases. Further validation through large, multicenter clinical trials is essential. Furthermore, identifying reliable predictive biomarkers is a critical step toward realizing personalized immunotherapy strategies for OS.
11 Conclusion: challenges and future directions in OSCC research
Notwithstanding considerable endeavors by researchers and clinicians, the 5-year survival rate for OSCC remains unacceptably low, chiefly because of recurrences and metastases. The EMT process is a significant factor in the metastasis of OSCC, wherein OSCC cells gradually adopt the traits and activities of mesenchymal cells (323). A thorough understanding of the predisposing factors and molecular pathways associated with the initiation, development, and advancement of OSCC is crucial for developing effective strategies to battle this disease. This can be accomplished by prioritizing specific and selective therapies aimed at modulating these pathways. The main treatment targets encompass inflammatory pathways, including JAK/STAT and NF-κB, alongside cell growth and survival pathways such as RAS/RAF/MAPK, EGFR, and PI3-K/Akt/mTOR.
Another hurdle that healthcare providers and researchers face is determining the appropriate treatment modality based on the personalized individual characteristics and the molecular manifestations of the disease. Studies of oral microbiome in OSCC prove the implication of bacterial antigens as potential confounders that can interact and become involved in various developmental, apoptotic, oncogenic, mutagenesis, angiogenesis, and cell proliferation pathways (324–326). Conversely, these studies lack standardization in study design and methodology. This heterogeneity in data collection and analysis methods poses challenges for meta-analyses and the identification of reliable biomarkers. Future directions should focus on unifying and conducting comprehensive microbiome analyses to better reveal its relation and effect on OSCC. Challenges in identifying reliable epigenetic biomarkers remain an obstacle to be solved. The interaction among many pathways in OSCC is of considerable importance. This review consolidates recent literature to create a core framework for prospective clinical applications. Moreover, investigating the functions of inflammatory proteins and commensal organisms in regulating apoptosis and cancer cell growth offers a promising direction for future research. Despite numerous unresolved inquiries, our research establishes a foundation for clinical translation and implementation. We underscore the significance of cultivating strong interaction between research and clinical practice, promoting the active application of research findings in clinical environments, and advancing the evolution of personalized medicine.
Author contributions
SS: Conceptualization, Writing – review & editing, Investigation, Visualization, Validation, Methodology, Writing – original draft. NA: Writing – original draft, Validation, Visualization, Writing – review & editing, Methodology, Conceptualization, Investigation. EY: Methodology, Conceptualization, Writing – review & editing, Validation, Investigation, Writing – original draft, Visualization. DM: Writing – review & editing, Conceptualization, Investigation, Writing – original draft, Validation, Methodology, Visualization. FT: Writing – review & editing, Supervision, Conceptualization, Project administration. LA: Conceptualization, Project administration, Writing – review & editing, Supervision. AE: Supervision, Conceptualization, Writing – review & editing, Project administration. HA: Supervision, Writing – review & editing, Project administration, Conceptualization. ME: Conceptualization, Project administration, Supervision, Writing – review & editing. AA: Writing – original draft, Investigation, Supervision, Writing – review & editing, Conceptualization, Methodology, Project administration, Funding acquisition, Resources, Validation.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was made possible with the support of the Qatar National Research Fund (Qatar Research Development and Innovation Council) [grant No. ARG01-0601-230451]. S.S. and D.E.M. are supported by Ph.D. graduate assistantships from the Office of Graduate Studies (Qatar University). N.A. is a recipient of a Graduate Sponsorship Research Award from the Qatar National Research Fund [Award No. GSRA11-L-1-0530-2408]. The statements made herein are solely the responsibility of the authors.
Acknowledgments
Figures are created in BioRender. Licenses are obtained.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Fatima J, Fatima E, Mehmood F, Ishtiaq I, Khan MA, Khurshid HMS, et al. Comprehensive analysis of oral squamous cell carcinomas: clinical, epidemiological, and histopathological insights with a focus on prognostic factors and survival time. Cureus. (2024) 16:e54394. doi: 10.7759/cureus.54394
3. Tan Y, Wang Z, Xu M, Li B, Huang Z, Qin S, et al. Oral squamous cell carcinomas: state of the field and emerging directions. Int J Oral sci. (2023) 15:44. doi: 10.1038/s41368-023-00249-w
4. Linsen SS, Gellrich N-C, and Krüskemper G. Age-and localization-dependent functional and psychosocial impairments and health related quality of life six months after OSCC therapy. Oral Oncol. (2018) 81:61–8. doi: 10.1016/j.oraloncology.2018.04.011
5. Meier J, Schuderer J, Zeman F, Klingelhöffer C, Hullmann M, Spanier G, et al. Health-related quality of life: a retrospective study on local vs. microvascular reconstruction in patients with oral cancer. BMC Oral Health. (2019) 19:1–8. doi: 10.1186/s12903-019-0760-2
6. Bushi G, Khatib MN, Singh MP, Pattanayak M, Vishwakarma T, Ballal S, et al. Prevalence of suicidal ideation, attempts and associated risk factors in oral cancer patients: a systematic review and meta-analysis. BMC Oral Health. (2025) 25:140. doi: 10.1186/s12903-025-05511-7
7. Nokovitch L, Maquet C, Crampon F, Taihi I, Roussel LM, Obongo R, et al. Oral cavity squamous cell carcinoma risk factors: state of the art. J Clin Med. (2023) 12. doi: 10.3390/jcm12093264
8. Weber ME, Yiannias JA, Hougeir FG, Kyle A, Noble BN, Landry AM, et al. Intraoral metal contact allergy as a possible risk factor for oral squamous cell carcinoma. Ann Otol Rhinol Laryngol. (2012) 121:389–94. doi: 10.1177/000348941212100605
9. Singhvi HR, Malik A, and Chaturvedi P. The role of chronic mucosal trauma in oral cancer: A review of literature. Indian J Med Paediatr Oncol. (2017) 38:44–50. doi: 10.4103/0971-5851.203510
10. Suri S, Boora GS, Kaur R, Chauhan A, Ghoshal S, and Pal A. Recent advances in minimally invasive biomarkers of OSCC: from generalized to personalized approach. Front Oral Health. (2024) 5:1426507. doi: 10.3389/froh.2024.1426507
11. Jin L, Lamster I, Greenspan J, Pitts N, Scully C, and Warnakulasuriya S. Global burden of oral diseases: emerging concepts, management and interplay with systemic health. Oral diseases. (2016) 22:609–19. doi: 10.1111/odi.12428
12. Badwelan M, Muaddi H, Ahmed A, Lee KT, and Tran SD. Oral squamous cell carcinoma and concomitant primary tumors, what do we know? A review of the literature. Curr Oncol. (2023) 30:3721–34. doi: 10.3390/curroncol30040283
13. Dong L, Xue L, Cheng W, Tang J, Ran J, and Li Y. Comprehensive survival analysis of oral squamous cell carcinoma patients undergoing initial radical surgery. BMC Oral Health. (2024) 24:919. doi: 10.1186/s12903-024-04690-z
14. Capote-Moreno A, Brabyn P, Muñoz-Guerra MF, Sastre-Pérez J, Escorial-Hernandez V, Rodríguez-Campo FJ, et al. Oral squamous cell carcinoma: epidemiological study and risk factor assessment based on a 39-year series. Int J Oral Maxillofac Surg. (2020) 49:1525–34. doi: 10.1016/j.ijom.2020.03.009
15. Ali K. Oral cancer - the fight must go on against all odds…. Evidence-Based Dentistry. (2022) 23:4–5. doi: 10.1038/s41432-022-0243-1
16. Lakshmipriya T and Gopinath SCB. Monitoring changes in the P53 gene mutation to diagnose oral cancer. Oral Oncol Rep. (2024) 10:100513. doi: 10.1016/j.oor.2024.100513
17. Abbas Z and Rehman S. An overview of cancer treatment modalities. Neoplasm. (2018) 1:139–57. doi: 10.5772/intechopen.76558
18. Vogelstein B, Lane D, and Levine AJ. Surfing the p53 network. Nature. (2000) 408:307–10. doi: 10.1038/35042675
19. Nakagaki T, Tamura M, Kobashi K, Koyama R, Fukushima H, Ohashi T, et al. Profiling cancer-related gene mutations in oral squamous cell carcinoma from Japanese patients by targeted amplicon sequencing. Oncotarget. (2017) 8:59113–22. doi: 10.18632/oncotarget.19262
20. Chen X, Zhao W, Chen S, and Yu D. Mutation profiles of oral squamous cell carcinoma cells. Adv Oral Maxillofac Surg. (2021) 2:100026. doi: 10.1016/j.adoms.2021.100026
21. Mendoza MC, Er EE, and Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci. (2011) 36:320–8. doi: 10.1016/j.tibs.2011.03.006
22. Murugan AK, Munirajan AK, and Tsuchida N. Genetic deregulation of the PIK3CA oncogene in oral cancer. Cancer Lett. (2013) 338:193–203. doi: 10.1016/j.canlet.2013.04.005
23. Arunkumar G, Murugan AK, Nagarajan M, Ajay C, Rajaraman R, and Munirajan AK. Absence of the frequently reported PIK3CA, CASP8, and NOTCH1 mutations in South Indian oral cancers. Oral Dis. (2017) 23:669–73. doi: 10.1111/odi.12655
24. Lyu H, Li M, Jiang Z, Liu Z, and Wang X. Correlate the TP53 mutation and the HRAS mutation with immune signatures in head and neck squamous cell cancer. Comput Struct Biotechnol J. (2019) 17:1020–30. doi: 10.1016/j.csbj.2019.07.009
25. Yoshida R, Nagata M, Nakayama H, Niimori-Kita K, Hassan W, Tanaka T, et al. The pathological significance of Notch1 in oral squamous cell carcinoma. Lab Invest. (2013) 93:1068–81. doi: 10.1038/labinvest.2013.95
26. Yi Y, Tian Z, Ju H, Ren G, and Hu J. A novel NOTCH3 mutation identified in patients with oral cancer by whole exome sequencing. Int J Mol Med. (2017) 39:1541–7. doi: 10.3892/ijmm.2017.2965
27. Bolos V, Grego-Bessa J, and de la Pompa JL. Notch signaling in development and cancer. Endocr Rev. (2007) 28:339–63. doi: 10.1210/er.2006-0046
28. Song X, Xia R, Li J, Long Z, Ren H, Chen W, et al. Common and complex Notch1 mutations in Chinese oral squamous cell carcinoma. Clin Cancer Res. (2014) 20:701–10. doi: 10.1158/1078-0432.CCR-13-1050
29. Izumchenko E, Sun K, Jones S, Brait M, Agrawal N, Koch W, et al. Notch1 mutations are drivers of oral tumorigenesis. Cancer Prev Res (Phila). (2015) 8:277–86. doi: 10.1158/1940-6207.CAPR-14-0257
30. Dotto GP and Rustgi AK. Squamous cell cancers: A unified perspective on biology and genetics. Cancer Cell. (2016) 29:622–37. doi: 10.1016/j.ccell.2016.04.004
31. Patel K, Bhat FA, Patil S, Routray S, Mohanty N, Nair B, et al. Whole-exome sequencing analysis of oral squamous cell carcinoma delineated by tobacco usage habits. Front Oncol. (2021) 11:660696. doi: 10.3389/fonc.2021.660696
32. Lan T, Ge Q, Zheng K, Huang L, Yan Y, Zheng L, et al. FAT1 upregulates in oral squamous cell carcinoma and promotes cell proliferation via cell cycle and DNA repair. Front Oncol. (2022) 12:870055. doi: 10.3389/fonc.2022.870055
33. Hayes TF, Benaich N, Goldie SJ, Sipila K, Ames-Draycott A, Cai W, et al. Integrative genomic and functional analysis of human oral squamous cell carcinoma cell lines reveals synergistic effects of FAT1 and CASP8 inactivation. Cancer Lett. (2016) 383:106–14. doi: 10.1016/j.canlet.2016.09.014
34. Harbison RA, Kubik M, Konnick EQ, Zhang Q, Lee SG, Park H, et al. The mutational landscape of recurrent versus nonrecurrent human papillomavirus-related oropharyngeal cancer. JCI Insight. (2018) 3. doi: 10.1172/jci.insight.99327
35. Cancer Genome Atlas N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. (2015) 517:576–82. doi: 10.1038/nature14129
36. Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. (2011) 333:1157–60. doi: 10.1126/science.1208130
37. Nohata N, Goto Y, and Gutkind JS. Onco-GPCR signaling and dysregulated expression of microRNAs in human cancer. J Hum Genet. (2017) 62:87–96. doi: 10.1038/jhg.2016.124
38. Irimie AI, Ciocan C, Gulei D, Mehterov N, Atanasov AG, Dudea D, et al. Current insights into oral cancer epigenetics. Int J Mol Sci. (2018) 19. doi: 10.3390/ijms19030670
39. Viet CT, Yu G, Asam K, Thomas CM, Yoon AJ, Wongworawat YC, et al. The REASON score: an epigenetic and clinicopathologic score to predict risk of poor survival in patients with early stage oral squamous cell carcinoma. biomark Res. (2021) 9:42. doi: 10.1186/s40364-021-00292-x
40. González-Ramírez I, García-Cuellar C, Sánchez-Pérez Y, and Granados-García M. DNA methylation in oral squamous cell carcinoma: molecular mechanisms and clinical implications. Oral Dis. (2011) 17:771–8. doi: 10.1111/j.1601-0825.2011.01833.x
41. Jithesh PV, Risk JM, Schache AG, Dhanda J, Lane B, Liloglou T, et al. The epigenetic landscape of oral squamous cell carcinoma. Br J Cancer. (2013) 108:370–9. doi: 10.1038/bjc.2012.568
42. Mesgari H, Esmaelian S, Nasiri K, Ghasemzadeh S, Doroudgar P, and Payandeh Z. Epigenetic regulation in oral squamous cell carcinoma microenvironment: A comprehensive review. Cancers (Basel). (2023) 15. doi: 10.3390/cancers15235600
43. Liu Q, Yu S, Zhao W, Qin S, Chu Q, and Wu K. EGFR-TKIs resistance via EGFR-independent signaling pathways. Mol Cancer. (2018) 17:53. doi: 10.1186/s12943-018-0793-1
44. Zhou Y, Tang Y, Luo J, Yang Y, Zang H, Ma J, et al. High expression of HSP60 and survivin predicts poor prognosis for oral squamous cell carcinoma patients. BMC Oral Health. (2023) 23:629. doi: 10.1186/s12903-023-03311-5
45. Yang H, Jin X, Dan H, and Chen Q. Histone modifications in oral squamous cell carcinoma and oral potentially Malignant disorders. Oral Dis. (2020) 26:719–32. doi: 10.1111/odi.13115
46. Campos-Fernández E, Matsuo FS, Andrade MF, Servato JPS, Loyola AM, Cardoso SV, et al. Prognostic value of histone H3 serine 10 phosphorylation and histone H4 lysine 12 acetylation in oral squamous cell carcinoma. Histopathology. (2019) 74:227–38. doi: 10.1111/his.13713
47. Lu Y, Yang J, Zhu J, Shu Y, Zou X, Ruan Q, et al. Advances in the histone acetylation modification in the oral squamous cell carcinoma. J Oncol. (2023) 2023:4616682. doi: 10.1155/2023/4616682
48. Hema KN, Smitha T, Sheethal HS, and Mirnalini SA. Epigenetics in oral squamous cell carcinoma. J Oral Maxillofac Pathol. (2017) 21:252–9. doi: 10.4103/jomfp.JOMFP_150_17
49. Tanaka M, Harada H, and Kimura H. The role of H3K9me3 in oral squamous cell carcinoma. Biochem Biophys Res Commun. (2023) 640:56–63. doi: 10.1016/j.bbrc.2022.11.102
50. Xu Z, Gu Y, Chen J, Chen X, Song Y, Fan J, et al. Epigenome-wide gene-age interaction study reveals reversed effects of MORN1 DNA methylation on survival between young and elderly oral squamous cell carcinoma patients. Front Oncol. (2022) 12:941731. doi: 10.3389/fonc.2022.941731
51. Mao W, Wang B, Huang R, Sun Z, Yan M, and Dong P. Histone modifications in head and neck squamous cell carcinoma. Front Oncol. (2024) 14:1427725. doi: 10.3389/fonc.2024.1427725
52. Rajan C, Roshan VGD, Khan I, Manasa VG, Himal I, Kattoor J, et al. MiRNA expression profiling and emergence of new prognostic signature for oral squamous cell carcinoma. Sci Rep. (2021) 11:7298. doi: 10.1038/s41598-021-86316-w
53. Manikandan M, Deva Magendhra Rao AK, Arunkumar G, Manickavasagam M, Rajkumar KS, Rajaraman R, et al. Oral squamous cell carcinoma: microRNA expression profiling and integrative analyses for elucidation of tumourigenesis mechanism. Mol Cancer. (2016) 15:28. doi: 10.1186/s12943-016-0512-8
54. Di Stasio D, Romano A, Boschetti CE, Montella M, Mosca L, and Lucchese A. Salivary miRNAs Expression in Potentially Malignant Disorders of the Oral Mucosa and Oral Squamous Cell Carcinoma: A Pilot Study on miR-21, miR-27b, and miR-181b. Cancers (Basel). (2022) 15. doi: 10.3390/cancers15010291
55. Shiiba M, Uzawa K, and Tanzawa H. MicroRNAs in head and neck squamous cell carcinoma (HNSCC) and oral squamous cell carcinoma (OSCC). Cancers (Basel). (2010) 2:653–69. doi: 10.3390/cancers2020653
56. Tranby EP, Heaton LJ, Tomar SL, Kelly AL, Fager GL, Backley M, et al. Oral cancer prevalence, mortality, and costs in medicaid and commercial insurance claims data. Cancer Epidemiol Biomarkers Prev. (2022) 31:1849–57. doi: 10.1158/1055-9965.EPI-22-0114
57. D’souza S and Addepalli V. Preventive measures in oral cancer: An overview. Biomed Pharmacother. (2018) 107:72–80. doi: 10.1016/j.biopha.2018.07.114
58. Ghantous Y and Elnaaj A. Global incidence and risk factors of oral cancer. Harefuah. (2017) 156:645–9.
59. Jiang X, Wu J, Wang J, and Huang R. Tobacco and oral squamous cell carcinoma: A review of carcinogenic pathways. Tobacco induced Dis. (2019) 17. doi: 10.18332/tid/105844
60. Muhammad JS, Khan MR, and Ghias K. DNA methylation as an epigenetic regulator of gallbladder cancer: An overview. Int J Surg. (2018) 53:178–83. doi: 10.1016/j.ijsu.2018.03.053
61. Zygogianni AG, Kyrgias G, Karakitsos P, Psyrri A, Kouvaris J, Kelekis N, et al. Oral squamous cell cancer: early detection and the role of alcohol and smoking. Head Neck Oncol. (2011) 3:2. doi: 10.1186/1758-3284-3-2
62. Abdelrahman H and Mahmoud S. Navigating the perilous intersection of alcohol consumption and oral squamous cell carcinoma: A multidisciplinary imperative. Int J Appl Health Care Analytics. (2024) 9:33–43.
63. Yao Y, Shen X, Zhou M, and Tang B. Periodontal pathogens promote oral squamous cell carcinoma by regulating ATR and NLRP3 inflammasome. Front Oncol. (2021) 11:722797. doi: 10.3389/fonc.2021.722797
64. Roura A-J, Szadkowska P, Poleszak K, Dabrowski MJ, Ellert-Miklaszewska A, Wojnicki K, et al. Regulatory networks driving expression of genes critical for glioblastoma are controlled by the transcription factor c-Jun and the pre-existing epigenetic modifications. Clin Epigenetics. (2023) 15:29. doi: 10.1186/s13148-023-01446-4
65. Fialka F, Gruber RM, Hitt R, Opitz L, Brunner E, Schliephake H, et al. CPA6, FMO2, LGI1, SIAT1 and TNC are differentially expressed in early-and late-stage oral squamous cell carcinoma–a pilot study. Oral Oncol. (2008) 44:941–8. doi: 10.1016/j.oraloncology.2007.10.011
66. Gingerich MA, Smith JD, Michmerhuizen NL, Ludwig M, Devenport S, Matovina C, et al. Comprehensive review of genetic factors contributing to head and neck squamous cell carcinoma development in low-risk, nontraditional patients. Head neck. (2018) 40:943–54. doi: 10.1002/hed.25057
67. Tharani Kumar S, Prasanna Devi S, Krithika C, and Raghavan RN. Review of metallic biomaterials in dental applications. J Pharm Bioallied Sci. (2020) 12:S14–s9. doi: 10.4103/jpbs.JPBS_88_20
68. Messer R and Wataha J. Dental materials: biocompatibility. In: Encyclopedia of Materials: Science and Technology. Amsterdam, Netherlands: Elsevier. (2002). p. 1–10.
69. Senevirathna K, Mahakapuge TAN, Ileperuma P, Jayawardana NU, Jayarathne L, Weerasekara R, et al. Correlation between serum heavy metals and the risk of oral squamous cell carcinoma and oral potentially Malignant disorders. Sci Rep. (2024) 14:19029. doi: 10.1038/s41598-024-70057-7
70. Stohs SJ, Bagchi D, Hassoun E, and Bagchi M. Oxidative mechanisms in the toxicity of chromium and cadmium ions. J Environ Pathol Toxicol Oncol. (2001) 20:77–88. doi: 10.1615/JEnvironPatholToxicolOncol.v20.i2.10
71. Durgo K, Orešić S, Rinčić Mlinarić M, Fiket Ž, and Jurešić G. Toxicity of metal ions released from a fixed orthodontic appliance to gastrointestinal tract cell lines. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24129940
72. Albagieh H, Alshehri AZ, Alduraywishi AS, Aldaws A, AlBalawi SS, Shaqqaf HFA, et al. Evaluation of salivary diagnostics: applications, benefits, challenges, and future prospects in dental and systemic disease detection. Cureus. (2025) 17. doi: 10.7759/cureus.77520
73. Al-Jamaei AAH, van Dijk BAC, Helder MN, Forouzanfar T, Leemans CR, and de Visscher JGAM. A population-based study of the epidemiology of oral squamous cell carcinoma in the Netherlands 1989–2018, with emphasis on young adults. Int J Oral Maxillofac Surg. (2022) 51:18–26. doi: 10.1016/j.ijom.2021.03.006
74. Meurman JH. Infectious and dietary risk factors of oral cancer. Oral Oncol. (2010) 46:411–3. doi: 10.1016/j.oraloncology.2010.03.003
75. Marron M, Boffetta P, Zhang Z-F, Zaridze D, Wünsch-Filho V, Winn DM, et al. Cessation of alcohol drinking, tobacco smoking and the reversal of head and neck cancer risk. Int J Epidemiol. (2010) 39:182–96. doi: 10.1093/ije/dyp291
76. Lewandowska U, Szewczyk K, Hrabec E, Janecka A, and Gorlach S. Overview of metabolism and bioavailability enhancement of polyphenols. J Agric Food Chem. (2013) 61:12183–99. doi: 10.1021/jf404439b
77. Narotzki B, Reznick AZ, Aizenbud D, and Levy Y. Green tea: a promising natural product in oral health. Arch Oral Biol. (2012) 57:429–35. doi: 10.1016/j.archoralbio.2011.11.017
78. Agar N and Patel R. Early detection, causes and screening of oral cancer. JSM Dent. (2014) 2:1039.
79. Hashibe M, Boffetta P, Zaridze D, Shangina O, Szeszenia-Dabrowska N, Mates D, et al. Evidence for an important role of alcohol-and aldehyde-metabolizing genes in cancers of the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev. (2006) 15:696–703. doi: 10.1158/1055-9965.EPI-05-0710
80. Huang W, Jiang M, Lin Y, Qi Y, and Li B. Crosstalk between cancer cells and macrophages promotes OSCC cell migration and invasion through a CXCL1/EGF positive feedback loop. Discov Oncol. (2024) 15:145. doi: 10.1007/s12672-024-00972-8
81. Jing F, Zhu L, Zhang J, Zhou X, Bai J, Li X, et al. Multi-omics reveals lactylation-driven regulatory mechanisms promoting tumor progression in oral squamous cell carcinoma. Genome Biol. (2024) 25:272. doi: 10.1186/s13059-024-03383-8
82. Sable MN and Kane SV. Molecular carcinopathogenesis in oral squamous cell carcinoma. In: Microbes and Oral Squamous Cell Carcinoma: A Network Spanning Infection and Inflammation. Singapore: Springer (2022). p. 41–53.
83. Uddin S, Singh A, Mishra V, Agrawal N, Gooi Z, and Izumchenko E. Molecular drivers of oral cavity squamous cell carcinoma in non-smoking and non-drinking patients: what do we know so far? Oncol Rev. (2022) 16. doi: 10.4081/oncol.2022.549
84. Vatsa PP, Jindal Y, Bhadwalkar J, Chamoli A, Upadhyay V, and Mandoli A. Role of epigenetics in OSCC: an understanding above genetics. Med Oncol. (2023) 40:122. doi: 10.1007/s12032-023-01992-0
85. Patel S, Alam A, Pant R, and Chattopadhyay S. Wnt signaling and its significance within the tumor microenvironment: novel therapeutic insights. Front Immunol. (2019) 10:2872. doi: 10.3389/fimmu.2019.02872
86. MacDonald BT, Tamai K, and He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. (2009) 17:9–26. doi: 10.1016/j.devcel.2009.06.016
87. Wang Y, Cao Z, Liu F, and Ou Y. Clinical significance of activated Wnt/β-catenin signaling in apoptosis inhibition of oral cancer. Open Life Sci. (2021) 16:1045–52. doi: 10.1515/biol-2021-0104
88. Iwai S, Yonekawa A, Harada C, Hamada M, Katagiri W, Nakazawa M, et al. Involvement of the Wnt-β-catenin pathway in invasion and migration of oral squamous carcinoma cells. Int J Oncol. (2010) 37:1095–103. doi: 10.3892/ijo_00000761
89. Xie J, Huang L, Lu YG, and Zheng DL. Roles of the wnt signaling pathway in head and neck squamous cell carcinoma. Front Mol Biosci. (2020) 7:590912. doi: 10.3389/fmolb.2020.590912
90. Rhee CS, Sen M, Lu D, Wu C, Leoni L, Rubin J, et al. Wnt and frizzled receptors as potential targets for immunotherapy in head and neck squamous cell carcinomas. Oncogene. (2002) 21:6598–605. doi: 10.1038/sj.onc.1205920
91. Li Y, Huang L, Hu Q, Zheng K, Yan Y, Lan T, et al. WNT7B promotes cancer progression via WNT/β-catenin signaling pathway and predicts a poor prognosis in oral squamous cell carcinoma. BMC Oral Health. (2024) 24:1335. doi: 10.1186/s12903-024-05113-9
92. Xie H, Ma Y, Li J, Chen H, Xie Y, Chen M, et al. WNT7A promotes EGF-induced migration of oral squamous cell carcinoma cells by activating β-catenin/MMP9-mediated signaling. Front Pharmacol. (2020) 11:98. doi: 10.3389/fphar.2020.00098
93. Huang Q, Xiao Y, Lan T, Lu Y, Huang L, and Zheng D. WNT7A promotes tumorigenesis of head and neck squamous cell carcinoma via activating FZD7/JAK1/STAT3 signaling. Int J Oral Sci. (2024) 16:7. doi: 10.1038/s41368-024-00279-y
94. Shen Y, Chen Y, Lin Y, Li Y, Liu P, Zhang B, et al. CDK5RAP2 is a Wnt target gene and promotes stemness and progression of oral squamous cell carcinoma. Cell Death Disease. (2023) 14:107. doi: 10.1038/s41419-023-05652-z
95. Prgomet Z, Andersson T, and Lindberg P. Higher expression of WNT5A protein in oral squamous cell carcinoma compared with dysplasia and oral mucosa with a normal appearance. Eur J Oral Sci. (2017) 125:237–46. doi: 10.1111/eos.12352
96. Lequerica-Fernández P, Rodríguez-Santamarta T, García-García E, Blanco-Lorenzo V, Torres-Rivas HE, Rodrigo JP, et al. Prognostic significance of β-catenin in relation to the tumor immune microenvironment in oral cancer. Biomedicines. (2023) 11:2675. doi: 10.3390/biomedicines11102675
97. Di Paola FJ, Alquati C, Conti G, Calafato G, Turroni S, D’Amico F, et al. Interplay between WNT/PI3K-mTOR axis and the microbiota in APC-driven colorectal carcinogenesis: data from a pilot study and possible implications for CRC prevention. J Transl Med. (2024) 22:631. doi: 10.1186/s12967-024-05305-5
98. Bennedsen ALB, Furbo S, Bjarnsholt T, Raskov H, Gögenur I, and Kvich L. The gut microbiota can orchestrate the signaling pathways in colorectal cancer. APMIS. (2022) 130:121–39. doi: 10.1111/apm.13206
99. Yi S, Jin X, Liu B, Wu P, Xiao W, and Chen W. Portulaca oleracea extract reduces gut microbiota imbalance and inhibits colorectal cancer progression via inactivation of the Wnt/β-catenin signaling pathway. Phytomedicine. (2022) 105:154279. doi: 10.1016/j.phymed.2022.154279
100. Li Z, Liu Y, Huang X, Wang Q, Fu R, Wen X, et al. F. Nucleatum enhances oral squamous cell carcinoma proliferation via E-cadherin/β-Catenin pathway. BMC Oral Health. (2024) 24:518. doi: 10.1186/s12903-024-04252-3
101. Cai L, Zhu H, Mou Q, Wong PY, Lan L, Ng CWK, et al. Integrative analysis reveals associations between oral microbiota dysbiosis and host genetic and epigenetic aberrations in oral cavity squamous cell carcinoma. NPJ Biofilms Microbiomes. (2024) 10:39. doi: 10.1038/s41522-024-00511-x
102. Zhou J, Hu Z, Wang L, Hu Q, Chen Z, Lin T, et al. Tumor-colonized Streptococcus mutans metabolically reprograms tumor microenvironment and promotes oral squamous cell carcinoma. Microbiome. (2024) 12:193. doi: 10.1186/s40168-024-01907-9
103. Alam M and Mishra R. Role of PI3K and EGFR in oral cancer progression and drug resistance. Int J Res Appl Sci Biotechnol (IJRASB). (2020) 7:85–9. doi: 10.31033/ijrasb.7.6.14
104. Cívico-Ortega JL, González-Ruiz I, Ramos-García P, Cruz-Granados D, Samayoa-Descamps V, and González-Moles MÁ. Prognostic and clinicopathological significance of epidermal growth factor receptor (EGFR) expression in oral squamous cell carcinoma: systematic review and meta-analysis. Int J Mol Sci. (2023) 24:11888. doi: 10.3390/ijms241511888
105. Onda M, Ota A, Ito K, Ono T, Karnan S, Kato M, et al. Inhibition of VEGFR2 and EGFR signaling cooperatively suppresses the proliferation of oral squamous cell carcinoma. Cancer Med. (2023) 12:16416–30. doi: 10.1002/cam4.6282
106. Hutchinson MND, Mierzwa M, and D’Silva NJ. Radiation resistance in head and neck squamous cell carcinoma: dire need for an appropriate sensitizer. Oncogene. (2020) 39:3638–49. doi: 10.1038/s41388-020-1250-3
107. Saravani S, Parsamanesh N, and Miri-Moghaddam E. Role of EGFR gene polymorphisms in oral squamous cell carcinoma patients of Southeast Iran: A case-control study. Caspian J Intern Med. (2020) 11:391–7. doi: 10.22088/cjim.11.4.391
108. Saintigny P, William WN Jr., Foy J-P, Papadimitrakopoulou V, Lang W, Zhang L, et al. Met receptor tyrosine kinase and chemoprevention of oral cancer. JNCI: J Natl Cancer Institute. (2018) 110:250–7. doi: 10.1093/jnci/djx186
109. Yokokawa M, Morita Ki, Oikawa Y, Kayamori K, Sakamoto K, Ikeda T, et al. Co-expression of EGFR and MET has a synergistic effect on the prognosis of patients with oral squamous cell carcinoma. J Oral Pathol Med. (2020) 49:235–42. doi: 10.1111/jop.12977
110. Cheng Y, Chen J, Shi Y, Fang X, and Tang Z. MAPK signaling pathway in oral squamous cell carcinoma: biological function and targeted therapy. Cancers. (2022) 14:4625. doi: 10.3390/cancers14194625
111. Wee P and Wang Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers (Basel). (2017) 9. doi: 10.3390/cancers9050052
112. Bahar ME, Kim HJ, and Kim DR. Targeting the RAS/RAF/MAPK pathway for cancer therapy: from mechanism to clinical studies. Signal Transduct Target Ther. (2023) 8:455. doi: 10.1038/s41392-023-01705-z
113. Hussain MR, Baig M, Mohamoud HS, Ulhaq Z, Hoessli DC, Khogeer GS, et al. BRAF gene: From human cancers to developmental syndromes. Saudi J Biol Sci. (2015) 22:359–73. doi: 10.1016/j.sjbs.2014.10.002
114. Peng Q, Deng Z, Pan H, Gu L, Liu O, and Tang Z. Mitogen-activated protein kinase signaling pathway in oral cancer. Oncol Lett. (2018) 15:1379–88. doi: 10.3892/ol.2017.7491
115. Niklander SE. Inflammatory mediators in oral cancer: pathogenic mechanisms and diagnostic potential. Front Oral Health. (2021) 2:642238. doi: 10.3389/froh.2021.642238
116. Papa S, Bubici C, Zazzeroni F, Pham C, Kuntzen C, Knabb J, et al. The NF-κB-mediated control of the JNK cascade in the antagonism of programmed cell death in health and disease. Cell Death Differentiation. (2006) 13:712–29. doi: 10.1038/sj.cdd.4401865
117. Tampa M, Mitran MI, Mitran CI, Sarbu MI, Matei C, Nicolae I, et al. Mediators of inflammation–a potential source of biomarkers in oral squamous cell carcinoma. J Immunol Res. (2018) 2018:1061780. doi: 10.1155/2018/1061780
118. Jimi E, Kokabu S, Matsubara T, Nakatomi C, Matsuo K, and Watanabe S. NF-κB acts as a multifunctional modulator in bone invasion by oral squamous cell carcinoma. Oral Sci Int. (2016) 13:1–6. doi: 10.1016/S1348-8643(15)00038-5
119. Postler TS and Ghosh S. Bridging the gap: a regulator of NF-κB linking inflammation and cancer. J Oral biosciences. (2015) 57:143–7. doi: 10.1016/j.job.2015.05.001
120. Yang X, Cheng H, Chen J, Wang R, Saleh A, Si H, et al. Head and neck cancers promote an inflammatory transcriptome through coactivation of classic and alternative NF-κB pathways. Cancer Immunol Res. (2019) 7:1760–74. doi: 10.1158/2326-6066.CIR-18-0832
121. Zhang J and Peng B. NF-κB promotes iNOS and VEGF expression in salivary gland adenoid cystic carcinoma cells and enhances endothelial cell motility in vitro. Cell proliferation. (2009) 42:150–61. doi: 10.1111/j.1365-2184.2009.00588.x
122. Ikebe T, Takeuchi H, Jimi E, Beppu M, Shinohara M, and Shirasuna K. Involvement of proteasomes in migration and matrix metalloproteinase-9 production of oral squamous cell carcinoma. Int J cancer. (1998) 77:578–85. doi: 10.1002/(SICI)1097-0215(19980812)77:4<578::AID-IJC18>3.0.CO;2-2
123. Nakayama H, Ikebe T, Beppu M, and Shirasuna K. High expression levels of nuclear factor κB, IκB kinase α and Akt kinase in squamous cell carcinoma of the oral cavity. Cancer. (2001) 92:3037–44. doi: 10.1002/1097-0142(20011215)92:12<3037::AID-CNCR10171>3.0.CO;2-#
124. Shaulian E and Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. (2002) 4:E131–E6. doi: 10.1038/ncb0502-e131
125. Bunjobpol W, Dulloo I, Igarashi K, Concin N, Matsuo K, and Sabapathy K. Suppression of acetylpolyamine oxidase by selected AP-1 members regulates DNp73 abundance: mechanistic insights for overcoming DNp73-mediated resistance to chemotherapeutic drugs. Cell Death Differentiation. (2014) 21:1240–9. doi: 10.1038/cdd.2014.41
126. Song D, Lian Y, and Zhang L. The potential of activator protein 1 (AP-1) in cancer targeted therapy. Front Immunol. (2023) 14:1224892. doi: 10.3389/fimmu.2023.1224892
127. Alam M, Kashyap T, Pramanik KK, Singh AK, Nagini S, and Mishra R. The elevated activation of NFκB and AP-1 is correlated with differential regulation of Bcl-2 and associated with oral squamous cell carcinoma progression and resistance. Clin Oral Investigations. (2017) 21:2721–31. doi: 10.1007/s00784-017-2074-6
128. Schindler C and Darnell JE Jr. Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem. (1995) 64:621–51. doi: 10.1146/annurev.bi.64.070195.003201
129. Boudny V and Kovarik J. JAK/STAT signaling pathways and cancer. Janus kinases/signal transducers and activators of transcription. Neoplasma. (2002) 49:349–55.
130. Yu H, Pardoll D, and Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. (2009) 9:798–809. doi: 10.1038/nrc2734
131. Xiao L, Li X, Cao P, Fei W, Zhou H, Tang N, et al. Interleukin-6 mediated inflammasome activation promotes oral squamous cell carcinoma progression via JAK2/STAT3/Sox4/NLRP3 signaling pathway. J Exp Clin Cancer Res. (2022) 41:166. doi: 10.1186/s13046-022-02376-4
132. Masuda M, Suzui M, Yasumatu R, Nakashima T, Kuratomi Y, Azuma K, et al. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. (2002) 62:3351–5.
133. Pena JC, Thompson CB, Recant W, Vokes EE, and Rudin CM. Bcl-xL and Bcl-2 expression in squamous cell carcinoma of the head and neck. Cancer. (1999) 85:164–70. doi: 10.1002/(SICI)1097-0142(19990101)85:1<164::AID-CNCR23>3.0.CO;2-Q
134. Gao X, Leone GW, and Wang H. Cyclin D-CDK4/6 functions in cancer. Adv Cancer Res. (2020) 148:147–69. doi: 10.1016/bs.acr.2020.02.002
135. Mishra R and Das BR. Cyclin D1 expression and its possible regulation in chewing tobacco mediated oral squamous cell carcinoma progression. Arch Oral Biol. (2009) 54:917–23. doi: 10.1016/j.archoralbio.2009.07.003
136. Mandal R, Becker S, and Strebhardt K. Targeting CDK9 for anti-cancer therapeutics. Cancers (Basel). (2021) 13. doi: 10.3390/cancers13092181
137. Jiang M and Li B. STAT3 and its targeting inhibitors in oral squamous cell carcinoma. Cells. (2022) 11. doi: 10.3390/cells11193131
138. Niklander S, Bordagaray MJ, Fernández A, and Hernández M. Vascular endothelial growth factor: A translational view in oral non-communicable diseases. Biomolecules. (2021) 11. doi: 10.3390/biom11010085
139. Lien MY, Tsai HC, Chang AC, Tsai MH, Hua CH, Wang SW, et al. Chemokine CCL4 induces vascular endothelial growth factor C expression and lymphangiogenesis by miR-195-3p in oral squamous cell carcinoma. Front Immunol. (2018) 9:412. doi: 10.3389/fimmu.2018.00412
140. Klein JD, Sano D, Sen M, Myers JN, Grandis JR, and Kim S. STAT3 oligonucleotide inhibits tumor angiogenesis in preclinical models of squamous cell carcinoma. PloS One. (2014) 9:e81819. doi: 10.1371/journal.pone.0081819
141. Gao P, Niu N, Wei T, Tozawa H, Chen X, Zhang C, et al. The roles of signal transducer and activator of transcription factor 3 in tumor angiogenesis. Oncotarget. (2017) 8:69139–61. doi: 10.18632/oncotarget.19932
142. Bid HK, Oswald D, Li C, London CA, Lin J, and Houghton PJ. Anti-angiogenic activity of a small molecule STAT3 inhibitor LLL12. PloS One. (2012) 7:e35513. doi: 10.1371/journal.pone.0035513
143. Wang X, Crowe PJ, Goldstein D, and Yang JL. STAT3 inhibition, a novel approach to enhancing targeted therapy in human cancers (review). Int J Oncol. (2012) 41:1181–91. doi: 10.3892/ijo.2012.1568
144. Wang Y, Guo W, Li Z, Wu Y, Jing C, Ren Y, et al. Corrigendum] Role of the EZH2/miR−200 axis in STAT3−mediated OSCC invasion. Int J Oncol. (2023) 63. doi: 10.3892/ijo.2023.5528
145. Liu C, Zhou J, Zhang S, Fu J, Li Y, Hao Y, et al. Mesenchymal stem cells-derived IL-6 promotes invasion and metastasis of oral squamous cell carcinoma via JAK-STAT3 signalling. Oral Dis. (2024) 30:2097–109. doi: 10.1111/odi.14617
146. Bu LL, Yu GT, Deng WW, Mao L, Liu JF, Ma SR, et al. Targeting STAT3 signaling reduces immunosuppressive myeloid cells in head and neck squamous cell carcinoma. Oncoimmunology. (2016) 5:e1130206. doi: 10.1080/2162402X.2015.1130206
147. Boutilier AJ and Elsawa SF. Macrophage polarization states in the tumor microenvironment. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22136995
148. Gao J, Liang Y, and Wang L. Shaping polarization of tumor-associated macrophages in cancer immunotherapy. Front Immunol. (2022) 13:888713. doi: 10.3389/fimmu.2022.888713
149. Cui H, Lan Z, Zou KL, Zhao YY, and Yu GT. STAT3 promotes differentiation of monocytes to MDSCs via CD39/CD73-adenosine signal pathway in oral squamous cell carcinoma. Cancer Immunol Immunother. (2023) 72:1315–26. doi: 10.1007/s00262-022-03336-9
150. Venkatesiah SS, Augustine D, Mishra D, Gujjar N, Haragannavar VC, Awan KH, et al. Immunology of oral squamous cell carcinoma-A comprehensive insight with recent concepts. Life (Basel). (2022) 12. doi: 10.3390/life12111807
151. Caponio VCA, Zhurakivska K, Lo Muzio L, Troiano G, and Cirillo N. The immune cells in the development of oral squamous cell carcinoma. Cancers (Basel). (2023) 15. doi: 10.3390/cancers15153779
152. Shah NG, Trivedi TI, Tankshali RA, Goswami JA, Jetly DH, Kobawala TP, et al. Stat3 expression in oral squamous cell carcinoma: association with clinicopathological parameters and survival. Int J Biol Markers. (2006) 21:175–83. doi: 10.1177/172460080602100307
153. Cui B, Chen J, Luo M, Liu Y, Chen H, Lü D, et al. PKD3 promotes metastasis and growth of oral squamous cell carcinoma through positive feedback regulation with PD-L1 and activation of ERK-STAT1/3-EMT signalling. Int J Oral Sci. (2021) 13:8. doi: 10.1038/s41368-021-00112-w
154. Dave K, Ali A, and Magalhaes M. Increased expression of PD-1 and PD-L1 in oral lesions progressing to oral squamous cell carcinoma: a pilot study. Sci Rep. (2020) 10:9705. doi: 10.1038/s41598-020-66257-6
155. Sami A, Elimairi I, Stanton C, Ross RP, and Ryan CA. The role of the microbiome in oral squamous cell carcinoma with insight into the microbiome-treatment axis. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21218061
156. Vyhnalova T, Danek Z, Gachova D, and Linhartova PB. The role of the oral microbiota in the etiopathogenesis of oral squamous cell carcinoma. Microorganisms. (2021) 9. doi: 10.3390/microorganisms9081549
157. Goertzen C, Mahdi H, Laliberte C, Meirson T, Eymael D, Gil-Henn H, et al. Oral inflammation promotes oral squamous cell carcinoma invasion. Oncotarget. (2018) 9:29047–63. doi: 10.18632/oncotarget.25540
158. Rai AK, Panda M, Das AK, Rahman T, Das R, Das K, et al. Dysbiosis of salivary microbiome and cytokines influence oral squamous cell carcinoma through inflammation. Arch Microbiol. (2021) 203:137–52. doi: 10.1007/s00203-020-02011-w
159. La Rosa GRM, Gattuso G, Pedullà E, Rapisarda E, Nicolosi D, and Salmeri M. Association of oral dysbiosis with oral cancer development. Oncol Lett. (2020) 19:3045–58. doi: 10.3892/ol.2020.11441
160. Wang X, He X, and Zhong B. Oral microbiota: the overlooked catalyst in cancer initiation and progression. Front Cell Dev Biol. (2025) 12. doi: 10.3389/fcell.2024.1479720
161. Groeger S and Meyle J. Oral mucosal epithelial cells. Front Immunol. (2019) 10. doi: 10.3389/fimmu.2019.00208
162. Sukmana BI, Saleh RO, Najim MA, Al-Ghamdi HS, Achmad H, Al-Hamdani MM, et al. Oral microbiota and oral squamous cell carcinoma: a review of their relation and carcinogenic mechanisms. Front Oncol. (2024) 14:1319777. doi: 10.3389/fonc.2024.1319777
163. Al-Hebshi NN, Nasher AT, Maryoud MY, Homeida HE, Chen T, Idris AM, et al. Inflammatory bacteriome featuring Fusobacterium nucleatum and Pseudomonas aeruginosa identified in association with oral squamous cell carcinoma. Sci Rep. (2017) 7:1834. doi: 10.1038/s41598-017-02079-3
164. Neuzillet C, Marchais M, Vacher S, Hilmi M, Schnitzler A, Meseure D, et al. Prognostic value of intratumoral Fusobacterium nucleatum and association with immune-related gene expression in oral squamous cell carcinoma patients. Sci Rep. (2021) 11:7870. doi: 10.1038/s41598-021-86816-9
165. Yang K, Wang Y, Zhang S, Zhang D, Hu L, Zhao T, et al. Oral microbiota analysis of tissue pairs and saliva samples from patients with oral squamous cell carcinoma - A pilot study. Front Microbiol. (2021) 12:719601. doi: 10.3389/fmicb.2021.719601
166. Zhang Z, Yang J, Feng Q, Chen B, Li M, Liang C, et al. Compositional and functional analysis of the microbiome in tissue and saliva of oral squamous cell carcinoma. Front Microbiol. (2019) 10:1439. doi: 10.3389/fmicb.2019.01439
167. Chen G, Gao C, Jiang S, Cai Q, Li R, Sun Q, et al. Fusobacterium nucleatum outer membrane vesicles activate autophagy to promote oral cancer metastasis. J Advanced Res. (2024) 56:167–79. doi: 10.1016/j.jare.2023.04.002
168. van Dijk MC, Petersen JF, Raber-Durlacher JE, Epstein JB, and Laheij A. Diversity and compositional differences in the oral microbiome of oral squamous cell carcinoma patients and healthy controls: a scoping review. Front Oral Health. (2024) 5:1366153. doi: 10.3389/froh.2024.1366153
169. Liu Y, Baba Y, Ishimoto T, Tsutsuki H, Zhang T, Nomoto D, et al. Fusobacterium nucleatum confers chemoresistance by modulating autophagy in oesophageal squamous cell carcinoma. Br J Cancer. (2021) 124:963–74. doi: 10.1038/s41416-020-01198-5
170. Su SC, Chang LC, Huang HD, Peng CY, Chuang CY, Chen YT, et al. Oral microbial dysbiosis and its performance in predicting oral cancer. Carcinogenesis. (2021) 42:127–35. doi: 10.1093/carcin/bgaa062
171. Marttila E, Uittamo J, Rusanen P, Lindqvist C, Salaspuro M, and Rautemaa R. Site-specific acetaldehyde production and microbial colonization in relation to oral squamous cell carcinoma and oral lichenoid disease. Oral Surg Oral Med Oral Pathol Oral Radiol. (2015) 119:697–9. doi: 10.1016/j.oooo.2015.01.019
172. Ohshima M, Sugahara K, Kasahara K, and Katakura A. Metabolomic analysis of the saliva of Japanese patients with oral squamous cell carcinoma. Oncol Rep. (2017) 37:2727–34. doi: 10.3892/or.2017.5561
173. de Sá Alves M, de Sá Rodrigues N, Bandeira CM, Chagas JFS, Pascoal MBN, Nepomuceno G, et al. Identification of possible salivary metabolic biomarkers and altered metabolic pathways in South American patients diagnosed with oral squamous cell carcinoma. Metabolites. (2021) 11. doi: 10.3390/metabo11100650
174. Saikia PJ, Pathak L, Mitra S, and Das B. The emerging role of oral microbiota in oral cancer initiation, progression and stemness. Front Immunol. (2023) 14:1198269. doi: 10.3389/fimmu.2023.1198269
175. Guo ZC, Jing SL, Jumatai S, and Gong ZC. Porphyromonas gingivalis promotes the progression of oral squamous cell carcinoma by activating the neutrophil chemotaxis in the tumour microenvironment. Cancer Immunol Immunother. (2023) 72:1523–39. doi: 10.1007/s00262-022-03348-5
176. McIlvanna E, Linden GJ, Craig SG, Lundy FT, and James JA. Fusobacterium nucleatum and oral cancer: a critical review. BMC Cancer. (2021) 21:1212. doi: 10.1186/s12885-021-08903-4
177. Zhang M, Liang J, Yang Y, Liang H, Jia H, and Li D. Current trends of targeted drug delivery for oral cancer therapy. Front Bioeng Biotechnol. (2020) 8:618931. doi: 10.3389/fbioe.2020.618931
178. Szturz P and Vermorken JB. Management of recurrent and metastatic oral cavity cancer: Raising the bar a step higher. Oral Oncol. (2020) 101:104492. doi: 10.1016/j.oraloncology.2019.104492
179. Liu L, Chen J, Cai X, Yao Z, and Huang J. Progress in targeted therapeutic drugs for oral squamous cell carcinoma. Surg Oncol. (2019) 31:90–7. doi: 10.1016/j.suronc.2019.09.001
180. Altamura G and Borzacchiello G. Anti-EGFR monoclonal antibody Cetuximab displays potential anti-cancer activities in feline oral squamous cell carcinoma cell lines. Front Vet Sci. (2022) 9:1040552. doi: 10.3389/fvets.2022.1040552
181. Naruse T, Yanamoto S, Matsushita Y, Sakamoto Y, Morishita K, Ohba S, et al. Cetuximab for the treatment of locally advanced and recurrent/metastatic oral cancer: An investigation of distant metastasis. Mol Clin Oncol. (2016) 5:246–52. doi: 10.3892/mco.2016.928
182. Mehra R, Cohen RB, and Burtness BA. The role of cetuximab for the treatment of squamous cell carcinoma of the head and neck. Clin Adv Hematol Oncol. (2008) 6:742–50.
183. Moral M and Paramio JM. Akt pathway as a target for therapeutic intervention in HNSCC. Histol Histopathol. (2008) 23:1269–78. doi: 10.14670/HH-23.1269
184. Ramakrishnan MS, Eswaraiah A, Crombet T, Piedra P, Saurez G, Iyer H, et al. Nimotuzumab, a promising therapeutic monoclonal for treatment of tumors of epithelial origin. MAbs. (2009) 1:41–8. doi: 10.4161/mabs.1.1.7509
185. Guan M, Zhang D, Zhao Y, Mao M, Shen K, Wang X, et al. Nimotuzumab combined with radiotherapy+/- chemotherapy for definitive treatment of locally advanced squamous cell carcinoma of head and neck: a metanalysis of randomized controlled trials. Front Oncol. (2024) 14:1380428. doi: 10.3389/fonc.2024.1380428
186. Dickerson H, Diab A, and Al Musaimi O. Epidermal growth factor receptor tyrosine kinase inhibitors in cancer: current use and future prospects. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms251810008
187. Mak MP and William WN Jr. Targeting the epidermal growth factor receptor for head and neck cancer chemoprevention. Oral Oncol. (2014) 50:918–23. doi: 10.1016/j.oraloncology.2013.12.024
188. Gupta D and Rao R. OP0012 Chemotherapy with gefitinib in recurrent and metastatic head and neck cancer. Eur J Cancer. (2014) 50:e4. doi: 10.1016/j.ejca.2014.03.030
189. William WN Jr., Tsao AS, Feng L, Ginsberg LE, Lee JJ, Kies MS, et al. Single arm, phase II study of cisplatin, docetaxel, and erlotinib in patients with recurrent and/or metastatic head and neck squamous cell carcinomas. Oncologist. (2018) 23:526–e49. doi: 10.1634/theoncologist.2017-0661
190. Siu LL, Soulieres D, Chen EX, Pond GR, Chin SF, Francis P, et al. Phase I/II trial of erlotinib and cisplatin in patients with recurrent or metastatic squamous cell carcinoma of the head and neck: a Princess Margaret Hospital phase II consortium and National Cancer Institute of Canada Clinical Trials Group Study. J Clin Oncol. (2007) 25:2178–83. doi: 10.1200/JCO.2006.07.6547
191. Seiwert TY, Fayette J, Cupissol D, Del Campo JM, Clement PM, Hitt R, et al. A randomized, phase II study of afatinib versus cetuximab in metastatic or recurrent squamous cell carcinoma of the head and neck. Ann Oncol. (2014) 25:1813–20. doi: 10.1093/annonc/mdu216
192. Hanahan D and Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. (1996) 86:353–64. doi: 10.1016/S0092-8674(00)80108-7
193. Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. (1995) 1:27–31. doi: 10.1038/nm0195-27
194. Daniele G, Raspagliesi F, Scambia G, Pisano C, Colombo N, Frezzini S, et al. Bevacizumab, carboplatin, and paclitaxel in the first line treatment of advanced ovarian cancer patients: the phase IV MITO-16A/MaNGO-OV2A study. Int J Gynecol Cancer. (2021) 31:875–82. doi: 10.1136/ijgc-2021-002434
195. Lin Y, Qin S, Li Z, Yang H, Fu W, Li S, et al. Apatinib vs placebo in patients with locally advanced or metastatic, radioactive iodine-refractory differentiated thyroid cancer: the REALITY randomized clinical trial. JAMA Oncol. (2022) 8:242–50. doi: 10.1001/jamaoncol.2021.6268
196. Gyanchandani R, Sano D, Ortega Alves MV, Klein JD, Knapick BA, Oh S, et al. Interleukin-8 as a modulator of response to bevacizumab in preclinical models of head and neck squamous cell carcinoma. Oral Oncol. (2013) 49:761–70. doi: 10.1016/j.oraloncology.2013.03.452
197. Fury MG, Xiao H, Sherman EJ, Baxi S, Smith-Marrone S, Schupak K, et al. Phase II trial of bevacizumab + cetuximab + cisplatin with concurrent intensity-modulated radiation therapy for patients with stage III/IVB head and neck squamous cell carcinoma. Head Neck. (2016) 38 Suppl 1:E566–70. doi: 10.1002/hed.24041
198. Argiris A, Karamouzis MV, Gooding WE, Branstetter BF, Zhong S, Raez LE, et al. Phase II trial of pemetrexed and bevacizumab in patients with recurrent or metastatic head and neck cancer. J Clin Oncol. (2011) 29:1140–5. doi: 10.1200/JCO.2010.33.3591
199. Yao M, Galanopoulos N, Lavertu P, Fu P, Gibson M, Argiris A, et al. Phase II study of bevacizumab in combination with docetaxel and radiation in locally advanced squamous cell carcinoma of the head and neck. Head Neck. (2015) 37:1665–71. doi: 10.1002/hed.23813
200. Ibrahim N, Yu Y, Walsh WR, and Yang JL. Molecular targeted therapies for cancer: sorafenib mono-therapy and its combination with other therapies (review). Oncol Rep. (2012) 27:1303–11. doi: 10.3892/or.2012.1675
201. Möckelmann N, Kriegs M, Dikomey E, and Knecht R. 2 Effects of sorafenib treatment on HNSCC cells in combination with cisplatin based-chemoradiation. Oral Oncol. (2015) 51:e27–e8. doi: 10.1016/j.oraloncology.2015.02.006
202. Zhou G, Hasina R, Wroblewski K, Mankame TP, Doçi CL, and Lingen MW. Dual inhibition of vascular endothelial growth factor receptor and epidermal growth factor receptor is an effective chemopreventive strategy in the mouse 4-NQO model of oral carcinogenesis. Cancer Prev Res (Phila). (2010) 3:1493–502. doi: 10.1158/1940-6207.CAPR-10-0135
203. Sano D, Matsumoto F, Valdecanas DR, Zhao M, Molkentine DP, Takahashi Y, et al. Vandetanib restores head and neck squamous cell carcinoma cells’ sensitivity to cisplatin and radiation in vivo and in vitro. Clin Cancer Res. (2011) 17:1815–27. doi: 10.1158/1078-0432.CCR-10-2120
204. Naruse T, Yanamoto S, Yamada S, Rokutanda S, Kawakita A, Kawasaki G, et al. Anti-tumor effect of the mammalian target of rapamycin inhibitor everolimus in oral squamous cell carcinoma. Pathol Oncol Res. (2015) 21:765–73. doi: 10.1007/s12253-014-9888-1
205. Geiger JL, Bauman JE, Gibson MK, Gooding WE, Varadarajan P, Kotsakis A, et al. Phase II trial of everolimus in patients with previously treated recurrent or metastatic head and neck squamous cell carcinoma. Head Neck. (2016) 38:1759–64. doi: 10.1002/hed.24501
206. Pellarin I, Dall’Acqua A, Favero A, Segatto I, Rossi V, Crestan N, et al. Cyclin-dependent protein kinases and cell cycle regulation in biology and disease. Signal Transduct Target Ther. (2025) 10:11. doi: 10.1038/s41392-024-02080-z
207. Zhang J, Di Y, Zhang B, Li T, Li D, and Zhang H. CDK1 and CCNA2 play important roles in oral squamous cell carcinoma. Med (Baltimore). (2024) 103:e37831. doi: 10.1097/MD.0000000000037831
208. Laderian B and Fojo T. CDK4/6 Inhibition as a therapeutic strategy in breast cancer: palbociclib, ribociclib, and abemaciclib. Semin Oncol. (2017) 44:395–403. doi: 10.1053/j.seminoncol.2018.03.006
209. Chohan TA, Qayyum A, Rehman K, Tariq M, and Akash MSH. An insight into the emerging role of cyclin-dependent kinase inhibitors as potential therapeutic agents for the treatment of advanced cancers. BioMed Pharmacother. (2018) 107:1326–41. doi: 10.1016/j.biopha.2018.08.116
210. Mendes RA, Carvalho JF, and Waal I. An overview on the expression of cyclooxygenase-2 in tumors of the head and neck. Oral Oncol. (2009) 45:e124–8. doi: 10.1016/j.oraloncology.2009.03.016
211. Yang CC and Chang KW. Eicosanoids and HB-EGF/EGFR in cancer. Cancer Metastasis Rev. (2018) 37:385–95. doi: 10.1007/s10555-018-9746-9
212. Qian M, Qian D, Jing H, Li Y, Ma C, and Zhou Y. Combined cetuximab and celecoxib treatment exhibits a synergistic anticancer effect on human oral squamous cell carcinoma in vitro and in vivo. Oncol Rep. (2014) 32:1681–8. doi: 10.3892/or.2014.3334
213. Li WZ, Huo QJ, Wang XY, and Xu F. Inhibitive effect of celecoxib on the adhesion and invasion of human tongue squamous carcinoma cells to extracellular matrix via down regulation of MMP-2 expression. Prostaglandins Other Lipid Mediat. (2010) 93:113–9. doi: 10.1016/j.prostaglandins.2010.08.001
214. Chen Z, Zhang X, Li M, Wang Z, Wieand HS, Grandis JR, et al. Simultaneously targeting epidermal growth factor receptor tyrosine kinase and cyclooxygenase-2, an efficient approach to inhibition of squamous cell carcinoma of the head and neck. Clin Cancer Res. (2004) 10:5930–9. doi: 10.1158/1078-0432.CCR-03-0677
215. Du X, Ji Y, Qin W, and Wei J. Clinical efficacy of Endostar continuous infusion combined with concurrent chemoradiotherapy in the treatment of oesophageal squamous cell carcinoma. Front Med (Lausanne). (2024) 11:1463041. doi: 10.3389/fmed.2024.1463041
216. Liao Z, Zhang C, Yang T, Liu H, Yang S, Li T, et al. Chemotherapy combined with recombinant human endostatin (Endostar) significantly improves the progression-free survival of stage IV soft tissue sarcomas. Front Oncol. (2021) 11:778774. doi: 10.3389/fonc.2021.778774
217. Mohamad I, Glaun MD, Prabhash K, Busheri A, Lai SY, Noronha V, et al. Current treatment strategies and risk stratification for oral carcinoma. Am Soc Clin Oncol Educ Book. (2023) 43:e389810. doi: 10.1200/EDBK_389810
218. Jagadeesan D, Sathasivam KV, Fuloria NK, Balakrishnan V, Khor GH, Ravichandran M, et al. Comprehensive insights into oral squamous cell carcinoma: diagnosis, pathogenesis, and therapeutic advances. Pathology-Research Pract. (2024) 261:155489. doi: 10.1016/j.prp.2024.155489
219. Flausino CS, Daniel FI, and Modolo F. DNA methylation in oral squamous cell carcinoma: from its role in carcinogenesis to potential inhibitor drugs. Crit Rev Oncol Hematol. (2021) 164:103399. doi: 10.1016/j.critrevonc.2021.103399
220. Ghosh RD, Pattatheyil A, and Roychoudhury S. Functional landscape of dysregulated microRNAs in oral squamous cell carcinoma: clinical implications. Front Oncol. (2020) 10:619. doi: 10.3389/fonc.2020.00619
221. Zeng Q, Tao X, Huang F, Wu T, Wang J, Jiang X, et al. Overexpression of miR-155 promotes the proliferation and invasion of oral squamous carcinoma cells by regulating BCL6/cyclin D2. Int J Mol Med. (2016) 37:1274–80. doi: 10.3892/ijmm.2016.2529
222. Asgar MA, Senawong G, Sripa B, and Senawong T. Synergistic anticancer effects of cisplatin and histone deacetylase inhibitors (SAHA and TSA) on cholangiocarcinoma cell lines. Int J Oncol. (2016) 48:409–20. doi: 10.3892/ijo.2015.3240
223. Wang J, Lv N, Lu X, Yuan R, Chen Z, and Yu J. Diagnostic and therapeutic role of microRNAs in oral cancer (Review). Oncol Rep. (2021) 45:58–64. doi: 10.3892/or.2020.7854
224. Waldman AD, Fritz JM, and Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. (2020) 20:651–68. doi: 10.1038/s41577-020-0306-5
225. Andtbacka RHI, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. (2015) 33:2780–8. doi: 10.1200/JCO.2014.58.3377
226. Kai M, Marx AN, Liu DD, Shen Y, Gao H, Reuben JM, et al. A phase II study of talimogene laherparepvec for patients with inoperable locoregional recurrence of breast cancer. Sci Rep. (2021) 11:22242. doi: 10.1038/s41598-021-01473-2
227. Kohlhapp FJ and Kaufman HL. Molecular pathways: mechanism of action for talimogene laherparepvec, a new oncolytic virus immunotherapy. Clin Cancer Res. (2016) 22:1048–54. doi: 10.1158/1078-0432.CCR-15-2667
228. Ferris RL, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. (2016) 375:1856–67. doi: 10.1056/NEJMoa1602252
229. Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. (2016) 17:956–65. doi: 10.1016/S1470-2045(16)30066-3
230. Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. (2019) 394:1915–28. doi: 10.1016/S0140-6736(19)32591-7
231. Harrington KJ, Burtness B, Greil R, Soulières D, Tahara M, Castro Gd, et al. Pembrolizumab with or without chemotherapy in recurrent or metastatic head and neck squamous cell carcinoma: updated results of the phase III KEYNOTE-048 study. J Clin Oncol. (2023) 41:790–802. doi: 10.1200/JCO.21.02508
232. Harrington KJ, Kong A, Mach N, Chesney JA, Fernandez BC, Rischin D, et al. Talimogene laherparepvec and pembrolizumab in recurrent or metastatic squamous cell carcinoma of the head and neck (MASTERKEY-232): A multicenter, phase 1b study. Clin Cancer Res. (2020) 26:5153–61. doi: 10.1158/1078-0432.CCR-20-1170
233. Mell LK, Brumund KT, Daniels GA, Advani SJ, Zakeri K, Wright ME, et al. Phase I trial of intravenous oncolytic vaccinia virus (GL-ONC1) with cisplatin and radiotherapy in patients with locoregionally advanced head and neck carcinoma. Clin Cancer Res. (2017) 23:5696–702. doi: 10.1158/1078-0432.CCR-16-3232
234. Moss B. Poxvirus entry and membrane fusion. Virology. (2006) 344:48–54. doi: 10.1016/j.virol.2005.09.037
235. Smith GL, Vanderplasschen A, and Law M. The formation and function of extracellular enveloped vaccinia virus. J Gen Virol. (2002) 83:2915–31. doi: 10.1099/0022-1317-83-12-2915
236. Raja K. Comments on “Oncolytic virotherapy for oral squamous cell carcinoma using replication-competent viruses. Oral Oncol. (2024) 155:106897. doi: 10.1016/j.oraloncology.2024.106897
237. Gohara S, Shinohara K, Yoshida R, Kariya R, Tazawa H, Hashimoto M, et al. An oncolytic virus as a promising candidate for the treatment of radioresistant oral squamous cell carcinoma. Mol Ther - Oncolytics. (2022) 27:141–56. doi: 10.1016/j.omto.2022.10.001
238. Shah MA, Eads JR, Sarkar S, Khan S, Sharaiha R, Carr-Locke D, et al. Phase II study of telomelysin (OBP-301) in combination with pembrolizumab in gastroesophageal (GEA) adenocarcinoma. J Clin Oncol. (2023) 41:4052–. doi: 10.1200/JCO.2023.41.16_suppl.4052
239. Hegde PS and Chen DS. Top 10 challenges in cancer immunotherapy. Immunity. (2020) 52:17–35. doi: 10.1016/j.immuni.2019.12.011
240. Tang J, Shalabi A, and Hubbard-Lucey VM. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol. (2018) 29:84–91. doi: 10.1093/annonc/mdx755
241. Khalil A and Jameson MJ. The EGFR inhibitor gefitinib enhanced the response of human oral squamous cell carcinoma to cisplatin in vitro. Drugs R D. (2017) 17:545–55. doi: 10.1007/s40268-017-0204-x
242. Costa V, Kowalski LP, Coutinho-Camillo CM, Begnami MD, Calsavara VF, Neves JI, et al. EGFR amplification and expression in oral squamous cell carcinoma in young adults. Int J Oral Maxillofac Surg. (2018) 47:817–23. doi: 10.1016/j.ijom.2018.01.002
243. Li H, Zhang Y, Xu M, and Yang D. Current trends of targeted therapy for oral squamous cell carcinoma. J Cancer Res Clin Oncol. (2022) 148:2169–86. doi: 10.1007/s00432-022-04028-8
244. El-Hamid ESA, Gamal-Eldeen AM, and Sharaf Eldeen AM. Liposome-coated nano doxorubicin induces apoptosis on oral squamous cell carcinoma CAL-27 cells. Arch Oral Biol. (2019) 103:47–54. doi: 10.1016/j.archoralbio.2019.05.011
245. Yan X, Chen YR, Song YF, Ye J, Yang M, Xu BB, et al. Advances in the application of supramolecular hydrogels for stem cell delivery and cartilage tissue engineering. Front Bioeng Biotechnol. (2020) 8:847. doi: 10.3389/fbioe.2020.00847
246. Silva JPN, Pinto B, Monteiro L, Silva PMA, and Bousbaa H. Combination therapy as a promising way to fight oral cancer. Pharmaceutics. (2023) 15. doi: 10.3390/pharmaceutics15061653
247. Cheng Y, Li S, Gao L, Zhi K, and Ren W. The molecular basis and therapeutic aspects of cisplatin resistance in oral squamous cell carcinoma. Front Oncol. (2021) 11:761379. doi: 10.3389/fonc.2021.761379
248. He Y, Tai S, Deng M, Fan Z, Ping F, He L, et al. Metformin and 4SC-202 synergistically promote intrinsic cell apoptosis by accelerating ΔNp63 ubiquitination and degradation in oral squamous cell carcinoma. Cancer Med. (2019) 8:3479–90. doi: 10.1002/cam4.2206
249. Satgunaseelan L, Porazinski S, Strbenac D, Istadi A, Willet C, Chew T, et al. Oral squamous cell carcinoma in young patients show higher rates of EGFR amplification: implications for novel personalized therapy. Front Oncol. (2021) 11:750852. doi: 10.3389/fonc.2021.750852
250. Wang N, Ma T, and Yu B. Targeting epigenetic regulators to overcome drug resistance in cancers. Signal Transduct Target Ther. (2023) 8:69. doi: 10.1038/s41392-023-01341-7
251. Mascitti M, Orsini G, Tosco V, Monterubbianesi R, Balercia A, Putignano A, et al. An overview on current non-invasive diagnostic devices in oral oncology. Front Physiol. (2018) 9:1510. doi: 10.3389/fphys.2018.01510
252. Menditti D, Santagata M, Imola G, Staglianò S, Vitagliano R, Boschetti CE, et al. Personalized medicine in oral oncology: imaging methods and biological markers to support diagnosis of oral squamous cell carcinoma (OSCC): A narrative literature review. J Pers Med. (2023) 13. doi: 10.3390/jpm13091397
253. Bissinger O, Kolk A, Drecoll E, Straub M, Lutz C, Wolff KD, et al. EGFR and Cortactin: Markers for potential double target therapy in oral squamous cell carcinoma. Exp Ther Med. (2017) 14:4620–6. doi: 10.3892/etm.2017.5120
254. Awawdeh MA, Sasikumar R, Aboalela AA, Siddeeqh S, Gopinathan PA, Sawair F, et al. Evaluation of Prognostic Significance of the Expression of p53, Cyclin D1, EGFR in Advanced Oral Squamous Cell Carcinoma after Chemoradiation—A Systematic Review. Appl Sci. (2023) 13. doi: 10.3390/app13095292
255. Kadashetti V, Patil N, Datkhile K, Kanetakar S, and Shivakumar KM. Analysis of expression of p53, p63 and proliferating cell nuclear antigen proteins in odontogenic keratocyst: An immunohistochemical study. J Oral Maxillofac Pathol. (2020) 24:273–8. doi: 10.4103/jomfp.JOMFP_203_19
256. Liu R, Sun K, Wang Y, Jiang Y, Kang J, and Ma H. The effects of proliferating cell nuclear antigen and p53 in patients with oral squamous cell carcinoma: a systematic review and meta-analysis. Ann Transl Med. (2021) 9:1739. doi: 10.21037/atm-21-6133
257. Kang JJ, Ko A, Kil SH, Mallen-St Clair J, Shin DS, Wang MB, et al. EGFR pathway targeting drugs in head and neck cancer in the era of immunotherapy. Biochim Biophys Acta Rev Cancer. (2023) 1878:188827. doi: 10.1016/j.bbcan.2022.188827
258. Bossi P, Resteghini C, Paielli N, Licitra L, Pilotti S, and Perrone F. Prognostic and predictive value of EGFR in head and neck squamous cell carcinoma. Oncotarget. (2016) 7:74362–79. doi: 10.18632/oncotarget.11413
259. Saawarn S, Astekar M, Saawarn N, Dhakar N, and Gomateshwar Sagari S. Cyclin d1 expression and its correlation with histopathological differentiation in oral squamous cell carcinoma. ScientificWorldJournal. (2012) 2012:978327. doi: 10.1100/2012/978327
260. Huang SF, Cheng SD, Chuang WY, Chen IH, Liao CT, Wang HM, et al. Cyclin D1 overexpression and poor clinical outcomes in Taiwanese oral cavity squamous cell carcinoma. World J Surg Oncol. (2012) 10:40. doi: 10.1186/1477-7819-10-40
261. Shin YJ, Vu H, Lee JH, and Kim HD. Diagnostic and prognostic ability of salivary MMP-9 for oral squamous cell carcinoma: A pre-/post-surgery case and matched control study. PloS One. (2021) 16:e0248167. doi: 10.1371/journal.pone.0248167
262. Shreya Reddy CS, Usman P.P AS, Ganapathy DM, K.P A, and Sekar D. MicroRNA-21 as a biomarker in terminal stage oral squamous cell carcinoma (OSCC) in the South Indian population. Oral Oncol Rep. (2024) 9:100139. doi: 10.1016/j.oor.2023.100139
263. Yoon AJ, Wang S, Shen J, Robine N, Philipone E, Oster MW, et al. Prognostic value of miR-375 and miR-214-3p in early stage oral squamous cell carcinoma. Am J Transl Res. (2014) 6:580–92.
264. Lopes VKM, Jesus AS, Souza LL, Miyahara LAN, Guimaraes DM, Pontes HAR, et al. Ki-67 protein predicts survival in oral squamous carcinoma cells: an immunohistochemical study. Braz Oral Res. (2017) 31:e66. doi: 10.1590/1807-3107bor-2017.vol31.0066
265. Govindaraj PK, Kallarakkal TG, Mohd Zain R, Tilakaratne WM, and Lew HL. Expression of Ki-67, Cornulin and ISG15 in non-involved mucosal surgical margins as predictive markers for relapse in oral squamous cell carcinoma (OSCC). PloS One. (2021) 16:e0261575. doi: 10.1371/journal.pone.0261575
266. Kawaguchi H, El-Naggar AK, Papadimitrakopoulou V, Ren H, Fan YH, Feng L, et al. Podoplanin: a novel marker for oral cancer risk in patients with oral premalignancy. J Clin Oncol. (2008) 26:354–60. doi: 10.1200/JCO.2007.13.4072
267. Lenouvel D, Gonzalez-Moles MA, Ruiz-Avila I, Chamorro-Santos C, Gonzalez-Ruiz L, Gonzalez-Ruiz I, et al. Clinicopathological and prognostic significance of PD-L1 in oral cancer: A preliminary retrospective immunohistochemistry study. Oral Dis. (2021) 27:173–82. doi: 10.1111/odi.13509
268. Ries J, Agaimy A, Wehrhan F, Baran C, Bolze S, Danzer E, et al. Importance of the PD-1/PD-L1 axis for Malignant transformation and risk assessment of oral leukoplakia. Biomedicines. (2021) 9. doi: 10.3390/biomedicines9020194
269. Christianto S, Li KY, Huang TH, and Su YX. The prognostic value of human papilloma virus infection in oral cavity squamous cell carcinoma: A meta-analysis. Laryngoscope. (2022) 132:1760–70. doi: 10.1002/lary.29996
270. Doll C, Steffen C, Beck-Broichsitter B, Richter M, Neumann K, Pohrt A, et al. The Prognostic Significance of p16 and its Role as a Surrogate Marker for Human Papilloma Virus in Oral Squamous Cell Carcinoma: An Analysis of 281 Cases. Anticancer Res. (2022) 42:2405–13. doi: 10.21873/anticanres.15719
271. Alsafadi R and Manadili A. The correlation between CD44 and angiogenesis in oral squamous cell carcinoma induced in Buccal pouch in Syrian hamster that underwent radiotherapy. Asian Pac J Cancer Prev. (2022) 23:3571–6. doi: 10.31557/APJCP.2022.23.10.3571
272. Arora R, Haynes L, Kumar M, McNeil R, Ashkani J, Nakoneshny SC, et al. NCBP2 and TFRC are novel prognostic biomarkers in oral squamous cell carcinoma. Cancer Gene Ther. (2023) 30:752–65. doi: 10.1038/s41417-022-00578-8
273. Li M, Wei Y, Huang W, Wang C, He S, Bi S, et al. Identifying prognostic biomarkers in oral squamous cell carcinoma: an integrated single-cell and bulk RNA sequencing study on mitophagy-related genes. Sci Rep. (2024) 14:19992. doi: 10.1038/s41598-024-70498-0
274. Luan CW, Yang HY, Tsai YT, Hsieh MC, Chou HH, and Chen KS. Prognostic value of C-reactive protein-to-albumin ratio in head and neck cancer: A meta-analysis. Diagnostics (Basel). (2021) 11. doi: 10.3390/diagnostics11030403
275. Nalecz D, Swietek A, Hudy D, Wiczkowski K, Zlotopolska Z, and Strzelczyk JK. Assessment of concentration KRT6 proteins in tumor and matching surgical margin from patients with head and neck squamous cell carcinoma. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms25137356
276. Ghuwalewala S, Ghatak D, Das P, Dey S, Sarkar S, Alam N, et al. CD44(high)CD24(low) molecular signature determines the Cancer Stem Cell and EMT phenotype in Oral Squamous Cell Carcinoma. Stem Cell Res. (2016) 16:405–17. doi: 10.1016/j.scr.2016.02.028
277. Mankapure PK, Barpande SR, Bhavthankar JD, and Mandale M. Serum big endothelin-1 as a biomarker in oral squamous cell carcinoma patients: an analytical study. J Appl Oral Sci. (2015) 23:491–6. doi: 10.1590/1678-775720150125
278. Hu S, Arellano M, Boontheung P, Wang J, Zhou H, Jiang J, et al. Salivary proteomics for oral cancer biomarker discovery. Clin Cancer Res. (2008) 14:6246–52. doi: 10.1158/1078-0432.CCR-07-5037
279. Salunkhe V, Mahajan A, Prakash N, Pradeep GL, Patil R, and Gajdhar SK. Galectin-1 expression in oral squamous cell carcinoma: An immunohistochemical study. J Oral Maxillofac Pathol. (2020) 24:186. doi: 10.4103/jomfp.JOMFP_240_19
280. Chen IH, Liao CT, Wang HM, Huang JJ, Kang CJ, and Huang SF. Using SCC antigen and CRP levels as prognostic biomarkers in recurrent oral cavity squamous cell carcinoma. PloS One. (2014) 9:e103265. doi: 10.1371/journal.pone.0103265
281. Roi A, Rusu LC, Roi CI, Luca RE, Boia S, and Munteanu RI. A new approach for the diagnosis of systemic and oral diseases based on salivary biomolecules. Dis Markers. (2019) 2019:8761860. doi: 10.1155/2019/8761860
282. Zhang CZ, Cheng XQ, Li JY, Zhang P, Yi P, Xu X, et al. Saliva in the diagnosis of diseases. Int J Oral Sci. (2016) 8:133–7. doi: 10.1038/ijos.2016.38
283. Moskovitz JM and Ferris RL. Tumor immunology and immunotherapy for head and neck squamous cell carcinoma. J Dent Res. (2018) 97:622–6. doi: 10.1177/0022034518759464
284. Cramer JD, Burtness B, and Ferris RL. Immunotherapy for head and neck cancer: Recent advances and future directions. Oral Oncol. (2019) 99:104460. doi: 10.1016/j.oraloncology.2019.104460
285. Emancipator K, Huang L, Aurora-Garg D, Bal T, Cohen EEW, Harrington K, et al. Comparing programmed death ligand 1 scores for predicting pembrolizumab efficacy in head and neck cancer. Mod Pathol. (2021) 34:532–41. doi: 10.1038/s41379-020-00710-9
286. Zandberg DP, Algazi AP, Jimeno A, Good JS, Fayette J, Bouganim N, et al. Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: Results from a single-arm, phase II study in patients with ≥25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur J Cancer. (2019) 107:142–52. doi: 10.1016/j.ejca.2018.11.015
287. Rebelatto MC, Midha A, Mistry A, Sabalos C, Schechter N, Li X, et al. Development of a programmed cell death ligand-1 immunohistochemical assay validated for analysis of non-small cell lung cancer and head and neck squamous cell carcinoma. Diagn Pathol. (2016) 11:95. doi: 10.1186/s13000-016-0545-8
288. Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. (2018) 362. doi: 10.1126/science.aar3593
289. Strickler JH, Hanks BA, and Khasraw M. Tumor mutational burden as a predictor of immunotherapy response: is more always better? Clin Cancer Res. (2021) 27:1236–41. doi: 10.1158/1078-0432.CCR-20-3054
290. Almangush A, Bello IO, Heikkinen I, Hagstrom J, Haglund C, Kowalski LP, et al. Stromal categorization in early oral tongue cancer. Virchows Arch. (2021) 478:925–32. doi: 10.1007/s00428-020-02930-5
291. Almangush A, Leivo I, and Mäkitie AA. Overall assessment of tumor-infiltrating lymphocytes in head and neck squamous cell carcinoma: time to take notice. Acta Otolaryngol. (2020) 140:246–8. doi: 10.1080/00016489.2020.1720284
292. Almangush A, Leivo I, and Makitie AA. Biomarkers for immunotherapy of oral squamous cell carcinoma: current status and challenges. Front Oncol. (2021) 11:616629. doi: 10.3389/fonc.2021.616629
293. Cao M, Shi E, Wang H, Mao L, Wu Q, Li X, et al. Personalized targeted therapeutic strategies against oral squamous cell carcinoma. An evidence-based review of literature. Int J Nanomed. (2022) 17:4293–306. doi: 10.2147/IJN.S377816
294. Nagaraju R and Patil D. Personalised precision medicine - A novel approach for oral cancer management. In: Sridharan G, editor. Oral Cancer - Current Concepts and Future Perspectives. IntechOpen, Rijeka (2021).
295. Babu S, Manavalan MJ, jasmine Sh, and Krishnan M. Tumor microenvironment in oral squamous cell carcinoma: Implications for novel therapies. Oral Oncol Rep. (2024) 12:100666. doi: 10.1016/j.oor.2024.100666
296. Li C, Dong X, and Li B. Tumor microenvironment in oral squamous cell carcinoma. Front Immunol. (2024) 15:1485174. doi: 10.3389/fimmu.2024.1485174
297. Yang X, Xu X, Zhang C, Ji T, Wan T, and Liu W. The diagnostic value and prospects of gene mutations in circulating tumor DNA for head and neck cancer monitoring. Oral Oncol. (2022) 128:105846. doi: 10.1016/j.oraloncology.2022.105846
298. Wang H, Wu Q, Liu Z, Luo X, Fan Y, Liu Y, et al. Downregulation of FAP suppresses cell proliferation and metastasis through PTEN/PI3K/AKT and Ras-ERK signaling in oral squamous cell carcinoma. Cell Death Disease. (2014) 5:e1155–e. doi: 10.1038/cddis.2014.122
299. Chauhan VP, Chen IX, Tong R, Ng MR, Martin JD, Naxerova K, et al. Reprogramming the microenvironment with tumor-selective angiotensin blockers enhances cancer immunotherapy. Proc Natl Acad Sci. (2019) 116:10674–80. doi: 10.1073/pnas.1819889116
300. Zhang C, Fei Y, Wang H, Hu S, Liu C, Hu R, et al. CAFs orchestrates tumor immune microenvironment-A new target in cancer therapy? Front Pharmacol. (2023) 14:1113378. doi: 10.3389/fphar.2023.1113378
301. Li T, Liu T, Zhu W, Xie S, Zhao Z, Feng B, et al. Targeting MDSC for immune-checkpoint blockade in cancer immunotherapy: current progress and new prospects. Clin Med Insights Oncol. (2021) 15:11795549211035540. doi: 10.1177/11795549211035540
302. Wu B, Zhang B, Li B, Wu H, and Jiang M. Cold and hot tumors: from molecular mechanisms to targeted therapy. Signal Transduction Targeted Ther. (2024) 9:274. doi: 10.1038/s41392-024-01979-x
303. Chen D, Song Z, and Cheng G. Clinical efficacy of first-generation EGFR-TKIs in patients with advanced non-small-cell lung cancer harboring EGFR exon 20 mutations. Onco Targets Ther. (2016) 9:4181–6. doi: 10.2147/OTT.S108242
304. Veeraraghavan VP, Daniel S, Dasari AK, Aileni KR, Patil C, and Patil SR. Emerging targeted therapies in oral oncology: Focus on EGFR inhibition. Oral Oncol Rep. (2024) 11:100592. doi: 10.1016/j.oor.2024.100592
305. Zubair T and Bandyopadhyay D. Small molecule EGFR inhibitors as anti-cancer agents: discovery, mechanisms of action, and opportunities. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24032651
306. Tao G and Chityala PK. Epidermal growth factor receptor inhibitor-induced diarrhea: clinical incidence, toxicological mechanism, and management. Toxicol Res (Camb). (2021) 10:476–86. doi: 10.1093/toxres/tfab026
307. Recuero JK, Fitz JR, Pereira AA, and Bonamigo RR. EGFR inhibitors: clinical aspects, risk factors and biomarkers for acneiform eruptions and other mucosal and cutaneous adverse effects. Bras Dermatol. (2023) 98:429–39. doi: 10.1016/j.abd.2022.10.004
308. Milano G, Spano JP, and Leyland-Jones B. EGFR-targeting drugs in combination with cytotoxic agents: from bench to bedside, a contrasted reality. Br J Cancer. (2008) 99:1–5. doi: 10.1038/sj.bjc.6604373
309. Cuneo KC, Nyati MK, Ray D, and Lawrence TS. EGFR targeted therapies and radiation: Optimizing efficacy by appropriate drug scheduling and patient selection. Pharmacol Ther. (2015) 154:67–77. doi: 10.1016/j.pharmthera.2015.07.002
310. Saba NF, Chen ZG, Haigentz M, Bossi P, Rinaldo A, Rodrigo JP, et al. Targeting the EGFR and immune pathways in squamous cell carcinoma of the head and neck (SCCHN): forging a new alliance. Mol Cancer Ther. (2019) 18:1909–15. doi: 10.1158/1535-7163.MCT-19-0214
311. Bayat Mokhtari R, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B, et al. Combination therapy in combating cancer. Oncotarget. (2017) 8:38022–43. doi: 10.18632/oncotarget.16723
312. Ju W-t, Xia R-h, Zhu D-w, Dou S-j, Zhu G-p, Dong M-j, et al. A pilot study of neoadjuvant combination of anti-PD-1 camrelizumab and VEGFR2 inhibitor apatinib for locally advanced resectable oral squamous cell carcinoma. Nat Commun. (2022) 13:5378. doi: 10.1038/s41467-022-33080-8
313. Adkins D, Oppelt PJ, Ley JC, Myneni PC, Gogula VR, Kanumuri S, et al. Oral NRC-2694-A in combination with paclitaxel as therapy for recurrent and/or metastatic head and neck squamous cell carcinoma (R/M-HNSCC) that progressed on or after an immune checkpoint inhibitor (ICI): A multi-center, single-arm, phase 2 trial. J Clin Oncol. (2023) 41:TPS6108–TPS. doi: 10.1200/JCO.2023.41.16_suppl.TPS6108
314. Hsieh CY, Lein MY, Yang SN, Wang YC, Lin YJ, Lin CY, et al. Dose-dense TPF induction chemotherapy for locally advanced head and neck cancer: a phase II study. BMC Cancer. (2020) 20:832. doi: 10.1186/s12885-020-07347-6
315. Kartini D, Taher A, Panigoro SS, Setiabudy R, Jusman SW, Haryana SM, et al. Effect of melatonin supplementation in combination with neoadjuvant chemotherapy to miR-210 and CD44 expression and clinical response improvement in locally advanced oral squamous cell carcinoma: a randomized controlled trial. J Egypt Natl Canc Inst. (2020) 32:12. doi: 10.1186/s43046-020-0021-0
316. Liu HM, Xiong XP, Yu ZL, Shao Z, Chen GL, Liu YT, et al. Neoadjuvant immunotherapy with or without chemotherapy in locally advanced oral squamous cell carcinoma: Randomized, two-arm, phase 2 trial. Cell Rep Med. (2025) 6:101930. doi: 10.1016/j.xcrm.2025.101930
317. Zhong L-P, Huang Y-y, Sun J-j, Li J, Dong M-j, Zhu G, et al. Neoadjuvant trial with toripalimab, albumin paclitaxel, and cisplatin on pathological response in locally advanced resectable oral squamous cell carcinoma (Illuminate Trial). J Clin Oncol. (2022) 40:e18067–e.
318. Tao Y, Aupérin A, Sun X, Sire C, Martin L, Coutte A, et al. Avelumab-cetuximab-radiotherapy versus standards of care in locally advanced squamous-cell carcinoma of the head and neck: The safety phase of a randomised phase III trial GORTEC 2017-01 (REACH). Eur J Cancer. (2020) 141:21–9. doi: 10.1016/j.ejca.2020.09.008
319. Knochelmann HM, Horton JD, Liu S, Armeson K, Kaczmar JM, Wyatt MM, et al. Neoadjuvant presurgical PD-1 inhibition in oral cavity squamous cell carcinoma. Cell Rep Med. (2021) 2:100426. doi: 10.1016/j.xcrm.2021.100426
320. Miura KI, Otsuru M, Fukushima H, Omori K, Naruse T, Umeda M, et al. Clinical study on postoperative treatment for patients at high risk of oral squamous cell carcinoma recurrence. Cureus. (2025) 17:e78395. doi: 10.7759/cureus.78395
321. Chen M, Huang Y, Zhang S, Zheng Y, Zeng T, Chen C, et al. Camrelizumab in combination with chemotherapy versus concurrent chemoradiotherapy for the conversion of locally advanced unresectable oesophageal squamous carcinoma: protocol for a two-arm, open-label phase II trial. BMJ Open. (2024) 14:e075421. doi: 10.1136/bmjopen-2023-075421
322. Yoon AJ, Carvajal RD, Graboyes EM, Kaczmar JM, Albergotti WG, Kejner AE, et al. Pilot clinical trial of neoadjuvant toll-like receptor 7 agonist (Imiquimod) immunotherapy in early-stage oral squamous cell carcinoma. Front Immunol. (2025) 16. doi: 10.3389/fimmu.2025.1530262
323. Ling Z, Cheng B, and Tao X. Epithelial-to-mesenchymal transition in oral squamous cell carcinoma: Challenges and opportunities. Int J Cancer. (2021) 148:1548–61. doi: 10.1002/ijc.33352
324. Karpiński TM. Role of oral microbiota in cancer development. Microorganisms. (2019) 7. doi: 10.3390/microorganisms7010020
325. Chakraborty R, Vickery K, Darido C, Ranganathan S, and Hu H. Bacterial antigens reduced the inhibition effect of capsaicin on Cal 27 oral cancer cell proliferation. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22168686
Keywords: oral squamous cell carcinoma (OSCC), molecular pathways, genetic predisposition, immunotherapy, therapeutic challenges
Citation: Shebbo S, Alateyah N, Yassin E, Mahmoud DES, Tamimi F, Anweigi L, Elhissi A, Abou-Saleh H, Elrayess MA and Agouni A (2025) Unravelling molecular mechanism of oral squamous cell carcinoma and genetic landscape: an insight into disease complexity, available therapies, and future considerations. Front. Immunol. 16:1626243. doi: 10.3389/fimmu.2025.1626243
Received: 10 May 2025; Accepted: 21 July 2025;
Published: 13 August 2025.
Edited by:
Abdullah Saeed, City of Hope National Medical Center, United StatesReviewed by:
Jin-Fen Xiao, University of Texas MD Anderson Cancer Center, United StatesViji Remadevi, University of Kansas Medical Center, United States
Copyright © 2025 Shebbo, Alateyah, Yassin, Mahmoud, Tamimi, Anweigi, Elhissi, Abou-Saleh, Elrayess and Agouni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdelali Agouni, YWFnb3VuaUBxdS5lZHUucWE=
†These authors have contributed equally to this work and share first authorship
‡ORCID: Abdelali Agouni, orcid.org/0000-0002-8363-1582
 Salima Shebbo
Salima Shebbo Nooralhuda Alateyah2†
Nooralhuda Alateyah2† Doaa El Sayed Mahmoud
Doaa El Sayed Mahmoud Faleh Tamimi
Faleh Tamimi Lamyia Anweigi
Lamyia Anweigi Haissam Abou-Saleh
Haissam Abou-Saleh Mohamed A. Elrayess
Mohamed A. Elrayess Abdelali Agouni
Abdelali Agouni