- 1Department of Translational Medical Sciences, University of Naples Federico II, Naples, Italy
- 2Istituti Clinici Scientifici Maugeri-Scientific Institute for Research Hospitalization and Healthcare (IRCCS) Scientific Institute of Telese Terme, Benevento, Italy
- 3World Allergy Organization (WAO), Center of Excellence (CoE), Naples, Italy
- 4Center for Basic and Clinical Immunology Research (CISI), University of Naples Federico II, Naples, Italy
- 5Benaroya Research Institute, Seattle, WA, United States
- 6Division of Allergy and Infectious Diseases, University of Washington, Seattle, WA, United States
- 7Department of Immunology, University of Washington, Seattle, WA, United States
Thymic stromal lymphopoietin (TSLP) is an alarmin cytokine possessing a plethora of pleiotropic properties. Human and mouse TSLP exerts their activity via a heterodimeric complex composed of TSLP receptor (TSLPR) chain and IL-7Rα. TSLP is predominantly expressed by epithelial cells and keratinocytes but can also be produced by several immune cells and some cancers. TSLP activates a plethora of immune cells implicated in inflammation, angiogenesis and tumorigenesis. In addition to its role in barrier immunity, recent studies have a role for TSLP in cancer development. This includes both human hematologic cancers and several solid tumors (largely carcinomas). The role of TSLP in human and experimental cancers has been the focus of several studies, with somewhat contradictory findings. In this Review, we will highlight recent advances in TSLP immunobiology in the context of human and experimental cancers. We will also discuss recent findings demonstrating that an anti-TSLP monoclonal antibody (mAb) can exert a protective effect in a mouse model of colorectal cancer. The recent approval of an anti-TSLP mAb for asthma treatment also emphasizes the urgent need for additional research on the role of TSLP, a Janus cytokine, in tumorigenesis.
Introduction
Thymic stromal lymphopoietin (TSLP) is a member of the 4-helix bundle cytokine family, and a distant paralog of IL-7 (1). As the name suggests, TSLP was first identified in the supernatant of a mouse thymic stromal cell line for its activity in supporting immature B cell proliferation and development (2–4). A human TSLP homolog was subsequently identified in humans using in silico methods (5, 6). Several groups isolated a TSLP-binding protein in both humans and mice [referred to as TSLP receptor (TSLPR) in mice and cytokine receptor-like factor 2 (CRLF2) in humans] (7–10). Sequence analysis found that TSLPR was most closely related to the common gamma chain (γc) (7). It is now known that the functional, high affinity, TSLPR complex is a heterodimer of TSLPR and interleukin 7 receptor alpha (IL-7Rα; Figure 1) (7, 8). Cross-species homology for both the cytokine and its receptor is relatively low (~40% for each), although functionally they appear to be quite similar. Thus, the role of this cytokine axis is conserved between human and mouse despite of a loss of sequence identity.
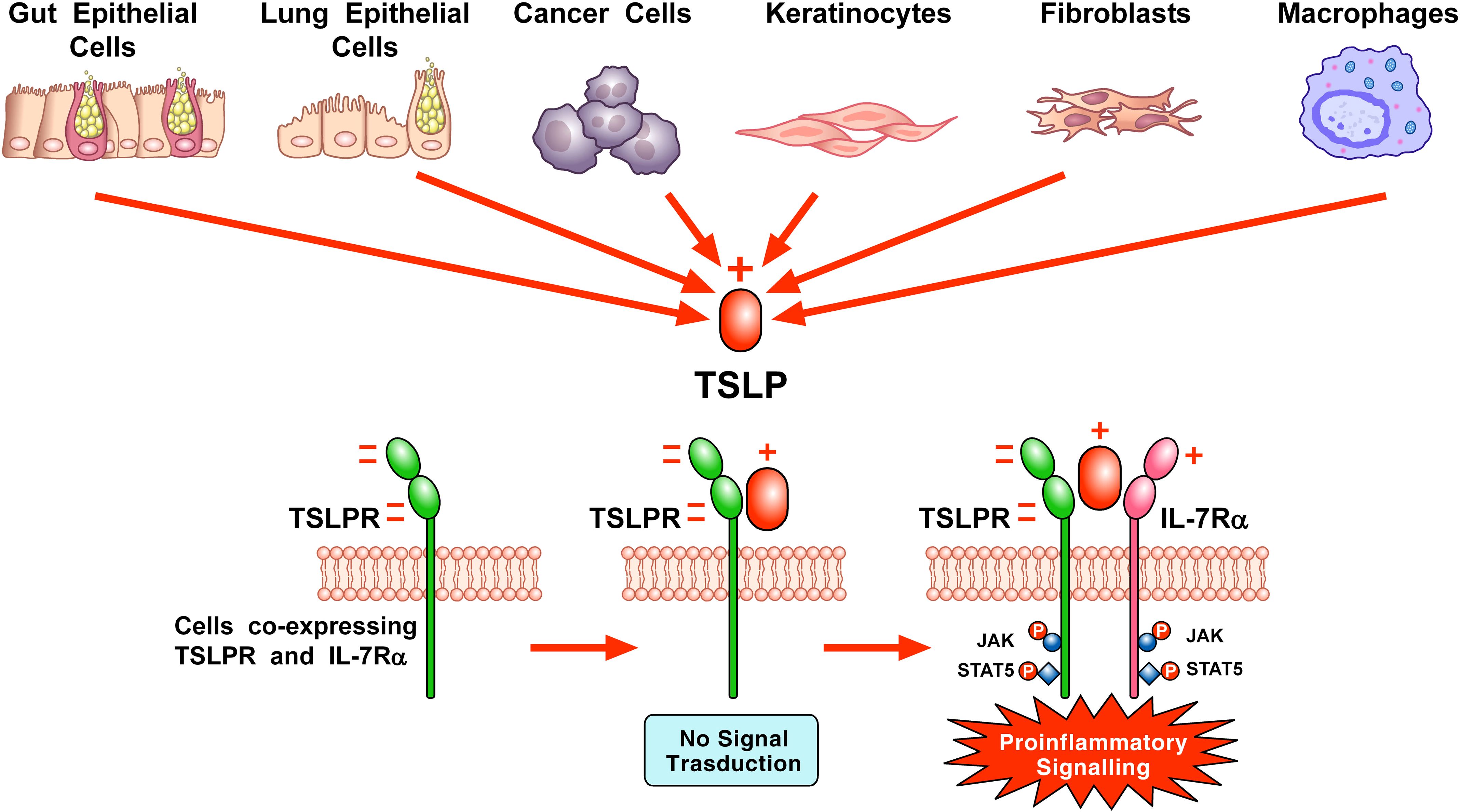
Figure 1. Schematic representation of TSLP-mediated receptor activation and signaling on the surface of cellular targets. A variety of triggers (e.g., cytokines, cigarette smoke extracts, viral, bacterial and fungal products, allergens, and tryptase) can stimulate the production and release of TSLP by lung and gut epithelial cells, cancer cells, keratinocytes, and/or macrophages. TSLP, positively charged, binds to the highly negatively charged TSLP receptor (TSLPR). Then, IL-7Rα, positively charged, can be recruited to the TSLPR: TSLP binary complex to form the ternary TSLPR-TSLP-IL-7Rα complex (11). This receptor complex phosphorylates Janus kinases (JAKs) and signal transducers and activators of transcription 5 (STAT5) to initiate proinflammatory signaling in target cells (Adapted from Varricchi et al., Front. Immunol 2018).
A primary cellular target for TSLP are dendritic cells (DCs), which upregulate OX40L, CD80, and CD86 in response to TSLP, and TSLP-treated DCs can drive IL-4, IL-5, and IL-13 production from naïve CD4+ T cells upon co-culture (12–15). In addition to its effects on Th2 cell polarization through antigen-presenting cells, TSLP can also act directly on CD4+ and CD8+ T cells, and Treg cells (16–18). TSLP can also promote Th2 cytokine responses through its actions on mast cells, innate lymphoid cells (ILCs), epithelial cells, macrophages, and basophils (19–23). Finally, TSLP was found to play an important role in mouse basophil biology, where in vitro, TSLP could induce basophil maturation from bone marrow precursors in an IL-3 independent manner. Furthermore, TSLP-elicited basophils in vivo were phenotypically distinct from IL-3-elicited basophils (24).
TSLP is expressed at basal levels at mucosal surfaces (e.g., gut and lung), as well as in the skin (5, 25–27). Its expression can be further enhanced through exposure to viral, bacterial, or parasitic pathogens as well as Toll-like receptor (TLR) agonists (22, 28, 29). A link between TSLP expression and atopic disease was first established by Soumelis et al. who showed elevated expression in the lesional skin of individuals with atopic dermatitis (AD) (30). Following that finding, TSLP expression was found in the airways of patients with asthma and in the nasal lavages of individuals with allergic rhinitis (31–33). TSLP levels in asthmatic airways correlated with Th2-attracting chemokine expression and disease severity (33). In eosinophilic esophagitis (EoE), a gain-of-function polymorphism in TSLP is associated with disease in pediatric subjects (34, 35), and TSLP expression was higher in esophageal biopsy samples from children with active EoE compared to subjects with inactive EoE (36).
Historically, physicians have noted that Type(T)-2 inflammatory disorders often develop in an individual patient in a typical sequential order, with AD occurring first, followed by food allergy and then upper and lower airway disease (37). This sequence, often referred to as the “atopic march” (38), highlights the potential role of TSLP and the other epithelial cytokines as initiators and propagators of allergic disease. Studies over the past 20 years have shown TSLP to be an important driver of the atopic march in both humans and rodents. Previous clinical and experimental studies concluded that the role of TSLP-TSLPR axis in cancer was controversial (39–41). Since then, several experimental and clinical studies have shed light on the different mechanisms of the protumorigenic role of TSLP and its isoforms in cancer. In this Review, we will summarize the work on TSLP immunobiology, emerging data regarding TSLP isoforms and a new-found role for TSLP in a wide variety of cancers.
TSLP in type-2 inflammation
Epithelial-derived cytokines, including TSLP, IL-33, IL-25, and TL1A, play critical roles in the development of allergic responses at barrier surfaces (42). These alarmins have been implicated in the pathogenesis of T2 inflammatory diseases, including AD (43), food hypersensitivity reactions (44), asthma (45, 46), CRSwNPs (47) and chronic obstructive pulmonary disease (COPD) (48, 49). The release of these alarmins is stimulated by epithelial exposure to allergens (particularly those rich in proteases), microbes (viruses, bacteria, parasites), and inorganic chemicals. Although the inducing stimuli, cellular sources, target populations and functions of alarmins share similarities, several differences characterize the three epithelial-derived cytokines (23, 42). Actually, there is some evidence that TSLP and IL-33 can synergistically enhance certain aspects of innate T2 airway inflammation (50).
TSLP has diverse effects in Type 2 (T2) inflammation. The most proximal effect of TSLP in this regard, shared with IL-33, is the upregulation of DC expression of OX40L, CD80, and CD86, which are required for T helper T2 cell (TH2) polarization (12). While expression of the IL-33 receptor ST2 on TH2 cells requires prior cell activation, TSLPR expression does not require TH2 cell activation and can be identified on naïve CD4+ T cells (51, 52), suggesting a possibly earlier role for TSLP. There are a number of other significant effects of TSLP on a broad range of cell types, including increased proliferation of T cells (18) and TH2 cells (53) and release of TH2 cytokines and chemokines from mast cells (54), ILCs (21), and macrophages (55) (48, 56). While the role of TSLP in human basophil activation is controversial (23, 57), mouse basophils appear to play an important role in the induction of TSLP-mediated TH2 inflammation (24, 58). Using a mouse model that employed the vitamin D analog MC903 to induce TSLP release from keratinocytes, investigators demonstrated that TSLP-activated DCs prime CD4+ T cells via OX40L signaling to produce IL-3, leading to recruitment of basophils. As these events precede the induction of IL-4 production by T cells, mouse basophils may provide an initial source of IL-4 early in the course of TH2 immune responses, suggesting that this sequential cascade of DCs, T cells, and basophils is critical to T cell expansion and TH2 priming.
The clear role of TSLP in atopic diseases led to the development of a neutralizing anti-TSLP human monoclonal antibody, referred to as tezepelumab. Tezepelumab has been used in clinical trials to treat a variety of T2 conditions, including AD (59), EoE (NCT05583227), asthma (60) and chronic rhinosinusitis with nasal polyps (CRSwNPs) (61). In a small study of patients with moderate-to-severe AD, treatment with tezepelumab resulted in a numerical, but not statistically significant improvement in eczema severity scores, likely due to the use of background medication during the trial (59). Tezepelumab has been extensively tested in patients with severe asthma. A large Phase III trial using tezepelumab in severe asthmatics to decrease exacerbations showed a clear benefit in glucocorticoid-resistant asthma compared to the placebo group (60). Importantly, the frequencies and types of adverse events did not differ between the two groups. Based on these results, tezepelumab has been approved by the American FDA in 2021 and the European EMA in 2023 for treatment of severe asthma. Recently, tezepelumab significantly reduced nasal polyp size, nasal symptoms and the need for nasal polyp surgery or systemic glucocorticoids in severe CRSwNPs compared to placebo (61).
Structural basis of TSLP-mediated receptor activation and signaling
X-ray crystallographic analysis of human TSLP showed that this cytokine has a four-helix bundle structure with four alpha helices (αA, αB, αC, and αD) arranged in an alternating ‘up-up-down-down’ configuration (11, 62). The TSLP four-helix bundle is threaded by three loops (a BC-, AB-, and CD- loop). Human TSLP contains six cysteine residues forming three disulfide bonds (11, 63).
TSLP engages a heterodimeric complex comprising the TSLPR, a type I cytokine receptor, and IL7Rα, a receptor also engaged by IL-7, on several target cells (7, 8). TSLPR, highly negative, binds TSLP containing several positively charged amino acids with high affinity (Kd = 32 nM). Although IL-7Rα does not interact with TSLPR alone, IL-7Rα associates with high affinity (Kd = 29 nM) to the TSLP: TSLPR binary complex (11, 62). TSLP binding induces the dimerization of these receptor chains, triggering Janus kinases (JAKs) and signal transducers and activators of transcription 5 (STAT5) signaling, leading to the transcription of genes in several targets cells (5, 6, 64, 65) (Figure 1).
The interaction of TSLP with TSLPR (site I) is mediated by electrostatic attraction, with a positively charged region on TSLP interfacing with a negatively charged area on TSLPR. This interaction establishes a binary complex with a negative charge, priming it for the addition of IL-7Rα, which has a positive electrostatic potential. Critical contact points for the amino acids involved in TSLP: TSLPR interactions are located in the C-terminal region of αD helix and AB-loop region undergoing conformational changes. The AB- loop offers a link to the αA helix, playing a crucial role in the engagement with IL-7Rα at site II. This interaction is essential for conferring an entropic benefit that facilitates the assembly of a stable T-shaped ternary complex. In addition to the αA helix’s role, the hydrophobic surface of IL-7Rα engages with various outward-facing residues on TSLP’s αC helix, further stabilizing the interaction (11).
TSLP isoforms
Harada et al. first discovered two TSLP isoforms in human bronchial epithelial cells (66, 67). The long form TSLP (lfTSLP), which is the homolog of mouse TSLP, is a small protein of 159 amino acids, which has a signal peptide encoded in the first 28 amino acids at the N-terminal portion of the protein (1, 6) (Figure 2). The amino acids sequence spanning 63 residues in the short form TSLP (sfTSLP) shares homology with the C-terminal segment of the long form. The mRNA encoding sfTSLP was shown to be initiated from an internal promoter in intron 2 of the TSLP gene (66). The relevance of sfTSLP is unclear for a variety of reasons (49). First, sfTSLP mRNA appears to be human specific and there are no reports of a similar variant in other species (40). Second, while there is evidence that the sfTSLP mRNA is constitutively expressed in a variety of tissues, including bronchial and colonic epithelial cells, keratinocytes, and lung fibroblasts (66, 68–72), there is no evidence for expression of a sfTSLP protein (49). This is further complicated by the lack of anti-sfTSLP antibody reagents. Thus, the biological role, if any, of sfTSLP remains largely unknown. Previous research has largely overlooked the application of analytical methods to investigate the differential expression patterns and roles of the two distinct isoforms of TSLP in different cancers.
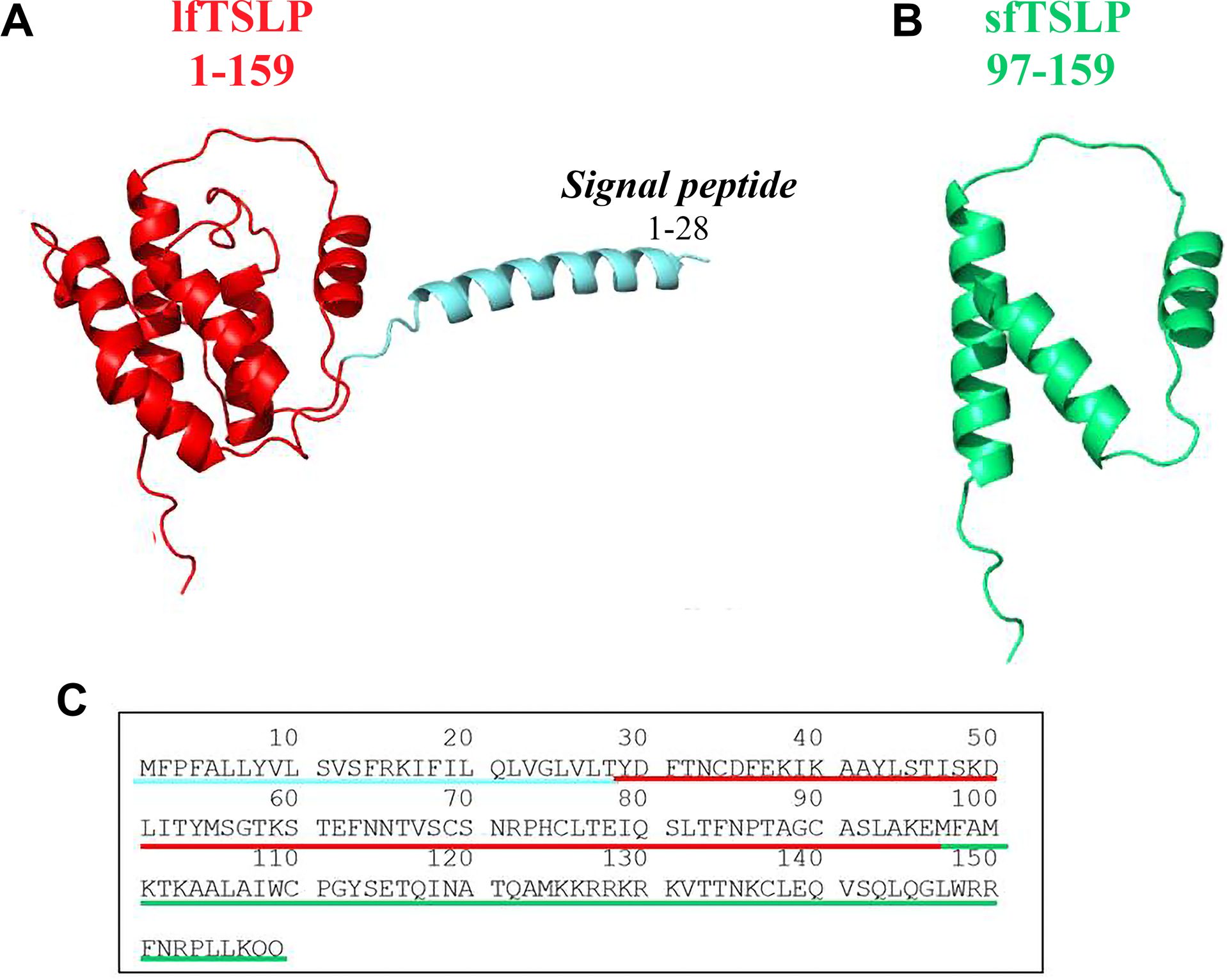
Figure 2. Three-dimensional (3D) structure of human long form TSLP (lfTSLP) and short form TSLP (sfTSLP). (A) TSLP is a small protein of 159 amino acids, which has a signal peptide of 28 amino acids at the N-portion of the protein (6). (B) The sequence of the 63 amino acids of sfTSLP is homologous to the C-terminal portion of the lfTSLP. (C) Amino acid sequence of lfTSLP (underlined in red), the signal peptide (underlined in light blue), and sfTSLP (underlined in green).
There is an additional level of complexity in studying the pathophysiological role of TSLP due to its post-translational cleavage. The protease furin can cleave TSLP, generating fragments of 10 and 4 kDa with different activity on human peripheral blood mononuclear cells compared with the mature cytokine (73). Carboxypeptidase N can also cleave TSLP to form two peptides, which strongly activate human DCs (63). Mast cell-derived tryptase and chymase rapidly cleave TSLP to generate several peptides without apparent biological activity on human lung macrophages (48, 74). These findings emphasize the need for additional studies on the role of post-transcriptionally cleaved products of TSLP in tumor biology.
Immune cellular targets of TSLP
TSLP can modulate the activation of various immune cell populations, including DCs (11, 63, 75, 76), CD4+ T cells and Th2 cells (18, 51). In particular, TSLP signaling in CD4+ T cells programs a pathogenic Th2 cell state (77). TSLP limits primary and recall responses of CD8+ T cell (78), which play a critical role in cancer immunity (79). TSLP is a critical mediator acting on ILC2s (63, 80, 81), and drives the development of Th2 cells (51). TSLP provides critical signals for human (82) and mouse B cell proliferation (83) and also expands bone marrow B cell precursors to support lung metastasis in a breast cancer model (84). TSLP-activated DCs promotes Tfh differentiation from naïve CD4+ T cells (75). Tfh cells are important constituents of tertiary lymphoid structure in human breast cancer (85). Moreover, TSLP influences regulatory T cells (Tregs) (86–88).
Initial studies demonstrated co-expression of TSLP receptor (TSLPR) and IL-7 receptor α chain (IL-7Rα) mRNA in human monocytes, with TSLP stimulation inducing CCL17 production (5). Borriello et al. (89) demonstrated that freshly isolated monocytes do not express detectable levels of TSLPR or IL-7Rα, as assessed by flow cytometry, nor do they exhibit STAT5 phosphorylation in response to TSLP. Exposure to lipopolysaccharide (LPS) induced expression of the TSLPR complex in a subset of monocytes. These results highlighted an unrecognized phenotypic and functional heterogeneity within the human monocyte compartment based on TSLPR expression.
In vivo administration of TSLP modulates the differentiation of alternatively activated macrophages (55). Interestingly, TSLP potentiated CCL17 production induced by IL-4 from murine macrophages. We presented novel evidence demonstrating the constitutive intracellular presence of TSLP within the cytoplasm of human lung macrophages (HLMs) (48). Upon stimulation with both type 2 (T2)-high and T2-low inflammatory stimuli, HLMs secreted TSLP (56, 74). Moreover, the long isoform of TSLP (lfTSLP) stimulated the release of vascular endothelial growth factor A (VEGF-A) from HLMs (48). In contrast, the short isoform of TSLP (sfTSLP) neither induced VEGF-A production nor inhibited the stimulatory effect of lfTSLP. These findings reveal a previously unrecognized feedback loop between HLMs and TSLP that may contribute to the regulation of inflammatory and tumor angiogenesis (48, 90).
Both TSLPR and IL-7Rα are expressed at the mRNA and protein levels in CD34+ progenitor-derived mast cells as well as in mast cells isolated from human lung tissue (91). TSLP, alone or in combination with proinflammatory cytokines such as IL-1β or TNF-α, did not induce mast cell degranulation or the release of lipid mediators (91, 92). Nonetheless, when co-stimulated with IL-1β or TNF-α, TSLP promoted the secretion of multiple cytokines and chemokines (91, 93, 94). Additionally, TSLP has been shown to enhance prostaglandin D2 (PGD2) production in human mast cells in the presence of IL-33 (95). TSLP promoted MRGPRX2-triggered degranulation of human skin mast cells (96, 97).
A notable interspecies divergence between human and murine basophils pertains to their responsiveness to TSLP. In line with previous studies (57, 98), we confirmed that human basophils did not exhibit cytokine release (i.e., IL-4 and IL-13) upon exposure to TSLP (23). Moreover, TSLP stimulation also failed to induce CXCL8 secretion in human basophils. In contrast, murine basophils responded to TSLP with upregulation of mRNA expression and subsequent release of IL-4, IL-13, CXCL1, and CXCL2 (23). These results reinforce the role of TSLP in promoting the differentiation and activation of basophils in various mouse models (24, 36, 99, 100). TSLP induced chemotaxis and the formation of eosinophil DNA extracellular traps from human eosinophils (101, 102). This observation is relevant because there is emerging evidence that eosinophils and their DNA extracellular traps play a role in cancer initiation and growth (103, 104).
Figure 3 shows the constellation of immune and structural cells that can be activated by TSLP.
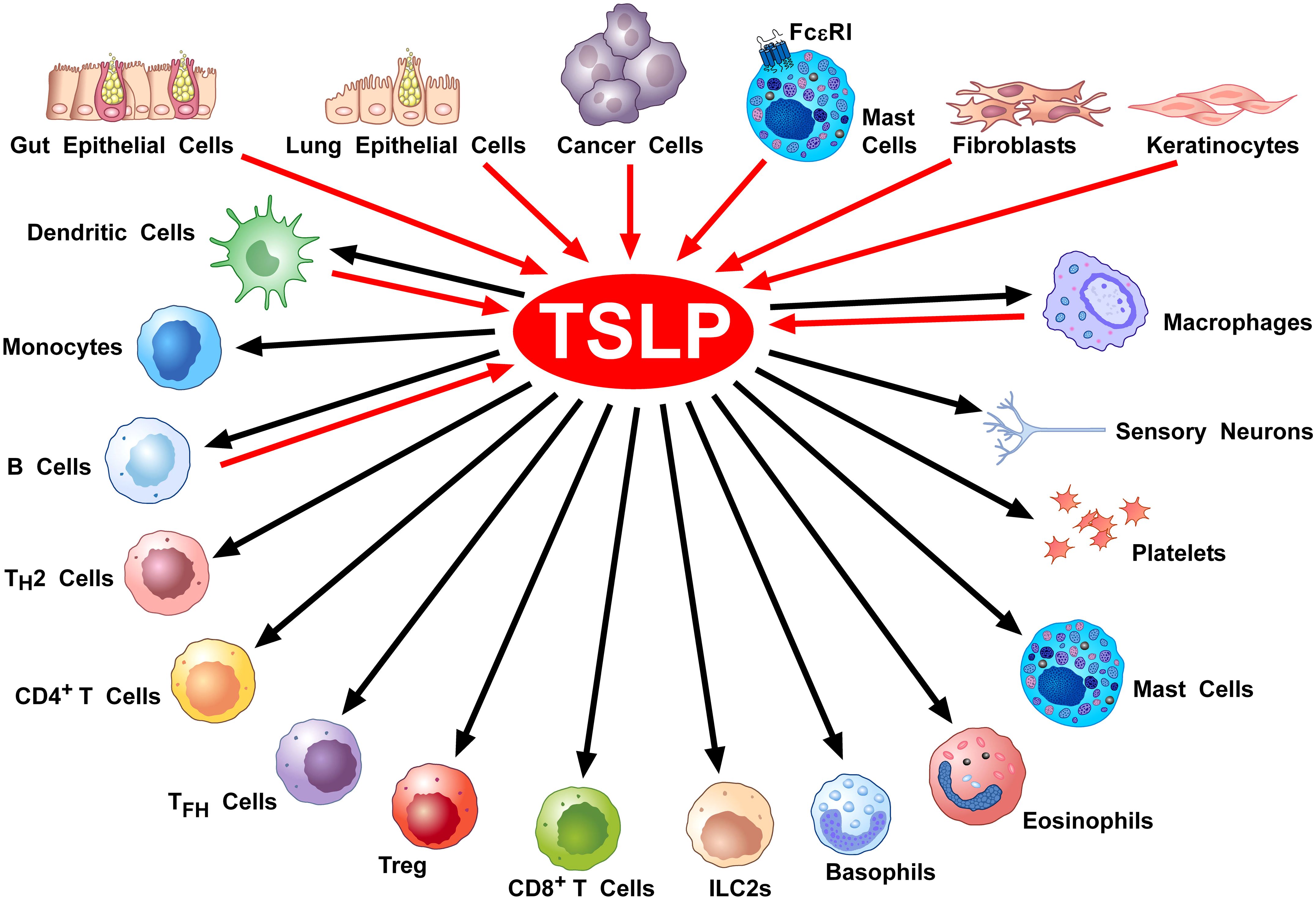
Figure 3. Cellular Sources and Targets of TSLP. A diverse array of triggers can activate lung (28, 29, 91, 105–107) and gut epithelial cells (66, 108–111), keratinocytes (30, 68, 70, 71, 112, 113) and cancer cells (114–118) to release TSLP. This alarmin can also be produced by mast cells (33, 92, 119, 120), DCs (121, 122), lung macrophages (48, 56, 74), and monocytes (56). Tryptase, released by mast cells can activate the protease-activated receptor 2 (PAR2) on fibroblasts (123, 124) and keratinocytes (123) to release TSLP. TSLP activates DCs (11, 63, 75, 76), CD4+ T and Th2 cells (18, 51, 77), ILC2 (63, 80, 81), NKT cells (125), CD8+ T cells (78, 126) and B cells (4, 82), Treg cells (86–88), murine (24) but not human basophils (23, 57), mast cells (91, 93–95), eosinophils (101, 102), macrophages (48, 55, 74), monocytes (48, 89), platelets (127, 128), and sensory neurons (123).
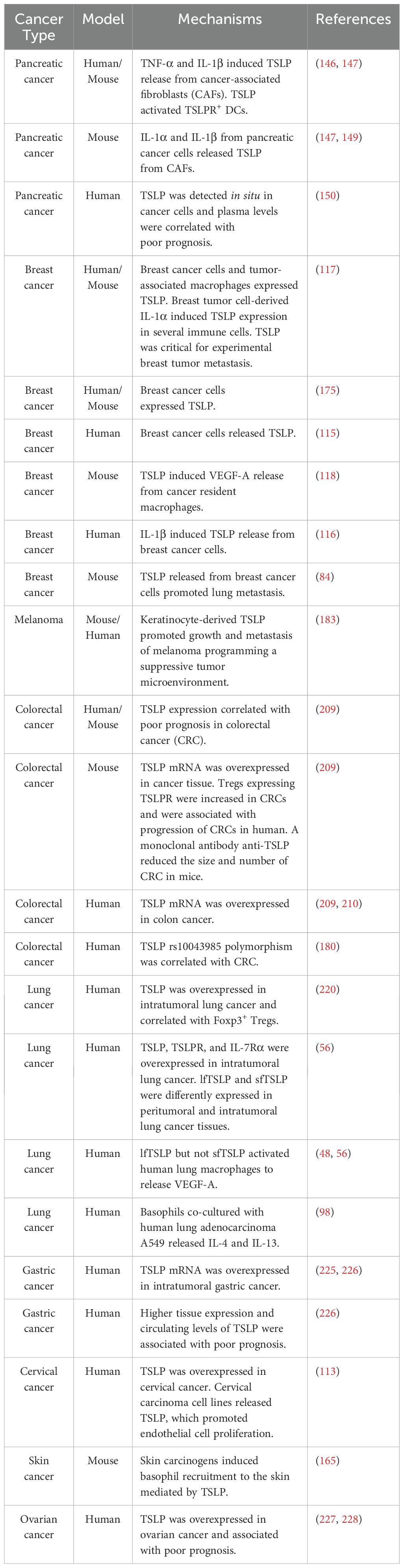
Protumorigenic role of TSLP in hematologic cancers
As previously emphasized, TSLP exerts several pleiotropic effects on cells of innate and adaptive immune system (40) that are directly and/or indirectly involved in the initiation and progression of tumors, angiogenesis and lymphangiogenesis (129–131). Hence, it is not surprising that TSLP would have a significant direct or indirect role in the regulation of experimental and human cancers (39–41).
Figure 4 schematically illustrates the protumorigenic role of TSLP in different hematologic and solid cancers.
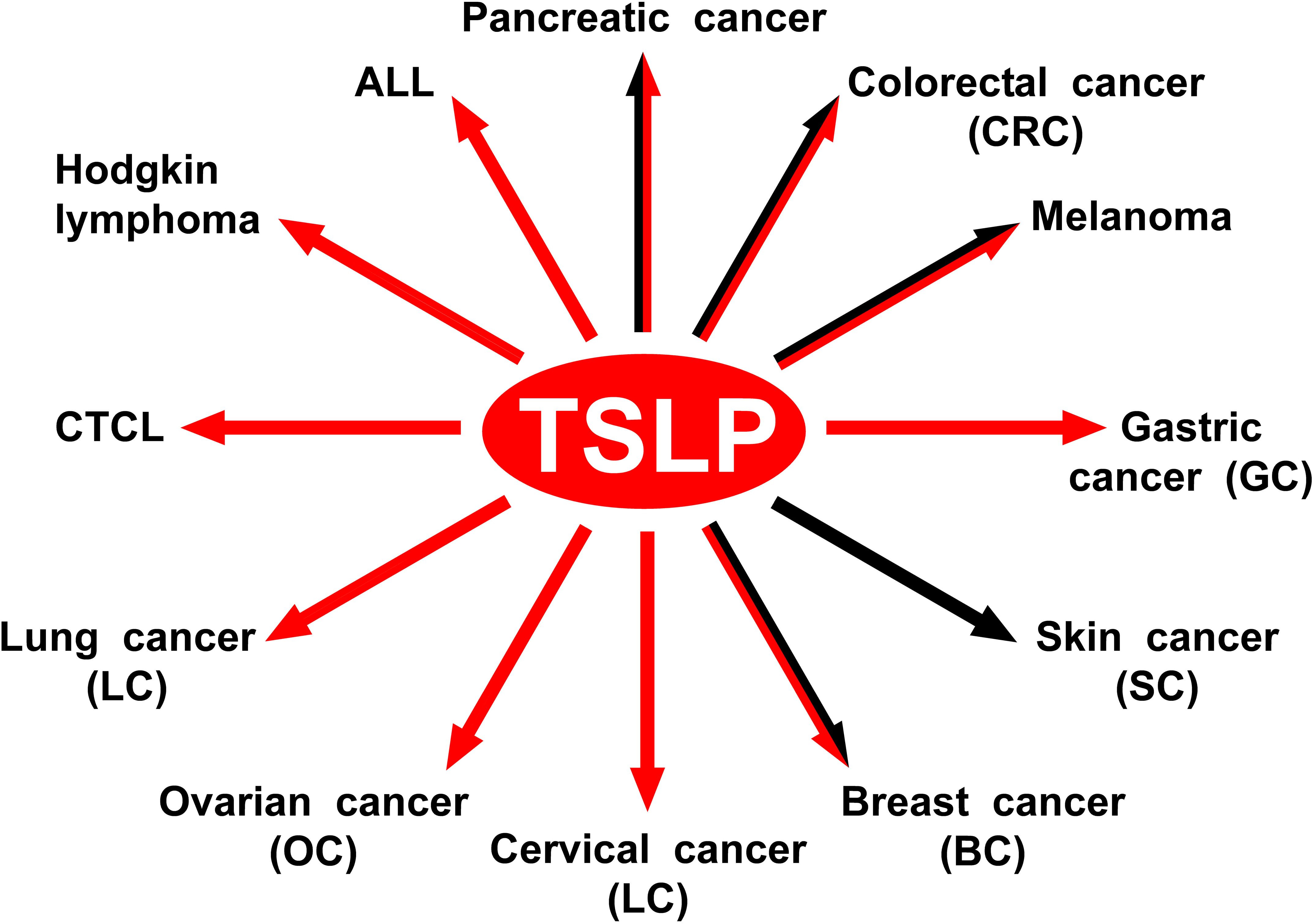
Figure 4. The protumorigenic role of TSLP in different hematologic and solid cancers. The red arrows indicate the human tumors in which TSLP plays a protumorigenic role. The black arrows indicate the experimental tumors in which TSLP appears to play a protumorigenic role.
The cytokine receptor-like factor 2 (CRLF2) locus encodes for human TSLPR (7). Russell et al. first identified genetic rearrangements and mutations in the TSLPR gene in a percentage of pediatric patients with acute lymphoblastic leukemia (ALL) (132). Subsequent studies confirmed and extended the previous observation demonstrating rearrangement of CRLF2 in approximately 15% of both pediatric and adult B-cell ALL (133, 134). A more recent study found CRLF2 rearrangement in approximately 50% of pediatric B-cell ALL (135). In this study, TSLPR was absent in normal precursor B cells, but variably expressed in B-cell ALL by flow cytometry (135, 136). Rearrangements including deletions and translocations of TSLPR can be associated in the majority of B-ALL with activating mutations in the gene encoding the tyrosine kinase JAK2, which signals downstream of the TSLP receptor complex (133–135, 137). TSLP enhanced proliferation of long-term cultures of B-ALL cells (136). CRLF2 overexpression was associated with a poor prognosis among children and adults with B-cell ALL (133, 134, 137–140). A recent study reported CRLF2 rearrangement in 30% of Russian children with B-cell ALL and 72% of CRLF+ were TSLPR+ by flow cytometry (141). Approximately 80% CRLF2 rearranged patients had translocation involving P2RY8, a known indicator of relapse in ALL. A study in a large cohort of 630 pediatric Chinese patients with B-ALL reported a low percentage of P2RYB-CRLF2 (3.33%) and CRLF2 (5.71%) overexpression. P2RYB-CRLF2 identified only a subset of pediatric patients with poor prognosis (142).
TSLP concentrations are increased in plasma and overexpressed in lymph nodes of patients with Hodgkin lymphoma (143). TSLP mRNA is overexpressed in lesional skin and cutaneous T-cell lymphoma (CTCL) (144). TSLP induced the production of Th2 cytokines (e.g., IL-4 and IL-13) from CTCL cell lines and proliferation of CTCL cells through the activation of STAT5.
Studies supporting the protumorigenic role of TSLP in hematologic cancers are outlined in Table 1.
Protumorigenic role of TSLP in solid cancers
Pancreatic cancer
Pancreatic cancer is a very aggressive disease characterized by a predominant Th2 (GATA3+) lymphoid infiltrate (145). Protti and collaborators first demonstrated that human pancreatic cancer [pancreatic ductal adenocarcinoma (PDAC)]-derived TNF-α and IL-1 induced the release of TSLP from cancer-associated fibroblasts (CAFs) (146). This observation was extended showing that TSLP released from CAFs activated TSLPR+ DCs to drive Th2 differentiation mediated by IL-4 released from basophils (147). The translational relevance of these findings was provided showing that IL4 expressing basophils increased in tumor-draining lymph nodes (TDLN) of PDAC patients (148). Basophils in TDLN correlated with Th2 phenotype in tumors and were a negative prognostic marker of patient survival. Studies in a mouse model of pancreatic cancer confirmed a role for basophils during pancreatic cancer progression (147). Collectively, these results demonstrate that TSLP released from CAFs activates DCs, which induce T cells to secrete IL-3. Monocytes resident in TDLN secrete CCL7, which recruits basophils that are activated by IL-3 to release IL-4. This cytokine favors GATA3 expression in Th2 cells. A recent study identified IL-1α and IL-1β released by pancreatic cancer cells and tumor-associated macrophages as relevant stimuli for TSLP release from CAFs (149). The protumorigenic role of TSLP in PDAC was extended by showing that TSLP levels are detected in situ in tumor cells and systematically in advanced cancer patients (150). Moreover, elevated plasma TSLP concentrations were correlated with reduced overall patient survival (150). Although basophils account for 1% or less of the circulating leukocytes both in humans and mice, they have the propensity to infiltrate into the sites of inflammation (151). Basophils share some morphological and functional characteristics with mast cells, but these cells are distinct in many aspects (152). TSLP influences the development (24, 100) and activation of mouse basophils (23). Different models have uncovered unique roles for basophils in Th2 inflammatory responses (152–154) and parasitic infections (155–158). Moreover, there is growing evidence supporting the significant roles of basophils in cancer (159–163).
TSLP can induce mouse basophil maturation in an IL-3-independent manner and TSLP-elicited basophils in vivo were phenotypically distinct from IL-3-elicited basophils (24). TSLP caused the production of these cytokines/chemokines (IL-4, IL-13, CXCL1, and CXCL2) from mouse basophils (23), but did not induce cytokine release from human basophils (23). Basophils are present in the tumor microenvironment (TME) of various human (148, 161, 164, 165) and mouse experimental cancers (99, 148, 165, 166). Their involvement is increasingly recognized as influential in the onset and progression of both solid tumors and hematologic cancers (159, 162, 163, 167).
These cells play protumorigenic roles through different mechanisms. TSLP-activated mouse and human basophils are a major source of IL-4 and IL-13 (23, 57), which favor the polarization towards Th2 and M2 phenotypes (168). Moreover, basophils can release vascular endothelial growth factor-A (VEGF-A) (169) and cysteinyl leukotriene C4 (LTC4) (170, 171), which are implicated in the mechanisms of angiogenesis, tumorigenesis, and metastasis (172, 173).
Breast cancer
Breast cancer is the most common malignancy in women and the second leading cause of cancer-related mortality in females (174). Mouse and human breast cancer cells express TSLP, which promotes Th2 differentiation of CD4+ T cells (175). Human breast cancer is heavily infiltrated by Th2 cells driven by OX40L-expressing DCs in response to cancer-derived TSLP (115). In a mouse model of breast cancer, TSLP activated resident macrophages to release VEGF-A, the most potent proangiogenic factor (118). Macrophages are a major anatomical and functional component of the TME, where they either promote or inhibit tumorigenesis and metastasis depending on their functional state (176, 177).
For decades, macrophages were simplistically classified into two groups, referred to as “classically activated M1” or “alternatively activated M2” endotypes (168). M2-like phenotype is mostly the phenotype of tumor-associated macrophages (TAMs) (168). Different subpopulations of TAMs promote angiogenesis, tumor invasion, suppress cytotoxic T-cell responses and promote the formation of metastasis (178). Single-cell analyses have identified several subsets of TAMs in human cancers (165). T2 cytokines (i.e., IL-4 and IL-13) drive the differentiation of macrophages into alternatively activated macrophages (131, 179). TSLP changes the phenotype of macrophages toward an M2-like phenotype during TSLP-induced airway inflammation (55). This differentiation of macrophages was IL-13-, but not IL-4-dependent. These results demonstrate that TSLP/TSLPR plays a significant role in the amplification of alternatively activated macrophage polarization (55).
Kuan and Ziegler demonstrated that TSLPR is expressed by human breast cancer cells and mouse TAM expressed TSLP (117). Interestingly, non-tumor breast tissue did not express TSLPR. Moreover, Tslp mRNA was increased in TAM, monocytes, and neutrophils from both breast cancer patients and mice. They also demonstrated that TSLP from non-tumor derived sources (i.e., IL-1α-activated neutrophils) is critical for breast tumor metastasis in lungs (117). The authors concluded that a breast-myeloid cell axis, mediated via TSLP and IL-1α, promotes the progression of breast cancer and metastasis formation (117).
Activation of primary breast cancer tissues, as well as surrounding tissue, released several proinflammatory cytokines (i.e., IL-1α, IL-1β, IL-18, and IL-33) (116). The secretion of cytokines was higher in breast cancer tissues than in non-malignant ones. cCD11c+ myeloid cells, including monocytes and DCs, were the main source of IL-1β in human breast cancer. IL-1β selectively induced TSLP secretion from breast cancer cells. These findings suggest that Th2 inflammation in breast cancer is dependent on IL-1β via TSLP induction. Importantly, neutralization of IL-1β prevented breast cancer progression in a humanized mouse model (116).
In a mouse model, TSLP released from breast cancer downregulates the receptors, CXCR4 and α4β1 integrin, which physiologically keep B-cell precursors in bone marrow (84). Using mouse and human bone marrow aspirates incubated with metastatic 4T1 breast cancer cells, the authors demonstrated that this was the result of TSLP release from cancer cells. The loss of CXCR4 signaling or α4β1 integrin binding to VCAM-expressing stromal cells, caused the exit of B-cell precursors from the bone marrow. It was suggested that these cells can differentiate into Bregs or suppressive B cells in TME, favoring lung metastasis (84). Finally, TSLP is overexpressed by immunohistochemistry in breast cancer compared to normal breast tissue and is associated with an increased risk in breast cancer in Saudi women (180).
Melanoma
Malignant melanoma continues to be a major health concern despite the developments of immunotherapy and targeted therapy (181, 182). Yao and collaborators used genetically engineered models of melanoma and tumor cell grafting combined with TSLP knockout or overexpression, to identify a crosstalk between keratinocytes, immune cells, and melanoma cells in TME (183). Melanoma cell-derived factors in Braf/Pten mice activated keratinocytes to release TSLP, which engaged TSLPR on DCs. These cells promoted the activation of GATA3+ Foxp3- Th2 cells to release IL-4 and IL-13. At the same time, TSLP-activated DCs promoted GATA3+ Foxp3- Treg cells showing suppressive activity on CD8+ T cell proliferation and IFN-γ production. Interestingly, a similar population of GATA3+ Tregs was also found in human melanoma. A similar subset of GATA3+ Tregs was also found in skin biopsies from patients with primary human melanoma. This study highlights the role of TSLP in programming a protumoral immune microenvironment in melanoma (183). Collectively, these results highlight a novel circuit involving keratinocytes-derived TSLP, which activates DCs and CD4+ cells to release IL-4 and IL-13, promoting the growth and metastasis of melanoma (183).
Eosinophils are present in the TME of several human solid (184–188) and hematologic tumors (189), and experimental cancers (190). Eosinophils release a plethora of mediators that individually have positive or negative effects on various immune cells (191). Studies addressing the potential functions of eosinophils in experimental and human tumors have provided conflicting results (192–194). In experimental studies, a protective role of eosinophils was found in melanoma (23, 195–199), Hodgkin’s lymphoma (200), hepatocellular carcinoma (201), and prostate cancer (202). IL-33 administration in mice-bearing melanoma resulted in tumor growth delay and prevented pulmonary metastasis (196, 199). On the other side, human eosinophils produce several proangiogenic factors such as VEGF-A (203), fibroblast growth factor (FGF-2) (195, 204), and CXCL8/IL-8 (205). Eosinophils release chemokines (CCL5, CCL9, CXCL10) important for the attraction of CD8+ T cells in TME (195).
Association studies have revealed that a higher presence of basophils (i.e., CD123+, CCR3+, FcεRI+) within tumors is correlated with improved overall survival (161). In a mouse melanoma model, basophils released CCL3 and CCL4, which played a crucial role in attracting CD8+ T cells to the tumor site, thereby promoting tumor rejection (161, 206, 207). Although the mechanisms by which basophils contribute to tumor suppression are not fully understood, certain mediators (e.g., granzyme B and TNF-α) released by these cells have tumor-killing properties. Moreover, basophils secrete chemokines (e.g., CCL3 and CCL4) involved in attracting cytotoxic CD8+ T cells into the TME (163).
Colorectal cancer
Colorectal cancer (CRC) is the third most common type of cancer and the second leading cause of malignancy-related mortality among the global population (208). Obata-Ninomiya and collaborators analyzed six independent databases and found that TSLP expression correlated with CRC and was a marker of poor prognosis (209). The expression of TSLP mRNA in colon cancer tissue was increased compared to normal colon from the same patients (209, 210). These findings were extended by showing increased expression of TSLP, TSLPR, and IL-7Rα by immunohistochemistry in colon cancer tissues compared to normal colon. The authors also found that TSLP rs10043985 polymorphism was strongly correlated with CRC in Saudi patients (210). The latter finding suggests that this mutation in the promoter region of TSLP might play a detrimental role in CRC.
In a mouse model of colitis associated with CRC, TSLP mRNA was overexpressed in colon cancer compared to non-tumor sites and control mice (209). The number of tumors in Tslp-/- mice was reduced compared to Tslp+/+ mice, suggesting that TSLP plays a protumorigenic role in this model of CRC. The frequency of Treg expressing TSLPR (TSLPR+ Tregs) was increased in colon cancer and TSLPR+ Tregs exhibited stronger immunosuppressive activity compared to TSLPR- Tregs in vitro and in vivo. TSLPR+ Tregs subset coexpressed ST2, CTLA-4, PD-1 that are associated with CRC in humans and mice (211–213). Collectively, these results indicated that TSLPR+ ST2+ Treg subset was involved in CRC development and progression (209). Although ST2 detection on Tregs had no effect on tumor number and size, double deficiency of TSLPR and ST2 on Tregs reduced tumor progression. These results suggested that TSLPR signaling rather than ST2 signaling by TSLPR+ ST2+ Tregs is important in tumor growth. The latter finding suggested that TSLPR blockade signaling could be effective for the treatment of CRC. In fact, the administration of an anti-TSLP monoclonal antibody reduced the size and number of CRC (209). This treatment was associated with decreased TSLPR+ ST2+ Tregs in colon and lymph nodes and increased Th1 cells in colon. Collectively, these findings demonstrate for the first time that an anti-TSLP antibody is effective in a mouse model of colitis-associated CRC.
These results have translational relevance in colorectal tumors in humans. The frequency of intratumor TSLPR+ ST2+ Foxp3+ CD25hi Tregs was increased in patients with CRC, compared to adjacent normal colon from the same donor. The frequency of this Tregs subset was also increased in peripheral blood from these patients (209). These results are consistent with those observed in the murine model supporting the notion that TSLPR+ ST2+ Tregs promote a protumorigenic microenvironment during CRC initiation and progression.
Lung cancer
Lung cancer is the leading cause of cancer mortality in men and the second in women, behind breast cancer (214, 215). Non-small cell lung cancer (NSLC) comprises 85% of lung cancers and 40% of those are adenocarcinomas (216). The human lung is particularly rich in a variety of cells of innate and adaptive immune system (217, 218), and tumor-infiltrating myeloid cells are key regulators of lung cancer initiation and progression (217, 219).
TSLP expression, examined by immunohistochemistry, was increased in intratumoral lung cancer compared to non-cancer tissue and benign lesions (220). The number of Foxp3+ Tregs in lung cancer tissue was increased compared to non-cancer tissue, particularly in the group of TSLP+ cancers. TSLP induced the differentiation of CD4+ CD25- T cells into Tregs (220). Recently, we have found that TSLP, TSLPR, and IL-7Rα expression, examined by immunohistochemistry, was higher in the intratumoral lung cancer compared to the peritumoral area (56). Total TSLP protein was also increased in intratumoral compared to peritumoral lung tissue. We also examined the expression of the two TSLP isoforms (lfTSLP and sfTSLP), TSLPR, and IL-7Rα mRNAs in peritumoral and intratumoral lung cancer. The proinflammatory lfTSLP mRNA was higher in peritumoral tissue, whereas the sfTSLP mRNA was overexpressed in intratumoral compared to peritumoral lung cancer. The TSLPR mRNA was equally expressed in both compartments. The IL-7Rα mRNA was highly expressed in intratumoral lung tissue (56). These results provide the first evidence that the protein and molecular expression of the different components of the TSLP/TSLPR network differ at the intra- and peritumoral levels in cancer. Furthermore, these results provide the first demonstration that the molecular expression of the two isoforms of TSLP is differentially expressed at peri- and intratumoral levels in human lung cancer. These results suggest that the expression and the pathogenic role(s) of the two isoforms of TSLP should be carefully investigated in the initiation and progression of other human cancers.
In the same study, it was demonstrated that macrophages purified from macroscopically normal lung parenchyma of patients with lung cancer constitutively express TSLP, TSLPR, and IL-7Rα (56). Activation of human lung macrophages (HLMs) with IL-4, alone and in combination with IL-13, induced the overexpression of lfTSLP mRNA and TSLP release (56). Moreover, lipopolysaccharide (LPS), a promoter of metastatic cells (221), was a potent stimulus for the release of TSLP from HLMs. Finally, LPS synergistically potentiated TSLP release induced by IL-4 from HLMs (56). More recently, it was demonstrated that TSLP, but not sfTSLP, can activate HLMs to release VEGF-A, the most potent angiogenic factor. Interestingly, sfTSLP did not induce nor interfere with the activating property of lfTSLP on HLMs (48). These results unveil an intriguing interplay between TSLP and HLMs that might be relevant in lung cancer. Th2-like cytokine in TME and LPS can induce TSLP release from HLMs. TSLP, but not sfTSLP, can feedback on TSLPR on HLMs to induce the release of angiogenic factors that can contribute to lung cancer growth. In conclusion, TSLP released by lung macrophages can play a role in the autocrine circuit that could favor lung cancer progression.
Human basophils co-cultured with the human lung adenocarcinoma cell line A549, release copious amounts of IL-4 and IL-13 (98). In human and mouse NSCLC, IL-4 derived from bone marrow basophils and eosinophils promoted the development of immunosuppressive tumor-promoting myeloid cells (162). Depletion of basophils and the administration of dupilumab, IL-4Rα blocking antibody (222), reduced tumor growth (162). Collectively, these results further suggest that basophils may contribute to tumor progression through the release of copious amounts of Th2-like cytokines (163, 223).
Gastric cancer
Gastric cancer is the fifth most prevalent malignancy and the fourth leading cause of cancer death worldwide (224). TSLP mRNA was overexpressed in the majority of gastric cancer patients compared to distant tumor-free samples (225). A significant association was reported between TSLP overexpression and lymph node metastasis. In another study, the expression of TSLP examined by immunohistochemistry was higher in cancer tissue compared to non-tumor sites (226). Higher tissue expression of TSLP and higher circulating levels of this cytokine were associated with a poor prognosis of gastric cancer (226).
Cervical cancer
Cervical cancer is one of the most common gynecological malignancies with high rates of morbidity and mortality (227). TSLP examined by immunohistochemistry was overexpressed in human cervical cancer compared to cervicitis (114). Cervical carcinoma HeLa and CaSki cells released TSLP in vitro. TSLP induced proliferation of human umbilical vein endothelial cells (HUVEC) expressing TSLPR and cervical carcinoma cell-derived TSLP promoted HUVEC proliferation. The authors concluded that TSLP released from human cervical cancer can promote tumor angiogenesis through the activation of TSLPR on endothelial cells (114). This group extended the previous findings showing that TSLP released from cervical cancer cells can activate eosinophils to produce proinflammatory cytokines (187). A more recent study reported that TSLP stimulates the proliferation and invasion of HeLa and SiHa cells by downregulating the expression of miR-132 (228).
Skin cancer
Human (68, 112, 229) and mouse keratinocytes (113) are a major source of TSLP. In a mouse model, repeated topical exposure to environmental carcinogens induced skin inflammation and enhanced the circulating and local levels of polyclonal IgE (99). IgE increase was accompanied by skin infiltration of basophils releasing Th2 cytokines (IL-4, IL-6, and IL-13). Basophil-derived conditioned media promoted proliferation of epithelial cells and the expression of inflammatory cytokines (i.e., IL-1α, IL-18, and IL-31). Basophil recruitment to the inflamed skin was dependent on TSLP/IL-3-mediated upregulation of CXCR4 in basophils (99). TSLP, abundantly expressed in inflamed skin, induced the transport of CXCR4 to the basophil surface. These results suggest that TSLP and IL-3 produced at site of skin inflammation drive the expression of CXCR4 on basophils, allowing recruitment to the skin in response to increased levels of CXCL12. In this model of inflammation-driven epithelial carcinogenesis, TSLP plays a key role in the promotion of epithelial hyperplasia and tumor growth (99).
Ovarian cancer
TSLP mRNA was overexpressed in human epithelial ovarian carcinoma (EOC) compared to adjacent normal tissues (230). TSLP protein overexpression was found in approximately 60% of 144 patients with EOC and 16% of benign cases. Patients with TSLP overexpression were associated with worse survival and lower overall survival (OS) (230). It has been reported that sfTSLP mRNA was selectively expressed by human ovarian cancers (231). Overexpression of sfTSLP in TSLP ovarian and endometrial cancer cells promoted tumor growth in vitro. The authors concluded that sfTSLP was predominantly expressed in human ovarian cancers and promoted tumor growth in vitro. These intriguing results emphasize the need for further studies to investigate the expression and role(s) of the two TSLP isoforms in human cancers.
Studies supporting the protumorigenic role of TSLP in human and experimental solid cancers are outlined in Table 2.
Figure 5 schematically illustrates the possible mechanisms by which TSLP plays a protumorigenic role in different human and experimental cancers.
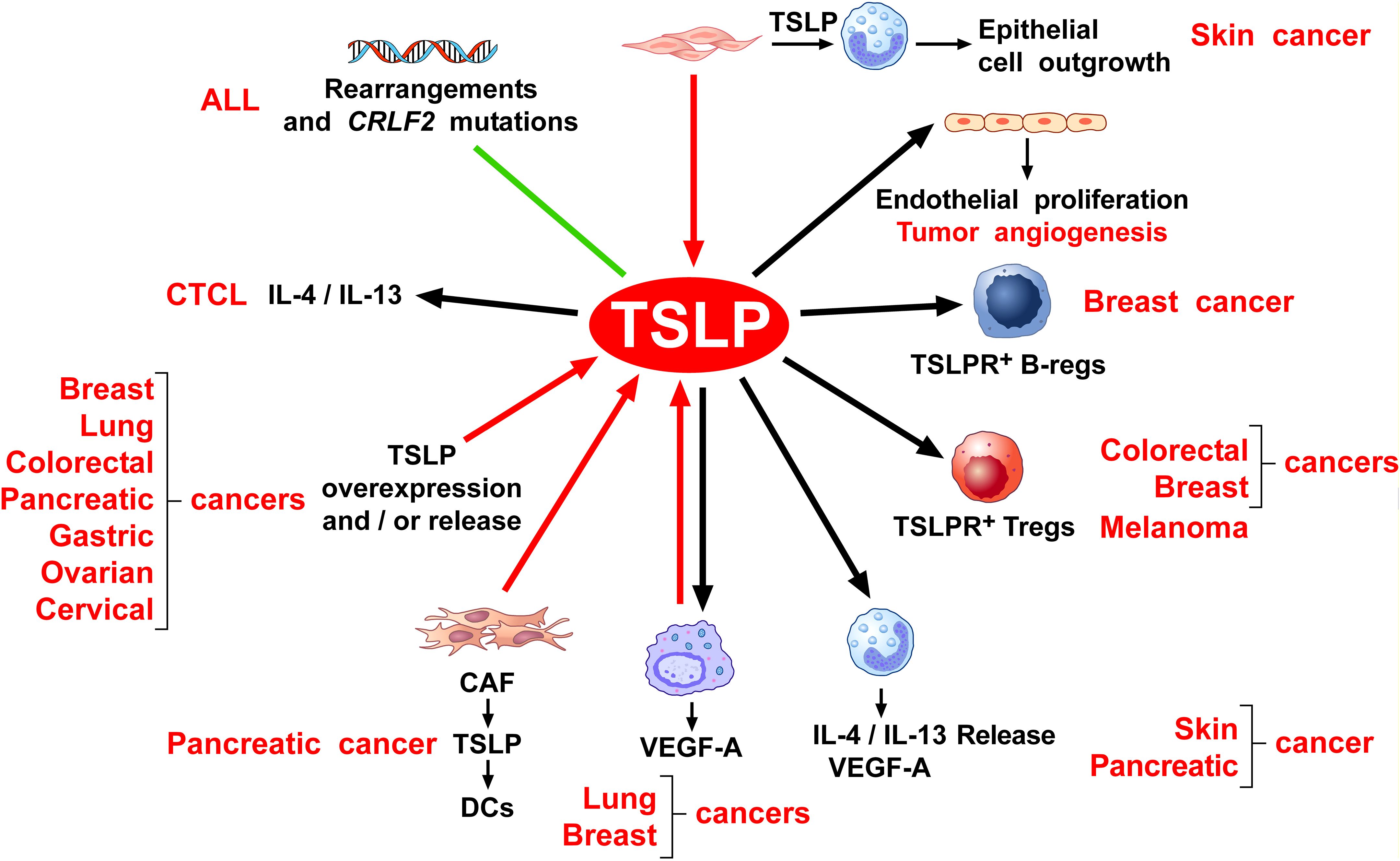
Figure 5. Possible mechanisms by which TSLP may play a protumorigenic role. Several lines of evidence suggest that thymic stromal lymphopoietin (TSLP) contributes to tumor development and progression through various mechanisms. Rearrangement and mutation of the cytokine receptor-like factor 2 (CRLF2) locus which encodes for human TSLPR are found in a variable percentage of children and adult patients with acute lymphoblastic leukemia (ALL) (132–142). TSLP induces the production of Th2 cytokines (e.g., IL-4, IL-13) from cutaneous T-cell lymphoma (CTCL) (144), thereby contributing to a protumorigenic immune milieu. Beyond hematologic malignancies, TSLP has also been implicated in a variety of solid tumors. Several human (56, 114, 117, 150, 175, 187, 209, 210, 225, 230) and mouse cancers (209) overexpress and/or release TSLP. Within the tumor microenvironment (TME), TSLP released from cancer-associated fibroblasts (CAFs) from pancreatic cancer activates TSLPR+ DCs to drive Th2 and macrophage M2 phenotypes (146, 148), contributing to a protumorigenic immune microenvironment. Similarly, tumor-associated macrophages (TAMs) from lung cancer patients express and release TSLP (48, 56, 74). Once activated by TSLP, TAMs release vascular endothelial growth factor-A (VEGF-A), a key mediator of angiogenesis (48, 56). Consistently, in a mouse model of breast cancer, TSLP can activate macrophages to release VEGF-A (118). TSLP can also exert direct pro-angiogenic effects. In human cervical cancer, TSLP can promote tumor angiogenesis through the activation of TSLPR+ endothelial cells (114). Additionally, TSLPR+ Tregs exhibit strong immunosuppressive activity in both human and experimental models of colorectal (209) and breast cancer (175) and melanoma (183), helping tumors evade immune surveillance. In a mouse model, TSLP released from breast cancer cells promotes the differentiation of B-cell precursors into Bregs or immunosuppressive B cells in tumor microenvironment (TME) (84). Furthermore, both mouse and human basophils activated by TSLP are a major source of T2 cytokines (IL-4, IL-13) (23, 57), which promote Th2 and M2-skewed immune responses (168). Finally, keratinocyte-derived TSLP promotes growth and metastasis of human and experimental melanoma by activating TSLPR+ DCs which induce Tregs and immunosuppression in TME (183). In a mouse model of chronic skin inflammation, basophils are recruited to inflamed skin via TSLP (99), promoting epithelial cell outgrowth harboring oncogenic mutations.
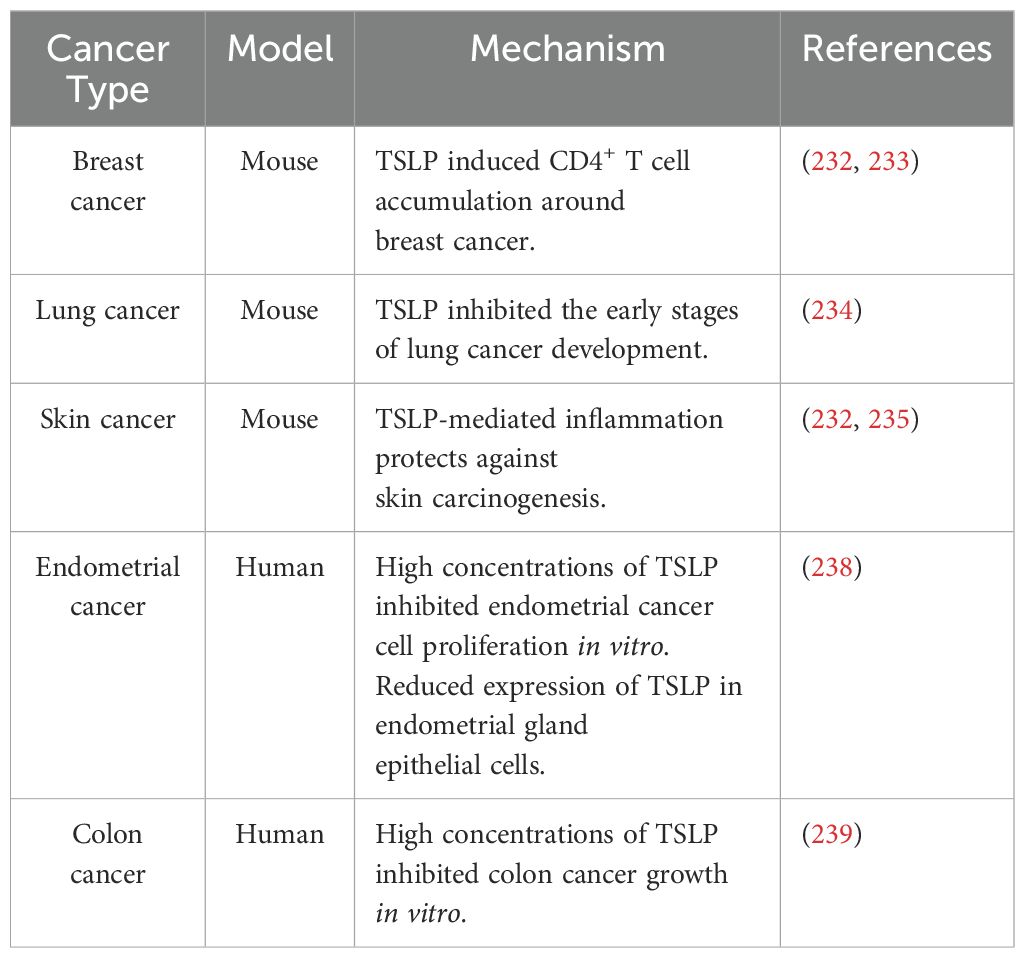
Antitumorigenic role of TSLP in solid cancers
Breast cancer
In a mouse model, TSLP overexpression in the skin leads to inflammation, which was associated with inhibition of early stages of breast carcinogenesis (232). TSLP-induced breast cancer suppression was associated with CD4+ T cell accumulation around breast cancer (232). The same group also examined the possible role of TSLP induction during breast cancer development using the PyMt cell line model in Tslptg mice (233). In an orthotopic breast tumor model, primary breast cancer cells from PyMttg mice or PyMt cell line were implanted into the mammary fat pad of Tslptg and wild-type (WT) controls. Tslptg mice receiving PyMt primary cells had delayed tumor growth and smaller tumors compared with WT mice. Tslptg mice receiving PyM cell line also showed delayed tumor growth. Analysis of PyMt cell line-derived breast tumor revealed increasing CD4+ T cells in Tslptg compared with WT mice. TSLP-activated CD4+ T cells sorted from the tumors inhibited the growth of PyMt cells in vitro. TNF-α and IFN-γ present in supernatants of TSLP-activated CD4+ T cells were required for PyMt tumor suppression. The authors concluded that TNF-α and IFN-γ produced by TSLP-stimulated CD4+ T cells play a major role in providing antitumor immunity against experimental breast cancer (233).
Lung cancer
To evaluate the role of TSLP on early lung carcinogenesis, a mouse model of spontaneous lung adenocarcinoma, Kras+/GI2D (KrasGI2D) was crossed with K14-TSLPtg (Tslptg) mice. Tslptg KrasGI2D mice developed a lower lung tumor burden compared to KrasGI2D mice. Tslptg KrasGI2D lung tumors were composed of lower-grade atypical alveolar hyperplasia and adenoma compared to adenocarcinoma in KrasGI2D lung (234). CD4+ T cell depletion inhibited the proliferative impact of TSLP against lung carcinogenesis in TSLP overexpressing mice. The authors suggested that in this experimental model of lung carcinogenesis, TSLP inhibits the early stages of lung cancer development.
Skin cancer
In a mouse model of Notch-deficient skin carcinogenesis, it has been proposed that TSLP-mediated inflammation protects against carcinogenesis (235). TSLP-mediated tumor protection was mediated by CD8+ and CD4+ T cells. The protective effect of TSLPR signalling was also confirmed in a model of Notch-independent skin cancer (235). Demeri et al. extended the previous findings showing that Notch-deficient mice develop severe skin inflammation caused by epidermal TSLP overexpression. Blocking TSLP signalling in Notch-deficient animals resulted in skin carcinogenesis. The authors concluded that upregulation of epidermal TSLP can generate anti-tumor CD4+ T cell response in a Th2 inflammatory microenvironment (236). Studies in humans appear necessary to clarify the possible role of TSLP/TSLPR network in skin carcinogenesis.
Endometrial cancer
Endometrial cancer is one of the most common types of gynecologic cancers worldwide (237). A recent study reported that the expression of TSLP (measured by Western blot) was reduced in several human endometrial cancer cell lines compared to normal human endometrial cells (238). Micrograms of TSLP partially inhibited the proliferation of two endometrial cancer cell lines. High concentrations of TSLP alone had no effect on the in vitro proliferation of an endometrial cancer cell line, but slightly enhanced the inhibitory effect of progesterone (238). The authors concluded that the loss of TSLP in endometrial gland epithelial cells may contribute to endometrial cancer development. The concentrations of TSLP used in these experiments exceed by several logarithms the pathophysiological levels of this cytokine making the results of difficult interpretation.
Colon cancer
Yue et al. observed a reduction in TSLP expression in human colon cancer, and there was an inverse relationship between TSLP levels and the clinical stage of the cancer (239). TSLP promoted apoptosis of colon cancer cells through the engagement of TSLPR. Using a xenograft mouse model, the authors reported that peritumoral administration of TSLP reduced tumor growth.
Studies supporting the antitumorigenic role of TSLP in experimental and human cancers are outlined in Table 3.
Conclusions and future perspectives
Previous reviews started to highlight the controversial nature of the TSLP–TSLPR axis in both experimental models and human cancers (39–41). Since then, several clinical and experimental studies have extended the intriguing observation that in different neoplasias TSLP can play a protumorigenic role or protective effects depending on the tumor context. In human hematologic cancers, such as ALL, Hodgkin disease and CTCL, TSLP appears to promote tumor progression (Figure 6).
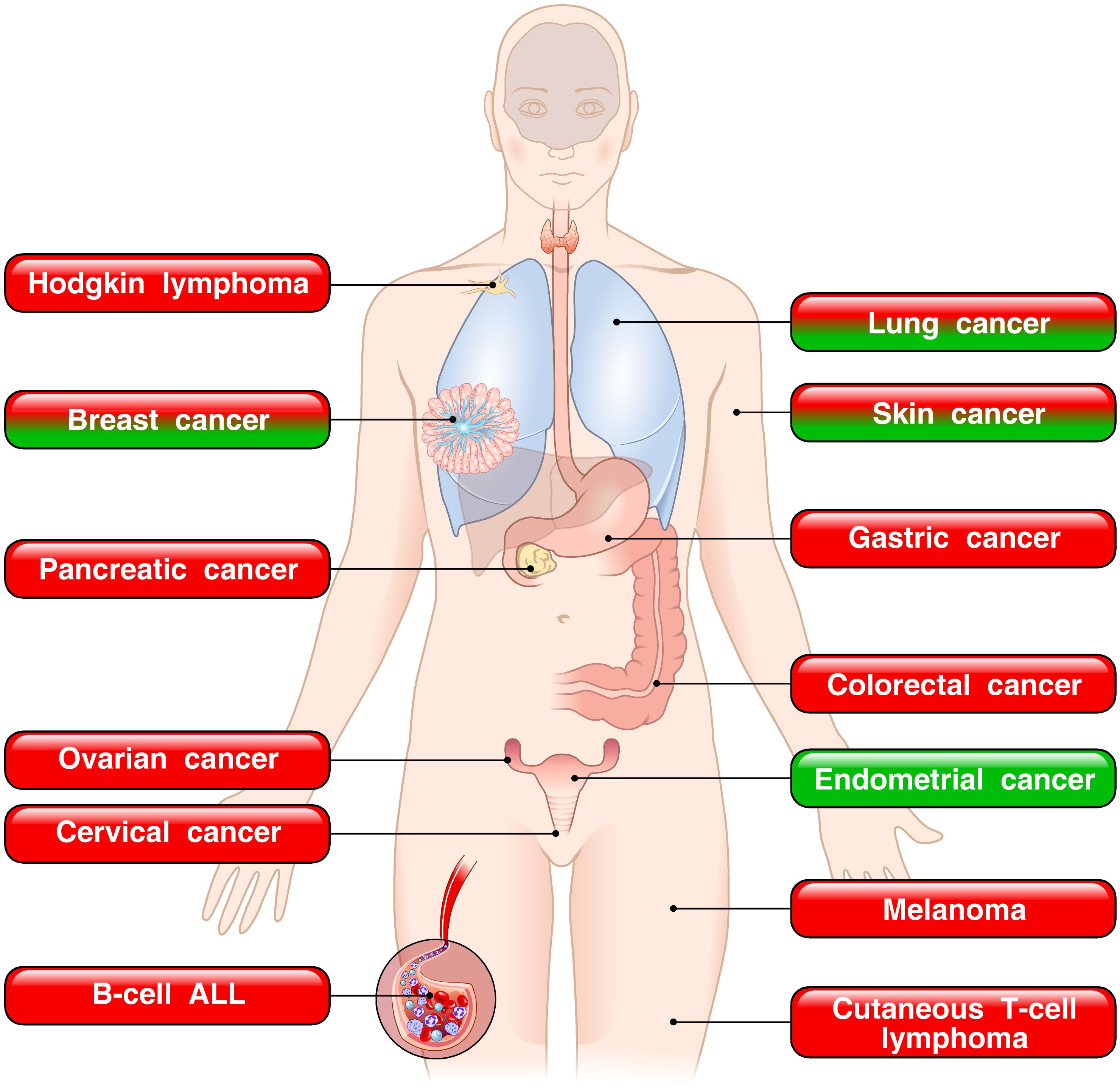
Figure 6. Dual role of TSLP in human tumors. The red boxes indicate the tumors in which TSLP is implicated in promoting tumor growth. The green boxes represent tumors in which TSLP appears to play a protective role. The mixed red/green boxes depict tumors in which TSLP plays both pro- and antitumorigenic roles in various experimental and human cancers.
By contrast, in a variety of human solid cancers, TSLP can play a protumorigenic, an antitumorigenic role, or both (Figure 6). In the vast majority of cancers (pancreatic, ovarian, cervical, gastric, colorectal cancers and melanoma), TSLP has been found to promote cancer initiation and growth. By contrast, in a model of sex hormone-dependent endometrial cancer, TSLP seems to play a protective role (238). In this study, industrial concentrations of TSLP inhibited cancer cell proliferation (238). It is intriguing that in certain tumors (breast, lung and skin cancers), different studies reported opposing views of TSLP in tumorigenesis. A possible explanation of these apparently different results is that the majority of studies showing an antitumorigenic effect of TSLP were performed in different mouse experimental models (232–236). Notably, the protumorigenic effects of TSLP were demonstrated in several human and experimental models of different cancers. The development of appropriate and specific animal models appears necessary to better understanding of the underlying mechanisms of TSLP-driven tumorigenesis in cancers.
In human cancers, the role of TSLP isoforms, which occur only in this species, has not been thoroughly investigated. There is preliminary evidence that the two variants of TSLP (lfTSLP and sfTSLP mRNAs) are differentially expressed at peri- and intratumoral levels in human lung cancer (56). Moreover, there is some evidence that sfTSLP is selectively expressed in human ovarian cancer (231). These preliminary results demand that the roles of the two TSLP isoforms should be examined during the initiation and progression of other human cancers.
The results of several studies have suggested that TSLP can exert a protumorigenic role through different mechanisms. For instance, TSLP can favor Th2 and M2 polarization in several cancers, including pancreatic cancer (146–148), melanoma (183), skin cancer (99), breast cancer (115–117, 175), and CTCL (144). TSLP can also increase the frequency of Tregs (209) in experimental and human colorectal cancer (209) and melanoma (183). In breast cancer, the protumorigenic mechanism is dependent on IL-1β released by cancer cells that activate myeloid cells in TME. The latter cells release TSLP, which promotes tumor cell proliferation (116). Finally, it has been shown in a mouse model of breast cancer that TSLP can activate resident macrophages to release VEGF-A (118). We have extended the latter observation showing that TSLP, but not sfTSLP, can induce the release of VEGF-A and VEGF-C from macrophages isolated from patients with lung cancer (48, 56). There is also the possibility that TSLP released from cancer cells can directly activate endothelial cells expressing TSLPR (114).
From a translational perspective, a deeper understanding of the tumor context-dependent effects of TSLP isoforms may encourage the identification of reliable biomarkers to stratify patients who might benefit from therapeutic targeting of the TSLP–TSLPR axis. Indeed, the role of TSLP in cancer initiation and growth has significant implications, especially considering the recent approval of an anti-TSLP monoclonal antibody (tezepeleumab) for the treatment of asthma, a common inflammatory disease of the respiratory system (60). On one side, it has been demonstrated that the administration of an anti-TSLP antibody decreased colorectal cancer in a mouse model (209). On the other side, if TSLP plays an antitumorigenic role in certain tumors, the administration of biological therapies targeting TSLP/TSLP receptor network could lead to negative effects.
Finally, considering the proposed homeostatic and anti-inflammatory functions of sfTSLP (71), these characteristics warrant careful consideration in the development of targeted therapies for cancer initiation and progression. In conclusion, the above considerations emphasize the urgency of further investigating the role of TSLP and its isoforms in the onset and progression of human and experimental cancers. A deeper understanding of the immunological and molecular determinants driving the dual behavior of TSLP in the tumor microenvironment will be essential to support the development of precision immunomodulatory strategies in oncology.
Author contributions
RP: Conceptualization, Data curation, Project administration, Visualization, Writing – original draft. GM: Writing – review & editing, Investigation, Supervision. SFZ: Investigation, Supervision, Writing – review & editing. GV: Conceptualization, Funding acquisition, Investigation, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Supported in part by grants from the CISI-Lab Project (University of Naples Federico II), TIMING Project and Campania Bioscience (Regione Campania), to GM and GV. RP is a recipient of grants from Associazione Italiana Pneumologi Ospedalieri (AIPO) 2023 and Società Italiana di Medicina Interna (SIMI) 2024.
Acknowledgments
The authors thank Dr. Gjada Criscuolo for her excellent managerial assistance in preparing this manuscript and the administrative staff (Dr. Roberto Bifulco, Dr. Anna Ferraro, and Dr. Gabriella Rusciano) and the medical graphic artist Fabrizio Fiorbianco for the elaboration of figures. We thank Dr. Leonardo Cristinziano for his valuable support in the preparation of Figure 2.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ALL, acute lymphoblastic leukemia; ANGPT2, angiopoietin 2; APC, antigen-presenting cell; ASM, airway smooth muscle; CAF, cancer-associated fibroblasts; CHR, cytokine binding homology region; COPD, chronic obstructive pulmonary disease; CRC, colorectal cancer; CRLF2, cytokine receptor-like factor 2; CRSwNP, severe chronic rhinosinusitis with nasal polyps; CTCL, cutaneous T-cell lymphoma; DC, dendritic cell; EMA, European-Medicine Agency; EOC, epithelial ovarian carcinoma; FDA, Food and Drug Administration; FGF, fibroblast growth factor; GC, germinal center; HLM, human lung macrophage; HUVEC, human umbilical vein endothelial cell; ICOSL, inducible T cell costimulatory ligand; ILC2, innate lymphoid cells type 2; IL-7Rα, interleukin 7 receptor-α; IM, interstitial macrophage; IPF, idiopathic pulmonary fibrosis; JAK, Janus kinase; lfTSLP, long form TSLP; LCMV, lymphocytic choriomeningitis; LPS, lipopolysaccharide; MRGPRX2, Mas-related G-protein coupled receptor member X2; NK cell, natural killer cell; NSLC, non-small cell lung cancer; OS, overall survival; OX40L, OX40 ligand; PBMC, peripheral blood mononuclear cell; PDAC, pancreatic cancer; pDC, plasmacytoid DC; sfTSLP, short form TSLP; STAT, signal transducers and activators of transcription; TAM, tumor associated macrophage; TCR, T cell receptor; TDLN, tumor-draining lymph nodes; Tfh cell, T follicular helper cell; TL1A, Tumor Necrosis Factor-like Ligand 1A; TLR3, toll-like receptor 3; TME, tumor microenvironment; Treg cell, regulatory T cell; TSLP, Thymic stromal lymphopoietin; TSLPR, TSLP receptor; VEGF-A, vascular endothelial growth factor-A; WT, wild-type.
References
1. Sims JE, Williams DE, Morrissey PJ, Garka K, Foxworthe D, Price V, et al. Molecular cloning and biological characterization of a novel murine lymphoid growth factor. J Exp Med. (2000) 192:671–80. doi: 10.1084/jem.192.5.671
2. Friend SL, Hosier S, Nelson A, Foxworthe D, Williams DE, and Farr A. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol. (1994) 22:321–8.
3. Ray RJ, Furlonger C, Williams DE, and Paige CJ. Characterization of thymic stromal-derived lymphopoietin (TSLP) in murine B cell development in vitro. Eur J Immunol. (1996) 26:10–6. doi: 10.1002/eji.1830260103
4. Levin SD, Koelling RM, Friend SL, Isaksen DE, Ziegler SF, Perlmutter RM, et al. Thymic stromal lymphopoietin: a cytokine that promotes the development of IgM+ B cells in vitro and signals via a novel mechanism. J Immunol. (1999) 162:677–83. doi: 10.4049/jimmunol.162.2.677
5. Reche PA, Soumelis V, Gorman DM, Clifford T, Liu M, Travis M, et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. (2001) 167:336–43. doi: 10.4049/jimmunol.167.1.336
6. Quentmeier H, Drexler HG, Fleckenstein D, Zaborski M, Armstrong A, Sims JE, et al. Cloning of human thymic stromal lymphopoietin (TSLP) and signaling mechanisms leading to proliferation. Leukemia. (2001) 15:1286–92. doi: 10.1038/sj.leu.2402175
7. Park LS, Martin U, Garka K, Gliniak B, Di Santo JP, Muller W, et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med. (2000) 192:659–70. doi: 10.1084/jem.192.5.659
8. Pandey A, Ozaki K, Baumann H, Levin SD, Puel A, Farr AG, et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. (2000) 1:59–64. doi: 10.1038/76923
9. Fujio K, Mosaka T, Kojima T, Kawashima T, Yahata T, Copeland NG, et al. Molecular cloning of a novel type 1 cytokine receptor similar to the common gamma chain. Blood. (2000) 95:2210. doi: 10.1182/blood.V95.7.2204
10. Tonozuka Y, Fujio K, Sugiyama T, Nosaka T, Hirai M, and Kitamura T. Molecular cloning of a human novel type I cytokine receptor related to delta1/TSLPR. Cytogenet Cell Genet. (2001) 93:23–5. doi: 10.1159/000056941
11. Verstraete K, Peelman F, Braun H, Lopez J, Van Rompaey D, Dansercoer A, et al. Structure and antagonism of the receptor complex mediated by human TSLP in allergy and asthma. Nat Commun. (2017) 8:14937. doi: 10.1038/ncomms14937
12. Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. (2005) 202:1213–23. doi: 10.1084/jem.20051135
13. Kitajima M, Kubo M, Ziegler SF, and Suzuki H. Critical role of TSLP receptor on CD4 T cells for exacerbation of skin inflammation. J Immunol. (2020) 205:27–35. doi: 10.4049/jimmunol.1900758
14. Kitajima M and Ziegler SF. Cutting edge: identification of the thymic stromal lymphopoietin-responsive dendritic cell subset critical for initiation of type 2 contact hypersensitivity. J Immunol. (2013) 191:4903–7. doi: 10.4049/jimmunol.1302175
15. Liu YJ. Thymic stromal lymphopoietin and OX40 ligand pathway in the initiation of dendritic cell-mediated allergic inflammation. J Allergy Clin Immunol. (2007) 120:238–44. doi: 10.1016/j.jaci.2007.06.004
16. Rochman Y and Leonard WJ. The role of thymic stromal lymphopoietin in CD8+ T cell homeostasis. J Immunol. (2008) 181:7699–705. doi: 10.4049/jimmunol.181.11.7699
17. Rochman I, Watanabe N, Arima K, Liu YJ, and Leonard WJ. Cutting edge: direct action of thymic stromal lymphopoietin on activated human CD4+ T cells. J Immunol. (2007) 178:6720–4. doi: 10.4049/jimmunol.178.11.6720
18. Omori M and Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol. (2007) 178:1396–404. doi: 10.4049/jimmunol.178.3.1396
19. Allakhverdi Z, Comeau MR, Jessup HK, and Delespesse G. Thymic stromal lymphopoietin as a mediator of crosstalk between bronchial smooth muscles and mast cells. J Allergy Clin Immunol. (2009) 123:958–60 e2. doi: 10.1016/j.jaci.2009.01.059
20. Kabata H, Flamar AL, Mahlakoiv T, Moriyama S, Rodewald HR, Ziegler SF, et al. Targeted deletion of the TSLP receptor reveals cellular mechanisms that promote type 2 airway inflammation. Mucosal Immunol. (2020) 13:626–36. doi: 10.1038/s41385-020-0266-x
21. Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. (2013) 5:170ra. doi: 10.1126/scitranslmed.3005374
22. Miazgowicz MM, Elliott MS, Debley JS, and Ziegler SF. Respiratory syncytial virus induces functional thymic stromal lymphopoietin receptor in airway epithelial cells. J Inflammation Res. (2013) 6:53–61. doi: 10.2147/JIR.S42381
23. Gambardella AR, Poto R, Tirelli V, Schroeder JT, Marone G, Mattei F, et al. Differential effects of alarmins on human and mouse basophils. Front Immunol. (2022) 13:894163. doi: 10.3389/fimmu.2022.894163
24. Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. (2011) 477:229–33. doi: 10.1038/nature10329
25. Zhang K, Shan L, Rahman MS, Unruh H, Halayko AJ, and Gounni AS. Constitutive and inducible thymic stromal lymphopoietin expression in human airway smooth muscle cells: role in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. (2007) 293:L375–82. doi: 10.1152/ajplung.00045.2007
26. Watanabe N, Hanabuchi S, Soumelis V, Yuan W, Ho S, Waal-Malefyt R, et al. Human thymic stromal lymphopoietin promotes dendritic cell-mediated CD4+ T cell homeostatic expansion. Nat Immunol. (2004) 5:426–34. doi: 10.1038/ni1048
27. Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, et al. Intestinal immune homeostasis is regulated by the corsstalk between epithelial cells and dendritic cells. Nat Immunol. (2005) 6:507–14. doi: 10.1038/ni1192
28. Kato A, Favoreto S Jr., Avila PC, and Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. (2007) 179:1080–7. doi: 10.4049/jimmunol.179.2.1080
29. Lee HC and Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc Natl Acad Sci U.S.A. (2007) 104:914–9. doi: 10.1073/pnas.0607305104
30. Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. (2002) 3:673–80. doi: 10.1038/ni805
31. Mou Z, Xia J, Tan Y, Wang X, Zhang Y, Zhou B, et al. Overexpression of thymic stromal lymphopoietin in allergic rhinitis. Acta Otolaryngol. (2009) 129:297–301. doi: 10.1080/00016480802225884
32. Bunyavanich S, Melen E, Wilk JB, Granada M, Soto-Quiros ME, Avila L, et al. Thymic stromal lymphopoietin (TSLP) is associated with allergic rhinitis in children with asthma. Clin Mol Allergy. (2011) 9:1. doi: 10.1186/1476-7961-9-1
33. Ying S, O’Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. (2005) 174:8183–90. doi: 10.4049/jimmunol.174.12.8183
34. Rothenberg ME, Spergel JM, Sherrill JD, Annaiah K, Martin LJ, Cianferoni A, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet. (2010) 42:289–91. doi: 10.1038/ng.547
35. Sherrill JD, Gao PS, Stucke EM, Blanchard C, Collins MH, Putnam PE, et al. Variants of thymic stromal lymphopoietin and its receptor associate with eosinophilic esophagitis. J Allergy Clin Immunol. (2010) 126:160–5. doi: 10.1016/j.jaci.2010.04.037
36. Noti M, Wojno EDT, Kim BS, Siracusa MC, Giacomin PR, Nair MG, et al. Thymic stromal lymphopoietin–elicited basophil responses promote eosinophilic esophagitis. Nat Med. (2013) 19:1005. doi: 10.1038/nm.3281
37. von Mutius E. The microbial environment and its influence on asthma prevention in early life. J Allergy Clin Immunol. (2016) 137:680–9. doi: 10.1016/j.jaci.2015.12.1301
38. Peters AS, Kellberger J, Vogelberg C, Dressel H, Windstetter D, Weinmayr G, et al. Prediction of the incidence, recurrence, and persistence of atopic dermatitis in adolescence: a prospective cohort study. J Allergy Clin Immunol. (2010) 126:590–5 e1-3. doi: 10.1016/j.jaci.2010.06.020
39. Lo Kuan E and Ziegler SF. Thymic stromal lymphopoietin and cancer. J Immunol. (2014) 193:4283–8. doi: 10.4049/jimmunol.1400864
40. Varricchi G, Pecoraro A, Marone G, Criscuolo G, Spadaro G, Genovese A, et al. Thymic stromal lymphopoietin isoforms, inflammatory disorders, and cancer. Front Immunol. (2018) 9:1595. doi: 10.3389/fimmu.2018.01595
41. Corren J and Ziegler SF. TSLP: from allergy to cancer. Nat Immunol. (2019) 20:1603–9. doi: 10.1038/s41590-019-0524-9
42. Varricchi G, Poto R, Criscuolo G, Strisciuglio C, Nair P, and Marone G. TL1A, a novel alarmin in airway, intestinal, and autoimmune disorders. J Allergy Clin Immunol. (2025) 155:1420–34. doi: 10.1016/j.jaci.2025.02.018
43. Nygaard U, Hvid M, Johansen C, Buchner M, Folster-Holst R, Deleuran M, et al. TSLP, IL-31, IL-33 and sST2 are new biomarkers in endophenotypic profiling of adult and childhood atopic dermatitis. J Eur Acad Dermatol Venereol. (2016) 30:1930–8. doi: 10.1111/jdv.13679
44. Muto T, Fukuoka A, Kabashima K, Ziegler SF, Nakanishi K, Matsushita K, et al. The role of basophils and proallergic cytokines, TSLP and IL-33, in cutaneously sensitized food allergy. Int Immunol. (2014) 26:539–49. doi: 10.1093/intimm/dxu058
45. Hammad H and Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity. (2015) 43:29–40. doi: 10.1016/j.immuni.2015.07.007
46. Varricchi G, Brightling CE, Grainge C, Lambrecht BN, and Chanez P. Airway remodelling in asthma and the epithelium: on the edge of a new era. Eur Respir J. (2024) 63:2301619. doi: 10.1183/13993003.01619-2023
47. Pelaia C, Pelaia G, Maglio A, Tinello C, Gallelli L, Lombardo N, et al. Pathobiology of type 2 inflammation in asthma and nasal polyposis. J Clin Med. (2023) 12:3371. doi: 10.3390/jcm12103371
48. Cane L, Poto R, Palestra F, Pirozzi M, Parashuraman S, Iacobucci I, et al. TSLP is localized in and released from human lung macrophages activated by T2-high and T2-low stimuli: relevance in asthma and COPD. Eur J Intern Med. (2024) 124:89. doi: 10.1016/j.ejim.2024.02.020
49. Poto R, Marone G, and Varricchi G. The Intriguing Role of Short-Form TSLP by Zeitvogel et al. J Med Virol. (2025) 97:e70224. doi: 10.1002/jmv.70224
50. Toki S, Goleniewska K, Zhang J, Zhou W, Newcomb DC, Zhou B, et al. TSLP and IL-33 reciprocally promote each other’s lung protein expression and ILC2 receptor expression to enhance innate type-2 airway inflammation. Allergy. (2020) 75:1606–17. doi: 10.1111/all.14196
51. Ochiai S, Jagot F, Kyle RL, Hyde E, White RF, Prout M, et al. Thymic stromal lymphopoietin drives the development of IL-13(+) Th2 cells. Proc Natl Acad Sci U.S.A. (2018) 115:1033–8. doi: 10.1073/pnas.1714348115
52. Lohning M, Stroehmann A, Coyle AJ, Grogan JL, Lin S, Gutierrez-Ramos JC, et al. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc Natl Acad Sci U.S.A. (1998) 95:6930–5. doi: 10.1073/pnas.95.12.6930
53. Kitajima M, Lee HC, Nakayama T, and Ziegler SF. TSLP enhances the function of helper type 2 cells. Eur J Immunol. (2011) 41:1862–71. doi: 10.1002/eji.201041195
54. Astrakhan A, Omori M, Nguyen T, Becker-Herman S, Iseki M, Aye T, et al. Local increase in thymic stromal lymphopoietin induces systemic alterations in B cell development. Nat Immunol. (2007) 8:522–31. doi: 10.1038/ni1452
55. Han H, Headley MB, Xu W, Comeau MR, Zhou B, and Ziegler SF. Thymic stromal lymphopoietin amplifies the differentiation of alternatively activated macrophages. J Immunol. (2013) 190:904–12. doi: 10.4049/jimmunol.1201808
56. Braile M, Fiorelli A, Sorriento D, Di Crescenzo RM, Galdiero MR, Marone G, et al. Human lung-resident macrophages express and are targets of thymic stromal lymphopoietin in the tumor microenvironment. Cells. (2021) 10:2012. doi: 10.3390/cells10082012
57. Salabert-Le Guen N, Hemont C, Delbove A, Poli C, Braudeau C, Fantou A, et al. Thymic stromal lymphopoietin does not activate human basophils. J Allergy Clin Immunol. (2018) 141:1476–1479 e6. doi: 10.1016/j.jaci.2017.11.012
58. Leyva-Castillo JM, Hener P, Michea P, Karasuyama H, Chan S, Soumelis V, et al. Skin thymic stromal lymphopoietin initiates Th2 responses through an orchestrated immune cascade. Nat Commun. (2013) 4:2847–7. doi: 10.1038/ncomms3847
59. Simpson EL, Parnes JR, She D, Crouch S, Rees W, Mo M, et al. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J Am Acad Dermatol. (2019) 80:1013–21. doi: 10.1016/j.jaad.2018.11.059
60. Menzies-Gow A, Corren J, Bourdin A, Chupp G, Israel E, Wechsler ME, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med. (2021) 384:1800–9. doi: 10.1056/NEJMoa2034975
61. Lipworth BJ, Han JK, Desrosiers M, Hopkins C, Lee SE, Mullol J, et al. Tezepelumab in adults with severe chronic rhinosinusitis with nasal polyps. N Engl J Med. (2025) 392:1178–88. doi: 10.1056/NEJMoa2414482
62. Verstraete K, van Schie L, Vyncke L, Bloch Y, Tavernier J, Pauwels E, et al. Structural basis of the proinflammatory signaling complex mediated by TSLP. Nat Struct Mol Biol. (2014) 21:375–82. doi: 10.1038/nsmb.2794
63. Poposki JA, Klingler AI, Stevens WW, Peters AT, Hulse KE, Grammer LC, et al. Proprotein convertases generate a highly functional heterodimeric form of thymic stromal lymphopoietin in humans. J Allergy Clin Immunol. (2017) 139:1559–1567 e8. doi: 10.1016/j.jaci.2016.08.040
64. Isaksen DE, Baumann H, Trobridge PA, Farr AG, Levin SD, and Ziegler SF. Requirement for stat5 in thymic stromal lymphopoietin-mediated signal transduction. J Immunol. (1999) 163:5971–7. doi: 10.4049/jimmunol.163.11.5971
65. Wohlmann A, Sebastian K, Borowski A, Krause S, and Friedrich K. Signal transduction by the atopy-associated human thymic stromal lymphopoietin (TSLP) receptor depends on Janus kinase function. Biol Chem. (2010) 391:181–6. doi: 10.1515/bc.2010.029
66. Harada M, Hirota T, Jodo AI, Doi S, Kameda M, Fujita K, et al. Functional analysis of the thymic stromal lymphopoietin variants in human bronchial epithelial cells. Am J Respir Cell Mol Biol. (2009) 40:368–74. doi: 10.1165/rcmb.2008-0041OC
67. Harada M, Hirota T, Jodo AI, Hitomi Y, Sakashita M, Tsunoda T, et al. Thymic stromal lymphopoietin gene promoter polymorphisms are associated with susceptibility to bronchial asthma. Am J Respir Cell Mol Biol. (2011) 44:787–93. doi: 10.1165/rcmb.2009-0418OC
68. Xie Y, Takai T, Chen X, Okumura K, and Ogawa H. Long TSLP transcript expression and release of TSLP induced by TLR ligands and cytokines in human keratinocytes. J Dermatol Sci. (2012) 66:233–7. doi: 10.1016/j.jdermsci.2012.03.007
69. Datta A, Alexander R, Sulikowski MG, Nicholson AG, Maher TM, Scotton CJ, et al. Evidence for a functional thymic stromal lymphopoietin signaling axis in fibrotic lung disease. J Immunol. (2013) 191:4867–79. doi: 10.4049/jimmunol.1300588
70. Fornasa G, Tsilingiri K, Caprioli F, Botti F, Mapelli M, Meller S, et al. Dichotomy of short and long thymic stromal lymphopoietin isoforms in inflammatory disorders of the bowel and skin. J Allergy Clin Immunol. (2015) 136:413–22. doi: 10.1016/j.jaci.2015.04.011
71. Bjerkan L, Schreurs O, Engen SA, Jahnsen FL, Baekkevold ES, Blix IJ, et al. The short form of TSLP is constitutively translated in human keratinocytes and has characteristics of an antimicrobial peptide. Mucosal Immunol. (2015) 8:49–56. doi: 10.1038/mi.2014.41
72. Martin Mena A, Langlois A, Speca S, Schneider L, Desreumaux P, Dubuquoy L, et al. The expression of the short isoform of thymic stromal lymphopoietin in the colon is regulated by the nuclear receptor peroxisome proliferator activated receptor-gamma and is impaired during ulcerative colitis. Front Immunol. (2017) 8:1052. doi: 10.3389/fimmu.2017.01052
73. Nagarkar DR, Poposki JA, Tan BK, Comeau MR, Peters AT, Hulse KE, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. (2013) 132:593–600 e12. doi: 10.1016/j.jaci.2013.04.005
74. Cane L, Poto R, Palestra F, Iacobucci I, Pirozzi M, Parashuraman S, et al. Thymic stromal lymphopoietin (TSLP) is cleaved by human mast cell tryptase and chymase. Int J Mol Sci. (2024) 25:3371. doi: 10.3390/ijms25074049
75. Pattarini L, Trichot C, Bogiatzi S, Grandclaudon M, Meller S, Keuylian Z, et al. TSLP-activated dendritic cells induce human T follicular helper cell differentiation through OX40-ligand. J Exp Med. (2017) 214:1529–46. doi: 10.1084/jem.20150402
76. Kummola L, Ortutay Z, Chen X, Caucheteux S, Hamalainen S, Aittomaki S, et al. IL-7Ralpha expression regulates murine dendritic cell sensitivity to thymic stromal lymphopoietin. J Immunol. (2017) 198:3909–18. doi: 10.4049/jimmunol.1600753
77. Rochman Y, Dienger-Stambaugh K, Richgels PK, Lewkowich IP, Kartashov AV, Barski A, et al. TSLP signaling in CD4(+) T cells programs a pathogenic T helper 2 cell state. Sci Signal. (2018) 11:eaam8858. doi: 10.1126/scisignal.aam8858
78. Ebina-Shibuya R, West EE, Spolski R, Li P, Oh J, Kazemian M, et al. Thymic stromal lymphopoietin limits primary and recall CD8(+) T-cell anti-viral responses. Elife. (2021) 10:e61912. doi: 10.7554/eLife.61912
79. Giles JR, Globig AM, Kaech SM, and Wherry EJ. CD8(+) T cells in the cancer-immunity cycle. Immunity. (2023) 56:2231–53. doi: 10.1016/j.immuni.2023.09.005
80. Halim TY, Krauss RH, Sun AC, and Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. (2012) 36:451–63. doi: 10.1016/j.immuni.2011.12.020
81. Kabata H, Moro K, Fukunaga K, Suzuki Y, Miyata J, Masaki K, et al. Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat Commun. (2013) 4:2675. doi: 10.1038/ncomms3675
82. Milford TA, Su RJ, Francis OL, Baez I, Martinez SR, Coats JS, et al. TSLP or IL-7 provide an IL-7Ralpha signal that is critical for human B lymphopoiesis. Eur J Immunol. (2016) 46:2155–61. doi: 10.1002/eji.201646307
83. Iseki M, Omori-Miyake M, Xu W, Sun X, Takaki S, Rawlings DJ, et al. Thymic stromal lymphopoietin (TSLP)-induced polyclonal B-cell activation and autoimmunity are mediated by CD4+ T cells and IL-4. Int Immunol. (2012) 24:183–95. doi: 10.1093/intimm/dxr113
84. Ragonnaud E, Moritoh K, Bodogai M, Gusev F, Garaud S, Chen C, et al. Tumor-derived thymic stromal lymphopoietin expands bone marrow B-cell precursors in circulation to support metastasis. Cancer Res. (2019) 79:5826–38. doi: 10.1158/0008-5472.CAN-19-1058
85. Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, et al. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. (2013) 123:2873–92. doi: 10.1172/JCI67428
86. Watanabe N, Wang YH, Lee HK, Ito T, Cao W, and Liu YJ. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. (2005) 436:1181–5. doi: 10.1038/nature03886
87. Leichner TM, Satake A, Harrison VS, Tanaka Y, Archambault AS, Kim BS, et al. Skin-derived TSLP systemically expands regulatory T cells. J Autoimmun. (2017) 79:39–52. doi: 10.1016/j.jaut.2017.01.003
88. Gurram RK, Li P, Oh J, Chen X, Spolski R, Yao X, et al. TSLP acts on regulatory T cells to maintain their identity and limit allergic inflammation. Sci Immunol. (2025) 10:eadk0073. doi: 10.1126/sciimmunol.adk0073
89. Borriello F, Iannone R, Di Somma S, Vastolo V, Petrosino G, Visconte F, et al. Lipopolysaccharide-elicited TSLPR expression enriches a functionally discrete subset of human CD14(+) CD1c(+) monocytes. J Immunol. (2017) 198:3426–35. doi: 10.4049/jimmunol.1601497
90. Poto R, Loffredo S, Palestra F, Marone G, Patella V, and Varricchi G. Angiogenesis, lymphangiogenesis, and inflammation in chronic obstructive pulmonary disease (COPD): few certainties and many outstanding questions. Cells. (2022) 11:1720. doi: 10.3390/cells11101720
91. Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. (2007) 204:253–8. doi: 10.1084/jem.20062211
92. Allakhverdi Z, Comeau MR, Armant M, Agrawal R, Woodfolk JA, Sehmi R, et al. Mast cell-activated bone marrow mesenchymal stromal cells regulate proliferation and lineage commitment of CD34(+) progenitor cells. Front Immunol. (2013) 4:461. doi: 10.3389/fimmu.2013.00461
93. Han NR, Oh HA, Nam SY, Moon PD, Kim DW, Kim HM, et al. TSLP induces mast cell development and aggravates allergic reactions through the activation of MDM2 and STAT6. J Invest Dermatol. (2014) 134:2521–30. doi: 10.1038/jid.2014.198
94. Kaur D, Doe C, Woodman L, Heidi Wan WY, Sutcliffe A, Hollins F, et al. Mast cell-airway smooth muscle crosstalk: the role of thymic stromal lymphopoietin. Chest. (2012) 142:76–85. doi: 10.1378/chest.11-1782
95. Buchheit KM, Cahill KN, Katz HR, Murphy KC, Feng C, Lee-Sarwar K, et al. Thymic stromal lymphopoietin controls prostaglandin D2 generation in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. (2016) 137:1566–76. doi: 10.1016/j.jaci.2015.10.020
96. Babina M, Wang Z, Franke K, and Zuberbier T. Thymic stromal lymphopoietin promotes MRGPRX2-triggered degranulation of skin mast cells in a STAT5-dependent manner with further support from JNK. Cells. (2021) 10:102. doi: 10.3390/cells10010102
97. Hazzan T, Eberle J, Worm M, Babina M, et al. Thymic stromal lymphopoietin interferes with the apoptosis of human skin mast cells by a dual strategy involving STAT5/mcl-1 and JNK/bcl-x(L). Cells. (2019) 8:829. doi: 10.3390/cells8080829
98. Schroeder JT and Bieneman AP. Activation of human basophils by A549 lung epithelial cells reveals a novel igE-dependent response independent of allergen. J Immunol. (2017) 199:855–65. doi: 10.4049/jimmunol.1700055
99. Hayes MD, Ward S, Crawford G, Seoane RC, Jackson WD, Kipling D, et al. Inflammation-induced IgE promotes epithelial hyperplasia and tumour growth. Elife. (2020) 9:e51862. doi: 10.7554/eLife.51862
100. Giacomin PR, Siracusa MC, Walsh KP, Grencis RK, Kubo M, Comeau MR, et al. Thymic stromal lymphopoietin-dependent basophils promote Th2 cytokine responses following intestinal helminth infection. J Immunol. (2012) 189:4371–8. doi: 10.4049/jimmunol.1200691
101. Wong CK, Hu S, Cheung PF, and Lam CW. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: implications in allergic inflammation. Am J Respir Cell Mol Biol. (2010) 43:305–15. doi: 10.1165/rcmb.2009-0168OC
102. Morshed M, Yousefi S, Stockle C, Simon HU, and Simon D. Thymic stromal lymphopoietin stimulates the formation of eosinophil extracellular traps. Allergy. (2012) 67:1127–37. doi: 10.1111/j.1398-9995.2012.02868.x
103. Varricchi G, Galdiero MR, Loffredo S, Lucarini V, Marone G, Mattei F, et al. Eosinophils: The unsung heroes in cancer? Oncoimmunology. (2018) 7:e1393134. doi: 10.1080/2162402X.2017.1393134
104. Grisaru-Tal S, Itan M, Klion AD, and Munitz A. A new dawn for eosinophils in the tumour microenvironment. Nat Rev Cancer. (2020) 20:594–607. doi: 10.1038/s41568-020-0283-9
105. Lee HC, Headley MB, Loo YM, Berlin A, Gale M Jr., Debley JS, et al. Thymic stromal lymphopoietin is induced by respiratory syncytial virus-infected airway epithelial cells and promotes a type 2 response to infection. J Allergy Clin Immunol. (2012) 130:1187–96. doi: 10.1016/j.jaci.2012.07.031
106. Calven J, Yudina Y, Hallgren O, Westergren-Thorsson G, Davies DE, Brandelius A, et al. Viral stimuli trigger exaggerated thymic stromal lymphopoietin expression by chronic obstructive pulmonary disease epithelium: role of endosomal TLR3 and cytosolic RIG-I-like helicases. J Innate Immun. (2012) 4:86–99. doi: 10.1159/000329131
107. Nagarkar DR, Poposki JA, Comeau MR, Biyasheva A, Avila PC, Schleimer RP, et al. Airway epithelial cells activate TH2 cytokine production in mast cells through IL-1 and thymic stromal lymphopoietin. J Allergy Clin Immunol. (2012) 130:225–32. doi: 10.1016/j.jaci.2012.04.019
108. Iliev ID, Spadoni I, Mileti E, Matteoli G, Sonzogni A, Sampietro GM, et al. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut. (2009) 58:1481–9. doi: 10.1136/gut.2008.175166
109. Cultrone A, de Wouters T, Lakhdari O, Kelly D, Mulder I, Logan E, et al. The NF-kappaB binding site located in the proximal region of the TSLP promoter is critical for TSLP modulation in human intestinal epithelial cells. Eur J Immunol. (2013) 43:1053–62. doi: 10.1002/eji.201142340
110. Collison AM, Sokulsky LA, Sherrill JD, Nightingale S, Hatchwell L, Talley NJ, et al. TNF-related apoptosis-inducing ligand (TRAIL) regulates midline-1, thymic stromal lymphopoietin, inflammation, and remodeling in experimental eosinophilic esophagitis. J Allergy Clin Immunol. (2015) 136:971–82. doi: 10.1016/j.jaci.2015.03.031
111. Biancheri P, Di Sabatino A, Rescigno M, Giuffrida P, Fornasa G, Tsilingiri K, et al. Abnormal thymic stromal lymphopoietin expression in the duodenal mucosa of patients with coeliac disease. Gut. (2016) 65:1670–80. doi: 10.1136/gutjnl-2014-308876
112. Vu AT, Baba T, Chen X, Le TA, Kinoshita H, Xie Y, et al. Staphylococcus aureus membrane and diacylated lipopeptide induce thymic stromal lymphopoietin in keratinocytes through the Toll-like receptor 2-Toll-like receptor 6 pathway. J Allergy Clin Immunol. (2010) 126:985–93, 993 e1-3. doi: 10.1016/j.jaci.2010.09.002
113. Li M, Hener P, Zhang Z, Kato S, Metzger D, and Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci U S A. (2006) 103:11736–41. doi: 10.1073/pnas.0604575103
114. Xie F, Meng YH, Liu LB, Chang KK, Li H, Li MQ, et al. Cervical carcinoma cells stimulate the angiogenesis through TSLP promoting growth and activation of vascular endothelial cells. Am J Reprod Immunol. (2013) 70:69–79. doi: 10.1111/aji.12104
115. Pedroza-Gonzalez A, Xu K, Wu TC, Aspord C, Tindle S, Marches F, et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J Exp Med. (2011) 208:479–90. doi: 10.1084/jem.20102131
116. Wu TC, Xu K, Martinek J, Young RR, Banchereau R, George J, et al. IL1 receptor antagonist controls transcriptional signature of inflammation in patients with metastatic breast cancer. Cancer Res. (2018) 78:5243–58. doi: 10.1158/0008-5472.CAN-18-0413
117. Kuan EL and Ziegler SF. A tumor-myeloid cell axis, mediated via the cytokines IL-1alpha and TSLP, promotes the progression of breast cancer. Nat Immunol. (2018) 19:366–74. doi: 10.1038/s41590-018-0066-6
118. Burkard-Mandel L, O’Neill R, Colligan S, Seshadri M, and Abrams SI. Tumor-derived thymic stromal lymphopoietin enhances lung metastasis through an alveolar macrophage-dependent mechanism. Oncoimmunology. (2018) 7:e1419115. doi: 10.1080/2162402X.2017.1419115
119. Okayama Y, Okumura S, Sagara H, Yuki K, Sasaki T, Watanabe N, et al. FcepsilonRI-mediated thymic stromal lymphopoietin production by interleukin-4-primed human mast cells. Eur Respir J. (2009) 34:425–35. doi: 10.1183/09031936.00121008
120. Shikotra A, Choy DF, Ohri CM, Doran E, Butler C, Hargadon B, et al. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol. (2012) 129:104–11. doi: 10.1016/j.jaci.2011.08.031
121. Kashyap M, Rochman Y, Spolski R, Samsel L, and Leonard WJ. Thymic stromal lymphopoietin is produced by dendritic cells. J Immunol. (2011) 187:1207–11. doi: 10.4049/jimmunol.1100355
122. Spadoni I, Iliev ID, Rossi G, and Rescigno M. Dendritic cells produce TSLP that limits the differentiation of Th17 cells, fosters Treg development, and protects against colitis. Mucosal Immunol. (2012) 5:184–93. doi: 10.1038/mi.2011.64
123. Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. (2013) 155:285–95. doi: 10.1016/j.cell.2013.08.057
124. McLarty JL, Melendez GC, Brower GL, Janicki JS, and Levick SP. Tryptase/Protease-activated receptor 2 interactions induce selective mitogen-activated protein kinase signaling and collagen synthesis by cardiac fibroblasts. Hypertension. (2011) 58:264–70. doi: 10.1161/HYPERTENSIONAHA.111.169417
125. Nagata Y, Kamijuku H, Taniguchi M, Ziegler S, and Seino K. Differential role of thymic stromal lymphopoietin in the induction of airway hyperreactivity and Th2 immune response in antigen-induced asthma with respect to natural killer T cell function. Int Arch Allergy Immunol. (2007) 144:305–14. doi: 10.1159/000106319
126. Akamatsu T, Watanabe N, Kido M, Saga K, Tanaka J, Kuzushima K, et al. Human TSLP directly enhances expansion of CD8+ T cells. Clin Exp Immunol. (2008) 154:98–106. doi: 10.1111/j.1365-2249.2008.03731.x
127. Wang B, Peng Y, Dong J, Lin J, Wu C, Su Y, et al. Human platelets express functional thymic stromal lymphopoietin receptors: a potential role in platelet activation in acute coronary syndrome. Cell Physiol Biochem. (2013) 32:1741–50. doi: 10.1159/000356608
128. Dong J, Lin J, Wang B, He S, Wu C, Kushwaha KK, et al. Inflammatory cytokine TSLP stimulates platelet secretion and potentiates platelet aggregation via a TSLPR-dependent PI3K/Akt signaling pathway. Cell Physiol Biochem. (2015) 35:160–74. doi: 10.1159/000369684
129. Varricchi G, Galdiero MR, Marone G, Granata F, Borriello F, and Marone G. Controversial role of mast cells in skin cancers. Exp Dermatol. (2017) 26:11–7. doi: 10.1111/exd.13107
130. Varricchi G, Galdiero MR, Loffredo S, Marone G, Iannone R, Marone G, et al. Are mast cells MASTers in cancer? Front Immunol. (2017) 8:424. doi: 10.3389/fimmu.2017.00424
131. Staiano RI, Loffredo S, Borriello F, Iannotti FA, Piscitelli F, Orlando P, et al. Human lung-resident macrophages express CB1 and CB2 receptors whose activation inhibits the release of angiogenic and lymphangiogenic factors. J Leukoc Biol. (2016) 99:531–40. doi: 10.1189/jlb.3HI1214-584R
132. Russell LJ, Capasso M, Vater I, Akasaka T, Bernard OA, Calasanz MJ, et al. Deregulated expression of cytokine receptor gene, CRLF2, is involved in lymphoid transformation in B-cell precursor acute lymphoblastic leukemia. Blood. (2009) 114:2688–98. doi: 10.1182/blood-2009-03-208397
133. Harvey RC, Mullighan CG, Chen IM, Wharton W, Mikhail FM, Carroll AJ, et al. Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood. (2010) 115:5312–21. doi: 10.1182/blood-2009-09-245944
134. Yoda A, Yoda Y, Chiaretti S, Bar-Natan M, Mani K, Rodig SJ, et al. Functional screening identifies CRLF2 in precursor B-cell acute lymphoblastic leukemia. Proc Natl Acad Sci U.S.A. (2010) 107:252–7. doi: 10.1073/pnas.0911726107
135. Konoplev S, Lu X, Konopleva M, Jain N, Ouyang J, Goswami M, et al. CRLF2-positive B-cell acute lymphoblastic leukemia in adult patients: A single-institution experience. Am J Clin Pathol. (2017) 147:357–63. doi: 10.1093/ajcp/aqx005
136. Vetter T, Borowski A, Wohlmann A, Ranjan N, Kuepper M, Badura S, et al. Blockade of thymic stromal lymphopoietin (TSLP) receptor inhibits TSLP-driven proliferation and signalling in lymphoblasts from a subset of B-precursor ALL patients. Leuk Res. (2016) 40:38–43. doi: 10.1016/j.leukres.2015.10.003
137. Chiaretti S, Brugnoletti F, Messina M, Paoloni F, Fedullo AL, Piciocchi A, et al. CRLF2 overexpression identifies an unfavourable subgroup of adult B-cell precursor acute lymphoblastic leukemia lacking recurrent genetic abnormalities. Leuk Res. (2016) 41:36–42. doi: 10.1016/j.leukres.2015.11.018
138. Palmi C, Vendramini E, Silvestri D, Longinotti G, Frison D, Cario G, et al. Poor prognosis for P2RY8-CRLF2 fusion but not for CRLF2 over-expression in children with intermediate risk B-cell precursor acute lymphoblastic leukemia. Leukemia. (2012) 26:2245–53. doi: 10.1038/leu.2012.101
139. Jia M, Wang ZJ, Zhao HZ, Shen HP, Cheng YP, Luo ZB, et al. Prognostic significance of cytokine receptor-like factor 2 alterations in acute lymphoblastic leukemia: a meta-analysis. World J Pediatr. (2015) 11:126–33. doi: 10.1007/s12519-015-0019-1
140. Reshmi SC, Harvey RC, Roberts KG, Stonerock E, Smith A, Jenkins H, et al. Targetable kinase gene fusions in high-risk B-ALL: a study from the Children’s Oncology Group. Blood. (2017) 129:3352–61. doi: 10.1182/blood-2016-12-758979
141. Demina I, Zerkalenkova E, Soldatkina O, Kazakova A, Semchenkova A, Goncharova M, et al. Correlation of the surface expression of thymic stromal lymphopoietin receptor with the presence of CRLF2 gene rearrangements in children with B-lineage acute lymphoblastic leukemia. Int J Lab Hematol. (2023) 45:337–43. doi: 10.1111/ijlh.14028
142. Wang Y, Li J, Xue TL, Tian S, Yue ZX, Liu SG, et al. Clinical, biological, and outcome features of P2RY8-CRLF2 and CRLF2 over-expression in pediatric B-cell precursor acute lymphoblastic leukemia according to the CCLG-ALL 2008 and 2018 protocol. Eur J Haematol. (2023) 110:669–79. doi: 10.1111/ejh.13948
143. Ferretti E, Hohaus S, Di Napoli A, Belmonte B, Cuccaro A, Cupelli E, et al. Interleukin-31 and thymic stromal lymphopoietin expression in plasma and lymph node from Hodgkin lymphoma patients. Oncotarget. (2017) 8:85263–75. doi: 10.18632/oncotarget.19665
144. Takahashi N, Sugaya M, Suga H, Oka T, Kawaguchi M, Miyagaki T, et al. Thymic stromal chemokine TSLP acts through th2 cytokine production to induce cutaneous T-cell lymphoma. Cancer Res. (2016) 76:6241–52. doi: 10.1158/0008-5472.CAN-16-0992
145. Tassi E, Gavazzi F, Albarello L, Senyukov V, Longhi R, Dellabona P, et al. Carcinoembryonic antigen-specific but not antiviral CD4+ T cell immunity is impaired in pancreatic carcinoma patients. J Immunol. (2008) 181:6595–603. doi: 10.4049/jimmunol.181.9.6595
146. De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. (2011) 208:469–78. doi: 10.1084/jem.20101876
147. Protti MP and De Monte L. Cross-talk within the tumor microenvironment mediates Th2-type inflammation in pancreatic cancer. Oncoimmunology. (2012) 1:89–91. doi: 10.4161/onci.1.1.17939
148. De Monte L, Wormann S, Brunetto E, Heltai S, Magliacane G, Reni M, et al. Basophil recruitment into tumor-draining lymph nodes correlates with th2 inflammation and reduced survival in pancreatic cancer patients. Cancer Res. (2016) (2016)76:1792–803. doi: 10.1158/0008-5472.CAN-15-1801-T
149. Brunetto E, De Monte L, Balzano G, Camisa B, Laino V, Riba M, et al. The IL-1/IL-1 receptor axis and tumor cell released inflammasome adaptor ASC are key regulators of TSLP secretion by cancer associated fibroblasts in pancreatic cancer. J Immunother Cancer. (2019) 7:45. doi: 10.1186/s40425-019-0521-4
150. Vizio B, Boita M, Cristiano C, Mazibrada J, Bosco O, Novarino A, et al. Thymic stromal lymphopoietin in human pancreatic ductal adenocarcinoma: expression and prognostic significance. Oncotarget. (2018) 9:32795–809. doi: 10.18632/oncotarget.25997
151. Marone G, Borriello F, Varricchi G, Genovese A, and Granata F. Basophils: historical reflections and perspectives. Chem Immunol Allergy. (2014) 100:172–92. doi: 10.1159/000358734
152. Varricchi G, Raap U, Rivellese F, Marone G, and Gibbs BF. Human mast cells and basophils-How are they similar how are they different? Immunol Rev. (2018) 282:8–34. doi: 10.1111/imr.12627
153. Miyake K, Shibata S, Yoshikawa S, and Karasuyama H. Basophils and their effector molecules in allergic disorders. Allergy. (2021) 76:1693–706. doi: 10.1111/all.14662
154. Nakashima C, Otsuka A, and Kabashima K. Recent advancement in the mechanism of basophil activation. J Dermatol Sci. (2018) 91:3–8. doi: 10.1016/j.jdermsci.2018.03.007
155. Eberle JU and Voehringer D. Role of basophils in protective immunity to parasitic infections. Semin Immunopathol. (2016) 38:605–13. doi: 10.1007/s00281-016-0563-3
156. Karasuyama H, Miyake K, and Yoshikawa S. Immunobiology of acquired resistance to ticks. Front Immunol. (2020) 11:601504. doi: 10.3389/fimmu.2020.601504
157. Peng J and Siracusa MC. Basophils in antihelminth immunity. Semin Immunol. (2021) 53:101529. doi: 10.1016/j.smim.2021.101529
158. Donnelly EL, Cespedes N, Hansten G, Wagers D, Briggs AM, Lowder C, et al. Basophil depletion alters host immunity, intestinal permeability, and mammalian host-to-mosquito transmission in malaria. Immunohorizons. (2022) 6:581–99. doi: 10.4049/immunohorizons.2200055
159. Marone G, Schroeder JT, Mattei F, Loffredo S, Gambardella AR, Poto R, et al. Is there a role for basophils in cancer? Front Immunol. (2020) 11:2103. doi: 10.3389/fimmu.2020.02103
160. Mukaida N, Tanabe Y, and Baba T. Cancer non-stem cells as a potent regulator of tumor microenvironment: a lesson from chronic myeloid leukemia. Mol BioMed. (2021) 2:7. doi: 10.1186/s43556-021-00030-7
161. Chauhan J, Stavraka C, Grandits M, Palhares L, Josephs DH, Lacy KE, et al. Clinical and translational significance of basophils in patients with cancer. Cells. (2022) 11:438. doi: 10.3390/cells11030438
162. LaMarche NM, Hegde S, Park MD, Maier BB, Troncoso L, Le Berichel J, et al. An IL-4 signalling axis in bone marrow drives pro-tumorigenic myelopoiesis. Nature. (2024) 625:166–74. doi: 10.1038/s41586-023-06797-9
163. Poto R, Gambardella AR, Marone G, Schroeder JT, Mattei F, Schiavoni G, et al. Basophils from allergy to cancer. Front Immunol. (2022) 13:1056838. doi: 10.3389/fimmu.2022.1056838
164. Stankovic B, Bjorhovde HAK, Skarshaug R, Aamodt H, Frafjord A, Muller E, et al. Immune cell composition in human non-small cell lung cancer. Front Immunol. (2018) 9:3101. doi: 10.3389/fimmu.2018.03101
165. Lavin Y, Kobayashi S, Leader A, Amir ED, Elefant N, Bigenwald C, et al. Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell. (2017) 169:750–765 e17. doi: 10.1016/j.cell.2017.04.014
166. Crawford G, Hayes MD, Seoane RC, Ward S, Dalessandri T, Lai C, et al. Epithelial damage and tissue gammadelta T cells promote a unique tumor-protective IgE response. Nat Immunol. (2018) 19:859–70. doi: 10.1038/s41590-018-0161-8
167. Varricchi G, Ameri P, Cadeddu C, Ghigo A, Madonna R, Marone G, et al. Antineoplastic drug-induced cardiotoxicity: A redox perspective. Front Physiol. (2018) 9:167. doi: 10.3389/fphys.2018.00167
168. Locati M, Curtale G, and Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. (2020) 15:123–47. doi: 10.1146/annurev-pathmechdis-012418-012718
169. de Paulis A, Prevete N, Fiorentino I, Rossi FW, Staibano S, Montuori N, et al. Expression and functions of the vascular endothelial growth factors and their receptors in human basophils. J Immunol. (2006) 177:7322–31. doi: 10.4049/jimmunol.177.10.7322
170. Ochensberger B, Tassera L, Bifrare D, Rihs S, and Dahinden CA. Regulation of cytokine expression and leukotriene formation in human basophils by growth factors, chemokines and chemotactic agonists. Eur J Immunol. (1999) 29:11–22. doi: 10.1002/(SICI)1521-4141(199901)29:01<11::AID-IMMU11>3.0.CO;2-B
171. Miura K and MacGlashan DW Jr. Dual phase priming by IL-3 for leukotriene C4 generation in human basophils: difference in characteristics between acute and late priming effects. J Immunol. (2000) 164:3026–34. doi: 10.4049/jimmunol.164.6.3026
172. Duah E, Teegala LR, Kondeti V, Adapala RK, Keshamouni VG, Kanaoka Y, et al. Cysteinyl leukotriene 2 receptor promotes endothelial permeability, tumor angiogenesis, and metastasis. Proc Natl Acad Sci U.S.A. (2019) 116:199–204. doi: 10.3389/fimmu.2020.02103
173. Loffredo S, Bova M, Suffritti C, Borriello F, Zanichelli A, Petraroli A, et al. Elevated plasma levels of vascular permeability factors in C1 inhibitor-deficient hereditary angioedema. Allergy. (2016) 71:989–96. doi: 10.1111/all.12862
174. Khaled N and Bidet Y. New insights into the implication of epigenetic alterations in the EMT of triple negative breast cancer. Cancers (Basel). (2019) 11:559. doi: 10.3390/cancers11040559
175. Olkhanud PB, Rochman Y, Bodogai M, Malchinkhuu E, Wejksza K, Xu M, et al. Thymic stromal lymphopoietin is a key mediator of breast cancer progression. J Immunol. (2011) 186:5656–62. doi: 10.4049/jimmunol.1100463
176. DeNardo DG and Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. (2019) 19:369–82. doi: 10.1038/s41577-019-0127-6
177. Mantovani A, Marchesi F, Malesci A, Laghi L, and Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. (2017) 14:399–416. doi: 10.1038/nrclinonc.2016.217
178. Cassetta L and Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov. (2018) 17:887–904. doi: 10.1038/nrd.2018.169
179. Balestrieri B, Granata F, Loffredo S, Petraroli A, Scalia G, Morabito P, et al. Phenotypic and functional heterogeneity of low-density and high-density human lung macrophages. Biomedicines. (2021) 9:505. doi: 10.3390/biomedicines9050505
180. Semlali A, Almutairi M, Reddy Parine N, Al Amri A, Almeer R, Alanazi MS, et al. Expression and allele frequencies of Thymic stromal lymphopoietin are a key factor of breast cancer risk. Mol Genet Genomic Med. (2019) 7:e813. doi: 10.1002/mgg3.813
181. Klobuch S, Seijkens TTP, Schumacher TN, and Haanen J. Tumour-infiltrating lymphocyte therapy for patients with advanced-stage melanoma. Nat Rev Clin Oncol. (2024) 21:173–84. doi: 10.1038/s41571-023-00848-w
182. Tocchetti CG, Galdiero MR, and Varricchi G. Cardiac toxicity in patients treated with immune checkpoint inhibitors: it is now time for cardio-immuno-oncology. J Am Coll Cardiol. (2018) 71:1765–7. doi: 10.1016/j.jacc.2018.02.038
183. Yao W, German B, Chraa D, Braud A, Hugel C, Meyer P, et al. Keratinocyte-derived cytokine TSLP promotes growth and metastasis of melanoma by regulating the tumor-associated immune microenvironment. JCI Insight. (2022) 7:e161438. doi: 10.1172/jci.insight.161438
184. Looi LM. Tumor-associated tissue eosinophilia in nasopharyngeal carcinoma. A pathologic study of 422 primary and 138 metastatic tumors. Cancer. (1987) 59:466–70. doi: 10.1002/1097-0142(19870201)59:3<466::AID-CNCR2820590319>3.0.CO;2-P
185. Caruso RA, Parisi A, Quattrocchi E, Scardigno M, Branca G, Parisi C, et al. Ultrastructural descriptions of heterotypic aggregation between eosinophils and tumor cells in human gastric carcinomas. Ultrastruct Pathol. (2011) 35:145–9. doi: 10.3109/01913123.2011.578233
186. Harbaum L, Pollheimer MJ, Kornprat P, Lindtner RA, Bokemeyer C, and Langner C. Peritumoral eosinophils predict recurrence in colorectal cancer. Mod Pathol. (2015) 28:403–13. doi: 10.1038/modpathol.2014.104
187. Xie F, Liu LB, Shang WQ, Chang KK, Meng YH, Mei J, et al. The infiltration and functional regulation of eosinophils induced by TSLP promote the proliferation of cervical cancer cell. Cancer Lett. (2015) 364:106–17. doi: 10.1016/j.canlet.2015.04.029
188. Prizment AE, Vierkant RA, Smyrk TC, Tillmans LS, Lee JJ, Sriramarao P, et al. Tumor eosinophil infiltration and improved survival of colorectal cancer patients: Iowa Women’s Health Study. Mod Pathol. (2016) 29:516–27. doi: 10.1038/modpathol.2016.42
189. Molin D, Glimelius B, Sundstrom C, Venge P, and Enblad G. The serum levels of eosinophil cationic protein (ECP) are related to the infiltration of eosinophils in the tumours of patients with Hodgkin’s disease. Leuk Lymphoma. (2001) 42:457–65. doi: 10.3109/10428190109064602
190. Cormier SA, Taranova AG, Bedient C, Nguyen T, Protheroe C, Pero R, et al. Pivotal Advance: eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J Leukoc Biol. (2006) 79:1131–9. doi: 10.1189/jlb.0106027
191. Varricchi G, Bagnasco D, Borriello F, Heffler E, and Canonica GW. Interleukin-5 pathway inhibition in the treatment of eosinophilic respiratory disorders: evidence and unmet needs. Curr Opin Allergy Clin Immunol. (2016) 16:186–200. doi: 10.1097/ACI.0000000000000251
192. Lotfi R, Lee JJ, and Lotze MT. Eosinophilic granulocytes and damage-associated molecular pattern molecules (DAMPs): role in the inflammatory response within tumors. J Immunother. (2007) 30:16–28. doi: 10.1097/01.cji.0000211324.53396.f6
193. Davis BP and Rothenberg ME. Eosinophils and cancer. Cancer Immunol Res. (2014) 2:1–8. doi: 10.1158/2326-6066.CIR-13-0196
194. Gatault S, Legrand F, Delbeke M, Loiseau S, and Capron M. Involvement of eosinophils in the anti-tumor response. Cancer Immunol Immunother. (2012) 61:1527–34. doi: 10.1007/s00262-012-1288-3
195. Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, and Hammerling GJ. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol. (2015) 16:609–17. doi: 10.1038/ni.3159
196. Lucarini V, Ziccheddu G, Macchia I, La Sorsa V, Peschiaroli F, Buccione C, et al. IL-33 restricts tumor growth and inhibits pulmonary metastasis in melanoma-bearing mice through eosinophils. Oncoimmunology. (2017) 6:e1317420. doi: 10.1080/2162402X.2017.1317420
197. Ikutani M, Yanagibashi T, Ogasawara M, Tsuneyama K, Yamamoto S, Hattori Y, et al. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. J Immunol. (2012) 188:703–13. doi: 10.4049/jimmunol.1101270
198. Mattes J, Hulett M, Xie W, Hogan S, Rothenberg ME, Foster P, et al. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: an eotaxin and STAT6-dependent process. J Exp Med. (2003) 197:387–93. doi: 10.1084/jem.20021683
199. Gambardella AR, Antonucci C, Zanetti C, Noto F, Andreone S, Vacca D, et al. IL-33 stimulates the anticancer activities of eosinophils through extracellular vesicle-driven reprogramming of tumor cells. J Exp Clin Cancer Res. (2024) 43:209. doi: 10.1186/s13046-024-03129-1
200. Glimelius I, Rubin J, Fischer M, Molin D, Amini RM, Venge P, et al. Effect of eosinophil cationic protein (ECP) on Hodgkin lymphoma cell lines. Exp Hematol. (2011) 39:850–8. doi: 10.1016/j.exphem.2011.05.006
201. Kataoka S, Konishi Y, Nishio Y, Fujikawa-Adachi K, and Tominaga A. Antitumor activity of eosinophils activated by IL-5 and eotaxin against hepatocellular carcinoma. DNA Cell Biol. (2004) 23:549–60. doi: 10.1089/dna.2004.23.549
202. Furbert-Harris P, Parish-Gause D, Laniyan I, Hunter KA, Okomo-Awich J, Vaughn TR, et al. Inhibition of prostate cancer cell growth by activated eosinophils. Prostate. (2003) 57:165–75. doi: 10.1002/pros.10286
203. Nissim Ben Efraim AH and Levi-Schaffer F. Roles of eosinophils in the modulation of angiogenesis. Chem Immunol Allergy. (2014) 99:138–54. doi: 10.1159/000353251
204. Hoshino M, Takahashi M, and Aoike N. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin immunoreactivity in asthmatic airways and its relationship to angiogenesis. J Allergy Clin Immunol. (2001) 107:295–301. doi: 10.1067/mai.2001.111928
205. Yousefi S, Hemmann S, Weber M, Holzer C, Hartung K, Blaser K, et al. IL-8 is expressed by human peripheral blood eosinophils. Evidence for increased secretion in asthma. J Immunol. (1995) 154:5481–90. doi: 10.4049/jimmunol.154.10.5481
206. Sektioglu IM, Carretero R, Bulbuc N, Bald T, Tuting T, Rudensky AY, et al. Basophils promote tumor rejection via chemotaxis and infiltration of CD8+ T cells. Cancer Res. (2017) 77:291–302. doi: 10.1158/0008-5472.CAN-16-0993
207. Bax HJ, Chauhan J, Stavraka C, Khiabany A, Nakamura M, Pellizzari G, et al. Basophils from cancer patients respond to immune stimuli and predict clinical outcome. Cells. (2020) 9:1631. doi: 10.3390/cells9071631
208. Xi Y and Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. (2021) 14:101174. doi: 10.1016/j.tranon.2021.101174
209. Obata-Ninomiya K, de Jesus Carrion S, Hu A, and Ziegler SF. Emerging role for thymic stromal lymphopoietin-responsive regulatory T cells in colorectal cancer progression in humans and mice. Sci Transl Med. (2022) 14:eabl6960. doi: 10.1126/scitranslmed.abl6960
210. Semlali A, Almutairi MH, Alamri A, Reddy Parine N, Arafah M, Almadi MA, et al. Expression and polymorphism of TSLP/TSLP receptors as potential diagnostic markers of colorectal cancer progression. Genes (Basel). (2021) 12:1386. doi: 10.3390/genes12091386
211. Ameri AH, Moradi Tuchayi S, Zaalberg A, Park JH, Ngo KH, Li T, et al. IL-33/regulatory T cell axis triggers the development of a tumor-promoting immune environment in chronic inflammation. Proc Natl Acad Sci U.S.A. (2019) 116:2646–51. doi: 10.1073/pnas.1815016116
212. Svensson H, Olofsson V, Lundin S, Yakkala C, Bjorck S, Borjesson L, et al. Accumulation of CCR4(+)CTLA-4 FOXP3(+)CD25(hi) regulatory T cells in colon adenocarcinomas correlate to reduced activation of conventional T cells. PloS One. (2012) 7:e30695. doi: 10.1371/journal.pone.0030695
213. Pastille E, Wasmer MH, Adamczyk A, Vu VP, Mager LF, Phuong NNT, et al. The IL-33/ST2 pathway shapes the regulatory T cell phenotype to promote intestinal cancer. Mucosal Immunol. (2019) 12:990–1003. doi: 10.1038/s41385-019-0176-y
214. Leiter A, Veluswamy RR, and Wisnivesky JP. The global burden of lung cancer: current status and future trends. Nat Rev Clin Oncol. (2023) 20:624–39. doi: 10.1038/s41571-023-00798-3
215. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
216. Schabath MB and Cote ML. Cancer progress and priorities: lung cancer. Cancer Epidemiol Biomarkers Prev. (2019) 28:1563–79. doi: 10.1158/1055-9965.EPI-19-0221
217. Cheng S, Li Z, Gao R, Xing B, Gao Y, Yang Y, et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell. (2021) 184:792–809 e23. doi: 10.1016/j.cell.2021.01.010
218. Travaglini KJ, Nabhan AN, Penland L, Sinha R, Gillich A, Sit RV, et al. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature. (2020) 587:619–25. doi: 10.1038/s41586-020-2922-4
219. Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, et al. The immune landscape of cancer. Immunity. (2018) 48:812–830 e14. doi: 10.1016/j.immuni.2018.03.023
220. Li H, Zhao H, Yu J, Su Y, Cao S, An X, et al. Increased prevalence of regulatory T cells in the lung cancer microenvironment: a role of thymic stromal lymphopoietin. Cancer Immunol Immunother. (2011) 60:1587–96. doi: 10.1007/s00262-011-1059-6
221. Barkan D, El Touny LH, Michalowski AM, Smith JA, Chu I, Davis AS, et al. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer Res. (2010) 70:5706–16. doi: 10.1158/0008-5472.CAN-09-2356
222. Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. (2018) 378:2486–96. doi: 10.1056/NEJMoa1804092
223. Poto R, Loffredo S, Marone G, Di Salvatore A, de Paulis A, Schroeder JT, et al. Basophils beyond allergic and parasitic diseases. Front Immunol. (2023) 14:1190034. doi: 10.3389/fimmu.2023.1190034
224. Morgan E, Arnold M, Camargo MC, Gini A, Kunzmann AT, Matsuda T, et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020-40: A population-based modelling study. EClinicalMedicine. (2022) 47:101404. doi: 10.1016/j.eclinm.2022.101404
225. Barooei R, Mahmoudian RA, Abbaszadegan MR, Mansouri A, and Gholamin M. Evaluation of thymic stromal lymphopoietin (TSLP) and its correlation with lymphatic metastasis in human gastric cancer. Med Oncol. (2015) 32:217. doi: 10.1007/s12032-015-0653-4
226. Watanabe J, Saito H, Miyatani K, Ikeguchi M, and Umekita Y. TSLP expression and high serum TSLP level indicate a poor prognosis in gastric cancer patients. Yonago Acta Med. (2015) 58:137–43.
227. Cohen PA, Jhingran A, Oaknin A, and Denny L. Cervical cancer. Lancet. (2019) 393:169–82. doi: 10.1016/S0140-6736(18)32470-X
228. Zhou WJ, Yang HL, Chang KK, Meng Y, Wang MY, Yuan MM, et al. Human thymic stromal lymphopoietin promotes the proliferation and invasion of cervical cancer cells by downregulating microRNA-132 expression. Oncol Lett. (2017) 14:7910–6. doi: 10.3892/ol.2017.7260
229. Kuroda Y, Yuki T, Takahashi Y, Sakaguchi H, Matsunaga K, and Itagaki H. Long form of thymic stromal lymphopoietin of keratinocytes is induced by protein allergens. J Immunotoxicol. (2017) 14:178–87. doi: 10.1080/1547691X.2017.1349220
230. Xu L, Guo Y, Xu N, Chen L, Zhu J, Liu N, et al. Overexpression of thymic stromal lymphopoietin is correlated with poor prognosis in epithelial ovarian carcinoma. Biosci Rep. (2019) 39:BSR20190116. doi: 10.1042/BSR20190116
231. Chan LKY, Lau TS, Chung KY, Tam C, Cheung TH, Yim SF, et al. Short-form thymic stromal lymphopoietin (sfTSLP) is the predominant isoform expressed by gynaecologic cancers and promotes tumour growth. Cancers (Basel). (2021) 13:980. doi: 10.3390/cancers13050980
232. Demehri S, Cunningham TJ, Manivasagam S, Ngo KH, Moradi Tuchayi S, Reddy R, et al. Thymic stromal lymphopoietin blocks early stages of breast carcinogenesis. J Clin Invest. (2016) 126:1458–70. doi: 10.1172/JCI83724
233. Boieri M, Marchese E, Pham QM, Azin M, Steidl LE, Malishkevich A, et al. Thymic stromal lymphopoietin-stimulated CD4(+) T cells induce senescence in advanced breast cancer. Front Cell Dev Biol. (2022) 10:1002692. doi: 10.3389/fcell.2022.1002692
234. Guennoun R, Hojanazarova J, Trerice KE, Azin M, McGoldrick MT, Schiferle EB, et al. Thymic stromal lymphopoietin induction suppresses lung cancer development. Cancers (Basel). (2022) 14, 2173. doi: 10.3390/cancers14092173
235. Di Piazza M, Nowell CS, Koch U, Durham AD, and Radtke F. Loss of cutaneous TSLP-dependent immune responses skews the balance of inflammation from tumor protective to tumor promoting. Cancer Cell. (2012) 22:479–93. doi: 10.1016/j.ccr.2012.08.016
236. Demehri S, Turkoz A, Manivasagam S, Yockey LJ, Turkoz M, and Kopan R. Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell. (2012) 22:494–505. doi: 10.1016/j.ccr.2012.08.017
237. Crosbie EJ, Kitson SJ, McAlpine JN, Mukhopadhyay A, Powell ME, and Singh N. Endometrial cancer. Lancet. (2022) 399:1412–28. doi: 10.1016/S0140-6736(22)00323-3
238. Lv M, Xu Y, Chen P, Li J, Qin Z, Huang B, et al. TSLP enhances progestin response in endometrial cancer via androgen receptor signal pathway. Br J Cancer. (2024) 130:585. doi: 10.1038/s41416-023-02545-y
Keywords: alarmin, cancer immunity, cytokine, TSLP, TSLP isoforms, tumor microenvironment
Citation: Poto R, Marone G, Ziegler SF and Varricchi G (2025) TSLP: contrasting roles in cancer. Front. Immunol. 16:1627235. doi: 10.3389/fimmu.2025.1627235
Received: 12 May 2025; Accepted: 21 July 2025;
Published: 12 August 2025.
Edited by:
Paula Lam, Independent Researcher, Singapore, SingaporeReviewed by:
Inigo Martinez, UiT The Arctic University of Norway, NorwayZhihao Wu, Germinal Biotech Pte Ltd, Singapore
Copyright © 2025 Poto, Marone, Ziegler and Varricchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steven F. Ziegler, c3ppZWdsZXJAYmVuYXJveWFyZXNlYXJjaC5vcmc=; Gilda Varricchi, Z2lsZGFuZXRAZ21haWwuY29t
 Remo Poto
Remo Poto Gianni Marone
Gianni Marone Steven F. Ziegler
Steven F. Ziegler Gilda Varricchi
Gilda Varricchi