- 1Institute of Animal Nutrition, Sichuan Agricultural University, Key Laboratory of Animal Disease-Resistance Nutrition, Ministry of Education, and Key Laboratory of Animal Disease-Resistant Nu-trition, Chengdu, Sichuan, China
- 2Animal and Plant Health & Nutrition, Chr. Hansen A/S, Hoersholm, Denmark
Purpose: SOLVENS (SLV) is a zootechnical feed additive based on viable spores of Bacillus licheniformis and Bacillus subtilis. This study aimed to evaluate the effects of SLV on the intestinal health of weaned piglets.
Methods: A total of 360 healthy 24-day-old weaned Duroc × Landrace × Yorkshire piglets were allocated to three treatment groups based on body weight and sex: T1 (Control, CON), T2 (SLV 200, 6.5×108 CFU per kg feed), and T3 (SLV 400, 1.3×109 CFU per kg feed). Each treatment consisted of 30 replicates with four pigs per replicate, and the experiment lasted 42 days. Piglets were fed mash pre-starter feed (days 1–14) and mash starter feed (days 15–42). Growth performance, fecal microorganisms, serum immunity, and intestinal barrier function were assessed. Experimental data were analyzed using SPSS 27.0 for one-way ANOVA, and multiple comparisons were made using the DUNCAN method.
Results: Compared with the control, SLV 200 and SLV 400 significantly reduced diarrhea rate (P < 0.05). SLV 200 increased fecal Lactobacilli and decreased Escherichia coli on day 14 (P < 0.05), while SLV 400 elevated Lactobacilli on days 14 and 42 and reduced E. coli on day 14 (P < 0.05). SLV 200 increased fecal sIgA and serum IgG on day 42 (P < 0.05), whereas SLV 400 elevated serum IgG and IgM on day 14 (P < 0.05) and serum IgA on day 42 (P < 0.05). Additionally, SLV 200 downregulated ileal interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) gene expression (P < 0.05), and SLV 400 reduced TNF-α expression (P < 0.05).
Conclusion: Dietary supplementation with SLV improved intestinal health by modulating gut microbiota and enhancing immunity in weaned piglets.
1 Introduction
Immune function and the digestive system of piglets at weaning are not fully developed, and weaning stress causes disturbances in gut microbiota and mucosal immune dysfunction (1, 2). Weaning stress often causes post-weaning diarrhea and slows down the growth of piglets (3). In addition, weaning stress damages intestinal structure and function, induces various diseases, and can even lead to death (4, 5).
In the past, livestock industry used antibiotics to alleviate post-weaning diarrhea, but due to the many negative effects of antibiotic use, many countries have banned the use of antibiotics in feed. Therefore, new feed additives are urgently needed in the swine industry to solve this problem. During the last decade, the application of probiotics in animal feed has increased. Studies have shown that probiotics can improve intestinal health and reduce the negative impact of weaning stress on piglets (6–9). According to previous studies, dietary supplementation with Bacillus subtilis in weaned piglets can increase the number of goblet cells and upregulate the expression of antimicrobial peptides, thereby enhancing intestinal mucosal barrier and improving mucosal immunity (10). This helps to inhibit Escherichia coli-induced intestinal damage, reduce diarrhea incidence, and improve growth performance in piglets (11, 12). Bacillus licheniformis functions to balance gut microbiota, promoting development of immune organs as well as improving immune function. (13–15). However, the effects of a single probiotic on the health of piglets are limited (16). Complex probiotics may have synergistic effects on piglet health, but relevant research is limited and needs further research.
The present experiment was designed to evaluate the effects of dietary addition of SOLVENS (SLV), a complex probiotic composed of Bacillus subtilis DSM5750, Bacillus subtilis DSM27273, and Bacillus licheniformis DSM5749. Our results will provide a new solution to alleviate post-weaning diarrhea for pig industry in the post-antibiotic era.
2 Materials and methods
This experiment was conducted at the Animal Experiment Center of Sichuan Agricultural University, Ya’an, China. All animal procedures associated with this study were approved by the Animal Care and Use Committee, Sichuan Agricultural University (Ethical Approval Code: SICAUAC20220506; Ya’an,China).
Comprehensive probiotic SLV is provided by Chr. Hansen A/S (Hoersholm, Denmark). SLV is composed of Bacillus subtilis DSM5750, Bacillus subtilis DSM27273, and Bacillus licheniformis DSM5749, in a ratio of 2:1:1. The total number of bacteria is 3.25×109 CFU/g.
2.1 Experimental design and animal management
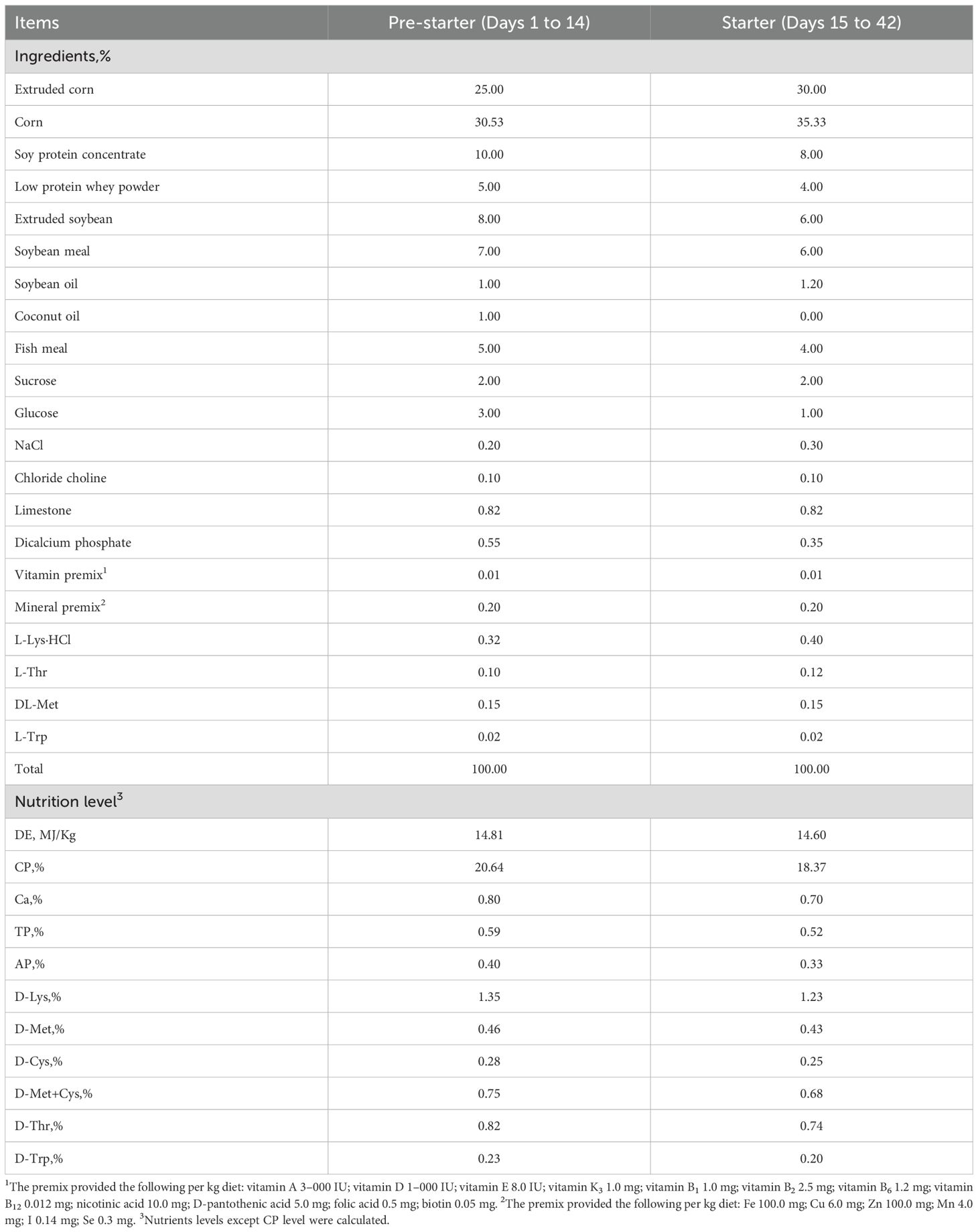
A total of 360 healthy 24-day-old weaned Duroc × Landrace × Yorkshire piglets (initial body weight: 7.79 ± 0.25kg, mean ± SEM) were selected and allocated to 3 treatments according to their initial body weight and sex: CON (a 2-period basal diet, Control); SLV 200 (adding 200 mg/kg SLV); T3: SLV 400 (adding 400 mg/kg SLV). Each treatment consisted of 30 replicate pens, with four pigs (2 male and 2 female) per pen. Therefore, each treatment contains a total of 120 piglets. The basal diets (Table 1) were formulated to meet nutrient requirements based on the National Research Council (17). The experiment lasted for 42 days.
All weaned piglets were fed the treatment diets 4 times per day at 08:00, 12:00, 16:00, and 20:00, and water was provided ad libitum. All piglets were housed in a controlled room with temperature maintained at 28 ± 1 °C and relative humidity controlled at 65–75%.
In the mornings of days 0, 14, and 42, after 12 hours of fasting, all piglets were weighed. Daily feed intake per pen was accurately recorded throughout the trial, and ADG, ADFI, and FCR were calculated.
From day 1 to day 42, the health status and diarrhea of all experimental piglets were checked every morning and evening continuously. Fecal scores of each piglet were observed and recorded daily by the same investigator according to a standardized scoring system. The scoring system was:
0 = normal, firm feces;
1 = soft feces, possible slight diarrhea;
2 = feces that are formless and semifluid, moderate diarrhea;
3 = very watery and frothy feces, severe diarrhea.
The fecal score ≥2 was defined as diarrhea. Diarrhea incidence was calculated as follows: Diarrhea incidence (%) = (total number of pigs per pen with diarrhea)/(number of pigs per pen × test period) × 100.
2.2 Sample collection
Fecal samples were collected from female piglets of 15 pens of each treatment on days 0, 14, and 42, respectively. Feces were collected directly from the anus to avoid contamination. Fecal samples were collected into sterile centrifuge tubes, placed in ice boxes, transported back to the laboratory, and immediately stored at -80°C.
Blood samples were collected from the anterior vena cava of the piglets that were the same individuals used for fecal sampling. After centrifugation for 10 minutes at 3500 rpm, the serum was carefully separated and stored at -20°C for testing of relevant indicators. A total of 45 blood samples were collected.
On day 42 at 08:00, piglets selected for fecal and serum sample collection (n=15) were euthanized by intravenous pentobarbital sodium (200 mg/kg BW) and then slaughtered by exsanguination using the protocol approved by the Sichuan Agricultural University Animal Care Advisory Committee. The abdominal cavity was immediately opened, and the ileal intestinal segments were removed according to the procedure described by a previous study (18). Cut the middle part of the ileum, remove its contents, rinse it with normal saline, and put it in an ice bag. Following scraping of the mucosa with a sterile glass slide, the sample was snap frozen in liquid nitrogen and stored at -80°C. Additionally, an ileal segment (about 3cm) was placed in 4% neutral formalin solution and processed for histology.
2.3 Feces sample analysis
The number of Lactobacilli (MRS agar plates), E. coli (blood agar plates to identify hemolytic colonies and MacConkey for Enterobacteriaceae) and C. perfringens (using the ISO-GRID membrane filtration, with TSC Agar medium supplemented with D-Cycloserine and in anaerobiosis conditions) in the feces of the piglets on days 0, 14, and 42 was determined using the bacterial enumeration method. Secretory immunoglobulin A (sIgA) was measured via enzyme-linked immunosorbent assay (ELISA) (Jiangsu Enzyme Free Industry Co., Ltd, Jiangsu, China), and the concentration of myeloperoxidase (MPO) was determined by enzymatic colorimetric methods (Nanjing Jian cheng Bioengineering Institute, Nanjing, China). Determination of dry matter content in feces was carried out by the constant weight method (19).
2.4 Blood sample analysis
Serum immunoglobulin A (IgA), immunoglobulin M (IgM), immunoglobulin G (IgG), C-reactive protein (CRP), and Haptoglobin (HPT) were measured via enzyme-linked immunosorbent assay (ELISA) (Jiangsu Enzyme Free Industry Co., Ltd, Jiangsu, China). The manufacturer’s instructions were followed for all procedures.
2.5 Intestinal morphology analysis
After fixation with 4% paraformaldehyde, samples of ileal segments were dehydrated, embedded, sectioned, and stained. The target area of tissue was then selected for imaging using an Eclipse Ci-L camera microscope. Analyzing images with Image-Pro Plus 6.0, and in each section, five intestinal villus heights and five crypt depths were measured using a 40×field of view, and the villus height/crypt depth (V/C) was calculated. Selection of target areas using a 100×field of view was then carried out, and measurement of the height of villi at each of five locations and a count of the number of Goblet cells and Peyer’s patches were made.
2.6 Gene expression in intestinal mucosa
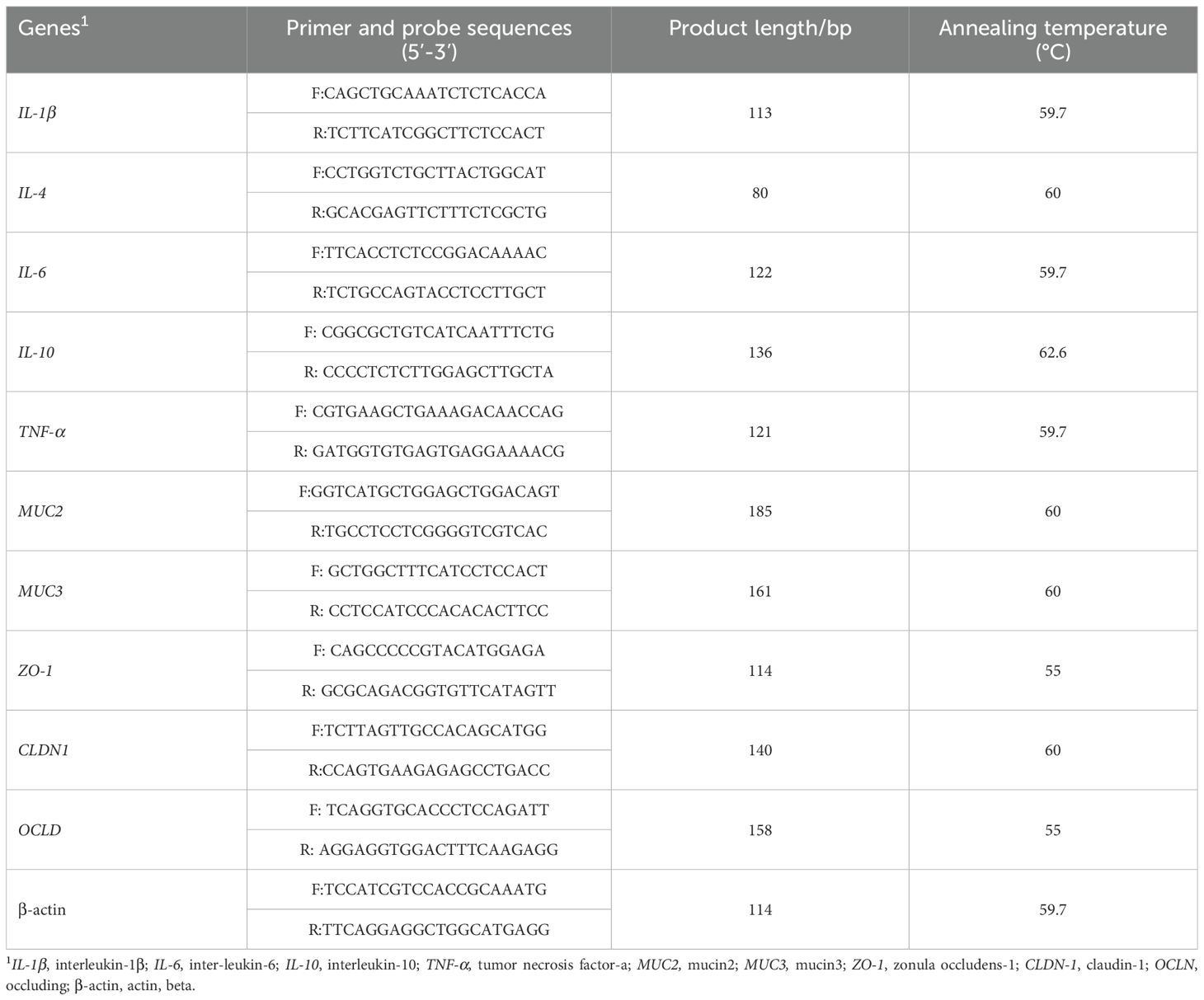
Trizol Reagent (TaKaRa, Dalian, China) was used to extract total RNA from the ileal mucosa, and a spectrophotometer was used to measure concentration and purity of total RNA (NanoDrop, Gene Company Limited, Guangzhou, China) at 260 and 280 nm according to the manufacturer’s directions. Reverse transcription was performed according to the manufacturer’s instructions using the Prime Script RT reagent kit (TaKaRa, Dalian, China). With the help of a CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA) and SYBR Green PCR reagents (TaKaRa, Dalian, China), real-time PCR reactions were performed. IL-1β, IL-4, IL-6, IL-10, TNF-α, MUC2, MUC3, ZO-1, CLDN-1, and OCLN gene expression in the ileal mucosal tissue were measured. Analyses of gene expression data in replicate samples were conducted using the 2 −▲▲CT method (20). Table 2 shows the sequence of primers used.
2.7 Statistical analysis
The pen was designated as the experimental unit for growth performance and diarrhea rate analysis, with 30 replicates per treatment group (n=30). For fecal, blood, and tissue sample collections, one piglet per pen was selected as the sampling unit, maintaining 15 replicates per treatment (n=15). Data were expressed as mean and standard error. Descriptive statistics were performed to evaluate whether data were normally distributed with statistical software SPSS 27.0 (IBM, USA). Then, a one-way ANOVA test was used to compare the differences in normally distributed data among groups, followed by Duncan’s multiple-range test. The diarrhea rate was analyzed by the chi-square test. Correlations between variables were calculated using Spearman rank correlation in GraphPad Prism v7.0. A marginally significant difference was considered at 0.05 ≤ P ≤ 0.10, a statistically significant difference was considered at P < 0.05, a highly significant difference at P < 0.01, and an extremely significant difference at P < 0.001.
3 Results
3.1 Effects of SLV on growth performance and diarrhea rate of weaned piglets
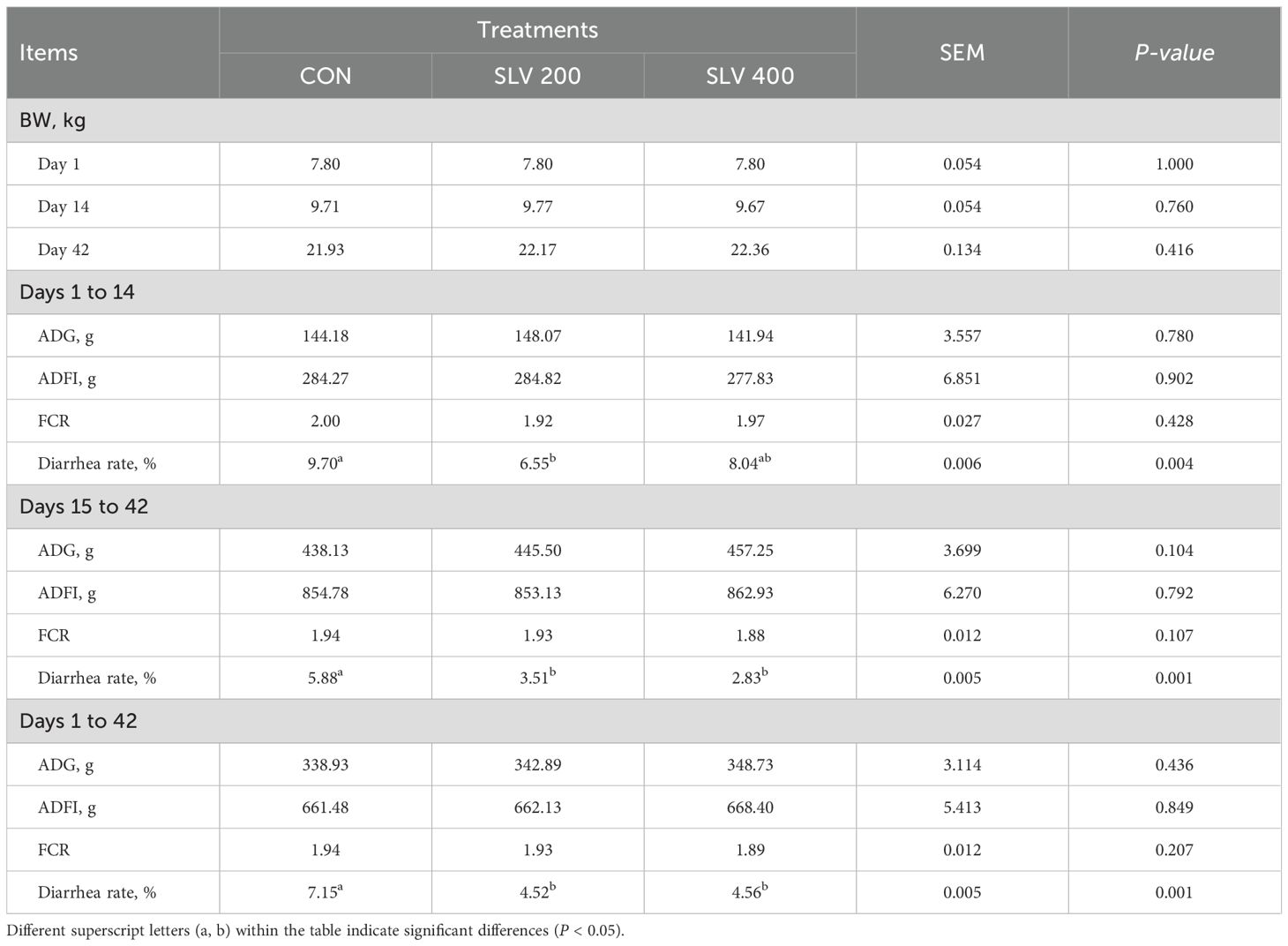
Results of growth performance and diarrhea rate are shown in Table 3. Compared with the control group, the FCR of piglets in the SLV400 group was decreased by 0.05 during the whole trial period (P > 0.05). Furthermore, the SLV 200 and SLV 400 groups both reduced diarrhea in the starter and the entire period (P < 0.05). The SLV 200 group also reduced diarrhea in the pre-starter period (P < 0.05).
3.2 Effects of SLV on fecal indexes of weaned piglets
As shown in Table 4, compared with the control group, SLV had no significant effects on either dry matter or MPO in feces of weaned piglets (P > 0.05).
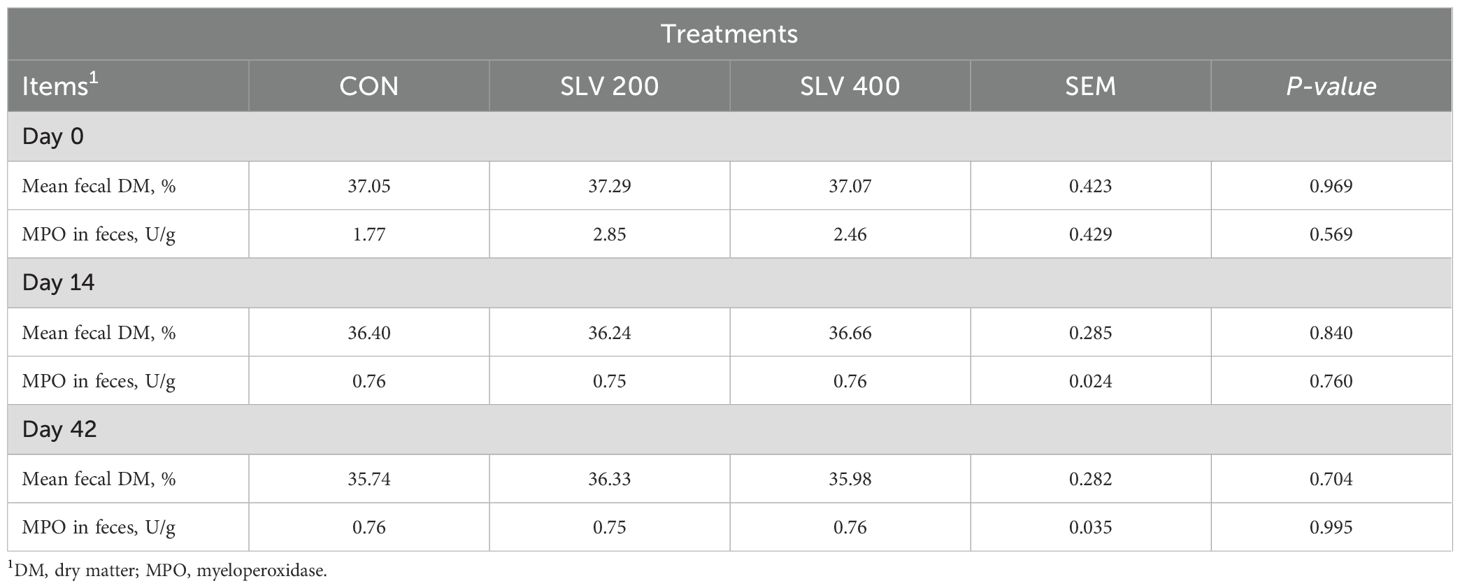
Table 4. Effects of dietary SLV supplementation on the dry matter and myeloperoxidase in feces of weaned piglet.
As shown in Figure 1, compared with the control group, SLV 200 increased the number of Lactobacilli and reduced the number of E. coli in feces on day 14 (P < 0.05). SLV 400 also increased the number of Lactobacilli in feces on day 14 and day 42 (P < 0.05) and reduced the number of E. coli in feces on day 14 (P < 0.05). However, SLV did not affect the number of C. perfringens in piglet feces (P > 0.05).
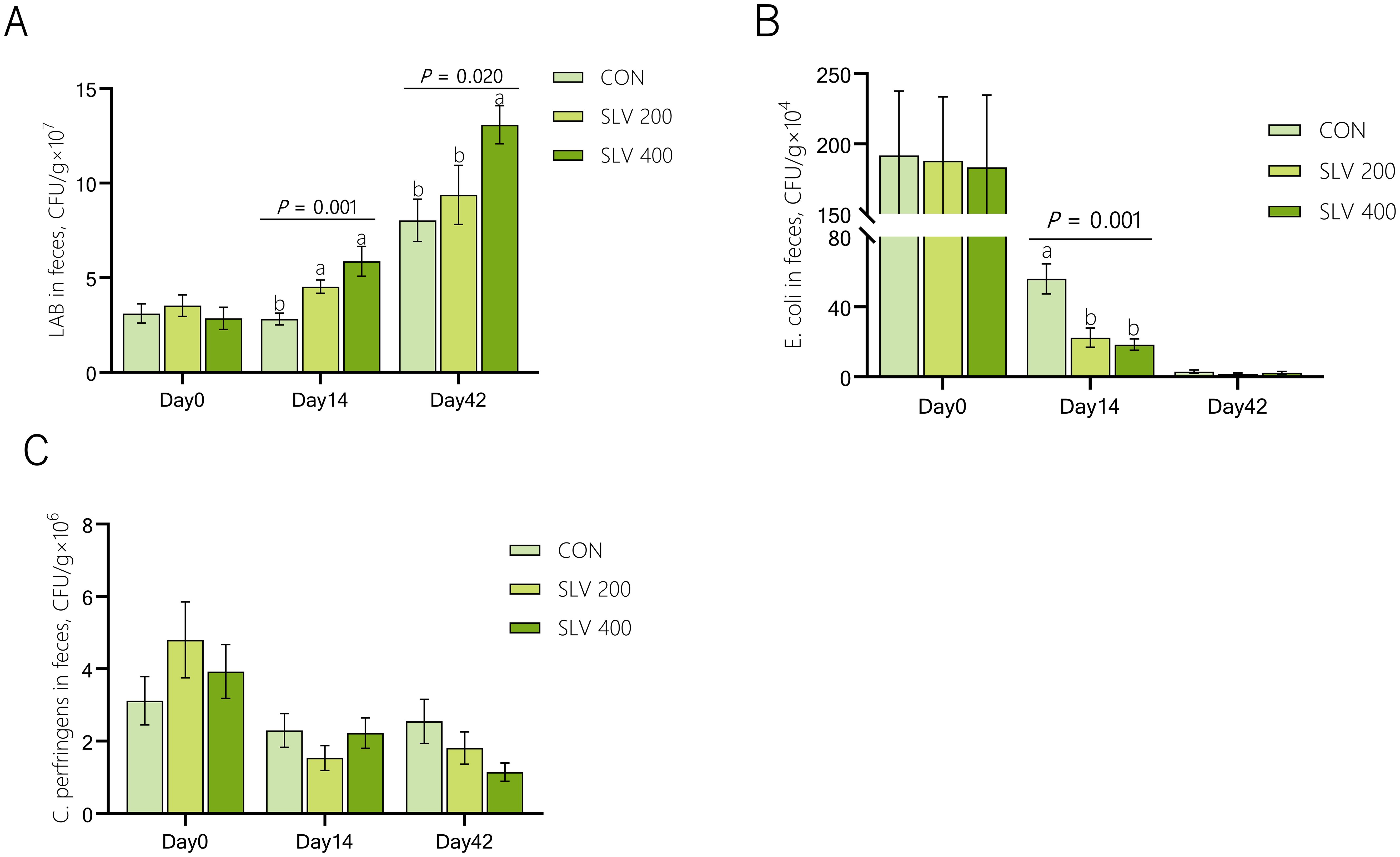
Figure 1. Effects of SLV supplementation on fecal microflora of weaned piglets: (A) Lactobacillus; (B) Escherichia coli; (C) Clostridium perfringens. 1Letters above the bars (a, b) indicate significant differences (P < 0.05) and x and y indicate a trend (0.05 ≤ P < 0.10) between the 3 treatments. (n=15). CON, control group; SLV 200, SOLVENS 200 (6.5×108 CFU per kg feed); SLV 400, SOLVENS 400 (1.3×109 CFU per kg feed). The same figure below. 2LAB, Lactobacillus; E. coli, Escherichia coli; C. perfringens, Clostridium perfringens.
As shown in Figure 2, compared with the control group, SLV200 and SLV400 tended to increase sIgA levels in feces on day 14 (P < 0.10), and SLV200 increased sIgA levels in feces on day 42 (P < 0.05).
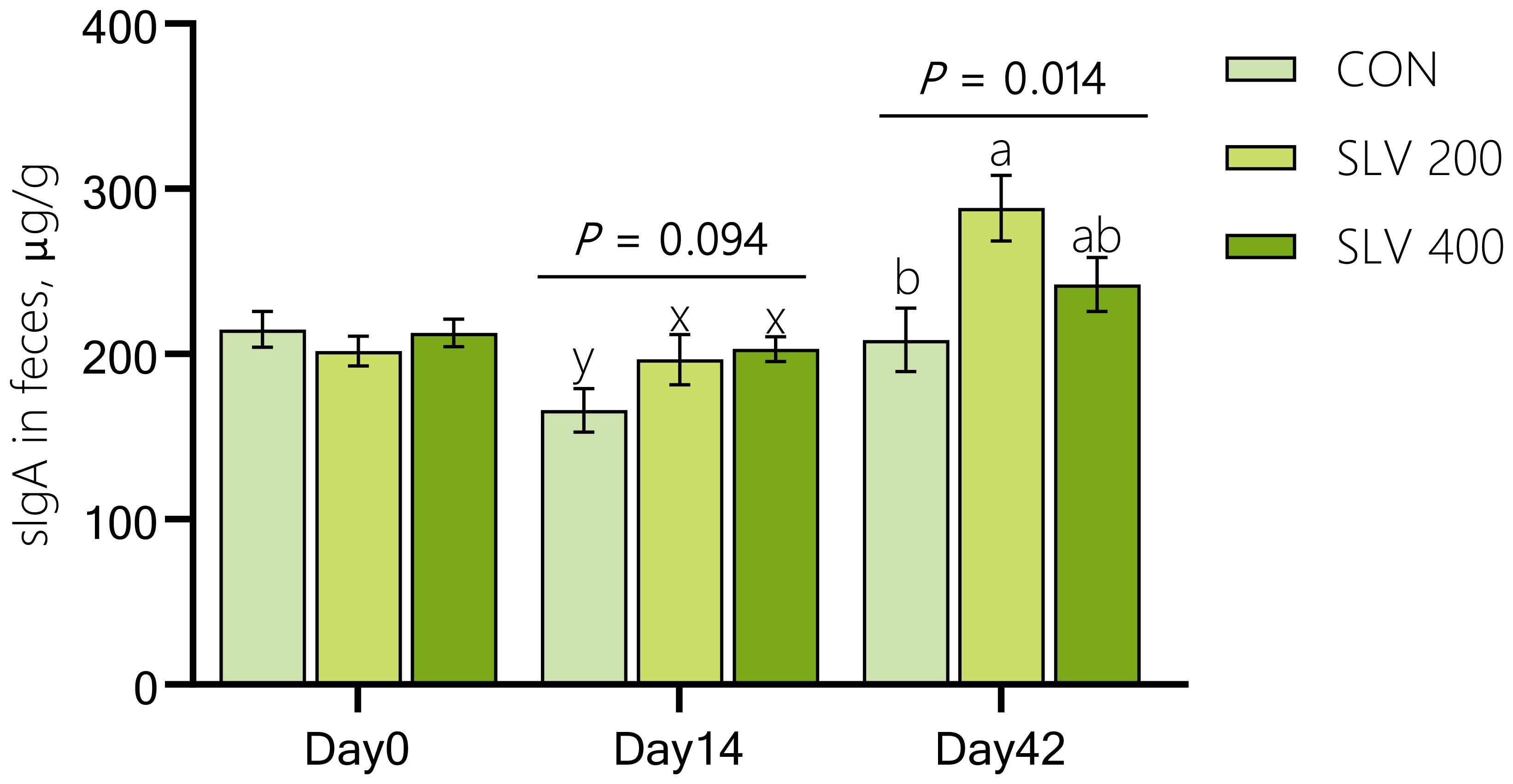
Figure 2. Effects of SLV supplementation on secretory immunoglobulin A in feces of piglets. sIgA, secretory Immunoglobulin A Letters above the bars (a, b) indicate significant differences (P < 0.05) and x and y indicate a trend (0.05 ≤ P < 0.10) between the 3 treatments. (n=15).
3.3 Effects of SLV on blood indexes of weaned piglets
Effects of SLV on serum immunoglobulin of piglets are shown in Figure 3. Compared with the control group, SLV 200 increased serum IgG on Day 42 (P < 0.05), and SLV 400 increased serum IgG and IgM on day 14 and increased the serum IgA on day 42 (P < 0.05).
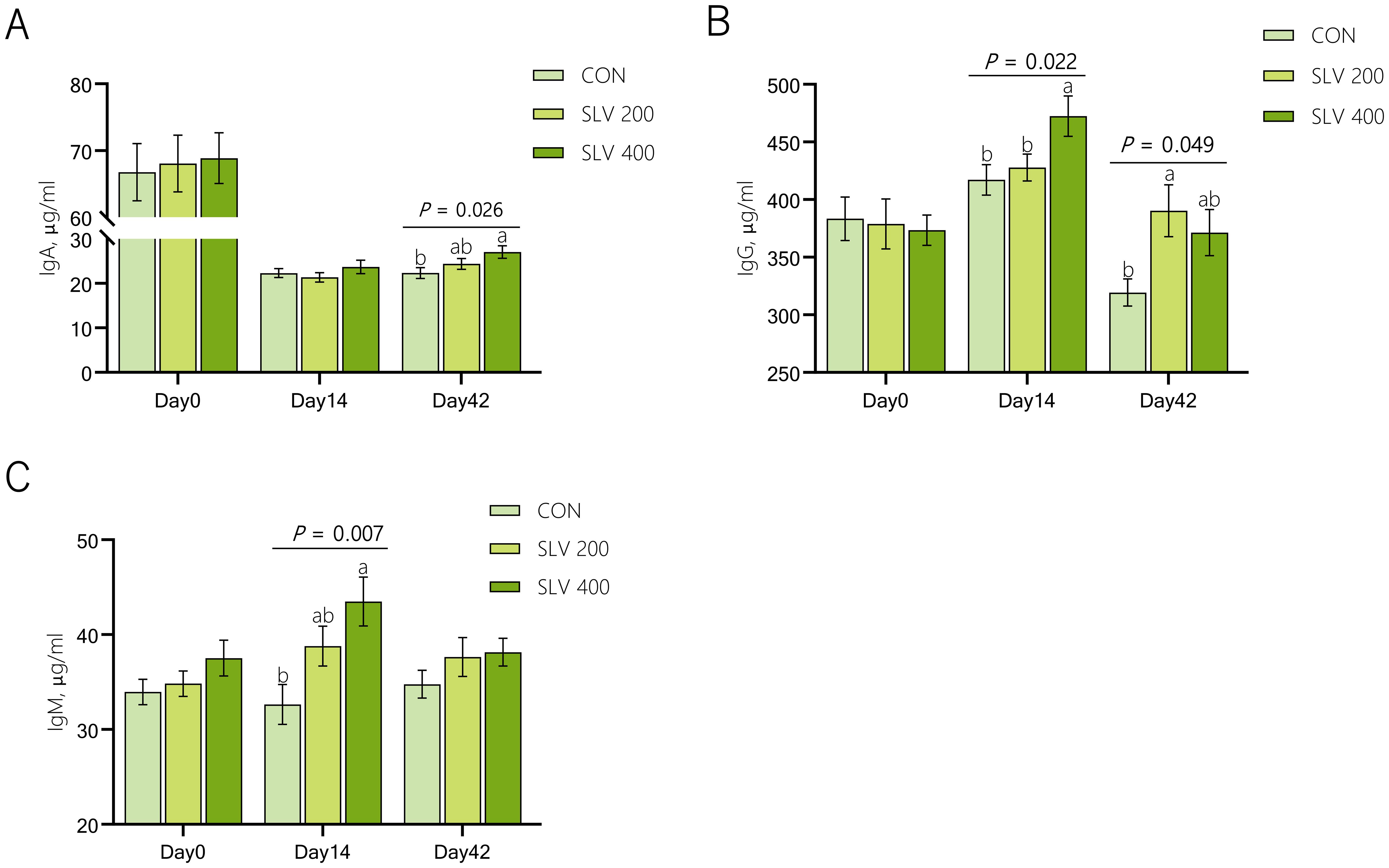
Figure 3. Effects of SLV supplementation on serum immunoglobulin of piglets: (A) immunoglobulin A; (B) immunoglobulin G; (C) immunoglobulin M. 1IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M. Letters above the bars (a, b) indicate significant differences (P < 0.05). (n=15).
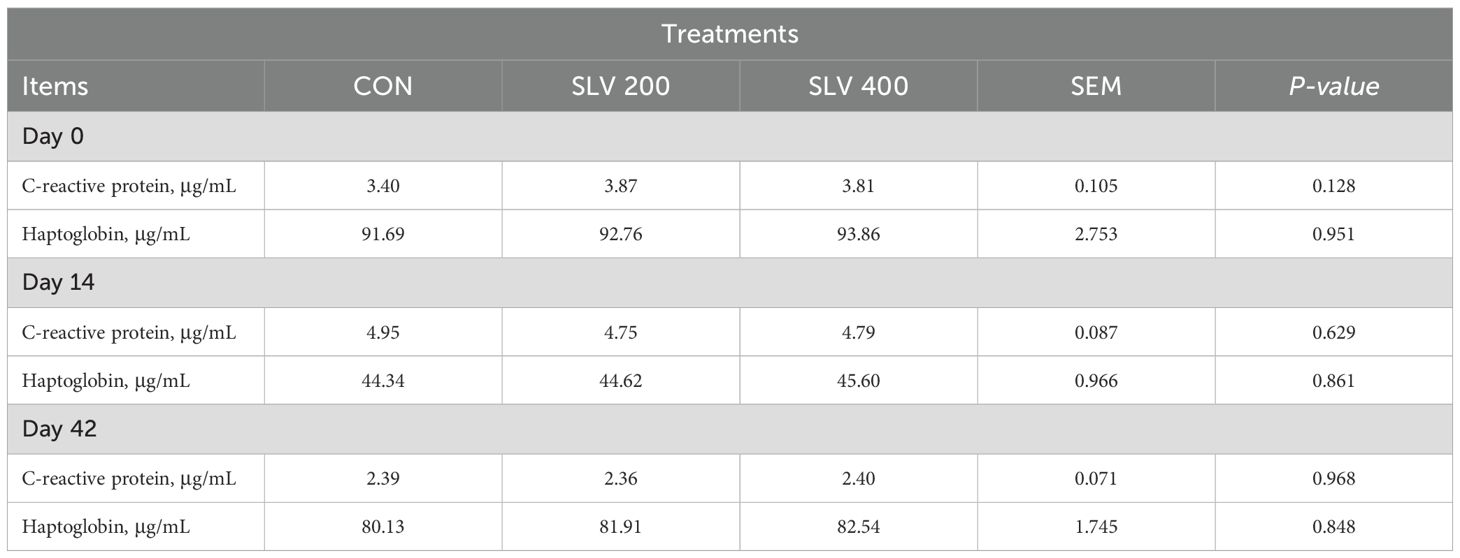
As shown in Table 5, dietary supplementation of SLV did not affect the concentration of C-reactive protein and haptoglobin in serum compared to the control group (P > 0.05).
3.4 Correlation between diarrhea rate, immunoglobulins, and fecal microorganisms
We further evaluated the correlations among diarrhea rate, serum immunoglobulins, fecal secretory IgA (sIgA), and fecal microbiota at different stages. As shown in Figure 4, during days 0–14, diarrhea rate was significantly positively correlated with Escherichia coli abundance (r = 0.53, P < 0.001). Fecal sIgA was positively correlated with serum IgM (r = 0.67, P < 0.001), and negatively correlated with fecal E. coli (r = –0.38, P < 0.05). On day 42, fecal sIgA was positively correlated with serum IgA (r = 0.42, P < 0.01), IgG (r = 0.64, P < 0.001), and IgM (r = 0.47, P < 0.01), and negatively correlated with fecal Clostridium perfringens (r = –0.35, P < 0.05).
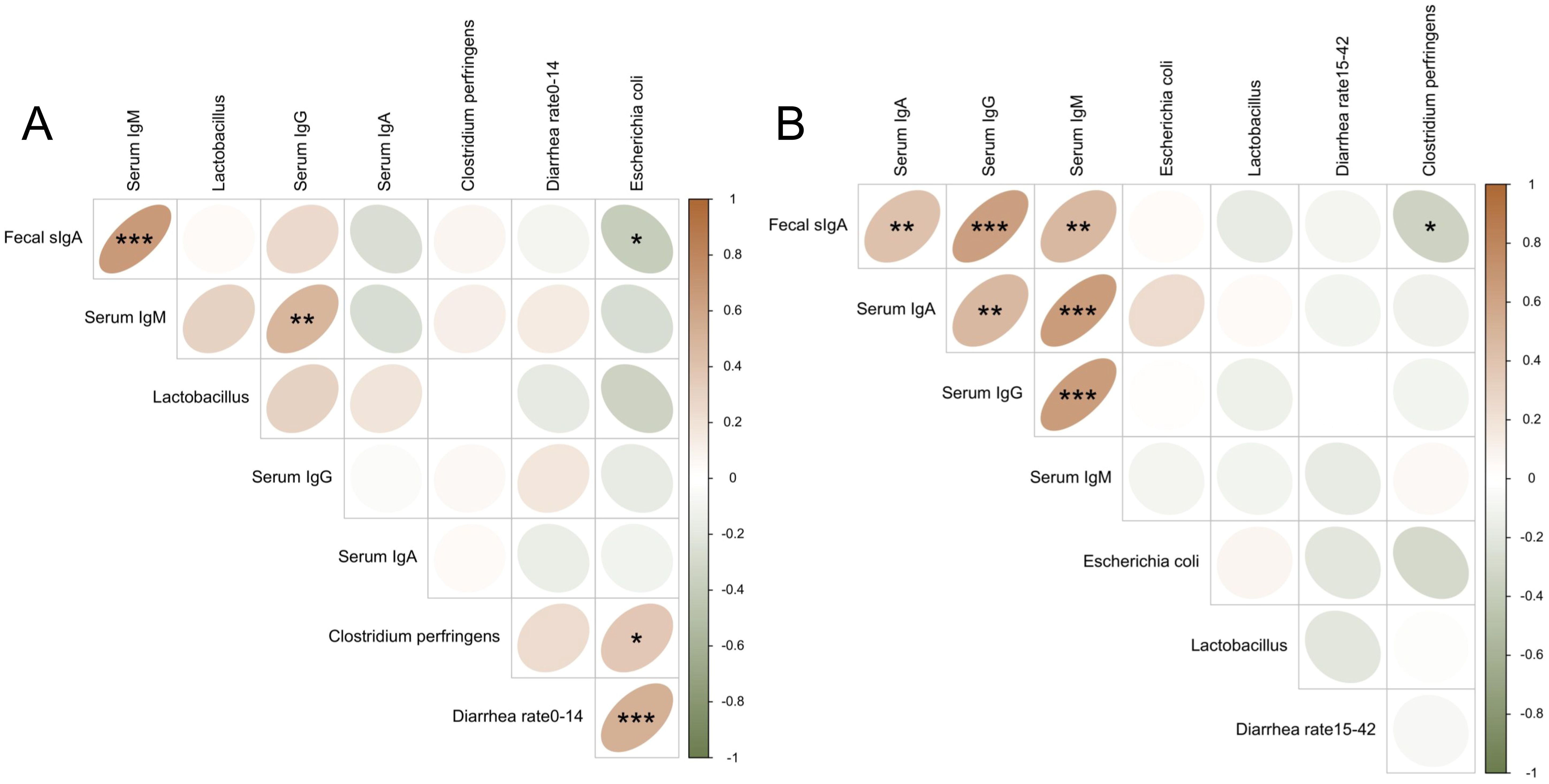
Figure 4. Spearman correlation among immunoglobulins, fecal microbiota, and diarrhea rate. Brown indicates a positive correlation, and green indicates a negative correlation. (A) Spearman correlation analysis for day 14 (or days 0–14); (B) Spearman correlation analysis for day 42 (or days 15–42). *, **, and *** indicate statistically significant correlations at P < 0.05, P < 0.01, and P < 0.001.
3.5 Effects of SLV on intestinal health in weaned piglets
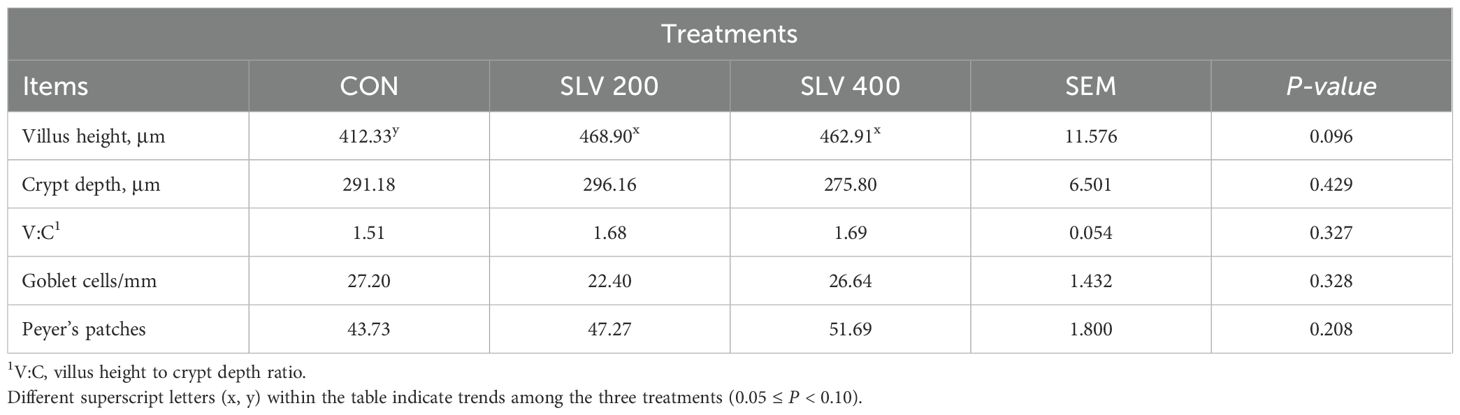
Results of SLV treatment on intestinal morphology of piglets are shown in Table 6 and Figure 5. Compared with the control group, adding SLV trended to increase ileal villus height (P < 0.10). There was no significant effect on crypt depth, villus height to crypt ratio, and goblet cell and Peyer’s patch density (P > 0.05).
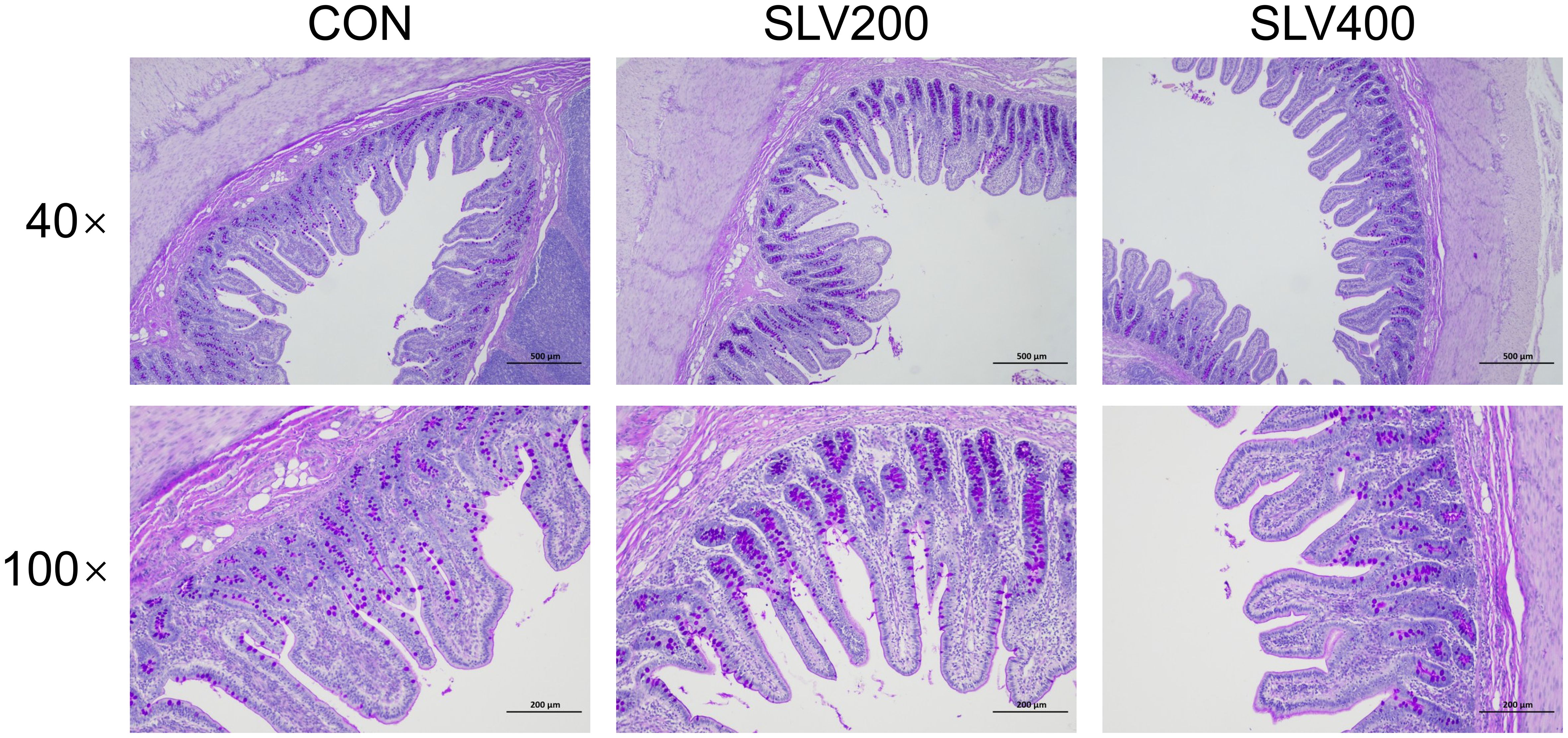
Figure 5. Effects of SLV supplementation on the ileal morphology of weaned piglets. Representative cross-sectional staining of the ileum with periodic acid-Schiff (PAS).
Results of the effect of SLV on gene expression in the ileal mucosa of piglets are shown in Figure 6. Compared with the control group, SLV 200 decreased gene expression of IL-1β and TNF-α in the ileal mucosa (P < 0.05), and SLV 400 reduced gene expression of TNF-α in the ileal mucosa (P < 0.05). There were no significant effects on the expression of MUC2 and MUC3, and tight junction proteins OCLD and CLDN-1 in the ileal mucosa (P > 0.05). The control group tended to increase expression of ZO-1 in the ileal mucosa compared with the SLV groups (P < 0.10).
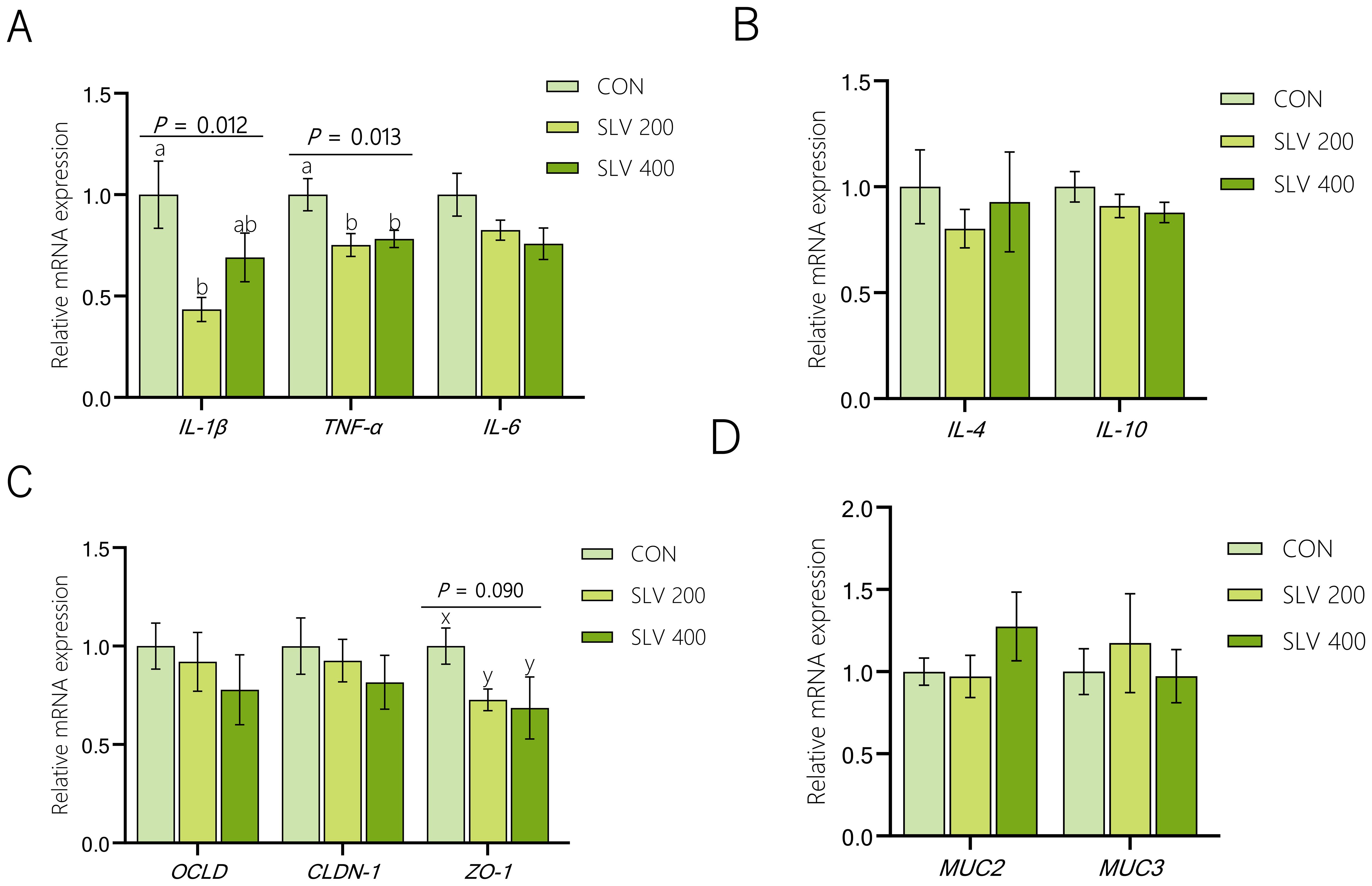
Figure 6. Effect of SLV on gene expression in the ileal mucosa of weaned piglets: (A) Relative expression of pro-inflammatory factors IL-1β, TNF-α and IL-6 in the ileum of weaned piglets; (B) Relative expression of anti-inflammatory factors IL-4, IL-10 in the ileum of weaned piglets; (C) Relative expression of tight junction proteins OCLD, CLDN-1 and ZO-1 in the ileum of weaned piglets; (D) Relative expression of mucins MUC2, MUC3 in the ileum of weaned piglets. 1IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-a; IL-6, inter-leukin-6; IL-4, inter-leukin-4; IL-10, interleukin-10; ZO-1, zonula occludens-1; CLDN-1, claudin-1; OCLD, occluding; MUC2, mucin 2; MUC3, mucin 3. Letters above the bars (a, b) indicate significant differences (P < 0.05) and x and y indicate a trend (0.05 ≤ P < 0.10) between the 3 treatments. (n=15).
4 Discussion
Weaning in piglets causes stress and diarrhea, which results in a decrease in feed intake and average daily gain. Probiotics can reduce piglet diarrhea and improve the growth performance of weaned piglets (11, 12, 21–23). Our results indicated that both SLV 200 and SLV 400 effectively reduced diarrhea rate. Furthermore, SLV 400 demonstrated numerically superior average daily gain (ADG) and feed conversion ratio (FCR) in weaned piglets during the 15 to 42-day period, though these differences did not reach statistical significance. The mechanisms underlying the alleviation of diarrhea and improved growth performance in piglets by dietary SLV supplementation may be attributed to the following aspects.
Firstly, supplementation with SLV in the diets of weaned piglets showed a tendency to improve intestinal morphology. Previous studies have demonstrated that weaning stress leads to damage to the structure of intestinal villi (4, 5). As the primary site for nutrient digestion and absorption, intestinal villi are essential for growth and development (24, 25). Damage to villi has been reported to increase the risk of diarrhea and growth retardation (24, 26–28). Tight junction proteins play a crucial role in maintaining normal tissue morphology of the intestine and contributing to the integrity and permeability of the intestinal barrier (29–31). Previous studies have shown that dietary supplementation with Bacillus subtilis or Bacillus licheniformis can increase intestinal villus height, promote the expression of tight junction proteins, and enhance structural integrity of intestinal morphology in pigs (32–34). The results of this study showed that dietary supplementation with 200 mg/kg and 400 mg/kg SLV increased ileal villus height in piglets by 13.73% and 12.27%, respectively, exhibiting a marginally significant statistical effect. However, it did not affect the gene expression of tight junction protein OCLD and CLDN-1. This discrepancy may be attributed to variations in the physiological and health status of piglets, where the efficacy of SLV could potentially exert more pronounced effects under pathological conditions, such as inflammatory states (e.g., E. coli-infected piglets) or intestinal damage. Future studies should validate this hypothesis in challenged models.
Secondly, SLV supplementation balanced the abundance of intestinal Lactobacillus, Escherichia coli, and Clostridium perfringens of piglets. Gut microbiota plays a crucial role in the intestinal health of weaned piglets. Dysbiosis of gut microbiota usually leads to a series of chain reactions such as diarrhea post-weaning, damage to the intestinal barrier, smaller intestinal villi, shrinkage of intestinal cells, reduced digestive capacity, and slower weight gain (35). In this study, SLV supplementation increased the number of Lactobacilli in the feces of weaned piglets on days 14 and 42, and reduced the number of E. coli in the feces of piglets on day 14. Previous studies also found that supplementation with probiotics could reduce diarrhea rates by regulating the balance of the intestinal community (13–15, 22, 36). Our findings revealed that dietary SLV supplementation reduced the abundance of pathogenic bacteria while increasing beneficial bacteria in the fecal microbiota. This modulatory effect on gut microbiota constitutes one mechanism through which SLV enhances intestinal health and reduces diarrhea incidence in piglets.
Thirdly, SLV supplementation enhanced immunity of piglets. Immunoglobulins are a critical component of immune system and play an essential role in protecting against diarrhea caused by intestinal infections (37–39). Previous studies have found that adding Bacillus subtilis to piglet diets increased the levels of IgA and IgM in serum of weaned piglets (36, 40–43). Results of our study showed that dietary supplementation of SLV to feed increased serum levels of IgA, IgG, and IgM in piglets on days 14 and 42 after weaning. These findings are consistent with previous studies (36, 42), indicating that SLV enhances immune function of weaned piglets and may help prevent pathogen invasion. Pro-inflammatory factors, such as IL-1β, IL-6, and TNF-α, can mediate host’s inflammatory response by rapidly generating an immune response after pathogen infection. In contrast, anti-inflammatory factors, including IL-4 and IL-10, can regulate body’s inflammatory response and enhance immune function. Previous studies have found that supplementation of Bacillus in feed can increase the expression of anti-inflammatory factors IL-4 and IL-10 and reduce the expression of pro-inflammatory factors IL-1β, IL-6, and TNF-α (44–46). Our study showed that SLV supplementation significantly reduced the expression of pro-inflammatory factors in the ileal mucosa of piglets. However, it did not affect gene expression of anti-inflammatory factors IL-4 and IL-10 in the ileal mucosa. These results support the notion that SLV enhances immune function and prevents disruption of intestinal immune homeostasis by pathogens before the onset of inflammation. Increased levels of fecal sIgA further support this interpretation. SIgA is secreted by B lymphocytes in Peyer’s patches. As the main component of intestinal immune barrier, sIgA is the most crucial immunoglobulin for maintaining intestinal mucosal immunity and preventing various infections (47). Studies have shown that sIgA can recognize and bind to Escherichia coli through its fragment crystallizable (Fc) region, secretory component (SC), and N-glycosylation (48), thereby preventing bacterial translocation across the intestinal epithelial barrier and maintaining mucosal integrity (49). Our study showed that dietary supplementation of SLV did not affect the number of ileal Peyer’s patches but could increase the concentration of sIgA in feces of weaned piglets. Spearman correlation analysis revealed a significant negative correlation between fecal sIgA levels and Escherichia coli abundance, while the abundance of E. coli was positively correlated with diarrhea rate. These findings are consistent with previous studies and further suggest that SLV enhances immune capacity of weaned piglets by increasing serum immunoglobulin levels and fecal secretory IgA (sIgA) concentrations, thereby preventing the onset of inflammation. This immune enhancement likely contributes to improved disease resistance and reduced diarrhea incidence observed in weaned piglets.
5 Conclusions
In conclusion, this study demonstrates that dietary supplementation with SLV significantly reduces diarrhea in weaned piglets. This effect is primarily attributed to the enhancement of intestinal barrier function, improvement of immune responses, reduction in the abundance of pathogenic bacteria, and enrichment of beneficial microbes in feces. This study elucidated the benefits of probiotics composed of Bacillus subtilis DSM5750, Bacillus subtilis DSM27273, and Bacillus licheniformis DSM5749 on intestinal health in weaned piglets and suggested an antibiotic-free effective strategy to alleviate diarrhea in weaned piglets.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The animal study was approved by Animal Care and Use Committee, Sichuan Agricultural University (Ethical Approval Code: SICAUAC20220506; Ya’an, China). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
MW: Formal analysis, Writing – review & editing. XZ: Formal analysis, Investigation, Writing – original draft. LH: Writing – original draft. YS: Writing – original draft. BY: Data curation, Writing – review & editing. JH: Data curation, Writing – review & editing. JY: Data curation, Writing – review & editing. PZ: Conceptualization, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Science and Technology Support Program of Sichuan Province (2021ZDZX0009), and Chr. Hansen A/S, Denmark project (80857).
Acknowledgments
We thank Huifen Wang and Quyuan Wang for their help during the experiments.
Conflict of interest
Authors LH and YS were employed by the company Chr. Hansen A/S.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cao S, Hou L, Sun L, Gao J, Gao K, Yang X, et al. Intestinal morphology and immune profiles are altered in piglets by early-weaning. Int Immunopharmacol. (2022) 105:108520. doi: 10.1016/j.intimp.2022.108520
2. Li Y, Guo Y, Wen Z, Jiang X, Ma X, and Han X. Weaning stress perturbs gut microbiome and its metabolic profile in piglets. Sci Rep. (2018) 8:18068. doi: 10.1038/s41598-018-33649-8
3. Ming D, Wang W, Huang C, Wang Z, Shi C, Ding J, et al. Effects of weaning age at 21 and 28 days on growth performance, intestinal morphology and redox status in piglets. Animals: an Open Access J MDPI. (2021) 11(8):2169. doi: 10.3390/ani11082169
4. Tang X, Xiong K, Fang R, and Li M. Weaning stress and intestinal health of piglets: A review. Front Immunol. (2022) 13:1042778. doi: 10.3389/fimmu.2022.1042778
5. Zheng L, Duarte ME, Sevarolli Loftus A, and Kim SW. Intestinal health of pigs upon weaning: challenges and nutritional intervention. Front Vet Sci. (2021) 8:628258. doi: 10.3389/fvets.2021.628258
6. Betancur C, Martínez Y, Merino-Guzman R, Hernandez-Velasco X, Castillo R, Rodríguez R, et al. Evaluation of oral administration of lactobacillus plantarum CAM6 strain as an alternative to antibiotics in weaned pigs. Animals: an Open Access J MDPI. (2020) 10(7):1218. doi: 10.3390/ani10071218
7. Fu J, Wang T, Xiao X, Cheng Y, Wang F, Jin M, et al. Clostridium butyricum ZJU-F1 benefits the intestinal barrier function and immune response associated with its modulation of gut microbiota in weaned piglets. Cells. (2021) 10(3):527. doi: 10.3390/cells10030527
8. Han Y, Tang C, Li Y, Yu Y, Zhan T, Zhao Q, et al. Effects of dietary supplementation with clostridium butyricum on growth performance, serum immunity, intestinal morphology, and microbiota as an antibiotic alternative in weaned piglets. Animals: an Open Access J MDPI. (2020) 10(12):2287. doi: 10.3390/ani10122287
9. Liang J, Kou S, Chen C, Raza SHA, Wang S, Ma X, et al. Effects of Clostridium butyricum on growth performance, metabonomics and intestinal microbial differences of weaned piglets. BMC Microbiol. (2021) 21:85. doi: 10.1186/s12866-021-02143-z
10. Liu P, Zuo J, Lu H, Zhang B, and Wu C. Bacillus subtilis fed to sows promotes intestinal development and regulates mucosal immunity in offspring. Vet Sci. (2025) 12(5):489. doi: 10.3389/fcimb.2017.00526
11. Liu Y, Cao L, Yu C, Zhou Q, Li H, Zhang R, et al. Dietary supplementation with Bacillus subtilis PB6 alleviates diarrhea and improves growth performance and immune function in weaned piglets fed a high-protein diet. Front Vet Sci. (2025) 12:1525354. doi: 10.3389/fvets.2025.1525354
12. Qin J, Liu Y, Cao M, Zhang Y, Bai G, and Shi B. Bacillus subtilis MZ-01 alleviates diarrhea caused by ETEC K88 by reducing inflammation and promoting intestinal health. J Appl Microbiol. (2025) 136(2):lxaf018. doi: 10.1093/jambio/lxaf018
13. Wongsamart R, Somboonna N, Cheibchalard T, Klankeo P, Ruampatana J, and Nuntapaitoon M. Probiotic Bacillus licheniformis DSMZ 28710 improves sow milk microbiota and enhances piglet health outcomes. Sci Rep. (2025) 15:17. doi: 10.1038/s41598-024-84573-z
14. Xu Y, Yu Y, Shen Y, Li Q, Lan J, Wu Y, et al. Effects of Bacillus subtilis and Bacillus licheniformis on growth performance, immunity, short chain fatty acid production, antioxidant capacity, and cecal microflora in broilers. Poult Sci. (2021) 100:101358. doi: 10.1016/j.psj.2021.101358
15. Yu X, Cui Z, Qin S, Zhang R, Wu Y, Liu J, et al. Effects of bacillus licheniformis on growth performance, diarrhea incidence, antioxidant capacity, immune function, and fecal microflora in weaned piglets. Anim (Basel). (2022) 12(13):1609. doi: 10.3390/ani12131609
16. Deng B, Wu J, Li X, Zhang C, Men X, and Xu Z. Effects of Bacillus subtilis on growth performance, serum parameters, digestive enzyme, intestinal morphology, and colonic microbiota in piglets. AMB Express. (2020) 10:212. doi: 10.1186/s13568-020-01150-z
18. Zheng P, Yu B, He J, Yu J, Mao X, Luo Y, et al. Arginine metabolism and its protective effects on intestinal health and functions in weaned piglets under oxidative stress induced by diquat. Br J Nutr. (2017) 117:1495–502. doi: 10.1017/S0007114517001519
19. AOAC. Official method of analysis of AOAC international. 18th ed. Gaithersburg, MD: AOAC Int (2007) 2007.
20. Fleige S, Walf V, Huch S, Prgomet C, Sehm J, and Pfaffl MW. Comparison of relative mRNA quantification models and the impact of RNA integrity in quantitative real-time RT-PCR. Biotechnol Lett. (2006) 28:1601–13. doi: 10.1007/s10529-006-9127-2
21. Cao S, Muniyappan M, Shi H, and Kim IH. Dietary inclusion of -based probiotic complex improved average daily weight gain and gain-to-feed ratio and reduced faecal noxious gas emission in weaned pigs. J Anim Feed Sci. (2025) 34:200–8. doi: 10.22358/jafs/193471/2025
22. Huaman SOB, de Souza FA, Bonato MA, Dias CP, Callegari MA, Oba A, et al. Effects of prebiotic and multispecies probiotic supplementation on the gut microbiota, immune function, and growth performance of weaned piglets. PloS One. (2024) 19:e0313475. doi: 10.1371/journal.pone.0313475
23. Qin S, Yuan X, Cheng Z, Zhang B, Ge Y, Yang L, et al. Effects of glucose oxidase and probiotics on growth performance, intestinal microvillus morphology and microfold cells in nursery pigs. J Anim Feed Sci. (2025) 34:189–99. doi: 10.22358/jafs/193241/2025
24. Bandyopadhyay T, Deswal S, Maria A, Phulware RH, Das P, and Ahuja A. Microvillous inclusion disease as a cause of severe congenital diarrhea in a newborn. J Matern Fetal Neonatal Med. (2022) 35:6226–8. doi: 10.1080/14767058.2021.1910656
25. Yu J, Song Y, Yu B, He J, Zheng P, Mao X, et al. Tannic acid prevents post-weaning diarrhea by improving intestinal barrier integrity and function in weaned piglets. J Anim Sci Biotechnol. (2020) 11:87. doi: 10.1186/s40104-020-00496-5
26. Ahsan MK, dos Reis DC, Barbieri A, Sumigray K, Nottoli T, Salas PJ, et al. Serum and glucocorticoid-inducible kinase 1 (SGK1): an important contributor to diarrhea and malabsorption in microvillus inclusion disease (MVID). FASEB J. (2022) 11(14):4179. doi: 10.1096/fasebj.2022.36.S1.R5730
27. Fang T, Tian G, Chen D, He J, Zheng P, Mao X, et al. Endoplasmic reticulum stress contributes to intestinal injury in intrauterine growth restriction newborn piglets. Anim (Basel). (2024) 14(18):2677. doi: 10.3390/ani14182677
28. Zhang Y, Tian X, Dong Y, Li R, Shen M, Yi D, et al. Bacillus coagulans prevents the decline in average daily feed intake in young piglets infected with enterotoxigenic Escherichia coli K88 by reducing intestinal injury and regulating the gut microbiota. Front Cell Infect Microbiol. (2023) 13:1284166. doi: 10.3389/fcimb.2023.1284166
29. Chen XL, Chen LL, Jia G, Zhao H, Liu GM, and Huang ZQ. L-theanine improves intestinal barrier functions by increasing tight junction protein expression and attenuating inflammatory reaction in weaned piglets. J Funct Foods. (2023) 100:10. doi: 10.1016/j.jff.2022.105400
30. Horowitz A, Chanez-Paredes SD, Haest X, and Turner JR. Paracellular permeability and tight junction regulation in gut health and disease. Nat Rev Gastroenterol Hepatol. (2023) 20:417–32. doi: 10.1038/s41575-023-00766-3
31. Paradis T, Begue H, Basmaciyan L, Dalle F, and Bon F. Tight junctions as a key for pathogens invasion in intestinal epithelial cells. Int J Mol Sci. (2021) 22(5):2506. doi: 10.3390/ijms22052506
32. Gonzalez F, Cervantes M, Morales A, Valle JA, Camacho RL, Ngelica Morales-Becerra A, et al. Effect of supplementing a Bacillus subtilis-based probiotic on performance, intestinal integrity, and serum antioxidant capacity and metabolites concentrations of heat-stressed growing pigs. J Anim Sci. (2024) 102:skae012. doi: 10.1093/jas/skae012
33. Li RL, Liu JW, Liu YH, Cao LT, Qiu WF, and Qin M. Probiotic effects of on growth performance and intestinal microecological balance of growing-to-finishing pigs. J Food Biochem. (2023) 2023:16. doi: 10.1155/2023/7150917
34. Sun W, Chen W, Meng K, Cai L, Li G, Li X, et al. Dietary Supplementation with Probiotic Bacillus licheniformis S6 Improves Intestinal Integrity via Modulating Intestinal Barrier Function and Microbial Diversity in Weaned Piglets. Biol (Basel). (2023) 12(2):238. doi: 10.3390/biology12020238
35. Wang M, Huang H, Hu Y, Huang J, Yang H, Wang L, et al. Effects of dietary microencapsulated tannic acid supplementation on the growth performance, intestinal morphology, and intestinal microbiota in weaning piglets. Journal of Animal Science (2020) 99:5. doi: 10.1093/jas/skaa112
36. Xue L, Long S, Cheng B, Song Q, Zhang C, Hansen LHB, et al. Dietary triple-strain bacillus-based probiotic supplementation improves performance, immune function, intestinal morphology, and microbial community in weaned pigs. Microorganisms. (2024) 12(8):1536. doi: 10.3390/microorganisms12081536
37. Alexander E, Hommeida S, Stephens MC, Manini ML, and Absah I. The role of oral administration of immunoglobulin in managing diarrheal illness in immunocompromised children. Paediatr Drugs. (2020) 22:331–4. doi: 10.1007/s40272-020-00389-0
38. Karamzadeh-Dehaghani A, Towhidi A, Zhandi M, Mojgani N, and Fouladi-Nashta A. Combined effect of probiotics and specific immunoglobulin Y directed against Escherichia coli on growth performance, diarrhea incidence, and immune system in calves. Animal. (2021) 15:100124. doi: 10.1016/j.animal.2020.100124
39. Nussbaum O, Gross JJ, Bruckmaier RM, and Eicher R. Efficacy of oral administration of specific immunoglobulins in preventing neonatal calf diarrhoea in dairy herds. Vet Rec. (2023) 193:e3559. doi: 10.1002/vetr.3559
40. Kongpanna P, Doerr JA, Jamikorn U, and Nilubol D. Impact of maternal parity and direct-fed microbial supplementation on reproductive performance, digestibility, and milk quality from early gestation to lactation in sows. Anim (Basel). (2025) 15(9):1191. doi: 10.3390/ani15091191
41. Li F, Wu D, Ma K, Wei T, Wu J, Zhou S, et al. Effect of dietary supplementation of Bacillus subtilis QST 713 on constipation, reproductive performance and offspring growth performance of sows. Anim Reprod Sci. (2025) 274:107785. doi: 10.1016/j.anireprosci.2025.107785
42. Liu J, Yang DA, Qu H, Liu D, and Huang K. Bacillus subtilis Feed Supplementation Combined with Oral E. coli Immunization in Sows as a Tool to Reduce Neonatal Diarrhea in Piglets. Anim (Basel). (2024) 14(13):1978. doi: 10.3390/ani14131978
43. Min H, Cho HS, Lee HS, Park YT, Lee HJ, and Park HS. Oral Bacillus subtilis spores-based vaccine for mass vaccination against porcine reproductive and respiratory syndrome. Sci Rep. (2024) 14:27742. doi: 10.1038/s41598-024-79387-y
44. Cao G, Tao F, Hu Y, Li Z, Zhang Y, Deng B, et al. Positive effects of a Clostridium butyricum-based compound probiotic on growth performance, immune responses, intestinal morphology, hypothalamic neurotransmitters, and colonic microbiota in weaned piglets. Food Funct. (2019) 10:2926–34. doi: 10.1039/c8fo02370k
45. Fu R, Liang C, Chen D, Yan H, Tian G, Zheng P, et al. Effects of dietary Bacillus coagulans and yeast hydrolysate supplementation on growth performance, immune response and intestinal barrier function in weaned piglets. J Anim Physiol Anim Nutr. (2021) 105:898–907. doi: 10.1111/jpn.13529
46. Li Q, Li L, Chen Y, Yu C, Azevedo P, Gong J, et al. Bacillus licheniformis PF9 improves barrier function and alleviates inflammatory responses against enterotoxigenic Escherichia coli F4 infection in the porcine intestinal epithelial cells. J Anim Sci Biotechnol. (2022) 13:86. doi: 10.1186/s40104-022-00746-8
47. Zhao Y, Wang J, Wang H, Huang Y, Qi M, Liao S, et al. Effects of GABA supplementation on intestinal SIgA secretion and gut microbiota in the healthy and ETEC-infected weanling piglets. Mediators Inflammation. (2020) 2020:7368483. doi: 10.1155/2020/7368483
48. Raskova Kafkova L, Brokesova D, Krupka M, Stehlikova Z, Dvorak J, Coufal S, et al. Secretory IgA N-glycans contribute to the protection against E. coli O55 infection of germ-free piglets. Mucosal Immunol. (2021) 14:511–22. doi: 10.1038/s41385-020-00345-8
Keywords: compound probiotics, weaned piglets, growth performance, intestinal health, immune modulation
Citation: Wang M, Zhou X, Hübertz Birch Hansen L, Sheng Y, Yu B, He J, Yu J and Zheng P (2025) Complex probiotics can reduce diarrhea by boosting immunity and balancing gut microbiota in weaned piglets. Front. Immunol. 16:1629044. doi: 10.3389/fimmu.2025.1629044
Received: 15 May 2025; Accepted: 16 July 2025;
Published: 01 August 2025.
Edited by:
Nallely Bueno-Hernández, General Hospital of Mexico, MexicoReviewed by:
Ming Qin, Shandong Agricultural University, ChinaEric Auclair, Phileo Lesaffre Animal Care, France
Copyright © 2025 Wang, Zhou, Hübertz Birch Hansen, Sheng, Yu, He, Yu and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Zheng, enBpbmQwNUAxNjMuY29t
†These authors have contributed equally to this work
 Mingyu Wang1†
Mingyu Wang1† Bing Yu
Bing Yu Jun He
Jun He Ping Zheng
Ping Zheng