- 1Department of Gastric Surgery, Liaoning Cancer Hospital and Institute, Cancer Hospital of China Medical University, Shenyang, China
- 2Department of Bone and Soft Tissue Tumor Surgery, Cancer Hospital of China Medical University, Liaoning Cancer Hospital & Institute, Shenyang, China
Melanoma, an aggressive malignancy originating from melanocytes, is characterized by rapid metastasis and dismal prognosis in advanced stages, with a 5-year survival rate of only 16% for stage IV disease. Despite breakthroughs in immune checkpoint inhibitors (ICIs) targeting CTLA-4 and PD-1/PD-L1, therapeutic challenges persist, including heterogeneous response rates, acquired resistance, and immune-related toxicities, underscoring the need for strategies to augment immunogenicity and overcome immune evasion. Programmed cell death (PCD) pathways—ferroptosis, pyroptosis, and necroptosis—have emerged as critical regulators of antitumor immunity. Ferroptosis, driven by iron-dependent lipid peroxidation (LPO), enhances immunogenicity through damage-associated molecular pattern (DAMP) release and depletion of immunosuppressive cells. Pyroptosis, mediated by gasdermin (GSDM) pore formation, promotes CD8+ T cell infiltration via pro-inflammatory cytokine secretion, while necroptosis, governed by Receptor-Interacting Protein Kinase 1 (RIPK1)/RIPK3-MLKL signaling, facilitates antigen cross-presentation and adaptive immune memory. In melanoma, dysregulation of these pathways contributes to tumor progression and immunosuppression, yet their targeted activation reshapes the tumor microenvironment (TME) to synergize with ICIs. Current challenges, including metabolic plasticity and off-target effects, highlight the necessity for precision approaches. This review delineates the mechanistic interplay of ferroptosis, pyroptosis, and necroptosis in melanoma immunotherapy, emphasizing advances in pharmacological induction, nanotechnology-driven delivery systems, and rational combination with ICIs. By integrating preclinical insights and clinical perspectives, we propose that co-targeting these immunogenic cell death (ICD) pathways offers a transformative strategy to enhance therapeutic efficacy, circumvent resistance, and achieve durable remission in melanoma.
1 Introduction
Melanoma, a malignancy originating from the transformation of pigment-producing melanocytes, stands as the most aggressive and lethal form of skin cancer (1, 2). It is characterized by a high propensity for rapid metastatic spread to distant organs and, consequently, a historically high mortality rate. While early-stage, localized melanoma is often curable through surgical resection, the prognosis for patients with advanced or metastatic disease remains a formidable therapeutic challenge (3, 4). For individuals with stage IV disease, the 5-year survival rate is starkly low, estimated at approximately 16%. For decades, the therapeutic armamentarium for advanced melanoma was profoundly limited. Systemic treatments were largely confined to conventional cytotoxic chemotherapy regimens and high-dose interleukin-2 (IL-2) therapy (5, 6). These approaches, however, were plagued by significant toxicities while offering only marginal survival benefits to a very small subset of patients, leaving a critical unmet need for effective and durable treatment strategies.
The last fifteen years have witnessed a revolutionary transformation in the management of advanced melanoma, shifting the treatment paradigm away from non-specific cytotoxic agents toward molecularly targeted and immune-based strategies. This revolution began with the advent of targeted therapy, which leverages small molecule inhibitors to block the specific driver mutations fueling cancer growth. Concurrent with these advances, a deeper understanding of tumor immunology heralded the era of immune checkpoint inhibitors (ICIs), a development that has fundamentally reshaped the prognosis for many patients. ICIs, such as anti-Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) agents (e.g., ipilimumab) and, more prominently, anti-Programmed Cell Death Protein 1 (PD-1) agents (e.g., nivolumab, pembrolizumab) and their ligands (anti-PD-L1), function by releasing the brakes on the host’s immune system, thereby reactivating cytotoxic T cells to recognize and eliminate tumor cells (7, 8) (Figure 1). Melanoma’s inherent immunogenicity, characterized by a high tumor mutational burden (TMB) often driven by ultraviolet (UV) radiation-induced DNA damage, makes it particularly susceptible to this class of therapy. The clinical success of ICIs has been unprecedented, with combination therapies such as ipilimumab plus nivolumab achieving durable, long-term survival in a significant portion of patients with advanced disease (9, 10). Adoptive cell therapy, which involves the infusion of genetically modified or expanded tumor-specific T cells, has shown promise, particularly in cases of refractory disease (11, 12). Oncolytic virus therapy uses genetically modified viruses to selectively infect and kill tumor cells while stimulating an immune response against the tumor (13–15). Cancer vaccines, designed to stimulate the immune system against melanoma-specific antigens, are another area of active investigation (16, 17).
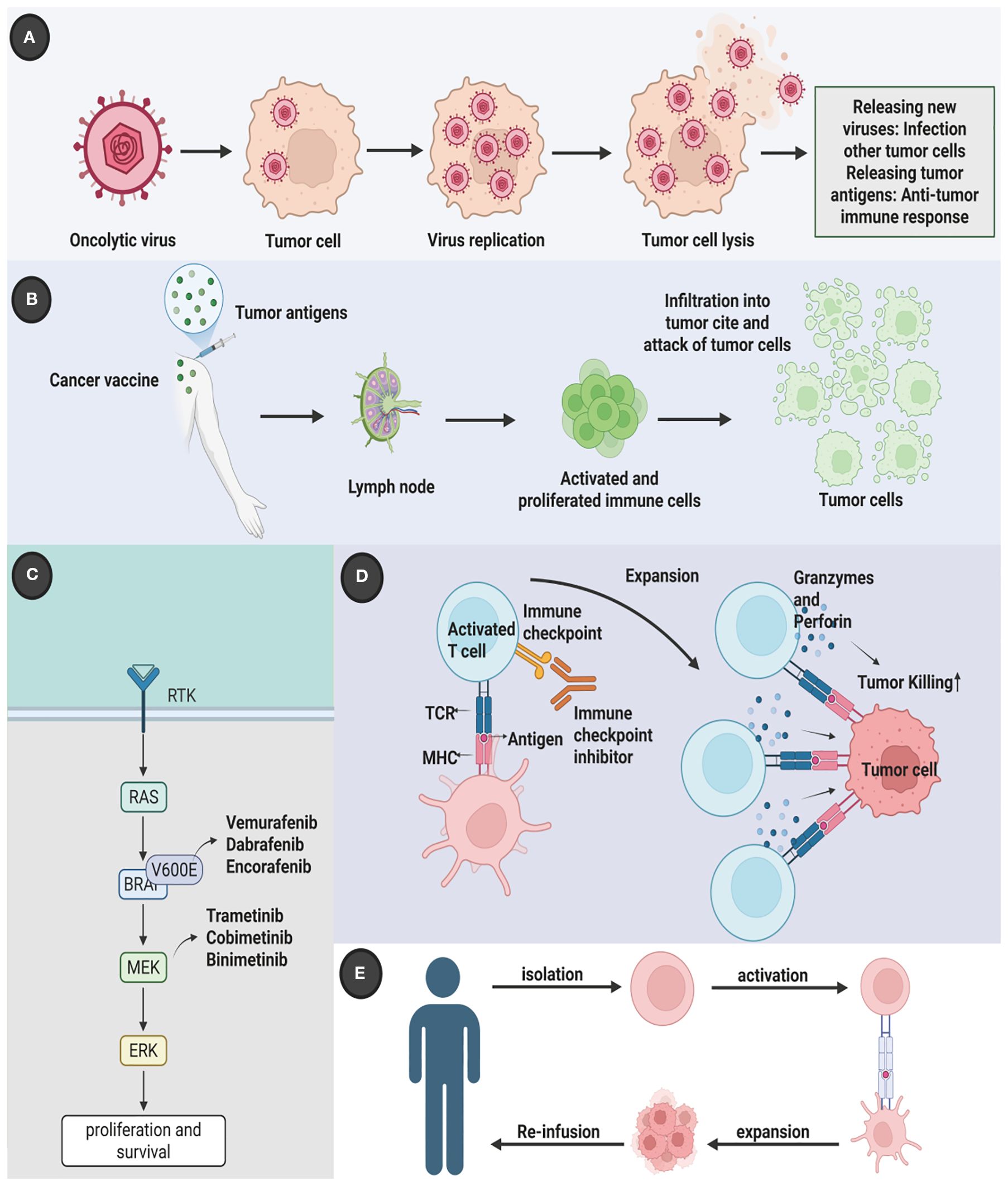
Figure 1. Multidimensional therapeutic innovation in melanoma: integrating molecular targeting, immune modulation, and biological precision. Contemporary melanoma management has evolved beyond conventional therapies to incorporate five strategic dimensions. (A) Molecular-targeted interventions counter oncogenic MAPK pathway activation through BRAF/NRAS mutation suppression; (B) Dual immune checkpoint blockade disrupts CTLA-4/B7-mediated T-cell anergy and PD-1/PD-L1-driven immune evasion, synergistically restoring antitumor surveillance; (C) Adoptive TIL therapy leverages ex vivo expanded tumor-reactive lymphocytes for precision tumor eradication; (D) Oncolytic virotherapy employs ICP34.5-deleted HSV-1 (T-VEC) for selective intralesional oncolysis; (E) Next-generation vaccine platforms coordinate cellular and nucleic acid vectors to establish durable tumor-specific immunity through effector T-cell activation and memory formation. This therapeutic matrix demonstrates enhanced tumor selectivity and reduced systemic toxicity compared to traditional cytotoxic approaches. Created by Biorender.com.
Despite this remarkable progress, significant challenges persist. A substantial proportion of patients either exhibit primary resistance to ICIs or develop acquired resistance over time, leading to eventual disease progression. Moreover, the clinical utility of ICIs is often constrained by immune-related adverse events (irAEs), which can affect any organ system and range from mild to life-threatening (18, 19). Addressing these hurdles requires a shift towards greater therapeutic personalization, which hinges on the identification and validation of robust predictive biomarkers. Existing biomarkers, such as PD-L1 expression and TMB, have proven to have limited predictive power and are not consistently reliable for guiding individual patient decisions. Consequently, intensive research efforts are focused on discovering novel biomarkers. Emerging candidates in 2025 include non-invasive, blood-based markers like circulating tumor DNA (ctDNA), which can provide real-time insights into tumor dynamics and response. Furthermore, deep interrogation of the tumor microenvironment has revealed that the presence of organized immune aggregates known as tertiary lymphoid structures (TLS) is strongly associated with favorable responses to immunotherapy, positioning TLS as a highly promising tissue-based biomarker. Other innovative approaches include analyzing circulating immune cell metabolic signatures and leveraging artificial intelligence to integrate multi-omics data for more accurate response prediction. Understanding these mechanisms of immune evasion and identifying reliable biomarkers are paramount to overcoming resistance, optimizing patient selection, and developing the next generation of therapies and combinations.
Ferroptosis, pyroptosis, and necroptosis have emerged as key regulators of tumor progression and antitumor immunity. Ferroptosis, driven by iron-dependent lipid peroxidation (LPO), enhances immunogenicity by exposing calreticulin and ATP, which promote antigen presentation and the activation of immune cells. Pyroptosis, mediated by gasdermin (GSDM) pore formation, triggers the release of pro-inflammatory cytokines, facilitating CD8+ T cell infiltration and amplifying Th1 immune responses (20–22). Necroptosis, initiated by Receptor-Interacting Protein Kinase 1 (RIPK1)/RIPK3-MLKL signaling, leads to the release of High Mobility Group Box 1 (HMGB1) and supports cross-presentation of tumor antigens, thus linking cell death to immune surveillance. In melanoma, these pathways are frequently dysregulated, with ferroptosis and pyroptosis playing a role in tumor suppression, while necroptosis may contribute to chronic inflammation and immune evasion. The ability of these lytic forms of programmed cell death (PCD) to modulate immune responses highlights their therapeutic potential in cancer immunotherapy (23, 24). By inducing immunogenic cell death (ICD), ferroptosis, pyroptosis, and necroptosis can reshape the tumor microenvironment (TME) to enhance the efficacy of immune checkpoint inhibitors (ICIs). For example, ferroptosis can deplete immunosuppressive regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), while pyroptosis recruits neutrophils and activates macrophages, transforming immunologically “cold” tumors into inflamed niches (24–27). Necroptosis further promotes antigen spreading and the formation of durable T cell memory. Despite preclinical advances, challenges remain in translating these findings to the clinic due to issues such as off-target toxicity, tumor metabolic plasticity, and the need for predictive biomarkers (28, 29). However, future strategies that combine Regulated Cell Death (RCD) inducers with immune checkpoint blockade (ICB) and use innovative technologies, such as tumor-penetrating nanocarriers, hold great promise for overcoming resistance and improving therapeutic outcomes in melanoma.
Overall, this review explores how the strategic targeting of ferroptosis, pyroptosis, and necroptosis can redefine melanoma immunotherapy, translating mechanistic insights into actionable clinical strategies aimed at achieving durable remissions. By bridging cutting-edge research on ICD with innovative therapeutic approaches, we anticipate a future where combinatory therapies targeting these death pathways will enhance the efficacy of immunotherapies, offering new hope for melanoma patients (30, 31).
2 Overview of ferroptosis, necroptosis, and pyroptosis
2.1 Overview of ferroptosis
Ferroptosis is an iron-dependent form of RCD driven by excessive LPO, leading to plasma membrane rupture (32–35). Key molecular mechanisms involve iron accumulation, mediated by transferrin receptor (TFRC)-dependent iron uptake and autophagy-driven degradation of iron storage proteins (36–38), which elevate intracellular labile iron pools. This iron catalyzes the production of reactive oxygen species (ROS) through Fenton reactions and activates enzymes like arachidonate lipoxygenases (ALOXs) (39–41). These enzymes oxidize polyunsaturated fatty acids (PUFAs) into phospholipid hydroperoxides (PL-PUFA-OOH) via Acyl-CoA Synthetase Long Chain Family Member 4 (ACSL4) and Lysophosphatidylcholine Acyltransferase 3 (LPCAT3), culminating in membrane damage (42–50). The glutathione GSH peroxidase 4 (Gpx4) antioxidant axis is central to ferroptosis suppression: system Xc- imports cysteine for GSH synthesis, while GPX4 neutralizes lipid peroxides (51–59). Dysregulation of this system—through oncogenic pathways or p53-mediated inhibition of SLC7A11—renders cells susceptible to ferroptosis (60–66). Autophagy further amplifies ferroptosis by degrading GPX4, lipid droplets, and iron storage proteins, highlighting its role as a metabolic vulnerability in cancer (67–71).
Emerging evidence highlights ferroptosis as a pivotal regulator of antitumor immunity, offering novel strategies to overcome therapeutic resistance. In pancreatic ductal adenocarcinoma (PDAC), circTRIP12 (cTRIP12) drives ferroptosis resistance by binding O-GlcNAc transferase (OGT), elevating O-GlcNAcylation to stabilize ferritin heavy chain (FTH) and PD-L1, thereby suppressing ICD and promoting immune evasion (72). Similarly, Kelch-like ECH-associated protein 1 (KEAP1)-mutant tumors exhibit ferroptosis resistance via NRF2-mediated upregulation of NQO1, which attenuates LPO and dampens immunotherapy responses; targeting NQO1 restores ferroptosis sensitivity and triggers antitumor immunity (73). Advances in nanotechnology further amplify ferroptosis-immunotherapy synergy. For small cell lung cancer (SCLC), a cationic liposome co-delivering paclitaxel and PFKFB4-targeting siRNA induces ferroptosis, reprograms the immunosuppressive microenvironment, and enhances PD-L1 blockade efficacy (74–76). In glioblastoma (GBM), magnetic exosomes co-loaded with arsenic trioxide and IR780 disrupt redox balance, augmenting photodynamic therapy to activate ferroptosis, polarize macrophages, and reinvigorate T cell responses (77–81). Hepatocellular carcinoma (HCC) studies demonstrate that lactate depletion via engineered nanoparticles sensitizes tumors to erastin-induced ferroptosis, while Fe²+/Fe³+-mediated chemodynamic therapy synergizes with αPD-L1 to suppress metastasis (82–85). Collectively, these findings underscore the dual role of ferroptosis in directly killing tumor cells and indirectly remodeling immune landscapes, with targeted delivery systems and metabolic interventions bridging mechanistic insights to clinical translation. Molecular orchestrations governing of ferroptosis was shown in Figure 2A.
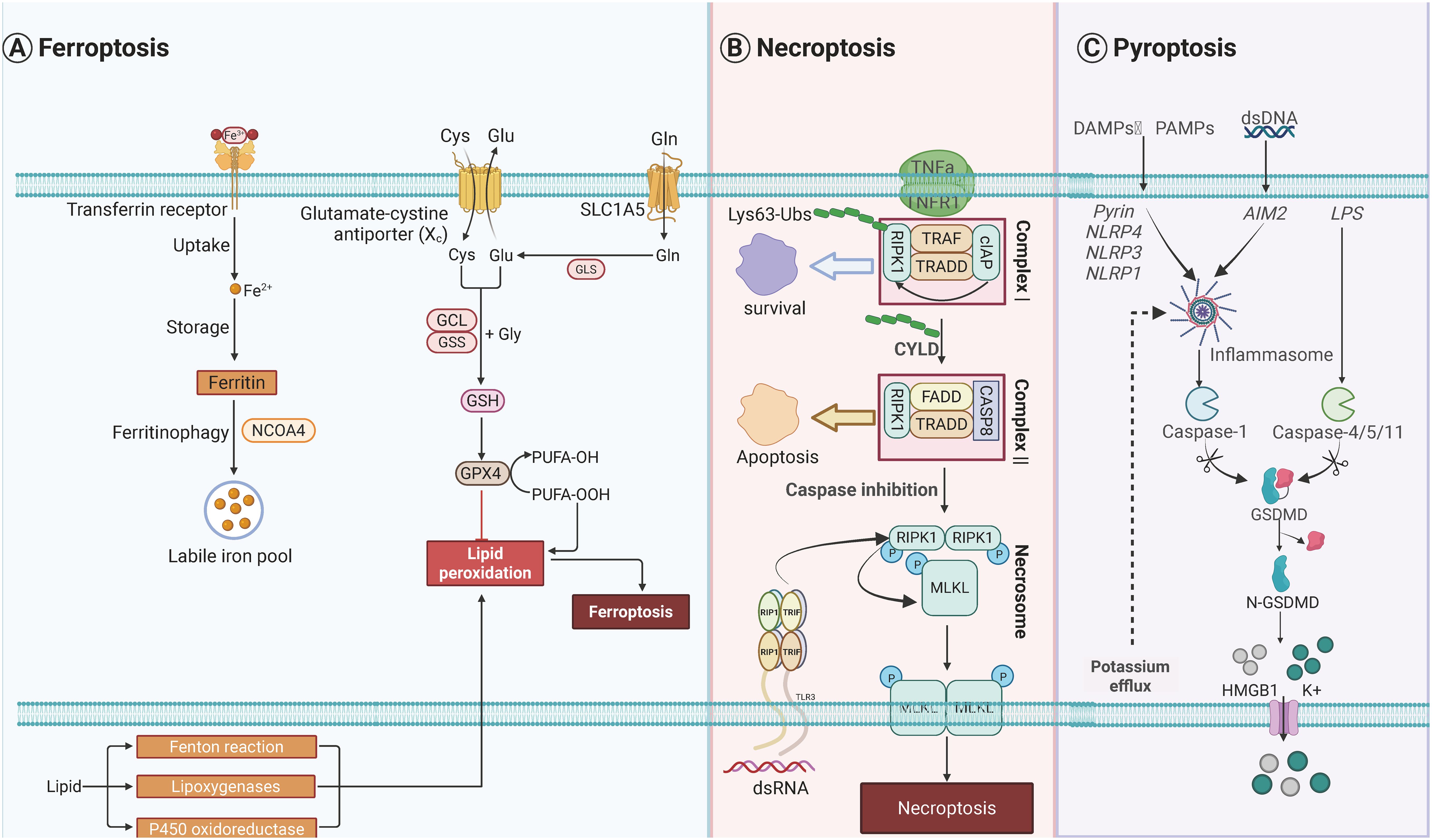
Figure 2. Molecular orchestrations governing three distinct programmed cell death modalities. (A) Pyroptotic signaling pathways are initiated through pattern recognition receptors including NLR family sensors (NLRP1/3/4), AIM2 detectors, and Pyrin proteins upon detecting pathogenic or danger signals. These sensors nucleate ASC-mediated inflammasome assembly, facilitating caspase-1 activation. Non-canonical pathway activation occurs through cytoplasmic LPS recognition by inflammatory caspases (CASP4/5/11). Both pathways converge on gasdermin-D proteolysis, generating pore-forming N-terminal fragments that execute lytic cell death. Concomitant potassium ion efflux during membrane permeabilization promotes HMGB1 liberation and extracellular potassium elevation. (B) Ferroptotic cell demise arises from dual metabolic dysregulation: iron homeostasis disruption and peroxidative membrane damage. Intracellular iron overload is mediated through enhanced TFRC-mediated uptake, impaired ferroportin export, and ferritin degradation via autophagy-lysosomal pathways. Lipid peroxidation cascades are driven by ACSL4-LPCAT3 enzymatic coupling facilitating ALOX-mediated polyunsaturated fatty acid oxidation, with RAB7A-regulated lipid droplet autophagy supplying peroxidation substrates. Cellular defenses against oxidative collapse include GPX4-glutathione redox cycling, AIFM2-CoQ10 antioxidant pairs, GTP cyclohydrolase-dependent radical scavenging systems, and ESCRT-III-mediated membrane repair mechanisms. (C) Necroptotic execution follows TNF receptor engagement and sequential signaling complex assembly. Initial membrane-proximal Complex I formation involves cIAP-mediated RIPK1 K63-ubiquitination, promoting NF-κB survival signaling. CYLD-dependent deubiquitination triggers cytosolic Complex II formation, where caspase-8 inhibition redirects signaling to RIPK1-RIPK3-MLKL necrosome assembly. Sequential kinase activation culminates in MLKL phosphorylation-induced oligomerization, forming plasma membrane-disrupting pores that mediate lytic cell death. Created by Biorender.com.
2.2 Overview of necroptosis
Necroptosis represents a caspase-independent, lytic form of regulated cell death (RCD). It is primarily initiated by the activation of death receptors or pathogen-sensing receptors, serving as a critical cellular response pathway (86, 87). A well-characterized trigger of necroptosis is the binding of tumor necrosis factor-α (TNF-α) to its cognate receptor. Upon this interaction, receptor-interacting serine/threonine-protein kinase 1 (RIPK1) undergoes recruitment of RIPK3, leading to the formation of a multiprotein complex termed the necrosome (88–93). The necrosome then mediates the phosphorylation of mixed-lineage kinase domain-like protein (MLKL); once phosphorylated, MLKL undergoes oligomerization and translocates to the plasma membrane. At the membrane, oligomerized MLKL forms transmembrane pores, which disrupt membrane integrity and drive the release of damage-associated molecular patterns (DAMPs)—a hallmark of lytic cell death (94–100). This process is often triggered when apoptosis is inhibited, serving as a backup defense against pathogens. Necroptosis plays dual roles in cancer: it acts as a tumor suppressor in colorectal and hepatocellular carcinomas (101–107), where RIPK3/MLKL downregulation correlates with poor prognosis, but may promote inflammation-driven malignancy in specific contexts (108–113). Epigenetic silencing of RIPK3 in tumors can be reversed by hypomethylating agents, restoring chemosensitivity (114, 115). Key regulators include Cylindromatosis (CYLD) (116–119), which deubiquitinates RIPK1 to facilitate necrosome assembly, and Z-DNA Binding Protein 1 (ZBP1), which senses viral RNA/DNA to activate RIPK3-MLKL signaling independently of death receptors (120, 121).
Necroptosis has emerged as a potent ICD pathway to amplify antitumor immunity. In PDAC, MLKL-driven necroptosis recruit macrophages, upregulates CD47 to evade phagocytosis, and triggers CXCL8-mediated epithelial-mesenchymal transition (EMT) and liver metastasis. Combining MLKL inhibitor GW806742X with CD47 blockade suppresses metastatic dissemination, highlighting necroptosis as a dual-edged sword in PDAC progression (122–128). To leverage its immunogenicity, nanotechnology platforms have been designed to induce tumor-specific necroptosis. For instance, a sulfate radical (SO4·−)-based in situ vaccine triggers MLKL-dependent necroptosis in acidic TMEs, releasing DAMPs to activate STING signaling and synergize with ICIs against distant tumors (129–135). Similarly, Mn²+-enriched shikonin-loaded nanoparticles induce necroptosis in head and neck squamous cell carcinoma (HNSCC), disrupting mismatch repair to activate cGAS-STING-dependent IFN responses and enhance PD-1 blockade efficacy by promoting DC maturation and cytotoxic T cell infiltration (136–140). Beyond canonical RIPK1/RIPK3 pathways, the natural compound OSW-1 induces a RIP1/RIP3-independent necroptosis in colorectal cancer via p53-PUMA-CamKIIδ-MLKL signaling. This mechanism not only kills tumor cells but also sensitizes tumors to anti-PD-1 therapy by enhancing immunogenic antigen release (141). In postoperative non-small cell lung cancer (NSCLC), a photothermal hydrogel induces necroptosis in residual cancer cells, elevating DAMPs to polarize M1 macrophages and activate CD8+ T cell immunity, thereby preventing recurrence and infection (142). These advances underscore necroptosis as a versatile tool to reprogram immunosuppressive microenvironments and potentiate ICB through targeted induction strategies. Molecular orchestrations governing of necroptosis was shown in Figure 2B.
2.3 Overview of pyroptosis
Pyroptosis is an inflammatory RCD pathway characterized by GSDM family pore formation, which releases proinflammatory cytokines and induces cell lysis (63, 143, 144). Caspase-1, activated by canonical inflammasomes, cleaves Gasdermin D (GSDMD) to generate N-terminal fragments that oligomerize and perforate membranes (145–147). Noncanonical pathways involve caspase-4/5/11 or caspase-11 directly cleaving GSDMD upon cytosolic LPS detection (148–150). Apoptotic caspases can also trigger pyroptosis by cleaving GSDME, converting immunologically silent apoptosis into inflammatory death (151–153). In cancer, pyroptosis exhibits dual roles: GSDMD overexpression in NSCLC correlates with tumor aggressiveness (154–156), while NLRP3 activation in HCC suppresses metastasis (157–159). Therapeutic strategies leverage pyroptosis for antitumor immunity, inducing immunogenic death (72, 160). Additionally, GSDMB cleavage by granzyme in natural killer (NK) cells enhances tumor clearance, underscoring its potential in immunotherapy (161).
Pyroptosis has emerged as a potent strategy to remodel immunosuppressive TMEs and enhance ICB efficacy. In triple-negative breast cancer (TNBC), the ER stress sensor IRE1α suppresses taxane-induced immunogenicity by degrading double-stranded RNA (dsRNA) via regulated IRE1-dependent decay (RIDD), thereby inhibiting NLRP3 inflammasome-dependent pyroptosis. Pharmacological inhibition of IRE1α unleashes ZBP1-mediated recognition of dsRNA, activating NLRP3-GSDMD pyroptosis and converting PD-L1-negative tumors into immunogenic niches responsive to ICB (162). Similarly, in HCC, selective HDAC1/2/3 inhibition synergizes with ICB to enhance chromatin accessibility of IFNγ-responsive genes, promoting STAT1-driven pyroptosis via GSDME cleavage and amplifying cytotoxic T cell infiltration (157). Nanotechnology further expands pyroptosis applications: a tumor-specific nanoparticle undergoes charge reversal and nanofiber formation within lysosomes, disrupting lysosomal integrity to trigger GSDMD-mediated pyroptosis, which reverses immunosuppression and potentiates PD-L1 blockade in aggressive breast and pancreatic tumors (163). In gastric cancer (GC), transcriptional activation of GSDMD by HIC1 induces pyroptosis, releasing inflammatory cytokines to recruit CD8+ T cells, while combinatorial HIC1 overexpression with PD-L1 antibodies synergistically suppresses tumor growth (164). Natural compounds also harness pyroptosis: the Huangqin Houpo-derived combination induces GSDME-dependent pyroptosis, sensitizing colorectal cancer to anti-PD-1 therapy (165). Additionally, microwave-responsive AlEu-MOFs amplify NLR Family Pyrin Domain Containing 3 (NLRP3) inflammasome activation through HSP90 upregulation and ROS generation, achieving targeted pyroptosis to inhibit primary and metastatic breast tumors (166). These findings collectively underscore pyroptosis as a multifaceted immunomodulator, bridging innate immune activation with adaptive antitumor responses across diverse malignancies. Molecular orchestrations governing of pyroptosis was shown in Figure 2C.
2.4 Cross-talk among ferroptosis, necroptosis, and pyroptosis
The interplay between RCD pathways—necroptosis, pyroptosis, ferroptosis, and cuproptosis—synergistically amplifies ICD and reshapes tumor-immune dynamics (167–169). For instance, ZBP1 activation by viral RNA simultaneously triggers necroptosis via RIPK3-MLKL signaling (170) and pyroptosis through NLRP3 inflammasome activation (171), with MLKL pores releasing potassium to further enhance NLRP3-driven IL-1β secretion (172, 173). Similarly, copper ionophores like elesclomol disrupt mitochondrial copper homeostasis to induce cuproptosis while depleting GSH and downregulating Solute Carrier Family 7 Member 11 (SLC7A11), sensitizing cells to ferroptosis (174). Metabolic crosstalk further links these pathways: ACSL4-driven LPO in ferroptosis alters membrane composition to suppress pyroptosis, whereas necroptosis-induced DAMPs prime DCs to recognize pyroptosis-derived antigens. Therapeutically, co-targeting RIPK1 and GPX4 (ferroptosis) synergizes with PD-1 blockade to overcome immunotherapy resistance, while pyroptosis-inducing nanoparticles recruit neutrophils to enhance ferroptosis inducers within the TME. These interactions underscore the complexity of RCD networks and their potential to redefine cancer treatment through multimodal immunomodulation.
2.5 Emerging RCD pathways in melanoma
Malignant melanoma remains a formidable clinical challenge due to its high metastatic potential and profound resistance to conventional therapies, which primarily induce apoptosis. This resistance has catalyzed a paradigm shift in cancer research, moving the focus towards non-apoptotic forms of regulated cell death (RCD). Among these, ferroptosis, necroptosis, and pyroptosis have emerged as critical mechanisms that not only execute tumor cell killing but also actively modulate the tumor microenvironment (TME). Understanding the intricate molecular cross-talk among these pathways is proving essential for developing next-generation therapeutic strategies to overcome melanoma’s notorious plasticity.
Melanoma cells are susceptible to several non-apoptotic death modalities, each governed by a distinct molecular machinery. Ferroptosis is an iron-dependent form of RCD characterized by the overwhelming accumulation of lipid reactive oxygen species (ROS). Its execution is centrally regulated by the inhibition of glutathione peroxidase 4 (GPX4), a key enzyme that neutralizes lipid peroxides. Inducing ferroptosis has shown significant promise in overcoming resistance to targeted therapies and immunotherapy in melanoma models. Necroptosis, a form of programmed necrosis, is orchestrated by a signaling complex involving receptor-interacting protein kinase 1 (RIPK1), RIPK3, and the terminal executioner protein, mixed lineage kinase domain-like pseudokinase (MLKL). Upon activation, MLKL oligomerizes and translocates to the plasma membrane, forming pores that lead to cell lysis and the release of damage-associated molecular patterns (DAMPs). While some melanoma subtypes exhibit low sensitivity to necroptosis due to the downregulation of key components its induction can provoke a potent anti-tumor immune response, synergizing effectively with checkpoint blockade therapies.
Pyroptosis is a highly inflammatory RCD pathway mediated by the gasdermin (GSDM) family of pore-forming proteins. It is typically triggered by inflammasome activation, which leads to the cleavage of GSDMs by inflammatory caspases (e.g., caspase-1). The resulting pores cause cell swelling and lysis, accompanied by the massive release of pro-inflammatory cytokines such as IL-1β and IL-18, thereby robustly reshaping the TME. These RCD pathways do not operate in isolation but are extensively interconnected, forming a complex and adaptable cell death network. Key proteins function as nodes that integrate signals and dictate the ultimate cellular fate. RIPK1, the canonical initiator of necroptosis, also plays a crucial role in activating the NLRP3 inflammasome, directly linking necroptotic signaling to caspase-1 activation and pyroptosis. Furthermore, cellular stress signals like ROS serve as a common currency connecting these pathways. The intense lipid ROS production during ferroptosis can amplify necroptotic or pyroptotic signaling while mitochondrial dysfunction is a shared feature across all three RCDs. Caspases also act as critical regulators; for instance, caspase-8 can cleave and inactivate RIPK1 and RIPK3, thereby inhibiting necroptosis and potentially shunting the cell towards an alternative fate.
The profound cross-talk among ferroptosis, necroptosis, and pyroptosis presents novel therapeutic opportunities. The focus is shifting from inducing a single RCD pathway to orchestrating a multi-pronged attack that leverages their synergistic interactions. A particularly promising strategy involves the simultaneous induction of multiple immunogenic cell death pathways to maximize the anti-tumor immune response. Recent preclinical studies from 2023 and 2024 have showcased innovative approaches, such as nano-MOFs (metal-organic frameworks) designed to co-induce ferroptosis and pyroptosis in melanoma. This dual induction amplifies ROS production, enhances DAMP release, and potently stimulates an immune response, significantly improving the efficacy of immunotherapy. As of 2025, developing therapies that precisely manipulate these interconnected RCD nodes represents a cutting-edge approach to dismantle the formidable defenses of malignant melanoma.
3 Targeting ferroptosis, pyroptosis and necroptosis for cancer immunotherapy in melanoma
3.1 Targeting ferroptosis for cancer immunotherapy in melanoma
Ferroptosis, driven by iron-dependent LPO, has emerged as a pivotal therapeutic target in melanoma, with its regulation intricately linked to both tumor cell vulnerability and immune modulation. This section synthesizes key molecular mechanisms and innovative strategies—from pharmacological agents to nanotechnology platforms—that harness ferroptosis to disrupt immunosuppression and enhance antitumor immunity. The synergy of ferroptosis inducers with ICB further highlights their potential to overcome therapeutic resistance. Figure 3 and Table 1 summarized these pathways and interventions, setting the stage for detailed exploration of ferroptosis-driven immunotherapy in melanoma.
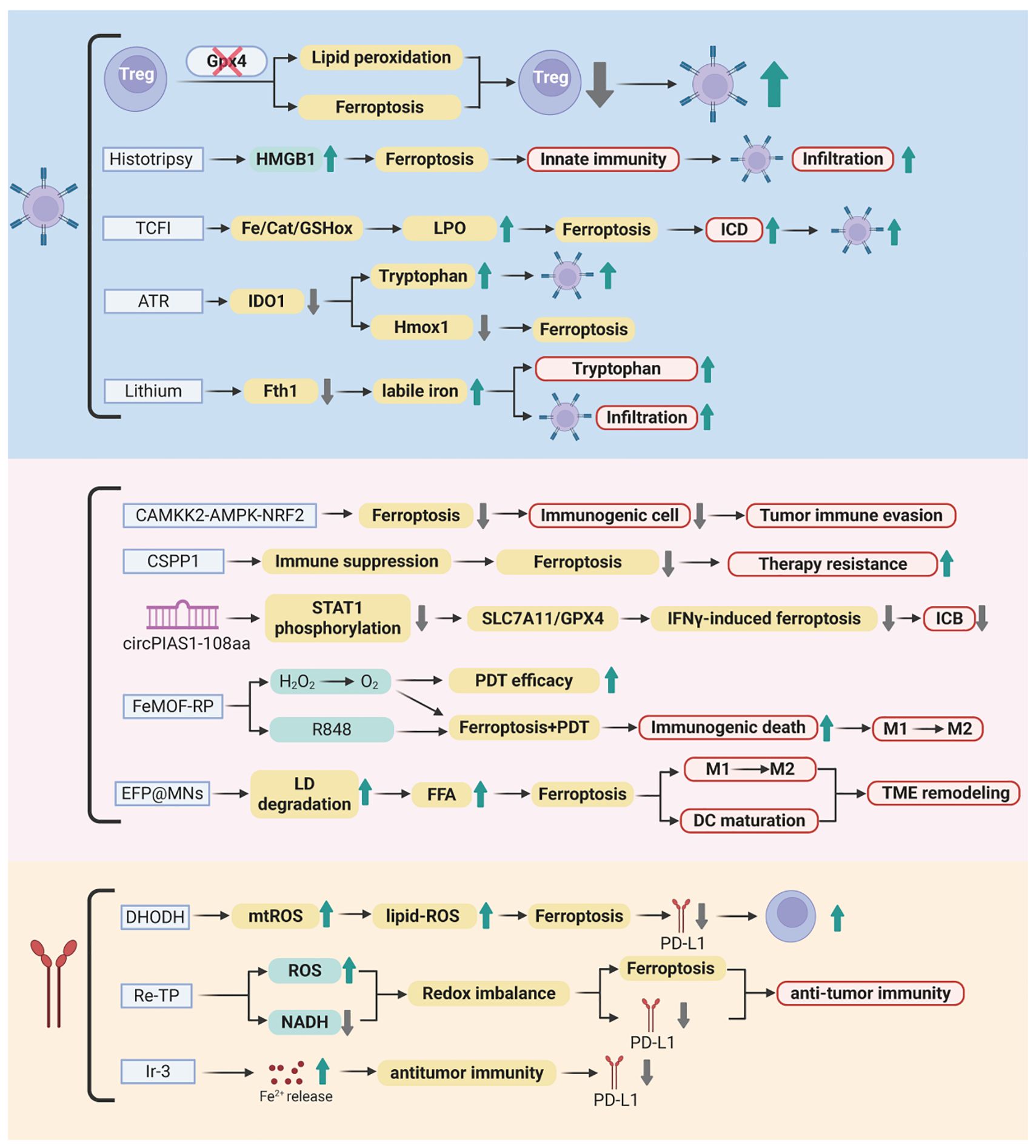
Figure 3. Ferroptosis-mediated immunotherapeutic landscape in melanoma. This figure illustrates the molecular interplay between ferroptosis induction and antitumor immunity. Key regulators including Gpx4, CAMKK2, and CSPP1 control lipid peroxidation, redox balance, and immune suppression within the tumor microenvironment. Pharmacological agents (B2, ART, lithium) and nanotechnology platforms (TCFI, FeMOF-RP, EFP@MNs) trigger iron-dependent cell death through ROS amplification, metabolic reprogramming, and immune checkpoint modulation. Synergistic integration with immune checkpoint inhibitors (anti-PD-1/PD-L1) enhances CD8+ T-cell activation, macrophage polarization, and systemic antitumor responses, overcoming therapeutic resistance. Created by Biorender.com.
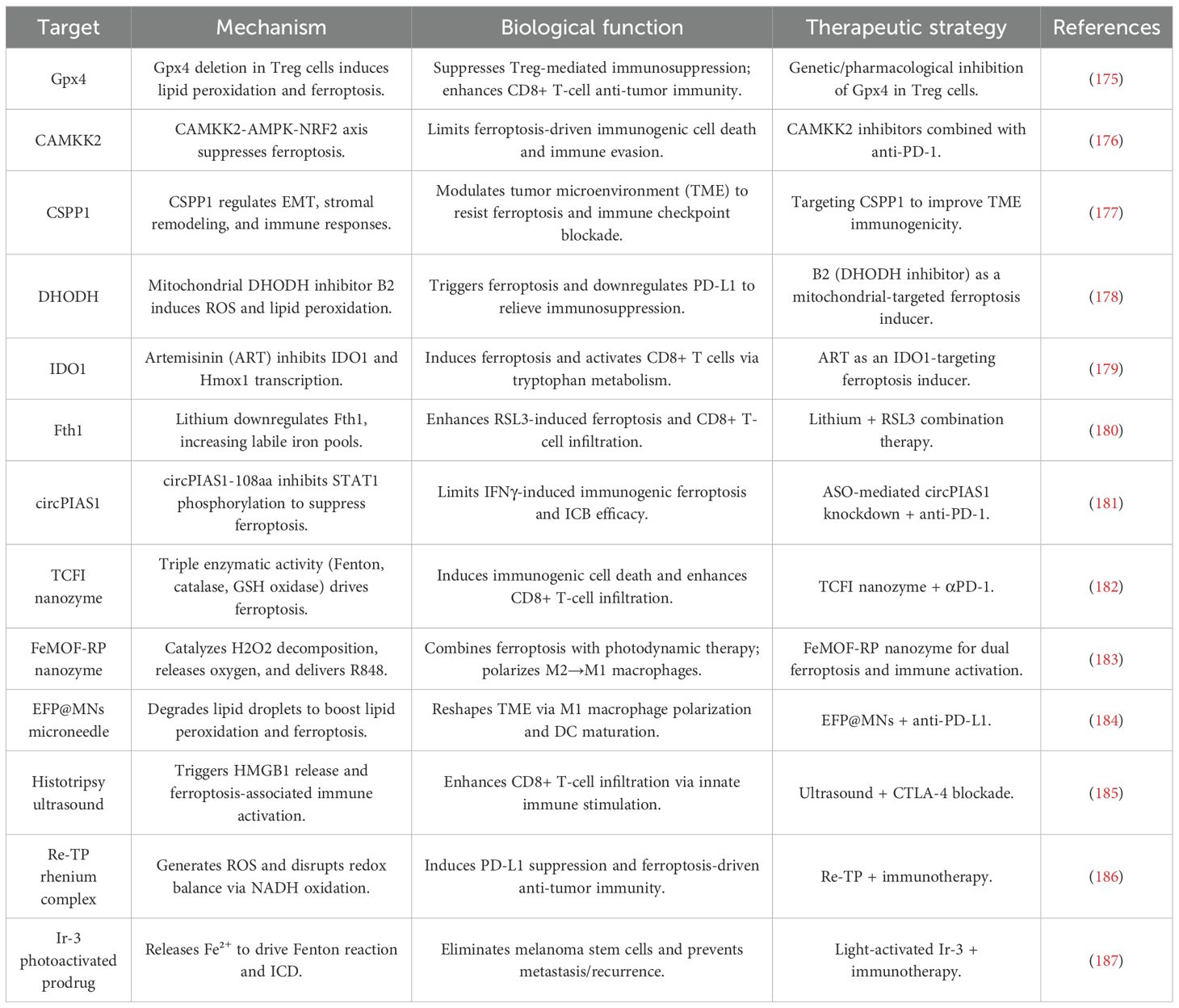
Table 1. Molecular targets and therapeutic strategies for ferroptosis-driven immunotherapy in melanoma.
3.1.1 Molecular and immune mechanisms of ferroptosis regulation
Ferroptosis has garnered increasing interest as a potential therapeutic target in melanoma due to its complex regulation by both molecular and immune mechanisms. Notably, Gpx4 plays a pivotal role in protecting activated Treg cells from ferroptotic cell death, enabling these cells to maintain immune tolerance and suppress antitumor immunity. The genetic ablation of Gpx4 in Treg cells leads to the disruption of their immunosuppressive functions, enhancement of Th17 responses, and promotion of CD8+ T-cell-mediated tumor suppression. These findings underscore Gpx4 as a promising target for therapeutic intervention in melanoma (175).
Similarly, the kinase Calcium/Calmodulin-Dependent Protein Kinase Kinase 2 (CAMKK2), which functions through the AMPK-NRF2 axis, has been shown to negatively regulate ferroptosis in melanoma cells. Inhibition of CAMKK2 not only facilitates ferroptosis but also potentiates the efficacy of anti-PD-1 therapy, overcoming resistance to ICB (176). In addition to kinases, centrosome-associated protein CSPP1 emerges as a multifaceted regulator of ferroptosis and TME dynamics. Dysregulated CSPP1 expression is associated with EMT, stromal interactions, and immune checkpoint responsiveness, positioning CSPP1 as both a diagnostic biomarker and a potential therapeutic target (177).
Pharmacological strategies further illuminate the mechanistic pathways underlying ferroptosis regulation. The mitochondrial-targeted DHODH inhibitor B2 induces ferroptosis by generating ROS and promoting LPO, while simultaneously downregulating PD-L1 to alleviate immune suppression (178). Natural compounds such as artemisinin (ART) demonstrate dual functionality by directly targeting IDO1 to induce ferroptosis and enhancing CD8+ T-cell activity through modulation of tryptophan metabolism and PD-1 suppression (179). Lithium, a well-known pharmacological agent, sensitizes melanoma cells to ferroptosis by downregulating FTH, thereby increasing labile iron pools and synergizing with RSL3 to inhibit tumor growth and promote CD8+ T-cell infiltration (180). Additionally, circular RNA circPIAS1 acts as a barrier to ferroptosis-driven immunotherapy by encoding a peptide that suppresses STAT1 phosphorylation and reactivates the SLC7A11/GPX4 axis. Antisense oligonucleotide-mediated knockdown of circPIAS1 restores ferroptosis sensitivity and enhances the efficacy of PD-1 blockade, suggesting its potential as a therapeutic target (181).
3.1.2 Therapeutic strategies for ferroptosis induction
Innovative therapeutic strategies have been developed to exploit ferroptosis induction in melanoma, offering new avenues for enhancing tumor immunotherapy. One promising approach involves the use of the TCFI nanozyme, which exhibits Fenton-like, catalase-like, and GSH oxidase-like activities. This nanozyme triggers LPO, alleviates hypoxia, and downregulates the GSH/GPX4 axis, synergizing with photodynamic therapy to enhance ROS generation and ICD. The TCFI nanozyme significantly increases CD8+ T-cell infiltration and interferon-γ (IFNγ) secretion, leading to potent suppression of both primary and metastatic tumors (182).
Another advanced strategy involves the FeMOF-RP nanosystem, which integrates photodynamic therapy with R848 delivery to catalyze intratumoral H2O2 decomposition. This process amplifies ferroptosis and promotes the polarization of immunosuppressive M2 macrophages toward an immunostimulatory M1 phenotype, thereby improving therapeutic outcomes (183). To address metabolic limitations within the TME, the EFP@MNs microneedle patch employs a lipolysis strategy to degrade lipid droplets and release free fatty acids (FFAs), fueling LPO. When combined with photothermal remodeling of the TME, this approach enhances DC maturation and synergizes with anti-PD-L1 therapy to achieve robust tumor ablation (184).
Non-chemical approaches have also demonstrated efficacy in triggering ferroptosis. Histotripsy, an ultrasound-based technique, induces ferroptosis-associated HMGB1 release and CD8+ T-cell infiltration. When combined with CTLA-4 blockade, histotripsy enhances antitumor effects (185). Metal complexes such as Re-TP exemplify the convergence of ferroptosis and immunotherapy. Re-TP, upon light activation, generates ROS, disrupts redox balance, and triggers PD-L1-related immune responses, leading to the eradication of both primary and metastatic tumors in murine models (186). Furthermore, the photoactivatable iridium complex Ir-3 releases Fe2+ to drive Fenton reactions and eliminate melanoma stem cells, thus preventing metastasis and recurrence (187).
3.1.3 Combination with ICB
The integration of ferroptosis inducers with ICB has emerged as a promising strategy to enhance the efficacy of cancer immunotherapy. For instance, inhibition of CAMKK2 enhances ferroptosis and synergizes with anti-PD-1 therapy, effectively reversing immune evasion and overcoming therapeutic resistance (176). The TCFI nanozyme, when combined with αPD-1 antibodies, induces complete regression of primary tumors and suppresses distant metastases, highlighting the potential of nanotechnology-ICB combinations in melanoma therapy (182). Similarly, the EFP@MNs microneedle patch, when combined with anti-PD-L1 therapy, leverages lipolysis-driven ferroptosis and photothermal remodeling of the TME to enhance therapeutic outcomes (184).
Metal-based agents such as Re-TP further amplify the efficacy of ICB by inducing metabolic reprogramming, promoting ferroptosis and ICD while simultaneously suppressing PD-L1 expression (186). Collectively, these studies underscore the potential of ferroptosis-ICB combination therapies to achieve durable antitumor immunity and address the challenges of tumor heterogeneity and resistance mechanisms.
3.2 Targeting necroptosis for cancer immunotherapy in melanoma
Necroptosis, a lytic and ICD pathway, has emerged as a key driver of antitumor immunity in melanoma by releasing DAMPs and activating DC-mediated T cell responses. This section highlights molecular regulators and therapeutic strategies—from repurposed drugs to mRNA-based MLKL delivery and hybrid nanovesicles—that induce necroptosis to overcome immune evasion. Synergy with ICB further enhances T cell infiltration and suppresses metastasis. Figure 4 and Table 2 summarized these mechanisms and interventions, setting the stage for exploring necroptosis-centered immunotherapies in melanoma.
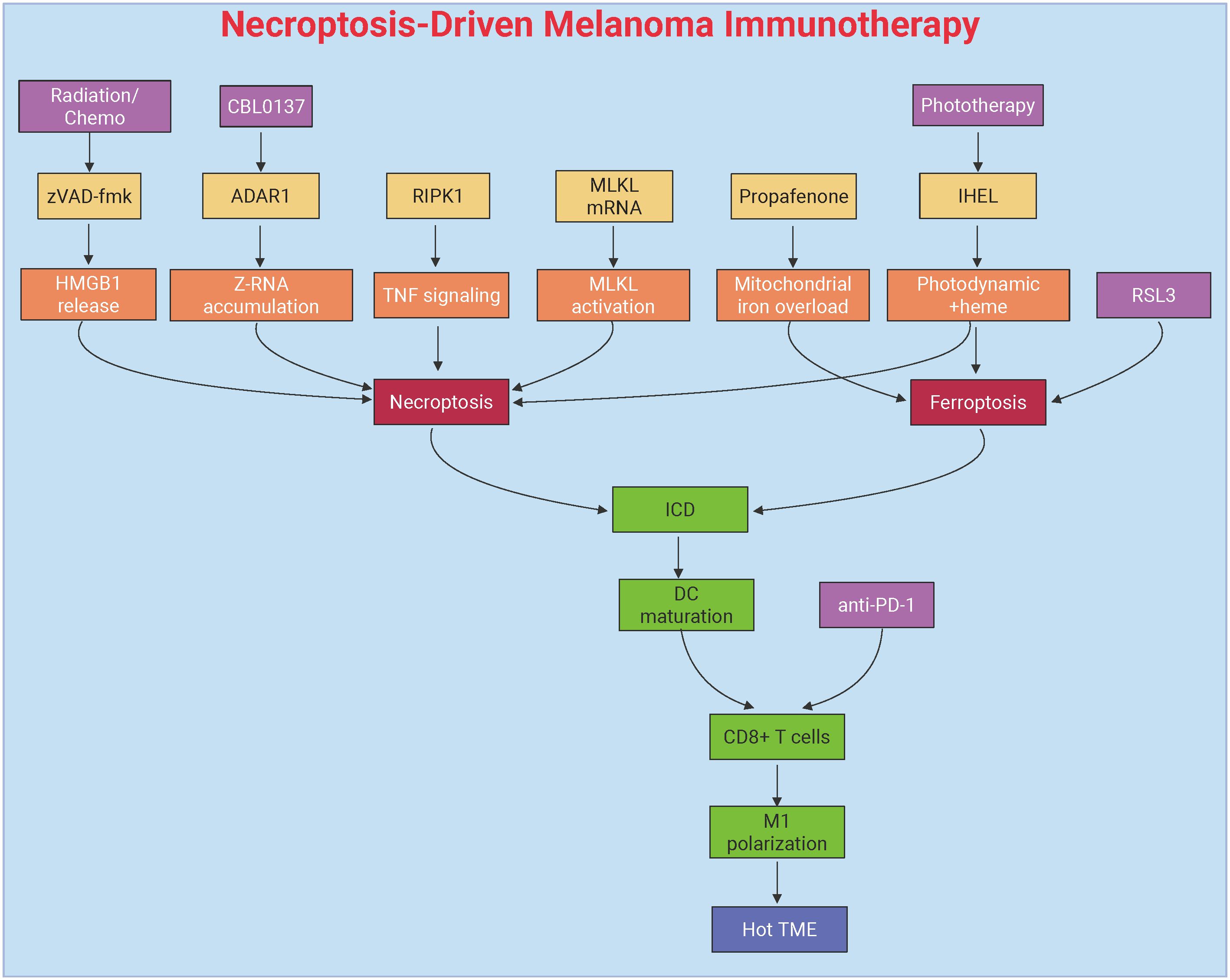
Figure 4. Necroptosis-mediated immunotherapeutic modulation in melanoma. This figure delineates necroptosis-driven antitumor immunity through DAMPs release and dendritic cell activation. Key interventions include pharmacological agents (propafenone) inducing JNK/JUN-mediated iron overload, mRNA-based MLKL delivery bypassing upstream signaling defects, and IHEL nanovesicles combining phototherapy with heme-induced necroptosis. Synergy with immune checkpoint blockade (anti-PD-1/PD-L1) amplifies T-cell infiltration, remodels the immunosuppressive tumor microenvironment, and suppresses metastasis through RIPK1-dependent immunogenic cell death and macrophage polarization. Created by Biorender.com.
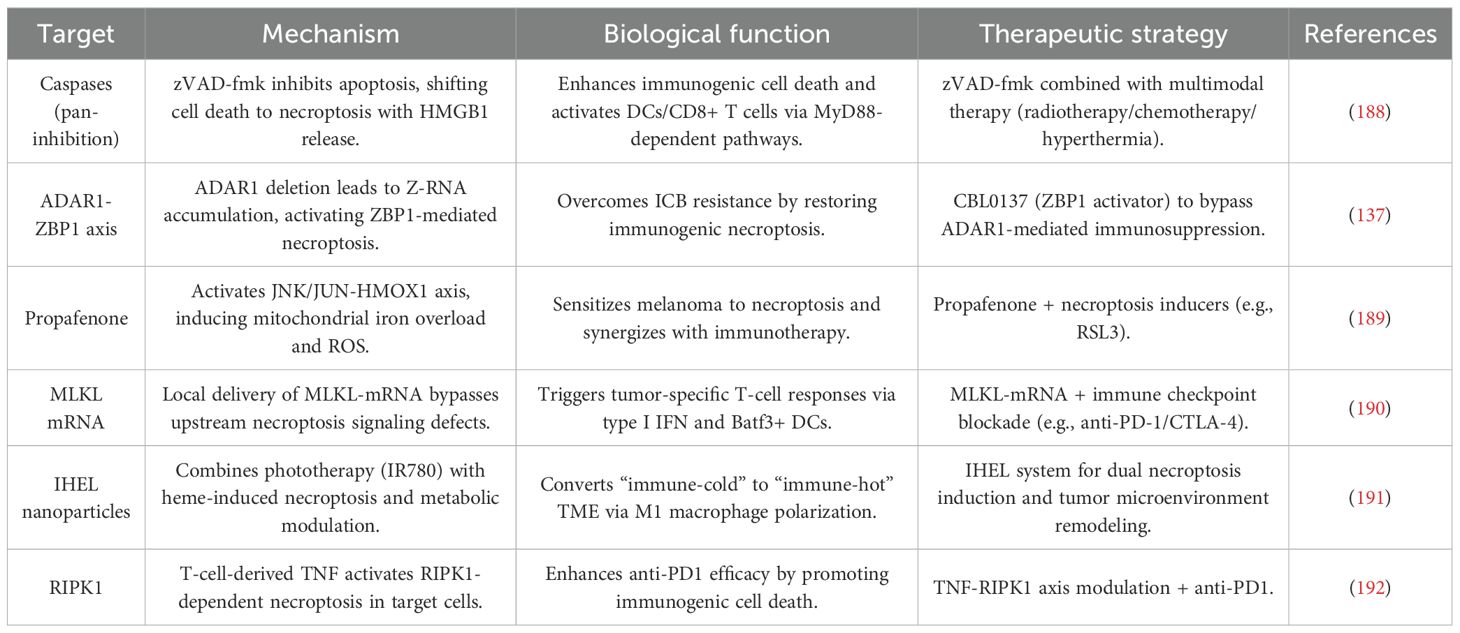
Table 2. Molecular targets and therapeutic strategies for necroptosis-driven immunotherapy in melanoma.
3.2.1 Molecular mechanisms of necroptosis-driven immune activation
Necroptosis has gained significant attention as a mechanism to stimulate antitumor immunity in melanoma. This process is characterized by the activation of several key immune pathways, making it a promising strategy for overcoming immune evasion in melanoma. One of the critical factors influencing necroptosis in melanoma is the pan-caspase inhibitor Z-Val-Ala-Asp(OMe)-FMK (zVAD-fmk). This compound enhances the immunogenicity of tumor cell death by shifting the cell death pathway from apoptosis to necroptosis, leading to increased release of DAMPs, such as HMGB1. These DAMPs, in turn, activate macrophages and DCs via MyD88-, nucleotide-, and T cell-dependent mechanisms. In murine melanoma models, the combination of zVAD-fmk with multimodal therapies has been shown to significantly reduce tumor growth, decrease regulatory T cell infiltration, and promote the recruitment of CD8+ T cells along with enhanced IFNγ expression. These effects indicate that necroptosis can remodel the immunosuppressive TME, paving the way for more effective cancer immunotherapy (188).
Another critical regulator of necroptosis-driven immune activation is the RNA-editing enzyme Adenosine Deaminase Acting on RNA 1 (ADAR1), which suppresses the accumulation of endogenous Z-RNA. ADAR1 deficiency, by allowing the accumulation of Z-RNA, triggers necroptosis through the Z-RNA sensor Z-DNA Binding Protein 1 (ZBP1), effectively bypassing resistance mechanisms to ICB. Pharmacological activation of ZBP1 using the small molecule CBL0137 has been shown to restore ICB responsiveness in melanoma. This is achieved by inducing the expression of IFN-stimulated genes (ISGs) and promoting necroptotic cell death, highlighting ADAR1 as a potential therapeutic target to overcome immune evasion in melanoma (137).
3.2.2 Therapeutic strategies to induce necroptosis
Targeted induction of necroptosis has shown significant promise in overcoming therapy resistance in melanoma. One such approach involves the use of the antiarrhythmic drug propafenone, which sensitizes melanoma cells to necroptosis by activating the JNK/JUN signaling pathway, upregulating mitochondrial heme oxygenase 1 (HMOX1), and inducing iron overload and ROS accumulation. Propafenone has been shown to synergize with necroptosis inducers, such as RSL3, resulting in near-complete tumor regression in xenograft models. Additionally, propafenone enhances ICB efficacy by promoting tumor cell necroptosis and increasing T cell infiltration. Clinically, melanoma patients exhibiting elevated necroptosis-related signatures, such as JUN and HMOX1 expression, show improved responses to ICB therapy and prolonged progression-free survival (189).
mRNA-based therapies, such as intratumoral delivery of MLKL-encoding mRNA, represent another promising strategy for inducing necroptosis. This approach bypasses upstream signaling defects in necroptosis and directly triggers robust antitumor immunity. MLKL-mRNA therapy induces tumor neoantigen-specific T cell responses, which are dependent on type I IFN signaling and Batf3+ DCs. When combined with ICB, MLKL-mRNA therapy effectively suppresses both primary and metastatic tumors, highlighting its potential as a valuable adjunct to existing immunotherapeutic strategies (190).
Hybrid exosome/liposome nanovesicles are another innovative strategy that integrates multiple therapeutic modalities, combining phototherapy with heme-induced necroptosis to overcome apoptosis resistance. IHEL nanovesicles not only induce necroptosis but also remodel the TME by alleviating hypoxia, inhibiting glycolysis, and polarizing macrophages towards an immunostimulatory M1 phenotype. These effects contribute to enhanced antitumor immunity, making IHEL a versatile platform for necroptosis-centered therapies (191).
3.2.3 Synergy with ICB
The combination of necroptosis induction with ICB represents a promising therapeutic approach for melanoma. T cell-derived TNFα has been shown to activate RIPK1-dependent necroptosis in target cells, a critical mechanism for accelerating allograft rejection and amplifying the antitumor effects of anti-PD1 therapy. In melanoma models, RIPK1-dependent necroptosis works synergistically with ICB by promoting ICD and enhancing T cell activation, thus amplifying the efficacy of ICIs (192).
MLKL-mRNA therapy further exemplifies this synergy, as its combination with ICB leads to enhanced CD8+ T cell-mediated tumor clearance and the establishment of durable immune memory. This approach leverages both direct tumor cell killing and the priming of antitumor immunity to improve therapeutic outcomes (190). Similarly, propafenone-induced necroptosis has been shown to reshape the TME to favor ICB responsiveness. By linking necroptotic cell death with adaptive immune activation, propafenone enhances the antitumor effects of ICB therapy (189).
These findings collectively underscore the dual role of necroptosis in both directly eliminating tumor cells and indirectly enhancing antitumor immunity. By modulating the TME and promoting immune activation, necroptosis is poised to be a cornerstone of next-generation combination therapies that aim to overcome resistance and improve the durability of cancer treatments.
3.3 Targeting pyroptosis for cancer immunotherapy in melanoma
Pyroptosis, an inflammatory and ICD pathway driven by GSDM family pore formation, has emerged as a pivotal strategy to reignite antitumor immunity in melanoma by releasing DAMPs and activating adaptive immune responses. This section outlines key molecular regulators and therapeutic innovations—from pharmacological agents to CRISPR-based platforms and biomimetic nanoparticles—that leverage pyroptosis to counter immune evasion. Synergy with ICB and targeted therapies further amplifies CD8+ T cell infiltration and remodels the immunosuppressive TME. Prognostic models like the pyroptosis score (PScore) provide insights into patient stratification and ICB responsiveness. Figure 5 and Table 3 summarize these pathways and interventions, setting the stage for detailed exploration of pyroptosis-driven immunotherapies in melanoma.
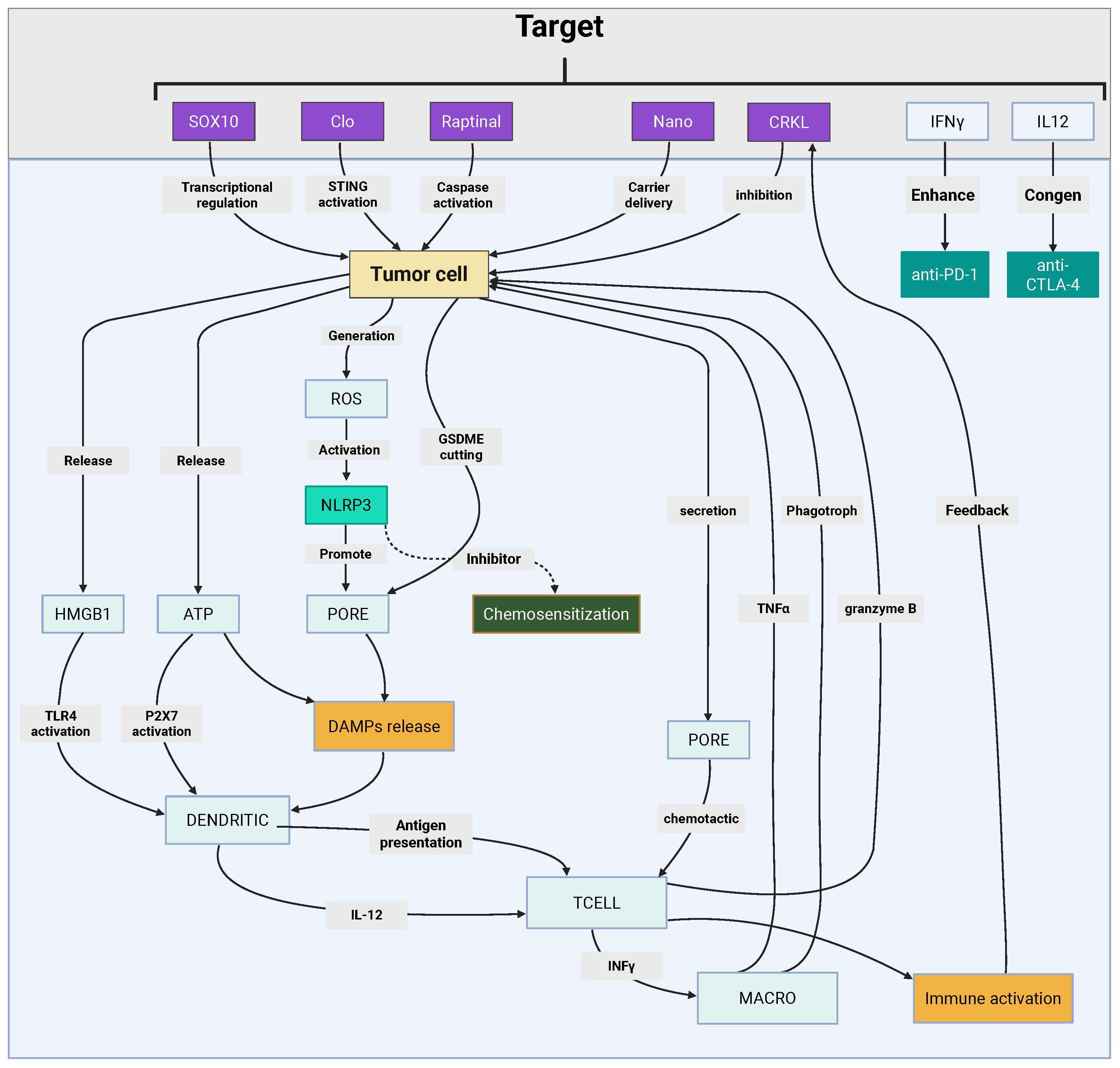
Figure 5. Pyroptosis-centric immunotherapeutic integration in melanoma. This figure depicts pyroptosis-mediated antitumor immunity through gasdermin pore-driven DAMPs release and adaptive immune activation. Key strategies include pharmacological activators (clofarabine, raptinal) modulating STING-NF-κB/GSDME pathways, nanotechnology platforms (R@AZOH, Nano-CD) coupling CRISPR-based GSDME induction with innate immunity priming, and biomimetic nanoparticles enhancing chemotherapeutic pyroptosis. Synergy with BRAF/MEK inhibitors and immune checkpoint blockade amplifies CD8+ T-cell infiltration via HMGB1-mediated immunogenicity while remodeling immunosuppressive niches. Prognostic pyroptosis scoring (PScore) stratifies patients by immune microenvironment features and therapeutic responsiveness, guiding precision immunotherapy design. Created by Biorender.com.
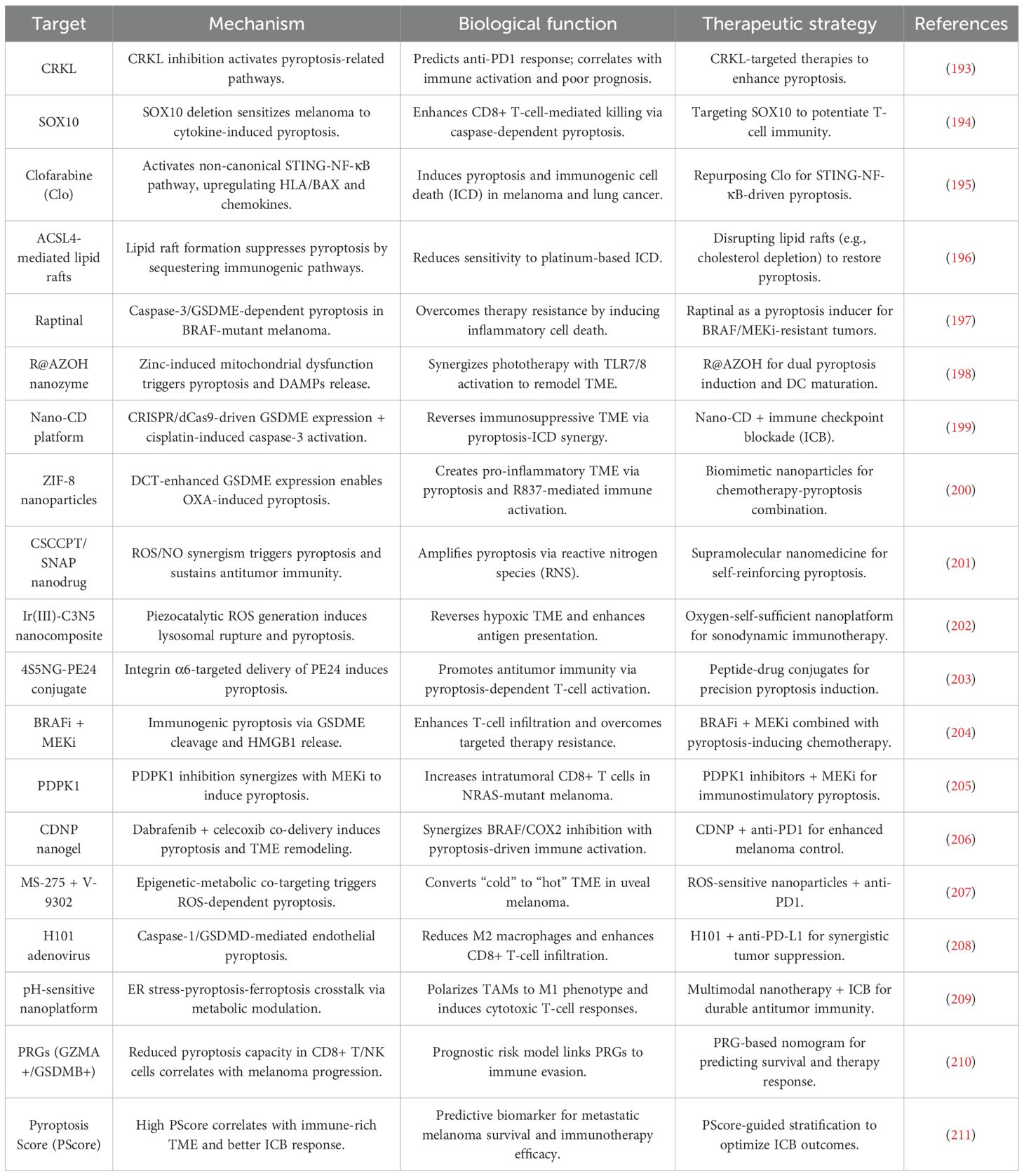
Table 3. Molecular targets and therapeutic strategies for pyroptosis-driven immunotherapy in melanoma.
3.3.1 Molecular mechanisms and regulatory pathways
Pyroptosis, a lytic and inflammatory form of PCD, is intricately regulated by molecular pathways that significantly intersect with tumor immunity in melanoma. One of the key regulators identified is the proto-oncogene C-reaK-tyrosine kinAse like (CRKL), which plays a critical role in melanoma progression. Overexpression of CRKL has been shown to correlate with poor prognosis and reduced responsiveness to anti-PD1 therapy, positioning it as both a prognostic biomarker and a potential therapeutic target. Suppression of CRKL activates pyroptosis-related pathways, highlighting its pivotal role in melanoma immune evasion (193).
Similarly, the transcription factor (Sex Determining Region Y)-box 10 (SOX10) modulates melanoma cell susceptibility to T cell-mediated killing. SOX10 deficiency sensitizes melanoma cells to pyroptosis induced by TNFα and IFNγ, triggering caspase-dependent mechanisms that enhance ICD. This process reshapes the melanoma tumor-immune interaction, promoting the activation of antitumor immunity (194).
Pharmacological agents such as clofarabine (Clo) have been identified as key enhancers of the link between apoptosis and pyroptosis. Clo activates the non-canonical STING-NF-κB pathway, inducing pyroptosis and upregulating HLA molecules and chemokines such as CCL5 and CXCL10. These changes amplify the recruitment and activation of CD8+ T cells, thereby enhancing antitumor immunity (195). Conversely, the formation of lipid rafts through ACSL4 activity has been shown to suppress platinum-induced pyroptosis. Notably, cholesterol depletion can restore ICD, enhancing the efficacy of chemotherapy (196). Collectively, these findings underscore the complex interplay between pyroptosis regulators and immune evasion mechanisms, highlighting potential therapeutic opportunities.
3.3.2 Therapeutic strategies for pyroptosis induction
A variety of innovative therapeutic platforms have been developed to exploit pyroptosis in melanoma treatment. One such approach involves the use of the caspase-3 activator raptinal, which induces pyroptosis in BRAF-mutant melanoma through GSDME cleavage. This mechanism overcomes resistance to BRAF/MEK inhibitors (BRAFi/MEKi) and delays tumor growth, suggesting its potential as a therapeutic strategy in resistant melanoma (197).
Nanotechnology-based strategies, such as R848-loaded AlZn hydroxide (R@AZOH), combine pyroptosis induction with innate immune activation. R@AZOH triggers zinc-dependent mitochondrial dysfunction, promoting the release of DAMPs and supporting DC maturation. This promotes sustained adaptive immunity, further strengthening the antitumor response (198).
CRISPR-based strategies also offer a promising approach to enhancing pyroptosis in melanoma. The Nano-CD platform exploits endogenous GSDME expression via tumor-supplied CRISPR/dCas9 systems. When combined with cisplatin-induced caspase-3 activation, Nano-CD induces robust pyroptosis, reverses the immunosuppressive TME, and synergizes with ICB (199).
Additionally, biomimetic nanoparticles, such as ZIF-8 coated with cancer cell membranes, enhance oxaliplatin delivery and GSDME-mediated pyroptosis by inducing GSDME upregulation through DNA methyltransferase inhibition (200). Advanced nanomedicines like CSCCPT/SNAP, which generate reactive nitrogen species (RNS), also amplify pyroptosis. Furthermore, iridium (III)-C3N5 nanocomposites induce lysosomal rupture and pyroptosis via sonodynamic therapy, effectively overcoming hypoxia and enhancing the therapeutic response (201, 202).
Targeted delivery systems, such as the integrin α6-specific cell-penetrating peptide 4S5NG, enable precise induction of pyroptosis in melanoma cells while sparing normal tissues, thereby increasing the safety and efficacy of treatment (203). These diverse strategies highlight the versatility and potential of pyroptosis-centered approaches in melanoma immunotherapy.
3.3.3 Combination therapies with immunotherapy/targeted agents
The combination of pyroptosis inducers with targeted therapies or ICB has shown transformative potential in melanoma treatment. BRAFi/MEKi induce pyroptosis via GSDME cleavage and HMGB1 release, activating CD8+ T cell-dependent antitumor immunity. However, resistance to BRAFi/MEKi therapy is often associated with diminished pyroptosis markers, suggesting that enhancing pyroptosis could overcome therapeutic escape and improve treatment outcomes (204).
Similarly, PDPK1 inhibition has been shown to synergize with MEK inhibitors in NRAS-mutant melanoma, enhancing pyroptosis and promoting CD8+ T cell infiltration in immunocompetent models (205). Nanotherapeutic platforms, such as the BRAFi/COX2i-loaded nanogel Cyclic di-nucleotide phosphodiesterase (CDNP), induce pyroptosis and remodel the TME, leading to superior tumor control when combined with anti-PD1 therapy (206).
ROS-sensitive nanoparticles, co-delivering HDAC and glutamine metabolism inhibitors, have also been shown to convert “immunologically cold” tumors into “hot” tumors by triggering pyroptosis and enhancing PD-1 blockade efficacy (207). Additionally, viral therapies such as the oncolytic adenovirus H101 induce endothelial pyroptosis via caspase-1/GSDMD activation, synergizing with anti-PD-L1 therapy to suppress tumor growth (208). Pioneering pH-sensitive nanoplatforms demonstrate multimodal induction of pyroptosis and ferroptosis, polarizing tumor-associated macrophages (TAMs) and enhancing ICB responses (209). These studies illustrate the potential of combining pyroptosis with immune modulation to overcome therapeutic resistance and improve treatment outcomes in melanoma.
3.3.4 Prognostic models and TME insights
Single-cell transcriptomic analyses have provided valuable insights into the dysregulated expression of pyroptosis-related genes (PRGs) in melanoma, particularly within CD8+ T and NK cells. The reduced presence of GZMA+ CD8+ T cells and GSDMB+ NK cells in tumors is associated with a diminished pyroptotic capacity, indicating that immune cell pyroptosis plays a role in melanoma progression (210).
The PScore, a computational model integrating PRG expression, has emerged as a potential tool for predicting patient survival and response to ICB therapy in metastatic melanoma. Tumors with a high PScore are characterized by an immune-enriched TME and show better responses to ICB therapy, while low PScore tumors are associated with fibrotic or immune-exhausted microenvironments. Notably, monocytes exhibit the highest PScore, contrasting with malignant cells and fibroblasts, which are resistant to pyroptosis (211). These prognostic models provide a framework for stratifying patients and tailoring pyroptosis-centered immunotherapies, offering a more personalized approach to melanoma treatment.
3.4 Targeting cross-mechanism interactions for immunomodulation in melanoma
The interplay of RCD pathways and metabolic reprogramming offers a multidimensional strategy to enhance antitumor immunity in melanoma. This section explores how synergistic activation of ICD, single-cell-guided insights into tumor heterogeneity, and metabolic interventions reshape the immunosuppressive microenvironment. By integrating these cross-mechanism approaches, novel therapeutic strategies aim to overcome resistance and amplify immunotherapy efficacy. Table 4 summarized these interactions, setting the stage for detailed discussion in the following sections to highlight the synergies and challenges of co-targeting multiple PCD pathways.
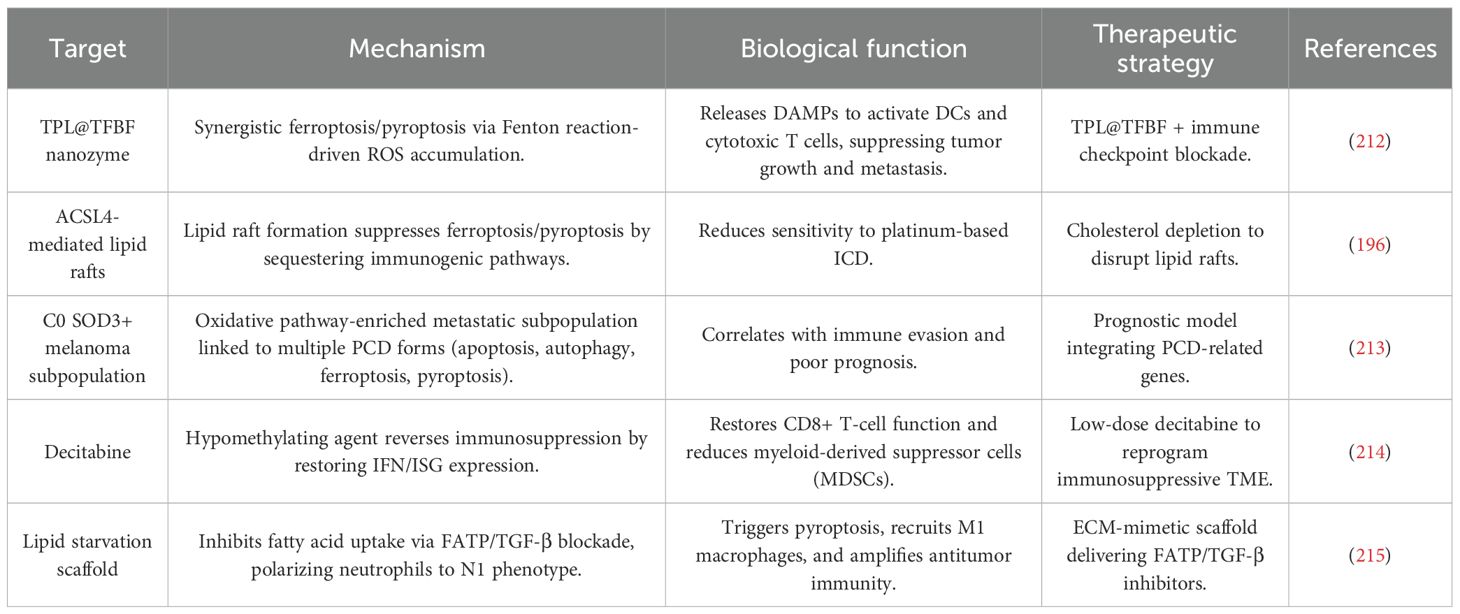
Table 4. Molecular targets and therapeutic strategies for cross-mechanism immunomodulation in melanoma.
3.4.1 The diverse landscape of regulated cell death in melanoma
Melanoma is characterized by its profound resistance to conventional cell death mechanisms, a hallmark of its aggressive nature. While apoptosis, or Type I programmed cell death (PCD), is the most studied pathway, melanoma cells frequently upregulate anti-apoptotic machinery, rendering them insensitive to therapies designed to trigger it. This inherent resistance has driven researchers to explore and exploit non-apoptotic forms of regulated cell death (RCD) as alternative therapeutic avenues. These pathways not only offer direct tumor-killing capabilities but can also be highly immunogenic, fundamentally altering the dialogue between the dying cancer cell and the host immune system (212).
Several non-apoptotic RCD pathways have emerged as critical players in melanoma biology and therapy.
3.4.1.1 Necroptosis
A caspase-independent form of programmed necrosis orchestrated by the RIPK1/RIPK3/MLKL signaling axis. Unlike the immunologically silent process of apoptosis, necroptosis results in cell lysis and the release of damage-associated molecular patterns (DAMPs), which can powerfully stimulate an anti-tumor immune response. Inducing necroptosis has been shown to synergize with immunotherapy to enhance anti-melanoma immunity. For instance, direct intratumoral delivery of MLKL mRNA can inhibit melanoma growth and boost the efficacy of immune checkpoint inhibitors (ICIs).
3.4.1.2 Ferroptosis
An iron-dependent form of cell death characterized by the overwhelming accumulation of lipid peroxides. This pathway is intricately linked to cellular metabolism, particularly lipid and iron homeostasis, making it a unique target in metabolically rewired cancer cells. Ferroptosis has demonstrated significant potential in overcoming therapy resistance in melanoma, including resistance to BRAF inhibitors and immunotherapy. Combining ferroptosis inducers with ICIs like anti-PD-1/PD-L1 antibodies has shown synergistic anti-tumor effects.
3.4.1.3 Pyroptosis
A highly inflammatory form of programmed cell death mediated by the gasdermin protein family, leading to cell swelling, lysis, and the release of pro-inflammatory cytokines. This robust inflammatory response can elicit a strong adaptive immune response, promoting the infiltration of immune cells into the tumor and correlating with a favorable prognosis in melanoma patients.
3.4.1.4 PANoptosis
A more recently described inflammatory cell death pathway that integrates key features of pyroptosis, apoptosis, and necroptosis. This pathway can be activated by the sensor protein ZBP1 and has been shown to repress melanoma growth and improve responsiveness to immune checkpoint blockade.
The ability to trigger these diverse death modalities provides a strategic advantage, allowing for tailored approaches that bypass melanoma’s specific anti-apoptotic defenses (196).
3.4.2 Immunogenic cell death: transforming tumors into endogenous vaccines
The ultimate goal of many modern cancer therapies is not just to kill tumor cells but to do so in a way that stimulates a lasting, systemic anti-tumor immune response. This is the central concept of Immunogenic Cell Death (ICD). ICD is a form of RCD where dying cancer cells release a specific set of DAMPs that act as potent adjuvants, effectively turning the tumor into an in-situ vaccine (213).
The canonical process of ICD involves several key steps: (1) Exposure of “Eat-Me” Signals: Dying cells expose calreticulin (CRT) on their surface, signaling phagocytic cells like dendritic cells (DCs) to engulf them. (2)Release of “Find-Me” Signals: The release of ATP attracts antigen-presenting cells (APCs) to the tumor site. (3)Activation of Immune Sensors: The release of High Mobility Group Box 1 (HMGB1) protein binds to Toll-like receptor 4 (TLR4) on DCs, promoting their maturation and antigen presentation capabilities.
This cascade of events transforms an immunosuppressive tumor microenvironment (TME) into an immunostimulatory one. Mature DCs migrate to draining lymph nodes, where they present tumor antigens to naive T cells, priming a robust, tumor-specific cytotoxic T lymphocyte (CTL) response. These CTLs then infiltrate the tumor, recognize and eliminate remaining cancer cells, and can form a long-term immunological memory.
Therapeutic strategies including certain chemotherapies, radiotherapy, and photodynamic therapy are known ICD inducers. The true power of ICD, however, lies in its synergy with immunotherapy. By generating a fresh wave of tumor-specific T cells, ICD can sensitize “cold” tumors (lacking immune infiltrate) to ICIs like anti-PD-1 and anti-CTLA-4 antibodies, which work by “releasing the brakes” on already-present T cells. This combination can overcome primary and acquired resistance to immunotherapy, a major clinical challenge in melanoma treatment (214).
3.4.3 Metabolic reprogramming: the engine of malignancy and an immunosuppressive force
Disordered metabolism is a core hallmark of cancer, and melanoma is a prime example of a tumor that extensively rewires its metabolic pathways to support rapid proliferation, survival, and metastasis. This metabolic reprogramming, often driven by oncogenic mutations such as in the BRAF gene, is not just a cell-intrinsic process; it profoundly shapes the TME and orchestrates immune evasion.
Melanoma cells exhibit high rates of aerobic glycolysis (the Warburg effect), diverting glucose away from oxidative phosphorylation (OXPHOS) to produce lactate and biosynthetic precursors. This metabolic shift creates a TME that is acidic and nutrient-deprived (e.g., low in glucose and tryptophan), which directly impairs the function of effector immune cells like T cells and NK cells, while favoring the function of immunosuppressive cells like regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs). Beyond glycolysis, melanoma cells also exhibit altered lipid, amino acid, and glutathione metabolism, all of which contribute to both tumor growth and the suppression of antitumor immunity.
This deep connection between metabolism and immunity presents a therapeutic vulnerability. Targeting key metabolic enzymes or pathways is emerging as a promising strategy to not only directly inhibit tumor growth but also to normalize the TME, thereby restoring the efficacy of immune cells and enhancing the effects of immunotherapy (215).
4 Discussion and future direction
The therapeutic landscape for advanced melanoma has been fundamentally reshaped over the past decade by the advent of immunotherapy, particularly immune checkpoint inhibitors (ICIs). Despite these transformative successes, a significant portion of patients exhibit primary or acquired resistance, creating an urgent need for novel strategies that can overcome these limitations (62, 63, 72). A promising new frontier in oncology involves the deliberate induction of specific forms of regulated cell death (RCD) to not only eliminate tumor cells directly but also to potently stimulate a robust and durable anti-tumor immune response.
This report focuses on the emerging paradigm of simultaneously targeting three distinct, non-apoptotic RCD pathways—ferroptosis, pyroptosis, and necroptosis—as a comprehensive strategy to enhance cancer immunotherapy in melanoma. These pathways, once studied in isolation, are now understood to be interconnected components of a broader “cell death network” that can be manipulated to convert an immunologically “cold” tumor microenvironment (TME) into a “hot” one, thereby sensitizing melanoma to immune-mediated destruction (216, 217). We will delve into the mechanistic underpinnings of each pathway, explore the rationale for their combined targeting, analyze the technological advancements facilitating this research, and critically evaluate the significant challenges and future directions on the path to clinical translation (53, 218–221).
Recent studies have demonstrated the potential of combining different forms of PCD, such as ferroptosis, pyroptosis, and necroptosis, to enhance ICD and overcome resistance to conventional therapies. By targeting these pathways simultaneously, researchers have designed multifunctional nanoparticles, small-molecule combinations, and metabolic interventions that not only induce tumor cell death but also reshape the immunosuppressive TME. For example, combining ferroptosis inducers with agents that activate pyroptosis has been shown to release DAMPs and cytokines, which in turn recruit DCs and CTLs, thereby enhancing systemic antitumor immunity (222, 223). Similarly, metabolic interventions that target lipid or iron homeostasis can deplete essential tumor resources while reprogramming immune cells toward pro-inflammatory phenotypes, presenting a promising avenue to augment therapeutic efficacy.
Additionally, cutting-edge technologies such as single-cell omics and spatial transcriptomics have offered an in-depth understanding of melanoma’s heterogeneity and its immune landscape (32, 46, 224–226). These approaches have revealed rare subpopulations of therapy-resistant melanoma cells and highlighted the dynamic shifts in immune cell states during treatment. This high-resolution analysis has facilitated the design of precision therapies aimed at vulnerabilities in both malignant and stromal compartments. Moreover, the repurposing of FDA-approved drugs, such as decitabine and propafenone, to modulate epigenetic and metabolic pathways underscores the potential to translate mechanistic insights into clinically actionable therapies (189, 227, 228).
Despite these promising developments, there are several barriers that need to be addressed before preclinical findings can be successfully translated into clinical practice. One of the key challenges is the incomplete understanding of how the different PCD pathways interact with each other. For instance, LPO, a hallmark of ferroptosis, may inadvertently suppress pyroptosis by altering membrane fluidity or sequestering essential mediators like GSDMs. Additionally, the role of necroptosis in modulating T cell exhaustion or myeloid cell polarization remains unclear, complicating the rational design of combination therapies. Furthermore, most preclinical studies rely on murine models or cell lines that do not fully capture the genetic diversity or immunosuppressive microenvironments of human melanoma, limiting the predictability and relevance of these findings. The lack of standardized models for therapy-resistant niches, such as brain metastases, further complicates the translation of preclinical data into clinical success.
Another significant hurdle lies in the translation of nanotherapy from preclinical studies to clinical use. While nanotechnology platforms, including MOFs and lipid-coated nanoparticles, have shown promising results in preclinical studies (229–232), their clinical application faces challenges such as variability in batch production, poor tumor penetration in desmoplastic tissues, and potential toxicity from metal ion leakage. Additionally, the absence of scalable manufacturing processes for these complex nanocarriers raises concerns regarding cost-effectiveness and regulatory approval. Moreover, current biomarkers, such as PD-L1 expression and TMB, have demonstrated limited predictive value for immunogenic therapies, highlighting the need for the development of more precise biomarkers to guide treatment selection. For example, patients with high PScore may benefit from ICD-inducing agents, but no validated assays currently exist to guide such stratification. The influence of factors such as gut microbiota, systemic metabolism, and host genetics on therapy outcomes remains underexplored, contributing to inconsistent results in clinical trials.
5 Technological and preclinical frontiers
Addressing these complex questions requires sophisticated tools. Cutting-edge technologies such as single-cell omics and spatial transcriptomics have offered an in-depth understanding of melanoma’s heterogeneity and its immune landscape. These approaches have revealed rare subpopulations of therapy-resistant melanoma cells and highlighted the dynamic shifts in immune cell states during treatment. This high-resolution analysis, powered by computational methods like BayesSpace for clustering spatial data, has facilitated the design of precision therapies aimed at vulnerabilities in both malignant and stromal compartments. However, a key challenge remains in integrating multi-modal data to enhance resolution, as spatial transcriptomics alone can sometimes lack single-cell precision.
Artificial intelligence (AI) and machine learning (ML) are also poised to play a transformative role. AI/ML algorithms can be applied to analyze vast datasets to predict the efficacy of novel drug combinations, identify new therapeutic targets, and optimize treatment regimens. For example, neural networks can be trained to predict cell viability and dose-response curves for complex combination therapies, guiding preclinical development. This computational power will be essential for navigating the immense complexity of targeting multiple RCD pathways simultaneously in heterogeneous patient populations.
6 Future directions and the path to clinical translation
The journey from compelling preclinical concepts to effective clinical therapies is fraught with challenges that must be systematically addressed.
6.1 Developing predictive biomarkers and optimizing clinical trials
A significant barrier to clinical translation is the lack of biomarker panels to monitor patient response to therapies targeting multiple RCD pathways. Current clinical protocols for melanoma incorporate biomarkers for targeted therapy (e.g., BRAF mutation status) and immunotherapy (e.g., PD-L1 expression) but none are established for this novel approach. Developing assays to measure the activation state of ferroptosis, pyroptosis, and necroptosis within tumors is a critical first step for personalizing treatment and assessing therapeutic efficacy.
Furthermore, clinical trial designs must be adapted to evaluate these complex combination strategies. Traditional linear trial designs may be inadequate. Instead, adaptive trial designs, such as Sequential, Multiple Assignment, Randomized Trial (SMART) designs, will be necessary to flexibly test different combinations, doses, and schedules based on emerging data, thereby optimizing the path to regulatory approval.
6.2 Overcoming manufacturing and delivery hurdles
For strategies involving multifunctional nanoparticles, significant translational barriers exist, including challenges in manufacturing at scale, ensuring batch-to-batch consistency, predicting and managing toxicity, and overcoming biological barriers to ensure effective tumor delivery. The gap between promising results in preclinical animal models and outcomes in human patients remains a major hurdle for nanomedicine in general.
6.3 Expanding the mechanistic framework to include the microbiome
The gut microbiome has emerged as a critical modulator of response to immunotherapy in melanoma patients. However, its role in modulating the response to therapies that induce ferroptosis, pyroptosis, and necroptosis is entirely unknown. Future research must investigate whether the microbiome influences the activation of these RCD pathways or the subsequent immune response, potentially opening avenues for microbiome-based co-therapies to enhance treatment efficacy (233, 234).
7 Conclusion
The strategy of targeting ferroptosis, pyroptosis, and necroptosis in concert represents a paradigm shift in cancer immunotherapy for melanoma. It moves beyond single-target approaches to embrace the complexity of tumor biology, aiming to induce a multi-faceted, immunologically potent form of cell death that can overcome intrinsic and acquired resistance. While the preclinical rationale is strong and supported by emerging evidence, the path forward requires a concerted, interdisciplinary effort. Key priorities must include elucidating the intricate molecular cross-talk between these pathways, developing robust biomarkers for patient selection and response monitoring, and designing innovative clinical trials capable of navigating the complexities of combination therapies. By addressing these fundamental challenges, the immense therapeutic potential of this triad approach may be unlocked, offering new hope for patients with advanced melanoma.
Author contributions
ZS: Writing – original draft, Writing – review & editing. FL: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
We thank the generous support by Liaoning Cancer Hospital & Institute (Shenyang) and Dalian University of Technology (Dalian).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
IL-2: interleukin-2
ICIs: immune checkpoint inhibitors
BRAF: B-Raf proto-oncogene, serine/threonine kinase
MEK: Mitogen-Activated Protein Kinase
CTLA-4: Cytotoxic T-Lymphocyte Antigen 4
TMB: tumor mutational burden
irAEs: immune-related adverse events
PCD: Programmed cell death
DAMPs: damage-associated molecular patterns
DCs: dendritic cells
CTLs: cytotoxic T lymphocytes
RIPK1: Receptor-Interacting Protein Kinase 1
HMGB1: High Mobility Group Box 1
ICD: immunogenic cell death
TME: tumor microenvironment
Tregs: regulatory T cells
MDSCs: myeloid-derived suppressor cells
RCD: Regulated Cell Death
TFRC: transferrin receptor
ROS: reactive oxygen species
ALOXs: arachidonate lipoxygenases
PUFAs: polyunsaturated fatty acids
PL-PUFA-OOH: phospholipid hydroperoxides
ACSL4: Acyl-CoA Synthetase Long Chain Family Member 4
LPCAT3: Lysophosphatidylcholine Acyltransferase 3
GSH: glutathione
PDAC: pancreatic ductal adenocarcinoma
cTRIP12: circTRIP12
OGT: O-GlcNAc transferase
FTH: ferritin heavy chain
KEAP1: Kelch-like ECH-associated protein 1
SCLC: small cell lung cancer
GBM: glioblastoma
HCC: Hepatocellular carcinoma
MLKL: mixed-lineage kinase domain-like
CYLD: Cylindromatosis
ZBP1: Z-DNA Binding Protein 1
EMT: epithelial-mesenchymal transition
HNSCC: head and neck squamous cell carcinoma
NSCLC: non-small cell lung cancer
GSDM: gasdermin
GSDMD: Gasdermin D
NK: natural killer
ICB: immune checkpoint blockade
TNBC: triple-negative breast cancer
dsRNA: double-stranded RNA
RIDD: regulated IRE1-dependent decay
GC: gastric cancer
NLRP3: NLR Family Pyrin Domain Containing 3
SLC7A11: Solute Carrier Family 7 Member 11
Gpx4: GSH peroxidase 4
ART: artemisinin
FFAs: free fatty acids
CAMKK2: Calcium/Calmodulin-Dependent Protein Kinase Kinase 2
zVAD-fmk: Z-Val-Ala-Asp(OMe)-FMK
IFNγ: interferon-γ
ADAR1: Adenosine Deaminase Acting on RNA 1
ZBP1: Z-DNA Binding Protein 1
ISGs: interferon-stimulated genes
HMOX1: heme oxygenase 1
PScore: pyroptosis score
CRKL: C-reaK-tyrosine kinAse like
SOX10: (Sex Determining Region Y)-box 10
Clo: clofarabine
R@AZOH: R848-loaded AlZn hydroxide
RNS: reactive nitrogen species
BRAFi: BRAF inhibitors
MEKi: MEK inhibitors
CDNP: Cyclic di-nucleotide phosphodiesterase
TAMs: tumor-associated macrophages
PRGs: pyroptosis-related genes
MOF: metal-organic framework
LPO: lipid peroxidation
scRNA-seq: single-cell RNA sequencing
GEMM: genetically engineered mouse model
IFN: interferon
ISGs: IFN-stimulated genes
ECM: extracellular matrix
FATP: fatty acid transport protein
TANs: tumor-associated neutrophils
TILs: tumor-infiltrating lymphocytes
References
1. Long GV, Swetter SM, Menzies AM, Gershenwald JE, and Scolyer RA. Cutaneous melanoma. Lancet. (2023) 402:485–502. doi: 10.1016/S0140-6736(23)00821-8
2. Haggenmuller S, Wies C, Abels J, Winterstein JT, Heinlein L, Nogueira Garcia C, et al. Discordance, accuracy and reproducibility study of pathologists’ diagnosis of melanoma and melanocytic tumors. Nat Commun. (2025) 16:789. doi: 10.1038/s41467-025-56160-x
3. Kasheri E, Sa B, Kraft G, Vattigunta M, Scheinkman R, and Nouri K. Mapping melanoma mortality hot spots: A geographic spatial analysis of socioeconomic and provider disparities. J Am Acad Dermatol. (2025) 93:477–9. doi: 10.1016/j.jaad.2025.04.045
4. Aristizabal MA, Evans LA, Pincelli TP, Degesys CA, and Barbosa NS. Cutaneous melanoma trends and disparities in Florida: A decade of incidence, mortality, and workforce data. J Am Acad Dermatol. (2025) 92:1452–4. doi: 10.1016/j.jaad.2025.02.054
5. Whiteman DC. Melanoma in England: incidence is up, mortality is down. Br J Dermatol. (2025) 193:588. doi: 10.1093/bjd/ljaf175
6. Schurink AW, Verhoef C, Waalboer-Spuij R, Mooyaart A, and Grunhagen DJ. Evaluation of residual tumour in wide local excision for melanoma: A nationwide population-based study. Eur J Cancer. (2025) 220:115364. doi: 10.1016/j.ejca.2025.115364
7. Garbe C, Amaral T, Peris K, Hauschild A, Arenberger P, Basset-Seguin N, et al. R. @ the European Organization for, C. Treatment of, European consensus-based interdisciplinary guideline for melanoma. Part 2: Treat - Update 2024 Eur J Cancer. (2025) 215:115153. doi: 10.1016/j.ejca.2024.115153
8. Green AC, Hughes MCB, Williams GM, Malt M, Beesley VL, Khosrotehrani K, et al. Increased melanoma recurrence in patients with multiple primary invasive melanomas. J Am Acad Dermatol. (2022) 86:1366–9. doi: 10.1016/j.jaad.2021.05.025
9. Davar D, Morrison RM, Dzutsev AK, Karunamurthy A, Chauvin JM, Amatore F, et al. Neoadjuvant vidutolimod and nivolumab in high-risk resecta ble melanoma: A prospective phase II trial. Cancer Cell. (2024) 42:1898–1918 e12. doi: 10.1016/j.ccell.2024.10.007
10. Martin-Lluesma S, Dafni U, Vervita K, Karlis D, Dimopoulou G, Tsourti Z, et al. Safety of adoptive therapy with tumor-infiltrating lymphocytes and high-dose recombinant interleukin-2 in advanced cutaneous melanoma: a systematic review and meta-analysis. Ann Oncol. (2025) 36:909–19. doi: 10.1016/j.annonc.2025.04.001
11. Martin-Lluesma S, Svane IM, Dafni U, Vervita K, Karlis D, Dimopoulou G, et al. Efficacy of TIL therapy in advanced cutaneous melanoma in the current immuno-oncology era: updated systematic review and meta-analysis. Ann Oncol. (2024) 35:860–72. doi: 10.1016/j.annonc.2024.07.723
12. Carvajal RD, Sacco JJ, Jager MJ, Eschelman DJ, Olofsson Bagge R, Harbour JW, et al. Advances in the clinical management of uveal melanoma. Nat Rev Clin Oncol. (2023) 20:99–115. doi: 10.1038/s41571-022-00714-1
13. Shen H, Huang F, Zhang X, Ojo OA, Li Y, Trummell HQ, et al. Selective suppression of melanoma lacking IFN-gamma pathway by JAK inhibition depends on T cells and host TNF signaling. Nat Commun. (2022) 13:5013. doi: 10.1038/s41467-022-32754-7
14. Tasdogan A, Sullivan RJ, Katalinic A, Lebbe C, Whitaker D, Puig S, et al. Cutaneous melanoma. Nat Rev Dis Primers. (2025) 11:23. doi: 10.1038/s41572-025-00603-8
15. Desaunay M and Poulikakos PI. Overcoming melanoma therapy resistance with RAF-MEK and FAK inhibition. Cancer Cell. (2025) 43:330–2. doi: 10.1016/j.ccell.2025.02.012
16. Weiss SA, Djureinovic D, Wei W, Tran T, Austin M, Markowitz J, et al. Phase II trial of pembrolizumab in combination with bevacizumab for untreated melanoma brain metastases. J Clin Oncol. (2025) 43:JCO2402219. doi: 10.1200/JCO-24-02219
17. Liang L, Kuang X, He Y, Zhu L, Lau P, Li X, et al. Alterations in PD-L1 succinylation shape anti-tumor immune responses in melanoma. Nat Genet. (2025) 57:680–93. doi: 10.1038/s41588-025-02077-6
18. Klobuch S, Seijkens TTP, Schumacher TN, and Haanen J. Tumour-infiltrating lymphocyte therapy for patients with advanced-stage melanoma. Nat Rev Clin Oncol. (2024) 21:173–84. doi: 10.1038/s41571-023-00848-w
19. Borgers JSW, Lenkala D, Kohler V, Jackson EK, Linssen MD, Hymson S, et al. Personalized, autologous neoantigen-specific T cell therapy in metastatic melanoma: a phase 1 trial. Nat Med. (2025) 31:881–93. doi: 10.1038/s41591-024-03418-4
20. Shalhout SZ, Miller DM, Emerick KS, and Kaufman HL. Therapy with oncolytic viruses: progress and challenges. Nat Rev Clin Oncol. (2023) 20:160–77. doi: 10.1038/s41571-022-00719-w
21. Dummer R, Robert C, Scolyer RA, Taube JM, Tetzlaff MT, Menzies AM, et al. Neoadjuvant anti-PD-1 alone or in combination with anti-TIGIT or an oncolytic virus in resecta ble stage IIIB-D melanoma: a phase 1/2 trial. Nat Med. (2025) 31:144–51. doi: 10.1038/s41591-024-03411-x
22. Moura LI, Malfanti A, Matos AI, Peres C, Arminan A, Duro-Castano A, et al. Off-the-shelf multivalent nanoconjugate cancer vaccine rescues host immune response against melanoma. Adv Mater. (2025) 37:e2417348. doi: 10.1002/adma.202417348
23. Cheng Q, Zhang T, Wang Q, Wu X, Li L, Lin R, et al. Photocatalytic carbon dots-triggered pyroptosis for whole cancer cell vaccines. Adv Mater. (2024) 36:e2408685. doi: 10.1002/adma.202408685
24. Menzies AM, Lo SN, Saw RPM, Gonzalez M, Ch’ng S, Nieweg OE, et al. Five-year analysis of neoadjuvant dabrafenib and trametinib for stage III melanoma. Ann Oncol. (2024) 35:739–46. doi: 10.1016/j.annonc.2024.05.002
25. Long GV, Hauschild A, Santinami M, Kirkwood JM, Atkinson V, Mandala M, et al. Final results for adjuvant dabrafenib plus trametinib in stage III melanoma. N Engl J Med. (2024) 391:1709–20. doi: 10.1056/NEJMoa2404139
26. Lubrano S, Cervantes-Villagrana RD, Faraji F, Ramirez S, Sato K, Adame-Garcia SR, et al. FAK inhibition combined with the RAF-MEK clamp avutometinib overcomes resistance to targeted and immune therapies in BRAF V600E melanoma. Cancer Cell. (2025) 43:428–445 e6. doi: 10.1016/j.ccell.2025.02.001
27. Wu S, Deng Y, Sun H, Liu X, Zhou S, Zhao H, et al. BRAF inhibitors enhance erythropoiesis and treat anemia through paradoxical activation of MAPK signaling. Signal Transduct Target Ther. (2024) 9:338. doi: 10.1038/s41392-024-02033-6
28. Borbenyi-Galambos K, Erdelyi K, Ditroi T, Juranyi EP, Szanto N, Szatmari R, et al. Realigned transsulfuration drives BRAF-V600E-targeted therapy resistance in melanoma. Cell Metab. (2025) 37:1171–1188 e9. doi: 10.1016/j.cmet.2025.01.021
29. Ponzone L, Audrito V, Landi C, Moiso E, Levra Levron C, Ferrua S, et al. RICTOR/mTORC2 downregulation in BRAF(V600E) melanoma cells promotes resistance to BRAF/MEK inhibition. Mol Cancer. (2024) 23:105. doi: 10.1186/s12943-024-02010-1
30. Blank CU, Lucas MW, Scolyer RA, Wiel BA, Menzies AM, Lopez-Yurda M, et al. Neoadjuvant nivolumab and ipilimumab in resectable stage III melanoma. N Engl J Med. (2024) 391:1696–708. doi: 10.1056/NEJMoa2402604
31. Long GV, Carlino MS, McNeil C, Ribas A, Gaudy-Marqueste C, Schachter J, et al. Pembrolizumab versus ipilimumab for advanced melanoma: 10-year follow-up of the phase III KEYNOTE-006 study. Ann Oncol. (2024) 35:1191–9. doi: 10.1016/j.annonc.2024.08.2330
32. Wang K, Coutifaris P, Brocks D, Wang G, Azar T, Solis S, et al. Combination anti-PD-1 and anti-CTLA-4 therapy generates waves of clonal responses that include progenitor-exhausted CD8(+) T cells. Cancer Cell. (2024) 42:1582–1597 e10. doi: 10.1016/j.ccell.2024.08.007
33. Wolchok JD, Chiarion-Sileni V, Rutkowski P, Cowey CL, SChadendorf D, Wagstaff J, et al. Final, 10-year outcomes with nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. (2025) 392:11–22. doi: 10.1056/NEJMoa2407417
34. Long GV, Carlino MS, Au-Yeung G, Spillane AJ, Shannon KF, Gyorki DE, et al. Neoadjuvant pembrolizumab, dabrafenib and trametinib in BRAF(V600)-mutant resectab le melanoma: the randomized phase 2 NeoTrio trial. Nat Med. (2024) 30:2540–8. doi: 10.1038/s41591-024-03077-5
35. Sun L, Wei X, Zhao Q, Mao L, Bai X, Li C, et al. Dynamic change of PD-L2 on circulating plasma extracellular vesicles as a predictor of treatment response in melanoma patients receiving anti-PD-1 therapy. J Extracell Vesicles. (2025) 14:e70054. doi: 10.1002/jev2.70054
36. Wermke M, Araujo DM, Chatterjee M, Tsimberidou AM, Holderried TAW, Jazaeri AA, et al. Autologous T cell therapy for PRAME(+) advanced solid tumors in HLA-A*02(+) patients: a phase 1 trial. Nat Med. (2025) 31:2365–74. doi: 10.1038/s41591-025-03650-6
37. Srivastava J, Yadav VK, Jimenez RV, Phadatare PR, Inamdar NA, Young MM, et al. Blocking nitrosylation induces immunogenic cell death by sensitizing NRAS-mutant melanoma to MEK inhibitors. Cancer Res. (2025) 85:2268–87. doi: 10.1158/0008-5472.29326849
38. Tao H, Jin C, Zhou L, Deng Z, Li X, Dang W, et al. PRMT1 inhibition activates the interferon pathway to potentiate antitumor immunity and enhance checkpoint blockade efficacy in melanoma. Cancer Res. (2024) 84:419–33. doi: 10.1158/0008-5472.CAN-23-1082
39. Monberg TJ, Pakola SA, Albieri B, Ellebaek E, Donia M, Eefsen RL, et al. Safety and efficacy of combined treatment with tumor-infiltrating lymphocytes and oncolytic adenovirus TILT-123 in metastatic melanoma. Cell Rep Med. (2025) 6:102016. doi: 10.1016/j.xcrm.2025.102016
40. Xu QH, Yin XY, Chen ZQ, Huang EK, Yao X, Li X, et al. Construction of in situ personalized cancer vaccines by bioorthogonal catalytic microneedles for augmented melanoma immunotherapy. Small. (2025) 21:e2500015. doi: 10.1002/smll.202500015
41. Kim YA, Jeong H, Kim H, Lee S, Kim KS, and Na K. Lipid nanoparticles with prazole adjuvant to enhance the efficacy of mRNA cancer vaccines. J Control Release. (2025) 383:113756. doi: 10.1016/j.jconrel.2025.113756
42. van Dorst DCH, Uyl TJJ, van der Veldt AAM, Andrawes T, Joosse A, Oomen-De Hoop E, et al. Onset and progression of atherosclerosis in patients with melanoma treated with immune checkpoint inhibitors. J Immunother Cancer. (2025) 13:e011226. doi: 10.1136/jitc-2024-011226
43. Kohanbash G, Frederico SC, and Raphael I. NK cells link immune-checkpoint blockade immunotherapy and response in melanoma brain metastases. J Immunother Cancer. (2025) 13:e011581. doi: 10.1136/jitc-2025-011581
44. Takahashi H, Perez-Villarroel P, Falahat R, and Mule JJ. Targeting MARCO in combination with anti-CTLA-4 leads to enhanced melanoma regression and immune cell infiltration via macrophage reprogramming. J Immunother Cancer. (2025) 13:e011030. doi: 10.1136/jitc-2024-011030
45. Tenstad HB, Ruhlmann CH, Moller S, Kjaer S, Bastholt L, Just SA, et al. Poorer survival for patients with inflammatory arthritis treated with immune checkpoint inhibitors for melanoma. J Autoimmun. (2025) 152:103400. doi: 10.1016/j.jaut.2025.103400
46. Cohen Shvefel S, Pai JA, Cao Y, Pal LR, Bartok O, Levy R, et al. Temporal genomic analysis of homogeneous tumor models reveals key regulators of immune evasion in melanoma. Cancer Discov. (2024). doi: 10.1158/2159-8290
47. Robertson BM, Fane ME, Weeraratna AT, and Rebecca VW. Determinants of resistance and response to melanoma therapy. Nat Cancer. (2024) 5:964–82. doi: 10.1038/s43018-024-00794-1
48. Liu S, Dharanipragada P, Lomeli SH, Wang Y, Zhang X, Yang Z, et al. Multi-organ landscape of therapy-resistant melanoma. Nat Med. (2023) 29:1123–34. doi: 10.1038/s41591-023-02304-9
49. Schiantarelli J, Benamar M, Park J, Sax HE, Oliveira G, Bosma-Moody A, et al. Genomic mediators of acquired resistance to immunotherapy in metastatic melanoma. Cancer Cell. (2025) 43:308–316.e6. doi: 10.1016/j.ccell.2025.01.009
50. Verheijden RJ, de Groot JS, Fabriek BO, Hew MN, May AM, and Suijkerbuijk KPM. Corticosteroids for immune-related adverse events and checkpoint inhibitor efficacy: analysis of six clinical trials. J Clin Oncol. (2024) 42:3713–24. doi: 10.1200/JCO.24.00191
51. Hijaz BA, Braun N, Reynolds KL, Semenov YR, LeBoeuf NR, and Chen ST. Nivolumab/relatlimab-associated cutaneous immune-related adverse events in patients treated for advanced melanoma: A multicenter retrospective cohort study. J Am Acad Dermatol. (2025) 93:250–2. doi: 10.1016/j.jaad.2025.02.080
52. Hoeijmakers LL, Reijers ILM, and Blank CU. Biomarker-driven personalization of neoadjuvant immunotherapy in melanoma. Cancer Discov. (2023) 13:2319–38. doi: 10.1158/2159-8290.CD-23-0352
53. Pozniak J and Marine JC. Decoding melanoma’s cellular mosaic to unlock immunotherapy potential. Trends Cell Biol. (2025). doi: 10.1016/j.tcb.2025.01.009
54. Qian S, Long Y, Tan G, Li X, Xiang B, Tao Y, et al. Programmed cell death: molecular mechanisms, biological functions, diseases, and therapeutic targets. MedComm (2020). (2024) 5:e70024. doi: 10.1002/mco2.70024
55. Bosch M and Franklin-Tong V. Regulating programmed cell death in plant cells: Intracellular acidification plays a pivotal role together with calcium signaling. Plant Cell. (2024) 36:4692–702. doi: 10.1093/plcell/koae245
56. Wang Y, Weng L, Wu X, and Du B. The role of programmed cell death in organ dysfunction induced by opportunistic pathogens. Crit Care. (2025) 29:43. doi: 10.1186/s13054-025-05278-x
57. Chen Y, Li X, Yang M, and Liu SB. Research progress on morphology and mechanism of programmed cell death. Cell Death Dis. (2024) 15:327. doi: 10.1038/s41419-024-06712-8
58. Gao Y, Lin H, Tang T, Wang Y, Chen W, and Li L. Circular RNAs in programmed cell death: Regulation mechanisms and potential clinical applications in cancer: A review. Int J Biol Macromol 280(Pt. (2024) 2):135659. doi: 10.1016/j.ijbiomac.2024.135659
59. Qian S, Tan G, Lei G, Zhang X, and Xie Z. Programmed cell death in nasopharyngeal carcinoma: Mechanisms and therapeutic targets. Biochim Biophys Acta Rev Cancer. (2025) 1880:189265. doi: 10.1016/j.bbcan.2025.189265
60. Wei C, Liu M, and Zhang W. Programmed cell death protein 1 in cancer cells. Cell Commun Signal. (2025) 23:185. doi: 10.1186/s12964-025-02155-6
61. Man SM and Kanneganti TD. Innate immune sensing of cell death in disease and therapeutics. Nat Cell Biol. (2024) 26:1420–33. doi: 10.1038/s41556-024-01491-y
62. Nadendla EK, Tweedell RE, Kasof G, and Kanneganti TD. Caspases: structural and molecular mechanisms and functions in cell death. innate immunity disease Cell Discov. (2025) 11:42. doi: 10.1038/s41421-025-00791-3
63. Min R, Bai Y, Wang NR, and Liu X. Gasdermins in pyroptosis, inflammation, and cancer. Trends Mol Med. (2025) 31:860–75. doi: 10.1016/j.molmed.2025.04.003
64. Chen P, Wang H, Tang Z, Shi J, Cheng L, Zhao C, et al. Selective depletion of CCR8+Treg cells enhances the antitumor immunity of cytotoxic T cells in lung cancer by dendritic cells. J Thorac Oncol. (2025) 20:1050–74. doi: 10.1016/j.jtho.2025.02.029
65. Meng H, Wang J, Wen H, Xu Z, Luo L, Lin W, et al. Evoking simultaneous ferroptosis and apoptosis by a dual-locked platinum (IV) prodrug for synergistic chemo-immunotherapy. Angew Chem Int Ed Engl. (2025) e202505930. doi: 10.1002/anie.202505930
66. Yan Z, Bai Y, Zhang S, Kong L, Wang Y, Sun H, et al. Quasi Fe MIL-53 nanozyme inducing ferroptosis and immunogenic cell death for cancer immunotherapy. Nat Commun. (2025) 16:2290. doi: 10.1038/s41467-025-57542-x
67. Reddy NR, Maachi H, Xiao Y, Simic MS, Yu W, Tonai Y, et al. Engineering synthetic suppressor T cells that execute locally targeted immunoprotective programs. Science. (2024) 386:eadl4793. doi: 10.1126/science.adl4793
68. Jiang X, Stockwell BR, and Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. (2021) 22:266–82. doi: 10.1038/s41580-020-00324-8
69. Hadian K and Stockwell BR. SnapShot: ferroptosis. Cell. (2020) 181:1188–1188 e1. doi: 10.1016/j.cell.2020.04.039
70. Sun S, Shen J, Jiang J, Wang F, and Min J. Targeting ferroptosis opens new avenues for the development of novel therapeutics. Signal Transduct Target Ther. (2023) 8:372. doi: 10.1038/s41392-023-01606-1
71. Zheng J and Conrad M. Ferroptosis: when metabolism meets cell death. Physiol Rev. (2025) 105:651–706. doi: 10.1152/physrev.00031.2024
72. Huang C, Li J, Wu R, Li Y, and Zhang C. Targeting pyroptosis for cancer immunotherapy: mechanistic insights and clinical perspectives. Mol Cancer. (2025) 24:131. doi: 10.1186/s12943-025-02344-4
73. Weinlich R, Oberst A, Beere HM, and Green DR. Necroptosis in development, inflammation and disease. Nat Rev Mol Cell Biol. (2017) 18:127–36. doi: 10.1038/nrm.2016.149
74. Simpson J, Loh Z, Ullah MA, Lynch JP, Werder RB, Collinson N, et al. Respiratory syncytial virus infection promotes necroptosis and HMGB1 release by airway epithelial cells. Am J Respir Crit Care Med. (2020) 201:1358–71. doi: 10.1164/rccm.201906-1149OC
75. Kamiya M, Mizoguchi F, Kawahata K, Wang D, Nishibori M, Day J, et al. Targeting necroptosis in muscle fibers ameliorates inflammatory myopathies. Nat Commun. (2022) 13:166. doi: 10.1038/s41467-021-27875-4
76. Luo S, Zeng Y, Chen B, Yan J, Ma F, Zhuang G, et al. GPX4 cooperatively protect treg cells from ferroptosis and alleviate intestinal inflammatory damage in necrotizing enterocolitis. Redox Biol. (2024) 75:103303. doi: 10.1016/j.redox.2024.103303
77. Kim R, Hashimoto A, Markosyan N, Tyurin VA, Tyurina YY, Kar G, et al. Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature. (2022) 612:338–46. doi: 10.1038/s41586-022-05443-0
78. Watanabe-Kusunoki K, Li C, Bandeira Honda TS, Zhao D, Kusunoki Y, Ku J, et al. Gasdermin D drives focal crystalline thrombotic microangiopathy by accelerating immunothrombosis and necroinflammation. Blood. (2024) 144:308–22. doi: 10.1182/blood.2023021949
79. Qin W, Qiao L, Wang Q, Gao M, Zhou M, Sun Q, et al. Advancing precision: A controllable self-synergistic nanoplatform initiating pyroptosis-based immunogenic cell death cascade for targeted tumor therapy. ACS Nano. (2024) 18:1582–98. doi: 10.1021/acsnano.3c09499
80. Gao F, Qiu X, Baddi S, He S, Wang S, Zhao C, et al. Chiral nanofibers of camptothecin trigger pyroptosis for enhanced immunotherapy. Angew Chem Int Ed Engl. (2025) 64:e202423446. doi: 10.1002/anie.202423446
81. Liu Q, Ma T, Zhang Z, Nan J, Liu G, Yang Y, et al. Fused extracellular vesicles from M(2) macrophages and human umbilical cord mesenchymal stem cells for the targeted regulation of macrophage pyroptosis in periprosthetic osteolysis. J Extracell Vesicles. (2024) 13:e70028. doi: 10.1002/jev2.70028
82. Shi W, Feng W, Li S, Cui Y, Liu S, Jiang H, et al. Ferroptosis and necroptosis produced autologous tumor cell lysates co-delivering with combined immnoadjuvants as personalized in situ nanovaccines for antitumor immunity. ACS Nano. (2023) 17:14475–93. doi: 10.1021/acsnano.3c00901
83. Xiang Q, Yang X, Zhang Z, Yang J, Li Y, Du J, et al. Fe/mo-based lipid peroxidation nanoamplifier combined with adenosine immunometabolism regulation to augment anti-breast cancer immunity. Adv Mater. (2025) 37:e2419120. doi: 10.1002/adma.202419120
84. Mou Y, Wang J, Wu J, He D, Zhang C, Duan C, et al. Ferroptosis, a new form of cell death: opportunities and challenges in cancer, J. Hematol Oncol. (2019) 12:34. doi: 10.1186/s13045-019-0720-y
85. Tang D, Chen X, Kang R, and Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. (2021) 31:107–25. doi: 10.1038/s41422-020-00441-1
86. Liang C, Zhang X, Yang M, and Dong X. Recent progress in ferroptosis inducers for cancer therapy. Adv Mater. (2019) 31:e1904197. doi: 10.1002/adma.201904197
87. Liang D, Minikes AM, and Jiang X. Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol Cell. (2022) 82:2215–27. doi: 10.1016/j.molcel.2022.03.022
88. Caneque T, Baron L, Muller S, Carmona A, Colombeau L, Versini A, et al. Activation of lysosomal iron triggers ferroptosis in cancer, Nature. (2025) 642:492–500. doi: 10.1038/s41586-025-08974-4
89. Yapici FI, Seidel E, Dahlhaus A, Weber J, Schmidt C, Filho A, et al. An atlas of ferroptosis-induced secretomes. Cell Death Differ. (2025). doi: 10.1038/s41418-025-01517-4
90. Zhu H, Liu Q, Meng Q, Zhang L, Ju S, Lang J, et al. CCT3/ACTN4/TFRC axis protects hepatocellular carcinoma cells from ferroptosis by inhibiting iron endocytosis. J Exp Clin Cancer Res. (2024) 43:245. doi: 10.1186/s13046-024-03169-7
91. Gong F, Zheng X, Xu W, Xie R, Liu W, Pei L, et al. H3K14la drives endothelial dysfunction in sepsis-induced ARDS by promoting SLC40A1/transferrin-mediated ferroptosis. MedComm (2020). (2025) 6:e70049. doi: 10.1002/mco2.70049
92. Zhou X, Wang Y, Li X, Zhou J, Yang W, Wang X, et al. O-GlcNAcylation regulates the stability of transferrin receptor (TFRC) to control the ferroptosis in hepatocellular carcinoma cells. Redox Biol. (2024) 73:103182. doi: 10.1016/j.redox.2024.103182
93. Park E and Chung SW. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. (2019) 10:822. doi: 10.1038/s41419-019-2064-5
94. Xiang Z, Zhang P, Jia C, Xu R, Cao D, Xu Z, et al. Piezo1 channel exaggerates ferroptosis of nucleus pulposus cells by mediating mechanical stress-induced iron influx. Bone Res. (2024) 12:20. doi: 10.1038/s41413-024-00317-9
95. Yang X, Chen Y, Guo J, Li J, Zhang P, Yang H, et al. Polydopamine nanoparticles targeting ferroptosis mitigate intervertebral disc degeneration via reactive oxygen species depletion, iron ions chelation, and GPX4 ubiquitination suppression. Adv Sci (Weinh). (2023) 10:e2207216. doi: 10.1002/advs.202207216
96. Zou Y, Henry WS, Ricq EL, Graham ET, Phadnis VV, Maretich P, et al. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature. (2020) 585:603–8. doi: 10.1038/s41586-020-2732-8
97. Qiu B, Zandkarimi F, Bezjian CT, Reznik E, Soni RK, Gu W, et al. Phospholipids with two polyunsaturated fatty acyl tails promote ferroptosis. Cell. (2024) 187:1177–1190 e18. doi: 10.1016/j.cell.2024.01.030
98. Zhang Y, Jing Y, He J, Dong R, Li T, Li F, et al. Bile acid receptor FXR promotes intestinal epithelial ferroptosis and subsequent ILC3 dysfunction in neonatal necrotizing enterocolitis. Immunity. (2025) 58:683–700.e10. doi: 10.1016/j.immuni.2025.02.003
99. Rousselle A, Lodka D, Sonnemann J, Kling L, Kettritz R, and Schreiber A. Endothelial but not systemic ferroptosis inhibition protects from antineutrophil cytoplasmic antibody-induced crescentic glomerulonephritis, Kidney Int. (2025) 107:1037–50. doi: 10.1016/j.kint.2025.02.023
100. Freitas-Cortez MA, Masrorpour F, Jiang H, Mahmud I, Lu Y, Huang A, et al. Cancer cells avoid ferroptosis induced by immune cells via fatty acid binding proteins. Mol Cancer. (2025) 24:40. doi: 10.1186/s12943-024-02198-2
101. Liu X, Chen Z, Yan Y, Zandkarimi F, Nie L, Li Q, et al. Proteomic analysis of ferroptosis pathways reveals a role of CEPT1 in suppressing ferroptosis. Protein Cell. (2024) 15:686–703. doi: 10.1093/procel/pwae004
102. Zhang M, Zheng H, Zhu X, Liu S, Jin H, Chen Y, et al. Synchronously evoking disulfidptosis and ferroptosis via systematical glucose deprivation targeting SLC7A11/GSH/GPX4 antioxidant axis. ACS Nano. (2025) 19:14233–48. doi: 10.1021/acsnano.5c00730
103. Ye Y, Chen A, Li L, Liang Q, Wang S, Dong Q, et al. Repression of the antiporter SLC7A11/glutathione/glutathione peroxidase 4 axis drives ferroptosis of vascular smooth muscle cells to facilitate vascular calcification. Kidney Int. (2022) 102:1259–75. doi: 10.1016/j.kint.2022.07.034
104. Jiang C, Li X, Wan S, Ji S, Wang Q, Hu S, et al. Copper-doped polydopamine nanoparticles-mediated GSH/GPX4-depleted ferroptosis and cuproptosis sensitizes lung tumor to checkpoint blockade immunotherapy. Small. (2025) 21:e2503208. doi: 10.1002/smll.202503208
105. Zhang H, Pan J, Huang S, Chen X, Chang ACY, Wang C, et al. Hydrogen sulfide protects cardiomyocytes from doxorubicin-induced ferroptosis through the SLC7A11/GSH/GPx4 pathway by Keap1 S-sulfhydration and Nrf2 activation. Redox Biol. (2024) 70:103066. doi: 10.1016/j.redox.2024.103066
106. Wang J, Zeng L, Wu N, Liang Y, Jin J, Fan M, et al. Inhibition of phosphoglycerate dehydrogenase induces ferroptosis and overcomes enzalutamide resistance in castration-resistant prostate cancer cells. Drug Resist Update. (2023) 70:100985. doi: 10.1016/j.drup.2023.100985
107. Xin Z, Hu C, Zhang C, Liu M, Li J, Sun X, et al. LncRNA-HMG incites colorectal cancer cells to chemoresistance via repressing p53-mediated ferroptosis. Redox Biol. (2024) 77:103362. doi: 10.1016/j.redox.2024.103362
108. Wang X, Chen T, Chen S, Zhang J, Cai L, Liu C, et al. STING aggravates ferroptosis-dependent myocardial ischemia-reperfusion injury by targeting GPX4 for autophagic degradation. Signal Transduct Target Ther. (2025) 10:136. doi: 10.1038/s41392-025-02216-9
109. Xue Q, Yan D, Chen X, Li X, Kang R, Klionsky DJ, et al. Copper-dependent autophagic degradation of GPX4 drives ferroptosis. Autophagy. (2023) 19:1982–96. doi: 10.1080/15548627.2023.2165323
110. Chen X, Li J, Kang R, Klionsky DJ, and Tang D. Ferroptosis: machinery and regulation. Autophagy. (2021) 17:2054–81. doi: 10.1080/15548627.2020.1810918
111. Lin H, Zhu S, Chen Y, Lu J, Xie C, Liao C, et al. Targeting cTRIP12 counteracts ferroptosis resistance and augments sensitivity to immunotherapy in pancreatic cancer. Drug Resist Update. (2025) 81:101240. doi: 10.1016/j.drup.2025.101240
112. Yuan Z, Wang X, Qin B, Hu R, Miao R, Zhou Y, et al. Targeting NQO1 induces ferroptosis and triggers anti-tumor immunity in immunotherapy-resistant KEAP1-deficient cancers. Drug Resist Update. (2024) 77:101160. doi: 10.1016/j.drup.2024.101160
113. Liu X, He J, Ying H, Chen C, Zheng C, Luo P, et al. Targeting PFKFB4 biomimetic codelivery system synergistically enhances ferroptosis to suppress small cell lung cancer and augments the efficacy of anti-PD-L1 immunotherapy. Adv Sci (Weinh). (2025) 12:e2417374. doi: 10.1002/advs.202417374
114. Zhang H, Feng K, Han M, Shi Y, Zhang Y, Wu J, et al. Homologous magnetic targeted immune vesicles for amplifying immunotherapy via ferroptosis activation augmented photodynamic therapy against glioblastoma. J Control Release. (2025) 383:113816. doi: 10.1016/j.jconrel.2025.113816
115. Chen X, Zhang F, Lu C, Wu R, Yang B, Liao T, et al. Lactate-fueled theranostic nanoplatforms for enhanced MRI-guided ferroptosis synergistic with immunotherapy of hepatocellular carcinoma. ACS Appl Mater Interfaces. (2025) 17:9155–72. doi: 10.1021/acsami.4c21890
116. Chen D, Zhao Z, Hong R, Yang D, Gong Y, Wu Q, et al. Harnessing the FGFR2/NF2/YAP signaling-dependent necroptosis to develop an FGFR2/IL-8 dual blockade therapeutic strategy. Nat Commun. (2025) 16:4128. doi: 10.1038/s41467-025-59318-9
117. Zhang N, Liu J, Guo R, Yan L, Yang Y, Shi C, et al. Palmitoylation licenses RIPK1 kinase activity and cytotoxicity in the TNF pathway. Mol Cell. (2024) 84:4419–4435 e10. doi: 10.1016/j.molcel.2024.10.002
118. Culver-Cochran AE, Hassan A, Hueneman K, Choi K, Ma A, VanCauwenbergh B, et al. Chemotherapy resistance in acute myeloid leukemia is mediated by A20 suppression of spontaneous necroptosis. Nat Commun. (2024) 15:9189. doi: 10.1038/s41467-024-53629-z
119. Liu J, Wu XL, Zhang J, Li B, Wang HY, Wang J, et al. The structure of mouse RIPK1 RHIM-containing domain as a homo-amyloid and in RIPK1/RIPK3 complex. Nat Commun. (2024) 15:6975. doi: 10.1038/s41467-024-51303-y
120. Rojas-Rivera D, Beltran S, Munoz-Carvajal F, Ahumada-Montalva P, Abarzua L, Gomez L, et al. The autophagy protein RUBCNL/PACER represses RIPK1 kinase-dependent apoptosis and necroptosis. Autophagy. (2024) 20:2444–59. doi: 10.1080/15548627.2024.2367923
121. Wu X, Zhao X, Li F, Wang Y, Ou Y, Zhang H, et al. MLKL-mediated endothelial necroptosis drives vascular damage and mortality in systemic inflammatory response syndrome. Cell Mol Immunol. (2024) 21:1309–21. doi: 10.1038/s41423-024-01217-y
122. Murai S, Yamaguchi Y, Shirasaki Y, Yamagishi M, Shindo R, Hildebrand JM, et al. A FRET biosensor for necroptosis uncovers two different modes of the release of DAMPs. Nat Commun. (2018) 9:4457. doi: 10.1038/s41467-018-06985-6
123. Frank D and Vince JE. Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ. (2019) 26:99–114. doi: 10.1038/s41418-018-0212-6
124. Seo J, Nam YW, Kim S, Oh DB, and Song J. Necroptosis molecular mechanisms: Recent findings regarding novel necroptosis regulators. Exp Mol Med. (2021) 53:1007–17. doi: 10.1038/s12276-021-00634-7
125. He GW, Gunther C, Thonn V, Yu YQ, Martini E, Buchen B, et al. Regression of apoptosis-resistant colorectal tumors by induction of necroptosis in mice. J Exp Med. (2017) 214:1655–62. doi: 10.1084/jem.20160442
126. Liu ZY, Zheng M, Li YM, Fan XY, Wang JC, Li ZC, et al. RIP3 promotes colitis-associated colorectal cancer by controlling tumor cell proliferation and CXCL1-induced immune suppression. Theranostics. (2019) 9:3659–73. doi: 10.7150/thno.32126
127. Zhu J, Li J, Yang K, Chen Y, Wang J, He Y, et al. NR4A1 depletion inhibits colorectal cancer progression by promoting necroptosis via the RIG-I-like receptor pathway. Cancer Lett. (2024) 585:216693. doi: 10.1016/j.canlet.2024.216693
128. Seehawer M, Heinzmann F, D’Artista L, Harbig J, Roux PF, Hoenicke L, et al. Necroptosis microenvironment directs lineage commitment in liver cancer. Nature. (2018) 562:69–75. doi: 10.1038/s41586-018-0519-y
129. Vucur M, Ghallab A, Schneider AT, Adili A, Cheng M, Castoldi M, et al. Sublethal necroptosis signaling promotes inflammation and liver cancer. Immunity. (2023) 56:1578–1595.e8. doi: 10.1016/j.immuni.2023.05.017
130. Cho MG, Kumar RJ, Lin CC, Boyer JA, Shahir JA, Fagan-Solis K, et al. MRE11 liberates cGAS from nucleosome sequestration during tumorigenesis. Nature. (2024) 625:585–92. doi: 10.1038/s41586-023-06889-6
131. Yu L, Guo Q, Li Y, Mao M, Liu Z, Li T, et al. CHMP4C promotes pancreatic cancer progression by inhibiting necroptosis via the RIPK1/RIPK3/MLKL pathway. J Adv Res. (2025). doi: 10.1016/j.jare.2025.01.040
132. Mohanty S, Yadav P, Lakshminarayanan H, Sharma P, Vivekanandhan A, and Karunagaran D. RETRA induces necroptosis in cervical cancer cells through RIPK1, RIPK3, MLKL and increased ROS production. Eur J Pharmacol. (2022) 920:174840. doi: 10.1016/j.ejphar.2022.174840
133. Tan Y, Sementino E, Cheung M, Peri S, Menges CW, Kukuyan AM, et al. Somatic epigenetic silencing of RIPK3 inactivates necroptosis and contributes to chemoresistance in Malignant mesothelioma. Clin Cancer Res. (2021) 27:1200–13. doi: 10.1158/1078-0432.CCR-18-3683
134. Sun Y, Zhai L, Ma S, Zhang C, Zhao L, Li N, et al. Down-regulation of RIP3 potentiates cisplatin chemoresistance by triggering HSP90-ERK pathway mediated DNA repair in esophageal squamous cell carcinoma. Cancer Lett. (2018) 418:97–108. doi: 10.1016/j.canlet.2018.01.022
135. Bonnet MC, Preukschat D, Welz PS, van Loo G, Ermolaeva MA, Bloch W, et al. The adaptor protein FADD protects epidermal keratinocytes from necroptosis in vivo and prevents skin inflammation. Immunity. (2011) 35:572–82. doi: 10.1016/j.immuni.2011.08.014
136. O’Donnell MA, Perez-Jimenez E, Oberst A, Ng A, Massoumi R, Xavier R, et al. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat Cell Biol. (2011) 13:1437–42. doi: 10.1038/ncb2362
137. Zhang T, Yin C, Fedorov A, Qiao L, Bao H, Beknazarov N, et al. ADAR1 masks the cancer immunotherapeutic promise of ZBP1-driven necroptosis. Nature. (2022) 606:594–602. doi: 10.1038/s41586-022-04753-7
138. Jiao H, Wachsmuth L, Kumari S, Schwarzer R, Lin J, Eren RO, et al. Publisher Correction: Z-nucleic-acid sensing triggers ZBP1-dependent necroptosis and inflammation. Nature. (2020) 580:E10. doi: 10.1038/s41586-020-2207-y
139. Liao CY, Li G, Kang FP, Lin CF, Xie CK, Wu YD, et al. Necroptosis enhances ‘don’t eat me’ signal and induces macrophage extracellular traps to promote pancreatic cancer liver metastasis. Nat Commun. (2024) 15:6043. doi: 10.1038/s41467-024-50450-6
140. Huang Y, Zou J, Huo J, Zhang M, and Yang Y. Sulfate radical based in situ vaccine boosts systemic antitumor immunity via concurrent activation of necroptosis and STING pathway. Adv Mater. (2024) 36:e2407914. doi: 10.1002/adma.202407914
141. Yu S, Li J, Zhang J, Zeng G, Zeng B, Song S, et al. Nanosized shikonin disrupts tumor-cell mismatch repair and synergizes with manganese to sensitize squamous carcinoma to immunotherapy. ACS Nano. (2025) 19:13889–905. doi: 10.1021/acsnano.4c17090
142. Lu X, Chen D, Wang M, Song X, Ermine K, Hao S, et al. Depletion of oxysterol-binding proteins by OSW-1 triggers RIP1/RIP3-independent necroptosis and sensitization to cancer immunotherapy. Cell Death Differ. (2025). doi: 10.1038/s41418-025-01521-8
143. Wang M, Cai R, Zhang Z, Feng L, Lei Z, Wang F, et al. NIR-responsive CN-Pt-GEM hydrogel induces necroptosis and immunotherapeutic responses prevent postoperative recurrence and wound infection in lung carcinoma. J Nanobiotechnology. (2024) 22:355. doi: 10.1186/s12951-024-02568-4
144. Shang DF, Xu WQ, Zhao Q, Zhao CL, Wang SY, Han YL, et al. Molecular mechanisms of pyroptosis in non-alcoholic steatohepatitis and feasible diagnosis and treatment strategies. Pharmacol Res. (2025) 216:107754. doi: 10.1016/j.phrs.2025.107754
145. Xu W, Huang Y, and Zhou R. NLRP3 inflammasome in neuroinflammation and central nervous system diseases. Cell Mol Immunol. (2025) 22:341–55. doi: 10.1038/s41423-025-01275-w
146. Shi Y, Hu Y, Li J, Chen H, Zhong Q, Ma X, et al. Inhibition of caspase-1 suppresses GSDMD-mediated peritoneal mesothelial cell pyroptosis and inflammation in peritoneal fibrosis. Small. (2025) 21:e2409362. doi: 10.1002/smll.202409362
147. Wang J, Zhang C, Qin J, An N, Bai M, Du RH, et al. Direct inhibition of the TXNIP-NLRP3-GSDMD pathway reduces pyroptosis in colonocytes and alleviates ulcerative colitis in mice by the small compound PEITC, Acta Pharmacol Sin. (2025) 46:2436–49. doi: 10.1038/s41401-025-01549-z
148. Toldo S and Abbate A. The role of the NLRP3 inflammasome and pyroptosis in cardiovascular diseases. Nat Rev Cardiol. (2024) 21:219–37. doi: 10.1038/s41569-023-00946-3
149. Elias EE, Lyons B, and Muruve DA. Gasdermins and pyroptosis in the kidney. Nat Rev Nephrol. (2023) 19:337–50. doi: 10.1038/s41581-022-00662-0
150. Wei X, Xie F, Zhou X, Wu Y, Yan H, Liu T, et al. Role of pyroptosis in inflammation and cancer. Cell Mol Immunol. (2022) 19:971–92. doi: 10.1038/s41423-022-00905-x
151. Tang D, Kang R, Berghe TV, Vandenabeele P, and Kroemer G. The molecular machinery of regulated cell death. Cell Res. (2019) 29:347–64. doi: 10.1038/s41422-019-0164-5
152. Van Opdenbosch N and Lamkanfi M. Caspases in cell death. Inflammation Disease Immun. (2019) 50:1352–64. doi: 10.1016/j.immuni.2019.05.020
153. Hou J, Hsu JM, and Hung MC. Molecular mechanisms and functions of pyroptosis in inflammation and antitumor immunity. Mol Cell. (2021) 81:4579–90. doi: 10.1016/j.molcel.2021.09.003
154. Peng Z, Wang P, Song W, Yao Q, Li Y, Liu L, et al. GSDME enhances Cisplatin sensitivity to regress non-small cell lung carcinoma by mediating pyroptosis to trigger antitumor immunocyte infiltration. Signal Transduct Target Ther. (2020) 5:159. doi: 10.1038/s41392-020-00274-9
155. Yuan R, Zhao W, Wang QQ, He J, Han S, Gao H, et al. Cucurbitacin B inhibits non-small cell lung cancer in vivo and in vitro by triggering TLR4/NLRP3/GSDMD-dependent pyroptosis. Pharmacol Res. (2021) 170:105748. doi: 10.1016/j.phrs.2021.105748
156. Wang F, Liu W, Ning J, Wang J, Lang Y, Jin X, et al. Simvastatin suppresses proliferation and migration in non-small cell lung cancer via pyroptosis. Int J Biol Sci. (2018) 14:406–17. doi: 10.7150/ijbs.23542
157. Tu Y, Wu H, Zhong C, Liu Y, Xiong Z, Chen S, et al. Pharmacological activation of STAT1-GSDME pyroptotic circuitry reinforces epigenetic immunotherapy for hepatocellular carcinoma. Gut. (2025) 74:613–27. doi: 10.1136/gutjnl-2024-332281
158. Zhang X, Zhang P, An L, Sun N, Peng L, Tang W, et al. Miltirone induces cell death in hepatocellular carcinoma cell through GSDME-dependent pyroptosis. Acta Pharm Sin B. (2020) 10:1397–413. doi: 10.1016/j.apsb.2020.06.015
159. Ling Y, Liang X, Yan K, Zeng G, Zhu X, Jiang J, et al. Bimetallic ca/zn nanoagonist remould the immunosuppressive hepatocellular carcinoma microenvironment following incomplete microwave ablation via pyroptosis and the STING signaling pathway. Adv Sci (Weinh). (2025) 12:e2500670. doi: 10.1002/advs.202500670
160. Fontana P, Du G, Zhang Y, Zhang H, Vora SM, Hu JJ, et al. Small-molecule GSDMD agonism in tumors stimulates antitumor immunity without toxicity. Cell. (2024) 187:6165–6181 e22. doi: 10.1016/j.cell.2024.08.007
161. Zhou Z, He H, Wang K, Shi X, Wang Y, Su Y, et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells, Science. (2020) 368:eaaz7548. doi: 10.1126/science.aaz7548
162. Xu L, Peng F, Luo Q, Ding Y, Yuan F, Zheng L, et al. IRE1alpha silences dsRNA to prevent taxane-induced pyroptosis in triple-negative breast cancer. Cell. (2024) 187:7248–7266 e34. doi: 10.1016/j.cell.2024.09.032
163. Zhang J, Hu Y, Wen X, Yang Z, Wang Z, Feng Z, et al. Tandem-controlled lysosomal assembly of nanofibres induces pyroptosis for cancer immunotherapy. Nat Nanotechnol. (2025) 20:563–74. doi: 10.1038/s41565-025-01857-9
164. Kang M, Du W, Ding L, Wu M, and Pei D. HIC1 suppresses tumor progression and enhances CD8(+) T cells infiltration through promoting GSDMD-induced pyroptosis in gastric cancer. Adv Sci (Weinh). (2025) 12:e2412083. doi: 10.1002/advs.202412083
165. Gao Q, Sheng Q, Yang Z, Zhu Z, Li L, Xu L, et al. Honokiol-magnolol-baicalin possesses synergistic anticancer potential and enhances the efficacy of anti-PD-1 immunotherapy in colorectal cancer by triggering GSDME-dependent pyroptosis. Adv Sci (Weinh). (2025) 12:e2417022. doi: 10.1002/advs.202417022
166. Wu Q, Zhao L, Tan L, Ren X, Fu C, Chen Z, et al. Microwave-responsive alEu-MOFs potentiate NLRP3-mediated pyroptosis via a “Triple initiating” Tactic for breast cancer microwave-immunotherapy. Small. (2025) 21:e2501157. doi: 10.1002/smll.202501157
167. Galluzzi L, Guilbaud E, Schmidt D, Kroemer G, and Marincola FM. Targeting immunogenic cell stress and death for cancer therapy. Nat Rev Drug Discov. (2024) 23:445–60. doi: 10.1038/s41573-024-00920-9
168. Clucas J and Meier P. Roles of RIPK1 as a stress sentinel coordinating cell survival and immunogenic cell death. Nat Rev Mol Cell Biol. (2023) 24:835–52. doi: 10.1038/s41580-023-00623-w
169. Zhang Y, Doan BT, and Gasser G. Metal-based photosensitizers as inducers of regulated cell death mechanisms. Chem Rev. (2023) 123:10135–55. doi: 10.1021/acs.chemrev.3c00161
170. Newton K, Wickliffe KE, Maltzman A, Dugger DL, Strasser A, Pham VC, et al. RIPK1 inhibits ZBP1-driven necroptosis during development. Nature. (2016) 540:129–33. doi: 10.1038/nature20559
171. Li T, Zhang Y, Li C, Song Y, Jiang T, Yin Y, et al. Microbial photosynthetic oxygenation and radiotherapeutic sensitization enables pyroptosis induction for combinatorial cancer therapy. Adv Mater. (2025) 37:e2503138. doi: 10.1002/adma.202503138
172. Kang TB, Yang SH, Toth B, Kovalenko A, and Wallach D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity. (2013) 38:27–40. doi: 10.1016/j.immuni.2012.09.015
173. Lawlor KE, Khan N, Mildenhall A, Gerlic M, Croker BA, D’Cruz AA, et al. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Commun. (2015) 6:6282. doi: 10.1038/ncomms7282
174. Liu Y, Stockwell BR, Jiang X, and Gu W. p53-regulated non-apoptotic cell death pathways and their relevance in cancer and other diseases, Nat Rev Mol Cell Biol. (2025) 26:600–14. doi: 10.1038/s41580-025-00842-3
175. Xu C, Sun S, Johnson T, Qi R, Zhang S, Zhang J, et al. The glutathione peroxidase Gpx4 prevents lipid peroxidation and ferroptosis to sustain Treg cell activation and suppression of antitumor immunity. Cell Rep. (2021) 35:109235. doi: 10.1016/j.celrep.2021.109235
176. Emmons MF and Smalley KSM. Ironing-out the details: new strategies for combining ferroptosis inhibitors with immunotherapy in melanoma. J Invest Dermatol. (2022) 142:18–20. doi: 10.1016/j.jid.2021.06.014
177. Wang W, Zhang J, Wang Y, Xu Y, and Zhang S. Identifies microtubule-binding protein CSPP1 as a novel cancer biomarker associated with ferroptosis and tumor microenvironment. Comput Struct Biotechnol J. (2022) 20:3322–35. doi: 10.1016/j.csbj.2022.06.046
178. Hai Y, Fan R, Zhao T, Lin R, Zhuang J, Deng A, et al. A novel mitochondria-targeting DHODH inhibitor induces robust ferroptosis and alleviates immune suppression. Pharmacol Res. (2024) 202:107115. doi: 10.1016/j.phrs.2024.107115
179. Liu W, Zhou H, Lai W, Hu C, Wang Q, Yuan C, et al. Artesunate induces melanoma cell ferroptosis and augments antitumor immunity through targeting Ido1. Cell Commun Signal. (2024) 22:378. doi: 10.1186/s12964-024-01759-8
180. Zhu B, Yang C, Hua S, Li K, Shang P, Chen X, et al. Lithium Enhances Ferroptosis sensitivity in melanoma cells and promotes CD8(+) T Cell infiltration and differentiation. Free Radic Biol Med. (2025) 227:233–45. doi: 10.1016/j.freeradbiomed.2024.12.012
181. Zang X, He XY, Xiao CM, Lin Q, Wang MY, Liu CY, et al. Circular RNA-encoded oncogenic PIAS1 variant blocks immunogenic ferroptosis by modulating the balance between SUMOylation and phosphorylation of STAT1. Mol Cancer. (2024) 23:207. doi: 10.1186/s12943-024-02124-6
182. Hou G, Qian J, Wang Y, Xu W, Guo M, Li Z, et al. Hydrazide/metal/indocyanine green coordinated nanoplatform for potentiating reciprocal ferroptosis and immunity against melanoma. ACS Appl Mater Interfaces. (2023) 15:37143–56. doi: 10.1021/acsami.3c05580
183. Fan Z, Wu S, Deng H, Li G, Huang L, and Liu H. Light-triggered nanozymes remodel the tumor hypoxic and immunosuppressive microenvironment for ferroptosis-enhanced antitumor immunity. ACS Nano. (2024) 18:12261–75. doi: 10.1021/acsnano.4c00844
184. Wang W, Zhong Z, Peng S, Fu J, Chen M, Lang T, et al. All-in-one” metal polyphenol network nanocapsules integrated microneedle patches for lipophagy fueled ferroptosis-mediated multimodal therapy. J Control Release. (2024) 373:599–616. doi: 10.1016/j.jconrel.2024.07.063
185. Pepple AL, Guy JL, McGinnis R, Felsted AE, Song B, Hubbard R, et al. Spatiotemporal local and abscopal cell death and immune responses to histotripsy focused ultrasound tumor ablation. Front Immunol. (2023) 14:1012799. doi: 10.3389/fimmu.2023.1012799
186. Li D, Wen G, Wang H, Ren Q, Wang D, Dao A, et al. Photoredox-mediated immunotherapy utilizing rhenium(I) photocatalysts with electron donor-acceptor-donor configuration. J Med Chem. (2025) 68:3749–63. doi: 10.1021/acs.jmedchem.4c02836
187. Lie Q, Jiang H, Lu X, Chen Z, Liang J, Zhang Y, et al. Photo-activated ferrocene-iridium(III) prodrug induces immunogenic cell death in melanoma stem cells. J Med Chem. (2025) 68:8894–906. doi: 10.1021/acs.jmedchem.5c00533
188. Werthmoller N, Frey B, Wunderlich R, Fietkau R, and Gaipl US. Modulation of radiochemoimmunotherapy-induced B16 melanoma cell death by the pan-caspase inhibitor zVAD-fmk induces anti-tumor immunity in a HMGB1-, nucleotide- and T-cell-dependent manner. Cell Death Dis. (2015) 6:e1761. doi: 10.1038/cddis.2015.129
189. Zhou Q, Dian Y, He Y, Yao L, Su H, Meng Y, et al. Propafenone facilitates mitochondrial-associated ferroptosis and synergizes with immunotherapy in melanoma, J Immunother Cancer 12(11). (2024) 12:e009805. doi: 10.1136/jitc-2024-009805
190. Hoecke LV, Lint SV, Roose K, Parys AV, Vandenabeele P, Grooten J, et al. Treatment with mRNA coding for the necroptosis mediator MLKL induces antitumor immunity directed against neo-epitopes. Nat Commun. (2018) 9:3417. doi: 10.1038/s41467-018-05979-8
191. Zhou H, Zhu C, Li Y, Zhao F, Feng Q, Liu S, et al. Exosome/liposome hybrid nanovesicles for enhanced phototherapy and boosted anti-tumor immunity against melanoma. Eur J Med Chem. (2025) 289:117485. doi: 10.1016/j.ejmech.2025.117485
192. Chun N, Ang RL, Chan M, Fairchild RL, Baldwin WM, Horwitz 3JK, et al. T cell-derived tumor necrosis factor induces cytotoxicity by activating RIPK1-dependent target cell death. JCI Insight. (2021) 6:e011030. doi: 10.1172/jci.insight.148643
193. Li Z, Wu X, Chen S, Zhong J, Qiu X, Kpegah J, et al. Identification of CRKL as an oncogenic biomarker for prognosis and immunotherapy in melanoma. its potential Mol mechanism Genomics. (2023) 115:110634. doi: 10.1016/j.ygeno.2023.110634
194. Rosenbaum SR, Caksa S, Stefanski CD, Trachtenberg IV, Wilson HP, Wilski NA, et al. SOX10 loss sensitizes melanoma cells to cytokine-mediated inflammatory cell death. Mol Cancer Res. (2024) 22:209–20. doi: 10.1158/1541-7786.MCR-23-0290
195. Wu J, Liu N, Chen J, Tao Q, Lu C, Li Q, et al. Clofarabine induces tumor cell apoptosis, GSDME-related pyroptosis, and CD8(+) T-cell antitumor activity via the non-canonical P53/STING pathway, J Immunother Cancer 13(2). (2025) 13:e010252. doi: 10.1136/jitc-2024-010252
196. Zhao X, Zhao Z, Li B, Huan S, Li Z, Xie J, et al. ACSL4-mediated lipid rafts prevent membrane rupture and inhibit immunogenic cell death in melanoma. Cell Death Dis. (2024) 15:695. doi: 10.1038/s41419-024-07098-3
197. Vernon M, Wilski NA, Kotas D, Cai W, Pomante D, Tiago M, et al. Raptinal induces gasdermin E-dependent pyroptosis in naive and therapy-resistant melanoma. Mol Cancer Res. (2022) 20:1811–21. doi: 10.1158/1541-7786.MCR-22-0040
198. Wu J, Liu Z, Wang L, Pei Z, Han Z, Cui X, et al. Hydrotalcites-induced pyroptosis combined with toll-like receptor activation elicited dual stimulation of innate and adaptive immunity. ACS Nano. (2025) 19:8070–84. doi: 10.1021/acsnano.4c16281
199. Wang N, Liu C, Li Y, Huang D, Wu X, Kou X, et al. A cooperative nano-CRISPR scaffold potentiates immunotherapy via activation of tumour-intrinsic pyroptosis. Nat Commun. (2023) 14:779. doi: 10.1038/s41467-023-36550-9
200. Sun S, He Y, Xu J, Leng S, Liu Y, Wan H, et al. Enhancing cell pyroptosis with biomimetic nanoparticles for melanoma chemo-immunotherapy. J Control Release. (2024) 367:470–85. doi: 10.1016/j.jconrel.2024.01.057
201. Ye B, Hu W, Yu G, Yang H, Gao B, Ji J, et al. A cascade-amplified pyroptosis inducer: optimizing oxidative stress microenvironment by self-supplying reactive nitrogen species enables potent cancer immunotherapy. ACS Nano. (2024) 18:16967–81. doi: 10.1021/acsnano.4c03172
202. Wu X, Liang J, Shu J, Li Z, Yin T, Zhang X, et al. Narrow-bandgap iridium(III)-C(3)N(5) nanocomplex as an oxygen self-sufficient piezo-sonosensitizer for hypoxic tumor sonodynamic immunotherapy. J Am Chem Soc. (2025) 147:15329–43. doi: 10.1021/jacs.5c00843
203. Zhao Z, Li ZQ, Huang YB, Liu MM, Cao F, Bu GL, et al. An optimized integrin alpha6-targeted peptide capable of delivering toxins for melanoma treatment. J Transl Med. (2025) 23:495. doi: 10.1186/s12967-025-06511-5
204. Erkes DA, Cai W, Sanchez IM, Purwin TJ, Rogers C, Field CO, et al. Mutant BRAF and MEK inhibitors regulate the tumor immune microenvironment via pyroptosis. Cancer Discov. (2020) 10:254–69. doi: 10.1158/2159-8290.CD-19-0672
205. Cai W, Nguyen MQ, Wilski NA, Purwin TJ, Vernon M, Tiago M, et al. A genome-wide screen identifies PDPK1 as a target to enhance the efficacy of MEK1/2 inhibitors in NRAS mutant melanoma. Cancer Res. (2022) 82:2625–39. doi: 10.1158/0008-5472.CAN-21-3217
206. Zhang MJ, Liang MY, Yang SC, Ma XB, Wan SC, Yang QC, et al. Bioengineering of BRAF and COX2 inhibitor nanogels to boost the immunotherapy of melanoma via pyroptosis. Chem Commun (Camb). (2023) 59:932–5. doi: 10.1039/D2CC05498A
207. Ren H, Wu Z, Tan J, Tao H, Zou W, Cao Z, et al. Co-delivery nano system of MS-275 and V-9302 induces pyroptosis and enhances anti-tumor immunity against uveal melanoma. Adv Sci (Weinh). (2024) 11:e2404375. doi: 10.1002/advs.202404375
208. Wang ZM, Li MK, Yang QL, Duan SX, Lou XY, Yang XY, et al. Recombinant human adenovirus type 5 promotes anti-tumor immunity via inducing pyroptosis in tumor endothelial cells. Acta Pharmacol Sin. (2024) 45:2646–56. doi: 10.1038/s41401-024-01349-x
209. Guo Y, Li Y, Zhang M, Ma R, Wang Y, Weng X, et al. Polymeric nanocarrier via metabolism regulation mediates immunogenic cell death with spatiotemporal orchestration for cancer immunotherapy. Nat Commun. (2024) 15:8586. doi: 10.1038/s41467-024-53010-0
210. Zhang Y, Bai Y, Ma XX, Song JK, Luo Y, Fei XY, et al. Clinical-mediated discovery of pyroptosis in CD8(+) T cell and NK cell reveals melanoma heterogeneity by single-cell and bulk sequence. Cell Death Dis. (2023) 14:553. doi: 10.1038/s41419-023-06068-5
211. Chen W, He Y, Zhou G, Chen X, Ye Y, Zhang G, et al. Multiomics characterization of pyroptosis in the tumor microenvironment and therapeutic relevance in metastatic melanoma. BMC Med. (2024) 22:24. doi: 10.1186/s12916-023-03175-0
212. Wang S, Guo Q, Xu R, Lin P, Deng G, and Xia X. Combination of ferroptosis and pyroptosis dual induction by triptolide nano-MOFs for immunotherapy of Melanoma. J Nanobiotechnology. (2023) 21:383. doi: 10.1186/s12951-023-02146-0
213. An Y, Zhao F, Jia H, Meng S, Zhang Z, Li S, et al. Inhibition of programmed cell death by melanoma cell subpopulations reveals mechanisms of melanoma metastasis and potential therapeutic targets. Discov Oncol. (2025) 16:62. doi: 10.1007/s12672-025-01789-9
214. Zhang Y, Naderi Yeganeh P, Zhang H, Wang SY, Li Z, Gu B, et al. Tumor editing suppresses innate and adaptive antitumor immunity and is reversed by inhibiting DNA methylation. Nat Immunol. (2024) 25:1858–70. doi: 10.1038/s41590-024-01932-8
215. Zhao H, Niu M, Guo Y, Li Q, Wang Y, Jiang Q, et al. A lipid starvation strategy-synergized neutrophil activation for postoperative melanoma immunotherapy. J Control Release. (2025) 380:860–74. doi: 10.1016/j.jconrel.2025.02.027
216. Goenka A, Khan F, Verma B, Sinha P, Dmello CC, Jogalekar MP, et al. Tumor microenvironment signaling and therapeutics in cancer progression. Cancer Commun (Lond). (2023) 43:525–61. doi: 10.1002/cac2.12416
217. Zhang L, Kuca K, You L, Zhao Y, Musilek K, Nepovimova E, et al. Signal transducer and activator of transcription 3 signaling in tumor immune evasion. Pharmacol Ther. (2022) 230:107969. doi: 10.1016/j.pharmthera.2021.107969
218. Patel SP, Sheth RA, Davis C, and Medina T. Combination immunotherapy with nivolumab plus ipilimumab in melanoma of unknown primary. J Clin Oncol. (2025) 43:907–11. doi: 10.1200/JCO-24-01802
219. Wawrzyniak P and Hartman ML. Dual role of interferon-gamma in the response of melanoma patients to immunotherapy with immune checkpoint inhibitors. Mol Cancer. (2025) 24:89. doi: 10.1186/s12943-025-02294-x
220. Rafiq Z, Kang M, Barsoumian HB, Manzar GS, Hu Y, Leuschner C, et al. Enhancing immunotherapy efficacy with synergistic low-dose radiation in metastatic melanoma: current insights and prospects. J Exp Clin Cancer Res. (2025) 44:31. doi: 10.1186/s13046-025-03281-2
221. Topalian SL and Pardoll DM. Neoadjuvant anti-PD-1-based immunotherapy: evolving a new standard of care. J Immunother Cancer. (2025) 13:e010833. doi: 10.1136/jitc-2024-010833
222. Hu Y, Yu Q, Li X, Wang J, Guo L, Huang L, et al. Nanoformula design for inducing non-apoptotic cell death regulation: A powerful booster for cancer immunotherapy. Adv Healthc Mater. (2025) 14:e2403493. doi: 10.1002/adhm.202403493
223. Duan M, Liu H, Xu S, Yang Z, Zhang F, Wang G, et al. IGF2BPs as novel m(6)A readers: Diverse roles in regulating cancer cell biological functions, hypoxia adaptation, metabolism, and immunosuppressive tumor microenvironment. Genes Dis. (2024) 11:890–920. doi: 10.1016/j.gendis.2023.06.017
224. Rodriguez-Baena FJ, Marquez-Galera A, Ballesteros-Martinez P, Castillo A, Diaz E, Moreno-Bueno G, et al. Microglial reprogramming enhances antitumor immunity and immunotherapy response in melanoma brain metastases. Cancer Cell. (2025) 43:413–427 e9. doi: 10.1016/j.ccell.2025.01.008
225. Karras P, Bordeu I, Pozniak J, Nowosad A, Pazzi C, Van Raemdonck N, et al. A cellular hierarchy in melanoma uncouples growth and metastasis. Nature. (2022) 610:190–8. doi: 10.1038/s41586-022-05242-7
226. Liu H, Gao J, Feng M, Cheng J, Tang Y, Cao Q, et al. Integrative molecular and spatial analysis reveals evolutionary dynamics and tumor-immune interplay of in situ and invasive acral melanoma. Cancer Cell. (2024) 42:1067–85.e11. doi: 10.1016/j.ccell.2024.04.012
227. Tawbi HA, Beumer JH, Tarhini AA, Moschos S, Buch SC, Egorin MJ, et al. Safety and efficacy of decitabine in combination with temozolomide in metastatic melanoma: a phase I/II study and pharmacokinetic analysis. Ann Oncol. (2013) 24:1112–9. doi: 10.1093/annonc/mds591
228. Wei J, Li Y, Li R, Chen X, Yang T, Liao L, et al. Drug repurposing of propafenone to discover novel anti-tumor agents by impairing homologous recombination to delay DNA damage recovery of rare disease conjunctival melanoma. Eur J Med Chem. (2023) 250:115238. doi: 10.1016/j.ejmech.2023.115238
229. Wang HL, Li YL, Zou HH, Liang FP, and Zhu ZH. Smart lanthanide metal-organic frameworks with multicolor luminescence switching induced by the dynamic adaptive antenna effect of molecular rotors. Adv Mater. (2025) 37:e2502742. doi: 10.1002/adma.202502742
230. Li S, Liu W, Shi Y, Wang T, Liu T, Xue X, et al. Ligand-rich oxygen evolution electrocatalysts reconstructed from metal-organic frameworks for anion-exchange membrane water electrolysis. Sci Bull (Beijing). (2025) 70:1976–85. doi: 10.1016/j.scib.2025.04.037
231. McClain SM, Milchberg MH, Rienstra CM, and Murphy CJ. Biologically representative lipid-coated gold nanoparticles and phospholipid vesicles for the study of alpha-synuclein/membrane interactions. ACS Nano. (2023) 17:20387–401. doi: 10.1021/acsnano.3c06606
232. Kim WJ, Kim BS, Kim HJ, Cho YD, Shin HL, Yoon HI, et al. Intratesticular peptidyl prolyl isomerase 1 protein delivery using cationic lipid-coated fibroin nanoparticle complexes rescues male infertility in mice. ACS Nano. (2020) 14:13217–31. doi: 10.1021/acsnano.0c04936
233. Wang Y, Hu M, Cao J, Wang F, Han JR, Wu TW, et al. ACSL4 and polyunsaturated lipids support metastatic extravasation and colonization. Cell. (2025) 188:412–429.e27. doi: 10.1016/j.cell.2024.10.047
Keywords: melanoma, cancer immunotherapy, ferroptosis, pyroptosis, necroptosis
Citation: Shan Z and Liu F (2025) Targeting ferroptosis, pyroptosis and necroptosis for cancer immunotherapy in melanoma: mechanistic insights and clinical perspectives. Front. Immunol. 16:1629620. doi: 10.3389/fimmu.2025.1629620
Received: 16 May 2025; Accepted: 19 September 2025;
Published: 29 October 2025.
Edited by:
Pedro Berraondo, Cima Universidad de Navarra, SpainReviewed by:
Jinchun Wu, Zhongshan Hospital Affiliated to Fudan University (QingPu Branch), ChinaShuai Ping, Huazhong University of Science and Technology, China
Copyright © 2025 Shan and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Liu, bGl1ZmVpMDFAY2FuY2VyaG9zcC1sbi1jbXUuY29t
 Zexing Shan1
Zexing Shan1 Fei Liu
Fei Liu