- Department of Orthopedics, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
Malignant melanoma brain metastases (MBM) represent one of the deadliest complications of melanoma, with an incidence rate of 7.3%. Among patients with acral and mucosal melanoma, the cumulative 5-year incidence can reach 19.5%, accompanied by poor prognosis. The blood-brain barrier (BBB), an immunosuppressive central nervous system (CNS) microenvironment, and tumor immune evasion collectively limit the efficacy of traditional therapies. Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1), as critical immune checkpoints, play pivotal roles in the progression of MBM. This study systematically analyzes the synergistic mechanisms, clinical outcomes, and challenges of CTLA-4 and PD-1 combined blockade therapy in MBM. The findings indicate that this combination therapy leverages a “priming and boosting” biological mechanism: CTLA-4 blockade broadens T-cell responses during the initial activation phase, while PD-1 blockade sustains T-cell activity during the effector phase, significantly improving intracranial response rates (46%, compared to 20% for monotherapy). Furthermore, the combination therapy increases the CD8+/Treg ratio and promotes memory CD8+ T-cell formation, enabling durable antitumor immune surveillance. However, challenges such as a 54% incidence rate of grade 3–4 adverse events and suboptimal therapeutic regimens remain. To address these issues, this study proposes a multi-tiered adverse event management system, personalized risk assessment models, and treatment optimization strategies based on real-time monitoring and dynamic adjustments. Future directions include developing precision stratified therapies based on immunogenomics, exploring multi-target synergistic approaches, and implementing intelligent adverse event prediction and management systems to maximize therapeutic efficacy and minimize toxicity, providing more effective treatment for MBM patients.
1 Introduction
Malignant melanoma is a highly aggressive skin cancer with a steadily increasing incidence. Among melanoma patients, brain metastases occur in approximately 7.3% of cases (1), with a cumulative 5-year incidence of up to 19.5% in acral and mucosal melanoma patients (2). Brain metastases progress rapidly, with 16.7% of patients diagnosed with CNS metastases during follow-up (3). Survival outcomes remain poor, particularly in male patients (4, 5). As one of the most lethal complications of melanoma, MBM has become a critical focus of oncological research.
MBM presents a unique immune microenvironment, posing significant challenges for immune therapy strategies. First, the BBB limits the penetration of most drugs into the CNS, reducing therapeutic efficacy (6). Second, the immunosuppressive microenvironment in the brain facilitates tumor immune evasion (7). Lastly, brain metastases often exhibit molecular and immune profiles distinct from their primary tumors (8). Traditional treatments such as surgical resection, whole-brain radiotherapy, and chemotherapy, while providing some benefit, are associated with limited efficacy and significant side effects, failing to improve long-term survival outcomes for patients (9). Therefore, developing more effective therapies for MBM is of paramount importance.
The advent of immune checkpoint inhibitors (ICIs) has revolutionized MBM treatment, particularly inhibitors targeting CTLA-4 and PD-1 (10). While these agents have shown efficacy in some MBM patients, the overall objective response rates remain suboptimal, with significant challenges related to primary and acquired resistance (11). To address these limitations, the combined blockade of CTLA-4 and PD-1, leveraging their distinct roles in different phases of T-cell activation, offers a promising therapeutic strategy with synergistic effects (12).
This paper focuses on the following key questions:
1. How does the immune microenvironment of brain metastases differ from primary tumors? What roles do CTLA-4 and PD-1 play in MBM progression? How do they modulate the tumor microenvironment and immune cell functions?
2. What clinical trial data support the application of combined therapy in MBM? How does it compare to monotherapy? How can immune-related adverse events (irAEs), particularly CNS toxicities, be effectively managed?
3. How can real-time biomarker monitoring be leveraged to optimize treatment dosing, sequencing, and duration to balance efficacy and toxicity? Under the guidance of immunogenomics, how might combination strategies involving CTLA-4/PD-1 inhibitors and small-molecule agents, next-generation immune checkpoint inhibitors, oncolytic viruses, cellular therapies, and vaccines reshape the therapeutic landscape of melanoma brain metastases?
2 Immune resistance and the basis of blockade therapy
A major challenge in the treatment of melanoma brain metastases (MBM) is the restrictive nature of the blood–brain barrier (BBB). Composed of tightly connected brain endothelial cells, a basal membrane, and astrocyte endfeet, the BBB effectively prevents most circulating immune cells and macromolecular drugs from entering the central nervous system (CNS) (6). Its integrity varies with metastatic lesion size: while micrometastases (<0.25 mm) typically maintain an intact BBB, larger lesions exhibit increased permeability yet still suffer from heterogeneous drug distribution (13). This spatial heterogeneity significantly impairs drug delivery and remains a key obstacle in MBM therapy. Melanoma cells further modulate BBB permeability by secreting vascular endothelial growth factor (VEGF) (14) and exosomes (15), promoting localized BBB disruption. However, single-agent immune checkpoint inhibitors (ICIs) often fail to efficiently penetrate the BBB. In contrast, combination therapy can reshape the immune microenvironment, enhance T cell activation and infiltration (16), and exploit BBB disruptions around metastatic foci (17), facilitating improved drug accumulation—an effect not consistently observed with monotherapy (16).
The MBM microenvironment poses additional barriers. TGF-β promotes immunosuppression by upregulating PD-1 and CTLA-4 on T cells, while IL-10 enhances CTLA-4-mediated inhibition by suppressing CD28 co-stimulation (18). Microglia interact with melanoma cells to promote malignant phenotypes (19), and astrocytes secrete proinflammatory cytokines such as IL-23, further driving tumor invasiveness (20). These interactions contribute to immune evasion and therapeutic resistance. However, dual checkpoint blockade can help overcome these mechanisms (21). For instance, PD-1/CTLA-4 combination therapy induces phenotypic changes in dendritic cells within brain metastases, increasing co-stimulatory molecules and proinflammatory cytokine expression, thereby mitigating resistance (22)—a benefit less evident with monotherapy (22).
PD-L1 upregulation also contributes to therapeutic resistance. Its expression in MBM is driven by multiple factors, including TGF-β, IFN-γ, and the EGFR–STAT3 pathway (23), leading to substantial intertumoral and intratumoral heterogeneity (24). Importantly, CTLA-4 and PD-1/PD-L1 pathways are mechanistically intertwined. CTLA-4 inhibition enhances T cell activation and IFN-γ secretion, which in turn upregulates PD-L1 expression on tumor cells, undermining CTLA-4 monotherapy efficacy (23). Dual blockade prevents this adaptive resistance, increases the CD8+/Treg ratio, and enhances therapeutic efficacy (25). Tumor cells can also evade immune surveillance by downregulating MHC expression and suppressing co-stimulatory signaling (26); yet, combination blockade effectively counters these escape mechanisms, restoring antitumor immunity (27).
3 Synergistic mechanisms of CTLA-4 and PD-1 combination therapy
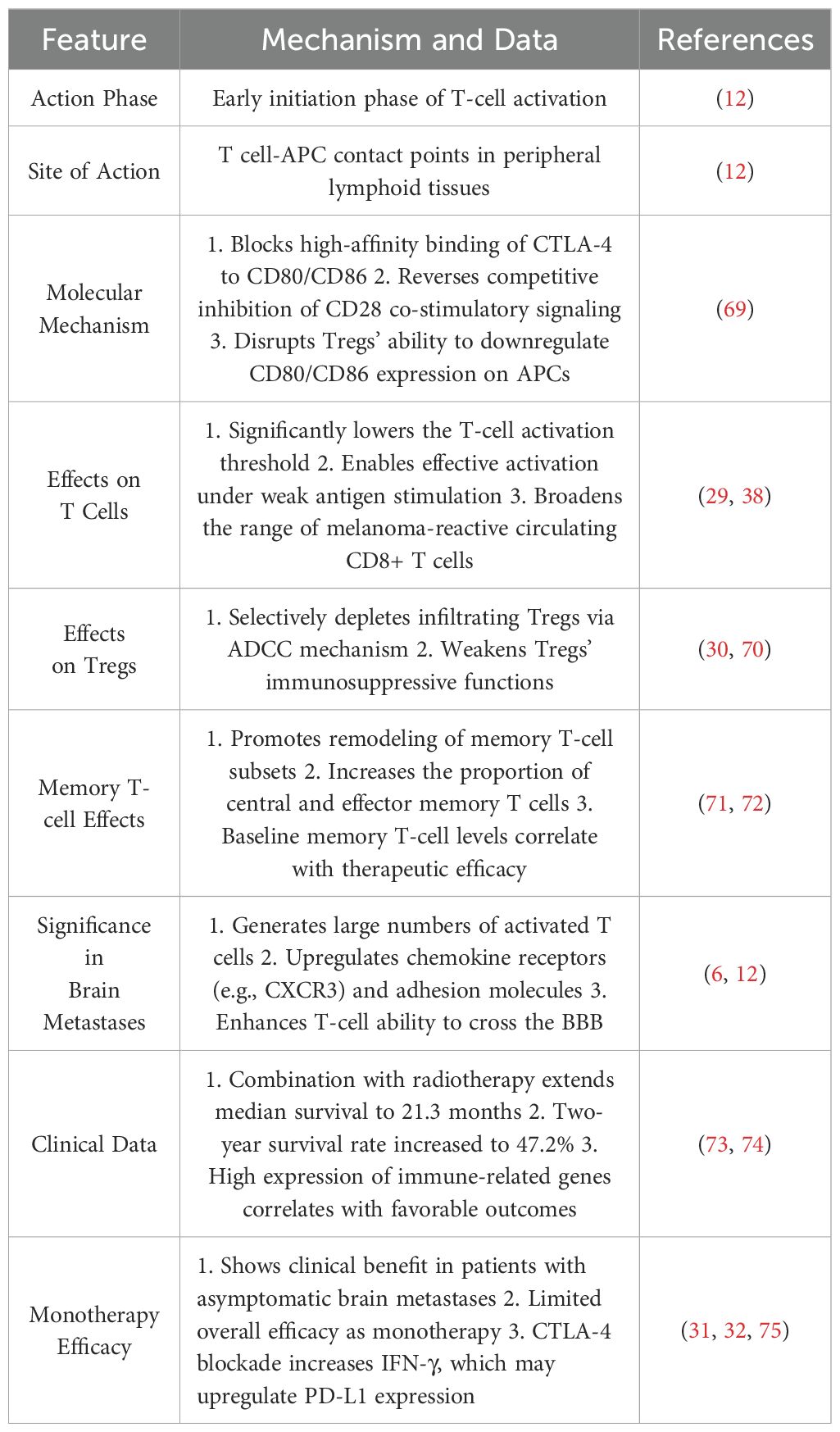
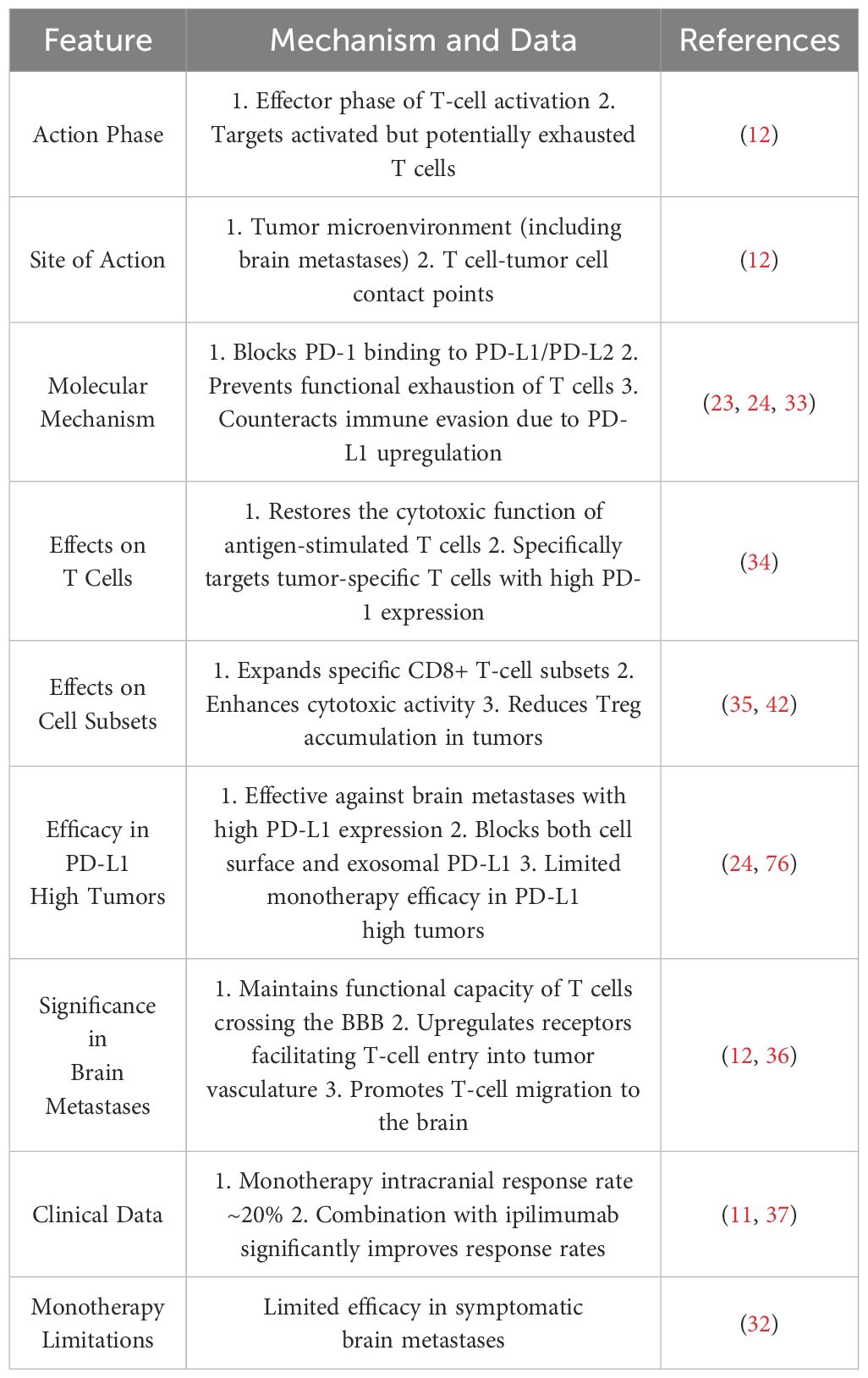
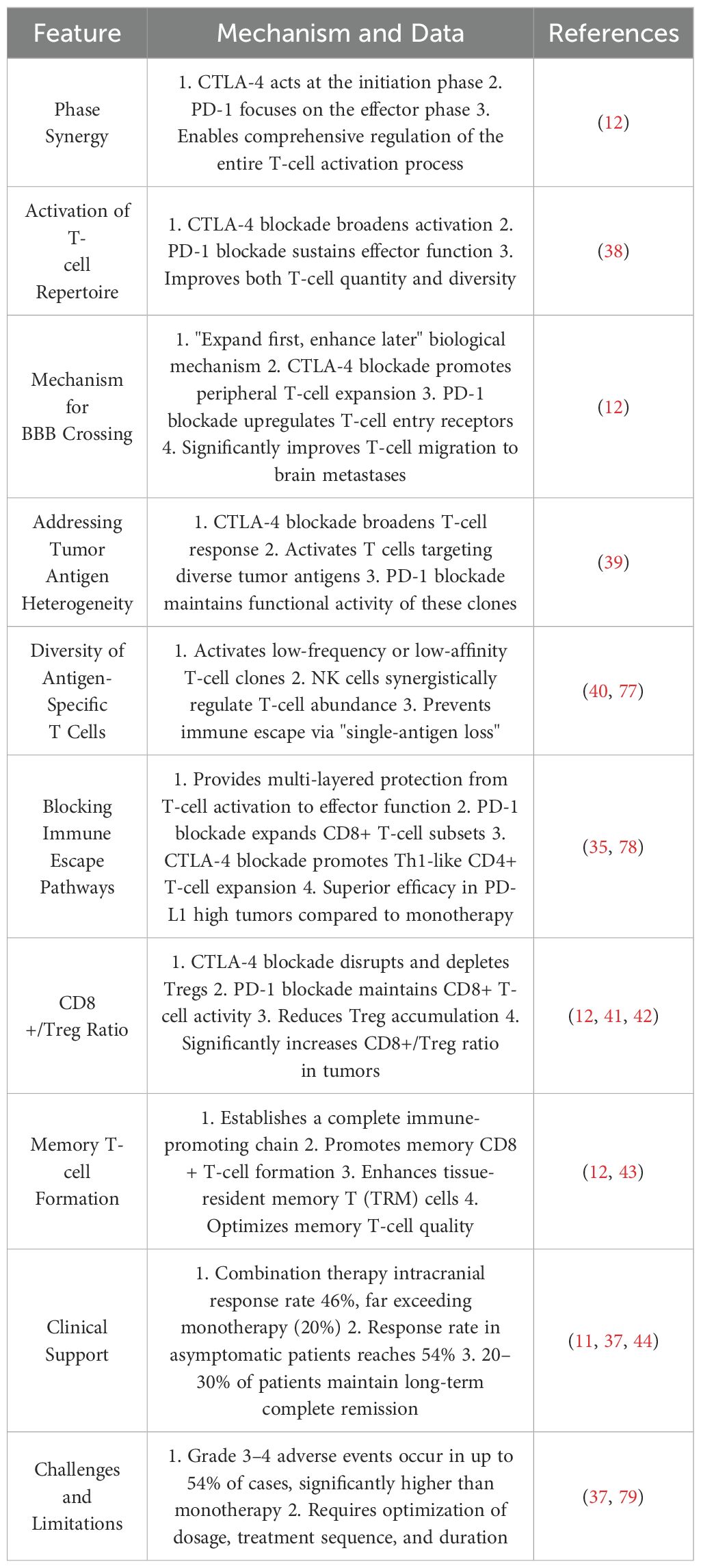
CTLA-4 and PD-1 dual blockade has demonstrated robust synergy in MBM treatment. Table 1 outlines the mechanisms and clinical data of CTLA-4 blockade, Table 2 summarizes the characteristics of PD-1 inhibitors, and Table 3 highlights their synergistic therapeutic advantages. Figure 1 illustrates their differential roles during T cell activation stages.
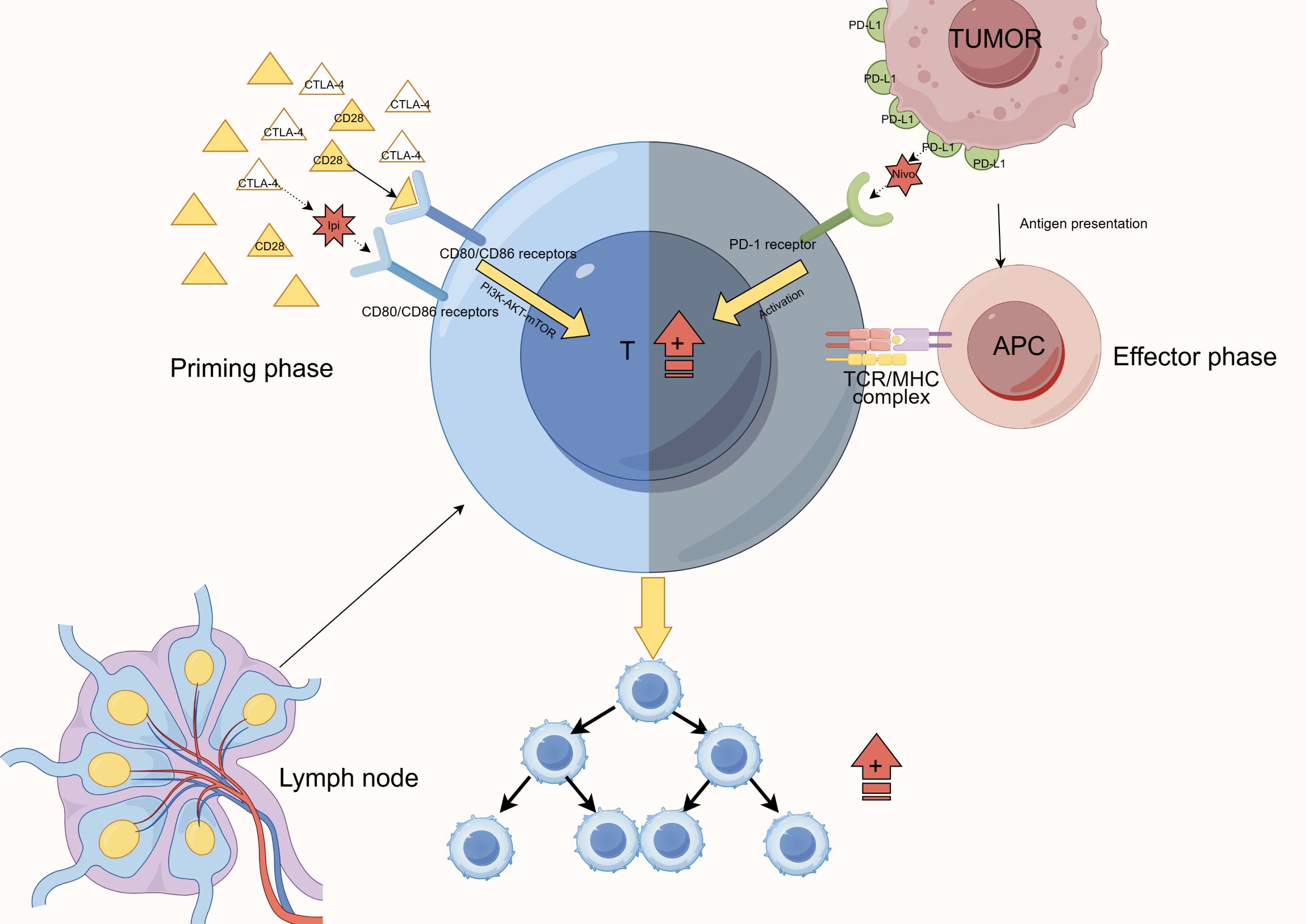
Figure 1. Mechanism of CTLA-4 and PD-1 blockade at different stages of T-cell activation. This figure illustrates the complementary actions of immune checkpoint blockade. Anti-CTLA-4 agents act at the initiation phase in lymph nodes, blocking CTLA-4-CD80/CD86 interactions to promote initial T-cell activation. In contrast, anti-PD-1 agents function at the effector phase in the tumor microenvironment, preventing PD-1-PD-L1 interactions to sustain T-cell functionality. Together, these agents achieve comprehensive regulation of the T-cell activation and effector processes, ultimately enhancing T-cell proliferation and function.
CTLA-4 is highly expressed on naïve and regulatory T cells (Tregs), where it competitively binds CD80/CD86 on antigen-presenting cells (APCs), thereby impeding full T cell activation (28). In Tregs, CTLA-4 also actively reduces CD80/CD86 expression on APCs, establishing a multilayered immunosuppressive network (28). Ipilimumab, a CTLA-4 inhibitor, lowers the T cell activation threshold and expands melanoma-reactive CD8+ T cell populations (29), while selectively depleting tumor-infiltrating Tregs via antibody-dependent cellular cytotoxicity (ADCC) (30). Although it can reprogram memory T cell subsets and enhance their BBB-penetrating ability (6), its efficacy as a monotherapy in brain metastases remains limited (31), likely due to CTLA-4 blockade-induced IFN-γ release, which upregulates PD-L1 expression and triggers adaptive resistance (32).
PD-1, in contrast, is predominantly expressed on activated T cells and plays a critical role at the tumor–T cell interface within brain metastases (12, 24). Blocking PD-1–PD-L1/PD-L2 interactions restores the cytotoxic function of antigen-experienced T cells, prevents exhaustion under chronic antigen exposure, and expands tumor-specific CD8+ T cell subsets (33–35). In MBM, PD-1 inhibitors preserve the effector function of T cells that have crossed the BBB and promote their migration into CNS lesions (36). However, the intracranial response rate to PD-1 monotherapy remains low—around 20%—especially in symptomatic MBM patients (32, 37).
Dual blockade of CTLA-4 and PD-1 overcomes these monotherapy limitations through a sequential synergy often described as “priming then sustaining.” CTLA-4 inhibition facilitates early T cell priming, increasing response breadth and clonal diversity, while PD-1 inhibition maintains effector T cell functionality during the response phase (12, 38). This strategy activates T cell clones against a broader range of tumor antigens, including low-affinity or low-frequency clones (39, 40). In MBM, this translates to improved BBB traversal and enhanced T cell infiltration into brain lesions (12). Furthermore, combination therapy significantly increases the CD8+/Treg ratio (41, 42), supports memory CD8+ T cell formation, and promotes tissue-resident memory T cells (TRMs), reinforcing long-term immune surveillance (43).
Clinical data corroborate these mechanistic insights: dual blockade yields intracranial response rates of up to 46%, markedly higher than monotherapy, and rates can reach 54% in asymptomatic patients. Moreover, 20–30% of patients achieve durable complete remission lasting multiple years (11, 37, 44).
4 Challenges
4.1 Adverse events
Dual blockade of CTLA-4 and PD-1 is associated with a high incidence (up to 54%) of grade 3–4 adverse events (AEs) (37), significantly exceeding those observed with monotherapies (Table 3). Mechanistically, this stems from the disruption of multiple immune tolerance checkpoints. CTLA-4 inhibition lowers the activation threshold of peripheral T cells, promoting polyclonal T cell expansion. Concurrently, PD-1 blockade enhances the effector function of these activated T cells, thereby increasing their cytotoxic potential against self-tissues. This “dual immune unleashing” effect compromises intrinsic immune tolerance, leading to a higher frequency and severity of immune-related adverse events (irAEs).
Studies have shown that combination therapy more frequently induces irAEs involving the skin, colon, endocrine organs, and liver (45). Neurologic irAEs (n-irAEs)—including meningitis, encephalitis, demyelinating syndromes, vasculitis, and peripheral neuropathies—are also more common with combined therapy (46). Additionally, immune checkpoint blockade alters the gut microbiome (47), which may further increase the likelihood of systemic autoimmune-like reactions (48).
Due to the severity of irAEs, systemic corticosteroids are often required for management (49). Although dual therapy offers improved intracranial response rates and prolonged survival in selected patients (37), treatment interruptions due to toxicity may compromise therapeutic efficacy and patient adherence (45). Notably, the increased incidence of neurologic irAEs has been associated with reduced overall survival (46). Therefore, effective AE management is not only essential for safety but also critical to optimizing clinical outcomes.
4.2 Challenges in treatment optimization
Another significant challenge is the suboptimal refinement of treatment regimens. Although the CTLA-4 and PD-1 combination demonstrates synergistic effects (9), current protocols lack personalization and fail to account for heterogeneity in disease presentation. Future studies must focus on tailoring regimens based on patient-specific factors to enhance therapeutic precision (50).
Furthermore, the temporal and functional differences between CTLA-4 and PD-1 regulation of T cell activity remain underutilized. While dual therapy improves intracranial responses and survival (9), it also increases irAE risk, including neurologic complications (46). Given that CTLA-4 and PD-1 modulate T cells at distinct stages of activation (12), rational treatment design should align with the drugs’ pharmacodynamic profiles rather than apply fixed combination regimens indiscriminately.
Interindividual variability in treatment response further underscores the need for precision medicine. Clinical markers such as tumor burden, lesion location, and serum lactate dehydrogenase (LDH) levels may guide treatment stratification (51). Notably, personalized strategies incorporating stereotactic radiosurgery (SRS) with PD-1 and CTLA-4 blockade have demonstrated durable intracranial control with manageable toxicity in MBM patients (50), suggesting a promising path toward maximizing efficacy while minimizing treatment-related harm.
5 Discussion
5.1 Strategies for managing adverse events
Given the high incidence of severe immune-related adverse events (irAEs), establishing a multi-tiered management framework is of paramount importance. Timely diagnosis and intervention are critical to prevent symptom escalation and the development of complications (52). This necessitates close monitoring and prompt action by experienced clinicians (49), alongside personalized treatment and management strategies tailored to individual patient factors, such as PD-L1 expression and medical history (52).
Due to the increased incidence of neurologic toxicities (53) and multi-organ irAEs associated with combination therapy (49), corticosteroids (e.g., methylprednisolone) are commonly employed as a first-line treatment. However, for severe or refractory cases, additional immunosuppressive agents may be required (49).
In parallel, the development of individualized risk assessment models has emerged as a promising direction to optimize therapy. Key biomarkers—such as MHC protein expression, CTLA-4 promoter methylation status, and immune cell profiling—can serve as predictive indicators of treatment response (54), potentially reducing therapy discontinuation and improving overall prognosis.
Moreover, optimizing combination dosing and scheduling is essential for mitigating toxicity. While dual checkpoint blockade offers substantial efficacy benefits (55), it is also associated with greater toxicity. Therefore, rational dose modulation and precise timing of drug administration are critical to achieve durable intracranial control with manageable toxicity levels in melanoma brain metastasis (MBM) patients (50).
5.2 Strategies for optimizing treatment regimens
To optimize therapeutic regimens, attention must be directed toward refining dosage, sequencing, and duration. First, the implementation of a “real-time monitoring–adaptive adjustment” strategy can be facilitated through dynamic biomarkers such as circulating tumor DNA (ctDNA) kinetics (56), the peripheral effector T cell to regulatory T cell ratio (57), and cytokine levels like IFN-γ (57). These indicators enable responsive dose adjustments during therapy.
Second, although concurrent CTLA-4 and PD-1 inhibition significantly improves outcomes in MBM (9), sequential regimens may further enhance efficacy and tolerability. Administering a CTLA-4 inhibitor as induction therapy followed by maintenance with PD-1 blockade can maximize treatment durability, improve quality of life, and balance efficacy with safety (51, 58).
Finally, tailoring dose intensity and treatment duration can strike a more favorable balance between therapeutic benefit and adverse effects, thereby achieving a more personalized and cost-effective treatment approach (59) that maximizes clinical benefit for patients.
5.3 Future perspectives
In the next 5–10 years, precision stratification systems guided by immunogenomic profiling are expected to become a prevailing trend. For instance, the density of CD16+ macrophages has been correlated with favorable responses to combination immunotherapy and may serve as a key biomarker for treatment stratification (60). Through immune microenvironment modulation, these approaches aim to enhance both the intracranial recruitment and peripheral expansion of CD8+ T cells, thereby improving the efficacy of combinatorial treatments (12). Consequently, the integration of CTLA-4/PD-1 inhibitors with small-molecule agents, antibodies, cellular therapies, and vaccines is emerging as a promising strategy for melanoma brain metastases (MBM) and warrants validation through prospective clinical trials incorporating stratified brain metastasis cohorts or dedicated MBM arms (see Table 4).
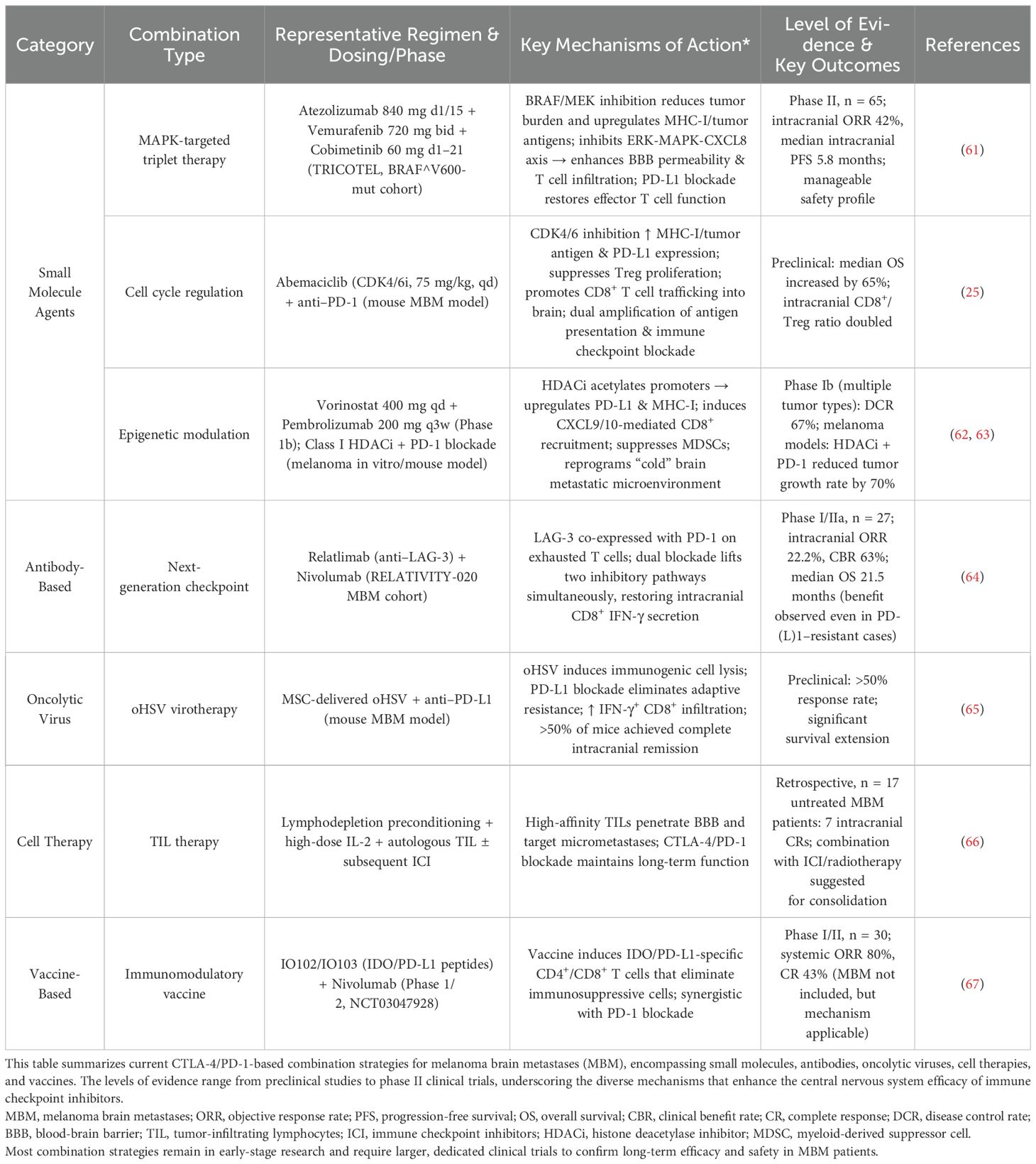
Table 4. Summary of mechanism-based CTLA-4/PD-1 combination strategies in melanoma brain metastases (MBM).
Among small-molecule agents, BRAF/MEK inhibitors (e.g., Vemurafenib/Cobimetinib) combined with PD-L1 blockade (Atezolizumab) exert dual effects: rapid tumor burden reduction via MAPK pathway inhibition and enhanced T cell infiltration by upregulating tumor antigens and MHC-I expression, thus improving blood-brain barrier (BBB) permeability. This “inhibition-then-counterattack” strategy achieves an intracranial objective response rate (ORR) of up to 42%, with a median progression-free survival (PFS) of 5.8 months (61).
CDK4/6 inhibitors (e.g., Abemaciclib) enhance immunogenicity by increasing MHC-I and tumor antigen expression while suppressing regulatory T cell (Treg) proliferation, resulting in a higher CD8+/Treg ratio and improved synergy with PD-1 blockade, ultimately extending survival (25).
HDAC inhibitors (e.g., Vorinostat), via epigenetic modulation, can upregulate PD-L1 and MHC-I, promote T cell recruitment, and suppress myeloid-derived suppressor cells (MDSCs). When combined with PD-1 inhibitors (e.g., Pembrolizumab), these agents help reprogram the “cold” brain metastasis microenvironment, inhibit tumor progression, and achieve high disease control rates (62, 63).
For immune checkpoint antibody-based therapies, co-targeting LAG-3 alongside CTLA-4 and PD-1 blockade may enhance efficacy while reducing toxicity (52). Dual inhibition of LAG-3 and PD-1 pathways effectively restores CD8+ IFN-γ production within the CNS (64), thereby improving intracranial T cell activation and migration (12). This multi-target synergistic blockade is a promising strategy to maximize clinical benefit while minimizing immune-related adverse events (irAEs) (48).
Oncolytic virus therapy utilizing mesenchymal stem cell (MSC)-delivered oHSV mediates immunogenic tumor cell lysis and releases damage/pathogen-associated molecular patterns (DAMPs/PAMPs). This activates type I interferon responses, and when combined with PD-L1 antibodies, can overcome adaptive immune resistance. In preclinical models, this strategy resulted in complete intracranial remission in the majority of treated mice (65).
In the realm of cellular therapy, tumor-infiltrating lymphocytes (TILs) infused after lymphodepleting preconditioning and supported by high-dose IL-2 can penetrate the BBB and eradicate micrometastases. Subsequent maintenance with CTLA-4/PD-1 inhibitors promotes persistence and durable responses. Notably, in a trial of 17 untreated MBM patients, 7 achieved long-term complete remission with TIL therapy alone, highlighting the potential for broader benefit when combined with ICIs (66).
With respect to immunomodulatory vaccines, the IDO/PD-L1 peptide vaccine (IO102/IO103) in combination with Nivolumab can elicit IDO/PD-L1-specific CD4+ and CD8+ T cell responses, targeting and eliminating immunosuppressive IDO+/PD-L1+ cells and converting the tumor microenvironment from “cold” to “hot.” This strategy achieved an ORR of up to 80% and a CR rate of 43%, offering a novel immunotherapeutic avenue for brain metastases (67).
Finally, the development of AI-driven systems for adverse event prediction and management may significantly reduce irAEs (68), facilitating safer and broader clinical implementation of these advanced combination strategies.
5.3.1 Permission to reuse and copyright
Permission must be obtained for use of copyrighted material from other sources (including the web). Please note that it is compulsory to follow figure instructions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
JW: Writing – original draft, Investigation, Conceptualization. YF: Project administration, Visualization, Writing – review & editing, Supervision, Conceptualization. JL: Investigation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
This is a short text to acknowledge the contributions of specific colleagues, institutions, or agencies that aided the efforts of the authors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kalluri A, Kramer P, Parker M, Jiang K, Materi J, Azad T, et al. Epid-29. Epidemiological characterization of brain metastases in melanoma: a large population based-study from 2014-2024. Neuro Oncol. (2024) 26:viii144–viii144. doi: 10.1093/neuonc/noae165.0565
2. Wang Y, Lian B, Si L, Mao L, Chi Z, Sheng X, et al. Cumulative incidence and risk factors of brain metastasis for acral and mucosal melanoma patients with stages I-III. Eur J Cancer. (2022) 175:196–203. doi: 10.1016/j.ejca.2022.08.008
3. Haydu LE, Lo SN, McQuade JL, Amaria RN, Wargo J, Ross MI, et al. Cumulative incidence and predictors of CNS metastasis for patients with American joint committee on cancer 8th edition stage III melanoma. J Clin Oncol. (2020) 38:1429–41. doi: 10.1200/JCO.19.01508
4. Spagnolo F, Picasso V, Lambertini M, Ottaviano V, Dozin B, Queirolo P, et al. Survival of patients with metastatic melanoma and brain metastases in the era of MAP-kinase inhibitors and immunologic checkpoint blockade antibodies: A systematic review. Cancer Treat Rev. (2016) 45:38–45. doi: 10.1016/j.ctrv.2016.03.003
5. Cioffi G, Ascha MS, Waite KA, Dmukauskas M, Wang X, Royce TJ, et al. Sex differences in odds of brain metastasis and outcomes by brain metastasis status after advanced melanoma diagnosis. Cancers (Basel). (2024) 16:1771. doi: 10.3390/cancers16091771
6. Saltarin F, Wegmüller A, Bejarano L, Ildiz ES, Zwicky P, Vianin A, et al. Compromised blood-brain barrier junctions enhance melanoma cell intercalation and extravasation. Cancers (Basel). (2023) 15:5071. doi: 10.3390/cancers15205071
7. Davies MA. Abstract IA05: Distinct immune and molecular features of melanoma brain metastases. Cancer Res. (2020) 80:IA05–5. doi: 10.1158/1538-7445.MEL2019-IA05
8. Fischer GM, Jalali A, Kircher DA, Lee WC, McQuade JL, Haydu LE, et al. Molecular profiling reveals unique immune and metabolic features of melanoma brain metastases. Cancer Discov. (2019) 9:628–45. doi: 10.1158/2159-8290.CD-18-1489
9. Dohm AE, Nakashima JY, Kalagotla H, Jiang SX, Tang JD, Bhandari M, et al. Stereotactic radiosurgery and anti-PD-1 + CTLA-4 therapy, anti-PD-1 therapy, anti-CTLA-4 therapy, BRAF/MEK inhibitors, BRAF inhibitors, or conventional chemotherapy for the management of melanoma brain metastases. Eur J Cancer. (2023) 192:113287. doi: 10.1016/j.ejca.2023.113287
10. Akinleye A and Rasool Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol. (2019) 12:92. doi: 10.1186/s13045-019-0779-5
11. Tawbi HA, Forsyth PA, Algazi A, Hamid O, Hodi FS, Moschos SJ, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. (2018) 379:722–30. doi: 10.1056/NEJMoa1805453
12. Taggart D, Andreou T, Scott KJ, Williams J, Rippaus N, Brownlie RJ, et al. Anti-PD-1/anti-CTLA-4 efficacy in melanoma brain metastases depends on extracranial disease and augmentation of CD8(+) T cell trafficking. Proc Natl Acad Sci U.S.A. (2018) 115:E1540–9. doi: 10.1073/pnas.1714089115
13. Wilhelm I, Molnár J, Fazakas C, Haskó J, and Krizbai IA. Role of the blood-brain barrier in the formation of brain metastases. Int J Mol Sci. (2013) 14:1383–411. doi: 10.3390/ijms14011383
14. Küsters B, Leenders WP, Wesseling P, Smits D, Verrijp K, Ruiter DJ, et al. Vascular endothelial growth factor-A(165) induces progression of melanoma brain metastases without induction of sprouting angiogenesis. Cancer Res. (2002) 62:341–5. Available online at: https://pubmed.ncbi.nlm.nih.gov/11809675/ (Accessed June 22, 2025).
15. Wang P, Wu Y, Chen W, Zhang M, and Qin J. Malignant melanoma-derived exosomes induce endothelial damage and glial activation on a human BBB chip model. Biosensors (Basel). (2022) 12:89. doi: 10.3390/bios12020089
16. Alvarez-Breckenridge C, Markson SC, Stocking JH, Nayyar N, Lastrapes M, Strickland MR, et al. Microenvironmental landscape of human melanoma brain metastases in response to immune checkpoint inhibition. Cancer Immunol Res. (2022) 10:996–1012. doi: 10.1158/2326-6066.CIR-21-0870
17. Arvanitis CD, Ferraro GB, and Jain RK. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat Rev Cancer. (2020) 20:26–41. doi: 10.1038/s41568-019-0205-x
18. Wu A, Wei J, Kong L, Wang Y, Priebe W, Qiao W, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. (2010) 12:1113–25. doi: 10.1093/neuonc/noq082
19. Izraely S, Ben-Menachem S, Sagi-Assif O, Telerman A, Zubrilov I, Ashkenazi O, et al. The metastatic microenvironment: Melanoma-microglia cross-talk promotes the Malignant phenotype of melanoma cells. Int J Cancer. (2019) 144:802–17. doi: 10.1002/ijc.31745
20. Klein A, Schwartz H, Sagi-Assif O, Meshel T, Izraely S, Ben Menachem S, et al. Astrocytes facilitate melanoma brain metastasis via secretion of IL-23. J Pathol. (2015) 236:116–27. doi: 10.1002/path.4509
21. Ungefroren H. Blockade of TGF-β signaling: a potential target for cancer immunotherapy? Expert Opin Ther Targets. (2019) 23:679–93. doi: 10.1080/14728222.2019.1636034
22. Biermann J, Melms JC, Amin AD, Wang Y, Caprio LA, Karz A, et al. Dissecting the treatment-naive ecosystem of human melanoma brain metastasis. Cell. (2022) 185:2591–2608.e30. doi: 10.1016/j.cell.2022.06.007
23. Chen S, Crabill GA, Pritchard TS, McMiller TL, Wei P, Pardoll DM, et al. Mechanisms regulating PD-L1 expression on tumor and immune cells. J Immunother Cancer. (2019) 7:305. doi: 10.1186/s40425-019-0770-2
24. Wang JJ, Burger P, Taube J, Soni A, Chaichana K, Sheu M, et al. PD-L1, PD-1, LAG-3, and TIM-3 in melanoma: expression in brain metastases compared to corresponding extracranial tumors. Cureus. (2019) 11:e6352. doi: 10.7759/cureus.6352
25. Nayyar N, Sauvage MA, Chuprin J, Sullivan EM, Singh M, Torrini C, et al. CDK4/6 inhibition sensitizes intracranial tumors to PD-1 blockade in preclinical models of brain metastasis. Clin Cancer Res. (2024) 30:420–35. doi: 10.1158/1078-0432.CCR-23-0433
26. Benboubker V, Boivin F, Dalle S, and Caramel J. Cancer cell phenotype plasticity as a driver of immune escape in melanoma. Front Immunol. (2022) 13:873116. doi: 10.3389/fimmu.2022.873116
27. Frangieh CJ, Melms JC, Thakore PI, Geiger-Schuller KR, Ho P, Luoma AM, et al. Multimodal pooled Perturb-CITE-seq screens in patient models define mechanisms of cancer immune evasion. Nat Genet. (2021) 53:332–41. doi: 10.1038/s41588-021-00779-1
28. Ovcinnikovs V, Ross EM, Petersone L, Edner NM, Heuts F, Ntavli E, et al. CTLA-4-mediated transendocytosis of costimulatory molecules primarily targets migratory dendritic cells. Sci Immunol. (2019) 4:eaaw0902. doi: 10.1126/sciimmunol.aaw0902
29. Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. (2014) 371:2189–99. doi: 10.1056/NEJMoa1406498
30. Romano E, Kusio-Kobialka M, Foukas PG, Bichat H, Baumgaertner P, Meyer C, et al. FcgRIIIA (CD16)-expressing monocytes mediate the depletion of tumor-infiltrating Tregs via Ipilimumab-dependent ADCC in melanoma patients. J ImmunoTherapy Cancer. (2014) 2:O14. doi: 10.1186/2051-1426-2-S3-O14
31. Rishi A and Yu HM. Current treatment of melanoma brain metastasis. Curr Treat Options Oncol. (2020) 21:45. doi: 10.1007/s11864-020-00733-z
32. Manacorda S, Carmena Toro De M, Malone C, Le HML, Furness AJS, Larkin J, et al. Ipilimumab plus nivolumab in patients with symptomatic melanoma brain metastasis requiring corticosteroids. Eur J Cancer. (2023) 188:98–107. doi: 10.1016/j.ejca.2023.04.018
33. Kluger HM, Zito CR, Barr ML, Baine MK, Chiang VLS, Sznol M, et al. Characterization of PD-L1 expression and associated T-cell infiltrates in metastatic melanoma samples from variable anatomic sites. Clin Cancer Res. (2015) 21:3052–60. doi: 10.1158/1078-0432.CCR-14-3073
34. Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. (2009) 114:1537–44. doi: 10.1182/blood-2008-12-195792
35. Wei SC, Levine JH, Cogdill AP, Zhao Y, Anang N-AS, Andrews MC, et al. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell. (2017) 170:1120–1133.e17. doi: 10.1016/j.cell.2017.07.024
36. Alvarez-Breckenridge C, Markson S, Stocking J, Lastrapes M, Nayyar N, Bertalan M, et al. Immu-01. Single cell sequencing of melanoma brain metastases unveils heterogeneity of the tumor microenvironment in response to immune checkpoint blockade. Neuro Oncol. (2020) 22:ii104–4. doi: 10.1093/neuonc/noaa215.432
37. Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. (2018) 19:672–81. doi: 10.1016/S1470-2045(18)30139-6
38. Gangaev A, Rozeman EA, Rohaan MW, Philips D, Patiwael S, Berg den van JH, et al. Differential effects of PD-1 and CTLA-4 blockade on the melanoma-reactive CD8 T cell response. bioRxiv. (2020) 2020:12. doi: 10.1101/2020.12.15.422827
39. Huang AY, Vokes N, Ricker C, Aprati T, Robitschek E, Yang J, et al. Abstract 3271: Multiomic meta-analysis of differential response to PD-1 and CTLA-4 blockade in metastatic melanoma. Cancer Res. (2023) 83:3271–1. doi: 10.1158/1538-7445.AM2023-3271
40. Fife C, Williams J, James F, Gregory S, Andreou T, Sunderland A, et al. Natural killer cells are required for the recruitment of CD8+ T cells and the efficacy of immune checkpoint blockade in melanoma brain metastases. J Immunother Cancer. (2024) 12:e009522. doi: 10.1136/jitc-2024-009522
41. Tekguc M, Wing JB, Osaki M, Long J, and Sakaguchi S. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc Natl Acad Sci U.S.A. (2021) 118:e2023739118. doi: 10.1073/pnas.2023739118
42. Geels SN, Moshensky A, Sousa RS, Murat C, Bustos MA, Walker BL, et al. Interruption of the intratumor CD8(+) T cell:Treg crosstalk improves the efficacy of PD-1 immunotherapy. Cancer Cell. (2024) 42:1051–1066.e7. doi: 10.1016/j.ccell.2024.05.013
43. Ribas A, Shin DS, Zaretsky J, Frederiksen J, Cornish A, Avramis E, et al. PD-1 blockade expands intratumoral memory T cells. Cancer Immunol Res. (2016) 4:194–203. doi: 10.1158/2326-6066.CIR-15-0210
44. Nayyar N, Singh M, Stocking J, Brehm M, and Brastianos P. Tmod-05. Extracranial tumors influence intracranial response to immune checkpoint inhibitors in pre-clinical models of melanoma brain metastasis. Neuro Oncol. (2020) 22:ii228–8. doi: 10.1093/neuonc/noaa215.956
45. Carlino MS, Larkin J, and Long GV. Immune checkpoint inhibitors in melanoma. Lancet. (2021) 398:1002–14. doi: 10.1016/S0140-6736(21)01206-X
46. Das N, Dhamija R, Kaelber D, Kelly M, Xie P, Reddy D, et al. Ncmp-07. Adverse neurologic complications following immune checkpoint inhibitors for treatment of melanoma: comparative analysis of pd-1 inhibitor monotherapy to combination therapy with ctla-4 inhibitors. Neuro Oncol. (2024) 26:viii218–viii218. doi: 10.1093/neuonc/noae165.0860
47. Andrews MC, Duong CPM, Gopalakrishnan V, Iebba V, Chen W-S, Derosa L, et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat Med. (2021) 27:1432–41. doi: 10.1038/s41591-021-01406-6
48. Willsmore ZN, Coumbe BGT, Crescioli S, Reci S, Gupta A, Harris RJ, et al. Combined anti-PD-1 and anti-CTLA-4 checkpoint blockade: Treatment of melanoma and immune mechanisms of action. Eur J Immunol. (2021) 51:544–56. doi: 10.1002/eji.202048747
49. Hassel JC, Heinzerling L, Aberle J, Bähr O, Eigentler TK, Grimm M-O, et al. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): Evaluation and management of adverse drug reactions. Cancer Treat Rev. (2017) 57:36–49. doi: 10.1016/j.ctrv.2017.05.003
50. Tang J, Dohm A, Kalagotla H, Bhandari M, Kim Y, Graham J, et al. Radt-11. Clinical outcomes in the management of melanoma brain metastases treated with stereotactic radiosurgery and anti-pd-1+ctla-4. Neuro Oncol. (2022) 24:vii51–1. doi: 10.1093/neuonc/noac209.201
51. Phadke MS, Li J, Chen Z, Rodriguez PC, Mandula JK, Karapetyan L, et al. Differential requirements for CD4+ T cells in the efficacy of the anti-PD-1+LAG-3 and anti-PD-1+CTLA-4 combinations in melanoma flank and brain metastasis models. J Immunother Cancer. (2023) 11:e007239. doi: 10.1136/jitc-2023-007239
52. Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res. (2019) 38:255. doi: 10.1186/s13046-019-1259-z
53. Das N, Dhamija R, Kaelber DC, Kalagotla H, Reddy D, Vogelbaum M, et al. Adverse neurologic events of immune checkpoint inhibitor monotherapy vs. Combination therapy for melanoma. Neuro-Oncology Adv. (2025) 7(1):vdaf030. doi: 10.1093/noajnl/vdaf030
54. Subrahmanyam PB, Dong Z, Gusenleitner D, Giobbie-Hurder A, Severgnini M, Zhou J, et al. Distinct predictive biomarker candidates for response to anti-CTLA-4 and anti-PD-1 immunotherapy in melanoma patients. J Immunother Cancer. (2018) 6:18. doi: 10.1186/s40425-018-0328-8
55. Wu K, Yi M, Qin S, Chu Q, Zheng X, and Wu K. The efficacy and safety of combination of PD-1 and CTLA-4 inhibitors: a meta-analysis. Exp Hematol Oncol. (2019) 8:26. doi: 10.1186/s40164-019-0150-0
56. Forschner A, Battke F, Hadaschik D, Schulze M, Weißgraeber S, Han C-T, et al. Tumor mutation burden and circulating tumor DNA in combined CTLA-4 and PD-1 antibody therapy in metastatic melanoma - results of a prospective biomarker study. J Immunother Cancer. (2019) 7:180. doi: 10.1186/s40425-019-0659-0
57. Curran MA, Montalvo W, Yagita H, and Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U.S.A. (2010) 107:4275–80. doi: 10.1073/pnas.0915174107
58. Li S, Duan R, Tang B, Sheng X, Si L, Cui C, et al. A phase I/II study of KD6001, a novel fully human anti-CTLA4 IgG1 monoclonal antibody, in combination with toripalimab in patients with advanced melanoma. J Clin Oncol. (2024) 42:9527–7. doi: 10.1200/JCO.2024.42.16_suppl.9527
59. Wang K, Coutifaris P, Brocks D, Wang G, Azar T, Solis S, et al. Combination anti-PD-1 and anti-CTLA-4 therapy generates waves of clonal responses that include progenitor-exhausted CD8(+) T cells. Cancer Cell. (2024) 42:1582–1597.e10. doi: 10.1016/j.ccell.2024.08.007
60. Lee H, Ferguson AL, Quek C, Vergara IA, Silva Pires I, Allen R, et al. Intratumoral CD16+ Macrophages are associated with clinical outcomes of patients with metastatic melanoma treated with combination anti-PD-1 and anti-CTLA-4 therapy. Clin Cancer Res. (2023) 29:2513–24. doi: 10.1158/1078-0432.CCR-22-2657
61. Dummer R, Queirolo P, Guijarro Abajo AM, Hu Y, Wang D, Azevedo SJ, et al. Atezolizumab, vemurafenib, and cobimetinib in patients with melanoma with CNS metastases (TRICOTEL): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. (2023) 24:e461–71. doi: 10.1016/S1470-2045(23)00334-0
62. Gray JE, Saltos A, Tanvetyanon T, Haura EB, Creelan B, Antonia SJ, et al. Phase I/ib study of pembrolizumab plus vorinostat in advanced/metastatic non-small cell lung cancer. Clin Cancer Res. (2019) 25:6623–32. doi: 10.1158/1078-0432.CCR-19-1305
63. Woods DM, Sodré AL, Villagra A, Sarnaik A, Sotomayor EM, and Weber J. HDAC inhibition upregulates PD-1 ligands in melanoma and augments immunotherapy with PD-1 blockade. Cancer Immunol Res. (2015) 3:1375–85. doi: 10.1158/2326-6066.CIR-15-0077-T
64. Cortese T. Nivolumab/relatlimab elicit IC activity in anti–PD-L1–refractory melanoma brain mets. Cancer Network. (2025). Available online at: https://www.cancernetwork.com/view/nivolumab-relatlimab-elicit-ic-activity-in-anti-pd-l1-refractory-melanoma-brain-mets (Accessed June 22, 2025).
65. Chen C-Y, Hutzen B, Wedekind MF, and Cripe TP. Oncolytic virus and PD-1/PD-L1 blockade combination therapy. Oncolytic Virother. (2018) 7:65–77. doi: 10.2147/OV.S145532
66. Mehta GU, Malekzadeh P, Shelton T, White DE, Butman JA, Yang JC, et al. Outcomes of adoptive cell transfer with tumor-infiltrating lymphocytes for metastatic melanoma patients with and without brain metastases. J Immunother. (2018) 41:241–7. doi: 10.1097/CJI.0000000000000223
67. Kjeldsen JW, Lorentzen CL, Martinenaite E, Ellebaek E, Donia M, Holmström RB, et al. A phase 1/2 trial of an immune-modulatory vaccine against IDO/PD-L1 in combination with nivolumab in metastatic melanoma. Nat Med. (2021) 27:2212–23. doi: 10.1038/s41591-021-01544-x
68. Ahmed KA, Abuodeh YA, Echevarria MI, Arrington JA, Stallworth DG, Hogue C, et al. Clinical outcomes of melanoma brain metastases treated with stereotactic radiosurgery and anti-PD-1 therapy, anti-CTLA-4 therapy, BRAF/MEK inhibitors, BRAF inhibitor, or conventional chemotherapy. Ann Oncol. (2016) 27:2288–94. doi: 10.1093/annonc/mdw417
69. Buchbinder E and Hodi FS. Cytotoxic T lymphocyte antigen-4 and immune checkpoint blockade. J Clin Invest. (2015) 125:3377–83. doi: 10.1172/JCI80012
70. Peggs KS, Quezada SA, Chambers CA, Korman AJ, and Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. (2009) 206:1717–25. doi: 10.1084/jem.20082492
71. Felix J, Lambert J, Roelens M, Maubec E, Guermouche H, Pages C, et al. Ipilimumab reshapes T cell memory subsets in melanoma patients with clinical response. Oncoimmunology. (2016) 5:1136045. doi: 10.1080/2162402X.2015.1136045
72. Tietze JKT, Angelova D, Heppt MV, Reinholz M, Murphy WJ, Spannagl M, et al. The proportion of circulating CD45RO(+)CD8(+) memory T cells is correlated with clinical response in melanoma patients treated with ipilimumab. Eur J Cancer. (2017) 75:268–79. doi: 10.1016/j.ejca.2016.12.031
73. Knisely JPS, Yu JB, Flanigan J, Sznol M, Kluger HM, Chiang VLS, et al. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg. (2012) 117:227–33. doi: 10.3171/2012.5.JNS111929
74. Ji R-R, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother. (2012) 61:1019–31. doi: 10.1007/s00262-011-1172-6
75. Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. (2012) 13:459–65. doi: 10.1016/S1470-2045(12)70090-6
76. Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. (2018) 560:382–6. doi: 10.1038/s41586-018-0392-8
77. Hirschhorn D, Budhu S, Kraehenbuehl L, Gigoux M, Schröder D, Chow A, et al. T cell immunotherapies engage neutrophils to eliminate tumor antigen escape variants. Cell. (2023) 186:1432–1447.e17. doi: 10.1016/j.cell.2023.03.007
78. Chae YK, Arya A, Iams W, Cruz MR, Chandra S, Choi J, et al. Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC). J Immunother Cancer. (2018) 6:39. doi: 10.1186/s40425-018-0349-3
Keywords: melanoma brain metastases, CTLA-4, PD-1, combined blockade therapy, immune checkpoint inhibitors, adverse event management, precision therapy
Citation: Wang J-W, Feng Y-F and Liu J-H (2025) CTLA-4 and PD-1 combined blockade therapy for malignant melanoma brain metastases: mechanisms, challenges, and prospects. Front. Immunol. 16:1629879. doi: 10.3389/fimmu.2025.1629879
Received: 16 May 2025; Accepted: 13 June 2025;
Published: 01 July 2025.
Edited by:
Dimitrios Ziogas, National and Kapodistrian University of Athens, GreeceReviewed by:
Kevinn Eddy, Calder Biosciences, Inc, United StatesCopyright © 2025 Wang, Feng and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying-Fa Feng, NDgyMDMxOTJAaGVibXUuZWR1LmNu
 Jia-Wen Wang
Jia-Wen Wang Ying-Fa Feng
Ying-Fa Feng