- 1Department of Nephrology, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2Traditional Chinese Medicine (TCM) Institute of Kidney Disease of Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 3Key Laboratory of Liver and Kidney Diseases, Ministry of Education, Shanghai, China
- 4Shanghai Key Laboratory of Traditional Chinese Clinical Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 5Department of Gastroenterology, The Affiliated Hospital of Southwest Medical University, Luzhou, China
- 6Department of Hematology, Shanghai Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 7Department of Nephrology, Shanghai Tenth People’s Hospital Affiliated to Tongji University, Shanghai, China
Renal cell carcinoma (RCC) is a highly vascularized and immunogenic malignancy with a complex tumor microenvironment (TME) that shapes disease progression and therapeutic resistance. Despite advances in immune checkpoint inhibitors (ICIs) and targeted therapies, clinical responses remain heterogeneous, underscoring the need for a deeper understanding of RCC immunobiology. This review comprehensively examines the immunosuppressive TME of RCC, emphasizing the roles of cytotoxic and immunosuppressive immune cells, carcinoma-associated fibroblasts (CAFs), abnormal vasculature, and extracellular matrix (ECM) remodeling in fostering immune evasion. This review summarized emerging biomarkers—including PD-L1 expression, tumor mutational burden (TMB), gene mutations, and immune-based subtypes—that may predict ICI response. Furthermore, we evaluate current immunotherapeutic strategies, such as ICIs, combination therapies, and novel approaches targeting immunosuppressive cells and metabolic pathways. While combination therapies have improved outcomes, challenges like toxicity and resistance persist, necessitating biomarker-driven patient stratification and optimized treatment sequencing. Future directions should focus on deciphering TME heterogeneity and developing precision immunotherapy strategies to enhance clinical efficacy in RCC.
1 Introduction
Renal cell carcinoma (RCC), a lethal genitourinary tumor originating from renal tubular epithelial cells, ranks among the top fifteen cancers globally (1). It exhibits a 30–40% mortality rate, with higher prevalence in males. Risk factors include obesity, hypertension, smoking, and chronic kidney disease (2). Early-stage RCC is often asymptomatic; however, advances in CT, MRI, PET-CT, and genetic testing have improved detection, with over 60% of cases diagnosed incidentally. While early-stage patients benefit from surgery, 30% present with metastasis at diagnosis. Post-surgical recurrence occurs in 30–40% of advanced cases, and 50% develop distant metastases, leading to poor prognosis (3, 4).
Clear cell RCC (ccRCC) is the most common subtype and dominates metastatic RCC (mRCC) pathology. Due to RCC’s resistance to radiation/chemotherapy, targeted therapy has been the first-line treatment, though drug resistance remains inevitable (5, 6). Immune checkpoint inhibitors (ICI) show efficacy, but only a subset of patients show response, potentially due to the immunosuppressive tumor microenvironment (TME) (7, 8). RCC TME features extensive immune infiltration, vascularity, and fibrosis, enabling immunotherapy but also influencing treatment resistance via complex interactions (9, 10). Recent therapeutic strategies for advanced RCC have evolved from targeted therapy to combined targeted/immunotherapy approaches (11, 12). This review discusses RCC TME crosstalk, clinical immunotherapy progress, and emerging TME-based biomarkers/therapeutic targets.
2 Characteristics of the TME in renal cell carcinoma
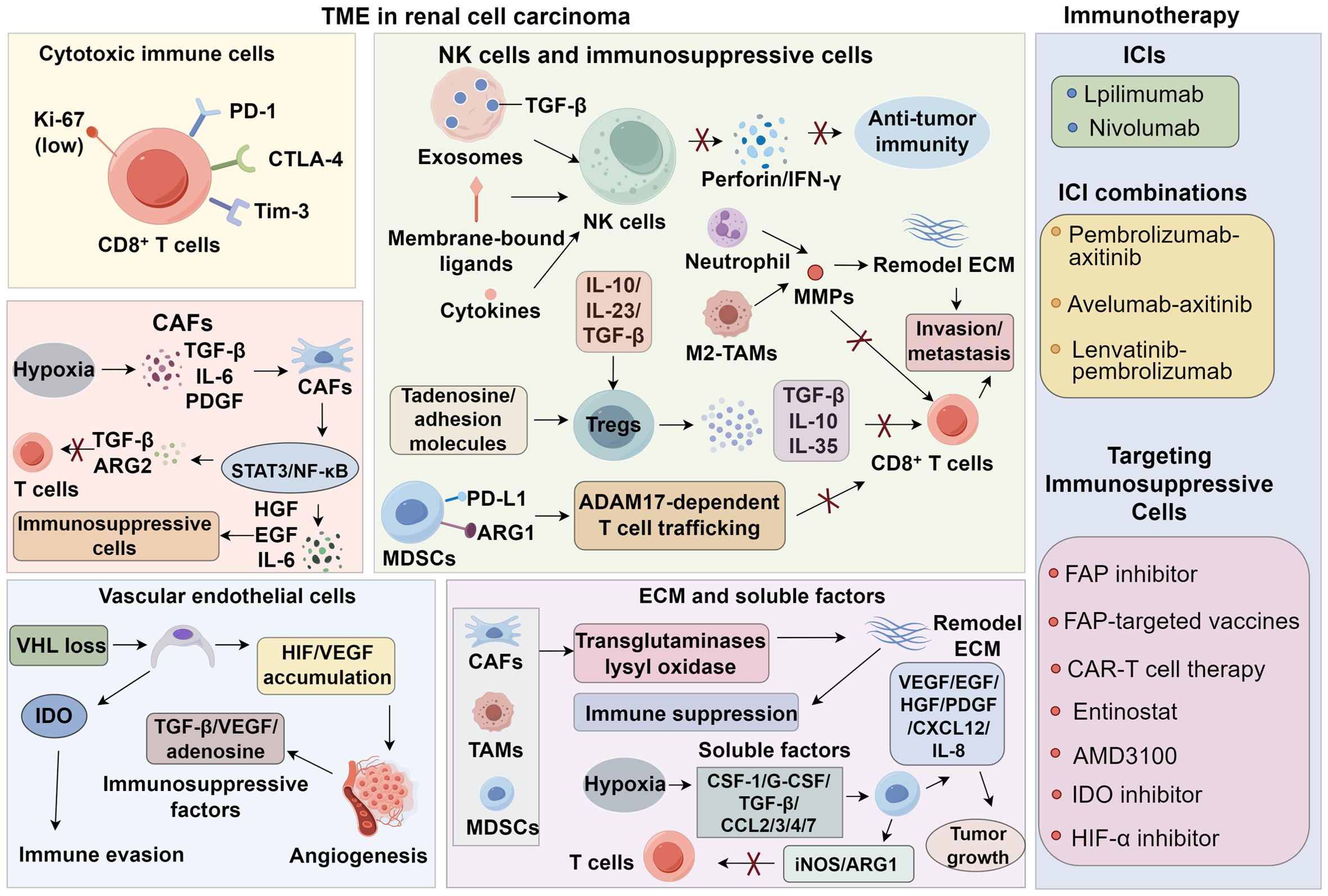
2.1 Cytotoxic immune cells
CD8+ T cells infiltrating the RCC TME frequently exhibit high expression of inhibitory checkpoint receptors—including programmed death-1 (PD-1), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), and T cell immunoglobulin and mucin domain-containing protein 3 (Tim-3)—alongside low levels of proliferative markers such as Ki-67, suggesting a state of dysfunction and exhaustion (13, 14). In a meta-analysis of 124 studies, Fridman et al. (15) reported that, unlike in most solid tumors, CD8+ T cell infiltration in RCC correlates with poorer prognosis. While the underlying mechanism remains unclear, one hypothesis is that prolonged exposure to immunosuppressive cells and factors within the RCC TME impairs CD8+ T cells’ ability to recognize antigens, proliferate, and secrete interleukin-2 (IL-2), ultimately abrogating their cytotoxic functions (16). Recent mechanistic studies indicate that chronic antigen stimulation activates NFAT in the absence of AP−1, which drives the transcription of TOX and WNK1, committing CD8+ T cells to an exhausted phenotype (17–19). In RCC, tumor-derived PD-L1 binds PD-1 on CD8+ T cells, recruiting SLC11A1 and inactivating ZAP70 and PI3K/AKT signaling, while CTLA−4 competes for B7 ligands on APCs to prevent costimulation (20–24). Additionally, Tim−3–Galectin−9 interactions promote Batf expression, further enforcing the exhausted transcriptional program (25). These events converge to reduce granzyme B production, IFN-γ secretion, and proliferative capacity. Single-cell RNA-seq studies in melanoma and RCC now reveal distinct subsets of exhausted T cells, characterized by high expression of PD−1, TOX, and CXCL13, suggesting specialized niches where these exhausted cells localize (26, 27). Therefore, the functional status of CD8+ T cells is pivotal in determining both patient prognosis and the efficacy of immunotherapies. Recent advances in single-cell RNA sequencing (scRNA-seq) have enabled detailed profiling of exhausted CD8+ T cells, and this technology has already been applied successfully in melanoma studies (28). Implementing scRNA-seq to characterize RCC-specific TME features may help elucidate the long-observed inverse association between CD8+ T cell infiltration and clinical outcomes in RCC (29).
2.2 Natural killer cells and immunosuppressive cells
NK cells are another major cytotoxic population capable of mediating anti-tumor immunity through perforin and interferon-γ (IFN-γ) release without prior sensitization (30). Remark et al. (31) demonstrated a positive correlation between NK cell infiltration and favorable prognosis in RCC. However, soluble cytokines, membrane-bound ligands, and TGF-β-enriched exosomes derived from tumor cells and immunosuppressive cells can inhibit NK cell degranulation and cytotoxicity (32–34). In addition to soluble TGF−β, RCC-derived exosomes carry TGF−β and immunomodulatory miRNAs (miR−23a, miR−146a), which are internalized by NK cells and lead to the downregulation of activating receptors such as NKG2D, NKp30, and NKp44 (35–38). This receptor loss reduces their ability to recognize and lyse tumor cells. Furthermore, CAFs secrete abundant prostaglandin E2 (PGE2), which acts on EP2/EP4 receptors expressed by NK cells (39, 40). Engagement of these receptors triggers the cAMP–PKA–CREB signaling cascade, suppressing the transcription of genes involved in cytotoxic granule formation and IFN−γ production (41–43). The net effect is impaired NK cell proliferation, decreased granule exocytosis, and weakened target cell killing capacity (44). These mechanisms, combined with other immunosuppressive metabolites (adenosine), synergistically dampen NK cell cytotoxicity within the RCC TME (45). Mechanistically, TGF−β binds TGFβRII on NK cells, activating SMAD2/3, which downregulates NKG2D and perforin expression; tumor-derived adenosine acts via A2A receptors to activate PKA signaling, suppressing NK metabolism and granule release (46–48). As a result, the functional capacity of tumor-infiltrating NK cells is often compromised. Therefore, strategies aimed at restoring NK cell activity are critical for enhancing the efficacy of ICIs in RCC (49).
Regulatory T cells (Tregs), a CD4+ T cell subset with immunosuppressive function, are essential for immune homeostasis but promote immune evasion in the RCC TME (50). Tumor and stromal cells secrete IL-10, IL-23, TGF-β, adenosine, and adhesion molecules to recruit Tregs, which suppress CD8+ T cells via TGF-β, IL-10, and IL-35 (51, 52). Although associated with poor prognosis, the precise role of Tregs in RCC remains unclear and requires further elucidation (53). Tumor-associated macrophages (TAMs), the dominant myeloid population in RCC, polarize into pro-inflammatory M1 and immunosuppressive M2 phenotypes (54). Elevated M2 or M2/M1 ratios correlate with poor outcomes (55). CSF1/CSF1R and IL−4/STAT6 signaling are major inducers of M2 polarization (56, 57). M2 TAMs secrete IL-10, CCL17/22, and VEGF, while activating PI3K/AKT and STAT3 pathways in tumor cells, which promotes proliferation and immune evasion (58–60). RCC-derived M-CSF promotes M2 polarization, comprising up to 20.9% of immune cells (61). M2-TAMs inhibit CD8+ T cell cytotoxicity, recruit suppressive cells, and remodel the ECM via MMPs, aiding invasion and metastasis (62). Chevrier et al. identified 17 TAM states, linking CD38+M5 TAMs to T cell exhaustion and Tregs, while high M11/M13 and low M5 TAM levels predicted shorter progression-free survival, suggesting therapeutic potential in TAM modulation. Myeloid-derived suppressor cells (MDSCs) inhibit CD8+ T cells through PD-L1 expression, ARG1-mediated amino acid depletion, and ADAM17-dependent T cell trafficking. MDSCs also promote immunosuppressive ECM remodeling via MMPs and iNOS (63, 64). ARG1 depletes arginine, limiting TCR ζ-chain expression; iNOS-derived NO leads to nitration of TCR complexes, impairing signal transduction, while NF-κB signaling within MDSCs maintains their suppressive function (65–67). Tie2-expressing monocytes (TEMs) facilitate angiogenesis and RCC progression (68). Neutrophil proteases also remodel the ECM via PAD4-mediated chromatin decondensation, promote invasion, induce T cell exclusion/exhaustion, and contribute to TKI resistance and poor prognosis in RCC (69).
2.3 Carcinoma-associated fibroblasts
CAFs, the most abundant stromal cell type in RCC, are central to tumor growth, metastasis, drug resistance, and immune evasion (70, 71). Under hypoxia and oxidative stress, tumor cells secrete TGF-β, IL-6, and platelet-derived growth factor (PDGF), activating CAF precursors, which upregulate fibroblast activation protein (FAP) (72). Activated CAFs stimulate pro-inflammatory signaling pathways such as STAT3 and NF-κB, and secrete hepatocyte growth factor (HGF), epidermal growth factor (EGF), and IL-6 to recruit Treg and activate immunosuppressive cells (73–75). Through secretion of TGF−β and ARG2, CAFs induce M2 polarization of TAMs and expansion of Tregs, while CXCL12 produced by CAFs engages CXCR4 on T cells, forming a “chemokine barrier” that excludes CD8+ T cells from tumor nests (76, 77). CAFs can also directly inhibit cytotoxic immune cells via TGF-β and ARG2 secretion (78). As “architects” of the TME, CAFs produce ECM components and facilitate tumor progression and metastasis (79, 80). The immunosuppressive nature of CAFs underlies the poor responsiveness of fibrotic tumors to therapy, yet their ubiquity offers multiple therapeutic targets (81, 82). Although anti-CAF therapies have shown promise in breast and pancreatic cancers, CAF heterogeneity across tumor types necessitates further investigation into RCC-specific CAF-targeting strategies (83).
2.4 Vascular endothelial cells
RCC is among the most vascularized tumors, a feature strongly associated with early biallelic inactivation of the tumor suppressor gene von Hippel–Lindau (VHL) (84). VHL negatively regulates hypoxia-inducible factor (HIF), and its loss leads to HIF accumulation and subsequent overproduction of vascular endothelial growth factor (VEGF), promoting tumor angiogenesis (85). VEGF binds VEGFR2 on endothelial cells, activating PI3K–AKT and MAPK/ERK pathways to promote angiogenesis. The resulting abnormal vessels express FasL and downregulate adhesion molecules (ICAM-1, VCAM-1), creating a physical and biochemical barrier to immune (86–88). Abnormal vasculature impairs perfusion, leading to hypoxia, acidosis, and reduced drug penetration. These conditions further induce immunosuppressive factors such as TGF-β, VEGF, and adenosine, and downregulate endothelial adhesion molecules, impeding immune cell adhesion, trafficking, and infiltration (89). While microvascular density serves as a prognostic indicator in cancers such as oral cancer, its prognostic value in RCC remains controversial due to variability in vascular morphology and differentiation (90, 91). Moreover, endothelial cells in RCC express high levels of indoleamine 2,3-dioxygenase (IDO) under IFN-γ stimulation, triggering tryptophan catabolism via the kynurenine pathway. Kynurenine activates aryl hydrocarbon receptor (AHR) in T cells, inducing FOXP3 expression and generating Tregs, further promoting immunosuppression (92).
2.5 Extracellular matrix and soluble factors
RCC progression involves extensive ECM deposition, providing structural support, biomechanical signaling, and regulation of cell behavior (93). The ECM, primarily secreted by CAFs, consists of collagens, laminins, glycoproteins, fibronectin, proteoglycans, and polysaccharides (94). Matrix remodeling is mediated by enzymes such as MMP2/9 and lysyl oxidase (LOX), which are activated by TGF−β and hypoxia (HIF−1α). These pathways stiffen the ECM and impair immune cell infiltration (95–97). TAMs, MDSCs, and CAFs secrete transglutaminases and lysyl oxidase, remodeling the ECM to induce collagen rearrangement, matrix stiffening, and reduced permeability, forming a barrier against cytotoxic immune infiltration (98). ECM remodeling also causes mechanical stress, impairing vascular function and promoting immune suppression (99). The remodeled ECM harbors abundant soluble mediators that facilitate bidirectional communication between tumor epithelial and stromal compartments, thereby promoting RCC invasion and metastasis (100, 101). Hypoxia and necrosis in rapidly growing tumors trigger the release of CSF-1, G-CSF, TGF-β, and chemokines (CCL2/3/4/7), recruiting myeloid cells (102–105). These cells, in turn, secrete VEGF, EGF, HGF, PDGF, CXCL12, and IL-8 to sustain tumor growth, angiogenesis, and immune infiltration (106, 107). In addition to amino acid depletion via iNOS and arginase-1, metabolic reprogramming driven by HIF signaling profoundly affects the immunosuppressive milieu (108, 109). RCC cells preferentially undergo aerobic glycolysis, resulting in excess lactate production and extracellular acidification (110). Elevated lactate concentrations reduce the glycolytic capacity of CD8+ T cells, suppress mTOR signaling, and promote a state of metabolic exhaustion (111). Lactate also enhances histone lactylation, which epigenetically upregulates PD−1 expression, thereby intensifying T cell dysfunction in synergy with PD−1/PD−L1 signaling (112, 113). Moreover, lactate accumulation favors the expansion of Tregs and M2-polarized macrophages, creating a positive feedback loop that reinforces immune evasion (114, 115). These mechanisms intersect with IDO- and arginase-mediated nutrient depletion, collectively dampening T cell activation and effector function within the RCC TME. Depletion of specific soluble factors also plays a critical role in immune evasion. Tumor cells consume large quantities of glucose and glutamine, the latter being essential for T-bet expression and CD4+ T cell differentiation (116). Enzymes such as iNOS and ARG1 from myeloid cells and CAFs and IDO from endothelial cells deplete essential amino acids and generate toxic metabolites, directly impairing T cell function (117) (Table 1).
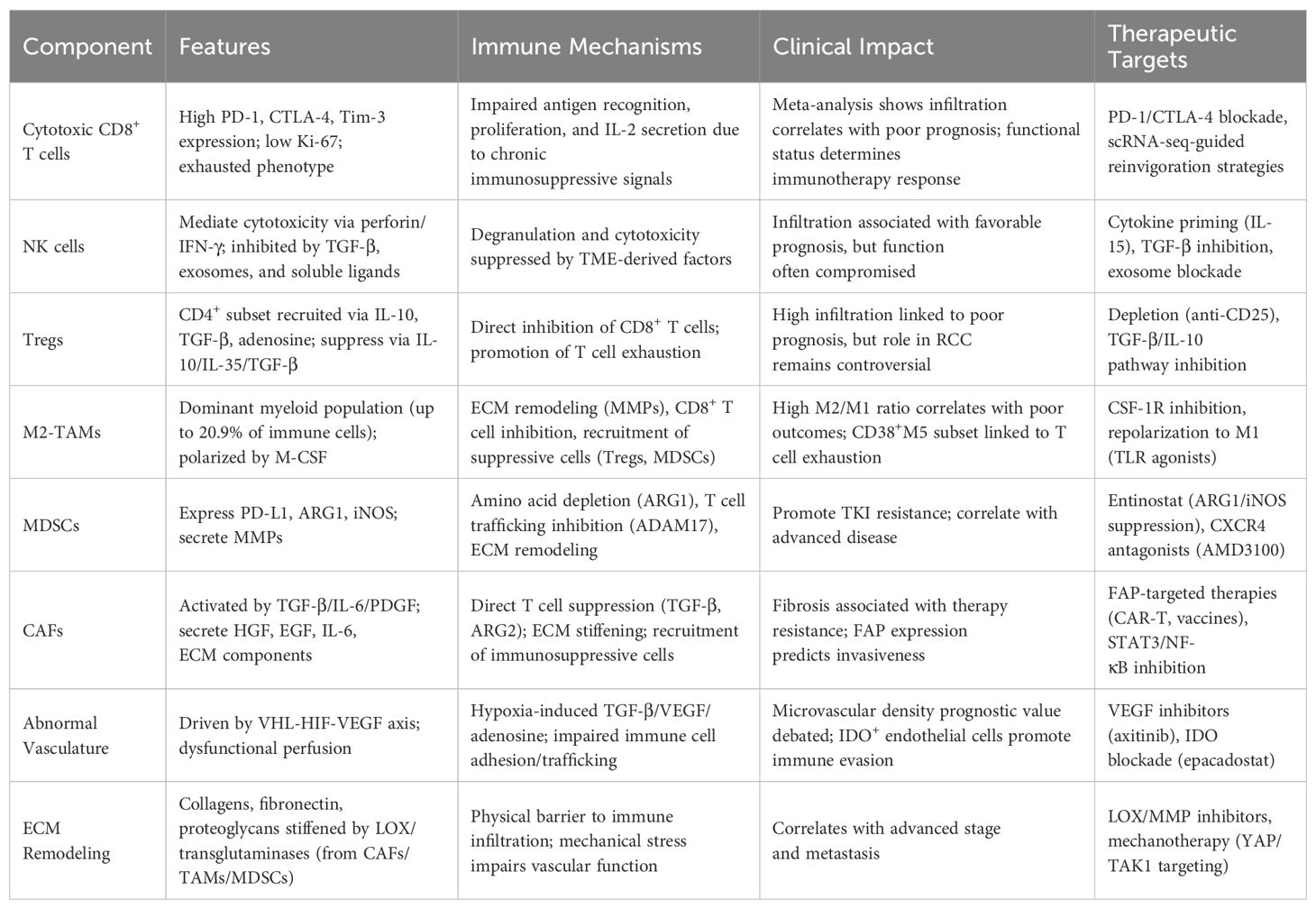
Table 1. Immunosuppressive components of the RCC tumor microenvironment and their roles in immune evasion.
3 Biomarkers for immunotherapy
3.1 PD-L1 expression and tumor-infiltrating lymphocytes
Numerous clinical trials in RCC have reported that only a small subset of patients can achieve complete response and tolerate long-term immunotherapy, while the majority experience disease progression (118, 119). Therefore, the identification of reliable biomarkers capable of predicting immunotherapeutic response is critical for selecting patients most likely to benefit from such treatments (120, 121). Tumor PD-L1 expression is the most widely used biomarker for predicting responses to PD-1/PD-L1 blockade therapy and one of the earliest predictive indicators studied in RCC (122). Although high PD-L1 expression in RCC tissues has been associated with poor prognosis, PD-L1 alone is insufficient to predict therapeutic efficacy (123). Stenzel et al. (124) demonstrated that tumor tissues from patients with ccRCC who responded favorably to ICIs exhibited significantly higher CD8+ T cell infiltration and PD-L1 positivity compared to non-responders. ICIs can reinvigorate pre-existing Th1 cells within the TME, enabling cytotoxic responses against tumor cells (125, 126). This seemingly paradoxical relationship between PD-L1 expression, poor prognosis, and ICI responsiveness may reflect both the spatial heterogeneity of PD-L1 expression in tumor cells and its dynamic regulation: inducible PD-L1 upregulation by IFN−γ released during an active anti-tumor immune response versus constitutive PD-L1 expression driven by HIF−1α in hypoxic regions (127). These mechanisms highlight that PD-L1 expression must be interpreted in the context of the tumor microenvironment and cellular localization (128, 129). Therefore, patients with this immune phenotype are more likely to benefit from ICI therapy.
3.2 Gene mutations
TMB and microsatellite instability (MSI) are well-established predictive biomarkers for ICI efficacy across several malignancies (130, 131). It is generally accepted that tumor-specific neoantigens generated by somatic mutations facilitate immune infiltration, a prerequisite for ICI responsiveness (132, 133). Despite the high immune infiltration in RCC, TMB levels are significantly lower compared to other immunogenic tumors such as lung adenocarcinoma and melanoma (134). A pan-cancer analysis of 19 malignancies by Turajlic et al. (135) using The Cancer Genome Atlas (TCGA) data revealed that RCC harbors the highest frequency and count of insertion or deletion (indel) mutations—over twice the average observed in other cancers. Further RNA sequencing of 329 RCC samples confirmed that indel mutations are associated with heightened immunogenicity, suggesting that indels may serve as superior predictive biomarkers compared to TMB in RCC. Over 90% of sporadic ccRCC cases involve chromosomal translocations at 3p, leading to frequent mutations in VHL, PBRM1, BAP1, and SETD2. Consequently, RCC is considered a disease defined by genomic rearrangements (136). Messai et al. (137) reported a positive correlation between VHL mutations and PD-L1 expression in ccRCC, which may influence patient responses to immunotherapy. In a prospective study, Miao et al. (138) performed whole-exome sequencing on tumor tissues from 35 untreated mRCC patients and found that loss-of-function mutations in PBRM1 were associated with enhanced responsiveness to ICIs, a finding subsequently validated in independent cohorts. A retrospective analysis of the CheckMate 025 trial further demonstrated that PBRM1-mutant RCC patients experienced significantly prolonged progression-free survival (PFS) and overall survival (OS) following anti-PD-1 therapy (139). Mechanistically, loss of PBRM1 disrupts the SWI/SNF chromatin remodeling complex, leading to changes in nucleosome positioning and transcriptional accessibility of interferon-stimulated genes (140). This epigenetic reprogramming can activate the STING–type I interferon pathway, increasing tumor immunogenicity and chemokine production (CXCL10, CCL5), thereby enhancing dendritic cell recruitment and T cell priming (141, 142). Additionally, PBRM1 deficiency has been associated with increased expression of MHC II molecules and components of the antigen-processing machinery, potentially improving tumor antigen presentation and amplifying CD8+ T cell responses (143, 144).
3.3 Emerging biomarkers
Clark et al. (136) utilized xCell to analyze the immune and stromal components of 103 ccRCC samples, integrating transcriptomic and proteomic data to classify ccRCC into four distinct subtypes: CD8+ inflamed tumors, CD8− inflamed tumors, VEGF-high immune desert tumors, and metabolically active immune desert tumors. CD8+ inflamed tumors are characterized by extensive CD8+ T cell infiltration and elevated expression of inhibitory receptors such as PD-1, PD-L1, and CTLA-4, conferring poor prognosis but high potential for immunotherapy response. CD8− inflamed tumors exhibit infiltration by CAFs and innate immune cells such as TAMs. VEGF-high immune desert tumors display pronounced vascularization due to elevated VEGF expression. Metabolically active immune desert tumors, with the lowest immune and stromal scores, exhibit upregulated expression of metabolic enzymes such as pyruvate kinase M (PKM) and peroxiredoxin-4 (PRDX4), along with activation of MYC and mTOR signaling pathways, indicative of tumor metabolic reprogramming. The Lung Immune Prognostic Index (LIPI) has recently emerged as a novel biomarker for immunotherapy, offering a valuable tool for risk stratification and personalized treatment decision-making across various malignancies. Initially applied in non-small cell lung cancer, melanoma, small cell lung cancer, head and neck squamous cell carcinoma, and bladder cancer, LIPI has also shown prognostic relevance in advanced RCC (145). Low-density lipoprotein receptor-related protein 6 (LRP6), a co-receptor in the Wnt/β-catenin signaling pathway involved in cell proliferation, inflammation, and transformation, has been correlated with drug sensitivity in clear cell RCC, suggesting its potential as a therapeutic target (146). Additionally, modulation of carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) signaling has been proposed as a novel approach in cancer immunotherapy. CEACAM1 expression is associated with disease progression, prognosis, and immune cell infiltration in clear cell RCC, highlighting its promise as both a predictive biomarker and a therapeutic target (147).
4 Immunotherapy
4.1 Immune checkpoint inhibitors
Preclinical studies have delineated the biological roles of PD-1, PD-L1, and CTLA-4, enabling clinical trials of ICIs for advanced RCC (148–150). CTLA-4, expressed on activated T cells, binds B7 molecules on antigen-presenting cells (APCs), inhibiting T cell activation (151). Ipilimumab, an anti-CTLA-4 antibody, restores T cell function by blocking CD80/CD86 interactions but has limited clinical utility due to a narrow therapeutic window (152). PD-1, another inhibitory checkpoint, binds PD-L1/PD-L2 on tumor cells, suppressing T cell activity. Nivolumab, a PD-1 inhibitor, showed superior OS and objective response rate (ORR) versus everolimus in the CheckMate-025 trial, leading to FDA approval for mRCC (150). Beyond PD-1/CTLA-4, other checkpoints like TIM-3, LAG-3, KIRs, and TIGIT modulate T cell function via distinct mechanisms, potentially compromising immunotherapy efficacy (153). Targeting these pathways is under clinical investigation in RCC (154). To enhance immunotherapy efficacy, clinical trials have investigated combining anti-PD-1/PD-L1 antibodies with anti-CTLA-4 antibodies or TKIs as first-line RCC treatments, demonstrating superior outcomes to TKI monotherapy (44). While both PD-1 and CTLA-4 inhibit T cell activation, CTLA-4 acts early in T cell priming, whereas PD-1 suppresses CD8+ T cell effector function in the TME (155). Dual blockade synergistically boosts CD8+ T cell activation and accumulation (156). The CheckMate-214 trial showed ipilimumab-nivolumab improved PFS, ORR, and OS in intermediate-/high-risk RCC versus sunitinib, leading to its approval for these patients (157).
In breast cancer models, ICIs activate CD8+ T cells, inducing tumor vessel normalization, which alleviates TME immunosuppression, enhancing T cell infiltration and cytotoxicity—a positive feedback loop underpinning ICI combinations (158). The KEYNOTE-426 trial reported pembrolizumab-axitinib outperformed sunitinib across risk groups and PD-L1 levels (159), while JAVELIN Renal-101 showed avelumab-axitinib improved PFS by 6.6 months versus axitinib alone (160). These results led to FDA approval of both ICI-TKI regimens. The CLEAR study revealed lenvatinib-pembrolizumab provided durable survival benefits over sunitinib (160). Despite their frontline status, combination therapies are not universally effective and may cause severe toxicity. In KEYNOTE-426, pembrolizumab-axitinib frequently induced diarrhea, hypertension, and hepatic toxicity, with 30.5% discontinuing at least one drug due to adverse events (159). Biomarker-driven patient stratification is crucial to mitigate toxicity and costs, alongside deeper investigation of drug interactions to guide monotherapy or sequential approaches when appropriate.
4.2 Targeting immunosuppressive cells
Current therapeutic strategies targeting immunosuppressive cells in the RCC TME can be broadly categorized into three types (161). The first strategy involves depleting immunosuppressive cells to restore CD8+ T cell infiltration and enhance anti-tumor immunity. Fibroblast activation protein (FAP), a surface marker broadly expressed by CAFs in epithelial tumors, is a strong predictor of tumor invasiveness. Agents that inhibit FAP activity, anti-FAP antibodies, FAP-targeted vaccines, and CAR-T cell therapy have shown efficacy in depleting CAFs in preclinical models of malignancies such as mesothelioma (162). The second strategy aims to normalize immunosuppressive cells by inducing CAF quiescence, promoting MDSC maturation, or repolarizing M2-type TAMs. In murine RCC models, entinostat suppressed the immunosuppressive activity of MDSCs by inhibiting ARG1 and iNOS, thereby enhancing CD8+ T cell infiltration (16). The combination of entinostat with atezolizumab and bevacizumab is currently being tested in clinical trials for advanced RCC (NCT03024437). The third strategy focuses on modulating downstream pathways of immunosuppressive cells. The CXCR4–CXCL12 axis plays a critical role in the recruitment of MDSCs and Tregs to the RCC TME. The CXCR4 antagonist AMD3100 has been shown to impair the immunosuppressive function of these cells and improve anti-tumor immune responses (163). Given the frequent occurrence of mutations in metabolism-related genes, RCC is also considered a metabolic disease. Metabolic reprogramming in RCC involves aerobic glycolysis, fatty acid metabolism, and the utilization of tryptophan, glutamine, and arginine, enabling tumor cells to adapt to hypoxia and nutrient depletion while evading immune surveillance (164). IDO contributes to local tryptophan depletion in the TME via the kynurenine pathway, leading to T cell exhaustion and apoptosis. Thus, IDO inhibition can relieve local immune suppression and enhance T cell activity (165). A phase I/II clinical trial is currently evaluating the combination of the IDO inhibitor epacadostat with the anti-PD-1 antibody pembrolizumab in various solid tumors, including RCC, with promising results previously reported in melanoma (166–168). Inhibitors of HIF-α and glutaminase have also entered clinical trials for RCC (169, 170) (Figure 1).
4.3 Clinical strategies to overcome resistance and manage toxicity
The clinical application of immunotherapy in RCC is constrained by tumor heterogeneity, acquired resistance, and treatment-related toxicity, and overcoming these challenges requires an integrated approach (171). Recent progress emphasizes adaptive and biomarker-driven trial designs which stratify patients according to PD−L1 expression, PBRM1 mutation status, or immune subtype to achieve precision therapy (172–174). Another key strategy is sequencing therapy rather than administering agents concurrently; for example, initiating treatment with TKIs to normalize aberrant vasculature and subsequently introducing ICIs can enhance immune cell infiltration while reducing overlapping toxicities (175). Efforts to counteract resistance also include the incorporation of novel agents such as TAM-reprogramming compounds, selective HIF−2α inhibitors, and metabolic modulators into combination regimens to disrupt pro-tumorigenic pathways (176, 177). Equally important is the proactive management of immune-related adverse events, which relies on early recognition, multidisciplinary collaboration, and the use of standardized treatment algorithms with corticosteroids or selective immunosuppressants to preserve antitumor activity (178, 179). Together, these strategies are shaping current and future clinical trials and provide clinicians with practical guidance to optimize therapeutic outcomes while minimizing toxicity in patients with RCC.
5 Conclusion
The immunosuppressive TME of RCC remains a major barrier to durable therapeutic responses, despite significant progress in immunotherapy. The interplay between cytotoxic immune cells and immunosuppressive components creates a permissive niche for tumor progression. While ICIs and combination therapies have revolutionized treatment, their efficacy is limited by intrinsic and acquired resistance, as well as toxicity. Biomarkers such as PD-L1, TMB, and PBRM1 mutations offer predictive insights but lack universal applicability, highlighting the need for multi-parametric profiling. Emerging strategies, including TAM repolarization, CAF depletion, metabolic modulation, and targeting novel immune checkpoints, hold promise but require further validation in clinical trials.
Looking ahead, advanced technologies will be pivotal in overcoming these limitations. Single-cell multi-omics and spatial transcriptomics enable high-resolution mapping of cellular states, lineage trajectories, and intercellular communication within the RCC TME, providing insights that bulk analyses cannot capture. These approaches will help to identify novel cellular subsets, spatially restricted immunosuppressive niches, and potential therapeutic targets. Additionally, artificial intelligence and machine learning are increasingly being applied to integrate multi-dimensional datasets, including genomics, transcriptomics, imaging, and clinical data, to develop predictive models for patient stratification and to discover novel biomarkers. Together, these emerging technologies hold great promise for bridging existing knowledge gaps, enabling real-time monitoring of TME evolution, and guiding the development of precision immunotherapies tailored to individual RCC patients. Moving forward, integrating these innovations with multi-omics profiling and optimizing treatment sequencing will be critical to overcoming resistance and improving outcomes. Ultimately, a precision medicine approach, guided by TME dynamics and predictive biomarkers, will be essential to unlocking the full potential of immunotherapy in RCC.
Author contributions
HW: Writing – original draft. SZ: Writing – original draft. XZ: Writing – original draft. LW: Writing – review & editing, Writing – original draft. DC: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74(3):12–49. doi: 10.3322/caac.21834
2. Bahadoram S, Davoodi M, Hassanzadeh S, Bahadoram M, Barahman M, and Mafakher L. Renal cell carcinoma: an overview of the epidemiology, diagnosis, and treatment. G Ital Nefrol. (2022) 39:2022–vol3.
3. Barata PC and Rini BI. Treatment of renal cell carcinoma: Current status and future directions. CA Cancer J Clin. (2017) 67:507–24. doi: 10.3322/caac.21411
4. Lin G, Yang Y, Feng Q, Zhan F, Sun C, Niu Y, et al. Prognostic implication and immunotherapy response prediction of a costimulatory molecule signature in kidney renal clear cell carcinoma. Immunogenetics. (2022) 74:285–301. doi: 10.1007/s00251-021-01246-1
5. Heo J, Jo Y, and Yoon M. Enhanced anti-tumor effects of combined electric fields, cabozantinib, and radiation therapy in metastatic renal cell carcinoma. Clin Transl Oncol. (2025) 40126769. doi: 10.1007/s12094-025-03898-x
6. Torrisi M, Giannini L, Tummineri R, Deantoni CL, and Fodor A. Excellent outcomes with stereotactic body radiotherapy in an elderly patient with locally progressive immunotherapy-resistant renal cell carcinoma. Cureus. (2025) 17:e85399. doi: 10.7759/cureus.85399
7. Xu W, Wu Y, Liu W, Anwaier A, Tian X, Su J, et al. Tumor-associated macrophage-derived chemokine CCL5 facilitates the progression and immunosuppressive tumor microenvironment of clear cell renal cell carcinoma. Int J Biol Sci. (2022) 18:4884–900. doi: 10.7150/ijbs.74647
8. Xu W, Lu J, Liu WR, Anwaier A, Wu Y, Tian X, et al. Heterogeneity in tertiary lymphoid structures predicts distinct prognosis and immune microenvironment characterizations of clear cell renal cell carcinoma. J Immunother Cancer. (2023) 11:e006667. doi: 10.1136/jitc-2023-006667
9. Decruyenaere A, Christine G, Sylvie R, Annouschka L, Seront E, Everaert E, et al. Optimal treatment duration in metastatic renal cell carcinoma patients responding to immune checkpoint inhibitors: should we treat beyond two years? Acta Oncol. (2025) 64:979–88. doi: 10.2340/1651-226X.2025.43876
10. Au L, Hatipoglu E, Robert de Massy M, Litchfield K, Beattie G, Rowan A, et al. Determinants of anti-PD-1 response and resistance in clear cell renal cell carcinoma. Cancer Cell. (2021) 39:1497–1518.e1411. doi: 10.1016/j.ccell.2021.10.001
11. Yanagisawa T, Schmidinger M, Kawada T, Bekku K, Kimura T, and Shariat SF. Radical nephrectomy after immune checkpoint inhibitors for metastatic renal cell carcinoma. Eur Urol Focus. (2023) 9:275–7. doi: 10.1016/j.euf.2023.01.022
12. Saliby RM, Labaki C, Jammihal TR, Xie W, Sun M, Shah V, et al. Impact of renal cell carcinoma molecular subtypes on immunotherapy and targeted therapy outcomes. Cancer Cell. (2024) 42:732–5. doi: 10.1016/j.ccell.2024.03.002
13. Zhang MX, Jing LY, Tan HT, Dai ZR, Long DZ, Liu HC, et al. MIAT promotes tumor-infiltrating CD8(+) T-cell exhaustion and Malignant progression of renal cell carcinoma via activating JAK3/STAT3 pathway. J Immunother Cancer. (2025) 13:e011162. doi: 10.1136/jitc-2024-011162
14. Granier C, Dariane C, Combe P, Verkarre V, Urien S, Badoual C, et al. Tim-3 expression on tumor-infiltrating PD-1(+)CD8(+) T cells correlates with poor clinical outcome in renal cell carcinoma. Cancer Res. (2017) 77:1075–82. doi: 10.1158/0008-5472.CAN-16-0274
15. Fridman WH, Pagès F, Sautès-Fridman C, and Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. (2012) 12:298–306. doi: 10.1038/nrc3245
16. Deleuze A, Saout J, Dugay F, Peyronnet B, Mathieu R, Verhoest G, et al. Immunotherapy in renal cell carcinoma: the future is now. Int J Mol Sci. (2020) 21:2532. doi: 10.3390/ijms21072532
17. Pontrelli P, Gigante M, Spadaccino F, Netti GS, Saldarelli M, Balducci L, et al. CD40 cross-linking induces migration of renal tumor cell through nuclear factor of activated T cells (NFAT) activation. Int J Mol Sci. (2021) 22:8871. doi: 10.3390/ijms22168871
18. Zhang C, Gou X, Lai G, Li K, Zhu X, Liu N, et al. Single-nucleus sequencing unveils heterogeneity in renal cell carcinomas microenvironment: Insights into pathogenic origins and treatment-responsive cellular subgroups. Cancer Lett. (2024) 604:217259. doi: 10.1016/j.canlet.2024.217259
19. Zapała Ł, Kunc M, Sharma S, Pęksa R, Popęda M, Biernat W, et al. Immune checkpoint receptor VISTA on immune cells is associated with expression of T-cell exhaustion marker TOX and worse prognosis in renal cell carcinoma with venous tumor thrombus. J Cancer Res Clin Oncol. (2023) 149:4131–9. doi: 10.1007/s00432-022-04329-y
20. Zhu Z, Jin Y, Zhou J, Chen F, Chen M, Gao Z, et al. PD1/PD-L1 blockade in clear cell renal cell carcinoma: mechanistic insights, clinical efficacy, and future perspectives. Mol Cancer. (2024) 23:146. doi: 10.1186/s12943-024-02059-y
21. Wu D, Zhou Y, Shi X, Yi X, Sheng Z, Fan L, et al. SLC11A1 promotes kidney renal clear cell carcinoma (KIRC) progression by remodeling the tumor microenvironment. Toxicol Appl Pharmacol. (2024) 487:116975. doi: 10.1016/j.taap.2024.116975
22. Cimadamore A, Boixareu C, Sharp A, Beltran H, and de Bono JS. Novel therapeutic strategies for metastatic prostate cancer care. Eur Urol. (2025) 19:S0302-2838(25)00357-4. doi: 10.1016/j.eururo.2025.06.013
23. Kawase K, Kawashima S, Nishi T, Inozume T, Morinaga T, Kawazu M, et al. PI3K/Akt signaling pathway regulates CD155 expression involved in resistance to cancer immunotherapy. Cancer Immunol Res. (2025) 40742385. doi: 10.1158/2326-6066.CIR-24-0853
24. Yochum ZA and Braun DA. Immunotherapy for renal cell carcinoma-what more is to come? Target Oncol. (2025) 20:467–83. doi: 10.1007/s11523-025-01143-7
25. Andrzejczak A, Tupikowski K, Tomkiewicz A, Małkiewicz B, Ptaszkowski K, Domin A, et al. The variations’ in genes encoding TIM-3 and its ligand, galectin-9, influence on ccRCC risk and prognosis. Int J Mol Sci. (2023) 24:2042. doi: 10.3390/ijms24032042
26. Zhang J, Peng Q, Fan J, Liu F, Chen H, Bi X, et al. Single-cell and spatial transcriptomics reveal SPP1-CD44 signaling drives primary resistance to immune checkpoint inhibitors in RCC. J Transl Med. (2024) 22:1157. doi: 10.1186/s12967-024-06018-5
27. Ning K, Peng Y, Jiang Y, Li Z, Luo X, Lin L, et al. Sex differences in renal cell carcinoma: a single-cell analysis reveals exhausted CD8(+) T-cells highly infiltrated in males. Biol Sex Differ. (2023) 14:58. doi: 10.1186/s13293-023-00540-9
28. Chen WJ, Cao H, Cao JW, Zuo L, Qu FJ, Xu D, et al. Heterogeneity of tumor microenvironment is associated with clinical prognosis of non-clear cell renal cell carcinoma: a single-cell genomics study. Cell Death Dis. (2022) 13:50. doi: 10.1038/s41419-022-04501-9
29. Liu X, Jiang R, Xu Y, Xu X, Fang L, Gao G, et al. Dual cytokine-engineered macrophages rejuvenate the tumor microenvironment and enhance anti-PD-1 therapy in renal cell carcinoma. Int Immunopharmacol. (2025) 156:114725. doi: 10.1016/j.intimp.2025.114725
30. Liu S, Galat V, Galat Y, Lee YKA, Wainwright D, and Wu J. NK cell-based cancer immunotherapy: from basic biology to clinical development. J Hematol Oncol. (2021) 14:7. doi: 10.1186/s13045-020-01014-w
31. Remark R, Alifano M, Cremer I, Lupo A, Dieu-Nosjean MC, Riquet M, et al. Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: influence of tumor origin. Clin Cancer Res. (2013) 19:4079–91. doi: 10.1158/1078-0432.CCR-12-3847
32. Xia Y, Zhang Q, Zhen Q, Zhao Y, Liu N, Li T, et al. Negative regulation of tumor-infiltrating NK cell in clear cell renal cell carcinoma patients through the exosomal pathway. Oncotarget. (2017) 8:37783–95. doi: 10.18632/oncotarget.16354
33. Jia H, Yang H, Xiong H, and Luo KQ. NK cell exhaustion in the tumor microenvironment. Front Immunol. (2023) 14:1303605. doi: 10.3389/fimmu.2023.1303605
34. Hosseini R, Sarvnaz H, Arabpour M, Ramshe SM, Asef-Kabiri L, Yousefi H, et al. Cancer exosomes and natural killer cells dysfunction: biological roles, clinical significance and implications for immunotherapy. Mol Cancer. (2022) 21:15. doi: 10.1186/s12943-021-01492-7
35. Shen T, Miao S, Zhou Y, Yi X, Xue S, Du B, et al. Exosomal AP000439.2 from clear cell renal cell carcinoma induces M2 macrophage polarization to promote tumor progression through activation of STAT3. Cell Commun Signal. (2022) 20:152. doi: 10.1186/s12964-022-00957-6
36. Berchem G, Noman MZ, Bosseler M, Paggetti J, Baconnais S, Le Cam E, et al. Hypoxic tumor-derived microvesicles negatively regulate NK cell function by a mechanism involving TGF-β and miR23a transfer. Oncoimmunology. (2016) 5:e1062968. doi: 10.1080/2162402X.2015.1062968
37. Shaffer TM, Aalipour A, Schürch CM, and Gambhir SS. PET imaging of the natural killer cell activation receptor NKp30. J Nucl Med. (2020) 61:1348–54. doi: 10.2967/jnumed.119.233163
38. Trotta AM, Santagata S, Zanotta S, D’Alterio C, Napolitano M, Rea G, et al. Mutated Von Hippel-Lindau-renal cell carcinoma (RCC) promotes patients specific natural killer (NK) cytotoxicity. J Exp Clin Cancer Res. (2018) 37:297. doi: 10.1186/s13046-018-0952-7
39. Zhang Y, Thayele Purayil H, Black JB, Fetto F, Lynch LD, Masannat JN, et al. Prostaglandin E2 receptor 4 mediates renal cell carcinoma intravasation and metastasis. Cancer Lett. (2017) 391:50–8. doi: 10.1016/j.canlet.2017.01.007
40. Park G, Song NY, Kim DH, Lee SJ, and Chun KS. Thymoquinone suppresses migration of human renal carcinoma caki-1 cells through inhibition of the PGE(2)-mediated activation of the EP2 receptor pathway. Biomol Ther (Seoul). (2021) 29:64–72. doi: 10.4062/biomolther.2020.048
41. Yan C, Yang Z, Chen P, Yeh Y, Sun C, Xie T, et al. GPR65 sensing tumor-derived lactate induces HMGB1 release from TAM via the cAMP/PKA/CREB pathway to promote glioma progression. J Exp Clin Cancer Res. (2024) 43:105. doi: 10.1186/s13046-024-03025-8
42. Liu L, Wang Y, Fan Y, Li CL, and Chang ZL. IFN-gamma activates cAMP/PKA/CREB signaling pathway in murine peritoneal macrophages. J Interferon Cytokine Res. (2004) 24:334–42. doi: 10.1089/107999004323142196
43. Hu S, Chen B, Zhou J, Liu F, Mao T, Pathak JL, et al. Dental pulp stem cell-derived exosomes revitalize salivary gland epithelial cell function in NOD mice via the GPER-mediated cAMP/PKA/CREB signaling pathway. J Transl Med. (2023) 21:361. doi: 10.1186/s12967-023-04198-0
44. Zhao W, Huang Y, Liu Z, Cao BB, Peng YP, and Qiu YH. Dopamine receptors modulate cytotoxicity of natural killer cells via cAMP-PKA-CREB signaling pathway. PloS One. (2013) 8:e65860. doi: 10.1371/journal.pone.0065860
45. Monjaras-Avila CU, Lorenzo-Leal AC, Luque-Badillo AC, D’Costa N, Chavez-Muñoz C, and Bach H. The tumor immune microenvironment in clear cell renal cell carcinoma. Int J Mol Sci. (2023) 24:7946. doi: 10.3390/ijms24097946
46. Kajdaniuk D, Hudy D, Strzelczyk JK, Młynarek K, Słomian S, Potyka A, et al. Transforming growth factors β and their signaling pathway in renal cell carcinoma and peritumoral space-transcriptome analysis. Clin Transl Oncol. (2024) 26:1229–39. doi: 10.1007/s12094-023-03350-y
47. Wang Y, Ding W, Hao W, Gong L, Peng Y, Zhang J, et al. CXCL3/TGF-β-mediated crosstalk between CAFs and tumor cells augments RCC progression and sunitinib resistance. iScience. (2024) 27:110224. doi: 10.1016/j.isci.2024.110224
48. Xu S, Zhang ZH, Fu L, Song J, Xie DD, Yu DX, et al. Calcitriol inhibits migration and invasion of renal cell carcinoma cells by suppressing Smad2/3-, STAT3- and β-catenin-mediated epithelial-mesenchymal transition. Cancer Sci. (2020) 111:59–71. doi: 10.1111/cas.14237
49. Li X, Zhang Y, Ye Y, Xiao W, Liu L, and Zhang X. NK cells in renal cell carcinoma and its implications for CAR-NK therapy. Front Cell Dev Biol. (2025) 13:1532491. doi: 10.3389/fcell.2025.1532491
50. Li Z, Ma J, Xu M, Duan Y, Huang C, Dai Q, et al. Regulatory T cells in renal cell carcinoma: tumor-promoting mechanisms and emerging therapeutic strategies. Int Immunopharmacol. (2025) 163:115322. doi: 10.1016/j.intimp.2025.115322
51. Santagata S, Rea G, Bello AM, Capiluongo A, Napolitano M, Desicato S, et al. Targeting CXCR4 impaired T regulatory function through PTEN in renal cancer patients. Br J Cancer. (2024) 130:2016–26. doi: 10.1038/s41416-024-02702-x
52. Sasidharan Nair V and Elkord E. Immune checkpoint inhibitors in cancer therapy: a focus on T-regulatory cells. Immunol Cell Biol. (2018) 96:21–33. doi: 10.1111/imcb.1003
53. Song X, Zhu Y, Geng W, Jiao J, Liu H, Chen R, et al. Spatial and single-cell transcriptomics reveal cellular heterogeneity and a novel cancer-promoting Treg cell subset in human clear-cell renal cell carcinoma. J Immunother Cancer. (2025) 13:e010183. doi: 10.1136/jitc-2024-010183
54. Zhang X, Sun Y, Ma Y, Gao C, Zhang Y, Yang X, et al. Tumor-associated M2 macrophages in the immune microenvironment influence the progression of renal clear cell carcinoma by regulating M2 macrophage-associated genes. Front Oncol. (2023) 13:1157861. doi: 10.3389/fonc.2023.1157861
55. Liu H, Lv Z, Zhang G, Yan Z, Bai S, Dong D, et al. Molecular understanding and clinical aspects of tumor-associated macrophages in the immunotherapy of renal cell carcinoma. J Exp Clin Cancer Res. (2024) 43:242. doi: 10.1186/s13046-024-03164-y
56. Zhou Y, Zeng J, Tu Y, Li L, Du S, Zhu L, et al. CSF1/CSF1R-mediated crosstalk between choroidal vascular endothelial cells and macrophages promotes choroidal neovascularization. Invest Ophthalmol Vis Sci. (2021) 62:37. doi: 10.1167/iovs.62.3.37
57. Liang W, Wu H, Long Q, Lin H, Lv X, Ma W, et al. LKB1 activated by NaB inhibits the IL-4/STAT6 axis and ameliorates renal fibrosis through the suppression of M2 macrophage polarization. Life Sci. (2025) 370:123564. doi: 10.1016/j.lfs.2025.123564
58. Zhao S, Mi Y, Guan B, Zheng B, Wei P, Gu Y, et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J Hematol Oncol. (2020) 13:156. doi: 10.1186/s13045-020-00991-2
59. Li D, Zhang Q, Li L, Chen K, Yang J, Dixit D, et al. β2-microglobulin maintains glioblastoma stem cells and induces M2-like polarization of tumor-associated macrophages. Cancer Res. (2022) 82:3321–34. doi: 10.1158/0008-5472.CAN-22-0507
60. Lv J, Liu C, Chen FK, Feng ZP, Jia L, Liu PJ, et al. M2−like tumour−associated macrophage−secreted IGF promotes thyroid cancer stemness and metastasis by activating the PI3K/AKT/mTOR pathway. Mol Med Rep. (2021) 24:604. doi: 10.3892/mmr.2021.12249
61. Pan Q, Wang L, Chai S, Zhang H, and Li B. The immune infiltration in clear cell Renal Cell Carcinoma and their clinical implications: A study based on TCGA and GEO databases. J Cancer. (2020) 11:3207–15. doi: 10.7150/jca.37285
62. Yang Y, Li S, To KKW, Zhu S, Wang F, and Fu L. Tumor-associated macrophages remodel the suppressive tumor immune microenvironment and targeted therapy for immunotherapy. J Exp Clin Cancer Res. (2025) 44:145. doi: 10.1186/s13046-025-03377-9
63. Groth C, Hu X, Weber R, Fleming V, Altevogt P, Utikal J, et al. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br J Cancer. (2019) 120:16–25. doi: 10.1038/s41416-018-0333-1
64. Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. (2009) 69:1553–60. doi: 10.1158/0008-5472.CAN-08-1921
65. Zeng QL, Yang B, Sun HQ, Feng GH, Jin L, Zou ZS, et al. Myeloid-derived suppressor cells are associated with viral persistence and downregulation of TCR ζ chain expression on CD8(+) T cells in chronic hepatitis C patients. Mol Cells. (2014) 37:66–73. doi: 10.14348/molcells.2014.2282
66. He S, Tian W, Zhao J, Gong R, Wang T, and Ma L. Carfilzomib inhibits the proliferation and apoptosis of multiple myeloma cells by inhibiting STAT1/COX-2/iNOS signaling pathway. Transl Cancer Res. (2022) 11:206–16. doi: 10.21037/tcr-21-2534
67. Song W, Li D, Tao L, Luo Q, and Chen L. Solute carrier transporters: the metabolic gatekeepers of immune cells. Acta Pharm Sin B. (2020) 10:61–78. doi: 10.1016/j.apsb.2019.12.006
68. Zhang X, Ji J, Zhang G, Fang C, Jiang F, Ma S, et al. Expression and significance of B7-H3 and Tie-2 in the tumor vasculature of clear cell renal carcinoma. Onco Targets Ther. (2017) 10:5417–24. doi: 10.2147/OTT.S147041
69. Carnevale R, Leopizzi M, Dominici M, d’Amati G, Bartimoccia S, Nocella C, et al. PAD4-induced NETosis via cathepsin G-mediated platelet-neutrophil interaction in chAdOx1 vaccine-induced thrombosis-brief report. Arterioscler Thromb Vasc Biol. (2023) 43:e396–403. doi: 10.1161/ATVBAHA.123.319522
70. Ding M, Zhao X, Chen X, Diao W, Kan Y, Cao W, et al. Cancer-associated fibroblasts promote the stemness and progression of renal cell carcinoma via exosomal miR-181d-5p. Cell Death Discov. (2022) 8:439. doi: 10.1038/s41420-022-01219-7
71. Kraxner A, Braun F, Cheng WY, Yang TO, Pipaliya S, Canamero M, et al. Investigating the complex interplay between fibroblast activation protein α-positive cancer associated fibroblasts and the tumor microenvironment in the context of cancer immunotherapy. Front Immunol. (2024) 15:1352632. doi: 10.3389/fimmu.2024.1352632
72. Yan J, Xiao G, Yang C, Liu Q, Lv C, Yu X, et al. Cancer-associated fibroblasts promote lymphatic metastasis in cholangiocarcinoma via the PDGF-BB/PDGFR-β Mediated paracrine signaling network. Aging Dis. (2024) 15:369–89. doi: 10.14336/AD.2023.0420
73. Ma J, Cao D, Zhang Y, Sun Y, Wu Y, and Wang J. Bruceine D inhibits CAF-promoted angiogenesis of breast cancer via suppressing IL-6-mediated activation of the STAT3/Notch1/VEGFR2 axis. Eur J Pharmacol. (2025) 1003:177994. doi: 10.1016/j.ejphar.2025.177994
74. Song X, Li T, Zhou W, Feng C, Zhou Z, Chen Y, et al. CAF-derived exosomal miR-196b-5p after androgen deprivation therapy promotes epithelial-mesenchymal transition in prostate cancer cells through HOXC8/NF-κB signaling pathway. Biol Direct. (2025) 20:80. doi: 10.1186/s13062-025-00667-2
75. Li Y, Zheng H, Luo Y, Lin Y, An M, Kong Y, et al. An HGF-dependent positive feedback loop between bladder cancer cells and fibroblasts mediates lymphangiogenesis and lymphatic metastasis. Cancer Commun (Lond). (2023) 43:1289–311. doi: 10.1002/cac2.12470
76. Luo F, Mei Y, Li Y, Yang J, Xi S, Cao E, et al. CAF-derived LRRC15 orchestrates macrophage polarization and limits PD-1 immunotherapy efficacy in glioblastoma. Neuro Oncol. (2025) 26:noaf157. doi: 10.1093/neuonc/noaf157
77. Aronovich A, Moyal L, Gorovitz B, Amitay-Laish I, Naveh HP, Forer Y, et al. Cancer-associated fibroblasts in mycosis fungoides promote tumor cell migration and drug resistance through CXCL12/CXCR4. J Invest Dermatol. (2021) 141:619–627.e612. doi: 10.1016/j.jid.2020.06.034
78. Peng D, Fu M, Wang M, Wei Y, and Wei X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol Cancer. (2022) 21:104. doi: 10.1186/s12943-022-01569-x
79. Arpinati L, Carradori G, and Scherz-Shouval R. CAF-induced physical constraints controlling T cell state and localization in solid tumours. Nat Rev Cancer. (2024) 24:676–93. doi: 10.1038/s41568-024-00740-4
80. Zhang R and Liu F. Cancer-associated fibroblast-derived gene signatures predict radiotherapeutic survival in prostate cancer patients. J Transl Med. (2022) 20:453. doi: 10.1186/s12967-022-03656-5
81. Xu C, Zhang K, Yang F, Zhou X, Liu S, Li Y, et al. CD248(+) cancer-associated fibroblasts: A novel prognostic and therapeutic target for renal cell carcinoma. Front Oncol. (2021) 11:773063. doi: 10.3389/fonc.2021.773063
82. Mao X, Xu J, Wang W, Liang C, Hua J, Liu J, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. (2021) 20:131. doi: 10.1186/s12943-021-01428-1
83. Lavie D, Ben-Shmuel A, Erez N, and Scherz-Shouval R. Cancer-associated fibroblasts in the single-cell era. Nat Cancer. (2022) 3:793–807. doi: 10.1038/s43018-022-00411-z
84. Rose TL and Kim WY. Renal cell carcinoma: A review. Jama. (2024) 332:1001–10. doi: 10.1001/jama.2024.12848
85. Li Q, Zeng K, Chen Q, Han C, Wang X, Li B, et al. Atractylenolide I inhibits angiogenesis and reverses sunitinib resistance in clear cell renal cell carcinoma through ATP6V0D2-mediated autophagic degradation of EPAS1/HIF2α. Autophagy. (2025) 21:619–38. doi: 10.1080/15548627.2024.2421699
86. Tumkur Sitaram R, Landström M, Roos G, and Ljungberg B. Significance of PI3K signalling pathway in clear cell renal cell carcinoma in relation to VHL and HIF status. J Clin Pathol. (2021) 74:216–22. doi: 10.1136/jclinpath-2020-206693
87. Lei Y, Chen X, Mo JL, Lv LL, Kou ZW, and Sun FY. Vascular endothelial growth factor promotes transdifferentiation of astrocytes into neurons via activation of the MAPK/Erk-Pax6 signal pathway. Glia. (2023) 71:1648–66. doi: 10.1002/glia.24361
88. Chen J, Gu Z, Wu M, Yang Y, Zhang J, Ou J, et al. C-reactive protein can upregulate VEGF expression to promote ADSC-induced angiogenesis by activating HIF-1α via CD64/PI3k/Akt and MAPK/ERK signaling pathways. Stem Cell Res Ther. (2016) 7:114. doi: 10.1186/s13287-016-0377-1
89. Vaupel P and Multhoff G. Accomplices of the hypoxic tumor microenvironment compromising antitumor immunity: adenosine, lactate, acidosis, vascular endothelial growth factor, potassium ions, and phosphatidylserine. Front Immunol. (2017) 8:1887. doi: 10.3389/fimmu.2017.01887
90. Batistella EA, Miguel AFP, Nascimento NL, Horta MCR, Vieira DSC, and Rivero ERC. Microvascular density analysis and histological parameters of oral cancer progression. Oral Dis. (2024) 30:2110–21. doi: 10.1111/odi.14694
91. Denize T, Farah S, Cimadamore A, Flaifel A, Walton E, Sticco-Ivins MA, et al. Biomarkers of angiogenesis and clinical outcomes to cabozantinib and everolimus in patients with metastatic renal cell carcinoma from the phase III METEOR trial. Clin Cancer Res. (2022) 28:748–55. doi: 10.1158/1078-0432.CCR-21-3088
92. Seeber A, Klinglmair G, Fritz J, Steinkohl F, Zimmer KC, Aigner F, et al. High IDO-1 expression in tumor endothelial cells is associated with response to immunotherapy in metastatic renal cell carcinoma. Cancer Sci. (2018) 109:1583–91. doi: 10.1111/cas.13560
93. Zhou X, Li R, Lai M, and Lai C. Exploring molecular and cellular mechanisms of Pre-Metastatic niche in renal cell carcinoma. Mol Cancer. (2025) 24:121. doi: 10.1186/s12943-025-02315-9
94. Wiśniowski T, Bryda J, Domosud J, and Wątroba SJ. Extracellular matrix metalloproteinases in pathophysiology, diagnostics and treatment of renal cell carcinoma - current state of knowledge and future perspectives. Ann Agric Environ Med. (2025) 32:27–45. doi: 10.26444/aaem/192555
95. Wu TK, Hung TW, Chen YS, Pan YR, Hsieh YH, and Tsai JP. Corosolic acid inhibits metastatic response of human renal cell carcinoma cells by modulating ERK/MMP2 signaling. Environ Toxicol. (2024) 39:857–68. doi: 10.1002/tox.23999
96. Ma G, Zhang B, Fu S, Lu J, Zhang L, Shang P, et al. Formin-related protein 1 facilitates proliferation and aggressive phenotype of clear cell renal cell carcinoma through MAPK/MMP2 pathway. Mol Cell Probes. (2023) 71:101921. doi: 10.1016/j.mcp.2023.101921
97. Rajamani K, Thirugnanasambandan SS, Natesan C, Subramaniam S, Thangavel B, and Aravindan N. Squalene deters drivers of RCC disease progression beyond VHL status. Cell Biol Toxicol. (2021) 37:611–31. doi: 10.1007/s10565-020-09566-w
98. Santi A, Kugeratski FG, and Zanivan S. Cancer associated fibroblasts: the architects of stroma remodeling. Proteomics. (2018) 18:e1700167. doi: 10.1002/pmic.201700167
99. Yang Y, Fan R, Zhang B, and Liu K. COL6A2 in clear cell renal cell carcinoma: a multifaceted driver of tumor progression, immune evasion, and drug sensitivity. J Transl Med. (2025) 23:875. doi: 10.1186/s12967-025-06793-9
100. Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, and Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun. (2020) 11:5120. doi: 10.1038/s41467-020-18794-x
101. Bhat P, Tamboli P, Sircar K, and Kannan K. Spatial distribution of tumor cells in clear cell renal cell carcinoma is associated with metastasis and a matrisome gene expression signature. Cancers (Basel). (2025) 17:249. doi: 10.3390/cancers17020249
102. Zhang Y, Wang X, Gu Y, Liu T, Zhao X, Cheng S, et al. Complement C3 of tumor-derived extracellular vesicles promotes metastasis of RCC via recruitment of immunosuppressive myeloid cells. Proc Natl Acad Sci U.S.A. (2025) 122:e2420005122. doi: 10.1073/pnas.2420005122
103. Cong X, Li X, Xu K, Yin L, Liang G, Sun R, et al. HIF-1α/m(6)A/NF-κB/CCL3 axis-mediated immunosurveillance participates in low level benzene-related erythrohematopoietic development toxicity. Environ Int. (2024) 184:108493. doi: 10.1016/j.envint.2024.108493
104. Silva Paiva R, Gomes I, Casimiro S, Fernandes I, and Costa L. c-Met expression in renal cell carcinoma with bone metastases. J Bone Oncol. (2020) 25:100315. doi: 10.1016/j.jbo.2020.100315
105. Forde AJ, Kolter J, Zwicky P, Baasch S, Lohrmann F, Eckert M, et al. Metabolic rewiring tunes dermal macrophages in staphylococcal skin infection. Sci Immunol. (2023) 8:eadg3517. doi: 10.1126/sciimmunol.adg3517
106. Hirsch L, Flippot R, Escudier B, and Albiges L. Immunomodulatory roles of VEGF pathway inhibitors in renal cell carcinoma. Drugs. (2020) 80:1169–81. doi: 10.1007/s40265-020-01327-7
107. Díaz-Montero CM, Rini BI, and Finke JH. The immunology of renal cell carcinoma. Nat Rev Nephrol. (2020) 16:721–35. doi: 10.1038/s41581-020-0316-3
108. Grobben Y. Targeting amino acid-metabolizing enzymes for cancer immunotherapy. Front Immunol. (2024) 15:1440269. doi: 10.3389/fimmu.2024.1440269
109. Kumari A, Syeda S, Rawat K, Kumari R, and Shrivastava A. Melatonin modulates L-arginine metabolism in tumor-associated macrophages by targeting arginase 1 in lymphoma. Naunyn Schmiedebergs Arch Pharmacol. (2024) 397:1163–79. doi: 10.1007/s00210-023-02676-2
110. Li J, Zhang Q, Guan Y, Liao D, Jiang D, Xiong H, et al. Circular RNA circVAMP3 promotes aerobic glycolysis and proliferation by regulating LDHA in renal cell carcinoma. Cell Death Dis. (2022) 13:443. doi: 10.1038/s41419-022-04863-0
111. Xin X, Li Z, Yan X, Liu T, Li Z, Chen Z, et al. Hepatocyte-specific Smad4 deficiency inhibits hepatocarcinogenesis by promoting CXCL10/CXCR3-dependent CD8(+)- T cell-mediated anti-tumor immunity. Theranostics. (2024) 14:5853–68. doi: 10.7150/thno.97276
112. Zhu R, Ye X, Lu X, Xiao L, Yuan M, Zhao H, et al. ACSS2 acts as a lactyl-CoA synthetase and couples KAT2A to function as a lactyltransferase for histone lactylation and tumor immune evasion. Cell Metab. (2025) 37:361–376.e367. doi: 10.1016/j.cmet.2024.10.015
113. Ma Z, Yang J, Jia W, Li L, Li Y, Hu J, et al. Histone lactylation-driven B7-H3 expression promotes tumor immune evasion. Theranostics. (2025) 15:2338–59. doi: 10.7150/thno.105947
114. Zhang Y, Huang Y, Hong Y, Lin Z, Zha J, Zhu Y, et al. Lactate acid promotes PD-1(+) Tregs accumulation in the bone marrow with high tumor burden of Acute myeloid leukemia. Int Immunopharmacol. (2024) 130:111765. doi: 10.1016/j.intimp.2024.111765
115. Jin X, Zhang N, Yan T, Wei J, Hao L, Sun C, et al. Lactate-mediated metabolic reprogramming of tumor-associated macrophages: implications for tumor progression and therapeutic potential. Front Immunol. (2025) 16:1573039. doi: 10.3389/fimmu.2025.1573039
116. Liu Y, Zhou Y, Zhang J, Li J, and Zou L. Regulation of CD4 + T cell differentiation and function by glucose metabolism. Genes Immun. (2025) 26:287–96. doi: 10.1038/s41435-025-00340-8
117. Mier JW. The tumor microenvironment in renal cell cancer. Curr Opin Oncol. (2019) 31:194–9. doi: 10.1097/CCO.0000000000000512
118. Calvo E, Boni V, Dumas O, Shin SJ, Rosen SD, Chaudhry A, et al. Nemvaleukin alfa monotherapy in patients with advanced melanoma and renal cell carcinoma: results from the phase 1/2 non-randomized ARTISTRY-1 trial. J Immunother Cancer. (2025) 13:e010777. doi: 10.1136/jitc-2024-010777
119. De Vries-Brilland M, Hamilou Z, Ghosh S, Heng DYC, Wood LA, Basappa NS, et al. Real-world assessment of clinical outcomes of first-line treatment in metastatic papillary Renal Cell Carcinoma. Oncologist. (2025) 4:oyaf240. doi: 10.1093/oncolo/oyaf240
120. Huang T, Peng Y, Liu R, Ma B, Chen J, Wei W, et al. Prognostic significance of immune evasion-related genes in clear cell renal cell carcinoma immunotherapy. Int Immunopharmacol. (2024) 142:113106. doi: 10.1016/j.intimp.2024.113106
121. Jin W, Yang Q, Chi H, Wei K, Zhang P, Zhao G, et al. Ensemble deep learning enhanced with self-attention for predicting immunotherapeutic responses to cancers. Front Immunol. (2022) 13:1025330. doi: 10.3389/fimmu.2022.1025330
122. Jahangir M, Yazdani O, Kahrizi MS, Soltanzadeh S, Javididashtbayaz H, Mivefroshan A, et al. Clinical potential of PD-1/PD-L1 blockade therapy for renal cell carcinoma (RCC): a rapidly evolving strategy. Cancer Cell Int. (2022) 22:401. doi: 10.1186/s12935-022-02816-3
123. Kammerer-Jacquet SF, Deleuze A, Saout J, Mathieu R, Laguerre B, Verhoest G, et al. Targeting the PD-1/PD-L1 pathway in renal cell carcinoma. Int J Mol Sci. (2019) 20:1692. doi: 10.3390/ijms20071692
124. Stenzel PJ, Schindeldecker M, Tagscherer KE, Foersch S, Herpel E, Hohenfellner M, et al. Prognostic and predictive value of tumor-infiltrating leukocytes and of immune checkpoint molecules PD1 and PDL1 in clear cell renal cell carcinoma. Transl Oncol. (2020) 13:336–45. doi: 10.1016/j.tranon.2019.11.002
125. Horzum U, Yanik H, Taskiran EZ, and Esendagli G. Effector Th1 cells under PD-1 and CTLA-4 checkpoint blockade abrogate the upregulation of multiple inhibitory receptors and by-pass exhaustion. Immunology. (2022) 167:640–50. doi: 10.1111/imm.13560
126. Wang Q, Xie B, Liu S, Shi Y, Tao Y, Xiao D, et al. What happens to the immune microenvironment after PD-1 inhibitor therapy? Front Immunol. (2021) 12:773168. doi: 10.3389/fimmu.2021.773168
127. van Duijn A, Willemsen KJ, van Uden NOP, Hoyng L, Erades S, Koster J, et al. A secondary role for hypoxia and HIF1 in the regulation of (IFNγ-induced) PD-L1 expression in melanoma. Cancer Immunol Immunother. (2022) 71:529–40. doi: 10.1007/s00262-021-03007-1
128. Tziakou P, Theodoropoulos G, Tsiambas E, Zizi-Sermpetzoglou A, Peschos D, Mastronikoli S, et al. Impact of PD-L1 protein expression on renal cell carcinoma histo-differentiation. Anticancer Res. (2021) 41:3809–13. doi: 10.21873/anticanres.15173
129. Chen S, Crabill GA, Pritchard TS, McMiller TL, Wei P, Pardoll DM, et al. Mechanisms regulating PD-L1 expression on tumor and immune cells. J Immunother Cancer. (2019) 7:305. doi: 10.1186/s40425-019-0770-2
130. Guo JN, Chen D, Deng SH, Huang JR, Song JX, Li XY, et al. Identification and quantification of immune infiltration landscape on therapy and prognosis in left- and right-sided colon cancer. Cancer Immunol Immunother. (2022) 71:1313–30. doi: 10.1007/s00262-021-03076-2
131. Holder AM, Dedeilia A, Sierra-Davidson K, Cohen S, Liu D, Parikh A, et al. Defining clinically useful biomarkers of immune checkpoint inhibitors in solid tumours. Nat Rev Cancer. (2024) 24:498–512. doi: 10.1038/s41568-024-00705-7
132. Hu Z, Leet DE, Allesøe RL, Oliveira G, Li S, Luoma AM, et al. Personal neoantigen vaccines induce persistent memory T cell responses and epitope spreading in patients with melanoma. Nat Med. (2021) 27:515–25. doi: 10.1038/s41591-020-01206-4
133. Chen H, Li Z, Qiu L, Dong X, Chen G, Shi Y, et al. Personalized neoantigen vaccine combined with PD-1 blockade increases CD8(+) tissue-resident memory T-cell infiltration in preclinical hepatocellular carcinoma models. J Immunother Cancer. (2022) 10:e004389. doi: 10.1136/jitc-2021-004389
134. Lee M, Samstein RM, Valero C, Chan TA, and Morris LGT. Tumor mutational burden as a predictive biomarker for checkpoint inhibitor immunotherapy. Hum Vaccin Immunother. (2020) 16:112–5. doi: 10.1080/21645515.2019.1631136
135. Turajlic S, Litchfield K, Xu H, Rosenthal R, McGranahan N, Reading JL, et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. Lancet Oncol. (2017) 18:1009–21. doi: 10.1016/S1470-2045(17)30516-8
136. Clark DJ, Dhanasekaran SM, Petralia F, Pan J, Song X, Hu Y, et al. Integrated proteogenomic characterization of clear cell renal cell carcinoma. Cell. (2019) 179:964–983.e931. doi: 10.1016/j.cell.2019.10.007
137. Messai Y, Gad S, Noman MZ, Le Teuff G, Couve S, Janji B, et al. Renal cell carcinoma programmed death-ligand 1, a new direct target of hypoxia-inducible factor-2 alpha, is regulated by von hippel-lindau gene mutation status. Eur Urol. (2016) 70:623–32. doi: 10.1016/j.eururo.2015.11.029
138. Miao D, Margolis CA, Gao W, Voss MH, Li W, Martini DJ, et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science. (2018) 359:801–6. doi: 10.1126/science.aan5951
139. Braun DA, Ishii Y, Walsh AM, Van Allen EM, Wu CJ, Shukla SA, et al. Clinical validation of PBRM1 alterations as a marker of immune checkpoint inhibitor response in renal cell carcinoma. JAMA Oncol. (2019) 5:1631–3. doi: 10.1001/jamaoncol.2019.3158
140. da Costa WH, Rezende M, Carneiro FC, Rocha RM, da Cunha IW, Carraro DM, et al. Polybromo-1 (PBRM1), a SWI/SNF complex subunit is a prognostic marker in clear cell renal cell carcinoma. BJU Int. (2014) 113:E157–163. doi: 10.1111/bju.12426
141. Maxwell MB, Hom-Tedla MS, Yi J, Li S, Rivera SA, Yu J, et al. ARID1A suppresses R-loop-mediated STING-type I interferon pathway activation of anti-tumor immunity. Cell. (2024) 187:3390–3408.e3319. doi: 10.1016/j.cell.2024.04.025
142. Mondal I, Das O, Sun R, Gao J, Yu B, Diaz A, et al. PP2Ac deficiency enhances tumor immunogenicity by activating STING-type I interferon signaling in glioblastoma. Cancer Res. (2023) 83:2527–42. doi: 10.1158/0008-5472.CAN-22-3382
143. Braun DA, Hou Y, Bakouny Z, Ficial M, Sant’ Angelo M, Forman J, et al. Interplay of somatic alterations and immune infiltration modulates response to PD-1 blockade in advanced clear cell renal cell carcinoma. Nat Med. (2020) 26:909–18. doi: 10.1038/s41591-020-0839-y
144. Li BE, Li GY, Cai W, Zhu Q, Seruggia D, Fujiwara Y, et al. In vivo CRISPR/Cas9 screening identifies Pbrm1 as a regulator of myeloid leukemia development in mice. Blood Adv. (2023) 7:5281–93. doi: 10.1182/bloodadvances.2022009455
145. Benitez JC, Recondo G, Rassy E, and Mezquita L. The LIPI score and inflammatory biomarkers for selection of patients with solid tumors treated with checkpoint inhibitors. Q J Nucl Med Mol Imaging. (2020) 64:162–74. doi: 10.23736/S1824-4785.20.03250-1
146. Lu L, Lei Y, Li Y, and Wang L. LRP6 is a potential biomarker of kidney clear cell carcinoma related to prognosis and immune infiltration. Aging (Albany NY). (2024) 16:1484–95. doi: 10.18632/aging.205440
147. Yang L, Liu Y, Zhang B, Yu M, Huang F, Zeng J, et al. CEACAM1 is a prognostic biomarker and correlated with immune cell infiltration in clear cell renal cell carcinoma. Dis Markers. (2023) 2023:3606362. doi: 10.1155/2023/3606362
148. Klümper N, Ralser DJ, Zarbl R, Schlack K, Schrader AJ, Rehlinghaus M, et al. CTLA4 promoter hypomethylation is a negative prognostic biomarker at initial diagnosis but predicts response and favorable outcome to anti-PD-1 based immunotherapy in clear cell renal cell carcinoma. J Immunother Cancer. (2021) 9:e002949. doi: 10.1136/jitc-2021-002949
149. Roy AM and George S. Emerging resistance vs. losing response to immune check point inhibitors in renal cell carcinoma: two differing phenomena. Cancer Drug Resist. (2023) 6:642–55. doi: 10.20517/cdr.2023.47
150. Grimm MO, Esteban E, Barthélémy P, Schmidinger M, Busch J, Valderrama BP, et al. Tailored immunotherapy approach with nivolumab with or without nivolumab plus ipilimumab as immunotherapeutic boost in patients with metastatic renal cell carcinoma (TITAN-RCC): a multicentre, single-arm, phase 2 trial. Lancet Oncol. (2023) 24:1252–65. doi: 10.1016/S1470-2045(23)00449-7
151. Schoenfeld DA, Djureinovic D, Su DG, Zhang L, Lu BY, Kamga L, et al. Decoy-resistant IL-18 reshapes the tumor microenvironment and enhances rejection by anti-CTLA-4 in renal cell carcinoma. JCI Insight. (2024) 10:e184545. doi: 10.1172/jci.insight.184545
152. Bergmann L, Albiges L, Ahrens M, Gross-Goupil M, Boleti E, Gravis G, et al. Prospective randomized phase-II trial of ipilimumab/nivolumab versus standard of care in non-clear cell renal cell cancer - results of the SUNNIFORECAST trial. Ann Oncol. (2025) 36:796–806. doi: 10.1016/j.annonc.2025.03.016
153. Takamatsu K, Tanaka N, Hakozaki K, Takahashi R, Teranishi Y, Murakami T, et al. Profiling the inhibitory receptors LAG-3, TIM-3, and TIGIT in renal cell carcinoma reveals Malignancy. Nat Commun. (2021) 12:5547. doi: 10.1038/s41467-021-25865-0
154. Lin CC, Garralda E, Schöffski P, Hong DS, Siu LL, Martin M, et al. A phase 2, multicenter, open-label study of anti-LAG-3 ieramilimab in combination with anti-PD-1 spartalizumab in patients with advanced solid Malignancies. Oncoimmunology. (2024) 13:2290787. doi: 10.1080/2162402X.2023.2290787
155. Topalian SL, Drake CG, and Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. (2015) 27:450–61. doi: 10.1016/j.ccell.2015.03.001
156. Wang K, Coutifaris P, Brocks D, Wang G, Azar T, Solis S, et al. Combination anti-PD-1 and anti-CTLA-4 therapy generates waves of clonal responses that include progenitor-exhausted CD8(+) T cells. Cancer Cell. (2024) 42:1582–1597.e1510. doi: 10.1016/j.ccell.2024.08.007
157. Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. (2018) 378:1277–90. doi: 10.1056/NEJMoa1712126
158. Tian L, Goldstein A, Wang H, Ching Lo H, Sun Kim I, Welte T, et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature. (2017) 544:250–4. doi: 10.1038/nature21724
159. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. (2019) 380:1116–27. doi: 10.1056/NEJMoa1816714
160. Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. (2019) 380:1103–15. doi: 10.1056/NEJMoa1816047
161. Drake CG and Stein MN. The immunobiology of kidney cancer. J Clin Oncol. (2018) 29:Jco2018792648. doi: 10.1200/JCO.2018.79.2648
162. Wang LC, Lo A, Scholler J, Sun J, Majumdar RS, Kapoor V, et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol Res. (2014) 2:154–66. doi: 10.1158/2326-6066.CIR-13-0027
163. Santagata S, Napolitano M, D’Alterio C, Desicato S, Maro SD, Marinelli L, et al. Targeting CXCR4 reverts the suppressive activity of T-regulatory cells in renal cancer. Oncotarget. (2017) 8:77110–20. doi: 10.18632/oncotarget.20363
164. Wettersten HI, Aboud OA, Lara PN Jr., and Weiss RH. Metabolic reprogramming in clear cell renal cell carcinoma. Nat Rev Nephrol. (2017) 13:410–9. doi: 10.1038/nrneph.2017.59
165. Riesenberg R, Weiler C, Spring O, Eder M, Buchner A, Popp T, et al. Expression of indoleamine 2,3-dioxygenase in tumor endothelial cells correlates with long-term survival of patients with renal cell carcinoma. Clin Cancer Res. (2007) 13:6993–7002. doi: 10.1158/1078-0432.CCR-07-0942
166. Lara PN Jr., Villanueva L, Ibanez C, Erman M, Lee JL, Heinrich D, et al. A randomized, open-label, phase 3 trial of pembrolizumab plus epacadostat versus sunitinib or pazopanib as first-line treatment for metastatic renal cell carcinoma (KEYNOTE-679/ECHO-302). BMC Cancer. (2024) 23:1253. doi: 10.1186/s12885-023-10971-7
167. Long GV, Dummer R, Hamid O, Gajewski TF, Caglevic C, Dalle S, et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol. (2019) 20:1083–97. doi: 10.1016/S1470-2045(19)30274-8
168. Gien LT, Enserro DM, Block MS, Waggoner S, Duska LR, Wahner-Hendrickson AE, et al. Phase II trial of pembrolizumab and epacadostat in recurrent clear cell carcinoma of the ovary: An NRG oncology study GY016. Gynecol Oncol. (2024) 186:61–8. doi: 10.1016/j.ygyno.2024.03.027
169. Nguyen CB, Oh E, Bahar P, Vaishampayan UN, Else T, and Alva AS. Novel approaches with HIF-2α Targeted therapies in metastatic renal cell carcinoma. Cancers (Basel). (2024) 16:601. doi: 10.3390/cancers16030601
170. Meric-Bernstam F, Tannir NM, Iliopoulos O, Lee RJ, Telli ML, Fan AC, et al. Telaglenastat plus cabozantinib or everolimus for advanced or metastatic renal cell carcinoma: an open-label phase I trial. Clin Cancer Res. (2022) 28:1540–8. doi: 10.1158/1078-0432.CCR-21-2972
171. Mou W, Deng Z, Zhu L, Jiang A, Lin A, Xu L, et al. Intratumoral mycobiome heterogeneity influences the tumor microenvironment and immunotherapy outcomes in renal cell carcinoma. Sci Adv. (2025) 11:eadu1727. doi: 10.1126/sciadv.adu1727
172. Zarrabi KK, Lanade O, and Geynisman DM. Determining front-line therapeutic strategy for metastatic clear cell renal cell carcinoma. Cancers (Basel). (2022) 14:4607. doi: 10.3390/cancers14194607
173. Nguyen Duc A, Heinzmann D, Berge C, and Wolbers M. A pragmatic adaptive enrichment design for selecting the right target population for cancer immunotherapies. Pharm Stat. (2021) 20:202–11. doi: 10.1002/pst.2066
174. Braun DA, Moranzoni G, Chea V, McGregor BA, Blass E, Tu CR, et al. A neoantigen vaccine generates antitumour immunity in renal cell carcinoma. Nature. (2025) 639:474–82. doi: 10.1038/s41586-024-08507-5
175. Barragan-Carrillo R, Saad E, Saliby RM, Sun M, Albiges L, Bex A, et al. First and second-line treatments in metastatic renal cell carcinoma. Eur Urol. (2025) 87:143–54. doi: 10.1016/j.eururo.2024.10.019
176. O’Connell BC, Hubbard C, Zizlsperger N, Fitzgerald D, Kutok JL, Varner J, et al. Eganelisib combined with immune checkpoint inhibitor therapy and chemotherapy in frontline metastatic triple-negative breast cancer triggers macrophage reprogramming, immune activation and extracellular matrix reorganization in the tumor microenvironment. J Immunother Cancer. (2024) 12:e009160. doi: 10.1136/jitc-2024-009160
177. Motzer RJ, Schmidinger M, Eto M, Suarez C, Figlin R, Liu Y, et al. LITESPARK-011: belzutifan plus lenvatinib vs cabozantinib in advanced renal cell carcinoma after anti-PD-1/PD-L1 therapy. Future Oncol. (2023) 19:113–21. doi: 10.2217/fon-2022-0802
178. Zhang Y, Chen J, Liu H, Dai J, Zhao J, Zhu S, et al. The incidence of immune-related adverse events (irAEs) and their association with clinical outcomes in advanced renal cell carcinoma and urothelial carcinoma patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Cancer Treat Rev. (2024) 129:102787. doi: 10.1016/j.ctrv.2024.102787
179. Washino S, Shirotake S, Takeshita H, Inoue M, Miura Y, Hyodo Y, et al. Association between immune-related adverse events and survival in patients with renal cell carcinoma treated with nivolumab plus ipilimumab: immortal time bias-corrected analysis. Int J Clin Oncol. (2023) 28:1651–8. doi: 10.1007/s10147-023-02406-x
Keywords: renal cell carcinoma, tumor microenvironment, immunosuppressive cells, biomarkers, immunotherapy, combined targeted/immunotherapy
Citation: Wen H, Zheng S, Zhu X, Wang L and Chen D (2025) Characteristics of the tumor microenvironment and potential immunotherapy strategies in renal cell carcinoma. Front. Immunol. 16:1643533. doi: 10.3389/fimmu.2025.1643533
Received: 09 June 2025; Accepted: 15 August 2025;
Published: 02 September 2025.
Edited by:
Zhe Pei, Virginia Tech, United StatesReviewed by:
Tingting Huang, Guangxi Medical University, ChinaQian Yang, Chongqing Medical University, China
Copyright © 2025 Wen, Zheng, Zhu, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongping Chen, MTM3NjQzNjI1NjlAMTYzLmNvbQ==; Ling Wang, bm93YXhAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Hui Wen
Hui Wen Shi Zheng
Shi Zheng Xiaoqin Zhu6†
Xiaoqin Zhu6† Dongping Chen
Dongping Chen