- Department of Neurology, Tianjin Neurological Institute, Tianjin Medical University General Hospital, Tianjin, China
Background: Neuromyelitis optica spectrum disorder (NMOSD) is a rare autoimmune disease of the central nervous system, primarily characterized by anti-AQP4 antibodies. While, treatment for preventing recurrence of NMOSD predominantly focuses on the production of anti-AQP4 antibodies and the subsequent inflammatory response, one effective strategy is targeting B cells, investigating the status of T lymphocytes during NMOSD onset holds significant importance for elucidating disease mechanisms and identifying potential novel therapeutic approaches.
Methods: Peripheral blood samples were collected from NMOSD patients with acute exacerbation. The NMOSD patients were divided into the pre- glucocorticoid treatment NMOSD patient group (PRE) and the glucocorticoid treatment NMOSD patient group (GC) based on whether they received glucocorticoid therapy. Healthy controls were included at the same time. Flow cytometry was employed to analyze differences in T cell compartment.
Results: Multivariate linear regression analysis adjusted for age revealed that the GC group had fewer CD8+ TEM cells than controls (β=-14.96, P=0.002). In addition, the PRE group had higher frequencies of HLA-DR+CD38+ CD4+ cells and HLA-DR+ CD4+ T cells compared to the control group (P= 0.005, P= 0.004, respectively), the frequency of HLA-DR+ CD4+ T cells in PRE group was higher than the GC group (P= 0.007). The multivariate analysis results showed that in CD4+ T cells, the frequency of Th1Th17 cells in the PRE patient group was higher than that in the control group (β= 6.37, 98.33% CI:1.96-10.78, P = 0.001), and the frequency of Th2 cells in the PRE group was lower than that in the control group (β=-11.41, 98.33% CI: -22.26– -0.55, P = 0.012).
Conclusion: These findings underscore the pivotal role of Th1Th17 and Th17 cells in NMOSD pathogenesis. Exploring intervention strategies targeting Th17 cells or T cell activation (e.g., HLA-DR-targeted therapies) may hold clinical relevance.
1 Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is a rare autoimmune-mediated demyelinating disease of the central nervous system characterized by pathogenic antibodies targeting aquaporin-4 (AQP4) on astrocytes (1). The disorder manifests with distinctive clinical features including optic neuritis, longitudinally extensive transverse myelitis, area postrema syndrome, and acute brainstem syndrome (2). Epidemiologically, NMOSD demonstrates considerable geographic and ethnic variation, with reported incidence rates ranging from 0.037 to 0.71 per 100,000 person-years and prevalence rates between 0.7 and 10 per 100,000 person-years worldwide. A striking female predominance is observed, with women affected at more than twice the rate of men (3). The disease follows a relapsing course with cumulative, irreversible disability progression, underscoring the critical need for elucidating its immunopathogenic mechanisms to develop novel therapeutic strategies (4).
The immunopathogenesis of NMOSD involves a complex interplay between humoral and cellular immunity. In AQP4-IgG seropositive patients, peripherally activated plasma cells produce pathogenic autoantibodies that cross the compromised blood-brain barrier. These AQP4-IgG antibodies initiate astrocytic injury through antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity, subsequently leading to secondary oligodendrocyte damage and demyelination (5). Importantly, T lymphocytes play a pivotal role in this process by facilitating B cell activation, differentiation, and antibody production. Experimental evidence from murine models demonstrates that AQP4-reactive T cells can induce NMOSD-like pathology even in the absence of AQP4-IgG, highlighting their autonomous pathogenic potential (6).
CD4+ and CD8+ T lymphocytes can be classified into functionally distinct subsets based on CD45RA and CCR7 expression profiles: naive (TN), central memory (TCM), effector memory (TEM), and terminally differentiated effector memory cells re-expressing CD45RA (TEMRA). Naive T cells (TN) of the innate immune system can migrate to the T cell area of secondary lymphoid organs to search for antigen-presenting dendritic cells (7). After antigen contact, they are activated and start to proliferate. Study found that compared with healthy controls, the frequencies of CD8+ TN (CD62LhiCD45RO-) cells in NMOSD and multiple sclerosis (MS) patients were significantly decreased, while the frequencies of CD8+ TE/M (CD62LloCD45RO+) cells were significantly increased. In NMOSD patients receiving immunotherapy, the frequencies of CD8+ TN increased and those of CD8+ TE/M decreased (8). Moreover, MS patients show an age-related abnormal increase in activated (HLA-DR+CD38+) and cytotoxic CD4 T cells (9). However, these have rarely been reported in NMOSD patients. Additionally, Helper T lymphocytes (Th) cells, especially Th17 cells, have gradually attracted attention in immune-related neurological diseases (10).
To better characterize T lymphocyte dynamics during acute NMOSD episodes and identify potential therapeutic targets, we conducted an immunological analysis in patients presenting with acute attack.
2 Methods
2.1 Patients and controls
This study recruited patients diagnosed with NMOSD in the acute phase, admitted to the Department of Neurology at Tianjin Medical University General Hospital between January 2024 and May 2025. Inclusion criteria: 1. Diagnosed with NMOSD, 2. Acute stage defined as a new or recurrent neurological symptom that lasts for at least 24 hours, without fever, infection or other autoimmune diseases, with symptoms persisting or worsening from the onset of this disease to the time of admission, 3. No immunomodulatory treatment (such as rituximab, tocilizumab, infliximab, etc.) since the onset of this disease. Exclusion criteria: 1. Pregnant or lactating, 2. Active infection, 3. Severe liver or kidney dysfunction, abnormal coagulation function, history of tumor, 4. Currently participating in other clinical trials. Patients with NMOSD had not yet undergone glucocorticoid therapy were assigned to the pre- glucocorticoid treatment group (PER), while those had received one week of glucocorticoid treatment were included in the glucocorticoid treatment group (GC).
Age- and sex-matched healthy controls were recruited from the hospital’s health examination center. Inclusion criteria: No immune system-related diseases, no history of tumors, no severe coagulation function abnormalities, no organ dysfunction (liver dysfunction, kidney dysfunction, etc.), no history of immunosuppressant use. Exclusion criteria: 1. Pregnant or lactating, 2. Active infection, 3. Currently participating in other clinical trials. A total of 9 patients with NMOSD before treatment, 9 patients with NMOSD treated with glucocorticoids and 25 healthy controls were included in this study.
2.2 Collection of basic information
Demographic characteristics, including age and sex, were recorded for all study participants. For NMOSD patients, serum anti-aquaporin-4 (AQP4) antibody levels were measured, and neurological disability was assessed using the Expanded Disability Status Scale (EDSS).
2.3 Flow cytometry
Peripheral blood samples were collected via antecubital venipuncture from all study participants. PBMCs were isolated using red blood cell lysing solution (BD FACS Lysing Solution, 349202) and were stained with two panels. Panel 1: The cells were stained with PerCP/Cyanine5.5 anti-human CD3 (300430, BioLegend), Brilliant Violet 510™ anti-human CD4 (317444, BioLegend), APC/Cyanine7 anti-human CD8 (344714, BioLegend), PE/Cyanine7 anti-human CD45RA (304126, BioLegend), PE anti-human CCR7 (353204, BioLegend), APC anti-human HLA-DR (307610, BioLegend), Brilliant Violet 421™ anti-human CD38 (303526, BioLegend). Panel 2: The cells were stained with PerCP/Cyanine5.5 anti-human CD3 (300430, BioLegend), Brilliant Violet 510™ anti-human CD4 (317444, BioLegend), FITC anti-human CXCR3 (353704, BioLegend), APC/Cyanine7 anti-human CCR6 (353432, BioLegend), PE/Cyanine7 anti-human CD45RA (304126, BioLegend), Brilliant Violet 421™ anti-human CCR7(353208, BioLegend). PBMCs were incubated with above-mentioned antibody cocktails for 30 min at room temperature. The cells were then washed twice in cold phosphate buffered saline (PBS). Finally, the cells were resuspended in 500μl PBS and acquired using a FACS Aria III (BD Biosciences, San Jose, CA, USA). The results were analyzed using FlowJo v10 software.
2.4 Data analysis and statistics
Univariate analysis among the three groups was conducted using ANOVA and Bonferroni multiple comparison correction, and the significance of the adjusted P value was 0.05. Linear regression was used to analyze the effects of age and different groups on T cell compartment. Due to multiple comparisons the significance level (0.05/3 = 0.017) and confidence intervals (98.33% CI) were adjusted. For age, the significance level was 0.05, and the 95% confidence interval was used. Statistical analysis was performed using SPSS 22.0 software. Graphs were performed using GraphPad Prism 6.
2.5 Ethics
This study was approved by the Ethics Committee of Tianjin Medical University General Hospital (IRB2025-YX-113-01). All the subjects included in the study gave their informed consent either by themselves or through their legal representatives.
3 Results
3.1 Basic information of the participants
This study included 9 research subjects in the group pre-glucocorticoid treatment (PRE), 9 research subjects in the glucocorticoid treatment group (GC), and 25 research subjects in the control group. The average age of the PRE group, the GC group and the control group was 51.56 years, 56.22 years and 51.00 years respectively. All the research subjects were female. 17 NMOSD patients were anti-AQP4 antibody positive, while the median EDSS scores for both the PRE group and the GC group were 2 (Table 1).
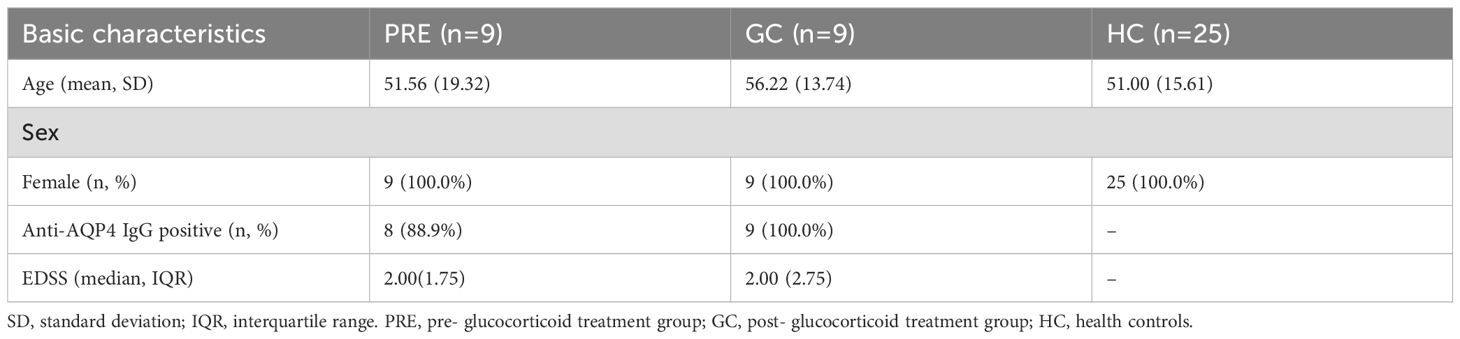
Table 1. Basic information among the pre- glucocorticoid treatment group, the glucocorticoid treatment group and the healthy controls.
3.2 Differences in lymphocyte functional subsets among the pre- glucocorticoid treatment group, the glucocorticoid treatment group and the healthy controls
ANOVA analysis showed that the GC group patients had significantly lower CD8+ TEM cell frequencies compared to healthy controls (21.71 vs. 35.03, adjust P=0.033). (Figure 1; Supplementary Table S1).
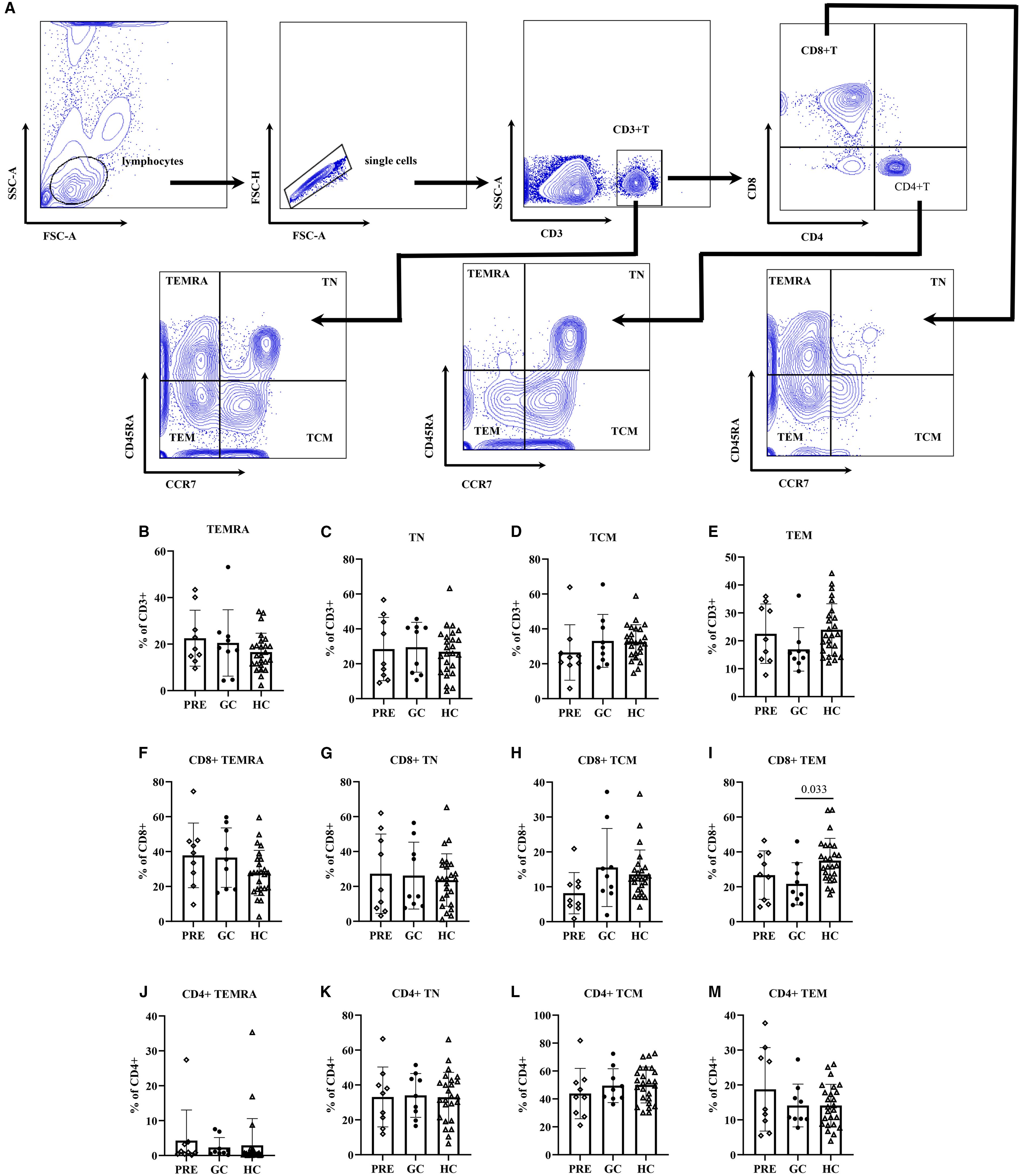
Figure 1. The flow cytometry gating strategy (A). Frequencies of lymphocyte functional subsets among the pre- glucocorticoid treatment group, the glucocorticoid treatment group and the healthy controls (B–M).
Multivariate linear regression analysis adjusted for age revealed that the GC group had fewer CD8+ TEM cells than controls (β=-14.96, 98.33% CI: -26.13 – -3.79; P=0.002). In addition, regression analysis also indicated that TN cells, CD8+ TN cells, and CD4+ TN cell frequencies decreased with age (P<0.001, P<0.001, P=0.003, respectively). In contrast, TEM and CD8+TEM cell frequencies increased with age: each additional year was associated with 0.23 increase in TEM cells (P=0.009), and 0.38 increase in CD8+ TEM cells (P=0.002). Similar trends were observed in CD8+ TCM and CD4+ TEMRA cell subsets, with each year of age corresponding to 0.15 increase in CD8+ TCM cells (P=0.045) and 0.14 increase in CD4+ TEMRA cells (P=0.047) (Table 2).
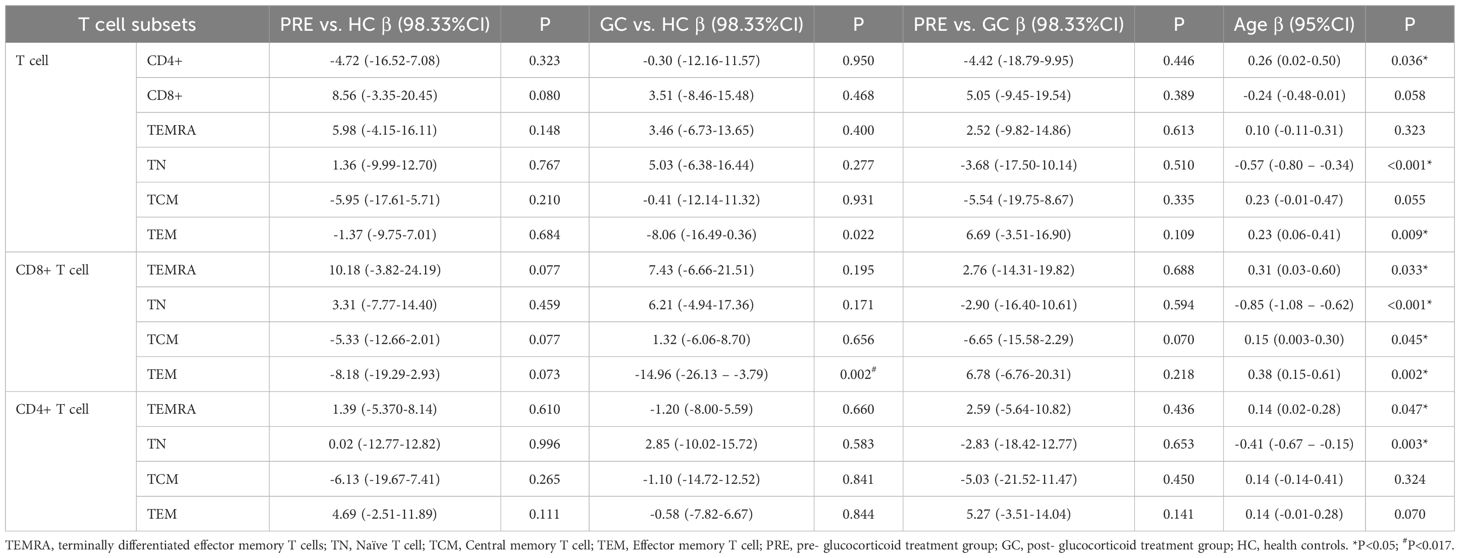
Table 2. The linear regression of lymphocyte functional subsets among the pre- glucocorticoid treatment group, the glucocorticoid treatment group and the healthy controls.
3.3 Differences in activated T lymphocyte subsets among the pre- glucocorticoid treatment group, the glucocorticoid treatment group and the healthy controls
ANOVA analysis showed that the frequency of HLA-DR+ CD38+ CD4+T cells in PRE group was higher than that in the control group (2.15 vs. 1.20, adjust P = 0.014). The frequency of HLA-DR+ CD4+ T cells in PRE group was higher than those in both the GC group and the control group, being 3.39 vs. 1.70, adjust P = 0.016, and 3.39 vs. 2.05, adjust P = 0.022, respectively (Figure 2; Supplementary Table S2).
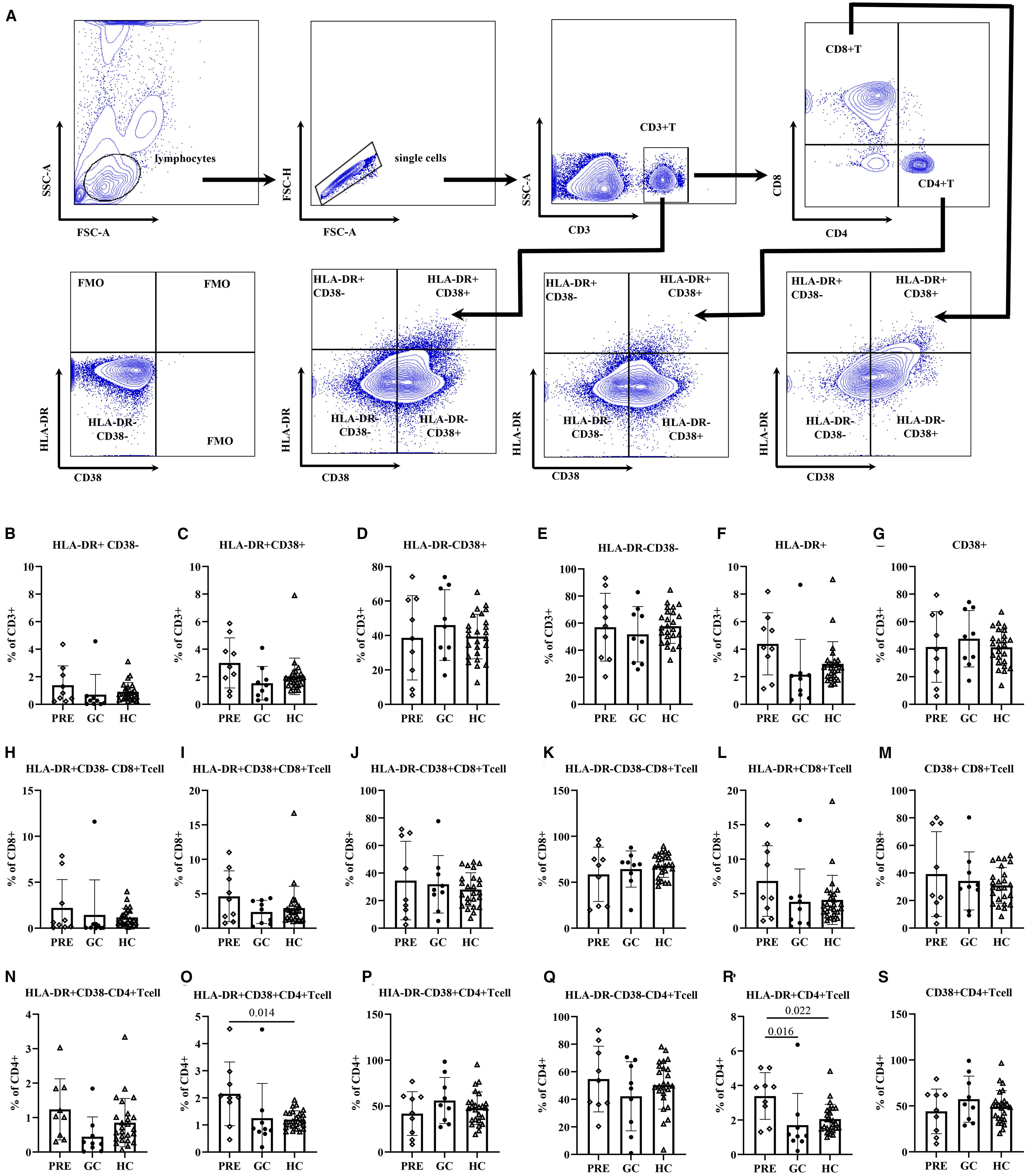
Figure 2. The flow cytometry gating strategy (A). Frequencies of activated T lymphocyte subsets among the pre- glucocorticoid treatment group, the glucocorticoid treatment group and the healthy controls (B–S).
Multivariate regression analysis adjusted for age revealed that the PRE group had higher frequencies of HLA-DR+CD38+ CD4+ cells compared to the control group (β=0.96, 98.33% CI: 0.16-1.75, P= 0.005). In addition, the frequency of HLA-DR+ CD4+ T cells in PRE group was higher than those in both the GC group and the control group (β=1.34, 98.33% CI: 0.17-2.51, P= 0.007, β=1.77, 98.33% CI: 0.34-3.19, P= 0.004, respectively). The frequencies of CD4+ HLA-DR-CD38+ T cells and CD4+ CD38+ T cells exhibited a significant decline with advancing age (P = 0.039, and P = 0.044, respectively). For each additional year of age, the frequencies of CD4+ HLA-DR− CD38− T cells increased by 0.40 (P=0.047) (Table 3).
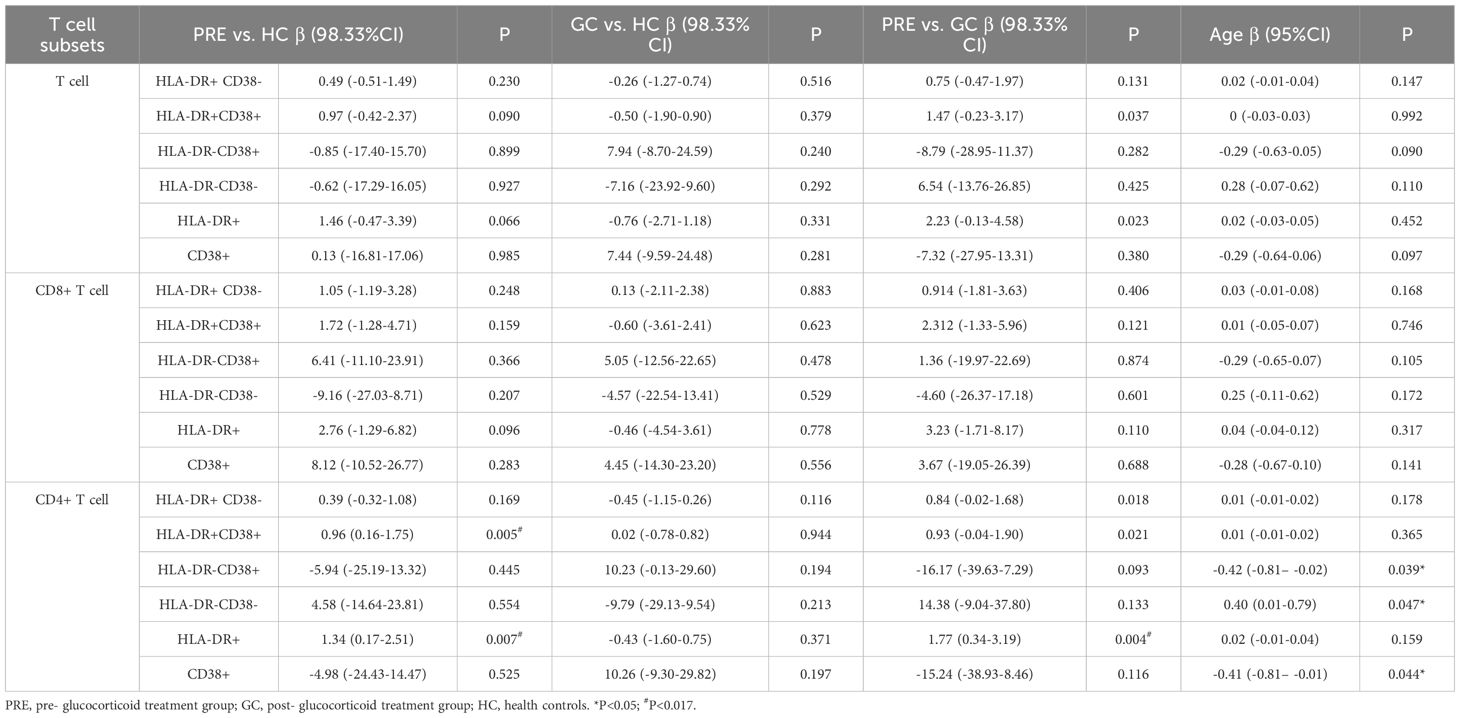
Table 3. The linear regression of activated T lymphocyte subsets among the pre- glucocorticoid treatment group, the glucocorticoid treatment group and the healthy controls.
3.4 Differences in helper T lymphocyte subsets among the pre- glucocorticoid treatment group, the glucocorticoid treatment group and the healthy controls
ANOVA analysis showed that in CD4+ T cells, the PRE group had a higher frequency of Th1Th17 cells compared to the control group (15.15 vs. 8.81, adjust P= 0.003), while the frequency of Th2 cells was lower in the PRE group than in controls (33.23 vs. 44.62, adjust P= 0.034). In CD4+ TCM cells, the GC group exhibited a lower frequency compared to controls, respectively, 28.18 vs. 38.05 (adjust P= 0.003). Conversely, the frequency of Th17 cells in the GC patient group was 26.43 (5.20), which was higher than that in the control group 17.97 (5.99) (adjust P = 0.002). In the CD4+TEM cells, the frequency of Th17 cells in the GC group was 19.01 (8.05), which was higher than that in controls 11.28 (4.44) (adjust P = 0.004) (Figure 3, Supplementary Table S3).
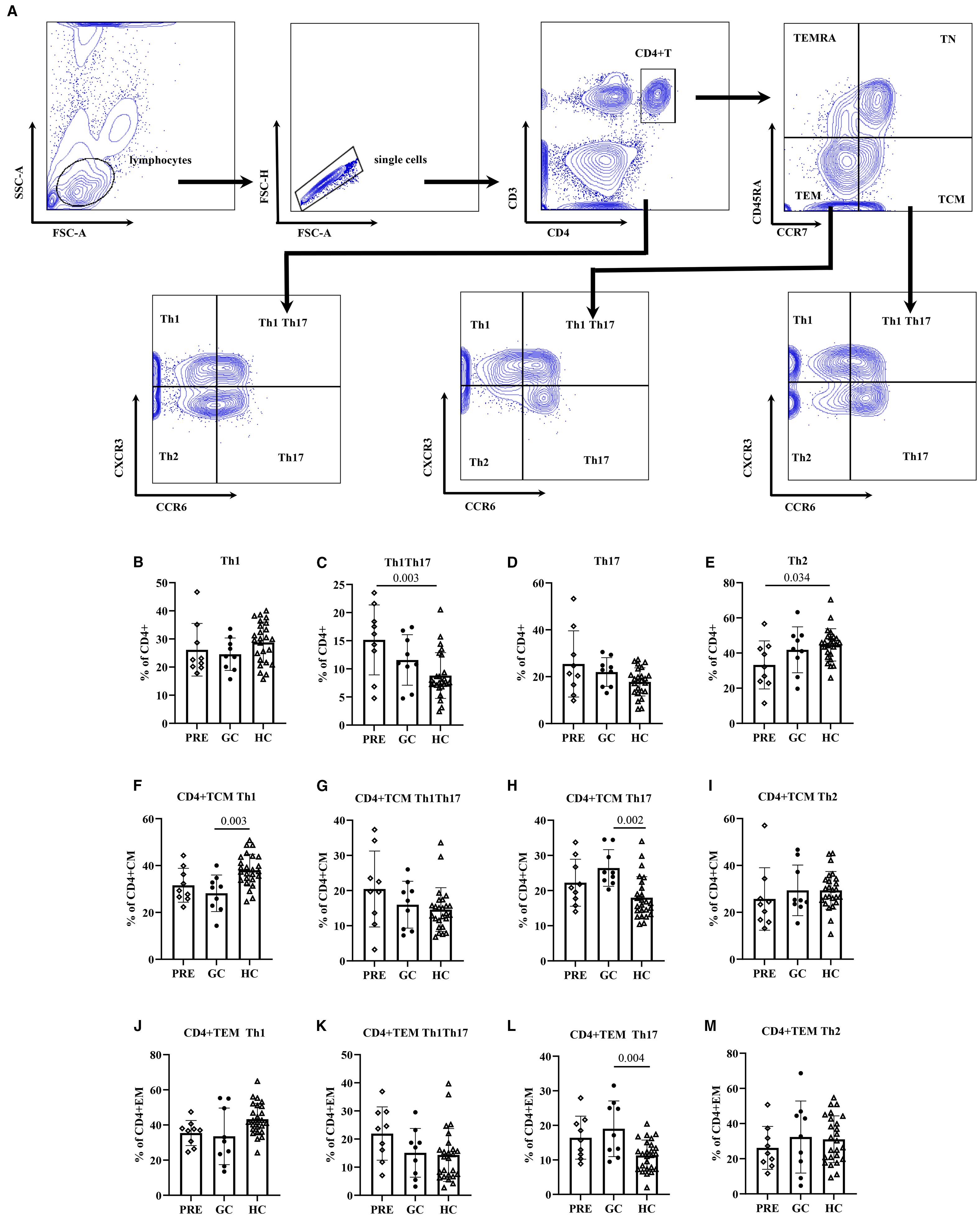
Figure 3. The flow cytometry gating strategy (A). Frequencies of helper T lymphocyte subsets among the pre- glucocorticoid treatment group, the glucocorticoid treatment group and the healthy controls (B–M).
The multivariate analysis results showed that in CD4+ T cells, the frequency of Th1Th17 cells in the PRE patient group was higher than that in the control group (β=6.37, 98.33% CI:1.96-10.78, P = 0.001), and the frequency of Th2 cells in the PRE group was lower than that in the control group (β=-11.41, 98.33% CI: -22.26– -0.55, P = 0.012). In CD4+ TCM cells, the frequency of Th1 cells in the GC group was 9.57 lower than that in the control group (P = 0.001), and the frequency of Th17 cells in the GC group was higher than that in the control group (β=8.22, 98.33% CI: 2.36-14.09, P = 0.001). In CD4+ TEM cells, the frequency of Th17 cells in the GC group was higher than that in the control group (β=7.93, 98.33% CI: 2.33-13.52, P = 0.001) (Table 4).
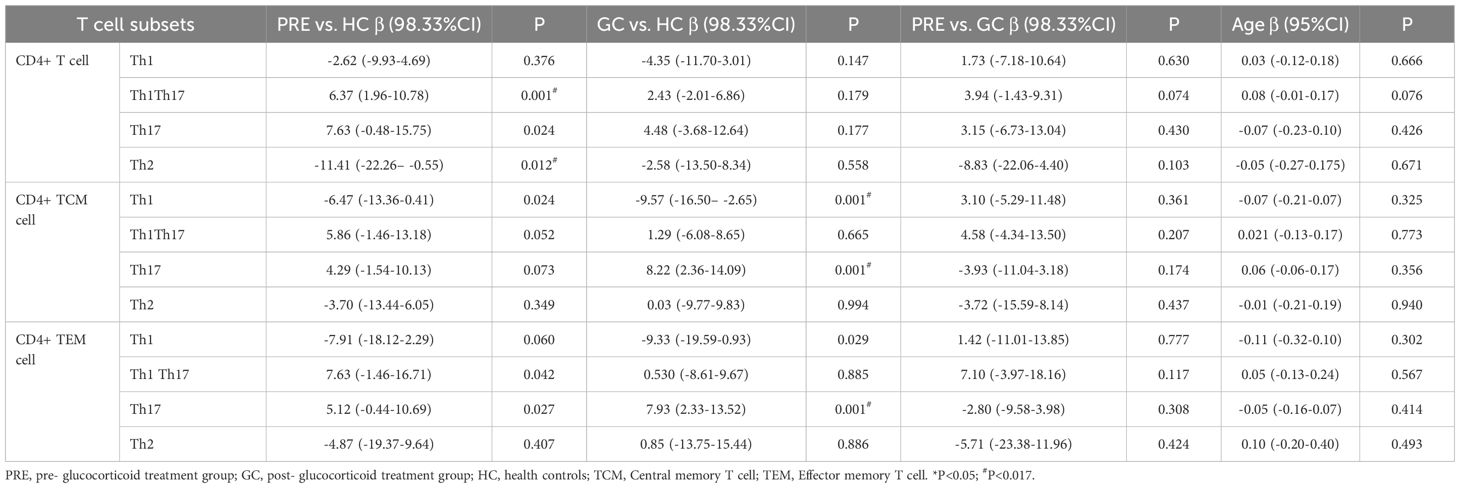
Table 4. The linear regression of helper T lymphocyte subsets among the pre- glucocorticoid treatment group, the glucocorticoid treatment group and the healthy controls.
4 Discussion
This study found that, compared to healthy controls, patients with untreated NMOSD in the acute phase exhibited higher frequency of CD4+ Th1Th17 and fewer CD4+ Th2 cells. Patients with NMOSD who received glucocorticoid treatment had fewer frequency of CD8+TEM cells compared to healthy controls. Additionally, NMOSD patients in the acute phase before glucocorticoid treatment showed higher frequencies of CD4+HLA-DR+CD38+ T cells and CD4+HLA-DR+ T cells, while glucocorticoid therapy reduced the frequencies of CD4+HLA-DR+ T cells.
Naïve T cells are mature T cells that have not been exposed to antigens, which have a high proliferative potential. When they encounter antigens for the first time, they are activated and differentiate into effector cells or memory cells. Memory T cells can be classified into TCM cells (central memory T cells), TEM cells (effector memory T cells), and TEMRA cells (terminally differentiated effector memory T cells), each exhibiting distinct homing and effector functions. When an infection or re-exposure to the same antigen occurs, TCM cells can rapidly proliferate and differentiate into effector T cells, promoting and maintaining the immune response against the specific pathogen (11). Compared to TCM cells, TEM cells have a weaker proliferative capacity. They can migrate to different tissues in the body after infection and exert their effector functions by receiving appropriate antigen signals (12). TEMRA is in the terminal differentiation stage and has strong effector functions but extremely low proliferation capacity. TN cells gradually decline with age, while TCM and TEM cells increase (7, 13, 14). Memory T cells play a crucial role in processes such as infection, organ transplant rejection and tolerance (15), autoimmune diseases (16, 17), and tumors (18). TEM cells play a crucial protective role against viral pathogens (19).
The research conducted by Shi et al. showed that, compared with healthy controls, the CD8 + TN (CD62LhiCD45RO-) cells in patients with NMOSD and MS were significantly decreased, while the CD8 + TE/M (CD62LloCD45RO+) cells were significantly increased. The CD8 + TN in NMOSD patients who received immunotherapy increased, and the CD8 + TE/M decreased, which was related to the treatment (8). Previous findings in MS on CD8+ TEM cells have yielded conflicting results: Pender et al. reported a deficiency of peripheral CD8+ TEM and TEMRA cells in MS patients, suggesting a possible link to EBV-infected B cells and impaired CD8+ T-cell responses (20). This study found that the frequency of CD8+TEM cells in the GC group were significantly lower than those in the control group, but there was no statistical significance when compared with the PRE group. Glucocorticoids may exert immunomodulatory effects by inhibiting the activation and expansion of TEM cells, thereby alleviating the inflammatory response in autoimmune diseases. However, the correlation between viral infections and the onset of NMOSD is not clear. Currently, most of the related studies are small-sample and retrospective in nature (21–23). This study revealed a downward trend in TEM cells in untreated patients with NMOSD, but this trend was not statistically significant. This suggests that there may be a connection between the onset of NMOSD and viral infection, but further exploration is needed through larger sample size longitudinal studies.
HLA-DR and CD38 are classic markers of T cell activation, and their upregulation may be associated with enhanced antigen presentation, increased pro-inflammatory cytokine release, and exacerbation of autoimmune responses. In pediatric patients with immune thrombocytopenia, the frequency of CD4+HLA-DR+ T cells was significantly higher than in healthy controls (24). HLA-DR+CD38+ T cells have also been identified in MS (9, 25, 26). Currently, there are few clinical studies on peripheral activated T-cell subsets in NMOSD patients. This study found that untreated NMOSD patients in the acute phase exhibited higher frequencies of CD4+HLA-DR+CD38+ and CD4+HLA-DR+ T cells, suggesting a highly activated state of T cells. This finding supports the notion that widespread T-cell activation occurs during the acute phase of NMOSD, which may drive disease exacerbation. Furthermore, glucocorticoid treatment significantly reduced the frequency of CD4+HLA-DR+ T cells in patients with NMOD. Previous studies have shown that glucocorticoids have a certain immunosuppressive effect (27). Glucocorticoids selectively induce the apoptosis of activated T cells by upregulating the pro-apoptotic protein Bim and inhibiting Bcl-2, which may lead to a reduction in HLA-DR+ T cells (28). Glucocorticoids can inhibit the functions of dendritic cells and the anti-inflammatory effects of macrophages. They indirectly affect the differentiation and activation of T cells through antigen presentation and cytokines, and may reduce T cell activation and HLA-DR expression (29).
Th1 cells mediate the immune response against intracellular pathogens and are also involved in the induction of some autoimmune diseases. Their main cytokine products are IFNγ and IL-2 (30). The production of IL-2 is of great significance for CD4+ T cell memory. IL-2 is crucial for stimulating the formation of CD8+ T cell memory during the initiation stage of CD8 cells (31). Th2 cells mediate host defense against extracellular parasites including helminths. They play an important role in the induction and persistence of asthma and other allergic diseases. Th2 cells produce cytokines such as IL-4, IL-5, IL-9, IL-10, IL-13, and IL-25 (30). Th17 cells have the function in the clearance of specific types of pathogens that require a massive inflammatory response, including Gram-positive and Gram-negative bacteria, and fungi (such as Candida albicans), which can trigger a strong Th17 response. The main cytokines secreted by Th17 cells include IL-17, IL-22, and IL-23 (32). They play an important role in autoimmune diseases and some bacterial and fungal infections (33). Th1Th17 cells are a subset identified by CXCR3+CCR6+, sharing proinflammatory features of both Th1 and Th17 cells. Th1Th17 cells have been found to be associated with the onset of autoimmune diseases such as multiple sclerosis (34, 35).
The study by Cao et al. found that Th1 levels in NMOSD patients during the acute phase were higher than those in the remission-phase cohort and healthy controls (36). Another study observed Th1 predominance only in MS patients, with no Th1/Th2 imbalance detected in NMO patients (37). This study observed a decreased frequency of CD4+ Th2 cells in pre-glucocorticoid treatment acute-phase NMOSD patients compare to the controls. The GC group showed a significantly lower frequency of Th1 cells in CD4+ TCM cells compared to the control group, but there was no difference when compared to the PRE group, suggesting that glucocorticoid therapy may reduce Th1 cell frequencies.
Previous histopathological studies have indicated that CD4+ T cell infiltration is prominent in the lesions of NMOSD during the acute phase and decreases during remission, suggesting the involvement of helper T lymphocytes in the pathogenesis of NMOSD (38). Research on the pathogenesis of NMO suggests that Th17 cells and their effector molecules play a significant role. A meta-analysis reported eight studies on the frequency of Th17 cells among CD4+ T cells in peripheral blood, revealing that NMOSD patients had a higher frequency of Th17 cells compared to controls. Additionally, NMOSD patients exhibited elevated levels of IL-1β, IL-6, IL-17, and IL-21 in cerebrospinal fluid and plasma, as well as higher serum levels of IL-6, IL-21, IL-22, and IL-23 compared to controls. However, due to high heterogeneity among multiple study results, it remains inconclusive whether Th17 cells are reliable biomarkers of NMOSD disease activity (39). This study found an increase in Th17 cells (including CD4+ Th17 and Th1Th17 subsets) in NMOSD patients during the acute phase. The frequency of Th1Th17 cells weas significantly higher in the PRE group compared to the control group. The Th17 cells showed a trend of being higher in the PRE group than in the control group, but the difference was not significant. However, in the GC group, the Th17 cells within CD4+TCM cells and CD4+TEM cells were significantly higher than in the control group, suggesting that short-term glucocorticoid treatment did not significantly reduce the circulating Th17 levels. These findings do not conflict with previous studies and further support the crucial role of Th17 cells, especially Th1Th17, in NMOSD pathogenesis.
Th17 cells were discovered and characterized based on their production of IL-17A (40, 41). IL-17A secreted by Th17 cells can activate endothelial cells and astrocytes, inducing the release of pro-inflammatory cytokines (e.g., IL-6, TNF-α) and chemokines, thereby recruiting myeloid cells such as neutrophils to infiltrate the central nervous system (CNS) and exacerbate inflammation (42). IL-17A disrupts tight junction proteins, increasing blood-brain barrier (BBB) permeability and facilitating the entry of autoantibodies (e.g., AQP4-IgG) and CD4+ lymphocytes into the CNS (10). IL-17A can directly activate astrocytes, making them more susceptible to AQP4-IgG-mediated complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity (43, 44). Furthermore, IL-6. a key factor in NMOSD, promotes Th17 differentiation, while IL-17 and IL-21 secreted by Th17 cells further stimulate IL-6 production, amplifying inflammation (45). In summary, Th17 cells contribute to BBB disruption, CNS inflammatory infiltration, and autoantibody production. In the EAE model, CD4+ T cells co-secreting IFN-γ and IL-17 (Th1Th17 cells) infiltrated the brain before the onset of clinical symptoms, whereas significant Th1 cell infiltration was only detected after clinical disease progression. This suggests that Th1Th17 cells exhibit stronger pro-inflammatory activation than Th1 cells, potentially mediating CNS inflammation through pro-inflammatory cytokines and microglial activation (46). However, research on NMOSD remains limited. Most current studies suggest that the Th1/Th17 balance plays a more significant pro-inflammatory role in NMOSD than the Th1/Th2 balance, and further exploration of this issue is warranted in future studies (10).
This study has certain limitations. First, NMOSD is a rare neurological disease with low incidence. As a single-center study with a limited number of enrolled cases, the findings should be validated in larger sample studies in the future. Second, this is a cross-sectional study. Longitudinal follow-up to monitor changes in various T cell compartment in NMOSD patients would be of great significance for both pathogenesis research and treatment.
5 Conclusion
The findings of this study further emphasize the importance of Th17 cells, especially Th1Th17, in NMOSD pathogenesis and suggest potential therapeutic targets. Exploring intervention strategies targeting Th17 cells (e.g., IL-17 inhibitors) or T cell activation (e.g., HLA-DR-targeted therapies) may help optimize immunomodulatory treatments for NMOSD. Additionally, the regulatory effects of glucocorticoids on TEM cells highlight their value in acute-phase management. However, long-term use may lead to adverse effects, necessitating the integration of more precise immunomodulatory approaches to achieve long-term disease control.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Tianjin Medical University General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YS: Writing – original draft, Methodology, Conceptualization. T-FT: Formal analysis, Writing – review & editing. L-JZ: Writing – review & editing. NZ: Writing – review & editing. LY: Writing – review & editing. Q-XZ: Writing – review & editing, Methodology, Conceptualization, Project administration.
Funding
The authors declare financial support was received for the research and/or publication of this article. This research was funded by National Natural Science Foundation of China (grant no.82401574).
Acknowledgments
We thank all participants for their valuable contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1654533/full#supplementary-material
Abbreviations
NMOSD, Neuromyelitis optica spectrum disorder; AQP4, anti-aquaporin-4; TEMRA, Terminally differentiated effector memory T cells; TN, Naïve T cell; TCM: Central memory T cell; TEM, Effector memory T cell; EDSS, Expanded Disability Status Scale; SD, standard deviation; IQR, interquartile range.
References
1. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. (2015) 85. doi: 10.1212/WNL.0000000000001729
2. Liu C, Shi M, Zhu M, Chu F, Jin T, and Zhu J. Comparisons of clinical phenotype, radiological and laboratory features, and therapy of neuromyelitis optica spectrum disorder by regions: update and challenges. Autoimmun Rev. (2022) 21:102921. doi: 10.1016/j.autrev.2021.102921
3. Papp V, Magyari M, Aktas O, Berger T, Broadley SA, Cabre P, et al. Worldwide incidence and prevalence of neuromyelitis optica: A systematic review. Neurology. (2021) 96:59–77. doi: 10.1212/WNL.0000000000011153
4. Huang W, ZhangBao J, Chang X, Wang L, Zhao C, Lu J, et al. Neuromyelitis optica spectrum disorder in China: Quality of life and medical care experience. Mult Scler Relat Disord. (2020) 46:102542. doi: 10.1016/j.msard.2020.102542
5. Siriratnam P, Huda S, Butzkueven H, van der Walt A, Jokubaitis V, and Monif M. A comprehensive review of the advances in neuromyelitis optica spectrum disorder. Autoimmun Rev. (2023) 22:103465. doi: 10.1016/j.autrev.2023.103465
6. Afzali AM, Nirschl L, Sie C, Pfaller M, Ulianov O, Hassler T, et al. B cells orchestrate tolerance to the neuromyelitis optica autoantigen AQP4. Nature. (2024) 627:407–15. doi: 10.1038/s41586-024-07079-8
7. Aiello A, Farzaneh F, Candore G, Caruso C, Davinelli S, Gambino CM, et al. Immunosenescence and its hallmarks: how to oppose aging strategically? A review of potential options for therapeutic intervention. Front Immunol. (2019) 10:2247. doi: 10.3389/fimmu.2019.02247
8. Shi Z, Qiu Y, Zhao Z, Wen D, Chen H, Du Q, et al. CD8+ T cell subpopulations and pro-inflammatory cytokines in neuromyelitis optica spectrum disorder. Ann Clin Transl Neurol. (2021) 8:43–53. doi: 10.1002/acn3.51241
9. Zuroff L, Rezk A, Shinoda K, Espinoza DA, Elyahu Y, Zhang B, et al. Immune aging in multiple sclerosis is characterized by abnormal CD4 T cell activation and increased frequencies of cytotoxic CD4 T cells with advancing age. eBioMedicine. (2022) 82:104179. doi: 10.1016/j.ebiom.2022.104179
10. Dos Passos GR, Sato DK, Becker J, and Fujihara K. Th17 cells pathways in multiple sclerosis and neuromyelitis optica spectrum disorders: pathophysiological and therapeutic implications. Mediators Inflammation. (2016) 2016:5314541. doi: 10.1155/2016/5314541
11. Zhang Q and Lakkis FG. Memory T cell migration. Front Immunol. (2015) 6:504. doi: 10.3389/fimmu.2015.00504
12. Mueller SN, Gebhardt T, Carbone FR, and Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. (2013) 31:137–61. doi: 10.1146/annurev-immunol-032712-095954
13. Hong MS, Dan JM, Choi J-Y, and Kang I. Age-associated changes in the frequency of naïve, memory and effector CD8+ T cells. Mech Ageing Dev. (2004) 125:615–8. doi: 10.1016/j.mad.2004.07.001
14. Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint J-P, and Labalette M. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech Ageing Dev. (2006) 127:274–81. doi: 10.1016/j.mad.2005.11.001
15. Benichou G, Gonzalez B, Marino J, Ayasoufi K, and Valujskikh A. Role of memory T cells in allograft rejection and tolerance. Front Immunol. (2017) 8:170. doi: 10.3389/fimmu.2017.00170
16. Deng G, Zhang Y, Song J, Zhang Y, Zheng Q, Luo Y, et al. The role and therapeutic strategies for tissue-resident memory T cells, central memory T cells, and effector memory T cells in psoriasis. Immunology. (2024) 173:470–80. doi: 10.1111/imm.13843
17. Casciano F, Severi P, Marongiu L, Caproni A, Terranova C, Spitilli A, et al. Highly differentiated T cells link systemic and vascular inflammation in a mouse model of recurrent psoriasis. Front Immunol. (2025) 16:1574455. doi: 10.3389/fimmu.2025.1574455
18. Gémes N, Balog JÁ, Neuperger P, Schlegl E, Barta I, Fillinger J, et al. Single-cell immunophenotyping revealed the association of CD4+ central and CD4+ effector memory T cells linking exacerbating chronic obstructive pulmonary disease and NSCLC. Front Immunol. (2023) 14:1297577. doi: 10.3389/fimmu.2023.1297577
19. Tian Y, Babor M, Lane J, Seumois G, Liang S, Goonawardhana NDS, et al. Dengue-specific CD8+ T cell subsets display specialized transcriptomic and TCR profiles. J Clin Invest. (2019) 129:1727–41. doi: 10.1172/JCI123726
20. Pender MP, Csurhes PA, Pfluger CM, and Burrows SR. Deficiency of CD8+ effector memory T cells is an early and persistent feature of multiple sclerosis. Mult Scler. (2014) 20:1825–32. doi: 10.1177/1352458514536252
21. Shi Z, Kong L, Wang R, Wang X, Wang Z, Luo W, et al. Cytomegalovirus and Epstein-Barr virus infections in patients with neuromyelitis optica spectrum disorder. J Neurol. (2024) 271:6089–95. doi: 10.1007/s00415-024-12571-2
22. Ou Yang W-Y, Tsai Y-S, Liu Y-H, Wang Y-F, Hsiao C-T, Lai K-L, et al. Preceding hepatitis B virus infection is highly prevalent in patients with neuromyelitis optica spectrum disorder in Taiwan. Mult Scler Relat Disord. (2024) 92:105923. doi: 10.1016/j.msard.2024.105923
23. Apiwattanakul M, Ounmuang C, and Aungsumart S. Impact of COVID-19 and vaccination on multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD). Mult Scler Relat Disord. (2025) 95:106316. doi: 10.1016/j.msard.2025.106316
24. Chen Y, Zhou Y, Chen P, Zhang P, Jia M, and Tang Y. Excessive expressions of T cell activation markers in pediatric immune thrombocytopenia. Thromb Res. (2019) 180:1–9. doi: 10.1016/j.thromres.2019.05.010
25. Ramaglia V, Sheikh-Mohamed S, Legg K, Park C, Rojas OL, Zandee S, et al. Multiplexed imaging of immune cells in staged multiple sclerosis lesions by mass cytometry. Elife. (2019) 8:e48051. doi: 10.7554/eLife.48051
26. Verstegen NJM, Hagen RR, Kreher C, Kuijper LH, van den Dijssel J, Ashhurst T, et al. T cell activation markers CD38 and HLA-DR indicative of non-seroconversion in anti-CD20-treated patients with multiple sclerosis following SARS-CoV-2 mRNA vaccination. J Neurol Neurosurg Psychiatry. (2024) 95:855–64. doi: 10.1136/jnnp-2023-332224
27. Besedovsky HO and del Rey A. Immune-neuro-endocrine interactions: facts and hypotheses. Endocr Rev. (1996) 17:64–102. doi: 10.1210/edrv-17-1-64
28. Herold MJ, McPherson KG, and Reichardt HM. Glucocorticoids in T cell apoptosis and function. Cell Mol Life Sci. (2006) 63:60–72. doi: 10.1007/s00018-005-5390-y
29. Coutinho AE and Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. (2011) 335:2–13. doi: 10.1016/j.mce.2010.04.005
30. Zhu J and Paul WE. CD4 T cells: fates, functions, and faults. Blood. (2008) 112:1557–69. doi: 10.1182/blood-2008-05-078154
31. Williams MA, Tyznik AJ, and Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. (2006) 441:890–3. doi: 10.1038/nature04790
32. Korn T, Bettelli E, Oukka M, and Kuchroo VK. IL-17 and th17 cells. Annu Rev Immunol. (2009) 27:485–517. doi: 10.1146/annurev.immunol.021908.132710
33. Kirkham BW, Lassere MN, Edmonds JP, Juhasz KM, Bird PA, Lee CS, et al. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort). Arthritis Rheum. (2006) 54:1122–31. doi: 10.1002/art.21749
34. van Langelaar J, van der Vuurst de Vries RM, Janssen M, Wierenga-Wolf AF, Spilt IM, Siepman TA, et al. T helper 17.1 cells associate with multiple sclerosis disease activity: perspectives for early intervention. Brain. (2018) 141:1334–49. doi: 10.1093/brain/awy069
35. Ayass MA, Tripathi T, Zhu K, Nair RR, Melendez K, Zhang J, et al. T helper (Th) cell profiles and cytokines/chemokines in characterization, treatment, and monitoring of autoimmune diseases. Methods. (2023) 220:115–25. doi: 10.1016/j.ymeth.2023.11.003
36. Cao F, Wang Y, Wei R, Li C, Cheng Y, Zhou Y, et al. Peripheral blood th1/th17 immune cell shift is associated with disease activity and severity of AQP4 antibody sero-positive neuromyelitis optica spectrum disorder. Neuropsychiatr Dis Treat. (2023) 19:2413–21. doi: 10.2147/NDT.S425759
37. Uzawa A, Mori M, Hayakawa S, Masuda S, Nomura F, and Kuwabara S. Expression of chemokine receptors on peripheral blood lymphocytes in multiple sclerosis and neuromyelitis optica. BMC Neurol. (2010) 10:113. doi: 10.1186/1471-2377-10-113
38. Takai Y, Misu T, Suzuki H, Takahashi T, Okada H, Tanaka S, et al. Staging of astrocytopathy and complement activation in neuromyelitis optica spectrum disorders. Brain. (2021) 144:2401–15. doi: 10.1093/brain/awab102
39. Hou M-M, Li Y-F, He L-L, Li X-Q, Zhang Y, Zhang S-X, et al. Proportions of Th17 cells and Th17-related cytokines in neuromyelitis optica spectrum disorders patients: A meta-analysis. Int Immunopharmacol. (2019) 75:105793. doi: 10.1016/j.intimp.2019.105793
40. Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. (2005) 6:1123–32. doi: 10.1038/ni1254
41. Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang Y-H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. (2005) 6:1133–41. doi: 10.1038/ni1261
42. Rostami A and Ciric B. Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination. J Neurol Sci. (2013) 333:76–87. doi: 10.1016/j.jns.2013.03.002
43. Zhou Z, Lin T, Liu Z, Ding Q, Ma Z, Li W, et al. IL-17A Mediates Demyelination by Activating A1 Astrocytes via SOCS3 During Angiostrongylus cantonensis Infection. Front Immunol. (2022) 13:845011. doi: 10.3389/fimmu.2022.845011
44. Luo Q, Liu Y, Shi K, Shen X, Yang Y, Liang X, et al. An autonomous activation of interleukin-17 receptor signaling sustains inflammation and promotes disease progression. Immunity. (2023) 56:2006–2020.e6. doi: 10.1016/j.immuni.2023.06.012
45. Khan AW, Farooq M, Hwang M-J, Haseeb M, and Choi S. Autoimmune neuroinflammatory diseases: role of interleukins. Int J Mol Sci. (2023) 24:7960. doi: 10.3390/ijms24097960
Keywords: neuromyelitis optica spectrum disorder, T lymphocytes, T helper cells, activated T cells, effector memory T cells
Citation: Shao Y-Z, Tan T-F, Zhang L-J, Zhao N, Yang L and Zhang Q-X (2025) Changes in T lymphocyte subsets in patients with acute neuromyelitis optica spectrum disorder. Front. Immunol. 16:1654533. doi: 10.3389/fimmu.2025.1654533
Received: 26 June 2025; Accepted: 27 August 2025;
Published: 22 September 2025.
Edited by:
Gunnar Houen, University of Copenhagen, DenmarkReviewed by:
Anne Haney Cross, Washington University in St. Louis, United StatesMonique Anderson, Mass General Brigham, United States
Copyright © 2025 Shao, Tan, Zhang, Zhao, Yang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiu-Xia Zhang, emhhbmdxaXV4aWFAdG11LmVkdS5jbg==
†These authors have contributed equally to this work
 Ying-Zhe Shao
Ying-Zhe Shao Tao-Feng Tan†
Tao-Feng Tan† Lin-Jie Zhang
Lin-Jie Zhang Li Yang
Li Yang