- 1Department of Neurology, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nürnberg, Erlangen, Germany
- 2Deutsches Zentrum Immuntherapie (DZI), University Hospital Erlangen, Erlangen, Germany
Smoldering inflammation and neurodegeneration, primarily driven by intraparenchymal immune cell activation and glial dysfunction, remains a major therapeutic challenge in Multiple Sclerosis (MS) and contributes largely to disability progression. Current disease-modifying therapies effectively decrease relapse rate and, to a lesser extent, disease progression by targeting peripheral immune cells. However, they largely fail to address Central-Nervous-System-(CNS)-intrinsic pathological processes – especially glial dysfunction – thus leaving a critical gap relevant to disease progression and therapeutic intervention. In this context, neurotrophic factors (NTF) are secreted proteins central for development and maintenance of the CNS. They promote anti-inflammatory, protective phenotypes in astrocytes and microglia, support remyelination by enhancing oligodendrocyte precursor recruitment, maturation and survival, and exert direct neuroprotective effects. Exploring their role in MS offers a novel perspective on neuroimmune crosstalk and prevention of progressive neurodegeneration. In this article, we summarize relevant findings on NTFs in MS, and give an outlook on opportunities and challenges of using these mediators as next-generation disease-modifying therapies.
1 Introduction
Multiple Sclerosis (MS) is a chronic inflammatory, demyelinating autoimmune disease of the central nervous system. The disease manifests with neurologic symptoms in a relapsing-remitting (RRMS) as well as a primary (PPMS) or secondary progressive (SPMS) form. It is characterized by the presence and development of multifocal inflammatory lesions in brain and spinal cord (1). T cells play an important role in the disease as they pass the blood-brain-barrier, recognize Central-Nervous-System-(CNS)-specific autoantigens and cause focal demyelinating lesions with axonal damage and reactive astrogliosis (2) characterized by the activation of astrocytes and microglia as well as deposition of extracellular matrix. Recently, long thought beliefs that neurodegeneration typically happens later in the disease course have been increasingly challenged. Research indicates that neurodegeneration occurs early in the disease (3, 4) and contributes largely to disability progression (5, 6). Indeed, the relevance of CNS-intrinsic glial cells in this process has become evident. Glial cells such as astrocytes can lead to the promotion of inflammation, but also contribute to its alleviation by membrane-bound as well as secreted mediators as outlined in a recent review (7).
Effective therapies targeting peripheral immune cells have been successfully established in recent years. However, their efficacy in the progressive course of the disease is limited (2) as progression seems to be mostly associated with intraparenchymal processes within the CNS (8). These intraparenchymal mechanisms are mostly driven by resident glial cells, e.g. astrocytes, microglia and oligodendrocytes, and their complex crosstalk (9). Glial cells are essential for support and protection of neurons and their axons, intercellular communication in the CNS, facilitation of synapse formation and neurotransmission as well as the detection of inflammation and tissue damage (10).
While the proinflammatory and neurotoxic properties of glial cells in MS have been of interest for a long time, only recently has their protective potential become a topic of interest (7, 11). Microglia play an important role in the phagocytosis of myelin debris following demyelination and promote recovery and remyelination by secretion of anti-inflammatory and immunomodulatory cytokines as well as neurotrophic factors (12, 13). In MS, they have been shown to play beneficial roles by formation of a physical barrier at the lesion site as a result of astrogliosis and providing trophic support for neurons and oligodendrocytes by secretion of neurotrophic factors, neuropoietic cytokines and growth factors (7).
Thus, glial cells exert part of their protective functions by the release of so-called neurotrophic factors (7). For reasons of clarity, conciseness and volume, this review focusses on an overview of glial neurotrophic factors, which might be of relevance for future therapeutic and translational strategies. But what exactly are neurotrophic factors?
1.1 Neurotrophic factors
Neurotrophic factors (NTFs) comprise a family of secreted proteins which are essential for the development, survival and function of neurons in the nervous system (14). In the prenatal brain, the survival of neurons depends on access to NTFs, while in the adult CNS, they provide a balance between degeneration and regeneration (15). NTFs can be broadly classified into the four neurotrophins, which are Brain-derived neurotrophic factor (BDNF), Nerve growth factor (NGF), neurotrophin 3 and neurotrophin 4, the Glia derived neurotrophic factor (GDNF)-family, members of the Ciliary neurotrophic factor (CNTF)-family as well as the more recently discovered factors Mesencephalic Astrocyte-Derived Neurotrophic Factor (MANF) and Cerebral Dopamine Neurotrophic Factor (CDNF). Several members of growth factor families, e.g. the epidermal growth factor family (EGF), have also been shown to support neuronal survival, but there is no consensus whether these factors fulfill the criteria of classical neurotrophic factors (16).
Neurotrophins are initially synthesized in the endoplasmic reticulum as precursors called proneurotrophins which are then processed into mature neurotrophins (17). The family of neurotrophins exert their effects by binding with high affinity to tropomyosin receptor kinases (Trk, namely TrkA, TrkB and TrkC) as well as the low-affinity p75 neurotrophin receptor (p75NTR). Trk-receptors are transmembrane proteins, consisting of an extracellular domain, a transmembrane region and an intracellular region, which contains the tyrosine kinase (18). Each of the four neurotrophins has a binding specificity for a distinct Trk-receptor. NGF binds to TrkA, BDNF and neurotrophin 4 to TrkB while neurotrophin 4 can bind to all Trk but has the highest affinity for TrkC (18). Trk are widely expressed in neurons, however they are also expressed in glial cells, especially TrkB (19–22) Most other neurotrophic factors also bind to and activate receptor tyrosin kinases (14). In the CNS, neurotrophic factors are secreted by neurons as well as glial cells (23, 24). Neurotrophic factors are implicated in a number of diseases of the CNS, which involve inflammation and neurodegeneration like Parkinson’s disease, Alzheimer’s disease, Huntington’s disease and neuropsychiatric disorders (25–28).
As outlined, NTFs play an important role in modifying inflammation in the CNS and controlling neurodegeneration, which both are central in MS. Therefore, investigating their role in the disease offers a deeper understanding on the pathophysiology of MS, especially in the progressive phase and their neuroprotective and myelinating properties may inspire the development of novel disease modifying drugs. Therefore, in this review, we will outline the current knowledge on the most relevant NTFs in MS. To allow for a more condensed, in depth analysis, we will focus on four classical neurotrophic factors – the neurotrophins BNDF and NGF, GDNF, CNTF as well as the growth factor Hb-EGF with an emphasis on recent data. While Hb-EGF is not a classical neurotrophic factor, it exerts a number of neurotrophic-like functions like tissue regeneration and promotion of neuronal survival and has been studied in the context of inflammatory brain disease (29, 30). Other factors like Neurotrophin-3/-4, Neurturin might also be of interest in MS in the future, their role has been less extensively studied and their functional relevance in MS remains less well designed.
1.1.1 BDNF
Brain-derived neurotrophic factor (BDNF) is one of the most common neurotrophins in the CNS and plays important roles for synaptic development and plasticity, neuronal differentiation, -transmission and -protection (31). In the CNS, it is expressed by neurons, astrocytes, oligodendrocytes and microglia (32–35). Glial expression in the healthy CNS is low (36), however, astrocytes significantly upregulate its expression during inflammatory conditions (7, 37). BDNF is first synthesized in the endoplasmic reticulum as preproBDNF and then cleaved into proBDNF in the Golgi apparatus. BDNF binds its specific receptor TrkB with high affinity, while BDNF, its precursor proBDNF as well as the other neurotrophins of the neurotrophin family bind to p75NTR (38) (Figure 1A). Engagement of BDNF with TrkB mainly regulates neuronal survival, differentiation and plasticity, while p75NTR can act pro-apoptotic but can also promote cell survival (39). TrkB is widely expressed in the central nervous system, mainly in neurons, astrocytes and oligodendroglia (36, 40). BDNF signaling regulates the cross-talk between astrocytes and microglia, thus influencing inflammatory mechanisms and exerting anti-inflammatory effects (41). BDNF-activity is altered in some patients with a distinct single-nucleotide polymorphism, where valine is exchanged with methionine at codon 66 in the BDNF gene pro-domain encoding region (Val66Met-polymorphism). This polymorphism has been studied in several disorders, including MS, however, data remains inconclusive as to its disease-exacerbating or protective effects in MS (42, 43). A recent study found no difference in clinical characteristics between the Val66Met polymorphism in MS (44). However, at disease onset, Val66Met-carriers showed reduced cortical thickness (44). Still, the influence of the polymorphism on MRI structural measures remains controversial, as other studies reported beneficial effects on gray matter volume in MS (45–47). Evidence points to epigenetic mechanisms, namely the level of methylation of the BDNF gene playing a more important role than the prevalence of the Val66Met-polymorphism itself. Genetic analysis of 209 MS patients revealed no influence of the polymorphism on the disease course, however, hypomethylation of the BDNF gene in the exonic CpG-site affected by the polymorphism was associated with a higher burden of disability. This indicates an upregulation of BDNF expression as a response to higher inflammatory/disease activity (48).
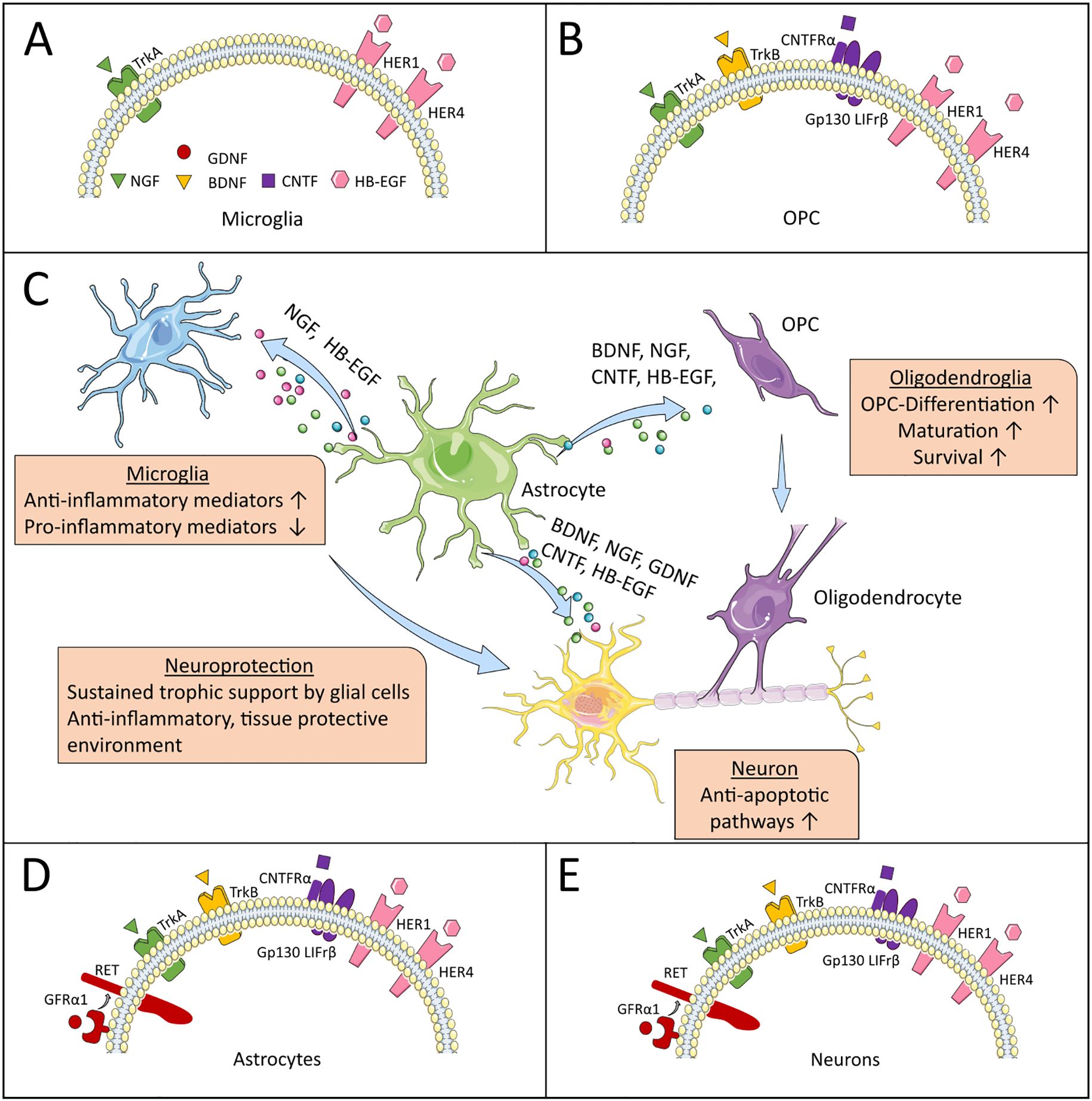
Figure 1. Neurotrophic factors and their receptors. (C) highlights astrocytes as central producers of NTFs, illustrating their beneficial effects on specific CNS-resident cells in MS. (A, B, D, E) depict the expression patterns of NTF receptors on these target cells, through which the NTFs mediate their supportive functions. The artwork used in this figure was adapted from Servier Medical Art (http://https://smart.servier.com/). Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License.
Indeed, data on BDNF levels in MS-patients showed conflicting results, as some authors described increased, decreased and some unchanged levels in the disease (49–57). A recent meta-analysis, which included thirteen studies with 689 MS-patients, concluded that circulating levels of BDNF are decreased in MS with disease duration correlating negatively with BDNF-levels (58). In clinical relapse, most studies reported an increase of BDNF-concentration (43, 52, 59). However, most of the data is over ten years old and relies on BDNF-levels in peripheral blood mononuclear cells (PBMC) or serum, as BDNF-levels in Cerebrospinal fluid (CSF) are low and ultra-sensitive immunoassays are necessary for its reliable detection (60). A more recent study found a positive correlation between serum BDNF-concentration at baseline and improvement in MS-disability-score EDSS 12 months after relapse (61). In a study with 28 patients with RRMS and 28 healthy controls, serum levels of BDNF negatively correlated with the number of T2-hyperintense lesions in MRI. However, no association was found between T1-positive lesions and Gadolinium-enhancing lesions. Comini-Frota, Rodrigues (62) - the authors of the study - speculate that BDNF controls acute repair processes and is therefore associated with acute demyelination as indicated by T2-lesions. T1-lesions, which mostly account for chronic lesions with chronic neuronal loss, can therefore not be influenced by BDNF. However, this finding cannot explain the missing association with Gad+-lesions in the data, which represent acute demyelination.
BDNF expressing cells are more numerous in actively demyelinating areas and TrkB is upregulated near MS plaques as was shown in a human post-mortem study (63). Preclinical, in vivo studies demonstrate that in the absence of BDNF, mice experienced a stronger disease course in experimental autoimmune encephalomyelitis, EAE, the animal model of MS (64, 65). This effect was more pronounced when BDNF was deleted early in the disease (65). This suggests that neurodegeneration already occurs early in the disease and could be ameliorated by neuroprotective agents like BDNF.
BDNF has been shown to play an important role in remyelination. Astrocytes promote oligodendrocyte progenitor cell (OPC) maturation and oligodendrogenesis by secretion of BDNF after white matter damage (66). Knock-down of TrkB in mice reduced myelination/myelin thickness and decreased the proliferative potential of OPCs, highlighting the potential for BDNF in remyelination (21). In accordance with this study, a BDNF-mimetic induced oligodendrocyte differentiation and myelin repair via TrkB in the cuprizone-induced model of toxin-induced demyelination (67). In another study, astrocytes were shown as key players in BDNF-dependent remyelination using the cuprizone model (37). The injection of 2-chloro-5-hydroxyphenylglycine (CHPG), an agonist of the astrocytic metabotropic glutamate receptor 5 enhanced myelination via astrocytic release of BDNF after cuprizone-induced injury in mice (68). As BDNF can hardly pass the Blood Brain Barrier (BBB), Kopec, Kiptoo (69) combined intravenous BDNF-injections with application of the BBB-modulator ADTC5. The combination reduced clinical disease progression and induced oligodendroglia-activation and remyelination in EAE mice (69).
Several immunotherapies have been shown to enhance BDNF levels. Glatiramer acetate, a synthetic mixture of 4 amino acids intended to mimic myelin basic protein, one of the major myelin autoantigens in MS (70), increases BDNF levels (71–73) as well as Interferon-beta (55, 74), even though these agents do not readily cross the blood brain barrier, indicating indirect effects founding these observations. Yet, Fingolimod, which can cross the blood-brain barrier after phosphorylation, has been shown to increase BDNF-levels in patients with MS (75, 76).
In summary, the current data suggests that BDNF controls remyelination after autoimmune inflammatory attacks and is an important factor in glial-derived neuroprotection. Therefore, a better understanding of its role in MS might offer new therapeutic avenues particularly for progressive disease stages characterized by compartmentalized inflammation.
1.1.2 NGF
Nerve growth factor (NGF) was the first neurotrophin discovered in 1950 (77). Its mature form is cleaved from its precursor proNGF (78). NGF mediates its biological function through two receptors, the high-affinity receptor TrkA and the low-affinity p75-receptor (79) (Figure 1A). While TrkA mainly activates growth and survival pathways and affects synaptic plasticity modulation, the effects of p75NTR are context specific and the receptor can form complexes with various other receptors, which mediate a great number of different effects, including pro-apoptotic pathways (80, 81). In the CNS, NGF is expressed in neurons, microglia, oligodendrocytes and astrocytes (81–83). In the inflamed brain, NGF-overexpression is induced in neurons and glia cells (84). In EAE NGF, TrkA and p75NTR are upregulated in inflammatory lesions of the spinal cord (85).
NGF mediates protective functions for oligodendroglia, ultimately protecting from demyelination. NGF can act directly on oligodendrocytes, which have been shown to express NGF-receptors TrkA and p75NTR (86, 87). Through activation of the Erk1/2-MAPK-pathway, NGF preserves myelin thickness and leads to its maintenance (88, 89). The synthetic micro-neurotrophin BNN27, which binds specifically to TrkA and p75NTR, protects mature oligodendrocytes against cell death in a cuprizone-induced model of demyelination in a TrkA-dependent manner in vitro and protects against myelin loss and reduces microgliosis and astrocytosis in an in vivo mouse model. (86). Another recent study, using mixed neural stem cell-derived OPC/astrocyte cultures showed that NGF induces OPC differentiation, maturation and protects them against cell death in the context of oxygen and glucose deprivation. In this mixed culture, astrocytes were the main producers of NGF (87). In microglia, NGF induces a neuroprotective phenotype (90). In accordance with these results, NGF and its receptors co-localize with anti-inflammatory microglia, but not pro-inflammatory microglia phenotypes in the EAE model (85). NGF also induces neuroprotection in astrocytes, as reduced levels of NGF induce a neurotoxic phenotype (91). In a recent study, artificial microvesicles carrying NGF showed beneficial effects in EAE by reducing neurogliosis and stimulating axon regeneration (92).
Taken together, NGF induces protective mechanisms in glial cells and can help protect against demyelination by protecting oligodendroglia.
1.1.3 GDNF
Glia derived neurotrophic factor (GDNF) is a member of the GDNF-family consisting of GDNF, neurturin, artemin and persepin. All four members are part of the TGF-ß superfamily. They exert their effects by activating the transmembrane receptor tyrosine kinase “rearranged during transfection” (RET), which regulates cell survival, differentiation, proliferation, migration, chemotaxis, branching, neurite outgrowth and synaptic plasticity (93) (Figures 1D, E). In the “healthy” brain, GDNF secretion is usually low and with neurons as its main cellular source. However, under autoimmune inflammation, its expression is upregulated in glial cells, e.g. astrocytes, microglia and infiltrating macrophages (94–96). So far, studies have shown beneficial effects of GDNF on dopaminergic neurons in Parkinson’s disease (97), striatal projection neurons and cortical neurons in Huntington’s disease (98) and motor neurons, which degenerate in amyotrophic lateral sclerosis (99, 100).
Recent data on GDNF in MS is scarce. In a current study, Sokolowski, Kucharska-Lusina (101) showed reduced gene expression and protein concentration of GDNF in the plasma of MS-patients, indicating impaired neuroprotection. Exposure of astrocytes to the CSF of MS-patients resulted in activation of astrocytes and increased expression of neurotrophic factors GDNF, BDNF and VEGF (102). Interestingly, Jin, Zhang (103) revealed the novel role of SARM1/GDNF-signaling in EAE. SARM1, short for Sterile Alpha and TIR Motif Containing 1 is a member of the Toll/interleukin 1 receptor junction family, is highly expressed in the CNS and is involved in mediating neuronal cell death and axonal degeneration (103). Astrocytic SARM1 promotes neuroinflammation and axonal demyelination in EAE by inhibiting the expression of GDNF and knock-out of SARM1 delayed disease onset and reduced inflammation, demyelination and neurodegeneration by up-regulation of GDNF (103).
Thus, GDNF serves as a neuroprotective agent in the CNS and might offer therapeutic potential to protect against neurodegeneration. However, further studies on the role of GDNF are needed to provide a broader understanding of its roles in MS.
1.1.4 CNTF
Ciliary neurotrophic factor (CNTF) forms part of the IL-6 cytokine family (104). In the CNS, it is primarily expressed by astrocytes, but not by neurons (105, 106). It provides protection for several types of neurons (sensory, sympathetic, motor neurons) and glial cells and plays an important role in brain development and neuronal differentiation (107–109). CNTF has been shown to induce the maturation of astrocytes and oligodendrocytes (7, 110, 111). Its receptor is composed of three components, the non-signaling CNTFRα, which is specific for CNTF, gp130 and LIFrβ, that are shared with other members of the IL-6 family including LIF and IL-6 (112). Binding of CNTF to CNTFRα induces the ß-receptors gp130 and LIFrβ, which then activate signaling cascades including JAK/STAT, Ras/MAP kinase and the phosphatidylinositol 3-kinase pathway (113) (Figures 1B, D, E).
CNTF has long been a protein of interest in motoneuron disease due to its protective effect on motoneurons (114). Recent studies investigating CNTF in MS are scarce. In an older study, CNTF receptor expression was enhanced in post-mortem cortical neurons of MS patients and CNTF expression was increased in neurons and astrocytes (108). CNTF has been shown to be expressed in a biphasic manner by astrocytes in a cuprizone model of demyelination, during early demyelination and then again in remyelination (115). It provides protection against demyelination in CNS-disease with more severe EAE-phenotype in CNTF-deficient mice with a strong decrease in OPC count and increase in oligodendrocyte death (116). Intraventricular injection of CNTF in EAE mice reduced expression of inflammatory cytokines TNF-α and interferon-γ and decreased demyelination and neurodegeneration (117). Overexpression of CNTF in Mesenchymal Stem Cells (MSC) and subsequent intravenous injection in EAE mice reduced demyelination, lowered levels of pro-inflammatory cytokines and ameliorated clinical disease course. However, MSC-CNTF were only found in the spinal cord, but not in the brain (118).
In summary, some studies showed a beneficial role of CNTF in MS disease pathology, however, more detailed research of recent time is mostly lacking.
1.1.5 HB-EGF
Heparin-binding epidermal growth factor-like growth factor (HB-EGF) is a member of the epidermal growth factor (EGF) family, which also includes EGF and TGF-α. Its early form is a transmembrane protein called pro-HB-EGF, which is then cleaved by a number of proteases (e.g. ADAMs, MMPs), generating soluble HB-EGF (29). It was first isolated in macrophage-like cells in the early 1990s (119). HB-EGF is a potent stimulator of cell proliferation and migration and targets a number of cells, including peripheral cells including epithelia cells, smooth muscle cells and fibroblasts (120). HB-EGF binds to epidermal growth factor receptors HER1 and HER4, which activate a tyrosine kinase triggering a series of signaling cascades including MAPK and AKT pathways (29) (Figure 1A). It is involved in development, homeostasis and tissue growth but also modulates inflammatory functions (121).
HB-EGF is produced by astrocytes during acute stages of neuroinflammation and decreases in later stages of the disease in EAE and in the CSF of patients with RRMS (30, 122). Its expression is induced by hypoxia, whereafter it exerts neuroprotective functions and stimulates neurogenesis (30, 123). Activation of the S1P-receptor, for example by S1P-receptor modulator Fingolimod, induces upregulation of astrocyte-derived Hb-EGF and - among other factors like LIF - protects cultured neurons against excitotoxic cell death (124, 125). In a cuprizone-induced model of demyelination of mice of different ages, intracisternal co-injection of EGF and Hb-EGF increased the proliferation of neural progenitor cells (NPC) in the ventricular-subventricular zone of the CNS, which increased the number of oligodendrogenic NPCs available for remyelination. The production of NPC-derived oligodendrocytes was increased in the corpus callosum following the injection, indicating an important role of HB-EGF in myelination. However, the total density of oligodendrocytes and myelin abundancy remained stable in this study, indicating an equilibrium with other oligodendrocyte sources and a consecutive downregulation of these other sources (126). Linnerbauer, Lößlein (30) could limit neuroinflammation by intranasal delivery of HB-EGF in the EAE model. HB-EGF reduced neuroinflammation through effects on multiple CNS-resident cell types like microglia, protected neurons against the pro-apoptotic effects of TNFα and was shown to support OPC survival and myelination (30). On the other hand, inflammatory conditions induced epigenetic changes in astrocytes by HB-EGF promoter hypermethylation. This suppressed HB-EGF expression and therefore hinders tissue-protective programs, offering a possible explanation for the progressive decrease of HB-EGF in advanced disease stages of MS and EAE (30). Of note, reduced HB-EGF expression in an inflammatory environment could be reversed by treatment with 5-Aza(-cytidine), a clinically approved chemotherapeutic agent and DNA methyltransferase inhibitor, confirming EAE-ameliorating effects of the drug in earlier studies (30, 127, 128).
Thus, HB-EGF might represent a promising factor relevant to disease progression and might serve as a novel therapeutic factor worthy of further study, either as a downstream target by selective demethylation strategies or as a drug itself.
2 Translational potential of neurotrophic factors
MS, especially in the progressive disease phase, poses therapeutic challenge as current disease modifying therapies fail to address the complex crosstalk between glial cells, neurons and CNS-infiltrating cells as well as their highly dynamic functions (2, 9).
Highlighting the functional dynamics of astrocytes, depletion of reactive astrocytes in early neuroinflammation increased disease severity in EAE, while depletion of reactive astrocytes during the chronic disease phase ameliorated disease pathogenesis (129, 130). Moreover, astrocytes represent a diverse population of cells with strong spatial and environment-dependent signatures (131). Similarly, microglia are well known for their proinflammatory and neurotoxic roles in neuroinflammation, however, they as well exert a number of protective effects, e.g. by phagocytosis of myelin debris, maintaining myelin health, limiting neurodegeneration, secretion of growth factors and elimination of destructive T-cells (13, 132–134). Considering the diversity of glial responses in MS, specific therapeutic interventions, which modulate distinct glial subsets are needed, since an overly broad approach as well as drug-application at an improper time-point might worsen clinical outcome by also affecting protective glial functions (2). Yet, neurotrophic factors are key players in this neuroimmune crosstalk (7). In the paragraphs above, we have outlined their beneficial effects on inflammation and neurodegeneration in MS (Table 1, Figure 1C). Their ability to activate anti-inflammatory pathways and to modulate glial cells into a neuroprotective and anti-inflammatory phenotype (90, 91) therefore makes neurotrophic factors interesting candidates for the development of new therapeutic approaches.
Yet, several challenges, however, must be addressed before their clinical application. Indeed, most data on neurotrophic factors stems from in vitro or animal models, underlining the need for human-derived data. Diagnosing NTF dynamics in MS patients is complex. Taking BDNF as an example: although it is stored in blood and platelets and therefore measurable in serum, its diagnostic utility for CNS-intrinsic mechanisms is limited (57). In contrast to Neurofilament Light Chain (NFL), a promising biomarker for neurodegeneration increasingly used in MS whose serum levels correlate well with CSF levels, it is unclear whether BDNF levels in serum reflect those in CSF (57). As a result, serum BDNF provides only limited insight into CNS-intrinsic mechanisms. Moreover, NTFs like BDNF and NGF are typically present in the CSF at very low concentrations, often below the detection limits of conventional ELISAs (60, 135). More sensitive assays are necessary for obtaining reproducible and biologically meaningful results, such as Single Molecule Arrays (SIMOA) (60). In clinical practice, CSF sampling via lumbar puncture is generally conducted only during the initial diagnostic work-up when most patients show a relapsing remitting form of the disease. Over time, as more patients transition to SPMS, CNS-intrinsic mechanisms primarily driven by resident glial cells become dominant (9). In a rare case of a MS-patient with available longitudinal CSF-samples Linnerbauer, Lößlein (30) found a continuous decline of HB-EGF levels over the disease course whereas initial HB-EGF CSF-levels in RRMS-patients were increased. This highlights a key limitation: most available CSF-samples are collected during early disease stages, potentially missing critical NTF alterations relevant to the progressive phase of MS. Repeated spinal taps for scientific purposes pose a challenge considering the invasive nature of the procedure (136, 137). Even when obtained, CSF-concentrations reflect global changes across the CNS and cannot resolve cell type-specific or region-specific dynamics in NTF expression. This is a significant limitation, especially given that MS is characterized by focal inflammatory lesions. To address this, human brain tissue samples offer superior spatial and cellular resolution. However, access to high-quality human brain tissue remains limited (138). The increasing establishment of brain banks – which systematically collect and store post-mortem brain tissue – provides valuable opportunities for studying MS-specific mechanisms in human CNS (139). Nonetheless, the success of such efforts depends critically on the quality of sample preservation, particularly for protein and gene expression analyses (139). Therapeutic interventions that target CNS-resident cells have been limited by the challenge of delivering drugs across the BBB, as NTFs pass the BBB only to a limited extent (140, 141). New application strategies might solve this problem. An interesting approach to passing the BBB is intranasal drug delivery, which involves transport via the trigeminal and olfactory nerve systems as direct connectors between the nasal cavity and the CNS (142) (Figure 2A). Relevant mechanisms include passive diffusion, paracellular transport, carrier transport and receptor mediated transcytosis (141, 145) (Figure 2B). Indeed, neurotrophic factors applied nasally pass the BBB and reach relevant concentrations in the CNS by this method of application (141),. Linnerbauer, Lößlein (30) could limit neuroinflammation in EAE-mice by intranasal delivery of HB-EGF (Figure 2D). There are additional examples of successful modification of glial - and specifically astrocyte - dysregulation in EAE via intranasal drug administration. Interferon-ß does not cross the BBB when administered peripherally and exerts its neuromodulatory effects mainly on T-cells and monocytes (146). Intranasal application however, ameliorated EAE disease scores by limiting CNS inflammation by acting on the aryl hydrocarbon receptor (AhR) on astrocytes (147). Similarly, intranasal delivery of pleiotrophin, an astrocyte-derived mediator that reduces pro-inflammatory signaling in astrocytes and microglia and exerts neuroprotective effects reduced disease severity in late EAE (148). Highlighting the potential of this approach, several recent translational and clinical studies beyond the field of MS used this method of delivery and were able to clear intracellular tau pathology in tauopathy mice by intranasal application of an anti-tau-antibody (144) or reduce apathy in frontotemporal dementia patients by intranasal application of oxytocin (149) (Figure 2C). Intranasal delivery can further be improved by combination with nanoparticle formulations like nanoemulsions, lipids or polymer particles. This was done in the aforementioned study of an intranasal anti-tau-antibody, which was loaded in micelles (144, 150). Another possible approach might be the use of synthetic nanoparticles specifically targeting Trk-receptor-dependent pathways in the CNS. Drug-carrying nanoparticles can significantly improve the delivery of drugs through the BBB and their biodistribution in the CNS (151, 152). Moreover, the modification of cells to express neurotrophic factors is another possible method of drug-delivery into the brain (153). Stem cells possess a high affinity to sites of injury with active migration from their respective origin. They can cross the BBB, especially during inflammation and have immunomodulatory properties (154–156) (Figure 2E). Their homing to inflamed areas can further be improved by modifying homing ligands, either by DNA-transfection or – which is potentially more scalable and cost-effective – pre-treatment with small molecules (157, 158). Pre-treatment of MSCs with Ro-31-8425, a selective protein kinase C (PKC) inhibitor, increased surface expression of CD11a in MSC and improved ICAM-1-dependent cell adhesion at inflammatory sites in mice (157). As PKC-dependent pathways also play a central role in T-cell activation, systemic administration of MSC loaded with Ro-31–8425 further ameliorated disease course in EAE mice compared to naive MSC or free Ro-31–8425 by suppressing antigen-specific proliferation of CD4+ T-cells (159). This further underscores the synergistic potential of stem-cell based drug delivery systems. Intravenous application of genetically engineered MSC overexpressing BDNF successfully increased BDNF-concentration in the CNS of EAE-mice and reduced disease severity (160) (Figure 2C). Finally, viral vector-mediated gene delivery might permanently upregulate the cellular production of certain NTFs (153).
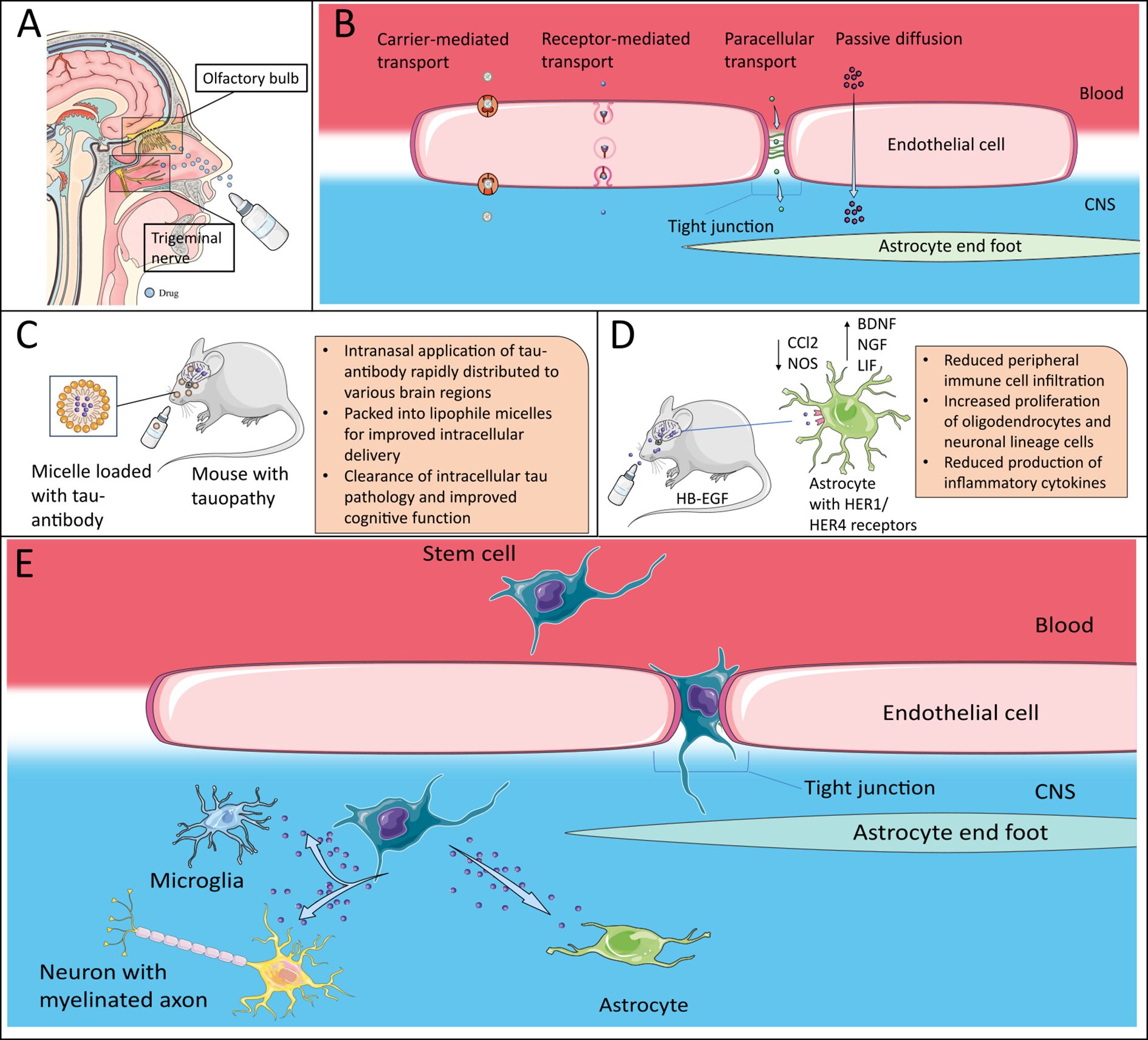
Figure 2. Therapeutic potential of neurotrophic factors. (A) Intranasal delivery of drugs via the olfactory nerve system or the trigeminal nerve to bypass the BBB. Efficacy can be further improved by use of nanoparticle formulations. Adapted after (143), which is licensed under CC BY 4.0. (B) Nanoparticles can pass the BBB via different mechanisms, which include carrier-mediated transport, receptor-mediated transcytosis and paracellular transport across tight junctions as well as passive diffusion due to their small size. (C) Packed into lipophile micelles, intranasal application of an tau-antibody was able to clear intracellular tau pathology (144). (D) Intranasal delivery of HB-EGF in mouse model successfully ameliorated EAE-disease course by inducing an anti-inflammatory phenotype in astrocytes, inducing trophic effects in CNS-intrinsic cells and reducing peripheral immune cell infiltration (30) (E) Stem cells, e.g. mesenchymal stem cells can be modified to overexpress NTFs. They can cross the BBB, especially in the inflamed brain and release their cargo. The artwork used in this figure was adapted from Servier Medical Art (http://https://smart.servier.com/). Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License.
Taken together, the translational potential for clinical applications for NTFs remains exciting, however, while strategies how to pass the BBB are on the horizon, little is known about potential deleterious consequences of NTFs in the complex neuroimmune crosstalk in MS for an early use in clinical studies. Further studies in vitro, in bio databases and in animal models will need to dive deeper into the complex glial-glial and glial-neuronal interactions mediated by NTFs in MS.
Author contributions
FG: Visualization, Conceptualization, Writing – review & editing, Writing – original draft. VR: Resources, Writing – review & editing, Conceptualization, Validation, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. VR was funded by an ERC Starting Grant by the European research Council (HICI 851693). VR was supported by a Heisenberg fellowship and Sachmittel support provided by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG, RO4866-3/1, RO4866-4/1; -1/1, 2/-1, 3/1, 4/1, 5/1, 6/1 Project ID 401772351) as well as transregional and collaborative research centers provided by the German Research Foundation (DFG, Project ID 408885537-TRR274, Project ID 261193037-CRC1181, Project ID 270949263-GRK2162, Project ID 405969122-FOR2886, Project ID 505539112-GB.com).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Berthele A and Hemmer B. S2k-Leitlinie: Diagnose und Therapie der Multiplen Sklerose, Neuromyelitis-optica-Spektrum-Erkrankungen und MOG-IgG-assoziierten Erkrankungen. DGNeurologie. (2021) 4:251–75. doi: 10.1007/s42451-021-00334-6
2. Charabati M, Wheeler MA, Weiner HL, and Quintana FJ. Multiple sclerosis: Neuroimmune crosstalk and therapeutic targeting. Cell. (2023) 186:1309–27. doi: 10.1016/j.cell.2023.03.008
3. Tur C, Carbonell-Mirabent P, Cobo-Calvo Á, Otero-Romero S, Arrambide G, Midaglia L, et al. Association of early progression independent of relapse activity with long-term disability after a first demyelinating event in multiple sclerosis. JAMA Neurol. (2023) 80:151–60. doi: 10.1001/jamaneurol.2022.4655
4. Lambe J, Fitzgerald KC, Murphy OC, Filippatou AG, Sotirchos ES, Kalaitzidis G, et al. Association of spectral-domain OCT with long-term disability worsening in multiple sclerosis. Neurology. (2021) 96:e2058–e69. doi: 10.1212/WNL.0000000000011788
5. Kappos L, Wolinsky JS, Giovannoni G, Arnold DL, Wang Q, Bernasconi C, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. (2020) 77:1132–40. doi: 10.1001/jamaneurol.2020.1568
6. Cagol A, Schaedelin S, Barakovic M, Benkert P, Todea RA, Rahmanzadeh R, et al. Association of brain atrophy with disease progression independent of relapse activity in patients with relapsing multiple sclerosis. JAMA Neurol. (2022) 79:682–92. doi: 10.1001/jamaneurol.2022.1025
7. Linnerbauer M and Rothhammer V. Protective functions of reactive astrocytes following central nervous system insult. Front Immunol. (2020) 11:573256. doi: 10.3389/fimmu.2020.573256
8. Thompson A and Ciccarelli O. Towards treating progressive multiple sclerosis. Nat Rev Neurol. (2020) 16:589–90. doi: 10.1038/s41582-020-00421-4
9. Healy LM, Stratton JA, Kuhlmann T, and Antel J. The role of glial cells in multiple sclerosis disease progression. Nat Rev Neurol. (2022) 18:237–48. doi: 10.1038/s41582-022-00624-x
10. Theophanous S, Sargiannidou I, and Kleopa KA. Glial cells as key regulators in neuroinflammatory mechanisms associated with multiple sclerosis. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms25179588
11. Aharoni R, Eilam R, and Arnon R. Astrocytes in multiple sclerosis-essential constituents with diverse multifaceted functions. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22115904
12. Liddelow SA and Barres BA. Reactive astrocytes: production, function, and therapeutic potential. Immunity. (2017) 46:957–67. doi: 10.1016/j.immuni.2017.06.006
13. Mado H, Adamczyk-Sowa M, and Sowa P. Role of microglial cells in the pathophysiology of MS: synergistic or antagonistic? Int J Mol Sci. (2023) 24(3):20230117. doi: 10.3390/ijms24031861
14. Skaper SD. Neurotrophic factors: an overview. In: Skaper SD, editor. Neurotrophic Factors: Methods and Protocols. Springer New York, New York, NY (2018). p. 1–17.
15. Palasz E, Wilkaniec A, Stanaszek L, Andrzejewska A, and Adamczyk A. Glia-neurotrophic factor relationships: possible role in pathobiology of neuroinflammation-related brain disorders. Int J Mol Sci. (2023) 24:6321. doi: 10.3390/ijms24076321
16. Castrén E. Trophic factors: neurotrophic factors. In: Pfaff DW, editor. Neuroscience in the 21st Century: From Basic to Clinical. Springer New York, New York, NY (2013). p. 1555–89.
17. Hernández-del Caño C, Varela-Andrés N, Cebrián-León A, and Deogracias R. Neurotrophins and their receptors: BDNF’s role in GABAergic neurodevelopment and disease. Int J Mol Sci. (2024) 25(15):20240730. doi: 10.3390/ijms25158312
18. Amatu A, Sartore-Bianchi A, Bencardino K, Pizzutilo EG, Tosi F, and Siena S. Tropomyosin receptor kinase (TRK) biology and the role of NTRK gene fusions in cancer. Ann Oncol. (2019) 30:viii5–viii15. doi: 10.1093/annonc/mdz383
19. Muragaki Y, Timothy N, Leight S, Hempstead BL, Chao MV, Trojanowski JQ, et al. Expression of trk receptors in the developing and adult human central and peripheral nervous system. J Comp Neurol. (1995) 356:387–97. doi: 10.1002/cne.903560306
20. Colombo E, Cordiglieri C, Melli G, Newcombe J, Krumbholz M, Parada LF, et al. Stimulation of the neurotrophin receptor TrkB on astrocytes drives nitric oxide production and neurodegeneration. J Exp Med. (2012) 209:521–35. doi: 10.1084/jem.20110698
21. Wong AW, Xiao J, Kemper D, Kilpatrick TJ, and Murray SS. Oligodendroglial expression of trkB independently regulates myelination and progenitor cell proliferation. J Neurosci. (2013) 33:4947–57. doi: 10.1523/JNEUROSCI.3990-12.2013
22. Wang Y, Hagel C, Hamel W, Müller S, Kluwe L, and Westphal M. Trk A, B, and C are commonly expressed in human astrocytes and astrocytic gliomas but not by human oligodendrocytes and oligodendroglioma. Acta Neuropathol. (1998) 96:357–64. doi: 10.1007/s004010050906
23. Pöyhönen S, Er S, Domanskyi A, and Airavaara M. Effects of neurotrophic factors in glial cells in the central nervous system: expression and properties in neurodegeneration and injury. Front Physiol. (2019) 10. doi: 10.3389/fphys.2019.00486
24. Thoenen H. Neurotrophins and neuronal plasticity. Science. (1995) 270:593–8. doi: 10.1126/science.270.5236.593
25. Bathina S and Das UN. Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci. (2015) 11:1164–78. doi: 10.5114/aoms.2015.56342
26. Zhang XY, Liang J, Chen DC, Xiu MH, Yang FD, Kosten TA, et al. Low BDNF is associated with cognitive impairment in chronic patients with schizophrenia. Psychopharmacol (Berl). (2012) 222:277–84. doi: 10.1007/s00213-012-2643-y
27. Xue Y, Liang H, Yang R, Deng K, Tang M, and Zhang M. The role of pro- and mature neurotrophins in the depression. Behav Brain Res. (2021) 404:113162. doi: 10.1016/j.bbr.2021.113162
28. Lübke JH, Idoon F, Mohasel-Roodi M, Alipour F, Hami J, Ehteshampour A, et al. Neurotrophic factors in Alzheimer’s disease: pathogenesis and therapy. Acta Neurobiol Exp (Wars). (2021) 81(4):314–27.
29. Dao DT, Anez-Bustillos L, Adam RM, Puder M, and Bielenberg DR. Heparin-binding epidermal growth factor-like growth factor as a critical mediator of tissue repair and regeneration. Am J Pathol. (2018) 188:2446–56. doi: 10.1016/j.ajpath.2018.07.016
30. Linnerbauer M, Lößlein L, Vandrey O, Peter A, Han Y, Tsaktanis T, et al. The astrocyte-produced growth factor HB-EGF limits autoimmune CNS pathology. Nat Immunol. (2024) 25:432–47. doi: 10.1038/s41590-024-01756-6
31. Colucci-D’Amato L, Speranza L, and Volpicelli F. Neurotrophic factor BDNF, physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21207777
32. Schwartz JP and Nishiyama N. Neurotrophic factor gene expression in astrocytes during development and following injury. Brain Res Bull. (1994) 35:403–7. doi: 10.1016/0361-9230(94)90151-1
33. Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR 3rd, Lafaille JJ, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. (2013) 155:1596–609. doi: 10.1016/j.cell.2013.11.030
34. Andreska T, Aufmkolk S, Sauer M, and Blum R. High abundance of BDNF within glutamatergic presynapses of cultured hippocampal neurons. Front Cell Neurosci. (2014) 8:107. doi: 10.3389/fncel.2014.00107
35. Kinboshi M, Mukai T, Nagao Y, Matsuba Y, Tsuji Y, Tanaka S, et al. Inhibition of inwardly rectifying potassium (Kir) 4.1 channels facilitates brain-derived neurotrophic factor (BDNF) expression in astrocytes. Front Mol Neurosci. (2017) 10:408. doi: 10.3389/fnmol.2017.00408
36. Niu C, Yue X, An JJ, Bass R, Xu H, and Xu B. Genetic dissection of BDNF and trkB expression in glial cells. Biomolecules. (2024) 14:91. doi: 10.3390/biom14010091
37. Fulmer CG, VonDran MW, Stillman AA, Huang Y, Hempstead BL, and Dreyfus CF. Astrocyte-derived BDNF supports myelin protein synthesis after cuprizone-induced demyelination. J Neurosci. (2014) 34:8186–96. doi: 10.1523/JNEUROSCI.4267-13.2014
38. Arévalo JC and Deogracias R. Mechanisms controlling the expression and secretion of BDNF. Biomolecules. (2023) 13:789. doi: 10.3390/biom13050789
39. Albini M, Krawczun-Rygmaczewska A, and Cesca F. Astrocytes and brain-derived neurotrophic factor (BDNF). Neurosci Res. (2023) 197:42–51. doi: 10.1016/j.neures.2023.02.001
40. Wei X, Browning JL, and Olsen ML. Neuron and astrocyte specific 5mC and 5hmC signatures of BDNF’s receptor, TrkB. Front Mol Neurosci. (2024) 17:1463437. doi: 10.3389/fnmol.2024.1463437
41. Lai SW, Chen JH, Lin HY, Liu YS, Tsai CF, Chang PC, et al. Regulatory effects of neuroinflammatory responses through brain-derived neurotrophic factor signaling in microglial cells. Mol Neurobiol. (2018) 55:7487–99. doi: 10.1007/s12035-018-0933-z
42. Nociti V and Romozzi M. The role of BDNF in multiple sclerosis neuroinflammation. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24098447
43. Maiworm M. The relevance of BDNF for neuroprotection and neuroplasticity in multiple sclerosis. Front Neurol. (2024) 15:1385042. doi: 10.3389/fneur.2024.1385042
44. Dolcetti E, Bruno A, Azzolini F, Gilio L, Moscatelli A, De Vito F, et al. The BDNF val66Met polymorphism (rs6265) modulates inflammation and neurodegeneration in the early phases of multiple sclerosis. Genes (Basel). (2022) 13. doi: 10.3390/genes13020332
45. Zivadinov R, Weinstock-Guttman B, Benedict R, Tamaño-Blanco M, Hussein S, Abdelrahman N, et al. Preservation of gray matter volume in multiple sclerosis patients with the Met allele of the rs6265 (Val66Met) SNP of brain-derived neurotrophic factor. Hum Mol Genet. (2007) 16:2659–68. doi: 10.1093/hmg/ddm189
46. Ramasamy DP, Ramanathan M, Cox JL, Antulov R, Weinstock-Guttman B, Bergsland N, et al. Effect of Met66 allele of the BDNF rs6265 SNP on regional gray matter volumes in patients with multiple sclerosis: A voxel-based morphometry study. Pathophysiology. (2011) 18:53–60. doi: 10.1016/j.pathophys.2010.04.006
47. Dinacci D, Tessitore A, Russo A, De Bonis ML, Lavorgna L, Picconi O, et al. BDNF Val66Met polymorphism and brain volumes in multiple sclerosis. Neurol Sci. (2011) 32:117–23. doi: 10.1007/s10072-010-0433-z
48. Nociti V, Santoro M, Quaranta D, Losavio FA, De Fino C, Giordano R, et al. BDNF rs6265 polymorphism methylation in Multiple Sclerosis: A possible marker of disease progression. PLoS One. (2018) 13:e0206140. doi: 10.1371/journal.pone.0206140
49. Gielen A, Khademi M, Muhallab S, Olsson T, and Piehl F. Increased brain-derived neurotrophic factor expression in white blood cells of relapsing-remitting multiple sclerosis patients. Scand J Immunol. (2003) 57:493–7. doi: 10.1046/j.1365-3083.2003.01260.x
50. Azoulay D, Vachapova V, Shihman B, Miler A, and Karni A. Lower brain-derived neurotrophic factor in serum of relapsing remitting MS: reversal by glatiramer acetate. J Neuroimmunol. (2005) 167:215–8. doi: 10.1016/j.jneuroim.2005.07.001
51. Sarchielli P, Zaffaroni M, Floridi A, Greco L, Candeliere A, Mattioni A, et al. Production of brain-derived neurotrophic factor by mononuclear cells of patients with multiple sclerosis treated with glatiramer acetate, interferon-beta 1a, and high doses of immunoglobulins. Mult Scler. (2007) 13:313–31. doi: 10.1177/1352458506070146
52. Frota ER, Rodrigues DH, Donadi EA, Brum DG, Maciel DR, and Teixeira AL. Increased plasma levels of brain derived neurotrophic factor (BDNF) after multiple sclerosis relapse. Neurosci Lett. (2009) 460:130–2. doi: 10.1016/j.neulet.2009.05.057
53. Liguori M, Fera F, Patitucci A, Manna I, Condino F, Valentino P, et al. A longitudinal observation of brain-derived neurotrophic factor mRNA levels in patients with relapsing-remitting multiple sclerosis. Brain Res. (2009) 1256:123–8. doi: 10.1016/j.brainres.2008.11.047
54. Damasceno A, Damasceno BP, Cendes F, Damasceno A, Moraes AS, Farias A, et al. Serum BDNF levels are not reliable correlates of neurodegeneration in MS patients. Multiple Sclerosis Related Disord. (2015) 4:65–6. doi: 10.1016/j.msard.2014.11.003
55. Mehrpour M, Akhoundi FH, Delgosha M, Keyvani H, Motamed MR, Sheibani B, et al. Increased serum brain-derived neurotrophic factor in multiple sclerosis patients on interferon-β and its impact on functional abilities. Neurologist. (2015) 20:57–60. doi: 10.1097/NRL.0000000000000053
56. Islas-Hernandez A, Aguilar-Talamantes HS, Bertado-Cortes B, Mejia-delCastillo GJ, Carrera-Pineda R, Cuevas-Garcia CF, et al. BDNF and Tau as biomarkers of severity in multiple sclerosis. biomark Med. (2018) 12:717–26. doi: 10.2217/bmm-2017-0374
57. Naegelin Y, Saeuberli K, Schaedelin S, Dingsdale H, Magon S, Baranzini S, et al. Levels of brain-derived neurotrophic factor in patients with multiple sclerosis. Ann Clin Transl Neurol. (2020) 7:2251–61. doi: 10.1002/acn3.51215
58. Karimi N, Ashourizadeh H, Akbarzadeh Pasha B, Haghshomar M, Jouzdani T, Shobeiri P, et al. Blood levels of brain-derived neurotrophic factor (BDNF) in people with multiple sclerosis (MS): A systematic review and meta-analysis. Mult Scler Relat Disord. (2022) 65:103984. doi: 10.1016/j.msard.2022.103984
59. Sarchielli P, Greco L, Stipa A, Floridi A, and Gallai V. Brain-derived neurotrophic factor in patients with multiple sclerosis. J Neuroimmunology. (2002) 132:180–8. doi: 10.1016/S0165-5728(02)00319-3
60. Ou ZA, Byrne LM, Rodrigues FB, Tortelli R, Johnson EB, Foiani MS, et al. Brain-derived neurotrophic factor in cerebrospinal fluid and plasma is not a biomarker for Huntington’s disease. Sci Rep. (2021) 11:3481. doi: 10.1038/s41598-021-83000-x
61. Yalachkov Y, Anschütz V, Maiworm M, Jakob J, Schaller-Paule MA, Schäfer JH, et al. Serum and cerebrospinal fluid BDNF concentrations are associated with neurological and cognitive improvement in multiple sclerosis: A pilot study. Multiple Sclerosis Related Disord. (2023) 71:104567. doi: 10.1016/j.msard.2023.104567
62. Comini-Frota ER, Rodrigues DH, Miranda EC, Brum DG, Kaimen-Maciel DR, Donadi EA, et al. Serum levels of brain-derived neurotrophic factor correlate with the number of T2 MRI lesions in multiple sclerosis. Braz J Med Biol Res. (2012) 45:68–71. doi: 10.1590/S0100-879X2011007500165
63. Stadelmann C, Kerschensteiner M, Misgeld T, Brück W, Hohlfeld R, and Lassmann H. BDNF and gp145trkB in multiple sclerosis brain lesions: neuroprotective interactions between immune and neuronal cells? Brain. (2002) 125:75–85. doi: 10.1093/brain/awf015
64. Linker RA, Lee DH, Demir S, Wiese S, Kruse N, Siglienti I, et al. Functional role of brain-derived neurotrophic factor in neuroprotective autoimmunity: therapeutic implications in a model of multiple sclerosis. Brain. (2010) 133:2248–63. doi: 10.1093/brain/awq179
65. Lee DH, Geyer E, Flach AC, Jung K, Gold R, Flügel A, et al. Central nervous system rather than immune cell-derived BDNF mediates axonal protective effects early in autoimmune demyelination. Acta Neuropathol. (2012) 123:247–58. doi: 10.1007/s00401-011-0890-3
66. Miyamoto N, Maki T, Shindo A, Liang AC, Maeda M, Egawa N, et al. Astrocytes Promote Oligodendrogenesis after White Matter Damage via Brain-Derived Neurotrophic Factor. J Neurosci. (2015) 35:14002–8. doi: 10.1523/JNEUROSCI.1592-15.2015
67. Fletcher JL, Wood RJ, Nguyen J, Norman EML, Jun CMK, Prawdiuk AR, et al. Targeting trkB with a brain-derived neurotrophic factor mimetic promotes myelin repair in the brain. J Neurosci. (2018) 38:7088–99. doi: 10.1523/JNEUROSCI.0487-18.2018
68. Saitta KS, Lercher LD, Sainato DM, Patel A, Huang Y, McAuliffe G, et al. CHPG enhances BDNF and myelination in cuprizone-treated mice through astrocytic metabotropic glutamate receptor 5. Glia. (2021) 69:1950–65. doi: 10.1002/glia.24003
69. Kopec BM, Kiptoo P, Zhao L, Rosa-Molinar E, and Siahaan TJ. Noninvasive brain delivery and efficacy of BDNF to stimulate neuroregeneration and suppression of disease relapse in EAE mice. Mol Pharm. (2020) 17(2):404–16. doi: 10.1021/acs.molpharmaceut.9b00644
70. Arnon R and Aharoni R. Mechanism of action of glatiramer acetate in multiple sclerosis and its potential for the development of new applications. Proc Natl Acad Sci U S A. (2004) 101 Suppl 2:14593–8. doi: 10.1073/pnas.0404887101
71. Ziemssen T, Kümpfel T, Schneider H, Klinkert WEF, Neuhaus O, and Hohlfeld R. Secretion of brain-derived neurotrophic factor by glatiramer acetate-reactive T-helper cell lines: Implications for multiple sclerosis therapy. J Neurological Sci. (2005) 233:109–12. doi: 10.1016/j.jns.2005.03.010
72. Chen M, Valenzuela RM, and Dhib-Jalbut S. Glatiramer acetate-reactive T cells produce brain-derived neurotrophic factor. J Neurol Sci. (2003) 215:37–44. doi: 10.1016/S0022-510X(03)00177-1
73. Blanco Y, Moral EA, Costa M, Gómez-Choco M, Torres-Peraza JF, Alonso-Magdalena L, et al. Effect of glatiramer acetate (Copaxone®) on the immunophenotypic and cytokine profile and BDNF production in multiple sclerosis: A longitudinal study. Neurosci Letters. (2006) 406:270–5. doi: 10.1016/j.neulet.2006.07.043
74. Azoulay D, Mausner-Fainberg K, Urshansky N, Fahoum F, and Karni A. Interferon-β therapy up-regulates BDNF secretion from PBMCs of MS patients through a CD40-dependent mechanism. J Neuroimmunology. (2009) 211:114–9. doi: 10.1016/j.jneuroim.2009.04.004
75. Smith PA, Schmid C, Zurbruegg S, Jivkov M, Doelemeyer A, Theil D, et al. Fingolimod inhibits brain atrophy and promotes brain-derived neurotrophic factor in an animal model of multiple sclerosis. J Neuroimmunology. (2018) 318:103–13. doi: 10.1016/j.jneuroim.2018.02.016
76. Golan M, Mausner-Fainberg K, Ibrahim B, Benhamou M, Wilf-Yarkoni A, Kolb H, et al. Fingolimod increases brain-derived neurotrophic factor level secretion from circulating T cells of patients with multiple sclerosis. CNS Drugs. (2019) 33:1229–37. doi: 10.1007/s40263-019-00675-7
77. Levi-Montalcini R and Hamburger V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J Exp Zool. (1951) 116:321–61. doi: 10.1002/jez.1401160206
78. Barker PA, Mantyh P, Arendt-Nielsen L, Viktrup L, and Tive L. Nerve growth factor signaling and its contribution to pain. J Pain Res. (2020) 13:1223–41. doi: 10.2147/JPR.S247472
79. Aloe L, Rocco ML, Balzamino BO, and Micera A. Nerve growth factor: A focus on neuroscience and therapy. Curr Neuropharmacol. (2015) 13:294–303. doi: 10.2174/1570159X13666150403231920
80. Iulita MF and Cuello AC. Nerve growth factor metabolic dysfunction in Alzheimer’s disease and Down syndrome. Trends Pharmacol Sci. (2014) 35:338–48. doi: 10.1016/j.tips.2014.04.010
81. Tonev D and Momchilova A. Therapeutic plasma exchange and multiple sclerosis dysregulations: focus on the removal of pathogenic circulatory factors and altering nerve growth factor and sphingosine-1-phosphate plasma levels. Curr Issues Mol Biol. (2023) 45:7749–74. doi: 10.3390/cimb45100489
82. Du Y and Dreyfus CF. Oligodendrocytes as providers of growth factors. J Neurosci Res. (2002) 68:647–54. doi: 10.1002/jnr.10245
83. Tiberi A, Capsoni S, and Cattaneo A. A microglial function for the nerve growth factor: predictions of the unpredictable. Cells. (2022) 11. doi: 10.3390/cells11111835
84. Minnone G, De Benedetti F, and Bracci-Laudiero L. NGF and its receptors in the regulation of inflammatory response. Int J Mol Sci. (2017) 18. doi: 10.3390/ijms18051028
85. Delivanoglou N, Boziki M, Theotokis P, Kesidou E, Touloumi O, Dafi N, et al. Spatio-temporal expression profile of NGF and the two-receptor system, TrkA and p75NTR, in experimental autoimmune encephalomyelitis. J Neuroinflammation. (2020) 17:41. doi: 10.1186/s12974-020-1708-9
86. Bonetto G, Charalampopoulos I, Gravanis A, and Karagogeos D. The novel synthetic microneurotrophin BNN27 protects mature oligodendrocytes against cuprizone-induced death, through the NGF receptor TrkA. Glia. (2017) 65:1376–94. doi: 10.1002/glia.23170
87. Baldassarro VA, Cescatti M, Rocco ML, Aloe L, Lorenzini L, Giardino L, et al. Nerve growth factor promotes differentiation and protects the oligodendrocyte precursor cells from in vitro hypoxia/ischemia. Front Neurosci. (2023) 17. doi: 10.3389/fnins.2023.1111170
88. Alcover-Sanchez B, Garcia-Martin G, Wandosell F, and Cubelos B. R-ras GTPases signaling role in myelin neurodegenerative diseases. Int J Mol Sci. (2020) 21:5911. doi: 10.3390/ijms21165911
89. Ishii A, Furusho M, Dupree JL, and Bansal R. Role of ERK1/2 MAPK signaling in the maintenance of myelin and axonal integrity in the adult CNS. J Neurosci. (2014) 34:16031–45. doi: 10.1523/JNEUROSCI.3360-14.2014
90. Rizzi C, Tiberi A, Giustizieri M, Marrone MC, Gobbo F, Carucci NM, et al. NGF steers microglia toward a neuroprotective phenotype. Glia. (2018) 66:1395–416. doi: 10.1002/glia.23312
91. Tiberi A, Carucci NM, Testa G, Rizzi C, Pacifico P, Borgonovo G, et al. Reduced levels of NGF shift astrocytes toward a neurotoxic phenotype. Front Cell Dev Biol. (2023) 11. doi: 10.3389/fcell.2023.1165125
92. Alatrash R, Golubenko M, Martynova E, Garanina E, Mukhamedshina Y, Khaiboullina S, et al. Genetically engineered artificial microvesicles carrying nerve growth factor restrains the progression of autoimmune encephalomyelitis in an experimental mouse model. Int J Mol Sci. (2023) 24:8332. doi: 10.3390/ijms24098332
93. Razavi S, Nazem G, Mardani M, Esfandiari E, Salehi H, and Esfahani SH. Neurotrophic factors and their effects in the treatment of multiple sclerosis. Adv BioMed Res. (2015) 4:53. doi: 10.4103/2277-9175.151570
94. Iravani MM, Sadeghian M, Leung CC, Jenner P, and Rose S. Lipopolysaccharide-induced nigral inflammation leads to increased IL-1β tissue content and expression of astrocytic glial cell line-derived neurotrophic factor. Neurosci Lett. (2012) 510:138–42. doi: 10.1016/j.neulet.2012.01.022
95. Ahn M, Jin JK, Moon C, Matsumoto Y, Koh CS, and Shin T. Glial cell line-derived neurotrophic factor is expressed by inflammatory cells in the sciatic nerves of Lewis rats with experimental autoimmune neuritis. J Peripher Nerv Syst. (2010) 15:104–12. doi: 10.1111/j.1529-8027.2010.00258.x
96. Duarte Azevedo M, Sander S, and Tenenbaum L. GDNF, A neuron-derived factor upregulated in glial cells during disease. J Clin Med. (2020) 9. doi: 10.3390/jcm9020456
97. Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. (2000) 290:767–73. doi: 10.1126/science.290.5492.767
98. McBride JL, Ramaswamy S, Gasmi M, Bartus RT, Herzog CD, Brandon EP, et al. Viral delivery of glial cell line-derived neurotrophic factor improves behavior and protects striatal neurons in a mouse model of Huntington’s disease. Proc Natl Acad Sci. (2006) 103:9345–50. doi: 10.1073/pnas.0508875103
99. Wang L-J, Lu Y-Y, Muramatsu S-i, Ikeguchi K, Fujimoto K-i, Okada T, et al. Neuroprotective effects of glial cell line-derived neurotrophic factor mediated by an adeno-associated virus vector in a transgenic animal model of amyotrophic lateral sclerosis. J Neurosci. (2002) 22:6920–8. doi: 10.1523/JNEUROSCI.22-16-06920.2002
100. Sandhu JK, Gardaneh M, Iwasiow R, Lanthier P, Gangaraju S, Ribecco-Lutkiewicz M, et al. Astrocyte-secreted GDNF and glutathione antioxidant system protect neurons against 6OHDA cytotoxicity. Neurobiol Disease. (2009) 33:405–14. doi: 10.1016/j.nbd.2008.11.016
101. Sokolowski I, Kucharska-Lusina A, Miller E, Poplawski T, and Majsterek I. Exploring the gene expression and plasma protein levels of HSP90, HSP60, and GDNF in multiple sclerosis patients and healthy controls. Curr Issues Mol Biol. (2024) 46:11668–80. doi: 10.3390/cimb46100693
102. Imraish A, Abu Thiab T, Alsalem M, Dahbour S, Khleif H, Abu-Irmaileh B, et al. The neuroprotective effect of human primary astrocytes in multiple sclerosis: In vitro model. PLoS One. (2024) 19:e0300203. doi: 10.1371/journal.pone.0300203
103. Jin L, Zhang J, Hua X, Xu X, Li J, Wang J, et al. Astrocytic SARM1 promotes neuroinflammation and axonal demyelination in experimental autoimmune encephalomyelitis through inhibiting GDNF signaling. Cell Death Disease. (2022) 13:759. doi: 10.1038/s41419-022-05202-z
104. Kang S, Narazaki M, Metwally H, and Kishimoto T. Historical overview of the interleukin-6 family cytokine. J Exp Med. (2020) 217. doi: 10.1084/jem.20190347
105. Wang H-T, Lu S-T, Xia Z-H, Xu T, Zou W-Y, and Sun M-Q. Ciliary neurotrophic factor activation of astrocytes mediates neuronal damage via the IL−6/IL−6R pathway. Mol Med Rep. (2025) 31(2):32. doi: 10.3892/mmr.2024.13396
106. Dallner C, Woods AG, Deller T, Kirsch M, and Hofmann HD. CNTF and CNTF receptor alpha are constitutively expressed by astrocytes in the mouse brain. Glia. (2002) 37:374–8. doi: 10.1002/glia.10048
107. Liu X, Clark AF, and Wordinger RJ. Expression of ciliary neurotrophic factor (CNTF) and its tripartite receptor complex by cells of the human optic nerve head. Mol Vis. (2007) 13:758–63.
108. Dutta R, McDonough J, Chang A, Swamy L, Siu A, Kidd GJ, et al. Activation of the ciliary neurotrophic factor (CNTF) signalling pathway in cortical neurons of multiple sclerosis patients. Brain. (2007) 130:2566–76. doi: 10.1093/brain/awm206
109. Oppenheim RW, Prevette D, Qin-Wei Y, Collins F, and MacDonald J. Control of embryonic motoneuron survival in vivo by ciliary neurotrophic factor. Science. (1991) 251:1616–8. doi: 10.1126/science.2011743
110. Hughes SM, Lillien LE, Raff MC, Rohrer H, and Sendtner M. Ciliary neurotrophic factor induces type-2 astrocyte differentiation in culture. Nature. (1988) 335:70–3. doi: 10.1038/335070a0
111. Stankoff B, Aigrot M-S, Noël F, Wattilliaux A, Zalc B, and Lubetzki C. Ciliary neurotrophic factor (CNTF) enhances myelin formation: A novel role for CNTF and CNTF-related molecules. J Neurosci. (2002) 22:9221–7. doi: 10.1523/JNEUROSCI.22-21-09221.2002
112. Stahl N and Yancopoulos GD. The tripartite CNTF receptor complex: activation and signaling involves components shared with other cytokines. J Neurobiol. (1994) 25:1454–66. doi: 10.1002/neu.480251111
113. Schuster B, Kovaleva M, Sun Y, Regenhard P, Matthews V, Grötzinger J, et al. Signaling of human ciliary neurotrophic factor (CNTF) revisited: THE INTERLEUKIN-6 RECEPTOR CAN SERVE AS AN &x3b1;-RECEPTOR FOR CNTF *. J Biol Chem. (2003) 278:9528–35. doi: 10.1074/jbc.M210044200
114. Stansberry WM and Pierchala BA. Neurotrophic factors in the physiology of motor neurons and their role in the pathobiology and therapeutic approach to amyotrophic lateral sclerosis. Front Mol Neurosci. (2023) 16. doi: 10.3389/fnmol.2023.1238453
115. Gudi V, Škuljec J, Yildiz Ö, Frichert K, Skripuletz T, Moharregh-Khiabani D, et al. Spatial and temporal profiles of growth factor expression during CNS demyelination reveal the dynamics of repair priming. PLoS One. (2011) 6:e22623. doi: 10.1371/journal.pone.0022623
116. Linker RA, Mäurer M, Gaupp S, Martini R, Holtmann B, Giess R, et al. CNTF is a major protective factor in demyelinating CNS disease: A neurotrophic cytokine as modulator in neuroinflammation. Nat Med. (2002) 8:620–4. doi: 10.1038/nm0602-620
117. Fang M, He D, Zhang F, Hu Z, Yang J, Jiang H, et al. Antineuroinflammatory and neurotrophic effects of CNTF and C16 peptide in an acute experimental autoimmune encephalomyelitis rat model. Front Neuroanat. (2013) 7. doi: 10.3389/fnana.2013.00044
118. Lu Z, Hu X, Zhu C, Wang D, Zheng X, and Liu Q. Overexpression of CNTF in Mesenchymal Stem Cells reduces demyelination and induces clinical recovery in experimental autoimmune encephalomyelitis mice. J Neuroimmunol. (2009) 206:58–69. doi: 10.1016/j.jneuroim.2008.10.014
119. Higashiyama S, Abraham JA, Miller J, Fiddes JC, and Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. (1991) 251:936–9. doi: 10.1126/science.1840698
120. Nishi E and Klagsbrun M. Heparin-binding epidermal growth factor-like growth factor (HB-EGF) is a mediator of multiple physiological and pathological pathways. Growth Factors. (2004) 22:253–60. doi: 10.1080/08977190400008448
121. Miyata K, Yotsumoto F, Nam SO, Kuroki M, and Miyamoto S. Regulatory mechanisms of the HB-EGF autocrine loop in inflammation, homeostasis, development and cancer. Anticancer Res. (2012) 32(6):2347–52.
122. Schenk GJ, Dijkstra S, Van Het Hof AJ, van der Pol SMA, Drexhage JAR, van der Valk P, et al. Roles for HB-EGF and CD9 in multiple sclerosis. Glia. (2013) 61:1890–905. doi: 10.1002/glia.22565
123. Jin K, Mao XO, Sun Y, Xie L, Jin L, Nishi E, et al. Heparin-binding epidermal growth factor-like growth factor: hypoxia-inducible expression in vitro and stimulation of neurogenesis in vitro and in vivo. J Neurosci. (2002) 22:5365–73. doi: 10.1523/JNEUROSCI.22-13-05365.2002
124. Hoffmann FS, Hofereiter J, Rübsamen H, Melms J, Schwarz S, Faber H, et al. Fingolimod induces neuroprotective factors in human astrocytes. J Neuroinflammation. (2015) 12:184. doi: 10.1186/s12974-015-0393-6
125. Tran C, Heng B, Teo JD, Humphrey SJ, Qi Y, Couttas TA, et al. Sphingosine 1-phosphate but not Fingolimod protects neurons against excitotoxic cell death by inducing neurotrophic gene expression in astrocytes. J Neurochemistry. (2020) 153:e14917. doi: 10.1111/jnc.14917
126. Moradi K, Mitew S, Xing YL, and Merson TD. HB-EGF and EGF infusion following CNS demyelination mitigates age-related decline in regeneration of oligodendrocytes from neural precursor cells originating in the ventricular-subventricular zone. bioRxiv. (2024). doi: 10.1101/2024.02.26.582092
127. Palii SS, Van Emburgh BO, Sankpal UT, Brown KD, and Robertson KD. DNA methylation inhibitor 5-Aza-2’-deoxycytidine induces reversible genome-wide DNA damage that is distinctly influenced by DNA methyltransferases 1 and 3B. Mol Cell Biol. (2008) 28:752–71. doi: 10.1128/MCB.01799-07
128. Chan MW, Chang CB, Tung CH, Sun J, Suen JL, and Wu SF. Low-dose 5-aza-2’-deoxycytidine pretreatment inhibits experimental autoimmune encephalomyelitis by induction of regulatory T cells. Mol Med. (2014) 20:248–56. doi: 10.2119/molmed.2013.00159
129. Mayo L, Trauger SA, Blain M, Nadeau M, Patel B, Alvarez JI, et al. Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat Med. (2014) 20:1147–56. doi: 10.1038/nm.3681
130. Toft-Hansen H, Füchtbauer L, and Owens T. Inhibition of reactive astrocytosis in established experimental autoimmune encephalomyelitis favors infiltration by myeloid cells over T cells and enhances severity of disease. Glia. (2011) 59:166–76. doi: 10.1002/glia.21088
131. Khakh BS and Deneen B. The emerging nature of astrocyte diversity. Annu Rev Neurosci. (2019) 42:187–207. doi: 10.1146/annurev-neuro-070918-050443
132. McNamara NB, Munro DAD, Bestard-Cuche N, Uyeda A, Bogie JFJ, Hoffmann A, et al. Microglia regulate central nervous system myelin growth and integrity. Nature. (2023) 613:120–9. doi: 10.1038/s41586-022-05534-y
133. Dong Y, D’Mello C, Pinsky W, Lozinski BM, Kaushik DK, Ghorbani S, et al. Oxidized phosphatidylcholines found in multiple sclerosis lesions mediate neurodegeneration and are neutralized by microglia. Nat Neurosci. (2021) 24:489–503. doi: 10.1038/s41593-021-00801-z
134. Wasser B, Luchtman D, Löffel J, Robohm K, Birkner K, Stroh A, et al. CNS-localized myeloid cells capture living invading T cells during neuroinflammation. J Exp Med. (2020) 217. doi: 10.1084/jem.20190812
135. Massaro AR, Soranzo C, Bigon E, Battiston S, Morandi A, Carnevale A, et al. Nerve growth factor (NGF) in cerebrospinal fluid (CSF) from patients with various neurological disorders. Ital J Neurological Sci. (1994) 15:105–8. doi: 10.1007/BF02340121
136. Hemmer B and Gehring K. Diagnose und Therapie der Multiplen Sklerose, Neuromyelitis-optica-Spektrum-Erkrankungen und MOG-IgG-assoziierten Erkrankungen, S2k-Leitlinie. Deutsche Gesellschaft für Neurologie (Hrsg), Leitlinien für Diagnostik und Therapie in der Neurologie. (2024). Available at: https://www.dgn.org/leitlinien (Accessed August 21, 2025).
137. Ciubotaru A, Smihor MI, Grosu C, Alexa D, Covali R, Anicăi RC, et al. Neurodegenerative biomarkers in multiple sclerosis: at the interface between research and clinical practice. Diagnostics (Basel). (2025) 15. doi: 10.3390/diagnostics15091178
138. Vornholt E, Luo D, Qiu W, McMichael GO, Liu Y, Gillespie N, et al. Postmortem brain tissue as an underutilized resource to study the molecular pathology of neuropsychiatric disorders across different ethnic populations. Neurosci Biobehav Rev. (2019) 102:195–207. doi: 10.1016/j.neubiorev.2019.04.015
139. Gomez-Nicola D and Boche D. Post-mortem analysis of neuroinflammatory changes in human Alzheimer’s disease. Alzheimers Res Ther. (2015) 7:42. doi: 10.1186/s13195-015-0126-1
140. Lee H-G, Wheeler MA, and Quintana FJ. Function and therapeutic value of astrocytes in neurological diseases. Nat Rev Drug Discovery. (2022) 21:339–58. doi: 10.1038/s41573-022-00390-x
141. Alcalá-Barraza SR, Lee MS, Hanson LR, McDonald AA, Frey WH 2nd, and McLoon LK. Intranasal delivery of neurotrophic factors BDNF, CNTF, EPO, and NT-4 to the CNS. J Drug Targeting. (2010) 18:179–90. doi: 10.3109/10611860903318134
142. Jin L, Nie L, Deng Y, Khana GJ, and He N. The application of polymeric nanoparticles as drug delivery carriers to cells in neurodegenerative diseases. Cell Proliferation. (2025) 58(8):e13804. doi: 10.1111/cpr.13804
143. Li J, Long Q, Ding H, Wang Y, Luo D, Li Z, et al. Progress in the treatment of central nervous system diseases based on nanosized traditional chinese medicine. Advanced Science. (2024) 11:2308677. doi: 10.1002/advs.202308677
144. Gaikwad S, Puangmalai N, Sonawane M, Montalbano M, Price R, Iyer MS, et al. Nasal tau immunotherapy clears intracellular tau pathology and improves cognitive functions in aged tauopathy mice. Sci Trans Med. (2024) 16:eadj5958. doi: 10.1126/scitranslmed.adj5958
145. Hersh AM, Alomari S, and Tyler BM. Crossing the blood-brain barrier: advances in nanoparticle technology for drug delivery in neuro-oncology. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23084153
146. Ross TM, Martinez PM, Renner JC, Thorne RG, Hanson LR, and Frey WH. Intranasal administration of interferon beta bypasses the blood–brain barrier to target the central nervous system and cervical lymph nodes: a non-invasive treatment strategy for multiple sclerosis. J Neuroimmunology. (2004) 151:66–77. doi: 10.1016/j.jneuroim.2004.02.011
147. Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. (2016) 22:586–97. doi: 10.1038/nm.4106
148. Linnerbauer M, Lößlein L, Farrenkopf D, Vandrey O, Tsaktanis T, Naumann U, et al. Astrocyte-derived pleiotrophin mitigates late-stage autoimmune CNS inflammation. Front Immunol. (2022) 12. doi: 10.3389/fimmu.2021.800128
149. Coleman KKL, Berry S, Cummings J, Hsiung G-YR, Laforce R, Huey E, et al. Intranasal oxytocin for apathy in people with frontotemporal dementia (FOXY): a multicentre, randomised, double-blind, placebo-controlled, adaptive, crossover, phase 2a/2b superiority trial. Lancet Neurology. (2025) 24:128–39. doi: 10.1016/S1474-4422(24)00456-3
150. Wang Z, Xiong G, Tsang WC, Schätzlein AG, and Uchegbu IF. Nose-to-brain delivery. J Pharmacol Exp Ther. (2019) 370:593–601. doi: 10.1124/jpet.119.258152
151. Zhang F, Lin Y-A, Kannan S, and Kannan RM. Targeting specific cells in the brain with nanomedicines for CNS therapies. J Controlled Release. (2016) 240:212–26. doi: 10.1016/j.jconrel.2015.12.013
152. Kreuter J. Drug delivery to the central nervous system by polymeric nanoparticles: What do we know? Advanced Drug Delivery Rev. (2014) 71:2–14. doi: 10.1016/j.addr.2013.08.008
153. El Ouaamari Y, Van den Bos J, Willekens B, Cools N, and Wens I. Neurotrophic factors as regenerative therapy for neurodegenerative diseases: current status, challenges and future perspectives. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24043866
154. Do AD, Kurniawati I, Hsieh CL, Wong TT, Lin YL, and Sung SY. Application of mesenchymal stem cells in targeted delivery to the brain: potential and challenges of the extracellular vesicle-based approach for brain tumor treatment. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms222011187
155. Tashima T. Mesenchymal Stem Cell (MSC)-Based Drug Delivery into the Brain across the Blood–Brain Barrier. Pharmaceutics. (2024) 16:289. doi: 10.3390/pharmaceutics16020289
156. de Girolamo L, Lucarelli E, Alessandri G, Avanzini MA, Bernardo ME, Biagi E, et al. Mesenchymal stem/stromal cells: a new ‘‘cells as drugs’’ paradigm. Efficacy and critical aspects in cell therapy. Curr Pharm Des. (2013) 19:2459–73. doi: 10.2174/1381612811319130015
157. Levy O, Mortensen LJ, Boquet G, Tong Z, Perrault C, Benhamou B, et al. A small-molecule screen for enhanced homing of systemically infused cells. Cell Rep. (2015) 10:1261–8. doi: 10.1016/j.celrep.2015.01.057
158. Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, et al. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. (2008) 14:181–7. doi: 10.1038/nm1703
159. Levy O, Rothhammer V, Mascanfroni I, Tong Z, Kuai R, De Biasio M, et al. A cell-based drug delivery platform for treating central nervous system inflammation. J Mol Med (Berl). (2021) 99:663–71. doi: 10.1007/s00109-020-02003-9
Keywords: astrocytes, protective, neurotrophic factor, neuroinflammation, multiple sclerosis, BDNF, NGF, HB-EGF
Citation: Güner F and Rothhammer V (2025) Neurotrophic factors in multiple sclerosis. Front. Immunol. 16:1654603. doi: 10.3389/fimmu.2025.1654603
Received: 26 June 2025; Accepted: 13 August 2025;
Published: 27 August 2025.
Edited by:
Valentina Tomassini, University of Studies G. d’Annunzio Chieti and Pescara, ItalyReviewed by:
Marta Olah, Columbia University, United StatesOliver W. Gramlich, University of Alabama at Birmingham, United States
Copyright © 2025 Güner and Rothhammer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Veit Rothhammer, dmVpdC5yb3RoaGFtbWVyQHVrLWVybGFuZ2VuLmRl
 Fabian Güner
Fabian Güner Veit Rothhammer
Veit Rothhammer