- 1Department of Endocrinology, The Affiliated People’s Hospital of Jiangsu University, Zhenjiang, China
- 2Department of Laboratory Medicine, The Affiliated Hospital of Jiangsu University, Zhenjiang, China
- 3Department of Laboratory Medicine, The Affiliated People’s Hospital of Jiangsu University, Zhenjiang, China
Autoimmune thyroid disease (AITD), which includes Graves’ disease and Hashimoto’s thyroiditis, poses a substantial global health burden due to its strong environmental influences. Among environmental factors, trace elements are increasingly recognized for their dual roles in regulating thyroid physiology and immune function. This narrative review synthesizes current evidence on the roles of key trace elements-iodine, selenium, vitamin D, zinc, iron, copper, and magnesium-in AITD pathogenesis. We detail their essential functions in thyroid hormone synthesis and metabolism, and critically examine how imbalances (deficiency or excess) can disrupt immune homeostasis, thereby promoting autoimmunity via mechanisms like oxidative stress, aberrant immune cell differentiation, and loss of self-tolerance. This review highlights complex dose-response relationships, such as the U-shaped curve for iodine, and the protective roles of selenium and vitamin D through antioxidant and immunomodulatory pathways. For other elements, including zinc, iron, copper, and magnesium, emerging associations with AITD have been identified, but the mechanistic understanding remains limited. We conclude that imbalances in trace elements are pivotal environmental triggers for AITD. Future research should prioritize elucidating molecular mechanisms, investigating interactions among elements, and conducting long-term interventional studies to translate these findings into precise nutritional strategies for AITD prevention and management.
Introduction
Autoimmune thyroid disease (AITD) is an organ-specific disease of the thyroid gland characterized by immune-mediated thyroid dysfunction, thyroid autoantibody production, and lymphocytic infiltration (1). AITD affects 1-5% of the global population, with a 5-10-fold higher incidence in female. In China, the prevalence of hypothyroidism is about 1.02%, whereas subclinical hypothyroidism affects 12.93%, with autoimmune etiologies accounting for > 60% of cases (2). The most common types of AITD include Graves’ disease (GD) (3) and Hashimoto’s thyroiditis (HT), sharing histopathological features of lymphocytic infiltration (4). While genetic predisposition (e.g., HLA-DR and CTLA-4 polymorphisms) accounts for 50-60% of disease susceptibility (5), environmental triggers-particularly trace elements-have garnered increasing attention due to their dual roles in thyroid physiology and immune regulation (6, 7).
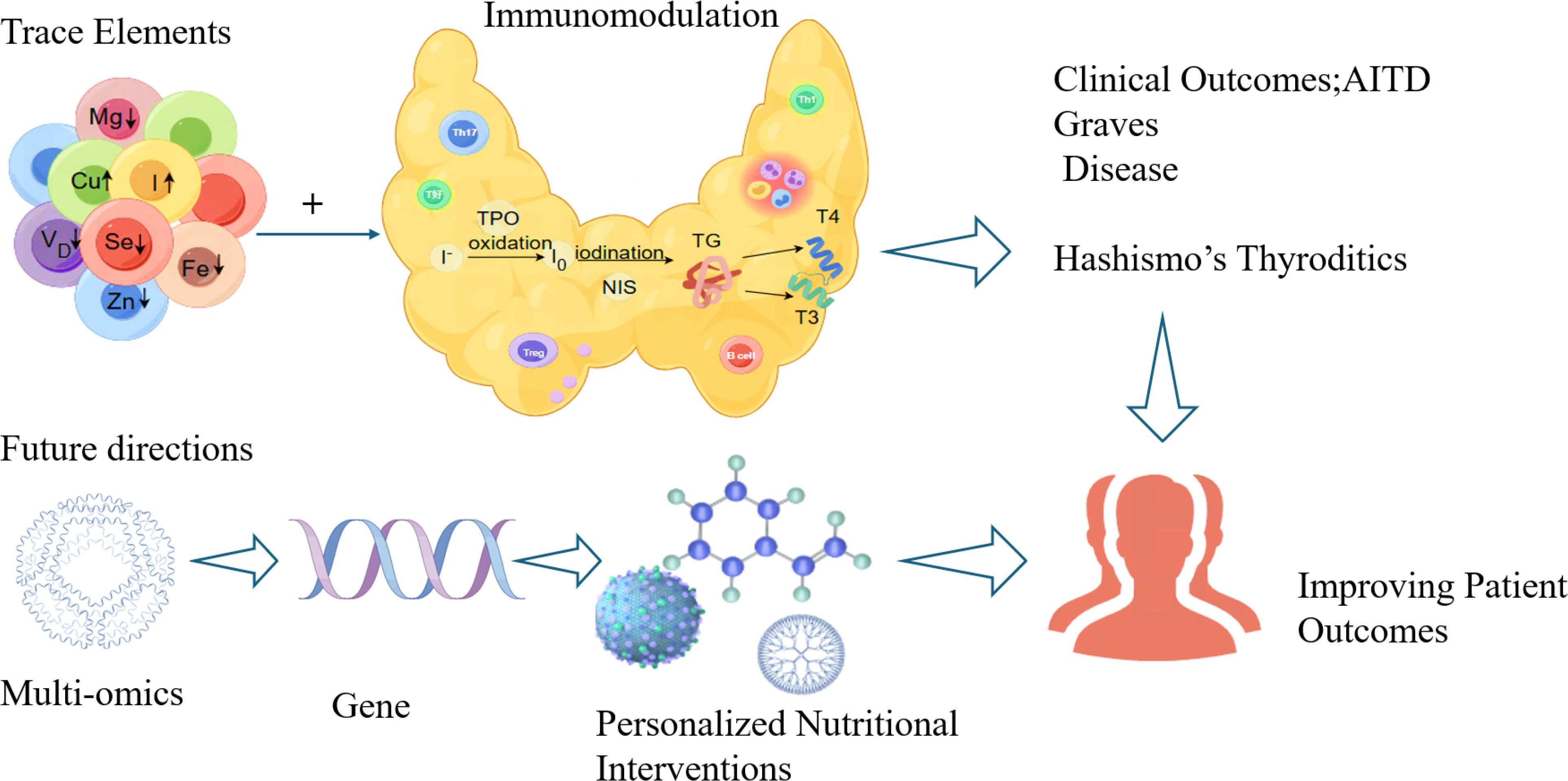
Despite minimal bodily concentrations, trace elements exert critical physiological influences (8). Dietary sources often prove insufficient to maintain optimal levels, predisposing individuals to deficiencies (9). Imbalances in these micronutrients can disrupt systemic functions and contribute to disease pathogenesis. Notably, certain trace elements modulate immune responses, cellular proliferation, and tissue repair (10, 11). Their impact on thyroid homeostasis is multifaceted: they regulate hormone synthesis, metabolism, and immune activity, with dysregulation linked to thyrotoxicosis, hypothyroidism, AITD (Graves’ disease and Hashimoto’s thyroiditis), and thyroid malignancies (12). Given the high prevalence and chronic nature of AITD-which imposes substantial socioeconomic burdens on healthcare systems and compromises patients’ quality of life-integrating trace element assessment into clinical practice offers a cost-effective approach for risk stratification and personalized management. This narrative review synthesizes current evidence regarding the roles of key trace elements (iodine, selenium, vitamin D, zinc, iron, copper, and magnesium) in AITD pathogenesis and discusses their potential implications for clinical management and future research.
The role of trace elements in AITD
Iodine and AITD
As an essential trace nutrient for the human body, iodine plays a crucial role in growth and development, metabolic regulation, and neurocognitive function. Iodine is an essential raw material for synthesizing thyroid hormones (bioactive triiodothyronine T3, thyroxine T4). Within the thyroid follicular lumen, it combines with tyrosine residues on thyroglobulin under the catalysis of thyroid peroxidase (TPO), ultimately converting into biologically active T3 and T4 (13–15). Since Baumann first discovered the phenomenon of iodine accumulation in the thyroid gland (16), numerous epidemiological studies have shown that iodine intake is associated with a U-shaped curve relationship with the risk of thyroid disease (17). Notably, in iodine-sufficient regions, Hashimoto’s thyroiditis has become the primary cause of hypothyroidism (18), while in high-iodine intake areas, the incidence of AITD significantly increases, particularly with marked elevations in thyroid peroxidase antibodies (TPOAb) and thyroid globulin antibodies (TgAb) levels (19–21).
While these epidemiological observations establish a clear association between iodine exposure and AITD risk, a deeper mechanistic understanding is required to elucidate the causal pathways involved. Mechanistic studies have elucidated the process by which excessive iodine intake disrupts thyroid homeostasis and triggers autoimmunity. At the cellular level, excessive iodine directly inhibits thyroid peroxidase (TPO) activity and sodium-iodide symporter (NIS) expression, thereby disrupting thyroid hormone synthesis (20). It also induces reactive oxygen species (ROS) generation in thyroid epithelial cells, leading to oxidative damage, suppression of protective autophagy, and ultimately, cellular apoptosis (22, 23). These cellular injuries are hypothesized to expose neo-antigens, thereby initiating an immune response. Supporting evidence from animal models of experimental autoimmune thyroiditis shows that high iodine intake amplifies the autoimmune cascade. Specifically, it promotes pro-inflammatory T helper 17 (Th17) cell proliferation, alters peripheral lymphocyte function, and disrupts systemic antioxidant balance and cytokine profiles (24, 25). Collectively, evidence from in vitro and in vivo studies delineates a coherent pathway from initial cellular damage and thyroid dysfunction to the breakdown of immune tolerance.
These findings reveal a complex dose-response relationship between iodine intake and the development of AITD. While adequate iodine intake is crucial for maintaining thyroid function, excessive intake may increase the risk of AITD through mechanisms such as oxidative stress and abnormal immune regulation. A deeper understanding of these mechanisms not only provides new insights into the etiology of AITD but also offers potential targets for the development of clinical prevention and treatment strategies.
Selenium and AITD
In 1817, Swedish chemist J.J. Berzelius discovered the element selenium—one of the essential trace elements in the human body (26). Selenium serves as a fundamental element for the normal functioning of human and animal organisms. It accumulates in the form of inorganic selenate compounds (IV) or (VI), which are then converted into organic forms to synthesize selenoproteins (27). These selenoproteins participate in various physiological, biochemical, and metabolic processes within human tissue cells, exhibiting antioxidant, anti-inflammatory, and immune-regulatory functions (28). Current research has identified 25 human-encoded selenoproteins, with the most closely associated with thyroid diseases being the glutathione peroxidase (GPx) family, thioredoxin reductase (TrxR), and iodothyronine deiodinase (DIO) (29).
Selenoproteins play a multifaceted role in the development of thyroid diseases (30–32). Firstly, selenoproteins such as GPx can remove hydrogen peroxide (H2O2) from thyroid cells, thereby protecting cell membranes. When selenium is deficient in the body, GPx function is inhibited, leading to reduced antioxidant stress resistance in thyroid cells and increased cellular damage and apoptosis. This damage may impair thyroid hormone synthesis. Second, selenium is a core component of selenoproteins and a key regulator of thyroid hormone metabolism. It facilitates the conversion of T4 to T3 through deiodinases (DIO1/DIO2) (33–36). Consequently, selenium deficiency lowers deiodinase activity, which impairs T4-to-T3 conversion, leads to aberrant thyroid hormone levels, and disrupts normal physiological functions. Additionally, thioredoxin in selenium proteins is an important component of cysteine residues in nuclear transcription factors, responsible for regulating thyroid cell differentiation and proliferation. Finally, selenium proteins also possess immune regulatory functions, participating in the activation of T cell proliferation, differentiation, and redox metabolism. Furthermore, selenium is involved in reducing excessive immune responses and chronic inflammation (37). Mice on a high-selenium diet not only increased T Cell Receptor (TCR) signal transduction in CD4+ T cells but also increased IL-2 expression, shifting the T Helper 1 Cell/T Helper 2 Cell (Th1/Th2 cell) balance toward the Th1 phenotype, with higher levels of interferon-γ (IFN-γ) and CD40 ligand (38), thereby inhibiting immune damage to thyroid cells and reducing the incidence of AITD. Therefore, it can be concluded that selenium plays a crucial regulatory role in the synthesis, conversion, metabolism, and associated diseases of thyroid hormones.
Selenium is both an antioxidant and an immune stabilizer, it can protect thyroid cells from oxidative stress damage, regulate immune function, and prevent thyroid tissue from being attacked by the immune system (39).In animal studies, selenium supplementation for eight weeks in a high-iodine diet-induced experimental autoimmune thyroiditis (EAT) mouse model significantly attenuated thyroid follicular destruction and lymphocyte infiltration, and improved thyroid hormone and autoantibody levels (40). These animal data provide strong evidence for selenium’s immunomodulatory effects. However, extrapolation to human disease requires caution due to differences in immune responses, disease progression, and selenium metabolism between mice and humans.
In human cross-sectional studies (as shown as Table 1), serum selenium levels are negatively correlated with free T4 (FT4) and TSH in GD patients without GO. Similarly, in GD patients with GO, serum selenium levels were negatively correlated with FT3, FT4, and TSH (41). Studies across regions with varying selenium intake further support this, demonstrating that GD patients exhibit lower selenium levels, which correlate with GO clinical manifestations. Lower selenium levels are associated with increased GO risk, and were identified as an independent risk factor for moderate-to-severe GO (42, 43). Moreover, the prevalence of autoimmune thyroiditis (AIT) was higher in selenium-deficient areas than in selenium-adequate areas, suggesting that low selenium is a potential risk factor for AIT (44). In an iodine-sufficient region, serum selenium levels were significantly lower in newly diagnosed GD (97.68 μg/l) and HT (104.36 μg/l) patients compared to healthy controls (122.63 μg/l, p < 0.001) (45). However, observational studies can only indicate associations rather than establish causality, and are susceptible to confounding by factors such as other nutrient intakes, environmental exposures, or genetic background.
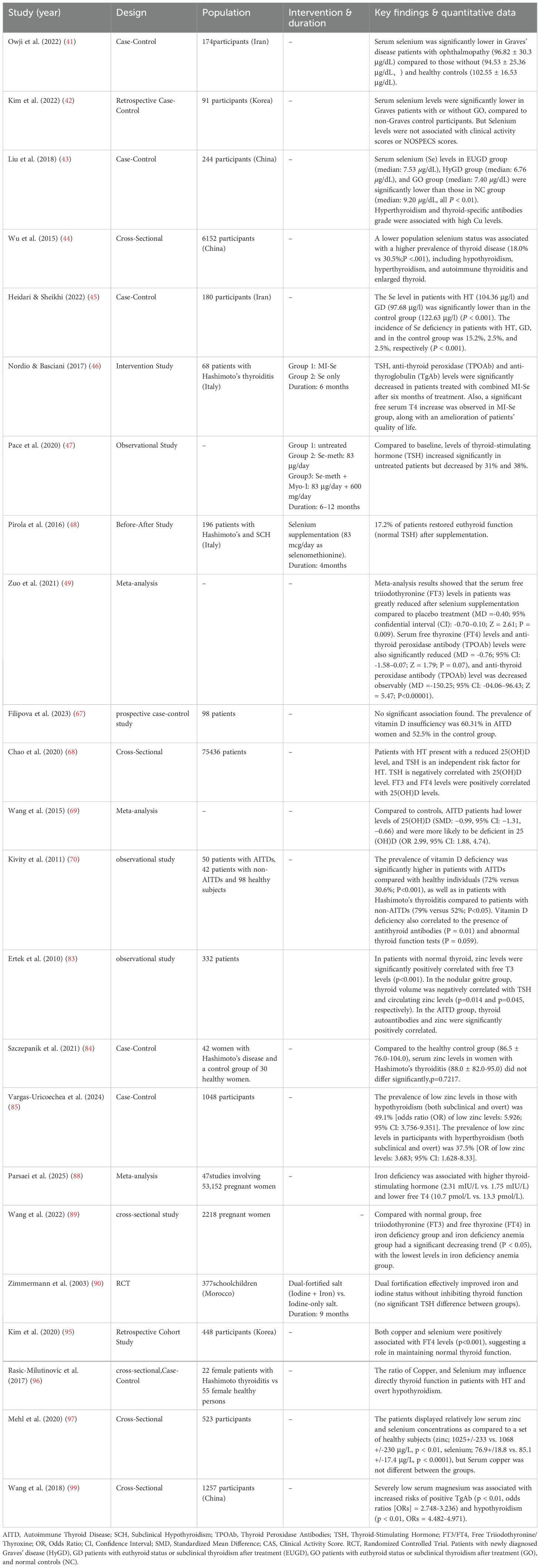
Table 1. Summary of selected clinical studies on trace elements supplementation in autoimmune thyroid diseases (AITD).
Based on these associations, several selenium supplementation trials have been conducted. After six months of combined myo-inositol and selenium supplementation, the six-month combined MI-Se regimen significantly reduced serum levels of thyroid-stimulating hormone, anti-thyroid peroxidase antibodies, and anti-thyroglobulin antibodies, indicating reduced antibody titers and prevention of thyroid function deterioration (46, 47).In another trial,4 months of selenium monotherapy (sodium selenite, 83 μg/day) normalized TSH levels in 17.2% (33/192) of subjects. Responders were significantly more frequent among Cases than Controls (30/96 [31.3%] vs. 3/96 [3.1%],resulting in a highly significant intergroup difference (p < 0.0001 (48). Additionally, clinical trials demonstrated that selenium supplementation significantly reduced FT3, FT4, and TPOAb levels in AITD patients (49).
Immunologically, T-cell subsets are key factors in AITD pathogenesis. Studies in autoimmune thyroid models show reduced CD4+CD25+Foxp3+ cell numbers and decreased Foxp3 mRNA expression in splenocytes. Selenium supplementation increases regulatory T cell (Treg) numbers (40, 50). CD4+CD25+Foxp3+ Tregs are essential immune components that suppress autoimmune responses; their deficiency promotes self-reactive immunity and tissue damage (51). Thus, selenium may inhibit antibody production by upregulating Tregs. Selenium supplementation increases Treg proportion and function, suppresses pro-inflammatory cytokine secretion, and delays thyroid follicular cell apoptosis (52). Selenium may regulate immune function in AITD by modulating Th1/Th2 cytokine expression. Th1 cytokines activate macrophages, stimulate complement and antibody production, and exert cytotoxic effects that promote thyroid disease progression. In contrast, Th2 cytokines inhibit Th1 cytokine production (53, 54). Th17 lymphocytes, which share pro-inflammatory properties with Th1 cells, have also been recently implicated (55). In early-stage HT, Th17-dependent responses predominate, whereas Th1 activity increases in later stages (56). A study showed that selenium supplementation modulates interferon-γ and interleukin-1β levels, suggesting it regulates the Th1/Th2/Th17-Treg balance (54). These studies provide evidence for selenium’s immunomodulatory potential in AITD treatment. However, most clinical trials are limited by small sample sizes, short follow-up, and inconsistent selenium forms and dosages, restricting result generalizability and comparability. Furthermore, variations in disease stage and baseline selenium status across studies may confound efficacy assessments.
In summary, evidence from in vitro, animal, and human studies progressively constructs a framework for selenium’s protective role in AITD, particularly through regulating T-cell subset balance. However, human studies, particularly intervention trials, have methodological limitations. Future research requires larger, longer-term, rigorously designed randomized controlled trials to determine the optimal timing and target populations for selenium supplementation in AITD prevention and treatment. Furthermore, elucidating the molecular mechanisms of selenium-mediated immune cell differentiation will provide a foundation for its application in AITD precision medicine.
Vitamin D and AITD
Vitamin D is a fat-soluble vitamin and a derivative of steroids, with multiple biological forms. The two most important fat-soluble steroids for humans are vitamin D2 and vitamin D3 (57). It can be naturally synthesized through sun exposure or obtained through diet, and is converted in the liver into 25-hydroxyvitamin D/25(OH)D, and in the kidneys into 1,25-dihydroxyvitamin D/1,25(OH)2D/calcitriol (58). 1,25(OH)2D is the primary bioactive metabolite, i.e., the hormonal form of vitamin D, whose primary function is to maintain calcium and phosphorus homeostasis and regulate bone metabolism (59). Furthermore, by binding to the vitamin D receptor (VDR), it modulates the expression of thyroid-related genes and is significantly inversely correlated with TSH levels (60–62). VDR is widely expressed in cells and organs such as vascular endothelial cells, immune cells, and endocrine glands. Notably, immune cells can also synthesize 1α-hydroxylase, which is associated with vitamin D activation. 25-hydroxy vitamin D [25-hydroxy vitamin D, 25-(OH)D] is converted into biologically active 1,25-(OH)2D by hydroxylase and exerts its biological functions through autocrine or paracrine mechanisms. This confirms the role of vitamin D as an immunomodulator in the development of various autoimmune diseases (63). Current research also confirms that vitamin D deficiency is associated with the onset of various autoimmune diseases, including rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, and type 1 diabetes (64). Recent domestic and international studies have found that vitamin D also plays a role in diabetes, the intestines, kidneys, metabolism, immunity, and tumors, regulating hormone secretion, immune function, and cell proliferation and differentiation (65).
The association between vitamin D and autoimmune thyroid disease (AITD), while substantiated by clinical evidence, reveals a complex picture when examined across different levels of investigation. Clinical studies (as shown as Table 1) consistently demonstrate an epidemiological link: AITD patients overall have a higher prevalence of vitamin D deficiency, AITD patients had lower levels of 25(OH)D compared to controls (66–69). A significant inverse correlation is often reported between vitamin D levels and anti-thyroid antibody titers (70). However, these observational data are inherently limited by confounding factors such as seasonal variation, sun exposure, and BMI, which preclude causal inference regarding whether vitamin D deficiency is a cause or a consequence of AITD.
The mechanistic basis for vitamin D’s immunomodulatory role is well-established in the majority of studies. The nuclear vitamin D receptor (VDR) is expressed on immune cells, including dendritic cells (DCs), monocytes, and lymphocytes (71), plays a crucial role in initiating immune responses and are the most potent antigen-presenting cells (72). 1,25-(OH)2D3 regulates gene expression by binding to intracellular VDR, participating in immune regulation, forming the cellular and molecular basis for vitamin D’s involvement in the pathogenesis of AITD (65). Vitamin D enhances innate immune responses while suppressing adaptive immune responses, increasing immune tolerance, and reducing the incidence of autoimmune diseases (73). Tregs cells play a crucial role in establishing and maintaining immune tolerance and suppressing autoimmune diseases, thereby regulating the normal functioning of the immune system. Studies have shown that vitamin D enhances Treg cell function, promotes Th2 cell differentiation and maturation, and inhibits Th1 cell activation (74). First, 1,25-(OH)2D3 inhibits B cell proliferation and differentiation into plasma cells, reducing the secretion of autoantibodies targeting thyroid cells; second, it directly or indirectly promotes plasma cell apoptosis through the Th cell pathway, preventing complement activation-mediated thyroid cell death. Exogenous antigens stimulate DC activation, initiating the primary immune response. DCs recognize self-antigens to induce immune tolerance in T lymphocytes, thereby suppressing autoimmune reactions that may harm the host. 1,25-(OH)2D3 inhibits the secretion of cytokines IL-12 and IL-23, which are associated with DC differentiation and maturation, promoting T cell differentiation toward the Th2 cell phenotype. Concurrently, it enhances DC-mediated Treg cell secretion of IL-10, suppressing Th1 cell activity, inhibiting CD4+ T cell differentiation into Th17 cells, and blocking Th17 cell secretion of IL-17, thereby preventing cell-mediated cytotoxic destruction of thyroid tissue. Additionally, 1,25-(OH)2D3 blocks nuclear factor κB activation and binding to nuclear factor κB consensus sequences, downregulates IL-8 and IL-12 cytokine secretion, weakens DC antigen presentation and immune response activation functions, and reduces T cell numbers and activity in thyroid tissue (75). These effects collectively form the immune protective network of vitamin D in AITD.
Although existing evidence suggests that 1,25-(OH)2D3, as an immunomodulator, multi-pathway inhibits reactive T lymphocytes from attacking self-antigens, suppresses abnormal immune responses, reduces pro-inflammatory factor production, regulates apoptosis to protect thyroid tissue, and supports the potential value of vitamin D in the prevention and treatment of AITD, further research is needed to address the following key issues: (1) Determining the optimal dose and timing of vitamin D supplementation therapy; (2) Elucidating the synergistic mechanisms between vitamin D and other trace elements (such as selenium); (3) Exploring the differential regulatory effects of vitamin D on different AITD subtypes (such as GD and HT). Future research should focus on establishing the causal relationship between vitamin D status and clinical outcomes in AITD to provide more reliable evidence for clinical intervention.
Zinc and AITD
Zinc serves as a cofactor for more than 300 metalloenzymes. It contributes to maintaining thyroid hormone receptor activity and modulates the function of the hypothalamic-pituitary-thyroid (HPT) axis (76–79).Zinc plays a multifaceted role in thyroid function regulation as an essential component of the thyroid antioxidant system. It serves as a key cofactor for superoxide dismutase (SOD) (80) and is involved in regulating serum T3, T4, and TSH levels (81).
Zinc is closely related to the immune system and is an essential substance for lymphocyte differentiation and maturation. It mediates cellular immune function, induces the synthesis of T lymphocytes and cytokines such as IL-1, IL-6, and TNF-α, and enhances interactions between immune cells. The association between zinc status and autoimmune thyroid disease (AITD) is supported by clinical observations (as shown as Table 1) but remains contentious. While some human studies report a high prevalence of zinc deficiency in patients with thyroid dysfunction and a positive correlation between serum zinc and thyroid autoantibody titers in patients with autoimmune thyroiditis (82, 83). However, these findings are challenged by a well-controlled survey showing no significant difference in mean serum zinc concentrations between women with Hashimoto’s thyroiditis and matched healthy controls (84). This discrepancy may stem from variations in study populations, thyroid dysfunction stages, or comorbidities influencing zinc status. Notably, more robust case-control evidence indicates a significantly higher incidence of hypozincemia in patients with overt hypothyroidism and hyperthyroidism compared to healthy individuals (85), reinforcing the potential link between zinc deficiency and thyroid autoimmunity. However, the exact molecular mechanisms underlying this association and the potential of zinc supplementation as a therapeutic intervention require further elucidation through well-designed longitudinal and interventional studies.
Iron and AITD
Iron is an essential cofactor for TPO, fulfilling a dual role in thyroid hormone synthesis: it facilitates crucial redox reactions and maintains the enzyme’s catalytic activity (86). Consequently, iron deficiency impairs TPO function and thereby disrupts thyroid hormone synthesis. Additionally, iron serves as a key regulatory factor in epigenetic modification processes. Its deficiency may lead to genomic-level alterations, potentially triggering or exacerbating the onset and progression of autoimmune thyroid diseases. This underscores iron’s crucial role as a cofactor for thyroid peroxidase in hormone synthesis. Clinically, the correction of this deficiency is paramount, as timely iron supplementation is recognized as an indispensable component of managing Hashimoto’s thyroiditis (87). From a physiological perspective, substantial evidence from both animal models and human studies (as shown as Table 1) has established that nutritional iron deficiency impairs thyroid metabolism, consistently leading to reduced plasma T4 and T3 levels, diminished peripheral T4-to-T3 conversion, and elevated TSH (88–90). However, the association between iron deficiency and the autoimmune process itself is confounded by high rates of comorbidity. AITD patients frequently present with iron deficiency, largely attributable to coexisting autoimmune gastritis (impairing absorption) and celiac disease (causing loss) (91).This epidemiological link complicates the distinction between a mere comorbidity and a potential pathogenic driver of autoimmunity. Consequently, while the biochemical consequences of iron deficiency on thyroid hormone synthesis are well-documented, the specific immunological mechanisms by which it might initiate or exacerbate the autoimmune response in AITD remain an important area for future investigation.
Copper and AITD
Copper (Cu), an important immune-modulating trace element, exists in plasma primarily in the form of ceruloplasmin-bound copper, accounting for over 90% of its total plasma concentration. It plays a role in both innate and adaptive immune responses (92). Research indicates that copper deficiency can lead to reduced ability of neutrophils to produce reactive oxygen species, decreased T-cell proliferation, and lowered IL-2 secretion, significantly impairing the body’s immune defense functions (92). In the context of thyroid autoimmunity, Copper influences thyroid function through a dual mechanism: it modulates the activity of enzymes critical for thyroid hormone synthesis and interacts with the thyroid hormone receptor β (TRβ) signaling pathway (93, 94). Additionally, copper’s redox properties may influence the progression of AITD by regulating oxidative stress levels within thyroid cells.
Current evidence linking copper (Cu) to autoimmune thyroid disease remains predominantly associative, inconsistent, and lacking mechanistic depth. The available evidence, consisting solely of clinical observations, presents substantial contradictions. For example, one study reported a positive correlation between serum cobalt and thyroid autoantibodies (43),whereas a later study found no significant association (95).Similarly, although the copper-to-selenium (Cu/Se) ratio has been hypothesized to influence thyroid function in hypothyroidism (96),analysis of 323 patients revealed no significant difference in serum copper levels between hypothyroidism and autoimmune thyroiditis groups (97) (as shown as Table 1).These discrepancies likely arise from methodological limitations, including uncontrolled confounders such as systemic inflammation—which elevates serum copper levels as an acute-phase reactant—and heterogeneity in patient populations and dietary intake. Furthermore, the complete absence of experimental data from cellular or animal models precludes determination of causal roles versus epiphenomena. Establishing pathogenic roles for copper in AITD therefore requires a transition from observational studies to hypothesis-driven experimental research examining specific mechanisms, such as modulation of oxidative stress or immune cell function in the thyroid microenvironment.
Magnesium and AITD
Magnesium (Mg), an essential divalent cation, influences thyroid function by modulating the bioavailability and tissue distribution of selenium (98). This action indirectly supports the conversion of T4 to T3, as selenium is an essential cofactor for the deiodinase enzymes responsible for this metabolic step (92). Magnesium deficiency can lead to reduced serum selenium levels, thereby affecting the activity of selenium-dependent proteins such as GPx (98).
Although clinical correlations support an association between magnesium (Mg) status and AITD, mechanistic insights remain limited. Human studies provide evidence for this link at the clinical level. For example, serum Mg levels correlate with thyroid function parameters in TPOAb-positive subclinical Hashimoto’s thyroiditis (92). A large cross-sectional study (as shown as Table 1) further showed that severe hypomagnesemia (serum Mg < 0.55 mmol/L) is associated with higher TgAb positivity and increased risk of overt hypothyroidism (99). However, these epidemiological findings share a critical limitation: they cannot establish causality. The association may be confounded by dietary patterns, comorbidities, or medications influencing both Mg levels and thyroid function. Furthermore, experimental data from cellular or animal models are lacking. The molecular mechanisms of Mg in AITD-such as effects on T-regulatory cell function or oxidative stress in thyrocytes-remain speculative. Therefore, establishing causation requires integrated approaches combining mechanistic studies in cellular and animal models with prospective clinical cohorts.
Discussion
AITD substantially impair patients’ quality of life and impose a significant socioeconomic burden, attributable to the costs associated with long-term pharmacotherapy, frequent monitoring, and complication management. Accumulating evidence indicates that early assessment and correction of trace element imbalances-particularly involving iodine, selenium, and vitamin D-may provide a cost-effective adjunct strategy to slow disease progression and improve patient outcomes. This review systematically examines the roles of multiple trace elements in AITD pathogenesis. Their imbalances-whether deficient or excessive-can disrupt thyroid immune homeostasis and act as environmental triggers. However, significant variations exist in their mechanisms of action, clinical relevance, and the depth of supporting research.
Iodine exhibits a classic dual role in AITD, characterized by a U-shaped dose-response curve. Excessive iodine not only inhibits thyroid peroxidase activity and sodium-iodide symporter expression but also compromises immune tolerance by inducing reactive oxygen species, promoting Th17 cell proliferation, and suppressing autophagy. Critical knowledge gaps persist, particularly in defining the optimal iodine intake for genetically susceptible populations and understanding its interactions with other elements, such as selenium. Future studies should establish personalized iodine targets through prospective cohorts and elucidate underlying molecular pathways using experimental models.
Selenium exerts protective effects mainly via selenoproteins, which contribute to (1) antioxidant defense through hydrogen peroxide neutralization, (2) immunomodulation by supporting regulatory T cells and suppressing Th1/Th17 responses, and (3) hormone metabolism via deiodinases. Although selenium supplementation can reduce thyroid peroxidase antibody and thyroglobulin antibody titers, randomized trials are often limited by small sample sizes, short duration, and protocol heterogeneity, precluding consensus on its long-term efficacy and optimal dosing. Selenium’s dose–response may also follow a U-shaped curve, underscoring the need to avoid excess. Future research should prioritize large, long-term, multicenter randomized trials and explore molecular mechanisms such as epigenetic regulation of T cell differentiation.
Vitamin D modulates immunity primarily via the vitamin D receptor on immune cells, inhibiting dendritic cell maturation, promoting Th2 and Treg differentiation, suppressing Th1 and Th17 pathways, and reducing autoantibody production. Cross-sectional studies consistently associate lower serum vitamin D levels with AITD and higher autoantibody titers. However, the causal direction remains unclear, and optimal serum targets, dosage, and duration of supplementation are undefined. Future work should employ prospective cohorts and interventional trials to clarify causality and differential effects in Graves’ disease versus Hashimoto’s thyroiditis.
Research on zinc, iron, copper, and magnesium in AITD remains less developed. Zinc supports antioxidant defense and hormone receptor function, yet its immunomodulatory mechanisms are poorly understood. Iron deficiency impairs thyroid peroxidase activity, but comorbid conditions often confound its association with AITD. Copper may influence oxidative stress, although reported findings are inconsistent. The copper-to-selenium ratio may be more clinically relevant than the absolute concentration of either element. Magnesium indirectly affects thyroid function via selenium bioavailability, but direct molecular insights are lacking. Research on these elements commonly suffers from limitations such as small-scale observational designs, scarce mechanistic data, and unvalidated benefits of supplementation. Future studies should identify molecular targets in the thyroid immune microenvironment and evaluate combined supplementation strategies.
In summary, trace elements form a complex regulatory network in AITD pathophysiology. Current evidence is heterogeneous, primarily focusing on the population-level effects of individual elements. Substantial gaps remain in understanding individual variability, long-term outcomes, and interactions between different elements. However, this review has some limitations, including the predominance of observational studies, heterogeneity in populations and interventions, and insufficient exploration of trace element interactions and cumulative effects.
Clinically, assessing trace element status-such as selenium, vitamin D, ferritin, and zinc-should be considered in AITD management, especially in refractory or nutritionally at-risk cases. Correcting deficiencies may serve as adjuvant therapy: for example, selenium supplementation (e.g., 100-200 μg/day) can lower TPOAb titers in Hashimoto’s patients (100, 101), and The World Health Organization (WHO) has made recommendation on the dose of selenium for adults to be 30 to 40 μg/day and stated that daily intake up to 400 μg selenium shall be considered safe (102), and vitamin D should be supplemented to achieve serum 25(OH)D > 30 ng/mL (103). Dietary recommendations should emphasize a balanced intake of specific nutrients: adequate but not excessive iodine from iodized salt; selenium from nuts and fish; and zinc and iron from sources such as lean meat, legumes, red meat, and spinach.
Future research should define precise reference ranges and validate supplementation efficacy through rigorous trials. Mechanistic studies ought to clarify the roles of trace elements in thyroid tissue and immune cells, particularly their regulation of key signaling pathways. Integrated multi-omics approaches-metagenomics, transcriptomics, proteomics, metabolomics and genetics-will help uncover pathogenesis mechanisms and immune-regulatory networks. Long-term prospective studies and well-designed interventions are essential to evaluate effects on thyroid function, immune response, and patient-centered outcomes. Ultimately, personalized nutritional strategies-incorporating genetic background, trace element status, and gut microbiota composition-represent a promising direction for the precise prevention and management of AITD (Figure 1).
Author contributions
SL: Writing – original draft. QX: Writing – original draft. SW: Writing – review & editing. HP: Project administration, Writing – review & editing, Conceptualization, Funding acquisition. YL: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by Jiangsu Provincial Medical Key Discipline Cultivation Unit (Grant No. JSDW202241), the Research Project of Jiangsu Commission of Health (Grant No. H2023053), Zhenjiang science and technology planning project (Grant No. SH2025033).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Antonelli A, Ferrari SM, Corrado A, Di Domenicantonio A, and Fallahi P. Autoimmune thyroid disorders. Autoimmun Rev. (2015) 14:174–80. doi: 10.1016/j.autrev.2014.10.016
2. Ma C, Zhong J, Zou Y, Liu Z, Li H, Pang J, et al. Establishment of Reference Intervals for Thyroid-Associated Hormones Using refineR Algorithm in Chinese Population at High-Altitude Areas. Front Endocrinol (Lausanne). (2022) 13:816970. doi: 10.3389/fendo.2022.816970
3. Liu H, Liu H, Liu C, Shang M, Wei T, and Yin P. Gut Microbiome and the Role of Metabolites in the Study of Graves' Disease. Front Mol Biosci. (2022) 9:841223. doi: 10.3389/fmolb.2022.841223
4. Petranović Ovčariček P, Görges R, and Giovanella L. Autoimmune Thyroid Diseases. Semin Nucl Med. (2024) 54:219–36. doi: 10.1053/j.semnuclmed.2023.11.002
5. Minelli R, Gaiani F, Kayali S, Di Mario F, Fornaroli F, Leandro G, et al. Thyroid and celiac disease in pediatric age: a literature review. Acta BioMed. (2018) 89:11–6. doi: 10.23750/abm.v89i9-S.7872
6. Ferrari SM, Fallahi P, Antonelli A, and Benvenga S. Environmental Issues in Thyroid Diseases. Front Endocrinol (Lausanne). (2017) 8:50. doi: 10.3389/fendo.2017.00050
7. Hasham A and Tomer Y. Genetic and epigenetic mechanisms in thyroid autoimmunity. Immunol Res. (2012) 54:204–13. doi: 10.1007/s12026-012-8302-x
8. Liakopoulos V, Roumeliotis S, Bozikas A, Eleftheriadis T, and Dounousi E. Antioxidant Supplementation in Renal Replacement Therapy Patients: Is There Evidence? Oxid Med Cell Longev. (2019) 2019:9109473. doi: 10.1155/2019/9109473
9. Sun J, Xu S, Du Y, Yu K, Jiang Y, Weng H, et al. Accumulation and Enrichment of Trace Elements by Yeast Cells and Their Applications: A Critical Review. Microorganisms. (2022) 10:1746. doi: 10.3390/microorganisms10091746
10. Jin J, Mulesa L, and Carrilero Rouillet M. Trace Elements in Parenteral Nutrition: Considerations for the Prescribing Clinician. Nutrients. (2017) 9:440. doi: 10.3390/nu9050440
11. Di Nardo V and Lotti T. New therapeutic vision of nutrition in dermatology: Integrative nutrition. Dermatol Ther. (2019) 32:e12746. doi: 10.1111/dth.12746
12. Zhou Q, Xue S, Zhang L, and Chen G. Trace elements and the thyroid. Front Endocrinol (Lausanne). (2022) 13:904889. doi: 10.3389/fendo.2022.904889
13. Zhao J, Su Y, Zhang JA, Fang M, Liu X, Jia X, et al. Inverse Association Between Iodine Status and Prevalence of Metabolic Syndrome: A Cross-Sectional Population-Based Study in a Chinese Moderate Iodine Intake Area. Diabetes Metab Syndr Obes. (2021) 14:3691–701. doi: 10.2147/DMSO.S322296
14. Leung A, Pearce EN, and Braverman LE. Role of iodine in thyroid physiology. Expert Rev Endocrinol Metab. (2010) 5:593–602. doi: 10.1586/eem.10.40
15. Sorrenti S, Baldini E, Pironi D, Lauro A, D'Orazi V, Tartaglia F, et al. Iodine: Its Role in Thyroid Hormone Biosynthesis and Beyond. Nutrients. (2021) 13:4469. doi: 10.3390/nu13124469
16. Markou K, Georgopoulos N, Kyriazopoulou V, and Vagenakis AG. Iodine-Induced hypothyroidism. Thyroid. (2001) 11:501–10. doi: 10.1089/105072501300176462
17. Vigone MC, Capalbo D, Weber G, and Salerno M. Mild Hypothyroidism in Childhood: Who, When, and How Should Be Treated? J Endocr Soc. (2018) 2:1024–39. doi: 10.1210/js.2017-00471
18. Laurberg P, Cerqueira C, Ovesen L, Rasmussen LB, Perrild H, Andersen S, et al. Iodine intake as a determinant of thyroid disorders in populations. Best Pract Res Clin Endocrinol Metab. (2010) 24:13–27. doi: 10.1016/j.beem.2009.08.013
19. Farebrother J, Zimmermann MB, and Andersson M. Excess iodine intake: sources, assessment, and effects on thyroid function. Ann N Y Acad Sci. (2019) 1446:44–65. doi: 10.1111/nyas.14041
20. Duntas LH. The catalytic role of iodine excess in loss of homeostasis in autoimmune thyroiditis. Curr Opin Endocrinol Diabetes Obes. (2018) 25:347–52. doi: 10.1097/MED.0000000000000425
21. Paparo SR, Ferrari SM, Patrizio A, Elia G, Ragusa F, Botrini C, et al. Myoinositol in Autoimmune Thyroiditis. Front Endocrinol (Lausanne). (2022) 13:930756. doi: 10.3389/fendo.2022.930756
22. Luo Y, Kawashima A, Ishido Y, Yoshihara A, Oda K, Hiroi N, et al. Iodine excess as an environmental risk factor for autoimmune thyroid disease. Int J Mol Sci. (2014) 15:12895–912. doi: 10.3390/ijms150712895
23. Horie I, Abiru N, Nagayama Y, Kuriya G, Saitoh O, Ichikawa T, et al. T helper type 17 immune response plays an indispensable role for development of iodine-induced autoimmune thyroiditis in nonobese diabetic-H2h4 mice. Endocrinology. (2009) 150:5135–42. doi: 10.1210/en.2009-0434
24. Xu C, Wu F, Mao C, Wang X, Zheng T, Bu L, et al. Excess iodine promotes apoptosis of thyroid follicular epithelial cells by inducing autophagy suppression and is associated with Hashimoto thyroiditis disease. J Autoimmun. (2016) 75:50–7. doi: 10.1016/j.jaut.2016.07.008
25. Saha A, Mukherjee S, Bhattacharjee A, Sarkar D, Chakraborty A, Banerjee A, et al. Excess iodine-induced lymphocytic impairment in adult rats. Toxicol Mech Methods. (2019) 29:110–8. doi: 10.1080/15376516.2018.1528647
26. Mao J, Pop VJ, Bath SC, Vader HL, Redman CW, and Rayman MP. Effect of low-dose selenium on thyroid autoimmunity and thyroid function in UK pregnant women with mild-to-moderate iodine deficiency. Eur J Nutr. (2016) 55:55–61. doi: 10.1007/s00394-014-0822-9
27. Kieliszek M. Selenium-Fascinating Microelement, Properties and Sources in Food. Molecules. (2019) 24:1298. doi: 10.3390/molecules24071298
28. De Waele E, Malbrain M, and Spapen H. Nutrition in Sepsis: A Bench-to-Bedside Review. Nutrients. (2020) 12:395. doi: 10.3390/nu12020395
29. Avery JC and Hoffmann PR. Selenium, Selenoproteins, and Immunity. Nutrients. (2018) 10:1203. doi: 10.3390/nu10091203
30. Wang P, Chen B, Huang Y, Li J, Cao D, Chen Z, et al. Selenium intake and multiple health-related outcomes: an umbrella review of meta-analyses. Front Nutr. (2023) 10:1263853. doi: 10.3389/fnut.2023.1263853
31. Rua RM, Nogales F, Carreras O, and Ojeda ML. Selenium, selenoproteins and cancer of the thyroid. J Trace Elem Med Biol. (2023) 76:127115. doi: 10.1016/j.jtemb.2022.127115
32. Wang F, Li C, Li S, Cui L, Zhao J, and Liao L. Selenium and thyroid diseases. Front Endocrinol (Lausanne). (2023) 14:1133000. doi: 10.3389/fendo.2023.1133000
33. Kawai M, Shoji Y, Onuma S, Etani Y, and Ida S. Thyroid hormone status in patients with severe selenium deficiency. Clin Pediatr Endocrinol. (2018) 27:67–74. doi: 10.1297/cpe.27.67
34. Mancini A, Di Segni C, Raimondo S, Olivieri G, Silvestrini A, Meucci E, et al. Thyroid Hormones, Oxidative Stress, and Inflammation. Mediators Inflammation. (2016) 2016:6757154. doi: 10.1155/2016/6757154
35. Leo M, Bartalena L, Rotondo Dottore G, Piantanida E, Premoli P, Ionni I, et al. Effects of selenium on short-term control of hyperthyroidism due to Graves' disease treated with methimazole: results of a randomized clinical trial. J Endocrinol Invest. (2017) 40:281–7. doi: 10.1007/s40618-016-0559-9
36. Sturniolo G and Mesa J. Selenium supplementation and autoimmune thyroid diseases. Endocrinol Nutr. (2013) 60:423–6. doi: 10.1016/j.endonu.2013.07.001
37. Weyh C, Krüger K, Peeling P, and Castell L. The Role of Minerals in the Optimal Functioning of the Immune System. Nutrients. (2022) 14:644. doi: 10.3390/nu14030644
38. Hoffmann FW, Hashimoto AC, Shafer LA, Dow S, Berry MJ, and Hoffmann PR. Dietary selenium modulates activation and differentiation of CD4+ T cells in mice through a mechanism involving cellular free thiols. J Nutr. (2010) 140:1155–61. doi: 10.3945/jn.109.120725
39. Samuel CG, Singh P, Abdullahi H, and Ibrahim I. Unlocking the Therapeutic Potential: Selenium and Myo-Inositol Supplementation in Thyroid Disorders-Efficacy and Future Directions. Life (Basel Switzerland). (2025) 15:1500. doi: 10.3390/life15101500
40. Wang W, Jiang QL, Xu Q, Zeng Y, Jiang R, and Jiang J. Selenium regulates T cell differentiation in experimental autoimmune thyroiditis in mice. Int Immunopharmacol. (2023) 124:110993. doi: 10.1016/j.intimp.2023.110993
41. Owji N, Moradi F, Khalili MR, and Jahanbani-Ardakani H. Serum Selenium Levels in Patients With Graves Disease With or Without Thyroid Ophthalmopathy. Endocr Pract. (2022) 28:1216–20. doi: 10.1016/j.eprac.2022.09.001
42. Kim TH, Ko J, Kim BR, Shin DY, Lee EJ, and Yoon JS. Serum Selenium Levels in Patients with Graves Disease: Associations with Clinical Activity and Severity in a Retrospective Case-control Study. Korean J Ophthalmol. (2022) 36:36–43. doi: 10.3341/kjo.2021.0146
43. Liu Y, Liu S, Mao J, Piao S, Qin J, Peng S, et al. Serum Trace Elements Profile in Graves' Disease Patients with or without Orbitopathy in Northeast China. BioMed Res Int. (2018) 2018:3029379. doi: 10.1155/2018/3029379
44. Wu Q, Rayman MP, Lv H, Schomburg L, Cui B, Gao C, et al. Low Population Selenium Status Is Associated With Increased Prevalence of Thyroid Disease. J Clin Endocrinol Metab. (2015) 100:4037–47. doi: 10.1210/jc.2015-2222
45. Heidari Z and Sheikhi V. Serum selenium status in Graves' disease and Hashimoto's thyroiditis in an iodine-sufficient area: A case-control study. J Res Med Sci. (2022) 27:87. doi: 10.4103/jrms.jrms_57_21
46. Nordio M and Basciani S. Myo-inositol plus selenium supplementation restores euthyroid state in Hashimoto's patients with subclinical hypothyroidism. Eur Rev Med Pharmacol Sci. (2017) 21:51–9.
47. Pace C, Tumino D, Russo M, Le Moli R, Naselli A, Borzì G, et al. Role of selenium and myo-inositol supplementation on autoimmune thyroiditis progression. Endocrine J. (2020) 67:1093–8. doi: 10.1507/endocrj.EJ20-0062
48. Pirola I, Gandossi E, Agosti B, Delbarba A, and Cappelli C. Selenium supplementation could restore euthyroidism in subclinical hypothyroid patients with autoimmune thyroiditis. Endokrynol Polska. (2016) 67:567–71. doi: 10.5603/EP.2016.0064
49. Zuo Y, Li Y, Gu X, and Lei Z. The correlation between selenium levels and autoimmune thyroid disease: a systematic review and meta-analysis. Ann Palliat Med. (2021) 10:4398–408. doi: 10.21037/apm-21-449
50. Wang Y, Zhao F, Rijntjes E, Wu L, Wu Q, Sui J, et al. Role of Selenium Intake for Risk and Development of Hyperthyroidism. J Clin Endocrinol Metab. (2019) 104:568–80. doi: 10.1210/jc.2018-01713
51. Zong Y, Deng K, and Chong WP. Regulation of Treg cells by cytokine signaling and co-stimulatory molecules. Front Immunol. (2024) 15:1387975. doi: 10.3389/fimmu.2024.1387975
52. Hu Y, Feng W, Chen H, Shi H, Jiang L, Zheng X, et al. Effect of selenium on thyroid autoimmunity and regulatory T cells in patients with Hashimoto's thyroiditis: A prospective randomized-controlled trial. Clin Transl Sci. (2021) 14:1390–402. doi: 10.1111/cts.12993
53. Colin IM, Isaac J, Dupret P, Ledant T, and D'Hautcourt JL. Functional lymphocyte subset assessment of the Th1/Th2 profile in patients with autoimmune thyroiditis by flowcytometric analysis of peripheral lymphocytes. J Biol Regul Homeost Agents. (2004) 18:72–6. doi: 10.1152/japplphysiol.01069.2003
54. Kryczyk-Kozioł J, Prochownik E, Błażewska-Gruszczyk A, Słowiaczek M, Sun Q, Schomburg L, et al. Assessment of the Effect of Selenium Supplementation on Production of Selected Cytokines in Women with Hashimoto's Thyroiditis. Nutrients. (2022) 14:2869. doi: 10.3390/nu14142869
55. Velázquez FE, Anastasiou M, Carrillo-Salinas FJ, Ngwenyama N, Salvador AM, Nevers T, et al. Sialomucin CD43 regulates T helper type 17 cell intercellular adhesion molecule 1 dependent adhesion, apical migration and transendothelial migration. Immunology. (2019) 157:52–69. doi: 10.1111/imm.13047
56. Konca Degertekin C, Aktas Yilmaz B, Balos Toruner F, Kalkanci A, Turhan Iyidir O, Fidan I, et al. Circulating Th17 cytokine levels are altered in Hashimoto's thyroiditis. Cytokine. (2016) 80:13–7. doi: 10.1016/j.cyto.2016.02.011
57. Pleić N, Babić Leko M, Gunjača I, Zemunik T, and Vitamin D. and thyroid function: A mendelian randomization study. PloS One. (2024) 19:e0304253. doi: 10.1371/journal.pone.0304253
58. Palanca A, Ampudia-Blasco FJ, and Real JT. The Controversial Role of Vitamin D in Thyroid Cancer Prevention. Nutrients. (2022) 14:2593. doi: 10.3390/nu14132593
59. Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. (2014) 21:319–29. doi: 10.1016/j.chembiol.2013.12.016
60. Gallo D, Mortara L, Gariboldi MB, Cattaneo SAM, Rosetti S, Gentile L, et al. Immunomodulatory effect of vitamin D and its potential role in the prevention and treatment of thyroid autoimmunity: a narrative review. J Endocrinol Invest. (2020) 43:413–29. doi: 10.1007/s40618-019-01123-5
61. Modi M and Garg P. Relationship between thyroid-stimulating hormone levels and the severity of vitamin D deficiency by age group. Clin Exp Reprod Med. (2025) 52:71–8. doi: 10.5653/cerm.2023.06779
62. Geng J, Qiu Y, Li Y, Li J, Liao R, Du H, et al. Associations Between 25-Hydroxyvitamin D, Kidney Function, and Insulin Resistance Among Adults in the United States of America. Front Nutr. (2021) 8:716878. doi: 10.3389/fnut.2021.716878
63. Charoenngam N and Holick MF. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients. (2020) 12:2097. doi: 10.3390/nu12072097
64. Yu Z, Cheng H, Liang Y, Ding T, Yan C, Gao C, et al. Decreased Serum 25-(OH)-D Level Associated With Muscle Enzyme and Myositis Specific Autoantibodies in Patients With Idiopathic Inflammatory Myopathy. Front Immunol. (2021) 12:642070. doi: 10.3389/fimmu.2021.642070
65. Durá-Travé T and Gallinas-Victoriano F. Autoimmune Thyroiditis and Vitamin D. Int J Mol Sci. (2024) 25:3154. doi: 10.3390/ijms25063154
66. Mazokopakis EE and Kotsiris DA. Hashimoto's autoimmune thyroiditis and vitamin D deficiency. Curr Aspects Hell J Nucl Med. (2014) 17:37–40. doi: 10.4149/BLL_2023_029
67. Filipova L, Lazurova Z, Fulop P, and Lazurova I. Vitamin D insufficiency is not associated with thyroid autoimmunity in Slovak women with Hashimoto´s disease. Bratisl Lek Listy. (2023) 124:182–6. doi: 10.4149/BLL_2023_029
68. Chao G, Zhu Y, and Fang L. Correlation Between Hashimoto's Thyroiditis-Related Thyroid Hormone Levels and 25-Hydroxyvitamin D. Front Endocrinol (Lausanne). (2020) 11:4. doi: 10.3389/fendo.2020.00004
69. Wang J, Lv S, Chen G, Gao C, He J, Zhong H, et al. Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients. (2015) 7:2485–98. doi: 10.3390/nu7042485
70. Kivity S, Agmon-Levin N, Zisappl M, Shapira Y, Nagy EV, Dankó K, et al. and autoimmune thyroid diseases. Cell Mol Immunol. (2011) 8:243–7. doi: 10.1038/cmi.2010.73
71. Skrobot A, Demkow U, and Wachowska M. Immunomodulatory Role of Vitamin D: A Review. Adv Exp Med Biol. (2018) 1108:13–23. doi: 10.1007/5584_2018_246
72. Wang Z, Chen L, Ma Y, Li X, Hu A, Wang H, et al. Peptide vaccine-conjugated mesoporous carriers synergize with immunogenic cell death and PD-L1 blockade for amplified immunotherapy of metastatic spinal. J Nanobiotechnol. (2021) 19:243. doi: 10.1186/s12951-021-00975-5
73. Miteva MZ, Nonchev BI, Orbetzova MM, Stoencheva SD, and Vitamin D. and Autoimmune Thyroid Diseases - a Review. Folia Med (Plovdiv). (2020) 62:223–9. doi: 10.3897/folmed.62.e47794
74. Zhao R, Zhang W, Ma C, Zhao Y, Xiong R, Wang H, et al. Immunomodulatory Function of Vitamin D and Its Role in Autoimmune Thyroid Disease. Front Immunol. (2021) 12:574967. doi: 10.3389/fimmu.2021.574967
75. Gorini F, Tonacci A, and Vitamin D. An Essential Nutrient in the Dual Relationship between Autoimmune Thyroid Diseases and Celiac Disease-A Comprehensive Review. Nutrients. (2024) 16:1762. doi: 10.3390/nu16111762
76. Baltaci AK, Mogulkoc R, and Baltaci SB. Review: The role of zinc in the endocrine system. Pak J Pharm Sci. (2019) 32:231–9.
77. Gustin K, Vahter M, Barman M, Jacobsson B, Skröder H, Filipsson Nyström H, et al. Assessment of Joint Impact of Iodine, Selenium, and Zinc Status on Women's Third-Trimester Plasma Thyroid Hormone Concentrations. J Nutr. (2022) 152:1737–46. doi: 10.1093/jn/nxac081
78. Lossow K, Renko K, Schwarz M, Schomburg L, Schwerdtle T, and Kipp AP. The Nutritional Supply of Iodine and Selenium Affects Thyroid Hormone Axis Related Endpoints in Mice. Nutrients. (2021) 13:3773. doi: 10.3390/nu13113773
79. Betsy A, Binitha M, and Sarita S. Zinc deficiency associated with hypothyroidism: an overlooked cause of severe alopecia. Int J Trichol. (2013) 5:40–2. doi: 10.4103/0974-7753.114714
80. Costa MI, Sarmento-Ribeiro AB, and Gonçalves AC. Zinc: From Biological Functions to Therapeutic Potential. Int J Mol Sci. (2023) 24:4822. doi: 10.3390/ijms24054822
81. Severo JS, Morais JBS, de Freitas TEC, Andrade ALP, Feitosa MM, Fontenelle LC, et al. The Role of Zinc in Thyroid Hormones Metabolism. Int J Vitam Nutr Res. (2019) 89:80–8. doi: 10.1024/0300-9831/a000262
82. Beserra JB, Morais JBS, Severo JS, Cruz KJC, De Oliveira ARS, Henriques GS, et al. Relation Between Zinc and Thyroid Hormones in Humans: a Systematic Review. Biol Trace Elem Res. (2021) 199:4092–100. doi: 10.1007/s12011-020-02562-5
83. Ertek S, Cicero AF, Caglar O, and Erdogan G. Relationship between serum zinc levels, thyroid hormones and thyroid volume following successful iodine supplementation. Hormones (Athens). (2010) 9:263–8. doi: 10.14310/horm.2002.1276
84. Szczepanik J, Podgórski T, and Domaszewska K. The Level of Zinc, Copper and Antioxidant Status in the Blood Serum of Women with Hashimoto's Thyroiditis. Int J Environ Res Public Health. (2021) 18:7805. doi: 10.3390/ijerph18157805
85. Vargas-Uricoechea H, Urrego-Noguera K, Vargas-Sierra H, and Pinzón-Fernández M. Zinc and Ferritin Levels and Their Associations with Functional Disorders and/or Thyroid Autoimmunity: A Population-Based Case-Control Study. Int J Mol Sci. (2024) 25:10217. doi: 10.3390/ijms251810217
86. Köhrle J. Selenium, Iodine and Iron-Essential Trace Elements for Thyroid Hormone Synthesis and Metabolism. Int J Mol Sci. (2023) 24:3393. doi: 10.3390/ijms24043393
87. Gierach M, Rudewicz M, and Junik R. Iron and ferritin deficiency in women with hypothyroidism and chronic lymphocytic thyroiditis - systematic review. Endokrynol Pol. (2024) 75:253–61. doi: 10.5603/ep.97860
88. Parsaei M, Dashtkoohi M, Amirkhalili E, Chashmyazdan M, Korevaar TIM, and Nazeri P. Association of iron status indicators with thyroid hormone concentrations during pregnancy: a systematic review and meta-analysis. Front Endocrinol (Lausanne). (2025) 16:1533169. doi: 10.3389/fendo.2025.1533169
89. Wang F, Zhang Y, Yuan Z, Li Y, Liu S, Zeng X, et al. The association between iron status and thyroid hormone levels during pregnancy. J Trace Elem Med Biol. (2022) 74:127047. doi: 10.1016/j.jtemb.2022.127047
90. Zimmermann MB, Zeder C, Chaouki N, Saad A, Torresani T, and Hurrell RF. Dual fortification of salt with iodine and microencapsulated iron: a randomized, double-blind, controlled trial in Moroccan schoolchildren. Am J Clin Nutr. (2003) 77:425–32. doi: 10.1093/ajcn/77.2.425
91. Rayman MP. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc Nutr Soc. (2019) 78:34–44. doi: 10.1017/S0029665118001192
92. Kravchenko V and Zakharchenko T. Thyroid hormones and minerals in immunocorrection of disorders in autoimmune thyroid diseases. Front Endocrinol (Lausanne). (2023) 14:1225494. doi: 10.3389/fendo.2023.1225494
93. Kazi TG, Kandhro GA, Afridi HI, Kazi N, Baig JA, Arain MB, et al. Interaction of copper with iron, iodine, and thyroid hormone status in goitrous patients. Biol Trace Elem Res. (2010) 134:265–79. doi: 10.1007/s12011-009-8478-7
94. Mittag J, Behrends T, Nordström K, Anselmo J, Vennström B, and Schomburg L. Serum copper as a novel biomarker for resistance to thyroid hormone. Biochem J. (2012) 443:103–9. doi: 10.1042/BJ20111817
95. Kim MJ, Kim SC, Chung S, Kim S, Yoon JW, and Park YJ. Exploring the role of copper and selenium in the maintenance of normal thyroid function among healthy Koreans. J Trace Elem Med Biol. (2020) 61:126558. doi: 10.1016/j.jtemb.2020.126558
96. Rasic-Milutinovic Z, Jovanovic D, Bogdanovic G, Trifunovic J, and Mutic J. Potential Influence of Selenium, Copper, Zinc and Cadmium on L-Thyroxine Substitution in Patients with Hashimoto Thyroiditis and Hypothyroidism. Exp Clin Endocrinol Diabetes. (2017) 125:79–85. doi: 10.1055/s-0042-116070
97. Mehl S, Sun Q, Görlich CL, Hackler J, Kopp JF, Renko K, et al. Cross-sectional analysis of trace element status in thyroid disease. J Trace Elem Med Biol. (2020) 58:126430. doi: 10.1016/j.jtemb.2019.126430
98. Shen F, Cai WS, Li JL, Feng Z, Cao J, and Xu B. The Association Between Serum Levels of Selenium, Copper, and Magnesium with Thyroid Cancer: a Meta-analysis. Biol Trace Elem Res. (2015) 167:225–35. doi: 10.1007/s12011-015-0304-9
99. Wang K, Wei H, Zhang W, Li Z, Ding L, Yu T, et al. Severely low serum magnesium is associated with increased risks of positive anti-thyroglobulin antibody and hypothyroidism: A cross-sectional study. Sci Rep. (2018) 8:9904. doi: 10.1038/s41598-018-28362-5
100. Wang LF, Sun RX, Li CF, and Wang XH. The effects of selenium supplementation on antibody titres in patients with Hashimoto's thyroiditis. Endokrynol Polska. (2021) 72:666–7. doi: 10.5603/EP.a2021.0074
101. Calissendorff J, Mikulski E, Larsen EH, and Möller M. A Prospective Investigation of Graves' Disease and Selenium: Thyroid Hormones, Auto-Antibodies and Self-Rated Symptoms. Eur Thyroid J. (2015) 4:93–8. doi: 10.1159/000381768
102. Kieliszek M and Błażejak S. Current Knowledge on the Importance of Selenium in Food for Living Organisms: A Review. Mol (Basel Switzerland). (2016) 21:609. doi: 10.3390/molecules21050609
Keywords: trace elements, micronutrients, immune regulation, autoimmune thyroid disease, hormones
Citation: Li S, Xu Q, Wang S, Peng H and Liu Y (2025) Recent advances of trace elements in autoimmune thyroid disease. Front. Immunol. 16:1662521. doi: 10.3389/fimmu.2025.1662521
Received: 09 July 2025; Accepted: 12 November 2025; Revised: 04 November 2025;
Published: 26 November 2025.
Edited by:
Dieter Kabelitz, University of Kiel, GermanyReviewed by:
George Paltoglou, National and Kapodistrian University of Athens, GreeceAnita Vergatti, University of Naples Federico II, Italy
Copyright © 2025 Li, Xu, Wang, Peng and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huiyong Peng, cGVuZ2h1aXlvbmczMzgxNUAxNjMuY29t; Yingzhao Liu, empsaXV5aW5nemhhb0AxMjYuY29t
 Shanshan Li
Shanshan Li Qian Xu
Qian Xu Shengjun Wang
Shengjun Wang Huiyong Peng
Huiyong Peng Yingzhao Liu
Yingzhao Liu