- Department of Orthopedics Surgery, Shengjing Hospital of China Medical University, Shenyang, China
Neurotrophic factors, once considered exclusive guardians of neuronal integrity, are increasingly recognised as pivotal regulators of osteosarcoma biology. Their paradoxical enhancement of malignant fitness and an immunosuppressive microenvironment complicates therapy, with metastatic survival remaining stubbornly low. Recent mechanistic studies reveal that ligand-dependent NGF–TrkA, BDNF–TrkB and GDNF–RET circuits intersect with MEK/ERK, PI3K/AKT and STAT3 pathways to ignite proliferation, invasion and metastatic spread. Concurrently, neurotrophin signalling recalibrates macrophage polarity, dampens cytotoxic T-cell function and orchestrates neural-immune feedback loops that shield tumours from surveillance. Harnessing this duality demands an integrative strategy. We synthesise tumour-intrinsic and extrinsic neurotrophic axes, delineate neuro-immune crosstalk, and highlight interventions—TRK/RET inhibitors, CSF1R blockade, β-adrenergic antagonists—aimed at converting this liability into therapeutic leverage. By framing neurotrophic factors as double-edged swords, this review provides a conceptual and practical roadmap for exploiting their vulnerabilities to improve outcomes in osteosarcoma.
1 Introduction
Osteosarcoma is the most prevalent primary malignant bone tumour in the paediatric and adolescent population, with a global incidence of roughly 3–5 cases per million each year and a sharp rise during periods of rapid skeletal growth (1–3). Despite multimodal refinements, five-year survival remains ~60–70% (localised) and <30% (metastatic) (4–6). This therapeutic plateau underscores the need for fresh biological insights that can guide innovative treatment strategies.
Classically, the neurotrophin family—NGF, BDNF, NT−3, NT−4/5 and GDNF—governs neuronal survival, axonal guidance and synaptic plasticity (7–9). These proteins signal via high−affinity TrkA/B/C isoforms, the low−affinity p75NTR, and RET–GFRα co−receptor complexes (10, 11). Recent transcriptomic and proteomic surveys of bone tumours have revealed that genes encoding both neurotrophins and their cognate receptors are expressed beyond the nervous system, prompting investigation into their oncological relevance (12, 13).
Accumulating evidence indicates that neurotrophic signalling has tumour-intrinsic consequences in osteosarcoma. HIF-1α drives TrkB transcription in U2OS cells, indicating that the osteogenic niche—defined by hypoxia, high extracellular calcium and constant mechanical remodelling—favours neurotrophin responsiveness via Ca2+-dependent CaMKIV/CREB and calcineurin–NFAT activity, while load-sensing integrin–FAK–YAP/TAZ signalling further primes TrkA/TrkB transcription under metabolic stress (14–16). Furthermore, activation of the BDNF–TrkB axis has been correlated with enhanced proliferation, resistance to apoptosis, and invasive behaviour in multiple malignancies, and similar molecular programmes have been described in experimental osteosarcoma systems (17, 18).
Neurotrophic factors also exert profound effects on non-malignant stromal and immune components. BDNF can skew macrophage polarisation toward an immunoregulatory M2 phenotype and modulate cytokine production, changes that are conducive to tumour immune evasion (19, 20). More broadly, neurotrophin-mediated cross-talk between peripheral nerves and immune cells has been implicated in shaping the inflammatory milieu of several solid tumours, a concept that is increasingly explored in bone sarcomas (21–23).
These observations highlight the context-dependent, bifunctional behaviour of neurotrophic factors in osteosarcoma: they can directly enhance malignant cell fitness while simultaneously reprogramming the host immune landscape. Deciphering this duality is therefore critical for the rational deployment of targeted and immunomodulatory therapies. We define tumour−intrinsic edges as neurotrophin actions within cancer cells and tumour−extrinsic edges as effects on immune or stromal cells; this review accordingly addresses (1) intrinsic drivers, (2) extrinsic modulation, and (3) neuro−immune feedback. By integrating these dimensions, we aim to identify therapeutic vulnerabilities that may convert this biological liability into a clinical opportunity. We searched PubMed and ClinicalTrials.gov (2010-June 2025) using ‘osteosarcoma’, ‘neurotrophin/NGF/BDNF/RET/Trk’, and ‘immunity’; English; prioritised primary OS data then mechanistic studies in other tumours; excluded reviews/abstract-only reports; key outcomes included pathway activity, immune metrics, and clinical responses.
2 Tumour-intrinsic edge: neurotrophic drivers of osteosarcoma growth, survival and metastasis
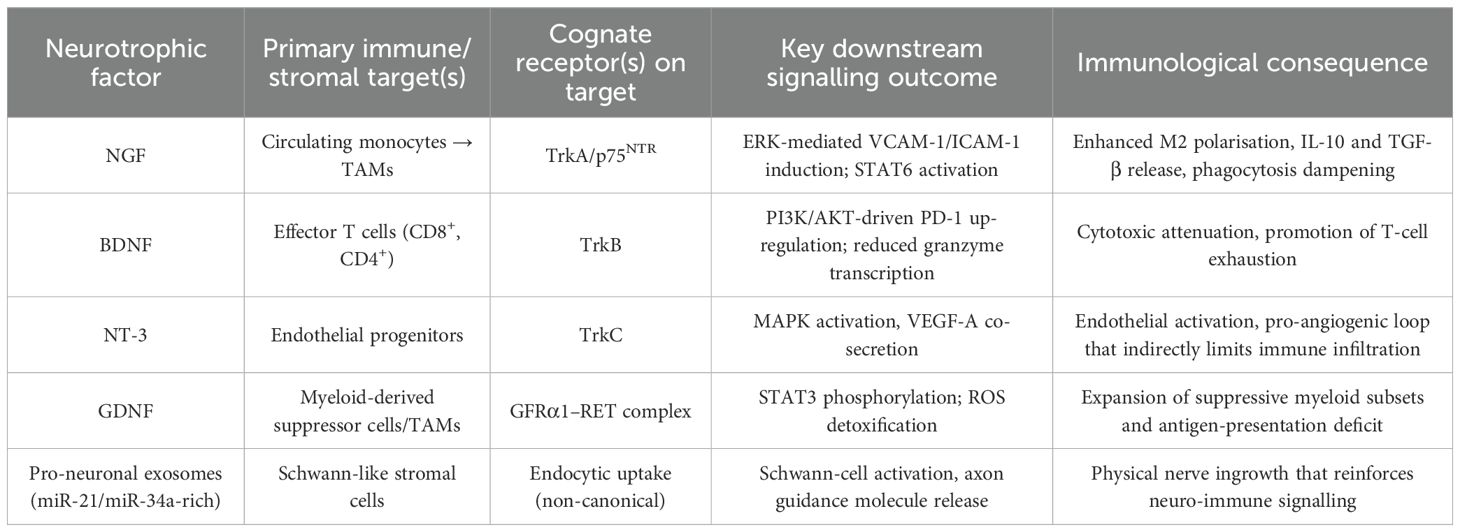
Large-scale interrogation of TARGET-OS and tissue micro-arrays shows osteosarcoma cells actively express neurotrophic ligands and cognate receptors (24, 25). NGF–TrkA shows highest expression; BDNF–TrkB and GDNF–GFRα/RET are variable, whereas NT−3/TrkC is low in osteosarcoma but prominent in other bone sarcomas, hence our emphasis (26–28). Expression patterns correlate with clinicopathological variables such as stage and metastatic propensity, indicating biological rather than incidental relevance.
The NGF–TrkA axis exemplifies an autocrine–paracrine circuit that reinforces malignant fitness. NGF stimulation in 143B and MG-63 cells triggers rapid MEK/ERK phosphorylation followed by transcriptional up-regulation of MMP-2, thereby accelerating wound closure, Transwell invasion and experimental lung colonisation (29, 30). Pharmacologic Trk blockade or MEK/ERK silencing abrogates these effects in vitro and reduces metastatic burden in orthotopic xenografts, underscoring pathway druggability (31, 32). Mechanistically, NGF lowers miR-92a-1-5p, lifting repression of MMP-2 and creating a feed-forward loop that promotes extracellular-matrix remodelling (33, 34). Such findings extend earlier observations that hypoxia inducible factors maintain TrkA transcription under metabolic stress and suggest that NGF signalling provides a selectable advantage in the poorly vascularised bone niche.
Although expressed at lower levels, BDNF-TrkB signalling confers distinct survival benefits. Epithelial models show TrkB-driven anoikis resistance via PI3K/AKT; osteosarcoma evidence remains preclinical, with similar signatures in cell lines and xenografts (35, 36). Osteosarcoma cells appear to reuse this programme: exogenous BDNF enhances clonogenicity, mitigates chemotherapy-induced apoptosis and promotes a spindle-shaped, vimentin-positive phenotype, features that collectively align with heightened metastatic risk (37, 38). Down-stream, TrkB engages PI3K/AKT, PLCγ/PKC and, in nutrient-limited settings, STAT3, integrating prosurvival and metabolic rewiring (39, 40). The plasticity of this circuitry rationalises investigations combining conventional cytotoxics with ATP-competitive TRK inhibitors or AKT antagonists.
The GDNF–GFRα1–RET module adds another layer of complexity. Osteosarcoma sub-clones with elevated GFRα1 expression demonstrate increased motility, drug tolerance and mesenchymal marker expression; conversely, GFRα1 interference restores chemosensitivity (41, 42). Soluble GFRα1, released by adjacent Schwann cells, can activate RET in trans, linking perineural niche signalling to malignant dissemination, a phenomenon already recognised in pancreas and prostate cancer (43, 44). RET activation funnels into the MAPK and JAK/STAT cascades, converging on transcriptional regulators that control cell-cycle progression and oxidative-stress resilience, characteristics advantageous for survival in circulation and at secondary sites.
Genomic alterations further amplify neurotrophic signalling. Oncogenic NTRK1/2/3 fusions—often retaining an active kinase domain—are documented but rare in osteosarcoma cohorts. These lesions confer exquisite sensitivity to first-line TRK inhibitors such as larotrectinib and repotrectinib, mirroring tissue-agnostic responses observed across solid malignancies (45, 46). The occurrence of such fusions, together with ligand-dependent activation described above, positions neurotrophin-RTK signalling as a central vulnerability that can be exploited both genomically and pharmacodynamically.
Taken together, NGF-TrkA, BDNF-TrkB and GDNF–RET circuits endow osteosarcoma cells with proliferative, anti-apoptotic and pro-metastatic properties. Convergence on MEK/ERK, PI3K/AKT/mTOR and JAK/STAT modules offers multiple pharmacological checkpoints, while the demonstrable efficacy of TRK inhibitors in fusion-positive disease highlights the clinical translational potential.
3 Tumour-extrinsic edge: neurotrophic remodelling of the osteosarcoma immune microenvironment
The cellular and acellular constituents of the osteosarcoma milieu are highly sensitive to neurotrophic cues that originate from malignant cells, infiltrating nerves and, to a lesser extent, resident stromal elements (47, 48). Single-cell deconvolution of primary tumours, coupled with spatial omics, reveals TrkA/TrkB/RET programmes in TAMs, dendritic and endothelial cells and maps their adjacency to nerve fibres and malignant cells, indicating that the immune landscape is intrinsically wired to perceive nerve-derived growth factors (49, 50). Functionally, NGF released by osteosarcoma cells up-regulates ICAM-1/VCAM-1 on circulating monocytes, accelerates extravasation, and skews differentiation toward an M2 phenotype via TrkA–ERK signalling (51, 52). BDNF–TrkB engagement on CD8+ T cells (shown in other tumours) dampens effector cytokines and increases PD-1 via PI3K/AKT-dependent re-programming; OS data are limited (53, 54). GDNF signalling through the GFRα1/RET complex in myeloid cells activates STAT3, enhances IL-10 secretion and impairs antigen presentation, a constellation that favours immune escape (55, 56). These observations mirror broader oncological evidence that neurotrophic factors orchestrate a permissive, low-immunogenic niche by synchronising axonogenesis with immune suppression. The principal mechanisms operative in osteosarcoma are summarised in Table 1.
Neurotrophin-responsive immune cells constitute a feed-forward circuit in which axonal infiltration, cytokine skewing and checkpoint induction converge to insulate osteosarcoma from effective immunosurveillance. Because PD-1/PD-L1 monotherapy yields <10% responses in relapsed osteosarcoma, interrupting this circuit via TRK/RET inhibition or macrophage re-education may unlock checkpoint synergy.
4 Bidirectional crosstalk: feedback loops linking neurotrophic signalling and immunity
Emerging evidence indicates that neurotrophins and immune mediators in osteosarcoma engage in tightly inter-connected positive feedback loops that reinforce both malignant cell fitness and micro-environmental immune suppression (57, 58). Osteosarcoma cells secrete NGF, BDNF and related cues that attract peripheral nerves and condition infiltrating leukocytes; reciprocally, cytokines and chemokines released by those stromal elements amplify neurotrophic signalling, thereby establishing a self-perpetuating circuit rather than a unidirectional pathway (59, 60). Dense axonal ingrowth driven by tumour-derived NGF has been associated with increased PD-1 expression on local T lymphocytes and a rise in M2-polarised macrophages, illustrating how neuronal inputs synchronise with immune checkpoints to maintain an immunosuppressive milieu.
As shown in Figure 1, cytokine induction of neurotrophins represents a first tier of reciprocity. Interleukin−6 from Schwann−like stromal cells or M2 macrophages activates STAT3, which partners with phosphorylated TrkB at BDNF enhancers, forming a feed−forward loop that amplifies neurotrophin output and tumour aggressiveness (61, 62). Similar IL-1β and TNF-α inputs have been reported to modulate NGF and BDNF levels in bone-associated tumours, suggesting a broader principle whereby inflammatory mediators act as upstream rheostats of neurotrophic output.
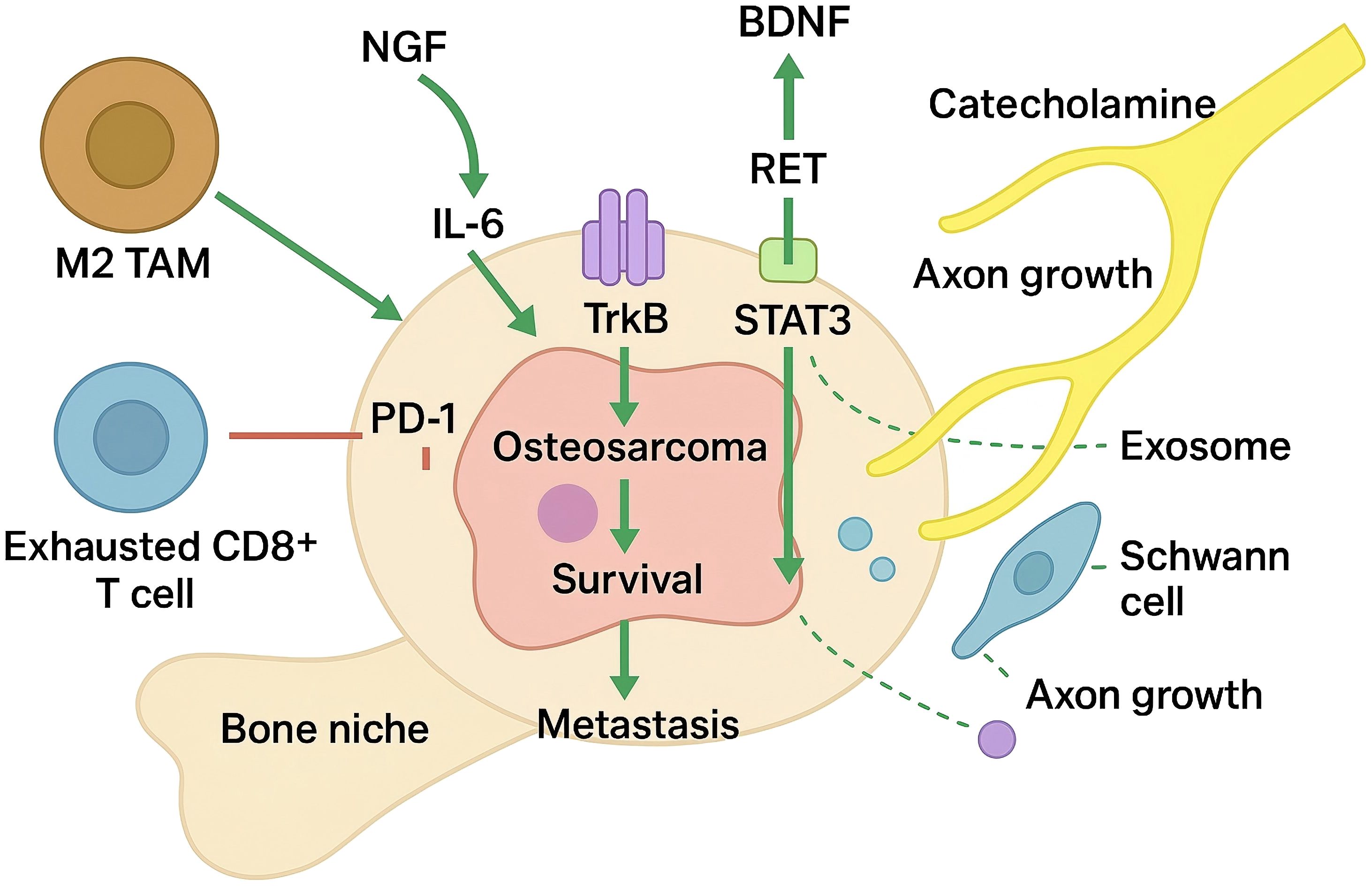
Figure 1. Neurotrophic factor-driven neuro-immune feed-forward loops in the osteosarcoma bone niche.
A second tier comprises neurotrophin-driven chemokine gradients that reinforce immune cell influx. BDNF/TrkB activation in solid tumours triggers JNK-dependent CCL2, recruiting CCR2+ monocytes that become TAMs and secrete IL-10, TGF-β and IL-6, sustaining TrkB phosphorylation; OS validation is pending (63, 64). Psychological stress elevates norepinephrine/epinephrine that engage β2-adrenergic receptors (ADRB2) on tumour and immune cells, raising cAMP to activate PKA and Epac→Rap1/JNK: PKA-phospho-CREB transactivates BDNF promoter IV, while JNK/AP-1 and STAT3 signalling increase CD274 (PD-L1) transcription; ADRB2 blockade (e.g., propranolol) reverses these changes and attenuates CCL2-mediated myeloid influx.
Macrophage‐derived NGF adds an additional layer of amplification. In the osteogenic bone niche, tumour-conditioned macrophages and osteoclast precursors can synthesise NGF, which in turn enhances ICAM-1-mediated monocyte adhesion and supports further macrophage infiltration (65, 66). The ensuing rise in local NGF concentration potentiates TrkA signalling in both malignant cells and nerve terminals, escalating axonal density and reinforcing macrophage tropism in a feed-forward manner.
Extracellular vesicles act as mobile amplifiers within these circuits. Osteosarcoma-derived exosomes enriched in miR-21 and miR-34a—often accompanied by miR-181a/miR-222/miR-146a—activate Schwann cells (via ERK–c-Jun) to secrete guidance cues and proNGF/GFRα1, stimulating neurite extension; in immune cells, exosomal miR-21 targets PTEN/PDCD4 and miR-146a modulates TRAF6–NF-κB to skew TAMs toward suppressive states, while Schwann-cell CCL2/CXCL5 recruits CCR2+ myeloid populations that stabilise the neuro-immune niche (67, 68). These multilayered feedback loops integrate neuronal, immune and malignant compartments into a cohesive signalling circuit that simultaneously accelerates osteosarcoma progression and dampens effective antitumour immunity. Therapeutic disruption of any single node—such as β-adrenergic blockade, Trk or RET inhibition, STAT3 antagonism, or CCL2/CCR2 axis interference—may therefore propagate inhibitory effects throughout the entire network, offering a rationale for combinatorial strategies that target both neurotrophic and immunological facets of the disease.
5 Harnessing neurotrophic duality: integrative therapies targeting tumour growth and immune remodelling
Pharmacological blockade of neurotrophin receptors now constitutes the most direct strategy to exploit the tumour-intrinsic arm of the NGF–TrkA/BDNF–TrkB axis while simultaneously attenuating the immunoregulatory feedback loops described above (69, 70). Larotrectinib, an ATP-competitive inhibitor with nanomolar affinity for all Trk isoforms, curtailed orthotopic osteosarcoma expansion and abolished experimental lung metastases in a murine model driven by NGF over-expression, an effect accompanied by reduced MEK/ERK activity and restoration of miR-92a-1-5p levels (71, 72). Although approvals are fusion-agnostic, ligand-dependent OS may also benefit; this remains hypothesis-generating and should be tested with pharmacodynamic readouts (e.g., p-TRK/RET) while accounting for niche modifiers (hypoxia, Ca2+, YAP/TAZ) (73, 74). RET-selective inhibitors such as selpercatinib have demonstrated durable responses in solid tumours bearing RET rearrangements; pre-screening of relapsed osteosarcoma for rarer NTRK or RET fusions therefore represents a rational enrichment strategy for precision trials that marry cytostatic and immunomodulatory endpoints.
Targeting neurotrophin-conditioned myeloid compartments is equally critical. In vivo administration of the CSF1R inhibitor pexidartinib (PLX3397) depleted M2-polarised macrophages, increased intratumoural CD8+ T-cell density and suppressed both primary tumour growth and pulmonary dissemination in orthotopic and patient-derived xenografts of osteosarcoma (75, 76). CSF1R blockade de-phosphorylates ERK and flips ARG1+ M2 macrophages to iNOS+ M1 via STAT6 loss, restoring MHC-II, CD86 and IL-12, enhancing cross-presentation to CD8+ T cells and thereby potentiating PD-1/PD-L1 antibodies in addition to reinforcing TRK inhibition through MAPK suppression and increased phagocytosis (77, 78). Given that NGF and BDNF transcription correlate positively with CSF1 expression in bulk RNA-seq datasets, sequential or combined Trk/CSF1R inhibition could interrupt two nodes of the same positive-feedback circuit and warrants formal evaluation.
Stress-responsive β-adrenergic signalling represents a third actionable layer linking neural inputs to immune suppression. Non-selective β-blockade with propranolol reduced osteosarcoma proliferation, impaired angiogenesis and potentiated low-dose cisplatin in xenograft models. Beyond cytostasis, propranolol enhanced T-cell infiltration and lowered myeloid-derived suppressor cell burden in syngeneic soft-tissue sarcoma, thereby amplifying the efficacy of anti-CTLA-4 therapy without additive toxicity (79, 80). Because catecholamine release is heightened by tumour-induced neo-innervation, β-adrenergic blockade may act upstream of the neurotrophin–immune loop, providing a low-cost adjunct to both kinase inhibition and checkpoint blockade.
These observations argue for multi-axis regimens (hypothesis-generating) that attenuate neurotrophin receptors, re-educate macrophages and dampen sympathetic inputs. Proposed sequence (for testing): molecular screening; short-course TRK/RET inhibition to debulk; add CSF1R blockade to shift macrophages; consider β-blockade plus PD-1/PD-L1 for maintenance; monitor neuropathy, hepatic AEs, QT, and paediatric growth-plate changes. Pharmacodynamic read-outs—p-TRK/RET, M2/M1 ratios, intratumoural catecholamines, IFN-γ signatures—should guide early-phase trial ordering and dosing. OS cohorts report low PD-1/PD-L1 monotherapy responses, rare NTRK/RET fusions, and ongoing OS-relevant trials testing TRK/RET inhibitors, CSF1R agents, and β-blockers with immunotherapy. The availability of paediatric-friendly formulations of larotrectinib and selpercatinib, the manageable safety profile of propranolol, and emerging oral CSF1R inhibitors create a realistic path toward combination protocols that convert neurotrophic duality from a liability into a therapeutic lever.
6 Translational outlook: targeting the double-edged sword for therapeutic gain
Understanding that neurotrophic factors can simultaneously accelerate malignant growth and subvert antitumour immunity places their signalling nodes among the most attractive, yet challenging, translational targets in osteosarcoma. The first prerequisite for clinical progress is molecular stratification that distinguishes tumours driven by ligand-dependent NGF-TrkA, BDNF-TrkB or GDNF–RET loops from those harbouring activating rearrangements or kinase-domain point mutations. Immunohistochemistry, RNA-seq–derived expression scores and DNA-based fusion panels are already feasible in routine pathology and should be embedded prospectively in early-phase studies to enrich for pharmacologically tractable subsets and to provide correlative datasets linking pathway dependence with immunological contexture (60, 65).
Drug-development pipelines are advancing beyond first-generation ATP-competitive inhibitors. Next−gen agents such as selitrectinib and zurletrectinib remain active against xDFG and solvent−front (G595R, G667C) TRK mutations while retaining CNS penetration (72, 77). Parallel efforts are refining highly selective RET inhibitors with favourable paediatric safety profiles and negligible off-target VEGFR blockade, a characteristic that may attenuate dose-limiting hypertension and thrombotic events often observed with multikinase agents (41). Adaptive designs with real-time PD (p-TRK/RET suppression, circulating neurotrophins, TAM polarisation) are recommended.
Translational leverage will increase further when kinase inhibition is combined with rational immunomodulation. CSF1R blockade remodels macrophage composition, reduces IL-10/TGF-β output and indirectly attenuates Trk-driven ERK activity in malignant cells, providing a mechanistic basis for dual CSF1R–TRK schedules (46). β-adrenergic antagonists diminish catecholamine-induced BDNF up-regulation and enhance CD8+ T-cell infiltration, making them logical, low-toxicity partners for neurotrophin-axis inhibitors or for anti-PD-1/PD-L1 antibodies once target engagement has curtailed tumour-intrinsic growth signals (18, 29). Because STAT3 integrates both neurotrophic and cytokine cues, early introduction of selective STAT3 degraders might suppress parallel survival pathways and blunt feedback re-activation observed with single-agent kinase therapy (70). Selecting the optimal sequence—kinase inhibition to debulk disease, macrophage re-education to disarm immune suppression, checkpoint blockade to sustain cytotoxic surveillance—will likely require window-of-opportunity trials with paired biopsies and multiplex spatial profiling.
Heterogeneity of neuro-immune coupling mandates preclinical test systems that faithfully recapitulate bone, neural and immune compartments. Three-dimensional tri-culture platforms and biomimetic scaffolds permit interrogation of neurite outgrowth, macrophage plasticity and T-cell trafficking under defined gradients of NGF, BDNF and catecholamines (58). Integration of single-cell and spatial transcriptomics from primary tumours and matched patient-derived xenografts exposes micro-niches in which neurotrophic signalling is dominant and predicts regional variability in drug response (9, 48). Such datasets will be invaluable for constructing computational models that nominate combination regimens and identify early biomarkers of benefit or resistance.
Toxicity management must evolve in parallel with therapeutic complexity. Careful neuro−cognitive and growth−plate monitoring is essential because paediatric TRK or RET inhibitor trials report mostly grade−1/2 neuropathy, transient liver enzyme rise, weight gain, physeal thickening and occasional hypertension—usually reversible with dose adjustment (5, 26). Off-tumour macrophage depletion, catecholamine withdrawal and cytokine shifts will necessitate longitudinal immune monitoring to pre-empt opportunistic infections or auto-inflammatory sequelae.
These considerations delineate a roadmap in which precise molecular selection, next-generation kinase inhibitors, and context-specific immunomodulators converge to neutralise both arms of the neurotrophic double-edged sword. Successful implementation will hinge on integrative trial designs that capture pharmacodynamic, immunological and functional imaging endpoints, enabling rapid iteration toward durable, low-toxicity control of osteosarcoma.
Author contributions
PL: Conceptualization, Data curation, Visualization, Writing – original draft. LL: Conceptualization, Funding acquisition, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by Liaoning Provincial Department of Science and Technology (Grant No. 2022-42).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Liu P, Lv H, Li Y, Liu Z, Yang X, Hu H, et al. Global bone cancer incidence and death rate analysis at 40 years. Discov Oncol. (2025) 16:1087. doi: 10.1007/s12672-025-02917-1
2. Yang J, Lou S, and Yao T. Trends in primary Malignant bone cancer incidence and mortality in the United States, 2000–2017: A population-based study. J Bone Oncol. (2024) 46:100607. doi: 10.1016/j.jbo.2024.100607
3. Boyland R, Amin S, Shostrom V, Zheng C, Allison J, Lin C, et al. Comparison of overall survival of adult and pediatric osteosarcoma patients using the national cancer database. BMC Cancer. (2025) 25:290. doi: 10.1186/s12885-025-13496-3
4. Feng L, Chen Y, Ye T, Shao Z, Ye C, Chen J, et al. Development and validation of an online prognostic nomogram for osteosarcoma after surgery: a retrospective study based on the SEER database and external validation with single-center data. Trans Cancer Res. (2022) 11:3156. doi: 10.21037/tcr-21-2756
5. Hou CH, Chen WL, and Lin CY. Targeting nerve growth factor-mediated osteosarcoma metastasis: mechanistic insights and therapeutic opportunities using larotrectinib. Cell Death Dis. (2024) 15:381. doi: 10.1038/s41419-024-06752-0
6. Kot EF, Goncharuk SA, Franco ML, McKenzie DM, Arseniev AS, Benito-Martínez A, et al. Structural basis for the transmembrane signalling and antidepressant-induced activation of the receptor tyrosine kinase TrkB. Nat Commun. (2024) 15:9316. doi: 10.1038/s41467-024-53710-7
7. Li Y, Wei C, Wang W, Li Q, and Wang ZC. Tropomyosin receptor kinase B (TrkB) signalling: targeted therapy in neurogenic tumours. J Pathol: Clin Res. (2023) 9:89–99. doi: 10.1002/cjp2.307
8. Li Q, Cao Z, and Zhao S. The emerging portrait of glial cell line-derived neurotrophic factor family receptor alpha (GFRα) in cancers. Int J Med Sci. (2022) 19:659. doi: 10.7150/ijms.64133
9. Tang H, Cai Y, Yang M, Tang S, Huang Q, Li H, et al. Single-cell and spatial transcriptomics reveals the key role of MCAM+ tip-like endothelial cells in osteosarcoma metastasis. NPJ Precis Oncol. (2025) 9:104. doi: 10.1038/s41698-025-00896-8
10. Chen Z, Han F, Du Y, Shi H, and Zhou W. Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. (2023) 8:70. doi: 10.1038/s41392-023-01332-8
11. Voissière A, Gomez-Roca C, Chabaud S, Rodriguez C, Nkodia A, Berthet J, et al. The CSF-1R inhibitor pexidartinib affects FLT3-dependent DC differentiation and may antagonize durvalumab effect in patients with advanced cancers. Sci Trans Med. (2024) 16:eadd1834. doi: 10.1126/scitranslmed.add1834
12. Solernó LM, Sobol NT, Gottardo MF, Capobianco CS, Ferrero MR, Vásquez L, et al. Propranolol blocks osteosarcoma cell cycle progression, inhibits angiogenesis and slows xenograft growth in combination with cisplatin-based chemotherapy. Sci Rep. (2022) 12:15058. doi: 10.1038/s41598-022-18324-3
13. Mascarenhas L, DuBois SG, Albert CM, Bielack S, Orbach D, Federman N, et al. Elective discontinuation of larotrectinib in pediatric patients with TRK fusion sarcomas and related mesenchymal tumours. J Clin Oncol. (2025) 43:1180–7. doi: 10.1200/JCO.24.00848
14. Pezzella M, Quintarelli C, Quadraccia MC, Sarcinelli A, Manni S, Iaffaldano L, et al. Tumour-derived G-CSF induces an immunosuppressive microenvironment in an osteosarcoma model, reducing response to CAR. GD2 T-cells. J Hematol Oncol. (2024) 17:127. doi: 10.1186/s13045-024-01641-7
15. Chi H, Huang J, Yan Y, Jiang C, Zhang S, Chen H, et al. Unraveling the role of disulfidptosis-related LncRNAs in colon cancer: a prognostic indicator for immunotherapy response, chemotherapy sensitivity, and insights into cell death mechanisms. Front Mol Biosci. (2023) 10:1254232. doi: 10.3389/fmolb.2023.1254232
16. Bansal S, Hedau ST, Sengupta S, Sakhuja P, Agarwal A, Singh S, et al. Oncogenic role of the brain-derived neurotrophic factor-TrkB axis with a focus on gallbladder cancer. Mol Med Rep. (2025) 32:205. doi: 10.3892/mmr.2025.13570
17. Komori T, Okamura K, Ikehara M, Yamamuro K, Endo N, Okumura K, et al. Brain-derived neurotrophic factor from microglia regulates neuronal development in the medial prefrontal cortex and its associated social behavior. Mol Psychiatry. (2024) 29:1338–49. doi: 10.1038/s41380-024-02413-y
18. Fjæstad KY, Rømer AMA, Goitea V, Johansen AZ, Thorseth ML, Carretta M, et al. Blockade of beta-adrenergic receptors reduces cancer growth and enhances the response to anti-CTLA4 therapy by modulating the tumor microenvironment. Oncogene. (2022) 41:1364–75. doi: 10.1038/s41388-021-02170-0
19. Malekan M, Nezamabadi SS, Samami E, Mohebalizadeh M, Saghazadeh A, Rezaei N, et al. BDNF and its signaling in cancer. J Cancer Res Clin Oncol. (2023) 149:2621–36. doi: 10.1007/s00432-022-04365-8
20. Laetsch TW, Voss S, Ludwig K, Hall D, Barkauskas DA, DuBois SG, et al. Larotrectinib for newly diagnosed infantile fibrosarcoma and other pediatric NTRK fusion–positive solid tumors (Children's oncology group ADVL1823). J Clin Oncol. (2025) 43:1188–97. doi: 10.1200/JCO-24-01854
21. Truong DD, Weistuch C, Murgas KA, Admane P, King BL, Chauviere Lee J, et al. Mapping the single-cell differentiation landscape of osteosarcoma. Clin Cancer Res. (2024) 30:3259–72. doi: 10.1158/1078-0432.CCR-24-0563
22. Song G, Peng G, Zhang J, Song B, Yang J, Xie X, et al. Uncovering the potential role of oxidative stress in the development of periodontitis and establishing a stable diagnostic model via combining single-cell and machine learning analysis. Front Immunol. (2023) 14:1181467. doi: 10.3389/fimmu.2023.1181467
23. Assi A, Farhat M, Hachem MCR, Zalaquett Z, Aoun M, Daher M, et al. Tyrosine kinase inhibitors in osteosarcoma: Adapting treatment strategiesa. J Bone Oncol. (2023) 43:100511. doi: 10.1016/j.jbo.2023.100511
24. Chen Y, Gao Z, Mohd-Ibrahim I, Yang H, Wu L, Fu Y, et al. Pan-cancer analyses of bromodomain containing 9 as a novel therapeutic target reveals its diagnostic, prognostic potential and biological mechanism in human tumours. Clin Trans Med. (2024) 14:e1543. doi: 10.1002/ctm2.1543
25. Manji GA, Stanton LJ, Hirbe AC, Ge L, Sta Ana S, Titus S, et al. Phase II study of pexidartinib plus sirolimus in unresectable Malignant peripheral nerve sheath tumors identifies M2 macrophage activation. JCO Oncol Adv. (2025) 2:e2400083. doi: 10.1200/OA-24-00083
26. Laetsch TW, DuBois SG, Mascarenhas L, Turpin B, Federman N, Albert CM, et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. (2018) 19:705–14. doi: 10.1016/S1470-2045(18)30119-0
27. Zhang L, Pan Y, Pan F, Huang S, Wang F, Zeng Z, et al. MATN4 as a target gene of HIF-1α promotes the proliferation and metastasis of osteosarcoma. Aging (Albany NY). (2024) 16:10462. doi: 10.18632/aging.205941
28. Liu YN, Chen WY, Liu MK, Yeh HL, Chen WH, Jiang KC, et al. Immunosuppressive role of BDNF in therapy-induced neuroendocrine prostate cancer. Mol Oncol. (2024) 18:1665–86. doi: 10.1002/1878-0261.13614
29. Thuya WL, Cao Y, Ho PC, Wong AL, Wang L, Zhou J, et al. Insights into IL-6/JAK/STAT3 signaling in the tumor microenvironment: Implications for cancer therapy. Cytokine Growth Factor Rev. (2025) 85(1):26–42. doi: 10.1016/j.cytogfr.2025.01.003
30. Chen S, Saeed AFUH, Liu Q, Jiang Q, Xu H, Xiao GG, et al. Macrophages in immunoregulation and therapeutics. Signal Transduct Target Ther. (2023) 8:207. doi: 10.1038/s41392-023-01452-1
31. Khan MN, Choudhary D, Mehan S, Khan Z, Gupta GD, Narula AS, et al. Molecular mechanisms of GDNF/GFRA1/RET and PI3K/AKT/ERK signaling interplay in neuroprotection: Therapeutic strategies for treating neurological disorders. Neuropeptides. (2025) 102516. doi: 10.1016/j.npep.2025.102516
32. Lin SL, Yang SY, Tsai CH, Fong YC, Chen WL, Liu JF, et al. Nerve growth factor promote VCAM-1-dependent monocyte adhesion and M2 polarization in osteosarcoma microenvironment: Implications for larotrectinib therapy. Int J Biol Sci. (2024) 20:4114. doi: 10.7150/ijbs.95463
33. Liu X, Ye J, Guo W, and Wang J. Significance of exosomes in osteosarcoma research: a systematic review and meta-analysis of a singular clinical investigation. Front Cell Dev Biol. (2024) 12:1473044. doi: 10.3389/fcell.2024.1473044
34. Zhang Y, Yu F, Ouyang J, Liu P, Dai Y, Wang Y, et al. ADRB2 inhibition suppresses cancer immune evasion by regulating tumor SOX10-PD-L1 axis and T cell function. J Immunother Cancer. (2025) 13:e011611. doi: 10.1136/jitc-2025-011611
35. Peng TJ, Chang Wang CC, Tang SJ, Sun GH, and Sun KH. Neurotrophin-3 facilitates stemness properties and associates with poor survival in lung cancer. Neuroendocrinology. (2024) 114:921–33. doi: 10.1159/000539815
36. Zhang K, Zakeri A, Alban T, Dong J, Ta HM, Zalavadia AH, et al. VISTA promotes the metabolism and differentiation of myeloid-derived suppressor cells by STAT3 and polyamine-dependent mechanisms. Cell Rep. (2024) 43:113661. doi: 10.1016/j.celrep.2023.113661
37. Duke ES, Bradford D, Marcovitz M, Amatya AK, Mishra-Kalyani PS, Nguyen E, et al. FDA approval summary: selpercatinib for the treatment of advanced RET fusion-positive solid tumors. Clin Cancer Res. (2023) 29:3573–8. doi: 10.1158/1078-0432.CCR-23-0459
38. Miao J, Chen S, Cao H, Ding Z, Li Y, Wang W, et al. Bruceantinol targeting STAT3 exerts promising antitumor effects in in vitro and in vivo osteosarcoma models. Mol Carcinog. (2024) 63:1133–45. doi: 10.1002/mc.23714
39. Qin Y, Maggio A, Hawkins D, Beaudry L, Kim A, Pan D, et al. Whole-genome bisulfite sequencing data analysis learning module on Google Cloud Platform. Briefings Bioinf. (2024) 25:bbae236. doi: 10.1093/bib/bbae236
40. Rogovskii V. Tumor-produced immune regulatory factors as a therapeutic target in cancer treatment. Front Immunol. (2024) 15:1416458. doi: 10.3389/fimmu.2024.1416458
41. Tomuleasa C, Tigu AB, Munteanu R, Moldovan CS, Kegyes D, Onaciu A, et al. Therapeutic advances of targeting receptor tyrosine kinases in cancer. Signal Transduct Target Ther. (2024) 9:201. doi: 10.1038/s41392-024-01899-w
42. Su K, Wang F, Li X, Chi H, Zhang J, He K, et al. Effect of external beam radiation therapy versus transcatheter arterial chemoembolization for non-diffuse hepatocellular carcinoma (≥ 5 cm): a multicenter experience over a ten-year period. Front Immunol. (2023) 14:1265959. doi: 10.3389/fimmu.2023.1265959
43. Qin Q, Ramesh S, Li Z, Zhong L, Cherief M, Archer M, et al. TrkA+ sensory neurons regulate osteosarcoma proliferation and vascularization to promote disease progression. BioRxiv. (2024), 2024.06.20.599869. doi: 10.1101/2024.06.20.599869
44. Li H, Guo L, Su K, Li C, Jiang Y, Wang P, et al. Construction and validation of TACE therapeutic efficacy by ALR score and nomogram: a large, multicenter study. J Hepatocell Carcinoma. (2023), 1009–17. doi: 10.2147/JHC.S414926
45. Rahmadiani N, Norahmawati E, Endharti AT, Hambalie AO, and Isma SPP. PD-L1, STAT3, IL6, and EGFR immunoexpressions in high-grade osteosarcoma. Adv Orthop. (2024) 2024:9036225. doi: 10.1155/2024/9036225
46. Zhang P, Zhang H, Tang J, Ren Q, Zhang J, Chi H, et al. The integrated single-cell analysis developed an immunogenic cell death signature to predict lung adenocarcinoma prognosis and immunotherapy. Aging (Albany NY). (2023) 15:10305. doi: 10.18632/aging.205077
47. Gelderblom H, Razak AA, Taylor MH, Bauer TM, Wilky B, Martin-Broto J, et al. CSF1R inhibition in patients with advanced solid tumors or tenosynovial giant cell tumor: a phase i study of vimseltinib. Clin Cancer Res. (2024) 30:3996–4004. doi: 10.1158/1078-0432.CCR-24-0103
48. Wu X, Deng W, Zhao Q, and Xiong J. Single-cell analysis links DCUN1D5 to immune remodeling and cisplatin resistance in recurrent osteosarcoma. Commun Biol. (2025) 8:1019. doi: 10.1038/s42003-025-08409-w
49. Matos AAM and Scheff NN. Sensory neurotransmission and pain in solid tumor progression. Trends Cancer. (2025) 11:309–20. doi: 10.1016/j.trecan.2025.01.003
50. Wang K, Zhang Y, Shu R, Yuan L, Tu H, Wang S, et al. GPR37 activation alleviates bone cancer pain via the inhibition of osteoclastogenesis and neuronal hyperexcitability. Adv Sci. (2025) 12:2417367. doi: 10.1002/advs.202417367
51. Sheng J, Chen H, Fu B, Pan H, Wang J, Han W, et al. BPI-28592 as a novel second generation inhibitor for NTRK fusion tumors. NPJ Precis Oncol. (2024) 8:198. doi: 10.1038/s41698-024-00686-8
52. Liu R, Liu J, Cao Q, Chu Y, Chi H, Zhang J, et al. Identification of crucial genes through WGCNA in the progression of gastric cancer. J Cancer. (2024) 15:3284. doi: 10.7150/jca.95757
53. Liu Y, Liu L, Wei X, Xiong Y, Han Q, Gong T, et al. Identification of M2 macrophage markers for predicting outcome and therapeutic response in osteosarcoma: Integrated analysis of single-cell and bulk RNA-sequencing. J Cancer. (2025) 16:1873. doi: 10.7150/jca.104855
54. Cao Q, Wu X, Chen Y, Wei Q, You Y, Qiang Y, et al. The impact of concurrent bacterial lung infection on immunotherapy in patients with non-small cell lung cancer: a retrospective cohort study. Front Cell Infect Microbiol. (2023) 13:1257638. doi: 10.3389/fcimb.2023.1257638
55. McGee LE, Pereira JS, McEachron TA, Mazcko C, LeBlanc AK, Beck JA, et al. The tumor microenvironment of metastatic osteosarcoma in the human and canine lung. Commun Biol. (2025) 8:756. doi: 10.1038/s42003-025-07992-2
56. Chen Y, You Y, Wei M, Yang P, Zhang Q, Li X, et al. Exploration of physical activity, sedentary behavior and insulin level among short sleepers. Front Endocrinol. (2024) 15:1371682. doi: 10.3389/fendo.2024.1371682
57. Zeng J, Wang D, Tong Z, Li Z, Wang G, Du Y, et al. Development of a prognostic model for osteosarcoma based on macrophage polarization-related genes using machine learning: implications for personalized therapy. Clin Exp Med. (2025) 25:146. doi: 10.1007/s10238-024-01530-w
58. You Y, Chen Y, You Y, Zhang Q, and Cao Q. Evolutionary game analysis of artificial intelligence such as the generative pre-trained transformer in future education. Sustainability. (2023) 15:9355. doi: 10.3390/su15129355
59. Marcus L, Donoghue M, Aungst S, Myers CE, Helms WS, Shen G, et al. FDA approval summary: entrectinib for the treatment of NTRK gene fusion solid tumors. Clin Cancer Res. (2021) 27:928–32. doi: 10.1158/1078-0432.CCR-20-2771
60. Harada G and Drilon A. TRK inhibitor activity and resistance in TRK fusion-positive cancers in adults. Cancer Genet. (2022) 264:33–9. doi: 10.1016/j.cancergen.2022.03.002
61. Tian H, Cao J, Li B, Nice EC, Mao H, Zhang Y, et al. Managing the immune microenvironment of osteosarcoma: the outlook for osteosarcoma treatment. Bone Res. (2023) 11:11. doi: 10.1038/s41413-023-00246-z
62. Petrosiute A, Musvicait J, Petroška D, Šcerbavicien? A, Arnold S, Matulien? J, et al. CCL2-CCR2 axis inhibition in osteosarcoma cell model: the impact of oxygen level on cell phenotype. J Cell Physiol. (2025) 240:e31489. doi: 10.1002/jcp.31489
63. Li Y and Bai X. Naringenin induces ferroptosis in osteosarcoma cells through the STAT3-MGST2 signaling pathway. J Bone Oncol. (2025) 50:100657. doi: 10.1016/j.jbo.2024.100657
64. Cao Q, Wang Q, Wu X, Zhang Q, Huang J, Chen Y, et al. A literature review: mechanisms of antitumor pharmacological action of leonurine alkaloid. Front Pharmacol. (2023) 14:1272546. doi: 10.3389/fphar.2023.1272546
65. Gupta R, Dittmeier M, Wohlleben G, Nickl V, Bischler T, Luzak V, et al. Atypical cellular responses mediated by intracellular constitutive active TrkB (NTRK2) kinase domains and a solely intracellular NTRK2-fusion oncogene. Cancer Gene Ther. (2024) 31:1357–79. doi: 10.1038/s41417-024-00809-0
66. Jiang L, Ren X, Yang J, Chen H, Zhang S, Zhou X, et al. Mitophagy and clear cell renal cell carcinoma: insights from single-cell and spatial transcriptomics analysis. Front Immunol. (2024) 15:1400431. doi: 10.3389/fimmu.2024.1400431
67. Cavalu S, Saber S, Amer AE, Hamad RS, Abdel-Reheim MA, Elmorsy EA, et al. The multifaceted role of beta-blockers in overcoming cancer progression and drug resistance: extending beyond cardiovascular disorders. FASEB J. (2024) 38:e23813. doi: 10.1096/fj.202400725RR
68. Wu X, Zhou Z, Cao Q, Chen Y, Gong J, Zhang Q, et al. Reprogramming of Treg cells in the inflammatory microenvironment during immunotherapy: a literature review. Front Immunol. (2023) 14:1268188. doi: 10.3389/fimmu.2023.1268188
69. Liu W, Hu H, Shao Z, Lv X, Zhang Z, Deng X, et al. Characterizing the tumor microenvironment at the single-cell level reveals a novel immune evasion mechanism in osteosarcoma. Bone Res. (2023) 11:4. doi: 10.1038/s41413-022-00237-6
70. Ji T, Shi Q, Mei S, Xu J, Liang H, Xie L, et al. Integrated analysis of single-cell and bulk RNA sequencing data reveals an immunostimulatory microenvironment in tumor thrombus of osteosarcoma. Oncogenesis. (2023) 12:31. doi: 10.1038/s41389-023-00474-2
71. Huang Z, Chen P, and Liu Y. WTAP-mediated m6A modification of circ_0032463 promotes osteosarcoma progression by sponging miR-145-5p and regulating GFRA1 expression. J Biochem Mol Toxicol. (2024) 38:e23833. doi: 10.1002/jbt.23833
72. Rogers C, Morrissette JJD, and Sussman RT. NTRK point mutations and their functional consequences. Cancer Genet. (2022) 262:5–15. doi: 10.1016/j.cancergen.2021.12.002
73. Zhang H, Liu Y, Liu J, Chen J, Wang J, Hua H, et al. cAMP-PKA/EPAC signaling and cancer: the interplay in tumor microenvironment. J Hematol Oncol. (2024) 17:5. doi: 10.1186/s13045-024-01524-x
74. Li X, Peng X, Yang S, Wei S, Fan Q, Liu J, et al. Targeting tumor innervation: premises, promises, and challenges. Cell Death Discov. (2022) 8:131. doi: 10.1038/s41420-022-00930-9
75. Yaniv D, Mattson B, Talbot S, Gleber-Netto FO, and Amit M. Targeting the peripheral neural-tumour microenvironment for cancer therapy. Nat Rev Drug Discov. (2024) 23:780–96. doi: 10.1038/s41573-024-01017-z
76. Pu T, Sun J, Ren G, and Li H. Neuro-immune crosstalk in cancer: mechanisms and therapeutic implications. Signal Transduct Target Ther. (2025) 10:176. doi: 10.1038/s41392-025-02241-8
77. Xiang S and Lu X. Selective type II TRK inhibitors overcome xDFG mutation mediated acquired resistance to the second-generation inhibitors selitrectinib and repotrectinib. Acta Pharm Sin B. (2024) 14:517–32. doi: 10.1016/j.apsb.2023.11.010
78. Huang S, Zhu J, Yu L, Huang Y, and Hu Y. Cancer-nervous system crosstalk: from biological mechanism to therapeutic opportunities. Mol Cancer. (2025) 24:133. doi: 10.1186/s12943-025-02336-4
79. Shrestha P, Shrestha R, Zhou Y, Zielinski R, Priebe W, Kleinerman ES, et al. STAT3 inhibition in combination with CD47 blockade inhibits osteosarcoma lung metastasis. Front Immunol. (2025) 16:1608375. doi: 10.3389/fimmu.2025.1608375
Keywords: neurotrophic factors, osteosarcoma, immune microenvironment, neuro-immune crosstalk, targeted therapy, immunotherapy
Citation: Lei P and Li L (2025) Neurotrophic factors as double-edged swords in osteosarcoma: drivers of tumour growth and immune remodelling. Front. Immunol. 16:1666343. doi: 10.3389/fimmu.2025.1666343
Received: 15 July 2025; Accepted: 23 September 2025;
Published: 09 October 2025.
Edited by:
Qihang Yuan, Dalian Medical University, ChinaReviewed by:
Dmitry Aleksandrovich Zinovkin, Gomel State Medical University, BelarusAgnieszka Śmieszek, Wroclaw University of Environmental and Life Sciences, Poland
Zhe Pei, Virginia Tech, United States
Copyright © 2025 Lei and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Li, bGlsZWlzcGluZUAxMjYuY29t
 Puzhou Lei
Puzhou Lei Lei Li
Lei Li