- 1Department of Neurology, The Second Hospital & Clinical Medical School, Lanzhou University, Lanzhou, China
- 2Department of Respiratory, The Second Hospital & Clinical Medical School, Lanzhou University, Lanzhou, China
Epilepsy, as a chronic neurological disorder marked by recurrent seizures, is closely linked to neuroinflammation and immune dysregulation. Exosomes, extracellular vesicles with potent immunomodulatory properties, have emerged as key players in mitigating epilepsy-associated inflammation by targeting glial activation and balancing pro- and anti-inflammatory cytokine release. Their ability to cross the blood-brain barrier (BBB) enables targeted delivery of anti-inflammatory cargo, such as miRNAs and proteins, offering promise for diagnosing and treating drug-resistant epilepsy. This review highlights exosomes’ dual role as biomarkers of inflammatory pathways and therapeutic vehicles for immune modulation. By suppressing neuroinflammation and restoring neuronal homeostasis, exosome-based strategies may revolutionize epilepsy management, though clinical translation requires further optimization of isolation and engineering techniques.
1 Introduction
Epilepsy is a chronic neurological disorder characterized by transient brain dysfunction caused by recurrent, sudden, and excessive hypersynchronous discharges of cortical neurons (1). Accumulating evidence implicates localized neuroinflammation within specific regions of the central nervous system (CNS) as a hallmark pathological feature (2). During seizures, glutamate receptor overactivation, oxidative stress, and elevated pro-inflammatory cytokines disrupt the integrity of the blood–brain barrier (BBB), which facilitates both central and peripheral immune cell infiltration, thereby intensifying seizure susceptibility (3). Among emerging regulatory elements, exosomes have garnered attention for their anti-inflammatory capacity (4, 5). Through suppressing glial cell activation, modulating immune responses, and dampening neuronal excitability, exosomes hold promise in attenuating seizure initiation. Their intrinsic lipid bilayer and capacity to cross the BBB further underscore their translational potential as therapeutic delivery platforms in epilepsy (6).
Despite advances in diagnostics and pharmacotherapy, up to 25% of cases are misdiagnosed (7), and nearly 30% of patients suffer from drug-refractory epilepsy (DRE) (8), reflecting an urgent need for alternative interventions. Although seizure control is achieved in approximately 70% of individuals via monotherapy or polytherapy, the remainder often progresses to DRE, which markedly elevates disease burden and mortality (9). Notably, compromised BBB function has been closely linked to antiepileptic drug resistance (10), and enhancing drug penetration across the BBB may significantly improve therapeutic efficacy (11). With growing recognition of their therapeutic and diagnostic utility, cs have become a focal point in epilepsy research (12). This review outlines the roles of exosomes in modulating epilepsy-associated neuroinflammation, highlights their mechanistic involvement in seizure suppression, and evaluates their utility in overcoming drug resistance, offering a conceptual framework for next-generation epilepsy management.
2 Overview of exosomes
Exosomes are nanoscale extracellular vesicles (EVs), typically 50 – 150 nm in diameter, that encapsulate a rich cargo of nucleic acids, proteins, and lipids (13, 14). They are secreted by virtually all cell types and are detectable in a variety of biological fluids, including blood, urine, and cerebrospinal fluid (15, 16). Far from being inert cellular byproducts, exosomes have emerged as dynamic mediators of intercellular communication, orchestrating diverse physiological and pathological responses to environmental cues (17). In the CNS, exosomes have gained increasing attention for their multifaceted roles in neurodevelopment, synaptic plasticity, and immune surveillance (18). Their pathological relevance is underscored by mounting evidence implicating them in the onset and progression of various neurological diseases (19). For example, microglia-derived exosomal miR-124-3p has been shown to exert neuroprotective effects by targeting the Rela/ApoE signaling axis, thereby mitigating neuronal damage and neuroinflammation (20). This axis is critical, as RELA (p65), a subunit of NF-κB, modulates inflammatory gene expression, while ApoE is involved in lipid transport and neural repair. In major depressive disorder (MDD), Kuwano et al. (21) identified exosomes enriched with IL - 34 and the tetraspanin CD81 as potential diagnostic biomarkers. IL - 34 is known to promote microglial survival and neurogenesis, suggesting that exosomal IL - 34/CD81 signatures may reflect active neuroinflammatory or neurotrophic processes in MDD. Exosomes also function as vectors for therapeutic repair. In a rat model of focal cerebral ischemia, systemically administered exosomes were shown to localize to the ischemic brain, where they fuse with recipient cells, deliver functional biomolecules, and promote neuroregeneration (22). Mechanistically, this may involve the modulation of PI3K/AKT and MAPK signaling cascades, which are central to cell survival and axonal remodeling (23). Moreover, exosomes exhibit anti-inflammatory potential in perinatal brain injury by suppressing pro-inflammatory cytokine production and fostering a reparative microenvironment through the delivery of anti-inflammatory miRNAs and immunomodulatory proteins such as TGF-β (24). Emerging studies also suggest that exosomes derived from brain-metastatic cancer cells can precondition the brain microenvironment to support tumor growth (25). Together, these studies underscore the dualistic nature of exosomes in the CNS, as both mediators of repair and drivers of pathology, highlighting their potential as diagnostic biomarkers and therapeutic targets across a broad spectrum of neurological conditions.
3 Exosomes in epilepsy-associated neuroinflammation
3.1 Astrocytes and exosomes
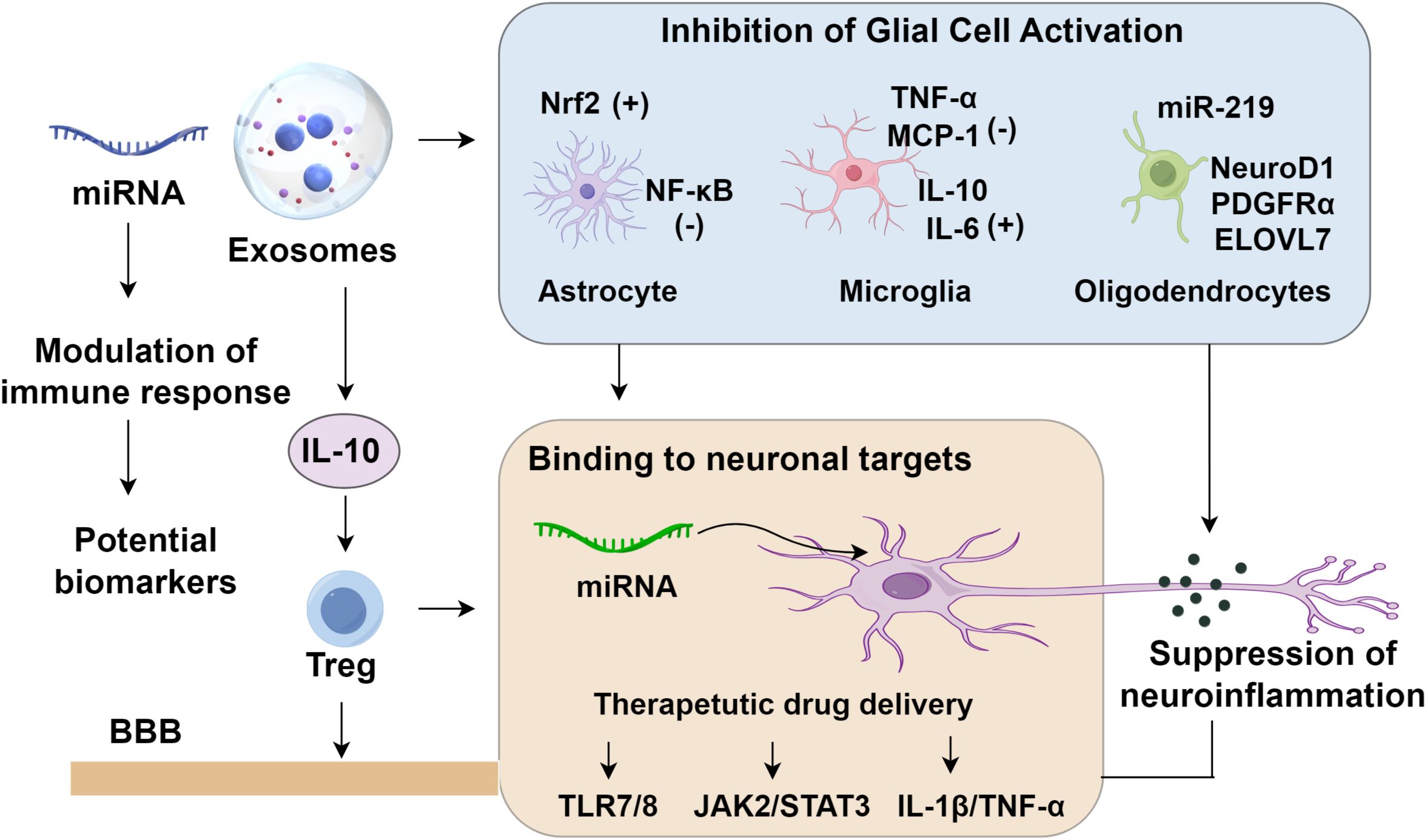
Glial cell activation is a hallmark of neuroinflammatory responses in epilepsy (26). Astrocyte proliferation is often more persistent than microglial activation, contributing to more sustained inflammatory damage (27, 28). Astrocytes perform diverse roles, including neuroprotection, neurotransmitter regulation, extracellular ion buffering, synaptic signaling, and maintenance of the BBB (29). Upon CNS injury, reactive astrocytosis occurs, accompanied by glutamate excitotoxicity, altered gap junctions, and mitochondrial dysfunction. Mesenchymal stem cell (MSC)-derived exosomes alleviate pilocarpine-induced epilepsy in mice by reducing expression of glial fibrillary acidic protein (GFAP) and complement component 3 (C3) in bilateral hippocampi, dampening IL - 1β and TNF-α secretion, and mitigating intracellular Ca²+ influx (30). These changes were associated with improved spatial learning and memory, indicating a restoration of astrocytic function and mitochondrial homeostasis. The underlying mechanism involves the modulation of the Nrf2/NF-κB signaling axis, where Nrf2 suppresses oxidative stress-induced inflammation, while NF-κB promotes glial proliferation and cytokine release (31). Exosomes appear to upregulate Nrf2 nuclear translocation while inhibiting NF-κB activity, thus attenuating reactive astrocyte activation (30).
In particular, MSC-derived exosomes suppress the formation of neurotoxic A1 astrocytes, induced by pro-inflammatory cytokines such as IL - 1α, TNF-α, and C1q, by downregulating C3, a key molecular marker of the A1 phenotype (32). Inhibition of the C3-C3aR signaling axis by exosomal miRNAs and proteins attenuates synaptotoxic effects and neuronal death (33). Moreover, exosomes modulate STAT3 phosphorylation, a pathway implicated in A2 astrocyte differentiation and neuroprotection, thus promoting a phenotypic shift toward anti-inflammatory, pro-repair states (34–36). Targeting reactive astrocytes, particularly the neurotoxic A1 phenotype, offers a promising therapeutic strategy in epilepsy (37). A1 astrocytes, which are induced during inflammation, disrupt synapses and exhibit neurotoxicity (38, 39). Exosomes not only suppress proliferation of A1 astrocytes but may also promote their phenotypic reversion to non-reactive states via Nrf2-NF-κB signaling (40). In addition to molecular inhibition of A1 activation, exosomes restore cellular metabolism by improving mitochondrial membrane potential and reducing ROS, thereby promoting phenotypic reversion to homeostatic astrocytes (41). Furthermore, MSC-derived exosomes restore mitochondrial membrane potential, reduce Ca²+ influx, and reverse abnormal calcium signaling in hippocampal astrocytes (42–44). Compared to MSCs, their exosomes—containing anti-inflammatory mRNA, miRNA, and proteins, exhibit lower immunogenicity, greater stability, and ease of storage, making them ideal vectors for targeted drug delivery to astrocytes within the hippocampus (45).
3.2 Microglia and exosomes
The degree of microglial activation is positively correlated with the duration and severity of epilepsy (46). As key immune cells in the brain, microglia orchestrate inflammatory responses, which are implicated in neurodegeneration, hippocampal inflammation, and BBB disruption following status epilepticus (SE) (47). Long et al. (45) showed that A1-type MSC-derived exosomes administered after SE reduce neurodegeneration, dampen hippocampal inflammation, and preserve neurogenesis and cognitive function. Elevated glutamate levels and persistent neuroinflammation following SE are major contributors to excitotoxicity and neuronal apoptosis (48, 49). Moreover, GABAergic inhibition is suppressed during seizures, disrupting the excitatory-inhibitory balance. Exosomes help restore this balance by directly protecting GABAergic neurons through multiple mechanisms, including suppression of microglial activation and modulation of cytokine profiles (50, 51). Specifically, exosomes downregulate pro-inflammatory cytokines such as TNF-α and MCP - 1 while upregulating IL - 10, an anti-inflammatory cytokine that promotes neuronal survival (52, 53). This shift in the cytokine milieu reduces oxidative stress, attenuates neuroinflammation, and limits excitotoxic injury to GABAergic interneurons, which are essential for seizure containment (54, 55). While resting microglia aid in debris clearance and neuroprotection, their hyperactivation leads to the release of pro-inflammatory and cytotoxic agents that drive neuronal loss (56). In SE mice, A1 exosomes attenuated microglial activation and suppressed pro-inflammatory cytokines (TNF-α, MCP - 1) while enhancing anti-inflammatory mediators (IL - 10, IL - 6). These changes were associated with the preservation of GABAergic neurons, reduced apoptotic markers, and improved synaptic integrity (45). Inflammatory oxidative stress in the hippocampus exacerbates cognitive deficits and memory loss via ROS-induced synaptic dysregulation (57, 58). Thus, A1 exosomes, by maintaining GABA-glutamate homeostasis, suppressing microglial overactivation, and preserving interneurons, attenuate SE-induced neurodegeneration and prevent progression to chronic epilepsy (45).
3.3 Oligodendrocytes and exosomes
Oligodendrocytes serve as the principal myelinating cells within the central nervous system (CNS), playing an indispensable role in maintaining neuronal conductivity and structural integrity (59). In pathological contexts characterized by demyelination, oligodendrocyte viability is compromised, particularly due to inflammation-induced apoptosis, which severely impairs the capacity for remyelination and functional recovery (60). Notably, demyelinating lesions are frequently observed in individuals with epilepsy, a phenomenon that may arise from disrupted autonomic regulation and altered neuronal-glial dynamics (61). Low-dose interferon-γ (IFN-γ) stimulation of dendritic cells induces the secretion of exosomes enriched with remyelination-associated microRNAs, including miR-219, miR-335, and miR-494-3p (62–64). These exosomes exhibited preferential tropism for oligodendrocytes, followed sequentially by uptake in microglia and astrocytes, suggesting a degree of cell-specific targeting that holds translational relevance for therapeutic interventions in demyelinating diseases (65, 66). Mechanistically, dendritic cell–derived exosomes have been shown to modulate the expression of pivotal oligodendrocyte lineage regulators, including NeuroD1, PDGFRα, and ELOVL7, predominantly via miR-219-mediated gene regulation (67). Continued exploration of these exosome–oligodendrocyte interactions may yield novel strategies for enhancing remyelination in epilepsy and related demyelinating diseases.
3.4 Exosomes and the immune response
The immune system exerts a pivotal influence on the onset and progression of epilepsy (68). Inflammatory responses represent transient manifestations of immune activation, encompassing both pro- and anti-inflammatory mediators. Infiltrating immune cells release cytokines such as TNF-α and IL - 18, thereby amplifying neuroinflammation (69); conversely, anti-inflammatory cytokines like IL - 10 contribute to the resolution of the immune response (70). Under physiological conditions, the blood–brain barrier (BBB) maintains neural immune privilege by restricting peripheral immune cell infiltration. However, during inflammation, endothelial dysfunction compromises BBB integrity and enhances its permeability, subsequently lowering neuronal firing thresholds and heightening seizure susceptibility. While moderate inflammation may facilitate central nervous system repair and synaptic remodeling, sustained or dysregulated immune activation promotes epileptogenesis. The convergence of BBB disruption and increased neuronal excitability constitutes a fundamental axis in the pathophysiology of epilepsy. Exosomes actively participate in immune regulation through mechanisms such as antigen presentation (71), immune suppression (72), immune surveillance (73), and intercellular signaling. For instance, MSC-derived exosomes upregulate IL - 10 and other immunosuppressive molecules, thereby promoting Tregs proliferation and conferring potent immunomodulatory effects.
4 Exosomes and their cargo as potential biomarkers in epilepsy
Exosomes derived from various cell types encapsulate proteins, mRNAs, and miRNAs (74), reflecting the diverse and complex nature of their molecular cargo. This heterogeneity forms the foundation for their utility as biomarkers in epilepsy (75). Notably, the dysregulation of specific miRNAs is closely associated with epileptogenesis. For instance, miR-23b-3p, a critical regulator of neuronal excitability, is markedly reduced in epilepsy, and its loss can precipitate fatal seizures (76). Alterations in circulating exosomal miRNAs have been observed in patients with epilepsy. Emerging evidence from serum miRNA profiling has revealed marked dysregulation of circulating microRNAs in individuals with epilepsy compared to healthy controls. Notably, several miRNAs, including miR-27a-3p, miR-181a-5p, miR-134, miR-221, miR-155, and miR-146—are significantly upregulated, whereas others such as miR-132, miR-125a-5p, and miR-34c-5p exhibit reduced expression levels (77). Besides, miR-106b-5p demonstrated a sensitivity of 80% and specificity of 81%, suggesting strong potential as a novel diagnostic biomarker. Beyond circulating miRNAs, exosomal dynamics have also garnered attention as pathophysiological hallmarks of epileptogenesis. Batool et al. (78) reported that elevated levels of exosomal secretion persisted in epileptic mice two weeks after status epilepticus (SE), suggesting a sustained involvement of exosomes in the chronic phase of epilepsy.
5 Exosomes in epilepsy therapy
5.1 Exosomes suppress neuroinflammation
Inflammation serves both as an initiator and a consequence of epileptic seizures, forming a self-perpetuating loop that amplifies seizure frequency and severity. Seizure activity induces excessive glutamate receptor activation, oxidative stress, and elevated levels of pro-inflammatory cytokines, all of which compromise the integrity of the BBB. Mounting evidence implicates exosomes as key modulators of neuroinflammatory processes. These vesicles mitigate glial activation, modulate immune signaling, suppress neuronal hyperexcitability, selectively interact with neuronal targets, and limit neuronal loss (79). Notably, exosomes derived from epileptogenic tubers in tuberous sclerosis complex were investigated using small RNA sequencing, revealing microRNAs enriched within these vesicles that are capable of activating toll-like receptors TLR7/8 (80). This activation initiated a neuroinflammatory cascade, substantially upregulating pro-inflammatory cytokines and heightening seizure susceptibility, thereby identifying a novel therapeutic target for drug-resistant epilepsy associated with tuberous sclerosis (80). Intravenous administration of astrocyte-derived exosomes has been shown to attenuate microglial activation in epileptic rats by suppressing the JAK2/STAT3 signaling pathway, thereby mitigating neuroinflammation and neuronal apoptosis and contributing to effective seizure control (81–83). In a separate study, mesenchymal stem cell (MSC)-derived exosomes delivered via intracerebroventricular injection in pilocarpine-induced status epilepticus (SE) mice promoted astrocyte proliferation and alleviated neuroinflammatory responses, resulting in improved cognitive performance. Consistently, bone marrow MSC-derived exosomes were reported to rapidly accumulate in the hippocampus following muscarine-induced SE, highlighting their capacity for targeted delivery to affected brain regions (45). The treatment preserved glutamatergic and GABAergic neurons, alleviated hippocampal inflammation, and ameliorated SE-induced cognitive impairments. Collectively, these findings illustrate the multifaceted therapeutic potential of exosomes in epilepsy through neuroinflammatory modulation and cognitive restoration, offering promising avenues for drug-resistant epilepsy (Figure 1).
5.2 Neuroprotective functions of exosomes
MSC-derived exosomes serve as pivotal mediators of neural repair in epilepsy, exerting their effects through four principal mechanisms: first, directly enhancing the survival and differentiation of damaged neurons; second, indirectly attenuating glial activation, thereby mitigating neuroinflammation and oxidative stress while promoting neurovascular regeneration; third, restoring systemic metabolic homeostasis; and fourth, modulating immune responses via the secretion of cytokines and the presentation of distinctive membrane-associated molecules (84). Exosomes derived from bone marrow MSCs have been shown to confer robust neuroprotection in epilepsy models. One study (85) reported that these exosomes significantly alleviated neuronal loss in the dentate gyrus and CA1 regions of the hippocampus in mice with status epilepticus. Supporting these findings, intranasal administration of MSC-derived exosomes enabled effective hippocampal targeting, reduced brain injury, preserved neurogenesis, and sustained cognitive performance (86). Importantly, this approach also suppressed pro-inflammatory cytokine-induced iNOS expression, thereby mitigating neuronal damage. Complementarily, systemic injection of microglia-derived exosomes in a transient middle cerebral artery occlusion model reduced brain atrophy, improved neuronal function, promoted oligodendrocyte regeneration, and ameliorated white matter injury (87). These therapeutic effects were mediated through intercellular signaling pathways that modulate inflammation and coordinate immune responses to facilitate neural repair. Collectively, this body of evidence underscores the multifaceted neuroprotective roles of MSC-derived exosomes and illuminates their translational potential for epilepsy therapy.
5.3 Exosomes as drug delivery vehicles in epilepsy therapy
Current epilepsy management primarily relies on antiepileptic drugs (AEDs) such as phenytoin, carbamazepine, and valproate. Despite the availability of approximately 30 AEDs targeting diverse molecular mechanisms, clinical challenges persist, including drug resistance (88) and adverse effects (89). Nearly one-third of patients remain refractory to current therapies. One critical barrier in AED efficacy is the BBB. Exosomes, owing to their dual capacity to participate in neuroimmune communication and traverse the BBB via receptor-mediated endocytosis or fusion, hold significant promise for targeted CNS drug delivery. Moreover, exosomes can bypass the P-glycoprotein efflux system, enabling stable and sustained drug delivery across the BBB (90). Exosomes serve as versatile vectors for delivering proteins, small and large molecules, and nucleic acids (91). Their intrinsic targeting capability, driven by membrane proteins and glycan structures that recognize specific receptors on recipient cells, enables precise delivery. Additionally, exosomes can protect therapeutic agents from enzymatic degradation and ensure efficient BBB penetration and tissue-specific accumulation. Various engineering techniques such as surface modification, transfection, electroporation, ultrasound, extrusion, and freeze–thaw cycles have been developed to enhance drug encapsulation and delivery.
Previous studies have predominantly focused on the role of endogenous exosomal miRNAs in modulating neuroinflammation in epilepsy (30, 45). Although clinical studies using exosomes as drug carriers for epilepsy remain lacking, advances from other neurodegenerative disorders offer instructive parallels. For example, Haney et al. (92) successfully delivered catalase-loaded exosomes intranasally, achieving significant CNS accumulation and neuroprotection in murine models without provoking immune rejection. Curcumin, known for its antioxidant, anti-inflammatory, lipid-lowering, and anti-aggregatory effects (93), suffers from poor bioavailability due to low absorption and rapid metabolism. Kalani et al. (94) encapsulated curcumin into embryonic stem cell–derived exosomes, enhancing its bioavailability and reducing glial fibrillary acidic protein expression, thereby limiting astrogliosis. Dad et al. (95) proposed an innovative strategy for treating post-epileptic depression by transfecting miR-219 and miR-338 into synthetic polyvalent antibodies and utilizing exosomes as carriers. This approach effectively suppressed immune responses while promoting axonal regeneration and remyelination in the injured CNS. Extending this concept, researchers proposed loading therapeutic mRNA, miRNA, and proteins into exosomes to enhance their capacity to traverse the blood–brain barrier, thereby offering adjunctive benefits in the aftermath of status epilepticus by reducing seizure burden (96). Moreover, brain-derived exosomes carrying acid sphingomyelinase (ASM) or functional miRNAs have been shown to modulate neuronal excitability through targeted regulation of gene expression and signaling pathways (97), providing a conceptual framework for precision-targeted epilepsy interventions. In summary, exosomes represent a powerful drug delivery platform for addressing drug-resistant epilepsy. Their ability to traverse the BBB and deliver therapeutic agents directly to target tissues underscores their potential to transform epilepsy treatment paradigms.
6 Conclusion
In summary, exosomes represent a transformative avenue for epilepsy diagnosis and therapy, leveraging their innate ability to modulate neuroinflammation, cross the BBB, and deliver bioactive cargo. By suppressing glial activation, regulating immune responses, and serving as biomarkers or drug carriers, exosomes address critical gaps in managing drug-resistant epilepsy. Specifically, exosomal miRNAs such as miR-124, miR-219, and miR-146a have demonstrated significant promise in experimental models due to their ability to suppress neuroinflammation, promote remyelination, and modulate microglial and astrocytic activity. These molecules merit further investigation as potential therapeutic candidates.
Nonetheless, several key engineering and translational hurdles remain. These include the development of scalable and reproducible exosome isolation techniques, standardization of cargo loading methods, real-time tracking of in vivo distribution, and improving cell-type-specific targeting to minimize off-target effects. Future research must prioritize mechanistic validation of exosomal miRNAs/proteins, optimization of delivery systems, and rigorous clinical trials to harness their full potential. Addressing these challenges will be essential to position exosome-based strategies as a cornerstone of precision medicine in epilepsy and related neuroinflammatory disorders.
Author contributions
BL: Writing – original draft. YZ: Writing – original draft. LC: Writing – original draft. JC: Writing – original draft. ZZ: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Falco-Walter J. Epilepsy-definition, classification, pathophysiology, and epidemiology. Semin Neurol. (2020) 40:617–23. doi: 10.1055/s-0040-1718719
2. Bartolini L, Libbey JE, Ravizza T, Fujinami RS, Jacobson S, and Gaillard WD. Viral triggers and inflammatory mechanisms in pediatric epilepsy. Mol Neurobiol. (2019) 56:1897–907. doi: 10.1007/s12035-018-1215-5
3. Wang Q, Pan L, Chen S, Zhang Y, Liu G, Wu Y, et al. BBB proteomic analysis reveals that complex febrile seizures in infancy enhance susceptibility to epilepsy in adulthood through dysregulation of ECM-receptor interaction signaling pathway. Fluids Barriers CNS. (2025) 22:49. doi: 10.1186/s12987-025-00660-x
4. E VB, Ramesh D, Shaju MC, Kumar A, Pandey S, Nayak R, et al. Biological, pathological, and multifaceted therapeutic functions of exosomes to target cancer. Oncol Res. (2023) 32:73–94. doi: 10.32604/or.2023.030401
5. Wang Y, Li X, Liu D, Wang Z, Xia J, Wang L, et al. Research progress on the role of adipocyte exosomes in cancer progression. Oncol Res. (2024) 32:1649–60. doi: 10.32604/or.2024.043482
6. Yuan D, Zhao Y, Banks WA, Bullock KM, Haney M, Batrakova E, et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. (2017) 142:1–12. doi: 10.1016/j.biomaterials.2017.07.011
7. Lin Z, Gu Y, Zhou R, Wang M, Guo Y, Chen Y, et al. Serum exosomal proteins F9 and TSP - 1 as potential diagnostic biomarkers for newly diagnosed epilepsy. Front Neurosci. (2020) 14:737. doi: 10.3389/fnins.2020.00737
8. O’Connell BK, Gloss D, and Devinsky O. Cannabinoids in treatment-resistant epilepsy: A review. Epilepsy Behav. (2017) 70:341–8. doi: 10.1016/j.yebeh.2016.11.012
9. Sheng J, Liu S, Qin H, Li B, and Zhang X. Drug-resistant epilepsy and surgery. Curr Neuropharmacol (2018) 16:17–28. doi: 10.2174/1570159X15666170504123316
10. Löscher W and Friedman A. Structural, molecular, and functional alterations of the blood-brain barrier during epileptogenesis and epilepsy: A cause, consequence, or both? Int J Mol Sci. (2020) 21:591. doi: 10.3390/ijms21020591
11. Han LJP. Modulation of the blood–brain barrier for drug delivery to brain. Pharmaceutics (2021) 13:2024. doi: 10.3390/pharmaceutics13122024
12. Derisfard F, Jafarinezhad Z, Azarpira N, Namavar MR, and Aligholi H. Exosomes obtained from human adipose-derived stem cells alleviate epileptogenesis in the pentylenetetrazol model of epilepsy. Neuroreport. (2025) 36:161–8. doi: 10.1097/WNR.0000000000002133
13. Xiong J, Chi H, Yang G, Zhao S, Zhang J, Tran LJ, et al. Revolutionizing anti-tumor therapy: unleashing the potential of B cell-derived exosomes. Front Immunol. (2023) 14:1188760. doi: 10.3389/fimmu.2023.1188760
14. Gong X, Chi H, Strohmer DF, Teichmann AT, Xia Z, and Wang Q. Exosomes: A potential tool for immunotherapy of ovarian cancer. Front Immunol. (2022) 13:1089410. doi: 10.3389/fimmu.2022.1089410
15. Cheng J, Meng J, Zhu L, and Peng Y. Exosomal noncoding RNAs in Glioma: biological functions and potential clinical applications. Mol Cancer. (2020) 19:66. doi: 10.1186/s12943-020-01189-3
16. Sun T, Song Q, and Liu H. CAF-derived exosome-miR-3124-5p promotes Malignant biological processes in NSCLC via the TOLLIP/TLR4-MyD88-NF-κB pathway. Oncol Res. (2025) 33:133–48. doi: 10.32604/or.2024.054141
17. Xiao L, Hareendran S, and Loh YP. Function of exosomes in neurological disorders and brain tumors. Extracell Vesicles Circ Nucl Acids. (2021) 2:55–79. doi: 10.20517/evcna.2021.04
18. Hastuti S, Idroes R, Imran I, Ramli Y, Abas AH, and Tallei TE. hUMSC vs. hUMSC-Exosome: Which One Is Better for Epilepsy? Pharm (Basel). (2022) 15:1247. doi: 10.3390/ph15101247
19. Wang ZY, Wen ZJ, Xu HM, Zhang Y, and Zhang YF. Exosomal noncoding RNAs in central nervous system diseases: biological functions and potential clinical applications. Front Mol Neurosci. (2022) 15:1004221. doi: 10.3389/fnmol.2022.1004221
20. Ge X, Guo M, Hu T, Li W, Huang S, Yin Z, et al. Increased Microglial Exosomal miR-124-3p Alleviates Neurodegeneration and Improves Cognitive Outcome after rmTBI. Mol Ther. (2020) 28:503–22. doi: 10.1016/j.ymthe.2019.11.017
21. Kuwano N, Kato TA, Mitsuhashi M, Sato-Kasai M, Shimokawa N, Hayakawa K, et al. Neuron-related blood inflammatory markers as an objective evaluation tool for major depressive disorder: An exploratory pilot case-control study. J Affect Disord. (2018) 240:88–98. doi: 10.1016/j.jad.2018.07.040
22. Zhang ZG, Buller B, and Chopp M. Exosomes - beyond stem cells for restorative therapy in stroke and neurological injury. Nat Rev Neurol. (2019) 15:193–203. doi: 10.1038/s41582-018-0126-4
23. Yu H, Wang B, Li Z, Liu K, Chen W, Zhao S, et al. Tβ4-exosome-loaded hemostatic and antibacterial hydrogel to improve vascular regeneration and modulate macrophage polarization for diabetic wound treatment. Mater Today Bio. (2025) 31:101585. doi: 10.1016/j.mtbio.2025.101585
24. Thomi G, Surbek D, Haesler V, Joerger-Messerli M, and Schoeberlein A. Exosomes derived from umbilical cord mesenchymal stem cells reduce microglia-mediated neuroinflammation in perinatal brain injury. Stem Cell Res Ther. (2019) 10:105. doi: 10.1186/s13287-019-1207-z
25. Rodrigues G, Hoshino A, Kenific CM, Matei IR, Steiner L, Freitas D, et al. Tumour exosomal CEMIP protein promotes cancer cell colonization in brain metastasis. Nat Cell Biol. (2019) 21:1403–12. doi: 10.1038/s41556-019-0404-4
26. Kalsariya RA, Kavila D, Shorter S, Negi D, Goodall ICA, Boussios S, et al. Molecular biomarkers of glial activation and injury in epilepsy. Drug Discov Today. (2025) 30:104289. doi: 10.1016/j.drudis.2025.104289
27. Li W, Zhou H, Li X, Hu G, and Wei D. Astrocytic acid-sensing ion channel 1a contributes to the development of epileptic cognitive impairment. Biomolecules. (2025) 15:142. doi: 10.3390/biom15010142
28. Bedetta M, Pizzo P, and Lia A. The multifaceted role of P2X7R in microglia and astrocytes. Neurochem Res. (2025) 50:239. doi: 10.1007/s11064-025-04502-y
29. Kwon HS and Koh SH. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener. (2020) 9:42. doi: 10.1186/s40035-020-00221-2
30. Xian P, Hei Y, Wang R, Wang T, Yang J, Li J, et al. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics. (2019) 9:5956–75. doi: 10.7150/thno.33872
31. Bellezza I, Giambanco I, Minelli A, and Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. (2018) 1865:721–33. doi: 10.1016/j.bbamcr.2018.02.010
32. Das M, Mayilsamy K, Mohapatra SS, and Mohapatra S. Mesenchymal stem cell therapy for the treatment of traumatic brain injury: progress and prospects. Rev Neurosci. (2019) 30:839–55. doi: 10.1515/revneuro-2019-0002
33. Chen ZP, Zhao X, Wang S, Cai R, Liu Q, Ye H, et al. GABA-dependent microglial elimination of inhibitory synapses underlies neuronal hyperexcitability in epilepsy. Nat Neurosci. (2025) 28:1404–17. doi: 10.1038/s41593-025-01979-2
34. Tang W, Wang A, Liu S, Wen G, Qi H, Gu Y, et al. Calycosin regulates astrocyte reactivity and astrogliosis after spinal cord injury by targeting STAT3 phosphorylation. J Neuroimmunol. (2025) 400:578535. doi: 10.1016/j.jneuroim.2025.578535
35. Janik K, Jin LQ, Kyzy KZ, Kaminski R, Smith GM, and Krynska B. Neural tube defects induce abnormal astrocyte development by activation and epigenetic permissiveness of STAT3. Exp Neurol. (2025) 389:115231. doi: 10.1016/j.expneurol.2025.115231
36. Jin N, Lee J, Park SY, and Han JS. NOTCH1-STAT3 signaling axis regulates astrocytic differentiation of hippocampal neural stem/progenitor cells. Biochem Biophys Res Commun. (2025) 765:151844. doi: 10.1016/j.bbrc.2025.151844
37. Vargas-Sánchez K, Mogilevskaya M, Rodríguez-Pérez J, Rubiano MG, Javela JJ, and González-Reyes RE. Astroglial role in the pathophysiology of status epilepticus: an overview. Oncotarget. (2018) 9:26954–76. doi: 10.18632/oncotarget.25485
38. Escartin C, Galea E, Lakatos A, O’Callaghan JP, Petzold GC, Serrano-Pozo A, et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci. (2021) 24:312–25. doi: 10.1038/s41593-020-00783-4
39. Zhang Y, Li K, Wang X, Ding Y, Ren Z, Fang J, et al. CSE-derived H(2)S inhibits reactive astrocytes proliferation and promotes neural functional recovery after cerebral ischemia/reperfusion injury in mice via inhibition of rhoA/ROCK(2) pathway. ACS Chem Neurosci. (2021) 12:2580–90. doi: 10.1021/acschemneuro.0c00674
40. Cardanho-Ramos C and Morais VA. Mitochondrial biogenesis in neurons: how and where. Int J Mol Sci. (2021) 22:13059. doi: 10.3390/ijms222313059
41. Bierhansl L, Gola L, Narayanan V, Dik A, Meuth SG, Wiendl H, et al. Neuronal mitochondrial calcium uniporter (MCU) deficiency is neuroprotective in hyperexcitability by modulation of metabolic pathways and ROS balance. Mol Neurobiol. (2024) 61:9529–38. doi: 10.1007/s12035-024-04148-x
42. Wu H, Wang Q, Liao Y, and Wang S. MSC-derived exosomes deliver ZBTB4 to mediate transcriptional repression of ITIH3 in astrocytes in spinal cord injury. Brain Res Bull. (2024) 212:110954. doi: 10.1016/j.brainresbull.2024.110954
43. Zhongxin S, Zhijie C, Feng D, Qin GM, Ya Z, Jinxin H, et al. Astragaloside IV increases PDHA1 in mesenchymal stem cell exosomes to treat myocardial infarction. Sci Rep. (2025) 15:25461. doi: 10.1038/s41598-025-08628-5
44. Guo XF, Gu SS, Wang J, Sun H, Zhang YJ, Yu PF, et al. Protective effect of mesenchymal stem cell-derived exosomal treatment of hippocampal neurons against oxygen-glucose deprivation/reperfusion-induced injury. World J Emerg Med. (2022) 13:46–53. doi: 10.5847/wjem.j.1920-8642.2022.015
45. Long Q, Upadhya D, Hattiangady B, Kim DK, An SY, Shuai B, et al. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc Natl Acad Sci U.S.A. (2017) 114:E3536–e3545. doi: 10.1073/pnas.1703920114
46. Bogdanović RM, Syvänen S, Michler C, Russmann V, Eriksson J, Windhorst AD, et al. (R)-[11C]PK11195 brain uptake as a biomarker of inflammation and antiepileptic drug resistance: evaluation in a rat epilepsy model. Neuropharmacology. (2014) 85:104–12. doi: 10.1016/j.neuropharm.2014.05.002
47. Hanak TJ, Libbey JE, Doty DJ, Sim JT, DePaula-Silva AB, and Fujinami RS. Positive modulation of mGluR5 attenuates seizures and reduces TNF-α(+) macrophages and microglia in the brain in a murine model of virus-induced temporal lobe epilepsy. Exp Neurol. (2019) 311:194–204. doi: 10.1016/j.expneurol.2018.10.006
48. Jin P, Deng S, Tian M, Lenahan C, Wei P, Wang Y, et al. INT - 777 prevents cognitive impairment by activating Takeda G protein-coupled receptor 5 (TGR5) and attenuating neuroinflammation via cAMP/PKA/CREB signaling axis in a rat model of sepsis. Exp Neurol. (2021) 335:113504. doi: 10.1016/j.expneurol.2020.113504
49. Sarlo GL and Holton KF. Brain concentrations of glutamate and GABA in human epilepsy: A review. Seizure. (2021) 91:213–27. doi: 10.1016/j.seizure.2021.06.028
50. Chen C, Lan Z, Tang X, Chen W, Zhou X, Su H, et al. Human-derived induced GABAergic progenitor cells improve cognitive function in mice and inhibit astrocyte activation with anti-inflammatory exosomes. Ann Neurol. (2024) 96:488–507. doi: 10.1002/ana.27001
51. Gitaí DLG, Dos Santos YDR, Upadhya R, Kodali M, Madhu LN, and Shetty AK. Extracellular Vesicles in the Forebrain Display Reduced miR-346 and miR-331-3p in a Rat Model of Chronic Temporal Lobe Epilepsy. Mol Neurobiol. (2020) 57:1674–87. doi: 10.1007/s12035-019-01797-1
52. Wang C, Cheng F, Han Z, Yan B, Liao P, Yin Z, et al. Human-induced pluripotent stem cell-derived neural stem cell exosomes improve blood-brain barrier function after intracerebral hemorrhage by activating astrocytes via PI3K/AKT/MCP-1 axis. Neural Regener Res. (2025) 20:518–32. doi: 10.4103/NRR.NRR-D-23-01889
53. Akbari-Gharalari N, Aliyari-Serej Z, Ghahremani-Nasab M, Zangbar HS, Yahyavi Y, Nezhadshahmohammad F, et al. Cerebrolysin-loaded platelet-rich plasma exosomes: Restoring immune homeostasis via TNF-α/IL-10 modulation and apoptosis targeting for spinal cord injury repair. J Spinal Cord Med. (2025) 2025:1–13. doi: 10.1080/10790268.2025.2503053
54. Ruffolo G, Alfano V, Romagnolo A, Zimmer T, Mills JD, Cifelli P, et al. GABA(A) receptor function is enhanced by Interleukin-10 in human epileptogenic gangliogliomas and its effect is counteracted by Interleukin-1β. Sci Rep. (2022) 12:17956. doi: 10.1038/s41598-022-22806-9
55. Suryanarayanan A, Carter JM, Landin JD, Morrow AL, Werner DF, and Spigelman I. Role of interleukin-10 (IL - 10) in regulation of GABAergic transmission and acute response to ethanol. Neuropharmacology. (2016) 107:181–8. doi: 10.1016/j.neuropharm.2016.03.027
56. Rafiei M, Shojaei A, and Chau Y. Machine learning-assisted design of immunomodulatory lipid nanoparticles for delivery of mRNA to repolarize hyperactivated microglia. Drug Delivery. (2025) 32:2465909. doi: 10.1080/10717544.2025.2465909
57. Li HR, Liu Q, Zhu CL, Sun XY, Sun CY, Yu CM, et al. β-Nicotinamide mononucleotide activates NAD+/SIRT1 pathway and attenuates inflammatory and oxidative responses in the hippocampus regions of septic mice. Redox Biol. (2023) 63:102745. doi: 10.1016/j.redox.2023.102745
58. Elias A, Padinjakara N, and Lautenschlager NT. Effects of intermittent fasting on cognitive health and Alzheimer’s disease. Nutr Rev. (2023) 81:1225–33. doi: 10.1093/nutrit/nuad021
59. Ma Z, Zhang W, Wang C, Su Y, Yi C, and Niu J. A new acquaintance of oligodendrocyte precursor cells in the central nervous system. Neurosci Bull. (2024) 40:1573–89. doi: 10.1007/s12264-024-01261-8
60. Kuhn S, Gritti L, Crooks D, and Dombrowski Y. Oligodendrocytes in development, myelin generation and beyond. Cells. (2019) 8:1424. doi: 10.3390/cells8111424
61. de Curtis M, Garbelli R, and Uva L. A hypothesis for the role of axon demyelination in seizure generation. Epilepsia. (2021) 62:583–95. doi: 10.1111/epi.16824
62. Pusic KM, Kraig RP, and Pusic AD. IFNγ-stimulated dendritic cell extracellular vesicles can be nasally administered to the brain and enter oligodendrocytes. PloS One. (2021) 16:e0255778. doi: 10.1371/journal.pone.0255778
63. Cao Z, Wu Y, Yu L, Zou L, Yang L, Lin S, et al. Exosomal miR-335 derived from mature dendritic cells enhanced mesenchymal stem cell-mediated bone regeneration of bone defects in athymic rats. Mol Med. (2021) 27:20. doi: 10.1186/s10020-021-00268-5
64. Liu H, Zhang Y, Yuan J, Gao W, Zhong X, Yao K, et al. Dendritic cell−derived exosomal miR−494−3p promotes angiogenesis following myocardial infarction. Int J Mol Med. (2021) 47:315–25. doi: 10.3892/ijmm.2020.4776
65. Zhang H, Xie XH, Xu SX, Wang C, Sun S, Song X, et al. Oligodendrocyte-derived exosomes-containing SIRT2 ameliorates depressive-like behaviors and restores hippocampal neurogenesis and synaptic plasticity via the AKT/GSK-3β pathway in depressed mice. CNS Neurosci Ther. (2024) 30:e14661. doi: 10.1111/cns.14661
66. Li T, Tan X, Li S, Al-Nusaif M, and Le W. Role of glia-derived extracellular vesicles in neurodegenerative diseases. Front Aging Neurosci. (2021) 13:765395. doi: 10.3389/fnagi.2021.765395
67. Pusic AD, Pusic KM, Clayton BL, and Kraig RP. IFNγ-stimulated dendritic cell exosomes as a potential therapeutic for remyelination. J Neuroimmunol. (2014) 266:12–23. doi: 10.1016/j.jneuroim.2013.10.014
68. Steriade C, Titulaer MJ, Vezzani A, Sander JW, and Thijs RD. The association between systemic autoimmune disorders and epilepsy and its clinical implications. Brain. (2021) 144:372–90. doi: 10.1093/brain/awaa362
69. Palomino-Antolin A, Narros-Fernández P, Farré-Alins V, Sevilla-Montero J, Decouty-Pérez C, Lopez-Rodriguez AB, et al. Time-dependent dual effect of NLRP3 inflammasome in brain ischaemia. Br J Pharmacol. (2022) 179:1395–410. doi: 10.1111/bph.15732
70. Mishra B, Bachu M, Yuan R, Wingert C, Chaudhary V, Brauner C, et al. IL - 10 targets IRF transcription factors to suppress IFN and inflammatory response genes by epigenetic mechanisms. Nat Immunol. (2025) 26:748–59. doi: 10.1038/s41590-025-02137-3
71. Lindenbergh MFS and Stoorvogel W. Antigen presentation by extracellular vesicles from professional antigen-presenting cells. Annu Rev Immunol. (2018) 36:435–59. doi: 10.1146/annurev-immunol-041015-055700
72. Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. (2018) 560:382–6. doi: 10.1038/s41586-018-0392-8
73. Taha EA, Ono K, and Eguchi T. Roles of extracellular HSPs as biomarkers in immune surveillance and immune evasion. Int J Mol Sci. (2019) 20:4588. doi: 10.3390/ijms20184588
74. Mathivanan S, Fahner CJ, Reid GE, and Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. (2012) 40:D1241–1244. doi: 10.1093/nar/gkr828
75. Yu Y, Hou K, Ji T, Wang X, Liu Y, Zheng Y, et al. The role of exosomal microRNAs in central nervous system diseases. Mol Cell Biochem. (2021) 476:2111–24. doi: 10.1007/s11010-021-04053-0
76. Feng YB, Lin YT, Han YX, Pang YJ, Xu JJ, Xue Y, et al. 2R,4R-APDC, a metabotropic glutamate receptor agonist, reduced neuronal apoptosis by upregulating microRNA-128 in a rat model after seizures. Neurochem Res. (2018) 43:591–9. doi: 10.1007/s11064-017-2453-z
77. Shyam M, Bm O, Srirangan P, N N, and Sabina EP. Targeted miRNA delivery in epilepsy: mechanisms, advances, and therapeutic potential. Mol Biol Rep. (2025) 52:368. doi: 10.1007/s11033-025-10436-z
78. Batool A, Hill TDM, Nguyen NT, Langa E, Diviney M, Mooney C, et al. Altered biogenesis and microRNA content of hippocampal exosomes following experimental status epilepticus. Front Neurosci. (2019) 13:1404. doi: 10.3389/fnins.2019.01404
79. Löscher W. Epilepsy and alterations of the blood–brain barrier: cause or consequence of epileptic seizures or both? In: Physiology, pharmacology and Pathology of the Blood-Brain Barrier. Springer (2020). p. 331–50. doi: 10.1007/164_2020_406
80. Cukovic D, Bagla S, Ukasik D, Stemmer PM, Jena BP, Naik AR, et al. Exosomes in epilepsy of tuberous sclerosis complex: Carriers of pro-inflammatory microRNAs. Noncoding RNA (2021) 7:40. doi: 10.3390/ncrna7030040
81. Ding W, Zhao Z, Zheng Y, Wang R, Zhang Z, Zhang Z, et al. Exposure to short-chain chlorinated paraffins induces astrocyte activation via JAK2/STAT3 signaling pathway. Ecotoxicol Environ Saf. (2022) 248:114268. doi: 10.1016/j.ecoenv.2022.114268
82. Abjean L, Ben Haim L, Riquelme-Perez M, Gipchtein P, Derbois C, Palomares MA, et al. Reactive astrocytes promote proteostasis in Huntington’s disease through the JAK2-STAT3 pathway. Brain. (2023) 146:149–66. doi: 10.1093/brain/awac068
83. Yang Z, Liang Z, Rao J, Xie H, Zhou M, Xu X, et al. Hypoxic-preconditioned mesenchymal stem cell-derived small extracellular vesicles promote the recovery of spinal cord injury by affecting the phenotype of astrocytes through the miR-21/JAK2/STAT3 pathway. CNS Neurosci Ther. (2024) 30:e14428. doi: 10.1111/cns.14428
84. Li Y, Wu H, Jiang X, Dong Y, Zheng J, and Gao J. New idea to promote the clinical applications of stem cells or their extracellular vesicles in central nervous system disorders: Combining with intranasal delivery. Acta Pharm Sin B (2022) 12:3215–32. doi: 10.1016/j.apsb.2022.04.001
85. Xin H, Katakowski M, Wang F, Qian J-Y, Liu XS, Ali MM, et al. MicroRNA-17–92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke (2017) 48:747–53. doi: 10.1161/STROKEAHA.116.015204
86. Liang Y, Xu X, Li X, Xiong J, Li B, Duan L, et al. Chondrocyte-targeted microRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl Mater Interfaces. (2020) 12:36938–47. doi: 10.1021/acsami.0c10458
87. Li Y, Liu Z, Song Y, Pan JJ, Jiang Y, Shi X, et al. M2 microglia-derived extracellular vesicles promote white matter repair and functional recovery via miR-23a-5p after cerebral ischemia in mice. Theranostics. (2022) 12:3553–73. doi: 10.7150/thno.68895
88. Janson MT and Bainbridge JL. Continuing burden of refractory epilepsy. Ann Pharmacother. (2021) 55:406–8. doi: 10.1177/1060028020948056
89. Wang Y and Chen Z. An update for epilepsy research and antiepileptic drug development: Toward precise circuit therapy. Pharmacol Ther (2019) 201:77–93. doi: 10.1016/j.pharmthera.2019.05.010
90. Saint-Pol J, Gosselet F, Duban-Deweer S, Pottiez G, and Karamanos Y. Targeting and crossing the blood-brain barrier with extracellular vesicles. Cells (2020) 9:851. doi: 10.3390/cells9040851
91. Liang Y, Iqbal Z, Lu J, Wang J, Zhang H, Chen X, et al. Cell-derived nanovesicle-mediated drug delivery to the brain: principles and strategies for vesicle engineering. Mol Ther (2023) 31:1207–24. doi: 10.1016/j.ymthe.2022.10.008
92. Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release (2015) 207:18–30. doi: 10.1016/j.jconrel.2015.03.033
93. Manarin G, Anderson D, e Silva JM, da Silva Coppede J, Roxo-Junior P, Pereira AMS, et al. Curcuma longa L. ameliorates asthma control in children and adolescents: A randomized, double-blind, controlled trial. J Ethnopharmacol (2019) 238:111882. doi: 10.1016/j.jep.2019.111882
94. Anuradha Kalani AK, Pankaj Chaturvedi PC, Kamat P, Maldonado C, Bauer P, Joshua I, et al. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. (2016) 79:360–9. doi: 10.1016/j.biocel.2016.09.002
95. Dad HA, Gu T-W, Zhu A-Q, Huang L-Q, and Peng L-H. Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Mol Ther (2021) 29:13–31. doi: 10.1016/j.ymthe.2020.11.030
96. Batrakova EV and Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release (2015) 219:396–405. doi: 10.1016/j.jconrel.2015.07.030
Keywords: epilepsy, exosomes, blood-brain barrier, neuroinflammation, drug-resistance, drug delivery
Citation: Li B, Zhu Y, Chen L, Cui J and Zhang Z (2025) Exosomes in epilepsy: bridging neuroinflammation, diagnosis, and therapeutic delivery. Front. Immunol. 16:1667122. doi: 10.3389/fimmu.2025.1667122
Received: 16 July 2025; Accepted: 26 August 2025;
Published: 25 September 2025.
Edited by:
Jin Bin, Shandong University, ChinaReviewed by:
Zhijia Xia, Ludwig Maximilian University of Munich, GermanyCopyright © 2025 Li, Zhu, Chen, Cui and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenchang Zhang, MTM4OTM2NDc1OTVAMTYzLmNvbQ==
 Bin Li
Bin Li Yanping Zhu1
Yanping Zhu1