- 1Public Relations Section, Shenzhen Longhua District Central Hospital, Shenzhen, China
- 2Department of Emergency, Shenzhen Longhua District Central Hospital, Shenzhen, China
- 3Chronic Disease Management Department, The First Hospital of Hunan University of Chinese Medicine, Changsha, China
- 4Department of Pharmacy, Shenzhen Longhua District Central Hospital, Affiliated Longhua Hospital of Shenzhen University, Shenzhen, China
- 5Department of Geriatric Medicine, Shenzhen Longhua District Central Hospital, Shenzhen, China
- 6Beijing University of Chinese Medicine Affiliated Shenzhen Hospital (Longgang), Shenzhen, China
Inflammatory bowel disease (IBD), encompassing ulcerative colitis (UC) and Crohn’s disease (CD), represents a group of chronic, relapsing intestinal inflammatory disorders with incompletely understood etiology. Depression and anxiety, as prevalent psychiatric conditions, exhibit rising incidence rates; notably, IBD patients demonstrate heightened susceptibility to these disorders compared to the general population, thereby exacerbating disease burden and increasing risks of adverse clinical outcomes. Emerging evidence reveals shared pathophysiological mechanisms between IBD and depression/anxiety. This review specifically addresses depression and anxiety within the IBD disease context, integrating recent epidemiological evidence and risk factors. Centered on the gut-brain axis framework, we examine mechanistic underpinnings through two interconnected pathways: gut dysbiosis and neuroimmune interactions mediated by inflammatory cytokines and neurotransmitters. Finally, we explore therapeutic interventions for depression and anxiety in IBD based on these mechanistic insights, aiming to advance clinical and public health management strategies.
1 Introduction
Inflammatory Bowel Disease (IBD) is a group of chronic, relapsing inflammatory bowel diseases whose etiology has not been fully clarified; it primarily includes Ulcerative Colitis (UC) and Crohn’s Disease (CD) (1). The characteristic pathological changes of IBD involve impaired intestinal mucosal barrier function, abnormal activation of the immune system, and persistent inflammatory responses, ultimately leading to disturbances in intestinal structure and function (2, 3). UC is characterized by continuous superficial inflammation that primarily involves the large intestine (colon), typically originating from the rectum and extending proximally in a contiguous pattern (4). In contrast, Crohn’s disease (CD) can affect any segment of the gastrointestinal tract, most commonly presenting as a patchy distribution with preferential involvement of the small intestine, particularly the terminal ileum. In some cases, distinguishing between CD and UC may be challenging, resulting in an interim diagnosis of “indeterminate” or “unclassified” colitis and potential delays in treatment (5, 6).
Over the past decade, the incidence and prevalence of IBD have been on an upward trend (7, 8). IBD patients are more susceptible to mental disorders, particularly anxiety and depression, compared to the general population, and these comorbidities exacerbate the disease burden by increasing healthcare resource utilization, hospitalization risks, and readmission rates (9, 10). The bidirectional comorbidity mechanisms between IBD and anxiety/depression are complex, involving genetic correlations between IBD and anxiety/depression, induction of anxiety and depression through hormonal and inflammatory signaling pathways, dysregulation of the gut-microbiota-brain axis, and gut-immune-brain axis cascades—such as systemic inflammation triggered by chronic bowel inflammation breaching the intestinal barrier, which transmits signals to the central nervous system (11, 12). However, mental health issues in IBD, including depression and anxiety symptoms, have become a global public health concern, urgently demanding the development of prevention and management strategies for mental disorders in IBD (13, 14). Therefore, deeply understanding the mechanisms underlying depression and anxiety in IBD based on the gut-microbiota-brain axis theory can help provide comprehensive mental health guidance for primary healthcare policymakers and formulate more holistic and effective diagnostic and therapeutic strategies to improve patients’ overall health.
This study aims to review existing research, analyze the prevalence and risk factors of depression and anxiety in IBD, and explore the mechanisms of their occurrence in IBD patients from two aspects: gut microbiota dysbiosis and neuroimmune interactions mediated by inflammatory cytokines and neurotransmitters, thereby offering new insights and directions for mental health prevention and management in the IBD population.
2 Depression and anxiety in IBD: prevalence and bidirectional association
Depression and anxiety are common comorbidities in IBD. Recent meta-analyses reveal that among 300 IBD participants, 39.0% reported symptoms of common mental disorders, with 35.7% exhibiting anxiety and 15.7% depression (15). Younger age, female sex, tobacco use, longer duration of pre-diagnostic symptoms, higher gastrointestinal symptom-specific anxiety, and stressful life events within the past 12 months were significantly associated with increased likelihood of these psychiatric symptoms (15, 16). During over 150,000 person-years of follow-up, IBD patients demonstrated elevated risks for anxiety (OR 1.4; 95% CI 1.2–1.7) and depression (OR 1.4; 95% CI 1.3–1.6), commencing at least five years before IBD diagnosis and persisting for at least a decade post-diagnosis (anxiety HR 1.3; 95% CI 1.1–1.5; depression HR 1.5; 95% CI 1.4–1.7) (17). A study of 48,799 newly diagnosed IBD cases indicated significantly higher psychiatric incidence versus healthy controls: anxiety IRR 1.17 (1.11–1.24) and depression IRR 1.36 (1.31–1.42) (18). CD patients showed particularly pronounced risks: anxiety HR 1.38 (1.16–1.65) and depression HR 1.36 (1.26–1.47), with peak mental disorder risk occurring within the first year post-IBD diagnosis (18). These findings robustly support the high prevalence of depression and anxiety in IBD.
Studies indicate a complex bidirectional association between depression and IBD. On one hand, individuals with depression exhibit a significantly elevated risk of developing IBD (19–21), and depression exacerbates clinical symptoms in IBD patients, increasing flare-ups, rehospitalizations, and surgical risks (22). Conversely, antidepressant therapy demonstrates selective protective effects against IBD, with differential efficacy across antidepressant classes for CD and UC (20). Genetic research further elucidates potential mechanisms: genome-wide association studies confirm a causal effect of depression on IBD (23), while evidence for reverse causality remains weaker (24). These results suggest that although genetic factors play a crucial role in depression-mediated IBD pathogenesis, the precise mechanisms and objective influencing factors underlying anxiety/depression development in IBD patients require further clarification. In-depth elucidation of these pathophysiological mechanisms will provide novel intervention targets and therapeutic strategies for comprehensive management of psychological comorbidities in IBD.
3 Mechanisms underlying depression and anxiety in IBD
The chronic disease burden and uncertainty in IBD patients may lead to psychological stress, where maladaptive coping mechanisms can increase vulnerability to depression (25). Patients’ perceptions and understanding of their illness—termed illness cognition—also impact mental health, with negative illness perceptions correlating with reduced quality of life and elevated depression levels (26). Personality traits (e.g., neuroticism) and external psychosocial stressors (e.g., pandemics) may modulate mental health outcomes in IBD patients (27). For instance, the COVID-19 pandemic significantly exacerbated psychological distress in this population (28, 29). During active disease phases, IBD patients exhibit higher depression prevalence than those in remission (30). Analyses from the Swiss IBD Cohort Study (SIBDCS) confirm that depressive symptoms strongly correlate with intestinal inflammatory activity and serve as critical predictors of clinical deterioration (31). A 12-month longitudinal study revealed that depressed IBD patients experienced significantly higher rates of: Disease flare-ups, Glucocorticoid usage, Treatment escalation, Hospitalizations or intestinal resections (32). Numerous studies demonstrate escalating depression and anxiety incidence with worsening IBD severity (33). Consequently, IBD patients face heightened susceptibility to these psychiatric comorbidities, mediated by the aforementioned psychological shifts and disease activity factors.
From a pathophysiological perspective, the development of depression and anxiety in IBD is governed by structural and molecular mechanisms (34). Comprehensive understanding of these biological substrates—including neuroimmune interactions, gut-brain axis dysregulation, and inflammatory cascades—is essential for developing targeted pharmacological interventions and precision management strategies.
3.1 Brain structural changes
The dextran sulfate sodium (DSS) and 2,4,6-trinitrobenzene sulfonic acid (TNBS) models exhibit distinct pathophysiological profiles (35): DSS induces Th2-mediated UC-like inflammation with superficial mucosal damage through direct epithelial toxicity (36), whereas TNBS triggers Th1-driven CD-like pathology characterized by transmural inflammation and granuloma formation (37). These divergent peripheral inflammatory patterns are mirrored in the central nervous system, with the prefrontal cortex (PFC) emerging as a critical mediator of IBD-associated neuropsychiatric symptoms (38). Comparative studies reveal model-specific mPFC remodeling: DSS exposure leads to reduced microglial immunoreactivity (Iba1/CD68 downregulation) and myelin protein depletion with concomitant Ranvier node disorganization, while TNBS challenge induces P2Y12 receptor upregulation and microglial hyperactivation (38, 39). Clinically, UC patients demonstrate mPFC hyperstability correlating with depression severity, alongside γ-aminobutyric acid (GABA)/Glx (a combination of glutamate and glutamine) metabolic deficits inversely linked to depressive symptoms (40). These findings collectively implicate mPFC dysfunction in IBD neuropsychiatric comorbidities through microglial priming and myelin disruption (38, 41).
Neuroimaging studies uncover widespread brain structural remodeling characteristics in IBD patients (42). Compared to healthy controls, patients exhibit reduced gray matter volume (GMV) in multiple emotion-related brain regions, including the insula, thalamus, anterior knee of the cingulate gyrus, hippocampal complex, amygdala, and temporal pole, with more pronounced changes in active-stage patients (43, 44). Notably, disease duration negatively correlates with GMV in several brain regions (43). These findings suggest that, beyond the prefrontal cortex, structural alterations in the limbic system (e.g., hippocampus, amygdala) and cingulate gyrus collectively form the neurobiological basis for comorbid anxiety and depression in IBD patients (Figure 1A). These insights provide crucial clues for understanding the mechanisms of IBD and psychiatric symptom comorbidities.
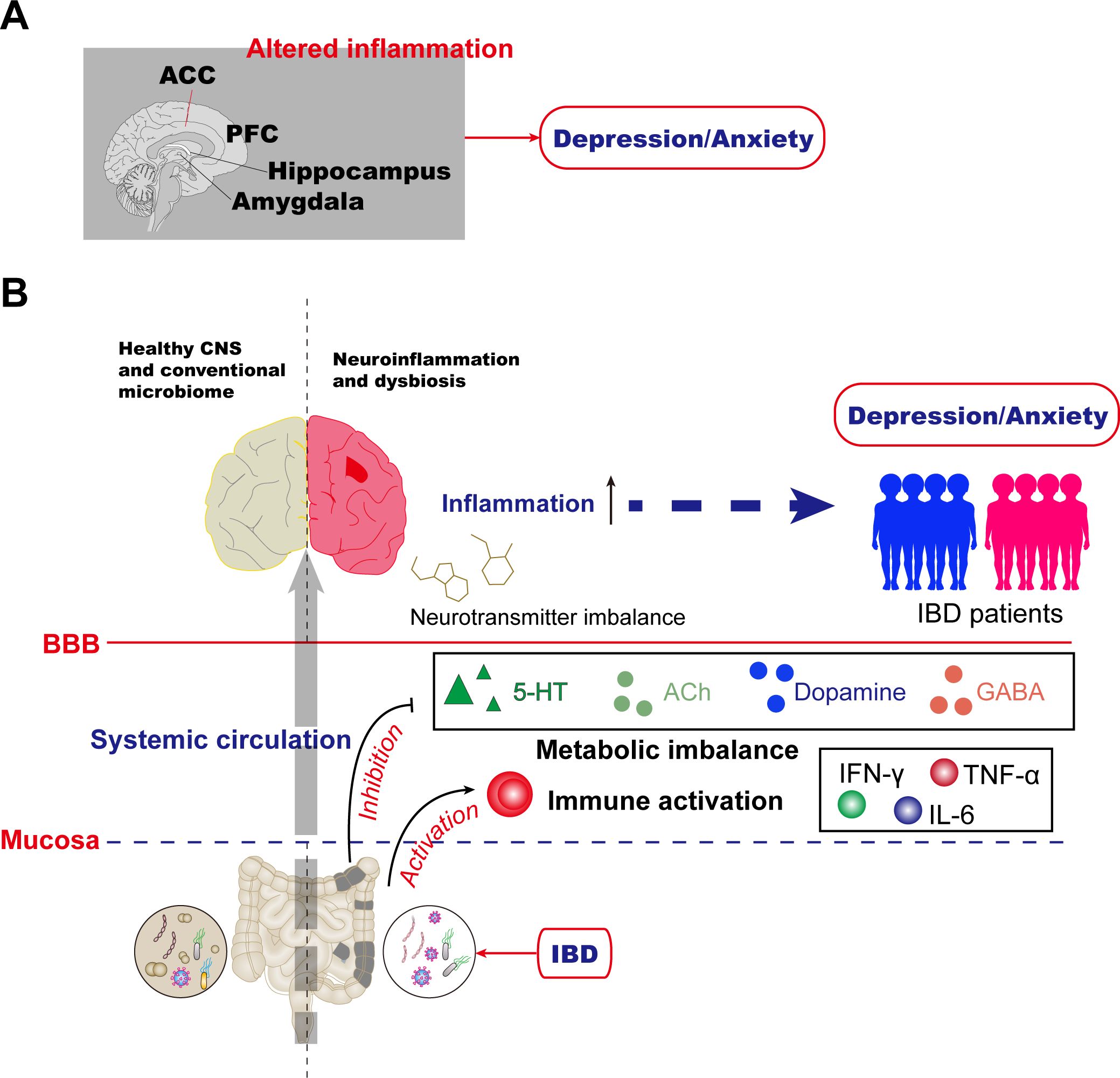
Figure 1. Mechanisms for anxiety and depression in inflammatory bowel disease. (A) Altered inflammation in the PFC, anterior cingulate cortex (ACC), amygdala, hippocampus, and other brain areas has been reported in humans. (B) The gut microbiome is essential for host immune functions and actively regulates mental health through microbiota-derived components and metabolites such as indoxyl sulfate, ACh, and norepinephrine (NE). In IBD patients, aberrations in gut microbiota homeostasis, or dysbiosis, are a common comorbidity that can lead to changes in metabolic imbalance and immune activation, resulting in altered neurotransmitter, and neuroinflammation. Collectively, these changes orchestrate the depression/anxiety observed in gut-brain interactions during IBD disease.
3.2 Increased blood-brain barrier permeability
The BBB serves as a critical regulator of neuroimmune interactions, constituting a dynamic interface composed of brain microvascular endothelial cells, pericytes, neurons, astrocytes, and extracellular matrix (12, 45). Endothelial cells restrict paracellular diffusion of water-soluble substances by expressing tight junction proteins, solute carriers, and receptors, while facilitating selective transport of nutrients and metabolites from blood to the brain (46). Under physiological conditions, the BBB functions as an essential physical barrier limiting interactions between the peripheral immune system and the central nervous system (CNS) (47). In pathological states involving BBB dysfunction, increased permeability may exacerbate neuroinflammatory responses through immune cell infiltration and proinflammatory signaling (48).
Elevated levels of inflammatory cytokines—such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6)—in IBD patients can cross the BBB to directly affect neurotransmitter synthesis, release, and metabolism, thereby disrupting emotional regulation (49, 50). The chronic inflammatory state in IBD patients promotes the translocation of peripheral proinflammatory cytokines (TNF-α, IL-1β, IL-6) across the BBB, activating microglia and astrocytes in the brain (51). This leads to neuroinflammation and impairs synaptic plasticity (particularly in the hippocampus, a region crucial for emotional regulation), further aggravating depressive symptoms.
3.3 Alterations in gut microbiota and metabolites
Gut microbiota play a pivotal role in regulating the gut-brain axis (52, 53). IBD patients typically exhibit dysbiosis characterized by reduced microbial diversity, decreased beneficial bacteria, and increased harmful bacteria (54). Activation of microglia by gut-derived inflammatory signals could contribute to emotional dysregulation, a pathway supported by preclinical models of neuroinflammation (55, 56). Specific alterations (e.g., Bacteroides, Faecalibacterium prausnitzii, Prevotella spp. reduction (57) or Proteobacteria (58, 59), Campylobacter concisus (60) increase) promote the release of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) (61–63), activating microglia (immune cells in the brain) and thereby disrupting emotional regulation (12, 64–66). A meta-analysis of 16S rRNA sequencing data from 1,200 IBD patients, which explicitly demonstrates dysbiosis patterns including reduced microbial diversity and depletion of beneficial genera (e.g., Faecalibacterium) in human IBD cohorts (54). Roseburia genus might harbor protective function against CD onset (57) and established IBD (67).
The gut microbiota plays a pivotal role in synthesizing neurotransmitters essential for mood regulation, such as serotonin (5-HT), acetylcholine (ACh), dopamine (DA), and gamma-aminobutyric acid (GABA) (68–70). Microbial dysbiosis can significantly impair neurotransmitter production or disrupt their metabolic pathways (71–73), thereby directly altering emotional states (Figure 1B). Notably, 5-HT secreted by enteroendocrine cells engages in bidirectional communication with specific gut bacteria, including Turicibacter sanguinis, which possesses serotonin uptake mechanisms critical for both microbial colonization and host physiological function (71). Beyond direct neurotransmitter synthesis, the gut microbiota modulates mood through the tryptophan metabolic pathway (74). As the primary precursor for 5-HT production, tryptophan metabolism is particularly vulnerable to microbial imbalance. Dysbiosis not only diminishes 5-HT synthesis but also promotes the accumulation of neurotoxic metabolites such as kynurenine (75). This phenomenon is clinically evident in IBD patients, where intestinal inflammation triggers excessive activation of the kynurenine (KYN) pathway, leading to elevated neurotoxic metabolites that significantly contribute to depressive symptomatology (76–78).
Short-chain fatty acids (SCFAs)—produced by microbial fermentation of dietary fiber—exert multifaceted benefits, including immune modulation and maintenance of gut barrier integrity (79). Reduced SCFA production in IBD patients compromises gut and brain health (80). Notably, butyrate and propionate possess anti-inflammatory properties and regulate microglial function; their deficiency potentiates neuroinflammation (81).
3.4 Intestinal inflammatory responses
The neural regulation of the gastrointestinal tract involves a complex multi-tiered nervous system (82). The gut is innervated by the CNS, autonomic nervous system (ANS), and enteric nervous system (ENS) (83, 84). The ENS—comprising the myenteric plexus and submucosal plexus—exhibits autonomous regulatory capacity and operates with relative independence from the CNS (85). The vagus nerve, a key component of the parasympathetic nervous system, plays a pivotal role in gastrointestinal modulation (86).
During IBD pathogenesis, intestinal inflammation activates the ENS (87). Inflammatory signals are transmitted to the brain via the vagus nerve, directly disrupting the synthesis and release of neurotransmitters (e.g., serotonin, dopamine) (88). These signals first reach the nucleus tractus solitarius (NTS) in the brainstem, then propagate to emotional regulatory centers such as the hypothalamus and amygdala (89). Chronic inflammatory stimulation amplifies pro-inflammatory signaling, impairing emotional regulation and inducing or exacerbating depression-like behaviors (90).
Increased intestinal permeability in IBD patients facilitates the translocation of lipopolysaccharide (LPS, an endotoxin from Gram-negative bacteria) into the bloodstream (50). This activates the peripheral immune system, triggering the release of pro-inflammatory cytokines (TNF-α, IL-6). These cytokines bind to receptors expressed on vagal afferents, altering central neurotransmitter release and behavior (33, 91–93). Studies indicate that administering pro-inflammatory cytokines to healthy volunteers or animals induces depression through this mechanism. Conversely, cytokine antagonists—such as anti-TNF therapy in IBD—alleviate disease-associated anxiety and depressive behaviors (94).
3.5 Impaired anti-inflammatory function of the vagus nerve
As a core conduit of the gut-brain axis, the vagus nerve exerts bidirectional regulatory effects in IBD-depression comorbidity (95). Its afferent fibers transduce intestinal inflammatory signals into neuroelectrochemical signals transmitted to the CNS, while its efferent fibers activate the cholinergic anti-inflammatory pathway (CAP) (96). CAP mediates the release of ACh, which binds to the α7 nicotinic acetylcholine receptor (α7nAChR) on immune cells, potently suppressing the release of pro-inflammatory cytokines such as TNF-α and establishing a neuro-immune negative feedback loop (97).
Clinical studies reveal that IBD patients commonly exhibit reduced vagal tone, impairing this anti-inflammatory function (98). This deficiency not only exacerbates intestinal inflammation but also induces depressive symptoms by disrupting hippocampal neuroplasticity (99).
3.6 HPA axis dysregulation
The hypothalamic-pituitary-adrenal (HPA) axis is a complex neuroendocrine system that maintains cortisol (CORT) homeostasis through negative feedback mechanisms, where cortisol inhibits hypothalamic and pituitary activity to reduce adrenocorticotropic hormone (ACTH) secretion, thereby regulating its own synthesis and release (100). This balanced system enables the body to adapt to various internal and external environmental changes, ensuring physiological stability. In DSS-induced colitis mice, HPA axis activation was observed to enhance pathogen clearance during the acute phase while inducing persistent inflammation during remission (101). Stress-induced hyperactivity of the HPA axis leads to prolonged glucocorticoid elevation, which causes synaptic structural remodeling and disrupts negative feedback regulation - both of which are implicated in depression (102, 103). Notably, anxiety and stress can further exacerbate colitis by activating the HPA axis. In experimental models, dexamethasone (DEX) administration to simulate CORT secretion resulted in increased IL-6/TNF-α expression and significant downregulation of tight junction proteins occludin/ZO-1 (104). TNBS-induced colitis rats exhibited markedly elevated serum ACTH and CORT levels, though electroacupuncture (EA) treatment effectively alleviated both HPA axis hyperactivity and anxiety/depression-like behaviors (105). Collectively, these findings demonstrate intricate connections between the HPA axis, anxiety/depression, and inflammatory bowel diseases, though further research is warranted to elucidate the specific mechanisms underlying HPA axis involvement in IBD patients with comorbid psychiatric symptoms.
3.7 Tryptophan metabolic dysregulation
Recent research by Kennedy et al. confirms significant disruptions in tryptophan metabolism among IBD patients, characterized by an elevated kynurenine/tryptophan ratio. Under chronic inflammation, pro-inflammatory cytokines (e.g., IFN-γ, IL-6) induce indoleamine 2,3-dioxygenase (IDO) expression, redirecting approximately 95% of tryptophan toward the kynurenine pathway (106, 107). This depletes substrates essential for 5-HT synthesis.
The metabolic imbalance exerts dual detrimental effects: it directly reduces levels of neuroprotective brain-derived neurotrophic factor (BDNF), while concurrently enabling kynurenine metabolites to cross the BBB (108). These metabolites activate microglia and trigger neuroinflammation.
Notably, gut microbiota dysbiosis further disrupts tryptophan homeostasis (109), establishing a self-perpetuating vicious cycle of “gut inflammation - dysbiosis - neurotransmitter abnormalities”. This mechanistic framework provides novel insights into the high prevalence of mood disorders in IBD patients.
4 Emerging therapies for depression and anxiety in IBD
4.1 Microbial-gut-brain axis-targeted therapies
The orally administered hydrogel strategy (SP@Rh-gel) developed by Zhejiang University researchers co-delivers Spirulina platensis and rhein, significantly enhancing intestinal drug retention. This system inhibits the NF-κB-Caspase-1 inflammatory pathway, repairs the intestinal barrier, and reduces pro-inflammatory cytokines crossing into the brain. Preclinical studies confirm its dual efficacy in alleviating IBD symptoms and anxiety-depression behaviors by modulating the microbiome-gut-brain axis (MGBA) (110). SP@Rh-gel enhances drug solubility, controlled release, and intestinal retention, thereby improving oral bioavailability. It also rebalances disrupted gut microbiota and maintains intestinal barrier integrity, blocking pro-inflammatory cytokines (e.g., TNF-α, IL-6) and endotoxins (e.g., LPS) from entering the hippocampus via the BBB, thus suppressing neuroinflammation and preserving neural plasticity.
Recent clinical studies highlight specific bacterial strains (e.g., SCFA producers) as key regulators of gut-brain signaling. Restoring microbiota-derived metabolites (e.g., butyrate) enhances vagal neurotransmission and reduces central neuroinflammation (53, 111). German research identified inverse correlations between depression severity and abundances of SCFA-producing genera (e.g., Odoribacter, Anaerostipes) in IBD patients. Targeted supplementation with these bacteria modulates glycosaminoglycan (GAG) metabolic pathways, alleviating fatigue and depressive symptoms (80).
4.2 Neuromodulation technologies
Neuromodulation technologies provide innovative solutions for this clinical challenge by targeting the “gut-brain axis” (112). Among these, invasive vagus nerve stimulation (VNS)—approved by the U.S. FDA for treatment-resistant depression—involves implanting electrodes to directly stimulate cervical vagus nerves. This effectively regulates serotonergic neuronal activity in brain regions such as the locus coeruleus. A 2023 multicenter clinical study reported significant anxiety improvement in 58% of IBD patients receiving VNS, with therapeutic effects sustained over a 6-month follow-up period (113). While VNS offers stable neurotransmitter modulation, its surgical risks require careful evaluation.
In contrast, non-invasive VNS (nVNS) employs transcutaneous electrical stimulation to activate auricular (taVNS) or cervical (tcVNS) vagal branches. This technique delivers dual regulatory effects. Direct modulation of emotional circuits in the central nervous system. Improvement of intestinal inflammation via gut-brain-axis-mediated immunoregulation (114). Such dual neuromodulatory and immunomodulatory properties position nVNS as a potential therapy for IBD with comorbid refractory depression. However, current neuromodulation primarily targets depressive/anxiety symptoms, with limited clinical evidence specific to IBD populations (115).
4.3 Innovations in pharmacotherapy combination strategies
NE and 5-HT serve as critical neuroregulators in mood modulation for IBD patients. Human studies demonstrate significantly reduced colonic NE and 5-HT levels in the inflamed mucosa of both CD and UC patients compared to non-diseased controls (116, 117). The elevation of tissue NE/5-HT levels induced by SSRIs (selective 5-HT reuptake inhibitors) or SNRIs provides a mechanistic explanation for their observed protective effects in CD and UC management (4). Anti-inflammatory/antidepressant combination therapy synergistically alleviates mood symptoms through dual-pathway modulation1: Duloxetine (SNRI) combined with anti-TNF-α agents concurrently blocks peripheral inflammatory cytokines from crossing into the brain while inhibiting central monoamine reuptake, thereby achieving simultaneous intestinal mucosal healing and reduced Hamilton Depression Rating Scale (HAMD) scores (118); concurrently, microbial metabolite formulations such as butyrate sustained-release capsules activate intestinal epithelial FFAR receptors to enhance brain-derived neurotrophic factor (BDNF) expression, with Phase II clinical trials confirming their efficacy in alleviating depressive symptoms and reducing IL-1β levels, as illustrated in Figure 2A.
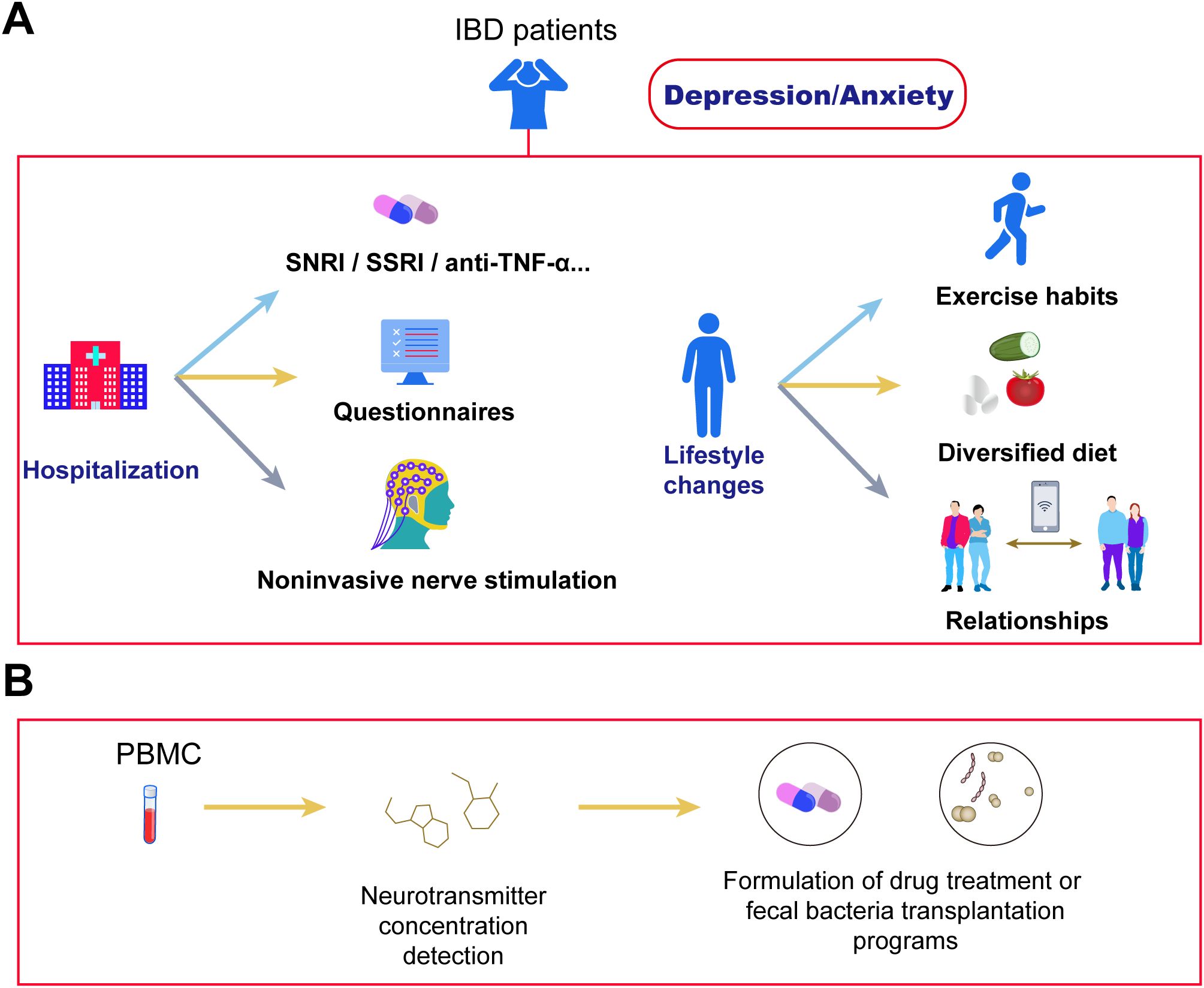
Figure 2. Cutting-edge therapies for depression and anxiety in IBD. (A) IBD patients with depression/anxiety can address symptoms through hospitalization or lifestyle adjustments. During hospitalization, symptoms improve via antidepressant/anxiolytic medications, questionnaire assessments, and non-invasive stimulation therapies. Lifestyle improvements include establishing regular exercise habits, adopting a diversified diet, and expanding social networks. (B) Blood neurotransmitter assays enable precise evaluation of depression/anxiety symptoms in IBD patients, facilitating targeted interventions. Examples include department-specific medication protocols to modulate neurotransmitter concentrations, and microbiota-targeted approaches (e.g., fecal microbiota transplantation or specialized formulations) that selectively modify gut microbiota structure to regulate neurotransmitter balance.
4.4 Complementary therapies
Various complementary therapies can synergize with primary interventions. Research indicates that anti-inflammatory diets and increased physical activity (PA) significantly alleviate anxiety and depressive symptoms in UC patients (119). Multiple studies confirm that higher anxiety/depression scores in IBD patients correlate strongly with sedentary behavior tendencies (120), while moderate-intensity leisure exercise improves psychological states (121); increasing activity levels (e.g., transitioning from moderate to high activity) reduces the CD activity index by an average of 25.3 points (122) (Figure 2A). Additionally, the high prevalence of anxiety and depression in IBD patients may deteriorate healthy eating beliefs into pathological pursuits of “pure” foods (123), with studies revealing a strong negative correlation between anxiety/depression and food-related quality of life (FR-QoL)—individuals with low FR-QoL exhibit reduced fiber intake (124, 125). Although animal experiments demonstrate anti-inflammatory effects from SCFAs produced by gut microbiota metabolism of dietary fiber, in IBD patients during active phases, frozen high-fiber foods may trigger gastrointestinal discomfort, and certain fiber types could exacerbate anxiety symptoms; thus, their efficacy for psychological symptom improvement requires further clinical validation.
Beyond dietary and exercise interventions, adjunct therapies like sleep regulation demonstrate potential value in IBD management despite limited current evidence (112, 115, 118). Monitoring peripheral blood neurotransmitter levels enables a three-tier prevention system: alterations in 5-HT and GABA levels facilitate early screening for psychological disorder risks (126); tracking dopamine dysfunction and acetylcholine/NE balance guides personalized treatments (127); and post-supplementation recovery of neurotransmitter levels (e.g., after SCFAs therapy) or enhanced 5-HT reuptake efficiency following SSRI administration serve as efficacy indicators (128). This framework offers novel insights for integrated management of comorbid psychological disorders in IBD (Figure 2B).
5 Summary and future directions
The review systematically delineates the epidemiological characteristics, pathological mechanisms, and clinical management strategies for anxiety and depression comorbidities in IBD patients. Research demonstrates that the prevalence of psychiatric disorders in IBD patients significantly exceeds that in the general population, with psychiatric symptoms and disease activity forming a vicious cycle. Genetic evidence indicates the first year post-IBD diagnosis represents the peak period for mental disorder risk. Pathological mechanisms involve: gut microbiota dysbiosis impairing neural function via reduced SCFAs and disrupted tryptophan metabolism; proinflammatory cytokines altering central nervous system activity through vagus nerve signaling; and systemic inflammation potentially compromising the blood-brain barrier and exacerbating neurological damage. Interventional approaches reveal that anti-inflammatory diets and exercise therapy significantly alleviate symptoms, while a neurotransmitter-monitored three-tier prevention system offers novel avenues for precision treatment.
This review has several key limitations that need to be addressed. Firstly, the clinical translation of mechanistic research remains inadequate, with targeted therapies such as microbial interventions and vagus nerve stimulation primarily confined to animal studies or small-scale clinical trials, lacking validation through large-scale randomized controlled trials (RCTs). Secondly, therapeutic strategies lack personalization, as multimodal interventions fail to provide stratified recommendations based on IBD subtypes, disease activity levels, or psychological symptom severity. Finally, there is insufficient interdisciplinary integration, with emerging advancements in behavioral psychology and digital health technologies not being adequately incorporated, creating a disconnect from the current “biopsychosocial” comprehensive treatment model. These gaps highlight the need for improved translational research, more rigorous methodologies, and better interdisciplinary collaboration in addressing IBD-related psychiatric comorbidities.
Future studies should focus on elucidating the mechanisms by which specific microbial strains regulate the gut-brain axis, developing microbiota-targeted dietary interventions and novel therapies such as vagus nerve stimulation, and optimizing personalized antidepressant regimens. Clinically, multidisciplinary collaboration should be enhanced to integrate mental health assessments into routine IBD care, thereby simultaneously improving both physical and psychological symptoms. These efforts will advance personalized and precision management of IBD comorbidities.
Author contributions
YQ: Writing – original draft, Formal analysis, Validation, Investigation, Methodology. YC: Investigation, Methodology, Writing – original draft. LL: Investigation, Methodology, Writing – original draft. TW: Conceptualization, Formal analysis, Data curation, Writing – original draft. XC: Conceptualization, Project administration, Data curation, Writing – review & editing. GM: Supervision, Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Guangdong Basic and Applied Basic Research Foundation (No. 2023A1515111116), the Shenzhen Foundation of Science and Technology (No. JCYJ20230807151308018), the Zhanjiang Science and Technology Project (2023B01176), the Shenzhen Health Economics Society Project (Nos. 202521 and 2025211), the Sanming Project of Medicine in Shenzhen (No.SZZYSM202311018), the Longgang District Science and Technology Innovation Bureau, Longgang District Medical and Health Technology Research Support Project (LGKCYLWS2024-22), the Key Clinical Specialties in Shenzhen Longhua District Central Hospital, the Shenzhen Longhua District Science and Technology Innovation Fund Projects (No. 2022045), and the Research Foundation of Shenzhen Longhua District Central Hospital (No. 202203).
Acknowledgments
We sincerely appreciate Dr. Xiaokang Wang’s invaluable assistance throughout the manuscript submission and revision process. We also extend our heartfelt gratitude to all reviewers and editorial board members for their insightful suggestions and constructive feedback.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Stemmer E, Zahavi T, Kellerman M, Sinberger LA, Shrem G, and Salmon-Divon M. Exploring potential biomarkers and therapeutic targets in inflammatory bowel disease: insights from a mega-analysis approach. Front Immunol. (2024) 15:1353402. doi: 10.3389/fimmu.2024.1353402
2. Zhou D, Wu C, Li C, Li M, Li Z, Li J, et al. SLAMF7 regulates goblet cell mucus production and negatively impacts gut homeostasis and commensalism. Gut Microbes. (2025) 17:2527857. doi: 10.1080/19490976.2025.2527857
3. Gao J, Zhao X, Hu S, Huang Z, Hu M, Jin S, et al. Gut microbial DL-endopeptidase alleviates Crohn’s disease via the NOD2 pathway. Cell Host Microbe. (2022) 30:1435–49. doi: 10.1016/j.chom.2022.08.002
4. Neurath MF. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat Immunol. (2019) 20:970–9. doi: 10.1038/s41590-019-0415-0
5. Strober W, Fuss I, and Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. (2007) 117:514–21. doi: 10.1172/JCI30587
6. Kiesslich R, Duckworth CA, Moussata D, Gloeckner A, Lim LG, Goetz M, et al. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut. (2012) 61:1146–53. doi: 10.1136/gutjnl-2011-300695
7. Gros B and Kaplan GG. Ulcerative colitis in adults: A review. JAMA. (2023) 330:951–65. doi: 10.1001/jama.2023.15389
8. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. (2023) 402:203–34.
9. Zhou MS, Zhang WR, Dang Y, Xu F, Xu CY, Wang Z, et al. Plasma inflammation-related proteins associated with anxiety and depression disorders in IBD patients. Sci Rep. (2025) 15:18445. doi: 10.1038/s41598-025-03543-1
10. Roderburg C, Yaqubi K, Konrad M, May P, Luedde T, Kostev K, et al. Association between inflammatory bowel disease and subsequent depression or anxiety disorders - A retrospective cohort study of 31,728 outpatients. J Psychiatr Res. (2024) 169:231–7. doi: 10.1016/j.jpsychires.2023.11.026
11. Gracie DJ, Hamlin PJ, and Ford AC. The influence of the brain-gut axis in inflammatory bowel disease and possible implications for treatment. Lancet Gastroenterol Hepatol. (2019) 4:632–42. doi: 10.1016/S2468-1253(19)30089-5
12. Zhao S, Fu D, Lin Y, Sun X, Wang X, Wu X, et al. The role of the microbiome on immune homeostasis of the host nervous system. Front Immunol. (2025) 16:1609960. doi: 10.3389/fimmu.2025.1609960
13. Dibley L, Khoshaba B, Artom M, Van Loo V, Sweeney L, Syred J, et al. Patient strategies for managing the vicious cycle of fatigue, pain and urgency in inflammatory bowel disease: impact, planning and support. Dig Dis Sci. (2021) 66:3330–42. doi: 10.1007/s10620-020-06698-1
14. Tandon P, Huang V, Feig DS, Saskin R, Maxwell C, Fell DB, et al. Health care utilization of children born to women with and without inflammatory bowel disease in the first 5 years of life: A population-cohort study. Am J Gastroenterol. (2024). doi: 10.14309/ajg.0000000000003280
15. Riggott C, Gracie DJ, and Ford AC. Prevalence and predictors of symptoms of anxiety or depression at diagnosis in patients with inflammatory bowel disease: an inception cohort. Aliment Pharmacol Ther. (2025) 62(8):788–98. doi: 10.1111/apt.70248
16. Xie Y, Yu X, Wu X, Zhang W, Feng Z, Xiao F, et al. Association between the guardians’ educational levels and unintentional injuries in children aged 6–18 in Shenzhen. China BMC Public Health. (2024) 24:2344. doi: 10.1186/s12889-024-19748-4
17. Bisgaard TH, Poulsen G, Allin KH, Keefer L, Ananthakrishnan AN, and Jess T. Longitudinal trajectories of anxiety, depression, and bipolar disorder in inflammatory bowel disease: a population-based cohort study. EClinicalMedicine. (2023) 59:101986. doi: 10.1016/j.eclinm.2023.101986
18. Umar N, King D, Chandan JS, Bhala N, Nirantharakumar K, Adderley N, et al. The association between inflammatory bowel disease and mental ill health: a retrospective cohort study using data from UK primary care. Aliment Pharmacol Ther. (2022) 56:814–22. doi: 10.1111/apt.17110
19. Bisgaard TH, Allin KH, Elmahdi R, and Jess T. The bidirectional risk of inflammatory bowel disease and anxiety or depression: A systematic review and meta-analysis. Gen Hosp Psychiatry. (2023) 83:109–16. doi: 10.1016/j.genhosppsych.2023.05.002
20. Frolkis AD, Vallerand IA, Shaheen A-A, Lowerison MW, Swain MG, Barnabe C, et al. Depression increases the risk of inflammatory bowel disease, which may be mitigated by the use of antidepressants in the treatment of depression. Gut. (2019) 68:1606–12. doi: 10.1136/gutjnl-2018-317182
21. Blackwell J, Saxena S, Petersen I, Hotopf M, Creese H, Bottle A, et al. Depression in individuals who subsequently develop inflammatory bowel disease: a population-based nested case-control study. Gut. (2021) 70:1642–8. doi: 10.1136/gutjnl-2020-322308
22. Ji Y, Li H, Dai G, Zhang X, and Ju W. Systematic review and meta-analysis: Impact of depression on prognosis in inflammatory bowel disease. J Gastroenterol Hepatol. (2024) 39:1476–88. doi: 10.1111/jgh.16568
23. Zhou S, Zi J, Hu Y, Wang X, Cheng G, and Xiong J. Genetic correlation, pleiotropic loci and shared risk genes between major depressive disorder and gastrointestinal tract disorders. J Affect Disord. (2025) 374:84–90. doi: 10.1016/j.jad.2025.01.048
24. Luo J, Xu Z, Noordam R, van Heemst D, and Li-Gao R. Depression and inflammatory bowel disease: A bidirectional two-sample Mendelian randomization study. J Crohns Colitis. (2022) 16:633–42. doi: 10.1093/ecco-jcc/jjab191
25. Jordi SBU, Botte F, Lang BM, Greuter T, Krupka N, Auschra B, et al. Type D personality is associated with depressive symptoms and clinical activity in inflammatory bowel disease. Aliment Pharmacol Ther. (2021) 54:53–67. doi: 10.1111/apt.16365
26. Wynne B, McHugh L, Gao W, Keegan D, Byrne K, Rowan C, et al. Acceptance and commitment therapy reduces psychological stress in patients with inflammatory bowel diseases. Gastroenterology. (2019) 156:935–945.e931. doi: 10.1053/j.gastro.2018.11.030
27. Brzozowski B, Mazur-Bialy A, Pajdo R, Kwiecien S, Bilski J, Zwolinska-Wcislo M, et al. Mechanisms by which stress affects the experimental and clinical inflammatory bowel disease (IBD): role of brain-gut axis. Curr Neuropharmacol. (2016) 14:892–900. doi: 10.2174/1570159X14666160404124127
28. Wang H, Tu L, Li Y, Bai T, Zou K, Xiao F, et al. The symptoms and medications of patients with inflammatory bowel disease in Hubei province after COVID-19 epidemic. J Immunol Res. (2020) 2020:2847316. doi: 10.1155/2020/2847316
29. Shaffer SR. How did COVID-19 affect mental health and access to care in persons with inflammatory bowel disease. J Can Assoc Gastroenterol. (2024) 7:115–20. doi: 10.1093/jcag/gwad033
30. Kim YJ, Lee SG, Lee JS, Choi YJ, and Son CG. Comparative characteristics of fatigue in irritable bowel syndrome and inflammatory bowel disease: A systematic review and meta-analysis. J Psychosom Res. (2024) 177:111589. doi: 10.1016/j.jpsychores.2024.111589
31. Jordi SBU, Lang BM, Auschra B, von Känel R, Biedermann L, Greuter T, et al. Depressive symptoms predict clinical recurrence of inflammatory bowel disease. Inflammation Bowel Dis. (2022) 28:560–71. doi: 10.1093/ibd/izab136
32. Schreiner P, Rossel J-B, Biedermann L, Valko PO, Baumann CR, Greuter T, et al. Fatigue in inflammatory bowel disease and its impact on daily activities. Aliment Pharmacol Ther. (2021) 53:138–49. doi: 10.1111/apt.16145
33. Tiles-Sar N, Neuser J, de Sordi D, Baltes A, Preiss JC, Moser G, et al. Psychological interventions for treatment of inflammatory bowel disease. Cochrane Database Syst Rev. (2025) 4:CD006913.
34. Wang J, Liu G, Xu K, Ai K, Huang W, and Zhang J. The role of neurotransmitters in mediating the relationship between brain alterations and depressive symptoms in patients with inflammatory bowel disease. Hum Brain Mapp. (2023) 44:5357–71. doi: 10.1002/hbm.26439
35. Xiong H, Xue G, Zhang Y, Wu S, Zhao Q, Zhao R, et al. Effect of exogenous galectin-9, a natural TIM-3 ligand, on the severity of TNBS- and DSS-induced colitis in mice. Int Immunopharmacol. (2023) 115:109645. doi: 10.1016/j.intimp.2022.109645
36. Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. (2010) 59:1192–9. doi: 10.1136/gut.2009.197822
37. Chen G, Ran X, Li B, Li Y, He D, Huang B, et al. Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS-induced inflammatory bowel disease mice model. EBioMedicine. (2018) 30:317–25. doi: 10.1016/j.ebiom.2018.03.030
38. Wang H, Labus JS, Griffin F, Gupta A, Bhatt RR, Sauk JS, et al. Functional brain rewiring and altered cortical stability in ulcerative colitis. Mol Psychiatry. (2022) 27:1792–804. doi: 10.1038/s41380-021-01421-6
39. Li Y, Zhang H, Yang J, Zhan M, Hu X, Liu Y, et al. P2Y12 receptor as a new target for electroacupuncture relieving comorbidity of visceral pain and depression of inflammatory bowel disease. Chin Med. (2021) 16:139. doi: 10.1186/s13020-021-00553-9
40. Takahashi K, Hong L, Kurokawa K, Miyagawa K, Mochida-Saito A, Takeda H, et al. Brexpiprazole prevents colitis-induced depressive-like behavior through myelination in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. (2023) 121:110666. doi: 10.1016/j.pnpbp.2022.110666
41. Filipovic BR and Filipovic BF. Psychiatric comorbidity in the treatment of patients with inflammatory bowel disease. World J Gastroenterol. (2014) 20:3552–63. doi: 10.3748/wjg.v20.i13.3552
42. Chojnacki AK, Navaneetha Krishnan S, Jijon H, Shutt TE, Colarusso P, and McKay DM. Tissue imaging reveals disruption of epithelial mitochondrial networks and loss of mitochondria-associated cytochrome-C in inflamed human and murine colon. Mitochondrion. (2023) 68:44–59. doi: 10.1016/j.mito.2022.10.004
43. Zhang S, Chen F, Wu J, Liu C, Yang G, Piao R, et al. Regional gray matter volume changes in brains of patients with ulcerative colitis. Inflammation Bowel Dis. (2022) 28:599–610. doi: 10.1093/ibd/izab252
44. Goodyear BG, Heidari F, Ingram RJM, Cortese F, Sharifi N, Kaplan GG, et al. Multimodal brain MRI of deep gray matter changes associated with inflammatory bowel disease. Inflammation Bowel Dis. (2023) 29:405–16. doi: 10.1093/ibd/izac089
45. Yang T, Velagapudi R, and Terrando N. Neuroinflammation after surgery: from mechanisms to therapeutic targets. Nat Immunol. (2020) 21:1319–26. doi: 10.1038/s41590-020-00812-1
46. Pérez-López A, Torres-Suárez AI, Martín-Sabroso C, and Aparicio-Blanco J. An overview of in vitro 3D models of the blood-brain barrier as a tool to predict the in vivo permeability of nanomedicines. Adv Drug Delivery Rev. (2023) 196:114816. doi: 10.1016/j.addr.2023.114816
47. Gao Y, Tang S, Liu J, Yang X, Chen H, Li J, et al. The administration of circulating extracellular vesicles modified by anesthesia and surgery induces delirium-like behaviors in aged mice. CNS Neurosci Ther. (2025) 31:e70483. doi: 10.1111/cns.70483
48. Huang X, Wei P, Fang C, Yu M, Yang S, Qiu L, et al. Compromised endothelial Wnt/β-catenin signaling mediates the blood-brain barrier disruption and leads to neuroinflammation in endotoxemia. J Neuroinflamm. (2024) 21:265. doi: 10.1186/s12974-024-03261-x
49. Prossin AR, Zalcman SS, Heitzeg MM, Koch AE, Campbell PL, Phan KL, et al. Dynamic interactions between plasma IL-1 family cytokines and central endogenous opioid neurotransmitter function in humans. Neuropsychopharmacol: Off Publ Am Coll Neuropsychopharmacol. (2015) 40:554–65. doi: 10.1038/npp.2014.202
50. Xu S, Tian Y, Chen X, Zhang W, Mei Y, Zhang Q, et al. Quantitative distribution of theobromine in mouse brain regions and its mitigation of nicotine withdrawal-induced anxiety and depression through neuroinflammation suppression. Drug Metab Dispos. (2025) 53:100084. doi: 10.1016/j.dmd.2025.100084
51. Aliyu M, Saboor-Yaraghi AA, Getso MI, and Zohora FT. Immuno-nutritional therapy in experimental autoimmune encephalomyelitis: a translational pathway to multiple sclerosis management. Inflammopharmacology. (2025). doi: 10.1007/s10787-025-01804-z
52. Zhang R, Ding N, Feng X, and Liao W. The gut microbiome, immune modulation, and cognitive decline: insights on the gut-brain axis. Front Immunol. (2025) 16:1529958. doi: 10.3389/fimmu.2025.1529958
53. Rao J, Qiu P, Zhang Y, and Wang X. Gut microbiota trigger host liver immune responses that affect drug-metabolising enzymes. Front Immunol. (2024) 15:1511229. doi: 10.3389/fimmu.2024.1511229
54. Gonzalez CG, Mills RH, Zhu Q, Sauceda C, Knight R, Dulai PS, et al. Location-specific signatures of Crohn’s disease at a multi-omics scale. Microbiome. (2022) 10:133. doi: 10.1186/s40168-022-01331-x
55. Chen X, Gan Y, Zhang K, Wu Y, Li Y, Lan T, et al. MicroRNA-204-5p Deficiency within the vmPFC Region Contributes to Neuroinflammation and Behavioral Disorders via the JAK2/STAT3 Signaling Pathway in Rats. Adv Sci (Weinh). (2025) 12:e2403080. doi: 10.1002/advs.202403080
56. Fu Q, Qiu R, Yao T, Liu L, Li Y, Li X, et al. Music therapy as a preventive intervention for postpartum depression: modulation of synaptic plasticity, oxidative stress, and inflammation in a mouse model. Transl Psychiatry. (2025) 15:143. doi: 10.1038/s41398-025-03370-y
57. Raygoza Garay JA, Turpin W, Lee S-H, Smith MI, Goethel A, Griffiths AM, et al. Gut microbiome composition is associated with future onset of Crohn’s disease in healthy first-degree relatives. Gastroenterology. (2023) 165:670–81. doi: 10.1053/j.gastro.2023.05.032
58. Rizzatti G, Lopetuso LR, Gibiino G, Binda C, and Gasbarrini A. Proteobacteria: A common factor in human diseases. BioMed Res Int. (2017) 2017:9351507. doi: 10.1155/2017/9351507
59. Shin N-R, Whon TW, and Bae J-W. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. (2015) 33:496–503. doi: 10.1016/j.tibtech.2015.06.011
60. Park J-D, Lee SR, Dhennezel C, Taylor N, Dame A, Kadoki M, et al. Elucidating the role of Campylobacter concisus-derived indole metabolites in gut inflammation and immune modulation. Proc Natl Acad Sci U.S.A. (2025) 122:e2514071122. doi: 10.1073/pnas.2514071122
61. Jarne-Ferrer J, Griñán-Ferré C, Jora B, Codony S, Miró L, Rosell-Cardona C, et al. Soluble epoxide hydrolase inhibition improves Alzheimer’s disease hallmarks: correlation with peripheral inflammation and gut microbiota modulation. Aging Dis. (2025).
62. Fang C, Chen Q, Zheng G, Zhang F, Li Z, Mei J, et al. Cellulose-like chitosan microfibrils facilitate targeted release and enhance the prolonged residence time of quercetin-selenium nanoparticles for Alzheimer’s disease treatment. Int J Pharm. (2024) 670:125129. doi: 10.1016/j.ijpharm.2024.125129
63. Mancilla VJ, Braden-Kuhle PN, Brice KN, Mann AE, Williams MT, Zhang Y, et al. A synthetic formula amino acid diet leads to microbiome dysbiosis, reduced colon length, inflammation, and altered locomotor activity in C57BL/6J mice. Microorganisms. (2023) 11. doi: 10.3390/microorganisms11112694
64. Talley S, Valiauga R, Anderson L, Cannon AR, Choudhry MA, and Campbell EM. DSS-induced inflammation in the colon drives a proinflammatory signature in the brain that is ameliorated by prophylactic treatment with the S100A9 inhibitor paquinimod. J Neuroinflamm. (2021) 18:263. doi: 10.1186/s12974-021-02317-6
65. Zhou L, Wu Q, Jiang L, Rao J, Gao J, Zhao F, et al. Role of the microbiota in inflammation-related related psychiatric disorders. Front Immunol. (2025) 16:1613027. doi: 10.3389/fimmu.2025.1613027
66. Tang A, Jiang H, Li J, Chen Y, Zhang J, Wang D, et al. Gut microbiota links to cognitive impairment in bipolar disorder via modulating synaptic plasticity. BMC Med. (2025) 23:470. doi: 10.1186/s12916-025-04313-6
67. Zhu C, Song K, Shen Z, Quan Y, Tan B, Luo W, et al. Roseburia intestinalis inhibits interleukin−17 excretion and promotes regulatory T cells differentiation in colitis. Mol Med Rep. (2018) 17:7567–74. doi: 10.3892/mmr.2018.8833
68. Matta T, Bishnoi M, Gupta S, Aggarwal A, Chopra K, and Kondepudi KK. GABA-producing levilactobacillus brevis LAB6 mitigates pentylenetetrazol-induced gut-brain dysregulation in SD rats. J Neurochemistry. (2025) 169:e70141. doi: 10.1111/jnc.70141
69. Łuc M, Misiak B, Pawłowski M, Stańczykiewicz B, Zabłocka A, Szcześniak D, et al. Gut microbiota in dementia. Critical review of novel findings and their potential application. Prog Neuropsychopharmacol Biol Psychiatry. (2021) 104:110039. doi: 10.1016/j.pnpbp.2020.110039
70. Wang X, Hu X, Ye C, Zhao J, Tan SC, Zhou L, et al. Astragalus Polysaccharide Enhances Voriconazole Metabolism under Inflammatory Conditions through the Gut Microbiota. J Clin Trans Hepatol. (2024) 12:481–95. doi: 10.14218/JCTH.2024.00024
71. Fung TC, Vuong HE, Luna CDG, Pronovost GN, Aleksandrova AA, Riley NG, et al. Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat Microbiol. (2019) 4:2064–73. doi: 10.1038/s41564-019-0540-4
72. Barrett E, Ross RP, O’Toole PW, Fitzgerald GF, and Stanton C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. (2012) 113:411–7. doi: 10.1111/j.1365-2672.2012.05344.x
73. Li X, Jin R, Wang Z, Niu C, Song Z, Liu X, et al. TSP50 deficiency in neural stem cells aggravates colitis in mice by altering intestinal microbiome. NPJ Biofilms Microbiomes. (2025) 11:93. doi: 10.1038/s41522-025-00737-3
74. Cheng S, Zhu Z, Li H, Wang W, Jiang Z, Pan F, et al. Rifaximin ameliorates depression-like behaviour in chronic unpredictable mild stress rats by regulating intestinal microbiota and hippocampal tryptophan metabolism. J Affect Disord. (2023) 329:30–41. doi: 10.1016/j.jad.2023.02.086
75. Trevelline BK and Kohl KD. The gut microbiome influences host diet selection behavior. Proc Natl Acad Sci U.S.A. (2022) 119:e2117537119. doi: 10.1073/pnas.2117537119
76. Sofia MA, Ciorba MA, Meckel K, Lim CK, Guillemin GJ, Weber CR, et al. Tryptophan metabolism through the kynurenine pathway is associated with endoscopic inflammation in ulcerative colitis. Inflammation Bowel Dis. (2018) 24:1471–80. doi: 10.1093/ibd/izy103
77. Guloksuz S, Wichers M, Kenis G, Russel MGVM, Wauters A, Verkerk R, et al. Depressive symptoms in Crohn’s disease: relationship with immune activation and tryptophan availability. PloS One. (2013) 8:e60435. doi: 10.1371/journal.pone.0060435
78. Abautret-Daly Á, Dempsey E, Riestra S, de Francisco-García R, Parra-Blanco A, Rodrigo L, et al. Association between psychological measures with inflammatory anddisease-related markers of inflammatory bowel disease. Int J Psychiatry Clin Pract. (2017) 21:221–30. doi: 10.1080/13651501.2017.1306081
79. Patel P, Jin C, Nookaew I, Robeson M, Malipatlolla DK, Devarakonda S, et al. Oat bran fiber protects against radiation-induced disruption of gut barrier dynamics and mucosal damage. NPJ Biofilms Microbiomes. (2025) 11:128. doi: 10.1038/s41522-025-00759-x
80. Thomann AK, Wüstenberg T, Wirbel J, Knoedler L-L, Thomann PA, Zeller G, et al. Depression and fatigue in active IBD from a microbiome perspective-a Bayesian approach to faecal metagenomics. BMC Med. (2022) 20:366. doi: 10.1186/s12916-022-02550-7
81. Jing Y, Yang D, Bai F, Wang Q, Zhang C, Yan Y, et al. Spinal cord injury-induced gut dysbiosis influences neurological recovery partly through short-chain fatty acids. NPJ Biofilms Microbiomes. (2023) 9:99. doi: 10.1038/s41522-023-00466-5
82. Larsson JW, Olofsson PS, and Sundman E. The innervated gut and critical illness. Curr Opin Crit Care. (2025) 31:198–203. doi: 10.1097/MCC.0000000000001260
83. Jones MP, Dilley JB, Drossman D, and Crowell MD. Brain-gut connections in functional GI disorders: anatomic and physiologic relationships. Neurogastroenterol Motil. (2006) 18:91–103. doi: 10.1111/j.1365-2982.2005.00730.x
84. Hamnett R, Bendrick JL, Saha Z, Marciano JH, Zhao ET, Kaltschmidt JA, et al. Enteric glutamatergic interneurons regulate intestinal motility. Neuron. (2025) 113:1019–1035.e1016. doi: 10.1016/j.neuron.2025.01.014
85. Wisén O and Hellström PM. Gastrointestinal motility in obesity. J Internal Med. (1995) 237:411–8. doi: 10.1111/j.1365-2796.1995.tb01195.x
86. Asker M, Krieger JP, Maric I, Bedel E, Steen J, Börchers S, et al. Vagal oxytocin receptors are necessary for esophageal motility and function. JCI Insight. (2025) 10. doi: 10.1172/jci.insight.190108
87. Rahman AA, Stavely R, Pan W, Ott L, Ohishi K, Ohkura T, et al. Optogenetic activation of cholinergic enteric neurons reduces inflammation in experimental colitis. Cell Mol Gastroenterol Hepatol. (2024) 17:907–21. doi: 10.1016/j.jcmgh.2024.01.012
88. Nijhuis LEJ, Olivier BJ, and de Jonge WJ. Neurogenic regulation of dendritic cells in the intestine. Biochem Pharmacol. (2010) 80:2002–8. doi: 10.1016/j.bcp.2010.06.034
89. Bonaz BL and Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology. (2013) 144:36–49. doi: 10.1053/j.gastro.2012.10.003
90. Baillie S, Saxena S, Jayasooriya N, Bottle A, Petersen I, Blackwell J, et al. Pain and sedative medication use among individuals with inflammatory bowel disease: A nationwide population-based cohort study. Aliment Pharmacol Ther. (2025). doi: 10.1111/apt.70247
91. Eisenberger NI, Inagaki TK, Mashal NM, and Irwin MR. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun. (2010) 24:558–63. doi: 10.1016/j.bbi.2009.12.009
92. Loftis JM, Huckans M, and Morasco BJ. Neuroimmune mechanisms of cytokine-induced depression: current theories and novel treatment strategies. Neurobiol Dis. (2010) 37:519–33. doi: 10.1016/j.nbd.2009.11.015
93. Ghia J-E, Blennerhassett P, Deng Y, Verdu EF, Khan WI, and Collins SM. Reactivation of inflammatory bowel disease in a mouse model of depression. Gastroenterology. (2009) 136:2280–8. doi: 10.1053/j.gastro.2009.02.069
94. Dolovich C, Bernstein CN, Singh H, Nugent Z, Tennakoon A, Shafer LA, et al. Anxiety and depression leads to anti-tumor necrosis factor discontinuation in inflammatory bowel disease. Clin Gastroenterol Hepatol. (2021) 19. doi: 10.1016/j.cgh.2020.07.013
95. Pope BS and Wood SK. Advances in understanding mechanisms and therapeutic targets to treat comorbid depression and cardiovascular disease. Neurosci Biobehav Rev. (2020) 116:337–49. doi: 10.1016/j.neubiorev.2020.06.031
96. Fang YT, Lin YT, Tseng WL, Tseng P, Hua GL, Chao YJ, et al. Neuroimmunomodulation of vagus nerve stimulation and the therapeutic implications. Front Aging Neurosci. (2023) 15:1173987. doi: 10.3389/fnagi.2023.1173987
97. Liu H, Zhang X, Shi P, Yuan J, Jia Q, Pi C, et al. α7 Nicotinic acetylcholine receptor: a key receptor in the cholinergic anti-inflammatory pathway exerting an antidepressant effect. J Neuroinflamm. (2023) 20:84. doi: 10.1186/s12974-023-02768-z
98. Hesampour F, Bernstein CN, and Ghia JE. Brain-gut axis: invasive and noninvasive vagus nerve stimulation, limitations, and potential therapeutic approaches. Inflammation Bowel Dis. (2024) 30:482–95. doi: 10.1093/ibd/izad211
99. Chen B, Jin K, Dong J, Cheng S, Kong L, Hu S, et al. Hypocretin-1/hypocretin receptor 1 regulates neuroplasticity and cognitive function through hippocampal lactate homeostasis in depressed model. Adv Sci (Weinh). (2024) 11:e2405354. doi: 10.1002/advs.202405354
100. Keenan EL and Granstein RD. Proinflammatory cytokines and neuropeptides in psoriasis, depression, and anxiety. Acta Physio (Oxford England). (2025) 241:e70019. doi: 10.1111/apha.70019
101. Liao X, Liu H, Li Y, Zhang W, Dai Q, Wei H, et al. Dual role of α-MSH in colitis progression: mediating neutrophil differentiation via bone marrow. J Inflammation Res. (2025) 18:2011–29. doi: 10.2147/JIR.S503621
102. Cui L, Li S, Wang S, Wu X, Liu Y, Yu W, et al. Major depressive disorder: hypothesis, mechanism, prevention and treatment. Signal Transduct Target Ther. (2024) 9:30. doi: 10.1038/s41392-024-01738-y
103. Keller J, Gomez R, Williams G, Lembke A, Lazzeroni L, Murphy GM, et al. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry. (2016) 22:527–36. doi: 10.1038/mp.2016.120
104. Feng B, Lin L, Li L, Long X, Liu C, Zhao Z, et al. Glucocorticoid induced group 2 innate lymphoid cell overactivation exacerbates experimental colitis. Front Immunol. (2022) 13:863034. doi: 10.3389/fimmu.2022.863034
105. Zhou F, Jiang H, Kong N, Lin J, Zhang F, Mai T, et al. Electroacupuncture attenuated anxiety and depression-like behavior via inhibition of hippocampal inflammatory response and metabolic disorders in TNBS-induced IBD rats. Oxid Med Cell Longev. (2022) 2022:8295580. doi: 10.1155/2022/8295580
106. Dounay AB, Tuttle JB, and Verhoes PR. t challenges and opportunities in the discovery of new therapeutics targeting the kynurenine pathway. J Med Chem. (2015) 58:8762–82. doi: 10.1021/acs.jmedchem.5b00461
107. Schefold JC, Fritschi N, Fusch G, Bahonjic A, Doehner W, von Haehling S, et al. Influence of core body temperature on Tryptophan metabolism, kynurenines, and estimated IDO activity in critically ill patients receiving target temperature management following cardiac arrest. Resuscitation. (2016) 107:107–14. doi: 10.1016/j.resuscitation.2016.07.239
108. Souza LC, Jesse CR, Del Fabbro L, de Gomes MG, Gomes NS, Filho CB, et al. Aging exacerbates cognitive and anxiety alterations induced by an intracerebroventricular injection of amyloid-β(1-42) peptide in mice. Mol Cell Neurosci. (2018) 88:93–106. doi: 10.1016/j.mcn.2018.01.005
109. Wang G, Fan Y, Zhang G, Cai S, Ma Y, Yang L, et al. Microbiota-derived indoles alleviate intestinal inflammation and modulate microbiome by microbial cross-feeding. Microbiome. (2024) 12:59. doi: 10.1186/s40168-024-01750-y
110. Zhong D, Jin K, Wang R, Chen B, Zhang J, Ren C, et al. Microalgae-based hydrogel for inflammatory bowel disease and its associated anxiety and depression. Adv Mater. (2024) 36:e2312275. doi: 10.1002/adma.202312275
111. Wang X, Xu G, Ma H, Deng X, and Ma G. Emerging frontiers in epigenetic-targeted therapeutics for pediatric neuroblastoma. Front Immunol. (2025) 16:1637626. doi: 10.3389/fimmu.2025.1637626
112. Vergallito A, Gallucci A, Pisoni A, Punzi M, Caselli G, Ruggiero GM, et al. Effectiveness of noninvasive brain stimulation in the treatment of anxiety disorders: a meta-analysis of sham or behaviour-controlled studies. J Psychiatry Neurosci. (2021) 46:E592–614. doi: 10.1503/jpn.210050
113. Fornaro R, Actis GC, Caviglia GP, Pitoni D, and Ribaldone DG. Inflammatory bowel disease: role of vagus nerve stimulation. J Clin Med. (2022) 11. doi: 10.3390/jcm11195690
114. Atalar K, Alim E, Yigman Z, Belen HB, Erten F, Sahin K, et al. Transauricular vagal nerve stimulation suppresses inflammatory responses in the gut and brain in an inflammatory bowel disease model. J Anat. (2025) 246:602–15. doi: 10.1111/joa.14178
115. Razza LB, Palumbo P, Moffa AH, Carvalho AF, Solmi M, Loo CK, et al. A systematic review and meta-analysis on the effects of transcranial direct current stimulation in depressive episodes. Depress Anxiety. (2020) 37:594–608. doi: 10.1002/da.23004
116. Magro F, Vieira-Coelho MA, Fraga S, Serrão MP, Veloso FT, Ribeiro T, et al. Impaired synthesis or cellular storage of norepinephrine, dopamine, and 5-hydroxytryptamine in human inflammatory bowel disease. Dig Dis Sci. (2002) 47:216–24. doi: 10.1023/A:1013256629600
117. Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. (2004) 126:1657–64. doi: 10.1053/j.gastro.2004.03.013
118. Hung Y-Y, Tsai C-Y, Lee C-T, Fu H-C, Chou C-K, Yang Y-C, et al. Targeting TNIP1 as a new therapeutic avenue for major depressive disorder. Brain Behav Immun. (2025) 126:214–24. doi: 10.1016/j.bbi.2025.02.015
119. DuBois KE, Blake CE, Rudisill C, Harrison SE, Wirth MD, and Hébert JR. Ulcerative colitis is associated with diet-related inflammation and physical activity in the IBD partners E-cohort. Inflammation Bowel Dis. (2024) 30:273–80. doi: 10.1093/ibd/izad139
120. Sciberras M, Karmiris K, Nascimento C, Tabone T, Nikolaou P, Theodoropoulou A, et al. Mental health, work presenteeism, and exercise in inflammatory bowel disease. J Crohns Colitis. (2022) 16:1197–201. doi: 10.1093/ecco-jcc/jjac037
121. Sun S, Chen J, Zheng M, Zhou M, Ying X, Shen Y, et al. Impact of exercise on outcomes among Chinese patients with Crohn’s disease: a mixed methods study based on social media and the real world. BMC Gastroenterol. (2024) 24:441. doi: 10.1186/s12876-024-03533-z
122. Griffin AC, Mentch L, Lin F-C, and Chung AE. mHealth physical activity and patient-reported outcomes in patients with inflammatory bowel diseases: cluster analysis. J Med Internet Res. (2024) 26:e48020. doi: 10.2196/48020
123. Saintila J, Carranza-Cubas SP, Serpa-Barrientos A, Carranza Esteban RF, Cunza-Aranzábal DF, and Calizaya-Milla YE. Depression, anxiety, emotional eating, and body mass index among self-reported vegetarians and non-vegetarians: A cross-sectional study in Peruvian adults. Nutrients. (2024) 16. doi: 10.3390/nu16111663
124. Tian M, Li D, Ma C, Feng Y, Hu X, and Chen F. Barley leaf insoluble dietary fiber alleviated dextran sulfate sodium-induced mice colitis by modulating gut microbiota. Nutrients. (2021) 13. doi: 10.3390/nu13030846
125. Ito K, Hosoki H, Kasai Y, Sasaki H, Haraguchi A, Shibata, et al. A cellulose-rich diet disrupts gut homeostasis and leads to anxiety through the gut-brain axis. ACS Pharmacol Transl Sci. (2024) 7:3071–85. doi: 10.1021/acsptsci.4c00270
126. Zivko C, Sagar R, Xydia A, Lopez-Montes A, Mintzer J, Rosenberg PB, et al. iPSC-derived hindbrain organoids to evaluate escitalopram oxalate treatment responses targeting neuropsychiatric symptoms in Alzheimer’s disease. Mol Psychiatry. (2024) 29:3644–52. doi: 10.1038/s41380-024-02629-y
127. Li Q, Zhang T, Sun J, Lu Z, Chen D, Ma Y, et al. Dynamic analysis of protracted withdrawal symptoms, neurotransmitter, cytokine content and psychological status of heroin addicts in Yunnan Province. Behav Brain Res. (2025), 115708. doi: 10.1016/j.bbr.2025.115708
Keywords: depression and anxiety, gut microbiome, inflammatory bowel disease, prevention and treatment, public health management
Citation: Qian Y, Chen Y, Liu L, Wu T, Chen X and Ma G (2025) Depression and anxiety in inflammatory bowel disease: mechanisms and emerging therapeutics targeting the microbiota-gut-brain axis. Front. Immunol. 16:1676160. doi: 10.3389/fimmu.2025.1676160
Received: 30 July 2025; Accepted: 22 October 2025;
Published: 07 November 2025.
Edited by:
Lei Huang, Newcastle University, United KingdomReviewed by:
Pavlos G. Doulidis, University of Veterinary Medicine Vienna, AustriaCopyright © 2025 Qian, Chen, Liu, Wu, Chen and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiehui Chen, eGhjaGVuNjZAMTI2LmNvbQ==; Guiping Ma, Nzg3MTA5MDA4QHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yan Qian1†
Yan Qian1† Yang Chen
Yang Chen Xiehui Chen
Xiehui Chen